User login
Study evaluates Zika syndrome with joint contractures
Congenital Zika syndrome should be added to the differential diagnosis of congenital infections and arthrogryposis (joint contractures), results from a case series study in Brazil suggest.
“Brain impairment in the presence of microcephaly is the main characteristic of a congenital Zika virus syndrome,” researchers led by Vanessa van der Linden, MD, wrote in a study published online Aug. 9 in the BMJ. “However, little is still known about this condition and its clinical spectrum, which also concerns newborns with a normal head circumference. Two studies have described the association between arthrogryposis and microcephaly in newborns presumed to have congenital Zika virus infection [See Morb Mortal Wkly. Rep. 2016;65:59-62 and Ultrasound Obstet Gynecol. 2016;47:6-7].” The authors went on to note that while arthrogryposis might be considered more of a sign than a specific disease, “it might be associated with several disorders. However, there are no reports in the literature about other congenital infections in humans associated with arthrogryposis.”
Dr. van der Linden, a pediatric neurologist with the Association for Assistance of Disabled Children, Recife, Brazil, and her associates retrospectively evaluated the medical records of seven patients with arthrogryposis associated with congenital infection believed to be caused by Zika virus, during the Brazilian microcephaly epidemic (BMJ. 2016;354:i3899). The main outcomes of interest were clinical, radiologic, and electromyographic findings, and likely collaboration between clinical and primary neurological abnormalities.
The researchers reported that brain images of all seven children revealed characteristics of congenital infection and arthrogryposis. Two children (29%) tested positive for IgM antibody for Zika virus in the cerebrospinal fluid, while arthrogryposis was present in the arms and legs of six children (86%) and in the legs of one child (14%). In addition, hip x-rays showed bilateral dislocation in all seven children and subluxation of the knee associated with genu valgus in three (43%). No evidence of abnormalities was seen on high-definition ultrasonography of the joints, but moderate signs of remodeling of the motor units and a reduced recruitment pattern were found on needle electromyography. Results from brain computed tomography conducted in all seven patients and magnetic resonance imaging conducted in five revealed malformations of cortical development, calcifications predominantly in the cortex and subcortical white matter, reduction in brain volume, ventriculomegaly, and hypoplasia of the brainstem and cerebellum. Spinal MRI conducted in four children showed apparent thinning of the cord and reduced ventral roots.
“Further research is needed with a larger number of cases to study the neurological abnormalities behind arthrogryposis, including histopathology of autopsy samples or tissues from stillborn babies,” the researchers concluded. “As we do not know the potential implications of congenital Zika virus infection as it evolves, children must receive orthopedic follow-up, even those with a standard first orthopedic evaluation, because they could develop musculoskeletal deformities secondary to neurological impairment, central or peripheral, or both, as these occur in patients with cerebral palsy and other chronic encephalopathies.”
The researchers reported having no financial disclosures.
Congenital Zika syndrome should be added to the differential diagnosis of congenital infections and arthrogryposis (joint contractures), results from a case series study in Brazil suggest.
“Brain impairment in the presence of microcephaly is the main characteristic of a congenital Zika virus syndrome,” researchers led by Vanessa van der Linden, MD, wrote in a study published online Aug. 9 in the BMJ. “However, little is still known about this condition and its clinical spectrum, which also concerns newborns with a normal head circumference. Two studies have described the association between arthrogryposis and microcephaly in newborns presumed to have congenital Zika virus infection [See Morb Mortal Wkly. Rep. 2016;65:59-62 and Ultrasound Obstet Gynecol. 2016;47:6-7].” The authors went on to note that while arthrogryposis might be considered more of a sign than a specific disease, “it might be associated with several disorders. However, there are no reports in the literature about other congenital infections in humans associated with arthrogryposis.”
Dr. van der Linden, a pediatric neurologist with the Association for Assistance of Disabled Children, Recife, Brazil, and her associates retrospectively evaluated the medical records of seven patients with arthrogryposis associated with congenital infection believed to be caused by Zika virus, during the Brazilian microcephaly epidemic (BMJ. 2016;354:i3899). The main outcomes of interest were clinical, radiologic, and electromyographic findings, and likely collaboration between clinical and primary neurological abnormalities.
The researchers reported that brain images of all seven children revealed characteristics of congenital infection and arthrogryposis. Two children (29%) tested positive for IgM antibody for Zika virus in the cerebrospinal fluid, while arthrogryposis was present in the arms and legs of six children (86%) and in the legs of one child (14%). In addition, hip x-rays showed bilateral dislocation in all seven children and subluxation of the knee associated with genu valgus in three (43%). No evidence of abnormalities was seen on high-definition ultrasonography of the joints, but moderate signs of remodeling of the motor units and a reduced recruitment pattern were found on needle electromyography. Results from brain computed tomography conducted in all seven patients and magnetic resonance imaging conducted in five revealed malformations of cortical development, calcifications predominantly in the cortex and subcortical white matter, reduction in brain volume, ventriculomegaly, and hypoplasia of the brainstem and cerebellum. Spinal MRI conducted in four children showed apparent thinning of the cord and reduced ventral roots.
“Further research is needed with a larger number of cases to study the neurological abnormalities behind arthrogryposis, including histopathology of autopsy samples or tissues from stillborn babies,” the researchers concluded. “As we do not know the potential implications of congenital Zika virus infection as it evolves, children must receive orthopedic follow-up, even those with a standard first orthopedic evaluation, because they could develop musculoskeletal deformities secondary to neurological impairment, central or peripheral, or both, as these occur in patients with cerebral palsy and other chronic encephalopathies.”
The researchers reported having no financial disclosures.
Congenital Zika syndrome should be added to the differential diagnosis of congenital infections and arthrogryposis (joint contractures), results from a case series study in Brazil suggest.
“Brain impairment in the presence of microcephaly is the main characteristic of a congenital Zika virus syndrome,” researchers led by Vanessa van der Linden, MD, wrote in a study published online Aug. 9 in the BMJ. “However, little is still known about this condition and its clinical spectrum, which also concerns newborns with a normal head circumference. Two studies have described the association between arthrogryposis and microcephaly in newborns presumed to have congenital Zika virus infection [See Morb Mortal Wkly. Rep. 2016;65:59-62 and Ultrasound Obstet Gynecol. 2016;47:6-7].” The authors went on to note that while arthrogryposis might be considered more of a sign than a specific disease, “it might be associated with several disorders. However, there are no reports in the literature about other congenital infections in humans associated with arthrogryposis.”
Dr. van der Linden, a pediatric neurologist with the Association for Assistance of Disabled Children, Recife, Brazil, and her associates retrospectively evaluated the medical records of seven patients with arthrogryposis associated with congenital infection believed to be caused by Zika virus, during the Brazilian microcephaly epidemic (BMJ. 2016;354:i3899). The main outcomes of interest were clinical, radiologic, and electromyographic findings, and likely collaboration between clinical and primary neurological abnormalities.
The researchers reported that brain images of all seven children revealed characteristics of congenital infection and arthrogryposis. Two children (29%) tested positive for IgM antibody for Zika virus in the cerebrospinal fluid, while arthrogryposis was present in the arms and legs of six children (86%) and in the legs of one child (14%). In addition, hip x-rays showed bilateral dislocation in all seven children and subluxation of the knee associated with genu valgus in three (43%). No evidence of abnormalities was seen on high-definition ultrasonography of the joints, but moderate signs of remodeling of the motor units and a reduced recruitment pattern were found on needle electromyography. Results from brain computed tomography conducted in all seven patients and magnetic resonance imaging conducted in five revealed malformations of cortical development, calcifications predominantly in the cortex and subcortical white matter, reduction in brain volume, ventriculomegaly, and hypoplasia of the brainstem and cerebellum. Spinal MRI conducted in four children showed apparent thinning of the cord and reduced ventral roots.
“Further research is needed with a larger number of cases to study the neurological abnormalities behind arthrogryposis, including histopathology of autopsy samples or tissues from stillborn babies,” the researchers concluded. “As we do not know the potential implications of congenital Zika virus infection as it evolves, children must receive orthopedic follow-up, even those with a standard first orthopedic evaluation, because they could develop musculoskeletal deformities secondary to neurological impairment, central or peripheral, or both, as these occur in patients with cerebral palsy and other chronic encephalopathies.”
The researchers reported having no financial disclosures.
FROM BMJ
Key clinical point: The differential diagnosis of congenital infections and arthrogryposis should include congenital Zika syndrome.
Major finding: All seven children revealed characteristics of congenital infection and arthrogryposis. Two children (29%) tested positive for IgM antibody for Zika virus in the cerebrospinal fluid, while arthrogryposis was present in the arms and legs of six children (86%) and in the legs of one child (14%).
Data source: A retrospective case series study of seven children with arthrogryposis associated with congenital infection believed to be caused by Zika virus.
Disclosures: The researchers reported having no financial disclosures.
Surgical Technique for Morcellating Hard Fibroids Hysteroscopically
This video is sponsored by Hologic, Inc.
Michael D. Randell, MD, FACOG
Division of Gynecology
Department of Surgery
Emory Saint Joseph’s Hospital
Atlanta, Georgia
This video is sponsored by Hologic, Inc.
Michael D. Randell, MD, FACOG
Division of Gynecology
Department of Surgery
Emory Saint Joseph’s Hospital
Atlanta, Georgia
This video is sponsored by Hologic, Inc.
Michael D. Randell, MD, FACOG
Division of Gynecology
Department of Surgery
Emory Saint Joseph’s Hospital
Atlanta, Georgia
Obstetrics Moonshots: 50 years of discoveries
In 1961 before Congress, and in 1962 at Rice University, Houston, President John F. Kennedy called on America to land a man on the moon and bring him back safely, and to look beyond the moon as well, and pursue an ambitious space exploration program. He challenged the country to think and act boldly, telling Americans in his speech at Rice that “we choose to go the moon in this decade and do the other things, not because they are easy, but because they are hard.”
When Neil Armstrong and Buzz Aldrin set foot on the moon in 1969 – even before President Kennedy’s 10-year deadline had arrived – the country’s primary moonshot was realized. The President had inspired the nation, teams of engineers and others had collectively met daunting technological challenges, and space consequently was more open to us than ever before.
In looking at the field of obstetrics and how far it has come in the past 50 years, since the 1960s, it is similarly astonishing and inspiring to reflect on what extraordinary advances we have made. Who would have thought that the fetus would become such a visible and intimate patient – one who, like the mother, can be interrogated, monitored, and sometimes treated before birth? Who would have thought we would be utilizing genomic studies in a now well-established field of prenatal diagnosis, or that fetal therapy would become a field in and of itself?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Our specialty has advanced through a series of moonshots that have been inspired and driven by technological advancement and by our continually bold goals and vision for the health and well-being of women and their offspring. We have taken on ambitious challenges, achieved many goals, and embraced advancements in practice only to then set new targets that previously were unimaginable.
Yet just as our country’s space exploration program has faced disappointments, so has our field. It is sobering, for instance, that we have made only incremental improvements in prematurity and infant mortality, and that the age-old maternal problem of preeclampsia is still with us. We also face new challenges, such as the rising rate of maternal obesity and diabetes, which threaten both maternal and fetal health.
President Kennedy spoke of having “examined where we are strong, and where we are not.” Such self-reflection and assessment is a critical underpinning of advancement in fields across all of science, medicine, and health care, and in our specialty, it is a process that has driven ambitious new research efforts to improve fetal and maternal health.
A step back to more in-depth fundamental research on the biomolecular mechanisms of premature labor and diabetes-associated birth defects, for instance, as well as new efforts to approach fetal surgery less invasively, are positioning us to both conquer our disappointments and achieve ambitious new moonshots.
The fetus as our patient
Fifty years ago, in 1966, a seminal paper in the Lancet reported that amniotic fluid cells could be cultured and were suitable for karyotyping (1[7434]:383-5). The tapping and examination of amniotic fluid had been reported on sporadically for many decades, for various clinical purposes, but by and large the fetal compartment was not invaded or directly examined. The fetus was instead the hopeful beneficiary of pregnancy care that focused on the mother. Fetal outcome was clouded in mystery, known only at birth.
With the Lancet report, prenatal detection of chromosomal disorders began to feel achievable, and the 1960s marked the beginning of a journey first through invasive methods of prenatal diagnosis and then through increasingly non-invasive approaches.
In 1970, just several years after the report on chromosome analysis of amniotic-fluid cells, another landmark paper in the New England Journal of Medicine described 162 amniocenteses performed between the 13th and 18th weeks of gestation and the detection of 10 cases of Down syndrome, as well as a few other cases of metabolic and other disorders (282[11]:596-9). This report provided an impetus for broader use of the procedure to detect neural tube defects, Down syndrome, and other abnormalities.
The adoption of amniocentesis for prenatal diagnosis still took some time, however. The procedure was used primarily early on to determine fetal lung maturity, and to predict the ability of the fetus to survive after delivery.
At the time, it was widely praised as an advanced method for evaluating the fetus. Yet, looking back, the early years of the procedure seem primitive. The procedure was done late in pregnancy and it was performed blindly, with the puncture site located either with external palpation of the uterus or with the assistance of static ultrasound. Patients who had scans would usually visit the radiologist, who would mark on the patient’s abdomen a suggested location for needle insertion. Upon the patient’s return, the obstetrician would then insert a needle into that spot, blindly and likely after the fetus had moved.
The development and adoption of real-time ultrasound was a revolutionary achievement. Ultrasound-guided amniocentesis was first described in 1972, 14 years after Ian Donald’s seminal paper introducing obstetric ultrasound was published in the Lancet (1958 Jun 7;1[7032]:1188-95).
As real-time ultrasound made its way into practice, it marked the true realization of a moonshot for obstetrics.
Not only could we simultaneously visualize the needle tip and place the needle safety, but we could see the real-time movement of the fetus, its activity, and the surrounding pockets of fluid. It was like looking up into the sky and seeing the stars for the first time. We could see fetal arrhythmia – not only hear it. With this window into the fetal compartment, we could visualize the fetal bowel migrating into the chest cavity due to a hole (hernia) in the diaphragm. We could visualize other malformations as well.
Chorionic villus sampling (CVS) was technically more difficult and took longer to evolve. For years, through the early 1980s, it was performed only at select centers throughout the country. Patients traveled for the procedure and faced relatively significant risks of complications.
By the end of the 1980s, however, with successive improvements in equipment and technique (including development of a transabdominal approach in addition to transvaginal) the procedure was deemed safe, effective, and acceptable for routine use. Fetoscopy, pioneered by John Hobbins, MD, and his colleagues at Yale University, New Haven, Conn., had also advanced and was being used to diagnose sickle cell anemia, Tay-Sachs disease, congenital fetal skin diseases, and other disorders.
With these advances and with our newfound ability to obtain and analyze a tissue sample earlier in pregnancy – even before a woman shared the news of her pregnancy, in some cases – it seemed that we had achieved our goals and may have even reached past the moon.
Yet there were other moonshots being pursued, including initiatives to make prenatal diagnosis less invasive. The discovery in 1997 of cell-free fetal DNA in maternal plasma and serum, for instance, was a pivotal development that opened the door for noninvasive prenatal testing.
This, and other advances in areas from biochemistry to ultrasound to genomic analysis, led to an array of prenatal diagnostic tools that today enable women and their physicians to assess the genetic, chromosomal, and biophysical aspects of their fetus considerably before the time of viability, and from both the maternal side and directly in the fetal compartment.
First-trimester screening is a current option, and we now have the ability to more selectively perform amniocentesis and CVS based on probability testing, and not solely on maternal age. Ultrasound technology now encompasses color Doppler, 3D and 4D imaging, and other techniques that can be used to assess the placenta, various structures inside the brain, and the heart, as well as blood flow through the ductus venosus.
Parents have called for and welcomed having the option of assessing the fetus in greater detail, and of having either assurance when anomalies are excluded or the opportunity to plan and make decisions when anomalies are detected.
Fetal surgery has been a natural extension of our unprecedented access to the fetus. Our ability to visualize malformations and their evolution led to animal studies that advanced our interest in arresting, correcting, or reversing fetal anomalies through in-utero interventions. In 1981, surgeons performed the first human open fetal surgery to correct congenital hydronephrosis.
Today, we can employ endoscopic laser ablation or laser coagulation to treat severe twin-to-twin syndrome, for instance, as well as other surgical techniques to repair defects such as congenital diaphragmatic hernia, lower urinary tract obstruction, and myelomeningocele. Such advances were unimaginable decades ago.
Old foes and new threats
Despite these advances in diagnosis and care, obstetrics faces unrealized moonshots – lingering challenges that, 50 years ago, we would have predicted would have been solved. Who would have thought that we would still have as high an infant mortality rate as we do, and that we would not be further along in solving the problem of prematurity? Our progress has been only incremental.
Fifty years ago, we lacked an understanding of the basic biology of preterm labor. Prematurity was viewed simply as term labor occurring too early, and many efforts were made over the years to halt the premature labor process through the use of various drugs and other therapeutics, with variable and minimally impactful levels of success.
In the last 25 years, and especially in the last decade, we have made greater efforts to better understand the biology of premature labor – to elucidate how and why it occurs – and we have come to understand that premature labor is very different physiologically from term labor.
Thanks to the work at the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), led by Roberto Romero, MD, attention has consequently shifted toward prediction, identification of women at highest risk, and prevention of the onset of premature labor among those deemed to be at highest risk.
Cervical length in the mid-trimester is now a well-verified predictor of preterm birth, and vaginal progesterone has been shown to benefit women without other known risk factors who are diagnosed with a shortened cervical length.
We have consequently seen the preterm birth rate decline a bit. In 2013, the last year for which we have complete data, the preterm birth rate dropped to 11.4%, down from a high of 12.8% in 2006, according to the Centers for Disease Control and Prevention.
Infant mortality similarly remains unacceptably high, due largely to the high preterm birth rate and to our failure to significantly alter the prevalence of birth defects. In 2010, according to the CDC, the infant mortality rate in the U.S. was 6.1 deaths per 1,000 live births (compared with 6.87 in 2005), and the United States ranked 26th in infant mortality among countries belonging to the Organisation for Economic Co-operation and Development, despite the fact that we spend a significant portion of our gross domestic product (17.5% in 2014) on health care.
Birth defects have taken over as a leading cause of infant mortality after early newborn life, and while we’ve made some advancements in understanding and diagnosing them, the majority of causes of birth defects are still unknown.
On the maternal side of obstetrical care, our progress has similarly been more modest than we have hoped for. Preeclampsia remains a problem, for instance. Despite decades of research into its pathogenesis, our advancements have been only incremental, and the condition – particularly its severe form – continues to be a vexing and high-risk problem.
Added to such age-old foes, moreover, are the growing threats of maternal obesity and diabetes, two closely related and often chronic conditions that affect not only the health of the mother but the in-utero environment and the health of the fetus. Today, more than one-third of all adults in the U.S., and 34% of women aged 20-39 years, are obese, and almost 10% of the U.S. population has diabetes.
Both conditions are on the rise, and obstetrics is confronting an epidemic of “diabesity” that would not necessarily have been predicted 50 years ago. It is particularly alarming given our growing knowledge of how obesity can be programmed in-utero and essentially passed on from generation to generation, of how diabetes can negatively affect perinatal outcomes, and of how the two conditions can have an additive effect on fetal complications.
Achieving new moonshots
Concerted efforts in the past several decades to step back and try to understand the basic biology and physiology of term labor and of premature labor have better positioned our specialty to achieve the moonshot of significantly reducing the incidence of preterm birth.
Establishment in the mid-1980s of the NICHD’s Perinatology Research Branch was a major development in this regard, helping to build and direct research efforts, including basic laboratory science, toward questions about what triggers and propagates labor. There has been notable progress in the past decade, in particular, and our specialty is now on the right path toward development of therapeutic interventions for preventing prematurity.
Additionally, the NICHD’s recently launched Human Placenta Project is building upon the branch-sponsored animal and cell culture model systems of the placenta to allow researchers, for the first time, to monitor human placental health in real time. By more fully understanding the role of the placenta in health and disease, we will be able to better evaluate pregnancy risks and improve pregnancy outcomes.
We also are learning through research in the University of Maryland Birth Defects Research Laboratory, which I am privileged to direct, and at other facilities, that maternal hyperglycemia is a teratogen, creating insults that can trigger a series of developmental fetal defects. By studying the biomolecular mechanisms of hyperglycemia-induced birth defects and developing “molecular maps,” we expect to be able to develop strategies for preventing or mitigating the development of such anomalies. I hope and expect that these future advancements, combined with reductions in prematurity, will significantly impact the infant mortality rate.
Fetal therapy and surgery will also continue to advance, with a much more minimally invasive approach taken in the next 50 years to addressing the fetal condition without putting the mother at increased risk. Just as surgery in other fields has moved from open laparotomy to minimally invasive techniques, I believe we will develop endoscopic or laparoscopic means of correcting the various problems in-utero, such as the repair of neural tube defects and diaphragmatic hernias. It already appears likely that a fetoscopic approach to treating myelomeningocele can reduce maternal morbidity while achieving infant neurological outcomes that are at least as good as outcomes achieved with open fetal surgery.
We’re in a much different position than we were 50 years ago in that we have two patients – the mother and the fetus – with whom we can closely work. We also have a relatively new and urgent obligation to place our attention not only on women’s reproductive health, but on the general gynecologic state. Ob.gyns. often are the only primary care physicians whom women see for routine care, and the quality of our attention to their weight and their diabetes risk factors will have far-reaching consequences, both for them and for their offspring.
As we have since the 1960s, we will continue to set new moonshots and meet new challenges, working with each other and with our patients to evaluate where we are strong and where we must improve. We will persistently harness the power of technology, choosing to do the things that “are hard,” while stepping back as needed to ask and address fundamental questions.
As a result, I can envision the next 50 years as a revolutionary time period for obstetrics – a time in which current problems and disorders are abated or eliminated through a combination of genomics, microbiomics, and other technological advances. Someday in the future, we will look back on some of our many achievements and marvel at how we have transformed the unimaginable to reality.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Select advances through the years
1960s
1965: Siemens Corp. introduces first real-time ultrasound scanner.
1966: Lancet paper reports that amniotic fluid cells can be cultured and karyotyped.
1970s
1970: New England Journal of Medicine paper describes mid-trimester amniocenteses and detection of Down syndrome cases.
1972: Ultrasound-guided amniocentesis first described.
1973: Fetoscopy introduced.
1980s
1981: First human open fetal surgery to correct congenital hydronephrosis.
Early 1980s: Chorionic villus sampling introduced at select centers.
1985: Color Doppler incorporated into ultrasound.
1990s
1990: Embryoscopy first described.
Mid-1990s: 3D/4D ultrasound begins to assume major role in ob.gyn. imaging.1997: Discovery of cell-free fetal DNA in maternal plasma.
2000s
2003: MOMS (Management of Myelomeningocele Study) was launched.
2010s
2012: The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine support cell-free DNA screening for women at increased risk of fetal aneuploidy.
2013: Preterm birth rate drops to 11.4%
2014: Diabetes incidence marks a 4-fold increase since 1980.
In 1961 before Congress, and in 1962 at Rice University, Houston, President John F. Kennedy called on America to land a man on the moon and bring him back safely, and to look beyond the moon as well, and pursue an ambitious space exploration program. He challenged the country to think and act boldly, telling Americans in his speech at Rice that “we choose to go the moon in this decade and do the other things, not because they are easy, but because they are hard.”
When Neil Armstrong and Buzz Aldrin set foot on the moon in 1969 – even before President Kennedy’s 10-year deadline had arrived – the country’s primary moonshot was realized. The President had inspired the nation, teams of engineers and others had collectively met daunting technological challenges, and space consequently was more open to us than ever before.
In looking at the field of obstetrics and how far it has come in the past 50 years, since the 1960s, it is similarly astonishing and inspiring to reflect on what extraordinary advances we have made. Who would have thought that the fetus would become such a visible and intimate patient – one who, like the mother, can be interrogated, monitored, and sometimes treated before birth? Who would have thought we would be utilizing genomic studies in a now well-established field of prenatal diagnosis, or that fetal therapy would become a field in and of itself?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Our specialty has advanced through a series of moonshots that have been inspired and driven by technological advancement and by our continually bold goals and vision for the health and well-being of women and their offspring. We have taken on ambitious challenges, achieved many goals, and embraced advancements in practice only to then set new targets that previously were unimaginable.
Yet just as our country’s space exploration program has faced disappointments, so has our field. It is sobering, for instance, that we have made only incremental improvements in prematurity and infant mortality, and that the age-old maternal problem of preeclampsia is still with us. We also face new challenges, such as the rising rate of maternal obesity and diabetes, which threaten both maternal and fetal health.
President Kennedy spoke of having “examined where we are strong, and where we are not.” Such self-reflection and assessment is a critical underpinning of advancement in fields across all of science, medicine, and health care, and in our specialty, it is a process that has driven ambitious new research efforts to improve fetal and maternal health.
A step back to more in-depth fundamental research on the biomolecular mechanisms of premature labor and diabetes-associated birth defects, for instance, as well as new efforts to approach fetal surgery less invasively, are positioning us to both conquer our disappointments and achieve ambitious new moonshots.
The fetus as our patient
Fifty years ago, in 1966, a seminal paper in the Lancet reported that amniotic fluid cells could be cultured and were suitable for karyotyping (1[7434]:383-5). The tapping and examination of amniotic fluid had been reported on sporadically for many decades, for various clinical purposes, but by and large the fetal compartment was not invaded or directly examined. The fetus was instead the hopeful beneficiary of pregnancy care that focused on the mother. Fetal outcome was clouded in mystery, known only at birth.
With the Lancet report, prenatal detection of chromosomal disorders began to feel achievable, and the 1960s marked the beginning of a journey first through invasive methods of prenatal diagnosis and then through increasingly non-invasive approaches.
In 1970, just several years after the report on chromosome analysis of amniotic-fluid cells, another landmark paper in the New England Journal of Medicine described 162 amniocenteses performed between the 13th and 18th weeks of gestation and the detection of 10 cases of Down syndrome, as well as a few other cases of metabolic and other disorders (282[11]:596-9). This report provided an impetus for broader use of the procedure to detect neural tube defects, Down syndrome, and other abnormalities.
The adoption of amniocentesis for prenatal diagnosis still took some time, however. The procedure was used primarily early on to determine fetal lung maturity, and to predict the ability of the fetus to survive after delivery.
At the time, it was widely praised as an advanced method for evaluating the fetus. Yet, looking back, the early years of the procedure seem primitive. The procedure was done late in pregnancy and it was performed blindly, with the puncture site located either with external palpation of the uterus or with the assistance of static ultrasound. Patients who had scans would usually visit the radiologist, who would mark on the patient’s abdomen a suggested location for needle insertion. Upon the patient’s return, the obstetrician would then insert a needle into that spot, blindly and likely after the fetus had moved.
The development and adoption of real-time ultrasound was a revolutionary achievement. Ultrasound-guided amniocentesis was first described in 1972, 14 years after Ian Donald’s seminal paper introducing obstetric ultrasound was published in the Lancet (1958 Jun 7;1[7032]:1188-95).
As real-time ultrasound made its way into practice, it marked the true realization of a moonshot for obstetrics.
Not only could we simultaneously visualize the needle tip and place the needle safety, but we could see the real-time movement of the fetus, its activity, and the surrounding pockets of fluid. It was like looking up into the sky and seeing the stars for the first time. We could see fetal arrhythmia – not only hear it. With this window into the fetal compartment, we could visualize the fetal bowel migrating into the chest cavity due to a hole (hernia) in the diaphragm. We could visualize other malformations as well.
Chorionic villus sampling (CVS) was technically more difficult and took longer to evolve. For years, through the early 1980s, it was performed only at select centers throughout the country. Patients traveled for the procedure and faced relatively significant risks of complications.
By the end of the 1980s, however, with successive improvements in equipment and technique (including development of a transabdominal approach in addition to transvaginal) the procedure was deemed safe, effective, and acceptable for routine use. Fetoscopy, pioneered by John Hobbins, MD, and his colleagues at Yale University, New Haven, Conn., had also advanced and was being used to diagnose sickle cell anemia, Tay-Sachs disease, congenital fetal skin diseases, and other disorders.
With these advances and with our newfound ability to obtain and analyze a tissue sample earlier in pregnancy – even before a woman shared the news of her pregnancy, in some cases – it seemed that we had achieved our goals and may have even reached past the moon.
Yet there were other moonshots being pursued, including initiatives to make prenatal diagnosis less invasive. The discovery in 1997 of cell-free fetal DNA in maternal plasma and serum, for instance, was a pivotal development that opened the door for noninvasive prenatal testing.
This, and other advances in areas from biochemistry to ultrasound to genomic analysis, led to an array of prenatal diagnostic tools that today enable women and their physicians to assess the genetic, chromosomal, and biophysical aspects of their fetus considerably before the time of viability, and from both the maternal side and directly in the fetal compartment.
First-trimester screening is a current option, and we now have the ability to more selectively perform amniocentesis and CVS based on probability testing, and not solely on maternal age. Ultrasound technology now encompasses color Doppler, 3D and 4D imaging, and other techniques that can be used to assess the placenta, various structures inside the brain, and the heart, as well as blood flow through the ductus venosus.
Parents have called for and welcomed having the option of assessing the fetus in greater detail, and of having either assurance when anomalies are excluded or the opportunity to plan and make decisions when anomalies are detected.
Fetal surgery has been a natural extension of our unprecedented access to the fetus. Our ability to visualize malformations and their evolution led to animal studies that advanced our interest in arresting, correcting, or reversing fetal anomalies through in-utero interventions. In 1981, surgeons performed the first human open fetal surgery to correct congenital hydronephrosis.
Today, we can employ endoscopic laser ablation or laser coagulation to treat severe twin-to-twin syndrome, for instance, as well as other surgical techniques to repair defects such as congenital diaphragmatic hernia, lower urinary tract obstruction, and myelomeningocele. Such advances were unimaginable decades ago.
Old foes and new threats
Despite these advances in diagnosis and care, obstetrics faces unrealized moonshots – lingering challenges that, 50 years ago, we would have predicted would have been solved. Who would have thought that we would still have as high an infant mortality rate as we do, and that we would not be further along in solving the problem of prematurity? Our progress has been only incremental.
Fifty years ago, we lacked an understanding of the basic biology of preterm labor. Prematurity was viewed simply as term labor occurring too early, and many efforts were made over the years to halt the premature labor process through the use of various drugs and other therapeutics, with variable and minimally impactful levels of success.
In the last 25 years, and especially in the last decade, we have made greater efforts to better understand the biology of premature labor – to elucidate how and why it occurs – and we have come to understand that premature labor is very different physiologically from term labor.
Thanks to the work at the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), led by Roberto Romero, MD, attention has consequently shifted toward prediction, identification of women at highest risk, and prevention of the onset of premature labor among those deemed to be at highest risk.
Cervical length in the mid-trimester is now a well-verified predictor of preterm birth, and vaginal progesterone has been shown to benefit women without other known risk factors who are diagnosed with a shortened cervical length.
We have consequently seen the preterm birth rate decline a bit. In 2013, the last year for which we have complete data, the preterm birth rate dropped to 11.4%, down from a high of 12.8% in 2006, according to the Centers for Disease Control and Prevention.
Infant mortality similarly remains unacceptably high, due largely to the high preterm birth rate and to our failure to significantly alter the prevalence of birth defects. In 2010, according to the CDC, the infant mortality rate in the U.S. was 6.1 deaths per 1,000 live births (compared with 6.87 in 2005), and the United States ranked 26th in infant mortality among countries belonging to the Organisation for Economic Co-operation and Development, despite the fact that we spend a significant portion of our gross domestic product (17.5% in 2014) on health care.
Birth defects have taken over as a leading cause of infant mortality after early newborn life, and while we’ve made some advancements in understanding and diagnosing them, the majority of causes of birth defects are still unknown.
On the maternal side of obstetrical care, our progress has similarly been more modest than we have hoped for. Preeclampsia remains a problem, for instance. Despite decades of research into its pathogenesis, our advancements have been only incremental, and the condition – particularly its severe form – continues to be a vexing and high-risk problem.
Added to such age-old foes, moreover, are the growing threats of maternal obesity and diabetes, two closely related and often chronic conditions that affect not only the health of the mother but the in-utero environment and the health of the fetus. Today, more than one-third of all adults in the U.S., and 34% of women aged 20-39 years, are obese, and almost 10% of the U.S. population has diabetes.
Both conditions are on the rise, and obstetrics is confronting an epidemic of “diabesity” that would not necessarily have been predicted 50 years ago. It is particularly alarming given our growing knowledge of how obesity can be programmed in-utero and essentially passed on from generation to generation, of how diabetes can negatively affect perinatal outcomes, and of how the two conditions can have an additive effect on fetal complications.
Achieving new moonshots
Concerted efforts in the past several decades to step back and try to understand the basic biology and physiology of term labor and of premature labor have better positioned our specialty to achieve the moonshot of significantly reducing the incidence of preterm birth.
Establishment in the mid-1980s of the NICHD’s Perinatology Research Branch was a major development in this regard, helping to build and direct research efforts, including basic laboratory science, toward questions about what triggers and propagates labor. There has been notable progress in the past decade, in particular, and our specialty is now on the right path toward development of therapeutic interventions for preventing prematurity.
Additionally, the NICHD’s recently launched Human Placenta Project is building upon the branch-sponsored animal and cell culture model systems of the placenta to allow researchers, for the first time, to monitor human placental health in real time. By more fully understanding the role of the placenta in health and disease, we will be able to better evaluate pregnancy risks and improve pregnancy outcomes.
We also are learning through research in the University of Maryland Birth Defects Research Laboratory, which I am privileged to direct, and at other facilities, that maternal hyperglycemia is a teratogen, creating insults that can trigger a series of developmental fetal defects. By studying the biomolecular mechanisms of hyperglycemia-induced birth defects and developing “molecular maps,” we expect to be able to develop strategies for preventing or mitigating the development of such anomalies. I hope and expect that these future advancements, combined with reductions in prematurity, will significantly impact the infant mortality rate.
Fetal therapy and surgery will also continue to advance, with a much more minimally invasive approach taken in the next 50 years to addressing the fetal condition without putting the mother at increased risk. Just as surgery in other fields has moved from open laparotomy to minimally invasive techniques, I believe we will develop endoscopic or laparoscopic means of correcting the various problems in-utero, such as the repair of neural tube defects and diaphragmatic hernias. It already appears likely that a fetoscopic approach to treating myelomeningocele can reduce maternal morbidity while achieving infant neurological outcomes that are at least as good as outcomes achieved with open fetal surgery.
We’re in a much different position than we were 50 years ago in that we have two patients – the mother and the fetus – with whom we can closely work. We also have a relatively new and urgent obligation to place our attention not only on women’s reproductive health, but on the general gynecologic state. Ob.gyns. often are the only primary care physicians whom women see for routine care, and the quality of our attention to their weight and their diabetes risk factors will have far-reaching consequences, both for them and for their offspring.
As we have since the 1960s, we will continue to set new moonshots and meet new challenges, working with each other and with our patients to evaluate where we are strong and where we must improve. We will persistently harness the power of technology, choosing to do the things that “are hard,” while stepping back as needed to ask and address fundamental questions.
As a result, I can envision the next 50 years as a revolutionary time period for obstetrics – a time in which current problems and disorders are abated or eliminated through a combination of genomics, microbiomics, and other technological advances. Someday in the future, we will look back on some of our many achievements and marvel at how we have transformed the unimaginable to reality.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Select advances through the years
1960s
1965: Siemens Corp. introduces first real-time ultrasound scanner.
1966: Lancet paper reports that amniotic fluid cells can be cultured and karyotyped.
1970s
1970: New England Journal of Medicine paper describes mid-trimester amniocenteses and detection of Down syndrome cases.
1972: Ultrasound-guided amniocentesis first described.
1973: Fetoscopy introduced.
1980s
1981: First human open fetal surgery to correct congenital hydronephrosis.
Early 1980s: Chorionic villus sampling introduced at select centers.
1985: Color Doppler incorporated into ultrasound.
1990s
1990: Embryoscopy first described.
Mid-1990s: 3D/4D ultrasound begins to assume major role in ob.gyn. imaging.1997: Discovery of cell-free fetal DNA in maternal plasma.
2000s
2003: MOMS (Management of Myelomeningocele Study) was launched.
2010s
2012: The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine support cell-free DNA screening for women at increased risk of fetal aneuploidy.
2013: Preterm birth rate drops to 11.4%
2014: Diabetes incidence marks a 4-fold increase since 1980.
In 1961 before Congress, and in 1962 at Rice University, Houston, President John F. Kennedy called on America to land a man on the moon and bring him back safely, and to look beyond the moon as well, and pursue an ambitious space exploration program. He challenged the country to think and act boldly, telling Americans in his speech at Rice that “we choose to go the moon in this decade and do the other things, not because they are easy, but because they are hard.”
When Neil Armstrong and Buzz Aldrin set foot on the moon in 1969 – even before President Kennedy’s 10-year deadline had arrived – the country’s primary moonshot was realized. The President had inspired the nation, teams of engineers and others had collectively met daunting technological challenges, and space consequently was more open to us than ever before.
In looking at the field of obstetrics and how far it has come in the past 50 years, since the 1960s, it is similarly astonishing and inspiring to reflect on what extraordinary advances we have made. Who would have thought that the fetus would become such a visible and intimate patient – one who, like the mother, can be interrogated, monitored, and sometimes treated before birth? Who would have thought we would be utilizing genomic studies in a now well-established field of prenatal diagnosis, or that fetal therapy would become a field in and of itself?
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Our specialty has advanced through a series of moonshots that have been inspired and driven by technological advancement and by our continually bold goals and vision for the health and well-being of women and their offspring. We have taken on ambitious challenges, achieved many goals, and embraced advancements in practice only to then set new targets that previously were unimaginable.
Yet just as our country’s space exploration program has faced disappointments, so has our field. It is sobering, for instance, that we have made only incremental improvements in prematurity and infant mortality, and that the age-old maternal problem of preeclampsia is still with us. We also face new challenges, such as the rising rate of maternal obesity and diabetes, which threaten both maternal and fetal health.
President Kennedy spoke of having “examined where we are strong, and where we are not.” Such self-reflection and assessment is a critical underpinning of advancement in fields across all of science, medicine, and health care, and in our specialty, it is a process that has driven ambitious new research efforts to improve fetal and maternal health.
A step back to more in-depth fundamental research on the biomolecular mechanisms of premature labor and diabetes-associated birth defects, for instance, as well as new efforts to approach fetal surgery less invasively, are positioning us to both conquer our disappointments and achieve ambitious new moonshots.
The fetus as our patient
Fifty years ago, in 1966, a seminal paper in the Lancet reported that amniotic fluid cells could be cultured and were suitable for karyotyping (1[7434]:383-5). The tapping and examination of amniotic fluid had been reported on sporadically for many decades, for various clinical purposes, but by and large the fetal compartment was not invaded or directly examined. The fetus was instead the hopeful beneficiary of pregnancy care that focused on the mother. Fetal outcome was clouded in mystery, known only at birth.
With the Lancet report, prenatal detection of chromosomal disorders began to feel achievable, and the 1960s marked the beginning of a journey first through invasive methods of prenatal diagnosis and then through increasingly non-invasive approaches.
In 1970, just several years after the report on chromosome analysis of amniotic-fluid cells, another landmark paper in the New England Journal of Medicine described 162 amniocenteses performed between the 13th and 18th weeks of gestation and the detection of 10 cases of Down syndrome, as well as a few other cases of metabolic and other disorders (282[11]:596-9). This report provided an impetus for broader use of the procedure to detect neural tube defects, Down syndrome, and other abnormalities.
The adoption of amniocentesis for prenatal diagnosis still took some time, however. The procedure was used primarily early on to determine fetal lung maturity, and to predict the ability of the fetus to survive after delivery.
At the time, it was widely praised as an advanced method for evaluating the fetus. Yet, looking back, the early years of the procedure seem primitive. The procedure was done late in pregnancy and it was performed blindly, with the puncture site located either with external palpation of the uterus or with the assistance of static ultrasound. Patients who had scans would usually visit the radiologist, who would mark on the patient’s abdomen a suggested location for needle insertion. Upon the patient’s return, the obstetrician would then insert a needle into that spot, blindly and likely after the fetus had moved.
The development and adoption of real-time ultrasound was a revolutionary achievement. Ultrasound-guided amniocentesis was first described in 1972, 14 years after Ian Donald’s seminal paper introducing obstetric ultrasound was published in the Lancet (1958 Jun 7;1[7032]:1188-95).
As real-time ultrasound made its way into practice, it marked the true realization of a moonshot for obstetrics.
Not only could we simultaneously visualize the needle tip and place the needle safety, but we could see the real-time movement of the fetus, its activity, and the surrounding pockets of fluid. It was like looking up into the sky and seeing the stars for the first time. We could see fetal arrhythmia – not only hear it. With this window into the fetal compartment, we could visualize the fetal bowel migrating into the chest cavity due to a hole (hernia) in the diaphragm. We could visualize other malformations as well.
Chorionic villus sampling (CVS) was technically more difficult and took longer to evolve. For years, through the early 1980s, it was performed only at select centers throughout the country. Patients traveled for the procedure and faced relatively significant risks of complications.
By the end of the 1980s, however, with successive improvements in equipment and technique (including development of a transabdominal approach in addition to transvaginal) the procedure was deemed safe, effective, and acceptable for routine use. Fetoscopy, pioneered by John Hobbins, MD, and his colleagues at Yale University, New Haven, Conn., had also advanced and was being used to diagnose sickle cell anemia, Tay-Sachs disease, congenital fetal skin diseases, and other disorders.
With these advances and with our newfound ability to obtain and analyze a tissue sample earlier in pregnancy – even before a woman shared the news of her pregnancy, in some cases – it seemed that we had achieved our goals and may have even reached past the moon.
Yet there were other moonshots being pursued, including initiatives to make prenatal diagnosis less invasive. The discovery in 1997 of cell-free fetal DNA in maternal plasma and serum, for instance, was a pivotal development that opened the door for noninvasive prenatal testing.
This, and other advances in areas from biochemistry to ultrasound to genomic analysis, led to an array of prenatal diagnostic tools that today enable women and their physicians to assess the genetic, chromosomal, and biophysical aspects of their fetus considerably before the time of viability, and from both the maternal side and directly in the fetal compartment.
First-trimester screening is a current option, and we now have the ability to more selectively perform amniocentesis and CVS based on probability testing, and not solely on maternal age. Ultrasound technology now encompasses color Doppler, 3D and 4D imaging, and other techniques that can be used to assess the placenta, various structures inside the brain, and the heart, as well as blood flow through the ductus venosus.
Parents have called for and welcomed having the option of assessing the fetus in greater detail, and of having either assurance when anomalies are excluded or the opportunity to plan and make decisions when anomalies are detected.
Fetal surgery has been a natural extension of our unprecedented access to the fetus. Our ability to visualize malformations and their evolution led to animal studies that advanced our interest in arresting, correcting, or reversing fetal anomalies through in-utero interventions. In 1981, surgeons performed the first human open fetal surgery to correct congenital hydronephrosis.
Today, we can employ endoscopic laser ablation or laser coagulation to treat severe twin-to-twin syndrome, for instance, as well as other surgical techniques to repair defects such as congenital diaphragmatic hernia, lower urinary tract obstruction, and myelomeningocele. Such advances were unimaginable decades ago.
Old foes and new threats
Despite these advances in diagnosis and care, obstetrics faces unrealized moonshots – lingering challenges that, 50 years ago, we would have predicted would have been solved. Who would have thought that we would still have as high an infant mortality rate as we do, and that we would not be further along in solving the problem of prematurity? Our progress has been only incremental.
Fifty years ago, we lacked an understanding of the basic biology of preterm labor. Prematurity was viewed simply as term labor occurring too early, and many efforts were made over the years to halt the premature labor process through the use of various drugs and other therapeutics, with variable and minimally impactful levels of success.
In the last 25 years, and especially in the last decade, we have made greater efforts to better understand the biology of premature labor – to elucidate how and why it occurs – and we have come to understand that premature labor is very different physiologically from term labor.
Thanks to the work at the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), led by Roberto Romero, MD, attention has consequently shifted toward prediction, identification of women at highest risk, and prevention of the onset of premature labor among those deemed to be at highest risk.
Cervical length in the mid-trimester is now a well-verified predictor of preterm birth, and vaginal progesterone has been shown to benefit women without other known risk factors who are diagnosed with a shortened cervical length.
We have consequently seen the preterm birth rate decline a bit. In 2013, the last year for which we have complete data, the preterm birth rate dropped to 11.4%, down from a high of 12.8% in 2006, according to the Centers for Disease Control and Prevention.
Infant mortality similarly remains unacceptably high, due largely to the high preterm birth rate and to our failure to significantly alter the prevalence of birth defects. In 2010, according to the CDC, the infant mortality rate in the U.S. was 6.1 deaths per 1,000 live births (compared with 6.87 in 2005), and the United States ranked 26th in infant mortality among countries belonging to the Organisation for Economic Co-operation and Development, despite the fact that we spend a significant portion of our gross domestic product (17.5% in 2014) on health care.
Birth defects have taken over as a leading cause of infant mortality after early newborn life, and while we’ve made some advancements in understanding and diagnosing them, the majority of causes of birth defects are still unknown.
On the maternal side of obstetrical care, our progress has similarly been more modest than we have hoped for. Preeclampsia remains a problem, for instance. Despite decades of research into its pathogenesis, our advancements have been only incremental, and the condition – particularly its severe form – continues to be a vexing and high-risk problem.
Added to such age-old foes, moreover, are the growing threats of maternal obesity and diabetes, two closely related and often chronic conditions that affect not only the health of the mother but the in-utero environment and the health of the fetus. Today, more than one-third of all adults in the U.S., and 34% of women aged 20-39 years, are obese, and almost 10% of the U.S. population has diabetes.
Both conditions are on the rise, and obstetrics is confronting an epidemic of “diabesity” that would not necessarily have been predicted 50 years ago. It is particularly alarming given our growing knowledge of how obesity can be programmed in-utero and essentially passed on from generation to generation, of how diabetes can negatively affect perinatal outcomes, and of how the two conditions can have an additive effect on fetal complications.
Achieving new moonshots
Concerted efforts in the past several decades to step back and try to understand the basic biology and physiology of term labor and of premature labor have better positioned our specialty to achieve the moonshot of significantly reducing the incidence of preterm birth.
Establishment in the mid-1980s of the NICHD’s Perinatology Research Branch was a major development in this regard, helping to build and direct research efforts, including basic laboratory science, toward questions about what triggers and propagates labor. There has been notable progress in the past decade, in particular, and our specialty is now on the right path toward development of therapeutic interventions for preventing prematurity.
Additionally, the NICHD’s recently launched Human Placenta Project is building upon the branch-sponsored animal and cell culture model systems of the placenta to allow researchers, for the first time, to monitor human placental health in real time. By more fully understanding the role of the placenta in health and disease, we will be able to better evaluate pregnancy risks and improve pregnancy outcomes.
We also are learning through research in the University of Maryland Birth Defects Research Laboratory, which I am privileged to direct, and at other facilities, that maternal hyperglycemia is a teratogen, creating insults that can trigger a series of developmental fetal defects. By studying the biomolecular mechanisms of hyperglycemia-induced birth defects and developing “molecular maps,” we expect to be able to develop strategies for preventing or mitigating the development of such anomalies. I hope and expect that these future advancements, combined with reductions in prematurity, will significantly impact the infant mortality rate.
Fetal therapy and surgery will also continue to advance, with a much more minimally invasive approach taken in the next 50 years to addressing the fetal condition without putting the mother at increased risk. Just as surgery in other fields has moved from open laparotomy to minimally invasive techniques, I believe we will develop endoscopic or laparoscopic means of correcting the various problems in-utero, such as the repair of neural tube defects and diaphragmatic hernias. It already appears likely that a fetoscopic approach to treating myelomeningocele can reduce maternal morbidity while achieving infant neurological outcomes that are at least as good as outcomes achieved with open fetal surgery.
We’re in a much different position than we were 50 years ago in that we have two patients – the mother and the fetus – with whom we can closely work. We also have a relatively new and urgent obligation to place our attention not only on women’s reproductive health, but on the general gynecologic state. Ob.gyns. often are the only primary care physicians whom women see for routine care, and the quality of our attention to their weight and their diabetes risk factors will have far-reaching consequences, both for them and for their offspring.
As we have since the 1960s, we will continue to set new moonshots and meet new challenges, working with each other and with our patients to evaluate where we are strong and where we must improve. We will persistently harness the power of technology, choosing to do the things that “are hard,” while stepping back as needed to ask and address fundamental questions.
As a result, I can envision the next 50 years as a revolutionary time period for obstetrics – a time in which current problems and disorders are abated or eliminated through a combination of genomics, microbiomics, and other technological advances. Someday in the future, we will look back on some of our many achievements and marvel at how we have transformed the unimaginable to reality.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of this column. Contact him at [email protected].
Select advances through the years
1960s
1965: Siemens Corp. introduces first real-time ultrasound scanner.
1966: Lancet paper reports that amniotic fluid cells can be cultured and karyotyped.
1970s
1970: New England Journal of Medicine paper describes mid-trimester amniocenteses and detection of Down syndrome cases.
1972: Ultrasound-guided amniocentesis first described.
1973: Fetoscopy introduced.
1980s
1981: First human open fetal surgery to correct congenital hydronephrosis.
Early 1980s: Chorionic villus sampling introduced at select centers.
1985: Color Doppler incorporated into ultrasound.
1990s
1990: Embryoscopy first described.
Mid-1990s: 3D/4D ultrasound begins to assume major role in ob.gyn. imaging.1997: Discovery of cell-free fetal DNA in maternal plasma.
2000s
2003: MOMS (Management of Myelomeningocele Study) was launched.
2010s
2012: The American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine support cell-free DNA screening for women at increased risk of fetal aneuploidy.
2013: Preterm birth rate drops to 11.4%
2014: Diabetes incidence marks a 4-fold increase since 1980.
Smartphone App Helps Decrease Depression Symptoms in Pregnancy
BETHESDA, MD. – A smartphone application helped decrease depressive symptoms and improve confidence in self care for low-income pregnant women in their third trimester, a pilot study has shown.
“There is a difficulty in bringing mental health into the OB setting, particularly for underserved communities, in part because of too much to accomplish during a visit or because some women don’t think it’s the appropriate place to talk about their mental health concerns,” Liisa Hantsoo, PhD, a researcher at the Penn Center for Women’s Behavioral Wellness in Philadelphia, said during the annual National Institute of Mental Health Conference on Mental Health Services Research.
However, in a single academic site pilot study of 64 pregnant women, most of whom were covered under Medicaid, Dr. Hantsoo and her colleagues found that when the women were given access to their obstetrician’s office via a smartphone app integrated into the practice, they were significantly more likely to open up about their mental health concerns, spend more time in conversation with their clinician when symptoms increased, and experience fewer symptoms of depression and anxiety.
“Participants used the app frequently, they reported feeling more positive about their emotions, and they reported feeling more confident about taking care of their own health during their third trimester,” Dr. Hantsoo said.
All women in the study were assessed for depression using the Patient Health Questionnaire depression module (PHQ-9). Women with scores of 5 or higher who were no more than 32 weeks pregnant were included in the study. The women – more than half of whom had a prior history of mental illness – were also assessed using the Generalized Anxiety Disorder 7-item scale (GAD-7). And they were asked to rate their satisfaction levels with their OB care at baseline, including whether they believed their care team connected with them as individuals. The study participants were all in their mid-20s and had previously given birth.
Twenty-two women were randomly assigned to use a control app, which only allowed self-initiated communication with the practice through an established patient portal not designed specifically for mental health. Another 23 women were assigned to the same control app plus an app designed by Ginger.io for mental health self-care and symptom tracking. The study app included daily cognitive-behavioral therapy messages, other behavioral health educational messages, and prompts to record self-assessments of mood that were monitored daily by a care coordinator. The remaining 19 women were assigned to both apps and received additional prompts throughout the day to record their thoughts and mood, which were also monitored. If a patient’s depressive symptoms increased, the care coordinator alerted a physician in the practice, who then contacted the patient.
By week 8, the study app users had significantly decreased PHQ-9 scores (P = .001) and significantly decreased GAD-7 scores (P = .003). The combined study cohorts (women using the study app and those with the study app plus prompts to record mood) also self-reported significantly improved mood ratings at week 8 (P = .03). The combined study groups also reported more confidence in their ability to care for themselves, particularly in their third trimester, compared with women using only the control app (P = .002).
The difference is likely because of the ways that the study app was integrated into care, Dr. Hantsoo said. “Apps allow self-monitoring and identify your patterns over time, but they are also limited in that they aren’t often integrated into treatment or care, leaving a person hanging in distress if they enter their data, but then not having it seem to go anywhere. This app allowed patients to interact with their providers.”
Use of any app did not significantly affect how participants rated their overall care, although at least half of all study app users reported feeling more confident in their ability to assess and manage their moods and their overall health, particularly in their third trimester.
As for the physicians who participated in the study, they reported needing more time each week to respond to app-triggered patient needs. “It was a bit of a disruption, but they did report they thought it was worthwhile to do,” Dr. Hantsoo said in an interview.
Support for this study was provided by Ginger.io and by the Penn Medicine Center for Health Care Innovation. Dr. Hantsoo reported having no relevant financial
BETHESDA, MD. – A smartphone application helped decrease depressive symptoms and improve confidence in self care for low-income pregnant women in their third trimester, a pilot study has shown.
“There is a difficulty in bringing mental health into the OB setting, particularly for underserved communities, in part because of too much to accomplish during a visit or because some women don’t think it’s the appropriate place to talk about their mental health concerns,” Liisa Hantsoo, PhD, a researcher at the Penn Center for Women’s Behavioral Wellness in Philadelphia, said during the annual National Institute of Mental Health Conference on Mental Health Services Research.
However, in a single academic site pilot study of 64 pregnant women, most of whom were covered under Medicaid, Dr. Hantsoo and her colleagues found that when the women were given access to their obstetrician’s office via a smartphone app integrated into the practice, they were significantly more likely to open up about their mental health concerns, spend more time in conversation with their clinician when symptoms increased, and experience fewer symptoms of depression and anxiety.
“Participants used the app frequently, they reported feeling more positive about their emotions, and they reported feeling more confident about taking care of their own health during their third trimester,” Dr. Hantsoo said.
All women in the study were assessed for depression using the Patient Health Questionnaire depression module (PHQ-9). Women with scores of 5 or higher who were no more than 32 weeks pregnant were included in the study. The women – more than half of whom had a prior history of mental illness – were also assessed using the Generalized Anxiety Disorder 7-item scale (GAD-7). And they were asked to rate their satisfaction levels with their OB care at baseline, including whether they believed their care team connected with them as individuals. The study participants were all in their mid-20s and had previously given birth.
Twenty-two women were randomly assigned to use a control app, which only allowed self-initiated communication with the practice through an established patient portal not designed specifically for mental health. Another 23 women were assigned to the same control app plus an app designed by Ginger.io for mental health self-care and symptom tracking. The study app included daily cognitive-behavioral therapy messages, other behavioral health educational messages, and prompts to record self-assessments of mood that were monitored daily by a care coordinator. The remaining 19 women were assigned to both apps and received additional prompts throughout the day to record their thoughts and mood, which were also monitored. If a patient’s depressive symptoms increased, the care coordinator alerted a physician in the practice, who then contacted the patient.
By week 8, the study app users had significantly decreased PHQ-9 scores (P = .001) and significantly decreased GAD-7 scores (P = .003). The combined study cohorts (women using the study app and those with the study app plus prompts to record mood) also self-reported significantly improved mood ratings at week 8 (P = .03). The combined study groups also reported more confidence in their ability to care for themselves, particularly in their third trimester, compared with women using only the control app (P = .002).
The difference is likely because of the ways that the study app was integrated into care, Dr. Hantsoo said. “Apps allow self-monitoring and identify your patterns over time, but they are also limited in that they aren’t often integrated into treatment or care, leaving a person hanging in distress if they enter their data, but then not having it seem to go anywhere. This app allowed patients to interact with their providers.”
Use of any app did not significantly affect how participants rated their overall care, although at least half of all study app users reported feeling more confident in their ability to assess and manage their moods and their overall health, particularly in their third trimester.
As for the physicians who participated in the study, they reported needing more time each week to respond to app-triggered patient needs. “It was a bit of a disruption, but they did report they thought it was worthwhile to do,” Dr. Hantsoo said in an interview.
Support for this study was provided by Ginger.io and by the Penn Medicine Center for Health Care Innovation. Dr. Hantsoo reported having no relevant financial
BETHESDA, MD. – A smartphone application helped decrease depressive symptoms and improve confidence in self care for low-income pregnant women in their third trimester, a pilot study has shown.
“There is a difficulty in bringing mental health into the OB setting, particularly for underserved communities, in part because of too much to accomplish during a visit or because some women don’t think it’s the appropriate place to talk about their mental health concerns,” Liisa Hantsoo, PhD, a researcher at the Penn Center for Women’s Behavioral Wellness in Philadelphia, said during the annual National Institute of Mental Health Conference on Mental Health Services Research.
However, in a single academic site pilot study of 64 pregnant women, most of whom were covered under Medicaid, Dr. Hantsoo and her colleagues found that when the women were given access to their obstetrician’s office via a smartphone app integrated into the practice, they were significantly more likely to open up about their mental health concerns, spend more time in conversation with their clinician when symptoms increased, and experience fewer symptoms of depression and anxiety.
“Participants used the app frequently, they reported feeling more positive about their emotions, and they reported feeling more confident about taking care of their own health during their third trimester,” Dr. Hantsoo said.
All women in the study were assessed for depression using the Patient Health Questionnaire depression module (PHQ-9). Women with scores of 5 or higher who were no more than 32 weeks pregnant were included in the study. The women – more than half of whom had a prior history of mental illness – were also assessed using the Generalized Anxiety Disorder 7-item scale (GAD-7). And they were asked to rate their satisfaction levels with their OB care at baseline, including whether they believed their care team connected with them as individuals. The study participants were all in their mid-20s and had previously given birth.
Twenty-two women were randomly assigned to use a control app, which only allowed self-initiated communication with the practice through an established patient portal not designed specifically for mental health. Another 23 women were assigned to the same control app plus an app designed by Ginger.io for mental health self-care and symptom tracking. The study app included daily cognitive-behavioral therapy messages, other behavioral health educational messages, and prompts to record self-assessments of mood that were monitored daily by a care coordinator. The remaining 19 women were assigned to both apps and received additional prompts throughout the day to record their thoughts and mood, which were also monitored. If a patient’s depressive symptoms increased, the care coordinator alerted a physician in the practice, who then contacted the patient.
By week 8, the study app users had significantly decreased PHQ-9 scores (P = .001) and significantly decreased GAD-7 scores (P = .003). The combined study cohorts (women using the study app and those with the study app plus prompts to record mood) also self-reported significantly improved mood ratings at week 8 (P = .03). The combined study groups also reported more confidence in their ability to care for themselves, particularly in their third trimester, compared with women using only the control app (P = .002).
The difference is likely because of the ways that the study app was integrated into care, Dr. Hantsoo said. “Apps allow self-monitoring and identify your patterns over time, but they are also limited in that they aren’t often integrated into treatment or care, leaving a person hanging in distress if they enter their data, but then not having it seem to go anywhere. This app allowed patients to interact with their providers.”
Use of any app did not significantly affect how participants rated their overall care, although at least half of all study app users reported feeling more confident in their ability to assess and manage their moods and their overall health, particularly in their third trimester.
As for the physicians who participated in the study, they reported needing more time each week to respond to app-triggered patient needs. “It was a bit of a disruption, but they did report they thought it was worthwhile to do,” Dr. Hantsoo said in an interview.
Support for this study was provided by Ginger.io and by the Penn Medicine Center for Health Care Innovation. Dr. Hantsoo reported having no relevant financial
AT THE ANNUAL NIMH CONFERENCE ON MENTAL HEALTH SERVICES RESEARCH
Smartphone app helps decrease depression symptoms in pregnancy
BETHESDA, MD. – A smartphone application helped decrease depressive symptoms and improve confidence in self care for low-income pregnant women in their third trimester, a pilot study has shown.
“There is a difficulty in bringing mental health into the OB setting, particularly for underserved communities, in part because of too much to accomplish during a visit or because some women don’t think it’s the appropriate place to talk about their mental health concerns,” Liisa Hantsoo, PhD, a researcher at the Penn Center for Women’s Behavioral Wellness in Philadelphia, said during the annual National Institute of Mental Health Conference on Mental Health Services Research.
However, in a single academic site pilot study of 64 pregnant women, most of whom were covered under Medicaid, Dr. Hantsoo and her colleagues found that when the women were given access to their obstetrician’s office via a smartphone app integrated into the practice, they were significantly more likely to open up about their mental health concerns, spend more time in conversation with their clinician when symptoms increased, and experience fewer symptoms of depression and anxiety.
“Participants used the app frequently, they reported feeling more positive about their emotions, and they reported feeling more confident about taking care of their own health during their third trimester,” Dr. Hantsoo said.
All women in the study were assessed for depression using the Patient Health Questionnaire depression module (PHQ-9). Women with scores of 5 or higher who were no more than 32 weeks pregnant were included in the study. The women – more than half of whom had a prior history of mental illness – were also assessed using the Generalized Anxiety Disorder 7-item scale (GAD-7). And they were asked to rate their satisfaction levels with their OB care at baseline, including whether they believed their care team connected with them as individuals. The study participants were all in their mid-20s and had previously given birth.
Twenty-two women were randomly assigned to use a control app, which only allowed self-initiated communication with the practice through an established patient portal not designed specifically for mental health. Another 23 women were assigned to the same control app plus an app designed by Ginger.io for mental health self-care and symptom tracking. The study app included daily cognitive-behavioral therapy messages, other behavioral health educational messages, and prompts to record self-assessments of mood that were monitored daily by a care coordinator. The remaining 19 women were assigned to both apps and received additional prompts throughout the day to record their thoughts and mood, which were also monitored. If a patient’s depressive symptoms increased, the care coordinator alerted a physician in the practice, who then contacted the patient.
By week 8, the study app users had significantly decreased PHQ-9 scores (P = .001) and significantly decreased GAD-7 scores (P = .003). The combined study cohorts (women using the study app and those with the study app plus prompts to record mood) also self-reported significantly improved mood ratings at week 8 (P = .03). The combined study groups also reported more confidence in their ability to care for themselves, particularly in their third trimester, compared with women using only the control app (P = .002).
The difference is likely because of the ways that the study app was integrated into care, Dr. Hantsoo said. “Apps allow self-monitoring and identify your patterns over time, but they are also limited in that they aren’t often integrated into treatment or care, leaving a person hanging in distress if they enter their data, but then not having it seem to go anywhere. This app allowed patients to interact with their providers.”
Use of any app did not significantly affect how participants rated their overall care, although at least half of all study app users reported feeling more confident in their ability to assess and manage their moods and their overall health, particularly in their third trimester.
As for the physicians who participated in the study, they reported needing more time each week to respond to app-triggered patient needs. “It was a bit of a disruption, but they did report they thought it was worthwhile to do,” Dr. Hantsoo said in an interview.
Support for this study was provided by Ginger.io and by the Penn Medicine Center for Health Care Innovation. Dr. Hantsoo reported having no relevant financial
On Twitter @whitneymcknight
BETHESDA, MD. – A smartphone application helped decrease depressive symptoms and improve confidence in self care for low-income pregnant women in their third trimester, a pilot study has shown.
“There is a difficulty in bringing mental health into the OB setting, particularly for underserved communities, in part because of too much to accomplish during a visit or because some women don’t think it’s the appropriate place to talk about their mental health concerns,” Liisa Hantsoo, PhD, a researcher at the Penn Center for Women’s Behavioral Wellness in Philadelphia, said during the annual National Institute of Mental Health Conference on Mental Health Services Research.
However, in a single academic site pilot study of 64 pregnant women, most of whom were covered under Medicaid, Dr. Hantsoo and her colleagues found that when the women were given access to their obstetrician’s office via a smartphone app integrated into the practice, they were significantly more likely to open up about their mental health concerns, spend more time in conversation with their clinician when symptoms increased, and experience fewer symptoms of depression and anxiety.
“Participants used the app frequently, they reported feeling more positive about their emotions, and they reported feeling more confident about taking care of their own health during their third trimester,” Dr. Hantsoo said.
All women in the study were assessed for depression using the Patient Health Questionnaire depression module (PHQ-9). Women with scores of 5 or higher who were no more than 32 weeks pregnant were included in the study. The women – more than half of whom had a prior history of mental illness – were also assessed using the Generalized Anxiety Disorder 7-item scale (GAD-7). And they were asked to rate their satisfaction levels with their OB care at baseline, including whether they believed their care team connected with them as individuals. The study participants were all in their mid-20s and had previously given birth.
Twenty-two women were randomly assigned to use a control app, which only allowed self-initiated communication with the practice through an established patient portal not designed specifically for mental health. Another 23 women were assigned to the same control app plus an app designed by Ginger.io for mental health self-care and symptom tracking. The study app included daily cognitive-behavioral therapy messages, other behavioral health educational messages, and prompts to record self-assessments of mood that were monitored daily by a care coordinator. The remaining 19 women were assigned to both apps and received additional prompts throughout the day to record their thoughts and mood, which were also monitored. If a patient’s depressive symptoms increased, the care coordinator alerted a physician in the practice, who then contacted the patient.
By week 8, the study app users had significantly decreased PHQ-9 scores (P = .001) and significantly decreased GAD-7 scores (P = .003). The combined study cohorts (women using the study app and those with the study app plus prompts to record mood) also self-reported significantly improved mood ratings at week 8 (P = .03). The combined study groups also reported more confidence in their ability to care for themselves, particularly in their third trimester, compared with women using only the control app (P = .002).
The difference is likely because of the ways that the study app was integrated into care, Dr. Hantsoo said. “Apps allow self-monitoring and identify your patterns over time, but they are also limited in that they aren’t often integrated into treatment or care, leaving a person hanging in distress if they enter their data, but then not having it seem to go anywhere. This app allowed patients to interact with their providers.”
Use of any app did not significantly affect how participants rated their overall care, although at least half of all study app users reported feeling more confident in their ability to assess and manage their moods and their overall health, particularly in their third trimester.
As for the physicians who participated in the study, they reported needing more time each week to respond to app-triggered patient needs. “It was a bit of a disruption, but they did report they thought it was worthwhile to do,” Dr. Hantsoo said in an interview.
Support for this study was provided by Ginger.io and by the Penn Medicine Center for Health Care Innovation. Dr. Hantsoo reported having no relevant financial
On Twitter @whitneymcknight
BETHESDA, MD. – A smartphone application helped decrease depressive symptoms and improve confidence in self care for low-income pregnant women in their third trimester, a pilot study has shown.
“There is a difficulty in bringing mental health into the OB setting, particularly for underserved communities, in part because of too much to accomplish during a visit or because some women don’t think it’s the appropriate place to talk about their mental health concerns,” Liisa Hantsoo, PhD, a researcher at the Penn Center for Women’s Behavioral Wellness in Philadelphia, said during the annual National Institute of Mental Health Conference on Mental Health Services Research.
However, in a single academic site pilot study of 64 pregnant women, most of whom were covered under Medicaid, Dr. Hantsoo and her colleagues found that when the women were given access to their obstetrician’s office via a smartphone app integrated into the practice, they were significantly more likely to open up about their mental health concerns, spend more time in conversation with their clinician when symptoms increased, and experience fewer symptoms of depression and anxiety.
“Participants used the app frequently, they reported feeling more positive about their emotions, and they reported feeling more confident about taking care of their own health during their third trimester,” Dr. Hantsoo said.
All women in the study were assessed for depression using the Patient Health Questionnaire depression module (PHQ-9). Women with scores of 5 or higher who were no more than 32 weeks pregnant were included in the study. The women – more than half of whom had a prior history of mental illness – were also assessed using the Generalized Anxiety Disorder 7-item scale (GAD-7). And they were asked to rate their satisfaction levels with their OB care at baseline, including whether they believed their care team connected with them as individuals. The study participants were all in their mid-20s and had previously given birth.
Twenty-two women were randomly assigned to use a control app, which only allowed self-initiated communication with the practice through an established patient portal not designed specifically for mental health. Another 23 women were assigned to the same control app plus an app designed by Ginger.io for mental health self-care and symptom tracking. The study app included daily cognitive-behavioral therapy messages, other behavioral health educational messages, and prompts to record self-assessments of mood that were monitored daily by a care coordinator. The remaining 19 women were assigned to both apps and received additional prompts throughout the day to record their thoughts and mood, which were also monitored. If a patient’s depressive symptoms increased, the care coordinator alerted a physician in the practice, who then contacted the patient.
By week 8, the study app users had significantly decreased PHQ-9 scores (P = .001) and significantly decreased GAD-7 scores (P = .003). The combined study cohorts (women using the study app and those with the study app plus prompts to record mood) also self-reported significantly improved mood ratings at week 8 (P = .03). The combined study groups also reported more confidence in their ability to care for themselves, particularly in their third trimester, compared with women using only the control app (P = .002).
The difference is likely because of the ways that the study app was integrated into care, Dr. Hantsoo said. “Apps allow self-monitoring and identify your patterns over time, but they are also limited in that they aren’t often integrated into treatment or care, leaving a person hanging in distress if they enter their data, but then not having it seem to go anywhere. This app allowed patients to interact with their providers.”
Use of any app did not significantly affect how participants rated their overall care, although at least half of all study app users reported feeling more confident in their ability to assess and manage their moods and their overall health, particularly in their third trimester.
As for the physicians who participated in the study, they reported needing more time each week to respond to app-triggered patient needs. “It was a bit of a disruption, but they did report they thought it was worthwhile to do,” Dr. Hantsoo said in an interview.
Support for this study was provided by Ginger.io and by the Penn Medicine Center for Health Care Innovation. Dr. Hantsoo reported having no relevant financial
On Twitter @whitneymcknight
AT THE ANNUAL NIMH CONFERENCE ON MENTAL HEALTH SERVICES RESEARCH
Key clinical point: Smartphone technology could improve mental health outcomes in the ob.gyn. setting.
Major finding: A smartphone app integrated into obstetrics practice was associated with significantly decreased PHQ-9 scores (P = .001) and significantly decreased GAD-7 scores (P = .003).
Data source: A single-site pilot study of 64 pregnant women.
Disclosures: Support for the study was provided by Ginger.io, and by the Penn Medicine Center for Health Care Innovation. Dr. Hantsoo reported having no relevant financial disclosures.
Territories now have U.S. majority of pregnant women with Zika
The total number of pregnant women with evidence of Zika virus infection reported in the U.S. territories surpassed that of the 50 states and the District of Columbia during the week ending July 28, 2016, according to the Centers for Disease Control and Prevention.
There were 71 new cases of Zika in pregnant women reported in U.S. territories that week, bringing the total for the year to 493. The states and D.C. reported 46 new cases, for a total of 479 for the year, which puts the United States as a whole at 972 cases of confirmed Zika virus infection in pregnant women for 2016, the CDC reported Aug. 4.
Among the territories, the overwhelming majority of Zika cases are in Puerto Rico, which has reported 5,482 cases so far, compared with 44 in American Samoa and 22 in the U.S. Virgin Islands. In all, there have been 1,825 cases reported in the states and D.C., the CDC reported.
The territories, so far, have mostly avoided Zika-related pregnancy losses and birth defects, with only one case of pregnancy loss and no infants born with birth defects in 2016. Two more cases of infants born with birth defects were reported, however, in the states and D.C. for the week ending July 28, bringing the state/D.C. total to 15 for the year, but no new pregnancy losses with Zika-related birth defects were added to the six reported so far, the CDC announced.
“These outcomes occurred in pregnancies with laboratory evidence of Zika virus infection,” the CDC noted, and it is not known “whether they were caused by Zika virus infection or other factors.”
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The total number of pregnant women with evidence of Zika virus infection reported in the U.S. territories surpassed that of the 50 states and the District of Columbia during the week ending July 28, 2016, according to the Centers for Disease Control and Prevention.
There were 71 new cases of Zika in pregnant women reported in U.S. territories that week, bringing the total for the year to 493. The states and D.C. reported 46 new cases, for a total of 479 for the year, which puts the United States as a whole at 972 cases of confirmed Zika virus infection in pregnant women for 2016, the CDC reported Aug. 4.
Among the territories, the overwhelming majority of Zika cases are in Puerto Rico, which has reported 5,482 cases so far, compared with 44 in American Samoa and 22 in the U.S. Virgin Islands. In all, there have been 1,825 cases reported in the states and D.C., the CDC reported.
The territories, so far, have mostly avoided Zika-related pregnancy losses and birth defects, with only one case of pregnancy loss and no infants born with birth defects in 2016. Two more cases of infants born with birth defects were reported, however, in the states and D.C. for the week ending July 28, bringing the state/D.C. total to 15 for the year, but no new pregnancy losses with Zika-related birth defects were added to the six reported so far, the CDC announced.
“These outcomes occurred in pregnancies with laboratory evidence of Zika virus infection,” the CDC noted, and it is not known “whether they were caused by Zika virus infection or other factors.”
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The total number of pregnant women with evidence of Zika virus infection reported in the U.S. territories surpassed that of the 50 states and the District of Columbia during the week ending July 28, 2016, according to the Centers for Disease Control and Prevention.
There were 71 new cases of Zika in pregnant women reported in U.S. territories that week, bringing the total for the year to 493. The states and D.C. reported 46 new cases, for a total of 479 for the year, which puts the United States as a whole at 972 cases of confirmed Zika virus infection in pregnant women for 2016, the CDC reported Aug. 4.
Among the territories, the overwhelming majority of Zika cases are in Puerto Rico, which has reported 5,482 cases so far, compared with 44 in American Samoa and 22 in the U.S. Virgin Islands. In all, there have been 1,825 cases reported in the states and D.C., the CDC reported.
The territories, so far, have mostly avoided Zika-related pregnancy losses and birth defects, with only one case of pregnancy loss and no infants born with birth defects in 2016. Two more cases of infants born with birth defects were reported, however, in the states and D.C. for the week ending July 28, bringing the state/D.C. total to 15 for the year, but no new pregnancy losses with Zika-related birth defects were added to the six reported so far, the CDC announced.
“These outcomes occurred in pregnancies with laboratory evidence of Zika virus infection,” the CDC noted, and it is not known “whether they were caused by Zika virus infection or other factors.”
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
NIH launches trial of Zika vaccine candidate
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
Zika virus update: A rapidly moving target
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.
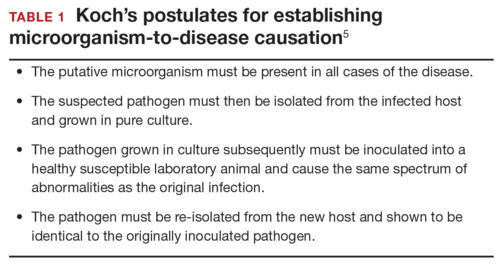
Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.
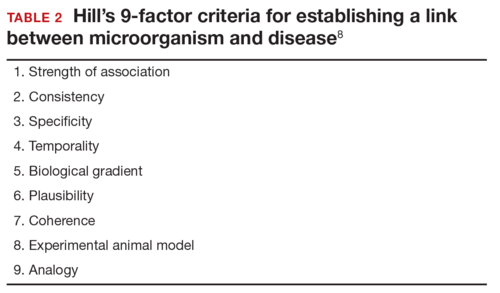
How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.
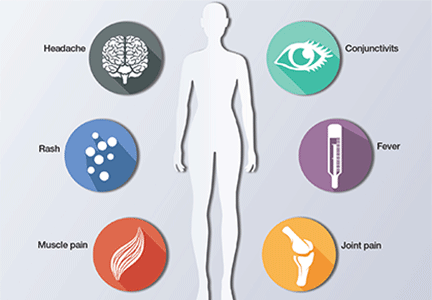
How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.

Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.

How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.

How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
We recently reviewed the most current information on the epidemiology, clinical manifestations, and diagnosis of maternal and congenital Zika virus (ZV) infection.1 We also offered tentative recommendations for reducing the risk of infection and for managing the treatment of women exposed to the virus.
In this update, we present new information on the broadened spectrum of anomalies now known to be causally related to congenital ZV infection and on the increasing number of serious neurologic complications directly related to ZV infection in adults. We also update recommendations for diagnosing maternal, fetal, and neonatal infection and present guidelines for preventing sexual transmission of ZV infection.
CASE Woman from Brazil gives birth to stillborn baby with microcephaly
A 23-year-old woman (G2P1) recently emigrated from Pernambuco in Brazil to the United States and now presents to the hospital in advanced labor. Based on results of first-trimester ultrasonography performed in Brazil, it is determined that she is at 39 weeks’ gestation. The patient has not had any prenatal care since early in the second trimester because of low income and lack of medical insurance. She reports no serious illness before or during the pregnancy.
In the labor and delivery suite, she rapidly delivers a stillborn female infant—5 pounds 3 ounces, growth restricted, with multiple congenital anomalies. Postmortem examination reveals microcephaly, ventriculomegaly, extensive brain atrophy, intracranial calcifications, cerebellar agenesis, cataracts, ocular calcifications, redundant scalp tissue, and multiple joint contractures.
What is the most likely cause of these multiple anomalies?
The patient’s findings are most consistent with a diagnosis of severe intrauterine infection. Possible pathogenic organisms include rubella virus, cytomegalovirus, lymphocytic choriomeningitis virus, toxoplasmosis, and ZV.2 Given the patient’s recent move from Pernambuco in northeastern Brazil, the epicenter of the ZV epidemic in the Americas, the most likely diagnosis is congenital ZV infection.
The initial reports of congenital anomalies associated with ZV infection focused on microcephaly, usually defined as head circumference less than 3 standard deviations below the mean, or less than the third or fifth percentile for gestational age. Subsequent reports have linked many other serious central nervous system (CNS) anomalies to the virus. In a retrospective case series, de Fatima Vasco Aragao and colleagues3 described neuroimaging findings in 23 infants with presumed congenital ZV infection. Of the 22 with computed tomography scans, all had calcifications at the junction of cortical and subcortical white matter, 21 (95%) had disordered cortical development, 20 (91%) had a significant decrease in brain volume, 19 (86%) had ventriculomegaly, and half had distinct hypoplasia of either cerebellum or brainstem. In addition, of the 8 infants with magnetic resonance imaging (MRI) studies, 7 (88%) had an enlarged cisterna magna, 7 (88%) had delayed myelination, 6 (75%) had a simplified gyral pattern, and 3 (38%) had hypoplasia of corpus callosum.
De Paula Freitas and colleagues4 recently found congenital ZV infection associated with severe ocular abnormalities. Comprehensive ophthalmologic examination of 29 infants with microcephaly, presumed caused by congenital ZV infection, revealed 10 (35%) had abnormalities, which included focal pigment mottling, chorioretinal atrophy, hypoplasia and cupping of optic disk, loss of foveal reflex, macular atrophy, lens subluxation, and coloboma of iris.
Other conditions linked to congenital ZV infection include intrauterine growth restriction, redundant scalp tissue, contractures of multiple joints, and clubfoot.2
Bottom line. Although the ocular abnormalities are undetectable by prenatal ultrasound, many of the CNS and skeletal anomalies can be identified antenatally. Therefore, serial ultrasound examinations should be performed on adults who have a clinical illness consistent with ZV infection or who have traveled to an endemic area or have a sexual partner who has been in an endemic area. Patients should be assessed for possible microcephaly, ventriculomegaly, agenesis of corpus callosum, hypoplasia of cerebellum, and skeletal deformities.
Zika virus has been shown to be a direct cause of microcephaly
To make the determination that Zika virus (ZV) causes microcephaly, Rasmussen and colleagues1 very recently evaluated Shepard’s 7 criteria,2 published in 1994, for establishing a cause between a microorganism and a specific clinical condition. These 7 criteria are:
- There must be a proven exposure at one or more critical times during prenatal development.
Rasmussen and colleagues1 pointed to case reports, case series, and epidemiologic studies showing a clear association between ZV exposure and microcephaly. Although exposure at any time during pregnancy may cause congenital infection, exposure in the late first and early second trimesters seems to pose the most risk for severe central nervous system (CNS) injury. - There must be consistent findings in 2 or more high-quality epidemiologic studies.
The studies must control for important confounding variables and include an appropriate number of patients to clearly identify an association between a given exposure and specific fetal anomalies. Rasmussen and colleagues1 cited 2 important epidemiologic studies. The first, a prospective cohort investigation of women in Brazil, found that 29% of those with ZV infection had abnormalities on prenatal ultrasound.3
In the second investigation, a retrospective study of 8 infants in French Polynesia, the mathematical modeling performed by the authors4 suggested microcephaly occurred in 1% of infants born to women with first-trimester ZV infection. Using a different mathematical model, Johansson and colleagues5 found that the risk of fetal microcephaly associated with first-trimester infection may range from as low as 1% to as high as 13%.
Although these studies are helpful in quantifying the risk of congenital infection, they only partially satisfy Shepard’s second criterion. - The suspected microorganism must produce a specific defect or clearly delineated syndrome.
Rasmussen and colleagues1 argued that this criterion has been fulfilled. Zika virus infection causes a distinct phenotype that includes microcephaly, multiple other CNS anomalies, redundant scalp skin, ocular abnormalities, joint contractures (arthrogryposis), and clubfoot.6,7 - The observed birth defect must be associated with a rare environmental exposure.
This criterion also has been met, Rasmussen and colleagues1 reported. They noted that congenital microcephaly is rare in the United States (only about 6 cases in 10,000 liveborn infants) but that the number of cases in Brazil and French Polynesia is much in excess of what would be predicted in the absence of the ZV epidemic. - Teratogenicity should be demonstrated in laboratory animals.
Shepard indicated that this criterion is important but not essential to prove causation. As there is yet no animal model for ZV infection, this criterion has not been fulfilled. - The association between the exposure and the observed anomaly or spectrum of anomalies should be biologically plausible.
Rasmussen and colleagues1 demonstrated that the findings linked to maternal ZV infection are similar to those described for at least 2 other viral pathogens, rubella virus and cytomegalovirus. Animal models also have clearly shown that the ZV is neurotropic. Moreover, ZV has been clearly identified in the brains of infants with microcephaly.8 - Shepard’s seventh criterion relates to a medication or chemical exposure and is not relevant to a microorganism.
References
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Brasil P, Pereira JP Jr, Raja Gabaglia C, et al. Zika virus infection in pregnant women in Rio de Janeiro—preliminary report [published online ahead of print March 4, 2016]. N Engl J Med. doi:10.1056/NEJMoa1602412.
- Cauchemez S, Besnard M, Bompard P, et al. Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet. 2016;387(10033):2125–2132.
- Johansson MA, Mier-Y-Teran-Romero L, Reefhuis J, Gilboa SM, Hills SL. Zika and the risk of microcephaly [published online ahead of print May 25, 2016; updated June 9, 2016]. N Engl J Med. 2016;375:1–4. doi:10.1056/NEJMp1605367.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374(16):1552–1563.
- Mlakar J, Korva M, Tul N, et al. Zika virus associated with microcephaly. N Engl J Med. 2016;374(10):951–958.
Did ZV cause these anomalies?
How certain can we be that the anomalies present in the case patient’s baby were caused by ZV? In the past, and for many years, scientists relied on Koch’s 4 postulates (TABLE 1) to answer this question and establish a causal relationship between a microorganism and a specific clinical disease.5 Koch’s postulates have not been satisfied for the relationship between maternal ZV infection and congenital anomalies. Today’s more relevant standards for determining causality of a teratogen were published in 1994 by Shepard.6 In 2016, Rasmussen and colleagues7 found that the critical components of these criteria are fulfilled and concluded that there is little doubt ZV is a proven and extremely dangerous teratogen. See “Zika virus has been shown to be a direct cause of microcephaly”.

Rasmussen and colleagues7 also used Hill’s criteria to assess the evidence for causation. Hill’s systematic assessment is based on 9 factors (TABLE 2)8, and Rasmussen and colleagues7 concluded that the necessary 7 of these 9 criteria have been met (the experimental animal model criterion was not satisfied, and the biological gradient criterion was not applicable). Given their assessment of Shepard’s criteria,6 the authors argued that the link between maternal ZV infection and severe congenital anomalies has risen from association to well-defined causation.

How should ZV infection be confirmed in adults and newborns?
After our first review was published in March 2016,1 the testing algorithm recommended by the US Centers for Disease Control and Prevention (CDC) was revised.9 Now, according to the CDC, if a patient has had symptoms of ZV infection for less than 5 days, serum and urine should be obtained for reverse transcriptase–polymerase chain reaction (RT-PCR) testing. If symptoms have been present for 5 to 14 days, urine should be tested by RT-PCR because urine samples appear to remain positive for virus longer than serum samples do. If RT-PCR is performed within the appropriate period and the result is negative, ZV infection is excluded; if the result is positive, acute ZV infection is confirmed, and additional testing is not indicated. RT-PCR can be performed by 2 commercial laboratories (Quest Diagnostics and LabCorp), state health departments, and the CDC.
If serum or urine is collected more than 5 days after symptom onset and the RT-PCR result is negative, the patient should have an immunoglobulin M (IgM) assay for ZV. If the assay result is negative, infection is excluded; if the result is positive or equivocal, additional testing is needed to ensure that the presence of the antibody does not reflect a cross-reaction to dengue or chikungunya virus. The confirmatory plaque reduction neutralization test (PRNT) is performed only by the CDC. To be considered positive, the PRNT result must be at least 4-fold higher than the dengue virus neutralizing antibody titer.
In patients with suspected Guillain-Barré syndrome (GBS), RT-PCR can be performed on cerebrospinal fluid. For suspected fetal or neonatal infection, RT-PCR can be performed on amniotic fluid, umbilical cord blood, and fetal and placental tissue.
CASE 2 Nonpregnant woman with possible Zika virus exposure presents to ED with neurologic symptoms
A 31-year-old nulligravid woman presents to the emergency department (ED) for evaluation of numbness, tingling, and weakness in the lower extremities and difficulty walking. She reports having had a low-grade fever and a fine disseminated macular rash 1 week earlier. She denies recent travel and exposure to friends or relatives with illness, but she says her husband travels extensively and was living and working in Puerto Rico. The patient has no other neurologic symptoms.
Serum and cerebrospinal fluid chemistries and MRI findings are normal. However, the ZV IgM assay is positive, and nerve conduction study results are consistent with GBS. The patient is admitted to the hospital, treated with intravenous immunoglobulin and given supportive care. Over 10 days, her neurologic condition gradually improves.
What is the link between ZV infection and serious neurologic complications in adults?
ZV infection has been associated with serious neurologic complications in adults. Investigators in several countries have reported dramatic increases in GBS cases during the ZV outbreak.10
GBS is an acute, immune-mediated, demyelinating peripheral neuropathy that can vary in presentation but most commonly manifests as a rapidly ascending paralysis. The disorder often is preceded by an immunization or live viral infection. In some patients, paralysis severely weakens the respiratory muscles and even the cranial nerves, and affected individuals may require intubation, ventilator support, and parenteral or enteral alimentation.
In a case-control study conducted duringthe 2013–2014 outbreak in French Polynesia, the association between ZV infection and GBS was evaluated in 3 groups of patients: 42 patients with GBS, 98 control patients, and 70 patients with ZV infection but no neurologic complications.11 Symptoms of ZV infection were present in about 88% of the patients with GBS, and the median interval from viral infection to onset of neurologic symptoms was 6 days. The ZV IgM assay was positive in 93% of GBS cases. Nerve conduction study results were consistent with the acute motor axonal neuropathy of GBS. All patients were treated with intravenous immunoglobulin; 38% of patients had to be admitted to the intensive care unit, and 29% needed respiratory support. There were no fatalities. The overall incidence of GBS was 2.4 cases per 10,000 ZV infections.
Other neurologic complications that have been associated with ZV infection are meningoencephalitis,12 brain ischemia,13 and myelitis.14
Bottom line. ZV infection may cause serious neurologic complications in adults. The most devastating complication is GBS, which can result in respiratory muscle paralysis and cranial nerve palsies.

How can patients prevent sexual transmission of ZV infection?
The ZV can be transmitted by sexual contact, including vaginal, anal, and oral sex.15 It is known to persist longer in semen than in blood or urine, though the exact duration remains unknown. Atkinson and colleagues16 reported RT-PCR detection of ZV RNA in semen about 62 days after onset of febrile illness—long after the virus became undetectable in blood.15
Mansuy and colleagues17 found that the viral load in semen was more than 100,000 times that in blood and urine more than 2 weeks after symptom onset.16 The ZV has been detected in saliva, urine, and breast milk. Although it has not been identified in vaginal secretions in humans, it has been detected in the vaginal secretions of nonhuman primates up to 7 days after subcutaneous inoculation of virus.18 In addition, the first case of female-to-male sexual transmission of ZV infection was just reported.19 In this report, transmission seems to have occurred on day 3 of the woman’s symptomatic illness, when she had unprotected vaginal intercourse with her partner. The partner became symptomatic 7 days after sexual exposure. To date, there is no evidence that infection is spread through kissing or breastfeeding.
The most recent recommendations from the CDC are that a man with symptomatic ZV infection wait at least 6 months before having unprotected sexual contact. In addition, a man who is asymptomatic after ZV exposure should wait at least 8 weeks before having unprotected sexual contact.17
A woman planning a pregnancy should know there is no evidence that prior ZV infection increases the risk of birth defects. However, a woman with a proven ZV infection should wait at least 8 weeks after symptom onset before trying to conceive. Even an asymptomatic woman with possible exposure should wait at least 8 weeks after the last exposure before attempting conception. In addition, given the risks associated with maternal and fetal infection, a man who has been exposed to the virus and who has a pregnant partner should abstain from unprotected sexual contact for the duration of the pregnancy.20
Key takeaways
- Zika virus has now been clearly established as the cause of severe fetal malformations, particularly microcephaly.
- The risk of fetal injury appears to be greater when maternal infection occurs in the first trimester of pregnancy.
- Zika virus has now been established as the cause of Guillain-Barré syndrome in adults.
- Although most cases of Zika virus infection are transmitted as the result of mosquito bites, patients can acquire the infection through sexual contact. Both male-to-female and female-to-male transmission have been documented.
- If symptoms have been present for 5 to 14 days, only the urine RT-PCR test should be performed.
- If symptoms have been present for more than 14 days, the patient should have an immunoglobulin M assay for Zika virus. If this test is equivocal or positive, a plaque reduction neutralization test should be performed to exclude infection caused by dengue or chikungunya virus.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
- Chelliah A, Duff P. Zika virus: counseling considerations for this emerging perinatal threat. OBG Manag. 2016;28(3):28–34.
- Meaney-Delman D, Rasmussen SA, Staples JE, et al. Zika virus and pregnancy: what obstetric health care providers need to know. Obstet Gynecol. 2016;127(4):642–648.
- de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901.
- de Paula Freitas B, de Oliveira Dias JR, Prazeres J, et al. Ocular findings in infants with microcephaly associated with presumed Zika virus congenital infection in Salvador, Brazil [published online ahead of print February 9, 2016]. JAMA Ophthalmol. doi:10.1001/jamaophthalmol.2016.0267.
- Segen JC. Concise Dictionary of Modern Medicine. New York, NY: McGraw-Hill; 2002.
- Shepard TH. “Proof” of human teratogenicity. Teratology. 1994;50(2):97–98.
- Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med. 2016;374(20):1981–1987.
- Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32–37.
- Florida Department of Health. Zika fever: sample submission guidance for county health departments (CHDs). Version 2.0. http://www.floridahealth.gov/diseases-and-conditions/disease-reporting-and-management/disease-reporting-and-surveillance/_documents/zika-fever-sample-submission-guidance-for-chds.pdf. Published June 7, 2016. Accessed July 8, 2016.
- European Centre for Disease Prevention and Control. Zika virus disease epidemic: potential association with microcephaly and Guillain-Barré syndrome (first update). http://ecdc.europa.eu/en/publications/Publications/rapid-risk-assessment-zika-virus-first-update-jan-2016.pdf. Published January 21, 2016. Accessed January 25, 2016.
- Cao-Lormeau VM, Blake A, Mons S, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387(10027):1531–1539.
- Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374(16):1595–1596.
- Baud D, Van Mieghem T, Musso D, Truttmann AC, Panchaud A, Vouga M. Clinical management of pregnant women exposed to Zika virus [published online ahead of print April 4, 2016]. Lancet Infect Dis. 2016;16(5):523. doi:10.1016/S1473-3099(16)30008-1.
- Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387(10026):1481.
- Oster AM, Russell K, Stryker JE, et al. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):323–325.
- Atkinson B, Hearn P, Afrough B, et al. Detection of Zika virus in semen. Emerg Infect Dis. 2016;22(5):940.
- Mansuy JM, Dutertre M, Mengelle C, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16(4):405.
- Dudley DM, Aliota MT, Mohr EL, et al. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204.
- Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus-New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016; 65(28):716-717.
- Petersen EE, Polen KN, Meaney-Delman D, et al. Update: interim guidance for health care providers caring for women of reproductive age with possible Zika virus exposure—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(12):315–322.
In this Article
- Confirming Zika virus infection
- Zika virus and Guillain-Barré syndrome
- Preventing sexual transmission
STOP using instruments to assist with delivery of the head at cesarean
Rates of cesarean delivery in the second stage of labor have increased dramatically over the past few years.1 Compared with cesarean delivery prior to labor, second-stage labor cesarean is associated with a higher risk to both the mother and the fetus; risks include excessive bleeding, lower uterine segment extensions, injuries to the maternal ureters or bladder, and injury to the fetus.2−4 The risk is increased even further if the fetal head is deeply impacted in the pelvis. What can we do to avoid and manage such situations?
Anticipate an impacted fetal headThe true incidence of an impacted fetal head at the time of cesarean is not known, although a number of risk factors have been described (TABLE). Obstetric care providers should be aware of these risk factors and anticipate the likelihood of a difficult delivery of the fetal head at cesarean.
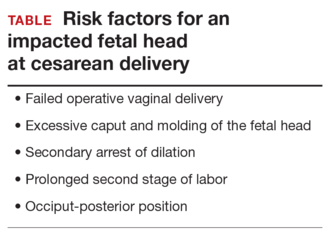
Options for managing an impacted fetal head at cesareanSeveral techniques have been reported in the literature for managing the delivery of a deeply engaged head, including:
Using an assistant to push the fetus’s head up using a hand in the vagina (“push” technique). This can cause trauma to the fetus, since the force required to push the fetus up from below is uncontrolled.5,6
The reverse breech extraction (“pull” technique) involves pulling the infant out feet first through the uterine incision.7
Use of an instrument. The most common instrument used is a vacuum extractor,8 although a number of other devices have been developed, including the Murless fetal head extractor (an instrument with a hinged shaft and sliding collar lock),9 the C-Snorkel impacted fetal head release device (the device’s tip contains ventilation ports to facilitate airflow and release of the vacuum/suction created by the impacted fetal head),10 and the Fetal Pillow (a balloon device inserted in the vagina and inflated with sterile saline to disimpact an engaged fetal head before cesarean delivery).11
While all of these techniques can cause injury to the mother and the fetus, available data favor use of the reverse breech extraction (pull) technique, since it is associated with fewer maternal risks, including lower rates of uterine incision extension, infection, and postpartum hemorrhage and a shorter operative time.12−18
Stop use of vacuum to deliver the fetal head at cesarean
Placement of a vacuum can be effective in assisting with delivery of the fetal head at cesarean. For this reason, vacuum-assisted deliveries at cesarean are becoming more common. While the rate of complications caused by vacuum extraction of the fetal head at cesarean is not known, injuries have been reported.19,20 As such, routine use of vacuum extraction at the time of cesarean delivery cannot be recommended.
Start disengaging the fetal head prior to cesarean
One useful technique in planning a cesarean in the second stage of labor or when an impacted fetal head is anticipated is to disengage the fetal head vaginally prior to skin incision. This can be done in the delivery room or in the operating room immediately prior to surgery with the help of an assistant.
While supporting the patient’s legs, the assistant inserts a hand into the vagina and pushes upward on the fetal head with gentle, sustained effort. The assistant should use a cupped hand or the palm of the hand while attempting to both elevate and flex the fetal head. It is best to avoid using 1 or 2 fingers to elevate the head, as this may cause excessive pressure at a single point and lead to injury, such as a skull fracture (FIGURE). The assistant should disengage his or her hand only when the operating surgeon is able to reach down and secure the fetal head from above.
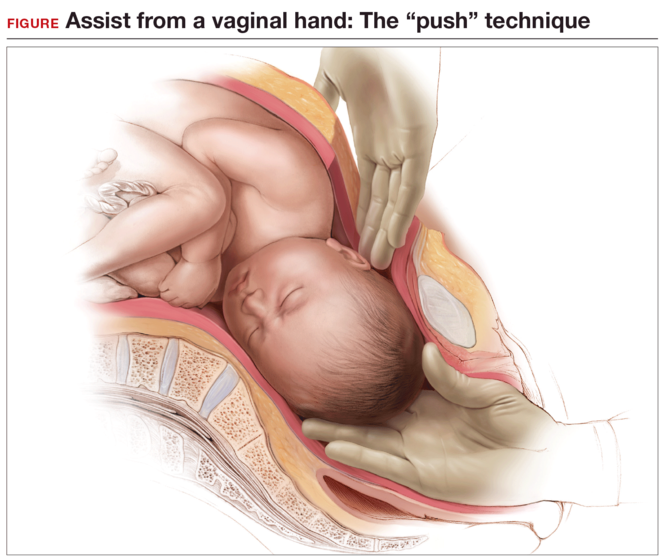
Elevating the fetal head prior to skin incision offers 3 major advantages:
- It avoids the embarrassing situation of having the fetus deliver vaginally before it can be pulled out through the abdominal incision. Although rare, this has been known to happen, because the dense regional anesthesia further relaxes the pelvic floor musculature, leading to flexion and rotation of the fetal head, which then descends and delivers. Performing a final bimanual examination in the operating room after the establishment of surgical level anesthesia and immediately prior to skin incision will avoid this situation.
- It elevates the fetal head, thereby creating additional space between the bony pelvis and fetal presenting part for the provider’s hand to fit. This helps minimize injury to the fetus and to the maternal soft tissues at the time of cesarean.
- Lastly, it provides additional information about the extent to which the fetal head is impacted in the pelvis and may influence decision making around the time of cesarean. For example, if the fetal head were deeply impacted in the pelvis and could not be disimpacted vaginally, the surgeon may choose to make a different uterine incision (such as a low vertical hysterotomy), administer a uterine relaxant (an inhaled anesthetic agent or nitric oxide), ask for additional instrumentation, and/or ask an assistant to be ready to elevate the fetal head vaginally should this be necessary.21
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Spencer C, Murphy D, Bewley S. Caesarean delivery in the second stage of labour. BMJ. 2006;333(7569):613–614.
- Häger RM, Daltviet AK, Hofoss D, et al. Complications of cesarean deliveries: rates and risk factors. Am J Obstet Gynecol. 2004;190(2):428–434.
- Murphy DJ, Liebling RE, Verity L, Swingler R, Patel R. Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: a cohort study. Lancet. 2001;358(9289):1203–1207.
- Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
- Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the midcavity. Arch Gynecol. 1983;234(1):59–60.
- Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707–710.
- Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynaecol Obstet. 1997;56(2):183–184.
- Arad I, Linder N, Bercovici B. Vacuum extraction at cesarean section—neonatal outcome. J Perinat Med. 1986;14(2):137–140.
- Murless BC. Lower-segment caesarean section; a new head extractor. BMJ. 1948;1(4564):1234.
- C-Snorkle impacted fetal head release device. Clinical Innovations website. http://clinicalinnovations.com /portfolio-items/c-snorkel/. Accessed July 22, 2016.
- Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J, Onwude JL. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133(2):178–182.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375–378.
- Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):24–26.
- Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scand. 2009;88(10):1163–1166.
- Veisi F, Zangeneh M, Malekkhosravi S, Rezavand N. Comparison of “push” and “pull” methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):4–6.
- Bastani P, Pourabolghasem S, Abbasalizadeh F, Motvalli L. ComparisonColor/Black of neonatal and maternal outcomes associated with head-pushing and head-pulling methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):1–3.
- Waterfall H, Grivell RM, Dodd JM. Techniques for assisting difficult delivery at caesarean section. Cochrane Database Syst Rev. 2016;1:CD004944.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123(3): 337–345.
- Clark SL, Vines VL, Belfort MA. Fetal injury associated with routine vacuum use during cesarean delivery. Am J Obstet Gynecol. 2008;198(4):e4.
- Fareeduddin R, Schifrin BS. Subgaleal hemorrhage after the use of a vacuum extractor during elective cesarean delivery: a case report. J Reprod Med. 2008;53(10):809–810.
- Barbieri RL. Difficult fetal extraction at cesarean delivery: What should you do? OBG Manag. 2012;24(1):8–12.
Rates of cesarean delivery in the second stage of labor have increased dramatically over the past few years.1 Compared with cesarean delivery prior to labor, second-stage labor cesarean is associated with a higher risk to both the mother and the fetus; risks include excessive bleeding, lower uterine segment extensions, injuries to the maternal ureters or bladder, and injury to the fetus.2−4 The risk is increased even further if the fetal head is deeply impacted in the pelvis. What can we do to avoid and manage such situations?
Anticipate an impacted fetal headThe true incidence of an impacted fetal head at the time of cesarean is not known, although a number of risk factors have been described (TABLE). Obstetric care providers should be aware of these risk factors and anticipate the likelihood of a difficult delivery of the fetal head at cesarean.

Options for managing an impacted fetal head at cesareanSeveral techniques have been reported in the literature for managing the delivery of a deeply engaged head, including:
Using an assistant to push the fetus’s head up using a hand in the vagina (“push” technique). This can cause trauma to the fetus, since the force required to push the fetus up from below is uncontrolled.5,6
The reverse breech extraction (“pull” technique) involves pulling the infant out feet first through the uterine incision.7
Use of an instrument. The most common instrument used is a vacuum extractor,8 although a number of other devices have been developed, including the Murless fetal head extractor (an instrument with a hinged shaft and sliding collar lock),9 the C-Snorkel impacted fetal head release device (the device’s tip contains ventilation ports to facilitate airflow and release of the vacuum/suction created by the impacted fetal head),10 and the Fetal Pillow (a balloon device inserted in the vagina and inflated with sterile saline to disimpact an engaged fetal head before cesarean delivery).11
While all of these techniques can cause injury to the mother and the fetus, available data favor use of the reverse breech extraction (pull) technique, since it is associated with fewer maternal risks, including lower rates of uterine incision extension, infection, and postpartum hemorrhage and a shorter operative time.12−18
Stop use of vacuum to deliver the fetal head at cesarean
Placement of a vacuum can be effective in assisting with delivery of the fetal head at cesarean. For this reason, vacuum-assisted deliveries at cesarean are becoming more common. While the rate of complications caused by vacuum extraction of the fetal head at cesarean is not known, injuries have been reported.19,20 As such, routine use of vacuum extraction at the time of cesarean delivery cannot be recommended.
Start disengaging the fetal head prior to cesarean
One useful technique in planning a cesarean in the second stage of labor or when an impacted fetal head is anticipated is to disengage the fetal head vaginally prior to skin incision. This can be done in the delivery room or in the operating room immediately prior to surgery with the help of an assistant.
While supporting the patient’s legs, the assistant inserts a hand into the vagina and pushes upward on the fetal head with gentle, sustained effort. The assistant should use a cupped hand or the palm of the hand while attempting to both elevate and flex the fetal head. It is best to avoid using 1 or 2 fingers to elevate the head, as this may cause excessive pressure at a single point and lead to injury, such as a skull fracture (FIGURE). The assistant should disengage his or her hand only when the operating surgeon is able to reach down and secure the fetal head from above.

Elevating the fetal head prior to skin incision offers 3 major advantages:
- It avoids the embarrassing situation of having the fetus deliver vaginally before it can be pulled out through the abdominal incision. Although rare, this has been known to happen, because the dense regional anesthesia further relaxes the pelvic floor musculature, leading to flexion and rotation of the fetal head, which then descends and delivers. Performing a final bimanual examination in the operating room after the establishment of surgical level anesthesia and immediately prior to skin incision will avoid this situation.
- It elevates the fetal head, thereby creating additional space between the bony pelvis and fetal presenting part for the provider’s hand to fit. This helps minimize injury to the fetus and to the maternal soft tissues at the time of cesarean.
- Lastly, it provides additional information about the extent to which the fetal head is impacted in the pelvis and may influence decision making around the time of cesarean. For example, if the fetal head were deeply impacted in the pelvis and could not be disimpacted vaginally, the surgeon may choose to make a different uterine incision (such as a low vertical hysterotomy), administer a uterine relaxant (an inhaled anesthetic agent or nitric oxide), ask for additional instrumentation, and/or ask an assistant to be ready to elevate the fetal head vaginally should this be necessary.21
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Rates of cesarean delivery in the second stage of labor have increased dramatically over the past few years.1 Compared with cesarean delivery prior to labor, second-stage labor cesarean is associated with a higher risk to both the mother and the fetus; risks include excessive bleeding, lower uterine segment extensions, injuries to the maternal ureters or bladder, and injury to the fetus.2−4 The risk is increased even further if the fetal head is deeply impacted in the pelvis. What can we do to avoid and manage such situations?
Anticipate an impacted fetal headThe true incidence of an impacted fetal head at the time of cesarean is not known, although a number of risk factors have been described (TABLE). Obstetric care providers should be aware of these risk factors and anticipate the likelihood of a difficult delivery of the fetal head at cesarean.

Options for managing an impacted fetal head at cesareanSeveral techniques have been reported in the literature for managing the delivery of a deeply engaged head, including:
Using an assistant to push the fetus’s head up using a hand in the vagina (“push” technique). This can cause trauma to the fetus, since the force required to push the fetus up from below is uncontrolled.5,6
The reverse breech extraction (“pull” technique) involves pulling the infant out feet first through the uterine incision.7
Use of an instrument. The most common instrument used is a vacuum extractor,8 although a number of other devices have been developed, including the Murless fetal head extractor (an instrument with a hinged shaft and sliding collar lock),9 the C-Snorkel impacted fetal head release device (the device’s tip contains ventilation ports to facilitate airflow and release of the vacuum/suction created by the impacted fetal head),10 and the Fetal Pillow (a balloon device inserted in the vagina and inflated with sterile saline to disimpact an engaged fetal head before cesarean delivery).11
While all of these techniques can cause injury to the mother and the fetus, available data favor use of the reverse breech extraction (pull) technique, since it is associated with fewer maternal risks, including lower rates of uterine incision extension, infection, and postpartum hemorrhage and a shorter operative time.12−18
Stop use of vacuum to deliver the fetal head at cesarean
Placement of a vacuum can be effective in assisting with delivery of the fetal head at cesarean. For this reason, vacuum-assisted deliveries at cesarean are becoming more common. While the rate of complications caused by vacuum extraction of the fetal head at cesarean is not known, injuries have been reported.19,20 As such, routine use of vacuum extraction at the time of cesarean delivery cannot be recommended.
Start disengaging the fetal head prior to cesarean
One useful technique in planning a cesarean in the second stage of labor or when an impacted fetal head is anticipated is to disengage the fetal head vaginally prior to skin incision. This can be done in the delivery room or in the operating room immediately prior to surgery with the help of an assistant.
While supporting the patient’s legs, the assistant inserts a hand into the vagina and pushes upward on the fetal head with gentle, sustained effort. The assistant should use a cupped hand or the palm of the hand while attempting to both elevate and flex the fetal head. It is best to avoid using 1 or 2 fingers to elevate the head, as this may cause excessive pressure at a single point and lead to injury, such as a skull fracture (FIGURE). The assistant should disengage his or her hand only when the operating surgeon is able to reach down and secure the fetal head from above.

Elevating the fetal head prior to skin incision offers 3 major advantages:
- It avoids the embarrassing situation of having the fetus deliver vaginally before it can be pulled out through the abdominal incision. Although rare, this has been known to happen, because the dense regional anesthesia further relaxes the pelvic floor musculature, leading to flexion and rotation of the fetal head, which then descends and delivers. Performing a final bimanual examination in the operating room after the establishment of surgical level anesthesia and immediately prior to skin incision will avoid this situation.
- It elevates the fetal head, thereby creating additional space between the bony pelvis and fetal presenting part for the provider’s hand to fit. This helps minimize injury to the fetus and to the maternal soft tissues at the time of cesarean.
- Lastly, it provides additional information about the extent to which the fetal head is impacted in the pelvis and may influence decision making around the time of cesarean. For example, if the fetal head were deeply impacted in the pelvis and could not be disimpacted vaginally, the surgeon may choose to make a different uterine incision (such as a low vertical hysterotomy), administer a uterine relaxant (an inhaled anesthetic agent or nitric oxide), ask for additional instrumentation, and/or ask an assistant to be ready to elevate the fetal head vaginally should this be necessary.21
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Spencer C, Murphy D, Bewley S. Caesarean delivery in the second stage of labour. BMJ. 2006;333(7569):613–614.
- Häger RM, Daltviet AK, Hofoss D, et al. Complications of cesarean deliveries: rates and risk factors. Am J Obstet Gynecol. 2004;190(2):428–434.
- Murphy DJ, Liebling RE, Verity L, Swingler R, Patel R. Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: a cohort study. Lancet. 2001;358(9289):1203–1207.
- Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
- Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the midcavity. Arch Gynecol. 1983;234(1):59–60.
- Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707–710.
- Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynaecol Obstet. 1997;56(2):183–184.
- Arad I, Linder N, Bercovici B. Vacuum extraction at cesarean section—neonatal outcome. J Perinat Med. 1986;14(2):137–140.
- Murless BC. Lower-segment caesarean section; a new head extractor. BMJ. 1948;1(4564):1234.
- C-Snorkle impacted fetal head release device. Clinical Innovations website. http://clinicalinnovations.com /portfolio-items/c-snorkel/. Accessed July 22, 2016.
- Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J, Onwude JL. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133(2):178–182.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375–378.
- Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):24–26.
- Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scand. 2009;88(10):1163–1166.
- Veisi F, Zangeneh M, Malekkhosravi S, Rezavand N. Comparison of “push” and “pull” methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):4–6.
- Bastani P, Pourabolghasem S, Abbasalizadeh F, Motvalli L. ComparisonColor/Black of neonatal and maternal outcomes associated with head-pushing and head-pulling methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):1–3.
- Waterfall H, Grivell RM, Dodd JM. Techniques for assisting difficult delivery at caesarean section. Cochrane Database Syst Rev. 2016;1:CD004944.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123(3): 337–345.
- Clark SL, Vines VL, Belfort MA. Fetal injury associated with routine vacuum use during cesarean delivery. Am J Obstet Gynecol. 2008;198(4):e4.
- Fareeduddin R, Schifrin BS. Subgaleal hemorrhage after the use of a vacuum extractor during elective cesarean delivery: a case report. J Reprod Med. 2008;53(10):809–810.
- Barbieri RL. Difficult fetal extraction at cesarean delivery: What should you do? OBG Manag. 2012;24(1):8–12.
- Spencer C, Murphy D, Bewley S. Caesarean delivery in the second stage of labour. BMJ. 2006;333(7569):613–614.
- Häger RM, Daltviet AK, Hofoss D, et al. Complications of cesarean deliveries: rates and risk factors. Am J Obstet Gynecol. 2004;190(2):428–434.
- Murphy DJ, Liebling RE, Verity L, Swingler R, Patel R. Early maternal and neonatal morbidity associated with operative delivery in second stage of labour: a cohort study. Lancet. 2001;358(9289):1203–1207.
- Pergialiotis V, Vlachos DG, Rodolakis A, Haidopoulos D, Thomakos N, Vlachos GD. First versus second stage C/S maternal and neonatal morbidity: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;175:15–24.
- Lippert TH. Bimanual delivery of the fetal head at cesarean section with the fetal head in the midcavity. Arch Gynecol. 1983;234(1):59–60.
- Landesman R, Graber EA. Abdominovaginal delivery: modification of the cesarean section operation to facilitate delivery of the impacted head. Am J Obstet Gynecol. 1984;148(6):707–710.
- Fong YF, Arulkumaran S. Breech extraction—an alternative method of delivering a deeply engaged head at cesarean section. Int J Gynaecol Obstet. 1997;56(2):183–184.
- Arad I, Linder N, Bercovici B. Vacuum extraction at cesarean section—neonatal outcome. J Perinat Med. 1986;14(2):137–140.
- Murless BC. Lower-segment caesarean section; a new head extractor. BMJ. 1948;1(4564):1234.
- C-Snorkle impacted fetal head release device. Clinical Innovations website. http://clinicalinnovations.com /portfolio-items/c-snorkel/. Accessed July 22, 2016.
- Seal SL, Dey A, Barman SC, Kamilya G, Mukherji J, Onwude JL. Randomized controlled trial of elevation of the fetal head with a fetal pillow during cesarean delivery at full cervical dilatation. Int J Gynaecol Obstet. 2016;133(2):178–182.
- Fasubaa OB, Ezechi OC, Orji EO, et al. Delivery of the impacted head of the fetus at caesarean section after prolonged obstructed labour: a randomised comparative study of two methods. J Obstet Gynaecol. 2002;22(4):375–378.
- Levy R, Chernomoretz T, Appelman Z, Levin D, Or Y, Hagay ZJ. Head pushing versus reverse breech extraction in cases of impacted fetal head during Cesarean section. Eur J Obstet Gynecol Reprod Biol. 2005;121(1):24–26.
- Chopra S, Bagga R, Keepanasseril A, Jain V, Kalra J, Suri V. Disengagement of the deeply engaged fetal head during cesarean section in advanced labor: conventional method versus reverse breech extraction. Acta Obstet Gynecol Scand. 2009;88(10):1163–1166.
- Veisi F, Zangeneh M, Malekkhosravi S, Rezavand N. Comparison of “push” and “pull” methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):4–6.
- Bastani P, Pourabolghasem S, Abbasalizadeh F, Motvalli L. ComparisonColor/Black of neonatal and maternal outcomes associated with head-pushing and head-pulling methods for impacted fetal head extraction during cesarean delivery. Int J Gynaecol Obstet. 2012;118(1):1–3.
- Waterfall H, Grivell RM, Dodd JM. Techniques for assisting difficult delivery at caesarean section. Cochrane Database Syst Rev. 2016;1:CD004944.
- Jeve YB, Navti OB, Konje JC. Comparison of techniques used to deliver a deeply impacted fetal head at full dilation: a systematic review and meta-analysis. BJOG. 2016;123(3): 337–345.
- Clark SL, Vines VL, Belfort MA. Fetal injury associated with routine vacuum use during cesarean delivery. Am J Obstet Gynecol. 2008;198(4):e4.
- Fareeduddin R, Schifrin BS. Subgaleal hemorrhage after the use of a vacuum extractor during elective cesarean delivery: a case report. J Reprod Med. 2008;53(10):809–810.
- Barbieri RL. Difficult fetal extraction at cesarean delivery: What should you do? OBG Manag. 2012;24(1):8–12.
In this Article
- Risk factors for impacted fetal head
- Advantages to elevating fetal head
Protecting the newborn brain—the final frontier in obstetric and neonatal care
During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.
The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.
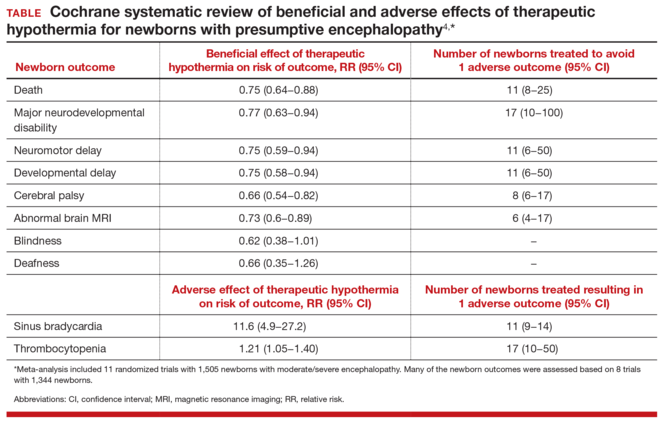
Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.
During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.

The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.

Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

During the past 40 years neonatologists have discovered new treatments to improve pulmonary and cardiovascular care of preterm newborns, resulting in a dramatic reduction in newborn mortality and childhood morbidity. Important advances include glucocorticoid administration to mothers at risk for preterm birth, surfactant and nitric oxide administration to the newborn, kangaroo (or skin-to-skin) care, continuous positive airway pressure, and high-frequency ventilation.1 In 1960, only 5% of 1,000-g newborns survived. In 2000, 95% of 1,000-g newborns survive.1
The successes in pulmonary and cardiovascular care have revealed a new frontier in neonatal care: the prevention of long-term neurologic disability by the early treatment of newborn encephalpathy with therapeutic hypothermia. This novel undertaking is an important one; approximately 1 in 300 newborns are diagnosed with encephalopathy.2
Until recently there were no proven treatments for newborns with encephalopathy. However, therapeutic hypothermia now has been proven to be an effective intervention for the treatment of moderate and severe encephalopathy,3,4 and its use is expanding to include mild cases.
This increased use can lead to more complex situations arising for obstetricians, for when a neonatologist decides to initiate therapeutic hypothermia of a newborn the parents may wonder if the obstetrician’s management of labor and delivery was suboptimal, contributing to their baby’s brain injury.
Therapeutic hypothermia: The basics
First, we need to define therapeutic hypothermia. Both head hypothermia and whole-body hypothermia are effective techniques for the treatment of newborn encephalopathy.3,4 Most centers use whole-body (FIGURE) rather than head, hypothermia because it facilitates access to the head for placement of electroencephalogram (EEG) sensors.

The key principles of therapeutic hypothermia include5,6:
- Initiate hypothermia within 6 hours of birth.
- Cool the newborn to a core temperature of 33.5° to 34.5°C (92.3° to 94.1°F). Some centers focus on achieving consistent core temperatures of 33.5°C (92.3°F).
- Monitor core temperature every 5 to 15 minutes.
- Cool the newborn for 72 hours.
- Obtain head ultrasonography to detect intracranial hemorrhage.
- Initiate continuous or intermittent EEG monitoring.
- Treat seizures with phenobarbital, lorazepam, or phenytoin.
- Obtain blood cultures, a complete blood count, blood gas concentrations, alactate coagulation profile, and liver function tests.
- Sedate the newborn, if necessary.
- Minimize oral feedings during the initial phase of hypothermia.
- Obtain sequential magnetic resonance imaging (MRI) studies to assess brain structure and function.
- For all newborns with suspected encephalopathy, the placenta should be sent to pathology for histologic study.7
The data on therapy effectivenessTwo recent meta-analyses independently reported that therapeutic hypothermia reduced the risk of newborn death and major neurodevelopmental disability.3,4 The Cochrane meta-analysis reported that the therapy reduced the risk of neuromotor delay, developmental delay, cerebral palsy, and abnormal MRI results (TABLE).4 The study authors also reported that therapeutic hypothermia reduced the risk of blindness and deafness, although these effects did not reach statistical significance.4 Therapeutic hypothermia did increase the risk of newborn sinus bradycardia and thrombocytopenia.3,4 Compared with usual care, the therapy increased the average survival rate with a normal neurologic outcome at 18 months from 23% to 40%.3 It should be noted that even with therapeutic hypothermia treatment, many newborns with moderate to severe encephalopathy have long-term neurologic disabilities.

Indications for therapeutic hypothermia are expandingIn the initial clinical trials of therapeutic hypothermia, newborns with moderate to severe encephalopathy were enrolled. Typical inclusion criteria were: gestational age ≥35 or 36 weeks, initiation of therapeutic hypothermia within 6 hours of birth, pH ≤7.0 or base deficit of ≥16 mEq/L, 10-minute Apgar score <5 or ongoing resuscitation for 10 minutes, and moderate to severe encephalopathy on clinical examination.3,4 Typical exclusion criteria were: intrauterine growth restriction with birth weight less than 1,750 g, severe congenital anomalies or severe genetic or metabolic syndromes, major intracranial hemorrhage, sepsis, or persistent coagulopathy.
Given the success of therapeutic hypothermia for moderate to severe newborn encephalopathy, many neonatologists are expanding the indications for treatment. In some centers current indications for initiation of hypothermia include the following:
- gestational age ≥34 weeks
- suspicion of encephalopathy or a seizure event
- any obstetric sentinel event (including a bradycardia, umbilical cord prolapse, uterine rupture, placental abruption, Apgar score ≤5 at 10 minutes, pH ≤7.1 or base deficit of ≥10 mEq/L or Category III tracing, or fetal tachycardia with recurrent decelerations or fetal heart rate with minimal variability and recurrent decelerations).
Suspicion for encephalopathy might be triggered by any of a large number of newborn behaviors: lethargy, decreased activity, hypotonia, weak suck or incomplete Moro reflexes, constricted pupils, bradycardia, periodic breathing or apnea, hyperalertness, or irritability.8
Coordinate neonatology and obstetric communication with the familyGiven the expanding indications for therapeutic hypothermia, an increasing number of newborns will receive this treatment. This scenario makes enhanced communication vital. Consider this situation:
CASE Baby rushed for therapeutic hypothermia upon birthA baby is born limp and blue without a cry. Her hypotonia raises a concern for encephalopathy, and she is whisked off to the neonatal intensive care unit for 72 hours of therapeutic hypothermia. Stunned, the parents begin to wonder, “Will our baby be O.K.?” and “What went wrong?”
When neonatologists recommend therapeutic hypothermia for the newborn with presumptive encephalopathy, they may explain the situation to the parents with words such as brain injury, encephalopathy, hypoxia, and ischemia. Intrapartum events such as a Category II or III fetal heart rate tracing, operative vaginal delivery, or maternal sepsis or abruption might be mentioned as contributing factors. A consulting neurologist may mention injury of the cerebral cortex, subcortical white matter, or lateral thalami. The neonatologists and neurologists might not mention that less than 50% of cases of newborn encephalopathy are thought to be due to the management of labor.2
The obstetrician, as stunned by the events as the parents, may be at a loss about how to communicate effectively with their patient about the newborn’s encephalopathy. Obstetricians can help assure the parents of their continued involvement in the care and reinforce that the hospital’s neonatologists are superb clinicians who will do their best for the baby.
Challenges exist to effective communication. It is often difficult to optimally coordinate and align the communications of the neonatologists, neurologist, nurses, and obstetrician with the family. Communication with the family can be uncoordinated because interactions occur between the family and multiple specialists with unique perspectives and vocabularies. These conversations occur in sequence, separated in time and place. The communication between family and neonatologists typically occurs in the neonatal intensive care unit. Interactions between obstetrician and mother typically occur in the postpartum unit. The neonatologists and obstetricians are assigned to the hospital in rotating coverage shifts, increasing the number of hand-offs and physicians involved in the hospital care of the mother and newborn dyad.
A joint family meeting with the neonatologists, obstetrician, and family early in the course of newborn care might be an optimal approach to coordinating communication with the parents. Conflicting obligations certainly may make a joint meeting difficult to arrange, however.
Reducing the risk of permanent injury to the central and peripheral nervous system of the newborn is the goal of all obstetricians and neonatologists. Many authorities believe that therapeutic hypothermia can reduce the risk of death and major neurodevelopmental disorders in newborns with encephalopathy. Initial data are promising. If long-term follow-up studies prove that this therapy reduces neurologic disability, the treatment represents a major advance in maternal-child care. As we learn more about this novel, and potentially effective therapy, it should be on the minds of those involved with newborn care to involve the ObGyn in coordinated communication with the family and other medical staff.

- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.
- Philip AG. The evolution of neonatology. Pediatr Res. 2005;58(4):799−815.
- Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischemic encephalopathy. Early Hum Dev. 2010;86(6):329−338.
- Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558−566.
- Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Syst Rev. 2013;(1):CD003311.
- Papile LA, Baley JE, Benitz W, et al; American Academy of Pediatrics Committee on Fetus and Newborn. Hypothermia and neonatal encephalopathy. Pediatrics. 2014;133(6):1146−1150.
- Azzopardi D, Strohm B, Edwards AD, et al; Steering Group and TOBY Cooling Register participants. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: how cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed. 2009;94(4):F260−F264.
- Mir IN, Johnson-Welch SF, Nelson DB, Brown LS, Rosenfeld CR, Chalak LF. Placental pathology is associated with severity of neonatal encephalopathy and adverse developmental outcomes following hypothermia. Am J Obstet Gynecol. 2015;213(6):849.e1−e7.
- Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 1997;86(7):757−761.