User login
FDA approves topical roflumilast for psoriasis in children aged 6-11
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
On Oct. 6, the This marks an expanded indication for the drug, which was first approved for the same indication in July, 2022, for individuals aged 12 and older.
Roflumilast cream 0.3% is a phosphodiesterase-4 inhibitor approved for once-daily topical treatment of mild, moderate, and severe plaque psoriasis. According to a press release from the manufacturer, Arcutis Biotherapeutics, approval of the expanded indication is based on data from a 4-week Maximal Usage Systemic Exposure (MUSE) study in children ages 6-11 years with plaque psoriasis. It stated that pharmacokinetic, safety, tolerability, and efficacy data from this study were “generally consistent” with data from the DERMIS-1 and DERMIS-2 pivotal phase 3 trials in adults.
According to the press release, a future FDA review is planned for the results from a second MUSE study in children ages 2-5 years, as well as data from an ongoing open-label extension study evaluating the long-term safety of roflumilast cream in individuals with plaque psoriasis aged 2 years and older. The company markets topical roflumilast as Zoryve.
These adverse events linked to improved cancer prognosis
TOPLINE:
.
METHODOLOGY:
- Emerging evidence suggests that the presence of cutaneous immune-related adverse events may be linked with favorable outcomes among patients with cancer who receive ICIs.
- Researchers conducted a systematic review and meta-analysis that included 23 studies and a total of 22,749 patients with cancer who received ICI treatment; studies compared outcomes among patients with and those without cutaneous immune-related adverse events.
- The major outcomes evaluated in the analysis were overall survival and progression-free survival (PFS); subgroup analyses assessed cutaneous immune-related adverse event type, cancer type, and other factors.
TAKEAWAY:
- The occurrence of cutaneous immune-related adverse events was associated with improved PFS (hazard ratio, 0.52; P < .001) and overall survival (HR, 0.61; P < .001).
- In the subgroup analysis, patients with eczematous (HR, 0.69), lichenoid or lichen planus–like skin lesions (HR, 0.51), pruritus without rash (HR, 0.70), psoriasis (HR, 0.63), or vitiligo (HR, 0.30) demonstrated a significant overall survival advantage. Vitiligo was the only adverse event associated with a PFS advantage (HR, 0.28).
- Among patients with melanoma, analyses revealed a significant association between the incidence of cutaneous immune-related adverse events and improved overall survival (HR, 0.51) and PFS (HR, 0.45). The authors highlighted similar findings among patients with non–small cell lung cancer (HR, 0.50 for overall survival and 0.61 for PFS).
IN PRACTICE:
“These data suggest that [cutaneous immune-related adverse events] may have useful prognostic value in ICI treatment,” the authors concluded.
SOURCE:
The analysis, led by Fei Wang, MD, Zhong Da Hospital, School of Medicine, Southeast University, Nanjing, China, was published online in JAMA Dermatology.
LIMITATIONS:
Most of the data came from retrospective studies, and there were limited data on specific patient subgroups. The Egger tests, used to assess potential publication bias in meta-analyses, revealed publication bias.
DISCLOSURES:
No disclosures were reported. The study was supported by a grant from the Postgraduate Research and Practice Innovation Program of Jiangsu Province.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Emerging evidence suggests that the presence of cutaneous immune-related adverse events may be linked with favorable outcomes among patients with cancer who receive ICIs.
- Researchers conducted a systematic review and meta-analysis that included 23 studies and a total of 22,749 patients with cancer who received ICI treatment; studies compared outcomes among patients with and those without cutaneous immune-related adverse events.
- The major outcomes evaluated in the analysis were overall survival and progression-free survival (PFS); subgroup analyses assessed cutaneous immune-related adverse event type, cancer type, and other factors.
TAKEAWAY:
- The occurrence of cutaneous immune-related adverse events was associated with improved PFS (hazard ratio, 0.52; P < .001) and overall survival (HR, 0.61; P < .001).
- In the subgroup analysis, patients with eczematous (HR, 0.69), lichenoid or lichen planus–like skin lesions (HR, 0.51), pruritus without rash (HR, 0.70), psoriasis (HR, 0.63), or vitiligo (HR, 0.30) demonstrated a significant overall survival advantage. Vitiligo was the only adverse event associated with a PFS advantage (HR, 0.28).
- Among patients with melanoma, analyses revealed a significant association between the incidence of cutaneous immune-related adverse events and improved overall survival (HR, 0.51) and PFS (HR, 0.45). The authors highlighted similar findings among patients with non–small cell lung cancer (HR, 0.50 for overall survival and 0.61 for PFS).
IN PRACTICE:
“These data suggest that [cutaneous immune-related adverse events] may have useful prognostic value in ICI treatment,” the authors concluded.
SOURCE:
The analysis, led by Fei Wang, MD, Zhong Da Hospital, School of Medicine, Southeast University, Nanjing, China, was published online in JAMA Dermatology.
LIMITATIONS:
Most of the data came from retrospective studies, and there were limited data on specific patient subgroups. The Egger tests, used to assess potential publication bias in meta-analyses, revealed publication bias.
DISCLOSURES:
No disclosures were reported. The study was supported by a grant from the Postgraduate Research and Practice Innovation Program of Jiangsu Province.
A version of this article first appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Emerging evidence suggests that the presence of cutaneous immune-related adverse events may be linked with favorable outcomes among patients with cancer who receive ICIs.
- Researchers conducted a systematic review and meta-analysis that included 23 studies and a total of 22,749 patients with cancer who received ICI treatment; studies compared outcomes among patients with and those without cutaneous immune-related adverse events.
- The major outcomes evaluated in the analysis were overall survival and progression-free survival (PFS); subgroup analyses assessed cutaneous immune-related adverse event type, cancer type, and other factors.
TAKEAWAY:
- The occurrence of cutaneous immune-related adverse events was associated with improved PFS (hazard ratio, 0.52; P < .001) and overall survival (HR, 0.61; P < .001).
- In the subgroup analysis, patients with eczematous (HR, 0.69), lichenoid or lichen planus–like skin lesions (HR, 0.51), pruritus without rash (HR, 0.70), psoriasis (HR, 0.63), or vitiligo (HR, 0.30) demonstrated a significant overall survival advantage. Vitiligo was the only adverse event associated with a PFS advantage (HR, 0.28).
- Among patients with melanoma, analyses revealed a significant association between the incidence of cutaneous immune-related adverse events and improved overall survival (HR, 0.51) and PFS (HR, 0.45). The authors highlighted similar findings among patients with non–small cell lung cancer (HR, 0.50 for overall survival and 0.61 for PFS).
IN PRACTICE:
“These data suggest that [cutaneous immune-related adverse events] may have useful prognostic value in ICI treatment,” the authors concluded.
SOURCE:
The analysis, led by Fei Wang, MD, Zhong Da Hospital, School of Medicine, Southeast University, Nanjing, China, was published online in JAMA Dermatology.
LIMITATIONS:
Most of the data came from retrospective studies, and there were limited data on specific patient subgroups. The Egger tests, used to assess potential publication bias in meta-analyses, revealed publication bias.
DISCLOSURES:
No disclosures were reported. The study was supported by a grant from the Postgraduate Research and Practice Innovation Program of Jiangsu Province.
A version of this article first appeared on Medscape.com.
Concurrent Atopic Dermatitis and Psoriasis Successfully Treated With Dual Biologic Therapy
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.
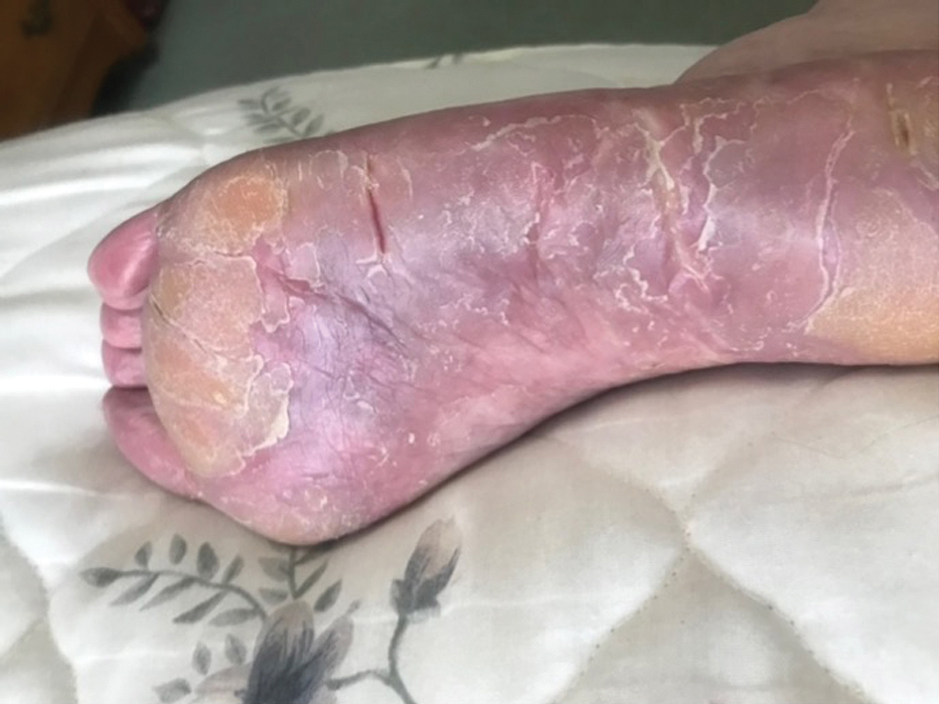
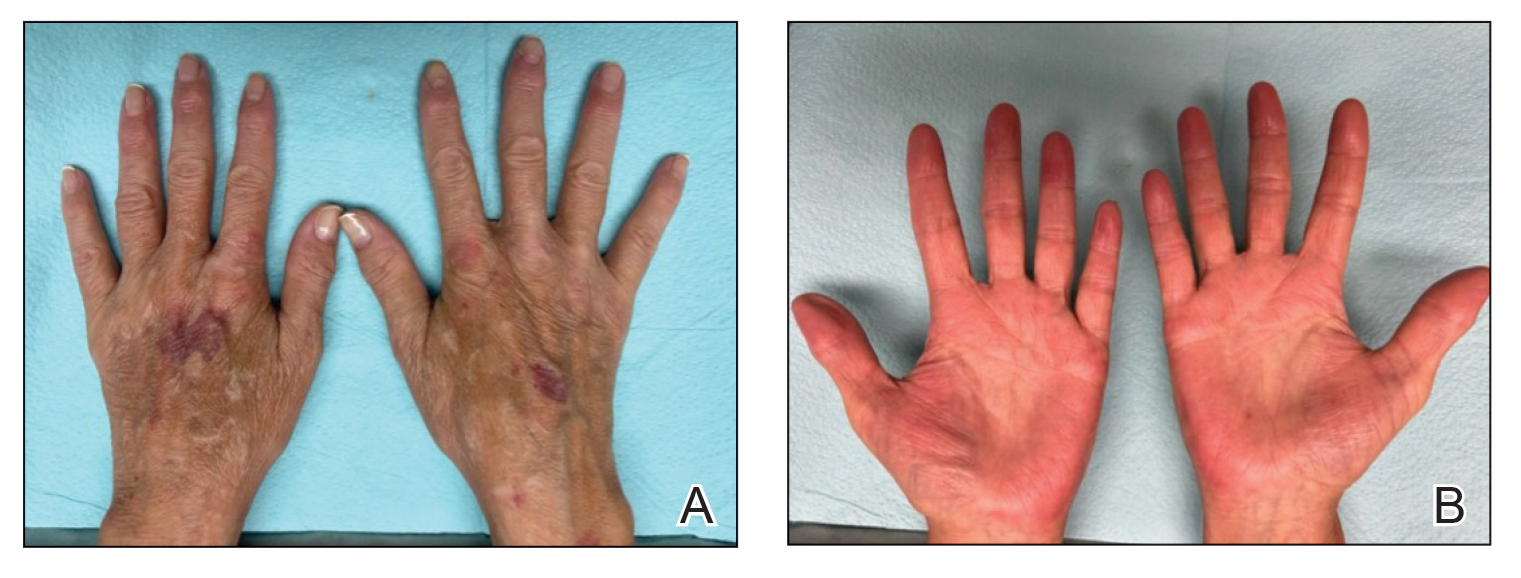
Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.
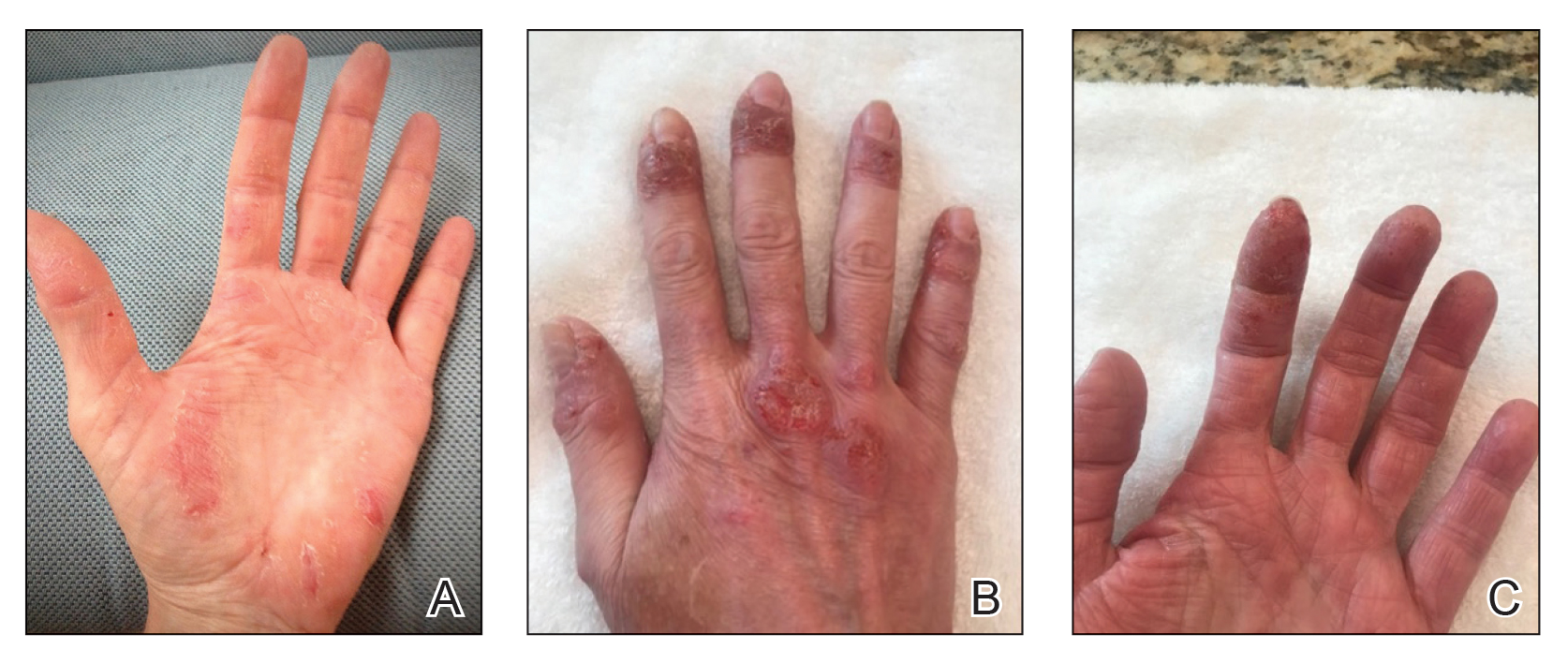
After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.
Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
Atopic dermatitis (AD) and psoriasis are common skin diseases in which dysfunction of the epidermal barrier leads to skin inflammation and altered expression of proinflammatory cytokines.1 There often is overlap in the clinical and histopathologic features of AD and psoriasis, which can make diagnosis a challenge. Persistent late-stage AD can present with psoriasiform lichenified changes, and psoriasis lesions in the acute stage can have an eczematous appearance.2 Histologically, chronic psoriasis lesions share many overlapping features with AD, and some subsets of AD with IL-17 predominance (ie, intrinsic, pediatric, presentation in Asian patients) exhibit a psoriasiform appearance.3,4
Atopic dermatitis and psoriasis are considered 2 distinct conditions because AD is a helper T cell (TH2)–driven disease with subsequent overproduction of IL-4 and IL-13 and psoriasis is a TH17 cell–driven disease with overproduction of IL-173; however, the shared features of AD and psoriasis represent an underlying immunopathological spectrum2,5,6 in which one condition can develop following treatment of the other condition (immunological shift in pathways), both conditions can occur at different times in a patient’s life with alternating cycles of disease flares, or both conditions can coexist as an overlapping syndrome.1,2 A retrospective study from 2012 to 2019 estimated the prevalence of concomitant AD and psoriasis in the United States at 1.3%, with AD following the diagnosis of psoriasis in 67% of cases.1 Concurrent AD and psoriasis—when both diseases flaresimultaneously—is the rarest scenario.2,5
Treatment modalities for AD include topical corticosteroids, which act on immune cells to suppress the release of proinflammatory cytokines, as well as dupilumab, which offers targeted blockade of involved cytokines IL-4 and IL-13. Psoriasis can be treated with multiple immune modulators, including topical corticosteroids and vitamin D analogs, as well as systemic medications that reduce T-cell activation and inflammatory cytokines through targeting of IFN-γ, IL-2, tumor necrosis factor α, IL-17, and IL-23.7,8
We present the case of a patient with long-standing concurrent, treatment-resistant AD and psoriasis who was successfully treated with dual biologic therapy with guselkumab and dupilumab.
Case Report
A 62-year-old woman presented to our dermatology clinic with red itchy scales and painful fissures on the palms, hands, and soles of more than 12 years’ duration. Her medical history included an allergy to amoxicillin-clavulanate as well as an allergy to both dog and cat dander on prick testing. Her family history included dyshidrotic eczema in her mother. A complete blood cell count with differential was within reference range. A shave biopsy of the right dorsal hand performed at the onset of symptoms at an outside facility revealed hyperkeratotic acanthotic epidermis with a mild perivascular lymphocytic infiltrate.
Results of patch testing indicated contact hypersensitivity to the botanical rosin colophonium (or colophony); carba mix (1, 3-diphenylguanidine, zinc dibutyldithiocarbamate, and zinc diethydithiocarbamate); thiuram mix (tetramethylthiuram disulfide, tetramethylthiuram monosulfide, and tetraethylthiuram disulfide); n,n-diphenylguanidine; and tixocortol-21-pivalate. Our patient was given guidance on avoiding these agents, as it was suspected that exposure may be exacerbating the psoriasis. The psoriasis was treated with topical corticosteroids, keratolytics, and calcineurin inhibitors, all of which offered minimal or no relief. Trials of systemic agents, including methotrexate (discontinued because transaminitis developed), etanercept, adalimumab, and apremilast for 6 to 10 months did not provide improvement.

Two years prior to the current presentation, our patient had been treated with the IL-23 inhibitor guselkumab, which provided moderate improvement. When she presented to our clinic, physical examination while she was taking guselkumab demonstrated prurigo with excoriations of the extremities, hyperkeratosis with scaling and fissures of the soles, erythematous scaly plaques on the palms and dorsal surface of the hands, and mild onycholysis of the nails (Figures 1 and 2). Because we were concerned about concomitant intrinsic AD, dupilumab was initiated in conjunction with guselkumab. A second biopsy was considered but deferred in favor of clinical monitoring.

After 1 year of dual biologic therapy, the patient experienced near-complete resolution of symptoms. The psoriasis completely resolved from an initial body surface area of 5%, and the AD body surface area decreased from 30% to 2% (Figure 3). The patient reported no adverse effects from treatment.

Comment
Atopic dermatitis and psoriasis involve complex immunopathology and a spectrum of cytokines that might explain the overlap in their clinical and histopathologic presentations.
Atopic dermatitis—Atopic dermatitis involves TH1, TH2, TH9, TH17, and TH22 cells; TH2 cells release IL-4, IL-5, and IL-13, all of which are key cytokines in the inflammatory pathway of AD.9,10 Activation of the helper T-cell subset and the release of cytokines differ slightly based on the subcategory of AD and the stage of exacerbation. In addition to TH2-cell activation, TH1 cells and TH22 cells—which release IL-12 and IL-22, respectively—are active in both intrinsic and extrinsic AD. TH17 cells and TH9 cells—which release IL-17 and IL-9, respectively—are more prominent in the intrinsic pathway than in the extrinsic pathway.9 Intrinsic AD is recognized by a lack of eosinophilia, female predominance, and delayed onset compared to extrinsic AD; there also is a lack of history of atopy.1 Extrinsic AD is characterized by eosinophilia as well as a personal and family history of atopy.11 Our patient—a female with onset in older adulthood, lack of eosinophilia, and a family history of atopy—displayed features of both intrinsic and extrinsic AD.
Psoriasis—The immunopathology of psoriasis involves stimulation of dendritic cells, which activate TH17 cells through IL-23. TH17 cells then release IL-17 and IL-22. Therefore, both AD and psoriasis involve activation of TH22 and TH1 cells, with increased IL-17 and IL-22 production.3,10,12 IL-17 and IL-22 induce epidermal hyperplasia; IL-22 also contributes to skin barrier dysfunction.12 Therefore, it might be reasonable to consider psoriasis and AD as diseases that exist across a T-cell axis spectrum, thereby accounting for some overlap in disease characteristics.3
Dual Biologic Therapy—Dupilumab blocks the IL-4 receptor α subunit, a receptor for IL-4 and IL-13, which are key cytokines in the pathogenesis of AD.10 Guselkumab inhibits IL-23, thus blocking the inflammatory cascade of TH17 cell activation and release of IL-17 and IL-22 in the psoriasis pathway.13 Although an immunopathological spectrum exists between the 2 diseases, the continued presence of AD symptoms after blocking the IL-23 cascade suggests that additional blockade of TH2 cells is required to control AD in patients with true concurrent disease.
Accurate diagnosis of AD and/or psoriasis is important when considering targeted treatment of these conditions with biologics. The use of dual biologics is limited by a paucity of data regarding the safety of these agents when given in combination. A recent meta-analysis of dual biologic therapy in patients with inflammatory bowel disease demonstrated acceptable safety results with a pooled adverse reaction rate of 31%.14
Anchoring Bias—Anchoring bias can occur when a clinician’s decisions are influenced by a particular event or reference point, which might cause them to disregard subsequent evidence. Our case illustrates the importance of critically assessing the response to treatment and being mindful of the potential influence of anchoring bias on the differential diagnosis. Although overcoming biases in conditions with clinical overlap can be challenging, it is important to consider coexisting AD and psoriasis in patients with extensive hand involvement when multiple treatments have failed and only a partial response to targeted pathways has been achieved. In our case, the patient also had contact hypersensitivity to tixocortol-21-pivalate, which indicates hypersensitivity to many prescription topical corticosteroids, oral prednisone, and over-the-counter hydrocortisone; however, topical corticosteroids continued to be prescribed for her, which might have contributed to the lack of improvement and even exacerbated the rash.
Future Considerations—A consideration for the future in this case is discontinuing guselkumab to observe whether symptoms recur. We discussed this option with the patient, but she opted to continue treatment with dupilumab and guselkumab because of the symptom resolution.
Conclusion
Concomitant disease can present as an overlapping pattern in the same area, whereas other regions might have geographically isolated disease. Our patient’s overlap of symptoms, the failure of multiple treatments, and the partial improvement she experienced on guselkumab made diagnosis and management challenging; however, dual biologic therapy was successful.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
- Barry K, Zancanaro P, Casseres R, et al. Concomitant atopic dermatitis and psoriasis—a retrospective review. J Dermatolog Treat. 2021;32:716-720. doi:10.1080/09546634.2019.1702147
- Bozek A, Zajac M, Krupka M. Atopic dermatitis and psoriasis as overlapping syndromes. Mediators Inflamm. 2020;2020:7527859. doi:10.1155/2020/7527859
- Guttman-Yassky E, Krueger JG. Atopic dermatitis and psoriasis: two different immune diseases or one spectrum? Curr Opin Immunol. 2017;48:68-73. doi:10.1016/j.coi.2017.08.008
- De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(suppl 1):46-48. doi:10.4081/reumatismo.2007.1s.46
- Docampo A, MJ, I, et al. Response to letter to the editor: ‘psoriasis dermatitis: an overlap condition of psoriasis and atopic dermatitis in children.’ J Eur Acad Dermatol Venereol. 2019;33:E410-E412. doi:10.1111/jdv.15716
- Johnson MC, Bowers NL, Strowd LC. Concurrent atopic dermatitis and psoriasis vulgaris: implications for targeted biologic therapy. Cutis. 2022;109:110-112. doi:10.12788/cutis.0453
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Klonowska J, Glen J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci. 2018;19:3086. doi:10.3390/ijms19103086
- Ratchataswan T, Banzon TM, Thyssen JP, et al. Biologics for treatment of atopic dermatitis: current status and future prospect. J Allergy Clin Immunol Pract. 2021;9:1053-1065. doi:10.1016/j.jaip.2020.11.034
- Czarnowicki T, He H, Krueger JG, et al. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1-11. doi:10.1016/j.jaci.2018.10.032
- Tokuyama M, Mabuchi T. New treatment addressing the pathogenesis of psoriasis. Int J Mol Sci. 2020;21:7488. doi:10.3390/ijms21207488
- Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139:2437-2446.e1. doi:10.1016/j.jid.2019.05.016
- Gold SL, Steinlauf AF. Efficacy and safety of dual biologic therapy in patients with inflammatory bowel disease: a review of the literature. Gastroenterol Hepatol (N Y). 2021;17:406-414.
Practice Points
- Atopic dermatitis and psoriasis can share clinical and histopathologic features, which represents their underlying immunopathologic spectrum.
- Atopic dermatitis and psoriasis can coexist in a single patient, which may be suspected from a clinical picture of treatment-resistant disease, a partial response to targeted therapies, or extensive hand involvement.
Severe psoriasis linked to a higher risk for heart disease, study confirms
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Prior studies with small sample sizes have shown that CMD predicts poor cardiovascular outcomes in patients with severe psoriasis.
- In a prospective multicenter study, researchers enrolled 448 patients with moderate to severe psoriasis with no documented clinical cardiovascular disease who underwent transthoracic Doppler echocardiography to evaluate coronary microcirculation.
- The outcome variable of interest was CMD, defined as a coronary flow rate of 2.5 mL or less.
- The researchers used multivariable linear regression to model the associations of the characteristics of patients with psoriasis with CMD.
TAKEAWAY:
- Of the 448 patients, 141 (31.5%) showed CMD.
- Multivariable regression revealed four variables independently associated with CMD: higher Psoriasis Area Severity Index (PASI) score (per unit, odds ratio, 1.058; P < .001), duration of psoriasis (per year; OR, 1.046; P < .001), the presence of psoriatic arthritis (OR, 1.938; P = .015), and hypertension (OR, 2.169; P = .010).
- An increase of 1 point in the PASI score and 1 year of psoriasis duration were associated with a 5.8% and a 4.6% increased risk for CMD, respectively.
IN PRACTICE:
“We should diagnose and actively search for microvascular dysfunction in patients with psoriasis, as this population is at particularly high risk,” the researchers wrote.
SOURCE:
Stefano Piaserico, MD, PhD, of the University of Padova (Italy), led the research. The study was published in the Journal of Investigative Dermatology.
LIMITATIONS:
A small proportion of patients in the study were being treated for psoriasis, and other tools for assessing CMD were not used, such as PET-CT and cardiovascular MRI.
DISCLOSURES:
The authors reported having no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
Effect of COVID-19 Vaccination on Disease Severity in Patients With Stable Plaque Psoriasis: A Cross-sectional Study
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
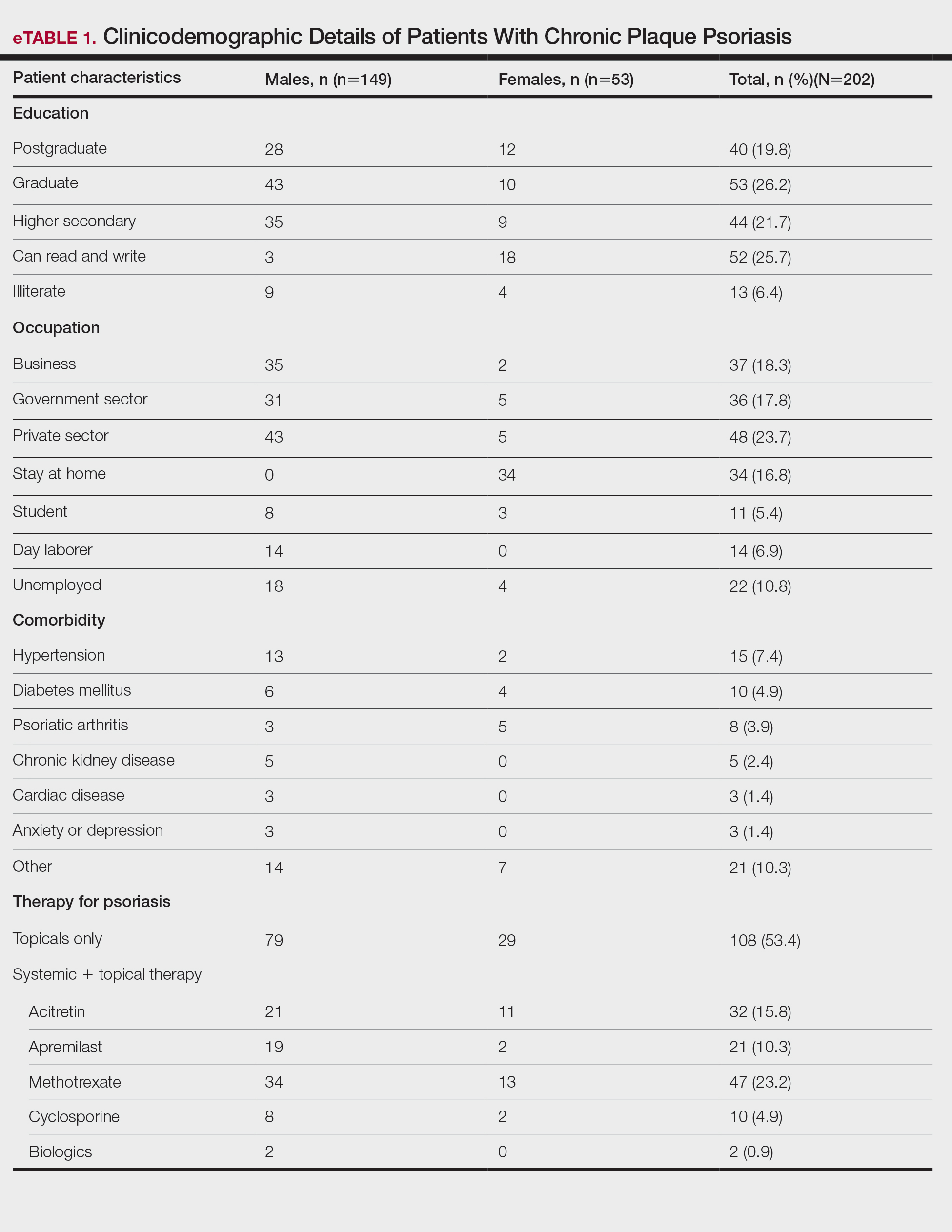
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.
The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.

The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
To the Editor:
COVID-19 infection has resulted in 6.9 million deaths worldwide. India has the third highest mortality from COVID-19 infection after the United States and Brazil.1 Vaccination plays a crucial role in containing COVID-19 infection and reducing its severity. At present, 11 vaccines have been approved by the World Health Organization. India started its vaccination program on January 16, 2021, with approval for use of Covaxin (Bharat Biotech) and Covishield (Oxford/AstraZeneca formulation)(Serum Institute of India). More than 2 billion doses have been administered since then.2,3
Patients with psoriasis are prone to develop a severe form of COVID-19 due to comorbidities and the intake of immunosuppressive drugs.4 These patients often are hesitant to receive the vaccine without an expert opinion. COVID-19 vaccines are considered to increase tumor necrosis factor α (TNF-α) and IFN-γ production by CD4+ T cells. Tumor necrosis factor α is a key proinflammatory cytokine implicated in the pathogenesis of psoriasis. COVID-19 messenger RNA vaccines induce elevation of IL-6 and helper T cells (TH17), which can induce a flare of psoriasis in a subset of patients.5The International Psoriasis Council recommends that patients with psoriasis receive one of the vaccines approved to prevent COVID-19 infection as soon as possible.6 Reports of new-onset psoriasis and flare of psoriasis after the use of COVID-19 vaccines, such as those manufactured by Pfizer-BioNTech, Moderna, and AstraZeneca, have been published from different parts of the world.7 India used locally developed whole virion inactivated BBV152 (Covaxin) and nonreplicating viral vaccine ChAdOx1 nCoV-19 (Covishield) in its vaccination program and exported them to other developing countries. There is a dearth of data on the safety of these vaccines in patients with psoriasis, which needs to be assessed. Later, Covaxin, ZyCoV-D (DNA plasmid vaccine; Cadila Healthcare), and CorbeVax (protein subunit vaccine; Biological E) were approved for usage in children.8 We conducted a cross-sectional study using the direct interview method.
Patients with psoriasis who attended the outpatient department of the Postgraduate Institute of Medical Education and Research (Chandigarh, India) from April 2022 to June 2022 were invited to participate in the study after written informed consent was received. Patients 18 years and older with chronic plaque psoriasis who had received a COVID-19 vaccine dose in the last 90 days were enrolled. Data on demographics, comorbidities, treatment received for psoriasis, vaccination concerns, history of COVID-19 infection, type of vaccine received with doses, adverse effects, and psoriasis flare after receiving the vaccine (considered up to 2 weeks from the date of vaccination) were collected. Ordinal logistic regression was used to identify factors associated with a psoriasis flare following vaccination. P<.05 was considered statistically significant.
A total of 202 patients with chronic plaque psoriasis who received either Covaxin or Covishield were enrolled during the study period. The mean age (SD) was 40.3 (13.1) years, and 149 (73.8%) patients were male. One hundred thirty-five (66.8%) patients completed 2 doses of the vaccine. eTable 1 provides the clinicodemographic details of the patients. Eighty-three (41.1%) patients had a fear of psoriasis flare after vaccination. Seventy-two (35.6%) patients received the vaccine after clearance from their treating physician/dermatologist. One hundred sixty-four (81.2%) patients received the Covishield vaccine, and 38 (18.8%) patients received Covaxin. Eighty-three (41.1%) patients reported flulike symptoms, such as fever, myalgia, or body pain, within the first week of vaccination. Sixty-one (30.2%) patients reported a psoriasis flare after vaccination in the form of new lesions or worsening of pre-existing lesions. Of these patients, 51 reported a flare after receiving the first dose of vaccine, 8 patients reported a flare after receiving the second dose of vaccine, and 2 patients reported a flare after receiving both doses of vaccine. The mean (SD) flare onset was 8.1 (3.4) days after the vaccination. Eighteen patients considered the flare to be severe. Seventeen (8.4%) patients reported a positive history of COVID-19 infection before vaccination. None of the patients reported breakthrough COVID-19 infection or pustular aggravation of psoriasis following the vaccination.

The self-reported psoriasis flare after receiving the COVID-19 vaccine was significantly higher in patients who experienced immediate adverse effects (P=.005), which included fever, myalgia, joint pain, and injection-site reaction. The reported postvaccination psoriasis flare was not significantly associated with patient sex, history of COVID-19 infection, type of vaccine received, comorbidities, or therapy for psoriasis (eTable 2).

Nearly 30% of our patients reported a postvaccination psoriasis flare, which was more common after the first vaccine dose. Sotiriou et al7 reported 14 cases of psoriasis flare in patients after receiving Pfizer-BioNTech, Moderna, and AstraZeneca COVID-19 vaccines. These patients experienced an exacerbation of disease soon after the second dose of vaccine (mean [SD], 10.36 [7.71] days), and 21% of the 713 enrolled patients wanted to forego the immunization due to concern of a postvaccination psoriasis flare.7 In another report, 14 (27%) patients developed a psoriasis flare after COVID-19 vaccination; the mean (SD) flare onset was 9.3 (4.3) days after vaccination.9
Data on the safety of the COVID-19 vaccine in patients using immunosuppressive drugs are limited. We did not find a significant association between the psoriasis flare and use of immunosuppressive drugs or type of vaccine received. Huang and Tsai9 observed similar results, with no association between psoriasis flare and use of immunosuppressive drugs or biologics, while Damiani et al10 demonstrated a protective role of biologics in preventing vaccine-induced psoriasis flare.
Similar to another study from India,11 the immediate adverse effects due to immunization with Covaxin and Covishield were mild in our study and resolved within a week. The incidence of psoriasis flare was significantly higher in patients who reported adverse effects (P=.005). Activation of immune response after vaccination leads to the release of proinflammatory and pyrogenic cytokines (ie, IL-1, IL-6, TNF-α), which may explain the higher incidence of psoriasis flare in patients experiencing adverse effects to vaccination.12
Our study showed approximately 30% of patients developed a psoriasis flare after COVID-19 vaccination, with no patients experiencing any vaccine-related serious adverse events, which suggests that Covaxin and Covishield are safe for patients with psoriasis in India. Limitations of our study include potential inaccuracy of the patient’s self-assessment of symptoms and disease flare, recall bias that may lead to errors in estimating patient-reported outcomes, the flare of psoriasis potentially being a part of disease fluctuation, and flare being enhanced by the psychological stress of vaccination.
Considering a high risk for severe COVID-19 infection in patients with psoriasis with comorbidities and those using immunosuppressive drugs, Covaxin and Covishield can be safely recommended in India. However, caution needs to be exercised when vaccinating patients with an unstable disease or severe psoriasis.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
- COVID-19 coronavirus pandemic: weekly trends. Worldometer. Accessed August 21, 2023. https://www.worldometers.info/coronavirus/
- National COVID-19 vaccination programme meets its goals by overcoming R&D and logistical challenges, says economic survey 2022-23. Government of India Press Information Bureau website. Published January 31, 2023. Accessed August 24, 2023. https://pib.gov.in/PressReleasePage.aspx?PRID=1894907
- Ministry of Health and Family Welfare. CoWIN. Accessed August 21, 2023. https://www.cowin.gov.in/
- Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
- Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: anemerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368-370.
- International Psoriasis Council. Revised IPC statement on COVID-19. Published December 19, 2022. Accessed August 24, 2023. https://psoriasiscouncil.org/covid-19/revised-statement-covid-19/
- Sotiriou E, Tsentemeidou A, Bakirtzi K, et al. Psoriasis exacerbation after COVID-19 vaccination: a report of 14 cases from a single centre. J Eur Acad Dermatol Venereol. 2021;35:E857-E859.
- Kaul R. India clears 2 vaccines for kids under 12 years. Hindustan Times. Published April 27, 2022. Accessed August 24, 2023. https://www.hindustantimes.com/india-news/india-clears-2-vaccines-for-kids-under-12-years-101650998027336.html
- Huang YW, Tsai TF. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med (Lausanne). 2021;8:812010.
- Damiani G, Allocco F, Young Dermatologists Italian Network, et al. COVID-19 vaccination and patients with psoriasis under biologics: real-life evidence on safety and effectiveness from Italian vaccinated healthcare workers. Clin Exp Dermatol. 2021;460:1106-1108.
- Joshi RK, Muralidharan CG, Gulati DS, et al. Higher incidence of reported adverse events following immunisation (AEFI) after first dose of COVID-19 vaccine among previously infected health care workers. Med J Armed Forces India. 2021;77(suppl 2):S505-S507.
- Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. NPJ Vaccines. 2019;4:39.
Practice Points
- Vaccines are known to induce a psoriasis flare.
- Given the high risk for severe COVID infection in individuals with psoriasis who have comorbidities, vaccination with Covaxin and Covishield can be safely recommended in India for this population.
Medicare announces 10 drugs targeted for price cuts in 2026
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
People on Medicare may in 2026 see prices drop for 10 medicines, including pricey diabetes, cancer, blood clot, and arthritis treatments, if advocates for federal drug-price negotiations can implement their plans amid tough opposition.
It’s unclear at this time, though, how these negotiations will play out. The Chamber of Commerce has sided with pharmaceutical companies in bids to block direct Medicare negotiation of drug prices. Many influential Republicans in Congress oppose this plan, which has deep support from both Democrats and AARP.
While facing strong opposition to negotiations, the Centers for Medicare & Medicaid Services sought in its announcement to illustrate the high costs of the selected medicines.
CMS provided data on total Part D costs for selected medicines for the period from June 2022 to May 2023, along with tallies of the number of people taking these drugs. The 10 selected medicines are as follows:
- Eliquis (generic name: apixaban), used to prevent and treat serious blood clots. It is taken by about 3.7 million people through Part D plans. The estimated cost is $16.4 billion.
- Jardiance (generic name: empagliflozin), used for diabetes and heart failure. It is taken by almost 1.6 million people through Part D plans. The estimated cost is $7.06 billion.
- Xarelto (generic name: rivaroxaban), used for blood clots. It is taken by about 1.3 million people through Part D plans. The estimated cost is $6 billion.
- Januvia (generic name: sitagliptin), used for diabetes. It is taken by about 869,00 people through Part D plans. The estimated cost is $4.1 billion.
- Farxiga (generic name: dapagliflozin), used for diabetes, heart failure, and chronic kidney disease. It is taken by about 799,000 people through Part D plans. The estimated cost is almost $3.3 billion.
- Entresto (generic name: sacubitril/valsartan), used to treat heart failure. It is taken by 587,000 people through Part D plans. The estimated cost is $2.9 billion.
- Enbrel( generic name: etanercept), used for rheumatoid arthritis, psoriasis, and psoriatic arthritis. It is taken by 48,000 people through Part D plans. The estimated cost is $2.8 billion.
- Imbruvica (generic name: ibrutinib), used to treat some blood cancers. It is taken by about 20,000 people in Part D plans. The estimated cost is $2.7 billion.
- Stelara (generic name: ustekinumab), used to treat plaque psoriasis, psoriatic arthritis, or certain bowel conditions (Crohn’s disease, ulcerative colitis). It is used by about 22,000 people through Part D plans. The estimated cost is $2.6 billion.
- Fiasp; Fiasp FlexTouch; Fiasp PenFill; NovoLog; NovoLog FlexPen; NovoLog PenFill. These are forms of insulin used to treat diabetes. They are used by about 777,000 people through Part D plans. The estimated cost is $2.6 billion.
A vocal critic of Medicare drug negotiations, Joel White, president of the Council for Affordable Health Coverage, called the announcement of the 10 drugs selected for negotiation “a hollow victory lap.” A former Republican staffer on the House Ways and Means Committee, Mr. White aided with the development of the Medicare Part D plans and has kept tabs on the pharmacy programs since its launch in 2006.
“No one’s costs will go down now or for years because of this announcement” about Part D negotiations, Mr. White said in a statement.
According to its website, CAHC includes among its members the American Academy of Ophthalmology as well as some patient groups, drugmakers, such as Johnson & Johnson, and insurers and industry groups, such as the National Association of Manufacturers.
Separately, the influential Chamber of Commerce is making a strong push to at least delay the implementation of the Medicare Part D drug negotiations. On Aug. 28, the chamber released a letter sent to the Biden administration, raising concerns about a “rush” to implement the provisions of the Inflation Reduction Act.
The chamber also has filed suit to challenge the drug negotiation provisions of the Inflation Reduction Act, requesting that the court issue a preliminary injunction by Oct. 1, 2023.
Other pending legal challenges to direct Medicare drug negotiations include suits filed by Merck, Bristol-Myers Squibb, Johnson & Johnson, Boehringer Ingelheim, and AstraZeneca, according to an email from Pharmaceutical Research and Manufacturers of America. PhRMA also said it is a party to a case.
In addition, the three congressional Republicans with most direct influence over Medicare policy issued on Aug. 29 a joint statement outlining their objections to the planned negotiations on drug prices.
This drug-negotiation proposal is “an unworkable, legally dubious scheme that will lead to higher prices for new drugs coming to market, stifle the development of new cures, and destroy jobs,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-Wash.), House Ways and Means Committee Chair Jason Smith (R-Mo.), and Senate Finance Committee Ranking Member Mike Crapo (R-Idaho).
Democrats were equally firm and vocal in their support of the negotiations. Senate Finance Chairman Ron Wyden (D-Ore.) issued a statement on Aug. 29 that said the release of the list of the 10 drugs selected for Medicare drug negotiations is part of a “seismic shift in the relationship between Big Pharma, the federal government, and seniors who are counting on lower prices.
“I will be following the negotiation process closely and will fight any attempt by Big Pharma to undo or undermine the progress that’s been made,” Mr. Wyden said.
In addition, AARP issued a statement of its continued support for Medicare drug negotiations.
“The No. 1 reason seniors skip or ration their prescriptions is because they can’t afford them. This must stop,” said AARP executive vice president and chief advocacy and engagement officer Nancy LeaMond in the statement. “The big drug companies and their allies continue suing to overturn the Medicare drug price negotiation program to keep up their price gouging. We can’t allow seniors to be Big Pharma’s cash machine anymore.”
A version of this article first appeared on Medscape.com.
Rheumatology trials seem vulnerable to unblinding: Report
Until more is known about the potential for unblinding, clinicians need to keep in mind that patients and physicians could often guess accurately who was getting placebo or active drug, first author Cody Bruggemeyer, MD, a resident at the Medical College of Wisconsin, Milwaukee, said in an interview.
“It’s important that rheumatologists be aware of this potential issue and use their clinical reasoning and their ability to critically assess papers to evaluate the study design” of research on treatments, he said in an interview.
Dr. Bruggemeyer and coauthors at the Medical College of Wisconsin presented their assessment of the potential for unblinding in a Viewpoint article in The Lancet Rheumatology.
A sample of pivotal clinical trials
The authors selected a sample of pivotal studies of 14 commonly prescribed drugs for rheumatic conditions for which double-blind randomized controlled trials (RCTs) that compared the active ingredient with a placebo were available.
The 14 trials involved treatments classified as disease-modifying antirheumatic drugs (DMARDs), some of which were likely to produce side effects that placebos would not mimic, such as injection site and infusion reactions and difference in readings in lab reports, the authors wrote.
In their analysis, Dr. Bruggemeyer and colleagues evaluated discrepancies in the rates of adverse events reported between active drugs and placebos and classified the 14 studies as follows:
- High unblinding risk: Nine studies had a high estimated risk of unblinding, including trials of adalimumab with citrate (Humira), anakinra (Kineret), anifrolumab (Saphnelo), apremilast (Otezla), ixekizumab (Taltz), leflunomide (Arava), methotrexate, risankizumab (Skyrizi) and tofacitinib (Xeljanz).
- Moderate unblinding risk: Three studies had a moderate estimated risk of unblinding, including trials of azathioprine (Imuran), mycophenolate mofetil and tocilizumab (Actemra).
- Low unblinding risk: Two studies had a low estimated risk of unblinding. These involved tests of belimumab (Benlysta) and rituximab (Rituxan).
Many of the effectiveness measurements of treatments used in rheumatology depend on patients’ reports of relief of pain and other disease symptoms. For example, the widely used American College of Rheumatology 20% response for rheumatoid arthritis includes components that rely on patient and physician assessment of disease activity.
Unblinding risk to clinical trial validity
CTs are the highest level of evidence to establish efficacy, because the study design aims to mask whether the experimental treatment is a drug or placebo. In cases where patients and physicians are more likely to correctly detect use of an active drug, there can be biases that skew results toward reports of symptom improvement. Other patients’ views of their treatment may be distorted by accurate guesses that they have been given placebo, Dr. Bruggemeyer and coauthors wrote.
“The degree of these effects cannot be predicted, but they tend to erroneously inflate the perceived benefit of novel interventions,” they wrote.
The consequences of this unblinding may be minimal in cases where there’s a clear difference between the placebo and active drug, they said. As an example, they cited trials of interleukin-23 inhibitors for psoriasis, where skin clearance as measured by the Psoriatic Area and Severity Index 75 differed by more than 50% in absolute terms between the treatment and placebo groups.
But in other cases, there needs to be more attention paid to the potential role of unblinding, they wrote.
“Studies where effect sizes were small, contradictory, or dependent on subgroup analyses might be especially problematic, but commentary rarely reflects this issue or acknowledges the potential influence of unblinding,” they wrote.
In the paper, they call for more analysis of previous trials to look for unreported assessments of unblinding, while also asking that researchers consider surveying participants in future trials to evaluate the degree to which unblinding occurs.
“Advocacy from professional societies and the U.S. Food and Drug Administration itself might be necessary, but in the interim, rheumatologists should assume unblinding has occurred to some degree in most trials,” they wrote.
Unblinding measure needs validation
In an interview, Roy M. Fleischmann, MD, co–medical director of the Metroplex Clinical Research Center in Dallas, raised some objections to the paper. The paper addresses an interesting question about unblinding, but there should have been more work done, such as finding “a measure that is validated that can say whether you’ve been unblinded or not.”
He added that he was surprised the paper on unblinding in rheumatology trials was published in its current form.
“I would have sent it for a major rewrite” if asked to review this paper before publication, said Dr. Fleischmann, who as a reviewer for Lancet Rheumatology. “I would have said: ‘Okay, 90% of this paper is okay, but your gist is not correct.’ It should be: ‘Is this a problem?’ ”
Dr. Fleischmann said he would have recommended a different perspective to the paper. “That is, this could occur. Should we be looking at this, and how would we look at this?”
In the paper, the authors acknowledge their approach has not been validated, “but it highlights the potential effect of idiosyncratic adverse events,” they wrote.
There’s less funding in general for meta-research than for studies involving treatments, so researchers look for approaches that can be handled without requiring significant funding, and much of the research on the quality of research is conducted like this analysis of rheumatology trials, Michael Putman, MD, the corresponding author and is a rheumatologist and an assistant professor at the Medical College of Wisconsin, said in an interview.
“You’re mostly doing on a shoestring budget with yourself and trainees,” he said. Dr. Putman is an associate editor at the journal Rheumatology and also involved in meta-research, or efforts to understand how studies and trials answer questions about how medical treatments work.
In an Aug. 16 tweet, Dr. Putman said this issue of unintentional unblinding with rheumatology trials was something he’d “been ruminating about for awhile; took two all star trainees to push it over the top!”
One of the barriers to funding of meta-research is a tendency for major funding for medical studies to be focused on specific diseases or targets. With meta-research, it may be more difficult to explain how a specific project will advance efforts to treat or prevent a certain disease, Dr. Putman said.
“It’s a little more esoteric and maybe not quite as clear how these projects will move things forward,” Dr. Putman said.
In addition, the nature of meta-research is to question and often be critical of work that’s already been published, adding another hurdle in attempts to secure funding, he said.
Dr. Putman is supported by a Rheumatology Research Foundation Scientist Development Grant, receives research funding related to clinical trials by AbbVie and AstraZeneca, and consulting fees from Novartis. The other authors declared no competing interests.
Until more is known about the potential for unblinding, clinicians need to keep in mind that patients and physicians could often guess accurately who was getting placebo or active drug, first author Cody Bruggemeyer, MD, a resident at the Medical College of Wisconsin, Milwaukee, said in an interview.
“It’s important that rheumatologists be aware of this potential issue and use their clinical reasoning and their ability to critically assess papers to evaluate the study design” of research on treatments, he said in an interview.
Dr. Bruggemeyer and coauthors at the Medical College of Wisconsin presented their assessment of the potential for unblinding in a Viewpoint article in The Lancet Rheumatology.
A sample of pivotal clinical trials
The authors selected a sample of pivotal studies of 14 commonly prescribed drugs for rheumatic conditions for which double-blind randomized controlled trials (RCTs) that compared the active ingredient with a placebo were available.
The 14 trials involved treatments classified as disease-modifying antirheumatic drugs (DMARDs), some of which were likely to produce side effects that placebos would not mimic, such as injection site and infusion reactions and difference in readings in lab reports, the authors wrote.
In their analysis, Dr. Bruggemeyer and colleagues evaluated discrepancies in the rates of adverse events reported between active drugs and placebos and classified the 14 studies as follows:
- High unblinding risk: Nine studies had a high estimated risk of unblinding, including trials of adalimumab with citrate (Humira), anakinra (Kineret), anifrolumab (Saphnelo), apremilast (Otezla), ixekizumab (Taltz), leflunomide (Arava), methotrexate, risankizumab (Skyrizi) and tofacitinib (Xeljanz).
- Moderate unblinding risk: Three studies had a moderate estimated risk of unblinding, including trials of azathioprine (Imuran), mycophenolate mofetil and tocilizumab (Actemra).
- Low unblinding risk: Two studies had a low estimated risk of unblinding. These involved tests of belimumab (Benlysta) and rituximab (Rituxan).
Many of the effectiveness measurements of treatments used in rheumatology depend on patients’ reports of relief of pain and other disease symptoms. For example, the widely used American College of Rheumatology 20% response for rheumatoid arthritis includes components that rely on patient and physician assessment of disease activity.
Unblinding risk to clinical trial validity
CTs are the highest level of evidence to establish efficacy, because the study design aims to mask whether the experimental treatment is a drug or placebo. In cases where patients and physicians are more likely to correctly detect use of an active drug, there can be biases that skew results toward reports of symptom improvement. Other patients’ views of their treatment may be distorted by accurate guesses that they have been given placebo, Dr. Bruggemeyer and coauthors wrote.
“The degree of these effects cannot be predicted, but they tend to erroneously inflate the perceived benefit of novel interventions,” they wrote.
The consequences of this unblinding may be minimal in cases where there’s a clear difference between the placebo and active drug, they said. As an example, they cited trials of interleukin-23 inhibitors for psoriasis, where skin clearance as measured by the Psoriatic Area and Severity Index 75 differed by more than 50% in absolute terms between the treatment and placebo groups.
But in other cases, there needs to be more attention paid to the potential role of unblinding, they wrote.
“Studies where effect sizes were small, contradictory, or dependent on subgroup analyses might be especially problematic, but commentary rarely reflects this issue or acknowledges the potential influence of unblinding,” they wrote.
In the paper, they call for more analysis of previous trials to look for unreported assessments of unblinding, while also asking that researchers consider surveying participants in future trials to evaluate the degree to which unblinding occurs.
“Advocacy from professional societies and the U.S. Food and Drug Administration itself might be necessary, but in the interim, rheumatologists should assume unblinding has occurred to some degree in most trials,” they wrote.
Unblinding measure needs validation
In an interview, Roy M. Fleischmann, MD, co–medical director of the Metroplex Clinical Research Center in Dallas, raised some objections to the paper. The paper addresses an interesting question about unblinding, but there should have been more work done, such as finding “a measure that is validated that can say whether you’ve been unblinded or not.”
He added that he was surprised the paper on unblinding in rheumatology trials was published in its current form.
“I would have sent it for a major rewrite” if asked to review this paper before publication, said Dr. Fleischmann, who as a reviewer for Lancet Rheumatology. “I would have said: ‘Okay, 90% of this paper is okay, but your gist is not correct.’ It should be: ‘Is this a problem?’ ”
Dr. Fleischmann said he would have recommended a different perspective to the paper. “That is, this could occur. Should we be looking at this, and how would we look at this?”
In the paper, the authors acknowledge their approach has not been validated, “but it highlights the potential effect of idiosyncratic adverse events,” they wrote.
There’s less funding in general for meta-research than for studies involving treatments, so researchers look for approaches that can be handled without requiring significant funding, and much of the research on the quality of research is conducted like this analysis of rheumatology trials, Michael Putman, MD, the corresponding author and is a rheumatologist and an assistant professor at the Medical College of Wisconsin, said in an interview.
“You’re mostly doing on a shoestring budget with yourself and trainees,” he said. Dr. Putman is an associate editor at the journal Rheumatology and also involved in meta-research, or efforts to understand how studies and trials answer questions about how medical treatments work.
In an Aug. 16 tweet, Dr. Putman said this issue of unintentional unblinding with rheumatology trials was something he’d “been ruminating about for awhile; took two all star trainees to push it over the top!”
One of the barriers to funding of meta-research is a tendency for major funding for medical studies to be focused on specific diseases or targets. With meta-research, it may be more difficult to explain how a specific project will advance efforts to treat or prevent a certain disease, Dr. Putman said.
“It’s a little more esoteric and maybe not quite as clear how these projects will move things forward,” Dr. Putman said.
In addition, the nature of meta-research is to question and often be critical of work that’s already been published, adding another hurdle in attempts to secure funding, he said.
Dr. Putman is supported by a Rheumatology Research Foundation Scientist Development Grant, receives research funding related to clinical trials by AbbVie and AstraZeneca, and consulting fees from Novartis. The other authors declared no competing interests.
Until more is known about the potential for unblinding, clinicians need to keep in mind that patients and physicians could often guess accurately who was getting placebo or active drug, first author Cody Bruggemeyer, MD, a resident at the Medical College of Wisconsin, Milwaukee, said in an interview.
“It’s important that rheumatologists be aware of this potential issue and use their clinical reasoning and their ability to critically assess papers to evaluate the study design” of research on treatments, he said in an interview.
Dr. Bruggemeyer and coauthors at the Medical College of Wisconsin presented their assessment of the potential for unblinding in a Viewpoint article in The Lancet Rheumatology.
A sample of pivotal clinical trials
The authors selected a sample of pivotal studies of 14 commonly prescribed drugs for rheumatic conditions for which double-blind randomized controlled trials (RCTs) that compared the active ingredient with a placebo were available.
The 14 trials involved treatments classified as disease-modifying antirheumatic drugs (DMARDs), some of which were likely to produce side effects that placebos would not mimic, such as injection site and infusion reactions and difference in readings in lab reports, the authors wrote.
In their analysis, Dr. Bruggemeyer and colleagues evaluated discrepancies in the rates of adverse events reported between active drugs and placebos and classified the 14 studies as follows:
- High unblinding risk: Nine studies had a high estimated risk of unblinding, including trials of adalimumab with citrate (Humira), anakinra (Kineret), anifrolumab (Saphnelo), apremilast (Otezla), ixekizumab (Taltz), leflunomide (Arava), methotrexate, risankizumab (Skyrizi) and tofacitinib (Xeljanz).
- Moderate unblinding risk: Three studies had a moderate estimated risk of unblinding, including trials of azathioprine (Imuran), mycophenolate mofetil and tocilizumab (Actemra).
- Low unblinding risk: Two studies had a low estimated risk of unblinding. These involved tests of belimumab (Benlysta) and rituximab (Rituxan).
Many of the effectiveness measurements of treatments used in rheumatology depend on patients’ reports of relief of pain and other disease symptoms. For example, the widely used American College of Rheumatology 20% response for rheumatoid arthritis includes components that rely on patient and physician assessment of disease activity.
Unblinding risk to clinical trial validity
CTs are the highest level of evidence to establish efficacy, because the study design aims to mask whether the experimental treatment is a drug or placebo. In cases where patients and physicians are more likely to correctly detect use of an active drug, there can be biases that skew results toward reports of symptom improvement. Other patients’ views of their treatment may be distorted by accurate guesses that they have been given placebo, Dr. Bruggemeyer and coauthors wrote.
“The degree of these effects cannot be predicted, but they tend to erroneously inflate the perceived benefit of novel interventions,” they wrote.
The consequences of this unblinding may be minimal in cases where there’s a clear difference between the placebo and active drug, they said. As an example, they cited trials of interleukin-23 inhibitors for psoriasis, where skin clearance as measured by the Psoriatic Area and Severity Index 75 differed by more than 50% in absolute terms between the treatment and placebo groups.
But in other cases, there needs to be more attention paid to the potential role of unblinding, they wrote.
“Studies where effect sizes were small, contradictory, or dependent on subgroup analyses might be especially problematic, but commentary rarely reflects this issue or acknowledges the potential influence of unblinding,” they wrote.
In the paper, they call for more analysis of previous trials to look for unreported assessments of unblinding, while also asking that researchers consider surveying participants in future trials to evaluate the degree to which unblinding occurs.
“Advocacy from professional societies and the U.S. Food and Drug Administration itself might be necessary, but in the interim, rheumatologists should assume unblinding has occurred to some degree in most trials,” they wrote.
Unblinding measure needs validation
In an interview, Roy M. Fleischmann, MD, co–medical director of the Metroplex Clinical Research Center in Dallas, raised some objections to the paper. The paper addresses an interesting question about unblinding, but there should have been more work done, such as finding “a measure that is validated that can say whether you’ve been unblinded or not.”
He added that he was surprised the paper on unblinding in rheumatology trials was published in its current form.
“I would have sent it for a major rewrite” if asked to review this paper before publication, said Dr. Fleischmann, who as a reviewer for Lancet Rheumatology. “I would have said: ‘Okay, 90% of this paper is okay, but your gist is not correct.’ It should be: ‘Is this a problem?’ ”
Dr. Fleischmann said he would have recommended a different perspective to the paper. “That is, this could occur. Should we be looking at this, and how would we look at this?”
In the paper, the authors acknowledge their approach has not been validated, “but it highlights the potential effect of idiosyncratic adverse events,” they wrote.
There’s less funding in general for meta-research than for studies involving treatments, so researchers look for approaches that can be handled without requiring significant funding, and much of the research on the quality of research is conducted like this analysis of rheumatology trials, Michael Putman, MD, the corresponding author and is a rheumatologist and an assistant professor at the Medical College of Wisconsin, said in an interview.
“You’re mostly doing on a shoestring budget with yourself and trainees,” he said. Dr. Putman is an associate editor at the journal Rheumatology and also involved in meta-research, or efforts to understand how studies and trials answer questions about how medical treatments work.
In an Aug. 16 tweet, Dr. Putman said this issue of unintentional unblinding with rheumatology trials was something he’d “been ruminating about for awhile; took two all star trainees to push it over the top!”
One of the barriers to funding of meta-research is a tendency for major funding for medical studies to be focused on specific diseases or targets. With meta-research, it may be more difficult to explain how a specific project will advance efforts to treat or prevent a certain disease, Dr. Putman said.
“It’s a little more esoteric and maybe not quite as clear how these projects will move things forward,” Dr. Putman said.
In addition, the nature of meta-research is to question and often be critical of work that’s already been published, adding another hurdle in attempts to secure funding, he said.
Dr. Putman is supported by a Rheumatology Research Foundation Scientist Development Grant, receives research funding related to clinical trials by AbbVie and AstraZeneca, and consulting fees from Novartis. The other authors declared no competing interests.
FROM THE LANCET RHEUMATOLOGY
Financial Insecurity Among US Adults With Psoriasis
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.

Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).
Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
To the Editor:
Approximately 3% of the US population, or 6.9 million adults, is affected by psoriasis.1 Psoriasis has a substantial impact on quality of life and is associated with increased health care expenses and medication costs. In 2013, it was reported that the estimated US annual cost—direct, indirect, intangible, and comorbidity costs—of psoriasis for adults was $112 billion.2 We investigated the prevalence and sociodemographic characteristics of adult psoriasis patients (aged ≥20 years) with financial insecurity utilizing the 2009–2014 National Health and Nutrition Examination Survey (NHANES) data.3
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database to evaluate financial insecurity. Financial insecurity was evaluated by 2 outcome variables. The primary outcome variable was assessed by the question “Are you covered by health insurance or some other kind of health care plan (including health insurance obtained through employment or purchased directly as well as government programs like Medicare and Medicaid that provide medical care or help pay medical bills)?”3 Our secondary outcome variable was evaluated by a reported annual household income of less than $20,000. P values in Table 1 were calculated using Pearson χ2 tests. In Table 2, multivariate logistic regressions were performed using Stata/MP 17 (StataCorp LLC) to analyze associations between outcome variables and sociodemographic characteristics. Additionally, we controlled for age, race/ethnicity, sex, education, marital status, US citizenship status, and tobacco use. Subsequently, relationships with P<.05 were considered statistically significant.


Our analysis comprised 480 individuals with psoriasis; 40 individuals were excluded from our analysis because they did not report annual household income and health insurance status (Table 1). Among the 480 individuals with psoriasis, approximately 16% (weighted) reported a lack of health insurance, and approximately 17% (weighted) reported an annual household income of less than $20,000. Among those who reported an annual household income of less than $20,000, approximately 38% (weighted) of them reported that they did not have health insurance.
Multivariate logistic regression analyses revealed that elderly individuals (aged >60 years), college graduates, married individuals, and US citizens had decreased odds of lacking health insurance (Table 2). Additionally, those with a history of tobacco use (adjusted odds ratio [AOR] 2.02; 95% CI, 1.00-4.05) were associated with lacking health insurance. Non-Hispanic Black individuals (AOR 2.26; 95% CI, 1.09-4.71) and US citizens (AOR 5.01; 95% CI, 1.28-19.63) had a significant association with an annual household income of less than $20,000 (P<.05). Lastly, males, those with education beyond ninth grade, and married individuals had a significantly decreased odds of having an annual household income of less than $20,000 (P<.05)(Table 2).


Our findings indicate that certain sociodemographic groups of psoriasis patients have an increased risk for being financially insecure. It is important to evaluate the cost of treatment, number of necessary visits to the office, and cost of transportation, as these factors can serve as a major economic burden to patients being managed for psoriasis.4 Additionally, the cost of biologics has been increasing over time.5 Taking all of this into account when caring for psoriasis patients is crucial, as understanding the financial status of patients can assist with determining appropriate individualized treatment regimens.
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
- Liu J, Thatiparthi A, Martin A, et al. Prevalence of psoriasis among adults in the US 2009-2010 and 2013-2014 National Health and Nutrition Examination Surveys. J Am Acad Dermatol. 2021;84:767-769. doi:10.1016/j.jaad.2020.10.035
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658. doi:10.1001/jamadermatol.2014.3593
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Maya-Rico AM, Londoño-García Á, Palacios-Barahona AU, et al. Out-of-pocket costs for patients with psoriasis in an outpatient dermatology referral service. An Bras Dermatol. 2021;96:295-300. doi:10.1016/j.abd.2020.09.004
- Cheng J, Feldman SR. The cost of biologics for psoriasis is increasing. Drugs Context. 2014;3:212266. doi:10.7573/dic.212266
Practice Points
- The economic burden on patients with psoriasis has been rising over time, as the disease impacts many aspects of patients’ lives.
- Various sociodemographic groups among patients with psoriasis are financially insecure. Knowing which groups are at higher risk for poor outcomes due to financial insecurity can assist with appropriate treatment regimens.
New evidence suggests genetic risk factors in hidradenitis suppurativa
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition with lesions that include deep-seated nodules and abscesses, draining tracts, and fibrotic scars, and has been reported to be highly heritable.
A genomewide association study (GWAS) that involved a meta-analysis of data from three large biobanks (UK Biobank, FinnGen, and BioVU) identified one significant locus and two suggestive loci. The researchers were able to replicate the association with HS for two loci near the SOX9 and KLF5 genes in BioVU.
In addition, they also looked at genetic correlations between HS and autoimmune and inflammatory diseases, and results suggested a positive association with inflammatory bowel disease, psoriasis, and type 2 diabetes.
However, while plausible, the variant associations near candidate genes do not prove a causal effect of these variants or genes on disease risk, and further study is needed.
“It’s possible that these genes aren’t the ones affected by the variations we describe, but both are very strong candidates,” said study author Christopher J. Sayed, MD, associate professor of dermatology, University of North Carolina at Chapel Hill. “This improves our understanding of HS as a disease that is likely related to genetic changes that result in dysregulated hair follicle maintenance, inflammation, and wound healing.”
In turn, this will allow clinicians to educate patients about the underlying genetic influences on HS, he explained, as opposed to stigmatizing misconceptions focusing only on weight, smoking, and hygiene.
“Larger studies are underway and will be needed to find variants that may predict things like disease severity and response to treatment, but this is a big first step in the right direction,” Dr. Sayed added.
The study was published online in JAMA Dermatology.
Loci identified
GWASs have been limited in HS, and previous research has not identified significant risk loci. In the current study, Dr. Sayed and colleagues sought to identify underlying genes and genetic mechanisms that may underlie pathogenesis in HS.
They performed a GWAS in a cohort of 720 patients who were part of the Hidradenitis Suppurativa Program for Research and Care Excellence (HS ProCARE) at the UNC department of dermatology, and controls from the National Longitudinal Study of Adolescent to Adult Health (Add Health) study, a U.S.-based study following adolescents through adulthood.
The UK Biobank (UKB) is a prospective biobank with about 500,000 individuals aged 40-69 years, and FinnGen collects and analyzes genome and health data from Finnish biobank participants. To increase power to detect associations, a GWAS was performed using participants from the UKB (247 HS cases, 453,048 controls). The HS ProCARE GWAS results were meta-analyzed with UKB, along with data from FinnGen (673 HS cases, 297,544 controls). This three-way meta-analysis revealed one genomewide significant locus and two suggestive loci.
The authors found that the most strongly associated variant, rs10512572 located on chromosome 17, showed the strongest association in FinnGen; at the second locus, the most strongly associated variant was rs17090189 located on chromosome 13 and also showed the strongest association in FinnGen; and at the third locus, the most strongly associated variant, rs5792315, located on chromosome 11, showed the strongest association in HS ProCARE.
Next, they tested the 10 most strongly associated variants at the three loci in the BioVU biobank, which has 290 HS cases, including 189 individuals of European ancestry and 101 individuals of African ancestry, with 64,234 and 12,105 controls, respectively. The locus on chromosome 17 was replicated in BioVU in the same direction of effect, while one variant at the chromosome 13 locus showed nominal evidence of association in the same direction.
In a four-way meta-analysis of BioVU combined with the other three studies, the chromosome locus became more significant and the chromosome 13 locus exceeded the genomewide significance threshold. In contrast, the chromosome 11 locus was not replicated in BioVU (P = .27).
The authors pointed out that variants at these loci are located in keratinocyte regulatory elements near the genes SOX9 and KLF5, which play a role in skin and follicular inflammation, but have not previously been associated with HS pathogenesis.
Finally, they looked to see if there were shared genetic components between HS and autoimmune and inflammatory diseases. A nominally significant genetic correlation was observed between HS and inflammatory bowel disease (P = .04), psoriasis (P = .03), and type 2 diabetes (P = .04), although none reached significance.
Different manifestation with genetic variant
In a related study, researchers assessed the prevalence of the NCSTN:c.671_682del variant among individuals with HS in Malta, the island country in the Mediterranean, which has a high prevalence of HS and its associated risk factors, particularly obesity.
Led by Dillon Mintoff, MD, of the department of pathology at the University of Malta, Msida, they conducted a cross-sectional study of 113 adults with HS. In this group, 14 (12.39%) were found to be heterozygous for the NCSTN:c.671_682del variant. Individuals who had this variant were more likely to develop symptoms earlier and to manifest them in atypical skin sites, including the scalp, neck, torso, and antecubital fossae. Additionally, even though their symptoms weren’t more severe than those without the variant, patients with the variant were more likely to require treatment with biologic agents.
Studies move genetics in HS forward
Writing in an accompanying editorial, Atlas Khan, PhD, and Lynn Petukhova, PhD, from Columbia University, New York, noted that both of these HS genetic studies “set a solid foundation for future studies aimed at understanding the biological and clinical relevance of new HS genetic evidence.”
“Each study suggests a series of experiments that will allow us to gain new knowledge about HS,” they wrote, including coming closer to providing patients with a genetic diagnosis.
In addition, evidence from the GWAS paper suggests that with “larger HS GWASs we will be able to better prioritize drug-repurposing studies,” concluded the editorialists. “Both of these goals will help to improve health outcomes for patients with HS and their family members.”
Dr. Sayed reported grants and/or personal fees from AbbVie, Novartis, UCB, Incyte, InflaRx, Alumis, and ChemoCentryx outside the submitted work; and serving as a volunteer member of the board of the HS Foundation and member of the European HS Foundation. The study in Malta was funded by the Government of Malta’s Tertiary Education Scholarship Scheme and Institutional Funds from the University of Malta. Dr. Mintoff reported grants from the Government of Malta Ministry for Education, Sport, Youth, Research and Innovation during the conduct of the study. Dr. Khan reported receiving grants from the National Institute of Diabetes and Digestive Kidney Diseases. Dr. Petukhova reported receiving grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Columbia University’s Precision Medicine Initiative, Herbert Irving Comprehensive Cancer Center, and Data Science Institute.
FROM JAMA DERMATOLOGY
PsA biomarkers move researchers closer to predictive test
In a new study, researchers report that they have found epigenetic methylation markers on 15 genes that appear to foreshadow psoriatic arthritis (PsA), a development that could bring scientists closer to developing a DNA test to predict which patients with psoriasis will develop the condition.
While no predictive test is in sight yet, the findings published in Arthritis & Rheumatology mark an important step, study lead author Omar F. Cruz-Correa, PhD, of the Psoriatic Arthritis Research Program in the University Health Network, Toronto, said in an interview. “In the future, markers like these could be measured by dermatologists and even general practitioners to help identify new psoriasis patients at a high risk of developing PsA,” he said. “Then both the health care team and the patients themselves could be more aware of their increased risk and the pressing need of closer monitoring for musculoskeletal symptoms. Once the first symptoms appear, treatment can be initiated early on, helping to prevent permanent joint damage.”
An estimated 30% of patients with psoriasis will develop PsA, too, putting them at higher risk of disability and death. According to Dr. Cruz-Correa, “one of the more pressing matters in PsA is the lack of means of predicting which psoriasis patients will develop PsA.”
DNA methylation, the topic of the new study, has already been linked to psoriasis and PsA. It’s “relatively easy to measure and helps regulate gene expression in response to environmental effects,” Dr. Cruz-Correa said. “DNA methylation is also appealing because it serves as an intermediary between environment and genetic factors as it’s transmitted between generations of cells and influenced by external factors.”
For the new study, researchers examined the DNA of 117 patients with psoriasis – 58 who went on to develop PsA (“converters”) and another 59 who were matched to converters but did not develop PsA (“nonconverters”). The patients were in a larger group of 700 patients with psoriasis who had the disease for a mean of about 17 years at the time of blood sampling.
Samples from converters were taken an average of 5.16 years (± 12.77 years) before PsA set in.
The researchers report that they found “36 highly relevant methylation markers … across 15 genes and several intergenic regions. A classification model relying on these markers identified converters and nonconverters with an area under the ROC curve of 0.9644.”
Statistically, this number is high and means that “the DNA methylation markers are really good at identifying psoriasis patients who will develop PsA and those that will not,” at least in this specific patient group, Dr. Cruz-Correa said.
At this point, the number of markers is a bit too high to develop a feasible DNA test to predict PsA, he said. “However, the results from our study have also pointed us toward some interesting metabolic pathways that may warrant further study.”
What’s next?
The first step forward “is the validation of these predictive DNA methylation markers in a wider population of patients with varied clinical and demographic characteristics. This would help assess the potential for generalization of such a test,” Dr. Cruz-Correa said. “A second step is to assess the potential impact of these methylation markers on disease activity and treatment response, which are clinical outcomes of great importance to patients.”
Meanwhile, he said, “there are ongoing efforts to shed light into how DNA methylation integrates with other epigenetic mechanisms like micro-RNAs to regulate gene expression in concert with one another. An integrative look into these mechanisms may be able to give insight into the pathogenesis of psoriatic disease in a way that has not been possible before.”
In an interview, Johann E. Gudjonsson, MD, PhD, professor of skin molecular immunology at the University of Michigan, Ann Arbor, said the study “is interesting and important as it indicates that there are changes in the blood that occur before the development of psoriatic arthritis. However, it does not provide much in terms of novel insights into the mechanisms involved and is still a long way away from being useful as a clinical predictor or biomarker.”
The National Psoriasis Foundation, Krembil Foundation, and Canadian Institutes of Health Research provided support for the study. Dr. Cruz-Correa reports support from the National Psoriasis Foundation and the Arthritis Society. Dr. Gudjonsson has no relevant financial relationships.
In a new study, researchers report that they have found epigenetic methylation markers on 15 genes that appear to foreshadow psoriatic arthritis (PsA), a development that could bring scientists closer to developing a DNA test to predict which patients with psoriasis will develop the condition.
While no predictive test is in sight yet, the findings published in Arthritis & Rheumatology mark an important step, study lead author Omar F. Cruz-Correa, PhD, of the Psoriatic Arthritis Research Program in the University Health Network, Toronto, said in an interview. “In the future, markers like these could be measured by dermatologists and even general practitioners to help identify new psoriasis patients at a high risk of developing PsA,” he said. “Then both the health care team and the patients themselves could be more aware of their increased risk and the pressing need of closer monitoring for musculoskeletal symptoms. Once the first symptoms appear, treatment can be initiated early on, helping to prevent permanent joint damage.”
An estimated 30% of patients with psoriasis will develop PsA, too, putting them at higher risk of disability and death. According to Dr. Cruz-Correa, “one of the more pressing matters in PsA is the lack of means of predicting which psoriasis patients will develop PsA.”
DNA methylation, the topic of the new study, has already been linked to psoriasis and PsA. It’s “relatively easy to measure and helps regulate gene expression in response to environmental effects,” Dr. Cruz-Correa said. “DNA methylation is also appealing because it serves as an intermediary between environment and genetic factors as it’s transmitted between generations of cells and influenced by external factors.”
For the new study, researchers examined the DNA of 117 patients with psoriasis – 58 who went on to develop PsA (“converters”) and another 59 who were matched to converters but did not develop PsA (“nonconverters”). The patients were in a larger group of 700 patients with psoriasis who had the disease for a mean of about 17 years at the time of blood sampling.
Samples from converters were taken an average of 5.16 years (± 12.77 years) before PsA set in.
The researchers report that they found “36 highly relevant methylation markers … across 15 genes and several intergenic regions. A classification model relying on these markers identified converters and nonconverters with an area under the ROC curve of 0.9644.”
Statistically, this number is high and means that “the DNA methylation markers are really good at identifying psoriasis patients who will develop PsA and those that will not,” at least in this specific patient group, Dr. Cruz-Correa said.
At this point, the number of markers is a bit too high to develop a feasible DNA test to predict PsA, he said. “However, the results from our study have also pointed us toward some interesting metabolic pathways that may warrant further study.”
What’s next?
The first step forward “is the validation of these predictive DNA methylation markers in a wider population of patients with varied clinical and demographic characteristics. This would help assess the potential for generalization of such a test,” Dr. Cruz-Correa said. “A second step is to assess the potential impact of these methylation markers on disease activity and treatment response, which are clinical outcomes of great importance to patients.”
Meanwhile, he said, “there are ongoing efforts to shed light into how DNA methylation integrates with other epigenetic mechanisms like micro-RNAs to regulate gene expression in concert with one another. An integrative look into these mechanisms may be able to give insight into the pathogenesis of psoriatic disease in a way that has not been possible before.”
In an interview, Johann E. Gudjonsson, MD, PhD, professor of skin molecular immunology at the University of Michigan, Ann Arbor, said the study “is interesting and important as it indicates that there are changes in the blood that occur before the development of psoriatic arthritis. However, it does not provide much in terms of novel insights into the mechanisms involved and is still a long way away from being useful as a clinical predictor or biomarker.”
The National Psoriasis Foundation, Krembil Foundation, and Canadian Institutes of Health Research provided support for the study. Dr. Cruz-Correa reports support from the National Psoriasis Foundation and the Arthritis Society. Dr. Gudjonsson has no relevant financial relationships.
In a new study, researchers report that they have found epigenetic methylation markers on 15 genes that appear to foreshadow psoriatic arthritis (PsA), a development that could bring scientists closer to developing a DNA test to predict which patients with psoriasis will develop the condition.
While no predictive test is in sight yet, the findings published in Arthritis & Rheumatology mark an important step, study lead author Omar F. Cruz-Correa, PhD, of the Psoriatic Arthritis Research Program in the University Health Network, Toronto, said in an interview. “In the future, markers like these could be measured by dermatologists and even general practitioners to help identify new psoriasis patients at a high risk of developing PsA,” he said. “Then both the health care team and the patients themselves could be more aware of their increased risk and the pressing need of closer monitoring for musculoskeletal symptoms. Once the first symptoms appear, treatment can be initiated early on, helping to prevent permanent joint damage.”
An estimated 30% of patients with psoriasis will develop PsA, too, putting them at higher risk of disability and death. According to Dr. Cruz-Correa, “one of the more pressing matters in PsA is the lack of means of predicting which psoriasis patients will develop PsA.”
DNA methylation, the topic of the new study, has already been linked to psoriasis and PsA. It’s “relatively easy to measure and helps regulate gene expression in response to environmental effects,” Dr. Cruz-Correa said. “DNA methylation is also appealing because it serves as an intermediary between environment and genetic factors as it’s transmitted between generations of cells and influenced by external factors.”
For the new study, researchers examined the DNA of 117 patients with psoriasis – 58 who went on to develop PsA (“converters”) and another 59 who were matched to converters but did not develop PsA (“nonconverters”). The patients were in a larger group of 700 patients with psoriasis who had the disease for a mean of about 17 years at the time of blood sampling.
Samples from converters were taken an average of 5.16 years (± 12.77 years) before PsA set in.
The researchers report that they found “36 highly relevant methylation markers … across 15 genes and several intergenic regions. A classification model relying on these markers identified converters and nonconverters with an area under the ROC curve of 0.9644.”
Statistically, this number is high and means that “the DNA methylation markers are really good at identifying psoriasis patients who will develop PsA and those that will not,” at least in this specific patient group, Dr. Cruz-Correa said.
At this point, the number of markers is a bit too high to develop a feasible DNA test to predict PsA, he said. “However, the results from our study have also pointed us toward some interesting metabolic pathways that may warrant further study.”
What’s next?
The first step forward “is the validation of these predictive DNA methylation markers in a wider population of patients with varied clinical and demographic characteristics. This would help assess the potential for generalization of such a test,” Dr. Cruz-Correa said. “A second step is to assess the potential impact of these methylation markers on disease activity and treatment response, which are clinical outcomes of great importance to patients.”
Meanwhile, he said, “there are ongoing efforts to shed light into how DNA methylation integrates with other epigenetic mechanisms like micro-RNAs to regulate gene expression in concert with one another. An integrative look into these mechanisms may be able to give insight into the pathogenesis of psoriatic disease in a way that has not been possible before.”
In an interview, Johann E. Gudjonsson, MD, PhD, professor of skin molecular immunology at the University of Michigan, Ann Arbor, said the study “is interesting and important as it indicates that there are changes in the blood that occur before the development of psoriatic arthritis. However, it does not provide much in terms of novel insights into the mechanisms involved and is still a long way away from being useful as a clinical predictor or biomarker.”
The National Psoriasis Foundation, Krembil Foundation, and Canadian Institutes of Health Research provided support for the study. Dr. Cruz-Correa reports support from the National Psoriasis Foundation and the Arthritis Society. Dr. Gudjonsson has no relevant financial relationships.
FROM ARTHRITIS & RHEUMATOLOGY