User login
14-year-old girl • history of bullying • lack of social support • multiple linear scars on breasts • Dx?
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
THE CASE
A 14-year-old girl with no significant medical history presented to the office accompanied by her mother for a routine well-adolescent visit. She attended school online due to a history of severe bullying and, when interviewed alone, admitted to a lack of a social life as a result. On questioning, she denied tobacco, alcohol, or illicit drug use. Her gender identity was female. Her sexual orientation was toward both males and females, but she was not sexually active. She denied exposure to physical or emotional violence at home and said she did not feel depressed or think about suicide.
Physical examination revealed multiple erythematous linear scars surrounding the areola of both breasts. When questioned about these lesions, she admitted to cutting herself on the breasts during the past several months but again denied suicidal intent. She believed that her behavior was a normal coping mechanism.
The physical exam was otherwise normal. Lab results, including thyroid-stimulating hormone and complete blood count, were within normal limits.
THE DIAGNOSIS
The physical exam findings and the patient’s self report pointed to a diagnosis of nonsuicidal self-injurious (NSSI) behavior involving cutting.
DISCUSSION
The NSSI behavior displayed by this patient is a common biopsychosocial disorder observed in adolescents. Self-injury is defined as the deliberate injuring of body tissues without suicidal intent.1 Self-injurious behavior typically begins when patients are 13 to 16 years of age, and cutting is the most common form. Most acts occur on the arms, legs, wrists, and stomach.2 Studies have shown that the prevalence of this behavior is on the rise among adolescents, from about 7% in 2014 to between 14% and 24% in 2015.3
Risk for suicide. Although a feature of NSSI is the lack of suicidal intent, this type of high-risk behavior is associated with past, present, and future suicide attempts. It is important for physicians to identify NSSI in an adolescent, as it is linked to a 7-fold increased risk for a suicide attempt.3
Screening for NSSI. Less than one-fifth of adolescents who injure themselves come to the attention of health care providers.4 We propose that primary care physicians add NSSI to the list of risky behaviors—including drug abuse, sexual activity, and depression—for which they screen during well-child visits.
Continue to: Identifying risk factors
Identifying risk factors. The case patient experienced bullying and reported a nonheterosexual orientation, both of which have been demonstrated as strong risk factors for NSSI.5 Female gender has also been identified as a risk factor for NSSI.3
In adolescent psychiatric samples, prevalence rates of NSSI were found to be as high as 60% for 1 incident of NSSI and around 50% for repetitive NSSI.6 NSSI coincides with other psychiatric comorbidities, including eating disorders, mood disorders (depression), anxiety disorders, posttraumatic stress disorder, and borderline personality disorder.3 In a study of 93 subjects, each of whom was a self-reported abuse survivor with a history of self-injury, 96% were in therapy for diagnoses that included posttraumatic stress disorder (73%), dissociative disorder (40%), borderline personality disorder (37%), and multiple personality disorder (29%).7
The experience of adverse childhood events also increases risk for NSSI. This includes parental neglect, abuse, or deprivation.6 Insecure paternal attachment and parental neglect are significant predictors for women, while childhood separation is a primary predictor for men.8 Indirect childhood maltreatment, such as witnessing domestic violence or increased parental critique, is also associated with NSSI.8 NSSI is also more prevalent among young people who identify with a subculture such as gothic or emo.6
Why they do it and how to help
In multiple studies aimed at identifying reasons for self-injury, converging evidence suggests that nearly all patients act with the intent of alleviating negative affect.9 Patients self-harm to regulate distress, anxiety, and frustration that they perceive to be intolerable.9 They may self-harm to generate feeling when emotionally empty or to avert suicidal intent.9 For others, self-harm is a way to communicate their distress.
How to proceed. After a physician identifies NSSI, the patient should be assessed for suicidality and medical severity of the injury.3 Factors associated with higher likelihood of suicidality in patients with NSSI include multiple self-injurious methods and locations, early age of onset, longer history of NSSI, recent worsening of the injuries, simultaneous substance use, and the perception that the patient is addicted to self-injury.10
Continue to: It is also important...
It is also important to ask the patient whether she or he has told anyone about the behavior. Participation in NSSI communities may reinforce it.3
Treatment found to be effective for NSSI involves dialectical behavioral therapy, cognitive behavioral therapy, and mentalization-based therapy.11
Our patient was admitted to the hospital several weeks after her well visit because she expressed suicidal ideation. After being discharged, she was referred to outpatient Psychiatry with a treatment plan that included cognitive behavioral therapy.
THE TAKEAWAY
While our patient may have concealed her self-injurious experience because of stigma and concern about others’ reactions, there were several risk factors for NSSI in her history that prompted further investigation with a skin exam.
If a patient presents with 1 or more risk factors, an initial assessment for possible NSSI should be performed with detailed history-taking and a skin exam. Once NSSI is identified, the initial response and tone of questioning toward the patient need to convey a sense of genuine curiosity about the patient’s experience. From there, the physician can avail the patient to the proper treatment modalities.
NSSI patients can be resistant to sharing and participating in support groups. However, a referred counselor can follow up with a stepwise approach to slowly gain the trust of the individual, find the root cause, and get the patient to a point where she or he is ready to start the necessary treatment.
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
1. Klonsky ED, Glenn CR. Resisting urges to self-injure. Behav Cogn Psychother. 2008;36:211-220. doi: 10.1017/S1352465808004128
2. Whitlock J, Eckenrode J, Silverman D. Self-injurious behaviors in a college population. Pediatrics. 2006;117:1939-1948. doi: 10.1542/peds.2005-2543
3. Lewis SP, Heath NL. Non-suicidal self-injury among youth. J Pediatr. 2015;166:526-630. doi: 10.1016/j.jpeds.2014.11.062
4. Ystgaard M, Arensman E, Hawton K, et al. Deliberate self-harm in adolescents: comparison between those who receive help following self-harm and those who do not. J Adolesc. 2009;32: 875-891.
5. Lereya ST, Copeland WE, Costello EJ, et al. Adult mental health consequences of peer bullying and maltreatment in childhood: two cohorts in two countries. Lancet Psychiatry. 2015;2:524-531. doi: 10.1016/S2215-0366(15)00165-0
6. Brown RC, Plener PL. Non-suicidal self-injury in adolescence. Curr Psychiatry Rep. 2017;19:20. doi: 10.1007/s11920-017-0767-9
7. Briere J, Gil E. Self-mutilation in clinical and general population samples: prevalence, correlates, and functions. Am J Orthopsychiatry. 1998;68:609-620. doi:10.1037/h0080369
8. Gratz KL, Conrad SD, Roemer L. Risk factors for deliberate self-harm among college students. Am J Orthopsychiatry. 2002;1:128-140. doi: 10.1037//0002-9432.72.1.128
9. Klonsky ED. The functions of deliberate self-injury: a review of the evidence. Clin Psychol Rev. 2007;27:226-239.
10. Nock MK, Joiner Jr. TE, Gordon KH, et al. Non-suicidal self-injury among adolescents: diagnostic correlates and relation to suicide attempts. Psychiatry Res. 2006;144:65-72. doi: 10.1016/j.psychres.2006.05.010
11. Lewis SP, Baker TG. The possible risks of self-injury websites: a content analysis. Arch Suicide Res. 2011;15:390-396. doi: 10.1080/13811118.2011.616154
Conservative or surgical management for that shoulder dislocation?
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
The shoulder, or glenohumeral joint, is the most commonly dislocated large joint; dislocation occurs at a rate of 23.9 per 100,000 person/years.1,2 There are 2 types of dislocation: traumatic anterior dislocation, which accounts for roughly 90% of dislocations, and posterior dislocation (10%).3 Anterior dislocation typically occurs when the patient’s shoulder is forcefully abducted and externally rotated.
The diagnosis is made after review of the history and mechanism of injury and performance of a complete physical exam with imaging studies—the most critical component of diagnosis.4 Standard radiographs (anteroposterior, axillary, and scapular Y) can confirm the presence of a dislocation; once the diagnosis is confirmed, closed reduction of the joint should be performed.1 (Methods of reduction are beyond the scope of this article but have been recently reviewed.5)
Risk for recurrence drives management choices
Following an initial shoulder dislocation, the risk of recurrence is high.6,7 Rates vary based on age, pathology after dislocation, activity level, type of immobilization, and whether surgery was performed. Overall, age is the strongest predictor of recurrence: 72% of patients ages 12 to 22 years, 56% of those ages 23 to 29 years, and 27% of those older than 30 years experience recurrence.6 Patients who have recurrent dislocations are at risk for arthropathy, fear of instability, and worsening surgical outcomes.6
Reducing the risk of a recurrent shoulder dislocation has been the focus of intense study. Proponents of surgical stabilization argue that surgery—rather than a trial of conservative treatment—is best when you consider the high risk of recurrence in young athletes (the population primarily studied), the soft-tissue and bony damage caused by recurrent instability, and the predictable improvement in quality of life following surgery.
In a recent systematic review and meta-analysis, there was evidence that, for first-time traumatic shoulder dislocations, early surgery led to fewer repeat shoulder dislocations (number needed to treat [NNT] = 2-4.7). However, a significant number of patients primarily treated nonoperatively did not experience a repeat shoulder dislocation within 2 years.2
The conflicting results from randomized trials comparing operative intervention to conservative management have led surgeons and physicians in other specialties to take different approaches to the management of shoulder dislocation.2 In this review, we aim to summarize considerations for conservative vs surgical management and provide clinical guidance for primary care physicians.
When to try conservative management
Although the initial treatment after a traumatic anterior shoulder dislocation has been debated, a recent meta-analysis of randomized controlled trials showed that at least half of first-time dislocations are successfully treated with conservative management.2 Management can include immobilization for comfort and/or physical therapy. Age will play a role, as mentioned earlier; in general, patients older than 30 have a significant decrease in recurrence rate and are good candidates for conservative therapy.6 It should be noted that much of the research with regard to management of shoulder dislocations has been done in an athletic population.
Continue to: Immobilization may benefit some
Immobilization may benefit some
Recent evidence has determined that the duration of immobilization in internal rotation does not impact recurrent instability.8,9 In patients older than 30, the rate of repeat dislocation is lower, and early mobilization after 1 week is advocated to avoid joint stiffness and minimize the risk of adhesive capsulitis.10
Arm position during immobilization remains controversial.11 In a classic study by Itoi et al, immobilization for 3 weeks in internal rotation vs 10° of external rotation was associated with a recurrence rate of 42% vs 26%, respectively.12 In this study, immobilization in 10° of external rotation was especially beneficial for patients ages 30 years or younger.12
Cadaveric and magnetic resonance imaging (MRI) studies have shown external rotation may improve the odds of labral tear healing by positioning the damaged and intact parts of the glenoid labrum in closer proximity.13 While this is theoretically plausible, a recent Cochrane review found insufficient evidence to determine whether immobilization in external rotation has any benefits beyond those offered by internal rotation.14 A recent systematic review and meta-analysis found that immobilization in external rotation vs internal rotation after a first-time traumatic shoulder dislocation did not change outcomes.2 With that said, most would prefer to immobilize in the internal rotation position for ease.
More research is needed. A Cochrane review highlighted the need for continued research.14 Additionally, most of the available randomized controlled trials to date have consisted of young men, with the majority of dislocations related to sports activities. Women, nonathletes, and older patients have been understudied to date; extrapolating current research to those groups of patients may not be appropriate and should be a focus for future research.2
Physical therapy: The conservative standard of care
Rehabilitation after glenohumeral joint dislocation is the current standard of care in conservative management to reduce the risk for repeat dislocation.15 Depending on the specific characteristics of the instability pattern, the approach may be adapted to the patient. A recent review focused on the following 4 key points: (1) restoration of rotator cuff strength, focusing on the eccentric capacity of the external rotators, (2) normalization of rotational range of motion with particular focus on internal range of motion, (3) optimization of the flexibility and muscle performance of the scapular muscles, and (4) increasing the functional sport-specific load on the shoulder girdle.
Continue to: A common approach to the care of...
A common approach to the care of a patient after a glenohumeral joint dislocation is to place the patient’s shoulder in a sling for comfort, with permitted pain-free isometric exercise along with passive and assisted elevation up to 100°.16 This is followed by a nonaggressive rehabilitation protocol for 2 months until full recovery, which includes progressive range of motion, strength, proprioception, and return to functional activities.16
More aggressive return-to-play protocols with accelerated timelines and functional progression have been studied, including in a multicenter observational study that followed 45 contact intercollegiate athletes prospectively after in-season anterior glenohumeral instability. Thirty-three of 45 (73%) athletes returned to sport for either all or part of the season after a median 5 days lost from competition, with 12 athletes (27%) successfully completing the season without recurrence. Athletes with a subluxation event were 5.3 times more likely to return to sport during the same season, compared with those with dislocations.17
Dynamic bracing may also allow for a safe and quicker return to sport in athletes18 but recently was shown to not impact recurrent dislocation risk.19
Return to play should be based on subjective assessment as well as objective measurements of range of motion, strength, and dynamic function.15 Patients who continue to have significant weakness and pain at 2 to 3 weeks post injury despite physical therapy should be re-evaluated with an MRI for concomitant rotator cuff tears and need for surgical referral.20
When to consider surgical intervention
In a recent meta-analysis, recurrent dislocation and instability occurred at a rate of 52.9% following nonsurgical treatment.2 The decision to perform surgical intervention is typically made following failure of conservative management. Other considerations include age, gender, bone loss, and cartilage defect.21,22 Age younger than 30 years, participation in competition, contact sports, and male gender have been associated with an increased risk of recurrence.23-25 For this reason, obtaining an MRI at time of first dislocation can help facilitate surgical decisions if the patient is at high risk for surgical need.26
Continue to: An increasing number...
An increasing number of dislocations portends a poor outcome with nonoperative treatment. Kao et al demonstrated a second dislocation leads to another dislocation in 19.6% of cases, while 44.3% of those with a third dislocation event will sustain another dislocation.24 Surgery should be considered for patients with recurrent instability events to prevent persistent instability and decrease the amount of bone loss that can occur with repetitive dislocations.
What are the surgical options?
Several surgical options exist to remedy the unstable shoulder. Procedures can range from an arthroscopic repair to an open stabilization combined with structural bone graft to replace a bone defect caused by repetitive dislocations.
Arthroscopic techniques have become the mainstay of treatment and account for 71% of stabilization procedures performed.21 These techniques cause less pain in the early postoperative period and provide for a faster return to work.27 Arthroscopy has the additional advantage of allowing for complete visualization of the glenohumeral joint to identify and address concomitant pathology, such as intra-articular loose bodies or rotator cuff tears.
Open repair was the mainstay of treatment prior to development of arthroscopic techniques. Some surgeons still prefer this method—especially in high-risk groups—because of a lower risk of recurrent disloca-tion.28 Open techniques often involve detachment and repair of the upper subscapularis tendon and are more likely to produce long-term losses in external rotation range of motion.28
Which one is appropriate for your patient? The decision to pursue an open or arthroscopic procedure and to augment with bone graft depends on the amount of glenoid and humeral head bone loss, patient activity level, risk of recurrent dislocation, and surgeon preference.
Continue to: For the nonathletic population...
For the nonathletic population, the timing of injury is less critical and surgery is typically recommended after conservative treatment has failed. In an athletic population, the timing of injury is a necessary consideration. An injury midseason may be “rehabbed” in hopes of returning to play. Individuals with injuries occurring at the end of a season, who are unable to regain desired function, and/or with peri-articular fractures or associated full-thickness rotator cuff tears may benefit from sooner surgical intervention.21
Owens et al have described appropriate surgical indications and recommendations for an in-season athlete.21 In this particular algorithm, the authors suggest obtaining an MRI for decision making, but this is specific to in-season athletes wishing to return to play. In general, an MRI is not always indicated for patients who wish to receive conservative therapy but would be indicated for surgical considerations. The algorithm otherwise uses bone and soft-tissue injury, recurrent instability, and timing in the season to help determine management.21
Outcomes: Surgery has advantages …
Recurrence rates following surgical intervention are considerably lower than with conservative management, especially among young, active individuals. A recent systematic review by Donohue et al demonstrated recurrent instability rates following surgical intervention as low as 2.4%.29 One study comparing the outcome of arthroscopic repair vs conservative management showed that the risk of postoperative instability was reduced by 20% compared to other treatments.7 Furthermore, early surgical fixation can improve quality of life, produce better functional outcomes, decrease time away from activity, increase patient satisfaction, and slow the development of glenohumeral osteoarthritis produced from recurrent instability.2,7
Complications. Surgery does carry inherent risks of infection, anesthesia effects, surgical complications, and surgical failure. Recurrent instability is the most common complication following surgical shoulder stabilization. Rates of recurrent instability after surgical stabilization depend on patient age, activity level, and amount of bone loss: males younger than 18 years who participate in contact competitive sports and have significant bone loss are more likely to have recurrent dislocation after surgery.23 The type of surgical procedure selected may decrease this risk.
While the open procedures decrease risk of postoperative instability, these surgeries can pose a significant risk of complications. Major complications for specific open techniques have been reported in up to 30% of patients30 and are associated with lower levels of surgeon experience.31 While the healing of bones and ligaments is always a concern, 1 of the most feared complications following stabilization surgery is iatrogenic nerve injury. Because of the axillary nerve’s close proximity to the inferior glenoid, this nerve can be injured without meticulous care and can result in paralysis of the deltoid muscle. This injury poses a major impediment to normal shoulder function. Some procedures may cause nerve injuries in up to 10% of patients, although most injuries are transient.32
Continue to: Bottom line
Bottom line
Due to the void of evidence-based guidelines for conservative vs surgical management of primary shoulder dislocation, it would be prudent to have a risk-benefit discussion with patients regarding treatment options.
Patients older than 30 years and those with uncomplicated injuries are best suited for conservative management of primary shoulder dislocations. Immobilization is debated and may not change outcomes, but a progressive rehabilitative program after the initial acute injury is helpful. Risk factors for failing conservative management include recurrent dislocation, subsequent arthropathy, and additional concomitant bone or soft-tissue injuries.
Patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability, and highly physically active individuals are best suited for surgical management. Shoulder arthroscopy has become the mainstay of surgical treatment for shoulder dislocations. Outcomes are favorable and dislocation recurrence is low after surgical repair. Surgery does carry its own inherent risks of infection, anesthesia effects, complications during surgery, and surgical failure leading to recurrent instability.
Cayce Onks, DO, MS, ATC, Penn State Hershey, Milton S. Hershey Medical Center, Penn State College of Medicine, Family and Community Medicine H154, 500 University Drive, PO Box 850, Hershey, PA 17033-0850; [email protected]
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
1. Lin K, James E, Spitzer E, et al. Pediatric and adolescent anterior shoulder instability: clinical management of first time dislocators. Curr Opin Pediatr. 2018;30:49-56.
2. Kavaja L, Lähdeoja T, Malmivaara A, et al. Treatment after traumatic shoulder dislocation: a systematic review with a network meta-analysis. Br J Sports Med. 2018;52:1498-1506.
3. Brelin A, Dickens JF. Posterior shoulder instability. Sports Med Arthrosc Rev. 2017;25:136-143.
4. Galvin JW, Ernat JJ, Waterman BR, et al. The epidemiology and natural history of anterior shoulder dislocation. Curr Rev Musculoskelet Med. 2017;10:411-424.
5. Rozzi SL, Anderson JM, Doberstein ST, et al. National Athletic Trainers’ Association position statement: immediate management of appendicular joint dislocations. J Athl Train. 2018;53:1117-1128.
6. Hovelius L, Saeboe M. Arthropathy after primary anterior shoulder dislocation: 223 shoulders prospectively followed up for twenty-five years. J Shoulder Elbow Surg. 2009;18:339-347.
7. Polyzois I, Dattani R, Gupta R, et al. Traumatic first time shoulder dislocation: surgery vs non-operative treatment. Arch Bone Jt Surg. 2016;4:104-108.
8. Cox CL, Kuhn JE. Operative versus nonoperative treatment of acute shoulder dislocation in the athlete. Curr Sports Med Rep. 2008;7:263-268.
9. Kuhn JE. Treating the initial anterior shoulder dislocation—an evidence-based medicine approach. Sports Med Arthrosc Rev. 2006;14:192-198.
10. Smith TO. Immobilization following traumatic anterior glenohumeral joint dislocation: a literature review. Injury. 2006;37:228-237.
11. Liavaag S, Brox JI, Pripp AH, et al. Immobilization in external rotation after primary shoulder dislocation did not reduce the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:897-904.
12. Itoi E, Hatakeyama Y, Sato T, et al. Immobilization in external rotation after shoulder dislocation reduces the risk of recurrence: a randomized controlled trial. J Bone Joint Surg Am. 2007;89:2124-2131.
13. Miller BS, Sonnabend DH, Hatrick C, et al. Should acute anterior dislocations of the shoulder be immobilized in external rotation? A cadaveric study. J Shoulder Elbow Surg. 2004;13:589-592.
14. Hanchard NCA, Goodchild LM, Kottam L. Conservative management following closed reduction of traumatic anterior dislocation of the shoulder. Cochrane Database Syst Rev. 2014;(4):CD004962.
15. Cools AM, Borms D, Castelein B, et al. Evidence-based rehabilitation of athletes with glenohumeral instability. Knee Surg Sports Traumatol Arthrosc. 2016;24:382-389.
16. Lafuente JLA, Marco SM, Pequerul JMG. Controversies in the management of the first time shoulder dislocation. Open Orthop J. 2017;11:1001-1010.
17. Dickens JF, Owens BD, Cameron KL, et al. Return to play and recurrent instability after in-season anterior shoulder instability: a prospective multicenter study. Am J Sports Med. 2014;42:2842-2850.
18. Conti M, Garofalo R, Castagna A, et al. Dynamic brace is a good option to treat first anterior shoulder dislocation in season. Musculoskelet Surg. 2017;101(suppl 2):169-173.
19. Shanley E, Thigpen C, Brooks J, et al. Return to sport as an outcome measure for shoulder instability. Am J Sports Med. 2019;47:1062-1067.
20. Gombera MM, Sekiya JK. Rotator cuff tear and glenohumeral instability. Clin Orthop Relat Res. 2014;472:2448-2456.
21. Owens BD, Dickens JF, Kilcoyne KG, et al. Management of mid-season traumatic anterior shoulder instability in athletes. J Am Acad Orthop Surg. 2012;20:518-526.
22. Ozturk BY, Maak TG, Fabricant P, et al. Return to sports after arthroscopic anterior stabilization in patients aged younger than 25 years. Arthroscopy. 2013;29:1922-1931.
23. Balg F, Boileau P. The instability severity index score. A simple preoperative score to select patients for arthroscopic or open shoulder stabilisation. J Bone Joint Surg Br. 2007;89:1470-1477.
24. Kao J-T, Chang C-L, Su W-R, et al. Incidence of recurrence after shoulder dislocation: a nationwide database study. J Shoulder Elbow Surg. 2018;27:1519-1525.
25. Porcillini G, Campi F, Pegreffi F, et al. Predisposing factors for recurrent shoulder dislocation after arthroscopic treatment. J Bone Joint Surg Am. 2009;91:2537-2542.
26. Magee T. 3T MRI of the shoulder: is MR arthrography necessary? AJR Am J Roentgenol. 2009;192:86-92.
27. Green MR, Christensen KP. Arthroscopic versus open Bankart procedures: a comparison of early morbidity and complications. Arthroscopy. 1993;9:371-374.
28. Khatri K, Arora H, Chaudhary S, et al. Meta-analysis of randomized controlled trials involving anterior shoulder instability. Open Orthop J. 2018;12:411-418.
29. Donohue MA, Owens BD, Dickens JF. Return to play following anterior shoulder dislocations and stabilization surgery. Clin Sports Med. 2016;35:545-561.
30. Griesser MJ, Harris JD, McCoy BW, et al. Complications and re-operations after Bristow-Latarjet shoulder stabilization: a systematic review. J Shoulder Elbow Surg. 2013;22:286-292.
31. Ekhtiari S, Horner NS, Bedi A, et al. The learning curve for the Latarjet procedure: a systematic review. Orthop J Sports Med. 2018;6:2325967118786930.
32. Shah AA, Butler RB, Romanowski J, et al. Short-term complications of the Latarjet procedure. J Bone Joint Surg Am. 2012;94:495-501.
PRACTICE RECOMMENDATIONS
› Start with conservative management of shoulder dislocation in patients older than 30 years and those with uncomplicated injuries. B
› Discourage strict immobilization; its utility is debated and it may not change outcomes. B
› Recommend a progressive rehabilitative program after the initial acute shoulder injury. B
› Consider surgical management for patients younger than 30 years who have complicated injuries with bone or cartilage loss, rotator cuff tears, or recurrent instability or for the highly physically active individual. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Functional neurological disorder: A practical guide to an elusive Dx
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.
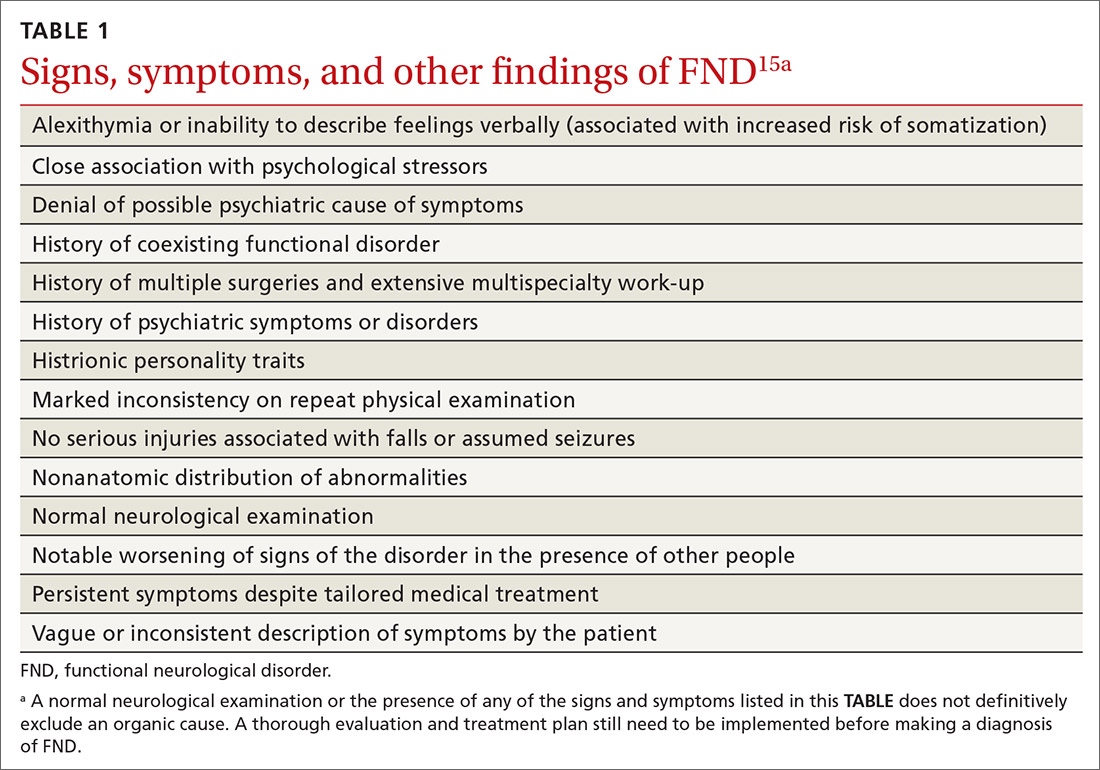
Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.
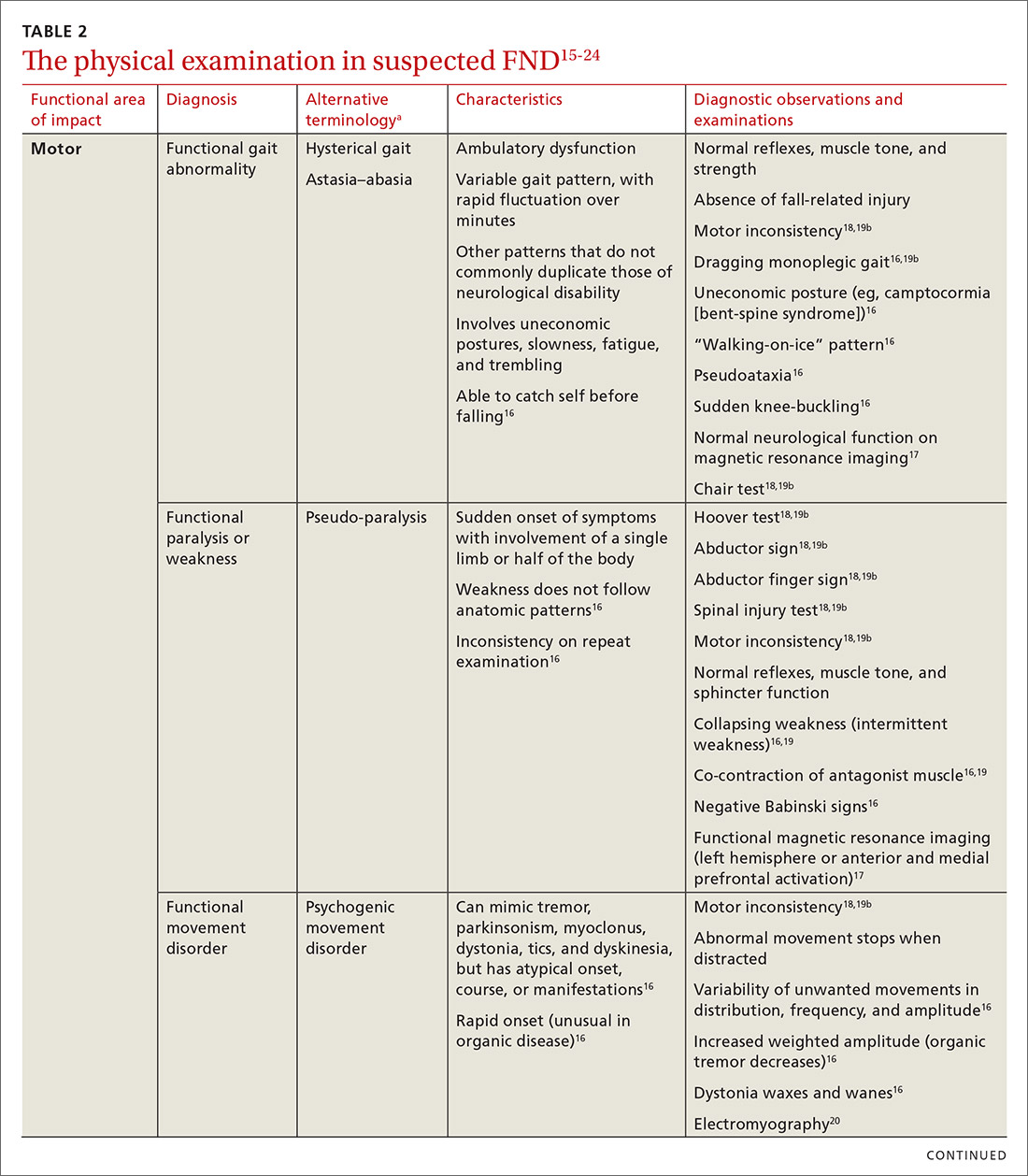
Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.
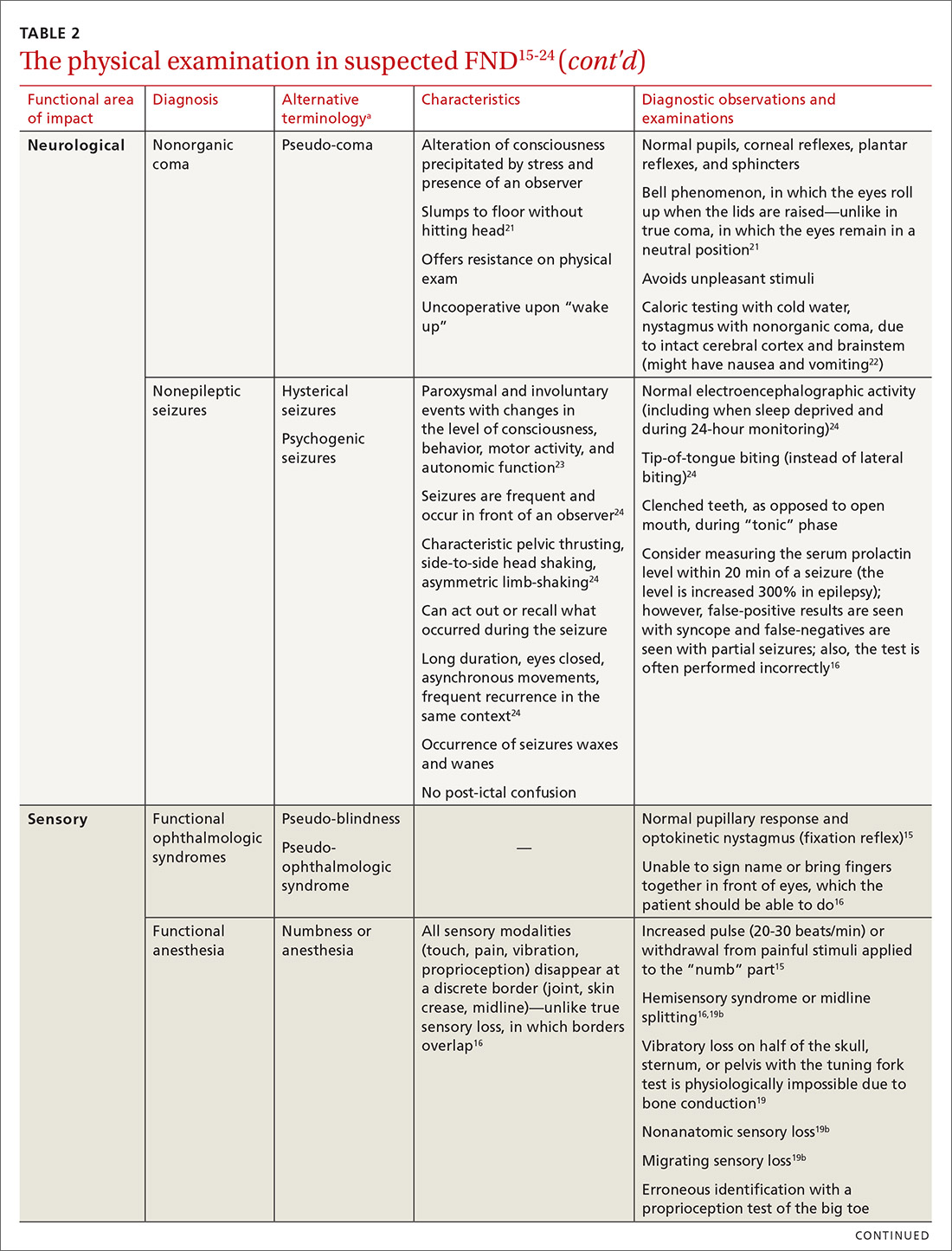
The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.
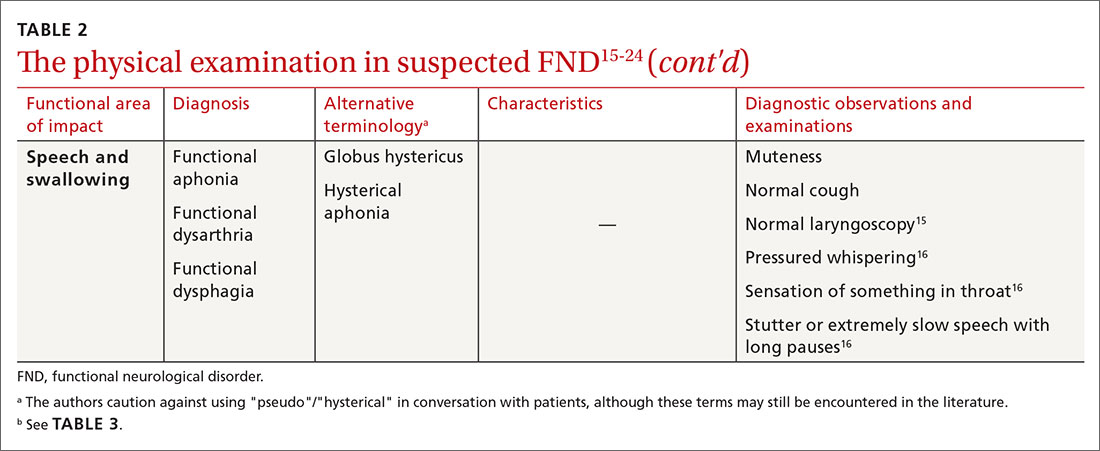
Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.
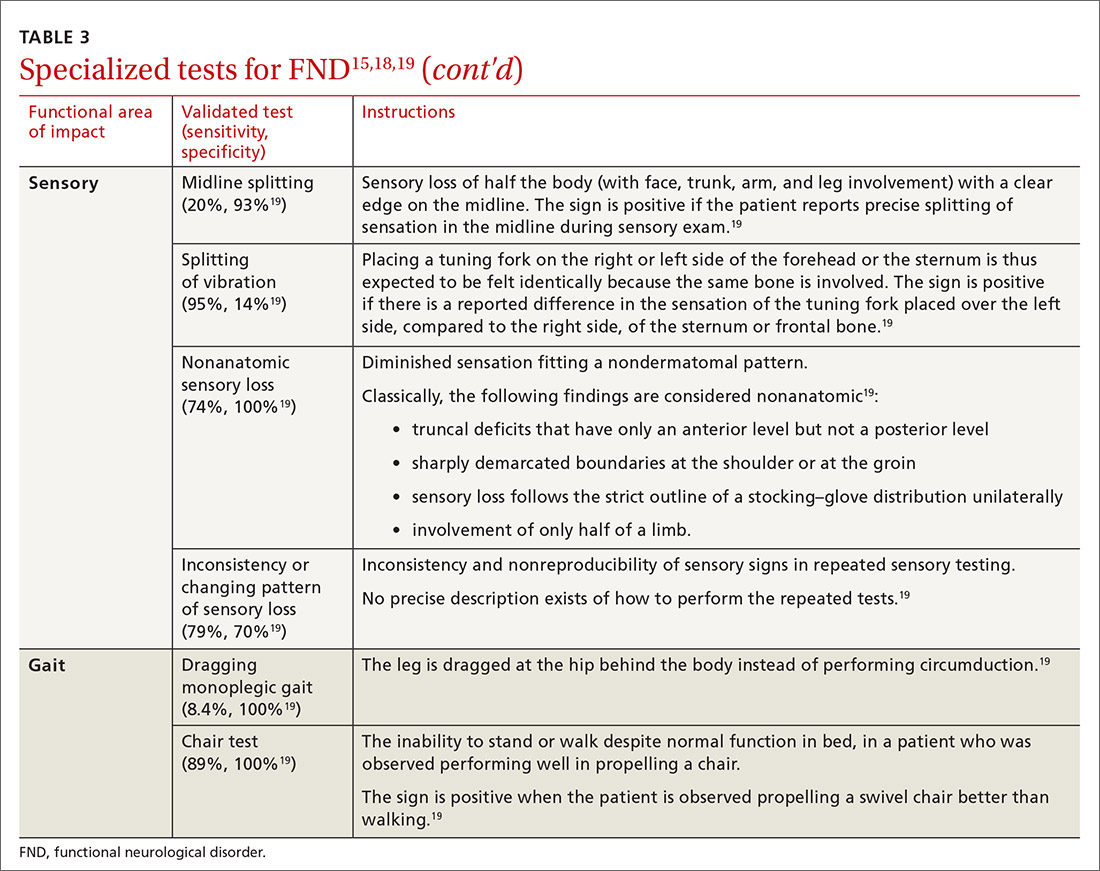
Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.
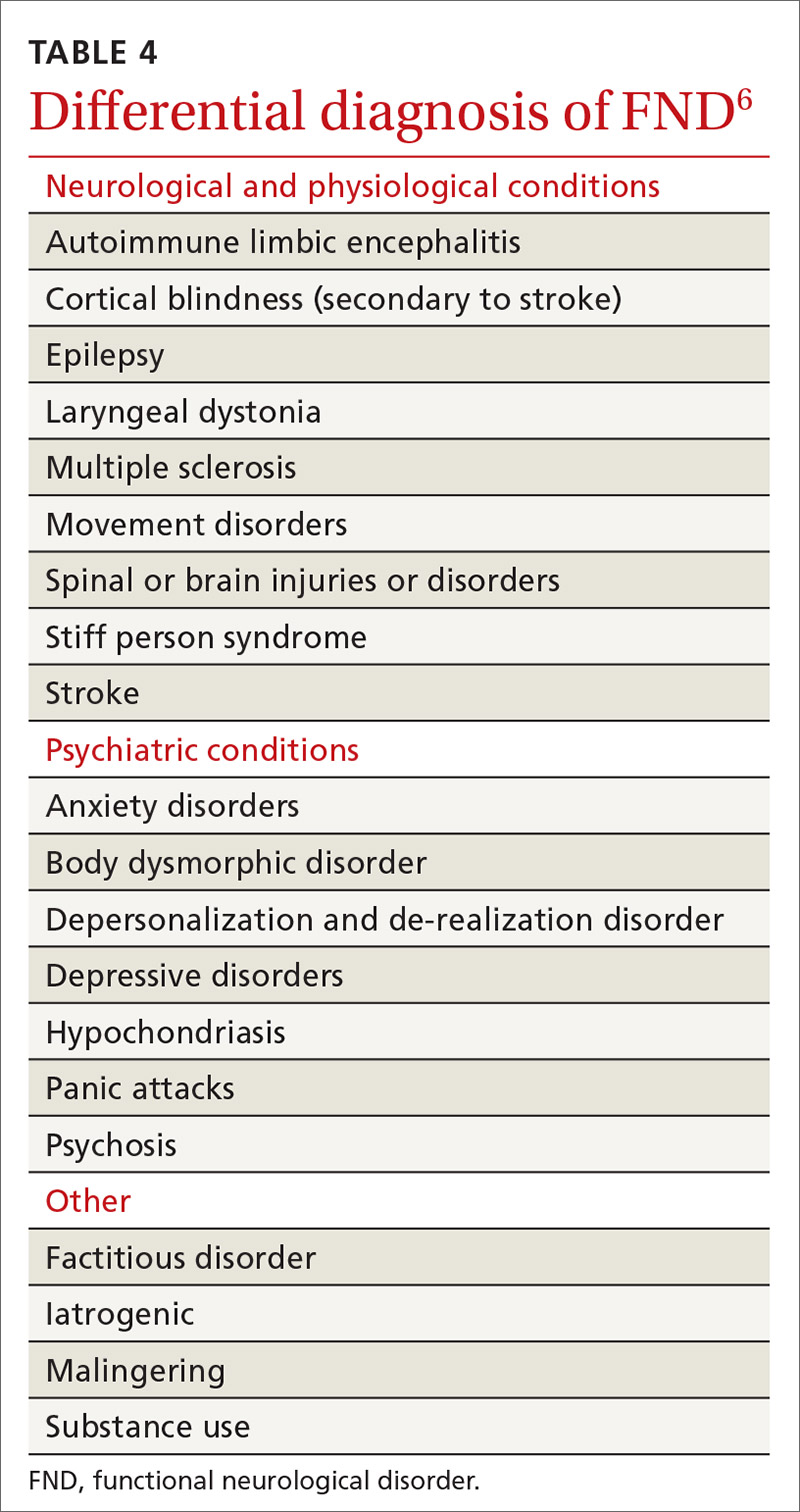
A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.

Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.

Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.

The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.

Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.

Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.

A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
CASE
John D,* a 25-year-old patient with an otherwise unremarkable medical history, describes 2 months of daily headache, lower-extremity weakness, and unsteady gait that began fairly suddenly during his first deployment in the US Army. He explains that these symptoms affected his ability to perform his duties and necessitated an early return stateside for evaluation and treatment.
Mr. D denies precipitating trauma or unusual environmental exposures. He reports that, stateside now, symptoms continue to affect his ability to work and attend to personal and family responsibilities.
Asked about stressors, Mr. D notes the birth of his first child approximately 3 months ago, while he was deployed, and marital stressors. He denies suicidal or homicidal ideation.
* The patient’s name has been changed to protect his identity.
The challenge of identifying and managing FND
A functional neurological disorder (FND) is a constellation of psychological, physiological, and neurological symptoms, without an identifiable organic etiology, a conscious decision, or secondary gain for the patient,1 that adversely impacts functioning in 1 or more significant life domains.
Given the high throughput of patients in primary care practices, family physicians can expect to encounter suspected cases of FND in their practices. Regrettably, however, a lack of familiarity with the disorder and its related problems (eg, nonorganic paralysis, sensory loss, nonepileptic seizures, and abnormal movements) can add as much as $20,000 in excess direct and indirect costs of care for every such patient.1 In this article, we synthesize the recent literature on FND so that family physicians can expand their acumen in understanding, identifying, and evaluating patients whose presentation suggests FND.
An underrecognized entity
A precise estimate of the prevalence of FND is difficult to determine because the disorder is underrecognized and misdiagnosed and because it is often accompanied by the confounding of psychological and physiological comorbidities. A 2012 study estimated the annual incidence of FND to be 4 to 12 cases for every 100,000 people2; in primary care and outpatient neurology settings, prevalence is 6% to 22% of all patients.3,4 Stone and colleagues identified functional neurological symptoms as the second most common reason for outpatient neurology consultation,5 with 1 nonepileptic seizure patient seen for every 6 epileptic patients, and functional weakness presenting at the same rate as multiple sclerosis.6
Continue to: Demographics of patients with FND...
Demographics of patients with FND vary, depending on presenting neurological symptoms and disorder subtype. Existing data indicate a correlation between FND and younger age, female sex, physical disability,7 and a history of abuse or trauma.3,8 A challenge in concretely ascertaining the prevalence of FND is that conditions such as fibromyalgia, chronic pelvic pain, globus hystericus, and nonepileptic seizures can also be characterized as medically unexplained functional disorders, even within the network of neurology care.4
Misdiagnosis and bias are not uncommon
Ambiguity in classifying and evaluating FND can affect physicians’ perceptions, assessment, and care of patients with suggestive presenting symptoms. A major early challenge in diagnosing FND is the inconsistency of characterizing terminology (pseudoneurological, somatic, dissociative, conversion, psychogenic, hysterical, factitious, functional, medically unexplained9,10) and definitions in the literature. Neurological symptoms of unidentifiable organic cause can greatly diminish quality of life4; FND is a scientifically and clinically useful diagnosis for many combinations of nonrandomly co-occurring symptoms and clinical signs.
The pitfall of misdiagnosis. Remain cautious about making a diagnosis of FND by exclusion, which might yield an incorrect or false-negative finding because of an atypical presentation. It is important to avoid misdiagnosis by prematurely closing the differential diagnosis; instead, keep in mind that a medically unexplained diagnosis might be better explained by conducting a robust social and medical history and obtaining additional or collateral data, or both, along with appropriate consultation.4,9
Misdiagnosis can lead to a circuitous and costly work-up, with the potential to increase the patient’s distress. You can reduce this burden with early recognition of FND and centralized management of multidisciplinary care, which are more likely to lead to an accurate and timely diagnosis—paramount to empowering patients with access to the correct information and meaningful support needed to enhance treatment and self-care.9
Bias, haste, and dismissal are unproductive. Even with a clear definition of FND, it is not uncommon for a physician to rapidly assess a patient’s clinical signs, make a diagnosis of “unknown etiology,” or openly question the veracity of complaints. Furthermore, be aware of inadvertently characterizing FND using the prefix “pseudo” or the term “hysterical,” which can be psychologically discomforting for many patients, who legitimately experience inexplicable symptoms. Such pejoratives can lead to stigmatizing and misleading assessments and treatment paths4—courses of action that can cause early and, possibly, irreparable harm to the patient–physician relationship and increase the patient’s inclination to go “doctor-shopping,” with associated loss of continuity of care.
Why is it difficult to diagnose FND?
The latest (5th) edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) describes conversion, somatoform disorder, and FND synonymously.DSM-5 diagnostic criteria for conversion disorderare11:
- a specified type of symptom or deficit of altered voluntary motor or sensory function (eg, weakness, difficulty swallowing, slurred speech, seizures)
- clinical evidence of the incompatibility of the symptom or deficit and any recognized neurological or medical disorder
- incapability of better explaining the symptom or deficit as another medical or mental disorder.
- The symptom or deficit causes distress or impairment that (1) is clinically significant in occupational, social, or other important areas of function or (2) warrants medical evaluation.
The overarching feature of these criteria is the inconsistency of symptoms with recognized neurological, physiological, or psychiatric conditions. Although identification of psychological factors can help clarify and provide a treatment direction, such identification is not essential for making a diagnosis of FND. Malingering does not need to be refuted as part of establishing the diagnosis.12
Continue to: In contrast...
In contrast, the World Health Organization’s ICD-10 Classification of Mental and Behavioural Disorders groups diagnostic criteria for FND among the dissociative disorders13:
- Clinical features are specified for the individual dissociative disorder (motor, sensory, convulsions, mixed).
- Evidence is absent of a physical disorder that might explain symptoms.
- Evidence of psychological causation is present in clear temporal association with stressful events and problems or disturbed relationships, even if the patient denies such association.
Note the emphasis on psychological causation and exclusion of purposeful simulation of symptoms, as opposed to a primarily unconscious disconnection from the patient’s body or environment.
ICD-10 guidelines acknowledge the difficulty of finding definitive evidence of a psychological cause and recommend provisional diagnosis of FND if psychological factors are not readily apparent.14 Of note, many patients with FND are affected psychologically by their condition, with an impact on mood, behaviors, and interpersonal interactions, although not necessarily to a clinically diagnostic degree. Therefore, a psychiatric diagnosis alone is not a necessary precursor for the diagnosis of an FND.
CASE
History. Mr. D’s history is positive for light alcohol consumption (“2 or 3 cans of beer on weekends”) and chewing tobacco (he reports stopping 6 months earlier) and negative for substance abuse. The family history is positive for maternal hypertension and paternal suicide when the patient was 10 years old (no other known paternal history).
Physical findings. The review of systems is positive for intermittent palpitations, lower-extremity weakness causing unsteady gait, and generalized headache.
Vital signs are within normal limits, including blood pressure (120/82 mm Hg) and heart rate (110 beats/min). The patient is not in acute distress; he is awake, alert, and oriented × 3. No murmurs are heard; lungs are clear bilaterally to auscultation. There is no tenderness on abdominal palpation, and no hepatomegaly or splenomegaly; bowel sounds are normal. No significant bruising or lacerations are noted.
Neurology exam. Cranial nerves II-XII are intact. Pupils are equal and reactive to light. Reflexes are 2+ bilaterally. Muscle strength and tone are normal; no tremors are noted. Babinski signs are normal. A Romberg test is positive (swaying).
Continue to: Mr. D has an antalgic gait...
Mr. D has an antalgic gait with significant swaying (without falling); bent posture; and unsteadiness that requires a cane. However, he is able to get up and off the exam table without assistance, and to propel himself, by rolling a chair forward and backward, without difficulty.
Conducting a diagnostic examination
Taking the history. Certain clues can aid in the diagnosis of FND (TABLE 1).15 For example, the patient might have been seen in multiple specialty practices for a multitude of vague symptoms indicative of potentially related conditions (eg, chronic fatigue, allergies and sensitivities, fibromyalgia, and other chronic pain). The history might include repeated surgeries to investigate those symptoms (eg, laparoscopy, or hysterectomy at an early age). Taking time and care to explore all clinical clues, patient reports, and collateral data are therefore key to making an accurate diagnosis.

Note any discrepancies between the severity of reported symptoms and functional ability. A technique that can help elucidate a complex or ambiguous medical presentation is to ask the patient to list all their symptoms at the beginning of the interview. This has threefold benefit: You get a broad picture of the problem; the patient is unburdened of their concerns and experiences your validation; and a long list of symptoms can be an early clue to a diagnosis of FND.

Other helpful questions to determine the impact of symptoms on the patient’s well-being include inquiries about16:
- functional impairment
- onset and course of symptoms
- potential causal or correlating events
- dissociative episodes
- previous diagnoses and treatments
- the patient’s perceptions of, and emotional response to, their illness
- a history of abuse.

The physical examination to determine the presence of FND varies, depending on the functional area of impact (eg, motor, neurological, sensory, speech and swallowing). Pay particular attention to presenting signs and clues, and balance them with the patient’s report (or lack of report). Endeavor to demonstrate positive functional signs, such as a positive Hoover test, which relies on the principle of synergistic muscle contraction. You might see evidence of inconsistency, such as weakness or a change in gait, under observation, that seemingly resolves when the patient is getting on and off the exam table.16Table 215-24 describes areas affected by FND, characteristics of the disorder, and related diagnostic examinations.

Table 315,18,19 reviews validated special exams that can aid in making the diagnosis. Additional special tests are discussed in the literature.15-24 These tests can be helpful in narrowing the differential diagnosis but have not been validated and should be used with caution.

Some clinical signs associated with FND might be affected by other factors, including socioeconomic status, limited access to health care, low health literacy, poor communication skills, and physician bias. Keep these factors in mind during the visit, to avoid contributing further to health disparities among groups of patients affected by these problems.

Continue to: CASE
CASE
The work-up over the next month for Mr. D includes numerous studies, all yielding results that are negative or within normal limits: visual acuity; electrocardiography and an event monitor; laboratory testing (including a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, vitamin B12, folate, and vitamin D); magnetic resonance imaging of the brain and lumbar spine; lumbar puncture; and electromyography.
The score on the 9-item Patient Health Questionnaire for depression is 4 (severity: “none or minimal”); on the 7-item Generalized Anxiety Disorder scale, 0 (“no anxiety disorder”).
Referral. A neurology work-up of headache, lower extremity weakness, and unsteady gait to address several diagnostic possibilities, including migraine and multiple sclerosis, is within normal limits. A cardiology work-up of palpitations is negative for arrhythmias and other concerning findings.
Mr. D declines psychiatric and psychological evaluations.
Building a differential diagnosisis a formidable task
The differential diagnosis of FND is vast. It includes neurological, physiological, and psychiatric symptoms and disorders; somatization; and malingering (Table 4).6 Any disorder or condition in these areas that is in the differential diagnosis can be precipitated or exacerbated by stress; most, however, do not involve loss of physical function.12 In addition, the diagnosis of an FND does not necessarily exclude an organic disorder.

A patient’s presentation becomes complicated—and more difficult to treat—when functional symptoms and an unrelated underlying or early-stage neurological condition coexist. For example, a patient with epilepsy might also have dissociative seizures atop their organic disorder. Neurological disease is considered a risk factor for an overlying FND—just as the risk of depression or anxiety runs concurrently with other chronic diseases.14
Focus on clinical signs to narrow the differential. A thorough social and medical history and physical examination, as discussed earlier, help narrow the differential diagnosis of organic and medically unexplained disorders. Well-defined imaging or laboratory protocols do not exist to guide physicians to a definitive diagnosis, however.
Continue to: Psychiatric conditions
Psychiatric conditions can coexist with the diagnosis of FND, but might be unrelated. A systematic review of the literature showed that 17% to 42% of patients with FND had a concurrent anxiety disorder. Depression disorders were co-diagnosed in 19% to 71% of patients with FND; dissociative and personality disorders were noted, as well.25 However, coexisting psychiatric diagnosis might more likely be associated with distress from the presenting functional neurological symptoms, not linked to the FND diagnosis itself.12 This shift in understanding is reflected in the description of FND in the DSM-5.11
CASE
Mr. D reports debilitating headaches at return office visits. Trials of abortive triptans provide no relief; neither do control medications (beta-blockers, coenzyme Q10, magnesium, onabotulinumtoxinA [Botox], topiramate, and valproate). Lower-extremity weakness and unsteadiness are managed with supportive devices, including a cane, and physical therapy.
Importance of establishing a multidisciplinary approach
The complexity of FND lends itself to a multidisciplinary approach during evaluation and, eventually, for treatment. The assessment and diagnostic intervention that you provide, along with the contributions of consulted specialists (including neurology, physical and occupational therapy, psychiatry, psychology, and other mental health professionals) establishes a team-based approach that can increase the patient’s sense of support and reduce excessive testing and unnecessary medications, surgeries, and other treatments.26
Family physicians are in the ideal position to recognize the patient’s functional capacity and the quality of symptoms and to provide timely referral (eg, to Neurology and Psychiatry) for confirmation of the diagnosis and then treatment.
Evidence-based treatment options include:
- psychotherapy, with an emphasis on cognitive behavioral therapy
- physical therapy
- psychopharmacology
- promising combinations of physical and psychological treatment to improve long-term functionality.27
A promising diagnostic tool
The most significant update in the FND literature is on functional neuroimaging for assessing the disorder. Early findings suggest an intricate relationship between mind and body regarding the pathological distortion in FND. And, there is clear evidence that neuroimaging—specifically, functional magnetic resonance imaging—shows changes in brain activity that correspond to the patient’s symptom report. That said, imaging is not the recommended standard of care in the initial work-up of FND because of its cost and the fact that the diagnosis is principally a clinical undertaking.17,28
Call to action
Offer a generous ear. Begin the diagnostic pursuit by listening carefully and fully to the patient’s complaints, without arriving at a diagnosis with unwarranted bias or haste. This endeavor might require support from other clinical staff (eg, nurses, social workers, case managers) because the diagnostic process can be arduous and lengthy.
Continue to: Convey the diagnosis with sensitivity
Convey the diagnosis with sensitivity. Inquire about the patient’s perceptions and impairments to best personalize your diagnostic explanations. Delivery of the diagnosis might affect the patient’s acceptance and compliance with further testing and treatment of what is generally a persistent and treatment-resistant disorder; poor delivery of diagnostic information can impair the patient–physician relationship and increase the risk of disjointed care. Many patients find that improved patient–physician communication is therapeutic.29
Let the patient know that you’re taking her seriously. Validate patient concerns with a nonstigmatizing diagnostic label; discuss the diagnostic parameters and cause of symptoms in layman’s terms; and emphasize the potential for reversibility.30 Some patients are not satisfied with having a diagnosis of FND until they are reassured with normal results of testing and provided with referral; even then, some seek further reassurance.
Key tenets of managing care for patients who have been given a diagnosis of FND include:
- nonjudgmental, positive regard
- meaningful expression of empathy
- multidisciplinary coordination
- avoidance of unnecessary testing and harmful treatments
- descriptive and contextual explanations of the diagnosis.
Last, keep in mind that the course of treatment for FND is potentially prolonged and multilayered.
CASE
After many visits with his family physician and the neurology and cardiology specialists, as well as an extensive work-up, the physician approaches Mr. D with the possibility of a diagnosis of FND and proposes a multidisciplinary plan that includes:
- a course of physical and occupational therapy
- development of individualized cognitive behavioral tools
- weekly personal and marital counseling
- initiation of a selective serotonin reuptake inhibitor for anxiety
- monthly visits with his family physician.
Months after his return from deployment for evaluation and treatment, Mr. D is able to return to military duty. He reports that his quality of life has improved.
CORRESPONDENCE
Roselyn W. Clemente Fuentes, MD, FAAFP, Eglin Family Medicine Residency, 307 Boatner Road, Eglin AFB, FL 32547; [email protected].
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
1. Konnopka A, Schaefert R, Heinrich S, et al. Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom. 2012;81:265-275.
2. Carson AJ, Brown R, David AS, et al; on behalf of UK-FNS. Functional (conversion) neurological symptoms: research since the millennium. J Neurol Neurosurg Psychiatry. 2012;83:842-850.
3. Stone J, Carson A, Duncan R, et al. Who is referred to neurology clinics?—the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. 2010;112:747-751.
4. Evens A, Vendetta L, Krebs K, et al. Medically unexplained neurologic symptoms: a primer for physicians who make the initial encounter. Am J Med. 2015;128:1059-1064.
5. Stone J, Reuber M, Carson A. Functional symptoms in neurology: mimics and chameleons. Pract Neurol. 2013;13:104-113.
6. Stone J, Warlow C, Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537-1551.
7. Carson A, Stone J, Hibberd C, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. 2011;82:810-813.
8. Fink P, Hansen MS, Oxhøj M-L. The prevalence of somatoform disorders among internal medical inpatients. J Psychosom Res. 2004;56:413-418.
9. Thomas LE. Are your patient’s medically unexplained symptoms really “all in her head”? Med Hypotheses. 2012;78:542-547.
10. Ding JM, Kanaan RAA. What should we say to patients with unexplained neurological symptoms? How explanation affects offence. J Psychosom Res. 2016;91:55-60.
11. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. American Psychiatric Association; 2013.
12. Stone J, LaFrance WC Jr, Levenson JL, et al. Issues for DSM-5: Conversion disorder. Am J Psychiatry. 2010;167:626-627.
13. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1994. Accessed January 21, 2021. www.who.int/classifications/icd/en/bluebook.pdf
14. Stone J, Carson A, Duncan R, et al. Which neurological diseases are most likely to be associated with “symptoms unexplained by organic disease.” J Neurol. 2012;259:33-38.
15. Shaibani A, Sabbagh M. Pseudoneurologic syndromes: recognition and diagnosis. Am Fam Physician. 1998;57:2485-2494.
16. Stone J, Carson A, Sharpe M. Functional symptoms and signs in neurology: assessment and diagnosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i2-i12.
17. Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol Clin. 2014;44:323-337.
18. McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics. 2018;59:358-368.
19. Daum C, Hubschmid M, Aybek S. The value of ‘positive’ clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J Neurol Neurosurg Psychiatry. 2014;85:180-190.
20. Brown P, Thompson PD. Electrophysiological aids to the diagnosis of psychogenic jerks, spasms, and tremor. Mov Disord. 2001;16:595-599.
21. Ludwig L, McWhirter L, Williams S, et al. Functional coma. In: Hallett M, Stone J, Carson A, eds. Handbook of Clinical Neurology: Volume 139: Functional Neurologic Disorders. 1st ed. Academic Press; 2016:313.
22. Miller NR, Subramanian PS, Patel VR. Walsh and Hoyt’s Clinical Neuro-ophthalmology. 3rd ed. Wolters Kluwer; 2016:512-513.
23. Takazaki K, Stransky AD, Miller G. Psychogenic nonepileptic seizures: diagnosis, management, and bioethics. Pediatr Neurol. 2016;62:3-8.
24. Sahaya K, Dholakia SA, Sahota PK. Psychogenic non-epileptic seizures: a challenging entity. J Clin Neurosci. 2011;18:1602-1607.
25. Gelauff J, Stone J, Edwards M, et al. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220-226.
26. Kranick SM, Gorrindo T, Hallett M. Psychogenic movement disorders and motor conversion: a roadmap for collaboration between neurology and psychiatry. Psychosomatics. 2011;52:109-116.
27. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. 2012;11:250-260.
28. Burgmer M, Kugel H, Pfleiderer B, et al. The mirror neuron system under hypnosis—brain substrates of voluntary and involuntary motor activation in hypnotic paralysis. Cortex. 2013;49:437-445.
29. van Bokhoven MA, Koch H, van der Weijden T, et al. Influence of watchful waiting on satisfaction and anxiety among patients seeking care for unexplained complaints, Ann Fam Med. 2009;7:112-120.
30. Stone J, Carson A, Hallet M. Explanation as treatment for functional neurologic disorders. Handb Clin Neurol. 2016;139:543-553.
PRACTICE RECOMMENDATIONS
› Avoid using stigmatizing terminology (eg, adding the prefix “pseudo” or the adjective “hysterical”) to characterize a suspected functional neurological disorder (FND) or a medically unexplained disorder. C
› Refrain from ordering functional magnetic resonance imaging as part of the routine evaluation of suspected FND. C
› Validate the patient‘s concerns with an appropriate diagnostic label; use layman’s terms to discuss the diagnostic parameters of FND and the cause of symptoms; and emphasize treatment possibilities and plans. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Opioid use common for pain in multiple sclerosis
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
, new research shows.
“This high level of opioid use supports that better pain management treatment options, including nonpharmacological options, are needed for people with MS and pain,” wrote the authors of the study, which was presented at ACTRIMS Forum 2021, held by the Americas Committee for Treatment and Research in Multiple Sclerosis.
Previous research has shown that more than 50% of people with MS report chronic pain that is serious enough to interfere with daily activities, employment, and quality of life. Many with MS report that pain is one of their worst symptoms, the authors noted.
With surprisingly few studies evaluating opioid use in the MS population, Cinda L. Hugos, PT, associate professor of neurology with the VA Portland Health Care System and the department of neurology, Oregon Health and Science University, Portland, and colleagues investigated the issue in a sample of patients participating in a U.S. multisite MS fatigue management trial conducted between 2013 and 2014.
Of the 281 participants with MS in the study, 58 patients (20.6%) reported using prescription opioids. Among them, most – 44 (76%) – reported regular daily use, 10 (17%) reported using the drugs only as needed, 3 (5%) reported only short-term use, including after recent injury or dental surgery, and 1 provided incomplete information.
Those who reported opioid use had significantly worse fatigue scores on the Modified Fatigue Impact Scale (P = .015) and worse pain scores (P < .0001).
There were no significant differences in terms of age (mean age, 53 years), gender (69% were female), or race (in both groups, about 76% were White). No significant differences were seen in disability or depression scores in the opioid users versus nonusers.
“In this sample of people with multiple sclerosis who self-reported fatigue and volunteered to join an MS fatigue management research study, more than one in five reported using prescription opioids and nearly one in six used opioids daily,” the authors wrote. “Opioid users had more pain and fatigue than nonusers.”
Commenting on the study, Jeffrey Cohen, MD, president of ACTRIMS, said that the findings are consistent with his observations that “in the general population, opioids often are used to treat chronic pain in people with MS.”
But they’re not getting the drugs from his clinic. “We do not prescribe opioids in our clinic, referring such patients to a chronic pain program,” Dr. Cohen said. “However, there clearly is need for better treatment options.”
A previous study on opioid use by people with MS, published in 2015, found even higher rates – 42% reported having ever used opioids, and 38% reported currently using opioids.
Although reports of opioid use by patients with MS have been lacking, more has been published on the emerging use of cannabis-related products. One recent study showed that nearly half of people with MS reported using a cannabis-based therapy for nerve-based pain and sleep disturbances.
Although cannabis is considered safer than opioids, the authors noted that it has its own significant drawback – a “paucity of provider guidance.”
“The range of perceived benefits and potential differential effects of THC and cannabinoid highlight the need for personalized, evidence-based guidelines regarding cannabinoid use,” they wrote.
Stretching program for spasticity shows benefits
With spasticity representing a key contributor to MS pain and affecting more than 80% of people with MS, Ms. Hugos and colleagues are developing an alternative to medication – a nonpharmacologic stretching regimen called Spasticity: Take Control” (STC).
Based on evidence-based strategies for the treatment of spasticity in MS, the program involves exercises with daily routines of 15-20 minutes over 6 months.
In a pilot study of 66 patients, also presented at the ACTRIMS meeting, the investigators reported that the program showed significant reductions in pain severity and interference, measured with the Brief Pain Inventory–Short Form, compared with a control consisting of range of motion instruction over 6 months.
The study also offered insights on the specific areas of pain. Among those who reported chronic pain (42% in the STC group and 63.3% in the range-of-motion group), the pain was most frequently reported in the lower back (74.3%), legs (68.6%), or lower back and legs (88.6%).
Ms. Hugos noted that the findings suggest a potentially important nonpharmacologic alternative to spasticity-related pain in MS.
“Stretching is the cornerstone treatment for spasticity from all causes, but there is very little information on stretching exercises in MS or any other conditions,” Ms. Hugos said. “[Our] pilot study is the first and only study using a standardized, daily stretching exercise program to treat MS spasticity,” she said.
“A fully powered study is needed to better understand the impact of different types of exercise on pain severity and interference in multiple sclerosis,” she noted.
Ms. Hugos has received consulting fees from Greenwich Biosciences, Evidera, and Techspert.io. Dr. Cohen has received personal compensation for consulting for Adamas, Atara, Bristol-Myers Squibb, Convelo, MedDay, and Mylan.
A version of this article first appeared on Medscape.com.
FROM ACTRIMS FORUM 2021
How to convince patients muscle pain isn’t a statin Achilles heel: StatinWISE
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Another randomized trial, on the heels of the recently published SAMSON, has concluded – many would say confirmed – that .
Affected patients who sorely doubt that conclusion might possibly embrace statins, researchers say, if the new trial’s creative methodology could somehow be applied to them in clinical practice.
The recent SAMSON trial made waves in November 2020 by concluding, with some caveats, that about 90% of the burden of muscle symptoms reported by patients on statins may be attributable to a nocebo effect; that is, they are attributed to the drugs – perhaps because of negative expectations – but not actually caused by them.
The new trial, StatinWISE (Statin Web-based Investigation of Side Effects), triple the size but similar in design and conducted parallel to SAMSON, similarly saw no important differences in patient-reported muscle symptom prevalence or severity during administration of atorvastatin 20 mg/day or placebo, in withdrawal from the study because of such symptoms, or in patient quality of life.
The findings also support years of observational evidence that argues against a statin effect on muscle symptoms except in rare cases of confirmed myopathy, as well as results from randomized trials like ODYSSEY ALTERNATIVE and GAUSS-3, in which significant muscle symptoms in “statin-intolerant” patients were unusual, note StatinWISE investigators in their report, published online Feb. 24 in BMJ, with lead author Emily Herrett, MSc, PhD, London School of Hygiene and Tropical Medicine.
“I’m hoping it can change minds a bit and reassure people. That was part of the reason we did it, to inform this debate about harms and benefits of statins,” principal investigator Liam Smeeth, MBChB, MSc, PhD, from the same institution, said during a virtual press conference on the trial conducted by the U.K. nonprofit Science Media Centre.
“In thinking through whether to take a statin or not, people can be reassured that these muscle symptoms are rare; they aren’t common. Aches and pains are common, but are not caused by statins,” said Dr. Smeeth, who is senior author on the trial publication.
Another goal of the 200-patient study, he said, was to explore whether patients who had experienced muscle symptoms on a statin but were willing to explore whether the statin was to blame could be convinced – depending on what they learned in the trial – to stay on the drugs.
It seemed to work; two-thirds of the participants who finished the study “decided that they would actually want to try starting statins again, which was quite amazing.”
But there was a “slight caveat,” Dr. Smeeth observed. “To join our trial, yes, you had to have had a bad experience with statins, but you probably had to be a little bit open to the idea of trying them again. So, I can’t claim that that two-thirds would apply to everybody in the population.”
Because StatinWISE entered only patients who had reported severe muscle symptoms on a statin but hadn’t showed significant enzymatic evidence of myopathy, all had either taken themselves off the statin or were “considering” it. And the study had excluded anyone with “persistent, generalized, unexplained muscle pain” regardless of any statin therapy.
“This was very deliberately a select group of people who had serious problems taking statins. This was not a random sample by any means,” Dr. Smeeth said.
“The patients in the study were willing to participate and take statins again,” suggesting they “may not be completely representative of all those who believe they experience side effects with statins, as anyone who refused to take statins ever again would not have been recruited,” observed Tim Chico, MBChB, MD, University of Sheffield (England) in a Science Media Centre press release on StatinWISE.
Still, even among this “supersaturated group of people” selected for having had muscle symptoms on statins, Dr. Smeeth said at the briefing, “in almost all cases, their pains and aches were no worse on statins than they were on placebo. We’re not saying that anyone is making up their aches and pains. These are real aches and pains. What we’re showing very clearly is that those aches and pains are no worse on statins than they are on placebo.”
Rechallenge is possible
Some people are more likely than others to experience adverse reactions to any drug, “and that’s true of statins,” Neil J. Stone, MD, Northwestern University, Chicago, told this news organization. But StatinWISE underscores that many patients with muscle symptoms on the drugs can be convinced to continue with them rather than stop them entirely.
“The study didn’t say that everybody who has symptoms on a statin is having a nocebo effect,” said Dr. Stone, vice chair for the multisociety 2018 Guideline on the Management of Blood Cholesterol, who was not involved with StatinWISE.
“It simply said,” allowing for some caveats, “that a significant number of patients may have symptoms that don’t preclude them from being rechallenged with a statin again, once they understand what this nocebo effect is.”
And, Dr. Stone said, “it amplifies the 2018 guidelines, with their emphasis on the clinician-patient discussion before starting therapy,” by showing that statin-associated muscle pain isn’t necessarily caused by the drugs and isn’t a reason to stop them.
“That there is a second study confirming SAMSON is helpful, and the results are helpful because they say many of these patients, once they are shown the results, can be rechallenged and will then tolerate statins,” Steven E. Nissen, MD, Cleveland Clinic, said in an interview.
“They were able to get two-thirds of those completing the trial into long-term treatment, which I think is obviously very admirable and very important,” said Dr. Nissen, who was GAUSS-3 principal investigator but not associated with StatinWISE.
“I think it is important, however, that we not completely dismiss patients who complain of adverse effects. Because, in fact, there probably are some people who do have muscle-related symptoms,” he said. “But you know, to really call somebody statin intolerant, they really should fail three statins, which would be a very good standard.”
In his experience, said Patrick M. Moriarty, MD, who directs the Atherosclerosis & Lipoprotein-Apheresis Center at the University of Kansas Medical Center, Kansas City, perhaps 80%-90% of patients who believe they are statin intolerant because of muscle symptoms are actually not statin intolerant at all.
“I think a massive amount of it is supratentorial,” Dr. Moriarty, who was not part of StatinWISE, told this news organization. It comes directly from “what they heard, what they read, or what they were told – and at their age, they’re going to have aches and pains.”
Value of the n-of-1 trial
Dr. Smeeth and colleagues framed StatinWISE in part as a test of a strategy for overcoming nocebo-based aversion to statins. One goal was to see whether these methods might be helpful in practice for convincing patients who want to reject statins because of muscle symptoms to give the drugs another chance.
In StatinWISE, patients were individually assigned to take atorvastatin or placebo in randomized order with multiple blinding during each of six successive 2-month periods, so that they were on one or the other agent half the time. They rated their symptoms at the end of each period.
So the trial in composite was, as the publication states, “a series of randomized, placebo-controlled n-of-1 trials.” SAMSON followed a similar scheme, except – as previously reported – it had specified 4 months of atorvastatin, 4 months of placebo, and 4 months with patients on neither statin nor placebo.
StatinWISE “provides a useful approach (the n = 1 study) that could be used in real life to help patients understand the cause of their own possible side effects, which could also be applied to medications other than statins,” Dr. Chico added in the Science Media Centre release.
“I often encounter people who have a firmly held view that statins cause muscle pains, even when they haven’t taken these medications themselves, and I hope that this study may help change this view and make them willing to try such an ‘experiment,’ ” he said.
Others aren’t sure an experiment resembling an n-of-1 trial would be practical or effective when conducted in routine practice.
More efficient and useful, Dr. Moriarty noted, would be for physicians to nurture a close relationship with patients, one that could help transform their negative feelings about statins into a willingness to accept the drugs. “This is a trust you have to build; these are human beings.”
He said getting the patient’s confidence is critical. “You have to explain the pluses and minuses of getting treatment, of the 30% reduction in cardiovascular events that occur with the statin. You don’t go ‘testing this and that.’ I think it’s more about getting them on board.”
No statin effect on muscle symptoms
Patients in StatinWISE were recruited from 50 primary care practices in England and Wales from December 2016 to April 2018, the report notes; their mean age was 69 years, and 58% were men. Of the 200 patients, 151 recorded muscle-symptom scores for at least one statin period and one placebo period, and so were included in the primary-endpoint assessment.
The mean muscle symptom score was lower on statin therapy than on placebo (1.68 vs. 2.57), but there was no significant difference in adjusted analysis (mean difference, –0.11 (95% confidence interval, –0.36 to 0.14; P = .40).
Statins showed no significant effect on development of muscle symptoms overall, it was reported, with an odds ratio of 1.11 (99% confidence interval, 0.62-1.99). Nor was there an effect on “muscle symptoms that could not be attributed to another cause,” (OR, 1.22; 95% CI, 0.77-1.94).
Of the 80 withdrawals during the study for any reason, 43% occurred when the patient was on the statin, 49% when the patient was on placebo, and 9% after randomization but before either statin or placebo had been initiated. Of those, 33 were because of “intolerable muscle symptoms,” says the report. But withdrawal occurred about as often on statin therapy as off the drug – 9% and 7%, respectively – throughout the 1-year study.
“This study provides further evidence through the lived experience of individuals that muscle pains often attributed to statins are not due to the drug,” said Sir Nilesh J. Samani, MBChB, MD, medical director for the British Heart Foundation, as quoted in the Science Media Centre press release.
“The use of each patient as their own control in the trial provides a powerful way of distinguishing the effect of a statin from that of taking a pill,” he said. “The findings should give confidence to patients who may be concerned about taking statins.”
StatinWISE was funded by the National Institute for Health Research-Health Technology Program and sponsored by the London School of Hygiene and Tropical Medicine. The authors declare that they have “no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.” Dr. Smeeth reports receiving grants from GlaxoSmithKline, and personal fees for advisory work from AstraZeneca and GlaxoSmithKline. Dr. Stone reports no industry relationships or other disclosures. Dr. Nissen reports that his center has received funding for clinical trials from AbbVie, Amgen, AstraZeneca, Cerenis, Eli Lilly, Esperion, Medtronic, MyoKardia, Novartis, Orexigen, Pfizer, Takeda, The Medicines Company, and Silence Therapeutics; that he is involved in these trials but receives no personal remuneration; and that he consults for many pharmaceutical companies but requires them to donate all honoraria or fees directly to charity so that he receives neither income nor a tax deduction. Dr. Chico had no conflicts. Dr. Moriarty declared no relevant conflicts of interest. Dr. Samani had no disclosures.
A version of this article first appeared on Medscape.com.
Painful hand and foot plaques
This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

This patient had hand and foot psoriasis with the classic thick scale and erythema on his palms and soles. Additionally, in the area of the sole toward the heel, he had hyperpigmented macules called mahogany spots that are another hallmark of psoriasis. Pitting and distal onycholysis were also visible on his right ring finger.
This case illustrates how the painful plaques seen in hand and foot psoriasis—and other forms of psoriasis—can interfere with work and usual daily activities. UVA or narrowband UVB light therapy is a treatment option but requires 3 visits per week, which is not conducive to most people’s work schedules. Acitretin can be prescribed to decrease the abnormal proliferation of keratinocytes; however, adverse reactions can be expected, like this patient’s dry skin and itching. Furthermore, acitretin is a retinoid, like isotretinoin, which can cause severe birth defects, as well as hypertriglyceridemia and transaminitis. Pregnancy needs to be avoided for 3 years due to the teratogenicity and long washout period, so it should not be used in women with reproductive potential.1
This patient was initially treated with topical calcipotriene (a vitamin D derivative) and clobetasol (high-potency topical steroid) bid but did not have adequate improvement. Screening lab tests showed elevated liver enzymes, precluding treatment with methotrexate (and acitretin, which he’d received previously). He was started on apremilast, an oral phosphodiesterase inhibitor, because his insurance denied adalimumab. Apremilast can cause diarrhea, depression, nausea, and headache. Other than some loose stools, the patient tolerated apremilast well and showed significant improvement in his psoriasis at his 3-month follow-up visit.
Photo and text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
1. Kaushik SB, Lebwohl MG. Review of safety and efficacy of approved systemic psoriasis therapies. Int J Dermatol. 2019;58:649-658. doi: 10.1111/ijd.14246.
Endometriosis-associated ovarian cancer
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Endometriosis, which affects 1 in 10 women, is one of the most common conditions that gynecologists treat. It is known to cause pain, pelvic adhesive disease, endometriotic cyst formation, and infertility. However, even more sinister, it also increases a woman’s risk for the development of epithelial ovarian cancer (known as endometriosis-associated ovarian cancer or EAOC). A woman with endometriosis has a two- to threefold increased risk of developing epithelial ovarian cancer, compared with nonaffected women.1 This risk appears to be concentrated in the premenopausal age group, particularly the fifth decade of life. After menopause their risk of developing cancer returns to a baseline level.
EAOC classically presents as clear cell or endometrioid adenocarcinomas, rather than high-grade serous carcinomas. However, low-grade serous carcinomas are also frequently observed in this cohort.2,3 Unlike high-grade serous carcinoma, EAOC is more likely to be diagnosed at an early stage, with the majority at stage I or II, and prognosis is better. After matching for age and stage with cases of high-grade serous carcinoma, there is improved disease-free and overall survival observed among cases of EAOC of clear cell and endometrioid histologic cell types.4 The phenomenon of dual primaries (synchronous endometrial and ovarian cancer) occurs more frequently in EAOC than it does in patients with nonendometriosis-related high-grade serous cancer (25% vs. 4%).
The genomics of these endometriosis-associated cancers are quite distinct. Similar to benign endometriosis implants, EAOC is associated with genomic mutations in ARID1A, PIK3CA, and PTEN, as well as progesterone resistance.1,2 Multiple studies have shown that the adjacent eutopic endometrium carries similar gene mutations as those found in both benign endometriotic implants and EAOC.2 This may explain the higher incidence (twofold) of endometrial cancer in patients with endometriosis as well as the increased incidence of dual ovarian and endometrial cancer primaries.
Just as there are multiple theories regarding the mechanism of benign endometriosis, we have theories rather than conclusions regarding the origins of EAOC. One such theory is that it develops from malignant transformation in an existing endometriotic cyst.5 Endometriotic cysts provide an iron-rich environment which promotes reactive oxygen species that promote carcinogenesis by inducing gene mutations and epigenetic alterations. However, if prolonged exposure to oxidative stress within endometriotic cysts were to be the cause for EAOC, we would expect to see a progressively increasing incidence of ovarian cancer over time in patients with expectantly managed cysts. However, in cases of expectant management, an initial, early, increased risk for cancer within the first 5 years is followed by a subsequent decreasing incidence over time.6 This early incidence spike suggests that some endometriotic cysts may have been misclassified as benign, then rapidly declare themselves as malignant during the observation period rather than a transformation into malignancy from a benign endometrioma over time.
An alternative, and favored, theory for the origins of EAOC are that endometrial cells with carcinogenic genomic alterations reflux through the fallopian tubes during menstruation and settle onto the ovarian epithelium which itself is damaged from recent ovulation thus providing an environment that is highly suitable for oncogenesis.2 Genomic analyses of both the eutopic endometrium and malignant cells in patients with EAOC have shown that both tissues contain the same genomic alterations.1 Given that menstruation, including retrograde menstruation, ends after menopause, this mechanism supports the observation that EAOC is predominantly a malignancy of premenopausal women. Additionally, salpingectomy and hysterectomy confers a protective effect on the development of EAOC, theoretically by preventing the retrograde transfer of these mutant progenitor endometrial cells. Furthermore, the factors that increase the number of menstrual cycles (such as an early age of menarche and delayed or nonchildbearing states) increases the risk for EAOC and factors that inhibit menstruation, such as oral contraceptive pill use, appear to decrease its risk.
EAOC most commonly arises in the ovary, and not in the deep endometriosis implants of adjacent pelvic structures (such as the anterior and posterior cul de sac and pelvic peritoneum). It is suggested that the ovary itself provides a uniquely favorable environment for carcinogenesis. As stated above, it is hypothesized that refluxed endometrial cells, carrying important progenitor mutations, may become trapped in the tissues of traumatized ovarian epithelium, ripe with inflammatory changes, post ovulation.2 This microenvironment may promote the development of malignancy.
Given these theories and their supporting evidence, how can we attempt to reduce the incidence of this cancer for our patients with endometriosis? Despite their increased risk for ovarian and endometrial cancers, current recommendations do not support routine cancer screening in women with endometriosis.7 However, risk-mitigation strategies can still be pursued. Hormonal contraceptives to decrease ovulation and menstrual cycling are protective against ovarian cancer and are also helpful in mitigating the symptoms of endometriosis. While removal of endometriotic cysts may not, in and of itself, be a strategy to prevent EAOC, it is still generally recommended because these cysts are commonly a source of pain and infertility. While they do not appear to undergo malignant transformation, it can be difficult to definitively rule out an early ovarian cancer in these complex ovarian cysts, particularly as they are often associated with tumor marker abnormalities such as elevations in CA 125. Therefore, if surgical excision of an endometriotic cyst is not performed, it should be closely followed for at least 5 years to ensure it is a benign structure. If surgery is pursued and ovarian preservation is desired, removal of the fallopian tubes and uterus can help mitigate the risk for EAOC.8
Endometriosis is a morbid condition for many young women. In addition to causing pain and infertility it increases a woman’s risk for ovarian and endometrial cancer, particularly ovarian clear cell, endometrioid, and low-grade serous cancers and synchronous endometrial and ovarian cancers. Endometriotic cysts should be removed or closely monitored, and clinicians should discuss treatment options that minimize frequency of ovulation and menstruation events as a preventative strategy.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Endocrinology. 2019;160(3):626-38.
2. Cancers. 2020;12(6):1676.
3. Lancet Oncol. 2012;13:385-94.
4. Gynecol Oncol. 2014;132(3):760-6.
5. Redox Rep. 2016;21:119-26.
6. Int. J Clin Oncol. 2020;25:51-8.
7. Hum Reprod. 2013;28:1552-68.
8. J Natl Cancer Inst. 2019;111:1097-103.
Opioids prescribed for diabetic neuropathy pain, against advice
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
Prescriptions for opioids as a first-line treatment for painful diabetic peripheral neuropathy (DPN) outnumbered those for other medications between 2014 and 2018, despite the fact that the former is not recommended, new research indicates.
“We know that for any kind of chronic pain, opioids are not ideal. They’re not very effective for chronic pain in general, and they’re definitely not safe,” senior author Rozalina G. McCoy, MD, an endocrinologist and primary care clinician at the Mayo Clinic in Rochester, Minn., told this news organization.
That’s true even for severe DPN pain or painful exacerbations, she added.
“There’s a myth that opioids are the strongest pain meds possible ... For painful neuropathic pain, duloxetine [Cymbalta], pregabalin [Lyrica], and gabapentin [Neurontin] are the most effective pain medications based on multiple studies and extensive experience using them,” she explained. “But I think the public perception is that opioids are the strongest. When a patient comes with severe pain, I think there’s that kind of gut feeling that if the pain is severe, I need to give opioids.”
What’s more, she noted, “evidence is emerging for other harms, not only the potential for dependency and potential overdose, but also the potential for opioid-induced hyperalgesia. Opioids themselves can cause chronic pain. When we think about using opioids for chronic pain, we are really shooting ourselves in the foot. We’re going to harm patients.”
The American Diabetes Association DPN guidelines essentially say as much, advising opioids only as a tertiary option for refractory pain, she observed.
The new findings, from a retrospective study of Mayo Clinic electronic health data, were published online in JAMA Network Open by Jungwei Fan, PhD, also of Mayo Clinic, and colleagues.
Are fewer patients with DPN receiving any treatment now?
The data also reveal that, while opioid prescribing dropped over the study period, there wasn’t a comparable rise in prescriptions of recommended pain medications, suggesting that recent efforts to minimize opioid prescribing may have resulted in less overall treatment of significant pain. (The study had to be stopped in 2018 when Mayo switched to a new electronic health record system, Dr. McCoy explained.)
“The proportion of opioids among new prescriptions has been decreasing. I’m hopeful that the rates are even lower now than they were 2 years ago. What was concerning to me was the proportion of people receiving treatment overall had gone down,” Dr. McCoy noted.
“So, while it’s great that opioids aren’t being used, it’s doubtful that people with DPN are any less symptomatic. So I worry that there’s a proportion of patients who have pain who aren’t getting the treatment they need just because we don’t want to give them opioids. There are other options,” Dr. McCoy said, including nonpharmacologic approaches.
Opioids dominated in new-onset DPN prescribing during 2014-2018
The study involved 3,495 adults with newly diagnosed DPN from all three Mayo Clinic locations in Rochester, Minn.; Phoenix, Ariz.; and Jacksonville, Fla. during the period 2014-2018. Of those, 40.2% (1,406) were prescribed a new pain medication after diagnosis. However, that proportion dropped from 45.6% in 2014 to 35.2% in 2018.
The odds of initiating any treatment were significantly greater among patients with depression (odds ratio, 1.61), arthritis (OR, 1.21), and back pain (OR, 1.34), but decreased over time among all patients.
Among those receiving drug treatment, opioids were prescribed to 43.8%, whereas guideline-recommended medications (gabapentin, pregabalin, and serotonin norepinephrine reuptake inhibitors including duloxetine) were prescribed to 42.9%.
Another 20.6% received medications deemed “acceptable” for treating neuropathic pain, including topical analgesics, tricyclic antidepressants, and other anticonvulsants.
Males were significantly more likely than females to receive opioids (OR, 1.26), while individuals diagnosed with comorbid fibromyalgia were less likely (OR, 0.67). Those with comorbid arthritis were less likely to receive recommended DPN medications (OR, 0.76).
Use of opioids was 29% less likely in 2018, compared with 2014, although this difference did not achieve significance. Similarly, use of recommended medications was 25% more likely in 2018, compared with 2014, also not a significant difference.
Dr. McCoy offers clinical pearls for treating pain in DPN
Clinically, Dr. McCoy said that she individualizes treatment for painful DPN.
“I tend to use duloxetine if the patient also has a mood disorder including depression or anxiety, because it can also help with that. Gabapentin can also be helpful for radiculopathy or for chronic low-back pain. It can even help with degenerative joint disease like arthritis of the knees. So, you maximize benefit if you use one drug to treat multiple things.”
All three recommended medications are generic now, although pregabalin still tends to be more expensive, she noted. Gabapentin can cause drowsiness, which makes it ideal for a patient with insomnia but much less so for a long-haul truck driver. Duloxetine doesn’t cause sleepiness. Pregabalin can, but less so than gabapentin.
“I think that’s why it’s so important to talk to your patient and ask how the neuropathy is affecting them. What other comorbidities do they have? What is their life like? I think you have to figure out what drug works for each individual person.”
Importantly, she advised, if one of the three doesn’t work, stop it and try another. “It doesn’t mean that none of these meds work. All three should be tried to see if they give relief.”
Nonpharmacologic measures such as cognitive behavioral therapy, acupuncture, or physical therapy may help some patients as well.
Supplements such as vitamin B12 – which can also help with metformin-induced B12 deficiency – or alpha-lipoic acid may also be worth a try as long as the patient is made aware of potential risks, she noted.
Dr. McCoy hopes to repeat this study using national data. “I don’t think this is isolated to Mayo ... I think it affects all practices,” she said.
Since the study, “we [Mayo Clinic] have implemented practice changes to limit use of opioids for chronic pain ... so I hope it’s getting better. It’s important to be aware of our patterns in prescribing.”
The study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. McCoy reported receiving grants from the AARP Quality Measure Innovation program through a collaboration with OptumLabs and the Mayo Clinic’s Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
A version of this article first appeared on Medscape.com.
The true measure of cluster headache
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
Patients with cluster headache face a double whammy: Physicians too often fail to recognize it, and their condition is among the most severe and debilitating among headache types. In fact,
The study’s comparison of cluster headaches to other common painful experiences can help nonsufferers relate to the experience, said Larry Schor, PhD, a coauthor of the paper. “Headache is a terrible word. Bee stings sting, burns burn. [A cluster headache] doesn’t ache. It’s a piercing intensity like you just can’t believe,” said Dr. Schor, professor of psychology at the University of West Georgia, Carrollton, and a cluster headache patient since he first experienced an attack at the age of 21.
The study was published in the January 2021 issue of Headache.
Ranking cluster headaches as worse than experiences such as childbirth or kidney stones is “kind of eye opening, and helps to describe the experience in terms that more people can relate to. I think it helps to share the experience of cluster headache more broadly, because we’re in a situation where cluster headache remains underfunded, and we don’t have enough treatments for it. I think one way to overcome that is to spread awareness of what this problem is, and the impact it has on human life,” said Rashmi Halker Singh, MD, associate professor of neurology at the Mayo Clinic in Scottsdale, Ariz., and deputy editor of Headache. She was not involved in the study.
Dr. Schor called for physicians to consider cluster headache an emergency, because of the severity of pain and also the potential for suicidality. Treatments remain comparatively sparse, but high-flow oxygen can help some patients, and intranasal or intravenous triptans can treat acute pain. In 2018, the Food and Drug Administration approved galcanezumab (Eli Lilly) for prevention of episodic cluster headaches.
But cluster headaches are often misdiagnosed. For many patients, it takes more than a year or even as long as 5 years to get an accurate diagnosis, according to Dr. Schor. Women may be particularly vulnerable to misdiagnosis, because migraines are more common in women. It doesn’t help that many neurologists are taught that cluster headache is primarily a male disease. “Because that idea is so ingrained, I think a lot of women who have cluster headache are probably missed and told they have migraine instead. There are a lot of women who have cluster headache, and that gender difference might not be as big a difference as we were initially taught. We need to do a better job of recognizing cluster headache to better understand what the true prevalence is,” said Dr. Halker Singh.
She noted that patients with side-locked headache should be evaluated for cluster headache, and asked how long the pain lasts in the absence of medication. “Also ask about the presence of cranial autonomic symptoms, and if they occur in the context of headache pain, and if they are side-locked to the side of the headache. Those are important questions that can tease out cluster headache from other conditions,” said Dr. Halker Singh.
For the survey, the researchers asked 1,604 patients with cluster headache patients to rate pain on a scale of 1 to 10. Cluster headache ranked highest at 9.7, then labor pain (7.2), pancreatitis (7.0), and nephrolithiasis (6.9). Cluster headache pain was ranked at 10.0 by 72.1% of respondents. Those reporting maximal pain or were more likely to have cranial autonomic features in comparison with patients who reported less pain, including conjunctival injection or lacrimation (91% versus 85%), eyelid edema (77% versus 66%), forehead/facial sweating (60% versus 49%), fullness in the ear (47% versus 35%), and miosis or ptosis (85% versus 75%). They had more frequent attacks (4.0 versus 3.5 per day), higher Hopelessness Depression Symptom Questionnaire scores (24.5 versus 21.1), and reduced effectiveness of calcium channel blockers (2.2 versus 2.5 on a 5-point Likert scale). They were more often female (34% versus 24%). (P < .001 for all).
The study received funding from Autonomic Technologies and Cluster Busters. Dr. Schor and Dr. Halker Singh had no relevant financial disclosures.
FROM HEADACHE
Researchers examine factors associated with opioid use among migraineurs
Among patients with migraine who use prescription medications, the increasing use of prescription opioids is associated with chronic migraine, more severe disability, and anxiety and depression, according to an analysis published in the January issue of Headache . The use of prescription opioids also is associated with treatment-related variables such as poor acute treatment optimization and treatment in a pain clinic. The results indicate the continued need to educate patients and clinicians about the potential risks of opioids for migraineurs, according to the researchers.
In the Migraine in America Symptoms and Treatment (MAST) study, which the researchers analyzed for their investigation, one-third of migraineurs who use acute prescriptions reported using opioids. Among opioid users, 42% took opioids on 4 or more days per month. “These findings are like [those of] a previous report from the American Migraine Prevalence and Prevention study and more recent findings from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study,” said Richard Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine in the Bronx, New York. “High rates of opioid use are problematic because opioid use is associated with worsening of migraine over time.”
Opioids remain in widespread use for migraine, even though guidelines recommend against this treatment. Among migraineurs, opioid use is associated with more severe headache-related disability and greater use of health care resources. Opioid use also increases the risk of progressing from episodic migraine to chronic migraine.
A review of MAST data
Dr. Lipton and colleagues set out to identify the variables associated with the frequency of opioid use in people with migraine. Among the variables that they sought to examine were demographic characteristics, comorbidities, headache characteristics, medication use, and patterns of health care use. Dr. Lipton’s group hypothesized that migraine-related severity and burden would increase with increasing frequency of opioid use.
To conduct their research, the investigators examined data from the MAST study, a nationwide sample of American adults with migraine. They focused specifically on participants who reported receiving prescription acute medications. Participants eligible for this analysis reported 3 or more headache days in the previous 3 months and at least 1 monthly headache day in the previous month. In all, 15,133 participants met these criteria.
Dr. Lipton and colleagues categorized participants into four groups based on their frequency of opioid use. The groups had no opioid use, 3 or fewer monthly days of opioid use, 4 to 9 monthly days of opioid use, and 10 or more days of monthly opioid use. The last category is consistent with the International Classification of Headache Disorders-3 criteria for overuse of opioids in migraine.
At baseline, MAST participants provided information about variables such as gender, age, marital status, smoking status, education, and income. Participants also reported how many times in the previous 6 months they had visited a primary care doctor, a neurologist, a headache specialist, or a pain specialist. Dr. Lipton’s group calculated monthly headache days using the number of days during the previous 3 months affected by headache. The Migraine Disability Assessment (MIDAS) questionnaire was used to measure headache-related disability. The four-item Patient Health Questionnaire (PHQ-4) was used to screen for anxiety and depression, and the Migraine Treatment Optimization Questionnaire (mTOQ-4) evaluated participants’ treatment optimization.
Men predominated among opioid users
The investigators included 4,701 MAST participants in their analysis. The population’s mean age was 45 years, and 71.6% of participants were women. Of the entire sample, 67.5% reported no opioid use, and 32.5% reported opioid use. Of the total study population, 18.7% of patients took opioids 3 or fewer days per month, 6.5% took opioids 4 to 9 days per month, and 7.3% took opioids on 10 or more days per month.
Opioid users did not differ from nonusers on race or marital status. Men were overrepresented among all groups of opioid users, however. In addition, opioid use was more prevalent among participants with fewer than 4 years of college education (34.9%) than among participants with 4 or more years of college (30.8%). The proportion of participants with fewer than 4 years of college increased with increasing monthly opioid use. Furthermore, opioid use increased with decreasing household income. As opioid use increased, rates of employment decreased. Approximately 33% of the entire sample were obese, and the proportion of obese participants increased with increasing days per month of opioid use.
The most frequent setting during the previous 6 months for participants seeking care was primary care (49.7%). The next most frequent setting was neurology units (20.9%), pain clinics (8.3%), and headache clinics (7.7%). The prevalence of opioid use was 37.5% among participants with primary care visits, 37.3% among participants with neurologist visits, 43.0% among participants with headache clinic visits, and 53.5% with pain clinic visits.
About 15% of the population had chronic migraine. The prevalence of chronic migraine increased with increasing frequency of opioid use. About 49% of the sample had allodynia, and the prevalence of allodynia increased with increasing frequency of opioid use. Overall, disability was moderate to severe in 57.3% of participants. Participants who used opioids on 3 or fewer days per month had the lowest prevalence of moderate to severe disability (50.2%), and participants who used opioids on 10 or more days per month had the highest prevalence of moderate to severe disability (83.8%).
Approximately 21% of participants had anxiety or depression. The lowest prevalence of anxiety or depression was among participants who took opioids on 3 or fewer days per month (17.4%), and the highest prevalence was among participants who took opioids on 10 or more days per month (43.2%). About 39% of the population had very poor to poor treatment optimization. Among opioid nonusers, 35.6% had very poor to poor treatment optimization, and 59.4% of participants who used opioids on 10 or more days per month had very poor to poor treatment optimization.
Dr. Lipton and colleagues also examined the study population’s use of triptans. Overall, 51.5% of participants reported taking triptans. The prevalence of triptan use was highest among participants who did not use opioids (64.1%) and lowest among participants who used opioids on 3 or fewer days per month (20.5%). Triptan use increased as monthly days of opioid use increased.
Pain clinics and opioid prescription
“In the general population, women are more likely to receive opioids than men,” said Dr. Lipton. “This [finding] could reflect, in part, that women have more pain disorders than men and are more likely to seek medical care for pain than men.” In the current study, however, men with migraine were more likely to receive opioid prescriptions than were women with migraine. One potential explanation for this finding is that men with migraine are less likely to receive a migraine diagnosis, which might attenuate opioid prescribing, than women with migraine. “It may be that opioids are perceived to be serious drugs for serious pain, and that some physicians may be more likely to prescribe opioids to men because the disorder is taken more seriously in men than women,” said Dr. Lipton.
The observation that opioids were more likely to be prescribed for people treated in pain clinics “is consistent with my understanding of practice patterns,” he added. “Generally, neurologists strive to find effective acute treatment alternatives to opioids. The emergence of [drug classes known as] gepants and ditans provides a helpful set of alternatives to tritpans.”
Dr. Lipton and his colleagues plan further research into the treatment of migraineurs. “In a claims analysis, we showed that when people with migraine fail a triptan, they are most likely to get an opioid as their next drug,” he said. “Reasonable [clinicians] might disagree on the next step. The next step, in the absence of contraindications, could be a different oral triptan, a nonoral triptan, or a gepant or ditan. We are planning a randomized trial to probe this question.”
Why are opioids still being used?
The study’s reliance on patients’ self-report and its retrospective design are two of its weaknesses, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews. One strength, however, is that the stratified sampling methodology produced a study population that accurately reflects the demographic characteristics of the U.S. adult population, he added. Another strength is the investigators’ examination of opioid use by patient characteristics such as marital status, education, income, obesity, and smoking.
Given the harmful effects of opioids in migraine, it is hard to understand why as much as one-third of study participants using acute care medication for migraine were using opioids, said Dr. Rapoport. Using opioids for the acute treatment of migraine attacks often indicates inadequate treatment optimization, which leads to ongoing headache. As a consequence, patients may take more medication, which can increase headache frequency and lead to diagnoses of chronic migraine and medication overuse headache. Although the study found an association between the increased use of opioids and decreased household income and increased unemployment, smoking, and obesity, “it is not possible to assign causality to any of these associations, even though some would argue that decreased socioeconomic status was somehow related to more headache, disability, obesity, smoking, and unemployment,” he added.
“The paper suggests that future research should look at the risk factors for use of opioids and should determine if depression is a risk factor for or a consequence of opioid use,” said Dr. Rapoport. “Interventional studies designed to improve the acute care of migraine attacks might be able to reduce the use of opioids. I have not used opioids or butalbital-containing medication in my office for many years.”
This study was funded and sponsored by Dr. Reddy’s Laboratories group of companies, Princeton, N.J. Dr. Lipton has received grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund. He serves as a consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Biohaven, Dr. Reddy’s Laboratories, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff’s Headache, 8th Edition (New York: Oxford University Press, 2009) and holds stock options in eNeura Therapeutics and Biohaven.
SOURCE: Lipton RB, et al. Headache. https://doi.org/10.1111/head.14018. 2020;61(1):103-16.
Among patients with migraine who use prescription medications, the increasing use of prescription opioids is associated with chronic migraine, more severe disability, and anxiety and depression, according to an analysis published in the January issue of Headache . The use of prescription opioids also is associated with treatment-related variables such as poor acute treatment optimization and treatment in a pain clinic. The results indicate the continued need to educate patients and clinicians about the potential risks of opioids for migraineurs, according to the researchers.
In the Migraine in America Symptoms and Treatment (MAST) study, which the researchers analyzed for their investigation, one-third of migraineurs who use acute prescriptions reported using opioids. Among opioid users, 42% took opioids on 4 or more days per month. “These findings are like [those of] a previous report from the American Migraine Prevalence and Prevention study and more recent findings from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study,” said Richard Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine in the Bronx, New York. “High rates of opioid use are problematic because opioid use is associated with worsening of migraine over time.”
Opioids remain in widespread use for migraine, even though guidelines recommend against this treatment. Among migraineurs, opioid use is associated with more severe headache-related disability and greater use of health care resources. Opioid use also increases the risk of progressing from episodic migraine to chronic migraine.
A review of MAST data
Dr. Lipton and colleagues set out to identify the variables associated with the frequency of opioid use in people with migraine. Among the variables that they sought to examine were demographic characteristics, comorbidities, headache characteristics, medication use, and patterns of health care use. Dr. Lipton’s group hypothesized that migraine-related severity and burden would increase with increasing frequency of opioid use.
To conduct their research, the investigators examined data from the MAST study, a nationwide sample of American adults with migraine. They focused specifically on participants who reported receiving prescription acute medications. Participants eligible for this analysis reported 3 or more headache days in the previous 3 months and at least 1 monthly headache day in the previous month. In all, 15,133 participants met these criteria.
Dr. Lipton and colleagues categorized participants into four groups based on their frequency of opioid use. The groups had no opioid use, 3 or fewer monthly days of opioid use, 4 to 9 monthly days of opioid use, and 10 or more days of monthly opioid use. The last category is consistent with the International Classification of Headache Disorders-3 criteria for overuse of opioids in migraine.
At baseline, MAST participants provided information about variables such as gender, age, marital status, smoking status, education, and income. Participants also reported how many times in the previous 6 months they had visited a primary care doctor, a neurologist, a headache specialist, or a pain specialist. Dr. Lipton’s group calculated monthly headache days using the number of days during the previous 3 months affected by headache. The Migraine Disability Assessment (MIDAS) questionnaire was used to measure headache-related disability. The four-item Patient Health Questionnaire (PHQ-4) was used to screen for anxiety and depression, and the Migraine Treatment Optimization Questionnaire (mTOQ-4) evaluated participants’ treatment optimization.
Men predominated among opioid users
The investigators included 4,701 MAST participants in their analysis. The population’s mean age was 45 years, and 71.6% of participants were women. Of the entire sample, 67.5% reported no opioid use, and 32.5% reported opioid use. Of the total study population, 18.7% of patients took opioids 3 or fewer days per month, 6.5% took opioids 4 to 9 days per month, and 7.3% took opioids on 10 or more days per month.
Opioid users did not differ from nonusers on race or marital status. Men were overrepresented among all groups of opioid users, however. In addition, opioid use was more prevalent among participants with fewer than 4 years of college education (34.9%) than among participants with 4 or more years of college (30.8%). The proportion of participants with fewer than 4 years of college increased with increasing monthly opioid use. Furthermore, opioid use increased with decreasing household income. As opioid use increased, rates of employment decreased. Approximately 33% of the entire sample were obese, and the proportion of obese participants increased with increasing days per month of opioid use.
The most frequent setting during the previous 6 months for participants seeking care was primary care (49.7%). The next most frequent setting was neurology units (20.9%), pain clinics (8.3%), and headache clinics (7.7%). The prevalence of opioid use was 37.5% among participants with primary care visits, 37.3% among participants with neurologist visits, 43.0% among participants with headache clinic visits, and 53.5% with pain clinic visits.
About 15% of the population had chronic migraine. The prevalence of chronic migraine increased with increasing frequency of opioid use. About 49% of the sample had allodynia, and the prevalence of allodynia increased with increasing frequency of opioid use. Overall, disability was moderate to severe in 57.3% of participants. Participants who used opioids on 3 or fewer days per month had the lowest prevalence of moderate to severe disability (50.2%), and participants who used opioids on 10 or more days per month had the highest prevalence of moderate to severe disability (83.8%).
Approximately 21% of participants had anxiety or depression. The lowest prevalence of anxiety or depression was among participants who took opioids on 3 or fewer days per month (17.4%), and the highest prevalence was among participants who took opioids on 10 or more days per month (43.2%). About 39% of the population had very poor to poor treatment optimization. Among opioid nonusers, 35.6% had very poor to poor treatment optimization, and 59.4% of participants who used opioids on 10 or more days per month had very poor to poor treatment optimization.
Dr. Lipton and colleagues also examined the study population’s use of triptans. Overall, 51.5% of participants reported taking triptans. The prevalence of triptan use was highest among participants who did not use opioids (64.1%) and lowest among participants who used opioids on 3 or fewer days per month (20.5%). Triptan use increased as monthly days of opioid use increased.
Pain clinics and opioid prescription
“In the general population, women are more likely to receive opioids than men,” said Dr. Lipton. “This [finding] could reflect, in part, that women have more pain disorders than men and are more likely to seek medical care for pain than men.” In the current study, however, men with migraine were more likely to receive opioid prescriptions than were women with migraine. One potential explanation for this finding is that men with migraine are less likely to receive a migraine diagnosis, which might attenuate opioid prescribing, than women with migraine. “It may be that opioids are perceived to be serious drugs for serious pain, and that some physicians may be more likely to prescribe opioids to men because the disorder is taken more seriously in men than women,” said Dr. Lipton.
The observation that opioids were more likely to be prescribed for people treated in pain clinics “is consistent with my understanding of practice patterns,” he added. “Generally, neurologists strive to find effective acute treatment alternatives to opioids. The emergence of [drug classes known as] gepants and ditans provides a helpful set of alternatives to tritpans.”
Dr. Lipton and his colleagues plan further research into the treatment of migraineurs. “In a claims analysis, we showed that when people with migraine fail a triptan, they are most likely to get an opioid as their next drug,” he said. “Reasonable [clinicians] might disagree on the next step. The next step, in the absence of contraindications, could be a different oral triptan, a nonoral triptan, or a gepant or ditan. We are planning a randomized trial to probe this question.”
Why are opioids still being used?
The study’s reliance on patients’ self-report and its retrospective design are two of its weaknesses, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews. One strength, however, is that the stratified sampling methodology produced a study population that accurately reflects the demographic characteristics of the U.S. adult population, he added. Another strength is the investigators’ examination of opioid use by patient characteristics such as marital status, education, income, obesity, and smoking.
Given the harmful effects of opioids in migraine, it is hard to understand why as much as one-third of study participants using acute care medication for migraine were using opioids, said Dr. Rapoport. Using opioids for the acute treatment of migraine attacks often indicates inadequate treatment optimization, which leads to ongoing headache. As a consequence, patients may take more medication, which can increase headache frequency and lead to diagnoses of chronic migraine and medication overuse headache. Although the study found an association between the increased use of opioids and decreased household income and increased unemployment, smoking, and obesity, “it is not possible to assign causality to any of these associations, even though some would argue that decreased socioeconomic status was somehow related to more headache, disability, obesity, smoking, and unemployment,” he added.
“The paper suggests that future research should look at the risk factors for use of opioids and should determine if depression is a risk factor for or a consequence of opioid use,” said Dr. Rapoport. “Interventional studies designed to improve the acute care of migraine attacks might be able to reduce the use of opioids. I have not used opioids or butalbital-containing medication in my office for many years.”
This study was funded and sponsored by Dr. Reddy’s Laboratories group of companies, Princeton, N.J. Dr. Lipton has received grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund. He serves as a consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Biohaven, Dr. Reddy’s Laboratories, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff’s Headache, 8th Edition (New York: Oxford University Press, 2009) and holds stock options in eNeura Therapeutics and Biohaven.
SOURCE: Lipton RB, et al. Headache. https://doi.org/10.1111/head.14018. 2020;61(1):103-16.
Among patients with migraine who use prescription medications, the increasing use of prescription opioids is associated with chronic migraine, more severe disability, and anxiety and depression, according to an analysis published in the January issue of Headache . The use of prescription opioids also is associated with treatment-related variables such as poor acute treatment optimization and treatment in a pain clinic. The results indicate the continued need to educate patients and clinicians about the potential risks of opioids for migraineurs, according to the researchers.
In the Migraine in America Symptoms and Treatment (MAST) study, which the researchers analyzed for their investigation, one-third of migraineurs who use acute prescriptions reported using opioids. Among opioid users, 42% took opioids on 4 or more days per month. “These findings are like [those of] a previous report from the American Migraine Prevalence and Prevention study and more recent findings from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study,” said Richard Lipton, MD, Edwin S. Lowe professor and vice chair of neurology at Albert Einstein College of Medicine in the Bronx, New York. “High rates of opioid use are problematic because opioid use is associated with worsening of migraine over time.”
Opioids remain in widespread use for migraine, even though guidelines recommend against this treatment. Among migraineurs, opioid use is associated with more severe headache-related disability and greater use of health care resources. Opioid use also increases the risk of progressing from episodic migraine to chronic migraine.
A review of MAST data
Dr. Lipton and colleagues set out to identify the variables associated with the frequency of opioid use in people with migraine. Among the variables that they sought to examine were demographic characteristics, comorbidities, headache characteristics, medication use, and patterns of health care use. Dr. Lipton’s group hypothesized that migraine-related severity and burden would increase with increasing frequency of opioid use.
To conduct their research, the investigators examined data from the MAST study, a nationwide sample of American adults with migraine. They focused specifically on participants who reported receiving prescription acute medications. Participants eligible for this analysis reported 3 or more headache days in the previous 3 months and at least 1 monthly headache day in the previous month. In all, 15,133 participants met these criteria.
Dr. Lipton and colleagues categorized participants into four groups based on their frequency of opioid use. The groups had no opioid use, 3 or fewer monthly days of opioid use, 4 to 9 monthly days of opioid use, and 10 or more days of monthly opioid use. The last category is consistent with the International Classification of Headache Disorders-3 criteria for overuse of opioids in migraine.
At baseline, MAST participants provided information about variables such as gender, age, marital status, smoking status, education, and income. Participants also reported how many times in the previous 6 months they had visited a primary care doctor, a neurologist, a headache specialist, or a pain specialist. Dr. Lipton’s group calculated monthly headache days using the number of days during the previous 3 months affected by headache. The Migraine Disability Assessment (MIDAS) questionnaire was used to measure headache-related disability. The four-item Patient Health Questionnaire (PHQ-4) was used to screen for anxiety and depression, and the Migraine Treatment Optimization Questionnaire (mTOQ-4) evaluated participants’ treatment optimization.
Men predominated among opioid users
The investigators included 4,701 MAST participants in their analysis. The population’s mean age was 45 years, and 71.6% of participants were women. Of the entire sample, 67.5% reported no opioid use, and 32.5% reported opioid use. Of the total study population, 18.7% of patients took opioids 3 or fewer days per month, 6.5% took opioids 4 to 9 days per month, and 7.3% took opioids on 10 or more days per month.
Opioid users did not differ from nonusers on race or marital status. Men were overrepresented among all groups of opioid users, however. In addition, opioid use was more prevalent among participants with fewer than 4 years of college education (34.9%) than among participants with 4 or more years of college (30.8%). The proportion of participants with fewer than 4 years of college increased with increasing monthly opioid use. Furthermore, opioid use increased with decreasing household income. As opioid use increased, rates of employment decreased. Approximately 33% of the entire sample were obese, and the proportion of obese participants increased with increasing days per month of opioid use.
The most frequent setting during the previous 6 months for participants seeking care was primary care (49.7%). The next most frequent setting was neurology units (20.9%), pain clinics (8.3%), and headache clinics (7.7%). The prevalence of opioid use was 37.5% among participants with primary care visits, 37.3% among participants with neurologist visits, 43.0% among participants with headache clinic visits, and 53.5% with pain clinic visits.
About 15% of the population had chronic migraine. The prevalence of chronic migraine increased with increasing frequency of opioid use. About 49% of the sample had allodynia, and the prevalence of allodynia increased with increasing frequency of opioid use. Overall, disability was moderate to severe in 57.3% of participants. Participants who used opioids on 3 or fewer days per month had the lowest prevalence of moderate to severe disability (50.2%), and participants who used opioids on 10 or more days per month had the highest prevalence of moderate to severe disability (83.8%).
Approximately 21% of participants had anxiety or depression. The lowest prevalence of anxiety or depression was among participants who took opioids on 3 or fewer days per month (17.4%), and the highest prevalence was among participants who took opioids on 10 or more days per month (43.2%). About 39% of the population had very poor to poor treatment optimization. Among opioid nonusers, 35.6% had very poor to poor treatment optimization, and 59.4% of participants who used opioids on 10 or more days per month had very poor to poor treatment optimization.
Dr. Lipton and colleagues also examined the study population’s use of triptans. Overall, 51.5% of participants reported taking triptans. The prevalence of triptan use was highest among participants who did not use opioids (64.1%) and lowest among participants who used opioids on 3 or fewer days per month (20.5%). Triptan use increased as monthly days of opioid use increased.
Pain clinics and opioid prescription
“In the general population, women are more likely to receive opioids than men,” said Dr. Lipton. “This [finding] could reflect, in part, that women have more pain disorders than men and are more likely to seek medical care for pain than men.” In the current study, however, men with migraine were more likely to receive opioid prescriptions than were women with migraine. One potential explanation for this finding is that men with migraine are less likely to receive a migraine diagnosis, which might attenuate opioid prescribing, than women with migraine. “It may be that opioids are perceived to be serious drugs for serious pain, and that some physicians may be more likely to prescribe opioids to men because the disorder is taken more seriously in men than women,” said Dr. Lipton.
The observation that opioids were more likely to be prescribed for people treated in pain clinics “is consistent with my understanding of practice patterns,” he added. “Generally, neurologists strive to find effective acute treatment alternatives to opioids. The emergence of [drug classes known as] gepants and ditans provides a helpful set of alternatives to tritpans.”
Dr. Lipton and his colleagues plan further research into the treatment of migraineurs. “In a claims analysis, we showed that when people with migraine fail a triptan, they are most likely to get an opioid as their next drug,” he said. “Reasonable [clinicians] might disagree on the next step. The next step, in the absence of contraindications, could be a different oral triptan, a nonoral triptan, or a gepant or ditan. We are planning a randomized trial to probe this question.”
Why are opioids still being used?
The study’s reliance on patients’ self-report and its retrospective design are two of its weaknesses, said Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, and editor-in-chief of Neurology Reviews. One strength, however, is that the stratified sampling methodology produced a study population that accurately reflects the demographic characteristics of the U.S. adult population, he added. Another strength is the investigators’ examination of opioid use by patient characteristics such as marital status, education, income, obesity, and smoking.
Given the harmful effects of opioids in migraine, it is hard to understand why as much as one-third of study participants using acute care medication for migraine were using opioids, said Dr. Rapoport. Using opioids for the acute treatment of migraine attacks often indicates inadequate treatment optimization, which leads to ongoing headache. As a consequence, patients may take more medication, which can increase headache frequency and lead to diagnoses of chronic migraine and medication overuse headache. Although the study found an association between the increased use of opioids and decreased household income and increased unemployment, smoking, and obesity, “it is not possible to assign causality to any of these associations, even though some would argue that decreased socioeconomic status was somehow related to more headache, disability, obesity, smoking, and unemployment,” he added.
“The paper suggests that future research should look at the risk factors for use of opioids and should determine if depression is a risk factor for or a consequence of opioid use,” said Dr. Rapoport. “Interventional studies designed to improve the acute care of migraine attacks might be able to reduce the use of opioids. I have not used opioids or butalbital-containing medication in my office for many years.”
This study was funded and sponsored by Dr. Reddy’s Laboratories group of companies, Princeton, N.J. Dr. Lipton has received grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund. He serves as a consultant, serves as an advisory board member, or has received honoraria from Alder, Allergan, American Headache Society, Autonomic Technologies, Biohaven, Dr. Reddy’s Laboratories, Eli Lilly, eNeura Therapeutics, Merck, Novartis, Pfizer, and Teva, Inc. He receives royalties from Wolff’s Headache, 8th Edition (New York: Oxford University Press, 2009) and holds stock options in eNeura Therapeutics and Biohaven.
SOURCE: Lipton RB, et al. Headache. https://doi.org/10.1111/head.14018. 2020;61(1):103-16.
FROM HEADACHE