User login
A pediatrician notices empty fields
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
The high school football team here in Brunswick has had winning years and losing years but the school has always fielded a competitive team. It has been state champion on several occasions and has weathered the challenge when soccer became the new and more popular sport shortly after it arrived in town several decades ago. But this year, on the heels of a strong winning season last year, the numbers are down significantly. The school is in jeopardy of not having enough players to field a junior varsity team.
This dearth of student athletes is a problem not just here in Brunswick. Schools across the state of Maine are being forced to shift to an eight man football format. Nor is it unique to football here in vacationland. A recent article in a Hudson Valley, N.Y., newspaper chronicles a broad-based decline in participation in high school sports including field hockey, tennis, and cross country (‘Covid,’ The Journal News, Nancy Haggerty, Sept. 5, 2021). In many situations the school may have enough players to field a varsity team but too few to play a junior varsity schedule. Without a supply of young talent coming up from the junior varsity, the future of any varsity program is on a shaky legs. Some of the coaches are referring to the decline in participation as a “COVID hangover” triggered in part by season disruptions, cancellations, and fluctuating remote learning formats.
I and some other coaches argue that the participation drought predates the pandemic and is the result of a wide range of unfortunate trends. First, is the general malaise and don’t-give-a-damn-about-anything attitude that has settled on the young people of this country, the causes of which are difficult to define. It may be that after years of sitting in front of a video screen, too many children have settled into the role of being spectators and find the energy it takes to participate just isn’t worth the effort.
Another contributor to the decline in participation is the heavy of emphasis on early specialization. Driven in many cases by unrealistic parental dreams, children are shepherded into elite travel teams with seasons that often stretch to lengths that make it difficult if not impossible for a child to participate in other sports. The child who may simply be a late bloomer or whose family can’t afford the time or money to buy into the travel team ethic quickly finds himself losing ground. Without the additional opportunities for skill development, many of the children noon travel teams eventually wonder if it is worth trying to catch up. Ironically, the trend toward early specialization is short-sighted because many college and professional coaches report that their best athletes shunned becoming one-trick ponies and played a variety of sports growing up.
Parental concerns about injury, particularly concussion, probably play a role in the trend of falling participation in sports, even those with minimal risk of head injury. Certainly our new awareness of the long-term effects of multiple concussions is long overdue. However, we as pediatricians must take some of the blame for often emphasizing the injury risk inherent in sports in general while neglecting to highlight the positive benefits of competitive sports such as fitness and team building. Are there situations where our emphasis on preparticipation physicals is acting as a deterrent?
There are exceptions to the general trend of falling participation, lacrosse being the most obvious example. However, as lacrosse becomes more popular across the country there are signs that it is already drifting into the larger and counterproductive elite travel team model. There have always been communities in which an individual coach or parent has created a team culture that is both inclusive and competitive. The two are not mutually exclusive.
Sadly, these exceptional programs are few and far between. I’m not sure where we can start to turn things around so that more children choose to be players rather than observers. But, we pediatricians certainly can play a more positive role in emphasizing the benefits of team play.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Hormone agonist therapy disrupts bone density in transgender youth
The use of gonadotropin-releasing hormone agonists has a negative effect on bone mass in transgender youth, according to data from 172 individuals.
The onset of puberty and pubertal hormones contributes to the development of bone mass and body composition in adolescence, wrote Behdad Navabi, MD, and colleagues at Children’s Hospital of Eastern Ontario, Canada. Although the safety and efficacy of gonadotropin-releasing hormone agonists (GnRHa) has been described in short-term studies of youth with gender dysphoria, concerns persist about suppression of bone mass accrual from extended use of GnRHas in this population, they noted.
In a study published in Pediatrics, the researchers reviewed data from 172 youth younger than 18 years of age who were treated with GNRHa and underwent at least one baseline dual-energy radiograph absorptiometry (DXA) measurement between January 2006 and April 2017 at a single center. The standard treatment protocol started with three doses of 7.5 mg leuprolide acetate, given intramuscularly every 4 weeks, followed by 11.25 mg intramuscularly every 12 weeks after puberty suppression was confirmed both clinically and biochemically. Areal bone mineral density (aBMD) measurement z scores were based on birth-assigned sex, age, and ethnicity, and assessed at baseline and every 12 months. In addition, volumetric bone mineral density was calculated as bone mineral apparent density (BMAD) at the lower spine, and the z score based on age-matched, birth-assigned gender BMAD.
Overall, 55.2% of the youth were vitamin D deficient or insufficient at baseline, but 87.3% were sufficient by the time of a third follow-up visit after treatment with 1,000-2,000 IU of vitamin D daily; no cases of vitamin D toxicity were reported.
At baseline, transgender females had lower z scores for the LS aBMD and BMAD compared to transgender males, reflecting a difference seen in previous studies of transgender youth and adult females, the researchers noted.
The researchers analyzed pre- and posttreatment DXA data in a subgroup of 36 transgender females and 80 transgender males to identify any changes associated with GnRHa. The average time between the DXA scans was 407 days. In this population, aBMD z scores at the lower lumbar spine (LS), left total hip (LTH), and total body less head (TBLH) decreased significantly from baseline in transgender males and females.
Among transgender males, LS bone mineral apparent density (BMAD) z scores also decreased significantly from baseline, but no such change occurred among transgender females. The most significant decrease in z scores occurred in the LS aBMD and BMAD of transgender males, with changes that reflect findings from previous studies and may be explained by decreased estrogen, the researchers wrote.
In terms of body composition, no significant changes occurred in body mass index z score from baseline to follow-up in transgender males or females, the researchers noted, and changes in both gynoid and android fat percentages were consistent with the individuals’ affirmed genders. No vertebral fractures were detected.
However, GnRHa was significantly associated with a decrease in total body fat percentage and a decrease in lean body mass (LBM) in transgender females.
The study findings were limited by several factors, including the lack of consistent baseline physical activity records, and limited analysis at follow-up of the possible role of physical activity in bone health and body composition, the researchers noted. However, the results were strengthened by the relatively large study population with baseline assessments, and by the pre- and posttreatment analysis, they added.
“Evidence on GnRHa-associated changes in body composition and BMD will help health care professionals involved in the care of youth with GD [gender dysphoria] to counsel appropriately and optimize their bone health,” the researchers said. “Given the absence of vertebral fractures detected in those with significant decreases in their LS z scores, the significance of BMD effects of GnRHa in transgender youth needs further study, as well as whether future spine radiographs are needed on the basis of BMD trajectory,” they concluded.
Balance bone health concerns with potential benefits
The effect of estrogen and testosterone on bone geometry in puberty varies, and the increase in the use of GnRHa as part of a multidisciplinary gender transition plan makes research on the skeletal impact of this therapy in transgender youth a top priority, Laura K. Bachrach, MD, of Stanford (Calif.) University, and Catherine M. Gordon of Harvard Medical School, Boston, wrote in an accompanying editorial.
The decrease in areal bone mineral density and in bone mineral apparent density (BMAD) z scores in the current study is not unexpected, but the key question is how much bone density recovers once the suppression therapy ends and transgender sex steroid use begins, they said. “Follow-up studies of young adults treated with GnRHa for precocious puberty in childhood are reassuring,” they wrote. “It is premature, however, to extrapolate from these findings to transgender youth,” because the impact of gender-affirming sex steroid therapy on the skeleton at older ages and stages of maturity are unclear, they emphasized.
In the absence of definitive answers, the editorial authors advised clinicians treating youth with gender dysphoria to provide a balanced view of the risks and benefits of hormone therapy, and encourage adequate intake of dietary vitamin D and calcium, along with weight-bearing physical activity, to promote general bone health. “Transgender teenagers and their parents should be reassured that some recovery from decreases in aBMD during pubertal suppression with GnRHa is likely,” the authors noted. Bone health should be monitored throughout all stages of treatment in transgender youth, but concerns about transient bone loss should not discourage gender transition therapy, they emphasized. “In this patient group, providing a pause in pubertal development offers a life-changing and, for some, a life-saving intervention,” they concluded.
Comparison to cisgender controls would add value
“This study is important because one of the major side effects of GnRH agonists is decreased bone density, especially the longer that patients are on them,” M. Brett Cooper, MD, of UT Southwestern Medical Center, said in an interview. The findings add to existing data to underscore the importance of screening for low bone density and low vitamin D levels, Dr. Cooper added.
Dr. Cooper said that he was not surprised by the study findings. “I think that this study supported what clinicians already knew, which is that GnRH agonists do potentially cause a decline in bone mineral density and thus, you need to support these patients as best you can with calcium, vitamin D, and weight-bearing exercise,” he noted.
Dr. Cooper emphasized two main take-home points from the study. “First, clinicians who prescribe GnRH agonists need to ensure that they are checking bone density and vitamin D measurements, and then optimizing these appropriately,” he said. “Second, when a bone density is found to be low or a vitamin level is low, clinicians need to ensure that they are monitored and treated appropriately.” Clinicians need to use these data when deciding when to start gender-affirming hormones so their patients have the best chance to recover bone density, he added.
“I think one confounding factor on this study is the ranges they used for vitamin D deficiency,” Dr. Cooper noted. “This study was done in Canada, and the scale used was in nmol/L, while most labs in the U.S. use ng/mL,” he said. “Most pediatric and adolescent societies in the United States use < 20 ng/mL as an indicator of vitamin D deficient and between 20 and 29 ng/mL as insufficient,” he explained, citing the position statement on recommended vitamin D intake for adolescents published by The Society for Adolescent Health and Medicine. In this study, the results converted to < 12 ng/mL as deficient and between 12 and 20 ng/mL as insufficient, respectively, on the U.S. scale, said Dr. Cooper.
“Therefore, I can see that there are cases where someone may have been labeled vitamin D insufficient in this study using their range, whereas in the U.S. these patients would be labeled as vitamin D deficient and treated with higher-dose supplementation,” he said. In addition, individuals with levels between 20 ng/mL and 29 ng/mL in the U.S. would still be treated with vitamin D supplementation, “whereas in their study those individuals would have been labeled as normal,” he noted.
As for future research, it would be useful to study whether bone mass in transgender young people differs from age- and gender-matched controls who are not gender diverse (cisgender), Dr. Cooper added. “It may be possible that the youth in this study are not different from their peers and maybe the GnRH agonist is not the culprit,” he said.
The study received no outside funding. The researchers, editorial authors, and Dr. Cooper had no financial conflicts to disclose.
The use of gonadotropin-releasing hormone agonists has a negative effect on bone mass in transgender youth, according to data from 172 individuals.
The onset of puberty and pubertal hormones contributes to the development of bone mass and body composition in adolescence, wrote Behdad Navabi, MD, and colleagues at Children’s Hospital of Eastern Ontario, Canada. Although the safety and efficacy of gonadotropin-releasing hormone agonists (GnRHa) has been described in short-term studies of youth with gender dysphoria, concerns persist about suppression of bone mass accrual from extended use of GnRHas in this population, they noted.
In a study published in Pediatrics, the researchers reviewed data from 172 youth younger than 18 years of age who were treated with GNRHa and underwent at least one baseline dual-energy radiograph absorptiometry (DXA) measurement between January 2006 and April 2017 at a single center. The standard treatment protocol started with three doses of 7.5 mg leuprolide acetate, given intramuscularly every 4 weeks, followed by 11.25 mg intramuscularly every 12 weeks after puberty suppression was confirmed both clinically and biochemically. Areal bone mineral density (aBMD) measurement z scores were based on birth-assigned sex, age, and ethnicity, and assessed at baseline and every 12 months. In addition, volumetric bone mineral density was calculated as bone mineral apparent density (BMAD) at the lower spine, and the z score based on age-matched, birth-assigned gender BMAD.
Overall, 55.2% of the youth were vitamin D deficient or insufficient at baseline, but 87.3% were sufficient by the time of a third follow-up visit after treatment with 1,000-2,000 IU of vitamin D daily; no cases of vitamin D toxicity were reported.
At baseline, transgender females had lower z scores for the LS aBMD and BMAD compared to transgender males, reflecting a difference seen in previous studies of transgender youth and adult females, the researchers noted.
The researchers analyzed pre- and posttreatment DXA data in a subgroup of 36 transgender females and 80 transgender males to identify any changes associated with GnRHa. The average time between the DXA scans was 407 days. In this population, aBMD z scores at the lower lumbar spine (LS), left total hip (LTH), and total body less head (TBLH) decreased significantly from baseline in transgender males and females.
Among transgender males, LS bone mineral apparent density (BMAD) z scores also decreased significantly from baseline, but no such change occurred among transgender females. The most significant decrease in z scores occurred in the LS aBMD and BMAD of transgender males, with changes that reflect findings from previous studies and may be explained by decreased estrogen, the researchers wrote.
In terms of body composition, no significant changes occurred in body mass index z score from baseline to follow-up in transgender males or females, the researchers noted, and changes in both gynoid and android fat percentages were consistent with the individuals’ affirmed genders. No vertebral fractures were detected.
However, GnRHa was significantly associated with a decrease in total body fat percentage and a decrease in lean body mass (LBM) in transgender females.
The study findings were limited by several factors, including the lack of consistent baseline physical activity records, and limited analysis at follow-up of the possible role of physical activity in bone health and body composition, the researchers noted. However, the results were strengthened by the relatively large study population with baseline assessments, and by the pre- and posttreatment analysis, they added.
“Evidence on GnRHa-associated changes in body composition and BMD will help health care professionals involved in the care of youth with GD [gender dysphoria] to counsel appropriately and optimize their bone health,” the researchers said. “Given the absence of vertebral fractures detected in those with significant decreases in their LS z scores, the significance of BMD effects of GnRHa in transgender youth needs further study, as well as whether future spine radiographs are needed on the basis of BMD trajectory,” they concluded.
Balance bone health concerns with potential benefits
The effect of estrogen and testosterone on bone geometry in puberty varies, and the increase in the use of GnRHa as part of a multidisciplinary gender transition plan makes research on the skeletal impact of this therapy in transgender youth a top priority, Laura K. Bachrach, MD, of Stanford (Calif.) University, and Catherine M. Gordon of Harvard Medical School, Boston, wrote in an accompanying editorial.
The decrease in areal bone mineral density and in bone mineral apparent density (BMAD) z scores in the current study is not unexpected, but the key question is how much bone density recovers once the suppression therapy ends and transgender sex steroid use begins, they said. “Follow-up studies of young adults treated with GnRHa for precocious puberty in childhood are reassuring,” they wrote. “It is premature, however, to extrapolate from these findings to transgender youth,” because the impact of gender-affirming sex steroid therapy on the skeleton at older ages and stages of maturity are unclear, they emphasized.
In the absence of definitive answers, the editorial authors advised clinicians treating youth with gender dysphoria to provide a balanced view of the risks and benefits of hormone therapy, and encourage adequate intake of dietary vitamin D and calcium, along with weight-bearing physical activity, to promote general bone health. “Transgender teenagers and their parents should be reassured that some recovery from decreases in aBMD during pubertal suppression with GnRHa is likely,” the authors noted. Bone health should be monitored throughout all stages of treatment in transgender youth, but concerns about transient bone loss should not discourage gender transition therapy, they emphasized. “In this patient group, providing a pause in pubertal development offers a life-changing and, for some, a life-saving intervention,” they concluded.
Comparison to cisgender controls would add value
“This study is important because one of the major side effects of GnRH agonists is decreased bone density, especially the longer that patients are on them,” M. Brett Cooper, MD, of UT Southwestern Medical Center, said in an interview. The findings add to existing data to underscore the importance of screening for low bone density and low vitamin D levels, Dr. Cooper added.
Dr. Cooper said that he was not surprised by the study findings. “I think that this study supported what clinicians already knew, which is that GnRH agonists do potentially cause a decline in bone mineral density and thus, you need to support these patients as best you can with calcium, vitamin D, and weight-bearing exercise,” he noted.
Dr. Cooper emphasized two main take-home points from the study. “First, clinicians who prescribe GnRH agonists need to ensure that they are checking bone density and vitamin D measurements, and then optimizing these appropriately,” he said. “Second, when a bone density is found to be low or a vitamin level is low, clinicians need to ensure that they are monitored and treated appropriately.” Clinicians need to use these data when deciding when to start gender-affirming hormones so their patients have the best chance to recover bone density, he added.
“I think one confounding factor on this study is the ranges they used for vitamin D deficiency,” Dr. Cooper noted. “This study was done in Canada, and the scale used was in nmol/L, while most labs in the U.S. use ng/mL,” he said. “Most pediatric and adolescent societies in the United States use < 20 ng/mL as an indicator of vitamin D deficient and between 20 and 29 ng/mL as insufficient,” he explained, citing the position statement on recommended vitamin D intake for adolescents published by The Society for Adolescent Health and Medicine. In this study, the results converted to < 12 ng/mL as deficient and between 12 and 20 ng/mL as insufficient, respectively, on the U.S. scale, said Dr. Cooper.
“Therefore, I can see that there are cases where someone may have been labeled vitamin D insufficient in this study using their range, whereas in the U.S. these patients would be labeled as vitamin D deficient and treated with higher-dose supplementation,” he said. In addition, individuals with levels between 20 ng/mL and 29 ng/mL in the U.S. would still be treated with vitamin D supplementation, “whereas in their study those individuals would have been labeled as normal,” he noted.
As for future research, it would be useful to study whether bone mass in transgender young people differs from age- and gender-matched controls who are not gender diverse (cisgender), Dr. Cooper added. “It may be possible that the youth in this study are not different from their peers and maybe the GnRH agonist is not the culprit,” he said.
The study received no outside funding. The researchers, editorial authors, and Dr. Cooper had no financial conflicts to disclose.
The use of gonadotropin-releasing hormone agonists has a negative effect on bone mass in transgender youth, according to data from 172 individuals.
The onset of puberty and pubertal hormones contributes to the development of bone mass and body composition in adolescence, wrote Behdad Navabi, MD, and colleagues at Children’s Hospital of Eastern Ontario, Canada. Although the safety and efficacy of gonadotropin-releasing hormone agonists (GnRHa) has been described in short-term studies of youth with gender dysphoria, concerns persist about suppression of bone mass accrual from extended use of GnRHas in this population, they noted.
In a study published in Pediatrics, the researchers reviewed data from 172 youth younger than 18 years of age who were treated with GNRHa and underwent at least one baseline dual-energy radiograph absorptiometry (DXA) measurement between January 2006 and April 2017 at a single center. The standard treatment protocol started with three doses of 7.5 mg leuprolide acetate, given intramuscularly every 4 weeks, followed by 11.25 mg intramuscularly every 12 weeks after puberty suppression was confirmed both clinically and biochemically. Areal bone mineral density (aBMD) measurement z scores were based on birth-assigned sex, age, and ethnicity, and assessed at baseline and every 12 months. In addition, volumetric bone mineral density was calculated as bone mineral apparent density (BMAD) at the lower spine, and the z score based on age-matched, birth-assigned gender BMAD.
Overall, 55.2% of the youth were vitamin D deficient or insufficient at baseline, but 87.3% were sufficient by the time of a third follow-up visit after treatment with 1,000-2,000 IU of vitamin D daily; no cases of vitamin D toxicity were reported.
At baseline, transgender females had lower z scores for the LS aBMD and BMAD compared to transgender males, reflecting a difference seen in previous studies of transgender youth and adult females, the researchers noted.
The researchers analyzed pre- and posttreatment DXA data in a subgroup of 36 transgender females and 80 transgender males to identify any changes associated with GnRHa. The average time between the DXA scans was 407 days. In this population, aBMD z scores at the lower lumbar spine (LS), left total hip (LTH), and total body less head (TBLH) decreased significantly from baseline in transgender males and females.
Among transgender males, LS bone mineral apparent density (BMAD) z scores also decreased significantly from baseline, but no such change occurred among transgender females. The most significant decrease in z scores occurred in the LS aBMD and BMAD of transgender males, with changes that reflect findings from previous studies and may be explained by decreased estrogen, the researchers wrote.
In terms of body composition, no significant changes occurred in body mass index z score from baseline to follow-up in transgender males or females, the researchers noted, and changes in both gynoid and android fat percentages were consistent with the individuals’ affirmed genders. No vertebral fractures were detected.
However, GnRHa was significantly associated with a decrease in total body fat percentage and a decrease in lean body mass (LBM) in transgender females.
The study findings were limited by several factors, including the lack of consistent baseline physical activity records, and limited analysis at follow-up of the possible role of physical activity in bone health and body composition, the researchers noted. However, the results were strengthened by the relatively large study population with baseline assessments, and by the pre- and posttreatment analysis, they added.
“Evidence on GnRHa-associated changes in body composition and BMD will help health care professionals involved in the care of youth with GD [gender dysphoria] to counsel appropriately and optimize their bone health,” the researchers said. “Given the absence of vertebral fractures detected in those with significant decreases in their LS z scores, the significance of BMD effects of GnRHa in transgender youth needs further study, as well as whether future spine radiographs are needed on the basis of BMD trajectory,” they concluded.
Balance bone health concerns with potential benefits
The effect of estrogen and testosterone on bone geometry in puberty varies, and the increase in the use of GnRHa as part of a multidisciplinary gender transition plan makes research on the skeletal impact of this therapy in transgender youth a top priority, Laura K. Bachrach, MD, of Stanford (Calif.) University, and Catherine M. Gordon of Harvard Medical School, Boston, wrote in an accompanying editorial.
The decrease in areal bone mineral density and in bone mineral apparent density (BMAD) z scores in the current study is not unexpected, but the key question is how much bone density recovers once the suppression therapy ends and transgender sex steroid use begins, they said. “Follow-up studies of young adults treated with GnRHa for precocious puberty in childhood are reassuring,” they wrote. “It is premature, however, to extrapolate from these findings to transgender youth,” because the impact of gender-affirming sex steroid therapy on the skeleton at older ages and stages of maturity are unclear, they emphasized.
In the absence of definitive answers, the editorial authors advised clinicians treating youth with gender dysphoria to provide a balanced view of the risks and benefits of hormone therapy, and encourage adequate intake of dietary vitamin D and calcium, along with weight-bearing physical activity, to promote general bone health. “Transgender teenagers and their parents should be reassured that some recovery from decreases in aBMD during pubertal suppression with GnRHa is likely,” the authors noted. Bone health should be monitored throughout all stages of treatment in transgender youth, but concerns about transient bone loss should not discourage gender transition therapy, they emphasized. “In this patient group, providing a pause in pubertal development offers a life-changing and, for some, a life-saving intervention,” they concluded.
Comparison to cisgender controls would add value
“This study is important because one of the major side effects of GnRH agonists is decreased bone density, especially the longer that patients are on them,” M. Brett Cooper, MD, of UT Southwestern Medical Center, said in an interview. The findings add to existing data to underscore the importance of screening for low bone density and low vitamin D levels, Dr. Cooper added.
Dr. Cooper said that he was not surprised by the study findings. “I think that this study supported what clinicians already knew, which is that GnRH agonists do potentially cause a decline in bone mineral density and thus, you need to support these patients as best you can with calcium, vitamin D, and weight-bearing exercise,” he noted.
Dr. Cooper emphasized two main take-home points from the study. “First, clinicians who prescribe GnRH agonists need to ensure that they are checking bone density and vitamin D measurements, and then optimizing these appropriately,” he said. “Second, when a bone density is found to be low or a vitamin level is low, clinicians need to ensure that they are monitored and treated appropriately.” Clinicians need to use these data when deciding when to start gender-affirming hormones so their patients have the best chance to recover bone density, he added.
“I think one confounding factor on this study is the ranges they used for vitamin D deficiency,” Dr. Cooper noted. “This study was done in Canada, and the scale used was in nmol/L, while most labs in the U.S. use ng/mL,” he said. “Most pediatric and adolescent societies in the United States use < 20 ng/mL as an indicator of vitamin D deficient and between 20 and 29 ng/mL as insufficient,” he explained, citing the position statement on recommended vitamin D intake for adolescents published by The Society for Adolescent Health and Medicine. In this study, the results converted to < 12 ng/mL as deficient and between 12 and 20 ng/mL as insufficient, respectively, on the U.S. scale, said Dr. Cooper.
“Therefore, I can see that there are cases where someone may have been labeled vitamin D insufficient in this study using their range, whereas in the U.S. these patients would be labeled as vitamin D deficient and treated with higher-dose supplementation,” he said. In addition, individuals with levels between 20 ng/mL and 29 ng/mL in the U.S. would still be treated with vitamin D supplementation, “whereas in their study those individuals would have been labeled as normal,” he noted.
As for future research, it would be useful to study whether bone mass in transgender young people differs from age- and gender-matched controls who are not gender diverse (cisgender), Dr. Cooper added. “It may be possible that the youth in this study are not different from their peers and maybe the GnRH agonist is not the culprit,” he said.
The study received no outside funding. The researchers, editorial authors, and Dr. Cooper had no financial conflicts to disclose.
FROM PEDIATRICS
Researchers warn young adults are at highest risk of obesity
Individuals aged 18-24 years are at the highest risk of weight gain and developing overweight or obesity over the next 10 years, compared with all other adults, and should be a target for obesity prevention policies, say U.K. researchers.
The research, published online Sept. 2, 2021, in The Lancet Diabetes and Endocrinology, showed that factors more traditionally associated with obesity – such as socioeconomic status and ethnicity – play less of a role than age.
“Our results show clearly that age is the most important sociodemographic factor for BMI [body mass index] change,” lead author Michail Katsoulis, PhD, Institute of Health Informatics, University College London, said in a press release.
Cosenior author Claudia Langenberg, PhD, agreed, adding young people “go through big life changes. They may start work, go to university, or leave home for the first time,” and the habits formed during these years “may stick through adulthood.”
Current obesity prevention guidelines are mainly directed at individuals who already have obesity, the researchers said in their article.
“As the evidence presented in our study suggests, the opportunity to modify weight gain is greatest in individuals who are young and do not yet have obesity,” they observed.
“If we are serious about preventing obesity, then we should develop interventions that can be targeted and are relevant for young adults,” added Dr. Langenberg, of the MRC Epidemiology Unit, University of Cambridge, (England), and Berlin Institute of Health.
Risks for higher BMI substantially greater in the youngest adults
The researchers gathered data on more than 2 million adults aged 18-74 years registered with general practitioners in England. Participants had BMI and weight measurements recorded between Jan. 1, 1998, and June 30, 2016, with at least 1 year of follow-up. Overall, 58% were women, 76% were White, 9% had prevalent cardiovascular disease, and 4% had prevalent cancer.
Changes in BMI were assessed at 1 year, 5 years, and 10 years.
At 10 years, adults aged 18-24 years had the highest risk of transitioning from normal weight to overweight or obesity, compared with adults aged 65-74 years, at a greatest absolute risk of 37% versus 24% (odds ratio, 4.22).
Moreover, the results showed that adults aged 18-24 years who were already overweight or obese had a greater risk of transitioning to a higher BMI category during follow-up versus the oldest participants.
They had an absolute risk of 42% versus 18% of transitioning from overweight to class 1 and 2 obesity (OR, 4.60), and an absolute risk of transitioning from class 1 and 2 obesity to class 3 obesity of 22% versus 5% (OR, 5.87).
Online risk calculator and YouTube video help explain findings
While factors other than age were associated with transitioning to a higher BMI category, the association was less pronounced.
For example, the OR of transitioning from normal weight to overweight or obesity in the most socially deprived versus the least deprived areas was 1.23 in men and 1.12 in women. The OR for making the same transition in Black versus White individuals was 1.13.
The findings allowed the researchers to develop a series of nomograms to determine an individual’s absolute risk of transitioning to a higher BMI category over 10 years based on their baseline BMI category, age, sex, and Index of Multiple Deprivation quintile.
“We show that, within each stratum, the risks for transitioning to higher BMI categories were substantially higher in the youngest adult age group than in older age groups,” the team writes.
From this, they developed an open-access online risk calculator to help individuals calculate their risk of weight change over the next 1, 5, and 10 years. The calculator takes into account current weight, height, age, sex, ethnicity, and socioeconomic-area characteristics.
They have also posted a video on YouTube to help explain their findings.
COVID and obesity pandemics collide
Cosenior author Harry Hemingway, MD, PhD, also of University College London, believes that focusing on this young age group is especially critical now because of the COVID-19 pandemic.
“Calculating personal risk of transitioning to a higher weight category is important” as COVID-19 “collides with the obesity pandemic,” he said, noting that “people are exercising less and finding it harder to eat healthy diets during lockdowns.
“Health systems like the NHS [National Health Service] need to identify new ways to prevent obesity and its consequences,” he continued. “This study demonstrates that NHS data collected over time in primary care holds an important key to unlocking new insights for public health action.”
The study was funded by the British Heart Foundation, Health Data Research UK, the UK Medical Research Council, and the National Institute for Health Research. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals aged 18-24 years are at the highest risk of weight gain and developing overweight or obesity over the next 10 years, compared with all other adults, and should be a target for obesity prevention policies, say U.K. researchers.
The research, published online Sept. 2, 2021, in The Lancet Diabetes and Endocrinology, showed that factors more traditionally associated with obesity – such as socioeconomic status and ethnicity – play less of a role than age.
“Our results show clearly that age is the most important sociodemographic factor for BMI [body mass index] change,” lead author Michail Katsoulis, PhD, Institute of Health Informatics, University College London, said in a press release.
Cosenior author Claudia Langenberg, PhD, agreed, adding young people “go through big life changes. They may start work, go to university, or leave home for the first time,” and the habits formed during these years “may stick through adulthood.”
Current obesity prevention guidelines are mainly directed at individuals who already have obesity, the researchers said in their article.
“As the evidence presented in our study suggests, the opportunity to modify weight gain is greatest in individuals who are young and do not yet have obesity,” they observed.
“If we are serious about preventing obesity, then we should develop interventions that can be targeted and are relevant for young adults,” added Dr. Langenberg, of the MRC Epidemiology Unit, University of Cambridge, (England), and Berlin Institute of Health.
Risks for higher BMI substantially greater in the youngest adults
The researchers gathered data on more than 2 million adults aged 18-74 years registered with general practitioners in England. Participants had BMI and weight measurements recorded between Jan. 1, 1998, and June 30, 2016, with at least 1 year of follow-up. Overall, 58% were women, 76% were White, 9% had prevalent cardiovascular disease, and 4% had prevalent cancer.
Changes in BMI were assessed at 1 year, 5 years, and 10 years.
At 10 years, adults aged 18-24 years had the highest risk of transitioning from normal weight to overweight or obesity, compared with adults aged 65-74 years, at a greatest absolute risk of 37% versus 24% (odds ratio, 4.22).
Moreover, the results showed that adults aged 18-24 years who were already overweight or obese had a greater risk of transitioning to a higher BMI category during follow-up versus the oldest participants.
They had an absolute risk of 42% versus 18% of transitioning from overweight to class 1 and 2 obesity (OR, 4.60), and an absolute risk of transitioning from class 1 and 2 obesity to class 3 obesity of 22% versus 5% (OR, 5.87).
Online risk calculator and YouTube video help explain findings
While factors other than age were associated with transitioning to a higher BMI category, the association was less pronounced.
For example, the OR of transitioning from normal weight to overweight or obesity in the most socially deprived versus the least deprived areas was 1.23 in men and 1.12 in women. The OR for making the same transition in Black versus White individuals was 1.13.
The findings allowed the researchers to develop a series of nomograms to determine an individual’s absolute risk of transitioning to a higher BMI category over 10 years based on their baseline BMI category, age, sex, and Index of Multiple Deprivation quintile.
“We show that, within each stratum, the risks for transitioning to higher BMI categories were substantially higher in the youngest adult age group than in older age groups,” the team writes.
From this, they developed an open-access online risk calculator to help individuals calculate their risk of weight change over the next 1, 5, and 10 years. The calculator takes into account current weight, height, age, sex, ethnicity, and socioeconomic-area characteristics.
They have also posted a video on YouTube to help explain their findings.
COVID and obesity pandemics collide
Cosenior author Harry Hemingway, MD, PhD, also of University College London, believes that focusing on this young age group is especially critical now because of the COVID-19 pandemic.
“Calculating personal risk of transitioning to a higher weight category is important” as COVID-19 “collides with the obesity pandemic,” he said, noting that “people are exercising less and finding it harder to eat healthy diets during lockdowns.
“Health systems like the NHS [National Health Service] need to identify new ways to prevent obesity and its consequences,” he continued. “This study demonstrates that NHS data collected over time in primary care holds an important key to unlocking new insights for public health action.”
The study was funded by the British Heart Foundation, Health Data Research UK, the UK Medical Research Council, and the National Institute for Health Research. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Individuals aged 18-24 years are at the highest risk of weight gain and developing overweight or obesity over the next 10 years, compared with all other adults, and should be a target for obesity prevention policies, say U.K. researchers.
The research, published online Sept. 2, 2021, in The Lancet Diabetes and Endocrinology, showed that factors more traditionally associated with obesity – such as socioeconomic status and ethnicity – play less of a role than age.
“Our results show clearly that age is the most important sociodemographic factor for BMI [body mass index] change,” lead author Michail Katsoulis, PhD, Institute of Health Informatics, University College London, said in a press release.
Cosenior author Claudia Langenberg, PhD, agreed, adding young people “go through big life changes. They may start work, go to university, or leave home for the first time,” and the habits formed during these years “may stick through adulthood.”
Current obesity prevention guidelines are mainly directed at individuals who already have obesity, the researchers said in their article.
“As the evidence presented in our study suggests, the opportunity to modify weight gain is greatest in individuals who are young and do not yet have obesity,” they observed.
“If we are serious about preventing obesity, then we should develop interventions that can be targeted and are relevant for young adults,” added Dr. Langenberg, of the MRC Epidemiology Unit, University of Cambridge, (England), and Berlin Institute of Health.
Risks for higher BMI substantially greater in the youngest adults
The researchers gathered data on more than 2 million adults aged 18-74 years registered with general practitioners in England. Participants had BMI and weight measurements recorded between Jan. 1, 1998, and June 30, 2016, with at least 1 year of follow-up. Overall, 58% were women, 76% were White, 9% had prevalent cardiovascular disease, and 4% had prevalent cancer.
Changes in BMI were assessed at 1 year, 5 years, and 10 years.
At 10 years, adults aged 18-24 years had the highest risk of transitioning from normal weight to overweight or obesity, compared with adults aged 65-74 years, at a greatest absolute risk of 37% versus 24% (odds ratio, 4.22).
Moreover, the results showed that adults aged 18-24 years who were already overweight or obese had a greater risk of transitioning to a higher BMI category during follow-up versus the oldest participants.
They had an absolute risk of 42% versus 18% of transitioning from overweight to class 1 and 2 obesity (OR, 4.60), and an absolute risk of transitioning from class 1 and 2 obesity to class 3 obesity of 22% versus 5% (OR, 5.87).
Online risk calculator and YouTube video help explain findings
While factors other than age were associated with transitioning to a higher BMI category, the association was less pronounced.
For example, the OR of transitioning from normal weight to overweight or obesity in the most socially deprived versus the least deprived areas was 1.23 in men and 1.12 in women. The OR for making the same transition in Black versus White individuals was 1.13.
The findings allowed the researchers to develop a series of nomograms to determine an individual’s absolute risk of transitioning to a higher BMI category over 10 years based on their baseline BMI category, age, sex, and Index of Multiple Deprivation quintile.
“We show that, within each stratum, the risks for transitioning to higher BMI categories were substantially higher in the youngest adult age group than in older age groups,” the team writes.
From this, they developed an open-access online risk calculator to help individuals calculate their risk of weight change over the next 1, 5, and 10 years. The calculator takes into account current weight, height, age, sex, ethnicity, and socioeconomic-area characteristics.
They have also posted a video on YouTube to help explain their findings.
COVID and obesity pandemics collide
Cosenior author Harry Hemingway, MD, PhD, also of University College London, believes that focusing on this young age group is especially critical now because of the COVID-19 pandemic.
“Calculating personal risk of transitioning to a higher weight category is important” as COVID-19 “collides with the obesity pandemic,” he said, noting that “people are exercising less and finding it harder to eat healthy diets during lockdowns.
“Health systems like the NHS [National Health Service] need to identify new ways to prevent obesity and its consequences,” he continued. “This study demonstrates that NHS data collected over time in primary care holds an important key to unlocking new insights for public health action.”
The study was funded by the British Heart Foundation, Health Data Research UK, the UK Medical Research Council, and the National Institute for Health Research. The authors reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PHM 2021: Leading through adversity
PHM 2021 session
Leading through adversity
Presenter
Ilan Alhadeff, MD, MBA, SFHM, CLHM
Session summary
As the VP of hospitalist services and a practicing hospitalist in Boca Raton, Fla., Dr. Alhadeff shared an emotional journey where the impact of lives lost has led to organizational innovation and advocacy. He started this journey on the date of the Parkland High School shooting, Feb. 14, 2018. On this day, he lost his 14 year-old daughter Alyssa and described subsequent emotions of anger, sadness, hopelessness, and feeling the pressure to be the protector of his family. Despite receiving an outpouring of support through memorials, texts, letters, and social media posts, he was immersed in “survival mode.” He likens this to the experience many of us may be having during the pandemic. He described caring for patients with limited empathy and the impact this likely had on patient care. During this challenging time, the strongest supports became those that stated they couldn’t imagine how this event could have impacted Dr. Alhadeff’s life but offered support in any way needed – true empathic communication.
“It ain’t about how hard you hit. It’s about how hard you can get hit and keep moving forward.” – Rocky Balboa (2006)
Despite the above, he and his wife founded Make Our Schools Safe (MOSS), a student-forward organization that promotes a culture of safety where all involved are counseled, “If you see something, say something.” Students are encouraged to use social media as an anonymous reporting tool. Likewise, this organization supports efforts for silent safety alerts in schools and fencing around schools to allow for 1-point entry. Lessons Dr. Alhadeff learned that might impact any pediatric hospitalist include the knowledge that mental health concerns aren’t going away; for example, after a school shooting any student affected should be provided counseling services as needed, the need to prevent triggering events, and turning grief into action can help.
“Life is like riding a bicycle. To keep your balance, you must keep moving.” – Albert Einstein (1930)
Dr. Alhadeff then described the process of “moving on” for him and his family. For his children, this initially meant “busying” their lives. They then gradually eased into therapy, and ultimately adopted a support dog. He experienced recurrent loss with his father passing away in March 2019, and he persevered in legislative advocacy in New Jersey and Florida and personal/professional development with work toward his MBA degree. Through this work, he collaborated with many legislators and two presidents. He describes resiliency as the ability to bounce back from adversity, with components including self-awareness, mindfulness, self-care, positive relationships, and purpose. While many of us have not had the great personal losses and challenge experienced by Dr. Alhadeff, we all are experiencing an once-in-a-lifetime transformation of health care with political and social interference. It is up to each of us to determine our role and how we can use our influence for positive change.
As noted by Dr. Alhadeff, “We are not all in the same boat. We ARE in the same storm.”
Key takeaways
- How PHM can promote MOSS: Allow children to be part of the work to keep schools safe. Advocate for local MOSS chapters. Support legislative advocacy for school safety.
- Despite adversity, we have the ability to demonstrate resilience. We do so through development of self-awareness, mindfulness, engagement in self-care, nurturing positive relationships, and continuing to pursue our greater purpose.
Dr. King is a pediatric hospitalist at Children’s MN and the director of medical education, an associate program director for the Pediatrics Residency program at the University of Minnesota. She received her medical degree from Wright State University Boonshoft School of Medicine and completed pediatric residency and chief residency at the University of Minnesota.
PHM 2021 session
Leading through adversity
Presenter
Ilan Alhadeff, MD, MBA, SFHM, CLHM
Session summary
As the VP of hospitalist services and a practicing hospitalist in Boca Raton, Fla., Dr. Alhadeff shared an emotional journey where the impact of lives lost has led to organizational innovation and advocacy. He started this journey on the date of the Parkland High School shooting, Feb. 14, 2018. On this day, he lost his 14 year-old daughter Alyssa and described subsequent emotions of anger, sadness, hopelessness, and feeling the pressure to be the protector of his family. Despite receiving an outpouring of support through memorials, texts, letters, and social media posts, he was immersed in “survival mode.” He likens this to the experience many of us may be having during the pandemic. He described caring for patients with limited empathy and the impact this likely had on patient care. During this challenging time, the strongest supports became those that stated they couldn’t imagine how this event could have impacted Dr. Alhadeff’s life but offered support in any way needed – true empathic communication.
“It ain’t about how hard you hit. It’s about how hard you can get hit and keep moving forward.” – Rocky Balboa (2006)
Despite the above, he and his wife founded Make Our Schools Safe (MOSS), a student-forward organization that promotes a culture of safety where all involved are counseled, “If you see something, say something.” Students are encouraged to use social media as an anonymous reporting tool. Likewise, this organization supports efforts for silent safety alerts in schools and fencing around schools to allow for 1-point entry. Lessons Dr. Alhadeff learned that might impact any pediatric hospitalist include the knowledge that mental health concerns aren’t going away; for example, after a school shooting any student affected should be provided counseling services as needed, the need to prevent triggering events, and turning grief into action can help.
“Life is like riding a bicycle. To keep your balance, you must keep moving.” – Albert Einstein (1930)
Dr. Alhadeff then described the process of “moving on” for him and his family. For his children, this initially meant “busying” their lives. They then gradually eased into therapy, and ultimately adopted a support dog. He experienced recurrent loss with his father passing away in March 2019, and he persevered in legislative advocacy in New Jersey and Florida and personal/professional development with work toward his MBA degree. Through this work, he collaborated with many legislators and two presidents. He describes resiliency as the ability to bounce back from adversity, with components including self-awareness, mindfulness, self-care, positive relationships, and purpose. While many of us have not had the great personal losses and challenge experienced by Dr. Alhadeff, we all are experiencing an once-in-a-lifetime transformation of health care with political and social interference. It is up to each of us to determine our role and how we can use our influence for positive change.
As noted by Dr. Alhadeff, “We are not all in the same boat. We ARE in the same storm.”
Key takeaways
- How PHM can promote MOSS: Allow children to be part of the work to keep schools safe. Advocate for local MOSS chapters. Support legislative advocacy for school safety.
- Despite adversity, we have the ability to demonstrate resilience. We do so through development of self-awareness, mindfulness, engagement in self-care, nurturing positive relationships, and continuing to pursue our greater purpose.
Dr. King is a pediatric hospitalist at Children’s MN and the director of medical education, an associate program director for the Pediatrics Residency program at the University of Minnesota. She received her medical degree from Wright State University Boonshoft School of Medicine and completed pediatric residency and chief residency at the University of Minnesota.
PHM 2021 session
Leading through adversity
Presenter
Ilan Alhadeff, MD, MBA, SFHM, CLHM
Session summary
As the VP of hospitalist services and a practicing hospitalist in Boca Raton, Fla., Dr. Alhadeff shared an emotional journey where the impact of lives lost has led to organizational innovation and advocacy. He started this journey on the date of the Parkland High School shooting, Feb. 14, 2018. On this day, he lost his 14 year-old daughter Alyssa and described subsequent emotions of anger, sadness, hopelessness, and feeling the pressure to be the protector of his family. Despite receiving an outpouring of support through memorials, texts, letters, and social media posts, he was immersed in “survival mode.” He likens this to the experience many of us may be having during the pandemic. He described caring for patients with limited empathy and the impact this likely had on patient care. During this challenging time, the strongest supports became those that stated they couldn’t imagine how this event could have impacted Dr. Alhadeff’s life but offered support in any way needed – true empathic communication.
“It ain’t about how hard you hit. It’s about how hard you can get hit and keep moving forward.” – Rocky Balboa (2006)
Despite the above, he and his wife founded Make Our Schools Safe (MOSS), a student-forward organization that promotes a culture of safety where all involved are counseled, “If you see something, say something.” Students are encouraged to use social media as an anonymous reporting tool. Likewise, this organization supports efforts for silent safety alerts in schools and fencing around schools to allow for 1-point entry. Lessons Dr. Alhadeff learned that might impact any pediatric hospitalist include the knowledge that mental health concerns aren’t going away; for example, after a school shooting any student affected should be provided counseling services as needed, the need to prevent triggering events, and turning grief into action can help.
“Life is like riding a bicycle. To keep your balance, you must keep moving.” – Albert Einstein (1930)
Dr. Alhadeff then described the process of “moving on” for him and his family. For his children, this initially meant “busying” their lives. They then gradually eased into therapy, and ultimately adopted a support dog. He experienced recurrent loss with his father passing away in March 2019, and he persevered in legislative advocacy in New Jersey and Florida and personal/professional development with work toward his MBA degree. Through this work, he collaborated with many legislators and two presidents. He describes resiliency as the ability to bounce back from adversity, with components including self-awareness, mindfulness, self-care, positive relationships, and purpose. While many of us have not had the great personal losses and challenge experienced by Dr. Alhadeff, we all are experiencing an once-in-a-lifetime transformation of health care with political and social interference. It is up to each of us to determine our role and how we can use our influence for positive change.
As noted by Dr. Alhadeff, “We are not all in the same boat. We ARE in the same storm.”
Key takeaways
- How PHM can promote MOSS: Allow children to be part of the work to keep schools safe. Advocate for local MOSS chapters. Support legislative advocacy for school safety.
- Despite adversity, we have the ability to demonstrate resilience. We do so through development of self-awareness, mindfulness, engagement in self-care, nurturing positive relationships, and continuing to pursue our greater purpose.
Dr. King is a pediatric hospitalist at Children’s MN and the director of medical education, an associate program director for the Pediatrics Residency program at the University of Minnesota. She received her medical degree from Wright State University Boonshoft School of Medicine and completed pediatric residency and chief residency at the University of Minnesota.
Urticaria and edema in a 2-year-old boy
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
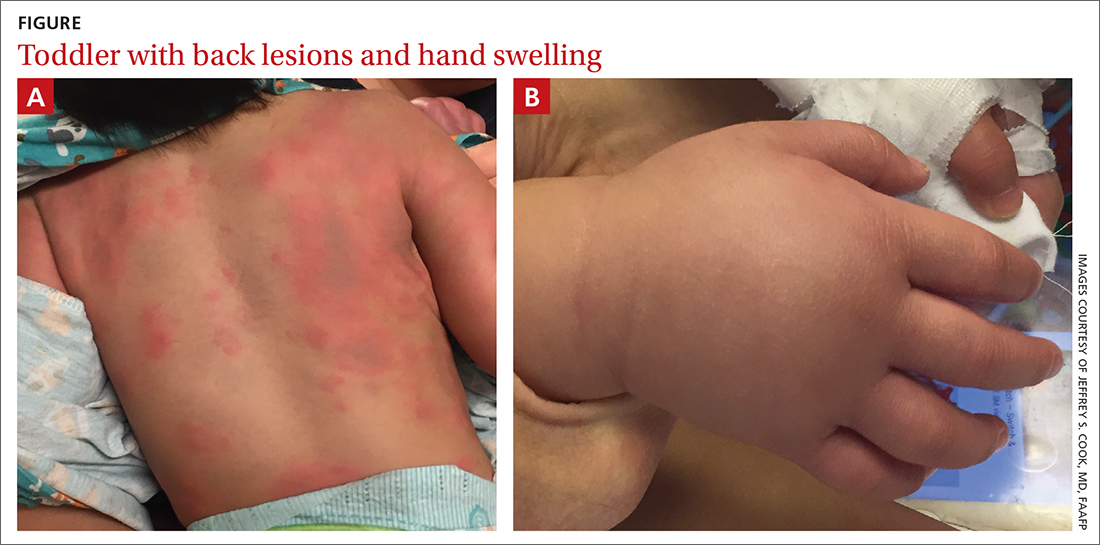
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
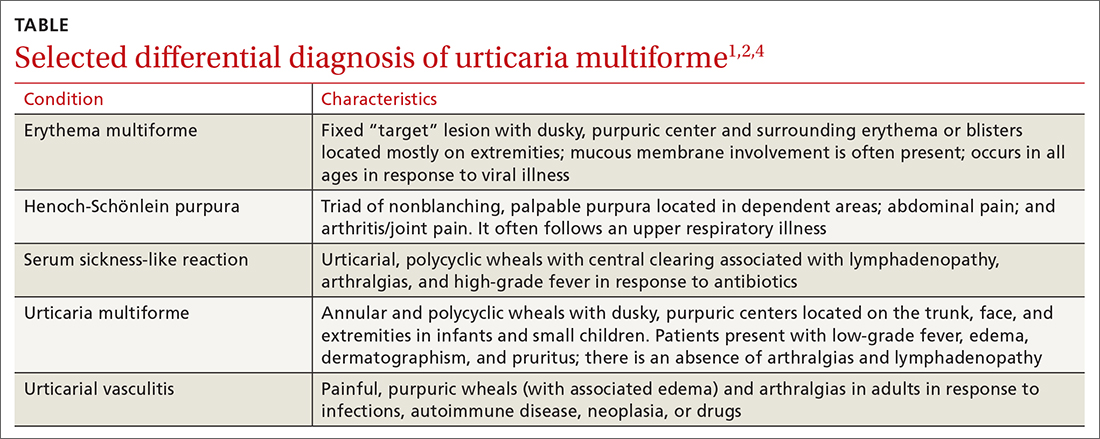
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).
Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).

Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
A 2-YEAR-OLD BOY presented to the emergency room with a 1-day history of a diffuse, mildly pruritic rash and swelling of his knees, ankles, and feet following treatment of acute otitis media with amoxicillin for the previous 8 days. He was mildly febrile and consolable, but he was refusing to walk. His medical history was unremarkable.
Physical examination revealed erythematous annular wheals on his chest, face, back, and extremities. Lymphadenopathy and mucous membrane involvement were not present. A complete blood count (CBC) with differential, inflammatory marker tests, and a comprehensive metabolic panel were ordered. Given the joint swelling and rash, the patient was admitted for observation.
During his second day in the hospital, his skin lesions enlarged and several formed dusky blue centers (FIGURE 1A). He also developed swelling of his hands (FIGURE 1B).

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Urticaria multiforme
The patient’s lab work came back within normal range, except for an elevated white blood cell count (19,700/mm3; reference range, 4500-13,500/mm3). His mild systemic symptoms, skin lesions without blistering or necrosis, acral edema, and the absence of lymphadenopathy pointed to a diagnosis of urticaria multiforme.
Urticaria multiforme, also called acute annular urticaria or acute urticarial hypersensitivity syndrome, is a histamine-mediated hypersensitivity reaction characterized by transient annular, polycyclic, urticarial lesions with central ecchymosis. The incidence and prevalence are not known. Urticaria multiforme is considered common, but it is frequently misdiagnosed.1 It typically manifests in children ages 4 months to 4 years and begins with small erythematous macules, papules, and plaques that progress to large blanchable wheals with dusky blue centers.1-3 Lesions are usually located on the face, trunk, and extremities and are often pruritic (60%-94%).1-3 Individual lesions last less than 24 hours, but new ones may appear. The rash generally lasts 2 to 12 days.1,3
Patients often report a preceding viral illness, otitis media, recent use of antibiotics, or recent immunizations. Dermatographism due to mast cell–mediated cutaneous hypersensitivity at sites of minor skin trauma is common (44%).
The diagnosis is made clinically and should not require a skin biopsy or extensive laboratory testing.When performed, laboratory studies, including CBC, erythrocyte sedimentation rate, C-reactive protein, and urinalysis are routinely normal.
Erythema multiforme and urticarial vasculitis are part of the differential
The differential diagnosis in this case includes erythema multiforme, Henoch-Schönlein purpura, serum sickness-like reaction, and urticarial vasculitis (TABLE1,2,4).

Continue to: Erythema multiforme
Erythema multiforme is a common misdiagnosis in patients with urticaria multiforme.1,2 The erythema multiforme rash has a “target” lesion with outer erythema and central ecchymosis, which may develop blisters or necrosis. Lesions are fixed and last 2 to 3 weeks. Unlike urticaria multiforme, patients with erythema multiforme commonly have mucous membrane erosions and occasionally ulcerations. Facial and acral edema is rare. Treatment is largely symptomatic and can include glucocorticoids. Antiviral medications may be used to treat recurrences.1,2
Henoch-Schönlein purpura is an immunoglobulin A–mediated vasculitis that affects the skin, gastrointestinal tract, and joints.4,5 Patients often present with arthralgias, gastrointestinal symptoms such as abdominal pain and bleeding, and a nonpruritic, erythematous rash that progresses to palpable purpura in dependent areas of the body. Treatment is generally symptomatic, but steroids may be used in severe cases.4,5
Serum sickness-like reaction can manifest with angioedema and a similar urticarial rash (with central clearing) that lasts 1 to 6 weeks.1,2,6,7 However, patients tend to have a high-grade fever, arthralgias, myalgias, and lymphadenopathy while dermatographism is absent. Treatment includes discontinuing the offending agent and the use of H1 and H2 antihistamines and steroids, in severe cases.
Urticarial vasculitis manifests as plaques or wheals lasting 1 to 7 days that may cause burning and pain but not pruritis.2,5 Purpura or hypopigmentation may develop as the hives resolve. Angioedema and arthralgias are common, but dermatographism is not present. Triggers include infections, autoimmune disease, malignancy, and the use of certain medications. H1 and H2 blockers and nonsteroidal anti-inflammatory agents are first-line therapy.2
Step 1: Discontinue offending agents; Step 2: Recommend antihistamines
Treatment consists of discontinuing any offending agent (if suspected) and using systemic H1 or H2 antihistamines for symptom relief. Systemic steroids should only be given in refractory cases.
Continue to: Our patient's amoxicillin
Our patient’s amoxicillin was discontinued, and he was started on a 14-day course of cetirizine 5 mg bid and hydroxyzine 10 mg at bedtime. He was also started on triamcinolone 0.1% cream to be applied twice daily for 1 week. During his 3-day hospital stay, his fever resolved and his rash and edema improved.
During an outpatient follow-up visit with a pediatric dermatologist 2 weeks after discharge, the patient’s rash was still present and dermatographism was noted. In light of this, his parents were instructed to continue giving the cetirizine and hydroxyzine once daily for an additional 2 weeks and to return as needed.
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
1. Shah KN, Honig PJ, Yan AC. “Urticaria multiforme”: a case series and review of acute annular urticarial hypersensitivity syndromes in children. Pediatrics. 2007;119:e1177-e1183. doi: 10.1542/peds.2006-1553
2. Emer JJ, Bernardo SG, Kovalerchik O, et al. Urticaria multiforme. J Clin Aesthet Dermatol. 2013;6:34-39.
3. Starnes L, Patel T, Skinner RB. Urticaria multiforme – a case report. Pediatr Dermatol. 2011; 28:436-438. doi: 10.1111/j.1525-1470.2011.01311.x
4. Reamy BV, Williams PM, Lindsay TJ. Henoch-Schönlein purpura. Am Fam Physician. 2009;80:697-704.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby, Elsevier Inc; 2016.
6. King BA, Geelhoed GC. Adverse skin and joint reactions associated with oral antibiotics in children: the role of cefaclor in serum sickness-like reactions. J Paediatr Child Health. 2003;39:677-681. doi: 10.1046/j.1440-1754.2003.00267.x
7. Misirlioglu ED, Duman H, Ozmen S, et al. Serum sickness-like reaction in children due to cefditoren. Pediatr Dermatol. 2011;29:327-328. doi: 10.1111/j.1525-1470.2011.01539.x
Children and COVID: New cases down slightly from record high
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
PHM 2021: Achieving gender equity in medicine
PHM 2021 session
Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion
Presenter
Julie Silver, MD
Session summary
Gender inequity in medicine has been well documented and further highlighted by the tremendous impact of the COVID-19 pandemic on women in medicine. While more women than men are entering medical schools across the U.S., women still struggle to reach the highest levels of academic rank, achieve leadership positions of power and influence, receive fair equitable pay, attain leadership roles in national societies, and receive funding from national agencies. They also continue to face discrimination and implicit and explicit biases. Women of color or from other minority backgrounds face even greater barriers and biases. Despite being a specialty in which women represent almost 70% of the workforce, pediatrics is not immune to these disparities.
In her PHM21 plenary on Aug. 3, 2021, Dr. Julie Silver, a national expert in gender equity disparities, detailed the landscape for women in medicine and proposed some solutions to accelerate systemic change for gender equity. In order to understand and mitigate gender inequity, Dr. Silver encouraged the PHM community to identify influential “gatekeepers” of promotion, advancement, and salary compensation. In academic medicine medical schools, funding agencies, professional societies, and journals are the gatekeepers to advancement and compensation for women. Women are traditionally underrepresented as members and influential leaders of these gatekeeping organizations and in their recognition structures, therefore their advancement, compensation, and wellbeing are hindered.
Key takeaways
- Critical mass theory will not help alleviate gender inequity in medicine, as women make up a critical mass in pediatrics and are still experiencing stark inequities. Critical actor leaders are needed to highlight disparities and drive change even once a critical mass is reached.
- Our current diversity, equity, and inclusion (DEI) efforts are ineffective and are creating an “illusion of fairness that causes majority group members to become less sensitive to recognizing discrimination against minorities.” Many of the activities that are considered citizenship, including committees focused on DEI efforts, should be counted as scholarship, and appropriately compensated to ensure promotion of our women and minority colleagues.
- Male allies are critical to documenting, disseminating, and addressing gender inequality. Without the support of men in the field, we will see little progress.
- While there are numerous advocacy angles we can take when advocating for gender equity, the most effective will be the financial angle. Gender pay gaps at the start of a career can lead to roughly 2 million dollars of salary loss for a woman over the course of her career. In order to alleviate those salary pay gaps our institutions must not expect women to negotiate for fair pay, make salary benchmarks transparent, continue to monitor and conduct research on compensation disparities, and attempt to alleviate the weight of educational debt.
- COVID-19 is causing immense stress on women in medicine, and the impact could be disastrous. We must recognize and reward the “4th shift” women are working for COVID-19–related activities at home and at work, and put measures in place to #GiveHerAReasonToStay in health care.
- Men and other women leaders have a responsibility to sponsor the many and well-qualified women in medicine for awards, committees, and speaking engagements. These opportunities are key markers of success in academic medicine and are critical to advancement and salary compensation.
Dr. Casillas is the internal medicine-pediatric chief resident for the University of Cincinnati/Cincinnati Children’s Internal Medicine-Pediatric program. His career goal is to serve as a hospitalist for children and adults, and he is interested in health equity and Latinx health. Dr. O’Toole is a pediatric and adult hospitalist at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center, and a professor of pediatrics and internal medicine at the University of Cincinnati College of Medicine. She serves as program director of Cincinnati’s Combined Internal Medicine and Pediatrics Residency Program.
PHM 2021 session
Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion
Presenter
Julie Silver, MD
Session summary
Gender inequity in medicine has been well documented and further highlighted by the tremendous impact of the COVID-19 pandemic on women in medicine. While more women than men are entering medical schools across the U.S., women still struggle to reach the highest levels of academic rank, achieve leadership positions of power and influence, receive fair equitable pay, attain leadership roles in national societies, and receive funding from national agencies. They also continue to face discrimination and implicit and explicit biases. Women of color or from other minority backgrounds face even greater barriers and biases. Despite being a specialty in which women represent almost 70% of the workforce, pediatrics is not immune to these disparities.
In her PHM21 plenary on Aug. 3, 2021, Dr. Julie Silver, a national expert in gender equity disparities, detailed the landscape for women in medicine and proposed some solutions to accelerate systemic change for gender equity. In order to understand and mitigate gender inequity, Dr. Silver encouraged the PHM community to identify influential “gatekeepers” of promotion, advancement, and salary compensation. In academic medicine medical schools, funding agencies, professional societies, and journals are the gatekeepers to advancement and compensation for women. Women are traditionally underrepresented as members and influential leaders of these gatekeeping organizations and in their recognition structures, therefore their advancement, compensation, and wellbeing are hindered.
Key takeaways
- Critical mass theory will not help alleviate gender inequity in medicine, as women make up a critical mass in pediatrics and are still experiencing stark inequities. Critical actor leaders are needed to highlight disparities and drive change even once a critical mass is reached.
- Our current diversity, equity, and inclusion (DEI) efforts are ineffective and are creating an “illusion of fairness that causes majority group members to become less sensitive to recognizing discrimination against minorities.” Many of the activities that are considered citizenship, including committees focused on DEI efforts, should be counted as scholarship, and appropriately compensated to ensure promotion of our women and minority colleagues.
- Male allies are critical to documenting, disseminating, and addressing gender inequality. Without the support of men in the field, we will see little progress.
- While there are numerous advocacy angles we can take when advocating for gender equity, the most effective will be the financial angle. Gender pay gaps at the start of a career can lead to roughly 2 million dollars of salary loss for a woman over the course of her career. In order to alleviate those salary pay gaps our institutions must not expect women to negotiate for fair pay, make salary benchmarks transparent, continue to monitor and conduct research on compensation disparities, and attempt to alleviate the weight of educational debt.
- COVID-19 is causing immense stress on women in medicine, and the impact could be disastrous. We must recognize and reward the “4th shift” women are working for COVID-19–related activities at home and at work, and put measures in place to #GiveHerAReasonToStay in health care.
- Men and other women leaders have a responsibility to sponsor the many and well-qualified women in medicine for awards, committees, and speaking engagements. These opportunities are key markers of success in academic medicine and are critical to advancement and salary compensation.
Dr. Casillas is the internal medicine-pediatric chief resident for the University of Cincinnati/Cincinnati Children’s Internal Medicine-Pediatric program. His career goal is to serve as a hospitalist for children and adults, and he is interested in health equity and Latinx health. Dr. O’Toole is a pediatric and adult hospitalist at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center, and a professor of pediatrics and internal medicine at the University of Cincinnati College of Medicine. She serves as program director of Cincinnati’s Combined Internal Medicine and Pediatrics Residency Program.
PHM 2021 session
Accelerating Patient Care and Healthcare Workforce Diversity and Inclusion
Presenter
Julie Silver, MD
Session summary
Gender inequity in medicine has been well documented and further highlighted by the tremendous impact of the COVID-19 pandemic on women in medicine. While more women than men are entering medical schools across the U.S., women still struggle to reach the highest levels of academic rank, achieve leadership positions of power and influence, receive fair equitable pay, attain leadership roles in national societies, and receive funding from national agencies. They also continue to face discrimination and implicit and explicit biases. Women of color or from other minority backgrounds face even greater barriers and biases. Despite being a specialty in which women represent almost 70% of the workforce, pediatrics is not immune to these disparities.
In her PHM21 plenary on Aug. 3, 2021, Dr. Julie Silver, a national expert in gender equity disparities, detailed the landscape for women in medicine and proposed some solutions to accelerate systemic change for gender equity. In order to understand and mitigate gender inequity, Dr. Silver encouraged the PHM community to identify influential “gatekeepers” of promotion, advancement, and salary compensation. In academic medicine medical schools, funding agencies, professional societies, and journals are the gatekeepers to advancement and compensation for women. Women are traditionally underrepresented as members and influential leaders of these gatekeeping organizations and in their recognition structures, therefore their advancement, compensation, and wellbeing are hindered.
Key takeaways
- Critical mass theory will not help alleviate gender inequity in medicine, as women make up a critical mass in pediatrics and are still experiencing stark inequities. Critical actor leaders are needed to highlight disparities and drive change even once a critical mass is reached.
- Our current diversity, equity, and inclusion (DEI) efforts are ineffective and are creating an “illusion of fairness that causes majority group members to become less sensitive to recognizing discrimination against minorities.” Many of the activities that are considered citizenship, including committees focused on DEI efforts, should be counted as scholarship, and appropriately compensated to ensure promotion of our women and minority colleagues.
- Male allies are critical to documenting, disseminating, and addressing gender inequality. Without the support of men in the field, we will see little progress.
- While there are numerous advocacy angles we can take when advocating for gender equity, the most effective will be the financial angle. Gender pay gaps at the start of a career can lead to roughly 2 million dollars of salary loss for a woman over the course of her career. In order to alleviate those salary pay gaps our institutions must not expect women to negotiate for fair pay, make salary benchmarks transparent, continue to monitor and conduct research on compensation disparities, and attempt to alleviate the weight of educational debt.
- COVID-19 is causing immense stress on women in medicine, and the impact could be disastrous. We must recognize and reward the “4th shift” women are working for COVID-19–related activities at home and at work, and put measures in place to #GiveHerAReasonToStay in health care.
- Men and other women leaders have a responsibility to sponsor the many and well-qualified women in medicine for awards, committees, and speaking engagements. These opportunities are key markers of success in academic medicine and are critical to advancement and salary compensation.
Dr. Casillas is the internal medicine-pediatric chief resident for the University of Cincinnati/Cincinnati Children’s Internal Medicine-Pediatric program. His career goal is to serve as a hospitalist for children and adults, and he is interested in health equity and Latinx health. Dr. O’Toole is a pediatric and adult hospitalist at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center, and a professor of pediatrics and internal medicine at the University of Cincinnati College of Medicine. She serves as program director of Cincinnati’s Combined Internal Medicine and Pediatrics Residency Program.
Study: Use urine sampling more broadly to rule out pediatric UTI
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.
Flu and COVID-19 vaccines can be given on the same day: CDC and AAP
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
Infants breathe better when pregnant moms exercise
Lung function in early infancy may be influenced by the mother’s level of physical activity during pregnancy, results of a study from Sweden suggest.
Low-lung function at 3 months of age, as measured by the ratio of time to peak tidal expiratory flow to expiratory time (tPTEF/tE), was more frequent among children whose mothers were physically inactive during the first half of pregnancy compared with those who exercised either moderately or strenuously, reported Hrefna Katrin Gudmundsdottir, MD, a pediatrician and PhD candidate at the University of Oslo, Norway. The results were based on a prospective observational study of 841 mother-child pairs.
“The potential link between maternal inactivity and low lung function in infancy adds to the importance of advising pregnant women and women of childbearing age on physical activity,” she said in an oral abstract presented during the virtual European Respiratory Society (ERS) International Congress.
Jonathan Grigg, MD, professor of pediatric respiratory and environmental medicine at Queen Mary University of London, who was not involved in the study, commented that it “offers a fascinating hint that increased physical activity of mothers is associated with better lung function in their babies and, therefore, possibly their health in later life. More research is needed to confirm this link, but it is important that women feel supported by their health care providers to be active in a way that is comfortable and accessible to them.”
Impaired lung function in infancy is associated with wheezing and asthma in childhood, and lower lung function later in life, Dr. Gudmundsdottir said. She also noted that impaired lung function begins in utero and is related to fetal and infant size, family history of asthma, and/or maternal smoking.
Physical activity during pregnancy has been demonstrated to reduce the risk of preterm birth and cesarean birth and of children being born either abnormally small or abnormally large for their gestational age, she explained.
To see where physical inactivity in the first half of pregnancy is associated with lower lung function in otherwise healthy 3-month old infants, Dr. Gudmundsdottir and colleagues looked at data on a mother-child cohort from the prospective population-based PreventADALL study, which was designed to study prevention of atopic dermatitis and allergies in children in Norway and Sweden.
A total of 814 infants (49% female) had available measures of tidal flow volume in the awake state at 3 months, as well as mother-reported data on physical activity at 18 weeks of pregnancy.
The investigators categorized the mothers as inactive, with either no or only low-intensity physical activity, “fairly” active, or “very” active based on self reporting.
The average tPTEF/tE value among all infants in the study was 0.391. The average value for 290 infants born to inactive mothers was 0.387, compared with 0.394 for 299 infants born to very active mothers, a difference that was not statistically significant.
Maternal physical activity level was not significantly associated with continuous tPTEF/tE, but the investigators did find that the offspring of inactive mothers were significantly more likely than the children of fairly or very active mothers to have a tPTEF/tE below 0.25 in both univariate analysis (odds ratio, 2.15; P = .011), and in multivariate analysis controlling for maternal age, education, parity, prepregnancy body-mass index, parental atopy, and in-utero exposure to nicotine (OR, 2.18; P = .013).
In univariate but not multivariate analysis, children of inactive mothers were significantly more likely than infants of more active mothers to have tPTEF/tE values below the 50th percentile (OR, 1.35; P = .042).
“We observed a trend that adds to the importance of advising women of childbearing age and pregnant women about physical activity. However, there may be factors that affect both maternal physical activity and lung function in offspring that we have not accounted for and could affect the results, so more research is needed,” Dr. Gudmundsdottir said in a statement.
Dr. Grigg pointed out that “it’s also worth keeping in mind that the single most important thing that mothers can do for their own health and that of their baby is to ensure that they do not smoke or use other tobacco products before, during, and after pregnancy. A smoke-free home has the biggest impact on lung function and health in childhood and later life.”
The study was supported by the University of Oslo. Dr. Gudmundsdottir and Dr. Grigg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lung function in early infancy may be influenced by the mother’s level of physical activity during pregnancy, results of a study from Sweden suggest.
Low-lung function at 3 months of age, as measured by the ratio of time to peak tidal expiratory flow to expiratory time (tPTEF/tE), was more frequent among children whose mothers were physically inactive during the first half of pregnancy compared with those who exercised either moderately or strenuously, reported Hrefna Katrin Gudmundsdottir, MD, a pediatrician and PhD candidate at the University of Oslo, Norway. The results were based on a prospective observational study of 841 mother-child pairs.
“The potential link between maternal inactivity and low lung function in infancy adds to the importance of advising pregnant women and women of childbearing age on physical activity,” she said in an oral abstract presented during the virtual European Respiratory Society (ERS) International Congress.
Jonathan Grigg, MD, professor of pediatric respiratory and environmental medicine at Queen Mary University of London, who was not involved in the study, commented that it “offers a fascinating hint that increased physical activity of mothers is associated with better lung function in their babies and, therefore, possibly their health in later life. More research is needed to confirm this link, but it is important that women feel supported by their health care providers to be active in a way that is comfortable and accessible to them.”
Impaired lung function in infancy is associated with wheezing and asthma in childhood, and lower lung function later in life, Dr. Gudmundsdottir said. She also noted that impaired lung function begins in utero and is related to fetal and infant size, family history of asthma, and/or maternal smoking.
Physical activity during pregnancy has been demonstrated to reduce the risk of preterm birth and cesarean birth and of children being born either abnormally small or abnormally large for their gestational age, she explained.
To see where physical inactivity in the first half of pregnancy is associated with lower lung function in otherwise healthy 3-month old infants, Dr. Gudmundsdottir and colleagues looked at data on a mother-child cohort from the prospective population-based PreventADALL study, which was designed to study prevention of atopic dermatitis and allergies in children in Norway and Sweden.
A total of 814 infants (49% female) had available measures of tidal flow volume in the awake state at 3 months, as well as mother-reported data on physical activity at 18 weeks of pregnancy.
The investigators categorized the mothers as inactive, with either no or only low-intensity physical activity, “fairly” active, or “very” active based on self reporting.
The average tPTEF/tE value among all infants in the study was 0.391. The average value for 290 infants born to inactive mothers was 0.387, compared with 0.394 for 299 infants born to very active mothers, a difference that was not statistically significant.
Maternal physical activity level was not significantly associated with continuous tPTEF/tE, but the investigators did find that the offspring of inactive mothers were significantly more likely than the children of fairly or very active mothers to have a tPTEF/tE below 0.25 in both univariate analysis (odds ratio, 2.15; P = .011), and in multivariate analysis controlling for maternal age, education, parity, prepregnancy body-mass index, parental atopy, and in-utero exposure to nicotine (OR, 2.18; P = .013).
In univariate but not multivariate analysis, children of inactive mothers were significantly more likely than infants of more active mothers to have tPTEF/tE values below the 50th percentile (OR, 1.35; P = .042).
“We observed a trend that adds to the importance of advising women of childbearing age and pregnant women about physical activity. However, there may be factors that affect both maternal physical activity and lung function in offspring that we have not accounted for and could affect the results, so more research is needed,” Dr. Gudmundsdottir said in a statement.
Dr. Grigg pointed out that “it’s also worth keeping in mind that the single most important thing that mothers can do for their own health and that of their baby is to ensure that they do not smoke or use other tobacco products before, during, and after pregnancy. A smoke-free home has the biggest impact on lung function and health in childhood and later life.”
The study was supported by the University of Oslo. Dr. Gudmundsdottir and Dr. Grigg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Lung function in early infancy may be influenced by the mother’s level of physical activity during pregnancy, results of a study from Sweden suggest.
Low-lung function at 3 months of age, as measured by the ratio of time to peak tidal expiratory flow to expiratory time (tPTEF/tE), was more frequent among children whose mothers were physically inactive during the first half of pregnancy compared with those who exercised either moderately or strenuously, reported Hrefna Katrin Gudmundsdottir, MD, a pediatrician and PhD candidate at the University of Oslo, Norway. The results were based on a prospective observational study of 841 mother-child pairs.
“The potential link between maternal inactivity and low lung function in infancy adds to the importance of advising pregnant women and women of childbearing age on physical activity,” she said in an oral abstract presented during the virtual European Respiratory Society (ERS) International Congress.
Jonathan Grigg, MD, professor of pediatric respiratory and environmental medicine at Queen Mary University of London, who was not involved in the study, commented that it “offers a fascinating hint that increased physical activity of mothers is associated with better lung function in their babies and, therefore, possibly their health in later life. More research is needed to confirm this link, but it is important that women feel supported by their health care providers to be active in a way that is comfortable and accessible to them.”
Impaired lung function in infancy is associated with wheezing and asthma in childhood, and lower lung function later in life, Dr. Gudmundsdottir said. She also noted that impaired lung function begins in utero and is related to fetal and infant size, family history of asthma, and/or maternal smoking.
Physical activity during pregnancy has been demonstrated to reduce the risk of preterm birth and cesarean birth and of children being born either abnormally small or abnormally large for their gestational age, she explained.
To see where physical inactivity in the first half of pregnancy is associated with lower lung function in otherwise healthy 3-month old infants, Dr. Gudmundsdottir and colleagues looked at data on a mother-child cohort from the prospective population-based PreventADALL study, which was designed to study prevention of atopic dermatitis and allergies in children in Norway and Sweden.
A total of 814 infants (49% female) had available measures of tidal flow volume in the awake state at 3 months, as well as mother-reported data on physical activity at 18 weeks of pregnancy.
The investigators categorized the mothers as inactive, with either no or only low-intensity physical activity, “fairly” active, or “very” active based on self reporting.
The average tPTEF/tE value among all infants in the study was 0.391. The average value for 290 infants born to inactive mothers was 0.387, compared with 0.394 for 299 infants born to very active mothers, a difference that was not statistically significant.
Maternal physical activity level was not significantly associated with continuous tPTEF/tE, but the investigators did find that the offspring of inactive mothers were significantly more likely than the children of fairly or very active mothers to have a tPTEF/tE below 0.25 in both univariate analysis (odds ratio, 2.15; P = .011), and in multivariate analysis controlling for maternal age, education, parity, prepregnancy body-mass index, parental atopy, and in-utero exposure to nicotine (OR, 2.18; P = .013).
In univariate but not multivariate analysis, children of inactive mothers were significantly more likely than infants of more active mothers to have tPTEF/tE values below the 50th percentile (OR, 1.35; P = .042).
“We observed a trend that adds to the importance of advising women of childbearing age and pregnant women about physical activity. However, there may be factors that affect both maternal physical activity and lung function in offspring that we have not accounted for and could affect the results, so more research is needed,” Dr. Gudmundsdottir said in a statement.
Dr. Grigg pointed out that “it’s also worth keeping in mind that the single most important thing that mothers can do for their own health and that of their baby is to ensure that they do not smoke or use other tobacco products before, during, and after pregnancy. A smoke-free home has the biggest impact on lung function and health in childhood and later life.”
The study was supported by the University of Oslo. Dr. Gudmundsdottir and Dr. Grigg have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.