User login
Psoriatic Arthritis Treatment: The Dermatologist’s Role
Resolution of Psoriatic Lesions on the Gingiva and Hard Palate Following Administration of Adalimumab for Cutaneous Psoriasis
Psoriasis is a chronic, relapsing, inflammatory systemic disorder of the skin with an incidence of 2% to 3% and is estimated to affect 125 million individuals worldwide.1 Environmental triggers of disease modulation may include cutaneous microbiota, smoking, alcohol use, drugs (ie, beta-blockers, lithium, antimalarials), stress, and trauma.2 Comorbidities associated with cutaneous lesions include psoriatic arthritis, Crohn disease, type 2 diabetes mellitus, metabolic syndrome, stroke, and cardiovascular disease.3 In some studies, patients with psoriasis also had a 24% to 27% increased propensity for periodontal bone loss versus 10% of controls.4,5
Oral psoriasis is rare and case reports have been preferentially published in dental journals, usually with regard to glossal lesions, leaving gingival and palatal psoriatic involvement infrequently reported in the dermatologic literature.6,7 In fact, oral assessments involving 535 psoriatic patients from a dermatology center only yielded cases of geographic and fissured tongue.8 Another study at a psoriasis clinic found 3.8% (21/547) of patients with geographic tongue, 3.1% (17/547) with buccal mucosal plaques, and only 0.4% (2/547) with palatal lesions.9 To extend the knowledge of oral psoriasis, we provide the clinical and histopathologic findings of a patient with synchronous oral and cutaneous psoriatic lesions that responded well to the administration of adalimumab for management of recurrent cutaneous disease.
Case Report
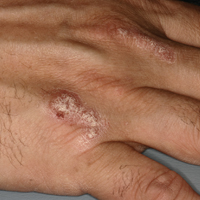
A 51-year-old man presented to the attending periodontist for comprehensive treatment of multiple quadrants of gingival recession. His medical history was remarkable for psoriasis; Prinzmetal angina, which led to myocardial infarction; and diverticulitis. The cutaneous psoriasis began approximately 18 years prior to the current presentation and was initially managed with various topical therapeutics. At an 11-year follow-up, the patient was experiencing poor lesional control as well as severe pruritus and was prescribed etanercept by a dermatologist. His inconsistent compliance with frequency and dosing failed to achieve satisfactory disease suppression and etanercept was discontinued after approximately 2.5 years. Two years later the patient was switched to adalimumab by a dermatologist, and around this time he had developed psoriatic arthritis of the hands and knees and pitting of the nail plates. The patient elected to discontinue adalimumab usage after 3 years due to successful management of the skin lesions, cost considerations, and his perception that the psoriasis could “remain in remission.” After a 6-month lapse, the patient resumed adalimumab due to cutaneous lesional recurrence (Figure 1A).
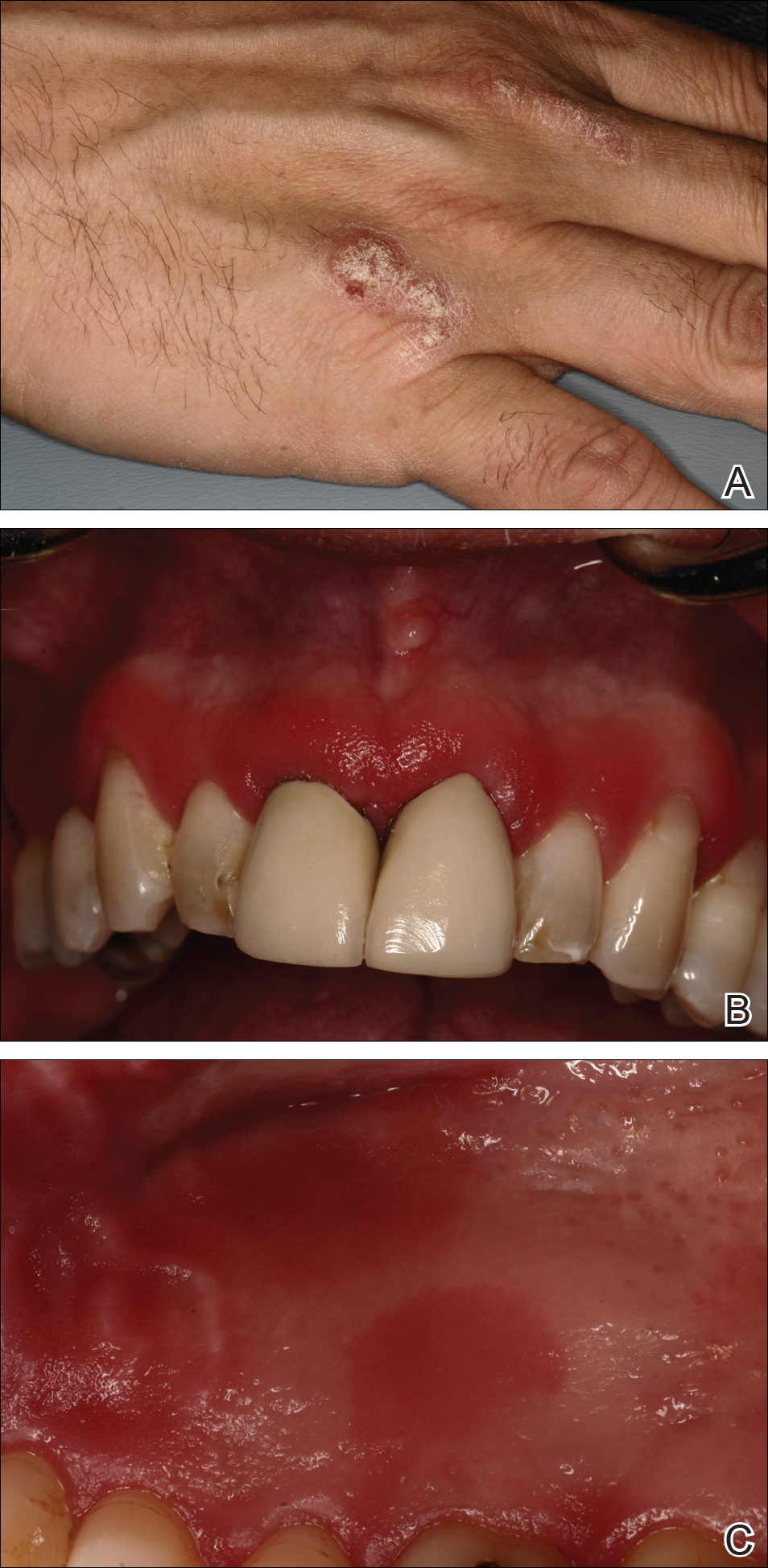
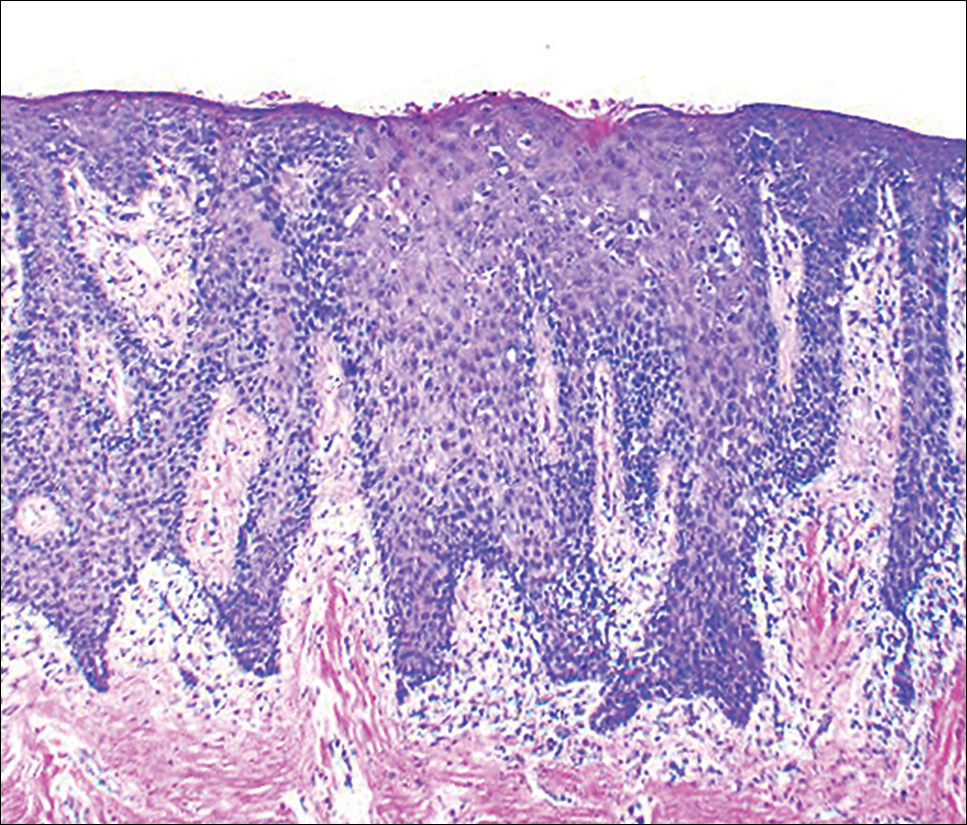
At the current presentation, an oral examination performed 2 days after the reinstitution of adalim-umab revealed generalized severe gingivitis with an atypical inflammatory response that extended from just beyond the mucogingival junction to the marginal gingiva. The gingiva also appeared edematous with a conspicuously granular surface (Figure 1B). The hard palate displayed multiple red macules of varying sizes (Figure 1C). A maxillary gingival biopsy demonstrated hyperkeratosis, parakeratosis, spongiosis, acanthosis, elongation of the rete ridges, numerous collections of neutrophils (Munro microabscesses), and abundant lymphocytes in the subjacent connective tissue (Figure 2). Periodic acid–Schiff staining was negative for fungal hyphae. These features were consistent with oral mucosal psoriasis.
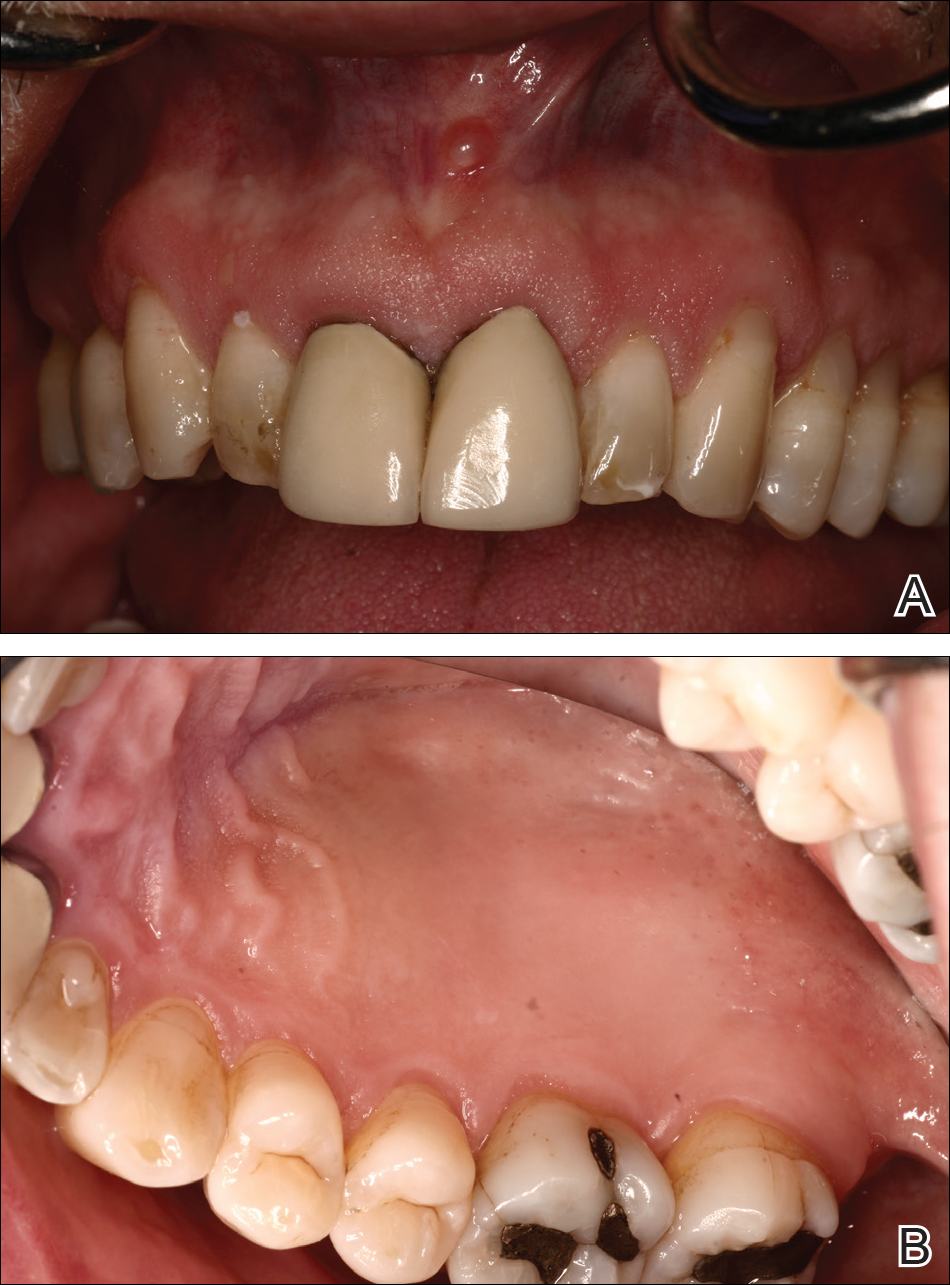
At a 2-month follow-up, the biopsy site had healed without incident and without loss of the gingival architecture. There was an almost-complete resolution of the gingival erythema (Figure 3A) and the patient has since noticed a lack of bleeding using floss. Additionally, the red macules on the palate were no longer present (Figure 3B). The cutaneous plaques were greatly reduced in size and the patient experienced a proportionate decline in pruritus. Based on the uneventful surgical biopsy procedure, the patient was advised to undergo gingival grafting and has not returned for periodontal care.
Comment
Psoriasis of the oral cavity is rare and typically occurs on the tongue and less frequently on the hard palate, lip, buccal mucosa, and gingiva.2,7 The lesions are almost always concordant with cutaneous psoriasis, and only sporadic examples exclusive to the oral mucosa have been recognized.7,10 Gingival psoriasis usually is described as intensely erythematous and occasionally laced with white scaly streaks involving the marginal gingiva that extend toward the mucogingival junction. In general, the erythematous presentation of gingival psoriasis may not be commensurate with the degree of inflammation induced by dental plaque-based periodontal disease. Doben11 documented gingival psoriasis as appearing “deeply stippled and grainy” and commented that the tissue was “friable” and incapable of maintaining a “clean incision line” during periodontal surgery. In our patient, the gingiva also had exhibited a granular surface. Patients with oral psoriasis often report soreness or a burning sensation of the gingiva, which may easily bleed on manipulation or brushing the teeth, whereas other patients are asymptomatic,12 as in our case. Psoriasis of the hard palate usually presents as multiple painless red macules. Unlike cutaneous psoriasis, oral lesions rarely evoke pruritus.10 Histopathologically, oral psoriasis bears a striking resemblance to its cutaneous counterpart. The epithelium has a pronounced parakeratinized surface with elongated rete ridges and aggregations of Munro microabscesses. The connective tissue often is composed of dilated capillaries that closely approximate the epithelium as well as infiltrations of lymphocytes. Specimens suspected for oral psoriasis should routinely be stained with periodic acid–Schiff to rule out candidiasis coinfection. The microscopic findings of our patient were congruent with prior reports of oral psoriasis.7,10-12 Some clinicians have questioned if psoriasis can actually occur in the oral cavity, but most authorities in the field have recognized its true existence, as evidenced by various shared HLA antigens, specifically HLA-Cw.13
Another group of oral lesions collectively referred to as psoriasiform mucositis, notably geographic tongue (benign migratory glossitis, erythema migrans) and its extraglossal variant geographic stomatitis,14,15 have histopathologic features and HLAs similar to those seen in cutaneous psoriasis.13 Interestingly, geographic tongue has been found in 3.8% to 9.1% of cohorts with cutaneous psoriasis,8,9 but in the extant population, the vast majority of patients with oral psoriasiform mucositis do not have cutaneous psoriasis. Other differential diagnoses for gingival psoriasis are lichen planus, human immunodeficiency virus–associated periodontitis, desquamative gingivitis, plasma cell gingivitis, erythematous candidiasis, mucous membrane pemphigoid, pemphigus vulgaris, leukemia, systemic lupus erythematosus, granulomatosis with polyangiitis, orofacial granulomatosis, localized juvenile spongiotic gingivitis hyperplasia, and primary gingivostomatitis.
Management of gingival psoriasis focuses on strategies to reduce inflammation and discomfort and measures to achieve meticulous oral plaque control. Judicious efforts should be exercised to avoid oral soft-tissue injury when performing periodontal scaling, although it has not been established whether gingival psoriasis is associated with the Köbner phenomenon, as seen with cutaneous lesions. Adjunctive measures employed for symptomatic patients have involved the use of corticosteroids (eg, lesional injection, oral rinse, systemic) and oral rinses with retinoic acid, chlorhexidine gluconate, and warm saline.7,10,16 Prolonged utilization of corticosteroids, however, may necessitate supplemental administration of antifungal agents.
This case report represents a rare documentation of a successful outcome of gingival and palatal psoriasis subsequent to the reinstitution of adalimumab solely for treatment of recurrent cutaneous disease. There likely is a pharmacologic basis for the amelioration of oral psoriasis in our patient. Adalimumab is a bivalent IgG monoclonal antibody that binds to activated dermal dendritic cell receptors of tumor necrosis factor α, thereby attenuating a cytokine-derived inflammatory response and apoptosis.17 In fact, patients with rheumatoid arthritis showed notable reductions in both gingival inflammation and bleeding following a 3-month regimen of adalimumab.18
Conclusion
Practitioners should be aware of the phenotypic overlap of cutaneous and oral psoriasis, particularly involving the gingiva and palate. It is recommended that psoriasis patients routinely receive a dental prophylaxis and engage in oral hygiene efforts to reduce the presence of oral microbiota. Furthermore, it is emphasized that psoriatic patients who maintain an atypical erythematous presentation on the oral mucosa undergo a biopsy for identification of the lesions and correlation with disease dissemination. Prospective studies are needed to characterize the clinical courses of oral psoriasis, ascertain their correlative behavior with cutaneous flares, and determine if lesional improvement can be achieved with the use of biologic agents or other therapeutic modalities.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Younai FS, Phelan JA. Oral mucositis with features of psoriasis: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:61-67.
- Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167:1345-1350.
- Lazaridou E, Tsikrikoni A, Fotiadou C, et al. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. J Eur Acad Dermatol Venereol. 2013;27:967-972.
- Skudutyte-Rysstad R, Slevolden EM, Hansen BF, et al. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139.
- Zunt SL, Tomich CE. Erythema migrans—a psoriasiform lesion of the oral mucosa. J Dermatol Surg Oncol. 1989;15:1067-1070.
- Reis V, Artico G, Seo J, et al. Psoriasiform mucositis on the gingival and palatal mucosae treated with retinoic-acid mouthwash. Int J Dermatol. 2013;52:113-115.
- Germi L, De Giorgi V, Bergamo F, et al. Psoriasis and oral lesions: multicentric study of oral mucosa diseases Italian group (GIPMO). Dermatol Online J. 2012;18:11.
- Kaur I, Handa S, Kumar B. Oral lesions in psoriasis. Int J Dermatol. 1997;36:78-79.
- Brayshaw HA, Orban B. Psoriasis gingivae. J Periodontol. 1953;24:156-160.
- Doben DI. Psoriasis of the attached gingiva. J Periodontol. 1976;47:38-40.
- Mattsson U, Warfvinge G, Jontell M. Oral psoriasis—a diagnostic dilemma: a report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e183-e189.
- Dermatologic diseases. In: Neville BW, Damm DD, Allen CM, et al, eds. Oral and Maxillofacial Pathology. 3rd ed. St. Louis, MO: Saunders/Elsevier; 2009:792-794.
- Brooks JK, Balciunas BA. Geographic stomatitis: review of the literature and report of five cases. J Am Dent Assoc. 1987;115:421-424.
- Brooks JK, Nikitakis NG. Multiple mucosal lesions. erythema migrans. Gen Dent. 2007;55:160, 163.
- Ulmansky M, Michelle R, Azaz B. Oral psoriasis: report of six new cases. J Oral Pathol Med. 1995;24:42-45.
- Lis K, Kuzawinska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Kobayashi T, Yokoyama T, Ito S, et al. Periodontal and serum protein profiles in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitor adalimumab. J Periodontol. 2014;85:1480-1488.
Psoriasis is a chronic, relapsing, inflammatory systemic disorder of the skin with an incidence of 2% to 3% and is estimated to affect 125 million individuals worldwide.1 Environmental triggers of disease modulation may include cutaneous microbiota, smoking, alcohol use, drugs (ie, beta-blockers, lithium, antimalarials), stress, and trauma.2 Comorbidities associated with cutaneous lesions include psoriatic arthritis, Crohn disease, type 2 diabetes mellitus, metabolic syndrome, stroke, and cardiovascular disease.3 In some studies, patients with psoriasis also had a 24% to 27% increased propensity for periodontal bone loss versus 10% of controls.4,5
Oral psoriasis is rare and case reports have been preferentially published in dental journals, usually with regard to glossal lesions, leaving gingival and palatal psoriatic involvement infrequently reported in the dermatologic literature.6,7 In fact, oral assessments involving 535 psoriatic patients from a dermatology center only yielded cases of geographic and fissured tongue.8 Another study at a psoriasis clinic found 3.8% (21/547) of patients with geographic tongue, 3.1% (17/547) with buccal mucosal plaques, and only 0.4% (2/547) with palatal lesions.9 To extend the knowledge of oral psoriasis, we provide the clinical and histopathologic findings of a patient with synchronous oral and cutaneous psoriatic lesions that responded well to the administration of adalimumab for management of recurrent cutaneous disease.
Case Report
A 51-year-old man presented to the attending periodontist for comprehensive treatment of multiple quadrants of gingival recession. His medical history was remarkable for psoriasis; Prinzmetal angina, which led to myocardial infarction; and diverticulitis. The cutaneous psoriasis began approximately 18 years prior to the current presentation and was initially managed with various topical therapeutics. At an 11-year follow-up, the patient was experiencing poor lesional control as well as severe pruritus and was prescribed etanercept by a dermatologist. His inconsistent compliance with frequency and dosing failed to achieve satisfactory disease suppression and etanercept was discontinued after approximately 2.5 years. Two years later the patient was switched to adalimumab by a dermatologist, and around this time he had developed psoriatic arthritis of the hands and knees and pitting of the nail plates. The patient elected to discontinue adalimumab usage after 3 years due to successful management of the skin lesions, cost considerations, and his perception that the psoriasis could “remain in remission.” After a 6-month lapse, the patient resumed adalimumab due to cutaneous lesional recurrence (Figure 1A).

At the current presentation, an oral examination performed 2 days after the reinstitution of adalim-umab revealed generalized severe gingivitis with an atypical inflammatory response that extended from just beyond the mucogingival junction to the marginal gingiva. The gingiva also appeared edematous with a conspicuously granular surface (Figure 1B). The hard palate displayed multiple red macules of varying sizes (Figure 1C). A maxillary gingival biopsy demonstrated hyperkeratosis, parakeratosis, spongiosis, acanthosis, elongation of the rete ridges, numerous collections of neutrophils (Munro microabscesses), and abundant lymphocytes in the subjacent connective tissue (Figure 2). Periodic acid–Schiff staining was negative for fungal hyphae. These features were consistent with oral mucosal psoriasis.

At a 2-month follow-up, the biopsy site had healed without incident and without loss of the gingival architecture. There was an almost-complete resolution of the gingival erythema (Figure 3A) and the patient has since noticed a lack of bleeding using floss. Additionally, the red macules on the palate were no longer present (Figure 3B). The cutaneous plaques were greatly reduced in size and the patient experienced a proportionate decline in pruritus. Based on the uneventful surgical biopsy procedure, the patient was advised to undergo gingival grafting and has not returned for periodontal care.

Comment
Psoriasis of the oral cavity is rare and typically occurs on the tongue and less frequently on the hard palate, lip, buccal mucosa, and gingiva.2,7 The lesions are almost always concordant with cutaneous psoriasis, and only sporadic examples exclusive to the oral mucosa have been recognized.7,10 Gingival psoriasis usually is described as intensely erythematous and occasionally laced with white scaly streaks involving the marginal gingiva that extend toward the mucogingival junction. In general, the erythematous presentation of gingival psoriasis may not be commensurate with the degree of inflammation induced by dental plaque-based periodontal disease. Doben11 documented gingival psoriasis as appearing “deeply stippled and grainy” and commented that the tissue was “friable” and incapable of maintaining a “clean incision line” during periodontal surgery. In our patient, the gingiva also had exhibited a granular surface. Patients with oral psoriasis often report soreness or a burning sensation of the gingiva, which may easily bleed on manipulation or brushing the teeth, whereas other patients are asymptomatic,12 as in our case. Psoriasis of the hard palate usually presents as multiple painless red macules. Unlike cutaneous psoriasis, oral lesions rarely evoke pruritus.10 Histopathologically, oral psoriasis bears a striking resemblance to its cutaneous counterpart. The epithelium has a pronounced parakeratinized surface with elongated rete ridges and aggregations of Munro microabscesses. The connective tissue often is composed of dilated capillaries that closely approximate the epithelium as well as infiltrations of lymphocytes. Specimens suspected for oral psoriasis should routinely be stained with periodic acid–Schiff to rule out candidiasis coinfection. The microscopic findings of our patient were congruent with prior reports of oral psoriasis.7,10-12 Some clinicians have questioned if psoriasis can actually occur in the oral cavity, but most authorities in the field have recognized its true existence, as evidenced by various shared HLA antigens, specifically HLA-Cw.13
Another group of oral lesions collectively referred to as psoriasiform mucositis, notably geographic tongue (benign migratory glossitis, erythema migrans) and its extraglossal variant geographic stomatitis,14,15 have histopathologic features and HLAs similar to those seen in cutaneous psoriasis.13 Interestingly, geographic tongue has been found in 3.8% to 9.1% of cohorts with cutaneous psoriasis,8,9 but in the extant population, the vast majority of patients with oral psoriasiform mucositis do not have cutaneous psoriasis. Other differential diagnoses for gingival psoriasis are lichen planus, human immunodeficiency virus–associated periodontitis, desquamative gingivitis, plasma cell gingivitis, erythematous candidiasis, mucous membrane pemphigoid, pemphigus vulgaris, leukemia, systemic lupus erythematosus, granulomatosis with polyangiitis, orofacial granulomatosis, localized juvenile spongiotic gingivitis hyperplasia, and primary gingivostomatitis.
Management of gingival psoriasis focuses on strategies to reduce inflammation and discomfort and measures to achieve meticulous oral plaque control. Judicious efforts should be exercised to avoid oral soft-tissue injury when performing periodontal scaling, although it has not been established whether gingival psoriasis is associated with the Köbner phenomenon, as seen with cutaneous lesions. Adjunctive measures employed for symptomatic patients have involved the use of corticosteroids (eg, lesional injection, oral rinse, systemic) and oral rinses with retinoic acid, chlorhexidine gluconate, and warm saline.7,10,16 Prolonged utilization of corticosteroids, however, may necessitate supplemental administration of antifungal agents.
This case report represents a rare documentation of a successful outcome of gingival and palatal psoriasis subsequent to the reinstitution of adalimumab solely for treatment of recurrent cutaneous disease. There likely is a pharmacologic basis for the amelioration of oral psoriasis in our patient. Adalimumab is a bivalent IgG monoclonal antibody that binds to activated dermal dendritic cell receptors of tumor necrosis factor α, thereby attenuating a cytokine-derived inflammatory response and apoptosis.17 In fact, patients with rheumatoid arthritis showed notable reductions in both gingival inflammation and bleeding following a 3-month regimen of adalimumab.18
Conclusion
Practitioners should be aware of the phenotypic overlap of cutaneous and oral psoriasis, particularly involving the gingiva and palate. It is recommended that psoriasis patients routinely receive a dental prophylaxis and engage in oral hygiene efforts to reduce the presence of oral microbiota. Furthermore, it is emphasized that psoriatic patients who maintain an atypical erythematous presentation on the oral mucosa undergo a biopsy for identification of the lesions and correlation with disease dissemination. Prospective studies are needed to characterize the clinical courses of oral psoriasis, ascertain their correlative behavior with cutaneous flares, and determine if lesional improvement can be achieved with the use of biologic agents or other therapeutic modalities.
Psoriasis is a chronic, relapsing, inflammatory systemic disorder of the skin with an incidence of 2% to 3% and is estimated to affect 125 million individuals worldwide.1 Environmental triggers of disease modulation may include cutaneous microbiota, smoking, alcohol use, drugs (ie, beta-blockers, lithium, antimalarials), stress, and trauma.2 Comorbidities associated with cutaneous lesions include psoriatic arthritis, Crohn disease, type 2 diabetes mellitus, metabolic syndrome, stroke, and cardiovascular disease.3 In some studies, patients with psoriasis also had a 24% to 27% increased propensity for periodontal bone loss versus 10% of controls.4,5
Oral psoriasis is rare and case reports have been preferentially published in dental journals, usually with regard to glossal lesions, leaving gingival and palatal psoriatic involvement infrequently reported in the dermatologic literature.6,7 In fact, oral assessments involving 535 psoriatic patients from a dermatology center only yielded cases of geographic and fissured tongue.8 Another study at a psoriasis clinic found 3.8% (21/547) of patients with geographic tongue, 3.1% (17/547) with buccal mucosal plaques, and only 0.4% (2/547) with palatal lesions.9 To extend the knowledge of oral psoriasis, we provide the clinical and histopathologic findings of a patient with synchronous oral and cutaneous psoriatic lesions that responded well to the administration of adalimumab for management of recurrent cutaneous disease.
Case Report
A 51-year-old man presented to the attending periodontist for comprehensive treatment of multiple quadrants of gingival recession. His medical history was remarkable for psoriasis; Prinzmetal angina, which led to myocardial infarction; and diverticulitis. The cutaneous psoriasis began approximately 18 years prior to the current presentation and was initially managed with various topical therapeutics. At an 11-year follow-up, the patient was experiencing poor lesional control as well as severe pruritus and was prescribed etanercept by a dermatologist. His inconsistent compliance with frequency and dosing failed to achieve satisfactory disease suppression and etanercept was discontinued after approximately 2.5 years. Two years later the patient was switched to adalimumab by a dermatologist, and around this time he had developed psoriatic arthritis of the hands and knees and pitting of the nail plates. The patient elected to discontinue adalimumab usage after 3 years due to successful management of the skin lesions, cost considerations, and his perception that the psoriasis could “remain in remission.” After a 6-month lapse, the patient resumed adalimumab due to cutaneous lesional recurrence (Figure 1A).

At the current presentation, an oral examination performed 2 days after the reinstitution of adalim-umab revealed generalized severe gingivitis with an atypical inflammatory response that extended from just beyond the mucogingival junction to the marginal gingiva. The gingiva also appeared edematous with a conspicuously granular surface (Figure 1B). The hard palate displayed multiple red macules of varying sizes (Figure 1C). A maxillary gingival biopsy demonstrated hyperkeratosis, parakeratosis, spongiosis, acanthosis, elongation of the rete ridges, numerous collections of neutrophils (Munro microabscesses), and abundant lymphocytes in the subjacent connective tissue (Figure 2). Periodic acid–Schiff staining was negative for fungal hyphae. These features were consistent with oral mucosal psoriasis.

At a 2-month follow-up, the biopsy site had healed without incident and without loss of the gingival architecture. There was an almost-complete resolution of the gingival erythema (Figure 3A) and the patient has since noticed a lack of bleeding using floss. Additionally, the red macules on the palate were no longer present (Figure 3B). The cutaneous plaques were greatly reduced in size and the patient experienced a proportionate decline in pruritus. Based on the uneventful surgical biopsy procedure, the patient was advised to undergo gingival grafting and has not returned for periodontal care.

Comment
Psoriasis of the oral cavity is rare and typically occurs on the tongue and less frequently on the hard palate, lip, buccal mucosa, and gingiva.2,7 The lesions are almost always concordant with cutaneous psoriasis, and only sporadic examples exclusive to the oral mucosa have been recognized.7,10 Gingival psoriasis usually is described as intensely erythematous and occasionally laced with white scaly streaks involving the marginal gingiva that extend toward the mucogingival junction. In general, the erythematous presentation of gingival psoriasis may not be commensurate with the degree of inflammation induced by dental plaque-based periodontal disease. Doben11 documented gingival psoriasis as appearing “deeply stippled and grainy” and commented that the tissue was “friable” and incapable of maintaining a “clean incision line” during periodontal surgery. In our patient, the gingiva also had exhibited a granular surface. Patients with oral psoriasis often report soreness or a burning sensation of the gingiva, which may easily bleed on manipulation or brushing the teeth, whereas other patients are asymptomatic,12 as in our case. Psoriasis of the hard palate usually presents as multiple painless red macules. Unlike cutaneous psoriasis, oral lesions rarely evoke pruritus.10 Histopathologically, oral psoriasis bears a striking resemblance to its cutaneous counterpart. The epithelium has a pronounced parakeratinized surface with elongated rete ridges and aggregations of Munro microabscesses. The connective tissue often is composed of dilated capillaries that closely approximate the epithelium as well as infiltrations of lymphocytes. Specimens suspected for oral psoriasis should routinely be stained with periodic acid–Schiff to rule out candidiasis coinfection. The microscopic findings of our patient were congruent with prior reports of oral psoriasis.7,10-12 Some clinicians have questioned if psoriasis can actually occur in the oral cavity, but most authorities in the field have recognized its true existence, as evidenced by various shared HLA antigens, specifically HLA-Cw.13
Another group of oral lesions collectively referred to as psoriasiform mucositis, notably geographic tongue (benign migratory glossitis, erythema migrans) and its extraglossal variant geographic stomatitis,14,15 have histopathologic features and HLAs similar to those seen in cutaneous psoriasis.13 Interestingly, geographic tongue has been found in 3.8% to 9.1% of cohorts with cutaneous psoriasis,8,9 but in the extant population, the vast majority of patients with oral psoriasiform mucositis do not have cutaneous psoriasis. Other differential diagnoses for gingival psoriasis are lichen planus, human immunodeficiency virus–associated periodontitis, desquamative gingivitis, plasma cell gingivitis, erythematous candidiasis, mucous membrane pemphigoid, pemphigus vulgaris, leukemia, systemic lupus erythematosus, granulomatosis with polyangiitis, orofacial granulomatosis, localized juvenile spongiotic gingivitis hyperplasia, and primary gingivostomatitis.
Management of gingival psoriasis focuses on strategies to reduce inflammation and discomfort and measures to achieve meticulous oral plaque control. Judicious efforts should be exercised to avoid oral soft-tissue injury when performing periodontal scaling, although it has not been established whether gingival psoriasis is associated with the Köbner phenomenon, as seen with cutaneous lesions. Adjunctive measures employed for symptomatic patients have involved the use of corticosteroids (eg, lesional injection, oral rinse, systemic) and oral rinses with retinoic acid, chlorhexidine gluconate, and warm saline.7,10,16 Prolonged utilization of corticosteroids, however, may necessitate supplemental administration of antifungal agents.
This case report represents a rare documentation of a successful outcome of gingival and palatal psoriasis subsequent to the reinstitution of adalimumab solely for treatment of recurrent cutaneous disease. There likely is a pharmacologic basis for the amelioration of oral psoriasis in our patient. Adalimumab is a bivalent IgG monoclonal antibody that binds to activated dermal dendritic cell receptors of tumor necrosis factor α, thereby attenuating a cytokine-derived inflammatory response and apoptosis.17 In fact, patients with rheumatoid arthritis showed notable reductions in both gingival inflammation and bleeding following a 3-month regimen of adalimumab.18
Conclusion
Practitioners should be aware of the phenotypic overlap of cutaneous and oral psoriasis, particularly involving the gingiva and palate. It is recommended that psoriasis patients routinely receive a dental prophylaxis and engage in oral hygiene efforts to reduce the presence of oral microbiota. Furthermore, it is emphasized that psoriatic patients who maintain an atypical erythematous presentation on the oral mucosa undergo a biopsy for identification of the lesions and correlation with disease dissemination. Prospective studies are needed to characterize the clinical courses of oral psoriasis, ascertain their correlative behavior with cutaneous flares, and determine if lesional improvement can be achieved with the use of biologic agents or other therapeutic modalities.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Younai FS, Phelan JA. Oral mucositis with features of psoriasis: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:61-67.
- Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167:1345-1350.
- Lazaridou E, Tsikrikoni A, Fotiadou C, et al. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. J Eur Acad Dermatol Venereol. 2013;27:967-972.
- Skudutyte-Rysstad R, Slevolden EM, Hansen BF, et al. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139.
- Zunt SL, Tomich CE. Erythema migrans—a psoriasiform lesion of the oral mucosa. J Dermatol Surg Oncol. 1989;15:1067-1070.
- Reis V, Artico G, Seo J, et al. Psoriasiform mucositis on the gingival and palatal mucosae treated with retinoic-acid mouthwash. Int J Dermatol. 2013;52:113-115.
- Germi L, De Giorgi V, Bergamo F, et al. Psoriasis and oral lesions: multicentric study of oral mucosa diseases Italian group (GIPMO). Dermatol Online J. 2012;18:11.
- Kaur I, Handa S, Kumar B. Oral lesions in psoriasis. Int J Dermatol. 1997;36:78-79.
- Brayshaw HA, Orban B. Psoriasis gingivae. J Periodontol. 1953;24:156-160.
- Doben DI. Psoriasis of the attached gingiva. J Periodontol. 1976;47:38-40.
- Mattsson U, Warfvinge G, Jontell M. Oral psoriasis—a diagnostic dilemma: a report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e183-e189.
- Dermatologic diseases. In: Neville BW, Damm DD, Allen CM, et al, eds. Oral and Maxillofacial Pathology. 3rd ed. St. Louis, MO: Saunders/Elsevier; 2009:792-794.
- Brooks JK, Balciunas BA. Geographic stomatitis: review of the literature and report of five cases. J Am Dent Assoc. 1987;115:421-424.
- Brooks JK, Nikitakis NG. Multiple mucosal lesions. erythema migrans. Gen Dent. 2007;55:160, 163.
- Ulmansky M, Michelle R, Azaz B. Oral psoriasis: report of six new cases. J Oral Pathol Med. 1995;24:42-45.
- Lis K, Kuzawinska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Kobayashi T, Yokoyama T, Ito S, et al. Periodontal and serum protein profiles in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitor adalimumab. J Periodontol. 2014;85:1480-1488.
- Gupta R, Debbaneh MG, Liao W. Genetic epidemiology of psoriasis. Curr Dermatol Rep. 2014;3:61-78.
- Younai FS, Phelan JA. Oral mucositis with features of psoriasis: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:61-67.
- Xu T, Zhang YH. Association of psoriasis with stroke and myocardial infarction: meta-analysis of cohort studies. Br J Dermatol. 2012;167:1345-1350.
- Lazaridou E, Tsikrikoni A, Fotiadou C, et al. Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. J Eur Acad Dermatol Venereol. 2013;27:967-972.
- Skudutyte-Rysstad R, Slevolden EM, Hansen BF, et al. Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 2014;14:139.
- Zunt SL, Tomich CE. Erythema migrans—a psoriasiform lesion of the oral mucosa. J Dermatol Surg Oncol. 1989;15:1067-1070.
- Reis V, Artico G, Seo J, et al. Psoriasiform mucositis on the gingival and palatal mucosae treated with retinoic-acid mouthwash. Int J Dermatol. 2013;52:113-115.
- Germi L, De Giorgi V, Bergamo F, et al. Psoriasis and oral lesions: multicentric study of oral mucosa diseases Italian group (GIPMO). Dermatol Online J. 2012;18:11.
- Kaur I, Handa S, Kumar B. Oral lesions in psoriasis. Int J Dermatol. 1997;36:78-79.
- Brayshaw HA, Orban B. Psoriasis gingivae. J Periodontol. 1953;24:156-160.
- Doben DI. Psoriasis of the attached gingiva. J Periodontol. 1976;47:38-40.
- Mattsson U, Warfvinge G, Jontell M. Oral psoriasis—a diagnostic dilemma: a report of two cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:e183-e189.
- Dermatologic diseases. In: Neville BW, Damm DD, Allen CM, et al, eds. Oral and Maxillofacial Pathology. 3rd ed. St. Louis, MO: Saunders/Elsevier; 2009:792-794.
- Brooks JK, Balciunas BA. Geographic stomatitis: review of the literature and report of five cases. J Am Dent Assoc. 1987;115:421-424.
- Brooks JK, Nikitakis NG. Multiple mucosal lesions. erythema migrans. Gen Dent. 2007;55:160, 163.
- Ulmansky M, Michelle R, Azaz B. Oral psoriasis: report of six new cases. J Oral Pathol Med. 1995;24:42-45.
- Lis K, Kuzawinska O, Bałkowiec-Iskra E. Tumor necrosis factor inhibitors—state of knowledge. Arch Med Sci. 2014;10:1175-1185.
- Kobayashi T, Yokoyama T, Ito S, et al. Periodontal and serum protein profiles in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitor adalimumab. J Periodontol. 2014;85:1480-1488.
Practice Points
- A subset of patients with cutaneous psoriasis may be associated with oral psoriatic outbreaks.
- Oral psoriasis presents as an atypical inflammatory response, and histopathologic assessment is recommended for lesional identity.
- Use of adalimumab for management of cutaneous psoriasis may demonstrate efficacy for oral psoriasis.
Biosimilars in Psoriasis: The Future or Not?
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19
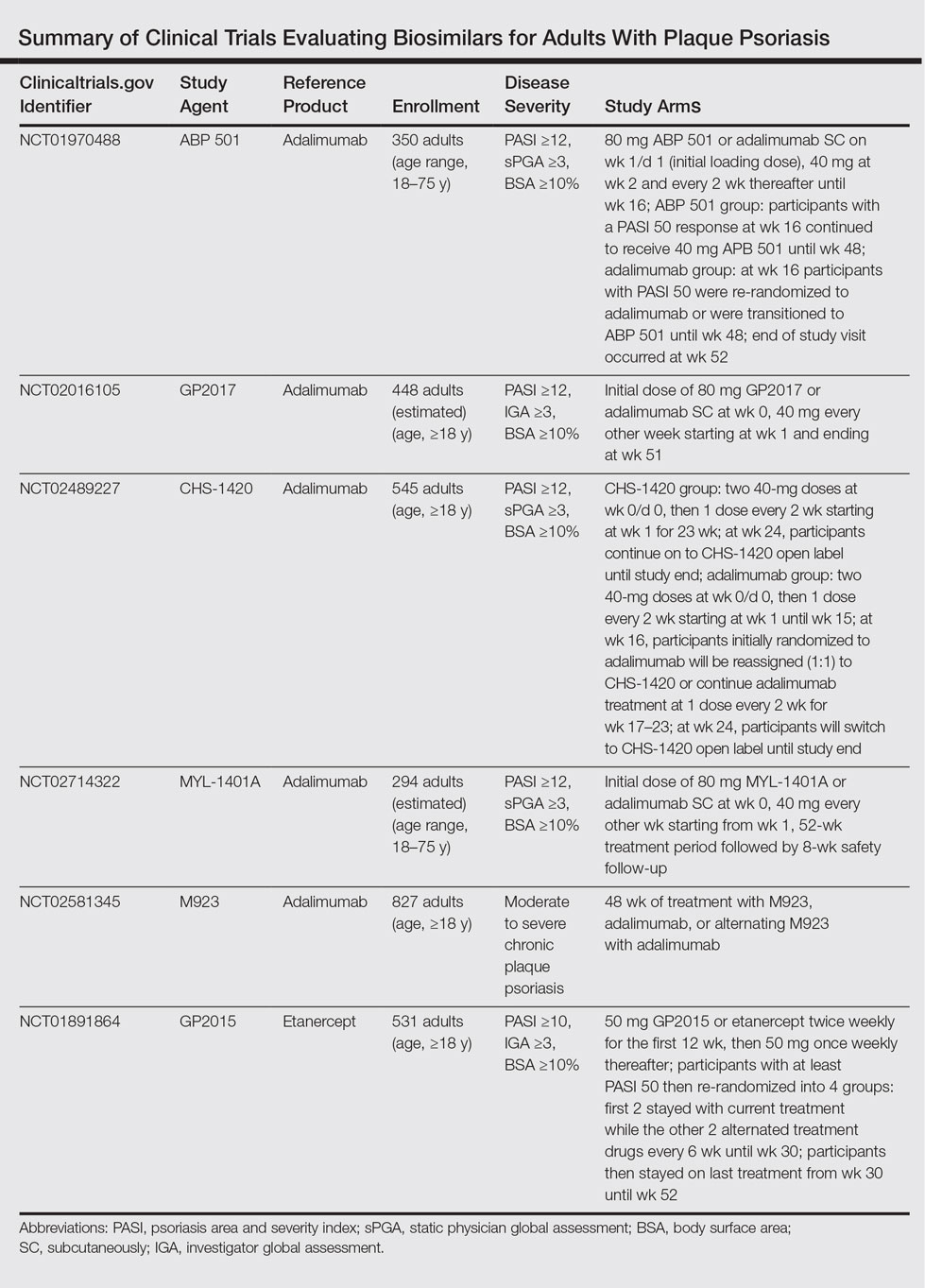
A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19

A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
According to the US Food and Drug Administration (FDA), a biosimilar is “highly similar to an FDA-approved biological product, . . . and has no clinically meaningful differences in terms of safety and effectiveness.”1 The Biologics Price Competition and Innovation (BPCI) Act of 2009 created an expedited pathway for the approval of products shown to be biosimilar to FDA-licensed reference products.2 In 2013, the European Medicines Agency approved the first biosimilar modeled on infliximab (Remsima [formerly known as CT-P13], Celltrion Healthcare Co, Ltd) for the same indications as its reference product.3 In 2016, the FDA approved Inflectra (Hospira, a Pfizer Company), an infliximab biosimilar; Erelzi (Sandoz, a Novartis Division), an etanercept biosimilar; and Amjevita (Amgen Inc), an adalimumab biosimilar, all for numerous clinical indications including plaque psoriasis and psoriatic arthritis.4-6
There has been a substantial amount of distrust surrounding the biosimilars; however, as the patents for the biologic agents expire, new biosimilars will undoubtedly flood the market. In this article, we provide information that will help dermatologists understand the need for and use of these agents.
Biosimilars Versus Generic Drugs
Small-molecule generics can be made in a process that is relatively inexpensive, reproducible, and able to yield identical products with each lot.7 In contrast, biosimilars are large complex proteins made in living cells. They differ from their reference product because of changes that occur during manufacturing (eg, purification system, posttranslational modifications).7-9 Glycosylation is particularly sensitive to manufacturing and can affect the immunogenicity of the product.9 The impact of manufacturing can be substantial; for example, during phase 3 trials for efalizumab, a change in the manufacturing facility affected pharmacokinetic properties to such a degree that the FDA required a repeat of the trials.10
FDA Guidelines on Biosimilarity
The FDA outlines the following approach to demonstrate biosimilarity.2 The first step is structural characterization to evaluate the primary, secondary, tertiary, and quaternary structures and posttranslational modifications. The next step utilizes in vivo and/or in vitro functional assays to compare the biosimilar and reference product. The third step is a focus on toxicity and immunogenicity. The fourth step involves clinical studies to study pharmacokinetic and pharmacodynamic data, immunogenicity, safety, and efficacy. After the biosimilar has been approved, there must be a system in place to monitor postmarketing safety. If a biosimilar is tested in one patient population (eg, patients with plaque psoriasis), a request can be made to approve the drug for all the conditions that the reference product was approved for, such as plaque psoriasis, rheumatoid arthritis, and inflammatory bowel disease, even though clinical trials were not performed in all of these patient populations.2 The BPCI Act leaves it up to the FDA to determine how much and what type of data (eg, in vitro, in vivo, clinical) are required.11
Extrapolation and Interchangeability
Once a biosimilar has been approved, 2 questions must be answered: First, can its use be extrapolated to all indications for the reference product? The infliximab biosimilar approved by the European Medicines Agency and the FDA had only been studied in patients with ankylosing spondylitis12 and rheumatoid arthritis,13 yet it was granted all the indications for infliximab, including severe plaque psoriasis.14 As of now, the various regulatory agencies differ on their policies regarding extrapolation. Extrapolation is not automatically bestowed on a biosimilar in the United States but can be requested by the manufacturer.2
Second, can the biosimilar be seamlessly switched with its reference product at the pharmacy level? The BPCI Act allows for the substitution of biosimilars that are deemed interchangeable without notifying the provider, yet individual states ultimately can pass laws regarding this issue.15,16 An interchangeable agent would “produce the same clinical result as the reference product,” and “the risk in terms of safety or diminished efficacy of alternating or switching between use of the biological product and the reference product is not greater than the risk of using the reference product.”15 Generic drugs are allowed to be substituted without notifying the patient or prescriber16; however, biosimilars that are not deemed interchangeable would require permission from the prescriber before substitution.11
Biosimilars for Psoriasis
In April 2016, an infliximab biosimilar (Inflectra) became the second biosimilar approved by the FDA.4 Inflectra was studied in clinical trials for patients with ankylosing spondylitis17 and rheumatoid arthritis,18 and in both trials the biosimilar was found to have similar efficacy and safety profiles to that of the reference product. In August 2016, an etanercept biosimilar (Erelzi) was approved,5 and in September 2016, an adalimumab biosimilar (Amjevita) was approved.6
The Table summarizes clinical trials (both completed and ongoing) evaluating biosimilars in adults with plaque psoriasis; thus far, there are 2464 participants enrolled across 5 different studies of adalimumab biosimilars (registered at www.clinicaltrials.gov with the identifiers NCT01970488, NCT02016105, NCT02489227, NCT02714322, NCT02581345) and 531 participants in an etanercept biosimilar study (NCT01891864).
A phase 3 double-blind study compared adalimumab to an adalimumab biosimilar (ABP 501) in 350 adults with plaque psoriasis (NCT01970488). Participants received an initial loading dose of adalimumab (n=175) or ABP 501 (n=175) 80 mg subcutaneously on week 1/day 1, followed by 40 mg at week 2 every 2 weeks thereafter. At week 16, participants with psoriasis area and severity index (PASI) 50 or greater remained in the study for up to 52 weeks; those who were receiving adalimumab were re-randomized to receive either ABP 501 or adalimumab. Participants receiving ABP 501 continued to receive the biosimilar. The mean PASI improvement at weeks 16, 32, and 50 was 86.6, 87.6, and 87.2, respectively, in the ABP 501/ABP 501 group (A/A) compared to 88.0, 88.2, and 88.1, respectively, in the adalimumab/adalimumab group (B/B).19 Autoantibodies developed in 68.4% of participants in the A/A group compared to 74.7% in the B/B group. The incidence of treatment-emergent adverse events (TEAEs) was 86.2% in the A/A group and 78.5% in the B/B group. The most common TEAEs were nasopharyngitis, headache, and upper respiratory tract infection. The incidence of serious TEAEs was 4.6% in the A/A group compared to 5.1% in the B/B group. Overall, the efficacy, safety, and immunogenicity of the adalimumab biosimilar was comparable to the reference product.19

A second phase 3 trial (ADACCESS) evaluated the adalimumab biosimilar GP2017 (NCT02016105). Participants received an initial dose of 80 mg subcutaneously of either GP2017 or adalimumab at week 0, followed by 40 mg every other week starting at week 1 and ending at week 51. The study has been completed but results are not yet available.
The third trial is evaluating the adalimumab biosimilar CHS-1420 (NCT02489227). Participants in the experimental arm receive two 40-mg doses of CHS-1420 at week 0/day 0, and then 1 dose every 2 weeks from week 1 for 23 weeks. At week 24, participants continue with an open-label study. Participants in the adalimumab group receive two 40-mg doses at week 0/day 0, and then 1 dose every 2 weeks from week 1 to week 15. At week 16, participants will be re-randomized (1:1) to continue adalimumab or start CHS-1420 at one 40-mg dose every 2 weeks during weeks 17 to 23. At week 24, participants will switch to CHS-1420 open label until the end of the study. Study results are not yet available; the study is ongoing but not recruiting.
The fourth ongoing trial is evaluating the adalimumab biosimilar MYL-1401A (NCT02714322). Participants receive an initial dose of 80 mg subcutaneously of either MYL-1401A or adalimumab (2:1), followed by 40 mg every other week starting 1 week after the initial dose. After the 52-week treatment period, there is an 8-week safety follow-up period. Study results are not yet available; the study is ongoing but not recruiting.
A fifth adalimumab biosimilar, M923, also is currently being tested in clinical trials (NCT02581345). Participants receive either M923, adalimumab, or alternate between the 2 agents. Although the study is still ongoing, data released from the manufacturer state that the proportion of participants who achieved PASI 75 after 16 weeks of treatment was equivalent in the 2 treatment groups. The proportion of participants who achieved PASI 90, as well as the type, frequency, and severity of adverse events, also were comparable.20
The EGALITY trial, completed in March 2015, compared the etanercept biosimilar GP2015 to etanercept over a 52-week period (NCT01891864). Participants received either GP2015 or etanercept 50 mg twice weekly for the first 12 weeks. Participants with at least PASI 50 were then re-randomized into 4 groups: the first 2 groups stayed with their current treatments while the other 2 groups alternated treatments every 6 weeks until week 30. Participants then stayed on their last treatment from week 30 to week 52. The adjusted PASI 75 response rate at week 12 was 73.4% in the group receiving GP2015 and 75.7% in the group receiving etanercept.21 The percentage change in PASI score at all time points was found to be comparable from baseline until week 52. Importantly, the incidence of TEAEs up to week 52 was comparable and no new safety issues were reported. Additionally, switching participants from etanercept to the biosimilar during the subsequent treatment periods did not cause an increase in formation of antidrug antibodies.21
There are 2 upcoming studies involving biosimilars that are not yet recruiting patients. The first (NCT02925338) will analyze the characteristics of patients treated with Inflectra as well as their response to treatment. The second (NCT02762955) will be comparing the efficacy and safety of an adalimumab biosimilar (BCD-057, BIOCAD) to adalimumab.
Economic Advantages of Biosimilars
The annual economic burden of psoriasis in the United States is substantial, with estimates between $35.2 billion22 and $112 billion.23 Biosimilars can be 25% to 30% cheaper than their reference products9,11,24 and have the potential to save the US health care system billions of dollars.25 Furthermore, the developers of biosimilars could offer patient assistance programs.11 That being said, drug developers can extend patents for their branded drugs; for instance, 2 patents for Enbrel (Amgen Inc) could protect the drug until 2029.26,27
Although cost is an important factor in deciding which medications to prescribe for patients, it should never take precedence over safety and efficacy. Manufacturers can develop new drugs with greater efficacy, fewer side effects, or more convenient dosing schedules,26,27 or they could offer co-payment assistance programs.26,28 Physicians also must consider how the biosimilars will be integrated into drug formularies. Would patients be required to use a biosimilar before a branded drug?11,29 Will patients already taking a branded drug be grandfathered in?11 Would they have to pay a premium to continue taking their drug? And finally, could changes in formularies and employer-payer relationships destabilize patient regimens?30
Conclusion
Preliminary results suggest that biosimilars can have similar safety, efficacy, and immunogenicity data compared to their reference products.19,21 Biosimilars have the potential to greatly reduce the cost burden associated with psoriasis. However, how similar is “highly similar”? Although cost is an important consideration in selecting drug therapies, the reason for using a biosimilar should never be based on cost alone.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
- Information on biosimilars. US Food and Drug Administration website. http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/. Updated May 10, 2016. Accessed July 5, 2016.
- US Department of Health and Human Services. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product: Guidance for Industry. Silver Spring, MD: US Food and Drug Administration; 2015.
- McKeage K. A review of CT-P13: an infliximab biosimilar. BioDrugs. 2014;28:313-321.
- FDA approves Inflectra, a biosimilar to Remicade [news release]. Silver Spring, MD: US Food and Drug Administration; April 5, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494227.htm. Updated April 20, 2016. Accessed January 23, 2017.
- FDA approves Erelzi, a biosimilar to Enbrel [news release]. Silver Spring, MD: US Food and Drug Administration; August 30, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm518639.htm. Accessed January 23, 2017.
- FDA approves Amjevita, a biosimilar to Humira [news release]. Silver Spring, MD: US Food and Drug Administration; September 23, 2016. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm522243.htm. Accessed January 23, 2017.
- Scott BJ, Klein AV, Wang J. Biosimilar monoclonal antibodies: a Canadian regulatory perspective on the assessment of clinically relevant differences and indication extrapolation [published online June 26, 2014]. J Clin Pharmacol. 2015;55(suppl 3):S123-S132.
- Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars [published online September 14, 2007]. Ann Oncol. 2008;19:411-419.
- Puig L. Biosimilars and reference biologics: decisions on biosimilar interchangeability require the involvement of dermatologists [published online October 2, 2013]. Actas Dermosifiliogr. 2014;105:435-437.
- Strober BE, Armour K, Romiti R, et al. Biopharmaceuticals and biosimilars in psoriasis: what the dermatologist needs to know. J Am Acad Dermatol. 2012;66:317-322.
- Falit BP, Singh SC, Brennan TA. Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers. Health Aff (Millwood). 2015;34:294-301.
- Park W, Hrycaj P, Jeka S, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72:1605-1612.
- Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72:1613-1620.
- Carretero Hernandez G, Puig L. The use of biosimilar drugs in psoriasis: a position paper. Actas Dermosifiliogr. 2015;106:249-251.
- Regulation of Biological Products, 42 USC §262 (2013).
- Ventola CL. Evaluation of biosimilars for formulary inclusion: factors for consideration by P&T committees. P T. 2015;40:680-689.
- Park W, Yoo DH, Jaworski J, et al. Comparable long-term efficacy, as assessed by patient-reported outcomes, safety and pharmacokinetics, of CT-P13 and reference infliximab in patients with ankylosing spondylitis: 54-week results from the randomized, parallel-group PLANETAS study. Arthritis Res Ther. 2016;18:25.
- Yoo DH, Racewicz A, Brzezicki J, et al. A phase III randomized study to evaluate the efficacy and safety of CT-P13 compared with reference infliximab in patients with active rheumatoid arthritis: 54-week results from the PLANETRA study. Arthritis Res Ther. 2015;18:82.
- Strober B, Foley P, Philipp S, et al. Evaluation of efficacy and safety of ABP 501 in a phase 3 study in subjects with moderate to severe plaque psoriasis: 52-week results. J Am Acad Dermatol. 2016;74(5, suppl 1):AB249.
- Momenta Pharmaceuticals announces positive top-line phase 3 results for M923, a proposed Humira (adalimumab) biosimilar [news release]. Cambridge, MA: Momenta Pharmaceuticals, Inc; November 29, 2016. http://ir.momentapharma.com/releasedetail.cfm?ReleaseID=1001255. Accessed January 25, 2017.
- Griffiths CE, Thaci D, Gerdes S, et al. The EGALITY study: a confirmatory, randomised, double-blind study comparing the efficacy, safety and immunogenicity of GP2015, a proposed etanercept biosimilar, versus the originator product in patients with moderate to severe chronic plaque-type psoriasis [published online October 27, 2016]. Br J Dermatol. doi:10.1111/bjd.15152.
- Vanderpuye-Orgle J, Zhao Y, Lu J, et al. Evaluating the economic burden of psoriasis in the United States [published online April 14, 2015]. J Am Acad Dermatol. 2015;72:961-967.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Menter MA, Griffiths CE. Psoriasis: the future. Dermatol Clin. 2015;33:161-166.
- Hackbarth GM, Crosson FJ, Miller ME. Report to the Congress: improving incentives in the Medicare program. Medicare Payment Advisory Commission, Washington, DC; 2009.
- Lovenworth SJ. The new biosimilar era: the basics, the landscape, and the future. Bloomberg website. http://about.bloomberglaw.com/practitioner-contributions/the-new-biosimilar-era-the-basics-the-landscape-and-the-future. Published September 21, 2012. Accessed July 6, 2016.
- Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478.
- Calvo B, Zuniga L. The US approach to biosimilars: the long-awaited FDA approval pathway. BioDrugs. 2012;26:357-361.
- Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70:2004-2017.
- Barriers to access attributed to formulary changes. Manag Care. 2012;21:41.
Practice Points
- Three biosimilars have been approved by the US Food and Drug Administration to treat adult patients with plaque psoriasis and psoriatic arthritis.
- By virtue of their production, biosimilars are not identical to their reference products, and we must ensure that their safety is comparable.
VIDEO: Coffee break at the Hawaii Dermatology Seminar
WAILEA, HAWAII – A few attendees at the Hawaii Dermatology Seminar discussed some of the highlights of the presentations during a coffee break at the meeting.
New treatment protocols for psoriasis, details about skin disease in children, managing medication side effects, and promising data about biologics are discussed in this video, as are updates on the latest tips for treating atopic dermatitis.
The interviewees had no conflicts to disclose.
The meeting is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – A few attendees at the Hawaii Dermatology Seminar discussed some of the highlights of the presentations during a coffee break at the meeting.
New treatment protocols for psoriasis, details about skin disease in children, managing medication side effects, and promising data about biologics are discussed in this video, as are updates on the latest tips for treating atopic dermatitis.
The interviewees had no conflicts to disclose.
The meeting is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – A few attendees at the Hawaii Dermatology Seminar discussed some of the highlights of the presentations during a coffee break at the meeting.
New treatment protocols for psoriasis, details about skin disease in children, managing medication side effects, and promising data about biologics are discussed in this video, as are updates on the latest tips for treating atopic dermatitis.
The interviewees had no conflicts to disclose.
The meeting is provided by Global Academy for Medical Education/Skin Disease Education Foundation. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
VIDEO: Distinctive features define pediatric psoriasis
WAILEA, HAWAII – Plaque type psoriasis continues to be the most common type of psoriasis in children, but there are other presentations that should be considered, said Wynnis Tom, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
“We certainly see a form of what some people would call napkin dermatitis,” or “diaper psoriasis,” affecting the diaper area in young infants, Dr. Tom said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
So when a child has a more refractory diaper rash, “look around to see if there are other lesions in the surrounding area that might be more typical for psoriasis,” she noted.
“We also see a lot more guttate disease” in children with psoriasis, which is more likely to be related to infections and triggers, Dr. Tom said. The face and scalp are often affected in children, and it is important to attend to these areas early to help avoid social stigma, she added.
Dr. Tom disclosed financial relationships with companies including Celgene, Janssen, and Promius. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Plaque type psoriasis continues to be the most common type of psoriasis in children, but there are other presentations that should be considered, said Wynnis Tom, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
“We certainly see a form of what some people would call napkin dermatitis,” or “diaper psoriasis,” affecting the diaper area in young infants, Dr. Tom said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
So when a child has a more refractory diaper rash, “look around to see if there are other lesions in the surrounding area that might be more typical for psoriasis,” she noted.
“We also see a lot more guttate disease” in children with psoriasis, which is more likely to be related to infections and triggers, Dr. Tom said. The face and scalp are often affected in children, and it is important to attend to these areas early to help avoid social stigma, she added.
Dr. Tom disclosed financial relationships with companies including Celgene, Janssen, and Promius. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, HAWAII – Plaque type psoriasis continues to be the most common type of psoriasis in children, but there are other presentations that should be considered, said Wynnis Tom, MD, a pediatric dermatologist at the University of California, San Diego, and Rady Children’s Hospital, San Diego.
“We certainly see a form of what some people would call napkin dermatitis,” or “diaper psoriasis,” affecting the diaper area in young infants, Dr. Tom said in a video interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
So when a child has a more refractory diaper rash, “look around to see if there are other lesions in the surrounding area that might be more typical for psoriasis,” she noted.
“We also see a lot more guttate disease” in children with psoriasis, which is more likely to be related to infections and triggers, Dr. Tom said. The face and scalp are often affected in children, and it is important to attend to these areas early to help avoid social stigma, she added.
Dr. Tom disclosed financial relationships with companies including Celgene, Janssen, and Promius. SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
Clinical Pearl: Early Diagnosis of Nail Psoriasis and Psoriatic Arthritis
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).
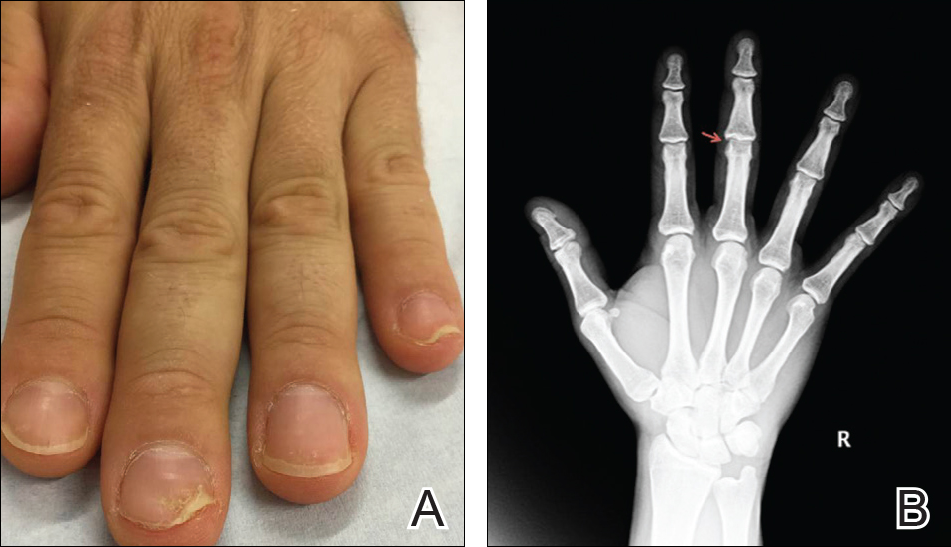
Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).

Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
Practice Gap
Early diagnosis of nail psoriasis is challenging because nail changes, including pitting, subungual hyperkeratosis, crumbling, oil spots, salmon patches, onycholysis, and splinter hemorrhages, may be subtle and nonspecific. Furthermore, 5% to 10% of psoriasis patients do not have skin findings, making the diagnosis of nail psoriasis even more difficult. Psoriatic arthritis (PsA) is more common in patients with nail psoriasis than in those with cutaneous psoriasis, and early joint damage may be asymptomatic.1 Both nail psoriasis and PsA may progress rapidly, leading to functional impairment with poor quality of life.2
Diagnostic Tool
A 36-year-old man presented with a 4-year history of abnormal fingernails. He denied nail pain but stated that the nails felt sensitive at times and it was difficult to pick up small objects. His medical history was notable for type 2 diabetes mellitus, hypertension, and attention deficit disorder. He denied joint pain or skin rash.
Physical examination revealed pitting and onycholysis of the fingernails (Figure, A) without involvement of the toenails. A nail clipping was negative for fungus but revealed an incompletely keratinized nail plate with subungual parakeratotic scale, consistent with nail psoriasis. A radiograph showed erosive changes of the third finger of the right hand that were compatible with PsA (Figure, B).

Practice Implications
A nail clipping may be performed to diagnose nail psoriasis. Imaging and/or referral to a rheumatologist should be performed in all patients with isolated nail psoriasis to evaluate for early arthritic changes. If present, appropriate therapy is initiated to prevent further joint damage. In patients with nail psoriasis with or without associated joint pain, dermatologists should consider using radiograph imaging to screen patients for PsA.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
- 1. Balestri R, Rech G, Rossi E, et al. Natural history of isolated nail psoriasis and its role as a risk factor for the development of psoriatic arthritis: a single center cross sectional study [published online September 2, 2016]. Br J Dermatol. doi:10.1111/bjd.15026.
- Klaassen KM, van de Kerkhof PC, Pasch MC. Nail psoriasis, the unknown burden of disease [published online January 15, 2014]. J Eur Acad Dermatol Venereol. 2014;28:1690-1695.
Cost of Diagnosing Psoriasis and Rosacea for Dermatologists Versus Primary Care Physicians
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i

Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
Growing incentives to control health care costs may cause accountable care organizations (ACOs) to reconsider how diseases are best managed. Few studies have examined the cost difference between primary care providers (PCPs) and specialists in managing the same disease. Limited data have suggested that management of some diseases by a PCP may be less costly compared to a specialist1,2; however, it is not clear if this finding extends to skin disease. This study sought to assess the cost of seeing a dermatologist versus a PCP for diagnosis of the common skin diseases psoriasis and rosacea.
Methods
Patient data were obtained from the Humana database, a large commercial data set for claims and reimbursed costs encompassing 18,162,539 patients covered between January 2007 and December 2014. Our study population consisted of 3,944,465 patients with claims that included International Classification of Diseases, Ninth Revision (ICD-9), codes for dermatological diagnoses (680.0–709.9). We searched by ICD-9 code for US patients with primary diagnoses of psoriasis (696.1) and rosacea (695.3). We narrowed the search to include patients aged 30 to 64 years, as the diagnoses for these diseases are most common in patients older than 30 years. Patients who were older than 64 years were not included in the study, as most are covered by Medicare and therefore costs covered by Humana in this age group would not be as representative as in younger age groups. Total and average diagnosis-related costs per patient were compared between dermatologists and PCPs. Diagnosis-related costs encompassed physician reimbursement; laboratory and imaging costs, including skin biopsies; inpatient hospitalization cost; and any other charge that could be coded or billed by providers and reimbursed by the insurance company. To be eligible for reimbursement from Humana, dermatologists and PCPs must be registered with the insurer according to specialty board certification and practice credentialing, and they are reimbursed differently based on specialty. Drug costs, which would possibly skew the data toward providers using more expensive systemic medications (ie, dermatologists), were not included in this study, as the discussion is better reserved for long-term management of disease rather than diagnosis-related costs. All diagnoses of psoriasis were included in the study, which likely includes all severities of psoriasis, though we did not have the ability to further break down these diagnoses by severity.
Results
We identified 30,217 psoriasis patients and 37,561 rosacea patients. Of those patients with a primary diagnosis of psoriasis, 26,112 (86%) were seen by a dermatologist and 4105 (14%) were seen by a PCP (Table). Of those patients with a primary diagnosis of rosacea, 34,694 (92%) were seen by a dermatologist and 2867 (8%) were seen by a PCP (Table). There was little difference in the average diagnosis-related cost per patient for psoriasis in males (dermatologists, $638; PCPs, $657) versus females (dermatologists, $592; PCPs, $586) or between specialties (Figure). Findings were similar for rosacea in males (dermatologists, $179; PCPs, $168) versus females (dermatologists, $157; PCPs, $161). For these skin diseases, i


Comment
For the management of common skin disorders such as psoriasis and rosacea, there is little cost difference in seeing a dermatologist versus a PCP. Through extensive training and repeated exposure to many skin diseases, dermatologists are expected to be more comfortable in diagnosing and managing psoriasis and rosacea. Compared to PCPs, dermatologists have demonstrated increased diagnostic accuracy and efficiency when examining pigmented lesions and other dermatologic diseases in several studies.3-6 Although the current study shows that diagnosis-related costs for psoriasis and rosacea are essentially equal between dermatologists and PCPs, it actually may be less expensive for patients to see a dermatologist, as unnecessary tests, biopsies, or medications are more likely to be ordered/prescribed when there is less clinical diagnostic certainty.7,8 Additionally, seeing a PCP for diagnosis of a skin disease may be inefficient if subsequent referral to a dermatologist is needed, a common scenario that occurs when patients see a PCP for skin conditions.9
Our study had limitations, which is typical of a study using a claims database. We used ICD-9 codes recorded in patients’ medical claims to determine diagnosis of psoriasis and rosacea; therefore, our study and data are subject to coding errors. We could not assess the severity of disease, only the presence of disease. Further confirmation of diagnosis could have been made through searching for a second ICD-9 code in the patient’s history. Our data also are from a limited time period and may not represent costs from other time periods.
Conclusion
Given the lack of cost difference between both specialties, we conclude that ACOs should consider encouraging patients to seek care for dermatologic diseases by dermatologists who generally are more accurate and efficient skin diagnosticians, particularly if there is a shortage of PCPs within the ACO network.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
- Wimo A, Religa D, Spångberg K, et al. Costs of diagnosing dementia: results from SveDem, the Swedish Dementia Registry. Int J Geriatr Psychiatry. 2013;28:1039-1044.
- Grunfeld E, Fitzpatrick R, Mant D, et al. Comparison of breast cancer patient satisfaction with follow-up in primary care versus specialist care: results from a randomized controlled trial. Br J Gen Pract. 1999;49:705-710.
- Chen SC, Pennie ML, Kolm P, et al. Diagnosing and managing cutaneous pigmented lesions: primary care physicians versus dermatologists. J Gen Intern Med. 2006;21:678-682.
- Federman D, Hogan D, Taylor JR, et al. A comparison of diagnosis, evaluation, and treatment of patients with dermatologic disorders. J Am Acad Dermatol. 1995;32:726-729.
- Feldman SR, Fleischer AB, Young AC, et al. Time-efficiency of nondermatologists compared with dermatologists in the care of skin disease. J Am Acad Dermatol. 1999;40:194-199.
- Feldman SR, Peterson SR, Fleischer AB Jr. Dermatologists meet the primary care standard for first contact management of skin disease. J Am Acad Dermatol. 1998;39(2, pt 1):182-186.
- Smith ES, Fleischer AB, Feldman SR. Nondermatologists are more likely than dermatologists to prescribe antifungal/corticosteroid products: an analysis of office visits for cutaneous fungal infections, 1990-1994. J Am Acad Dermatol. 1998;39:43-47.
- Shaffer MP, Feldman SR, Fleischer AB. Use of clotrimazole/betamethasone diproprionate by family physicians. Fam Med. 2000;32:561-565.
- Feldman SR, Fleischer AB, Chen JG. The gatekeeper model is inefficient for the delivery of dermatologic services. J Am Acad Dermatol. 1999;40:426-432.
Practice Points
- Growing health care costs are causing accountable care organizations (ACOs) to reconsider how to best manage skin disease.
- There is little difference in average diagnosis-related cost between primary care physicians and dermatologists in diagnosing psoriasis or rosacea.
- With diagnosis costs essentially equal and increased dermatologist diagnostic accuracy, ACOs may encourage skin disease to be managed by dermatologists.
New Biologics in Psoriasis: An Update on IL-23 and IL-17 Inhibitors
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.
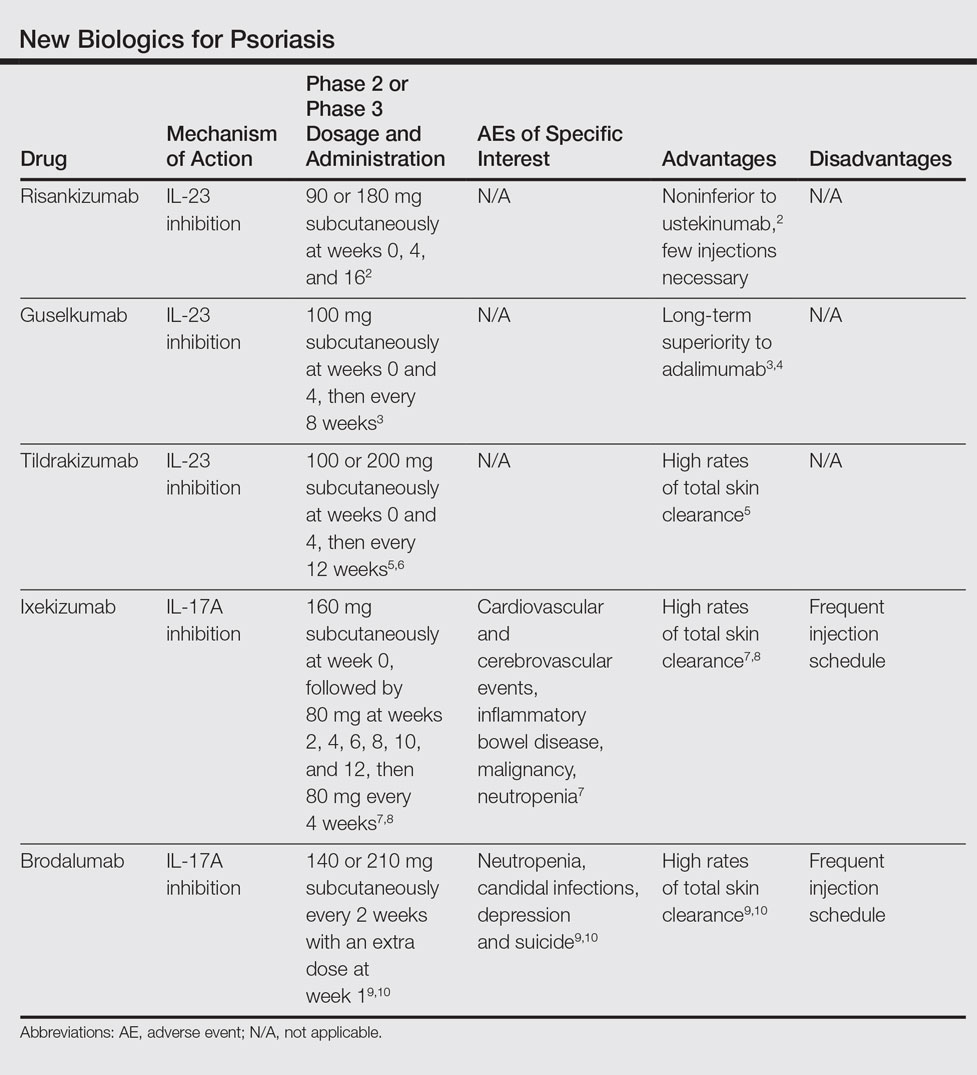
IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.

IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
The role of current biologic therapies in psoriasis predicates on the pathogenic role of upregulated, immune-related mechanisms that result in the activation of myeloid dendritic cells, which release IL-17, IL-23, and other cytokines to activate T cells, including helper T cell TH17. Along with other immune cells, TH17 produces IL-17. This proinflammatory cascade results in keratinocyte proliferation, angiogenesis, and migration of immune cells toward psoriatic lesions.1 Thus, the newest classes of biologics target IL-12, IL-23, and IL-17 to disrupt this inflammatory cascade.
We provide an updated review of the most recent clinical efficacy and safety data on the newest IL-23 and IL-17 inhibitors in the pipeline or approved for psoriasis, including risankizumab, guselkumab, tildrakizumab, ixekizumab, and brodalumab (Table). Ustekinumab and adalimumab, which have been previously approved by the US Food and Drug Administration (FDA), will be discussed here only as comparators.

IL-23 Inhibitors
Risankizumab
Risankizumab (formerly known as BI 655066)(Boehringer Ingelheim) is a selective human monoclonal antibody targeting the p19 subunit of IL-23 and currently is undergoing phase 3 trials for psoriasis. A proof-of-concept phase 1 study of 39 participants demonstrated efficacy after 12 weeks of treatment at varying subcutaneous and intravenous doses with placebo control.11 At week 12, 87% (27/31)(P<.001) of all risankizumab-treated participants achieved 75% reduction in psoriasis area and severity index (PASI) score compared to 0% of 8 placebo-treated participants. Common adverse effects (AEs) occurred in 65% (20/31) of risankizumab-treated participants, including non–dose-dependent upper respiratory tract infections, nasopharyngitis, and headache. Serious adverse events (SAEs) that occurred were considered unrelated to the study medication.11
A phase 2 trial of 166 participants compared 3 dosing regimens of subcutaneous risankizumab (single 18-mg dose at week 0; single 90-mg dose at weeks 0, 4, and 16; or single 180-mg dose at weeks 0, 4, and 16) and ustekinumab (weight-based single 45- or 90-mg dose at weeks 0, 4, and 16), demonstrating noninferiority at higher doses of risankizumab.2 Preliminary primary end point results at week 12 showed PASI 90 in 32.6% (P=.4667), 73.2% (P=.0013), 81.0% (P<.0001), and 40.0% of the treatment groups, respectively. Participants in the 180-mg risankizumab group achieved PASI 90 eight weeks faster than those on ustekinumab, lasting more than 2 months longer. Adverse effects were similar across all treatment groups and SAEs were unrelated to the study medications.2
Guselkumab
Guselkumab (Janssen Biotech, Inc) is a selective human monoclonal antibody against the p19 subunit of IL-23. The 52-week phase 2 X-PLORE trial compared dose-ranging subcutaneous guselkumab (5 mg at weeks 0 and 4, then every 12 weeks; 15 mg every 8 weeks; 50 mg at weeks 0 and 4, then every 12 weeks; 100 mg every 8 weeks; or 200 mg at weeks 0 and 4, then every 12 weeks), adalimumab (80-mg loading dose, followed by 40 mg at week 1, then every other week), and placebo in 293 randomized participants.4 At week 16, 34% (P=.002) of participants in the 5-mg guselkumab group, 61% (P<.001) in the 15-mg group, 79% (P<.001) in the 50-mg group, 86% (P<.001) in the 100-mg group, 83% (P<.001) in the 200-mg group, and 58% (P<.001) in the adalimumab group achieved physician global assessment (PGA) scores of 0 (clear) or 1 (minimal psoriasis) compared to 7% of the placebo group. Achievement of PASI 75 similarly favored the guselkumab (44% [P<.001]; 76% [no P value given]; 81% [P<.001]; 79% [P<.001]; and 81% [P<.001], respectively) and adalimumab treatment arms (70% [P<.001]) compared to 5% in the placebo group. In longer-term comparisons to week 40, participants in the 50-, 100-, and 200-mg guselkumab groups showed significantly greater remission of psoriatic lesions, measured by a PGA score of 0 or 1, than participants in the adalimumab group (71% [P=.05]; 77% [P=.005]; 81% [P=.01]; and 49%, respectively).4
Preliminary results from VOYAGE 1 (N=837), the first of several phase 3 trials, further demonstrate the superiority of guselkumab 100 mg at weeks 0 and 4 and then every 8 weeks over adalimumab (standard dosing) and placebo; at week 16, 73.3% (P<.001 for both comparisons) versus 49.7% and 2.9% of participants, respectively, achieved PASI 90, with sustained superiority of skin clearance in guselkumab-treated participants compared to adalimumab and placebo through week 48.3
Long-term safety data showed no dose dependence or trend from 0 to 16 weeks and 16 to 52 weeks of treatment regarding rates of AEs, SAEs, or serious infections.4 Between weeks 16 and 52, 48.9% of all guselkumab-treated participants exhibited AEs compared to 60.5% of adalimumab-treated participants and 51.3% of placebo participants. Overall infection rates also were lowest in the guselkumab group at 29.8% compared to 36.8% and 35.9%, respectively. Three participants treated with guselkumab had major cardiovascular events, including a fatal myocardial infarction. No cases of tuberculosis or serious opportunistic infections were reported.4
Tildrakizumab
Tildrakizumab (formerly known as MK-3222)(Sun Pharmaceutical Industries Ltd) is a human monoclonal antibody also targeting the p19 subunit of IL-23. In a phase 2 study of 355 participants with chronic plaque psoriasis, participants received 5-, 25-, 100-, or 200-mg subcutaneous tildrakizumab or placebo at weeks 0 and 4 and then every 12 weeks for a total of 52 weeks.6 At week 16, PASI 75 results were 33.3%, 64.4%, 66.3%, 74.4%, and 4.4%, respectively (P<.001 for each comparison). Improvement began within the first month of treatment, with median times to PASI 75 of 57 days at 200-mg dosing and 84 days at 100-mg dosing. Of those participants achieving PASI 75 by drug discontinuation at week 52, 96% of the 100-mg group and 93% of the 200-mg group maintained PASI 75 through week 72, suggesting low relapse rates after treatment cessation.6
In October 2016, the efficacy results of 2 pivotal phase 3 trials (reSURFACE 1 and reSURFACE 2) involving more than 1800 participants combined revealed PASI 90 achievement in an average of 54% of participants on tildrakizumab 100 mg and 59% of participants on tildrakizumab 200 mg at week 28.5 Achievement of PASI 100 occurred in 24% and 30% of participants at week 28, respectively. The second of these trials included an etanercept comparison group and demonstrated head-to-head superiority of 100 and 200 mg subcutaneous tildrakizumab at week 12 by end point measures.5
Treatment-related AEs occurred at rates of 25% in tildrakizumab-treated participants and 22% in placebo-treated participants, most frequently nasopharyngitis and headache.6 At least 1 AE occurred in 64% of tildrakizumab-treated participants without dose dependence compared to 69% of placebo-treated participants. Severe AEs thought to be drug treatment related were bacterial arthritis, lymphedema, melanoma, stroke, and epiglottitis.6
IL-17 Inhibitors
Ixekizumab
Ixekizumab (Eli Lilly and Company), a monoclonal inhibitor of IL-17A, is the most recently approved psoriasis biologic on the market and has been cleared for use in adults with moderate to severe plaque psoriasis. Recommended dosing is 160 mg (given in two 80-mg subcutaneous injections via an autoinjector or prefilled syringe) at week 0, followed by an 80-mg injection at weeks 2, 4, 6, 8, 10, and 12, and then 80 mg every 4 weeks thereafter. The FDA approved ixekizumab in March 2016 following favorable results of several phase 3 trials: UNCOVER-1, UNCOVER-2, and UNCOVER-3.7,8
In UNCOVER-1, 1296 participants were randomized to 1 of 2 ixekizumab treatment arms—160 mg starting dose at week 0, 80 mg every 2 or 4 weeks thereafter—or placebo.7 At week 12, 89.1%, 82.6%, and 3.9% achieved PASI 75, respectively (P<.001 for both). Importantly, high numbers of participants also achieved PASI 90 (70.9% in the 2-week group and 64.6% in the 4-week group vs 0.5% in the placebo group [P<.001]) and PASI 100 (35.3% and 33.6% vs 0%, respectively [P<.001]), suggesting high rates of disease clearance.7
UNCOVER-2 (N=1224) and UNCOVER-3 (N=1346) investigated the same 2 dosing regimens of ixekizumab compared to etanercept 50 mg biweekly and placebo.8 At week 12, the percentage of participants achieving PASI 90 in UNCOVER-2 was 70.7%, 59.7%, 18.7%, and 0.6%, respectively, and 68.1%, 65.3%, 25.7%, and 3.1%, respectively, in UNCOVER-3 (P<.0001 for all comparisons to placebo and etanercept). At week 12, PASI 100 results also showed striking superiority, with 40.5%, 30.8%, 5.3%, and 0.6% of participants, respectively, in UNCOVER-2, and 37.7%, 35%, 7.3%, and 0%, respectively, in UNCOVER-3, achieving complete clearance of disease (P<.0001 for all comparisons to placebo and etanercept). Responses to ixekizumab were observed as early as weeks 1 and 2, while no participants in the etanercept and placebo treatment groups achieved comparative efficiency.8
In an extension of UNCOVER-3, efficacy increased from week 12 to week 60 according to PASI 90 (68%–73% in the 2-week group; 65%–72% in the 4-week group) and PASI 100 measures (38%–55% in the 2-week group; 35%–52% in the 4-week group).7
The most common AEs associated with ixekizumab treatment from weeks 0 to 12 occurred at higher rates in the 2-week and 4-week ixekizumab groups compared to placebo, including nasopharyngitis (9.5% and 9% vs 8.7%, respectively), upper respiratory tract infection (4.4% and 3.9% vs 3.5%, respectively), injection-site reaction (10% and 7.7% vs 1%, respectively), arthralgia (4.4% and 4.3% vs 2.9%, respectively), and headache (2.5% and 1.9% vs 2.1%, respectively). Infections, including candidal, oral, vulvovaginal, and cutaneous, occurred in 27% of the 2-week dosing group and 27.4% of the 4-week dosing group compared to 22.9% of the placebo group during weeks 0 to 12, with candidal infections in particular occurring more frequently in the active treatment groups and exhibiting dose dependence. Other AEs of special interest that occurred among all ixekizumab-treated participants (n=3736) from weeks 0 to 60 were cardiovascular and cerebrovascular events (22 [0.6%]), inflammatory bowel disease (11 [0.3%]), non–skin cancer malignancy (14 [0.4%]), and nonmelanoma skin cancer (20 [0.5%]). Neutropenia occurred at higher rates in ixekizumab-treated participants (9.3% in the 2-week group and 8.6% in the 4-week group) compared to placebo (3.3%) and occurred in 11.5% of all ixekizumab participants over 60 weeks.7
Brodalumab
Brodalumab (Valeant Pharmaceuticals International, Inc) is a human monoclonal antibody targeting the IL-17A receptor currently under review for FDA approval after undergoing phase 3 trials. The first of these trials, AMAGINE-1, showed efficacy of subcutaneous brodalumab (140 or 210 mg administered every 2 weeks with an extra dose at week 1) compared to placebo in 661 participants.9 At week 12, 60%, 83%, and 3%, respectively, achieved PASI 75; 43%, 70%, and 1%, respectively, achieved PASI 90; and 23%, 42%, and 1%, respectively, achieved PASI 100 (P<.001 for all respective comparisons to placebo). These effects were retained through 52 weeks of treatment. The median time to complete disease clearance in participants reaching PASI 100 was 12 weeks. Conversely, participants who were re-randomized to placebo after week 12 of brodalumab treatment relapsed within weeks to months.9
AMAGINE-2 and AMAGINE-3 further demonstrated the efficacy of brodalumab (140 or 210 mg every 2 weeks with extra dose at week 1) compared to ustekinumab (45 or 90 mg weight-based standard dosing) and placebo in 1831 participants, respectively.10 In AMAGINE-2, 49% of participants in the 140-mg group (P<.001 vs placebo), 70% in the 210-mg group (P<.001 vs placebo), 47% in the ustekinumab group, and 3% in the placebo group achieved PASI 90 at week 12. Similarly, in AMAGINE-3, 52% of participants in the 140-mg group (P<.001), 69% in the 210-mg group (P<.001), 48% in the ustekinumab group, and 2% in the placebo group achieved PASI 90. Impressively, complete clearance (PASI 100) at week 12 occurred in 26% of the 140-mg group (P<.001 vs placebo), 44% of the 210-mg group (P<.001 vs placebo), and 22% of the ustekinumab group compared to 2% of the placebo group in AMAGINE-2, with similar rates in AMAGINE-3. Brodalumab was significantly superior to ustekinumab at the 210-mg dose by PASI 90 measures (P<.001) in both studies and at the 140-mg dose by PASI 100 measures (P=.007) in AMAGINE-3 only.10
Common AEs were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia, all occurring at grossly similar rates (49%–60%) across all experimental groups in AMAGINE-1, AMAGINE-2, and AMAGINE-3 during the first 12-week treatment period.9,10 Brodalumab treatment groups had high rates of specific interest AEs compared to ustekinumab and placebo groups, including neutropenia (0.8%, 1.1%, 0.3%, and 0%, respectively) and candidal infections (0.8%, 1.3%, 0.3%, and 0.3%, respectively). Induction phase (weeks 0–12) depression rates were concerning, with 6 cases each in AMAGINE-2 (4 [0.7%] in the 140-mg group, 2 [0.3%] in the 210-mg group) and AMAGINE-3 (4 [0.6%] in the 140-mg group, 2 [0.3%] in the 210-mg group). Cases of neutropenia were mild, were not associated with major infection, and were transient or reversible. Depression rates after 52 weeks of treatment were 1.7% (23/1567) of brodalumab participants in AMAGINE-2 and 1.8% (21/1613) in AMAGINE-3. Three participants, all on constant 210-mg dosing through week 52, attempted suicide with 1 completion10; however, because no other IL-17 inhibitors were associated with depression or suicide in other trials, it has been suggested that these cases were incidental and not treatment related.12 An FDA advisory panel recommended approval of brodalumab in July 2016 despite ongoing concerns of depression and suicide.13
Conclusion
The robust investigation into IL-23 and IL-17 inhibitors to treat plaque psoriasis has yielded promising results, including the unprecedented rates of PASI 100 achievement with these new biologics. Risankizumab, ixekizumab, and brodalumab have demonstrated superior efficacy in trials compared to ustekinumab. Tildrakizumab has shown low disease relapse after drug cessation. Ixekizumab and brodalumab have shown high rates of total disease clearance. Thus far, safety findings for these pipeline biologics have been consistent with those of ustekinumab. With ixekizumab approved in 2016 and brodalumab under review, new options in biologic therapy will offer patients and clinicians greater choices in treating severe and recalcitrant psoriasis.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
- Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496-509.
- Papp K, Menter A, Sofen H, et al. Efficacy and safety of different dose regimens of a selective IL-23p19 inhibitor (BI 655066) compared with ustekinumab in patients with moderate-to-severe plaque psoriasis with and without psoriatic arthritis. Paper presented at: 2015 American College of Rheumatology/Association of Rheumatology Health Professionals Annual Meeting; November 6-11, 2015; San Francisco, CA.
- New phase 3 data show significant efficacy versus placebo and superiority of guselkumab versus Humira in treatment of moderate to severe plaque psoriasis [press release]. Vienna, Austria; Janssen Research & Development, LLC: October 1, 2016.
- Gordon KB, Duffin KC, Bissonnette R, et al. A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med. 2015;373:136-144.
- Sun Pharma to announce late-breaking results for investigational IL-23p19 inhibitor, Tildrakizumab, achieves primary end point in both phase-3 studies in patients with moderate-to-severe plaque psoriasis [press release]. Mumbai, India; Sun Pharmaceutical Industries Ltd: October 1, 2016.
- Papp K, Thaci D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930-939.
- Gordon KB, Blauvelt A, Papp KA, et al; UNCOVER-1 Study Group, UNCOVER-2 Study Group, UNCOVER-3 Study Group. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Griffiths CE, Reich K, Lebwohl M, et al. Comparison of ixekizumab with etanercept or placebo in moderate-to-severe psoriasis (UNCOVER-2 and UNCOVER-3): results from two phase 3 randomised trials. Lancet. 2015;386:541-551.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis [published online June 23, 2016]. Br J Dermatol. 2016;175:273-286.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-1328.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial [published online March 1, 2015]. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Chiricozzi A, Romanelli M, Saraceno R, et al. No meaningful association between suicidal behavior and the use of IL-17A-neutralizing or IL-17RA-blocking agents [published online August 31, 2016]. Expert Opin Drug Saf. 2016;15:1653-1659.
- FDA advisory committee recommends approval of brodalumab for treatment of moderate-to-severe plaque psoriasis [news release]. Laval, Quebec: Valeant Pharmaceuticals International, Inc; July 19, 2016.
Practice Points
- The newest biologics for treatment of moderate to severe plaque psoriasis are IL-23 and IL-17 inhibitors with unprecedented efficacy of complete skin clearance compared to older biologics.
- Risankizumab, guselkumab, and tildrakizumab are new IL-23 inhibitors currently in phase 3 trials with promising early efficacy and safety results.
- Ixekizumab, which recently was approved, and brodalumab, which is pending US Food and Drug Administration review, are new IL-17 inhibitors that achieved total skin clearance in more than one-quarter of phase 3 participants after 12 weeks of treatment.
VIDEO: Pediatric psoriasis patients prepare for biologics
WAILEA, Hawaii – The first approval of a biologic for pediatric psoriasis, and ongoing clinical trials of other biologics in children with psoriasis, are among the encouraging therapeutic developments for this patient population, Wynnis Tom, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“We are incredibly excited ... as pediatric dermatologists that we’re finally seeing breakthroughs” in terms of Food and Drug Administration activity regarding the use of biologics for treating psoriasis in children, Dr. Tom said in a video interview at the seminar.
Etanercept (Enbrel) is now approved for children with psoriasis, the first biologic indicated for pediatric psoriasis, and clinical trials of other biologics that have been available for adults and nonbiologic products for pediatric psoriasis are underway, she said.
However, getting insurance coverage can still be a challenge, although having long-term efficacy and safety data helps, noted Dr. Tom of the department of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego. Also helpful is sending letters to insurers on behalf of the patient, describing the patient’s quality of life, descriptions of treatments that have been unsuccessful, and even photos documenting the disease in the child, she added.
Dr. Tom disclosed ties with Promius, Celgene, and Janssen.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, Hawaii – The first approval of a biologic for pediatric psoriasis, and ongoing clinical trials of other biologics in children with psoriasis, are among the encouraging therapeutic developments for this patient population, Wynnis Tom, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“We are incredibly excited ... as pediatric dermatologists that we’re finally seeing breakthroughs” in terms of Food and Drug Administration activity regarding the use of biologics for treating psoriasis in children, Dr. Tom said in a video interview at the seminar.
Etanercept (Enbrel) is now approved for children with psoriasis, the first biologic indicated for pediatric psoriasis, and clinical trials of other biologics that have been available for adults and nonbiologic products for pediatric psoriasis are underway, she said.
However, getting insurance coverage can still be a challenge, although having long-term efficacy and safety data helps, noted Dr. Tom of the department of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego. Also helpful is sending letters to insurers on behalf of the patient, describing the patient’s quality of life, descriptions of treatments that have been unsuccessful, and even photos documenting the disease in the child, she added.
Dr. Tom disclosed ties with Promius, Celgene, and Janssen.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WAILEA, Hawaii – The first approval of a biologic for pediatric psoriasis, and ongoing clinical trials of other biologics in children with psoriasis, are among the encouraging therapeutic developments for this patient population, Wynnis Tom, MD, said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
“We are incredibly excited ... as pediatric dermatologists that we’re finally seeing breakthroughs” in terms of Food and Drug Administration activity regarding the use of biologics for treating psoriasis in children, Dr. Tom said in a video interview at the seminar.
Etanercept (Enbrel) is now approved for children with psoriasis, the first biologic indicated for pediatric psoriasis, and clinical trials of other biologics that have been available for adults and nonbiologic products for pediatric psoriasis are underway, she said.
However, getting insurance coverage can still be a challenge, although having long-term efficacy and safety data helps, noted Dr. Tom of the department of dermatology and pediatrics, University of California, San Diego, and Rady Children’s Hospital, San Diego. Also helpful is sending letters to insurers on behalf of the patient, describing the patient’s quality of life, descriptions of treatments that have been unsuccessful, and even photos documenting the disease in the child, she added.
Dr. Tom disclosed ties with Promius, Celgene, and Janssen.
SDEF and this news organization are owned by the same parent company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT SDEF HAWAII DERMATOLOGY SEMINAR
The Role of Biologic Therapy for Psoriasis in Cardiovascular Risk Reduction
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
The cardiovascular comorbidities associated with psoriasis have been well documented; however, the mechanism by which psoriasis increases the risk for cardiovascular disease (CVD) remains unclear. Elevated systemic inflammatory cytokines and mediators may play a key role in their association, which prompts the questions: Do systemic medications have a protective effect? Do patients on systemic antipsoriatic treatment have a decreased risk for major adverse cardiovascular events (MACEs) compared with untreated patients?
We believe the shared inflammatory processes involved in psoriasis and atherosclerosis formation are potential targets for therapy in reducing the incidence of CVD and its associated complications. A growing amount of evidence suggests cardioprotective effects associated with antipsoriatic treatments such as tumor necrosis factor (TNF) inhibitors and methotrexate. Gkalpakiotis et al1 demonstrated a reduction in serum E-selectin (mean [standard deviation], 53.04 [23.54] ng/mL vs 35.32 [8.70] ng/mL; P<.001) and IL-22 (25.11 [19.9] pg/mL vs 12.83 [8.42] pg/mL; P<.001) after 3 months of adalimumab administration in patients with moderate to severe psoriasis. Both E-selectin and IL-22 are associated with the development of atherosclerosis, endothelial dysfunction, and an increased incidence of CVD. Similarly, Wu et al2 demonstrated a statistically significant reduction (–5.04 mg/dL [95% confidence interval [CI], –8.24 to –2.12; P<.01) in C-reactive protein in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis after concurrent use of methotrexate and TNF inhibitors.
Solomon et al3 compared the rate of newly diagnosed diabetes mellitus among psoriasis and rheumatoid arthritis patients treated with TNF inhibitors, methotrexate, hydroxychloroquine, and other nonbiologic disease-modifying antirheumatic drugs. The authors’ findings suggest that those who take a TNF inhibitor (hazard ratio [HR], 0.62; 95% CI, 0.42-0.91) and hydroxychloroquine (HR, 0.54; 95% CI, 0.36-0.80) are at lower risk for diabetes mellitus compared to those treated with nonbiologic disease-modifying antirheumatic drugs. Conversely, the methotrexate (HR, 0.77; 95% CI, 0.53-1.13) cohort did not show a statistically significant reduction in diabetes risk.3
Pina et al4 revealed improvement in endothelial function after 6 months of adalimumab use in patients with moderate to severe psoriasis. To evaluate the presence of subclinical endothelial dysfunction, the authors assessed brachial artery reactivity by measuring flow-mediated dilation and carotid artery stiffness by pulse wave velocity. Patients showed an increase in flow-mediated dilation (mean [SD], 6.19% [2.44%] vs 7.46% [2.43%]; P=.008) and reduction in pulse wave velocity (6.28 [1.04] m/s vs 5.69 [1.31] m/s; P=.03) compared to baseline measurements, indicating an improvement of endothelial function.4
Ahlehoff et al5 observed for improvements in subclinical left ventricular dysfunction in psoriasis patients after treatment with biologics. Using echocardiography, they assessed for changes in diastolic function and left ventricular systolic deformation (defined by global longitudinal strain). Of patients who received 3 months of biologic therapy (TNF inhibitor orIL-12/23 inhibitor) and maintained at minimum a psoriasis area and severity index 50 response, all demonstrated an improvement in diastolic function (mean [SD], 8.1 [2.1] vs 6.7 [1.9]; P<.001) and global longitudinal strain (mean [SD], –16.8% [2.1%] vs –18.3% [2.3%]; P<.001). Of note, patients who did not achieve a psoriasis area and severity index 50 response at follow-up did not exhibit an improvement in subclinical myocardial function.5
Moreover, a Danish nationwide study with up to 5-year follow-up evaluated the risk for MACE (ie, cardiovascular death, myocardial infarction, stroke) in patients with severe psoriasis receiving systemic anti-inflammatory medications and nonsystemic therapies including topical treatments, phototherapy, and climate therapy.6 Compared to nonsystemic therapies, methotrexate use (HR, 0.53; 95% CI, 0.34-0.83) was associated with a decreased risk for cardiovascular events. However, a protective decreased risk was not found among patients who used systemic cyclosporine (HR, 1.06; 95% CI, 0.26-4.27) or retinoids (HR, 1.80; 95% CI, 1.03-2.96). Any biological drug use had a comparable but nonsignificant reduction of cardiovascular events (HR, 0.58; 95% CI, 0.30-1.10). After multivariable adjustment, TNF inhibitors were associated with a statistically significant decreased risk for cardiovascular events (HR, 0.46; 95% CI, 0.22-0.98; P=.04) compared to nonsystemic therapies. The IL-12/23 inhibitor did not demonstrate this relationship (HR, 1.52; 95% CI, 0.47-4.94).6
Lastly, Wu et al7 compared the risk for MACE (ie, myocardial infarction, stroke, unstable angina, transient ischemic attack) between patients with psoriasis who received TNF inhibitors or methotrexate. The TNF inhibitor and methotrexate cohorts were observed for a median of 12 months and 9 months, respectively. After adjusting for potential confounding factors, they found a 45% reduction (HR, 0.55; 95% CI, 0.45-0.67) in cardiovascular event risk in the TNF inhibitor cohort compared with the methotrexate cohort. Notably, analyses also showed comparatively fewer cardiovascular events in the TNF inhibitor cohort throughout all time points—6, 12, 18, 24, 60 months—in the observation period. Regression analysis revealed an 11% reduction in cardiovascular events (HR, 0.89; 95% CI, 0.80-0.98) with each additional 6 months of cumulative TNF inhibitor exposure.
The current sum of evidence suggests cardioprotective effects of TNF inhibitor and methotrexate use. However, given the cumulative systemic toxicity and inferior cutaneous efficacy of methotrexate, TNF inhibitors will likely play a more significant role going forward. The role of methotrexate may be for its simultaneous use with biologic therapies to limit immunogenicity. Newer biologic agents such as IL-12/23 and IL-17 inhibitors have not yet been as extensively studied for their effects on cardiovascular risk as their TNF inhibitor counterparts. However, because of their shared ability to target specific immunological pathways, it is plausible that IL-12/23 and IL-17 agents may exhibit cardioprotective effects.8
Patients with psoriasis should be counseled and educated about the increased risk for CVD and its associated morbidity and mortality risk. Screening for modifiable risk factors and recommending therapeutic lifestyle changes also is appropriate. Future studies should help define the role of specific systemic drugs in reducing the risk for CVD in patients with psoriasis.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.
- Gkalpakiotis S, Arenbergerova M, Gkalpakioti P, et al. Impact of adalimumab treatment on cardiovascular risk biomarkers in psoriasis: results of a pilot study [published online October 24, 2016]. J Dermatol. doi:10.1111/1346-8138.13661.
- Wu JJ, Rowan CG, Bebchuk JD, et al. Association between tumor necrosis factor inhibitor (TNFi) therapy and changes in C-reactive protein (CRP), blood pressure, and alanine aminotransferase (ALT) among patients with psoriasis, psoriatic arthritis, or rheumatoid arthritis [published online March 5, 2015]. J Am Acad Dermatol. 2015;72:917-919.
- Solomon DH, Massarotti E, Garg R, et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305:2525-2531.
- Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
- Ahlehoff O, Hansen PR, Gislason GH, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study [published online April 6, 2015]. J Eur Acad Dermatol Venereol. 2016;30:819-823.
- Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort [published online October 10, 2014]. J Eur Acad Dermatol Venereol. 2015;29:1128-1134.
- Wu JJ, Guérin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-α inhibitors versus methotrexate [published online October 26, 2016]. J Am Acad Dermatol. 2017;76:81-90.
- Egeberg A, Skov L. Management of cardiovascular disease in patients with psoriasis. Expert Opin Pharmacother. 2016;17:1509-1516.