User login
Vaginal cleansing protocol curbs deep SSIs after cesarean
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
reported Johanna Quist-Nelson, MD, of the University of North Carolina, Chapel Hill.
“Surgical site infections after a cesarean delivery are more common if the patient is in labor or has ruptured membranes,” she said at the 2020 virtual meeting of the American College of Obstetricians and Gynecologists..
Two options to decrease the risk of SSIs after cesarean for those patients in labor or with ruptured membranes are vaginal cleansing and azithromycin, given in addition to preoperative antibiotics, Dr. Quist-Nelson said. She and her colleagues conducted a quality improvement study of the effects of a stepwise implementation of vaginal cleansing and azithromycin to reduce SSIs at cesarean delivery in this high-risk population. The data were collected from 2016 to 2019 at Thomas Jefferson University, Philadelphia.
“We aimed to decrease our SSI rate by 30% by adopting an intervention of cleansing followed by azithromycin,” she said.
The researchers added vaginal cleansing to the SSI prevention protocol in January 2017, with the addition of azithromycin in March 2018. Vaginal cleansing involved 30 seconds of anterior to posterior cleaning prior to urinary catheter placement. Azithromycin was given at a dose of 500 mg intravenously in addition to preoperative antibiotics and within an hour of cesarean delivery.
A total of 1,033 deliveries qualified for the study by being in labor or with ruptured membranes; of these 291 were performed prior to the interventions, 335 received vaginal cleansing only, and 407 received vaginal cleansing and azithromycin. The average age of the participants was 30 years; approximately 42% were Black, and 32% were White.
Cleansing protocol reduces SSIs
Overall, the rate of SSIs was 22% in the standard care group, 17% in the vaginal cleansing group, and 15% in the vaginal cleansing plus azithromycin group. When broken down by infection type, no deep SSI occurred in the vaginal cleansing or cleansing plus azithromycin group, compared with 2% of the standard care group (P = .009). In addition, endometritis, which is an organ-space SSI, was significantly lower in the cleansing group (10%) and the cleansing plus azithromycin group (11%), compared with the standard care group (16%).
The study findings were limited by factors including the use of EMRs for collection of data, and given that it is a quality improvement study, there is a potential lack of generalizability to other institutions. The study focused on patients at high risk for SSI and the use of the Plan-Do-Study-Act (PDSA) method of conducting the research, Dr. Quist-Nelson said. Compared with standard care, the implementation of vaginal cleansing reduced the SSI rate by 33%, with no significantly further change in SSI after the addition of azithromycin, she concluded.
Data sharing boosts compliance
In a question-and-answer session, Dr. Quist-Nelson noted that povidone iodine (Betadine) was chosen for vaginal cleansing because it was easily accessible at her institution, but that patients with allergies were given chlorhexidine. The cleansing itself was “primarily vaginal, not a full vulvar cleansing,” she clarified. The cleansing was performed immediately before catheter placement and included the urethra.
When asked about strategies to increase compliance, Dr. Quist-Nelson noted that sharing data was valuable, namely “reporting to our group the current compliance,” as well as sharing information by email and discussing it during multidisciplinary rounds.
The study was a quality improvement project and not a randomized trial, so the researchers were not able to tease out the impact of vaginal cleansing from the impact of azithromycin, Dr. Quist-Nelson said.
Based on her results, Dr. Quist-Nelson said she would recommend the protocol for use in patients who require cesarean delivery after being in labor or having ruptured membranes, and that “there are trials to support the use of both interventions.”
The results suggest opportunities for further randomized trials, including examination of the use of oral versus IV azithromycin, she added.
The study received no outside funding. Dr. Quist-Nelson had no financial conflicts to disclose.
FROM ACOG 2020
Treating insomnia, anxiety in a pandemic
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
Since the start of the pandemic, we have been conducting an extra hour of Virtual Rounds at the Center for Women’s Mental Health. Virtual Rounds has been an opportunity to discuss cases around a spectrum of clinical management issues with respect to depression, bipolar disorder, and a spectrum of anxiety disorders like obsessive-compulsive disorder (OCD), posttraumatic stress disorder (PTSD), and generalized anxiety disorder. How to apply the calculus of risk-benefit decision-making around management of psychiatric disorder during pregnancy and the postpartum period has been the cornerstone of the work at our center for over 2 decades.
When we went virtual at our center in the early Spring, we decided to keep the format of our faculty rounds the way they have been for years and to sustain cohesiveness of our program during the pandemic. But we thought the needs of pregnant and postpartum women warranted being addressed in a context more specific to COVID-19, and also that reproductive psychiatrists and other clinicians could learn from each other about novel issues coming up for this group of patients during the pandemic. With that backdrop, Marlene Freeman, MD, and I founded “Virtual Rounds at the Center” to respond to queries from our colleagues across the country; we do this just after our own rounds on Wednesdays at 2:00 p.m.
As the pandemic has progressed, Virtual Rounds has blossomed into a virtual community on the Zoom platform, where social workers, psychologists, nurse prescribers, psychiatrists, and obstetricians discuss the needs of pregnant and postpartum women specific to COVID-19. Frequently, our discussions involve a review of the risks and benefits of treatment before, during, and after pregnancy.
Seemingly, week to week, more and more colleagues raise questions about the treatment of anxiety and insomnia during pregnancy and the postpartum period. I’ve spoken in previous columns about the enhanced use of telemedicine. Telemedicine not only facilitates efforts like Virtual Rounds and our ability to reach out to colleagues across the country and share cases, but also has allowed us to keep even closer tabs on the emotional well-being of our pregnant and postpartum women during COVID-19.
The question is not just about the effects of a medicine that a woman might take to treat anxiety or insomnia during pregnancy, but the experience of the pandemic per se, which we are measuring in multiple studies now using a variety of psychological instruments that patients complete. The pandemic is unequivocally taking a still unquantified toll on the mental health of Americans and potentially on the next generation to come.
Midcycle awakening during pregnancy
Complaints of insomnia and midcycle awakening during pregnancy are not new – it is the rule, rather than the exception for many pregnant women, particularly later in pregnancy. We have unequivocally seen a worsening of complaints of sleep disruption including insomnia and midcycle awakening during the pandemic that is greater than what we have seen previously. Both patients and colleagues have asked us the safest ways to manage it. One of the first things we consider when we hear about insomnia is whether it is part of an underlying mood disorder. While we see primary insomnia clinically, it really is important to remember that insomnia can be part and parcel of an underlying mood disorder.
With that in mind, what are the options? During the pandemic, we’ve seen an increased use of digital cognitive behavioral therapy for insomnia (CBT-I) for patients who cannot initiate sleep, which has a very strong evidence base for effectiveness as a first-line intervention for many.
If a patient has an incomplete response to CBT-I, what might be pursued next? In our center, we have a low threshold for using low doses of benzodiazepines, such as lorazepam or clonazepam, because the majority of data do not support an increased risk of major congenital malformations even when used in the first trimester. It is quite common to see medicines such as newer nonbenzodiazepine sedative hypnotics such as Ambien CR (zolpidem) or Lunesta (eszopiclone) used by our colleagues in ob.gyn. The reproductive safety data on those medicines are particularly sparse, and they may have greater risk of cognitive side effects the next day, so we tend to avoid them.
Another sometimes-forgotten option to consider is using low doses of tricyclic antidepressants (i.e., 10-25 mg of nortriptyline at bedtime), with tricyclics having a 40-year history and at least one pooled analysis showing the absence of increased risk for major congenital malformations when used. This may be a very easy way of managing insomnia, with low-dose tricyclics having an anxiolytic effect as well.
Anxiety during pregnancy
The most common rise in symptoms during COVID-19 for women who are pregnant or post partum has been an increase in anxiety. Women present with a spectrum of concerns leading to anxiety symptoms in the context of the pandemic. Earlier on in the pandemic, concerns focused mostly on how to stay healthy, and how to mitigate risk and not catch SARS-CoV-2 during pregnancy, as well as the very complex issues that were playing out in real time as hospital systems were figuring out how to manage pregnant women in labor and to keep both them and staff safe. Over time, anxiety has shifted to still staying safe during the pandemic and the potential impact of SARS-CoV-2 infection on pregnancy outcomes. The No. 1 concern is what the implications of COVID-19 disease are on mother and child. New mothers also are anxious about how they will practically navigate life with a newborn in the postpartum setting.
Early on in the pandemic, some hospital systems severely limited who was in the room with a woman during labor, potentially impeding the wishes of women during delivery who would have wanted their loved ones and/or a doula present, as an example. With enhanced testing available now, protocols have since relaxed in many hospitals to allow partners – but not a team – to remain in the hospital during the labor process. Still, the prospect of delivering during a pandemic is undoubtedly a source of anxiety for some women.
This sort of anxiety, particularly in patients with preexisting anxiety disorders, can be particularly challenging. Fortunately, there has been a rapid increase over the last several years of digital apps to mitigate anxiety. While many of them have not been systematically studied, the data on biobehavioral intervention for anxiety is enormous, and this should be used as first-line treatment for patients with mild to moderate symptoms; so many women would prefer to avoid pharmacological intervention during pregnancy, if possible, to avoid fetal drug exposure. For patients who meet criteria for frank anxiety disorder, other nonpharmacologic interventions such as CBT have been shown to be effective.
Frequently, we see women who are experiencing levels of anxiety where nonpharmacological interventions have an incomplete response, and colleagues have asked about the safest way to treat these patients. As has been discussed in multiple previous columns, selective serotonin reuptake inhibitors (SSRIs) should be thought of sooner rather than later, particularly with medicines with good reproductive safety data such as sertraline, citalopram, or fluoxetine.
We also reported over 15 years ago that at least 30%-40% of women presenting with histories of recurrent major depression at the beginning of pregnancy had comorbid anxiety disorders, and that the use of benzodiazepines in that population in addition to SSRIs was exceedingly common, with doses of approximately 0.5-1.5 mg of clonazepam or lorazepam being standard fare. Again, this is very appropriate treatment to mitigate anxiety symptoms because now have enough data as a field that support the existence of adverse outcomes associated with untreated anxiety during pregnancy in terms of both adverse obstetric and neonatal outcomes, higher rates of preterm birth, and other obstetric complications. Hence, managing anxiety during pregnancy should be considered like managing a toxic exposure – the same way that one would be concerned about anything else that a pregnant woman could be exposed to.
Lastly, although no atypical antipsychotic has been approved for the treatment of anxiety, its use off label is extremely common. More and more data support the absence of a signal of teratogenicity across the family of molecules including atypical antipsychotics. Beyond potential use of atypical antipsychotics, at Virtual Rounds last week, a colleague asked about the use of gabapentin in a patient who was diagnosed with substance use disorder and who had inadvertently conceived on gabapentin, which was being used to treat both anxiety and insomnia. We have typically avoided the use of gabapentin during pregnancy because prospective data have been limited to relatively small case series and one report, with a total of exposures in roughly the 300 range.
However, our colleagues at the Harvard School of Public Health have recently published an article that looked at the United States Medicaid Analytic eXtract (MAX) dataset, which has been used to publish other articles addressing atypical antipsychotics, SSRIs, lithium, and pharmacovigilance investigations among other important topics. In this study, the database was used to look specifically at 4,642 pregnancies with gabapentin exposure relative to 1,744,447 unexposed pregnancies, without a significant finding for increased risk for major congenital malformations.
The question of an increased risk of cardiac malformations and of increased risk for obstetric complications are difficult to untangle from anxiety and depression, as they also are associated with those same outcomes. With that said, the analysis is a welcome addition to our knowledge base for a medicine used more widely to treat symptoms such as anxiety and insomnia in the general population, with a question mark around where it may fit into the algorithm during pregnancy.
In our center, gabapentin still would not be used as a first-line treatment for the management of anxiety or insomnia during pregnancy. But these new data still are reassuring for patients who come in, frequently with unplanned pregnancies. It is an important reminder to those of us taking care of patients during the pandemic to review use of contraception, because although data are unavailable specific to the period of the pandemic, what is clear is that, even prior to COVID-19, 50% of pregnancies in America were unplanned. Addressing issues of reliable use of contraception, particularly during the pandemic, is that much more important.
In this particular case, our clinician colleague in Virtual Rounds decided to continue gabapentin across pregnancy in the context of these reassuring data, but others may choose to discontinue or pursue some of the other treatment options noted above.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email Dr. Cohen at [email protected].
The fourth trimester: Achieving improved postpartum care
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.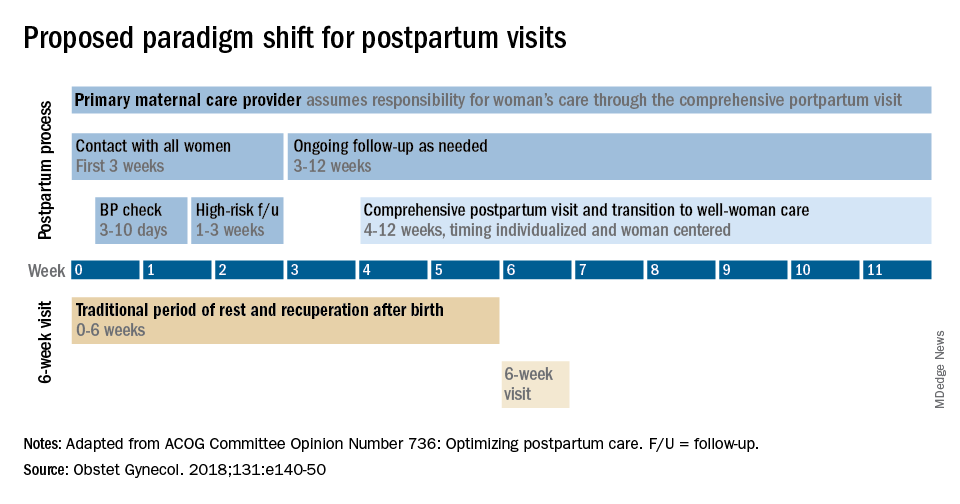
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
The field of ob.gyn. has long focused significantly more attention on the prenatal period – on determining the optimal frequency of ultrasound examinations, for instance, and on screening for diabetes and other conditions – than on women’s health and well-being after delivery.
The traditional 6-week postpartum visit has too often been a quick and cursory visit, with new mothers typically navigating the preceding postpartum transitions on their own.
The need to redefine postpartum care was a central message of Haywood Brown, MD, who in 2017 served as the president of the America College of Obstetricians and Gynecologists. Dr. Brown established a task force whose work resulted in important guidance for taking a more comprehensive and patient-centered approach to postpartum care.1
Improved care in the “fourth trimester,” as it has come to be known, is comprehensive and includes ensuring that our patients have a solid transition to health care beyond the pregnancy. We also hope that it will help us to reduce maternal mortality, given that more than half of pregnancy-related deaths occur after delivery.
Timing and frequency of contact
Historically, we’ve had a single 6-week postpartum visit, with little or no maternal support or patient contact before this visit unless the patient reported a complication. In the new paradigm, as described in the ACOG committee opinion on optimizing postpartum care, maternal care should be an ongoing process.1
so that any questions or concerns may be addressed and support can be provided.
This should be followed by individualized, ongoing care until a comprehensive postpartum visit covering physical, social, and psychological well-being is conducted by 12 weeks after birth – anytime between 4 and 12 weeks.
By stressing the importance of postpartum care during prenatal visits – and by talking about some of its key elements such as mental health, breastfeeding, and chronic disease management – we can let our patients know that postpartum care is not just an afterthought, but that it involves planning backed by evidence and expert opinion. Currently, as many as 40% of women do not attend a postpartum visit; early discussion, it is hoped, will increase attendance.
Certain high-risk groups should be seen or screened earlier than 3 weeks post partum. For instance, women who have hypertensive disorders of pregnancy should be evaluated no later than 7-10 days post partum, and women with severe hypertension should be seen within 72 hours, according to ACOG.
Early blood pressure checks – and follow-up as necessary – are critical for reducing the risk of postpartum stroke and other complications. I advocate uniformly checking blood pressure within several days after hospital discharge for all women who have hypertension at the end of their pregnancy.
Other high-risk conditions requiring early follow-up include diabetes and autoimmune conditions such as lupus, multiple sclerosis, and psoriasis that may flare in the postpartum period. Women with a history of postpartum depression similarly may benefit from early contact; they are at higher risk of having depression again, and there are clearly effective treatments, both medication and psychotherapy based.
In between the initial early contact (by 7-10 days post partum or by 3 weeks post partum) and the comprehensive visit between 4 and 12 weeks, the need for and timing of patient contact can be individualized. Some women will need only a brief contact and a visit at 8-10 weeks, while others will need much more. Our goal, as in all of medicine, is to provide individualized, patient-centered care.
Methods of contact
With the exception of the final comprehensive visit, postpartum care need not occur in person. Some conditions require an early office visit, but in general, as ACOG states, the usefulness of an in-person visit should be weighed against the burden of traveling to and attending that visit.
For many women, in-person visits are difficult, and we must be creative in utilizing telemedicine and phone support, text messaging, and app-based support. Having practiced during this pandemic, we are better positioned than ever before to make it relatively easy for new mothers to obtain ongoing postpartum care.
Notably, research is demonstrating that the use of technology may allow us to provide improved care and monitoring of hypertension in the postpartum period. For example, a randomized trial published in 2018 of over 200 women with a hypertensive disorder of pregnancy found that text-based surveillance with home blood pressure monitoring was more effective than usual in-person blood pressure checks in meeting clinical guidelines for postpartum monitoring.2
Women in the texting group were significantly more likely to have a single blood pressure obtained in the first 10 days post partum than women in the office group.
Postpartum care is also not a completely physician-driven endeavor. Much of what is needed to help women successfully navigate the fourth trimester can be provided by certified nurse midwives, advanced practice nurses, and other members of our maternal care teams.
Components of postpartum care
The postpartum care plan should be comprehensive, and having a checklist to guide one through initial and comprehensive visits may be helpful. The ACOG committee opinion categorizes the components of postpartum care into seven domains: mood and emotional well-being; infant care and feeding; sexuality, contraception, and birth spacing; sleep and fatigue; physical recovery from birth; chronic disease management; and health maintenance.1
The importance of screening for depression and anxiety cannot be emphasized enough. Perinatal depression is highly prevalent: It affects as many as one in seven women and can result in adverse short- and long-term effects on both the mother and child.
The U.S. Preventive Services Task Force has offered guidance for years, most recently in 2019 with its recommendations that clinicians refer pregnant and postpartum women who are at increased risk for depression to counseling interventions such as cognitive behavioral therapy and interpersonal therapy.3 There is evidence that some form of treatment for women who screen positive reduces the risk of perinatal depression.
Additionally, there is emerging evidence that postpartum PTSD may be as prevalent as postpartum depression.4 As ACOG points out, trauma is “in the eye of the beholder,” and an estimated 3%-16% of women have PTSD related to a traumatic birth experience. Complications like shoulder dystocia or postpartum hemorrhage, in which delivery processes rapidly change course, can be experienced as traumatic by women even though they and their infants are healthy. The risk of posttraumatic stress should be on our radar screen.
Interpregnancy intervals similarly are not discussed enough. We do not commonly talk to patients about how pregnancy and breastfeeding are nutritionally depleting and how it takes time to replenish these stores – yet birth spacing is so important.
Compared with interpregnancy intervals of at least 18 months, intervals shorter than 6 months were associated in a meta-analysis with increased risks of preterm birth, low birth weight, and small for gestational age.5 Optimal birth spacing is one of the few low-cost interventions available for reducing pregnancy complications in the future.
Finally, that chronic disease management is a domain of postpartum care warrants emphasis. We must work to ensure that patients have a solid plan of care in place for their diabetes, hypertension, lupus, or other chronic conditions. This includes who will provide that ongoing care, as well as when medical management should be restarted.
Some women are aware of the importance of timely care – of not waiting for 12 months, for instance, to see an internist or specialist – but others are not.
Again, certain health conditions such as multiple sclerosis and RA necessitate follow-up within a couple weeks after delivery so that medications can be restarted or dose adjustments made. The need for early postpartum follow-up can be discussed during prenatal visits, along with anticipatory guidance about breastfeeding, the signs and symptoms of perinatal depression and anxiety, and other components of the fourth trimester.
Dr. Macones has no relevant financial disclosures.
References
1. Obstet Gynecol. 2018 May;131(5):e140-50.
2. BMJ Qual Saf. 2018 Apr 27;27(11):871-7.
3. JAMA. 2019 Feb 12;321(6):580-7.
4. Clin Psychol Rev. 2014 Jul;34:389-401.JAMA. 2006 Apr 19;295(15):1809-23.
The fourth trimester
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
*This version has been updated to correct an erroneous byline, photo, and bio.
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
*This version has been updated to correct an erroneous byline, photo, and bio.
As we approach the end of this year, one of the most surreal times in human history, we will look back on the many things we taught ourselves, the many things we took for granted, the many things we were grateful for, the many things we missed, and the many things we plan to do once we can do things again. Among the many things 2020 taught us to appreciate was the very real manifestation of the old adage, “prevention is the best medicine.” To prevent transmission of SARS-CoV-2, we wore masks, we sanitized everything, we avoided crowds, we traded in-person meetings for virtual meetings, we learned how to homeschool our children, and we delayed seeing relatives and friends.
Ob.gyns. in small and large practices around the world had the tremendous challenge of balancing necessary in-person prenatal care services with keeping their patients and babies safe. Labor and delivery units had even greater demands to keep women and neonates free of SARS-CoV-2 infection. Practices quickly put into place new treatment protocols and new management strategies to maintain the health of their staff while ensuring a high quality of care.
While we have focused much of our attention on greater precautions during pregnancy and childbirth, an important component of care is the immediate postpartum period – colloquially referred to as the “fourth trimester” – which remains critical to maintaining physical and mental health and well-being.
Despite concerns regarding COVID-19 safety, we should continue monitoring our patients during these crucial first weeks after childbirth. This year of social isolation, financial strain, and incredible uncertainty has created additional stress in many women’s lives. The usual support that some women would receive from family members, friends, and other mothers in the early days post partum may not be available. The pandemic also has further highlighted inequities in access to health care for vulnerable groups. In addition, restrictions have increased the incidence of intimate partner violence as many women and children have needed to shelter with their abusers. Perhaps now more than any time previously, ob.gyns. must be attuned to their patients’ needs and be ready to provide compassionate and sensitive care.
In this final month of the year, we have invited George A. Macones, MD, professor and chair of the department of women’s health at the University of Texas, Austin, to address the importance of care in the final “trimester” of pregnancy – the first 3 months post partum.
Dr. Reece, who specializes in maternal-fetal medicine, is executive vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. He is the medical editor of this column. He said he had no relevant financial disclosures. Contact him at [email protected].
*This version has been updated to correct an erroneous byline, photo, and bio.
Metformin improves most outcomes for T2D during pregnancy
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
including reduced weight gain, reduced insulin doses, and fewer large-for-gestational-age babies, suggest the results of a randomized controlled trial.
However, the drug was associated with an increased risk of small-for-gestational-age babies, which poses the question as to risk versus benefit of metformin on the health of offspring.
“Better understanding of the short- and long-term implications of these effects on infants will be important to properly advise patients with type 2 diabetes contemplating use of metformin during pregnancy,” said lead author Denice S. Feig, MD, Mount Sinai Hospital, Toronto.
The research was presented at the Diabetes UK Professional Conference: Online Series on Nov. 17 and recently published in The Lancet Diabetes & Endocrinology.
Summing up, Dr. Feig said that, on balance, she would be inclined to give metformin to most pregnant women with type 2 diabetes, perhaps with the exception of those who may have risk factors for small-for-gestational-age babies; for example, women who’ve had intrauterine growth restriction, who are smokers, and have significant renal disease, or have a lower body mass index.
Increased prevalence of type 2 diabetes in pregnancy
Dr. Feig said that across the developed world there have been huge increases in the prevalence of type 2 diabetes in pregnancy in recent years.
Insulin is the standard treatment for the management of type 2 diabetes in pregnancy, but these women have marked insulin resistance that worsens in pregnancy, which means their insulin requirements increase, leading to weight gain, painful injections, high cost, and noncompliance.
So despite treatment with insulin, these women continue to face increased rates of adverse maternal and fetal outcomes.
And although metformin is increasingly being used in women with type 2 diabetes during pregnancy, there is a scarcity of data on the benefits and harms of metformin use on pregnancy outcomes in these women.
The MiTy trial was therefore undertaken to determine whether metformin could improve outcomes.
The team recruited 502 women from 29 sites in Canada and Australia who had type 2 diabetes prior to pregnancy or were diagnosed during pregnancy, before 20 weeks’ gestation. The women were randomized to metformin 1 g twice daily or placebo, in addition to their usual insulin regimen, at between 6 and 28 weeks’ gestation.
Type 2 diabetes was diagnosed prior to pregnancy in 83% of women in the metformin group and in 90% of those assigned to placebo. The mean hemoglobin A1c level at randomization was 47 mmol/mol (6.5%) in both groups.
The average maternal age at baseline was approximately 35 years and mean gestational age at randomization was 16 weeks. Mean prepregnancy BMI was approximately 34 kg/m2.
Of note, only 30% were of European ethnicity.
Less weight gain, lower A1c, less insulin needed with metformin
Dr. Feig reported that there was no significant difference between the treatment groups in terms of the proportion of women with the composite primary outcome of pregnancy loss, preterm birth, birth injury, respiratory distress, neonatal hypoglycemia, or admission to neonatal intensive care lasting more than 24 hours (P = 0.86).
However, women in the metformin group had significantly less overall weight gain during pregnancy than did those in the placebo group, at –1.8 kg (P < .0001).
They also had a significantly lower last A1c level in pregnancy, at 41 mmol/mol (5.9%) versus 43.2 mmol/mol (6.1%) in those given placebo (P = .015), and required fewer insulin doses, at 1.1 versus 1.5 units/kg/day (P < .0001), which translated to a reduction of almost 44 units/day.
Women given metformin were also less likely to require Cesarean section delivery, at 53.4% versus 62.7% in the placebo group (P = .03), although there was no difference between groups in terms of gestational hypertension or preeclampsia.
The most common adverse events were gastrointestinal complications, which occurred in 27.3% of women in the metformin group and 22.3% of those given placebo.
There were no significant differences between the metformin and placebo groups in rates of pregnancy loss (P = .81), preterm birth (P = .16), birth injury (P = .37), respiratory distress (P = .49), and congenital anomalies (P = .16).
Average birth weight lower with metformin
However, Dr. Feig showed that the average birth weight was lower for offspring of women given metformin than those assigned to placebo, at 3.2 kg (7.05 lb) versus 3.4 kg (7.4 lb) (P = .002).
Women given metformin were also less likely to have a baby with a birth weight of 4 kg (8.8 lb) or more, at 12.1% versus 19.2%, or a relative risk of 0.65 (P = .046), and a baby that was extremely large for gestational age, at 8.6% versus 14.8%, or a relative risk of 0.58 (P = .046).
But of concern, metformin was associated with an increased risk of small-for-gestational-age babies, at 12.9% versus 6.6% with placebo, or a relative risk of 1.96 (P = .03).
Dr. Feig suggested that this may be due to a direct effect of metformin “because as we know metformin inhibits the mTOR pathway,” which is a “primary nutrient sensor in the placenta” and could “attenuate nutrient flux and fetal growth.”
She said it is not clear whether the small-for-gestational-age babies were “healthy or unhealthy.”
To investigate further, the team has launched the MiTy Kids study, which will follow the offspring in the MiTy trial to determine whether metformin during pregnancy is associated with a reduction in adiposity and improvement in insulin resistance in the babies at 2 years of age.
Who should be given metformin?
During the discussion, Helen R. Murphy, MD, PhD, Norwich Medical School, University of East Anglia, England, asked whether Dr. Feig would recommend continuing metformin in pregnancy if it was started preconception for fertility issues rather than diabetes.
She replied: “If they don’t have diabetes and it’s simply for PCOS [polycystic ovary syndrome], then I have either stopped it as soon as they got pregnant or sometimes continued it through the first trimester, and then stopped.
“If the person has diabetes, however, I think given this work, for most people I would continue it,” she said.
The study was funded by the Canadian Institutes of Health Research, Lunenfeld-Tanenbaum Research Institute, and the University of Toronto. The authors have reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pregnancy outcomes ‘favorable’ after BRCA breast cancer treatment
said researchers reporting a review of more than 1,000 young women with breast cancer, mostly in Europe but also Israel and North and South America.
It’s been known that pregnancy after breast cancer treatment, even for hormone receptor–positive disease, is safe overall, the team commented. However, there have been concerns about women who have BRCA mutations because of a lack of data.
The new findings “provide reassurance to patients with BRCA-mutated breast cancer interested in future fertility” and are of “paramount importance for health care providers involved in counseling young patients,” said the researchers, led by Matteo Lambertini, MD, PhD, a medical oncologist at the University of Genoa, Italy.
The review was published in September in the Journal of Clinical Oncology.
The team reviewed reproductive outcomes among 1,252 women who were no older than 40 years when diagnosed with stage I-III BRCA-mutated invasive breast cancer between January 2000 and December 2012.
More than half (65%; n = 811) had BRCA1 mutations, 430 women (34%) had BRCA2 mutations, and 11 women had both.
Overall, 195 women became pregnant, at a median of 4.5 years after the breast cancer diagnosis and at a median age of 36 years.
The miscarriage rate was 10.3%, lower than expected in the general population.
Among the 150 patients who gave birth to 170 infants, delivery complications occurred in 13 of the 112 pregnancies with available data (11.6%), and congenital anomalies were seen in just 2 pregnancies (1.8%). This is a lower rate of anomalies than expected in the general population, the team noted. The rate of preterm delivery was 9.2%, similar to the general population.
There was no difference between the women who became pregnant and those who did not in either disease-free survival (adjusted hazard ratio, 0.87; P = .41) or overall survival (aHR, 0.88; P = .66), over a median follow-up of 8.3 years from diagnosis. In addition to BRCA mutations, the analysis adjusted for age at diagnosis, tumor size, nodal status, hormone receptor status, type of endocrine therapy, and breast surgery.
Over 80% of the subjects had ductal carcinoma, and over 90% of women were HER2-negative. More women in the pregnancy cohort had tumor diameters of 2 cm or less (47.2% vs. 40.9%) and a higher percentage had breast conserving surgery (59% vs. 45.9%).
Chemotherapy was administered to 95.3% of the subjects, most commonly anthracycline and taxane based, and more than 90% received endocrine therapy, most often tamoxifen alone among women who did not become pregnant and tamoxifen plus a luteinizing hormone-releasing hormone agonist among those who did. Endocrine therapy was shorter among women who became pregnant (median, 50 vs. 60 months; P < .001).
The findings held when 176 pregnant cases were matched to 528 nonpregnant controls for year of diagnosis, nodal status, hormone receptor status, and type of BRCA mutation. However, disease-free survival was improved among pregnant women (HR, 0.71; P = .045) who were younger at diagnosis, with median ages of 31 years vs. 36 years (P < .001).
The study was funded by the Italian Association for Cancer Research, among others. Dr. Lambertini reports acting as a consultant for Roche and Novartis and as a speaker for Theramex, Takeda, Roche, Eli Lilly, Novartis. Several coauthors also report relationships with pharmaceutical companies, as detailed in the original article.
A version of this article originally appeared on Medscape.com.
said researchers reporting a review of more than 1,000 young women with breast cancer, mostly in Europe but also Israel and North and South America.
It’s been known that pregnancy after breast cancer treatment, even for hormone receptor–positive disease, is safe overall, the team commented. However, there have been concerns about women who have BRCA mutations because of a lack of data.
The new findings “provide reassurance to patients with BRCA-mutated breast cancer interested in future fertility” and are of “paramount importance for health care providers involved in counseling young patients,” said the researchers, led by Matteo Lambertini, MD, PhD, a medical oncologist at the University of Genoa, Italy.
The review was published in September in the Journal of Clinical Oncology.
The team reviewed reproductive outcomes among 1,252 women who were no older than 40 years when diagnosed with stage I-III BRCA-mutated invasive breast cancer between January 2000 and December 2012.
More than half (65%; n = 811) had BRCA1 mutations, 430 women (34%) had BRCA2 mutations, and 11 women had both.
Overall, 195 women became pregnant, at a median of 4.5 years after the breast cancer diagnosis and at a median age of 36 years.
The miscarriage rate was 10.3%, lower than expected in the general population.
Among the 150 patients who gave birth to 170 infants, delivery complications occurred in 13 of the 112 pregnancies with available data (11.6%), and congenital anomalies were seen in just 2 pregnancies (1.8%). This is a lower rate of anomalies than expected in the general population, the team noted. The rate of preterm delivery was 9.2%, similar to the general population.
There was no difference between the women who became pregnant and those who did not in either disease-free survival (adjusted hazard ratio, 0.87; P = .41) or overall survival (aHR, 0.88; P = .66), over a median follow-up of 8.3 years from diagnosis. In addition to BRCA mutations, the analysis adjusted for age at diagnosis, tumor size, nodal status, hormone receptor status, type of endocrine therapy, and breast surgery.
Over 80% of the subjects had ductal carcinoma, and over 90% of women were HER2-negative. More women in the pregnancy cohort had tumor diameters of 2 cm or less (47.2% vs. 40.9%) and a higher percentage had breast conserving surgery (59% vs. 45.9%).
Chemotherapy was administered to 95.3% of the subjects, most commonly anthracycline and taxane based, and more than 90% received endocrine therapy, most often tamoxifen alone among women who did not become pregnant and tamoxifen plus a luteinizing hormone-releasing hormone agonist among those who did. Endocrine therapy was shorter among women who became pregnant (median, 50 vs. 60 months; P < .001).
The findings held when 176 pregnant cases were matched to 528 nonpregnant controls for year of diagnosis, nodal status, hormone receptor status, and type of BRCA mutation. However, disease-free survival was improved among pregnant women (HR, 0.71; P = .045) who were younger at diagnosis, with median ages of 31 years vs. 36 years (P < .001).
The study was funded by the Italian Association for Cancer Research, among others. Dr. Lambertini reports acting as a consultant for Roche and Novartis and as a speaker for Theramex, Takeda, Roche, Eli Lilly, Novartis. Several coauthors also report relationships with pharmaceutical companies, as detailed in the original article.
A version of this article originally appeared on Medscape.com.
said researchers reporting a review of more than 1,000 young women with breast cancer, mostly in Europe but also Israel and North and South America.
It’s been known that pregnancy after breast cancer treatment, even for hormone receptor–positive disease, is safe overall, the team commented. However, there have been concerns about women who have BRCA mutations because of a lack of data.
The new findings “provide reassurance to patients with BRCA-mutated breast cancer interested in future fertility” and are of “paramount importance for health care providers involved in counseling young patients,” said the researchers, led by Matteo Lambertini, MD, PhD, a medical oncologist at the University of Genoa, Italy.
The review was published in September in the Journal of Clinical Oncology.
The team reviewed reproductive outcomes among 1,252 women who were no older than 40 years when diagnosed with stage I-III BRCA-mutated invasive breast cancer between January 2000 and December 2012.
More than half (65%; n = 811) had BRCA1 mutations, 430 women (34%) had BRCA2 mutations, and 11 women had both.
Overall, 195 women became pregnant, at a median of 4.5 years after the breast cancer diagnosis and at a median age of 36 years.
The miscarriage rate was 10.3%, lower than expected in the general population.
Among the 150 patients who gave birth to 170 infants, delivery complications occurred in 13 of the 112 pregnancies with available data (11.6%), and congenital anomalies were seen in just 2 pregnancies (1.8%). This is a lower rate of anomalies than expected in the general population, the team noted. The rate of preterm delivery was 9.2%, similar to the general population.
There was no difference between the women who became pregnant and those who did not in either disease-free survival (adjusted hazard ratio, 0.87; P = .41) or overall survival (aHR, 0.88; P = .66), over a median follow-up of 8.3 years from diagnosis. In addition to BRCA mutations, the analysis adjusted for age at diagnosis, tumor size, nodal status, hormone receptor status, type of endocrine therapy, and breast surgery.
Over 80% of the subjects had ductal carcinoma, and over 90% of women were HER2-negative. More women in the pregnancy cohort had tumor diameters of 2 cm or less (47.2% vs. 40.9%) and a higher percentage had breast conserving surgery (59% vs. 45.9%).
Chemotherapy was administered to 95.3% of the subjects, most commonly anthracycline and taxane based, and more than 90% received endocrine therapy, most often tamoxifen alone among women who did not become pregnant and tamoxifen plus a luteinizing hormone-releasing hormone agonist among those who did. Endocrine therapy was shorter among women who became pregnant (median, 50 vs. 60 months; P < .001).
The findings held when 176 pregnant cases were matched to 528 nonpregnant controls for year of diagnosis, nodal status, hormone receptor status, and type of BRCA mutation. However, disease-free survival was improved among pregnant women (HR, 0.71; P = .045) who were younger at diagnosis, with median ages of 31 years vs. 36 years (P < .001).
The study was funded by the Italian Association for Cancer Research, among others. Dr. Lambertini reports acting as a consultant for Roche and Novartis and as a speaker for Theramex, Takeda, Roche, Eli Lilly, Novartis. Several coauthors also report relationships with pharmaceutical companies, as detailed in the original article.
A version of this article originally appeared on Medscape.com.
Report may inform first dietary guidelines for Americans from birth to 24 months
The U.S. Department of Agriculture and the Department of Health & Human Services aim to release new dietary guidelines by the end of 2020.
An advisory committee submitted to the agencies a scientific report that examines relationships between diet and health at various life stages. Four chapters focus on dietary considerations for infants and toddlers, and two chapters focus on diet during pregnancy and lactation.
The report may inform the development of the new guidelines. The advisory committee’s recommendations include introducing infants to foods that are rich in zinc and iron at about age 6 months and having women who are lactating eat sources of omega-3 and omega-6 fatty acids, such as fish, to improve the fatty acid status of infants.
Ahead of the release of the 2020-2025 Dietary Guidelines for Americans, Joan Younger Meek, MD, discussed parts of the scientific report at the annual meeting of the American Academy of Pediatrics, held virtually this year.
While the 2015-2020 guidelines use ChooseMyPlate to help people implement the recommendations, it is not known how the new guidelines will be presented to the public, she said. “Many of you will remember the pyramids earlier and different food groups before that.”
Promote healthy dietary patterns
The advisory committee’s report notes that diet in the first years of life contributes to long-term health and shapes taste preferences, said Dr. Meek, professor of clinical sciences at Florida State University, Orlando. Human milk or infant formula are primary sources of nutrition until approximately 6 months, when families may introduce complementary foods and beverages. Between 6 months and 24 months, children transition to the typical family diet.
Dr. Meek highlighted some of the advisory committee’s findings and recommendations.
- Infants who are ever breastfed have a reduced risk of overweight or obesity, type 1 diabetes, and asthma. Likewise, longer duration of breastfeeding is associated with lower risk of type 1 diabetes and asthma, and exclusive breastfeeding is associated with lower risk of type 1 diabetes.
- Complementary foods and beverages should not be introduced before age 4 months. Limited evidence indicates that their introduction before 4 months may be associated with increased odds of overweight or obesity. Introducing complementary foods or beverages at 4 or 5 months, compared with 6 months, is not associated with long-term advantages or disadvantages.
- Introducing peanut and egg after age 4 months may reduce the risk of food allergies.
- From age 12 months to 24 months, children should consume a variety of nutrient-rich protein sources from animals – including meat, poultry, seafood, eggs, and dairy – plus nuts, seeds, fruits, vegetables, and grains.
- The report prioritizes oils over solid fats, and whole grains over refined grains. It also discourages added sugars, particularly from sugar-sweetened beverages. Other sources of added sugars include sweets, baked goods, and sweetened dairy products.
The report acknowledges that dietary guidelines should accommodate cultural preferences and cost considerations.
Recommendations during pregnancy
Healthy dietary patterns before or during pregnancy may modestly reduce the odds of gestational diabetes, hypertensive disorders of pregnancy, and preterm birth, according to the report.
The report recommends that during pregnancy women consume 8-12 ounces per week of seafood with high levels of omega-3 fatty acids and low levels of methylmercury, consistent with existing recommendations.
Egg and milk consumption during pregnancy does not influence the risk of food allergy, asthma, or atopic disease in the child, according to the report.
The advisory committee recommended universal folic acid supplementation during pregnancy.
Addressing a gap
The Agricultural Act of 2014 required that infants and toddlers and women who are pregnant or lactating be included in the 2020-2025 guidelines. Covering these populations in the scientific report was a substantial undertaking, said Kathryn Dewey, PhD, of the Institute for Global Nutrition at the University of California, Davis. Dr. Dewey chaired the subcommittee on birth to 24 months for the 2020 Dietary Guidelines Advisory Committee.
“Given that this age group had not been covered before, we could not rely on previous dietary guidelines’ reports,” Dr. Dewey said in an interview.
Outlining food patterns for infants and toddlers proved challenging. The committee explored models that considered various scenarios including children who consumed human milk, children who consumed formula, and those with vegetarian diets. Future research should clarify dietary reference intakes for these age groups, Dr. Dewey said.
Dr. Dewey sees the committee’s report on dietary guidance for birth to 24 months as a starting point and not necessarily an exhaustive look at the subject.
For one, the committee focused more on what to feed infants and toddlers rather than on how to feed them. Information about how to feed children is considered more in depth in a 2020 report from the National Academies of Sciences, Engineering, and Medicine. That report summarizes existing guidance from various organizations on feeding infants and children from birth to 24 months. Dr. Dewey chaired the committee that created the National Academies report.
Sharing the new USDA and HHS guidelines after they are released could be the next important step. “The public does not necessarily know about the guidelines or they do not necessarily seek them out unless there is a very well-constructed strategy for dissemination and implementation,” Dr. Dewey said.
To that end, health care providers can play a role, Dr. Meek said. “Be aware of changes in guidance, adopt those new recommendations, and then advocate those with our patients as well as with the public at large.”
Dr. Meek and Dr. Dewey had no relevant financial disclosures.
The U.S. Department of Agriculture and the Department of Health & Human Services aim to release new dietary guidelines by the end of 2020.
An advisory committee submitted to the agencies a scientific report that examines relationships between diet and health at various life stages. Four chapters focus on dietary considerations for infants and toddlers, and two chapters focus on diet during pregnancy and lactation.
The report may inform the development of the new guidelines. The advisory committee’s recommendations include introducing infants to foods that are rich in zinc and iron at about age 6 months and having women who are lactating eat sources of omega-3 and omega-6 fatty acids, such as fish, to improve the fatty acid status of infants.
Ahead of the release of the 2020-2025 Dietary Guidelines for Americans, Joan Younger Meek, MD, discussed parts of the scientific report at the annual meeting of the American Academy of Pediatrics, held virtually this year.
While the 2015-2020 guidelines use ChooseMyPlate to help people implement the recommendations, it is not known how the new guidelines will be presented to the public, she said. “Many of you will remember the pyramids earlier and different food groups before that.”
Promote healthy dietary patterns
The advisory committee’s report notes that diet in the first years of life contributes to long-term health and shapes taste preferences, said Dr. Meek, professor of clinical sciences at Florida State University, Orlando. Human milk or infant formula are primary sources of nutrition until approximately 6 months, when families may introduce complementary foods and beverages. Between 6 months and 24 months, children transition to the typical family diet.
Dr. Meek highlighted some of the advisory committee’s findings and recommendations.
- Infants who are ever breastfed have a reduced risk of overweight or obesity, type 1 diabetes, and asthma. Likewise, longer duration of breastfeeding is associated with lower risk of type 1 diabetes and asthma, and exclusive breastfeeding is associated with lower risk of type 1 diabetes.
- Complementary foods and beverages should not be introduced before age 4 months. Limited evidence indicates that their introduction before 4 months may be associated with increased odds of overweight or obesity. Introducing complementary foods or beverages at 4 or 5 months, compared with 6 months, is not associated with long-term advantages or disadvantages.
- Introducing peanut and egg after age 4 months may reduce the risk of food allergies.
- From age 12 months to 24 months, children should consume a variety of nutrient-rich protein sources from animals – including meat, poultry, seafood, eggs, and dairy – plus nuts, seeds, fruits, vegetables, and grains.
- The report prioritizes oils over solid fats, and whole grains over refined grains. It also discourages added sugars, particularly from sugar-sweetened beverages. Other sources of added sugars include sweets, baked goods, and sweetened dairy products.
The report acknowledges that dietary guidelines should accommodate cultural preferences and cost considerations.
Recommendations during pregnancy
Healthy dietary patterns before or during pregnancy may modestly reduce the odds of gestational diabetes, hypertensive disorders of pregnancy, and preterm birth, according to the report.
The report recommends that during pregnancy women consume 8-12 ounces per week of seafood with high levels of omega-3 fatty acids and low levels of methylmercury, consistent with existing recommendations.
Egg and milk consumption during pregnancy does not influence the risk of food allergy, asthma, or atopic disease in the child, according to the report.
The advisory committee recommended universal folic acid supplementation during pregnancy.
Addressing a gap
The Agricultural Act of 2014 required that infants and toddlers and women who are pregnant or lactating be included in the 2020-2025 guidelines. Covering these populations in the scientific report was a substantial undertaking, said Kathryn Dewey, PhD, of the Institute for Global Nutrition at the University of California, Davis. Dr. Dewey chaired the subcommittee on birth to 24 months for the 2020 Dietary Guidelines Advisory Committee.
“Given that this age group had not been covered before, we could not rely on previous dietary guidelines’ reports,” Dr. Dewey said in an interview.
Outlining food patterns for infants and toddlers proved challenging. The committee explored models that considered various scenarios including children who consumed human milk, children who consumed formula, and those with vegetarian diets. Future research should clarify dietary reference intakes for these age groups, Dr. Dewey said.
Dr. Dewey sees the committee’s report on dietary guidance for birth to 24 months as a starting point and not necessarily an exhaustive look at the subject.
For one, the committee focused more on what to feed infants and toddlers rather than on how to feed them. Information about how to feed children is considered more in depth in a 2020 report from the National Academies of Sciences, Engineering, and Medicine. That report summarizes existing guidance from various organizations on feeding infants and children from birth to 24 months. Dr. Dewey chaired the committee that created the National Academies report.
Sharing the new USDA and HHS guidelines after they are released could be the next important step. “The public does not necessarily know about the guidelines or they do not necessarily seek them out unless there is a very well-constructed strategy for dissemination and implementation,” Dr. Dewey said.
To that end, health care providers can play a role, Dr. Meek said. “Be aware of changes in guidance, adopt those new recommendations, and then advocate those with our patients as well as with the public at large.”
Dr. Meek and Dr. Dewey had no relevant financial disclosures.
The U.S. Department of Agriculture and the Department of Health & Human Services aim to release new dietary guidelines by the end of 2020.
An advisory committee submitted to the agencies a scientific report that examines relationships between diet and health at various life stages. Four chapters focus on dietary considerations for infants and toddlers, and two chapters focus on diet during pregnancy and lactation.
The report may inform the development of the new guidelines. The advisory committee’s recommendations include introducing infants to foods that are rich in zinc and iron at about age 6 months and having women who are lactating eat sources of omega-3 and omega-6 fatty acids, such as fish, to improve the fatty acid status of infants.
Ahead of the release of the 2020-2025 Dietary Guidelines for Americans, Joan Younger Meek, MD, discussed parts of the scientific report at the annual meeting of the American Academy of Pediatrics, held virtually this year.
While the 2015-2020 guidelines use ChooseMyPlate to help people implement the recommendations, it is not known how the new guidelines will be presented to the public, she said. “Many of you will remember the pyramids earlier and different food groups before that.”
Promote healthy dietary patterns
The advisory committee’s report notes that diet in the first years of life contributes to long-term health and shapes taste preferences, said Dr. Meek, professor of clinical sciences at Florida State University, Orlando. Human milk or infant formula are primary sources of nutrition until approximately 6 months, when families may introduce complementary foods and beverages. Between 6 months and 24 months, children transition to the typical family diet.
Dr. Meek highlighted some of the advisory committee’s findings and recommendations.
- Infants who are ever breastfed have a reduced risk of overweight or obesity, type 1 diabetes, and asthma. Likewise, longer duration of breastfeeding is associated with lower risk of type 1 diabetes and asthma, and exclusive breastfeeding is associated with lower risk of type 1 diabetes.
- Complementary foods and beverages should not be introduced before age 4 months. Limited evidence indicates that their introduction before 4 months may be associated with increased odds of overweight or obesity. Introducing complementary foods or beverages at 4 or 5 months, compared with 6 months, is not associated with long-term advantages or disadvantages.
- Introducing peanut and egg after age 4 months may reduce the risk of food allergies.
- From age 12 months to 24 months, children should consume a variety of nutrient-rich protein sources from animals – including meat, poultry, seafood, eggs, and dairy – plus nuts, seeds, fruits, vegetables, and grains.
- The report prioritizes oils over solid fats, and whole grains over refined grains. It also discourages added sugars, particularly from sugar-sweetened beverages. Other sources of added sugars include sweets, baked goods, and sweetened dairy products.
The report acknowledges that dietary guidelines should accommodate cultural preferences and cost considerations.
Recommendations during pregnancy
Healthy dietary patterns before or during pregnancy may modestly reduce the odds of gestational diabetes, hypertensive disorders of pregnancy, and preterm birth, according to the report.
The report recommends that during pregnancy women consume 8-12 ounces per week of seafood with high levels of omega-3 fatty acids and low levels of methylmercury, consistent with existing recommendations.
Egg and milk consumption during pregnancy does not influence the risk of food allergy, asthma, or atopic disease in the child, according to the report.
The advisory committee recommended universal folic acid supplementation during pregnancy.
Addressing a gap
The Agricultural Act of 2014 required that infants and toddlers and women who are pregnant or lactating be included in the 2020-2025 guidelines. Covering these populations in the scientific report was a substantial undertaking, said Kathryn Dewey, PhD, of the Institute for Global Nutrition at the University of California, Davis. Dr. Dewey chaired the subcommittee on birth to 24 months for the 2020 Dietary Guidelines Advisory Committee.
“Given that this age group had not been covered before, we could not rely on previous dietary guidelines’ reports,” Dr. Dewey said in an interview.
Outlining food patterns for infants and toddlers proved challenging. The committee explored models that considered various scenarios including children who consumed human milk, children who consumed formula, and those with vegetarian diets. Future research should clarify dietary reference intakes for these age groups, Dr. Dewey said.
Dr. Dewey sees the committee’s report on dietary guidance for birth to 24 months as a starting point and not necessarily an exhaustive look at the subject.
For one, the committee focused more on what to feed infants and toddlers rather than on how to feed them. Information about how to feed children is considered more in depth in a 2020 report from the National Academies of Sciences, Engineering, and Medicine. That report summarizes existing guidance from various organizations on feeding infants and children from birth to 24 months. Dr. Dewey chaired the committee that created the National Academies report.
Sharing the new USDA and HHS guidelines after they are released could be the next important step. “The public does not necessarily know about the guidelines or they do not necessarily seek them out unless there is a very well-constructed strategy for dissemination and implementation,” Dr. Dewey said.
To that end, health care providers can play a role, Dr. Meek said. “Be aware of changes in guidance, adopt those new recommendations, and then advocate those with our patients as well as with the public at large.”
Dr. Meek and Dr. Dewey had no relevant financial disclosures.
FROM AAP 2020
Fertility delay varied with contraceptive method in study
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
according to a new prospective cohort study.
Women who used hormonal intrauterine devices, copper intrauterine devices, and implants had the shortest delays, based on the same research project, which involved analyzing data from approximately 18,000 women in North America and Denmark.
“Most research on the use of contraceptives and fertility has focused on the effect of oral contraceptives on fecundability,” and data on the association between fertility and other contraceptive methods are limited, wrote Jennifer J. Yland, MS, of Boston University School of Public Health and colleagues.
“Given the increasing popularity of long acting reversible contraceptive methods and other alternatives to oral contraceptives, more research into their short- and long-term effects on fertility is needed,” the researchers noted.
In the study, which was published in the BMJ, the researchers reviewed data from a total of 17,954 women from three cohort studies of individuals planning pregnancies between 2007 and 2019. Participants reported their contraceptive use and typical menstrual cycle at baseline, then responded to questionnaires every 2 months for up to a year or until pregnancy.
On average, users of injectable contraceptives had the longest delay in return of normal fertility (five to eight menstrual cycles), compared with four cycles for patch contraceptives, three cycles for oral and ring contraceptives, and two cycles for hormonal and copper intrauterine devices and implants.
A total of 10,729 pregnancies were reported within 66,759 menstrual cycles; approximately 77% of the women conceived within 12 months, and 56% conceived within 6 months.
Oral contraceptives were the most common method of contraception (38%), followed by barrier methods (31%), natural methods (15%), and long-acting reversible contraceptives (13%). Intrauterine devices were the most frequently used of long-acting reversible contraceptives (8% hormonal, 4% copper).
The time until fertility returned after discontinuing contraceptives was not associated with duration of contraceptive use.
The study findings were limited by several factors including the potential misclassification of menstrual cycles and the use of self-reports for the time of contraceptive discontinuation, especially for users of injectable contraceptives, the researchers noted.
However, the results were strengthened by the large study size and show “little or no lasting effect” of long-term use of any of the reported contraceptive methods on fertility, the researchers noted. “Understanding the comparative effects of different contraceptives on fecundity is essential for family planning, counseling for contraception, and management of infertility,” they said.
Comparison of contraceptives can inform counseling
The study is important because the use of long-acting reversible contraceptive (LARC) methods (IUDs, implants, patches, and injectable contraceptives) has become increasingly common worldwide, corresponding author Jennifer J. Yland, MS, said in an interview. “Many women are concerned about the potential effects of contraception on future fertility. However, previous research on this topic has focused mostly on oral contraceptives,” she said.
Ms. Yland said that the findings on oral and injectable contraceptives were consistent with previous publications. However, “we were surprised to find that women who had recently used the hormonal IUD had a shorter time to pregnancy, compared with women who used barrier methods,” she said.
The take-home message for clinicians is that delays in the return to normal fertility were temporary for all hormonal contraceptive methods, Ms. Yland emphasized. “However, delays in the return of fertility after discontinuing certain hormonal methods, such as injectables, were considerably longer than that shown for oral contraceptives. These findings should be taken into account when women are considering contraceptive choice in the context of family planning and infertility management,” she noted.
“Future research should evaluate the potential associations between recent use of hormonal contraceptives and perinatal outcomes,” she added.
Managing expectations helps patients plan
“The question of return to fertility is one that many patients who use contraception have unless they have completed their child bearing,” said Sarah W. Prager, MD, of the University of Washington, Seattle, in an interview. “For patients who want to plan a pregnancy, knowing what to expect in terms of return to fertility is important so they can make sure they are in the space and place they want to be with their health, life, job, and partner,” she said.
Dr. Prager said she was not surprised by the study findings because they agree with previously published data. “Overall, except for the injection, people using any form of contraception are back to their baseline fertility within a few months,” she noted. “It also makes perfect sense for return to fertility to be longer with the injection, as it is designed to prevent pregnancy for 16 weeks after the injection is given. Unlike all the other methods, it cannot be removed from the body once given,” she said.
“Clinicians should continue to advise patients that their return to baseline fertility is relatively rapid with any contraception other than the Depo-Provera injection,” said Dr. Prager. “There are no data to support a benefit in switching from an IUD or implant to a combination hormonal method (pills, patch, ring) before starting to try to conceive,” she said.
“This study tries to account for differences in baseline fertility for people using the different methods, but since the choice of method was not randomized, there could still be baseline differences that were not measured or accounted for,” Dr. Prager noted. “A randomized study would certainly eliminate some of these biases; however, I don’t think the differences found in this study are so profound as to require such study,” she said. “Generally speaking, almost 80% of people using any form of contraception were able to conceive within 1 year of trying, which has been the stated fertility data for decades,” she said.
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Lead author Ms. Yland had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose.
SOURCE: Yland JJ et al. BMJ. 2020 Nov 12. doi: 10.1136/bmj.m3966.
FROM THE BMJ
Employment protections now include sexual orientation, but our role in LGBTQIA+ equality continues
The state of Tennessee, where I worked and attended medical school, did not have legislation in place prohibiting termination of employment based on sexual orientation alone. As a lesbian, I never felt safe at work knowing that I could be fired at any time simply because of who I loved and how I identified. When I started medical school in rural Appalachia, I decided I would be “out” but remained cautious. That meant inspecting everyone I encountered for signs of acceptance and safety before sharing details about my life. As a third-year medical student, I started wearing a rainbow triangle on my white coat. One of the first patients I cared for cried and thanked me for wearing the pin. She then proceeded to tell me about her partner, her own struggles with depression, and the secrets she had to keep from her community. It was overwhelming and, yet, so familiar. I was struck by how wearing this pin, a small gesture, made this patient feel safe enough to come out to me and seek help for her depression. Although I found a supportive community in Tennessee, it was only after I moved to Massachusetts for residency—where antidiscrimination laws protected lesbian, gay, bisexual, transgender, queer/questioning, intersex, asexual, plus all other gender and sexual minority (LGBTQIA+) identified people—did I feel safe to freely share about my partner and our life together.
A landmark decision in the Supreme Court
This past June, in a 6 to 3 decision, the US Supreme Court ruled in the case of Bostock v Clayton County that Title VII’s ban on discrimination also protects LGBTQIA+ employees. Title VII is a federal law that protects employees from discrimination based on race, color, national origin, sex, and religion.1 In this decision, the court determined that “sex” cannot be differentiated from sexual orientation. Justice Neil Gorsuch, who wrote the majority opinion, stated, “It is impossible… to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex.”2 Title VII not only protects employees in hiring and firing practices but also protects against harassment and retaliation. Prior to this ruling, there were no federal antidiscrimination laws for LGBTQIA+ individuals, and only 22 states and the District of Columbia had laws in place that specified antidiscrimination protection for this community.3 Because of this landmark decision, Title VII now protects all employees in all states from discrimination, including due to an individual’s sexual orientation.
This is a huge victory in the battle for equality; however, the fight is not over. Justice Gorsuch stated, “We do not purport to address bathrooms, locker rooms or anything else of the kind…whether other policies and practices might or might not qualify as unlawful discrimination or find justifications under other provisions of Title VII are questions for future cases, not these.”2 This victory sets a new precedent and will continue to be further defined with more court cases as states and employers push back against these protections.
Continue to: A worrying shift in the Court...
A worrying shift in the Court
We have already started to see the repercussions of this ruling from Supreme Court justices themselves. Justice Clarence Thomas, who dissented in the Obergefell v Hodges decision in 2015, which established the constitutional right for marriage equality, recently wrote a petition to have the Supreme Court reconsider that ruling. He wrote “Obergefell enables courts and governments to brand religious adherents who believe that marriage is between one man and one woman as bigots, making their religious liberty concerns that much easier to dismiss.”3 After the passing of Justice Ruth Bader Ginsburg, the Supreme Court became decidedly more conservative with the appointment of Judge Amy Coney Barrett, whose mentor was the late Justice Antonin Scalia, who also dissented in the 2015 case.
As we celebrate this huge win for equality in this June decision, we also must recognize that LGBTQIA+ rights are still at risk.
LGBTQIA+ patients at higher risk for litany of conditions
Even with the Bostock v Clayton County ruling, we must not forget that discrimination will continue to exist. As health care providers, we have a responsibility to advocate on behalf of our LGBTQIA+ colleagues and patients. According to the Healthy People 2020 survey, there are higher rates of obesity, tobacco dependence, and sexually transmitted infection, as well as lower adherence to cancer screening recommendations in the LGBTQIA+ community.4 These disparities are a result of systemic, legal, and social factors, including limited access to affirming and inclusive health care.5 The LGBTQIA+ community deserves better.
Take action
In the coming months and years, as the US Supreme Court hears more cases that will threaten the rights of the LGBTQIA+ community, I challenge all clinicians to take action. Even the smallest of gestures, such as wearing a rainbow pin, can be transformative for our patients and within our communities.
- Advocate for your state to enact nondiscrimination laws protecting the LGBTQIA+ community. Find out if your state has a law.
- Support your LGBTQIA+ colleagues by establishing an employee support group.
- Educate yourself and your colleagues on LGBTQIA+ inclusive medical practices.
- US Equal Employment Opportunity Commission. Title VII of the Civil Rights Act of 1964. https://www.eeoc.gov/statutes/title-vii-civil-rights-act-1964. Accessed November 4, 2020.
- Bostock v Clayton County, 590 US ___ (2020).
- Petition for Writ of Certiorari, Clarence Thomas. October 2020. https://www.supremecourt.gov/orders/courtorders/100520zor_3204.pdf. Accessed November 11, 2020.
- US Department of Health and Human Services. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Accessed November 4, 2020.
- Ard KL, Makadon HJ. Improving the health of lesbian, gay, bisexual and transgender people: understanding and eliminating health disparities. The National LGBT Health Education Center website. https://www.lgbtqiahealtheducation.org/wp-content/uploads/Improving-the-Health-of-LGBT-People.pdf. Accessed November 4, 2020.
The state of Tennessee, where I worked and attended medical school, did not have legislation in place prohibiting termination of employment based on sexual orientation alone. As a lesbian, I never felt safe at work knowing that I could be fired at any time simply because of who I loved and how I identified. When I started medical school in rural Appalachia, I decided I would be “out” but remained cautious. That meant inspecting everyone I encountered for signs of acceptance and safety before sharing details about my life. As a third-year medical student, I started wearing a rainbow triangle on my white coat. One of the first patients I cared for cried and thanked me for wearing the pin. She then proceeded to tell me about her partner, her own struggles with depression, and the secrets she had to keep from her community. It was overwhelming and, yet, so familiar. I was struck by how wearing this pin, a small gesture, made this patient feel safe enough to come out to me and seek help for her depression. Although I found a supportive community in Tennessee, it was only after I moved to Massachusetts for residency—where antidiscrimination laws protected lesbian, gay, bisexual, transgender, queer/questioning, intersex, asexual, plus all other gender and sexual minority (LGBTQIA+) identified people—did I feel safe to freely share about my partner and our life together.
A landmark decision in the Supreme Court
This past June, in a 6 to 3 decision, the US Supreme Court ruled in the case of Bostock v Clayton County that Title VII’s ban on discrimination also protects LGBTQIA+ employees. Title VII is a federal law that protects employees from discrimination based on race, color, national origin, sex, and religion.1 In this decision, the court determined that “sex” cannot be differentiated from sexual orientation. Justice Neil Gorsuch, who wrote the majority opinion, stated, “It is impossible… to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex.”2 Title VII not only protects employees in hiring and firing practices but also protects against harassment and retaliation. Prior to this ruling, there were no federal antidiscrimination laws for LGBTQIA+ individuals, and only 22 states and the District of Columbia had laws in place that specified antidiscrimination protection for this community.3 Because of this landmark decision, Title VII now protects all employees in all states from discrimination, including due to an individual’s sexual orientation.
This is a huge victory in the battle for equality; however, the fight is not over. Justice Gorsuch stated, “We do not purport to address bathrooms, locker rooms or anything else of the kind…whether other policies and practices might or might not qualify as unlawful discrimination or find justifications under other provisions of Title VII are questions for future cases, not these.”2 This victory sets a new precedent and will continue to be further defined with more court cases as states and employers push back against these protections.
Continue to: A worrying shift in the Court...
A worrying shift in the Court
We have already started to see the repercussions of this ruling from Supreme Court justices themselves. Justice Clarence Thomas, who dissented in the Obergefell v Hodges decision in 2015, which established the constitutional right for marriage equality, recently wrote a petition to have the Supreme Court reconsider that ruling. He wrote “Obergefell enables courts and governments to brand religious adherents who believe that marriage is between one man and one woman as bigots, making their religious liberty concerns that much easier to dismiss.”3 After the passing of Justice Ruth Bader Ginsburg, the Supreme Court became decidedly more conservative with the appointment of Judge Amy Coney Barrett, whose mentor was the late Justice Antonin Scalia, who also dissented in the 2015 case.
As we celebrate this huge win for equality in this June decision, we also must recognize that LGBTQIA+ rights are still at risk.
LGBTQIA+ patients at higher risk for litany of conditions
Even with the Bostock v Clayton County ruling, we must not forget that discrimination will continue to exist. As health care providers, we have a responsibility to advocate on behalf of our LGBTQIA+ colleagues and patients. According to the Healthy People 2020 survey, there are higher rates of obesity, tobacco dependence, and sexually transmitted infection, as well as lower adherence to cancer screening recommendations in the LGBTQIA+ community.4 These disparities are a result of systemic, legal, and social factors, including limited access to affirming and inclusive health care.5 The LGBTQIA+ community deserves better.
Take action
In the coming months and years, as the US Supreme Court hears more cases that will threaten the rights of the LGBTQIA+ community, I challenge all clinicians to take action. Even the smallest of gestures, such as wearing a rainbow pin, can be transformative for our patients and within our communities.
- Advocate for your state to enact nondiscrimination laws protecting the LGBTQIA+ community. Find out if your state has a law.
- Support your LGBTQIA+ colleagues by establishing an employee support group.
- Educate yourself and your colleagues on LGBTQIA+ inclusive medical practices.
The state of Tennessee, where I worked and attended medical school, did not have legislation in place prohibiting termination of employment based on sexual orientation alone. As a lesbian, I never felt safe at work knowing that I could be fired at any time simply because of who I loved and how I identified. When I started medical school in rural Appalachia, I decided I would be “out” but remained cautious. That meant inspecting everyone I encountered for signs of acceptance and safety before sharing details about my life. As a third-year medical student, I started wearing a rainbow triangle on my white coat. One of the first patients I cared for cried and thanked me for wearing the pin. She then proceeded to tell me about her partner, her own struggles with depression, and the secrets she had to keep from her community. It was overwhelming and, yet, so familiar. I was struck by how wearing this pin, a small gesture, made this patient feel safe enough to come out to me and seek help for her depression. Although I found a supportive community in Tennessee, it was only after I moved to Massachusetts for residency—where antidiscrimination laws protected lesbian, gay, bisexual, transgender, queer/questioning, intersex, asexual, plus all other gender and sexual minority (LGBTQIA+) identified people—did I feel safe to freely share about my partner and our life together.
A landmark decision in the Supreme Court
This past June, in a 6 to 3 decision, the US Supreme Court ruled in the case of Bostock v Clayton County that Title VII’s ban on discrimination also protects LGBTQIA+ employees. Title VII is a federal law that protects employees from discrimination based on race, color, national origin, sex, and religion.1 In this decision, the court determined that “sex” cannot be differentiated from sexual orientation. Justice Neil Gorsuch, who wrote the majority opinion, stated, “It is impossible… to discriminate against a person for being homosexual or transgender without discriminating against that individual based on sex.”2 Title VII not only protects employees in hiring and firing practices but also protects against harassment and retaliation. Prior to this ruling, there were no federal antidiscrimination laws for LGBTQIA+ individuals, and only 22 states and the District of Columbia had laws in place that specified antidiscrimination protection for this community.3 Because of this landmark decision, Title VII now protects all employees in all states from discrimination, including due to an individual’s sexual orientation.
This is a huge victory in the battle for equality; however, the fight is not over. Justice Gorsuch stated, “We do not purport to address bathrooms, locker rooms or anything else of the kind…whether other policies and practices might or might not qualify as unlawful discrimination or find justifications under other provisions of Title VII are questions for future cases, not these.”2 This victory sets a new precedent and will continue to be further defined with more court cases as states and employers push back against these protections.
Continue to: A worrying shift in the Court...
A worrying shift in the Court
We have already started to see the repercussions of this ruling from Supreme Court justices themselves. Justice Clarence Thomas, who dissented in the Obergefell v Hodges decision in 2015, which established the constitutional right for marriage equality, recently wrote a petition to have the Supreme Court reconsider that ruling. He wrote “Obergefell enables courts and governments to brand religious adherents who believe that marriage is between one man and one woman as bigots, making their religious liberty concerns that much easier to dismiss.”3 After the passing of Justice Ruth Bader Ginsburg, the Supreme Court became decidedly more conservative with the appointment of Judge Amy Coney Barrett, whose mentor was the late Justice Antonin Scalia, who also dissented in the 2015 case.
As we celebrate this huge win for equality in this June decision, we also must recognize that LGBTQIA+ rights are still at risk.
LGBTQIA+ patients at higher risk for litany of conditions
Even with the Bostock v Clayton County ruling, we must not forget that discrimination will continue to exist. As health care providers, we have a responsibility to advocate on behalf of our LGBTQIA+ colleagues and patients. According to the Healthy People 2020 survey, there are higher rates of obesity, tobacco dependence, and sexually transmitted infection, as well as lower adherence to cancer screening recommendations in the LGBTQIA+ community.4 These disparities are a result of systemic, legal, and social factors, including limited access to affirming and inclusive health care.5 The LGBTQIA+ community deserves better.
Take action
In the coming months and years, as the US Supreme Court hears more cases that will threaten the rights of the LGBTQIA+ community, I challenge all clinicians to take action. Even the smallest of gestures, such as wearing a rainbow pin, can be transformative for our patients and within our communities.
- Advocate for your state to enact nondiscrimination laws protecting the LGBTQIA+ community. Find out if your state has a law.
- Support your LGBTQIA+ colleagues by establishing an employee support group.
- Educate yourself and your colleagues on LGBTQIA+ inclusive medical practices.
- US Equal Employment Opportunity Commission. Title VII of the Civil Rights Act of 1964. https://www.eeoc.gov/statutes/title-vii-civil-rights-act-1964. Accessed November 4, 2020.
- Bostock v Clayton County, 590 US ___ (2020).
- Petition for Writ of Certiorari, Clarence Thomas. October 2020. https://www.supremecourt.gov/orders/courtorders/100520zor_3204.pdf. Accessed November 11, 2020.
- US Department of Health and Human Services. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Accessed November 4, 2020.
- Ard KL, Makadon HJ. Improving the health of lesbian, gay, bisexual and transgender people: understanding and eliminating health disparities. The National LGBT Health Education Center website. https://www.lgbtqiahealtheducation.org/wp-content/uploads/Improving-the-Health-of-LGBT-People.pdf. Accessed November 4, 2020.
- US Equal Employment Opportunity Commission. Title VII of the Civil Rights Act of 1964. https://www.eeoc.gov/statutes/title-vii-civil-rights-act-1964. Accessed November 4, 2020.
- Bostock v Clayton County, 590 US ___ (2020).
- Petition for Writ of Certiorari, Clarence Thomas. October 2020. https://www.supremecourt.gov/orders/courtorders/100520zor_3204.pdf. Accessed November 11, 2020.
- US Department of Health and Human Services. Lesbian, gay, bisexual, and transgender health. https://www.healthypeople.gov/2020/topics-objectives/topic/lesbian-gay-bisexual-and-transgender-health. Accessed November 4, 2020.
- Ard KL, Makadon HJ. Improving the health of lesbian, gay, bisexual and transgender people: understanding and eliminating health disparities. The National LGBT Health Education Center website. https://www.lgbtqiahealtheducation.org/wp-content/uploads/Improving-the-Health-of-LGBT-People.pdf. Accessed November 4, 2020.
Pregnancy can be safe with interstitial lung disease
Pregnant women with interstitial lung disease (ILD) related to autoimmune disease may not need to terminate their pregnancies if they have close monitoring before, during, and after pregnancy with a multidisciplinary team of physicians, new research suggests.
Senior author Megan Clowse, MD, MPH, associate professor of medicine in the division of rheumatology at Duke University, Durham, N.C., explained during a press conference at the virtual annual meeting of the American College of Rheumatology that women with ILD are often advised by obstetricians or rheumatologists to avoid conception or terminate their pregnancies, though evidence for that has been based on small studies of 9-15 patients that have had mixed results.
“Many of these pregnancies were delivered 20-30 years ago, definitely with different rheumatic and obstetric care than we can provide now,” she said. “It’s really time to rethink our approach to interstitial lung disease and pregnancy.”
This study showed that while adverse pregnancy outcomes are common in these women, overall maternal morbidity and mortality are low.
ILD may be a secondary disease in people who have scleroderma, lupus, and sarcoidosis.
Largest study to date
This Pfizer-sponsored retrospective study of 67 pregnant women is the largest to date, and it analyzed 94 pregnancies (including five sets of twins).
Sarah Rae Easter, MD, maternal-fetal medicine doctor in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston, called the work “exciting” as the researchers were able to look back at a large number of cases for a rare condition for more than 20 years.
“Their data provides much-needed evidence to provide some reassurance for women affected by this type of pulmonary disease regarding the relative safety of pregnancy,” she said in an interview.
Study spanned 23 years
The researchers reviewed pregnancy records in patients diagnosed with ILD secondary to autoimmune disease at Duke University Health System from January 1996 to July 2019.
They classified the severity of ILD based on two standard breathing tests – forced vital capacity and diffusion capacity for carbon monoxide.
Overall, 69% of the women were diagnosed with sarcoidosis and the remaining 31% had a connective tissue disease associated with ILD (CTD-ILD). Of those measured for ILD severity, 11% were severe, 25% were moderate, 50% were mild, and 14% were normal. Their average maternal age was 32.1 and 83% were Black.
While 70% of the pregnancies resulted in live births, 9% were terminated. The remainder resulted in miscarriage or stillbirth.
Researchers reported a 15% rate of preeclampsia, a 34% rate of the composite measure PROMISSE-Adverse Pregnancy Outcome (APO), and a 15% rate of PROMISSE-APO SEVERE. Patients with severe disease had the highest rates of PROMISSE-APO (P = .03 across groups).
(PROMISSE stands for the Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus study.)
None of the women died
Dr. Clowse said it was a pleasant surprise to find that none of the women died, though patients with severe ILD had more adverse outcomes. Only 2.1% were treated in an intensive care unit during or soon after delivery. In 4.2%, ILD patients had significant shortness of breath due to fluid volume overload around the time of delivery.
For the women who had normal-to-moderate lung disease, Dr. Clowse said, “they really had remarkably good outcomes, really pretty comparable to the general population. About 15% delivered preterm and about 20% suffered a pregnancy loss.”
Dr. Easter, who was not involved with the study, noted the large number of Black women in the cohort.
“Focusing in on improving outcomes for Black and Brown women related to pregnancy in our country is a much-needed undertaking,” Dr. Easter said.
Being able to quote percentages from this research, based on a good-sized study “at least gives people a benchmark about what kind of risk they are willing to assume for themselves,” she said.
For providers, being able to place this rare disease within the spectrum of other diseases where there is more data is also very helpful, she said.
Dr. Clowse said in an interview that the preponderance of Black women in the study was a surprise but may be explained by two factors: Sarcoidosis is seen more frequently in Black women and in the study area in North Carolina there is a large population of Black women.
“Also, our patients with more severe lupus, the ones who are more likely to have interstitial lung disease, are often Black and that’s likely contributing as well,” she said.
Multidisciplinary teams advised
Dr. Clowse emphasized that women with ILD need multidisciplinary teams in pregnancy and should be managed at tertiary care centers where there is a full complement of obstetric and internal medicine experts.
“We do recommend evaluating the severity of their lungs and their heart disease around the time of pregnancy and during pregnancy if they have shortness of breath,” she said.
“We currently recommend that these patients with moderate or severe disease stay in the hospital for up to a week, just for monitoring,” she said.
Dr. Easter said having that kind of access to a large academic healthcare center should be an important part of the decision-making.
Patients need to think about whether they would have access to care similar to what the researchers are describing when they are making the decision to pursue or continue pregnancy, she said.
The study was sponsored by Pfizer Inc. Dr. Clowse reported relationships with UCB, GlaxoSmithKline, AstraZeneca, and Pfizer. Dr. Easter has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pregnant women with interstitial lung disease (ILD) related to autoimmune disease may not need to terminate their pregnancies if they have close monitoring before, during, and after pregnancy with a multidisciplinary team of physicians, new research suggests.
Senior author Megan Clowse, MD, MPH, associate professor of medicine in the division of rheumatology at Duke University, Durham, N.C., explained during a press conference at the virtual annual meeting of the American College of Rheumatology that women with ILD are often advised by obstetricians or rheumatologists to avoid conception or terminate their pregnancies, though evidence for that has been based on small studies of 9-15 patients that have had mixed results.
“Many of these pregnancies were delivered 20-30 years ago, definitely with different rheumatic and obstetric care than we can provide now,” she said. “It’s really time to rethink our approach to interstitial lung disease and pregnancy.”
This study showed that while adverse pregnancy outcomes are common in these women, overall maternal morbidity and mortality are low.
ILD may be a secondary disease in people who have scleroderma, lupus, and sarcoidosis.
Largest study to date
This Pfizer-sponsored retrospective study of 67 pregnant women is the largest to date, and it analyzed 94 pregnancies (including five sets of twins).
Sarah Rae Easter, MD, maternal-fetal medicine doctor in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston, called the work “exciting” as the researchers were able to look back at a large number of cases for a rare condition for more than 20 years.
“Their data provides much-needed evidence to provide some reassurance for women affected by this type of pulmonary disease regarding the relative safety of pregnancy,” she said in an interview.
Study spanned 23 years
The researchers reviewed pregnancy records in patients diagnosed with ILD secondary to autoimmune disease at Duke University Health System from January 1996 to July 2019.
They classified the severity of ILD based on two standard breathing tests – forced vital capacity and diffusion capacity for carbon monoxide.
Overall, 69% of the women were diagnosed with sarcoidosis and the remaining 31% had a connective tissue disease associated with ILD (CTD-ILD). Of those measured for ILD severity, 11% were severe, 25% were moderate, 50% were mild, and 14% were normal. Their average maternal age was 32.1 and 83% were Black.
While 70% of the pregnancies resulted in live births, 9% were terminated. The remainder resulted in miscarriage or stillbirth.
Researchers reported a 15% rate of preeclampsia, a 34% rate of the composite measure PROMISSE-Adverse Pregnancy Outcome (APO), and a 15% rate of PROMISSE-APO SEVERE. Patients with severe disease had the highest rates of PROMISSE-APO (P = .03 across groups).
(PROMISSE stands for the Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus study.)
None of the women died
Dr. Clowse said it was a pleasant surprise to find that none of the women died, though patients with severe ILD had more adverse outcomes. Only 2.1% were treated in an intensive care unit during or soon after delivery. In 4.2%, ILD patients had significant shortness of breath due to fluid volume overload around the time of delivery.
For the women who had normal-to-moderate lung disease, Dr. Clowse said, “they really had remarkably good outcomes, really pretty comparable to the general population. About 15% delivered preterm and about 20% suffered a pregnancy loss.”
Dr. Easter, who was not involved with the study, noted the large number of Black women in the cohort.
“Focusing in on improving outcomes for Black and Brown women related to pregnancy in our country is a much-needed undertaking,” Dr. Easter said.
Being able to quote percentages from this research, based on a good-sized study “at least gives people a benchmark about what kind of risk they are willing to assume for themselves,” she said.
For providers, being able to place this rare disease within the spectrum of other diseases where there is more data is also very helpful, she said.
Dr. Clowse said in an interview that the preponderance of Black women in the study was a surprise but may be explained by two factors: Sarcoidosis is seen more frequently in Black women and in the study area in North Carolina there is a large population of Black women.
“Also, our patients with more severe lupus, the ones who are more likely to have interstitial lung disease, are often Black and that’s likely contributing as well,” she said.
Multidisciplinary teams advised
Dr. Clowse emphasized that women with ILD need multidisciplinary teams in pregnancy and should be managed at tertiary care centers where there is a full complement of obstetric and internal medicine experts.
“We do recommend evaluating the severity of their lungs and their heart disease around the time of pregnancy and during pregnancy if they have shortness of breath,” she said.
“We currently recommend that these patients with moderate or severe disease stay in the hospital for up to a week, just for monitoring,” she said.
Dr. Easter said having that kind of access to a large academic healthcare center should be an important part of the decision-making.
Patients need to think about whether they would have access to care similar to what the researchers are describing when they are making the decision to pursue or continue pregnancy, she said.
The study was sponsored by Pfizer Inc. Dr. Clowse reported relationships with UCB, GlaxoSmithKline, AstraZeneca, and Pfizer. Dr. Easter has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Pregnant women with interstitial lung disease (ILD) related to autoimmune disease may not need to terminate their pregnancies if they have close monitoring before, during, and after pregnancy with a multidisciplinary team of physicians, new research suggests.
Senior author Megan Clowse, MD, MPH, associate professor of medicine in the division of rheumatology at Duke University, Durham, N.C., explained during a press conference at the virtual annual meeting of the American College of Rheumatology that women with ILD are often advised by obstetricians or rheumatologists to avoid conception or terminate their pregnancies, though evidence for that has been based on small studies of 9-15 patients that have had mixed results.
“Many of these pregnancies were delivered 20-30 years ago, definitely with different rheumatic and obstetric care than we can provide now,” she said. “It’s really time to rethink our approach to interstitial lung disease and pregnancy.”
This study showed that while adverse pregnancy outcomes are common in these women, overall maternal morbidity and mortality are low.
ILD may be a secondary disease in people who have scleroderma, lupus, and sarcoidosis.
Largest study to date
This Pfizer-sponsored retrospective study of 67 pregnant women is the largest to date, and it analyzed 94 pregnancies (including five sets of twins).
Sarah Rae Easter, MD, maternal-fetal medicine doctor in the department of obstetrics and gynecology at Brigham and Women’s Hospital, Boston, called the work “exciting” as the researchers were able to look back at a large number of cases for a rare condition for more than 20 years.
“Their data provides much-needed evidence to provide some reassurance for women affected by this type of pulmonary disease regarding the relative safety of pregnancy,” she said in an interview.
Study spanned 23 years
The researchers reviewed pregnancy records in patients diagnosed with ILD secondary to autoimmune disease at Duke University Health System from January 1996 to July 2019.
They classified the severity of ILD based on two standard breathing tests – forced vital capacity and diffusion capacity for carbon monoxide.
Overall, 69% of the women were diagnosed with sarcoidosis and the remaining 31% had a connective tissue disease associated with ILD (CTD-ILD). Of those measured for ILD severity, 11% were severe, 25% were moderate, 50% were mild, and 14% were normal. Their average maternal age was 32.1 and 83% were Black.
While 70% of the pregnancies resulted in live births, 9% were terminated. The remainder resulted in miscarriage or stillbirth.
Researchers reported a 15% rate of preeclampsia, a 34% rate of the composite measure PROMISSE-Adverse Pregnancy Outcome (APO), and a 15% rate of PROMISSE-APO SEVERE. Patients with severe disease had the highest rates of PROMISSE-APO (P = .03 across groups).
(PROMISSE stands for the Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and Systemic Lupus Erythematosus study.)
None of the women died
Dr. Clowse said it was a pleasant surprise to find that none of the women died, though patients with severe ILD had more adverse outcomes. Only 2.1% were treated in an intensive care unit during or soon after delivery. In 4.2%, ILD patients had significant shortness of breath due to fluid volume overload around the time of delivery.
For the women who had normal-to-moderate lung disease, Dr. Clowse said, “they really had remarkably good outcomes, really pretty comparable to the general population. About 15% delivered preterm and about 20% suffered a pregnancy loss.”
Dr. Easter, who was not involved with the study, noted the large number of Black women in the cohort.
“Focusing in on improving outcomes for Black and Brown women related to pregnancy in our country is a much-needed undertaking,” Dr. Easter said.
Being able to quote percentages from this research, based on a good-sized study “at least gives people a benchmark about what kind of risk they are willing to assume for themselves,” she said.
For providers, being able to place this rare disease within the spectrum of other diseases where there is more data is also very helpful, she said.
Dr. Clowse said in an interview that the preponderance of Black women in the study was a surprise but may be explained by two factors: Sarcoidosis is seen more frequently in Black women and in the study area in North Carolina there is a large population of Black women.
“Also, our patients with more severe lupus, the ones who are more likely to have interstitial lung disease, are often Black and that’s likely contributing as well,” she said.
Multidisciplinary teams advised
Dr. Clowse emphasized that women with ILD need multidisciplinary teams in pregnancy and should be managed at tertiary care centers where there is a full complement of obstetric and internal medicine experts.
“We do recommend evaluating the severity of their lungs and their heart disease around the time of pregnancy and during pregnancy if they have shortness of breath,” she said.
“We currently recommend that these patients with moderate or severe disease stay in the hospital for up to a week, just for monitoring,” she said.
Dr. Easter said having that kind of access to a large academic healthcare center should be an important part of the decision-making.
Patients need to think about whether they would have access to care similar to what the researchers are describing when they are making the decision to pursue or continue pregnancy, she said.
The study was sponsored by Pfizer Inc. Dr. Clowse reported relationships with UCB, GlaxoSmithKline, AstraZeneca, and Pfizer. Dr. Easter has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.









