User login
Seniors face higher risk of other medical conditions after COVID-19
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
The findings of the observational study, which were published in the BMJ, show the risk of a new condition being triggered by COVID is more than twice as high in seniors, compared with younger patients. Plus, the researchers observed an even higher risk among those who were hospitalized, with nearly half (46%) of patients having developed new conditions after the acute COVID-19 infection period.
Respiratory failure with shortness of breath was the most common postacute sequela, but a wide range of heart, kidney, lung, liver, cognitive, mental health, and other conditions were diagnosed at least 3 weeks after initial infection and persisted beyond 30 days.
This is one of the first studies to specifically describe the incidence and severity of new conditions triggered by COVID-19 infection in a general sample of older adults, said study author Ken Cohen MD, FACP, executive director of translational research at Optum Labs and national senior medical director at Optum Care.
“Much of what has been published on the postacute sequelae of COVID-19 has been predominantly from a younger population, and many of the patients had been hospitalized,” Dr. Cohen noted. “This was the first study to focus on a large population of seniors, most of whom did not require hospitalization.”
Dr. Cohen and colleagues reviewed the health insurance records of more than 133,000 Medicare beneficiaries aged 65 or older who were diagnosed with COVID-19 before April 2020. They also matched individuals by age, race, sex, hospitalization status, and other factors to comparison groups without COVID-19 (one from 2020 and one from 2019), and to a group diagnosed with other lower respiratory tract viral infections before the pandemic.
Risk of developing new conditions was higher in hospitalized
After acute COVID-19 infection, 32% of seniors sought medical care for at least one new medical condition in 2020, compared with 21% of uninfected people in the same year.
The most commonly observed conditions included:
- Respiratory failure (7.55% higher risk).
- Fatigue (5.66% higher risk).
- High blood pressure (4.43% higher risk).
- Memory problems (2.63% higher risk).
- Kidney injury (2.59% higher risk).
- Mental health diagnoses (2.5% higher risk).
- Blood-clotting disorders (1.47 % higher risk).
- Heart rhythm disorders (2.9% higher risk).
The risk of developing new conditions was even higher among those 23,486 who were hospitalized in 2020. Those individuals showed a 23.6% higher risk for developing at least one new condition, compared with uninfected seniors in the same year. Also, patients older than 75 had a higher risk for neurological disorders, including dementia, encephalopathy, and memory problems. The researchers also found that respiratory failure and kidney injury were significantly more likely to affect men and Black patients.
When those who had COVID were compared with the group with other lower respiratory viral infections before the pandemic, only the risks of respiratory failure (2.39% higher), dementia (0.71% higher), and fatigue (0.18% higher) were higher.
Primary care providers can learn from these data to better evaluate and manage their geriatric patients with COVID-19 infection, said Amit Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, in an interview.
“We must assess older patients who have had COVID-19 for more than just improvement from the respiratory symptoms of COVID-19 in post-COVID follow-up visits,” he said. “Older individuals with frailty have vulnerability to subsequent complications from severe illnesses and it is common to see post-illness diagnoses, such as new diagnosis of delirium; dementia; or renal, respiratory, or cardiac issues that is precipitated by the original illness. This study confirms that this is likely the case with COVID-19 as well.
“Primary care physicians should be vigilant for these complications, including attention to the rehabilitation needs of older patients with longer-term postviral fatigue from COVID-19,” Dr. Shah added.
Data predates ‘Omicron wave’
It remains uncertain whether sequelae will differ with the Omicron variant, but the findings remain applicable, Dr. Cohen said.
“We know that illness from the Omicron variant is on average less severe in those that have been vaccinated. However, throughout the Omicron wave, individuals who have not been vaccinated continue to have significant rates of serious illness and hospitalization,” he said.
“Our findings showed that serious illness with hospitalization was associated with a higher rate of sequelae. It can therefore be inferred that the rates of sequelae seen in our study would continue to occur in unvaccinated individuals who contract Omicron, but might occur less frequently in vaccinated individuals who contract Omicron and have less severe illness.”
Dr. Cohen serves as a consultant for Pfizer. Dr. Shah has disclosed no relevant financial relationships.
FROM BMJ
Substantial numbers of U.S. youth report vaping cannabis
Adolescents and young adults who use e-cigarettes reported vaping cannabis, according to selected data from the national Population Assessment of Tobacco and Health (PATH) study.
Ruoyan Sun, PhD, an assistant professor at the University of Alabama at Birmingham, and colleagues examined results of PATH’s wave5 survey conducted from December 2018 to November 2019. PATH is a National Institutes of Health–Food and Drug Administration collaboration begun in 2013.
Their analysis, published online Feb. 7, 2022, in JAMA Pediatrics, evaluated the frequency of cannabis vaping across several age groups: 164 respondents ages 12-14; 919 participants ages 15-17; and 3,038 participants ages 18-24. Respondents included for analysis reported electronic nicotine product consumption in the past 30 days. In response to the question “When you have used an electronic product, how often were you using it to smoke marijuana, marijuana concentrates, marijuana waxes, THC, or hash oils?” 35.0% (95% confidence interval, 29.3%-41.2%) of current e-smokers aged 12-14 years said they had done so, as did 51.3% (95% CI, 47.7%-54.9%) of those aged 15-17 years and 54.6% (95% CI, 52.5%-56.7%) of young adults aged 18-24.
The prevalence of those who reported vaping cannabis every time they vaped was 3.1% (95% CI, 1.3%-6.9%) of youths aged 12-14 years, 6.7% (95% CI, 5.3%-8.6%) of youths aged 15-17 years, and 10.3% (95% CI, 9.0%-11.6%) of young adults aged 18-24.
Among children ages 12-14, 65% said they never vaped cannabis, while 48.7% and 45.4%, respectively, in the two older groups said they did.
“This is a very important finding and it mirrors what some of us have already seen in practice,” said pediatric pulmonologist S. Christy Sadreameli, MD, MHS, an assistant professor of pediatrics at John Hopkins University, Baltimore. “It is important for pediatricians to realize that dual use of cannabis and nicotine vaping, and exclusive use of cannabis vaping, are not uncommon. It informs how we ask questions and how we counsel our patients.” Dr. Sadreameli was not involved in the PATH study.
Overall, the survey participants were 56% male, with 24% of respondents identifying as Hispanic, 8% as non-Hispanic Black, 58% as non-Hispanic White, and 10% as of other race/ethnicity. The weighted proportion of current e-cigarette use was 3.0% (95% CI, 2.6%-3.4%) in youths ages 12-14 years, 14.4% (95% CI, 13.5%-15.3%) in those 15-17 years, and 26.2% (95% CI, 25.3%-27.1%) in young adults.
Other recent national surveys such as the National Institute on Drug Abuses’s Monitoring the Future are reporting a growing prevalence of youth cannabis vaping, Dr. Sun said. For example, the prevalence of cannabis vaping in the past 12-month period among grade 12 students grew from 9.5% in 2017 to 22.1% in 2020. Vaping cannabis was more prevalent among Hispanic teens than other ethnicities.
Vaping devices such as e-cigarettes, vaping pens, e-cigars, and e-hookahs can be used to inhale multiple substances, including nicotine, cannabis, and opium, Dr. Sun noted in an interview. “So in addition to asking about the behavior of vaping itself, pediatricians could pay more attention to what is being vaped in these devices.”
According to Dr. Sadreameli, vaping more than one substance at a time could potentially work synergistically to cause more harm, compared with one product alone. “The other aspect to consider is that vaping multiple types of products may increase the chance of harm from other components of the mixture,” she said. For instance, a lot of the e-cigarette or vaping use-associated lung injury (EVALI) cases have been linked to vitamin E acetate, which was found in certain cannabis formulations. “Anecdotally, most EVALI patients I’ve met seemed to report use of multiple products, including cannabis-containing and nicotine-containing products.”
Dr. Sadreameli added that some cannabis vapers will have other issues. “For example, there is a severe vomiting syndrome I’ve seen, which is induced by cannabis and improved by cessation from cannabis,” she said. “It is important for pediatricians to ask the right questions of their patients in order to better understand what they may be experiencing, provide counseling, and to help them.”
A related issue is cessation, she said. “For those working to achieve cessation from nicotine-based products, sometimes nicotine replacement therapies are helpful. However, cessation from cannabis-containing products is going to look different.”
Although the study did not yield information on the prevalence simultaneous nicotine/cannabis vaping, the authors suggested that some vapers may be combining substances. Previous studies may have modestly overestimated the prevalence of nicotine vaping given their finding that some current e-cigarette users reported vaping cannabis every time they vaped and may be vaping cannabis exclusively. “However, if some current users vaped nicotine and cannabis simultaneously, then overestimation of nicotine vaping would be smaller,” they wrote.
Future surveys on this area should contain detailed questions on nicotine and cannabis vaping, including the substance being vaped and the frequency and intensity of use, Dr. Sun said. “In addition, these surveys could examine some other substances that are being vaped, such as opium and cocaine.”
The PATH study is supported by the NIH, National Institute on Drug Abuse, Department of Health & Human Services, and the FDA’s Center for Tobacco Products. The authors and Dr. Sadreameli had no competing interests to disclose.
Adolescents and young adults who use e-cigarettes reported vaping cannabis, according to selected data from the national Population Assessment of Tobacco and Health (PATH) study.
Ruoyan Sun, PhD, an assistant professor at the University of Alabama at Birmingham, and colleagues examined results of PATH’s wave5 survey conducted from December 2018 to November 2019. PATH is a National Institutes of Health–Food and Drug Administration collaboration begun in 2013.
Their analysis, published online Feb. 7, 2022, in JAMA Pediatrics, evaluated the frequency of cannabis vaping across several age groups: 164 respondents ages 12-14; 919 participants ages 15-17; and 3,038 participants ages 18-24. Respondents included for analysis reported electronic nicotine product consumption in the past 30 days. In response to the question “When you have used an electronic product, how often were you using it to smoke marijuana, marijuana concentrates, marijuana waxes, THC, or hash oils?” 35.0% (95% confidence interval, 29.3%-41.2%) of current e-smokers aged 12-14 years said they had done so, as did 51.3% (95% CI, 47.7%-54.9%) of those aged 15-17 years and 54.6% (95% CI, 52.5%-56.7%) of young adults aged 18-24.
The prevalence of those who reported vaping cannabis every time they vaped was 3.1% (95% CI, 1.3%-6.9%) of youths aged 12-14 years, 6.7% (95% CI, 5.3%-8.6%) of youths aged 15-17 years, and 10.3% (95% CI, 9.0%-11.6%) of young adults aged 18-24.
Among children ages 12-14, 65% said they never vaped cannabis, while 48.7% and 45.4%, respectively, in the two older groups said they did.
“This is a very important finding and it mirrors what some of us have already seen in practice,” said pediatric pulmonologist S. Christy Sadreameli, MD, MHS, an assistant professor of pediatrics at John Hopkins University, Baltimore. “It is important for pediatricians to realize that dual use of cannabis and nicotine vaping, and exclusive use of cannabis vaping, are not uncommon. It informs how we ask questions and how we counsel our patients.” Dr. Sadreameli was not involved in the PATH study.
Overall, the survey participants were 56% male, with 24% of respondents identifying as Hispanic, 8% as non-Hispanic Black, 58% as non-Hispanic White, and 10% as of other race/ethnicity. The weighted proportion of current e-cigarette use was 3.0% (95% CI, 2.6%-3.4%) in youths ages 12-14 years, 14.4% (95% CI, 13.5%-15.3%) in those 15-17 years, and 26.2% (95% CI, 25.3%-27.1%) in young adults.
Other recent national surveys such as the National Institute on Drug Abuses’s Monitoring the Future are reporting a growing prevalence of youth cannabis vaping, Dr. Sun said. For example, the prevalence of cannabis vaping in the past 12-month period among grade 12 students grew from 9.5% in 2017 to 22.1% in 2020. Vaping cannabis was more prevalent among Hispanic teens than other ethnicities.
Vaping devices such as e-cigarettes, vaping pens, e-cigars, and e-hookahs can be used to inhale multiple substances, including nicotine, cannabis, and opium, Dr. Sun noted in an interview. “So in addition to asking about the behavior of vaping itself, pediatricians could pay more attention to what is being vaped in these devices.”
According to Dr. Sadreameli, vaping more than one substance at a time could potentially work synergistically to cause more harm, compared with one product alone. “The other aspect to consider is that vaping multiple types of products may increase the chance of harm from other components of the mixture,” she said. For instance, a lot of the e-cigarette or vaping use-associated lung injury (EVALI) cases have been linked to vitamin E acetate, which was found in certain cannabis formulations. “Anecdotally, most EVALI patients I’ve met seemed to report use of multiple products, including cannabis-containing and nicotine-containing products.”
Dr. Sadreameli added that some cannabis vapers will have other issues. “For example, there is a severe vomiting syndrome I’ve seen, which is induced by cannabis and improved by cessation from cannabis,” she said. “It is important for pediatricians to ask the right questions of their patients in order to better understand what they may be experiencing, provide counseling, and to help them.”
A related issue is cessation, she said. “For those working to achieve cessation from nicotine-based products, sometimes nicotine replacement therapies are helpful. However, cessation from cannabis-containing products is going to look different.”
Although the study did not yield information on the prevalence simultaneous nicotine/cannabis vaping, the authors suggested that some vapers may be combining substances. Previous studies may have modestly overestimated the prevalence of nicotine vaping given their finding that some current e-cigarette users reported vaping cannabis every time they vaped and may be vaping cannabis exclusively. “However, if some current users vaped nicotine and cannabis simultaneously, then overestimation of nicotine vaping would be smaller,” they wrote.
Future surveys on this area should contain detailed questions on nicotine and cannabis vaping, including the substance being vaped and the frequency and intensity of use, Dr. Sun said. “In addition, these surveys could examine some other substances that are being vaped, such as opium and cocaine.”
The PATH study is supported by the NIH, National Institute on Drug Abuse, Department of Health & Human Services, and the FDA’s Center for Tobacco Products. The authors and Dr. Sadreameli had no competing interests to disclose.
Adolescents and young adults who use e-cigarettes reported vaping cannabis, according to selected data from the national Population Assessment of Tobacco and Health (PATH) study.
Ruoyan Sun, PhD, an assistant professor at the University of Alabama at Birmingham, and colleagues examined results of PATH’s wave5 survey conducted from December 2018 to November 2019. PATH is a National Institutes of Health–Food and Drug Administration collaboration begun in 2013.
Their analysis, published online Feb. 7, 2022, in JAMA Pediatrics, evaluated the frequency of cannabis vaping across several age groups: 164 respondents ages 12-14; 919 participants ages 15-17; and 3,038 participants ages 18-24. Respondents included for analysis reported electronic nicotine product consumption in the past 30 days. In response to the question “When you have used an electronic product, how often were you using it to smoke marijuana, marijuana concentrates, marijuana waxes, THC, or hash oils?” 35.0% (95% confidence interval, 29.3%-41.2%) of current e-smokers aged 12-14 years said they had done so, as did 51.3% (95% CI, 47.7%-54.9%) of those aged 15-17 years and 54.6% (95% CI, 52.5%-56.7%) of young adults aged 18-24.
The prevalence of those who reported vaping cannabis every time they vaped was 3.1% (95% CI, 1.3%-6.9%) of youths aged 12-14 years, 6.7% (95% CI, 5.3%-8.6%) of youths aged 15-17 years, and 10.3% (95% CI, 9.0%-11.6%) of young adults aged 18-24.
Among children ages 12-14, 65% said they never vaped cannabis, while 48.7% and 45.4%, respectively, in the two older groups said they did.
“This is a very important finding and it mirrors what some of us have already seen in practice,” said pediatric pulmonologist S. Christy Sadreameli, MD, MHS, an assistant professor of pediatrics at John Hopkins University, Baltimore. “It is important for pediatricians to realize that dual use of cannabis and nicotine vaping, and exclusive use of cannabis vaping, are not uncommon. It informs how we ask questions and how we counsel our patients.” Dr. Sadreameli was not involved in the PATH study.
Overall, the survey participants were 56% male, with 24% of respondents identifying as Hispanic, 8% as non-Hispanic Black, 58% as non-Hispanic White, and 10% as of other race/ethnicity. The weighted proportion of current e-cigarette use was 3.0% (95% CI, 2.6%-3.4%) in youths ages 12-14 years, 14.4% (95% CI, 13.5%-15.3%) in those 15-17 years, and 26.2% (95% CI, 25.3%-27.1%) in young adults.
Other recent national surveys such as the National Institute on Drug Abuses’s Monitoring the Future are reporting a growing prevalence of youth cannabis vaping, Dr. Sun said. For example, the prevalence of cannabis vaping in the past 12-month period among grade 12 students grew from 9.5% in 2017 to 22.1% in 2020. Vaping cannabis was more prevalent among Hispanic teens than other ethnicities.
Vaping devices such as e-cigarettes, vaping pens, e-cigars, and e-hookahs can be used to inhale multiple substances, including nicotine, cannabis, and opium, Dr. Sun noted in an interview. “So in addition to asking about the behavior of vaping itself, pediatricians could pay more attention to what is being vaped in these devices.”
According to Dr. Sadreameli, vaping more than one substance at a time could potentially work synergistically to cause more harm, compared with one product alone. “The other aspect to consider is that vaping multiple types of products may increase the chance of harm from other components of the mixture,” she said. For instance, a lot of the e-cigarette or vaping use-associated lung injury (EVALI) cases have been linked to vitamin E acetate, which was found in certain cannabis formulations. “Anecdotally, most EVALI patients I’ve met seemed to report use of multiple products, including cannabis-containing and nicotine-containing products.”
Dr. Sadreameli added that some cannabis vapers will have other issues. “For example, there is a severe vomiting syndrome I’ve seen, which is induced by cannabis and improved by cessation from cannabis,” she said. “It is important for pediatricians to ask the right questions of their patients in order to better understand what they may be experiencing, provide counseling, and to help them.”
A related issue is cessation, she said. “For those working to achieve cessation from nicotine-based products, sometimes nicotine replacement therapies are helpful. However, cessation from cannabis-containing products is going to look different.”
Although the study did not yield information on the prevalence simultaneous nicotine/cannabis vaping, the authors suggested that some vapers may be combining substances. Previous studies may have modestly overestimated the prevalence of nicotine vaping given their finding that some current e-cigarette users reported vaping cannabis every time they vaped and may be vaping cannabis exclusively. “However, if some current users vaped nicotine and cannabis simultaneously, then overestimation of nicotine vaping would be smaller,” they wrote.
Future surveys on this area should contain detailed questions on nicotine and cannabis vaping, including the substance being vaped and the frequency and intensity of use, Dr. Sun said. “In addition, these surveys could examine some other substances that are being vaped, such as opium and cocaine.”
The PATH study is supported by the NIH, National Institute on Drug Abuse, Department of Health & Human Services, and the FDA’s Center for Tobacco Products. The authors and Dr. Sadreameli had no competing interests to disclose.
FROM JAMA PEDIATRICS
Cystic fibrosis in retreat, but still unbeaten
In 1938, the year that cystic fibrosis (CF) was first described clinically, four of five children born with the disease did not live past their first birthdays.
In 2019, the median age at death for patients enrolled in the Cystic Fibrosis Foundation (CFF) registry was 32 years, and the predicted life expectancy for patients with CF who were born from 2015 through 2019 was 46 years.
Those numbers reflect the remarkable progress made in the past 4 decades in the care of patients with CF, but also highlight the obstacles ahead, given that the predicted life expectancy for the overall U.S. population in 2019 (pre–COVID-19) was 78.9 years.
Julie Desch, MD, is a CF survivor who has beaten the odds and then some. At age 61, the retired surgical pathologist is a CF patient advocate, speaker, and a board member of the Cystic Fibrosis Research Institute, a not-for-profit organization that funds CF research and offers education, advocacy, and psychosocial support for persons with CF and their families and caregivers.
In an interview, Dr. Desch said that while there has been remarkable progress in her lifetime in the field of CF research and treatment, particularly in the development of drugs that modulate function of the underlying cause of approximately 90% of CF cases, there are still many CF patients who cannot benefit from these therapies.
“There are still 10% of people who don’t make a protein to be modified, so that’s a huge unmet need,” she said.
Genetic disorder
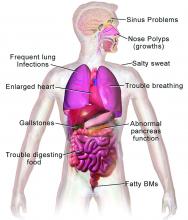
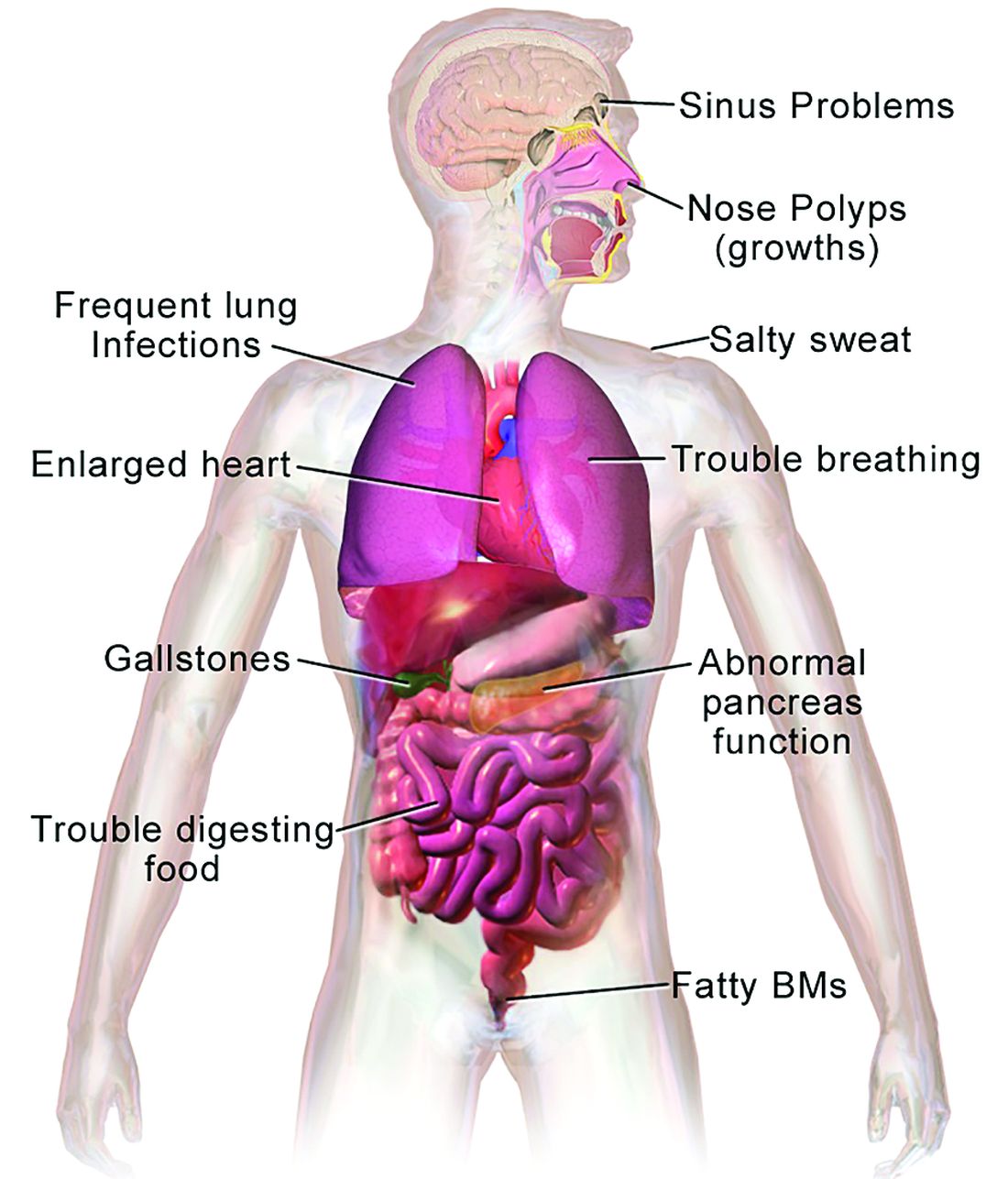
CF is a chronic autosomal recessive disorder with multiorgan and multisystem manifestations. It is caused by mutations in the CFTR gene, which codes for the protein CF transmembrane conductance regulator. CFTR controls transport of chloride ions across cell membranes, specifically the apical membrane of epithelial cells in tissues of the airways, intestinal tract, pancreas, kidneys, sweat glands, and the reproductive system, notably the vas deferens in males.
The F508 deletion (F508del) mutation is the most common, occurring in approximately 70% of persons with CF. It is a class 2-type protein processing mutation, leading to defects in cellular processing, protein stability, and chloride channel gating defects.
The CFTR protein also secretes bicarbonate to regulate the pH of airway surface liquid, and inhibits the epithelial sodium channel, which mediates passive sodium transport across apical membranes of sodium-absorbing epithelial cells in the kidneys, intestine, and airways.
CF typically presents with the buildup in the lungs of abnormally viscous and sticky mucus leading to frequent, severe infections, particularly with Pseudomonas aeruginosa, progressive lung damage and, prior to the development of effective disease management, to premature death. The phenotype often includes malnutrition due to malabsorption, and failure to thrive.
Diagnosis
In all 50 U.S. states and the District of Columbia, newborns are screened for CF with an assay for immunoreactive trypsinogen (IRT) an indirect marker for pancreatic injury that is elevated in serum in most newborns with CF, but also detected in premature infants or those delivered under stressful circumstances. In some states newborns are tested only for IRT, with a diagnosis confirmed with a sweat chloride test and/or a CFTR mutation panel.
Treatment
There is no cure for CF, but the discovery of the gene in 1989 by Canadian and U.S. investigators has led to life-prolonging therapeutic interventions, specifically the development of CFTR modulators.
CFTR modulators include potentiators such as ivacaftor (Kalydeco), and correctors such as lumacaftor and tezacaftor (available in the combination Orkambi), and most recently in the triple combination of elexacaftor, tezacaftor, and ivacaftor (Trikafta; ETI).
Neil Sweezey, MD, FRCPC, a CF expert at The Hospital for Sick Children (SickKids) in Toronto, told this news organization that the ideal therapy for CF, genetic correction of the underlying mutations, is still not feasible, but that CFTR modulators are a close second.
“For 90% of patients, the three-drug combination Trikafta has been shown to be quite safe, quite tolerable, and quite remarkably beneficial,” he said.
In a study reported at CHEST 2021 by investigators from Nationwide Children’s Hospital in Columbus, Ohio, 32 adults who were started on the triple combination had significantly improved in forced expiratory volume in 1 second (FEV1), gain in body mass index, decreased sweat chloride and decreased colonization by Pseudomonas species. In addition, patients had significant improvements in blood inflammatory markers.
Christopher H. Goss, MD, FCCP, professor of pulmonary critical care and sleep medicine and professor of pediatrics at the University of Washington in Seattle, agreed that with the availability of the triple combination, “these are extraordinary times. An astounding fact is that most patients have complete resolution of cough, and the exacerbation rates have just plummeted,” he said in an interview.
Some of the reductions in exacerbations may be attributable to the COVID-19 pandemic, he noted, because patients in isolation have less exposure to circulating respiratory viruses.
“But it has been miraculous, and the clinical effect is certainly still more astounding than the effects of ivacaftor, which was the first truly breakthrough drug. Weight goes up, well-being increases, and the population lung function has shifted up to better grade lung function, in the entire population,” he said.
In addition, the need for lung and heart transplantation has sharply declined.
“I had a patient who had decided to forgo transplantation, despite absolutely horrible lung function, and he’s now bowling and leading a very productive life, when before he had been preparing for end of life,” Dr. Goss said.
Dr. Sweezey emphasized that as with all medications, patients being started on the triple combination require close monitoring for potential adverse events that might require dose modification or, for a small number of patients, withdrawal.
Burden of care
CFTR modulators have reduced but not eliminated the need for some patients to have mucolytic therapy, which may include dornase alfa, a recombinant human deoxyribonuclease (DNase) that reduces the viscosity of lung secretions, hypertonic saline inhaled twice daily (for patients 12 and older), mannitol, and physical manipulations to help patients clear mucus. This can include both manual percussion and the use of devices for high-frequency chest wall oscillation.
The complex nature of CF often requires a combination of other therapies to address comorbidities. These therapies may include infection prophylaxis and treatment with antibiotics and antifungals, nutrition support, and therapy for CF-related complications, including gastrointestinal issues, liver diseases, diabetes, and osteopenia that may be related to poor nutrient absorption, chronic inflammation, or other sequelae of CF.
In addition, patients often require frequent CF care center visits – ideally a minimum of every 3 months – which can result in significant loss of work or school time.
“Outcomes for patients in the long run have been absolutely proven to be best if they’re followed in big, established, multidisciplinary well-organized CF centers,” Dr. Sweezey said. “In the United States and Canada if you’re looked after on a regular basis, which means quarterly, every 3 months – whether you need it or not, you really do need it – and if the patients are seen and assessed and checked every 3 months all of their lives, they have small changes caught early, whether it’s an infection you can slap down with medication or a nutrition problem that may be affecting a child’s growth and development.”
“We’re really kind of at a pivotal moment in CF, where we realize things are changing,” said A. Whitney Brown, MD, senior director for clinical affairs at the Cystic Fibrosis Foundation, and an adult CF and lung transplant physician in the Inova Advanced Lung Disease Program in Falls Church, Va.
“Patient needs and interest have evolved, because of the pandemic and because of the highly effective modulator therapy, but we want to take great effort to study it in a rigorous way, to make sure that as we are agile and adapt the care model, that we can maintain the same quality outcomes that we have traditionally done,” she said in an interview.
The Lancet Respiratory Medicine Commission on the future of CF care states that models of care “need to consider management approaches (including disease monitoring) to maintain health and delay lung transplantation, while minimizing the burden of care for patients and their families.”
‘A great problem to have’
One of the most significant changes in CF care has been the growing population of CF patients like Dr. Desch who are living well into adulthood, with some approaching Medicare eligibility.
With the advent of triple therapy and CFTR modulators being started earlier in life, lung function can be preserved, damage to other organs can be minimized, and life expectancy for patients with CF will continue to improve.
“We’re anticipating that there may be some needs in the aging CF population that are different than what we have historically had,” Dr. Brown said. “Will there be geriatric providers that need to become experts in CF care? That’s a great problem to have,” she said.
Dr. Goss agreed, noting that CF is steadily shifting from a near uniformly fatal disease to a chronic disorder that in many cases can be managed “with a complex regimen of novel drugs, much like HIV.”
He noted that there are multiple drug interactions with the triple combination, “so it’s really important that people don’t start a CF patient on a drug without consulting a pharmacist, because you can totally inactivate ETI, or augment it dramatically, and we’ve seen both happen.”
Cost and access
All experts interviewed for this article agreed that while the care of patients with CF has improved exponentially over the last few decades, there are still troubling inequities in care.
One of the largest impediments is the cost of care, with the triple combination costing more than $300,000 per year.
“Clearly patients aren’t paying that, but insurance companies are, and that’s causing all kinds of trickle-down effects that definitely affect patients. The patients like myself who are able to have insurance that covers it benefit, but there are so many people that don’t,” Dr. Desch said.
Dr. Sweezey noted that prior to the advent of ETI, patients with CF in Canada had better outcomes and longer life expectancy than did similar patients in the United States because of universal access to care and coordinated services under Canada’s health care system, compared with the highly fragmented and inefficient U.S. system. He added that the wider availability of ETI in the United States vs. Canada may begin to narrow that gap, however.
As noted before, there is a substantial proportion of patients – an estimated 10% – who have CFTR mutations that are not correctable by currently available CFTR modulators, and these patients are at significant risk for irreversible airway complications and lung damage.
In addition, although CF occurs most frequently among people of White ancestry, the disease does not respect distinctions of race or ethnicity.
“It’s not just [Whites] – a lot of people from different racial backgrounds, ethnic backgrounds, are not being diagnosed or are not being diagnosed soon enough to have effective care early enough,” Dr. Desch said.
That statement is supported by the Lancet Respiratory Medicine Commission on the future of cystic fibrosis care, whose members noted in 2019 that “epidemiological studies in the past 2 decades have shown that cystic fibrosis occurs and is more frequent than was previously thought in populations of non-European descent, and the disease is now recognized in many regions of the world.”
The commission members noted that the costs of adequate CF care may be beyond the reach of many patients in developing nations.
Still, if the substantial barriers of cost and access can be overcome, the future will continue to look brighter for patients with CF. As Dr. Sweezey put it: “There are studies that are pushing lower age limits for using these modulators, and as the evidence builds for the efficacy and safety at younger ages, I think all of us are hoping that we’ll end up being able to use either the current or future modulators to actually prevent trouble in CF, rather than trying to come along and fix it after it’s been there.”
Dr. Brown disclosed advisory board activity for Vertex that ended prior to her joining the CF Foundation. Dr. Desch, Dr. Goss, and Dr. Sweezey reported no relevant conflicts of interest.
In 1938, the year that cystic fibrosis (CF) was first described clinically, four of five children born with the disease did not live past their first birthdays.
In 2019, the median age at death for patients enrolled in the Cystic Fibrosis Foundation (CFF) registry was 32 years, and the predicted life expectancy for patients with CF who were born from 2015 through 2019 was 46 years.
Those numbers reflect the remarkable progress made in the past 4 decades in the care of patients with CF, but also highlight the obstacles ahead, given that the predicted life expectancy for the overall U.S. population in 2019 (pre–COVID-19) was 78.9 years.
Julie Desch, MD, is a CF survivor who has beaten the odds and then some. At age 61, the retired surgical pathologist is a CF patient advocate, speaker, and a board member of the Cystic Fibrosis Research Institute, a not-for-profit organization that funds CF research and offers education, advocacy, and psychosocial support for persons with CF and their families and caregivers.
In an interview, Dr. Desch said that while there has been remarkable progress in her lifetime in the field of CF research and treatment, particularly in the development of drugs that modulate function of the underlying cause of approximately 90% of CF cases, there are still many CF patients who cannot benefit from these therapies.
“There are still 10% of people who don’t make a protein to be modified, so that’s a huge unmet need,” she said.
Genetic disorder
CF is a chronic autosomal recessive disorder with multiorgan and multisystem manifestations. It is caused by mutations in the CFTR gene, which codes for the protein CF transmembrane conductance regulator. CFTR controls transport of chloride ions across cell membranes, specifically the apical membrane of epithelial cells in tissues of the airways, intestinal tract, pancreas, kidneys, sweat glands, and the reproductive system, notably the vas deferens in males.
The F508 deletion (F508del) mutation is the most common, occurring in approximately 70% of persons with CF. It is a class 2-type protein processing mutation, leading to defects in cellular processing, protein stability, and chloride channel gating defects.
The CFTR protein also secretes bicarbonate to regulate the pH of airway surface liquid, and inhibits the epithelial sodium channel, which mediates passive sodium transport across apical membranes of sodium-absorbing epithelial cells in the kidneys, intestine, and airways.
CF typically presents with the buildup in the lungs of abnormally viscous and sticky mucus leading to frequent, severe infections, particularly with Pseudomonas aeruginosa, progressive lung damage and, prior to the development of effective disease management, to premature death. The phenotype often includes malnutrition due to malabsorption, and failure to thrive.
Diagnosis
In all 50 U.S. states and the District of Columbia, newborns are screened for CF with an assay for immunoreactive trypsinogen (IRT) an indirect marker for pancreatic injury that is elevated in serum in most newborns with CF, but also detected in premature infants or those delivered under stressful circumstances. In some states newborns are tested only for IRT, with a diagnosis confirmed with a sweat chloride test and/or a CFTR mutation panel.
Treatment
There is no cure for CF, but the discovery of the gene in 1989 by Canadian and U.S. investigators has led to life-prolonging therapeutic interventions, specifically the development of CFTR modulators.
CFTR modulators include potentiators such as ivacaftor (Kalydeco), and correctors such as lumacaftor and tezacaftor (available in the combination Orkambi), and most recently in the triple combination of elexacaftor, tezacaftor, and ivacaftor (Trikafta; ETI).
Neil Sweezey, MD, FRCPC, a CF expert at The Hospital for Sick Children (SickKids) in Toronto, told this news organization that the ideal therapy for CF, genetic correction of the underlying mutations, is still not feasible, but that CFTR modulators are a close second.
“For 90% of patients, the three-drug combination Trikafta has been shown to be quite safe, quite tolerable, and quite remarkably beneficial,” he said.
In a study reported at CHEST 2021 by investigators from Nationwide Children’s Hospital in Columbus, Ohio, 32 adults who were started on the triple combination had significantly improved in forced expiratory volume in 1 second (FEV1), gain in body mass index, decreased sweat chloride and decreased colonization by Pseudomonas species. In addition, patients had significant improvements in blood inflammatory markers.
Christopher H. Goss, MD, FCCP, professor of pulmonary critical care and sleep medicine and professor of pediatrics at the University of Washington in Seattle, agreed that with the availability of the triple combination, “these are extraordinary times. An astounding fact is that most patients have complete resolution of cough, and the exacerbation rates have just plummeted,” he said in an interview.
Some of the reductions in exacerbations may be attributable to the COVID-19 pandemic, he noted, because patients in isolation have less exposure to circulating respiratory viruses.
“But it has been miraculous, and the clinical effect is certainly still more astounding than the effects of ivacaftor, which was the first truly breakthrough drug. Weight goes up, well-being increases, and the population lung function has shifted up to better grade lung function, in the entire population,” he said.
In addition, the need for lung and heart transplantation has sharply declined.
“I had a patient who had decided to forgo transplantation, despite absolutely horrible lung function, and he’s now bowling and leading a very productive life, when before he had been preparing for end of life,” Dr. Goss said.
Dr. Sweezey emphasized that as with all medications, patients being started on the triple combination require close monitoring for potential adverse events that might require dose modification or, for a small number of patients, withdrawal.
Burden of care
CFTR modulators have reduced but not eliminated the need for some patients to have mucolytic therapy, which may include dornase alfa, a recombinant human deoxyribonuclease (DNase) that reduces the viscosity of lung secretions, hypertonic saline inhaled twice daily (for patients 12 and older), mannitol, and physical manipulations to help patients clear mucus. This can include both manual percussion and the use of devices for high-frequency chest wall oscillation.
The complex nature of CF often requires a combination of other therapies to address comorbidities. These therapies may include infection prophylaxis and treatment with antibiotics and antifungals, nutrition support, and therapy for CF-related complications, including gastrointestinal issues, liver diseases, diabetes, and osteopenia that may be related to poor nutrient absorption, chronic inflammation, or other sequelae of CF.
In addition, patients often require frequent CF care center visits – ideally a minimum of every 3 months – which can result in significant loss of work or school time.
“Outcomes for patients in the long run have been absolutely proven to be best if they’re followed in big, established, multidisciplinary well-organized CF centers,” Dr. Sweezey said. “In the United States and Canada if you’re looked after on a regular basis, which means quarterly, every 3 months – whether you need it or not, you really do need it – and if the patients are seen and assessed and checked every 3 months all of their lives, they have small changes caught early, whether it’s an infection you can slap down with medication or a nutrition problem that may be affecting a child’s growth and development.”
“We’re really kind of at a pivotal moment in CF, where we realize things are changing,” said A. Whitney Brown, MD, senior director for clinical affairs at the Cystic Fibrosis Foundation, and an adult CF and lung transplant physician in the Inova Advanced Lung Disease Program in Falls Church, Va.
“Patient needs and interest have evolved, because of the pandemic and because of the highly effective modulator therapy, but we want to take great effort to study it in a rigorous way, to make sure that as we are agile and adapt the care model, that we can maintain the same quality outcomes that we have traditionally done,” she said in an interview.
The Lancet Respiratory Medicine Commission on the future of CF care states that models of care “need to consider management approaches (including disease monitoring) to maintain health and delay lung transplantation, while minimizing the burden of care for patients and their families.”
‘A great problem to have’
One of the most significant changes in CF care has been the growing population of CF patients like Dr. Desch who are living well into adulthood, with some approaching Medicare eligibility.
With the advent of triple therapy and CFTR modulators being started earlier in life, lung function can be preserved, damage to other organs can be minimized, and life expectancy for patients with CF will continue to improve.
“We’re anticipating that there may be some needs in the aging CF population that are different than what we have historically had,” Dr. Brown said. “Will there be geriatric providers that need to become experts in CF care? That’s a great problem to have,” she said.
Dr. Goss agreed, noting that CF is steadily shifting from a near uniformly fatal disease to a chronic disorder that in many cases can be managed “with a complex regimen of novel drugs, much like HIV.”
He noted that there are multiple drug interactions with the triple combination, “so it’s really important that people don’t start a CF patient on a drug without consulting a pharmacist, because you can totally inactivate ETI, or augment it dramatically, and we’ve seen both happen.”
Cost and access
All experts interviewed for this article agreed that while the care of patients with CF has improved exponentially over the last few decades, there are still troubling inequities in care.
One of the largest impediments is the cost of care, with the triple combination costing more than $300,000 per year.
“Clearly patients aren’t paying that, but insurance companies are, and that’s causing all kinds of trickle-down effects that definitely affect patients. The patients like myself who are able to have insurance that covers it benefit, but there are so many people that don’t,” Dr. Desch said.
Dr. Sweezey noted that prior to the advent of ETI, patients with CF in Canada had better outcomes and longer life expectancy than did similar patients in the United States because of universal access to care and coordinated services under Canada’s health care system, compared with the highly fragmented and inefficient U.S. system. He added that the wider availability of ETI in the United States vs. Canada may begin to narrow that gap, however.
As noted before, there is a substantial proportion of patients – an estimated 10% – who have CFTR mutations that are not correctable by currently available CFTR modulators, and these patients are at significant risk for irreversible airway complications and lung damage.
In addition, although CF occurs most frequently among people of White ancestry, the disease does not respect distinctions of race or ethnicity.
“It’s not just [Whites] – a lot of people from different racial backgrounds, ethnic backgrounds, are not being diagnosed or are not being diagnosed soon enough to have effective care early enough,” Dr. Desch said.
That statement is supported by the Lancet Respiratory Medicine Commission on the future of cystic fibrosis care, whose members noted in 2019 that “epidemiological studies in the past 2 decades have shown that cystic fibrosis occurs and is more frequent than was previously thought in populations of non-European descent, and the disease is now recognized in many regions of the world.”
The commission members noted that the costs of adequate CF care may be beyond the reach of many patients in developing nations.
Still, if the substantial barriers of cost and access can be overcome, the future will continue to look brighter for patients with CF. As Dr. Sweezey put it: “There are studies that are pushing lower age limits for using these modulators, and as the evidence builds for the efficacy and safety at younger ages, I think all of us are hoping that we’ll end up being able to use either the current or future modulators to actually prevent trouble in CF, rather than trying to come along and fix it after it’s been there.”
Dr. Brown disclosed advisory board activity for Vertex that ended prior to her joining the CF Foundation. Dr. Desch, Dr. Goss, and Dr. Sweezey reported no relevant conflicts of interest.
In 1938, the year that cystic fibrosis (CF) was first described clinically, four of five children born with the disease did not live past their first birthdays.
In 2019, the median age at death for patients enrolled in the Cystic Fibrosis Foundation (CFF) registry was 32 years, and the predicted life expectancy for patients with CF who were born from 2015 through 2019 was 46 years.
Those numbers reflect the remarkable progress made in the past 4 decades in the care of patients with CF, but also highlight the obstacles ahead, given that the predicted life expectancy for the overall U.S. population in 2019 (pre–COVID-19) was 78.9 years.
Julie Desch, MD, is a CF survivor who has beaten the odds and then some. At age 61, the retired surgical pathologist is a CF patient advocate, speaker, and a board member of the Cystic Fibrosis Research Institute, a not-for-profit organization that funds CF research and offers education, advocacy, and psychosocial support for persons with CF and their families and caregivers.
In an interview, Dr. Desch said that while there has been remarkable progress in her lifetime in the field of CF research and treatment, particularly in the development of drugs that modulate function of the underlying cause of approximately 90% of CF cases, there are still many CF patients who cannot benefit from these therapies.
“There are still 10% of people who don’t make a protein to be modified, so that’s a huge unmet need,” she said.
Genetic disorder
CF is a chronic autosomal recessive disorder with multiorgan and multisystem manifestations. It is caused by mutations in the CFTR gene, which codes for the protein CF transmembrane conductance regulator. CFTR controls transport of chloride ions across cell membranes, specifically the apical membrane of epithelial cells in tissues of the airways, intestinal tract, pancreas, kidneys, sweat glands, and the reproductive system, notably the vas deferens in males.
The F508 deletion (F508del) mutation is the most common, occurring in approximately 70% of persons with CF. It is a class 2-type protein processing mutation, leading to defects in cellular processing, protein stability, and chloride channel gating defects.
The CFTR protein also secretes bicarbonate to regulate the pH of airway surface liquid, and inhibits the epithelial sodium channel, which mediates passive sodium transport across apical membranes of sodium-absorbing epithelial cells in the kidneys, intestine, and airways.
CF typically presents with the buildup in the lungs of abnormally viscous and sticky mucus leading to frequent, severe infections, particularly with Pseudomonas aeruginosa, progressive lung damage and, prior to the development of effective disease management, to premature death. The phenotype often includes malnutrition due to malabsorption, and failure to thrive.
Diagnosis
In all 50 U.S. states and the District of Columbia, newborns are screened for CF with an assay for immunoreactive trypsinogen (IRT) an indirect marker for pancreatic injury that is elevated in serum in most newborns with CF, but also detected in premature infants or those delivered under stressful circumstances. In some states newborns are tested only for IRT, with a diagnosis confirmed with a sweat chloride test and/or a CFTR mutation panel.
Treatment
There is no cure for CF, but the discovery of the gene in 1989 by Canadian and U.S. investigators has led to life-prolonging therapeutic interventions, specifically the development of CFTR modulators.
CFTR modulators include potentiators such as ivacaftor (Kalydeco), and correctors such as lumacaftor and tezacaftor (available in the combination Orkambi), and most recently in the triple combination of elexacaftor, tezacaftor, and ivacaftor (Trikafta; ETI).
Neil Sweezey, MD, FRCPC, a CF expert at The Hospital for Sick Children (SickKids) in Toronto, told this news organization that the ideal therapy for CF, genetic correction of the underlying mutations, is still not feasible, but that CFTR modulators are a close second.
“For 90% of patients, the three-drug combination Trikafta has been shown to be quite safe, quite tolerable, and quite remarkably beneficial,” he said.
In a study reported at CHEST 2021 by investigators from Nationwide Children’s Hospital in Columbus, Ohio, 32 adults who were started on the triple combination had significantly improved in forced expiratory volume in 1 second (FEV1), gain in body mass index, decreased sweat chloride and decreased colonization by Pseudomonas species. In addition, patients had significant improvements in blood inflammatory markers.
Christopher H. Goss, MD, FCCP, professor of pulmonary critical care and sleep medicine and professor of pediatrics at the University of Washington in Seattle, agreed that with the availability of the triple combination, “these are extraordinary times. An astounding fact is that most patients have complete resolution of cough, and the exacerbation rates have just plummeted,” he said in an interview.
Some of the reductions in exacerbations may be attributable to the COVID-19 pandemic, he noted, because patients in isolation have less exposure to circulating respiratory viruses.
“But it has been miraculous, and the clinical effect is certainly still more astounding than the effects of ivacaftor, which was the first truly breakthrough drug. Weight goes up, well-being increases, and the population lung function has shifted up to better grade lung function, in the entire population,” he said.
In addition, the need for lung and heart transplantation has sharply declined.
“I had a patient who had decided to forgo transplantation, despite absolutely horrible lung function, and he’s now bowling and leading a very productive life, when before he had been preparing for end of life,” Dr. Goss said.
Dr. Sweezey emphasized that as with all medications, patients being started on the triple combination require close monitoring for potential adverse events that might require dose modification or, for a small number of patients, withdrawal.
Burden of care
CFTR modulators have reduced but not eliminated the need for some patients to have mucolytic therapy, which may include dornase alfa, a recombinant human deoxyribonuclease (DNase) that reduces the viscosity of lung secretions, hypertonic saline inhaled twice daily (for patients 12 and older), mannitol, and physical manipulations to help patients clear mucus. This can include both manual percussion and the use of devices for high-frequency chest wall oscillation.
The complex nature of CF often requires a combination of other therapies to address comorbidities. These therapies may include infection prophylaxis and treatment with antibiotics and antifungals, nutrition support, and therapy for CF-related complications, including gastrointestinal issues, liver diseases, diabetes, and osteopenia that may be related to poor nutrient absorption, chronic inflammation, or other sequelae of CF.
In addition, patients often require frequent CF care center visits – ideally a minimum of every 3 months – which can result in significant loss of work or school time.
“Outcomes for patients in the long run have been absolutely proven to be best if they’re followed in big, established, multidisciplinary well-organized CF centers,” Dr. Sweezey said. “In the United States and Canada if you’re looked after on a regular basis, which means quarterly, every 3 months – whether you need it or not, you really do need it – and if the patients are seen and assessed and checked every 3 months all of their lives, they have small changes caught early, whether it’s an infection you can slap down with medication or a nutrition problem that may be affecting a child’s growth and development.”
“We’re really kind of at a pivotal moment in CF, where we realize things are changing,” said A. Whitney Brown, MD, senior director for clinical affairs at the Cystic Fibrosis Foundation, and an adult CF and lung transplant physician in the Inova Advanced Lung Disease Program in Falls Church, Va.
“Patient needs and interest have evolved, because of the pandemic and because of the highly effective modulator therapy, but we want to take great effort to study it in a rigorous way, to make sure that as we are agile and adapt the care model, that we can maintain the same quality outcomes that we have traditionally done,” she said in an interview.
The Lancet Respiratory Medicine Commission on the future of CF care states that models of care “need to consider management approaches (including disease monitoring) to maintain health and delay lung transplantation, while minimizing the burden of care for patients and their families.”
‘A great problem to have’
One of the most significant changes in CF care has been the growing population of CF patients like Dr. Desch who are living well into adulthood, with some approaching Medicare eligibility.
With the advent of triple therapy and CFTR modulators being started earlier in life, lung function can be preserved, damage to other organs can be minimized, and life expectancy for patients with CF will continue to improve.
“We’re anticipating that there may be some needs in the aging CF population that are different than what we have historically had,” Dr. Brown said. “Will there be geriatric providers that need to become experts in CF care? That’s a great problem to have,” she said.
Dr. Goss agreed, noting that CF is steadily shifting from a near uniformly fatal disease to a chronic disorder that in many cases can be managed “with a complex regimen of novel drugs, much like HIV.”
He noted that there are multiple drug interactions with the triple combination, “so it’s really important that people don’t start a CF patient on a drug without consulting a pharmacist, because you can totally inactivate ETI, or augment it dramatically, and we’ve seen both happen.”
Cost and access
All experts interviewed for this article agreed that while the care of patients with CF has improved exponentially over the last few decades, there are still troubling inequities in care.
One of the largest impediments is the cost of care, with the triple combination costing more than $300,000 per year.
“Clearly patients aren’t paying that, but insurance companies are, and that’s causing all kinds of trickle-down effects that definitely affect patients. The patients like myself who are able to have insurance that covers it benefit, but there are so many people that don’t,” Dr. Desch said.
Dr. Sweezey noted that prior to the advent of ETI, patients with CF in Canada had better outcomes and longer life expectancy than did similar patients in the United States because of universal access to care and coordinated services under Canada’s health care system, compared with the highly fragmented and inefficient U.S. system. He added that the wider availability of ETI in the United States vs. Canada may begin to narrow that gap, however.
As noted before, there is a substantial proportion of patients – an estimated 10% – who have CFTR mutations that are not correctable by currently available CFTR modulators, and these patients are at significant risk for irreversible airway complications and lung damage.
In addition, although CF occurs most frequently among people of White ancestry, the disease does not respect distinctions of race or ethnicity.
“It’s not just [Whites] – a lot of people from different racial backgrounds, ethnic backgrounds, are not being diagnosed or are not being diagnosed soon enough to have effective care early enough,” Dr. Desch said.
That statement is supported by the Lancet Respiratory Medicine Commission on the future of cystic fibrosis care, whose members noted in 2019 that “epidemiological studies in the past 2 decades have shown that cystic fibrosis occurs and is more frequent than was previously thought in populations of non-European descent, and the disease is now recognized in many regions of the world.”
The commission members noted that the costs of adequate CF care may be beyond the reach of many patients in developing nations.
Still, if the substantial barriers of cost and access can be overcome, the future will continue to look brighter for patients with CF. As Dr. Sweezey put it: “There are studies that are pushing lower age limits for using these modulators, and as the evidence builds for the efficacy and safety at younger ages, I think all of us are hoping that we’ll end up being able to use either the current or future modulators to actually prevent trouble in CF, rather than trying to come along and fix it after it’s been there.”
Dr. Brown disclosed advisory board activity for Vertex that ended prior to her joining the CF Foundation. Dr. Desch, Dr. Goss, and Dr. Sweezey reported no relevant conflicts of interest.
PAH care turns corner with new therapies, intensified monitoring
Aggressive up-front combination therapy, more lofty treatment goals, and earlier and more frequent reassessments to guide treatment are improving care of patients with pulmonary arterial hypertension (PAH) while at the same time making it more complex.
A larger number of oral and generic treatment options have in some respects ushered in more management ease. But overall, “I don’t know if management of these patients has ever been more complicated, given the treatment options and strategies,” said Murali M. Chakinala, MD, professor of medicine at Washington University, St. Louis. “We’re always thinking through approaches.”
Diagnosis continues to be challenging given the rarity of PAH and its nonspecific presentation – and in some cases it’s now harder. Experts such as Dr. Chakinala are seeing increasing number of aging patients with left heart disease, chronic kidney disease, and other comorbidities who have significant precapillary pulmonary hypertension and who exhibit hemodynamics consistent with PAH, or group 1 PH.
The question experts face is, do such patients have “true PAH,” as do a reported 25-50 people per million, or do they have another type of PH in the classification schema – or a mixture?
Deciding which patients “really fit into group 1 and should be managed like group 1,” Dr. Chakinala said, requires clinical acumen and has important implications, as patients with PAH are the main beneficiaries of vasodilator therapy. Most other patients with PH will not respond to or tolerate such treatment.
“These older patients may be getting PAH through different mechanisms than our younger patients, but because we define PAH through hemodynamic criteria and by ruling out other obvious explanations, they all get lumped together,” said Dr. Chakinala. “We need to parse these patients out better in the future, much like our oncology colleagues are doing.”
Personalized medicine hopefully is the next horizon for this condition, characterized by severe remodeling of the distal pulmonary arteries. Researchers are pushing to achieve deep phenotyping, identify biomarkers and improve risk assessment tools.
And with 80 or so centers now accredited by the Pulmonary Hypertension Association as Pulmonary Hypertension Care Centers, referred patients are accessing clinical trials of new nonvasodilatory drugs. Currently available therapies improve hemodynamics and symptoms, and can slow disease progression, but are not truly disease modifying, sources say.
“The endothelin, nitric oxide, and prostacyclin pathways have been exhaustively studied and we now have great drugs for those pathways,” said Dr. Chakinala, who leads the PHA’s scientific leadership council. But “we’re not going to put a greater dent into this disease until we have new drugs that work on different biologic pathways.”
Diagnostic challenges
The diagnosis of PAH – a remarkably heterogeneous condition that encompasses heritable forms and idiopathic forms, and that comprises a broad mix of predisposing conditions and exposures, from scleroderma to methamphetamine use – is still too often missed or delayed. Delayed diagnoses and misdiagnoses of PAH and other types of PH have been reported in up to 85% of at-risk patients, according to a 2016 literature review.
Being able to pivot from thinking about common pulmonary ailments or heart failure to considering PAH is a key part of earlier diagnosis and better treatment outcomes. “If someone has unexplained dyspnea or if they’re treated for other lung diseases and are not improving, think about a screening echocardiogram,” said Timothy L. Williamson, MD, vice president of quality and safety and a pulmonary and critical care physician at the University of Kansas Health Center, Kansas City.
One of the most common reasons Dr. Chakinala sees for missed diagnoses are right heart catheterizations that are incomplete or misinterpreted. (Right heart catheterizations are required to confirm the diagnosis.) “One can’t simply measure pressures and stop,” he said. “We need the full hemodynamic profile to know that it’s truly precapillary PAH ... and we need proper interpretation of [elements like] the waveforms.”
The 2019 World Symposium on Pulmonary Hypertension shifted the definition of PH from an arbitrarily defined mean pulmonary arterial pressure of at least 25 mm Hg at rest (as measured by right heart catheterization) to a more scientifically determined mPAP of at least 20 mm Hg.
The classification document also requires pulmonary vascular resistance (PVR) of at least 3 Wood units in the definition of all forms of precapillary PH. PAH specifically is defined as the presence of mPAP of at least 20 mm Hg, PVR of at least 3 Wood units, and pulmonary arterial wedge pressure 15 mm Hg or less.
Trends in treatment
The value of initial combination therapy with an endothelin receptor antagonist (ERA) and a phosphodiesterase-5 (PDE5) inhibitor in treatment-naive PAH was cemented in 2015 by the AMBITION trial. The primary endpoint (death, PAH hospitalization, or unsatisfactory clinical response) occurred in 18%, 34%, and 28% of patients who were randomized, respectively, to combination therapy, monotherapy with the ERA ambrisentan, or monotherapy with the PDE-5 inhibitor tadalafil – and in 31% of the two monotherapy groups combined.
The trial reported a 50% reduction in the primary endpoint in the combination-therapy group versus the pooled monotherapy group, as well as greater reductions in N-terminal of the prohormone brain natriuretic peptide levels, more satisfactory clinical response and greater improvement in 6-minute walking distance.
In practice, a minority of patients – typically older patients with multiple comorbidities – still receive initial monotherapy with sequential add-on therapies based on tolerance, but “for the most part PAH patients will start on combination therapy, most commonly with a ERA and PDE5 inhibitor,” Dr. Chakinala said.
For patients who are not improving on the ERA-PDE5 inhibitor approach – typically those who remain in the intermediate-risk category for intermediate-term mortality – substitution of the PDE5 inhibitor with the soluble guanylate cyclase stimulator riociguat may be considered, he and Dr. Williamson said. Clinical improvement with this substitution was demonstrated in the REPLACE trial.
Experts at PH care centers are also utilizing triple therapy for patients who do not improve to low-risk status after 2-4 months of dual combination therapy. The availability of oral prostacyclin analogues (selexipag and treprostinil) makes it easier to consider adding these agents early on, Dr. Chakinala and Dr. Richardson said.
Patients who fall into the high-risk category, at any point, are still best managed with parenteral prostacyclin analogues, Dr. Chakinala said.
In general, said Dr. Williamson, who also directs the University of Kansas Pulmonary Hypertension Comprehensive Care Center, “the PH community tends to be fairly aggressive up front, and with a low threshold for using prostacyclin analogues.”
The agents are “always part of the picture for someone who is really ill, in functional class IV, or has really impaired right ventricular function,” he said. “And we’re finding increased roles in patients who are not as ill but still have decompensated right ventricular dysfunction. It’s something we now consider.”
Recently published research on up-front oral triple therapy suggests possible benefit for some patients – but it’s far from conclusive, said Dr. Chakinala. The TRITON study randomized treatment-naive patients to the traditional ERA-PDE5 combination and either oral selexipag (a selective prostacyclin receptor agonist) or placebo as a third agent. It found no significant difference in reduction in PVR, the primary outcome, at week 26. However, the authors reported a “possible signal” for improved long-term outcomes with triple therapy.
“Based on this best evidence from a randomized clinical trial, I think it’s unfair to say that all patients should be on triple combination therapy right out of the gate,” he said. “Having said that, more recent [European] data showed that two drugs fell short of the mark in some patients, with high rates of clinical progression. And even in AMBITION, there were a number of patients in the combination arm who didn’t have a robust response.”
A 2021 retrospective analysis from the French Pulmonary Hypertension Registry – one of the European studies – assessed survival with monotherapy, dual therapy, or triple-combination therapy (two orals with a parenteral prostacyclin), and found no difference between monotherapy and dual therapy in high-risk patients.
Experts have been upping the ante, therefore, on early assessment and frequent reassessment of treatment response. Not long ago, patients were typically reassessed 6-12 months after the initiation of treatment. Now, experts at the PH care centers want to assess patients at 3-4 months and adjust or intensify treatment regimens for those who don’t yet qualify as low risk using a multidimensional risk score calculator.
The REVEAL (Registry to Evaluate Early and Long-Term PAH Management) risk score calculator, for instance, predicts the probability of 1-year survival and assigns patients to a strata of risk level based on either 12 or 6 variables (for the full or “lite” versions).]
Even better monitoring and risk assessment is needed, however, to “help sift out which patients are not improving enough on initial therapy or who are starting to fall off after being on a regimen for a period of time,” Dr. Chakinala said.
Today, with a network of accredited centers of expertise and a desire and need for many patients to remain close to home, Dr. Chakinala encourages finding a balance. Well-resourced clinicians can strive for early diagnosis and management – potentially initiating ERA–PDE-5 inhibitor combination therapy – but still should collaborate with PH experts.
“It’s a good idea to comanage these patients and let the experts see them periodically to help you determine when your patient may be declining,” he said. “The timetable for reassessment, the complexity of the reassessment, and the need to escalate to more advanced therapies has never been more important.”
Research highlights
Therapies that target inflammation and altered metabolism – including metformin – are among those being investigated for PAH. So are therapies targeting dysfunctional bone morphogenetic protein pathway signaling, which has been shown to be associated with hereditary, idiopathic, and likely other forms of PAH; one such drug, called sotatercept, is currently at the phase 3 trial stage.
Most promising for PAH may be the research efforts involving deep phenotyping, said Andrew J. Sweatt, MD, of Stanford (Calif.) University and the Vera Moulton Wall Center for Pulmonary Vascular Disease.
“It’s where a lot of research is headed – deep phenotyping to deconstruct the molecular and clinical heterogeneity that exists within PAH ... to detect distinct subphenotypes of patients who would respond to particular therapies,” said Dr. Sweatt, who led a review of PH clinical research presented at the 2020 American Thoracic Society International Conference
“Right now, we largely treat all patients the same ... [while] we know that patients have a wide response to therapies and there’s a lot of clinical heterogeneity in how their disease evolves over time,” he said.
Data from a large National Institutes of Health–funded multicenter phenotyping study of PH is being analyzed and should yield findings and publications starting this year, said Anna R. Hemnes, MD, associate professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn., and an investigator with the initiative, coined “Redefining Pulmonary Hypertension through Pulmonary Disease Phenomics (PVDOMICS).”
Patients have undergone advanced imaging (for example, echocardiography, cardiac MRI, chest CT, ventilation/perfusion scans), advanced testing through right heart catheterization, body composition testing, quality of life questionnaires, and blood draws that have been analyzed for DNA and RNA expression, proteomics, and metabolomics, said Dr. Hemnes, assistant director of Vanderbilt’s Pulmonary Vascular Center.
The initiative aims to refine the classification of all kinds of PH and “to bring precision medicine to the field so we’re no longer characterizing somebody [based on imaging] and right heart catheterization, but we also incorporating molecular pieces and biomarkers into the diagnostic evaluation,” she said.
In the short term, the results of deep phenotyping should “allow us to be more effective with our therapy recommendations,” Dr. Hemnes said. “Then hopefully in the longer term, [identified biomarkers] will help us to develop new, more effective therapies.”
Dr. Sweatt and Dr. Williamson reported that they have no relevant financial disclosures. Dr. Hemnes reported that she holds stock in Tenax (which is studying a tyrosine kinase inhibitor for PAH) and serves as a consultant for Acceleron, Bayer, GossamerBio, United Therapeutics, and Janssen. She also receives research funding from Imara. Dr. Chakinala reported that he is an investigator on clinical trials for a number of pharmaceutical companies. He also serves on advisory boards for Phase Bio, Liquidia/Rare Gen, Bayer, Janssen, Trio Health Analytics, and Aerovate.
Aggressive up-front combination therapy, more lofty treatment goals, and earlier and more frequent reassessments to guide treatment are improving care of patients with pulmonary arterial hypertension (PAH) while at the same time making it more complex.
A larger number of oral and generic treatment options have in some respects ushered in more management ease. But overall, “I don’t know if management of these patients has ever been more complicated, given the treatment options and strategies,” said Murali M. Chakinala, MD, professor of medicine at Washington University, St. Louis. “We’re always thinking through approaches.”
Diagnosis continues to be challenging given the rarity of PAH and its nonspecific presentation – and in some cases it’s now harder. Experts such as Dr. Chakinala are seeing increasing number of aging patients with left heart disease, chronic kidney disease, and other comorbidities who have significant precapillary pulmonary hypertension and who exhibit hemodynamics consistent with PAH, or group 1 PH.
The question experts face is, do such patients have “true PAH,” as do a reported 25-50 people per million, or do they have another type of PH in the classification schema – or a mixture?
Deciding which patients “really fit into group 1 and should be managed like group 1,” Dr. Chakinala said, requires clinical acumen and has important implications, as patients with PAH are the main beneficiaries of vasodilator therapy. Most other patients with PH will not respond to or tolerate such treatment.
“These older patients may be getting PAH through different mechanisms than our younger patients, but because we define PAH through hemodynamic criteria and by ruling out other obvious explanations, they all get lumped together,” said Dr. Chakinala. “We need to parse these patients out better in the future, much like our oncology colleagues are doing.”
Personalized medicine hopefully is the next horizon for this condition, characterized by severe remodeling of the distal pulmonary arteries. Researchers are pushing to achieve deep phenotyping, identify biomarkers and improve risk assessment tools.
And with 80 or so centers now accredited by the Pulmonary Hypertension Association as Pulmonary Hypertension Care Centers, referred patients are accessing clinical trials of new nonvasodilatory drugs. Currently available therapies improve hemodynamics and symptoms, and can slow disease progression, but are not truly disease modifying, sources say.
“The endothelin, nitric oxide, and prostacyclin pathways have been exhaustively studied and we now have great drugs for those pathways,” said Dr. Chakinala, who leads the PHA’s scientific leadership council. But “we’re not going to put a greater dent into this disease until we have new drugs that work on different biologic pathways.”
Diagnostic challenges
The diagnosis of PAH – a remarkably heterogeneous condition that encompasses heritable forms and idiopathic forms, and that comprises a broad mix of predisposing conditions and exposures, from scleroderma to methamphetamine use – is still too often missed or delayed. Delayed diagnoses and misdiagnoses of PAH and other types of PH have been reported in up to 85% of at-risk patients, according to a 2016 literature review.
Being able to pivot from thinking about common pulmonary ailments or heart failure to considering PAH is a key part of earlier diagnosis and better treatment outcomes. “If someone has unexplained dyspnea or if they’re treated for other lung diseases and are not improving, think about a screening echocardiogram,” said Timothy L. Williamson, MD, vice president of quality and safety and a pulmonary and critical care physician at the University of Kansas Health Center, Kansas City.
One of the most common reasons Dr. Chakinala sees for missed diagnoses are right heart catheterizations that are incomplete or misinterpreted. (Right heart catheterizations are required to confirm the diagnosis.) “One can’t simply measure pressures and stop,” he said. “We need the full hemodynamic profile to know that it’s truly precapillary PAH ... and we need proper interpretation of [elements like] the waveforms.”
The 2019 World Symposium on Pulmonary Hypertension shifted the definition of PH from an arbitrarily defined mean pulmonary arterial pressure of at least 25 mm Hg at rest (as measured by right heart catheterization) to a more scientifically determined mPAP of at least 20 mm Hg.
The classification document also requires pulmonary vascular resistance (PVR) of at least 3 Wood units in the definition of all forms of precapillary PH. PAH specifically is defined as the presence of mPAP of at least 20 mm Hg, PVR of at least 3 Wood units, and pulmonary arterial wedge pressure 15 mm Hg or less.
Trends in treatment
The value of initial combination therapy with an endothelin receptor antagonist (ERA) and a phosphodiesterase-5 (PDE5) inhibitor in treatment-naive PAH was cemented in 2015 by the AMBITION trial. The primary endpoint (death, PAH hospitalization, or unsatisfactory clinical response) occurred in 18%, 34%, and 28% of patients who were randomized, respectively, to combination therapy, monotherapy with the ERA ambrisentan, or monotherapy with the PDE-5 inhibitor tadalafil – and in 31% of the two monotherapy groups combined.
The trial reported a 50% reduction in the primary endpoint in the combination-therapy group versus the pooled monotherapy group, as well as greater reductions in N-terminal of the prohormone brain natriuretic peptide levels, more satisfactory clinical response and greater improvement in 6-minute walking distance.
In practice, a minority of patients – typically older patients with multiple comorbidities – still receive initial monotherapy with sequential add-on therapies based on tolerance, but “for the most part PAH patients will start on combination therapy, most commonly with a ERA and PDE5 inhibitor,” Dr. Chakinala said.
For patients who are not improving on the ERA-PDE5 inhibitor approach – typically those who remain in the intermediate-risk category for intermediate-term mortality – substitution of the PDE5 inhibitor with the soluble guanylate cyclase stimulator riociguat may be considered, he and Dr. Williamson said. Clinical improvement with this substitution was demonstrated in the REPLACE trial.
Experts at PH care centers are also utilizing triple therapy for patients who do not improve to low-risk status after 2-4 months of dual combination therapy. The availability of oral prostacyclin analogues (selexipag and treprostinil) makes it easier to consider adding these agents early on, Dr. Chakinala and Dr. Richardson said.
Patients who fall into the high-risk category, at any point, are still best managed with parenteral prostacyclin analogues, Dr. Chakinala said.
In general, said Dr. Williamson, who also directs the University of Kansas Pulmonary Hypertension Comprehensive Care Center, “the PH community tends to be fairly aggressive up front, and with a low threshold for using prostacyclin analogues.”
The agents are “always part of the picture for someone who is really ill, in functional class IV, or has really impaired right ventricular function,” he said. “And we’re finding increased roles in patients who are not as ill but still have decompensated right ventricular dysfunction. It’s something we now consider.”
Recently published research on up-front oral triple therapy suggests possible benefit for some patients – but it’s far from conclusive, said Dr. Chakinala. The TRITON study randomized treatment-naive patients to the traditional ERA-PDE5 combination and either oral selexipag (a selective prostacyclin receptor agonist) or placebo as a third agent. It found no significant difference in reduction in PVR, the primary outcome, at week 26. However, the authors reported a “possible signal” for improved long-term outcomes with triple therapy.
“Based on this best evidence from a randomized clinical trial, I think it’s unfair to say that all patients should be on triple combination therapy right out of the gate,” he said. “Having said that, more recent [European] data showed that two drugs fell short of the mark in some patients, with high rates of clinical progression. And even in AMBITION, there were a number of patients in the combination arm who didn’t have a robust response.”
A 2021 retrospective analysis from the French Pulmonary Hypertension Registry – one of the European studies – assessed survival with monotherapy, dual therapy, or triple-combination therapy (two orals with a parenteral prostacyclin), and found no difference between monotherapy and dual therapy in high-risk patients.
Experts have been upping the ante, therefore, on early assessment and frequent reassessment of treatment response. Not long ago, patients were typically reassessed 6-12 months after the initiation of treatment. Now, experts at the PH care centers want to assess patients at 3-4 months and adjust or intensify treatment regimens for those who don’t yet qualify as low risk using a multidimensional risk score calculator.
The REVEAL (Registry to Evaluate Early and Long-Term PAH Management) risk score calculator, for instance, predicts the probability of 1-year survival and assigns patients to a strata of risk level based on either 12 or 6 variables (for the full or “lite” versions).]
Even better monitoring and risk assessment is needed, however, to “help sift out which patients are not improving enough on initial therapy or who are starting to fall off after being on a regimen for a period of time,” Dr. Chakinala said.
Today, with a network of accredited centers of expertise and a desire and need for many patients to remain close to home, Dr. Chakinala encourages finding a balance. Well-resourced clinicians can strive for early diagnosis and management – potentially initiating ERA–PDE-5 inhibitor combination therapy – but still should collaborate with PH experts.
“It’s a good idea to comanage these patients and let the experts see them periodically to help you determine when your patient may be declining,” he said. “The timetable for reassessment, the complexity of the reassessment, and the need to escalate to more advanced therapies has never been more important.”
Research highlights
Therapies that target inflammation and altered metabolism – including metformin – are among those being investigated for PAH. So are therapies targeting dysfunctional bone morphogenetic protein pathway signaling, which has been shown to be associated with hereditary, idiopathic, and likely other forms of PAH; one such drug, called sotatercept, is currently at the phase 3 trial stage.
Most promising for PAH may be the research efforts involving deep phenotyping, said Andrew J. Sweatt, MD, of Stanford (Calif.) University and the Vera Moulton Wall Center for Pulmonary Vascular Disease.
“It’s where a lot of research is headed – deep phenotyping to deconstruct the molecular and clinical heterogeneity that exists within PAH ... to detect distinct subphenotypes of patients who would respond to particular therapies,” said Dr. Sweatt, who led a review of PH clinical research presented at the 2020 American Thoracic Society International Conference
“Right now, we largely treat all patients the same ... [while] we know that patients have a wide response to therapies and there’s a lot of clinical heterogeneity in how their disease evolves over time,” he said.
Data from a large National Institutes of Health–funded multicenter phenotyping study of PH is being analyzed and should yield findings and publications starting this year, said Anna R. Hemnes, MD, associate professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn., and an investigator with the initiative, coined “Redefining Pulmonary Hypertension through Pulmonary Disease Phenomics (PVDOMICS).”
Patients have undergone advanced imaging (for example, echocardiography, cardiac MRI, chest CT, ventilation/perfusion scans), advanced testing through right heart catheterization, body composition testing, quality of life questionnaires, and blood draws that have been analyzed for DNA and RNA expression, proteomics, and metabolomics, said Dr. Hemnes, assistant director of Vanderbilt’s Pulmonary Vascular Center.
The initiative aims to refine the classification of all kinds of PH and “to bring precision medicine to the field so we’re no longer characterizing somebody [based on imaging] and right heart catheterization, but we also incorporating molecular pieces and biomarkers into the diagnostic evaluation,” she said.
In the short term, the results of deep phenotyping should “allow us to be more effective with our therapy recommendations,” Dr. Hemnes said. “Then hopefully in the longer term, [identified biomarkers] will help us to develop new, more effective therapies.”
Dr. Sweatt and Dr. Williamson reported that they have no relevant financial disclosures. Dr. Hemnes reported that she holds stock in Tenax (which is studying a tyrosine kinase inhibitor for PAH) and serves as a consultant for Acceleron, Bayer, GossamerBio, United Therapeutics, and Janssen. She also receives research funding from Imara. Dr. Chakinala reported that he is an investigator on clinical trials for a number of pharmaceutical companies. He also serves on advisory boards for Phase Bio, Liquidia/Rare Gen, Bayer, Janssen, Trio Health Analytics, and Aerovate.
Aggressive up-front combination therapy, more lofty treatment goals, and earlier and more frequent reassessments to guide treatment are improving care of patients with pulmonary arterial hypertension (PAH) while at the same time making it more complex.
A larger number of oral and generic treatment options have in some respects ushered in more management ease. But overall, “I don’t know if management of these patients has ever been more complicated, given the treatment options and strategies,” said Murali M. Chakinala, MD, professor of medicine at Washington University, St. Louis. “We’re always thinking through approaches.”
Diagnosis continues to be challenging given the rarity of PAH and its nonspecific presentation – and in some cases it’s now harder. Experts such as Dr. Chakinala are seeing increasing number of aging patients with left heart disease, chronic kidney disease, and other comorbidities who have significant precapillary pulmonary hypertension and who exhibit hemodynamics consistent with PAH, or group 1 PH.
The question experts face is, do such patients have “true PAH,” as do a reported 25-50 people per million, or do they have another type of PH in the classification schema – or a mixture?
Deciding which patients “really fit into group 1 and should be managed like group 1,” Dr. Chakinala said, requires clinical acumen and has important implications, as patients with PAH are the main beneficiaries of vasodilator therapy. Most other patients with PH will not respond to or tolerate such treatment.
“These older patients may be getting PAH through different mechanisms than our younger patients, but because we define PAH through hemodynamic criteria and by ruling out other obvious explanations, they all get lumped together,” said Dr. Chakinala. “We need to parse these patients out better in the future, much like our oncology colleagues are doing.”
Personalized medicine hopefully is the next horizon for this condition, characterized by severe remodeling of the distal pulmonary arteries. Researchers are pushing to achieve deep phenotyping, identify biomarkers and improve risk assessment tools.
And with 80 or so centers now accredited by the Pulmonary Hypertension Association as Pulmonary Hypertension Care Centers, referred patients are accessing clinical trials of new nonvasodilatory drugs. Currently available therapies improve hemodynamics and symptoms, and can slow disease progression, but are not truly disease modifying, sources say.
“The endothelin, nitric oxide, and prostacyclin pathways have been exhaustively studied and we now have great drugs for those pathways,” said Dr. Chakinala, who leads the PHA’s scientific leadership council. But “we’re not going to put a greater dent into this disease until we have new drugs that work on different biologic pathways.”
Diagnostic challenges
The diagnosis of PAH – a remarkably heterogeneous condition that encompasses heritable forms and idiopathic forms, and that comprises a broad mix of predisposing conditions and exposures, from scleroderma to methamphetamine use – is still too often missed or delayed. Delayed diagnoses and misdiagnoses of PAH and other types of PH have been reported in up to 85% of at-risk patients, according to a 2016 literature review.
Being able to pivot from thinking about common pulmonary ailments or heart failure to considering PAH is a key part of earlier diagnosis and better treatment outcomes. “If someone has unexplained dyspnea or if they’re treated for other lung diseases and are not improving, think about a screening echocardiogram,” said Timothy L. Williamson, MD, vice president of quality and safety and a pulmonary and critical care physician at the University of Kansas Health Center, Kansas City.
One of the most common reasons Dr. Chakinala sees for missed diagnoses are right heart catheterizations that are incomplete or misinterpreted. (Right heart catheterizations are required to confirm the diagnosis.) “One can’t simply measure pressures and stop,” he said. “We need the full hemodynamic profile to know that it’s truly precapillary PAH ... and we need proper interpretation of [elements like] the waveforms.”
The 2019 World Symposium on Pulmonary Hypertension shifted the definition of PH from an arbitrarily defined mean pulmonary arterial pressure of at least 25 mm Hg at rest (as measured by right heart catheterization) to a more scientifically determined mPAP of at least 20 mm Hg.
The classification document also requires pulmonary vascular resistance (PVR) of at least 3 Wood units in the definition of all forms of precapillary PH. PAH specifically is defined as the presence of mPAP of at least 20 mm Hg, PVR of at least 3 Wood units, and pulmonary arterial wedge pressure 15 mm Hg or less.
Trends in treatment
The value of initial combination therapy with an endothelin receptor antagonist (ERA) and a phosphodiesterase-5 (PDE5) inhibitor in treatment-naive PAH was cemented in 2015 by the AMBITION trial. The primary endpoint (death, PAH hospitalization, or unsatisfactory clinical response) occurred in 18%, 34%, and 28% of patients who were randomized, respectively, to combination therapy, monotherapy with the ERA ambrisentan, or monotherapy with the PDE-5 inhibitor tadalafil – and in 31% of the two monotherapy groups combined.
The trial reported a 50% reduction in the primary endpoint in the combination-therapy group versus the pooled monotherapy group, as well as greater reductions in N-terminal of the prohormone brain natriuretic peptide levels, more satisfactory clinical response and greater improvement in 6-minute walking distance.
In practice, a minority of patients – typically older patients with multiple comorbidities – still receive initial monotherapy with sequential add-on therapies based on tolerance, but “for the most part PAH patients will start on combination therapy, most commonly with a ERA and PDE5 inhibitor,” Dr. Chakinala said.
For patients who are not improving on the ERA-PDE5 inhibitor approach – typically those who remain in the intermediate-risk category for intermediate-term mortality – substitution of the PDE5 inhibitor with the soluble guanylate cyclase stimulator riociguat may be considered, he and Dr. Williamson said. Clinical improvement with this substitution was demonstrated in the REPLACE trial.
Experts at PH care centers are also utilizing triple therapy for patients who do not improve to low-risk status after 2-4 months of dual combination therapy. The availability of oral prostacyclin analogues (selexipag and treprostinil) makes it easier to consider adding these agents early on, Dr. Chakinala and Dr. Richardson said.
Patients who fall into the high-risk category, at any point, are still best managed with parenteral prostacyclin analogues, Dr. Chakinala said.
In general, said Dr. Williamson, who also directs the University of Kansas Pulmonary Hypertension Comprehensive Care Center, “the PH community tends to be fairly aggressive up front, and with a low threshold for using prostacyclin analogues.”
The agents are “always part of the picture for someone who is really ill, in functional class IV, or has really impaired right ventricular function,” he said. “And we’re finding increased roles in patients who are not as ill but still have decompensated right ventricular dysfunction. It’s something we now consider.”
Recently published research on up-front oral triple therapy suggests possible benefit for some patients – but it’s far from conclusive, said Dr. Chakinala. The TRITON study randomized treatment-naive patients to the traditional ERA-PDE5 combination and either oral selexipag (a selective prostacyclin receptor agonist) or placebo as a third agent. It found no significant difference in reduction in PVR, the primary outcome, at week 26. However, the authors reported a “possible signal” for improved long-term outcomes with triple therapy.
“Based on this best evidence from a randomized clinical trial, I think it’s unfair to say that all patients should be on triple combination therapy right out of the gate,” he said. “Having said that, more recent [European] data showed that two drugs fell short of the mark in some patients, with high rates of clinical progression. And even in AMBITION, there were a number of patients in the combination arm who didn’t have a robust response.”
A 2021 retrospective analysis from the French Pulmonary Hypertension Registry – one of the European studies – assessed survival with monotherapy, dual therapy, or triple-combination therapy (two orals with a parenteral prostacyclin), and found no difference between monotherapy and dual therapy in high-risk patients.
Experts have been upping the ante, therefore, on early assessment and frequent reassessment of treatment response. Not long ago, patients were typically reassessed 6-12 months after the initiation of treatment. Now, experts at the PH care centers want to assess patients at 3-4 months and adjust or intensify treatment regimens for those who don’t yet qualify as low risk using a multidimensional risk score calculator.
The REVEAL (Registry to Evaluate Early and Long-Term PAH Management) risk score calculator, for instance, predicts the probability of 1-year survival and assigns patients to a strata of risk level based on either 12 or 6 variables (for the full or “lite” versions).]
Even better monitoring and risk assessment is needed, however, to “help sift out which patients are not improving enough on initial therapy or who are starting to fall off after being on a regimen for a period of time,” Dr. Chakinala said.
Today, with a network of accredited centers of expertise and a desire and need for many patients to remain close to home, Dr. Chakinala encourages finding a balance. Well-resourced clinicians can strive for early diagnosis and management – potentially initiating ERA–PDE-5 inhibitor combination therapy – but still should collaborate with PH experts.
“It’s a good idea to comanage these patients and let the experts see them periodically to help you determine when your patient may be declining,” he said. “The timetable for reassessment, the complexity of the reassessment, and the need to escalate to more advanced therapies has never been more important.”
Research highlights
Therapies that target inflammation and altered metabolism – including metformin – are among those being investigated for PAH. So are therapies targeting dysfunctional bone morphogenetic protein pathway signaling, which has been shown to be associated with hereditary, idiopathic, and likely other forms of PAH; one such drug, called sotatercept, is currently at the phase 3 trial stage.
Most promising for PAH may be the research efforts involving deep phenotyping, said Andrew J. Sweatt, MD, of Stanford (Calif.) University and the Vera Moulton Wall Center for Pulmonary Vascular Disease.
“It’s where a lot of research is headed – deep phenotyping to deconstruct the molecular and clinical heterogeneity that exists within PAH ... to detect distinct subphenotypes of patients who would respond to particular therapies,” said Dr. Sweatt, who led a review of PH clinical research presented at the 2020 American Thoracic Society International Conference
“Right now, we largely treat all patients the same ... [while] we know that patients have a wide response to therapies and there’s a lot of clinical heterogeneity in how their disease evolves over time,” he said.
Data from a large National Institutes of Health–funded multicenter phenotyping study of PH is being analyzed and should yield findings and publications starting this year, said Anna R. Hemnes, MD, associate professor of medicine at Vanderbilt University Medical Center, Nashville, Tenn., and an investigator with the initiative, coined “Redefining Pulmonary Hypertension through Pulmonary Disease Phenomics (PVDOMICS).”
Patients have undergone advanced imaging (for example, echocardiography, cardiac MRI, chest CT, ventilation/perfusion scans), advanced testing through right heart catheterization, body composition testing, quality of life questionnaires, and blood draws that have been analyzed for DNA and RNA expression, proteomics, and metabolomics, said Dr. Hemnes, assistant director of Vanderbilt’s Pulmonary Vascular Center.
The initiative aims to refine the classification of all kinds of PH and “to bring precision medicine to the field so we’re no longer characterizing somebody [based on imaging] and right heart catheterization, but we also incorporating molecular pieces and biomarkers into the diagnostic evaluation,” she said.
In the short term, the results of deep phenotyping should “allow us to be more effective with our therapy recommendations,” Dr. Hemnes said. “Then hopefully in the longer term, [identified biomarkers] will help us to develop new, more effective therapies.”
Dr. Sweatt and Dr. Williamson reported that they have no relevant financial disclosures. Dr. Hemnes reported that she holds stock in Tenax (which is studying a tyrosine kinase inhibitor for PAH) and serves as a consultant for Acceleron, Bayer, GossamerBio, United Therapeutics, and Janssen. She also receives research funding from Imara. Dr. Chakinala reported that he is an investigator on clinical trials for a number of pharmaceutical companies. He also serves on advisory boards for Phase Bio, Liquidia/Rare Gen, Bayer, Janssen, Trio Health Analytics, and Aerovate.
No COVID vax, no transplant: Unfair or good medicine?
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Chronic respiratory conditions occur more often in RSV vs. flu
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
Hospitalized intensive care patients with respiratory syncytial virus were significantly more likely to be immunocompromised and to have chronic respiratory conditions than those with influenza infections, but in-hospital mortality rates were similar, based on data from 618 adults.
Respiratory syncytial virus is common in adults, but characteristics of RSV patients requiring ICU care have not been explored, despite routine testing for RSV in critically ill patients in many institutions, Julien Coussement, PhD, of Université Libre de Bruxelles, Brussels, and colleagues wrote.
“Influenza is another respiratory virus routinely tested for in ICU patients with respiratory symptoms because of its well-known morbidity and mortality, but there are no data specifically comparing RSV and influenza infections in adult ICU patients,” they noted.
In a retrospective, multicenter study published in the journal CHEST, the researchers analyzed data from 309 adult ICU patients with RSV infection and 309 with influenza infection between November 2011 and April 2018 from 17 sites in France and Belgium. Each RSV patient was matched to a flu patient according to institution and date of diagnosis.
The primary objective was a comparison of in-hospital mortality between the groups, defined as death from any cause during an index hospital stay in acute care. Secondary objectives were comparisons of the clinical and biological characteristics of patients with RSV versus flu.
Overall, in-hospital mortality was not significantly different between the RSV and influenza groups (23.9% vs. 25.6%, P = .63).
However, patients with RSV infection were significantly more likely than those with flu to have an underlying chronic respiratory condition (60.2% vs. 40.1%, P < .001) and to be immunocompromised (35% vs. 26.2%, P = .02). Very few of the patients overall (39 patients, 6.3%) were considered young and healthy prior to hospitalization; and significantly fewer of these were in the RSV group than in the influenza group (9 patients and 30 patients, respectively).
Airway obstruction at the time of diagnosis was significantly more common in the RSV patients than in influenza patients (49.5% vs. 39.5%, P = .01), but influenza patients were significantly more likely to have acute respiratory distress syndrome at the time of diagnosis (21.7% vs. 14.6%, P = .02). Rates of coinfections were similar between the groups, and approximately 60% of coinfected patients received at least 72 hours of therapeutic antibiotics. Overall length of hospital stay, ICU stay, and duration of mechanical ventilation were similar between the groups.
The results show that severe RSV occurs mainly in older patients with comorbidities, and these results reflect data from previous studies, the researchers wrote in their discussion. In addition, “patients with influenza infection were significantly more likely to have fever, myalgia, increased CPK level, thrombocytopenia and transaminitis at diagnosis than were those with RSV infection. Whether these differences may be used to guide patient management remains to be determined.”
The study findings were limited by several factors including the retrospective design, and testing for respiratory viruses on symptomatic patients only, rather than all ICU patients, the researchers noted. Other limitations include the inability to show a causal link between viral infections and patient outcomes and the heterogenous management of patients among different centers.
However, the results were strengthened by the large sample size and multivariate analysis, and support the need for interventions to prevent and treat severe RSV, they concluded.
The study received no outside funding. Lead author Dr. Coussement disclosed serving on advisory board for Sanofi.
FROM CHEST
Withholding anticoagulation for isolated subsegmental pulmonary embolism – Houston, we have a problem
All else being equal, I’d prefer to do nothing. Whether this is nihilism, laziness, or experience is a matter of debate. The American College of Chest Physicians (CHEST) Guidelines on therapy for venous thromboembolism (VTE) opened a door for withholding treatment for isolated subsegmental pulmonary embolism (ISSPE) in 2016 and kept it open in 2021. I was happy to walk through it and withhold therapy if it wasn’t indicated.
ISSPE is truly a conundrum. With advances in technology, the distal vessels in the lung became visible on commercial CT a little more than 10 years ago. The subsegmental branches are located after the fourth bifurcation of the pulmonary arterial system, and the new technology offered resolution adequate to identify clot in these vessels. But the new technology told us nothing about how to manage clot isolated to the subsegmental vasculature.
Autopsy data say clot in these vessels is common, even in patients who were never diagnosed with VTE while they were alive. To some degree then, the pulmonary arterial system is thought to serve as a filter to prevent clot from crossing to the systemic circulation and causing stroke. This led some to speculate that the subsegmental pulmonary arteries are supposed to contain clot and that we simply couldn’t see it before now. If this theory is correct, the practice of providing anticoagulation for ISSPE could increase bleeding without reducing the risk for VTE recurrence.
Management studies generally supported this concept. In 2007, a trial that was published in JAMA randomized patients to two different diagnostic strategies: ventilation-perfusion (VQ) and CT. CT detected more clot than VQ did, so more anticoagulation was given in the CT arm. Yet, the VTE rate during follow-up was not significantly different between arms. The implication? Some of the clots detected by CT were of lesser clinical significance and didn’t need to be treated.
Meta-analytic data from management trials also suggested that some pulmonary emboli (PE) need not be treated. Data also show when compared with patients who have more proximal PE, those with ISSPE have lower pretest probability for VTE, are less symptomatic, and have a lower burden of coexistent lower extremity thrombosis (deep vein thrombosis [DVT]).
In response to this data, the CHEST Guidelines began cautiously providing the option for withholding therapy in patients who were diagnosed with ISSPE in 2016. Their recommendations stated that patients should be stratified for recurrence risk and have lower extremity ultrasonography performed to rule out DVT. A patient with ISSPE, a low recurrence risk, and a negative ultrasound can have anticoagulation withheld. This made perfect sense to me based on what I thought I knew at the time.
Recently published data cast doubt on my nihilism. The first prospective study designed specifically to assess the safety of withholding therapy for ISSPE suggests that this practice could be dangerous. How did this happen? The trial was very well done, and the authors enrolled the right population. All of the patients had ISSPE, low recurrence risk, and negative lower extremity ultrasound. The authors were anticipating a 1% VTE rate at 90 days based on prior data but instead found a rate of 3.1% (1.6%-6.1%). They point out that this rate is not different from those seen in patients with more proximal PE who are treated with anticoagulation. However, they acknowledge that it is higher than what’s considered acceptable and warrants therapeutic anticoagulation.
So what should we do now? We treat ISSPE, that’s what. All the arguments for withholding therapy remain valid, the recurrence rate is reasonably low, and none of the recurrent VTEs in the new study were fatal. There’s still no doubt that some patients with PE won’t benefit from anticoagulation. Unfortunately, we currently lack the tools to identify them. The risk-benefit ratio for recurrence versus bleeding will be tighter with ISSPE, particularly when there’s only one clot. Unless the bleeding risk is elevated though, the ratio still favors treatment.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center.
A version of this article first appeared on Medscape.com.
All else being equal, I’d prefer to do nothing. Whether this is nihilism, laziness, or experience is a matter of debate. The American College of Chest Physicians (CHEST) Guidelines on therapy for venous thromboembolism (VTE) opened a door for withholding treatment for isolated subsegmental pulmonary embolism (ISSPE) in 2016 and kept it open in 2021. I was happy to walk through it and withhold therapy if it wasn’t indicated.
ISSPE is truly a conundrum. With advances in technology, the distal vessels in the lung became visible on commercial CT a little more than 10 years ago. The subsegmental branches are located after the fourth bifurcation of the pulmonary arterial system, and the new technology offered resolution adequate to identify clot in these vessels. But the new technology told us nothing about how to manage clot isolated to the subsegmental vasculature.
Autopsy data say clot in these vessels is common, even in patients who were never diagnosed with VTE while they were alive. To some degree then, the pulmonary arterial system is thought to serve as a filter to prevent clot from crossing to the systemic circulation and causing stroke. This led some to speculate that the subsegmental pulmonary arteries are supposed to contain clot and that we simply couldn’t see it before now. If this theory is correct, the practice of providing anticoagulation for ISSPE could increase bleeding without reducing the risk for VTE recurrence.
Management studies generally supported this concept. In 2007, a trial that was published in JAMA randomized patients to two different diagnostic strategies: ventilation-perfusion (VQ) and CT. CT detected more clot than VQ did, so more anticoagulation was given in the CT arm. Yet, the VTE rate during follow-up was not significantly different between arms. The implication? Some of the clots detected by CT were of lesser clinical significance and didn’t need to be treated.
Meta-analytic data from management trials also suggested that some pulmonary emboli (PE) need not be treated. Data also show when compared with patients who have more proximal PE, those with ISSPE have lower pretest probability for VTE, are less symptomatic, and have a lower burden of coexistent lower extremity thrombosis (deep vein thrombosis [DVT]).
In response to this data, the CHEST Guidelines began cautiously providing the option for withholding therapy in patients who were diagnosed with ISSPE in 2016. Their recommendations stated that patients should be stratified for recurrence risk and have lower extremity ultrasonography performed to rule out DVT. A patient with ISSPE, a low recurrence risk, and a negative ultrasound can have anticoagulation withheld. This made perfect sense to me based on what I thought I knew at the time.
Recently published data cast doubt on my nihilism. The first prospective study designed specifically to assess the safety of withholding therapy for ISSPE suggests that this practice could be dangerous. How did this happen? The trial was very well done, and the authors enrolled the right population. All of the patients had ISSPE, low recurrence risk, and negative lower extremity ultrasound. The authors were anticipating a 1% VTE rate at 90 days based on prior data but instead found a rate of 3.1% (1.6%-6.1%). They point out that this rate is not different from those seen in patients with more proximal PE who are treated with anticoagulation. However, they acknowledge that it is higher than what’s considered acceptable and warrants therapeutic anticoagulation.
So what should we do now? We treat ISSPE, that’s what. All the arguments for withholding therapy remain valid, the recurrence rate is reasonably low, and none of the recurrent VTEs in the new study were fatal. There’s still no doubt that some patients with PE won’t benefit from anticoagulation. Unfortunately, we currently lack the tools to identify them. The risk-benefit ratio for recurrence versus bleeding will be tighter with ISSPE, particularly when there’s only one clot. Unless the bleeding risk is elevated though, the ratio still favors treatment.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center.
A version of this article first appeared on Medscape.com.
All else being equal, I’d prefer to do nothing. Whether this is nihilism, laziness, or experience is a matter of debate. The American College of Chest Physicians (CHEST) Guidelines on therapy for venous thromboembolism (VTE) opened a door for withholding treatment for isolated subsegmental pulmonary embolism (ISSPE) in 2016 and kept it open in 2021. I was happy to walk through it and withhold therapy if it wasn’t indicated.
ISSPE is truly a conundrum. With advances in technology, the distal vessels in the lung became visible on commercial CT a little more than 10 years ago. The subsegmental branches are located after the fourth bifurcation of the pulmonary arterial system, and the new technology offered resolution adequate to identify clot in these vessels. But the new technology told us nothing about how to manage clot isolated to the subsegmental vasculature.
Autopsy data say clot in these vessels is common, even in patients who were never diagnosed with VTE while they were alive. To some degree then, the pulmonary arterial system is thought to serve as a filter to prevent clot from crossing to the systemic circulation and causing stroke. This led some to speculate that the subsegmental pulmonary arteries are supposed to contain clot and that we simply couldn’t see it before now. If this theory is correct, the practice of providing anticoagulation for ISSPE could increase bleeding without reducing the risk for VTE recurrence.
Management studies generally supported this concept. In 2007, a trial that was published in JAMA randomized patients to two different diagnostic strategies: ventilation-perfusion (VQ) and CT. CT detected more clot than VQ did, so more anticoagulation was given in the CT arm. Yet, the VTE rate during follow-up was not significantly different between arms. The implication? Some of the clots detected by CT were of lesser clinical significance and didn’t need to be treated.
Meta-analytic data from management trials also suggested that some pulmonary emboli (PE) need not be treated. Data also show when compared with patients who have more proximal PE, those with ISSPE have lower pretest probability for VTE, are less symptomatic, and have a lower burden of coexistent lower extremity thrombosis (deep vein thrombosis [DVT]).
In response to this data, the CHEST Guidelines began cautiously providing the option for withholding therapy in patients who were diagnosed with ISSPE in 2016. Their recommendations stated that patients should be stratified for recurrence risk and have lower extremity ultrasonography performed to rule out DVT. A patient with ISSPE, a low recurrence risk, and a negative ultrasound can have anticoagulation withheld. This made perfect sense to me based on what I thought I knew at the time.
Recently published data cast doubt on my nihilism. The first prospective study designed specifically to assess the safety of withholding therapy for ISSPE suggests that this practice could be dangerous. How did this happen? The trial was very well done, and the authors enrolled the right population. All of the patients had ISSPE, low recurrence risk, and negative lower extremity ultrasound. The authors were anticipating a 1% VTE rate at 90 days based on prior data but instead found a rate of 3.1% (1.6%-6.1%). They point out that this rate is not different from those seen in patients with more proximal PE who are treated with anticoagulation. However, they acknowledge that it is higher than what’s considered acceptable and warrants therapeutic anticoagulation.
So what should we do now? We treat ISSPE, that’s what. All the arguments for withholding therapy remain valid, the recurrence rate is reasonably low, and none of the recurrent VTEs in the new study were fatal. There’s still no doubt that some patients with PE won’t benefit from anticoagulation. Unfortunately, we currently lack the tools to identify them. The risk-benefit ratio for recurrence versus bleeding will be tighter with ISSPE, particularly when there’s only one clot. Unless the bleeding risk is elevated though, the ratio still favors treatment.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center.
A version of this article first appeared on Medscape.com.
Role and Experience of a Subintensive Care Unit in Caring for Patients With COVID-19 in Italy: The CO-RESP Study
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.
Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).
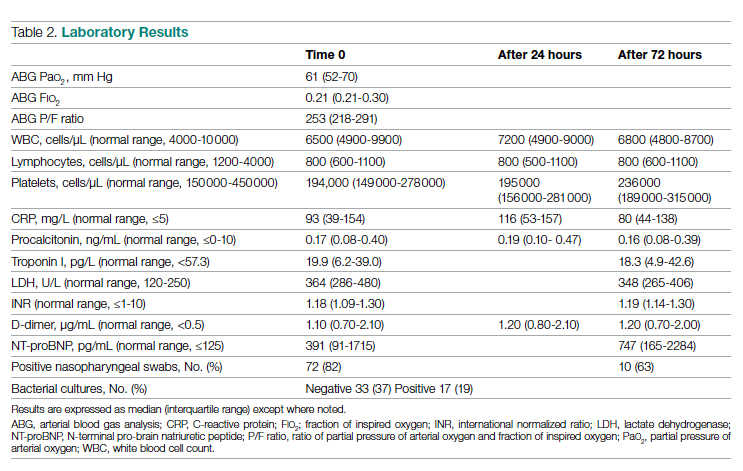
Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.
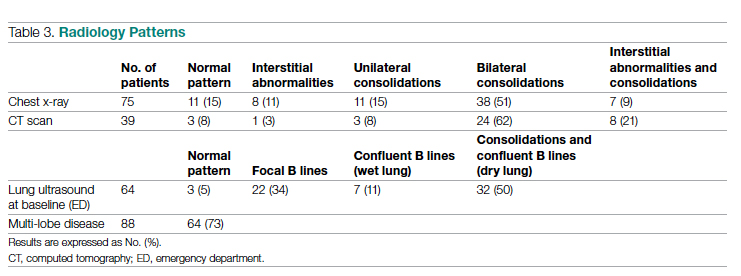
Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.
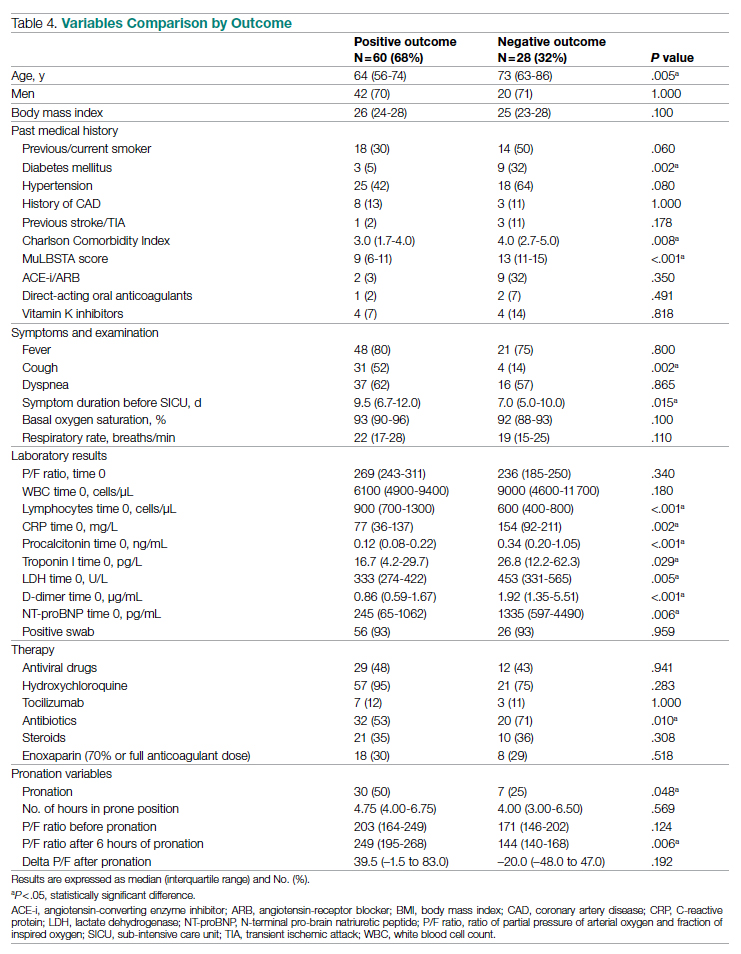
Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.

Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).

Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.

Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.

Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
From the Department of Emergency Medicine, Santa Croce e Carle Hospital, Cuneo, Italy (Drs. Abram, Tosello, Emanuele Bernardi, Allione, Cavalot, Dutto, Corsini, Martini, Sciolla, Sara Bernardi, and Lauria). From the School of Emergency Medicine, University of Turin, Turin, Italy (Drs. Paglietta and Giamello).
Objective: This retrospective and prospective cohort study was designed to describe the characteristics, treatments, and outcomes of patients with SARS-CoV-2 infection (COVID-19) admitted to subintensive care units (SICU) and to identify the variables associated with outcomes. SICUs have been extremely stressed during the pandemic, but most data regarding critically ill COVID-19 patients come from intensive care units (ICUs). Studies about COVID-19 patients in SICUs are lacking.
Setting and participants: The study included 88 COVID-19 patients admitted to our SICU in Cuneo, Italy, between March and May 2020.
Measurements: Clinical and ventilatory data were collected, and patients were divided by outcome. Multivariable logistic regression analysis examined the variables associated with negative outcomes (transfer to the ICU, palliation, or death in a SICU).
Results: A total of 60 patients (68%) had a positive outcome, and 28 patients (32%) had a negative outcome; 69 patients (78%) underwent continuous positive airway pressure (CPAP). Pronation (n = 37 [42%]) had been more frequently adopted in patients who had a positive outcome vs a negative outcome (n = 30 [50%] vs n = 7 [25%]; P = .048), and the median (interquartile range) Pa
Conclusion: SICUs have a fundamental role in the treatment of critically ill patients with COVID-19, who require long-term CPAP and pronation cycles. Diabetes, lymphopenia, and high D-dimer and LDH levels are associated with negative outcomes.
Keywords: emergency medicine, noninvasive ventilation, prone position, continuous positive airway pressure.
The COVID-19 pandemic has led to large increases in hospital admissions. Subintensive care units (SICUs) are among the wards most under pressure worldwide,1 dealing with the increased number of critically ill patients who need noninvasive ventilation, as well as serving as the best alternative to overfilled intensive care units (ICUs). In Italy, SICUs are playing a fundamental role in the management of COVID-19 patients, providing early treatment of respiratory failure by continuous noninvasive ventilation in order to reduce the need for intubation.2-5 Nevertheless, the great majority of available data about critically ill COVID-19 patients comes from ICUs. Full studies about outcomes of patients in SICUs are lacking and need to be conducted.
We sought to evaluate the characteristics and outcomes of patients admitted to our SICU for COVID-19 to describe the treatments they needed and their impact on prognosis, and to identify the variables associated with patient outcomes.
Methods
Study Design
This cohort study used data from patients who were admitted in the very first weeks of the pandemic. Data were collected retrospectively as well as prospectively, since the ethical committee approved our project. The quality and quantity of data in the 2 groups were comparable.
Data were collected from electronic and written medical records gathered during the patient’s entire stay in our SICU. Data were entered in a database with limited and controlled access. This study complied with the Declaration of Helsinki and was approved by the local ethics committees (ID: MEDURG10).
Study Population
Clinical Data
The past medical history and recent symptoms description were obtained by manually reviewing medical records. Epidemiological exposure was defined as contact with SARS-CoV-2–positive people or staying in an epidemic outbreak area. Initial vital parameters, venous blood tests, arterial blood gas analysis, chest x-ray, as well as the result of the nasopharyngeal swab were gathered from the emergency department (ED) examination. (Additional swabs could be requested when the first one was negative but clinical suspicion for COVID-19 was high.) Upon admission to the SICU, a standardized panel of blood tests was performed, which was repeated the next day and then every 48 hours. Arterial blood gas analysis was performed when clinically indicated, at least twice a day, or following a scheduled time in patients undergoing pronation. Charlson Comorbidity Index7 and MuLBSTA score8 were calculated based on the collected data.
Imaging
Chest ultrasonography was performed in the ED at the time of hospitalization and once a day in the SICU. Pulmonary high-resolution computed tomography (HRCT) was performed when clinically indicated or when the results of nasopharyngeal swabs and/or x-ray results were discordant with COVID-19 clinical suspicion. Contrast CT was performed when pulmonary embolism was suspected.
Medical Therapy
Hydroxychloroquine, antiviral agents, tocilizumab, and ruxolitinib were used in the early phase of the pandemic, then were dismissed after evidence of no efficacy.9-11 Steroids and low-molecular-weight heparin were used afterward. Enoxaparin was used at the standard prophylactic dosage, and 70% of the anticoagulant dosage was also adopted in patients with moderate-to-severe COVID-19 and D-dimer values >3 times the normal value.12-14 Antibiotics were given when a bacterial superinfection was suspected.
Oxygen and Ventilatory Therapy
Oxygen support or noninvasive ventilation were started based on patients’ respiratory efficacy, estimated by respiratory rate and the ratio of partial pressure of arterial oxygen and fraction of inspired oxygen (P/F ratio).15,16 Oxygen support was delivered through nasal cannula, Venturi mask, or reservoir mask. Noninvasive ventilation was performed by continuous positive airway pressure (CPAP) when the P/F ratio was <250 or the respiratory rate was >25 breaths per minute, using the helmet interface.5,17 Prone positioning during CPAP18-20 was adopted in patients meeting the acute respiratory distress syndrome (ARDS) criteria21 and having persistence of respiratory distress and P/F <300 after a 1-hour trial of CPAP.
The prone position was maintained based on patient tolerance. P/F ratio was measured before pronation (T0), after 1 hour of prone position (T1), before resupination (T2), and 6 hours after resupination (T3). With the same timing, the patient was asked to rate their comfort in each position, from 0 (lack of comfort) to 10 (optimal comfort). Delta P/F was defined as the difference between P/F at T3 and basal P/F at T0.
Outcomes
Statistical Analysis
Continuous data are reported as median and interquartile range (IQR); normal distribution of variables was tested using the Shapiro-Wilk test. Categorical variables were reported as absolute number and percentage. The Mann-Whitney test was used to compare continuous variables between groups, and chi-square test with continuity correction was used for categorical variables. The variables that were most significantly associated with a negative outcome on the univariate analysis were included in a stepwise logistic regression analysis, in order to identify independent predictors of patient outcome. Statistical analysis was performed using JASP (JASP Team) software.
Results
Study Population
Of the 88 patients included in the study, 70% were male; the median age was 66 years (IQR, 60-77). In most patients, the diagnosis of COVID-19 was derived from a positive SARS-CoV-2 nasopharyngeal swab. Six patients, however, maintained a negative swab at all determinations but had clinical and imaging features strongly suggesting COVID-19. No patients met the exclusion criteria. Most patients came from the ED (n = 58 [66%]) or general wards (n = 22 [25%]), while few were transferred from the ICU (n = 8 [9%]). The median length of stay in the SICU was 4 days (IQR, 2-7). An epidemiological link to affected persons or a known virus exposure was identifiable in 37 patients (42%).
Clinical, Laboratory, and Imaging Data
The clinical and anthropometric characteristics of patients are shown in Table 1. Hypertension and smoking habits were prevalent in our population, and the median Charlson Comorbidity Index was 3. Most patients experienced fever, dyspnea, and cough during the days before hospitalization.

Laboratory data showed a marked inflammatory milieu in all studied patients, both at baseline and after 24 and 72 hours. Lymphopenia was observed, along with a significant increase of lactate dehydrogenase (LDH), C-reactive protein (CPR), and D-dimer, and a mild increase of procalcitonin. N-terminal pro-brain natriuretic peptide (NT-proBNP) values were also increased, with normal troponin I values (Table 2).

Chest x-rays were obtained in almost all patients, while HRCT was performed in nearly half of patients. Complete bedside pulmonary ultrasonography data were available for 64 patients. Heterogeneous pulmonary alterations were found, regardless of the radiological technique, and multilobe infiltrates were the prevalent radiological pattern (73%) (Table 3). Seven patients (8%) were diagnosed with associated pulmonary embolism.

Medical Therapy
Most patients (89%) received hydroxychloroquine, whereas steroids were used in one-third of the population (36%). Immunomodulators (tocilizumab and ruxolitinib) were restricted to 12 patients (14%). Empirical antiviral therapy was introduced in the first 41 patients (47%). Enoxaparin was the default agent for thromboembolism prophylaxis, and 6 patients (7%) received 70% of the anticoagulating dose.
Oxygen and Ventilatory Therapy
Outcomes
A total of 28 patients (32%) had a negative outcome in the SICU: 8 patients (9%) died, having no clinical indication for higher-intensity care; 6 patients (7%) were transferred to general wards for palliation; and 14 patients (16%) needed an upgrade of cure intensity and were transferred to the ICU. Of these 14 patients, 9 died in the ICU. The total in-hospital mortality of COVID-19 patients, including patients transferred from the SICU to general wards in fair condition, was 27% (n = 24). Clinical, laboratory, and therapeutic characteristics between the 2 groups are shown in Table 4.

Patients who had a negative outcome were significantly older and had more comorbidities, as suggested by a significantly higher prevalence of diabetes and higher Charlson Comorbidity scores (reflecting the mortality risk based on age and comorbidities). The median MuLBSTA score, which estimates the 90-day mortality risk from viral pneumonia, was also higher in patients who had a negative outcome (9.33%). Symptom occurrence was not different in patients with a negative outcome (apart from cough, which was less frequent), but these patients underwent hospitalization earlier—since the appearance of their first COVID-19 symptoms—compared to patients who had a positive outcome. No difference was found in antihypertensive therapy with angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers among outcome groups.
More pronounced laboratory abnormalities were found in patients who had a negative outcome, compared to patients who had a positive outcome: lower lymphocytes and higher C-reactive protein (CRP), procalcitonin, D-dimer, LDH, and NT-proBNP. We found no differences in the radiological distribution of pulmonary involvement in patients who had negative or positive outcomes, nor in the adopted medical treatment.
Data showed no difference in CPAP implementation in the 2 groups. However, prone positioning had been more frequently adopted in the group of patients who had a positive outcome, compared with patients who had a negative outcome. No differences of basal P/F were found in patients who had a negative or positive outcome, but the median P/F after 6 hours of prone position was significantly lower in patients who had a negative outcome. The delta P/F ratio did not differ in the 2 groups of patients.
Discussion
Role of Subintensive Units and Mortality
The novelty of our report is its attempt to investigate the specific group of COVID-19 patients admitted to a SICU. In Italy, SICUs receive acutely ill, spontaneously breathing patients who need (invasive) hemodynamic monitoring, vasoactive medication, renal replacement therapy, chest- tube placement, thrombolysis, and respiratory noninvasive support. The nurse-to-patient ratio is higher than for general wards (usually 1 nurse to every 4 or 5 patients), though lower than for ICUs. In northern Italy, a great number of COVID-19 patients have required this kind of high-intensity care during the pandemic: Noninvasive ventilation support had to be maintained for several days, pronation maneuvers required a high number of people 2 or 3 times a day, and strict monitoring had to be assured. The SICU setting allows patients to buy time as a bridge to progressive reduction of pulmonary involvement, sometimes preventing the need for intubation.
The high prevalence of negative outcomes in the SICU underlines the complexity of COVID-19 patients in this setting. In fact, published data about mortality for patients with severe COVID-19 pneumonia are similar to ours.22,23
Clinical, Laboratory, and Imaging Data
Our analysis confirmed a high rate of comorbidities in COVID-19 patients24 and their prognostic role with age.25,26 A marked inflammatory milieu was a negative prognostic indicator, and associated concomitant bacterial superinfection could have led to a worse prognosis (procalcitonin was associated with negative outcomes).27 The cardiovascular system was nevertheless stressed, as suggested by higher values of NT-proBNP in patients with negative outcomes, which could reflect sepsis-related systemic involvement.28
It is known that the pulmonary damage caused by SARS-CoV-2 has a dynamic radiological and clinical course, with early areas of subsegmental consolidation, and bilateral ground-glass opacities predominating later in the course of the disease.29 This could explain why in our population we found no specific radiological pattern leading to a worse outcome.
Medical Therapy
No specific pharmacological therapy was found to be associated with a positive outcome in our study, just like antiviral and immunomodulator therapies failed to demonstrate effectiveness in subsequent pandemic surges. The low statistical power of our study did not allow us to give insight into the effectiveness of steroids and heparin at any dosage.
PEEP Support and Prone Positioning
Continuous positive airway pressure was initiated in the majority of patients and maintained for several days. This was an absolute novelty, because we rarely had to keep patients in helmets for long. This was feasible thanks to the SICU’s high nurse-to-patient ratio and the possibility of providing monitored sedation. Patients who could no longer tolerate CPAP helmets or did not improve with CPAP support were evaluated with anesthetists for programming further management. No initial data on respiratory rate, level of hypoxemia, or oxygen support need (level of PEEP and F
Prone positioning during CPAP was implemented in 42% of our study population: P/F ratio amelioration after prone positioning was highly variable, ranging from very good P/F ratio improvements to few responses or no response. No significantly greater delta P/F ratio was seen after the first prone positioning cycle in patients who had a positive outcome, probably due to the small size of our population, but we observed a clear positive trend. Interestingly, patients showing a negative outcome had a lower percentage of long-term responses to prone positioning: 6 hours after resupination, they lost the benefit of prone positioning in terms of P/F ratio amelioration. Similarly, a greater number of patients tolerating prone positioning had a positive outcome. These data give insight on the possible benefits of prone positioning in a noninvasively supported cohort of patients, which has been mentioned in previous studies.30,31
Outcomes and Variables Associated With Negative Outcomes
After correction for age and sex, we found in multiple regression analysis that higher D-dimer and LDH values, lymphopenia, and history of diabetes were independently associated with a worse outcome. Although our results had low statistical significance, we consider the trend of the obtained odds ratios important from a clinical point of view. These results could lead to greater attention being placed on COVID-19 patients who present with these characteristics upon their arrival to the ED because they have increased risk of death or intensive care need. Clinicians should consider SICU admission for these patients in order to guarantee closer monitoring and possibly more aggressive ventilatory treatments, earlier pronation, or earlier transfer to the ICU.
Limitations
The major limitation to our study is undoubtedly its statistical power, due to its relatively low patient population. Particularly, the small number of patients who underwent pronation did not allow speculation about the efficacy of this technique, although preliminary data seem promising. However, ours is among the first studies regarding patients with COVID-19 admitted to a SICU, and these preliminary data truthfully describe the Italian, and perhaps international, experience with the first surge of the pandemic.
Conclusions
Our data highlight the primary role of the SICU in COVID-19 in adequately treating critically ill patients who have high care needs different from intubation, and who require noninvasive ventilation for prolonged times as well as frequent pronation cycles. This setting of care may represent a valid, reliable, and effective option for critically ill respiratory patients. History of diabetes, lymphopenia, and high D-dimer and LDH values are independently associated with negative outcomes, and patients presenting with these characteristics should be strictly monitored.
Acknowledgments: The authors thank the Informatica System S.R.L., as well as Allessando Mendolia for the pro bono creation of the ISCovidCollect data collecting app.
Corresponding author: Sara Abram, MD, via Coppino, 12100 Cuneo, Italy; [email protected].
Disclosures: None.
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
1. Plate JDJ, Leenen LPH, Houwert M, Hietbrink F. Utilisation of intermediate care units: a systematic review. Crit Care Res Pract. 2017;2017:8038460. doi:10.1155/2017/8038460
2. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35(1):18-25. doi:10.1097/01.CCM.0000251821.44259.F3
3. Patel BK, Wolfe KS, Pohlman AS, Hall JB, Kress JP. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2016;315(22):2435-2441. doi:10.1001/jama.2016.6338
4. Mas A, Masip J. Noninvasive ventilation in acute respiratory failure. Int J Chron Obstruct Pulmon Dis. 2014;9:837-852. doi:10.2147/COPD.S42664
5. Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74(11):651-656.
6. Lomoro P, Verde F, Zerboni F, et al. COVID-19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single-center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. doi:10.1016/j.ejro.2020.100231
7. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. doi:10.1016/0021-9681(87)90171-8
8. Guo L, Wei D, Zhang X, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi:10.3389/fmicb.2019.02752
9. Lombardy Section Italian Society Infectious and Tropical Disease. Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Infez Med. 2020;28(2):143-152.
10. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271. doi:10.1038/s41422-020-0282-0
11. Cao B, Wang Y, Wen D, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787-1799. doi:10.1056/NEJMoa2001282
12. Stone JH, Frigault MJ, Serling-Boyd NJ, et al; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333-2344. doi:10.1056/NEJMoa2028836
13. Shastri MD, Stewart N, Horne J, et al. In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin. PLoS One. 2015;10(5):e0126763. doi:10.1371/journal.pone.0126763
14. Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparin sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221-13230. doi:10.1128/JVI.02078-14
15. Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D’Amico R; COVID-19 HD Study Group. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials. 2020;21(1):574. doi:10.1186/s13063-020-04475-z
16. Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995;23(10):1638-1652. doi:10.1097/00003246-199510000-00007
17. Sinha P, Calfee CS. Phenotypes in acute respiratory distress syndrome: moving towards precision medicine. Curr Opin Crit Care. 2019;25(1):12-20. doi:10.1097/MCC.0000000000000571
18. Lucchini A, Giani M, Isgrò S, Rona R, Foti G. The “helmet bundle” in COVID-19 patients undergoing non-invasive ventilation. Intensive Crit Care Nurs. 2020;58:102859. doi:10.1016/j.iccn.2020.102859
19. Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24(1):28. doi:10.1186/s13054-020-2738-5
20. Scaravilli V, Grasselli G, Castagna L, et al. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30(6):1390-1394. doi:10.1016/j.jcrc.2015.07.008
21. Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27(5):375-378. doi:10.1111/acem.13994
22. ARDS Definition Task Force; Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526-2533. doi:10.1001/jama.2012.5669
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi:10.1136/bmj.m1966
24. Docherty AB, Harrison EM, Green CA, et al; ISARIC4C investigators. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi:10.1136/bmj.m1985
25. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052-2059. doi:10.1001/jama.2020.6775
26. Muniyappa R, Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am J Physiol Endocrinol Metab. 2020;318(5):E736-E741. doi:10.1152/ajpendo.00124.2020
27. Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319. doi:10.1002/dmrr.3319
28. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. doi:10.1016/S0140-6736(20)30211-7
29. Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the Department of Radiology should know. J Am Coll Radiol. 2020;17(4):447-451. doi:10.1016/j.jacr.2020.02.008
30. Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med. 2020;8(8):765-774. doi:10.1016/S2213-2600(20)30268-X
31. Weatherald J, Solverson K, Zuege DJ, Loroff N, Fiest KM, Parhar KKS. Awake prone positioning for COVID-19 hypoxemic respiratory failure: a rapid review. J Crit Care. 2021;61:63-70. doi:10.1016/j.jcrc.2020.08.018
CDC issues new pneumococcal vaccine recommendations for adults
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The recommendations, voted on by the CDC’s Advisory Committee on Immunization Practices (ACIP) in October and made final in January with publication in the agency’s Morbidity and Mortality Weekly Report (MMWR), call for use of the 15-valent pneumococcal conjugate vaccine (PCV15; Vaxneuvance, Merck Sharp & Dohme) or 20-valent PCV (PREVNAR20; Wyeth Pharmaceuticals).
The recommendations apply to PCV-naive adults in the United States who are either aged 65 years or older, or who are aged 19-64 years and have underlying conditions such as diabetes, chronic heart or liver disease, or HIV, and have not previously received a PCV or whose previous vaccination history is unknown.
If the PCV15 vaccine is used, a subsequent dose of the 23-valent pneumococcal polysaccharide vaccine (PPSV23; Pneumovax23, Merck Sharp & Dohme) should be provided, typically at least 1 year later, under the recommendations.
As reported by this news organization, PCV15 and PREVNAR20 received approval from the Food and Drug Administration last July.
Those approvals provided an impetus for the revised recommendations, “offer[ing] an opportunity to review the existing recommendations and available data,” Miwako Kobayashi, MD, first author of the MMWR report and a medical epidemiologist with the National Center for Immunization and Respiratory Diseases, CDC, in Atlanta, said in an interview.
“As part of that process, ACIP strived to simplify the recommendations,” she said.
The previous recommendations called for the PCV13 vaccine and the PPSV23 and had varying conditions (depending on certain age and risk groups) that added complexity to the process. Under the new approach, the same recommendation applies regardless of specific medical conditions or other risk factors.
“With the simplified recommendation for adults 19 through 64, we expect coverage may increase among this population,” Dr. Kobayashi said.
Compared with the PCV13 vaccine, PREVNAR20 protects against seven additional serotypes involved in cases of invasive pneumococcal disease (IPD) and pneumonia, which are responsible for up to 40% of all cases of pneumococcal disease and related deaths in the United States.
While the PREVNAR20 includes five more pneumococcal serotypes than PCV15, the
CDC does not recommend one over the other, Dr. Kobayashi noted.
More than 90% of cases of adult IPD involve older adults and adults with chronic medical conditions or immunocompromising conditions, cerebrospinal fluid leaks, or cochlear implants, the MMWR report notes.
Commenting on the recommendations, Amit A. Shah, MD, a geriatrician with the Mayo Clinic in Phoenix, Ariz., underscored the need for clinicians to be proactive in recommending the vaccines to those patients.
“Despite only needing one vaccine dose after turning 65 to be considered vaccinated, only about 70% of people in this group have received any pneumococcal vaccination,” he said in an interview. “This percentage has not increased much over the past several years.”
The new approach should help change that, he said.
“These new recommendations are a significant simplification from the prior confusing and challenging-to-implement recommendations from 2019,” Dr. Shah explained.
Among the 2019 recommendations was a stipulation for “shared decision-making” with PCV13, and a conversation that often only complicated matters, he noted.
“Patients and providers alike had confusion about this since it was not a clear-cut ‘yes, give it’ or ‘no, do not give it any longer’ recommendation.”
“Now that this new recommendation will require no extra time for a discussion in the clinic, and just a simple ‘it’s time for your pneumonia shot’ offer, this may become more feasible,” Dr. Shah added. “In addition, removal of the shared decision-making stipulation allows for this immunization to be easily protocolized in the clinic, similar to automatic offers to the flu vaccine for patients each year.”
According to the CDC, pneumococcal pneumonia causes an estimated 150,000 hospitalizations each year in the United States, while pneumococcal meningitis and bacteremia killed approximately 3,250 people in the United States in 2019.
“Clinicians are patients’ most trusted resource when it comes to vaccine recommendations,” Dr. Kobayashi said. “We encourage all clinicians to recommend pneumococcal vaccines when indicated.”
Dr. Kobayashi and Dr. Shah have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Pandemic pushed death rates to historic highs
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
Excess mortality is a way of quantifying the impact of a pandemic, based on overall mortality from nonpandemic periods. Mortality data over long periods of time are not available for many countries, but Switzerland, Sweden, and Spain have accumulated death count data for an uninterrupted period of more than 100 years.
In a study published in the Annals of Internal Medicine, Kaspar Staub, PhD, of the University of Zurich led a team of researchers in reviewing data on monthly excess deaths from all causes for Switzerland, Sweden, and Spain for 2020 to 2021. Dr. Staub and colleagues also compared these numbers to other pandemic and nonpandemic periods since the end of the 19th century. The starting years were 1877 for Switzerland, 1851 for Sweden, and 1908 for Spain.
The researchers collected data for monthly all-cause deaths from the statistical offices of each country and determined excess mortality by comparing these numbers to population size and age structure.
They found that 2020 showed the highest number of excess deaths since 1918, with relative excess of deaths of 12.5% in Switzerland, 8.5% in Sweden, and 17.3 % in Spain.
To put it another way, the number of excess deaths per 100,000 people was 100 for Switzerland, 75 for Sweden, and 155 for Spain.
“Our findings suggest that the pandemic led to the second-largest mortality disaster driven by a viral infection in more than 100 years in the three countries we studied, second only to the 1918 influenza pandemic,” the researchers wrote.
They explained that the excess mortality for the year 1918 was six to seven times higher than the 2020 numbers, but that the 2020 numbers might have been higher without the strong public health interventions taken worldwide to mitigate the impact of the COVID-19 pandemic.
“Early estimates suggest that vaccination prevented approximately 470,000 deaths in persons aged 60 years or older across 33 European countries between December 2019 and November 2021,” they wrote. However, because the COVID-19 pandemic is ongoing, “a more conclusive assessment will have to wait,” they added.
The 2020 numbers also were higher than most mortality rates since 1918, including peak years of previous influenza pandemics that occurred in 1957, 1968, 1977, and, most recently, the swine flu pandemic of 2009 which was caused by a novel strain of the H1N1 influenza virus.
The study findings had some limitations. For example, only three countries were included. Also, monthly death numbers according to sex, age, and cause of death were available only for the past 60 years, and data from years before the 20th century may not be reliable, the researchers said.
The new study does not account for the long-term effects of patients suffering from long COVID, they noted.
Study findings support strong public health response
“With the COVID-19 pandemic ongoing, this study reinforces the historic magnitude of the problem in terms of mortality and could add to the justification for ongoing public health measures such as vaccination drives and vaccine mandates to curb deaths,” said Suman Pal, MD, an internal medicine physician at the University of New Mexico, Albuquerque, in an interview.
“The results are surprising because when we view the rapid advancement in medical science over the last few decades, which have led to a decline in mortality from many previously fatal diseases, the scale of excess mortality from COVID-19 seems to have offset many such gains in the past 2 years.”
Prior studies of United States mortality data have estimated that excess deaths in the United States in 2020 exceeded the deaths attributed to COVID-19, said Dr. Pal. “The findings of this study could help clinicians in their discussion of the need for COVID-19 prevention measures with their patients” and inform discussions between doctors and patients about prevention strategies, he explained.
“Emphasizing that this pandemic is the second-largest cause of death due to a viral infection in a century could help patients understand the need for public health measures that may be viewed as unprecedented, such as government-imposed lockdowns, contact tracing, mask requirements, restrictions on travel, and vaccine mandates,” Dr. Pal noted. Better understanding of the evidence behind such measures may decrease the public’s resistance to following them, he added.As for additional research, “region-specific analysis of excess deaths may help estimate the impact of COVID-19 better, especially in regions where data reporting may be unreliable.”
Dr. F. Perry Wilson's take on study
“All-cause mortality is a key metric to assess the impact of the pandemic, because each death is treated equally,” said F. Perry Wilson, MD, of Yale University, in an interview. “With this type of analysis, there is no vague definition of a death from COVID or with COVID,” he explained. “A death is a death, and more deaths than expected is, of course, a bad thing. These analyses give a high-level view of the true human cost of the pandemic,” he said.
Dr. Wilson said he was not surprised by the findings. “There have been multiple studies, across multiple countries including the United States, which show similar findings—that observed deaths during this pandemic are substantially higher than expected,” he said. The current study findings are unique in that they compare the current pandemic to death rates in a nearly unbroken chain into the last century using data that only a few countries can provide, he noted.
The mortality data are “quite similar to what we see in the United States, with the exception that Spain was particularly hard-hit in the first COVID-19 wave in April 2020, said Dr. Wilson. By contrast, “the U.S. had substantially more excess deaths in the recent Delta wave, presumably due to lower vaccination uptake,” he added.
The current study is important for clinicians and their patients, said Dr. Wilson. “Data like these can help cut through some of the misinformation, such as the idea that only people who would have died anyway die of COVID, or that COVID is not severe,” he emphasized. “Overall death data are quite clear that far more people, millions more people, died over the last 22 months than could possibly be explained except by a global-level mortality event,” he said.
“One thing this study reminds us of is the value of high-quality data,” said Dr. Wilson. “Few countries have near complete vital statistics records on their entire populations and these can be so crucial to understand the true impact of pandemics and other disasters,” he explained. Of course, mortality data also serve as a reminder “that COVID is a serious disease: a once-in-a-century (we hope) pandemic,” he added.
The current study showed that excess death rates were similar, but not the same, from country to country, Dr. Wilson noted. “Moving forward, we need to learn what factors, from vaccination to social distancing strategies,” saved lives around the world,” he said.
The study was supported by the Foundation for Research in Science and the Humanities at the University of Zurich, the Swiss National Science Foundation, and the U.S. National Institute of Allergy and Infectious Diseases. The researchers, Dr. Pal, and Dr. Wilson had no financial conflicts.
*This article was updated on 2/1/2022.
FROM ANNALS OF INTERNAL MEDICINE