User login
Darkening and Eruptive Nevi During Treatment With Erlotinib
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
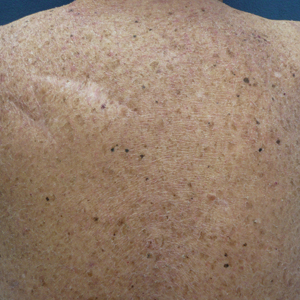
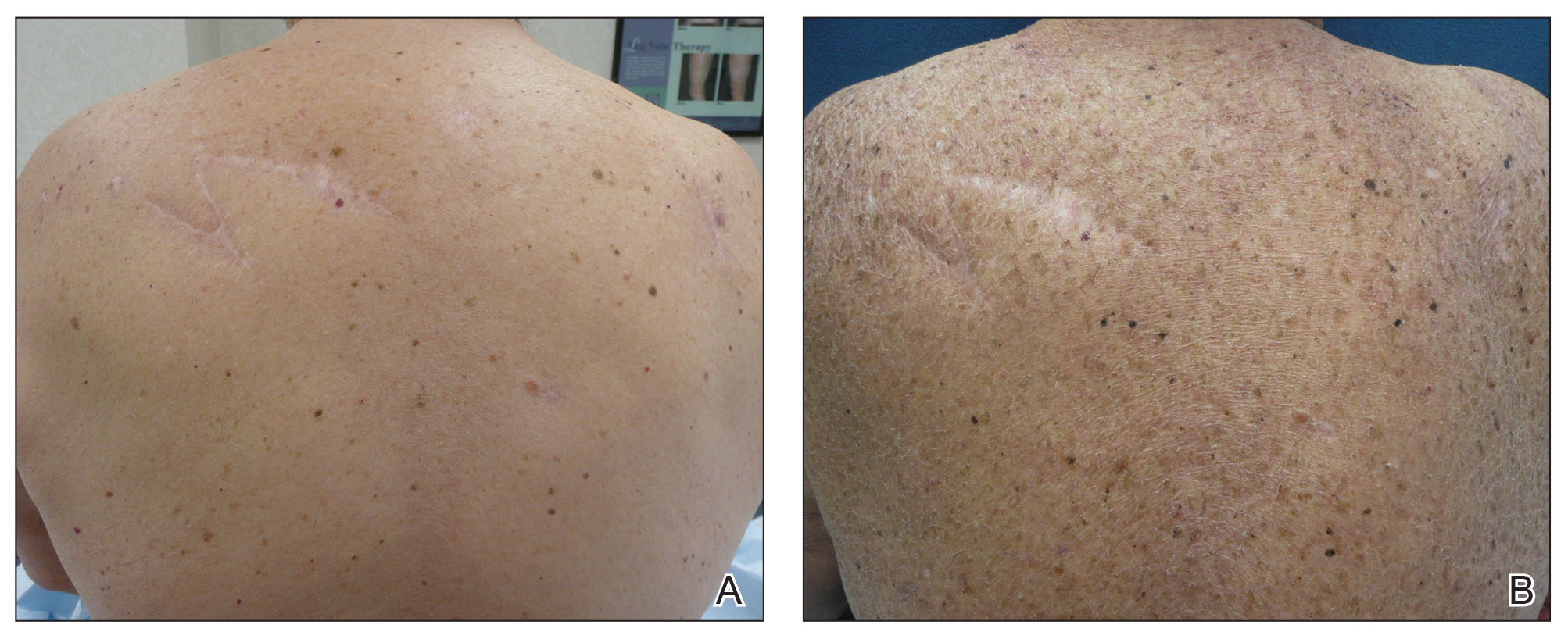
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).
The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
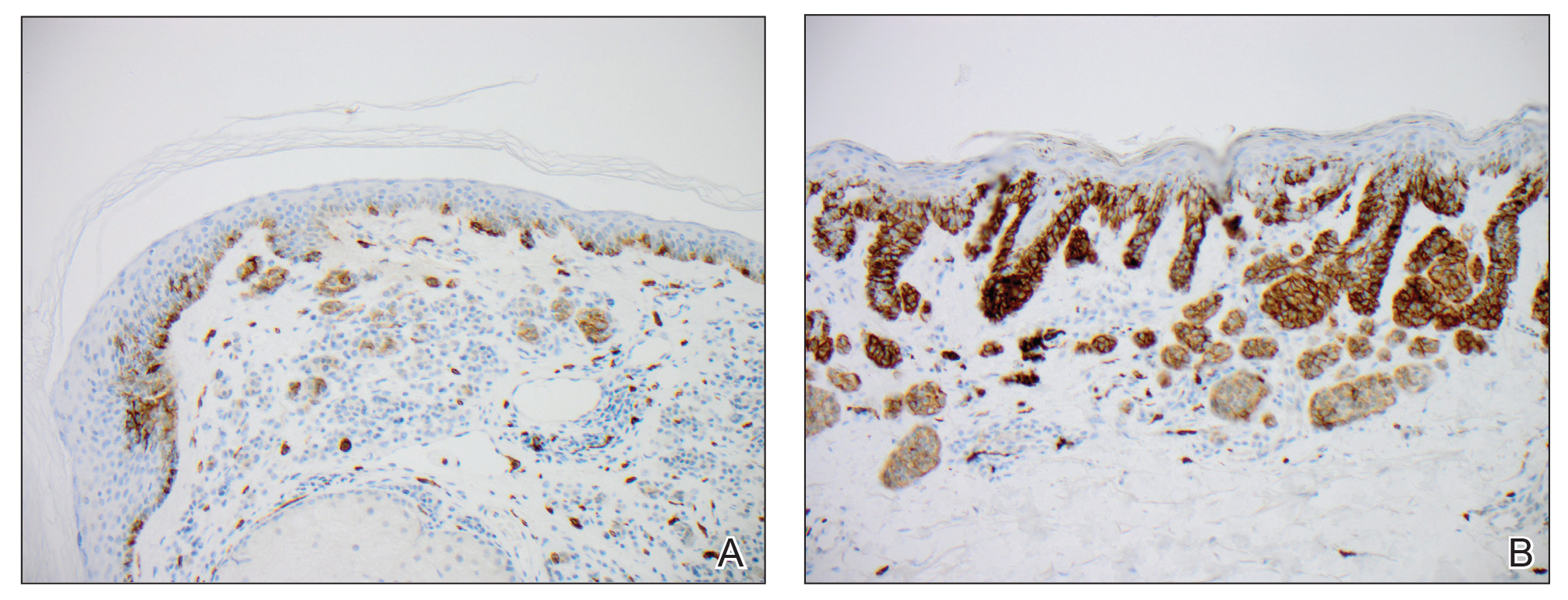
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.
In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).

The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.

In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).

The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.

In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
Practice Points
- Cutaneous side effects of erlotinib include acneform eruption, xerosis, paronychia, and pruritus.
- Clinicians should monitor patients for darkening and/or eruptive nevi as well as melanoma during treatment with erlotinib.
Topical ruxolitinib looks good for facial vitiligo, in phase 2 study
MILAN – Targeting the Janus kinase (JAK) 1 and 2 pathways in vitiligo resulted in significant reduction of facial depigmentation after 24 weeks of treatment, in a phase 2b trial of topical ruxolitinib cream.
compared with vehicle alone, said David Rosmarin, MD, speaking in a late-breaking abstracts session at the World Congress of Dermatology.
The highest response rate was seen with a higher dose: Among patients receiving ruxolitinib cream 1.5% once daily, 50% met the 50% clearing mark at 24 weeks, as did 45.5% of those with twice-daily 1.5% dosing of the 1.5% formulation. At 24 weeks, 3.1% of those receiving vehicle had 50% facial vitiligo resolution (P less than .0001, compared with vehicle for both doses).
Vitiligo affects about 3,000,000 people in the United States, and it is a plausible treatment target for the JAK inhibitor ruxolitinib, explained Dr. Rosmarin, a dermatologist at Tufts University, Boston. “Interferon-gamma, signaling through JAK1 and JAK2, is central to the pathogenesis of vitiligo,” he said. “Ruxolitinib is a potent inhibitor of JAK1 and JAK2, so it made sense to investigate it as a treatment for vitiligo.”
The 24-month randomized, double-blind, vehicle-controlled phase 2 study of ruxolitinib cream for vitiligo compared the vehicle to four different concentrations of ruxolitinib during the first phase of the study. For the first 24 weeks, patients were randomized to receive vehicle twice daily, or various doses of ruxolitinib ranging from 0.15% once daily to 1.5% twice daily.
At this point, the study’s primary endpoint was assessed, with investigators comparing the proportion of patients treated with ruxolitinib who had at least 50% improvement in facial repigmentation from baseline on the Facial Vitiligo Area Scoring Index (F-VASI50) compared with those who received vehicle. A secondary endpoint, also assessed at week 24, was the proportion of patients who were clear, or almost clear, of facial vitiligo; safety and tolerability were also assessed.
In addition to the F-VASI50 measure, Dr. Rosmarin and his coinvestigators also tracked 75% facial clearing (F-VASI75). Here, the 1.5% twice daily regimen topped the others, with 30% of those receiving that dose achieving F-VASI75, compared with almost 10%-17% of those on other doses.
Using another measure, More than one-third of patients using ruxolitinib (35.3%) had clear (no signs of vitiligo) or almost clear (only specks of depigmentation) facial skin at week 24, according to a clinician assessment tool. No patients on placebo had clear or almost clear facial skin at that point. “It is my hope that with continued use beyond week 24, more patients will meet this very stringent endpoint,” Dr. Rosmarin said.
The safety profile was good, with no serious treatment-related adverse events, and no application site reactions that reached clinical significance, although numerically more patients reported acne with ruxolitinib than with vehicle alone.
In the trial, patients aged 18-75 years with vitiligo were eligible if they had facial depigmentation that constituted at least half of their body surface area (BSA), as well as depigmentation of at least 3% of BSA on nonfacial areas. Patients were excluded if they had another dermatologic disease, infection, prior JAK inhibitor therapy, or recent use of biologic or experimental drugs, laser or light-based treatments, or immunomodulators. Of the 157 patients who were randomized, 18 patients (11.5%) had discontinued treatment by week 24, with 3 patients stopping for adverse events, 3 for protocol deviation or noncompliance, and 10 withdrawals. Two patients were lost to follow-up; all patients were included in analysis of the primary and secondary endpoints.
In the second year of the study, investigators rerandomized patients who had been receiving vehicle to an active arm of the study, and patients who had less than 25% improvement on a facial vitiligo scoring scale were rerandomized to one of the different doses. Twenty-eight weeks after rerandomization, all participants were given the opportunity to participate in a year-long open-label extension, receiving 1.5% ruxolitinib cream twice daily. Phototherapy was allowed in the extension arm, but not in the first year of the study.
Data beyond 24 weeks have not yet been reported, and the 2-year study plan acknowledged that “repigmentation takes a while,” Dr. Rosmarin said. He added that patients were allowed to use the study drug on body vitiligo as well, and many saw improvement there, although these results weren’t tracked in the study. “This isn’t a drug that’s meant just for the face,” he said.
Dr. Rosmarin and his coauthors reported financial arrangements with several pharmaceutical companies, including Incyte, which funded the study. An oral formulation of ruxolitinib (Jakafi), marketed by Incyte, was approved by the Food and Drug Administration in 2011, for myelofibrosis, and was recently approved for steroid-refractory acute graft-versus-host disease in adults and children aged 12 years and older.
MILAN – Targeting the Janus kinase (JAK) 1 and 2 pathways in vitiligo resulted in significant reduction of facial depigmentation after 24 weeks of treatment, in a phase 2b trial of topical ruxolitinib cream.
compared with vehicle alone, said David Rosmarin, MD, speaking in a late-breaking abstracts session at the World Congress of Dermatology.
The highest response rate was seen with a higher dose: Among patients receiving ruxolitinib cream 1.5% once daily, 50% met the 50% clearing mark at 24 weeks, as did 45.5% of those with twice-daily 1.5% dosing of the 1.5% formulation. At 24 weeks, 3.1% of those receiving vehicle had 50% facial vitiligo resolution (P less than .0001, compared with vehicle for both doses).
Vitiligo affects about 3,000,000 people in the United States, and it is a plausible treatment target for the JAK inhibitor ruxolitinib, explained Dr. Rosmarin, a dermatologist at Tufts University, Boston. “Interferon-gamma, signaling through JAK1 and JAK2, is central to the pathogenesis of vitiligo,” he said. “Ruxolitinib is a potent inhibitor of JAK1 and JAK2, so it made sense to investigate it as a treatment for vitiligo.”
The 24-month randomized, double-blind, vehicle-controlled phase 2 study of ruxolitinib cream for vitiligo compared the vehicle to four different concentrations of ruxolitinib during the first phase of the study. For the first 24 weeks, patients were randomized to receive vehicle twice daily, or various doses of ruxolitinib ranging from 0.15% once daily to 1.5% twice daily.
At this point, the study’s primary endpoint was assessed, with investigators comparing the proportion of patients treated with ruxolitinib who had at least 50% improvement in facial repigmentation from baseline on the Facial Vitiligo Area Scoring Index (F-VASI50) compared with those who received vehicle. A secondary endpoint, also assessed at week 24, was the proportion of patients who were clear, or almost clear, of facial vitiligo; safety and tolerability were also assessed.
In addition to the F-VASI50 measure, Dr. Rosmarin and his coinvestigators also tracked 75% facial clearing (F-VASI75). Here, the 1.5% twice daily regimen topped the others, with 30% of those receiving that dose achieving F-VASI75, compared with almost 10%-17% of those on other doses.
Using another measure, More than one-third of patients using ruxolitinib (35.3%) had clear (no signs of vitiligo) or almost clear (only specks of depigmentation) facial skin at week 24, according to a clinician assessment tool. No patients on placebo had clear or almost clear facial skin at that point. “It is my hope that with continued use beyond week 24, more patients will meet this very stringent endpoint,” Dr. Rosmarin said.
The safety profile was good, with no serious treatment-related adverse events, and no application site reactions that reached clinical significance, although numerically more patients reported acne with ruxolitinib than with vehicle alone.
In the trial, patients aged 18-75 years with vitiligo were eligible if they had facial depigmentation that constituted at least half of their body surface area (BSA), as well as depigmentation of at least 3% of BSA on nonfacial areas. Patients were excluded if they had another dermatologic disease, infection, prior JAK inhibitor therapy, or recent use of biologic or experimental drugs, laser or light-based treatments, or immunomodulators. Of the 157 patients who were randomized, 18 patients (11.5%) had discontinued treatment by week 24, with 3 patients stopping for adverse events, 3 for protocol deviation or noncompliance, and 10 withdrawals. Two patients were lost to follow-up; all patients were included in analysis of the primary and secondary endpoints.
In the second year of the study, investigators rerandomized patients who had been receiving vehicle to an active arm of the study, and patients who had less than 25% improvement on a facial vitiligo scoring scale were rerandomized to one of the different doses. Twenty-eight weeks after rerandomization, all participants were given the opportunity to participate in a year-long open-label extension, receiving 1.5% ruxolitinib cream twice daily. Phototherapy was allowed in the extension arm, but not in the first year of the study.
Data beyond 24 weeks have not yet been reported, and the 2-year study plan acknowledged that “repigmentation takes a while,” Dr. Rosmarin said. He added that patients were allowed to use the study drug on body vitiligo as well, and many saw improvement there, although these results weren’t tracked in the study. “This isn’t a drug that’s meant just for the face,” he said.
Dr. Rosmarin and his coauthors reported financial arrangements with several pharmaceutical companies, including Incyte, which funded the study. An oral formulation of ruxolitinib (Jakafi), marketed by Incyte, was approved by the Food and Drug Administration in 2011, for myelofibrosis, and was recently approved for steroid-refractory acute graft-versus-host disease in adults and children aged 12 years and older.
MILAN – Targeting the Janus kinase (JAK) 1 and 2 pathways in vitiligo resulted in significant reduction of facial depigmentation after 24 weeks of treatment, in a phase 2b trial of topical ruxolitinib cream.
compared with vehicle alone, said David Rosmarin, MD, speaking in a late-breaking abstracts session at the World Congress of Dermatology.
The highest response rate was seen with a higher dose: Among patients receiving ruxolitinib cream 1.5% once daily, 50% met the 50% clearing mark at 24 weeks, as did 45.5% of those with twice-daily 1.5% dosing of the 1.5% formulation. At 24 weeks, 3.1% of those receiving vehicle had 50% facial vitiligo resolution (P less than .0001, compared with vehicle for both doses).
Vitiligo affects about 3,000,000 people in the United States, and it is a plausible treatment target for the JAK inhibitor ruxolitinib, explained Dr. Rosmarin, a dermatologist at Tufts University, Boston. “Interferon-gamma, signaling through JAK1 and JAK2, is central to the pathogenesis of vitiligo,” he said. “Ruxolitinib is a potent inhibitor of JAK1 and JAK2, so it made sense to investigate it as a treatment for vitiligo.”
The 24-month randomized, double-blind, vehicle-controlled phase 2 study of ruxolitinib cream for vitiligo compared the vehicle to four different concentrations of ruxolitinib during the first phase of the study. For the first 24 weeks, patients were randomized to receive vehicle twice daily, or various doses of ruxolitinib ranging from 0.15% once daily to 1.5% twice daily.
At this point, the study’s primary endpoint was assessed, with investigators comparing the proportion of patients treated with ruxolitinib who had at least 50% improvement in facial repigmentation from baseline on the Facial Vitiligo Area Scoring Index (F-VASI50) compared with those who received vehicle. A secondary endpoint, also assessed at week 24, was the proportion of patients who were clear, or almost clear, of facial vitiligo; safety and tolerability were also assessed.
In addition to the F-VASI50 measure, Dr. Rosmarin and his coinvestigators also tracked 75% facial clearing (F-VASI75). Here, the 1.5% twice daily regimen topped the others, with 30% of those receiving that dose achieving F-VASI75, compared with almost 10%-17% of those on other doses.
Using another measure, More than one-third of patients using ruxolitinib (35.3%) had clear (no signs of vitiligo) or almost clear (only specks of depigmentation) facial skin at week 24, according to a clinician assessment tool. No patients on placebo had clear or almost clear facial skin at that point. “It is my hope that with continued use beyond week 24, more patients will meet this very stringent endpoint,” Dr. Rosmarin said.
The safety profile was good, with no serious treatment-related adverse events, and no application site reactions that reached clinical significance, although numerically more patients reported acne with ruxolitinib than with vehicle alone.
In the trial, patients aged 18-75 years with vitiligo were eligible if they had facial depigmentation that constituted at least half of their body surface area (BSA), as well as depigmentation of at least 3% of BSA on nonfacial areas. Patients were excluded if they had another dermatologic disease, infection, prior JAK inhibitor therapy, or recent use of biologic or experimental drugs, laser or light-based treatments, or immunomodulators. Of the 157 patients who were randomized, 18 patients (11.5%) had discontinued treatment by week 24, with 3 patients stopping for adverse events, 3 for protocol deviation or noncompliance, and 10 withdrawals. Two patients were lost to follow-up; all patients were included in analysis of the primary and secondary endpoints.
In the second year of the study, investigators rerandomized patients who had been receiving vehicle to an active arm of the study, and patients who had less than 25% improvement on a facial vitiligo scoring scale were rerandomized to one of the different doses. Twenty-eight weeks after rerandomization, all participants were given the opportunity to participate in a year-long open-label extension, receiving 1.5% ruxolitinib cream twice daily. Phototherapy was allowed in the extension arm, but not in the first year of the study.
Data beyond 24 weeks have not yet been reported, and the 2-year study plan acknowledged that “repigmentation takes a while,” Dr. Rosmarin said. He added that patients were allowed to use the study drug on body vitiligo as well, and many saw improvement there, although these results weren’t tracked in the study. “This isn’t a drug that’s meant just for the face,” he said.
Dr. Rosmarin and his coauthors reported financial arrangements with several pharmaceutical companies, including Incyte, which funded the study. An oral formulation of ruxolitinib (Jakafi), marketed by Incyte, was approved by the Food and Drug Administration in 2011, for myelofibrosis, and was recently approved for steroid-refractory acute graft-versus-host disease in adults and children aged 12 years and older.
REPORTING FROM WCD2019
Visual examinations yield signs to guide vitiligo treatment
MILAN – Subtle signs beyond depigmentation alone can guide management of vitiligo, Michelle Rodrigues, MBBS, said at the World Congress of Dermatology.
Signs of high disease activity can be visually observed and, when found, can compel urgent treatment, Dr. Rodrigues said. “If we identify and understand these [signs, they] can change our management plan, and the patient’s outcomes ... picking these up quickly, getting the best response you can, can help our patients tremendously.”
To assess clinical signs of severity in vitiligo, “use the tools that you have in your practice – your dermatoscope, your Wood’s lamp.”
Showing an image of the leg of a patient with vitiligo, Dr. Rodrigues said, “I know this patient’s vitiligo is very, very active. Why?” Clues come when there are areas of hypopigmentation at the rim of lesions, with depigmentation at the center. The presence of pigmentation, hypopigmentation, and depigmentation within the same lesion indicates high disease activity. This finding is the trichrome sign, also called the “blurry borders” sign in some regions, said Dr. Rodrigues, a dermatologist in Melbourne and the founder of Chroma Dermatology, which specializes in treating pigment problems and diagnosing and managing skin conditions in patients with skin of color.
Next, Dr. Rodrigues said, look at hair growth within the vitiliginous area. “If you’re unable to see that clinically, it’s really important to get that dermatoscope onto the patient, and look within a patch, to see whether or not you can actually see white hairs or normal colored hairs,” she said. This finding will help to determine both treatment plan and prognosis, since leukotrichia is a marker of disease severity in vitiligo.
Be alert to Koebnerization, said Dr. Rodrigues; the presentation may be subtle. As an example, she shared an image of a patient with depigmented patches on the dorsum of each foot. It wasn’t until the patient removed her foot gear – rubber slide-type sandals with a single broad strap over the dorsum – that Dr. Rodrigues recognized that “there was clear Koebnerization from the constant friction as a result of the wearing of the shoes.
“This can also be seen when patients scratch themselves, as can be seen with the itch that vitiligo can sometimes cause,” she said.
She noted that about 10% of patients with vitiligo have pruritus as a prominent symptom. Here, she said, is where a Wood’s lamp can be helpful as well. “Sometimes we can’t appreciate the very, very subtle Koebnerization, especially in patients with lighter skin. Getting out that Wood’s lamp and looking at other areas of involvement is really important,” she said. Areas of high disease activity and signs of progression that might otherwise be missed will be more obvious under the ultraviolet light.
It’s important to look beyond the obvious patches of vitiligo to examine the surrounding skin. Searching for “confetti depigmentation” – tiny white dots of depigmentation scattered over the otherwise normally pigmented skin – also marks high disease activity. An area with these dots – each often only a few millimeters in diameter – is likely destined for rapid depigmentation unless aggressive treatment is started. “We know that without treating these areas there will be very, very rapid and aggressive depigmentation. And remember that in areas that have a paucity of hair follicles, it might be irreversible ... so recognizing these signs is absolutely critical.”
The final clue to highly active disease that’s likely to move quickly without intervention can be found at the border of a vitiligo lesion. Look for a fine rim of erythema and some scale, Dr. Rodrigues said. This sign is common, and often seen early in the disease course. When this erythematous region is biopsied, ”You’ll see an intense inflammatory response, with an interface dermatitis. Again, this tells us that the patient may have a poorer prognosis if we don’t commence treatment early on.”
As a final clinical tip, Dr. Rodrigues reminded attendees that when one sign of disease activity is seen, others are often present. A thorough clinical examination is needed to document aggressive disease. “Please make sure that if you find one, you’re looking for other signs of disease severity as well.”
Dr. Rodrigues reported that she had no disclosures relevant to her presentation.
MILAN – Subtle signs beyond depigmentation alone can guide management of vitiligo, Michelle Rodrigues, MBBS, said at the World Congress of Dermatology.
Signs of high disease activity can be visually observed and, when found, can compel urgent treatment, Dr. Rodrigues said. “If we identify and understand these [signs, they] can change our management plan, and the patient’s outcomes ... picking these up quickly, getting the best response you can, can help our patients tremendously.”
To assess clinical signs of severity in vitiligo, “use the tools that you have in your practice – your dermatoscope, your Wood’s lamp.”
Showing an image of the leg of a patient with vitiligo, Dr. Rodrigues said, “I know this patient’s vitiligo is very, very active. Why?” Clues come when there are areas of hypopigmentation at the rim of lesions, with depigmentation at the center. The presence of pigmentation, hypopigmentation, and depigmentation within the same lesion indicates high disease activity. This finding is the trichrome sign, also called the “blurry borders” sign in some regions, said Dr. Rodrigues, a dermatologist in Melbourne and the founder of Chroma Dermatology, which specializes in treating pigment problems and diagnosing and managing skin conditions in patients with skin of color.
Next, Dr. Rodrigues said, look at hair growth within the vitiliginous area. “If you’re unable to see that clinically, it’s really important to get that dermatoscope onto the patient, and look within a patch, to see whether or not you can actually see white hairs or normal colored hairs,” she said. This finding will help to determine both treatment plan and prognosis, since leukotrichia is a marker of disease severity in vitiligo.
Be alert to Koebnerization, said Dr. Rodrigues; the presentation may be subtle. As an example, she shared an image of a patient with depigmented patches on the dorsum of each foot. It wasn’t until the patient removed her foot gear – rubber slide-type sandals with a single broad strap over the dorsum – that Dr. Rodrigues recognized that “there was clear Koebnerization from the constant friction as a result of the wearing of the shoes.
“This can also be seen when patients scratch themselves, as can be seen with the itch that vitiligo can sometimes cause,” she said.
She noted that about 10% of patients with vitiligo have pruritus as a prominent symptom. Here, she said, is where a Wood’s lamp can be helpful as well. “Sometimes we can’t appreciate the very, very subtle Koebnerization, especially in patients with lighter skin. Getting out that Wood’s lamp and looking at other areas of involvement is really important,” she said. Areas of high disease activity and signs of progression that might otherwise be missed will be more obvious under the ultraviolet light.
It’s important to look beyond the obvious patches of vitiligo to examine the surrounding skin. Searching for “confetti depigmentation” – tiny white dots of depigmentation scattered over the otherwise normally pigmented skin – also marks high disease activity. An area with these dots – each often only a few millimeters in diameter – is likely destined for rapid depigmentation unless aggressive treatment is started. “We know that without treating these areas there will be very, very rapid and aggressive depigmentation. And remember that in areas that have a paucity of hair follicles, it might be irreversible ... so recognizing these signs is absolutely critical.”
The final clue to highly active disease that’s likely to move quickly without intervention can be found at the border of a vitiligo lesion. Look for a fine rim of erythema and some scale, Dr. Rodrigues said. This sign is common, and often seen early in the disease course. When this erythematous region is biopsied, ”You’ll see an intense inflammatory response, with an interface dermatitis. Again, this tells us that the patient may have a poorer prognosis if we don’t commence treatment early on.”
As a final clinical tip, Dr. Rodrigues reminded attendees that when one sign of disease activity is seen, others are often present. A thorough clinical examination is needed to document aggressive disease. “Please make sure that if you find one, you’re looking for other signs of disease severity as well.”
Dr. Rodrigues reported that she had no disclosures relevant to her presentation.
MILAN – Subtle signs beyond depigmentation alone can guide management of vitiligo, Michelle Rodrigues, MBBS, said at the World Congress of Dermatology.
Signs of high disease activity can be visually observed and, when found, can compel urgent treatment, Dr. Rodrigues said. “If we identify and understand these [signs, they] can change our management plan, and the patient’s outcomes ... picking these up quickly, getting the best response you can, can help our patients tremendously.”
To assess clinical signs of severity in vitiligo, “use the tools that you have in your practice – your dermatoscope, your Wood’s lamp.”
Showing an image of the leg of a patient with vitiligo, Dr. Rodrigues said, “I know this patient’s vitiligo is very, very active. Why?” Clues come when there are areas of hypopigmentation at the rim of lesions, with depigmentation at the center. The presence of pigmentation, hypopigmentation, and depigmentation within the same lesion indicates high disease activity. This finding is the trichrome sign, also called the “blurry borders” sign in some regions, said Dr. Rodrigues, a dermatologist in Melbourne and the founder of Chroma Dermatology, which specializes in treating pigment problems and diagnosing and managing skin conditions in patients with skin of color.
Next, Dr. Rodrigues said, look at hair growth within the vitiliginous area. “If you’re unable to see that clinically, it’s really important to get that dermatoscope onto the patient, and look within a patch, to see whether or not you can actually see white hairs or normal colored hairs,” she said. This finding will help to determine both treatment plan and prognosis, since leukotrichia is a marker of disease severity in vitiligo.
Be alert to Koebnerization, said Dr. Rodrigues; the presentation may be subtle. As an example, she shared an image of a patient with depigmented patches on the dorsum of each foot. It wasn’t until the patient removed her foot gear – rubber slide-type sandals with a single broad strap over the dorsum – that Dr. Rodrigues recognized that “there was clear Koebnerization from the constant friction as a result of the wearing of the shoes.
“This can also be seen when patients scratch themselves, as can be seen with the itch that vitiligo can sometimes cause,” she said.
She noted that about 10% of patients with vitiligo have pruritus as a prominent symptom. Here, she said, is where a Wood’s lamp can be helpful as well. “Sometimes we can’t appreciate the very, very subtle Koebnerization, especially in patients with lighter skin. Getting out that Wood’s lamp and looking at other areas of involvement is really important,” she said. Areas of high disease activity and signs of progression that might otherwise be missed will be more obvious under the ultraviolet light.
It’s important to look beyond the obvious patches of vitiligo to examine the surrounding skin. Searching for “confetti depigmentation” – tiny white dots of depigmentation scattered over the otherwise normally pigmented skin – also marks high disease activity. An area with these dots – each often only a few millimeters in diameter – is likely destined for rapid depigmentation unless aggressive treatment is started. “We know that without treating these areas there will be very, very rapid and aggressive depigmentation. And remember that in areas that have a paucity of hair follicles, it might be irreversible ... so recognizing these signs is absolutely critical.”
The final clue to highly active disease that’s likely to move quickly without intervention can be found at the border of a vitiligo lesion. Look for a fine rim of erythema and some scale, Dr. Rodrigues said. This sign is common, and often seen early in the disease course. When this erythematous region is biopsied, ”You’ll see an intense inflammatory response, with an interface dermatitis. Again, this tells us that the patient may have a poorer prognosis if we don’t commence treatment early on.”
As a final clinical tip, Dr. Rodrigues reminded attendees that when one sign of disease activity is seen, others are often present. A thorough clinical examination is needed to document aggressive disease. “Please make sure that if you find one, you’re looking for other signs of disease severity as well.”
Dr. Rodrigues reported that she had no disclosures relevant to her presentation.
EXPERT ANALYSIS FROM WCD2019
Timolol shortens propranolol use in infantile hemangioma
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
according to a study published in Pediatric Dermatology.
Diana B. Mannschreck, BSN, of Johns Hopkins University, Baltimore, and colleagues performed a retrospective chart review of 559 patients with infantile hemangioma seen in the dermatology clinic at Johns Hopkins between December 2008 and January 2018. Patients received any of five courses of treatment, including oral propranolol followed by topical timolol, propranolol only, and timolol only. Of the courses evaluated, propranolol followed by timolol had the shortest duration of propranolol therapy – a median of 2.2 months shorter than propranolol-only therapy (P = .0006). This sequential regimen also was associated with no reinitiations of propranolol therapy following tapering, whereas 13% of those receiving propranolol alone had to reinitiate it after tapering.
This is of interest because oral beta-blockers, including propranolol, have been associated with rare but serious adverse events, such as bronchospasm, hypotension, and hypoglycemia.
Limitations of the study include its retrospective and single-center nature. There was no funding or disclosure information given.
SOURCE: Mannschreck DB et al. Pediatr Dermatol. 2019 Apr 9. doi: 10.1111/pde.13816.
FROM PEDIATRIC DERMATOLOGY
Topical calcineurin inhibitors prove beneficial for patients with vitiligo
Though responses to topical calcineurin inhibitors (TCIs) plus phototherapy were found to be higher than TCI monotherapy, a meta-analysis of studies on TCI therapy found that both should be used in treatment for patients with vitiligo.
“In addition, the proactive use of TCIs to maintain remission of vitiligo could be promising, considering its high recurrence rate,” wrote Ji Hae Lee, MD, PhD, of the Catholic University of Korea, Seoul, and coauthors in JAMA Dermatology.
To assess TCIs as treatment for vitiligo, the researchers undertook a systematic review and analysis of 56 relevant studies. Eleven of the studies were on the TCI mechanism; 36 were on TCI monotherapy; 12 were on TCI plus phototherapy; and 1 was on TCI maintenance therapy. Treatment responses for each study were measured via the degree of repigmentation on a quartile scale: an at least mild response (25% or greater repigmentation), at least moderate response (50% or greater repigmentation), and marked response (75% or greater repigmentation).
In regard to TCI monotherapy, an at least mild response was achieved in 55% (95% confidence interval, 42.2%-67.8%) of 560 patients in 21 studies. An at least moderate response was achieved in 38.5% (95% CI, 28.2%-48.8%) of 619 patients in 23 studies, and there was a marked response in 18.1% (95% CI, 13.2%-23.1%) of 520 patients in 19 studies.
For TCI plus phototherapy, an at least mild response was achieved in 89.5% (95% CI, 81.1%-97.9%) of 433 patients in eight studies. An at least moderate response was achieved in 72.9% (95% CI, 57.6%-88.2%) of 486 patients in 10 studies, and a marked response was achieved in 47.5% (95% CI, 30.6%-64.4%) of 490 patients in 9 studies.
The authors noted several limitations with their review, including a level of heterogeneity in the study designs, characteristics of the patients, and protocols. They also acknowledged that the quartile scale may be somewhat arbitrary in nature, though they added that it has been the “most commonly used measure and would have been one of the best estimates of the treatment response at this time.”
The authors report no conflicts of interest.
SOURCE: Lee JH et al. Jama Dermatol. 2019 May 29. doi: 10.1001/Jamadermatol.2019.0696.
Though responses to topical calcineurin inhibitors (TCIs) plus phototherapy were found to be higher than TCI monotherapy, a meta-analysis of studies on TCI therapy found that both should be used in treatment for patients with vitiligo.
“In addition, the proactive use of TCIs to maintain remission of vitiligo could be promising, considering its high recurrence rate,” wrote Ji Hae Lee, MD, PhD, of the Catholic University of Korea, Seoul, and coauthors in JAMA Dermatology.
To assess TCIs as treatment for vitiligo, the researchers undertook a systematic review and analysis of 56 relevant studies. Eleven of the studies were on the TCI mechanism; 36 were on TCI monotherapy; 12 were on TCI plus phototherapy; and 1 was on TCI maintenance therapy. Treatment responses for each study were measured via the degree of repigmentation on a quartile scale: an at least mild response (25% or greater repigmentation), at least moderate response (50% or greater repigmentation), and marked response (75% or greater repigmentation).
In regard to TCI monotherapy, an at least mild response was achieved in 55% (95% confidence interval, 42.2%-67.8%) of 560 patients in 21 studies. An at least moderate response was achieved in 38.5% (95% CI, 28.2%-48.8%) of 619 patients in 23 studies, and there was a marked response in 18.1% (95% CI, 13.2%-23.1%) of 520 patients in 19 studies.
For TCI plus phototherapy, an at least mild response was achieved in 89.5% (95% CI, 81.1%-97.9%) of 433 patients in eight studies. An at least moderate response was achieved in 72.9% (95% CI, 57.6%-88.2%) of 486 patients in 10 studies, and a marked response was achieved in 47.5% (95% CI, 30.6%-64.4%) of 490 patients in 9 studies.
The authors noted several limitations with their review, including a level of heterogeneity in the study designs, characteristics of the patients, and protocols. They also acknowledged that the quartile scale may be somewhat arbitrary in nature, though they added that it has been the “most commonly used measure and would have been one of the best estimates of the treatment response at this time.”
The authors report no conflicts of interest.
SOURCE: Lee JH et al. Jama Dermatol. 2019 May 29. doi: 10.1001/Jamadermatol.2019.0696.
Though responses to topical calcineurin inhibitors (TCIs) plus phototherapy were found to be higher than TCI monotherapy, a meta-analysis of studies on TCI therapy found that both should be used in treatment for patients with vitiligo.
“In addition, the proactive use of TCIs to maintain remission of vitiligo could be promising, considering its high recurrence rate,” wrote Ji Hae Lee, MD, PhD, of the Catholic University of Korea, Seoul, and coauthors in JAMA Dermatology.
To assess TCIs as treatment for vitiligo, the researchers undertook a systematic review and analysis of 56 relevant studies. Eleven of the studies were on the TCI mechanism; 36 were on TCI monotherapy; 12 were on TCI plus phototherapy; and 1 was on TCI maintenance therapy. Treatment responses for each study were measured via the degree of repigmentation on a quartile scale: an at least mild response (25% or greater repigmentation), at least moderate response (50% or greater repigmentation), and marked response (75% or greater repigmentation).
In regard to TCI monotherapy, an at least mild response was achieved in 55% (95% confidence interval, 42.2%-67.8%) of 560 patients in 21 studies. An at least moderate response was achieved in 38.5% (95% CI, 28.2%-48.8%) of 619 patients in 23 studies, and there was a marked response in 18.1% (95% CI, 13.2%-23.1%) of 520 patients in 19 studies.
For TCI plus phototherapy, an at least mild response was achieved in 89.5% (95% CI, 81.1%-97.9%) of 433 patients in eight studies. An at least moderate response was achieved in 72.9% (95% CI, 57.6%-88.2%) of 486 patients in 10 studies, and a marked response was achieved in 47.5% (95% CI, 30.6%-64.4%) of 490 patients in 9 studies.
The authors noted several limitations with their review, including a level of heterogeneity in the study designs, characteristics of the patients, and protocols. They also acknowledged that the quartile scale may be somewhat arbitrary in nature, though they added that it has been the “most commonly used measure and would have been one of the best estimates of the treatment response at this time.”
The authors report no conflicts of interest.
SOURCE: Lee JH et al. Jama Dermatol. 2019 May 29. doi: 10.1001/Jamadermatol.2019.0696.
FROM JAMA Dermatology
Leukemia Cutis–Associated Leonine Facies and Eyebrow Loss
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
To the Editor:
I read with interest the informative Cutis case report by Krooks and Weatherall1 in which the authors not only described the case of a 66-year-old man whose diagnosis of bone marrow biopsy–confirmed acute myeloid leukemia (AML) presented concurrently with skin biopsy–confirmed leukemia cutis but also discussed the poor prognosis of individuals with acute myelogenous leukemia cutis. Their patient died within 5 weeks of establishing the diagnosis. In addition, lateral and frontal photographs of the patient’s face demonstrated diffuse infiltrative plaques of leukemia cutis; he had swollen eyelids and lips with distortion of the nose secondary to dermal infiltration of leukemic myeloid cells.1 Although not emphasized by the authors, the patient appeared to have a leonine facies and at least partial loss of the lateral eyebrows.
Malignancy-associated leonine facies resulting from infiltration of the skin by neoplastic cells has been reported in a patient with metastatic breast carcinoma.2,3 However, it predominantly occurs in patients with hematologic dyscrasias such as leukemia cutis, lymphoma (ie, cutaneous B cell, cutaneous T cell, Hodgkin), plasmacytoma, and systemic mastocytosis.3,4 The report by Krooks and Weatherall1 adds AML-associated leukemia cutis to the previously observed types of leukemia cutis–related leonine facies in patients with acute lymphocytic leukemia, acute myelomonocytic leukemia, and chronic lymphocytic leukemia.3,4
Partial or complete loss of eyebrows in the setting of leonine facies has a limited differential diagnosis.3,5 In addition to cancer, the associated disorders include adnexal mucin deposition (alopecia mucinosis), granulomatous conditions (sarcoidosis), infectious diseases (leprosy), inherited syndromes (Setleis syndrome), photoallergic dermatoses (actinic reticuloid), and viral conditions (viral-associated trichodysplasia).3-9 Neoplasms associated with leonine facies and eyebrow loss include lymphomas (mycosis fungoides and unspecified cutaneous T-cell lymphoma), systemic mastocytosis and leukemia cutis secondary to acute lymphocytic leukemia, acute myelomonocytic leukemia, and now AML.1,3-5
The eyebrow loss associated with leonine facies often is not reversible once the causative cell of the associated condition (eg, granulomas of mycobacteria-infected histiocytes in leprosy, neoplastic lymphocytes in cutaneous T-cell lymphoma) has infiltrated the area of the eyebrows and abolished the preexisting hair follicles; however, follow-up descriptions of patients after treatment of other conditions that cause eyebrow loss usually are not reported. Indeed, there was partial reappearance of the eyebrows in a woman with systemic mastocytosis–associated loss of the eyebrows after malignancy-related treatment was reinitiated and the infiltrative facial plaques that had created her leonine facies had decreased in size.5 It is reasonable to speculate that the eyebrows may have reappeared in the patient reported by Krooks and Weatherall1 and his leonine facies–associated facial plaques may have resolved if he had underwent and responded to treatment with antineoplastic chemotherapy.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
- Krooks JA, Weatherall AG. Leukemia cutis in acute myeloid leukemia signifies a poor prognosis. Cutis. 2018;102:266, 271-272.
- Jin CC, Martinelli PT, Cohen PR. What are these erythematous skin lesions? leukemia cutis. The Dermatologist. 2012;20:46-50.
- Chodkiewicz HM, Cohen PR. Systemic mastocytosis-associated leonine facies and eyebrow loss. South Med J. 2011;104:236-238.
- Cohen PR, Rapini RP, Beran M. Infiltrated blue-gray plaques in a patient with leukemia. Chloroma (granulocytic sarcoma). Arch Dermatol. 1987;123:251, 254.
- Cohen PR. Leonine facies associated with eyebrow loss. Int J Dermatol. 2014;53:e148-e149.
- Ravic-Nikolic A, Milicic V, Ristic G, et al. Actinic reticuloid presented as facies leonine. Int J Dermatol. 2012;51:234-236.
- Jacob Raja SA, Raja JJ, Vijayashree R, et al. Evaluation of oral and periodontal status of leprosy patients in Dindigul district. J Pharm Bioallied Sci. 2016;8(suppl 1):S119-S121.
- McGaughran J, Aftimos S. Setleis syndrome: three new cases and a review of the literature. Am J Med Genet. 2002;111:376-380.
- Benoit T, Bacelieri R, Morrell DS, et al. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871-874.
Pigmented Fungiform Papillae of the Tongue in an Indian Male
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.
Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.

Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
To the Editor:
The tongue is composed of 4 different types of papillae: fungiform, foliate, circumvallate, and filiform. Fungiform papillae, primarily located on the tip and sides of the tongue, are mushroom-shaped epithelial elevations composed of taste buds at the upper surface overlying a core of connective tissue.1 Foliate and circumvallate papillae are likewise associated with taste buds, while the filiform papillae are hypothesized to exclusively provide a frictional surface for proper food manipulation. Pigmented fungiform papillae of the tongue (PFPT) was first reported by Leonard2 in 1905, who described discrete hyperpigmentation present only on the surface of fungiform papillae, mainly in black patients. Although they have been primarily described in black individuals, PFPT also has been occasionally reported in Asian and Middle Eastern individuals as well as Indian women.3-6
A 36-year-old Indian man initially presented to his primary care provider with brown discoloration of the dorsolateral aspects of the tongue that had been present since childhood. His primary care provider was concerned about a potential syndrome or systemic illness and referred the patient to dermatology for further evaluation. The patient denied any oral mucosal bleeding or discomfort, and a review of systems was unremarkable. His medical and family history were otherwise noncontributory, and he denied a history of tobacco use.
Physical examination of the tongue and oral mucosa revealed numerous 0.5- to 1.0-mm brown papillae in a symmetric distribution, primarily located on the tip and lateral aspects of the tongue (Figure). No hyperpigmentation was present on the posterior aspect of the tongue or on any other mucosal surface. Routine laboratory values were notable for mild elevations in aspartate aminotransferase and alanine aminotransferase (47 U/L [reference range, 10–30 U/L] and 64 U/L [reference range, 10–40 U/L], respectively) and mild hyperbilirubinemia (total bilirubin, 1.8 mg/dL [reference range, 0.3–1.2 mg/dL]). A complete blood cell count and electrolytes were within reference range. Based on the clinical appearance of the lesions and their presence since childhood, the patient was diagnosed with PFPT. No intervention was undertaken, and the patient was reassured of the benign nature of the lesions.

Pigmented fungiform papillae of the tongue presents in 3 variants. The first variant involves hyperpigmentation of all fungiform papillae located on the lateral and frontal aspects of the tongue and is the most common manifestation of PFPT.3 Our patient falls into this category. The second and third variants involve the dorsal surface, with the former involving only a few fungiform papillae on the dorsal aspect of the tongue and the latter variant involving all papillae.3 In 1974, Holzwanger et al3 conducted a survey of 300 random individuals, finding that 30% of black women and 25% of black men had some hyperpigmentation of the tongue, while only 1 white individual demonstrated lingual pigmentation. The physiology of PFPT remains largely unknown. Dermoscopic evaluation often demonstrates elevations with pigmented borders in a rose petal shape.7 Histopathologic evaluation reveals melanophages without inflammation that are positive for melanin on Fontana-Masson silver staining but negative for iron on Prussian blue staining.8
Despite the fact that PFPT is not a rare condition, the diagnosis remains notably missing from many standard dermatology textbooks and online dermatology resources, making it a potentially overlooked clinical entity.4-6 The tongue has a number of normal variations that are unlikely to be fully appreciated or acknowledged by dermatologists on routine physical examination but may cause distress to patients and raise concerns from primary care providers. Given that PFPT are benign, physicians should be aware of this diagnosis so as to provide reassurance to patients and avoid unnecessary testing. However, because the tongue can represent a harbinger of systemic disease, the differential diagnosis for the hyperpigmented lesions must always be considered, including Peutz-Jeghers syndrome, hemochromatosis, Addison disease, and Laugier-Hunziker syndrome (a rarer condition causing pigmented lesions on the lips, palate, and tongue), particularly if the hyperpigmented lesions extend beyond the fungiform papillae and do not fit into the 3 categories of PFPT.9
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
- Ross MH, Pawlina W. Digestive system I: oral cavity and associated structures. In: Ross MH, Pawlina W. Histology: A Text and Atlas, With Correlated Cell and Molecular Biology. 6th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2010:526-567.
- Leonard TMR. Ankylostomiasis or uncinariasis. JAMA. 1905;45:588-594.
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408.
- Tan C, Liu Y, Min ZS, et al. A clinical analysis of 58 Chinese cases of pigmented fungiform papillae of the tongue. J Eur Acad Dermatol Venereol. 2014;28:242-245.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399.
- Millington GW, Shah SN. A case of pigmented fungiform lingual papillae in an Indian woman. J Eur Acad Dermatol Venereol. 2007;21:705.
- Mukamal LV, Ormiga P, Ramos ESM. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399.
- Werchniak AE, Storm CA, Dinulos JG. Hyperpigmented patches on the tongue of a young girl. Pigmented fungiform papillae of the tongue. Arch Dermatol. 2004;140:1275-1280.
- Urbina F, Sudy E. Pigmented fungiform papillae of the tongue in Laugier disease or Laugier-Hunziker syndrome. Actas Dermosifiliogr. 2013;104:173-174.
Practice Points
- Pigmented fungiform papillae of the tongue are common lingual hyperpigmented macules in patients with skin of color.
- It is important to be aware of this benign entity to provide reassurance to patients and avoid unnecessary testing.
Prurigo Pigmentosa Induced by Ketosis: Resolution Through Dietary Modification
To the Editor:
A 40-year-old white woman presented with a waxing and waning erythematous pruritic rash on the chest, back, and axillae of 3 years’ duration. The appearance of the rash coincided with an intentional weight loss of more than 100 lb, achieved through various diets, most recently a Paleolithic (paleo) diet that was high in protein; low in carbohydrates; and specifically restricted dairy, cereal grains, refined sugars, processed foods, white potatoes, salt, refined oils, and legumes.1 The patient had been monitoring blood glucose and ketone levels. Prior to presentation, she received various treatments including clotrimazole cream and topical steroids with no improvement.
On physical examination, there were scaly, pink-red, reticulated papules and plaques coexisting with tan reticulated patches that were symmetrically distributed on the central back, lateral and central chest (Figure 1A), breasts, and inframammary areas. During the most severe flare-up, the blood ketones measured 1 mmol/L. There was no relevant medical history. She was of Spanish and Italian descent.
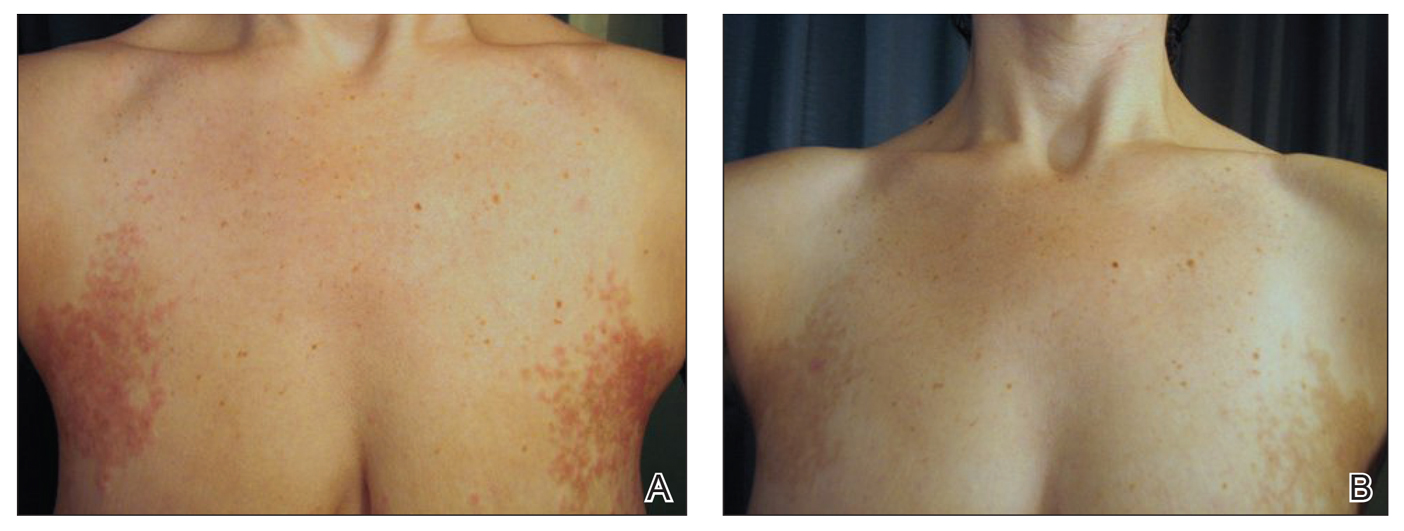
Histologic sections showed a sparse infiltrate of lymphocytes surrounding superficial dermal vessels and a mildly acanthotic epidermis with a focally parakeratotic stratum corneum (Figure 2A). Pigmentary incontinence and subtle interface changes were apparent, including rare necrotic keratinocytes (Figure 2B). No eosinophils or neutrophils were present.
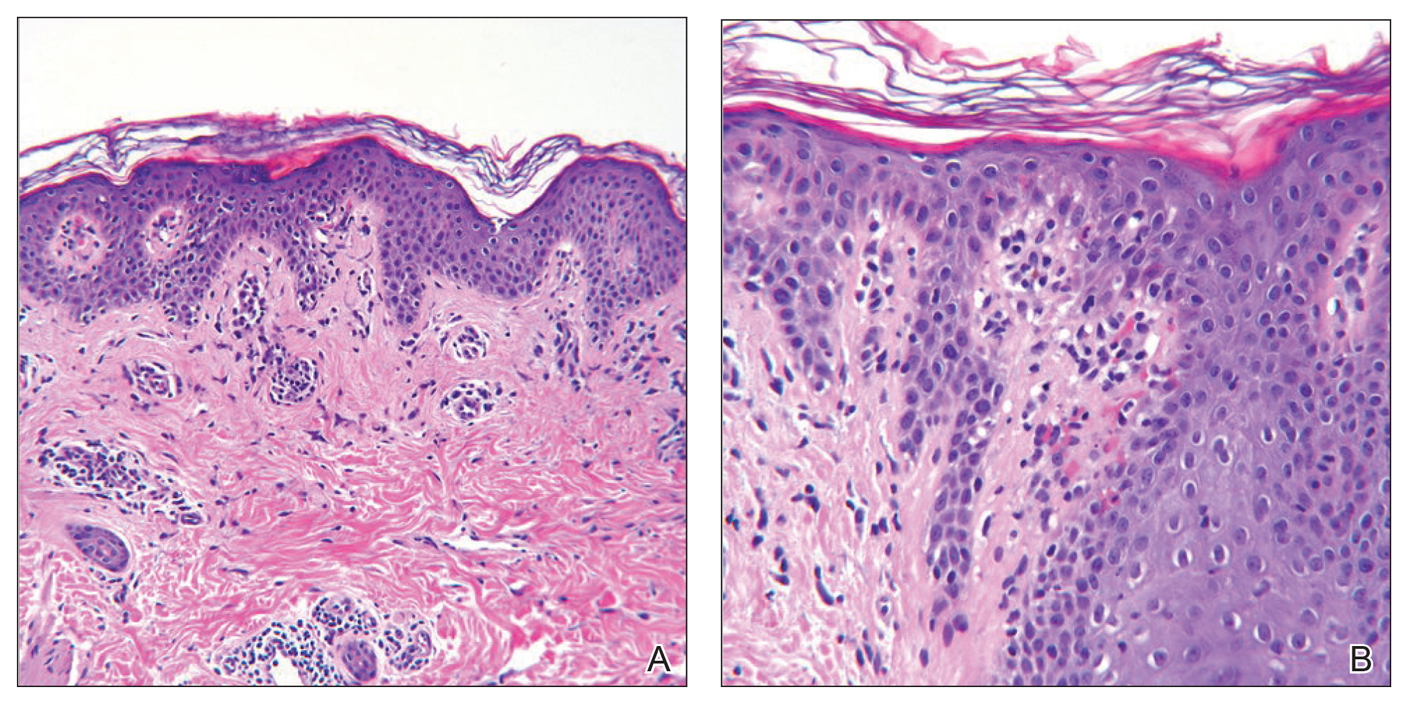
After the initial presentation, carbohydrates were added back into her diet and both the ketosis and eruption remarkably resolved. When carbohydrate restriction was rechallenged, she again entered ketosis (0.5 mmol/L), followed by subsequent recurrence of the pruritic lesions. With re-introduction of carbohydrates, the eruption and ketosis once more resolved, leaving only postinflammatory reticulated hyperpigmentation (Figure 1B). Based on the clinical presentation, supportive histopathologic findings, and interesting response to ketones and diet modification, the patient was diagnosed with prurigo pigmentosa (PP).
Prurigo pigmentosa is a rare inflammatory dermatosis that was initially described in 1971 as “a peculiar pruriginous dermatosis with gross reticular pigmentation” by Nagashima et al.2 Prurigo pigmentosa is most frequently diagnosed in Japan, and since its discovery, it has been reported in more than 300 cases worldwide.2-4
Fewer than 50 non-Japanese cases have been reported, with the possibility of an additional ethnic predisposition among the Turkish and Sicilian populations, though only 6 cases have been reported in the United States.3-6 Prurigo pigmentosa tends to occur in the spring and summer months and is most common among young females, with a mean age of 24 years. The typical lesions of PP are symmetrically distributed on the trunk with a tendency to localize on the upper back, nape of the neck, and intermammary and inframammary regions. Eruptions have been reported to occur on additional areas; however, mucus membranes are always spared.6
Individual lesions differ in appearance depending on the stage of presentation and are categorized as early, fully developed, resolving, and late lesions.6 Pruritic macules and papules are present early in the disease state and resolve into crusted and/or scaly papules followed by pigmented macules. Early lesions tend to be intensely pruritic with signs of excoriation, while resolving lesions lack symptoms. Lesions last approximately 1 week but tend to reappear at the site where they were previously present, which allows for lesions of different ages to coexist, appearing in a reticular arrangement with hyperpigmented mottling lasting from a few weeks to months.6
Just as the clinical picture transpires rapidly within 1 week, so do the histopathologic findings.6 Early lesions are categorized by a superficial perivascular and interstitial infiltrate of neutrophils, spongiosis, ballooning, and necrotic keratinocytes. These early lesions are present for less than 48 hours, and these histopathologic findings are diagnostic of PP. Within 2 days, lymphocytes predominate in the dermal infiltrate, and a patchy lichenoid aspect is established in the fully developed lesion along with reticular and vacuolar alterations. Late lesions show a parakeratotic and hyperpigmented epidermis with melanophages present in the papillary and reticular dermis. At this last stage, the histopathologic features of PP are indistinguishable from any other disease that results in postinflammatory hyperpigmentation, making diagnosis difficult.6
A variety of therapeutic options are used in the treatment of PP, with the most effective agents being oral antibiotics including dapsone, minocycline, and doxycycline, all of which limit the local tissue inflammatory response and cytotoxic effects. Topical and systemic antihistamines as well as corticosteroids are ineffective and have not been shown to prevent the postinflammatory reticular pigmentation.6-10
Various underlying factors have been associated with PP, including friction, heat, sunlight, sweating, allergic contact sensitization, and ketosis due to nutritional deficiency or diabetes mellitus; however; the exact etiology remains ambiguous.2-7 The association with ketosis and nutrition is of particular interest in this case. Onset of PP has been reported to coincide with dieting, fasting, weight loss, anorexia nervosa, and diabetes mellitus.3,6-9 Roughly 50 patients with PP had ketosis subsequent to these metabolic disturbances.3,6-10 As of now, the only reported correlation between ketosis and PP is that upon diet modification, lesions resolved following ketone normalization, as was observed in our patient.3,6-8 Reports of PP in diabetic patients while in ketoacidosis describe resolution of lesions with insulin administration.6-9 The pathophysiology of ketosis and its association with PP is unclear; however, the similarities seen in the immune response of PP and that stimulated by ketosis may expose an associated mechanism.
Ketosis is a temporary condition characterized by elevated serum ketones that are used as an alternative energy source when blood glucose is low or insulin is deficient.11 The most common causes of ketosis are the physiologic responses to fasting, prolonged exercise, or a high-protein/low-carbohydrate diet, though pathologic causes include insulin-dependent diabetes mellitus, alcoholism, and salicylate overdose.11 In healthy individuals, blood ketone levels rarely approach 0.5 mmol/L. Prolonged fasting or restricting intake of carbohydrates to less than 40 g daily can induce mild ketosis that resolves with re-introduction of carbohydrates.11
Ketone bodies pass from the circulating blood into tissues or remain near the blood vessels, inducing cytotoxic effects and perivascular inflammation.10,11 Increased ketone bodies have been shown to upregulate intercellular adhesion molecule 1 (ICAM-1) and leukocyte function-associated antigen 1 (LFA-1), a phenomenon also seen in lesional keratinocytes of PP.12,13 Teraki et al13 observed that epidermal keratinocytes exhibited increased expression of ICAM-1 as well as intense expression of LFA-1 on dermal and epidermotropic leukocytes, which was thought to be due to cell-mediated cytotoxicity. Not only do increased ketone bodies upregulate ICAM-1 and LFA-1, but they also are involved in increasing many proinflammatory mediators that may be capable of inducing the response seen in PP keratinocytes.12,13
Intercellular adhesion molecule 1 is important in initiating cellular interactions in the immune response and is the ligand for LFA-1 found on most leukocytes.14 Increased ICAM-1/LFA-1 interaction is thought to be the major pathway by which leukocytes are able to attach to keratinocytes and endothelial cells, allowing for leukocyte tissue migration and specific immunologic reactions, including leukocyte-mediated cytotoxicity. Interestingly, glucocorticoids are ineffective in reducing the expression of ICAM-1 in cultured keratinocytes.14 This connection between ketosis and inflammation that results in leukocyte migration and ultimately keratinocyte cytotoxicity may well be fundamental to the pathophysiology of PP and may provide a possible explanation for the ineffectiveness of corticosteroid treatment.
Middleton and Norris15 observed that individual keratinocyte strains show considerable variability in ICAM-1 expression that was found to be attributable to genetic polymorphisms. The presence of a particular polymorphism affecting ICAM-1 expression on human keratinocytes may explain the apparent ethnogeographic predisposition of PP as well as the ease at which ICAM-1 is expressed in the presence of ketones.
We describe a case of a 40-year-old white woman who was diagnosed with PP that was prompted by a 100-lb weight loss and self-induced ketosis while following a paleo diet with carbohydrate restriction. Successful treatment was attained through diet modification alone. This interesting case was another instance in which the pathophysiology of PP was attributed to ketosis. Because not all patients that are in ketosis have PP, larger prospective cohort studies are needed to further elucidate the association of PP and ketosis.
- What is the paleo diet? The Paleo Diet website. http://thepaleodiet.com/the-paleo-diet-premise. Accessed March 9, 2019.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation [in Japanese]. Japanese J Dermatol. 1971;81:38-39.
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet [published online December 30, 2013]. Pediatr Dermatol. 2015;32:248-251.
- Baykal C, Buyukbabani N, Akinturk S, et al. Prurigo pigmentosa: not an uncommon disease in the Turkish population. Int J Dermatol. 2006;45:1164-1168.
- Whang T, Kirkorian Y, Krishtul A, et al. Prurigo pigmentosa: report of two cases in the United States and review of the literature. Dermatology Online J. 2011;17:2.
- Böer A, Ackerman AB. Prurigo Pigmentosa (Nagashima Disease): Textbook and Atlas of a Distinctive Inflammatory Disease of the Skin. New York, NY: Ardor Scribendi Ltd; 2004.
- Teraki Y, Teraki E, Kawashima M, at al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511.
- Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2011;26:1149-1153.
- Yokozeki M, Watanabe J, Hotsubo T, et al. Prurigo pigmentosa disappeared following improvement of diabetic ketosis by insulin. J Dermatol. 2003;30:257-258.
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
- VanItallie TB, Nufert TH. Ketones: metabolism’s ugly duckling. Annu Rev Nutr. 2003;61:327-341.
- Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:e298-e306.
- Teraki Y, Shiohara T, Nagashima M, et al. Prurigo pigmentosa: role of ICAM-1 in the localization of the eruption. Br J Dermatol. 1991;125:360-363.
- Kashihara-Sawami M, Norris DA. The state of differentiation of cultured human keratinocytes determines the level of intercellular adhesion molecule-1 (ICAM-1) expression induced by gamma interferon. J Invest Dermatol. 1992;98:741-747.
- Middleton MH, Norris DA. Cytokine-induced ICAM-1 expression in human keratinocytes is highly variable in keratinocyte strains from different donors. J Invest Dermatol. 1995;104:489-496.
To the Editor:
A 40-year-old white woman presented with a waxing and waning erythematous pruritic rash on the chest, back, and axillae of 3 years’ duration. The appearance of the rash coincided with an intentional weight loss of more than 100 lb, achieved through various diets, most recently a Paleolithic (paleo) diet that was high in protein; low in carbohydrates; and specifically restricted dairy, cereal grains, refined sugars, processed foods, white potatoes, salt, refined oils, and legumes.1 The patient had been monitoring blood glucose and ketone levels. Prior to presentation, she received various treatments including clotrimazole cream and topical steroids with no improvement.
On physical examination, there were scaly, pink-red, reticulated papules and plaques coexisting with tan reticulated patches that were symmetrically distributed on the central back, lateral and central chest (Figure 1A), breasts, and inframammary areas. During the most severe flare-up, the blood ketones measured 1 mmol/L. There was no relevant medical history. She was of Spanish and Italian descent.

Histologic sections showed a sparse infiltrate of lymphocytes surrounding superficial dermal vessels and a mildly acanthotic epidermis with a focally parakeratotic stratum corneum (Figure 2A). Pigmentary incontinence and subtle interface changes were apparent, including rare necrotic keratinocytes (Figure 2B). No eosinophils or neutrophils were present.

After the initial presentation, carbohydrates were added back into her diet and both the ketosis and eruption remarkably resolved. When carbohydrate restriction was rechallenged, she again entered ketosis (0.5 mmol/L), followed by subsequent recurrence of the pruritic lesions. With re-introduction of carbohydrates, the eruption and ketosis once more resolved, leaving only postinflammatory reticulated hyperpigmentation (Figure 1B). Based on the clinical presentation, supportive histopathologic findings, and interesting response to ketones and diet modification, the patient was diagnosed with prurigo pigmentosa (PP).
Prurigo pigmentosa is a rare inflammatory dermatosis that was initially described in 1971 as “a peculiar pruriginous dermatosis with gross reticular pigmentation” by Nagashima et al.2 Prurigo pigmentosa is most frequently diagnosed in Japan, and since its discovery, it has been reported in more than 300 cases worldwide.2-4
Fewer than 50 non-Japanese cases have been reported, with the possibility of an additional ethnic predisposition among the Turkish and Sicilian populations, though only 6 cases have been reported in the United States.3-6 Prurigo pigmentosa tends to occur in the spring and summer months and is most common among young females, with a mean age of 24 years. The typical lesions of PP are symmetrically distributed on the trunk with a tendency to localize on the upper back, nape of the neck, and intermammary and inframammary regions. Eruptions have been reported to occur on additional areas; however, mucus membranes are always spared.6
Individual lesions differ in appearance depending on the stage of presentation and are categorized as early, fully developed, resolving, and late lesions.6 Pruritic macules and papules are present early in the disease state and resolve into crusted and/or scaly papules followed by pigmented macules. Early lesions tend to be intensely pruritic with signs of excoriation, while resolving lesions lack symptoms. Lesions last approximately 1 week but tend to reappear at the site where they were previously present, which allows for lesions of different ages to coexist, appearing in a reticular arrangement with hyperpigmented mottling lasting from a few weeks to months.6
Just as the clinical picture transpires rapidly within 1 week, so do the histopathologic findings.6 Early lesions are categorized by a superficial perivascular and interstitial infiltrate of neutrophils, spongiosis, ballooning, and necrotic keratinocytes. These early lesions are present for less than 48 hours, and these histopathologic findings are diagnostic of PP. Within 2 days, lymphocytes predominate in the dermal infiltrate, and a patchy lichenoid aspect is established in the fully developed lesion along with reticular and vacuolar alterations. Late lesions show a parakeratotic and hyperpigmented epidermis with melanophages present in the papillary and reticular dermis. At this last stage, the histopathologic features of PP are indistinguishable from any other disease that results in postinflammatory hyperpigmentation, making diagnosis difficult.6
A variety of therapeutic options are used in the treatment of PP, with the most effective agents being oral antibiotics including dapsone, minocycline, and doxycycline, all of which limit the local tissue inflammatory response and cytotoxic effects. Topical and systemic antihistamines as well as corticosteroids are ineffective and have not been shown to prevent the postinflammatory reticular pigmentation.6-10
Various underlying factors have been associated with PP, including friction, heat, sunlight, sweating, allergic contact sensitization, and ketosis due to nutritional deficiency or diabetes mellitus; however; the exact etiology remains ambiguous.2-7 The association with ketosis and nutrition is of particular interest in this case. Onset of PP has been reported to coincide with dieting, fasting, weight loss, anorexia nervosa, and diabetes mellitus.3,6-9 Roughly 50 patients with PP had ketosis subsequent to these metabolic disturbances.3,6-10 As of now, the only reported correlation between ketosis and PP is that upon diet modification, lesions resolved following ketone normalization, as was observed in our patient.3,6-8 Reports of PP in diabetic patients while in ketoacidosis describe resolution of lesions with insulin administration.6-9 The pathophysiology of ketosis and its association with PP is unclear; however, the similarities seen in the immune response of PP and that stimulated by ketosis may expose an associated mechanism.
Ketosis is a temporary condition characterized by elevated serum ketones that are used as an alternative energy source when blood glucose is low or insulin is deficient.11 The most common causes of ketosis are the physiologic responses to fasting, prolonged exercise, or a high-protein/low-carbohydrate diet, though pathologic causes include insulin-dependent diabetes mellitus, alcoholism, and salicylate overdose.11 In healthy individuals, blood ketone levels rarely approach 0.5 mmol/L. Prolonged fasting or restricting intake of carbohydrates to less than 40 g daily can induce mild ketosis that resolves with re-introduction of carbohydrates.11
Ketone bodies pass from the circulating blood into tissues or remain near the blood vessels, inducing cytotoxic effects and perivascular inflammation.10,11 Increased ketone bodies have been shown to upregulate intercellular adhesion molecule 1 (ICAM-1) and leukocyte function-associated antigen 1 (LFA-1), a phenomenon also seen in lesional keratinocytes of PP.12,13 Teraki et al13 observed that epidermal keratinocytes exhibited increased expression of ICAM-1 as well as intense expression of LFA-1 on dermal and epidermotropic leukocytes, which was thought to be due to cell-mediated cytotoxicity. Not only do increased ketone bodies upregulate ICAM-1 and LFA-1, but they also are involved in increasing many proinflammatory mediators that may be capable of inducing the response seen in PP keratinocytes.12,13
Intercellular adhesion molecule 1 is important in initiating cellular interactions in the immune response and is the ligand for LFA-1 found on most leukocytes.14 Increased ICAM-1/LFA-1 interaction is thought to be the major pathway by which leukocytes are able to attach to keratinocytes and endothelial cells, allowing for leukocyte tissue migration and specific immunologic reactions, including leukocyte-mediated cytotoxicity. Interestingly, glucocorticoids are ineffective in reducing the expression of ICAM-1 in cultured keratinocytes.14 This connection between ketosis and inflammation that results in leukocyte migration and ultimately keratinocyte cytotoxicity may well be fundamental to the pathophysiology of PP and may provide a possible explanation for the ineffectiveness of corticosteroid treatment.
Middleton and Norris15 observed that individual keratinocyte strains show considerable variability in ICAM-1 expression that was found to be attributable to genetic polymorphisms. The presence of a particular polymorphism affecting ICAM-1 expression on human keratinocytes may explain the apparent ethnogeographic predisposition of PP as well as the ease at which ICAM-1 is expressed in the presence of ketones.
We describe a case of a 40-year-old white woman who was diagnosed with PP that was prompted by a 100-lb weight loss and self-induced ketosis while following a paleo diet with carbohydrate restriction. Successful treatment was attained through diet modification alone. This interesting case was another instance in which the pathophysiology of PP was attributed to ketosis. Because not all patients that are in ketosis have PP, larger prospective cohort studies are needed to further elucidate the association of PP and ketosis.
To the Editor:
A 40-year-old white woman presented with a waxing and waning erythematous pruritic rash on the chest, back, and axillae of 3 years’ duration. The appearance of the rash coincided with an intentional weight loss of more than 100 lb, achieved through various diets, most recently a Paleolithic (paleo) diet that was high in protein; low in carbohydrates; and specifically restricted dairy, cereal grains, refined sugars, processed foods, white potatoes, salt, refined oils, and legumes.1 The patient had been monitoring blood glucose and ketone levels. Prior to presentation, she received various treatments including clotrimazole cream and topical steroids with no improvement.
On physical examination, there were scaly, pink-red, reticulated papules and plaques coexisting with tan reticulated patches that were symmetrically distributed on the central back, lateral and central chest (Figure 1A), breasts, and inframammary areas. During the most severe flare-up, the blood ketones measured 1 mmol/L. There was no relevant medical history. She was of Spanish and Italian descent.

Histologic sections showed a sparse infiltrate of lymphocytes surrounding superficial dermal vessels and a mildly acanthotic epidermis with a focally parakeratotic stratum corneum (Figure 2A). Pigmentary incontinence and subtle interface changes were apparent, including rare necrotic keratinocytes (Figure 2B). No eosinophils or neutrophils were present.

After the initial presentation, carbohydrates were added back into her diet and both the ketosis and eruption remarkably resolved. When carbohydrate restriction was rechallenged, she again entered ketosis (0.5 mmol/L), followed by subsequent recurrence of the pruritic lesions. With re-introduction of carbohydrates, the eruption and ketosis once more resolved, leaving only postinflammatory reticulated hyperpigmentation (Figure 1B). Based on the clinical presentation, supportive histopathologic findings, and interesting response to ketones and diet modification, the patient was diagnosed with prurigo pigmentosa (PP).
Prurigo pigmentosa is a rare inflammatory dermatosis that was initially described in 1971 as “a peculiar pruriginous dermatosis with gross reticular pigmentation” by Nagashima et al.2 Prurigo pigmentosa is most frequently diagnosed in Japan, and since its discovery, it has been reported in more than 300 cases worldwide.2-4
Fewer than 50 non-Japanese cases have been reported, with the possibility of an additional ethnic predisposition among the Turkish and Sicilian populations, though only 6 cases have been reported in the United States.3-6 Prurigo pigmentosa tends to occur in the spring and summer months and is most common among young females, with a mean age of 24 years. The typical lesions of PP are symmetrically distributed on the trunk with a tendency to localize on the upper back, nape of the neck, and intermammary and inframammary regions. Eruptions have been reported to occur on additional areas; however, mucus membranes are always spared.6
Individual lesions differ in appearance depending on the stage of presentation and are categorized as early, fully developed, resolving, and late lesions.6 Pruritic macules and papules are present early in the disease state and resolve into crusted and/or scaly papules followed by pigmented macules. Early lesions tend to be intensely pruritic with signs of excoriation, while resolving lesions lack symptoms. Lesions last approximately 1 week but tend to reappear at the site where they were previously present, which allows for lesions of different ages to coexist, appearing in a reticular arrangement with hyperpigmented mottling lasting from a few weeks to months.6
Just as the clinical picture transpires rapidly within 1 week, so do the histopathologic findings.6 Early lesions are categorized by a superficial perivascular and interstitial infiltrate of neutrophils, spongiosis, ballooning, and necrotic keratinocytes. These early lesions are present for less than 48 hours, and these histopathologic findings are diagnostic of PP. Within 2 days, lymphocytes predominate in the dermal infiltrate, and a patchy lichenoid aspect is established in the fully developed lesion along with reticular and vacuolar alterations. Late lesions show a parakeratotic and hyperpigmented epidermis with melanophages present in the papillary and reticular dermis. At this last stage, the histopathologic features of PP are indistinguishable from any other disease that results in postinflammatory hyperpigmentation, making diagnosis difficult.6
A variety of therapeutic options are used in the treatment of PP, with the most effective agents being oral antibiotics including dapsone, minocycline, and doxycycline, all of which limit the local tissue inflammatory response and cytotoxic effects. Topical and systemic antihistamines as well as corticosteroids are ineffective and have not been shown to prevent the postinflammatory reticular pigmentation.6-10
Various underlying factors have been associated with PP, including friction, heat, sunlight, sweating, allergic contact sensitization, and ketosis due to nutritional deficiency or diabetes mellitus; however; the exact etiology remains ambiguous.2-7 The association with ketosis and nutrition is of particular interest in this case. Onset of PP has been reported to coincide with dieting, fasting, weight loss, anorexia nervosa, and diabetes mellitus.3,6-9 Roughly 50 patients with PP had ketosis subsequent to these metabolic disturbances.3,6-10 As of now, the only reported correlation between ketosis and PP is that upon diet modification, lesions resolved following ketone normalization, as was observed in our patient.3,6-8 Reports of PP in diabetic patients while in ketoacidosis describe resolution of lesions with insulin administration.6-9 The pathophysiology of ketosis and its association with PP is unclear; however, the similarities seen in the immune response of PP and that stimulated by ketosis may expose an associated mechanism.
Ketosis is a temporary condition characterized by elevated serum ketones that are used as an alternative energy source when blood glucose is low or insulin is deficient.11 The most common causes of ketosis are the physiologic responses to fasting, prolonged exercise, or a high-protein/low-carbohydrate diet, though pathologic causes include insulin-dependent diabetes mellitus, alcoholism, and salicylate overdose.11 In healthy individuals, blood ketone levels rarely approach 0.5 mmol/L. Prolonged fasting or restricting intake of carbohydrates to less than 40 g daily can induce mild ketosis that resolves with re-introduction of carbohydrates.11
Ketone bodies pass from the circulating blood into tissues or remain near the blood vessels, inducing cytotoxic effects and perivascular inflammation.10,11 Increased ketone bodies have been shown to upregulate intercellular adhesion molecule 1 (ICAM-1) and leukocyte function-associated antigen 1 (LFA-1), a phenomenon also seen in lesional keratinocytes of PP.12,13 Teraki et al13 observed that epidermal keratinocytes exhibited increased expression of ICAM-1 as well as intense expression of LFA-1 on dermal and epidermotropic leukocytes, which was thought to be due to cell-mediated cytotoxicity. Not only do increased ketone bodies upregulate ICAM-1 and LFA-1, but they also are involved in increasing many proinflammatory mediators that may be capable of inducing the response seen in PP keratinocytes.12,13
Intercellular adhesion molecule 1 is important in initiating cellular interactions in the immune response and is the ligand for LFA-1 found on most leukocytes.14 Increased ICAM-1/LFA-1 interaction is thought to be the major pathway by which leukocytes are able to attach to keratinocytes and endothelial cells, allowing for leukocyte tissue migration and specific immunologic reactions, including leukocyte-mediated cytotoxicity. Interestingly, glucocorticoids are ineffective in reducing the expression of ICAM-1 in cultured keratinocytes.14 This connection between ketosis and inflammation that results in leukocyte migration and ultimately keratinocyte cytotoxicity may well be fundamental to the pathophysiology of PP and may provide a possible explanation for the ineffectiveness of corticosteroid treatment.
Middleton and Norris15 observed that individual keratinocyte strains show considerable variability in ICAM-1 expression that was found to be attributable to genetic polymorphisms. The presence of a particular polymorphism affecting ICAM-1 expression on human keratinocytes may explain the apparent ethnogeographic predisposition of PP as well as the ease at which ICAM-1 is expressed in the presence of ketones.
We describe a case of a 40-year-old white woman who was diagnosed with PP that was prompted by a 100-lb weight loss and self-induced ketosis while following a paleo diet with carbohydrate restriction. Successful treatment was attained through diet modification alone. This interesting case was another instance in which the pathophysiology of PP was attributed to ketosis. Because not all patients that are in ketosis have PP, larger prospective cohort studies are needed to further elucidate the association of PP and ketosis.
- What is the paleo diet? The Paleo Diet website. http://thepaleodiet.com/the-paleo-diet-premise. Accessed March 9, 2019.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation [in Japanese]. Japanese J Dermatol. 1971;81:38-39.
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet [published online December 30, 2013]. Pediatr Dermatol. 2015;32:248-251.
- Baykal C, Buyukbabani N, Akinturk S, et al. Prurigo pigmentosa: not an uncommon disease in the Turkish population. Int J Dermatol. 2006;45:1164-1168.
- Whang T, Kirkorian Y, Krishtul A, et al. Prurigo pigmentosa: report of two cases in the United States and review of the literature. Dermatology Online J. 2011;17:2.
- Böer A, Ackerman AB. Prurigo Pigmentosa (Nagashima Disease): Textbook and Atlas of a Distinctive Inflammatory Disease of the Skin. New York, NY: Ardor Scribendi Ltd; 2004.
- Teraki Y, Teraki E, Kawashima M, at al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511.
- Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2011;26:1149-1153.
- Yokozeki M, Watanabe J, Hotsubo T, et al. Prurigo pigmentosa disappeared following improvement of diabetic ketosis by insulin. J Dermatol. 2003;30:257-258.
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
- VanItallie TB, Nufert TH. Ketones: metabolism’s ugly duckling. Annu Rev Nutr. 2003;61:327-341.
- Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:e298-e306.
- Teraki Y, Shiohara T, Nagashima M, et al. Prurigo pigmentosa: role of ICAM-1 in the localization of the eruption. Br J Dermatol. 1991;125:360-363.
- Kashihara-Sawami M, Norris DA. The state of differentiation of cultured human keratinocytes determines the level of intercellular adhesion molecule-1 (ICAM-1) expression induced by gamma interferon. J Invest Dermatol. 1992;98:741-747.
- Middleton MH, Norris DA. Cytokine-induced ICAM-1 expression in human keratinocytes is highly variable in keratinocyte strains from different donors. J Invest Dermatol. 1995;104:489-496.
- What is the paleo diet? The Paleo Diet website. http://thepaleodiet.com/the-paleo-diet-premise. Accessed March 9, 2019.
- Nagashima M, Ohshiro A, Shimizu N. A peculiar pruriginous dermatosis with gross reticular pigmentation [in Japanese]. Japanese J Dermatol. 1971;81:38-39.
- Michaels JD, Hoss E, DiCaudo DJ, et al. Prurigo pigmentosa after a strict ketogenic diet [published online December 30, 2013]. Pediatr Dermatol. 2015;32:248-251.
- Baykal C, Buyukbabani N, Akinturk S, et al. Prurigo pigmentosa: not an uncommon disease in the Turkish population. Int J Dermatol. 2006;45:1164-1168.
- Whang T, Kirkorian Y, Krishtul A, et al. Prurigo pigmentosa: report of two cases in the United States and review of the literature. Dermatology Online J. 2011;17:2.
- Böer A, Ackerman AB. Prurigo Pigmentosa (Nagashima Disease): Textbook and Atlas of a Distinctive Inflammatory Disease of the Skin. New York, NY: Ardor Scribendi Ltd; 2004.
- Teraki Y, Teraki E, Kawashima M, at al. Ketosis is involved in the origin of prurigo pigmentosa. J Am Acad Dermatol. 1996;34:509-511.
- Oh YJ, Lee MH. Prurigo pigmentosa: a clinicopathologic study of 16 cases. J Eur Acad Dermatol Venereol. 2011;26:1149-1153.
- Yokozeki M, Watanabe J, Hotsubo T, et al. Prurigo pigmentosa disappeared following improvement of diabetic ketosis by insulin. J Dermatol. 2003;30:257-258.
- Kim JK, Chung WK, Chang SE, et al. Prurigo pigmentosa: clinicopathological study and analysis of 50 cases in Korea. J Dermatol. 2012;39:891-897.
- VanItallie TB, Nufert TH. Ketones: metabolism’s ugly duckling. Annu Rev Nutr. 2003;61:327-341.
- Rains JL, Jain SK. Hyperketonemia increases monocyte adhesion to endothelial cells and is mediated by LFA-1 expression in monocytes and ICAM-1 expression in endothelial cells. Am J Physiol Endocrinol Metab. 2011;301:e298-e306.
- Teraki Y, Shiohara T, Nagashima M, et al. Prurigo pigmentosa: role of ICAM-1 in the localization of the eruption. Br J Dermatol. 1991;125:360-363.
- Kashihara-Sawami M, Norris DA. The state of differentiation of cultured human keratinocytes determines the level of intercellular adhesion molecule-1 (ICAM-1) expression induced by gamma interferon. J Invest Dermatol. 1992;98:741-747.
- Middleton MH, Norris DA. Cytokine-induced ICAM-1 expression in human keratinocytes is highly variable in keratinocyte strains from different donors. J Invest Dermatol. 1995;104:489-496.
Practice Points
- Ketosis can be associated with a specific rash known as prurigo pigmentosa (PP).
- Resolution of PP is related to re-introduction of carbohydrates into the diet.
- Consider asking about dietary modifications in patients presenting with a new rash.
Whether diet, vitamins, or supplements can benefit patients with vitiligo remains unclear
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
WASHINGTON – Many patients with vitiligo are interested in treating their condition with vitamins, supplements, or a modified diet, but research on whether these measures have an impact remains limited, Nada Elbuluk, MD, said at the annual meeting of the American Academy of Dermatology.
, “we need more well designed, controlled studies in the future to know where this belongs in our treatment armamentarium,” said Dr. Elbuluk of the department of dermatology at the University of Southern California, Los Angeles.
During a session at the AAD meeting, Dr. Elbuluk, who is also director of the pigmentary disorders clinic at USC, reviewed the evidence for the use of these adjunctive therapies in patients with vitiligo.
Vitamins
The pathogenesis of vitiligo includes the overproduction of reactive oxygen species and oxidative stress, factors that contribute to melanocyte damage and death. In addition, many patients with vitiligo are deficient in certain vitamins and minerals, the basis of the hypothesis that supplementation could be beneficial, according to Dr. Elbuluk.
Vitamin B12 and folic acid contribute to DNA repair, synthesis, and methylation, and researchers have hypothesized that these vitamins also play a role in melanin synthesis. In a review of the literature, Dr. Elbuluk and her colleagues found four studies that evaluated vitamin B12 and folic acid in vitiligo. In one study, a controlled trial in which patients took B12 and folic acid with and without phototherapy, the investigators observed no significant difference in repigmentation between groups. The other three studies were uncontrolled and thus provide an insufficient understanding of the effect of B12 and folic acid, said Dr. Elbuluk.
Vitamin D is involved in melanocyte and keratinocyte growth and differentiation, and inhibits T cell activation. Data indicate that low vitamin D levels are common in patients with vitiligo and comorbid autoimmune diseases. In one study, patients who received narrow-band UVB had an increase in vitamin D levels that could contribute to photo-induced melanogenesis, and an open-label study indicated that patients who took vitamin D daily (without phototherapy) for 6 months had an increase of repigmentation over time. “Topical vitamin D analogs have also been used in vitiligo treatment with varying success,” Dr. Elbuluk noted.
“I check vitamin D levels on my patients and make sure that they are within normal range. But I think the degree of supplementation and its role in vitiligo needs to be further elucidated,” she said. And because vitamin D is fat soluble, there is a risk of toxicity if a patient takes too much.
Vitamin C, vitamin E, and alpha-lipoic acid have antioxidant properties. In a double-blind, randomized, controlled trial, one group of patients took vitamins C and E and alpha-lipoic acid for 2 months before and during treatment with narrow-band UVB twice per week (Clin Exp Dermatol. 2007 Nov;32[6]:631-6). Another group underwent phototherapy without supplementation. A significantly greater proportion of patients who received the antioxidants obtained more than 75% repigmentation compared with those who did not. In another study, 73% of patients who received oral vitamin E and narrow band UVB phototherapy had marked to excellent repigmentation, compared with 55.6% of those who had phototherapy only (J Clin Pharmacol. 2009 Jul;49[7]:852-5).
The results of these studies support the idea that antioxidants can stabilize disease, reduce oxidative stress, and improve the effect of phototherapy, Dr. Elbuluk said.
Herbal supplements
Several research teams have examined Ginkgo biloba as a possible treatment for vitiligo. This plant is native to China and has antioxidant and anti-inflammatory properties; its most common side effect is gastrointestinal distress. Because it entails a risk of coagulopathy, it may not be appropriate for patients receiving anticoagulant treatment, Dr. Elbuluk pointed out. In a double-blinded, randomized, controlled trial comparing ginkgo biloba alone with placebo in patients with vitiligo, treatment was associated with cessation of active disease in most patients, and more than 40% of patients receiving ginkgo biloba had 75% repigmentation or more.
Polypodium leucotomos, a fern native to Central and South America, protects against UV radiation damage, modulates the immune system, and has anti-inflammatory and antioxidant effects. It has a good safety profile and is well tolerated at a dose of 240 mg/day, she said. It sometimes causes gastrointestinal discomfort or pruritus. Several randomized, controlled trials in patients with vitiligo showed that supplementation with polypodium leucotomos improves repigmentation, particularly in photo-exposed areas, she noted.
Khellin is an extract from the Mediterranean khella plant that is thought to stimulate melanocyte proliferation and melanogenesis. Several studies have examined khellin supplementation in combination with phototherapy. Khellin can be administered orally or topically and appears to be more beneficial than sunlight or phototherapy alone in stabilizing disease or inducing repigmentation. Oral khellin can cause many side effects, including nausea, transaminitis, and hypotension, so researchers have been more interested in using topical khellin as a liposomal vehicle to improve drug delivery, Dr. Elbuluk said.
Minerals
Some patients with vitiligo have deficiencies in zinc and copper. Zinc is an antioxidant that aids wound healing, protects against free radicals, supports melanogenesis, and possibly prevents melanocyte death, but can cause gastrointestinal irritation. Copper, too, is an antioxidant and coenzyme involved in melanogenesis. One study compared topical steroid treatment with and without oral zinc supplementation. Dual treatment was associated with greater repigmentation, but the difference was not statistically significant. No studies have examined copper supplementation, she said.
L-phenylalanine, diet, and green tea
Investigators have proposed that the amino acid L-phenylalanine, a precursor to tyrosine in the pathway of melanin synthesis, might interfere with antibody production against melanocytes. This supplement is administered orally by weight, typically in conjunction with phototherapy or sunlight. Various studies have observed positive outcomes of L-phenylalanine combined with phototherapy or sunlight. L-phenylalanine tends to be safe and has been administered to children with vitiligo.
Many patients with vitiligo “have already tried diets by the time they come to me,” said Dr. Elbuluk. No controlled studies have analyzed the role of diet in the prevention or treatment of vitiligo, but case reports describe gluten-free diets in this population, including one report of a patient with celiac disease whose vitiligo improved after adoption of such a diet. Another case report described a patient without celiac disease who had refractory acrofacial vitiligo, which improved after the adoption of a gluten-free diet. Evidence supports a gluten-free diet for patients with celiac disease, but does not support this challenging diet for people without celiac disease, she pointed out.
Green tea includes catechins, which have antioxidant and anti-inflammatory properties. Its main component is epigallocatechin gallate (EGCG), which is thought to modulate T cell mediated responses. In one animal study, administration of EGCG delayed the onset of vitiligo and decreased the area of depigmentation in a mouse model. Although these findings are promising, clinical trials are needed to determine whether EGCG is beneficial in humans with vitiligo, said Dr. Elbuluk.
The literature on diets and supplementation as treatments for vitiligo has several shortcomings, with studies that used heterogeneous methodologies, and many that used nonstandard outcome measures that have not been validated. Sample sizes often are small, and many trials are uncontrolled. “These limitations make it harder to make sense of the data and have take-home conclusions,” Dr. Elbuluk said.
She had no disclosures.
SOURCE: Elbuluk N. AAD 19, Session S002.
REPORTING FROM AAD 19
Firing patients
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
After last month’s
One might assume that, just as patients are free to choose or reject their doctors, physicians have an equal right to reject their patients; and to a certain extent, that’s true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, sex, sexual orientation, and so on. However, our ethical obligations to “do no harm” and to place our patients’ welfare above our own self-interests dictate that dismissing a patient should be the absolute last resort, after all other options have been exhausted.
First, to avoid charges of arbitrary termination, you should draw up a specific list of situations that could merit a dismissal from your office, and add it to your office manual. Every list will probably differ in some respects, but for the sake of example, here is mine:
- Threats or violence toward physicians or staff.
- Inappropriate sexual advances toward physicians or staff.
- Providing false or misleading medical history.
- Repeated rude or disruptive behavior.
- Demands for unapproved, unindicated, or inappropriate treatments or medications (particularly controlled substances).
- Refusal to adhere to agreed-upon treatment plans.
- Repeated failure to keep scheduled appointments.
- Repeated failure to pay medical bills.
As with pretty much everything in a private practice, accurate and written documentation of dismissible behavior is essential. Record all incidents and assemble as much material evidence as possible from all available sources.
In most cases (except the first two infractions on our list, for which we have zero tolerance), we make every effort to resolve the problem amicably. We communicate with the patients in question, explain our concerns, and discuss options for resolution. I also may send a letter, repeating my concerns and proposed solutions, as further documentation of our efforts to achieve an amicable resolution. All verbal and written warnings are, of course, documented as well. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating these could lead to intervention by your state licensing board. There also is the risk of civil litigation, which typically is not covered by malpractice policies and may not be covered by your general liability policy either.
Patients who feel that termination was unjustified also may respond with negative reviews on social media, which I’ve discussed in recent columns, and will again, soon.
If something untrue is posted about you on a doctor-rating site, take action. Reputable sites have their own reputations to protect and can usually be persuaded to remove anything that is demonstrably false, although you may need a lawyer’s letter to get their attention. Try to get the error removed entirely or corrected within the original posting. An erratum on some distant page of the website is likely to be ignored, and will leave the false information online, intact.
Unfair comments are unlikely to be removed unless they are blatantly libelous; but many sites allow you to post a response, giving your side of the story. (More on that in the near future.) Also, there is nothing wrong with encouraging happy patients to write favorable reviews on those same sites. Sauce for the goose, and all that.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].