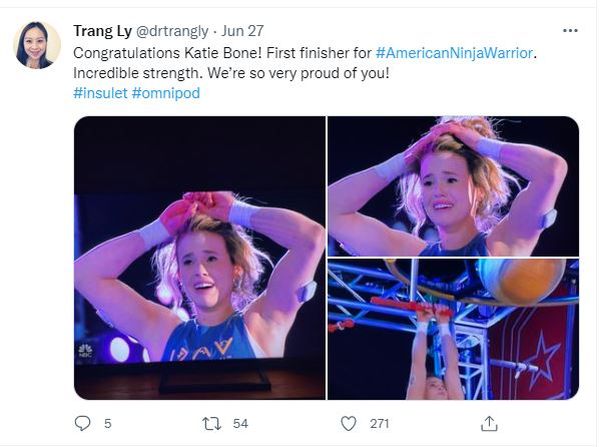User login
Vitamin D supplements during pregnancy may protect infants from atopic eczema
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to results of a clinical trial.
“Our data provide the first randomized controlled trial evidence of a protective effect of antenatal cholecalciferol supplementation on risk of infantile atopic eczema, with the effect only seen in infants that were breastfed for more than 1 month,” lead study author Sarah El-Heis, MRCP, DM, and colleagues wrote.
“The findings support a developmental influence on infantile atopic eczema and point to gestational cholecalciferol supplementation as a preventive strategy to reduce the burden of atopic eczema during infancy,” Dr. El-Heis, an academic clinical lecturer in dermatology at the Medical Research Council Lifecourse Epidemiology Center of the University of Southampton (England), said in a presentation at the annual meeting of the Society for Investigative Dermatology.
The study also was published in the British Journal of Dermatology.
Dr. El-Heis and colleagues analyzed data from one of the three U.K. study sites involved in the double-blind Maternal Vitamin D Osteoporosis Study (MAVIDOS), which enrolled participants between 2008 and 2014.
The women enrolled at the University of Southampton site were of age 18 or older, and had a singleton pregnancy. Serum 25-hydroxy vitamin D (25[OH]D) levels were 25-100 nmol/L, and calcium levels were less than 2.75 mmol/L.
Those who had metabolic bone disease, kidney stones, hyperparathyroidism, or hypercalciuria or who were taking more than 400 IU/day of vitamin D supplements or medication known to interfere with fetal growth or whose fetus had a major anomaly were excluded.
The study included 1,134 women. Half of the participants were randomly assigned to receive cholecalciferol 1,000 IU/day from around 14 weeks’ gestation until delivery, and half were assigned to receive placebo. Their babies were assessed for atopic eczema at 12, 24, and 48 months of age.
The maternal and infant characteristics were similar in both groups, but the treatment group tended to breastfeed longer.
Infants appear to be protected up to 1 year of age
Using logistic regression, the researchers analyzed links between maternal cholecalciferol 1,000 IU/day supplements or placebo and atopic eczema risk in their offspring.
After adjustments for breastfeeding duration, among the 636 infants assessed at 12 months, those whose mothers received cholecalciferol had lower odds ratios of atopic eczema than those whose mothers received placebo (OR, 0.55; 95% confidence interval, 0.32-0.97).
The risk of atopic eczema at 12 months was reduced only for children in the treatment group who were breastfed longer than 1 month (OR, 0.48; 95% CI, 0.24-0.94), further analysis showed. Those who were breastfed for less than 1 month showed no reduced risk.
The combined effect of vitamin D and breastfeeding for longer than 1 month weakened after 1 year and was not statistically significant among the 611 children assessed at 24 months and the 450 children assessed at 48 months. The ORs of atopic eczema in the treatment group and in the control group increased to 0.76 (95% CI, 0.47-1.23) and 0.75 (95% CI, 0.37-1.52), respectively.
At baseline, the mean maternal serum 25(OH)D levels in the treatment group (46.0 nmol/L) and in the control group (44.7 nmol/L) were similar. But by late pregnancy, maternal serum 25(OH)D levels in the treatment group were higher (67.4 nmol/L) than in the control group (42.4 nmol/L).
The authors note that strengths of the study include its design, the uniformity of criteria used to diagnose atopic eczema, and the similarity of both pregnant groups in their intake of vitamin D during the study.
Limitations included the lack of ultraviolet B light exposure data, the lack of non-White women in the study, the lack of measurement of cord blood and offspring 25(OH)D levels, and the exclusion of women with baseline 25(OH)D concentrations less than 25 nmol/L.
“This is an interesting study that brings up the possibility that maternal factors during pregnancy may impact atopic dermatitis,” Kalyani S. Marathe, MD, MPH, the director of the division of dermatology at Cincinnati Children’s Hospital Medical Center, told this news organization.
The results are mixed, though, she noted.
“While some impact on the risk of eczema is seen at 1 year of age, that protective effect is gone by 2 years and 4 years,” Dr. Marathe, who was not involved in the study, said in an email. “So if maternal supplementation does improve eczema, the effect is not long-lasting.
“The other complicating factor is that the babies who showed reduction in eczema were also the ones who were breastfed longer than 1 month,” she added. “We know that breastfeeding is associated with several factors, including socioeconomic status, so it is difficult to tease out the relationships here.
“Vitamin D has become a very hot topic lately and seems to have protective effects in many areas of health care,” Dr. Marathe said. “These results may motivate pregnant women to be compliant with their prenatal vitamins that contain the amount of vitamin D studied here.”
The study received grant support. Several authors disclosed financial relationships with pharmaceutical and nutritional products industries. Dr. El-Heis and Dr. Marathe reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SID 2022
Meet a champion climber with type 1 diabetes
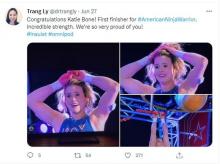
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Managing type 1 diabetes is never easy. But if you ask 16-year-old climbing star Katie Bone, she’ll tell you that she will never let this disease get in the way of her goals.
“My motto is the same one as Bethany Hamilton’s – the surfer who lost her arm in a shark attack: ‘I don’t need easy, I just need possible,” said Ms. Bone, who lives in Albuquerque and has been a competitive rock climber since she was 8 years old. “That really stuck with me.”
Just watching her compete on NBC’s hit reality show American Ninja Warrior in June is proof of that. Not only did the nationally ranked climber fly through the obstacles with grace and grit, but she proudly showed off her two monitoring devices: a glucose monitor on one arm and a tubeless insulin pump on the other.
“I specifically decided to keep my devices visible when I went on the show,” she said. “It’s part of my life, and I wanted to show that I’m not ashamed to wear medical devices.”
Still, it has been a long journey since Bone was diagnosed in 2017. She was just 11 years old at the time and had recently done a climbing competition when she started feeling ill.
“I didn’t perform well,” she said. “I needed to go to the bathroom a lot and felt really nauseous. Three days later, we ended up in urgent care.”
When her doctor first told her she had diabetes, she started crying.
“My grandma had type 1 and was extremely sick and died from complications,” she said. “That was all I knew about diabetes, and it was scary to think my life could be like that.”
But her outlook brightened when her doctor assured her that she could keep climbing.
“When I was told that I could keep competing, a switch flipped for me and I made a decision that nothing would hold me back,” she says.
But every day isn’t easy.
“It’s sometimes really hard to manage my diabetes during competitions,” she said. “When we climb, for example, we’re not allowed to have our phones, and I manage my [glucose monitor] through my phone. This means accommodations have to be made for me.”
And managing her diabetes can be unpredictable at times.
“If my blood sugar is low or high, I might be put last in a competition,” she said. “That messes up my warm-up and my mental game. It’s a never-ending battle.”
Ultimately, Ms. Bone’s goal is to inspire others and advocate for diabetes awareness. She says she’s been overwhelmed by viewer responses to her appearance on the show.
“I heard from so many parents and kids,” she said. “I want the world to know that wearing a pump on your arm only makes you more amazing.”
She also draws inspiration from others with diabetes.
“Everyone with this disease is a role model for me, since everyone is fighting their own battles,” she said. “Diabetes is different for everyone, and seeing how people can do what they do despite the diagnosis has been incredibly inspiring.”
For now, the rising high school junior plans to continue training and competing.
“My goal is to make the 2024 Olympic climbing team in Paris,” she said. “I’ve always wanted to compete in the Olympics since I was a little kid. Nothing can stop me.”
A version of this article first appeared on WebMD.com.
Experts: EPA should assess risk of sunscreens’ UV filters
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.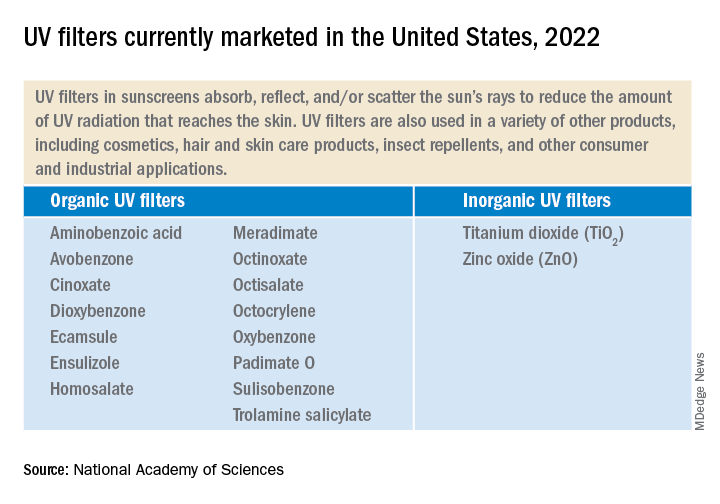
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The , an expert panel of the National Academies of Sciences, Engineering, and Medicine (NAS) said on Aug. 9.
The assessment is urgently needed, the experts said, and the results should be shared with the Food and Drug Administration, which oversees sunscreens.
In its 400-page report, titled the Review of Fate, Exposure, and Effects of Sunscreens in Aquatic Environments and Implications for Sunscreen Usage and Human Health, the panel does not make recommendations but suggests that such an EPA risk assessment should highlight gaps in knowledge.
“We are teeing up the critical information that will be used to take on the challenge of risk assessment,” Charles A. Menzie, PhD, chair of the committee that wrote the report, said at a media briefing Aug. 9 when the report was released. Dr. Menzie is a principal at Exponent, Inc., an engineering and scientific consulting firm. He is former executive director of the Society of Environmental Toxicology and Chemistry.
The EPA sponsored the study, which was conducted by a committee of the National Academy of Sciences, a nonprofit, nongovernmental organization authorized by Congress that studies issues related to science, technology, and medicine.
Balancing aquatic, human health concerns
Such an EPA assessment, Dr. Menzie said in a statement, will help inform efforts to understand the environmental effects of UV filters as well as clarify a path forward for managing sunscreens. For years, concerns have been raised about the potential toxicity of sunscreens regarding many marine and freshwater aquatic organisms, especially coral. That concern, however, must be balanced against the benefits of sunscreens, which are known to protect against skin cancer. A low percentage of people use sunscreen regularly, Dr. Menzie and other panel members said.
“Only about a third of the U.S. population regularly uses sunscreen,” Mark Cullen, MD, vice chair of the NAS committee and former director of the Center for Population Health Sciences, Stanford (Calif.) University, said at the briefing. About 70% or 80% of people use it at the beach or outdoors, he said.
Report background, details
UV filters are the active ingredients in physical as well as chemical sunscreen products. They decrease the amount of UV radiation that reaches the skin. They have been found in water, sediments, and marine organisms, both saltwater and freshwater.
Currently, 17 UV filters are used in U.S. sunscreens; 15 of those are organic, such as oxybenzone and avobenzone, and are used in chemical sunscreens. They work by absorbing the rays before they damage the skin. In addition, two inorganic filters, which are used in physical sunscreens, sit on the skin and as a shield to block the rays.
UV filters enter bodies of water by direct release, as when sunscreens rinse off people while swimming or while engaging in other water activities. They also enter bodies of water in storm water runoff and wastewater.
Lab toxicity tests, which are the most widely used, provide effects data for ecologic risk assessment. The tests are more often used in the study of short-term, not long-term exposure. Test results have shown that in high enough concentrations, some UV filters can be toxic to algal, invertebrate, and fish species.
But much information is lacking, the experts said. Toxicity data for many species, for instance, are limited. There are few studies on the longer-term environmental effects of UV filter exposure. Not enough is known about the rate at which the filters degrade in the environment. The filters accumulate in higher amounts in different areas. Recreational water areas have higher concentrations.
The recommendations
The panel is urging the EPA to complete a formal risk assessment of the UV filters “with some urgency,” Dr. Cullen said. That will enable decisions to be made about the use of the products. The risks to aquatic life must be balanced against the need for sun protection to reduce skin cancer risk.
The experts made two recommendations:
- The EPA should conduct ecologic risk assessments for all the UV filters now marketed and for all new ones. The assessment should evaluate the filters individually as well as the risk from co-occurring filters. The assessments should take into account the different exposure scenarios.
- The EPA, along with partner agencies, and sunscreen and UV filter manufacturers should fund, support, and conduct research and share data. Research should include study of human health outcomes if usage and availability of sunscreens change.
Dermatologists should “continue to emphasize the importance of protection from UV radiation in every way that can be done,” Dr. Cullen said, including the use of sunscreen as well as other protective practices, such as wearing long sleeves and hats, seeking shade, and avoiding the sun during peak hours.
A dermatologist’s perspective
“I applaud their scientific curiosity to know one way or the other whether this is an issue,” said Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, DC. “I welcome this investigation.”
The multitude of studies, Dr. Friedman said, don’t always agree about whether the filters pose dangers. He noted that the concentration of UV filters detected in water is often lower than the concentrations found to be harmful in a lab setting to marine life, specifically coral.
However, he said, “these studies are snapshots.” For that reason, calling for more assessment of risk is desirable, Dr. Friedman said, but “I want to be sure the call to do more research is not an admission of guilt. It’s very easy to vilify sunscreens – but the facts we know are that UV light causes skin cancer and aging, and sunscreen protects us against this.”
Dr. Friedman has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Stopping JIA drugs? Many can regain control after a flare
About two-thirds of children with juvenile idiopathic arthritis (JIA) were able to return to an inactive disease state within 12 months after a flare occurred when they took a break from medication, and slightly more than half – 55% – reached this state within 6 months, according to findings from registry data examined in a study published in Arthritis Care & Research.
Sarah Ringold, MD, MS, of the Seattle Children’s Hospital, and coauthors used data from participants in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry to track what happened to patients when they took a break from antirheumatic drugs. They described their paper as being the first to use a large multicenter database such as the CARRA Registry to focus on JIA outcomes after medication discontinuation and flare, to describe flare severity after medication discontinuation, and to report patterns of medication use for flares.
“To date, JIA studies have established that flares after medication discontinuation are common but have generated conflicting data regarding flare risk factors,” Dr. Ringold and coauthors wrote. “Since it is not yet possible to predict reliably which children will successfully discontinue medication, families and physicians face uncertainty when deciding to stop medications, and there is significant variation in approach.”
The study will be “very helpful” to physicians working with parents and patients to make decisions about discontinuing medications, said Grant Schulert, MD, PhD, of Cincinnati Children’s Hospital, who was not involved with the study.
“It gives some numbers to help us have those conversations,” he said in an interview.
But interpreting those numbers still will present parents with a challenge, Dr. Schulert said.
“You can say: ‘The glass is half full; 55% of them could go back into remission in 6 months, a little bit higher in a year,’ ” he said. “Or the glass is half empty; some of them, even at a year, are still not back in remission.”
But “patients aren’t a statistic. They’re each one person,” he said. “They’re going to be in one of those two situations.”
There are many challenges in explaining the potential advantages and disadvantages of medication breaks to patients and families, said the study’s senior author, Daniel B. Horton, MD, MSCE, of Rutgers Robert Wood Johnson Medical School and the Rutgers Center for Pharmacoepidemiology and Treatment Science, both in New Brunswick, N.J., and the department of biostatistics and epidemiology at Rutgers School of Public Health, Piscataway, N.J.
“One of the challenges of explaining the pros and cons about stopping medicines is the uncertainty – not knowing if and when a flare will occur, if and when a flare would be well controlled, and, for treatments that are continued, if and when complications of that treatment could occur,” Dr. Horton said in an interview. “Many patients and families are afraid about what the medicines might do long-term and want to stop treatment as soon as possible, despite the risks of stopping. Another challenge is that we do not yet have accurate, widely available tests that help us predict these various outcomes. Still, it is important for clinicians to explain the risks of continuing treatment and of stopping treatment, and to give patients and families time to ask questions and share their own values and preferences. If these conversations don’t happen, patients or families may just stop the medicines even if stopping is not warranted or is likely to lead to a poor outcome.”
Study details
Of the 367 patients studied, 270 (74%) were female. Half of all patients in the study had extended oligoarticular/rheumatoid factor (RF)–negative polyarticular JIA, and the second most common category was persistent oligoarthritis at 25%.The median age at disease onset was 4, with a range of 2-9 years.
The median age at disease flare was 11.3, with a range of 7.5-15.7 years. At the time of flare, children had a median disease duration of 5.1 years and had been off systemic disease-modifying antirheumatic drugs (DMARDs) for a median of 205 days. In addition, at the time of flare, the median active joint count was 1 and the maximum active joint count was 33, and approximately 13% of children had 5 or more active joints.
Conventional synthetic DMARDs were the most commonly stopped medications (48%), and tumor necrosis factor inhibitors (TNFi) were second (42%), Dr. Ringold and coauthors wrote.
Independent predictors of successful recapture of inactive disease included TNFi as recapture medication and history of a non-TNFi biologic use.
Dr. Ringold and coauthors noted limitations of the registry-based study. This is “a convenience sample of patients who are cared for and consented at academic sites, and additional study may be needed to understand how these results generalize to other countries and health systems,” they wrote.
And there may have been misclassification and inclusion of patients who stopped medications for self-perceived well-controlled disease, they wrote.
“Although the intent was to include children who stopped their medications at their physician’s direction due to physician-confirmed inactive disease, patients who had been previously enrolled in the registry were included if inactive disease was listed as the reason for medication discontinuation,” they said.
Still, these results should serve as a “benchmark for future studies of medication discontinuation” in JIA, the researchers wrote.
‘Fortunate challenge’
In an accompanying editorial, Melissa L. Mannion, MD, MSPH, and Randy Q. Cron, MD, PhD, of the University of Alabama at Birmingham noted that pediatric rheumatologists now face what they call the “fortunate challenge” of helping patients and parents decide whether treatments can be stopped in cases where there’s been a sustained period of inactive disease.
“Once a patient has reached the goal of inactive disease, why would patients or providers want to stop medications?” Dr. Mannion and Dr. Cron wrote. “We tell our patients that we want them to be like everyone else and have no limitations on their goals. However, the burden of chronic medication to achieve that goal is a constant reminder that they are different from their peers.”
In their article, Dr. Mannion and Dr. Cron noted what they called “interesting” results observed among children with different forms of JIA in the study.
Children with “systemic JIA had the highest recapture rates at 6 or 12 months, perhaps reflecting the high percentage use of [biologic] DMARDs targeting interleukin-1 and IL-6, or maybe the timeliness of recognition (e.g., fever, rash) of disease flare,” Dr. Mannion and Dr. Cron wrote. “Conversely, children with JIA enthesitis-related arthritis (ERA) had the lowest recapture rate at 6 months (27.6%, even lower than RF-positive polyarticular JIA, 42.9%).”
Still, the editorial authors said that “additional well-controlled studies are needed to move pediatric rheumatology deeper into the realm of precision medicine and the ability to decide whether or not to wean DMARD therapy for those with clinically inactive disease.”
Pamela Weiss, MD, of Children’s Hospital of Philadelphia, said in a comment that the study by Dr. Ringold and colleagues, as well as others that address similar questions, “are critically needed to move our field towards a personalized medicine approach.” But she added that while the paper from Dr. Ringold and colleagues addresses an important question, it “should be interpreted with some caution.”
She noted, for example, that “disease flare,” which prompted reinitiation of treatment and study entry, was not always aligned with a registry visit, which makes determination of the primary exposure less stringent. The rate of recapture across JIA categories differed by as much as 20% depending upon which inactive disease assessment outcome was used – either the study’s novel but unvalidated primary outcome or the validated secondary outcome of using the clinical Juvenile Arthritis Disease Activity Score based on 10 joints. The resulting difference was marked for some JIA categories and minimal for others.
“The flare and recapture rates are likely to be vastly different for JIA categories with distinct pathophysiology – namely systemic JIA, psoriatic arthritis, and enthesitis-related arthritis,” Dr. Weiss said. “While numbers for these categories were too small to make meaningful conclusions, grouping them with the other JIA categories has limitations.”
The research was funded by a Rheumatology Research Foundation Innovative Research Award.
Dr. Ringold’s current employment is through Janssen Research & Development. She changed primary employment from Seattle Children’s to Janssen during completion of the analyses and preparation of the manuscript. She has maintained her affiliation with Seattle Children’s. Dr. Schulert has consulting for Novartis. Dr. Cron reported speaker fees, consulting fees, and grant support from Sobi, consulting fees from Sironax and Novartis, speaker fees from Lilly, and support from Pfizer for working on a committee adjudicating clinical trial side effects.
* This article was updated on 8/11/2022.
About two-thirds of children with juvenile idiopathic arthritis (JIA) were able to return to an inactive disease state within 12 months after a flare occurred when they took a break from medication, and slightly more than half – 55% – reached this state within 6 months, according to findings from registry data examined in a study published in Arthritis Care & Research.
Sarah Ringold, MD, MS, of the Seattle Children’s Hospital, and coauthors used data from participants in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry to track what happened to patients when they took a break from antirheumatic drugs. They described their paper as being the first to use a large multicenter database such as the CARRA Registry to focus on JIA outcomes after medication discontinuation and flare, to describe flare severity after medication discontinuation, and to report patterns of medication use for flares.
“To date, JIA studies have established that flares after medication discontinuation are common but have generated conflicting data regarding flare risk factors,” Dr. Ringold and coauthors wrote. “Since it is not yet possible to predict reliably which children will successfully discontinue medication, families and physicians face uncertainty when deciding to stop medications, and there is significant variation in approach.”
The study will be “very helpful” to physicians working with parents and patients to make decisions about discontinuing medications, said Grant Schulert, MD, PhD, of Cincinnati Children’s Hospital, who was not involved with the study.
“It gives some numbers to help us have those conversations,” he said in an interview.
But interpreting those numbers still will present parents with a challenge, Dr. Schulert said.
“You can say: ‘The glass is half full; 55% of them could go back into remission in 6 months, a little bit higher in a year,’ ” he said. “Or the glass is half empty; some of them, even at a year, are still not back in remission.”
But “patients aren’t a statistic. They’re each one person,” he said. “They’re going to be in one of those two situations.”
There are many challenges in explaining the potential advantages and disadvantages of medication breaks to patients and families, said the study’s senior author, Daniel B. Horton, MD, MSCE, of Rutgers Robert Wood Johnson Medical School and the Rutgers Center for Pharmacoepidemiology and Treatment Science, both in New Brunswick, N.J., and the department of biostatistics and epidemiology at Rutgers School of Public Health, Piscataway, N.J.
“One of the challenges of explaining the pros and cons about stopping medicines is the uncertainty – not knowing if and when a flare will occur, if and when a flare would be well controlled, and, for treatments that are continued, if and when complications of that treatment could occur,” Dr. Horton said in an interview. “Many patients and families are afraid about what the medicines might do long-term and want to stop treatment as soon as possible, despite the risks of stopping. Another challenge is that we do not yet have accurate, widely available tests that help us predict these various outcomes. Still, it is important for clinicians to explain the risks of continuing treatment and of stopping treatment, and to give patients and families time to ask questions and share their own values and preferences. If these conversations don’t happen, patients or families may just stop the medicines even if stopping is not warranted or is likely to lead to a poor outcome.”
Study details
Of the 367 patients studied, 270 (74%) were female. Half of all patients in the study had extended oligoarticular/rheumatoid factor (RF)–negative polyarticular JIA, and the second most common category was persistent oligoarthritis at 25%.The median age at disease onset was 4, with a range of 2-9 years.
The median age at disease flare was 11.3, with a range of 7.5-15.7 years. At the time of flare, children had a median disease duration of 5.1 years and had been off systemic disease-modifying antirheumatic drugs (DMARDs) for a median of 205 days. In addition, at the time of flare, the median active joint count was 1 and the maximum active joint count was 33, and approximately 13% of children had 5 or more active joints.
Conventional synthetic DMARDs were the most commonly stopped medications (48%), and tumor necrosis factor inhibitors (TNFi) were second (42%), Dr. Ringold and coauthors wrote.
Independent predictors of successful recapture of inactive disease included TNFi as recapture medication and history of a non-TNFi biologic use.
Dr. Ringold and coauthors noted limitations of the registry-based study. This is “a convenience sample of patients who are cared for and consented at academic sites, and additional study may be needed to understand how these results generalize to other countries and health systems,” they wrote.
And there may have been misclassification and inclusion of patients who stopped medications for self-perceived well-controlled disease, they wrote.
“Although the intent was to include children who stopped their medications at their physician’s direction due to physician-confirmed inactive disease, patients who had been previously enrolled in the registry were included if inactive disease was listed as the reason for medication discontinuation,” they said.
Still, these results should serve as a “benchmark for future studies of medication discontinuation” in JIA, the researchers wrote.
‘Fortunate challenge’
In an accompanying editorial, Melissa L. Mannion, MD, MSPH, and Randy Q. Cron, MD, PhD, of the University of Alabama at Birmingham noted that pediatric rheumatologists now face what they call the “fortunate challenge” of helping patients and parents decide whether treatments can be stopped in cases where there’s been a sustained period of inactive disease.
“Once a patient has reached the goal of inactive disease, why would patients or providers want to stop medications?” Dr. Mannion and Dr. Cron wrote. “We tell our patients that we want them to be like everyone else and have no limitations on their goals. However, the burden of chronic medication to achieve that goal is a constant reminder that they are different from their peers.”
In their article, Dr. Mannion and Dr. Cron noted what they called “interesting” results observed among children with different forms of JIA in the study.
Children with “systemic JIA had the highest recapture rates at 6 or 12 months, perhaps reflecting the high percentage use of [biologic] DMARDs targeting interleukin-1 and IL-6, or maybe the timeliness of recognition (e.g., fever, rash) of disease flare,” Dr. Mannion and Dr. Cron wrote. “Conversely, children with JIA enthesitis-related arthritis (ERA) had the lowest recapture rate at 6 months (27.6%, even lower than RF-positive polyarticular JIA, 42.9%).”
Still, the editorial authors said that “additional well-controlled studies are needed to move pediatric rheumatology deeper into the realm of precision medicine and the ability to decide whether or not to wean DMARD therapy for those with clinically inactive disease.”
Pamela Weiss, MD, of Children’s Hospital of Philadelphia, said in a comment that the study by Dr. Ringold and colleagues, as well as others that address similar questions, “are critically needed to move our field towards a personalized medicine approach.” But she added that while the paper from Dr. Ringold and colleagues addresses an important question, it “should be interpreted with some caution.”
She noted, for example, that “disease flare,” which prompted reinitiation of treatment and study entry, was not always aligned with a registry visit, which makes determination of the primary exposure less stringent. The rate of recapture across JIA categories differed by as much as 20% depending upon which inactive disease assessment outcome was used – either the study’s novel but unvalidated primary outcome or the validated secondary outcome of using the clinical Juvenile Arthritis Disease Activity Score based on 10 joints. The resulting difference was marked for some JIA categories and minimal for others.
“The flare and recapture rates are likely to be vastly different for JIA categories with distinct pathophysiology – namely systemic JIA, psoriatic arthritis, and enthesitis-related arthritis,” Dr. Weiss said. “While numbers for these categories were too small to make meaningful conclusions, grouping them with the other JIA categories has limitations.”
The research was funded by a Rheumatology Research Foundation Innovative Research Award.
Dr. Ringold’s current employment is through Janssen Research & Development. She changed primary employment from Seattle Children’s to Janssen during completion of the analyses and preparation of the manuscript. She has maintained her affiliation with Seattle Children’s. Dr. Schulert has consulting for Novartis. Dr. Cron reported speaker fees, consulting fees, and grant support from Sobi, consulting fees from Sironax and Novartis, speaker fees from Lilly, and support from Pfizer for working on a committee adjudicating clinical trial side effects.
* This article was updated on 8/11/2022.
About two-thirds of children with juvenile idiopathic arthritis (JIA) were able to return to an inactive disease state within 12 months after a flare occurred when they took a break from medication, and slightly more than half – 55% – reached this state within 6 months, according to findings from registry data examined in a study published in Arthritis Care & Research.
Sarah Ringold, MD, MS, of the Seattle Children’s Hospital, and coauthors used data from participants in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry to track what happened to patients when they took a break from antirheumatic drugs. They described their paper as being the first to use a large multicenter database such as the CARRA Registry to focus on JIA outcomes after medication discontinuation and flare, to describe flare severity after medication discontinuation, and to report patterns of medication use for flares.
“To date, JIA studies have established that flares after medication discontinuation are common but have generated conflicting data regarding flare risk factors,” Dr. Ringold and coauthors wrote. “Since it is not yet possible to predict reliably which children will successfully discontinue medication, families and physicians face uncertainty when deciding to stop medications, and there is significant variation in approach.”
The study will be “very helpful” to physicians working with parents and patients to make decisions about discontinuing medications, said Grant Schulert, MD, PhD, of Cincinnati Children’s Hospital, who was not involved with the study.
“It gives some numbers to help us have those conversations,” he said in an interview.
But interpreting those numbers still will present parents with a challenge, Dr. Schulert said.
“You can say: ‘The glass is half full; 55% of them could go back into remission in 6 months, a little bit higher in a year,’ ” he said. “Or the glass is half empty; some of them, even at a year, are still not back in remission.”
But “patients aren’t a statistic. They’re each one person,” he said. “They’re going to be in one of those two situations.”
There are many challenges in explaining the potential advantages and disadvantages of medication breaks to patients and families, said the study’s senior author, Daniel B. Horton, MD, MSCE, of Rutgers Robert Wood Johnson Medical School and the Rutgers Center for Pharmacoepidemiology and Treatment Science, both in New Brunswick, N.J., and the department of biostatistics and epidemiology at Rutgers School of Public Health, Piscataway, N.J.
“One of the challenges of explaining the pros and cons about stopping medicines is the uncertainty – not knowing if and when a flare will occur, if and when a flare would be well controlled, and, for treatments that are continued, if and when complications of that treatment could occur,” Dr. Horton said in an interview. “Many patients and families are afraid about what the medicines might do long-term and want to stop treatment as soon as possible, despite the risks of stopping. Another challenge is that we do not yet have accurate, widely available tests that help us predict these various outcomes. Still, it is important for clinicians to explain the risks of continuing treatment and of stopping treatment, and to give patients and families time to ask questions and share their own values and preferences. If these conversations don’t happen, patients or families may just stop the medicines even if stopping is not warranted or is likely to lead to a poor outcome.”
Study details
Of the 367 patients studied, 270 (74%) were female. Half of all patients in the study had extended oligoarticular/rheumatoid factor (RF)–negative polyarticular JIA, and the second most common category was persistent oligoarthritis at 25%.The median age at disease onset was 4, with a range of 2-9 years.
The median age at disease flare was 11.3, with a range of 7.5-15.7 years. At the time of flare, children had a median disease duration of 5.1 years and had been off systemic disease-modifying antirheumatic drugs (DMARDs) for a median of 205 days. In addition, at the time of flare, the median active joint count was 1 and the maximum active joint count was 33, and approximately 13% of children had 5 or more active joints.
Conventional synthetic DMARDs were the most commonly stopped medications (48%), and tumor necrosis factor inhibitors (TNFi) were second (42%), Dr. Ringold and coauthors wrote.
Independent predictors of successful recapture of inactive disease included TNFi as recapture medication and history of a non-TNFi biologic use.
Dr. Ringold and coauthors noted limitations of the registry-based study. This is “a convenience sample of patients who are cared for and consented at academic sites, and additional study may be needed to understand how these results generalize to other countries and health systems,” they wrote.
And there may have been misclassification and inclusion of patients who stopped medications for self-perceived well-controlled disease, they wrote.
“Although the intent was to include children who stopped their medications at their physician’s direction due to physician-confirmed inactive disease, patients who had been previously enrolled in the registry were included if inactive disease was listed as the reason for medication discontinuation,” they said.
Still, these results should serve as a “benchmark for future studies of medication discontinuation” in JIA, the researchers wrote.
‘Fortunate challenge’
In an accompanying editorial, Melissa L. Mannion, MD, MSPH, and Randy Q. Cron, MD, PhD, of the University of Alabama at Birmingham noted that pediatric rheumatologists now face what they call the “fortunate challenge” of helping patients and parents decide whether treatments can be stopped in cases where there’s been a sustained period of inactive disease.
“Once a patient has reached the goal of inactive disease, why would patients or providers want to stop medications?” Dr. Mannion and Dr. Cron wrote. “We tell our patients that we want them to be like everyone else and have no limitations on their goals. However, the burden of chronic medication to achieve that goal is a constant reminder that they are different from their peers.”
In their article, Dr. Mannion and Dr. Cron noted what they called “interesting” results observed among children with different forms of JIA in the study.
Children with “systemic JIA had the highest recapture rates at 6 or 12 months, perhaps reflecting the high percentage use of [biologic] DMARDs targeting interleukin-1 and IL-6, or maybe the timeliness of recognition (e.g., fever, rash) of disease flare,” Dr. Mannion and Dr. Cron wrote. “Conversely, children with JIA enthesitis-related arthritis (ERA) had the lowest recapture rate at 6 months (27.6%, even lower than RF-positive polyarticular JIA, 42.9%).”
Still, the editorial authors said that “additional well-controlled studies are needed to move pediatric rheumatology deeper into the realm of precision medicine and the ability to decide whether or not to wean DMARD therapy for those with clinically inactive disease.”
Pamela Weiss, MD, of Children’s Hospital of Philadelphia, said in a comment that the study by Dr. Ringold and colleagues, as well as others that address similar questions, “are critically needed to move our field towards a personalized medicine approach.” But she added that while the paper from Dr. Ringold and colleagues addresses an important question, it “should be interpreted with some caution.”
She noted, for example, that “disease flare,” which prompted reinitiation of treatment and study entry, was not always aligned with a registry visit, which makes determination of the primary exposure less stringent. The rate of recapture across JIA categories differed by as much as 20% depending upon which inactive disease assessment outcome was used – either the study’s novel but unvalidated primary outcome or the validated secondary outcome of using the clinical Juvenile Arthritis Disease Activity Score based on 10 joints. The resulting difference was marked for some JIA categories and minimal for others.
“The flare and recapture rates are likely to be vastly different for JIA categories with distinct pathophysiology – namely systemic JIA, psoriatic arthritis, and enthesitis-related arthritis,” Dr. Weiss said. “While numbers for these categories were too small to make meaningful conclusions, grouping them with the other JIA categories has limitations.”
The research was funded by a Rheumatology Research Foundation Innovative Research Award.
Dr. Ringold’s current employment is through Janssen Research & Development. She changed primary employment from Seattle Children’s to Janssen during completion of the analyses and preparation of the manuscript. She has maintained her affiliation with Seattle Children’s. Dr. Schulert has consulting for Novartis. Dr. Cron reported speaker fees, consulting fees, and grant support from Sobi, consulting fees from Sironax and Novartis, speaker fees from Lilly, and support from Pfizer for working on a committee adjudicating clinical trial side effects.
* This article was updated on 8/11/2022.
FROM ARTHRITIS CARE & RESEARCH
In one state, pandemic tamped down lice and scabies cases
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
.
When COVID-19 was declared a public health emergency by the World Health Organization in March 2020, many countries including the United States enacted lockdown and isolation measures to help contain the spread of the disease. Since scabies and lice are both spread by direct contact, “we hypothesized that the nationwide lockdown would influence the transmission of these two conditions among individuals,” wrote Marianne Bonanno, MD, of the University of North Carolina, Chapel Hill, and colleagues.
“The pandemic created a unique opportunity for real-life observations following physical distancing measures being put in place,” coauthor Christopher Sayed, MD, associate professor of dermatology at UNC, said in an interview. “It makes intuitive sense that since lice and scabies spread by cost physical contact that rates would decrease with school closures and other physical distancing measures. Reports from other countries in which extended families more often live together and were forced to spend more time in close quarters saw increased rates so it was interesting to see this contrast,” he noted.
In the study, the researchers reviewed data from 1,858 cases of adult scabies, 893 cases of pediatric scabies, and 804 cases of pediatric lice reported in North Carolina between March 2017 and February 2021. They compared monthly cases of scabies and lice, and prescriptions during the period before the pandemic (March 2017 to February 2020), and during the pandemic (March 2020 to February 2021).
Pediatric lice cases decreased by 60.6% over the study period (P < .001). Significant decreases also occurred in adult scabies (31.1%, P < .001) and pediatric scabies (39%, P < .01).
The number of prescriptions for lice and scabies also decreased significantly (P < .01) during the study period, although these numbers differed from the actual cases. Prescriptions decreased by 41.4%, 29.9%, and 69.3% for pediatric scabies, adult scabies, and pediatric lice, respectively.
Both pediatric scabies and pediatric lice showed a greater drop in prescriptions than in cases, while the drop in prescriptions for adult scabies was slightly less than the drop in cases.
The difference in the decreased numbers between cases and prescriptions may stem from the decrease in close contacts during the pandemic, which decreased the need for multiple prescriptions, but other potential explanations could be examined in future studies, the researchers wrote in their discussion.
The study findings were limited by several factors including the cross-sectional design and potential underdiagnosis and underreporting, as well as the focus only on a population in a single state, which may limit generalizability, the researchers noted.
However, the results offer preliminary insights on the impact of COVID-19 restrictions on scabies and lice, and suggest the potential value of physical distancing to reduce transmission of both conditions, especially in settings such as schools and prisons, to help contain future outbreaks, they concluded.
The study findings reinforce physical contact as the likely route of disease transmission, for lice and scabies, Dr. Sayed said in the interview. “It’s possible distancing measures on a small scale could be considered for outbreaks in institutional settings, though the risks of these infestations are much lower than with COVID-19,” he said. “It will be interesting to observe trends as physical distancing measures end to see if cases rebound in the next few years,” he added.
Drop in cases likely temporary
“Examining the epidemiology of different infectious diseases over time is an interesting and important area of study,” said Sheilagh Maguiness, MD, associate professor of dermatology and pediatrics at the University of Minnesota, Minneapolis, who was asked to comment on the results.
“The pandemic dramatically altered the daily lives of adults and children across the globe, and we can learn a lot from studying how social distancing and prolonged masking has made an impact on the incidence and prevalence of different infectious illnesses in the country and across the world,” she said in an interview.
Dr. Maguiness said she was not surprised by the study findings. “In fact, other countries have published similar studies documenting a reduction in both head lice and scabies infestations during the time of the pandemic,” she said. “In France, it was noted that during March to December 2020, there was a reduction in sales for topical head lice and scabies treatments of 44% and 14%, respectively. Similarly, a study from Argentina documented a decline in head lice infestations by about 25% among children,” she said.
“I personally noted a marked decrease in both of these diagnoses among children in my own clinic,” she added.
“Since both of these conditions are spread through close physical contact with others, it makes sense that there would be a steep decline in ectoparasitic infections during times of social distancing. However, anecdotally we are now diagnosing and treating these infestations again more regularly in our clinic,” said Dr. Maguiness. “As social distancing relaxes, I would expect that the incidence of both head lice and scabies will again increase.”
The study received no outside funding. The researchers and Dr. Maguiness had no financial conflicts to disclose.
FROM PEDIATRIC DERMATOLOGY
Children and COVID: Severe illness rising as vaccination effort stalls
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
After new child cases jumped by 22% during the week of July 15-21, the two successive weeks have produced increases of 3.9% (July 22-29) and 1.2% (July 30-Aug. 4). The latest weekly count from all states and territories still reporting was 96,599, the AAP and CHA said in their weekly COVID report, noting that several states have stopped reporting child cases and that others are reporting every other week.
The deceleration in new cases, however, does not apply to emergency department visits and hospital admissions. The proportion of ED visits with diagnosed COVID rose steadily throughout June and July, as 7-day averages went from 2.6% on June 1 to 6.3% on July 31 for children aged 0-11 years, from 2.1% to 3.1% for children aged 12-15, and from 2.4% to 3.5% for 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
The rate of new admissions with confirmed COVID, which reached 0.46 per 100,000 population for children aged 0-17 years on July 30, has more than tripled since early April, when it had fallen to 0.13 per 100,000 in the wake of the Omicron surge, the CDC reported on its COVID Data Tracker.
A smaller but more detailed sample of children from the COVID-19–Associated Hospitalization Network (COVID-NET), which covers nearly 100 counties in 14 states, indicates that the increase in new admissions is occurring almost entirely among children aged 0-4 years, who had a rate of 5.6 per 100,000 for the week of July 17-23, compared with 0.8 per 100,000 for 5- to 11-year-olds and 1.5 per 100,000 for those aged 12-17, the CDC said.
Vaccine’s summer rollout gets lukewarm reception
As a group, children aged 0-4 years have not exactly flocked to the COVID-19 vaccine. As of Aug. 2 – about 6 weeks since the vaccine was authorized for children aged 6 months to 4 years – just 3.8% of those eligible had received at least one dose. Among children aged 5-11 the corresponding number on Aug. 2 was 37.4%, and for those aged 12-17 years it was 70.3%, the CDC data show.
That 3.8% of children aged less than 5 years represents almost 756,000 initial doses. That compares with over 6 million children aged 5-11 years who had received at least one dose through the first 6 weeks of their vaccination experience and over 5 million children aged 12-15, according to the COVID Data Tracker.
Jury out on synbiotics for kids with GI disorders
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
That’s the conclusion of a position paper from the European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) special interest group on gut microbiota and modifications and its working group for pre- and probiotics.
Based on their review of available data, the group could not offer a recommendation on use of any specific synbiotic preparation for treatment of acute gastroenteritis, Helicobacter pylori (H. pylori) infection, inflammatory bowel disease (IBD), infantile colic, functional abdominal pain, irritable bowel syndrome (IBS), or constipation.
No recommendation can be formulated on their use in the prevention of food allergies, the group also says.
The same goes for prevention of necrotizing enterocolitis (NEC) in preterm infants and newborns with cyanotic congenital heart disease, as well as prevention of food allergies.
The position paper was published online in the Journal of Pediatric Gastroenterology and Nutrition.
Few studies, major limitations
A synbiotic mixture comprises probiotics and prebiotics selectively utilized by host microorganisms that confers a health benefit on the host.
While the number of studies evaluating the effect of different synbiotics is increasing, the results to date are “ambiguous,” report first author Iva Hojsak, with Children’s Hospital Zagreb, Croatia, and colleagues. Well-designed studies using the same outcome measures for specific clinical indications are needed to allow comparison between studies, they write.
To gauge their effect on pediatric GI disorders, the group searched the literature for studies in English that compared the use of synbiotics, in all delivery vehicles and formulations, at any dose, with no synbiotic (placebo or no treatment or other interventions).
They found very few studies that addressed the specific indications of interest, ranging from two randomized controlled trials (RCTs) each for infantile colic and IBD to five RCTs for acute gastroenteritis.
There were only two indications (acute gastroenteritis and H. pylori) where two synbiotic preparations were tested.
The studies often included a limited number of participants, had significant methodological biases, scarcely reported on side effects or adverse events, and reported different outcomes, making inter-study comparisons tough.
“Comparison of studies was further limited by the synbiotic preparation used, where dose effect was not assessed,” the group writes. Also, few studies used the same synbiotic preparation for a specific clinical indication or the same amount of live bacteria and prebiotic in the preparation.
The authors made note of the newly stringent recommendations for direct evidence proposed by the International Scientific Association for Probiotics and Prebiotics, which state RCTs need to compare the synergistic synbiotic combination, the substrate alone, the live microorganisms alone, and a control.
Outside experts weigh in
Offering outside perspective, Gail Cresci, PhD, microbiome researcher, department of pediatric gastroenterology, hepatology, and nutrition, Cleveland Clinic Children’s Hospital, said what’s “most notable with this review is that there is an issue with studies that incorporate a prebiotic and probiotic, in that there is much heterogeneity with the probiotic strains and prebiotic substrates that are investigated.”
Dr. Cresci also noted that “both pre- and probiotics have specific mechanisms of action based on the substrate and strain, respectively, so to pull the trials together and analyze as a ‘synbiotic’ treating all the combinations the same is not accurate [and] also limits the ability to do meta-analyses and make recommendations in a position paper.”
Geoffrey Preidis, MD, PhD, spokesperson for the American Gastroenterological Association (AGA), also reviewed the paper for this news organization.
He noted that few studies tested the exact same synbiotic preparation for a given clinical indication.
“For the majority of GI disorders examined in this review, the total number of studies testing a particular synbiotic formulation is exactly one. Clinical recommendations rarely can be made based on the results of a single trial,” said Dr. Preidis, a pediatric gastroenterologist with Texas Children’s Hospital, Houston.
“Perhaps most importantly, most studies do not report safety data as rigorously as these data are reported in pharmaceutical trials, so the risk of side effects could be higher than we think,” he noted.
“Millions of Americans take probiotics. They are the third most commonly used dietary supplement behind vitamins and minerals,” Dr. Preidis added. “Prebiotics and synbiotics also are increasing in popularity. They can be found almost everywhere – in supermarkets, drugstores, health food stores, and online – in pill or powder form and in some foods and beverages.
None of these products have been approved by the [U.S. Food and Drug Administration] to treat, cure, or prevent any disease. In most circumstances, there is not enough clinical evidence to suggest a clear value to be gained for most consumers,” he said.
Dr. Preidis said he agrees with the conclusions of this “thoughtfully written position paper” on whether synbiotics have a role in the management of GI disorders in children.
“Synbiotics should not be given routinely to infants or children to manage GI disorders at this time,” he said in an interview. “Potential beneficial effects are not yet confirmed in multiple well-designed, adequately powered trials that test the same synbiotic combination and dose, measure the same outcomes, and rigorously document all adverse effects.”
This research had no specific funding. Dr. Hojsak received payment/honorarium for lectures from BioGaia, Nutricia, Biocodex, AbelaPharm, Nestle, Abbott, Sandoz, Oktal Pharma, and Takeda. Dr. Cresci and Dr. Preidis report no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM JOURNAL OF PEDIATRIC GASTROENTEROLOGY AND NUTRITION
ACR makes changes to adult, pediatric vaccinations guidance
Patients with rheumatic and musculoskeletal diseases may need additional vaccines or different versions of vaccines they were not previously recommended to receive, according to updated guidelines from the American College of Rheumatology (ACR) on vaccinations for these patients. The new guidelines pertain to routine vaccinations for adults and children and are based on the most current evidence. They include recommendations on whether to hold certain medications before or after vaccination. They do not include recommendations regarding COVID-19 vaccines.
For guidance on COVID-19 vaccine timing and frequency, the ACR directs physicians to the CDC’s recommendations for people with mild or severe immunosuppression and the ACR’s previous clinical guidance summary on the topic, last revised in February 2022. The recommendations in the new guidance differ from ACR’s guidance on COVID-19 vaccines on whether and when to hold immunosuppressive medications when patients receive nonlive vaccines. The new guidelines now align more closely with those of EULAR, the Infectious Diseases Society of America, and the CDC’s recommendations for human papillomavirus (HPV), pneumococcal, and shingles vaccines.
Vaccinations in this population are particularly important because “a leading cause of morbidity and mortality in those with rheumatic diseases is infections, due to the detrimental impact immunosuppression has on the ability for the patient to properly clear the pathogen,” Alfred Kim, MD, PhD, professor of rheumatology at Washington University, St. Louis, told this news organization. While immunosuppressive medications are the most common reason patients with these conditions may have impaired immune function, “some of our patients with autoimmune disease also have a preexisting immunodeficiency that can inherently blunt immune responses to either infection or vaccination,” Dr. Kim explained.
“The authors of the guidelines have done a really nice job of making distinct recommendations based on the mechanism of action of various immunosuppressive medications,” Dr. Kim said. “This helps simplify the process of deciding the timing of vaccination for the health provider, especially for those on multiple immunosuppressives who represent an important proportion of our patients with rheumatic diseases.”
The main change to the guidelines for children, aside from those related to flu vaccination, is in regard to rotavirus vaccination for infants exposed to tumor necrosis factor (TNF) inhibitors or rituximab in utero. Infants prenatally exposed to rituximab should not receive the rotavirus vaccine until they are older than 6 months. Those exposed prenatally to TNF inhibitors should receive the rotavirus vaccine on time, according to the CDC schedule for all infants.
The new rotavirus recommendations follow data showing that immune responses to rotavirus are blunted in those with infliximab exposure, according to Dr. Kim.
“Thus, this poses a serious theoretical risk in newborns with mothers on [a TNF inhibitor] of ineffective clearance of rotavirus infections,” Dr. Kim said in an interview. “While rotavirus infections are quite common with typically self-limiting disease, sometimes requiring hydration to counteract diarrhea-induced dehydration, this can become severe in these newborns that have [a TNF inhibitor] in their system.”
For adults, the ACR issued the following expanded indications for four vaccines for patients currently taking immunosuppressive medication:
- Patients aged 18 and older should receive the recombinant zoster vaccine against shingles.
- For patients aged 27-44 who weren’t previously vaccinated against HPV, the HPV vaccine is “conditionally recommended.”
- Patients younger than 65 should receive the pneumococcal vaccine.
- Patients aged 19-64 are conditionally recommended to receive the high-dose or adjuvanted flu vaccine rather than the regular-dose flu vaccine.
The guidelines also conditionally recommend that all patients aged 65 and older who have rheumatic or musculoskeletal diseases receive the high-dose or adjuvanted flu vaccine, regardless of whether they are taking immunosuppressive medication. Another new conditional recommendation is to give multiple vaccinations to patients on the same day, rather than give individual vaccines on different days.
The guidelines make conditional recommendations regarding flu and nonlive attenuated vaccines for those taking methotrexate, rituximab, or glucocorticoids. Methotrexate should be held for 2 weeks after flu vaccination as long as disease activity allows it, but patients who are taking methotrexate should continue taking it for any other nonlive attenuated vaccinations.
“Non-rheumatology providers, such as general pediatricians and internists, are encouraged to give the influenza vaccination and then consult with the patient’s rheumatology provider about holding methotrexate to avoid a missed vaccination opportunity,” the guidelines state.
Patients taking rituximab should receive the flu vaccine on schedule and continue taking rituximab. However, for these patients, the guidelines recommend to “delay any subsequent rituximab dosing for at least two weeks after influenza vaccination if disease activity allows.”
“Because of the relatively short time period between the rollout of the influenza vaccine and its season, we can’t always wait to time the B-cell depletion dosage,” Dr. Kim said. “Also, it is not always easy to synchronize the patient’s B-cell depletion dosing schedule to the influenza vaccine rollout. Thus, we now just recommend getting the influenza vaccine regardless of the patient’s last B-cell depletion dosage despite its known strong attenuation of optimal immune responses.”
For other nonlive attenuated vaccines, providers should time vaccination for when the next rituximab dose is due and then hold the drug for at least 2 weeks thereafter, providing time for the B cells to mount a response before rituximab depletes B cells again.
Patients taking less than 20 mg of prednisone daily should still receive the flu vaccine and other nonlive attenuated vaccines. Those taking 20 mg or more of prednisone each day should still receive the flu vaccine, but other vaccines should be deferred until their dose of glucocorticoids has been tapered down to less than 20 mg daily.
Patients taking all other immunosuppressive medications should continue taking them for the flu vaccine and other nonlive attenuated vaccinations, but it is conditionally recommended that live attenuated vaccines be deferred. For any patient with a rheumatic and musculoskeletal disease, regardless of disease activity, it is conditionally recommended that all routine nonlive attenuated vaccines be administered.
For live attenuated virus vaccines, the ACR provides a chart on which immunosuppressive medications to hold and for how long. Glucocorticoids, methotrexate, azathioprine, leflunomide, mycophenolate mofetil, calcineurin inhibitors, and oral cyclophosphamide should all be held 4 weeks before and 4 weeks after administration of a live attenuated vaccine. For those taking JAK inhibitors, the medication should be halted 1 week before administration of a live vaccine and should continue to be withheld for 4 weeks after.
For most other biologics, the ACR recommends holding the medication for one dosing interval before the live vaccine and 4 weeks thereafter. The main exception is rituximab, which should be held for 6 months before a live vaccine and then for 4 more weeks thereafter.
For patients receiving intravenous immunoglobulin, the drug should be held for 8-11 months before they are administered a live attenuated vaccine, depending on the dosage, and then 4 weeks after vaccination, regardless of dosage.
To reassure people with rheumatic disease who may have anxiety or concerns about receiving immunizations, whether taking immunosuppressive medication or not, Dr. Kim said it’s important to provide lots of education to patients.
“Fear and emotion have replaced facts, and data as a leading factor in decision-making, as seen with COVID-19,” Dr. Kim said. “The reality is that a small minority of people will have any issues with most vaccines, which include disease flares, adverse events, or acquisition of an autoimmune disease. We are not saying there is zero risk, rather, that the risk is quite small. This is where shared decision-making between the health care provider and the patient must be done effectively to enable the patient to properly weigh risk versus benefit.”
Dr. Kim has relationships with GlaxoSmithKline, Aurinia Pharmaceuticals, Kypha, Pfizer, Alexion Pharmaceuticals, AstraZeneca, Exagen Diagnostics, and Foghorn Therapeutics.
A version of this article first appeared on Medscape.com.
Patients with rheumatic and musculoskeletal diseases may need additional vaccines or different versions of vaccines they were not previously recommended to receive, according to updated guidelines from the American College of Rheumatology (ACR) on vaccinations for these patients. The new guidelines pertain to routine vaccinations for adults and children and are based on the most current evidence. They include recommendations on whether to hold certain medications before or after vaccination. They do not include recommendations regarding COVID-19 vaccines.
For guidance on COVID-19 vaccine timing and frequency, the ACR directs physicians to the CDC’s recommendations for people with mild or severe immunosuppression and the ACR’s previous clinical guidance summary on the topic, last revised in February 2022. The recommendations in the new guidance differ from ACR’s guidance on COVID-19 vaccines on whether and when to hold immunosuppressive medications when patients receive nonlive vaccines. The new guidelines now align more closely with those of EULAR, the Infectious Diseases Society of America, and the CDC’s recommendations for human papillomavirus (HPV), pneumococcal, and shingles vaccines.
Vaccinations in this population are particularly important because “a leading cause of morbidity and mortality in those with rheumatic diseases is infections, due to the detrimental impact immunosuppression has on the ability for the patient to properly clear the pathogen,” Alfred Kim, MD, PhD, professor of rheumatology at Washington University, St. Louis, told this news organization. While immunosuppressive medications are the most common reason patients with these conditions may have impaired immune function, “some of our patients with autoimmune disease also have a preexisting immunodeficiency that can inherently blunt immune responses to either infection or vaccination,” Dr. Kim explained.
“The authors of the guidelines have done a really nice job of making distinct recommendations based on the mechanism of action of various immunosuppressive medications,” Dr. Kim said. “This helps simplify the process of deciding the timing of vaccination for the health provider, especially for those on multiple immunosuppressives who represent an important proportion of our patients with rheumatic diseases.”
The main change to the guidelines for children, aside from those related to flu vaccination, is in regard to rotavirus vaccination for infants exposed to tumor necrosis factor (TNF) inhibitors or rituximab in utero. Infants prenatally exposed to rituximab should not receive the rotavirus vaccine until they are older than 6 months. Those exposed prenatally to TNF inhibitors should receive the rotavirus vaccine on time, according to the CDC schedule for all infants.
The new rotavirus recommendations follow data showing that immune responses to rotavirus are blunted in those with infliximab exposure, according to Dr. Kim.
“Thus, this poses a serious theoretical risk in newborns with mothers on [a TNF inhibitor] of ineffective clearance of rotavirus infections,” Dr. Kim said in an interview. “While rotavirus infections are quite common with typically self-limiting disease, sometimes requiring hydration to counteract diarrhea-induced dehydration, this can become severe in these newborns that have [a TNF inhibitor] in their system.”
For adults, the ACR issued the following expanded indications for four vaccines for patients currently taking immunosuppressive medication:
- Patients aged 18 and older should receive the recombinant zoster vaccine against shingles.
- For patients aged 27-44 who weren’t previously vaccinated against HPV, the HPV vaccine is “conditionally recommended.”
- Patients younger than 65 should receive the pneumococcal vaccine.
- Patients aged 19-64 are conditionally recommended to receive the high-dose or adjuvanted flu vaccine rather than the regular-dose flu vaccine.
The guidelines also conditionally recommend that all patients aged 65 and older who have rheumatic or musculoskeletal diseases receive the high-dose or adjuvanted flu vaccine, regardless of whether they are taking immunosuppressive medication. Another new conditional recommendation is to give multiple vaccinations to patients on the same day, rather than give individual vaccines on different days.
The guidelines make conditional recommendations regarding flu and nonlive attenuated vaccines for those taking methotrexate, rituximab, or glucocorticoids. Methotrexate should be held for 2 weeks after flu vaccination as long as disease activity allows it, but patients who are taking methotrexate should continue taking it for any other nonlive attenuated vaccinations.
“Non-rheumatology providers, such as general pediatricians and internists, are encouraged to give the influenza vaccination and then consult with the patient’s rheumatology provider about holding methotrexate to avoid a missed vaccination opportunity,” the guidelines state.
Patients taking rituximab should receive the flu vaccine on schedule and continue taking rituximab. However, for these patients, the guidelines recommend to “delay any subsequent rituximab dosing for at least two weeks after influenza vaccination if disease activity allows.”
“Because of the relatively short time period between the rollout of the influenza vaccine and its season, we can’t always wait to time the B-cell depletion dosage,” Dr. Kim said. “Also, it is not always easy to synchronize the patient’s B-cell depletion dosing schedule to the influenza vaccine rollout. Thus, we now just recommend getting the influenza vaccine regardless of the patient’s last B-cell depletion dosage despite its known strong attenuation of optimal immune responses.”
For other nonlive attenuated vaccines, providers should time vaccination for when the next rituximab dose is due and then hold the drug for at least 2 weeks thereafter, providing time for the B cells to mount a response before rituximab depletes B cells again.
Patients taking less than 20 mg of prednisone daily should still receive the flu vaccine and other nonlive attenuated vaccines. Those taking 20 mg or more of prednisone each day should still receive the flu vaccine, but other vaccines should be deferred until their dose of glucocorticoids has been tapered down to less than 20 mg daily.
Patients taking all other immunosuppressive medications should continue taking them for the flu vaccine and other nonlive attenuated vaccinations, but it is conditionally recommended that live attenuated vaccines be deferred. For any patient with a rheumatic and musculoskeletal disease, regardless of disease activity, it is conditionally recommended that all routine nonlive attenuated vaccines be administered.
For live attenuated virus vaccines, the ACR provides a chart on which immunosuppressive medications to hold and for how long. Glucocorticoids, methotrexate, azathioprine, leflunomide, mycophenolate mofetil, calcineurin inhibitors, and oral cyclophosphamide should all be held 4 weeks before and 4 weeks after administration of a live attenuated vaccine. For those taking JAK inhibitors, the medication should be halted 1 week before administration of a live vaccine and should continue to be withheld for 4 weeks after.
For most other biologics, the ACR recommends holding the medication for one dosing interval before the live vaccine and 4 weeks thereafter. The main exception is rituximab, which should be held for 6 months before a live vaccine and then for 4 more weeks thereafter.
For patients receiving intravenous immunoglobulin, the drug should be held for 8-11 months before they are administered a live attenuated vaccine, depending on the dosage, and then 4 weeks after vaccination, regardless of dosage.
To reassure people with rheumatic disease who may have anxiety or concerns about receiving immunizations, whether taking immunosuppressive medication or not, Dr. Kim said it’s important to provide lots of education to patients.
“Fear and emotion have replaced facts, and data as a leading factor in decision-making, as seen with COVID-19,” Dr. Kim said. “The reality is that a small minority of people will have any issues with most vaccines, which include disease flares, adverse events, or acquisition of an autoimmune disease. We are not saying there is zero risk, rather, that the risk is quite small. This is where shared decision-making between the health care provider and the patient must be done effectively to enable the patient to properly weigh risk versus benefit.”
Dr. Kim has relationships with GlaxoSmithKline, Aurinia Pharmaceuticals, Kypha, Pfizer, Alexion Pharmaceuticals, AstraZeneca, Exagen Diagnostics, and Foghorn Therapeutics.
A version of this article first appeared on Medscape.com.
Patients with rheumatic and musculoskeletal diseases may need additional vaccines or different versions of vaccines they were not previously recommended to receive, according to updated guidelines from the American College of Rheumatology (ACR) on vaccinations for these patients. The new guidelines pertain to routine vaccinations for adults and children and are based on the most current evidence. They include recommendations on whether to hold certain medications before or after vaccination. They do not include recommendations regarding COVID-19 vaccines.
For guidance on COVID-19 vaccine timing and frequency, the ACR directs physicians to the CDC’s recommendations for people with mild or severe immunosuppression and the ACR’s previous clinical guidance summary on the topic, last revised in February 2022. The recommendations in the new guidance differ from ACR’s guidance on COVID-19 vaccines on whether and when to hold immunosuppressive medications when patients receive nonlive vaccines. The new guidelines now align more closely with those of EULAR, the Infectious Diseases Society of America, and the CDC’s recommendations for human papillomavirus (HPV), pneumococcal, and shingles vaccines.
Vaccinations in this population are particularly important because “a leading cause of morbidity and mortality in those with rheumatic diseases is infections, due to the detrimental impact immunosuppression has on the ability for the patient to properly clear the pathogen,” Alfred Kim, MD, PhD, professor of rheumatology at Washington University, St. Louis, told this news organization. While immunosuppressive medications are the most common reason patients with these conditions may have impaired immune function, “some of our patients with autoimmune disease also have a preexisting immunodeficiency that can inherently blunt immune responses to either infection or vaccination,” Dr. Kim explained.
“The authors of the guidelines have done a really nice job of making distinct recommendations based on the mechanism of action of various immunosuppressive medications,” Dr. Kim said. “This helps simplify the process of deciding the timing of vaccination for the health provider, especially for those on multiple immunosuppressives who represent an important proportion of our patients with rheumatic diseases.”
The main change to the guidelines for children, aside from those related to flu vaccination, is in regard to rotavirus vaccination for infants exposed to tumor necrosis factor (TNF) inhibitors or rituximab in utero. Infants prenatally exposed to rituximab should not receive the rotavirus vaccine until they are older than 6 months. Those exposed prenatally to TNF inhibitors should receive the rotavirus vaccine on time, according to the CDC schedule for all infants.
The new rotavirus recommendations follow data showing that immune responses to rotavirus are blunted in those with infliximab exposure, according to Dr. Kim.
“Thus, this poses a serious theoretical risk in newborns with mothers on [a TNF inhibitor] of ineffective clearance of rotavirus infections,” Dr. Kim said in an interview. “While rotavirus infections are quite common with typically self-limiting disease, sometimes requiring hydration to counteract diarrhea-induced dehydration, this can become severe in these newborns that have [a TNF inhibitor] in their system.”
For adults, the ACR issued the following expanded indications for four vaccines for patients currently taking immunosuppressive medication:
- Patients aged 18 and older should receive the recombinant zoster vaccine against shingles.
- For patients aged 27-44 who weren’t previously vaccinated against HPV, the HPV vaccine is “conditionally recommended.”
- Patients younger than 65 should receive the pneumococcal vaccine.
- Patients aged 19-64 are conditionally recommended to receive the high-dose or adjuvanted flu vaccine rather than the regular-dose flu vaccine.
The guidelines also conditionally recommend that all patients aged 65 and older who have rheumatic or musculoskeletal diseases receive the high-dose or adjuvanted flu vaccine, regardless of whether they are taking immunosuppressive medication. Another new conditional recommendation is to give multiple vaccinations to patients on the same day, rather than give individual vaccines on different days.
The guidelines make conditional recommendations regarding flu and nonlive attenuated vaccines for those taking methotrexate, rituximab, or glucocorticoids. Methotrexate should be held for 2 weeks after flu vaccination as long as disease activity allows it, but patients who are taking methotrexate should continue taking it for any other nonlive attenuated vaccinations.
“Non-rheumatology providers, such as general pediatricians and internists, are encouraged to give the influenza vaccination and then consult with the patient’s rheumatology provider about holding methotrexate to avoid a missed vaccination opportunity,” the guidelines state.
Patients taking rituximab should receive the flu vaccine on schedule and continue taking rituximab. However, for these patients, the guidelines recommend to “delay any subsequent rituximab dosing for at least two weeks after influenza vaccination if disease activity allows.”
“Because of the relatively short time period between the rollout of the influenza vaccine and its season, we can’t always wait to time the B-cell depletion dosage,” Dr. Kim said. “Also, it is not always easy to synchronize the patient’s B-cell depletion dosing schedule to the influenza vaccine rollout. Thus, we now just recommend getting the influenza vaccine regardless of the patient’s last B-cell depletion dosage despite its known strong attenuation of optimal immune responses.”
For other nonlive attenuated vaccines, providers should time vaccination for when the next rituximab dose is due and then hold the drug for at least 2 weeks thereafter, providing time for the B cells to mount a response before rituximab depletes B cells again.
Patients taking less than 20 mg of prednisone daily should still receive the flu vaccine and other nonlive attenuated vaccines. Those taking 20 mg or more of prednisone each day should still receive the flu vaccine, but other vaccines should be deferred until their dose of glucocorticoids has been tapered down to less than 20 mg daily.
Patients taking all other immunosuppressive medications should continue taking them for the flu vaccine and other nonlive attenuated vaccinations, but it is conditionally recommended that live attenuated vaccines be deferred. For any patient with a rheumatic and musculoskeletal disease, regardless of disease activity, it is conditionally recommended that all routine nonlive attenuated vaccines be administered.
For live attenuated virus vaccines, the ACR provides a chart on which immunosuppressive medications to hold and for how long. Glucocorticoids, methotrexate, azathioprine, leflunomide, mycophenolate mofetil, calcineurin inhibitors, and oral cyclophosphamide should all be held 4 weeks before and 4 weeks after administration of a live attenuated vaccine. For those taking JAK inhibitors, the medication should be halted 1 week before administration of a live vaccine and should continue to be withheld for 4 weeks after.
For most other biologics, the ACR recommends holding the medication for one dosing interval before the live vaccine and 4 weeks thereafter. The main exception is rituximab, which should be held for 6 months before a live vaccine and then for 4 more weeks thereafter.
For patients receiving intravenous immunoglobulin, the drug should be held for 8-11 months before they are administered a live attenuated vaccine, depending on the dosage, and then 4 weeks after vaccination, regardless of dosage.
To reassure people with rheumatic disease who may have anxiety or concerns about receiving immunizations, whether taking immunosuppressive medication or not, Dr. Kim said it’s important to provide lots of education to patients.
“Fear and emotion have replaced facts, and data as a leading factor in decision-making, as seen with COVID-19,” Dr. Kim said. “The reality is that a small minority of people will have any issues with most vaccines, which include disease flares, adverse events, or acquisition of an autoimmune disease. We are not saying there is zero risk, rather, that the risk is quite small. This is where shared decision-making between the health care provider and the patient must be done effectively to enable the patient to properly weigh risk versus benefit.”
Dr. Kim has relationships with GlaxoSmithKline, Aurinia Pharmaceuticals, Kypha, Pfizer, Alexion Pharmaceuticals, AstraZeneca, Exagen Diagnostics, and Foghorn Therapeutics.
A version of this article first appeared on Medscape.com.
Underweight in early childhood persists
The association was most pronounced for girls, as well as for children with lower growth rates, write the authors of the prospective Canadian cohort study published in JAMA Network Open.
The findings “highlight the importance of preventing underweight in early life,” because this can have “lasting effects” in later childhood, senior author Jonathon L. Maguire, MD, from St Michael’s Hospital Pediatric Clinic, and the University of Toronto said in an interview.
Methods and results
The study recruited 5,803 healthy children, mean age 4.07 months, between February 2008 and September 2020 during well-child visits at clinics in The Applied Research Group for Kids! (TARGet Kids!) practice-based research network in Canada. The study’s exclusion criteria included a premature birth, or a health condition affecting growth.
The primary outcome of the study was the child’s age- and sex-adjusted weight, also known as the body mass index z score (zBMI), between the ages of 2 and 10 years.
At baseline, a total of 550 children (9.5%) were classified as underweight, based on the World Health Organization definition of zBMI less than –2. Underweight children were more likely to be younger, have lower birth weight, and to report Asian maternal ethnicity, the researchers observed.
The study found that, compared with children with normal weight, those who were underweight in the first 2 years had lower zBMI at ages 5 and 10 years (–0.49 and –0.39 respectively). This meant that at 10 years old, they were a mean of 1.23 kg lighter than 10-year-olds who had been normal weight at age 2 years.
Height-for-age z score (HAZ) was also lower for underweight 2-year-olds (–0.24), making them a mean of 0.68 cm shorter than normal-weight 2-year-olds. This difference was attenuated at age 5 years.
Growth rate modified the association of underweight with both zBMI and HAZ. Among children who were underweight in the first 2 years, those with lower growth rate had lower zBMI at 10 years (–0.64) compared with those with average (–0.38) or high growth rate (0.11). Similarly, children who were underweight and had a lower growth rate at age 2 years also a lower HAZ at age 10 years (–0.12), compared with those with average (0.02) or high growth rates (0.16). These effects were more pronounced in girls.
Increased health risks linked with chronic underweight
This study did not assess the reasons for early underweight, Dr. Maguire commented in an interview. But, he cited challenges with dietary transitions as a possible explanation.
“Considerable dietary changes happen around 2 years of age with increasing diversity of foods as children transition from primarily liquid foods to primarily solid foods,” he noted.
Asked for comment on the study, Colleen Spees, PhD, associate professor in the division of medical dietetics and director of Hope lab at the Ohio State University, Columbus, said that “at age 10, it’s not surprising to see a lower zBMI and height-for-age in those that were underweight at age 2 with poor growth trajectories.”
Although, this is the first study she is aware of to document these findings in a Canadian cohort, “the results align with what we know about low birth weight and underweight infants and children in terms of linear growth trajectories from child stunting studies,” Dr. Spees said.
She said child stunting, which is more common in less developed countries where children have lower birth weights and greater socioeconomic and environmental risk factors, is defined by the WHO as impaired linear growth with adverse functional consequences.
“In short, a chronic underweight status in infants and young children can lead to greater risk of malnutrition, vitamin and mineral deficiencies, decreased immune function, as well as physical growth and development issues,” she said. “Hence, the most recent 2020-2025 Dietary Guidelines for Americans now includes both pregnancy, breastfeeding, and the first 2 years of life (referred to as the “first 1,000 days”) in their recommendations.”
She added that, if caregivers are concerned about their child’s weight, they should consult with their pediatrician to rule out any medical issues. If no medical issues are identified, they should ask for a referral to a pediatric dietitian.
The study was funded by the Canadian Institute of Health Research. Dr Maguire reported receiving grants from the CIHR, Physician Services, Ontario SPOR Support Unit, and Dairy Farmers of Canada during the conduct of the study and nonfinancial support from DDrops outside the submitted work. Other authors of the paper reported receiving grants from various institutions. Dr. Spees reported no relevant disclosures.
The association was most pronounced for girls, as well as for children with lower growth rates, write the authors of the prospective Canadian cohort study published in JAMA Network Open.
The findings “highlight the importance of preventing underweight in early life,” because this can have “lasting effects” in later childhood, senior author Jonathon L. Maguire, MD, from St Michael’s Hospital Pediatric Clinic, and the University of Toronto said in an interview.
Methods and results
The study recruited 5,803 healthy children, mean age 4.07 months, between February 2008 and September 2020 during well-child visits at clinics in The Applied Research Group for Kids! (TARGet Kids!) practice-based research network in Canada. The study’s exclusion criteria included a premature birth, or a health condition affecting growth.
The primary outcome of the study was the child’s age- and sex-adjusted weight, also known as the body mass index z score (zBMI), between the ages of 2 and 10 years.
At baseline, a total of 550 children (9.5%) were classified as underweight, based on the World Health Organization definition of zBMI less than –2. Underweight children were more likely to be younger, have lower birth weight, and to report Asian maternal ethnicity, the researchers observed.
The study found that, compared with children with normal weight, those who were underweight in the first 2 years had lower zBMI at ages 5 and 10 years (–0.49 and –0.39 respectively). This meant that at 10 years old, they were a mean of 1.23 kg lighter than 10-year-olds who had been normal weight at age 2 years.
Height-for-age z score (HAZ) was also lower for underweight 2-year-olds (–0.24), making them a mean of 0.68 cm shorter than normal-weight 2-year-olds. This difference was attenuated at age 5 years.
Growth rate modified the association of underweight with both zBMI and HAZ. Among children who were underweight in the first 2 years, those with lower growth rate had lower zBMI at 10 years (–0.64) compared with those with average (–0.38) or high growth rate (0.11). Similarly, children who were underweight and had a lower growth rate at age 2 years also a lower HAZ at age 10 years (–0.12), compared with those with average (0.02) or high growth rates (0.16). These effects were more pronounced in girls.
Increased health risks linked with chronic underweight
This study did not assess the reasons for early underweight, Dr. Maguire commented in an interview. But, he cited challenges with dietary transitions as a possible explanation.
“Considerable dietary changes happen around 2 years of age with increasing diversity of foods as children transition from primarily liquid foods to primarily solid foods,” he noted.
Asked for comment on the study, Colleen Spees, PhD, associate professor in the division of medical dietetics and director of Hope lab at the Ohio State University, Columbus, said that “at age 10, it’s not surprising to see a lower zBMI and height-for-age in those that were underweight at age 2 with poor growth trajectories.”
Although, this is the first study she is aware of to document these findings in a Canadian cohort, “the results align with what we know about low birth weight and underweight infants and children in terms of linear growth trajectories from child stunting studies,” Dr. Spees said.
She said child stunting, which is more common in less developed countries where children have lower birth weights and greater socioeconomic and environmental risk factors, is defined by the WHO as impaired linear growth with adverse functional consequences.
“In short, a chronic underweight status in infants and young children can lead to greater risk of malnutrition, vitamin and mineral deficiencies, decreased immune function, as well as physical growth and development issues,” she said. “Hence, the most recent 2020-2025 Dietary Guidelines for Americans now includes both pregnancy, breastfeeding, and the first 2 years of life (referred to as the “first 1,000 days”) in their recommendations.”
She added that, if caregivers are concerned about their child’s weight, they should consult with their pediatrician to rule out any medical issues. If no medical issues are identified, they should ask for a referral to a pediatric dietitian.
The study was funded by the Canadian Institute of Health Research. Dr Maguire reported receiving grants from the CIHR, Physician Services, Ontario SPOR Support Unit, and Dairy Farmers of Canada during the conduct of the study and nonfinancial support from DDrops outside the submitted work. Other authors of the paper reported receiving grants from various institutions. Dr. Spees reported no relevant disclosures.
The association was most pronounced for girls, as well as for children with lower growth rates, write the authors of the prospective Canadian cohort study published in JAMA Network Open.
The findings “highlight the importance of preventing underweight in early life,” because this can have “lasting effects” in later childhood, senior author Jonathon L. Maguire, MD, from St Michael’s Hospital Pediatric Clinic, and the University of Toronto said in an interview.
Methods and results
The study recruited 5,803 healthy children, mean age 4.07 months, between February 2008 and September 2020 during well-child visits at clinics in The Applied Research Group for Kids! (TARGet Kids!) practice-based research network in Canada. The study’s exclusion criteria included a premature birth, or a health condition affecting growth.
The primary outcome of the study was the child’s age- and sex-adjusted weight, also known as the body mass index z score (zBMI), between the ages of 2 and 10 years.
At baseline, a total of 550 children (9.5%) were classified as underweight, based on the World Health Organization definition of zBMI less than –2. Underweight children were more likely to be younger, have lower birth weight, and to report Asian maternal ethnicity, the researchers observed.
The study found that, compared with children with normal weight, those who were underweight in the first 2 years had lower zBMI at ages 5 and 10 years (–0.49 and –0.39 respectively). This meant that at 10 years old, they were a mean of 1.23 kg lighter than 10-year-olds who had been normal weight at age 2 years.
Height-for-age z score (HAZ) was also lower for underweight 2-year-olds (–0.24), making them a mean of 0.68 cm shorter than normal-weight 2-year-olds. This difference was attenuated at age 5 years.
Growth rate modified the association of underweight with both zBMI and HAZ. Among children who were underweight in the first 2 years, those with lower growth rate had lower zBMI at 10 years (–0.64) compared with those with average (–0.38) or high growth rate (0.11). Similarly, children who were underweight and had a lower growth rate at age 2 years also a lower HAZ at age 10 years (–0.12), compared with those with average (0.02) or high growth rates (0.16). These effects were more pronounced in girls.
Increased health risks linked with chronic underweight
This study did not assess the reasons for early underweight, Dr. Maguire commented in an interview. But, he cited challenges with dietary transitions as a possible explanation.
“Considerable dietary changes happen around 2 years of age with increasing diversity of foods as children transition from primarily liquid foods to primarily solid foods,” he noted.
Asked for comment on the study, Colleen Spees, PhD, associate professor in the division of medical dietetics and director of Hope lab at the Ohio State University, Columbus, said that “at age 10, it’s not surprising to see a lower zBMI and height-for-age in those that were underweight at age 2 with poor growth trajectories.”
Although, this is the first study she is aware of to document these findings in a Canadian cohort, “the results align with what we know about low birth weight and underweight infants and children in terms of linear growth trajectories from child stunting studies,” Dr. Spees said.
She said child stunting, which is more common in less developed countries where children have lower birth weights and greater socioeconomic and environmental risk factors, is defined by the WHO as impaired linear growth with adverse functional consequences.
“In short, a chronic underweight status in infants and young children can lead to greater risk of malnutrition, vitamin and mineral deficiencies, decreased immune function, as well as physical growth and development issues,” she said. “Hence, the most recent 2020-2025 Dietary Guidelines for Americans now includes both pregnancy, breastfeeding, and the first 2 years of life (referred to as the “first 1,000 days”) in their recommendations.”
She added that, if caregivers are concerned about their child’s weight, they should consult with their pediatrician to rule out any medical issues. If no medical issues are identified, they should ask for a referral to a pediatric dietitian.
The study was funded by the Canadian Institute of Health Research. Dr Maguire reported receiving grants from the CIHR, Physician Services, Ontario SPOR Support Unit, and Dairy Farmers of Canada during the conduct of the study and nonfinancial support from DDrops outside the submitted work. Other authors of the paper reported receiving grants from various institutions. Dr. Spees reported no relevant disclosures.
FROM JAMA NETWORK OPEN
Topical ruxolitinib quickly relieves atopic dermatitis itch in Black patients
“Ruxolitinib cream monotherapy over 8 weeks was associated with rapid and considerable itch relief in Black or African American patients with AD and was well tolerated,” the study authors wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology.
AD can behave differently in different racial groups and can be especially bothersome in Black patients. AD has a prevalence of about 20% in Black children and 5%-10% in Black adults. Black children are roughly twice as likely to be diagnosed with AD, and to have severe AD, than White children, according to the authors.
Lead author Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and colleagues used pooled data from two identically designed phase 3 studies to describe the effects of the cream formulation of the Janus kinase (JAK) 1 and JAK 2 inhibitor ruxolitinib on itch in Black patients.
Topical ruxolitinib (Opzelura), 1.5%, was approved last September for treating AD in non-immunocompromised patients with mild to moderate AD, ages 12 years and older. In July 2022, it was approved for the treatment of nonsegmental vitiligo in the same age group.
FDA approval for AD was based on the results of the TRuE-AD1 and TRuE-AD2 double-blind randomized trials, which enrolled about 1,200 patients over age 12 with AD. These patients included 292 Black teenagers and adults between aged 12-71 years who had AD for 2 years or longer, with an Investigator’s Global Assessment (IGA) score of 2 or 3, with 3%-20% affected body surface area, excluding the scalp.
Of the 292 patients, those in the two treatment groups (n = 231) applied ruxolitinib cream twice a day for 8 weeks (0.75% in 118 patients and 1.5% in 113 patients) and 61 applied the vehicle. They used electronic diaries to record the worst level of itch they had experienced each day, from 0 (no itch) to 10 (worst imaginable itch). The main results were as follows:
- Mean itch numerical rating scale (NRS) scores at baseline were 5.3 and 5.4 for ruxolitinib cream 0.75% and 1.5%, respectively, and 5.7 for vehicle. Within about 12 hours of first application, mean itch NRS scores dropped –0.6 and –0.7 from baseline among those treated with ruxolitinib cream 0.75% and 1.5%, respectively, compared with –0.2 for those on the vehicle. At day 4, the decreases were –1.4 and –1.6 for ruxolitinib cream 0.75% and 1.5%, respectively, versus –0.6 for the vehicle (P = .026 and P = .005, respectively, vs. vehicle).
- At day 2, among the 187 patients with a baseline itch NRS score 4 or higher, more patients achieved 4-point or greater itch NRS improvement: 6.1% and 16.4% for ruxolitinib cream 0.75% and 1.5%, respectively versus 0% for vehicle. At day 7, the differences were 15.9% and 26.6% versus 3%, respectively. And by week 8, they increased to 30.1% and 43.2% versus 17.5% (P = .212 and P = .009), respectively.
- At week 2, 19% of patients in the 0.75% formulation group and 19.4% of patients in the 1.5% formulation group, compared with 5.3% in the vehicle group, reported no days of itch on question 1 of the Patient-Oriented Eczema Measure (POEM) questionnaire that evaluated various aspects of the disease over the previous week. By week 8, the differences grew to 34% and 30.8% versus 12.2%, respectively.
- Adverse events, reported by 14.4% and 22.1% of patients on 0.75% and 1.5% ruxolitinib, respectively, and by 32.8% of patients who received the vehicle, were headaches, upper respiratory tract infection, and application site pain.
Ruxolitinib may be an alternative to systemic immunosuppressives
Asked to comment on the results, Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, N.C., called itch “one of the major life disruptors in atopic dermatitis.”
Providers often assume that patients of different races respond similarly to treatment, but that is not always true, she noted in an email.
“This study proves ruxolitinib’s effectiveness in Black patients, who often have more severe atopic dermatitis signs and symptoms,” said Dr. McMichael, who was not involved in the study. “The fact that atopic dermatitis in patients of color has been singled out to examine efficacy is a great way to show that the findings are not just in those who have thinner plaques and potentially less longstanding thickening of the skin from scratching (lichenification),” she added.
Dr. McMichael welcomed the lack of systemic side effects and quick relief of itch with this treatment, noting that the effect on itch “is rare with other treatments and extremely rare with other topical medications.”
The effect of topical ruxolitinib on pruritus “was interesting and surprising because very few available topical medications can control itch,” she explained. “The strongest topical steroids can help with pruritus, but they have the risk for skin thinning (atrophy),” while topical ruxolitinib is not associated with skin atrophy.
“After topical steroids fail as first-line treatment, it is likely that more patients will be given this topical medication rather than be moved to immunosuppressive systemic medications,” she noted.
All study authors report relevant relationships with Incyte Corporation, which manufactures ruxolitinib and funded the study, and several authors report employment and shareholding interests in the company. Dr. McMichael reports no relevant relationship with the study.
A version of this article first appeared on Medscape.com.
“Ruxolitinib cream monotherapy over 8 weeks was associated with rapid and considerable itch relief in Black or African American patients with AD and was well tolerated,” the study authors wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology.
AD can behave differently in different racial groups and can be especially bothersome in Black patients. AD has a prevalence of about 20% in Black children and 5%-10% in Black adults. Black children are roughly twice as likely to be diagnosed with AD, and to have severe AD, than White children, according to the authors.
Lead author Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and colleagues used pooled data from two identically designed phase 3 studies to describe the effects of the cream formulation of the Janus kinase (JAK) 1 and JAK 2 inhibitor ruxolitinib on itch in Black patients.
Topical ruxolitinib (Opzelura), 1.5%, was approved last September for treating AD in non-immunocompromised patients with mild to moderate AD, ages 12 years and older. In July 2022, it was approved for the treatment of nonsegmental vitiligo in the same age group.
FDA approval for AD was based on the results of the TRuE-AD1 and TRuE-AD2 double-blind randomized trials, which enrolled about 1,200 patients over age 12 with AD. These patients included 292 Black teenagers and adults between aged 12-71 years who had AD for 2 years or longer, with an Investigator’s Global Assessment (IGA) score of 2 or 3, with 3%-20% affected body surface area, excluding the scalp.
Of the 292 patients, those in the two treatment groups (n = 231) applied ruxolitinib cream twice a day for 8 weeks (0.75% in 118 patients and 1.5% in 113 patients) and 61 applied the vehicle. They used electronic diaries to record the worst level of itch they had experienced each day, from 0 (no itch) to 10 (worst imaginable itch). The main results were as follows:
- Mean itch numerical rating scale (NRS) scores at baseline were 5.3 and 5.4 for ruxolitinib cream 0.75% and 1.5%, respectively, and 5.7 for vehicle. Within about 12 hours of first application, mean itch NRS scores dropped –0.6 and –0.7 from baseline among those treated with ruxolitinib cream 0.75% and 1.5%, respectively, compared with –0.2 for those on the vehicle. At day 4, the decreases were –1.4 and –1.6 for ruxolitinib cream 0.75% and 1.5%, respectively, versus –0.6 for the vehicle (P = .026 and P = .005, respectively, vs. vehicle).
- At day 2, among the 187 patients with a baseline itch NRS score 4 or higher, more patients achieved 4-point or greater itch NRS improvement: 6.1% and 16.4% for ruxolitinib cream 0.75% and 1.5%, respectively versus 0% for vehicle. At day 7, the differences were 15.9% and 26.6% versus 3%, respectively. And by week 8, they increased to 30.1% and 43.2% versus 17.5% (P = .212 and P = .009), respectively.
- At week 2, 19% of patients in the 0.75% formulation group and 19.4% of patients in the 1.5% formulation group, compared with 5.3% in the vehicle group, reported no days of itch on question 1 of the Patient-Oriented Eczema Measure (POEM) questionnaire that evaluated various aspects of the disease over the previous week. By week 8, the differences grew to 34% and 30.8% versus 12.2%, respectively.
- Adverse events, reported by 14.4% and 22.1% of patients on 0.75% and 1.5% ruxolitinib, respectively, and by 32.8% of patients who received the vehicle, were headaches, upper respiratory tract infection, and application site pain.
Ruxolitinib may be an alternative to systemic immunosuppressives
Asked to comment on the results, Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, N.C., called itch “one of the major life disruptors in atopic dermatitis.”
Providers often assume that patients of different races respond similarly to treatment, but that is not always true, she noted in an email.
“This study proves ruxolitinib’s effectiveness in Black patients, who often have more severe atopic dermatitis signs and symptoms,” said Dr. McMichael, who was not involved in the study. “The fact that atopic dermatitis in patients of color has been singled out to examine efficacy is a great way to show that the findings are not just in those who have thinner plaques and potentially less longstanding thickening of the skin from scratching (lichenification),” she added.
Dr. McMichael welcomed the lack of systemic side effects and quick relief of itch with this treatment, noting that the effect on itch “is rare with other treatments and extremely rare with other topical medications.”
The effect of topical ruxolitinib on pruritus “was interesting and surprising because very few available topical medications can control itch,” she explained. “The strongest topical steroids can help with pruritus, but they have the risk for skin thinning (atrophy),” while topical ruxolitinib is not associated with skin atrophy.
“After topical steroids fail as first-line treatment, it is likely that more patients will be given this topical medication rather than be moved to immunosuppressive systemic medications,” she noted.
All study authors report relevant relationships with Incyte Corporation, which manufactures ruxolitinib and funded the study, and several authors report employment and shareholding interests in the company. Dr. McMichael reports no relevant relationship with the study.
A version of this article first appeared on Medscape.com.
“Ruxolitinib cream monotherapy over 8 weeks was associated with rapid and considerable itch relief in Black or African American patients with AD and was well tolerated,” the study authors wrote in a poster presented at the annual meeting of the Society for Investigative Dermatology.
AD can behave differently in different racial groups and can be especially bothersome in Black patients. AD has a prevalence of about 20% in Black children and 5%-10% in Black adults. Black children are roughly twice as likely to be diagnosed with AD, and to have severe AD, than White children, according to the authors.
Lead author Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, and colleagues used pooled data from two identically designed phase 3 studies to describe the effects of the cream formulation of the Janus kinase (JAK) 1 and JAK 2 inhibitor ruxolitinib on itch in Black patients.
Topical ruxolitinib (Opzelura), 1.5%, was approved last September for treating AD in non-immunocompromised patients with mild to moderate AD, ages 12 years and older. In July 2022, it was approved for the treatment of nonsegmental vitiligo in the same age group.
FDA approval for AD was based on the results of the TRuE-AD1 and TRuE-AD2 double-blind randomized trials, which enrolled about 1,200 patients over age 12 with AD. These patients included 292 Black teenagers and adults between aged 12-71 years who had AD for 2 years or longer, with an Investigator’s Global Assessment (IGA) score of 2 or 3, with 3%-20% affected body surface area, excluding the scalp.
Of the 292 patients, those in the two treatment groups (n = 231) applied ruxolitinib cream twice a day for 8 weeks (0.75% in 118 patients and 1.5% in 113 patients) and 61 applied the vehicle. They used electronic diaries to record the worst level of itch they had experienced each day, from 0 (no itch) to 10 (worst imaginable itch). The main results were as follows:
- Mean itch numerical rating scale (NRS) scores at baseline were 5.3 and 5.4 for ruxolitinib cream 0.75% and 1.5%, respectively, and 5.7 for vehicle. Within about 12 hours of first application, mean itch NRS scores dropped –0.6 and –0.7 from baseline among those treated with ruxolitinib cream 0.75% and 1.5%, respectively, compared with –0.2 for those on the vehicle. At day 4, the decreases were –1.4 and –1.6 for ruxolitinib cream 0.75% and 1.5%, respectively, versus –0.6 for the vehicle (P = .026 and P = .005, respectively, vs. vehicle).
- At day 2, among the 187 patients with a baseline itch NRS score 4 or higher, more patients achieved 4-point or greater itch NRS improvement: 6.1% and 16.4% for ruxolitinib cream 0.75% and 1.5%, respectively versus 0% for vehicle. At day 7, the differences were 15.9% and 26.6% versus 3%, respectively. And by week 8, they increased to 30.1% and 43.2% versus 17.5% (P = .212 and P = .009), respectively.
- At week 2, 19% of patients in the 0.75% formulation group and 19.4% of patients in the 1.5% formulation group, compared with 5.3% in the vehicle group, reported no days of itch on question 1 of the Patient-Oriented Eczema Measure (POEM) questionnaire that evaluated various aspects of the disease over the previous week. By week 8, the differences grew to 34% and 30.8% versus 12.2%, respectively.
- Adverse events, reported by 14.4% and 22.1% of patients on 0.75% and 1.5% ruxolitinib, respectively, and by 32.8% of patients who received the vehicle, were headaches, upper respiratory tract infection, and application site pain.
Ruxolitinib may be an alternative to systemic immunosuppressives
Asked to comment on the results, Amy J. McMichael, MD, professor of dermatology at Wake Forest University School of Medicine, Winston-Salem, N.C., called itch “one of the major life disruptors in atopic dermatitis.”
Providers often assume that patients of different races respond similarly to treatment, but that is not always true, she noted in an email.
“This study proves ruxolitinib’s effectiveness in Black patients, who often have more severe atopic dermatitis signs and symptoms,” said Dr. McMichael, who was not involved in the study. “The fact that atopic dermatitis in patients of color has been singled out to examine efficacy is a great way to show that the findings are not just in those who have thinner plaques and potentially less longstanding thickening of the skin from scratching (lichenification),” she added.
Dr. McMichael welcomed the lack of systemic side effects and quick relief of itch with this treatment, noting that the effect on itch “is rare with other treatments and extremely rare with other topical medications.”
The effect of topical ruxolitinib on pruritus “was interesting and surprising because very few available topical medications can control itch,” she explained. “The strongest topical steroids can help with pruritus, but they have the risk for skin thinning (atrophy),” while topical ruxolitinib is not associated with skin atrophy.
“After topical steroids fail as first-line treatment, it is likely that more patients will be given this topical medication rather than be moved to immunosuppressive systemic medications,” she noted.
All study authors report relevant relationships with Incyte Corporation, which manufactures ruxolitinib and funded the study, and several authors report employment and shareholding interests in the company. Dr. McMichael reports no relevant relationship with the study.
A version of this article first appeared on Medscape.com.
FROM SID 2022