User login
Are operative vaginal delivery discharge instructions needed?
In order to identify the prevalence of concerns among postpartum women and the factors associated with them, the University of California–San Francisco (UCSF) began calling all of its obstetric patients through an automated phone call within 72 hours after hospital discharge.
Study details
All postpartum women from March to June 2017 were contacted after discharge via an automated call. Calls were considered successful if the woman engaged with the automated system; those who reported concerns were contacted by a nurse. UCSF researchers compared call success and presence of concerns by mode of delivery, insurance type (public or private), parity, pregnancy complication (diabetes, hypertension, hemorrhage), and neonatal intensive care (ICN) admission using univariate analyses and multivariable logistic regression.
A total of 881 women were called, and 730 (83%) were successfully contacted (meaning they engaged with the automated system through to the end of the call). About one-third of women (224 / 29%) reported a concern. Women with operative vaginal delivery were more likely to report an issue than spontaneous vaginal and cesarean delivery (42% vs 28%; P = .04). Nulliparous women also were more likely to report an issue (32% vs 25%; P = .05). They also were more likely to answer the call (86% vs 79%; P = .004). Women with public insurance were less likely to be successfully contacted (68% vs 84%; P = .003), but the frequency of concerns were equivalent (28% vs 29%). Women with neonates in the ICN were less likely to be successfully contacted. When controlling for confounders, nulliparity (odds ratio [OR], 1.5; 95% confidence interval [CI], 1.1–2.2) and private insurance (OR, 1.9; 95% CI, 1.1–3.8) both were independently associated with successful contact.
What do the results mean for practice?
Nulliparous women and women with operative vaginal deliveries may benefit from additional discharge support, concluded the researchers. “For most patients we can’t predict in advance if they will have an operative vaginal delivery but I do think that we could do more counseling in the antepartum period about different options or mode of delivery and include operative vaginal deliveries in that bucket, especially as we are doing more of them,” said Dr. Molly Siegel, Resident at UCSF. “In the postpartum period we probably should be thinking more about our instructions to those patients because we have cesarean delivery and vaginal delivery discharge instructions, and I think there needs to be something specific for operative vaginal delivery. Ultimately the goal is to improve our counseling of patients so that they don’t have as many questions after they leave the hospital.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In order to identify the prevalence of concerns among postpartum women and the factors associated with them, the University of California–San Francisco (UCSF) began calling all of its obstetric patients through an automated phone call within 72 hours after hospital discharge.
Study details
All postpartum women from March to June 2017 were contacted after discharge via an automated call. Calls were considered successful if the woman engaged with the automated system; those who reported concerns were contacted by a nurse. UCSF researchers compared call success and presence of concerns by mode of delivery, insurance type (public or private), parity, pregnancy complication (diabetes, hypertension, hemorrhage), and neonatal intensive care (ICN) admission using univariate analyses and multivariable logistic regression.
A total of 881 women were called, and 730 (83%) were successfully contacted (meaning they engaged with the automated system through to the end of the call). About one-third of women (224 / 29%) reported a concern. Women with operative vaginal delivery were more likely to report an issue than spontaneous vaginal and cesarean delivery (42% vs 28%; P = .04). Nulliparous women also were more likely to report an issue (32% vs 25%; P = .05). They also were more likely to answer the call (86% vs 79%; P = .004). Women with public insurance were less likely to be successfully contacted (68% vs 84%; P = .003), but the frequency of concerns were equivalent (28% vs 29%). Women with neonates in the ICN were less likely to be successfully contacted. When controlling for confounders, nulliparity (odds ratio [OR], 1.5; 95% confidence interval [CI], 1.1–2.2) and private insurance (OR, 1.9; 95% CI, 1.1–3.8) both were independently associated with successful contact.
What do the results mean for practice?
Nulliparous women and women with operative vaginal deliveries may benefit from additional discharge support, concluded the researchers. “For most patients we can’t predict in advance if they will have an operative vaginal delivery but I do think that we could do more counseling in the antepartum period about different options or mode of delivery and include operative vaginal deliveries in that bucket, especially as we are doing more of them,” said Dr. Molly Siegel, Resident at UCSF. “In the postpartum period we probably should be thinking more about our instructions to those patients because we have cesarean delivery and vaginal delivery discharge instructions, and I think there needs to be something specific for operative vaginal delivery. Ultimately the goal is to improve our counseling of patients so that they don’t have as many questions after they leave the hospital.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In order to identify the prevalence of concerns among postpartum women and the factors associated with them, the University of California–San Francisco (UCSF) began calling all of its obstetric patients through an automated phone call within 72 hours after hospital discharge.
Study details
All postpartum women from March to June 2017 were contacted after discharge via an automated call. Calls were considered successful if the woman engaged with the automated system; those who reported concerns were contacted by a nurse. UCSF researchers compared call success and presence of concerns by mode of delivery, insurance type (public or private), parity, pregnancy complication (diabetes, hypertension, hemorrhage), and neonatal intensive care (ICN) admission using univariate analyses and multivariable logistic regression.
A total of 881 women were called, and 730 (83%) were successfully contacted (meaning they engaged with the automated system through to the end of the call). About one-third of women (224 / 29%) reported a concern. Women with operative vaginal delivery were more likely to report an issue than spontaneous vaginal and cesarean delivery (42% vs 28%; P = .04). Nulliparous women also were more likely to report an issue (32% vs 25%; P = .05). They also were more likely to answer the call (86% vs 79%; P = .004). Women with public insurance were less likely to be successfully contacted (68% vs 84%; P = .003), but the frequency of concerns were equivalent (28% vs 29%). Women with neonates in the ICN were less likely to be successfully contacted. When controlling for confounders, nulliparity (odds ratio [OR], 1.5; 95% confidence interval [CI], 1.1–2.2) and private insurance (OR, 1.9; 95% CI, 1.1–3.8) both were independently associated with successful contact.
What do the results mean for practice?
Nulliparous women and women with operative vaginal deliveries may benefit from additional discharge support, concluded the researchers. “For most patients we can’t predict in advance if they will have an operative vaginal delivery but I do think that we could do more counseling in the antepartum period about different options or mode of delivery and include operative vaginal deliveries in that bucket, especially as we are doing more of them,” said Dr. Molly Siegel, Resident at UCSF. “In the postpartum period we probably should be thinking more about our instructions to those patients because we have cesarean delivery and vaginal delivery discharge instructions, and I think there needs to be something specific for operative vaginal delivery. Ultimately the goal is to improve our counseling of patients so that they don’t have as many questions after they leave the hospital.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Making a difference: ACOG’s guidance on low-dose aspirin for preventing superimposed preeclampsia
Investigators at Thomas Jefferson University found that low-dose aspirin therapy in pregnant women with chronic hypertension—as recommended by the American College of Obstetricians and Gynecologists (ACOG) in 20161—was associated with a 57% decrease in superimposed preeclampsia. Chaitra Banala, BS, presented the study’s results in a poster presentation at the ACOG 2018 annual meeting (April 27–30, 2018, Austin, Texas).2

The study’s goal was to evaluate the incidence of superimposed preeclampsia in women with chronic hypertension in the periods before and after the ACOG recommendation was published.
Study participants. Pregnant women with chronic hypertension who delivered at Thomas Jefferson University Hospital from January 2008 to July 2017 were included in this retrospective cohort study. Women with multiple gestations were excluded.
The cohort included 715 pregnant patients with chronic hypertension divided into 2 groups: 635 pre-ACOG patients and 80 post-ACOG patients (that is, patients who delivered before and after the ACOG recommendation). The investigators offered daily low-dose (81 mg) aspirin.
The cohort was further stratified by additional risk factors for superimposed preeclampsia, including a history of preeclampsia and pregestational diabetes.
Outcomes. The primary outcome was the incidence of superimposed preeclampsia. Secondary outcomes included the incidence of superimposed preeclampsia with severe features (SIPSF), small for gestational age, and preterm birth.
Findings. The incidence of superimposed preeclampsia in women with chronic hypertension was 20 (25%) in the post-ACOG group versus 232 (37%) in the pre-ACOG group (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.26–0.73).
In the subgroup of women with chronic hypertension who did not have other risk factors, superimposed preeclampsia and SIPSF were significantly decreased: 4/41 (10%) versus 106/355 (30%) (OR, 0.25 [95% CI, 0.08–0.73]) and 2/41 (5%) versus 65/355 (18%) (OR, 0.22 [95% CI, 0.54–0.97]), respectively. The maternal demographics and secondary outcomes did not differ significantly.
After the ACOG guidance was released, low-dose aspirin decreased superimposed preeclampsia by 57% in all women with chronic hypertension. Of those with chronic hypertension without other risk factors, there were decreases of 75% in superimposed preeclampsia and 78% in SIPSF.
Final thoughts. Ms. Banala said in an interview with OBG Management following her presentation, “When we stratified the cohort based on their risk factors, we found that aspirin had the highest benefit in patients with only chronic hypertension, so without other risk factors. And we found that there was a benefit in patients with chronic hypertension who were not on antihypertensive medication. So overall our study concluded that this guideline has made a significant impact in decreasing the frequency of superimposed preeclampsia.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed May 3, 2018.
- Banala C, Cruz Y, Moreno C, Schoen C, Berghella V, Roman A. Impact of ACOG guideline regarding low dose aspirin for prevention of superimposed preeclampsia [abstract 27O]. Obstet Gynecol. 2018;131(suppl 1):170S.
Investigators at Thomas Jefferson University found that low-dose aspirin therapy in pregnant women with chronic hypertension—as recommended by the American College of Obstetricians and Gynecologists (ACOG) in 20161—was associated with a 57% decrease in superimposed preeclampsia. Chaitra Banala, BS, presented the study’s results in a poster presentation at the ACOG 2018 annual meeting (April 27–30, 2018, Austin, Texas).2

The study’s goal was to evaluate the incidence of superimposed preeclampsia in women with chronic hypertension in the periods before and after the ACOG recommendation was published.
Study participants. Pregnant women with chronic hypertension who delivered at Thomas Jefferson University Hospital from January 2008 to July 2017 were included in this retrospective cohort study. Women with multiple gestations were excluded.
The cohort included 715 pregnant patients with chronic hypertension divided into 2 groups: 635 pre-ACOG patients and 80 post-ACOG patients (that is, patients who delivered before and after the ACOG recommendation). The investigators offered daily low-dose (81 mg) aspirin.
The cohort was further stratified by additional risk factors for superimposed preeclampsia, including a history of preeclampsia and pregestational diabetes.
Outcomes. The primary outcome was the incidence of superimposed preeclampsia. Secondary outcomes included the incidence of superimposed preeclampsia with severe features (SIPSF), small for gestational age, and preterm birth.
Findings. The incidence of superimposed preeclampsia in women with chronic hypertension was 20 (25%) in the post-ACOG group versus 232 (37%) in the pre-ACOG group (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.26–0.73).
In the subgroup of women with chronic hypertension who did not have other risk factors, superimposed preeclampsia and SIPSF were significantly decreased: 4/41 (10%) versus 106/355 (30%) (OR, 0.25 [95% CI, 0.08–0.73]) and 2/41 (5%) versus 65/355 (18%) (OR, 0.22 [95% CI, 0.54–0.97]), respectively. The maternal demographics and secondary outcomes did not differ significantly.
After the ACOG guidance was released, low-dose aspirin decreased superimposed preeclampsia by 57% in all women with chronic hypertension. Of those with chronic hypertension without other risk factors, there were decreases of 75% in superimposed preeclampsia and 78% in SIPSF.
Final thoughts. Ms. Banala said in an interview with OBG Management following her presentation, “When we stratified the cohort based on their risk factors, we found that aspirin had the highest benefit in patients with only chronic hypertension, so without other risk factors. And we found that there was a benefit in patients with chronic hypertension who were not on antihypertensive medication. So overall our study concluded that this guideline has made a significant impact in decreasing the frequency of superimposed preeclampsia.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Investigators at Thomas Jefferson University found that low-dose aspirin therapy in pregnant women with chronic hypertension—as recommended by the American College of Obstetricians and Gynecologists (ACOG) in 20161—was associated with a 57% decrease in superimposed preeclampsia. Chaitra Banala, BS, presented the study’s results in a poster presentation at the ACOG 2018 annual meeting (April 27–30, 2018, Austin, Texas).2

The study’s goal was to evaluate the incidence of superimposed preeclampsia in women with chronic hypertension in the periods before and after the ACOG recommendation was published.
Study participants. Pregnant women with chronic hypertension who delivered at Thomas Jefferson University Hospital from January 2008 to July 2017 were included in this retrospective cohort study. Women with multiple gestations were excluded.
The cohort included 715 pregnant patients with chronic hypertension divided into 2 groups: 635 pre-ACOG patients and 80 post-ACOG patients (that is, patients who delivered before and after the ACOG recommendation). The investigators offered daily low-dose (81 mg) aspirin.
The cohort was further stratified by additional risk factors for superimposed preeclampsia, including a history of preeclampsia and pregestational diabetes.
Outcomes. The primary outcome was the incidence of superimposed preeclampsia. Secondary outcomes included the incidence of superimposed preeclampsia with severe features (SIPSF), small for gestational age, and preterm birth.
Findings. The incidence of superimposed preeclampsia in women with chronic hypertension was 20 (25%) in the post-ACOG group versus 232 (37%) in the pre-ACOG group (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.26–0.73).
In the subgroup of women with chronic hypertension who did not have other risk factors, superimposed preeclampsia and SIPSF were significantly decreased: 4/41 (10%) versus 106/355 (30%) (OR, 0.25 [95% CI, 0.08–0.73]) and 2/41 (5%) versus 65/355 (18%) (OR, 0.22 [95% CI, 0.54–0.97]), respectively. The maternal demographics and secondary outcomes did not differ significantly.
After the ACOG guidance was released, low-dose aspirin decreased superimposed preeclampsia by 57% in all women with chronic hypertension. Of those with chronic hypertension without other risk factors, there were decreases of 75% in superimposed preeclampsia and 78% in SIPSF.
Final thoughts. Ms. Banala said in an interview with OBG Management following her presentation, “When we stratified the cohort based on their risk factors, we found that aspirin had the highest benefit in patients with only chronic hypertension, so without other risk factors. And we found that there was a benefit in patients with chronic hypertension who were not on antihypertensive medication. So overall our study concluded that this guideline has made a significant impact in decreasing the frequency of superimposed preeclampsia.”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed May 3, 2018.
- Banala C, Cruz Y, Moreno C, Schoen C, Berghella V, Roman A. Impact of ACOG guideline regarding low dose aspirin for prevention of superimposed preeclampsia [abstract 27O]. Obstet Gynecol. 2018;131(suppl 1):170S.
- American College of Obstetricians and Gynecologists. Practice advisory on low-dose aspirin and prevention of preeclampsia: updated recommendations. https://www.acog.org/Clinical-Guidance-and-Publications/Practice-Advisories/Practice-Advisory-Low-Dose-Aspirin-and-Prevention-of-Preeclampsia-Updated-Recommendations. Published July 11, 2016. Accessed May 3, 2018.
- Banala C, Cruz Y, Moreno C, Schoen C, Berghella V, Roman A. Impact of ACOG guideline regarding low dose aspirin for prevention of superimposed preeclampsia [abstract 27O]. Obstet Gynecol. 2018;131(suppl 1):170S.
MDedge Daily News: How to handle opioid constipation
Bath emollients are a washout for childhood eczema. Does warfarin cause acute kidney injury? And there may be a new option for postpartum depression.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Bath emollients are a washout for childhood eczema. Does warfarin cause acute kidney injury? And there may be a new option for postpartum depression.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Bath emollients are a washout for childhood eczema. Does warfarin cause acute kidney injury? And there may be a new option for postpartum depression.
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Did unsafe oxytocin dose cause uterine rupture? $3.5M settlement
Did unsafe oxytocin dose cause uterine rupture? $3.5M settlement
When a mother presented to the hospital in labor, the on-call ObGyn ordered oxytocin with the dosage to be increased by 2 mU/min until she was receiving 30 mU/min or until an adequate contraction pattern was achieved and maintained. Over the next few hours, the labor and delivery nurse increased the dosage of the infusion several times.
As the patient began to push, a trickle of bright red blood was seen coming from her vagina and the baby's heart tones were temporarily lost. When the fetal heart tones were restored, his heart rate was approximately 50 bpm. After vaginal delivery was attempted using vacuum extraction and forceps, an emergency cesarean delivery was performed, leading to the finding that the mother's uterus had ruptured.
The baby suffered a permanent brain injury due to hypoxic-ischemic encephalopathy.
PATIENT'S CLAIM: The mother sued the hospital and on-call ObGyn. She alleged that the health care providers breached the standard of care by negligently increasing and maintaining the oxytocin at unsafe levels, which caused the mother's uterus to be overworked and eventually rupture. The rupture led to the child's hypoxia. An expert ObGyn noted that the patient's contractions were adequate by the time the oxytocin dose reached 14 mU/min, but the dosage continued to be increased.
DEFENDANTS' DEFENSE: The case was settled during the trial.
VERDICT: A $3.5 million Kansas settlement was reached.
When did the bowel injury occur?
One day after undergoing a hysterectomy, a woman went to the emergency department (ED) because she was feeling ill. She received a diagnosis of a pulmonary embolism for which she was given anticoagulant medications. The patient's symptoms persisted. Computed tomography (CT) imaging showed a bowel injury, and, 17 days after the initial surgery, an emergency laparotomy was performed.
PATIENT'S CLAIM: The patient sued the surgeon and the hospital. The hospital settled before the trial and the case continued against the surgeon.
The patient's early symptoms after surgery were evidence of a bowel injury, but imaging was not undertaken for several days. If the imaging had been undertaken earlier, the bowel injury would have been detected before it caused a rectovaginal fistula. An expert pathologist testified that the microscopic findings he detected postlaparotomy could only exist if a bowel perforation had been there for a significant period of time before the fistula developed. The patient's experts argued that the injury was not a "free perforation," but had been contained by her body, preventing the spread of the infection.
DEFENDANTS' DEFENSE: The surgeon maintained that the injury did not occur during the hysterectomy but developed in the days just before it was discovered. Over time, a collection of infected fluid at the vaginal cuff eroded into the bowel above it, creating an entryway for stool to pass through. Continuous leakage from the bowel for 17 days (the length of time between development of symptoms and discovery of the bowel injury) would have likely resulted in the patient's death.
VERDICT: A Missouri defense verdict was returned.
Did improper delivery techniques caused brachial plexus injury? $950,000 settlement
In April, a woman began receiving prenatal care for her 7th pregnancy. Her history of maternal obesity and diabetes mellitus, physical examinations, and tests suggested that she was at increased risk for having a macrosomic baby and encountering shoulder dystocia during vaginal delivery.
At 37 weeks' gestation, the mother was admitted to the hospital for induction of labor. Shoulder dystocia was encountered during delivery. At birth, the baby weighed 9 lb 10 oz and her right arm was limp. She was found to have a right brachial plexus injury involving C5‒C8 nerve roots and muscles. Two nerve root avulsions were evident on MRI and visualized by the surgeon during an extensive nerve graft operation. Arm function and range of motion improved after surgery, but the child has not recovered normal use of her arm.
PARENTS' CLAIM: Under the standard of care, the ObGyn was required to obtain informed consent, including a discussion of the risks of vaginal delivery (shoulder dystocia and brachial plexus injury), and the option of cesarean delivery. The patient claimed that the ObGyn neither obtained informed consent nor discussed these risks with her.
The labor and delivery nurse reported that the ObGyn told her, before delivery, that he was expecting a large baby and, perhaps, shoulder dystocia.
The ObGyn deviated from the standard of care by applying more than gentle traction to the fetal head while the shoulder was still impacted. The injury to the baby's right brachial plexus resulted from excessive lateral traction used by the ObGyn. The injury would not have occurred if a cesarean delivery had been performed. The mechanism of maternal forces injuring a brachial plexus nerve has never been visualized by any physician and is an unproven hypothesis.
PHYSICIAN'S DEFENSE: The ObGyn reported using standard maneuvers to deliver the baby. He applied traction on the fetal head 3 times: once after McRoberts maneuver, once after suprapubic pressure, and once after delivery of the posterior arm. He dictated into his notes that "We had to be careful to avoid excessive tractive forces." He claimed that shoulder dystocia is an unpredictable and unpreventable obstetric emergency, and that the injury was caused by the maternal forces of labor.
VERDICT: A $950,000 Virginia settlement was reached.
Wrongful death claim
On March 13, a 76-year-old woman went to her primary care physician's office because of a vaginal discharge. A nurse practitioner (NP) diagnosed a urinary tract infection (UTI) and prescribed cefixime (Suprax). Four days later, the patient began to experience severe diarrhea and blamed the medication. At a follow-up visit on March 20, the NP switched the patient to sulfamethoxazole-trimethoprim (Bactrim).
The following day, the patient was found unresponsive on her bathroom floor and was taken to the ED. It was determined that she had Clostridium difficile colitis (C difficile) and was admitted to the hospital. She developed acute renal failure, metabolic acidosis, hypovolemia, hypotension, and tachycardia. When she went into cardiac arrest, attempts to resuscitate her failed. She died on March 22.
ESTATE'S CLAIM: The estate sued the NP, claiming that the patient's symptoms did not meet the criteria for a UTI. If appropriate tests had been performed, the correct diagnosis would have been made and she could have received potentially life-saving treatment.
DEFENDANTS' DEFENSE: The NP claimed there was no negligence. Her diagnosis and treatment of the patient's condition were appropriate in all respects. The development of C difficile is a risk of any antibiotic.
VERDICT: An Indiana defense verdict was returned.
Nuchal cord: Undisclosed settlement
During delivery, the labor and delivery nurses lost the fetal heart-rate (FHR) monitor tracing, resulting in their being unaware of increasing signs of fetal intolerance to labor. The nurses continued to administer oxytocin to induce labor.
At birth, a nuchal cord was identified. The baby was born without signs of life but was successfully resuscitated by hospital staff.
The baby was found to have sustained severe brain damage as a result of profound fetal hypoxia. The child will require 24-hour nursing and supportive care for as long as she lives.
PARENTS' CLAIM: The nurses and ObGyn breached the standard of care resulting in her child's severe brain damage. The hospital nurses failed to continuously monitor the FHR. Profound brain damage was preventable in this case.
DEFENDANTS' DEFENSE: The nurses continuously monitored by listening to sounds coming out of the bedside monitor even though no taping of FHR was occurring on the central monitors or FHR monitor strip. A nuchal cord is an unforeseeable medical emergency; nothing different could have been done to change the outcome.
VERDICT: An undisclosed Texas settlement was reached.
Surgical needle left near bladder
The patient underwent a hysterectomy on July 9. Because of an injury sustained during the operation, bladder repair surgery was performed on July 19. After that surgery, she reported bleeding and urinary incontinence. Results of a computed tomography (CT) scan showed that the bladder repair was not successful, a vesicovaginal fistula had developed, and a 13-mm C-shaped needle was found near her bladder. A third operation to remove the needle from her abdomen took place on August 16.
PATIENT'S CLAIM: The needle left behind after the second surgery caused a fistula to develop. The patient suffered mental and emotional distress from knowing the needle was in her abdomen. Foreign objects left in a patient during surgery are strong evidence of negligence.
DEFENDANTS' DEFENSE: The primary defense claim was the absence of causation--any negligence did not cause the injury. Defense experts testified that the needle was on top of the bladder and did move anywhere to cause damage. The patient developed a vesicovaginal fistula due to complications from the bladder repair operation and not from the needle. In addition, there was testimony that the third surgery was unnecessary because the needle would eventually flush out of the abdomen without causing damage.
VERDICT: A Michigan defense verdict was returned.
Claims cancer diagnosis was delayed
On October 1, 2008, a woman saw her family physician (FP) for routine care. Blood work results showed an elevated white blood cell (WBC) count. The patient claimed that the FP did not inform her of these test results.
One year later, the patient went to an urgent care facility where blood work was performed; the results showed a high WBC count. After a work-up, the patient was given a presumptive diagnosis of mantel cell lymphoma. By December 9, she had undergone the first round of chemotherapy. Subsequent tests revealed that the presumptive diagnosis was incorrect; she actually had low-grade lymphoproliferative disorder.
In lieu of this new diagnosis, the medical team offered the patient the option of discontinuing chemotherapy. She decided, however, to continue the treatment. Chemotherapy was followed by 2 years of rituximab/hyaluronidase human maintenance therapy.
PATIENT'S CLAIM: The patient presented her case to a medical review board. The course of treatment (chemotherapy plus maintenance therapy) left her with permanent heart damage and an elevated risk of developing secondary cancer.
The patient claimed that none of this would have happened if her FP had informed her of the October 2008 test results and recommended appropriate follow-up studies. The results of those studies would have given her the correct diagnosis and allowed her to receive prompt, proper treatment. The medical review board responded unanimously that the FP's conduct constituted a breach of the standard of care, but concluded that the breach was not a factor in the patient's damages.
The patient filed suit against the FP. An expert in internal medicine commented that, based on the 2008 WBC count, the tests should have been repeated and the patient should have been referred to a hematologist/oncologist. Failure to do so increased the patient's risk of developing cancer in the future.
DEFENDANTS' DEFENSE: The FP denied any breach of standard of care. According to her notes, she had shared test results with the patient on November 26 and recommended following up with repeat blood work. The FP blamed the patient for failing to follow-up as recommended.
VERDICT: An Indiana defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Did unsafe oxytocin dose cause uterine rupture? $3.5M settlement
When a mother presented to the hospital in labor, the on-call ObGyn ordered oxytocin with the dosage to be increased by 2 mU/min until she was receiving 30 mU/min or until an adequate contraction pattern was achieved and maintained. Over the next few hours, the labor and delivery nurse increased the dosage of the infusion several times.
As the patient began to push, a trickle of bright red blood was seen coming from her vagina and the baby's heart tones were temporarily lost. When the fetal heart tones were restored, his heart rate was approximately 50 bpm. After vaginal delivery was attempted using vacuum extraction and forceps, an emergency cesarean delivery was performed, leading to the finding that the mother's uterus had ruptured.
The baby suffered a permanent brain injury due to hypoxic-ischemic encephalopathy.
PATIENT'S CLAIM: The mother sued the hospital and on-call ObGyn. She alleged that the health care providers breached the standard of care by negligently increasing and maintaining the oxytocin at unsafe levels, which caused the mother's uterus to be overworked and eventually rupture. The rupture led to the child's hypoxia. An expert ObGyn noted that the patient's contractions were adequate by the time the oxytocin dose reached 14 mU/min, but the dosage continued to be increased.
DEFENDANTS' DEFENSE: The case was settled during the trial.
VERDICT: A $3.5 million Kansas settlement was reached.
When did the bowel injury occur?
One day after undergoing a hysterectomy, a woman went to the emergency department (ED) because she was feeling ill. She received a diagnosis of a pulmonary embolism for which she was given anticoagulant medications. The patient's symptoms persisted. Computed tomography (CT) imaging showed a bowel injury, and, 17 days after the initial surgery, an emergency laparotomy was performed.
PATIENT'S CLAIM: The patient sued the surgeon and the hospital. The hospital settled before the trial and the case continued against the surgeon.
The patient's early symptoms after surgery were evidence of a bowel injury, but imaging was not undertaken for several days. If the imaging had been undertaken earlier, the bowel injury would have been detected before it caused a rectovaginal fistula. An expert pathologist testified that the microscopic findings he detected postlaparotomy could only exist if a bowel perforation had been there for a significant period of time before the fistula developed. The patient's experts argued that the injury was not a "free perforation," but had been contained by her body, preventing the spread of the infection.
DEFENDANTS' DEFENSE: The surgeon maintained that the injury did not occur during the hysterectomy but developed in the days just before it was discovered. Over time, a collection of infected fluid at the vaginal cuff eroded into the bowel above it, creating an entryway for stool to pass through. Continuous leakage from the bowel for 17 days (the length of time between development of symptoms and discovery of the bowel injury) would have likely resulted in the patient's death.
VERDICT: A Missouri defense verdict was returned.
Did improper delivery techniques caused brachial plexus injury? $950,000 settlement
In April, a woman began receiving prenatal care for her 7th pregnancy. Her history of maternal obesity and diabetes mellitus, physical examinations, and tests suggested that she was at increased risk for having a macrosomic baby and encountering shoulder dystocia during vaginal delivery.
At 37 weeks' gestation, the mother was admitted to the hospital for induction of labor. Shoulder dystocia was encountered during delivery. At birth, the baby weighed 9 lb 10 oz and her right arm was limp. She was found to have a right brachial plexus injury involving C5‒C8 nerve roots and muscles. Two nerve root avulsions were evident on MRI and visualized by the surgeon during an extensive nerve graft operation. Arm function and range of motion improved after surgery, but the child has not recovered normal use of her arm.
PARENTS' CLAIM: Under the standard of care, the ObGyn was required to obtain informed consent, including a discussion of the risks of vaginal delivery (shoulder dystocia and brachial plexus injury), and the option of cesarean delivery. The patient claimed that the ObGyn neither obtained informed consent nor discussed these risks with her.
The labor and delivery nurse reported that the ObGyn told her, before delivery, that he was expecting a large baby and, perhaps, shoulder dystocia.
The ObGyn deviated from the standard of care by applying more than gentle traction to the fetal head while the shoulder was still impacted. The injury to the baby's right brachial plexus resulted from excessive lateral traction used by the ObGyn. The injury would not have occurred if a cesarean delivery had been performed. The mechanism of maternal forces injuring a brachial plexus nerve has never been visualized by any physician and is an unproven hypothesis.
PHYSICIAN'S DEFENSE: The ObGyn reported using standard maneuvers to deliver the baby. He applied traction on the fetal head 3 times: once after McRoberts maneuver, once after suprapubic pressure, and once after delivery of the posterior arm. He dictated into his notes that "We had to be careful to avoid excessive tractive forces." He claimed that shoulder dystocia is an unpredictable and unpreventable obstetric emergency, and that the injury was caused by the maternal forces of labor.
VERDICT: A $950,000 Virginia settlement was reached.
Wrongful death claim
On March 13, a 76-year-old woman went to her primary care physician's office because of a vaginal discharge. A nurse practitioner (NP) diagnosed a urinary tract infection (UTI) and prescribed cefixime (Suprax). Four days later, the patient began to experience severe diarrhea and blamed the medication. At a follow-up visit on March 20, the NP switched the patient to sulfamethoxazole-trimethoprim (Bactrim).
The following day, the patient was found unresponsive on her bathroom floor and was taken to the ED. It was determined that she had Clostridium difficile colitis (C difficile) and was admitted to the hospital. She developed acute renal failure, metabolic acidosis, hypovolemia, hypotension, and tachycardia. When she went into cardiac arrest, attempts to resuscitate her failed. She died on March 22.
ESTATE'S CLAIM: The estate sued the NP, claiming that the patient's symptoms did not meet the criteria for a UTI. If appropriate tests had been performed, the correct diagnosis would have been made and she could have received potentially life-saving treatment.
DEFENDANTS' DEFENSE: The NP claimed there was no negligence. Her diagnosis and treatment of the patient's condition were appropriate in all respects. The development of C difficile is a risk of any antibiotic.
VERDICT: An Indiana defense verdict was returned.
Nuchal cord: Undisclosed settlement
During delivery, the labor and delivery nurses lost the fetal heart-rate (FHR) monitor tracing, resulting in their being unaware of increasing signs of fetal intolerance to labor. The nurses continued to administer oxytocin to induce labor.
At birth, a nuchal cord was identified. The baby was born without signs of life but was successfully resuscitated by hospital staff.
The baby was found to have sustained severe brain damage as a result of profound fetal hypoxia. The child will require 24-hour nursing and supportive care for as long as she lives.
PARENTS' CLAIM: The nurses and ObGyn breached the standard of care resulting in her child's severe brain damage. The hospital nurses failed to continuously monitor the FHR. Profound brain damage was preventable in this case.
DEFENDANTS' DEFENSE: The nurses continuously monitored by listening to sounds coming out of the bedside monitor even though no taping of FHR was occurring on the central monitors or FHR monitor strip. A nuchal cord is an unforeseeable medical emergency; nothing different could have been done to change the outcome.
VERDICT: An undisclosed Texas settlement was reached.
Surgical needle left near bladder
The patient underwent a hysterectomy on July 9. Because of an injury sustained during the operation, bladder repair surgery was performed on July 19. After that surgery, she reported bleeding and urinary incontinence. Results of a computed tomography (CT) scan showed that the bladder repair was not successful, a vesicovaginal fistula had developed, and a 13-mm C-shaped needle was found near her bladder. A third operation to remove the needle from her abdomen took place on August 16.
PATIENT'S CLAIM: The needle left behind after the second surgery caused a fistula to develop. The patient suffered mental and emotional distress from knowing the needle was in her abdomen. Foreign objects left in a patient during surgery are strong evidence of negligence.
DEFENDANTS' DEFENSE: The primary defense claim was the absence of causation--any negligence did not cause the injury. Defense experts testified that the needle was on top of the bladder and did move anywhere to cause damage. The patient developed a vesicovaginal fistula due to complications from the bladder repair operation and not from the needle. In addition, there was testimony that the third surgery was unnecessary because the needle would eventually flush out of the abdomen without causing damage.
VERDICT: A Michigan defense verdict was returned.
Claims cancer diagnosis was delayed
On October 1, 2008, a woman saw her family physician (FP) for routine care. Blood work results showed an elevated white blood cell (WBC) count. The patient claimed that the FP did not inform her of these test results.
One year later, the patient went to an urgent care facility where blood work was performed; the results showed a high WBC count. After a work-up, the patient was given a presumptive diagnosis of mantel cell lymphoma. By December 9, she had undergone the first round of chemotherapy. Subsequent tests revealed that the presumptive diagnosis was incorrect; she actually had low-grade lymphoproliferative disorder.
In lieu of this new diagnosis, the medical team offered the patient the option of discontinuing chemotherapy. She decided, however, to continue the treatment. Chemotherapy was followed by 2 years of rituximab/hyaluronidase human maintenance therapy.
PATIENT'S CLAIM: The patient presented her case to a medical review board. The course of treatment (chemotherapy plus maintenance therapy) left her with permanent heart damage and an elevated risk of developing secondary cancer.
The patient claimed that none of this would have happened if her FP had informed her of the October 2008 test results and recommended appropriate follow-up studies. The results of those studies would have given her the correct diagnosis and allowed her to receive prompt, proper treatment. The medical review board responded unanimously that the FP's conduct constituted a breach of the standard of care, but concluded that the breach was not a factor in the patient's damages.
The patient filed suit against the FP. An expert in internal medicine commented that, based on the 2008 WBC count, the tests should have been repeated and the patient should have been referred to a hematologist/oncologist. Failure to do so increased the patient's risk of developing cancer in the future.
DEFENDANTS' DEFENSE: The FP denied any breach of standard of care. According to her notes, she had shared test results with the patient on November 26 and recommended following up with repeat blood work. The FP blamed the patient for failing to follow-up as recommended.
VERDICT: An Indiana defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Did unsafe oxytocin dose cause uterine rupture? $3.5M settlement
When a mother presented to the hospital in labor, the on-call ObGyn ordered oxytocin with the dosage to be increased by 2 mU/min until she was receiving 30 mU/min or until an adequate contraction pattern was achieved and maintained. Over the next few hours, the labor and delivery nurse increased the dosage of the infusion several times.
As the patient began to push, a trickle of bright red blood was seen coming from her vagina and the baby's heart tones were temporarily lost. When the fetal heart tones were restored, his heart rate was approximately 50 bpm. After vaginal delivery was attempted using vacuum extraction and forceps, an emergency cesarean delivery was performed, leading to the finding that the mother's uterus had ruptured.
The baby suffered a permanent brain injury due to hypoxic-ischemic encephalopathy.
PATIENT'S CLAIM: The mother sued the hospital and on-call ObGyn. She alleged that the health care providers breached the standard of care by negligently increasing and maintaining the oxytocin at unsafe levels, which caused the mother's uterus to be overworked and eventually rupture. The rupture led to the child's hypoxia. An expert ObGyn noted that the patient's contractions were adequate by the time the oxytocin dose reached 14 mU/min, but the dosage continued to be increased.
DEFENDANTS' DEFENSE: The case was settled during the trial.
VERDICT: A $3.5 million Kansas settlement was reached.
When did the bowel injury occur?
One day after undergoing a hysterectomy, a woman went to the emergency department (ED) because she was feeling ill. She received a diagnosis of a pulmonary embolism for which she was given anticoagulant medications. The patient's symptoms persisted. Computed tomography (CT) imaging showed a bowel injury, and, 17 days after the initial surgery, an emergency laparotomy was performed.
PATIENT'S CLAIM: The patient sued the surgeon and the hospital. The hospital settled before the trial and the case continued against the surgeon.
The patient's early symptoms after surgery were evidence of a bowel injury, but imaging was not undertaken for several days. If the imaging had been undertaken earlier, the bowel injury would have been detected before it caused a rectovaginal fistula. An expert pathologist testified that the microscopic findings he detected postlaparotomy could only exist if a bowel perforation had been there for a significant period of time before the fistula developed. The patient's experts argued that the injury was not a "free perforation," but had been contained by her body, preventing the spread of the infection.
DEFENDANTS' DEFENSE: The surgeon maintained that the injury did not occur during the hysterectomy but developed in the days just before it was discovered. Over time, a collection of infected fluid at the vaginal cuff eroded into the bowel above it, creating an entryway for stool to pass through. Continuous leakage from the bowel for 17 days (the length of time between development of symptoms and discovery of the bowel injury) would have likely resulted in the patient's death.
VERDICT: A Missouri defense verdict was returned.
Did improper delivery techniques caused brachial plexus injury? $950,000 settlement
In April, a woman began receiving prenatal care for her 7th pregnancy. Her history of maternal obesity and diabetes mellitus, physical examinations, and tests suggested that she was at increased risk for having a macrosomic baby and encountering shoulder dystocia during vaginal delivery.
At 37 weeks' gestation, the mother was admitted to the hospital for induction of labor. Shoulder dystocia was encountered during delivery. At birth, the baby weighed 9 lb 10 oz and her right arm was limp. She was found to have a right brachial plexus injury involving C5‒C8 nerve roots and muscles. Two nerve root avulsions were evident on MRI and visualized by the surgeon during an extensive nerve graft operation. Arm function and range of motion improved after surgery, but the child has not recovered normal use of her arm.
PARENTS' CLAIM: Under the standard of care, the ObGyn was required to obtain informed consent, including a discussion of the risks of vaginal delivery (shoulder dystocia and brachial plexus injury), and the option of cesarean delivery. The patient claimed that the ObGyn neither obtained informed consent nor discussed these risks with her.
The labor and delivery nurse reported that the ObGyn told her, before delivery, that he was expecting a large baby and, perhaps, shoulder dystocia.
The ObGyn deviated from the standard of care by applying more than gentle traction to the fetal head while the shoulder was still impacted. The injury to the baby's right brachial plexus resulted from excessive lateral traction used by the ObGyn. The injury would not have occurred if a cesarean delivery had been performed. The mechanism of maternal forces injuring a brachial plexus nerve has never been visualized by any physician and is an unproven hypothesis.
PHYSICIAN'S DEFENSE: The ObGyn reported using standard maneuvers to deliver the baby. He applied traction on the fetal head 3 times: once after McRoberts maneuver, once after suprapubic pressure, and once after delivery of the posterior arm. He dictated into his notes that "We had to be careful to avoid excessive tractive forces." He claimed that shoulder dystocia is an unpredictable and unpreventable obstetric emergency, and that the injury was caused by the maternal forces of labor.
VERDICT: A $950,000 Virginia settlement was reached.
Wrongful death claim
On March 13, a 76-year-old woman went to her primary care physician's office because of a vaginal discharge. A nurse practitioner (NP) diagnosed a urinary tract infection (UTI) and prescribed cefixime (Suprax). Four days later, the patient began to experience severe diarrhea and blamed the medication. At a follow-up visit on March 20, the NP switched the patient to sulfamethoxazole-trimethoprim (Bactrim).
The following day, the patient was found unresponsive on her bathroom floor and was taken to the ED. It was determined that she had Clostridium difficile colitis (C difficile) and was admitted to the hospital. She developed acute renal failure, metabolic acidosis, hypovolemia, hypotension, and tachycardia. When she went into cardiac arrest, attempts to resuscitate her failed. She died on March 22.
ESTATE'S CLAIM: The estate sued the NP, claiming that the patient's symptoms did not meet the criteria for a UTI. If appropriate tests had been performed, the correct diagnosis would have been made and she could have received potentially life-saving treatment.
DEFENDANTS' DEFENSE: The NP claimed there was no negligence. Her diagnosis and treatment of the patient's condition were appropriate in all respects. The development of C difficile is a risk of any antibiotic.
VERDICT: An Indiana defense verdict was returned.
Nuchal cord: Undisclosed settlement
During delivery, the labor and delivery nurses lost the fetal heart-rate (FHR) monitor tracing, resulting in their being unaware of increasing signs of fetal intolerance to labor. The nurses continued to administer oxytocin to induce labor.
At birth, a nuchal cord was identified. The baby was born without signs of life but was successfully resuscitated by hospital staff.
The baby was found to have sustained severe brain damage as a result of profound fetal hypoxia. The child will require 24-hour nursing and supportive care for as long as she lives.
PARENTS' CLAIM: The nurses and ObGyn breached the standard of care resulting in her child's severe brain damage. The hospital nurses failed to continuously monitor the FHR. Profound brain damage was preventable in this case.
DEFENDANTS' DEFENSE: The nurses continuously monitored by listening to sounds coming out of the bedside monitor even though no taping of FHR was occurring on the central monitors or FHR monitor strip. A nuchal cord is an unforeseeable medical emergency; nothing different could have been done to change the outcome.
VERDICT: An undisclosed Texas settlement was reached.
Surgical needle left near bladder
The patient underwent a hysterectomy on July 9. Because of an injury sustained during the operation, bladder repair surgery was performed on July 19. After that surgery, she reported bleeding and urinary incontinence. Results of a computed tomography (CT) scan showed that the bladder repair was not successful, a vesicovaginal fistula had developed, and a 13-mm C-shaped needle was found near her bladder. A third operation to remove the needle from her abdomen took place on August 16.
PATIENT'S CLAIM: The needle left behind after the second surgery caused a fistula to develop. The patient suffered mental and emotional distress from knowing the needle was in her abdomen. Foreign objects left in a patient during surgery are strong evidence of negligence.
DEFENDANTS' DEFENSE: The primary defense claim was the absence of causation--any negligence did not cause the injury. Defense experts testified that the needle was on top of the bladder and did move anywhere to cause damage. The patient developed a vesicovaginal fistula due to complications from the bladder repair operation and not from the needle. In addition, there was testimony that the third surgery was unnecessary because the needle would eventually flush out of the abdomen without causing damage.
VERDICT: A Michigan defense verdict was returned.
Claims cancer diagnosis was delayed
On October 1, 2008, a woman saw her family physician (FP) for routine care. Blood work results showed an elevated white blood cell (WBC) count. The patient claimed that the FP did not inform her of these test results.
One year later, the patient went to an urgent care facility where blood work was performed; the results showed a high WBC count. After a work-up, the patient was given a presumptive diagnosis of mantel cell lymphoma. By December 9, she had undergone the first round of chemotherapy. Subsequent tests revealed that the presumptive diagnosis was incorrect; she actually had low-grade lymphoproliferative disorder.
In lieu of this new diagnosis, the medical team offered the patient the option of discontinuing chemotherapy. She decided, however, to continue the treatment. Chemotherapy was followed by 2 years of rituximab/hyaluronidase human maintenance therapy.
PATIENT'S CLAIM: The patient presented her case to a medical review board. The course of treatment (chemotherapy plus maintenance therapy) left her with permanent heart damage and an elevated risk of developing secondary cancer.
The patient claimed that none of this would have happened if her FP had informed her of the October 2008 test results and recommended appropriate follow-up studies. The results of those studies would have given her the correct diagnosis and allowed her to receive prompt, proper treatment. The medical review board responded unanimously that the FP's conduct constituted a breach of the standard of care, but concluded that the breach was not a factor in the patient's damages.
The patient filed suit against the FP. An expert in internal medicine commented that, based on the 2008 WBC count, the tests should have been repeated and the patient should have been referred to a hematologist/oncologist. Failure to do so increased the patient's risk of developing cancer in the future.
DEFENDANTS' DEFENSE: The FP denied any breach of standard of care. According to her notes, she had shared test results with the patient on November 26 and recommended following up with repeat blood work. The FP blamed the patient for failing to follow-up as recommended.
VERDICT: An Indiana defense verdict was returned.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
MDedge Daily News: Fewer smokes mean fewer strokes
Are biopsy’s days numbered for diagnosing celiac disease? The perils of endoscopic therapy for Barrett’s esophagus. And is your EHR preventing breastfeeding?
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Are biopsy’s days numbered for diagnosing celiac disease? The perils of endoscopic therapy for Barrett’s esophagus. And is your EHR preventing breastfeeding?
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Are biopsy’s days numbered for diagnosing celiac disease? The perils of endoscopic therapy for Barrett’s esophagus. And is your EHR preventing breastfeeding?
Listen to the MDedge Daily News podcast for all the details on today’s top news.
Maternal morbidity and BMI: A dose-response relationship
AUSTIN, TEX. – Women with the highest levels of obesity were at higher odds of experiencing a composite serious maternal morbidity outcome, while women at all levels of obesity experienced elevated risks of some serious complications of pregnancy, compared with women with a body mass index (BMI) in the normal range, according to a recent study.
Looking at individual indicators of severe maternal morbidity, Marissa Platner, MD, and her study coauthors saw that women who fell into the higher levels of obesity had significantly elevated odds of some complications.
“Those risks are really impressive, with odds ratios of two and three times that of a normal-weight patient,” said Dr. Platner in a video interview.
The adjusted odds ratio of acute renal failure for women with superobesity (BMI of 50 kg/m2 or more) was 3.62 (95% confidence interval, 1.75-7.52); odds ratios for renal failure were not significantly elevated for less-obese women.
Women with all levels of obesity had elevated risks of experiencing heart failure during a procedure or surgery, with adjusted odds ratios ranging from 1.68 (95% CI, 1.48-1.93) for women with class I obesity (BMI, 30-34.9 kg/m2) to 2.23 for women with superobesity (95% CI, 1.15-4.33).
Results from the retrospective cohort study were presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Platner and her colleagues examined 4 years of New York City delivery data that were linked to birth certificates, identifying those singleton live births for whom maternal prepregnancy BMI data were available.
From this group, they included women aged 15-50 years who delivered at 20-45 weeks’ gestational age. Women with prepregnancy BMIs less than 18.5 kg/m2 – those who were underweight – were excluded.
Dr. Platner and her coinvestigators used multivariable analysis to see what association the full range of obesity classes had with severe maternal morbidity, adjusting for many socioeconomic and demographic factors.
Of the 539,870 women included in the study, 3.3% experienced severe maternal morbidity, and 17.4% of patients met criteria for obesity. “Across all classes of obesity, there was a significantly greater risk of severe maternal morbidity, compared to nonobese women,” wrote Dr. Platner and her colleagues in the poster accompanying the presentation.
These risks climbed for women with the highest BMIs, however. “Women with higher levels of obesity, not surprisingly, are at increased risk” of severe maternal morbidity, said Dr. Platner. She and her colleagues noted in the poster that, “There is a significant dose-response relationship between increasing obesity class and risk of [severe maternal morbidity] at delivery hospitalization.”
It had been known that women with obesity are at increased risk of some serious complications of pregnancy, including severe maternal morbidity and mortality, and that those considered morbidly obese – with BMIs of 40 and above – are most likely to experience these complications, Dr. Platner said. However, she added, there’s a paucity of data to inform maternal risk stratification by level of obesity.
“We included the group of superobese women, which is significant in the surgical literature, and that’s a BMI of 50 and above ... we thought that would be an important subgroup to analyze in this population,” she said.
Dr. Platner said that she and her colleagues already had the clinical impressions that women with the highest BMIs were most likely to have serious complications. “I don’t think that these findings are particularly surprising,” she said. “This is what our hypothesis was in terms of why we did this study.”
The greater surprise, she said, was the magnitude of increased risk seen for serious morbidity with higher levels of obesity.
“Really, the risk is truly increased for those women with class III or superobesity, and when we start to stratify ... those are the women we need to be concerned about in terms of our prenatal counseling,” said Dr. Platner, a maternal-fetal medicine fellow at Yale University, New Haven, Conn.
“What can we do to intervene before we get there?” asked Dr. Platner. Although data are lacking about what specific interventions might be able to reduce the risk of these serious complications, she said she could envision such steps as acquiring predelivery baseline ECGs and cardiac ultrasounds in women with higher levels of obesity and being sure to follow renal function closely as well.
The findings also may help physicians provide more evidence-based preconception advice to women who are among the 35% of American adults who have obesity.
Dr. Platner reported no relevant financial disclosures.
SOURCE: Platner M et al. ACOG 2018, Abstract 39I.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AUSTIN, TEX. – Women with the highest levels of obesity were at higher odds of experiencing a composite serious maternal morbidity outcome, while women at all levels of obesity experienced elevated risks of some serious complications of pregnancy, compared with women with a body mass index (BMI) in the normal range, according to a recent study.
Looking at individual indicators of severe maternal morbidity, Marissa Platner, MD, and her study coauthors saw that women who fell into the higher levels of obesity had significantly elevated odds of some complications.
“Those risks are really impressive, with odds ratios of two and three times that of a normal-weight patient,” said Dr. Platner in a video interview.
The adjusted odds ratio of acute renal failure for women with superobesity (BMI of 50 kg/m2 or more) was 3.62 (95% confidence interval, 1.75-7.52); odds ratios for renal failure were not significantly elevated for less-obese women.
Women with all levels of obesity had elevated risks of experiencing heart failure during a procedure or surgery, with adjusted odds ratios ranging from 1.68 (95% CI, 1.48-1.93) for women with class I obesity (BMI, 30-34.9 kg/m2) to 2.23 for women with superobesity (95% CI, 1.15-4.33).
Results from the retrospective cohort study were presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Platner and her colleagues examined 4 years of New York City delivery data that were linked to birth certificates, identifying those singleton live births for whom maternal prepregnancy BMI data were available.
From this group, they included women aged 15-50 years who delivered at 20-45 weeks’ gestational age. Women with prepregnancy BMIs less than 18.5 kg/m2 – those who were underweight – were excluded.
Dr. Platner and her coinvestigators used multivariable analysis to see what association the full range of obesity classes had with severe maternal morbidity, adjusting for many socioeconomic and demographic factors.
Of the 539,870 women included in the study, 3.3% experienced severe maternal morbidity, and 17.4% of patients met criteria for obesity. “Across all classes of obesity, there was a significantly greater risk of severe maternal morbidity, compared to nonobese women,” wrote Dr. Platner and her colleagues in the poster accompanying the presentation.
These risks climbed for women with the highest BMIs, however. “Women with higher levels of obesity, not surprisingly, are at increased risk” of severe maternal morbidity, said Dr. Platner. She and her colleagues noted in the poster that, “There is a significant dose-response relationship between increasing obesity class and risk of [severe maternal morbidity] at delivery hospitalization.”
It had been known that women with obesity are at increased risk of some serious complications of pregnancy, including severe maternal morbidity and mortality, and that those considered morbidly obese – with BMIs of 40 and above – are most likely to experience these complications, Dr. Platner said. However, she added, there’s a paucity of data to inform maternal risk stratification by level of obesity.
“We included the group of superobese women, which is significant in the surgical literature, and that’s a BMI of 50 and above ... we thought that would be an important subgroup to analyze in this population,” she said.
Dr. Platner said that she and her colleagues already had the clinical impressions that women with the highest BMIs were most likely to have serious complications. “I don’t think that these findings are particularly surprising,” she said. “This is what our hypothesis was in terms of why we did this study.”
The greater surprise, she said, was the magnitude of increased risk seen for serious morbidity with higher levels of obesity.
“Really, the risk is truly increased for those women with class III or superobesity, and when we start to stratify ... those are the women we need to be concerned about in terms of our prenatal counseling,” said Dr. Platner, a maternal-fetal medicine fellow at Yale University, New Haven, Conn.
“What can we do to intervene before we get there?” asked Dr. Platner. Although data are lacking about what specific interventions might be able to reduce the risk of these serious complications, she said she could envision such steps as acquiring predelivery baseline ECGs and cardiac ultrasounds in women with higher levels of obesity and being sure to follow renal function closely as well.
The findings also may help physicians provide more evidence-based preconception advice to women who are among the 35% of American adults who have obesity.
Dr. Platner reported no relevant financial disclosures.
SOURCE: Platner M et al. ACOG 2018, Abstract 39I.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AUSTIN, TEX. – Women with the highest levels of obesity were at higher odds of experiencing a composite serious maternal morbidity outcome, while women at all levels of obesity experienced elevated risks of some serious complications of pregnancy, compared with women with a body mass index (BMI) in the normal range, according to a recent study.
Looking at individual indicators of severe maternal morbidity, Marissa Platner, MD, and her study coauthors saw that women who fell into the higher levels of obesity had significantly elevated odds of some complications.
“Those risks are really impressive, with odds ratios of two and three times that of a normal-weight patient,” said Dr. Platner in a video interview.
The adjusted odds ratio of acute renal failure for women with superobesity (BMI of 50 kg/m2 or more) was 3.62 (95% confidence interval, 1.75-7.52); odds ratios for renal failure were not significantly elevated for less-obese women.
Women with all levels of obesity had elevated risks of experiencing heart failure during a procedure or surgery, with adjusted odds ratios ranging from 1.68 (95% CI, 1.48-1.93) for women with class I obesity (BMI, 30-34.9 kg/m2) to 2.23 for women with superobesity (95% CI, 1.15-4.33).
Results from the retrospective cohort study were presented at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists.
Dr. Platner and her colleagues examined 4 years of New York City delivery data that were linked to birth certificates, identifying those singleton live births for whom maternal prepregnancy BMI data were available.
From this group, they included women aged 15-50 years who delivered at 20-45 weeks’ gestational age. Women with prepregnancy BMIs less than 18.5 kg/m2 – those who were underweight – were excluded.
Dr. Platner and her coinvestigators used multivariable analysis to see what association the full range of obesity classes had with severe maternal morbidity, adjusting for many socioeconomic and demographic factors.
Of the 539,870 women included in the study, 3.3% experienced severe maternal morbidity, and 17.4% of patients met criteria for obesity. “Across all classes of obesity, there was a significantly greater risk of severe maternal morbidity, compared to nonobese women,” wrote Dr. Platner and her colleagues in the poster accompanying the presentation.
These risks climbed for women with the highest BMIs, however. “Women with higher levels of obesity, not surprisingly, are at increased risk” of severe maternal morbidity, said Dr. Platner. She and her colleagues noted in the poster that, “There is a significant dose-response relationship between increasing obesity class and risk of [severe maternal morbidity] at delivery hospitalization.”
It had been known that women with obesity are at increased risk of some serious complications of pregnancy, including severe maternal morbidity and mortality, and that those considered morbidly obese – with BMIs of 40 and above – are most likely to experience these complications, Dr. Platner said. However, she added, there’s a paucity of data to inform maternal risk stratification by level of obesity.
“We included the group of superobese women, which is significant in the surgical literature, and that’s a BMI of 50 and above ... we thought that would be an important subgroup to analyze in this population,” she said.
Dr. Platner said that she and her colleagues already had the clinical impressions that women with the highest BMIs were most likely to have serious complications. “I don’t think that these findings are particularly surprising,” she said. “This is what our hypothesis was in terms of why we did this study.”
The greater surprise, she said, was the magnitude of increased risk seen for serious morbidity with higher levels of obesity.
“Really, the risk is truly increased for those women with class III or superobesity, and when we start to stratify ... those are the women we need to be concerned about in terms of our prenatal counseling,” said Dr. Platner, a maternal-fetal medicine fellow at Yale University, New Haven, Conn.
“What can we do to intervene before we get there?” asked Dr. Platner. Although data are lacking about what specific interventions might be able to reduce the risk of these serious complications, she said she could envision such steps as acquiring predelivery baseline ECGs and cardiac ultrasounds in women with higher levels of obesity and being sure to follow renal function closely as well.
The findings also may help physicians provide more evidence-based preconception advice to women who are among the 35% of American adults who have obesity.
Dr. Platner reported no relevant financial disclosures.
SOURCE: Platner M et al. ACOG 2018, Abstract 39I.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM ACOG 2018
Inhaled nitrous oxide for labor analgesia: Pearls from clinical experience
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
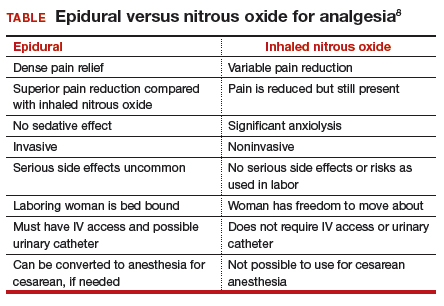
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.
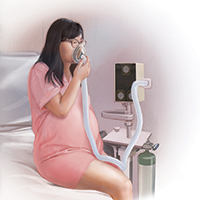
Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.

Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Nitrous oxide, a colorless, odorless gas, has long been used for labor analgesia in many countries, including the United Kingdom, Canada, throughout Europe, Australia, and New Zealand. Recently, interest in its use in the United States has increased, since the US Food and Drug Administration (FDA) approval in 2012 of simple devices for administration of nitrous oxide in a variety of locations. Being able to offer an alternative technique, other than parenteral opioids, for women who may not wish to or who cannot have regional analgesia, and for women who have delivered and need analgesia for postdelivery repair, conveys significant benefits. Risks to its use are very low, although the quality of pain relief is inferior to that offered by regional analgesic techniques. Our experience with its use since 2014 at Brigham and Women’s Hospital in Boston, Massachusetts, corroborates that reported in the literature and leads us to continue offering inhaled nitrous oxide and advocating that others do as well.1–7 When using nitrous oxide in your labor and delivery unit, or if considering its use, keep the following points in mind.
A successful inhaled nitrous oxide program requires proper patient selection
Inhaled nitrous oxide is not an epidural (TABLE).8 The pain relief is clearly inferior to that of an epidural. Inhaled nitrous oxide will not replace epidurals or even have any effect on the epidural rate at a particular institution.6 However, the use of inhaled nitrous oxide for labor analgesia has a long track record of safety (albeit with moderate efficacy for selected patients) for many years in many countries around the world. Inhaled nitrous oxide is a valuable addition to the options we can offer patients:
- who are poor responders to opioid medication or who have high opioid tolerance
- with certain disorders of coagulation
- with chronic pain or anxiety
- who for other reasons need to consider alternatives or adjuncts to neuraxial analgesia.

Although it is important to be realistic regarding the expectations of analgesia quality offered by this agent,7 compared with other agents we have tried, it has less adverse effects, is economically reasonable, and has no proven impact on neonatal outcomes.
No significant complications with inhaled nitrous oxide have been reported
Systematic reviews did not report any significant complications to either mother or newborn.1,2 Our personal experiences corroborate this, as no complications have been associated with its frequent use at Brigham and Women’s Hospital. Reported adverse effects are mild. The incidence of nausea is 13%, dizziness is 3% to 5%, and drowsiness is 4%; these rates are hard to detect over the baseline rates of those side effects associated with labor and delivery alone.1 Many other centers have now adopted the use of this agent, with several hundred locations now offering inhaled nitrous oxide for labor analgesia in the United States.
Practical use of inhaled nitrous oxide is relatively simple
Several vendors offer portable, user-friendly, cost-effective equipment that is appropriate for labor and delivery use. All devices are structured in demand-valve modality, meaning that the patient must initiate a breath in order to open a valve that allows gas to flow. Cessation of the inspiratory effort closes the valve, thus preventing the free flow of gas into the ambient atmosphere of the room. The devices generally include a tank with nitrous oxide as well as a source of oxygen. Most devices designed for labor and delivery provide a fixed mixture of 50% nitrous oxide and 50% oxygen, with fail-safe mechanisms to allow increased oxygen delivery in the event of failure or depletion of the nitrous supply. All modern, FDA–approved devices include effective scavenging systems, such that expired gases are vented outside (generally via room suction), which prevents occupational exposure to low levels of nitrous oxide.
Inhaled nitrous oxide for labor pain must be patient controlled
An essential feature of the use of inhaled nitrous oxide for labor analgesia is that it must be considered a patient-controlled system. Patients have an option to use either a mask or a mouthpiece, according to their preferences and comfort. The patient must hold the mask or mouthpiece herself; it is neither appropriate nor safe for anyone else, such as a nurse, family member, or labor support personnel, to assist with this task.
Some coordination with the nurse is essential for optimal timing of administration. Onset of a therapeutic level of pain relief is generally 30 to 60 seconds after inhalation has begun, with rapid resolution after cessation of the inhalation. The patient should thus initiate the inspiration of the gas at the earliest signs of onset of a contraction, so as to achieve maximal analgesia at the peak of the contraction. Waiting until the peak of the contraction to initiate inhalation of the nitrous oxide will not provide effective analgesia, yet will result in sedation after the contraction has ended.
Read about patient satisfaction with inhaled nitrous oxide.
No oversight by an anesthesiologist is required
The Centers for Medicare and Medicaid Services (CMS) produced a clarification statement for definitions of “anesthesia services” (42 CFR 482.52)9 that may be offered by a hospital, based on American Society of Anesthesiologists (ASA) definitions. CMS, consistent with ASA guidelines, does not define moderate or conscious sedation as “anesthesia,” thus direct oversight by an anesthesiologist is not required. Furthermore, the definition of “minimal sedation,” which is where 50% concentration delivery of inhaled nitrous oxide would be categorized, also does not meet this requirement by CMS.
Women who use inhaled nitrous oxide for labor pain typically are satisfied with its use
The use of analog pain scale measurements may not be appropriate in a setting where dissociation from pain might be the primary beneficial effect. Measurements of maternal satisfaction with their analgesic experience support this. The experiences at Vanderbilt University and Brigham and Women’s Hospital show that, while pain relief is limited, like reported in systematic reviews, maternal satisfaction scores for labor analgesia are not different among women who receive inhaled nitrous oxide analgesia, neuraxial analgesia, and those who transition from nitrous to neuraxial analgesia. In fact, published evidence supports extraordinarily high satisfaction in women who plan to use inhaled nitrous oxide, and actually successfully do so, despite only limited degrees of pain relief.10,11 Work to identify the characteristics of women who report success with inhaled nitrous oxide use needs to be performed so that patients can be better selected and informed when making analgesic choices.
Animal research on inhaled nitrous oxide may not translate well to human neonates
A very recent task force convened by the European Society of Anaesthesiology (ESA) addressed some of the potential concerns about inhaled nitrous oxide analgesia.12 Per their report:
“the potential teratogenic effect of N2O observed in experimental models cannot be extrapolated to humans. There is a lack of evidence for an association between N2O and reproductive toxicity. The incidence of health hazards and abortion was not shown to be higher in women exposed to, or spouses of men exposed to N2O than those who were not so exposed. Moreover, the incidence of congenital malformations was not higher among women who received N2O for anaesthesia during the first trimester of pregnancy nor during anaesthesia management for cervical cerclage, nor for surgery in the first two trimesters of pregnancy.”
There is a theoretical concern of an increase in neuronal apoptosis in neonates, demonstrated in laboratory animals in anesthetic concentrations, but the human relevance of this is not clear, since the data on animal developmental neurotoxicity is generally combined with data wherein potent inhalational anesthetic agents were also used, not nitrous oxide alone.13 The analgesic doses and time of exposure of inhaled nitrous oxide administered for labor analgesia are well below those required for these changes, as subanesthetic doses are associated with minimal changes, if any, in laboratory animals.
No labor analgesic is without the potential for fetal effects, and alternative labor analgesics such as systemic opioids in higher doses also may have potential adverse effects on the fetus, such as fetal heart rate effects or early tone, alertness, and breastfeeding difficulties. The low solubility and short half-life of inhaled nitrous oxide contribute to low absorption by tissues, thus contributing to the safety of this agent. Nitrous oxide via inhalation for sedation during elective cesarean has been reported to show no adverse effects on neonatal Apgar scores.14
Modern equipment keeps occupational exposure to nitrous oxide safe
One retrospective review of women exposed to high concentrations of inhaled nitrous oxide reported reduced fertility.15 However, the only effects on fertility were seen when nitrous was used without scavenging equipment, and in high concentrations. Moreover, that study examined dental offices, where nitrous was free flowing during procedures—quite a different setting than the intermittent inhalation, demand-valve modality as is used during labor—and when using appropriate modern, FDA-approved equipment, and scavenging devices. Per the recent ESA task force12:
“Members of the task force agreed that, despite theoretical concerns and laboratory data, there is no evidence indicating that the use of N2O in a clinically relevant setting would increase health risk in patients or providers exposed to this drug. With the ubiquitous availability of scavenging systems in the modern operating room, the health concern for medical staff has decreased dramatically. Properly operating scavenging systems reduce N2O concentrations by more than 70%, thereby efficiently keeping ambient N2O levels well below official limits.”
The ESA task force concludes: “An extensive amount of clinical evidence indicates that N2O can be used safely for procedural pain management, for labour pain, and for anxiolysis and sedation in dentistry.”12
Two important reminders
Inhaled nitrous oxide has been a central component of the labor pain relief menu in most of the rest of the world for decades, and the safety record is impeccable. This agent has now had extensive and growing experience in American maternity units. Remember 2 critical points: 1) patient selection is key, 2) analgesia is not like that provided by regional anesthetic techniques such as an epidural.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
- Likis FE, Andrews JC, Collins MR, et al. Nitrous oxide for the management of labor pain: a systematic review. Anesth Analg. 2014;118(1):153-167.
- Rosen MA. Nitrous oxide for relief of labor pain: a systematic review. Am J Obstet Gynecol. 2002;186(5 suppl nature):S110-S126.
- Angle P, Landy CK, Charles C. Phase 1 development of an index to measure the quality of neuraxial labour analgesia: exploring the perspectives of childbearing women. Can J Anaesth. 2010;57(5):468-478.
- Migliaccio L, Lawton R, Leeman L, Holbrook A. Initiating intrapartum nitrous oxide in an academic hospital: considerations and challenges. J Midwifery Womens Health. 2017;62(3):358-362.
- Markley JC, Rollins MD. Non-neuraxial labor analgesia: options. Clin Obstet Gynecol. 2017;60(2);350-364.
- Bobb LE, Farber MK, McGovern C, Camann W. Does nitrous oxide labor analgesia influence the pattern of neuraxial analgesia usage? An impact study at an academic medical center. J Clin Anesth. 2016;35:54-57.
- Sutton CD, Butwick AJ, Riley ET, Carvalho B. Nitrous oxide for labor analgesia: utilization and predictors of conversion to neuraxial analgesia. J Clin Anesth. 2017;40:40-45.
- Collins MR, Starr SA, Bishop JT, Baysinger CL. Nitrous oxide for labor analgesia: expanding analgesic options for women in the United States. Rev Obstet Gynecol. 2012;5(3-4):e126-e131.
- 42 CFR 482.52 - Condition of participation: Anesthesia services. US Government Publishing Office website. https://www.gpo.gov/fdsys/granule/CFR-2011-title42-vol5/CFR-2011-title42-vol5-sec482-52. Accessed April 16, 2018.
- Richardson MG, Lopez BM, Baysinger CL, Shotwell MS, Chestnut DH. Nitrous oxide during labor: maternal satisfaction does not depend exclusively on analgesic effectiveness. Anesth Analg. 2017;124(2):548-553.
- Camann W. Pain, pain relief, satisfaction, and excellence in obstetric anesthesia: a surprisingly complex relationship. Anesth Analg. 2017;124(2):383-385.
- European Society of Anaesthesiology Task Force on Use of Nitrous Oxide in Clinical Anaesthetic Practice. The current place of nitrous oxide in clinical practice: an expert opinion-based task force consensus statement of the European Society of Anaesthesiology. Eur J Anaesthesiol. 2015;32(8):517-520.
- Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. 2011;364(15):1387-1390.
- Vallejo MC, Phelps AL, Shepherd CJ, Kaul B, Mandell GL, Ramanathan S. Nitrous oxide anxiolysis for elective cesarean section. J Clin Anesth. 2005;17(7):543-548.
- Rowland AS, Baird DD, Weinberg CR, et al. Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. N Engl J Med. 1992;327(14):993-997.
Glyburide failed to show noninferiority in gestational diabetes
A randomized, multicenter trial failed to find glyburide noninferior to insulin for treatment of gestational diabetes, investigators reported.
The composite rate of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia was 27.6% with oral glyburide and 23.4% with subcutaneous insulin (P = .19) therapy, said Marie-Victoire Sénat, MD, PhD, of Hôpital Bicêtre in Paris, and her associates. The upper limit of the 97.5% confidence interval for the difference between groups was 10.5%, exceeding the prespecified noninferiority margin of 7%. “These findings do not justify the use of glyburide as first-line treatment,” the researchers wrote. The report was published online May 1 in JAMA.
Glyburide is a common add-on therapy for gestational diabetes in the United States but is not used regularly in Europe. The treatments exert similar glycemic control, but meta-analyses and recent studies have linked glyburide to increased rates of neonatal macrosomia and hypoglycemia. However, trials comparing glyburide with insulin focused on maternal glycemic control and thus “were not optimally designed to investigate neonatal complications,” the researchers wrote.
For the study, they randomly assigned 914 women whose gestational diabetes persisted despite dietary intervention to receive either 2.5 mg glyburide once daily or 4 IU to 20 IU insulin one to four times daily. Patients up-titrated treatment as needed based on self-measured blood glucose levels. Glyburide first was increased by 2.5 mg on day 4 and thereafter by 5 mg every 4 days in morning and evening doses to a daily maximum of 20 mg. Prandial insulin was increased by 2 IU every 2 days, while basal or intermediate insulin was dosed at 4 IU to 8 IU at bedtime and increased by 2 IU every 2 days.
The difference in the composite endpoint still exceeded 4% between groups even after the researchers controlled for multiparity and gestational age at treatment. Rates of each individual complication were higher with glyburide than with insulin, although only hypoglycemia reached statistical significance (12.2% for glyburide versus 7.2% for insulin; P = .02).
Maternal hypoglycemia affected 3.8% of the glyburide arm and 1% of the insulin arm (P = .02), and 72% of glyburide patients maintained good fasting glycemic control versus 63% of insulin recipients (P = .003). Also, 58% of glyburide recipients had good postprandial glucose control versus 49% of insulin recipients (P = .051).
Questionnaires indicated that patients were more likely to find glyburide tolerable and to report that they would use it again, if needed, during a future pregnancy (P less than .001 for between-group comparisons). “Although the data do not allow a conclusion that glyburide is not inferior to insulin in the prevention of perinatal complications, the results suggest that the increase in complications may be no more than 10.5% compared with insulin,” the investigators wrote. “This result should be balanced with the ease of use and better satisfaction with glyburide.”
Dr. Sénat reported having no conflicts of interest. One coinvestigator disclosed ties to Ferring Laboratories.
SOURCE: Sénat M-V et al. JAMA. 319(17):1773-80.
The researchers were “reasonable” to conclude that insulin should remain the first-line pharmacotherapy for gestational diabetes, according to Donald R. Coustan, MD, and Linda Barbour, MD, MSPH, whose editorial accompanied the study in JAMA.
“Use of glyburide may be most appropriate when insulin injections are not acceptable or practical,” they wrote. They suggested “frankly” counseling pregnant women about glyburide crossing the placenta and about “unanswered questions regarding long-term effects on offspring.”
Ideally, pregnant women should receive glyburide 1 hour before meals so that its effect peaks 3-4 hours later, according to the experts. But the study authors did not describe treatment timing with respect to meals, did not adjust initial dosing based on fasting or postprandial hyperglycemia, and only increased the dose every 4 days, they noted.
Although insulin was dosed much more flexibly, the glyburide group had better fasting glucose than did controls (72% vs. 63%; P = .003), the editorialists noted. Glyburide is most likely to succeed in younger women without fasting hyperglycemia and whose gestational diabetes begins later in pregnancy. Better dosing and patient selection might make glyburide more effective while also helping prevent maternal hypoglycemia and adverse perinatal outcomes, they contended.
Dr. Coustan is with Brown University, Providence, R.I. Dr. Barbour is with University of Colorado at Denver, Aurora. They reported having no conflicts of interest. These comments paraphrase their editorial ( JAMA. 2018;319[17]:1769-70 ).
The researchers were “reasonable” to conclude that insulin should remain the first-line pharmacotherapy for gestational diabetes, according to Donald R. Coustan, MD, and Linda Barbour, MD, MSPH, whose editorial accompanied the study in JAMA.
“Use of glyburide may be most appropriate when insulin injections are not acceptable or practical,” they wrote. They suggested “frankly” counseling pregnant women about glyburide crossing the placenta and about “unanswered questions regarding long-term effects on offspring.”
Ideally, pregnant women should receive glyburide 1 hour before meals so that its effect peaks 3-4 hours later, according to the experts. But the study authors did not describe treatment timing with respect to meals, did not adjust initial dosing based on fasting or postprandial hyperglycemia, and only increased the dose every 4 days, they noted.
Although insulin was dosed much more flexibly, the glyburide group had better fasting glucose than did controls (72% vs. 63%; P = .003), the editorialists noted. Glyburide is most likely to succeed in younger women without fasting hyperglycemia and whose gestational diabetes begins later in pregnancy. Better dosing and patient selection might make glyburide more effective while also helping prevent maternal hypoglycemia and adverse perinatal outcomes, they contended.
Dr. Coustan is with Brown University, Providence, R.I. Dr. Barbour is with University of Colorado at Denver, Aurora. They reported having no conflicts of interest. These comments paraphrase their editorial ( JAMA. 2018;319[17]:1769-70 ).
The researchers were “reasonable” to conclude that insulin should remain the first-line pharmacotherapy for gestational diabetes, according to Donald R. Coustan, MD, and Linda Barbour, MD, MSPH, whose editorial accompanied the study in JAMA.
“Use of glyburide may be most appropriate when insulin injections are not acceptable or practical,” they wrote. They suggested “frankly” counseling pregnant women about glyburide crossing the placenta and about “unanswered questions regarding long-term effects on offspring.”
Ideally, pregnant women should receive glyburide 1 hour before meals so that its effect peaks 3-4 hours later, according to the experts. But the study authors did not describe treatment timing with respect to meals, did not adjust initial dosing based on fasting or postprandial hyperglycemia, and only increased the dose every 4 days, they noted.
Although insulin was dosed much more flexibly, the glyburide group had better fasting glucose than did controls (72% vs. 63%; P = .003), the editorialists noted. Glyburide is most likely to succeed in younger women without fasting hyperglycemia and whose gestational diabetes begins later in pregnancy. Better dosing and patient selection might make glyburide more effective while also helping prevent maternal hypoglycemia and adverse perinatal outcomes, they contended.
Dr. Coustan is with Brown University, Providence, R.I. Dr. Barbour is with University of Colorado at Denver, Aurora. They reported having no conflicts of interest. These comments paraphrase their editorial ( JAMA. 2018;319[17]:1769-70 ).
A randomized, multicenter trial failed to find glyburide noninferior to insulin for treatment of gestational diabetes, investigators reported.
The composite rate of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia was 27.6% with oral glyburide and 23.4% with subcutaneous insulin (P = .19) therapy, said Marie-Victoire Sénat, MD, PhD, of Hôpital Bicêtre in Paris, and her associates. The upper limit of the 97.5% confidence interval for the difference between groups was 10.5%, exceeding the prespecified noninferiority margin of 7%. “These findings do not justify the use of glyburide as first-line treatment,” the researchers wrote. The report was published online May 1 in JAMA.
Glyburide is a common add-on therapy for gestational diabetes in the United States but is not used regularly in Europe. The treatments exert similar glycemic control, but meta-analyses and recent studies have linked glyburide to increased rates of neonatal macrosomia and hypoglycemia. However, trials comparing glyburide with insulin focused on maternal glycemic control and thus “were not optimally designed to investigate neonatal complications,” the researchers wrote.
For the study, they randomly assigned 914 women whose gestational diabetes persisted despite dietary intervention to receive either 2.5 mg glyburide once daily or 4 IU to 20 IU insulin one to four times daily. Patients up-titrated treatment as needed based on self-measured blood glucose levels. Glyburide first was increased by 2.5 mg on day 4 and thereafter by 5 mg every 4 days in morning and evening doses to a daily maximum of 20 mg. Prandial insulin was increased by 2 IU every 2 days, while basal or intermediate insulin was dosed at 4 IU to 8 IU at bedtime and increased by 2 IU every 2 days.
The difference in the composite endpoint still exceeded 4% between groups even after the researchers controlled for multiparity and gestational age at treatment. Rates of each individual complication were higher with glyburide than with insulin, although only hypoglycemia reached statistical significance (12.2% for glyburide versus 7.2% for insulin; P = .02).
Maternal hypoglycemia affected 3.8% of the glyburide arm and 1% of the insulin arm (P = .02), and 72% of glyburide patients maintained good fasting glycemic control versus 63% of insulin recipients (P = .003). Also, 58% of glyburide recipients had good postprandial glucose control versus 49% of insulin recipients (P = .051).
Questionnaires indicated that patients were more likely to find glyburide tolerable and to report that they would use it again, if needed, during a future pregnancy (P less than .001 for between-group comparisons). “Although the data do not allow a conclusion that glyburide is not inferior to insulin in the prevention of perinatal complications, the results suggest that the increase in complications may be no more than 10.5% compared with insulin,” the investigators wrote. “This result should be balanced with the ease of use and better satisfaction with glyburide.”
Dr. Sénat reported having no conflicts of interest. One coinvestigator disclosed ties to Ferring Laboratories.
SOURCE: Sénat M-V et al. JAMA. 319(17):1773-80.
A randomized, multicenter trial failed to find glyburide noninferior to insulin for treatment of gestational diabetes, investigators reported.
The composite rate of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia was 27.6% with oral glyburide and 23.4% with subcutaneous insulin (P = .19) therapy, said Marie-Victoire Sénat, MD, PhD, of Hôpital Bicêtre in Paris, and her associates. The upper limit of the 97.5% confidence interval for the difference between groups was 10.5%, exceeding the prespecified noninferiority margin of 7%. “These findings do not justify the use of glyburide as first-line treatment,” the researchers wrote. The report was published online May 1 in JAMA.
Glyburide is a common add-on therapy for gestational diabetes in the United States but is not used regularly in Europe. The treatments exert similar glycemic control, but meta-analyses and recent studies have linked glyburide to increased rates of neonatal macrosomia and hypoglycemia. However, trials comparing glyburide with insulin focused on maternal glycemic control and thus “were not optimally designed to investigate neonatal complications,” the researchers wrote.
For the study, they randomly assigned 914 women whose gestational diabetes persisted despite dietary intervention to receive either 2.5 mg glyburide once daily or 4 IU to 20 IU insulin one to four times daily. Patients up-titrated treatment as needed based on self-measured blood glucose levels. Glyburide first was increased by 2.5 mg on day 4 and thereafter by 5 mg every 4 days in morning and evening doses to a daily maximum of 20 mg. Prandial insulin was increased by 2 IU every 2 days, while basal or intermediate insulin was dosed at 4 IU to 8 IU at bedtime and increased by 2 IU every 2 days.
The difference in the composite endpoint still exceeded 4% between groups even after the researchers controlled for multiparity and gestational age at treatment. Rates of each individual complication were higher with glyburide than with insulin, although only hypoglycemia reached statistical significance (12.2% for glyburide versus 7.2% for insulin; P = .02).
Maternal hypoglycemia affected 3.8% of the glyburide arm and 1% of the insulin arm (P = .02), and 72% of glyburide patients maintained good fasting glycemic control versus 63% of insulin recipients (P = .003). Also, 58% of glyburide recipients had good postprandial glucose control versus 49% of insulin recipients (P = .051).
Questionnaires indicated that patients were more likely to find glyburide tolerable and to report that they would use it again, if needed, during a future pregnancy (P less than .001 for between-group comparisons). “Although the data do not allow a conclusion that glyburide is not inferior to insulin in the prevention of perinatal complications, the results suggest that the increase in complications may be no more than 10.5% compared with insulin,” the investigators wrote. “This result should be balanced with the ease of use and better satisfaction with glyburide.”
Dr. Sénat reported having no conflicts of interest. One coinvestigator disclosed ties to Ferring Laboratories.
SOURCE: Sénat M-V et al. JAMA. 319(17):1773-80.
FROM JAMA
Key clinical point: A large trial failed to justify the use of glyburide as first-line therapy for gestational diabetes.
Major finding: Combined rates of macrosomia, neonatal hypoglycemia, and hyperbilirubinemia were 27.6% in the glyburide group and 23.4% in the insulin group (P = .19). The upper limit of the confidence interval for the difference between groups was 10.5%, exceeding the prespecified noninferiority margin of 7%.
Study details: Multicenter randomized trial of 914 women with gestational diabetes.
Disclosures: Dr. Sénat reported having no conflicts of interest. One coinvestigator disclosed ties to Ferring Laboratories.
Source: Sénat M-V et al. JAMA. 319(17):1773-80.
VIDEO: Postpartum care gets a new look
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
AUSTIN, TEX. – While women may have a plethora of options for care during pregnancy, attention given to women after birth is seriously lacking, with detrimental effect.
Currently, postpartum care is limited to a follow-up appointment 6 weeks after pregnancy, but according to Alison Stuebe, MD, medical director of lactation services at the University of North Carolina, Chapel Hill, there is too much going on in those 6 weeks to continue this model.
To address preferred changes to this system of care, the .
“What we’d like to do with the new committee opinion is move from this one-off visit at 6 weeks where we tell people ‘you’re good to go, you can have sex, get out of my office,’ to a much more comprehensive approach that reaches out to moms in the first couple of weeks,” explained Dr. Stuebe. “Whether that’s by phone, by asynchronous communication, by in-person visit, [the physician] finds out what’s going on, and then makes appropriate recommendations to help her rather than waiting to see what’s left after 6 weeks,” she said at ACOG’s annual clinical and scientific meeting.
Paying for these services is a big barrier right now, said Dr. Stuebe, but some solutions have already shown signs of being cost effective.
One example, in Dr. Stuebe’s hometown of Durham County, N.C., is a program called Durham Connect, which puts nurses in contact with women at 3 weeks postpartum to make assessments of what care the mother needs, and then offers service referrals to help with those needs.
According to Dr. Stuebe, studies have found every dollar invested in the program would save $3 in emergency department visits for children.
As postpartum care evolves, the most important thing is to remember that when it comes to pregnancy and birth, just because the baby is out doesn’t mean the mother can be ignored, she said.
“When we think about the way postpartum care exists today, you think about the mom being the candy wrapper and the baby being the candy; when the candy’s out of the wrapper, we toss the wrapper,” said Dr. Stuebe. “What these new guidelines are saying is this wrapper is actually pretty important.”
The revised committee opinion states: “The comprehensive postpartum visit should include a full assessment of physical, social, and psychological well-being, including the following domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.”
Dr. Stuebe receives support from Janssen.
REPORTING FROM ACOG 2018
VIDEO: To boost newborn breastfeeding rates, hide the EHR formula order
AUSTIN, TEXAS – When the check box for ordering formula for newborns was removed as a standard newborn order option in the electronic health record (EHR), rates of exclusive breastfeeding climbed significantly in Los Angeles County hospitals, according to a recent study.
“The saying, ‘out of sight, out of mind’ cannot be overstated when it comes to physician order entry,” wrote Ramy Eskander, MD, and his colleagues in the poster accompanying the presentation at the annual clinical and scientific sessions of the American College of Obstetricians and Gynecologists.
In a video interview, Dr. Eskander said that he and his colleagues at the University of California, Los Angeles, were looking for an intervention that would use the EHR as a quality improvement tool.
What they decided to do was to see “how could we possibly ‘get in the way’ and have an intervention between the provider and the patient that didn’t necessarily involve much work on the provider’s end, that had a significant impact on the back end,” he said. What they ended up doing was remove the order to request formula for mothers from the physician order set in the EHR.
Study data were collected in three stages for the academic tertiary care hospital within the Los Angeles County Department of Health Services system.
First, Dr. Eskander and his colleagues collected baseline data from January to July of 2016. Then, data were collected from July to the end of 2016, while a campaign was underway to bring staff and patients up to speed on the benefits of exclusive breastfeeding. There were no statistically significant differences in the rates of exclusive breastfeeding on discharge between these two time periods, when rates hovered between 30% and 40%.
The final data collection period began in January 2017. At that time, the option to order formula for a newborn was removed as an option for the EHR newborn order set.
“When we did that, providers weren’t looking at the possibility of having that easy check box right there to fill in … and we know that when people have to go through more steps, they invariably don’t do it,” Dr. Eskander said.
He and his colleagues saw an almost immediate . Once the formula order was removed, breastfeeding rates rose from 40.57% to 53.90% (P less than .001). Rates have been sustained since the removal of the EHR option for formula.
There was no difference in how infants fared after the intervention, said Dr. Eskander. “The outcomes for those infants was identical. There were no increased NICU admissions, there were no increased poor outcomes.”
Length of stay remained the same as well. “The babies were being discharged in the same state of health, just more of them were getting breast milk only, and we know the benefits that tends to portend,” he added.
There was some initial grumbling when the formula order was pulled from the newborn order set, he conceded. “The providers were not very happy about having to look for the newborn order for formula.” However, it took just about a month for the new workflow to seem normal, he said.
Dr. Eskander envisions a future where the EHR is “smart” enough to prompt appropriate orders and interventions for serious conditions such as preeclampsia. The electronic record, he said, could recognize the maternal diagnosis “and immediately create a system and structure around that mother to be able to help protect her and her baby. ... then having those diagnoses be able to drive outcomes can be very significant.”
Dr. Eskander reported no relevant financial disclosures.
SOURCE: Eskander, R et al. ACOG 2018, Abstract 31I.
AUSTIN, TEXAS – When the check box for ordering formula for newborns was removed as a standard newborn order option in the electronic health record (EHR), rates of exclusive breastfeeding climbed significantly in Los Angeles County hospitals, according to a recent study.
“The saying, ‘out of sight, out of mind’ cannot be overstated when it comes to physician order entry,” wrote Ramy Eskander, MD, and his colleagues in the poster accompanying the presentation at the annual clinical and scientific sessions of the American College of Obstetricians and Gynecologists.
In a video interview, Dr. Eskander said that he and his colleagues at the University of California, Los Angeles, were looking for an intervention that would use the EHR as a quality improvement tool.
What they decided to do was to see “how could we possibly ‘get in the way’ and have an intervention between the provider and the patient that didn’t necessarily involve much work on the provider’s end, that had a significant impact on the back end,” he said. What they ended up doing was remove the order to request formula for mothers from the physician order set in the EHR.
Study data were collected in three stages for the academic tertiary care hospital within the Los Angeles County Department of Health Services system.
First, Dr. Eskander and his colleagues collected baseline data from January to July of 2016. Then, data were collected from July to the end of 2016, while a campaign was underway to bring staff and patients up to speed on the benefits of exclusive breastfeeding. There were no statistically significant differences in the rates of exclusive breastfeeding on discharge between these two time periods, when rates hovered between 30% and 40%.
The final data collection period began in January 2017. At that time, the option to order formula for a newborn was removed as an option for the EHR newborn order set.
“When we did that, providers weren’t looking at the possibility of having that easy check box right there to fill in … and we know that when people have to go through more steps, they invariably don’t do it,” Dr. Eskander said.
He and his colleagues saw an almost immediate . Once the formula order was removed, breastfeeding rates rose from 40.57% to 53.90% (P less than .001). Rates have been sustained since the removal of the EHR option for formula.
There was no difference in how infants fared after the intervention, said Dr. Eskander. “The outcomes for those infants was identical. There were no increased NICU admissions, there were no increased poor outcomes.”
Length of stay remained the same as well. “The babies were being discharged in the same state of health, just more of them were getting breast milk only, and we know the benefits that tends to portend,” he added.
There was some initial grumbling when the formula order was pulled from the newborn order set, he conceded. “The providers were not very happy about having to look for the newborn order for formula.” However, it took just about a month for the new workflow to seem normal, he said.
Dr. Eskander envisions a future where the EHR is “smart” enough to prompt appropriate orders and interventions for serious conditions such as preeclampsia. The electronic record, he said, could recognize the maternal diagnosis “and immediately create a system and structure around that mother to be able to help protect her and her baby. ... then having those diagnoses be able to drive outcomes can be very significant.”
Dr. Eskander reported no relevant financial disclosures.
SOURCE: Eskander, R et al. ACOG 2018, Abstract 31I.
AUSTIN, TEXAS – When the check box for ordering formula for newborns was removed as a standard newborn order option in the electronic health record (EHR), rates of exclusive breastfeeding climbed significantly in Los Angeles County hospitals, according to a recent study.
“The saying, ‘out of sight, out of mind’ cannot be overstated when it comes to physician order entry,” wrote Ramy Eskander, MD, and his colleagues in the poster accompanying the presentation at the annual clinical and scientific sessions of the American College of Obstetricians and Gynecologists.
In a video interview, Dr. Eskander said that he and his colleagues at the University of California, Los Angeles, were looking for an intervention that would use the EHR as a quality improvement tool.
What they decided to do was to see “how could we possibly ‘get in the way’ and have an intervention between the provider and the patient that didn’t necessarily involve much work on the provider’s end, that had a significant impact on the back end,” he said. What they ended up doing was remove the order to request formula for mothers from the physician order set in the EHR.
Study data were collected in three stages for the academic tertiary care hospital within the Los Angeles County Department of Health Services system.
First, Dr. Eskander and his colleagues collected baseline data from January to July of 2016. Then, data were collected from July to the end of 2016, while a campaign was underway to bring staff and patients up to speed on the benefits of exclusive breastfeeding. There were no statistically significant differences in the rates of exclusive breastfeeding on discharge between these two time periods, when rates hovered between 30% and 40%.
The final data collection period began in January 2017. At that time, the option to order formula for a newborn was removed as an option for the EHR newborn order set.
“When we did that, providers weren’t looking at the possibility of having that easy check box right there to fill in … and we know that when people have to go through more steps, they invariably don’t do it,” Dr. Eskander said.
He and his colleagues saw an almost immediate . Once the formula order was removed, breastfeeding rates rose from 40.57% to 53.90% (P less than .001). Rates have been sustained since the removal of the EHR option for formula.
There was no difference in how infants fared after the intervention, said Dr. Eskander. “The outcomes for those infants was identical. There were no increased NICU admissions, there were no increased poor outcomes.”
Length of stay remained the same as well. “The babies were being discharged in the same state of health, just more of them were getting breast milk only, and we know the benefits that tends to portend,” he added.
There was some initial grumbling when the formula order was pulled from the newborn order set, he conceded. “The providers were not very happy about having to look for the newborn order for formula.” However, it took just about a month for the new workflow to seem normal, he said.
Dr. Eskander envisions a future where the EHR is “smart” enough to prompt appropriate orders and interventions for serious conditions such as preeclampsia. The electronic record, he said, could recognize the maternal diagnosis “and immediately create a system and structure around that mother to be able to help protect her and her baby. ... then having those diagnoses be able to drive outcomes can be very significant.”
Dr. Eskander reported no relevant financial disclosures.
SOURCE: Eskander, R et al. ACOG 2018, Abstract 31I.
REPORTING FROM ACOG 2018