User login
FDA panel backs new COVID booster focusing only on variants
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
but questioned whether the population as a whole needs booster shots and how often they should be given.
The Vaccines and Related Biological Products Advisory Committee of the FDA voted 21-0 in favor of the recommendation about the strain to be used in the next crop of vaccines.
In the briefing document for the meeting, FDA staff said the available evidence suggests that a monovalent (single-strain) XBB-lineage vaccine “is warranted” for the 2023-2024 vaccination campaign and would replace the current bivalent vaccine, which targets the original version of the virus and two strains from the Omicron variant.
FDA staff also noted how such a shift would be in line with the World Health Organization toward targeting the XBB family of subvariants. European regulators have done this as well.
The FDA is not obligated to act on the panel’s recommendations. But the agency often does and is highly likely to do so in this case. Vaccine companies will need the recommendation from the FDA to begin making vaccines for the fall.
New shot every year?
The FDA asked its expert panel to vote only on the question about the makeup of future vaccines in terms of which strain to include.
But panelists also raised other questions during the meeting, including concerns about moves toward tying COVID vaccinations into the model of annual flu shots.
Paul Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia, argued for greater focus on the response of T cells after vaccination, even in light of the already recognized waning of antibody protection.
In a recent Substack article, Dr. Offit called T cells the “unsung hero” of the pandemic. They take longer to develop after infection or vaccination than the antibodies that first attack the virus, but immune memory cells called B and T cells “are long-lived,” and their “protection against severe disease often lasts for years and sometimes decades.”
Dr. Offit said he was concerned about using a blanket approach for future recommendations for COVID vaccinations, following the one now in place for influenza vaccines.
The Centers for Disease Control and Prevention recommends flu shots for everyone 6 months and older, with rare exceptions.
“We need to continue to define who those high-risk groups are and not make this a recommendation for everybody every season,” he said.
Dr. Offit offered his own experience as an example. While he had been vaccinated against the virus’s early Wuhan strain, he still was infected, most likely with a variant that emerged later.
“That was a drifted virus. That’s why I had a mild infection but I didn’t have a severe infection, because presumably I had T cells which prevented that severe infection, which may last for years,” Dr. Offit said.
Pfizer and Moderna, the two companies that make mRNA-based COVID vaccines, are working on experimental products meant to protect against both flu and SARS-COv-2 in one shot. Novavax, maker of a more traditional protein-based COVID shot, is doing the same.
The idea of these combination products is to make it more convenient for people to protect against both viruses, while also offering companies some marketing advantages.
But without referring to these drugmakers’ plans for future combo flu-COVID shots, members of the FDA panel raised objections to an assumption of routine annual vaccines against variants of SARS-CoV-2.
Among the panelists who expressed concerns was Henry H. Bernstein, DO, a former member of the CDC’s Advisory Committee on Immunization Practices.
Bernstein questioned the approach of dubbing these the “2023-2024 formulas,” as this approach conveyed a sense of an expectation for a need for annual vaccines, as happens with flu.
“It’s not clear to me that this is a seasonal virus yet,” said Dr. Bernstein, who is also a professor of pediatrics at Hofstra University, Hempstead, N.Y..
In response to Dr. Bernstein’s point, Arnold Monto, MD, the acting chair of the FDA panel, suggested such a pattern could emerge, while also agreeing that it’s too soon to say for sure.
A professor emeritus at the University of Michigan, Ann Arbor, Dr. Monto’s career included pandemic planning and emergency response to virus outbreaks, including the 1968 Hong Kong influenza pandemic, avian influenza, and the original SARS.
“I think it’s premature to say that this virus will not become seasonal,” Dr. Monto said about SARS-CoV-2. “I agree. We’re not there yet, but we may be.”
At the end of the meeting, Dr. Monto recapped the meeting’s key points, noting that there was a general consensus that the XBB.1.5 subvariant would be the best to use in future COVID shots.
He also noted that Novavax, which makes the more traditional protein-based vaccine, along with Pfizer and Moderna, already have honed in on this subvariant, which would allow for rapid development of updated COVID vaccines.
“The fact that most of the manufacturers are ready to work on an XBB 1.5 [vaccine] is an added reason to select this strain or this variant, given the immunologic data,” Dr. Monto said.
Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, said the demands involved in manufacturing vaccines tilts toward annual changes.
“Practically, we’re going to have one update per year, barring a heroic effort to deal with a strain that pops up that is essentially so different that it requires us to mobilize tremendous resources to address that strain change,” he said.
Dr. Marks questioned the panelists’ concerns about likening flu and COVID vaccination practices. The FDA staff’s intent was to try to help the public understand the need for follow-on vaccination.
“I’m really having trouble understanding that committee’s need to bristle against something that’s similar to influenza. People understand a yearly influenza vaccine,” Dr. Marks said.
And it’s not certain when another major change in the COVID virus will follow the XBB subvariant, but it’s likely one will – and soon, Dr. Marks said.
“It looks like, probably by next fall, there’ll be further drift from this,” he said.
Informing the public
Dr. Marks also stressed the need to better convey the benefits of vaccination to people in the United States.
CDC data estimate that 70% of the U.S. population completed an initial series of the original monovalent vaccines, with only 17% then getting bivalent shots. There’s even a decline among people ages 65 and older. CDC estimates 94% of this group completed their primary series, but only 43% got the bivalent booster dose.
“We have to do better because we have not done a good job today communicating to the American public what’s going on here,” Marks said.
Researchers also are still trying to determine the best timing for people to get additional COVID shots. Finding the “sweet spot” where people can maximize additional protection is tricky, with people most protected if they happen to get shot near the beginning of an uptick in viral spread, the CDC’s Ruth Link-Gelles, PhD, MPH, told the panel during a presentation.
“You’re going to get the best incremental benefit if it’s been longer since your last vaccine,” she said. “But of course, if you wait too long since your last vaccine, you’re left with very little protection, and so you’re at higher risk of severe illness.”
Like Dr. Marks, Dr. Link-Gelles stressed the need for persuading more people to get follow-on vaccines.
“Most Americans, at this point, haven’t even received the bivalent and so are a year or more out from their monovalent dose and so have relatively little protection left,” she said.
A version of this article first appeared on WebMD.com.
Latest data: COVID vaccine safety, protection, and breakthrough infections in inflammatory, autoimmune diseases
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
MILAN – The impact of the COVID-19 pandemic on patients with rheumatic and nonrheumatic autoimmune diseases is ongoing and not yet fully comprehended. New data presented at the annual European Congress of Rheumatology, primarily derived from the global COVID-19 in Autoimmune Diseases (COVAD) survey but not limited to it, provide reassurance regarding the protection and safety of COVID-19 vaccines for older and younger adults, as well as for pregnant and breastfeeding women. These data also explore the influence of underlying diseases and medications on breakthrough SARS-CoV-2 infections and infection outcomes.
Safety of vaccines in patients with autoimmune or immune-mediated diseases
Following vaccination, even with low levels of antibodies, the risk of severe COVID-19 remains relatively low for patients who receive immunosuppressive therapy for various immune-mediated inflammatory diseases (IMIDs). This encouraging finding comes from the Nor-vaC study, presented by Hilde Ørbo, MD, of the Center for Treatment of Rheumatic and Musculoskeletal Diseases, Diakonhjemmet Hospital, Oslo.
During the presentation, Dr. Ørbo stated: “We did not find any specific diagnosis or medication associated with a significantly higher risk of hospitalization.” Receiving booster doses of the vaccine, having high levels of anti-spike antibodies after vaccination, and achieving hybrid immunity are correlated with further reductions in the risk of breakthrough SARS-CoV-2 infections.
Between Feb. 15, 2021, and Feb. 15, 2023, COVID-19 affected a similar proportion among the 729 patients and 350 healthy control persons (67% and 68%, respectively). Among the patients, 22 reported severe COVID-19, whereas none of the healthy control persons did. However, there were no fatalities among the patients. The study cohort consisted of patients with various IMIDs; 70% had an inflammatory joint disease. The use of immunosuppressive medications also varied, with 63% of patients using tumor necrosis factor inhibitors, either as monotherapy or in combination with other treatments, and other patients taking medications such as methotrexate, interleukin inhibitors, Janus kinase inhibitors, vedolizumab (Entyvio), and others.
While being older than 70 years and the presence of comorbidities were identified as risk factors for severe COVID-19, there was a significant reduction in risk with each additional vaccine dose. These results support the protective role of repeated COVID-19 vaccination for patients with IMIDs who are receiving immunosuppressive therapies; they yield a favorable prognosis even with the Omicron variant.
The study further compared the risk of severe COVID-19 between a group with hybrid immunity (having received three vaccine doses and experiencing breakthrough infection with the Omicron variant) and a group that received a fourth vaccine dose within the same time frame. The difference was striking: Hybrid immunity was associated with a 5.8-fold decrease in risk, compared with four-dose vaccination (P < .0001).
The level of antibodies, measured 2-4 weeks after the last vaccination, was predictive of the risk of breakthrough COVID-19. An antibody level above 6000 binding antibody units/mL after vaccination was significantly associated with a reduction in risk. “We can conclude that patients who receive multiple vaccine doses have a lower risk of COVID-19,” Dr. Ørbo said. “In patients who recently experienced breakthrough infections, the administration of a booster vaccine dose might be delayed.”
“The virus has undergone changes throughout the pandemic, while the vaccines have remained relatively stable. Are we anticipating more infections over time?” asked Hendrik Schulze-Koops, MD, PhD, of Ludwig Maximilians University of Munich (Germany), the session moderator. In response, Dr. Ørbo stated that 85% of the recorded infections in the study occurred after the emergence of the Omicron variant, and time was considered a covariable in the analysis.
These data shed light on a topic discussed by Pedro Machado, MD, PhD, professor and consultant in rheumatology and neuromuscular diseases at University College London, during his scientific session talk entitled, “Unsolved Issues of COVID Vaccination and Re-vaccination.” Dr. Machado referred to the VROOM study published in 2022, which examined the interruption of methotrexate for 2 weeks following booster administration. Both groups demonstrated a significant antibody response, but the group that stopped taking methotrexate showed double the antibody titers.
However, he emphasized, “what remains unknown is the clinical relevance of these differences in terms of severe infection, hospitalization, or even death. The potential benefit of increased immunogenicity by interrupting conventional synthetic disease-modifying antirheumatic drugs [csDMARDs] such as methotrexate before or after vaccination needs to be balanced against the potential risk of disease flare. Ultimately, decision-making should be individualized based on factors such as comorbidities, disease activity, and other considerations.” The results presented by Dr. Ørbo suggest that, while there may be a clinical difference in terms of severe infection, the overall prognosis for vaccinated patients is reasonably good.
Regarding other DMARDs, such as biologics, the approach may differ. Dr. Machado suggested: “In patients using rituximab or other B cell–depleting therapies, SARS-CoV-2 vaccination should be scheduled in a way that optimizes vaccine immunogenicity. A minimum of 10 B cells/mcL of blood is likely a relevant threshold above which a sufficient cellular and immune response is established.”
COVID vaccines are safe for pregnant and breastfeeding women
According to data from the COVAD study, which comprised two global cross-sectional surveys conducted in 2021 and 2022, the COVID-19 vaccine appeared safe for pregnant and breastfeeding women with autoimmune diseases (AID).
Presenter Laura Andreoli, MD, PhD, of the University of Brescia (Italy), said that, although pregnant patients with AID reported more adverse events related to vaccination, these rates were not significantly higher than those among pregnant, healthy control persons who were without AID. No difference in adverse events was observed between breastfeeding women and healthy control persons, and the incidence of disease flares did not significantly differ among all groups.
“In summary, this study provides initial insights into the safety of COVID-19 vaccination during the gestational and postpartum periods in women with autoimmune diseases. These reassuring observations will hopefully improve clinician-patient communication and address hesitancy towards COVID-19 vaccination, as the benefits for the mother and fetus through passive immunization appear to outweigh potential risks,” Dr. Andreoli said in an interview.
“The large number of participants and the global geographical spread of the COVAD survey were very beneficial in gaining access to this important subset of patients,” added Dr. Andreoli. However, she acknowledged that patients with low socioeconomic status and/or high disability were likely underrepresented. While no data on pregnancy outcomes have been collected thus far, Dr. Andreoli expressed the desire to include them in the study’s follow-up.
The COVAD survey data also indicate that, in general, vaccine hesitancy among patients with AID is decreasing; from 2021 to 2022, it declined from 16.5% to 5.1%, as Dr. Machado indicated in his presentation.
Multiple factors contribute to breakthrough infections
The risk of breakthrough SARS-CoV-2 infections after vaccination varies among patients with rheumatoid arthritis and rheumatic or nonrheumatic autoimmune diseases, primarily depending on the underlying condition rather than the immunosuppressive medication. Environmental factors also appear to play a role. This complex landscape emerges from a further analysis of the COVAD survey dataset.
Alessia Alunno, MD, PhD, of the University of L’Aquila (Italy), presented a detailed and occasionally counterintuitive picture of similarities and differences among young adult patients (aged 18-35 years), mostly women, with various rheumatic and nonrheumatic diseases in relation to COVID-19. Most notably, the type of disease seemed to have more significance than the immunosuppression resulting from the treatment regimen. This held true for vaccine safety as well as for the risk of breakthrough COVID-19 and symptom profiles.
Patients with rheumatic disease (RMD) and nonrheumatic autoimmune disease (nr-AD) had significantly different therapeutic profiles on average. Before vaccination, 45% of patients with RMD used glucocorticoids (GC), and 91% used immunosuppressants (IS). In contrast, only 9.5% of nr-AD patients used GC, and 21% were taking IS.
Interestingly, the overall prevalence of reported SARS-CoV-2 infections was not influenced by medication and was practically identical (25% to 28%) across all groups. However, there were intriguing differences in the occurrence of infections before and after vaccination between disease groups. Prevaccine infections were less frequent among patients with RMD compared with healthy control persons (adjusted odds ratio, 0.6), while the rates were similar among patients with nr-AD and healthy control persons. On the other hand, breakthrough infections were more frequent in patients with RMD (aOR, 2.7), whereas the rate was similar between healthy control persons and patients with nr-AD.
Despite a much lower rate of GC/IS use, patients with nr-AD experienced repeated infections more frequently. In contrast, patients with RMD were less prone to multiple infections, even compared with healthy control persons (aOR, 0.5).
Regarding the disease profile, fewer than 5% of all infected patients required advanced therapies for SARS-CoV-2 infection. Notably, all SARS-CoV-2 infections in patients with nr-AD were symptomatic, whereas among patients with RMD and healthy control persons, the incidence of asymptomatic infections was 3%. The rate of hospital admissions was 4% for patients with RMD, compared with 2% for patients with nr-AD and 1% for control persons. The RMD group exhibited some differences between prevaccine infections and breakthrough infections, including a significantly lower frequency of loss of smell and taste during breakthrough infections. Overall, patients with RMD and COVID-19 experienced cough, runny nose, throat pain, nausea, and vomiting more frequently. In contrast, patients with nr-AD had a much higher risk of skin rashes during breakthrough infections (aOR, 8.7).
Vaccine adverse events (AEs) were also influenced by the underlying disease. Patients with RMD and those with nr-AD were more likely to experience mild AEs after the first or second dose, compared with healthy control persons (adjusted OR, 2.4 and 2.0, respectively). The most common early, mild AEs across all groups were injection-site pain, headache, and fatigue, but they occurred more frequently in the nr-AD group than in the RMD or healthy control group. Additionally, fever and chills occurred more frequently among the nr-AD group. Late, mild AEs and severe AEs were rare and affected all groups equally.
“The overall incidence of AEs was very low. Our results certainly do not undermine the safety of vaccines,” Dr. Alunno said.
Disease flares were more common after vaccination (10% with RMD and 7% with nr-AD) than after infection (5% with RMD and 1.5% with nr-AD). Furthermore, in many cases, after vaccination, flares required a change of medications, particularly for patients with RMD.
Additional results from the COVAD survey from January to July 2022, presented by Naveen Ravichandran, MD, DM, of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India, revealed a higher prevalence (OR, 1.2; P = .001) of breakthrough infections among patients with RA. A total of 22.6% of patients with RA experienced breakthrough infections, compared with 20.6% for patients with other autoimmune rheumatic diseases and 18.4% of healthy control persons. Hospitalizations and the need for advanced treatment were also more common among patients with RA (30.9%) than among healthy control persons (13.9%). Patients with RA who had breakthrough infections tended to be older (closer to 50 years of age on average) and female, and they were more likely to have comorbidities and mental disorders. The human development index of the patient’s country of residence also played a role. Further research is necessary to understand how breakthrough infection outcomes are affected by a patient’s socioeconomic situation.
According to Dr. Ravichandran, medication was not a significant factor, except for the use of steroids and rituximab, which were associated with a higher risk of severe COVID-19 and hospitalization. Patients using rituximab, in particular, faced significantly increased odds for hospitalization (OR, 3.4) and severe breakthrough COVID-19 (OR, 3.0).
Session moderator Kim Lauper, MD, of the University of Geneva, cautioned: “The roles of disease and medication are challenging to separate. Some diseases require a more aggressive immunosuppressive regimen. It’s possible that different diseases affect the immune system differently, but it is not easy to demonstrate.”
The complications observed in the data warrant further study, as mentioned by Dr. Schulze-Koops: “We have a problem tied to the time line of the pandemic, where we had different viruses, different population behaviors, different treatments, and different standards of care over time. We also have differences between ethnic communities and regions of the world. But most importantly, we have different viruses: From the original strain to Delta to Omicron, we know they have very different clinical outcomes. I believe we need more scientific research to unravel these factors.”
Dr. Ørbo, Dr. Ravichandran, Dr. Andreoli, and Dr. Alunno reported no relevant financial relationships. Dr. Machado has received grants and/or honoraria from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Orphazyme, Pfizer, Roche, and UCB.
A version of this article originally appeared on Medscape.com.
AT EULAR 2023
COVID vaccines safe for young children, study finds
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
TOPLINE:
COVID-19 vaccines from Moderna and Pfizer-BioNTech are safe for children under age 5 years, according to findings from a study funded by the Centers for Disease Control and Prevention.
METHODOLOGY:
- Data came from the Vaccine Safety Datalink, which gathers information from eight health systems in the United States.
- Analyzed data from 135,005 doses given to children age 4 and younger who received the Pfizer-BioNTech , and 112,006 doses given to children aged 5 and younger who received the Moderna version.
- Assessed for 23 safety outcomes, including myocarditis, pericarditis, and seizures.
TAKEAWAY:
- One case of hemorrhagic stroke and one case of pulmonary embolism occurred after vaccination but these were linked to preexisting congenital abnormalities.
IN PRACTICE:
“These results can provide reassurance to clinicians, parents, and policymakers alike.”
STUDY DETAILS:
The study was led by Kristin Goddard, MPH, a researcher at the Kaiser Permanente Vaccine Study Center in Oakland, Calif., and was funded by the Centers for Disease Control and Prevention.
LIMITATIONS:
The researchers reported low statistical power for early analysis, especially for rare outcomes. In addition, fewer than 25% of children in the database had received a vaccine at the time of analysis.
DISCLOSURES:
A coauthor reported receiving funding from Janssen Vaccines and Prevention for a study unrelated to COVID-19 vaccines. Another coauthor reported receiving grants from Pfizer in 2019 for clinical trials for coronavirus vaccines, and from Merck, GSK, and Sanofi Pasteur for unrelated research.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
The new vaccine your patients may not want
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Compared with the complicated and ever-changing recommended vaccine schedule for infants and children, vaccines for adults have been straightforward. Adults without compromised immunity who received all their childhood vaccinations are eligible for a tetanus and diphtheria (Td) or tetanus, diphtheria, and pertussis (Tdap) booster every 10 years, recombinant herpes zoster vaccine at age 50, and pneumococcal vaccines at age 65, along with annual influenza and (likely) COVID-19 vaccines. Last year, due to rising rates of acute hepatitis B, the Centers for Disease Control and Prevention first recommended universal hepatitis B vaccination for adults aged 19-59 years without a record of previous hepatitis B infection or vaccination.
An additional routine vaccine for adults is now on the horizon. The U.S. Food and Drug Administration recently approved Arexvy, a vaccine against respiratory syncytial virus (RSV) for adults aged 60 years or older. Two more RSV vaccines are in the final stages of development. Why should family physicians prioritize vaccinating older adults against RSV, and how can we incorporate this new vaccine into our practices and overcome patient hesitancy to receive yet another vaccine?
Clinicians tend to think of RSV as a serious disease in young children – which it is – but data suggest that in 2019, RSV infection led to more than 100,000 hospitalizations and 7,700 deaths in older adults in the United States. In a randomized controlled trial of 25,000 adults aged 60 years or older with a median of 6.7 months of follow-up, Arexvy reduced severe RSV disease by 94% and RSV-related acute respiratory infections by 71%, with similar effectiveness in adults with underlying health conditions. That’s considerably better protection than current influenza vaccines and comparable to COVID-19 mRNA vaccines before variants became widespread. Pain and fatigue were the most common side effects and usually resolved within 1-2 days.
Although the seasonal pattern of RSV shifted during the COVID-19 pandemic, RSV season historically begins in October, peaks in December, and ends in April. If the vaccine is recommended by the CDC and is widely available by fall, as the manufacturer, GSK, expects, it could be administered around the same time as influenza and COVID-19 vaccines.
The challenges of incorporating this new vaccine into practice will feel familiar: Many of our patients won’t have heard about it, may feel that they don’t need it, or may decline it because of concerns about side effects, real or imagined. (Of note, the FDA is requiring GSK to perform a postmarketing study to rule out associations with rare cases of Guillain-Barré syndrome and acute disseminated encephalomyelitis, and the company also plans to monitor the incidence of atrial fibrillation, which was slightly more common in the vaccine group than the placebo group.)
While a strong recommendation from a family physician is often enough to convince patients to accept vaccination, rampant misinformation during the pandemic may have worsened vaccine hesitancy for some. It may feel like a fruitless exercise to try to convince adults who have refused COVID-19 and influenza vaccines to accept a newer vaccine against a respiratory virus that causes less serious illness overall. But with other RSV vaccines and monoclonal antibodies for older adults and infants likely to be approved soon, it’s important for us to start laying the groundwork now by educating colleagues, staff, and patients about preventing serious illness caused by RSV.
Dr. Lin is an associate professor in the Department of Family Medicine at Georgetown University and a staff physician atMedStar Health Center, both in Washington. He has received income from UpToDate, Wiley-Blackwell, and the American Academy of Family Physicians.
A version of this article first appeared on Medscape.com.
Safety remains top parent concern for HPV vaccine
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
, according to a study published online in Pediatrics.
“Although HPV vaccination rates in the United States have steadily improved over the past decade, a sizable subset of parents remains highly hesitant about administering the vaccine to their adolescent children,” wrote Eric Adjei Boakye, PhD, of the departments of public health sciences and otolaryngology–head and neck surgery at the Henry Ford Health System, Detroit, and associates. But a silver lining in the study is the downward trend in parents not vaccinating their children against HPV because the child’s provider did not recommend it.
“Provider recommendation has been shown to be the single best predictor of HPV vaccine uptake and vaccine acceptability,” the authors wrote. They noted one previous study finding that provider recommendations for the vaccine had increased from 27% in 2012 to 49.3% in 2018.
Safety concerns increased while other concerns decreased
The findings were not surprising to Robert A. Bednarczyk, PhD, associate professor of global health at Emory University Rollins School of Public Health, Atlanta, who specializes in HPV vaccine research.
“We have seen over the years that vaccine safety concerns have been on the increase, notably recently in the context of the COVID-19 pandemic and vaccination program, but HPV vaccine safety, though well established, continues to be a major concern for parents,” Dr. Bednarczyk said in an interview. But he found it striking that parents’ other reasons for turning down the vaccine had declined. “This shows that the outreach around the need for HPV vaccination and efforts to improve provider recommendation strategies is likely having positive impacts on HPV vaccine attitudes.”
Top five reasons for not vaccinating
The researchers analyzed data from the National Immunization Survey–Teen for the years 2010 through 2020 to track the annual changes in the top five reasons cited for not planning to get the HPV vaccine. The data covered 119,695 teens aged 13-17.
The researchers identified parents’ five most commonly cited reasons for not planning to vaccinate their children against HPV: “not necessary,” “safety concerns,” “lack of recommendation,” “lack of knowledge,” and “not sexually active.”
Parents’ HPV vaccine hesitancy decreased by 5.5% each year from 2010 to 2012, but then it stagnated for the remaining years through 2020. Across most of that time, from 2010 to 2018, parents’ concerns about the vaccine’s safety and side effects increased by 15.6%. A major reason for this increase, the authors suggested, may include the widespread distribution of online misinformation, particularly given the 7.8 million increase in antivaccine social media accounts since 2019.
“Fear tactics are often used by antivaccine campaigners to dissuade parents from vaccinating their children. There have been several myths propagated about vaccines causing adverse reactions,” the authors wrote. “Although these myths have been scientifically debunked, they continue to circulate.”
In contrast to parents’ concerns, a study in 2021 found a downward trend in reports of nonserious adverse effects and no change in reports of serious adverse effects from the HPV vaccine between 2015 and 2018. Further, more than 95% of the adverse effect reports to the Vaccine Adverse Event Reporting System after HPV vaccination were nonserious.
Reducing perceived barriers
Meanwhile, however, parents’ other reasons for avoiding the vaccine became less prevalent throughout most of the study period. For each year between 2013 and 2020, the proportion of parents saying they didn’t intend to get their children the HPV vaccine because it was “not recommended” decreased by 6.8%.
Similarly, avoiding the vaccine due to “lack of knowledge” declined 9.9%, and avoidance because the child was “not sexually active” declined 5.9% each year from 2013 to 2020. No difference occurred during that time period regarding how frequently parents cited that the vaccine was “not necessary.”
“Decreases in the percentage of parents/guardians citing lack of provider recommendation, lack of knowledge, and child ‘not sexually active’ as the main reason for HPV vaccine hesitancy ... are encouraging and suggest that interventions have been successful in reducing perceived barriers to HPV vaccination,” the authors wrote.
Dr. Bednarczyk agreed that these findings were encouraging, underscoring that outreach and support for health care providers to give strong recommendations for the vaccine need to continue.
“But additionally, we need to find better ways to communicate about vaccine safety,” Dr. Bednarczyk said. “Seeing that the number of parents citing safety concerns as the primary barrier has not changed much between 2016 and 2020, but that the percent of parents having those concerns increased, likely means there is a stable part of the population with these safety concerns, and as more adolescents are getting vaccinated against HPV, the relative contribution of safety concerns is increasing.” A key way to address those concerns includes “engaging with our trusted community partners and giving them the tools to discuss the safety of HPV vaccination with members of the community,” he said.
Debunking misinformation
Like the authors, Dr. Bednarczyk pointed out several conditions that parents erroneously worry could be caused by the HPV vaccine, but he emphasized that simply telling parents those misconceptions are untrue is insufficient to allay fears.
“It’s important for both clinicians and community partners to recognize we cannot just present a list of facts and figures and statistics to parents to reassure them and hope that this works,” Dr. Bednarczyk said. “Effective communication, strong narratives to illustrate this knowledge, and engagement with not just clinicians but community partners and other trusted sources is needed.” Dr. Bednarczyk continues to support the evidence-based model of presumptive recommendations, which does not remove parental autonomy but simplifies vaccine messaging about what’s recommended, “but clinicians need to be prepared with both the data and effective ways to communicate it to address questions if they come up after the presumptive recommendation is given,” he added.
The researchers pointed out that their study data were collected before the pandemic, so “it is reasonable to expect that HPV vaccine–related safety concerns may continue to rise because of the plethora of misinformation surrounding coronavirus disease 2019 vaccination.”
Dr. Bednarczyk said it will be important to see in future research whether shifts in beliefs about the HPV vaccine have occurred in the midst of the pandemic and afterward.
“As the authors stated, it’s important to remember that HPV vaccination has consistently been shown to be safe and effective,” Dr. Bednarczyk said. “But those research findings are not seeming to resonate with parents, highlighting how we need to improve our outreach and communication work.”
The research did not receive external funding. A coauthor is a scientific adviser to Navigating Cancer. The other authors and Dr. Bednarczyk had no disclosures.
FROM PEDIATRICS
Severe rash after COVID-19 vaccination
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.
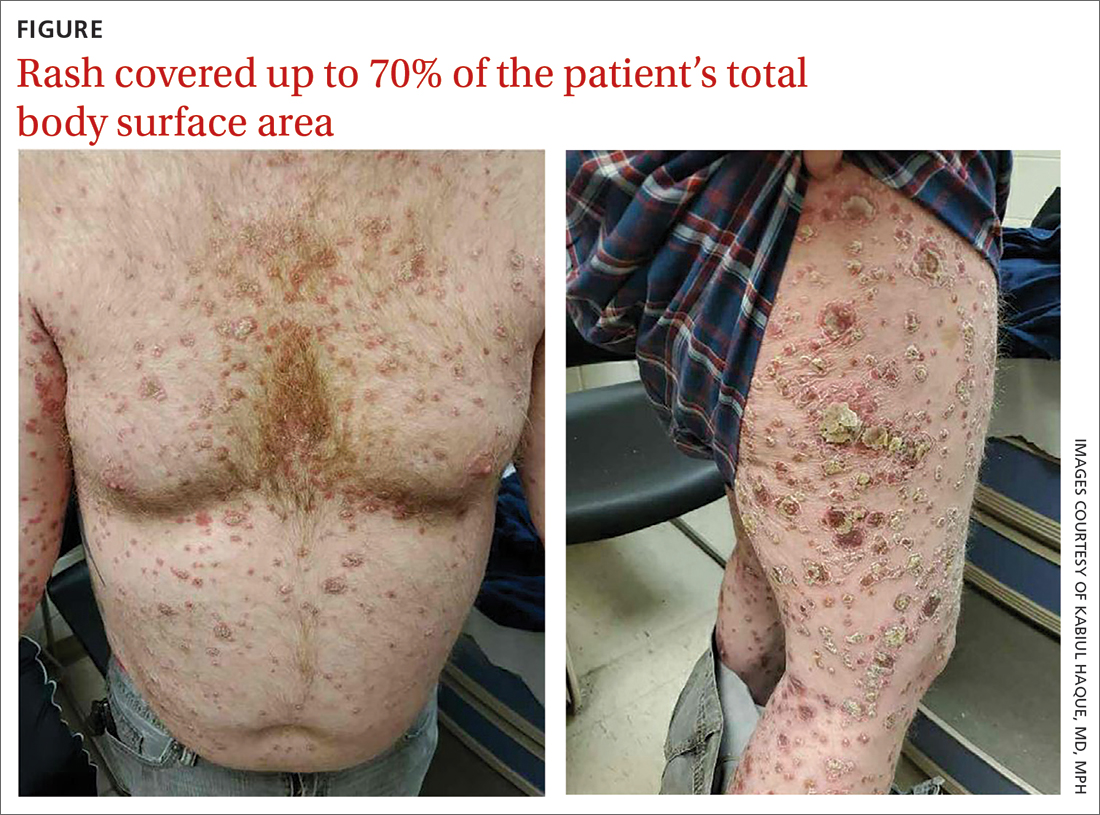
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
A 41-year-old man presented for evaluation of an extensive skin rash that had erupted more than a month earlier. The patient had received 2 doses of the Pfizer COVID-19 vaccine 3 weeks apart. Ten days after his second dose, the patient developed a rash all over his body. He described the rash as burning, itchy, and uncomfortable. The patient denied any triggers such as recent or previous infections, stressors, or drugs. The patient had no personal or family history of dermatologic disorders; his general medical history was unremarkable. The patient smoked and drank alcohol occasionally.
On physical exam, the patient had a diffuse rash, which initially had manifested on both of his hands, including the palms, and then spread to 60% to 70% of his total body surface area, including his face, ears, anterior and posterior chest, upper and lower extremities, and buttocks. The rash consisted of 10- to 15-mm white scaly plaques that did not bleed.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Guttate psoriasis
Punch biopsies were obtained, and histopathology revealed diffuse compact hyperkeratosis with broad zones of parakeratosis. There was attenuation of the granular layer and regular elongation of the rete ridges associated with thinning of the suprapapillary epidermis and mild spongiosis. These pathologic findings were consistent with a diagnosis of psoriasis. There were no drug-related skin eruption features, such as apoptotic keratinocytes, eosinophils, or interface dermatitis. Periodic acid-Schiff stains for fungal organisms were negative. The combined clinical presentation (itchy, teardrop-shaped, scaly lesions) and histologic impression were consistent with guttate psoriasis.
Psoriasis can be seen in various forms. Subtypes of psoriasis include guttate psoriasis, inverse psoriasis, erythrodermic psoriasis, nail psoriasis, and pustular psoriasis.1 Guttate psoriasis accounts for about 2% of psoriasis cases and usually is seen in patients younger than 30 years.2 Guttate psoriasis is characterized by 1- to 10-mm teardrop-shaped pink papules with fine scaling.3
Triggers for psoriasis. Vaccinations, medications, and infections (eg, group A beta-hemolytic streptococcal upper respiratory infections) can trigger guttate psoriasis.3 MRNA vaccines (eg, Moderna and Pfizer/BioNTech COVID-19 vaccines) have been associated with psoriasis episodes.1 Other vaccines such as influenza, rubella, bacillus Calmette-Guerin, tetanus-diphtheria, and pneumococcal polysaccharide also have been known to trigger psoriasis.4 Medications that can trigger psoriasis include beta-blockers, lithium, antimalarial drugs, and (in some cases) nonsteroidal anti-inflammatory drugs.5
The impact of COVID-19 vaccine. We are still learning about the incidence and prevalence of adverse effects (such as psoriasis) that can follow COVID-19 vaccination.
Psoriasis following vaccination. The pathologic mechanism for the new onset or flare of psoriasis after COVID-19 vaccination is unknown. What is known is that the dysregulation of Th-1 and Th-17 plays an important role in the pathogenesis of psoriasis.7 Previously, it was found that psoriasis can manifest after tetanus-diphtheria vaccines due to an increase in the production of Th-17 cells.7 Th-1 and Th-17 production also increases after influenza vaccine and can cause an onset or flare-up of psoriasis.8
Continue to: The differential includes syphilis and exfoliative dermatitis
The differential includes syphilis and exfoliative dermatitis
The differential diagnosis includes various forms of psoriasiform dermatitis, such as secondary syphilis, chronic spongiotic dermatitis, psoriasiform drug eruption, exfoliative dermatitis, and pityriasis rubra pilaris. A combination of clinical and histopathologic findings is used to zero in on the diagnosis. The summary below highlights the clinical findings.
Secondary syphilis manifests with symmetric papular eruptions primarily on the trunk and extremities with involvement on the palms and soles. Lesions are red or reddish brown, can be smooth, and are rarely pustular.
Chronic spongiotic dermatitis manifests with a shiny, glazed, cracked appearance and itchy reddish lesions on the soles.
Psoriasiform drug eruption manifests after drug administration with a psoriasis-like rash with erythematous, squamous, thick, dry, and plaque-type lesions.
Exfoliative dermatitis manifests with erythematous single or multiple pruritic patches on the trunk, head, and genitals.
Continue to: Pityriasis rubra pilaris
Pityriasis rubra pilaris manifests in various ways. Patients may have plaques that are erythematous, scaly, or follicular. Sometimes, it may manifest as erythroderma with an “island of sparing,” which is normal-looking skin in the affected areas.
How to make the diagnosis
Psoriasis can be diagnosed by physical examination. A skin biopsy is not usually necessary but can be helpful for complex cases.
There are no laboratory or genetic tests to confirm the diagnosis of psoriasis. Depending on the case, routine bloodwork (eg, complete blood count and metabolic panel) and infectious disease tests (eg, HIV, hepatitis panel, and
Starting with a low- to medium-potency steroid, such as betamethasone valerate 0.1% cream twice per day or triamcinolone acetonide 0.1% cream twice per day for 2 weeks, provides high safety and efficacy for localized disease.9 An appropriate-potency steroid should be chosen based on the disease severity, location, and patient’s preference and age. Topical vitamin D analogues often are used in conjunction with topical steroids to treat psoriasis.9
Depending on the severity, patient age, comorbidities, and availability of treatment, other treatment options for psoriasis include oral methotrexate (2.5 mg to 25 mg weekly, starting with a low dose), acitretin (10 mg to 50 mg daily), apremilast (10 mg daily, gradually increasing to 30 mg twice per day in a divided dose), biologics, and narrowband ultraviolet light.
In this case, betamethasone dipropionate 0.05% cream twice daily for 2 weeks was not sufficiently effective due to the extent of the psoriasis. Following consultation with a dermatologist, clobetasol propionate 0.05% cream twice per day and oral apremilast (10 mg once per day on the first day and 10 mg twice per day afterward) were prescribed for 2 weeks. The patient’s psoriasis improved somewhat after 2 weeks of the treatment, but many plaques remained. Therefore, apremilast was stopped and subcutaneous adalimumab was started (initial loading dose, 80 mg, then 40 mg every other week). The psoriasis lesions cleared over the next 2 to 3 months. The patient was maintained on the adalimumab to avoid a recurrence of lesions.
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
1. Wu PC, Huang IH, Wang CW, et al. New onset and exacerbations of psoriasis following COVID-19 vaccines: a systematic review. Am J Clin Dermatol. 2022;23:775-799. doi: 10.1007/s40257-022-00721-z
2. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850. doi: 10.1016/j.jaad.2008.02.039
3. Weigle N, McBane S. Psoriasis. Am Fam Physician. 2013;87:626-633.
4. Wei N, Kresch M, Elbogen E, et al. New onset and exacerbation of psoriasis after COVID-19 vaccination. JAAD Case Rep. 2022;19:74-77. doi: 10.1016/j.jdcr.2021.11.016
5. Piérard-Franchimont C, Piérard GE. L’iatrogénie psoriasique [Drug-related psoriasis]. Rev Med Liege. 2012;67:139-142. French.
6. Huang Y, Tsai T. Exacerbation of psoriasis following COVID-19 vaccination: report from a single center. Front Med. 8:812010. doi: 10.3389/fmed.2021.812010
7. Pesque D, Lopez-Trujillo E, Marcantonio O, et al. New-onset and exacerbation of psoriasis after mRNA COVID-19 vaccines: two sides of the same coin? J Eur Acad Dermatol Venereol. 2022;36:e80-e157 doi: 10.1111/jdv.17690
8. Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis. J Immunol Res. 2015;2015:258430. doi: 10.1155/2015/258430
9. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi: 10.1016/j.jaad.2020.07.087
Here’s how we can rebuild trust in vaccines
When people ask Paul Offit, MD, what worries him the most about the COVID-19 pandemic, he names two concerns. “One is the lack of socialization and education that came from keeping kids out of school for so long,” Dr. Offit said in a recent interview. “And I think vaccines have suffered.”
Dr. Offit is director of the Vaccine Education Center and a professor of pediatrics at Children’s Hospital of Philadelphia. He has watched with alarm as the American public appears to be losing faith in the lifesaving vaccines the public health community has worked hard to promote. The Centers for Disease Control and Prevention estimates that the proportion of kids entering kindergarten who have received state-required vaccines dipped to 94% in the 2020-2021 school year – a full point less than the year before the pandemic – then dropped by another percentage point, to 93%, the following year.
Although a couple of percentage points may sound trivial, were only 93% of kindergarteners to receive the vaccine against measles, mumps, and rubella (MMR), approximately 250,000 vulnerable 5-year-olds could spark the next big outbreak, such as the recent measles outbreaks in Ohio and Minnesota.
Dr. Offit is one of many public health officials and clinicians who are working to reverse the concerning trends in pediatric vaccinations.
“I just don’t want to see an outbreak of something that we could have avoided because we were not protected enough,” Judith Shlay, MD, associate director of the Public Health Institute at Denver Health, said.
Official stumbles in part to blame
Disruptions in health care from the COVID-19 pandemic certainly played a role in the decline. Parents were afraid to expose their children to other sick kids, providers shifted to a telehealth model, and routine preventive care was difficult to access.
But Dr. Offit also blamed erosion of trust on mistakes made by government and public health institutions for the alarming trend. “I think that health care professionals have lost some level of trust in the Food and Drug Administration and CDC.”
He cited as an example poor messaging during a large outbreak in Massachusetts in summer 2021, when the CDC published a report that highlighted the high proportion of COVID-19 cases among vaccinated people. Health officials called those cases “breakthrough” infections, although most were mild or asymptomatic.
Dr. Offit said the CDC should have focused the message instead on the low rate (1%) of hospitalizations and the low number of deaths from the infections. Instead, they had to walk back their promise that vaccinated people didn’t need to wear masks. At other times, the Biden administration pressured public health officials by promising to make booster shots available to the American public when the FDA and CDC felt they lacked evidence to recommend the injections.
Rupali Limaye, PhD, an associate professor of international health at the Bloomberg School of Public Health at Johns Hopkins University, Baltimore, studies vaccine behavior and decision-making. She would go a step further in characterizing the roots of worsening vaccine hesitancy.
“In the last 20 years, we’ve seen there’s less and less trust in health care providers in general,” Dr. Limaye said. “More people are turning to their social networks or social contacts for that kind of information.” In the maelstrom of the COVID-19 pandemic, digital social networks facilitated the spread of misinformation about COVID-19 faster than scientists could unravel the mysteries of the disease.
“There’s always been this underlying hesitancy for some people about vaccines,” Dr. Shlay said. But she has noticed more resistance to the COVID-19 vaccine from parents nervous about the new mRNA technology. “There was a lot of politicization of the vaccine, even though the mRNA vaccine technology has been around for a long time,” she said.
Multipronged approaches
Dr. Shlay is committed to restoring childhood vaccination uptake to prepandemic levels now that clinics are open again. To do so, she is relying on a combination of quality improvement strategies and outreach to undervaccinated populations.
Denver Health, for instance, offers vaccinations at any inpatient or outpatient visit – not just well-child visits – with the help of alerts built into their electronic health records that notify clinicians if a patient is due for a vaccine.
COVID-19 revealed marked health inequities in underserved communities as Black, Hispanic, and people from other minority communities experienced higher rates of COVID-19 cases and deaths, compared with White people. The Public Health Institute, which is part of Denver Health, has responded with vaccine outreach teams that go to schools, shelters, churches, and community-based organizations to vaccinate children. They focus their efforts on areas where immunization rates are low. Health centers in schools throughout Colorado vaccinate students, and the Public Health Institute partners with Denver-area public schools to provide vaccines to students in schools that don’t have such centers. (They also provide dental care and behavioral health services.)
But it is unlikely that restoring clinic operations and making vaccines more accessible will fill the gap. After 3 years of fear and mistrust, parents are still nervous about routine shots. To help clinicians facilitate conversations about vaccination, Denver Health trains providers in communication techniques using motivational interviewing (MI), a collaborative goal-oriented approach that encourages changes in health behaviors.
Dr. Shlay, who stressed the value of persistence, advised, “Through motivational interviewing, discussing things, talking about it, you can actually address most of the concerns.”
Giving parents a boost in the right direction
That spirit drives the work of Boost Oregon, a parent-led nonprofit organization founded in 2015 that helps parents make science-based decisions for themselves and their families. Even before the pandemic, primary care providers needed better strategies for addressing parents who had concerns about vaccines and found themselves failing in the effort while trying to see 20 patients a day.
For families that have questions about vaccines, Boost Oregon holds community meetings in which parents meet with clinicians, share their concerns with other parents, and get answers to their questions in a nonjudgmental way. The 1- to 2-hour sessions enable deeper discussions of the issues than many clinicians can manage in a 20-minute patient visit.
Boost Oregon also trains providers in communication techniques using MI. Ryan Hassan, MD, a pediatrician in private practice who serves as the medical director for the organization, has made the approach an integral part of his day. A key realization for him about the use of MI is that if providers want to build trust with parents, they need to accept that their role is not simply to educate but also to listen.
“Even if it’s the wildest conspiracy theory I’ve ever heard, that is my opportunity to show them that I’m listening and to empathize,” Dr. Hassan said.
His next step, a central tenet of MI, is to make reflective statements that summarize the parent’s concerns, demonstrate empathy, and help him get to the heart of their concerns. He then tailors his message to their issues.
Dr. Hassan tells people who are learning the technique to acknowledge that patients have the autonomy to make their own decisions. Coercing them into a decision is unhelpful and potentially counterproductive. “You can’t change anyone else’s mind,” he said. “You have to help them change their own mind.”
Dr. Limaye reinforced that message. Overwhelmed by conflicting messages on the internet, people are just trying to find answers. She trains providers not to dismiss patients’ concerns, because dismissal erodes trust.
“When you’re dealing with misinformation and conspiracy, to me, one thing to keep in mind is that it’s the long game,” Dr. Limaye said, “You’re not going to be able to sway them in one conversation.”
Can the powers of social media be harnessed for pro-vaccine messaging? Dr. Limaye has studied social media strategies to promote vaccine acceptance and has identified several elements that can be useful for swaying opinions about vaccine.
One is the messenger – as people trust their physicians less, “it’s important to find influencers that people might trust to actually spread a message,” she said. Another factor is that as society has become more polarized, interaction with the leadership of groups that hold influence has become key. To promote vaccine acceptance, for example, leaders of moms’ groups on Facebook could be equipped with evidence-based information.
“It’s important for us to reach out and engage with those that are leaders in those groups, because they kind of hold the power,” Dr. Limaye said.
Framing the message is critical. Dr. Limaye has found that personal narratives can be persuasive and that to influence vaccine behavior, it is necessary to tailor the approach to the specific audience. Danish researchers, for example, in 2017 launched a campaign to increase uptake of HPV vaccinations among teenagers. The researchers provided facts about the safety and effectiveness of the vaccine, cited posts by clinicians about the importance of immunization against the virus, and relayed personal stories, such as one about a father who chose to vaccinate his daughter and another about a blogger’s encounter with a woman with cervical cancer. The researchers found that the highest engagement rates were achieved through personal content and that such content generated the highest proportion of positive comments.
According to Dr. Limaye, to change behavior, social media messaging must address the issues of risk perception and self-efficacy. For risk perception regarding vaccines, a successful message needs to address the parents’ questions about whether their child is at risk for catching a disease, such as measles or pertussis, and if they are, whether the child will wind up in the hospital.
Self-efficacy is the belief that one can accomplish a task. An effective message would provide information on where to find free or low-cost vaccines and would identify locations that are easy to reach and that have expanded hours for working parents, Dr. Limaye said.
What’s the best approach for boosting vaccination rates in the post-pandemic era? In the 1850s, Massachusetts enacted the first vaccine mandate in the United States to prevent smallpox, and by the 1900s, similar laws had been passed in almost half of states. But recent polls suggest that support for vaccine mandates is dwindling. In a poll by the Kaiser Family Foundation last fall, 71% of adults said that healthy children should be required to be vaccinated against measles before entering school, which was down from 82% in a similar poll in 2019.
So perhaps a better approach for promoting vaccine confidence in the 21st century would involve wider use of MI by clinicians and more focus by public health agencies taking advantage of the potential power of social media. As Dr. Offit put it, “I think trust is the key thing.”
Dr. Offit, Dr. Limaye, Dr. Shlay, and Dr. Hassan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When people ask Paul Offit, MD, what worries him the most about the COVID-19 pandemic, he names two concerns. “One is the lack of socialization and education that came from keeping kids out of school for so long,” Dr. Offit said in a recent interview. “And I think vaccines have suffered.”
Dr. Offit is director of the Vaccine Education Center and a professor of pediatrics at Children’s Hospital of Philadelphia. He has watched with alarm as the American public appears to be losing faith in the lifesaving vaccines the public health community has worked hard to promote. The Centers for Disease Control and Prevention estimates that the proportion of kids entering kindergarten who have received state-required vaccines dipped to 94% in the 2020-2021 school year – a full point less than the year before the pandemic – then dropped by another percentage point, to 93%, the following year.
Although a couple of percentage points may sound trivial, were only 93% of kindergarteners to receive the vaccine against measles, mumps, and rubella (MMR), approximately 250,000 vulnerable 5-year-olds could spark the next big outbreak, such as the recent measles outbreaks in Ohio and Minnesota.
Dr. Offit is one of many public health officials and clinicians who are working to reverse the concerning trends in pediatric vaccinations.
“I just don’t want to see an outbreak of something that we could have avoided because we were not protected enough,” Judith Shlay, MD, associate director of the Public Health Institute at Denver Health, said.
Official stumbles in part to blame
Disruptions in health care from the COVID-19 pandemic certainly played a role in the decline. Parents were afraid to expose their children to other sick kids, providers shifted to a telehealth model, and routine preventive care was difficult to access.
But Dr. Offit also blamed erosion of trust on mistakes made by government and public health institutions for the alarming trend. “I think that health care professionals have lost some level of trust in the Food and Drug Administration and CDC.”
He cited as an example poor messaging during a large outbreak in Massachusetts in summer 2021, when the CDC published a report that highlighted the high proportion of COVID-19 cases among vaccinated people. Health officials called those cases “breakthrough” infections, although most were mild or asymptomatic.
Dr. Offit said the CDC should have focused the message instead on the low rate (1%) of hospitalizations and the low number of deaths from the infections. Instead, they had to walk back their promise that vaccinated people didn’t need to wear masks. At other times, the Biden administration pressured public health officials by promising to make booster shots available to the American public when the FDA and CDC felt they lacked evidence to recommend the injections.
Rupali Limaye, PhD, an associate professor of international health at the Bloomberg School of Public Health at Johns Hopkins University, Baltimore, studies vaccine behavior and decision-making. She would go a step further in characterizing the roots of worsening vaccine hesitancy.
“In the last 20 years, we’ve seen there’s less and less trust in health care providers in general,” Dr. Limaye said. “More people are turning to their social networks or social contacts for that kind of information.” In the maelstrom of the COVID-19 pandemic, digital social networks facilitated the spread of misinformation about COVID-19 faster than scientists could unravel the mysteries of the disease.
“There’s always been this underlying hesitancy for some people about vaccines,” Dr. Shlay said. But she has noticed more resistance to the COVID-19 vaccine from parents nervous about the new mRNA technology. “There was a lot of politicization of the vaccine, even though the mRNA vaccine technology has been around for a long time,” she said.
Multipronged approaches
Dr. Shlay is committed to restoring childhood vaccination uptake to prepandemic levels now that clinics are open again. To do so, she is relying on a combination of quality improvement strategies and outreach to undervaccinated populations.
Denver Health, for instance, offers vaccinations at any inpatient or outpatient visit – not just well-child visits – with the help of alerts built into their electronic health records that notify clinicians if a patient is due for a vaccine.
COVID-19 revealed marked health inequities in underserved communities as Black, Hispanic, and people from other minority communities experienced higher rates of COVID-19 cases and deaths, compared with White people. The Public Health Institute, which is part of Denver Health, has responded with vaccine outreach teams that go to schools, shelters, churches, and community-based organizations to vaccinate children. They focus their efforts on areas where immunization rates are low. Health centers in schools throughout Colorado vaccinate students, and the Public Health Institute partners with Denver-area public schools to provide vaccines to students in schools that don’t have such centers. (They also provide dental care and behavioral health services.)
But it is unlikely that restoring clinic operations and making vaccines more accessible will fill the gap. After 3 years of fear and mistrust, parents are still nervous about routine shots. To help clinicians facilitate conversations about vaccination, Denver Health trains providers in communication techniques using motivational interviewing (MI), a collaborative goal-oriented approach that encourages changes in health behaviors.
Dr. Shlay, who stressed the value of persistence, advised, “Through motivational interviewing, discussing things, talking about it, you can actually address most of the concerns.”
Giving parents a boost in the right direction
That spirit drives the work of Boost Oregon, a parent-led nonprofit organization founded in 2015 that helps parents make science-based decisions for themselves and their families. Even before the pandemic, primary care providers needed better strategies for addressing parents who had concerns about vaccines and found themselves failing in the effort while trying to see 20 patients a day.
For families that have questions about vaccines, Boost Oregon holds community meetings in which parents meet with clinicians, share their concerns with other parents, and get answers to their questions in a nonjudgmental way. The 1- to 2-hour sessions enable deeper discussions of the issues than many clinicians can manage in a 20-minute patient visit.
Boost Oregon also trains providers in communication techniques using MI. Ryan Hassan, MD, a pediatrician in private practice who serves as the medical director for the organization, has made the approach an integral part of his day. A key realization for him about the use of MI is that if providers want to build trust with parents, they need to accept that their role is not simply to educate but also to listen.
“Even if it’s the wildest conspiracy theory I’ve ever heard, that is my opportunity to show them that I’m listening and to empathize,” Dr. Hassan said.
His next step, a central tenet of MI, is to make reflective statements that summarize the parent’s concerns, demonstrate empathy, and help him get to the heart of their concerns. He then tailors his message to their issues.
Dr. Hassan tells people who are learning the technique to acknowledge that patients have the autonomy to make their own decisions. Coercing them into a decision is unhelpful and potentially counterproductive. “You can’t change anyone else’s mind,” he said. “You have to help them change their own mind.”
Dr. Limaye reinforced that message. Overwhelmed by conflicting messages on the internet, people are just trying to find answers. She trains providers not to dismiss patients’ concerns, because dismissal erodes trust.
“When you’re dealing with misinformation and conspiracy, to me, one thing to keep in mind is that it’s the long game,” Dr. Limaye said, “You’re not going to be able to sway them in one conversation.”
Can the powers of social media be harnessed for pro-vaccine messaging? Dr. Limaye has studied social media strategies to promote vaccine acceptance and has identified several elements that can be useful for swaying opinions about vaccine.
One is the messenger – as people trust their physicians less, “it’s important to find influencers that people might trust to actually spread a message,” she said. Another factor is that as society has become more polarized, interaction with the leadership of groups that hold influence has become key. To promote vaccine acceptance, for example, leaders of moms’ groups on Facebook could be equipped with evidence-based information.
“It’s important for us to reach out and engage with those that are leaders in those groups, because they kind of hold the power,” Dr. Limaye said.
Framing the message is critical. Dr. Limaye has found that personal narratives can be persuasive and that to influence vaccine behavior, it is necessary to tailor the approach to the specific audience. Danish researchers, for example, in 2017 launched a campaign to increase uptake of HPV vaccinations among teenagers. The researchers provided facts about the safety and effectiveness of the vaccine, cited posts by clinicians about the importance of immunization against the virus, and relayed personal stories, such as one about a father who chose to vaccinate his daughter and another about a blogger’s encounter with a woman with cervical cancer. The researchers found that the highest engagement rates were achieved through personal content and that such content generated the highest proportion of positive comments.
According to Dr. Limaye, to change behavior, social media messaging must address the issues of risk perception and self-efficacy. For risk perception regarding vaccines, a successful message needs to address the parents’ questions about whether their child is at risk for catching a disease, such as measles or pertussis, and if they are, whether the child will wind up in the hospital.
Self-efficacy is the belief that one can accomplish a task. An effective message would provide information on where to find free or low-cost vaccines and would identify locations that are easy to reach and that have expanded hours for working parents, Dr. Limaye said.
What’s the best approach for boosting vaccination rates in the post-pandemic era? In the 1850s, Massachusetts enacted the first vaccine mandate in the United States to prevent smallpox, and by the 1900s, similar laws had been passed in almost half of states. But recent polls suggest that support for vaccine mandates is dwindling. In a poll by the Kaiser Family Foundation last fall, 71% of adults said that healthy children should be required to be vaccinated against measles before entering school, which was down from 82% in a similar poll in 2019.
So perhaps a better approach for promoting vaccine confidence in the 21st century would involve wider use of MI by clinicians and more focus by public health agencies taking advantage of the potential power of social media. As Dr. Offit put it, “I think trust is the key thing.”
Dr. Offit, Dr. Limaye, Dr. Shlay, and Dr. Hassan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When people ask Paul Offit, MD, what worries him the most about the COVID-19 pandemic, he names two concerns. “One is the lack of socialization and education that came from keeping kids out of school for so long,” Dr. Offit said in a recent interview. “And I think vaccines have suffered.”
Dr. Offit is director of the Vaccine Education Center and a professor of pediatrics at Children’s Hospital of Philadelphia. He has watched with alarm as the American public appears to be losing faith in the lifesaving vaccines the public health community has worked hard to promote. The Centers for Disease Control and Prevention estimates that the proportion of kids entering kindergarten who have received state-required vaccines dipped to 94% in the 2020-2021 school year – a full point less than the year before the pandemic – then dropped by another percentage point, to 93%, the following year.
Although a couple of percentage points may sound trivial, were only 93% of kindergarteners to receive the vaccine against measles, mumps, and rubella (MMR), approximately 250,000 vulnerable 5-year-olds could spark the next big outbreak, such as the recent measles outbreaks in Ohio and Minnesota.
Dr. Offit is one of many public health officials and clinicians who are working to reverse the concerning trends in pediatric vaccinations.
“I just don’t want to see an outbreak of something that we could have avoided because we were not protected enough,” Judith Shlay, MD, associate director of the Public Health Institute at Denver Health, said.
Official stumbles in part to blame
Disruptions in health care from the COVID-19 pandemic certainly played a role in the decline. Parents were afraid to expose their children to other sick kids, providers shifted to a telehealth model, and routine preventive care was difficult to access.
But Dr. Offit also blamed erosion of trust on mistakes made by government and public health institutions for the alarming trend. “I think that health care professionals have lost some level of trust in the Food and Drug Administration and CDC.”
He cited as an example poor messaging during a large outbreak in Massachusetts in summer 2021, when the CDC published a report that highlighted the high proportion of COVID-19 cases among vaccinated people. Health officials called those cases “breakthrough” infections, although most were mild or asymptomatic.
Dr. Offit said the CDC should have focused the message instead on the low rate (1%) of hospitalizations and the low number of deaths from the infections. Instead, they had to walk back their promise that vaccinated people didn’t need to wear masks. At other times, the Biden administration pressured public health officials by promising to make booster shots available to the American public when the FDA and CDC felt they lacked evidence to recommend the injections.
Rupali Limaye, PhD, an associate professor of international health at the Bloomberg School of Public Health at Johns Hopkins University, Baltimore, studies vaccine behavior and decision-making. She would go a step further in characterizing the roots of worsening vaccine hesitancy.
“In the last 20 years, we’ve seen there’s less and less trust in health care providers in general,” Dr. Limaye said. “More people are turning to their social networks or social contacts for that kind of information.” In the maelstrom of the COVID-19 pandemic, digital social networks facilitated the spread of misinformation about COVID-19 faster than scientists could unravel the mysteries of the disease.
“There’s always been this underlying hesitancy for some people about vaccines,” Dr. Shlay said. But she has noticed more resistance to the COVID-19 vaccine from parents nervous about the new mRNA technology. “There was a lot of politicization of the vaccine, even though the mRNA vaccine technology has been around for a long time,” she said.
Multipronged approaches
Dr. Shlay is committed to restoring childhood vaccination uptake to prepandemic levels now that clinics are open again. To do so, she is relying on a combination of quality improvement strategies and outreach to undervaccinated populations.
Denver Health, for instance, offers vaccinations at any inpatient or outpatient visit – not just well-child visits – with the help of alerts built into their electronic health records that notify clinicians if a patient is due for a vaccine.
COVID-19 revealed marked health inequities in underserved communities as Black, Hispanic, and people from other minority communities experienced higher rates of COVID-19 cases and deaths, compared with White people. The Public Health Institute, which is part of Denver Health, has responded with vaccine outreach teams that go to schools, shelters, churches, and community-based organizations to vaccinate children. They focus their efforts on areas where immunization rates are low. Health centers in schools throughout Colorado vaccinate students, and the Public Health Institute partners with Denver-area public schools to provide vaccines to students in schools that don’t have such centers. (They also provide dental care and behavioral health services.)
But it is unlikely that restoring clinic operations and making vaccines more accessible will fill the gap. After 3 years of fear and mistrust, parents are still nervous about routine shots. To help clinicians facilitate conversations about vaccination, Denver Health trains providers in communication techniques using motivational interviewing (MI), a collaborative goal-oriented approach that encourages changes in health behaviors.
Dr. Shlay, who stressed the value of persistence, advised, “Through motivational interviewing, discussing things, talking about it, you can actually address most of the concerns.”
Giving parents a boost in the right direction
That spirit drives the work of Boost Oregon, a parent-led nonprofit organization founded in 2015 that helps parents make science-based decisions for themselves and their families. Even before the pandemic, primary care providers needed better strategies for addressing parents who had concerns about vaccines and found themselves failing in the effort while trying to see 20 patients a day.
For families that have questions about vaccines, Boost Oregon holds community meetings in which parents meet with clinicians, share their concerns with other parents, and get answers to their questions in a nonjudgmental way. The 1- to 2-hour sessions enable deeper discussions of the issues than many clinicians can manage in a 20-minute patient visit.
Boost Oregon also trains providers in communication techniques using MI. Ryan Hassan, MD, a pediatrician in private practice who serves as the medical director for the organization, has made the approach an integral part of his day. A key realization for him about the use of MI is that if providers want to build trust with parents, they need to accept that their role is not simply to educate but also to listen.
“Even if it’s the wildest conspiracy theory I’ve ever heard, that is my opportunity to show them that I’m listening and to empathize,” Dr. Hassan said.
His next step, a central tenet of MI, is to make reflective statements that summarize the parent’s concerns, demonstrate empathy, and help him get to the heart of their concerns. He then tailors his message to their issues.
Dr. Hassan tells people who are learning the technique to acknowledge that patients have the autonomy to make their own decisions. Coercing them into a decision is unhelpful and potentially counterproductive. “You can’t change anyone else’s mind,” he said. “You have to help them change their own mind.”
Dr. Limaye reinforced that message. Overwhelmed by conflicting messages on the internet, people are just trying to find answers. She trains providers not to dismiss patients’ concerns, because dismissal erodes trust.
“When you’re dealing with misinformation and conspiracy, to me, one thing to keep in mind is that it’s the long game,” Dr. Limaye said, “You’re not going to be able to sway them in one conversation.”
Can the powers of social media be harnessed for pro-vaccine messaging? Dr. Limaye has studied social media strategies to promote vaccine acceptance and has identified several elements that can be useful for swaying opinions about vaccine.
One is the messenger – as people trust their physicians less, “it’s important to find influencers that people might trust to actually spread a message,” she said. Another factor is that as society has become more polarized, interaction with the leadership of groups that hold influence has become key. To promote vaccine acceptance, for example, leaders of moms’ groups on Facebook could be equipped with evidence-based information.
“It’s important for us to reach out and engage with those that are leaders in those groups, because they kind of hold the power,” Dr. Limaye said.
Framing the message is critical. Dr. Limaye has found that personal narratives can be persuasive and that to influence vaccine behavior, it is necessary to tailor the approach to the specific audience. Danish researchers, for example, in 2017 launched a campaign to increase uptake of HPV vaccinations among teenagers. The researchers provided facts about the safety and effectiveness of the vaccine, cited posts by clinicians about the importance of immunization against the virus, and relayed personal stories, such as one about a father who chose to vaccinate his daughter and another about a blogger’s encounter with a woman with cervical cancer. The researchers found that the highest engagement rates were achieved through personal content and that such content generated the highest proportion of positive comments.
According to Dr. Limaye, to change behavior, social media messaging must address the issues of risk perception and self-efficacy. For risk perception regarding vaccines, a successful message needs to address the parents’ questions about whether their child is at risk for catching a disease, such as measles or pertussis, and if they are, whether the child will wind up in the hospital.
Self-efficacy is the belief that one can accomplish a task. An effective message would provide information on where to find free or low-cost vaccines and would identify locations that are easy to reach and that have expanded hours for working parents, Dr. Limaye said.
What’s the best approach for boosting vaccination rates in the post-pandemic era? In the 1850s, Massachusetts enacted the first vaccine mandate in the United States to prevent smallpox, and by the 1900s, similar laws had been passed in almost half of states. But recent polls suggest that support for vaccine mandates is dwindling. In a poll by the Kaiser Family Foundation last fall, 71% of adults said that healthy children should be required to be vaccinated against measles before entering school, which was down from 82% in a similar poll in 2019.
So perhaps a better approach for promoting vaccine confidence in the 21st century would involve wider use of MI by clinicians and more focus by public health agencies taking advantage of the potential power of social media. As Dr. Offit put it, “I think trust is the key thing.”
Dr. Offit, Dr. Limaye, Dr. Shlay, and Dr. Hassan report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Study of hospitalizations in Canada quantifies benefit of COVID-19 vaccine to reduce death, ICU admissions
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
A cohort study of more than 1.5 million hospital admissions in Canada through the first 2 years of the COVID-19 pandemic has quantified the benefit of vaccinations. Unvaccinated patients were found to be up to 15 times more likely to die from COVID-19 than fully vaccinated patients.
Investigators analyzed 1.513 million admissions at 155 hospitals across Canada from March 15, 2020, to May 28, 2022. The study included 51,679 adult admissions and 4,035 pediatric admissions for COVID-19. Although the share of COVID-19 admissions increased in the fifth and sixth waves, from Dec. 26, 2021, to March 19, 2022 – after the full vaccine rollout – to 7.73% from 2.47% in the previous four waves, the proportion of adults admitted to the intensive care unit was significantly lower, at 8.7% versus 21.8% (odds ratio, 0.35; 95% confidence interval, 0.32-0.36).
“The good thing about waves five and six was we were able to show the COVID cases tended to be less severe, but on the other hand, because the disease in the community was so much higher, the demands on the health care system were much higher than the previous waves,” study author Charles Frenette, MD, director of infection prevention and control at McGill University, Montreal, and chair of the study’s adult subgroup, said in an interview. “But here we were able to show the benefit of vaccinations, particularly the boosting dose, in protecting against those severe outcomes.”
The study, published in JAMA Network Open, used the Canadian Nosocomial Infection Surveillance Program database, which collects hospital data across Canada. It was activated in March 2020 to collect details on all COVID-19 admissions, co-author Nisha Thampi, MD, chair of the study’s pediatric subgroup, told this news organization.
“We’re now over 3 years into the pandemic, and CNISP continues to monitor COVID-19 as well as other pathogens in near real time,” said Dr. Thampi, an associate professor and infectious disease specialist at Children’s Hospital of Eastern Ontario.
“That’s a particular strength of this surveillance program as well. We would see this data on a biweekly basis, and that allows for [us] to implement timely protection and action.”
Tracing trends over six waves
The study tracked COVID-19 hospitalizations during six waves. The first lasted from March 15 to August 31, 2020, and the second lasted from Sept. 1, 2020, to Feb. 28, 2021. The wild-type variant was dominant during both waves. The third wave lasted from March 1 to June 30, 2021, and was marked by the mixed Alpha, Beta, and Gamma variants. The fourth wave lasted from July 1 to Dec. 25, 2021, when the Alpha variant was dominant. The Omicron variant dominated during waves five (Dec. 26, 2021, to March 19, 2022) and six (March 20 to May 28, 2022).
Hospitalizations reached a peak of 14,461 in wave five. ICU admissions, however, peaked at 2,164 during wave four, and all-cause deaths peaked at 1,663 during wave two.
The investigators also analyzed how unvaccinated patients fared, compared with the fully vaccinated and the fully vaccinated-plus (that is, patients with one or more additional doses). During waves five and six, unvaccinated patients were 4.3 times more likely to end up in the ICU than fully vaccinated patients and were 12.2 times more likely than fully vaccinated-plus patients. Likewise, the rate for all-cause in-hospital death for unvaccinated patients was 3.9 times greater than that for fully vaccinated patients and 15.1 times greater than that for fully vaccinated-plus patients.
The effect of vaccines emerged in waves three and four, said Dr. Frenette. “We started to see really, really significant protection and benefit from the vaccine, not only in incidence of admission but also in the incidence of complications of ICU care, ventilation, and mortality.”
Results for pediatric patients were similar to those for adults, Dr. Thampi noted. During waves five and six, overall admissions peaked, but the share of ICU admissions decreased to 9.4% from 18.1%, which was the rate during the previous four waves (OR, 0.47).
“What’s important is how pediatric hospitalizations changed over the course of the various waves,” said Dr. Thampi.
“Where we saw the highest admissions during the early Omicron dominance, we actually had the lowest numbers of hospitalizations with death and admissions into ICUs.”
Doing more with the data
David Fisman, MD, MPH, a professor of epidemiology at the University of Toronto, said, “This is a study that shows us how tremendously dramatic the effects of the COVID-19 vaccine were in terms of saving lives during the pandemic.” Dr. Fisman was not involved in the study.
But CNISP, which receives funding from Public Health Agency of Canada, could do more with the data it collects to better protect the public from COVID-19 and other nosocomial infections, Dr. Fisman said.
“The first problematic thing about this paper is that Canadians are paying for a surveillance system that looks at risks of acquiring infections, including COVID-19 infections, in the hospital, but that data is not fed back to the people paying for its production,” he said.
“So, Canadians don’t have the ability to really understand in real time how much risk they’re experiencing via going to the hospital for some other reason.”
The study was independently supported. Dr. Frenette and Dr. Thampi report no relevant financial relationships. Dr. Fisman has disclosed financial relationships with Pfizer, AstraZeneca, Sanofi, Seqirus, Merck, the Ontario Nurses Association, and the Elementary Teachers’ Federation of Ontario.
A version of this article first appeared on Medscape.com.
New drugs in primary care: Lessons learned from COVID-19
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
SAN DIEGO – – plus it has helped keep many patients out of the hospital, according to a presenter at the annual meeting of the American College of Physicians.
Nirmatrelvir-ritonavir was granted emergency use authorization by the FDA late in 2021 to prevent progression to severe disease when COVID-19 cases and deaths were surging, and the Delta and Omicron variants started to spread.
Gerald Smetana, MD, an internist at Beth Israel Deaconess Medical Center in Boston, discussed nirmatrelvir-ritonavir as an example of how new drugs relevant to primary care can have a profound impact on public health.
Understanding the mechanism of action
Nirmatrelvir is the active agent of this combination and inhibits the SARS-CoV-2 main protease (Mpro), which is required for viral replication. In contrast to the SARS-CoV-2 spike protein, Mpro is highly conserved in coronaviruses and rarely acquires mutations. Therefore, unlike monoclonal antibodies targeting the spike protein, nirmatrelvir is active against known Omicron variants and is predicted to remain active against new variants that may emerge. The HIV1 protease inhibitor ritonavir has no activity against SARS-CoV-2. It can help increase the serum concentration of nirmatrelvir by inhibiting its metabolization.
“Although the details are not important for prescribing internists, having a basic understanding of the mechanism of action can help [doctors] better understand for which patients the drugs are indicated,” said Dr. Smetana, also a professor of medicine at Harvard Medical School, Boston. This is particularly important for newly approved drugs with a lot of new information to digest.
“Knowing the mechanisms of action of new drugs can help us predict their efficacy and potential side effects,” said Hubertus Kiefl, MD, an internist at Beth Israel Deaconess Medical Center and a lecturer at Harvard Medical School, during an interview after the session.
Understanding how drugs work also can help clinicians make better decisions, such as avoiding the use of a monoclonal antibody during a surge of a new variant with mutations in surface proteins or carefully managing the use of nirmatrelvir-ritonavir in patients who take certain medications that would cause potentially serious drug-drug interactions, Dr. Kiefl added.
Nirmatrelvir-ritonavir reduces the risk of hospitalization – but only in high-risk patients.
Dr. Smetana presented published data from the EPIC-HR study, a pivotal phase 2-3 clinical trial in 2,246 adult patients with COVID-19, all of whom were unvaccinated. Additionally, all patients had at least one risk factor for progression to severe disease.
When initiated 5 days after symptom onset or earlier, treatment with 300 mg nirmatrelvir plus 100 mg ritonavir twice a day for 5 days led to an 89% relative risk reduction in COVID-19–related hospitalization or death through day 28, compared with placebo.
Subgroup analyses showed that some patients benefited more than others. The highest risk reduction after treatment with nirmatrelvir-ritonavir was observed in patients at least 65 years old.
“It is important to remember that all the patients of this study were unvaccinated and [had] not had prior SARS-CoV-2 infection. This study population isn’t representative of most patients we are seeing today,” said Dr. Smetana.
Unpublished data from a study of standard-risk patients showed a nonsignificant reduction in the risk of hospitalization or death, he said. The study was stopped because of the low rates of hospitalization and death.
Effective in real world, but less so than in clinical trials
The fact that the patient cohort in the EPIC-HR trial was different from the patients internists see today makes real-world data critical for determining the usefulness of nirmatrelvir-ritonavir in everyday practice, Dr. Smetana said.
A real-world study from Israel conducted during the first Omicron wave (January to March 2022) showed that treatment with nirmatrelvir alone substantially reduced the relative risk of hospitalization in adults older than 65, with no evidence of benefit in adults aged 40-65. Dr. Smetana highlighted that, unlike the EPIC-HR cohort, most patients in the Israeli study had prior immunity due to vaccination or prior SARS-CoV-2 infection.
Many drug-drug interactions, but they can be managed
Nirmatrelvir-ritonavir interacts with many drugs, some of which are commonly used by primary care patients.
To help internists identify drug-drug interactions, Dr. Smetana proposed the use of the Liverpool COVID-19 Drug Interactions Checker, an intuitive tool that can help prescribers identify potential drug-drug interactions, categorize them based on severity, and identify management strategies.
This tool is specific to COVID-19 drugs. The Liverpool group also offers online drug interaction checkers for HIV, hepatitis, and cancer. “We need more tools like this to help improve the safe use of new drugs,” Dr. Smetana said.
To manage drug interactions, according to Dr. Smetana, U.S. treatment guidelines offer the following three options:
- Prescribe an alternative COVID therapy.
- Temporarily withhold concomitant medication if clinically appropriate.
- Adjust the dose of concomitant medication and monitor for adverse effects.
Medication doses that are withheld or modified should be continued through 3 days after completing nirmatrelvir-ritonavir, he added.
Important considerations
Commenting on things to consider for patients with COVID-19, Dr. Smetana said that there is a short window after symptom onset when nirmatrelvir-ritonavir can be prescribed, and safety in pregnancy is not known. There is also uncertainty regarding funding of nirmatrelvir-ritonavir prescriptions after the state of emergency is lifted. He reminded attendees that, although nirmatrelvir-ritonavir is the preferred first-line treatment for high-risk patients, another antiviral agent, molnupiravir, is also available and might be more appropriate for some patients.
He also cautioned about prescribing new drugs off label for indications that are not yet FDA-approved. “We are often stewards of limited resources when new drugs first become available but are not yet in sufficient supply to meet demand. Limiting our prescribing to FDA-approved indications helps to ensure equitable access,” he said.
Dr. Smetana and Dr. Kiefl reported no disclosures.
AT INTERNAL MEDICINE 2023
FDA approves first RSV vaccine for older adults
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older, the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older, the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.
Arexvy, manufactured by GSK, is the world’s first RSV vaccine for adults aged 60 years and older, the company said in an announcement.
Every year, RSV is responsible for 60,000–120,000 hospitalizations and 6,000–10,000 deaths among U.S. adults older than age, according to the FDA. Older adults with underlying health conditions — such as diabetes, a weakened immune system, or lung or heart disease — are at high risk for severe disease. "Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States," said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in a statement.
The FDA approval of Arexvy was based on a clinical study of approximately 25,000 patients. Half of these patients received Arexvy, while the other half received a placebo. Researchers found that the RSV vaccine reduced RSV-associated lower respiratory tract disease (LRTD) by nearly 83% and reduced the risk of developing severe RSV-associated LRTD by 94%. The most commonly reported side effects were injection site pain, fatigue, muscle pain, headache, and joint stiffness/pain. Ten patients who received Arexvy and four patients who received placebo experienced atrial fibrillation within 30 days of vaccination. The company is planning to assess risk for atrial fibrillation in postmarking studies, the FDA said. The European Medicine Agency’s Committee for Medicinal Products for Human Use recommended approval of Arexvy on April 25, 2023, on the basis of data from the same clinical trial.
GSK said that the U.S. launch of Arexvy will occur sometime in the fall before the 2023/2024 RSV season, but the company did not provide exact dates. "Today marks a turning point in our effort to reduce the significant burden of RSV," said GSK’s chief scientific officer, Tony Wood, PhD, in a company statement. "Our focus now is to ensure eligible older adults in the U.S. can access the vaccine as quickly as possible and to progress regulatory review in other countries."
A version of this article first appeared on Medscape.com.