User login
Study validates use of new psoriatic arthritis prediction tool
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
Though it requires further validation, researchers led by rheumatologist Lihi Eder, MD, PhD, of the Women’s College Research Institute at Women’s College Hospital, Toronto, characterized the development and validation of PRESTO as “an important first step in the development and testing of interventional strategies that may ultimately halt disease progression,” they wrote in their study of the tool, which published in Arthritis & Rheumatology. Dr. Eder presented a summary of progress on the effort at the 2023 annual meeting of the Canadian Rheumatology Association.
To develop and validate the tool, the researchers evaluated 635 patients from the University of Toronto Psoriasis Cohort, which was launched in 2006 as a prospective longitudinal cohort study to examine risk factors for the development of PsA among patients with psoriasis. Patients enrolled in the cohort have a dermatologist-confirmed diagnosis of psoriasis and are assessed by a rheumatologist prior to enrollment to exclude those with inflammatory arthritis in the past or at the time of assessment.
To develop prediction models for PsA, Dr. Eder and colleagues used information from the patient cohort demographics, psoriasis characteristics, comorbidities, medications, and musculoskeletal symptoms. Next, they used multivariable logistic regression models adjusting for covariates, duration of psoriasis, and the log duration at risk to estimate the probability of developing PsA within 1-year and 5-year time windows from consecutive study visits.
The mean age of the study participants was 47 years, 76% were White, and 57% were male; and they had psoriasis for a mean of 16 years. The researchers found that 51 patients developed PsA during the 1-year follow-up, and 71 developed PsA during the 5-year follow-up. The risk of developing PsA within 1 year was associated with younger age, male sex, family history of psoriasis, back stiffness, nail pitting, joint stiffness, use of biologic medications, patient global health, and pain severity (area under the curve, 72.3).
In addition, the risk of developing PsA within 5 years was associated with morning stiffness, psoriatic nail lesions, psoriasis severity, fatigue, pain, and use of systemic non-biologic medication or phototherapy (AUC, 74.9). Calibration plots showed reasonable agreement between predicted and observed probabilities.
“Interestingly, several previously reported risk factors for PsA, such as HLA-B27, family history of PsA, uveitis, and flexural psoriasis, were not included in the risk prediction model due to their scarcity in our cohort,” the researchers wrote. “This finding may be due to immortal time bias which can complicate the development of risk prediction models for PsA. Genetic factors or their surrogates (e.g., family history of PsA) are associated with the development of PsA concurrently or shortly after the onset of psoriasis.”
They acknowledged certain limitations of the study, including its relatively small sample size and questionable generalizability of the study findings, “as most of the patients were recruited from dermatology clinics leading to overrepresentation of moderate-severe psoriasis. Therefore, PRESTO will require an external validation to assess its performance in other populations of psoriasis patients with different characteristics.”
Saakshi Khattri, MD, a board-certified dermatologist, rheumatologist, and internist at the Icahn School of Medicine at Mount Sinai, New York, who was not involved in the study and was asked to comment on the results, characterized the PRESTO tool as “an interesting step in the right direction, but it’s the first step.”
Since dermatologists are usually the first point of contact for psoriasis patients, she added, “a risk calculator can be helpful, but the question remains: When do we refer them to a rheumatologist? If the risk comes to 5%, is that a low risk that doesn’t need referral to rheumatology? I don’t think those questions have been answered here. From a rheumatology perspective, does the risk calculator help me decide when to intervene? At present, I’m not sure it does. Perhaps a higher score might make us intervene sooner if our clinical exam doesn’t show swollen or tender joints.”
Clinical exam findings and history she considers as a rheumatologist before making treatment recommendations include the following: Are there swollen and tender joints? Does the patient report morning stiffness for upwards of 30 minutes? Do they have enthesitis or dactylitis? Is there axial involvement? “Imaging can help if there isn’t anything on clinical exam and the history is compelling and/or the patient has risk factors for PsA,” she said.
The study’s finding of biologic use being associated with risk of developing PsA at year 1 but not at year 5 is “confusing,” Dr. Khattri added. “My concern is, will that now dissuade our moderate to severe psoriasis patients from using biologics to clear their psoriasis? We know that biologics are indicated for moderate to severe psoriasis. We also know psoriasis is associated with increased cardiovascular risk and there’s data to suggest that treatment with biologics with its resultant decrease in systemic inflammation can decrease cardiovascular risk.”
The study was supported by a New Investigator Grant from the Physician Services Incorporated Foundation. Dr. Eder disclosed that she is supported by the Canada Research Chair in Inflammatory Rheumatic Diseases. Dr. Khattri reported that she is a member of the advisory board for UCB, Janssen, AbbVie, Regeneron, Sanofi, Lilly, Argenx, and Arcutis. She has also received research funds from Incyte, AbbVie, Leo, Galderma, Pfizer, and Acelyrin.
FROM ARTHRITIS AND RHEUMATOLOGY
Roflumilast cream appears safe, effective for children with psoriasis, researchers report
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
In patients aged 2-11 years, roflumilast cream was well tolerated and improved signs and symptoms of psoriasis over 4 weeks, according to results from a pair of phase two studies.
“Limited topical treatments are approved for children younger than 12 years old with psoriasis,” researchers led by Adelaide A. Hebert, MD, wrote in their abstract. The results were presented during a poster session at the annual meeting of the Society for Pediatric Dermatology.
Roflumilast cream 0.3% (Zoryve) is a once-daily, topical nonsteroidal treatment from Arcutis Biotherapeutics. A phosphodiesterase-4 inhibitor, it was approved by the Food and Drug Administration in 2022 for mild, moderate and severe psoriasis in individuals aged 12 and older, including intertriginous psoriasis.
For the analysis, Dr. Hebert, chief of pediatric dermatology at the University of Texas, Houston, and colleagues conducted two 4-week, phase 2, open-label safety studies of roflumilast cream 0.3%.
One, study 216, enrolled 10 children aged 2-5, and all but one were Black. The other, study 215, enrolled 20 children aged 6-11, and half were Black and nearly half were White. At baseline, patients had 2% or greater body surface area (BSA) involvement and an Investigator Global Assessment (IGA) score of at least mild.
Caregivers applied roflumilast cream to all affected areas once daily for 28 days. The researchers collected pharmacokinetic samples at week 2 and week 4. The primary endpoints were pharmacokinetic, safety, and tolerability.
Efficacy was evaluated as exploratory endpoints: An IGA of clear or almost clear plus a 2-grade or more improvement from baseline, a 50% or greater improvement and a 75% or greater improvement on the Psoriasis Area and Severity Index (PASI-50 and PASI-75), a 4-point or greater reduction in the Worst Itch–Numeric Rating Scale (WI-NRS) in patients with a baseline score of 4 or greater, a mean change from baseline in BSA, and improvement in the Children’s Dermatology Life Quality Index (CDLQI).
At baseline, the mean BSA was similar for patients enrolled in studies 216 and 215 (9.6% and 8.8%, respectively), and 80% of all patients had baseline IGA of moderate. By week 2, the mean roflumilast and N-oxide predose plasma concentrations among patients in the younger group were 2.15 and 22.4 ng/mL, compared with 3.15 and 28.9 ng/mL among those in the older group. At week 4, the mean roflumilast and N-oxide predose concentrations were 2.04 and 15.8 ng/mL in the younger group (study 216), compared with 1.68 and 15.7 ng/mL in the older group (study 215).
As for efficacy, 90% and 40% of patients in studies 216 and 215 achieved IGA success at week 4, respectively, while 90% and 50% achieved PASI-75, 90% and 40% achieved WI-NRS success, and the mean BSA reductions at week 4 were 79.1% and 44.4%. Meanwhile, one younger patient in study 216 reported a treatment-emergent adverse event (TEAE) of headache, which was considered mild, while four older patients in study 215 reported 8 TEAEs, which were considered mild and ranged from back pain to nasal congestion.
“The rapid onset of action was surprising but exceedingly rewarding for the subjects enrolled in the study,” Dr. Hebert told this news organization after the meeting. “The PASI scores and itch scores were markedly improved at the end of the 4-week clinical trial. Patient and parents alike were pleased to use a steroid-free option with once-daily application and rapid onset of action to help control plaque psoriasis.”
In the poster abstract, she and her coauthors concluded that “under maximal use conditions in children aged 2-11 years, roflumilast cream 0.3% was well tolerated and improved signs and symptoms of psoriasis with measured improvements in IGA score, PASI score, BSA involvement, CDLQI, and WI-NRS. Overall, pharmacokinetics, safety, tolerability, and efficacy in patients aged 2-11 years were consistent with prior results in adults and adolescents.”
The study was funded by Arcutis Biotherapeutics. Dr. Hebert reported that she is an investigator for Arcutis. About half the coauthors are employees of Arcutis, and the other half disclosed grants, research funding and/or honoraria from the company. Research grants from the company for this study were paid to the McGovern Medical School at the University of Texas.
FROM SPD 2023
What factors cause multiple biologic failure in psoriasis?
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
, results from a prospective cohort demonstrated.
“Prior cross-sectional and single-center studies have primarily analyzed therapeutic failure of a single biologic or biologics within one class,” researchers led by Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, wrote in the study, published in the Journal of the American Academy of Dermatology. “However, failure of multiple biologics targeting different signaling pathways is common over the course of treatment. These ‘multiple biologic failure’ patients are not well-characterized, and the patterns of biologics attempted and sociodemographic or clinical features that may predict difficult treatment are incompletely studied.”
To bridge this gap, the researchers conducted a prospective cohort study from the CorEvitas Psoriasis Registry, which collected data from dermatologist-diagnosed patients with psoriasis who started or switched to a Food and Drug Administration (FDA)–approved systemic therapy for psoriasis during routine dermatology visits from April 15, 2015, to May 10, 2022. This period included data from 17,196 patients across 259 private and 209 academic sites from 580 physicians in the United States and Canada.
From this registry, Dr. Liao and colleagues identified 1,039 patients with 24 months or more of follow-up data, a confirmed index biologic start date, and valid baseline assessment data, and categorized them into three cohorts:
- 490 (47.2%) with good response (GR), defined as patients with 24 months or more of continued index biologic use by the last registry visit.
- 65 (6.3%) with multiple biologic failure (MBF), defined as patients administered two or more biologic agents of different mechanistic classes who discontinued these biologics because of physician-reported “inadequate initial response,” “failure to maintain initial response,” or “active disease” despite 90 or more days of use per biologic.
- 484 (46.6%) categorized as “other,” defined as patients failed by one biologic or who discontinued treatment for nonmedical reasons.
The researchers used multivariable logistic regression to identify sociodemographic, clinical, and patient-reported outcomes that differed between the MBF and GR groups. The mean age of the patients in the study was 49.1 years, 44.2% were female, 77.9% were White, 9.7% were Hispanic, and the mean duration of psoriasis was 11.5 years.
On multivariable logistic regression, factors associated with MBF, compared with those with GR, included female at birth (odds ratio [OR] = 2.29; confidence interval [CI], 1.11-4.72), history of hyperlipidemia (OR = 3.14; CI, 1.35-7.30), Medicaid insurance (OR = 4.53; CI, 1.40-14.60), prior nonbiologic systemic therapy (OR = 2.47; CI, 1.16-5.25), higher psoriasis duration (OR = 0.60 per standard deviation [SD]; CI, 0.38-0.94), and later index biologic initiation (OR = 0.37 per year; CI, 0.27-0.52). Sensitivity analysis revealed that the duration of prior nonbiologic systemic therapy use was not associated with MBF (OR = 0.99; CI, 0.94-1.02; P = 0.56).
“Interestingly, health-related behaviors (e.g., smoking, alcohol use) and location/extent of psoriasis were not important differentiators between MBF and GR,” the authors noted. “We might suspect these features to correlate with MBF, as numerous observational studies found associations between health-related behaviors or psoriasis severity and presence at difficult-to-treat locations, which often relates to biologic use.”
They acknowledged certain limitations of their study, including underrepresentation of ethnoracial minorities and male sex at birth relative to reported psoriasis epidemiology, “possibly reflecting participation bias and reduced access to specialty care, given that patients were enrolled into the registry by dermatologists,” they wrote. “Patient adherence to prescribed biologic regimens between registry visits was not evaluated.”
Raj Chovatiya, MD, PhD, assistant professor of dermatology at Northwestern University, Chicago, who was asked to comment on the study, said that despite the rapid expansion in biologic therapies for psoriasis, “analysis of real-world use patterns and patient characteristics has been limited – particularly for those who have failed multiple treatments. These findings suggest that there indeed may be some key differences between patients who have had to cycle through multiple biologics versus those who have had a sustained satisfactory response on a single therapy, such as disease duration and previous nonbiologic treatments.”
However, he added, “while this prospective study utilized a robust approach to gather standard-of-care data across multiple clinical sites, the absolute number of patients with multiple biologic failures was low, and additional data for these kinds of patients are still highly needed.”
The study was sponsored by CorEvitas and supported through a partnership between CorEvitas and the National Psoriasis Foundation. Dr. Liao disclosed that he has received research grant funding from AbbVie, Amgen, Janssen, Leo, Novartis, Pfizer, Regeneron, and TRex Bio. Dr. Chovatiya disclosed ties with several pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Could risk stratifying methotrexate users lead to less frequent testing?
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new model can predict which patients are more likely to experience side effects from long-term methotrexate (MTX) use, research suggests. Patients with a lower risk profile may benefit from less frequent testing, the authors hypothesize.
Most recommendations advise that patients initiating MTX therapy should get blood testing every 2-4 weeks to monitor for full blood count, liver function, urea electrolytes, and creatinine. After 6 months taking MTX, monitoring can be tapered to every 3 months. But Abhishek Abhishek, MD, PhD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust and colleagues argue that abnormal results after the initial 6 months of treatment are “infrequent,” and patients may benefit from fewer tests throughout the year.
“Unnecessary blood tests waste patients’ time and health care resources, including the time of general practitioners and phlebotomists,” Dr. Abhishek and associates write. “It would be beneficial to predict the risk of clinically significant abnormal blood test results during long-term methotrexate treatment to inform the frequency of testing for individuals.”
Stratifying risk
In the study, published in the BMJ, researchers used the UK’s Clinical Practice Research Datalink (CPRD) to identify the electronic medical records of over 37,000 adult patients with an immune-mediated inflammatory disease who were prescribed MTX during 2007-2019. All included patients were prescribed MTX for at least 6 months. The main outcome was discontinuation of methotrexate because of abnormal blood test results. Around 62% of patients had rheumatoid arthritis and 22% had psoriasis or psoriatic arthritis.
Using these anonymized data, the group developed a risk stratification model using 11 clinical predictors. “The factors that went in the model are simple things that most patients can self-report or doctors can get from their patient’s medical records,” Dr. Abhishek told this news organization, including methotrexate dose, age, sex, and comorbidities. Dr. Abhishek emphasized that the model should be used only in patients who have continued taking MTX for at least 6 months and have already undergone more frequent initial testing.
The strongest individual predictors were diabetes (hazard ratio, 1.25), chronic kidney disease stage 3 (HR, 2.01), and previous cytopenia or raised liver enzyme levels during the first 6 months of MTX therapy (HR, 2.97). However, Dr. Abhishek emphasized that the individual factors were less important, noting that the model sums the risks to predict outcomes more accurately. Most patients (68.4%) were sorted into the low-risk cohort, with a less than 10% estimated risk of discontinuing MTX over the next 5 years. About one-fifth (20.9%) were categorized as moderate risk (10%-20% estimated risk over 5 years), and 10.7% were high risk, with a greater than 20% estimated risk of discontinuing the drug over 5 years.
The authors argue that low-risk patients could receive less frequent testing – perhaps every 6 months or annually, while moderate-risk patients would continue to be tested every 3 months. High-risk patients could potentially be tested with even greater frequently.
More research needed
The research involved “incredibly sophisticated statistical analysis,” said Daniel E. Furst, MD, professor emeritus of medicine at the University of California, Los Angeles, who was not involved with the study. However, the data do not yet support altering blood testing frequency based on this model.
“The hypothesis that not all patients have to be examined so frequently is a very reasonable hypothesis,” Dr. Furst said in an interview, and additional research is needed to corroborate it. The model also needs to be validated in patient populations outside of the United Kingdom, he added.
Dr. Abhishek agreed that validating the model in other patient populations is an important next step. “When we develop a tool [using] a one-nation data set, we want other researchers to then validate it in other countries’ data sets to make sure there is nothing odd about patients in the U.K. that makes the tool work well here but not in [the] U.S., Europe, or Asia, for example,” he said. Doing so should be relatively easy, he said, as the model is publicly available, and the information required is routinely collected during clinic visits.
To understand if less frequent testing might be appropriate for some patients, researchers would need to look at data registries like the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry or CorEvitas registries “where the testing is done in a very regular way over the long haul,” Dr. Furst said. Analyzing these datasets, researchers could determine the testing intervals that would be most efficient for low- and high-risk patients.
A word of caution
While less frequent testing for long-term MTX therapy could likely have benefits, there is still some risk involved, cautioned Prabha Ranganathan, MD, professor of medicine at Washington University in St. Louis.
“Although most methotrexate toxicity occurs within the first 6 months of starting treatment, rare idiosyncratic toxicity can occur that does not correlate with the dose, duration, or method of how methotrexate is administered,” she wrote in an accompanying editorial. “Most rheumatologists can identify a handful of patients who receive methotrexate in their practice who develop sudden leukopenia or thrombocytopenia or transaminitis that is severe enough to warrant drug discontinuation.” While tools like this prediction model can be useful, clinicians need to consider each patient individually and use shared decision-making when monitoring for MTX toxicity, she advised.
“As in most of areas of medicine, the one-size-fits-all approach does not work for methotrexate users,” she noted.
This study was funded by the U.K. National Institute for Health and Care Research and Health Technology Assessment. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadila Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Furst and Dr. Ranganathan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE BMJ
Vitamin D deficiency linked to psoriasis severity
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
, suggesting that some people who increase their intake of the vitamin could better control this skin condition that affects up to 8 million people in the United States alone.
Brown University researchers studied almost 500 psoriasis cases taken from the National Health and Nutrition Examination Survey (NHANES), the scientists told attendees at the conference of the American Society for Nutrition. They compared the peoples’ reports on how much of their body surface was affected by psoriasis to vitamin D levels collected in blood samples.
“After adjusting for lifestyle factors such as smoking, the analysis showed that lower vitamin D levels and vitamin D deficiency were significantly associated with greater psoriasis severity,” the ASN said in a news release. “The researchers also found that patients with the least amount of body surface affected by psoriasis had the highest average vitamin D levels while those with the greatest affected area had the lowest average levels of vitamin D.”
The researchers said that people with psoriasis might improve their condition by getting more vitamin D in their diet and through supplements.
“Topical synthetic vitamin D creams are emerging as new therapies for psoriasis, but these usually require a doctor’s prescription,” said researcher Rachel K. Lim, an MD candidate at Brown University, Providence, R.I. “Our results suggest that a vitamin D–rich diet or oral vitamin D supplementation may also provide some benefit to psoriasis patients.”
The researchers said that vitamin D toxicity is rare but that people should consult with their medical caregivers before they start taking supplements.
A version of this article first appeared on WebMD.com.
FROM NUTRITION 2023
Humira biosimilars: Five things to know
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.
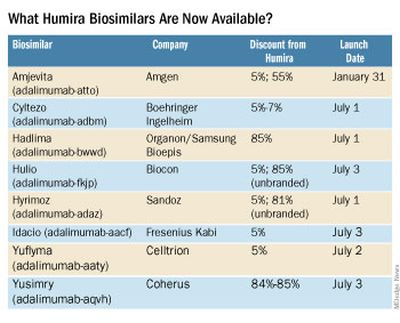
Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
Remote teams offer chance to improve difficult-to-treat PsA
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
DUBLIN – according to presenters at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
In the same session at the meeting, GRAPPA also announced a new initiative to define difficult-to-treat PsA.
Deepak Jadon, MBBCh, PhD, a rheumatologist with Cambridge (England) University Hospitals NHS Foundation Trust, described his experience of running a clinic for patients with difficult-to-treat PsA in eastern England, covering a catchment area of approximately 6 million people between six and seven hospitals. He discussed how the MDT in his region operates to discuss the management of such patients, whose treatment options may also have indications for comorbidities such as inflammatory bowel disease or uveitis, or have complicating factors such as metabolic syndrome.
“You have to have an interested and engaged colleague to form that collaboration,” Dr. Jadon said. “If you are working in isolation, without your colleagues in the same building, that becomes harder. We have been running remote multispecialty meetings without the patient being present, and I have had the good fortune of having medical students brought into our practice. We discussed approximately 220 patients, initially in our psoriasis-spondyloarthritis MDT and subsequently in our inflammatory bowel disease–spondyloarthritis MDT.”
There are also MDTs with hepatologist colleagues carried out on an ad hoc basis to discuss patients with nonalcoholic fatty liver disease, as well as patients with hepatitis or a transplanted liver, who have psoriatic disease.
This difficult-to-treat cohort is discussed in MDT meetings conducted on Zoom. At MDT meetings, carried out with frequencies ranging from monthly to bimonthly, Dr. Jadon said there would be two dermatologists, two rheumatologists, one to four dermatology and rheumatology trainees and fellows, one to four specialist nurses, one to three research nurses, and one biologics pharmacist. They record the meetings and discuss anywhere from 4 to 18 patients, reviewing items in their electronic medical record, calling or writing patients and/or their primary care clinician as needed. They take about an hour to meet, with a half hour of prep time and another 1.5 hours to undertake necessary actions.
“Generally, the question is, how can we change treatment to best cover the domains of disease?” Dr. Jadon said. “Progressively, more patients are being put onto biologics as a result of these conversations, and I do feel that it has helped our patients and us to consolidate their management plan. Naturally, as all clinicians do, we doubt ourselves and wonder if we are missing something. Is there an aspect of the disease [being missed]? Is there a treatment that I haven’t been using? [The meetings have] been reassuring in that regard. I also learn from my colleagues who have earlier access to treatments, especially in dermatology.”
In a small number of patients, some combinations of advanced therapies, such as combining a Janus kinase inhibitor with a biologic, have been used as a result of these collaborations, “and to discuss this in an MDT has been reassuring, including from a medico-legal perspective,” Dr. Jadon said. “One of the main things we found to be useful is having a brief referral pro forma. Usually, by the time patients reach this forum, they have used a lot of treatments, and it can be difficult to remember that on the spot. It is also important to focus on what the actual question is. Naturally, in these discussions, where you talk about the complexities and various facets of disease, you can get a bit lost and sometimes you actually don’t address the original question.”
He also said it has been very beneficial to use screen sharing in the remote MDTs so that different disciplines can review images together, such as with radiology colleagues. “There are varying skill sets among our colleagues, especially in radiology, and it has been quite nice to review their peripheral imaging, their axial imaging, laboratory markers, and skin lesions together.”
New GRAPPA project to provide clarity
A new GRAPPA project has been devised to help physicians identify and define difficult-to-treat and difficult-to-manage PsA in order to help physicians to categorize and treat these patients.
“We have a growing treatment armamentarium ... but we still do not reach all the patients that we would like to,” said Fabian Proft, MD, of Charité University Medicine, Berlin. “We set our targets, but we see in the real world that we are only reaching them in 40% or 50% of our patients. So, we need to do better, and in order to do better, we need to understand better.”
“We should not only make a definition of difficult-to-treat PsA, which is nonresponse to treatment with objective signs of inflammation, but also we need to address and acknowledge difficult-to-manage [patients],” Dr. Proft said. “We should not stop as soon as we come up with a definition. This will be a working definition and will need to be validated.”
The speakers reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT GRAPPA 2023
Keep depression, anxiety screening top of mind in patients with psoriatic disease
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
DUBLIN – , warranting routine screening and having community contacts for mental health professional referrals, Elizabeth Wallace, MD, said at the annual meeting of the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis.
Dr. Wallace, of Cherry Hills Dermatology, Englewood, Colo., discussed the complex interactions between mental illness and psoriatic disease and the potential pitfalls of this comorbidity for these patients.
The topic of mental health is “consistently at the top of our patients’ minds, and certainly our minds too,” said session comoderator and GRAPPA president-elect Joseph F. Merola, MD, MMSc.
“In the U.S., around 17% of people with psoriasis have depression vs. 9% in those without psoriasis,” Dr. Wallace explained. “Psoriasis patients are twice as likely to have depression, compared to those without psoriasis, and psoriasis patients are 33% more likely to attempt suicide and 20% more likely to complete suicide, compared to those without psoriasis.” More severe psoriasis and younger age of onset are also associated with a greater likelihood of suicidality, she added.
Mediators of depression
“The inflammatory mechanisms driving PsD can drive depression and anxiety, and vice-versa,” she said. “There are often also genetic links, for example genetic variations in serotonin receptors, and psychological issues in psoriatic disease are predictably worsened by feelings of stigmatization, embarrassment, and social isolation.”
There are also efforts underway in clinics to “normalize” screening for anxiety and depression among this patient cohort, Dr. Wallace said. “We know that our psoriasis patients face social stigma from the visibility of their disease, and that stress can lead to flares of their condition,” she told the attendees. “We also know that patients who experience stigma also have an increased risk of depressive symptoms. We all know now that psoriasis has well-established pathways with upregulated proinflammatory cytokines.
“Increased cytokines stimulate indoleamine 2,3-dioxygenase, which converts tryptophan to kynurenine. Kynurenine is metabolized to quinolinic acid, which is neurotoxic.” She explained that because serotonin derives from tryptophan, decreases in tryptophan lead to reduced serotonin, and therefore increased risk of depression.
Interleukin-6 is known to be upregulated in depression and downregulated with the use of antidepressant medications, Dr. Wallace said. Mouse models in research have shown that deletion of the IL-6 gene produces antidepressant effects, and studies in humans have shown that IL-6, more than any other serum cytokine, is found at higher levels in humans with depression and psoriatic disease.
IL-17 is also implicated in psoriatic disease and mental health problems, Dr. Wallace said. “With stress, you get upregulation of the Tc17 cells, which produce IL-17,” she explained. “IL-17, along with other inflammatory markers, can actually make the blood-brain barrier more permeable, and when you get more permeability to the blood-brain barrier, you get these cytokines that can cross from the periphery and into the brain.
“With this crossing into the brain, you get further activation of more Th17 [cells] and that, on neurons, leads to increased potassium production, which is directly neurotoxic, so you get neuron destruction.”
Talking about depression
“So, what can we share with our patients?” Dr. Wallace asked. “We can discuss with them that psoriatic patients in general are more likely to be depressed or to have higher rates of suicide. The literature consistently shows that patients whose psoriasis is successfully treated experience reduced depression, and we can provide an understandable review of systemic medications, with warnings on depression and/or suicidality.”
Dr. Wallace advised to screen for depression with the Patient Health Questionnaire-2 (PHQ-2), a validated, two-item tool that asks, “Over the past 2 weeks, how often have you been bothered by having little interest or pleasure in doing things?” and “Over the past 2 weeks, how often have you been bothered by feeling down, depressed, or hopeless?”
She presented a case study illustrative of the type of presentation she sees in her clinic. It involved a 32-year-old man with plaque psoriasis and a high degree of body surface affected. “It’s now July in Colorado, it’s getting warm, people want to wear their shorts and T-shirts, but he said he could no longer hide his psoriasis,” said Dr. Wallace. “Further, it’s in areas that he cannot hide, such as his scalp, his beard, and he also has nail disease. Often, these patients don’t want to shake hands with their bosses or their colleagues and that’s very embarrassing for them.”
Dr. Wallace explained that this patient had seen advertisements for biologic drugs and requested to commence a treatment course. “During the exam, and now that you are developing some rapport with him, you discover that he is feeling down, is embarrassed at work, and has started to avoid social situations.” This is illustrative of a patient who should be screened for mental health conditions, specifically using PHQ-2, she said.
“You can be the person at the front line to screen these patients for mental health conditions, and, specifically for depression, with PHQ-2,” she said. PHQ-2 scores range from 0 to 6, and a score of 3 or higher is considered a positive screen.
“This is where your relationship with another health provider who is most qualified to care for these patients and validate them for their mental health condition can be absolutely critical,” Dr. Wallace said.
Successful PsD treatment lessens the risk for mental health comorbidities, and this is also seen in psoriatic arthritis, Dr. Wallace pointed out. Patient education is critical regarding their increased risk for depression and potential suicidal ideation, she added.
“It’s our job as clinicians to provide patients with an understandable, easy-to-digest review of systemic medications and warnings on depression and suicidality so that they can be aware of these factors.”
Perspective from Dr. Merola
In an interview, Dr. Merola, a double board-certified dermatologist and rheumatologist at Brigham and Women’s Hospital, Boston, discussed the interactions between mental and physical illness.
“One of the things we are learning is that it’s very much a multifactorial issue, in that skin and joints contribute, in some obvious ways, to anxiety and depression, like the fact that somebody doesn’t feel good about their appearance, or they can’t complete daily activities,” he said. “Those are the more obvious ones. But there is data and evidence that there is a biology behind that as well – inflammatory cytokines that drive skin disease probably also have a direct impact on the CNS and probably also drive anxiety and depression.
“We know that disordered sleep contributes to anxiety – think about how we feel if we get a horrible night’s sleep ... it’s hard to pick apart: ‘Am I depressed, am I anxious because I am having too much coffee? Because I am fatigued?’ So, we get into these circles, but the point is, we have to break these cycles, and we have to do it in multiple places. Yes, we have to fix the skin and the joints, but we also have to have interventions and think about how to screen for anxiety and depression. We also have to think about identifying disordered sleep, and how we intervene there as well.”
These challenges require a collaborative approach among physicians. “We can help patients to build their team that gets them help for their skin, for their joints, for their anxiety or depression, their disordered sleep, for their nutritional disorders, their obesity, and so on. So, we are trying to pick apart and unpack those complexities,” he said.
In regard to the potential impacts of this holistic strategy on physician workloads, Dr. Merola acknowledged it is important to consider physician wellness. “There’s no question that we want to be doing the best we can for our colleagues, but we don’t want to overload our colleagues by saying, ‘By the way, not only should we be treating their skin and joints,’ which of course we should be doing, but ‘could you also manage their diabetes, their obesity, their disordered sleep, their anxiety, their depression, difficulties with insurance, getting access to treatments, etc.’
“This is where effective collaboration between physicians becomes important,” he stressed. “We can’t manage every single piece, but we can make sure our patients are informed, are aware, and assist them to get the help that they need.”
In the United States, there “is a real issue” with access to mental health care and greater awareness needs to be created around this issue, he added.
Dr. Wallace and Dr. Merola report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT GRAPPA 2023
Oral IL-23 receptor antagonist for psoriasis promising: Phase 2b study
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
SINGAPORE – across all doses, compared with placebo, according to results of the FRONTIER 1 trial.
In the 16-week phase 2b study, 255 adults with moderate to severe plaque psoriasis were randomly assigned into six treatment groups: placebo (n = 43), JNJ-2113 25 mg daily (n = 43), 25 mg twice daily (n = 41), 50 mg daily (n = 43), 100 mg daily (n = 43), or 100 mg twice daily (n = 42).
Of those who took the placebo, only 9.3% achieved the study’s primary endpoint of a 75% or greater improvement in the Psoriasis Area and Severity Index (PASI-75) by week 16. This was compared with 78.6% in the group that took the highest dose.
“Additionally, the onset of action was fairly fast: at week 4, more than 20% of patients had achieved PASI 75,” said Robert Bissonnette, MD, CEO of Innovaderm Research in Montreal, who presented the findings during a late-breaker session at the World Congress of Dermatology.
Patients in the remaining groups demonstrated a response that corresponded to dosing level: with 37.2%, 51.2%, 58.1%, and 65.1% achieving PASI-75 in the 25 mg daily, 25 mg twice-daily, 50 mg daily, and 100 mg daily groups, respectively.
“These results are very interesting because in terms of psoriasis treatment, if this is confirmed in phase 3, it would give us an oral alternative that would be selective for IL-23,” said Dr. Bissonnette, referring to the signaling pathway that plays a critical role in the pathogenesis of several immune-mediated inflammatory diseases, including plaque psoriasis.
Although rarely life-threatening, the skin disorder is often intractable to treatment. In recent years, therapies that block IL-23 signaling and downstream inflammatory cytokine production have proven useful. “We have on the market a number of biological agents targeting IL-23 that we use on a regular basis,” said Dr. Bissonnette. “However, there are currently no orally delivered therapies.”
If successful, JNJ-2113 – a first-in-class oral IL-23 antagonist peptide developed by Janssen – could change the treatment paradigm for patients with moderate to severe plaque psoriasis. “When I was first introduced to the concept, I thought it wouldn’t work as it’s a peptide, that it would be digested by the stomach,” he told the audience. “But because of its GI stability and its potency, when you administer it orally, you can detect pharmacological activity.”
A well-tolerated alternative
Participants in the FRONTIER 1 trial were on average about 44 years old and weighed 88.9 kg (195 lb). Most had been living with psoriasis for about 18 years, with a total PASI score of 19.05. In addition, 43.1% had been treated with phototherapy in the past, 22% with biologics, and 78.4% with systemics.
PASI 90 and 100 were among some of the secondary outcomes measured. Similar to the primary outcome of PASI 75, all treatment groups demonstrated a statistically significant dose-response in PASI 90, compared with placebo. For those on the highest dose of JNJ-2113, 59.5% and 40.5% achieved PASI 90 and PASI 100, respectively, by week 16. The corresponding figures for those receiving placebo were 2.3% and 0%.
The safety profile for JNJ-2113 across all doses was similar to that of placebo, with no evidence of a dose-dependent increase in the occurrence of adverse events (AEs). The most frequently reported AEs were COVID-19 and nasopharyngitis. There were three serious AEs (COVID-19, infected cyst, suicide attempt) among those on the active drug, but the investigators assessed that they were not related to the study intervention. No deaths, major adverse cardiac events, or malignancies were reported during the study.
Approached for an independent comment, Marius-Anton Ionescu, MD, PhD, from the University Hospital Saint Louis, Paris, who specializes in psoriasis, told this news organization that the new development with JNJ-2113 “is really promising.”
Treatment for plaque psoriasis has improved to the point where some biologics, such as risankizumab (Skyrizi), only require patients to have “four shots a year,” he says. “This is the future of psoriasis treatment; it might go down to two shots a year” – a regimen that will be easier than taking an oral medication once or twice a day.
“But it’s good to have an oral option because you will always have some patients who say: ‘Shots are not for me, I’m afraid,’ ” he says.
However, Dr. Ionescu noted that if JNJ-2113 were to pass phase 3 trials, it might face stiff competition from the selective tyrosine kinase 2 (TYK2) inhibitor deucravacitinib (Sotyktu), which the U.S. Food and Drug Administration approved for use in adults with moderate to severe plaque psoriasis last September. “It has very good results and is the first oral therapy that is comparable with biologics for plaque psoriasis,” he says.
But Dr. Bissonnette remains hopeful for the future. “I think JNJ-2113 goes way beyond psoriasis because this type of strategy using oral peptide–blocking receptors could be used in other immune-mediated diseases, including atopic dermatitis and other diseases outside of dermatology.” In addition to running a phase 3 study for moderate to severe plaque psoriasis, Janssen is planning to initiate a phase 2b clinical trial of JNJ-2113 in adults with ulcerative colitis.
The study was funded by Janssen. Dr. Bissonnette reports consulting and investigating for Janssen, and being on advisory panels and receiving research funding from multiple other pharmaceutical companies. Dr. Ionescu is an investigator for Psoriasis National Register France Psobioteq (no honoraria), and an investigator and speaker for Uriage cosmetics (honoraria).
A version of this article first appeared on Medscape.com.
AT WCD 2023
Study finds subcutaneous spesolimab reduces flares in patients with GPP
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
AT WCD 2023