User login
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19-era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome which was strengthened by every “there is nothing else we can offer your loved one at this time,” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic, Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today, you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician.
Dr. Persampiere is a 2nd-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
Antithrombotic therapy not warranted in COVID-19 outpatients
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Sepsis multiplies in-hospital mortality risk in COPD
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although slightly fewer than 1% of hospitalizations for chronic obstructive pulmonary disease (COPD) are complicated by sepsis, this complication increases the risk for in-hospital mortality fivefold, investigators who studied a representative national sample found.
Among nearly 7 million hospitalizations in which the primary diagnosis was COPD, nearly 65,000 (0.93%) patients experienced sepsis as a complication. In all, 31% of patients with COPD and sepsis were discharged from the hospital to another care facility, and 19% of patients died in hospital, report Harshil Shah, MD, from Guthrie Corning (N.Y.) Hospital and colleagues.
“Our study highlights the need for better risk stratification in patients with COPD developing sepsis to improve the outcomes. Further studies are warranted to consider factoring some of the modifiable factors into account and to ameliorate the outcomes of sepsis during COPD hospitalizations,” Dr. Shah and colleagues write in a poster presented during the at the annual meeting of the American College of Chest Physicians, held virtually this year.
COPD has been associated with increased risk for sepsis because of the use of corticosteroids, underlying comorbidities, and, potentially, because of impaired barrier function, the authors note.
Nationwide sample
To determine the effects of sepsis and predictors of poor outcomes among patients hospitalized for COPD, the investigators used standard diagnostic codes to identify patients with a primary diagnosis of COPD from the Nationwide Inpatient Sample for the period 2007 through 2018 and sepsis from codes in secondary fields in the International Classification of Diseases (9th/10th Editions) Clinical Modification.
They identified a total of 6,940,615 hospitalizations in which the primary diagnosis was COPD; in 64,748 of those cases, sepsis was a complication.
As noted, the in-hospital death rate, one of two primary outcomes, was 19% for patients with COPD and sepsis, and the rate of discharge to other facilities was 31%.
In analysis adjusted for confounding factors, sepsis was associated with an odds ratio for mortality of 4.9 (P < .01) and an OR for discharge to a facility of 2.2 (P < .01).
With regard to trends, the investigators saw that, although the adjusted odds for in-hospital mortality remained stable over time, discharge to facilities increased significantly. In 2007, the adjusted OR was 2.2, whereas in 2018, it was 2.6 (P for trend = .02).
Predictors of in-hospital mortality among patients with sepsis included increasing age (OR, not shown), White ethnicity (OR, 1.2), treatment in the Northeast region (OR, 1.4), disseminated intravascular coagulation (OR, 3.7), pneumococcal infection (OR, 1.2), congestive heart failure (OR, 1.2), and renal failure (OR, 1.4; P < .01 for all comparisons).
Mortality risk for many patients
A COPD specialist who was not involved in the study told this news organization that sepsis is an uncommon but serious complication, not just for patients with COPD but also for those with other severe illnesses.
“Sepsis has a high risk for mortality whether a person has COPD or not,” commented David M. Mannino III MD, FCCP, FERS, professor of medicine at the University of Kentucky, Lexington, and a cofounder and co–medical director of the COPD Foundation.
“It’s not surprising that sepsis is lethal in this population; the question is, if you have COPD, are you more likely to have sepsis? And I think the answer is probably yes. The connection there is that people with COPD have a higher risk for pneumonia, and pneumonia itself is probably one of the biggest risk factors, or certainly an important risk factor, for the development of sepsis,” he said in an interview.
It would be interesting to see the relationship between sepsis and in-hospital mortality for patients with other chronic diseases or people without COPD, he said, and he would have liked to have seen more detailed information about trends over time than Dr. Shah and colleagues provided.
No funding source for the study was reported. Dr. Shah and colleagues and Dr. Mannino have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
White House announces vaccination plans for younger children
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
States were allowed to begin preordering the shots this week. But they can’t be delivered into kids’ arms until the FDA and CDC sign off. The shots could be available in early November.
“We know millions of parents have been waiting for COVID-19 vaccine for kids in this age group, and should the FDA and CDC authorize the vaccine, we will be ready to get shots in arms,” Jeff Zients, the White House COVID-19 response coordinator, said at a briefing Oct. 20.
Asked whether announcing plans to deliver a vaccine to children might put pressure on the agencies considering the evidence for their use, Mr. Zients defended the Biden administration’s plans.
“This is the right way to do things: To be operationally ready,” he said. Mr. Zients said they had learned a lesson from the prior administration.
“The decision was made by the FDA and CDC, and the operations weren’t ready. And that meant that adults at the time were not able to receive their vaccines as efficiently, equitably as possible. And this will enable us to be ready for kids,” he said.
Pfizer submitted data to the FDA in late September from its test of the vaccine in 2,200 children. The company said the shots had a favorable safety profile and generated “robust” antibody responses.
An FDA panel is scheduled to meet on Oct. 26 to consider Pfizer’s application. The CDC’s Advisory Committee on Immunization Practices will meet the following week, on Nov. 2 and 3.
Laying the groundwork
Doctors applauded the advance planning.
“Laying this advance groundwork, ensuring supply is available at physician practices, and that a patient’s own physician is available to answer questions, is critical to the continued success of this rollout,” Gerald Harmon, MD, president of the American Medical Association, said in a written statement.
The shots planned for children are 10 micrograms, a smaller dose than is given to adults. To be fully immunized, kids get two doses, spaced about 21 days apart. Vaccines for younger children are packaged in smaller vials and injected through smaller needles, too.
The vaccine for younger children will roll out slightly differently than it has for adults and teens. While adults mostly got their COVID-19 vaccines through pop-up mass vaccination sites, health departments, and other community locations, the strategy to get children immunized against COVID is centered on the offices of pediatricians and primary care doctors.
The White House says 25,000 doctors have already signed up to give the vaccines.
The vaccination campaign will get underway at a tough moment for pediatricians.
The voicemail message at Roswell Pediatrics Center in the suburbs north of Atlanta, for instance, warns parents to be patient.
“Due to the current, new COVID-19 surge, we are experiencing extremely high call volume, as well as suffering from the same staffing shortages that most businesses are having,” the message says, adding that they’re working around the clock to answer questions and return phone calls.
Jesse Hackell, MD, says he knows the feeling. He’s the chief operating officer of Pomona Pediatrics in Pomona, N.Y., and a spokesperson for the American Academy of Pediatrics.
“We’re swamped now by kids who get sent home from school because they sneezed once and they have to be cleared before they can go back to school,” he said. “We’re seeing kids who we don’t need to see in terms of the degree of illness because the school requires them to be cleared [of COVID-19].”
Dr. Hackell has been offering the vaccines to kids ages 12 and up since May. He’s planning to offer it to younger children too.
“Adding the vaccines to it is going to be a challenge, but you know we’ll get up to speed and we’ll make it happen,” he said, adding that pediatricians have done many large-scale vaccination campaigns, like those for the H1N1 influenza vaccine in 2009.
Dr. Hackell helped to draft a new policy in New York that will require COVID-19 vaccines for schoolchildren once they are granted full approval from the FDA. Other states may follow with their own vaccination requirements.
He said ultimately, vaccinating school-age children is going to make them safer, will help prevent the virus from mutating and spreading, and will help society as a whole get back to normal.
“We’re the vaccine experts in pediatrics. This is what we do. It’s a huge part of our practice like no other specialty. If we can’t get it right, how can anyone else be expected to?” he said.
A version of this article first appeared on WebMD.com.
Fungal infection can mimic lung cancer metastases
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
A fungal infection typically seen in the lungs may have a variety of unusual clinical presentations elsewhere in the body, even raising suspicion of cancer in some cases, a medical resident reported at the annual meeting of the American College of Chest Physicians.
In one recent and unusual presentation, a 58-year-old woman with persistent headaches had skull lesions on computed tomography (CT) was eventually diagnosed with disseminated coccidioidomycosis (Valley fever), a fungal infection endemic to the Southwestern U.S.
The imaging pattern of her head CT was initially concerning for cancer metastasis, according to Sharjeel Israr, MD, a third-year internal medicine resident at Creighton University in Phoenix, Ariz.
However, the subsequent chest CT revealed a suspicious chest mass. A biopsy of that mass led to the correct diagnosis of disseminated coccidioidomycosis, according to Dr. Israr, who presented the case report in an e-poster at the CHEST meeting, which was held virtually this year.
Mistaken identity
Coccidioidomycosis, caused by the fungus Coccidioides, usually affects the lungs, according to the Centers for Disease Control and Prevention. However, in severe cases it can spread to other parts of the body. In those cases, it’s referred to as disseminated coccidioidomycosis.
Arizona accounted for about 10,000 out of 18,000 reported Valley fever cases in 2019, according to the latest statistics from the CDC.
Coccidioidomycosis is frequently mistaken not only for cancer, but also for rheumatic conditions and bacterial infections, according to Valley fever specialist John Galgiani, MD, director of the Valley Fever Center for Excellence at the University of Arizona in Tucson.
“Where Valley fever is common, it should very frequently be in the differential for masses that are thought to be cancer,” Dr. Galgiani said in an interview. “This case is a good example of that.”
Challenging case
In an interview, Dr. Israr said the case was challenging to crack despite the fact that Valley fever is very common in Phoenix.
“It was definitely on the differential from the get-go, but it was very, very low our differential, just based on the presentation that she had,” said Dr. Israr.
The patient had history of diabetes and presented with headaches for 4 weeks. However, she had no pulmonary symptoms or meningeal signs, according to Dr. Israr.
A head CT revealed multiple osseous skull lesions and a left temporal lobe lesion.
“The fact that this patient had lesions in the skull, specifically, is something that raised our initial red flags for cancer – especially since she presented with just a headache as her only complaint,” he said.
The imaging pattern was concerning for metastasis, according to Dr. Israr, particularly since a subsequent CT of the chest showed multiple pulmonary nodules plus a 7.7-cm mass in the right lower lobe.
Once the biopsy confirmed coccidioidomycosis, the patient was started on fluconazole 600 mg twice daily, according to Dr. Israr.
Although severe disseminated coccidioidomycosis can be difficult to treat, the lung lesion had decreased in size from 7.7 cm to 4.2 cm about 3 months later, Dr. Israr said.
“At the end of the day, she didn’t have cancer, and it’s something that we’re treating and she’s actually doing better right now,” Dr. Israr said in the interview.
Dr. Israr and coauthors of the case reported they had no relevant relationships to disclose.
REPORTING FROM CHEST 2021
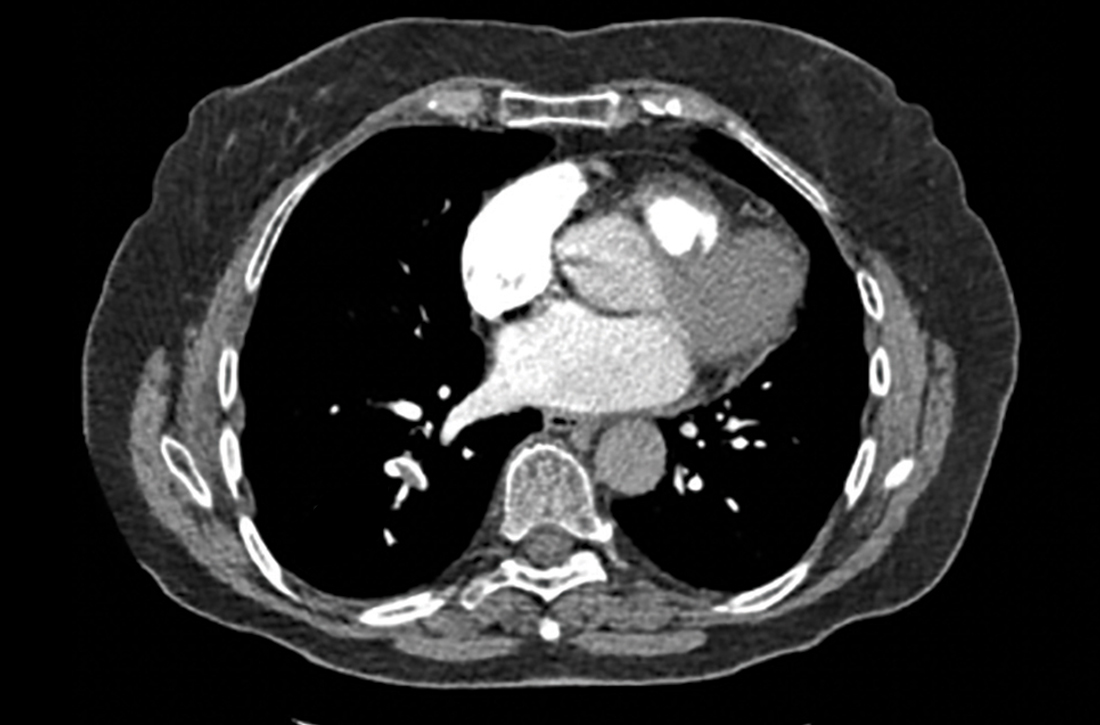
75-year-old woman • right-side rib pain • radiating shoulder pain • history of hypertension & hypercholesterolemia • Dx?
THE CASE
A 75-year-old woman presented to the primary care clinic with right-side rib pain. The patient said the pain started 1 week earlier, after she ate fried chicken for dinner, and had since been exacerbated by rich meals, lying supine, and taking a deep inspiratory breath. She also said that prior to coming to the clinic that day, the pain had been radiating to her right shoulder.
The patient denied experiencing associated fevers, chills, shortness of breath, chest pain, nausea, vomiting, constipation, diarrhea, or changes in stool color. She had a history of hypertension, for which she was taking lisinopril 20 mg/d, and hypercholesterolemia, for which she was on simvastatin 10 mg/d. She was additionally using timolol ophthalmic solution for her glaucoma.
During the examination, the patient’s vital signs were stable, with a pulse of 80 beats/min, a respiratory rate of 16 breaths/min, and an oxygen saturation of 98% on room air. The patient had no abdominal tenderness upon palpation, and the physical exam revealed no abnormalities. An in-office electrocardiogram was performed, with normal results. Additionally, a comprehensive metabolic panel, lipase test, and
THE DIAGNOSIS
Based on the lab results, a stat computed tomography pulmonary angiogram (CTPA) was ordered and showed a right segmental and subsegmental pulmonary embolism (PE; FIGURE 1).
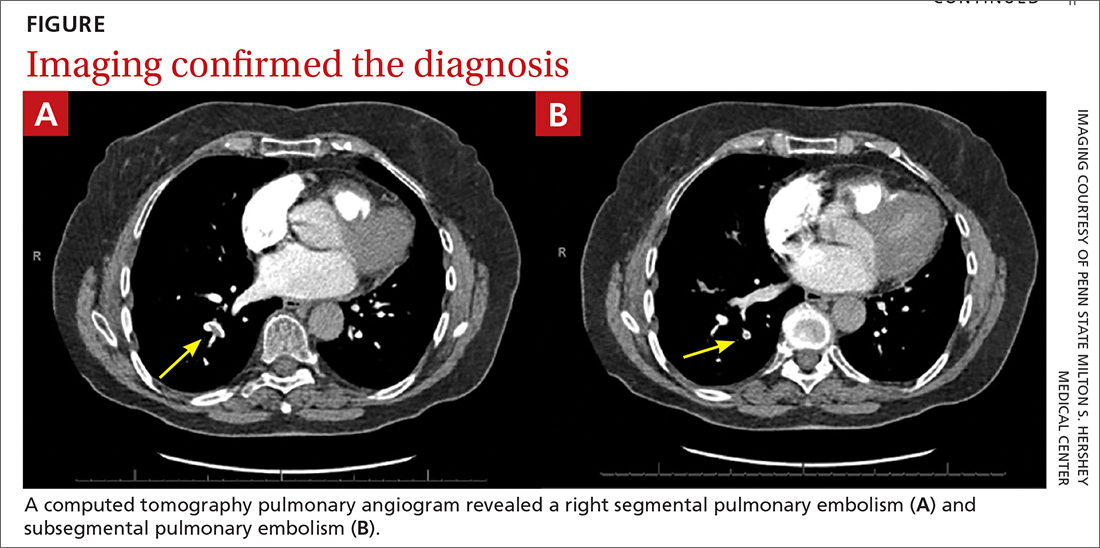
DISCUSSION
PE shares pathophysiologic mechanisms with deep vein thrombosis (DVT), and together these comprise venous thromboembolism (VTE). Risk factors for VTE include hypercoagulable disorders, use of estrogens, active malignancy, and immobilization.1 Unprovoked VTE occurs in the absence of identifiable risk factors and carries a higher risk of recurrence.2,3 While PE is classically thought to occur in the setting of a DVT, there is increasing literature describing de novo PE that can occur independent of a DVT.4
Common symptoms of PE include tachycardia, tachypnea, and pleuritic chest pain.5 Abdominal pain is a rare symptom described in some case reports.6,7 Thus, a high clinical suspicion is needed for diagnosis of PE.
The Wells criteria is an established model for risk stratifying patients presenting with possible VTE (TABLE).8 For patients with low pretest probability, as in this case, a
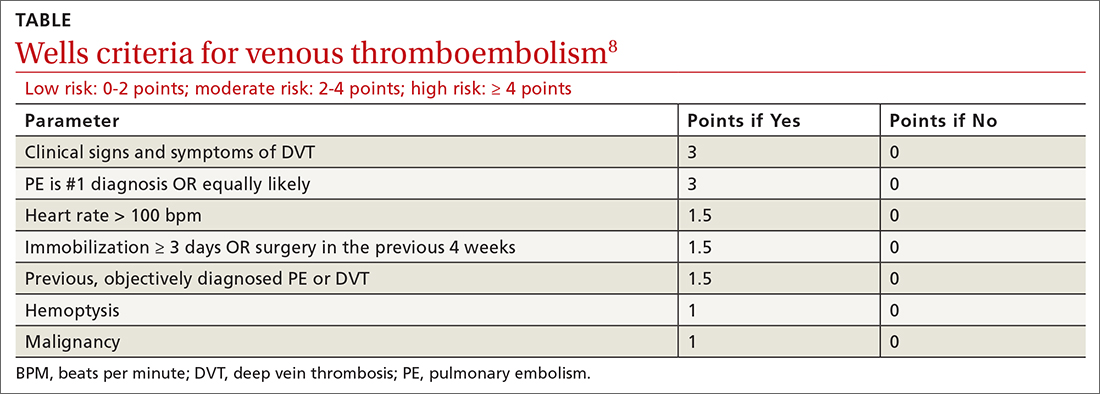
Continue to: Length of treatment depends on gender and etiology
Length of treatment depends on gender and etiology
The cornerstone treatment for stable patients with VTE is therapeutic anticoagulation. The new oral anticoagulants, which directly inhibit factor Xa or thrombin, have become increasingly popular for management of VTE, in part because they don’t require INR testing and monitoring.2
The duration of anticoagulation, particularly in unprovoked PE, is debatable. As noted earlier, patients with an unprovoked PE are at higher risk of recurrence than those with a reversible cause, so the question becomes whether these patients should have indefinite anticoagulation.2,3 Studies examining risk stratification of patients with a first, unprovoked VTE have found that men have the highest risk of recurrence, followed by women who were not taking estrogen during the index VTE, and lastly women who were taking estrogen therapy during the index VTE and subsequently discontinued it.2,3,10
Thus, it is reasonable to give women the option to discontinue anticoagulation in the setting of a negative
Our patient was directed to the emergency department for further monitoring following CT confirmation. She was discharged home after being deemed stable and prescribed apixaban 10 mg/d. A venous duplex ultrasound performed 12 days later for knee pain revealed no venous thrombosis. A CT of the abdomen performed 3 months later for other reasons revealed a normal gallbladder with no visible stones.
Apixaban was continued for 3 months and discontinued after discussion of risks and benefits of therapy cessation in the setting of a normal
Continue to: THE TAKEAWAY
THE TAKEAWAY
PE carries a significantly high mortality rate and can manifest with nonspecific and masquerading signs. A high index of suspicion is required to place PE on the differential diagnosis and carry out appropriate testing. Our patient presented with a history consistent with biliary colic but with pleuritic chest pain that warranted consideration of a PE.
CORRESPONDENCE
Alyssa Anderson, MD, 1 Continental Drive, Elizabethtown, PA 17022; [email protected]
1. Israel HL, Goldstein F. The varied clinical manifestations of pulmonary embolism. Ann Intern Med. 1957;47:202-226. doi: 10.7326/0003-4819-47-2-202
2. Rehman H, John E, Parikh P. Pulmonary embolism presenting as abdominal pain: an atypical presentation of a common diagnosis. Case Rep Emerg Med. 2016;2016:1-3. doi: 10.1155/2016/7832895
3. Park ES, Cho JY, Seo J-H, et al. Pulmonary embolism presenting with acute abdominal pain in a girl with stable ankle fracture and inherited antithrombin deficiency. Blood Res. 2018;53:81-83. doi: 10.5045/br.2018.53.1.81
4. Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358:1037-1052. doi: 10.1056/NEJMra072753
5. Agrawal V, Kim ESH. Risk of recurrent venous thromboembolism after an initial episode: risk stratification and implications for long-term treatment. Curr Cardiol Rep. 2019;21:24. doi: 10.1007/s11886-019-1111-2
6. Kearon C, Parpia S, Spencer FA, et al. Long‐term risk of recurrence in patients with a first unprovoked venous thromboembolism managed according to d‐dimer results; A cohort study. J Thromb Haemost. 2019;17:1144-1152. doi: 10.1111/jth.14458
7. Van Gent J-M, Zander AL, Olson EJ, et al. Pulmonary embolism without deep venous thrombosis. J Trauma Acute Care Surg. 2014;76:1270-1274. doi: 10.1097/TA.0000000000000233
8. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
9. Kline JA. Diagnosis and exclusion of pulmonary embolism. Thromb Res. 2018;163:207-220. doi: 10.1016/j.thromres.2017.06.002
10. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease. Chest. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
THE CASE
A 75-year-old woman presented to the primary care clinic with right-side rib pain. The patient said the pain started 1 week earlier, after she ate fried chicken for dinner, and had since been exacerbated by rich meals, lying supine, and taking a deep inspiratory breath. She also said that prior to coming to the clinic that day, the pain had been radiating to her right shoulder.
The patient denied experiencing associated fevers, chills, shortness of breath, chest pain, nausea, vomiting, constipation, diarrhea, or changes in stool color. She had a history of hypertension, for which she was taking lisinopril 20 mg/d, and hypercholesterolemia, for which she was on simvastatin 10 mg/d. She was additionally using timolol ophthalmic solution for her glaucoma.
During the examination, the patient’s vital signs were stable, with a pulse of 80 beats/min, a respiratory rate of 16 breaths/min, and an oxygen saturation of 98% on room air. The patient had no abdominal tenderness upon palpation, and the physical exam revealed no abnormalities. An in-office electrocardiogram was performed, with normal results. Additionally, a comprehensive metabolic panel, lipase test, and
THE DIAGNOSIS
Based on the lab results, a stat computed tomography pulmonary angiogram (CTPA) was ordered and showed a right segmental and subsegmental pulmonary embolism (PE; FIGURE 1).

DISCUSSION
PE shares pathophysiologic mechanisms with deep vein thrombosis (DVT), and together these comprise venous thromboembolism (VTE). Risk factors for VTE include hypercoagulable disorders, use of estrogens, active malignancy, and immobilization.1 Unprovoked VTE occurs in the absence of identifiable risk factors and carries a higher risk of recurrence.2,3 While PE is classically thought to occur in the setting of a DVT, there is increasing literature describing de novo PE that can occur independent of a DVT.4
Common symptoms of PE include tachycardia, tachypnea, and pleuritic chest pain.5 Abdominal pain is a rare symptom described in some case reports.6,7 Thus, a high clinical suspicion is needed for diagnosis of PE.
The Wells criteria is an established model for risk stratifying patients presenting with possible VTE (TABLE).8 For patients with low pretest probability, as in this case, a

Continue to: Length of treatment depends on gender and etiology
Length of treatment depends on gender and etiology
The cornerstone treatment for stable patients with VTE is therapeutic anticoagulation. The new oral anticoagulants, which directly inhibit factor Xa or thrombin, have become increasingly popular for management of VTE, in part because they don’t require INR testing and monitoring.2
The duration of anticoagulation, particularly in unprovoked PE, is debatable. As noted earlier, patients with an unprovoked PE are at higher risk of recurrence than those with a reversible cause, so the question becomes whether these patients should have indefinite anticoagulation.2,3 Studies examining risk stratification of patients with a first, unprovoked VTE have found that men have the highest risk of recurrence, followed by women who were not taking estrogen during the index VTE, and lastly women who were taking estrogen therapy during the index VTE and subsequently discontinued it.2,3,10
Thus, it is reasonable to give women the option to discontinue anticoagulation in the setting of a negative
Our patient was directed to the emergency department for further monitoring following CT confirmation. She was discharged home after being deemed stable and prescribed apixaban 10 mg/d. A venous duplex ultrasound performed 12 days later for knee pain revealed no venous thrombosis. A CT of the abdomen performed 3 months later for other reasons revealed a normal gallbladder with no visible stones.
Apixaban was continued for 3 months and discontinued after discussion of risks and benefits of therapy cessation in the setting of a normal
Continue to: THE TAKEAWAY
THE TAKEAWAY
PE carries a significantly high mortality rate and can manifest with nonspecific and masquerading signs. A high index of suspicion is required to place PE on the differential diagnosis and carry out appropriate testing. Our patient presented with a history consistent with biliary colic but with pleuritic chest pain that warranted consideration of a PE.
CORRESPONDENCE
Alyssa Anderson, MD, 1 Continental Drive, Elizabethtown, PA 17022; [email protected]
THE CASE
A 75-year-old woman presented to the primary care clinic with right-side rib pain. The patient said the pain started 1 week earlier, after she ate fried chicken for dinner, and had since been exacerbated by rich meals, lying supine, and taking a deep inspiratory breath. She also said that prior to coming to the clinic that day, the pain had been radiating to her right shoulder.
The patient denied experiencing associated fevers, chills, shortness of breath, chest pain, nausea, vomiting, constipation, diarrhea, or changes in stool color. She had a history of hypertension, for which she was taking lisinopril 20 mg/d, and hypercholesterolemia, for which she was on simvastatin 10 mg/d. She was additionally using timolol ophthalmic solution for her glaucoma.
During the examination, the patient’s vital signs were stable, with a pulse of 80 beats/min, a respiratory rate of 16 breaths/min, and an oxygen saturation of 98% on room air. The patient had no abdominal tenderness upon palpation, and the physical exam revealed no abnormalities. An in-office electrocardiogram was performed, with normal results. Additionally, a comprehensive metabolic panel, lipase test, and
THE DIAGNOSIS
Based on the lab results, a stat computed tomography pulmonary angiogram (CTPA) was ordered and showed a right segmental and subsegmental pulmonary embolism (PE; FIGURE 1).

DISCUSSION
PE shares pathophysiologic mechanisms with deep vein thrombosis (DVT), and together these comprise venous thromboembolism (VTE). Risk factors for VTE include hypercoagulable disorders, use of estrogens, active malignancy, and immobilization.1 Unprovoked VTE occurs in the absence of identifiable risk factors and carries a higher risk of recurrence.2,3 While PE is classically thought to occur in the setting of a DVT, there is increasing literature describing de novo PE that can occur independent of a DVT.4
Common symptoms of PE include tachycardia, tachypnea, and pleuritic chest pain.5 Abdominal pain is a rare symptom described in some case reports.6,7 Thus, a high clinical suspicion is needed for diagnosis of PE.
The Wells criteria is an established model for risk stratifying patients presenting with possible VTE (TABLE).8 For patients with low pretest probability, as in this case, a

Continue to: Length of treatment depends on gender and etiology
Length of treatment depends on gender and etiology
The cornerstone treatment for stable patients with VTE is therapeutic anticoagulation. The new oral anticoagulants, which directly inhibit factor Xa or thrombin, have become increasingly popular for management of VTE, in part because they don’t require INR testing and monitoring.2
The duration of anticoagulation, particularly in unprovoked PE, is debatable. As noted earlier, patients with an unprovoked PE are at higher risk of recurrence than those with a reversible cause, so the question becomes whether these patients should have indefinite anticoagulation.2,3 Studies examining risk stratification of patients with a first, unprovoked VTE have found that men have the highest risk of recurrence, followed by women who were not taking estrogen during the index VTE, and lastly women who were taking estrogen therapy during the index VTE and subsequently discontinued it.2,3,10
Thus, it is reasonable to give women the option to discontinue anticoagulation in the setting of a negative
Our patient was directed to the emergency department for further monitoring following CT confirmation. She was discharged home after being deemed stable and prescribed apixaban 10 mg/d. A venous duplex ultrasound performed 12 days later for knee pain revealed no venous thrombosis. A CT of the abdomen performed 3 months later for other reasons revealed a normal gallbladder with no visible stones.
Apixaban was continued for 3 months and discontinued after discussion of risks and benefits of therapy cessation in the setting of a normal
Continue to: THE TAKEAWAY
THE TAKEAWAY
PE carries a significantly high mortality rate and can manifest with nonspecific and masquerading signs. A high index of suspicion is required to place PE on the differential diagnosis and carry out appropriate testing. Our patient presented with a history consistent with biliary colic but with pleuritic chest pain that warranted consideration of a PE.
CORRESPONDENCE
Alyssa Anderson, MD, 1 Continental Drive, Elizabethtown, PA 17022; [email protected]
1. Israel HL, Goldstein F. The varied clinical manifestations of pulmonary embolism. Ann Intern Med. 1957;47:202-226. doi: 10.7326/0003-4819-47-2-202
2. Rehman H, John E, Parikh P. Pulmonary embolism presenting as abdominal pain: an atypical presentation of a common diagnosis. Case Rep Emerg Med. 2016;2016:1-3. doi: 10.1155/2016/7832895
3. Park ES, Cho JY, Seo J-H, et al. Pulmonary embolism presenting with acute abdominal pain in a girl with stable ankle fracture and inherited antithrombin deficiency. Blood Res. 2018;53:81-83. doi: 10.5045/br.2018.53.1.81
4. Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358:1037-1052. doi: 10.1056/NEJMra072753
5. Agrawal V, Kim ESH. Risk of recurrent venous thromboembolism after an initial episode: risk stratification and implications for long-term treatment. Curr Cardiol Rep. 2019;21:24. doi: 10.1007/s11886-019-1111-2
6. Kearon C, Parpia S, Spencer FA, et al. Long‐term risk of recurrence in patients with a first unprovoked venous thromboembolism managed according to d‐dimer results; A cohort study. J Thromb Haemost. 2019;17:1144-1152. doi: 10.1111/jth.14458
7. Van Gent J-M, Zander AL, Olson EJ, et al. Pulmonary embolism without deep venous thrombosis. J Trauma Acute Care Surg. 2014;76:1270-1274. doi: 10.1097/TA.0000000000000233
8. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
9. Kline JA. Diagnosis and exclusion of pulmonary embolism. Thromb Res. 2018;163:207-220. doi: 10.1016/j.thromres.2017.06.002
10. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease. Chest. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
1. Israel HL, Goldstein F. The varied clinical manifestations of pulmonary embolism. Ann Intern Med. 1957;47:202-226. doi: 10.7326/0003-4819-47-2-202
2. Rehman H, John E, Parikh P. Pulmonary embolism presenting as abdominal pain: an atypical presentation of a common diagnosis. Case Rep Emerg Med. 2016;2016:1-3. doi: 10.1155/2016/7832895
3. Park ES, Cho JY, Seo J-H, et al. Pulmonary embolism presenting with acute abdominal pain in a girl with stable ankle fracture and inherited antithrombin deficiency. Blood Res. 2018;53:81-83. doi: 10.5045/br.2018.53.1.81
4. Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358:1037-1052. doi: 10.1056/NEJMra072753
5. Agrawal V, Kim ESH. Risk of recurrent venous thromboembolism after an initial episode: risk stratification and implications for long-term treatment. Curr Cardiol Rep. 2019;21:24. doi: 10.1007/s11886-019-1111-2
6. Kearon C, Parpia S, Spencer FA, et al. Long‐term risk of recurrence in patients with a first unprovoked venous thromboembolism managed according to d‐dimer results; A cohort study. J Thromb Haemost. 2019;17:1144-1152. doi: 10.1111/jth.14458
7. Van Gent J-M, Zander AL, Olson EJ, et al. Pulmonary embolism without deep venous thrombosis. J Trauma Acute Care Surg. 2014;76:1270-1274. doi: 10.1097/TA.0000000000000233
8. Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001;135:98-107. doi: 10.7326/0003-4819-135-2-200107170-00010
9. Kline JA. Diagnosis and exclusion of pulmonary embolism. Thromb Res. 2018;163:207-220. doi: 10.1016/j.thromres.2017.06.002
10. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease. Chest. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
Sleep apnea has many faces
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Fortunately her problem stemmed from sleep apnea, and resolved with continuous positive airway pressure (CPAP) therapy.
Wallace and Bucks performed a meta analysis of 42 studies of memory in patients with sleep apnea and found sleep apnea patients were impaired when compared to healthy controls on verbal episodic memory (immediate recall, delayed recall, learning, and recognition) and visuospatial episodic memory (immediate and delayed recall).1 A meta-analysis by Olaithe and associates found an improvement in executive function in patients with sleep apnea who were treated with CPAP.2 I think this is worth considering especially in your patients who have subjective memory disturbances and do not appear to have a mild cognitive impairment or dementia.
About 15 years ago I saw a 74-year-old man for nocturia. He had seen two urologists and had a transurethral resection of the prostate (TURP) without any real change in his nocturia. I trialed him on all sorts of medications, and he seemed to improve temporarily a little on trazodone (went from seven episodes a night to four).
Eventually, after several years, I sent him for a sleep study. He had severe sleep apnea (Apnea Hypopnea Index, 65; O2 saturations as low as 60%). With treatment, his nocturia resolved. He went from seven episodes to two each night.
Zhou and colleagues performed a meta-analysis of 13 studies looking at the association of sleep apnea with nocturia.3 They found that men with sleep apnea have a high incidence of nocturia.
Miyazato and colleagues looked at the effect of CPAP treatment on nighttime urine production in patients with obstructive sleep apnea.4 In this small study of 40 patients, mean nighttime voiding episodes decreased from 2.1 to 1.2 (P < .01).
I have seen several patients with night sweats who ended up having sleep apnea. These patients have had a resolution of their night sweats with sleep apnea treatment.
Arnardottir and colleagues found that obstructive sleep apnea was associated with frequent nocturnal sweating.5 They found that 31% of men and 33% of women with OSA had nocturnal sweating, compared with about 10% of the general population.
When the OSA patients were treated with positive airway pressure, the prevalence of nocturnal sweating decreased to 11.5%, which is similar to general population numbers. Given how common both sleep apnea and night sweats are, this is an important consideration as you evaluate night sweats.
I have seen many patients who have had atrial fibrillation and sleep apnea. Shapira-Daniels and colleagues did a prospective study of 188 patients with atrial fibrillation without a history of sleep apnea who were referred for ablation.6 All patients had home sleep studies, and testing was consistent with sleep apnea in 82% of patients.
Kanagala and associates found that patients with untreated sleep apnea had a greater chance of recurrent atrial fibrillation after cardioversion.7 Recurrence of atrial fibrillation at 12 months was 82% in untreated OSA patients, higher than the 42% recurrence in the treated OSA group (P = .013) and the 53% recurrence in control patients.
I think sleep apnea evaluation should be strongly considered in patients with atrial fibrillation and should be done before referral for ablations.
Pearl: Consider sleep apnea as a possible cause of or contributing factor to the common primary care problems of cognitive concerns, nocturia, night sweats, and atrial fibrillation.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as 3rd-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose. Contact him at [email protected].
References
1. Wallace A and Bucks RS. Memory and obstructive sleep apnea: a meta-analysis. Sleep. 2013;36(2):203. Epub 2013 Feb 1.
2. Olaithe M and Bucks RS. Executive dysfunction in OSA before and after treatment: a meta-analysis. Sleep. 2013;36(9):1297. Epub 2013 Sep 1.
3. Zhou J et al. Association between obstructive sleep apnea syndrome and nocturia: a meta-analysis. Sleep Breath. 2020 Dec;24(4):1293-8.
4. Miyauchi Y et al. Effect of the continuous positive airway pressure on the nocturnal urine volume or night-time frequency in patients with obstructive sleep apnea syndrome. Urology 2015;85:333.
5. Arnardottir ES et al. Nocturnal sweating–a common symptom of obstructive sleep apnoea: the Icelandic sleep apnoea cohort. BMJ Open. 2013 May 14;3(5):e002795. BMJ Open 2013;3:e002795
6. Shapira-Daniels A et al. Prevalence of undiagnosed sleep apnea in patients with atrial fibrillation and its impact on therapy. JACC Clin Electrophysiol. 2020;6(12):1499. Epub 2020 Aug 12.
7. Kanagala R et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589. Epub 2003 May 12.
Life-threatening paradoxical bronchospasm may escape recognition in patients with COPD or asthma
according to a researcher who reviewed spirometry test results from U.S. military veterans.
Nearly 1.5% of the tests met the criteria for paradoxical bronchospasm, which refers to airway constriction that may rapidly occur after inhalation of a short-acting beta2 agonist (SABA) such as albuterol.
However, none of those reports alluded to paradoxical bronchospasm, said investigator Malvika Kaul, MD, fellow in the department of pulmonary and critical care at the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center, also in Chicago.
“Paradoxical bronchospasm was neither recognized nor reported in any spirometry test results,” Dr. Kaul said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
By recognizing paradoxical bronchospasm, health care providers could address its clinical implications and identify potential alternative management options, according to Dr. Kaul.
“We hope in the future, education of clinicians about this phenomena is emphasized,” Dr. Kaul said in her presentation.
Recognizing paradoxical bronchospasm
In an interview, Dr. Kaul said she began researching paradoxical bronchospasm after encountering a patient who had an acute reaction to albuterol during a pulmonary function test.
“I was not taught about it, and I wasn’t recognizing that pattern very frequently in my patients,” she said.
Prescribing information for Food and Drug Administration–approved SABAs include a warning that life-threatening paradoxical bronchospasm may occur, said Dr. Kaul.
If paradoxical bronchospasm occurs, the patient should discontinue the medication immediately and start on alternative therapy, according to the available prescribing information for albuterol sulfate.
Paradoxical bronchospasm has been linked to worsened respiratory outcomes, including more frequent exacerbations, in patients with obstructive lung diseases, according to Dr. Kaul.
Two previous large studies pegged the prevalence of paradoxical bronchospasm at around 4.5% in patients with COPD or asthma, but “it has not been reported or addressed in high-risk population, such as veterans who have high prevalence of obstructive lung diseases like COPD,” Dr. Kaul said.
Latest study results
Dr. Kaul described a retrospective analysis of 1,150 pre- and postbronchodilator spirometry tests conducted in patients with COPD or asthma at the Jesse Brown VA Medical Center between 2017 and 2020.
A positive paradoxical bronchodilator response was defined as a decrease of least 12% and 200 mL in forced expiratory volume in 1 second and forced vital capacity from baseline after four puffs of albuterol were inhaled, Dr. Kaul said.
Out of 18 reviewed spirometry results that met the criteria, none of the test results reported or recognized paradoxical bronchospasm, according to Dr. Kaul.
Those meeting the criteria were predominantly COPD patients, according to Dr. Kaul, who said 12 had an underlying diagnosis COPD, 4 had asthma, and 2 had COPD and asthma.
Of the 18 patients, 13 were African American, and all but 1 of the 18 patients had a current or past smoking history, according to reported data.
A history of obstructive sleep apnea was reported in nine patients, and history of gastroesophageal reflux disease was also reported in nine patients. Eleven patients had emphysema.
Greater awareness needed
Results of this study emphasize the need to recognize potential cases paradoxical bronchospasm in clinical practice, as well as a need for more research, according to Allen J. Blaivas, DO, FCCP, chair of the CHEST Airway Disorders NetWork.
“It’s something to be on the alert for, and certainly be aware that, if your patient is telling you that they feel worse, we shouldn’t just pooh-pooh it,” said Dr. Blaivas, who is medical director of the intensive care unit at the East Orange campus of the VA New Jersey Health Care System.
Further research could focus on breaking down whether patients with suspected paradoxical bronchospasm are using metered-dose inhalers or nebulizers, whether or not they are also taking inhaled corticosteroids, and whether prospective testing can confirm paradoxical bronchospasm in patients who report tightness after using a SABA, he said in an interview.
Dr. Kaul and coauthor Israel Rubinstein, MD had no relevant relationships to disclose. Dr. Blaivas had no relevant relationships to disclose.
according to a researcher who reviewed spirometry test results from U.S. military veterans.
Nearly 1.5% of the tests met the criteria for paradoxical bronchospasm, which refers to airway constriction that may rapidly occur after inhalation of a short-acting beta2 agonist (SABA) such as albuterol.
However, none of those reports alluded to paradoxical bronchospasm, said investigator Malvika Kaul, MD, fellow in the department of pulmonary and critical care at the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center, also in Chicago.
“Paradoxical bronchospasm was neither recognized nor reported in any spirometry test results,” Dr. Kaul said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
By recognizing paradoxical bronchospasm, health care providers could address its clinical implications and identify potential alternative management options, according to Dr. Kaul.
“We hope in the future, education of clinicians about this phenomena is emphasized,” Dr. Kaul said in her presentation.
Recognizing paradoxical bronchospasm
In an interview, Dr. Kaul said she began researching paradoxical bronchospasm after encountering a patient who had an acute reaction to albuterol during a pulmonary function test.
“I was not taught about it, and I wasn’t recognizing that pattern very frequently in my patients,” she said.
Prescribing information for Food and Drug Administration–approved SABAs include a warning that life-threatening paradoxical bronchospasm may occur, said Dr. Kaul.
If paradoxical bronchospasm occurs, the patient should discontinue the medication immediately and start on alternative therapy, according to the available prescribing information for albuterol sulfate.
Paradoxical bronchospasm has been linked to worsened respiratory outcomes, including more frequent exacerbations, in patients with obstructive lung diseases, according to Dr. Kaul.
Two previous large studies pegged the prevalence of paradoxical bronchospasm at around 4.5% in patients with COPD or asthma, but “it has not been reported or addressed in high-risk population, such as veterans who have high prevalence of obstructive lung diseases like COPD,” Dr. Kaul said.
Latest study results
Dr. Kaul described a retrospective analysis of 1,150 pre- and postbronchodilator spirometry tests conducted in patients with COPD or asthma at the Jesse Brown VA Medical Center between 2017 and 2020.
A positive paradoxical bronchodilator response was defined as a decrease of least 12% and 200 mL in forced expiratory volume in 1 second and forced vital capacity from baseline after four puffs of albuterol were inhaled, Dr. Kaul said.
Out of 18 reviewed spirometry results that met the criteria, none of the test results reported or recognized paradoxical bronchospasm, according to Dr. Kaul.
Those meeting the criteria were predominantly COPD patients, according to Dr. Kaul, who said 12 had an underlying diagnosis COPD, 4 had asthma, and 2 had COPD and asthma.
Of the 18 patients, 13 were African American, and all but 1 of the 18 patients had a current or past smoking history, according to reported data.
A history of obstructive sleep apnea was reported in nine patients, and history of gastroesophageal reflux disease was also reported in nine patients. Eleven patients had emphysema.
Greater awareness needed
Results of this study emphasize the need to recognize potential cases paradoxical bronchospasm in clinical practice, as well as a need for more research, according to Allen J. Blaivas, DO, FCCP, chair of the CHEST Airway Disorders NetWork.
“It’s something to be on the alert for, and certainly be aware that, if your patient is telling you that they feel worse, we shouldn’t just pooh-pooh it,” said Dr. Blaivas, who is medical director of the intensive care unit at the East Orange campus of the VA New Jersey Health Care System.
Further research could focus on breaking down whether patients with suspected paradoxical bronchospasm are using metered-dose inhalers or nebulizers, whether or not they are also taking inhaled corticosteroids, and whether prospective testing can confirm paradoxical bronchospasm in patients who report tightness after using a SABA, he said in an interview.
Dr. Kaul and coauthor Israel Rubinstein, MD had no relevant relationships to disclose. Dr. Blaivas had no relevant relationships to disclose.
according to a researcher who reviewed spirometry test results from U.S. military veterans.
Nearly 1.5% of the tests met the criteria for paradoxical bronchospasm, which refers to airway constriction that may rapidly occur after inhalation of a short-acting beta2 agonist (SABA) such as albuterol.
However, none of those reports alluded to paradoxical bronchospasm, said investigator Malvika Kaul, MD, fellow in the department of pulmonary and critical care at the University of Illinois at Chicago and the Jesse Brown Veterans Affairs Medical Center, also in Chicago.
“Paradoxical bronchospasm was neither recognized nor reported in any spirometry test results,” Dr. Kaul said in an online poster presentation at the annual meeting of the American College of Chest Physicians, held virtually this year.
By recognizing paradoxical bronchospasm, health care providers could address its clinical implications and identify potential alternative management options, according to Dr. Kaul.
“We hope in the future, education of clinicians about this phenomena is emphasized,” Dr. Kaul said in her presentation.
Recognizing paradoxical bronchospasm
In an interview, Dr. Kaul said she began researching paradoxical bronchospasm after encountering a patient who had an acute reaction to albuterol during a pulmonary function test.
“I was not taught about it, and I wasn’t recognizing that pattern very frequently in my patients,” she said.
Prescribing information for Food and Drug Administration–approved SABAs include a warning that life-threatening paradoxical bronchospasm may occur, said Dr. Kaul.
If paradoxical bronchospasm occurs, the patient should discontinue the medication immediately and start on alternative therapy, according to the available prescribing information for albuterol sulfate.
Paradoxical bronchospasm has been linked to worsened respiratory outcomes, including more frequent exacerbations, in patients with obstructive lung diseases, according to Dr. Kaul.
Two previous large studies pegged the prevalence of paradoxical bronchospasm at around 4.5% in patients with COPD or asthma, but “it has not been reported or addressed in high-risk population, such as veterans who have high prevalence of obstructive lung diseases like COPD,” Dr. Kaul said.
Latest study results
Dr. Kaul described a retrospective analysis of 1,150 pre- and postbronchodilator spirometry tests conducted in patients with COPD or asthma at the Jesse Brown VA Medical Center between 2017 and 2020.
A positive paradoxical bronchodilator response was defined as a decrease of least 12% and 200 mL in forced expiratory volume in 1 second and forced vital capacity from baseline after four puffs of albuterol were inhaled, Dr. Kaul said.
Out of 18 reviewed spirometry results that met the criteria, none of the test results reported or recognized paradoxical bronchospasm, according to Dr. Kaul.
Those meeting the criteria were predominantly COPD patients, according to Dr. Kaul, who said 12 had an underlying diagnosis COPD, 4 had asthma, and 2 had COPD and asthma.
Of the 18 patients, 13 were African American, and all but 1 of the 18 patients had a current or past smoking history, according to reported data.
A history of obstructive sleep apnea was reported in nine patients, and history of gastroesophageal reflux disease was also reported in nine patients. Eleven patients had emphysema.
Greater awareness needed
Results of this study emphasize the need to recognize potential cases paradoxical bronchospasm in clinical practice, as well as a need for more research, according to Allen J. Blaivas, DO, FCCP, chair of the CHEST Airway Disorders NetWork.
“It’s something to be on the alert for, and certainly be aware that, if your patient is telling you that they feel worse, we shouldn’t just pooh-pooh it,” said Dr. Blaivas, who is medical director of the intensive care unit at the East Orange campus of the VA New Jersey Health Care System.
Further research could focus on breaking down whether patients with suspected paradoxical bronchospasm are using metered-dose inhalers or nebulizers, whether or not they are also taking inhaled corticosteroids, and whether prospective testing can confirm paradoxical bronchospasm in patients who report tightness after using a SABA, he said in an interview.
Dr. Kaul and coauthor Israel Rubinstein, MD had no relevant relationships to disclose. Dr. Blaivas had no relevant relationships to disclose.
FROM CHEST 2021
Pandemic adds more weight to burden of obesity in children
according to a new report from the Robert Wood Johnson Foundation.
“Our nation’s safety net is fragile, outdated, and out of reach for millions of eligible kids and caregivers,” said Jamie Bussel, senior program officer at the RWJF, and senior author of the report. She added that the pandemic further fractured an already broken system that disproportionately overlooks “children of color and those who live farthest from economic opportunity”.
It’s time to think ‘bigger and better’
Ms. Bussel said, during a press conference, that congress responded to the pandemic with “an array of policy solutions,” but it’s now time to think ‘bigger and better.’
“There have been huge flexibilities deployed across the safety net program and these have been really important reliefs, but the fact is many of them are temporary emergency relief measures,” she explained.
For the past 3 years, the RWJF’s annual State of Childhood Obesity report has drawn national and state obesity data from large surveys including the National Survey of Children’s Health, the Youth Risk Behavior Surveillance System, the WIC Participant and Program Characteristics Survey, and the National Health and Nutrition Examination Survey.
Similar to in past years, this year’s data show that rates of obesity and overweight have remained relatively steady and have been highest among minority and low-income populations. For example, data from the 2019-2020 National Survey of Children’s Health, along with an analysis conducted by the Health Resources and Services Administration’s Maternal and Child Health Bureau, show that one in six – or 16.2% – of youth aged 10-17 years have obesity.
While non-Hispanic Asian children had the lowest obesity rate (8.1%), followed by non-Hispanic White children (12.1%), rates were significantly higher for Hispanic (21.4%), non-Hispanic Black (23.8%), and non-Hispanic American Indian/Alaska Native (28.7%) children, according to the report.
“Additional years of data are needed to assess whether obesity rates changed after the onset of the pandemic,” explained Ms. Bussel.
Digging deeper
Other studies included in this year’s report were specifically designed to measure the impact of the pandemic, and show a distinct rise in overweight and obesity, especially in younger children. For example, a retrospective cohort study using data from Kaiser Permanente Southern California showed the rate of overweight and obesity in children aged 5-11 years rose to 45.7% between March 2020 and January 2021, up from 36.2% before the pandemic.
Another of these studies, which was based on national electronic health records of more than 430,000 children, showed the obesity rate crept from 19.3% to 22.4% between August 2019 and August 2020.
“The lid we had been trying desperately to put on the obesity epidemic has come off again,” said Sandra G Hassink, MD, MSc, who is medical director of the American Academy of Pediatrics Institute for Healthy Childhood Weight.
“In the absence of COVID we had been seeing slow upticks in the numbers – and in some groups we’d been thinking maybe we were headed toward stabilization – but these numbers blow that out of the water ... COVID has escalated the rates,” she said in an interview.
“Unfortunately, these two crises – the COVID pandemic, the childhood obesity epidemic – in so many ways have exacerbated one another,” said Ms. Bussel. “It’s not a huge surprise that we’re seeing an increase in childhood obesity rates given the complete and utter disruption of every single system that circumscribes our lives.”
The systems that feed obesity
Addressing childhood obesity requires targeting far beyond healthy eating and physical activity, Ms. Bussel said.
“As important is whether that child has a safe place to call home. Does mom or dad or their care provider have a stable income? Is there reliable transportation? Is their access to health insurance? Is there access to high-quality health care? ... All of those factors influence the child and the family’s opportunities to live well, be healthy, and be at a healthy weight,” she noted.
The report includes a list of five main policy recommendations.
- Making free, universal school meal programs permanent.
- Extending eligibility for WIC, the Special Supplemental Nutrition Program for Women, Infants, and Children, to postpartum mothers and to children through age 6.
- Extending and expanding other programs, such as the Child Tax Credit.
- Closing the Medicaid coverage gap.
- Developing a consistent approach to collecting obesity data organized by race, ethnicity, and income level.
“Collectively, over at least the course of the last generation or two, our policy approach to obesity prevention has not been sufficient. But that doesn’t mean all of our policy approaches have been failures,” Ms. Bussel said during an interview. “Policy change does not always need to be dramatic to have a real impact on families.”
Fighting complacency
For Dr. Hassink, one of the barriers to change is society’s level of acceptance. She said an identifiable explanation for pandemic weight gain doesn’t mean society should simply shrug it off.
“If we regarded childhood obesity as the population level catastrophe that it is for chronic disease maybe people would be activated around these policy changes,” she said.
“We’re accepting a disease process that wreaks havoc on people,” noted Dr. Hassink, who was not involved in the new report. “I think it’s hard for people to realize the magnitude of the disease burden that we’re seeing. If you’re in a weight management clinic or any pediatrician’s office you would see it – you would see kids coming in with liver disease, 9-year-olds on [continuous positive airway pressure] for sleep apnea, kids needing their hips pinned because they had a hip fracture because of obesity.
“So, those of us that see the disease burden see what’s behind those numbers. The sadness of what we’re talking about is we know a lot about what could push the dial and help reduce this epidemic and we’re not doing what we already know,” added Dr. Hassink.
Ms. Bussel and Dr. Hassink reported no conflicts.
according to a new report from the Robert Wood Johnson Foundation.
“Our nation’s safety net is fragile, outdated, and out of reach for millions of eligible kids and caregivers,” said Jamie Bussel, senior program officer at the RWJF, and senior author of the report. She added that the pandemic further fractured an already broken system that disproportionately overlooks “children of color and those who live farthest from economic opportunity”.
It’s time to think ‘bigger and better’
Ms. Bussel said, during a press conference, that congress responded to the pandemic with “an array of policy solutions,” but it’s now time to think ‘bigger and better.’
“There have been huge flexibilities deployed across the safety net program and these have been really important reliefs, but the fact is many of them are temporary emergency relief measures,” she explained.
For the past 3 years, the RWJF’s annual State of Childhood Obesity report has drawn national and state obesity data from large surveys including the National Survey of Children’s Health, the Youth Risk Behavior Surveillance System, the WIC Participant and Program Characteristics Survey, and the National Health and Nutrition Examination Survey.
Similar to in past years, this year’s data show that rates of obesity and overweight have remained relatively steady and have been highest among minority and low-income populations. For example, data from the 2019-2020 National Survey of Children’s Health, along with an analysis conducted by the Health Resources and Services Administration’s Maternal and Child Health Bureau, show that one in six – or 16.2% – of youth aged 10-17 years have obesity.
While non-Hispanic Asian children had the lowest obesity rate (8.1%), followed by non-Hispanic White children (12.1%), rates were significantly higher for Hispanic (21.4%), non-Hispanic Black (23.8%), and non-Hispanic American Indian/Alaska Native (28.7%) children, according to the report.
“Additional years of data are needed to assess whether obesity rates changed after the onset of the pandemic,” explained Ms. Bussel.
Digging deeper
Other studies included in this year’s report were specifically designed to measure the impact of the pandemic, and show a distinct rise in overweight and obesity, especially in younger children. For example, a retrospective cohort study using data from Kaiser Permanente Southern California showed the rate of overweight and obesity in children aged 5-11 years rose to 45.7% between March 2020 and January 2021, up from 36.2% before the pandemic.
Another of these studies, which was based on national electronic health records of more than 430,000 children, showed the obesity rate crept from 19.3% to 22.4% between August 2019 and August 2020.
“The lid we had been trying desperately to put on the obesity epidemic has come off again,” said Sandra G Hassink, MD, MSc, who is medical director of the American Academy of Pediatrics Institute for Healthy Childhood Weight.
“In the absence of COVID we had been seeing slow upticks in the numbers – and in some groups we’d been thinking maybe we were headed toward stabilization – but these numbers blow that out of the water ... COVID has escalated the rates,” she said in an interview.
“Unfortunately, these two crises – the COVID pandemic, the childhood obesity epidemic – in so many ways have exacerbated one another,” said Ms. Bussel. “It’s not a huge surprise that we’re seeing an increase in childhood obesity rates given the complete and utter disruption of every single system that circumscribes our lives.”
The systems that feed obesity
Addressing childhood obesity requires targeting far beyond healthy eating and physical activity, Ms. Bussel said.
“As important is whether that child has a safe place to call home. Does mom or dad or their care provider have a stable income? Is there reliable transportation? Is their access to health insurance? Is there access to high-quality health care? ... All of those factors influence the child and the family’s opportunities to live well, be healthy, and be at a healthy weight,” she noted.
The report includes a list of five main policy recommendations.
- Making free, universal school meal programs permanent.
- Extending eligibility for WIC, the Special Supplemental Nutrition Program for Women, Infants, and Children, to postpartum mothers and to children through age 6.
- Extending and expanding other programs, such as the Child Tax Credit.
- Closing the Medicaid coverage gap.
- Developing a consistent approach to collecting obesity data organized by race, ethnicity, and income level.
“Collectively, over at least the course of the last generation or two, our policy approach to obesity prevention has not been sufficient. But that doesn’t mean all of our policy approaches have been failures,” Ms. Bussel said during an interview. “Policy change does not always need to be dramatic to have a real impact on families.”
Fighting complacency
For Dr. Hassink, one of the barriers to change is society’s level of acceptance. She said an identifiable explanation for pandemic weight gain doesn’t mean society should simply shrug it off.
“If we regarded childhood obesity as the population level catastrophe that it is for chronic disease maybe people would be activated around these policy changes,” she said.
“We’re accepting a disease process that wreaks havoc on people,” noted Dr. Hassink, who was not involved in the new report. “I think it’s hard for people to realize the magnitude of the disease burden that we’re seeing. If you’re in a weight management clinic or any pediatrician’s office you would see it – you would see kids coming in with liver disease, 9-year-olds on [continuous positive airway pressure] for sleep apnea, kids needing their hips pinned because they had a hip fracture because of obesity.
“So, those of us that see the disease burden see what’s behind those numbers. The sadness of what we’re talking about is we know a lot about what could push the dial and help reduce this epidemic and we’re not doing what we already know,” added Dr. Hassink.
Ms. Bussel and Dr. Hassink reported no conflicts.
according to a new report from the Robert Wood Johnson Foundation.
“Our nation’s safety net is fragile, outdated, and out of reach for millions of eligible kids and caregivers,” said Jamie Bussel, senior program officer at the RWJF, and senior author of the report. She added that the pandemic further fractured an already broken system that disproportionately overlooks “children of color and those who live farthest from economic opportunity”.
It’s time to think ‘bigger and better’
Ms. Bussel said, during a press conference, that congress responded to the pandemic with “an array of policy solutions,” but it’s now time to think ‘bigger and better.’
“There have been huge flexibilities deployed across the safety net program and these have been really important reliefs, but the fact is many of them are temporary emergency relief measures,” she explained.
For the past 3 years, the RWJF’s annual State of Childhood Obesity report has drawn national and state obesity data from large surveys including the National Survey of Children’s Health, the Youth Risk Behavior Surveillance System, the WIC Participant and Program Characteristics Survey, and the National Health and Nutrition Examination Survey.
Similar to in past years, this year’s data show that rates of obesity and overweight have remained relatively steady and have been highest among minority and low-income populations. For example, data from the 2019-2020 National Survey of Children’s Health, along with an analysis conducted by the Health Resources and Services Administration’s Maternal and Child Health Bureau, show that one in six – or 16.2% – of youth aged 10-17 years have obesity.
While non-Hispanic Asian children had the lowest obesity rate (8.1%), followed by non-Hispanic White children (12.1%), rates were significantly higher for Hispanic (21.4%), non-Hispanic Black (23.8%), and non-Hispanic American Indian/Alaska Native (28.7%) children, according to the report.
“Additional years of data are needed to assess whether obesity rates changed after the onset of the pandemic,” explained Ms. Bussel.
Digging deeper
Other studies included in this year’s report were specifically designed to measure the impact of the pandemic, and show a distinct rise in overweight and obesity, especially in younger children. For example, a retrospective cohort study using data from Kaiser Permanente Southern California showed the rate of overweight and obesity in children aged 5-11 years rose to 45.7% between March 2020 and January 2021, up from 36.2% before the pandemic.
Another of these studies, which was based on national electronic health records of more than 430,000 children, showed the obesity rate crept from 19.3% to 22.4% between August 2019 and August 2020.
“The lid we had been trying desperately to put on the obesity epidemic has come off again,” said Sandra G Hassink, MD, MSc, who is medical director of the American Academy of Pediatrics Institute for Healthy Childhood Weight.
“In the absence of COVID we had been seeing slow upticks in the numbers – and in some groups we’d been thinking maybe we were headed toward stabilization – but these numbers blow that out of the water ... COVID has escalated the rates,” she said in an interview.
“Unfortunately, these two crises – the COVID pandemic, the childhood obesity epidemic – in so many ways have exacerbated one another,” said Ms. Bussel. “It’s not a huge surprise that we’re seeing an increase in childhood obesity rates given the complete and utter disruption of every single system that circumscribes our lives.”
The systems that feed obesity
Addressing childhood obesity requires targeting far beyond healthy eating and physical activity, Ms. Bussel said.
“As important is whether that child has a safe place to call home. Does mom or dad or their care provider have a stable income? Is there reliable transportation? Is their access to health insurance? Is there access to high-quality health care? ... All of those factors influence the child and the family’s opportunities to live well, be healthy, and be at a healthy weight,” she noted.
The report includes a list of five main policy recommendations.
- Making free, universal school meal programs permanent.
- Extending eligibility for WIC, the Special Supplemental Nutrition Program for Women, Infants, and Children, to postpartum mothers and to children through age 6.
- Extending and expanding other programs, such as the Child Tax Credit.
- Closing the Medicaid coverage gap.
- Developing a consistent approach to collecting obesity data organized by race, ethnicity, and income level.
“Collectively, over at least the course of the last generation or two, our policy approach to obesity prevention has not been sufficient. But that doesn’t mean all of our policy approaches have been failures,” Ms. Bussel said during an interview. “Policy change does not always need to be dramatic to have a real impact on families.”
Fighting complacency
For Dr. Hassink, one of the barriers to change is society’s level of acceptance. She said an identifiable explanation for pandemic weight gain doesn’t mean society should simply shrug it off.
“If we regarded childhood obesity as the population level catastrophe that it is for chronic disease maybe people would be activated around these policy changes,” she said.
“We’re accepting a disease process that wreaks havoc on people,” noted Dr. Hassink, who was not involved in the new report. “I think it’s hard for people to realize the magnitude of the disease burden that we’re seeing. If you’re in a weight management clinic or any pediatrician’s office you would see it – you would see kids coming in with liver disease, 9-year-olds on [continuous positive airway pressure] for sleep apnea, kids needing their hips pinned because they had a hip fracture because of obesity.
“So, those of us that see the disease burden see what’s behind those numbers. The sadness of what we’re talking about is we know a lot about what could push the dial and help reduce this epidemic and we’re not doing what we already know,” added Dr. Hassink.
Ms. Bussel and Dr. Hassink reported no conflicts.
D-dimer unreliable for ruling out pulmonary embolism in COVID-19
The plasma D-dimer assay has been used, along with clinical prediction scores, to rule out pulmonary embolism (PE) in critically ill patients for decades, but a new study suggests it may not be the right test to use in hospitalized COVID-19 patients.
The results showed that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater, the cutoff point for the diagnosis.
“If using D-dimer to exclude patients with PE, the increased values we found among 92.3% of patients suggest that this assay would be less useful than in the populations in which it was originally validated, among which a minority of patients had increased D-dimer values,” the authors write. “Setting higher D-dimer thresholds was associated with improved specificity at the cost of an increased false-negative rate that could be associated with an unacceptable patient safety risk.”
The inclusion of patients with D-dimer and computed tomography pulmonary angiography (CTPA) was necessary to estimate diagnostic performance, they note, but “this may have introduced selection bias by excluding patients unable to undergo CTPA.”
“Nonetheless, given the high pretest probability of PE and low specificity observed in this and other studies, these results suggest that use of D-dimer levels to exclude PE among patients hospitalized with COVID-19 may be inappropriate and have limited clinical utility,” they conclude.
Led by Constantine N. Logothetis, MD, from Morsani College of Medicine, University of South Florida, Tampa, the study was published online Oct. 8 as a Research Letter in JAMA Network Open.
Uncertain utility
The authors note that the availability of D-dimer samples routinely collected from hospitalized COVID-19 patients – as well as the heterogeneity of early, smaller studies – generated uncertainty about the utility of this assay.
This uncertainty prompted them to test the diagnostic accuracy of the D-dimer assay among a sample of 1,541 patients who were hospitalized with COVID-19 at their institution between January 2020 and February 2021 for a possible PE.
They compared plasma D-dimer concentrations with CTPA, the criterion standard for diagnosing PE, in 287 of those patients.
Overall, 118 patients (41.1%) required care in the ICU, and 27 patients (9.4%) died during hospitalization.
The investigators looked at the ability of plasma D-dimer levels collected on the same day as CTPA to diagnose PE.
Thirty-seven patients (12.9%) had radiographic evidence of PE, and 250 patients (87.1%) did not.
Overall, the vast majority of patients (92.3%; n = 265 patients) had plasma D-dimer levels of 0.05 mcg/mL or more, including all patients with PE and 225 of 250 patients without PE (91.2%).
The median D-dimer values were 1.0 mcg/mL for 250 patients without PE and 6.1 mcg/mL for 37 patients with PE.
D-dimer values ranged from 0.2 mcg/mL to 128 mcg/mL among patients without PE, and from 0.5 mcg/mL to more than 10,000 mcg/mL among patients with PE. Patients without PE had statistically significantly decreased mean D-dimer values (8.7 mcg/mL vs. 1.2 mcg/mL; P < .001).
A D-dimer concentration of 0.05 mcg/mL was associated with a sensitivity of 100%, specificity of 8.8%, negative predictive value (NPV) of 100%, positive predictive value (PPV) of 13.9%, and a negative likelihood ratio (NLR) of less than 0.1.
The age-adjusted threshold was associated with a sensitivity of 94.6%, specificity of 22.8%, NPV of 96.6%, PPV of 13.9%, and NLR of 0.24.
The authors note that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater.
D-dimer in VTE may not extrapolate to COVID-19
“The D-dimer test, which is a measure of circulating byproducts of blood clot dissolution, has long been incorporated into diagnostic algorithms for venous thromboembolic [VTE] disease, including deep vein thrombosis and pulmonary embolism. It is uncertain whether this diagnostic use of D-dimer testing can be extrapolated to the context of COVID-19 – an illness we now understand to be associated itself with intravascular thrombosis and fibrinolysis,” Matthew Tomey, MD, a cardiologist at Mount Sinai Morningside, New York, said in an interview.
“The authors of this study sought to evaluate the test characteristics of the D-dimer assay for diagnosis of pulmonary embolism in a consecutive series of 287 hospitalized patients with COVID-19 who underwent computed tomography pulmonary angiography (CTPA). This was a selected group of patients representing less than 20% of the 1,541 patients screened. Exclusion of data on the more than 80% of screened patients who did not undergo CTPA is a significant limitation of the study,” Dr. Tomey said.
“In the highly selected, small cohort studied, representing a group of patients at high pretest probability of pulmonary embolism, there was no patient with pulmonary embolism who had a D-dimer value less than 0.5 mcg/mL. Yet broad ranges of D-dimer values were observed in COVID-19 patients with (0.5 to >10,000 mcg/mL) and without (0.2 to 128 mcg/mL) pulmonary embolism,” he added.
Based on the presented data, it is likely true that very low levels of D-dimer decrease the likelihood of finding a pulmonary embolus on a CTPA, if it is performed, Dr. Tomey noted.
“Yet the data confirm that a wide range of D-dimer values can be observed in COVID-19 patients with or without pulmonary embolism. It is not clear at this time that D-dimer levels should be used as gatekeepers to diagnostic imaging studies such as CTPA when pretest suspicion of pulmonary embolism is high,” he said.
“This issue becomes relevant as we consider evolving data on use of anticoagulation in treatment of hospitalized patients with COVID-19. We learned this year that in critically ill patients hospitalized with COVID-19, routine therapeutic anticoagulation (with heparin) was not beneficial and potentially harmful when compared with usual thromboprophylaxis,” he concluded.
“As we strive to balance competing risks of bleeding and thrombosis, accurate diagnosis of pulmonary embolism is important to guide decision-making about therapeutic anticoagulation, including in COVID-19.”
Dr. Logothetis and Dr. Tomey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The plasma D-dimer assay has been used, along with clinical prediction scores, to rule out pulmonary embolism (PE) in critically ill patients for decades, but a new study suggests it may not be the right test to use in hospitalized COVID-19 patients.
The results showed that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater, the cutoff point for the diagnosis.
“If using D-dimer to exclude patients with PE, the increased values we found among 92.3% of patients suggest that this assay would be less useful than in the populations in which it was originally validated, among which a minority of patients had increased D-dimer values,” the authors write. “Setting higher D-dimer thresholds was associated with improved specificity at the cost of an increased false-negative rate that could be associated with an unacceptable patient safety risk.”
The inclusion of patients with D-dimer and computed tomography pulmonary angiography (CTPA) was necessary to estimate diagnostic performance, they note, but “this may have introduced selection bias by excluding patients unable to undergo CTPA.”
“Nonetheless, given the high pretest probability of PE and low specificity observed in this and other studies, these results suggest that use of D-dimer levels to exclude PE among patients hospitalized with COVID-19 may be inappropriate and have limited clinical utility,” they conclude.
Led by Constantine N. Logothetis, MD, from Morsani College of Medicine, University of South Florida, Tampa, the study was published online Oct. 8 as a Research Letter in JAMA Network Open.
Uncertain utility
The authors note that the availability of D-dimer samples routinely collected from hospitalized COVID-19 patients – as well as the heterogeneity of early, smaller studies – generated uncertainty about the utility of this assay.
This uncertainty prompted them to test the diagnostic accuracy of the D-dimer assay among a sample of 1,541 patients who were hospitalized with COVID-19 at their institution between January 2020 and February 2021 for a possible PE.
They compared plasma D-dimer concentrations with CTPA, the criterion standard for diagnosing PE, in 287 of those patients.
Overall, 118 patients (41.1%) required care in the ICU, and 27 patients (9.4%) died during hospitalization.
The investigators looked at the ability of plasma D-dimer levels collected on the same day as CTPA to diagnose PE.
Thirty-seven patients (12.9%) had radiographic evidence of PE, and 250 patients (87.1%) did not.
Overall, the vast majority of patients (92.3%; n = 265 patients) had plasma D-dimer levels of 0.05 mcg/mL or more, including all patients with PE and 225 of 250 patients without PE (91.2%).
The median D-dimer values were 1.0 mcg/mL for 250 patients without PE and 6.1 mcg/mL for 37 patients with PE.
D-dimer values ranged from 0.2 mcg/mL to 128 mcg/mL among patients without PE, and from 0.5 mcg/mL to more than 10,000 mcg/mL among patients with PE. Patients without PE had statistically significantly decreased mean D-dimer values (8.7 mcg/mL vs. 1.2 mcg/mL; P < .001).
A D-dimer concentration of 0.05 mcg/mL was associated with a sensitivity of 100%, specificity of 8.8%, negative predictive value (NPV) of 100%, positive predictive value (PPV) of 13.9%, and a negative likelihood ratio (NLR) of less than 0.1.
The age-adjusted threshold was associated with a sensitivity of 94.6%, specificity of 22.8%, NPV of 96.6%, PPV of 13.9%, and NLR of 0.24.
The authors note that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater.
D-dimer in VTE may not extrapolate to COVID-19
“The D-dimer test, which is a measure of circulating byproducts of blood clot dissolution, has long been incorporated into diagnostic algorithms for venous thromboembolic [VTE] disease, including deep vein thrombosis and pulmonary embolism. It is uncertain whether this diagnostic use of D-dimer testing can be extrapolated to the context of COVID-19 – an illness we now understand to be associated itself with intravascular thrombosis and fibrinolysis,” Matthew Tomey, MD, a cardiologist at Mount Sinai Morningside, New York, said in an interview.
“The authors of this study sought to evaluate the test characteristics of the D-dimer assay for diagnosis of pulmonary embolism in a consecutive series of 287 hospitalized patients with COVID-19 who underwent computed tomography pulmonary angiography (CTPA). This was a selected group of patients representing less than 20% of the 1,541 patients screened. Exclusion of data on the more than 80% of screened patients who did not undergo CTPA is a significant limitation of the study,” Dr. Tomey said.
“In the highly selected, small cohort studied, representing a group of patients at high pretest probability of pulmonary embolism, there was no patient with pulmonary embolism who had a D-dimer value less than 0.5 mcg/mL. Yet broad ranges of D-dimer values were observed in COVID-19 patients with (0.5 to >10,000 mcg/mL) and without (0.2 to 128 mcg/mL) pulmonary embolism,” he added.
Based on the presented data, it is likely true that very low levels of D-dimer decrease the likelihood of finding a pulmonary embolus on a CTPA, if it is performed, Dr. Tomey noted.
“Yet the data confirm that a wide range of D-dimer values can be observed in COVID-19 patients with or without pulmonary embolism. It is not clear at this time that D-dimer levels should be used as gatekeepers to diagnostic imaging studies such as CTPA when pretest suspicion of pulmonary embolism is high,” he said.
“This issue becomes relevant as we consider evolving data on use of anticoagulation in treatment of hospitalized patients with COVID-19. We learned this year that in critically ill patients hospitalized with COVID-19, routine therapeutic anticoagulation (with heparin) was not beneficial and potentially harmful when compared with usual thromboprophylaxis,” he concluded.
“As we strive to balance competing risks of bleeding and thrombosis, accurate diagnosis of pulmonary embolism is important to guide decision-making about therapeutic anticoagulation, including in COVID-19.”
Dr. Logothetis and Dr. Tomey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The plasma D-dimer assay has been used, along with clinical prediction scores, to rule out pulmonary embolism (PE) in critically ill patients for decades, but a new study suggests it may not be the right test to use in hospitalized COVID-19 patients.
The results showed that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater, the cutoff point for the diagnosis.
“If using D-dimer to exclude patients with PE, the increased values we found among 92.3% of patients suggest that this assay would be less useful than in the populations in which it was originally validated, among which a minority of patients had increased D-dimer values,” the authors write. “Setting higher D-dimer thresholds was associated with improved specificity at the cost of an increased false-negative rate that could be associated with an unacceptable patient safety risk.”
The inclusion of patients with D-dimer and computed tomography pulmonary angiography (CTPA) was necessary to estimate diagnostic performance, they note, but “this may have introduced selection bias by excluding patients unable to undergo CTPA.”
“Nonetheless, given the high pretest probability of PE and low specificity observed in this and other studies, these results suggest that use of D-dimer levels to exclude PE among patients hospitalized with COVID-19 may be inappropriate and have limited clinical utility,” they conclude.
Led by Constantine N. Logothetis, MD, from Morsani College of Medicine, University of South Florida, Tampa, the study was published online Oct. 8 as a Research Letter in JAMA Network Open.
Uncertain utility
The authors note that the availability of D-dimer samples routinely collected from hospitalized COVID-19 patients – as well as the heterogeneity of early, smaller studies – generated uncertainty about the utility of this assay.
This uncertainty prompted them to test the diagnostic accuracy of the D-dimer assay among a sample of 1,541 patients who were hospitalized with COVID-19 at their institution between January 2020 and February 2021 for a possible PE.
They compared plasma D-dimer concentrations with CTPA, the criterion standard for diagnosing PE, in 287 of those patients.
Overall, 118 patients (41.1%) required care in the ICU, and 27 patients (9.4%) died during hospitalization.
The investigators looked at the ability of plasma D-dimer levels collected on the same day as CTPA to diagnose PE.
Thirty-seven patients (12.9%) had radiographic evidence of PE, and 250 patients (87.1%) did not.
Overall, the vast majority of patients (92.3%; n = 265 patients) had plasma D-dimer levels of 0.05 mcg/mL or more, including all patients with PE and 225 of 250 patients without PE (91.2%).
The median D-dimer values were 1.0 mcg/mL for 250 patients without PE and 6.1 mcg/mL for 37 patients with PE.
D-dimer values ranged from 0.2 mcg/mL to 128 mcg/mL among patients without PE, and from 0.5 mcg/mL to more than 10,000 mcg/mL among patients with PE. Patients without PE had statistically significantly decreased mean D-dimer values (8.7 mcg/mL vs. 1.2 mcg/mL; P < .001).
A D-dimer concentration of 0.05 mcg/mL was associated with a sensitivity of 100%, specificity of 8.8%, negative predictive value (NPV) of 100%, positive predictive value (PPV) of 13.9%, and a negative likelihood ratio (NLR) of less than 0.1.
The age-adjusted threshold was associated with a sensitivity of 94.6%, specificity of 22.8%, NPV of 96.6%, PPV of 13.9%, and NLR of 0.24.
The authors note that all hospitalized patients with COVID-19 and radiographic evidence of PE had plasma D-dimer levels of 0.05 mcg/mL or greater.
D-dimer in VTE may not extrapolate to COVID-19
“The D-dimer test, which is a measure of circulating byproducts of blood clot dissolution, has long been incorporated into diagnostic algorithms for venous thromboembolic [VTE] disease, including deep vein thrombosis and pulmonary embolism. It is uncertain whether this diagnostic use of D-dimer testing can be extrapolated to the context of COVID-19 – an illness we now understand to be associated itself with intravascular thrombosis and fibrinolysis,” Matthew Tomey, MD, a cardiologist at Mount Sinai Morningside, New York, said in an interview.
“The authors of this study sought to evaluate the test characteristics of the D-dimer assay for diagnosis of pulmonary embolism in a consecutive series of 287 hospitalized patients with COVID-19 who underwent computed tomography pulmonary angiography (CTPA). This was a selected group of patients representing less than 20% of the 1,541 patients screened. Exclusion of data on the more than 80% of screened patients who did not undergo CTPA is a significant limitation of the study,” Dr. Tomey said.
“In the highly selected, small cohort studied, representing a group of patients at high pretest probability of pulmonary embolism, there was no patient with pulmonary embolism who had a D-dimer value less than 0.5 mcg/mL. Yet broad ranges of D-dimer values were observed in COVID-19 patients with (0.5 to >10,000 mcg/mL) and without (0.2 to 128 mcg/mL) pulmonary embolism,” he added.
Based on the presented data, it is likely true that very low levels of D-dimer decrease the likelihood of finding a pulmonary embolus on a CTPA, if it is performed, Dr. Tomey noted.
“Yet the data confirm that a wide range of D-dimer values can be observed in COVID-19 patients with or without pulmonary embolism. It is not clear at this time that D-dimer levels should be used as gatekeepers to diagnostic imaging studies such as CTPA when pretest suspicion of pulmonary embolism is high,” he said.
“This issue becomes relevant as we consider evolving data on use of anticoagulation in treatment of hospitalized patients with COVID-19. We learned this year that in critically ill patients hospitalized with COVID-19, routine therapeutic anticoagulation (with heparin) was not beneficial and potentially harmful when compared with usual thromboprophylaxis,” he concluded.
“As we strive to balance competing risks of bleeding and thrombosis, accurate diagnosis of pulmonary embolism is important to guide decision-making about therapeutic anticoagulation, including in COVID-19.”
Dr. Logothetis and Dr. Tomey have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.