User login
One in three children fall short of sleep recommendations
Just over one-third of children in the United States get less sleep than recommended, with higher rates occurring among several racial/ethnic and socioeconomic groups, according to a report from the Centers for Disease Control and Prevention.
, Anne G. Wheaton, PhD, and Angelika H. Claussen, PhD, said in the Morbidity and Mortality Weekly Report.
Unlike previous reports, this analysis showed that adolescents were less likely than infants to have short sleep duration, 31.2% vs. 40.3%. These latest data are based on the 2016-2018 editions of the National Survey of Children’s Health, and the “difference might be explained by NSCH’s reliance on parent report rather than self-report with Youth Risk Behavior Surveys,” they suggested.
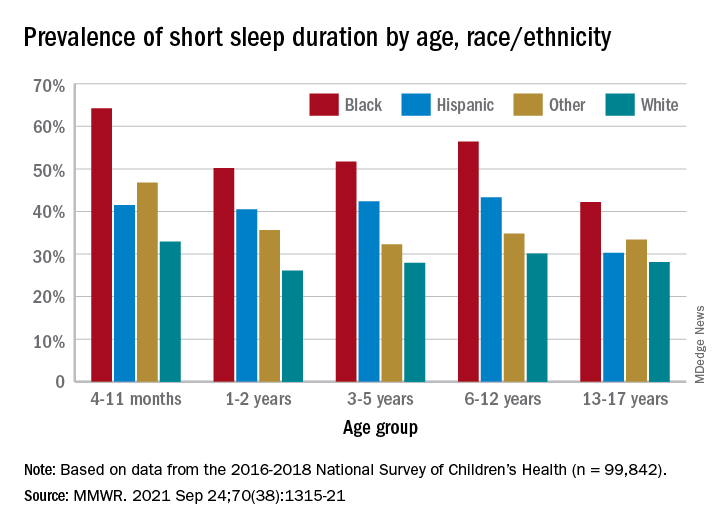
Black children had the highest prevalence of any group included in the study, as parents reported that 50.8% of all ages were not getting the recommended amount of sleep, compared with 39.1% among Hispanics, 34.6% for other races, and 28.8% for Whites. The figure for Black infants was 64.2%, almost double the prevalence for White infants (32.9%), said Dr. Wheaton and Dr. Claussen of the CDC.
Short sleep duration also was more common in children from lower-income families and among those with less educated parents. Geography had an effect as well, with prevalence “highest in the Southeast, similar to geographic variation in adequate sleep observed for adults,” they noted.
Previous research has shown that “sleep disparity was associated with various social determinants of health (e.g., poverty, food insecurity, and perceived racism), which can increase chronic and acute stress and result in environmental and psychological factors that negatively affect sleep duration and can compound long-term health risks,” the investigators wrote.
Short sleep duration by age group was defined as less the following amounts: Twelve hours for infants (4-11 months), 11 hours for children aged 1-2 years, 10 hours for children aged 3-5 years, 9 hours for children aged 6-12, and 8 hours for adolescents (13-17 years), they explained. Responses for the survey’s sleep-duration question totaled 99,842 for the 3 years included.
Just over one-third of children in the United States get less sleep than recommended, with higher rates occurring among several racial/ethnic and socioeconomic groups, according to a report from the Centers for Disease Control and Prevention.

, Anne G. Wheaton, PhD, and Angelika H. Claussen, PhD, said in the Morbidity and Mortality Weekly Report.
Unlike previous reports, this analysis showed that adolescents were less likely than infants to have short sleep duration, 31.2% vs. 40.3%. These latest data are based on the 2016-2018 editions of the National Survey of Children’s Health, and the “difference might be explained by NSCH’s reliance on parent report rather than self-report with Youth Risk Behavior Surveys,” they suggested.
Black children had the highest prevalence of any group included in the study, as parents reported that 50.8% of all ages were not getting the recommended amount of sleep, compared with 39.1% among Hispanics, 34.6% for other races, and 28.8% for Whites. The figure for Black infants was 64.2%, almost double the prevalence for White infants (32.9%), said Dr. Wheaton and Dr. Claussen of the CDC.
Short sleep duration also was more common in children from lower-income families and among those with less educated parents. Geography had an effect as well, with prevalence “highest in the Southeast, similar to geographic variation in adequate sleep observed for adults,” they noted.
Previous research has shown that “sleep disparity was associated with various social determinants of health (e.g., poverty, food insecurity, and perceived racism), which can increase chronic and acute stress and result in environmental and psychological factors that negatively affect sleep duration and can compound long-term health risks,” the investigators wrote.
Short sleep duration by age group was defined as less the following amounts: Twelve hours for infants (4-11 months), 11 hours for children aged 1-2 years, 10 hours for children aged 3-5 years, 9 hours for children aged 6-12, and 8 hours for adolescents (13-17 years), they explained. Responses for the survey’s sleep-duration question totaled 99,842 for the 3 years included.
Just over one-third of children in the United States get less sleep than recommended, with higher rates occurring among several racial/ethnic and socioeconomic groups, according to a report from the Centers for Disease Control and Prevention.

, Anne G. Wheaton, PhD, and Angelika H. Claussen, PhD, said in the Morbidity and Mortality Weekly Report.
Unlike previous reports, this analysis showed that adolescents were less likely than infants to have short sleep duration, 31.2% vs. 40.3%. These latest data are based on the 2016-2018 editions of the National Survey of Children’s Health, and the “difference might be explained by NSCH’s reliance on parent report rather than self-report with Youth Risk Behavior Surveys,” they suggested.
Black children had the highest prevalence of any group included in the study, as parents reported that 50.8% of all ages were not getting the recommended amount of sleep, compared with 39.1% among Hispanics, 34.6% for other races, and 28.8% for Whites. The figure for Black infants was 64.2%, almost double the prevalence for White infants (32.9%), said Dr. Wheaton and Dr. Claussen of the CDC.
Short sleep duration also was more common in children from lower-income families and among those with less educated parents. Geography had an effect as well, with prevalence “highest in the Southeast, similar to geographic variation in adequate sleep observed for adults,” they noted.
Previous research has shown that “sleep disparity was associated with various social determinants of health (e.g., poverty, food insecurity, and perceived racism), which can increase chronic and acute stress and result in environmental and psychological factors that negatively affect sleep duration and can compound long-term health risks,” the investigators wrote.
Short sleep duration by age group was defined as less the following amounts: Twelve hours for infants (4-11 months), 11 hours for children aged 1-2 years, 10 hours for children aged 3-5 years, 9 hours for children aged 6-12, and 8 hours for adolescents (13-17 years), they explained. Responses for the survey’s sleep-duration question totaled 99,842 for the 3 years included.
FROM MMWR
New fellowship, no problem
Using growth mindset to tackle fellowship in a new program
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
Using growth mindset to tackle fellowship in a new program
Using growth mindset to tackle fellowship in a new program
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
Growth mindset is a well-established phenomenon in childhood education that is now starting to appear in health care education literature.1 This concept emphasizes the capacity of individuals to change and grow through experience and that an individual’s basic qualities can be cultivated through hard work, open-mindedness, and help from others.2
Growth mindset opposes the concept of fixed mindset, which implies intelligence or other personal traits are set in stone, unable to be fundamentally changed.2 Individuals with fixed mindsets are less adept at coping with perceived failures and critical feedback because they view these as attacks on their own abilities.2 This oftentimes leads these individuals to avoid potential challenges and feedback because of fear of being exposed as incompetent or feeling inadequate. Conversely, individuals with a growth mindset embrace challenges and failures as learning opportunities and identify feedback as a critical element of growth.2 These individuals maintain a sense of resilience in the face of adversity and strive to become lifelong learners.
As the field of pediatric hospital medicine (PHM) continues to rapidly evolve, so too does the landscape of PHM fellowships. New programs are opening at a torrid pace to accommodate the increasing demand of residents looking to enter the field with new subspecialty accreditation. Most first-year PHM fellows in established programs enter with a clear precedent to follow, set forth by fellows who have come before them. For PHM fellows in new programs, however, there is often no beaten path to follow.
Entering fellowship as a first-year PHM fellow in a new program and blazing one’s own trail can be intriguing and exhilarating given the unique opportunities available. However, the potential challenges for both fellows and program directors during this transition cannot be understated. The role of new PHM fellows within the institutional framework may initially be unclear to others, which can lead to ambiguous expectations and disruptions to normal workflows. Furthermore, assessing and evaluating new fellows may prove difficult as a result of these unclear expectations and general uncertainties. Using the growth mindset can help both PHM fellows and program directors take a deliberate approach to the challenges and uncertainty that may accompany the creation of a new fellowship program.
One of the challenges new PHM fellows may encounter lies within the structure of the care team. Resident and medical student learners may express consternation that the new fellow role may limit their own autonomy. In addition, finding the right balance of autonomy and supervision between the attending-fellow dyad may prove to be difficult. However, using the growth mindset may allow fellows to see the inherent benefits of this new role.
Fellows should seize the opportunity to discuss the nuances and differing approaches to difficult clinical questions, managing a team and interpersonal dynamics, and balancing clinical and nonclinical responsibilities with an experienced supervising clinician; issues that are often less pressing as a resident. The fellow role also affords the opportunity to more carefully observe different clinical styles of practice to subsequently shape one’s own preferred style.
Finally, fellows should employ a growth mindset to optimize clinical time by discussing expectations with involved stakeholders prior to rotations and explicitly identifying goals for feedback and improvement. Program directors can also help stakeholders including faculty, residency programs, medical schools, and other health care professionals on the clinical teams prepare for this transition by providing expectations for the fellow role and by soliciting questions and feedback before and after fellows begin.
One of the key tenets of the growth mindset is actively seeking out constructive feedback and learning from failures to grow and improve. This can be a particularly useful practice for fellows during the course of their scholarly pursuits in clinical research, quality improvement, and medical education. From initial stages of idea development through the final steps of manuscript submission and peer review, fellows will undoubtedly navigate a plethora of challenges and setbacks along the way. Program directors and other core faculty members can promote a growth mindset culture by honestly discussing their own challenges and failures in career endeavors in addition to giving thoughtful constructive feedback.
Fellows should routinely practice explicitly identifying knowledge and skills gaps that represent areas for potential improvement. But perhaps most importantly, fellows must strive to see all feedback and perceived failures not as personal affronts or as commentaries on personal abilities, but rather as opportunities to strengthen their scholarly products and gain valuable experience for future endeavors.
Not all learners will come equipped with a growth mindset. So, what can fellows and program directors in new programs do to develop this practice and mitigate some of the inevitable uncertainty? To begin, program directors should think about how to create cultures of growth and development as the fixed and growth mindsets are not just limited to individuals.3 Program directors can strive to augment this process by committing to solicit feedback for themselves and acknowledging their own vulnerabilities and perceived weaknesses.
Fellows must have early, honest discussions with program directors and other stakeholders about expectations and goals. Program directors should consider creating lists of “must meet” individuals within the institution that can help fellows begin to carve out their roles in the clinical, educational, and research realms. Deliberately crafting a mentorship team that will encourage a commitment to growth and improvement is critical. Seeking out growth feedback, particularly in areas that prove challenging, should become common practice for fellows from the onset.
Most importantly, fellows should reframe uncertainty as opportunity for growth and progression. Seeing oneself as a work in progress provides a new perspective that prioritizes learning and emphasizes improvement potential.
Embodying this approach requires patience and practice. Being part of a newly created fellowship represents an opportunity to learn from personal challenges rather than leaning on the precedent set by previous fellows. And although fellows will often face uncertainty as a part of the novelty within a new program, they can ultimately succeed by practicing the principles of Dweck’s Growth Mindset: embracing challenges and failure as learning experiences, seeking out feedback, and pursuing the opportunities among ambiguity.
Dr. Herchline is a pediatric hospitalist at Cincinnati Children’s Hospital Medical Center and recent fellow graduate of the Children’s Hospital of Philadelphia. During fellowship, he completed a master’s degree in medical education at the University of Pennsylvania. His academic interests include graduate medical education, interprofessional collaboration and teamwork, and quality improvement.
References
1. Klein J et al. A growth mindset approach to preparing trainees for medical error. BMJ Qual Saf. 2017 Sep;26(9):771-4. doi: 10.1136/bmjqs-2016-006416.
2. Dweck C. Mindset: The new psychology of success. New York: Ballantine Books; 2006.
3. Murphy MC, Dweck CS. A culture of genius: How an organization’s lay theory shapes people’s cognition, affect, and behavior. Pers Soc Psychol Bull. 2010 Mar;36(3):283-96. doi: 10.1177/0146167209347380.
Feasibility of a Saliva-Based COVID-19 Screening Program in Abu Dhabi Primary Schools
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.
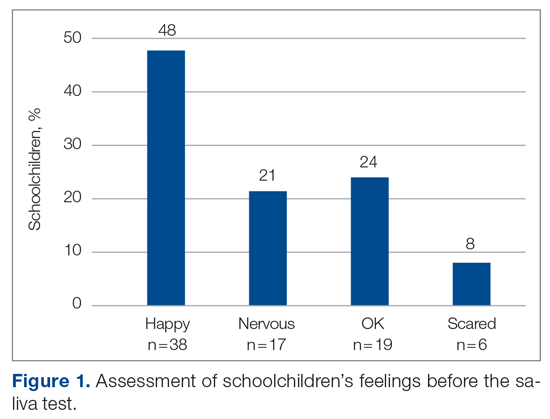
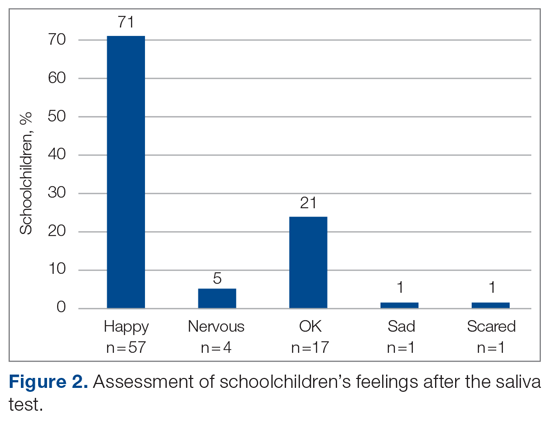
The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.
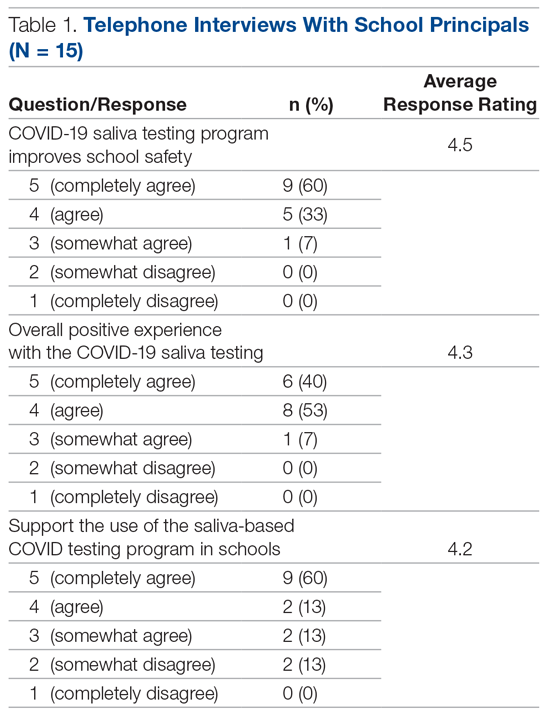
A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).
Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.

The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.

A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).

Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
From Health Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates (Dr. Virji and Aisha Al Hamiz), Public Health, Abu Dhabi Public Health Center, Abu Dhabi, United Arab Emirates (Drs. Al Hajeri, Al Shehhi, Al Memari, and Ahlam Al Maskari), College of Medicine and Health Sciences, Khalifa University, Abu Dhabi, United Arab Emirates, Department of Medicine, Sheikh Shakhbout Medical City, Abu Dhabi, United Arab Emirates (Dr. Alhajri), Public Health Research Center, New York University Abu Dhabi, Abu Dhabi, United Arab Emirates, Oxford University Hospitals NHS Foundation Trust, Oxford, England, and the MRC Epidemiology Unit, University of Cambridge, Cambridge, England (Dr. Ali).
Objective: The pandemic has forced closures of primary schools, resulting in loss of learning time on a global scale. In addition to face coverings, social distancing, and hand hygiene, an efficient testing method is important to mitigate the spread of COVID-19 in schools. We evaluated the feasibility of a saliva-based SARS-CoV-2 polymerase chain reaction testing program among 18 primary schools in the Emirate of Abu Dhabi, United Arab Emirates. Qualitative results show that children 4 to 5 years old had difficulty producing an adequate saliva specimen compared to those 6 to 12 years old.
Methods: A short training video on saliva collection beforehand helps demystify the process for students and parents alike. Informed consent was challenging yet should be done beforehand by school health nurses or other medical professionals to reassure parents and maximize participation.
Results: Telephone interviews with school administrators resulted in an 83% response rate. Overall, 93% of school administrators had a positive experience with saliva testing and felt the program improved the safety of their schools. The ongoing use of saliva testing for SARS-CoV-2 was supported by 73% of respondents.
Conclusion: On-campus saliva testing is a feasible option for primary schools to screen for COVID-19 in their student population to help keep their campuses safe and open for learning.
Keywords: COVID-19; saliva testing; mitigation; primary school.
The COVID-19 pandemic is a leading cause of morbidity and mortality worldwide and continues to exhaust health care resources on a large scale.1 Efficient testing is critical to identify cases early and to help mitigate the deleterious effects of the pandemic.2 Saliva polymerase chain reaction (PCR) nucleic acid amplification testing (NAAT) is more comfortable than nasopharyngeal (NP) NAAT and has been validated as a test for SARS-CoV-2.1 Although children are less susceptible to severe disease, primary schools are considered a vector for transmission and community spread.3 Efficient and scalable methods of routine testing are needed globally to help keep schools open. Saliva testing has proven a useful resource for this population.4,5
Abu Dhabi is the largest Emirate in the United Arab Emirates (UAE), with an estimated population of 2.5 million.6 The first case of COVID-19 was discovered in the UAE on January 29, 2020.7 The UAE has been recognized worldwide for its robust pandemic response. Along with the coordinated and swift application of public health measures, the country has one of the highest COVID-19 testing rates per capita and one of the highest vaccination rates worldwide.8,9 The Abu Dhabi Public Health Center (ADPHC) works alongside the Ministry of Education (MOE) to establish testing, quarantine, and general safety guidelines for primary schools. In December 2020, the ADPHC partnered with a local, accredited diagnostic laboratory to test the feasibility of a saliva-based screening program for COVID-19 directly on school campuses for 18 primary schools in the Emirate.
Saliva-based PCR testing for COVID-19 was approved for use in schools in the UAE on January 24, 2021.10 As part of a greater mitigation strategy to reduce both school-based transmission and, hence, community spread, the ADPHC focused its on-site testing program on children aged 4 to 12 years. The program required collaboration among medical professionals, school administrators and teachers, students, and parents. Our study evaluates the feasibility of implementing a saliva-based COVID-19 screening program directly on primary school campuses involving children as young as 4 years of age.
Methods
The ADPHC, in collaboration with G42 Biogenix Labs, conducted a saliva SARS-CoV-2 NAAT testing program in 18 primary schools in the Emirate. Schools were selected based on outbreak prevalence at the time and focused on “hot spot” areas. The school on-site saliva testing program included children aged 4 to 12 years old in a “bubble” attendance model during the school day. This model involved children being assigned to groups or “pods.” This allowed us to limit a potential outbreak to a single pod, as opposed to risk exposing the entire school, should a single student test positive. The well-established SalivaDirect protocol developed at Yale University was used for testing and included an RNA extraction-free, RT-qPCR method for SARS-CoV-2 detection.11
We conducted a qualitative study involving telephone interviews of school administrators to evaluate their experience with the ADPHC testing program at their schools. In addition, we interviewed the G42 Biogenix Lab providers to understand the logistics that supported on-campus collection of saliva specimens for this age group. We also gathered the attitudes of school children before and after testing. This study was reviewed and approved by the Abu Dhabi Health Research and Technology Committee and the Institutional Review Board (IRB), New York University Abu Dhabi (NYUAD).
Sample and recruitment
The original sample collection of saliva specimens was performed by the ADPHC in collaboration with G42 Biogenix Lab providers on school campuses between December 6 and December 10, 2020. During this time, schools operated in a hybrid teaching model, where learning took place both online and in person. Infection control measures were deployed based on ADPHC standards and guidelines. Nurses utilized appropriate patient protective equipment, frequent hand hygiene, and social distancing during the collection process. Inclusion criteria included asymptomatic students aged 4 to 12 years attending in-person classes on campus. Students with respiratory symptoms who were asked to stay home or those not attending in-person classes were excluded.
Data collection
Data with regard to school children’s attitudes before and after testing were compiled through an online survey sent randomly to participants postintervention. Data from school administrators were collected through video and telephone interviews between April 14 and April 29, 2021. We first interviewed G42 Biogenix Lab providers to obtain previously acquired qualitative and quantitative data, which were collected during the intervention itself. After obtaining this information, we designed a questionnaire and proceeded with a structured interview process for school officials.
We interviewed school principals and administrators to collect their overall experiences with the saliva testing program. Before starting each interview, we established the interviewees preferred language, either English or Arabic. We then introduced the meeting attendees and provided study details, aims, and objectives, and described collaborating entities. We obtained verbal informed consent from a script approved by the NYUAD IRB and then proceeded with the interview, which included 4 questions. The first 3 questions were answered on a 5-point Likert scale model that consisted of 5 answer options: 5 being completely agree, 4 agree, 3 somewhat agree, 2 somewhat disagree, and 1 completely disagree. The fourth question invited open-ended feedback and comments on the following statements:
- I believe the COVID-19 saliva testing program improved the safety for my school campus.
- Our community had an overall positive experience with the COVID saliva testing.
- We would like to continue a saliva-based COVID testing program on our school campus.
- Please provide any additional comments you feel important about the program.
During the interview, we transcribed the answers as the interviewee was answering. We then translated those in Arabic into English and collected the data in 1 Excel spreadsheet. School interviewees and school names were de-identified in the collection and storage process.
Results
A total of 2011 saliva samples were collected from 18 different primary school campuses. Samples were sent the same day to G42 Biogenix Labs in Abu Dhabi for COVID PCR testing. A team consisting of 5 doctors providing general oversight, along with 2 to 6 nurses per site, were able to manage the collection process for all 18 school campuses. Samples were collected between 8
Sample stations were set up in either the school auditorium or gymnasium to ensure appropriate crowd control and ventilation. Teachers and other school staff, including public safety, were able to manage lines and the shuttling of students back and forth from classes to testing stations, which allowed medical staff to focus on sample collection.
Informed consent was obtained by prior electronic communication to parents from school staff, asking them to agree to allow their child to participate in the testing program. Informed consent was identified as a challenge: Getting parents to understand that saliva testing was more comfortable than NP testing, and that the results were only being used to help keep the school safe, took time. School staff are used to obtaining consent from parents for field trips, but this was clearly more challenging for them.

The saliva collection process per child took more time than expected. Children fasted for 45 minutes before saliva collection. We used an active drool technique, which required children to pool saliva in their mouth then express it into a collection tube. Adults can generally do this on command, but we found it took 10 to 12 minutes per child. Saliva production was cued by asking the children to think about food, and by showing them pictures and TV commercials depicting food. Children 4 to 5 years old had more difficulty with the process despite active cueing, while those 6 to 12 years old had an easier time with the process. We collected data on a cohort of 80 children regarding their attitudes pre (Figure 1) and post collection (Figure 2). Children felt happier, less nervous, and less scared after collection than before collection. This trend reassured us that future collections would be easier for students.

A total of 15 of 18 school principals completed the telephone interview, yielding a response rate of 83%. Overall, 93% of the school principals agreed or completely agreed that the COVID-19 saliva testing program improved school safety; 93% agreed or completely agreed that they had an overall positive experience with the program; and 73% supported the ongoing use of saliva testing in their schools (Table 1). Administrators’ open-ended comments on their experience were positive overall (Table 2).

Discussion
By March 2020, many kindergarten to grade 12 public and private schools suspended in-person classes due to the pandemic and turned to online learning platforms. The negative impact of school closures on academic achievement is projected to be significant.7,12,13 Ensuring schools can stay open and run operations safely will require routine SARS-CoV-2 testing. Our study investigated the feasibility of routine saliva testing on children aged 4 to 12 years on their school campuses. The ADPHC school on-site saliva testing program involved bringing lab providers onto 18 primary school campuses and required cooperation among parents, students, school administrators, and health care professionals.

Children younger than 6 years had difficulty producing an adequate saliva specimen, whereas those 6 to 12 years did so with relative ease when cued by thoughts or pictures of food while waiting in line for collection. Schools considering on-site testing programs should consider the age range of 6 to 12 years as a viable age range for saliva screening. Children should fast for a minimum of 45 minutes prior to saliva collection and should be cued by thoughts of food, food pictures, or food commercials. Setting up a sampling station close to the cafeteria where students can smell meal preparation may also help.14,15 Sampling before breakfast or lunch, when children are potentially at their hungriest, should also be considered.
The greatest challenge was obtaining informed consent from parents who were not yet familiar with the reliability of saliva testing as a tool for SARS-CoV-2 screening or with the saliva collection process as a whole. Informed consent was initially done electronically, lacking direct human interaction to answer parents’ questions. Parents who refused had a follow-up call from the school nurse to further explain the logistics and rationale for saliva screening. Having medical professionals directly answer parents’ questions was helpful. Parents were reassured that the process was painless, confidential, and only to be used for school safety purposes. Despite school administrators being experienced in obtaining consent from parents for field trips, obtaining informed consent for a medical testing procedure is more complicated, and parents aren’t accustomed to providing such consent in a school environment. Schools considering on-site testing should ensure that their school nurse or other health care providers are on the front line obtaining informed consent and allaying parents’ fears.
School staff were able to effectively provide crowd control for testing, and children felt at ease being in a familiar environment. Teachers and public safety officers are well-equipped at managing the shuttling of students to class, to lunch, to physical education, and, finally, to dismissal. They were equally equipped at handling the logistics of students to and from testing, including minimizing crowds and helping students feel at ease during the process. This effective collaboration allowed the lab personnel to focus on sample collection and storage, while school staff managed all other aspects of the children’s safety and care.
Conclusion
Overall, school administrators had a positive experience with the testing program, felt the program improved the safety of their schools, and supported the ongoing use of saliva testing for SARS-CoV-2 on their school campuses. Children aged 6 years and older were able to provide adequate saliva samples, and children felt happier and less nervous after the process, indicating repeatability. Our findings highlight the feasibility of an integrated on-site saliva testing model for primary school campuses. Further research is needed to determine the scalability of such a model and whether the added compliance and safety of on-site testing compensates for the potential loss of learning time that testing during school hours would require.
Corresponding author: Ayaz Virji, MD, New York University Abu Dhabi, PO Box 129188, Abu Dhabi, United Arab Emirates; [email protected].
Financial disclosures: None.
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
1. Kuehn BM. Despite improvements, COVID-19’s health care disruptions persist. JAMA. 2021;325(23):2335. doi:10.1001/jama.2021.9134
2. National Institute on Aging. Why COVID-19 testing is the key to getting back to normal. September 4, 2020. Accessed September 8, 2021. https://www.nia.nih.gov/news/why-covid-19-testing-key-getting-back-normal
3. Centers for Disease Control and Prevention. Science brief: Transmission of SARS-CoV-2 in K-12 schools. Updated July 9, 2021. Accessed September 8, 2021. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html
4. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi:10.1001/jamainternmed.2020.8876
5. Al Suwaidi H, Senok A, Varghese R, et al. Saliva for molecular detection of SARS-CoV-2 in school-age children. Clin Microbiol Infect. 2021;27(9):1330-1335. doi:10.1016/j.cmi.2021.02.009
6. Abu Dhabi. Accessed September 8, 2021. https://u.ae/en/about-the-uae/the-seven-emirates/abu-dhabi
7. Alsuwaidi AR, Al Hosani FI, Al Memari S, et al. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol. 2021;50(4):1077-1090. doi:10.1093/ije/dyab077
8. Al Hosany F, Ganesan S, Al Memari S, et al. Response to COVID-19 pandemic in the UAE: a public health perspective. J Glob Health. 2021;11:03050. doi:10.7189/jogh.11.03050
9. Bremmer I. The best global responses to the COVID-19 pandemic, 1 year later. Time Magazine. Updated February 23, 2021. Accessed September 8, 2021. https://time.com/5851633/best-global-responses-covid-19/
10. Department of Health, Abu Dhabi. Laboratory diagnostic test for COVID-19: update regarding saliva-based testing using RT-PCR test. 2021.
11. Vogels C, Brackney DE, Kalinich CC, et al. SalivaDirect: RNA extraction-free SARS-CoV-2 diagnostics. Protocols.io. Accessed September 8, 2021. https://www.protocols.io/view/salivadirect-rna-extraction-free-sars-cov-2-diagno-bh6jj9cn?version_warning=no
12. Education Endowment Foundation. Impact of school closures on the attainment gap: rapid evidence assessment. June 2020. Accessed September 8, 2021. https://www.researchgate.net/publication/342501263_EEF_2020_-_Impact_of_School_Closures_on_the_Attainment_Gap
13. United Nations. Policy brief: Education during COVID-19 and beyond. Accessed September 8, 2021. https://www.un.org/development/desa/dspd/wp-content/uploads/sites/22/2020/08/sg_policy_brief_covid-19_and_education_august_2020.pdf
14. Schiffman SS, Miletic ID. Effect of taste and smell on secretion rate of salivary IgA in elderly and young persons. J Nutr Health Aging. 1999;3(3):158-164.
15. Lee VM, Linden RW. The effect of odours on stimulated parotid salivary flow in humans. Physiol Behav. 1992;52(6):1121-1125. doi:10.1016/0031-9384(92)90470-m
New virus causing ‘Alaskapox’ detected in two more cases
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.
Both people were diagnosed after receiving urgent care in a Fairbanks-area clinic. One was a child with a sore on the left elbow, along with fever and swollen lymph nodes. And the other was an unrelated middle-aged woman with a pox mark on her leg, swollen lymph nodes, and joint pain. In both cases, symptoms improved within 3 weeks.
This isn’t the first time the so-called Alaskapox virus has been detected in the region. In 2015, a woman living near Fairbanks turned up at her doctor’s office with a single reddened pox-like mark on her upper arm and a feeling of fatigue.
Sampling of the pox mark showed that it was caused by a previously unidentified virus of the same family as smallpox and cowpox.
Five years later, another woman showed up with similar signs and symptoms, and her pox also proved to be the result of what public health experts started calling the Alaskapox virus.
In both cases, the women recovered completely.
Smallpox-like illness
Public health sleuths figured out that in three of the four cases, the patients lived in a home with a cat or cats, and one of these cats was known to hunt small animals.
Experts already knew that cats mingling in cow pastures and sickened by cattle virus had helped cowpox make the leap from bovines to humans. And just as in the case of cowpox, they suspected that cats might again be spreading this new virus to people, too.
All four of the infected people lived in sparsely populated areas amid forests. Officials laid animal traps where some of the affected people lived and identified the virus in several species of small wild animals.
The animals that turned up most often with Alaskapox were small mouse-like voles. The rodents with rounded muzzles are known for burrowing in the region. And scientists suspect the Alaskapox virus makes its way from these wild animals to humans through their pet cats or possibly by direct exposure outdoors.
None of the four people identified so far with Alaskapox knew each other or interacted, so officials also suspect that there are more cases going unrecognized, possibly because the symptoms are mild or nonexistent.
There are no documented cases of person-to-person transmission of Alaskapox, according to public health officials monitoring the small number of cases. But other pox viruses can spread by direct contact with skin lesions, so clinicians are recommending that people cover wounds with bandages. Three of the people with Alaskapox mistook their lesions at first for a bite from a spider or insect.
A version of this article first appeared on WebMD.com.
Dopamine and reward: The story of social media
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
How often do you find yourself on social media? The first thing I do when I wake up is check my email and text messages, as well as my Facebook, Snapchat, and Instagram notifications.
Some 150,000 messages are shared on Facebook each minute; 293 million daily active users worldwide were recorded on Snapchat during the second quarter of 2021; 127.2 million monthly active users in the United States are projected to be on Instagram by 2023.
Social media has gained the hearts and wonder of many around the world. It’s absolutely incredible how ingrained it has become in our lives as a medium for creativity, outlet for communication, and platform for information. In fact, these online network tools have now become essential during COVID-19 to ensure productive workflow, keep in touch with our loved ones, and, overall, maintain social capital. Social media has truly emerged as a powerful form of living beyond our physical selves.
Yet, increased (and addictive) social media use is associated with negative health outcomes, especially among adolescents. For example, in a study reporting parent and adolescent accounts of social media use, it was reported that social media use was associated with hyperactivity/impulsivity, depression, anxiety, loneliness, and a fear of missing out. Furthermore, a meta-analysis investigating the relationship between social media use and depressive symptoms among adolescents found a small but significant and positive relationship between the two. However, additional research is required to elucidate this association.
Notwithstanding, the addictive nature of social media has previously been called out as analogous to the addictive nature of gambling. Let’s think about it. Whether you’re on Instagram, TikTok, or a similar platform, you can’t help but scroll from one video to the next. It’s one 5- to 10-second video after the next, and before you know it, you’ve spent the past hour going through random videos – but you can’t stop. Why is that so?
Social media actually “rewires” our brain such that we expect instant gratification. In other words, when we get a notification, message, like, or share, we expect fast and short-term pleasure/reward because the brain will produce a “hit of dopamine.” However, it is important to note that the reward system is not delimited to the dopaminergic pathway and, in fact, should be understood as a complex network system (i.e., governed by changes in brain morphology through addiction and excessive behavior). Given the quick pace of the social media world, the reward pathways in our brain change and there’s an increasing demand for attention, perpetuating an addictive mindset.
When we refresh our page, we expect instant gratification. But what happens when we don’t get a like, or a message, or some sort of “reward”? Recounts of social media use by adolescents have likened online attention to popularity. Accordingly, a lack of constant attention on social media has created a vicious cycle of anxiety, loneliness, and depression because of a failure to receive “virtual” reward. Taken together, social media may be harmful because it distorts our self-image, and while social media platforms help connect us, they can also ironically make us feel isolated, lower our self-confidence, and diminish our overall sense of well-being.
As the platforms for communication and information have evolved so rapidly over the past decade, there is a need to establish boundaries between what is beneficial and what is potentially detrimental to our mental health. While social media companies should play a role in mitigating addictive social network behavior, it would also seem counterintuitive to the general business model. In that case, who takes charge? This multifaceted problem requires a multidisciplinary approach.
Leanna M.W. Lui is an MSc candidate at the University of Toronto.
A version of this article first appeared on Medscape.com.
COVID-19 vaccines in pregnancy may protect baby, too
Women who receive COVID-19 vaccines during pregnancy pass antibodies to their babies, which could protect newborns from the disease, research has shown.
.
Researchers with New York University Langone Health conducted a study that included pregnant women who had received at least one dose of an mRNA COVID-19 vaccine (Pfizer/BioNTech or Moderna) by June 4.
All neonates had antibodies to the spike protein at high titers, the researchers found.
Unlike similar prior studies, the researchers also looked for antibodies to the nucleocapsid protein, which would have indicated the presence of antibodies from natural COVID-19 infection. They did not detect antibodies to the nucleocapsid protein, and the lack of these antibodies suggests that the antibodies to the spike protein resulted from vaccination and not from prior infection, the researchers said.
The participants had a median time from completion of the vaccine series to delivery of 13 weeks. The study was published online in the American Journal of Obstetrics & Gynecology MFM.
“The presence of these anti-spike antibodies in the cord blood should, at least in theory, offer these newborns some degree of protection,” said study investigator Ashley S. Roman, MD, director of the division of maternal-fetal medicine at NYU Langone Health. “While the primary rationale for vaccination during pregnancy is to keep moms healthy and keep moms out of the hospital, the outstanding question to us was whether there is any fetal or neonatal benefit conferred by receiving the vaccine during pregnancy.”
Questions remain about the degree and durability of protection for newborns from these antibodies. An ongoing study, MOMI-VAX, aims to systematically measure antibody levels in mothers who receive COVID-19 vaccines during pregnancy and in their babies over time.
The present study contributes welcome preliminary evidence suggesting a benefit to infants, said Emily Adhikari, MD, of the University of Texas Southwestern Medical Center in Dallas, who was not involved in the study.
Still, “the main concern and our priority as obstetricians is to vaccinate pregnant women to protect them from severe or critical illness,” she said.
Although most individuals infected with SARS-CoV-2 recover, a significant portion of pregnant women get seriously sick, Dr. Adhikari said. “With this recent Delta surge, we are seeing more pregnant patients who are sicker,” said Dr. Adhikari, who has published research from one hospital describing this trend.
When weighing whether patients should receive COVID-19 vaccines in pregnancy, the risks from infection have outweighed any risk from vaccination to such an extent that there is “not a comparison to make,” Dr. Adhikari said. “The risks of the infection are so much higher.
“For me, it is a matter of making sure that my patient understands that we have really good safety data on these vaccines and there is no reason to think that a pregnant person would be harmed by them. On the contrary, the benefit is to protect and maybe even save your life,” Dr. Adhikari said. “And now we have more evidence that the fetus may also benefit.”
The rationale for vaccinations during pregnancy can vary, Dr. Roman said. Flu shots in pregnancy mainly are intended to protect the mother, though they confer protection for newborns as well. With the whooping cough vaccine given in the third trimester, however, the primary aim is to protect the baby from whooping cough in the first months of life, Dr. Roman said.
“I think it is really important for pregnant women to understand that antibodies crossing the placenta is a good thing,” she added.
As patients who already have received COVID-19 vaccines become pregnant and may become eligible for a booster dose, Dr. Adhikari will offer it, she said, though she has confidence in the protection provided by the initial immune response.
Dr. Roman and Dr. Adhikari had no disclosures.
Women who receive COVID-19 vaccines during pregnancy pass antibodies to their babies, which could protect newborns from the disease, research has shown.
.
Researchers with New York University Langone Health conducted a study that included pregnant women who had received at least one dose of an mRNA COVID-19 vaccine (Pfizer/BioNTech or Moderna) by June 4.
All neonates had antibodies to the spike protein at high titers, the researchers found.
Unlike similar prior studies, the researchers also looked for antibodies to the nucleocapsid protein, which would have indicated the presence of antibodies from natural COVID-19 infection. They did not detect antibodies to the nucleocapsid protein, and the lack of these antibodies suggests that the antibodies to the spike protein resulted from vaccination and not from prior infection, the researchers said.
The participants had a median time from completion of the vaccine series to delivery of 13 weeks. The study was published online in the American Journal of Obstetrics & Gynecology MFM.
“The presence of these anti-spike antibodies in the cord blood should, at least in theory, offer these newborns some degree of protection,” said study investigator Ashley S. Roman, MD, director of the division of maternal-fetal medicine at NYU Langone Health. “While the primary rationale for vaccination during pregnancy is to keep moms healthy and keep moms out of the hospital, the outstanding question to us was whether there is any fetal or neonatal benefit conferred by receiving the vaccine during pregnancy.”
Questions remain about the degree and durability of protection for newborns from these antibodies. An ongoing study, MOMI-VAX, aims to systematically measure antibody levels in mothers who receive COVID-19 vaccines during pregnancy and in their babies over time.
The present study contributes welcome preliminary evidence suggesting a benefit to infants, said Emily Adhikari, MD, of the University of Texas Southwestern Medical Center in Dallas, who was not involved in the study.
Still, “the main concern and our priority as obstetricians is to vaccinate pregnant women to protect them from severe or critical illness,” she said.
Although most individuals infected with SARS-CoV-2 recover, a significant portion of pregnant women get seriously sick, Dr. Adhikari said. “With this recent Delta surge, we are seeing more pregnant patients who are sicker,” said Dr. Adhikari, who has published research from one hospital describing this trend.
When weighing whether patients should receive COVID-19 vaccines in pregnancy, the risks from infection have outweighed any risk from vaccination to such an extent that there is “not a comparison to make,” Dr. Adhikari said. “The risks of the infection are so much higher.
“For me, it is a matter of making sure that my patient understands that we have really good safety data on these vaccines and there is no reason to think that a pregnant person would be harmed by them. On the contrary, the benefit is to protect and maybe even save your life,” Dr. Adhikari said. “And now we have more evidence that the fetus may also benefit.”
The rationale for vaccinations during pregnancy can vary, Dr. Roman said. Flu shots in pregnancy mainly are intended to protect the mother, though they confer protection for newborns as well. With the whooping cough vaccine given in the third trimester, however, the primary aim is to protect the baby from whooping cough in the first months of life, Dr. Roman said.
“I think it is really important for pregnant women to understand that antibodies crossing the placenta is a good thing,” she added.
As patients who already have received COVID-19 vaccines become pregnant and may become eligible for a booster dose, Dr. Adhikari will offer it, she said, though she has confidence in the protection provided by the initial immune response.
Dr. Roman and Dr. Adhikari had no disclosures.
Women who receive COVID-19 vaccines during pregnancy pass antibodies to their babies, which could protect newborns from the disease, research has shown.
.
Researchers with New York University Langone Health conducted a study that included pregnant women who had received at least one dose of an mRNA COVID-19 vaccine (Pfizer/BioNTech or Moderna) by June 4.
All neonates had antibodies to the spike protein at high titers, the researchers found.
Unlike similar prior studies, the researchers also looked for antibodies to the nucleocapsid protein, which would have indicated the presence of antibodies from natural COVID-19 infection. They did not detect antibodies to the nucleocapsid protein, and the lack of these antibodies suggests that the antibodies to the spike protein resulted from vaccination and not from prior infection, the researchers said.
The participants had a median time from completion of the vaccine series to delivery of 13 weeks. The study was published online in the American Journal of Obstetrics & Gynecology MFM.
“The presence of these anti-spike antibodies in the cord blood should, at least in theory, offer these newborns some degree of protection,” said study investigator Ashley S. Roman, MD, director of the division of maternal-fetal medicine at NYU Langone Health. “While the primary rationale for vaccination during pregnancy is to keep moms healthy and keep moms out of the hospital, the outstanding question to us was whether there is any fetal or neonatal benefit conferred by receiving the vaccine during pregnancy.”
Questions remain about the degree and durability of protection for newborns from these antibodies. An ongoing study, MOMI-VAX, aims to systematically measure antibody levels in mothers who receive COVID-19 vaccines during pregnancy and in their babies over time.
The present study contributes welcome preliminary evidence suggesting a benefit to infants, said Emily Adhikari, MD, of the University of Texas Southwestern Medical Center in Dallas, who was not involved in the study.
Still, “the main concern and our priority as obstetricians is to vaccinate pregnant women to protect them from severe or critical illness,” she said.
Although most individuals infected with SARS-CoV-2 recover, a significant portion of pregnant women get seriously sick, Dr. Adhikari said. “With this recent Delta surge, we are seeing more pregnant patients who are sicker,” said Dr. Adhikari, who has published research from one hospital describing this trend.
When weighing whether patients should receive COVID-19 vaccines in pregnancy, the risks from infection have outweighed any risk from vaccination to such an extent that there is “not a comparison to make,” Dr. Adhikari said. “The risks of the infection are so much higher.
“For me, it is a matter of making sure that my patient understands that we have really good safety data on these vaccines and there is no reason to think that a pregnant person would be harmed by them. On the contrary, the benefit is to protect and maybe even save your life,” Dr. Adhikari said. “And now we have more evidence that the fetus may also benefit.”
The rationale for vaccinations during pregnancy can vary, Dr. Roman said. Flu shots in pregnancy mainly are intended to protect the mother, though they confer protection for newborns as well. With the whooping cough vaccine given in the third trimester, however, the primary aim is to protect the baby from whooping cough in the first months of life, Dr. Roman said.
“I think it is really important for pregnant women to understand that antibodies crossing the placenta is a good thing,” she added.
As patients who already have received COVID-19 vaccines become pregnant and may become eligible for a booster dose, Dr. Adhikari will offer it, she said, though she has confidence in the protection provided by the initial immune response.
Dr. Roman and Dr. Adhikari had no disclosures.
FROM AMERICAN JOURNAL OF OBSTETRICS & GYNECOLOGY MFM
Pandemic affected home life of nearly 70% of female physicians with children
The survey, conducted by the Robert Graham Center and the American Board of Family Medicine from May to June 2020, examined the professional and personal experiences of being a mother and a primary care physician during the pandemic.
“The pandemic was hard for everyone, but for women who had children in the home, and it didn’t really matter what age, it seemed like the emotional impact was much harder,” study author Yalda Jabbarpour, MD, said in an interview.
The results of the survey of 89 female physicians who worked in the primary care specialty were published in the Journal of Mother Studies.
Dr. Jabbapour and her colleagues found that 67% of female physicians with children said the pandemic had a great “impact” on their home life compared with 25% of those without children. Furthermore, 41% of physician moms said COVID-19 greatly affected their work life, as opposed to 17% of their counterparts without children.
“Women are going into medicine at much higher rates. In primary care, it’s becoming close to the majority,” said Dr. Jabbarpour, a family physician and medical director of the Robert Graham Center for Policy Studies. “That has important workforce implications. If we’re not supporting our female physicians and they are greater than 50% of the physician workforce and they’re burning out, who’s going to have a doctor anymore?”
Child care challenges
Researchers found that the emotional toll female physicians experienced early on in the pandemic was indicative of the challenges they were facing. Some of those challenges included managing anxiety, increased stress from both work and home, and social isolation from friends and family.
Another challenge physician mothers had to deal with was fulfilling child care and homeschooling needs, as many women didn’t know what to do with their children and didn’t have external support from their employers.
Child care options vanished for many people during the pandemic, Emily Kaye, MD, MPH, who was not involved in the study, said in an interview.
“I think it was incredibly challenging for everyone and uniquely challenging for women who were young mothers, specifically with respect to child care” said Dr. Kaye, assistant professor in the department of oncology at St. Jude Children’s Research Hospital. “Many women were expected to just continue plugging on in the absence of any reasonable or safe form of child care.”
Some of the changes physician-mothers said they were required to make at home or in their personal lives included physical changes related to their family safety, such as decontaminating themselves in their garages before heading home after a shift. Some also reported that they had to find new ways to maintain emotional and mental health because of social isolation from family and friends.
The survey results, which were taken early on in the pandemic, highlight the need for health policies that support physician mothers and families, as women shoulder the burden of parenting and domestic responsibilities in heterosexual relationships, the researchers said.
“I’m hoping that people pay attention and start to implement more family friendly policies within their workplaces,” Dr. Jabbarpour said. “But during a pandemic, it was essential for [female health care workers] to go in, and they had nowhere to put their kids. [Therefore], the choice became leaving young children alone at home, putting them into daycare facilities that did remain open without knowing if they were [safe], or quitting their jobs. None of those choices are good.”
Community support as a potential solution
Dr. Kaye said she believes that there should be a “long overdue investment” in community support, affordable and accessible child care, flexible spending, paid family leave, and other forms of caregiving support.
“In order to keep women physicians in the workforce, we need to have a significant increase in investment in the social safety net in this country,” Dr. Kaye said.
Researchers said more studies should evaluate the role the COVID-19 pandemic had on the primary care workforce in the U.S., “with a specific emphasis on how the pandemic impacted mothers, and should more intentionally consider the further intersections of race and ethnicity in the experiences of physician-mothers.”
“I think people are burning out and then there’s all this anti-science, anti-health sentiment out there, which makes it harder,” Dr. Jabbarpour said. “If we did repeat this study now, I think things would be even more dire in the voices of the women that we heard.”
Dr. Jabbarpour and Dr. Kaye reported no disclosures.
The survey, conducted by the Robert Graham Center and the American Board of Family Medicine from May to June 2020, examined the professional and personal experiences of being a mother and a primary care physician during the pandemic.
“The pandemic was hard for everyone, but for women who had children in the home, and it didn’t really matter what age, it seemed like the emotional impact was much harder,” study author Yalda Jabbarpour, MD, said in an interview.
The results of the survey of 89 female physicians who worked in the primary care specialty were published in the Journal of Mother Studies.
Dr. Jabbapour and her colleagues found that 67% of female physicians with children said the pandemic had a great “impact” on their home life compared with 25% of those without children. Furthermore, 41% of physician moms said COVID-19 greatly affected their work life, as opposed to 17% of their counterparts without children.
“Women are going into medicine at much higher rates. In primary care, it’s becoming close to the majority,” said Dr. Jabbarpour, a family physician and medical director of the Robert Graham Center for Policy Studies. “That has important workforce implications. If we’re not supporting our female physicians and they are greater than 50% of the physician workforce and they’re burning out, who’s going to have a doctor anymore?”
Child care challenges
Researchers found that the emotional toll female physicians experienced early on in the pandemic was indicative of the challenges they were facing. Some of those challenges included managing anxiety, increased stress from both work and home, and social isolation from friends and family.
Another challenge physician mothers had to deal with was fulfilling child care and homeschooling needs, as many women didn’t know what to do with their children and didn’t have external support from their employers.
Child care options vanished for many people during the pandemic, Emily Kaye, MD, MPH, who was not involved in the study, said in an interview.
“I think it was incredibly challenging for everyone and uniquely challenging for women who were young mothers, specifically with respect to child care” said Dr. Kaye, assistant professor in the department of oncology at St. Jude Children’s Research Hospital. “Many women were expected to just continue plugging on in the absence of any reasonable or safe form of child care.”
Some of the changes physician-mothers said they were required to make at home or in their personal lives included physical changes related to their family safety, such as decontaminating themselves in their garages before heading home after a shift. Some also reported that they had to find new ways to maintain emotional and mental health because of social isolation from family and friends.
The survey results, which were taken early on in the pandemic, highlight the need for health policies that support physician mothers and families, as women shoulder the burden of parenting and domestic responsibilities in heterosexual relationships, the researchers said.
“I’m hoping that people pay attention and start to implement more family friendly policies within their workplaces,” Dr. Jabbarpour said. “But during a pandemic, it was essential for [female health care workers] to go in, and they had nowhere to put their kids. [Therefore], the choice became leaving young children alone at home, putting them into daycare facilities that did remain open without knowing if they were [safe], or quitting their jobs. None of those choices are good.”
Community support as a potential solution
Dr. Kaye said she believes that there should be a “long overdue investment” in community support, affordable and accessible child care, flexible spending, paid family leave, and other forms of caregiving support.
“In order to keep women physicians in the workforce, we need to have a significant increase in investment in the social safety net in this country,” Dr. Kaye said.
Researchers said more studies should evaluate the role the COVID-19 pandemic had on the primary care workforce in the U.S., “with a specific emphasis on how the pandemic impacted mothers, and should more intentionally consider the further intersections of race and ethnicity in the experiences of physician-mothers.”
“I think people are burning out and then there’s all this anti-science, anti-health sentiment out there, which makes it harder,” Dr. Jabbarpour said. “If we did repeat this study now, I think things would be even more dire in the voices of the women that we heard.”
Dr. Jabbarpour and Dr. Kaye reported no disclosures.
The survey, conducted by the Robert Graham Center and the American Board of Family Medicine from May to June 2020, examined the professional and personal experiences of being a mother and a primary care physician during the pandemic.
“The pandemic was hard for everyone, but for women who had children in the home, and it didn’t really matter what age, it seemed like the emotional impact was much harder,” study author Yalda Jabbarpour, MD, said in an interview.
The results of the survey of 89 female physicians who worked in the primary care specialty were published in the Journal of Mother Studies.
Dr. Jabbapour and her colleagues found that 67% of female physicians with children said the pandemic had a great “impact” on their home life compared with 25% of those without children. Furthermore, 41% of physician moms said COVID-19 greatly affected their work life, as opposed to 17% of their counterparts without children.
“Women are going into medicine at much higher rates. In primary care, it’s becoming close to the majority,” said Dr. Jabbarpour, a family physician and medical director of the Robert Graham Center for Policy Studies. “That has important workforce implications. If we’re not supporting our female physicians and they are greater than 50% of the physician workforce and they’re burning out, who’s going to have a doctor anymore?”
Child care challenges
Researchers found that the emotional toll female physicians experienced early on in the pandemic was indicative of the challenges they were facing. Some of those challenges included managing anxiety, increased stress from both work and home, and social isolation from friends and family.
Another challenge physician mothers had to deal with was fulfilling child care and homeschooling needs, as many women didn’t know what to do with their children and didn’t have external support from their employers.
Child care options vanished for many people during the pandemic, Emily Kaye, MD, MPH, who was not involved in the study, said in an interview.
“I think it was incredibly challenging for everyone and uniquely challenging for women who were young mothers, specifically with respect to child care” said Dr. Kaye, assistant professor in the department of oncology at St. Jude Children’s Research Hospital. “Many women were expected to just continue plugging on in the absence of any reasonable or safe form of child care.”
Some of the changes physician-mothers said they were required to make at home or in their personal lives included physical changes related to their family safety, such as decontaminating themselves in their garages before heading home after a shift. Some also reported that they had to find new ways to maintain emotional and mental health because of social isolation from family and friends.
The survey results, which were taken early on in the pandemic, highlight the need for health policies that support physician mothers and families, as women shoulder the burden of parenting and domestic responsibilities in heterosexual relationships, the researchers said.
“I’m hoping that people pay attention and start to implement more family friendly policies within their workplaces,” Dr. Jabbarpour said. “But during a pandemic, it was essential for [female health care workers] to go in, and they had nowhere to put their kids. [Therefore], the choice became leaving young children alone at home, putting them into daycare facilities that did remain open without knowing if they were [safe], or quitting their jobs. None of those choices are good.”
Community support as a potential solution
Dr. Kaye said she believes that there should be a “long overdue investment” in community support, affordable and accessible child care, flexible spending, paid family leave, and other forms of caregiving support.
“In order to keep women physicians in the workforce, we need to have a significant increase in investment in the social safety net in this country,” Dr. Kaye said.
Researchers said more studies should evaluate the role the COVID-19 pandemic had on the primary care workforce in the U.S., “with a specific emphasis on how the pandemic impacted mothers, and should more intentionally consider the further intersections of race and ethnicity in the experiences of physician-mothers.”
“I think people are burning out and then there’s all this anti-science, anti-health sentiment out there, which makes it harder,” Dr. Jabbarpour said. “If we did repeat this study now, I think things would be even more dire in the voices of the women that we heard.”
Dr. Jabbarpour and Dr. Kaye reported no disclosures.
FROM JOURNAL OF MOTHER STUDIES
When children and teens with cancer get COVID-19
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Youth e-cigarette use: Assessing for, and halting, the hidden habit
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23
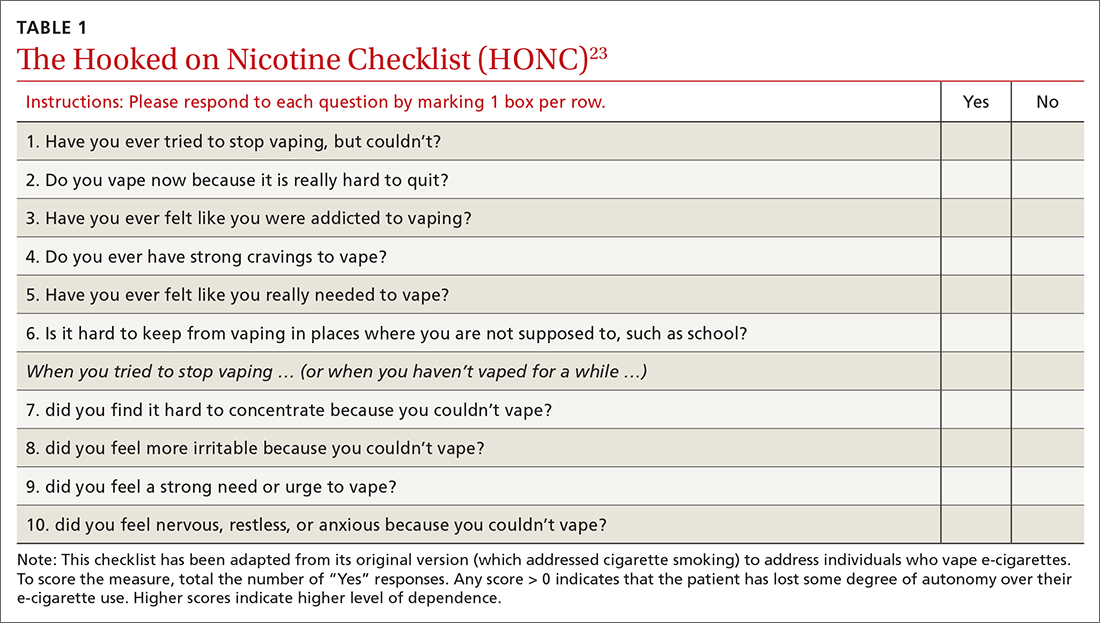
The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23

The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
THE CASE
Joe, an 18-year-old, has been your patient for many years and has an uncomplicated medical history. He presents for his preparticipation sports examination for the upcoming high school baseball season. Joe’s mother, who arrives at the office with him, tells you she’s worried because she found an e-cigarette in his backpack last week. Joe says that many of the kids at his school vape and he tried it a while back and now vapes “a lot.”
After talking further with Joe, you realize that he is vaping every day, using a 5% nicotine pod. Based on previous consults with the behavioral health counselor in your clinic, you know that this level of vaping is about the same as smoking 1 pack of cigarettes per day in terms of nicotine exposure. Joe states that he often vapes in the bathroom at school because he cannot concentrate in class if he doesn’t vape. He also reports that he had previously used 1 pod per week but had recently started vaping more to help with his cravings.
You assess his withdrawal symptoms and learn that he feels on edge when he is not able to vape and that he vapes heavily before going into school because he knows he will not be able to vape again until his third passing period.
●
Electronic cigarettes (e-cigarettes; also called “vapes”) are electronic nicotine delivery systems that heat and aerosolize e-liquid or “e-juice” that is inhaled by the user. The e-liquid is made up primarily of propylene glycol, vegetable glycerin, and flavorings, and often includes nicotine. Nicotine levels in e-cigarettes can range from 0 mg/mL to 60 mg/mL (regular cigarettes contain ~12 mg of nicotine). The nicotine level of the pod available from e-cigarette company JUUL (50 mg/mL e-liquid) is equivalent to about 1 pack of cigarettes.1 E-cigarette devices are relatively affordable; popular brands cost $10 to $20, while the replacement pods or e-liquid are typically about $4 each.
The e-cigarette market is quickly evolving and diversifying. Originally, e-cigarettes looked similar to cigarettes (cig-a-likes) but did not efficiently deliver nicotine to the user.2 E-cigarettes have evolved and some now deliver cigarette-like levels of nicotine to the user.3,4 Youth and young adults primarily use pod-mod e-cigarettes, which have a sleek design and produce less vapor than older e-cigarettes, making them easier to conceal. They can look like a USB flash-drive or have a teardrop shape. Pod-mod e-cigarettes dominate the current market, led by companies such as JUUL, NJOY, and Vuse.5
E-cigarette use is proliferating in the United States, particularly among young people and facilitated by the introduction of pod-based e-cigarettes in appealing flavors.6,7 While rates of current e-cigarette use by US adults is around 5.5%,8 recent data show that 32.7% of US high school students say they’ve used an e-cigarette in the past 30 days.9
Continue to: A double-edged sword
A double-edged sword. E-cigarettes are less harmful than traditional cigarettes in the short term and likely benefit adult smokers who completely substitute e-cigarettes for their tobacco cigarettes.10 In randomized trials of adult smokers, e-cigarette use resulted in moderate combustible-cigarette cessation rates that rival or exceed rates achieved with traditional nicotine replacement therapy (NRT).11-13 However, most e-cigarettes contain addictive nicotine, can facilitate transitions to more harmful forms of tobacco use,10,14,15 and have unknown long-term health effects. Therefore, youth, young adults, and those who are otherwise tobacco naïve should not initiate e-cigarette use.
Moreover, cases of e-cigarette or vaping product use–associated lung injury (EVALI)—a disease linked to vaping that causes cough, fever, shortness of breath, and death—were first identified in August 2019 and peaked in September 2019 before new cases decreased dramatically through January 2020.16 Since the initial cases of EVALI arose, product testing has shown that tetrahydrocannabinol (THC) and vitamin E acetate are the main ingredients linked to EVALI cases.17 For this reason, the Centers for Disease Control and Prevention and others strongly recommend against use of THC-containing e-cigarettes.18
Given the high rates of e-cigarette use among youth and young adults and its potential health harms, it is critical to inquire about e-cigarette use at primary care visits, and, as appropriate, to assess frequency and quantity of use. Patients who require intervention will be more likely to succeed in quitting if they are connected with behavioral health counseling and prescribed medication. This article offers evidence-based guidance to assess and advise teens and young adults regarding the potential health impact of e-cigarettes.
A NEW ICD-10-CM CODE AND A BRIEF ASSESSMENT TOOL
According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)19 and the International Classification of Diseases, 10th Revision (ICD-10-CM),20 a tobacco use disorder is a problematic pattern of use leading to clinically significant impairment or distress. Associated features and behavioral markers of frequency and quantity include use within 30 minutes of waking, daily use, and increasing use. However, with youth, consider intervention for use of any nicotine or tobacco product, including e-cigarettes, regardless of whether it meets the threshold for diagnosis.21
The new code.
Continue to: As with other tobacco use...
As with other tobacco use, assess e-cigarette use patterns by asking questions about the frequency, duration, and quantity of use. Additionally, determine the level of nicotine in the e-liquid (discussed earlier) and evaluate whether the individual displays signs of physiologic dependence (eg, failed attempts to reduce or quit e-cigarette use, increased use, nicotine withdrawal symptoms).
A useful assessment tool. While e-cigarette use is not often included on current substance use screening measures, the above questions can be added to the end of measures such as the CRAFFT (Car-Relax-Alone-Forget-Family and Friends-Trouble) test.22 Additionally, if an adolescent reports vaping, the American Academy of Pediatrics (AAP) recommends using a brief screening tool such as the Hooked on Nicotine Checklist (HONC) to establish his or her level of dependence (TABLE 1).23

The HONC is ideal for a primary care setting because it is brief and has a high level of sensitivity, minimizing false-negative reports24; a patient’s acknowledgement of any item indicates a loss of autonomy over nicotine. Establishing the level of nicotine dependence is particularly pertinent when making decisions regarding the course of treatment and whether to prescribe NRT (eg, nicotine patch, gum, lozenge). Alternatively, you can quickly assess level of dependence by determining the time to first e-cigarette use in the morning. Tobacco guidelines suggest that if time to first use is > 30 minutes, the individual is “moderately dependent”; if time to first use is < 30 minutes after waking, the individual is “severely dependent.”25
COMBINATION TREATMENT IS MOST SUCCESSFUL
Studies have shown that the most effective treatment for tobacco cessation is pairing behavioral treatment with combination NRT (eg, nicotine gum + patch).25,26 The literature on e-cigarette cessation remains in its infancy, but techniques from traditional smoking cessation can be applied because the behaviors differ only in their mode of nicotine delivery.
Behavioral treatment. There are several options for behavioral treatment for tobacco cessation—and thus, e-cigarette cessation. The first step will depend on the patient’s level of motivation. If the patient is not yet ready to quit, consider using brief motivational interviewing. Once the patient is willing to engage in treatment, options include setting a mutually agreed upon quit date or planning for a reduction in the frequency and duration of vaping.
Continue to: Referrals to the Quitline...
Referrals to the Quitline (800-QUIT-NOW) have long been standard practice and can be used to extend primary care treatment.25 Studies show that it is more effective to connect patients directly to the Quitline at their primary care appointment27 than asking them to call after the visit.28,29 We suggest providing direct assistance in the office to patients as they initiate treatment with the Quitline.
Finally, if the level of dependence is severe or the patient is not motivated to quit, connect them with a behavioral health provider in your clinic or with an outside therapist skilled in cognitive behavioral techniques related to tobacco cessation. Discuss with the patient that quitting nicotine use is difficult for many people and that the best option for success is the combination of counseling and medication.25
Nicotine replacement therapy for e-cigarette use. While over-the-counter NRT (nicotine gum, patches, lozenges) is approved by the US Food and Drug Administration only for sale to adults ≥ 18 years, the AAP issued guidance on prescribing NRT for those < 18 years who use e-cigarettes.30 While the AAP does not suggest a lower age limit for prescribing NRT, national data show that < 6% of middle schoolers report e-cigarette use and that e-cigarette use does not become common (~20% current use) until high school.31 It is therefore unlikely that a child < 14 years would require pharmacotherapy. On their fact sheet, the AAP includes the following guidance:
“Patients who are motivated to quit should use as much safe, FDA-approved NRT as needed to avoid smoking or vaping. When assessing a patient’s current level of nicotine use, it may be helpful to understand that using one JUUL pod per day is equivalent to one pack of cigarettes per day …. Pediatricians and other healthcare providers should work with each patient to determine a starting dosage of NRT that is most likely to help them quit successfully. Dosing is based on the patient’s level of nicotine dependence, which can be measured using a screening tool” (TABLE 123).32
The AAP NRT dosing guidelines can be found at downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf.32 Of note, the dosing guidelines for adolescents are the same as those for adults and are based on level of use and dependence. Moreover, the clinician and patient should work together to choose the initial dose and the plan for weaning NRT over time.
Continue to: THE CASE
Based on your conversation with Joe, you administer the HONC screening tool. He scores 9 out of 10, indicating significant loss of autonomy over nicotine. You consult with a behavioral health counselor, who believes that Joe would benefit from counseling and NRT. You discuss this treatment plan with Joe, who says he is ready to quit because he does not like feeling as if he depends on vaping. Your shared decision is to start the 21-mg patch and 4-mg gum with plans to step down from there.
Joe agrees to set a quit date in the following week. The behavioral health counselor then meets with Joe and they develop a quit plan, which is shared with you so you can follow up at the next visit. Joe also agrees to talk with his parents, who are unaware of his level of use and dependence. Everyone agrees on the quit plan, and a follow-up visit is scheduled.
At the follow-up visit 1 month later, Joe and his parents report that he has quit vaping but is still using the patch and gum. You instruct Joe to reduce his NRT use to the 14-mg patch and 2-mg gum and to stop using them over the next 2 to 3 weeks. Everyone is in agreement with the treatment plan. You also re-administer the HONC screening tool and see that Joe’s score has reduced by 7 points to just 2 out of 10. You recommend that Joe continue to see the behavioral health counselor and follow up as needed. (A noted benefit of having a behavioral health counselor in your clinic is the opportunity for informal briefings on patient progress.33,34)
Following each visit with Joe, you make sure to complete documentation on (1) tobacco/e-cigarette use assessment, (2) diagnoses, (3) discussion of benefits of quitting,(4) assessment of readiness to quit, (5) creation and support of a quit plan, and (6) connection with a behavioral health counselor and planned follow-up. (See TABLE 235 for details onbilling codes.)

CORRESPONDENCE
Eleanor L. S. Leavens, PhD, 3901 Rainbow Boulevard, Mail Stop 1008, Kansas City, KS 66160; [email protected]
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
1. Prochaska JJ, Vogel EA, Benowitz N. Nicotine delivery and cigarette equivalents from vaping a JUULpod. Tob Control. Published online March 24, 2021. doi: 10.1136/tobaccocontrol- 2020-056367
2. Rüther T, Hagedorn D, Schiela K, et al. Nicotine delivery efficiency of first-and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191-198. doi: 10.1016/j.ijheh.2017.10.012
3. Hajek P, Pittaccio K, Pesola F, et al. Nicotine delivery and users’ reactions to Juul compared with cigarettes and other e‐cigarette products. Addiction. 2020;115:1141-1148. doi: 10.1111/add.14936
4. Wagener TL, Floyd EL, Stepanov I, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob control. 2017;26:e23-e28. doi: 10.1136/tobaccocontrol-2016-053041
5. Herzog B, Kanada P. Nielsen: Tobacco all channel data thru 8/11 - cig vol decelerates. Published August 21, 2018. Accessed August 19, 2021. https://athra.org.au/wp-content/uploads/2018/09/Wells-Fargo-Nielsen-Tobacco-All-Channel-Report-Period-Ending-8.11.18.pdf
6. Harrell MB, Weaver SR, Loukas A, et al. Flavored e-cigarette use: characterizing youth, young adult, and adult users. Prev Med Rep. 2017;5:33-40. doi: 10.1016/j.pmedr.2016.11.001
7. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PloS One. 2018;13:e0189015. doi: 10.1371/journal.pone.0189015
8. Obisesan OH, Osei AD, Iftekhar Uddin SM, et al. Trends in e-cigarette use in adults in the United States, 2016-2018. JAMA Intern Med. 2020;180:1394-1398. doi: 10.1001/jamainternmed.2020.2817
9. Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:1013-1019. doi: 10.15585/mmwr.mm6845a2
10. NASEM. Public health consequences of e-cigarettes. National Academies Press; 2018. Accessed August 19, 2021. www.ncbi.nlm.nih.gov/books/NBK507171/
11. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380:629-637. doi: 10.1056/NEJMoa1808779
12. Pulvers K, Nollen NL, Rice M, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw Open. 2020;3:e2026324. doi: 10.1001/jamanetworkopen.2020.26324
13. Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. Am J Public Health. 2021;111:230-246. doi: 10.2105/AJPH.2020.305999
14. Barrington-Trimis JL, Urman R, Berhane K, et al. E-cigarettes and future cigarette use. Pediatrics. 2016;138:e20160379. doi: 10.1542/peds.2016-0379
15. Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults: a systematic review and meta-analysis. JAMA Pediatr. 2017;171:788-797. doi: 10.1001/jamapediatrics.2017.1488
16. Krishnasamy VP, Hallowell BD, Ko JY, et al. Update: characteristics of a nationwide outbreak of e-cigarette, or vaping, product use–associated lung injury—United States, August 2019–January 2020. MMWR Morb Mortal Wkly Rep. 2020;69:90-94. doi: 10.15585/mmwr.mm6903e2
17. Blount BC, Karwowski MP, Shields PG, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. N Engl J Med. 2020;382:697-705. doi: 10.1056/NEJMoa1916433
18. CDC. Outbreak of lung injury associated with use of e-cigarette, or vaping, products. Updated February 25, 2020. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
19. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
20. CDC. International Classification of Diseases, 10th Revision. Updated July 30, 2021. Accessed August 31, 2021. www.cdc.gov/nchs/icd/icd10cm.htm
21. CDC. Surgeon General’s advisory on e-cigarette use among youth. Reviewed April 9, 2019. Accessed August 19, 2021. www.cdc.gov/tobacco/basic_information/e-cigarettes/surgeon-general-advisory/index.html
22. Knight JR, Sherritt L, Shrier LA, et al. Validity of the CRAFFT substance abuse screening test among adolescent clinic patients. Arch Pediatr Adolesc Med. 2002;156:607-614. doi: 10.1001/archpedi.156.6.607
23. DiFranza JR, Savageau JA, Fletcher K, et al. Measuring the loss of autonomy over nicotine use in adolescents: the DANDY (Development and Assessment of Nicotine Dependence in Youths) study. Arch Pediatr Adolesc Med. 2002;156:397-403. doi: 10.1001/archpedi.156.4.397
24. Wellman RJ, Savageau JA, Godiwala S, et al. A comparison of the Hooked on Nicotine Checklist and the Fagerström Test for Nicotine Dependence in adult smokers. Nicotine Tob Res. 2006;8:575-580. doi: 10.1080/14622200600789965
25. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Published May 2008. Accessed August 19, 2021. www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
26. Shah SD, Wilken LA, Winkler SR, et al. Systematic review and meta-analysis of combination therapy for smoking cessation. J Am Pharm Assoc. 2008;48:659-665. doi: 10.1331/JAPhA.2008.07063
27. Vidrine JI, Shete S, Cao Y, et al. Ask-Advise-Connect: a new approach to smoking treatment delivery in health care settings. JAMA Intern Med. 2013;173:458-464. doi: 10.1001/jamainternmed.2013.3751
28. Bentz CJ, Bayley KB, Bonin KE, et al. The feasibility of connecting physician offices to a state-level tobacco quit line. Am J Prev Med. 2006;30:31-37. doi: 10.1016/j.amepre.2005.08.043
29. Borland R, Segan CJ. The potential of quitlines to increase smoking cessation. Drug Alcohol Rev. 2006;25:73-78. doi: 10.1080/09595230500459537
30. Farber HJ, Walley SC, Groner JA, et al. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017. doi: 10.1542/peds.2015-3108
31. Gentzke AS, Wang TW, Jamal A, et al. Tobacco product use among middle and high school students—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1881-1888. doi: 10.15585/mmwr.mm6950a1
32. AAP. Nicotine replacement therapy and adolescent patients: information for pediatricians. Updated November 2019. Accessed August 19, 2021. https://downloads.aap.org/RCE/NRT_and_Adolescents_Pediatrician_Guidance_factsheet.pdf
33. Blasi PR, Cromp D, McDonald S, et al. Approaches to behavioral health integration at high performing primary care practices. J Am Board Fam Med. 2018;31:691-701. doi: 10.3122/jabfm.2018.05.170468
34. Jacobs C, Brieler JA, Salas J, et al. Integrated behavioral health care in family medicine residencies a CERA survey. Fam Med. 2018;50:380-384. doi: 10.22454/FamMed.2018.639260
35. Oliverez M. Quick guide: billing for smoking cessation services. Capture Billing. Accessed August 26, 2021. https://capturebilling.com/how-bill-smoking-cessation-counseling-99406-99407/
Decline in child COVID may signal end of latest surge
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.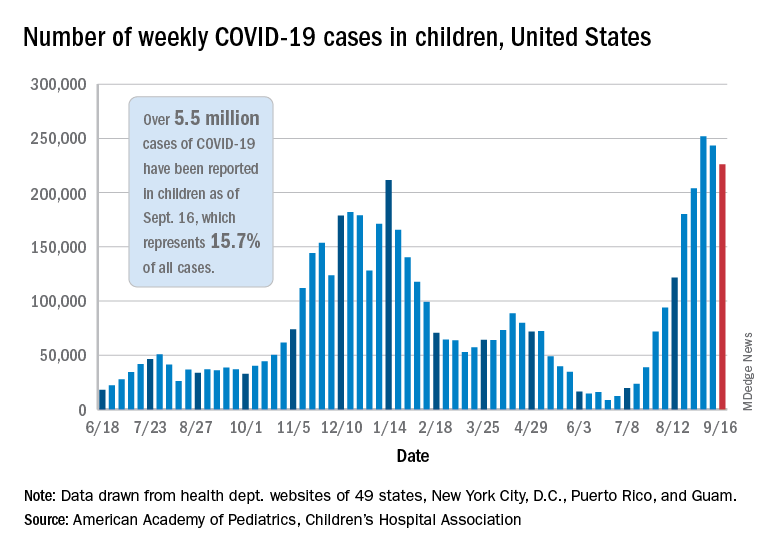
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.
A second consecutive week of falling COVID-19 cases in children, along with continued declines in new admissions, may indicate that the latest surge has peaked.
Children made up over 25% of all new cases each week over that 3-week period covering the end of August and the first half of September, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
New hospitalizations in children aged 0-17 years peaked on Sept. 4 – when the rate reached 0.51 per 100,000 population – and were down to 0.47 as of Sept. 11, the latest date for which data should be considered reliable, the Centers for Disease Control and Prevention said.
The CDC’s data largely agree with the AAP/CHA report, showing that cases peaked during the week of Aug. 22-28. Cases per 100,000 for children that week looked like this: 154.7 (age 0-4 years), 276.6 (5-11 years), 320.0 (12-15), and 334.1 (16-17). The highest rates that week among adults were 288.6 per 100,000 in 30- to 39-year-olds and 286.5 for those aged 18-29, the CDC said on its COVID Data Tracker.
By the week of Sept. 5-11 – reporting delays can affect more recent data – the rates in children were down more than 20% in each of the four age groups, according to the CDC.
Vaccinations among children, unfortunately, continue to decline. Vaccine initiations for 12- to 15-year-olds slipped from 199,000 (Sept. 7-13) to 179,000 during the week of Sept. 14-20, while the 16- to 17-year-olds went from almost 83,000 down to 75,000. Initiations have dropped for 6 straight weeks in both age groups, based on the CDC data.
Despite those declines, however, the 16- and 17-year-olds just passed a couple of vaccination milestones. More than 60% – 60.9%, to be exact – have now received at least one dose of COVID vaccine, and 50.3% can be considered fully vaccinated. For those aged 12-15, the corresponding figures are 53.1% and 42.0%, the CDC reported.
When children under age 12 years are included – through clinical trial involvement or incorrect birth dates – the CDC data put the total count of Americans under age 18 who have received at least one dose of vaccine at almost 12.8 million, with vaccination complete in 10.3 million.
Total cases, as calculated by the APA and CHA, are now over 5.5 million, although that figure includes cases in individuals as old as 20 years, since many states differ from the CDC on the age range for a child. The CDC’s COVID Data Tracker put the total for children aged 0-17 at nearly 4.6 million.
The total number of COVID-related deaths in children is 480 as of Sept. 16, the AAP and CHA said, based on data from 45 states, New York, City, Puerto Rico, and Guam, but the CDC provides a higher number, 548, since the pandemic began. Children aged 0-4 years represent the largest share (32.3%) of those 548 deaths, followed by the 12- to 15-year-olds (26.5%), based on the CDC data.





