User login
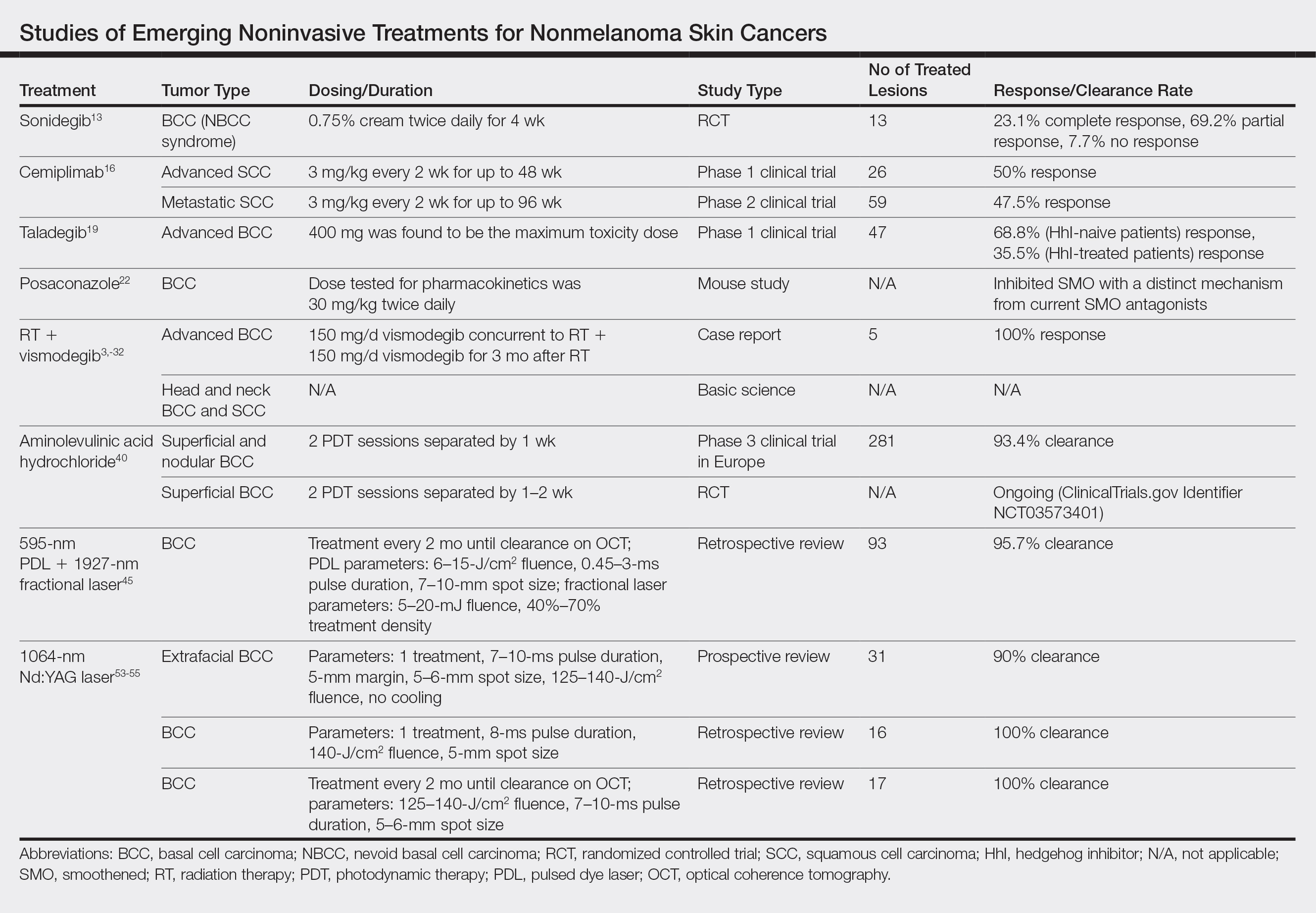
Emerging Noninvasive Treatments of Nonmelanoma Skin Cancers
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.

Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.

- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Practice Points
- As of 2018, there has been an increase in options for the noninvasive management of nonmelanoma skin cancers that should be considered.
- Recently, approved advances in treatment options have included not only advanced basal cell carcinoma but also advanced squamous cell carcinoma such as cemiplimab.
Firm Abdominal Papule
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).
Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).

Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
The Diagnosis: Cutaneous Metastatic Gastric Carcinoma
Cutaneous metastasis of primary gastric carcinoma is a rare occurrence, with the more common metastatic sites being the lymph nodes, liver, and peritoneal cavity. The incidence of visceral neoplasm metastasis to the skin ranges from 0.7% to 9% and is less than 1% for upper digestive tract carcinomas.1 Cutaneous metastases make up 2% of all tumors of the skin and commonly are located near the site of the primary tumor.2 The most common cutaneous metastasis sites for gastric carcinoma include the neck, chest, and head.3 One of the more typical sites of cutaneous metastasis from gastric cancer is the umbilicus (ie, Sister Mary Joseph nodule). Cutaneous metastases from gastric carcinoma commonly present as asymptomatic hyperpigmented nodules.1,3
In our patient, histopathologic sections showed diffuse infiltration of the dermis by atypical polygonal/round cells arranged in cords and small aggregates. Some of the neoplastic cells had signet ring morphology (Figure). Tumor cells demonstrated positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen; they were negative for S-100, MART-1 (melanoma-associated antigen recognized by T cells 1), leukocyte common antigen, gross cystic disease fluid protein 15, estrogen and progesterone receptor, and HER2/neu (human epidermal growth factor receptor 2).

Our patient's presentation was rare in that she developed an asymptomatic erythematous papule on the skin of the abdomen. However, her history of stage IIIB gastric adenocarcinoma in conjunction with the clinical picture and microscopic findings were most consistent with metastatic carcinoma of gastrointestinal origin. The histologic hallmarks of cutaneous metastatic gastric carcinoma include aggregates of neoplastic cells arranged in cords, sometimes forming glands, embedded in a fibrous stroma. Tumor cells may demonstrate signet ring morphology. These unique histologic findings, as well as positive immunostaining for CDX2, villin, CAM 5.2, and epithelial membrane antigen, rule out other potential diagnoses for an asymptomatic solitary papule.
Dermatofibrosarcoma protuberans presents as an asymptomatic, slow-growing, indurated papule or plaque that develops into a red or brownish nodule. Histologically, dermatofibrosarcoma protuberans is characterized by spindled cells, few mitotic figures, infiltration of the subcutaneous tissue in a honeycomblike pattern, and obliteration of the adnexal structures.4
Cutaneous B-cell lymphoma (CBCL) can present as single or multiple red papules or nodules located on the trunk, face, or extremities. Histologically, CBCL would show a nodular or diffuse infiltrate throughout the dermis, frequently with accentuation in the deep reticular dermis, sparing of the epidermis, and the presence of a grenz zone. The infiltrate in CBCL consists of CD20+, CD19+, and CD79a+ B cells. Identification of a monoclonal B-cell population either by immunohistochemistry or polymerase chain reaction would further support a diagnosis of CBCL.4 These specific histologic findings and the immunohistochemical staining pattern helped rule out CBCL as the diagnosis in our patient.
Amelanotic melanomas present as flesh-colored to light pink papules, making them especially challenging to diagnose clinically. Asymmetrical, poorly circumscribed nests of atypical melanocytes as well as single melanocytes within the epidermis and dermis are seen histologically; mitotic figures are common. Immunohistochemical staining for melanoma includes S-100, human melanoma black 45, MART-1/Melan-A, tyrosinase, and microphthalmia-associated transcription factor 1.4
Neurothekeomas can present as asymptomatic, solitary, flesh-colored papules located on the head, neck, and upper trunk. Histologically, neurothekeomas have a distinct appearance consisting of a well-defined mass composed of variable-sized lobules of spindled and epithelioid cells dispersed in a myxoid stroma within the reticular dermis.4 These specific histologic findings helped rule out neurothekeoma in our patient.
Following the diagnosis of cutaneous metastatic gastric carcinoma in our patient, positron emission tomography and computed tomography of the chest, abdomen, and pelvis were unremarkable for distant disease. Subsequently, the patient underwent surgical excision of the papule with clear margins, followed by a short course of radiation therapy. She currently is under close monitoring but remains in remission with no new cutaneous manifestations of the gastric carcinoma.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
- Erdemir A, Atilganoglu U, Onsun N, et al. Cutaneous metastases from gastric adenocarcinoma. Indian J Dermatol. 2011;56:236-237.
- Junqueira AL, Corbett AM, Oliveira Filho Jd, et al. Cutaneous metastasis from gastrointestinal adenocarcinoma of unknown primary origin. An Bras Dermatol. 2015;90:564-566.
- Cesaretti M, Malerba M, Basso V, et al. Cutaneous metastasis from primary gastric cancer: a case report and review of the literature. Cutis. 2014;93:E9-E13.
- Bolognia J, Jorizzo JL, Schaffer JV. Dermatology. Philadelphia, PA: Elsevier Saunders; 2012.
A 53-year-old woman with a history of melanoma on the right thigh, stage II Hodgkin lymphoma, and stage IIIB gastric adenocarcinoma treated with a distal gastrectomy presented with an asymptomatic but persistent skin lesion on the abdomen of 2 months' duration. The lesion arose spontaneously 6 months prior and had increased in size during that time. Physical examination revealed a 6-mm, solitary, firm, erythematous papule on the skin of the right upper quadrant of the abdomen. The patient was otherwise healthy, and a review of systems did not reveal any abnormalities. A punch biopsy was submitted for histopathologic review.
Angiosarcoma Imitating a Morpheaform Basal Cell Carcinoma
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.
This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.



This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
To the Editor:
Basal cell carcinoma (BCC) is the most common of the nonmelanoma skin cancers and is a highly curable skin growth.1,2 Conversely, angiosarcomas are aggressive vascular tumors of endothelial origin that classically appear as reddish purple patches or plaques that exhibit rapid growth and invasion.3 Sporadic cutaneous angiosarcomas are the most common type of this soft tissue tumor, occurring most often in the head and neck regions in men older than 70 years.4,5 Other types of angiosarcomas include those associated with radiation therapy and chronic lymphedema. Postradiation angiosarcomas have been most frequently reported after treatment of breast cancer and appear as infiltrative plaques over the irradiated area.4,5 Patients with chronic lymphedema, which most commonly is related to axillary lymph node dissection for breast cancer (90% of cases), may develop angiosarcoma presenting as a violaceous indurated plaque.5 Although angiosarcomas most often are seen with these distinct clinical characteristics, especially their violaceous color, they have been shown to mimic a few other skin disorders such as eczema and keratoacanthoma, but a limited number of cases of angiosarcoma mimicking BCC have been reported.1,6,7 We present a case of an elderly man with a unique presentation of a lesion that clinically appeared as a morpheaform BCC but was confirmed to be an angiosarcoma on histopathology.
A 75-year-old man was referred to our dermatology clinic for evaluation of a flesh-colored plaque on the face that initially had developed 2 years prior on the right central malar cheek. Computed tomography of the head and neck 1 year prior, which the patient reported was for workup of the lesion, was found to be negative; however, these medical records were not obtained for confirmation. The lesion had been stable in size and remained flesh colored until 6 months prior to the current presentation when it exhibited a rapid increase in size. An initial biopsy was performed 1 month prior to presentation by an outside dermatology office and had been read as an angiosarcoma.
Physical examination revealed a 6-cm, flesh-colored, indurated, ill-defined plaque distributed on the right malar cheek below the eye and extending to the nasal bridge (Figure 1). There was no cervical or facial lymphadenopathy. The clinical features resembled a morpheaform BCC, and the lesion did not exhibit any reddish or purple color indicating it was of vascular origin. However, due to the prior histopathology report and recent rapid enlargement, a repeat sampling with a larger punch biopsy was performed, which confirmed the diagnosis of angiosarcoma. Histopathology demonstrated multiple atypical vascular channels lined by hyperchromatic cells extending from the upper dermis to the base of the biopsy site (Figure 2). Large, oval, atypical nuclei were present in multiple endothelial cells in the vascular channels, with some forming irregularly contoured and slitlike formations (Figure 3). Immunochemical staining was intensely and uniformly positive for CD31 and CD34, both endothelial markers. Diffuse positive staining with CD31 is considered to have high sensitivity and specificity for the diagnosis of angiosarcoma.4 Other pertinent staining demonstrated 2+ positivity for factor VIII and 1+ positivity for D2-40; CD45, AE1/AE3, S-100, and human herpesvirus 8 were negative, consistent with angiosarcoma. The patient was referred to radiation oncology and otolaryngology at our Multidisciplinary Head and Neck Oncology Center for further investigation of the extent of the disease and discussion of treatment. Computed tomography of the head and neck region at this time showed extensive disease extending into the medial canthal area without metastasis. Due to the extent of disease and facial location, he was not deemed a candidate for surgery. He was treated with 6 weeks of targeted radiation therapy with concurrent chemotherapy. He tolerated this treatment with minimal side effects and was found to be free from clinical disease 1 year after diagnosis. He was followed for 20 months by our Multidisciplinary Oncology Clinic without recurrence of his disease but was then lost to follow-up.



This case illustrates a rare presentation of an angiosarcoma clinically mimicking a BCC, which has been described in a small number of case reports and retrospective reviews. One study of 656 patients diagnosed with BCC based on clinical features revealed that 48 of these lesions were proven to be a BCC-mimicking lesion and only 1 was an angiosarcoma.1 Cutaneous lesions that appear on physical examination to be a highly curable BCC may not induce the same urgency for treatment as an angiosarcoma. Although the clinical presentation may mimic a morpheaform BCC, our case demonstrates that it is imperative to include angiosarcoma in the differential diagnosis and underscores the utility of tissue sampling. Angiosarcoma has a poor overall 5-year survival rate, and patients often are found to have multiple metastatic lesions at diagnosis. However, diagnosis prior to metastasis may improve prognosis.8
Our patient’s angiosarcoma did not exhibit metastasis at the time of diagnosis, and he was able to achieve a favorable outcome. However, the 5-year survival rate is only 40%, and close clinical monitoring after diagnosis is required.8 Including angiosarcoma in the differential diagnosis for our patient, particularly upon lesion appearance 2 years prior, may have resulted in diagnosis antecedent to local invasion, possibly providing more treatment options. Employing a higher index of clinical suspicion for angiosarcoma may lead to decreased mortality in other patients due to increased detection.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Kim HS, Kim TW, Mun JH, et al. Basal cell carcinoma–mimicking lesions in Korean clinical settings. Ann Dermatol. 2014;26:431-436.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Goldsmith LA, Katz S, Gilchrest BA. Fitzpatrick’s Dermatology in General Medicine. New York, NY: McGraw Hill; 2012.
- Dosset LA, Harrington M, Cruse CW, et al. Cutaneous angiosarcoma. Curr Probl Cancer. 2015;39:258-263.
- North PE, Kincannon J. Vascular neoplasms and neoplastic-like proliferations. In: Bolognia J, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2012:1915-1942.
- Kong YL, Chandran NS, Goh SG, et al. Cutaneous angiosarcoma of the scalp mimicking a keratoacanthoma. Dermatol Online J. 2013;19:18566.
- Trinh NQ, Rashed I, Hutchens KA, et al. Unusual clinical presentation of cutaneous angiosarcoma masquerading as eczema: a case report and review of the literature. Case Rep Dermatol Med. 2013;2013:906426.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors. a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
Practice Points
- Angiosarcoma is an aggressive vascular tumor with a poor prognosis.
- Angiosarcomas can arise in the setting of chronic lymphedema or prior radiation therapy or can arise spontaneously.
- Classically, angiosarcoma presents as a violaceous patch or plaque but occasionally can exhibit atypical clinical features. Angiosarcomas should be considered on the differential for any changing plaque on the head or neck.
SCC survival remains poor in epidermolysis bullosa
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
LONDON – Median survival among patients with generalized severe recessive dystrophic epidermolysis bullosa (RDEB-GS) after a first diagnosis of mucocutaneous squamous cell carcinoma (SCC) was 2.4 years in an observational, retrospective study.
The study, conducted at St. Thomas’ Hospital and Great Ormond Street Hospital in London, was a review of all individuals with EB who had developed the skin cancer over a 28-year period, from 1991 to 2019.
A total of 44 subjects were identified who together had 221 primary SCCs. Considering all study subjects, the median age at first diagnosis of SCC was 32.6 years, with a mean of five tumors present. Almost 40% had metastatic tumors, and of the 57% who died during the observation period, 88% of deaths were attributable to the SCC.
“EB-associated SCCs differ from those in the general population,” the study’s investigators wrote in a poster presented at the EB World Congress, organized by the Dystrophic Epidermolysis Bullosa Association (debra). “They affect a younger age group, and there are often multiple primaries,” they added. Furthermore, “they behave aggressively and metastasize early despite being well differentiated.”
Most (31) of the study participants had RDEB-GS and tended to develop their first SCC at a younger age than the group overall, at a median of 29.5 years (compared with 32.6 years for the overall group). The mean number of tumors was 5.8 among those with RDEB-GS, with over half (53.4%) of the SCCs being well differentiated and located on the hands, upper arms, feet, and lower legs. Median survival after a first diagnosis in this group was 2.4 years. The short survival after a first diagnosis of SCC “underscores the poor prognosis in this group,” the researchers wrote.
“As the largest cohort of EB SCC patients with comprehensive data regarding clinical course and management to date, our data reinforce the need for regular clinical surveillance for SCCs in EB patients,” the team concluded. This surveillance should start in adolescence for those with the severe generalized RDEB subtype, they advise, and from the third or fourth decade for other at-risk groups.
These data also highlight “the pressing need for more effective treatments,” the investigators wrote. Most (86.4%) of the SCCs among the patients in the study had been surgically removed by wide local excision, with a few patients undergoing lymph node dissection, radiotherapy, chemotherapy, electrochemotherapy, or receiving targeted cancer therapies such as erlotinib, cetuximab, or cemiplimab.
Surgery may not be an option for many patients, Jemima Mellerio, MD explained in an oral presentation at the meeting. Dr. Mellerio, a consultant dermatologist and chief of St John’s Institute of Dermatology at Guy’s & St. Thomas’ NHS Foundation, London, noted that the location of the tumor was important, as sometimes it was not physically possible to excise it completely.
Guidelines on how to manage SCCs in patients with EB were published a few years ago (Br J Dermatol. 2016;174:56-67) and noted that the clinical detection of SCCs could be difficult because of chronic wound ulceration in these patients. The “possibility of malignancy should be borne in mind, with suspicious lesions biopsied for histological evaluation,” the document states. Evidence for many of the nonsurgical options – radiotherapy, conventional chemotherapy, biologic therapies – was poor, according to the guidelines, and effective nonsurgical options are still desperately needed.
Several avenues of research are being investigated, Dr. Mellerio noted, such as targeting the fibrotic process and perhaps using a micro-RNA inhibitor to stop the upregulation of certain microRNAs in fibroblasts. Targeting inflammatory mechanisms such as thrombospondin 1, which can lead to elevated levels of tumor necrosis factor–beta and contribute to extracellular matrix stiffness, also is under investigation. Raised interleukin-6 may be another target to consider.
Research shows that similar genes are mutated in EB-related and ultraviolet-related SCCs, Dr. Mellerio said. Indeed, mutations in HRAS, NOTCH1, TP53, and CDKN2A have been reported, but mutations in these genes occur much earlier in life in patients with EB. “Something else is going on,” she added, commenting that researchers are looking at apolipoprotein B editing complex (APOBEC) enzymes, which modulate DNA and can cause “particular types of genetic changes in EB cancers.”
One investigator who is studying the genetics of EB SCCs and how APOBEC enzymes might be involved is Andrew South, PhD, an associate professor at Thomas Jefferson University, Philadelphia. APOBEC enzymes are a very prominent source of mutations in RDEB. These mutations are found in 10%-20% of squamous cell carcinomas not associated with RDEB, and 80%-90% of head and neck cancers, he said during a separate talk at the meeting.
Dr. South observed that “RDEB squamous cell carcinoma does not show any particular somatic mutation or upregulation or downregulation of genes that differentiates it from other squamous cell carcinomas, which might be disappointing on the front of it, but actually it does mean that precision therapies that have been developed for other squamous cell carcinomas have application in RDEB.”
RDEB SCC shows the greatest similarity with head and neck SCC, Dr. South said. He also stressed that fibrosis is a major driver of cancer development, SCC tumors in RDEB are homogenous, and that frontline therapy is still unclear.
What is clear, however, is that interdisciplinary management of patients is crucial, said Leena Bruckner-Tuderman, MD, professor and chair of the department of dermatology at the University Medical Center, Albert Ludwig University of Freiburg, Germany.
“In severe RDEB, metastatic SCC is the leading cause of death at a young age. We need monitoring, careful diagnostics, and multidisciplinary treatment,” Dr. Bruckner-Tuderman said. The latter should be delivered by a coordinated team that consists of dermatologists, surgeons, radiologists, oncologists, pathologists, geneticists, and (molecular) tumor boards, she advised.
The study had no commercial funding. Dr. Mellerio disclosed financial relationships with Castle Creek Pharmaceuticals and ProQR Therapeutics, and acted as an unpaid advisor to Helpberby Therapeutics. Dr. South disclosed financial relationships with Krystal Biotech Inc. and Amryt Genetics and has been an advisory board member for Abeona Therapeutics and Sanofi Genzyme. Dr. Bruckner-Tuderman disclosed receiving grants or research support from Constant Pharmaceuticals/Tarix Orphan.
REPORTING FROM EB 2020
Banning indoor tanning devices could save lives and money
according to a study published in JAMA Dermatology.
The study also suggests a ban would result in a collective cost savings of $5.7 billion and productivity gains of $41.3 billion.
Compared with a ban on indoor tanning for minors, the benefits of a full ban on devices were 3.7-fold higher in the United States/Canada and 2.6-fold higher in Europe, according to study author Louisa G. Gordon, PhD, of the QIMR Berghofer Medical Research Institute in Brisbane, Australia, and colleagues.
The researchers noted that indoor tanning is regulated in more than 20 countries. Australia has instituted a ban on commercial indoor tanning devices, and Brazil has banned both commercial and private tanning devices.
In the United States, 19 states have banned the use of indoor tanning beds for minors, and 44 states as well as the District of Columbia have some regulation of tanning facilities for minors, according to the National Conference of State Legislatures.
With this study, Dr. Gordon and colleagues sought to explore what effect an outright ban on indoor tanning devices, a prohibition for minors only, or continuing current levels of indoor tanning would have on the health and economy of the United States, Canada, and Europe.
The researchers created a Markov cohort model of 110,932,523 individuals in the United States/Canada and 141,970,492 individuals in Europe, all aged 12-35 years.
The team used data from epidemiologic studies, cost reports, and official cancer registries to estimate the prevalence of indoor tanning, risk of developing melanoma, and mortality rates from skin cancer and other causes. The researchers also estimated health care costs of melanoma treatment in each region as well as the societal cost of dying prematurely from melanoma, adjusted to 2018 dollars.
Results
The model suggested a ban on indoor tanning in the United States and Canada would result in 244,347 fewer melanomas (–8.7%), 89,193 fewer deaths from melanoma (–6.9%), and 7.3 million fewer keratinocyte carcinomas (–7.8%) than continuing at the current levels of use. The ban would also save 428,781 life-years, have a cost savings of $3.5 billion, and confer productivity gains of $27.5 billion, the researchers said.
When applying the ban in Europe, the model estimated 203,736 fewer melanomas (–4.9%), 98,288 fewer deaths from melanoma (–4.4%), and 2.4 million fewer keratinocyte carcinomas (–4.4%). The researchers also noted that Europe would see a gain of 459,669 life-years, a cost savings of $2.1 billion, and a productivity gain of $13.7 billion.
Dr. Gordon and colleagues acknowledged that their model had some limitations, such as in estimating the prevalence of certain skin cancers across Europe, which can range from 10% to 56% depending on the country. In addition, the model did not account for the money spent in implementing a ban, which could include costs associated with regulation, compliance, and buy-back schemes for tanning devices.
Implications
In an interview, Dr. Gordon said the researchers conducted this study to stress the health benefits and cost savings of regulating indoor tanning devices in North America and Europe. She noted that she had previously published a report in 2009 that helped Australia make the decision to ban such devices there, but she said the tanning industry was in its infancy during that time, which factored into the decision to ban indoor tanning (Health Policy. 2009 Mar;89[3]:303-11).
Any ban by a regulatory agency “should include everyone,” Dr. Gordon said, because “banning minors is a halfway attempt to prevent skin cancers.” The danger isn’t just present in children. “People in their 20s and 30s are still very image conscious,” she said. “The pressure is enormous.”
Anyone interested in tanning should use tanning creams or sprays instead of using indoor tanning devices, Dr. Gordon said. “Consumers can control their UV exposure,” she noted. “Prevention is incredibly important, and skin cancer is one of a few cancers we can almost entirely prevent via protecting our skin. The same can’t be said for other horrible cancers.”
Adam Friedman, MD, a professor at George Washington University, Washington, who was not involved in this study, said it should come as no surprise to dermatologists that preventing artificial UVA heavy exposure reduces the incidence of skin cancer, but the “more compelling component of this study is cost.”
“The lay public is extremely health care cost conscientious,” he said. “This is a commonly debated topic for emerging politicians at every level; not to mention, no one enjoys bleeding money. Dermatologists can use the angle of, ‘save skin now, save money later,’ to target the financial burden of accelerated skin aging and skin cancer as a mechanism for persuading patients not to ‘shake and bake.’ ”
While the Food and Drug Administration has proposed restricting the use of indoor tanning devices for minors nationwide, it has not issued a final rule on the matter, and the prospect of an outright ban in the United States for the general population is less feasible, noted Dr. Friedman.
“I think it would be difficult to expand this [proposed] ban given the financial impact on numerous businesses,” he said. “It would likely take more evidence and support beyond the medical community to make this happen, but here’s hoping,”
This study was funded by the World Health Organization UV Radiation Programme and Cancer Council Victoria. One author disclosed personal fees from Cancer Council Victoria, and one disclosed grants from TrygFonden. The other authors and Dr. Friedman reported no relevant conflicts of interest.
SOURCE: Gordon L et al. JAMA Dermatol. 2020 Feb 19. doi: 10.1001/jamadermatol.2020.0001.
according to a study published in JAMA Dermatology.
The study also suggests a ban would result in a collective cost savings of $5.7 billion and productivity gains of $41.3 billion.
Compared with a ban on indoor tanning for minors, the benefits of a full ban on devices were 3.7-fold higher in the United States/Canada and 2.6-fold higher in Europe, according to study author Louisa G. Gordon, PhD, of the QIMR Berghofer Medical Research Institute in Brisbane, Australia, and colleagues.
The researchers noted that indoor tanning is regulated in more than 20 countries. Australia has instituted a ban on commercial indoor tanning devices, and Brazil has banned both commercial and private tanning devices.
In the United States, 19 states have banned the use of indoor tanning beds for minors, and 44 states as well as the District of Columbia have some regulation of tanning facilities for minors, according to the National Conference of State Legislatures.
With this study, Dr. Gordon and colleagues sought to explore what effect an outright ban on indoor tanning devices, a prohibition for minors only, or continuing current levels of indoor tanning would have on the health and economy of the United States, Canada, and Europe.
The researchers created a Markov cohort model of 110,932,523 individuals in the United States/Canada and 141,970,492 individuals in Europe, all aged 12-35 years.
The team used data from epidemiologic studies, cost reports, and official cancer registries to estimate the prevalence of indoor tanning, risk of developing melanoma, and mortality rates from skin cancer and other causes. The researchers also estimated health care costs of melanoma treatment in each region as well as the societal cost of dying prematurely from melanoma, adjusted to 2018 dollars.
Results
The model suggested a ban on indoor tanning in the United States and Canada would result in 244,347 fewer melanomas (–8.7%), 89,193 fewer deaths from melanoma (–6.9%), and 7.3 million fewer keratinocyte carcinomas (–7.8%) than continuing at the current levels of use. The ban would also save 428,781 life-years, have a cost savings of $3.5 billion, and confer productivity gains of $27.5 billion, the researchers said.
When applying the ban in Europe, the model estimated 203,736 fewer melanomas (–4.9%), 98,288 fewer deaths from melanoma (–4.4%), and 2.4 million fewer keratinocyte carcinomas (–4.4%). The researchers also noted that Europe would see a gain of 459,669 life-years, a cost savings of $2.1 billion, and a productivity gain of $13.7 billion.
Dr. Gordon and colleagues acknowledged that their model had some limitations, such as in estimating the prevalence of certain skin cancers across Europe, which can range from 10% to 56% depending on the country. In addition, the model did not account for the money spent in implementing a ban, which could include costs associated with regulation, compliance, and buy-back schemes for tanning devices.
Implications
In an interview, Dr. Gordon said the researchers conducted this study to stress the health benefits and cost savings of regulating indoor tanning devices in North America and Europe. She noted that she had previously published a report in 2009 that helped Australia make the decision to ban such devices there, but she said the tanning industry was in its infancy during that time, which factored into the decision to ban indoor tanning (Health Policy. 2009 Mar;89[3]:303-11).
Any ban by a regulatory agency “should include everyone,” Dr. Gordon said, because “banning minors is a halfway attempt to prevent skin cancers.” The danger isn’t just present in children. “People in their 20s and 30s are still very image conscious,” she said. “The pressure is enormous.”
Anyone interested in tanning should use tanning creams or sprays instead of using indoor tanning devices, Dr. Gordon said. “Consumers can control their UV exposure,” she noted. “Prevention is incredibly important, and skin cancer is one of a few cancers we can almost entirely prevent via protecting our skin. The same can’t be said for other horrible cancers.”
Adam Friedman, MD, a professor at George Washington University, Washington, who was not involved in this study, said it should come as no surprise to dermatologists that preventing artificial UVA heavy exposure reduces the incidence of skin cancer, but the “more compelling component of this study is cost.”
“The lay public is extremely health care cost conscientious,” he said. “This is a commonly debated topic for emerging politicians at every level; not to mention, no one enjoys bleeding money. Dermatologists can use the angle of, ‘save skin now, save money later,’ to target the financial burden of accelerated skin aging and skin cancer as a mechanism for persuading patients not to ‘shake and bake.’ ”
While the Food and Drug Administration has proposed restricting the use of indoor tanning devices for minors nationwide, it has not issued a final rule on the matter, and the prospect of an outright ban in the United States for the general population is less feasible, noted Dr. Friedman.
“I think it would be difficult to expand this [proposed] ban given the financial impact on numerous businesses,” he said. “It would likely take more evidence and support beyond the medical community to make this happen, but here’s hoping,”
This study was funded by the World Health Organization UV Radiation Programme and Cancer Council Victoria. One author disclosed personal fees from Cancer Council Victoria, and one disclosed grants from TrygFonden. The other authors and Dr. Friedman reported no relevant conflicts of interest.
SOURCE: Gordon L et al. JAMA Dermatol. 2020 Feb 19. doi: 10.1001/jamadermatol.2020.0001.
according to a study published in JAMA Dermatology.
The study also suggests a ban would result in a collective cost savings of $5.7 billion and productivity gains of $41.3 billion.
Compared with a ban on indoor tanning for minors, the benefits of a full ban on devices were 3.7-fold higher in the United States/Canada and 2.6-fold higher in Europe, according to study author Louisa G. Gordon, PhD, of the QIMR Berghofer Medical Research Institute in Brisbane, Australia, and colleagues.
The researchers noted that indoor tanning is regulated in more than 20 countries. Australia has instituted a ban on commercial indoor tanning devices, and Brazil has banned both commercial and private tanning devices.
In the United States, 19 states have banned the use of indoor tanning beds for minors, and 44 states as well as the District of Columbia have some regulation of tanning facilities for minors, according to the National Conference of State Legislatures.
With this study, Dr. Gordon and colleagues sought to explore what effect an outright ban on indoor tanning devices, a prohibition for minors only, or continuing current levels of indoor tanning would have on the health and economy of the United States, Canada, and Europe.
The researchers created a Markov cohort model of 110,932,523 individuals in the United States/Canada and 141,970,492 individuals in Europe, all aged 12-35 years.
The team used data from epidemiologic studies, cost reports, and official cancer registries to estimate the prevalence of indoor tanning, risk of developing melanoma, and mortality rates from skin cancer and other causes. The researchers also estimated health care costs of melanoma treatment in each region as well as the societal cost of dying prematurely from melanoma, adjusted to 2018 dollars.
Results
The model suggested a ban on indoor tanning in the United States and Canada would result in 244,347 fewer melanomas (–8.7%), 89,193 fewer deaths from melanoma (–6.9%), and 7.3 million fewer keratinocyte carcinomas (–7.8%) than continuing at the current levels of use. The ban would also save 428,781 life-years, have a cost savings of $3.5 billion, and confer productivity gains of $27.5 billion, the researchers said.
When applying the ban in Europe, the model estimated 203,736 fewer melanomas (–4.9%), 98,288 fewer deaths from melanoma (–4.4%), and 2.4 million fewer keratinocyte carcinomas (–4.4%). The researchers also noted that Europe would see a gain of 459,669 life-years, a cost savings of $2.1 billion, and a productivity gain of $13.7 billion.
Dr. Gordon and colleagues acknowledged that their model had some limitations, such as in estimating the prevalence of certain skin cancers across Europe, which can range from 10% to 56% depending on the country. In addition, the model did not account for the money spent in implementing a ban, which could include costs associated with regulation, compliance, and buy-back schemes for tanning devices.
Implications
In an interview, Dr. Gordon said the researchers conducted this study to stress the health benefits and cost savings of regulating indoor tanning devices in North America and Europe. She noted that she had previously published a report in 2009 that helped Australia make the decision to ban such devices there, but she said the tanning industry was in its infancy during that time, which factored into the decision to ban indoor tanning (Health Policy. 2009 Mar;89[3]:303-11).
Any ban by a regulatory agency “should include everyone,” Dr. Gordon said, because “banning minors is a halfway attempt to prevent skin cancers.” The danger isn’t just present in children. “People in their 20s and 30s are still very image conscious,” she said. “The pressure is enormous.”
Anyone interested in tanning should use tanning creams or sprays instead of using indoor tanning devices, Dr. Gordon said. “Consumers can control their UV exposure,” she noted. “Prevention is incredibly important, and skin cancer is one of a few cancers we can almost entirely prevent via protecting our skin. The same can’t be said for other horrible cancers.”
Adam Friedman, MD, a professor at George Washington University, Washington, who was not involved in this study, said it should come as no surprise to dermatologists that preventing artificial UVA heavy exposure reduces the incidence of skin cancer, but the “more compelling component of this study is cost.”
“The lay public is extremely health care cost conscientious,” he said. “This is a commonly debated topic for emerging politicians at every level; not to mention, no one enjoys bleeding money. Dermatologists can use the angle of, ‘save skin now, save money later,’ to target the financial burden of accelerated skin aging and skin cancer as a mechanism for persuading patients not to ‘shake and bake.’ ”
While the Food and Drug Administration has proposed restricting the use of indoor tanning devices for minors nationwide, it has not issued a final rule on the matter, and the prospect of an outright ban in the United States for the general population is less feasible, noted Dr. Friedman.
“I think it would be difficult to expand this [proposed] ban given the financial impact on numerous businesses,” he said. “It would likely take more evidence and support beyond the medical community to make this happen, but here’s hoping,”
This study was funded by the World Health Organization UV Radiation Programme and Cancer Council Victoria. One author disclosed personal fees from Cancer Council Victoria, and one disclosed grants from TrygFonden. The other authors and Dr. Friedman reported no relevant conflicts of interest.
SOURCE: Gordon L et al. JAMA Dermatol. 2020 Feb 19. doi: 10.1001/jamadermatol.2020.0001.
FROM JAMA DERMATOLOGY
Data back botulinum toxin for facial flushing, androgenetic alopecia
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – The list of Mark Rubin, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
There are data to support these uses, and there are data associating botulinum toxin treatment with improvement in depression, which suggest the effect may not be necessarily be related to improvement in appearance, said Dr. Rubin, who is in private practice in Beverly Hills, Calif., and is associate professor of dermatology at the University of California, San Diego.
Facial flushing: Very few people use botulinum toxin for facial flushing, but Dr. Rubin, who is among those who do not, described the data as “impressive.” Several trials, he noted, have found that very small doses can significantly reduce the amount of facial erythema, including an average 45% reduction after 60 days in one trial of 24 women (Acta Med Iran. 2016 Jul;54[7]:454-7).
In another study of 25 patients with facial erythema related to rosacea who were treated with 14-45 units intradermally to the nasal tip, bridge, and alae, there were statistically significant improvements in erythema 1, 2, and 3 months after treatment among the 15 with complete data (Dermatol Surg. 2015 Jan;41 Suppl 1:S9-16).
“If you’re using very small doses and they’re intradermal, there really is minimal risk you’re going to have a problem by inadvertently affecting musculature” in these patients, Dr. Rubin commented.
In another study of 9 patients with rosacea, treatment with incobotulinumtoxinA was associated with a significant reduction in erythema, papules, pustules, and telangiectasias, up to 15 weeks, compared with saline. The treatment patients also experienced less burning and stinging that did those who received saline (J Drugs Dermatol. 2017 Jun 1;16[6]:549-54.)
Menopausal hot flashes: Dr. Rubin described one study of 60 patients with severe hot flashes that compared saline with botulinum toxin, injected in 40 sites (2 units per site), including the neck, hairline, scalp, and chest. At 60 days’ follow-up, those treated with botulinum toxin had a significant reduction in sweating and in the number and severity of hot flashes; these women also had improved mood in terms of depression and irritability (Dermatol Surg. 2011 Nov;37[11]:1579-83).
Androgenetic alopecia: In a 60-week study of 50 men with androgenetic alopecia (Hamilton ratings of II-IV), 150 units of botulinum toxin A was injected into the scalp muscles (temporalis, frontalis, periauricular, and occipital), and repeated 6 months later (Plast Reconstr Surg. 2010 Nov;126[5]:246e-8e). Among the 40 patients who completed the trial, 75% had a response, and from baseline to 48 weeks, there was an 18% increase in mean hair counts in a 2 cm area, and a“profound” 39% reduction in hair loss (as measured by hair counts on the pillow in the morning), Dr. Rubin noted.
“Presumably, this is because if you’re relaxing the scalp muscles you’re getting increased blood flow into the scalp,” including increased oxygenation, which decreases the conversion of testosterone to dihydrotestosterone and increases the conversion of testosterone to estradiol, he said.
In another study, 8 of 10 patients with androgenic alopecia has “good to excellent” results 24 weeks after botulinum toxin injections with 5 units per site at 30 sites. Referring to the increasing popularity of platelet-rich plasma (PRP) injections for male pattern alopecia, Dr. Rubin said that in his opinion “PRP certainly doesn’t do any better” than botulinum toxin for male pattern alopecia and is a much more involved injection, “so this is definitely something worth considering if you have more people coming into your practice thinking about injections for male pattern alopecia.”
Pore size and sebum production: A 2019 review of published studies of botulinum toxin A looking at the effect on sebum and pore size, Dr. Rubin said, found that most studies “suggest it does actually reduce pore size and sebum production” (J Cosmet Dermatol. 2019 Apr;18[2]:451-7).
This can be considered an option for those patients concerned about pore size, who are not satisfied with results of retinoid or laser treatment, he commented. This approach may not have an effect in all patients, so he advised first treating a small trial area, and photographing patients to record their level of improvement. “It’s rarely profound, but it’s additive, it’s one more thing you can do.”
Depression: These data include a study of 30 patients with major depression, half who received one onabotulinumtoxinA injection in the glabellar area as adjunctive treatment of depression. After 6 weeks, those who were treated had an average of 47% reduction in depression scores on the Hamilton Depression Rating Scale, compared with an average 9% reduction among those on placebo (J Psychiatr Res. 2012 May;46[5]:574-81). Two recent studies have had similar results, according to Dr. Rubin.
Results of another study, he said, raise the question of whether patients are less depressed because they are pleased with the cosmetic effects or if there is another explanation (J Am Acad Dermatol. 2016 Jan;74[1]:171-3.e1). The study, which included 59 patients with depression treated in the glabellar areas with botulinum toxin injections, found no association between severity of the furrows and degree of depression or between the degree of furrow correction and degree of relief from depression after treatment. “So the patients who had the most improvement were not necessarily the ones who were the least depressed afterwards,” he said.
These data imply that something else may be occurring that is not necessarily muscle related, he said.
Dr. Rubin said he had no relevant disclosures. SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Psoriasis elevates cancer risk
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
Psoriasis patients are at increased risk for several types of cancer, notably lymphoma and keratinocyte cancer, based on data from a systematic review and meta-analysis of more than 2 million patients.
Previous studies have identified an increased overall cancer risk in psoriasis patients, compared with the general population or controls without psoriasis, and both lymphomas and keratinocyte cancers occur more often in psoriasis patients, compared with controls, but additional larger studies have been conducted since the last meta-analysis was published in 2013, wrote Sofie Vaengebjerg, MD, of the University of Copenhagen and colleagues.
To better identify the risk of cancer in psoriasis and psoriatic arthritis patients and to explore the impact of biologics, the researchers reviewed data from 112 studies totaling 2,053,932 patients in a study published in JAMA Dermatology.
Overall, the risk of any cancer was slightly higher in psoriasis patients (risk ratio, 1.21; 95% confidence interval, 1.11-1.33), compared with controls, with a prevalence of 4.78% and an incidence rate of 11.75 per 1,000 person-years. The most common cancer among psoriasis patients was keratinocyte cancer, with a risk ratio of 2.28 (95% CI, 1.73-3.01), a prevalence of 2.55%, and an incidence rate of 4.35 per 1,000 person-years.
Other cancers with significantly elevated risk among psoriasis patients were lymphomas (RR, 1.56; 95% CI, 1.37-1.78), lung cancer (RR, 1.26; 95% CI, 1.13-1.40), and bladder cancer (RR, 1.12; 95% CI, 1.04-1.19).
No increased risk of cancer was noted among psoriasis patients who were treated with biologics. “However, patients receiving biologic agents are selected and the results might be reliant on selection bias, and studies investigating long-term safety of these drugs are still limited,” the researchers wrote.
In addition, psoriatic arthritis was not associated with any overall increase in cancer risk, with the exception of three studies showing an increased risk for breast cancer, the researchers noted. The overall cancer prevalence for psoriatic arthritis patients was 5.74%, with an incidence rate of 6.44 per 1,000 person-years.
The study findings were limited by several factors, including the inconsistencies in study design and characteristics and the small amount of data on biologic agents and psoriatic arthritis, the researchers noted. However, the results were strengthened by the large number of patients, real-world study settings, inclusion of biologics, and analysis of cancer in psoriatic arthritis patients.
“Clinicians treating patients with psoriasis should be aware of this increased risk, especially for lymphomas, as immunogenic treatment might be associated with exacerbations,” and should be aware that more research is needed to assess cancer risk associated with biologics, they concluded.
The study received no outside funding. Lead author Dr. Vaengebjerg had no financial conflicts to disclose. Several coauthors disclosed relationships with multiple companies, including AbbVie, Janssen, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi.
SOURCE: Vaengebjerg S et al. JAMA Dermatol. 2020 Feb 19. doi:10.1001/jamadermatol.2020.0024.
FROM JAMA DERMATOLOGY
Bleeding Hand Mass in an Older Man
The Diagnosis: Epithelioid Angiosarcoma
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.

Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
The Diagnosis: Epithelioid Angiosarcoma
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.




Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
The Diagnosis: Epithelioid Angiosarcoma
Histopathology showed a large soft-tissue neoplasm with extensive hemorrhage (Figure 1). The epithelioid angiosarcoma (EA) consisted mostly of irregular slit-shaped vessels lined by sheets of atypical endothelial cells (Figure 2). At higher-power magnification, the cellular atypia was prominent and diffuse (Figure 3). Immunostaining of the tumor cells showed positive uptake for CD31, confirming vascular origin (Figure 4). Other vascular markers, including CD34 and factor VIII, as well as nuclear positivity for the erythroblast transformation-specific transcription factor gene, ERG, can be demonstrated by EA. Irregular, smooth muscle actin-positive spindle cells are distributed around some of the vessels. The human herpesvirus 8 stain is negative.




Compared to classic angiosarcomas, EAs have a predilection for the extremities rather than the head and scalp. Histopathologically, the cells are epithelioid and are strongly positive for vimentin and CD31, in addition to factor VIII, friend leukemia integration 1 transcription factor, and CD34.1,2 In contrast, epithelioid sarcomas more typically are seen in younger adults and less likely to be CD31 positive.3 An epithelioid hemangioendothelioma is more focal in cellular atypia and forms small nests and trabeculae rather than sheets of atypical cells. Melanoma cells stain positive for human melanoma black 45, Melan-A, and S-100 but not for CD31.3 Glomangiosarcomas typically stain positive for smooth muscle actin and muscle-specific actin.4
Epithelioid angiosarcomas are rare and aggressive malignancies of endothelial origin.3 They are more prevalent in men and have a peak incidence in the seventh decade of life. They most commonly occur in the deep soft tissues of the extremities but have been reported to form in a variety of primary sites, including the skin, bones, thyroid, and adrenal glands.3
Tumors tend to be highly aggressive and demonstrate early nodal and solid organ metastases.3 Our case demonstrated the aggressive nature of this high-grade malignancy by showing neoplastic invasion through a vascular wall. Within 2 to 3 years of diagnosis, 50% of patients die of the disease, and the 5-year survival rate is estimated to be 12% to 20%.3,5 The etiology remains unknown, but EA has been linked to prior exposure to toxic chemicals, irradiation, or Thorotrast contrast media, and it may arise in the setting of arteriovenous fistulae and chronic lymphedema.6
Although radiation therapy often is utilized, surgery is the primary treatment modality.5 Even with wide excision, local recurrence is common. Tumor size is one of the most important prognostic features, with a worse prognosis for tumors larger than 5 cm. Evidence suggests that paclitaxel-based chemotherapeutic regimens may improve survival, and a combination of paclitaxel and sorafenib has been reported to induce remission in metastatic angiosarcoma of parietal EA.5 Currently, no standardized treatment regimen for this condition exists.
Acknowledgment
The authors thank Amanda Marsch, MD (Chicago, Illinois), for obtaining outside pathology consultation.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
- Suchak R, Thway K, Zelger B, et al. Primary cutaneous epithelioid angiosarcoma: a clinicopathologic study of 13 cases of a rare neoplasm occurring outside the setting of conventional angiosarcomas and with predilection for the limbs. Am J Surg Pathol. 2011;35:60-69.
- Prescott RJ, Banerjee SS, Eyden BP, et al. Cutaneous epithelioid angiosarcoma: a clinicopathological study of four cases. Histopathology. 1994;25:421-429.
- Hart J, Mandavilli S. Epithelioid angiosarcoma: a brief diagnostic review and differential diagnosis. Arch Pathol Lab Med. 2011;135:268-272.
- Maselli AM, Jambhekar AV, Hunter JG. Glomangiosarcoma arising from a prior biopsy site. Plast Reconstr Surg Glob Open. 2017;5:e1219.
- Donghi D, Dummer R, Cozzio A. Complete remission in a patient with multifocal metastatic cutaneous angiosarcoma with a combination of paclitaxel and sorafenib. Br J Dermatol. 2010;162:697-699.
- Wu J, Li X, Liu X. Epithelioid angiosarcoma: a clinicopathological study of 16 Chinese cases. Int J Clin Exp Pathol. 2015;8:3901-3909.
A 72-year-old man presented for evaluation of a mass on the left hand that continued to grow over the last few months and eventually bled. The patient first noticed a small firm lump on the palm approximately 1 year prior to presentation, and it was originally diagnosed as a Dupuytren contracture by his primary care physician. Months later, the lesion grew and began to bleed. Magnetic resonance imaging showed large hematomas of the hand with areas of nodular enhancement. The mass was located between the third and fourth proximal phalanges and abutted the extensor tendon. Complete excision yielded a definitive diagnosis.
Smartphone apps for suspicious skin lesions unreliable
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
Smartphone applications ( said U.K. researchers reporting a systematic review.
These apps are providing information that could lead to “potentially life-or-death decisions,” commented co-lead author Hywel C. Williams, MD, from the Centre of Evidence Based Dermatology, University of Nottingham (England).
“The one thing you mustn’t do in a situation where early diagnosis can make a difference between life and death is you mustn’t miss the melanoma,” he said in an interview.
“These apps were missing melanomas, and that’s very worrisome,” he commented.
The review included nine studies of skin cancer smartphone apps, including two apps, SkinScan and SkinVision, that have been given Conformit Europenne (CE) marks, allowing them to be marketed across Europe. These apps are also available in Australia and New Zealand, but not in the United States.
The review found that SkinScan was not able to identify any melanomas in the one study that assessed this app, while SkinVision had a relatively low sensitivity and specificity, with 12% of cancerous or precancerous lesions missed and 21% of benign lesions wrongly identified as cancerous.
This means that among 1,000 people with a melanoma prevalence of 3%, 4 of 30 melanomas would be missed, and 200 people would be incorrectly told that a mole was of high concern, the authors estimated.
The research was published by The BMJ on Feb. 10.
“Although I was broad minded on the potential benefit of apps for diagnosing skin cancer, I am now worried given the results of our study and the overall poor quality of studies used to test these apps,” Dr. Williams commented in a statement.
Coauthor Jac Dinnes, PhD, from the Institute of Applied Health Research at the University of Birmingham (England), added it is “really disappointing that there is not better quality evidence available to judge the efficacy of these apps.”
“It is vital that health care professionals are aware of the current limitations both in the technologies and in their evaluations,” she added.
The results also highlight the limitations of the regulatory system governing smartphone apps in that they are currently not subject to assessment by bodies such as the U.K.’s Medicines and Healthcare Products Regulatory Agency (MHRA), the authors commented.
“Regulators need to become alert to the potential harm that poorly performing algorithm-based diagnostic or risk monitoring apps create,” said co-lead author Jonathan J. Deeks, PhD, also at the Institute of Applied Health Research.
“We rely on the CE mark as a sign of quality, but the current CE mark assessment processes are not fit for protecting the public against the risks that these apps present.”
Speaking in an interview, Williams lamented the poor quality of the research that had been conducted. “These studies were not good enough,” he said, adding that “there’s no excuse for really poor study design and poor reporting.”
He would like to see the regulations tightened around AI apps purporting to inform decision making for the general public and suggests that these devices should be assessed by the MHRA. “I really do think a CE mark is not enough,” he said.
The team noted that the skin cancer apps “all include disclaimers that the results should only be used as a guide and cannot replace health care advice,” through which the manufacturers “attempt to evade any responsibility for negative outcomes experienced by users.”
Nevertheless, the “poor and variable performance” of the apps revealed by their review indicates that they “have not yet shown sufficient promise to recommend their use,” they concluded.
The “official approval” implied by a CE mark “will give consumers the impression that the apps have been assessed as effective and safe,” wrote Ben Goldacre, DataLab director, Nuffield Department of Primary Care, University of Oxford (England), and colleagues in an accompanying editorial.
“The implicit assumption is that apps are similarly low-risk technology” to devices such as sticking plasters and reading glasses, they comment.
“But shortcomings in diagnostic apps can have serious implications,” they warn. The “risks include psychological harm from health anxiety or ‘cyberchondria,’ and physical harm from misdiagnosis or overdiagnosis; for clinicians there is a risk of increased workload, and changes to ethical or legal responsibilities around triage, referral, diagnosis, and treatment.” There is also potential for “inappropriate resource use, and even loss of credibility for digital technology in general.”
Details of the review
For their review, the authors searched the Cochrane Central Register on Controlled Trials, the MEDLNE, Embase, Cumulative Index to Nursing and Allied Health Literature, Conference Proceedings Citation index, Zetoc, and Science Citation Index databases, and online trial registers for studies published between August 2016 and April 2019.
From 80 studies identified, 9 met the eligibility criteria.
Of those, six studies, evaluating a total of 725 skin lesions, determined the accuracy of smartphone apps in risk stratifying suspicious skin lesions by comparing them against a histopathological reference standard diagnosis or expert follow-up.
Five of these studies aimed to detect only melanoma, while one sought to differentiate between malignant or premalignant lesions (including melanoma, basal cell carcinoma, and squamous cell carcinoma) and benign lesions.
The three remaining studies, which evaluated 407 lesions in all, compared smartphone app recommendations against a reference standard of expert recommendations for further investigation or intervention.
The researchers found the studies had a string of potential biases and limitations.
For example, only four studies recruited a consecutive sample of study participants and lesions, and only two included lesions selected by study participants, whereas five studies used lesions that had been selected by a clinician.
Three studies reported that it took 5-10 attempts to obtain an adequate image. In seven studies, it was the researchers and not the patients who used the app to photograph the lesions, and two studies used images obtained from dermatology databases.
This “raised concerns that the results of the studies were unlikely to be representative of real life use,” the authors comment.
In addition, the exclusion of unevaluable images “might have systematically inflated the diagnostic performance of the tested apps,” they add.
The independent research was supported by the National Institute for Health Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham and is an update of one of a collection of reviews funded by the NIHR through its Cochrane Systematic Review Programme Grant.
This article first appeared on Medscape.com.
FDA: Cell phones still look safe
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.
according to a review by the Food and Drug Administration.
The FDA reviewed the published literature from 2008 to 2018 and concluded that the data don’t support any quantifiable adverse health risks from RFR. However, the evidence is not without limitations.
The FDA’s evaluation included evidence from in vivo animal studies from Jan. 1, 2008, to Aug. 1, 2018, and epidemiologic studies in humans from Jan. 1, 2008, to May 8, 2018. Both kinds of evidence had limitations, but neither produced strong indications of any causal risks from cell phone use.
The FDA noted that in vivo animal studies are limited by variability of methods and RFR exposure, which make comparisons of results difficult. These studies are also impacted by the indirect effects of temperature increases (the only currently established biological effect of RFR) and stress experienced by the animals, which make teasing out the direct effects of RFR difficult.
The FDA noted that strong epidemiologic studies can provide more relevant and accurate information than in vivo studies, but epidemiologic studies are not without limitations. For example, most have participants track and self-report their cell phone use. There’s also no way to directly track certain factors of RFR exposure, such as frequency, duration, or intensity.
Even with those caveats in mind, the FDA wrote that, “based on the studies that are described in detail in this report, there is insufficient evidence to support a causal association between RFR exposure and tumorigenesis. There is a lack of clear dose-response relationship, a lack of consistent findings or specificity, and a lack of biological mechanistic plausibility.”
The full review is available on the FDA website.