User login
Apremilast shows promise for mild-to-moderate psoriasis in phase 3
Key clinical point: Apremilast demonstrated a significant and clinically meaningful improvement in overall psoriasis severity compared with placebo in patients with mild-to-moderate psoriasis with no new safety signals identified.
Major finding: At week 16, a significantly greater proportion of patients treated with apremilast vs placebo achieved static Physician Global Assessment score of 0 or 1 with 2-point or more reduction from baseline (21.6% vs 4.1%; P < .0001). Most common treatment-emergent adverse events with apremilast vs placebo were diarrhea (16.4% vs 5.1%), headache (13.1% vs 5.1%), and nausea (12.8% vs 4.4%).
Study details: ADVANCE, a phase 3 trial included 595 adults with mild-to-moderate psoriasis inadequately controlled or intolerant to 1 or more topical therapy who were randomly assigned to either apremilast or placebo.
Disclosures: This study was sponsored by Amgen Inc. Some of the authors reported receiving honoraria, grants, and/or research funding and serving as a speaker, investigator, and/or advisory board member for various sources including Amgen Inc. M Chen, M Paris, and Y Wang declared being current/former employees at Amgen Inc.
Source: Gold LS et al. J Am Acad Dermatol. 2021 Aug 2. doi: 10.1016/j.jaad.2021.07.040.
Key clinical point: Apremilast demonstrated a significant and clinically meaningful improvement in overall psoriasis severity compared with placebo in patients with mild-to-moderate psoriasis with no new safety signals identified.
Major finding: At week 16, a significantly greater proportion of patients treated with apremilast vs placebo achieved static Physician Global Assessment score of 0 or 1 with 2-point or more reduction from baseline (21.6% vs 4.1%; P < .0001). Most common treatment-emergent adverse events with apremilast vs placebo were diarrhea (16.4% vs 5.1%), headache (13.1% vs 5.1%), and nausea (12.8% vs 4.4%).
Study details: ADVANCE, a phase 3 trial included 595 adults with mild-to-moderate psoriasis inadequately controlled or intolerant to 1 or more topical therapy who were randomly assigned to either apremilast or placebo.
Disclosures: This study was sponsored by Amgen Inc. Some of the authors reported receiving honoraria, grants, and/or research funding and serving as a speaker, investigator, and/or advisory board member for various sources including Amgen Inc. M Chen, M Paris, and Y Wang declared being current/former employees at Amgen Inc.
Source: Gold LS et al. J Am Acad Dermatol. 2021 Aug 2. doi: 10.1016/j.jaad.2021.07.040.
Key clinical point: Apremilast demonstrated a significant and clinically meaningful improvement in overall psoriasis severity compared with placebo in patients with mild-to-moderate psoriasis with no new safety signals identified.
Major finding: At week 16, a significantly greater proportion of patients treated with apremilast vs placebo achieved static Physician Global Assessment score of 0 or 1 with 2-point or more reduction from baseline (21.6% vs 4.1%; P < .0001). Most common treatment-emergent adverse events with apremilast vs placebo were diarrhea (16.4% vs 5.1%), headache (13.1% vs 5.1%), and nausea (12.8% vs 4.4%).
Study details: ADVANCE, a phase 3 trial included 595 adults with mild-to-moderate psoriasis inadequately controlled or intolerant to 1 or more topical therapy who were randomly assigned to either apremilast or placebo.
Disclosures: This study was sponsored by Amgen Inc. Some of the authors reported receiving honoraria, grants, and/or research funding and serving as a speaker, investigator, and/or advisory board member for various sources including Amgen Inc. M Chen, M Paris, and Y Wang declared being current/former employees at Amgen Inc.
Source: Gold LS et al. J Am Acad Dermatol. 2021 Aug 2. doi: 10.1016/j.jaad.2021.07.040.
Bimekizumab approved in Europe for psoriasis treatment
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.
, according to a statement from the manufacturer.
Bimekizumab (Bimzelx), a humanized IgG1 monoclonal antibody, is the first approved treatment for moderate to severe plaque psoriasis that selectively inhibits interleukin (IL)–17A and IL-17F, the statement from UCB said.
In the United States, the Food and Drug Administration is expected to make a decision on approval of bimekizumab for treating psoriasis on Oct. 15.
Approval in the EU was based on data from three phase 3 trials including a total of 1,480 adult patients with moderate to severe psoriasis, which found that those treated with bimekizumab experienced significantly greater skin clearance, compared with placebo, ustekinumab, and adalimumab, with a favorable safety profile, according to the company.
In all three studies (BE VIVID, BE READY, and BE SURE), more than 80% of patients treated with bimekizumab showed improved skin clearance after 16 weeks, significantly more than those treated with ustekinumab, placebo, or adalimumab, based on an improvement of at least 90% in the Psoriasis Area & Severity Index (PASI 90) and an Investigator’s Global Assessment (IGA) response of clear or almost clear skin (IGA 0/1). In all three studies, these clinical responses persisted after 1 year.
The recommended dose of bimekizumab is 320 mg, given in two subcutaneous injections every 4 weeks to week 16, then every 8 weeks. However, for “some patients” weighing 120 kg or more who have not achieved complete skin clearance at 16 weeks, 320 mg every 4 weeks after that time may improve response to treatment, according to the company statement.
The most common treatment-related adverse events in the studies were upper respiratory tract infections (a majority of which were nasopharyngitis), reported by 14.5% of patients, followed by oral candidiasis, reported by 7.3%.
Results of BE READY and BE VIVID were published in The Lancet. Results of the BE SURE study were published in The New England Journal of Medicine.
Bimekizumab is contraindicated for individuals with clinically important active infections such as tuberculosis, and for individuals with any hypersensitivity to the active substance. More details on bimekizumab are available on the website of the European Medicines Agency.
Psoriatic arthritis health care costs continue to rise over time
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Annual health care costs for patients with psoriatic arthritis rose over recent 5-year periods across all categories of resource use to a significantly greater extent than among patients with psoriasis only or those without any psoriatic disease diagnoses, according to commercial insurance claims data.
Using an IBM MarketScan Commercial Database, researchers examined claims data for 208,434 patients with psoriasis, 47,274 with PsA, and 255,708 controls who had neither psoriasis nor PsA. Controls were matched for age and sex. Those with RA, ankylosing spondylitis, Crohn’s disease, or ulcerative colitis were excluded.
The investigators examined data for 2009-2020, following patients for 5 years within that period. They looked at hospitalizations, outpatient and pharmacy services, lab services, and office visits, Steven Peterson, director of market access for rheumatology at Janssen Pharmaceuticals, said in his presentation of the data at the Pan American League of Associations for Rheumatology 2021 annual meeting, held recently as a virtual event.
The research was also published online May 2, 2021, in Clinical Rheumatology.
Big differences between the groups were seen in the first year, when the average health care costs for the PsA group were $28,322, about half of which was outpatient drug costs. That compared with $12,039 for the psoriasis group and $6,672 for the control group.
The differences tended to widen over time. By the fifth year, average costs for the PsA group were $34,290, nearly 60% of which were drug costs. That compared with $12,877 for the psoriasis group and $8,569 for the control group. In each year examined, outpatient drug costs accounted for less than half of the expenses for the psoriasis group and about a quarter for the control group.
Researchers found that the PsA group needed 28.7 prescriptions per person per year, compared with 17.0 and 12.7 in the psoriasis and control groups, respectively, Mr. Peterson said. He also noted that patients with PsA and psoriasis tended to have higher rates of hypertension, depression, and anxiety.
“The cost and resource utilization disparity between these patient groups demonstrates the high remaining unmet medical need for patients with psoriasis and psoriatic arthritis,” Mr. Peterson said during the virtual proceedings.
Do findings reflect treatment advances?
Elaine Husni, MD, MPH, director of the Arthritis and Musculoskeletal Center at the Cleveland Clinic, where she studies health outcomes in PsA, said the findings are helpful in pointing to a trend across a large sample. But she added it’s important to remember that the increasing costs could reflect recent advances in PsA treatment, which include costly biologic drugs.
“There’s a ton more treatments for psoriasis and psoriatic arthritis than there were even just 5 years ago,” she said in an interview. She was not involved in the research.
Dr. Husni would like to see a more detailed look at the costs, from the categories of expenses to the patients who are incurring the highest costs.
“Is it just a couple of percent of really sick patients that are driving the psoriatic arthritis group?” she wondered.
She also pointed out that PsA is going to be more expensive by its very nature. PsA tends to develop 3-10 years after psoriasis, adding to the costs for someone who already has psoriasis and at a time when they are older and likely have higher health care costs because of comorbidities that develop with age.
Dr. Husni said she does think about treatment costs, in that a less expensive first-line drug might be more appropriate than going straight to a more expensive biologic, especially because they also tend to be safer. She said it’s not just a simple question of curbing costs.
“Is there a way that we can personalize medicine?” she asked. “Is there a way that we can be more accurate about which people may need the more expensive drugs, and which patients may need the less expensive drugs? Are we getting better at monitoring so we can avoid high-cost events?”
Mr. Peterson is an employee of Janssen Pharmaceuticals. Dr. Husni reported serving as a consultant to AbbVie, Amgen, Bristol-Myers Squibb, UCB, Novartis, Lilly, and Pfizer.
* Update, 9/28/21: The headline and parts of this story were updated to better reflect the study on which it reports.
A version of this article first appeared on Medscape.com.
Translating the 2019 AAD-NPF Guidelines of Care for Psoriasis With Attention to Comorbidities
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.

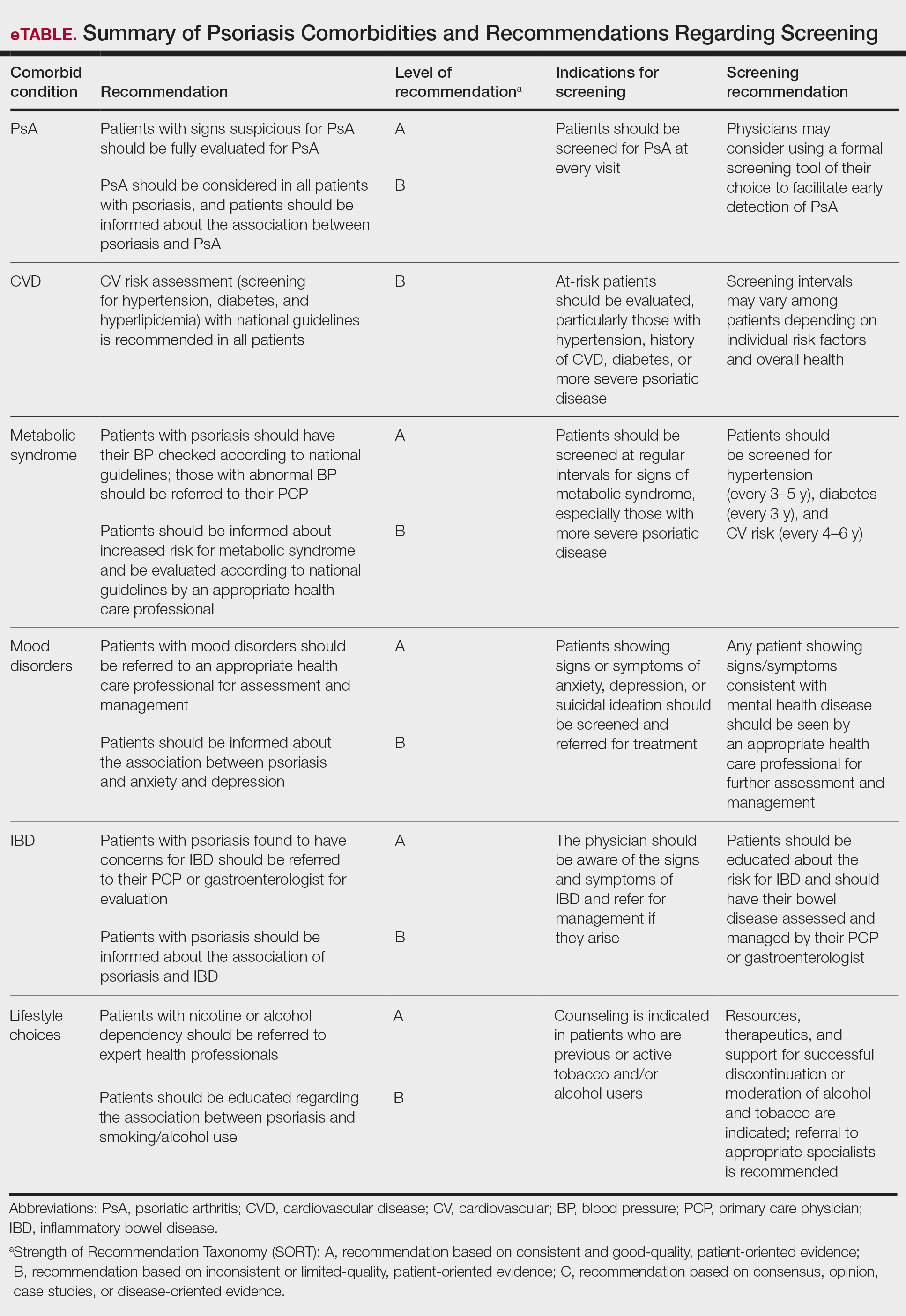
Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
Psoriasis is a chronic and relapsing systemic inflammatory disease that predisposes patients to a host of other conditions. It is believed that these widespread effects are due to chronic inflammation and cytokine activation, which affect multiple body processes and lead to the development of various comorbidities that need to be proactively managed.
In April 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released recommendation guidelines for managing psoriasis in adults with an emphasis on common disease comorbidities, including psoriatic arthritis (PsA), cardiovascular disease (CVD), inflammatory bowel disease (IBD), metabolic syndrome, and mood disorders. Psychosocial wellness, mental health, and quality of life (QOL) measures in relation to psoriatic disease also were discussed.1
The AAD-NPF guidelines address current screening, monitoring, education, and treatment recommendations for the management of psoriatic comorbidities. The Table and eTable summarize the screening recommendations. These guidelines aim to assist dermatologists with comprehensive disease management by addressing potential extracutaneous manifestations of psoriasis in adults.


Screening and Risk Assessment
Patients with psoriasis should receive a thorough history and physical examination to assess disease severity and risk for potential comorbidities. Patients with greater disease severity—as measured by body surface area (BSA) involvement and type of therapy required—have a greater risk for other disease-related comorbidities, specifically metabolic syndrome, renal disease, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea, uveitis, IBD, malignancy, and increased mortality.2 Because the likelihood of comorbidities is greatest with severe disease, more frequent monitoring is recommended for these patients.
Psoriatic Arthritis
Patients with psoriasis need to be evaluated for PsA at every visit. Patients presenting with signs and symptoms suspicious for PsA—joint swelling, peripheral joint involvement, and joint inflammation—warrant further evaluation and consultation. Early detection and treatment of PsA is essential for preventing unnecessary suffering and progressive joint destruction.3
There are several PsA screening questionnaires currently available, including the Psoriatic Arthritis Screening Evaluation, Psoriasis Epidemiology Screening Tool, and Toronto Psoriatic Arthritis Screen. No significant differences in sensitivity and specificity were found among these questionnaires when using the Classification Criteria for Psoriatic Arthritis as the gold standard. All 3 questionnaires—the Psoriatic Arthritis Screening Evaluation and the Psoriasis Epidemiology Screening Tool were developed for use in dermatology and rheumatology clinics, and the Toronto Psoriatic Arthritis Screen was developed for use in the primary care setting—were found to be effective in dermatology/rheumatology clinics and primary care clinics, respectively.3 False-positive results predominantly were seen in patients with degenerative joint disease or osteoarthritis. Dermatologists should conduct a thorough physical examination to distinguish PsA from degenerative joint disease. Imaging and laboratory tests to evaluate for signs of systemic inflammation (erythrocyte sedimentation rate, C-reactive protein) also can be helpful in distinguishing the 2 conditions; however, these metrics have not been shown to contribute to PsA diagnosis.1 Full rheumatologic consultation is warranted in challenging cases.
Cardiovascular Disease
Primary care physicians (PCPs) are recommended to screen patients for CVD risk factors using height, weight, blood pressure, blood glucose, hemoglobin A1C, lipid levels, abdominal circumference, and body mass index (BMI). Lifestyle modifications such as smoking cessation, exercise, and dietary changes are encouraged to achieve and maintain a normal BMI.
Dermatologists also need to give special consideration to comorbidities when selecting medications and/or therapies for disease management. Patients on TNF inhibitors have a lower risk for MI compared with patients using topical medications, phototherapy, and other oral agents.10 Additionally, patients on TNF inhibitors have a lower risk for occurrence of major adverse cardiovascular events compared with patients treated with methotrexate or phototherapy.11,12
Metabolic Syndrome
Numerous studies have demonstrated an association between psoriasis and metabolic syndrome. Patients with increased BSA involvement and
The association between psoriasis and weight loss has been analyzed in several studies. Weight loss, particularly in obese patients, has been shown to improve psoriasis severity, as measured by psoriasis area and severity index score and QOL measures.15 Another study found that gastric bypass was associated with a significant risk reduction in the development of psoriasis (P=.004) and the disease prognosis (P=.02 for severe psoriasis; P=.01 for PsA).16 Therefore, patients with moderate to severe psoriasis are recommended to have their obesity status determined according to national guidelines. For patients with a BMI above 40 kg/m2 and standard weight-loss measures fail, bariatric surgery is recommended. Additionally, the impact of psoriasis medications on weight has been studied. Apremilast has been associated with weight loss, whereas etanercept and infliximab have been linked to weight gain.17,18
An association between psoriasis and hypertension also has been demonstrated by studies, especially among patients with severe disease. Therefore, patients with moderate to severe psoriasis are recommended to have their blood pressure evaluated according to national guidelines, and those with a blood pressure of 140/90 mm Hg or higher should be referred to their PCP for assessment and treatment. Current evidence does not support restrictions on antihypertensive medications in patients with psoriasis. Physicians should be aware of the potential for cyclosporine to induce hypertension, which should be treated, specifically with amlodipine.19
Many studies have demonstrated an association between psoriasis and dyslipidemia, though the results are somewhat conflicting. In 2018, the American Heart Association and the American College of Cardiology deemed psoriasis as an atherosclerotic CVD risk-enhancing condition, favoring early initiation of statin therapy. Because dyslipidemia plays a prominent role in atherosclerosis and CVD, patients with moderate to severe psoriasis are recommended to undergo periodic screening with lipid tests (eg, fasting total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides).20 Patients with elevated fasting triglycerides or low-density lipoprotein cholesterol should be referred to their PCP for further management. Certain psoriasis medications also have been linked to dyslipidemia. Acitretin and cyclosporine are known to adversely affect lipid levels, so patients treated with either agent should undergo routine monitoring of serum lipid levels.
Psoriasis is strongly associated with diabetes mellitus. Because of the increased risk for diabetes in patients with severe disease, regular monitoring of fasting blood glucose and/or hemoglobin A1C levels in patients with moderate to severe psoriasis is recommended. Patients who meet criteria for prediabetes or diabetes should be referred to their PCP for further assessment and management.21,22
Mood Disorders
Psoriasis affects QOL and can have a major impact on patients’ interpersonal relationships. Studies have shown an association between psoriasis and mood disorders, specifically depression and anxiety. Unfortunately, patients with mood disorders are less likely to seek intervention for their skin disease, which poses a tremendous treatment barrier. Dermatologists should regularly monitor patients for psychiatric symptoms so that resources and treatments can be offered.
Certain psoriasis therapies have been shown to help alleviate associated depression and anxiety. Improvements in Beck Depression Inventory and Hamilton Depression Rating Scale scores were seen with etanercept.23 Adalimumab and ustekinumab showed improvement in Dermatology Life Quality Index compared with placebo.24,25 Patients receiving Goeckerman treatment also had improvement in anxiety and depression scores compared with conventional therapy.26 Biologic medications had the largest impact on improving depression symptoms compared with conventional systemic therapy and phototherapy.27 The recommendations support use of biologics and the Goeckerman regimen for the concomitant treatment of mood disorders and psoriasis.
Renal Disease
Studies have supported an association between psoriasis and chronic kidney disease (CKD), independent of risk factors including vascular disease, hypertension, and diabetes. The prevalence of moderate to advanced CKD also has been found to be directly related to increasing BSA affected by psoriasis.28 Patients should receive testing of blood urea nitrogen, creatinine, and urine microalbumin levels to assess for occult renal disease. In addition, physicians should be cautious when prescribing nephrotoxic drugs (nonsteroidal anti-inflammatory drugs and cyclosporine) and renally excreted agents (methotrexate and apremilast) because of the risk for underlying renal disease in patients with psoriasis. If newly acquired renal disease is suspected, physicians should withhold the offending agents. Patients with psoriasis with CKD are recommended to follow up with their PCP or nephrologist for evaluation and management.
Pulmonary Disease
Psoriasis also has an independent association with COPD. Patients with psoriasis have a higher likelihood of developing COPD (hazard ratio, 2.35; 95% CI, 1.42-3.89; P<.01) than controls.29 The prevalence of COPD also was found to correlate with psoriasis severity. Dermatologists should educate patients about the association between smoking and psoriasis as well as advise patients to discontinue smoking to reduce their risk for developing COPD and cancer.
Patients with psoriasis also are at an increased risk for obstructive sleep apnea. Obstructive sleep apnea should be considered in patients with risk factors including snoring, obesity, hypertension, or diabetes.
Inflammatory Bowel Disease
Patients with psoriasis have an increased risk for developing IBD. The prevalence ratios of both Crohn disease (2.49) and ulcerative colitis (1.64) are increased in patients with psoriasis relative to patients without psoriasis.30 Physicians need to be aware of the association between psoriasis and IBD and the effect that their coexistence may have on treatment choice for patients.
Adalimumab and infliximab are approved for the treatment of IBD, and certolizumab and ustekinumab are approved for Crohn disease. Use of TNF inhibitors in patients with IBD may cause psoriasiform lesions to develop.31 Nonetheless, treatment should be individualized and psoriasiform lesions treated with standard psoriasis measures. Psoriasis patients with IBD are recommended to avoid IL-17–inhibitor therapy, given its potential to worsen IBD flares.
Malignancy
Psoriasis patients aged 0 to 79 years have a greater overall risk for malignancy compared with patients without psoriasis.32 Patients with psoriasis have an increased risk for respiratory tract cancer, upper aerodigestive tract cancer, urinary tract cancer, and non-Hodgkin lymphoma.33 A mild association exists between PsA and lymphoma, nonmelanoma skin cancer (NMSC), and lung cancer.34 More severe psoriasis is associated with greater risk for lymphoma and NMSC. Dermatologists are recommended to educate patients on their risk for certain malignancies and to refer patients to specialists upon suspicion of malignancy.
Risk for malignancy has been shown to be affected by psoriasis treatments. Patients treated with UVB have reduced overall cancer rates for all age groups (hazard ratio, 0.52; P=.3), while those treated with psoralen plus UVA have an increased incidence of
Lifestyle Choices and QOL
A crucial aspect of successful psoriasis management is patient education. The strongest recommendations support lifestyle changes, such as smoking cessation and limitation of alcohol use. A tactful discussion regarding substance use, work productivity, interpersonal relationships, and sexual function can address substantial effects of psoriasis on QOL so that support and resources can be provided.
Final Thoughts
Management of psoriasis is multifaceted and involves screening, education, monitoring, and collaboration with PCPs and specialists. Regular follow-up with a dermatologist and PCP is strongly recommended for patients with psoriasis given the systemic nature of the disease. The 2019 AAD-NPF recommendations provide important information for dermatologists to coordinate care for complicated psoriasis cases, but clinical judgment is paramount when making medical decisions. The consideration of comorbidities is critical for developing a comprehensive treatment approach, and this approach will lead to better health outcomes and improved QOL for patients with psoriasis.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
- Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
- Gelfand JM, Troxel AB, Lewis JD, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. 2007;143:1493-1499.
- Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168:802-807.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168-3209.
- Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136:263-276.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Dunlay SM, Weston SA, Redfield MM, et al. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation. 2008;118:625-631.
- Russell SD, Saval MA, Robbins JL, et al. New York Heart Association functional class predicts exercise parameters in the current era. Am Heart J. 2009;158(4 suppl):S24-S30.
- Wu JJ, Poon K-YT, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
- Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
- Wu JJ, Sundaram M, Cloutier M, et al. The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol. 2018;79:60-68.
- Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403-414.
- Langan SM, Seminara NM, Shin DB, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. 2012;132:556-562.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Egeberg A, Sørensen JA, Gislason GH, et al. Incidence and prognosis of psoriasis and psoriatic arthritis in patients undergoing bariatric surgery. JAMA Surg. 2017;152:344-349.
- Crowley J, Thaçi D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for ≥156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1. doi:10.1016/j.jaad.2017.01.052
- Gisondi P, Del Giglio M, Di Francesco V, et al. Weight loss improves the response of obese patients with moderate-to-severe chronic plaque psoriasis to low-dose cyclosporine therapy: a randomized, controlled, investigator-blinded clinical trial. Am J Clin Nutr. 2008;88:1242-1247.
- Leenen FHH, Coletta E, Davies RA. Prevention of renal dysfunction and hypertension by amlodipine after heart transplant. Am J Cardiol. 2007;100:531-535.
- Goff DC Jr, Lloyd-Jones DM, Bennet G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation. 2014;129(suppl 2):S49-S73.
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14-S80.
- Ratner RE, Diabetes Prevention Program Research Group. An update on the diabetes prevention program. Endocr Pract. 2006;12(suppl 1):20-24.
- Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
- Kimball AB, Edson-Heredia E, Zhu B, et al. Understanding the relationship between pruritus severity and work productivity in patients with moderate-to-severe psoriasis: sleep problems are a mediating factor. J Drugs Dermatol. 2016;15:183-188.
- Langley RG, Tsai T-F, Flavin S, et al. Efficacy and safety of guselkumab in patients with psoriasis who have an inadequate response to ustekinumab: results of the randomized, double-blind, phase III NAVIGATE trial. Br J Dermatol. 2018;178:114-123.
- Chern E, Yau D, Ho J-C, et al. Positive effect of modified Goeckerman regimen on quality of life and psychosocial distress in moderate and severe psoriasis. Acta Derm Venereol. 2011;91:447-451.
- Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
- Wan J, Wang S, Haynes K, et al. Risk of moderate to advanced kidney disease in patients with psoriasis: population based cohort study. BMJ. 2013;347:f5961. doi:10.1136/bmj.f5961
- Chiang Y-Y, Lin H-W. Association between psoriasis and chronic obstructive pulmonary disease: a population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2012;26:59-65.
- Cohen AD, Dreiher J, Birkenfeld S. Psoriasis associated with ulcerative colitis and Crohn’s disease. J Eur Acad Dermatol Venereol. 2009;23:561-565.
- Denadai R, Teixeira FV, Saad-Hossne R. The onset of psoriasis during the treatment of inflammatory bowel diseases with infliximab: should biological therapy be suspended? Arq Gastroenterol. 2012;49:172-176.
- Chen Y-J, Wu C-Y, Chen T-J, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in Taiwan. J Am Acad Dermatol. 2011;65:84-91.
- Pouplard C, Brenaut E, Horreau C, et al. Risk of cancer in psoriasis: a systematic review and meta-analysis of epidemiological studies. J Eur Acad Dermatol Venereol. 2013;27(suppl 3):36-46.
- Chiesa Fuxench ZC, Shin DB, Ogdie Beatty A, et al. The risk of cancer in patients with psoriasis: a population-based cohort study in the health improvement network. JAMA Dermatol. 2016;152:282-290.
- Burmester GR, Panaccione R, Gordon KB, et al. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn’s disease. Ann Rheum Dis. 2013;72:517-524.
- Dommasch ED, Abuabara K, Shin DB, et al. The risk of infection and malignancy with tumor necrosis factor antagonists in adults with psoriatic disease: a systematic review and meta-analysis of randomized controlled trials. J Am Acad Dermatol. 2011;64:1035-1050.
- Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol. 2012;66:742-751.
Practice Points
- Educating patients about psoriasis and its extracutaneous manifestations, available treatment options, and the impact of lifestyle choices is advised to maximize their patient’s disease awareness and to promote a collaborative physician-patient partnership.
- Physicians are strongly recommended to screen patients with psoriasis for the presence of disease comorbidities to ensure comprehensive management of their disease.
- Managing psoriasis as a multisystem inflammatory disorder requires the combined effort of dermatologists and other specialists to prevent and treat disease comorbidities and enhance patients’ quality of life.
Psoriatic Arthritis Diagnosis and Management in the Era of Telehealth
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
With the rise of telehealth utilization during the COVID-19 pandemic, clinical care delivery has undergone a substantial shift. This is especially true in dermatology, as utilization of telehealth has jumped from under 15% to more than 95% of dermatologists after the COVID-19 pandemic.1 However, with this new form of care delivery, it is important to ensure that patients don’t get left behind, either due to socioeconomic/language barriers2 or hesitancy about the conditions being treated.
It may not be surprising to know that the idea of using telemedicine for rheumatology is not new. Indeed, a report from 20 years ago outlined the high level of both satisfaction with live interactive telehealth visits for rheumatologic conditions and diagnostic accuracy as compared to in-person visits.3 Through guided palpation and careful history taking, it is possible to conduct a thorough visit and even manage biologics, diagnose active arthritis/enthesitis via photographs, and evaluate pain through a visual analog scale.4 As far as dermatology is concerned, it is clear that certain situations seem to be better suited for teledermatology, such as follow-up visits for acne/rosacea.1 But what of psoriatic arthritis (PsA)? Does telehealth have the potential to mitigate our undertreatment of this important condition, which finds about half of patients being treated with only topical therapy or no treatment at all?5 Or can we modulate our visits to accommodate these patients, taking care of not only their visible psoriasis but also the underlying PsA?
Psoriasis is well suited for teledermatology management in general, especially once the diagnosis is made. Multiple studies have shown diagnostic equivalence with in-person care and even similar outcomes after treatment.6,7 However, most studies have looked at telemedicine primarily for cutaneous psoriasis, and translating this to screening for and management of PsA is paramount. After all, a delay of only 6 months in diagnosing and treating PsA has been associated with poor outcomes.8 Thankfully, we do have some tools that can help. There are 3 validated screening tools for PsA: the Psoriasis Epidemiology Screening Tool (PEST), the Psoriatic Arthritis Screening and Evaluation (PASE), and the Toronto Psoriatic Arthritis Screen (ToPAS) questionnaire.9 Of these, the PEST seems to be a reasonable option that is quick and easily deployed; it has shown strong performance in terms of sensitivity, specificity, and negative predictive value/positive predictive value when compared to similar screening tools.10 It also should be facile to direct patients to complete the screening tool, as an online version is available on the National Psoriasis Foundation’s website (https://www.psoriasis.org/psoriatic-arthritis-screening-test/) where patients can be directed to answer 5 simple questions and report back the outcome. For treatment decisions, this tool also can be used to help identify patients who are good candidates for systemic or biologic therapy or those who should see a rheumatologist. Of course, an in-depth discussion of joint pain, morning stiffness, and tender/swollen joints may be more fruitful but also more challenging to conduct. I would propose that this can be pared down to a more direct conversation about finger pain/tenderness, tenderness at the elbow/knee (lateral epicondyle/medial femoral condyle), or heel (Achilles) as more common sites of enthesitis, and questioning about back pain or stiffness that improves with movement.9 By combining the screening tool with these pointed questions, even via telehealth, we can greatly improve our yield in diagnosing PsA while only adding a minute or two to our visits. I’d argue that this is much more fruitful than asking the patient to contort their bodies and camera to show an obscure lesion!
It is interesting to consider areas in dermatology where we might make a notable impact on mortality and morbidity by expanding access to care. Earlier diagnosis of melanoma, for instance, certainly would be in consideration, especially in areas of the country where access to dermatologic care is challenging. Better management of PsA has to be up there on the list of conditions where we immediately can make a tangible difference; we have the tools to do so and excellent therapeutics that are safe and effective. Our colleagues in rheumatology have embraced telemedicine with a “how, not if” approach to embracing new technology,11 and it is about time that dermatology takes a similar attitude. The gap between access to dermatologic care in urban areas vs either nonmetropolitan or rural areas is increasing, and dermatology tends to be much more available in well-resourced, urban areas.12 There are patients who need our expertise, and if it takes the compromise of adopting a technology that sometimes gives us headaches (we’ve all been on video visits with a choppy signal and inadequate lighting), we still should try to figure out the best way to do it because it’s the right thing to do for these patients. If we don’t, the determination of how to conduct teledermatology care will be taken away from us and either insurance companies or corporations not guided by dermatologists may try to enter this health care void and decide how to provide these services.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
- Kennedy J, Arey S, Hopkins Z, et al. Dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Rodriguez JA, Saadi A, Schwamm LH, et al. Disparities in telehealth use among California patients with limited English proficiency. Health Aff (Millwood). 2021;40:487-495.
- Leggett P, Graham L, Steele K, et al. Telerheumatology—diagnostic accuracy and acceptability to patient, specialist, and general practitioner. Br J Gen Pract. 2001;51:746-748.
- Costa L, Tasso M, Scotti N, et al. Telerheumatology in COVID-19 era: a study from a psoriatic arthritis cohort [published online June 11, 2020]. Ann Rheum Dis. doi:10.1136/annrheumdis-2020-217806
- Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70:871-881; E871-E830.
- Armstrong AW, Chambers CJ, Maverakis E, et al. Effectiveness of online vs in-person care for adults with psoriasis: a randomized clinical trial. JAMA Netw Open. 2018;1:E183062.
- Koller S, Hofmann-Wellenhof R, Hayn D, et al. Teledermatological monitoring of psoriasis patients on biologic therapy. Acta Derm Venereol. 2011;91:680-685.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Gottlieb A, Merola JF. Psoriatic arthritis for dermatologists. J Dermatolog Treat. 2020;31:662-679.
- Urruticoechea-Arana A, Benavent D, Leon F, et al. Psoriatic arthritis screening: a systematic literature review and experts’ recommendations. PLoS One. 2021;16:E0248571.
- Bateman J, Cleaton N. Managing patients using telerheumatology: lessons from a pandemic. Best Pract Res Clin Rheumatol. 2021;35:101662.
- Feng H, Berk-Krauss J, Feng PW, et al. Comparison of dermatologist density between urban and rural counties in the United States. JAMA Dermatol. 2018;154:1265-1271.
Update on Biologics for Psoriasis in Clinical Practice
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
Biologics have transformed the management of moderate to severe psoriasis. There currently are 11 biologics approved by the US Food and Drug Administration (Table) for psoriasis treatment that have been affirmed by various clinical studies. This article provides dosing initiation, maintenance information, and updated clinical data using phase 3 studies (N=8) published between May 2020 and February 2021. Generic names of the 11 biologics were searched separately in the PubMed database within the specified date range. Subsequent results were reviewed by title and selected for phase 3 and 4 trials. Clinical data in this review focus on reducing patient disease burden by allocating a biologic best fit for each patient’s individual health profile.

IL-17A Inhibitors Update
Secukinumab is safe and efficacious for skin clearance in the presence of comorbidities and can be used for improving plaque psoriasis and palmoplantar pustular psoriasis. An extension of a phase 3 randomized controlled trial (RCT)—2PRECISE—evaluated the efficacy and safety of secukinumab dosing at 300 mg (n=79) and 150 mg (n=80) in adults with moderate to severe palmoplantar pustular psoriasis (palmoplantar psoriasis area and severity index [PPPASI] score ≥12 and dermatology life quality index [DLQI] ≥10) over 148 weeks.1 Extension patients were included from the 52-week 2PRECISE study per the investigator’s judgement of a meaningful clinical response (exact criteria not described). All treatment groups demonstrated a mean (SD) PPPASI of 22.7 (9.5) by the extension trial’s start. Results affirmed that clinical response waned after week 148 in all groups excluding placebo/secukinumab 150 mg, which maintained a mean (SD) PPPASI of 22.7 (9.5). The most frequent adverse events were nasopharyngitis, pustular psoriasis, headache, and pruritus.1
Comorbidities do not have a major impact on secukinumab’s efficacy. A post hoc analysis of 4 phase 3 RCTs—ERASURE, FIXTURE, FEATURE, and JUNCTURE—gathered data from adult patients (N=2401) to assess baseline comorbidities with efficacy and safety of secukinumab vs etanercept after 12 weeks of treatment.2 Sixty-one percent (n=1469) had at least 1 comorbidity, most frequently obesity, hypertension, psoriatic arthritis, hyperlipidemia, or diabetes mellitus. All patient groups had a greater likelihood of a psoriasis area and severity index (PASI) response with any dose of secukinumab vs patients with comorbidities who were taking etanercept or placebo (P<.05) at week 12. All groups had a greater likelihood of achieving investigator global assessment scores of 0/1 (clear/almost clear) vs patients with comorbidities taking etanercept or placebo (P<.05). Baseline comorbidities did not significantly affect treatment response, except obesity, which was associated with decreased probability of achieving all PASI and investigator global assessment (P<.01) responses. Secukinumab-treated patients with and without comorbidities had equivalent likelihood of treatment-emergent adverse events (TEAEs).2
Brodalumab is an effective biologic that has shown long-term safety with continuous administration. Continuous brodalumab and brodalumab after placebo demonstrated impactful skin clearance after 120 weeks in AMAGINE-1, a phase 3 RCT involving adults (N=442) with moderate to severe plaque psoriasis.3 Patients randomized to brodalumab 210 mg (n=222) or placebo (n=220) were rerandomized according to initial treatment response. In patients switching from brodalumab to placebo at week 12, 55% and 94% achieved PASI 75 at week 20 and week 120, respectively, and 75% reached PASI 100 at week 120. Of patients with static physician global assessment (sPGA) scores of 0/1 (clear/almost clear) at week 12 who were rerandomized to brodalumab, 96% and 80% (using observed data) achieved PASI 75 and PASI 100, respectively. Mean (SD) time to return of skin disease following withdrawal of brodalumab was 74.7 (50.5) days. Treatment-emergent adverse events included headaches, arthralgia, diarrhea, and nausea. Suicidal ideation was rare (this study had 1 completed suicide), and authors cited that no causal association has been made between brodalumab and suicidality. Brodalumab also demonstrated favorable treatment response in patients who underwent a lapse in treatment, offering real-world value, as intermittent treatment administration can occur because of personal or financial reasons.3
Ixekizumab is associated with more rapid skin clearance, better resolution of nail psoriasis, and superior improvement in quality-of-life measures when compared with guselkumab. The phase 3 study IXORA-R compared skin and nail clearance as well as patient-reported outcomes over 24 weeks with ixekizumab 80 mg (n=520) vs guselkumab 100 mg (n=507) in adults with moderate to severe plaque psoriasis.4 Ixekizumab (50%) was shown to be no worse than guselkumab (52%; difference, –2.3%) using a noninferiority test (noninferiority margin of –11.4%). The treatments exhibited similar efficacy, with no significant difference in proportion of patients reaching PASI 100 (P=.41). Ixekizumab patients tended to have skin clearance sooner than guselkumab patients, reaching PASI 50/75/90 and PASI 100 in a median time that was 2 weeks and 7.5 weeks earlier, respectively. More ixekizumab patients (52%) achieved clear nails vs guselkumab patients (31%; P=.007). Ixekizumab patients reported greater satisfaction with their skin disease affecting quality of life (DLQI), with more DLQI 0/1 (no effect at all on patient’s life) scores and being itch free (P<.05). Ixekizumab was associated with significantly more days of complete skin clearance (PASI 100) vs guselkumab (55.6 days vs 42.2 days; P<.001). Although an upper respiratory tract infection was the most common TEAE, the proportion of TEAEs was similar between treatments.4
IL-23 Inhibitors Update
Tildrakizumab has similar long-term skin clearance efficacy and safety in patients with psoriasis with and without comorbid metabolic syndrome (MetS). A post hoc analysis of 2 phase 2 RCTs (reSURFACE 1/2) involving adults (N=338 and N=307) with moderate to severe plaque psoriasis assessed long-term efficacy (3 years), drug survival, and safety for 5 years of continuous tildrakizumab 100 mg and 200 mg in adults with comorbid MetS.5 Although no difference in efficacy was concluded, greater body mass index of the MetS population was shown to be associated with lower biologic efficacy compared to the general population. The proportion of patients who achieved PASI 75 at week 52 was comparable in patients with MetS and patients without MetS (tildrakizumab 100 mg, 85% and 86% vs 86% and 94% for reSURFACE 1/2, respectively; tildrakizumab 200 mg, 76% and 87% vs 76% and 87% for reSURFACE 1/2, respectively).5
Tildrakizumab also demonstrated efficacy and safety for up to 5 years in 2 other phase 3 RCTs with no dose-related differences in frequency of injections and malignancies. Tildrakizumab 100 mg is the recommended dose. The 200-mg dose can be utilized in patients with a high burden of disease and disability. reSURFACE 1 and reSURFACE 2 involved adults with chronic moderate to severe plaque psoriasis randomized to tildrakizumab 100 mg, 200 mg, or placebo with the option of long-term extension to week 244 if patients reached 50% or greater improvement from baseline PASI score.6 Patients in reSURFACE 2 also were randomized to etanercept 50 mg with partial responders and nonresponders at week 28 switching to tildrakizumab 200 mg until week 244. Extension results showed PASI 75 achievement in 88.7% (95% CI, 84.6%-92.1%) of patients taking tildrakizumab 100 mg (n=235), 92.5% (95% CI, 88.1%-95.7%) of patients taking tildrakizumab 200 mg (n=176), and 81.3% (95% CI, 72.6%-88.2%) of patients taking etanercept/partial nonresponders (n=85). The most common TEAE was nasopharyngitis (10.5/100 patient-years for tildrakizumab 100 mg and 10.7/100 patient-years for tildrakizumab 200 mg). The frequency of severe infections (eg, diverticulitis, pneumonia, cellulitis, appendicitis) was 1.2 per 100 patient-years for tildrakizumab 100 mg and 1.3 per 100 patient-years for tildrakizumab 200 mg.6
Risankizumab and tildrakizumab require the lowest number of injections, thereby providing sustainable skin clearance with a convenient injection dosing schedule for patients. Risankizumab efficacy (8.2% with inferiority margin of 12%) was noninferior to secukinumab when assessing the proportion of PASI 90 responders at week 16 (after 2 doses of risankizumab vs 7 doses of secukinumab).7 IMMerge, an international phase 3 RCT, involved adults (N=327) with moderate to severe plaque psoriasis to compare the safety and efficacy of risankizumab 150 mg (n=164) vs secukinumab 300 mg (n=163) up to 52 weeks. A greater proportion of the risankizumab arm (86.6%) achieved PASI 90 in 52 weeks compared to the secukinumab arm (57.1%). Superior skin clearance (PASI 90) at week 52 was achieved after 5 doses with risankizumab vs 16 doses of secukinumab. Risankizumab TEAEs were nasopharyngitis, upper respiratory tract infection, headache, arthralgia, diarrhea, and bronchitis.7
Continuous risankizumab treatment shows substantially stronger skin clearing performance compared with intermittent treatment following drug withdrawal, demonstrating that treatment gaps minimize therapeutic response. IMMhance, an international phase 3 RCT involving adults (N=507) with moderate to severe plaque psoriasis, evaluated the safety and efficacy with risankizumab 150 mg after 52 weeks and 104 weeks.8 Part A randomized patients to risankizumab 150 mg (n=407) or placebo (n=100). Part B rerandomized patients at week 28 to continue risankizumab 150 mg or placebo (designated as withdrawal of treatment; later re-treated with risankizumab 150 mg if patients had sPGA ≥3). At week 52, significantly more patients reached sPGA score of 0/1 with risankizumab/risankizumab (n=97 [87.4%]) vs risankizumab/placebo (n=138 [61.3%]; P<.001). At week 104, significantly more patients reached an sPGA score of 0/1 with risankizumab/risankizumab (n=90 [81.1%]) vs risankizumab/placebo (n=16 [7.1%]; P<.001). Risankizumab exhibited longevity following withdrawal, as median time to loss of response and relapse was 42 weeks (sPGA ≥3). The extent of TEAEs was similar between risankizumab and placebo and included nasopharyngitis, upper respiratory tract infection, headache, and back pain.8
Final Thoughts
Biologics for psoriasis help produce intended results for skin disease clearance and are tools for precision medicine. Recent data demonstrate safe, durable, and continuous efficacy with biologics, which offer patients a better chance of treatment success. This guide may serve as a quick reference for biologic selection with special consideration of individual disease characteristics and comorbidities.
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
- Mrowietz U, Bachelez H, Burden AD, et al. Efficacy and safety of secukinumab in moderate to severe palmoplantar pustular psoriasis over 148 weeks: extension of the 2PRECISE study. J Am Acad Dermatol. 2021;84:552-554. doi:10.1016/j.jaad.2020.06.038
- Gottlieb AB, Wu JJ, Griffiths CEM, et al. Clinical efficacy and safety of secukinumab in patients with psoriasis and comorbidities: pooled analysis of 4 phase 3 clinical trials [published online October 21, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1832187
- Papp K, Menter A, Leonardi C, et al. Long-term efficacy and safety of brodalumab in psoriasis through 120 weeks and after withdrawal and retreatment: subgroup analysis of a randomized phase III trial (AMAGINE-1). Br J Dermatol. 2020;183:1037-1048. doi:10.1111/bjd.19132
- Blauvelt A, Leonardi C, Elewski B, et al. A head-to-head comparison of ixekizumab vs. guselkumab in patients with moderate-to-severe plaque psoriasis: 24-week efficacy and safety results from a randomized, double-blinded trial. Br J Dermatol. 2021;184:1047-1058. doi:10.1111/bjd.19509
- Lebwohl MG, Leonardi CL, Mehta NN, et al. Tildrakizumab efficacy, drug survival, and safety are comparable in patients with psoriasis with and without metabolic syndrome: long-term results from 2 phase 3 randomized controlled studies (reSURFACE 1 and reSURFACE 2). J Am Acad Dermatol. 2021;84:398-407. doi:10.1016/j.jaad.2020.09.047
- Thaci D, Piaserico S, Warren RB, et al. Five-year efficacy and safety of tildrakizumab in patients with moderate-to-severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2)[published online February 5, 2021]. Br J Dermatol. doi:10.1111/bjd.19866
- Warren RB, Blauvelt A, Poulin Y, et al. Efficacy and safety of risankizumab vs. secukinumab in patients with moderate-to-severe plaque psoriasis (IMMerge): results from a phase III, randomized, open-label, efficacy-assessor-blinded clinical trial. Br J Dermatol. 2021;184:50-59. doi:10.1111/bjd.19341
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Practice Points
- Choosing a biologic best fit for each patient’s individual health profile can reduce psoriasis disease burden.
- Clinicians should educate psoriasis patients that biologics are safe for most comorbidities, and conditions such as obesity have been associated with poorer therapeutic response.
- It is important to discuss possible side effects of biologics with patients and reassure them that mild side effects are the most common during therapy.
Anecdote Increases Patient Willingness to Take a Biologic Medication for Psoriasis
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).
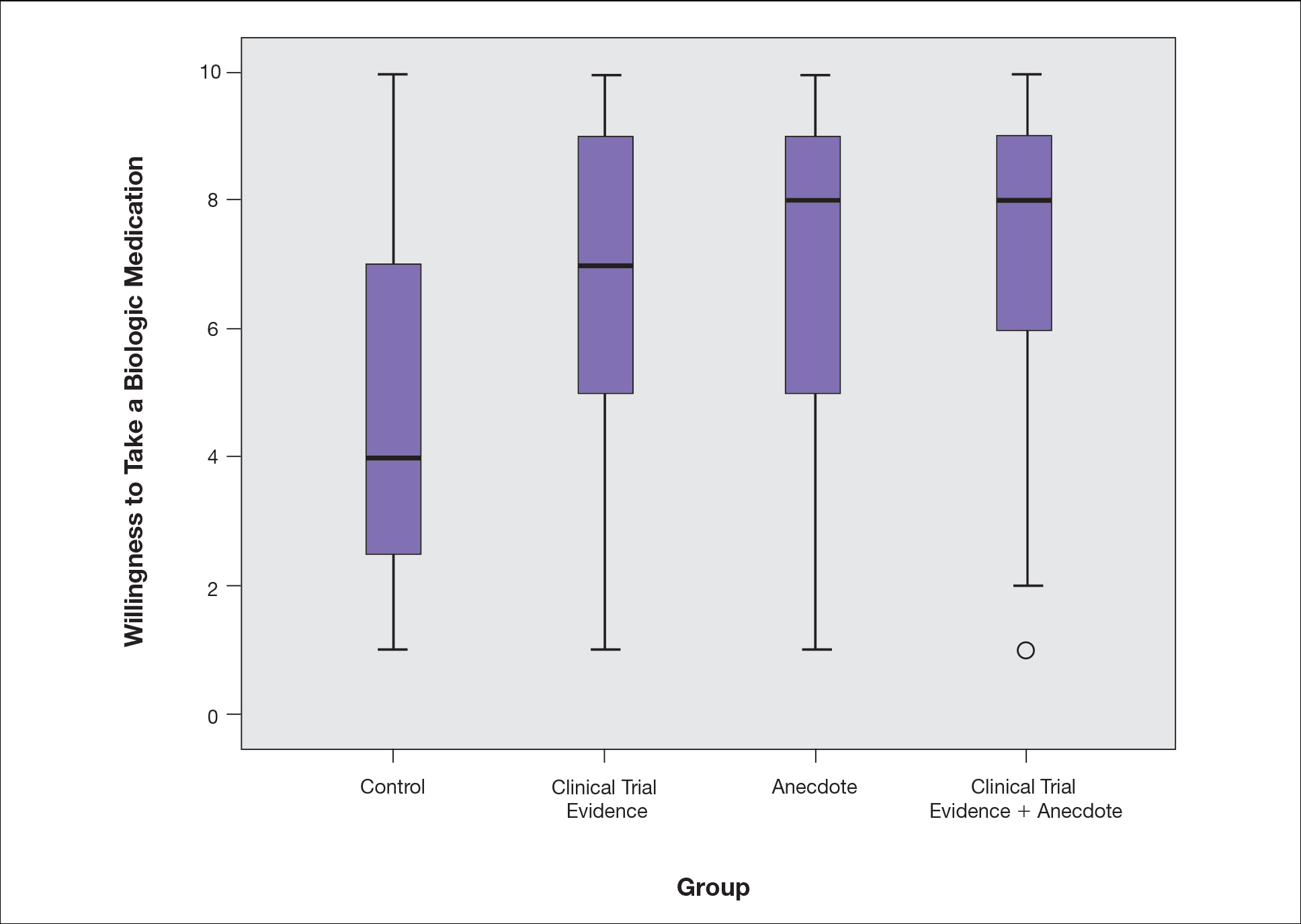
Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.
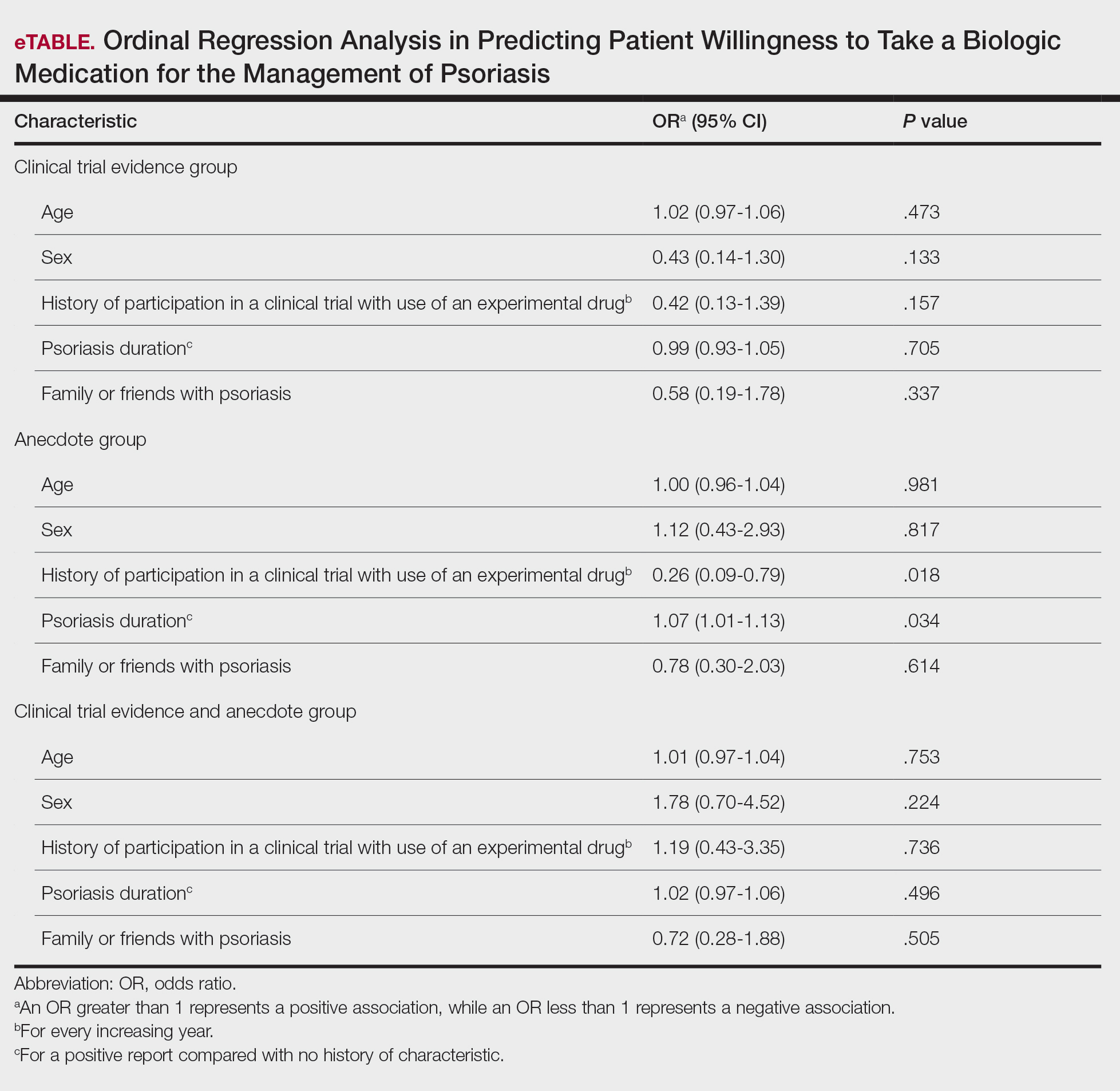
Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
Biologic medications are highly effective in treating moderate to severe psoriasis, yet many patients are apprehensive about taking a biologic medication for a variety of reasons, such as hearing negative information about the drug from friends or family, being nervous about injection, or seeing the drug or its side effects negatively portrayed in the media.1-3 Because biologic medications are costly, many patients may fear needing to discontinue use of the medication owing to lack of affordability, which may result in subsequent rebound of psoriasis. Because patients’ fear of a drug is inherently subjective, it can be modified with appropriate reassurance and presentation of evidence. By understanding what information increases patients’ confidence in their willingness to take a biologic medication, patients may be more willing to initiate use of the drug and improve treatment outcomes.
There are mixed findings about whether statistical evidence or an anecdote is more effective in persuasion.4-6 The specific context in which the persuasion takes place may be important in determining which method is superior. In most nonthreatening situations, people appear to be more easily persuaded by statistical evidence rather than an anecdote. However, in circumstances where emotional engagement is high, such as regarding one’s own health, an anecdote tends to be more persuasive compared to statistical evidence.7 The purpose of this study was to evaluate patients’ willingness to take a biologic medication for the management of their psoriasis if presented with either clinical trial evidence of the agent’s efficacy and safety, an anecdote of a single patient’s positive experience, or both.
Methods
Patient Inclusion Criteria
Following Wake Forest School of Medicine institutional review board approval, a prospective parallel-arm survey study was performed on eligible patients 18 years or older with a self-reported diagnosis of psoriasis. Patients were required to have a working knowledge of English and not have been previously prescribed a biologic medication for their psoriasis. If patients did not meet inclusion criteria after answering the survey eligibility screening questions, then they were unable to complete the remainder of the survey and were excluded from the analysis.
Survey Administration
A total of 222 patients were recruited through Amazon Mechanical Turk, an online crowdsourcing platform. (Amazon Mechanical Turk is a validated tool in conducting research in psychology and other social sciences and is considered as diverse as and perhaps more representative than traditional samples.8,9) Patients received a fact sheet and were taken to the survey hosted on Qualtrics, a secure web-based survey software that supports data collection for research studies. Amazon Mechanical Turk requires some amount of compensation to patients; therefore, recruited patients were compensated $0.03.
Statistical Analysis
Patients were randomized using SPSS Statistics version 23.0 (IBM) in a 1:1 ratio to assess how willing they would be to take a biologic medication for their psoriasis if presented with one of the following: (1) a control that queried patients about their willingness to take treatment without having been informed on its efficacy or safety, (2) clinical trial evidence of the agent’s efficacy and safety, (3) an anecdote of a single patient’s positive experience, or (4) both clinical trial evidence of the agent’s efficacy and safety and an anecdote of a single patient’s positive experience (Table 1). Demographic information including sex, age, ethnicity, and education level was collected, in addition to other baseline characteristics such as having friends or family with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis.

Outcome measures were recorded as patients’ responses regarding their willingness to take a biologic medication on a 10-point Likert scale (1=not willing; 10=completely willing). Scores were treated as ordinal data and evaluated using the Kruskal-Wallis test followed by the Dunn test. Descriptive statistics were tabulated on all variables. Baseline characteristics were analyzed using a 2-tailed, unpaired t test for continuous variables and the χ2 and Fisher exact tests for categorical variables. Ordinal linear regression analysis was performed to determine whether reported willingness to take a biologic medication was related to patients’ demographics, including age, sex, having family or friends with a history of psoriasis, history of participation in a clinical trial with use of an experimental drug, and the number of years since clinical diagnosis of psoriasis. Answers on the ordinal scale were binarized. The data were analyzed with SPSS Statistics version 23.0.
Results
There were no statistically significant differences among the baseline characteristics of the 4 information assignment groups (Table 2). Patients in the control group not given either clinical trial evidence of a biologic medication’s efficacy and safety or anecdote of a single patient’s positive experience had the lowest reported willingness to take treatment (median, 4.0)(Figure).


Based on regression analysis, age, sex, and having friends or family with a history of psoriasis were not significantly associated with patients’ responses (eTable). The number of years since clinical diagnosis of psoriasis (P=.034) and history of participation in a clinical trial with use of an experimental drug (P=.018) were significantly associated with the willingness of patients presented with an anecdote to take a biologic medication.

Comment
Anecdotal Reassurance
The presentation of clinical trial and/or anecdotal evidence had a strong effect on patients’ willingness to take a biologic medication for their psoriasis. Human perception of a treatment is inherently subjective, and such perceptions can be modified with appropriate reassurance and presentation of evidence.1 Across the population we studied, presenting a brief anecdote of a single patient’s positive experience is a quick and efficient means—and as or more effective as giving details on efficacy and safety—to help patients decide to take a treatment for their psoriasis.
Anecdotal reassurance is powerful. Both health care providers and patients have a natural tendency to focus on anecdotal experiences rather than statistical reasoning when making treatment decisions.10-12 Although negative anecdotal experiences may make patients unwilling to take a medication (or may make them overly desirous of an inappropriate treatment), clinicians can harness this psychological phenomenon to both increase patient willingness to take potentially beneficial treatments or to deter them from engaging in activities that can be harmful to their health, such as tanning and smoking.
Psoriasis Duration and Willingness to Take a Biologic Medication
In general, patient demographics did not appear to have an association with reported willingness to take a biologic medication for psoriasis. However, the number of years since clinical diagnosis of psoriasis had an effect on willingness to take a biologic medication, with patients with a longer personal history of psoriasis showing a higher willingness to take a treatment after being presented with an anecdote than patients with a shorter personal history of psoriasis. We can only speculate on the reasons why. Patients with a longer personal history of psoriasis may have tried and failed more treatments and therefore have a distrust in the validity of clinical trial evidence. These patients may feel their psoriasis is different than that of other clinical trial participants and thus may be more willing to rely on the success stories of individual patients.
Prior participation in a clinical trial with use of an experimental drug was associated with a lower willingness to choose treatment after being presented with anecdotal reassurance. This finding may be attributable to these patients understanding the subjective nature of anecdotes and preferring more objective information in the form of randomized clinical trials in making treatment decisions. Overall, the presentation of evidence about the efficacy and safety of biologic medications in the treatment of psoriasis has a greater impact on patient decision-making than patients’ age, sex, and having friends or family with a history of psoriasis.
Limitations
Limitations of the study were typical of survey-based research. With closed-ended questions, patients were not able to explain their responses. In addition, hypothetical informational statements of a biologic’s efficacy and safety may not always imitate clinical reality. However, we believe the study is valid in exploring the power of an anecdote in influencing patients’ willingness to take biologic medications for psoriasis. Furthermore, educational level and ethnicity were excluded from the ordinal regression analysis because the assumption of parallel lines was not met.
Ethics Behind an Anecdote
An important consideration is the ethical implications of sharing an anecdote to guide patients’ perceptions of treatment and behavior. Although clinicians rely heavily on the available data to determine the best course of treatment, providing patients with comprehensive information on all risks and benefits is rarely, if ever, feasible. Moreover, even objective clinical data will inevitably be subjectively interpreted by patients. For example, describing a medication side effect as occurring in 1 in 100 patients may discourage patients from pursuing treatment, whereas describing that risk as not occurring in 99 in 100 patients may encourage patients, despite these 2 choices being mathematically identical.13 Because the subjective interpretation of data is inevitable, presenting patients with subjective information in the form of an anecdote to help them overcome fears of starting treatment and achieve their desired clinical outcomes may be one of the appropriate approaches to present what is objectively the best option, particularly if the anecdote is representative of the expected treatment response. Clinicians can harness this understanding of human psychology to better educate patients about their treatment options while fulfilling their ethical duty to act in their patients’ best interest.
Conclusion
Using an anecdote to help patients overcome fears of starting a biologic medication may be appropriate if the anecdote is reasonably representative of an expected treatment outcome. Patients should have an accurate understanding of the common risks and benefits of a medication for purposes of shared decision-making.
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
- Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932-934. doi:10.1001/jamadermatol.2017.1271
- Brown KK, Rehmus WE, Kimball AB. Determining the relative importance of patient motivations for nonadherence to topical corticosteroid therapy in psoriasis. J Am Acad Dermatol. 2006;55:607-613. doi:10.1016/j.jaad.2005.12.021
- Im H, Huh J. Does health information in mass media help or hurt patients? Investigation of potential negative influence of mass media health information on patients’ beliefs and medication regimen adherence. J Health Commun. 2017;22:214-222. doi:10.1080/10810730.2016.1261970
- Hornikx J. A review of experimental research on the relative persuasiveness of anecdotal, statistical, causal, and expert evidence. Studies Commun Sci. 2005;5:205-216.
- Allen M, Preiss RW. Comparing the persuasiveness of narrative and statistical evidence using meta-analysis. Int J Phytoremediation Commun Res Rep. 1997;14:125-131. doi:10.1080/08824099709388654
- Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44:105-113. doi:10.1080/00913367.2015.1018467
- Freling TH, Yang Z, Saini R, et al. When poignant stories outweigh cold hard facts: a meta-analysis of the anecdotal bias. Organ Behav Hum Decis Process. 2020;160:51-67. doi:10.1016/j.obhdp.2020.01.006
- Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk. Perspect Psychol Sci. 2011;6:3-5. doi:10.1177/1745691610393980
- Berry K, Butt M, Kirby JS. Influence of information framing on patient decisions to treat actinic keratosis. JAMA Dermatol. 2017;153:421-426. doi:10.1001/jamadermatol.2016.5245
- Landon BE, Reschovsky J, Reed M, et al. Personal, organizational, and market level influences on physicians’ practice patterns: results of a national survey of primary care physicians. Med Care. 2001;39:889-905. doi:10.1097/00005650-200108000-00014
- Borgida E, Nisbett RE. The differential impact of abstract vs. concrete information on decisions. J Appl Soc Psychol. 1977;7:258-271. doi:10.1111/j.1559-1816.1977.tb00750.x
- Fagerlin A, Wang C, Ubel PA. Reducing the influence of anecdotal reasoning on people’s health care decisions: is a picture worth a thousand statistics? Med Decis Making. 2005;25:398-405. doi:10.1177/0272989X05278931
- Gurm HS, Litaker DG. Framing procedural risks to patients: Is 99% safe the same as a risk of 1 in 100? Acad Med. 2000;75:840-842. doi:10.1097/00001888-200008000-00018
Practice Points
- Patients often are apprehensive to start biologic medications for their psoriasis.
- Clinical trial evidence of a biologic medication’s efficacy and safety as well as anecdotes of patient experiences appear to be important factors for patients when considering taking a medication.
- The use of an anecdote—alone or in combination with clinical trial evidence—to help patients overcome fears of starting a biologic medication for their psoriasis may be an effective way to improve patients’ willingness to take treatment.
Western diet promoted skin, joint inflammation in preclinical study
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
A short-term Western diet facilitated the development of interleukin (IL)-23-mediated psoriasis-like skin and joint inflammation and caused shifts in the intestinal microbiota in a murine model – , say the investigators and other experts who reviewed the findings.
The mice did not become obese during the short duration of the multilayered study, which suggests that a Western diet (high sugar, moderate fat) can be impactful independent of obesity, Samuel T. Hwang, MD, PhD, professor and chair of dermatology at the University of California, Davis, and senior author of the study, said in an interview. The study was published in the Journal of Investigative Dermatology.
In an accompanying commentary, Renuka R. Nayak, MD, PhD, of the department of rheumatology at the University of California, San Francisco, wrote that the findings “add to the mounting evidence suggesting that diet has a prominent role in the treatment of psoriasis and [psoriatic arthritis] and raise the possibility that the microbiome may contribute to disease severity”.
Mice were fed a Western diet (WD) or conventional chow diet for 6 weeks and then injected with IL-23 minicircle (MC) DNA to induce systemic IL-23 overexpression – or a control minicircle DNA injection – and continued on these diets for another 4 weeks.
The mice in the WD/IL-23 MC DNA group developed erythema and scaling and increased epidermal thickness in the ears; such changes were “remarkably milder” or nonexistent in the other groups. Skin and joint immune cell populations, such as gamma delta T cells, neutrophils, and T helper type 17 cytokines were elevated in WD-fed mice, as were other markers of IL-23-mediated joint inflammation.
Recent research has suggested that the gut microbiota is dysbiotic in patients with psoriasis, and this new study found that WD-fed mice had less microbial diversity than that of mice fed a conventional diet. After IL-23 MC delivery, WD-fed reduced microbial diversity and pronounced dysbiosis.
“When we combined the Western diet and IL-23, we saw some very different microbes in abundance. The whole landscape changed,” Dr. Hwang said in the interview.
The data “suggest that WD and overexpression of IL-23 may contribute to gut microbiota dysbiosis in a synergistic and complex manner,” he and his coinvestigators wrote.
Treatment with broad-spectrum antibiotics suppressed IL-23-mediated skin and joint inflammation in the WD-fed mice – and moderately affected skin inflammation in conventionally-fed mice as well – which affirmed the role of dysbiosis.
And “notably,” in another layer of the study, mice that switched diets from a WD to a conventional diet had reduced skin and joint inflammation and increased diversity of gut microbiota. (Mice that were fed a WD for 6 weeks and given the IL-23 MC DNA were randomized to continue this diet for another 4 weeks or switch to a conventional diet.)
Commenting on the new research, Wilson Liao, MD, professor and vice chair of research in the department of dermatology at the University of California, San Francisco, said it “provides evidence” that diet can affect not only psoriasis, but psoriatic arthritis (PsA) as well, “through altering the ratio of good to bad bacteria in the gut.”
Going forward, better understanding “which specific gut bacteria and bacterial products lead to increased psoriatic inflammation, and the immunologic mechanism by which this occurs” will be important and could lead to novel treatments for psoriasis and PsA, said Dr. Liao, director of the UCSF Psoriasis and Skin Treatment Center.
Next on his research agenda, Dr. Hwang said, is the question of “how microbiota in the gut are actually able to influence inflammation at very distant sites in the joints and the skin.
“We want to understand the metabolic mechanisms,” he said, noting that “we invariably talk about cytokines, but there are other substances, like certain bile acids that are metabolized through the gut microbiome,” which may play a role.
The findings also offer a basis for treatment experiments in humans – of diet, probiotic therapy, or selective antibiotic modulation, for instance, Dr. Hwang said.
And in the meantime, the findings should encourage patients who are interested in making dietary changes, such as reducing sugar intake. “There’s wide interest – patients will ask, is there something I can change to make this better?” Dr. Hwang said. “Before, we could say it might be logical, but now we have some evidence. The message now is [high-sugar, moderate-fat] diets, apart from their ability to stimulate obesity, probably have some effects.”
Dietary change may not replace the need for other psoriasis treatments, he said, “but I think there’s good reason to believe that if you do change your diet, your treatment will be better than it would be without that dietary change,” he said.
In their discussion, Dr. Hwang and coauthors note that WD with IL-23 overexpression also decreased the mRNA expression of barrier-forming tight junction proteins, thus increasing intestinal permeability. This finding may be relevant, they wrote, because “leaky gut has been proposed as a pathogenic link between unhealthy diet, gut dysbiosis, and enhanced immune response,” and has been observed in a number of autoimmune diseases, including psoriasis.
Dr. Hwang, lead author Zhenrui Shi, MD, PhD, and coauthors reported no conflicts of interest. Their study was supported by the National Psoriasis Foundation, as well as the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the National Cancer Institute.
FROM THE JOURNAL OF INVESTIGATIVE DERMATOLOGY
The Top 100 Most-Cited Articles on Nail Psoriasis: A Bibliometric Analysis
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
To the Editor:
Nail psoriasis is highly prevalent in patients with cutaneous psoriasis and also may present as an isolated finding. There is a strong association between nail psoriasis and development of psoriatic arthritis (PsA). However, publications on nail psoriasis are sparse compared with articles describing cutaneous psoriasis.1 Our objectives were to analyze the nail psoriasis literature for content, citations, and media attention.
The Web of Science database was searched for the term nail psoriasis on April 27, 2020, and publications by year, subject, and article type were compiled. Total and average yearly citations were calculated to create a list of the top 100 most-cited articles (eTable). First and last authors, sex, and Altmetric Attention Scores were then recorded. The Wilcoxon rank sum test was calculated to compare the relationship of Altmetric scores between nail psoriasis–specific references and others on the list.
In our data set, the average total number of citations was 134.09 (range, 42–1617), with average yearly citations ranging from 2 to 108. Altmetric scores—measures of media attention of scholarly work—were available for 58 of 100 papers (58%), with an average score of 33.2 (range, 1–509).
Of the top 100 most-cited articles using the search term nail psoriasis, only 20% focused on nail psoriasis, with the remainder concentrating on psoriasis/PsA. Only 32% and 24% of first and last authors, respectively, were female. Fifty-two percent and 31% of the articles were published in dermatology and arthritis/rheumatology journals, respectively. There was no statistically significant difference in Altmetric scores between nail psoriasis–specific and other articles in our data set (P=.7551).
For the nail psoriasis–specific articles, all 20 highlighted a lack of nail clinical trials, a positive association with PsA, and a correlation of increased cutaneous psoriasis body surface area with increased onychodystrophy likelihood.2 Three of 20 (15%) articles stated that nail psoriasis often is overlooked, despite the negative impact on quality of life,1 and emphasized the importance of patient compliance owing to the chronic nature of the disease. Only 1 of 20 (5%) articles focused on nail psoriasis treatments.3 There was no overlap between the 100 most-cited psoriasis articles from 1970 to 2012 and our top 100 articles on nail psoriasis.4
Treatment recommendations for nail psoriasis by consensus were published by a nail expert group in 2019.5 For 3 or fewer nails involved, suggested first-line treatment is intralesional matrix injections with triamcinolone acetonide. For more than 3 affected nails, systemic treatment with oral or biologic therapy is recommended.5 Although this article is likely to change clinical practice, it did not qualify for our list because it did not garner sufficient citations in the brief period between its publication date and our search (July 2019–April 2020).
This study is subject to several limitations. Only the Web of Science database was utilized, and only the term nail psoriasis was searched, potentially excluding relevant articles. Using total citations biases toward older articles.
Our bibliometric analysis highlights a lack of publications on nail psoriasis, with most articles focusing on psoriasis and PsA. This deficiency in highly cited nail psoriasis references is likely to be a barrier to physicians in managing patients with nail disease. There is a need for controlled clinical trials and better mechanisms to disseminate information on management of nail psoriasis to practicing physicians.
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
- Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: nail disease in psoriatic arthritis—clinically important, potentially treatable and often overlooked. Rheumatology (Oxford). 2004;43:790-794. doi:10.1093/rheumatology/keh198
- Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(suppl 1):15-21. doi:10.1111/j.1468-3083.2009.03364.x
- de Berker D. Management of nail psoriasis. Clin Exp Dermatol. 2000;25:357-362. doi:10.1046/j.1365-2230.2000.00663.x
- Wu JJ, Choi YM, Marczynski W. The 100 most cited psoriasis articles in clinical dermatologic journals, 1970 to 2012. J Clin Aesthet Dermatol. 2014;7:10-19.
- Rigopoulos D, Baran R, Chiheb S, et al. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: a dermatologist and nail expert group consensus. J Am Acad Dermatol. 2019;81:228-240. doi:10.1016/j.jaad.2019.01.072
Use of Complementary Alternative Medicine and Supplementation for Skin Disease
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
Complementary alternative medicine (CAM) has been described by the National Center for Complementary and Integrative Medicine as “health care approaches that are not typically part of conventional medical care or that may have origins outside of usual Western practice.”1 Although this definition is broad, CAM encompasses therapies such as traditional Chinese medicine, herbal therapies, dietary supplements, and mind/body interventions. The use of CAM has grown, and according to a 2012 National Center for Complementary and Integrative Health survey, more than 30% of US adults and 12% of US children use health care approaches that are considered outside of conventional medical practice. In a survey study of US adults, at least 17.7% of respondents said they had taken a dietary supplement other than a vitamin or mineral in the last year.1 Data from the 2007 National Health Interview Survey showed that the prevalence of adults with skin conditions using CAM was 84.5% compared to 38.3% in the general population.2 In addition, 8.15 million US patients with dermatologic conditions reported using CAM over a 5-year period.3 Complementary alternative medicine has emerged as an alternative or adjunct to standard treatments, making it important for dermatologists to understand the existing literature on these therapies. Herein, we review the current evidence-based literature that exists on CAM for the treatment of atopic dermatitis (AD), psoriasis, and alopecia areata (AA).
Atopic Dermatitis
Atopic dermatitis is a chronic, pruritic, inflammatory skin condition with considerable morbidity.4,5 The pathophysiology of AD is multifactorial and includes aspects of barrier dysfunction, IgE hypersensitivity, abnormal cell-mediated immune response, and environmental factors.6 Atopic dermatitis also is one of the most common inflammatory skin conditions in adults, affecting more than 7% of the US population and up to 20% of the total population in developed countries. Of those affected, 40% have moderate or severe symptoms that result in a substantial impact on quality of life.7 Despite advances in understanding disease pathology and treatment, a subset of patients opt to defer conventional treatments such as topical and systemic corticosteroids, antibiotics, nonsteroidal immunomodulators, and biologics. Patients may seek alternative therapies when typical treatments fail or when the perceived side effects outweigh the benefits.5,8 The use of CAM has been well described in patients with AD; however, the existing evidence supporting its use along with its safety profile have not been thoroughly explored. Herein, we will discuss some of the most well-studied supplements for treatment of AD, including evening primrose oil (EPO), fish oil, and probiotics.5
Oral supplementation with polyunsaturated fatty acids commonly is reported in patients with AD.5,8 The idea that a fatty acid deficiency could lead to atopic skin conditions has been around since 1937, when it was suggested that patients with AD had lower levels of blood unsaturated fatty acids.9 Conflicting evidence regarding oral fatty acid ingestion and AD disease severity has emerged.10,11 One unsaturated fatty acid, γ-linolenic acid (GLA), has demonstrated anti-inflammatory properties and involvement in barrier repair.12 It is converted to dihomo-GLA in the body, which acts on cyclooxygenase enzymes to produce the inflammatory mediator prostaglandin E1. The production of GLA is mediated by the enzyme delta-6 desaturase in the metabolization of linoleic acid.12 However, it has been reported that in a subset of patients with AD, a malfunction of delta-6 desaturase may play a role in disease progression and result in lower baseline levels of GLA.10,12 Evening primrose oil and borage oil contain high amounts of GLA (8%–10% and 23%, respectively); thus, supplementation with these oils has been studied in AD.13
EPO for AD
Studies investigating EPO (Oenothera biennis) and its association with AD severity have shown mixed results. A Cochrane review reported that oral borage oil and EPO were not effective treatments for AD,14 while another larger randomized controlled trial (RCT) found no statistically significant improvement in AD symptoms.15 However, multiple smaller studies have found that clinical symptoms of AD, such as erythema, xerosis, pruritus, and total body surface area involved, did improve with oral EPO supplementation when compared to placebo, and the results were statistically significant (P=.04).16,17 One study looked at different dosages of EPO and found that groups ingesting both 160 mg and 320 mg daily experienced reductions in eczema area and severity index score, with greater improvement noted with the higher dosage.17 Side effects associated with oral EPO include an anticoagulant effect and transient gastrointestinal tract upset.8,14 There currently is not enough evidence or safety data to recommend this supplement to AD patients.
Although topical use of fatty acids with high concentrations of GLA, such as EPO and borage oil, have demonstrated improvement in subjective symptom severity, most studies have not reached statistical significance.10,11 One study used a 10% EPO cream for 2 weeks compared to placebo and found statistically significant improvement in patient-reported AD symptoms (P=.045). However, this study only included 10 participants, and therefore larger studies are necessary to confirm this result.18 Some RCTs have shown that topical coconut oil, sunflower seed oil, and sandalwood album oil improve AD symptom severity, but again, large controlled trials are needed.5 Unfortunately, many essential oils, including EPO, can cause a secondary allergic contact dermatitis and potentially worsen AD.19
Fish Oil for AD
Fish oil is a commonly used supplement for AD due to its high content of the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Omega-3 fatty acids exert anti-inflammatory effects by displacing arachidonic acid, a proinflammatory omega-6 fatty acid thought to increase IgE, as well as helper T cell (TH2) cytokines and prostaglandin E2.8,20 A 2012 Cochrane review found that, while some studies revealed mild improvement in AD symptoms with oral fish oil supplementation, these RCTs were of poor methodological quality.21 Multiple smaller studies have shown a decrease in pruritus, severity, and physician-rated clinical scores with fish oil use.5,8,20,22 One study with 145 participants reported that 6 g of fish oil once daily compared to isoenergetic corn oil for 16 weeks identified no statistically significant differences between the treatment groups.20 No adverse events were identified in any of the reported trials. Further studies should be conducted to assess the utility and dosing of fish oil supplements in AD patients.
Probiotics for AD
Probiotics consist of live microorganisms that enhance the microflora of the gastrointestinal tract.8,20 They have been shown to influence food digestion and also have demonstrated potential influence on the skin-gut axis.23 The theory that intestinal dysbiosis plays a role in AD pathogenesis has been investigated in multiple studies.23-25 The central premise is that low-fiber and high-fat Western diets lead to fundamental changes in the gut microbiome, resulting in fewer anti-inflammatory metabolites, such as short-chain fatty acids (SCFAs).23-25 These SCFAs are produced by microbes during the fermentation of dietary fiber and are known for their effect on epithelial barrier integrity and anti-inflammatory properties mediated through G protein–coupled receptor 43.25 Multiple studies have shown that the gut microbiome in patients with AD have higher proportions of Clostridium difficile, Escherichia coli, and Staphylococcus aureus and lower levels of Bifidobacterium, Bacteroidetes, and Bacteroides species compared to healthy controls.26,27 Metagenomic analysis of fecal samples from patients with AD have shown a reduction of Faecalibacterium prausnitzii species when compared to controls, along with a decreased SCFA production, leading to the hypothesis that the gut microbiome may play a role in epithelial barrier disruption.28,29 Systematic reviews and smaller studies have found that oral probiotic use does lead to AD symptom improvement.8,30,31 A systematic review of 25 RCTs with 1599 participants found that supplementation with oral probiotics significantly decreased the SCORAD (SCORing Atopic Dermatitis) index in adults and children older than 1 year with AD but had no effect on infants younger than 1 year (P<.001). They also found that supplementation with diverse microbes or Lactobacillus species showed greater benefit than Bifidobacterium species alone.30 Another study analyzed the effect of oral Lactobacillus fermentum (1×109 CFU twice daily) in 53 children with AD vs placebo for 16 weeks. This study found a statically significant decrease in SCORAD index between oral probiotics and placebo, with 92% (n=24) of participants supplementing with probiotics having a lower SCORAD index than baseline compared to 63% (n=17) in the placebo group (P=.01).31 However, the use of probiotics for AD treatment has remained controversial. Two recent systematic reviews, including 39 RCTs of 2599 randomized patients, found that the use of currently available oral probiotics made little or no difference in patient-rated AD symptoms, investigator-rated AD symptoms, or quality of life.32,33 No adverse effects were observed in the included studies. Unfortunately, the individual RCTs included were heterogeneous, and future studies with standardized probiotic supplementation should be undertaken before probiotics can be routinely recommended.
The use of topical probiotics in AD also has recently emerged. Multiple studies have shown that patients with AD have higher levels of colonization with S aureus, which is associated with T-cell dysfunction, more severe allergic skin reactions, and disruptions in barrier function.34,35 Therefore, altering the skin microbiota through topical probiotics could theoretically reduce AD symptoms and flares. Multiple RCTs and smaller studies have shown that topical probiotics can alter the skin microbiota, improve erythema, and decrease scaling and pruritus in AD patients.35-38 One study used a heat-treated Lactobacillus johnsonii 0.3% lotion twice daily for 3 weeks vs placebo in patients with AD with positive S aureus skin cultures. The S aureus load decreased in patients using the topical probiotic lotion, which correlated with lower SCORAD index that was statistically significant compared to placebo (P=.012).36 More robust studies are needed to determine if topical probiotics should routinely be recommended in AD.
Psoriasis
Psoriasis vulgaris is a chronic inflammatory skin condition characterized by pruritic, hyperkeratotic, scaly plaques.39,40 Keratinocyte hyperproliferation is central to psoriasis pathogenesis and is thought to be a T-cell–driven reaction to antigens or trauma in genetically predisposed individuals. Standard treatments for psoriasis currently include topical corticosteroids and anti-inflammatories, oral immunomodulatory therapy, biologic agents, and phototherapy.40 The use of CAM is highly prevalent among patients with psoriasis, with one study reporting that 51% (n=162) of psoriatic patients interviewed had used CAM.41 The most common reasons for CAM use included dissatisfaction with current treatment, adverse side effects of standard therapy, and patient-reported attempts at “trying everything to heal disease.”42 Herein, we will discuss some of the most frequently used supplements for treatment of psoriatic disease.39
Fish Oil for Psoriasis
One of the most common supplements used by patients with psoriasis is fish oil due to its purported anti-inflammatory qualities.20,39 The consensus on fish oil supplementation for psoriasis is mixed.43-45 Multiple RCTs have reported reductions in psoriasis area and severity index (PASI) scores or symptomatic improvement with variable doses of fish oil.44,46 One RCT found that using EPA 1.8 g once daily and DHA 1.2 g once daily for 12 weeks resulted in significant improvement in pruritus, scaling, and erythema (P<.05).44 Another study reported a significant decrease in erythema (P=.02) and total body surface area affected (P=.0001) with EPA 3.6 g once daily and DHA 2.4 g once daily supplementation compared to olive oil supplementation for 15 weeks.46 Alternatively, multiple studies have failed to show statistically significant improvement in psoriatic symptoms with fish oil supplementation at variable doses and time frames (14–216 mg daily EPA, 9–80 mg daily DHA, from 2 weeks to 9 months).40,47,48 Fish oil may impart anticoagulant properties and should not be started without the guidance of a physician. Currently, there are no data to make specific recommendations on the use of fish oil as an adjunct psoriatic treatment.
Curcumin for Psoriasis
Another supplement routinely utilized in patients with psoriasis is curcumin,40,49,50 a yellow phytochemical that is a major component of the spice turmeric. Curcumin has been shown to inhibit certain proinflammatory cytokines including IL-17, IL-6, IFN-γ, and tumor necrosis factor α and has been regarded as having immune-modulating, anti-inflammatory, and antibacterial properties.40,50 Curcumin also has been reported to suppress phosphorylase kinase, an enzyme that has increased activity in psoriatic plaques that correlates with markers of psoriatic hyperproliferation.50,51 When applied topically, turmeric microgel 0.5% has been reported to decrease scaling, erythema, and psoriatic plaque thickness over the course of 9 weeks.50 In a nonrandomized trial with 10 participants, researchers found that phosphorylase kinase activity levels in psoriatic skin biopsies of patients applying topical curcumin 1% were lower than placebo and topical calcipotriol applied in combination. The lower phosphorylase kinase levels correlated with level of disease severity, and topical curcumin 1% showed a superior outcome when compared to topical calcipotriol.40,49 Although these preliminary results are interesting, there still are not enough data at this time to recommend topical curcumin as a treatment of psoriasis. No known adverse events have been reported with the use of topical curcumin to date.
Oral curcumin has poor oral bioavailability, and 40% to 90% of oral doses are excreted, making supplementation a challenge.40 In one RCT, oral curcumin 2 g daily (using a lecithin-based delivery system to increase bioavailability) was administered in combination with topical methylprednisolone aceponate 0.1%, resulting in significant improvement in psoriatic symptoms and lower IL-22 compared to placebo and topical methylprednisolone aceponate (P<.05).52 Other studies also have reported decreased PASI scores with oral curcumin supplementation.53,54 Adverse effects reported with oral curcumin included gastrointestinal tract upset and hot flashes.53 Although there is early evidence that may support the use of oral curcumin supplementation for psoriasis, more data are needed before recommending this therapy.
Indigo Naturalis for Psoriasis
Topical indigo naturalis (IN) also has been reported to improve psoriasis symptoms.39,53,55 The antipsoriatic effects are thought to occur through the active ingredient in IN (indirubin), which is responsible for inhibition of keratinocyte proliferation.40 One study reported that topical IN 1.4% containing indirubin 0.16% with a petroleum ointment vehicle applied to psoriatic plaques over 12 weeks resulted in a significant decrease in PASI scores from 18.9 at baseline to 6.3 after IN treatment (P<.001).56 Another study found that over 8 weeks, topical application of IN 2.83% containing indirubin 0.24% to psoriatic plaques vs petroleum jelly resulted in 56.3% (n=9) of the treatment group achieving PASI 75 compared to 0% in the placebo group (n=24).55 One deterrent in topical IN treatment is the dark blue pigment it contains; however, no other adverse outcomes were found with topical IN treatment.56 Larger clinical trials are necessary to further explore IN as a potential adjunct treatment in patients with mild psoriatic disease. When taken orally, IN has caused gastrointestinal tract disturbance and elevated liver enzyme levels.57
Herbal Toxicities
It is important to consider that oral supplements including curcumin and IN are widely available over-the-counter and online without oversight by the US Food and Drug Administration.40 Herbal supplements typically are compounded with other ingredients and have been associated with hepatotoxicity as well as drug-supplement interactions, including abnormal bleeding and clotting.58 There exists a lack of general surveillance data, making the true burden of herbal toxicities more difficult to accurately discern. Although some supplements have been associated with anti-inflammatory qualities and disease improvement, other herbal supplements have been shown to possess immunostimulatory characteristics. Herbal supplements such as spirulina, chlorella, Aphanizomenon flos-aquae, and echinacea have been shown to upregulate inflammatory pathways in a variety of autoimmune skin conditions.59
Probiotics for Psoriasis
Data on probiotic use in patients with psoriasis are limited.23 A distinct pattern of dysbiosis has been identified in psoriatic patients, as there is thought to be depletion of beneficial bacteria such as Bifidobacterium, lactobacilli, and F prausnitzii and increased colonization with pathogenic organisms such as Salmonella, E coli, Heliobacter, Campylobacter, and Alcaligenes in psoriasis patients.23,59,60 Early mouse studies have supported this hypothesis, as mice fed with Lactobacillus pentosus have developed milder forms of imiquimod-induced psoriasis compared to placebo,55 and mice receiving probiotic supplementation have lower levels of psoriasis-related proinflammatory markers such as TH17-associated cytokines.61 Another study in humans found that daily oral Bifidobacterium infantis supplementation for 8 weeks in psoriatic patients resulted in lower C-reactive protein and tumor necrosis factor α levels compared to placebo.62 Studies on the use of topical probiotics in psoriasis have been limited, and more research is needed to explore this relationship.38 At this time, no specific recommendations can be made on the use of probiotics in psoriatic patients.
Alopecia Areata
Alopecia areata is nonscarring hair loss that can affect the scalp, face, or body.63,64 The pathophysiology of AA involves the attack of the hair follicle matrix epithelium by inflammatory cells without hair follicle stem cell destruction. The precise events that precipitate these episodes are unknown, but triggers such as emotional or physical stress, vaccines, or viral infections have been reported.65 There is no cure for AA, and current treatments such as topical minoxidil and corticosteroids (topical, intralesional, or oral) vary widely in efficacy.64 Although Janus kinase inhibitors recently have shown promising results in the treatment of AA, the need for prolonged therapy may be frustrating to patients.66 Severity of AA also can vary, with 30% of patients experiencing extensive hair loss.67 The use of CAM has been widely reported in AA due to high levels of dissatisfaction with existing therapies.68 Herein, we discuss the most studied alternative treatments used in AA
Garlic and Onion for Alopecia
One alternative treatment that has shown promising initial results is application of topical garlic and onion extracts to affected areas.64,69,70 Both garlic and onion belong to the Allium genus and are high in sulfur and phenolic compounds.70 They have been reported to possess bactericidal and vasodilatory activity,71 and it has been hypothesized that onion and garlic extracts may induce therapeutic effects through induction of a mild contact dermatitis.70 One single-blinded, controlled trial using topical crude onion juice reported that 86.9% (n=20) of patients had full regrowth of hair compared to 13.3% (n=2) of patients treated with a tap water placebo at 8 weeks (P<.0001). This study also noted that patients using onion juice had a higher rate of erythema at application site; unfortunately, the study was small with only 38 patients.70 Another double-blind RCT using garlic gel 5% with betamethasone valerate cream 0.1% compared to betamethasone valerate cream alone found that after 3 months, patients in the garlic gel group had increased terminal hairs and smaller patch sizes compared to the betamethasone valerate cream group.69 More studies are needed to confirm these results.
Aromatherapy With Essential Oils for Alopecia
Another alternative treatment in AA that has demonstrated positive results is aromatherapy skin massage with essential oils to patches of alopecia.72 Although certain essential oils, such as tea tree oil, have been reported to have specific antibacterial or anti-inflammatory properties, essential oils have been reported to cause allergic contact dermatitis and should be used with caution.73,74 For example, tea tree oil is a well-known cause of allergic contact dermatitis, and positive patch testing has ranged from 0.1% to 3.5% in studies assessing topical tea tree oil 5% application.75 Overall, there have been nearly 80 essential oils implicated in contact dermatitis, with high-concentration products being one of the highest risk factors for an allergic contact reaction.76 One RCT compared daily scalp massage with essential oils (rosemary, lavender, thyme, and cedarwood in a carrier oil) to daily scalp massage with a placebo carrier oil in AA patients. The results showed that at 7 months of treatment, 44% (n=19) of the aromatherapy group showed improvement compared to 15% (n=6) in the control group.77 Another study used a similar group of essential oils (thyme, rosemary, atlas cedar, lavender, and EPO in a carrier oil) with daily scalp massage and reported similar improvement of AA symptoms compared to control; the investigators also reported irritation at application site in 1 patient.78 There currently are not enough data to recommend aromatherapy skin massage for the treatment of AA, and this practice may cause harm to the patient by induction of allergic contact dermatitis.
There have been a few studies to suggest that the use of total glucosides of peony with compound glycyrrhizin and oral Korean red ginseng may have beneficial effects on AA treatment, but efficacy and safety data are lacking, and these therapies should not be recommended without more information.64,79,80
Final Thoughts
Dermatologic patients frequently are opting for CAM,2 and although some therapies may show promising initial results, alternative medicines also can drive adverse events.19,30 The lack of oversight from the US Food and Drug Administration on the products leads to many unknowns for true health risks with over-the-counter CAM supplements.40 As the use of CAM becomes increasingly common among dermatologic patients, it is important for dermatologists to understand the benefits and risks, especially for commonly treated conditions. More data is needed before CAM can be routinely recommended.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
- Complementary, alternative, or integrative health: what’s in a name? National Center for Complementary and Integrative Health website. Updated April 2021. Accessed April 25, 2021. https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name
- Fuhrmann T, Smith N, Tausk F. Use of complementary and alternative medicine among adults with skin disease: updated results from a national survey. J Am Acad Dermatol. 2010;63:1000-1005.
- Landis ET, Davis SA, Feldman SR, et al. Complementary and alternative medicine use in dermatology in the United States. J Altern Complement Med. 2014;20:392-398.
- Solman L, Lloyd‐Lavery A, Grindlay DJC, et al. What’s new in atopic eczema? an analysis of systematic reviews published in 2016. part 1: treatment and prevention. Clin Exp Dermatol. 2019;44:363-369.
- Vieira BL, Lim NR, Lohman ME, et al. Complementary and alternative medicine for atopic dermatitis: an evidence-based review. Am J Clin Dermatol. 2016;17:557-581.
- David Boothe W, Tarbox JA, Tarbox MB. Atopic dermatitis: pathophysiology. In: Fortson EA, Feldman SR, Strowd LC, eds. Management of Atopic Dermatitis: Methods and Challenges. Springer International Publishing; 2017:21-37.
- Atopic dermatitis in America. Asthma and Allergy Foundation of America website. Accessed July 30, 2021. https://www.aafa.org/atopic-dermatitis-in-america
- Schlichte MJ, Vandersall A, Katta R. Diet and eczema: a review of dietary supplements for the treatment of atopic dermatitis. Dermatol Pract Concept. 2016;6:23-29.
- Brown WR, Hansen AE. Arachidonic and linolic acid of the serum in normal and eczematous human subjects. Proc Soc Exp Bio Med. 1937;36:113-117.
- Lee J, Bielory L. Complementary and alternative interventions in atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:411-424.
- Ferreira MJ, Fiadeiro T, Silva M, et al. Topical γ-linolenic acid therapy in atopic dermatitis. Allergo J. 1998;7:213-216.
- Simon D, Eng PA, Borelli S, et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv Ther. 2014;31:180-188.
- Fan Y-Y, Chapkin RS. Importance of dietary γ-linolenic acid in human health and nutrition. J Nutr. 1998;128:1411-1414.
- Bamford JTM, Ray S, Musekiwa A, et al. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst Rev. 2013;4:CD004416.
- Williams H. Evening primrose oil for atopic dermatitis. BMJ. 2003;327:2.
- Schalin-Karrila M, Mattila L, Jansen CT, et al. Evening primrose oil in the treatment of atopic eczema: effect on clinical status, plasma phospholipid fatty acids and circulating blood prostaglandins. Br J Dermatol. 1987;117:11-19.
- Chung BY, Park SY, Jung MJ, et al. Effect of evening primrose oil on Korean patients with mild atopic dermatitis: a randomized, double-blinded, placebo-controlled clinical study. Ann Dermatol. 2018;30:409-416.
- Anstey A, Quigley M, Wilkinson JD. Topical evening primrose oil as treatment for atopic eczema. J Dermatolog Treat. 1990;1:199-201.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. Dermatitis. 2016;27:39-42.
- Reynolds KA, Juhasz MLW, Mesinkovska NA. The role of oral vitamins and supplements in the management of atopic dermatitis: a systematic review. Int J Dermatol. 2019;58:1371-1376.
- Bath-Hextall FJ, Jenkinson C, Humphreys R, et al. Dietary supplements for established atopic eczema [published online February 15, 2012]. Cochrane Database Syst Rev. Accessed July 22, 2021. doi:10.1002/14651858.CD005205.pub3
- Balic´ A, Vlašic´ D, Žužul K, et al. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int J Mol Sci. 2020;21:741.
- Salem I, Ramser A, Isham N, et al. The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol. 2018;9:1459.
- Agrawal R, Wisniewski JA, Woodfolk JA. The role of regulatory T cells in atopic dermatitis. Pathogenesis Manage Atopic Dermatitis. 2011;41:112-124.
- Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282-1286.
- Lee E, Lee S-Y, Kang M-J, et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91-92.e1.
- Nylund L, Nermes M, Isolauri E, et al. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy. 2015;70:241-244.
- Kim H-J, Kim HY, Lee S-Y, et al. Clinical efficacy and mechanism of probiotics in allergic diseases. Korean J Pediatr. 2013;56:369-376.
- Song H, Yoo Y, Hwang J, et al. Faecalibacterium prausnitzii subspecies-level dysbiosis in the human gut microbiome underlying atopic dermatitis. J Allergy Clin Immunol. 2016;137:852-860.
- Kim S-O, Ah Y-M, Yu YM, et al. Effects of probiotics for the treatment of atopic dermatitis: a meta-analysis of randomized controlled trials. Ann Allergy Asthma Immunol. 2014;113:217-226.
- Weston S, Halbert A, Richmond P, et al. Effects of probiotics on atopic dermatitis: a randomised controlled trial. Arch Dis Child. 2005;90:892-897.
- Huang R, Ning H, Shen M, et al. Probiotics for the treatment of atopic dermatitis in children: a systematic review and meta-analysis of randomized controlled trials. Front Cell Infect Microbiol. 2017;7:392.

- Makrgeorgou A, Leonardi-Bee J, Bath-Hextall FJ, et al. Probiotics for treating eczema. Cochrane Database Syst Rev. 2018;11:CD006135.
- Knackstedt R, Knackstedt T, Gatherwright J. The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp Dermatol. 2020;29:15-21.
- Woo TE, Sibley CD. The emerging utility of the cutaneous microbiome in the treatment of acne and atopic dermatitis. J Am Acad Dermatol. 2020;82:222-228.
- Blanchet-Réthoré S, Bourdès V, Mercenier A, et al. Effect of a lotion containing the heat-treated probiotic strain Lactobacillus johnsonii NCC 533 on Staphylococcus aureus colonization in atopic dermatitis. Clin Cosmet Investig Dermatol. 2017;10:249-257.
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nature Medicine. 2021;27:700-709.
- França K. Topical probiotics in dermatological therapy and skincare: a concise review. Dermatol Ther (Heidelb). 2020;11:71-77.
- Talbott W, Duffy N. Complementary and alternative medicine for psoriasis: what the dermatologist needs to know. Am J Clin Dermatol. 2015;16:147-165.
- Gamret AC, Price A, Fertig RM, et al. Complementary and alternative medicine therapies for psoriasis: a systematic review. JAMA Dermatol. 2018;154:1330-1337.
- Fleischer AB, Feldman SR, Rapp SR, et al. Alternative therapies commonly used within a population of patients with psoriasis. Cutis. 1996;58:216-220.
- Ben-Arye E, Ziv M, Frenkel M, et al. Complementary medicine and psoriasis: linking the patient’s outlook with evidence-based medicine. Dermatology. 2003;207:302-307.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Ford AR, Siegel M, Bagel J, et al. Dietary recommendations for adults with psoriasis or psoriatic arthritis from the medical board of the National Psoriasis Foundation: a Systematic review. JAMA Dermatol. 2018;154:934-950.
- Gupta AK, Ellis CN, Tellner DC, et al. Double-blind, placebo-controlled study to evaluate the efficacy of fish oil and low-dose UVB in the treatment of psoriasis. Br J Dermatol. 1989;120:801-807.
- Kristensen S, Schmidt EB, Schlemmer A, et al. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: a randomized, double blind, placebo-controlled trial. Scand J Rheumatol. 2018;47:27-36.
- Søyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Heng MCY, Song MK, Harker J, et al. Drug-induced suppression of phosphorylase kinase activity correlates with resolution of psoriasis as assessed by clinical, histological and immunohistochemical parameters. Br J Dermatol. 2000;143:937-949.
- Sarafian G, Afshar M, Mansouri P, et al. Topical turmeric microemulgel in the management of plaque psoriasis; a clinical evaluation. Iran J Pharm Res. 2015;14:865-876.
- Reddy S, Aggarwal BB. Curcumin is a non-competitive and selective inhibitor of phosphorylase kinase. FEBS Letters. 1994;341:19-22.
- Antiga E, Bonciolini V, Volpi W, et al. Oral curcumin (meriva) is effective as an adjuvant treatment and is able to reduce IL-22 serum levels in patients with psoriasis vulgaris. Biomed Res Int. 2015;2015:283634.
- Kurd SK, Smith N, VanVoorhees A, et al. Oral curcumin in the treatment of moderate to severe psoriasis vulgaris: a prospective clinical trial. J Am Acad Dermatol. 2008;58:625-631.
- Carrion-Gutierrez M, Ramirez-Bosca A, Navarro-Lopez V, et al. Effects of Curcuma extract and visible light on adults with plaque psoriasis. Eur J Dermatol. 2015;25:240-246.
- Cheng H-M, Wu Y-C, Wang Q, et al. Clinical efficacy and IL-17 targeting mechanism of indigo naturalis as a topical agent in moderate psoriasis. BMC Complement Altern Med. 2017;17:439.
- Lin Y-K, Chang C-J, Chang Y-C, et al. Clinical assessment of patients with recalcitrant psoriasis in a randomized, observer-blind, vehicle-controlled trial using indigo naturalis. Arch Dermatol. 2008;144:1457-1464.
- Naganuma M, Sugimoto S, Suzuki H, et al. Adverse events in patients with ulcerative colitis treated with indigo naturalis: a Japanese nationwide survey. J Gastroenterol. 2019;54:891-896.
- Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Alimentary Pharmacol Ther. 2013;37:3-17.
- Bax CE, Chakka S, Concha JSS, et al. The effects of immunostimulatory herbal supplements on autoimmune skin diseases. J Am Acad Dermatol. 2021;84:1051-1058.
- Scher JU, Ubeda C, Artacho A, et al. Decreased bacterial diversity characterizes an altered gut microbiota in psoriatic arthritis and resembles dysbiosis of inflammatory bowel disease. Arthritis Rheumatol. 2015;67:128-139.
- Chen Y-H, Wu C-S, Chao Y-H, et al. Lactobacillus pentosus GMNL-77 inhibits skin lesions in imiquimod-induced psoriasis-like mice. J Food Drug Anal. 2017;25:559-566.
- Groeger D, O’Mahony L, Murphy EF, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4:325-339.
- Hosking A-M, Juhasz M, Atanaskova Mesinkovska N. Complementary and alternative treatments for alopecia: a comprehensive review. Skin Appendage Disord. 2019;5:72-89.
- Tkachenko E, Okhovat J-P, Manjaly P, et al. Complementary & alternative medicine for alopecia areata: a systematic review [published online December 20, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.12.027
- Lepe K, Zito PM. Alopecia areata. In: StatPearls. StatPearls Publishing; 2021. Accessed July 22, 2021. https://pubmed.ncbi.nlm.nih.gov/30725685/
- Ismail FF, Sinclair R. JAK inhibition in the treatment of alopecia areata—a promising new dawn? Expert Rev Clin Pharmacol. 2020;13:43-51. doi:10.1080/17512433.2020.1702878
- van den Biggelaar FJHM, Smolders J, Jansen JFA. Complementary and alternative medicine in alopecia areata. AM J Clin Dermatol. 2010;11:11-20.
- Hussain ST, Mostaghimi A, Barr PJ, et al. Utilization of mental health resources and complementary and alternative therapies for alopecia areata: a U.S. survey. Int J Trichology. 2017;9:160-164.
- Hajheydari Z, Jamshidi M, Akbari J, et al. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29-32.
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343-346.
- Burian JP, Sacramento LVS, Carlos IZ. Fungal infection control by garlic extracts (Allium sativum L.) and modulation of peritoneal macrophages activity in murine model of sporotrichosis. Braz J Biol. 2017;77:848-855.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Lakshmi C, Srinivas CR. Allergic contact dermatitis following aromatherapy with valiya narayana thailam—an ayurvedic oil presenting as exfoliative dermatitis. Contact Dermatitis. 2009;61:297-298.
- Carson CF, Hammer KA, Riley TV. Melaleuca alternifolia (tea tree) oil: a review of antimicrobial and other medicinal properties. Clin Microbiol Rev. 2006;19:50-62.
- Groot AC de, Schmidt E. Tea tree oil: contact allergy and chemical composition. Contact Dermatitis. 2016;75:129-143.
- de Groot AC, Schmidt E. Essential oils, part I: introduction. dermatitis. 2016;27:39-42.
- Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
- Ozmen I, Caliskan E, Arca E, et al. Efficacy of aromatherapy in the treatment of localized alopecia areata: a double-blind placebo controlled study. Gulhane Med J. 2015;57:233.
- Oh GN, Son SW. Efficacy of Korean red ginseng in the treatment of alopecia areata. J Ginseng Res. 2012;36:391-395.
- Yang D-Q, You L-P, Song P-H, et al. A randomized controlled trial comparing total glucosides of paeony capsule and compound glycyrrhizin tablet for alopecia areata. Chin J Integr Med. 2012;18:621-625.
Practice Points
- Dermatologic patients are increasingly opting for alternative treatments in addition to or instead of standard therapies for many common skin conditions.
- Dermatologists should be aware of the emerging evidence regarding the risks and benefits of some of the most popular alternative treatments in common skin disorders.
- Counseling patients on the side effects that accompany many supplements and the lack of data to support others is a crucial component of patient care.





