User login
Innovations in pediatric chronic pain management
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
At the new Walnut Creek Clinic in the East Bay of the San Francisco Bay area, kids get a “Comfort Promise.”
The clinic extends the work of the Stad Center for Pediatric Pain, Palliative & Integrative Medicine beyond the locations in University of California San Francisco Benioff Children’s Hospitals in San Francisco and Oakland.
At Walnut Creek, clinical acupuncturists, massage therapists, and specialists in hypnosis complement advanced medical care with integrative techniques.
The “Comfort Promise” program, which is being rolled out at that clinic and other UCSF pediatric clinics through the end of 2024, is the clinicians’ pledge to do everything in their power to make tests, infusions, and vaccinations “practically pain free.”
Needle sticks, for example, can be a common source of pain and anxiety for kids. Techniques to minimize pain vary by age. Among the ways the clinicians minimize needle pain for a child 6- to 12-years-old are:
- Giving the child control options to pick which arm; and watch the injection, pause it, or stop it with a communication sign.
- Introducing memory shaping by asking the child about the experience afterward and presenting it in a positive way by praising the acts of sitting still, breathing deeply, or being brave.
- Using distractors such as asking the child to hold a favorite item from home, storytelling, coloring, singing, or using breathing exercises.
Stefan Friedrichsdorf, MD, chief of the UCSF division of pediatric pain, palliative & integrative medicine, said in a statement: “For kids with chronic pain, complex pain medications can cause more harm than benefit. Our goal is to combine exercise and physical therapy with integrative medicine and skills-based psychotherapy to help them become pain free in their everyday life.”
Bundling appointments for early impact
At Lurie Children’s Hospital of Chicago, the chronic pain treatment program bundles visits with experts in several disciplines, include social workers, psychologists, and physical therapists, in addition to the medical team, so that patients can complete a first round of visits with multiple specialists in a short period, as opposed to several months.
Natalie Weatherred, APRN-NP, CPNP-PC, a pediatric nurse practitioner in anesthesiology and the pain clinic coordinator, said in an interview that the up-front visits involve between four and eight follow-up sessions in a short period with everybody in the multidisciplinary team “to really help jump-start their pain treatment.”
She pointed out that many families come from distant parts of the state or beyond so the bundled appointments are also important for easing burden on families.
Sarah Duggan, APRN-NP, CPNP-PC, also a pediatric nurse practitioner in anesthesiology at Lurie’s, pointed out that patients at their clinic often have other chronic conditions as well, such as such as postural orthostatic tachycardia syndrome so the care integration is particularly important.
“We can get them the appropriate care that they need and the resources they need, much sooner than we would have been able to do 5 or 10 years ago,” Ms. Duggan said.
Virtual reality distraction instead of sedation
Henry Huang, MD, anesthesiologist and pain physician at Texas Children’s Hospital, Houston, said a special team there collaborates with the Chariot Program at Stanford (Calif.) University and incorporates virtual reality to distract children from pain and anxiety and harness their imaginations during induction for anesthesia, intravenous placement, and vaccinations.
“At our institution we’ve been recruiting patients to do a proof of concept to do virtual reality distraction for pain procedures, such as nerve blocks or steroid injections,” Dr. Huang said.
Traditionally, kids would have received oral or intravenous sedation to help them cope with the fear and pain.
“We’ve been successful in several cases without relying on any sedation,” he said. “The next target is to expand that to the chronic pain population.”
The distraction techniques are promising for a wide range of ages, he said, and the programming is tailored to the child’s ability to interact with the technology.
He said he is also part of a group promoting use of ultrasound instead of x-rays to guide injections to the spine and chest to reduce children’s exposure to radiation. His group is helping teach these methods to other clinicians nationally.
Dr. Huang said the most important development in chronic pediatric pain has been the growth of rehab centers that include the medical team, and practitioners from psychology as well as occupational and physical therapy.
“More and more hospitals are recognizing the importance of these pain rehab centers,” he said.
The problem, Dr. Huang said, is that these programs have always been resource intensive and involve highly specialized clinicians. The cost and the limited number of specialists make it difficult for widespread rollout.
“That’s always been the challenge from the pediatric pain world,” he said.
Recognizing the complexity of kids’ chronic pain
Angela Garcia, MD, a consulting physician for pediatric rehabilitation medicine at UPMC Children’s Hospital of Pittsburgh said
Techniques such as biofeedback and acupuncture are becoming more mainstream in pediatric chronic care, she said.
At the UPMC clinic, children and their families talk with a care team about their values and what they want to accomplish in managing the child’s pain. They ask what the pain is preventing the child from doing.
“Their goals really are our goals,” she said.
She said she also refers almost all patients to one of the center’s pain psychologists.
“Pain is biopsychosocial,” she said. “We want to make sure we’re addressing how to cope with pain.”
Dr. Garcia said she hopes nutritional therapy is one of the next approaches the clinic will incorporate, particularly surrounding how dietary changes can reduce inflammation “and heal the body from the inside out.”
She said the hospital is also looking at developing an inpatient pain program for kids whose functioning has changed so drastically that they need more intensive therapies.
Whatever the treatment approach, she said, addressing the pain early is critical.
“There is an increased risk of a child with chronic pain becoming an adult with chronic pain,” Dr. Garcia pointed out, “and that can lead to a decrease in the ability to participate in society.”
Ms. Weatherred, Ms. Duggan, Dr. Huang, and Dr. Garcia reported no relevant financial relationships.
Polio in the US? Yes, and it prompted ACIP to update its recs
The Advisory Committee on Immunization Practices (ACIP) recently issued new recommendations on polio vaccine for adults. The ACIP decided to update its previous recommendations (from 2000) in response to a case in New York that demonstrated the United States is at risk for poliovirus importation as long as the disease has not been eliminated worldwide.1
What happened in New York? In July 2022, a case of paralytic polio was confirmed in an unvaccinated adult in Rockland County, New York, an area that has low polio vaccine coverage. Subsequent testing of wastewater systems detected poliovirus in a total of 5 New York counties (including 2 in New York City).1
The Centers for Disease Control and Prevention estimates that this region of the state probably experienced 1000 to 2000 nonparalytic, mostly asymptomatic poliovirus infections. The virus detected in wastewater in New York is genetically linked to polioviruses collected in wastewater in Israel, the United Kingdom, and Canada. No poliovirus has been detected in these wastewater systems since late 2022.1,2
Why there’s reason for concern. Routine immunization against polio has been part of the immunization schedule for infants and children since the mid-1950s. As a result, endemic polio was eliminated in the United States in 1979 and in the Western Hemisphere in 1994.
However, adult vaccination until now has been recommended only for those at risk for exposure to poliovirus by way of travel or occupation. And while most adults in the United States are immune to polio due to childhood vaccination, unvaccinated adults remain susceptible if exposed to poliovirus—as demonstrated in the New York case.
What does the ACIP now recommend? Two recommendations were adopted by the ACIP this June to address this problem2:
- Adults who are known or suspected to be unvaccinated or incompletely vaccinated against polio should complete a primary vaccination series with inactivated polio vaccine (IPV).
- Adults who have received a primary series of oral polio vaccine (OPV) or IPV in any combination and who are at increased risk for poliovirus exposure may receive another dose of IPV. Available data do not indicate a need for > 1 lifetime booster.
A few details: To be considered fully vaccinated, a patient must have received a primary series of ≥ 3 doses of OPV or IPV (in any combination) given at least 4 weeks apart, with the last dose given on or after the 4th birthday and at least 6 months from the previous dose. Most adults who were born and raised in the United States can assume they were vaccinated against polio as children, unless there are specific reasons to suspect otherwise.2
Individuals considered to be at increased risk include: travelers who are going to countries where polio is epidemic or endemic; laboratory and health care workers who handle specimens that might contain polioviruses; and health care workers or other caregivers who have close contact with a person who could be infected with poliovirus.2
Take-home message. Be prepared to discuss and offer IPV (the only form of the vaccine currently in use in the United States) to adults, as either a one-time booster for those at increased risk for exposure to poliovirus or a complete series for those you know or suspect to be unvaccinated or incompletely vaccinated.
1. Ryerson AB, Lang D, Alazawi MA, et al; US Poliovirus Response Team. Wastewater testing and detection of poliovirus type 2 genetically linked to virus isolated from a paralytic polio case—New York, March 9-October 11, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1418-1424. doi: 10.15585/mmwr.mm7144e2
2. Kidd S. Adult polio vaccination. Presented to the ACIP on June 21, 2023. Accessed July 24, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-POLIO-Kidd-Jun-2023.pdf
The Advisory Committee on Immunization Practices (ACIP) recently issued new recommendations on polio vaccine for adults. The ACIP decided to update its previous recommendations (from 2000) in response to a case in New York that demonstrated the United States is at risk for poliovirus importation as long as the disease has not been eliminated worldwide.1
What happened in New York? In July 2022, a case of paralytic polio was confirmed in an unvaccinated adult in Rockland County, New York, an area that has low polio vaccine coverage. Subsequent testing of wastewater systems detected poliovirus in a total of 5 New York counties (including 2 in New York City).1
The Centers for Disease Control and Prevention estimates that this region of the state probably experienced 1000 to 2000 nonparalytic, mostly asymptomatic poliovirus infections. The virus detected in wastewater in New York is genetically linked to polioviruses collected in wastewater in Israel, the United Kingdom, and Canada. No poliovirus has been detected in these wastewater systems since late 2022.1,2
Why there’s reason for concern. Routine immunization against polio has been part of the immunization schedule for infants and children since the mid-1950s. As a result, endemic polio was eliminated in the United States in 1979 and in the Western Hemisphere in 1994.
However, adult vaccination until now has been recommended only for those at risk for exposure to poliovirus by way of travel or occupation. And while most adults in the United States are immune to polio due to childhood vaccination, unvaccinated adults remain susceptible if exposed to poliovirus—as demonstrated in the New York case.
What does the ACIP now recommend? Two recommendations were adopted by the ACIP this June to address this problem2:
- Adults who are known or suspected to be unvaccinated or incompletely vaccinated against polio should complete a primary vaccination series with inactivated polio vaccine (IPV).
- Adults who have received a primary series of oral polio vaccine (OPV) or IPV in any combination and who are at increased risk for poliovirus exposure may receive another dose of IPV. Available data do not indicate a need for > 1 lifetime booster.
A few details: To be considered fully vaccinated, a patient must have received a primary series of ≥ 3 doses of OPV or IPV (in any combination) given at least 4 weeks apart, with the last dose given on or after the 4th birthday and at least 6 months from the previous dose. Most adults who were born and raised in the United States can assume they were vaccinated against polio as children, unless there are specific reasons to suspect otherwise.2
Individuals considered to be at increased risk include: travelers who are going to countries where polio is epidemic or endemic; laboratory and health care workers who handle specimens that might contain polioviruses; and health care workers or other caregivers who have close contact with a person who could be infected with poliovirus.2
Take-home message. Be prepared to discuss and offer IPV (the only form of the vaccine currently in use in the United States) to adults, as either a one-time booster for those at increased risk for exposure to poliovirus or a complete series for those you know or suspect to be unvaccinated or incompletely vaccinated.
The Advisory Committee on Immunization Practices (ACIP) recently issued new recommendations on polio vaccine for adults. The ACIP decided to update its previous recommendations (from 2000) in response to a case in New York that demonstrated the United States is at risk for poliovirus importation as long as the disease has not been eliminated worldwide.1
What happened in New York? In July 2022, a case of paralytic polio was confirmed in an unvaccinated adult in Rockland County, New York, an area that has low polio vaccine coverage. Subsequent testing of wastewater systems detected poliovirus in a total of 5 New York counties (including 2 in New York City).1
The Centers for Disease Control and Prevention estimates that this region of the state probably experienced 1000 to 2000 nonparalytic, mostly asymptomatic poliovirus infections. The virus detected in wastewater in New York is genetically linked to polioviruses collected in wastewater in Israel, the United Kingdom, and Canada. No poliovirus has been detected in these wastewater systems since late 2022.1,2
Why there’s reason for concern. Routine immunization against polio has been part of the immunization schedule for infants and children since the mid-1950s. As a result, endemic polio was eliminated in the United States in 1979 and in the Western Hemisphere in 1994.
However, adult vaccination until now has been recommended only for those at risk for exposure to poliovirus by way of travel or occupation. And while most adults in the United States are immune to polio due to childhood vaccination, unvaccinated adults remain susceptible if exposed to poliovirus—as demonstrated in the New York case.
What does the ACIP now recommend? Two recommendations were adopted by the ACIP this June to address this problem2:
- Adults who are known or suspected to be unvaccinated or incompletely vaccinated against polio should complete a primary vaccination series with inactivated polio vaccine (IPV).
- Adults who have received a primary series of oral polio vaccine (OPV) or IPV in any combination and who are at increased risk for poliovirus exposure may receive another dose of IPV. Available data do not indicate a need for > 1 lifetime booster.
A few details: To be considered fully vaccinated, a patient must have received a primary series of ≥ 3 doses of OPV or IPV (in any combination) given at least 4 weeks apart, with the last dose given on or after the 4th birthday and at least 6 months from the previous dose. Most adults who were born and raised in the United States can assume they were vaccinated against polio as children, unless there are specific reasons to suspect otherwise.2
Individuals considered to be at increased risk include: travelers who are going to countries where polio is epidemic or endemic; laboratory and health care workers who handle specimens that might contain polioviruses; and health care workers or other caregivers who have close contact with a person who could be infected with poliovirus.2
Take-home message. Be prepared to discuss and offer IPV (the only form of the vaccine currently in use in the United States) to adults, as either a one-time booster for those at increased risk for exposure to poliovirus or a complete series for those you know or suspect to be unvaccinated or incompletely vaccinated.
1. Ryerson AB, Lang D, Alazawi MA, et al; US Poliovirus Response Team. Wastewater testing and detection of poliovirus type 2 genetically linked to virus isolated from a paralytic polio case—New York, March 9-October 11, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1418-1424. doi: 10.15585/mmwr.mm7144e2
2. Kidd S. Adult polio vaccination. Presented to the ACIP on June 21, 2023. Accessed July 24, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-POLIO-Kidd-Jun-2023.pdf
1. Ryerson AB, Lang D, Alazawi MA, et al; US Poliovirus Response Team. Wastewater testing and detection of poliovirus type 2 genetically linked to virus isolated from a paralytic polio case—New York, March 9-October 11, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1418-1424. doi: 10.15585/mmwr.mm7144e2
2. Kidd S. Adult polio vaccination. Presented to the ACIP on June 21, 2023. Accessed July 24, 2023. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/02-POLIO-Kidd-Jun-2023.pdf
Partial immunization leaves children and communities at risk, study finds
TOPLINE
A new American Academy of Pediatrics study reveals that 17.2% of toddlers started but did not finish at least one recommended early childhood vaccine series.
METHODOLOGY
- Examined data collected in 2019 from the National Immunization Survey – Child.
- 16,365 children ages 19-35 months were included.
- Vaccines for diphtheria, tetanus, acellular pertussis, pneumococcal infections, Haemophilus influenzae type b, hepatitis B, polio, measles, mumps, rubella, and varicella were included.
TAKEAWAY
- 72.9% of toddlers completed the seven-vaccine series.
- 17.2% initiated but did not complete one or more of a multidose vaccine series.
- The strongest association with not completing the vaccine series was moving across state lines and not having insurance.
- Children with more siblings at home were less likely to complete a vaccine series.
IN PRACTICE
The study suggests that the “children experienced structural barriers to vaccination,” and the authors urge an “increased focus on strategies to encourage multidose series completion ... to optimize protection from preventable diseases and achieve vaccination coverage goals.”
SOURCE
The study was funded by the National Institutes of Health and published online July 25 in Pediatrics. Sarah Y. Michels, an epidemiology specialist from the University of Montana in Missoula, was the lead author.
LIMITATIONS
Though the researchers studied the risk factors for series noncompletion, they did not have information on the specific reasons why children were missing vaccine doses. Children whose parents chose to participate in the National Immunization Survey – Child may have had higher vaccination coverage than children whose parents declined participation.
DISCLOSURES
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE
A new American Academy of Pediatrics study reveals that 17.2% of toddlers started but did not finish at least one recommended early childhood vaccine series.
METHODOLOGY
- Examined data collected in 2019 from the National Immunization Survey – Child.
- 16,365 children ages 19-35 months were included.
- Vaccines for diphtheria, tetanus, acellular pertussis, pneumococcal infections, Haemophilus influenzae type b, hepatitis B, polio, measles, mumps, rubella, and varicella were included.
TAKEAWAY
- 72.9% of toddlers completed the seven-vaccine series.
- 17.2% initiated but did not complete one or more of a multidose vaccine series.
- The strongest association with not completing the vaccine series was moving across state lines and not having insurance.
- Children with more siblings at home were less likely to complete a vaccine series.
IN PRACTICE
The study suggests that the “children experienced structural barriers to vaccination,” and the authors urge an “increased focus on strategies to encourage multidose series completion ... to optimize protection from preventable diseases and achieve vaccination coverage goals.”
SOURCE
The study was funded by the National Institutes of Health and published online July 25 in Pediatrics. Sarah Y. Michels, an epidemiology specialist from the University of Montana in Missoula, was the lead author.
LIMITATIONS
Though the researchers studied the risk factors for series noncompletion, they did not have information on the specific reasons why children were missing vaccine doses. Children whose parents chose to participate in the National Immunization Survey – Child may have had higher vaccination coverage than children whose parents declined participation.
DISCLOSURES
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
TOPLINE
A new American Academy of Pediatrics study reveals that 17.2% of toddlers started but did not finish at least one recommended early childhood vaccine series.
METHODOLOGY
- Examined data collected in 2019 from the National Immunization Survey – Child.
- 16,365 children ages 19-35 months were included.
- Vaccines for diphtheria, tetanus, acellular pertussis, pneumococcal infections, Haemophilus influenzae type b, hepatitis B, polio, measles, mumps, rubella, and varicella were included.
TAKEAWAY
- 72.9% of toddlers completed the seven-vaccine series.
- 17.2% initiated but did not complete one or more of a multidose vaccine series.
- The strongest association with not completing the vaccine series was moving across state lines and not having insurance.
- Children with more siblings at home were less likely to complete a vaccine series.
IN PRACTICE
The study suggests that the “children experienced structural barriers to vaccination,” and the authors urge an “increased focus on strategies to encourage multidose series completion ... to optimize protection from preventable diseases and achieve vaccination coverage goals.”
SOURCE
The study was funded by the National Institutes of Health and published online July 25 in Pediatrics. Sarah Y. Michels, an epidemiology specialist from the University of Montana in Missoula, was the lead author.
LIMITATIONS
Though the researchers studied the risk factors for series noncompletion, they did not have information on the specific reasons why children were missing vaccine doses. Children whose parents chose to participate in the National Immunization Survey – Child may have had higher vaccination coverage than children whose parents declined participation.
DISCLOSURES
The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CDC offers guidance on RSV vaccines for adults
Two newly approved respiratory syncytial virus (RSV) vaccines for adults aged 60 years and older may be able to prevent illness in those at risk for severe RSV disease.
Most adult RSV illness occurs among the older age group and results in an estimated 60,000-160,000 hospitalizations and 6,000-10,000 deaths per year among people aged at least 65 years.
Older adults deciding whether to get the vaccines should weigh risks and their own preferences and make the decision in consultation with their clinicians, said authors of a Centers for Disease Control and Prevention report.
Michael Melgar, MD, with the Coronavirus and Other Respiratory Viruses Division at the CDC, was lead author on the report, published in the Morbidity and Mortality Weekly Report.
Two new vaccines
In May, the Food and Drug Administration approved the first of two vaccines for preventing RSV lower respiratory tract disease for adults aged at least 60 years.
On June 21, the Advisory Committee on Immunization Practices (ACIP) recommended that people in that age group receive a single dose of RSV vaccine using shared decision-making.
The recommendation for shared decision-making makes the ACIP decision different from routine and risk-based vaccine recommendations. Rather than targeting all in a particular age group or risk group, the decision calls for consideration of a patients’ risk for disease and their characteristics, preferences, and values; the health care professional’s clinical discretion; and performance of the vaccine.
Dr. Melgar and colleagues reported that vaccination with one dose of the GSK or Pfizer RSV vaccines has proved moderately to highly effective in preventing symptomatic RSV-associated lower respiratory tract disease over two consecutive RSV seasons among people aged 60 and older.
The trials that led to approval weren’t powered to gauge efficacy against RSV-associated hospitalization and death. However, the authors wrote, the prevention of lower respiratory tract disease, including medically attended illness, suggests that the shots might prevent considerable morbidity from RSV disease among those aged 60 and older.
Both vaccines were generally well tolerated with a good safety profile. However, six cases of inflammatory neurologic events (including Guillain-Barré Syndrome, acute disseminated encephalomyelitis, and others) were reported in clinical trials after RSV vaccination.
“Whether these events occurred due to chance, or whether RSV vaccination increases the risk for inflammatory neurologic events, is currently unknown,” the authors wrote.
Postmarketing surveillance may help clarify the existence of any potential risk, but until those results are clearer, the CDC researchers said, RSV vaccinations should be targeted to older adults at highest risk for severe RSV and those most likely to benefit from the shots.
At higher risk
Some adults with certain medical conditions have a higher risk for RSV-associated hospitalization, according to the report.
Those conditions include chronic obstructive pulmonary disease, asthma, heart failure, coronary artery disease, cerebrovascular disease, diabetes mellitus, and chronic kidney disease.
People who are frail and of advanced age also are at higher risk for RSV hospitalization. That risk increases with age and the highest risk is for people aged at least 75 years.
The researchers added that RSV can cause severe disease in those with compromised immunity, including people who have received hematopoietic stem cell transplants and patients taking immunosuppressive drugs such as those used with solid organ transplants, cancer treatment, or other conditions.
As for when physicians should offer the vaccinations, shots are optimally given before the start of the RSV season.
However, the COVID-19 pandemic interrupted the seasonality and the timing has not yet returned to prepandemic patterns.
For the 2023-24 season, this report states, clinicians should offer RSV vaccination to adults aged at least 60 years using shared clinical decision-making as early as vaccine supply is available and should continue to offer vaccination to eligible adults who remain unvaccinated.
RSV vaccines can be administered with other adult vaccines during the same visit, the authors confirmed.
A version of this article first appeared on Medscape.com.
Two newly approved respiratory syncytial virus (RSV) vaccines for adults aged 60 years and older may be able to prevent illness in those at risk for severe RSV disease.
Most adult RSV illness occurs among the older age group and results in an estimated 60,000-160,000 hospitalizations and 6,000-10,000 deaths per year among people aged at least 65 years.
Older adults deciding whether to get the vaccines should weigh risks and their own preferences and make the decision in consultation with their clinicians, said authors of a Centers for Disease Control and Prevention report.
Michael Melgar, MD, with the Coronavirus and Other Respiratory Viruses Division at the CDC, was lead author on the report, published in the Morbidity and Mortality Weekly Report.
Two new vaccines
In May, the Food and Drug Administration approved the first of two vaccines for preventing RSV lower respiratory tract disease for adults aged at least 60 years.
On June 21, the Advisory Committee on Immunization Practices (ACIP) recommended that people in that age group receive a single dose of RSV vaccine using shared decision-making.
The recommendation for shared decision-making makes the ACIP decision different from routine and risk-based vaccine recommendations. Rather than targeting all in a particular age group or risk group, the decision calls for consideration of a patients’ risk for disease and their characteristics, preferences, and values; the health care professional’s clinical discretion; and performance of the vaccine.
Dr. Melgar and colleagues reported that vaccination with one dose of the GSK or Pfizer RSV vaccines has proved moderately to highly effective in preventing symptomatic RSV-associated lower respiratory tract disease over two consecutive RSV seasons among people aged 60 and older.
The trials that led to approval weren’t powered to gauge efficacy against RSV-associated hospitalization and death. However, the authors wrote, the prevention of lower respiratory tract disease, including medically attended illness, suggests that the shots might prevent considerable morbidity from RSV disease among those aged 60 and older.
Both vaccines were generally well tolerated with a good safety profile. However, six cases of inflammatory neurologic events (including Guillain-Barré Syndrome, acute disseminated encephalomyelitis, and others) were reported in clinical trials after RSV vaccination.
“Whether these events occurred due to chance, or whether RSV vaccination increases the risk for inflammatory neurologic events, is currently unknown,” the authors wrote.
Postmarketing surveillance may help clarify the existence of any potential risk, but until those results are clearer, the CDC researchers said, RSV vaccinations should be targeted to older adults at highest risk for severe RSV and those most likely to benefit from the shots.
At higher risk
Some adults with certain medical conditions have a higher risk for RSV-associated hospitalization, according to the report.
Those conditions include chronic obstructive pulmonary disease, asthma, heart failure, coronary artery disease, cerebrovascular disease, diabetes mellitus, and chronic kidney disease.
People who are frail and of advanced age also are at higher risk for RSV hospitalization. That risk increases with age and the highest risk is for people aged at least 75 years.
The researchers added that RSV can cause severe disease in those with compromised immunity, including people who have received hematopoietic stem cell transplants and patients taking immunosuppressive drugs such as those used with solid organ transplants, cancer treatment, or other conditions.
As for when physicians should offer the vaccinations, shots are optimally given before the start of the RSV season.
However, the COVID-19 pandemic interrupted the seasonality and the timing has not yet returned to prepandemic patterns.
For the 2023-24 season, this report states, clinicians should offer RSV vaccination to adults aged at least 60 years using shared clinical decision-making as early as vaccine supply is available and should continue to offer vaccination to eligible adults who remain unvaccinated.
RSV vaccines can be administered with other adult vaccines during the same visit, the authors confirmed.
A version of this article first appeared on Medscape.com.
Two newly approved respiratory syncytial virus (RSV) vaccines for adults aged 60 years and older may be able to prevent illness in those at risk for severe RSV disease.
Most adult RSV illness occurs among the older age group and results in an estimated 60,000-160,000 hospitalizations and 6,000-10,000 deaths per year among people aged at least 65 years.
Older adults deciding whether to get the vaccines should weigh risks and their own preferences and make the decision in consultation with their clinicians, said authors of a Centers for Disease Control and Prevention report.
Michael Melgar, MD, with the Coronavirus and Other Respiratory Viruses Division at the CDC, was lead author on the report, published in the Morbidity and Mortality Weekly Report.
Two new vaccines
In May, the Food and Drug Administration approved the first of two vaccines for preventing RSV lower respiratory tract disease for adults aged at least 60 years.
On June 21, the Advisory Committee on Immunization Practices (ACIP) recommended that people in that age group receive a single dose of RSV vaccine using shared decision-making.
The recommendation for shared decision-making makes the ACIP decision different from routine and risk-based vaccine recommendations. Rather than targeting all in a particular age group or risk group, the decision calls for consideration of a patients’ risk for disease and their characteristics, preferences, and values; the health care professional’s clinical discretion; and performance of the vaccine.
Dr. Melgar and colleagues reported that vaccination with one dose of the GSK or Pfizer RSV vaccines has proved moderately to highly effective in preventing symptomatic RSV-associated lower respiratory tract disease over two consecutive RSV seasons among people aged 60 and older.
The trials that led to approval weren’t powered to gauge efficacy against RSV-associated hospitalization and death. However, the authors wrote, the prevention of lower respiratory tract disease, including medically attended illness, suggests that the shots might prevent considerable morbidity from RSV disease among those aged 60 and older.
Both vaccines were generally well tolerated with a good safety profile. However, six cases of inflammatory neurologic events (including Guillain-Barré Syndrome, acute disseminated encephalomyelitis, and others) were reported in clinical trials after RSV vaccination.
“Whether these events occurred due to chance, or whether RSV vaccination increases the risk for inflammatory neurologic events, is currently unknown,” the authors wrote.
Postmarketing surveillance may help clarify the existence of any potential risk, but until those results are clearer, the CDC researchers said, RSV vaccinations should be targeted to older adults at highest risk for severe RSV and those most likely to benefit from the shots.
At higher risk
Some adults with certain medical conditions have a higher risk for RSV-associated hospitalization, according to the report.
Those conditions include chronic obstructive pulmonary disease, asthma, heart failure, coronary artery disease, cerebrovascular disease, diabetes mellitus, and chronic kidney disease.
People who are frail and of advanced age also are at higher risk for RSV hospitalization. That risk increases with age and the highest risk is for people aged at least 75 years.
The researchers added that RSV can cause severe disease in those with compromised immunity, including people who have received hematopoietic stem cell transplants and patients taking immunosuppressive drugs such as those used with solid organ transplants, cancer treatment, or other conditions.
As for when physicians should offer the vaccinations, shots are optimally given before the start of the RSV season.
However, the COVID-19 pandemic interrupted the seasonality and the timing has not yet returned to prepandemic patterns.
For the 2023-24 season, this report states, clinicians should offer RSV vaccination to adults aged at least 60 years using shared clinical decision-making as early as vaccine supply is available and should continue to offer vaccination to eligible adults who remain unvaccinated.
RSV vaccines can be administered with other adult vaccines during the same visit, the authors confirmed.
A version of this article first appeared on Medscape.com.
FROM THE MMWR
Screening for hepatitis B: Where the CDC and USPSTF diverge
The Centers for Disease Control and Prevention (CDC) recently published new recommendations on screening for hepatitis B infection.1 They recommend screening all adults (ages 18 years and older) at least once.
These recommendations differ in a few ways from those of the US Preventive Services Task Force (USPSTF).2 This Practice Alert will highlight these differences but also point out areas of agreement between the 2 sets of recommendations—and discuss why 2 separate agencies in the US Department of Health and Human Services reached different conclusions on some issues.
First, some background on hepatitis B
An estimated 580,000 to 2.4 million people in the United States have chronic hepatitis B (CHB) infection—and as many as two-thirds are unaware of it.3 In 2020, the Department of Health and Human Services published the Viral Hepatitis National Strategic Plan for the United States with a stated goal of increasing awareness of infection status among those with hepatitis B virus (HBV) from 32% to 90% by 2030.4 People living in the United States but born outside the country are at highest risk for CHB; they account for 69% of those with the infection.5
The incidence of acute HBV infection has declined markedly since the HBV vaccine was recommended for high-risk adults in 1982 and universally for infants in 1991.6,7 Overall rates of HBV infection declined fairly steadily starting around 1987—but in 2014, rates began to increase, especially in those ages 40 to 59 years.8,9 In 2019, 3192 cases were reported; but when one factors in underreporting, the CDC estimates that the number is likely closer to 20,700.10 This uptick is one reason the Advisory Committee on Immunization Practices changed its HBV vaccination recommendation for adults from a risk-based to a universal recommendation for all unvaccinated adults through age 60 years.10
Chronic hepatitis B infection has serious consequences
The proportion of those infected with HBV who develop CHB differs by age at infection: 80% to 90% if infected during infancy, 30% if infected before age 6 years, and 1% to 12% if infected as an older child or adult.8
CHB infection can lead to chronic liver disease, including cirrhosis of the liver, liver cancer, and liver failure. About 25% of those who develop CHB infection during childhood and 15% of those who develop chronic infection after childhood will die prematurely from cirrhosis or liver cancer.8
The American Association for the Study of Liver Diseases (AASLD) classifies CHB into 4 phases that reflect the rate of viral replication and the patient’s immune response.11 These phases are:
- immune-tolerant (minimal inflammation and fibrosis)
- hepatitis B e-antigen (HBeAg)-positive immune-active (moderate-to-severe inflammation or fibrosis)
- inactive CHB (minimal necroinflammation but variable fibrosis), and
- HBeAg-negative immune reactivation (moderate-to-severe inflammation or fibrosis).11
Continue to: The progression from one phase...
The progression from one phase to the next varies by patient, and not all patients will progress through each phase. The AASLD recommends periodically monitoring the HBV DNA and alanine aminotransferase (ALT) levels in those with CHB to track the progression from one phase to the next and to guide treatment decisions.
Treatment can be beneficial for those who meet criteria
The evidence report prepared for USPSTF found that antiviral treatment of those with CHB infection resulted in improved intermediate outcomes (histologic improvement, loss of hepatitis B surface antigen [HBsAg], loss of HBeAg, HBeAg seroconversion, virologic suppression, and normalization of ALT levels). The magnitude of benefit varied by location and study design.12
In addition, the evidence review found that antiviral therapy was associated with a decreased risk for overall mortality (relative risk [RR] = 0.15; 95% CI, 0.03-0.69), cirrhosis (RR = 0.72; 95% CI, 0.29-1.77), and hepatocellular carcinoma (RR = 0.60; 95% CI, 0.16-2.33). However, these results came from studies that were “limited due to small numbers of trials, few events, and insufficient duration of follow-up.”12
The USPSTF and the CDC both judged that the intermediate outcome results, as well as findings that improved intermediate outcomes lead to decreases in chronic liver disease, are strong enough evidence for their recommendations.
However, not all patients with CHB infection require treatment; estimates of patients with HBV infection meeting AASLD criteria for treatment range from 24% to 48%.1 The AASLD guideline on the treatment of CHB infection is an excellent resource that makes recommendations on the initial evaluation, ongoing monitoring, and treatment decisions for those with CHB.11
Continue to: How CDC and USPSTF guidance on HBV screeinng differs
How CDC and USPSTF guidance on HBV screening differs
The CDC and USPSTF recommendations for HBV screening differ in 3 aspects: whom to screen, whom to classify as at high risk for HBV infection, and what tests to use for screening.
Who should be screened?
The USPSTF recommends screening adults and adolescents who are at high risk for HBV. The CDC recommends screening all adults at least once. Both entities agree that those who are at increased risk should be screened periodically, although the optimal frequency has not been established. The USPSTF does not recommend against screening for the general population, so universal screening (as advocated by the CDC) is not in direct conflict with the USPSTF’s recommendations.
Who is at increased risk for HBV infection?
The CDC and the USPSTF differ slightly on the factors they consider to constitute increased risk for HBV infection. These are listed in TABLE 1.1,2

The CDC lists 6 categories that the USPSTF does not mention. However, 4 of these categories are mentioned indirectly in the USPSTF evidence report that accompanies the recommendations, via statements that certain settings have high proportions of people at risk for HBV infection: sexually transmitted infection clinics; HIV testing and treatment centers; health care settings that target services toward people who inject drugs and men who have sex with men; correctional facilities; hemodialysis facilities; and institutions and nonresidential daycare centers for developmentally disabled persons. People who are served at most of these facilities are also at risk for hepatitis C virus infection.
Three categories are listed by the CDC and not by the USPSTF, in either the recommendation or evidence report. These include a history of multiple sex partners; elevated ALT or aspartate aminotransferase levels of unknown origin; and patient request for testing (because they may not want to reveal risk factors).
Continue to: What test(s) should be ordered?
What test(s) should be ordered?
The USPSTF recommends screening using HBsAg. The CDC recommends using triple-panel screening: HBsAg, anti-hepatitis B surface antigen (anti-HBs), and total antibody to hepatitis B core antigen (anti-HBc).
HBsAg indicates HBV infection, either acute or chronic, or a recent dose of HBV vaccine. Anti-HBs indicate recovery from HBV infection, response to HBV vaccine, or recent receipt of hepatitis B immune globulin. Total anti-HBc develops in all HBV infections, resolved or current, and usually persists for life. Vaccine-induced immunity does not cause anti-HBc to develop.
The USPSTF’s rationale is that testing for HBsAg is more than 98% sensitive and specific for detecting HBV infections.2 The CDC recommends triple testing because it can detect those with asymptomatic active HBV infections (this would be a rare occurrence); those who have resolved infection and might be susceptible to reactivation (eg, those who are immunosuppressed); and those who are susceptible and need vaccination.
Interpretation of HBV test results and suggested actions are described in TABLE 2.1,8,13
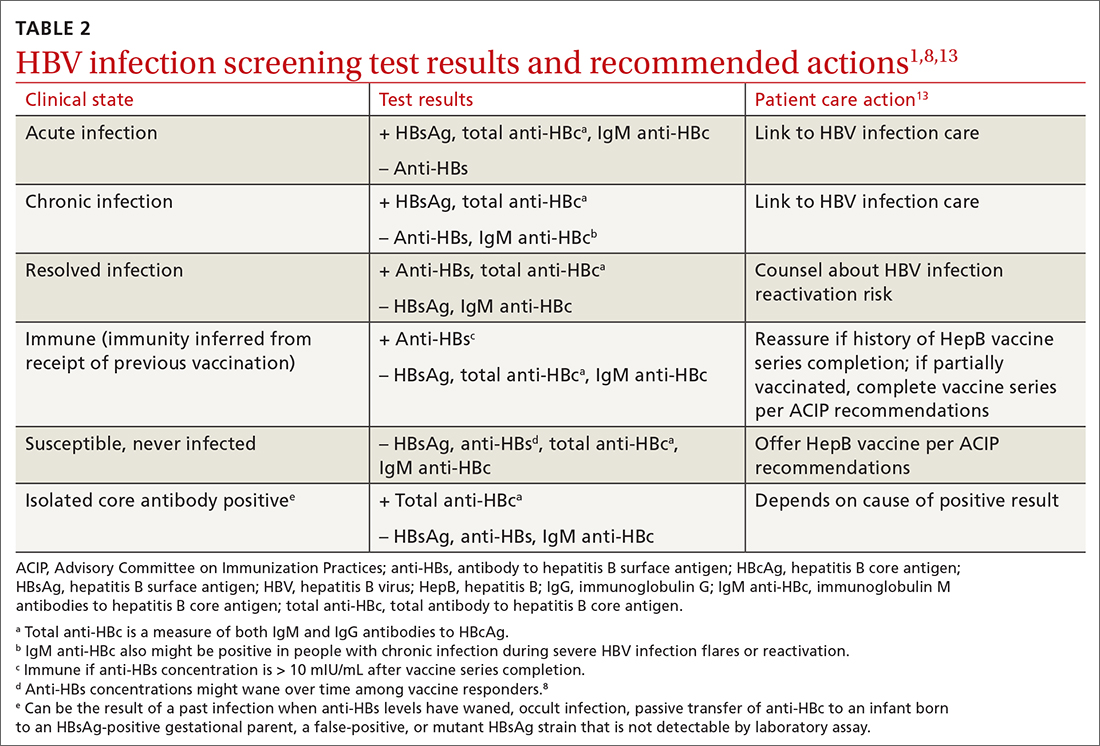
Why do the CDC and USPSTF differ?
While it would be optimal if the CDC and the USPSTF coordinated and harmonized recommendations, this is difficult to achieve given their different missions. The USPSTF is charged to make evidence-based recommendations about preventive services such as screenings, behavioral counseling, and preventive medications, which are provided by clinicians to individual patients. The Task Force uses a very strict evidence-based process and will not make recommendations unless there is adequate evidence of efficacy and safety. Members of the Task Force are primary care professionals, and their collaborating professional organizations are primary care focused.
The CDC takes a community-wide, public health perspective. The professionals that work there are not always clinicians. They strive to prevent as much illness as possible, using public health measures and making recommendations to clinicians. They collaborate with professional organizations; on topics such as hepatitis and other infectious diseases, they collaborate with specialty-oriented societies. Given the imperative to act with the best evidence available, their evidence assessment process is not as strict.
The result, at times, is slight differences in recommendations. However, the HBV screening recommendations from the CDC and the USPSTF agree more than they do not. Based on practice-specific characteristics, family physicians should decide if they want to screen all adults or only those at increased risk, and whether to use single- or triple-test screening.
1. Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and testing for hepatitis B virus infection: CDC recommendations—United States, 2023. MMWR Recomm Rep. 2023;72:1-25. doi: 10.15585/mmwr.rr7201a1
2. USPSTF. Hepatitis B virus infection in adolescents and adults: screening. Final recommendation statement. Published December 15, 2020. Access June 21, 2023. www.uspreventiveser vicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
3. Roberts H, Ly KN, Yin S, et al. Prevalence of HBV infection, vaccine-induced immunity, and susceptibility among at-risk populations: US households, 2013-2018. Hepatology. 2021;74:2353-2365. doi: 10.1002/hep.31991
4. US Department of Health and Human Services. Viral hepatitis national strategic plan for the United States: a roadmap to elimination (2021-2025). Published January 7, 2021. Accessed June 21, 2023. www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf
5. Wong RJ, Brosgart CL, Welch S, et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology. 2021;74:607-626. doi: 10.1002/hep.31782
6. CDC. Recommendation of the Immunization Practices Advisory Committee (ACIP): inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31:317-318, 327-288.
7. CDC. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee. MMWR Morb Mortal Wkly Rep. 1991;40:1-25.
8. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31. doi: 10.15585/mmwr.rr6701a1
9. CDC. Viral hepatitis surveillance 2019. Published July 2021. Accessed June 29, 2023. www.cdc.gov/hepatitis/statistics/2019surveillance/
10. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19-59 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:477-483. doi: 10.15585/mmwr.mm7113a1
11. Terrault NA, Bzowej NH, Chang KM, et al; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. doi: 10.1002/hep.28156
12. Chou R, Blazina I, Bougatsos C, et al. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;324:2423-2436. doi: 10.1001/jama.2020.19750
13. Abara WE, Qaseem A, Schillie S, et al. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167:794-804. doi: 10.7326/M17-110
The Centers for Disease Control and Prevention (CDC) recently published new recommendations on screening for hepatitis B infection.1 They recommend screening all adults (ages 18 years and older) at least once.
These recommendations differ in a few ways from those of the US Preventive Services Task Force (USPSTF).2 This Practice Alert will highlight these differences but also point out areas of agreement between the 2 sets of recommendations—and discuss why 2 separate agencies in the US Department of Health and Human Services reached different conclusions on some issues.
First, some background on hepatitis B
An estimated 580,000 to 2.4 million people in the United States have chronic hepatitis B (CHB) infection—and as many as two-thirds are unaware of it.3 In 2020, the Department of Health and Human Services published the Viral Hepatitis National Strategic Plan for the United States with a stated goal of increasing awareness of infection status among those with hepatitis B virus (HBV) from 32% to 90% by 2030.4 People living in the United States but born outside the country are at highest risk for CHB; they account for 69% of those with the infection.5
The incidence of acute HBV infection has declined markedly since the HBV vaccine was recommended for high-risk adults in 1982 and universally for infants in 1991.6,7 Overall rates of HBV infection declined fairly steadily starting around 1987—but in 2014, rates began to increase, especially in those ages 40 to 59 years.8,9 In 2019, 3192 cases were reported; but when one factors in underreporting, the CDC estimates that the number is likely closer to 20,700.10 This uptick is one reason the Advisory Committee on Immunization Practices changed its HBV vaccination recommendation for adults from a risk-based to a universal recommendation for all unvaccinated adults through age 60 years.10
Chronic hepatitis B infection has serious consequences
The proportion of those infected with HBV who develop CHB differs by age at infection: 80% to 90% if infected during infancy, 30% if infected before age 6 years, and 1% to 12% if infected as an older child or adult.8
CHB infection can lead to chronic liver disease, including cirrhosis of the liver, liver cancer, and liver failure. About 25% of those who develop CHB infection during childhood and 15% of those who develop chronic infection after childhood will die prematurely from cirrhosis or liver cancer.8
The American Association for the Study of Liver Diseases (AASLD) classifies CHB into 4 phases that reflect the rate of viral replication and the patient’s immune response.11 These phases are:
- immune-tolerant (minimal inflammation and fibrosis)
- hepatitis B e-antigen (HBeAg)-positive immune-active (moderate-to-severe inflammation or fibrosis)
- inactive CHB (minimal necroinflammation but variable fibrosis), and
- HBeAg-negative immune reactivation (moderate-to-severe inflammation or fibrosis).11
Continue to: The progression from one phase...
The progression from one phase to the next varies by patient, and not all patients will progress through each phase. The AASLD recommends periodically monitoring the HBV DNA and alanine aminotransferase (ALT) levels in those with CHB to track the progression from one phase to the next and to guide treatment decisions.
Treatment can be beneficial for those who meet criteria
The evidence report prepared for USPSTF found that antiviral treatment of those with CHB infection resulted in improved intermediate outcomes (histologic improvement, loss of hepatitis B surface antigen [HBsAg], loss of HBeAg, HBeAg seroconversion, virologic suppression, and normalization of ALT levels). The magnitude of benefit varied by location and study design.12
In addition, the evidence review found that antiviral therapy was associated with a decreased risk for overall mortality (relative risk [RR] = 0.15; 95% CI, 0.03-0.69), cirrhosis (RR = 0.72; 95% CI, 0.29-1.77), and hepatocellular carcinoma (RR = 0.60; 95% CI, 0.16-2.33). However, these results came from studies that were “limited due to small numbers of trials, few events, and insufficient duration of follow-up.”12
The USPSTF and the CDC both judged that the intermediate outcome results, as well as findings that improved intermediate outcomes lead to decreases in chronic liver disease, are strong enough evidence for their recommendations.
However, not all patients with CHB infection require treatment; estimates of patients with HBV infection meeting AASLD criteria for treatment range from 24% to 48%.1 The AASLD guideline on the treatment of CHB infection is an excellent resource that makes recommendations on the initial evaluation, ongoing monitoring, and treatment decisions for those with CHB.11
Continue to: How CDC and USPSTF guidance on HBV screeinng differs
How CDC and USPSTF guidance on HBV screening differs
The CDC and USPSTF recommendations for HBV screening differ in 3 aspects: whom to screen, whom to classify as at high risk for HBV infection, and what tests to use for screening.
Who should be screened?
The USPSTF recommends screening adults and adolescents who are at high risk for HBV. The CDC recommends screening all adults at least once. Both entities agree that those who are at increased risk should be screened periodically, although the optimal frequency has not been established. The USPSTF does not recommend against screening for the general population, so universal screening (as advocated by the CDC) is not in direct conflict with the USPSTF’s recommendations.
Who is at increased risk for HBV infection?
The CDC and the USPSTF differ slightly on the factors they consider to constitute increased risk for HBV infection. These are listed in TABLE 1.1,2

The CDC lists 6 categories that the USPSTF does not mention. However, 4 of these categories are mentioned indirectly in the USPSTF evidence report that accompanies the recommendations, via statements that certain settings have high proportions of people at risk for HBV infection: sexually transmitted infection clinics; HIV testing and treatment centers; health care settings that target services toward people who inject drugs and men who have sex with men; correctional facilities; hemodialysis facilities; and institutions and nonresidential daycare centers for developmentally disabled persons. People who are served at most of these facilities are also at risk for hepatitis C virus infection.
Three categories are listed by the CDC and not by the USPSTF, in either the recommendation or evidence report. These include a history of multiple sex partners; elevated ALT or aspartate aminotransferase levels of unknown origin; and patient request for testing (because they may not want to reveal risk factors).
Continue to: What test(s) should be ordered?
What test(s) should be ordered?
The USPSTF recommends screening using HBsAg. The CDC recommends using triple-panel screening: HBsAg, anti-hepatitis B surface antigen (anti-HBs), and total antibody to hepatitis B core antigen (anti-HBc).
HBsAg indicates HBV infection, either acute or chronic, or a recent dose of HBV vaccine. Anti-HBs indicate recovery from HBV infection, response to HBV vaccine, or recent receipt of hepatitis B immune globulin. Total anti-HBc develops in all HBV infections, resolved or current, and usually persists for life. Vaccine-induced immunity does not cause anti-HBc to develop.
The USPSTF’s rationale is that testing for HBsAg is more than 98% sensitive and specific for detecting HBV infections.2 The CDC recommends triple testing because it can detect those with asymptomatic active HBV infections (this would be a rare occurrence); those who have resolved infection and might be susceptible to reactivation (eg, those who are immunosuppressed); and those who are susceptible and need vaccination.
Interpretation of HBV test results and suggested actions are described in TABLE 2.1,8,13

Why do the CDC and USPSTF differ?
While it would be optimal if the CDC and the USPSTF coordinated and harmonized recommendations, this is difficult to achieve given their different missions. The USPSTF is charged to make evidence-based recommendations about preventive services such as screenings, behavioral counseling, and preventive medications, which are provided by clinicians to individual patients. The Task Force uses a very strict evidence-based process and will not make recommendations unless there is adequate evidence of efficacy and safety. Members of the Task Force are primary care professionals, and their collaborating professional organizations are primary care focused.
The CDC takes a community-wide, public health perspective. The professionals that work there are not always clinicians. They strive to prevent as much illness as possible, using public health measures and making recommendations to clinicians. They collaborate with professional organizations; on topics such as hepatitis and other infectious diseases, they collaborate with specialty-oriented societies. Given the imperative to act with the best evidence available, their evidence assessment process is not as strict.
The result, at times, is slight differences in recommendations. However, the HBV screening recommendations from the CDC and the USPSTF agree more than they do not. Based on practice-specific characteristics, family physicians should decide if they want to screen all adults or only those at increased risk, and whether to use single- or triple-test screening.
The Centers for Disease Control and Prevention (CDC) recently published new recommendations on screening for hepatitis B infection.1 They recommend screening all adults (ages 18 years and older) at least once.
These recommendations differ in a few ways from those of the US Preventive Services Task Force (USPSTF).2 This Practice Alert will highlight these differences but also point out areas of agreement between the 2 sets of recommendations—and discuss why 2 separate agencies in the US Department of Health and Human Services reached different conclusions on some issues.
First, some background on hepatitis B
An estimated 580,000 to 2.4 million people in the United States have chronic hepatitis B (CHB) infection—and as many as two-thirds are unaware of it.3 In 2020, the Department of Health and Human Services published the Viral Hepatitis National Strategic Plan for the United States with a stated goal of increasing awareness of infection status among those with hepatitis B virus (HBV) from 32% to 90% by 2030.4 People living in the United States but born outside the country are at highest risk for CHB; they account for 69% of those with the infection.5
The incidence of acute HBV infection has declined markedly since the HBV vaccine was recommended for high-risk adults in 1982 and universally for infants in 1991.6,7 Overall rates of HBV infection declined fairly steadily starting around 1987—but in 2014, rates began to increase, especially in those ages 40 to 59 years.8,9 In 2019, 3192 cases were reported; but when one factors in underreporting, the CDC estimates that the number is likely closer to 20,700.10 This uptick is one reason the Advisory Committee on Immunization Practices changed its HBV vaccination recommendation for adults from a risk-based to a universal recommendation for all unvaccinated adults through age 60 years.10
Chronic hepatitis B infection has serious consequences
The proportion of those infected with HBV who develop CHB differs by age at infection: 80% to 90% if infected during infancy, 30% if infected before age 6 years, and 1% to 12% if infected as an older child or adult.8
CHB infection can lead to chronic liver disease, including cirrhosis of the liver, liver cancer, and liver failure. About 25% of those who develop CHB infection during childhood and 15% of those who develop chronic infection after childhood will die prematurely from cirrhosis or liver cancer.8
The American Association for the Study of Liver Diseases (AASLD) classifies CHB into 4 phases that reflect the rate of viral replication and the patient’s immune response.11 These phases are:
- immune-tolerant (minimal inflammation and fibrosis)
- hepatitis B e-antigen (HBeAg)-positive immune-active (moderate-to-severe inflammation or fibrosis)
- inactive CHB (minimal necroinflammation but variable fibrosis), and
- HBeAg-negative immune reactivation (moderate-to-severe inflammation or fibrosis).11
Continue to: The progression from one phase...
The progression from one phase to the next varies by patient, and not all patients will progress through each phase. The AASLD recommends periodically monitoring the HBV DNA and alanine aminotransferase (ALT) levels in those with CHB to track the progression from one phase to the next and to guide treatment decisions.
Treatment can be beneficial for those who meet criteria
The evidence report prepared for USPSTF found that antiviral treatment of those with CHB infection resulted in improved intermediate outcomes (histologic improvement, loss of hepatitis B surface antigen [HBsAg], loss of HBeAg, HBeAg seroconversion, virologic suppression, and normalization of ALT levels). The magnitude of benefit varied by location and study design.12
In addition, the evidence review found that antiviral therapy was associated with a decreased risk for overall mortality (relative risk [RR] = 0.15; 95% CI, 0.03-0.69), cirrhosis (RR = 0.72; 95% CI, 0.29-1.77), and hepatocellular carcinoma (RR = 0.60; 95% CI, 0.16-2.33). However, these results came from studies that were “limited due to small numbers of trials, few events, and insufficient duration of follow-up.”12
The USPSTF and the CDC both judged that the intermediate outcome results, as well as findings that improved intermediate outcomes lead to decreases in chronic liver disease, are strong enough evidence for their recommendations.
However, not all patients with CHB infection require treatment; estimates of patients with HBV infection meeting AASLD criteria for treatment range from 24% to 48%.1 The AASLD guideline on the treatment of CHB infection is an excellent resource that makes recommendations on the initial evaluation, ongoing monitoring, and treatment decisions for those with CHB.11
Continue to: How CDC and USPSTF guidance on HBV screeinng differs
How CDC and USPSTF guidance on HBV screening differs
The CDC and USPSTF recommendations for HBV screening differ in 3 aspects: whom to screen, whom to classify as at high risk for HBV infection, and what tests to use for screening.
Who should be screened?
The USPSTF recommends screening adults and adolescents who are at high risk for HBV. The CDC recommends screening all adults at least once. Both entities agree that those who are at increased risk should be screened periodically, although the optimal frequency has not been established. The USPSTF does not recommend against screening for the general population, so universal screening (as advocated by the CDC) is not in direct conflict with the USPSTF’s recommendations.
Who is at increased risk for HBV infection?
The CDC and the USPSTF differ slightly on the factors they consider to constitute increased risk for HBV infection. These are listed in TABLE 1.1,2

The CDC lists 6 categories that the USPSTF does not mention. However, 4 of these categories are mentioned indirectly in the USPSTF evidence report that accompanies the recommendations, via statements that certain settings have high proportions of people at risk for HBV infection: sexually transmitted infection clinics; HIV testing and treatment centers; health care settings that target services toward people who inject drugs and men who have sex with men; correctional facilities; hemodialysis facilities; and institutions and nonresidential daycare centers for developmentally disabled persons. People who are served at most of these facilities are also at risk for hepatitis C virus infection.
Three categories are listed by the CDC and not by the USPSTF, in either the recommendation or evidence report. These include a history of multiple sex partners; elevated ALT or aspartate aminotransferase levels of unknown origin; and patient request for testing (because they may not want to reveal risk factors).
Continue to: What test(s) should be ordered?
What test(s) should be ordered?
The USPSTF recommends screening using HBsAg. The CDC recommends using triple-panel screening: HBsAg, anti-hepatitis B surface antigen (anti-HBs), and total antibody to hepatitis B core antigen (anti-HBc).
HBsAg indicates HBV infection, either acute or chronic, or a recent dose of HBV vaccine. Anti-HBs indicate recovery from HBV infection, response to HBV vaccine, or recent receipt of hepatitis B immune globulin. Total anti-HBc develops in all HBV infections, resolved or current, and usually persists for life. Vaccine-induced immunity does not cause anti-HBc to develop.
The USPSTF’s rationale is that testing for HBsAg is more than 98% sensitive and specific for detecting HBV infections.2 The CDC recommends triple testing because it can detect those with asymptomatic active HBV infections (this would be a rare occurrence); those who have resolved infection and might be susceptible to reactivation (eg, those who are immunosuppressed); and those who are susceptible and need vaccination.
Interpretation of HBV test results and suggested actions are described in TABLE 2.1,8,13

Why do the CDC and USPSTF differ?
While it would be optimal if the CDC and the USPSTF coordinated and harmonized recommendations, this is difficult to achieve given their different missions. The USPSTF is charged to make evidence-based recommendations about preventive services such as screenings, behavioral counseling, and preventive medications, which are provided by clinicians to individual patients. The Task Force uses a very strict evidence-based process and will not make recommendations unless there is adequate evidence of efficacy and safety. Members of the Task Force are primary care professionals, and their collaborating professional organizations are primary care focused.
The CDC takes a community-wide, public health perspective. The professionals that work there are not always clinicians. They strive to prevent as much illness as possible, using public health measures and making recommendations to clinicians. They collaborate with professional organizations; on topics such as hepatitis and other infectious diseases, they collaborate with specialty-oriented societies. Given the imperative to act with the best evidence available, their evidence assessment process is not as strict.
The result, at times, is slight differences in recommendations. However, the HBV screening recommendations from the CDC and the USPSTF agree more than they do not. Based on practice-specific characteristics, family physicians should decide if they want to screen all adults or only those at increased risk, and whether to use single- or triple-test screening.
1. Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and testing for hepatitis B virus infection: CDC recommendations—United States, 2023. MMWR Recomm Rep. 2023;72:1-25. doi: 10.15585/mmwr.rr7201a1
2. USPSTF. Hepatitis B virus infection in adolescents and adults: screening. Final recommendation statement. Published December 15, 2020. Access June 21, 2023. www.uspreventiveser vicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
3. Roberts H, Ly KN, Yin S, et al. Prevalence of HBV infection, vaccine-induced immunity, and susceptibility among at-risk populations: US households, 2013-2018. Hepatology. 2021;74:2353-2365. doi: 10.1002/hep.31991
4. US Department of Health and Human Services. Viral hepatitis national strategic plan for the United States: a roadmap to elimination (2021-2025). Published January 7, 2021. Accessed June 21, 2023. www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf
5. Wong RJ, Brosgart CL, Welch S, et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology. 2021;74:607-626. doi: 10.1002/hep.31782
6. CDC. Recommendation of the Immunization Practices Advisory Committee (ACIP): inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31:317-318, 327-288.
7. CDC. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee. MMWR Morb Mortal Wkly Rep. 1991;40:1-25.
8. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31. doi: 10.15585/mmwr.rr6701a1
9. CDC. Viral hepatitis surveillance 2019. Published July 2021. Accessed June 29, 2023. www.cdc.gov/hepatitis/statistics/2019surveillance/
10. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19-59 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:477-483. doi: 10.15585/mmwr.mm7113a1
11. Terrault NA, Bzowej NH, Chang KM, et al; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. doi: 10.1002/hep.28156
12. Chou R, Blazina I, Bougatsos C, et al. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;324:2423-2436. doi: 10.1001/jama.2020.19750
13. Abara WE, Qaseem A, Schillie S, et al. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167:794-804. doi: 10.7326/M17-110
1. Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and testing for hepatitis B virus infection: CDC recommendations—United States, 2023. MMWR Recomm Rep. 2023;72:1-25. doi: 10.15585/mmwr.rr7201a1
2. USPSTF. Hepatitis B virus infection in adolescents and adults: screening. Final recommendation statement. Published December 15, 2020. Access June 21, 2023. www.uspreventiveser vicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
3. Roberts H, Ly KN, Yin S, et al. Prevalence of HBV infection, vaccine-induced immunity, and susceptibility among at-risk populations: US households, 2013-2018. Hepatology. 2021;74:2353-2365. doi: 10.1002/hep.31991
4. US Department of Health and Human Services. Viral hepatitis national strategic plan for the United States: a roadmap to elimination (2021-2025). Published January 7, 2021. Accessed June 21, 2023. www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf
5. Wong RJ, Brosgart CL, Welch S, et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology. 2021;74:607-626. doi: 10.1002/hep.31782
6. CDC. Recommendation of the Immunization Practices Advisory Committee (ACIP): inactivated hepatitis B virus vaccine. MMWR Morb Mortal Wkly Rep. 1982;31:317-318, 327-288.
7. CDC. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee. MMWR Morb Mortal Wkly Rep. 1991;40:1-25.
8. Schillie S, Vellozzi C, Reingold A, et al. Prevention of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2018;67:1-31. doi: 10.15585/mmwr.rr6701a1
9. CDC. Viral hepatitis surveillance 2019. Published July 2021. Accessed June 29, 2023. www.cdc.gov/hepatitis/statistics/2019surveillance/
10. Weng MK, Doshani M, Khan MA, et al. Universal hepatitis B vaccination in adults aged 19-59 years: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:477-483. doi: 10.15585/mmwr.mm7113a1
11. Terrault NA, Bzowej NH, Chang KM, et al; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. doi: 10.1002/hep.28156
12. Chou R, Blazina I, Bougatsos C, et al. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;324:2423-2436. doi: 10.1001/jama.2020.19750
13. Abara WE, Qaseem A, Schillie S, et al. Hepatitis B vaccination, screening, and linkage to care: best practice advice from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2017;167:794-804. doi: 10.7326/M17-110
Pneumococcal vaccine label adds injection-site risk
No similar safety signal has been detected for the more recently approved 15-valent and 20-valent pneumococcal conjugate vaccines, explain the investigators, led by Brendan Day, MD, MPH, from the FDA’s Center for Biologics Evaluation and Research, in their report published online in JAMA Internal Medicine.
Reports of injection-site necrosis emerged after the vaccine (Pneumovax 23, Merck) had been approved by the FDA and was administered to a large, diverse, real-world population.
Rare safety events can emerge after FDA approval, as clinical trials may not be able to detect them in a study-group population.
Therefore, “postmarketing safety surveillance is critical to further characterize the safety profile of licensed vaccines,” the investigators point out.
The FDA and the Centers for Disease Control and Prevention monitor the postmarketing safety of licensed vaccines using the Vaccine Adverse Event Reporting System (VAERS), which relies on people who get the vaccines to report adverse events.
Real-world finding
After reports indicated a safety signal in 2020, the researchers conducted a case-series review, calculated the reporting rate, and did a PubMed search for similar reports.
They found that the reporting rate for injection-site necrosis was less than 0.2 cases per 1 million vaccine doses administered. The PubMed search yielded two cases of injection-site necrosis after the vaccine.
The 23-valent vaccine helps protect people from pneumococcus bacterial infection. The manufacturer reports that it is for people at least 50 years of age and for children who are at least 2 years of age with medical conditions that put them at elevated risk for infection.
The U.S. package insert has been updated, in the Post-Marketing Experience section, to include injection-site necrosis.
Of the 104 VAERS reports identified by the researchers, 48 met the case definition. Of those cases, most were for skin necrosis (n = 43), five of which also included fat necrosis. The remaining five cases of necrosis affected fascia (n = 2); fat and fascia (n = 1); fat, fascia, and muscle (n = 1); and muscle (n = 1).
In 23 of the 48 cases (47.9%), the reactions were serious and included one death (unrelated to vaccination).
Seventeen patients (35.4%) were hospitalized and 26 (54.2%) required surgery, most commonly debridement. Eight patients (16.7%) underwent multiple surgical procedures and three (6.3%) required a skin graft.
For patients with skin necrosis (n = 43), the median age was 67 years, and most patients were female (n = 36). Twelve patients were immunocompromised.
Concomitant vaccinations were reported in 10 patients, five of whom got the shot in the same arm as the 23-valent pneumococcal vaccine. A concurrent diagnosis of cellulitis was reported in 16 patients and an abscess was reported in three patients. There were too few cases of fat, fascia, or muscle necrosis to draw conclusions, the researchers report.
Often, skin necrosis was seen after a progression of symptoms, such as redness, pain, or swelling.
“These reports are consistent with published descriptions of injection-site necrosis, which has been reported as a rare complication for many vaccines and injectable drugs,” the investigators report.
Although the researchers couldn’t conclude from the VAERS reports alone that the vaccine injection caused the necrosis, “the timing and the location of reactions at the injection site suggest a possible causal association with the vaccine,” they explain. However, they add, patient comorbidities and poor injection technique may also be contributors.
A version of this article first appeared on Medscape.com.
No similar safety signal has been detected for the more recently approved 15-valent and 20-valent pneumococcal conjugate vaccines, explain the investigators, led by Brendan Day, MD, MPH, from the FDA’s Center for Biologics Evaluation and Research, in their report published online in JAMA Internal Medicine.
Reports of injection-site necrosis emerged after the vaccine (Pneumovax 23, Merck) had been approved by the FDA and was administered to a large, diverse, real-world population.
Rare safety events can emerge after FDA approval, as clinical trials may not be able to detect them in a study-group population.
Therefore, “postmarketing safety surveillance is critical to further characterize the safety profile of licensed vaccines,” the investigators point out.
The FDA and the Centers for Disease Control and Prevention monitor the postmarketing safety of licensed vaccines using the Vaccine Adverse Event Reporting System (VAERS), which relies on people who get the vaccines to report adverse events.
Real-world finding
After reports indicated a safety signal in 2020, the researchers conducted a case-series review, calculated the reporting rate, and did a PubMed search for similar reports.
They found that the reporting rate for injection-site necrosis was less than 0.2 cases per 1 million vaccine doses administered. The PubMed search yielded two cases of injection-site necrosis after the vaccine.
The 23-valent vaccine helps protect people from pneumococcus bacterial infection. The manufacturer reports that it is for people at least 50 years of age and for children who are at least 2 years of age with medical conditions that put them at elevated risk for infection.
The U.S. package insert has been updated, in the Post-Marketing Experience section, to include injection-site necrosis.
Of the 104 VAERS reports identified by the researchers, 48 met the case definition. Of those cases, most were for skin necrosis (n = 43), five of which also included fat necrosis. The remaining five cases of necrosis affected fascia (n = 2); fat and fascia (n = 1); fat, fascia, and muscle (n = 1); and muscle (n = 1).
In 23 of the 48 cases (47.9%), the reactions were serious and included one death (unrelated to vaccination).
Seventeen patients (35.4%) were hospitalized and 26 (54.2%) required surgery, most commonly debridement. Eight patients (16.7%) underwent multiple surgical procedures and three (6.3%) required a skin graft.
For patients with skin necrosis (n = 43), the median age was 67 years, and most patients were female (n = 36). Twelve patients were immunocompromised.
Concomitant vaccinations were reported in 10 patients, five of whom got the shot in the same arm as the 23-valent pneumococcal vaccine. A concurrent diagnosis of cellulitis was reported in 16 patients and an abscess was reported in three patients. There were too few cases of fat, fascia, or muscle necrosis to draw conclusions, the researchers report.
Often, skin necrosis was seen after a progression of symptoms, such as redness, pain, or swelling.
“These reports are consistent with published descriptions of injection-site necrosis, which has been reported as a rare complication for many vaccines and injectable drugs,” the investigators report.
Although the researchers couldn’t conclude from the VAERS reports alone that the vaccine injection caused the necrosis, “the timing and the location of reactions at the injection site suggest a possible causal association with the vaccine,” they explain. However, they add, patient comorbidities and poor injection technique may also be contributors.
A version of this article first appeared on Medscape.com.
No similar safety signal has been detected for the more recently approved 15-valent and 20-valent pneumococcal conjugate vaccines, explain the investigators, led by Brendan Day, MD, MPH, from the FDA’s Center for Biologics Evaluation and Research, in their report published online in JAMA Internal Medicine.
Reports of injection-site necrosis emerged after the vaccine (Pneumovax 23, Merck) had been approved by the FDA and was administered to a large, diverse, real-world population.
Rare safety events can emerge after FDA approval, as clinical trials may not be able to detect them in a study-group population.
Therefore, “postmarketing safety surveillance is critical to further characterize the safety profile of licensed vaccines,” the investigators point out.
The FDA and the Centers for Disease Control and Prevention monitor the postmarketing safety of licensed vaccines using the Vaccine Adverse Event Reporting System (VAERS), which relies on people who get the vaccines to report adverse events.
Real-world finding
After reports indicated a safety signal in 2020, the researchers conducted a case-series review, calculated the reporting rate, and did a PubMed search for similar reports.
They found that the reporting rate for injection-site necrosis was less than 0.2 cases per 1 million vaccine doses administered. The PubMed search yielded two cases of injection-site necrosis after the vaccine.
The 23-valent vaccine helps protect people from pneumococcus bacterial infection. The manufacturer reports that it is for people at least 50 years of age and for children who are at least 2 years of age with medical conditions that put them at elevated risk for infection.
The U.S. package insert has been updated, in the Post-Marketing Experience section, to include injection-site necrosis.
Of the 104 VAERS reports identified by the researchers, 48 met the case definition. Of those cases, most were for skin necrosis (n = 43), five of which also included fat necrosis. The remaining five cases of necrosis affected fascia (n = 2); fat and fascia (n = 1); fat, fascia, and muscle (n = 1); and muscle (n = 1).
In 23 of the 48 cases (47.9%), the reactions were serious and included one death (unrelated to vaccination).
Seventeen patients (35.4%) were hospitalized and 26 (54.2%) required surgery, most commonly debridement. Eight patients (16.7%) underwent multiple surgical procedures and three (6.3%) required a skin graft.
For patients with skin necrosis (n = 43), the median age was 67 years, and most patients were female (n = 36). Twelve patients were immunocompromised.
Concomitant vaccinations were reported in 10 patients, five of whom got the shot in the same arm as the 23-valent pneumococcal vaccine. A concurrent diagnosis of cellulitis was reported in 16 patients and an abscess was reported in three patients. There were too few cases of fat, fascia, or muscle necrosis to draw conclusions, the researchers report.
Often, skin necrosis was seen after a progression of symptoms, such as redness, pain, or swelling.
“These reports are consistent with published descriptions of injection-site necrosis, which has been reported as a rare complication for many vaccines and injectable drugs,” the investigators report.
Although the researchers couldn’t conclude from the VAERS reports alone that the vaccine injection caused the necrosis, “the timing and the location of reactions at the injection site suggest a possible causal association with the vaccine,” they explain. However, they add, patient comorbidities and poor injection technique may also be contributors.
A version of this article first appeared on Medscape.com.
FROM JAMA INTERNAL MEDICINE
FDA approves RSV monoclonal antibody for all infants
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
The monoclonal antibody Beyfortus (nirsevimab-alip), which already is approved for use in Europe and Canada, is indicated for newborns and infants born during or entering their first RSV season, and for children up to 24 months of age who are vulnerable to severe RSV through their second RSV season.
As many as 80,000 children under age 5 years are hospitalized with an RSV infection annually in the United States. Most cases are mild, but infants under 6 months, those born prematurely, and children with weakened immune systems or neuromuscular disorders are at an increased risk for severe illness, according to the Centers for Disease Control and Prevention.
The highly contagious virus is also a concern for immunocompromised adults and older people with underlying health conditions, who are at increased risk for severe disease.
Sanofi and AstraZeneca, which jointly developed the injectable agent, said in a press release that the companies plan to make it available by the fall of 2023. The long-acting antibody is given as a single intramuscular injection.
Beyfortus was approved in part based on data from the phase 3 MELODY trial, which found the shot reduced the incidence of medically attended lower respiratory tract infections associated with RSV by 74.9% versus placebo (95% confidence interval, 50.6-87.3; P < .001).
The phase 2/3 MEDLEY trial, conducted between July 2019 and May 2021, compared Beyfortus with palivizumab, another RSV antibody injection with more limited indications. The trial included more than 900 preterm infants less than 35 weeks’ gestational age and infants with congenital heart disease. Results were similar to the phase 3 MELODY trial, according to the manufacturers.
“Today’s approval marks an unprecedented moment for protecting infant health in the United States, following an RSV season that took a record toll on infants, their families, and the U.S. health care system,” said Thomas Triomphe, executive vice president for vaccines at Sanofi, in a press release about the FDA decision. “Beyfortus is the only monoclonal antibody approved for passive immunization to provide safe and effective protection for all infants during their first RSV season.”
A version of this article first appeared on Medscape.com.
Long COVID and vaccines: Separating facts from falsehoods
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.
The COVID-19 vaccines have been a game changer for millions of people worldwide in preventing death or disability from the virus. Research suggests that they offer significant protection against long COVID.
False and unfounded claims made by some antivaccine groups that the vaccines themselves may cause long COVID persist and serve as barriers to vaccination.
To help separate the facts from falsehoods, here’s a checklist for doctors on what scientific studies have determined about vaccination and long COVID.
What the research shows
Doctors who work in long COVID clinics have for years suspected that vaccination may help protect against the development of long COVID, noted Lawrence Purpura, MD, MPH, an infectious disease specialist at New York–Presbyterian/Columbia University Irving Medical Center, who treats patients with long COVID in his clinic.
Over the past year, several large, well-conducted studies have borne out that theory, including the following studies:
- In the RECOVER study, published in May in the journal Nature Communications, researchers examined the electronic health records of more than 5 million people who had been diagnosed with COVID and found that vaccination reduced the risk that they would develop long COVID. Although the researchers didn’t compare the effects of having boosters to being fully vaccinated without them, experts have suggested that having a full round of recommended shots may offer the most protection. “My thoughts are that more shots are better, and other work has shown compelling evidence that the protective effect of vaccination on COVID-19 wanes over time,” said study coauthor Daniel Brannock, MS, a research scientist at RTI International in Research Triangle Park, N.C. “It stands to reason that the same is true for long COVID.”
- A review published in February in BMJ Medicine concluded that 10 studies showed a significant reduction in the incidence of long COVID among vaccinated patients. Even one dose of a vaccine was protective.
- A meta-analysis of six studies published last December in Antimicrobial Stewardship and Healthcare Epidemiology found that one or more doses of a COVID-19 vaccine were 29% effective in preventing symptoms of long COVID.
- In a June meta-analysis published in JAMA Internal Medicine, researchers analyzed more than 40 studies that included 860,000 patients and found that two doses of a COVID-19 vaccine reduced the risk of long COVID by almost half.
The message? COVID vaccination is very effective in reducing the risk of long COVID.
“It’s important to emphasize that many of the risk factors [for long COVID] cannot be changed, or at least cannot be changed easily, but vaccination is a decision that can be taken by everyone,” said Vassilios Vassiliou, MBBS, PhD, clinical professor of cardiac medicine at Norwich Medical School in England, who coauthored the article in JAMA Internal Medicine.
Why vaccines may be protective
The COVID-19 vaccines work well to prevent serious illness from the virus, noted Aaron Friedberg, MD, clinical coleader of the Post COVID Recovery Program at the Ohio State University Wexner Medical Center. That may be a clue to why the vaccines help prevent long COVID symptoms.
“When you get COVID and you’ve been vaccinated, the virus may still attach in your nose and respiratory tract, but it’s less likely to spread throughout your body,” he explained. “It’s like a forest fire – if the ground is wet or it starts to rain, it’s less likely to create a great blaze. As a result, your body is less likely to experience inflammation and damage that makes it more likely that you’ll develop long COVID.”
Dr. Friedberg stressed that even for patients who have had COVID, it’s important to get vaccinated – a message he consistently delivers to his own patients.
“There is some protection that comes from having COVID before, but for some people, that’s not enough,” he said. “It’s true that after infection, your body creates antibodies that help protect you against the virus. But I explain to patients that these may be like old Velcro: They barely grab on enough to stay on for the moment, but they don’t last long term. You’re much more likely to get a reliable immune response from the vaccine.”
In addition, a second or third bout of COVID could be the one that gives patients long COVID, Dr. Friedberg adds.
“I have a number of patients in my clinic who were fine after their first bout of COVID but experienced debilitating long COVID symptoms after they developed COVID again,” he said. “Why leave it to chance?”
Vaccines and ‘long vax’
The COVID vaccines are considered very safe but have been linked to very rare side effects, such as blood clots and heart inflammation. There have also been anecdotal reports of symptoms that resemble long COVID – a syndrome that has come to be known as “long Vax” – an extremely rare condition that may or may not be tied to vaccination.
“I have seen people in my clinic who developed symptoms suggestive of long COVID that linger for months – brain fog, fatigue, heart palpitations – soon after they got the COVID-19 vaccine,” said Dr. Purpura. But no published studies have suggested a link, he cautions.
A study called LISTEN is being organized at Yale in an effort to better understand postvaccine adverse events and a potential link to long COVID.
Talking to patients
Discussions of vaccination with patients, including those with COVID or long COVID, are often fraught and challenging, said Dr. Purpura.
“There’s a lot of fear that they will have a worsening of their symptoms,” he explained. The conversation he has with his patients mirrors the conversation all physicians should have with their patients about COVID-19 vaccination, even if they don’t have long COVID. He stresses the importance of highlighting the following components:
- Show compassion and empathy. “A lot of people have strongly held opinions – it’s worth it to try to find out why they feel the way that they do,” said Dr. Friedberg.
- Walk them through side effects. “Many people are afraid of the side effects of the vaccine, especially if they already have long COVID,” explained Dr. Purpura. Such patients can be asked how they felt after their last vaccination, such a shingles or flu shot. Then explain that the COVID-19 vaccine is not much different and that they may experience temporary side effects such as fatigue, headache, or a mild fever for 24-48 hours.
- Explain the benefits. Eighty-five percent of people say their health care provider is a trusted source of information on COVID-19 vaccines, according to the Kaiser Family Foundation. That trust is conducive to talks about the vaccine’s benefits, including its ability to protect against long COVID.
Other ways to reduce risk of long COVID
Vaccines can lower the chances of a patient’s developing long COVID. So can the antiviral medication nirmatrelvir (Paxlovid). A March 2023 study published in JAMA Internal Medicine included more than 280,000 people with COVID. The researchers found that vaccination reduced the risk for developing the condition by about 25%.
“I mention that study to all of my long COVID patients who become reinfected with the virus,” said Dr. Purpura. “It not only appears protective against long COVID, but since it lowers levels of virus circulating in their body, it seems to help prevent a flare-up of symptoms.”
Another treatment that may help is the diabetes drug metformin, he added.
A June 2023 study published in The Lancet Infectious Diseases found that when metformin was given within 3 days of symptom onset, the incidence of long COVID was reduced by about 41%.
“We’re still trying to wrap our brains around this one, but the thought is it may help to lower inflammation, which plays a role in long COVID,” Dr. Purpura explained. More studies need to be conducted, though, before recommending its use.
A version of this article first appeared on Medscape.com.
HPV rates skyrocket despite safe, effective vaccine
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
An epidemic of sexually transmitted HPV is now swirling around the United States and the United Kingdom, with some serious cases leading to oropharyngeal cancer, which can affect the back of the throat, tonsils, and tongue.
HPV is the leading cause (70%) of this oropharyngeal cancer, according to the CDC. It is the most common sexually transmitted disease in the nation, and around 3.6% of women and 10% of men report oral HPV specifically. But over the past decade, oropharyngeal cases have been steadily falling a little under 4% and 2%, respectively, according to the National Cancer Institute.
HPV is often undetectable and can clear up within a few months. But unfortunately for some, serious disease, such as throat cancer, can develop.
Studies show the HPV vaccine to be extremely effective in lowering sexually transmitted HPV cases. Yet, only 54.5% of young people aged 13-15 have taken the recommended two to three doses, according to the National Cancer Institute.
Why aren’t more young people taking the vaccine?
Low public awareness of the dangers of HPV may be behind young people’s poor vaccination rates, according to Teresa Lee, MD, of the Fox Chase Cancer Center in Philadelphia. “For example, while the link with head and neck cancers has been well-studied, the FDA labeling was not changed to reflect this as an indication until 2020,” she said.
Other reasons can include one’s socioeconomic background, poor health literacy, cultural or religious stigmas around vaccines, and lack of quality, low-cost health care, says Emmanuel Aguh, MD, a board-certified family medicine physician. “Some individuals and families are still resistant to vaccines and the noted lack of uptake.”
Doctors and other health care professionals should also be sure to tell patients of all ages about the risks of HPV infection and how well the vaccine works, Dr. Lee said. “Not everyone who is now eligible may have been offered the vaccine as a child, and the first time young adults may receive counseling on this subject may not be until they are entering a very busy period of their lives with many responsibilities – when it may be hard to fit in things like health maintenance.”
How safe is the HPV vaccine?
The Food and Drug Administration and Centers for Disease Control and Prevention have studied the HPV vaccine for years to find out how safe it is and how well it works, Dr. Aguh said. No major side effects have been reported, and the most common side effect is soreness where you get the shot (which is normal after most vaccines). Some dizziness and fainting in adolescents can also occur, so young people are usually asked to sit or lie down during the shot and for 15 minutes afterward, he said.
“Serious adverse events have not been reported at higher rates than expected following HPV vaccination, meaning there is no clear evidence they are related to the vaccine,” Dr. Lee said. “The vaccine is highly effective in decreasing rates of detectable infection with the high-risk HPV strains responsible for HPV-associated cancers.”
The HPV vaccine is largely recommended for people aged 9-26, and sometimes up to age 45, depending on the individual, Dr. Aguh said. If you are over 26, talk to your doctor about whether you should consider getting the vaccine.
“It is usually given in two doses for complete protection if taken before the 15th birthday,” Dr. Aguh said. “If taken afterward, or in those with a weak immune system, they might require three doses to be fully protected.”
The vaccine produces antibodies that can stop HPV from infecting cells and lowers your chances of catching an HPV-related cancer, such as throat cancer or cancer of the cervix, he said.
While the vaccine is not guaranteed to protect you from the more than 100 strains of HPV, it can protect you from HPV 16 and HPV 18 – two high-risk strains that cause around 70% of cervical cancers.
What is fueling the rise of HPV cases?
A misconception that oral sex is somehow a “safe and risk-free” alternative to anal or vaginal sex could be one reason, Dr. Aguh said.
“It is important to know that, with oral sex, you are exposed to many of the risks associated with vaginal intercourse, especially if you do not take any measures to protect yourself and/or your partner,” Dr. Aguh said. “[With oral sex] it is possible to end up contracting an infection like chlamydia, gonorrhea, and even HPV, leading to an increased risk of HPV-associated oropharyngeal cancers.”
A lack of public awareness of what can cause throat cancer could also explain this phenomenon. The number of people you have oral sex with, along with the age you begin sexual activity, can greatly determine your risk of the disease, according to Dr. Lee. She echoes a report by Hisham Mehanna, PhD, in The Conversation.
“For oropharyngeal cancer, the main risk factor is the number of lifetime sexual partners, especially oral sex,” wrote Dr. Mehanna, a professor at the Institute of Cancer and Genomic Sciences at the University of Birmingham (England). “Those with six or more lifetime oral-sex partners are 8.5 times more likely to develop oropharyngeal cancer than those who do not practice oral sex.”
What are symptoms of oropharyngeal cancer?
Labored breathing or swallowing, a cough that won’t go away, and crackling or hoarseness of your voice could all be signs of throat cancer. Other symptoms include earaches, swelling of the head or neck, and enlarged lymph nodes, among others, Dr. Aguh said.
“The signs and symptoms of HPV-related throat cancers can be difficult to identify and recognize, as they can be vague and are also associated with other medical conditions. Sometimes, there are no signs at all, or they are not easily noticeable due to the location,” he said.
You should go see your doctor if you have any of these ailments for an extended period.
How to reduce your risk
In addition to having six or more oral-sex partners, smoking and drinking heavily could also raise your risk of throat cancer, said Dr. Lee. Proper dental health – like seeing your dentist regularly and practicing proper oral hygiene – can also shave your risk.
“[Good dental health] can help not just with head and neck cancer risk, but with many other inflammation-related diseases,” Dr. Lee said.
Using dental dams and condoms can also be a good method of protection, Dr. Aguh said. A dental dam is a stretchy sheet of latex, or polyurethane plastic, in the shape of a square that is made for blocking body fluid to lower your risk of contracting an STD via oral sex.
Keep in mind: Even with these protections, make sure you and your partner discuss each other’s sexual history, any prior or current STDs and their preferred protection from STDs, said Dr. Aguh.
If you or your partner is being treated for an STD, consider opting out of oral sex and consulting a doctor.
The HPV vaccine is another common method of protection. The shot is “approved for prevention of nine of the most high-risk strains of HPV,” or those that are most commonly linked to cancer, according to Dr. Lee. The vaccine “reduces the frequency of infection” with these viruses, which can ultimately lower the risk of cancers linked to HPV, including cervical, anal, and vulvar and vaginal cancers, she said.
“The best time to receive treatment for prevention of disease is prior to onset of sexual intercourse,” said Dr. Lee.
To get your HPV vaccine, head to your family doctor, school- or community-based health center, or state health department, suggests the CDC.
A version of this article originally appeared on WebMD.com.
CDC signs off on RSV vaccine for older adults
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.
The Centers for Disease Control and Prevention has given a green light to two new vaccines to protect against respiratory syncytial virus, or RSV, in older adults.
CDC Director Rochelle P. Walensky, MD, MPH, agreed with and endorsed the recommendations made earlier by CDC advisors that people age 60 and over may get one of two new vaccines for RSV. Decisions should be made based on discussions with one’s health care provider about whether the vaccine is right for them, the federal health agency said.
The new vaccines, the first licensed in the United States to protect against the respiratory illness, are expected to be available this fall.
On June 21, the CDC’s Advisory Committee on Immunization Practices (ACIP), an independent panel, stopped short of recommending the vaccines for everyone age 65 and above, which was the original question the committee was to consider. The experts amended that question, changing it to whether the panel should recommend the vaccine for those 65 and above if the person and their doctor agreed. The committee voted 9 to 5 in favor.
RSV vaccines
RSV leads to 6,000 to 10,000 deaths a year in the United States among those age 65 and older and 60,000 to 160,000 hospitalizations in that group. Seniors and infants are among the most vulnerable to the lower respiratory infection, marked by runny nose, wheezing, sneezing, decreased appetite, and fever.
The FDA in May approved two vaccines — GSK’s Arexvy and Pfizer’s Abrysvo — for adults age 60 and above.
The vote recommending shared decision-making about the vaccine, instead of a routine vaccination recommended for all, “is a weaker recommendation,” said William Schaffner, MD, an infectious disease specialist at Vanderbilt University Medical Center in Nashville and medical director of the National Foundation for Infectious Diseases. Dr. Schaffner is a non-voting member of ACIP. He attended the meeting.
He said the experts voiced concern about a number of issues, including what some saw as a lack of sufficient data from trials on the most vulnerable groups, such as nursing home residents.
Experts also wanted more information about the duration of protection and exactly when a second dose might be needed. At the meeting, a GSK official said its vaccine was 84.6% effective after one and a half seasons, down from 94.1% after one season. A Pfizer official said its vaccine decreased the risk of RSV with three or more symptoms by 78.6% after a season and a half, down from 88.9% after one season.
The panel also wanted more data on whether the RSV vaccines could be administered at the same time as other vaccines recommended for adults.
Both companies gave a range of cost estimates. Pfizer expects its vaccine to cost $180 to $270 but said it could not guarantee that range. GSK said it expects a price of $200 to $295. Under the Inflation Reduction Act, recommended vaccines are covered under Medicare for those with Part D plans, which 51 million of 65 million Medicare patients have. Commercial insurance is likely to cover the vaccines if the CDC recommends them.
A version of this article first appeared on WebMD.com.
This article was updated 7/5/23.


