User login
Antecedent Chronic Lymphocytic Leukemia May Be Associated With More Aggressive Mycosis Fungoides
To the Editor:
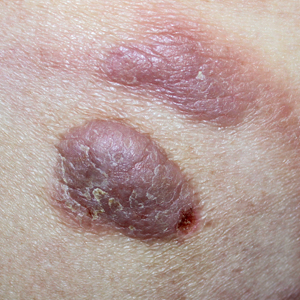
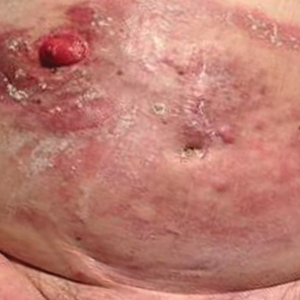
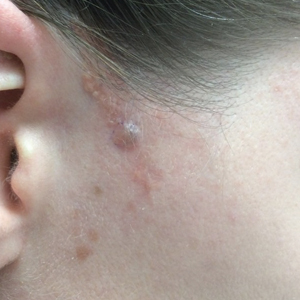
Mycosis fungoides (MF) is the most common form of primary cutaneous T-cell lymphoma. It has been associated with increased risk for other visceral and hematologic malignancies.1 Chronic lymphocytic leukemia (CLL) is one of the most common hematologic malignancies. In the United States, a patient’s lifetime risk for CLL is 0.6%. Chronic lymphocytic leukemia often is diagnosed as an incidental finding and typically is not detrimental to a patient’s health. Six cases of MF with antecedent or concomitant CLL were identified in a cohort of patients treated at the University of Minnesota (Minneapolis, Minnesota) from 2005 to 2017 (Table).
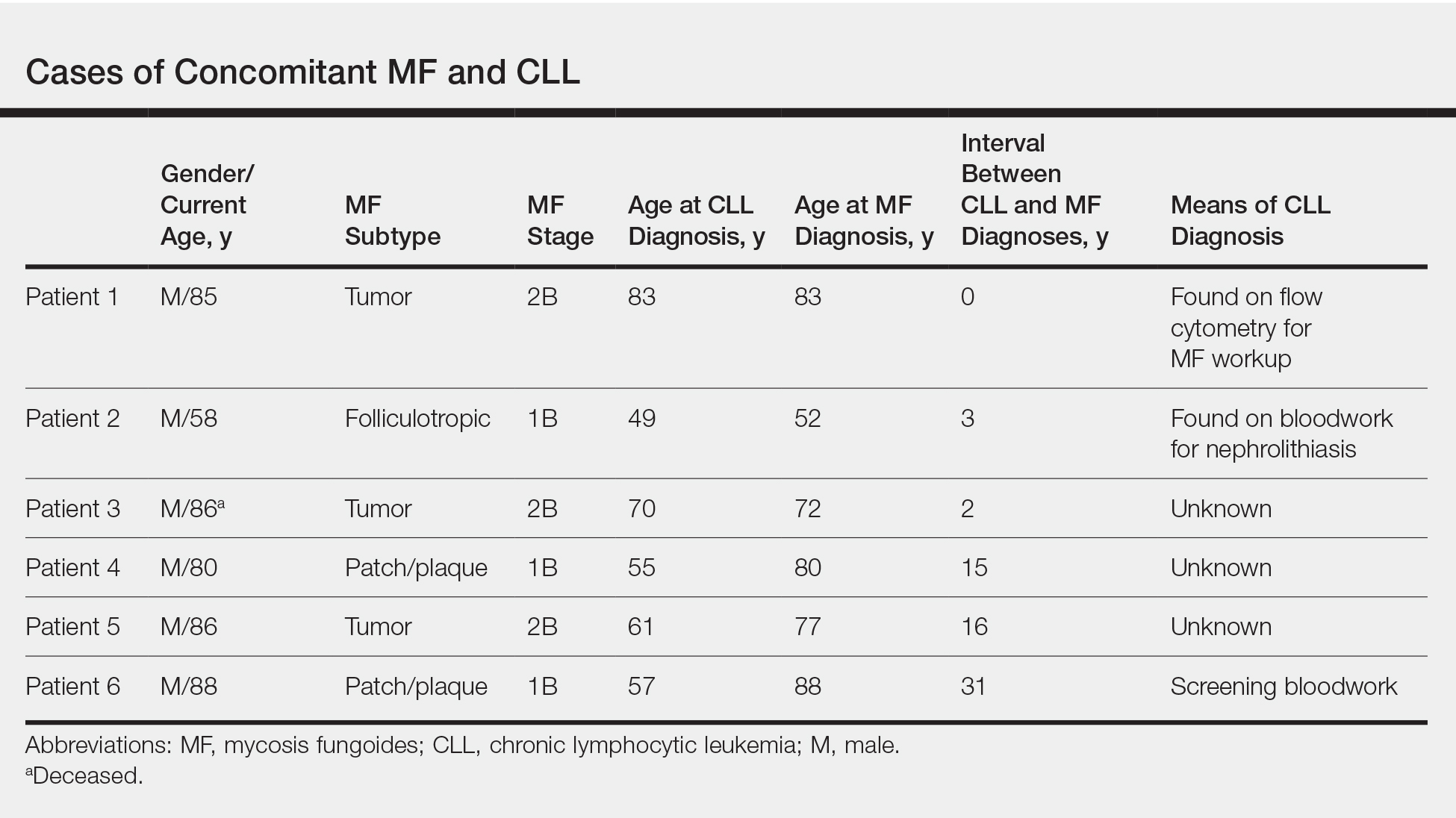
All 6 patients were male, with a mean age of 80.5 years. The mean age at CLL diagnosis was 62.5 years, while the mean age at MF diagnosis was 75.3 years. Three patients were younger than 60 years when their CLL was diagnosed: 49, 55, and 57 years. Notably, 4 patients had more aggressive types of MF: 3 with tumor-stage disease, and 1 with folliculotropic MF. Five patients were diagnosed with CLL before their MF was diagnosed (mean, 13.4 years prior; range, 3–31 years), and 1 was diagnosed as part of the initial MF workup.
Given the frequency of both MF and CLL, the co-occurrence of these diseases is not surprising, as other case reports and a larger case series have described the relationship between MF and malignancy.2 It is possible that CLL patients are more likely to be diagnosed with MF because of their regular hematology/oncology follow-up; however, none of our patients were referred from hematology/oncology to dermatology. Alternatively, patients with MF may be more likely to be diagnosed with CLL because of repeated bloodwork performed for diagnosis and screening, which occurred in only 1 of 6 cases. Most of the other patients were diagnosed with MF more than a decade after being diagnosed with CLL.
Does having CLL make patients more likely to develop MF? It is known that patients with CLL may experience immunodeficiency secondary to immune dysregulation, making them more susceptible to infection and secondary malignancies.3 Of our 6 cases, 4 had aggressive or advanced forms of MF, which is similar to the findings of Chang et al.2 In their report, of 8 patients with MF, 2 had tumor-stage disease and 2 had erythrodermic MF. They determined that these patients had worse overall survival.2 Our data corroborate the finding that patients with CLL may develop more severe MF, which leads to the conclusion that patients diagnosed with CLL before, concomitantly, or after their diagnosis of MF should be closely monitored. It is notable that patients with more advanced disease tend to be older at the time of diagnosis and that patients who are diagnosed at 57 years or older have been found to have worse disease-specific survival.4,5
This report is limited by the small sample size (6 cases), but it serves to draw attention to the phenomenon of co-occurrence of MF and CLL, and the concern that patients with CLL may develop more aggressive MF.
- Huang KP, Weinstock MA, Clarke CA, et al. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2007;143:45-50.
- Chang MB, Weaver AL, Brewer JD. Cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia: clinical characteristics, temporal relationships, and survival data in a series of 14 patients at Mayo Clinic. Int J Dermatol. 2014;53:966-970.
- Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49-62.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
To the Editor:
Mycosis fungoides (MF) is the most common form of primary cutaneous T-cell lymphoma. It has been associated with increased risk for other visceral and hematologic malignancies.1 Chronic lymphocytic leukemia (CLL) is one of the most common hematologic malignancies. In the United States, a patient’s lifetime risk for CLL is 0.6%. Chronic lymphocytic leukemia often is diagnosed as an incidental finding and typically is not detrimental to a patient’s health. Six cases of MF with antecedent or concomitant CLL were identified in a cohort of patients treated at the University of Minnesota (Minneapolis, Minnesota) from 2005 to 2017 (Table).

All 6 patients were male, with a mean age of 80.5 years. The mean age at CLL diagnosis was 62.5 years, while the mean age at MF diagnosis was 75.3 years. Three patients were younger than 60 years when their CLL was diagnosed: 49, 55, and 57 years. Notably, 4 patients had more aggressive types of MF: 3 with tumor-stage disease, and 1 with folliculotropic MF. Five patients were diagnosed with CLL before their MF was diagnosed (mean, 13.4 years prior; range, 3–31 years), and 1 was diagnosed as part of the initial MF workup.
Given the frequency of both MF and CLL, the co-occurrence of these diseases is not surprising, as other case reports and a larger case series have described the relationship between MF and malignancy.2 It is possible that CLL patients are more likely to be diagnosed with MF because of their regular hematology/oncology follow-up; however, none of our patients were referred from hematology/oncology to dermatology. Alternatively, patients with MF may be more likely to be diagnosed with CLL because of repeated bloodwork performed for diagnosis and screening, which occurred in only 1 of 6 cases. Most of the other patients were diagnosed with MF more than a decade after being diagnosed with CLL.
Does having CLL make patients more likely to develop MF? It is known that patients with CLL may experience immunodeficiency secondary to immune dysregulation, making them more susceptible to infection and secondary malignancies.3 Of our 6 cases, 4 had aggressive or advanced forms of MF, which is similar to the findings of Chang et al.2 In their report, of 8 patients with MF, 2 had tumor-stage disease and 2 had erythrodermic MF. They determined that these patients had worse overall survival.2 Our data corroborate the finding that patients with CLL may develop more severe MF, which leads to the conclusion that patients diagnosed with CLL before, concomitantly, or after their diagnosis of MF should be closely monitored. It is notable that patients with more advanced disease tend to be older at the time of diagnosis and that patients who are diagnosed at 57 years or older have been found to have worse disease-specific survival.4,5
This report is limited by the small sample size (6 cases), but it serves to draw attention to the phenomenon of co-occurrence of MF and CLL, and the concern that patients with CLL may develop more aggressive MF.
To the Editor:
Mycosis fungoides (MF) is the most common form of primary cutaneous T-cell lymphoma. It has been associated with increased risk for other visceral and hematologic malignancies.1 Chronic lymphocytic leukemia (CLL) is one of the most common hematologic malignancies. In the United States, a patient’s lifetime risk for CLL is 0.6%. Chronic lymphocytic leukemia often is diagnosed as an incidental finding and typically is not detrimental to a patient’s health. Six cases of MF with antecedent or concomitant CLL were identified in a cohort of patients treated at the University of Minnesota (Minneapolis, Minnesota) from 2005 to 2017 (Table).

All 6 patients were male, with a mean age of 80.5 years. The mean age at CLL diagnosis was 62.5 years, while the mean age at MF diagnosis was 75.3 years. Three patients were younger than 60 years when their CLL was diagnosed: 49, 55, and 57 years. Notably, 4 patients had more aggressive types of MF: 3 with tumor-stage disease, and 1 with folliculotropic MF. Five patients were diagnosed with CLL before their MF was diagnosed (mean, 13.4 years prior; range, 3–31 years), and 1 was diagnosed as part of the initial MF workup.
Given the frequency of both MF and CLL, the co-occurrence of these diseases is not surprising, as other case reports and a larger case series have described the relationship between MF and malignancy.2 It is possible that CLL patients are more likely to be diagnosed with MF because of their regular hematology/oncology follow-up; however, none of our patients were referred from hematology/oncology to dermatology. Alternatively, patients with MF may be more likely to be diagnosed with CLL because of repeated bloodwork performed for diagnosis and screening, which occurred in only 1 of 6 cases. Most of the other patients were diagnosed with MF more than a decade after being diagnosed with CLL.
Does having CLL make patients more likely to develop MF? It is known that patients with CLL may experience immunodeficiency secondary to immune dysregulation, making them more susceptible to infection and secondary malignancies.3 Of our 6 cases, 4 had aggressive or advanced forms of MF, which is similar to the findings of Chang et al.2 In their report, of 8 patients with MF, 2 had tumor-stage disease and 2 had erythrodermic MF. They determined that these patients had worse overall survival.2 Our data corroborate the finding that patients with CLL may develop more severe MF, which leads to the conclusion that patients diagnosed with CLL before, concomitantly, or after their diagnosis of MF should be closely monitored. It is notable that patients with more advanced disease tend to be older at the time of diagnosis and that patients who are diagnosed at 57 years or older have been found to have worse disease-specific survival.4,5
This report is limited by the small sample size (6 cases), but it serves to draw attention to the phenomenon of co-occurrence of MF and CLL, and the concern that patients with CLL may develop more aggressive MF.
- Huang KP, Weinstock MA, Clarke CA, et al. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2007;143:45-50.
- Chang MB, Weaver AL, Brewer JD. Cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia: clinical characteristics, temporal relationships, and survival data in a series of 14 patients at Mayo Clinic. Int J Dermatol. 2014;53:966-970.
- Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49-62.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Huang KP, Weinstock MA, Clarke CA, et al. Second lymphomas and other malignant neoplasms in patients with mycosis fungoides and Sézary syndrome. Arch Dermatol. 2007;143:45-50.
- Chang MB, Weaver AL, Brewer JD. Cutaneous T-cell lymphoma in patients with chronic lymphocytic leukemia: clinical characteristics, temporal relationships, and survival data in a series of 14 patients at Mayo Clinic. Int J Dermatol. 2014;53:966-970.
- Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49-62.
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
Practice Points
- Patients with mycosis fungoides (MF) are at increased risk for second hematologic malignancies, including chronic lymphocytic leukemia (CLL).
- Anecdotal information suggests that patients with CLL prior to developing MF may have more severe phenotypes of MF.
Cutaneous squamous cell cancer staging still requires refinement
LAS VEGAS – Cases of . But even as cSCC draws more attention, two popular staging systems still need refinement, a hematologist-oncologist told dermatologist colleagues.
Both staging systems can be helpful, however, Guilherme Rabinowits, MD, said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “The best thing to do is combine both,” he noted, referring to the staging system developed by the Brigham and Women’s Hospital (BWH) and staging systems released in the 7th and 8th editions of the American Joint Committee on Cancer Staging Manual (AJCC 7 and AJCC 8; the latter focuses on head and neck only).
Cutaneous SCC has become a “huge health care burden,” said Dr. Rabinowits of the Miami Cancer Institute/Baptist Health South Florida and Florida International University, Miami. He referred to Medicare data suggesting that cSCC has become as common as basal cell carcinoma (JAMA Dermatol. 2015 Oct;151[10]:1081-6).
While most patients with cSCC have a positive prognosis, he added, the 5-year survival rate among those with the most aggressive disease is only 25%.
As he pointed out, staging systems provide insight into prognosis and treatment, guide appropriate monitoring, and give a common language to clinicians around the world. “When I talk about stage 3, we all understand who we’re talking about,” he said.
Dr. Rabinowits said he formerly worked at BWH so he is especially comfortable with its staging system. It can be appropriate to use both the BWH and AJCC systems at once, he said, noting, however, that studies suggest that the BWH system is superior.
An analysis released in 2014, for example, found that most poor outcomes occurred in patients whose tumors had been rated at lower stages – suggesting less risk – in the AJCC 7 system (J Clin Oncol 2014 Feb 1;32[4]:327-4).
In contrast, the 5% of tumors that were rated at the highest stages in the BWH system – with the highest level of perceived risk – accounted for 70% of nodal metastases and 83% of disease-specific deaths. This group may deserve extra attention in terms of staging and adjuvant treatment in order to improve outcomes, he said.
A study published this year, which compared the BWH and AJCC 8 staging systems, found that they had the same negative predictive value (0.99), while the BWH system showed higher positive predictive value (0.30 vs. 0.17). “Use of BWH tumor classification may minimize the number of patients recommended for radiologic evaluation, close surveillance, and possible adjuvant therapy while still identifying most patients at risk for recurrence, metastasis, and death,” the authors wrote (JAMA Dermatol. 2019 Jul 1;155[7]: 819-25).
As for treatment of localized disease in high-risk patients, Dr. Rabinowits advised turning to a multidisciplinary team for guidance because there are limited prospective randomized data.
Dr. Rabinowits discloses scientific advisory board/consultant relationships (Merck, Regeneron, Sanofi, EMD Serono, Pfizer, and Castle) and shares in Regeneron and Syros Pharmaceuticals. He spoke during a forum on cutaneous malignancies at the meeting.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Cases of . But even as cSCC draws more attention, two popular staging systems still need refinement, a hematologist-oncologist told dermatologist colleagues.
Both staging systems can be helpful, however, Guilherme Rabinowits, MD, said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “The best thing to do is combine both,” he noted, referring to the staging system developed by the Brigham and Women’s Hospital (BWH) and staging systems released in the 7th and 8th editions of the American Joint Committee on Cancer Staging Manual (AJCC 7 and AJCC 8; the latter focuses on head and neck only).
Cutaneous SCC has become a “huge health care burden,” said Dr. Rabinowits of the Miami Cancer Institute/Baptist Health South Florida and Florida International University, Miami. He referred to Medicare data suggesting that cSCC has become as common as basal cell carcinoma (JAMA Dermatol. 2015 Oct;151[10]:1081-6).
While most patients with cSCC have a positive prognosis, he added, the 5-year survival rate among those with the most aggressive disease is only 25%.
As he pointed out, staging systems provide insight into prognosis and treatment, guide appropriate monitoring, and give a common language to clinicians around the world. “When I talk about stage 3, we all understand who we’re talking about,” he said.
Dr. Rabinowits said he formerly worked at BWH so he is especially comfortable with its staging system. It can be appropriate to use both the BWH and AJCC systems at once, he said, noting, however, that studies suggest that the BWH system is superior.
An analysis released in 2014, for example, found that most poor outcomes occurred in patients whose tumors had been rated at lower stages – suggesting less risk – in the AJCC 7 system (J Clin Oncol 2014 Feb 1;32[4]:327-4).
In contrast, the 5% of tumors that were rated at the highest stages in the BWH system – with the highest level of perceived risk – accounted for 70% of nodal metastases and 83% of disease-specific deaths. This group may deserve extra attention in terms of staging and adjuvant treatment in order to improve outcomes, he said.
A study published this year, which compared the BWH and AJCC 8 staging systems, found that they had the same negative predictive value (0.99), while the BWH system showed higher positive predictive value (0.30 vs. 0.17). “Use of BWH tumor classification may minimize the number of patients recommended for radiologic evaluation, close surveillance, and possible adjuvant therapy while still identifying most patients at risk for recurrence, metastasis, and death,” the authors wrote (JAMA Dermatol. 2019 Jul 1;155[7]: 819-25).
As for treatment of localized disease in high-risk patients, Dr. Rabinowits advised turning to a multidisciplinary team for guidance because there are limited prospective randomized data.
Dr. Rabinowits discloses scientific advisory board/consultant relationships (Merck, Regeneron, Sanofi, EMD Serono, Pfizer, and Castle) and shares in Regeneron and Syros Pharmaceuticals. He spoke during a forum on cutaneous malignancies at the meeting.
SDEF and this news organization are owned by the same parent company.
LAS VEGAS – Cases of . But even as cSCC draws more attention, two popular staging systems still need refinement, a hematologist-oncologist told dermatologist colleagues.
Both staging systems can be helpful, however, Guilherme Rabinowits, MD, said at Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar. “The best thing to do is combine both,” he noted, referring to the staging system developed by the Brigham and Women’s Hospital (BWH) and staging systems released in the 7th and 8th editions of the American Joint Committee on Cancer Staging Manual (AJCC 7 and AJCC 8; the latter focuses on head and neck only).
Cutaneous SCC has become a “huge health care burden,” said Dr. Rabinowits of the Miami Cancer Institute/Baptist Health South Florida and Florida International University, Miami. He referred to Medicare data suggesting that cSCC has become as common as basal cell carcinoma (JAMA Dermatol. 2015 Oct;151[10]:1081-6).
While most patients with cSCC have a positive prognosis, he added, the 5-year survival rate among those with the most aggressive disease is only 25%.
As he pointed out, staging systems provide insight into prognosis and treatment, guide appropriate monitoring, and give a common language to clinicians around the world. “When I talk about stage 3, we all understand who we’re talking about,” he said.
Dr. Rabinowits said he formerly worked at BWH so he is especially comfortable with its staging system. It can be appropriate to use both the BWH and AJCC systems at once, he said, noting, however, that studies suggest that the BWH system is superior.
An analysis released in 2014, for example, found that most poor outcomes occurred in patients whose tumors had been rated at lower stages – suggesting less risk – in the AJCC 7 system (J Clin Oncol 2014 Feb 1;32[4]:327-4).
In contrast, the 5% of tumors that were rated at the highest stages in the BWH system – with the highest level of perceived risk – accounted for 70% of nodal metastases and 83% of disease-specific deaths. This group may deserve extra attention in terms of staging and adjuvant treatment in order to improve outcomes, he said.
A study published this year, which compared the BWH and AJCC 8 staging systems, found that they had the same negative predictive value (0.99), while the BWH system showed higher positive predictive value (0.30 vs. 0.17). “Use of BWH tumor classification may minimize the number of patients recommended for radiologic evaluation, close surveillance, and possible adjuvant therapy while still identifying most patients at risk for recurrence, metastasis, and death,” the authors wrote (JAMA Dermatol. 2019 Jul 1;155[7]: 819-25).
As for treatment of localized disease in high-risk patients, Dr. Rabinowits advised turning to a multidisciplinary team for guidance because there are limited prospective randomized data.
Dr. Rabinowits discloses scientific advisory board/consultant relationships (Merck, Regeneron, Sanofi, EMD Serono, Pfizer, and Castle) and shares in Regeneron and Syros Pharmaceuticals. He spoke during a forum on cutaneous malignancies at the meeting.
SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Researchers working to squash recurrence of superficial BCCs
LAS VEGAS – For according to Ervin H. Epstein, MD.
In a noninferiority trial, researchers in the Netherlands randomized 601 patients with superficial BCCs to one of three medical treatments: two treatments of photodynamic therapy (PDT) 1 week apart (group 1), imiquimod 5 days per week for 6 weeks with no occlusion (group 2), and 5-fluorouracil (5-FU) twice per day for 4 weeks with no occlusion (group 3). They followed the patients for 5 years to see which tumors came back (J Invest Dermatol. 2018 Mar;138[3]:527-33). At 5 years, tumor-free survival was 63% in group 1, 81% in group 2, and 70% in group 3. Based on this analysis the authors concluded that imiquimod is the first choice for noninvasive treatment of most primary BCCs.
“Yes, imiquimod is helpful, but it’s not as good as surgery,” Dr. Epstein said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Researchers like Dr. Epstein, a dermatologist at Brown & Toland Physicians in Oakland, Calif., have been exploring ways to reduce the recurrence of BCCs in those at high risk. One approach is to stay out of the sun, but that is not practical for most people, “and the data are not very supportive,” he said. “Very few people can actually avoid sunlight. What about topical 5-FU? Suppose you put on 5-FU and then study people for the next year, to see what the likelihood is of them getting a BCC? Why it has taken us so long to do this kind of study is in some ways shameful.” He mentioned one study in which patients at risk of superficial BCC recurrence applied topical 5-FU twice a day for 2-4 weeks. The regimen led to an 11% decrease in the development of BCCs, a trend that did not reach statistical significance.
Another approach to warding off BCC recurrence is to take oral hedgehog pathway inhibitors, which are highly effective. “The problem is, hedgehog pathway inhibitors have side effects that are not catastrophic, but they’re annoying,” Dr. Epstein said. “Patients can lose their hair, they get muscle cramps, and can lose their taste.”
To date, there have been at least three trials evaluating the feasibility of a topical hedgehog pathway inhibitor in adult subjects. In one of the trials, researchers evaluated the efficacy of LDE225, a selective antagonist of Smoothened, in eight patients with nevoid basal cell carcinoma syndrome. They were instructed to apply LDE225 twice per day for 4 weeks (J Invest Dermatol 2011 Aug;131[8]:1735-44). Of 13 BCCs treated in the patients, 12 had a partial to complete response. In fact, eight BCCs had an average of 56% volume reduction but none had complete histologic clearing.
At PellePharm, a biotechnology that Dr. Epstein cofounded, researchers are developing patidegib topical gel, 2%, for the reduction of disease burden of persistently developing BCCs in subjects with Gorlin syndrome. In a phase 3 trial, which just completed recruitment, adults are instructed to apply the gel twice daily to the face for 12 months. The primary endpoint is a comparison between the two treatment arms of the number of new surgically eligible BCCs that develop over the 12-month period. “We want to see if we can reduce the number of surgically eligible BCCs,” Dr. Epstein said. “We’re very hopeful.”
SDEF and this news organization are owned by the same parent company. Dr. Epstein spoke during a forum on cutaneous malignancies at the meeting.
Dr. Epstein disclosed that he has been a consultant for Genentech, Novartis, Amgen, and Infinity. He is also cofounder/director and stockholder in PellePharm.
LAS VEGAS – For according to Ervin H. Epstein, MD.
In a noninferiority trial, researchers in the Netherlands randomized 601 patients with superficial BCCs to one of three medical treatments: two treatments of photodynamic therapy (PDT) 1 week apart (group 1), imiquimod 5 days per week for 6 weeks with no occlusion (group 2), and 5-fluorouracil (5-FU) twice per day for 4 weeks with no occlusion (group 3). They followed the patients for 5 years to see which tumors came back (J Invest Dermatol. 2018 Mar;138[3]:527-33). At 5 years, tumor-free survival was 63% in group 1, 81% in group 2, and 70% in group 3. Based on this analysis the authors concluded that imiquimod is the first choice for noninvasive treatment of most primary BCCs.
“Yes, imiquimod is helpful, but it’s not as good as surgery,” Dr. Epstein said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Researchers like Dr. Epstein, a dermatologist at Brown & Toland Physicians in Oakland, Calif., have been exploring ways to reduce the recurrence of BCCs in those at high risk. One approach is to stay out of the sun, but that is not practical for most people, “and the data are not very supportive,” he said. “Very few people can actually avoid sunlight. What about topical 5-FU? Suppose you put on 5-FU and then study people for the next year, to see what the likelihood is of them getting a BCC? Why it has taken us so long to do this kind of study is in some ways shameful.” He mentioned one study in which patients at risk of superficial BCC recurrence applied topical 5-FU twice a day for 2-4 weeks. The regimen led to an 11% decrease in the development of BCCs, a trend that did not reach statistical significance.
Another approach to warding off BCC recurrence is to take oral hedgehog pathway inhibitors, which are highly effective. “The problem is, hedgehog pathway inhibitors have side effects that are not catastrophic, but they’re annoying,” Dr. Epstein said. “Patients can lose their hair, they get muscle cramps, and can lose their taste.”
To date, there have been at least three trials evaluating the feasibility of a topical hedgehog pathway inhibitor in adult subjects. In one of the trials, researchers evaluated the efficacy of LDE225, a selective antagonist of Smoothened, in eight patients with nevoid basal cell carcinoma syndrome. They were instructed to apply LDE225 twice per day for 4 weeks (J Invest Dermatol 2011 Aug;131[8]:1735-44). Of 13 BCCs treated in the patients, 12 had a partial to complete response. In fact, eight BCCs had an average of 56% volume reduction but none had complete histologic clearing.
At PellePharm, a biotechnology that Dr. Epstein cofounded, researchers are developing patidegib topical gel, 2%, for the reduction of disease burden of persistently developing BCCs in subjects with Gorlin syndrome. In a phase 3 trial, which just completed recruitment, adults are instructed to apply the gel twice daily to the face for 12 months. The primary endpoint is a comparison between the two treatment arms of the number of new surgically eligible BCCs that develop over the 12-month period. “We want to see if we can reduce the number of surgically eligible BCCs,” Dr. Epstein said. “We’re very hopeful.”
SDEF and this news organization are owned by the same parent company. Dr. Epstein spoke during a forum on cutaneous malignancies at the meeting.
Dr. Epstein disclosed that he has been a consultant for Genentech, Novartis, Amgen, and Infinity. He is also cofounder/director and stockholder in PellePharm.
LAS VEGAS – For according to Ervin H. Epstein, MD.
In a noninferiority trial, researchers in the Netherlands randomized 601 patients with superficial BCCs to one of three medical treatments: two treatments of photodynamic therapy (PDT) 1 week apart (group 1), imiquimod 5 days per week for 6 weeks with no occlusion (group 2), and 5-fluorouracil (5-FU) twice per day for 4 weeks with no occlusion (group 3). They followed the patients for 5 years to see which tumors came back (J Invest Dermatol. 2018 Mar;138[3]:527-33). At 5 years, tumor-free survival was 63% in group 1, 81% in group 2, and 70% in group 3. Based on this analysis the authors concluded that imiquimod is the first choice for noninvasive treatment of most primary BCCs.
“Yes, imiquimod is helpful, but it’s not as good as surgery,” Dr. Epstein said at the Skin Disease Education Foundation’s annual Las Vegas Dermatology Seminar.
Researchers like Dr. Epstein, a dermatologist at Brown & Toland Physicians in Oakland, Calif., have been exploring ways to reduce the recurrence of BCCs in those at high risk. One approach is to stay out of the sun, but that is not practical for most people, “and the data are not very supportive,” he said. “Very few people can actually avoid sunlight. What about topical 5-FU? Suppose you put on 5-FU and then study people for the next year, to see what the likelihood is of them getting a BCC? Why it has taken us so long to do this kind of study is in some ways shameful.” He mentioned one study in which patients at risk of superficial BCC recurrence applied topical 5-FU twice a day for 2-4 weeks. The regimen led to an 11% decrease in the development of BCCs, a trend that did not reach statistical significance.
Another approach to warding off BCC recurrence is to take oral hedgehog pathway inhibitors, which are highly effective. “The problem is, hedgehog pathway inhibitors have side effects that are not catastrophic, but they’re annoying,” Dr. Epstein said. “Patients can lose their hair, they get muscle cramps, and can lose their taste.”
To date, there have been at least three trials evaluating the feasibility of a topical hedgehog pathway inhibitor in adult subjects. In one of the trials, researchers evaluated the efficacy of LDE225, a selective antagonist of Smoothened, in eight patients with nevoid basal cell carcinoma syndrome. They were instructed to apply LDE225 twice per day for 4 weeks (J Invest Dermatol 2011 Aug;131[8]:1735-44). Of 13 BCCs treated in the patients, 12 had a partial to complete response. In fact, eight BCCs had an average of 56% volume reduction but none had complete histologic clearing.
At PellePharm, a biotechnology that Dr. Epstein cofounded, researchers are developing patidegib topical gel, 2%, for the reduction of disease burden of persistently developing BCCs in subjects with Gorlin syndrome. In a phase 3 trial, which just completed recruitment, adults are instructed to apply the gel twice daily to the face for 12 months. The primary endpoint is a comparison between the two treatment arms of the number of new surgically eligible BCCs that develop over the 12-month period. “We want to see if we can reduce the number of surgically eligible BCCs,” Dr. Epstein said. “We’re very hopeful.”
SDEF and this news organization are owned by the same parent company. Dr. Epstein spoke during a forum on cutaneous malignancies at the meeting.
Dr. Epstein disclosed that he has been a consultant for Genentech, Novartis, Amgen, and Infinity. He is also cofounder/director and stockholder in PellePharm.
EXPERT ANALYSIS FROM THE SDEF LAS VEGAS DERMATOLOGY SEMINAR
Systemic Medications Linked to an Increased Risk for Skin Malignancy
Dermatologists are increasingly called on to evaluate patients with complex medical problems who are often taking many medications. Over the last several decades, many new drugs that target molecular pathways in carcinogenesis and the inflammatory immune system have been developed. Increased skin cancer risk has been reported in association with BRAF inhibitors, sonic hedgehog–inhibiting agents, Janus kinase (JAK) inhibitors, and phosphodiesterase 5 (PDE-5) inhibitors. We review the literature and data regarding the significance and strength of these associations and the molecular pathways by which these medications promote cutaneous tumorigenesis. The association of skin cancer with drugs that either induce photosensitivity—nonsteroidal anti-inflammatory drugs, antibiotics (eg, tetracyclines, fluoroquinolones, trimethoprim-sulfamethoxazole), voriconazole, thiazides—or suppress the immune system—certain biologics (eg, anti–tumor necrosis factor agents), calcineurin inhibitors, thiopurines, methotrexate, cyclosporine—is well known and is therefore not reviewed in this discussion.
BRAF Inhibitors
The mitogen-activated protein kinase (MAPK) pathway (also known as the RAS/RAF/MAPK signaling pathway) is important in growth factor–receptor signaling and plays a key role in cell differentiation, survival, and proliferation. Activating mutations in this pathway allow cells to grow and proliferate in a growth factor–independent manner. Twenty percent of human cancers harbor a mutation in the RAS oncogene, an upstream mediator of the pathway.1 Activating mutations in BRAF, a serine/threonine kinase, predominate in cutaneous melanoma and also have been found in 40% to 70% of papillary thyroid malignancies, 10% to 20% of cholangiocarcinomas, and 5% to 20% of colorectal carcinomas. The most common BRAF mutation in cutaneous melanoma is V600E, which involves a glutamic acid for valine substitution at codon 600. This mutation activates BRAF 500-fold and is present in approximately 50% of melanomas.1,2
Vemurafenib, a selective BRAF inhibitor, was approved by the US Food and Drug Administration (FDA) for the treatment of metastatic melanoma in the United States in 2011. Phase 3 trial data demonstrated that vemurafenib resulted in improved survival and decreased risk for disease progression compared to dacarbazine, the former best treatment.3 During phase 1 testing, it became apparent that vemurafenib treatment was associated with a 31% increased risk for squamous cell carcinoma (SCC), most commonly well-differentiated SCC, and keratoacanthomas (KAs).4 This association was confirmed in phase 2 and 3 studies, though the incidence was lower. McArthur et al5 reported a 19% incidence of cutaneous SCC with extended follow-up analysis of the phase 3 trial. Dabrafenib, another BRAF inhibitor, has been similarly associated with increasing the risk for SCC and KA.
In one study, the mean time to development of SCC after initiating vemurafenib therapy was 10 weeks, with lesions reported as early as 3 weeks. Most patients had clinical signs of chronically sun damaged skin; however, a history of SCC was present in only 17%. Most lesions (63%) were characterized as KAs.6
The mechanism for BRAF inhibitor–induced squamoproliferative growth is due to paradoxical activation of the MAPK pathway in cells with wild-type BRAF that harbor upstream-activating mutations in RAS or tyrosine kinase receptors.7 In the presence of a BRAF inhibitor, inactivated BRAF forms heterodimers with wild-type CRAF (a BRAF-CRAF heterodimer). The heterodimer forms a complex with the mutant RAS that leads to transactivation of the CRAF molecule,8,9 resulting in a paradoxical increase in MAPK signaling and consequent ERK phosphorylation and activation through CRAF signaling. RAS, particularly HRAS, mutations have been found in 60% of all vemurafenib-associated SCCs and KAs. For this reason, it is thought that vemurafenib potentiates tumorigenesis in subclinical lesions harboring upstream MAPK pathway mutations as opposed to inducing de novo lesions.6
Because BRAF inhibitors are remarkably efficacious in the treatment of metastatic melanomas harboring the V600E BRAF mutation, there are no restrictions on their use, despite the known increased risk for SCC. Squamous cell carcinomas tend to be low grade, and all tumors that developed in phase 1 to 3 trials were treated with simple excision. The development of SCC did not necessitate interruption of treatment. Furthermore, the addition of MEK inhibition to BRAF inhibitor therapy reduces the risk for SCC from 19% to 7%.7,10,11
In addition to SCC, second primary melanomas (SPMs) have been reported in patients treated with BRAF inhibitors. It has been shown that these melanomas occur in melanocytes with wild-type BRAF. It has been postulated that some of these tumors occur in cells that harbor upstream mutations in RAS, whereas others might result from alternate signaling through non-RAF oncogenic pathways.9,12
Zimmer et al1 reported 12 SPMs in 11 patients treated with BRAF inhibitor therapy. They reported a median delay of 8 weeks (range, 4–27 weeks) for SPM development. Tumors were detected in early stages; 1 tumor harbored an NRAS mutation.1
Dalle et al13 reported 25 SPMs in 120 vemurafenib-treated patients. Median delay in SPM development was 14 weeks (range, 4–42 weeks). All tumors were thin, ranging from in situ to 0.45-mm thick. Wild-type BRAF was detected in the 21 melanomas sampled; 1 lesion showed mutated NRAS.13
The exact incidence of SPM in the setting of BRAF inhibition is thought to be at least 10-fold less than SCC and KA.2 Patients on BRAF inhibitor therapy should have routine full-body skin examinations, given the increased risk for SPM and SCC.
Another drug belonging to the tyrosine kinase inhibitor family, sorafenib, is used in the treatment of solid tumors, particularly hepatocellular and renal cell carcinomas, and also has been associated with development of cutaneous SCC and KAs.14 Sorafenib is a multiple tyrosine kinase inhibitor that also inhibits the RAF serine/threonine kinases. Similar to vemurafenib and dabrafenib, SCCs and KAs associated with sorafenib tend to arise in patients with chronic actinic damage during the first 2 months of treatment. It has been hypothesized that inhibition of RAF kinases is pathogenic in inducing SCCs because these lesions have not been reported with sunitinib, another multiple tyrosine kinase inhibitor that lacks the ability to inhibit serine/threonine kinases.15,16 Although SCCs and KAs associated with sorafenib tend to be low grade, it is reasonable to consider sunitinib or an alternative tyrosine kinase inhibitor in patients who develop multiple SCCs while taking sorafenib.16
Sonic Hedgehog–Inhibiting Agents
Vismodegib, the first small molecule inhibitor of the signaling protein smoothened, gained FDA approval for the treatment of metastatic or locally advanced basal cell carcinoma (BCC) in 2012. A second agent with an identical mechanism of action, sonidegib, was approved by the FDA for locally advanced BCC in 2015. Approximately 90% of BCCs contain mutations in the sonic hedgehog pathway, which lead to constitutive smoothened activation and uncontrolled cell proliferation.17 The development of smoothened inhibitors introduced a much-needed treatment for inoperable or metastatic BCC,17,18 though long-term utility is limited by drug resistance with extended use in this patient population.19,20 Several case reports have documented the emergence of KA21 and cutaneous SCC following vismodegib treatment of advanced or metastatic BCC.22-24 A larger case-control study by Mohan et al25 showed that patients with BCC treated with vismodegib had an increased risk for non-BCC malignancy (hazard ratio [HR]=6.37), most of which were cutaneous SCC (HR=8.12).
The mechanism by which selective inhibition of smoothened leads to cutaneous SCC is unclear. A study found that patients on vismodegib who developed SCC within the original BCC site had elevated ERK levels within tumor tissue, suggesting that the RAS/RAF/MAPK pathway can become upregulated during hedgehog inhibition.26 Other studies looking at hedgehog inhibition in medulloblastoma models also have shown activated RAS/RAF/MAPK pathways.25 These findings suggest that tumors under smoothened inhibition might be able to bypass the sonic hedgehog pathway and continue to grow by upregulating alternative growth pathways, such as RAS/RAF/MAPK.25,26
The incidence of cutaneous SCC following vismodegib treatment is unknown. Chang and Oro27 examined BCC tumor regrowth from secondary (acquired) resistance to vismodegib and noted that lesions recurred within 1 cm of the original tumor 21% of the time. Although none of the 12 patients whose tumors regrew during treatment were reported to have developed SCC, several demonstrated different BCC subtypes than the pretreatment specimen. The authors proposed that regrowth of BCC was due to upregulated alternative pathways allowing tumors to bypass smoothened inhibition, which is similar to the proposed mechanism for SCC development in vismodegib patients.27
Prospective studies are needed to confirm the link between vismodegib and cutaneous SCC; establish the incidence of SCC development; and identify any pretreatment factors, tumor characteristics, or treatment details (eg, dosage, duration) that might contribute to SCC development. Furthermore, because Mohan et al25 observed that vismodegib-treated patients were less likely to develop SCC in situ than controls, it is unknown if these tumors are more aggressive than traditional SCC. At this point, careful surveillance and regular full-body skin examinations are advised for patients on vismodegib for treatment of advanced BCC.
JAK Inhibitors
Another class of medications potentially associated with increased development of nonmelanoma skin cancer (NMSC) is the JAK inhibitors (also known as jakinibs). Many proinflammatory signaling pathways converge on the JAK family of enzymes—JAK1, JAK2, JAK3, and TYK2. These enzymes operate in cytokine signal transduction by phosphorylating activated cytokine receptors, which allows for recruitment and activation by means of phosphorylation of transcription factors collectively known as signal transducers and activators of transcription (STATs). Phosphorylated STATs dimerize and translocate to the nucleus, acting as direct transcription promoters. Janus kinase inhibitors modulate the immune response by reducing the effect of interleukin and interferon signaling.
Ruxolitinib, a JAK1/JAK2 inhibitor, was the first JAK inhibitor approved by the FDA and is indicated for the treatment of myelofibrosis and polycythemia vera. Additionally, oral and topical JAK inhibitors have shown efficacy in the treatment of psoriasis, rheumatoid arthritis, alopecia areata, vitiligo, and pruritus from atopic dermatitis.28
The JAK-STAT pathway is complex, and the biological activity of the pathway is both proinflammatory and pro–cell survival and proliferation. Because signaling through the pathway can increase angiogenesis and inhibit apoptosis, inhibition of this pathway has been exploited for the treatment of some tumors. However, inhibition of interferon and proinflammatory interleukin signaling also can potentially promote tumor growth by means of inhibition of downstream cytotoxic T-cell signaling, theoretically increasing the risk for NMSC. A study examining the 5-year efficacy of ruxolitinib in myelofibrosis patients (COMFORT-II trial) found that 17.1% of patients developed NMSC compared to only 2.7% of those on the best available therapy. After adjustment by patient exposure, the NMSC rate was still doubled for ruxolitinib-treated patients compared to controls (6.1/100 patient-years and 3.0/100 patient-years, respectively).29 Eighty-week follow-up of the phase 3 clinical trial of ruxolitinib for the treatment of polycythemia vera also noted an increased incidence of NMSC, albeit a more conservative increase. Patients randomized to the ruxolitinib treatment group developed NMSC at a rate of 4.4/100 patient-years, whereas the rate for controls treated with best available therapy was 2.7/100 patient-years.30 In contrast, 5-year follow-up of the COMFORT-I trial, also examining the efficacy of ruxolitinib in myelofibrosis, showed no increased risk for NMSC between ruxolitinib-treated patients and placebo (2.7/100 patient-years and 3.9/100 patient-years, respectively).31
A 2017 case series described 5 patients with myelofibrosis who developed multiple skin cancers with aggressive features while receiving ruxolitinib.32 Duration of ruxolitinib therapy ranged from 4 months to 4 years; 3 patients had a history of hydroxyurea exposure, and only 1 patient had a history of NMSC. High-risk cutaneous SCC, undifferentiated pleomorphic sarcoma, and lentigo maligna melanoma (Breslow thickness, 0.45 mm) were among the tumors reported in this series. Although no definitive conclusion can be made regarding the causality of JAK inhibitors in promoting these tumors, the association warrants further investigation. Clinicians should be aware that ruxolitinib might amplify the risk for NMSC in patients with pre-existing genetic or exposure-related susceptibility. Interruption of drug therapy may be necessary in managing patients who develop an aggressive tumor.32
In contrast, tofacitinib, which specifically inhibits JAK3, carries very low risk, if any, for NMSC when used for the treatment of psoriasis and rheumatoid arthritis. Results from 2 phase 3 trials analyzing the efficacy of tofacitinib in psoriasis demonstrated that only 2 of 1486 patients treated developed NMSC compared to none in the control group.33 Furthermore, analysis of NMSC across the tofacitinib rheumatoid arthritis clinical program, which included a total of 15,103 patient-years of exposure, demonstrated that the overall NMSC incidence was 0.55 for every 100 patient-years. Of note, the risk in patients receiving high-dose treatment (10 mg vs 5 mg) was nearly doubled in long-term follow-up studies (0.79/100 patient-years and 0.41/100 patient-years, respectively). Overall, the study concluded that treatment with tofacitinib presents no greater increased risk for NMSC than treatment with tumor necrosis factor inhibitors.33
PDE-5 Inhibitors
Phosphodiesterase 5 inhibitors, such as sildenafil citrate, have been widely prescribed for the treatment of erectile dysfunction. Studies have shown that BRAF-activated melanomas, which occur in approximately 50% to 70% of melanomas, also result in reduced PDE-5 expression.34-36 In these melanomas, downregulation of PDE-5 results in increased intracellular calcium,36 which has been shown to induce melanoma invasion.36,37 Given this similarity in molecular pathway between BRAF-activated melanomas and PDE-5 inhibitors, there has been increased concern that PDE-5 inhibitors might be associated with an increased risk for melanoma.
In 2014, Li et al38 published a retrospective analysis suggesting an association with sildenafil and an increased risk for melanoma. Their study utilized the Health Professionals Follow-up Study to identify a statistically significant elevation in the risk for invasive melanoma with both recent sildenafil use (multivariate-adjusted HR=2.24) and use at any time (HR=1.92). These results controlled for confounding variables, such as presence of major chronic disease, use of other erectile dysfunction treatments, family history of melanoma, history of sun exposure, and UV index of the patient’s residence. Notably, the study also found that sildenafil did not affect the incidence of BCC or SCC.38
In 2015, Loeb et al39 also examined the potential association between PDE-5 inhibitors and melanoma. Review of several Swedish drug and cancer registries allowed for analysis of melanoma risk and PDE-5 inhibitor use, based on number of prescriptions filled and type of PDE-5 inhibitor prescribed. Their analysis showed that men developing melanoma were more likely than nonmelanoma controls to have taken a PDE-5 inhibitor (11% vs 8%). In a subgroup analysis, however, statistical significance was shown for men with only a single prescription filled (34% of cases; P<.05), whereas the difference for men with multiple filled prescriptions did not meet statistical significance. Furthermore, the study did not find increased risk with longer-acting tadalafil and vardenafil (odds ratio [OR]=1.16) compared to sildenafil (OR=1.14). Last, use of PDE-5 inhibitors was only associated with stage 0 (OR=1.49) and stage I (OR=1.21) tumors, not with stages II to IV (OR=0.83) tumors. Although there was a statistically significant association between PDE-5 inhibitors and malignant melanoma (P<.05), the subgroup analysis findings pointed away from a causal relationship and likely toward a confounding of variable(s).39
A 2016 study by Lian et al40 looked at the risk for melanoma in a cohort of patients diagnosed with erectile dysfunction. No association between PDE-5 inhibitors and melanoma risk was shown when comparing patients who received a PDE-5 inhibitor and those who did not receive a PDE-5 inhibitor. However, secondary analysis did show that melanoma risk was increased among patients receiving more pills (34%) and prescriptions (30%). The authors concluded that there was no association between PDE-5 inhibitor use and overall increased risk for melanoma, and the increased risk associated with a greater number of pills and prescriptions would require further study.40
In contrast, a 2017 meta-analysis by Tang et al41 of 5 studies (3 of which were the aforementioned trials38-40) concluded that use of PDE-5 inhibitors was associated with a small but significantly increased risk for melanoma (OR=1.12) and BCC (OR=1.14) but not SCC. Furthermore, the study found no evidence of dosage-dependent association between PDE-5 inhibitor use and melanoma risk.41
Overall, clinical studies have been inconclusive in determining the risk for melanoma in the setting of PDE-5 inhibitor use. Studies showing an increased rate of melanoma within patient cohorts receiving PDE-5 inhibitors are limited; results might be affected by confounding variables. However, given the similarity in mechanism between PDE-5 inhibitors and HRAS-activated melanomas, it is reasonable to continue research into this potential association.
Conclusion
Since the turn of the century, drugs targeting cell-signaling pathways have been developed to treat inflammatory, oncologic, and immune conditions. The role of immunosuppressants in promoting skin cancer is well established and supported by a vast literature base. However, associations are less clear with newer immunomodulatory and antineoplastic medications. Skin cancer has been reported in association with BRAF inhibitors, sonic hedgehog–inhibiting agents, JAK inhibitors, and PDE-5 inhibitors. In the case of JAK and PDE-5 inhibitors, the increased risk for melanoma and NMSC is somewhat inconclusive; risk is more firmly established for BRAF inhibitors and smoothened inhibitors. For the antineoplastic agents reviewed, the therapeutic effect of cancer regression is well documented, and benefits of continued therapy outweigh the increased risk for skin cancer promotion in nearly all cases. The value of early detection has been well documented for skin malignancy; therefore, increased skin surveillance and prompt management of suspicious lesions should be a priority for physicians treating patients undergoing therapy with these medications
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanoma in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
- Chapman PB, Hauschild A, Robert C, et al; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
- Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427-430.
- Ryan MB, Der CJ, Wang-Gillam A, et al. Targeting RAS-mutant cancers: is ERK the key? Trends Cancer. 2015;1:183-198.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Holderfield M, Nagel TE, Stuart DD. Mechanism and consequence of RAF kinase activation by small-molecule inhibitors. Br J Cancer. 2014;111:640-645.
- Dalle S, Poulalhon N, Debarbieux S, et al. Tracking of second primary melanomas in vemurafenib-treated patients. JAMA Dermatol. 2013;149:488-490.
- Williams VL, Cohen PR, Stewart DJ. Sorafenib-induced premalignant and malignant skin lesions. Int J Dermatol. 2011;50:396-402.
- Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59-e61.
- Smith KJ, Haley H, Hamza S, et al. Eruptive keratoacanthoma-type squamous cell carcinomas in patients taking sorafenib for the treatment of solid tumors. Dermatol Surg. 2009;35:1766-1770.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Demirci H, Worden F, Nelson CC, et al. Efficacy of vismodegib (Erivedge) for basal cell carcinoma involving the orbit and periocular area. Ophthalmic Plast Reconstr Surg. 2015;31:463-466.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Ridky TW, Cotsarelis G. Vismodegib resistance in basal cell carcinoma: not a smooth fit. Cancer Cell. 2015;27:315-316.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:e33-e34.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Zhao X, Ponomaryov T, Ornell KJ, et al. RAS/MAPK activation drives resistance to Smo inhibition, metastasis, and tumor evolution in Shh pathway-dependent tumors. Cancer Res. 2015;75:3623-3635.
- Chang AL, Oro AE. Initial assessment of tumor regrowth after vismodegib in advanced basal cell carcinoma. Arch Dermatol. 2012;148:1324-1325.
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76:736-744.
- Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30:1701-1707.
- Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101:821-829.
- Verstovsek S, Mesa RA, Gotlib J, et al; COMFORT-I investigators. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10:55.
- Blechman AB, Cabell CE, Weinberger CH, et al. Aggressive skin cancers occurring in patients treated with the Janus kinase inhibitor ruxolitinib. J Drugs Dermatol. 2017;16:508-511.
- Papp KA, Menter MA, Abe M, et al; OPT Pivotal 1 and OPT Pivotal 2 investigators. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949-961.
- Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875-885.
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851-857.
- Arozarena I, Sanchez-Laorden B, Packer L, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45-57.
- Houslay MD. Hard times for oncogenic BRAF-expressing melanoma cells. Cancer Cell. 2011;19:3-4.
- Li WQ, Qureshi AA, Robinson KC, et al. Sildenafil use and increased risk of incident melanoma in US men: a prospective cohort study. JAMA Intern Med. 2014;174:964-970.
- Loeb S, Folkvaljon Y, Lambe M, et al. Use of phosphodiesterase type 5 inhibitors for erectile dysfunction and risk of malignant melanoma. JAMA. 2015;313:2449-2455.
- Lian Y, Yin H, Pollak MN, et al. Phosphodiesterase type 5 inhibitors and the risk of melanoma skin cancer. Eur Urol. 2016;70:808-815.
- Tang H, Wu W, Fu S, et al. Phosphodiesterase type 5 inhibitors and risk of melanoma: a meta-analysis. J Am Acad Dermatol. 2017;77:480.e9-488.e9.
Dermatologists are increasingly called on to evaluate patients with complex medical problems who are often taking many medications. Over the last several decades, many new drugs that target molecular pathways in carcinogenesis and the inflammatory immune system have been developed. Increased skin cancer risk has been reported in association with BRAF inhibitors, sonic hedgehog–inhibiting agents, Janus kinase (JAK) inhibitors, and phosphodiesterase 5 (PDE-5) inhibitors. We review the literature and data regarding the significance and strength of these associations and the molecular pathways by which these medications promote cutaneous tumorigenesis. The association of skin cancer with drugs that either induce photosensitivity—nonsteroidal anti-inflammatory drugs, antibiotics (eg, tetracyclines, fluoroquinolones, trimethoprim-sulfamethoxazole), voriconazole, thiazides—or suppress the immune system—certain biologics (eg, anti–tumor necrosis factor agents), calcineurin inhibitors, thiopurines, methotrexate, cyclosporine—is well known and is therefore not reviewed in this discussion.
BRAF Inhibitors
The mitogen-activated protein kinase (MAPK) pathway (also known as the RAS/RAF/MAPK signaling pathway) is important in growth factor–receptor signaling and plays a key role in cell differentiation, survival, and proliferation. Activating mutations in this pathway allow cells to grow and proliferate in a growth factor–independent manner. Twenty percent of human cancers harbor a mutation in the RAS oncogene, an upstream mediator of the pathway.1 Activating mutations in BRAF, a serine/threonine kinase, predominate in cutaneous melanoma and also have been found in 40% to 70% of papillary thyroid malignancies, 10% to 20% of cholangiocarcinomas, and 5% to 20% of colorectal carcinomas. The most common BRAF mutation in cutaneous melanoma is V600E, which involves a glutamic acid for valine substitution at codon 600. This mutation activates BRAF 500-fold and is present in approximately 50% of melanomas.1,2
Vemurafenib, a selective BRAF inhibitor, was approved by the US Food and Drug Administration (FDA) for the treatment of metastatic melanoma in the United States in 2011. Phase 3 trial data demonstrated that vemurafenib resulted in improved survival and decreased risk for disease progression compared to dacarbazine, the former best treatment.3 During phase 1 testing, it became apparent that vemurafenib treatment was associated with a 31% increased risk for squamous cell carcinoma (SCC), most commonly well-differentiated SCC, and keratoacanthomas (KAs).4 This association was confirmed in phase 2 and 3 studies, though the incidence was lower. McArthur et al5 reported a 19% incidence of cutaneous SCC with extended follow-up analysis of the phase 3 trial. Dabrafenib, another BRAF inhibitor, has been similarly associated with increasing the risk for SCC and KA.
In one study, the mean time to development of SCC after initiating vemurafenib therapy was 10 weeks, with lesions reported as early as 3 weeks. Most patients had clinical signs of chronically sun damaged skin; however, a history of SCC was present in only 17%. Most lesions (63%) were characterized as KAs.6
The mechanism for BRAF inhibitor–induced squamoproliferative growth is due to paradoxical activation of the MAPK pathway in cells with wild-type BRAF that harbor upstream-activating mutations in RAS or tyrosine kinase receptors.7 In the presence of a BRAF inhibitor, inactivated BRAF forms heterodimers with wild-type CRAF (a BRAF-CRAF heterodimer). The heterodimer forms a complex with the mutant RAS that leads to transactivation of the CRAF molecule,8,9 resulting in a paradoxical increase in MAPK signaling and consequent ERK phosphorylation and activation through CRAF signaling. RAS, particularly HRAS, mutations have been found in 60% of all vemurafenib-associated SCCs and KAs. For this reason, it is thought that vemurafenib potentiates tumorigenesis in subclinical lesions harboring upstream MAPK pathway mutations as opposed to inducing de novo lesions.6
Because BRAF inhibitors are remarkably efficacious in the treatment of metastatic melanomas harboring the V600E BRAF mutation, there are no restrictions on their use, despite the known increased risk for SCC. Squamous cell carcinomas tend to be low grade, and all tumors that developed in phase 1 to 3 trials were treated with simple excision. The development of SCC did not necessitate interruption of treatment. Furthermore, the addition of MEK inhibition to BRAF inhibitor therapy reduces the risk for SCC from 19% to 7%.7,10,11
In addition to SCC, second primary melanomas (SPMs) have been reported in patients treated with BRAF inhibitors. It has been shown that these melanomas occur in melanocytes with wild-type BRAF. It has been postulated that some of these tumors occur in cells that harbor upstream mutations in RAS, whereas others might result from alternate signaling through non-RAF oncogenic pathways.9,12
Zimmer et al1 reported 12 SPMs in 11 patients treated with BRAF inhibitor therapy. They reported a median delay of 8 weeks (range, 4–27 weeks) for SPM development. Tumors were detected in early stages; 1 tumor harbored an NRAS mutation.1
Dalle et al13 reported 25 SPMs in 120 vemurafenib-treated patients. Median delay in SPM development was 14 weeks (range, 4–42 weeks). All tumors were thin, ranging from in situ to 0.45-mm thick. Wild-type BRAF was detected in the 21 melanomas sampled; 1 lesion showed mutated NRAS.13
The exact incidence of SPM in the setting of BRAF inhibition is thought to be at least 10-fold less than SCC and KA.2 Patients on BRAF inhibitor therapy should have routine full-body skin examinations, given the increased risk for SPM and SCC.
Another drug belonging to the tyrosine kinase inhibitor family, sorafenib, is used in the treatment of solid tumors, particularly hepatocellular and renal cell carcinomas, and also has been associated with development of cutaneous SCC and KAs.14 Sorafenib is a multiple tyrosine kinase inhibitor that also inhibits the RAF serine/threonine kinases. Similar to vemurafenib and dabrafenib, SCCs and KAs associated with sorafenib tend to arise in patients with chronic actinic damage during the first 2 months of treatment. It has been hypothesized that inhibition of RAF kinases is pathogenic in inducing SCCs because these lesions have not been reported with sunitinib, another multiple tyrosine kinase inhibitor that lacks the ability to inhibit serine/threonine kinases.15,16 Although SCCs and KAs associated with sorafenib tend to be low grade, it is reasonable to consider sunitinib or an alternative tyrosine kinase inhibitor in patients who develop multiple SCCs while taking sorafenib.16
Sonic Hedgehog–Inhibiting Agents
Vismodegib, the first small molecule inhibitor of the signaling protein smoothened, gained FDA approval for the treatment of metastatic or locally advanced basal cell carcinoma (BCC) in 2012. A second agent with an identical mechanism of action, sonidegib, was approved by the FDA for locally advanced BCC in 2015. Approximately 90% of BCCs contain mutations in the sonic hedgehog pathway, which lead to constitutive smoothened activation and uncontrolled cell proliferation.17 The development of smoothened inhibitors introduced a much-needed treatment for inoperable or metastatic BCC,17,18 though long-term utility is limited by drug resistance with extended use in this patient population.19,20 Several case reports have documented the emergence of KA21 and cutaneous SCC following vismodegib treatment of advanced or metastatic BCC.22-24 A larger case-control study by Mohan et al25 showed that patients with BCC treated with vismodegib had an increased risk for non-BCC malignancy (hazard ratio [HR]=6.37), most of which were cutaneous SCC (HR=8.12).
The mechanism by which selective inhibition of smoothened leads to cutaneous SCC is unclear. A study found that patients on vismodegib who developed SCC within the original BCC site had elevated ERK levels within tumor tissue, suggesting that the RAS/RAF/MAPK pathway can become upregulated during hedgehog inhibition.26 Other studies looking at hedgehog inhibition in medulloblastoma models also have shown activated RAS/RAF/MAPK pathways.25 These findings suggest that tumors under smoothened inhibition might be able to bypass the sonic hedgehog pathway and continue to grow by upregulating alternative growth pathways, such as RAS/RAF/MAPK.25,26
The incidence of cutaneous SCC following vismodegib treatment is unknown. Chang and Oro27 examined BCC tumor regrowth from secondary (acquired) resistance to vismodegib and noted that lesions recurred within 1 cm of the original tumor 21% of the time. Although none of the 12 patients whose tumors regrew during treatment were reported to have developed SCC, several demonstrated different BCC subtypes than the pretreatment specimen. The authors proposed that regrowth of BCC was due to upregulated alternative pathways allowing tumors to bypass smoothened inhibition, which is similar to the proposed mechanism for SCC development in vismodegib patients.27
Prospective studies are needed to confirm the link between vismodegib and cutaneous SCC; establish the incidence of SCC development; and identify any pretreatment factors, tumor characteristics, or treatment details (eg, dosage, duration) that might contribute to SCC development. Furthermore, because Mohan et al25 observed that vismodegib-treated patients were less likely to develop SCC in situ than controls, it is unknown if these tumors are more aggressive than traditional SCC. At this point, careful surveillance and regular full-body skin examinations are advised for patients on vismodegib for treatment of advanced BCC.
JAK Inhibitors
Another class of medications potentially associated with increased development of nonmelanoma skin cancer (NMSC) is the JAK inhibitors (also known as jakinibs). Many proinflammatory signaling pathways converge on the JAK family of enzymes—JAK1, JAK2, JAK3, and TYK2. These enzymes operate in cytokine signal transduction by phosphorylating activated cytokine receptors, which allows for recruitment and activation by means of phosphorylation of transcription factors collectively known as signal transducers and activators of transcription (STATs). Phosphorylated STATs dimerize and translocate to the nucleus, acting as direct transcription promoters. Janus kinase inhibitors modulate the immune response by reducing the effect of interleukin and interferon signaling.
Ruxolitinib, a JAK1/JAK2 inhibitor, was the first JAK inhibitor approved by the FDA and is indicated for the treatment of myelofibrosis and polycythemia vera. Additionally, oral and topical JAK inhibitors have shown efficacy in the treatment of psoriasis, rheumatoid arthritis, alopecia areata, vitiligo, and pruritus from atopic dermatitis.28
The JAK-STAT pathway is complex, and the biological activity of the pathway is both proinflammatory and pro–cell survival and proliferation. Because signaling through the pathway can increase angiogenesis and inhibit apoptosis, inhibition of this pathway has been exploited for the treatment of some tumors. However, inhibition of interferon and proinflammatory interleukin signaling also can potentially promote tumor growth by means of inhibition of downstream cytotoxic T-cell signaling, theoretically increasing the risk for NMSC. A study examining the 5-year efficacy of ruxolitinib in myelofibrosis patients (COMFORT-II trial) found that 17.1% of patients developed NMSC compared to only 2.7% of those on the best available therapy. After adjustment by patient exposure, the NMSC rate was still doubled for ruxolitinib-treated patients compared to controls (6.1/100 patient-years and 3.0/100 patient-years, respectively).29 Eighty-week follow-up of the phase 3 clinical trial of ruxolitinib for the treatment of polycythemia vera also noted an increased incidence of NMSC, albeit a more conservative increase. Patients randomized to the ruxolitinib treatment group developed NMSC at a rate of 4.4/100 patient-years, whereas the rate for controls treated with best available therapy was 2.7/100 patient-years.30 In contrast, 5-year follow-up of the COMFORT-I trial, also examining the efficacy of ruxolitinib in myelofibrosis, showed no increased risk for NMSC between ruxolitinib-treated patients and placebo (2.7/100 patient-years and 3.9/100 patient-years, respectively).31
A 2017 case series described 5 patients with myelofibrosis who developed multiple skin cancers with aggressive features while receiving ruxolitinib.32 Duration of ruxolitinib therapy ranged from 4 months to 4 years; 3 patients had a history of hydroxyurea exposure, and only 1 patient had a history of NMSC. High-risk cutaneous SCC, undifferentiated pleomorphic sarcoma, and lentigo maligna melanoma (Breslow thickness, 0.45 mm) were among the tumors reported in this series. Although no definitive conclusion can be made regarding the causality of JAK inhibitors in promoting these tumors, the association warrants further investigation. Clinicians should be aware that ruxolitinib might amplify the risk for NMSC in patients with pre-existing genetic or exposure-related susceptibility. Interruption of drug therapy may be necessary in managing patients who develop an aggressive tumor.32
In contrast, tofacitinib, which specifically inhibits JAK3, carries very low risk, if any, for NMSC when used for the treatment of psoriasis and rheumatoid arthritis. Results from 2 phase 3 trials analyzing the efficacy of tofacitinib in psoriasis demonstrated that only 2 of 1486 patients treated developed NMSC compared to none in the control group.33 Furthermore, analysis of NMSC across the tofacitinib rheumatoid arthritis clinical program, which included a total of 15,103 patient-years of exposure, demonstrated that the overall NMSC incidence was 0.55 for every 100 patient-years. Of note, the risk in patients receiving high-dose treatment (10 mg vs 5 mg) was nearly doubled in long-term follow-up studies (0.79/100 patient-years and 0.41/100 patient-years, respectively). Overall, the study concluded that treatment with tofacitinib presents no greater increased risk for NMSC than treatment with tumor necrosis factor inhibitors.33
PDE-5 Inhibitors
Phosphodiesterase 5 inhibitors, such as sildenafil citrate, have been widely prescribed for the treatment of erectile dysfunction. Studies have shown that BRAF-activated melanomas, which occur in approximately 50% to 70% of melanomas, also result in reduced PDE-5 expression.34-36 In these melanomas, downregulation of PDE-5 results in increased intracellular calcium,36 which has been shown to induce melanoma invasion.36,37 Given this similarity in molecular pathway between BRAF-activated melanomas and PDE-5 inhibitors, there has been increased concern that PDE-5 inhibitors might be associated with an increased risk for melanoma.
In 2014, Li et al38 published a retrospective analysis suggesting an association with sildenafil and an increased risk for melanoma. Their study utilized the Health Professionals Follow-up Study to identify a statistically significant elevation in the risk for invasive melanoma with both recent sildenafil use (multivariate-adjusted HR=2.24) and use at any time (HR=1.92). These results controlled for confounding variables, such as presence of major chronic disease, use of other erectile dysfunction treatments, family history of melanoma, history of sun exposure, and UV index of the patient’s residence. Notably, the study also found that sildenafil did not affect the incidence of BCC or SCC.38
In 2015, Loeb et al39 also examined the potential association between PDE-5 inhibitors and melanoma. Review of several Swedish drug and cancer registries allowed for analysis of melanoma risk and PDE-5 inhibitor use, based on number of prescriptions filled and type of PDE-5 inhibitor prescribed. Their analysis showed that men developing melanoma were more likely than nonmelanoma controls to have taken a PDE-5 inhibitor (11% vs 8%). In a subgroup analysis, however, statistical significance was shown for men with only a single prescription filled (34% of cases; P<.05), whereas the difference for men with multiple filled prescriptions did not meet statistical significance. Furthermore, the study did not find increased risk with longer-acting tadalafil and vardenafil (odds ratio [OR]=1.16) compared to sildenafil (OR=1.14). Last, use of PDE-5 inhibitors was only associated with stage 0 (OR=1.49) and stage I (OR=1.21) tumors, not with stages II to IV (OR=0.83) tumors. Although there was a statistically significant association between PDE-5 inhibitors and malignant melanoma (P<.05), the subgroup analysis findings pointed away from a causal relationship and likely toward a confounding of variable(s).39
A 2016 study by Lian et al40 looked at the risk for melanoma in a cohort of patients diagnosed with erectile dysfunction. No association between PDE-5 inhibitors and melanoma risk was shown when comparing patients who received a PDE-5 inhibitor and those who did not receive a PDE-5 inhibitor. However, secondary analysis did show that melanoma risk was increased among patients receiving more pills (34%) and prescriptions (30%). The authors concluded that there was no association between PDE-5 inhibitor use and overall increased risk for melanoma, and the increased risk associated with a greater number of pills and prescriptions would require further study.40
In contrast, a 2017 meta-analysis by Tang et al41 of 5 studies (3 of which were the aforementioned trials38-40) concluded that use of PDE-5 inhibitors was associated with a small but significantly increased risk for melanoma (OR=1.12) and BCC (OR=1.14) but not SCC. Furthermore, the study found no evidence of dosage-dependent association between PDE-5 inhibitor use and melanoma risk.41
Overall, clinical studies have been inconclusive in determining the risk for melanoma in the setting of PDE-5 inhibitor use. Studies showing an increased rate of melanoma within patient cohorts receiving PDE-5 inhibitors are limited; results might be affected by confounding variables. However, given the similarity in mechanism between PDE-5 inhibitors and HRAS-activated melanomas, it is reasonable to continue research into this potential association.
Conclusion
Since the turn of the century, drugs targeting cell-signaling pathways have been developed to treat inflammatory, oncologic, and immune conditions. The role of immunosuppressants in promoting skin cancer is well established and supported by a vast literature base. However, associations are less clear with newer immunomodulatory and antineoplastic medications. Skin cancer has been reported in association with BRAF inhibitors, sonic hedgehog–inhibiting agents, JAK inhibitors, and PDE-5 inhibitors. In the case of JAK and PDE-5 inhibitors, the increased risk for melanoma and NMSC is somewhat inconclusive; risk is more firmly established for BRAF inhibitors and smoothened inhibitors. For the antineoplastic agents reviewed, the therapeutic effect of cancer regression is well documented, and benefits of continued therapy outweigh the increased risk for skin cancer promotion in nearly all cases. The value of early detection has been well documented for skin malignancy; therefore, increased skin surveillance and prompt management of suspicious lesions should be a priority for physicians treating patients undergoing therapy with these medications
Dermatologists are increasingly called on to evaluate patients with complex medical problems who are often taking many medications. Over the last several decades, many new drugs that target molecular pathways in carcinogenesis and the inflammatory immune system have been developed. Increased skin cancer risk has been reported in association with BRAF inhibitors, sonic hedgehog–inhibiting agents, Janus kinase (JAK) inhibitors, and phosphodiesterase 5 (PDE-5) inhibitors. We review the literature and data regarding the significance and strength of these associations and the molecular pathways by which these medications promote cutaneous tumorigenesis. The association of skin cancer with drugs that either induce photosensitivity—nonsteroidal anti-inflammatory drugs, antibiotics (eg, tetracyclines, fluoroquinolones, trimethoprim-sulfamethoxazole), voriconazole, thiazides—or suppress the immune system—certain biologics (eg, anti–tumor necrosis factor agents), calcineurin inhibitors, thiopurines, methotrexate, cyclosporine—is well known and is therefore not reviewed in this discussion.
BRAF Inhibitors
The mitogen-activated protein kinase (MAPK) pathway (also known as the RAS/RAF/MAPK signaling pathway) is important in growth factor–receptor signaling and plays a key role in cell differentiation, survival, and proliferation. Activating mutations in this pathway allow cells to grow and proliferate in a growth factor–independent manner. Twenty percent of human cancers harbor a mutation in the RAS oncogene, an upstream mediator of the pathway.1 Activating mutations in BRAF, a serine/threonine kinase, predominate in cutaneous melanoma and also have been found in 40% to 70% of papillary thyroid malignancies, 10% to 20% of cholangiocarcinomas, and 5% to 20% of colorectal carcinomas. The most common BRAF mutation in cutaneous melanoma is V600E, which involves a glutamic acid for valine substitution at codon 600. This mutation activates BRAF 500-fold and is present in approximately 50% of melanomas.1,2
Vemurafenib, a selective BRAF inhibitor, was approved by the US Food and Drug Administration (FDA) for the treatment of metastatic melanoma in the United States in 2011. Phase 3 trial data demonstrated that vemurafenib resulted in improved survival and decreased risk for disease progression compared to dacarbazine, the former best treatment.3 During phase 1 testing, it became apparent that vemurafenib treatment was associated with a 31% increased risk for squamous cell carcinoma (SCC), most commonly well-differentiated SCC, and keratoacanthomas (KAs).4 This association was confirmed in phase 2 and 3 studies, though the incidence was lower. McArthur et al5 reported a 19% incidence of cutaneous SCC with extended follow-up analysis of the phase 3 trial. Dabrafenib, another BRAF inhibitor, has been similarly associated with increasing the risk for SCC and KA.
In one study, the mean time to development of SCC after initiating vemurafenib therapy was 10 weeks, with lesions reported as early as 3 weeks. Most patients had clinical signs of chronically sun damaged skin; however, a history of SCC was present in only 17%. Most lesions (63%) were characterized as KAs.6
The mechanism for BRAF inhibitor–induced squamoproliferative growth is due to paradoxical activation of the MAPK pathway in cells with wild-type BRAF that harbor upstream-activating mutations in RAS or tyrosine kinase receptors.7 In the presence of a BRAF inhibitor, inactivated BRAF forms heterodimers with wild-type CRAF (a BRAF-CRAF heterodimer). The heterodimer forms a complex with the mutant RAS that leads to transactivation of the CRAF molecule,8,9 resulting in a paradoxical increase in MAPK signaling and consequent ERK phosphorylation and activation through CRAF signaling. RAS, particularly HRAS, mutations have been found in 60% of all vemurafenib-associated SCCs and KAs. For this reason, it is thought that vemurafenib potentiates tumorigenesis in subclinical lesions harboring upstream MAPK pathway mutations as opposed to inducing de novo lesions.6
Because BRAF inhibitors are remarkably efficacious in the treatment of metastatic melanomas harboring the V600E BRAF mutation, there are no restrictions on their use, despite the known increased risk for SCC. Squamous cell carcinomas tend to be low grade, and all tumors that developed in phase 1 to 3 trials were treated with simple excision. The development of SCC did not necessitate interruption of treatment. Furthermore, the addition of MEK inhibition to BRAF inhibitor therapy reduces the risk for SCC from 19% to 7%.7,10,11
In addition to SCC, second primary melanomas (SPMs) have been reported in patients treated with BRAF inhibitors. It has been shown that these melanomas occur in melanocytes with wild-type BRAF. It has been postulated that some of these tumors occur in cells that harbor upstream mutations in RAS, whereas others might result from alternate signaling through non-RAF oncogenic pathways.9,12
Zimmer et al1 reported 12 SPMs in 11 patients treated with BRAF inhibitor therapy. They reported a median delay of 8 weeks (range, 4–27 weeks) for SPM development. Tumors were detected in early stages; 1 tumor harbored an NRAS mutation.1
Dalle et al13 reported 25 SPMs in 120 vemurafenib-treated patients. Median delay in SPM development was 14 weeks (range, 4–42 weeks). All tumors were thin, ranging from in situ to 0.45-mm thick. Wild-type BRAF was detected in the 21 melanomas sampled; 1 lesion showed mutated NRAS.13
The exact incidence of SPM in the setting of BRAF inhibition is thought to be at least 10-fold less than SCC and KA.2 Patients on BRAF inhibitor therapy should have routine full-body skin examinations, given the increased risk for SPM and SCC.
Another drug belonging to the tyrosine kinase inhibitor family, sorafenib, is used in the treatment of solid tumors, particularly hepatocellular and renal cell carcinomas, and also has been associated with development of cutaneous SCC and KAs.14 Sorafenib is a multiple tyrosine kinase inhibitor that also inhibits the RAF serine/threonine kinases. Similar to vemurafenib and dabrafenib, SCCs and KAs associated with sorafenib tend to arise in patients with chronic actinic damage during the first 2 months of treatment. It has been hypothesized that inhibition of RAF kinases is pathogenic in inducing SCCs because these lesions have not been reported with sunitinib, another multiple tyrosine kinase inhibitor that lacks the ability to inhibit serine/threonine kinases.15,16 Although SCCs and KAs associated with sorafenib tend to be low grade, it is reasonable to consider sunitinib or an alternative tyrosine kinase inhibitor in patients who develop multiple SCCs while taking sorafenib.16
Sonic Hedgehog–Inhibiting Agents
Vismodegib, the first small molecule inhibitor of the signaling protein smoothened, gained FDA approval for the treatment of metastatic or locally advanced basal cell carcinoma (BCC) in 2012. A second agent with an identical mechanism of action, sonidegib, was approved by the FDA for locally advanced BCC in 2015. Approximately 90% of BCCs contain mutations in the sonic hedgehog pathway, which lead to constitutive smoothened activation and uncontrolled cell proliferation.17 The development of smoothened inhibitors introduced a much-needed treatment for inoperable or metastatic BCC,17,18 though long-term utility is limited by drug resistance with extended use in this patient population.19,20 Several case reports have documented the emergence of KA21 and cutaneous SCC following vismodegib treatment of advanced or metastatic BCC.22-24 A larger case-control study by Mohan et al25 showed that patients with BCC treated with vismodegib had an increased risk for non-BCC malignancy (hazard ratio [HR]=6.37), most of which were cutaneous SCC (HR=8.12).
The mechanism by which selective inhibition of smoothened leads to cutaneous SCC is unclear. A study found that patients on vismodegib who developed SCC within the original BCC site had elevated ERK levels within tumor tissue, suggesting that the RAS/RAF/MAPK pathway can become upregulated during hedgehog inhibition.26 Other studies looking at hedgehog inhibition in medulloblastoma models also have shown activated RAS/RAF/MAPK pathways.25 These findings suggest that tumors under smoothened inhibition might be able to bypass the sonic hedgehog pathway and continue to grow by upregulating alternative growth pathways, such as RAS/RAF/MAPK.25,26
The incidence of cutaneous SCC following vismodegib treatment is unknown. Chang and Oro27 examined BCC tumor regrowth from secondary (acquired) resistance to vismodegib and noted that lesions recurred within 1 cm of the original tumor 21% of the time. Although none of the 12 patients whose tumors regrew during treatment were reported to have developed SCC, several demonstrated different BCC subtypes than the pretreatment specimen. The authors proposed that regrowth of BCC was due to upregulated alternative pathways allowing tumors to bypass smoothened inhibition, which is similar to the proposed mechanism for SCC development in vismodegib patients.27
Prospective studies are needed to confirm the link between vismodegib and cutaneous SCC; establish the incidence of SCC development; and identify any pretreatment factors, tumor characteristics, or treatment details (eg, dosage, duration) that might contribute to SCC development. Furthermore, because Mohan et al25 observed that vismodegib-treated patients were less likely to develop SCC in situ than controls, it is unknown if these tumors are more aggressive than traditional SCC. At this point, careful surveillance and regular full-body skin examinations are advised for patients on vismodegib for treatment of advanced BCC.
JAK Inhibitors
Another class of medications potentially associated with increased development of nonmelanoma skin cancer (NMSC) is the JAK inhibitors (also known as jakinibs). Many proinflammatory signaling pathways converge on the JAK family of enzymes—JAK1, JAK2, JAK3, and TYK2. These enzymes operate in cytokine signal transduction by phosphorylating activated cytokine receptors, which allows for recruitment and activation by means of phosphorylation of transcription factors collectively known as signal transducers and activators of transcription (STATs). Phosphorylated STATs dimerize and translocate to the nucleus, acting as direct transcription promoters. Janus kinase inhibitors modulate the immune response by reducing the effect of interleukin and interferon signaling.
Ruxolitinib, a JAK1/JAK2 inhibitor, was the first JAK inhibitor approved by the FDA and is indicated for the treatment of myelofibrosis and polycythemia vera. Additionally, oral and topical JAK inhibitors have shown efficacy in the treatment of psoriasis, rheumatoid arthritis, alopecia areata, vitiligo, and pruritus from atopic dermatitis.28
The JAK-STAT pathway is complex, and the biological activity of the pathway is both proinflammatory and pro–cell survival and proliferation. Because signaling through the pathway can increase angiogenesis and inhibit apoptosis, inhibition of this pathway has been exploited for the treatment of some tumors. However, inhibition of interferon and proinflammatory interleukin signaling also can potentially promote tumor growth by means of inhibition of downstream cytotoxic T-cell signaling, theoretically increasing the risk for NMSC. A study examining the 5-year efficacy of ruxolitinib in myelofibrosis patients (COMFORT-II trial) found that 17.1% of patients developed NMSC compared to only 2.7% of those on the best available therapy. After adjustment by patient exposure, the NMSC rate was still doubled for ruxolitinib-treated patients compared to controls (6.1/100 patient-years and 3.0/100 patient-years, respectively).29 Eighty-week follow-up of the phase 3 clinical trial of ruxolitinib for the treatment of polycythemia vera also noted an increased incidence of NMSC, albeit a more conservative increase. Patients randomized to the ruxolitinib treatment group developed NMSC at a rate of 4.4/100 patient-years, whereas the rate for controls treated with best available therapy was 2.7/100 patient-years.30 In contrast, 5-year follow-up of the COMFORT-I trial, also examining the efficacy of ruxolitinib in myelofibrosis, showed no increased risk for NMSC between ruxolitinib-treated patients and placebo (2.7/100 patient-years and 3.9/100 patient-years, respectively).31
A 2017 case series described 5 patients with myelofibrosis who developed multiple skin cancers with aggressive features while receiving ruxolitinib.32 Duration of ruxolitinib therapy ranged from 4 months to 4 years; 3 patients had a history of hydroxyurea exposure, and only 1 patient had a history of NMSC. High-risk cutaneous SCC, undifferentiated pleomorphic sarcoma, and lentigo maligna melanoma (Breslow thickness, 0.45 mm) were among the tumors reported in this series. Although no definitive conclusion can be made regarding the causality of JAK inhibitors in promoting these tumors, the association warrants further investigation. Clinicians should be aware that ruxolitinib might amplify the risk for NMSC in patients with pre-existing genetic or exposure-related susceptibility. Interruption of drug therapy may be necessary in managing patients who develop an aggressive tumor.32
In contrast, tofacitinib, which specifically inhibits JAK3, carries very low risk, if any, for NMSC when used for the treatment of psoriasis and rheumatoid arthritis. Results from 2 phase 3 trials analyzing the efficacy of tofacitinib in psoriasis demonstrated that only 2 of 1486 patients treated developed NMSC compared to none in the control group.33 Furthermore, analysis of NMSC across the tofacitinib rheumatoid arthritis clinical program, which included a total of 15,103 patient-years of exposure, demonstrated that the overall NMSC incidence was 0.55 for every 100 patient-years. Of note, the risk in patients receiving high-dose treatment (10 mg vs 5 mg) was nearly doubled in long-term follow-up studies (0.79/100 patient-years and 0.41/100 patient-years, respectively). Overall, the study concluded that treatment with tofacitinib presents no greater increased risk for NMSC than treatment with tumor necrosis factor inhibitors.33
PDE-5 Inhibitors
Phosphodiesterase 5 inhibitors, such as sildenafil citrate, have been widely prescribed for the treatment of erectile dysfunction. Studies have shown that BRAF-activated melanomas, which occur in approximately 50% to 70% of melanomas, also result in reduced PDE-5 expression.34-36 In these melanomas, downregulation of PDE-5 results in increased intracellular calcium,36 which has been shown to induce melanoma invasion.36,37 Given this similarity in molecular pathway between BRAF-activated melanomas and PDE-5 inhibitors, there has been increased concern that PDE-5 inhibitors might be associated with an increased risk for melanoma.
In 2014, Li et al38 published a retrospective analysis suggesting an association with sildenafil and an increased risk for melanoma. Their study utilized the Health Professionals Follow-up Study to identify a statistically significant elevation in the risk for invasive melanoma with both recent sildenafil use (multivariate-adjusted HR=2.24) and use at any time (HR=1.92). These results controlled for confounding variables, such as presence of major chronic disease, use of other erectile dysfunction treatments, family history of melanoma, history of sun exposure, and UV index of the patient’s residence. Notably, the study also found that sildenafil did not affect the incidence of BCC or SCC.38
In 2015, Loeb et al39 also examined the potential association between PDE-5 inhibitors and melanoma. Review of several Swedish drug and cancer registries allowed for analysis of melanoma risk and PDE-5 inhibitor use, based on number of prescriptions filled and type of PDE-5 inhibitor prescribed. Their analysis showed that men developing melanoma were more likely than nonmelanoma controls to have taken a PDE-5 inhibitor (11% vs 8%). In a subgroup analysis, however, statistical significance was shown for men with only a single prescription filled (34% of cases; P<.05), whereas the difference for men with multiple filled prescriptions did not meet statistical significance. Furthermore, the study did not find increased risk with longer-acting tadalafil and vardenafil (odds ratio [OR]=1.16) compared to sildenafil (OR=1.14). Last, use of PDE-5 inhibitors was only associated with stage 0 (OR=1.49) and stage I (OR=1.21) tumors, not with stages II to IV (OR=0.83) tumors. Although there was a statistically significant association between PDE-5 inhibitors and malignant melanoma (P<.05), the subgroup analysis findings pointed away from a causal relationship and likely toward a confounding of variable(s).39
A 2016 study by Lian et al40 looked at the risk for melanoma in a cohort of patients diagnosed with erectile dysfunction. No association between PDE-5 inhibitors and melanoma risk was shown when comparing patients who received a PDE-5 inhibitor and those who did not receive a PDE-5 inhibitor. However, secondary analysis did show that melanoma risk was increased among patients receiving more pills (34%) and prescriptions (30%). The authors concluded that there was no association between PDE-5 inhibitor use and overall increased risk for melanoma, and the increased risk associated with a greater number of pills and prescriptions would require further study.40
In contrast, a 2017 meta-analysis by Tang et al41 of 5 studies (3 of which were the aforementioned trials38-40) concluded that use of PDE-5 inhibitors was associated with a small but significantly increased risk for melanoma (OR=1.12) and BCC (OR=1.14) but not SCC. Furthermore, the study found no evidence of dosage-dependent association between PDE-5 inhibitor use and melanoma risk.41
Overall, clinical studies have been inconclusive in determining the risk for melanoma in the setting of PDE-5 inhibitor use. Studies showing an increased rate of melanoma within patient cohorts receiving PDE-5 inhibitors are limited; results might be affected by confounding variables. However, given the similarity in mechanism between PDE-5 inhibitors and HRAS-activated melanomas, it is reasonable to continue research into this potential association.
Conclusion
Since the turn of the century, drugs targeting cell-signaling pathways have been developed to treat inflammatory, oncologic, and immune conditions. The role of immunosuppressants in promoting skin cancer is well established and supported by a vast literature base. However, associations are less clear with newer immunomodulatory and antineoplastic medications. Skin cancer has been reported in association with BRAF inhibitors, sonic hedgehog–inhibiting agents, JAK inhibitors, and PDE-5 inhibitors. In the case of JAK and PDE-5 inhibitors, the increased risk for melanoma and NMSC is somewhat inconclusive; risk is more firmly established for BRAF inhibitors and smoothened inhibitors. For the antineoplastic agents reviewed, the therapeutic effect of cancer regression is well documented, and benefits of continued therapy outweigh the increased risk for skin cancer promotion in nearly all cases. The value of early detection has been well documented for skin malignancy; therefore, increased skin surveillance and prompt management of suspicious lesions should be a priority for physicians treating patients undergoing therapy with these medications
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanoma in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
- Chapman PB, Hauschild A, Robert C, et al; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
- Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427-430.
- Ryan MB, Der CJ, Wang-Gillam A, et al. Targeting RAS-mutant cancers: is ERK the key? Trends Cancer. 2015;1:183-198.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Holderfield M, Nagel TE, Stuart DD. Mechanism and consequence of RAF kinase activation by small-molecule inhibitors. Br J Cancer. 2014;111:640-645.
- Dalle S, Poulalhon N, Debarbieux S, et al. Tracking of second primary melanomas in vemurafenib-treated patients. JAMA Dermatol. 2013;149:488-490.
- Williams VL, Cohen PR, Stewart DJ. Sorafenib-induced premalignant and malignant skin lesions. Int J Dermatol. 2011;50:396-402.
- Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59-e61.
- Smith KJ, Haley H, Hamza S, et al. Eruptive keratoacanthoma-type squamous cell carcinomas in patients taking sorafenib for the treatment of solid tumors. Dermatol Surg. 2009;35:1766-1770.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Demirci H, Worden F, Nelson CC, et al. Efficacy of vismodegib (Erivedge) for basal cell carcinoma involving the orbit and periocular area. Ophthalmic Plast Reconstr Surg. 2015;31:463-466.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Ridky TW, Cotsarelis G. Vismodegib resistance in basal cell carcinoma: not a smooth fit. Cancer Cell. 2015;27:315-316.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:e33-e34.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Zhao X, Ponomaryov T, Ornell KJ, et al. RAS/MAPK activation drives resistance to Smo inhibition, metastasis, and tumor evolution in Shh pathway-dependent tumors. Cancer Res. 2015;75:3623-3635.
- Chang AL, Oro AE. Initial assessment of tumor regrowth after vismodegib in advanced basal cell carcinoma. Arch Dermatol. 2012;148:1324-1325.
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76:736-744.
- Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30:1701-1707.
- Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101:821-829.
- Verstovsek S, Mesa RA, Gotlib J, et al; COMFORT-I investigators. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10:55.
- Blechman AB, Cabell CE, Weinberger CH, et al. Aggressive skin cancers occurring in patients treated with the Janus kinase inhibitor ruxolitinib. J Drugs Dermatol. 2017;16:508-511.
- Papp KA, Menter MA, Abe M, et al; OPT Pivotal 1 and OPT Pivotal 2 investigators. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949-961.
- Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875-885.
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851-857.
- Arozarena I, Sanchez-Laorden B, Packer L, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45-57.
- Houslay MD. Hard times for oncogenic BRAF-expressing melanoma cells. Cancer Cell. 2011;19:3-4.
- Li WQ, Qureshi AA, Robinson KC, et al. Sildenafil use and increased risk of incident melanoma in US men: a prospective cohort study. JAMA Intern Med. 2014;174:964-970.
- Loeb S, Folkvaljon Y, Lambe M, et al. Use of phosphodiesterase type 5 inhibitors for erectile dysfunction and risk of malignant melanoma. JAMA. 2015;313:2449-2455.
- Lian Y, Yin H, Pollak MN, et al. Phosphodiesterase type 5 inhibitors and the risk of melanoma skin cancer. Eur Urol. 2016;70:808-815.
- Tang H, Wu W, Fu S, et al. Phosphodiesterase type 5 inhibitors and risk of melanoma: a meta-analysis. J Am Acad Dermatol. 2017;77:480.e9-488.e9.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanoma in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246.
- Chapman PB, Hauschild A, Robert C, et al; BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15:323-332.
- Su F, Viros A, Milagre C, et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med. 2012;366:207-215.
- Carlos G, Anforth R, Clements A, et al. Cutaneous toxic effects of BRAF inhibitors alone and in combination with MEK inhibitors for metastatic melanoma. JAMA Dermatol. 2015;151:1103-1109.
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427-430.
- Ryan MB, Der CJ, Wang-Gillam A, et al. Targeting RAS-mutant cancers: is ERK the key? Trends Cancer. 2015;1:183-198.
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. 2014;371:1877-1888.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Holderfield M, Nagel TE, Stuart DD. Mechanism and consequence of RAF kinase activation by small-molecule inhibitors. Br J Cancer. 2014;111:640-645.
- Dalle S, Poulalhon N, Debarbieux S, et al. Tracking of second primary melanomas in vemurafenib-treated patients. JAMA Dermatol. 2013;149:488-490.
- Williams VL, Cohen PR, Stewart DJ. Sorafenib-induced premalignant and malignant skin lesions. Int J Dermatol. 2011;50:396-402.
- Arnault JP, Wechsler J, Escudier B, et al. Keratoacanthomas and squamous cell carcinomas in patients receiving sorafenib. J Clin Oncol. 2009;27:e59-e61.
- Smith KJ, Haley H, Hamza S, et al. Eruptive keratoacanthoma-type squamous cell carcinomas in patients taking sorafenib for the treatment of solid tumors. Dermatol Surg. 2009;35:1766-1770.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Demirci H, Worden F, Nelson CC, et al. Efficacy of vismodegib (Erivedge) for basal cell carcinoma involving the orbit and periocular area. Ophthalmic Plast Reconstr Surg. 2015;31:463-466.
- Atwood SX, Sarin KY, Whitson RJ, et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342-353.
- Ridky TW, Cotsarelis G. Vismodegib resistance in basal cell carcinoma: not a smooth fit. Cancer Cell. 2015;27:315-316.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:e33-e34.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Mohan SV, Chang J, Li S, et al. Increased risk of cutaneous squamous cell carcinoma after vismodegib therapy for basal cell carcinoma. JAMA Dermatol. 2016;152:527-532.
- Zhao X, Ponomaryov T, Ornell KJ, et al. RAS/MAPK activation drives resistance to Smo inhibition, metastasis, and tumor evolution in Shh pathway-dependent tumors. Cancer Res. 2015;75:3623-3635.
- Chang AL, Oro AE. Initial assessment of tumor regrowth after vismodegib in advanced basal cell carcinoma. Arch Dermatol. 2012;148:1324-1325.
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76:736-744.
- Harrison CN, Vannucchi AM, Kiladjian JJ, et al. Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia. 2016;30:1701-1707.
- Verstovsek S, Vannucchi AM, Griesshammer M, et al. Ruxolitinib versus best available therapy in patients with polycythemia vera: 80-week follow-up from the RESPONSE trial. Haematologica. 2016;101:821-829.
- Verstovsek S, Mesa RA, Gotlib J, et al; COMFORT-I investigators. Long-term treatment with ruxolitinib for patients with myelofibrosis: 5-year update from the randomized, double-blind, placebo-controlled, phase 3 COMFORT-I trial. J Hematol Oncol. 2017;10:55.
- Blechman AB, Cabell CE, Weinberger CH, et al. Aggressive skin cancers occurring in patients treated with the Janus kinase inhibitor ruxolitinib. J Drugs Dermatol. 2017;16:508-511.
- Papp KA, Menter MA, Abe M, et al; OPT Pivotal 1 and OPT Pivotal 2 investigators. Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two randomized, placebo-controlled, phase III trials. Br J Dermatol. 2015;173:949-961.
- Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875-885.
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851-857.
- Arozarena I, Sanchez-Laorden B, Packer L, et al. Oncogenic BRAF induces melanoma cell invasion by downregulating the cGMP-specific phosphodiesterase PDE5A. Cancer Cell. 2011;19:45-57.
- Houslay MD. Hard times for oncogenic BRAF-expressing melanoma cells. Cancer Cell. 2011;19:3-4.
- Li WQ, Qureshi AA, Robinson KC, et al. Sildenafil use and increased risk of incident melanoma in US men: a prospective cohort study. JAMA Intern Med. 2014;174:964-970.
- Loeb S, Folkvaljon Y, Lambe M, et al. Use of phosphodiesterase type 5 inhibitors for erectile dysfunction and risk of malignant melanoma. JAMA. 2015;313:2449-2455.
- Lian Y, Yin H, Pollak MN, et al. Phosphodiesterase type 5 inhibitors and the risk of melanoma skin cancer. Eur Urol. 2016;70:808-815.
- Tang H, Wu W, Fu S, et al. Phosphodiesterase type 5 inhibitors and risk of melanoma: a meta-analysis. J Am Acad Dermatol. 2017;77:480.e9-488.e9.
Practice Points
- Patients should be educated about the increased risk for skin malignancy while undergoing treatment with BRAF inhibitors, sonic hedgehog–inhibiting agents, Janus kinase (JAK) inhibitors, and phosphodiesterase 5 (PDE-5) inhibitors.
- For BRAF inhibitors, sonic hedgehog–inhibiting agents, and JAK inhibitors, the increased risk for skin cancer warrants regular surveillance; however, given the indications for these medications, many patients will already be receiving regular skin screenings.
- The association between PDE-5 inhibitors and melanoma as well as nonmelanoma skin cancer remains questionable, and increased skin surveillance is not recommended at this time, unless patients have other risk factors for cutaneous malignancy.
Erythematous Plaques and Nodules on the Abdomen and Groin
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
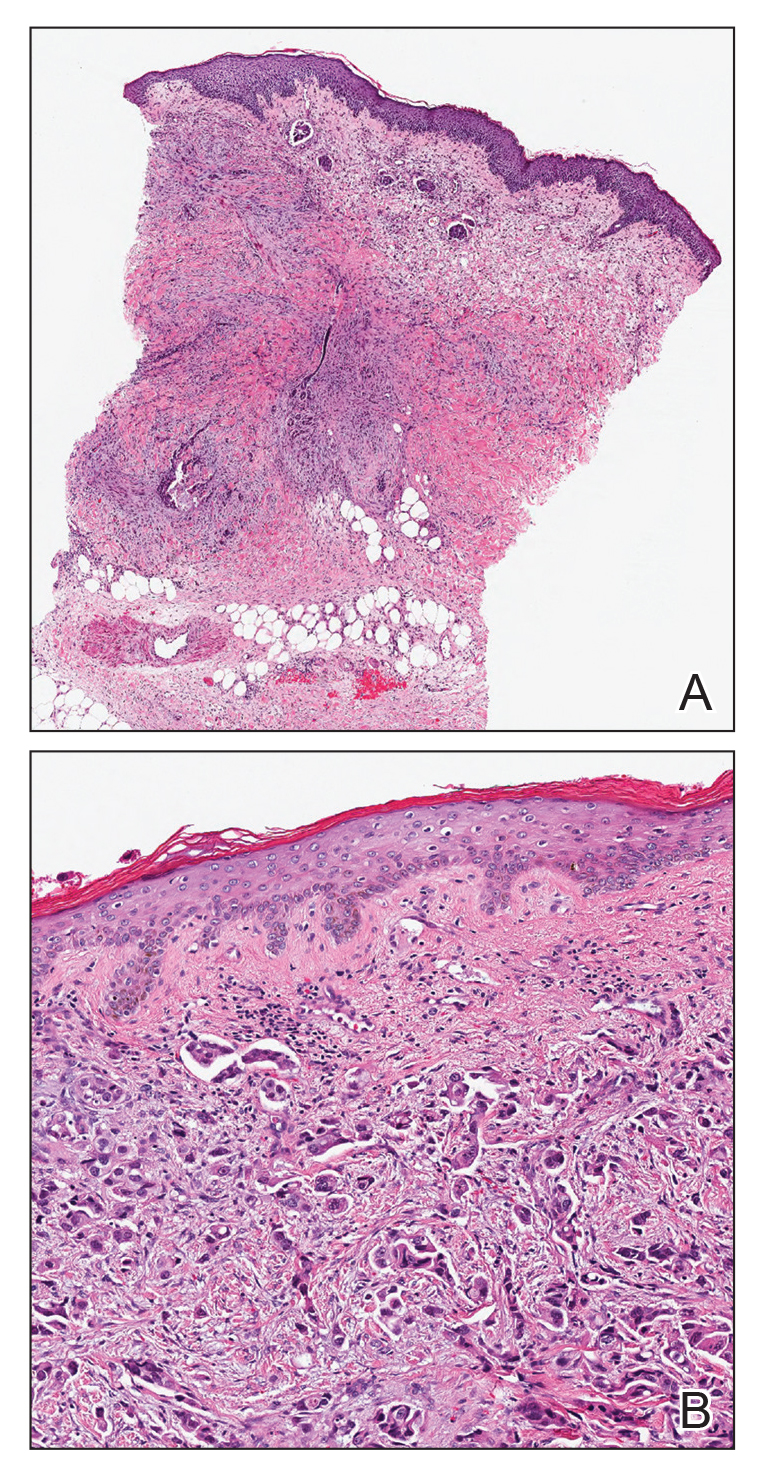
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.

The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.

The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
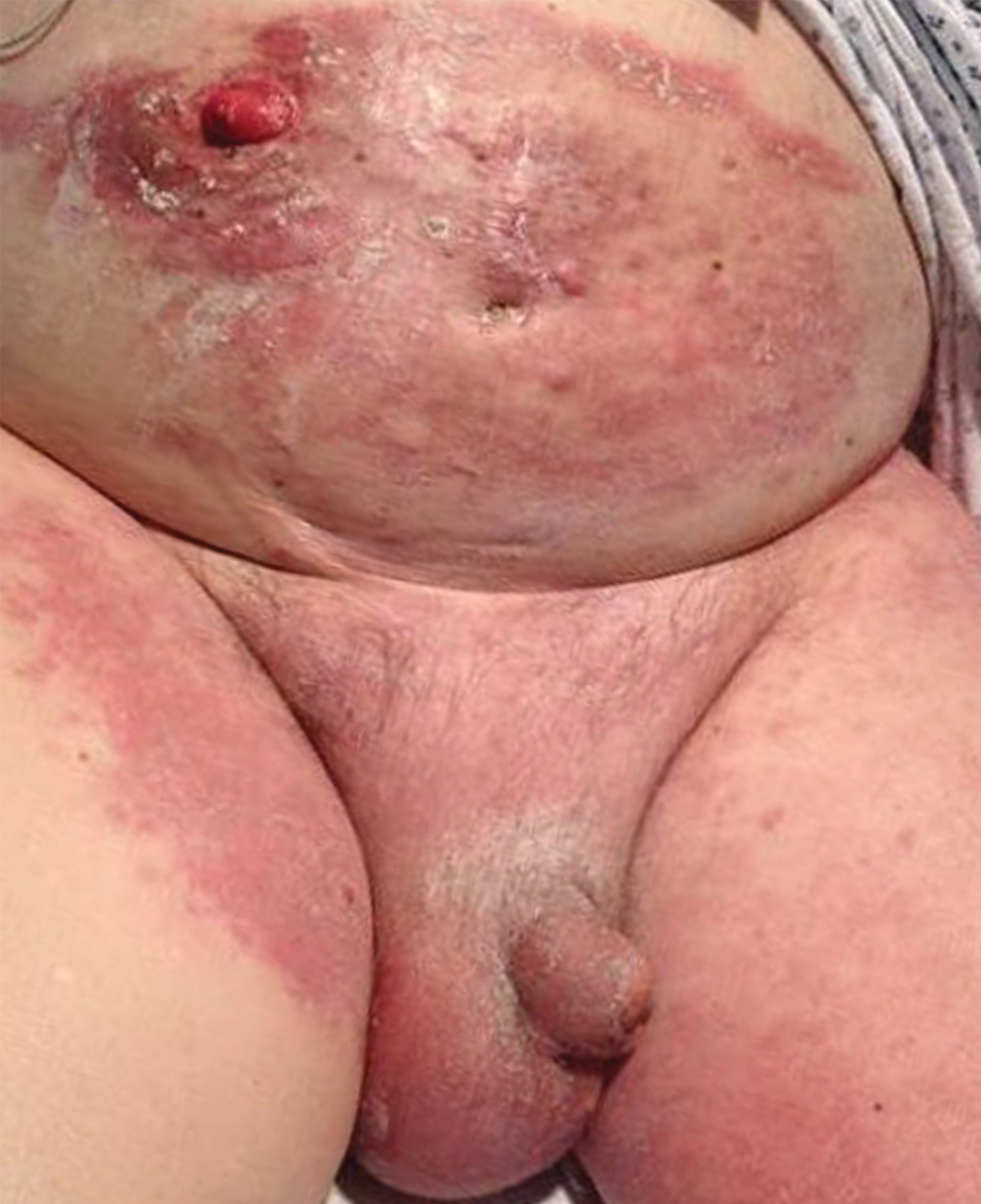
An 82-year-old man presented with acute abdominal pain and distension as well as an abdominal rash of 4 months' duration that was expanding despite treatment with topical miconazole. He had a history of melanoma and bladder cancer treated with cystoprostatectomy. He previously was diagnosed with candidiasis of his urostomy and was taking oral fluconazole. Physical examination revealed a large, well-demarcated, erythematous, smooth plaque covering the entire abdomen, scrotum, penis, inguinal folds, and bilateral upper thighs, with several satellite plaques and firm nodules clustered around the umbilicus. An 8-mm punch biopsy of a periumbilical nodule was performed.
Basal Cell Carcinoma Arising in Nevus Sebaceous During Pregnancy
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.

Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.

Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.

Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
Practice Points
- Benign neoplasms arise more frequently in nevus sebaceous (NS) lesions than do malignant neoplasms.
- The hormonal changes that occur during pregnancy and puberty appear to play a role in the development of neoplasms in NS lesions.
- Monitoring NS lesions more closely during periods of hormonal change may help diagnose malignant transformations in these patients.
Multiple Keratoacanthomas Arising Within Red Tattoo Pigment
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.

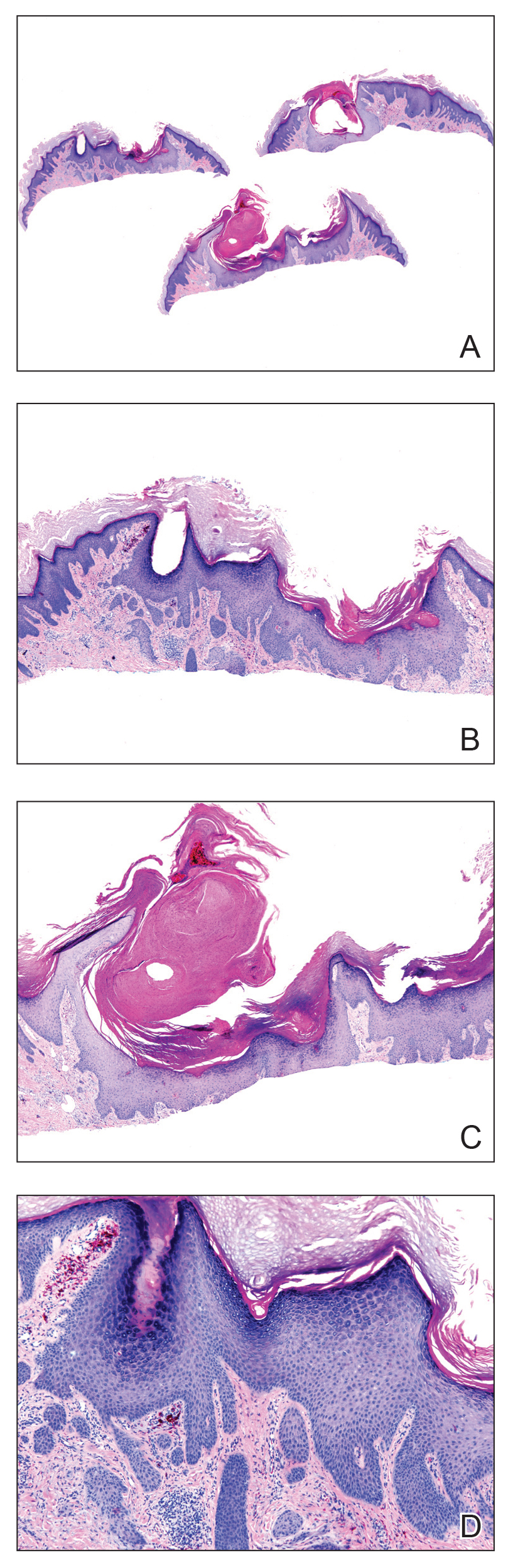
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.


Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.


Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
Practice Points
- Tattoo reactions range from infectious and inflammatory dermatoses to the development of malignant neoplasms.
- Red pigment is the most common cause of adverse tattoo reactions.
- The management of tattoo-associated keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) has not been widely published, but they can be approached similarly to nontattoo-associated KA-SCCs.
Skin Scores: A Review of Clinical Scoring Systems in Dermatology
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
The practice of dermatology is rife with bedside tools: swabs, smears, and scoring systems. First popularized in specialties such as emergency medicine and internal medicine, clinical scoring systems are now emerging in dermatology. These evidence-based scores can be calculated quickly at the bedside—often through a free smartphone app—to help guide clinical decision-making regarding diagnosis, prognosis, and management. As with any medical tool, scoring systems have limitations and should be used as a supplement, not substitute, for one’s clinical judgement. This article reviews 4 clinical scoring systems practical for dermatology residents.
SCORTEN Prognosticates Cases of Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Perhaps the best-known scoring system in dermatology, the SCORTEN is widely used to predict hospital mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis. The SCORTEN includes 7 variables of equal weight—age of 40 years or older, heart rate of 120 beats per minute or more, cancer/hematologic malignancy, involved body surface area (BSA) greater than 10%, serum urea greater than 10 mmol/L, serum bicarbonate less than 20 mmol/L, and serum glucose greater than 14 mmol/L—each contributing 1 point to the overall score if present.1 The involved BSA is defined as the sum of detached and detachable epidermis.1
The SCORTEN was developed and prospectively validated to be calculated at the end of the first 24 hours of admission; for this calculation, use the BSA affected at that time, and use the most abnormal values during the first 24 hours of admission for the other variables.1 In addition, a follow-up study including some of the original coauthors recommends recalculating the SCORTEN at the end of hospital day 3, having found that the score’s predictive value was better on this day than hospital days 1, 2, 4, or 5.2 Based on the original study, a SCORTEN of 0 to 1 corresponds to a mortality rate of 3.2%, 2 to 12.1%, 3 to 35.3%, 4 to 58.3%, and 5 or greater to 90.0%.1
Limitations of the SCORTEN include its ability to overestimate or underestimate mortality as demonstrated by 2 multi-institutional cohorts.3,4 Recently, the ABCD-10 score was developed as an alternative to the SCORTEN and was found to predict mortality similarly when validated in an internal cohort.5
PEST Screens for Psoriatic Arthritis
Dermatologists play an important role in screening for psoriatic arthritis, as an estimated 1 in 5 patients with psoriasis have psoriatic arthritis.6 To this end, several screening tools have been developed to help differentiate psoriatic arthritis from other arthritides. Joint guidelines from the American Academy of Dermatology and the National Psoriasis Foundation acknowledge that “. . . these screening tools have tended to perform less well when tested in groups of people other than those for which they were originally developed. As such, their usefulness in routine clinical practice remains controversial.”7 Nevertheless, the guidelines state, “[b]ecause screening and early detection of inflammatory arthritis are essential to optimize patient [quality of life] and reduce morbidity, providers may consider using a formal screening tool of their choice.”7
With these limitations in mind, I have found the Psoriasis Epidemiology Screening Tool (PEST) to be the most useful psoriatic arthritis screening tool. One study determined that the PEST has the best trade-off between sensitivity and specificity compared to 2 other psoriatic arthritis screening tools, the Psoriatic Arthritis Screening and Evaluation (PASE) and the Early Arthritis for Psoriatic Patients (EARP).8
The PEST is comprised of 5 questions: (1) Have you ever had a swollen joint (or joints)? (2) Has a doctor ever told you that you have arthritis? (3) Do your fingernails or toenails have holes or pits? (4) Have you had pain in your heel? (5) Have you had a finger or toe that was completely swollen and painful for no apparent reason? According to the PEST, a referral to a rheumatologist should be considered for patients answering yes to 3 or more questions, which is 97% sensitive and 79% specific for psoriatic arthritis.9 Patients who answer yes to fewer than 3 questions should still be referred to a rheumatologist if there is a strong clinical suspicion of psoriatic arthritis.10
The PEST can be accessed for free in 13 languages via the GRAPPA (Group for Research and Assessment of Psoriasis and Psoriatic Arthritis) app as well as downloaded for free from the National Psoriasis Foundation’s website (https://www.psoriasis.org/psa-screening/providers).
ALT-70 Differentiates Cellulitis From Pseudocellulitis
Overdiagnosing cellulitis in the United States has been estimated to result in up to 130,000 unnecessary hospitalizations and up to $515 million in avoidable health care spending.11 Dermatologists are in a unique position to help fix this issue. In one retrospective study of 1430 inpatient dermatology consultations, 74.32% of inpatients evaluated for presumed cellulitis by a dermatologist were instead diagnosed with a cellulitis mimicker (ie, pseudocellulitis), such as stasis dermatitis or contact dermatitis.12
The ALT-70 score was developed and prospectively validated to help differentiate lower extremity cellulitis from pseudocellulitis in adult patients in the emergency department (ED).13 In addition, the score has retrospectively been shown to function similarly in the inpatient setting when calculated at 24 and 48 hours after ED presentation.14 Although the ALT-70 score was designed for use by frontline clinicians prior to dermatology consultation, I also have found it helpful to calculate as a consultant, as it provides an objective measure of risk to communicate to the primary team in support of one diagnosis or another.
ALT-70 is an acronym for the score’s 4 variables: asymmetry, leukocytosis, tachycardia, and age of 70 years or older.15 If present, each variable confers a certain number of points to the final score: 3 points for asymmetry (defined as unilateral leg involvement), 1 point for leukocytosis (white blood cell count ≥10,000/μL), 1 point for tachycardia (≥90 beats per minute), and 2 points for age of 70 years or older. An ALT-70 score of 0 to 2 corresponds to an 83.3% or greater chance of pseudocellulitis, suggesting that the diagnosis of cellulitis be reconsidered. A score of 3 to 4 is indeterminate, and additional information such as a dermatology consultation should be pursued. A score of 5 to 7 corresponds to an 82.2% or greater chance of cellulitis, signifying that empiric treatment with antibiotics be considered.15
The ALT-70 score does not apply to cases involving areas other than the lower extremities; intravenous antibiotic use within 48 hours before ED presentation; surgery within the last 30 days; abscess; penetrating trauma; burn; or known history of osteomyelitis, diabetic ulcer, or indwelling hardware at the site of infection.15 The ALT-70 score is available for free via the MDCalc app and website (https://www.mdcalc.com/alt-70-score-cellulitis).
Mohs AUC Determines the Appropriateness of Mohs Micrographic Surgery
In 2012, the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery published appropriate use criteria (AUC) to guide the decision to pursue Mohs micrographic surgery (MMS) in the United States.16 Based on various tumor and patient characteristics, the Mohs AUC assign scores to 270 different clinical scenarios. A score of 1 to 3 signifies that MMS is inappropriate and generally not considered acceptable. A score 4 to 6 indicates that the appropriateness of MMS is uncertain. A score 7 to 9 means that MMS is appropriate and generally considered acceptable.16
Since publication, the Mohs AUC have been criticized for classifying most primary superficial basal cell carcinomas as appropriate for MMS17 (which an AUC coauthor18 and others19,20 have defended), excluding certain reasons for performing MMS (such as operating on multiple tumors on the same day),21 including counterintuitive scores,22 and omitting trials from Europe23 (which AUC coauthors also have defended24).
Final Thoughts
Scoring systems are emerging in dermatology as evidence-based bedside tools to help guide clinical decision-making. Despite their limitations, these scores have the potential to make a meaningful impact in dermatology as they have in other specialties.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Guegan S, Bastuji-Garin S, Poszepczynska-Guigne E, et al. Performance of the SCORTEN during the first five days of hospitalization to predict the prognosis of epidermal necrolysis. J Invest Dermatol. 2006;126:272-276.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective study of 377 adult patients from the United States. J Invest Dermatol. 2018;138:2315-2321.
- Sekula P, Liss Y, Davidovici B, et al. Evaluation of SCORTEN on a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis included in the RegiSCAR study. J Burn Care Res. 2011;32:237-245.
- Noe MH, Rosenbach M, Hubbard RA, et al. Development and validation of a risk prediction model for in-hospital mortality among patients with Stevens-Johnson syndrome/toxic epidermal necrolysis-ABCD-10. JAMA Dermatol. 2019;155:448-454.
- Alinaghi F, Calov M, Kristensen LE, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.e219.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Karreman MC, Weel A, van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology (Oxford). 2017;56:597-602.
- Ibrahim GH, Buch MH, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27:469-474.
- Zhang A, Kurtzman DJB, Perez-Chada LM, et al. Psoriatic arthritis and the dermatologist: an approach to screening and clinical evaluation. Clin Dermatol. 2018;36:551-560.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153:141-146.
- Strazzula L, Cotliar J, Fox LP, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Am Acad Dermatol. 2015;73:70-75.
- Li DG, Dewan AK, Xia FD, et al. The ALT-70 predictive model outperforms thermal imaging for the diagnosis of lower extremity cellulitis: a prospective evaluation. J Am Acad Dermatol. 2018;79:1076-1080.e1071.
- Singer S, Li DG, Gunasekera N, et al. The ALT-70 predictive model maintains predictive value at 24 and 48 hours after presentation [published online March 23, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.03.050.
- Raff AB, Weng QY, Cohen JM, et al. A predictive model for diagnosis of lower extremity cellulitis: a cross-sectional study. J Am Acad Dermatol. 2017;76:618-625.e2.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Montuno MA, Coldiron BM. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:394-395.
- MacFarlane DF, Perlis C. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395-396.
- Kantor J. Mohs appropriate use criteria for superficial basal cell carcinoma. JAMA Dermatol. 2019;155:395.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Connolly S, Baker D, Coldiron B, et al. Reply to “comment on 2012 appropriate use criteria for Mohs micrographic surgery.” J Am Acad Dermatol. 2013;69:318.
Resident Pearls
- Mortality from Stevens-Johnson syndrome/toxic epidermal necrolysis can be estimated by calculating the SCORTEN at the end of days 1 and 3 of hospitalization.
- The Psoriasis Epidemiology Screening Tool (PEST) assists with triaging which patients with psoriasis should be evaluated for psoriatic arthritis by a rheumatologist.
- The ALT-70 score is helpful to support one’s diagnosis of cellulitis or pseudocellulitis.
- The Mohs appropriate use criteria (AUC) score 270 different clinical scenarios as appropriate, uncertain, or inappropriate for Mohs micrographic surgery.
Lifetime indoor tanning raises risk of cutaneous squamous cell carcinoma
Women who accumulated more indoor tanning sessions over time significantly increased their risk for squamous cell cancer over women who never engaged in indoor tanning, based on data from 159,419 women.
Previous research has examined associations between indoor tanning and cutaneous melanoma, but an association between indoor tanning and squamous cell carcinoma (SCC) has not been well studied, wrote Simon Lergenmuller, MSc, of the University of Oslo, and colleagues.
In a prospective cohort study published in JAMA Dermatology, the researchers surveyed 159,419 women from the Norwegian Women and Cancer study. Of these, 95,552 women (69%) reported ever use of indoor tanning. The average age at study inclusion was 50 years. During an average of 17 years’ follow-up, 597 women developed SCC.
Overall, the risk of SCC increased with increasing numbers of indoor tanning sessions. The adjusted hazard ratio for most tanning sessions versus no tanning sessions was 1.83. “The association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation,” the researchers wrote.
The risk of SCC was significantly higher both among women with 10 years or less of tanning bed use and among those with more than 10 years of use, compared with never users (HRs, 1.41 and 1.43, respectively). Similarly, researchers found a significantly higher risk of SCC among women who started indoor tanning at age 30 years or older and those who started younger than 30 years, compared with never users (HRs, 1.36 and HR, 1.51, respectively).
No significant association appeared between age at initiation of indoor tanning and age at the time of SCC diagnosis.
The study findings were limited by several factors including the variation in UV radiation among tanning devices, the lack of data on men, and the retrospective collection of UV exposure data that likely led to some misclassification, the researchers noted.
However, the results were strengthened by the large sample size and support the association between increased exposure to indoor tanning and increased risk of SCC, they wrote. “Avoidance of indoor tanning may help prevent not only melanoma but also SCC, and our results support the development of policies that regulate indoor tanning.”
The study was supported by the Institute of Basic Medical Sciences, University of Oslo, and the Norwegian Cancer Society. The researchers had no financial conflicts to disclose.
SOURCE: Lergenmuller S et al. JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2681.
“There is a saying that ‘when prevention works, nothing happens,’ ” wrote Boris D. Lushniak, MD, in an accompanying editorial. Dermatology plays an important role in public health, most notably skin cancer prevention, and the specialty’s efforts to educate the public and raise awareness about skin cancer and its causes are paying off, in combination with government initiatives to reduce exposure to UV radiation. The current study adds to the data on risk factors for skin cancer, and is distinctive in its focus on artificial sources of UV radiation, specifically indoor tanning sources. The relationship between indoor tanning and basal cell carcinoma or squamous cell carcinoma has not been well studied, “so this study fills an important gap,” he noted.
Research on indoor tanning and other skin cancer risk factors can help expand skin cancer prevention efforts and support goals such as the Healthy People 2020 goal of “reducing the proportion of adolescents in grades 9-12 who report using artificial sources of UV light for tanning, and reducing the proportion of adults aged 18 years or older who report using artificial sources of UV light for tanning,” he wrote.
The first-ever Surgeon General’s Call to Action to Prevent Skin Cancer, released in 2014, made skin cancer prevention a priority and offered specific strategies including providing more sun protection in outdoor settings, educating the public about UV exposure, promoting polices to help prevent skin cancer, reducing harm from indoor tanning, and increasing the collection of data related to skin cancer prevention.
The strategies seem to be working, Dr. Lushniak wrote. Based on data from the 2015 Youth Risk Behavior Surveillance System, a significant linear decrease in the use of indoor tanning devices by youth occurred between 2009 and 2015. “Oftentimes we do not appreciate or celebrate the successes of prevention. ... Let’s keep on track on that bold and noble mission of preventing skin cancer,” he added (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2680).
Dr. Lushniak is affiliated with the University of Maryland School of Public Health, College Park. He served as acting U.S. Surgeon General from 2013 to 2014, and as U.S. Deputy Surgeon General from 2010 to 2015. He had no financial conflicts to disclose.
“There is a saying that ‘when prevention works, nothing happens,’ ” wrote Boris D. Lushniak, MD, in an accompanying editorial. Dermatology plays an important role in public health, most notably skin cancer prevention, and the specialty’s efforts to educate the public and raise awareness about skin cancer and its causes are paying off, in combination with government initiatives to reduce exposure to UV radiation. The current study adds to the data on risk factors for skin cancer, and is distinctive in its focus on artificial sources of UV radiation, specifically indoor tanning sources. The relationship between indoor tanning and basal cell carcinoma or squamous cell carcinoma has not been well studied, “so this study fills an important gap,” he noted.
Research on indoor tanning and other skin cancer risk factors can help expand skin cancer prevention efforts and support goals such as the Healthy People 2020 goal of “reducing the proportion of adolescents in grades 9-12 who report using artificial sources of UV light for tanning, and reducing the proportion of adults aged 18 years or older who report using artificial sources of UV light for tanning,” he wrote.
The first-ever Surgeon General’s Call to Action to Prevent Skin Cancer, released in 2014, made skin cancer prevention a priority and offered specific strategies including providing more sun protection in outdoor settings, educating the public about UV exposure, promoting polices to help prevent skin cancer, reducing harm from indoor tanning, and increasing the collection of data related to skin cancer prevention.
The strategies seem to be working, Dr. Lushniak wrote. Based on data from the 2015 Youth Risk Behavior Surveillance System, a significant linear decrease in the use of indoor tanning devices by youth occurred between 2009 and 2015. “Oftentimes we do not appreciate or celebrate the successes of prevention. ... Let’s keep on track on that bold and noble mission of preventing skin cancer,” he added (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2680).
Dr. Lushniak is affiliated with the University of Maryland School of Public Health, College Park. He served as acting U.S. Surgeon General from 2013 to 2014, and as U.S. Deputy Surgeon General from 2010 to 2015. He had no financial conflicts to disclose.
“There is a saying that ‘when prevention works, nothing happens,’ ” wrote Boris D. Lushniak, MD, in an accompanying editorial. Dermatology plays an important role in public health, most notably skin cancer prevention, and the specialty’s efforts to educate the public and raise awareness about skin cancer and its causes are paying off, in combination with government initiatives to reduce exposure to UV radiation. The current study adds to the data on risk factors for skin cancer, and is distinctive in its focus on artificial sources of UV radiation, specifically indoor tanning sources. The relationship between indoor tanning and basal cell carcinoma or squamous cell carcinoma has not been well studied, “so this study fills an important gap,” he noted.
Research on indoor tanning and other skin cancer risk factors can help expand skin cancer prevention efforts and support goals such as the Healthy People 2020 goal of “reducing the proportion of adolescents in grades 9-12 who report using artificial sources of UV light for tanning, and reducing the proportion of adults aged 18 years or older who report using artificial sources of UV light for tanning,” he wrote.
The first-ever Surgeon General’s Call to Action to Prevent Skin Cancer, released in 2014, made skin cancer prevention a priority and offered specific strategies including providing more sun protection in outdoor settings, educating the public about UV exposure, promoting polices to help prevent skin cancer, reducing harm from indoor tanning, and increasing the collection of data related to skin cancer prevention.
The strategies seem to be working, Dr. Lushniak wrote. Based on data from the 2015 Youth Risk Behavior Surveillance System, a significant linear decrease in the use of indoor tanning devices by youth occurred between 2009 and 2015. “Oftentimes we do not appreciate or celebrate the successes of prevention. ... Let’s keep on track on that bold and noble mission of preventing skin cancer,” he added (JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2680).
Dr. Lushniak is affiliated with the University of Maryland School of Public Health, College Park. He served as acting U.S. Surgeon General from 2013 to 2014, and as U.S. Deputy Surgeon General from 2010 to 2015. He had no financial conflicts to disclose.
Women who accumulated more indoor tanning sessions over time significantly increased their risk for squamous cell cancer over women who never engaged in indoor tanning, based on data from 159,419 women.
Previous research has examined associations between indoor tanning and cutaneous melanoma, but an association between indoor tanning and squamous cell carcinoma (SCC) has not been well studied, wrote Simon Lergenmuller, MSc, of the University of Oslo, and colleagues.
In a prospective cohort study published in JAMA Dermatology, the researchers surveyed 159,419 women from the Norwegian Women and Cancer study. Of these, 95,552 women (69%) reported ever use of indoor tanning. The average age at study inclusion was 50 years. During an average of 17 years’ follow-up, 597 women developed SCC.
Overall, the risk of SCC increased with increasing numbers of indoor tanning sessions. The adjusted hazard ratio for most tanning sessions versus no tanning sessions was 1.83. “The association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation,” the researchers wrote.
The risk of SCC was significantly higher both among women with 10 years or less of tanning bed use and among those with more than 10 years of use, compared with never users (HRs, 1.41 and 1.43, respectively). Similarly, researchers found a significantly higher risk of SCC among women who started indoor tanning at age 30 years or older and those who started younger than 30 years, compared with never users (HRs, 1.36 and HR, 1.51, respectively).
No significant association appeared between age at initiation of indoor tanning and age at the time of SCC diagnosis.
The study findings were limited by several factors including the variation in UV radiation among tanning devices, the lack of data on men, and the retrospective collection of UV exposure data that likely led to some misclassification, the researchers noted.
However, the results were strengthened by the large sample size and support the association between increased exposure to indoor tanning and increased risk of SCC, they wrote. “Avoidance of indoor tanning may help prevent not only melanoma but also SCC, and our results support the development of policies that regulate indoor tanning.”
The study was supported by the Institute of Basic Medical Sciences, University of Oslo, and the Norwegian Cancer Society. The researchers had no financial conflicts to disclose.
SOURCE: Lergenmuller S et al. JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2681.
Women who accumulated more indoor tanning sessions over time significantly increased their risk for squamous cell cancer over women who never engaged in indoor tanning, based on data from 159,419 women.
Previous research has examined associations between indoor tanning and cutaneous melanoma, but an association between indoor tanning and squamous cell carcinoma (SCC) has not been well studied, wrote Simon Lergenmuller, MSc, of the University of Oslo, and colleagues.
In a prospective cohort study published in JAMA Dermatology, the researchers surveyed 159,419 women from the Norwegian Women and Cancer study. Of these, 95,552 women (69%) reported ever use of indoor tanning. The average age at study inclusion was 50 years. During an average of 17 years’ follow-up, 597 women developed SCC.
Overall, the risk of SCC increased with increasing numbers of indoor tanning sessions. The adjusted hazard ratio for most tanning sessions versus no tanning sessions was 1.83. “The association between cumulative exposure to indoor tanning and SCC risk was the same regardless of duration of use and age at initiation,” the researchers wrote.
The risk of SCC was significantly higher both among women with 10 years or less of tanning bed use and among those with more than 10 years of use, compared with never users (HRs, 1.41 and 1.43, respectively). Similarly, researchers found a significantly higher risk of SCC among women who started indoor tanning at age 30 years or older and those who started younger than 30 years, compared with never users (HRs, 1.36 and HR, 1.51, respectively).
No significant association appeared between age at initiation of indoor tanning and age at the time of SCC diagnosis.
The study findings were limited by several factors including the variation in UV radiation among tanning devices, the lack of data on men, and the retrospective collection of UV exposure data that likely led to some misclassification, the researchers noted.
However, the results were strengthened by the large sample size and support the association between increased exposure to indoor tanning and increased risk of SCC, they wrote. “Avoidance of indoor tanning may help prevent not only melanoma but also SCC, and our results support the development of policies that regulate indoor tanning.”
The study was supported by the Institute of Basic Medical Sciences, University of Oslo, and the Norwegian Cancer Society. The researchers had no financial conflicts to disclose.
SOURCE: Lergenmuller S et al. JAMA Dermatol. 2019 Oct 2. doi: 10.1001/jamadermatol.2019.2681.
FROM JAMA DERMATOLOGY
Twitter Chat: Skin Cancer

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.

Join us on Tuesday, October 8, 2019, at 8:00 pm EST on Twitter at #MDedgeChats as we discuss skin cancer, and what’s new in sunscreen, skin of color, and melanoma.
Special guests include physicians with expertise in dermatology and skin cancer, Anthony Rossi, MD (@DrAnthonyRossi), Julie Amthor Croley, MD, 15k followers on IG (@Drskinandsmiles), and Candrice Heath, MD (@DrCandriceHeath). Background information about the chat can be found below.
What will the conversation cover?
Q1: What are the most common types of skin cancer?
Q2: What recent research findings can better inform patients about skin cancer risks?
Q3: What’s the difference between melanoma in fair skin vs. darker skin?
Q4: How does the risk of skin cancer differ in people with darker skin?
Q5: Why should sunscreen be used even in the fall and winter?

Follow us here: @MDedgeDerm | @MDedgeTweets | #MDedgeChats
About Dr. Rossi:
Dr. Anthony Rossi (@DrAnthonyRossi) is a board-certified dermatologist with fellowship training in Mohs micrographic surgery, cosmetic and laser surgery, and advanced cutaneous oncology at the Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College program, both in New York. He specializes in skin cancer surgery, cosmetic dermatologic surgery, and laser surgery.
His research includes quality of life in cancer survivors, the use of noninvasive imaging of the skin, and nonsurgical treatments of skin cancer. Additionally, Dr. Rossi is active in dermatologic organizations and advocacy for medicine.
Research and Publications by Dr. Rossi
About Dr. Heath:
Dr. Candrice Heath (@DrCandriceHeath) is Assistant Professor of Dermatology at the Lewis Katz School of Medicine at Temple University in Philadelphia, Pennsylvania with fellowship training in pediatric dermatology at Johns Hopkins University in Baltimore, Maryland. Dr. Heath is triple board certified in pediatrics, dermatology, and pediatric dermatology. She specializes in adult and pediatric dermatology, skin of color, acne, and eczema. Dr. Heath also enjoys educating primary care physicians on the front lines of health care and delivering easy to understand information to consumers.
Research and publications by Dr. Heath
Guest host of MDedge podcast: A sunscreen update with Dr. Vincent DeLeo.
About Dr. Croley:
Dr. Julie Amthor Croley (@Drskinandsmiles) also known as “Dr. Skin and Smiles” has 15,000 followers on Instagram, and is a Chief Dermatology Resident at the University of Texas Medical Branch in Galveston, Texas. She has a special interest in skin cancer and dermatological surgery and hopes to complete a fellowship in Mohs micrographic surgery after residency. In her free time, Dr. Croley enjoys spending time with her husband (an orthopedic surgeon), running and competing in marathons, and spending time on the beach.
Cutaneous melanoma is the most fatal form of skin cancer and is a considerable public health concern in the United States. Early detection and management of skin cancer can lead to decreased morbidity and mortality from skin cancer. As a result, the American Academy of Dermatology Association supports safe sun-protective practices and diligent self-screening for changing lesions.
Sunscreen use is an essential component of sun protection. New regulations from the US Food and Drug Administration (FDA) have left consumers concerned about the safety of sunscreens. According to a recent Cutis editorial from Vincent A. DeLeo, MD, “There is no question that, as physicians, we want to ‘first, do no harm,’ so we should all be interested in assuring our patients that our sunscreen recommendations are safe and we support the FDA proposal for additional data.”
Patients with skin of color experience disproportionately higher morbidity and mortality when diagnosed with melanoma. “Poor prognosis in patients with skin of color is multifactorial and may be due to poor use of sun protection, misconceptions about melanoma risk, atypical clinical presentation, impaired access to care, and delay in diagnosis,” according to a recent Cutis article.
Population-based skin cancer screening performed exclusively by dermatologists is not practical. Primary care physicians and other experts in melanoma and public health need to be involved in reducing melanoma mortality.
In this chat, we will provide expert recommendations on the diagnosis of skin cancer, preventive measures, and the latest research discussed among physicians.
- “Doctor, Do I Need a Skin Check?”
- Assessing the effectiveness of knowledge-based interventions in skin of color populations.
- Melanoma in US Hispanics
- Podcast: Sunscreen update from Dr. Vincent DeLeo
- Windshield and UV exposure
- Racial, ethnic minorities often don’t practice sun-protective behaviors.
- Sunscreen regulations and advice for your patients.