User login
Study links GLP-1R agonists, lower inflammatory biomarker levels
Patients with both type 2 diabetes and asthma who were on glucagonlike peptide receptor–1 (GLP-1R) agonists for glucose control had lower levels of a key biomarker of airway inflammation than similar patients on other types of glucose-control medications, according to results of a study to have been presented at the annual meeting of the American Academy of Asthma, Allergy, and Immunology. The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The findings from this study potentially replicated findings in humans that have been reported in preclinical trials.
“Our work showed that type 2 diabetics with asthma who were treated with GLP-1 receptor agonists had lower levels of periostin, and this provides really one of the first human data to show that these drugs may impact key inflammation pathways in the airway,” Dinah Foer, MD, of Brigham and Women’s Hospital, Boston, said in an interview. She described periostin as “a known critical inducer of airway mucous production and airway responsiveness.”
The study retrospectively evaluated serum samples from the Partners HealthCare Biobank of 161 adults with both asthma and type 2 diabetes, 42 of whom were on GLP-1R agonists and 119 of whom were taking non-GLP-1R agonist diabetes medications. The study used the Partners Healthcare EHR to identify eligible patients.
The study found that periostin levels were significantly decreased in GLP-1R agonist users: 19.1 ng/mL (standard deviation, +8.7) versus 27.4 ng/mL (SD, +14) in the non-GLP-1R agonist group (P = .001), Dr. Foer said. The other known mediators of asthma inflammatory pathways that were measured – interleukin-6, IL-8, sCD163, total IgE, and sST2 (soluble suppression of tumorigenesis–2) – showed no differences between the two groups, Dr. Foer said.
She said that this was the first human study to show similar results to preclinical models of asthma pathways. “What was interesting to us was that our findings were robust even when we controlled for covariates,” she added.
These findings lay the groundwork for further research into the potential therapeutic role GLP-1R agonists in asthma, Dr. Foer said. “This supports using periostin as a biomarker for novel therapeutic use of GLP-1R [agonists] in asthma,” she said. “At this point further study is needed to understand the clinical impact of GPL-1R [agonists] in asthma both for patients with type 2 diabetes and potentially in the future for patients who don’t have type 2 diabetes or metabolic disease.”
She added: “I don’t think we’re there yet; this is just one foot forward.”
The next step for researchers involves analyzing outcomes in asthmatics with type 2 diabetes on GLP-1R agonist therapy using a larger sample size as well as patients with asthma and metabolic disease, Dr. Foer said. The goal would be to identify corresponding biomarkers.
“There’s a terrific conversation in the field about the relationships between metabolism and asthma,” she said. “What our data contributes to that is, it suggests a role for metabolic pathways, specifically as it’s related GLP-1R [agonist] signaling pathways in regulating airway inflammation.”
Mark Moss, MD, associate professor of allergy & immunology at the University of Wisconsin–Madison, who was to serve as the moderator of the session, was positive about the GLP-1R agonist findings. He said in an interview: “This is promising research that provides a possible new target for the treatment of asthma.”
Dr. Foer disclosed that she has no relevant financial relationships.
SOURCE: Foer D et al. AAAAI Session 462, Abstract 784.
Patients with both type 2 diabetes and asthma who were on glucagonlike peptide receptor–1 (GLP-1R) agonists for glucose control had lower levels of a key biomarker of airway inflammation than similar patients on other types of glucose-control medications, according to results of a study to have been presented at the annual meeting of the American Academy of Asthma, Allergy, and Immunology. The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The findings from this study potentially replicated findings in humans that have been reported in preclinical trials.
“Our work showed that type 2 diabetics with asthma who were treated with GLP-1 receptor agonists had lower levels of periostin, and this provides really one of the first human data to show that these drugs may impact key inflammation pathways in the airway,” Dinah Foer, MD, of Brigham and Women’s Hospital, Boston, said in an interview. She described periostin as “a known critical inducer of airway mucous production and airway responsiveness.”
The study retrospectively evaluated serum samples from the Partners HealthCare Biobank of 161 adults with both asthma and type 2 diabetes, 42 of whom were on GLP-1R agonists and 119 of whom were taking non-GLP-1R agonist diabetes medications. The study used the Partners Healthcare EHR to identify eligible patients.
The study found that periostin levels were significantly decreased in GLP-1R agonist users: 19.1 ng/mL (standard deviation, +8.7) versus 27.4 ng/mL (SD, +14) in the non-GLP-1R agonist group (P = .001), Dr. Foer said. The other known mediators of asthma inflammatory pathways that were measured – interleukin-6, IL-8, sCD163, total IgE, and sST2 (soluble suppression of tumorigenesis–2) – showed no differences between the two groups, Dr. Foer said.
She said that this was the first human study to show similar results to preclinical models of asthma pathways. “What was interesting to us was that our findings were robust even when we controlled for covariates,” she added.
These findings lay the groundwork for further research into the potential therapeutic role GLP-1R agonists in asthma, Dr. Foer said. “This supports using periostin as a biomarker for novel therapeutic use of GLP-1R [agonists] in asthma,” she said. “At this point further study is needed to understand the clinical impact of GPL-1R [agonists] in asthma both for patients with type 2 diabetes and potentially in the future for patients who don’t have type 2 diabetes or metabolic disease.”
She added: “I don’t think we’re there yet; this is just one foot forward.”
The next step for researchers involves analyzing outcomes in asthmatics with type 2 diabetes on GLP-1R agonist therapy using a larger sample size as well as patients with asthma and metabolic disease, Dr. Foer said. The goal would be to identify corresponding biomarkers.
“There’s a terrific conversation in the field about the relationships between metabolism and asthma,” she said. “What our data contributes to that is, it suggests a role for metabolic pathways, specifically as it’s related GLP-1R [agonist] signaling pathways in regulating airway inflammation.”
Mark Moss, MD, associate professor of allergy & immunology at the University of Wisconsin–Madison, who was to serve as the moderator of the session, was positive about the GLP-1R agonist findings. He said in an interview: “This is promising research that provides a possible new target for the treatment of asthma.”
Dr. Foer disclosed that she has no relevant financial relationships.
SOURCE: Foer D et al. AAAAI Session 462, Abstract 784.
Patients with both type 2 diabetes and asthma who were on glucagonlike peptide receptor–1 (GLP-1R) agonists for glucose control had lower levels of a key biomarker of airway inflammation than similar patients on other types of glucose-control medications, according to results of a study to have been presented at the annual meeting of the American Academy of Asthma, Allergy, and Immunology. The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage.
The findings from this study potentially replicated findings in humans that have been reported in preclinical trials.
“Our work showed that type 2 diabetics with asthma who were treated with GLP-1 receptor agonists had lower levels of periostin, and this provides really one of the first human data to show that these drugs may impact key inflammation pathways in the airway,” Dinah Foer, MD, of Brigham and Women’s Hospital, Boston, said in an interview. She described periostin as “a known critical inducer of airway mucous production and airway responsiveness.”
The study retrospectively evaluated serum samples from the Partners HealthCare Biobank of 161 adults with both asthma and type 2 diabetes, 42 of whom were on GLP-1R agonists and 119 of whom were taking non-GLP-1R agonist diabetes medications. The study used the Partners Healthcare EHR to identify eligible patients.
The study found that periostin levels were significantly decreased in GLP-1R agonist users: 19.1 ng/mL (standard deviation, +8.7) versus 27.4 ng/mL (SD, +14) in the non-GLP-1R agonist group (P = .001), Dr. Foer said. The other known mediators of asthma inflammatory pathways that were measured – interleukin-6, IL-8, sCD163, total IgE, and sST2 (soluble suppression of tumorigenesis–2) – showed no differences between the two groups, Dr. Foer said.
She said that this was the first human study to show similar results to preclinical models of asthma pathways. “What was interesting to us was that our findings were robust even when we controlled for covariates,” she added.
These findings lay the groundwork for further research into the potential therapeutic role GLP-1R agonists in asthma, Dr. Foer said. “This supports using periostin as a biomarker for novel therapeutic use of GLP-1R [agonists] in asthma,” she said. “At this point further study is needed to understand the clinical impact of GPL-1R [agonists] in asthma both for patients with type 2 diabetes and potentially in the future for patients who don’t have type 2 diabetes or metabolic disease.”
She added: “I don’t think we’re there yet; this is just one foot forward.”
The next step for researchers involves analyzing outcomes in asthmatics with type 2 diabetes on GLP-1R agonist therapy using a larger sample size as well as patients with asthma and metabolic disease, Dr. Foer said. The goal would be to identify corresponding biomarkers.
“There’s a terrific conversation in the field about the relationships between metabolism and asthma,” she said. “What our data contributes to that is, it suggests a role for metabolic pathways, specifically as it’s related GLP-1R [agonist] signaling pathways in regulating airway inflammation.”
Mark Moss, MD, associate professor of allergy & immunology at the University of Wisconsin–Madison, who was to serve as the moderator of the session, was positive about the GLP-1R agonist findings. He said in an interview: “This is promising research that provides a possible new target for the treatment of asthma.”
Dr. Foer disclosed that she has no relevant financial relationships.
SOURCE: Foer D et al. AAAAI Session 462, Abstract 784.
SLIT tablet during pollen season improves symptoms of allergic rhinitis
according to recent research released as an abstract for the annual meeting of the American Academy of Allergy, Asthma & Immunology.
Tree pollen season is generally considered to be between February and June, with alder and hazel allergens affecting patients early and oak pollen affecting patients later in the season. Since a major birch allergen, Betula verrucosa 1 (Bet v 1), cross-reacts with alder, hazel, and oak allergens, some patients may experience allergies across the entire tree pollen season from members of this so-called birth homologous group, Hendrik Nolte, MD, senior vice president of research and development at ALK-Abello Americas and International, said in an interview.
According to the U.S. National Health and Nutrition Examination Survey 2005-2006, 16% of 8,086 participants 6 years or older with allergy had a specific immunoglobulin E (IgE) to birch, while 18% had a specific IgE to oak. Patients who reported having hay fever had a specific IgE to birch of 23% and a specific IgE to oak of 26% (J Allergy Clin Immunol. 2011 May;127[5]:1226-1235.e7).
“Patients who are allergic to birch pollen often experience symptoms in response to pollen from other members of the birch homologous group, which prolong the tree season and increase the symptom burden for these patients,” Dr. Nolte said. “Thus, treatment with SLIT-tablet immunotherapy may be an important treatment option for many allergy sufferers.”
Dr. Nolte and colleagues performed a randomized, double-blind, multinational trial of 634 patients before and during tree pollen season in which participants received a daily SLIT tablet or placebo. Patients were between ages 12 and 65 years with allergic rhinitis, and investigators enrolled patients or without conjunctivitis and with or without asthma. The investigations evaluated the patients’ daily symptom score and daily medication score, which was grouped into the total combined score. The patients were also allowed to use their rescue medications during the trial.
SLIT demonstrates symptom improvement
“Improvement in allergic rhinoconjunctivitis symptoms and reduction in symptom-relieving medication use with the tree SLIT-tablet during birch, alder/hazel, and oak pollen seasons were significant versus placebo and showed internal consistency across almost 4 months of birch and related tree pollen exposure,” Dr. Nolte said.
Patients showed relative improvements in their total combined score of 39.6% for birch, 29.7% for alder and hazel, 36.0% during oak pollen season, and 35.0% during the entire tree pollen season, compared with placebo (all P ≤ .002). Relative daily symptom scores also improved in the group that received oral SLIT, with 36.8% of patients showing improvement during birch season, 26.0% during alder and hazel season, 31.6% during oak season, and 31.6% across all pollen seasons, compared with those taking placebo (all P ≤ .003). A greater number of patients also achieved a relative improvement in daily medication score during birch season (49.2%), alder and hazel season (43.8%), oak season (45.9%) and during the whole of tree pollen season (45.3%), compared with placebo (P ≤ .002).
“The results support the clinical relevance of cross-reactivity between birch, alder/hazel, and oak pollen homologous allergens,” Dr. Nolte said. “Immunologic cross-reactivity is supported by alder, hazel, and oak specific IgE data and IgG4 in responses to the tree SLIT tablet.”
Dr. Nolte said the next step in his team’s research was to evaluate oral SLIT in a phase 3 trial for children aged 5-17 years.
This study was funded by ALK, and the authors received medical writing and editorial assistance from Scott Medical Communications. Dr. Nolte reported that he is a paid employee of ALK.
SOURCE: Nolte H et al. AAAAI, Abstract 267.
.
according to recent research released as an abstract for the annual meeting of the American Academy of Allergy, Asthma & Immunology.
Tree pollen season is generally considered to be between February and June, with alder and hazel allergens affecting patients early and oak pollen affecting patients later in the season. Since a major birch allergen, Betula verrucosa 1 (Bet v 1), cross-reacts with alder, hazel, and oak allergens, some patients may experience allergies across the entire tree pollen season from members of this so-called birth homologous group, Hendrik Nolte, MD, senior vice president of research and development at ALK-Abello Americas and International, said in an interview.
According to the U.S. National Health and Nutrition Examination Survey 2005-2006, 16% of 8,086 participants 6 years or older with allergy had a specific immunoglobulin E (IgE) to birch, while 18% had a specific IgE to oak. Patients who reported having hay fever had a specific IgE to birch of 23% and a specific IgE to oak of 26% (J Allergy Clin Immunol. 2011 May;127[5]:1226-1235.e7).
“Patients who are allergic to birch pollen often experience symptoms in response to pollen from other members of the birch homologous group, which prolong the tree season and increase the symptom burden for these patients,” Dr. Nolte said. “Thus, treatment with SLIT-tablet immunotherapy may be an important treatment option for many allergy sufferers.”
Dr. Nolte and colleagues performed a randomized, double-blind, multinational trial of 634 patients before and during tree pollen season in which participants received a daily SLIT tablet or placebo. Patients were between ages 12 and 65 years with allergic rhinitis, and investigators enrolled patients or without conjunctivitis and with or without asthma. The investigations evaluated the patients’ daily symptom score and daily medication score, which was grouped into the total combined score. The patients were also allowed to use their rescue medications during the trial.
SLIT demonstrates symptom improvement
“Improvement in allergic rhinoconjunctivitis symptoms and reduction in symptom-relieving medication use with the tree SLIT-tablet during birch, alder/hazel, and oak pollen seasons were significant versus placebo and showed internal consistency across almost 4 months of birch and related tree pollen exposure,” Dr. Nolte said.
Patients showed relative improvements in their total combined score of 39.6% for birch, 29.7% for alder and hazel, 36.0% during oak pollen season, and 35.0% during the entire tree pollen season, compared with placebo (all P ≤ .002). Relative daily symptom scores also improved in the group that received oral SLIT, with 36.8% of patients showing improvement during birch season, 26.0% during alder and hazel season, 31.6% during oak season, and 31.6% across all pollen seasons, compared with those taking placebo (all P ≤ .003). A greater number of patients also achieved a relative improvement in daily medication score during birch season (49.2%), alder and hazel season (43.8%), oak season (45.9%) and during the whole of tree pollen season (45.3%), compared with placebo (P ≤ .002).
“The results support the clinical relevance of cross-reactivity between birch, alder/hazel, and oak pollen homologous allergens,” Dr. Nolte said. “Immunologic cross-reactivity is supported by alder, hazel, and oak specific IgE data and IgG4 in responses to the tree SLIT tablet.”
Dr. Nolte said the next step in his team’s research was to evaluate oral SLIT in a phase 3 trial for children aged 5-17 years.
This study was funded by ALK, and the authors received medical writing and editorial assistance from Scott Medical Communications. Dr. Nolte reported that he is a paid employee of ALK.
SOURCE: Nolte H et al. AAAAI, Abstract 267.
.
according to recent research released as an abstract for the annual meeting of the American Academy of Allergy, Asthma & Immunology.
Tree pollen season is generally considered to be between February and June, with alder and hazel allergens affecting patients early and oak pollen affecting patients later in the season. Since a major birch allergen, Betula verrucosa 1 (Bet v 1), cross-reacts with alder, hazel, and oak allergens, some patients may experience allergies across the entire tree pollen season from members of this so-called birth homologous group, Hendrik Nolte, MD, senior vice president of research and development at ALK-Abello Americas and International, said in an interview.
According to the U.S. National Health and Nutrition Examination Survey 2005-2006, 16% of 8,086 participants 6 years or older with allergy had a specific immunoglobulin E (IgE) to birch, while 18% had a specific IgE to oak. Patients who reported having hay fever had a specific IgE to birch of 23% and a specific IgE to oak of 26% (J Allergy Clin Immunol. 2011 May;127[5]:1226-1235.e7).
“Patients who are allergic to birch pollen often experience symptoms in response to pollen from other members of the birch homologous group, which prolong the tree season and increase the symptom burden for these patients,” Dr. Nolte said. “Thus, treatment with SLIT-tablet immunotherapy may be an important treatment option for many allergy sufferers.”
Dr. Nolte and colleagues performed a randomized, double-blind, multinational trial of 634 patients before and during tree pollen season in which participants received a daily SLIT tablet or placebo. Patients were between ages 12 and 65 years with allergic rhinitis, and investigators enrolled patients or without conjunctivitis and with or without asthma. The investigations evaluated the patients’ daily symptom score and daily medication score, which was grouped into the total combined score. The patients were also allowed to use their rescue medications during the trial.
SLIT demonstrates symptom improvement
“Improvement in allergic rhinoconjunctivitis symptoms and reduction in symptom-relieving medication use with the tree SLIT-tablet during birch, alder/hazel, and oak pollen seasons were significant versus placebo and showed internal consistency across almost 4 months of birch and related tree pollen exposure,” Dr. Nolte said.
Patients showed relative improvements in their total combined score of 39.6% for birch, 29.7% for alder and hazel, 36.0% during oak pollen season, and 35.0% during the entire tree pollen season, compared with placebo (all P ≤ .002). Relative daily symptom scores also improved in the group that received oral SLIT, with 36.8% of patients showing improvement during birch season, 26.0% during alder and hazel season, 31.6% during oak season, and 31.6% across all pollen seasons, compared with those taking placebo (all P ≤ .003). A greater number of patients also achieved a relative improvement in daily medication score during birch season (49.2%), alder and hazel season (43.8%), oak season (45.9%) and during the whole of tree pollen season (45.3%), compared with placebo (P ≤ .002).
“The results support the clinical relevance of cross-reactivity between birch, alder/hazel, and oak pollen homologous allergens,” Dr. Nolte said. “Immunologic cross-reactivity is supported by alder, hazel, and oak specific IgE data and IgG4 in responses to the tree SLIT tablet.”
Dr. Nolte said the next step in his team’s research was to evaluate oral SLIT in a phase 3 trial for children aged 5-17 years.
This study was funded by ALK, and the authors received medical writing and editorial assistance from Scott Medical Communications. Dr. Nolte reported that he is a paid employee of ALK.
SOURCE: Nolte H et al. AAAAI, Abstract 267.
.
REPORTING FROM AAAAI
COVID-19 in pediatric patients: What the hospitalist needs to know
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
Coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization on March 11. This rapidly spreading disease is caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The infection has spread to more than 140 countries, including the United States. As of March 16, more than 170,400 people had tested positive for SARS-CoV-2 and more than 6,619 people have died across the globe.
The number of new COVID-19 cases appears to be decreasing in China, but the number of cases are rapidly increasing worldwide. Based on available data, primarily from China, children (aged 0-19 years) account for only about 2% of all cases. Despite the probable low virulence and incidence of infection in children, they could act as potential vectors and transmit infection to more vulnerable populations. As of March 16, approximately 3,823 cases and more than 67 deaths had been reported in the United States with few pediatric patients testing positive for the disease.
SARS-CoV2 transmission mainly occurs via respiratory route through close contact with infected individuals and through fomites. The incubation period ranges from 2-14 days with an average of about 5 days. Adult patients present with cough and fever, which may progress to lower respiratory tract symptoms, including shortness of breath. Approximately 10% of all patients develop severe disease and acute respiratory distress syndrome (ARDS), requiring mechanical ventilation.
COVID-19 carries a mortality rate of up to 3%, but has been significantly higher in the elderly population, and those with chronic health conditions. Available data so far shows that children are at lower risk and the severity of the disease has been milder compared to adults. The reasons for this are not clear at this time. As of March 16, there were no reported COVID-19 related deaths in children under age 9 years.
The pediatric population: Disease patterns and transmission
The epidemiology and spectrum of disease for COVID-19 is poorly understood in pediatrics because of the low number of reported pediatric cases and limited data available from these patients. Small numbers of reported cases in children has led some to believe that children are relatively immune to the infection by SARS-CoV-2. However, Oifang et al. found that children are equally as likely as adults to be infected.1
Liu et al. found that of 366 children admitted to a hospital in Wuhan with respiratory infections in January 2020, 1.6% (six patients) cases were positive for SARS-CoV-2.2 These six children were aged 1-7 years and had all been previously healthy; all six presented with cough and fever of 102.2° F or greater. Four of the children also had vomiting. Laboratory findings were notable for lymphopenia (six of six), leukopenia (four of six), and neutropenia (3/6) with mild to moderate elevation in C-reactive protein (6.8-58.8 mg/L). Five of six children had chest CT scans. One child’s CT scan showed “bilateral ground-glass opacities” (similar to what is reported in adults), three showed “bilateral patchy shadows,” and one was normal. One child (aged 3 years) was admitted to the ICU. All of the children were treated with supportive measures, empiric antibiotics, and antivirals (six of six received oseltamivir and four of six received ribavirin). All six children recovered completely and their median hospital stay was 7.5 days with a range of 5-13 days.
Xia et al. reviewed 20 children (aged 1 day to 14 years) admitted to a hospital in Wuhan during Jan. 23–Feb. 8.3 The study reported that fever and cough were the most common presenting symptoms (approximately 65%). Less common symptoms included rhinorrhea (15%), diarrhea (15%), vomiting (10%), and sore throat (5%). WBC count was normal in majority of children (70%) with leukopenia in 20% and leukocytosis in 10%. Lymphopenia was noted to be 35%. Elevated procalcitonin was noted in 80% of children, although the degree of elevation is unclear. In this study, 8 of 20 children were coinfected with other respiratory pathogens such as influenza, respiratory syncytial virus, mycoplasma, and cytomegalovirus. All children had chest CT scans. Ten of 20 children had bilateral pulmonary lesions, 6 of 20 had unilateral pulmonary lesions, 12 of 20 had ground-glass opacities and 10 of 20 had lung consolidations with halo signs.
Wei et al., retrospective chart review of nine infants admitted for COVID-19 found that all nine had at least one infected family member.4 This study reported that seven of nine were female infants, four of nine had fever, two had mild upper respiratory infection symptoms, and one had no symptoms. The study did report that two infants did not have any information available related to symptoms. None of the infants developed severe symptoms or required ICU admission.
The youngest patient to be diagnosed with COVID-19 was a newborn of less than 24 hours old from England, whose mother also tested positive for SARS-CoV-2. However, Chen et al. found no evidence of vertical transmission of the virus from infected pregnant women to their newborns.5
Although the risk of infection in children has been reported to be low, the infection has been shown to be particularly severe in adults with compromised immune systems and chronic health conditions. Thus immunocompromised children and those with chronic health conditions are thought to be at a higher risk for contracting the infection, with the probability for increased morbidity and mortality. Some of these risk groups include premature infants, young infants, immunocompromised children, and children with chronic health conditions like asthma, diabetes, and others. It is essential that caregivers, healthy siblings, and other family members are protected from contracting the infection in order to protect these vulnerable children. Given the high infectivity of SARS-CoV-2, the implications of infected children attending schools and daycares may be far reaching if there is delayed identification of the infection. For these reasons, it is important to closely monitor and promptly test children living with infected adults to prevent the spread. It may become necessary to close schools to mitigate transmission.
Schools and daycares should work with their local health departments and physicians in case of infected individuals in their community. In China, authorities closed schools and allowed students to receive virtual education from home, which may be a reasonable choice depending on resources.
Current challenges
Given the aggressive transmission of COVID-19, these numbers seem to be increasing exponentially with a significant impact on the life of the entire country. Therefore, we must focus on containing the spread and mitigating the transmission with a multimodality approach.
Some of the initial challenges faced by physicians in the United States were related to difficulty in access to testing in persons under investigation (PUI), which in turn resulted in a delay in diagnosis and infection control. At this time, the need is to increase surge testing capabilities across the country through a variety of innovative approaches including public-private partnerships with commercial labs through Emergency Use Authorization (EUA) issued by the Centers for Disease Control and Prevention and the Department of Health and Human Services. To minimize exposure to health care professionals, telemedicine and telehealth capabilities should be exploited. This will minimize the exposure to infected patients and reduce the need for already limited personal protective equipment (PPE). As the number of cases rise, hospitals should expect and prepare for a surge in COVID-19–related hospitalizations and health care utilization.
Conclusion
Various theories are being proposed as to why children are not experiencing severe disease with COVID-19. Children may have cross-protective immunity from infection with other coronaviruses. Children may not have the same exposures from work, travel, and caregiving that adults experience as they are typically exposed by someone in their home. At this time, not enough is known about clinical presentations in children as the situation continues to evolve across the globe.
Respiratory infections in children pose unique infection control challenges with respect to compliant hand hygiene, cough etiquette, and the use of PPE when indicated. There is also concern for persistent fecal shedding of virus in infected pediatric patients, which could be another mode of transmission.6 Children could, however, be very efficient vectors of COVID-19, similar to flu, and potentially spread the pathogen to very vulnerable populations leading to high morbidity and mortality. School closures are an effective social distancing measure needed to flatten the curve and avoid overwhelming the health care structure of the United States.
Dr. Konanki is a board-certified pediatrician doing inpatient work at Wellspan Chambersburg Hospital and outpatient work at Keystone Pediatrics in Chambersburg, Pa. He also serves as the physician member of the hospital’s Code Blue Jr. committee and as a member of Quality Metrics committee at Keystone Health. Dr. Tirupathi is the medical director of Keystone Infectious Diseases/HIV in Chambersburg, Pa., and currently chair of infection prevention at Wellspan Chambersburg and Waynesboro (Pa.) Hospitals. He also is the lead physician for antibiotic stewardship at these hospitals. Dr. Palabindala is hospital medicine division chief at the University of Mississippi Medical Center, Jackson.
References
1. Bi Q et al. Epidemiology and transmission of COVID-19 in Shenzhen China: Analysis of 391 cases and 1,286 of their close contacts. medRxiv 2020.03.03.20028423.
2. Liu W et al. Detection of Covid-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
3. Xia W et al. Clinical and CT features in pediatric patients with COVID‐19 infection: Different points from adults. Pediatr Pulmonol. 2020 Mar 5. doi: 10.1002/ppul.24718.
4. Wei M et al. Novel Coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020 Feb. 14. doi: 10.1001/jama.2020.2131.
5. Huijun C et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet. 2020 Mar 7 395;10226:809-15.
6. Xu Y et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 Mar 13. doi. org/10.1038/s41591-020-0817-4.
FDA provides flexibility to improve COVID-19 test availability
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
First, the FDA is giving states more flexibility to approve and implement testing for COVID-19.
“States can set up a system in which they take responsibility for authorizing such tests and the laboratories will not engage with the FDA,” agency Commissioner Stephen Hahn, MD, said in a March 16 statement announcing the policy updates. “Laboratories developing tests in these states can engage directly with the appropriate state authorities, instead of with the FDA.”
A copy of the updated guidance document can be found here.
Dr. Hahn added that laboratories working within this authority granted to states will not have to pursue an emergency use authorization (EUA). New York state was previously granted a waiver to allow for more state oversight over the introduction of diagnostic testing.
Second, the FDA is expanding guidance issued on Feb. 29 on who can develop diagnostic tests. Originally, the Feb. 29 guidance was aimed at labs certified to perform high-complexity testing consistent with requirements outlined in the Clinical Laboratory Improvement Amendments.
“Under the update published today, the agency does not intend to object to commercial manufacturers distributing and labs using new commercially developed tests prior to the FDA granting an EUA, under certain circumstances,” Commissioner Hahn said, adding that a number of commercial manufacturers are developing tests for the coronavirus with the intent of submitting an EUA request.
“During this public health emergency, the FDA does not intend to object to the distribution and use of these tests for specimen testing for a reasonable period of time after the manufacturer’s validation of the test while the manufacturer is preparing its EUA request,” he added.
The updated guidance also provides recommendations for test developers working on serologic tests for COVID-19.
During a March 16 conference call with reporters, Commissioner Hahn said the flexibility would add a “significant number of tests and we believe this will be a surge to meet the demand that we expect to see, although it is somewhat difficult” to quantify the number of tests this new flexibility will bring to the market.
CDC expert answers top COVID-19 questions
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
With new developments daily and lingering uncertainty about COVID-19, questions about testing and treatment for the coronavirus are at the forefront.
To address these top questions, Jay C. Butler, MD, deputy director for infectious diseases at the Centers for Disease Control and Prevention, sat down with JAMA editor Howard Bauchner, MD, to discuss the latest data on COVID-19 and to outline updated guidance from the agency. The following question-and-answer session was part of a live stream interview hosted by JAMA on March 16, 2020. The questions have been edited for length and clarity.
What test is being used to identify COVID-19?
In the United States, the most common and widely available test is the RT-polymerase chain reaction (rRT-PCR), which over the past few weeks has become available at public health labs across the country, Dr. Butler said during the JAMA interview. Capacity for the test is now possible in all 50 states and in Washington, D.C.
“More recently, there’s been a number of commercial labs that have come online to be able to do the testing,” Dr. Butler said. “Additionally, a number of academic centers are now able to run [Food and Drug Administration]–approved testing using slightly different PCR platforms.”
How accurate is the test?
Dr. Butler called PCR the “gold standard,” for testing COVID-19, and said it’s safe to say the test’s likelihood of identifying infection or past infection is extremely high. However, data on test sensitivity is limited.
“This may be frustrating to those of us who really like to know specifics of how to interpret the test results, but it’s important to keep in mind, we’re talking about a virus that we didn’t know existed 3 months ago,” he said.
At what point does a person with coronavirus test positive?
When exactly a test becomes positive is an unknown, Dr. Butler said. The assumption is that a patient who tests positive is more likely to be infectious, and data suggest the level of infectiousness is greatest after the onset of symptoms.
“There is at least some anecdotal reports that suggest that transmission could occur before onset of symptoms, but the data is still very limited,” he said. “Of course that has big implications in terms of how well we can really slow the spread of the virus.”
Who should get tested?
Dr. Butler said the focus should be individuals who are symptomatic with evidence of respiratory tract infection. People who are concerned about the virus and want a test are not the target.
“It’s important when talking to patients to help them to understand, this is different than a test for HIV or hepatitis C, where much of the message is: ‘Please get tested.’ ” he said. “This a situation where we’re trying to diagnose an acute infection. We do have a resource that may become limited again as some of the equipment required for running the test or collecting the specimen may come into short supply, so we want to focus on those people who are symptomatic and particularly on people who may be at higher risk of more severe illness.”
If a previously infected patient tests negative, can they still shed virus?
The CDC is currently analyzing how a negative PCR test relates to viral load, according to Dr. Butler. He added there have been situations in which a patient has twice tested negative for the virus, but a third swab resulted in a weakly positive result.
“It’s not clear if those are people who are actually infectious,” he said. “The PCR is detecting viral RNA, it doesn’t necessarily indicate there is viable virus present in the respiratory tract. So in general, I think it is safe to go back to work, but a positive test in a situation like that can be very difficult to interpret because we think it probably doesn’t reflect infectivity, but we don’t know for sure.”
Do we have an adequate supply of tests in the United States?
The CDC has addressed supply concerns by broadening the number of PCR platforms that can be used to run COVID-19 analyses, Dr. Butler said. Expansion of these platforms has been one way the government is furthering testing options and enabling consumer labs and academic centers to contribute to testing.
When can people who test positive go back to work?
The CDC is still researching that question and reviewing the data, Dr. Butler said. The current recommendation is that a patient who tests positive is considered clear to return to work after two negative tests at least 24 hours apart, following the resolution of symptoms. The CDC has not yet made an official recommendation on an exact time frame, but the CDC is considering a 14-day minimum of quarantine.
“The one caveat I’ll add is that someone who is a health care worker, even if they have resolved symptoms, it’s still a good idea to wear a surgical mask [when they return to work], just as an extra precaution.”
What do we know about immunity? Can patients get reinfected?
Long-term immunity after exposure and infection is virtually unknown, Dr. Butler said. Investigators know those with COVID-19 have an antibody response, but whether that is protective or not, is unclear. In regard to older coronaviruses, such as those that cause colds, patients generally develop an antibody response and may have a period of immunity, but that immunity eventually wanes and reinfection can occur.
What is the latest on therapies?
A number of trials are underway in China and in the United States to test possible therapies for COVID-19, Dr. Butler said. One of the candidate drugs is the broad spectrum antiviral drug remdesivir, which was developed for the treatment of the Ebola virus. Additionally, the National Institutes of Health is studying the potential for monoclonal antibodies to treat COVID-19.
“Of course these are drugs not yet FDA approved,” he said. “We all want to have them in our toolbox as soon as possible, but we want to make sure these drugs are going to benefit and not harm, and that they really do have the utility that we hope for.”
Is there specific guidance for healthcare workers about COVID-19?
Health care workers have a much higher likelihood of being exposed or exposing others who are at high risk of severe infection, Dr. Butler said. That’s why, if a health care worker becomes infected and recovers, it’s still important to take extra precautions when going back to work, such as wearing a mask.
“These are recommendations that are in-draft,” he said. “I want to be clear, I’m floating concepts out there that people can consider. ... I recognize as a former infection control medical director at a hospital that sometimes you have to adapt those guidelines based on your local conditions.”
Trump to governors: Don’t wait for feds on medical supplies
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
President Donald Trump has advised state governors not to wait on the federal government when it comes to ensuring readiness for a surge in patients from the COVID-19 outbreak.
“If they are able to get ventilators, respirators, if they are able to get certain things without having to go through the longer process of federal government,” they should order on their own and bypass the federal government ordering system, the president stated during a March 16 press briefing.
That being said, he noted that the federal government is “ordering tremendous numbers of ventilators, respirators, [and] masks,” although he could not give a specific number on how much has been ordered or how many has already been stockpiled.
“It is always going to be faster if they can get them directly, if they need them, and I have given them authorization to order directly,” President Trump said.
The comments came as the White House revised recommendations on gatherings. The new guidelines now limit gatherings to no more than 10 people. Officials are further advising Americans to self-quarantine for 2 weeks if they are sick, if someone in their house is sick, or if someone in their house has tested positive for COVID-19.
Additionally, the White House called on Americans to limit discretionary travel and to avoid eating and drinking in restaurants, bars, and food courts during the next 15 days, even if they are feeling healthy and are asymptomatic.
“With several weeks of focused action, we can turn the corner and turn it quickly,” the president said.
In terms of testing, the Food and Drug Administration has granted emergency use authorization to two commercial diagnostic tests: Thermo Fisher for its TaqPath COVID-19 Combo Kit and Roche for its cobas SARS-CoV-2 test. White House officials said up to 1 million tests will be available this week, with 2 million next week.
The president also announced that phase 1 testing of a vaccine has begun. The test involves more than 40 healthy volunteers in the Seattle area who will receive three shots over the trial period. Phase 1 testing is generally conducted to determine safety of a new therapeutic.
ESC says continue hypertension meds despite COVID-19 concern
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The European Society of Cardiology (ESC) has issued a statement urging physicians and patients to continue treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), in light of a newly described theory that those agents could increase the risk of developing COVID-19 and/or worsen its severity.
The concern arises from the observation that the new coronavirus SARS-CoV-2 causing COVID-19 binds to angiotensin-converting enzyme 2 (ACE2) to infect cells, and both ACE inhibitors and ARBs increase ACE2 levels.
This mechanism has been theorized as a possible risk factor for facilitating the acquisition of COVID-19 infection and worsening its severity. However, paradoxically, it has also been hypothesized to protect against acute lung injury from the disease.
Meanwhile, a Lancet Respiratory Medicine article was published March 11 entitled, “Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?”
“We ... hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” said the authors.
This prompted some media coverage in the United Kingdom and “social media-related amplification,” leading to concern and, in some cases, discontinuation of the drugs by patients.
But on March 13, the ESC Council on Hypertension dismissed the concerns as entirely speculative, in a statement posted to the ESC website.
It said that the council “strongly recommend that physicians and patients should continue treatment with their usual antihypertensive therapy because there is no clinical or scientific evidence to suggest that treatment with ACE inhibitors or ARBs should be discontinued because of the COVID-19 infection.”
The statement, signed by Council Chair Professor Giovanni de Simone, MD, on behalf of the nucleus members, also says that in regard to the theorized protective effect against serious lung complications in individuals with COVID-19, the data come only from animal, and not human, studies.
“Speculation about the safety of ACE-inhibitor or ARB treatment in relation to COVID-19 does not have a sound scientific basis or evidence to support it,” the ESC panel concludes.
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The European Society of Cardiology (ESC) has issued a statement urging physicians and patients to continue treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), in light of a newly described theory that those agents could increase the risk of developing COVID-19 and/or worsen its severity.
The concern arises from the observation that the new coronavirus SARS-CoV-2 causing COVID-19 binds to angiotensin-converting enzyme 2 (ACE2) to infect cells, and both ACE inhibitors and ARBs increase ACE2 levels.
This mechanism has been theorized as a possible risk factor for facilitating the acquisition of COVID-19 infection and worsening its severity. However, paradoxically, it has also been hypothesized to protect against acute lung injury from the disease.
Meanwhile, a Lancet Respiratory Medicine article was published March 11 entitled, “Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?”
“We ... hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” said the authors.
This prompted some media coverage in the United Kingdom and “social media-related amplification,” leading to concern and, in some cases, discontinuation of the drugs by patients.
But on March 13, the ESC Council on Hypertension dismissed the concerns as entirely speculative, in a statement posted to the ESC website.
It said that the council “strongly recommend that physicians and patients should continue treatment with their usual antihypertensive therapy because there is no clinical or scientific evidence to suggest that treatment with ACE inhibitors or ARBs should be discontinued because of the COVID-19 infection.”
The statement, signed by Council Chair Professor Giovanni de Simone, MD, on behalf of the nucleus members, also says that in regard to the theorized protective effect against serious lung complications in individuals with COVID-19, the data come only from animal, and not human, studies.
“Speculation about the safety of ACE-inhibitor or ARB treatment in relation to COVID-19 does not have a sound scientific basis or evidence to support it,” the ESC panel concludes.
This article first appeared on Medscape.com.
Editor’s note: Find the latest COVID-19 news and guidance in Medscape’s Coronavirus Resource Center.
The European Society of Cardiology (ESC) has issued a statement urging physicians and patients to continue treatment with angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), in light of a newly described theory that those agents could increase the risk of developing COVID-19 and/or worsen its severity.
The concern arises from the observation that the new coronavirus SARS-CoV-2 causing COVID-19 binds to angiotensin-converting enzyme 2 (ACE2) to infect cells, and both ACE inhibitors and ARBs increase ACE2 levels.
This mechanism has been theorized as a possible risk factor for facilitating the acquisition of COVID-19 infection and worsening its severity. However, paradoxically, it has also been hypothesized to protect against acute lung injury from the disease.
Meanwhile, a Lancet Respiratory Medicine article was published March 11 entitled, “Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection?”
“We ... hypothesize that diabetes and hypertension treatment with ACE2-stimulating drugs increases the risk of developing severe and fatal COVID-19,” said the authors.
This prompted some media coverage in the United Kingdom and “social media-related amplification,” leading to concern and, in some cases, discontinuation of the drugs by patients.
But on March 13, the ESC Council on Hypertension dismissed the concerns as entirely speculative, in a statement posted to the ESC website.
It said that the council “strongly recommend that physicians and patients should continue treatment with their usual antihypertensive therapy because there is no clinical or scientific evidence to suggest that treatment with ACE inhibitors or ARBs should be discontinued because of the COVID-19 infection.”
The statement, signed by Council Chair Professor Giovanni de Simone, MD, on behalf of the nucleus members, also says that in regard to the theorized protective effect against serious lung complications in individuals with COVID-19, the data come only from animal, and not human, studies.
“Speculation about the safety of ACE-inhibitor or ARB treatment in relation to COVID-19 does not have a sound scientific basis or evidence to support it,” the ESC panel concludes.
This article first appeared on Medscape.com.
Here’s what ICUs are putting up against COVID-19
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.
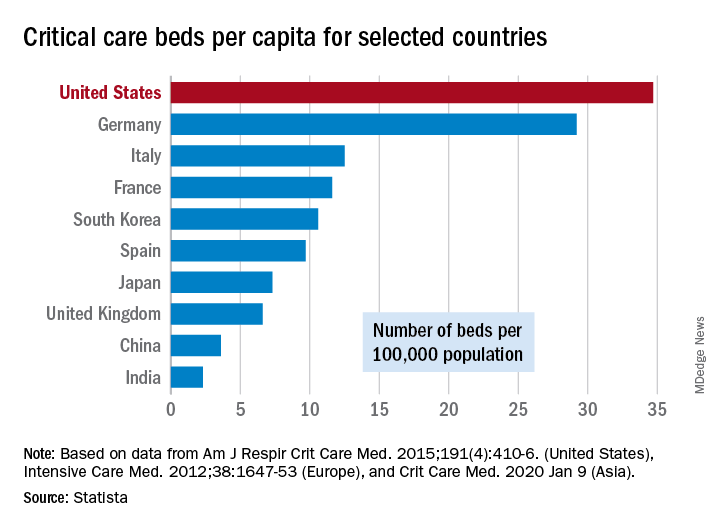
That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
As COVID-19 spreads across the United States, it is important to understand the extent of the nation’s ICU resources, according to the Society of Critical Care Medicine. The SCCM has updated its statistics on the resources available to care for what could become “an overwhelming number of critically ill patients, many of whom may require mechanical ventilation,” the society said in a blog post on March 13.

That overwhelming number was considered at an American Hospital Association webinar in February: Investigators projected that 4.8 million patients could be hospitalized with COVID-19, of whom 1.9 million would be admitted to ICUs and 960,000 would require ventilator support, Neil A. Halpern, MD, director of the critical care center at Memorial Sloan Kettering Cancer Center, New York, and Kay See Tan, PhD, of the hospital’s department of epidemiology and biostatistics, reported in that post.
As far as critical care beds are concerned, the United States is in better shape than are other countries dealing with the coronavirus. The United States’ 34.7 critical care beds per 100,000 population put it a good bit ahead of Germany, which has 29.2 beds per 100,000, while other countries in both Europe and Asia are well behind, Dr. Halpern and Dr. Tan noted.
More recent data from the AHA show that just over half of its registered community hospitals deliver ICU services and have at least 10 acute care beds and one ICU bed, they reported.
Those 2,704 hospitals have nearly 535,000 acute care beds, of which almost 97,000 are ICU beds. Almost 71% of those ICU beds are for adults, with the rest located in neonatal and pediatric units, data from an AHA 2018 survey show.
Since patients with COVID-19 are most often admitted to ICUs with severe hypoxic respiratory failure, the nation’s supply of ventilators also may be tested. U.S. acute care hospitals own about 62,000 full-featured mechanical ventilators and almost 99,000 older ventilators that “may not be capable of adequately supporting patients with severe acute respiratory failure,” Dr. Halpern and Dr. Tan said.
As U.S. hospitals reach the crisis levels anticipated in the COVID-19 pandemic, staffing shortages can be expected as well. Almost half (48%) of acute care hospitals have no intensivists, so “other physicians (e.g., pulmonologists, surgeons, anesthesiologists, etc) may be pressed into service as outpatient clinics and elective surgery are suspended,” they wrote.
The blog post includes a tiered staffing strategy that the SCCM “encourages hospitals to adopt in pandemic situations such as COVID-19.”
COVID-19 in children, pregnant women: What do we know?
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
A novel coronavirus, the causative agent of the current pandemic of viral respiratory illness and pneumonia, was first identified in Wuhan, Hubei, China. The disease has been given the name, coronavirus disease 2019 (COVID-19). The virus at last report has spread to more than 100 countries. Much of what we suspect about this virus comes from work on other severe coronavirus respiratory disease outbreaks – Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). MERS-CoV was a viral respiratory disease, first reported in Saudi Arabia, that was identified in more than 27 additional countries. The disease was characterized by severe acute respiratory illness, including fever, cough, and shortness of breath. Among 2,499 cases, only two patients tested positive for MERS-CoV in the United States. SARS-CoV also caused a severe viral respiratory illness. SARS was first recognized in Asia in 2003 and was subsequently reported in approximately 25 countries. The last case reported was in 2004.
As of March 13, there are 137,066 cases worldwide of COVID-19 and 1,701 in the United States, according to the John Hopkins University Coronavirus COVID-19 resource center.
What about children?
The remarkable observation is how few seriously ill children have been identified in the face of global spread. Unlike the H1N1 influenza epidemic of 2009, where older adults were relatively spared and children were a major target population, COVID-19 appears to be relatively infrequent in children or too mild to come to diagnosis, to date. Specifically, among China’s first approximately 44,000 cases, less than 2% were identified in children less than 20 years of age, and severe disease was uncommon with no deaths in children less than 10 years of age reported. One child, 13 months of age, with acute respiratory distress syndrome and septic shock was reported in China. According to the Centers for Disease Control and Prevention webcast , children present with fever in about 50% of cases, cough, fatigue, and subsequently some (3%-30%) progress to shortness of breath. Some children and adults have presented with gastrointestinal disease initially. Viral RNA has been detected in respiratory secretions, blood, and stool of affected children; however, the samples were not cultured for virus so whether stool is a potential source for transmission is unclear. In adults, the disease appears to be most severe – with development of pneumonia – in the second week of illness. In both children and adults, the chest x-ray findings are an interstitial pneumonitis, ground glass appearance, and/or patchy infiltrates.

Are some children at greater risk? Are children the source of community transmission? Will children become a greater part of the disease pattern as further cases are identified and further testing is available? We cannot answer many of these questions about COVID-19 in children as yet, but as you are aware, data are accumulating daily, and the Centers for Disease Control and Prevention and the National Institutes of Health are providing regular updates.
A report from China gave us some idea about community transmission and infection risk for children. The Shenzhen CDC identified 391 COVID-19 cases and 1,286 close contacts. Household contacts and those persons traveling with a case of the virus were at highest risk of acquisition. The secondary attack rates within households was 15%; children were as likely to become infected as adults (medRxiv preprint. 2020. doi: 10.1101/2020.03.03.20028423).
What about pregnant women?
The data on pregnant women are even more limited. The concern about COVID-19 during pregnancy comes from our knowledge of adverse outcomes from other respiratory viral infections. For example, respiratory viral infections such as influenza have been associated with increased maternal risk of severe disease, and adverse neonatal outcomes, including low birth weight and preterm birth. The experience with SARS also is concerning for excess adverse maternal and neonatal complications such as spontaneous miscarriage, preterm delivery, intrauterine growth restriction, admission to the ICU, renal failure, and disseminated intravascular coagulopathy all were reported as complications of SARS infection during pregnancy.
Two studies on COVID-19 in pregnancy have been reported to date. In nine pregnant women reported by Chen et al., COVID-19 pneumonia was identified in the third trimester. The women presented with fever, cough, myalgia, sore throat, and/or malaise. Fetal distress was reported in two; all nine infants were born alive. Apgar scores were 8-10 at 1 minute. Five were found to have lymphopenia; three had increases in hepatic enzymes. None of the infants developed severe COVID-19 pneumonia. Amniotic fluid, cord blood, neonatal throat swab, and breast milk samples from six of the nine patients were tested for the novel coronavirus 2019, and all results were negative (Lancet. 2020 Feb 12. doi: 10.1016/S0140-6736[20]30360-3)https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30360-3/fulltext.
In a study by Zhu et al., nine pregnant women with confirmed COVID-19 infection were identified during Jan. 20-Feb. 5, 2020. The onset of clinical symptoms in these women occurred before delivery in four cases, on the day of delivery in two cases, and after delivery in three cases. Of the 10 neonates (one set of twins) many had clinical symptoms, but none were proven to be COVID-19 positive in their pharyngeal swabs. Shortness of breath was observed in six, fever in two, tachycardia in one. GI symptoms such as feeding intolerance, bloating, GI bleed, and vomiting also were observed. Chest radiography showed abnormalities in seven neonates at admission. Thrombocytopenia and/or disseminated intravascular coagulopathy also was reported. Five neonates recovered and were discharged, one died, and four neonates remained in hospital in a stable condition. It is unclear if the illness in these infants was related to COVID-19 (Transl Pediatrics. 2020 Feb. doi: 10.21037/tp.2020.02.06)http://tp.amegroups.com/article/view/35919/28274.
In the limited experience to date, no evidence of virus has been found in the breast milk of women with COVID-19, which is consistent with the SARS experience. Current recommendations are to separate the infant from known COVID-19 infected mothers either in a different room or in the mother’s room using a six foot rule, a barrier curtain of some type, and mask and hand washing prior to any contact between mother and infant. If the mother desires to breastfeed her child, the same precautions – mask and hand washing – should be in place.
What about treatment?
There are no proven effective therapies and supportive care has been the mainstay to date. Clinical trials of remdesivir have been initiated both by Gilead (compassionate use, open label) and by the National Institutes of Health (randomized remdesivirhttps://www.drugs.com/history/remdesivir.html vs. placebo) in adults based on in vitro data suggesting activity again COVID-19. Lopinavir/ritonavir (combination protease inhibitors) also have been administered off label, but no results are available as yet.
Keeping up
I suggest several valuable resources to keep yourself abreast of the rapidly changing COVID-19 story. First the CDC website or your local Department of Health. These are being updated frequently and include advisories on personal protective equipment, clusters of cases in your local community, and current recommendations for mitigation of the epidemic. I have listened to Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and Robert R. Redfield, MD, the director of the CDC almost daily. I trust their viewpoints and transparency about what is and what is not known, as well as the why and wherefore of their guidance, remembering that each day brings new information and new guidance.
Dr. Pelton is professor of pediatrics and epidemiology at Boston University and public health and senior attending physician at Boston Medical Center. He has no relevant financial disclosures. Email him at [email protected].
Detection of COVID-19 in children in early January 2020 in Wuhan, China
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.
Clinical question: What were the clinical characteristics of children in Wuhan, China hospitalized with SARS-CoV-2?
Background: The coronavirus disease 2019 (COVID-19) was recently described by researchers in Wuhan, China.1 However, there has been limited discussion on how the disease has affected children. Based on the Chinese Center for Disease Control and Prevention report, Wu et al. found that 1% of the affected population was less than 10 years, and another 1% of the affected population was 10-19 years.2 However, little information regarding hospitalizations of children with viral infections was previously reported.
Study design: A retrospective analysis of hospitalized children.
Setting: Three sites of a multisite urban teaching hospital in central Wuhan, China.
Synopsis: Over an 8-day period, hospitalized pediatric patients were retrospectively enrolled into this study. The authors defined pediatric patients as those aged 16 years or younger. The patients had one throat swab specimen collected on admission. Throat swab specimens were tested for viral etiologies. In response to the COVID-19 outbreak, the throat samples were retrospectively tested for SARS-CoV-2. If two independent experiments and a clinically verified diagnostic test confirmed the SARS-CoV-2, the cases were confirmed as COVID-19 cases. During the 8-day period, 366 hospitalized pediatric patients were included in the study. Of the 366 patients, 6 tested positive for SARS-CoV-2, while 23 tested positive for influenza A and 20 tested positive for influenza B. The median age of the six patients was 3 years (range, 1-7 years), and all were previously healthy. All six pediatric patients with COVID-19 had high fevers (greater than 39°C), cough, and lymphopenia. Four of the six affected patients had vomiting and leukopenia, while three of the six patients had neutropenia. Four of the six affected patients had pneumonia, as diagnosed on CT scans. Of the six patients, one patient was admitted to the ICU and received intravenous immunoglobulin. The patient admitted to ICU underwent a CT scan which showed “patchy ground-glass opacities in both lungs,” while three of the five children requiring non-ICU hospitalization had chest radiographs showing “patchy shadows in both lungs.” The median length of stay in the hospital was 7.5 days (range, 5-13 days).
Bottom line: COVID-19 causes moderate to severe respiratory illness in pediatric patients with SARS-CoV-2, possibly leading to critical illness. During this time period of the Wuhan COVID-19 outbreak, pediatric patients were more likely to be hospitalized with influenza A or B, than they were with SARS-CoV-2.
Citation: Liu W et al. Detection of Covid-19 in Children in Early January 2020 in Wuhan, China. N Engl J Med. 2020 Mar 12. doi: 10.1056/NEJMc2003717.
Dr. Kumar is clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and a pediatric hospitalist at Cleveland Clinic Children’s. She is the pediatric editor of the Hospitalist.
References
1. Zhu N et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-33.
2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24 (Epub ahead of print).
From the Hospitalist editors: The pediatrics “In the Literature” series generally focuses on original articles. However, given the urgency to learn more about SARS-CoV-2/COVID-19 pandemic and the limited literature about hospitalized pediatric patients with the disease, the editors of the Hospitalist thought it was appropriate to share an article reviewing this letter that was recently published in the New England Journal of Medicine.