User login
Role of lasers and light sources in medicine continue to expand
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
suggests R. Rox Anderson, MD.
“I’ve been doing this in my practice for a number of years and it’s quite gratifying,” Dr. Anderson, a dermatologist who directs the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston, said during a virtual course on laser and aesthetic skin therapy. “You treat the periorbital skin, mostly under the eye, just as if you were treating telangiectasia rosacea. The meibomian glands under the upper eyelid that cause this disease are sebaceous glands, and most of the people with dry eye have rosacea.”
In a retrospective noncomparative, interventional case series, 78 patients with severe dry eye syndrome were treated with intense pulsed-light therapy and gland expression at a single outpatient clinic over 30 months. Physician-judged improvement in dry eye tear breakup time was found for 87% of patients with an average of seven treatment visits and four maintenance visits, while 93% of patients reported posttreatment satisfaction with the degree of dry eye syndrome symptoms. More information about the approach were published in Investigative Ophthalmology & Visual Science and Current Opinion in Ophthalmology.
“What’s gratifying here is that most patients will get about 2 months of relief after a single treatment,” Dr. Anderson said. “They are very happy – some of the happiest patients in my practice. Many ophthalmologists don’t have the technology, so I think you can do this depending on your local referral system.”
Light-based approaches are also making promising inroads in cancer treatment. A recent study led by Martin Purschke, PhD, at the Wellman Center evaluated the use of a novel radio-phototherapy approach for killing cancer cells. The center of solid tissue tumors that are treated with radiotherapy is hypoxic, Dr. Anderson explained, “and oxygen is typically located around the perimeter of the tumor. After a radiation therapy treatment, you kill only the outer portion of it, and then the remaining cells grow back, and you end up with the same tumor. This is why you have to do radiation therapy over and over again. In contrast, if you add scintillating nanoparticles, which are particles with a very high C number atoms in them that pick up the x-ray photon and then emit many UV photons from one x-ray photon, they are very efficient at converting x-ray energy to UV energy.” The x-ray, he added, “generates UV light, and the UV light kills the tumor. We’re hoping that we can make a dent in radiotherapy this way.”
Dr. Anderson predicted that fiber lasers, which are highly advanced for industrial applications, will play an increasing role in dermatology and in other areas of medicine. “There are not a new kid on the block anymore but fiber lasers are relatively new to medicine,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “We are seeing incredible capabilities with fiber lasers: essentially any wavelength, any power, any pulse duration you want. The lasers are efficient, small, rugged, and their lifetime exceeds your lifetime. They are likely to displace many of our old lasers in dermatology. I don’t know when, but I know it will happen.”
He reported having received research funding and/or consulting fees from numerous device and pharmaceutical companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
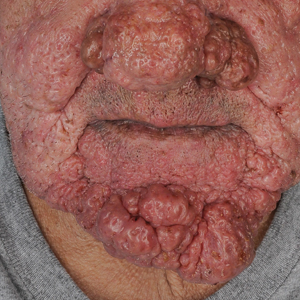
Treatment pipeline holds promise for rosacea
, according to Linda Stein Gold, MD, director of clinical research, in the department of dermatology, Henry Ford Hospital in Detroit.
In addition, topical minocycline has recently been approved by the Food and Drug Administration for the treatment of rosacea in a 1.5% foam formulation. “The reason it has taken so long to have a minocycline product is that it is challenging to deliver it topically,” she said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually. Studies of higher concentrations were not significantly better for rosacea, so development of the 1.5% foam was pursued, although a 4% foam is approved for the treatment of acne.
Dr. Stein Gold shared results from a pair of 12-week randomized trials in which significantly more patients treated with topical minocycline foam showed treatment success, compared with those on vehicle, based on Investigator’s Global Assessment (IGA) scores of clear or almost clear and a decrease of at least two grades from baseline: 52.1% versus 43.0% in one study and 49.1% versus 39.0% in the second, statistically significant differences. The product also was well tolerated, with most patients reporting no side effects or mild side effects.
Research on how to maximize effectiveness of available treatments such as ivermectin is ongoing, but several new treatments in the pipeline continue to show promising results, she noted.
An up-and-coming rosacea treatment is an old product used in a new way: Benzoyl peroxide in a microencapsulated form. “Benzoyl peroxide is encased in silica molecules that allow very slow release of the benzoyl peroxide into the skin and that leads to decreased irritation,” Dr. Stein Gold explained. The deposit of active ingredient on the skin appears to stay below the level of irritation.
Dr. Stein Gold and colleagues conducted two randomized, vehicle-controlled trials in which 733 adults with moderate to severe rosacea were treated with either the encapsulated benzoyl peroxide cream formulation or a vehicle applied once daily for 12 weeks.
At 12 weeks, IGA success increased over the course of the studies, and reached 43.5% in one and 50.1% in the other, compared with 16.1% and 25.9%, respectively, for the vehicle groups in those studies (P < .001 for both). Overall, she described this as “a nice improvement for a drug that we had not considered to be part of our treatment armamentarium for our rosacea patients.”
Dr. Stein Gold also shared data from a phase 2 study of low-dose oral minocycline in adults with papulopustular rosacea. A group of 200 patients used the drug or a placebo once daily for 16 weeks. The study examined 20-mg and 40-mg extended-release formulations, and found a significant improvement with the 40-mg dose over the 20-mg dose and over placebo, in terms of those who reached an IGA of 0 or 1, with a 2 grade improvement. While this is a phase 2 study, it may lead to oral minocycline as another treatment option, she said.
“It is an exciting time for the treatment of rosacea, with a variety of options and an active pipeline, so we can aim for clear skin for our patients,” she commented.
Dr. Stein Gold disclosed serving as an investigator and consultant for Galderma, Vyne, Sun, Sol Gel, and Almirall; she is a consultant and speaker for Ortho.
MedscapeLive and this news organization are owned by the same parent company.
, according to Linda Stein Gold, MD, director of clinical research, in the department of dermatology, Henry Ford Hospital in Detroit.
In addition, topical minocycline has recently been approved by the Food and Drug Administration for the treatment of rosacea in a 1.5% foam formulation. “The reason it has taken so long to have a minocycline product is that it is challenging to deliver it topically,” she said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually. Studies of higher concentrations were not significantly better for rosacea, so development of the 1.5% foam was pursued, although a 4% foam is approved for the treatment of acne.
Dr. Stein Gold shared results from a pair of 12-week randomized trials in which significantly more patients treated with topical minocycline foam showed treatment success, compared with those on vehicle, based on Investigator’s Global Assessment (IGA) scores of clear or almost clear and a decrease of at least two grades from baseline: 52.1% versus 43.0% in one study and 49.1% versus 39.0% in the second, statistically significant differences. The product also was well tolerated, with most patients reporting no side effects or mild side effects.
Research on how to maximize effectiveness of available treatments such as ivermectin is ongoing, but several new treatments in the pipeline continue to show promising results, she noted.
An up-and-coming rosacea treatment is an old product used in a new way: Benzoyl peroxide in a microencapsulated form. “Benzoyl peroxide is encased in silica molecules that allow very slow release of the benzoyl peroxide into the skin and that leads to decreased irritation,” Dr. Stein Gold explained. The deposit of active ingredient on the skin appears to stay below the level of irritation.
Dr. Stein Gold and colleagues conducted two randomized, vehicle-controlled trials in which 733 adults with moderate to severe rosacea were treated with either the encapsulated benzoyl peroxide cream formulation or a vehicle applied once daily for 12 weeks.
At 12 weeks, IGA success increased over the course of the studies, and reached 43.5% in one and 50.1% in the other, compared with 16.1% and 25.9%, respectively, for the vehicle groups in those studies (P < .001 for both). Overall, she described this as “a nice improvement for a drug that we had not considered to be part of our treatment armamentarium for our rosacea patients.”
Dr. Stein Gold also shared data from a phase 2 study of low-dose oral minocycline in adults with papulopustular rosacea. A group of 200 patients used the drug or a placebo once daily for 16 weeks. The study examined 20-mg and 40-mg extended-release formulations, and found a significant improvement with the 40-mg dose over the 20-mg dose and over placebo, in terms of those who reached an IGA of 0 or 1, with a 2 grade improvement. While this is a phase 2 study, it may lead to oral minocycline as another treatment option, she said.
“It is an exciting time for the treatment of rosacea, with a variety of options and an active pipeline, so we can aim for clear skin for our patients,” she commented.
Dr. Stein Gold disclosed serving as an investigator and consultant for Galderma, Vyne, Sun, Sol Gel, and Almirall; she is a consultant and speaker for Ortho.
MedscapeLive and this news organization are owned by the same parent company.
, according to Linda Stein Gold, MD, director of clinical research, in the department of dermatology, Henry Ford Hospital in Detroit.
In addition, topical minocycline has recently been approved by the Food and Drug Administration for the treatment of rosacea in a 1.5% foam formulation. “The reason it has taken so long to have a minocycline product is that it is challenging to deliver it topically,” she said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar, held virtually. Studies of higher concentrations were not significantly better for rosacea, so development of the 1.5% foam was pursued, although a 4% foam is approved for the treatment of acne.
Dr. Stein Gold shared results from a pair of 12-week randomized trials in which significantly more patients treated with topical minocycline foam showed treatment success, compared with those on vehicle, based on Investigator’s Global Assessment (IGA) scores of clear or almost clear and a decrease of at least two grades from baseline: 52.1% versus 43.0% in one study and 49.1% versus 39.0% in the second, statistically significant differences. The product also was well tolerated, with most patients reporting no side effects or mild side effects.
Research on how to maximize effectiveness of available treatments such as ivermectin is ongoing, but several new treatments in the pipeline continue to show promising results, she noted.
An up-and-coming rosacea treatment is an old product used in a new way: Benzoyl peroxide in a microencapsulated form. “Benzoyl peroxide is encased in silica molecules that allow very slow release of the benzoyl peroxide into the skin and that leads to decreased irritation,” Dr. Stein Gold explained. The deposit of active ingredient on the skin appears to stay below the level of irritation.
Dr. Stein Gold and colleagues conducted two randomized, vehicle-controlled trials in which 733 adults with moderate to severe rosacea were treated with either the encapsulated benzoyl peroxide cream formulation or a vehicle applied once daily for 12 weeks.
At 12 weeks, IGA success increased over the course of the studies, and reached 43.5% in one and 50.1% in the other, compared with 16.1% and 25.9%, respectively, for the vehicle groups in those studies (P < .001 for both). Overall, she described this as “a nice improvement for a drug that we had not considered to be part of our treatment armamentarium for our rosacea patients.”
Dr. Stein Gold also shared data from a phase 2 study of low-dose oral minocycline in adults with papulopustular rosacea. A group of 200 patients used the drug or a placebo once daily for 16 weeks. The study examined 20-mg and 40-mg extended-release formulations, and found a significant improvement with the 40-mg dose over the 20-mg dose and over placebo, in terms of those who reached an IGA of 0 or 1, with a 2 grade improvement. While this is a phase 2 study, it may lead to oral minocycline as another treatment option, she said.
“It is an exciting time for the treatment of rosacea, with a variety of options and an active pipeline, so we can aim for clear skin for our patients,” she commented.
Dr. Stein Gold disclosed serving as an investigator and consultant for Galderma, Vyne, Sun, Sol Gel, and Almirall; she is a consultant and speaker for Ortho.
MedscapeLive and this news organization are owned by the same parent company.
FROM THE MEDSCAPELIVE LAS VEGAS DERMATOLOGY SEMINAR
Dermatologists and the history of skin care and beauty devices: Part 4
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
Active Comparator Trial Designs Used to Promote Development of Innovative New Medications
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
Spending on medications is expected to grow from $344 billion in 2018 to $420 billion in 2023, largely driven by the introduction of new branded drugs.1 These costs place substantial financial burden on patients, with nearly 30% of patients not taking their prescriptions as directed because of costs. Although many new medications have transformed how we care for patients, others may not offer meaningful benefit over existing less-costly alternatives that are supported by declining effect sizes of conventional placebo-controlled trials.2 Most medications are approved based on placebo-controlled trial data that does not include an arm comparing the new drug to standard of care, leaving clinicians and patients unable to make meaningful comparisons when deciding on the most appropriate or cost-effective treatment. We consider ways in which clinicians, patients, payers, and regulators could compel more meaningful trials from industry.
Although we often look to the US Food and Drug Administration (FDA) to ensure rigorous and appropriate testing of new medications, the primary mission of the FDA is to ensure efficacy and safety. As a result, pharmaceutical companies seeking approval in the United States have little incentive to go beyond providing the minimal level of evidence required: placebo-controlled randomized trials. Although these trials provide important data on whether a treatment works and its associated risks, they do not provide data on comparative effectiveness. When relevant inexpensive medications are already on the market for the same indication, these placebo-controlled trials provide inadequate evidence to guide clinical decision-making. This issue is particularly relevant in dermatology given how easily topical medications can be combined or reformulated to pursue additional market exclusivity. The addition of an active comparator arm represents an important opportunity to improve the value of these studies.
In the pivotal trials of clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel for the treatment of acne, the experimental group was not only compared to vehicle but also the active comparator arms of clindamycin alone and benzoyl peroxide alone. The mean percentage change in total lesions was 47.9% with clindamycin phosphate 1.2%–benzoyl peroxide 2.5% gel, 41.6% with the active comparator arm of benzoyl peroxide alone, 40.4% with the active comparator arm of clindamycin alone, and 26.2% for vehicle.3 With these data in mind, clinicians and patients can decide whether the additional benefit of this new product over benzoyl peroxide alone is worth the increased cost.
In contrast, the trials of dapsone gel 7.5% for the treatment of acne did not include an active comparator. The mean percentage change in total lesions was 48.9% for dapsone gel and 43.2% for vehicle.4 Given these data, it is possible that dapsone gel may be no more effective, or possibly less effective, than alternatives such as benzoyl peroxide or other topical antibiotics. Nevertheless, dapsone annual sales were more than $200 million in 2016,5 suggesting that effectively marketed new products can achieve high sales even without convincing evidence of their value compared to standard of care. Although dapsone may be a useful treatment, we cannot effectively make patient-centered clinical decisions given the lack of an active comparator trial design.
This issue is not limited to acne. Phase 3 trials of halobetasol propionate foam 0.05% for psoriasis and crisaborole for atopic dermatitis also did not include an active comparator arm.6,7 Given that topical steroids—and calcineurin inhibitors for atopic dermatitis—are mainstays of treatment for each condition, it is difficult to determine whether these new treatments offer meaningful advantages over existing options and how to incorporate them into our management strategies.
Unfortunately, expensive new medications that are adopted without convincing evidence of their benefit above standard of care can put patients at risk for financial toxicity, either directly through higher out-of-pocket costs or indirectly through higher premiums. Given the impact of rising medication costs on clinicians, patients, and payers, we propose several approaches these stakeholders could adopt to encourage the use of active comparator trial designs.
Clinicians and patients can encourage these trials by remaining skeptical of new treatments that were only compared to vehicle or placebo. Because new medications often are more expensive, clinicians and patients could avoid using these treatments without evidence of either increased efficacy or improved safety and tolerability. In addition, health care institutions should consider reducing pharmaceutical representatives’ access to clinicians to encourage treatment decisions based on the published literature and comparative effectiveness data rather than marketing.
Payers, such as Medicare, also could play a role by requiring active comparator trials for coverage of new medications, particularly when there are already other effective treatments available or other medications in the same class. Payers also could give preferred coverage tier or step therapy status to medications that demonstrate value over existing options.
Although regulatory approaches to increase use of active comparator designs may be more politically challenging to introduce, these options would be more administratively robust. The FDA or a novel regulatory body could require that new treatments demonstrate value in addition to safety and efficacy. This approach would be similar to the role of The National Institute for Health and Care Excellence in the United Kingdom or the recommendations of the European Medicines Agency. Such a group also could provide independent adjudication to ensure appropriate selection of a relevant active comparator. Another approach would be to give extended market exclusivity to medications that are approved based on trials including an additional active comparator arm, an approach used by the European Medicines Agency.
Any approach that encourages increased use of active comparator trials is not without potential downsides. It will be important to avoid unintended consequences of reduced research for rare diseases with smaller markets that may not be able to support the increased cost of these trials. As a result, it would be reasonable to forgo active comparator designs for mediations indicated for rare and orphan diseases or for medications with novel mechanisms of action.
Another argument against including an active comparator arm is that it may stifle innovation by driving up the cost of conducting trials; however, if a product is so marginally innovative that it cannot demonstrate superior safety or efficacy to an existing product, such a new treatment may not be worth the increased cost. In addition, patients provide a notable contribution by participating in these trials, and it is important to ensure that their efforts result in the highest-quality data possible. Furthermore, given the adverse physical and psychosocial impact of a wide variety of dermatologic diseases, the inclusion of an active comparator arm reduces the likelihood that patients will receive placebo, which will make these trials more ethical when effective treatments are available.8 By raising the bar, we can encourage pharmaceutical companies to pursue novel approaches that are more likely to have a revolutionary impact rather than minor modifications or formulations that offer little to no benefit at substantially increased cost.
Although some recent clinical trials in dermatology have included active comparators, many new medications continue to be introduced without any evidence of how they compare to existing standards of care. Until clinicians, patients, payers, and regulators demand that pharmaceutical companies conduct the necessary trials to not only demonstrate whether a treatment is effective and safe but also how it provides value, there will be continued introduction of marginal innovations rather than revolutionary treatments that improve patients’ lives. The next time a new medication is approved, as clinicians, patients, and payers, we must ask ourselves, is this treatment worth it?
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
- Aitken M, Kleinrock M. Medicine Use and Spending in the U.S.: A Review of 2018 and Outlook to 2023. IQVIA Institute for Human Data Science. https://www.iqvia.com/insights/the-iqvia-institute/reports/medicine-use-and-spending-in-the-us-a-review-of-2018-and-outlook-to-2023. Published May 9, 2019. Accessed August 15, 2020.
- Olfson M, Marcus SC. Decline in placebo-controlled trial results suggests new directions for comparative effectiveness research. Health Aff Proj Hope. 2019;32:1116-1125.
- Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
- Eichenfield LF, Lain T, Frankel EH, et al. Efficacy and safety of once-daily dapsone gel, 7.5% for treatment of adolescents and adults with acne vulgaris: second of two identically designed, large, multicenter, randomized, vehicle-controlled trials. J Drugs Dermatol. 2016;15:962-969.
- Allergan. 2017 Form 10-K. https://www.abbvie.com/content/dam/abbvie-dotcom/uploads/PDFs/allergan/allergan-annual-report-form-10K-123117.pdf. Accessed August 19, 2020.
- Paller AS, Tom WL, Lebwohl MG, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75:494-503.e6.
- Bhatia N, Stein Gold L, Kircik LH, et al. Two multicenter, randomized, double-blind, parallel group comparison studies of a novel foam formulation of halobetasol propionate, 0.05% vs its vehicle in adult subjects with plaque psoriasis. J Drugs Dermatol. 2019;18:790-796.
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. part 1: ethical and scientific issues. Ann Intern Med. 2000;133:455-463.
Practice Points
- When evaluating a new treatment, it is important to consider not only whether it is effective but also whether it provides additional value compared to existing treatment options.
- Encouraging active comparator trials will provide clinicians and patients with important data to guide decision-making regarding the most appropriate treatment options.
Review finds evidence for beta-blockers for some rosacea symptoms
, while at the same time underscoring the paucity of evidence supporting their use, investigators reported.
“The evidence was highest for carvedilol and propranolol, two nonselective beta-blockers,” wrote the authors of the review, Jade G.M. Logger, MD, of the department of dermatology, Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. Their review is in the Journal of the American Academy of Dermatology.
The systematic review included a case control study of 53,927 patients and an equal number of controls that evaluated beta-blockers in general, but the remaining studies and case reports included only 106 patients in total. The largest was a prospective cohort study of propranolol in 63 patients. Other studies included a 15-patient randomized clinical trial of nadolol published 31 years ago and three single-patient case reports.
The studies included patients with a history of failed therapies; only a small number of beta-blockers were evaluated. Outcomes reported in the studies varied widely, which ruled out doing a meta-analysis. “Erythema and flushing were assessed by using a wide spectrum of mostly subjective clinical and patient-based scores, and method standardization was often missing,” the researchers stated.
“Most studies showed improved erythema and flushing after initiation of oral beta-blockers,” Dr. Logger and colleagues wrote. Treatment of facial erythema and flushing remains a clinical challenge despite approved therapies, for which poor response and reactivation are common. “Diminishing erythema and flushing in rosacea is challenging because it hardly responds to conventional anti-inflammatory treatment,” they noted.
“The study adds no new evidence to support the use of beta-blockers,” Diane M. Thiboutot, MD, professor of dermatology at Penn State University, Hershey, said in an interview. “As the authors point out, the nine studies reviewed were of low quality with a variety of outcome measures that precluded generation of a meta-analysis, which would have represented new information.”
Dr. Thiboutot is lead author of a 2019 update of management options for rosacea published by the National Rosacea Society Expert Committee last year.. Beta blockers are among the drugs that are sometimes prescribed off label to help rosacea-associated flushing, along with nonsteroidal anti-inflammatory drugs, antihistamines, and clonidine, according to the update.
Dr. Logger and coauthors noted that beta-blockers come with risks, and can aggravate asthma and psoriasis and are contraindicated in patients with heart failure, cardiogenic shock, and other cardiovascular diseases, along with hyperactive airway and Raynaud’s disease. “It is important to monitor patients for adverse effects, especially blood pressure and heart rate,” they stated. Carvedilol and propranolol may have more antioxidant and anti-inflammatory properties than other nonselective beta-blockers that may curtail rosacea manifestations, they wrote.
They called for large, prospective clinical trials to more accurately assess the efficacy of beta-blockers in rosacea patients. “Researchers should further focus on the determination of the optimal dosage, treatment duration, and long-term therapeutic effects for adequate treatment of erythema and flushing in rosacea,” they said.
Getting those trials is challenging, Dr. Thiboutot said. “Objective and even subjective measurement of transient and persistent facial erythema is extremely challenging, particularly in the setting of a prospective clinical trial.” The trials would have to control for a number of variables, including room conditions, patient diet, and timing of medication, and large trials require multiple sites,” which could add to the variability of the data,” she said in the interview. Funding such trials would be difficult because adding an indication for rosacea-related symptoms would have limited commercial potential, she added.
Nonetheless, the studies would be welcome, Dr. Thiboutot said. “If standardized outcome measures for facial erythema were to be developed, a study would be more feasible.”
Dr. Logger disclosed financial relationships with Galderma, AbbVie, Novartis, Janssen, and LEO Pharma; one author disclosed conducting clinical trials for AbbVie and Novartis; the third author disclosed relationships with Galderma, Cutanea Life Sciences, AbbVie, Novartis, and Janssen, with fees paid to his institution. Dr. Thiboutot disclosed a financial relationship with Galderma.
SOURCE: Logger JGM et al. J Am Acad Dermatol. 2020 Oct;83(4):1088-97.
, while at the same time underscoring the paucity of evidence supporting their use, investigators reported.
“The evidence was highest for carvedilol and propranolol, two nonselective beta-blockers,” wrote the authors of the review, Jade G.M. Logger, MD, of the department of dermatology, Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. Their review is in the Journal of the American Academy of Dermatology.
The systematic review included a case control study of 53,927 patients and an equal number of controls that evaluated beta-blockers in general, but the remaining studies and case reports included only 106 patients in total. The largest was a prospective cohort study of propranolol in 63 patients. Other studies included a 15-patient randomized clinical trial of nadolol published 31 years ago and three single-patient case reports.
The studies included patients with a history of failed therapies; only a small number of beta-blockers were evaluated. Outcomes reported in the studies varied widely, which ruled out doing a meta-analysis. “Erythema and flushing were assessed by using a wide spectrum of mostly subjective clinical and patient-based scores, and method standardization was often missing,” the researchers stated.
“Most studies showed improved erythema and flushing after initiation of oral beta-blockers,” Dr. Logger and colleagues wrote. Treatment of facial erythema and flushing remains a clinical challenge despite approved therapies, for which poor response and reactivation are common. “Diminishing erythema and flushing in rosacea is challenging because it hardly responds to conventional anti-inflammatory treatment,” they noted.
“The study adds no new evidence to support the use of beta-blockers,” Diane M. Thiboutot, MD, professor of dermatology at Penn State University, Hershey, said in an interview. “As the authors point out, the nine studies reviewed were of low quality with a variety of outcome measures that precluded generation of a meta-analysis, which would have represented new information.”
Dr. Thiboutot is lead author of a 2019 update of management options for rosacea published by the National Rosacea Society Expert Committee last year.. Beta blockers are among the drugs that are sometimes prescribed off label to help rosacea-associated flushing, along with nonsteroidal anti-inflammatory drugs, antihistamines, and clonidine, according to the update.
Dr. Logger and coauthors noted that beta-blockers come with risks, and can aggravate asthma and psoriasis and are contraindicated in patients with heart failure, cardiogenic shock, and other cardiovascular diseases, along with hyperactive airway and Raynaud’s disease. “It is important to monitor patients for adverse effects, especially blood pressure and heart rate,” they stated. Carvedilol and propranolol may have more antioxidant and anti-inflammatory properties than other nonselective beta-blockers that may curtail rosacea manifestations, they wrote.
They called for large, prospective clinical trials to more accurately assess the efficacy of beta-blockers in rosacea patients. “Researchers should further focus on the determination of the optimal dosage, treatment duration, and long-term therapeutic effects for adequate treatment of erythema and flushing in rosacea,” they said.
Getting those trials is challenging, Dr. Thiboutot said. “Objective and even subjective measurement of transient and persistent facial erythema is extremely challenging, particularly in the setting of a prospective clinical trial.” The trials would have to control for a number of variables, including room conditions, patient diet, and timing of medication, and large trials require multiple sites,” which could add to the variability of the data,” she said in the interview. Funding such trials would be difficult because adding an indication for rosacea-related symptoms would have limited commercial potential, she added.
Nonetheless, the studies would be welcome, Dr. Thiboutot said. “If standardized outcome measures for facial erythema were to be developed, a study would be more feasible.”
Dr. Logger disclosed financial relationships with Galderma, AbbVie, Novartis, Janssen, and LEO Pharma; one author disclosed conducting clinical trials for AbbVie and Novartis; the third author disclosed relationships with Galderma, Cutanea Life Sciences, AbbVie, Novartis, and Janssen, with fees paid to his institution. Dr. Thiboutot disclosed a financial relationship with Galderma.
SOURCE: Logger JGM et al. J Am Acad Dermatol. 2020 Oct;83(4):1088-97.
, while at the same time underscoring the paucity of evidence supporting their use, investigators reported.
“The evidence was highest for carvedilol and propranolol, two nonselective beta-blockers,” wrote the authors of the review, Jade G.M. Logger, MD, of the department of dermatology, Radboud University Medical Center in Nijmegen, the Netherlands, and coauthors. Their review is in the Journal of the American Academy of Dermatology.
The systematic review included a case control study of 53,927 patients and an equal number of controls that evaluated beta-blockers in general, but the remaining studies and case reports included only 106 patients in total. The largest was a prospective cohort study of propranolol in 63 patients. Other studies included a 15-patient randomized clinical trial of nadolol published 31 years ago and three single-patient case reports.
The studies included patients with a history of failed therapies; only a small number of beta-blockers were evaluated. Outcomes reported in the studies varied widely, which ruled out doing a meta-analysis. “Erythema and flushing were assessed by using a wide spectrum of mostly subjective clinical and patient-based scores, and method standardization was often missing,” the researchers stated.
“Most studies showed improved erythema and flushing after initiation of oral beta-blockers,” Dr. Logger and colleagues wrote. Treatment of facial erythema and flushing remains a clinical challenge despite approved therapies, for which poor response and reactivation are common. “Diminishing erythema and flushing in rosacea is challenging because it hardly responds to conventional anti-inflammatory treatment,” they noted.
“The study adds no new evidence to support the use of beta-blockers,” Diane M. Thiboutot, MD, professor of dermatology at Penn State University, Hershey, said in an interview. “As the authors point out, the nine studies reviewed were of low quality with a variety of outcome measures that precluded generation of a meta-analysis, which would have represented new information.”
Dr. Thiboutot is lead author of a 2019 update of management options for rosacea published by the National Rosacea Society Expert Committee last year.. Beta blockers are among the drugs that are sometimes prescribed off label to help rosacea-associated flushing, along with nonsteroidal anti-inflammatory drugs, antihistamines, and clonidine, according to the update.
Dr. Logger and coauthors noted that beta-blockers come with risks, and can aggravate asthma and psoriasis and are contraindicated in patients with heart failure, cardiogenic shock, and other cardiovascular diseases, along with hyperactive airway and Raynaud’s disease. “It is important to monitor patients for adverse effects, especially blood pressure and heart rate,” they stated. Carvedilol and propranolol may have more antioxidant and anti-inflammatory properties than other nonselective beta-blockers that may curtail rosacea manifestations, they wrote.
They called for large, prospective clinical trials to more accurately assess the efficacy of beta-blockers in rosacea patients. “Researchers should further focus on the determination of the optimal dosage, treatment duration, and long-term therapeutic effects for adequate treatment of erythema and flushing in rosacea,” they said.
Getting those trials is challenging, Dr. Thiboutot said. “Objective and even subjective measurement of transient and persistent facial erythema is extremely challenging, particularly in the setting of a prospective clinical trial.” The trials would have to control for a number of variables, including room conditions, patient diet, and timing of medication, and large trials require multiple sites,” which could add to the variability of the data,” she said in the interview. Funding such trials would be difficult because adding an indication for rosacea-related symptoms would have limited commercial potential, she added.
Nonetheless, the studies would be welcome, Dr. Thiboutot said. “If standardized outcome measures for facial erythema were to be developed, a study would be more feasible.”
Dr. Logger disclosed financial relationships with Galderma, AbbVie, Novartis, Janssen, and LEO Pharma; one author disclosed conducting clinical trials for AbbVie and Novartis; the third author disclosed relationships with Galderma, Cutanea Life Sciences, AbbVie, Novartis, and Janssen, with fees paid to his institution. Dr. Thiboutot disclosed a financial relationship with Galderma.
SOURCE: Logger JGM et al. J Am Acad Dermatol. 2020 Oct;83(4):1088-97.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Severe Phymatous Rosacea of the Nose, Cheeks, and Chin Treated With Hydrosurgery
Phymatous rosacea is a rare and severe form of rosacea that manifests as disfiguring soft-tissue hypertrophy and hyperplasia as well as fibrosis of the sebaceous glands. 1 Treatments for phymatous rosacea include pharmacotherapeutic and surgical modalities; most cases are treated surgically. Surgical modalities vary, ranging from cryosurgery to conventional excision, and consensus guidelines for surgical management do not exist because data are largely limited to case reports and small case series. 2 The Versajet II Hydrosurgery System (Smith-Nephew) is a high-pressure, pulsatile lavage system that has been used for phymatous rosacea and then only for rosacea of the nose (rhinophyma). We present the case of a patient with phymatous rosacea of the nose, cheeks, and chin who was successfully treated with the Versajet II Hydrosurgery System beyond just the nose region.
Case Report
A 75-year-old man presented to the dermatology clinic for evaluation of severe phymatous rosacea of the nose, cheeks, and chin that had been present for several years. Examination revealed verruciform, thickened, erythematous skin of the nose, cheeks, and chin; marked blue-gray hyperpigmentation on the neck and hands; generalized facial redness; and cystic and depressed scars (Figure 1). The patient had been treated with topical metronidazole without response, and isotretinoin worsened the symptoms. He also was taking minocycline but stopped it at our request because of concern that the drug was causing the blue-gray hyperpigmentation. The patient was referred to plastic surgery and tangential excision was recommended. Fractional ablative laser therapy was considered but deferred because the patient wanted quicker results.

The patient received tangential excision of the phymatous areas of the chin, bilateral cheeks, and nose with the Versajet II Hydrosurgery System until a pleasing contour was noted. At 1-month follow-up, the patient had an excellent contour of the nose, cheeks, and chin (Figure 2).
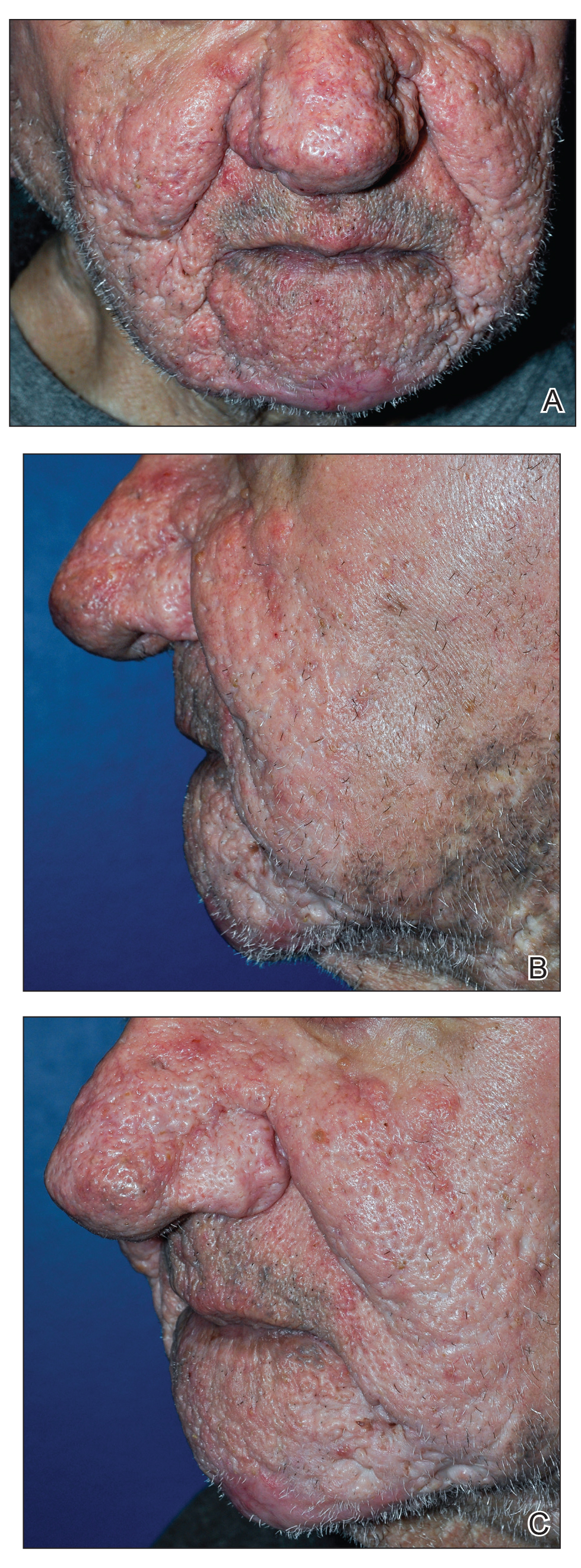
Comment
Phymatous rosacea is a rare disfiguring disease that most commonly presents on the nose but also can affect the chin, cheeks, eyelids, ears, and forehead. Incidence is greater in individuals of Scottish descent and in men due to the influence of androgens. The etiology of the condition is unknown.1
Aside from clinical findings of hyperplastic and fibrotic sebaceous glands in conjunction with enlargement of the affected facial areas, histopathologic findings of phymatous rosacea vary but typically include hypertrophy of subcutaneous tissue, enlarged sebaceous ducts filled with keratin and sebum, atrophy of the dermis, and abnormal vascular development in the form of telangiectases.
Phymatous rosacea adversely affects patients’ physical, mental, and social well-being. Left untreated, it can cause nasal obstruction and recurrent bacterial infections. Furthermore, because of the potential extent of facial deformity, phymatous rosacea can be highly stigmatizing.3 Nonmelanoma skin cancers have been reported within phymatous skin, but evidence of an association between the 2 diseases remains inconclusive.4 Excised tissue from our patient was not submitted to pathology for analysis.
Given the far-reaching physical and psychological consequences of phymatous rosacea, treatment is critical but, regrettably, challenging. Although medical and surgical interventions exist, surgery is the most common practice. Oral isotretinoin may help, but many cases are recalcitrant, as was the disease in our patient. Therefore, procedural remedies often are sought, including scalpel excision, cryosurgery, argon laser, CO2 laser, dermabrasion, and electrocautery.2
Our patient underwent Versajet II Hydrosurgery System treatment of the phymatous rosacea on the nose, cheeks, and chin. Versajet is not yet commonly used to treat phymatous rosacea, likely due to the upfront cost of obtaining a new device, lack of physician familiarity, and few reports of its use for phymatous skin. A search of PubMed, EMBASE, and the Web of Science using the terms Rosacea AND (Versajet OR Hydrosurgery) yielded only 6 cases of rosacea treated by hydrosurgery; all were limited to rhinophyma and reported excellent cosmetic and functional results.5-10 Our case was unique in that hydrosurgery was used to treat phymatous rosacea beyond the nose.
Hydrosurgery has many advantages in the treatment of phymatous rosacea and other conditions in which surgical debridement is necessary, such as burns and wounds. A randomized clinical trial demonstrated that hydrosurgery is more cost-effective than conventional excision because of decreased operative time and intraoperative blood loss, fewer debridement procedures, and fewer postoperative complications.11
Rennekampff et al12 showed that Versajet debridement is superior to conventional surgery in contouring facial and acral sites and has a lower probability of infection. They proposed that by running a highly pressurized constant stream of saline across the device, Versajet clears blood and debris from the surgical site during excision.12 Hydrosurgical debridement also has been shown to reduce Staphylococcus aureus inoculate levels from in vitro–contaminated equine models significantly more than conventional debridement methods (P<.05).13
Versajet surgery appears to be well tolerated, with side effects comparable to those of classic surgical excision. A randomized controlled trial in burn patients in which treatment with Versajet was compared to traditional debridement found no significant difference in postoperative pain, healing time, and contracture rate.13
Overall, tangential excision of our patient’s phymatous rosacea using the Versajet II Hydrosurgery System yielded excellent contouring. However, due to the paucity of literature on the subject, it is difficult to discern the optimal treatment modality. Therefore, more research—ideally randomized trials—should be pursued to examine the comparative effectiveness of different interventions for phymatous rosacea.
- Curnier A, Choudhary S. Rhinophyma: dispelling the myths. Plast Reconstr Surg. 2004;114:351-354.
- Sadick H, Goepel B, Bersch C, et al. Rhinophyma: diagnosis and treatment options for a disfiguring tumor of the nose. Ann Plast Surg. 2008;61:114-120.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey. Dermatol Ther (Heidelb). 2015;5:117-127.
- Lazzeri D, Colizzi L, Licata G, et al. Malignancies within rhinophyma: report of three new cases and review of the literature. Aesthetic Plast Surg. 2012;36:396-405.
- Dunne JA, Saleh DB, Rawlins JM. Management of rhinophyma with Versajet™ and ReCell®. Br J Oral Maxillofac Surg. 2013;51:e282-e284.
- Yildiz K, Kayan BR, Dulgeroglu T, et al. Treatment of rhinophyma with the Versajet™ Hydrosurgery System and autologous cell suspension (ReCELL®): a case report. J Cosmet Laser Ther. 2018;20:114-116.
- Nicolas J, Garmi R, Labbé D, et al. The role of Versajet in the surgical treatment of rhinophyma. case report. Ann Chir Plast Esthet. 2009;54:78-81.
- Novati FC, Franchi A, Roggio T, et al. Treatment of a double-giant rhinophyma with electrocautery and Versajet Hydrosurgery System. Ann Ital Chir. 2015;86. pii: S2239253X15023269.
- Taghizadeh R, Mackay SP, Gilbert PM. Treatment of rhinophyma with the Versajet Hydrosurgery System. J Plast Reconstr Aesthet Surg. 2008;61:330-333.
- Wong WL, Wong She R, Mathy JA. Rhinophyma treatment using Versajet Hydrosurgery. ANZ J Surg. 2017;87:E331-E332.
- Liu J, Ko JH, Secretov E, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12:456-461.
- Rennekampff H-O, Schaller H-E, Wisser D, et al. Debridement of burn wounds with a water jet surgical tool. Burns. 2006;32:64-69.
- Skarlina EM, Wilmink JM, Fall N, et al. Effectiveness of conventional and hydrosurgical debridement methods in reducing Staphylococcus aureus inoculation of equine muscle in vitro. Equine Vet J. 2015;47:218-222.
Phymatous rosacea is a rare and severe form of rosacea that manifests as disfiguring soft-tissue hypertrophy and hyperplasia as well as fibrosis of the sebaceous glands. 1 Treatments for phymatous rosacea include pharmacotherapeutic and surgical modalities; most cases are treated surgically. Surgical modalities vary, ranging from cryosurgery to conventional excision, and consensus guidelines for surgical management do not exist because data are largely limited to case reports and small case series. 2 The Versajet II Hydrosurgery System (Smith-Nephew) is a high-pressure, pulsatile lavage system that has been used for phymatous rosacea and then only for rosacea of the nose (rhinophyma). We present the case of a patient with phymatous rosacea of the nose, cheeks, and chin who was successfully treated with the Versajet II Hydrosurgery System beyond just the nose region.
Case Report
A 75-year-old man presented to the dermatology clinic for evaluation of severe phymatous rosacea of the nose, cheeks, and chin that had been present for several years. Examination revealed verruciform, thickened, erythematous skin of the nose, cheeks, and chin; marked blue-gray hyperpigmentation on the neck and hands; generalized facial redness; and cystic and depressed scars (Figure 1). The patient had been treated with topical metronidazole without response, and isotretinoin worsened the symptoms. He also was taking minocycline but stopped it at our request because of concern that the drug was causing the blue-gray hyperpigmentation. The patient was referred to plastic surgery and tangential excision was recommended. Fractional ablative laser therapy was considered but deferred because the patient wanted quicker results.

The patient received tangential excision of the phymatous areas of the chin, bilateral cheeks, and nose with the Versajet II Hydrosurgery System until a pleasing contour was noted. At 1-month follow-up, the patient had an excellent contour of the nose, cheeks, and chin (Figure 2).

Comment
Phymatous rosacea is a rare disfiguring disease that most commonly presents on the nose but also can affect the chin, cheeks, eyelids, ears, and forehead. Incidence is greater in individuals of Scottish descent and in men due to the influence of androgens. The etiology of the condition is unknown.1
Aside from clinical findings of hyperplastic and fibrotic sebaceous glands in conjunction with enlargement of the affected facial areas, histopathologic findings of phymatous rosacea vary but typically include hypertrophy of subcutaneous tissue, enlarged sebaceous ducts filled with keratin and sebum, atrophy of the dermis, and abnormal vascular development in the form of telangiectases.
Phymatous rosacea adversely affects patients’ physical, mental, and social well-being. Left untreated, it can cause nasal obstruction and recurrent bacterial infections. Furthermore, because of the potential extent of facial deformity, phymatous rosacea can be highly stigmatizing.3 Nonmelanoma skin cancers have been reported within phymatous skin, but evidence of an association between the 2 diseases remains inconclusive.4 Excised tissue from our patient was not submitted to pathology for analysis.
Given the far-reaching physical and psychological consequences of phymatous rosacea, treatment is critical but, regrettably, challenging. Although medical and surgical interventions exist, surgery is the most common practice. Oral isotretinoin may help, but many cases are recalcitrant, as was the disease in our patient. Therefore, procedural remedies often are sought, including scalpel excision, cryosurgery, argon laser, CO2 laser, dermabrasion, and electrocautery.2
Our patient underwent Versajet II Hydrosurgery System treatment of the phymatous rosacea on the nose, cheeks, and chin. Versajet is not yet commonly used to treat phymatous rosacea, likely due to the upfront cost of obtaining a new device, lack of physician familiarity, and few reports of its use for phymatous skin. A search of PubMed, EMBASE, and the Web of Science using the terms Rosacea AND (Versajet OR Hydrosurgery) yielded only 6 cases of rosacea treated by hydrosurgery; all were limited to rhinophyma and reported excellent cosmetic and functional results.5-10 Our case was unique in that hydrosurgery was used to treat phymatous rosacea beyond the nose.
Hydrosurgery has many advantages in the treatment of phymatous rosacea and other conditions in which surgical debridement is necessary, such as burns and wounds. A randomized clinical trial demonstrated that hydrosurgery is more cost-effective than conventional excision because of decreased operative time and intraoperative blood loss, fewer debridement procedures, and fewer postoperative complications.11
Rennekampff et al12 showed that Versajet debridement is superior to conventional surgery in contouring facial and acral sites and has a lower probability of infection. They proposed that by running a highly pressurized constant stream of saline across the device, Versajet clears blood and debris from the surgical site during excision.12 Hydrosurgical debridement also has been shown to reduce Staphylococcus aureus inoculate levels from in vitro–contaminated equine models significantly more than conventional debridement methods (P<.05).13
Versajet surgery appears to be well tolerated, with side effects comparable to those of classic surgical excision. A randomized controlled trial in burn patients in which treatment with Versajet was compared to traditional debridement found no significant difference in postoperative pain, healing time, and contracture rate.13
Overall, tangential excision of our patient’s phymatous rosacea using the Versajet II Hydrosurgery System yielded excellent contouring. However, due to the paucity of literature on the subject, it is difficult to discern the optimal treatment modality. Therefore, more research—ideally randomized trials—should be pursued to examine the comparative effectiveness of different interventions for phymatous rosacea.
Phymatous rosacea is a rare and severe form of rosacea that manifests as disfiguring soft-tissue hypertrophy and hyperplasia as well as fibrosis of the sebaceous glands. 1 Treatments for phymatous rosacea include pharmacotherapeutic and surgical modalities; most cases are treated surgically. Surgical modalities vary, ranging from cryosurgery to conventional excision, and consensus guidelines for surgical management do not exist because data are largely limited to case reports and small case series. 2 The Versajet II Hydrosurgery System (Smith-Nephew) is a high-pressure, pulsatile lavage system that has been used for phymatous rosacea and then only for rosacea of the nose (rhinophyma). We present the case of a patient with phymatous rosacea of the nose, cheeks, and chin who was successfully treated with the Versajet II Hydrosurgery System beyond just the nose region.
Case Report
A 75-year-old man presented to the dermatology clinic for evaluation of severe phymatous rosacea of the nose, cheeks, and chin that had been present for several years. Examination revealed verruciform, thickened, erythematous skin of the nose, cheeks, and chin; marked blue-gray hyperpigmentation on the neck and hands; generalized facial redness; and cystic and depressed scars (Figure 1). The patient had been treated with topical metronidazole without response, and isotretinoin worsened the symptoms. He also was taking minocycline but stopped it at our request because of concern that the drug was causing the blue-gray hyperpigmentation. The patient was referred to plastic surgery and tangential excision was recommended. Fractional ablative laser therapy was considered but deferred because the patient wanted quicker results.

The patient received tangential excision of the phymatous areas of the chin, bilateral cheeks, and nose with the Versajet II Hydrosurgery System until a pleasing contour was noted. At 1-month follow-up, the patient had an excellent contour of the nose, cheeks, and chin (Figure 2).

Comment
Phymatous rosacea is a rare disfiguring disease that most commonly presents on the nose but also can affect the chin, cheeks, eyelids, ears, and forehead. Incidence is greater in individuals of Scottish descent and in men due to the influence of androgens. The etiology of the condition is unknown.1
Aside from clinical findings of hyperplastic and fibrotic sebaceous glands in conjunction with enlargement of the affected facial areas, histopathologic findings of phymatous rosacea vary but typically include hypertrophy of subcutaneous tissue, enlarged sebaceous ducts filled with keratin and sebum, atrophy of the dermis, and abnormal vascular development in the form of telangiectases.
Phymatous rosacea adversely affects patients’ physical, mental, and social well-being. Left untreated, it can cause nasal obstruction and recurrent bacterial infections. Furthermore, because of the potential extent of facial deformity, phymatous rosacea can be highly stigmatizing.3 Nonmelanoma skin cancers have been reported within phymatous skin, but evidence of an association between the 2 diseases remains inconclusive.4 Excised tissue from our patient was not submitted to pathology for analysis.
Given the far-reaching physical and psychological consequences of phymatous rosacea, treatment is critical but, regrettably, challenging. Although medical and surgical interventions exist, surgery is the most common practice. Oral isotretinoin may help, but many cases are recalcitrant, as was the disease in our patient. Therefore, procedural remedies often are sought, including scalpel excision, cryosurgery, argon laser, CO2 laser, dermabrasion, and electrocautery.2
Our patient underwent Versajet II Hydrosurgery System treatment of the phymatous rosacea on the nose, cheeks, and chin. Versajet is not yet commonly used to treat phymatous rosacea, likely due to the upfront cost of obtaining a new device, lack of physician familiarity, and few reports of its use for phymatous skin. A search of PubMed, EMBASE, and the Web of Science using the terms Rosacea AND (Versajet OR Hydrosurgery) yielded only 6 cases of rosacea treated by hydrosurgery; all were limited to rhinophyma and reported excellent cosmetic and functional results.5-10 Our case was unique in that hydrosurgery was used to treat phymatous rosacea beyond the nose.
Hydrosurgery has many advantages in the treatment of phymatous rosacea and other conditions in which surgical debridement is necessary, such as burns and wounds. A randomized clinical trial demonstrated that hydrosurgery is more cost-effective than conventional excision because of decreased operative time and intraoperative blood loss, fewer debridement procedures, and fewer postoperative complications.11
Rennekampff et al12 showed that Versajet debridement is superior to conventional surgery in contouring facial and acral sites and has a lower probability of infection. They proposed that by running a highly pressurized constant stream of saline across the device, Versajet clears blood and debris from the surgical site during excision.12 Hydrosurgical debridement also has been shown to reduce Staphylococcus aureus inoculate levels from in vitro–contaminated equine models significantly more than conventional debridement methods (P<.05).13
Versajet surgery appears to be well tolerated, with side effects comparable to those of classic surgical excision. A randomized controlled trial in burn patients in which treatment with Versajet was compared to traditional debridement found no significant difference in postoperative pain, healing time, and contracture rate.13
Overall, tangential excision of our patient’s phymatous rosacea using the Versajet II Hydrosurgery System yielded excellent contouring. However, due to the paucity of literature on the subject, it is difficult to discern the optimal treatment modality. Therefore, more research—ideally randomized trials—should be pursued to examine the comparative effectiveness of different interventions for phymatous rosacea.
- Curnier A, Choudhary S. Rhinophyma: dispelling the myths. Plast Reconstr Surg. 2004;114:351-354.
- Sadick H, Goepel B, Bersch C, et al. Rhinophyma: diagnosis and treatment options for a disfiguring tumor of the nose. Ann Plast Surg. 2008;61:114-120.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey. Dermatol Ther (Heidelb). 2015;5:117-127.
- Lazzeri D, Colizzi L, Licata G, et al. Malignancies within rhinophyma: report of three new cases and review of the literature. Aesthetic Plast Surg. 2012;36:396-405.
- Dunne JA, Saleh DB, Rawlins JM. Management of rhinophyma with Versajet™ and ReCell®. Br J Oral Maxillofac Surg. 2013;51:e282-e284.
- Yildiz K, Kayan BR, Dulgeroglu T, et al. Treatment of rhinophyma with the Versajet™ Hydrosurgery System and autologous cell suspension (ReCELL®): a case report. J Cosmet Laser Ther. 2018;20:114-116.
- Nicolas J, Garmi R, Labbé D, et al. The role of Versajet in the surgical treatment of rhinophyma. case report. Ann Chir Plast Esthet. 2009;54:78-81.
- Novati FC, Franchi A, Roggio T, et al. Treatment of a double-giant rhinophyma with electrocautery and Versajet Hydrosurgery System. Ann Ital Chir. 2015;86. pii: S2239253X15023269.
- Taghizadeh R, Mackay SP, Gilbert PM. Treatment of rhinophyma with the Versajet Hydrosurgery System. J Plast Reconstr Aesthet Surg. 2008;61:330-333.
- Wong WL, Wong She R, Mathy JA. Rhinophyma treatment using Versajet Hydrosurgery. ANZ J Surg. 2017;87:E331-E332.
- Liu J, Ko JH, Secretov E, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12:456-461.
- Rennekampff H-O, Schaller H-E, Wisser D, et al. Debridement of burn wounds with a water jet surgical tool. Burns. 2006;32:64-69.
- Skarlina EM, Wilmink JM, Fall N, et al. Effectiveness of conventional and hydrosurgical debridement methods in reducing Staphylococcus aureus inoculation of equine muscle in vitro. Equine Vet J. 2015;47:218-222.
- Curnier A, Choudhary S. Rhinophyma: dispelling the myths. Plast Reconstr Surg. 2004;114:351-354.
- Sadick H, Goepel B, Bersch C, et al. Rhinophyma: diagnosis and treatment options for a disfiguring tumor of the nose. Ann Plast Surg. 2008;61:114-120.
- Dirschka T, Micali G, Papadopoulos L, et al. Perceptions on the psychological impact of facial erythema associated with rosacea: results of international survey. Dermatol Ther (Heidelb). 2015;5:117-127.
- Lazzeri D, Colizzi L, Licata G, et al. Malignancies within rhinophyma: report of three new cases and review of the literature. Aesthetic Plast Surg. 2012;36:396-405.
- Dunne JA, Saleh DB, Rawlins JM. Management of rhinophyma with Versajet™ and ReCell®. Br J Oral Maxillofac Surg. 2013;51:e282-e284.
- Yildiz K, Kayan BR, Dulgeroglu T, et al. Treatment of rhinophyma with the Versajet™ Hydrosurgery System and autologous cell suspension (ReCELL®): a case report. J Cosmet Laser Ther. 2018;20:114-116.
- Nicolas J, Garmi R, Labbé D, et al. The role of Versajet in the surgical treatment of rhinophyma. case report. Ann Chir Plast Esthet. 2009;54:78-81.
- Novati FC, Franchi A, Roggio T, et al. Treatment of a double-giant rhinophyma with electrocautery and Versajet Hydrosurgery System. Ann Ital Chir. 2015;86. pii: S2239253X15023269.
- Taghizadeh R, Mackay SP, Gilbert PM. Treatment of rhinophyma with the Versajet Hydrosurgery System. J Plast Reconstr Aesthet Surg. 2008;61:330-333.
- Wong WL, Wong She R, Mathy JA. Rhinophyma treatment using Versajet Hydrosurgery. ANZ J Surg. 2017;87:E331-E332.
- Liu J, Ko JH, Secretov E, et al. Comparing the hydrosurgery system to conventional debridement techniques for the treatment of delayed healing wounds: a prospective, randomised clinical trial to investigate clinical efficacy and cost-effectiveness. Int Wound J. 2015;12:456-461.
- Rennekampff H-O, Schaller H-E, Wisser D, et al. Debridement of burn wounds with a water jet surgical tool. Burns. 2006;32:64-69.
- Skarlina EM, Wilmink JM, Fall N, et al. Effectiveness of conventional and hydrosurgical debridement methods in reducing Staphylococcus aureus inoculation of equine muscle in vitro. Equine Vet J. 2015;47:218-222.
Practice Points
- Phymatous rosacea is a rare disfiguring disease that most commonly affects men and can have considerable effects on a patient’s physical, mental, and social well-being.
- Treatment of phymatous rosacea usually is surgical; however, no consensus guidelines exist for best surgical management.
- The Versajet II Hydrosurgery System can be useful and effective for the treatment of phymatous rosacea, not only on the nose but elsewhere on the face.
Today’s top news highlights: Remdesivir data dive, FDA approves contraceptive gel
:
Remdesivir trial data published
Weeks after topline remdesivir data appeared in the press, investigators published their full experience using the drug to treat COVID-19 patients. The study, published in the New England Journal of Medicine, showed the drug reduced recovery time from 15 to 11 days, compared with placebo. Patients receiving oxygen seemed to fare best from treatment with remdesivir. “There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.” READ MORE.
FDA approves contraceptive gel
The Food and Drug Administration approved Phexxi (lactic acid, citric acid, and potassium bitartrate) vaginal gel to prevent pregnancy in women of reproductive potential. It’s the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5. READ MORE.
COVID-19 lessons from one cancer center
Physicians at Levine Cancer Institute in Charlotte, N.C., largely have been able to keep hematologic oncology patients on their treatment regimens and continue to care for inpatients during the early months of the COVID-19 pandemic. How have they kept the situation managable? Strict infection control, liberal testing, and a proactive plan to defer and temporarily replace infusion care when medically appropriate were all part of the strategy. “My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious, even before the coronavirus, using distancing, masking, and meticulous hand hygiene,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in Levine Cancer Institute’s Department of Hematologic Oncology and Blood Disorders. READ MORE.
Convalescent plasma: Hope or hype?
There are currently more than two dozen trials of convalescent plasma in the United States and elsewhere but most are single-arm trials to determine if one infusion can decrease the need for intubation or help patients on a ventilator to improve. Others researchers are investigating whether convalescent plasma might be used before severe disease sets in. Meanwhile, about 2,200 hospitals are participating in an expanded access program being led by the Mayo Clinic nationwide. The National Institutes of Health recently said that “there are insufficient clinical data to recommend either for or against” its use for COVID-19. READ MORE.
New rosacea treatment guidelines
Patients with rosacea should receive treatments based on their phenotype and specific symptoms, rather than being assigned into distinct subtype categories, according to updated guidance published in the Journal of the American Academy of Dermatology. The update comes from the National Rosacea Society Expert Committee and is based on a review of the evidence. Patients “shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” said Diane Thiboutot, MD, lead author of the update and a professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey. READ MORE.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
:
Remdesivir trial data published
Weeks after topline remdesivir data appeared in the press, investigators published their full experience using the drug to treat COVID-19 patients. The study, published in the New England Journal of Medicine, showed the drug reduced recovery time from 15 to 11 days, compared with placebo. Patients receiving oxygen seemed to fare best from treatment with remdesivir. “There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.” READ MORE.
FDA approves contraceptive gel
The Food and Drug Administration approved Phexxi (lactic acid, citric acid, and potassium bitartrate) vaginal gel to prevent pregnancy in women of reproductive potential. It’s the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5. READ MORE.
COVID-19 lessons from one cancer center
Physicians at Levine Cancer Institute in Charlotte, N.C., largely have been able to keep hematologic oncology patients on their treatment regimens and continue to care for inpatients during the early months of the COVID-19 pandemic. How have they kept the situation managable? Strict infection control, liberal testing, and a proactive plan to defer and temporarily replace infusion care when medically appropriate were all part of the strategy. “My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious, even before the coronavirus, using distancing, masking, and meticulous hand hygiene,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in Levine Cancer Institute’s Department of Hematologic Oncology and Blood Disorders. READ MORE.
Convalescent plasma: Hope or hype?
There are currently more than two dozen trials of convalescent plasma in the United States and elsewhere but most are single-arm trials to determine if one infusion can decrease the need for intubation or help patients on a ventilator to improve. Others researchers are investigating whether convalescent plasma might be used before severe disease sets in. Meanwhile, about 2,200 hospitals are participating in an expanded access program being led by the Mayo Clinic nationwide. The National Institutes of Health recently said that “there are insufficient clinical data to recommend either for or against” its use for COVID-19. READ MORE.
New rosacea treatment guidelines
Patients with rosacea should receive treatments based on their phenotype and specific symptoms, rather than being assigned into distinct subtype categories, according to updated guidance published in the Journal of the American Academy of Dermatology. The update comes from the National Rosacea Society Expert Committee and is based on a review of the evidence. Patients “shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” said Diane Thiboutot, MD, lead author of the update and a professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey. READ MORE.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
:
Remdesivir trial data published
Weeks after topline remdesivir data appeared in the press, investigators published their full experience using the drug to treat COVID-19 patients. The study, published in the New England Journal of Medicine, showed the drug reduced recovery time from 15 to 11 days, compared with placebo. Patients receiving oxygen seemed to fare best from treatment with remdesivir. “There is clear and consistent evidence of clinically significant benefit for those hospitalized on oxygen but not yet requiring mechanical ventilation,” Daniel Kaul, MD, a professor of infectious diseases at the University of Michigan, Ann Arbor, said after seeing the published results. “Surprisingly, early dosing as measured from time to onset of symptoms did not seem to make a difference.” READ MORE.
FDA approves contraceptive gel
The Food and Drug Administration approved Phexxi (lactic acid, citric acid, and potassium bitartrate) vaginal gel to prevent pregnancy in women of reproductive potential. It’s the first nonhormonal, on-demand, vaginal pH regulator contraceptive designed to maintain vaginal pH within the range of 3.5-4.5. READ MORE.
COVID-19 lessons from one cancer center
Physicians at Levine Cancer Institute in Charlotte, N.C., largely have been able to keep hematologic oncology patients on their treatment regimens and continue to care for inpatients during the early months of the COVID-19 pandemic. How have they kept the situation managable? Strict infection control, liberal testing, and a proactive plan to defer and temporarily replace infusion care when medically appropriate were all part of the strategy. “My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious, even before the coronavirus, using distancing, masking, and meticulous hand hygiene,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in Levine Cancer Institute’s Department of Hematologic Oncology and Blood Disorders. READ MORE.
Convalescent plasma: Hope or hype?
There are currently more than two dozen trials of convalescent plasma in the United States and elsewhere but most are single-arm trials to determine if one infusion can decrease the need for intubation or help patients on a ventilator to improve. Others researchers are investigating whether convalescent plasma might be used before severe disease sets in. Meanwhile, about 2,200 hospitals are participating in an expanded access program being led by the Mayo Clinic nationwide. The National Institutes of Health recently said that “there are insufficient clinical data to recommend either for or against” its use for COVID-19. READ MORE.
New rosacea treatment guidelines
Patients with rosacea should receive treatments based on their phenotype and specific symptoms, rather than being assigned into distinct subtype categories, according to updated guidance published in the Journal of the American Academy of Dermatology. The update comes from the National Rosacea Society Expert Committee and is based on a review of the evidence. Patients “shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” said Diane Thiboutot, MD, lead author of the update and a professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey. READ MORE.
For more on COVID-19, visit our Resource Center. All of our latest news is available on MDedge.com.
New rosacea clinical management guidelines focus on symptomatology
, as was previously practiced, according to an update on options for managing rosacea published in the Journal of the American Academy of Dermatology.
The update, by the National Rosacea Society Expert Committee, is based on a review of the evidence, and is a follow-up to the classification system for rosacea that was updated in 2017, which recommended classification of rosacea based on phenotype (Am Acad Dermatol. 2018;78:148-155).
The key take-away is “that patients shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” lead author of the management update, Diane Thiboutot, MD, professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey, said in an interview.
“There is an opportunity for physicians to recognize that the symptom complex of rosacea differs widely and treatments should be selected to address the symptoms experienced by the patient, particularly with regard to ocular rosacea,” she said.
Until there were updated guidelines on rosacea classification, published in 2018, relying primarily on diagnostic subtypes “tended to limit consideration of the full range of potential signs and symptoms as well as the frequent simultaneous occurrence of more than one subtype or the potential progression from one subtype to another,” Dr. Thiboutot and coauthors wrote in the management update (J Am Acad Dermatol 2020;82:1501-10).
“The more we learn, the more complex rosacea becomes,” she said in the interview. “The clinical manifestations of rosacea are so varied, ranging from skin erythema, eye findings, papules and pustules to rhinophyma, [that] it calls into question, if all of these are actually one disease (rosacea) or if they represent localized reaction patterns to a multitude of stimuli that vary among individuals.”
Etiology and impact
Dr. Thiboutot and colleagues summarized the management options and recommendations from a committee of 27 experts who assessed the data on rosacea therapies using the updated standard classification system. They also highlighted the suspected systemic nature of rosacea etiology and its psychosocial impact on those with the condition.
“Recent studies have found an association between rosacea and increased risk of a growing number of systemic disorders, including cardiovascular, gastrointestinal, neurologic, and autoimmune diseases as well as certain types of cancer,” the authors wrote. “These findings further elevate the clinical significance of rosacea as growing evidence of its potential link with systemic inflammation is increasingly understood.”
Dr. Thiboutot said that research has implicated both the innate and adaptive immune systems and the neuromuscular system in rosacea’s underpinnings.
“Many of the triggers associated with clinical exacerbation of rosacea are known to activate the immune system and/or the neurovasculature, such as demodex, sunlight, alcohol, and changes in temperature,” she said, adding that therapies targeting the neurovascular effects of rosacea are particularly needed.
More than 50% of patients with rosacea have ocular manifestations, with symptoms such as “dryness, burning and stinging, light sensitivity, blurred vision, and foreign body sensation,” the authors reported.
Diagnosis and management
Without definitive laboratory tests, it’s essential that the clinical diagnosis takes into account not only the physical examination findings, but also patient history, the authors stressed, since “some features may not be visually evident or present at the time of the patient’s visit.”
The authors also recommend taking into account patients’ perception and response to their appearance and its effects on their emotional, social, and professional lives when selecting interventions.
“Rosacea’s unsightly and conspicuous appearance often has significant emotional ramifications, potentially resulting in depression or anxiety, and frequently interferes with social and occupational interactions,” the authors wrote. “It may also be advisable to remind patients that normalization of skin tone and color is the goal rather than complete eradication of facial coloration, which can leave the face with a sallow appearance.”
Therapy will often require multiple agents, such as topical and oral agents for inflammatory papules/pustules of rosacea. If insufficient, adjunctive therapy with oral antibiotics or retinoids may be options, though the latter requires prevention of pregnancy during treatment. The authors also discussed pharmacological treatments for facial erythema and flushing, with all these drugs organized in tables according to symptoms and their levels of evidence and effectiveness.
Despite limited clinical evidence, the authors noted success with two types of laser therapy – pulsed-dye and potassium titanyl phosphate – for telangiectasia and erythema. They also noted the option of intense pulsed light for flushing, ocular symptoms, and meibomian gland dysfunction, and of ablative lasers for rhinophymatous nose. But they highlighted the importance of being cautious when using these therapies on darker skin.
In their discussion of ocular rosacea, the authors described the various manifestations and the two “mainstays” of treatment: “eyelash hygiene and oral [omega-3] supplementation, followed by topical azithromycin or calcineurin inhibitors.” In addition to pharmacological and light therapy options, attention to environmental contributors and conscientious skin care regimens can benefit patients with rosacea as well.
“Clinicians may advise patients to keep a daily diary of lifestyle and environmental factors that appear to affect their rosacea to help identify and avoid their personal triggers,” the authors wrote. “The most common factors are sun exposure, emotional stress, hot weather, wind, heavy exercise, alcohol consumption, hot baths, cold weather, spicy foods, humidity, indoor heat, certain skin-care products, heated beverages, certain medications, medical conditions, certain fruits, marinated meats, certain vegetables, and dairy products.”
The paper also emphasizes the importance of gentle skin care given the highly sensitive and easily irritated skin of patients with rosacea. Sunscreen use, particularly with mineral sunscreens that provide physical barriers and reflect ultraviolet light, should be a key aspect of patients’ skin care, and clinicians should advise patients to seek out gentle, nonirritating cleansers.
Funding was provided by the National Rosacea Society, which receives funding from patients and corporations that include Aclaris Therapeutics, Allergan, Bayer, Cutanea Life Sciences, and Galderma Laboratories. Dr. Thiboutot consults for Galderma. Six of the other nine coauthors have financial links to industry through advisory boards, consulting, or research funding.
SOURCE: Thiboutot D et al. J Am Acad Dermatol. 2020;82:1501-10.
, as was previously practiced, according to an update on options for managing rosacea published in the Journal of the American Academy of Dermatology.
The update, by the National Rosacea Society Expert Committee, is based on a review of the evidence, and is a follow-up to the classification system for rosacea that was updated in 2017, which recommended classification of rosacea based on phenotype (Am Acad Dermatol. 2018;78:148-155).
The key take-away is “that patients shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” lead author of the management update, Diane Thiboutot, MD, professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey, said in an interview.
“There is an opportunity for physicians to recognize that the symptom complex of rosacea differs widely and treatments should be selected to address the symptoms experienced by the patient, particularly with regard to ocular rosacea,” she said.
Until there were updated guidelines on rosacea classification, published in 2018, relying primarily on diagnostic subtypes “tended to limit consideration of the full range of potential signs and symptoms as well as the frequent simultaneous occurrence of more than one subtype or the potential progression from one subtype to another,” Dr. Thiboutot and coauthors wrote in the management update (J Am Acad Dermatol 2020;82:1501-10).
“The more we learn, the more complex rosacea becomes,” she said in the interview. “The clinical manifestations of rosacea are so varied, ranging from skin erythema, eye findings, papules and pustules to rhinophyma, [that] it calls into question, if all of these are actually one disease (rosacea) or if they represent localized reaction patterns to a multitude of stimuli that vary among individuals.”
Etiology and impact
Dr. Thiboutot and colleagues summarized the management options and recommendations from a committee of 27 experts who assessed the data on rosacea therapies using the updated standard classification system. They also highlighted the suspected systemic nature of rosacea etiology and its psychosocial impact on those with the condition.
“Recent studies have found an association between rosacea and increased risk of a growing number of systemic disorders, including cardiovascular, gastrointestinal, neurologic, and autoimmune diseases as well as certain types of cancer,” the authors wrote. “These findings further elevate the clinical significance of rosacea as growing evidence of its potential link with systemic inflammation is increasingly understood.”
Dr. Thiboutot said that research has implicated both the innate and adaptive immune systems and the neuromuscular system in rosacea’s underpinnings.
“Many of the triggers associated with clinical exacerbation of rosacea are known to activate the immune system and/or the neurovasculature, such as demodex, sunlight, alcohol, and changes in temperature,” she said, adding that therapies targeting the neurovascular effects of rosacea are particularly needed.
More than 50% of patients with rosacea have ocular manifestations, with symptoms such as “dryness, burning and stinging, light sensitivity, blurred vision, and foreign body sensation,” the authors reported.
Diagnosis and management
Without definitive laboratory tests, it’s essential that the clinical diagnosis takes into account not only the physical examination findings, but also patient history, the authors stressed, since “some features may not be visually evident or present at the time of the patient’s visit.”
The authors also recommend taking into account patients’ perception and response to their appearance and its effects on their emotional, social, and professional lives when selecting interventions.
“Rosacea’s unsightly and conspicuous appearance often has significant emotional ramifications, potentially resulting in depression or anxiety, and frequently interferes with social and occupational interactions,” the authors wrote. “It may also be advisable to remind patients that normalization of skin tone and color is the goal rather than complete eradication of facial coloration, which can leave the face with a sallow appearance.”
Therapy will often require multiple agents, such as topical and oral agents for inflammatory papules/pustules of rosacea. If insufficient, adjunctive therapy with oral antibiotics or retinoids may be options, though the latter requires prevention of pregnancy during treatment. The authors also discussed pharmacological treatments for facial erythema and flushing, with all these drugs organized in tables according to symptoms and their levels of evidence and effectiveness.
Despite limited clinical evidence, the authors noted success with two types of laser therapy – pulsed-dye and potassium titanyl phosphate – for telangiectasia and erythema. They also noted the option of intense pulsed light for flushing, ocular symptoms, and meibomian gland dysfunction, and of ablative lasers for rhinophymatous nose. But they highlighted the importance of being cautious when using these therapies on darker skin.
In their discussion of ocular rosacea, the authors described the various manifestations and the two “mainstays” of treatment: “eyelash hygiene and oral [omega-3] supplementation, followed by topical azithromycin or calcineurin inhibitors.” In addition to pharmacological and light therapy options, attention to environmental contributors and conscientious skin care regimens can benefit patients with rosacea as well.
“Clinicians may advise patients to keep a daily diary of lifestyle and environmental factors that appear to affect their rosacea to help identify and avoid their personal triggers,” the authors wrote. “The most common factors are sun exposure, emotional stress, hot weather, wind, heavy exercise, alcohol consumption, hot baths, cold weather, spicy foods, humidity, indoor heat, certain skin-care products, heated beverages, certain medications, medical conditions, certain fruits, marinated meats, certain vegetables, and dairy products.”
The paper also emphasizes the importance of gentle skin care given the highly sensitive and easily irritated skin of patients with rosacea. Sunscreen use, particularly with mineral sunscreens that provide physical barriers and reflect ultraviolet light, should be a key aspect of patients’ skin care, and clinicians should advise patients to seek out gentle, nonirritating cleansers.
Funding was provided by the National Rosacea Society, which receives funding from patients and corporations that include Aclaris Therapeutics, Allergan, Bayer, Cutanea Life Sciences, and Galderma Laboratories. Dr. Thiboutot consults for Galderma. Six of the other nine coauthors have financial links to industry through advisory boards, consulting, or research funding.
SOURCE: Thiboutot D et al. J Am Acad Dermatol. 2020;82:1501-10.
, as was previously practiced, according to an update on options for managing rosacea published in the Journal of the American Academy of Dermatology.
The update, by the National Rosacea Society Expert Committee, is based on a review of the evidence, and is a follow-up to the classification system for rosacea that was updated in 2017, which recommended classification of rosacea based on phenotype (Am Acad Dermatol. 2018;78:148-155).
The key take-away is “that patients shouldn’t be classified as having a certain subtype of rosacea” since “many patients have features that overlap more than one subtype,” lead author of the management update, Diane Thiboutot, MD, professor of dermatology and associate dean of clinical and translational research education at Penn State University, Hershey, said in an interview.
“There is an opportunity for physicians to recognize that the symptom complex of rosacea differs widely and treatments should be selected to address the symptoms experienced by the patient, particularly with regard to ocular rosacea,” she said.
Until there were updated guidelines on rosacea classification, published in 2018, relying primarily on diagnostic subtypes “tended to limit consideration of the full range of potential signs and symptoms as well as the frequent simultaneous occurrence of more than one subtype or the potential progression from one subtype to another,” Dr. Thiboutot and coauthors wrote in the management update (J Am Acad Dermatol 2020;82:1501-10).
“The more we learn, the more complex rosacea becomes,” she said in the interview. “The clinical manifestations of rosacea are so varied, ranging from skin erythema, eye findings, papules and pustules to rhinophyma, [that] it calls into question, if all of these are actually one disease (rosacea) or if they represent localized reaction patterns to a multitude of stimuli that vary among individuals.”
Etiology and impact
Dr. Thiboutot and colleagues summarized the management options and recommendations from a committee of 27 experts who assessed the data on rosacea therapies using the updated standard classification system. They also highlighted the suspected systemic nature of rosacea etiology and its psychosocial impact on those with the condition.
“Recent studies have found an association between rosacea and increased risk of a growing number of systemic disorders, including cardiovascular, gastrointestinal, neurologic, and autoimmune diseases as well as certain types of cancer,” the authors wrote. “These findings further elevate the clinical significance of rosacea as growing evidence of its potential link with systemic inflammation is increasingly understood.”
Dr. Thiboutot said that research has implicated both the innate and adaptive immune systems and the neuromuscular system in rosacea’s underpinnings.
“Many of the triggers associated with clinical exacerbation of rosacea are known to activate the immune system and/or the neurovasculature, such as demodex, sunlight, alcohol, and changes in temperature,” she said, adding that therapies targeting the neurovascular effects of rosacea are particularly needed.
More than 50% of patients with rosacea have ocular manifestations, with symptoms such as “dryness, burning and stinging, light sensitivity, blurred vision, and foreign body sensation,” the authors reported.
Diagnosis and management
Without definitive laboratory tests, it’s essential that the clinical diagnosis takes into account not only the physical examination findings, but also patient history, the authors stressed, since “some features may not be visually evident or present at the time of the patient’s visit.”
The authors also recommend taking into account patients’ perception and response to their appearance and its effects on their emotional, social, and professional lives when selecting interventions.
“Rosacea’s unsightly and conspicuous appearance often has significant emotional ramifications, potentially resulting in depression or anxiety, and frequently interferes with social and occupational interactions,” the authors wrote. “It may also be advisable to remind patients that normalization of skin tone and color is the goal rather than complete eradication of facial coloration, which can leave the face with a sallow appearance.”
Therapy will often require multiple agents, such as topical and oral agents for inflammatory papules/pustules of rosacea. If insufficient, adjunctive therapy with oral antibiotics or retinoids may be options, though the latter requires prevention of pregnancy during treatment. The authors also discussed pharmacological treatments for facial erythema and flushing, with all these drugs organized in tables according to symptoms and their levels of evidence and effectiveness.
Despite limited clinical evidence, the authors noted success with two types of laser therapy – pulsed-dye and potassium titanyl phosphate – for telangiectasia and erythema. They also noted the option of intense pulsed light for flushing, ocular symptoms, and meibomian gland dysfunction, and of ablative lasers for rhinophymatous nose. But they highlighted the importance of being cautious when using these therapies on darker skin.
In their discussion of ocular rosacea, the authors described the various manifestations and the two “mainstays” of treatment: “eyelash hygiene and oral [omega-3] supplementation, followed by topical azithromycin or calcineurin inhibitors.” In addition to pharmacological and light therapy options, attention to environmental contributors and conscientious skin care regimens can benefit patients with rosacea as well.
“Clinicians may advise patients to keep a daily diary of lifestyle and environmental factors that appear to affect their rosacea to help identify and avoid their personal triggers,” the authors wrote. “The most common factors are sun exposure, emotional stress, hot weather, wind, heavy exercise, alcohol consumption, hot baths, cold weather, spicy foods, humidity, indoor heat, certain skin-care products, heated beverages, certain medications, medical conditions, certain fruits, marinated meats, certain vegetables, and dairy products.”
The paper also emphasizes the importance of gentle skin care given the highly sensitive and easily irritated skin of patients with rosacea. Sunscreen use, particularly with mineral sunscreens that provide physical barriers and reflect ultraviolet light, should be a key aspect of patients’ skin care, and clinicians should advise patients to seek out gentle, nonirritating cleansers.
Funding was provided by the National Rosacea Society, which receives funding from patients and corporations that include Aclaris Therapeutics, Allergan, Bayer, Cutanea Life Sciences, and Galderma Laboratories. Dr. Thiboutot consults for Galderma. Six of the other nine coauthors have financial links to industry through advisory boards, consulting, or research funding.
SOURCE: Thiboutot D et al. J Am Acad Dermatol. 2020;82:1501-10.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Energy-based therapy plus oxymetazoline proves safe for rosacea
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
FROM LASERS IN SURGERY AND MEDICINE
Studies add clarity to link between rosacea and Demodex, coffee
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
LAHAINA, HAWAII – Recent data on the roles of caffeinated coffee and two types of Demodex species play in rosacea were discussed by Linda Stein Gold, MD, at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
When considering rosacea triggers, the role of coffee has been difficult to determine, according to Dr. Stein Gold, director of dermatology research at the Henry Ford Health System in Detroit.
“We know that caffeine can vasoconstrict, it also has anti-inflammatory properties so ... that might help rosacea,” while the heat from a hot cup of coffee may cause vasodilation “and make rosacea worse,” she noted.
But a recent study of data from the Nurses’ Health Study II that evaluated intake of coffee, tea, soda, and chocolate every 4 years in over 82,000 women shed some light on the role coffee may play (JAMA Dermatol. 2018 Dec 1;154[12]:1394-1400). There were almost 5,000 cases of physician-diagnosed rosacea in the cohort. When the investigators looked at caffeinated coffee consumption, she said.
Those who consumed four or more servings of caffeinated coffee a day had a significantly lower risk of rosacea, compared with those who consumed one or fewer servings per month (hazard ratio, 0.77; 95% confidence interval, 0.69-0.87; P less than .001).
But there was no significant association with decaffeinated coffee or with edibles that contained caffeine such as tea, soda, and chocolate, “so something about caffeinated coffee seems to be protective for the development of rosacea,” Dr. Stein Gold said.
Demodex mites
A few years ago, “we really didn’t think much of Demodex, but now we know Demodex tends to be a key player” in people with rosacea, Dr. Stein Gold said.
In adults, the colonization rate of Demodex ranges from 70% to 100%, but the skin of people with rosacea have a particularly high density of Demodex: About 35%-50% of patients with rosacea have an increased Demodex load above 5 mites per cm2, as measured with a standard skin surface biopsy, she noted. The density of Demodex in the skin of patients with rosacea has been measured at sixfold higher, compared with age-matched controls.
There also are two different Demodex species: Demodex folliculorum, which are longer, and Demodex brevis, which are short, and there is evidence that each “may cause an individual reaction,” Dr. Stein Gold said.
She referred to a study that found a difference in the Demodex population in patients with highly inflammatory disease with a high level of Demodex, mild rosacea patients who did not have a lot of Demodex, and people with no rosacea (Dermatol Reports. 2019 Jan 23;11[1]:7675).
“Those people who had really severe, inflammatory rosacea had Demodex folliculorum,” and the patients with the more mild disease or those with clear skin had Demodex brevis, she said, so “different species of Demodex might cause a different inflammatory reaction within individual rosacea patients.”
Dr. Stein Gold reported that she has served as a consultant, investigator, or speaker for Galderma, Dermira, Foamix Pharmaceuticals, Valeant (now Bausch Health), Allergan, Actavis, and Roche.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR