User login
FDA approves viltolarsen (Viltepso) for Duchenne muscular dystrophy
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.
The FDA approved golodirsen (Vyondys 53, Sarepta Therapeutics) for this indication last year.
“The FDA is committed to fostering drug development for serious neurological disorders like Duchenne muscular dystrophy,” Billy Dunn, MD, director, Office of Neuroscience of the FDA’s Center for Drug Evaluation and Research, said in a statement.
The approval of viltolarsen provides “an important treatment option for Duchenne muscular dystrophy patients with this confirmed mutation,” Dr. Dunn said.
Viltolarsen is an antisense oligonucleotide that promotes production of functional dystrophin by masking exon 53 in the dystrophin gene. It was evaluated in two studies involving 32 male patients.
In one study of 16 patients, the increase in dystrophin production was established in eight patients receiving viltolarsen at the recommended dose. In this study, dystrophin levels increased, on average, from 0.6% of normal at baseline to 5.9% of normal at week 25.
The increase in dystrophin production is “reasonably likely to predict clinical benefit,” but a “clinical benefit of the drug has not been established,” the FDA said.
In making the decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease, and the lack of available therapies.
Viltolarsen was approved under the FDA’s accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally offer a meaningful advantage over existing treatments.
As part of the accelerated approval, the FDA requires the company to do a clinical trial to confirm the drug’s clinical benefit. If the trial fails to verify clinical benefit, the FDA may start proceedings to withdraw approval of the drug, the agency said.
The most common side effects with viltolarsen are upper respiratory tract infection, injection-site reaction, cough, and fever.
Kidney toxicity was not observed in the clinical studies, but the clinical experience with the drug is limited, and kidney toxicity, including potentially fatal glomerulonephritis, has been observed with some antisense oligonucleotides.
“Kidney function should be monitored in patients taking Viltepso,” the FDA advises.
A version of this article originally appeared on Medscape.com.
Sarcoidosis may raise long-term risk of heart failure and death
Patients with sarcoidosis have an increased risk of heart failure and other adverse outcomes, including all-cause mortality, according to a decade-long nationwide study of Danish patients with the inflammatory disease.
“Although these findings are suggestive of the need for regular monitoring of cardiac manifestations in patients with sarcoidosis, it is important to emphasize that no causal relationships can be established from an observational study. Further studies are therefore needed to confirm our findings,” said first author Adelina Yafasova, MB, of Copenhagen University Hospital in Denmark, in an interview. The study was published in the Journal of the American College of Cardiology.
To determine the long-term risk of cardiac outcomes, and beyond – including incident heart failure; a composite of implantable cardioverter-defibrillator (ICD) implantation, ventricular arrhythmias or cardiac arrest; and all-cause mortality – Dr. Yafasova and her colleagues analyzed data from all Danish residents 18 years or older who were diagnosed with sarcoidosis from 1996 to 2016. Patients with any history of cardiac events were excluded. Of the 12,883 diagnosed patients, 11,834 were matched with subjects from a nationwide background population of more than 47,000 based on age, sex, and comorbidity. The median age of both populations was 42.8 (33.1-55.8) and 54.3% were men.
Median follow-up was 8.2 years for the sarcoidosis population and 8.4 years for the background population. The absolute 10-year risk of heart failure was 3.18% (95% confidence interval, 2.83%-3.57%) for sarcoidosis patients and 1.72% (95% CI, 1.58%-1.86%) for their matched controls. The 10-year risk for the composite of ICD implantation, ventricular arrhythmias and cardiac arrest was 0.96% (95% CI, 0.77%-1.18%) for sarcoidosis patients and 0.45% (95% CI, 0.38%-0.53%) for the background population.
For all-cause mortality, the 10-year risk was 10.88% (95% CI, 10.23%-11.55%) for sarcoidosis patients and 7.43% (95% CI, 7.15%-7.72%) for the background population. In a secondary analysis that compared all-cause mortality between the 364 sarcoidosis patients who developed heart failure and the 1,456 patients with heart failure without a history of sarcoidosis, the sarcoidosis group had a 35% higher rate than the nonsarcoidosis group (adjusted hazard ratio 1.35; 95% CI, 1.10-1.64).
“It’s not necessarily surprising that sarcoidosis patients would have a higher rate of heart failure,” said Melissa A. Lyle, MD, of the Mayo Clinic in Jacksonville, Fla., in an interview. “But the key takeaway is that sarcoidosis was associated with a higher rate of all-cause mortality compared to patients with heart failure and no sarcoidosis. That was more of a surprise.”
“There’s been some discrepancy in previous studies describing the cardiovascular outcomes in sarcoidosis,” Dr. Lyle added, “so I think this study provides excellent information while also highlighting the need for additional large-scale studies. We need to have further data on cardiovascular outcomes, which will allow us to refine the consensus statements and guidelines for management and the diagnosis of cardiac sarcoidosis.”
Dr. Lyle and Leslie T. Cooper Jr., MD, also of the Mayo Clinic, extrapolated on those thoughts in an editorial that accompanied the study. In it, the two authors praised the size and lengthy follow-up of the study, while noting its limitations. Specifically, they stressed that the study’s Danish population “may not be representative of other general populations” because of notable differences in ethnicity, age, and comorbidities.
That said, they reinforced that the study did feature “important takeaways” and that its findings emphasize the “need for monitoring for cardiac manifestations in patients with systemic sarcoidosis.”
In addition to the limitations noted in the editorial, the study’s authors acknowledged that the observational nature limited its “assessment of cause-effect relationships” and that the diagnosis codes for sarcoidosis had not been validated in the Danish National Patient Registry.
The authors of both the study and the editorial reported no conflicts of interest.
SOURCE: Yafasova A et al. J Am Coll Cardiol. 2020 Aug 10. doi: 10.1016/j.jacc.2020.06.038.
Patients with sarcoidosis have an increased risk of heart failure and other adverse outcomes, including all-cause mortality, according to a decade-long nationwide study of Danish patients with the inflammatory disease.
“Although these findings are suggestive of the need for regular monitoring of cardiac manifestations in patients with sarcoidosis, it is important to emphasize that no causal relationships can be established from an observational study. Further studies are therefore needed to confirm our findings,” said first author Adelina Yafasova, MB, of Copenhagen University Hospital in Denmark, in an interview. The study was published in the Journal of the American College of Cardiology.
To determine the long-term risk of cardiac outcomes, and beyond – including incident heart failure; a composite of implantable cardioverter-defibrillator (ICD) implantation, ventricular arrhythmias or cardiac arrest; and all-cause mortality – Dr. Yafasova and her colleagues analyzed data from all Danish residents 18 years or older who were diagnosed with sarcoidosis from 1996 to 2016. Patients with any history of cardiac events were excluded. Of the 12,883 diagnosed patients, 11,834 were matched with subjects from a nationwide background population of more than 47,000 based on age, sex, and comorbidity. The median age of both populations was 42.8 (33.1-55.8) and 54.3% were men.
Median follow-up was 8.2 years for the sarcoidosis population and 8.4 years for the background population. The absolute 10-year risk of heart failure was 3.18% (95% confidence interval, 2.83%-3.57%) for sarcoidosis patients and 1.72% (95% CI, 1.58%-1.86%) for their matched controls. The 10-year risk for the composite of ICD implantation, ventricular arrhythmias and cardiac arrest was 0.96% (95% CI, 0.77%-1.18%) for sarcoidosis patients and 0.45% (95% CI, 0.38%-0.53%) for the background population.
For all-cause mortality, the 10-year risk was 10.88% (95% CI, 10.23%-11.55%) for sarcoidosis patients and 7.43% (95% CI, 7.15%-7.72%) for the background population. In a secondary analysis that compared all-cause mortality between the 364 sarcoidosis patients who developed heart failure and the 1,456 patients with heart failure without a history of sarcoidosis, the sarcoidosis group had a 35% higher rate than the nonsarcoidosis group (adjusted hazard ratio 1.35; 95% CI, 1.10-1.64).
“It’s not necessarily surprising that sarcoidosis patients would have a higher rate of heart failure,” said Melissa A. Lyle, MD, of the Mayo Clinic in Jacksonville, Fla., in an interview. “But the key takeaway is that sarcoidosis was associated with a higher rate of all-cause mortality compared to patients with heart failure and no sarcoidosis. That was more of a surprise.”
“There’s been some discrepancy in previous studies describing the cardiovascular outcomes in sarcoidosis,” Dr. Lyle added, “so I think this study provides excellent information while also highlighting the need for additional large-scale studies. We need to have further data on cardiovascular outcomes, which will allow us to refine the consensus statements and guidelines for management and the diagnosis of cardiac sarcoidosis.”
Dr. Lyle and Leslie T. Cooper Jr., MD, also of the Mayo Clinic, extrapolated on those thoughts in an editorial that accompanied the study. In it, the two authors praised the size and lengthy follow-up of the study, while noting its limitations. Specifically, they stressed that the study’s Danish population “may not be representative of other general populations” because of notable differences in ethnicity, age, and comorbidities.
That said, they reinforced that the study did feature “important takeaways” and that its findings emphasize the “need for monitoring for cardiac manifestations in patients with systemic sarcoidosis.”
In addition to the limitations noted in the editorial, the study’s authors acknowledged that the observational nature limited its “assessment of cause-effect relationships” and that the diagnosis codes for sarcoidosis had not been validated in the Danish National Patient Registry.
The authors of both the study and the editorial reported no conflicts of interest.
SOURCE: Yafasova A et al. J Am Coll Cardiol. 2020 Aug 10. doi: 10.1016/j.jacc.2020.06.038.
Patients with sarcoidosis have an increased risk of heart failure and other adverse outcomes, including all-cause mortality, according to a decade-long nationwide study of Danish patients with the inflammatory disease.
“Although these findings are suggestive of the need for regular monitoring of cardiac manifestations in patients with sarcoidosis, it is important to emphasize that no causal relationships can be established from an observational study. Further studies are therefore needed to confirm our findings,” said first author Adelina Yafasova, MB, of Copenhagen University Hospital in Denmark, in an interview. The study was published in the Journal of the American College of Cardiology.
To determine the long-term risk of cardiac outcomes, and beyond – including incident heart failure; a composite of implantable cardioverter-defibrillator (ICD) implantation, ventricular arrhythmias or cardiac arrest; and all-cause mortality – Dr. Yafasova and her colleagues analyzed data from all Danish residents 18 years or older who were diagnosed with sarcoidosis from 1996 to 2016. Patients with any history of cardiac events were excluded. Of the 12,883 diagnosed patients, 11,834 were matched with subjects from a nationwide background population of more than 47,000 based on age, sex, and comorbidity. The median age of both populations was 42.8 (33.1-55.8) and 54.3% were men.
Median follow-up was 8.2 years for the sarcoidosis population and 8.4 years for the background population. The absolute 10-year risk of heart failure was 3.18% (95% confidence interval, 2.83%-3.57%) for sarcoidosis patients and 1.72% (95% CI, 1.58%-1.86%) for their matched controls. The 10-year risk for the composite of ICD implantation, ventricular arrhythmias and cardiac arrest was 0.96% (95% CI, 0.77%-1.18%) for sarcoidosis patients and 0.45% (95% CI, 0.38%-0.53%) for the background population.
For all-cause mortality, the 10-year risk was 10.88% (95% CI, 10.23%-11.55%) for sarcoidosis patients and 7.43% (95% CI, 7.15%-7.72%) for the background population. In a secondary analysis that compared all-cause mortality between the 364 sarcoidosis patients who developed heart failure and the 1,456 patients with heart failure without a history of sarcoidosis, the sarcoidosis group had a 35% higher rate than the nonsarcoidosis group (adjusted hazard ratio 1.35; 95% CI, 1.10-1.64).
“It’s not necessarily surprising that sarcoidosis patients would have a higher rate of heart failure,” said Melissa A. Lyle, MD, of the Mayo Clinic in Jacksonville, Fla., in an interview. “But the key takeaway is that sarcoidosis was associated with a higher rate of all-cause mortality compared to patients with heart failure and no sarcoidosis. That was more of a surprise.”
“There’s been some discrepancy in previous studies describing the cardiovascular outcomes in sarcoidosis,” Dr. Lyle added, “so I think this study provides excellent information while also highlighting the need for additional large-scale studies. We need to have further data on cardiovascular outcomes, which will allow us to refine the consensus statements and guidelines for management and the diagnosis of cardiac sarcoidosis.”
Dr. Lyle and Leslie T. Cooper Jr., MD, also of the Mayo Clinic, extrapolated on those thoughts in an editorial that accompanied the study. In it, the two authors praised the size and lengthy follow-up of the study, while noting its limitations. Specifically, they stressed that the study’s Danish population “may not be representative of other general populations” because of notable differences in ethnicity, age, and comorbidities.
That said, they reinforced that the study did feature “important takeaways” and that its findings emphasize the “need for monitoring for cardiac manifestations in patients with systemic sarcoidosis.”
In addition to the limitations noted in the editorial, the study’s authors acknowledged that the observational nature limited its “assessment of cause-effect relationships” and that the diagnosis codes for sarcoidosis had not been validated in the Danish National Patient Registry.
The authors of both the study and the editorial reported no conflicts of interest.
SOURCE: Yafasova A et al. J Am Coll Cardiol. 2020 Aug 10. doi: 10.1016/j.jacc.2020.06.038.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
FDA approves first oral treatment for spinal muscular atrophy
This marks the first approval of an oral therapy for the rare and devastating condition.
Risdiplam, marketed by Roche and PTC Therapeutics, provides “an important treatment option for patients with SMA, following the approval of the first treatment for this devastating disease less than 4 years ago,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a release from the agency.
The approval was based on the results from two trials. In the open-label FIREFISH study of infantile-onset SMA, 7 (41%) of the 17 participants (mean baseline age, 6.7 months) were able to sit independently for more than 5 seconds after 12 months of treatment with risdiplam. This was a “meaningful difference from the natural progression of the disease because all untreated infants with infantile-onset SMA cannot sit independently,” the FDA noted. In addition, 81% of the participants were alive after 23 or more months of treatment – and without need of permanent ventilation.
The second study was the randomized controlled trial known as SUNFISH and included 180 patients with SMA between the ages of 2 and 25 years. Those who received the study drug had an average 1.36 increase from baseline on a motor function measure versus a 0.19 decrease in function for those who received placebo.
The FDA noted that the most common treatment-related adverse events (AEs) include fever, diarrhea, rash, ulcers of the mouth, arthralgia, and urinary tract infections. Additional AEs reported in some patients with infantile-onset SMA included upper respiratory tract infection, pneumonia, constipation, and vomiting.
The drug received fast track designation and priority review from the FDA, as well as orphan drug designation.
‘Eagerly awaited’
“Today marks an incredibly important moment for the broader SMA patient community that had been in dire need of safe and effective treatment options,” Stuart W. Peltz, PhD, chief executive officer of PTC Therapeutics, said in a company statement.
“Given [that] the majority of people with SMA in the U.S. remain untreated, we believe Evrysdi, with its favorable clinical profile and oral administration, may offer meaningful benefits for many living with this rare neurological disease,” Levi Garraway, MD, PhD, chief medical officer and head of global product development for Genentech, added in the company’s press release. Genentech is a member of the Roche Group.
The drug is continuing to be studied in more than 450 individuals as part of a “large and robust clinical trial program in SMA,” the company reports. These participants are between the ages of 2 months and 60 years.
“The approval of Evrysdi is an eagerly awaited milestone for our community. We appreciate Genentech’s commitment to … developing a treatment that can be administered at home,” Kenneth Hobby, president of the nonprofit Cure SMA, said in the same release.
In May 2019, the FDA approved the first gene therapy for SMA – the infusion drug onasemnogene abeparvovec-xioi (Zolgensma, AveXis Inc).
Genentech announced that the new oral drug will be available in the United States within 2 weeks “for direct delivery to patients’ homes.”
A version of this article originally appeared on Medscape.com.
This marks the first approval of an oral therapy for the rare and devastating condition.
Risdiplam, marketed by Roche and PTC Therapeutics, provides “an important treatment option for patients with SMA, following the approval of the first treatment for this devastating disease less than 4 years ago,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a release from the agency.
The approval was based on the results from two trials. In the open-label FIREFISH study of infantile-onset SMA, 7 (41%) of the 17 participants (mean baseline age, 6.7 months) were able to sit independently for more than 5 seconds after 12 months of treatment with risdiplam. This was a “meaningful difference from the natural progression of the disease because all untreated infants with infantile-onset SMA cannot sit independently,” the FDA noted. In addition, 81% of the participants were alive after 23 or more months of treatment – and without need of permanent ventilation.
The second study was the randomized controlled trial known as SUNFISH and included 180 patients with SMA between the ages of 2 and 25 years. Those who received the study drug had an average 1.36 increase from baseline on a motor function measure versus a 0.19 decrease in function for those who received placebo.
The FDA noted that the most common treatment-related adverse events (AEs) include fever, diarrhea, rash, ulcers of the mouth, arthralgia, and urinary tract infections. Additional AEs reported in some patients with infantile-onset SMA included upper respiratory tract infection, pneumonia, constipation, and vomiting.
The drug received fast track designation and priority review from the FDA, as well as orphan drug designation.
‘Eagerly awaited’
“Today marks an incredibly important moment for the broader SMA patient community that had been in dire need of safe and effective treatment options,” Stuart W. Peltz, PhD, chief executive officer of PTC Therapeutics, said in a company statement.
“Given [that] the majority of people with SMA in the U.S. remain untreated, we believe Evrysdi, with its favorable clinical profile and oral administration, may offer meaningful benefits for many living with this rare neurological disease,” Levi Garraway, MD, PhD, chief medical officer and head of global product development for Genentech, added in the company’s press release. Genentech is a member of the Roche Group.
The drug is continuing to be studied in more than 450 individuals as part of a “large and robust clinical trial program in SMA,” the company reports. These participants are between the ages of 2 months and 60 years.
“The approval of Evrysdi is an eagerly awaited milestone for our community. We appreciate Genentech’s commitment to … developing a treatment that can be administered at home,” Kenneth Hobby, president of the nonprofit Cure SMA, said in the same release.
In May 2019, the FDA approved the first gene therapy for SMA – the infusion drug onasemnogene abeparvovec-xioi (Zolgensma, AveXis Inc).
Genentech announced that the new oral drug will be available in the United States within 2 weeks “for direct delivery to patients’ homes.”
A version of this article originally appeared on Medscape.com.
This marks the first approval of an oral therapy for the rare and devastating condition.
Risdiplam, marketed by Roche and PTC Therapeutics, provides “an important treatment option for patients with SMA, following the approval of the first treatment for this devastating disease less than 4 years ago,” Billy Dunn, MD, director of the Office of Neuroscience in the Center for Drug Evaluation and Research at the FDA, said in a release from the agency.
The approval was based on the results from two trials. In the open-label FIREFISH study of infantile-onset SMA, 7 (41%) of the 17 participants (mean baseline age, 6.7 months) were able to sit independently for more than 5 seconds after 12 months of treatment with risdiplam. This was a “meaningful difference from the natural progression of the disease because all untreated infants with infantile-onset SMA cannot sit independently,” the FDA noted. In addition, 81% of the participants were alive after 23 or more months of treatment – and without need of permanent ventilation.
The second study was the randomized controlled trial known as SUNFISH and included 180 patients with SMA between the ages of 2 and 25 years. Those who received the study drug had an average 1.36 increase from baseline on a motor function measure versus a 0.19 decrease in function for those who received placebo.
The FDA noted that the most common treatment-related adverse events (AEs) include fever, diarrhea, rash, ulcers of the mouth, arthralgia, and urinary tract infections. Additional AEs reported in some patients with infantile-onset SMA included upper respiratory tract infection, pneumonia, constipation, and vomiting.
The drug received fast track designation and priority review from the FDA, as well as orphan drug designation.
‘Eagerly awaited’
“Today marks an incredibly important moment for the broader SMA patient community that had been in dire need of safe and effective treatment options,” Stuart W. Peltz, PhD, chief executive officer of PTC Therapeutics, said in a company statement.
“Given [that] the majority of people with SMA in the U.S. remain untreated, we believe Evrysdi, with its favorable clinical profile and oral administration, may offer meaningful benefits for many living with this rare neurological disease,” Levi Garraway, MD, PhD, chief medical officer and head of global product development for Genentech, added in the company’s press release. Genentech is a member of the Roche Group.
The drug is continuing to be studied in more than 450 individuals as part of a “large and robust clinical trial program in SMA,” the company reports. These participants are between the ages of 2 months and 60 years.
“The approval of Evrysdi is an eagerly awaited milestone for our community. We appreciate Genentech’s commitment to … developing a treatment that can be administered at home,” Kenneth Hobby, president of the nonprofit Cure SMA, said in the same release.
In May 2019, the FDA approved the first gene therapy for SMA – the infusion drug onasemnogene abeparvovec-xioi (Zolgensma, AveXis Inc).
Genentech announced that the new oral drug will be available in the United States within 2 weeks “for direct delivery to patients’ homes.”
A version of this article originally appeared on Medscape.com.
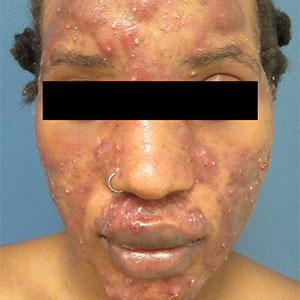
Large, painful facial cysts
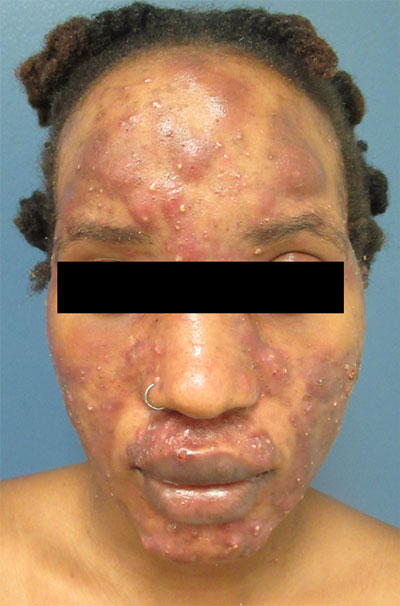
The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.

The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The abrupt onset of painful, violaceous coalescing papules, pustules, cysts, and nodules exclusively involving the centrofacial area with an overlying red-cyanotic erythema are the hallmarks of pyoderma faciale.
Pyoderma faciale is a rare disease that affects females in the second and third decades of life; 50% of these patients have a history of acne. The etiology of the condition remains unclear. Hormonal imbalance, inflammatory bowel disease, liver disease, and thyroid disease have been associated with the disorder. Ribavirin and interferon therapies for the treatment of hepatitis C along with high levels of vitamins (B6 and B12) have been identified as triggers. Culture of purulent drainage typically is sterile or may reveal commensal organisms.
The differential diagnosis includes acne fulminans and acne conglobata. Acne fulminans is not restricted to the face, as is pyoderma faciale, and it involves constitutional symptoms. Acne conglobata is a chronic process that affects males and females. It also involves purulent sinus tracts.
Prompt treatment of pyoderma faciale is essential to prevent widespread eruption, minimize the distress associated with the disfiguring nature of the disorder, and ultimately reduce scarring. Standard therapy consists of oral steroids (prednisone 1 mg/kg/d) for 1 week followed by a slow taper in combination with oral isotretinoin at a low dosage (0.2–0.5 mg/kg). The systemic retinoid is continued until all inflammatory lesions are healed.
In this case, a culture swab taken from the patient’s left cheek did not reveal any unexpected pathogens. The patient was started on oral doxycycline 100 mg bid and prednisone 50 mg/d tapered to 10 mg/d. She was counseled about the risks and benefits of isotretinoin and registered in the iPLEDGE system in anticipation of starting oral isotretinoin at 20 mg/d after negative pregnancy tests, 2 forms of contraception, and the 1-month qualification period.
Photo courtesy of Catherine N. Tchanque-Fossuo, MD, MS, and text courtesy of Catherine N. Tchanque-Fossuo, MD, MS, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.
Sharma RK, Pulimood S, Peter D, et al. A case report with review of literature on pyoderma faciale in pregnancy–a therapeutic dilemma. JDA Indian J Clin Dermatol. 2018;1:96-99.
ASCO says ‘no’ to home infusions of cancer treatment, with exceptions
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
in a new policy statement issued July 31.
At the same time, it supports exceptions: namely, when individual physicians and patients, having jointly discussed risks and benefits, agree to have treatments administered in the home.
The new policy is limited to intravenous infusions of anticancer agents such as chemotherapy, monoclonal antibodies, and other drugs — administered by health care personnel. It does not refer to injections.
The policy was prompted by regulatory flexibilities from the Centers for Medicare & Medicaid Services made in response to the accelerating COVID-19 pandemic. “Among these flexibilities were new provisions that enabled providers to deliver care in a setting most appropriate – and safest – for individual patient circumstances,” which has “opened the path for potential increases in use of home infusion for anticancer therapy,” says ASCO.
“We’re not ready to endorse [chemo at home] as a general policy until we have evidence that it’s safe. At the same time, the policy gives physicians and patients autonomy to respond to whatever situation they find themselves in,” Stephen Grubbs, MD, ASCO’s senior director of clinical affairs, said in an interview.
“Antineoplastic drugs are effective at treating cancer but can be extremely toxic to normal human cells,” reads the statement, which was written by a group of about 25 professionals, including Grubbs and other ASCO staff as well as independent advisers.
“There is a paucity of evidence directly comparing the safety of chemotherapy infusions in the home and outpatient settings,” the ASCO policy explains.
ASCO’s policy acknowledges that there are data “from other countries demonstrating that ... home infusion can be safe, well-tolerated, and may be preferred by some patients.” But such data are limited and only apply “to certain circumstances and for specific agents,” it adds.
One US cancer center (in Philadelphia) already has an established chemo-at-home program and has seen an increase in its use during the pandemic, as reported by Medscape Medical News. Approached for comment, Justin Bekelman, MD, director of the Penn Center for Cancer Care Innovation in Philadelphia, interpreted the new ASCO policy in a positive light.
“Physicians at the Abramson Cancer Center of the University of Pennsylvania and ASCO agree – home-based cancer therapy with oncologist oversight and well-designed safety protocols can be a safe option for patients with cancer,” he said in a statement.
ASCO says its existing safety standards “may be difficult to satisfy in the home infusion context,” including for safely resolving life-threatening emergencies.
Grubbs said that in the worst-case scenario, such as anaphylaxis, “you can die from [it] if you don’t manage it quickly and properly.”
“When I was practicing, we always had a physician present right next to the infusion area because these are severe reactions that happen very quickly,” he said, adding that “several a year” occurred when he practiced full-time.
Also, chemotherapy spills are a “big deal” in the home, as clean-up may be complex and difficult, added Grubbs.
Data from ASCO’s PracticeNET program show that in the first months (March and April) of the COVID-19 pandemic, chemotherapy visits to infusion suites were not reduced in a dataset of 16 US practices, he noted. However, there are exceptions and variance based on location, Grubbs said, such as “hot spots” including New York City in April.
While the pandemic has no end in sight, ASCO issued a set of six recommendations for use of anticancer therapies infused in the home. First, they call for independent, publicly funded research to evaluate the safety and effectiveness of home infusion of anticancer therapy.
Next in importance, ASCO wants the current temporary regulation change from CMS due to the pandemic to end.
“CMS should not extend the temporary flexibility related to home infusion for Part B cancer drugs that was approved as part of their response to the public health emergency,” they state.
Even before the pandemic, changes were afoot. Under the 21st Century Cures Act, which was passed in 2019 and will be implemented in 2021, CMS instituted a permanent home infusion therapy services benefit, which includes anticancer therapies. It “remains to be seen what, if any, shift away from outpatient infusion facilities will occur,” observes ASCO in its policy statement.
This article first appeared on Medscape.com.
Psychiatrists report rare case of woman who thinks she’s a chicken
LEUVEN, Belgium — A 54-year-old woman has suffered the delusion of thinking she is a chicken for 24 hours. This very rare condition, known as zoanthropy, in which people think they are an animal is often not recognised, say researchers from the University of Leuven.
Zoanthropy can include people believing they are, or behaving like, any kind of animal: from a dog, to a lion or tiger, crocodile, snake, or bee.
It’s important to recognise this as a potential symptom of something serious, say the researchers in the July issue of the Belgian Journal of Psychiatry, Tijdschrift voor Psychiatrie.
“Additional investigations with brain imaging and electroencephalogram are therefore advised,” say the authors.
Psychiatrists Need to Be Aware That Clinical Zoanthropy Exists
In their paper, they describe the case of the woman who briefly thought she was a chicken, which was followed by her having a generalized epileptic seizure.
“Clinically, we saw a lady who perspired profusely, trembled, blew up her cheeks, and ... seemed to imitate a chicken, [making noises] like clucking, cackling, and crowing like a rooster,” they say.
“After about 10 minutes she seemed to tighten her muscles for a few seconds, her face turned red and for a short time she didn’t react. These symptoms repeated themselves at intervals of a few minutes [and her] consciousness was fluctuating,” with the patient “disoriented in time and space.”
Lead author Dr Athena Beckers of University Psychiatric Centre, KU Leuven, Belgium, said in an interview with MediQuality: “With only 56 case descriptions in the medical literature from 1850 to the present day, the condition is rare. It amounts to about one description every 3 years.
“We suspect, however, that the delusion is not always noticed: the patient shows bizarre behaviour or makes animal sounds, it is probably often catalogued under the general term ‘psychosis’.”
Dr Beckers adds that it is important that the symptoms are recognised, because of the possible underlying causes which can include epilepsy. So this might require a different or complementary treatment “with, for example, antiepileptic drugs”.
“I myself have only seen this type of delusion once, but I ... heard anecdotal stories from other patients whose family member, for example with schizophrenia, sometimes thought he was a cow [during] ... a psychosis.
“After the publication of my article I was also contacted by someone who told me they had experienced the same thing 30 years ago – he thought he was a chicken.
“I think it’s a good thing that we psychiatrists are aware of the fact that clinical zoanthropy exists and may require additional research,” she observed.
Fortunately, this woman’s experience ended well. After about one year of disability, the patient was able to return to work progressively. Her mood remained stable and there were no more psychotic symptoms or any indication of epileptic episodes.
Such Delusions Are Rare
Dr Georges Otte, a recently retired neuropsychiatrist who formerly worked at Ghent University, Belgium, gave his thoughts to Mediquality: “The interface between neurology and psychiatry ... is a fertile meadow on which many crops thrive. But it is in the darkest corners of psychosis that one finds the most bizarre and also rarest excesses.”
There are a number of delusions of identity, said Dr Otte.
These include Cotard’s syndrome, a rare condition marked by the false belief that the person or their body parts are dead, dying, or don’t exist, or Capgras delusion, where the affected person believes that a spouse or close family member has been replaced with an imposter. Delusions can also occur as a result of substance abuse, for example after using psilocybin (magic mushrooms), he added.
“Delusions in which patients are convinced of ‘shape shifting’ (man to animal) are quite rare,” Dr Otte observed.
“In the literature we know that lycanthropy [a person thinks he or she is turning into a werewolf],” has been reported, and has “apparently inspired many authors of horror stories,” he added.
“But it’s not every day that as a psychiatrist, you will encounter such an extreme psychotic depersonalization as someone turning into a chicken.”
This article first appeared on Medscape.com.
LEUVEN, Belgium — A 54-year-old woman has suffered the delusion of thinking she is a chicken for 24 hours. This very rare condition, known as zoanthropy, in which people think they are an animal is often not recognised, say researchers from the University of Leuven.
Zoanthropy can include people believing they are, or behaving like, any kind of animal: from a dog, to a lion or tiger, crocodile, snake, or bee.
It’s important to recognise this as a potential symptom of something serious, say the researchers in the July issue of the Belgian Journal of Psychiatry, Tijdschrift voor Psychiatrie.
“Additional investigations with brain imaging and electroencephalogram are therefore advised,” say the authors.
Psychiatrists Need to Be Aware That Clinical Zoanthropy Exists
In their paper, they describe the case of the woman who briefly thought she was a chicken, which was followed by her having a generalized epileptic seizure.
“Clinically, we saw a lady who perspired profusely, trembled, blew up her cheeks, and ... seemed to imitate a chicken, [making noises] like clucking, cackling, and crowing like a rooster,” they say.
“After about 10 minutes she seemed to tighten her muscles for a few seconds, her face turned red and for a short time she didn’t react. These symptoms repeated themselves at intervals of a few minutes [and her] consciousness was fluctuating,” with the patient “disoriented in time and space.”
Lead author Dr Athena Beckers of University Psychiatric Centre, KU Leuven, Belgium, said in an interview with MediQuality: “With only 56 case descriptions in the medical literature from 1850 to the present day, the condition is rare. It amounts to about one description every 3 years.
“We suspect, however, that the delusion is not always noticed: the patient shows bizarre behaviour or makes animal sounds, it is probably often catalogued under the general term ‘psychosis’.”
Dr Beckers adds that it is important that the symptoms are recognised, because of the possible underlying causes which can include epilepsy. So this might require a different or complementary treatment “with, for example, antiepileptic drugs”.
“I myself have only seen this type of delusion once, but I ... heard anecdotal stories from other patients whose family member, for example with schizophrenia, sometimes thought he was a cow [during] ... a psychosis.
“After the publication of my article I was also contacted by someone who told me they had experienced the same thing 30 years ago – he thought he was a chicken.
“I think it’s a good thing that we psychiatrists are aware of the fact that clinical zoanthropy exists and may require additional research,” she observed.
Fortunately, this woman’s experience ended well. After about one year of disability, the patient was able to return to work progressively. Her mood remained stable and there were no more psychotic symptoms or any indication of epileptic episodes.
Such Delusions Are Rare
Dr Georges Otte, a recently retired neuropsychiatrist who formerly worked at Ghent University, Belgium, gave his thoughts to Mediquality: “The interface between neurology and psychiatry ... is a fertile meadow on which many crops thrive. But it is in the darkest corners of psychosis that one finds the most bizarre and also rarest excesses.”
There are a number of delusions of identity, said Dr Otte.
These include Cotard’s syndrome, a rare condition marked by the false belief that the person or their body parts are dead, dying, or don’t exist, or Capgras delusion, where the affected person believes that a spouse or close family member has been replaced with an imposter. Delusions can also occur as a result of substance abuse, for example after using psilocybin (magic mushrooms), he added.
“Delusions in which patients are convinced of ‘shape shifting’ (man to animal) are quite rare,” Dr Otte observed.
“In the literature we know that lycanthropy [a person thinks he or she is turning into a werewolf],” has been reported, and has “apparently inspired many authors of horror stories,” he added.
“But it’s not every day that as a psychiatrist, you will encounter such an extreme psychotic depersonalization as someone turning into a chicken.”
This article first appeared on Medscape.com.
LEUVEN, Belgium — A 54-year-old woman has suffered the delusion of thinking she is a chicken for 24 hours. This very rare condition, known as zoanthropy, in which people think they are an animal is often not recognised, say researchers from the University of Leuven.
Zoanthropy can include people believing they are, or behaving like, any kind of animal: from a dog, to a lion or tiger, crocodile, snake, or bee.
It’s important to recognise this as a potential symptom of something serious, say the researchers in the July issue of the Belgian Journal of Psychiatry, Tijdschrift voor Psychiatrie.
“Additional investigations with brain imaging and electroencephalogram are therefore advised,” say the authors.
Psychiatrists Need to Be Aware That Clinical Zoanthropy Exists
In their paper, they describe the case of the woman who briefly thought she was a chicken, which was followed by her having a generalized epileptic seizure.
“Clinically, we saw a lady who perspired profusely, trembled, blew up her cheeks, and ... seemed to imitate a chicken, [making noises] like clucking, cackling, and crowing like a rooster,” they say.
“After about 10 minutes she seemed to tighten her muscles for a few seconds, her face turned red and for a short time she didn’t react. These symptoms repeated themselves at intervals of a few minutes [and her] consciousness was fluctuating,” with the patient “disoriented in time and space.”
Lead author Dr Athena Beckers of University Psychiatric Centre, KU Leuven, Belgium, said in an interview with MediQuality: “With only 56 case descriptions in the medical literature from 1850 to the present day, the condition is rare. It amounts to about one description every 3 years.
“We suspect, however, that the delusion is not always noticed: the patient shows bizarre behaviour or makes animal sounds, it is probably often catalogued under the general term ‘psychosis’.”
Dr Beckers adds that it is important that the symptoms are recognised, because of the possible underlying causes which can include epilepsy. So this might require a different or complementary treatment “with, for example, antiepileptic drugs”.
“I myself have only seen this type of delusion once, but I ... heard anecdotal stories from other patients whose family member, for example with schizophrenia, sometimes thought he was a cow [during] ... a psychosis.
“After the publication of my article I was also contacted by someone who told me they had experienced the same thing 30 years ago – he thought he was a chicken.
“I think it’s a good thing that we psychiatrists are aware of the fact that clinical zoanthropy exists and may require additional research,” she observed.
Fortunately, this woman’s experience ended well. After about one year of disability, the patient was able to return to work progressively. Her mood remained stable and there were no more psychotic symptoms or any indication of epileptic episodes.
Such Delusions Are Rare
Dr Georges Otte, a recently retired neuropsychiatrist who formerly worked at Ghent University, Belgium, gave his thoughts to Mediquality: “The interface between neurology and psychiatry ... is a fertile meadow on which many crops thrive. But it is in the darkest corners of psychosis that one finds the most bizarre and also rarest excesses.”
There are a number of delusions of identity, said Dr Otte.
These include Cotard’s syndrome, a rare condition marked by the false belief that the person or their body parts are dead, dying, or don’t exist, or Capgras delusion, where the affected person believes that a spouse or close family member has been replaced with an imposter. Delusions can also occur as a result of substance abuse, for example after using psilocybin (magic mushrooms), he added.
“Delusions in which patients are convinced of ‘shape shifting’ (man to animal) are quite rare,” Dr Otte observed.
“In the literature we know that lycanthropy [a person thinks he or she is turning into a werewolf],” has been reported, and has “apparently inspired many authors of horror stories,” he added.
“But it’s not every day that as a psychiatrist, you will encounter such an extreme psychotic depersonalization as someone turning into a chicken.”
This article first appeared on Medscape.com.
IV gentamicin improves epidermolysis bullosa
In a pilot study, , Michelle Hao said at the virtual annual meeting of the American Academy of Dermatology.
Serial skin biopsies and immunofluorescent staining demonstrated the mechanism of benefit: The aminoglycoside promoted creation of new full-length functional collagen fibrils at the dermal-epidermal junction in affected patients, added Ms. Hao, a medical student at the University of Southern California, Los Angeles.
“Glycoside-mediated nonsense suppression therapy may provide a novel, low cost, and readily available treatment for RDEB [recessive dystrophic epidermolysis bullosa] patients harboring nonsense mutations,” she declared.
RDEB is a rare, incurable, life-threatening genetic skin disease which manifests as severe skin fragility and widespread blistering. The disease is caused by mutations in a gene coding for collagen type VII alpha 1 (COL7A1), the building block for the anchoring fibrils responsible for dermal-epidermal adherence. Roughly 30% of COL7A1 mutations are nonsense mutations, which result in truncated, nonfunctional collagen type VII.
Ms. Hao and her senior coinvestigators have previously shown that aminoglycoside antibiotics can override nonsense mutations to produce full-length, functioning protein. Indeed, they demonstrated that topical gentamicin in particular induces formation of new collagen type VII and improves wound closure in RDEB patients with nonsense mutations. However, RDEB skin lesions are so widespread that topical therapy becomes impractical. This was the impetus for the phase 1/2 clinical trial of IV gentamicin.
The open-label study included four patients with RDEB with nonsense mutations. All participants received IV gentamicin at 7.5 mg/kg/day for 2 weeks. Two of the four patients then got additional twice-weekly infusions at the same dose for another 3 months. Skin biopsies were obtained from two prospectively monitored open erosive wound sites and two intact skin sites at baseline and 1 and 3 months after treatment.
The primary endpoint was evidence of new collagen type VII at the dermal-epidermal junction post treatment. At baseline, patients averaged only 2% of the amount present in normal skin. One month post treatment, all four patients showed significant gains in expression of functioning collagen type VII, with levels 30%-130% of what’s present in normal skin. This effect proved durable 3 months post treatment.
At the same visits when biopsies were obtained, participants were assessed regarding wound closure, disease activity as measured using the validated Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), and quality of life as reflected in Skindex-16 scores. All four patients showed improved wound closure at 1 and 3 months post treatment at the monitored sites, as well as better EBDASI and Skindex-16 Symptoms and Skindex-16 Emotion scores, Ms. Hao continued.
Safety assessments revealed no evidence of oto- or nephrotoxicity in the gentamicin-treated patients. And no one developed autoantibodies to collagen type VII in skin or sera in response to the aminoglycoside-induced creation of new collagen type VII.
Ms. Hao said preliminary analysis of the study data suggests that the more convenient schedule of twice-weekly IV gentamicin was as effective with regard to wound closure as daily infusion therapy.
She reported having no financial conflicts regarding the study, supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the EB Research Partnership, and the EB Research Foundation.
In a pilot study, , Michelle Hao said at the virtual annual meeting of the American Academy of Dermatology.
Serial skin biopsies and immunofluorescent staining demonstrated the mechanism of benefit: The aminoglycoside promoted creation of new full-length functional collagen fibrils at the dermal-epidermal junction in affected patients, added Ms. Hao, a medical student at the University of Southern California, Los Angeles.
“Glycoside-mediated nonsense suppression therapy may provide a novel, low cost, and readily available treatment for RDEB [recessive dystrophic epidermolysis bullosa] patients harboring nonsense mutations,” she declared.
RDEB is a rare, incurable, life-threatening genetic skin disease which manifests as severe skin fragility and widespread blistering. The disease is caused by mutations in a gene coding for collagen type VII alpha 1 (COL7A1), the building block for the anchoring fibrils responsible for dermal-epidermal adherence. Roughly 30% of COL7A1 mutations are nonsense mutations, which result in truncated, nonfunctional collagen type VII.
Ms. Hao and her senior coinvestigators have previously shown that aminoglycoside antibiotics can override nonsense mutations to produce full-length, functioning protein. Indeed, they demonstrated that topical gentamicin in particular induces formation of new collagen type VII and improves wound closure in RDEB patients with nonsense mutations. However, RDEB skin lesions are so widespread that topical therapy becomes impractical. This was the impetus for the phase 1/2 clinical trial of IV gentamicin.
The open-label study included four patients with RDEB with nonsense mutations. All participants received IV gentamicin at 7.5 mg/kg/day for 2 weeks. Two of the four patients then got additional twice-weekly infusions at the same dose for another 3 months. Skin biopsies were obtained from two prospectively monitored open erosive wound sites and two intact skin sites at baseline and 1 and 3 months after treatment.
The primary endpoint was evidence of new collagen type VII at the dermal-epidermal junction post treatment. At baseline, patients averaged only 2% of the amount present in normal skin. One month post treatment, all four patients showed significant gains in expression of functioning collagen type VII, with levels 30%-130% of what’s present in normal skin. This effect proved durable 3 months post treatment.
At the same visits when biopsies were obtained, participants were assessed regarding wound closure, disease activity as measured using the validated Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), and quality of life as reflected in Skindex-16 scores. All four patients showed improved wound closure at 1 and 3 months post treatment at the monitored sites, as well as better EBDASI and Skindex-16 Symptoms and Skindex-16 Emotion scores, Ms. Hao continued.
Safety assessments revealed no evidence of oto- or nephrotoxicity in the gentamicin-treated patients. And no one developed autoantibodies to collagen type VII in skin or sera in response to the aminoglycoside-induced creation of new collagen type VII.
Ms. Hao said preliminary analysis of the study data suggests that the more convenient schedule of twice-weekly IV gentamicin was as effective with regard to wound closure as daily infusion therapy.
She reported having no financial conflicts regarding the study, supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the EB Research Partnership, and the EB Research Foundation.
In a pilot study, , Michelle Hao said at the virtual annual meeting of the American Academy of Dermatology.
Serial skin biopsies and immunofluorescent staining demonstrated the mechanism of benefit: The aminoglycoside promoted creation of new full-length functional collagen fibrils at the dermal-epidermal junction in affected patients, added Ms. Hao, a medical student at the University of Southern California, Los Angeles.
“Glycoside-mediated nonsense suppression therapy may provide a novel, low cost, and readily available treatment for RDEB [recessive dystrophic epidermolysis bullosa] patients harboring nonsense mutations,” she declared.
RDEB is a rare, incurable, life-threatening genetic skin disease which manifests as severe skin fragility and widespread blistering. The disease is caused by mutations in a gene coding for collagen type VII alpha 1 (COL7A1), the building block for the anchoring fibrils responsible for dermal-epidermal adherence. Roughly 30% of COL7A1 mutations are nonsense mutations, which result in truncated, nonfunctional collagen type VII.
Ms. Hao and her senior coinvestigators have previously shown that aminoglycoside antibiotics can override nonsense mutations to produce full-length, functioning protein. Indeed, they demonstrated that topical gentamicin in particular induces formation of new collagen type VII and improves wound closure in RDEB patients with nonsense mutations. However, RDEB skin lesions are so widespread that topical therapy becomes impractical. This was the impetus for the phase 1/2 clinical trial of IV gentamicin.
The open-label study included four patients with RDEB with nonsense mutations. All participants received IV gentamicin at 7.5 mg/kg/day for 2 weeks. Two of the four patients then got additional twice-weekly infusions at the same dose for another 3 months. Skin biopsies were obtained from two prospectively monitored open erosive wound sites and two intact skin sites at baseline and 1 and 3 months after treatment.
The primary endpoint was evidence of new collagen type VII at the dermal-epidermal junction post treatment. At baseline, patients averaged only 2% of the amount present in normal skin. One month post treatment, all four patients showed significant gains in expression of functioning collagen type VII, with levels 30%-130% of what’s present in normal skin. This effect proved durable 3 months post treatment.
At the same visits when biopsies were obtained, participants were assessed regarding wound closure, disease activity as measured using the validated Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI), and quality of life as reflected in Skindex-16 scores. All four patients showed improved wound closure at 1 and 3 months post treatment at the monitored sites, as well as better EBDASI and Skindex-16 Symptoms and Skindex-16 Emotion scores, Ms. Hao continued.
Safety assessments revealed no evidence of oto- or nephrotoxicity in the gentamicin-treated patients. And no one developed autoantibodies to collagen type VII in skin or sera in response to the aminoglycoside-induced creation of new collagen type VII.
Ms. Hao said preliminary analysis of the study data suggests that the more convenient schedule of twice-weekly IV gentamicin was as effective with regard to wound closure as daily infusion therapy.
She reported having no financial conflicts regarding the study, supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the EB Research Partnership, and the EB Research Foundation.
FROM AAD 2020
Terlipressin squeaks by FDA review for hepatorenal syndrome 1
The Food and Drug Administration’s Cardiovascular and Renal Drugs Advisory Committee narrowly recommended, by an 8-7 vote, that the agency grant marketing approval to terlipressin for the treatment of hepatorenal syndrome type 1, a severe, rare, and often rapidly lethal disease. No drugs are currently licensed in the United States for this indication.
The advisory committee’s discussion and vote on July 15 showcased the struggle the 15 members faced parsing data that hinted at efficacy but also featured clear flaws and limitations, with meager evidence showing clinically meaningful patient improvements.
Several advisory committee members voiced their dilemma balancing the desperation of patients and clinicians to have an effective agent to treat a frequently fatal condition against spotty evidence of efficacy.
Their uncertainty over benefit was exacerbated by the substantial rate of serious adverse events, compared with placebo. These events included respiratory failure, which occurred an absolute 9% more often among patients treated with terlipressin than among those who received placebo in the drug’s recent pivotal trial, and sepsis and septic shock, with an absolute 7% excess rate with terlipressin in comparison with placebo.
“This is an important, unmet need, and I want this drug, but the data are not clear that the benefits outweigh the risks,” commented Steven F. Solga, MD, a transplant hepatologist at the University of Pennsylvania, Philadelphia, who is a committee member.
“When you have sick patients with few treatment options, you grope for something to use, but I worry that this won’t help patients,” he said when explaining his vote against approval.
“I look forward to using this medication if I could figure out which patients could benefit from it,” he said.
‘Allow patients to decide if they want this treatment’
Experts estimate that the annual incidence of hepatorenal syndrome type 1 in the United States is about 35,000 patients.
“I would have liked to vote yes, because terlipressin was associated with a short-term increase in renal function, but there was also clear evidence for the risk of sepsis and respiratory failure, and no evidence that it improved survival,” said panel member Patrick H. Nachman, MD.
Dr. Nachman, professor of medicine and director of the division of nephrology and hypertension at the University of Minnesota, Minneapolis, voted against approval.
Several who voted in favor of terlipressin also shared these misgivings.
“The trend for benefit was quite small, I’m very worried about respiratory failure, and I’m uncomfortable with the postrandomization analyses” used by the developer of terlipressin (Mallinckrodt) to buttress the efficacy claims, explained panel member Paul M. Ridker, MD.
“So why did I vote yes? The problem is the enormous unmet need. Patients are in desperate shape, and the standard treatments are used off label, with no data. Here, we have data, and the primary endpoint was met,” said Dr. Ridker, who is professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
The effects of terlipressin appear to give clinicians a way to “stabilize renal function and buy time,” making it more feasible to try to rush the patient to liver transplantation or at least to “stop their downward spiral» as patients with decompensated liver failure develop inadequate renal blood flow that produces an acute fall in kidney function,” explained David N. Assis, MD, a hepatologist at Yale University, New Haven, Conn.
“The reality is, nothing else is available, aside from renal replacement therapy and pressors. There is a need for a treatment that buys time,” he said. He voted to recommend approval.
That sentiment was notably echoed in comments from the two nonclinical members of the advisory committee.
“This treatment addresses a major gap in care,” said Jacqueline D. Alikhaani, the panel’s consumer representative. “Allow patients to decide if they want this treatment,” said Daniel Bonner, the committee’s patient representative. Both voted in favor of FDA approval.
Terlipressin has been a long-standing linchpin for treating hepatorenal syndrome type 1 in Europe and other places outside the United States and Canada.
The most recent guidelines for managing patients with decompensated cirrhosis from the European Association for the Study of the Liver say that “[t]erlipressin plus albumin should be considered as the first-line therapeutic option for the treatment of hepatorenal syndrome and acute kidney injury” (J Hepatol. 2018 Aug 1;69).
According to company representatives who presented the case for terlipressin during the meeting, bringing the drug onto the U.S. market has been a 17-year journey, featuring three sequential trials.
- A 112-patient that the FDA accepted as the first of the two supportive trials needed for approval.
- A with 196 patients that tested terlipressin plus albumin against placebo plus albumin and showed a nominal benefit from terlipressin that failed to achieve statistical significance.
- The most recent trial, , which directly led to the advisory committee session. That trial enrolled 300 patients and met its primary endpoint. Data have not yet been published but have been at meetings.
One of the sources of controversy over the benefit from terlipressin centered on the primary endpoint used in CONFIRM, which required that the patient have two consecutive, low readings for serum creatinine, with levels no greater than 1.5 mg/dL while on treatment, and remain alive and free from need for renal replacement therapy for at least 10 days beyond this.
The FDA agreed to accept this as a primary endpoint but nonetheless considered it a surrogate.
According to FDA staffers who presented their take on the application, the agency accepted this primary endpoint “with the understanding that favorable trends in clinical outcomes, thought to be predicted by successful treatment of hepatic renal syndrome type 1, would be expected.”
The lack of many favorable trends in clinical outcomes helped foster the advisory committee’s divided response. The FDA’s staff uses its discretion when considering an advisory committee’s recommendations and making a final determination.
None of the advisory committee members disclosed any relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration’s Cardiovascular and Renal Drugs Advisory Committee narrowly recommended, by an 8-7 vote, that the agency grant marketing approval to terlipressin for the treatment of hepatorenal syndrome type 1, a severe, rare, and often rapidly lethal disease. No drugs are currently licensed in the United States for this indication.
The advisory committee’s discussion and vote on July 15 showcased the struggle the 15 members faced parsing data that hinted at efficacy but also featured clear flaws and limitations, with meager evidence showing clinically meaningful patient improvements.
Several advisory committee members voiced their dilemma balancing the desperation of patients and clinicians to have an effective agent to treat a frequently fatal condition against spotty evidence of efficacy.
Their uncertainty over benefit was exacerbated by the substantial rate of serious adverse events, compared with placebo. These events included respiratory failure, which occurred an absolute 9% more often among patients treated with terlipressin than among those who received placebo in the drug’s recent pivotal trial, and sepsis and septic shock, with an absolute 7% excess rate with terlipressin in comparison with placebo.
“This is an important, unmet need, and I want this drug, but the data are not clear that the benefits outweigh the risks,” commented Steven F. Solga, MD, a transplant hepatologist at the University of Pennsylvania, Philadelphia, who is a committee member.
“When you have sick patients with few treatment options, you grope for something to use, but I worry that this won’t help patients,” he said when explaining his vote against approval.
“I look forward to using this medication if I could figure out which patients could benefit from it,” he said.
‘Allow patients to decide if they want this treatment’
Experts estimate that the annual incidence of hepatorenal syndrome type 1 in the United States is about 35,000 patients.
“I would have liked to vote yes, because terlipressin was associated with a short-term increase in renal function, but there was also clear evidence for the risk of sepsis and respiratory failure, and no evidence that it improved survival,” said panel member Patrick H. Nachman, MD.
Dr. Nachman, professor of medicine and director of the division of nephrology and hypertension at the University of Minnesota, Minneapolis, voted against approval.
Several who voted in favor of terlipressin also shared these misgivings.
“The trend for benefit was quite small, I’m very worried about respiratory failure, and I’m uncomfortable with the postrandomization analyses” used by the developer of terlipressin (Mallinckrodt) to buttress the efficacy claims, explained panel member Paul M. Ridker, MD.
“So why did I vote yes? The problem is the enormous unmet need. Patients are in desperate shape, and the standard treatments are used off label, with no data. Here, we have data, and the primary endpoint was met,” said Dr. Ridker, who is professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
The effects of terlipressin appear to give clinicians a way to “stabilize renal function and buy time,” making it more feasible to try to rush the patient to liver transplantation or at least to “stop their downward spiral» as patients with decompensated liver failure develop inadequate renal blood flow that produces an acute fall in kidney function,” explained David N. Assis, MD, a hepatologist at Yale University, New Haven, Conn.
“The reality is, nothing else is available, aside from renal replacement therapy and pressors. There is a need for a treatment that buys time,” he said. He voted to recommend approval.
That sentiment was notably echoed in comments from the two nonclinical members of the advisory committee.
“This treatment addresses a major gap in care,” said Jacqueline D. Alikhaani, the panel’s consumer representative. “Allow patients to decide if they want this treatment,” said Daniel Bonner, the committee’s patient representative. Both voted in favor of FDA approval.
Terlipressin has been a long-standing linchpin for treating hepatorenal syndrome type 1 in Europe and other places outside the United States and Canada.
The most recent guidelines for managing patients with decompensated cirrhosis from the European Association for the Study of the Liver say that “[t]erlipressin plus albumin should be considered as the first-line therapeutic option for the treatment of hepatorenal syndrome and acute kidney injury” (J Hepatol. 2018 Aug 1;69).
According to company representatives who presented the case for terlipressin during the meeting, bringing the drug onto the U.S. market has been a 17-year journey, featuring three sequential trials.
- A 112-patient that the FDA accepted as the first of the two supportive trials needed for approval.
- A with 196 patients that tested terlipressin plus albumin against placebo plus albumin and showed a nominal benefit from terlipressin that failed to achieve statistical significance.
- The most recent trial, , which directly led to the advisory committee session. That trial enrolled 300 patients and met its primary endpoint. Data have not yet been published but have been at meetings.
One of the sources of controversy over the benefit from terlipressin centered on the primary endpoint used in CONFIRM, which required that the patient have two consecutive, low readings for serum creatinine, with levels no greater than 1.5 mg/dL while on treatment, and remain alive and free from need for renal replacement therapy for at least 10 days beyond this.
The FDA agreed to accept this as a primary endpoint but nonetheless considered it a surrogate.
According to FDA staffers who presented their take on the application, the agency accepted this primary endpoint “with the understanding that favorable trends in clinical outcomes, thought to be predicted by successful treatment of hepatic renal syndrome type 1, would be expected.”
The lack of many favorable trends in clinical outcomes helped foster the advisory committee’s divided response. The FDA’s staff uses its discretion when considering an advisory committee’s recommendations and making a final determination.
None of the advisory committee members disclosed any relevant financial relationships.
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration’s Cardiovascular and Renal Drugs Advisory Committee narrowly recommended, by an 8-7 vote, that the agency grant marketing approval to terlipressin for the treatment of hepatorenal syndrome type 1, a severe, rare, and often rapidly lethal disease. No drugs are currently licensed in the United States for this indication.
The advisory committee’s discussion and vote on July 15 showcased the struggle the 15 members faced parsing data that hinted at efficacy but also featured clear flaws and limitations, with meager evidence showing clinically meaningful patient improvements.
Several advisory committee members voiced their dilemma balancing the desperation of patients and clinicians to have an effective agent to treat a frequently fatal condition against spotty evidence of efficacy.
Their uncertainty over benefit was exacerbated by the substantial rate of serious adverse events, compared with placebo. These events included respiratory failure, which occurred an absolute 9% more often among patients treated with terlipressin than among those who received placebo in the drug’s recent pivotal trial, and sepsis and septic shock, with an absolute 7% excess rate with terlipressin in comparison with placebo.
“This is an important, unmet need, and I want this drug, but the data are not clear that the benefits outweigh the risks,” commented Steven F. Solga, MD, a transplant hepatologist at the University of Pennsylvania, Philadelphia, who is a committee member.
“When you have sick patients with few treatment options, you grope for something to use, but I worry that this won’t help patients,” he said when explaining his vote against approval.
“I look forward to using this medication if I could figure out which patients could benefit from it,” he said.
‘Allow patients to decide if they want this treatment’
Experts estimate that the annual incidence of hepatorenal syndrome type 1 in the United States is about 35,000 patients.
“I would have liked to vote yes, because terlipressin was associated with a short-term increase in renal function, but there was also clear evidence for the risk of sepsis and respiratory failure, and no evidence that it improved survival,” said panel member Patrick H. Nachman, MD.
Dr. Nachman, professor of medicine and director of the division of nephrology and hypertension at the University of Minnesota, Minneapolis, voted against approval.
Several who voted in favor of terlipressin also shared these misgivings.
“The trend for benefit was quite small, I’m very worried about respiratory failure, and I’m uncomfortable with the postrandomization analyses” used by the developer of terlipressin (Mallinckrodt) to buttress the efficacy claims, explained panel member Paul M. Ridker, MD.
“So why did I vote yes? The problem is the enormous unmet need. Patients are in desperate shape, and the standard treatments are used off label, with no data. Here, we have data, and the primary endpoint was met,” said Dr. Ridker, who is professor of medicine at Harvard Medical School and director of the Center for Cardiovascular Disease Prevention at Brigham and Women’s Hospital, both in Boston.
The effects of terlipressin appear to give clinicians a way to “stabilize renal function and buy time,” making it more feasible to try to rush the patient to liver transplantation or at least to “stop their downward spiral» as patients with decompensated liver failure develop inadequate renal blood flow that produces an acute fall in kidney function,” explained David N. Assis, MD, a hepatologist at Yale University, New Haven, Conn.
“The reality is, nothing else is available, aside from renal replacement therapy and pressors. There is a need for a treatment that buys time,” he said. He voted to recommend approval.
That sentiment was notably echoed in comments from the two nonclinical members of the advisory committee.
“This treatment addresses a major gap in care,” said Jacqueline D. Alikhaani, the panel’s consumer representative. “Allow patients to decide if they want this treatment,” said Daniel Bonner, the committee’s patient representative. Both voted in favor of FDA approval.
Terlipressin has been a long-standing linchpin for treating hepatorenal syndrome type 1 in Europe and other places outside the United States and Canada.
The most recent guidelines for managing patients with decompensated cirrhosis from the European Association for the Study of the Liver say that “[t]erlipressin plus albumin should be considered as the first-line therapeutic option for the treatment of hepatorenal syndrome and acute kidney injury” (J Hepatol. 2018 Aug 1;69).
According to company representatives who presented the case for terlipressin during the meeting, bringing the drug onto the U.S. market has been a 17-year journey, featuring three sequential trials.
- A 112-patient that the FDA accepted as the first of the two supportive trials needed for approval.
- A with 196 patients that tested terlipressin plus albumin against placebo plus albumin and showed a nominal benefit from terlipressin that failed to achieve statistical significance.
- The most recent trial, , which directly led to the advisory committee session. That trial enrolled 300 patients and met its primary endpoint. Data have not yet been published but have been at meetings.
One of the sources of controversy over the benefit from terlipressin centered on the primary endpoint used in CONFIRM, which required that the patient have two consecutive, low readings for serum creatinine, with levels no greater than 1.5 mg/dL while on treatment, and remain alive and free from need for renal replacement therapy for at least 10 days beyond this.
The FDA agreed to accept this as a primary endpoint but nonetheless considered it a surrogate.
According to FDA staffers who presented their take on the application, the agency accepted this primary endpoint “with the understanding that favorable trends in clinical outcomes, thought to be predicted by successful treatment of hepatic renal syndrome type 1, would be expected.”
The lack of many favorable trends in clinical outcomes helped foster the advisory committee’s divided response. The FDA’s staff uses its discretion when considering an advisory committee’s recommendations and making a final determination.
None of the advisory committee members disclosed any relevant financial relationships.
A version of this article originally appeared on Medscape.com.
New hope for ALS
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
. Both studies investigated potential benefits of suppressing the toxic activity in cells of a mutant gene (SOD1) that encodes superoxide dismutase 1 (SOD1) in patients with ALS.
One study investigated the antisense oligonucleotide (ASO) tofersen (Biogen); the other study examined viral vector–mediated gene suppression.
The studies’ promising results signal “the beginning of a new precision medicine–based approach towards treating ALS,” said Orla Hardiman, BSc, MB, BCh, BAO, MD, a consultant neurologist and professor of neurology at Trinity College and Beaumont Hospital in Dublin, Ireland. Dr. Hardiman co-authored an editorial that accompanied the two studies, which were published July 9 in the New England Journal of Medicine.
Genetic culprits
ALS is a disorder of progressive degeneration of upper and lower motor neurons. It typically leads to death from ventilatory failure within 5 years of symptom onset.
Genetic factors are responsible for about half the risk variance of ALS. In populations of European origin, variants in SOD1 account for an estimated 13% to 20% of familial ALS, although this rate varies around the world. Although SOD1 is not the most common variant in ALS, it is the one that researchers are most familiar with and has been studied in an animal model.
In the first study, investigators evaluated the safety, pharmacokinetics, and pharmacodynamics of the ASO tofersen in adults with ALS.
An ASO is a small piece of nucleic acid that enters neurons in the spinal cord and brain, explained co-investigator Toby A. Ferguson, MD, PhD, vice president and head of the neuromuscular development unit at Biogen.
ASO binds to the SOD1 gene and knocks down the SOD1 protein, which is the “toxic engine [that] drives the disease, kills neurons, and causes patients to have loss of function and eventually to die,” said Dr. Ferguson. “The ASO turns off the motor that produces that toxic protein,” he added.
Animal studies have shown that ASOs that target SOD1 messenger RNA transcripts prolong survival, improve motor performance, and reduce SOD1 protein concentrations.
The new phase 1/2 double-blind study included 50 adults at 18 sites in the United States, Canada, and four European countries. All had muscle weakness attributed to ALS and a documented SOD1 mutation. Participants were randomly assigned to receive one of four doses of tofersen—20, 40, 60, or 100 mg—or placebo. Treatment was administered via a lumbar intrathecal bolus injection. The study included a screening period followed by a 12-week intervention period and a 12-week follow-up.
Adverse events
A primary outcome was the incidence of adverse events (AEs) and serious AEs. Results showed that all participants reported one or more AEs. The most common AEs were headache, pain at the injection site, post–lumbar puncture syndrome, and falls. Three deaths occurred, one in the placebo group, one in the 20-mg dose group, and one in the 60-mg dose group. There were no serious AEs in the 100-mg group.
Although the investigators found an increase in cerebrospinal fluid (CSF) protein and white cell counts, there was no clear association between these observations and higher doses of tofersen or longer duration of exposure.
“We don’t know the implications of this, and it’s something we need to keep an eye on as we move these studies forward,” Dr. Ferguson said.
None of the AEs or CSF abnormalities led to trial discontinuation.
A secondary outcome was change in SOD1 protein concentration in CSF at day 85. The study showed that SOD1 concentrations decreased by 36% among the participants who received tofersen 100 mg and by lesser amounts in the patients who received lower doses. Concentrations in the placebo group were reduced by 3%.
The 36% reduction in the highest dose group is likely meaningful and “foundational to the concept of what this molecule can do,” Dr. Ferguson said.
“If the number one cause of SOD1 ALS is accumulation of toxic SOD1 protein, then the demonstration that we can reduce SOD1 protein in the CSF ... is saying that’s the first step on the way to showing the molecule is doing what it should do,” he added.
Emerging tool
In patients with ALS, neurofilament concentrations typically increase as the disease progresses. However, this study documented a reduction in these CSF concentrations. “One interpretation of that could be that there is less neurodegeneration or neuro injury” in patients treated with tofersen, Dr. Ferguson said.
He noted that neurofilament is “an emerging tool” for understanding neurodegeneration. It could also “be another sort of biochemical signal that the molecule is doing something important,” he added.
However, he noted that neurofilament concentration is still an exploratory marker.
Exploratory analyses suggested a possible slowing of functional loss, as measured by the ALS Functional Rating Scale–Revised (ALSFRS-R) score and the handheld dynamometry megascore. The latter assesses strength in 16 muscle groups in the arms and legs. The investigators noted that no conclusions can be drawn from these outcomes.
A post hoc analysis showed that among patients with SOD1 mutations associated with a fast-progressing disease course, the slope of clinical decline might have been gentler, and there was a greater decrease in CSF neurofilament concentration compared among those whose disease followed a slower course.
This suggests that “if you pick the right target,” even patients with severe disease can be treated, Dr. Ferguson said.
He acknowledged that in a relatively short study such as this one, it may be easier to see benefits in patients whose disease is progressing rapidly. However, he’s convinced that the treatment “would work for all SOD1 ALS patients, not just fast patients.”
Dr. Ferguson said the study investigators are encouraged by the new data, which “really suggest that we may be developing a meaningful treatment for SOD1 ALS.” However, “it’s still early” in terms of rolling out this therapy for patients with ALS, he said.
The safety and efficacy of tofersen are currently being evaluated in a phase 3, randomized, double-blind, placebo-controlled trial.
Limitations of the current study were the small number of participants, the short duration of treatment and follow-up, the exploratory nature of efficacy outcomes, and the post hoc methods for defining the fast-progressing subgroup.
Although an advantage of tofersen is that it can enter the nucleus of the cell, perhaps boosting effectiveness, a drawback might be that patients need several treatments administered via lumbar puncture. Following three initial doses, the drug is given every month.
An alternative approach might be a viral vector approach.
“Stunning” finding
In the second study, investigators assessed the safety of a single intrathecal infusion of a viral vector therapy designed to target SOD1 in two patients with familial ALS. The two patients were a 22-year-old man whose mother had died of ALS at age 45 and a 56-year-old man who had a family history of ALS.
The aim of the viral vector therapy is to continually suppress mutant gene activity, said study co-investigator Robert H. Brown, Jr, MD, professor of neurology, University of Massachusetts Medical School, Worcester.
“The virus essentially drops off a piece of DNA, and that DNA keeps making the agent that suppresses the gene,” Dr. Brown said.
He noted that the first patient had a mutation that causes a rapidly developing, “horribly devastating” disease.
Initially, the patient’s right leg, in which movement had been worsening over several weeks, “seemed to get stronger and remain strong for quite a long time. I’ve never seen that in this kind of mutation,” said Dr. Brown.
The patient died of ALS. At autopsy, there was evidence of suppression of SOD1 in the spinal cord. There was some preservation of motor neurons on the right side of the spinal cord, which Dr. Brown called a “stunning” finding.
“We have never seen preservation of motor neurons in an autopsy of a patient with this kind of mutation before,” he said.
Prior to the patient’s death, there were some initial signs of a decrease of SOD1 in CSF. However, the patient developed an inflammatory response in the lining of the CSF known as meningoradiculitis.
“In that setting, the SOD1 level went back up, so we could not say that we produced a significant lasting decrease,” Dr. Brown said.
One and done
Because meningoradiculitis occurred in the first patient, immunosuppressive drugs were administered to the second patient.
The functional status and vital capacity of the second patient were relatively stable during a 60-week period, a course that could be typical of the slow disease progression in patients with this SOD1 genotype.
As with the first patient, this man did not experience a substantial change in SOD1 protein levels in CSF, and he did not show clinical improvement.
The main advantage of a viral gene therapy is that it could be a one-time treatment; ideally, it could be used to replace a single missing gene in conditions such as cystic fibrosis. “The hope is that the virus will drop off the gene modulator or the gene itself of interest, depending on the disease, and that the gene will be there more or less indefinitely,” said Dr. Brown. “So the cliché is, ‘one and done’—if all goes well.”
This small study illustrates that gene therapy safely “turns off genes and that the extent of suppression of genes can be significant,” said Dr. Brown.
Most SOD1 mutations could be treated with this microRNA viral vector, he added. More than 180 such mutations have been identified in ALS.
Additional studies are now needed to determine the results of this method in a larger number of patients who have ALS with SOD1 mutations, the investigators wrote.
Within reach
Both studies are encouraging in that they show that a precision-medicine approach to ALS associated with single mutated genes “may be within reach,” said Dr. Hardiman.
She noted that gene therapies have been used successfully in other motor neuron conditions. For example, an ASO and a viral vector have “very significant efficacy” in a form of spinal muscular atrophy that occurs in infants. “So the underlying proof of principle is already there.”
The reduction in SOD1 levels among the highest-dose tofersen group in the first study indicates “target engagement,” Dr. Hardiman said.
In that study, the documented decreased protein in the CSF appeared to be dose related, as was the effect for neurofilaments, which is biomarker evidence of neuronal damage, she noted.
In the second study, the pathologic evidence from the first patient also suggests “evidence of target engagement,” Dr. Hardiman said.
However, she added, “We don’t know very much about the outcome of the second case other than immunosuppression seemed to be beneficial.”
New hope
Both studies have caveats, said Dr. Hardiman. For example, it is unclear whether the treatments would be beneficial for every variant in SOD1.
“These are very expensive therapies, and we will need to have some level of certainty in order to be able to determine whether this should be a treatment for a patient or not,” said Dr. Hardiman.
She also noted that the studies were not powered to provide evidence of efficacy and that they raise questions about the accuracy of the ALSFRS-R.
One issue is that the respiratory part of that scale is “very insensitive”; another is that the scale doesn’t capture nonmotor elements, such as cognition and behavior, she said.
Utilizing a combination of the ALSFRS-R slope and survival would “probably be more beneficial,” Dr. Hardiman said.
Understanding how to alter the genetic influence in a disorder is important to be able to identify successful treatments, Dr. Hardiman added. For example, the discovery of the BRCA gene led oncologists to develop a precision medicine approach to the treatment of breast cancer.
In regard to ALS, by starting with subgroups that have specific genomic features, “investigators are providing new hope for patients at genetic risk for this devastating fatal disease,” said Dr. Hardiman.
The first study was funded by Biogen. The second study was funded by a fellowship grant from the Alzheimer’s Association, a Jack Satter Foundation Award, the ALS Association, the Angel Fund for ALS Research, ALS Finding a Cure, ALS-One, Project ALS, the Massachusetts General Hospital, the Max Rosenfeld and Cellucci Funds for ALS Research, and several senior members of Bain Capital. Dr. Ferguson is employed by and holds stock in Biogen. Dr. Brown receives grant support from the National Institute of Neurological Disorders and Stroke. He is also co-founder of Apic Bio. Dr. Hardiman is the editor-in-chief of the Journal of Amyotrophic Lateral Sclerosis and Frontotemporal Degenerations, has consulted for Cytokinetics, Mitsubishi, and Wave, and holds research grants from Novartis and Merck. During the past 2 years, she has also been a principal investigator on ALS clinical trials sponsored by Orion and Cytokinetics and is currently on the data and safety monitoring board of Accelsior.
This article first appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
FDA approves new indications for pembrolizumab
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.
The Food and Drug Administration recently announced two new types of cancer that can be treated by the anti–PD-1 antibody pembrolizumab.
The new indications expand the use of pembrolizumab (Keytruda) to include treatment of patients with unresectable or metastatic tumor mutational burden–high (TMB-H) solid tumors as well as patients with cutaneous squamous cell carcinoma (cSCC). The FDA announced the new indications just 8 days apart, on June 16 and June 24.
In addition, on June 29, the FDA approved a third new indication for pembrolizumab, this time as first-line treatment for patients with unresectable or metastatic microsatellite instability–high or mismatch repair–deficient colorectal cancer.
The new approvals add to a wide range of oncology indications for which pembrolizumab can be used.
Accelerated approval to treat solid tumors
The FDA granted accelerated approval for pembrolizumab to treat children and adults with unresectable or metastatic TMB-H solid tumors that progressed after previous treatment or in instances where there are no satisfactory alternative treatment options.
The tumor mutational burden must be confirmed by an FDA-approved test. To that end, the FDA approved the FoundationOneCDx assay, which is designed to help physicians determine which patients meet the threshold for TMB-H malignancies (10 or more mutations per megabase).
The efficacy of pembrolizumab in TMB-H solid tumors was investigated in 10 cohorts from the multicenter, open-label KEYNOTE-158 trial. Participants received 200 mg of pembrolizumab intravenously every 3 weeks until their disease progressed or they experienced unacceptable toxicity.
Within this population, 102 patients had tumors that met the TMB-H definition. In this group, the overall response rate was 29%, including a 25% partial response rate and a 4% complete response rate.
The median duration of response was not reached, but 57% of participants experienced a response lasting 12 months or longer, and 50% had a response lasting 24 months or longer.
The most common adverse events associated with pembrolizumab in this trial were fatigue, musculoskeletal pain, decreased appetite, pruritus, diarrhea, nausea, rash, pyrexia, cough, dyspnea, constipation, pain, and abdominal pain. Pembrolizumab is associated with immune-mediated side effects, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, and skin adverse reactions, the FDA noted.
Safety and efficacy of pembrolizumab in pediatric patients with TMB-H central nervous system cancers have not been established.
New option for recurrent or metastatic cSCC
Physicians treating patients with cSCC that is not curable by surgery or radiation now have pembrolizumab to consider as another treatment option.
The cSCC approval is based on results of the multicenter, open-label KEYNOTE-629 trial. The dosage regimen was 200 mg of pembrolizumab intravenously every 3 weeks until cancer progressed, unacceptable toxicity arose, or 24 months of treatment were completed.
The objective response rate was 34%, and the median duration of response was not reached.
Adverse events were similar to those occurring in patients who received pembrolizumab as a single agent in other clinical trials, the FDA noted.