User login
Doctors more likely to prescribe opioids to COVID ‘long-haulers,’ raising addiction fears
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
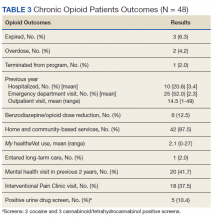
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
COVID-19 survivors are at risk from a possible second pandemic, this time of opioid addiction, given the high rate of painkillers being prescribed to these patients, health experts say.
A new study in Nature found alarmingly high rates of opioid use among COVID survivors with lingering symptoms at Veterans Affairs facilities. About 10% of COVID survivors develop “long COVID,” struggling with often disabling health problems even 6 months or longer after a diagnosis.
For every 1,000 long-COVID patients, known as “long-haulers,” who were treated at a VA facility, doctors wrote nine more prescriptions for opioids than they otherwise would have, along with 22 additional prescriptions for benzodiazepines, which include Xanax and other addictive pills used to treat anxiety.
Although previous studies have found many COVID survivors experience persistent health problems, the new article is the first to show they’re using more addictive medications, said Ziyad Al-Aly, MD, the paper’s lead author.
He’s concerned that even an apparently small increase in the inappropriate use of addictive pain pills will lead to a resurgence of the prescription opioid crisis, given the large number of COVID survivors. More than 3 million of the 31 million Americans infected with COVID develop long-term symptoms, which can include fatigue, shortness of breath, depression, anxiety, and memory problems known as “brain fog.”
The new study also found many patients have significant muscle and bone pain.
The frequent use of opioids was surprising, given concerns about their potential for addiction, said Dr. Al-Aly, chief of research and education service at the VA St. Louis Health Care System.
“Physicians now are supposed to shy away from prescribing opioids,” said Dr. Al-Aly, who studied more than 73,000 patients in the VA system. When Dr. Al-Aly saw the number of opioids prescriptions, he said, he thought to himself: “Is this really happening all over again?”
Doctors need to act now, before “it’s too late to do something,” Dr. Al-Aly said. “We must act now and ensure that people are getting the care they need. We do not want this to balloon into a suicide crisis or another opioid epidemic.”
As more doctors became aware of their addictive potential, new opioid prescriptions fell, by more than half since 2012. But said Andrew Kolodny, MD, medical director of opioid policy research at Brandeis University, Waltham, Mass.
Some patients who became addicted to prescription painkillers switched to heroin, either because it was cheaper or because they could no longer obtain opioids from their doctors. Overdose deaths surged in recent years as drug dealers began spiking heroin with a powerful synthetic opioid called fentanyl.
More than 88,000 Americans died from overdoses during the 12 months ending in August 2020, according to the Centers for Disease Control and Prevention. Health experts now advise doctors to avoid prescribing opioids for long periods.
The new study “suggests to me that many clinicians still don’t get it,” Dr. Kolodny said. “Many clinicians are under the false impression that opioids are appropriate for chronic pain patients.”
Hospitalized COVID patients often receive a lot of medication to control pain and anxiety, especially in ICUs, said Greg Martin, MD, president of the Society of Critical Care Medicine. Patients placed on ventilators, for example, are often sedated to make them more comfortable.
Martin said he’s concerned by the study’s findings, which suggest patients are unnecessarily continuing medications after leaving the hospital.
“I worry that COVID-19 patients, especially those who are severely and critically ill, receive a lot of medications during the hospitalization, and because they have persistent symptoms, the medications are continued after hospital discharge,” Dr. Martin said.
While some COVID patients are experiencing muscle and bone pain for the first time, others say the illness has intensified their preexisting pain.
Rachael Sunshine Burnett has suffered from chronic pain in her back and feet for 20 years, ever since an accident at a warehouse where she once worked. But Ms. Burnett, who first was diagnosed with COVID in April 2020, said the pain soon became 10 times worse and spread to the area between her shoulders and spine. Although she was already taking long-acting OxyContin twice a day, her doctor prescribed an additional opioid called oxycodone, which relieves pain immediately. She was reinfected with COVID in December.
“It’s been a horrible, horrible year,” said Ms. Burnett, 43, of Coxsackie, N.Y.
Doctors should recognize that pain can be a part of long COVID, Dr. Martin said. “We need to find the proper nonnarcotic treatment for it, just like we do with other forms of chronic pain,” he said.
The CDC recommends a number of alternatives to opioids – from physical therapy to biofeedback, over-the-counter anti-inflammatories, antidepressants, and antiseizure drugs that also relieve nerve pain.
The country also needs an overall strategy to cope with the wave of post-COVID complications, Dr. Al-Aly said.
“It’s better to be prepared than to be caught off guard years from now, when doctors realize: ‘Oh, we have a resurgence in opioids,’ ” Dr. Al-Aly said.
Dr. Al-Aly noted that his study may not capture the full complexity of post-COVID patient needs. Although women make up the majority of long-COVID patients in most studies, most patients in the VA system are men.
The study of VA patients makes it “abundantly clear that we are not prepared to meet the needs of 3 million Americans with long COVID,” said Eric Topol, MD, founder and director of the Scripps Research Translational Institute in San Diego. “We desperately need an intervention that will effectively treat these individuals.”
Dr. Al-Aly said COVID survivors may need care for years.
“That’s going to be a huge, significant burden on the health care system,” Dr. Al-Aly said. “Long COVID will reverberate in the health system for years or even decades to come.”
Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Sarcoidosis: An FP’s primer on an enigmatic disease
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
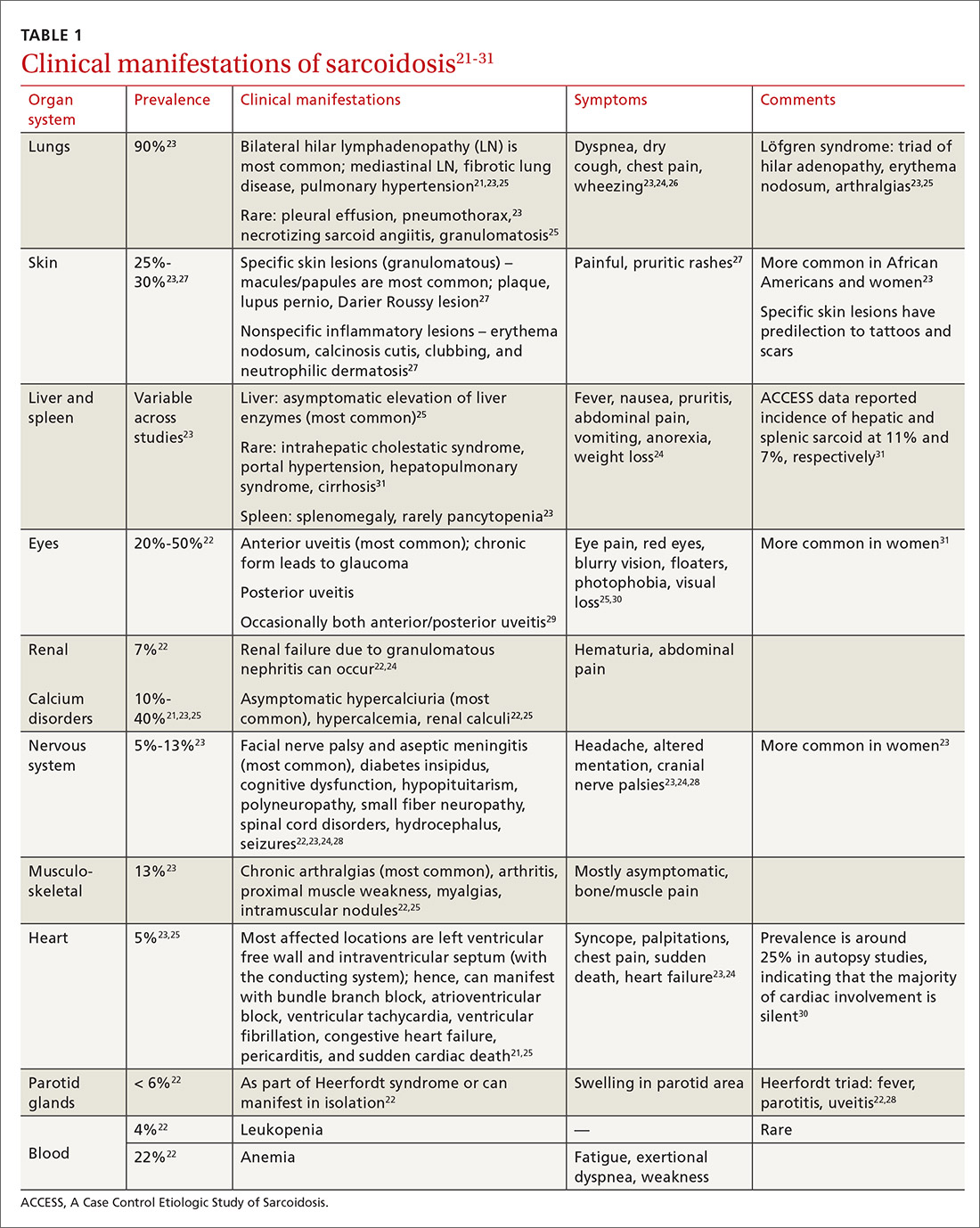
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22
Diagnostic work-up
Radiologic studies
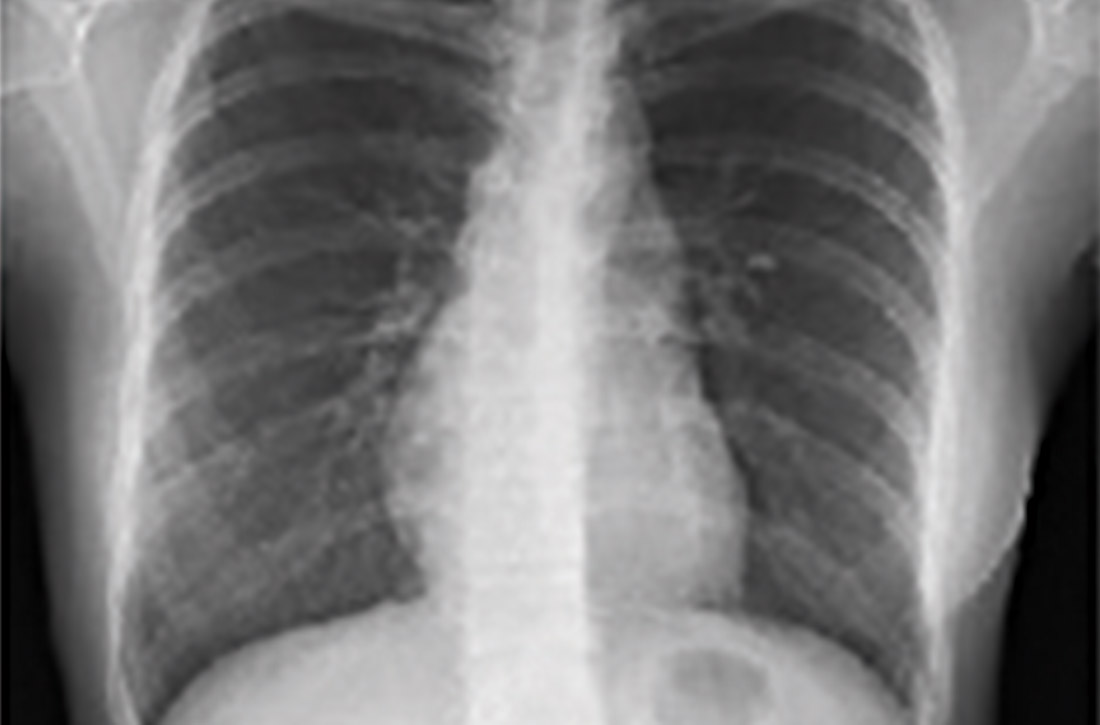
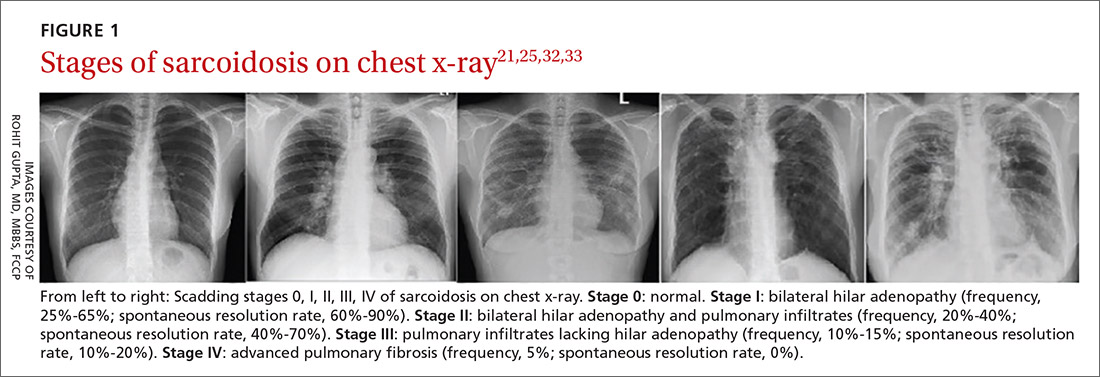
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.
Continue to: High-resoluton computed tomography
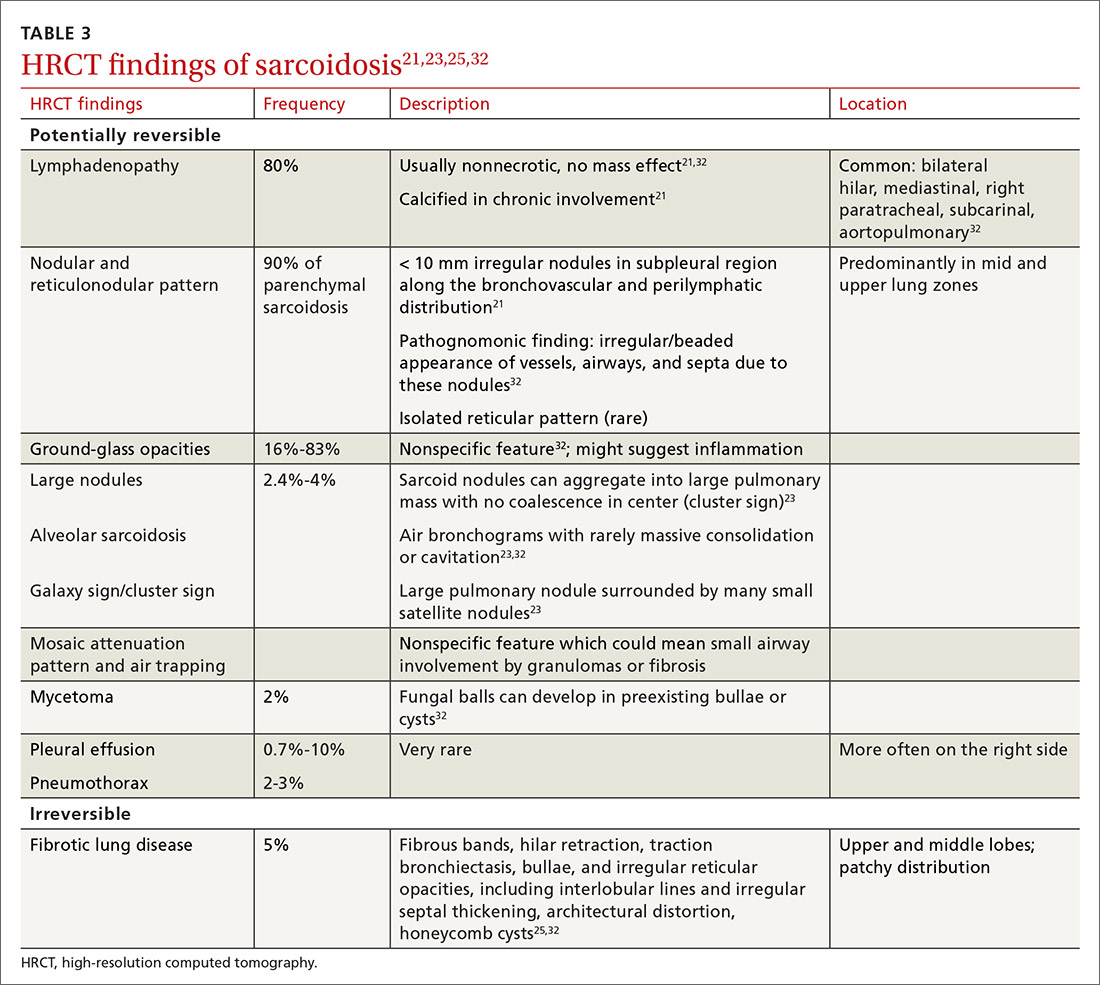
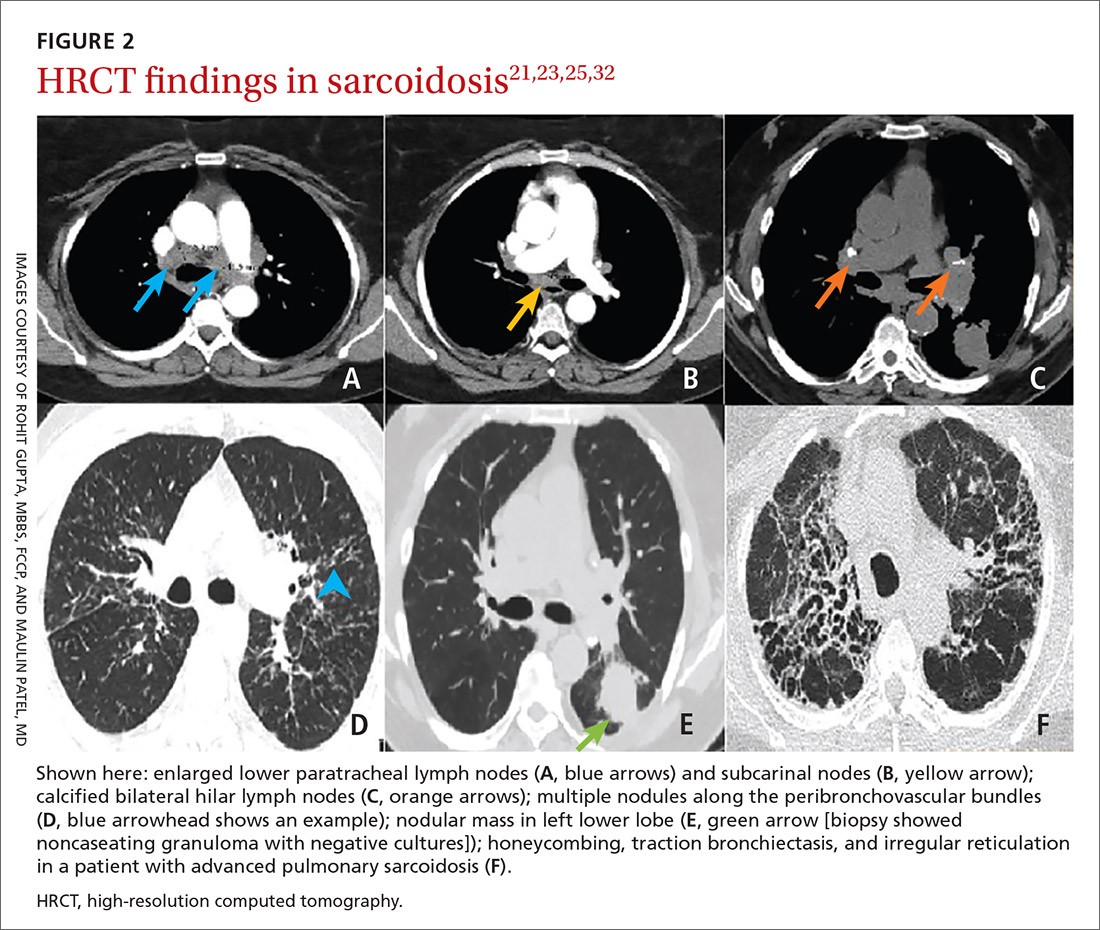
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.
Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.
Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
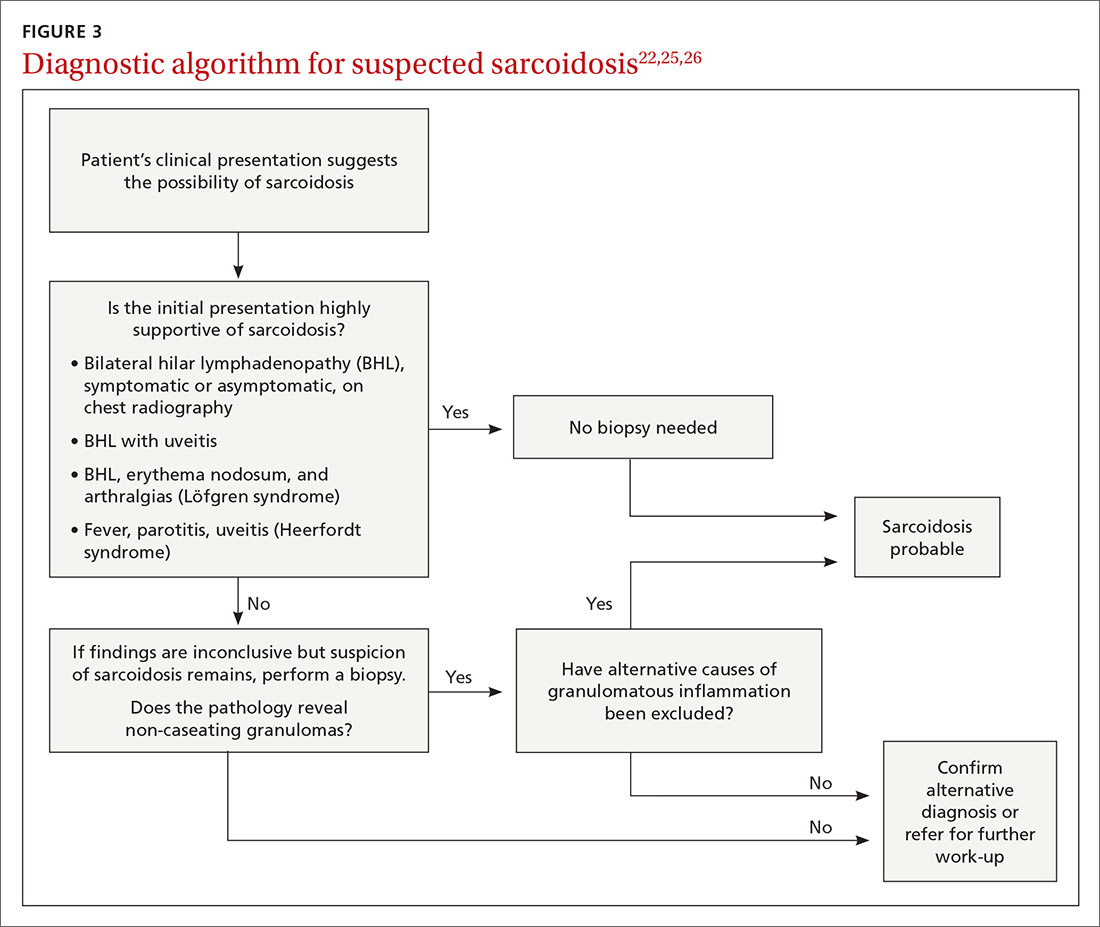
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26
Continue to: Laboratory studies
Laboratory studies
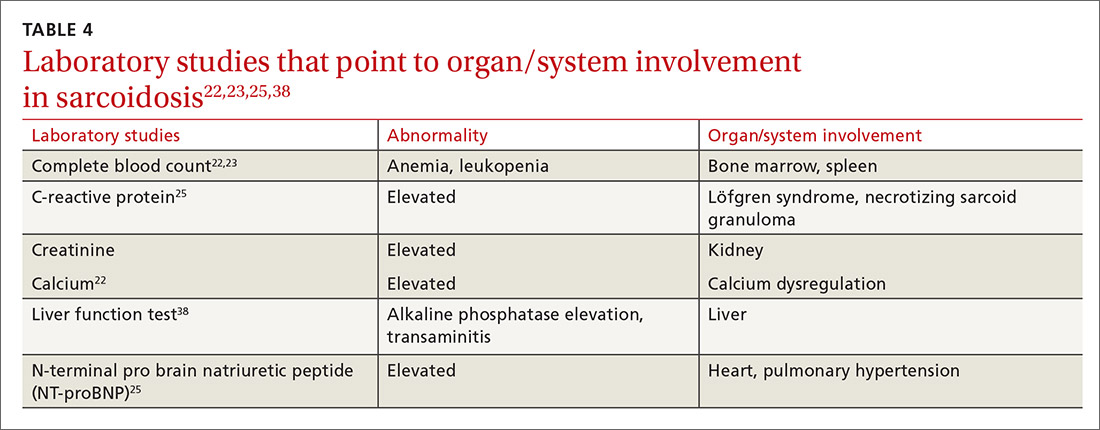
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21
Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
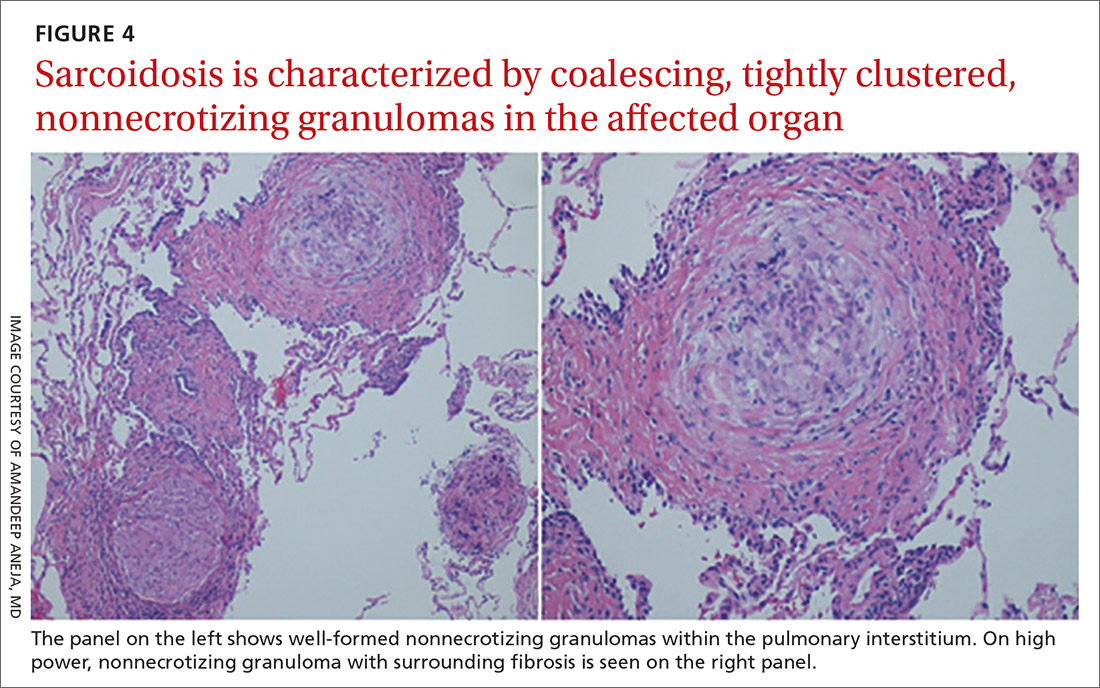
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.
In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81
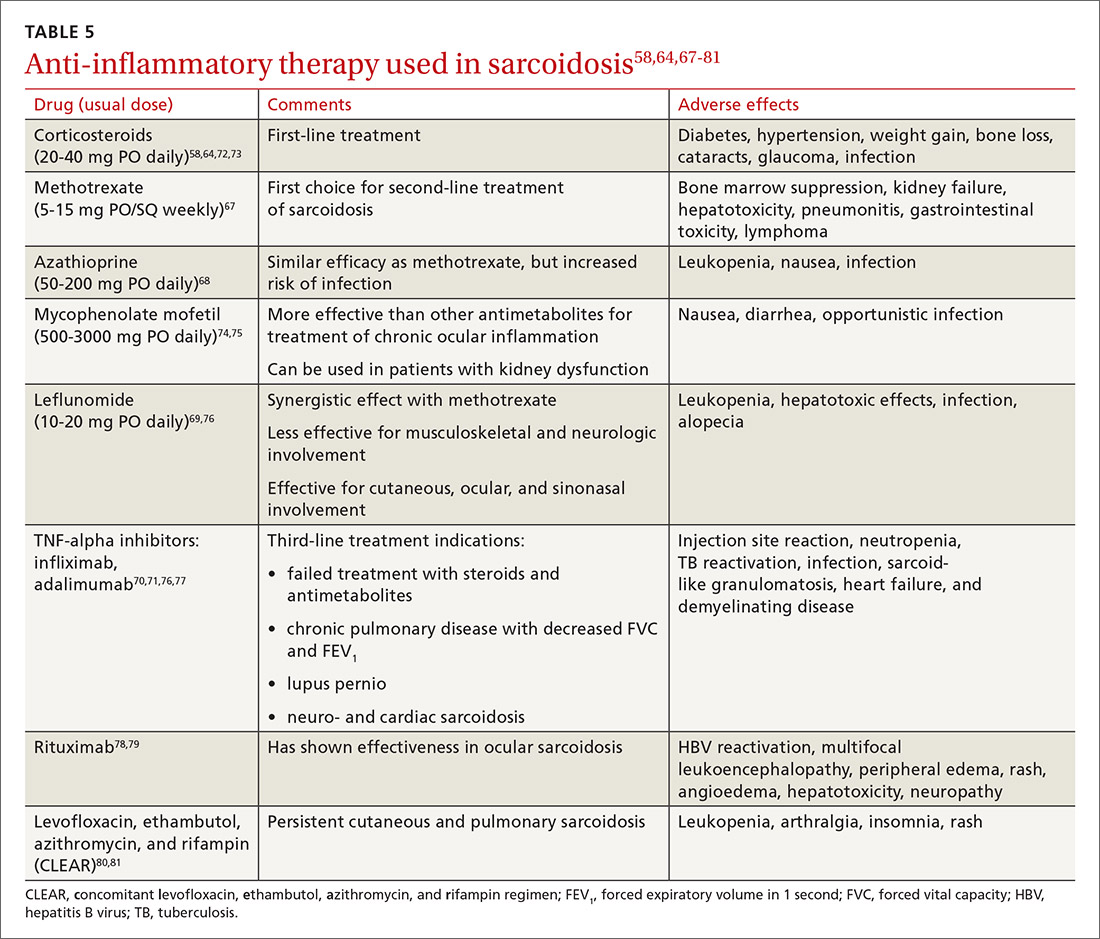
The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22

Diagnostic work-up

Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.

Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.

Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.

Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26

Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21

Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.

In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81

The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
Sarcoidosis is a multisystem inflammatory disease of unclear etiology that primarily affects the lungs. It can occur at any age but usually develops before the age of 50 years, with an initial peak incidence at 20 to 29 years and a second peak incidence after 50 years of age, especially among women in Scandinavia and Japan.1 Sarcoidosis affects men and women of all racial and ethnic groups throughout the world, but differences based on race, sex, and geography are noted.1
The highest rates are reported in northern European and African-American individuals, particularly in women.1,2 The adjusted annual incidence of sarcoidosis among African Americans is approximately 3 times that among White Americans3 and is more likely to be chronic and fatal in African Americans.3 The disease can be familial with a possible recessive inheritance mode with incomplete penetrance.4 Risk of sarcoidosis in monozygotic twins appears to be 80 times greater than that in the general population, which supports genetic factors accounting for two-thirds of disease susceptibility.5
Likely factors in the development of sarcoidosis
The exact cause of sarcoidosis is unknown, but we have insights into its pathogenesis and potential triggers.1,6-9 Genes involved are being identified: class I and II human leukocyte antigen (HLA) molecules are most consistently associated with risk of sarcoidosis. Environmental exposures can activate the innate immune system and precondition a susceptible individual to react to potential causative antigens in a highly polarized, antigen-specific Th1 immune response. The epithelioid granulomatous response involves local proinflammatory cytokine production and enhanced T-cell immunity at sites of inflammation.10 Granulomas generally form to confine pathogens, restrict inflammation, and protect surrounding tissue.11-13
ACCESS (A Case Control Etiologic Study of Sarcoidosis) identified several environmental exposures such as chemicals used in the agriculture industry, mold or mildew, and musty odors at work.14 Tobacco use was not associated with sarcoidosis.14 Recent studies have shown positive associations with service in the US Navy,15 metal working,16 firefighting,17 the handling of building supplies,18 and onsite exposure while assisting in rescue efforts at the World Trade Center disaster.19 Other data support the likelihood that specific environmental exposures associated with microbe-rich environments modestly increase the risk of sarcoidosis.14 Mycobacterial and propionibacterial DNA and RNA are potentially associated with sarcoidosis.20
Clinical manifestations are nonspecific
The diagnosis of sarcoidosis can be difficult and delayed due to diverse organ involvement and nonspecific presentations. TABLE 121-31 shows the diverse manifestations in a patient with suspected sarcoidosis. Around 50% of the patients are asymptomatic.23,24 Sarcoidosis is a diagnosis of exclusion, starting with a detailed history to rule out infections, occupational or environmental exposures, malignancies, and other possible disorders (TABLE 2).22

Diagnostic work-up

Radiologic studies
Chest x-ray (CXR) provides diagnostic and prognostic information in the evaluation of sarcoidosis using the Scadding classification system (FIGURE 1).21,25,32,33 Interobserver variability, especially between stages II and III and III and IV is the major limitation of this system.32 At presentation, radiographs are abnormal in approximately 90% of patients.34 Lymphadenopathy is the most common radiographic abnormality, occurring in more than two-thirds of cases, and pulmonary opacities (nodules and reticulation) with a middle to upper lobe predilection are present in 20% to 50% of patients.1,31,35 The nodules vary in size and can coalesce and cause alveolar collapse, thus producing consolidation.36 Linear opacities radiating laterally from the hilum into the middle and upper zones are characteristic in fibrotic disease.

Continue to: High-resoluton computed tomography
High-resolution computed tomography (HRCT). Micronodules in a perilymphatic distribution with upper lobe predominance combined with subcarinal and symmetrical hilar lymph node enlargement is practically diagnostic of sarcoidosis in the right clinical context. TABLE 321,23,25,32 and FIGURE 221,23,25,32 summarize the common CT chest findings of sarcoidosis.

Advanced imaging such as (18)F-fluorodeoxyglucose positron emission tomography (PET) and magnetic resonance imaging (MRI) are used in specialized settings for advanced pulmonary, cardiac, or neurosarcoidosis.

Tissue biopsy
Skin lesions (other than erythema nodosum), eye lesions, and peripheral lymph nodes are considered the safest extrapulmonary locations for biopsy.21,25 If pulmonary infiltrates or lymphadenopathy are present, or if extrapulmonary biopsy sites are not available, then flexible bronchoscopy with biopsy is the mainstay for tissue sampling.25
Bronchoalveolar lavage (BAL), transbronchial biopsy (TBB), endobronchial biopsy (EBB), and endobronchial ultrasound (EBUS) are invaluable modalities that have reduced the need for open lung biopsy. BAL in sarcoidosis can show lymphocytosis > 15% (nonspecific) and a CD4:CD8 lymphocyte ratio > 3.5 (specificity > 90%).21,22 TBB is more sensitive than EBB; however, sensitivity overall is heightened when both of them are combined. The advent of EBUS has increased the safety and efficiency of needle aspiration of mediastinal lymph nodes. Diagnostic yield of EBUS (~80%) is superior to that with TBB and EBB (~50%), especially in stage I and II sarcoidosis.37 The combination of EBUS with TBB improves the diagnostic yield to ~90%.37
The decision to obtain biopsy samples hinges on the nature of clinical and radiologic findings (FIGURE 3).22,25,26

Continue to: Laboratory studies
Laboratory studies
Multiple abnormalities may be seen in sarcoidosis, and specific lab tests may help support a diagnosis of sarcoidosis or detect organ-specific disease activity (TABLE 4).22,23,25,38 However, no consistently accurate biomarkers exist for use in clinical practice. An angiotensin-converting enzyme (ACE) level greater than 2 times the upper limit of normal may be helpful; however, sensitivity remains low, and genetic polymorphisms can influence the ACE level.25 Biomarkers sometimes used to assess disease activity are serum interleukin-2 receptor, neopterin, chitotriosidase, lysozyme, KL-6 glycoprotein, and amyloid A.21

Additional tests to assess specific features or organ involvement
Pulmonary function testing (PFT) is reviewed in detail below under “pulmonary sarcoidosis.”
Electrocardiogram (EKG)/transthoracic echocardiogram (TTE). EKG abnormalities—conduction disturbances, arrhythmias, or nonspecific ST segment and T-wave changes—are the most common nonspecific findings.30 TTE findings are also nonspecific but have value in assessing cardiac chamber size and function and myocardial involvement. TTE is indeed the most common screening modality for sarcoidosis-associated pulmonary hypertension (SAPH), which is definitively diagnosed by right heart catheterization (RHC). Further evaluation for cardiac sarcoidosis can be done with cardiac MRI or fluorodeoxyglucose PET in specialized settings.
Lumbar puncture (LP) may reveal lymphocytic infiltration in suspected neurosarcoidosis, but the finding is nonspecific and can reflect infection or malignancy. Oligoclonal bands may also be seen in about one-third of neurosarcoidosis cases, and it is imperative to rule out multiple sclerosis.28
Pulmonary sarcoidosis
Pulmonary sarcoidosis accounts for most of the morbidity, mortality, and health care use associated with sarcoidosis.39,40
Continue to: Pathology of early and advanced pulmonary sarcoidosis
Pathology of early and advanced pulmonary sarcoidosis
Sarcoidosis is characterized by coalescing, tightly clustered, nonnecrotizing granulomas in the lung (FIGURE 4), most often located along the lymphatic routes of the pleura, interlobular septa, and bronchovascular bundles.41 Granulomas contain epithelioid cells or multinucleated giant cells surrounded by a chronic lymphocytic infiltrate. Typically, intracytoplasmic inclusions, such as Schaumann bodies, asteroid bodies, and blue bodies of calcium oxalates are noted within giant cells.

In chronic disease, lymphocytic infiltrate vanishes and granulomas tend to become increasingly fibrotic and enlarge to form hyalinized nodules rich with densely eosinophilic collagen. In 10% to 30% of cases, the lungs undergo progressive fibrosis.40 Nonresolving inflammation appears to be the major cause of fibrosis and the peribronchovascular localization leading to marked bronchial distortion.
Clinical features, monitoring, and outcomes
Pulmonary involvement occurs in most patients with sarcoidosis, and subclinical pulmonary disease is generally present, even when extrathoracic manifestations predominate.23 Dry cough, dyspnea, and chest discomfort are the most common symptoms. Chest auscultation is usually unremarkable. Wheezing is more common in those with fibrosis and is attributed to airway-centric fibrosis.42 There is often a substantial delay between the onset of symptoms and the diagnosis of pulmonary sarcoidosis, as symptoms are nonspecific and might be mistaken for more common pulmonary diseases, such as asthma or chronic bronchitis.43
Since sarcoidosis can affect pulmonary parenchyma, interstitium, large and small airways, pulmonary vasculature, and respiratory muscles, the pattern of lung function impairment on PFT varies from normal to obstruction, restriction, isolated diffusion defect, or a combination of these. The typical physiologic abnormality is a restrictive ventilatory defect with a decreased diffusing capacity of the lung for carbon monoxide (DLCO). Extent of disease seen on HRCT correlates with level of restriction.44 Airway obstruction can be multifactorial and due to airway distortion (more likely to occur in fibrotic lung disease) and luminal disease.45-48 The 6-minute walk test and DLCO can also aid in the diagnosis of SAPH and advanced parenchymal lung disease.
While monitoring is done clinically and with testing (PFT and imaging) as needed, the optimal approach is unclear. Nevertheless, longitudinal monitoring with testing may provide useful management and prognostic information.40 Pulmonary function can remain stable in fibrotic sarcoidosis over extended periods and actually can improve in some patients.49 Serial spirometry, particularly forced vital capacity, is the most reliable tool for monitoring; when a decline in measurement occurs, chest radiography can elucidate the mechanism.50,51
Continue to: Because sarcoidosis is a multisystem disease...
Because sarcoidosis is a multisystem disease, caution needs to be exercised when evaluating a patient’s new or worsening respiratory symptoms to accurately determine the cause of symptoms and direct therapy accordingly. In addition to refractory inflammatory pulmonary disease, airway disease, infection, fibrosis, and SAPH, one needs to consider extrapulmonary involvement or complications such as cardiac or neurologic disease, musculoskeletal disease, depression, or fatigue. Adverse medication effects, deconditioning, or unrelated (or possibly related) disorders (eg pulmonary embolism) may be to blame.
Determining prognosis
Prognosis of sarcoidosis varies and depends on epidemiologic factors, clinical presentation, and course, as well as specific organ involvement. Patients may develop life-threatening pulmonary, cardiac, or neurologic complications. End-stage disease may require organ transplantation for eligible patients.
Most patients with pulmonary sarcoidosis experience clinical remission with minimal residual organ impairment and a favorable long-term outcome. Advanced pulmonary disease (known as APS) occurs in a small proportion of patients with sarcoidosis but accounts for most of the poor outcomes in sarcoidosis.40 APS is variably defined, but it generally includes pulmonary fibrosis, SAPH, and respiratory infection.
One percent to 5% of patients with sarcoidosis die from complications, and mortality is higher in women and African Americans.52 Mortality and morbidity may be increasing.53 The reasons behind these trends are unclear but could include true increases in disease incidence, better detection rates, greater severity of disease, or an aging population. Increased hospitalizations and health care use might be due to organ damage from granulomatous inflammation (and resultant fibrosis), complications associated with treatment, and psychosocial effects of the disease/treatment.
Management
Management consists primarily of anti-inflammatory or immunosuppressive therapies but can also include measures to address specific complications (such as fatigue) and organ transplant, as well as efforts to counter adverse medication effects. Other supportive and preventive measures may include, on a case-by-case basis, oxygen supplementation, vaccinations, or pulmonary rehabilitation. Details of these are found in other, more in-depth reviews on treatment; we will briefly review anti-inflammatory therapy, which forms the cornerstone of treatment in most patients with sarcoidosis.
Continue to: General approach to treatment decisions
General approach to treatment decisions. Anti-inflammatory therapy is used to reduce granulomatous inflammation, thereby preserving organ function and reducing symptoms. A decision to begin treatment is one shared with the patient and is based on symptoms and potential danger of organ system failure.54 Patients who are symptomatic or have progressive disease or physiologic impairment are generally candidates for treatment. Monitoring usually suffices for those who have minimal symptoms, stable disease, and preserved organ function.
Patients with pulmonary sarcoidosis at CXR stage 0 should not receive treatment, given that large, randomized trials have shown no meaningful benefit and that these patients have a high likelihood of spontaneous remission and excellent long-term prognosis.55-58 However, a subgroup of patients classified as stage 0/I on CXR may show parenchymal disease on HRCT,59 and, if more symptomatic, could be considered for treatment. For patients with stage II to IV pulmonary sarcoidosis with symptoms, there is good evidence that treatment may improve lung function and reduce dyspnea and fatigue.57,60-62
Corticosteroids are first-line treatment for most patients. Based on expert opinion, treatment of pulmonary sarcoidosis is generally started with oral prednisone (or an equivalent corticosteroid). A starting dose of 20 to 40 mg/d generally is sufficient for most patients. If the patient responds to initial treatment, prednisone dose is tapered over a period of months. If symptoms worsen during tapering, the minimum effective dose is maintained without further attempts at tapering. Treatment is continued for at least 3 to 6 months but it might be needed for longer durations; unfortunately, evidence-based guidelines are lacking.63 Once the patient goes into remission, close monitoring is done for possible relapses. Inhaled corticosteroids alone have not reduced symptoms or improved lung function in patients with pulmonary sarcoidosis.64-66
Steroid-sparing agents are added for many patients. For patients receiving chronic prednisone therapy (≥ 10 mg for > 6 months), steroid-sparing agents are considered to minimize the adverse effects of steroids or to better control the inflammatory activity of sarcoidosis. These agents must be carefully selected, and clinical and laboratory monitoring need to be done throughout therapy. TABLE 558,64,67-81

The management might be complicated for extrapulmonary, multi-organ, and advanced sarcoidosis (advanced pulmonary sarcoidosis, cardiac disease, neurosarcoidosis, lupus pernio, etc) when specialized testing, as well as a combination of corticosteroids and steroid-sparing agents (with higher doses or prolonged courses), might be needed. This should be performed at an expert sarcoidosis center, ideally in a multidisciplinary setting involving pulmonologists and/or rheumatologists, chest radiologists, and specialists as indicated, based on specific organ involvement.
Continue to: Research and future directions
Research and future directions
Key goals for research are identifying more accurate biomarkers of disease, improving diagnosis of multi-organ disease, determining validated endpoints of clinical trials in sarcoidosis, and developing treatments for refractory cases.
There is optimism and opportunity in the field of sarcoidosis overall. An example of an advancement is in the area of APS, as the severity and importance of this phenotype has been better understood. Worldwide registries and trials of pulmonary vasodilator therapy (bosentan, sildenafil, epoprostenol, and inhaled iloprost) in patients with SAPH without left ventricular dysfunction are promising.82-85 However, no benefit in survival has been shown.
RioSAPH is a double-blind, placebo-controlled trial of Riociguat (a stimulator of soluble guanylate cyclase) for SAPH (NCT02625558) that is closed to enrollment and undergoing data review. Similarly, results of the phase IV study of pirfenidone, an antifibrotic agent that was shown to decrease disease progression and deaths in idiopathic pulmonary fibrosis,86 are awaited in the near future.
Other potential directions being explored are multicenter patient registries and randomized controlled trials, analyses of existing databases, use of biobanking, and patient-centered outcome measures. Hopefully, the care of patients with sarcoidosis will become more evidence based with ongoing and upcoming research in this field.
CORRESPONDENCE
Rohit Gupta, MBBS, FCCP, 3401 North Broad Street, 7 Parkinson Pavilion, Philadelphia, PA 19140; [email protected]
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
1. Costabel U, Hunninghake G. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J. 1999;14:735-737.
2. Hillerdal G, Nöu E, Osterman K, et al. Sarcoidosis: epidemiology and prognosis. A 15-year European study. Am Rev Respir Dis. 1984;130:29-32.
3. Mirsaeidi M, Machado RF, Schraufnagel D, et al. Racial difference in sarcoidosis mortality in the United States. Chest. 2015;147:438-449.
4. Rybicki BA, Iannuzzi MC, Frederick MM, et al. Familial aggregation of sarcoidosis. A case-control etiologic study of sarcoidosis (ACCESS). Am J Resp Crit Care Med. 2001;164:2085-2091.
5. Sverrild A, Backer V, Kyvik KO, et al. Heredity in sarcoidosis:a registry-based twin study. Thorax. 2008;63:894.
6. Vuyst P, Dumortier P, Schandené L, et al. Sarcoidlike lung granulomatosis induced by aluminum dusts. Am Rev Respir Dis. 1987;135:493-497.
7. Werfel U, Schneider J, Rödelsperger K, et al. Sarcoid granulomatosis after zirconium exposure with multiple organ involvement. European Respir J. 1998;12:750.
8. Newman KL, Newman LS. Occupational causes of sarcoidosis. Curr Opin Allergy Clin Immunol. 2012;12:145-150.
9. Zissel G, Müller-Quernheim J. Specific antigen(s) in sarcoidosis:a link to autoimmunity? Eur Respir J. 2016;47:707-709.
10. Chen ES, Moller DR. Etiology of sarcoidosis. Clin Chest Med. 2008;29:365-377.
11. Agostini C, Adami F, Semenzato G. New pathogenetic insights into the sarcoid granuloma. Curr Opin Rheumatol. 2000;12:71-76.
12. Valentonyte R, Hampe J, Huse K, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet. 2005;37:357-364.
13. Rybicki BA, Walewski JL, Maliarik MJ, et al. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet. 2005;77:491-499.
14. Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324-1330.
15. Gorham ED, Garland CF, Garland FC, et al. Trends and occupational associations in incidence of hospitalized pulmonary sarcoidosis and other lung diseases in Navy personnel: a 27-year historical prospective study, 1975-2001. Chest. 2004;126:1431-1438.
16. Kucera GP, Rybicki BA, Kirkey KL, et al. Occupational risk factors for sarcoidosis in African-American siblings. Chest. 2003;123:1527-1535.
17. Prezant DJ, Dhala A, Goldstein A, et al. The incidence, prevalence, and severity of sarcoidosis in New York City firefighters. Chest. 1999;116:1183-1193.
18. Barnard J, Rose C, Newman L, et al. Job and industry classifications associated with sarcoidosis in A Case–Control Etiologic Study of Sarcoidosis (ACCESS). J Occup Environ Med. 2005;47:226-234.
19. Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414-1423.
20. Eishi Y, Suga M, Ishige I, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol. 2002;40:198-204.
21. Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet. 2014;383:1155-1167.
22. Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of sarcoidosis. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e26-51.
23. Judson MA, ed. Pulmonary Sarcoidosis: A Guide for the Practicing Clinician. Springer; 2014.
24. Govender P, Berman JS. The diagnosis of sarcoidosis. Clin Chest Med. 2015;36:585-602.
25. Valeyre D, Bernaudin J-F, Uzunhan Y, et al. Clinical presentation of sarcoidosis and diagnostic work-up. Semin Resp Crit Care Med. 2014;35:336-351.
26. Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol. 2015;49:63-78.
27. Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Clin Chest Med. 2015;36:685-702.
28. Culver DA, Neto ML, Moss BP, et al. Neurosarcoidosis. Semin Resp Crit Care Med. 2017;38:499-513.
29. Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36:669-683.
30. Sayah DM, Bradfield JS, Moriarty JM, et al. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Resp Crit Care Med. 2017;38:477-498.
31. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Resp Crit Care. 2012;164:1885-1889.
32. Keijsers RG, Veltkamp M, Grutters JC. Chest imaging. Clin Chest Med. 2015;36:603-619.
33. Scadding J. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Brit Med J. 1961;2:1165-1172.
34. Miller B, Putman C. The chest radiograph and sarcoidosis. Reevaluation of the chest radiograph in assessing activity of sarcoidosis: a preliminary communication. Sarcoidosis. 1985;2:85-90.
35. Loddenkemper R, Kloppenborg A, Schoenfeld N, et al. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis--results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft für die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis. 1998;15:178-182.
36. Calandriello L, Walsh SLF. Imaging for sarcoidosis. Semin Resp Crit Care Med. 2017;38:417-436.
37. Gupta D, Dadhwal DS, Agarwal R, et al. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146:547-556.
38. Baydur A. Recent developments in the physiological assessment of sarcoidosis: clinical implications. Curr Opin Pulm Med. 2012;18:499-505.
39. Jamilloux Y, Maucort-Boulch D, Kerever S, et al. Sarcoidosis-related mortality in France: a multiple-cause-of-death analysis. Eur Respir J. 2016;48:1700-1709.
40. Gupta R, Baughman RP. Advanced pulmonary sarcoidosis. Semin Respir Crit Care Med. 2020;41:700-715.
41. Rossi G, Cavazza A, Colby TV. Pathology of sarcoidosis. Clin Rev Allergy Immunol. 2015;49:36-44.
42. Hansell D, Milne D, Wilsher M, et al. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998;209:697-704.
43. Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406-412.
44. Müller NL, Mawson JB, Mathieson JR, et al. Sarcoidosis: correlation of extent of disease at CT with clinical, functional, and radiographic findings. Radiology. 1989;171:613-618.
45. Harrison BDW, Shaylor JM, Stokes TC, et al. Airflow limitation in sarcoidosis—a study of pulmonary function in 107 patients with newly diagnosed disease. Resp Med. 1991;85:59-64.
46. Polychronopoulos VS, Prakash UBS. Airway Involvement in sarcoidosis. Chest. 2009;136:1371-1380.
47. Chambellan A, Turbie P, Nunes H, et al. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005;127:472-481.
48. Handa T, Nagai S, Fushimi Y, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006;130:1851-1856.
49. Nardi A, Brillet P-Y, Letoumelin P, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011;38:1368-1373.
50. Zappala CJ, Desai SR, Copley SJ, et al. Accuracy of individual variables in the monitoring of long-term change in pulmonary sarcoidosis as judged by serial high-resolution CT scan data. Chest. 2014;145:101-107.
51. Gafà G, Sverzellati N, Bonati E, et al. Follow-up in pulmonary sarcoidosis: comparison between HRCT and pulmonary function tests. Radiol Med. 2012;117:968-978.
52. Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014;20:472-478.
53. Kearney GD, Obi ON, Maddipati V, et al. Sarcoidosis deaths in the United States: 1999–2016. Respir Med. 2019;149:30-35.
54. Baughman RP, Judson M, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis Vasc Diffuse Lung Dis. 2017;34:280-282.
55. Nagai S, Shigematsu M, Hamada K, et al. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med. 1999;5:293-298.
56. Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis: an analysis of 818 patients. Q J Med. 1983;52:525-533.
57. Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and the Irish Thoracic Society. Thorax. 2008;63(suppl 5):v1-v58.
58. Pietinalho A, Tukiainen P, Haahtela T, et al. Oral prednisolone followed by inhaled budesonide in newly diagnosed pulmonary sarcoidosis: a double-blind, placebo-controlled multicenter study. Finnish Pulmonary Sarcoidosis Group. Chest. 1999;116:424-431.
59. Oberstein A, von Zitzewitz H, Schweden F, et al. Non invasive evaluation of the inflammatory activity in sarcoidosis with high-resolution computed tomography. Sarcoidosis Vasc Diffuse Lung Dis. 1997;14:65-72.
60. Gibson G, Prescott RJ, Muers MF, et al. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax. 1996;51:238-247.
61. Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. 2012;8:95-103.
62. Pietinalho A, Tukiainen P, Haahtela T, et al. Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 2002;121:24-31.
63. Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29:190146.
64. Baughman RP, Iannuzzi MC, Lower EE, et al. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2002;19:198-204.
65. du Bois RM, Greenhalgh PM, Southcott AM, et al. Randomized trial of inhaled fluticasone propionate in chronic stable pulmonary sarcoidosis: a pilot study. Eur Respir J. 1999;13:1345-1350.
66. Milman N, Graudal N, Grode G, Munch E. No effect of high‐dose inhaled steroids in pulmonary sarcoidosis: a double‐blind, placebo‐controlled study. J Intern Med. 1994;236:285-290.
67. Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. 2000;17:60-66.
68. Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013;144:805-812.
69. Sahoo D, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011;38:1145-1150.
70. Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Resp Crit Care Med . 2006;174:795-802.
71. Rossman MD, Newman LS, Baughman RP, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2006;23:201-208.
72. Selroos O, Sellergren T. Corticosteroid therapy of pulmonary sarcoidosis. A prospective evaluation of alternate day and daily dosage in stage II disease. Scand J Respir Dis . 1979;60:215-221.
73. Israel HL, Fouts DW, Beggs RA. A controlled trial of prednisone treatment of sarcoidosis. Am Rev Respir Dis . 1973;107:609-614.
74. Hamzeh N, Voelker A, Forssén A, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med . 2014;108:1663-1669.
75. Brill A-K, Ott SR, Geiser T. Effect and safety of mycophenolate mofetil in chronic pulmonary sarcoidosis: a retrospective study. Respiration . 2013;86:376-383.
76. Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2004;21:43-48.
77. Sweiss NJ, Noth I, Mirsaeidi M, et al. Efficacy results of a 52-week trial of adalimumab in the treatment of refractory sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2014;31:46-54.
78. Sweiss NJ, Lower EE, Mirsaeidi M, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J . 2014;43:1525-1528.
79. Thatayatikom A, Thatayatikom S, White AJ. Infliximab treatment for severe granulomatous disease in common variable immunodeficiency: a case report and review of the literature. Ann Allergy Asthma Immunol . 2005;95:293-300.
80. Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. Jama Dermatol . 2013;149:1040-1049.
81. Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis . 2013;30:201-211.
82. Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest . 2014;145:810-817.
83. Baughman RP, Shlobin OA, Wells AU, et al. Clinical features of sarcoidosis associated pulmonary hypertension: results of a multi-national registry. Respir Med . 2018;139:72-78.
84. Fisher KA, Serlin DM, Wilson KC, et al. Sarcoidosis-associated pulmonary hypertension outcome with long-term epoprostenol treatment. Chest . 2006;130:1481-1488.
85. Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis . 2009;26:110-120.
86. King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med . 2014;370:2083-2092.
PRACTICE RECOMMENDATIONS
› Consider biopsy to aid in diagnosing sarcoidosis; it may be avoided with a high clinical suspicion for sarcoidosis (eg, Löfgren syndrome, lupus pernio, or Heerfordt syndrome). C
› Rule out alternative diagnoses such as infection, malignancy, collagen vascular disease, and vasculitis. C
› Identify extra-pulmonary organ involvement, as clinically indicated, by screening with a baseline eye examination; complete blood count; creatinine, alkaline phosphatase, and calcium levels; electrocardiogram, and other organ-specific studies. C
› Make a patient-centered decision whether to begin antiinflammatory treatment based on symptomatology and risk of organ failure or death. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A thoughtful approach to drug screening and addiction
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
Reading the excellent article on urine drug screening by Drs. Hayes and Fox reminds me of 2 important aspects of primary care: (1) Diagnosing and treating patients with drug addiction is an important service we provide, and (2) interpreting laboratory tests requires training, skill, and clinical judgment.
Drs. Hayes and Fox describe the proper use of urine drug testing in the management of patients for whom we prescribe opioids, whether for chronic pain or for addiction treatment. Combining a review of the literature with their own professional experience treating these patients, Drs. Hayes and Fox highlight the potential pitfalls in interpreting urine drug screening results and admonish us to use good clinical judgment in applying those results to patient care. They emphasize the need to avoid racial bias and blaming the patient.
This article is very timely because, amidst the COVID-19 pandemic, the opioid epidemic has continued unabated. The most recent data from the National Center for Health Statistics shows that the estimated number of opioid overdose deaths increased by a whopping 32%, from 47,772 for the 1-year period ending August 2019 to 62,972 for the 1-year period ending August 2020.1 Although this increase began in fall 2019, there can be little doubt that the COVID-19 pandemic is partly responsible. A positive sign, however, is that opioid prescribing in the United States is trending downward, reaching its lowest level in 14 years in 2019.2 In fact, use of cheap street fentanyl, rather than prescription drugs, accounts for nearly all of the increase in opioid overdose deaths.1
Despite this positive news, the number of deaths associated with opioid use remains sobering. The statistics continue to underscore the fact that there simply are not enough addiction treatment centers to manage all of those who need and want help. All primary care physicians are eligible to prescribe suboxone to treat patients with opioid addiction—a treatment that can be highly effective in reducing the use of street opioids and, therefore, reducing deaths from overdose. Fewer than 10% of primary care physicians prescribed suboxone in 2017.3 I hope that more of you will take the required training and become involved in assisting your patients who struggle with opioid addiction.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
1. National Center for Health Statistics. Provisional drug overdose death counts. Updated March 17, 2021. Accessed March 22, 2021. www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
2. CDC. US opioid dispensing rate maps. Updated December 7, 2020. Accessed March 22, 2021. www.cdc.gov/drugoverdose/maps/rxrate-maps.html
3. McBain RK, Dick A, Sorbero M, et al. Growth and distribution of buprenorphine-waivered providers in the United States, 2007-2017. Ann Intern Med. 2020;172:504-506.
Opioid Management in Older Adults: Lessons Learned From a Geriatric Patient-Centered Medical Home
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
The United States continues to confront an opioid crisis that also affects older adults. According to the Substance Abuse and Mental Health Services Administration from 1999 to 2010, there has been a 4-fold increase in opioid overdose deaths.1 Between 2010 and 2015, the rate of opioid-related inpatient stays and emergency department (ED) visits for people aged ≥ 65 years increased by 34% and 74%, respectively, and opioid-related overdose deaths continue to increase among older patients.1,2
Background
Chronic pain is estimated to affect 50 million US adults.3 Individuals receiving long-term opioid therapy may not have experienced relief with other medications or cannot take them for medical safety reasons. Losing access to opioid prescriptions can contribute to misuse of illicit opioids. Implementing best practices for prescription opioid management in older adults is challenging. Older adults have a high prevalence of chronic pain, which is linked to disability and loss of function, reduced mobility, falls, depression, anxiety, sleep disorders, social isolation, and suicide or suicidal ideation.4 Until recently, chronic pain in older adults was often treated primarily with long-term opioid prescriptions, despite little evidence for the effectiveness of that treatment for chronic conditions. The prevalence of long-term opioid use in adults has increased from 1.8% (1999-2000) to 5.4% (2013-2014), and 25% of adult long-term opioid users are aged ≥ 65 years.5
Older adults are especially vulnerable to developing adverse events (AEs) from opioid use, including constipation, confusion, nausea, falls, and overdose. These factors make safe prescribing more challenging even when opioids are an appropriate therapeutic choice. Older adults often have multiple chronic conditions and take multiple medications that increase risk of AEs due to drug-disease and drug-drug interactions. Finding appropriate alternatives for pain management can be challenging in the presence of dementia if other pharmacologic options are contraindicated or mobility issues limit access to other therapeutic options.
Pain treatment plans should be based on realistic functional goals using a shared decision-making approach accounting for patient and provider expectations. All reasonable nondrug and nonopioid treatments should be considered before opioids are initiated. A comprehensive, person-centered, approach to pain management in older adults that includes opioids, other medications, and complementary and integrative care could improve both pain control and function,and reduce the harms of unnecessary opioid exposure.6 A validated risk review should be performed and documented on all patients starting opioids except patients enrolled in hospice care.
In 2018, the US Department of Veterans Affairs (VA) required all facilities to complete case reviews for veterans identified in the Stratification Tool for Opioid Risk Mitigation (STORM) dashboard as being at particularly high risk for AEs among patients prescribed opioids.7 We present our experience with a 1-year management of 48 high-risk older patients receiving chronic prescription opioid therapy. These patients obtained all their care at the VA with complete record documentation.
Methods
The Tennessee Valley Healthcare System (TVHS) is an integrated VA health care system with > 100,000 veteran patients in middle Tennessee with 2 medical centers 40 miles apart, and 12 community-based outpatient clinics. In 2011, TVHS developed a geriatric patient-centered medical home model for geriatric primary care—the geriatric patient aligned care team (GeriPACT).8 GeriPACT consists of a GeriPACT primary care provider (geriatrician or geriatric nurse practitioner with a panel of about 800 outpatients), social worker, clinical pharmacist, registered nurse care manager, licensed vocational nurse, and clerical staff. GeriPACT is a special population PACT within primary care for complex geriatric and other high-risk vulnerable veterans providing integrated, interdisciplinary assessment and longitudinal management, and coordination of both VA and non-VA-funded (eg, Medicare and Medicaid) services for patients and caregivers. GeriPACT at the Nashville TVHS campus has an enrollment of 745 patients of whom 48 receive chronic prescription opioid therapy. The practice is supported by the VA Computerized Patients Record System (CPRS), including the electronic patient portal, My healtheVet, with telemedicine capabilities. Data were collected by chart review with operations data extracted from the Veterans Health Information System and Technology Architecture.
Best practices for prescription opioids for chronic pain follow the US Department of Health and Human Services Interagency Task Force pain management recommendations.4 These include: (1) Effective pain evaluation and management, including diagnostic evaluation and indicated referrals; (2) appropriately prescribed opioids when indicated; and (3) active management of opioid users to prevent AEs and misuse. Strategies used in GeriPACT were adopted from the pain management task force and designed to address needs and challenges associated with responsible chronic opioid prescribing (Table 1).
All 48 patients who were prescribed chronic opioid therapy received routine primary care at GeriPACT. A data tracking sheet was maintained from July 1, 2019 to June 30, 2020. Patients were presented for interdisciplinary collaboration and management at weekly GeriPACT where applicable continuous improvement processes were incorporated. Patients were seen every 3 to 6 months and offered dose reduction and alternative therapies at those times. All patients initiated monthly calls for medication refills and were monitored with an initial opioid contract and quarterly unannounced urine drug screens (UDSs) as well as a quarterly review of the prescription drug monitoring database (PDMD). Additionally, all patients received an Opioid Risk Tool assessment (scale 0-26; high risk ≥ 8) and a Risk Index for Overdose or Serious Opioid-Induced Respiratory Depression (RIOSORD) Score (scale 0-115).9,10 Patients with RIOSORD scores ≥ 25 (14% risk of opioid induced respiratory depression) were issued naloxone kits.
All VA patients additionally receive a risk stratification, the clinical assessment of need (CAN) score, which is a clinical predictor of hospitalization and death developed for VA populations.11 This methodology extracts predictors from 6 categories: social demographics, medical conditions, vital signs, prior year use of health services, medications, and laboratory tests and constructs logistic regression models to predict outcomes. CAN scores are on a 99-point scale, with higher scores corresponding to an increased probability of future health care events.
Our overall study was designed to meet standards for quality improvement reporting excellence (SQUIRE) criteria, and this report meets the quality improvement minimum quality criteria set (QI-MQCS) domains for reporting quality improvement work.12,13 The TVHS Institutional Review Board determined this study to be a quality improvement initiative.
Results
Chronic opioid patients comprised 6.4% of the GeriPACT population. These patients had many comorbidities, including diabetes mellitus (35%), pulmonary disease (25%), congestive heart failure (18.8%), and dementia (8%). There were 54% with estimated glomerular filtration rates (eGFR) < 60 mL/min, indicating at least stage 3 chronic kidney disease (Table 2). Patients had an average RIOSORD Score of 21 and a 14% risk of opioid induced respiratory depression, and 20% received mental health services.
The mean CAN score was 83.1, suggesting a 1-year risk of 20% for a major AE and 5% mortality risk. Many patients with chronic opioid use were transferred to GeriPACT from primary care due to presence of complex medical issues in addition to need for chronic pain management. In this population, 8% were coprescribed benzodiazepines and opioids. Consults were obtained from interventional pain for 37.5% of patients and palliative care for 27% of patients, the majority for goals of care related to chronic illness and advance directive discussions, and in 1 patient for pain and symptom management. The majority of patients (81%) had advance care planning documents or discussions documented in the electronic health record, and 87.5% of patients received home and community-based support in addition to GeriPACT care.
My healtheVet patient portal secure messaging was used a mean 2.1 times per patient (range 0-27) to maintain contact with GeriPACT providers and patients had a mean 14.5 outpatient visits yearly (range, 1-49) in addition to monthly clinic contact for opioid prescription refills (Table 3). One patient entered long-term care. Three patients expired due to congestive heart failure, sepsis, and complications following a hip fracture. Of the patients who expired, all had advance directives or hospice care involvement. The VA STORM risk tool identifies the highest risk patients: suicide risk, current opioid or substance use disorder, suicide attempt or overdose during the past year, and potential for opioid-related respiratory depression on the RIOSORD scale. None of the panel patients met high-risk criteria on the Opioid Risk Tool assessment or were identified on the facility’s highest risk category by the STORM risk tool.
Medication Reduction
Pharmacists routinely counseled patients regarding the appropriate timing of refills and made monthly calls to request refills of controlled drugs. Three patients agreed to opioid dose reduction due to improved clinical status. Two patients had 25% and 30% dose reductions, respectively, and 1 patient was able to be discontinue opioids. This was achieved through reduction of therapy and or substitution of alternative nonopioid pain medications. One patient was initiated on a slow benzodiazepine taper schedule after decades of benzodiazepine use in addition to engagement with a whole health coach and primary care mental health integration provider. Another patient was disenrolled from the clinic because of repeated nonadherence and positive UDSs for polysubstance use disorder.
Accidental Overdoses
There were 2 patients with accidental overdoses who survived, both on high morphine equivalent daily doses (MEDDs). One patient was admitted to the intensive care unit for increasing confusion after taking more than the prescribed opioids (120 mg MEDD) due to uncontrolled pain for 2 months following surgery. The second patient was taking 66 mg MEDD with multiple risk factors for respiratory depression (severe chronic obstructive pulmonary disease requiring oxygen, obstructive sleep apnea, and concomitant benzodiazepine use) who repeatedly refused tapering of opioids and benzodiazepines. He was found unresponsive in respiratory depression by home health staff. Both patients had naloxone kits in their home that were not administered.
Urine Drug Screening
There was an occasional negative opioid UDS attributed to patients on a low-dose opioid administered more than 24 hours before. Five patients (10.4%) had positive UDSs. Two patients were positive for cocaine, and because of chronic persistent pain and complex medical problems cared for in the clinic, counseled and continued on therapy with no repeat infractions. Two patients were positive for cannabinoids attributed to CBD oil products, which are legal in Tennessee. One patient had repeated positive UDSs for polysubstance abuse and was terminated from the clinic, preferring to use cannabinoids and other substances illegally. Meperidine, fentanyl, tramadol, and other synthetic opioids are not detected on a routine UDS.
Discussion
Primary care is critical in optimizing the prescribing and use of opioids in older adults. The patient-centered medical home can give health care providers the tools and support to provide evidence—based pain management for their older adult patients and to facilitate prescription and monitoring of appropriate opioid use to minimizing AEs and OUD risk. This includes a reliable health information technology monitoring system as part of a collaborative, person-centered care practice capable of managing frail older patients with multiple chronic conditions as well as social risk factors. GeriPACT was able to implement guideline—based evaluation and treatment of chronic pain patients through optimal management of opioids, risk reduction, and monitoring and management of AEs, misuse, and dose tapering using shared decision-making strategies when appropriate.
Complex older patients on chronic opioid treatment can do well and are best managed by an interdisciplinary team. Our panel had a high prevalence of chronic opioid patients and a high expected mortality based on the severity of comorbidities. Patients had frequent access to care with utilization of many support services. Patients received care for many chronic illnesses at the same time they received opioid therapy and generally were satisfied and adherent to their treatment plan. Published reports of the prevalence of coprescribing of benzodiazepines and opioids of 1.1 to 2.7% in the general population, may be lower than our veteran population.14 Despite the fact that nearly 20% of the population had a history of opioid misuse, only 1 patient was terminated from the clinic because of repeated episodes of polysubstance use disorder.
GeriPACT has the capability to be responsive to the changing needs of older chronic pain patients as a learning health system using continuous process improvement with frequent team meetings and interdisciplinary care.15 Our experience with chronic pain management demonstrates the feasibility and quality of guideline-based management and enhances our understanding of the intersection of care, chronic pain management, and opioid use disorder in older adults.
Limitations
Our experience with this population of older veterans may not be applicable to other geriatric populations. While all patients received their primary care at VA and patients were seen regularly, our data may not account for possible use of some community services, including hospitalization and long-term care.
Conclusions
Guideline-based patient-centered medical home management of patients with chronic pain treated with opioids can be an effective model to maintain and improve measures of health and well-being in older patients. Primary care management is critical in optimizing the prescribing and use of opioids in older adults. These complex older patients are best managed by an interdisciplinary team.
Acknowledgments
This work was supported in part by the Geriatric Workforce Enhancement Program, HRSA Grant: 1-U1Q-HP 033085-01-00.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.
1. Weiss AJ, Heslin KC, Barrett ML, Izar R, Bierman AS. Opioid-related inpatient stays and emergency department visits among patients aged 65 years and older, 2010 and 2015: Statistical Brief #244. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); September 18, 2018.
2. Centers for Disease Control and Prevention. New data show significant changes in drug overdose deaths. Published March 18, 2020. Accessed March 11, 2021. https://www.cdc.gov/media/releases/2020/p0318-data-show-changes-overdose-deaths.html
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001-1006. Published 2018 Sep 14. doi:10.15585/mmwr.mm6736a2
4. National Institutes of Health, Interagency Pain Research Coordinating Committee. National pain strategy overview. Updated March 11, 2021. Accessed March 11, 2021. https://www.iprcc.nih.gov/national-pain-strategy-overview
5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf. 2018;27(5):526-534. doi:10.1002/pds.4278
6. US Department of Health and Human Services. Pain management best practices inter-agency task force report: updates, gaps, inconsistencies, and recommendations. Published May 9, 2019. Accessed March 17, 2021.https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf
7. Oliva EM, Bowe T, Tavakoli S, et al. Development and applications of the Veterans Health Administration’s Stratification Tool for Opioid Risk Mitigation (STORM) to improve opioid safety and prevent overdose and suicide. Psychol Serv. 2017;14(1):34-49. doi:10.1037/ser0000099
8. US Department of Veterans Affairs, Veterans Health Administration. Geriatric patient aligned care team (Geri-PACT). Published June 15, 2015. Accessed March 11, 2021. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3115
9. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432-442. doi:10.1111/j.1526-4637.2005.00072.x
10. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans’ Health Administration patients. Pain Med. 2015;16(8):1566-1579. doi:10.1111/pme.12777
11. Wang L, Porter B, Maynard C, et al. Predicting risk of hospitalization or death among patients receiving primary care in the Veterans Health Administration. Med Care. 2013;51(4):368-373. doi:10.1097/MLR.0b013e31827da95a
12. Ogrinc G, Mooney SE, Estrada C, et al. The SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines for quality improvement reporting: explanation and elaboration. Qual Saf Health Care. 2008;17(suppl 1):i13-i32. doi:10.1136/qshc.2008.029058
13. Hempel S, Shekelle PG, Liu JL, et al. Development of the Quality Improvement Minimum Quality Criteria Set (QI-MQCS): a tool for critical appraisal of quality improvement intervention publications. BMJ Qual Saf. 2015;24(12):796-804. doi:10.1136/bmjqs-2014-003151
14. Rhee TG. Coprescribing of Benzodiazepines and Opioids in Older Adults: Rates, Correlates, and National Trends. J Gerontol A Biol Sci Med Sci. 2019;74(12):1910-1915. doi:10.1093/gerona/gly283
15. National Academy of Medicine. The Learning Healthcare System: Workshop Summary. The National Academies Press; 2007. doi:10.17226/11903.
Painful thickened breast lesion
Treatment was attempted for both a suspected spider bite (2 weeks of topical triamcinolone 0.1%) and presumed cellulitis (oral doxycycline 100 mg bid/5 d), but neither improved her condition. Concerned for the possibility of cutaneous breast cancer, a punch biopsy was ordered and revealed diffuse dermal angiomatosis (DDA).
DDA is an uncommon proliferation of cutaneous blood vessels causing a reticular blood vessel pattern, as seen in this image. Typically, DDA is associated with tissue hypoxia due to arterial insufficiency from peripheral artery disease. In recent years, there have been numerous case reports of painful ulcerated lesions and reticular blood vessels occurring in women with large, pendulous breasts, increased body mass index, and a history of smoking. One theory suggests that the weight of the breasts causes tissue to stretch, compressing the blood vessels. This, combined with smoking, leads to localized hypoxia and DDA.
Treatments have included oral isotretinoin, calcium channel blockers, aspirin, or pentoxifylline to help circulation. Smoking cessation is recommended, as well as reduction mammoplasty to decrease the stretch on the tissues and relieve the local hypoxia. Although invasive, breast reduction surgery has moved to the forefront of therapy, with reports having shown resolution of the ulcers and pain.1
Two important aspects of clinical medicine are highlighted by this case. First, nonhealing lesions that are not responding to prescribed therapies may require biopsy to rule out malignancy. Second, when there is difficulty making a diagnosis, especially with uncommon diseases, biopsy and input from a pathologist can be extremely helpful.
In this case, the patient was referred to Plastic Surgery and scheduled for reduction mammoplasty. The patient was advised to stop smoking for at least 4 weeks prior to the surgery to possibly improve her condition and reduce the likelihood of postoperative complications.
Photo courtesy of Michael Louie, MD, and text courtesy of Michael Louie, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Galambos J, Meuli-Simmen C, Schmid R, et al. Diffuse dermal angiomatosis of the breast: a distinct entity in the spectrum of cutaneous reactive angiomatoses—clinicopathologic study of two cases and comprehensive review of the literature. Case Rep Dermatol 2017;9:194-205. https://doi.org/10.1159/000480721

Treatment was attempted for both a suspected spider bite (2 weeks of topical triamcinolone 0.1%) and presumed cellulitis (oral doxycycline 100 mg bid/5 d), but neither improved her condition. Concerned for the possibility of cutaneous breast cancer, a punch biopsy was ordered and revealed diffuse dermal angiomatosis (DDA).
DDA is an uncommon proliferation of cutaneous blood vessels causing a reticular blood vessel pattern, as seen in this image. Typically, DDA is associated with tissue hypoxia due to arterial insufficiency from peripheral artery disease. In recent years, there have been numerous case reports of painful ulcerated lesions and reticular blood vessels occurring in women with large, pendulous breasts, increased body mass index, and a history of smoking. One theory suggests that the weight of the breasts causes tissue to stretch, compressing the blood vessels. This, combined with smoking, leads to localized hypoxia and DDA.
Treatments have included oral isotretinoin, calcium channel blockers, aspirin, or pentoxifylline to help circulation. Smoking cessation is recommended, as well as reduction mammoplasty to decrease the stretch on the tissues and relieve the local hypoxia. Although invasive, breast reduction surgery has moved to the forefront of therapy, with reports having shown resolution of the ulcers and pain.1
Two important aspects of clinical medicine are highlighted by this case. First, nonhealing lesions that are not responding to prescribed therapies may require biopsy to rule out malignancy. Second, when there is difficulty making a diagnosis, especially with uncommon diseases, biopsy and input from a pathologist can be extremely helpful.
In this case, the patient was referred to Plastic Surgery and scheduled for reduction mammoplasty. The patient was advised to stop smoking for at least 4 weeks prior to the surgery to possibly improve her condition and reduce the likelihood of postoperative complications.
Photo courtesy of Michael Louie, MD, and text courtesy of Michael Louie, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque

Treatment was attempted for both a suspected spider bite (2 weeks of topical triamcinolone 0.1%) and presumed cellulitis (oral doxycycline 100 mg bid/5 d), but neither improved her condition. Concerned for the possibility of cutaneous breast cancer, a punch biopsy was ordered and revealed diffuse dermal angiomatosis (DDA).
DDA is an uncommon proliferation of cutaneous blood vessels causing a reticular blood vessel pattern, as seen in this image. Typically, DDA is associated with tissue hypoxia due to arterial insufficiency from peripheral artery disease. In recent years, there have been numerous case reports of painful ulcerated lesions and reticular blood vessels occurring in women with large, pendulous breasts, increased body mass index, and a history of smoking. One theory suggests that the weight of the breasts causes tissue to stretch, compressing the blood vessels. This, combined with smoking, leads to localized hypoxia and DDA.
Treatments have included oral isotretinoin, calcium channel blockers, aspirin, or pentoxifylline to help circulation. Smoking cessation is recommended, as well as reduction mammoplasty to decrease the stretch on the tissues and relieve the local hypoxia. Although invasive, breast reduction surgery has moved to the forefront of therapy, with reports having shown resolution of the ulcers and pain.1
Two important aspects of clinical medicine are highlighted by this case. First, nonhealing lesions that are not responding to prescribed therapies may require biopsy to rule out malignancy. Second, when there is difficulty making a diagnosis, especially with uncommon diseases, biopsy and input from a pathologist can be extremely helpful.
In this case, the patient was referred to Plastic Surgery and scheduled for reduction mammoplasty. The patient was advised to stop smoking for at least 4 weeks prior to the surgery to possibly improve her condition and reduce the likelihood of postoperative complications.
Photo courtesy of Michael Louie, MD, and text courtesy of Michael Louie, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque
Galambos J, Meuli-Simmen C, Schmid R, et al. Diffuse dermal angiomatosis of the breast: a distinct entity in the spectrum of cutaneous reactive angiomatoses—clinicopathologic study of two cases and comprehensive review of the literature. Case Rep Dermatol 2017;9:194-205. https://doi.org/10.1159/000480721
Galambos J, Meuli-Simmen C, Schmid R, et al. Diffuse dermal angiomatosis of the breast: a distinct entity in the spectrum of cutaneous reactive angiomatoses—clinicopathologic study of two cases and comprehensive review of the literature. Case Rep Dermatol 2017;9:194-205. https://doi.org/10.1159/000480721
FDA warning letters target OTC cannabidiol product claims for pain relief
The Food and Drug Administration has warned two manufacturers about illegal marketing of drugs containing cannabidiol (CBD) for over-the-counter use without an approved new drug application, for using substandard manufacturing processes, and for failure to comply with current good manufacturing practices. These warnings add to 51 previous warning letters issued by the FDA since 2015 to other manufacturers of products containing CBD who were violating the Federal Food, Drug, and Cosmetic Act.
In a news release, the agency explained that its two most recent letters, sent to Honest Globe Inc. on March 15 and BioLyte Laboratories LLC on March 18, were issued because CBD has “known pharmacologic effects on humans, with demonstrated risks, it cannot be legally marketed as an inactive ingredient in OTC drug products that are not reviewed and approved by the FDA.” They also describe the companies’ failures to comply with current good manufacturing practices.
“The FDA continues to alert the public to potential safety and efficacy concerns with unapproved CBD products sold online and in stores across the country,” FDA Principal Deputy Commissioner Amy P. Abernethy, MD, PhD, said in the release. “It’s important that consumers understand that the FDA has only approved one drug containing CBD as an ingredient [Epidiolex]. These other, unapproved, CBD products may have dangerous health impacts and side effects. We remain focused on exploring potential pathways for CBD products to be lawfully marketed while also educating the public about these outstanding questions of CBD’s safety. Meanwhile, we will continue to monitor and take action, as needed, against companies that unlawfully market their products – prioritizing those that pose a risk to public health.”
The specific products from Santa Ana, Calif.–based Honest Globe that the FDA called unapproved new drugs and misbranded under the Federal Food, Drug, and Cosmetic Act included Elixicure Original Pain Relief and Elixicure Lavender Pain Relief, both of which were described as containing CBD. Products from Grand Rapids, Mich.–based BioLyte Laboratories LLC that the FDA similarly cited for violations included Silver Gel, Silver Gel with Aloe, Silver Liquid Supplement, Therapeutic Pain Gel, Pain Relief Cream, and Magnesium Oil Spray.
The agency has asked the two companies to respond to its letters within 15 working days, “stating how they will address these violations or providing their reasoning and supporting information as to why they believe these products are not in violation of the law. Failure to adequately address the violations promptly may result in legal action, including product seizure and/or injunction.”
The Food and Drug Administration has warned two manufacturers about illegal marketing of drugs containing cannabidiol (CBD) for over-the-counter use without an approved new drug application, for using substandard manufacturing processes, and for failure to comply with current good manufacturing practices. These warnings add to 51 previous warning letters issued by the FDA since 2015 to other manufacturers of products containing CBD who were violating the Federal Food, Drug, and Cosmetic Act.
In a news release, the agency explained that its two most recent letters, sent to Honest Globe Inc. on March 15 and BioLyte Laboratories LLC on March 18, were issued because CBD has “known pharmacologic effects on humans, with demonstrated risks, it cannot be legally marketed as an inactive ingredient in OTC drug products that are not reviewed and approved by the FDA.” They also describe the companies’ failures to comply with current good manufacturing practices.
“The FDA continues to alert the public to potential safety and efficacy concerns with unapproved CBD products sold online and in stores across the country,” FDA Principal Deputy Commissioner Amy P. Abernethy, MD, PhD, said in the release. “It’s important that consumers understand that the FDA has only approved one drug containing CBD as an ingredient [Epidiolex]. These other, unapproved, CBD products may have dangerous health impacts and side effects. We remain focused on exploring potential pathways for CBD products to be lawfully marketed while also educating the public about these outstanding questions of CBD’s safety. Meanwhile, we will continue to monitor and take action, as needed, against companies that unlawfully market their products – prioritizing those that pose a risk to public health.”
The specific products from Santa Ana, Calif.–based Honest Globe that the FDA called unapproved new drugs and misbranded under the Federal Food, Drug, and Cosmetic Act included Elixicure Original Pain Relief and Elixicure Lavender Pain Relief, both of which were described as containing CBD. Products from Grand Rapids, Mich.–based BioLyte Laboratories LLC that the FDA similarly cited for violations included Silver Gel, Silver Gel with Aloe, Silver Liquid Supplement, Therapeutic Pain Gel, Pain Relief Cream, and Magnesium Oil Spray.
The agency has asked the two companies to respond to its letters within 15 working days, “stating how they will address these violations or providing their reasoning and supporting information as to why they believe these products are not in violation of the law. Failure to adequately address the violations promptly may result in legal action, including product seizure and/or injunction.”
The Food and Drug Administration has warned two manufacturers about illegal marketing of drugs containing cannabidiol (CBD) for over-the-counter use without an approved new drug application, for using substandard manufacturing processes, and for failure to comply with current good manufacturing practices. These warnings add to 51 previous warning letters issued by the FDA since 2015 to other manufacturers of products containing CBD who were violating the Federal Food, Drug, and Cosmetic Act.
In a news release, the agency explained that its two most recent letters, sent to Honest Globe Inc. on March 15 and BioLyte Laboratories LLC on March 18, were issued because CBD has “known pharmacologic effects on humans, with demonstrated risks, it cannot be legally marketed as an inactive ingredient in OTC drug products that are not reviewed and approved by the FDA.” They also describe the companies’ failures to comply with current good manufacturing practices.
“The FDA continues to alert the public to potential safety and efficacy concerns with unapproved CBD products sold online and in stores across the country,” FDA Principal Deputy Commissioner Amy P. Abernethy, MD, PhD, said in the release. “It’s important that consumers understand that the FDA has only approved one drug containing CBD as an ingredient [Epidiolex]. These other, unapproved, CBD products may have dangerous health impacts and side effects. We remain focused on exploring potential pathways for CBD products to be lawfully marketed while also educating the public about these outstanding questions of CBD’s safety. Meanwhile, we will continue to monitor and take action, as needed, against companies that unlawfully market their products – prioritizing those that pose a risk to public health.”
The specific products from Santa Ana, Calif.–based Honest Globe that the FDA called unapproved new drugs and misbranded under the Federal Food, Drug, and Cosmetic Act included Elixicure Original Pain Relief and Elixicure Lavender Pain Relief, both of which were described as containing CBD. Products from Grand Rapids, Mich.–based BioLyte Laboratories LLC that the FDA similarly cited for violations included Silver Gel, Silver Gel with Aloe, Silver Liquid Supplement, Therapeutic Pain Gel, Pain Relief Cream, and Magnesium Oil Spray.
The agency has asked the two companies to respond to its letters within 15 working days, “stating how they will address these violations or providing their reasoning and supporting information as to why they believe these products are not in violation of the law. Failure to adequately address the violations promptly may result in legal action, including product seizure and/or injunction.”
Cannabinoids may pose death risk for older patients with COPD
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
, compared with nonusers, findings from a large study have shown.
Synthetic cannabinoids drugs, such as nabilone and dronabinol, have been approved by the Food and Drug Administration for nausea and vomiting caused by chemotherapy. But their off-label use by adults with COPD to help manage chronic musculoskeletal pain, insomnia, and refractory dyspnea is on the rise, wrote Nicholas T. Vozoris, MD, of the University of Toronto and colleagues.
Cannabinoids may actually contribute to negative respiratory outcomes among individuals with COPD through several possible mechanisms including causing sedation, inducing anxiety, and provoking respiratory muscle weakness, they said.
“Possible adverse respiratory effects of cannabinoids may occur with greater likelihood among older adults (in whom COPD is more prevalent), as this group is known to less efficiently metabolise drugs,” they noted.
In a retrospective, population-based cohort study published in Thorax the researchers identified 185,876 adults aged 66 years and older with COPD using health administrative database information from 2006 to 2016. New cannabinoid users (those starting nabilone or dronabinol) were matched with control nonusers (defined as new users of noncannabinoid drugs). Individuals receiving palliative care, or having a diagnosis of cancer or HIV, were excluded because these are settings where synthetic cannabinoids may be prescribed for nausea or vomiting, and these patients are more likely to be in a poorer state of health.
Overall, new cannabinoid users had significantly higher all-cause mortality rates, compared with nonusers (hazard ratio, 1.64). The effects was greater in high-dose users.
Daniel R. Ouellette, MD, associate professor of medicine at Wayne State University and a senior staff physician at Henry Ford Hospital, both in Detroit, commented that this study has value for clinicians. “Many states are liberalizing cannabinoid use, and it is important to know the health effects of this type of drug on patients with chronic respiratory disease,” he noted. “The study is somewhat surprising. While one might have expected adverse consequences in patients with COPD who inhaled smoke from cannabinoids, it is somewhat unexpected that oral use would be associated with adverse consequences.” He added, “Pain in older adults is a complex problem. Cannabinoids are often recommended for pain in the general community, but pain per se is not a primary symptom for most patients with COPD from their respiratory problems. Physicians treating patients with COPD should diagnose the cause of the pain and provide appropriate treatment.”
Dose makes a difference
All-cause mortality increased by 231% and hospitalization for COPD or pneumonia increased by 178% among new users of higher-dose cannabinoids, compared with nonusers. Higher dose was defined in this study as more than 1.5mg/day of nabilone. No significant differences appeared in new users vs. nonusers in hospitalization for COPD or pneumonia at lower doses, and no significant differences appeared overall in outpatient respiratory exacerbations, emergency department visits for COPD or pneumonia, or COPD- or pneumonia-related mortality.
Potential limitations and implications
“The fact that COPD- or pneumonia-related mortality was not observed to occur with significantly greater rates among cannabinoid users with COPD may suggest that the increased all-cause mortality finding was not being driven by adverse respiratory-related drug effects, as we hypothesized, and instead was possibly a result of unresolved confounding,” the researchers noted.
The study findings were limited by several factors including the inability to prove causation in an observational study, and the potential for confounding based on unmeasured differences between cannabinoid users and nonusers, the researchers said. “Our findings may not be generalizable to all individuals with COPD, as our study included only those aged 66 years and older, and our COPD identification algorithm, while highly specific, had modest sensitivity,” they added. However, the results were strengthened by the large study population and suggest that cannabinoids are not contraindicated for older adults with COPD, the researchers said. “There can be legitimate reasons for using cannabinoids in this population, such as to help treat chemotherapy-related nausea and vomiting, and possibly for end-of-life care,” they emphasized.
The study findings serve to inform clinicians of the significantly increased mortality risk when older adults with COPD initiate cannabinoids, and “this information should be discussed with patients and incorporated in prescribing decision-making and management plans,” along with consideration of using lower doses when possible to minimize adverse events, they concluded.
The study was supported by The Lung Association – Ontario Grant Review/Grant-In-Aid. The researchers had no financial conflicts to disclose.
FROM THORAX
ERRATUM
In the January 2019 article “Migraine: Expanding our Tx arsenal” (J Fam Pract. 2019;68:10-14,16-24), Table 2: Establishing the differential diagnosis of headache provided information that was incorrectly categorized. The table should not have included “Temporal arteritis” as a trigger for a headache caused by infection. Rather, the table should have listed “Temporal arteritis” among the triggers for a headache caused by an autoimmune disorder. In addition, “Acute and chronic sinusitis” and “Meningitis” should not have been listed as triggers for a headache with an iatrogenic or intoxication cause. Rather, they should have been the only triggers attributed to headaches with an infectious origin. The revised table can be found here.
In the January 2019 article “Migraine: Expanding our Tx arsenal” (J Fam Pract. 2019;68:10-14,16-24), Table 2: Establishing the differential diagnosis of headache provided information that was incorrectly categorized. The table should not have included “Temporal arteritis” as a trigger for a headache caused by infection. Rather, the table should have listed “Temporal arteritis” among the triggers for a headache caused by an autoimmune disorder. In addition, “Acute and chronic sinusitis” and “Meningitis” should not have been listed as triggers for a headache with an iatrogenic or intoxication cause. Rather, they should have been the only triggers attributed to headaches with an infectious origin. The revised table can be found here.
In the January 2019 article “Migraine: Expanding our Tx arsenal” (J Fam Pract. 2019;68:10-14,16-24), Table 2: Establishing the differential diagnosis of headache provided information that was incorrectly categorized. The table should not have included “Temporal arteritis” as a trigger for a headache caused by infection. Rather, the table should have listed “Temporal arteritis” among the triggers for a headache caused by an autoimmune disorder. In addition, “Acute and chronic sinusitis” and “Meningitis” should not have been listed as triggers for a headache with an iatrogenic or intoxication cause. Rather, they should have been the only triggers attributed to headaches with an infectious origin. The revised table can be found here.
Joint pain in patients with hemophilia may be neuropathic
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
Nearly one-third of persons with hemophilia had neuropathic pain or altered central pain mechanisms, investigators in a small study found.
Among 30 patients with hemophilia, 9 (30%) had scores of 4 or greater on the 10-point Diabetic Neuropathy 4 (DN4) scale, indicating significant neuropathic pain, reported Nathalie Roussel, PhD, from the University of Antwerp (Belgium), at the annual congress of the European Association for Haemophilia and Allied Disorders.
“The results of this study show us that a large difference exists in pain assessments when we have consecutive sample of patients with hemophilia. These results also show that there are subgroups of patients with altered central pain mechanisms and other subgroups with neuropathic pain, and patients with neuropathic pain have a significantly worse quality of life that is not associated with joint structure and joint function,” she said.
“This is a very good abstract in my opinion, and it deserves more study,” commented hemophilia specialist Rajiv K. Pruthi, MBBS, from the Mayo Clinic in Rochester, Minn., who was not involved in the study.
Structural and functional tests
To get a better understanding of the complexities of ankle pain in persons with hemophilia, Dr. Roussel and colleagues recruited 30 adults followed at their center for moderate or severe hemophilia A or B who were on replacement therapy with factor VIII or factor IX concentrate.
They used MRI without contrast to look for structural alterations in both the talocrural and subtalar joints of both ankles in all patients using the International Prophylaxis Study Group Score, adapted for subtalar joint assessment.
The investigators also used the hemophilia joint health score to assess joint funding, and tests for limits on physical activity, including the Timed Up and Go Test, 2-minute walk test, and Hemophilia Activities Lists.
In addition, they assessed pain with Quantitative Sensory Testing, a noninvasive method for evaluating patient responses to heat, cold, and mechanical pressure. Other measures included questionnaires regarding neuropathic pain and quality of life.
The participants included 23 patients with severe and 3 with moderate hemophilia A, and 1 patient with severe and 3 with moderate hemophilia B. The mean patient age was 39.4 years.
In all, 24 of the 30 patients (80%) were on prophylaxis, and 9 (30%) reported using pain medications; 25 patients reported having some degree of pain.
On MRI, 48/60 (80%) of talocrural joints imaged had pathological findings, as did 41 of 60 (68%) subtalar joints.
“Despite the fact that these patients do not all suffer from ankle joint pain, a lot of them have signs of joint pathology,” Dr. Roussel said.
On the Brief Pain Inventory, only 5 patients had no reported pain, but 14 patients reported either three, five, or six painful locations, and 20 out of 30 patients reported that their ankles were the most affected joints.
Although the sample size was not large enough for statistical comparisons, there were also large variations in pain perception across hemophilia severity.
“This is an important finding, that also patients with moderate hemophilia can have intense pain,” Dr. Roussel said.
On the DN4 questionnaire, nine patients had scores of 4 or greater, indicating that their pain was neuropathic in origin.
When they compared the patients with neuropathic pain with those suffering from nonneuropathic pain, the investigators observed similar structural and joint function between the groups, but significantly worse reported quality of life for patients with neuropathic pain.
“This is a finding that merits further attention,” she commented.
In correlation analyses, the investigators also found that MRI scores did not correlate significantly with either hemophilia joint health score, physical function, participation in activities, or pressure pain thresholds.
Why the discrepancies?
Dr. Pruthi said in an interview that he has seen evidence from other studies showing that some patients with hemophilia who were on prophylaxis had MRI evidence of joint damage, while others who used on-demand therapy had none.
“That opens up a whole can of worms as to what are we dealing with here. Why do some patients end up with damage and others don’t?” he asked.
He said that the finding that the origin of pain in a large proportion of patients was neuropathic rather than arthritic in origin was new to him.
“It raises a lot of good questions: maybe we need to be managing pain in these patients with nonnarcotic approaches, and in this day and age with the opioid crisis it’s even more important to do that,” he said.
He hypothesized that degenerative arthritis may irritate nearby nerves, resulting in neuropathic pain.
The study was funded by EAHAD, with support from participating institutions. Dr. Roussel and Dr. Pruthi reported no conflicts of interest to declare.
FROM EAHAD 2021
Amputation Care Quality and Satisfaction With Prosthetic Limb Services: A Longitudinal Study of Veterans With Upper Limb Amputation
Veterans with upper limb amputation (ULA) are a small, but important population, who have received more attention in the past decade due to the increased growth of the population of veterans with conflict-related amputation from recent military engagements. Among the 808 veterans with ULA receiving any care in the US Department of Veterans Affairs (VA) from 2010 to 2015 who participated in our national study, an estimated 28 to 35% had a conflict-related amputation.1 The care of these individuals with ULA is highly specialized, and there is a recognized shortage of experienced professionals in this area.2,3 The provision of high-quality prosthetic care is increasingly complex with advances in technology, such as externally powered devices with multiple functions.
The VA is a comprehensive, integrated health care system that serves more than 8.9 million veterans each year. Interdisciplinary amputation care is provided within the VA through a traditional clinic setting or by using one of several currently available virtual care modalities.4,5 In consultation with the veteran, VA health care providers (HCPs) prescribe prostheses and services based on the clinical needs and furnish authorized items and services to eligible veterans. Prescribed items and/or services are furnished either by internal VA resources or through a community-based prosthetist who is an authorized vendor or contractor. Although several studies have reported that the majority of veterans with ULA utilize VA services for at least some aspects of their health care, little is known about: (1) prosthetic limb care satisfaction or the quality of care that veterans receive; or (2) how care within the VA or Department of Defense (DoD) compares with care provided in the civilian sector.6-8
Earlier studies that examined the amputation rehabilitation services received by veterans with ULA pointed to quality gaps in care and differences in satisfaction in the VA and DoD when compared with the civilian sector but were limited in their scope and methodology.7,8 A 2008 study of veterans of the Vietnam War, Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF) compared satisfaction by location of care receipt (DoD only, VA only, private only, and multiple sources). That study dichotomized response categories for items related to satisfaction with care (satisfied/not), but did not estimate degree of satisfaction, calculate summary scores of the items, or utilize validated care satisfaction metrics. That study found that a greater proportion of Vietnam War veterans (compared with OIF/OEF veterans receiving care in the private sector) agreed that they “had a role in choosing prosthesis” and disagreed that they wanted to change their current prosthesis to another type.7 The assumption made by the authors is that much of this private sector care was actually VA-sponsored care prescribed and procured by the VA but delivered in the community. However, no data were collected to confirm or refute this assumption, and it is possible that some care was both VA sponsored and delivered, some was VA sponsored but commercially delivered, and in some cases, care was sponsored by other sources and delivered in still other facilities.
A 2012 VA Office of the Inspector General study of OIF, OEF, and Operation New Dawn (OND) veterans reported lower prosthetic satisfaction for veterans with upper limb when compared with lower limb amputation and described respondents concerns about lack of VA prosthetic expertise, difficulty with accessing VA services, and dissatisfaction with the sometimes lengthy approval process for obtaining fee-basis or VA contract care.8 Although this report suggested that there were quality gaps and areas for improvement, it did not employ validated metrics of prosthesis or care satisfaction and instead relied on qualitative data collected through telephone interviews.
Given the VA effort to enhance the quality and consistency of its amputation care services through the formal establishment of the Amputation System of Care, which began in 2008, further evaluation of care satisfaction and quality of care is warranted. In 2014 the VA and DoD released the first evidence-based clinical practice guidelines (CPGs) for the rehabilitation of persons with ULA.2 The CPG describes care paths to improve outcomes and basic tenets of amputation rehabilitation care and can be used to identify process activities that are essential aspects of quality care. However, the extent to which the CPG has impacted the quality and timeliness of care for veterans with ULA are presently unclear.
Thus, the purposes of this study were to: (1) measure veteran satisfaction with prosthetic limb care and identify factors associated with service satisfaction; (2) assess quality indicators that potentially reflect CPG) adoption; (3) compare care satisfaction and quality for those who received care in or outside of the VA or DoD; and (4) evaluate change in satisfaction over time.
Methods
The study was approved by the VA Central Institutional Review Board (IRB) (Study #16-20) and Human Research Protection Office, U.S. Army Medical Research and Development Command. The sampling frame consisted of veterans with major ULA who received care in the VA between 2010 and 2015 identified in VA Corporate Data Warehouse. We sent recruitment packages to nondeceased veterans who had current addresses and phone numbers. Those who did not opt out or inform us that they did not meet eligibility criteria were contacted by study interviewers. A waiver of documentation of written informed consent was obtained from the VA Central IRB. When reached by the study interviewer, Veterans provided oral informed consent. At baseline, 808 veterans were interviewed for a response rate of 47.7% as calculated by the American Association for Public Opinion Research (AAPOR) methodology.9 Follow-up interviews approximately 1 year later (mean [SD] 367 [16.8] days), were conducted with 585 respondents for a 72.4% response rate (Figure).
Survey Content
Development and pilot testing of the survey instrument previously was reported.1 The content of the survey drew from existing survey items and metrics, and included new items specifically designed to address patterns of amputation care, based on care goals within the CPG. All new and modified items were tested and refined through cognitive interviews with 10 participants, and tested with an additional 13 participants.
The survey collected data on demographics, amputation characteristics (year of amputation, level, laterality, and etiology), current prosthesis use, and type of prosthesis. This article focused on the sections of the survey pertaining to satisfaction with prosthetic care and indicators of quality of care. A description of the content of the full survey and a synopsis of overall findings are reported in a prior publication.1 The key independent, dependent, and other variables utilized in the analyses reported in this manuscript are described below.
Primary Independent Variables
In the follow-up survey, we asked respondents whether they had any amputation care in the prior 12 months, and if so to indicate where they had gone for care. We categorized respondents as having received VA/DoD care if they reported any care at the VA or DoD, and as having received non-VA/DoD care if they did not report care at the VA or DoD but indicated that they had received amputation care between baseline and follow-up.
Two primary outcomes were utilized; the Orthotics and Prosthetics User’s Survey (OPUS), client satisfaction with services scale (CSS), and a measure of care quality specifically developed for this study. The CSS is a measure developed specifically for orthotic and prosthesis users.10 This 11-item scale measures satisfaction with prosthetic limb services and contains items evaluating facets of care such as courtesy received from prosthetists and clinical staff, care coordination, appointment wait time, willingness of the prosthetist to listen to participant concerns, and satisfaction with prosthesis training. Items are rated on a 4-point scale (strongly agree [1] to strongly disagree [4]), thus higher CSS scores indicate worse satisfaction with services. The CSS was administered only to prosthesis users.
The Quality of Care assessment developed for this study contained items pertaining to amputation related care receipt and care quality. These items were generated by the study team in consultation with representatives from the VA/DoD Extremity Amputation Center of Excellence after review of the ULA rehabilitation CPG. Survey questions asked respondents about the clinicians visited for amputation related care in the past 12 months, whether they had an annual amputation-related checkup, whether any clinician had assessed their function, worked with them to identify goals, and/or to develop an amputation-related care plan. Respondents were also asked whether there had been family/caregiver involvement in their care and care coordination, whether a peer visitor was involved in their initial care, whether they had received information about amputation management in the prior year, and whether they had amputation-related pain. Those that indicated that they had amputation-related pain were subsequently asked whether their pain was well managed, whether they used medication for pain management, and whether they used any nonpharmaceutical strategies.
Quality of Care Index
We initially developed 15 indicator items of quality of care. We selected 7 of the items to create a summary index. We omitted 3 items about pain management, since these items were completed only by participants who indicated that they had experienced pain; however, we examined the 3 pain items individually given the importance of this topic. We omitted an additional 2 items from the summary index because they would not be sensitive to change because they pertained to the care in the year after initial amputation. One of these items asked whether caregivers were involved in initial amputation management and the other asked whether a peer visit occurred after amputation. Another 3 items were omitted because they only were completed by small subsamples due to intentional skip patterns in the survey. These items addressed whether clinical HCPs discussed amputation care goals in the prior year, worked to develop a care plan in the prior year, or helped to coordinate care after a move. Completion rates for all items considered for the index are shown in eAppendix 1 (Available at doi:10.12788/fp.0096). After item selection, we generated an index score, which was the number of reported “yes” responses to the seven relevant items.
Other Variables
We created a single variable called level/laterality which categorized ULA as unilateral or bilateral. We further categorized respondents with unilateral amputation by their amputation level. We categorized respondents as transradial for wrist joint or below the elbow amputations; transhumeral for at or above the elbow amputations; and shoulder for shoulder joint or forequarter amputations. Participants indicated the amputation etiology using 7 yes/no variables: combat injury, accident, burn, cancer, diabetes mellitus, and infection. Participants could select ≥ 1 etiology.
Primary prosthesis type was categorized as body powered, myoelectric/hybrid, cosmetic, other/unknown, or nonuser. The service era was classified based on amputation date as Before Vietnam, Vietnam War, After Vietnam to Gulf War, After Gulf War to September 10, 2001, and September 11, 2001 to present. For race, individuals with > 1 race were classified as other. We classified participants by region, using the station identification of the most recent VA medical center that they had visited between January 1, 2010 and December 30, 2015.
The survey also employed 2 measures of satisfaction with the prosthesis, the Trinity Amputation and Prosthetic Experience Scale (TAPES) satisfaction scale and the OPUS Client Satisfaction with Devices (CSD). TAPES consists of 10 items addressing color, shape, noise, appearance, weight, usefulness, reliability, fit, comfort and overall satisfaction.11 Items are rated on a 5-point Likert scale from very dissatisfied (1) to very satisfied (5). An 8-item version of the CSD scale was created for this study, after conducting a Rasch analysis (using Winsteps version 4.4.2) of the original 11-item CSD. The 8 items assess satisfaction with prosthesis fit, weight, comfort, donning ease, appearance, durability, skin contact, and pain. Items are rated on a 4-point scale from strongly agree (1) to strongly disagree (4); higher CSD scores indicate less satisfaction with devices. Psychometric analysis of the revised CSD score was reported in a prior publication.12 We also reported on the CSS using the original 10-item measure.
Data Analyses
We described characteristics of respondents at baseline and follow-up. We used baseline data to calculate CSS scores and described scores for all participants, for subgroups of unilateral and bilateral amputees, and for unilateral amputees stratified by amputation level. Wilcoxon rank sum tests were used to compare the CSS item and total scores of 461 prosthesis users with unilateral amputation and 29 with bilateral amputation. To identify factors that we hypothesized might be associated with CSS scores at baseline, we developed separate bivariate linear regression models. We added those factors that were associated with CSS scores at P ≤ .1 in bivariate analyses to a multivariable linear regression model of factors associated with CSS score. The P ≤ .1 threshold was used to ensure that relevant confounders were controlled for in regression models. We excluded 309 participants with no reported prosthesis use (who were not asked to complete the CSS), 20 participants with other/unknown prosthesis types, and 106 with missing data on amputation care in the prior year or on satisfaction metrics. We used baseline data for this analysis to maximize the sample size.
We compared CSS scores for those who reported receiving care within or outside of the VA or DoD in the prior year, using Wilcoxon Mann-Whitney rank tests. We also compared scores of individual quality of care items for these groups using Fisher exact tests. We chose to examine individual items rather than the full Index because several items specified care receipt within the VA and thus would be inappropriate to utilize in comparisons by site location; however, we described responses to all items. In these analyses, we excluded 2 respondents who had conflicting information regarding location of care. We used follow-up data for this analysis because it allowed us to identify location of care received in the prior year.
We also described the CSS scores, the 7-item Quality of Care Index and responses to other items related to quality of care at baseline and follow-up. To examine whether satisfaction with prosthetic care or aspects of care quality had changed over time, we compared baseline and follow-up CSS and quality of care scores for respondents who had measures at both times using Wilcoxon signed ranks tests. Individual items were compared using McNemar tests.
Results
Respondents were 97.4% male and included 776 unilateral amputees and 32 bilateral amputees with a mean (SD) age of 63.3 (14.1) years (Table 1). Respondents had lost their limbs a mean (SD) 31.4 (14.1) years prior, and half were transradial, 34.2% transhumeral, and 11.6% shoulder amputation. At baseline 185 (22.9%) participants received amputation-related care in the prior year and 118 (20.2%) participants received amputation-related care within 1 year of follow-up. Of respondents, 113 (19.3%) stated that their care was between baseline and follow-up and 89 (78.8%) of these received care at either the VA, the DoD or both; just 16 (14.2%) received care elsewhere.
Mean (SD) CSS scores were highest (lower satisfaction) for those with amputation at the shoulder and lowest for those with transhumeral amputation: 42.2 (20.0) vs 33.4 (20.8). Persons with bilateral amputation were less satisfied in almost every category when compared with those with unilateral amputation, although the total CSS score was not substantially different. Wilcoxon rank sum analyses revealed statistically significant differences in wait time satisfaction: bilateral amputees were less satisfied than unilateral amputees. Factors associated with overall CSS score in bivariate analyses were CSD score, TAPES score, amputation care receipt, prosthesis type, race, and region of care (eAppendix 2, available at doi:10.12788/fp.0096).
In the multivariate regression model of baseline CSS scores, only 2 variables were independently associated with CSS scores: CSD score and recent amputation care (Table 3). For each 1-point increase in CSD score there was a 0.7 point increase in CSS score. Those with amputation care in the prior year had higher satisfaction when compared with those who had not received care (P = .003).
For participants who indicated that they received amputation care between baseline and follow-up, CSS mean (SD) scores were better, but not statistically different, for those who reported care in the VA or DoD vs private care, 31.6 (22.6) vs 38.0 (17.7) (Table 4). When compared with community-based care, more participants who received care in the VA or DoD in the prior year had a functional assessment in that time period (33.7% vs 7.1%, P = .06), were contacted by HCPs outside of appointments (42.7% vs 18.8%, P = .07), and received information about amputation care in the prior year (41.6% vs 0%, P =.002). There was no difference in the proportion whose family/caregivers were involved in care in the prior year.
No statistically significant differences were observed in paired comparisons of the CSS and Quality of Care Index at baseline or follow-up for all participants with data at both time points (Table 5; eAppendix 3 available at doi:10.12788/fp.0096). Individual pain measures did not differ significantly between timepoints. Quality Index mean (SD) scores were 1.3 (1.5) and 1.2 (1.5) at baseline and follow-up, respectively (P = .07). For the 214 prosthesis users with longitudinal data, baseline CSS mean (SD) scores were generally worse at baseline than at follow-up: 34.4 (19.8) vs 32.5 (21.0) (P = .23). Family/caregiver involvement in amputation care was significantly higher in the year before baseline when compared with the year prior to follow-up (24.4% vs 17.7%, P = .001). There were no other statistically significant differences in Quality of Care items between baseline and follow-up.
Discussion
Our longitudinal study provides insights into the experiences of veterans with major ULA related to satisfaction with prosthetic limb care services and receipt of amputation-related care. We reported on the development and use of a new summary measure of amputation care quality, which we designed to capture some of the key elements of care quality as provided in the VA/DoD CPG.2
We used baseline data to identify factors independently associated with prosthetic limb care satisfaction as measured by a previously validated measure, the OPUS CSS. The CSS addresses satisfaction with prosthetic limb services and does not reflect satisfaction with other amputation care services. We found that persons who received amputation care in the prior year had CSS scores that were a mean 5.1 points better than those who had not received recent care. Although causality cannot be determined with this investigation, this finding highlights an important relationship between frequency of care and satisfaction, which can be leveraged by the VA in future care initiatives. Care satisfaction was also better by 0.7 points for every 1-point decrease (indicating higher satisfaction) in the OPUS CSD prosthetic satisfaction scale. This finding isn’t surprising, given that a major purpose of prosthetic limb care services is to procure and fit a satisfactory device. To determine whether these same relationships were observed in the smaller, longitudinal cohort data at follow-up, we repeated these models and found similar relationships between recent care receipt and prosthesis satisfaction and satisfaction with services. We believe that these findings are meaningful and emphasize the importance of both service and device satisfaction to the veteran with an ULA. Lower service satisfaction scores among those with amputations at the shoulder and those with bilateral limb loss suggest that these individuals may benefit from different service delivery approaches.
We did observe a difference in satisfaction scores by geographic region in the follow-up (but not the baseline) data with satisfaction higher in the Western vs the Southern region (data not shown). This finding suggests a need for continued monitoring of care satisfaction over time to determine whether differences by region persist. We grouped respondents into geographic region based on the location where they had received their most recent VA care of any type. Many veterans receive care at multiple VA locations. Thus, it is possible that some veterans received their amputation care at a non-VA facility or a VA facility in a different region.
Our findings related to prosthetic limb care services satisfaction are generalizable to veteran prosthesis users. Findings may not be generalizable to nonusers, because in our study, the CSS only was administered to prosthesis users. Thus, we were unable to identify factors associated with care satisfaction for persons who were not current users of an upper limb prosthesis.
The study findings confirmed that most veterans with ULA receive amputation-related care in the VA or DoD. We compared CSS and Quality of Care item scores for those who reported receiving care at the VA or DoD vs elsewhere. Amputation care within the VA is complex. Some services are provided at VA facilities and some are ordered by VA clinicians but provided by community-based HCPs. However, we found that better (though not statistically significantly different) CSS scores and several Quality of Care items were endorsed by a significantly more of those reporting care in the VA or DoD as compared to elsewhere. Given the dissemination of a rehabilitation of upper limb amputees CPG, we hypothesized that VA and DoD HCPs would be more aware of care guidelines and would provide better care. Overall, our findings supported this hypothesis while also suggesting that areas such as caregiver involvement and peer visitation may benefit from additional attention and program improvement.
We used longitudinal data to describe and compare CSS and Quality of Care Index scores. Our analyses did not detect any statistically significant differences between baseline and follow-up. This finding may reflect that this was a relatively stable population with regard to amputation experiences given the mean time since amputation was 31.4 years. However, we also recognize that our measures may not have captured all aspects of care satisfaction or quality. It is possible that there were other changes that had occurred over the course of the year that were not captured by the CSS or by the Quality of Care Index. It is also possible that some implementation and adoption of the CPG had happened prior to our baseline survey. Finally, it is possible that some elements of the CPG have not yet been fully integrated into clinical care. We believe that the latter is likely, given that nearly 80% of respondents did not report receiving any amputation care within the past year at follow-up, though the CPGs recommend an annual visit.
Aside from recall bias, 2 explanations must be considered relative to the low rate of adherence to the CPG recommendation for an annual follow-up. The first is that the CPG simply may not be widely adopted. The second is that the majority of patients with ULA who use prostheses use a body-powered system. These tend to be low maintenance, long-lasting systems and may ultimately not need annual maintenance and repair. Further, if the veteran’s body-powered system is functioning properly and health status has not changed, they may simply be opting out of an annual visit despite the CPG recommendation. Nonetheless, this apparent low rate of annual follow-up emphasizes the need for additional process improvement measures for the VA.
Strengths and Limitations
The VA provides a unique setting for a nationally representative study of amputation rehabilitation because it has centralized data sources that can be used to identify veterans with ULA. Our study had a strong response rate, and its prosthetic limb care satisfaction findings are generalizable to all veterans with major ULA who received VA care from 2010 to 2015. However, there are limits to generalizability outside of this population to civilians or to veterans who do not receive VA care. To examine possible nonresponse bias, which could limit generalizability, we compared the baseline characteristics of respondents and nonrespondents to the follow-up study (eAppendix 4 available at doi:10.12788/fp.0096). There were no significant differences in satisfaction, quality of care, or receipt of amputation-related care between those lost to follow-up and those with follow-up data. Although, we did find small differences in gender, race, and service era (defined by amputation date). We do not believe that these differences threaten the interpretation of findings at follow-up, but there may be limits to generalizability of these findings to the full baseline sample. The data were from a telephone survey of veterans. It is possible that some veterans did not recall their care receipt or did not understand some of the questions and thus may not have accurately answered questions related to type of care received or the timing of that care.
Our interpretation of findings comparing care received within the VA and DoD or elsewhere is also limited because we cannot say with certainty whether those who indicated no care in the VA or DoD actually had care that was sponsored by the VA or DoD as contract or fee-basis care. Just 8 respondents indicated that they had received care only outside of the VA or DoD in the prior year. There were also some limitations in the collection of data about care location. We asked about receipt of amputation care in the prior year and about location of any amputation care received between baseline and follow-up, and there were differences in responses. Thus, we used a combination of these items to identify location of care received in the prior year.
Despite these limitations, we believe that our study provides novel, important findings about the satisfaction with prosthetic limb care services and quality of amputation rehabilitation care for veterans. Findings from this study can be used to address amputation and prosthetic limb care satisfaction and quality weaknesses highlighted and to benchmark care satisfaction and CPG compliance. Other studies evaluating care guideline compliance have used indicators obtained from clinical records or data repositories.13-15 Future work could combine self-reported satisfaction and care quality measures with indicators obtained from clinical or repository sources to provide a more complete snapshot of care delivery.
Conclusions
We reported on a national survey of veterans with major upper limb loss that assessed satisfaction with prosthetic limb care services and quality of amputation care. Satisfaction with prosthetic limb care was independently associated with satisfaction with the prosthesis, and receipt of care within the prior year. Most of the veterans surveyed received care within the VA or DoD and reported receiving higher quality of care, when compared with those who received care outside of the VA or DoD. Satisfaction with care and quality of care were stable over the year of this study. Data presented in this study can serve to direct VA amputation care process improvement initiatives as benchmarks for future work. Future studies are needed to track satisfaction with and quality of care for veterans with ULA.
1. Resnik L, Ekerholm S, Borgia M, Clark MA. A national study of veterans with major upper limb amputation: Survey methods, participants, and summary findings. PLoS One. 2019;14(3):e0213578. Published 2019 Mar 14. doi:10.1371/journal.pone.0213578
2. US Department of Defense, US Department of Veterans Affairs, Management of Upper Extremity Amputation Rehabilitation Working Group. VA/DoD clinical practice guideline for the management of upper extremity amputation rehabilitation.Published 2014. Accessed February 18, 2021. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf
3. Jette AM. The Promise of Assistive Technology to Enhance Work Participation. Phys Ther. 2017;97(7):691-692. doi:10.1093/ptj/pzx054
4. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs amputations system of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
5. Scholten J, Poorman C, Culver L, Webster JB. Department of Veterans Affairs polytrauma telerehabilitation: twenty-first century care. Phys Med Rehabil Clin N Am. 2019;30(1):207-215. doi:10.1016/j.pmr.2018.08.003
6. Melcer T, Walker J, Bhatnagar V, Richard E. Clinic use at the Departments of Defense and Veterans Affairs following combat related amputations. Mil Med. 2020;185(1-2):e244-e253. doi:10.1093/milmed/usz149
7. Berke GM, Fergason J, Milani JR, et al. Comparison of satisfaction with current prosthetic care in veterans and servicemembers from Vietnam and OIF/OEF conflicts with major traumatic limb loss. J Rehabil Res Dev. 2010;47(4):361-371. doi:10.1682/jrrd.2009.12.0193
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection prosthetic limb care in VA facilities. Published March 8, 2012. Accessed February 18, 2021. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf 9. American Association for Public Opinion Research. Response rates - an overview. Accessed February 18, 2021. https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx
10. Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users’ Survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
11. Desmond DM, MacLachlan M. Factor structure of the Trinity Amputation and Prosthesis Experience Scales (TAPES) with individuals with acquired upper limb amputations. Am J Phys Med Rehabil. 2005;84(7):506-513. doi:10.1097/01.phm.0000166885.16180.63
12. Resnik L, Borgia M, Heinemann AW, Clark MA. Prosthesis satisfaction in a national sample of veterans with upper limb amputation. Prosthet Orthot Int. 2020;44(2):81-91. doi:10.1177/0309364619895201
13. Ho TH, Caughey GE, Shakib S. Guideline compliance in chronic heart failure patients with multiple comorbid diseases: evaluation of an individualised multidisciplinary model of care. PLoS One. 2014;9(4):e93129. Published 2014 Apr 8. doi:10.1371/journal.pone.0093129
14. Mitchell KB, Lin H, Shen Y, et al. DCIS and axillary nodal evaluation: compliance with national guidelines. BMC Surg. 2017;17(1):12. Published 2017 Feb 7. doi:10.1186/s12893-017-0210-5
15. Moesker MJ, de Groot JF, Damen NL, et al. Guideline compliance for bridging anticoagulation use in vitamin-K antagonist patients; practice variation and factors associated with non-compliance. Thromb J. 2019;17:15. Published 2019 Aug 5. doi:10.1186/s12959-019-0204-x
Veterans with upper limb amputation (ULA) are a small, but important population, who have received more attention in the past decade due to the increased growth of the population of veterans with conflict-related amputation from recent military engagements. Among the 808 veterans with ULA receiving any care in the US Department of Veterans Affairs (VA) from 2010 to 2015 who participated in our national study, an estimated 28 to 35% had a conflict-related amputation.1 The care of these individuals with ULA is highly specialized, and there is a recognized shortage of experienced professionals in this area.2,3 The provision of high-quality prosthetic care is increasingly complex with advances in technology, such as externally powered devices with multiple functions.
The VA is a comprehensive, integrated health care system that serves more than 8.9 million veterans each year. Interdisciplinary amputation care is provided within the VA through a traditional clinic setting or by using one of several currently available virtual care modalities.4,5 In consultation with the veteran, VA health care providers (HCPs) prescribe prostheses and services based on the clinical needs and furnish authorized items and services to eligible veterans. Prescribed items and/or services are furnished either by internal VA resources or through a community-based prosthetist who is an authorized vendor or contractor. Although several studies have reported that the majority of veterans with ULA utilize VA services for at least some aspects of their health care, little is known about: (1) prosthetic limb care satisfaction or the quality of care that veterans receive; or (2) how care within the VA or Department of Defense (DoD) compares with care provided in the civilian sector.6-8
Earlier studies that examined the amputation rehabilitation services received by veterans with ULA pointed to quality gaps in care and differences in satisfaction in the VA and DoD when compared with the civilian sector but were limited in their scope and methodology.7,8 A 2008 study of veterans of the Vietnam War, Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF) compared satisfaction by location of care receipt (DoD only, VA only, private only, and multiple sources). That study dichotomized response categories for items related to satisfaction with care (satisfied/not), but did not estimate degree of satisfaction, calculate summary scores of the items, or utilize validated care satisfaction metrics. That study found that a greater proportion of Vietnam War veterans (compared with OIF/OEF veterans receiving care in the private sector) agreed that they “had a role in choosing prosthesis” and disagreed that they wanted to change their current prosthesis to another type.7 The assumption made by the authors is that much of this private sector care was actually VA-sponsored care prescribed and procured by the VA but delivered in the community. However, no data were collected to confirm or refute this assumption, and it is possible that some care was both VA sponsored and delivered, some was VA sponsored but commercially delivered, and in some cases, care was sponsored by other sources and delivered in still other facilities.
A 2012 VA Office of the Inspector General study of OIF, OEF, and Operation New Dawn (OND) veterans reported lower prosthetic satisfaction for veterans with upper limb when compared with lower limb amputation and described respondents concerns about lack of VA prosthetic expertise, difficulty with accessing VA services, and dissatisfaction with the sometimes lengthy approval process for obtaining fee-basis or VA contract care.8 Although this report suggested that there were quality gaps and areas for improvement, it did not employ validated metrics of prosthesis or care satisfaction and instead relied on qualitative data collected through telephone interviews.
Given the VA effort to enhance the quality and consistency of its amputation care services through the formal establishment of the Amputation System of Care, which began in 2008, further evaluation of care satisfaction and quality of care is warranted. In 2014 the VA and DoD released the first evidence-based clinical practice guidelines (CPGs) for the rehabilitation of persons with ULA.2 The CPG describes care paths to improve outcomes and basic tenets of amputation rehabilitation care and can be used to identify process activities that are essential aspects of quality care. However, the extent to which the CPG has impacted the quality and timeliness of care for veterans with ULA are presently unclear.
Thus, the purposes of this study were to: (1) measure veteran satisfaction with prosthetic limb care and identify factors associated with service satisfaction; (2) assess quality indicators that potentially reflect CPG) adoption; (3) compare care satisfaction and quality for those who received care in or outside of the VA or DoD; and (4) evaluate change in satisfaction over time.
Methods
The study was approved by the VA Central Institutional Review Board (IRB) (Study #16-20) and Human Research Protection Office, U.S. Army Medical Research and Development Command. The sampling frame consisted of veterans with major ULA who received care in the VA between 2010 and 2015 identified in VA Corporate Data Warehouse. We sent recruitment packages to nondeceased veterans who had current addresses and phone numbers. Those who did not opt out or inform us that they did not meet eligibility criteria were contacted by study interviewers. A waiver of documentation of written informed consent was obtained from the VA Central IRB. When reached by the study interviewer, Veterans provided oral informed consent. At baseline, 808 veterans were interviewed for a response rate of 47.7% as calculated by the American Association for Public Opinion Research (AAPOR) methodology.9 Follow-up interviews approximately 1 year later (mean [SD] 367 [16.8] days), were conducted with 585 respondents for a 72.4% response rate (Figure).
Survey Content
Development and pilot testing of the survey instrument previously was reported.1 The content of the survey drew from existing survey items and metrics, and included new items specifically designed to address patterns of amputation care, based on care goals within the CPG. All new and modified items were tested and refined through cognitive interviews with 10 participants, and tested with an additional 13 participants.
The survey collected data on demographics, amputation characteristics (year of amputation, level, laterality, and etiology), current prosthesis use, and type of prosthesis. This article focused on the sections of the survey pertaining to satisfaction with prosthetic care and indicators of quality of care. A description of the content of the full survey and a synopsis of overall findings are reported in a prior publication.1 The key independent, dependent, and other variables utilized in the analyses reported in this manuscript are described below.
Primary Independent Variables
In the follow-up survey, we asked respondents whether they had any amputation care in the prior 12 months, and if so to indicate where they had gone for care. We categorized respondents as having received VA/DoD care if they reported any care at the VA or DoD, and as having received non-VA/DoD care if they did not report care at the VA or DoD but indicated that they had received amputation care between baseline and follow-up.
Two primary outcomes were utilized; the Orthotics and Prosthetics User’s Survey (OPUS), client satisfaction with services scale (CSS), and a measure of care quality specifically developed for this study. The CSS is a measure developed specifically for orthotic and prosthesis users.10 This 11-item scale measures satisfaction with prosthetic limb services and contains items evaluating facets of care such as courtesy received from prosthetists and clinical staff, care coordination, appointment wait time, willingness of the prosthetist to listen to participant concerns, and satisfaction with prosthesis training. Items are rated on a 4-point scale (strongly agree [1] to strongly disagree [4]), thus higher CSS scores indicate worse satisfaction with services. The CSS was administered only to prosthesis users.
The Quality of Care assessment developed for this study contained items pertaining to amputation related care receipt and care quality. These items were generated by the study team in consultation with representatives from the VA/DoD Extremity Amputation Center of Excellence after review of the ULA rehabilitation CPG. Survey questions asked respondents about the clinicians visited for amputation related care in the past 12 months, whether they had an annual amputation-related checkup, whether any clinician had assessed their function, worked with them to identify goals, and/or to develop an amputation-related care plan. Respondents were also asked whether there had been family/caregiver involvement in their care and care coordination, whether a peer visitor was involved in their initial care, whether they had received information about amputation management in the prior year, and whether they had amputation-related pain. Those that indicated that they had amputation-related pain were subsequently asked whether their pain was well managed, whether they used medication for pain management, and whether they used any nonpharmaceutical strategies.
Quality of Care Index
We initially developed 15 indicator items of quality of care. We selected 7 of the items to create a summary index. We omitted 3 items about pain management, since these items were completed only by participants who indicated that they had experienced pain; however, we examined the 3 pain items individually given the importance of this topic. We omitted an additional 2 items from the summary index because they would not be sensitive to change because they pertained to the care in the year after initial amputation. One of these items asked whether caregivers were involved in initial amputation management and the other asked whether a peer visit occurred after amputation. Another 3 items were omitted because they only were completed by small subsamples due to intentional skip patterns in the survey. These items addressed whether clinical HCPs discussed amputation care goals in the prior year, worked to develop a care plan in the prior year, or helped to coordinate care after a move. Completion rates for all items considered for the index are shown in eAppendix 1 (Available at doi:10.12788/fp.0096). After item selection, we generated an index score, which was the number of reported “yes” responses to the seven relevant items.
Other Variables
We created a single variable called level/laterality which categorized ULA as unilateral or bilateral. We further categorized respondents with unilateral amputation by their amputation level. We categorized respondents as transradial for wrist joint or below the elbow amputations; transhumeral for at or above the elbow amputations; and shoulder for shoulder joint or forequarter amputations. Participants indicated the amputation etiology using 7 yes/no variables: combat injury, accident, burn, cancer, diabetes mellitus, and infection. Participants could select ≥ 1 etiology.
Primary prosthesis type was categorized as body powered, myoelectric/hybrid, cosmetic, other/unknown, or nonuser. The service era was classified based on amputation date as Before Vietnam, Vietnam War, After Vietnam to Gulf War, After Gulf War to September 10, 2001, and September 11, 2001 to present. For race, individuals with > 1 race were classified as other. We classified participants by region, using the station identification of the most recent VA medical center that they had visited between January 1, 2010 and December 30, 2015.
The survey also employed 2 measures of satisfaction with the prosthesis, the Trinity Amputation and Prosthetic Experience Scale (TAPES) satisfaction scale and the OPUS Client Satisfaction with Devices (CSD). TAPES consists of 10 items addressing color, shape, noise, appearance, weight, usefulness, reliability, fit, comfort and overall satisfaction.11 Items are rated on a 5-point Likert scale from very dissatisfied (1) to very satisfied (5). An 8-item version of the CSD scale was created for this study, after conducting a Rasch analysis (using Winsteps version 4.4.2) of the original 11-item CSD. The 8 items assess satisfaction with prosthesis fit, weight, comfort, donning ease, appearance, durability, skin contact, and pain. Items are rated on a 4-point scale from strongly agree (1) to strongly disagree (4); higher CSD scores indicate less satisfaction with devices. Psychometric analysis of the revised CSD score was reported in a prior publication.12 We also reported on the CSS using the original 10-item measure.
Data Analyses
We described characteristics of respondents at baseline and follow-up. We used baseline data to calculate CSS scores and described scores for all participants, for subgroups of unilateral and bilateral amputees, and for unilateral amputees stratified by amputation level. Wilcoxon rank sum tests were used to compare the CSS item and total scores of 461 prosthesis users with unilateral amputation and 29 with bilateral amputation. To identify factors that we hypothesized might be associated with CSS scores at baseline, we developed separate bivariate linear regression models. We added those factors that were associated with CSS scores at P ≤ .1 in bivariate analyses to a multivariable linear regression model of factors associated with CSS score. The P ≤ .1 threshold was used to ensure that relevant confounders were controlled for in regression models. We excluded 309 participants with no reported prosthesis use (who were not asked to complete the CSS), 20 participants with other/unknown prosthesis types, and 106 with missing data on amputation care in the prior year or on satisfaction metrics. We used baseline data for this analysis to maximize the sample size.
We compared CSS scores for those who reported receiving care within or outside of the VA or DoD in the prior year, using Wilcoxon Mann-Whitney rank tests. We also compared scores of individual quality of care items for these groups using Fisher exact tests. We chose to examine individual items rather than the full Index because several items specified care receipt within the VA and thus would be inappropriate to utilize in comparisons by site location; however, we described responses to all items. In these analyses, we excluded 2 respondents who had conflicting information regarding location of care. We used follow-up data for this analysis because it allowed us to identify location of care received in the prior year.
We also described the CSS scores, the 7-item Quality of Care Index and responses to other items related to quality of care at baseline and follow-up. To examine whether satisfaction with prosthetic care or aspects of care quality had changed over time, we compared baseline and follow-up CSS and quality of care scores for respondents who had measures at both times using Wilcoxon signed ranks tests. Individual items were compared using McNemar tests.
Results
Respondents were 97.4% male and included 776 unilateral amputees and 32 bilateral amputees with a mean (SD) age of 63.3 (14.1) years (Table 1). Respondents had lost their limbs a mean (SD) 31.4 (14.1) years prior, and half were transradial, 34.2% transhumeral, and 11.6% shoulder amputation. At baseline 185 (22.9%) participants received amputation-related care in the prior year and 118 (20.2%) participants received amputation-related care within 1 year of follow-up. Of respondents, 113 (19.3%) stated that their care was between baseline and follow-up and 89 (78.8%) of these received care at either the VA, the DoD or both; just 16 (14.2%) received care elsewhere.
Mean (SD) CSS scores were highest (lower satisfaction) for those with amputation at the shoulder and lowest for those with transhumeral amputation: 42.2 (20.0) vs 33.4 (20.8). Persons with bilateral amputation were less satisfied in almost every category when compared with those with unilateral amputation, although the total CSS score was not substantially different. Wilcoxon rank sum analyses revealed statistically significant differences in wait time satisfaction: bilateral amputees were less satisfied than unilateral amputees. Factors associated with overall CSS score in bivariate analyses were CSD score, TAPES score, amputation care receipt, prosthesis type, race, and region of care (eAppendix 2, available at doi:10.12788/fp.0096).
In the multivariate regression model of baseline CSS scores, only 2 variables were independently associated with CSS scores: CSD score and recent amputation care (Table 3). For each 1-point increase in CSD score there was a 0.7 point increase in CSS score. Those with amputation care in the prior year had higher satisfaction when compared with those who had not received care (P = .003).
For participants who indicated that they received amputation care between baseline and follow-up, CSS mean (SD) scores were better, but not statistically different, for those who reported care in the VA or DoD vs private care, 31.6 (22.6) vs 38.0 (17.7) (Table 4). When compared with community-based care, more participants who received care in the VA or DoD in the prior year had a functional assessment in that time period (33.7% vs 7.1%, P = .06), were contacted by HCPs outside of appointments (42.7% vs 18.8%, P = .07), and received information about amputation care in the prior year (41.6% vs 0%, P =.002). There was no difference in the proportion whose family/caregivers were involved in care in the prior year.
No statistically significant differences were observed in paired comparisons of the CSS and Quality of Care Index at baseline or follow-up for all participants with data at both time points (Table 5; eAppendix 3 available at doi:10.12788/fp.0096). Individual pain measures did not differ significantly between timepoints. Quality Index mean (SD) scores were 1.3 (1.5) and 1.2 (1.5) at baseline and follow-up, respectively (P = .07). For the 214 prosthesis users with longitudinal data, baseline CSS mean (SD) scores were generally worse at baseline than at follow-up: 34.4 (19.8) vs 32.5 (21.0) (P = .23). Family/caregiver involvement in amputation care was significantly higher in the year before baseline when compared with the year prior to follow-up (24.4% vs 17.7%, P = .001). There were no other statistically significant differences in Quality of Care items between baseline and follow-up.
Discussion
Our longitudinal study provides insights into the experiences of veterans with major ULA related to satisfaction with prosthetic limb care services and receipt of amputation-related care. We reported on the development and use of a new summary measure of amputation care quality, which we designed to capture some of the key elements of care quality as provided in the VA/DoD CPG.2
We used baseline data to identify factors independently associated with prosthetic limb care satisfaction as measured by a previously validated measure, the OPUS CSS. The CSS addresses satisfaction with prosthetic limb services and does not reflect satisfaction with other amputation care services. We found that persons who received amputation care in the prior year had CSS scores that were a mean 5.1 points better than those who had not received recent care. Although causality cannot be determined with this investigation, this finding highlights an important relationship between frequency of care and satisfaction, which can be leveraged by the VA in future care initiatives. Care satisfaction was also better by 0.7 points for every 1-point decrease (indicating higher satisfaction) in the OPUS CSD prosthetic satisfaction scale. This finding isn’t surprising, given that a major purpose of prosthetic limb care services is to procure and fit a satisfactory device. To determine whether these same relationships were observed in the smaller, longitudinal cohort data at follow-up, we repeated these models and found similar relationships between recent care receipt and prosthesis satisfaction and satisfaction with services. We believe that these findings are meaningful and emphasize the importance of both service and device satisfaction to the veteran with an ULA. Lower service satisfaction scores among those with amputations at the shoulder and those with bilateral limb loss suggest that these individuals may benefit from different service delivery approaches.
We did observe a difference in satisfaction scores by geographic region in the follow-up (but not the baseline) data with satisfaction higher in the Western vs the Southern region (data not shown). This finding suggests a need for continued monitoring of care satisfaction over time to determine whether differences by region persist. We grouped respondents into geographic region based on the location where they had received their most recent VA care of any type. Many veterans receive care at multiple VA locations. Thus, it is possible that some veterans received their amputation care at a non-VA facility or a VA facility in a different region.
Our findings related to prosthetic limb care services satisfaction are generalizable to veteran prosthesis users. Findings may not be generalizable to nonusers, because in our study, the CSS only was administered to prosthesis users. Thus, we were unable to identify factors associated with care satisfaction for persons who were not current users of an upper limb prosthesis.
The study findings confirmed that most veterans with ULA receive amputation-related care in the VA or DoD. We compared CSS and Quality of Care item scores for those who reported receiving care at the VA or DoD vs elsewhere. Amputation care within the VA is complex. Some services are provided at VA facilities and some are ordered by VA clinicians but provided by community-based HCPs. However, we found that better (though not statistically significantly different) CSS scores and several Quality of Care items were endorsed by a significantly more of those reporting care in the VA or DoD as compared to elsewhere. Given the dissemination of a rehabilitation of upper limb amputees CPG, we hypothesized that VA and DoD HCPs would be more aware of care guidelines and would provide better care. Overall, our findings supported this hypothesis while also suggesting that areas such as caregiver involvement and peer visitation may benefit from additional attention and program improvement.
We used longitudinal data to describe and compare CSS and Quality of Care Index scores. Our analyses did not detect any statistically significant differences between baseline and follow-up. This finding may reflect that this was a relatively stable population with regard to amputation experiences given the mean time since amputation was 31.4 years. However, we also recognize that our measures may not have captured all aspects of care satisfaction or quality. It is possible that there were other changes that had occurred over the course of the year that were not captured by the CSS or by the Quality of Care Index. It is also possible that some implementation and adoption of the CPG had happened prior to our baseline survey. Finally, it is possible that some elements of the CPG have not yet been fully integrated into clinical care. We believe that the latter is likely, given that nearly 80% of respondents did not report receiving any amputation care within the past year at follow-up, though the CPGs recommend an annual visit.
Aside from recall bias, 2 explanations must be considered relative to the low rate of adherence to the CPG recommendation for an annual follow-up. The first is that the CPG simply may not be widely adopted. The second is that the majority of patients with ULA who use prostheses use a body-powered system. These tend to be low maintenance, long-lasting systems and may ultimately not need annual maintenance and repair. Further, if the veteran’s body-powered system is functioning properly and health status has not changed, they may simply be opting out of an annual visit despite the CPG recommendation. Nonetheless, this apparent low rate of annual follow-up emphasizes the need for additional process improvement measures for the VA.
Strengths and Limitations
The VA provides a unique setting for a nationally representative study of amputation rehabilitation because it has centralized data sources that can be used to identify veterans with ULA. Our study had a strong response rate, and its prosthetic limb care satisfaction findings are generalizable to all veterans with major ULA who received VA care from 2010 to 2015. However, there are limits to generalizability outside of this population to civilians or to veterans who do not receive VA care. To examine possible nonresponse bias, which could limit generalizability, we compared the baseline characteristics of respondents and nonrespondents to the follow-up study (eAppendix 4 available at doi:10.12788/fp.0096). There were no significant differences in satisfaction, quality of care, or receipt of amputation-related care between those lost to follow-up and those with follow-up data. Although, we did find small differences in gender, race, and service era (defined by amputation date). We do not believe that these differences threaten the interpretation of findings at follow-up, but there may be limits to generalizability of these findings to the full baseline sample. The data were from a telephone survey of veterans. It is possible that some veterans did not recall their care receipt or did not understand some of the questions and thus may not have accurately answered questions related to type of care received or the timing of that care.
Our interpretation of findings comparing care received within the VA and DoD or elsewhere is also limited because we cannot say with certainty whether those who indicated no care in the VA or DoD actually had care that was sponsored by the VA or DoD as contract or fee-basis care. Just 8 respondents indicated that they had received care only outside of the VA or DoD in the prior year. There were also some limitations in the collection of data about care location. We asked about receipt of amputation care in the prior year and about location of any amputation care received between baseline and follow-up, and there were differences in responses. Thus, we used a combination of these items to identify location of care received in the prior year.
Despite these limitations, we believe that our study provides novel, important findings about the satisfaction with prosthetic limb care services and quality of amputation rehabilitation care for veterans. Findings from this study can be used to address amputation and prosthetic limb care satisfaction and quality weaknesses highlighted and to benchmark care satisfaction and CPG compliance. Other studies evaluating care guideline compliance have used indicators obtained from clinical records or data repositories.13-15 Future work could combine self-reported satisfaction and care quality measures with indicators obtained from clinical or repository sources to provide a more complete snapshot of care delivery.
Conclusions
We reported on a national survey of veterans with major upper limb loss that assessed satisfaction with prosthetic limb care services and quality of amputation care. Satisfaction with prosthetic limb care was independently associated with satisfaction with the prosthesis, and receipt of care within the prior year. Most of the veterans surveyed received care within the VA or DoD and reported receiving higher quality of care, when compared with those who received care outside of the VA or DoD. Satisfaction with care and quality of care were stable over the year of this study. Data presented in this study can serve to direct VA amputation care process improvement initiatives as benchmarks for future work. Future studies are needed to track satisfaction with and quality of care for veterans with ULA.
Veterans with upper limb amputation (ULA) are a small, but important population, who have received more attention in the past decade due to the increased growth of the population of veterans with conflict-related amputation from recent military engagements. Among the 808 veterans with ULA receiving any care in the US Department of Veterans Affairs (VA) from 2010 to 2015 who participated in our national study, an estimated 28 to 35% had a conflict-related amputation.1 The care of these individuals with ULA is highly specialized, and there is a recognized shortage of experienced professionals in this area.2,3 The provision of high-quality prosthetic care is increasingly complex with advances in technology, such as externally powered devices with multiple functions.
The VA is a comprehensive, integrated health care system that serves more than 8.9 million veterans each year. Interdisciplinary amputation care is provided within the VA through a traditional clinic setting or by using one of several currently available virtual care modalities.4,5 In consultation with the veteran, VA health care providers (HCPs) prescribe prostheses and services based on the clinical needs and furnish authorized items and services to eligible veterans. Prescribed items and/or services are furnished either by internal VA resources or through a community-based prosthetist who is an authorized vendor or contractor. Although several studies have reported that the majority of veterans with ULA utilize VA services for at least some aspects of their health care, little is known about: (1) prosthetic limb care satisfaction or the quality of care that veterans receive; or (2) how care within the VA or Department of Defense (DoD) compares with care provided in the civilian sector.6-8
Earlier studies that examined the amputation rehabilitation services received by veterans with ULA pointed to quality gaps in care and differences in satisfaction in the VA and DoD when compared with the civilian sector but were limited in their scope and methodology.7,8 A 2008 study of veterans of the Vietnam War, Operation Iraqi Freedom (OIF), and Operation Enduring Freedom (OEF) compared satisfaction by location of care receipt (DoD only, VA only, private only, and multiple sources). That study dichotomized response categories for items related to satisfaction with care (satisfied/not), but did not estimate degree of satisfaction, calculate summary scores of the items, or utilize validated care satisfaction metrics. That study found that a greater proportion of Vietnam War veterans (compared with OIF/OEF veterans receiving care in the private sector) agreed that they “had a role in choosing prosthesis” and disagreed that they wanted to change their current prosthesis to another type.7 The assumption made by the authors is that much of this private sector care was actually VA-sponsored care prescribed and procured by the VA but delivered in the community. However, no data were collected to confirm or refute this assumption, and it is possible that some care was both VA sponsored and delivered, some was VA sponsored but commercially delivered, and in some cases, care was sponsored by other sources and delivered in still other facilities.
A 2012 VA Office of the Inspector General study of OIF, OEF, and Operation New Dawn (OND) veterans reported lower prosthetic satisfaction for veterans with upper limb when compared with lower limb amputation and described respondents concerns about lack of VA prosthetic expertise, difficulty with accessing VA services, and dissatisfaction with the sometimes lengthy approval process for obtaining fee-basis or VA contract care.8 Although this report suggested that there were quality gaps and areas for improvement, it did not employ validated metrics of prosthesis or care satisfaction and instead relied on qualitative data collected through telephone interviews.
Given the VA effort to enhance the quality and consistency of its amputation care services through the formal establishment of the Amputation System of Care, which began in 2008, further evaluation of care satisfaction and quality of care is warranted. In 2014 the VA and DoD released the first evidence-based clinical practice guidelines (CPGs) for the rehabilitation of persons with ULA.2 The CPG describes care paths to improve outcomes and basic tenets of amputation rehabilitation care and can be used to identify process activities that are essential aspects of quality care. However, the extent to which the CPG has impacted the quality and timeliness of care for veterans with ULA are presently unclear.
Thus, the purposes of this study were to: (1) measure veteran satisfaction with prosthetic limb care and identify factors associated with service satisfaction; (2) assess quality indicators that potentially reflect CPG) adoption; (3) compare care satisfaction and quality for those who received care in or outside of the VA or DoD; and (4) evaluate change in satisfaction over time.
Methods
The study was approved by the VA Central Institutional Review Board (IRB) (Study #16-20) and Human Research Protection Office, U.S. Army Medical Research and Development Command. The sampling frame consisted of veterans with major ULA who received care in the VA between 2010 and 2015 identified in VA Corporate Data Warehouse. We sent recruitment packages to nondeceased veterans who had current addresses and phone numbers. Those who did not opt out or inform us that they did not meet eligibility criteria were contacted by study interviewers. A waiver of documentation of written informed consent was obtained from the VA Central IRB. When reached by the study interviewer, Veterans provided oral informed consent. At baseline, 808 veterans were interviewed for a response rate of 47.7% as calculated by the American Association for Public Opinion Research (AAPOR) methodology.9 Follow-up interviews approximately 1 year later (mean [SD] 367 [16.8] days), were conducted with 585 respondents for a 72.4% response rate (Figure).
Survey Content
Development and pilot testing of the survey instrument previously was reported.1 The content of the survey drew from existing survey items and metrics, and included new items specifically designed to address patterns of amputation care, based on care goals within the CPG. All new and modified items were tested and refined through cognitive interviews with 10 participants, and tested with an additional 13 participants.
The survey collected data on demographics, amputation characteristics (year of amputation, level, laterality, and etiology), current prosthesis use, and type of prosthesis. This article focused on the sections of the survey pertaining to satisfaction with prosthetic care and indicators of quality of care. A description of the content of the full survey and a synopsis of overall findings are reported in a prior publication.1 The key independent, dependent, and other variables utilized in the analyses reported in this manuscript are described below.
Primary Independent Variables
In the follow-up survey, we asked respondents whether they had any amputation care in the prior 12 months, and if so to indicate where they had gone for care. We categorized respondents as having received VA/DoD care if they reported any care at the VA or DoD, and as having received non-VA/DoD care if they did not report care at the VA or DoD but indicated that they had received amputation care between baseline and follow-up.
Two primary outcomes were utilized; the Orthotics and Prosthetics User’s Survey (OPUS), client satisfaction with services scale (CSS), and a measure of care quality specifically developed for this study. The CSS is a measure developed specifically for orthotic and prosthesis users.10 This 11-item scale measures satisfaction with prosthetic limb services and contains items evaluating facets of care such as courtesy received from prosthetists and clinical staff, care coordination, appointment wait time, willingness of the prosthetist to listen to participant concerns, and satisfaction with prosthesis training. Items are rated on a 4-point scale (strongly agree [1] to strongly disagree [4]), thus higher CSS scores indicate worse satisfaction with services. The CSS was administered only to prosthesis users.
The Quality of Care assessment developed for this study contained items pertaining to amputation related care receipt and care quality. These items were generated by the study team in consultation with representatives from the VA/DoD Extremity Amputation Center of Excellence after review of the ULA rehabilitation CPG. Survey questions asked respondents about the clinicians visited for amputation related care in the past 12 months, whether they had an annual amputation-related checkup, whether any clinician had assessed their function, worked with them to identify goals, and/or to develop an amputation-related care plan. Respondents were also asked whether there had been family/caregiver involvement in their care and care coordination, whether a peer visitor was involved in their initial care, whether they had received information about amputation management in the prior year, and whether they had amputation-related pain. Those that indicated that they had amputation-related pain were subsequently asked whether their pain was well managed, whether they used medication for pain management, and whether they used any nonpharmaceutical strategies.
Quality of Care Index
We initially developed 15 indicator items of quality of care. We selected 7 of the items to create a summary index. We omitted 3 items about pain management, since these items were completed only by participants who indicated that they had experienced pain; however, we examined the 3 pain items individually given the importance of this topic. We omitted an additional 2 items from the summary index because they would not be sensitive to change because they pertained to the care in the year after initial amputation. One of these items asked whether caregivers were involved in initial amputation management and the other asked whether a peer visit occurred after amputation. Another 3 items were omitted because they only were completed by small subsamples due to intentional skip patterns in the survey. These items addressed whether clinical HCPs discussed amputation care goals in the prior year, worked to develop a care plan in the prior year, or helped to coordinate care after a move. Completion rates for all items considered for the index are shown in eAppendix 1 (Available at doi:10.12788/fp.0096). After item selection, we generated an index score, which was the number of reported “yes” responses to the seven relevant items.
Other Variables
We created a single variable called level/laterality which categorized ULA as unilateral or bilateral. We further categorized respondents with unilateral amputation by their amputation level. We categorized respondents as transradial for wrist joint or below the elbow amputations; transhumeral for at or above the elbow amputations; and shoulder for shoulder joint or forequarter amputations. Participants indicated the amputation etiology using 7 yes/no variables: combat injury, accident, burn, cancer, diabetes mellitus, and infection. Participants could select ≥ 1 etiology.
Primary prosthesis type was categorized as body powered, myoelectric/hybrid, cosmetic, other/unknown, or nonuser. The service era was classified based on amputation date as Before Vietnam, Vietnam War, After Vietnam to Gulf War, After Gulf War to September 10, 2001, and September 11, 2001 to present. For race, individuals with > 1 race were classified as other. We classified participants by region, using the station identification of the most recent VA medical center that they had visited between January 1, 2010 and December 30, 2015.
The survey also employed 2 measures of satisfaction with the prosthesis, the Trinity Amputation and Prosthetic Experience Scale (TAPES) satisfaction scale and the OPUS Client Satisfaction with Devices (CSD). TAPES consists of 10 items addressing color, shape, noise, appearance, weight, usefulness, reliability, fit, comfort and overall satisfaction.11 Items are rated on a 5-point Likert scale from very dissatisfied (1) to very satisfied (5). An 8-item version of the CSD scale was created for this study, after conducting a Rasch analysis (using Winsteps version 4.4.2) of the original 11-item CSD. The 8 items assess satisfaction with prosthesis fit, weight, comfort, donning ease, appearance, durability, skin contact, and pain. Items are rated on a 4-point scale from strongly agree (1) to strongly disagree (4); higher CSD scores indicate less satisfaction with devices. Psychometric analysis of the revised CSD score was reported in a prior publication.12 We also reported on the CSS using the original 10-item measure.
Data Analyses
We described characteristics of respondents at baseline and follow-up. We used baseline data to calculate CSS scores and described scores for all participants, for subgroups of unilateral and bilateral amputees, and for unilateral amputees stratified by amputation level. Wilcoxon rank sum tests were used to compare the CSS item and total scores of 461 prosthesis users with unilateral amputation and 29 with bilateral amputation. To identify factors that we hypothesized might be associated with CSS scores at baseline, we developed separate bivariate linear regression models. We added those factors that were associated with CSS scores at P ≤ .1 in bivariate analyses to a multivariable linear regression model of factors associated with CSS score. The P ≤ .1 threshold was used to ensure that relevant confounders were controlled for in regression models. We excluded 309 participants with no reported prosthesis use (who were not asked to complete the CSS), 20 participants with other/unknown prosthesis types, and 106 with missing data on amputation care in the prior year or on satisfaction metrics. We used baseline data for this analysis to maximize the sample size.
We compared CSS scores for those who reported receiving care within or outside of the VA or DoD in the prior year, using Wilcoxon Mann-Whitney rank tests. We also compared scores of individual quality of care items for these groups using Fisher exact tests. We chose to examine individual items rather than the full Index because several items specified care receipt within the VA and thus would be inappropriate to utilize in comparisons by site location; however, we described responses to all items. In these analyses, we excluded 2 respondents who had conflicting information regarding location of care. We used follow-up data for this analysis because it allowed us to identify location of care received in the prior year.
We also described the CSS scores, the 7-item Quality of Care Index and responses to other items related to quality of care at baseline and follow-up. To examine whether satisfaction with prosthetic care or aspects of care quality had changed over time, we compared baseline and follow-up CSS and quality of care scores for respondents who had measures at both times using Wilcoxon signed ranks tests. Individual items were compared using McNemar tests.
Results
Respondents were 97.4% male and included 776 unilateral amputees and 32 bilateral amputees with a mean (SD) age of 63.3 (14.1) years (Table 1). Respondents had lost their limbs a mean (SD) 31.4 (14.1) years prior, and half were transradial, 34.2% transhumeral, and 11.6% shoulder amputation. At baseline 185 (22.9%) participants received amputation-related care in the prior year and 118 (20.2%) participants received amputation-related care within 1 year of follow-up. Of respondents, 113 (19.3%) stated that their care was between baseline and follow-up and 89 (78.8%) of these received care at either the VA, the DoD or both; just 16 (14.2%) received care elsewhere.
Mean (SD) CSS scores were highest (lower satisfaction) for those with amputation at the shoulder and lowest for those with transhumeral amputation: 42.2 (20.0) vs 33.4 (20.8). Persons with bilateral amputation were less satisfied in almost every category when compared with those with unilateral amputation, although the total CSS score was not substantially different. Wilcoxon rank sum analyses revealed statistically significant differences in wait time satisfaction: bilateral amputees were less satisfied than unilateral amputees. Factors associated with overall CSS score in bivariate analyses were CSD score, TAPES score, amputation care receipt, prosthesis type, race, and region of care (eAppendix 2, available at doi:10.12788/fp.0096).
In the multivariate regression model of baseline CSS scores, only 2 variables were independently associated with CSS scores: CSD score and recent amputation care (Table 3). For each 1-point increase in CSD score there was a 0.7 point increase in CSS score. Those with amputation care in the prior year had higher satisfaction when compared with those who had not received care (P = .003).
For participants who indicated that they received amputation care between baseline and follow-up, CSS mean (SD) scores were better, but not statistically different, for those who reported care in the VA or DoD vs private care, 31.6 (22.6) vs 38.0 (17.7) (Table 4). When compared with community-based care, more participants who received care in the VA or DoD in the prior year had a functional assessment in that time period (33.7% vs 7.1%, P = .06), were contacted by HCPs outside of appointments (42.7% vs 18.8%, P = .07), and received information about amputation care in the prior year (41.6% vs 0%, P =.002). There was no difference in the proportion whose family/caregivers were involved in care in the prior year.
No statistically significant differences were observed in paired comparisons of the CSS and Quality of Care Index at baseline or follow-up for all participants with data at both time points (Table 5; eAppendix 3 available at doi:10.12788/fp.0096). Individual pain measures did not differ significantly between timepoints. Quality Index mean (SD) scores were 1.3 (1.5) and 1.2 (1.5) at baseline and follow-up, respectively (P = .07). For the 214 prosthesis users with longitudinal data, baseline CSS mean (SD) scores were generally worse at baseline than at follow-up: 34.4 (19.8) vs 32.5 (21.0) (P = .23). Family/caregiver involvement in amputation care was significantly higher in the year before baseline when compared with the year prior to follow-up (24.4% vs 17.7%, P = .001). There were no other statistically significant differences in Quality of Care items between baseline and follow-up.
Discussion
Our longitudinal study provides insights into the experiences of veterans with major ULA related to satisfaction with prosthetic limb care services and receipt of amputation-related care. We reported on the development and use of a new summary measure of amputation care quality, which we designed to capture some of the key elements of care quality as provided in the VA/DoD CPG.2
We used baseline data to identify factors independently associated with prosthetic limb care satisfaction as measured by a previously validated measure, the OPUS CSS. The CSS addresses satisfaction with prosthetic limb services and does not reflect satisfaction with other amputation care services. We found that persons who received amputation care in the prior year had CSS scores that were a mean 5.1 points better than those who had not received recent care. Although causality cannot be determined with this investigation, this finding highlights an important relationship between frequency of care and satisfaction, which can be leveraged by the VA in future care initiatives. Care satisfaction was also better by 0.7 points for every 1-point decrease (indicating higher satisfaction) in the OPUS CSD prosthetic satisfaction scale. This finding isn’t surprising, given that a major purpose of prosthetic limb care services is to procure and fit a satisfactory device. To determine whether these same relationships were observed in the smaller, longitudinal cohort data at follow-up, we repeated these models and found similar relationships between recent care receipt and prosthesis satisfaction and satisfaction with services. We believe that these findings are meaningful and emphasize the importance of both service and device satisfaction to the veteran with an ULA. Lower service satisfaction scores among those with amputations at the shoulder and those with bilateral limb loss suggest that these individuals may benefit from different service delivery approaches.
We did observe a difference in satisfaction scores by geographic region in the follow-up (but not the baseline) data with satisfaction higher in the Western vs the Southern region (data not shown). This finding suggests a need for continued monitoring of care satisfaction over time to determine whether differences by region persist. We grouped respondents into geographic region based on the location where they had received their most recent VA care of any type. Many veterans receive care at multiple VA locations. Thus, it is possible that some veterans received their amputation care at a non-VA facility or a VA facility in a different region.
Our findings related to prosthetic limb care services satisfaction are generalizable to veteran prosthesis users. Findings may not be generalizable to nonusers, because in our study, the CSS only was administered to prosthesis users. Thus, we were unable to identify factors associated with care satisfaction for persons who were not current users of an upper limb prosthesis.
The study findings confirmed that most veterans with ULA receive amputation-related care in the VA or DoD. We compared CSS and Quality of Care item scores for those who reported receiving care at the VA or DoD vs elsewhere. Amputation care within the VA is complex. Some services are provided at VA facilities and some are ordered by VA clinicians but provided by community-based HCPs. However, we found that better (though not statistically significantly different) CSS scores and several Quality of Care items were endorsed by a significantly more of those reporting care in the VA or DoD as compared to elsewhere. Given the dissemination of a rehabilitation of upper limb amputees CPG, we hypothesized that VA and DoD HCPs would be more aware of care guidelines and would provide better care. Overall, our findings supported this hypothesis while also suggesting that areas such as caregiver involvement and peer visitation may benefit from additional attention and program improvement.
We used longitudinal data to describe and compare CSS and Quality of Care Index scores. Our analyses did not detect any statistically significant differences between baseline and follow-up. This finding may reflect that this was a relatively stable population with regard to amputation experiences given the mean time since amputation was 31.4 years. However, we also recognize that our measures may not have captured all aspects of care satisfaction or quality. It is possible that there were other changes that had occurred over the course of the year that were not captured by the CSS or by the Quality of Care Index. It is also possible that some implementation and adoption of the CPG had happened prior to our baseline survey. Finally, it is possible that some elements of the CPG have not yet been fully integrated into clinical care. We believe that the latter is likely, given that nearly 80% of respondents did not report receiving any amputation care within the past year at follow-up, though the CPGs recommend an annual visit.
Aside from recall bias, 2 explanations must be considered relative to the low rate of adherence to the CPG recommendation for an annual follow-up. The first is that the CPG simply may not be widely adopted. The second is that the majority of patients with ULA who use prostheses use a body-powered system. These tend to be low maintenance, long-lasting systems and may ultimately not need annual maintenance and repair. Further, if the veteran’s body-powered system is functioning properly and health status has not changed, they may simply be opting out of an annual visit despite the CPG recommendation. Nonetheless, this apparent low rate of annual follow-up emphasizes the need for additional process improvement measures for the VA.
Strengths and Limitations
The VA provides a unique setting for a nationally representative study of amputation rehabilitation because it has centralized data sources that can be used to identify veterans with ULA. Our study had a strong response rate, and its prosthetic limb care satisfaction findings are generalizable to all veterans with major ULA who received VA care from 2010 to 2015. However, there are limits to generalizability outside of this population to civilians or to veterans who do not receive VA care. To examine possible nonresponse bias, which could limit generalizability, we compared the baseline characteristics of respondents and nonrespondents to the follow-up study (eAppendix 4 available at doi:10.12788/fp.0096). There were no significant differences in satisfaction, quality of care, or receipt of amputation-related care between those lost to follow-up and those with follow-up data. Although, we did find small differences in gender, race, and service era (defined by amputation date). We do not believe that these differences threaten the interpretation of findings at follow-up, but there may be limits to generalizability of these findings to the full baseline sample. The data were from a telephone survey of veterans. It is possible that some veterans did not recall their care receipt or did not understand some of the questions and thus may not have accurately answered questions related to type of care received or the timing of that care.
Our interpretation of findings comparing care received within the VA and DoD or elsewhere is also limited because we cannot say with certainty whether those who indicated no care in the VA or DoD actually had care that was sponsored by the VA or DoD as contract or fee-basis care. Just 8 respondents indicated that they had received care only outside of the VA or DoD in the prior year. There were also some limitations in the collection of data about care location. We asked about receipt of amputation care in the prior year and about location of any amputation care received between baseline and follow-up, and there were differences in responses. Thus, we used a combination of these items to identify location of care received in the prior year.
Despite these limitations, we believe that our study provides novel, important findings about the satisfaction with prosthetic limb care services and quality of amputation rehabilitation care for veterans. Findings from this study can be used to address amputation and prosthetic limb care satisfaction and quality weaknesses highlighted and to benchmark care satisfaction and CPG compliance. Other studies evaluating care guideline compliance have used indicators obtained from clinical records or data repositories.13-15 Future work could combine self-reported satisfaction and care quality measures with indicators obtained from clinical or repository sources to provide a more complete snapshot of care delivery.
Conclusions
We reported on a national survey of veterans with major upper limb loss that assessed satisfaction with prosthetic limb care services and quality of amputation care. Satisfaction with prosthetic limb care was independently associated with satisfaction with the prosthesis, and receipt of care within the prior year. Most of the veterans surveyed received care within the VA or DoD and reported receiving higher quality of care, when compared with those who received care outside of the VA or DoD. Satisfaction with care and quality of care were stable over the year of this study. Data presented in this study can serve to direct VA amputation care process improvement initiatives as benchmarks for future work. Future studies are needed to track satisfaction with and quality of care for veterans with ULA.
1. Resnik L, Ekerholm S, Borgia M, Clark MA. A national study of veterans with major upper limb amputation: Survey methods, participants, and summary findings. PLoS One. 2019;14(3):e0213578. Published 2019 Mar 14. doi:10.1371/journal.pone.0213578
2. US Department of Defense, US Department of Veterans Affairs, Management of Upper Extremity Amputation Rehabilitation Working Group. VA/DoD clinical practice guideline for the management of upper extremity amputation rehabilitation.Published 2014. Accessed February 18, 2021. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf
3. Jette AM. The Promise of Assistive Technology to Enhance Work Participation. Phys Ther. 2017;97(7):691-692. doi:10.1093/ptj/pzx054
4. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs amputations system of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
5. Scholten J, Poorman C, Culver L, Webster JB. Department of Veterans Affairs polytrauma telerehabilitation: twenty-first century care. Phys Med Rehabil Clin N Am. 2019;30(1):207-215. doi:10.1016/j.pmr.2018.08.003
6. Melcer T, Walker J, Bhatnagar V, Richard E. Clinic use at the Departments of Defense and Veterans Affairs following combat related amputations. Mil Med. 2020;185(1-2):e244-e253. doi:10.1093/milmed/usz149
7. Berke GM, Fergason J, Milani JR, et al. Comparison of satisfaction with current prosthetic care in veterans and servicemembers from Vietnam and OIF/OEF conflicts with major traumatic limb loss. J Rehabil Res Dev. 2010;47(4):361-371. doi:10.1682/jrrd.2009.12.0193
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection prosthetic limb care in VA facilities. Published March 8, 2012. Accessed February 18, 2021. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf 9. American Association for Public Opinion Research. Response rates - an overview. Accessed February 18, 2021. https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx
10. Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users’ Survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
11. Desmond DM, MacLachlan M. Factor structure of the Trinity Amputation and Prosthesis Experience Scales (TAPES) with individuals with acquired upper limb amputations. Am J Phys Med Rehabil. 2005;84(7):506-513. doi:10.1097/01.phm.0000166885.16180.63
12. Resnik L, Borgia M, Heinemann AW, Clark MA. Prosthesis satisfaction in a national sample of veterans with upper limb amputation. Prosthet Orthot Int. 2020;44(2):81-91. doi:10.1177/0309364619895201
13. Ho TH, Caughey GE, Shakib S. Guideline compliance in chronic heart failure patients with multiple comorbid diseases: evaluation of an individualised multidisciplinary model of care. PLoS One. 2014;9(4):e93129. Published 2014 Apr 8. doi:10.1371/journal.pone.0093129
14. Mitchell KB, Lin H, Shen Y, et al. DCIS and axillary nodal evaluation: compliance with national guidelines. BMC Surg. 2017;17(1):12. Published 2017 Feb 7. doi:10.1186/s12893-017-0210-5
15. Moesker MJ, de Groot JF, Damen NL, et al. Guideline compliance for bridging anticoagulation use in vitamin-K antagonist patients; practice variation and factors associated with non-compliance. Thromb J. 2019;17:15. Published 2019 Aug 5. doi:10.1186/s12959-019-0204-x
1. Resnik L, Ekerholm S, Borgia M, Clark MA. A national study of veterans with major upper limb amputation: Survey methods, participants, and summary findings. PLoS One. 2019;14(3):e0213578. Published 2019 Mar 14. doi:10.1371/journal.pone.0213578
2. US Department of Defense, US Department of Veterans Affairs, Management of Upper Extremity Amputation Rehabilitation Working Group. VA/DoD clinical practice guideline for the management of upper extremity amputation rehabilitation.Published 2014. Accessed February 18, 2021. https://www.healthquality.va.gov/guidelines/Rehab/UEAR/VADoDCPGManagementofUEAR121614Corrected508.pdf
3. Jette AM. The Promise of Assistive Technology to Enhance Work Participation. Phys Ther. 2017;97(7):691-692. doi:10.1093/ptj/pzx054
4. Webster JB, Poorman CE, Cifu DX. Guest editorial: Department of Veterans Affairs amputations system of care: 5 years of accomplishments and outcomes. J Rehabil Res Dev. 2014;51(4):vii-xvi. doi:10.1682/JRRD.2014.01.0024
5. Scholten J, Poorman C, Culver L, Webster JB. Department of Veterans Affairs polytrauma telerehabilitation: twenty-first century care. Phys Med Rehabil Clin N Am. 2019;30(1):207-215. doi:10.1016/j.pmr.2018.08.003
6. Melcer T, Walker J, Bhatnagar V, Richard E. Clinic use at the Departments of Defense and Veterans Affairs following combat related amputations. Mil Med. 2020;185(1-2):e244-e253. doi:10.1093/milmed/usz149
7. Berke GM, Fergason J, Milani JR, et al. Comparison of satisfaction with current prosthetic care in veterans and servicemembers from Vietnam and OIF/OEF conflicts with major traumatic limb loss. J Rehabil Res Dev. 2010;47(4):361-371. doi:10.1682/jrrd.2009.12.0193
8. US Department of Veterans Affairs, Office of Inspector General. Healthcare inspection prosthetic limb care in VA facilities. Published March 8, 2012. Accessed February 18, 2021. https://www.va.gov/oig/pubs/VAOIG-11-02138-116.pdf 9. American Association for Public Opinion Research. Response rates - an overview. Accessed February 18, 2021. https://www.aapor.org/Education-Resources/For-Researchers/Poll-Survey-FAQ/Response-Rates-An-Overview.aspx
10. Heinemann AW, Bode RK, O’Reilly C. Development and measurement properties of the Orthotics and Prosthetics Users’ Survey (OPUS): a comprehensive set of clinical outcome instruments. Prosthet Orthot Int. 2003;27(3):191-206. doi:10.1080/03093640308726682
11. Desmond DM, MacLachlan M. Factor structure of the Trinity Amputation and Prosthesis Experience Scales (TAPES) with individuals with acquired upper limb amputations. Am J Phys Med Rehabil. 2005;84(7):506-513. doi:10.1097/01.phm.0000166885.16180.63
12. Resnik L, Borgia M, Heinemann AW, Clark MA. Prosthesis satisfaction in a national sample of veterans with upper limb amputation. Prosthet Orthot Int. 2020;44(2):81-91. doi:10.1177/0309364619895201
13. Ho TH, Caughey GE, Shakib S. Guideline compliance in chronic heart failure patients with multiple comorbid diseases: evaluation of an individualised multidisciplinary model of care. PLoS One. 2014;9(4):e93129. Published 2014 Apr 8. doi:10.1371/journal.pone.0093129
14. Mitchell KB, Lin H, Shen Y, et al. DCIS and axillary nodal evaluation: compliance with national guidelines. BMC Surg. 2017;17(1):12. Published 2017 Feb 7. doi:10.1186/s12893-017-0210-5
15. Moesker MJ, de Groot JF, Damen NL, et al. Guideline compliance for bridging anticoagulation use in vitamin-K antagonist patients; practice variation and factors associated with non-compliance. Thromb J. 2019;17:15. Published 2019 Aug 5. doi:10.1186/s12959-019-0204-x