User login
Association Between Psoriasis and Obesity Among US Adults in the 2009-2014 National Health and Nutrition Examination Survey
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
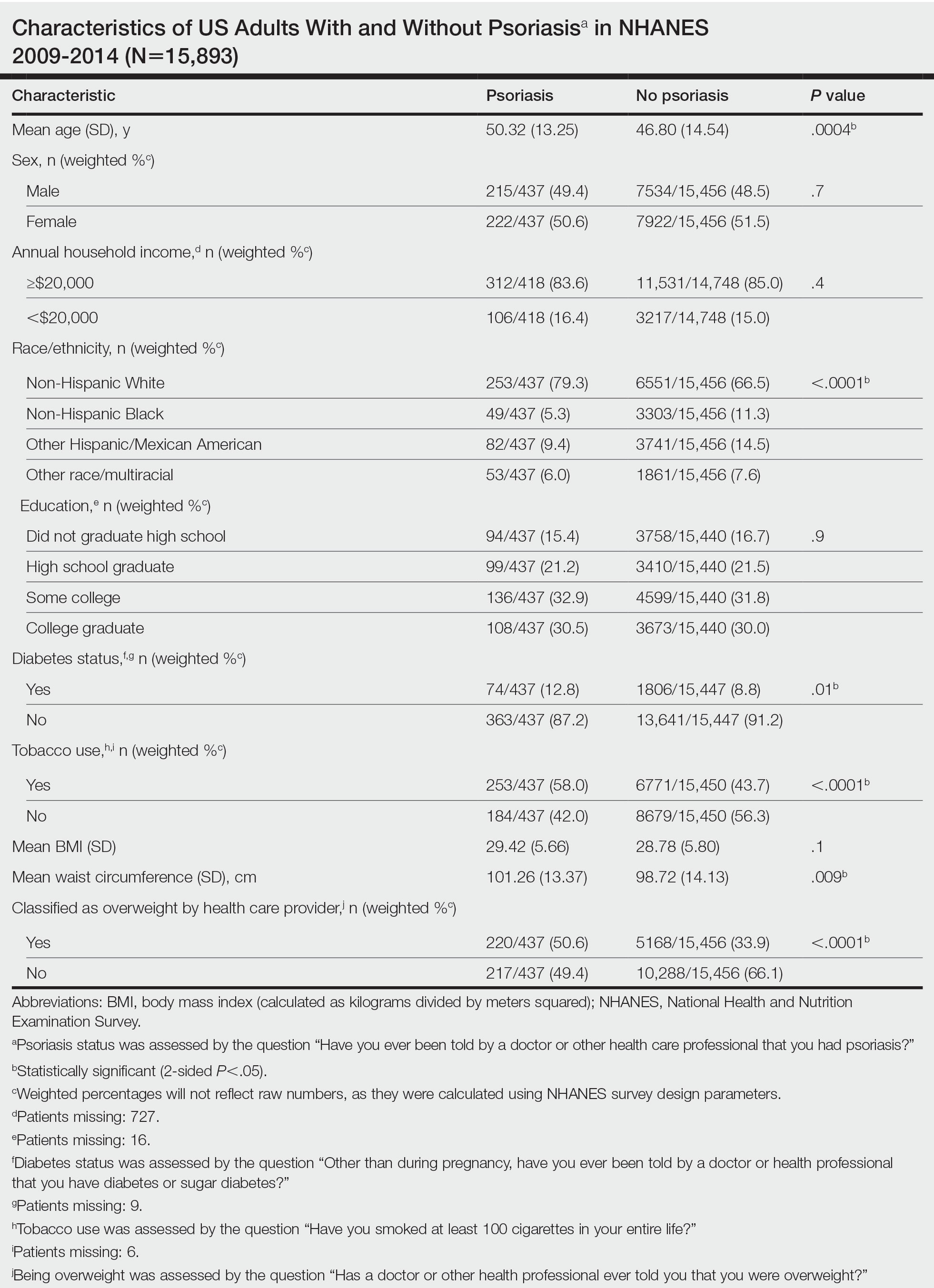
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”
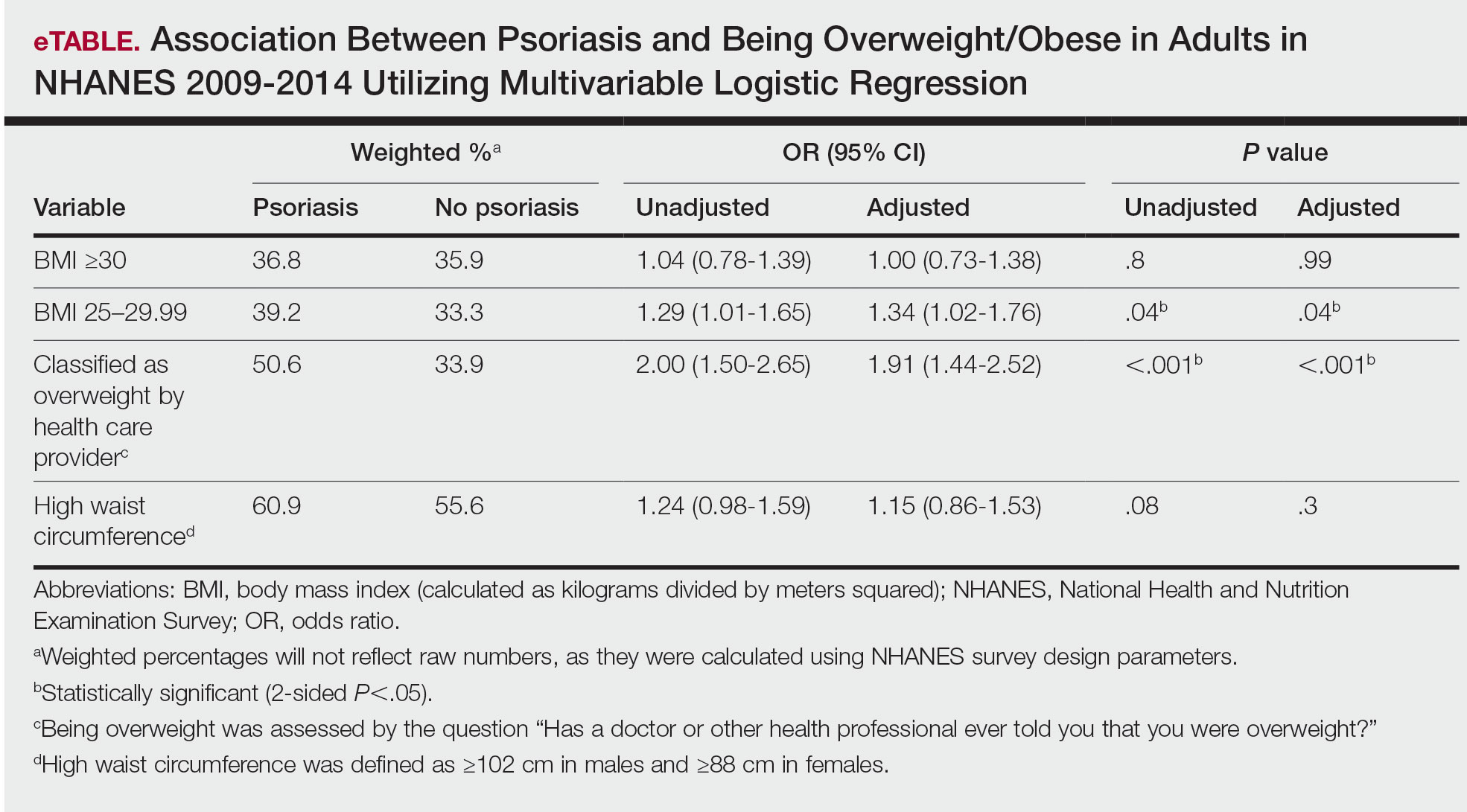
Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.
The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”

Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.

The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
To the Editor:
Psoriasis is an immune-mediated dermatologic condition that is associated with various comorbidities, including obesity.1 The underlying pathophysiology of psoriasis has been extensively studied, and recent research has discussed the role of obesity in IL-17 secretion.2 The relationship between being overweight/obese and having psoriasis has been documented in the literature.1,2 However, this association in a recent population is lacking. We sought to investigate the association between psoriasis and obesity utilizing a representative US population of adults—the 2009-2014 National Health and Nutrition Examination Survey (NHANES) data,3 which contains the most recent psoriasis data.
We conducted a population-based, cross-sectional study focused on patients 20 years and older with psoriasis from the 2009-2014 NHANES database. Three 2-year cycles of NHANES data were combined to create our 2009 to 2014 dataset. In the Table, numerous variables including age, sex, household income, race/ethnicity, education, diabetes status, tobacco use, body mass index (BMI), waist circumference, and being called overweight by a health care provider were analyzed using χ2 or t test analyses to evaluate for differences among those with and without psoriasis. Diabetes status was assessed by the question “Other than during pregnancy, have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes?” Tobacco use was assessed by the question “Have you smoked at least 100 cigarettes in your entire life?” Psoriasis status was assessed by a self-reported response to the question “Have you ever been told by a doctor or other health care professional that you had psoriasis?” Three different outcome variables were used to determine if patients were overweight or obese: BMI, waist circumference, and response to the question “Has a doctor or other health professional ever told you that you were overweight?” Obesity was defined as having a BMI of 30 or higher or waist circumference of 102 cm or more in males and 88 cm or more in females.4 Being overweight was defined as having a BMI of 25 to 29.99 or response of Yes to “Has a doctor or other health professional ever told you that you were overweight?”

Initially, there were 17,547 participants 20 years and older from 2009 to 2014, but 1654 participants were excluded because of missing data for obesity or psoriasis; therefore, 15,893 patients were included in our analysis. Multivariable logistic regressions were utilized to examine the association between psoriasis and being overweight/obese (eTable). Additionally, the models were adjusted based on age, sex, household income, race/ethnicity, diabetes status, and tobacco use. All data processing and analysis were performed in Stata/MP 17 (StataCorp LLC). P<.05 was considered statistically significant.

The Table shows characteristics of US adults with and without psoriasis in NHANES 2009-2014. We found that the variables of interest evaluating body weight that were significantly different on analysis between patients with and without psoriasis included waist circumference—patients with psoriasis had a significantly higher waist circumference (P=.009)—and being told by a health care provider that they are overweight (P<.0001), which supports the findings by Love et al,5 who reported abdominal obesity was the most common feature of metabolic syndrome exhibited among patients with psoriasis.
Multivariable logistic regression analysis (eTable) revealed that there was a significant association between psoriasis and BMI of 25 to 29.99 (adjusted odds ratio [AOR], 1.34; 95% CI, 1.02-1.76; P=.04) and being told by a health care provider that they are overweight (AOR, 1.91; 95% CI, 1.44-2.52; P<.001). After adjusting for confounding variables, there was no significant association between psoriasis and a BMI of 30 or higher (AOR, 1.00; 95% CI, 0.73-1.38; P=.99) or a waist circumference of 102 cm or more in males and 88 cm or more in females (AOR, 1.15; 95% CI, 0.86-1.53; P=.3).
Our findings suggest that a few variables indicative of being overweight or obese are associated with psoriasis. This relationship most likely is due to increased adipokine, including resistin, levels in overweight individuals, resulting in a proinflammatory state.6 It has been suggested that BMI alone is not a definitive marker for measuring fat storage levels in individuals. People can have a normal or slightly elevated BMI but possess excessive adiposity, resulting in chronic inflammation.6 Therefore, our findings of a significant association between psoriasis and being told by a health care provider that they are overweight might be a stronger measurement for possessing excessive fat, as this is likely due to clinical judgment rather than BMI measurement.
Moreover, it should be noted that the potential reason for the lack of association between BMI of 30 or higher and psoriasis in our analysis may be a result of BMI serving as a poor measurement for adiposity. Additionally, Armstrong and colleagues7 discussed that the association between BMI and psoriasis was stronger for patients with moderate to severe psoriasis. Our study consisted of NHANES data for self-reported psoriasis diagnoses without a psoriasis severity index, making it difficult to extrapolate which individuals had mild or moderate to severe psoriasis, which may have contributed to our finding of no association between BMI of 30 or higher and psoriasis.
The self-reported nature of the survey questions and lack of questions regarding psoriasis severity serve as limitations to the study. Both obesity and psoriasis can have various systemic consequences, such as cardiovascular disease, due to the development of an inflammatory state.8 Future studies may explore other body measurements that indicate being overweight or obese and the potential synergistic relationship of obesity and psoriasis severity, optimizing the development of effective treatment plans.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
- Jensen P, Skov L. Psoriasis and obesity. Dermatology. 2016;232:633-639.
- Xu C, Ji J, Su T, et al. The association of psoriasis and obesity: focusing on IL-17A-related immunological mechanisms. Int J Dermatol Venereol. 2021;4:116-121.
- National Center for Health Statistics. NHANES questionnaires, datasets, and related documentation. Centers for Disease Control and Prevention website. Accessed June 22, 2023. https://wwwn.cdc.govnchs/nhanes/Default.aspx
- Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. 2020;16:177-189.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- Paroutoglou K, Papadavid E, Christodoulatos GS, et al. Deciphering the association between psoriasis and obesity: current evidence and treatment considerations. Curr Obes Rep. 2020;9:165-178.
- Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:E54.
- Hamminga EA, van der Lely AJ, Neumann HAM, et al. Chronic inflammation in psoriasis and obesity: implications for therapy. Med Hypotheses. 2006;67:768-773.
Practice Points
- There are many comorbidities that are associated with psoriasis, making it crucial to evaluate for these diseases in patients with psoriasis.
- Obesity may be a contributing factor to psoriasis development due to the role of IL-17 secretion.
Palliative Care: Utilization Patterns in Inpatient Dermatology
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Practice Points
- Although severe dermatologic disease negatively impacts patients’ quality of life, palliative care may be underutilized in this population.
- Palliative care should be an integral part of caring for patients who are admitted to the hospital with serious dermatologic illnesses.
New guidelines for MTX use in pediatric inflammatory skin disease unveiled
While the typical dose of methotrexate (MTX) for inflammatory disease in pediatric patients varies in published studies, the maximum dose is considered to be 1 mg/kg and not to exceed 25 mg/week. In addition, test doses are not necessary for pediatric patients starting low dose (1 mg/kg or less) MTX for inflammatory skin disease, and the onset of efficacy with MTX may take 8-16 weeks.
and published online in Pediatric Dermatology.
“Methotrexate is a cost-effective, readily accessible, well-tolerated, useful, and time-honored option for children with a spectrum of inflammatory skin diseases,” project cochair Elaine C. Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, told this news organization. “Although considered an ‘immune suppressant’ by some, it is more accurately classified as an immune modulator and has been widely used for more than 50 years, and remains the standard of care when administered at very high doses and intrathecally in children with acute lymphoblastic leukemia – a practice that supports safety. But many details that support optimized treatment are not widely appreciated.”
In their guidelines document, Dr. Siegfried and her 22 coauthors noted that Food and Drug Administration labeling does not include approved indications for the use of MTX for many inflammatory skin diseases in pediatric patients, including morphea, psoriasis, atopic dermatitis, and alopecia areata. “Furthermore, some clinicians may be unfamiliar or uncomfortable prescribing medications off label for pediatric patients, causing delayed initiation, premature drug discontinuation, or use of less advantageous alternatives,” they wrote.
To address this unmet need, Dr. Siegfried and the other committee members used a modified Delphi process to reach agreement on recommendations related to five key topic areas: indications and contraindications, dosing, interactions with immunizations and medications, potential for and management of adverse effects, and monitoring needs. Consensus was predefined as at least 70% of participants rating a statement as 7-9 on the Likert scale. The effort to develop 46 recommendations has been a work in progress for almost 5 years, “somewhat delayed by the pandemic,” Dr. Siegfried, past president and director of the American Board of Dermatology, said in an interview. “But it remains relevant, despite the emergence of biologics and JAK inhibitors for treating inflammatory skin conditions in children. Although the mechanism-of-action of low-dose MTX is not clear, it may overlap with the newer small molecules.”
The guidelines contain several pearls to guide optimal dosing, including the following key points:
- MTX can be discontinued abruptly without adverse effects, other than the risk of disease worsening.
- Folic acid supplementation (starting at 1 mg/day, regardless of weight) is an effective approach to minimizing associated gastrointestinal adverse effects.
- Concomitant use of MTX and antibiotics (including trimethoprim-sulfamethoxazole) and NSAIDS are not contraindicated for most pediatric patients treated for inflammatory skin disease.
- Live virus vaccine boosters such as varicella-zoster virus (VZV) and measles, mumps, and rubella (MMR) are not contraindicated in patients taking MTX; there are insufficient data to make recommendations for or against primary immunization with MMR vaccine in patients taking MTX; inactivated vaccines should be given to patients taking MTX.
- Routine surveillance laboratory monitoring (i.e., CBC with differential, alanine transaminase, aspartate aminotransferase, creatinine) is recommended at baseline, after 1 month of treatment, and every 3-4 months thereafter.
- Transient transaminase elevation (≤ 3 upper limit normal for < 3 months) is not uncommon with low-dose MTX and does not usually require interruption of MTX. The most likely causes are concomitant viral infection, MTX dosing within 24 hours prior to phlebotomy, recent administration of other medications (such as acetaminophen), and/or recent alcohol consumption.
- Liver biopsy is not indicated for routine monitoring of pediatric patients taking low-dose MTX.
According to Dr. Siegfried, consensus of the committee members was lowest on the need for a test dose of MTX.
Overall, she said in the interview, helping to craft the guidelines caused her to reflect on how her approach to using MTX has evolved over the past 35 years, after treating “many hundreds” of patients. “I was gratified to confirm similar practice patterns among my colleagues,” she added.
The project’s other cochair was Heather Brandling-Bennett, MD, a dermatologist at Seattle Children’s Hospital. This work was supported by a grant from the Pediatric Dermatology Research Alliance (PeDRA), with additional funding from the National Eczema Association and the National Psoriasis Foundation. Dr. Siegfried disclosed ties with AbbVie, Boehringer Ingelheim, Incyte, LEO Pharma, Novan, Novartis, Pierre Fabre, Pfizer, Regeneron, Sanofi Genzyme, UCB, and Verrica. She has participated in contracted research for AI Therapeutics, and has served as principal investigator for Janssen. Many of the guideline coauthors disclosed having received grant support and other funding from pharmaceutical companies.
While the typical dose of methotrexate (MTX) for inflammatory disease in pediatric patients varies in published studies, the maximum dose is considered to be 1 mg/kg and not to exceed 25 mg/week. In addition, test doses are not necessary for pediatric patients starting low dose (1 mg/kg or less) MTX for inflammatory skin disease, and the onset of efficacy with MTX may take 8-16 weeks.
and published online in Pediatric Dermatology.
“Methotrexate is a cost-effective, readily accessible, well-tolerated, useful, and time-honored option for children with a spectrum of inflammatory skin diseases,” project cochair Elaine C. Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, told this news organization. “Although considered an ‘immune suppressant’ by some, it is more accurately classified as an immune modulator and has been widely used for more than 50 years, and remains the standard of care when administered at very high doses and intrathecally in children with acute lymphoblastic leukemia – a practice that supports safety. But many details that support optimized treatment are not widely appreciated.”
In their guidelines document, Dr. Siegfried and her 22 coauthors noted that Food and Drug Administration labeling does not include approved indications for the use of MTX for many inflammatory skin diseases in pediatric patients, including morphea, psoriasis, atopic dermatitis, and alopecia areata. “Furthermore, some clinicians may be unfamiliar or uncomfortable prescribing medications off label for pediatric patients, causing delayed initiation, premature drug discontinuation, or use of less advantageous alternatives,” they wrote.
To address this unmet need, Dr. Siegfried and the other committee members used a modified Delphi process to reach agreement on recommendations related to five key topic areas: indications and contraindications, dosing, interactions with immunizations and medications, potential for and management of adverse effects, and monitoring needs. Consensus was predefined as at least 70% of participants rating a statement as 7-9 on the Likert scale. The effort to develop 46 recommendations has been a work in progress for almost 5 years, “somewhat delayed by the pandemic,” Dr. Siegfried, past president and director of the American Board of Dermatology, said in an interview. “But it remains relevant, despite the emergence of biologics and JAK inhibitors for treating inflammatory skin conditions in children. Although the mechanism-of-action of low-dose MTX is not clear, it may overlap with the newer small molecules.”
The guidelines contain several pearls to guide optimal dosing, including the following key points:
- MTX can be discontinued abruptly without adverse effects, other than the risk of disease worsening.
- Folic acid supplementation (starting at 1 mg/day, regardless of weight) is an effective approach to minimizing associated gastrointestinal adverse effects.
- Concomitant use of MTX and antibiotics (including trimethoprim-sulfamethoxazole) and NSAIDS are not contraindicated for most pediatric patients treated for inflammatory skin disease.
- Live virus vaccine boosters such as varicella-zoster virus (VZV) and measles, mumps, and rubella (MMR) are not contraindicated in patients taking MTX; there are insufficient data to make recommendations for or against primary immunization with MMR vaccine in patients taking MTX; inactivated vaccines should be given to patients taking MTX.
- Routine surveillance laboratory monitoring (i.e., CBC with differential, alanine transaminase, aspartate aminotransferase, creatinine) is recommended at baseline, after 1 month of treatment, and every 3-4 months thereafter.
- Transient transaminase elevation (≤ 3 upper limit normal for < 3 months) is not uncommon with low-dose MTX and does not usually require interruption of MTX. The most likely causes are concomitant viral infection, MTX dosing within 24 hours prior to phlebotomy, recent administration of other medications (such as acetaminophen), and/or recent alcohol consumption.
- Liver biopsy is not indicated for routine monitoring of pediatric patients taking low-dose MTX.
According to Dr. Siegfried, consensus of the committee members was lowest on the need for a test dose of MTX.
Overall, she said in the interview, helping to craft the guidelines caused her to reflect on how her approach to using MTX has evolved over the past 35 years, after treating “many hundreds” of patients. “I was gratified to confirm similar practice patterns among my colleagues,” she added.
The project’s other cochair was Heather Brandling-Bennett, MD, a dermatologist at Seattle Children’s Hospital. This work was supported by a grant from the Pediatric Dermatology Research Alliance (PeDRA), with additional funding from the National Eczema Association and the National Psoriasis Foundation. Dr. Siegfried disclosed ties with AbbVie, Boehringer Ingelheim, Incyte, LEO Pharma, Novan, Novartis, Pierre Fabre, Pfizer, Regeneron, Sanofi Genzyme, UCB, and Verrica. She has participated in contracted research for AI Therapeutics, and has served as principal investigator for Janssen. Many of the guideline coauthors disclosed having received grant support and other funding from pharmaceutical companies.
While the typical dose of methotrexate (MTX) for inflammatory disease in pediatric patients varies in published studies, the maximum dose is considered to be 1 mg/kg and not to exceed 25 mg/week. In addition, test doses are not necessary for pediatric patients starting low dose (1 mg/kg or less) MTX for inflammatory skin disease, and the onset of efficacy with MTX may take 8-16 weeks.
and published online in Pediatric Dermatology.
“Methotrexate is a cost-effective, readily accessible, well-tolerated, useful, and time-honored option for children with a spectrum of inflammatory skin diseases,” project cochair Elaine C. Siegfried, MD, professor of pediatrics and dermatology at Saint Louis University, told this news organization. “Although considered an ‘immune suppressant’ by some, it is more accurately classified as an immune modulator and has been widely used for more than 50 years, and remains the standard of care when administered at very high doses and intrathecally in children with acute lymphoblastic leukemia – a practice that supports safety. But many details that support optimized treatment are not widely appreciated.”
In their guidelines document, Dr. Siegfried and her 22 coauthors noted that Food and Drug Administration labeling does not include approved indications for the use of MTX for many inflammatory skin diseases in pediatric patients, including morphea, psoriasis, atopic dermatitis, and alopecia areata. “Furthermore, some clinicians may be unfamiliar or uncomfortable prescribing medications off label for pediatric patients, causing delayed initiation, premature drug discontinuation, or use of less advantageous alternatives,” they wrote.
To address this unmet need, Dr. Siegfried and the other committee members used a modified Delphi process to reach agreement on recommendations related to five key topic areas: indications and contraindications, dosing, interactions with immunizations and medications, potential for and management of adverse effects, and monitoring needs. Consensus was predefined as at least 70% of participants rating a statement as 7-9 on the Likert scale. The effort to develop 46 recommendations has been a work in progress for almost 5 years, “somewhat delayed by the pandemic,” Dr. Siegfried, past president and director of the American Board of Dermatology, said in an interview. “But it remains relevant, despite the emergence of biologics and JAK inhibitors for treating inflammatory skin conditions in children. Although the mechanism-of-action of low-dose MTX is not clear, it may overlap with the newer small molecules.”
The guidelines contain several pearls to guide optimal dosing, including the following key points:
- MTX can be discontinued abruptly without adverse effects, other than the risk of disease worsening.
- Folic acid supplementation (starting at 1 mg/day, regardless of weight) is an effective approach to minimizing associated gastrointestinal adverse effects.
- Concomitant use of MTX and antibiotics (including trimethoprim-sulfamethoxazole) and NSAIDS are not contraindicated for most pediatric patients treated for inflammatory skin disease.
- Live virus vaccine boosters such as varicella-zoster virus (VZV) and measles, mumps, and rubella (MMR) are not contraindicated in patients taking MTX; there are insufficient data to make recommendations for or against primary immunization with MMR vaccine in patients taking MTX; inactivated vaccines should be given to patients taking MTX.
- Routine surveillance laboratory monitoring (i.e., CBC with differential, alanine transaminase, aspartate aminotransferase, creatinine) is recommended at baseline, after 1 month of treatment, and every 3-4 months thereafter.
- Transient transaminase elevation (≤ 3 upper limit normal for < 3 months) is not uncommon with low-dose MTX and does not usually require interruption of MTX. The most likely causes are concomitant viral infection, MTX dosing within 24 hours prior to phlebotomy, recent administration of other medications (such as acetaminophen), and/or recent alcohol consumption.
- Liver biopsy is not indicated for routine monitoring of pediatric patients taking low-dose MTX.
According to Dr. Siegfried, consensus of the committee members was lowest on the need for a test dose of MTX.
Overall, she said in the interview, helping to craft the guidelines caused her to reflect on how her approach to using MTX has evolved over the past 35 years, after treating “many hundreds” of patients. “I was gratified to confirm similar practice patterns among my colleagues,” she added.
The project’s other cochair was Heather Brandling-Bennett, MD, a dermatologist at Seattle Children’s Hospital. This work was supported by a grant from the Pediatric Dermatology Research Alliance (PeDRA), with additional funding from the National Eczema Association and the National Psoriasis Foundation. Dr. Siegfried disclosed ties with AbbVie, Boehringer Ingelheim, Incyte, LEO Pharma, Novan, Novartis, Pierre Fabre, Pfizer, Regeneron, Sanofi Genzyme, UCB, and Verrica. She has participated in contracted research for AI Therapeutics, and has served as principal investigator for Janssen. Many of the guideline coauthors disclosed having received grant support and other funding from pharmaceutical companies.
FROM PEDIATRIC DERMATOLOGY
For psoriasis, review finds several biosimilars as safe and effective as biologics
The effectiveness and safety of biosimilars for psoriasis appear to be similar to the originator biologics, reported the authors of a review of studies comparing the two.
“This systematic review found that there was no clinically or statistically significant difference in the efficacy and safety between biosimilars and originators of adalimumab, etanercept, infliximab, and ustekinumab for the treatment of psoriasis,” senior study author and clinical lecturer Zenas Z. N. Yiu, MBChB, PhD, and his colleagues at the University of Manchester, England, wrote in JAMA Dermatology.“The biosimilars evaluated in this study could be considered alongside originators for biologic-naive patients to improve the accessibility of biological treatments,” they added. “Switching patients currently on originators to biosimilars could be considered where clinically appropriate to reduce treatment costs.”
Biologics versus biosimilars
In contrast to most chemically synthesized drugs, biologics are created from living organisms, and they have complex structures that can vary slightly from batch to batch, Luigi Naldi, MD, director of the department of dermatology of Ospedale San Bortolo, Vicenza, Italy, and Antonio Addis, PharmD, researcher in the department of epidemiology, Regione Lazio, in Rome, wrote in an accompanying editorial.
Once the patent on the “originator” biologic expires, U.S. and European regulators allow other manufacturers to develop similar molecules – biosimilars – through an abbreviated approval process. If the results of a limited number of equivalence or noninferiority clinical trials are acceptable, registration for all the indications of the originator is allowed for its biosimilars. Referring to the expense of biologics, Dr. Naldi and Dr. Addis noted that in the United States, “biologics comprise less than 3% of the volume of drugs on the market, but account for more than one-third of all drug spending.”
Systematic review
Dr. Yiu and his colleagues queried standard medical research databases in August 2022, and included 14 randomized clinical trials (10 adalimumab, 2 etanercept, 1 infliximab, and 1 ustekinumab) and 3 cohort studies (1 adalimumab, 1 etanercept, 1 infliximab and etanercept) in their review.
Twelve trials compared biosimilars vs. originators in originator-naive patients, and 11 trials compared switching from originators to biosimilars vs. continuous treatment with the originator.
The researchers found the following:
At week 16, mean PASI75 (Psoriasis Area and Severity Index) response rates ranges from 60.7% to 90.6% for adalimumab biosimilars, vs. 61.5% to 91.7% for the originator. Mean PASI75 responses for the two etanercept biosimilars were 56.1% and 76.7% vs. 55.5% and 73.4% for the originator. In the ustekinumab study, mean PASI75 responses were 86.1% for the biosimilar vs. 84.0% for the originator.
At week 52, mean PASI75 responses were between 86.3% and 92.8% for adalimumab biosimilars vs. 84.9% and 93.9% for the originator. In the one comparison of an etanercept biosimilar, mean PAS175 responses were 80.9% for the biosimilar vs. 82.9% for the originator.
In studies involving patients switching from the originator to a biosimilar vs. continuing treatment with the originator, 32-week response rates ranged from 87.0% to 91.3% for adalimumab biosimilars and from 88.2% to 93.2% for the originator. In the one ustekinumab study, the 32-week mean PASI75 response was 92.6% after switching from the originator to a biosimilar vs. 92.9% with continuous treatment with the originator.
At week 52, mean PASI75 responses to adalimumab were between 84.2% and 94.8% for patients who switched to biosimilars and between 88.1% and 93.9% for those who stayed on the originator.
At week 52, in all the randomized trials, the incidence of adverse events and serious adverse events among those who switched to the biosimilar and those who continued with the originator were similar. Two cohort studies showed similar safety outcomes between originators and biosimilars, but one reported more adverse events in patients who switched to adalimumab biosimilars (P = .04).
Three clinical trials showed low risk for bias, 11 had moderate risk, and all cohort studies had moderate to high risk for bias.
Experts weigh in
Asked to comment on the study, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., told this news organization that he expects that the results will affect patient care.
However, he added, “I believe the decision of whether to use a biosimilar instead of the originator biologic may be more in the hands of the insurers than in the hands of physicians and patients.
“Biologics for psoriasis are so complicated that even the originator products vary from batch to batch. A biosimilar is basically like another batch of the innovative product,” explained Dr. Feldman, who was not involved in the study. “If we’re comfortable with patients being on different batches of the innovator product, we probably should be comfortable with them being on a biosimilar, as we have more evidence for the similarity of the biosimilar than we do for the current batch of the originator product.”
Aída Lugo-Somolinos, MD, professor of dermatology and director of the Contact Dermatitis Clinic at the University of North Carolina, Chapel Hill, said that “biologics have become the treatment of choice for moderate to severe psoriasis, and the use of biosimilars may be an alternative to reduce psoriasis treatment costs.
“Unfortunately, this study included a comparison of the existing biosimilars, which are drugs that are not the first line of treatment for psoriasis any longer,” added Dr. Lugo-Somolinos, who was not involved in the study.
Neil J. Korman, MD, PhD, professor of dermatology and codirector of the Skin Study Center at Case Western Reserve University, Cleveland, said the study was an important systematic review.
“This is a very timely publication because in the United States, several biosimilars are reaching the market in 2023,” he said. “The costs of the originator biologics are extraordinarily high, and the promise of biosimilars is that their costs will be significantly lower.”
Because all the studies were short term, Dr. Korman, who was not involved in the study, joins the study authors in recommending further related research into the long-term safety and efficacy of these agents.
Dr. Feldman, as well as one study author and one editorial author, reported relevant relationships with various pharmaceutical companies, including those that develop biosimilars. The remaining study authors, as well as Dr. Lugo-Somolinos and Dr. Korman, reported no relevant relationships. The study was funded by the Psoriasis Association and supported by the NIHR (National Institute for Health and Care Research) Manchester Biomedical Research Centre. All outside experts commented by email.
The effectiveness and safety of biosimilars for psoriasis appear to be similar to the originator biologics, reported the authors of a review of studies comparing the two.
“This systematic review found that there was no clinically or statistically significant difference in the efficacy and safety between biosimilars and originators of adalimumab, etanercept, infliximab, and ustekinumab for the treatment of psoriasis,” senior study author and clinical lecturer Zenas Z. N. Yiu, MBChB, PhD, and his colleagues at the University of Manchester, England, wrote in JAMA Dermatology.“The biosimilars evaluated in this study could be considered alongside originators for biologic-naive patients to improve the accessibility of biological treatments,” they added. “Switching patients currently on originators to biosimilars could be considered where clinically appropriate to reduce treatment costs.”
Biologics versus biosimilars
In contrast to most chemically synthesized drugs, biologics are created from living organisms, and they have complex structures that can vary slightly from batch to batch, Luigi Naldi, MD, director of the department of dermatology of Ospedale San Bortolo, Vicenza, Italy, and Antonio Addis, PharmD, researcher in the department of epidemiology, Regione Lazio, in Rome, wrote in an accompanying editorial.
Once the patent on the “originator” biologic expires, U.S. and European regulators allow other manufacturers to develop similar molecules – biosimilars – through an abbreviated approval process. If the results of a limited number of equivalence or noninferiority clinical trials are acceptable, registration for all the indications of the originator is allowed for its biosimilars. Referring to the expense of biologics, Dr. Naldi and Dr. Addis noted that in the United States, “biologics comprise less than 3% of the volume of drugs on the market, but account for more than one-third of all drug spending.”
Systematic review
Dr. Yiu and his colleagues queried standard medical research databases in August 2022, and included 14 randomized clinical trials (10 adalimumab, 2 etanercept, 1 infliximab, and 1 ustekinumab) and 3 cohort studies (1 adalimumab, 1 etanercept, 1 infliximab and etanercept) in their review.
Twelve trials compared biosimilars vs. originators in originator-naive patients, and 11 trials compared switching from originators to biosimilars vs. continuous treatment with the originator.
The researchers found the following:
At week 16, mean PASI75 (Psoriasis Area and Severity Index) response rates ranges from 60.7% to 90.6% for adalimumab biosimilars, vs. 61.5% to 91.7% for the originator. Mean PASI75 responses for the two etanercept biosimilars were 56.1% and 76.7% vs. 55.5% and 73.4% for the originator. In the ustekinumab study, mean PASI75 responses were 86.1% for the biosimilar vs. 84.0% for the originator.
At week 52, mean PASI75 responses were between 86.3% and 92.8% for adalimumab biosimilars vs. 84.9% and 93.9% for the originator. In the one comparison of an etanercept biosimilar, mean PAS175 responses were 80.9% for the biosimilar vs. 82.9% for the originator.
In studies involving patients switching from the originator to a biosimilar vs. continuing treatment with the originator, 32-week response rates ranged from 87.0% to 91.3% for adalimumab biosimilars and from 88.2% to 93.2% for the originator. In the one ustekinumab study, the 32-week mean PASI75 response was 92.6% after switching from the originator to a biosimilar vs. 92.9% with continuous treatment with the originator.
At week 52, mean PASI75 responses to adalimumab were between 84.2% and 94.8% for patients who switched to biosimilars and between 88.1% and 93.9% for those who stayed on the originator.
At week 52, in all the randomized trials, the incidence of adverse events and serious adverse events among those who switched to the biosimilar and those who continued with the originator were similar. Two cohort studies showed similar safety outcomes between originators and biosimilars, but one reported more adverse events in patients who switched to adalimumab biosimilars (P = .04).
Three clinical trials showed low risk for bias, 11 had moderate risk, and all cohort studies had moderate to high risk for bias.
Experts weigh in
Asked to comment on the study, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., told this news organization that he expects that the results will affect patient care.
However, he added, “I believe the decision of whether to use a biosimilar instead of the originator biologic may be more in the hands of the insurers than in the hands of physicians and patients.
“Biologics for psoriasis are so complicated that even the originator products vary from batch to batch. A biosimilar is basically like another batch of the innovative product,” explained Dr. Feldman, who was not involved in the study. “If we’re comfortable with patients being on different batches of the innovator product, we probably should be comfortable with them being on a biosimilar, as we have more evidence for the similarity of the biosimilar than we do for the current batch of the originator product.”
Aída Lugo-Somolinos, MD, professor of dermatology and director of the Contact Dermatitis Clinic at the University of North Carolina, Chapel Hill, said that “biologics have become the treatment of choice for moderate to severe psoriasis, and the use of biosimilars may be an alternative to reduce psoriasis treatment costs.
“Unfortunately, this study included a comparison of the existing biosimilars, which are drugs that are not the first line of treatment for psoriasis any longer,” added Dr. Lugo-Somolinos, who was not involved in the study.
Neil J. Korman, MD, PhD, professor of dermatology and codirector of the Skin Study Center at Case Western Reserve University, Cleveland, said the study was an important systematic review.
“This is a very timely publication because in the United States, several biosimilars are reaching the market in 2023,” he said. “The costs of the originator biologics are extraordinarily high, and the promise of biosimilars is that their costs will be significantly lower.”
Because all the studies were short term, Dr. Korman, who was not involved in the study, joins the study authors in recommending further related research into the long-term safety and efficacy of these agents.
Dr. Feldman, as well as one study author and one editorial author, reported relevant relationships with various pharmaceutical companies, including those that develop biosimilars. The remaining study authors, as well as Dr. Lugo-Somolinos and Dr. Korman, reported no relevant relationships. The study was funded by the Psoriasis Association and supported by the NIHR (National Institute for Health and Care Research) Manchester Biomedical Research Centre. All outside experts commented by email.
The effectiveness and safety of biosimilars for psoriasis appear to be similar to the originator biologics, reported the authors of a review of studies comparing the two.
“This systematic review found that there was no clinically or statistically significant difference in the efficacy and safety between biosimilars and originators of adalimumab, etanercept, infliximab, and ustekinumab for the treatment of psoriasis,” senior study author and clinical lecturer Zenas Z. N. Yiu, MBChB, PhD, and his colleagues at the University of Manchester, England, wrote in JAMA Dermatology.“The biosimilars evaluated in this study could be considered alongside originators for biologic-naive patients to improve the accessibility of biological treatments,” they added. “Switching patients currently on originators to biosimilars could be considered where clinically appropriate to reduce treatment costs.”
Biologics versus biosimilars
In contrast to most chemically synthesized drugs, biologics are created from living organisms, and they have complex structures that can vary slightly from batch to batch, Luigi Naldi, MD, director of the department of dermatology of Ospedale San Bortolo, Vicenza, Italy, and Antonio Addis, PharmD, researcher in the department of epidemiology, Regione Lazio, in Rome, wrote in an accompanying editorial.
Once the patent on the “originator” biologic expires, U.S. and European regulators allow other manufacturers to develop similar molecules – biosimilars – through an abbreviated approval process. If the results of a limited number of equivalence or noninferiority clinical trials are acceptable, registration for all the indications of the originator is allowed for its biosimilars. Referring to the expense of biologics, Dr. Naldi and Dr. Addis noted that in the United States, “biologics comprise less than 3% of the volume of drugs on the market, but account for more than one-third of all drug spending.”
Systematic review
Dr. Yiu and his colleagues queried standard medical research databases in August 2022, and included 14 randomized clinical trials (10 adalimumab, 2 etanercept, 1 infliximab, and 1 ustekinumab) and 3 cohort studies (1 adalimumab, 1 etanercept, 1 infliximab and etanercept) in their review.
Twelve trials compared biosimilars vs. originators in originator-naive patients, and 11 trials compared switching from originators to biosimilars vs. continuous treatment with the originator.
The researchers found the following:
At week 16, mean PASI75 (Psoriasis Area and Severity Index) response rates ranges from 60.7% to 90.6% for adalimumab biosimilars, vs. 61.5% to 91.7% for the originator. Mean PASI75 responses for the two etanercept biosimilars were 56.1% and 76.7% vs. 55.5% and 73.4% for the originator. In the ustekinumab study, mean PASI75 responses were 86.1% for the biosimilar vs. 84.0% for the originator.
At week 52, mean PASI75 responses were between 86.3% and 92.8% for adalimumab biosimilars vs. 84.9% and 93.9% for the originator. In the one comparison of an etanercept biosimilar, mean PAS175 responses were 80.9% for the biosimilar vs. 82.9% for the originator.
In studies involving patients switching from the originator to a biosimilar vs. continuing treatment with the originator, 32-week response rates ranged from 87.0% to 91.3% for adalimumab biosimilars and from 88.2% to 93.2% for the originator. In the one ustekinumab study, the 32-week mean PASI75 response was 92.6% after switching from the originator to a biosimilar vs. 92.9% with continuous treatment with the originator.
At week 52, mean PASI75 responses to adalimumab were between 84.2% and 94.8% for patients who switched to biosimilars and between 88.1% and 93.9% for those who stayed on the originator.
At week 52, in all the randomized trials, the incidence of adverse events and serious adverse events among those who switched to the biosimilar and those who continued with the originator were similar. Two cohort studies showed similar safety outcomes between originators and biosimilars, but one reported more adverse events in patients who switched to adalimumab biosimilars (P = .04).
Three clinical trials showed low risk for bias, 11 had moderate risk, and all cohort studies had moderate to high risk for bias.
Experts weigh in
Asked to comment on the study, Steven R. Feldman, MD, PhD, professor of dermatology at Wake Forest University, Winston-Salem, N.C., told this news organization that he expects that the results will affect patient care.
However, he added, “I believe the decision of whether to use a biosimilar instead of the originator biologic may be more in the hands of the insurers than in the hands of physicians and patients.
“Biologics for psoriasis are so complicated that even the originator products vary from batch to batch. A biosimilar is basically like another batch of the innovative product,” explained Dr. Feldman, who was not involved in the study. “If we’re comfortable with patients being on different batches of the innovator product, we probably should be comfortable with them being on a biosimilar, as we have more evidence for the similarity of the biosimilar than we do for the current batch of the originator product.”
Aída Lugo-Somolinos, MD, professor of dermatology and director of the Contact Dermatitis Clinic at the University of North Carolina, Chapel Hill, said that “biologics have become the treatment of choice for moderate to severe psoriasis, and the use of biosimilars may be an alternative to reduce psoriasis treatment costs.
“Unfortunately, this study included a comparison of the existing biosimilars, which are drugs that are not the first line of treatment for psoriasis any longer,” added Dr. Lugo-Somolinos, who was not involved in the study.
Neil J. Korman, MD, PhD, professor of dermatology and codirector of the Skin Study Center at Case Western Reserve University, Cleveland, said the study was an important systematic review.
“This is a very timely publication because in the United States, several biosimilars are reaching the market in 2023,” he said. “The costs of the originator biologics are extraordinarily high, and the promise of biosimilars is that their costs will be significantly lower.”
Because all the studies were short term, Dr. Korman, who was not involved in the study, joins the study authors in recommending further related research into the long-term safety and efficacy of these agents.
Dr. Feldman, as well as one study author and one editorial author, reported relevant relationships with various pharmaceutical companies, including those that develop biosimilars. The remaining study authors, as well as Dr. Lugo-Somolinos and Dr. Korman, reported no relevant relationships. The study was funded by the Psoriasis Association and supported by the NIHR (National Institute for Health and Care Research) Manchester Biomedical Research Centre. All outside experts commented by email.
FROM JAMA DERMATOLOGY
Does colchicine have a role in treating excess ASCVD risk in patients with chronic inflammatory conditions?
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
Methotrexate does not impair sperm quality, small study finds
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
After Yusimry’s steep discount, little clarity on future adalimumab biosimilar pricing
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
Adalimumab, sold under the brand name Humira, enjoyed a long run as one of the world’s best-selling medicines. But its 20-year, competition-free period has ended, and despite its best efforts to delay their arrival, drug manufacturer AbbVie now faces increasing competition from biosimilars entering the marketplace.
But one biosimilar about to be launched may be something of a game changer. Coherus BioSciences has announced plans to market its biosimilar Yusimry (adalimumab-aqvh) at a cost of $995 for two autoinjectors. This represents an approximate 85% discount over Humira’s sale list price of $6922.
This price, however, is slated to plunge even further as Coherus has also revealed that it will work with the Mark Cuban Cost Plus Drug Company (MCCPDC) to offer an even lower price. When Yusimry launches in July, it will sell for about $579 for two autoinjectors, making it the lowest-priced adalimumab biosimilar on the market.
“Coherus and Cost Plus Drug Company share a common mission, to increase access to high-quality medicine for patients at an affordable price,” said Dennis Lanfear, MBA, president, CEO and chairman of Coherus. “Mark Cuban and his team offer innovative solutions to health care problems, and Coherus is also a highly innovative company focused on unmet patient needs.”
He noted that, with adalimumab biosimilar pricing, this translates to a low list price approach. “We are pleased that Yusimry will be a part of that, as the first biologic they carry,” Mr. Lanfear said.
MCCPDC prices are based on the cost of ingredients and manufacturing plus 15% margin, a $3 pharmacy dispensing fee, and a $5 shipping fee. The company has expanded its inventory from 100 generics to more than 350 medications since it launched in January 2022. While MCCPDC is primarily directed to people who are paying cash for drugs, it does take insurance from select plans. And even for people who are covered by other insurers, the cost of drugs from Mr. Cuban’s company may be less than their out-of-pocket costs if they did go through their payer.
The low pricing of Yusimry is welcome, said Marcus Snow, MD, an assistant professor in the division of rheumatology at the University of Nebraska Medical Center, Omaha, but he pointed out that it is still a very expensive drug. “For patients who can’t afford Humira due to poor insurance coverage and high out-of-pocket costs, it is a welcome option. But it’s also unclear how many patients who lack adequate health insurance coverage can afford to pay $579 a month out of their own pockets.”
The biosimilars are coming
By early December 2022, the Food and Drug Administration had approved seven Humira biosimilars, and Amgen launched the first biosimilar to come on the market, Amjevita, soon afterward. By July 2023, half a dozen more are expected to enter the marketplace, said Steven Horvitz, managing director of EMC Analytics Group, a pharmaceutical research firm.
Mr. Horvitz agrees that the system is out of control, but it is unclear how much of an effect the low price tag on the Coherus product will have. “Some insurers may say, ‘we want the lowest price, and we don’t care about rebates,’ and will go with it,” he said. “PBMs [pharmacy benefit managers] are all about economics, so we have to see how many of their major clients will ask for the lowest price.”
Amgen has more or less followed the status quo on pricing for its biosimilar, but with a twist. It›s being offered at two different prices: $85,494 a year, which is only a 5% discount from Humira’s list price, or at $40,497 a year, a 55% discount. However, to date, the lower price has generally not been granted favorable formulary placement by PBMs. The plans that adopt the higher-priced biosimilar will get bigger rebates, but patients with coinsurance and deductibles will pay more out of pocket.
It is yet unknown how the pricing on Yusimry will affect the biosimilars ready to launch. “Will it give them pause for thought or not make any difference?” Mr. Horvitz said. “The companies do not reveal their pricing before the fact, so we have to wait and see.”
Large PBMs have not jumped at the opportunity to offer the Coherus biosimilar, but SmithRx, which bills itself as “next-generation pharmacy benefits management,” announced that it will offer Yusimry to its members at a discount of more than 90%.
“Unlike traditional PBMs, SmithRx prioritizes transparency and up-front cost savings. Humira is often an employer’s top drug expense so offering a low-cost alternative will have significant impact,” Jake Frenz, CEO and founder of SmithRx, said in a statement. “We’re excited to work with Cost Plus Drugs to bring this biosimilar to our members – and significantly reduce costs for them and their employers.”
A version of this article first appeared on Medscape.com.
How does psoriasis affect fertility and birth outcomes?
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
in a U.K. cohort study.
Those are key findings from what is believed to be one of the largest studies to investigate fertility and obstetric outcomes in patients with psoriasis.
“Studies that have examined fertility and pregnancy outcomes in women with psoriasis have reported conflicting findings,” lead author Teng-Chou Chen, PhD, of the Centre for Pharmacoepidemiology and Drug Safety at the University of Manchester (England), and colleagues from the Global Psoriasis Atlas wrote in the study, published in JAMA Dermatology. Most of the studies were small, with under 100 women, “and are thus likely underpowered to detect a difference in pregnancy outcomes. The majority of those studies used disease registry data or lacked a matched comparison group and hence were unable to estimate the association of fertility and adverse pregnancy outcomes in women with psoriasis when compared with the general population.”
To determine fertility rates and birth outcomes in female patients with psoriasis, compared with age- and practice-matched patients without psoriasis, the researchers evaluated EHR data from a large U.K. primary care database, the Clinical Practice Research Datalink GOLD, from 1998 to 2019. They limited the analysis to patients aged 15-44 years and used relevant codes from clinical consultations to identify those with psoriasis. Then, for each patient with psoriasis, the researchers selected five comparators without psoriasis from the same primary care practice and matched for year of birth.
Both sets of patients were followed from the index date to age 45 years, death, transfer out of practice, last date of data collection, or end of the study period (Dec. 31, 2019), whichever came first. Pregnancy records were extracted for both sets of patients, and birth outcomes were categorized as pregnancy loss, live birth, stillbirth, and preterm birth. Adverse pregnancy outcomes were also collected. Finally, Dr. Chen and colleagues used a negative binomial model to examine the association between psoriasis and the fertility rate, and they applied logistic regression to compare the association between psoriasis and obstetric outcomes.
The analysis included 63,681 patients with psoriasis and 318,405 comparators whose median age on the index date was 30 years and who were followed for a median of 4.1 years. Among patients with psoriasis, 5.1% met criteria for moderate to severe disease in the follow-up period. The researchers observed that, compared with their age- and practice-matched counterparts, patients with psoriasis were more likely to be current smokers, alcohol drinkers, or overweight on the index date. They were also more often diagnosed with diabetes, hypertension, inflammatory bowel disease, thyroid disorders, and respiratory diseases such as asthma and chronic obstructive pulmonary disease.
Fertility, birth outcomes
When they looked at fertility outcomes, the researchers found that, compared with their matched peers without psoriasis, those with psoriasis had higher rates of fertility (risk ratio, 1.30; 95% confidence interval, 1.27-1.33; P < .001). But after the researchers stratified patients based on psoriasis severity, those with moderate to severe disease had significantly lower rates of fertility (RR, 0.75; 95% CI, 0.69-0.83; P < .001), compared those who did not have psoriasis.
As for adverse birth outcomes, compared with their matched comparators, pregnancies in patients with psoriasis were less likely to end in a live birth (odds ratio, 0.91; 95% CI, 0.88-0.93; P < .001). They also had a higher risk of pregnancy loss (OR, 1.06; 95% CI, 1.03-1.10; P < .001), most during the first trimester, at a gestation period of under 91 days.
In addition to psoriasis, patients younger than age 20 (OR, 2.04; 95% CI, 1.94-2.15; P < .011) and those aged between 20 and 24 years (OR, 1.35; 95% CI, 1.31-1.40; P < .001) had a higher risk of pregnancy loss, compared with those aged between 25 and 34 years.
However, no increases in the risks of antenatal hemorrhage, preeclampsia, or gestational diabetes were observed in patients with psoriasis, and no statistically significant differences in the odds of stillbirth and preterm birth were found between patients with psoriasis and matched comparators who did not have psoriasis.
“The mechanism to link the higher risk of pregnancy loss in patients with psoriasis is not clear, but there might be potential explanations,” the researchers wrote. “Psoriasis is characterized by the increased activity of [interleukin]-17, IL-23, and tumor necrosis factor–alpha. Those proinflammatory cytokines may negatively affect the placenta and cause impaired fetal growth.”
They recommended that further studies “evaluate the effects of better management of psoriasis and close monitoring during pregnancy on pregnancy loss.” In particular, “patients with psoriasis were more likely to have comorbidities that may be related to poor pregnancy outcomes, and hence increased emphasis of managing comorbidities as part of the routine management plan is also warranted.”
Asked to comment on the study, Alexa B. Kimball, MD, MPH, who has been involved with research on this topic, said that she and other investigators had observed some years ago that fertility rates for women with moderate to severe psoriasis might be lower than expected.
This trend was observed in some psoriasis registries, some pregnancy registries, and in clinical practice, Dr. Kimball, professor of dermatology at Harvard Medical School, Boston, said in an interview. “This study clearly demonstrates that lower fertility rates in the moderate to severe psoriasis population occurs and compels further exploration of the reason why.” The reasons could be biologic, she continued, including difficulty conceiving or an increased risk of miscarriage, sociobehavioral issues, or a combination.
“Behavioral examples could include that some women with moderate to severe psoriasis can flare during pregnancy, which might affect their choice” to become pregnant, Dr. Kimball said. “Stigma may also play a role in how women with moderate to severe psoriasis form relationships. Now that there are much better treatments for moderate to severe psoriasis and better knowledge about managing psoriasis during pregnancy, it will also be important to explore whether these trends change over time.”
The study was funded by the International League of Dermatological Societies on behalf of the Global Psoriasis Atlas. Two of the study authors reported receiving consulting fees and grant support from many pharmaceutical companies. Dr. Kimball disclosed that she serves or has served on several Organization of Teratology Information Specialists advisory board pregnancy registries, is a consultant and investigator for Abbvie, Janssen, Lilly, Bristol-Myers Squibb, Moonlake, UCB, and Amgen; has fellowship funding from Janssen; and serves on the board of Almirall.
FROM JAMA DERMATOLOGY
Cell activity in psoriasis may predict disease severity and provide clues to comorbidities
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
The activity and clustering of certain cell types may distinguish mild and severe forms of psoriasis, with severe disease altering the cellular and metabolic composition of distal unaffected skin sites, according to a new analysis using single-cell transcriptomic technology.
On the surface, psoriasis severity is identified based on the visible lesions, Rochelle L. Castillo, MD, of the division of rheumatology and the NYU Psoriatic Arthritis Center, NYU Langone Health, New York, and colleagues wrote in their study, published in Science Immunology. Although cellular and molecular features of inflammatory skin diseases such as psoriasis have been characterized, activity at the tissue level and its systemic impact has not been explored.
“Our initial goal was to find measurable molecular signals that could tell us who is more likely to develop severe psoriasis, as well as who is at higher risk of developing related disorders that often accompany psoriasis, such as arthritis and cardiovascular disease,” study co–senior investigator Jose Scher, MD, director of the Psoriatic Arthritis Center and the Judith and Stewart Colton Center for Autoimmunity at NYU Langone Health, said in a press release accompanying the publication of the findings. “Having found signals with potential systemic consequences, we are now working to understand how skin inflammation can lead to widespread disease affecting other organs,”
In the study, the researchers used spatial transcriptomics, a technique that positions tissue sections onto genetic arrays to determine gene expression by cell type and histological location, helping to create a broad image-based map of where certain cell types are located in tissues and with what other cells they are communicating. They characterized the cell activity of skin samples from 11 men and women with mild to severe psoriasis/psoriatic arthritis, and three healthy adults who did not have psoriasis. They defined the cellular composition of 25 healthy skin biopsies and matched skin biopsies from psoriatic lesional and nonlesional skin, and identified 17 distinct clusters of cells, which they grouped into epidermal, dermis, pilosebaceous, and adipose categories.
The researchers found that cell activity associated with inflammation, as shown by clusters of fibroblasts and dermal macrophages, was more common in the upper layers of the skin in samples from patients with more severe psoriasis, compared with healthy control samples.
They also examined patterns of immune activity at the cellular level and found significant patterns around the upper follicle, around the perifollicular dermis, and within the hair follicle, where immune cells were enriched in healthy skin. Other cells enriched in these upper layer areas in healthy skin included dendritic cells, innate lymphoid cells, T helper cells, T cytotoxic cells, and myeloid cells.
Clusters of fibroblasts and macrophages, which are associated with inflammation, were clustered in psoriatic lesional skin, which also showed more inflammation at the dermal and suprabasal epidermal levels. B lymphocytes also were more prevalent in lesional skin.
The researchers then analyzed the skin samples according to disease severity; mild psoriasis was defined as a Psoriasis Area and Severity Index score less than 12; moderate to severe disease was defined as a PASI score of 12 or higher. The macrophage, fibroblast, and lymphatic endothelium–associated clusters distinguished mild and moderate to severe endotypes.
The pathology of moderate to severe psoriasis in lesional and nonlesional skin showed the extensive effects of psoriasis-related inflammation. Although nonlesional mild disease was clustered with healthy skin, in cases of moderate to severe disease, nonlesional and lesional groups were clustered together. This effect was segregated according to disease severity, independent of the presence of joint disease, and “was particularly evident in distal, nonlesional samples,” the researchers wrote.
The researchers also found evidence of increased gene activity in more than three dozen molecular pathways associated with metabolism and lipid levels in areas of lesional and nonlesional skin, Dr. Scher said.
The findings were limited by several factors including the small sample size and the limits of spatial transcriptomics technology resolution, the researchers wrote. “As this technology evolves, platforms with higher density, and by extension, resolution, of spatially barcoded beads will provide more granularity about cellular microenvironments in healthy and diseased states.”
The study was supported by the National Institutes of Health, the National Psoriasis Foundation, the NYU Colton Center for Autoimmunity, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, the Beatrice Snyder Foundation, The Riley Family Foundation, the Rheumatology Research Foundation, and the NY Stem Cell Foundation. Dr. Castillo had no financial conflicts to disclose. Dr. Scher has served as a consultant for Janssen, Abbvie, Novartis, Pfizer, Sanofi, UCB, and Bristol-Myers Squibb, and has received research funding from Janssen and Pfizer.
FROM SCIENCE IMMUNOLOGY
A Joint Effort to Save the Joints: What Dermatologists Need to Know About Psoriatic Arthritis
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
Nearly all dermatologists are aware that psoriatic arthritis (PsA) is one of the most prevalent comorbidities associated with psoriasis, yet we may lack the insight regarding how to utilize this information. After all, we specialize in the skin, not the joints, right?
When I graduated from residency in 2014, I began staffing our psoriasis clinic, where we care for the toughest, most complicated psoriasis patients, many of them struggling with both severe recalcitrant psoriasis as well as debilitating PsA. In 2016, we partnered with rheumatology to open a multidisciplinary psoriasis and PsA clinic, and I quickly began to appreciate how much PsA was being overlooked simply because patients with psoriasis were not being asked about their joints.
To start, let’s look at several facts:
- One quarter of patients with psoriasis also have PsA.1
- Skin disease most commonly develops before PsA.1
- Fifteen percent of PsA cases go undiagnosed, which dramatically increases the risk for deformed joints, erosions, osteolysis, sacroiliitis, and arthritis mutilans2 and also increases the cost of health care.3
- Everyone is crazy busy—rheumatology wait lists often are months long.
Given that dermatologists are the ones who already are seeing the majority of patients who develop PsA, we play a key role in screening for this debilitating comorbidity and starting therapy for patients with both psoriasis and PsA. We, too, are crazy busy; therefore, we need to make this process quick and efficient but also reliable. Fortunately, the Psoriasis Epidemiology Screening Tool (PEST) is effective, fast, and very easy. With only 5 questions and a sensitivity and specificity of around 70%,4 this short and simple questionnaire can be incorporated into an intake form or rooming note or can just be asked during the visit. The questions include whether the patient currently has or has had a swollen joint, nail pits, heel pain, and/or dactylitis, as well as if they have been told by a physician that they have arthritis. A score of 3 or higher is considered positive and a referral to rheumatology should be considered. At the bare minimum, I highly encourage all dermatologists to incorporate the PEST screening tool into their practice.
During the physical examination itself, be sure to look at the patient’s nails and also look for joint swelling and redness, especially in the hands. When palpating a swollen joint, the presence of inflammatory arthritis will feel spongy or boggy, while the osteophytes associated with osteoarthritis will feel hard. Radiography of the affected joint may be helpful, but keep in mind that bone changes are latter sequelae of PsA, and negative radiographs do not rule out PsA.
If you highly suspect PsA after using the PEST screening tool and palpating any swollen joints, then a rheumatology referral certainly is warranted. Medication that covers both psoriasis and PsA also can be initiated. Although methotrexate often is used for joints, higher doses (ie, >15 mg/wk) usually are needed. A 2019 Cochrane review found that low-dose methotrexate (ie, ≤15 mg/wk) may be only slightly more effective then placebo5—certainly not a ringing endorsement for its use in PsA. Additionally, quality data demonstrating methotrexate’s efficacy for enthesitis or axial spondyloarthritis is lacking, and methotrexate has not demonstrated an ability to slow the radiographic progression of joints. In contrast, the anti–tumor necrosis factor agents, including adalimumab, infliximab, etanercept, and certolizumab, as well as ustekinumab and the anti–IL-17 biologics secukinumab and ixekizumab have demonstrated efficacy in American College of Rheumatology (ACR) scores, enthesitis, dactylitis, and prevention of radiographic progression of joints.6,7 Although brodalumab, an anti–IL-17 receptor inhibitor, demonstrated improvement in ACR scores, enthesitis, and dactylitis, data on its effects on radiographic progression of joints were inconclusive given the phase III trial’s premature ending due to suicidal ideation and behavior in participants.8 Several of the anti–IL-23 agents also may help PsA, with trials demonstrating improvements in ACR scores, enthesitis, and dactylitis; however, only guselkumab 100 mg every 4 weeks decreased radiographic progression of joints.9 Additionally, with the age of the Janus kinase (JAK) inhibitor upon us, there are several JAK/TYK2 inhibitors that are approved by the US Food and Drug Administration for psoriasis (deucravacitinib) as well as for PsA (tofacitinib, upadacitinib), and there are more JAK inhibitors in the pipeline. These medications are effective; however, I do encourage caution and careful consideration in selecting the appropriate patient, as data demonstrated an increased risk for major adverse cardiovascular events and cancer in older (>50 years) rheumatoid arthritis patients who had at least 1 cardiovascular risk factor and were treated with tofacitinib.10 Although several other trials have not demonstrated this increased risk, further data are needed to determine risk for both pan-JAK inhibitors as well as selective JAK inhibitors and TYK2 inhibitors. Additionally, given psoriasis already is closely linked with many cardiovascular risk factors including heart disease, obesity, hypertension, hyperlipidemia, and diabetes mellitus,11 it will be important to have long-term safety information for JAK inhibitors in the psoriasis and PsA population.
Dermatologists are in a pivotal position to identify patients affected by PsA and start an appropriate systemic medication. We can help make an enormous impact on our patients’ lives as well as help decrease the economic impact of untreated disease. Let’s join the effort to save the joints!
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.
- Alinaghi F, Calov M, Kristensen L, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80:251-265.
- Villani A, Zouzaud M, Sevrain M, et al. Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol. 2015;73:242-248.
- Iragorri N, Hazlewood G, Manns B, et al. Model to determine the cost-effectiveness of screening psoriasis patients for psoriatic arthritis. Arth Car Res. 2021;73:266-274.
- Karreman M, Weel A, Van der Ven M, et al. Performance of screening tools for psoriatic arthritis: a cross-sectional study in primary care. Rheumatology. 2017;56:597-602.
- Wilsdon TD, Whittle SL, Thynne TR, et al. Methotrexate for psoriatic arthritis. Cochrane Database Syst Rev. 2019;1:CD012722. doi:10.1002/14651858.CD012722.pub2
- Mourad A, Gniadecki R. Treatment of dactylitis and enthesitis in psoriatic arthritis with biologic agents: a systematic review and metaanalysis. J Rheum. 2020;47:59-65.
- Wu D, Li C, Zhang S, et al. Effect of biologics on radiographic progression of peripheral joint in patients with psoriatic arthritis: meta-analysis. Rheumatology (Oxford). 2020;59:3172-3180.
- Mease P, Helliwell P, Fjellhaugen Hjuler K, et al. Brodalumab in psoriatic arthritis: results from the randomised phase III AMVISION-1 and AMVISION-2 trials. Ann Rheum Dis. 2021;80:185-193.
- McInnes I, Rahman P, Gottlieb A, et al. Long-term efficacy and safety of guselkumab, a monoclonal antibody specific to the p19 subunit of interleukin-23, through two years: results from a phase III, randomized, double-blind, placebo-controlled study conducted in biologic-naïve patients with active psoriatic arthritis. Arth Rheum. 2022;74:475-485.
- Ytterberg S, Bhatt D, Mikuls T, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- Miller I, Ellervik C, Yazdanyar S, et al. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. JAAD. 2013;69:1014-1024.