User login
Does Bariatric Surgery Also Improve Thyroid Function?
TOPLINE:
Metabolic/bariatric surgery (MBS) reduces thyroid-stimulating hormone (TSH), free triiodothyronine (fT3) levels, and thyroid hormone resistance indices in patients with obesity, changes strongly correlated with improvement in body composition.
METHODOLOGY:
- Recent studies have linked obesity with increased levels of TSH and thyroid hormones; however, the role that body fat distribution plays in this association remains unclear.
- This retrospective observational study evaluated the effects of MBS on thyroid hormone levels and thyroid hormone resistance in euthyroid individuals with obesity, focusing on the correlation with changes in body composition.
- Researchers included 470 patients with obesity (mean age, 33.4 years; mean body mass index [BMI], 37.9; 63.2% women) and 118 control individuals without obesity (mean BMI, 21.8), who had had normal levels of TSH, fT3, and free thyroxine.
- Among the patients with obesity, 125 underwent MBS and had thyroid tests both before and ≥ 3 months after surgery.
- Data on body composition and thyroid function were collected, and correlations between baseline and changes in thyroid function and body composition were assessed.
TAKEAWAY:
- Individuals with obesity had higher baseline TSH and fT3 levels (P < .001) and thyroid feedback quantile-based index (TFQI; P = .047) than those without obesity, with the values decreasing after MBS (all P < .001).
- Among individuals with obesity, preoperative TSH was positively correlated with the visceral fat area (VFA; P = .019) and body fat percentage (P = .013) and negatively correlated with skeletal muscle mass percentage (P = .024)
- The decrease in TSH post-surgery positively correlated with decreased VFA (P = .021) and decreased body fat percentage (P = .031).
- Decrease in VFA and body fat percentage after MBS was also associated with improved central thyroid hormone resistance indicated by TFQI.
IN PRACTICE:
“The relationship between obesity and [thyroid hormone] is bidirectional, indicating that addressing underlying thyroid disturbance could potentially benefit weight loss and metabolism,” the authors wrote.
SOURCE:
This study was led by Yu Yan, MD, Department of Pancreatic and Metabolic Surgery, Medical School of Southeast University, Nanjing Drum Tower Hospital, Nanjing, China, and published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The retrospective nature of this study limited the ability to definitively attribute changes in thyroid function and thyroid hormone resistance to changes in body composition. The relatively short duration of the study and the exclusion of individuals taking medications affecting thyroid function may also limit the generalizability of the findings.
DISCLOSURES:
This study was supported by the Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Nanjing University Medical School, Nanjing, China. The authors declared no potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Metabolic/bariatric surgery (MBS) reduces thyroid-stimulating hormone (TSH), free triiodothyronine (fT3) levels, and thyroid hormone resistance indices in patients with obesity, changes strongly correlated with improvement in body composition.
METHODOLOGY:
- Recent studies have linked obesity with increased levels of TSH and thyroid hormones; however, the role that body fat distribution plays in this association remains unclear.
- This retrospective observational study evaluated the effects of MBS on thyroid hormone levels and thyroid hormone resistance in euthyroid individuals with obesity, focusing on the correlation with changes in body composition.
- Researchers included 470 patients with obesity (mean age, 33.4 years; mean body mass index [BMI], 37.9; 63.2% women) and 118 control individuals without obesity (mean BMI, 21.8), who had had normal levels of TSH, fT3, and free thyroxine.
- Among the patients with obesity, 125 underwent MBS and had thyroid tests both before and ≥ 3 months after surgery.
- Data on body composition and thyroid function were collected, and correlations between baseline and changes in thyroid function and body composition were assessed.
TAKEAWAY:
- Individuals with obesity had higher baseline TSH and fT3 levels (P < .001) and thyroid feedback quantile-based index (TFQI; P = .047) than those without obesity, with the values decreasing after MBS (all P < .001).
- Among individuals with obesity, preoperative TSH was positively correlated with the visceral fat area (VFA; P = .019) and body fat percentage (P = .013) and negatively correlated with skeletal muscle mass percentage (P = .024)
- The decrease in TSH post-surgery positively correlated with decreased VFA (P = .021) and decreased body fat percentage (P = .031).
- Decrease in VFA and body fat percentage after MBS was also associated with improved central thyroid hormone resistance indicated by TFQI.
IN PRACTICE:
“The relationship between obesity and [thyroid hormone] is bidirectional, indicating that addressing underlying thyroid disturbance could potentially benefit weight loss and metabolism,” the authors wrote.
SOURCE:
This study was led by Yu Yan, MD, Department of Pancreatic and Metabolic Surgery, Medical School of Southeast University, Nanjing Drum Tower Hospital, Nanjing, China, and published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The retrospective nature of this study limited the ability to definitively attribute changes in thyroid function and thyroid hormone resistance to changes in body composition. The relatively short duration of the study and the exclusion of individuals taking medications affecting thyroid function may also limit the generalizability of the findings.
DISCLOSURES:
This study was supported by the Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Nanjing University Medical School, Nanjing, China. The authors declared no potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Metabolic/bariatric surgery (MBS) reduces thyroid-stimulating hormone (TSH), free triiodothyronine (fT3) levels, and thyroid hormone resistance indices in patients with obesity, changes strongly correlated with improvement in body composition.
METHODOLOGY:
- Recent studies have linked obesity with increased levels of TSH and thyroid hormones; however, the role that body fat distribution plays in this association remains unclear.
- This retrospective observational study evaluated the effects of MBS on thyroid hormone levels and thyroid hormone resistance in euthyroid individuals with obesity, focusing on the correlation with changes in body composition.
- Researchers included 470 patients with obesity (mean age, 33.4 years; mean body mass index [BMI], 37.9; 63.2% women) and 118 control individuals without obesity (mean BMI, 21.8), who had had normal levels of TSH, fT3, and free thyroxine.
- Among the patients with obesity, 125 underwent MBS and had thyroid tests both before and ≥ 3 months after surgery.
- Data on body composition and thyroid function were collected, and correlations between baseline and changes in thyroid function and body composition were assessed.
TAKEAWAY:
- Individuals with obesity had higher baseline TSH and fT3 levels (P < .001) and thyroid feedback quantile-based index (TFQI; P = .047) than those without obesity, with the values decreasing after MBS (all P < .001).
- Among individuals with obesity, preoperative TSH was positively correlated with the visceral fat area (VFA; P = .019) and body fat percentage (P = .013) and negatively correlated with skeletal muscle mass percentage (P = .024)
- The decrease in TSH post-surgery positively correlated with decreased VFA (P = .021) and decreased body fat percentage (P = .031).
- Decrease in VFA and body fat percentage after MBS was also associated with improved central thyroid hormone resistance indicated by TFQI.
IN PRACTICE:
“The relationship between obesity and [thyroid hormone] is bidirectional, indicating that addressing underlying thyroid disturbance could potentially benefit weight loss and metabolism,” the authors wrote.
SOURCE:
This study was led by Yu Yan, MD, Department of Pancreatic and Metabolic Surgery, Medical School of Southeast University, Nanjing Drum Tower Hospital, Nanjing, China, and published online in The Journal of Clinical Endocrinology & Metabolism.
LIMITATIONS:
The retrospective nature of this study limited the ability to definitively attribute changes in thyroid function and thyroid hormone resistance to changes in body composition. The relatively short duration of the study and the exclusion of individuals taking medications affecting thyroid function may also limit the generalizability of the findings.
DISCLOSURES:
This study was supported by the Fundings for Clinical Trials from the Affiliated Drum Tower Hospital, Nanjing University Medical School, Nanjing, China. The authors declared no potential conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Stones, Bones, Groans, and Moans: Could This Be Primary Hyperparathyroidism?
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Matthew F. Watto, MD: Welcome back to The Curbsiders. I’m Dr Matthew Frank Watto, here with my great friend and America’s primary care physician, Dr. Paul Nelson Williams.
Paul, we’re going to talk about our primary hyperparathyroidism podcast with Dr. Lindsay Kuo. It’s a topic that I feel much more clear on now.
Now, Paul, in primary care, you see a lot of calcium that is just slightly high. Can we just blame that on thiazide diuretics?
Paul N. Williams, MD: It’s a place to start. As you’re starting to think about the possible etiologies, primary hyperparathyroidism and malignancy are the two that roll right off the tongue, but it is worth going back to the patient’s medication list and making sure you’re not missing something.
Thiazides famously cause hypercalcemia, but in some of the reading I did for this episode, they may just uncover it a little bit early. Patients who are on thiazides who become hypercalcemic seem to go on to develop primary hyperthyroidism anyway. So I don’t think you can solely blame the thiazide.
Another medication that can be causative is lithium. So a good place to look first after you’ve repeated the labs and confirmed hypercalcemia is the patient’s medication list.
Dr. Watto: We’ve talked before about the basic workup for hypercalcemia, and determining whether it’s PTH dependent or PTH independent. On the podcast, we talk more about the full workup, but I wanted to talk about the classic symptoms. Our expert made the point that we don’t see them as much anymore, although we do see kidney stones. People used to present very late in the disease because they weren’t having labs done routinely.
The classic symptoms include osteoporosis and bone tumors. People can get nephrocalcinosis and kidney stones. I hadn’t really thought of it this way because we’re used to diagnosing it early now. Do you feel the same?
Dr. Williams: As labs have started routinely reporting calcium levels, this is more and more often how it’s picked up. The other aspect is that as we are screening for and finding osteoporosis, part of the workup almost always involves getting a parathyroid hormone and a calcium level. We’re seeing these lab abnormalities before we’re seeing symptoms, which is good.
But it also makes things more diagnostically thorny.
Dr. Watto: Dr. Lindsay Kuo made the point that when she sees patients before and after surgery, she’s aware of these nonclassic symptoms — the stones, bones, groans, and the psychiatric overtones that can be anything from fatigue or irritability to dysphoria.
Some people have a generalized weakness that’s very nonspecific. Dr. Kuo said that sometimes these symptoms will disappear after surgery. The patients may just have gotten used to them, or they thought these symptoms were caused by something else, but after surgery they went away.
There are these nonclassic symptoms that are harder to pin down. I was surprised by that.
Dr. Williams: She mentioned polydipsia and polyuria, which have been reported in other studies. It seems like it can be anything. You have to take a good history, but none of those things in and of themselves is an indication for operating unless the patient has the classic renal or bone manifestations.
Dr. Watto: The other thing we talked about is a normal calcium level in a patient with primary hyperparathyroidism, or the finding of a PTH level in the normal range but with a high calcium level that is inappropriate. Can you talk a little bit about those two situations?
Dr. Williams: They’re hard to say but kind of easy to manage because you treat them the same way as someone who has elevated calcium and PTH levels.
The normocalcemic patient is something we might stumble across with osteoporosis screening. Initially the calcium level is elevated, so you repeat it and it’s normal but with an elevated PTH level. You’re like, shoot. Now what?
It turns out that most endocrine surgeons say that the indications for surgery for the classic form of primary hyperparathyroidism apply to these patients as well, and it probably helps with the bone outcomes, which is one of the things they follow most closely. If you have hypercalcemia, you should have a suppressed PTH level, the so-called normohormonal hyperparathyroidism, which is not normal at all. So even if the PTH is in the normal range, it’s still relatively elevated compared with what it should be. That situation is treated in the same way as the classic elevated PTH and elevated calcium levels.
Dr. Watto: If the calcium is abnormal and the PTH is not quite what you’d expect it to be, you can always ask your friendly neighborhood endocrinologist to help you figure out whether the patient really has one of these conditions. You have to make sure that they don’t have a simple secondary cause like a low vitamin D level. In that case, you fix the vitamin D and then recheck the numbers to see if they’ve normalized. But I have found a bunch of these edge cases in which it has been helpful to confer with an endocrinologist, especially before you send someone to a surgeon to take out their parathyroid gland.
This was a really fantastic conversation. If you want to hear the full podcast episode, click here.
Dr. Watto, Clinical Assistant Professor, Department of Medicine, Perelman School of Medicine at University of Pennsylvania; Internist, Department of Medicine, Hospital Medicine Section, Pennsylvania Hospital, Philadelphia, Pennsylvania, has disclosed no relevant financial relationships. Dr. Williams, Associate Professor of Clinical Medicine, Department of General Internal Medicine, Lewis Katz School of Medicine; Staff Physician, Department of General Internal Medicine, Temple Internal Medicine Associates, Philadelphia, Pennsylvania, served as a director, officer, partner, employee, adviser, consultant, or trustee for The Curbsiders, and has received income in an amount equal to or greater than $250 from The Curbsiders.
A version of this article first appeared on Medscape.com.
A Simple Blood Test May Predict Cancer Risk in T2D
TOPLINE:
potentially enabling the identification of higher-risk individuals through a simple blood test.
METHODOLOGY:
- T2D is associated with an increased risk for obesity-related cancers, including breast, renal, uterine, thyroid, ovarian, and gastrointestinal cancers, as well as multiple myeloma, possibly because of chronic low-grade inflammation.
- Researchers explored whether the markers of inflammation IL-6, tumor necrosis factor alpha (TNF-alpha), and high-sensitivity C-reactive protein (hsCRP) can serve as predictive biomarkers for obesity-related cancers in patients recently diagnosed with T2D.
- They identified patients with recent-onset T2D and no prior history of cancer participating in the ongoing Danish Centre for Strategic Research in Type 2 Diabetes cohort study.
- At study initiation, plasma levels of IL-6 and TNF-alpha were measured using Meso Scale Discovery assays, and serum levels of hsCRP were measured using immunofluorometric assays.
TAKEAWAY:
- Among 6,466 eligible patients (40.5% women; median age, 60.9 years), 327 developed obesity-related cancers over a median follow-up of 8.8 years.
- Each SD increase in log-transformed IL-6 levels increased the risk for obesity-related cancers by 19%.
- The researchers did not find a strong association between TNF-alpha or hsCRP and obesity-related cancers.
- The addition of baseline IL-6 levels to other well-known risk factors for obesity-related cancers improved the performance of a cancer prediction model from 0.685 to 0.693, translating to a small but important increase in the ability to predict whether an individual would develop one of these cancers.
IN PRACTICE:
“In future, a simple blood test could identify those at higher risk of the cancers,” said the study’s lead author in an accompanying press release.
SOURCE:
The study was led by Mathilde D. Bennetsen, Steno Diabetes Center Odense, Odense University Hospital, Odense, Denmark, and published online on August 27 as an early release from the European Association for the Study of Diabetes (EASD) 2024 Annual Meeting.
LIMITATIONS:
No limitations were discussed in this abstract. However, the reliance on registry data may have introduced potential biases related to data accuracy and completeness.
DISCLOSURES:
The Danish Centre for Strategic Research in Type 2 Diabetes was supported by grants from the Danish Agency for Science and the Novo Nordisk Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
potentially enabling the identification of higher-risk individuals through a simple blood test.
METHODOLOGY:
- T2D is associated with an increased risk for obesity-related cancers, including breast, renal, uterine, thyroid, ovarian, and gastrointestinal cancers, as well as multiple myeloma, possibly because of chronic low-grade inflammation.
- Researchers explored whether the markers of inflammation IL-6, tumor necrosis factor alpha (TNF-alpha), and high-sensitivity C-reactive protein (hsCRP) can serve as predictive biomarkers for obesity-related cancers in patients recently diagnosed with T2D.
- They identified patients with recent-onset T2D and no prior history of cancer participating in the ongoing Danish Centre for Strategic Research in Type 2 Diabetes cohort study.
- At study initiation, plasma levels of IL-6 and TNF-alpha were measured using Meso Scale Discovery assays, and serum levels of hsCRP were measured using immunofluorometric assays.
TAKEAWAY:
- Among 6,466 eligible patients (40.5% women; median age, 60.9 years), 327 developed obesity-related cancers over a median follow-up of 8.8 years.
- Each SD increase in log-transformed IL-6 levels increased the risk for obesity-related cancers by 19%.
- The researchers did not find a strong association between TNF-alpha or hsCRP and obesity-related cancers.
- The addition of baseline IL-6 levels to other well-known risk factors for obesity-related cancers improved the performance of a cancer prediction model from 0.685 to 0.693, translating to a small but important increase in the ability to predict whether an individual would develop one of these cancers.
IN PRACTICE:
“In future, a simple blood test could identify those at higher risk of the cancers,” said the study’s lead author in an accompanying press release.
SOURCE:
The study was led by Mathilde D. Bennetsen, Steno Diabetes Center Odense, Odense University Hospital, Odense, Denmark, and published online on August 27 as an early release from the European Association for the Study of Diabetes (EASD) 2024 Annual Meeting.
LIMITATIONS:
No limitations were discussed in this abstract. However, the reliance on registry data may have introduced potential biases related to data accuracy and completeness.
DISCLOSURES:
The Danish Centre for Strategic Research in Type 2 Diabetes was supported by grants from the Danish Agency for Science and the Novo Nordisk Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
potentially enabling the identification of higher-risk individuals through a simple blood test.
METHODOLOGY:
- T2D is associated with an increased risk for obesity-related cancers, including breast, renal, uterine, thyroid, ovarian, and gastrointestinal cancers, as well as multiple myeloma, possibly because of chronic low-grade inflammation.
- Researchers explored whether the markers of inflammation IL-6, tumor necrosis factor alpha (TNF-alpha), and high-sensitivity C-reactive protein (hsCRP) can serve as predictive biomarkers for obesity-related cancers in patients recently diagnosed with T2D.
- They identified patients with recent-onset T2D and no prior history of cancer participating in the ongoing Danish Centre for Strategic Research in Type 2 Diabetes cohort study.
- At study initiation, plasma levels of IL-6 and TNF-alpha were measured using Meso Scale Discovery assays, and serum levels of hsCRP were measured using immunofluorometric assays.
TAKEAWAY:
- Among 6,466 eligible patients (40.5% women; median age, 60.9 years), 327 developed obesity-related cancers over a median follow-up of 8.8 years.
- Each SD increase in log-transformed IL-6 levels increased the risk for obesity-related cancers by 19%.
- The researchers did not find a strong association between TNF-alpha or hsCRP and obesity-related cancers.
- The addition of baseline IL-6 levels to other well-known risk factors for obesity-related cancers improved the performance of a cancer prediction model from 0.685 to 0.693, translating to a small but important increase in the ability to predict whether an individual would develop one of these cancers.
IN PRACTICE:
“In future, a simple blood test could identify those at higher risk of the cancers,” said the study’s lead author in an accompanying press release.
SOURCE:
The study was led by Mathilde D. Bennetsen, Steno Diabetes Center Odense, Odense University Hospital, Odense, Denmark, and published online on August 27 as an early release from the European Association for the Study of Diabetes (EASD) 2024 Annual Meeting.
LIMITATIONS:
No limitations were discussed in this abstract. However, the reliance on registry data may have introduced potential biases related to data accuracy and completeness.
DISCLOSURES:
The Danish Centre for Strategic Research in Type 2 Diabetes was supported by grants from the Danish Agency for Science and the Novo Nordisk Foundation. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Thyroid Resistance Ups Mortality in Euthyroid CKD Patients
TOPLINE:
An impaired central sensitivity to thyroid hormone may be associated with an increased risk for death in patients with chronic kidney disease (CKD) and normal thyroid function.
METHODOLOGY:
- Previous studies have shown that abnormal levels of thyroid-stimulating hormone (TSH) are associated with a higher mortality risk in patients with CKD, but whether the risk extends to those with normal thyroid function remains controversial.
- Researchers investigated the association between central sensitivity to thyroid hormone and the risk for all-cause mortality in 1303 euthyroid patients with CKD (mean age, 60 years; 59% women) from the National Health and Nutrition Examination Survey database (2007-2012).
- All participants had CKD stages I-IV, defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2 and/or a urinary albumin to urinary creatinine ratio ≥ 30 mg/g.
- The central sensitivity to thyroid hormone was primarily evaluated using a new central thyroid hormone resistance index, the Thyroid Feedback Quantile–based Index (TFQI), using free thyroxine and TSH concentrations.
- The participants were followed for a median duration of 115 months, during which 503 died.
TAKEAWAY:
- Patients with CKD who died during the follow-up period had a significantly higher TFQI (P < .001) than those who survived.
- The rates of all-cause mortality increased from 26.61% in the lowest TFQI tertile to 40.89% in the highest tertile (P = .001).
- A per unit increase in the TFQI was associated with a 40% increased risk for all-cause mortality (hazard ratio, 1.40; 95% CI, 1.10-1.79).
- This association between TFQI level and all-cause mortality persisted in all subgroups stratified by age, gender, race, body mass index, hypertension, diabetes, cardiovascular diseases, and CKD stages.
IN PRACTICE:
“Our study demonstrates that impaired sensitivity to thyroid hormone might be associated with all-cause mortality in CKD patients with normal thyroid function, independent of other traditional risk factors and comorbidities,” the authors wrote.
SOURCE:
This study was led by Qichao Yang and Ru Dong, Department of Endocrinology, Affiliated Wujin Hospital of Jiangsu University, Changzhou, China, and was published online on August 6, 2024, in BMC Public Health.
LIMITATIONS:
Thyroid function was measured only at baseline, and the changes in thyroid function over time were not measured. The study excluded people on thyroid hormone replacement therapy but did not consider other medication use that might have affected thyroid function, such as beta-blockers, steroids, and amiodarone. Thyroid-related antibodies, metabolic syndrome, and nonalcoholic fatty liver disease were not included in the analysis as possible confounding factors. The US-based sample requires further validation.
DISCLOSURES:
The study was supported by the Changzhou Health Commission. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An impaired central sensitivity to thyroid hormone may be associated with an increased risk for death in patients with chronic kidney disease (CKD) and normal thyroid function.
METHODOLOGY:
- Previous studies have shown that abnormal levels of thyroid-stimulating hormone (TSH) are associated with a higher mortality risk in patients with CKD, but whether the risk extends to those with normal thyroid function remains controversial.
- Researchers investigated the association between central sensitivity to thyroid hormone and the risk for all-cause mortality in 1303 euthyroid patients with CKD (mean age, 60 years; 59% women) from the National Health and Nutrition Examination Survey database (2007-2012).
- All participants had CKD stages I-IV, defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2 and/or a urinary albumin to urinary creatinine ratio ≥ 30 mg/g.
- The central sensitivity to thyroid hormone was primarily evaluated using a new central thyroid hormone resistance index, the Thyroid Feedback Quantile–based Index (TFQI), using free thyroxine and TSH concentrations.
- The participants were followed for a median duration of 115 months, during which 503 died.
TAKEAWAY:
- Patients with CKD who died during the follow-up period had a significantly higher TFQI (P < .001) than those who survived.
- The rates of all-cause mortality increased from 26.61% in the lowest TFQI tertile to 40.89% in the highest tertile (P = .001).
- A per unit increase in the TFQI was associated with a 40% increased risk for all-cause mortality (hazard ratio, 1.40; 95% CI, 1.10-1.79).
- This association between TFQI level and all-cause mortality persisted in all subgroups stratified by age, gender, race, body mass index, hypertension, diabetes, cardiovascular diseases, and CKD stages.
IN PRACTICE:
“Our study demonstrates that impaired sensitivity to thyroid hormone might be associated with all-cause mortality in CKD patients with normal thyroid function, independent of other traditional risk factors and comorbidities,” the authors wrote.
SOURCE:
This study was led by Qichao Yang and Ru Dong, Department of Endocrinology, Affiliated Wujin Hospital of Jiangsu University, Changzhou, China, and was published online on August 6, 2024, in BMC Public Health.
LIMITATIONS:
Thyroid function was measured only at baseline, and the changes in thyroid function over time were not measured. The study excluded people on thyroid hormone replacement therapy but did not consider other medication use that might have affected thyroid function, such as beta-blockers, steroids, and amiodarone. Thyroid-related antibodies, metabolic syndrome, and nonalcoholic fatty liver disease were not included in the analysis as possible confounding factors. The US-based sample requires further validation.
DISCLOSURES:
The study was supported by the Changzhou Health Commission. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
An impaired central sensitivity to thyroid hormone may be associated with an increased risk for death in patients with chronic kidney disease (CKD) and normal thyroid function.
METHODOLOGY:
- Previous studies have shown that abnormal levels of thyroid-stimulating hormone (TSH) are associated with a higher mortality risk in patients with CKD, but whether the risk extends to those with normal thyroid function remains controversial.
- Researchers investigated the association between central sensitivity to thyroid hormone and the risk for all-cause mortality in 1303 euthyroid patients with CKD (mean age, 60 years; 59% women) from the National Health and Nutrition Examination Survey database (2007-2012).
- All participants had CKD stages I-IV, defined as an estimated glomerular filtration rate < 60 mL/min/1.73 m2 and/or a urinary albumin to urinary creatinine ratio ≥ 30 mg/g.
- The central sensitivity to thyroid hormone was primarily evaluated using a new central thyroid hormone resistance index, the Thyroid Feedback Quantile–based Index (TFQI), using free thyroxine and TSH concentrations.
- The participants were followed for a median duration of 115 months, during which 503 died.
TAKEAWAY:
- Patients with CKD who died during the follow-up period had a significantly higher TFQI (P < .001) than those who survived.
- The rates of all-cause mortality increased from 26.61% in the lowest TFQI tertile to 40.89% in the highest tertile (P = .001).
- A per unit increase in the TFQI was associated with a 40% increased risk for all-cause mortality (hazard ratio, 1.40; 95% CI, 1.10-1.79).
- This association between TFQI level and all-cause mortality persisted in all subgroups stratified by age, gender, race, body mass index, hypertension, diabetes, cardiovascular diseases, and CKD stages.
IN PRACTICE:
“Our study demonstrates that impaired sensitivity to thyroid hormone might be associated with all-cause mortality in CKD patients with normal thyroid function, independent of other traditional risk factors and comorbidities,” the authors wrote.
SOURCE:
This study was led by Qichao Yang and Ru Dong, Department of Endocrinology, Affiliated Wujin Hospital of Jiangsu University, Changzhou, China, and was published online on August 6, 2024, in BMC Public Health.
LIMITATIONS:
Thyroid function was measured only at baseline, and the changes in thyroid function over time were not measured. The study excluded people on thyroid hormone replacement therapy but did not consider other medication use that might have affected thyroid function, such as beta-blockers, steroids, and amiodarone. Thyroid-related antibodies, metabolic syndrome, and nonalcoholic fatty liver disease were not included in the analysis as possible confounding factors. The US-based sample requires further validation.
DISCLOSURES:
The study was supported by the Changzhou Health Commission. The authors declared no competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
A New Focus for Cushing Syndrome Screening in Obesity
TOPLINE:
METHODOLOGY:
- Obesity is a key clinical feature of Cushing syndrome and shares many overlapping characteristics. An ongoing debate continues about the need to screen patients with obesity for the rare endocrine disease, but phenotypes known as metabolically healthy or unhealthy obesity may help better define an at-risk population.
- To assess the prevalence of Cushing syndrome by metabolic health status, researchers conducted a retrospective study of 1008 patients with obesity (mean age, 40 years; 83% women; body mass index ≥ 30) seen at an endocrinology outpatient clinic in Turkey between December 2020 and June 2022.
- They screened patients for Cushing syndrome with an overnight dexamethasone suppression test (1 mg DST), an oral dexamethasone dose given at 11 PM followed by a fasting blood sample for cortisol measurement the next morning. A serum cortisol level < 1.8 mcg/dL indicated normal suppression.
- Patients were categorized into those with metabolically healthy obesity (n = 229) or metabolically unhealthy obesity (n = 779) based on the absence or presence of comorbidities such as diabetes, prediabetes, coronary artery disease, hypertension, or dyslipidemia.
TAKEAWAY:
- The overall prevalence of Cushing syndrome in the study cohort was 0.2%, with only two patients definitively diagnosed after more tests and the remaining 10 classified as having subclinical hypercortisolism.
- Cortisol levels following the 1 mg DST were higher in the metabolically unhealthy obesity group than in the metabolically healthy obesity group (P = .001).
- Among the 12 patients with unsuppressed levels of cortisol, 11 belonged to the metabolically unhealthy obesity group, indicating a strong association between metabolic health and the levels of cortisol.
- The test demonstrated a specificity of 99% and sensitivity of 100% for screening Cushing syndrome in patients with obesity.
IN PRACTICE:
“Screening all patients with obesity for CS [Cushing syndrome] without considering any associated metabolic conditions appears impractical and unnecessary in everyday clinical practice,” the authors wrote. “However, it may be more reasonable and applicable to selectively screen the patients with obesity having comorbidities such as DM [diabetes mellitus], hypertension, dyslipidemia, or coronary artery disease, which lead to a metabolically unhealthy phenotype, rather than all individuals with obesity,” they added.
SOURCE:
The study, led by Sema Hepsen, Ankara Etlik City Hospital, Department of Endocrinology and Metabolism, Ankara, Turkey, was published online in the International Journal of Obesity.
LIMITATIONS:
The single-center design of the study and inclusion of patients from a single racial group may limit the generalizability of the findings. The retrospective design prevented the retrieval of all relevant data on clinical features and fat distribution.
DISCLOSURES:
The study was supported by an open access funding provided by the Scientific and Technological Research Council of Türkiye. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Obesity is a key clinical feature of Cushing syndrome and shares many overlapping characteristics. An ongoing debate continues about the need to screen patients with obesity for the rare endocrine disease, but phenotypes known as metabolically healthy or unhealthy obesity may help better define an at-risk population.
- To assess the prevalence of Cushing syndrome by metabolic health status, researchers conducted a retrospective study of 1008 patients with obesity (mean age, 40 years; 83% women; body mass index ≥ 30) seen at an endocrinology outpatient clinic in Turkey between December 2020 and June 2022.
- They screened patients for Cushing syndrome with an overnight dexamethasone suppression test (1 mg DST), an oral dexamethasone dose given at 11 PM followed by a fasting blood sample for cortisol measurement the next morning. A serum cortisol level < 1.8 mcg/dL indicated normal suppression.
- Patients were categorized into those with metabolically healthy obesity (n = 229) or metabolically unhealthy obesity (n = 779) based on the absence or presence of comorbidities such as diabetes, prediabetes, coronary artery disease, hypertension, or dyslipidemia.
TAKEAWAY:
- The overall prevalence of Cushing syndrome in the study cohort was 0.2%, with only two patients definitively diagnosed after more tests and the remaining 10 classified as having subclinical hypercortisolism.
- Cortisol levels following the 1 mg DST were higher in the metabolically unhealthy obesity group than in the metabolically healthy obesity group (P = .001).
- Among the 12 patients with unsuppressed levels of cortisol, 11 belonged to the metabolically unhealthy obesity group, indicating a strong association between metabolic health and the levels of cortisol.
- The test demonstrated a specificity of 99% and sensitivity of 100% for screening Cushing syndrome in patients with obesity.
IN PRACTICE:
“Screening all patients with obesity for CS [Cushing syndrome] without considering any associated metabolic conditions appears impractical and unnecessary in everyday clinical practice,” the authors wrote. “However, it may be more reasonable and applicable to selectively screen the patients with obesity having comorbidities such as DM [diabetes mellitus], hypertension, dyslipidemia, or coronary artery disease, which lead to a metabolically unhealthy phenotype, rather than all individuals with obesity,” they added.
SOURCE:
The study, led by Sema Hepsen, Ankara Etlik City Hospital, Department of Endocrinology and Metabolism, Ankara, Turkey, was published online in the International Journal of Obesity.
LIMITATIONS:
The single-center design of the study and inclusion of patients from a single racial group may limit the generalizability of the findings. The retrospective design prevented the retrieval of all relevant data on clinical features and fat distribution.
DISCLOSURES:
The study was supported by an open access funding provided by the Scientific and Technological Research Council of Türkiye. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Obesity is a key clinical feature of Cushing syndrome and shares many overlapping characteristics. An ongoing debate continues about the need to screen patients with obesity for the rare endocrine disease, but phenotypes known as metabolically healthy or unhealthy obesity may help better define an at-risk population.
- To assess the prevalence of Cushing syndrome by metabolic health status, researchers conducted a retrospective study of 1008 patients with obesity (mean age, 40 years; 83% women; body mass index ≥ 30) seen at an endocrinology outpatient clinic in Turkey between December 2020 and June 2022.
- They screened patients for Cushing syndrome with an overnight dexamethasone suppression test (1 mg DST), an oral dexamethasone dose given at 11 PM followed by a fasting blood sample for cortisol measurement the next morning. A serum cortisol level < 1.8 mcg/dL indicated normal suppression.
- Patients were categorized into those with metabolically healthy obesity (n = 229) or metabolically unhealthy obesity (n = 779) based on the absence or presence of comorbidities such as diabetes, prediabetes, coronary artery disease, hypertension, or dyslipidemia.
TAKEAWAY:
- The overall prevalence of Cushing syndrome in the study cohort was 0.2%, with only two patients definitively diagnosed after more tests and the remaining 10 classified as having subclinical hypercortisolism.
- Cortisol levels following the 1 mg DST were higher in the metabolically unhealthy obesity group than in the metabolically healthy obesity group (P = .001).
- Among the 12 patients with unsuppressed levels of cortisol, 11 belonged to the metabolically unhealthy obesity group, indicating a strong association between metabolic health and the levels of cortisol.
- The test demonstrated a specificity of 99% and sensitivity of 100% for screening Cushing syndrome in patients with obesity.
IN PRACTICE:
“Screening all patients with obesity for CS [Cushing syndrome] without considering any associated metabolic conditions appears impractical and unnecessary in everyday clinical practice,” the authors wrote. “However, it may be more reasonable and applicable to selectively screen the patients with obesity having comorbidities such as DM [diabetes mellitus], hypertension, dyslipidemia, or coronary artery disease, which lead to a metabolically unhealthy phenotype, rather than all individuals with obesity,” they added.
SOURCE:
The study, led by Sema Hepsen, Ankara Etlik City Hospital, Department of Endocrinology and Metabolism, Ankara, Turkey, was published online in the International Journal of Obesity.
LIMITATIONS:
The single-center design of the study and inclusion of patients from a single racial group may limit the generalizability of the findings. The retrospective design prevented the retrieval of all relevant data on clinical features and fat distribution.
DISCLOSURES:
The study was supported by an open access funding provided by the Scientific and Technological Research Council of Türkiye. The authors declared no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
A Step-by-Step Guide for Diagnosing Cushing Syndrome
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
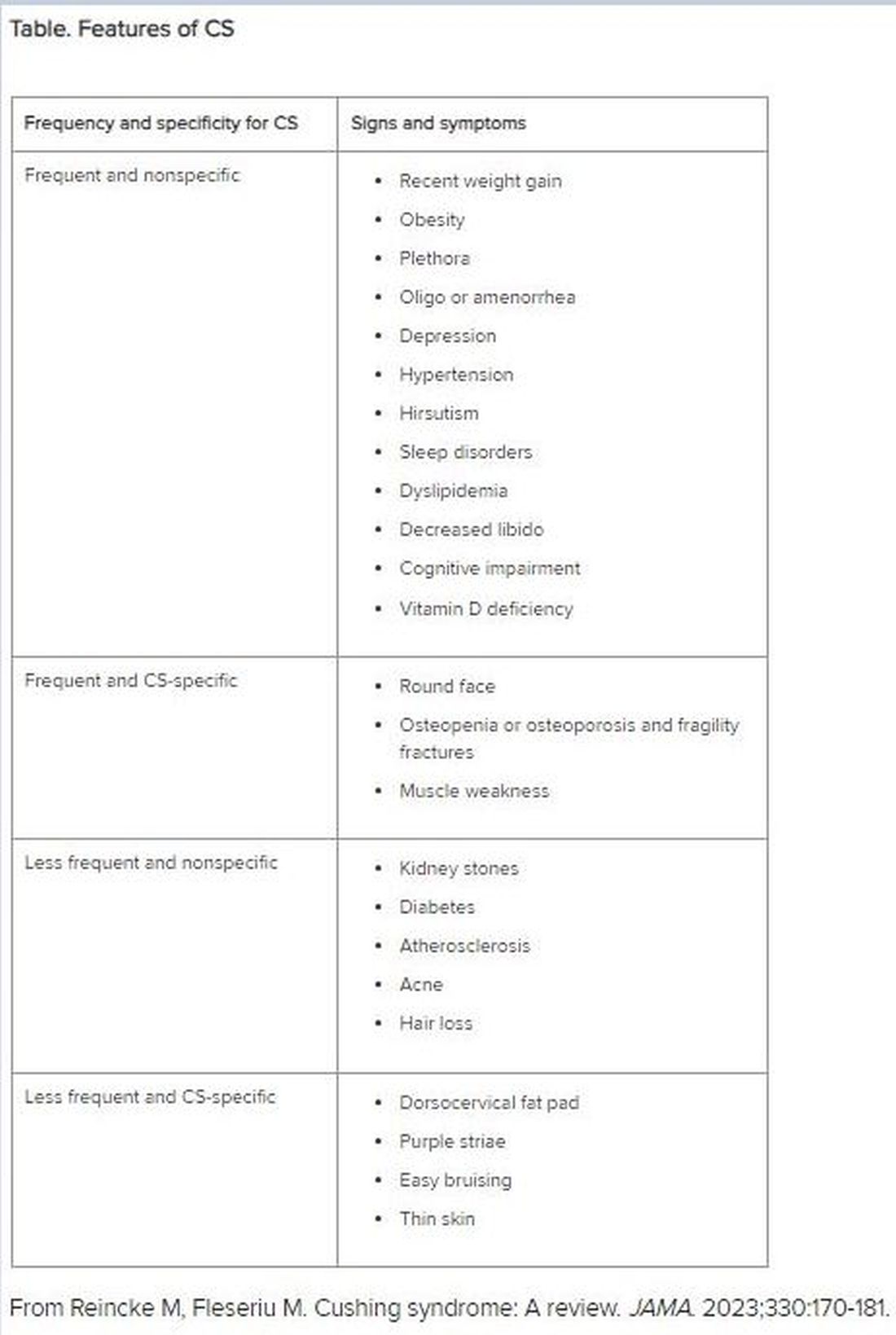
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
“Moon face” is a term that’s become popular on social media, used to describe people with unusually round faces who are purported to have high levels of cortisol. But the term “moon face” isn’t new. It was actually coined in the 1930s by neurosurgeon Harvey Cushing, MD, who identified patients with a constellation of clinical characteristics — a condition that came to bear his name — which included rapidly developing facial adiposity. And indeed, elevated cortisol is a hallmark feature of Cushing syndrome (CS), but there are other reasons for elevated cortisol and other manifestations of CS.
Today, the term “moon face” has been replaced with “round face,” which is considered more encompassing and culturally sensitive, said Maria Fleseriu, MD, professor of medicine and neurological surgery and director of the Pituitary Center at Oregon Health and Science University in Portland, Oregon.
Facial roundness can lead clinicians to be suspicious that their patient is experiencing CS. But because a round face is associated with several other conditions, it’s important to be familiar with its particular presentation in CS, as well as how to diagnose and treat CS.
Pathophysiology of CS
Dr. Fleseriu defined CS as “prolonged nonphysiologic increase in cortisol, due either to exogenous use of steroids (oral, topical, or inhaled) or to excess endogenous cortisol production.” She added that it’s important “to always exclude exogenous causes before conducting a further workup to determine the type and cause of cortisol excess.”
Dr. Fleseriu said. Other causes of CS are ectopic (caused by neuroendocrine tumors) or adrenal. CS affects primarily females and typically has an onset between ages 20 and 50 years, depending on the CS type.
Diagnosis of CS is “substantially delayed for most patients, due to metabolic syndrome phenotypic overlap and lack of a single pathognomonic symptom,” according to Dr. Fleseriu.
An accurate diagnosis should be on the basis of signs and symptoms, biochemical screening, other laboratory testing, and diagnostic imaging.
Look for Clinical Signs and Symptoms of CS
“CS mostly presents as a combination of two or more features,” Dr. Fleseriu stated. These include increased fat pads (in the face, neck, and trunk), skin changes, signs of protein catabolism, growth retardation and body weight increase in children, and metabolic dysregulations (Table).
“Biochemical screening should be performed in patients with a combination of symptoms, and therefore an increased pretest probability for CS,” Dr. Fleseriu advised.
A CS diagnosis requires not only biochemical confirmation of hypercortisolemia but also determination of the underlying cause of the excess endogenous cortisol production. This is a key step, as the management of CS is specific to its etiology.
Elevated plasma cortisol alone is insufficient for diagnosing CS, as several conditions can be associated with physiologic, nonneoplastic endogenous hypercortisolemia, according to the 2021 updated CS guidelines for which Dr. Fleseriu served as a coauthor. These include depression, alcohol dependence, glucocorticoid resistance, obesity, diabetes, pregnancy, prolonged physical exertion, malnutrition, and cortisol-binding globulin excess.
The diagnosis begins with the following screening tests:
- Late-night salivary cortisol (LNSC) to assess an abnormal circadian rhythm
According to the 2021 guideline, this is “based on the assumption that patients with CS lose the normal circadian nadir of cortisol secretion.”
- Overnight 1-mg dexamethasone suppression test (DST) to assess impaired glucocorticoid feedback
The authors noted that in healthy individuals, a supraphysiologic dexamethasone dose inhibits vasopressin and adrenocorticotropic hormone (ACTH) secretion, leading to decreased cortisol concentration. Cortisol concentrations of < 1-8 μg/dL in the morning (after administration of the dexamethasone between 11 p.m. and midnight) are considered “normal,” and a negative result “strongly predicts” the absence of CS. But false-positive and false-negative results can occur. Thus, “it is imperative that first-line testing is elected on the basis of physiologic conditions and drug intake — for example, use of CYP2A4/5 inhibitors or stimulators and oral estrogen — as well as laboratory quality control measure, and special attention to night shift workers,” Dr. Fleseriu emphasized.
- A 24-hour urinary free cortisol (UFC) test to assess increased bioavailable cortisol
The guideline encourages conducting several 24-hour urine collections to account for intra-patient variability.
Dr. Fleseriu recommended utilizing at least two of the three screening tests, all of which have reasonable sensitivity and specificity.
“Two normal test results usually exclude the presence of CS, except in rare cyclic CS,” she added.
Conduct Additional Laboratory Testing
Additional laboratory abnormalities suggestive of CS include:
- Increased leukocytes with decreased lymphocytes, eosinophils, monocytes, and basophils
- Elevated glucose and insulin levels
- Hypokalemia
- Increased triglycerides and total cholesterol levels
- Elevated liver enzymes
- Changes in activated thromboplastin time and plasma concentrations of pro- and anticoagulant factors
- Hypercalciuria, hypocalcemia (rare), hypophosphatemia, decreased phosphate maximum resorption, and increased alkaline phosphatase activity
Dr. Fleseriu noted that, in most cases, a final CS diagnosis can be reached after confirmation of biochemical hypercortisolism, which is done after an initial positive screening test.
She added that plasma ACTH levels are “instrumental” in distinguishing ACTH-depending forms of CS — such as Cushing disease and ectopic CS — from adrenal cases. Bilateral inferior petrosal sinus sampling is necessary in ACTH-dependent CS.
Utilize Diagnostic Imaging
There are several diagnostic imaging techniques that localize the origin of the hypercortisolism, thus informing the course of treatment.
- Pituitary MRI to detect corticotropin-secreting corticotroph adenomas, which are typically small lesions (< 6 mm in diameter)
- CT evaluation of the neck, thoracic cavity, and abdomen to diagnose ectopic CS, including lung neuroendocrine tumors and bronchial neuroendocrine tumors
- Cervical and thyroid ultrasonography to identify primary or metastatic medullary thyroid carcinoma, and PET scans, which have greater sensitivity in detecting tumors, compared with CT scans
- Contrast-enhanced CT scans to detect adrenal adenomas and adrenocortical carcinomas
Management of CS
“The primary aim of treatment is eucortisolemia, and in those with endogenous CS, complete surgical resection of the underlying tumor is the primary method,” Dr. Fleseriu said.
It’s critical to monitor for biochemical remission following surgery, utilizing 24-hour UFC, LNSC, and DST “because clinical manifestations may lag behind biochemical evidence.”
In Cushing disease, almost half of patients will have either persistent or recurrent hypercortisolemia after surgery. In those cases, individualized adjuvant treatments are recommended. These include repeat surgery, bilateral adrenalectomy, radiation, or medical treatments, including pituitary-directed drugs, adrenal steroidogenesis inhibitors, or glucocorticoid receptor-blocking agents. The last two groups are used for other types of CS.
Dr. Fleseriu pointed out that CS is “associated with increased metabolic, cardiovascular, psychiatric, infectious, and musculoskeletal morbidity, which are only partially reversible with successful [CS] treatment.” These comorbidities need to be addressed via individualized therapies. Moreover, long-term mortality is increased in all forms of CS. Thus, patients require lifelong follow-up to detect recurrence at an early stage and to treat comorbidities.
“It is likely that delayed diagnosis might explain the long-term consequences of CS, including increased morbidity and mortality despite remission,” she said.
Familiarity with the presenting signs and symptoms of CS and ordering recommended screening and confirmatory tests will enable appropriate management of the condition, leading to better outcomes.
Dr. Fleseriu reported receiving research grants from Sparrow Pharmaceuticals to Oregon Health and Science University as principal investigator and receiving occasional fees for scientific consulting/advisory boards from Sparrow Pharmaceuticals, Recordati Rare Diseases Inc., and Xeris Biopharma Holdings Inc.
A version of this article first appeared on Medscape.com.
After Rapid Weight Loss, Monitor Antiobesity Drug Dosing
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
A patient who developed atrial fibrillation resulting from the failure to adjust the levothyroxine dose after rapid, significant weight loss while on the antiobesity drug tirzepatide (Zepbound) serves as a key reminder in managing patients experiencing rapid weight loss, either from antiobesity medications or any other means: Patients taking medications with weight-based dosing need to have their doses closely monitored.
“Failing to monitor and adjust dosing of these [and other] medications during a period of rapid weight loss may lead to supratherapeutic — even toxic — levels, as was seen in this [case],” underscore the authors of an editorial regarding the Teachable Moment case, published in JAMA Internal Medicine.
Toxicities from excessive doses can have a range of detrimental effects. In terms of thyroid medicine, the failure to adjust levothyroxine treatment for hypothyroidism in cases of rapid weight loss can lead to thyrotoxicosis, and in older patients in particular, a resulting thyrotropin level < 0.1 mIU/L is associated with as much as a threefold increased risk for atrial fibrillation, as observed in the report.
Case Demonstrates Risks
The case involved a 62-year-old man with obesity, hypothyroidism, and type 1 diabetes who presented to the emergency department with palpitations, excessive sweating, confusion, fever, and hand tremors. Upon being diagnosed with atrial fibrillation, the patient was immediately treated.
His medical history revealed the underlying culprit: Six months earlier, the patient had started treatment with the gastric inhibitory polypeptide (GIP)/glucagon-like peptide (GLP) 1 dual agonist tirzepatide. As is typical with the drug, the patient’s weight quickly plummeted, dropping from a starting body mass index of 44.4 down to 31.2 after 6 months and a decrease in body weight from 132 kg to 93 kg (a loss of 39 kg [approximately 86 lb]).
When he was prescribed tirzepatide, 2.5 mg weekly, for obesity, the patient had been recommended to increase the dose every 4 weeks as tolerated and, importantly, to have a follow-up visit in a month. But because he lived in different states seasonally, the follow-up never occurred.
Upon his emergency department visit, the patient’s thyrotropin level had dropped from 1.9 mIU/L at the first visit 6 months earlier to 0.001 mIU/L (well within the atrial fibrillation risk range), and his free thyroxine level (fT4) was 7.26 ng/ dL — substantially outside of the normal range of about 0.9-1.7 ng/dL for adults.
“The patient had 4-times higher fT4 levels of the upper limit,” first author Kagan E. Karakus, MD, of the Barbara Davis Center for Diabetes, University of Colorado Anschutz Medical Campus, Aurora, told this news organization. “That is why he had experienced the adverse event of atrial fibrillation.”
Thyrotoxicosis Symptoms Can Be ‘Insidious,’ Levothyroxine Should Be Monitored
Although tirzepatide has not been approved by the US Food and Drug Administration for the treatment of type 1 diabetes, obesity is on the rise among patients with this disorder and recent research has shown a more than 10% reduction in body weight in 6 months and significant reductions in A1c with various doses.
Of note, in the current case, although the patient’s levothyroxine dose was not adjusted, his insulin dose was gradually self-decreased during his tirzepatide treatment to prevent hypoglycemia.
“If insulin treatment is excessive in diabetes, it causes hypoglycemia, [and] people with type 1 diabetes will recognize the signs of hypoglycemia related to excessive insulin earlier,” Dr. Karakus said.
If symptoms appear, patients can reduce their insulin doses on their own; however, the symptoms of thyrotoxicosis caused by excessive levothyroxine can be more insidious compared with hypoglycemia, he explained.
“Although patients can change their insulin doses, they cannot change the levothyroxine doses since it requires a blood test [thyroid-stimulating hormone; TSH] and a new prescription of the new dose.”
The key lesson is that “following levothyroxine treatment initiation or dose adjustment, 4-6 weeks is the optimal duration to recheck [the] thyrotropin level and adjust the dose as needed,” Dr. Karakus said.
Key Medications to Monitor
Other common outpatient medications that should be closely monitored in patients experiencing rapid weight loss, by any method, range from anticoagulants, anticonvulsants, and antituberculosis drugs to antibiotics and antifungals, the authors note.
Of note, medications with a narrow therapeutic index include phenytoin, warfarin, lithium carbonate, digoxin theophylline, tacrolimus, valproic acid, carbamazepine, and cyclosporine.
The failure to make necessary dose adjustments “is seen more often since the newer antiobesity drugs reduce a great amount of weight within months, almost as rapidly as bariatric surgery,” Dr. Karakus said.
“It is very important for physicians to be aware of the weight-based medications and narrow therapeutic index medications since their doses should be adjusted carefully, especially during weight loss,” he added.
Furthermore, “the patient should also know that weight reduction medication may cause adverse effects like nausea, vomiting and also may affect metabolism of other medications such that some medication doses should be adjusted regularly.”
In the editorial published with the study, Tyrone A. Johnson, MD, of the Department of Medicine, University of California, San Francisco, and colleagues note that the need for close monitoring is particularly important with older patients, who, in addition to having a higher likelihood of comorbidities, commonly have polypharmacy that could increase the potential for adverse effects.
Another key area concern is the emergence of direct-to-consumer avenues for GLP-1/GIP agonists for the many who either cannot afford or do not have access to the drugs, providing further opportunities for treatment without appropriate clinical oversight, they add.
Overall, the case “highlights the potential dangers underlying under-supervised prescribing of GLP-1/GIP receptor agonists and affirms the need for strong partnerships between patients and their clinicians during their use,” they wrote.
“These medications are best used in collaboration with continuity care teams, in context of a patient’s entire health, and in comprehensive risk-benefit assessment throughout the entire duration of treatment.”
A Caveat: Subclinical Levothyroxine Dosing
Commenting on the study, Matthew Ettleson, MD, a clinical instructor of medicine in the Section of Endocrinology, Diabetes, & Metabolism, University of Chicago, noted the important caveat that patients with hypothyroidism are commonly on subclinical doses, with varying dose adjustment needs.
“The patient in the case was clearly on a replacement level dose. However, many patients are on low doses of levothyroxine (75 µg or lower) for subclinical hypothyroidism, and, in general, I think the risks are lower with patients with subclinical hypothyroidism on lower doses of levothyroxine,” he told this news organization.
Because of that, “frequent TSH monitoring may be excessive in this population,” he said. “I would hesitate to empirically lower the dose with weight loss, unless it was clear that the patient was unlikely to follow up.
“Checking TSH at a more frequent interval and adjusting the dose accordingly should be adequate to prevent situations like this case.”
Dr. Karakus, Dr. Ettleson, and the editorial authors had no relevant disclosures to report.
A version of this article appeared on Medscape.com.
Could Dry Fasting Aid in Metabolic Disorders, Diabetes?
Dry fasting, the practice of going without food and water, has enthusiastic advocates on TikTok, X, YouTube, and other social media platforms. Devotees claim a wide range of health effects, but medical professionals advise caution to ensure that the practice does more good than harm, especially for individuals with diabetes.
Purported benefits and risks vary, depending on who is following the regimen and how long they abstain from food and water. Advocates on social media assert that dry fasting makes “intuition skyrocket” and puts autophagy on “overdrive.” Although such statements may rev up followers, there is little evidence to support these and many other dry-fasting claims. In fact, several physicians warned about unintended consequences.
“I had one patient who followed this fasting method often, and over time she developed kidney stones that led to a severe infection,” said Deena Adimoolam, MD, an endocrinologist in private practice in New York City and New Jersey. “Lack of both water and food can fuel hunger and increase the likelihood of overeating or binge eating once the fast is completed, which does not lead to weight loss. Untreated dehydration can lead to loss of consciousness.”
“For individuals with type 2 diabetes, dehydration can exacerbate hyperglycemia and increase the risk of complications such as diabetic ketoacidosis (DKA),” said Abeer Bader, lead clinical nutrition specialist at the Massachusetts General Hospital Weight Center in Boston. “Research also consistently shows that adequate hydration is crucial for maintaining physical and cognitive performance.”
, Ms. Bader noted. “Prolonged dry fasting can result in nutrient deficiencies. For individuals with diabetes, maintaining adequate nutrition is crucial to manage blood sugar levels and overall health. The lack of both food and water can exacerbate deficiencies.”
Joanne Bruno, MD, an endocrinologist at NYU Langone Health, added, “Certain medications used for the management of type 2 diabetes, such as SGLT2 inhibitors, can cause dehydration. It is critical that patients stay well hydrated while on these medications to avoid serious side effects such as euglycemic DKA.”
What Exactly Is Dry Fasting?
Defining dry fasting, like any kind of fasting, has remained a challenge, according to authors of the first international consensus on fasting terminology, published on July 25 in Cell Metabolism. The clinical terminology “has remained heterogeneous and often confusing, with similar terms being used to define different fasting regimens ... reflecting the manifold contexts in which fasting is practiced.”
Indeed, dry fasting was among the most discussed terms by the consensus panel and went through several rounds before the panelists came to agreement. A few experts were critical of the practice, whereas those familiar with religious fasting traditions, such as during Ramadan, were clear about the importance of including this term in the consensus process.
“The dissent was resolved by the clarification that this form of fasting has historical and geographical extensions and that the present consensus process did not aim at evaluating therapeutic effectiveness or safety for any term defined,” the authors wrote.
The panel concluded that dry fasting is not the same as total or complete fasting because the latter can include water (such as water-only fasting). Their final definition of dry fasting is ‘’a fasting regimen during which a voluntary abstinence from all foods and beverages, including water, is practiced for a certain period of time.’’
Different types of fasting regimens, such as intermittent fasting, may include dry fasting, in which case it is referred to as “intermittent dry fasting.” This is defined in the consensus as intermittent fasting regimens that involve abstaining from food and fluid intake during the fasting interval, which typically lasts 9-20 hours.
Most dry fasts, including religious ones, are maintained for a specific interval and are followed by a refeeding period. These fasts are not starvation, defined as no food or water intake for days.
What the Evidence Says
All that said, dry fasting by any other name remains dry fasting. “Abundant” evidence from animal studies suggests the potential of various types of fasting for disease prevention and treatment in humans, noted the authors of the consensus report, Along with the risks described above, small studies have explored short-term effects in people, all of which have yet to be established by larger and longer-term studies.
In a recent small study, researchers at Baylor College of Medicine, Houston, Texas, reported that dawn-to-dusk dry fasting for 30 days reduced levels of inflammatory cytokines in the 13 participants with a high body mass index. Earlier work by the group showed that dawn-to-dusk dry fasting for 30 days induced “anti-atherosclerotic, anti-inflammatory, and anti-tumorigenic proteome” in peripheral blood mononuclear cells of 14 individuals with metabolic syndrome (The researchers declined to comment for this article.)
Importantly, the health effects can vary among individuals for unknown reasons, found a recent cross-sectional study of fasting blood glucose (FBG) changes in 181 patients with type 2 diabetes during Ramadan intermittent fasting (RIF), which involves dry fasting during daylight hours for 1 month. The researchers classified participants into three groups: reduced average FBG levels (44%), no change in FBG levels (24%), and increased FBG levels (32%). The authors wrote that further studies are needed to identify factors associated with the differences and to identify “those who are great candidates for RIF.”
In contrast to some of the concerns expressed by clinicians, an exploratory study of daytime dry fasting among 34 healthy Baha’i volunteers in Germany concluded that the 19-day regimen “is safe, has no negative effects on hydration, can improve fat metabolism and can cause transient phase shifts of circadian rhythms.” The authors acknowledge that a larger number and more diverse participants are needed to validate the findings and assess the impact on long-term health.
What to Advise Patients
For patients who want to fast as part of their weight loss regimen or to help manage diabetes, clinicians can consider suggesting “alternate ways of eating that might achieve similar goals,” Ms. Bader said. One is intermittent fasting without dry fasting: the 16:8 method (16 hours of fasting, 8 hours of eating) or the 5:2 method (normal eating for 5 days, reduced calorie intake for 2 days), which can support improved insulin sensitivity and metabolic health.
Caloric restriction can also work if the patient maintains a balanced diet that includes all essential nutrients, she said. A low-carbohydrate diet that focuses on limiting carbohydrate intake while increasing consumption of lean proteins and healthy fats has been shown to lower blood sugar levels and improve insulin sensitivity.
Other healthy strategies for patients include the Mediterranean diet, which emphasizes whole grains, fruits, vegetables, nuts, seeds, olive oil, and lean proteins such as fish, or a similar plant-based diet with less animal protein. Ms. Bader advises cultivating mindful eating, which involves paying attention to hunger and fullness cues, making thoughtful food choices, and focusing on being present during meals.
“Each of these dietary strategies offers potential benefits for managing type 2 diabetes and improving overall health,” Ms. Bader said. “I have not had any patients who have tried dry fasting specifically. However, I have encountered scenarios where individuals abstained from food and beverages due to religious practices. In those cases, we focused on ensuring that they maintained proper hydration and balanced nutrition during their eating periods to manage their diabetes effectively and prevent complications.”
Overall, Dr. Adimoolam suggests that clinicians help patients find a weight-loss plan that works best for them based on understanding the calories in the foods they like and don’t like. For fasting regimens, patients can be encouraged to choose one with fluids when possible, as well as intervals of time to fast and eat that work best for their lifestyle.
Ms. Bader, Dr. Bruno, and Dr. Adimoolam report no relevant conflicts.
A version of this article appeared on Medscape.com.
Dry fasting, the practice of going without food and water, has enthusiastic advocates on TikTok, X, YouTube, and other social media platforms. Devotees claim a wide range of health effects, but medical professionals advise caution to ensure that the practice does more good than harm, especially for individuals with diabetes.
Purported benefits and risks vary, depending on who is following the regimen and how long they abstain from food and water. Advocates on social media assert that dry fasting makes “intuition skyrocket” and puts autophagy on “overdrive.” Although such statements may rev up followers, there is little evidence to support these and many other dry-fasting claims. In fact, several physicians warned about unintended consequences.
“I had one patient who followed this fasting method often, and over time she developed kidney stones that led to a severe infection,” said Deena Adimoolam, MD, an endocrinologist in private practice in New York City and New Jersey. “Lack of both water and food can fuel hunger and increase the likelihood of overeating or binge eating once the fast is completed, which does not lead to weight loss. Untreated dehydration can lead to loss of consciousness.”
“For individuals with type 2 diabetes, dehydration can exacerbate hyperglycemia and increase the risk of complications such as diabetic ketoacidosis (DKA),” said Abeer Bader, lead clinical nutrition specialist at the Massachusetts General Hospital Weight Center in Boston. “Research also consistently shows that adequate hydration is crucial for maintaining physical and cognitive performance.”
, Ms. Bader noted. “Prolonged dry fasting can result in nutrient deficiencies. For individuals with diabetes, maintaining adequate nutrition is crucial to manage blood sugar levels and overall health. The lack of both food and water can exacerbate deficiencies.”
Joanne Bruno, MD, an endocrinologist at NYU Langone Health, added, “Certain medications used for the management of type 2 diabetes, such as SGLT2 inhibitors, can cause dehydration. It is critical that patients stay well hydrated while on these medications to avoid serious side effects such as euglycemic DKA.”
What Exactly Is Dry Fasting?
Defining dry fasting, like any kind of fasting, has remained a challenge, according to authors of the first international consensus on fasting terminology, published on July 25 in Cell Metabolism. The clinical terminology “has remained heterogeneous and often confusing, with similar terms being used to define different fasting regimens ... reflecting the manifold contexts in which fasting is practiced.”
Indeed, dry fasting was among the most discussed terms by the consensus panel and went through several rounds before the panelists came to agreement. A few experts were critical of the practice, whereas those familiar with religious fasting traditions, such as during Ramadan, were clear about the importance of including this term in the consensus process.
“The dissent was resolved by the clarification that this form of fasting has historical and geographical extensions and that the present consensus process did not aim at evaluating therapeutic effectiveness or safety for any term defined,” the authors wrote.
The panel concluded that dry fasting is not the same as total or complete fasting because the latter can include water (such as water-only fasting). Their final definition of dry fasting is ‘’a fasting regimen during which a voluntary abstinence from all foods and beverages, including water, is practiced for a certain period of time.’’
Different types of fasting regimens, such as intermittent fasting, may include dry fasting, in which case it is referred to as “intermittent dry fasting.” This is defined in the consensus as intermittent fasting regimens that involve abstaining from food and fluid intake during the fasting interval, which typically lasts 9-20 hours.
Most dry fasts, including religious ones, are maintained for a specific interval and are followed by a refeeding period. These fasts are not starvation, defined as no food or water intake for days.
What the Evidence Says
All that said, dry fasting by any other name remains dry fasting. “Abundant” evidence from animal studies suggests the potential of various types of fasting for disease prevention and treatment in humans, noted the authors of the consensus report, Along with the risks described above, small studies have explored short-term effects in people, all of which have yet to be established by larger and longer-term studies.
In a recent small study, researchers at Baylor College of Medicine, Houston, Texas, reported that dawn-to-dusk dry fasting for 30 days reduced levels of inflammatory cytokines in the 13 participants with a high body mass index. Earlier work by the group showed that dawn-to-dusk dry fasting for 30 days induced “anti-atherosclerotic, anti-inflammatory, and anti-tumorigenic proteome” in peripheral blood mononuclear cells of 14 individuals with metabolic syndrome (The researchers declined to comment for this article.)
Importantly, the health effects can vary among individuals for unknown reasons, found a recent cross-sectional study of fasting blood glucose (FBG) changes in 181 patients with type 2 diabetes during Ramadan intermittent fasting (RIF), which involves dry fasting during daylight hours for 1 month. The researchers classified participants into three groups: reduced average FBG levels (44%), no change in FBG levels (24%), and increased FBG levels (32%). The authors wrote that further studies are needed to identify factors associated with the differences and to identify “those who are great candidates for RIF.”
In contrast to some of the concerns expressed by clinicians, an exploratory study of daytime dry fasting among 34 healthy Baha’i volunteers in Germany concluded that the 19-day regimen “is safe, has no negative effects on hydration, can improve fat metabolism and can cause transient phase shifts of circadian rhythms.” The authors acknowledge that a larger number and more diverse participants are needed to validate the findings and assess the impact on long-term health.
What to Advise Patients
For patients who want to fast as part of their weight loss regimen or to help manage diabetes, clinicians can consider suggesting “alternate ways of eating that might achieve similar goals,” Ms. Bader said. One is intermittent fasting without dry fasting: the 16:8 method (16 hours of fasting, 8 hours of eating) or the 5:2 method (normal eating for 5 days, reduced calorie intake for 2 days), which can support improved insulin sensitivity and metabolic health.
Caloric restriction can also work if the patient maintains a balanced diet that includes all essential nutrients, she said. A low-carbohydrate diet that focuses on limiting carbohydrate intake while increasing consumption of lean proteins and healthy fats has been shown to lower blood sugar levels and improve insulin sensitivity.
Other healthy strategies for patients include the Mediterranean diet, which emphasizes whole grains, fruits, vegetables, nuts, seeds, olive oil, and lean proteins such as fish, or a similar plant-based diet with less animal protein. Ms. Bader advises cultivating mindful eating, which involves paying attention to hunger and fullness cues, making thoughtful food choices, and focusing on being present during meals.
“Each of these dietary strategies offers potential benefits for managing type 2 diabetes and improving overall health,” Ms. Bader said. “I have not had any patients who have tried dry fasting specifically. However, I have encountered scenarios where individuals abstained from food and beverages due to religious practices. In those cases, we focused on ensuring that they maintained proper hydration and balanced nutrition during their eating periods to manage their diabetes effectively and prevent complications.”
Overall, Dr. Adimoolam suggests that clinicians help patients find a weight-loss plan that works best for them based on understanding the calories in the foods they like and don’t like. For fasting regimens, patients can be encouraged to choose one with fluids when possible, as well as intervals of time to fast and eat that work best for their lifestyle.
Ms. Bader, Dr. Bruno, and Dr. Adimoolam report no relevant conflicts.
A version of this article appeared on Medscape.com.
Dry fasting, the practice of going without food and water, has enthusiastic advocates on TikTok, X, YouTube, and other social media platforms. Devotees claim a wide range of health effects, but medical professionals advise caution to ensure that the practice does more good than harm, especially for individuals with diabetes.
Purported benefits and risks vary, depending on who is following the regimen and how long they abstain from food and water. Advocates on social media assert that dry fasting makes “intuition skyrocket” and puts autophagy on “overdrive.” Although such statements may rev up followers, there is little evidence to support these and many other dry-fasting claims. In fact, several physicians warned about unintended consequences.
“I had one patient who followed this fasting method often, and over time she developed kidney stones that led to a severe infection,” said Deena Adimoolam, MD, an endocrinologist in private practice in New York City and New Jersey. “Lack of both water and food can fuel hunger and increase the likelihood of overeating or binge eating once the fast is completed, which does not lead to weight loss. Untreated dehydration can lead to loss of consciousness.”
“For individuals with type 2 diabetes, dehydration can exacerbate hyperglycemia and increase the risk of complications such as diabetic ketoacidosis (DKA),” said Abeer Bader, lead clinical nutrition specialist at the Massachusetts General Hospital Weight Center in Boston. “Research also consistently shows that adequate hydration is crucial for maintaining physical and cognitive performance.”
, Ms. Bader noted. “Prolonged dry fasting can result in nutrient deficiencies. For individuals with diabetes, maintaining adequate nutrition is crucial to manage blood sugar levels and overall health. The lack of both food and water can exacerbate deficiencies.”
Joanne Bruno, MD, an endocrinologist at NYU Langone Health, added, “Certain medications used for the management of type 2 diabetes, such as SGLT2 inhibitors, can cause dehydration. It is critical that patients stay well hydrated while on these medications to avoid serious side effects such as euglycemic DKA.”
What Exactly Is Dry Fasting?
Defining dry fasting, like any kind of fasting, has remained a challenge, according to authors of the first international consensus on fasting terminology, published on July 25 in Cell Metabolism. The clinical terminology “has remained heterogeneous and often confusing, with similar terms being used to define different fasting regimens ... reflecting the manifold contexts in which fasting is practiced.”
Indeed, dry fasting was among the most discussed terms by the consensus panel and went through several rounds before the panelists came to agreement. A few experts were critical of the practice, whereas those familiar with religious fasting traditions, such as during Ramadan, were clear about the importance of including this term in the consensus process.
“The dissent was resolved by the clarification that this form of fasting has historical and geographical extensions and that the present consensus process did not aim at evaluating therapeutic effectiveness or safety for any term defined,” the authors wrote.
The panel concluded that dry fasting is not the same as total or complete fasting because the latter can include water (such as water-only fasting). Their final definition of dry fasting is ‘’a fasting regimen during which a voluntary abstinence from all foods and beverages, including water, is practiced for a certain period of time.’’
Different types of fasting regimens, such as intermittent fasting, may include dry fasting, in which case it is referred to as “intermittent dry fasting.” This is defined in the consensus as intermittent fasting regimens that involve abstaining from food and fluid intake during the fasting interval, which typically lasts 9-20 hours.
Most dry fasts, including religious ones, are maintained for a specific interval and are followed by a refeeding period. These fasts are not starvation, defined as no food or water intake for days.
What the Evidence Says
All that said, dry fasting by any other name remains dry fasting. “Abundant” evidence from animal studies suggests the potential of various types of fasting for disease prevention and treatment in humans, noted the authors of the consensus report, Along with the risks described above, small studies have explored short-term effects in people, all of which have yet to be established by larger and longer-term studies.
In a recent small study, researchers at Baylor College of Medicine, Houston, Texas, reported that dawn-to-dusk dry fasting for 30 days reduced levels of inflammatory cytokines in the 13 participants with a high body mass index. Earlier work by the group showed that dawn-to-dusk dry fasting for 30 days induced “anti-atherosclerotic, anti-inflammatory, and anti-tumorigenic proteome” in peripheral blood mononuclear cells of 14 individuals with metabolic syndrome (The researchers declined to comment for this article.)
Importantly, the health effects can vary among individuals for unknown reasons, found a recent cross-sectional study of fasting blood glucose (FBG) changes in 181 patients with type 2 diabetes during Ramadan intermittent fasting (RIF), which involves dry fasting during daylight hours for 1 month. The researchers classified participants into three groups: reduced average FBG levels (44%), no change in FBG levels (24%), and increased FBG levels (32%). The authors wrote that further studies are needed to identify factors associated with the differences and to identify “those who are great candidates for RIF.”
In contrast to some of the concerns expressed by clinicians, an exploratory study of daytime dry fasting among 34 healthy Baha’i volunteers in Germany concluded that the 19-day regimen “is safe, has no negative effects on hydration, can improve fat metabolism and can cause transient phase shifts of circadian rhythms.” The authors acknowledge that a larger number and more diverse participants are needed to validate the findings and assess the impact on long-term health.
What to Advise Patients
For patients who want to fast as part of their weight loss regimen or to help manage diabetes, clinicians can consider suggesting “alternate ways of eating that might achieve similar goals,” Ms. Bader said. One is intermittent fasting without dry fasting: the 16:8 method (16 hours of fasting, 8 hours of eating) or the 5:2 method (normal eating for 5 days, reduced calorie intake for 2 days), which can support improved insulin sensitivity and metabolic health.
Caloric restriction can also work if the patient maintains a balanced diet that includes all essential nutrients, she said. A low-carbohydrate diet that focuses on limiting carbohydrate intake while increasing consumption of lean proteins and healthy fats has been shown to lower blood sugar levels and improve insulin sensitivity.
Other healthy strategies for patients include the Mediterranean diet, which emphasizes whole grains, fruits, vegetables, nuts, seeds, olive oil, and lean proteins such as fish, or a similar plant-based diet with less animal protein. Ms. Bader advises cultivating mindful eating, which involves paying attention to hunger and fullness cues, making thoughtful food choices, and focusing on being present during meals.
“Each of these dietary strategies offers potential benefits for managing type 2 diabetes and improving overall health,” Ms. Bader said. “I have not had any patients who have tried dry fasting specifically. However, I have encountered scenarios where individuals abstained from food and beverages due to religious practices. In those cases, we focused on ensuring that they maintained proper hydration and balanced nutrition during their eating periods to manage their diabetes effectively and prevent complications.”
Overall, Dr. Adimoolam suggests that clinicians help patients find a weight-loss plan that works best for them based on understanding the calories in the foods they like and don’t like. For fasting regimens, patients can be encouraged to choose one with fluids when possible, as well as intervals of time to fast and eat that work best for their lifestyle.
Ms. Bader, Dr. Bruno, and Dr. Adimoolam report no relevant conflicts.
A version of this article appeared on Medscape.com.
Thyroid Hormone Balance Crucial for Liver Fat Reduction
TOPLINE:
Greater availability of peripheral tri-iodothyronine (T3), indicated by higher concentrations of free T3, T3, and T3/thyroxine (T4) ratio, is associated with increased liver fat content at baseline and a greater liver fat reduction following a dietary intervention known to reduce liver fat.
METHODOLOGY:
- Systemic hypothyroidism and subclinical hypothyroidism are proposed as independent risk factors for steatotic liver disease, but there are conflicting results in euthyroid individuals with normal thyroid function.
- Researchers investigated the association between thyroid function and intrahepatic lipids in 332 euthyroid individuals aged 50-80 years who reported limited alcohol consumption and had at least one condition for unhealthy aging (eg, cardiovascular disease).
- The analysis drew on a sub-cohort from the NutriAct trial, in which participants were randomly assigned to either an intervention group (diet rich in unsaturated fatty acids, plant protein, and fiber) or a control group (following the German Nutrition Society recommendations).
- The relationship between changes in intrahepatic lipid content and thyroid hormone parameters was evaluated in 243 individuals with data available at 12 months.
TAKEAWAY:
- Higher levels of free T3 and T3/T4 ratio were associated with increased liver fat content at baseline (P = .03 and P = .01, respectively).
- After 12 months, both the intervention and control groups showed reductions in liver fat content, along with similar reductions in free T3, total T3, T3/T4 ratio, and free T3/free T4 ratio (all P < .01).
- Thyroid stimulating hormone, T4, and free T4 levels remained stable in either group during the intervention.
- Participants who maintained higher T3 levels during the dietary intervention experienced a greater reduction in liver fat content over 12 months (Rho = −0.133; P = .039).
IN PRACTICE:
“A higher peripheral concentration of active THs [thyroid hormones] might reflect a compensatory mechanism in subjects with mildly increased IHL [intrahepatic lipid] content and early stages of MASLD [metabolic dysfunction–associated steatotic liver disease],” the authors wrote.
SOURCE:
The study was led by Miriam Sommer-Ballarini, Charité–Universitätsmedizin Berlin, Berlin, Germany. It was published online in the European Journal of Endocrinology.
LIMITATIONS:
Participants younger than 50 years of age and with severe hepatic disease, severe substance abuse, or active cancer were excluded, which may limit the generalizability of the findings. Because the study cohort had only mildly elevated median intrahepatic lipid content at baseline, it may not be suited to address the advanced stages of metabolic dysfunction–associated steatotic liver disease. The study’s findings are based on a specific dietary intervention, which may not be applicable to other dietary patterns or populations.
DISCLOSURES:
The Deutsche Forschungsgemeinschaft and German Federal Ministry for Education and Research funded this study. Some authors declared receiving funding, serving as consultants, or being employed by relevant private companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Greater availability of peripheral tri-iodothyronine (T3), indicated by higher concentrations of free T3, T3, and T3/thyroxine (T4) ratio, is associated with increased liver fat content at baseline and a greater liver fat reduction following a dietary intervention known to reduce liver fat.
METHODOLOGY:
- Systemic hypothyroidism and subclinical hypothyroidism are proposed as independent risk factors for steatotic liver disease, but there are conflicting results in euthyroid individuals with normal thyroid function.
- Researchers investigated the association between thyroid function and intrahepatic lipids in 332 euthyroid individuals aged 50-80 years who reported limited alcohol consumption and had at least one condition for unhealthy aging (eg, cardiovascular disease).
- The analysis drew on a sub-cohort from the NutriAct trial, in which participants were randomly assigned to either an intervention group (diet rich in unsaturated fatty acids, plant protein, and fiber) or a control group (following the German Nutrition Society recommendations).
- The relationship between changes in intrahepatic lipid content and thyroid hormone parameters was evaluated in 243 individuals with data available at 12 months.
TAKEAWAY:
- Higher levels of free T3 and T3/T4 ratio were associated with increased liver fat content at baseline (P = .03 and P = .01, respectively).
- After 12 months, both the intervention and control groups showed reductions in liver fat content, along with similar reductions in free T3, total T3, T3/T4 ratio, and free T3/free T4 ratio (all P < .01).
- Thyroid stimulating hormone, T4, and free T4 levels remained stable in either group during the intervention.
- Participants who maintained higher T3 levels during the dietary intervention experienced a greater reduction in liver fat content over 12 months (Rho = −0.133; P = .039).
IN PRACTICE:
“A higher peripheral concentration of active THs [thyroid hormones] might reflect a compensatory mechanism in subjects with mildly increased IHL [intrahepatic lipid] content and early stages of MASLD [metabolic dysfunction–associated steatotic liver disease],” the authors wrote.
SOURCE:
The study was led by Miriam Sommer-Ballarini, Charité–Universitätsmedizin Berlin, Berlin, Germany. It was published online in the European Journal of Endocrinology.
LIMITATIONS:
Participants younger than 50 years of age and with severe hepatic disease, severe substance abuse, or active cancer were excluded, which may limit the generalizability of the findings. Because the study cohort had only mildly elevated median intrahepatic lipid content at baseline, it may not be suited to address the advanced stages of metabolic dysfunction–associated steatotic liver disease. The study’s findings are based on a specific dietary intervention, which may not be applicable to other dietary patterns or populations.
DISCLOSURES:
The Deutsche Forschungsgemeinschaft and German Federal Ministry for Education and Research funded this study. Some authors declared receiving funding, serving as consultants, or being employed by relevant private companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Greater availability of peripheral tri-iodothyronine (T3), indicated by higher concentrations of free T3, T3, and T3/thyroxine (T4) ratio, is associated with increased liver fat content at baseline and a greater liver fat reduction following a dietary intervention known to reduce liver fat.
METHODOLOGY:
- Systemic hypothyroidism and subclinical hypothyroidism are proposed as independent risk factors for steatotic liver disease, but there are conflicting results in euthyroid individuals with normal thyroid function.
- Researchers investigated the association between thyroid function and intrahepatic lipids in 332 euthyroid individuals aged 50-80 years who reported limited alcohol consumption and had at least one condition for unhealthy aging (eg, cardiovascular disease).
- The analysis drew on a sub-cohort from the NutriAct trial, in which participants were randomly assigned to either an intervention group (diet rich in unsaturated fatty acids, plant protein, and fiber) or a control group (following the German Nutrition Society recommendations).
- The relationship between changes in intrahepatic lipid content and thyroid hormone parameters was evaluated in 243 individuals with data available at 12 months.
TAKEAWAY:
- Higher levels of free T3 and T3/T4 ratio were associated with increased liver fat content at baseline (P = .03 and P = .01, respectively).
- After 12 months, both the intervention and control groups showed reductions in liver fat content, along with similar reductions in free T3, total T3, T3/T4 ratio, and free T3/free T4 ratio (all P < .01).
- Thyroid stimulating hormone, T4, and free T4 levels remained stable in either group during the intervention.
- Participants who maintained higher T3 levels during the dietary intervention experienced a greater reduction in liver fat content over 12 months (Rho = −0.133; P = .039).
IN PRACTICE:
“A higher peripheral concentration of active THs [thyroid hormones] might reflect a compensatory mechanism in subjects with mildly increased IHL [intrahepatic lipid] content and early stages of MASLD [metabolic dysfunction–associated steatotic liver disease],” the authors wrote.
SOURCE:
The study was led by Miriam Sommer-Ballarini, Charité–Universitätsmedizin Berlin, Berlin, Germany. It was published online in the European Journal of Endocrinology.
LIMITATIONS:
Participants younger than 50 years of age and with severe hepatic disease, severe substance abuse, or active cancer were excluded, which may limit the generalizability of the findings. Because the study cohort had only mildly elevated median intrahepatic lipid content at baseline, it may not be suited to address the advanced stages of metabolic dysfunction–associated steatotic liver disease. The study’s findings are based on a specific dietary intervention, which may not be applicable to other dietary patterns or populations.
DISCLOSURES:
The Deutsche Forschungsgemeinschaft and German Federal Ministry for Education and Research funded this study. Some authors declared receiving funding, serving as consultants, or being employed by relevant private companies.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
GLP-1 Thyroid Warning Could Increase Overdiagnosis
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
ORLANDO, Florida — Clinicians should keep in mind concerns about overdiagnosis of thyroid cancer when prescribing glucagon-like peptide 1 (GLP-1) drugs, as the US boxed warning about this risk for this class of medicines for certain tumors in mice could trigger excess screening, an expert endocrinologist said.
Speaking at the annual American Diabetes Association (ADA) 84th Scientific Sessions, Elizabeth N. Pearce, MD, MSc, a professor of medicine at Boston University, Boston, reviewed the different approaches US and European regulators have taken for the GLP-1 drugs. She also explained the current concerns about the wide use of thyroid screening in general and how these intersect with the rapid uptake of the GLP-1 drugs.
said Dr. Pearce, who is also a former board president of the American Thyroid Association (ATA). “We do not want to contribute to this epidemic of overdiagnosis of thyroid cancer.”
The ATA and the US Preventive Services Task Force (USPSTF) are among the health organizations that have in recent years sought to boost public awareness of the potential risks for excess screening of thyroid nodules. In 2017, the USPSTF, which influences insurance coverage, recommended against routine screening for thyroid cancer in asymptomatic adults. At that time, the incidence of thyroid cancer detection had increased by 4.5% per year over a decade, faster than for any other cancer, but without a corresponding change in the mortality rate, USPSTF said.
“Unequivocally, the thyroid cancer mortality has not kept pace with thyroid cancer detection,” Dr. Pearce said at the ADA meeting. “We’ve been diagnosing a lot of small thyroid cancers that people would otherwise have been destined to die with and not die of.”
Dr. Pearce said clinicians should be careful not to overly restrict access to GLP-1 drugs due to concerns about thyroid cancer — and they should use care in screening nodules.
It’s possible that the weight loss experienced by people taking GLP-1 drugs may make preexisting thyroid nodules more prominent, Dr. Pearce said. It’s also likely that the US boxed warning on thyroid risk on GLP-1 drugs makes clinicians and patients more likely to look for these kinds of growths.
Dr. Pearce urged adherence to guidelines such as the ones the ATA published in 2015 for assessing nodules.
In an interview with this news organization, Dr. Pearce noted the frequency of CT scans in US medical practice in turning up many incidental thyroid nodules, a finding that can cause some panic for patients and their clinicians.
But it helps to put these findings in context, as by the age of 50, about 40% of women will have at least one thyroid nodule, making this a very common finding, she said.
“The vast majority are not malignant,” Dr. Pearce said. “When you explain this to patients, it alleviates anxiety.”
The US, European Union Differences
In the United States, the label for GLP-1 drugs starts with a boxed warning about thyroid C-cell tumors seen in rodents given these medicines in testing.
It’s unknown if the medicines could cause medullary thyroid carcinoma (MTC) in humans, the label adds. The drug is contraindicated in patients with a personal or family history of MTC or multiple endocrine neoplasia syndrome 2, the boxed warning says. This is based largely on data seen in laboratory rats.
“It’s a big black box warning that gets people’s attention,” Dr. Pearce said. “Important to note that if you practice in Europe, you will not be familiar with this labeling because it doesn’t exist there. They’ve never had this warning on the European package.”
The European Medicines Agency (EMA) does include information about the results of rodent studies as part of the discussion of known and potential risks for GLP-1 drugs but has not emphasized it in the same way as the US drug labels do.
For example, the public assessment report posted on the EMA website for semaglutide (Ozempic, Novo Nordisk) notes that nonlethal thyroid C-cell tumors “observed in rodents are a class effect for GLP-1 receptor agonists.” It’s possible that these may be due to a particular sensitivity in rodents, the report said.
“The relevance for humans is considered to be low but cannot be completely excluded,” the EMA report said in the product information section of the report.
There has been ongoing interest in the issue.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) in October concluded that the available evidence does not support a causal association between GLP-1 receptor agonists and thyroid cancer.
The EMA’s PRAC safety committee said it began assessing the evidence about a possible connection following the publication of a study in 2022 in the journal Diabetes Care. That paper reported on an analysis that suggested increased risk for all thyroid cancer and medullary thyroid cancer with the use of GLP-1 drugs, particularly after 1-3 years of treatment.
The EMA’s PRAC said that in making its decision, it also considered other published papers on this topic as well as clinical and postmarketing data on GLP-1 drugs.
In an email interview, Jean-Luc Faillie, MD, PhD, corresponding author of the Diabetes Care paper, called for continued “vigilance and prudence in clinical practice” with GLP-1 drugs.
His paper reported on a case-control analysis on the basis of reports from the French national healthcare insurance system database, looking at people who had taken GLP-1 drugs and similar people who had not.
Due to a lack of a specific diagnostic code for medullary thyroid cancers, the researchers used a composite definition combining thyroid cancer diagnosis with several calcitonin tests, a carcinoembryonic antigen test, or a specific treatment (vandetanib) to identify potential cases of this cancer.
It’s possible that this method could have led to overestimation of MTC among the cases of thyroid cancer, wrote Dr. Faillie, who is a professor at France’s Université de Montpellier, Montpellier, France, and part of its pharmacological vigilance service.
“Nevertheless, it’s crucial to emphasize that any potential overestimation of MTC cases would likely apply equally to both GLP-1 receptor agonist–exposed and unexposed groups,” Dr. Faillie wrote. “Therefore, it should not significantly impact our main findings regarding the suggested increased risk associated with GLP-1 receptor agonist use.”
Dr. Pearce disclosed honoraria for speaking at the Merck China Forum. Dr. Faille and his coauthors reported no conflicts of interest in the publication of their study. Their research was supported by the French Medicines Agency (Agence Nationale de Sécurité du Médicament et des Produits de Santé, grant 2019S015) in the context of a partnership with the Health Product Epidemiology Scientific Interest Group (EPI-PHARE). The study was part of France’s Drugs Systematized Assessment in Real-Life Environment (DRUGS-SAFEr) research program.
A version of this article first appeared on Medscape.com.
FROM ADA 2024