User login
ACOG lends support to bill promoting maternal mortality review committees
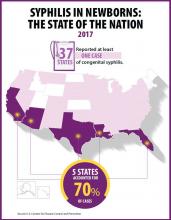
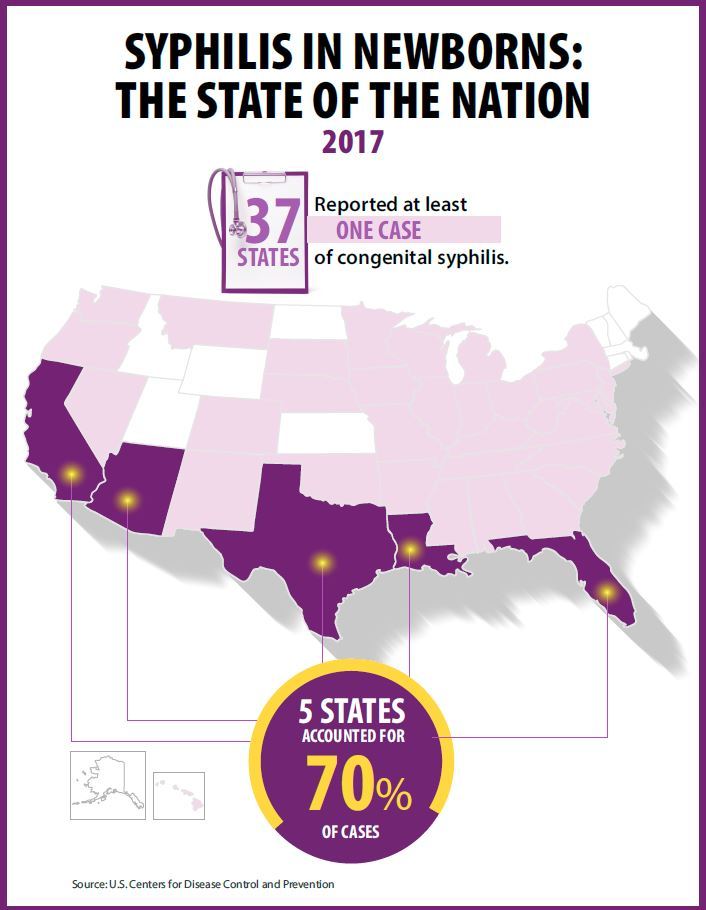
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
The Preventing Maternal Deaths Act of 2018 (H.R. 1318) was the subject of a Sept. 27 hearing of the House Energy and Commerce Health Subcommittee. The bill comes at a time when 700 women a year die as a result of pregnancy or pregnancy-related complications with a rate that is increasing, while 157 of 183 countries around the world are reporting decreasing rates of maternal mortality, according to ACOG.
The bill, authored by Rep. Jamie Herrera Beutler (R-Wash.) and Diana DeGette (D-Colo.) would allocate $58 million for each fiscal year from 2019 through 2023 to support the 33 existing states with maternal mortality review committees (MMRCs) and help the remaining 17 states develop them, as well as to standardize data collection across the nation.
The goal of having these committees in place is to “improve data collection and reporting around maternal mortality, and to develop or support surveillance systems at the local, state, and national level in order to better understand the burden of maternal complications,” a background memo on the hearing noted. “These surveillance efforts include identifying groups of women with disproportionately high rates of maternal mortality and identifying the determinants of disparities in maternal care, health risks, and health outcomes.”
Necessitating this legislation was a data point that was reiterated throughout the course of the hearing – that maternal mortality rates in the United States were on the rise.
“What’s both surprising and devastating is that, despite massive innovation and advances in health care and technology, we’ve experienced recent reports that have indicated that the number of women dying due to pregnancy complications is actually increasing,” Full Committee Chairman Greg Walden (R-Ore.) said in his opening remarks at the hearing. “According to the Centers for Disease Control and Prevention, maternal mortality rates in America have more than doubled since 1987. I think we are asking, how can that be? This is not a statistic any of us wants to hear.”
Chairman Walden acknowledged that there are questions as to whether the increase was a function of better data collection or whether it was an issue with the delivery of health care.
“The bill before us today will help us answer these really important questions and hopefully ensure that expectant newborn mothers receive even better care,” he said.
Lynne M. Coslett-Charlton, MD, ACOG Pennsylvania District legislative chair, offered the organization’s support for the bill.
MMRCs “are multidisciplinary groups of local experts in maternal and public health, as well as patient and community advocates, that closely examine maternal death cases and identify locally relevant ways to prevent future deaths,” she testified before the committee. “While traditional public health surveillance using vital statistics can tell us about trends and disparities, MMRCs are best positioned to comprehensively assess maternal deaths and identify opportunities for prevention.”
Dr. Coslett-Charlton added that to clearly understand why women are dying from preventable maternal complications, which she noted that 60% of maternal deaths are, “every state must have a robust MMRC. The Preventing Maternal Deaths Act will help us reach that goal, and ultimately improve maternal health across this nation,” as these committees review every maternal death and can make a determination as to whether they could have been preventable.
Additionally, the fact that black women face a significantly higher rate of maternal mortality was another data point highlighted during the hearing, further adding to the need for this bill that has bipartisan support and more than 170 cosponsors.
Rep. DeGette called it “one of the most striking aspects” that black women “are nearly four times as likely to experience a pregnancy-related death.”
Stacey D. Stewart, president of the March of Dimes, in her written testimony praised the inclusion in H.R. 1318 of a “demonstration project to determine how best to address disparities in maternal health outcomes.”
REPORTING FROM A HOUSE ENERGY AND COMMERCE HEALTH SUBCOMMITTEE HEARING
Breast cancer risk in type 2 diabetes related to adiposity
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
ORLANDO – findings from meta-analyses suggest.
In one meta-analysis of data from 21 prospective studies with a total of nearly 15.2 million women, 325,117 breast cancer cases, and a mean follow-up time of 8 years (nearly 33 million person-years), the risk of breast cancer was significantly greater among patients with diabetes than it was among patients without diabetes (summary relative risk, 1.11), Maria Bota reported at the annual scientific sessions of the American Diabetes Association.
However, there was substantial unexplained heterogeneity of results across the individual studies (I2 = 82%), said Ms. Bota, a faculty member at the International Prevention Research Institute, Lyon, France.
“When the analysis was restricted to the 12 studies that adjusted for [body mass index], the summary relative risk decreased to 1.09 and the heterogeneity also decreased to a moderate value of 32%; when the analysis was restricted to the 9 studies that did not adjust for BMI, the summary relative risk increased to 1.14 again, and the heterogeneity increased even more to 91%,” she said.
In an analysis that combined the results of the nine studies that did not adjust for BMI along with crude relative risks from studies that reported both crude and BMI-adjusted relative risks (17 studies in all), the SRR was 1.12, and heterogeneity among the studies was high at 84%.
Additionally, an analysis by menopausal status based on four studies that reported breast cancer in both pre- and postmenopausal women showed SRRs for breast cancer of 0.97 (a 3% decrease in risk) and 1.14 among diabetic vs. nondiabetic premenopausal women and postmenopausal women, respectively, she said, noting that heterogeneity was low (I2 = 0%) among the premenopausal breast cancer study groups and high (I2 = 70%) among the postmenopausal study groups.
The findings provide evidence for a moderately increased risk of breast cancer in women with T2DM, Ms. Bota said.
“However, the effect of the adjustment or lack of adjustment on the heterogeneity suggests that the higher risk of breast cancer in women with diabetes may not be due to diabetes itself, but to adiposity,” she said, adding that “this hypothesis is equally supported by our subgroup analysis according to menopausal status because we saw that the risk of breast cancer was only associated with diabetes in postmenopausal women and this pattern resembles the pattern of the risk of breast cancer associated with adiposity, which is also only increased in postmenopausal women.”
This study was limited by insufficient data for investigating the sources of heterogeneity, she said.
“Therefore we propose ... future pooled analyses based on individual data from good quality prospective studies in order to increase the study power and to do some detailed analysis of the links between adiposity, diabetes, and breast cancer,” she concluded, adding that new studies to examine those relationships only in premenopausal women are also needed
In a separate meta-analysis, she and her colleagues, including Peter Boyle, PhD, president of the International Prevention Research Institute, assessed the association between insulin treatment and breast cancer risk in patients with diabetes.
“The long-acting insulin analogues glargine and detemir have been shown in some studies to be associated with increased risk of breast cancer, and other studies have shown no association between the use of these two compounds and the risk of breast cancer,” Dr. Boyle said in a separate presentation at the ADA meeting. “It was important to sort out a little bit what was going on in the literature.”
Overall, the meta-analysis of data from 12 longitudinal cohort studies – including more than 6,000 cases of breast cancer – showed a slight increase in breast cancer risk in patients taking long-acting insulin (SRR, 1.13) with “a relatively reasonable” level of heterogeneity (I2 = 23%).
“But we see that the story is not that simple,” he said.
For example, some studies included only patients who were prescribed insulin for the first time after the study began (new users), some included only patients who were prescribed insulin before the study began (prevalent users), and some included both (ever users), which may have introduced bias in the results, he explained.
Studies of glargine included 4,168 breast cancer cases over a total of 1,418,743 person-years of observation, and studies of detemir included fewer than 2,047 breast cancer cases (not all studies reported case numbers). Among both new users of glargine and detemir, the SRR was 1.12, suggesting no real association between either glargine or detemir use and breast cancer, he said.
“One important take-home message is that you have to be careful that these pharmaco-epidemiological studies, even when working with the same database, may have conflicting results ... so we still need more robust standards in methods for [such] studies,” he concluded.
Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
SOURCE: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
REPORTING FROM ADA 2018
Key clinical point: Adiposity accounts for the increased risk of breast cancer among women with diabetes.
Major finding: An analysis of 12 studies that adjusted for BMI showed a summary relative risk for breast cancer of 1.09 in diabetic versus nondiabetic women, with moderate study heterogeneity.
Study details: Meta-analyses including 21 and 12 studies, respectively.
Disclosures: Ms. Bota reported having no disclosures. The study presented by Dr. Boyle was funded by Sanofi. Dr. Boyle is president of a charity that has received donations from Pfizer, Roche, Novartis, and Lilly.
Source: Bota M. ADA 2018, Abstract 180-OR; Boyle P. ADA 2018, Abstract 133-OR.
Zoledronate reduces fracture risk in elderly women with osteopenia
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
This trial reminds us that risk assessment and treatment decisions go well beyond bone mineral density and should focus particularly on age and a history of fractures.
Osteoporosis is defined as a T score below –2.5, but several longitudinal studies have shown that most fractures among postmenopausal women occur in those with osteopenia. Further, alendronate therapy did not reduce the risk of fractures among women with osteopenia which contributed to a treatment gap for women with osteopenic T scores but strong risk factors for an osteoporotic fracture.
In the current study, zoledronate was associated with a greater increase in bone mass and a lower fracture risk compared with placebo. Plus, zoledronate prevented fractures among women with an average T score of –1.27 at the total hip and –1.64 at the femoral neck. The positive data, coupled with the low number of adverse events over the 6-year study period, support the addition of zoledronate to the treatment options for osteoporosis. However, the average age of the patients in the current study was 3.5 years older than that of patients in previous alendronate studies. As a result, the findings should not be extrapolated to postmenopausal women under the age of 65 years with osteopenia.
Clifford J. Rosen, MD, is affiliated with the Maine Medical Center Research Institute, Scarborough, and serves as an associate editor at the New England Journal of Medicine. He made his remarks in an accompanying editorial (N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMe1812434). Dr. Rosen had no relevant financial conflicts to disclose.
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
Intravenous zoledronate therapy given once every 18 months, with minimal use of calcium supplements, was associated with an increase in bone mass and significantly reduced the risk of vertebral and nonvertebral fractures in postmenopausal women, compared with a placebo, based on data from a 6-year trial of 2,000 ambulatory women aged 65 and older with osteopenia.
The findings were presented at the annual meeting of the American Society for Bone and Mineral Research and published simultaneously in the New England Journal of Medicine.
Bisphosphonates have been shown to prevent fractures in osteoporosis patients, but their effectiveness has not been well studied in patients with osteopenia alone, noted Ian R. Reid, MD, of the University of Auckland, New Zealand, and his colleagues. “Many patients at high risk for fracture do not have T scores of less than –2.5 but rather have osteopenia in combination with other risk factors such as age.”
The researchers randomized 2,000 women aged 65 years and older with osteopenia to receive four infusions of zoledronate or a saline placebo every 18 months. A dietary intake of 1 g of calcium per day was advised, but calcium supplements were not provided; 2% of the women took supplements. Those not taking vitamin D before the trial were given a single 2.5-mg dose of cholecalciferol and a monthly 1.25-mg dose during the trial. Trial participants were followed for 6 years.
Demographic characteristics were similar between the groups, and their T scores ranged from –1.0 to –2.5 at the total hip or femoral neck. The primary endpoint was the time to a first fragility fracture, defined as nonvertebral fractures and vertebral fractures confirmed by radiography.
Overall, 122 women in the zoledronate group experienced 131 fractures, and 190 women in the placebo group experienced 227 fractures (hazard ratio 0.63, P less than .001). Differences in bone mineral density between the two groups were observed by 3 years.
The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
The findings were consistent with data on reduced fracture risk in osteoporosis patients treated with zoledronate. The study differed from other similar trials in its use of 18-month dosing intervals and low use of calcium supplements (2%), they noted.
The data were limited by the older age of the study individuals, so the results should not be extrapolated to younger women or individuals with normal bone mineral density, the researchers said. The results suggest that annual zoledronate dosing may be unnecessary, but further research is needed to explore longer dose intervals.
Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
SOURCE: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
REPORTING FROM ASBMR
Key clinical point: Vertebral and nonvertebral fracture risk was significantly lower in osteopenic women who received zoledronate, compared with those who received a placebo.
Major finding: Fragility fractures occurred in 122 women in a zoledronate group and 190 women in a placebo group. The number needed to treat to prevent a single fragility fracture was 10; the number needed to treat to prevent a symptomatic fracture was 20.
Study details: A 6-year randomized, double-blind trial of 2,000 women aged 65 years and older with osteopenia.
Disclosures: The study was supported in part by grants from the Health Research Council of New Zealand; Novartis provided the medication. Dr. Reid disclosed grants from Health Research Council of New Zealand, nonfinancial support from Novartis during the study, and financial relationships with Amgen, Merck, Novartis, and Eli Lilly unrelated to the study.
Source: Reid I et al. N Engl J Med. 2018 Oct 1. doi: 10.1056/NEJMoa1808082.
No significant VTE risk for women taking noncyclic COCs
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
Women who use combined oral contraceptives (COC) without hormone-free or low-dose hormone intervals have a slightly elevated, but not statistically significant, risk of venous thromboembolism (VTE), compared with women who use cyclic COCs, according to research published in JAMA Internal Medicine.
Jie Li, PhD, from the Center for Drug Evaluation and Research, and colleagues performed a retrospective cohort study of 733,007 women aged 18-50 years in the Sentinel Distributed Database from 2007 to 2015 who received low-dose extended and continuous cycle (210,691 women; mean age, 30 years) COCs or cyclic COCs (522,316 women; mean age, 29 years). Continuous cycle COCs were defined as an 84/7 cycle or a 365/0 cycle, while cyclic COCs were 21/7 cycles.
The researchers noted some baseline differences between the two groups, with gynecologic conditions occurring in 40% of the noncyclic group, compared with 32% in the cyclic group; cardiovascular and metabolic conditions occurring in 7% of noncyclic women, compared with 5% of cyclic women; inflammatory disease occurring in 3% of noncyclic women, compared with 2% of cyclic women; and a slightly higher rate of health care services use in the noncyclic group, compared with the cyclic group.
Dr. Li and associates found 228 cases of VTE in the noncyclic group and 297 cases in the cyclic group, with an incidence rate of 1.54 (95% confidence interval, 1.34-1.74) per 1,000 person-years for noncyclic users and 0.83 (95% CI, 0.74-0.93) per 1,000 person-years for cyclic users (crude hazard ratio, 1.84; 95% CI, 1.53-2.21).
However, propensity score matching lowered the incidence rate to 1.44 (95% CI, 1.24-1.64) per 1,000 person-years for the noncyclic group and raised it to 1.09 (95% CI, 0.92-1.27) per 1,000 person-years for the cyclic group, for an adjusted hazard ratio of 1.32 (95% CI, 1.07-1.64), which does not show “strong evidence” of VTE risk based on a small absolute risk difference of 0.27 cases per 1,000 persons, the researchers said. They added that there might be residual or unmeasured confounding, perhaps for potential concurrent medication use or incompletely measured covariates.
“Accordingly, we do not recommend selective prescribing of COCs based on the cyclic and continuous/extended type,” Dr. Li and colleagues wrote. “Clinicians should prescribe COCs based on patients’ individual risk factors and preferences.”
The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
SOURCE: Li J et al. JAMA Intern Med. 2018 Oct 1. doi: 10.1001/jamainternmed.2018.4251.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Continuous or extended cycle combined oral contraceptive (COC) use was associated with a slightly elevated, but not statistically significant, risk of venous thromboembolism.
Major finding: The adjusted hazard ratio for women taking continuous/extended COCs was 1.32 (95% confidence interval, 1.07-1.74), compared with women taking noncyclic COCs, but the absolute risk difference between the two groups was low (0.27 per 1,000 persons).
Study details: A retrospective cohort study of 210,691 women with continuous/extended COC use and 522,316 women with cyclic COC use.
Disclosures: The Sentinel Initiative is funded by a contract from the Department of Health and Human Services. The authors reported no relevant conflicts of interest.
Source: Li J et al. JAMA Intern Med. 2018 Oct 1. doi:10.1001/jamainternmed.2018.4251.
Genitourinary syndrome of menopause in breast cancer survivors: Treatments are available
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
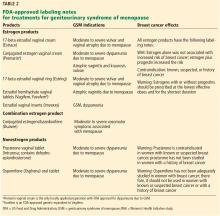
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
Many breast cancer survivors and women at high risk of breast cancer suffer from genitourinary syndrome of menopause (GSM), a term that encompasses any urinary, genital, or sexual dysfunction related to a hypoestrogenic state. Although GSM is usually caused by postmenopausal estrogen loss, it can also be caused by cancer treatments such as chemotherapy, radiation, and systemic endocrine therapy (eg, tamoxifen, aromatase inhibitors). These treatments can substantially decrease systemic estrogen levels, causing GSM symptoms that can profoundly worsen quality of life.
Managing GSM in these women poses a dilemma because systemic estrogen-containing therapies can increase the risk of breast cancer, and nonhormonal vaginal lubricants and moisturizers provide only minimal benefit. Fortunately, there are hormonal options, including locally applied estrogen, intravaginal dehydroepiandrosterone (DHEA), and estrogen receptor agonists/antagonists (ospemifene and bazedoxifene).
Here, we review the clinical management of GSM in breast cancer survivors and women at high risk of breast cancer and the efficacy and safety of available treatments, including their impact on breast cancer risk.
DRYNESS, IRRITATION, ATROPHY
The term GSM describes vulvovaginal and genitourinary symptoms associated with estrogen loss after menopause. Common symptoms are vaginal dryness, dyspareunia, irritation of genital skin, and pruritus.
LOCAL ESTROGEN THERAPY
Systemic estrogen therapy is widely used and effective for GSM, but there are concerns that it could increase the risk of breast cancer. After the Women’s Health Initiative in 2002 showed higher rates of cardiovascular disease and breast cancer with systemic estrogen-progestin use,5 the use of this hormone therapy declined by approximately 80%.6 Since then, healthcare providers have turned to local (ie, vaginal) estrogen therapies to manage GSM. These therapies have several advantages over systemic hormone therapy:
- Lower risk of adverse effects on the breast and cardiovascular system
- Greater efficacy in treating GSM
- In general, no need for progesterone when low-dose local estrogen is given to a woman with a uterus.7
Is locally applied estrogen systemically absorbed?
Despite these advantages, concerns remain as to whether vaginal estrogen therapy has adverse consequences associated with systemic absorption, particularly from atrophic vaginal tissues.
Santen,8 in a 2015 review of 33 studies, concluded that systemic absorption from low-dose vaginal estrogen is minimal, which provides some rationale for using it to treat vulvovaginal atrophy in postmenopausal women. This finding also suggests that the US Food and Drug Administration (FDA) “black box” warning of possible toxicities with vaginal estrogen is likely overstated, given that serum estrogen levels remained within normal postmenopausal levels.
Nevertheless, many providers are apprehensive about prescribing vaginal estrogen in women with a history of breast cancer because the threshold for systemic estrogen levels associated with breast cancer recurrence has not been established.
ACOG statement. In 2016, a committee of the American College of Obstetricians and Gynecologists cited data showing that low-dose vaginal estrogens do not result in sustained serum estrogen levels exceeding the normal postmenopausal range, and that the use of vaginal estrogens does not increase the risk of cancer recurrence.9 However, they recommend caution with vaginal estrogen use, especially in women with a history of estrogen-dependent breast cancer, reserving it for patients with GSM symptoms nonresponsive to nonhormonal treatment and specifying that it be used in low doses.
Vaginal estrogen formulations
Several types of locally applied estrogens are available, each with different properties and affinity for estrogen receptors. These include conjugated estrogens, 17-beta estradiol, estradiol acetate, and estradiol hemihydrate. Three delivery systems are FDA-approved: creams, rings, and tablets (Table 2).
Vaginal creams. Two vaginal creams are available, one (Estrace) containing 17-beta estradiol and the other (Premarin) containing conjugated estrogens.
The FDA-approved dosage for 17-beta estradiol is 2 to 4 g/day for 1 or 2 weeks, then gradually reduced to half the dose for a similar time. Maintenance dosing is 1 g 1 to 3 times per week. However, the ACOG statement notes that the FDA-approved dosages are higher than those proven to be effective and currently used in clinical practice, eg, 0.5 g twice a week.9
The FDA-approved dosage of conjugated estrogen cream for moderate to severe dyspareunia is 0.5 g daily for 21 days, then off for 7 days, or 0.5 g twice a week.
Vaginal tablets. The vaginal tablet Vagifem and its generic equivalent Yuvafem contain 10 µg of estradiol hemihydrate. The FDA-approved dosage is 10 µg daily for 2 weeks, followed by 10 µg twice a week, inserted into the lower third of the vagina. This dosage is significantly lower than that of estrogen creams.
Vaginal insert. A newly approved vaginal insert (Imvexxy) contains estradiol 4 µg (the lowest dose of vaginal estradiol available) or 10 µg, in a coconut oil vehicle. Its indications are for moderate to severe dyspareunia due to menopause and atrophic vaginitis due to menopause. A study cited in its package insert (www.accessdata.fda.gov/drugsatfda_docs/label/2018/208564s000lbl.pdf) showed that, in patients who used this product, systemic absorption of estradiol remained within the postmenopausal range. Its effects on breast cancer have not yet been studied.
Vaginal rings. Two vaginal rings are marketed. One (Estring) contains 17-beta estradiol, and the other (Femring) contains estradiol acetate. Only the 17-beta estradiol ring delivers a low dose to vaginal tissues, releasing 7.5 µg/day for 90 days. The estradiol acetate ring releases 0.05 mg/day or 0.10 mg/day and is a systemic treatment meant to be used with a progestin, not for local therapy.
VAGINAL ANDROGEN THERAPY: DHEA
After menopause, as the ovaries stop making estrogen from androstenedione, some production continues in other tissues, with DHEA as the primary precursor of androgens that are ultimately converted to estrogen. This has led to the theory that the cause of GSM is not estrogen deficiency but androgen deficiency. Evidence reviewed by Labrie et al11 shows that vulvovaginal atrophy is caused by decreased DHEA availability, which in turn causes sex steroid deficiency-related menopausal symptoms.11 Thus, it is reasonable to conclude that menopausal symptoms can be relieved by giving DHEA.
This theory has been borne out in clinical trials, in which DHEA in a vaginal tablet formulation increased the maturation of vaginal cells and lowered vaginal pH, leading to relief of GSM symptoms.12
The only DHEA product FDA-approved for treating GSM-related symptoms is prasterone (Intrarosa), indicated for moderate to severe dyspareunia due to vulvovaginal atrophy. The recommended dosing is a single 6.5-mg intravaginal tablet (0.5% prasterone) inserted nightly at bedtime. Its efficacy for treating hypoactive sexual desire disorder in postmenopausal women is being investigated.
Breast cancer implications
Because DHEA is converted to estrogen by aromatization, healthcare providers might hesitate to use it in women who have a history of hormone-sensitive cancer. Data on the safety of intravaginal DHEA use in breast cancer survivors are limited. However, studies have found that prasterone has highly beneficial effects on dyspareunia, vaginal dryness, and objective signs of vulvovaginal atrophy without significant drug-related adverse effects.12,13 In these studies, serum estrogen levels in women treated with DHEA were within the values observed in normal postmenopausal women. In addition, there are no aromatase enzymes in the endometrium, so even high doses of vaginal DHEA (in contrast to high doses of vaginal estrogen) will not stimulate the endometrium.
Clinically, this evidence indicates that DHEA exerts both estrogenic and androgenic activity in the vagina without increasing serum estrogen levels, making it a good alternative to topical estrogen therapy.
OSPEMIFENE: AN ESTROGEN RECEPTOR AGONIST/ANTAGONIST
Ospemifene (Osphena) is an estrogen receptor agonist/antagonist, a class of drugs previously called selective estrogen receptor modulators (SERMs). It is FDA-approved to treat moderate to severe dyspareunia secondary to vulvar and vaginal atrophy.
Ospemifene has unique estrogenic effects on the vaginal mucosa, having been shown to increase the number of epithelial cells, lower the vaginal pH, and decrease the percentage of parabasal cells seen on Papanicolaou smears after 12 weeks of use.14
Unlike tamoxifen, another drug of this class, ospemifene does not change the endometrial lining.14 Similarly, ospemifene acts as an estrogenic agonist in bone and, thus, has the potential for use in preventing and managing osteoporosis or for use in women at risk of fractures.
Breast cancer impact
In preclinical trials, ospemifene was found to have antiestrogenic effects on breast tissue, similar to those seen with tamoxifen.
In a model using human tumor grafts, ospemifene decreased tumor growth in mice implanted with estrogen receptor-positive breast cancer cells.15
In a mouse model using breast cancer cells that were biologically and histologically similar to those of humans, ospemifene had strong antiestrogenic effects on the breast tissue.16 The evidence suggests that ospemifene has a favorable effect on vulvar and vaginal atrophy.17
Ospemifene is FDA-approved to treat moderate to severe dyspareunia secondary to menopause. Recommended dosing is 60 mg/day orally with food.
Its antiestrogenic effects on breast tissue make it a promising option for women with a history of estrogen-receptor positive breast cancer. However, further study is needed to fully understand its effects on human breast tissue. According to the manufacturer’s package insert (www.osphena.com/files/pdf/osphena_prescribing_information.pdf), because the drug has not been adequately studied in women with breast cancer, it should not be used in women with known or suspected breast cancer or a history of breast cancer.
CONJUGATED ESTROGENS PLUS BAZEDOXIFENE
The combination of conjugated estrogens and bazedoxifene (Duavee) is a progesterone-free alternative for treating various menopausal symptoms. Bazedoxifene is another estrogen receptor agonist/antagonist, and it was added to counteract estrogen’s effects on the endometrium, thus replacing progesterone. This protective effect has been validated in clinical trials, which also found a favorable safety profile in breast tissue.18,19
SMART trials. The efficacy of this combination was studied in a series of large phase 3 multicenter trials called the SMART (Selective Estrogens, Menopause, and Response to Therapy) trials.20–23 Treated patients had markedly fewer vasomotor symptoms at 1 year, along with an increase in superficial cells and intermediate cells of the vaginal epithelium and a decrease in parabasal cells. They also had a substantial decrease in the incidence of dyspareunia.
Its effects on breast tissue were evaluated in the SMART-5 trial. Therapy had no net impact on breast density, suggesting that it has an estrogen-neutral effect on the breast.23
These results suggest that combined conjugated estrogens and bazedoxifene could be a noteworthy treatment option for GSM in women with a history of estrogen receptor-positive breast cancer, particularly in those with vasomotor symptoms and bone loss. However, the combination has not been studied specifically in breast cancer survivors.
Dosage. The FDA-approved dosing is 20 mg/0.45 mg per day orally to treat vasomotor symptoms, GSM, and osteoporosis in postmenopausal women with a uterus.
LASER THERAPY AND RADIOFREQUENCY HEAT: AN OFF-LABEL OPTION
Low-dose radiofrequency thermal therapy, delivered by carbon dioxide laser or by radiofrequency heat, has been used with some success to treat urinary stress incontinence and vaginal laxity in postpartum women. It may be an option for GSM, although it is not FDA-approved for this indication, and the FDA has recently issued a warning about it.24
Marketing literature promotes laser therapy as an effective option that stimulates vaginal connective tissue to produce new collagen, which then promotes improved blood flow and tissue regeneration for vaginal lubrication and elasticity.
A study comparing fractional carbon dioxide vaginal laser treatment and local estrogen therapy in postmenopausal women with vulvovaginal atrophy found that laser therapy was an effective treatment for vulvovaginal atrophy (dyspareunia, dryness, and burning), both alone and with local estrogen.25
Despite the promising effects of laser therapy for treating vulvovaginal atrophy in GSM, studies have not determined its short-term or long-term safety profile. Furthermore, laser therapy does not improve impaired sexual function, ie, decreased libido, arousal, and sexual satisfaction. Another important consideration is that the cost of laser therapy in 2017 was estimated to be $2,000 to $3,000 per treatment, not covered by healthcare insurance.
CLINICAL APPROACH
Symptoms of GSM are common in breast cancer survivors, both pre- and postmenopausal, especially those treated with tamoxifen or an aromatase inhibitor. Estimates are that 60% of postmenopausal breast cancer survivors and 40% of premenopausal breast cancer survivors suffer from GSM.26 Unfortunately, many women do not seek medical attention for their symptoms.
A variety of hormonal and nonhormonal options are available for these patients. We recommend an interdisciplinary approach to treatment, with the decision to use hormonal options made in collaboration with the patient’s oncologist and the patient herself, in an informed, shared decision-making process that takes into consideration the risks and possible benefits depending on the symptoms.
The first step in selecting a management plan for GSM symptoms in women with breast cancer is to conduct a thorough assessment to provide data for individualizing the care plan. The decision to use a hormonal option should be made in collaboration with a woman’s oncologist and should include an informed decision-making process during which the potential risks and benefits, including the breast cancer impact, are fully disclosed.
Alternatives to systemic estrogen
Vaginal estrogen is an effective and safe option to treat GSM in women with either estrogen receptor-negative or estrogen receptor-positive breast cancer. It often completely cures the symptoms without any noticeable increase in serum estrogen levels.
Vaginal DHEA therapy is a nonestrogen option shown to effectively treat GSM without increasing systemic levels of estrogen or testosterone. This profile makes vaginal DHEA therapy a particularly attractive treatment for symptoms of GSM in women at risk for breast cancer.
Use of an estrogen receptor agonist/antagonist in breast cancer survivors needs careful consideration. Ospemifene has antiestrogenic effects that make it a good option for women with bone loss and those at high risk for breast cancer, but it should not be used concurrently with tamoxifen or raloxifene. Additionally, ospemifene does not cause uterine hyperplasia, so it can be used in women with a uterus.
Although more study is needed, we do have options to improve the overall quality of life in breast cancer survivors with GSM.
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
- Lester J, Pahouja G, Andersen B, Lustberg M. Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 2015; 5(2):50–66. doi:10.3390/jpm5020050
- Chin SN, Trinkaus M, Simmons C, et al. Prevalence and severity of urogenital symptoms in postmenopausal women receiving endocrine therapy for breast cancer. Clin Breast Cancer 2009; 9(2):108–117. doi:10.3816/CBC.2009.n.020
- Fallowfield L, Cella D, Cuzick J, Francis S, Locker G, Howell A. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol 2004; 22(21):4261–4271. doi:10.1200/JCO.2004.08.029
- Cella D, Fallowfield LJ. Recognition and management of treatment-related side effects for breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer Res Treat 2008; 107(2):167–180. doi:10.1007/s10549-007-9548-1
- Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288(3):321–333. pmid:12117397
- Tsai SA, Stefanick ML, Stafford RS. Trends in menopausal hormone therapy use of US office-based physicians, 2000–2009. Menopause 2011; 18(4):385–392. doi:10.1097/gme.0b013e3181f43404
- North American Menopause Society. Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause 2013; 20(9):888–902. doi:10.1097/GME.0b013e3182a122c2
- Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. Climacteric 2015; 18(2):121–134. doi:10.3109/13697137.2014.947254
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice, Farrell R. ACOG Committee Opinion No. 659: the use of vaginal estrogen in women with a history of estrogen-dependent breast cancer. Obstet Gynecol 2016; 127(3):e93–e96. doi:10.1097/AOG.0000000000001351
- Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44(3):497–515. doi:10.1016/j.ecl.2015.05.001
- Labrie F, Archer DF, Martel C, Vaillancourt M, Montesino M. Combined data of intravaginal prasterone against vulvovaginal atrophy of menopause. Menopause 2017; 24(11):1246–1256. doi:10.1097/GME.0000000000000910
- Labrie F, Archer D, Bouchard C, et al. Serum steroid levels during 12-week intravaginal dehydroepiandrosterone administration. Menopause 2009; 16(5):897–906. doi:10.1097/gme.0b013e31819e8930
- Labrie F, Cusan L, Gomez JL, et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol 2008; 111(3-5):178–194. doi:10.1016/j.jsbmb.2008.06.003
- Soe LH, Wurz GT, Kao CJ, Degregorio MW. Ospemifene for the treatment of dyspareunia associated with vulvar and vaginal atrophy: potential benefits in bone and breast. Int J Womens Health 2013; 5:605–611. doi:10.2147/IJWH.S39146
- Taras TL, Wurz GT, DeGregorio MW. In vitro and in vivo biologic effects of ospemifene (FC-1271a) in breast cancer. J Steroid Biochem Mol Biol 2001; 77(4–5):271–279. pmid:11457665
- Wurz GT, Read KC, Marchisano-Karpman C, et al. Ospemifene inhibits the growth of dimethylbenzanthracene-induced mammary tumors in Sencar mice. J Steroid Biochem Mol Biol 2005; 97(3):230–240. doi:10.1016/j.jsbmb.2005.06.027
- Archer DF, Carr BR, Pinkerton JV, Taylor HS, Constantine GD. Effects of ospemifene on the female reproductive and urinary tracts: translation from preclinical models into clinical evidence. Menopause 2015; 22(7):786–796. doi:10.1097/GME.0000000000000365
- Mirkin S, Pickar JH. Management of osteoporosis and menopausal symptoms: focus on bazedoxifene/conjugated estrogen combination. Int J Womens Health 2013; 5:465–475. doi:10.2147/IJWH.S39455
- Kagan R, Goldstein SR, Pickar JH, Komm BS. Patient considerations in the management of menopausal symptoms: role of conjugated estrogens with bazedoxifene. Ther Clin Risk Manag 2016; 12:549–562. doi:10.2147/TCRM.S63833
- Lobo RA, Pinkerton JV, Gass ML, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril 2009; 92(3):1025–1038. doi:10.1016/j.fertnstert.2009.03.113
- Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16(6):1116–1124. doi:10.1097/gme.0b013e3181a7df0d
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo- and active-controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010; 17(2):281–289. doi:10.1097/GME.0b013e3181b7c65f
- Pinkerton JV, Harvey JA, Pan K, et al. Breast effects of bazedoxifene-conjugated estrogens: a randomized controlled trial. Obstet Gynecol 2013; 121(5):959–968. doi:10.1097/AOG.0b013e31828c5974
- FDA. U.S. Food & Drug Administration. FDA Statement. Statement from FDA Commissioner Scott Gottlieb, M.D., on efforts to safeguard women’s health from deceptive health claims and significant risks related to devices marketed for use in medical procedures for “vaginal rejuvenation.” www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm615130.htm. Accessed August 20, 2018.
- Cruz VL, Steiner ML, Pompei LM, et al. Randomized, double-blind, placebo-controlled clinical trial for evaluating the efficacy of fractional CO2 laser compared with topical estriol in the treatment of vaginal atrophy in postmenopausal women. Menopause 2018; 25(1):21–28. doi:10.1097/GME.0000000000000955
- Biglia N, Bounous VE, D’Alonzo M, et al. Vaginal atrophy in breast cancer survivors: attitude and approaches among oncologists. Clin Breast Cancer 2017; 17(8):611–617. doi:10.1016/j.clbc.2017.05.008
KEY POINTS
- In general, locally applied hormonal therapies relieve GSM symptoms without increasing breast cancer risk.
- DHEA relieves vaginal symptoms without increasing serum estrogen levels.
- Ospemifene has antiestrogenic effects on breast tissue that make it an attractive option for women with breast cancer.
- The combination of conjugated estrogens and bazedoxifene offers a progesterone-free treatment for GSM symptoms in women desiring systemic hormone therapy.
How long should we follow simple ovarian cysts with pelvic ultrasonography?
A 54-year-old postmenopausal woman presents with a 3-day history of left lower quadrant pain. Abdominal and pelvic computed tomography confirm the diagnosis of acute diverticulitis, and a left ovarian cyst is incidentally noted. Her abdominal discomfort resolves with antibiotics.
Transvaginal ultrasonography confirms the presence of a 4.5-cm simple left ovarian cyst. The radiologist recommends follow-up ultrasonography in 3 months “if clinically indicated.” The patient feels well and is anxious about having additional testing. What do you recommend?
HOW USEFUL IS ULTRASONOGRAPHY FOR OVARIAN CYSTS?
Ovarian cysts are common and may affect up to 20% of women at some time during their life.1 In a prospective study of almost 40,000 women enrolled in an ovarian cancer screening program, the prevalence of ovarian cysts was 15.3% in premenopausal women and 8.2% in postmenopausal women.2
Pelvic ultrasonography is the most effective way to evaluate incidentally noted cysts, and the transvaginal approach is preferred.3 The International Ovarian Tumor Analysis group has outlined morphologic features, referred to as “simple rules,” for predicting if a cyst is malignant or benign.4 In a prospective validation study, these simple rules were applied in 76% of cases, with a sensitivity of 95% and a specificity of 91%.4 However, it should be noted that these rules apply to examinations done by experienced gynecologic ultrasonographers, as accuracy of ultrasonography is both machine- and operator-dependent.
WHAT IS THE MALIGNANCY POTENTIAL OF A SIMPLE OVARIAN CYST?
A simple ovarian cyst is defined as an anechoic round or oval lesion, different from a unilocular cyst, which may contain septations, solid wall irregularities, or internal echoes.5 Overall, simple ovarian cysts have a very low likelihood of malignancy. In the large, multi-site Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, simple cysts were observed in 14% of postmenopausal women,6 but no cyst was associated with the development of ovarian cancer over 4 years of follow-up.
HOW OFTEN SHOULD IMAGING BE REPEATED?
In premenopausal women, most simple (thin-walled) ovarian cysts less than 5 cm in maximum diameter resolve in 2 to 3 menstrual cycles and do not require further intervention.3 Larger cysts (5–7 cm in diameter) should be followed with ultrasonography yearly. Cysts larger than 7 cm require advanced imaging or surgical intervention, and the patient should be referred to a gynecologist.3
In postmenopausal women, serum markers are combined with ultrasonography results to determine the risk of malignancy. Markers studied include cancer antigen 125 (CA-125), human epididymis protein 4, lactate dehydrogenase, alpha fetoprotein, and beta human chorionic gonadotropin (beta hCG).7
CA-125, the most studied marker, is elevated in more than 90% of advanced-stage ovarian cancers, but in only 50% of patients wth early-stage cancer.1,8 However, CA-125 may be elevated in a variety of other settings, including benign gynecologic disorders (pelvic infection, fibroids, endometriosis, adenomyosis) and nongynecologic disorders (liver disease, pancreatitis, and diverticulitis). Thus, it is unreliable for distinguishing benign from malignant ovarian masses in premenopausal women.1,3
Current guidelines recommend routine measurement of CA-125 in the initial evaluation of all postmenopausal women with an ovarian mass.7,8
Using a cutoff of 30 IU/mL, CA-125 has a sensitivity of 81% and a specificity of 75% for ovarian malignancy. However, serial measurements may be more useful for assessing ovarian cancer risk, especially in the setting of rapidly rising values.1,3
The Risk for Malignancy Index (RMI), which categorizes a cyst’s risk for malignancy, can be calculated based on the patient’s menopausal status, ultrasonographic characteristics (1 point each for multilocular cyst, solid area, metastasis, ascites, and bilateral lesions), and serum CA-125 level. The RMI has a sensitivity of 78% and a specificity of 87% for predicting ovarian cancer.8
Postmenopausal women with an asymptomatic small cyst (< 5 cm), a normal CA-125 level, and an RMI < 200 can be followed conservatively, with repeat ultrasonography in 4 to 6 months. At that time, if the cyst has not grown and the CA-125 level is normal, expectant management can continue, with reassessment in 4 to 6 months. If imaging remains unchanged and the CA-125 is persistently normal, the patient may be discharged from follow-up.8
If at any time during the evaluation the calculated RMI is greater than 200, there is an increased risk for malignancy, and the patient should be referred to a gynecologic oncologist for advanced imaging.
An algorithm from the Royal College of Obstetricians and Gynaecologists for managing ovarian cysts in postmenopausal women is available at www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf.8
CURRENT GUIDELINES ON REPEAT IMAGING
The American College of Radiology (ACR) has created a “Choosing Wisely” guideline to clarify when repeat imaging for ovarian cysts is indicated, to reduce both patient anxiety and healthcare costs.9 These guidelines highlight the distress women may experience from repeat testing due to concerns about cancer.
The guidelines also note that testing is often done during varying times of the menstrual cycle, thereby detecting new cysts, as opposed to monitoring previously detected cysts. Repeat ultrasonography may lead to surgical interventions that are not evidence-based, such as cystectomy or oophorectomy, in patients without radiologic features of malignancy or associated pelvic pain. And while ultrasonography is less expensive than other imaging tests, unnecessary imaging can mean additional costs to the patient, such as copayments, and possibly large payments for patients without insurance.
The American College of Obstetricians and Gynecologists (ACOG) and the ACR guidelines recommend against unnecessary repeat imaging for ovarian cysts.7,10 The ACOG Practice Bulletin on the Evaluation and Management of Adnexal Masses states, “Simple cysts up to 10 cm in diameter on transvaginal ultrasonography performed by experienced ultrasonographers are likely benign and may be safely monitored using repeat imaging without surgical intervention, even in postmenopausal patients.”7
The ideal frequency for repeat testing is yet to be determined. In postmenopausal women with a simple cyst smaller than 5 cm, ACOG guidelines recommend an interval of 4 to 6 months for initial repeat imaging. ACR guidelines recommend no follow-up imaging for simple cysts smaller than 5 cm detected by high-quality ultrasonography in asymptomatic women of reproductive age or for simple cysts smaller than 1 cm in postmenopausal women.10
THE CLINICAL BOTTOM LINE
Simple ovarian cysts can develop as part of the normal menstrual cycle, and although they are more common in premenopausal women, they have been detected in 1 out of 5 postmenopausal women.9 Simple ovarian cysts are typically not cancerous in women of any age. Therefore, most simple ovarian cysts in asymptomatic women either require no follow-up imaging or can be safely monitored with limited repeat ultrasonography for a defined length of time.
Our 54-year-old postmenopausal patient has a simple cyst smaller than 5 cm. Based on current guidelines, the CA-125 level should be measured, with subsequent calculation of the RMI. Assuming a normal CA-125 and RMI, she should be reassured that the risk of progression to malignancy is extremely low. Repeating ultrasonography 4 to 6 months after the initial imaging is warranted. At that time, if no change in cyst size or composition is detected, ultrasonography can be repeated at 1 year after initial detection. After that, assuming no changes of the cyst on repeat imaging, the patient does not require additional follow-up.
- van Nagell JR Jr, Miiler RW. Evaluation and management of ultrasonographically detected ovarian tumors in asymptomatic women. Obstet Gynecol 2016; 127(5):848–858. doi:10.1097/AOG.0000000000001384
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol 2013; 122(2 pt 1):210–217. doi:10.1097/AOG.0b013e318298def5
- Royal College of Obstetricians and Gynaecologists. Management of suspected ovarian masses in premenopausal women. Green-top guideline 2011; 62:1–14. www.rcog.org.uk/globalassets/documents/guidelines/gtg_62.pdf. Accessed August 16, 2018.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol 2008; 31(6):681–690. doi:10.1002/uog.5365
- Glanc P, Benacerraf B, Bourne T, et al. First international consensus report on adnexal masses: management recommendations. J Ultrasound Med 2017; 36(5):849–863. doi:10.1002/jum.14197
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202(4):373.e1–e9. doi:10.1016/j.ajog.2009.11.029
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet Gynecol 2016; 128(5):e210-226. doi:10.1097/AOG.0000000000001768
- Royal College of Obstetricians and Gynaecologists. The management of ovarian cysts in postmenopausal women. Green-top guideline 2016; 34:1–31. www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf. Accessed August 16, 2018.
- American College of Radiology Choosing Wisely. Imaging tests for ovarian cysts. When you need an ultrasound—and when you don’t. www.choosingwisely.org/wp-content/uploads/2012/09/ChoosingWiselyOvarianCystsACR-ER_Update.pdf. Accessed August 16, 2018.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256(3):943–954. doi:10.1148/radiol.10100213
A 54-year-old postmenopausal woman presents with a 3-day history of left lower quadrant pain. Abdominal and pelvic computed tomography confirm the diagnosis of acute diverticulitis, and a left ovarian cyst is incidentally noted. Her abdominal discomfort resolves with antibiotics.
Transvaginal ultrasonography confirms the presence of a 4.5-cm simple left ovarian cyst. The radiologist recommends follow-up ultrasonography in 3 months “if clinically indicated.” The patient feels well and is anxious about having additional testing. What do you recommend?
HOW USEFUL IS ULTRASONOGRAPHY FOR OVARIAN CYSTS?
Ovarian cysts are common and may affect up to 20% of women at some time during their life.1 In a prospective study of almost 40,000 women enrolled in an ovarian cancer screening program, the prevalence of ovarian cysts was 15.3% in premenopausal women and 8.2% in postmenopausal women.2
Pelvic ultrasonography is the most effective way to evaluate incidentally noted cysts, and the transvaginal approach is preferred.3 The International Ovarian Tumor Analysis group has outlined morphologic features, referred to as “simple rules,” for predicting if a cyst is malignant or benign.4 In a prospective validation study, these simple rules were applied in 76% of cases, with a sensitivity of 95% and a specificity of 91%.4 However, it should be noted that these rules apply to examinations done by experienced gynecologic ultrasonographers, as accuracy of ultrasonography is both machine- and operator-dependent.
WHAT IS THE MALIGNANCY POTENTIAL OF A SIMPLE OVARIAN CYST?
A simple ovarian cyst is defined as an anechoic round or oval lesion, different from a unilocular cyst, which may contain septations, solid wall irregularities, or internal echoes.5 Overall, simple ovarian cysts have a very low likelihood of malignancy. In the large, multi-site Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, simple cysts were observed in 14% of postmenopausal women,6 but no cyst was associated with the development of ovarian cancer over 4 years of follow-up.
HOW OFTEN SHOULD IMAGING BE REPEATED?
In premenopausal women, most simple (thin-walled) ovarian cysts less than 5 cm in maximum diameter resolve in 2 to 3 menstrual cycles and do not require further intervention.3 Larger cysts (5–7 cm in diameter) should be followed with ultrasonography yearly. Cysts larger than 7 cm require advanced imaging or surgical intervention, and the patient should be referred to a gynecologist.3
In postmenopausal women, serum markers are combined with ultrasonography results to determine the risk of malignancy. Markers studied include cancer antigen 125 (CA-125), human epididymis protein 4, lactate dehydrogenase, alpha fetoprotein, and beta human chorionic gonadotropin (beta hCG).7
CA-125, the most studied marker, is elevated in more than 90% of advanced-stage ovarian cancers, but in only 50% of patients wth early-stage cancer.1,8 However, CA-125 may be elevated in a variety of other settings, including benign gynecologic disorders (pelvic infection, fibroids, endometriosis, adenomyosis) and nongynecologic disorders (liver disease, pancreatitis, and diverticulitis). Thus, it is unreliable for distinguishing benign from malignant ovarian masses in premenopausal women.1,3
Current guidelines recommend routine measurement of CA-125 in the initial evaluation of all postmenopausal women with an ovarian mass.7,8
Using a cutoff of 30 IU/mL, CA-125 has a sensitivity of 81% and a specificity of 75% for ovarian malignancy. However, serial measurements may be more useful for assessing ovarian cancer risk, especially in the setting of rapidly rising values.1,3
The Risk for Malignancy Index (RMI), which categorizes a cyst’s risk for malignancy, can be calculated based on the patient’s menopausal status, ultrasonographic characteristics (1 point each for multilocular cyst, solid area, metastasis, ascites, and bilateral lesions), and serum CA-125 level. The RMI has a sensitivity of 78% and a specificity of 87% for predicting ovarian cancer.8
Postmenopausal women with an asymptomatic small cyst (< 5 cm), a normal CA-125 level, and an RMI < 200 can be followed conservatively, with repeat ultrasonography in 4 to 6 months. At that time, if the cyst has not grown and the CA-125 level is normal, expectant management can continue, with reassessment in 4 to 6 months. If imaging remains unchanged and the CA-125 is persistently normal, the patient may be discharged from follow-up.8
If at any time during the evaluation the calculated RMI is greater than 200, there is an increased risk for malignancy, and the patient should be referred to a gynecologic oncologist for advanced imaging.
An algorithm from the Royal College of Obstetricians and Gynaecologists for managing ovarian cysts in postmenopausal women is available at www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf.8
CURRENT GUIDELINES ON REPEAT IMAGING
The American College of Radiology (ACR) has created a “Choosing Wisely” guideline to clarify when repeat imaging for ovarian cysts is indicated, to reduce both patient anxiety and healthcare costs.9 These guidelines highlight the distress women may experience from repeat testing due to concerns about cancer.
The guidelines also note that testing is often done during varying times of the menstrual cycle, thereby detecting new cysts, as opposed to monitoring previously detected cysts. Repeat ultrasonography may lead to surgical interventions that are not evidence-based, such as cystectomy or oophorectomy, in patients without radiologic features of malignancy or associated pelvic pain. And while ultrasonography is less expensive than other imaging tests, unnecessary imaging can mean additional costs to the patient, such as copayments, and possibly large payments for patients without insurance.
The American College of Obstetricians and Gynecologists (ACOG) and the ACR guidelines recommend against unnecessary repeat imaging for ovarian cysts.7,10 The ACOG Practice Bulletin on the Evaluation and Management of Adnexal Masses states, “Simple cysts up to 10 cm in diameter on transvaginal ultrasonography performed by experienced ultrasonographers are likely benign and may be safely monitored using repeat imaging without surgical intervention, even in postmenopausal patients.”7
The ideal frequency for repeat testing is yet to be determined. In postmenopausal women with a simple cyst smaller than 5 cm, ACOG guidelines recommend an interval of 4 to 6 months for initial repeat imaging. ACR guidelines recommend no follow-up imaging for simple cysts smaller than 5 cm detected by high-quality ultrasonography in asymptomatic women of reproductive age or for simple cysts smaller than 1 cm in postmenopausal women.10
THE CLINICAL BOTTOM LINE
Simple ovarian cysts can develop as part of the normal menstrual cycle, and although they are more common in premenopausal women, they have been detected in 1 out of 5 postmenopausal women.9 Simple ovarian cysts are typically not cancerous in women of any age. Therefore, most simple ovarian cysts in asymptomatic women either require no follow-up imaging or can be safely monitored with limited repeat ultrasonography for a defined length of time.
Our 54-year-old postmenopausal patient has a simple cyst smaller than 5 cm. Based on current guidelines, the CA-125 level should be measured, with subsequent calculation of the RMI. Assuming a normal CA-125 and RMI, she should be reassured that the risk of progression to malignancy is extremely low. Repeating ultrasonography 4 to 6 months after the initial imaging is warranted. At that time, if no change in cyst size or composition is detected, ultrasonography can be repeated at 1 year after initial detection. After that, assuming no changes of the cyst on repeat imaging, the patient does not require additional follow-up.
A 54-year-old postmenopausal woman presents with a 3-day history of left lower quadrant pain. Abdominal and pelvic computed tomography confirm the diagnosis of acute diverticulitis, and a left ovarian cyst is incidentally noted. Her abdominal discomfort resolves with antibiotics.
Transvaginal ultrasonography confirms the presence of a 4.5-cm simple left ovarian cyst. The radiologist recommends follow-up ultrasonography in 3 months “if clinically indicated.” The patient feels well and is anxious about having additional testing. What do you recommend?
HOW USEFUL IS ULTRASONOGRAPHY FOR OVARIAN CYSTS?
Ovarian cysts are common and may affect up to 20% of women at some time during their life.1 In a prospective study of almost 40,000 women enrolled in an ovarian cancer screening program, the prevalence of ovarian cysts was 15.3% in premenopausal women and 8.2% in postmenopausal women.2
Pelvic ultrasonography is the most effective way to evaluate incidentally noted cysts, and the transvaginal approach is preferred.3 The International Ovarian Tumor Analysis group has outlined morphologic features, referred to as “simple rules,” for predicting if a cyst is malignant or benign.4 In a prospective validation study, these simple rules were applied in 76% of cases, with a sensitivity of 95% and a specificity of 91%.4 However, it should be noted that these rules apply to examinations done by experienced gynecologic ultrasonographers, as accuracy of ultrasonography is both machine- and operator-dependent.
WHAT IS THE MALIGNANCY POTENTIAL OF A SIMPLE OVARIAN CYST?
A simple ovarian cyst is defined as an anechoic round or oval lesion, different from a unilocular cyst, which may contain septations, solid wall irregularities, or internal echoes.5 Overall, simple ovarian cysts have a very low likelihood of malignancy. In the large, multi-site Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, simple cysts were observed in 14% of postmenopausal women,6 but no cyst was associated with the development of ovarian cancer over 4 years of follow-up.
HOW OFTEN SHOULD IMAGING BE REPEATED?
In premenopausal women, most simple (thin-walled) ovarian cysts less than 5 cm in maximum diameter resolve in 2 to 3 menstrual cycles and do not require further intervention.3 Larger cysts (5–7 cm in diameter) should be followed with ultrasonography yearly. Cysts larger than 7 cm require advanced imaging or surgical intervention, and the patient should be referred to a gynecologist.3
In postmenopausal women, serum markers are combined with ultrasonography results to determine the risk of malignancy. Markers studied include cancer antigen 125 (CA-125), human epididymis protein 4, lactate dehydrogenase, alpha fetoprotein, and beta human chorionic gonadotropin (beta hCG).7
CA-125, the most studied marker, is elevated in more than 90% of advanced-stage ovarian cancers, but in only 50% of patients wth early-stage cancer.1,8 However, CA-125 may be elevated in a variety of other settings, including benign gynecologic disorders (pelvic infection, fibroids, endometriosis, adenomyosis) and nongynecologic disorders (liver disease, pancreatitis, and diverticulitis). Thus, it is unreliable for distinguishing benign from malignant ovarian masses in premenopausal women.1,3
Current guidelines recommend routine measurement of CA-125 in the initial evaluation of all postmenopausal women with an ovarian mass.7,8
Using a cutoff of 30 IU/mL, CA-125 has a sensitivity of 81% and a specificity of 75% for ovarian malignancy. However, serial measurements may be more useful for assessing ovarian cancer risk, especially in the setting of rapidly rising values.1,3
The Risk for Malignancy Index (RMI), which categorizes a cyst’s risk for malignancy, can be calculated based on the patient’s menopausal status, ultrasonographic characteristics (1 point each for multilocular cyst, solid area, metastasis, ascites, and bilateral lesions), and serum CA-125 level. The RMI has a sensitivity of 78% and a specificity of 87% for predicting ovarian cancer.8
Postmenopausal women with an asymptomatic small cyst (< 5 cm), a normal CA-125 level, and an RMI < 200 can be followed conservatively, with repeat ultrasonography in 4 to 6 months. At that time, if the cyst has not grown and the CA-125 level is normal, expectant management can continue, with reassessment in 4 to 6 months. If imaging remains unchanged and the CA-125 is persistently normal, the patient may be discharged from follow-up.8
If at any time during the evaluation the calculated RMI is greater than 200, there is an increased risk for malignancy, and the patient should be referred to a gynecologic oncologist for advanced imaging.
An algorithm from the Royal College of Obstetricians and Gynaecologists for managing ovarian cysts in postmenopausal women is available at www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf.8
CURRENT GUIDELINES ON REPEAT IMAGING
The American College of Radiology (ACR) has created a “Choosing Wisely” guideline to clarify when repeat imaging for ovarian cysts is indicated, to reduce both patient anxiety and healthcare costs.9 These guidelines highlight the distress women may experience from repeat testing due to concerns about cancer.
The guidelines also note that testing is often done during varying times of the menstrual cycle, thereby detecting new cysts, as opposed to monitoring previously detected cysts. Repeat ultrasonography may lead to surgical interventions that are not evidence-based, such as cystectomy or oophorectomy, in patients without radiologic features of malignancy or associated pelvic pain. And while ultrasonography is less expensive than other imaging tests, unnecessary imaging can mean additional costs to the patient, such as copayments, and possibly large payments for patients without insurance.
The American College of Obstetricians and Gynecologists (ACOG) and the ACR guidelines recommend against unnecessary repeat imaging for ovarian cysts.7,10 The ACOG Practice Bulletin on the Evaluation and Management of Adnexal Masses states, “Simple cysts up to 10 cm in diameter on transvaginal ultrasonography performed by experienced ultrasonographers are likely benign and may be safely monitored using repeat imaging without surgical intervention, even in postmenopausal patients.”7
The ideal frequency for repeat testing is yet to be determined. In postmenopausal women with a simple cyst smaller than 5 cm, ACOG guidelines recommend an interval of 4 to 6 months for initial repeat imaging. ACR guidelines recommend no follow-up imaging for simple cysts smaller than 5 cm detected by high-quality ultrasonography in asymptomatic women of reproductive age or for simple cysts smaller than 1 cm in postmenopausal women.10
THE CLINICAL BOTTOM LINE
Simple ovarian cysts can develop as part of the normal menstrual cycle, and although they are more common in premenopausal women, they have been detected in 1 out of 5 postmenopausal women.9 Simple ovarian cysts are typically not cancerous in women of any age. Therefore, most simple ovarian cysts in asymptomatic women either require no follow-up imaging or can be safely monitored with limited repeat ultrasonography for a defined length of time.
Our 54-year-old postmenopausal patient has a simple cyst smaller than 5 cm. Based on current guidelines, the CA-125 level should be measured, with subsequent calculation of the RMI. Assuming a normal CA-125 and RMI, she should be reassured that the risk of progression to malignancy is extremely low. Repeating ultrasonography 4 to 6 months after the initial imaging is warranted. At that time, if no change in cyst size or composition is detected, ultrasonography can be repeated at 1 year after initial detection. After that, assuming no changes of the cyst on repeat imaging, the patient does not require additional follow-up.
- van Nagell JR Jr, Miiler RW. Evaluation and management of ultrasonographically detected ovarian tumors in asymptomatic women. Obstet Gynecol 2016; 127(5):848–858. doi:10.1097/AOG.0000000000001384
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol 2013; 122(2 pt 1):210–217. doi:10.1097/AOG.0b013e318298def5
- Royal College of Obstetricians and Gynaecologists. Management of suspected ovarian masses in premenopausal women. Green-top guideline 2011; 62:1–14. www.rcog.org.uk/globalassets/documents/guidelines/gtg_62.pdf. Accessed August 16, 2018.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol 2008; 31(6):681–690. doi:10.1002/uog.5365
- Glanc P, Benacerraf B, Bourne T, et al. First international consensus report on adnexal masses: management recommendations. J Ultrasound Med 2017; 36(5):849–863. doi:10.1002/jum.14197
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202(4):373.e1–e9. doi:10.1016/j.ajog.2009.11.029
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet Gynecol 2016; 128(5):e210-226. doi:10.1097/AOG.0000000000001768
- Royal College of Obstetricians and Gynaecologists. The management of ovarian cysts in postmenopausal women. Green-top guideline 2016; 34:1–31. www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf. Accessed August 16, 2018.
- American College of Radiology Choosing Wisely. Imaging tests for ovarian cysts. When you need an ultrasound—and when you don’t. www.choosingwisely.org/wp-content/uploads/2012/09/ChoosingWiselyOvarianCystsACR-ER_Update.pdf. Accessed August 16, 2018.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256(3):943–954. doi:10.1148/radiol.10100213
- van Nagell JR Jr, Miiler RW. Evaluation and management of ultrasonographically detected ovarian tumors in asymptomatic women. Obstet Gynecol 2016; 127(5):848–858. doi:10.1097/AOG.0000000000001384
- Pavlik EJ, Ueland FR, Miller RW, et al. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol 2013; 122(2 pt 1):210–217. doi:10.1097/AOG.0b013e318298def5
- Royal College of Obstetricians and Gynaecologists. Management of suspected ovarian masses in premenopausal women. Green-top guideline 2011; 62:1–14. www.rcog.org.uk/globalassets/documents/guidelines/gtg_62.pdf. Accessed August 16, 2018.
- Timmerman D, Testa AC, Bourne T, et al. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol 2008; 31(6):681–690. doi:10.1002/uog.5365
- Glanc P, Benacerraf B, Bourne T, et al. First international consensus report on adnexal masses: management recommendations. J Ultrasound Med 2017; 36(5):849–863. doi:10.1002/jum.14197
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202(4):373.e1–e9. doi:10.1016/j.ajog.2009.11.029
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. Practice Bulletin No. 174: Evaluation and Management of Adnexal Masses. Obstet Gynecol 2016; 128(5):e210-226. doi:10.1097/AOG.0000000000001768
- Royal College of Obstetricians and Gynaecologists. The management of ovarian cysts in postmenopausal women. Green-top guideline 2016; 34:1–31. www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_34.pdf. Accessed August 16, 2018.
- American College of Radiology Choosing Wisely. Imaging tests for ovarian cysts. When you need an ultrasound—and when you don’t. www.choosingwisely.org/wp-content/uploads/2012/09/ChoosingWiselyOvarianCystsACR-ER_Update.pdf. Accessed August 16, 2018.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256(3):943–954. doi:10.1148/radiol.10100213
Under new ACC-AHA criteria, more pregnant women at risk of hypertension, adverse outcomes
A total of 164 new diagnoses (5.6%) of lower range stage 1 hypertension were made when the revised American Heart Association and the American College of Cardiology (ACC-AHA) Task Force on Clinical Practice Guidelines for chronic hypertension in adults were applied to a population of 2,947 pregnant women.
These patients had a significantly increased risk of preeclampsia as well as an increased risk of gestational diabetes mellitus and preterm birth, compared with normotensive patients, according to a study published in Obstetrics & Gynecology.
Elizabeth F. Sutton, PhD, and her associates at Magee-Womens Research Institute in Pittsburgh, used data from a Eunice Kennedy Shriver National Institute of Child Health and Human Development study. It included 2,947 pregnant women with singleton pregnancies where 94% of women were normotensive prior to 25 weeks of gestation. The researchers identified 164 new cases (6%) of lower range stage 1 hypertension under the ACC-AHA guidelines. Patients were then randomized to receive 60 mg of aspirin (1,399 normotensive patients, 66 stage 1 hypertension patients) or placebo (1,384 normotensive patients, 98 stage 1 hypertension patients).
The women had their blood pressure (BP) measured at 25 weeks of gestation or prior, and those patients with a systolic BP between 130 mm Hg and 139 mm Hg or diastolic BP between 80 mm and 89 mm were reclassified as having stage 1 hypertension under the new ACC-AHA guidelines; outcomes were compared with normotensive women with a systolic BP of 130 mm Hg and diastolic BP of 80 mm Hg. The researchers also performed a sensitivity analysis that restricted enrollment to 1,661 women who were at 20 weeks of gestation or prior, they said.
“Of important note, as a result of eligibility criterion excluding women with chronic hypertension (greater than 135/85 mm Hg) at enrollment, the enrollment BP range within the stage 1 hypertension group was limited to lower range stage 1 hypertension, that is, 130-135, 80-85 mm Hg, or both,” Dr. Sutton and her colleagues wrote.
The researchers found a significantly increased risk of preeclampsia among hypertensive pregnant women in the placebo group (15%) compared with normotensive (5%) women (relative risk, 2.66; 95% confidence interval, 1.56-4.54; P less than .01). These patients also had an increased risk of gestational diabetes mellitus (6%) compared with normotensive (2.5%; P = .03) pregnant women as well as an increased risk of preterm birth (4% vs. 1%; P = .01). Among women with stage 1 hypertension who received low-dose aspirin, there were no significant differences regarding the rate of preeclampsia, gestational diabetes mellitus, or preterm birth risk.
“Although prepregnancy BPs would be ideal for diagnosis, prior studies have shown that women do not consistently seek primary care outside of pregnancy,” Dr. Sutton and her colleagues wrote. “In the United States, preconception care engagement rates are between 18% and 45% in reproductive-aged women; thus, early pregnancy BPs may be all that is available for the obstetrician.”
Limitations to the secondary analysis included the original study’s exclusion of BPs higher than 135/85 mm Hg and the small sample size of patients who met the new criteria for stage 1 hypertension. The researchers also noted an increased risk of preeclampsia among patients with intake systolic BP between 120 mm Hg and 129 mm Hg, “with a smaller magnitude of risk compared with stage 1 hypertension.
“These findings suggest preliminarily that when considering preeclampsia risk based on early pregnancy BP, perhaps BP could be considered as a continuous variable rather than categorical,” Dr. Sutton and her colleagues noted.
This study was supported in part by the American Heart Association and the Eunice Kennedy Shriver NICHD. The authors reported no relevant financial conflicts of interest.
SOURCE: Sutton EF et al. Obstet Gynecol. 2018 Oct doi: 10.1097/AOG.0000000000002870.
I have significant concerns that, were ob.gyns. to adopt the ACC-AHA guidelines for chronic hypertension in adults and apply them to pregnant women, it would have great implications, as about 25% of all pregnant women have these findings.
Under the new criteria, there would be an additional 1 million pregnant women diagnosed with prehypertension or stage 1 hypertension. The new criteria will not only overdiagnose prehypertension in these women, it will cause more women to receive antihypertension medications.
The ACC-AHA criteria apply only to patients who have their blood pressure (BP) recorded prior to pregnancy or prior to 20 weeks of gestation. This study included women at up to 25 weeks of gestation, which means some already had been developing the condition. The Sutton et al. data also do not follow the new ACC-AHA criteria because the criteria require two BP readings, and the current study shows only one elevation which is, in my opinion, more dangerous.
Any time new criteria and screening are introduced, there always should be preparations for what happens next. There is no doubt that the ACC-AHA criteria, performed using two BP readings, is associated with hypertension, but what should clinicians do with that information? Prospective studies aimed at evaluating whether a woman with hypertension prior to or early in pregnancy may benefit from more intensive screening and other interventions to prevent hypertensive disorders and gestational diabetes in pregnancy are needed.
Baha Sibai, MD, is a visiting professor in the department of obstetrics, gynecology, and reproductive sciences at The University of Texas Health Sciences Center at Houston. He was asked to comment on the study by Sutton et al. He reported no relevant financial conflicts of interest.
I have significant concerns that, were ob.gyns. to adopt the ACC-AHA guidelines for chronic hypertension in adults and apply them to pregnant women, it would have great implications, as about 25% of all pregnant women have these findings.
Under the new criteria, there would be an additional 1 million pregnant women diagnosed with prehypertension or stage 1 hypertension. The new criteria will not only overdiagnose prehypertension in these women, it will cause more women to receive antihypertension medications.
The ACC-AHA criteria apply only to patients who have their blood pressure (BP) recorded prior to pregnancy or prior to 20 weeks of gestation. This study included women at up to 25 weeks of gestation, which means some already had been developing the condition. The Sutton et al. data also do not follow the new ACC-AHA criteria because the criteria require two BP readings, and the current study shows only one elevation which is, in my opinion, more dangerous.
Any time new criteria and screening are introduced, there always should be preparations for what happens next. There is no doubt that the ACC-AHA criteria, performed using two BP readings, is associated with hypertension, but what should clinicians do with that information? Prospective studies aimed at evaluating whether a woman with hypertension prior to or early in pregnancy may benefit from more intensive screening and other interventions to prevent hypertensive disorders and gestational diabetes in pregnancy are needed.
Baha Sibai, MD, is a visiting professor in the department of obstetrics, gynecology, and reproductive sciences at The University of Texas Health Sciences Center at Houston. He was asked to comment on the study by Sutton et al. He reported no relevant financial conflicts of interest.
I have significant concerns that, were ob.gyns. to adopt the ACC-AHA guidelines for chronic hypertension in adults and apply them to pregnant women, it would have great implications, as about 25% of all pregnant women have these findings.
Under the new criteria, there would be an additional 1 million pregnant women diagnosed with prehypertension or stage 1 hypertension. The new criteria will not only overdiagnose prehypertension in these women, it will cause more women to receive antihypertension medications.
The ACC-AHA criteria apply only to patients who have their blood pressure (BP) recorded prior to pregnancy or prior to 20 weeks of gestation. This study included women at up to 25 weeks of gestation, which means some already had been developing the condition. The Sutton et al. data also do not follow the new ACC-AHA criteria because the criteria require two BP readings, and the current study shows only one elevation which is, in my opinion, more dangerous.
Any time new criteria and screening are introduced, there always should be preparations for what happens next. There is no doubt that the ACC-AHA criteria, performed using two BP readings, is associated with hypertension, but what should clinicians do with that information? Prospective studies aimed at evaluating whether a woman with hypertension prior to or early in pregnancy may benefit from more intensive screening and other interventions to prevent hypertensive disorders and gestational diabetes in pregnancy are needed.
Baha Sibai, MD, is a visiting professor in the department of obstetrics, gynecology, and reproductive sciences at The University of Texas Health Sciences Center at Houston. He was asked to comment on the study by Sutton et al. He reported no relevant financial conflicts of interest.
A total of 164 new diagnoses (5.6%) of lower range stage 1 hypertension were made when the revised American Heart Association and the American College of Cardiology (ACC-AHA) Task Force on Clinical Practice Guidelines for chronic hypertension in adults were applied to a population of 2,947 pregnant women.
These patients had a significantly increased risk of preeclampsia as well as an increased risk of gestational diabetes mellitus and preterm birth, compared with normotensive patients, according to a study published in Obstetrics & Gynecology.
Elizabeth F. Sutton, PhD, and her associates at Magee-Womens Research Institute in Pittsburgh, used data from a Eunice Kennedy Shriver National Institute of Child Health and Human Development study. It included 2,947 pregnant women with singleton pregnancies where 94% of women were normotensive prior to 25 weeks of gestation. The researchers identified 164 new cases (6%) of lower range stage 1 hypertension under the ACC-AHA guidelines. Patients were then randomized to receive 60 mg of aspirin (1,399 normotensive patients, 66 stage 1 hypertension patients) or placebo (1,384 normotensive patients, 98 stage 1 hypertension patients).
The women had their blood pressure (BP) measured at 25 weeks of gestation or prior, and those patients with a systolic BP between 130 mm Hg and 139 mm Hg or diastolic BP between 80 mm and 89 mm were reclassified as having stage 1 hypertension under the new ACC-AHA guidelines; outcomes were compared with normotensive women with a systolic BP of 130 mm Hg and diastolic BP of 80 mm Hg. The researchers also performed a sensitivity analysis that restricted enrollment to 1,661 women who were at 20 weeks of gestation or prior, they said.
“Of important note, as a result of eligibility criterion excluding women with chronic hypertension (greater than 135/85 mm Hg) at enrollment, the enrollment BP range within the stage 1 hypertension group was limited to lower range stage 1 hypertension, that is, 130-135, 80-85 mm Hg, or both,” Dr. Sutton and her colleagues wrote.
The researchers found a significantly increased risk of preeclampsia among hypertensive pregnant women in the placebo group (15%) compared with normotensive (5%) women (relative risk, 2.66; 95% confidence interval, 1.56-4.54; P less than .01). These patients also had an increased risk of gestational diabetes mellitus (6%) compared with normotensive (2.5%; P = .03) pregnant women as well as an increased risk of preterm birth (4% vs. 1%; P = .01). Among women with stage 1 hypertension who received low-dose aspirin, there were no significant differences regarding the rate of preeclampsia, gestational diabetes mellitus, or preterm birth risk.
“Although prepregnancy BPs would be ideal for diagnosis, prior studies have shown that women do not consistently seek primary care outside of pregnancy,” Dr. Sutton and her colleagues wrote. “In the United States, preconception care engagement rates are between 18% and 45% in reproductive-aged women; thus, early pregnancy BPs may be all that is available for the obstetrician.”
Limitations to the secondary analysis included the original study’s exclusion of BPs higher than 135/85 mm Hg and the small sample size of patients who met the new criteria for stage 1 hypertension. The researchers also noted an increased risk of preeclampsia among patients with intake systolic BP between 120 mm Hg and 129 mm Hg, “with a smaller magnitude of risk compared with stage 1 hypertension.
“These findings suggest preliminarily that when considering preeclampsia risk based on early pregnancy BP, perhaps BP could be considered as a continuous variable rather than categorical,” Dr. Sutton and her colleagues noted.
This study was supported in part by the American Heart Association and the Eunice Kennedy Shriver NICHD. The authors reported no relevant financial conflicts of interest.
SOURCE: Sutton EF et al. Obstet Gynecol. 2018 Oct doi: 10.1097/AOG.0000000000002870.
A total of 164 new diagnoses (5.6%) of lower range stage 1 hypertension were made when the revised American Heart Association and the American College of Cardiology (ACC-AHA) Task Force on Clinical Practice Guidelines for chronic hypertension in adults were applied to a population of 2,947 pregnant women.
These patients had a significantly increased risk of preeclampsia as well as an increased risk of gestational diabetes mellitus and preterm birth, compared with normotensive patients, according to a study published in Obstetrics & Gynecology.
Elizabeth F. Sutton, PhD, and her associates at Magee-Womens Research Institute in Pittsburgh, used data from a Eunice Kennedy Shriver National Institute of Child Health and Human Development study. It included 2,947 pregnant women with singleton pregnancies where 94% of women were normotensive prior to 25 weeks of gestation. The researchers identified 164 new cases (6%) of lower range stage 1 hypertension under the ACC-AHA guidelines. Patients were then randomized to receive 60 mg of aspirin (1,399 normotensive patients, 66 stage 1 hypertension patients) or placebo (1,384 normotensive patients, 98 stage 1 hypertension patients).
The women had their blood pressure (BP) measured at 25 weeks of gestation or prior, and those patients with a systolic BP between 130 mm Hg and 139 mm Hg or diastolic BP between 80 mm and 89 mm were reclassified as having stage 1 hypertension under the new ACC-AHA guidelines; outcomes were compared with normotensive women with a systolic BP of 130 mm Hg and diastolic BP of 80 mm Hg. The researchers also performed a sensitivity analysis that restricted enrollment to 1,661 women who were at 20 weeks of gestation or prior, they said.
“Of important note, as a result of eligibility criterion excluding women with chronic hypertension (greater than 135/85 mm Hg) at enrollment, the enrollment BP range within the stage 1 hypertension group was limited to lower range stage 1 hypertension, that is, 130-135, 80-85 mm Hg, or both,” Dr. Sutton and her colleagues wrote.
The researchers found a significantly increased risk of preeclampsia among hypertensive pregnant women in the placebo group (15%) compared with normotensive (5%) women (relative risk, 2.66; 95% confidence interval, 1.56-4.54; P less than .01). These patients also had an increased risk of gestational diabetes mellitus (6%) compared with normotensive (2.5%; P = .03) pregnant women as well as an increased risk of preterm birth (4% vs. 1%; P = .01). Among women with stage 1 hypertension who received low-dose aspirin, there were no significant differences regarding the rate of preeclampsia, gestational diabetes mellitus, or preterm birth risk.
“Although prepregnancy BPs would be ideal for diagnosis, prior studies have shown that women do not consistently seek primary care outside of pregnancy,” Dr. Sutton and her colleagues wrote. “In the United States, preconception care engagement rates are between 18% and 45% in reproductive-aged women; thus, early pregnancy BPs may be all that is available for the obstetrician.”
Limitations to the secondary analysis included the original study’s exclusion of BPs higher than 135/85 mm Hg and the small sample size of patients who met the new criteria for stage 1 hypertension. The researchers also noted an increased risk of preeclampsia among patients with intake systolic BP between 120 mm Hg and 129 mm Hg, “with a smaller magnitude of risk compared with stage 1 hypertension.
“These findings suggest preliminarily that when considering preeclampsia risk based on early pregnancy BP, perhaps BP could be considered as a continuous variable rather than categorical,” Dr. Sutton and her colleagues noted.
This study was supported in part by the American Heart Association and the Eunice Kennedy Shriver NICHD. The authors reported no relevant financial conflicts of interest.
SOURCE: Sutton EF et al. Obstet Gynecol. 2018 Oct doi: 10.1097/AOG.0000000000002870.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: gestational diabetes, and preterm birth.
Major finding: Pregnant women with newly diagnosed stage 1 hypertension not randomized to receive aspirin had a significantly higher risk of preeclampsia (15%) and a higher risk of gestational diabetes (6%) and preterm birth (4%), compared with normotensive pregnant women.
Study details: A secondary analysis of 2,947 women who were originally enrolled in a study by the Eunice Kennedy Shriver NICHD between 1989 and 1992.
Disclosures: This study was supported in part by the American Heart Association and the Eunice Kennedy Shriver NICHD. The authors reported no relevant financial conflicts of interest.
Source: Sutton EF et al. Obstet Gynecol. 2018 Oct doi:10.1097/AOG.0000000000002870.
Gestational weight outside guidelines adversely affects mothers, babies
Gestational weight gain above or below the level recommended by the Institute of Medicine (IOM) guidelines resulted in significantly worse outcomes for mothers and babies, according to data from nearly 30,000 women.
Previous studies of the relationship between gestational weight gain and maternal and neonatal outcomes have been limited by “small sample sizes, single sites, restricted reporting of outcomes, and a lack of racial-ethnic diversity,” Michelle A. Kominiarek, MD, of Northwestern University in Chicago and her colleagues wrote . To determine the effects of gestational weight gain on a large and more diverse population, the researchers conducted a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network’s Assessment of Perinatal Excellence study. The findings were published in Obstetrics & Gynecology.
Gestational weight gain above the amount recommended by IOM guidelines was significantly associated with adverse outcomes in neonates, including macrosomia (adjusted odds ratio, 2.66), shoulder dystocia (aOR, 1.74), and neonatal hypoglycemia (aOR, 1.60).
In further multivariate analysis, adverse maternal outcomes associated with gestational weight gain above that recommended by the guidelines included hypertensive diseases of pregnancy for any parity (aOR, 1.84) and increased risk of cesarean delivery in nulliparous and multiparous women (aORs, 1.44 and 1.26, respectively).
Gestational weight gain below the recommended amount was associated with both spontaneous (aOR, 1.50) and indicated (aOR, 1.34) preterm birth. Weight gain above the guidelines was associated with a greater risk of indicated preterm birth only (aOR, 1.24).
The study population included 29,861 women at 25 hospitals over a 3-year period. Of these, 51% had gestational weight gains above the amount recommended by the IOM guidelines and 21% had gestational weight gains below it. The researchers calculated gestational weight gain by subtracting prepregnancy weight from delivery weight or, if prepregnancy weight was not available, by subtracting weight at the first prenatal visit at 13 weeks of gestation or earlier from delivery weight.
The study findings were limited by the use of self-reported prepregnancy weight and the possible effects of changes to the guidelines with respect to obese patients, the researchers said. However, the results support those from previous studies, and the “noted strengths include analysis of 29,861 women representative of the United States with rigorous ascertainment of outcomes and calculation of gestational weight gain to account for the wide range of gestational ages at delivery,” Dr. Kominiarek and her associates wrote.
Overall, the data support efforts to educate women on health behaviors and how gestational weight gain affects them and their infants, and additional research is needed to help women meet their goals for appropriate gestational weight, the researchers concluded.
SOURCE: Kominiarek MA et al. Obstet Gynecol. 2018 Oct;132(4):875-81.
“We are struggling with an obesity epidemic in this country, and pregnancy accounts for a risk time for women to gain excessive weight,” Martina L. Badell, MD, said in an interview. “This is a very well-designed large study which attempted to systematically evaluate the adverse perinatal outcomes associated with inappropriate weight gain in pregnancy across a diverse group of women.”
She emphasized that “the take home message is the importance of counseling regarding weight gain in pregnancy and monitoring it closely in real time as the associated risks are significant and potentially avoidable. The first step to solving a problem is adequately quantifying it, and this study does just that. The next step is giving this information to pregnant women along with making weight gain a part of the discussion prior to pregnancy and at every prenatal visit.”
Dr. Badell added, “Ideally, the weight gain for an individual pregnant women would be tracked and discussed with her during each prenatal visit. If she is below or above the recommendations, the risks associated with this could be discussed along with strategies to get/stay on track. In an ideal world, women struggling with weight gain goals in pregnancy would have access to a dietitian. However, in reality, ob.gyn. offices will likely need to come up with patient education handouts or staff education.”
Another useful avenue for research would be assessing the effects of patient education, Dr. Badell said. “The next best step would be implementing a study to assess if education of women during pregnancy about their individual weight gain at each visit and discussion regarding perinatal risks affects ultimate weight gain and reduces risks. Additionally, education could begin in the preconception phase as this knowledge is likely important even prior to pregnancy. Finally, studies are needed on interventions such as working with dietitians or patient education classes once a woman has been identified as not being within weight gain goals to evaluate if these can alter weight gain and improve outcomes.”
Dr. Badell is a maternal-fetal medicine specialist in the department of gynecology and obstetrics at Emory University, Atlanta. She was asked to comment on the findings of Kominiarek MA et al. Dr. Badell had no relevant financial conflicts to disclose.
“We are struggling with an obesity epidemic in this country, and pregnancy accounts for a risk time for women to gain excessive weight,” Martina L. Badell, MD, said in an interview. “This is a very well-designed large study which attempted to systematically evaluate the adverse perinatal outcomes associated with inappropriate weight gain in pregnancy across a diverse group of women.”
She emphasized that “the take home message is the importance of counseling regarding weight gain in pregnancy and monitoring it closely in real time as the associated risks are significant and potentially avoidable. The first step to solving a problem is adequately quantifying it, and this study does just that. The next step is giving this information to pregnant women along with making weight gain a part of the discussion prior to pregnancy and at every prenatal visit.”
Dr. Badell added, “Ideally, the weight gain for an individual pregnant women would be tracked and discussed with her during each prenatal visit. If she is below or above the recommendations, the risks associated with this could be discussed along with strategies to get/stay on track. In an ideal world, women struggling with weight gain goals in pregnancy would have access to a dietitian. However, in reality, ob.gyn. offices will likely need to come up with patient education handouts or staff education.”
Another useful avenue for research would be assessing the effects of patient education, Dr. Badell said. “The next best step would be implementing a study to assess if education of women during pregnancy about their individual weight gain at each visit and discussion regarding perinatal risks affects ultimate weight gain and reduces risks. Additionally, education could begin in the preconception phase as this knowledge is likely important even prior to pregnancy. Finally, studies are needed on interventions such as working with dietitians or patient education classes once a woman has been identified as not being within weight gain goals to evaluate if these can alter weight gain and improve outcomes.”
Dr. Badell is a maternal-fetal medicine specialist in the department of gynecology and obstetrics at Emory University, Atlanta. She was asked to comment on the findings of Kominiarek MA et al. Dr. Badell had no relevant financial conflicts to disclose.
“We are struggling with an obesity epidemic in this country, and pregnancy accounts for a risk time for women to gain excessive weight,” Martina L. Badell, MD, said in an interview. “This is a very well-designed large study which attempted to systematically evaluate the adverse perinatal outcomes associated with inappropriate weight gain in pregnancy across a diverse group of women.”
She emphasized that “the take home message is the importance of counseling regarding weight gain in pregnancy and monitoring it closely in real time as the associated risks are significant and potentially avoidable. The first step to solving a problem is adequately quantifying it, and this study does just that. The next step is giving this information to pregnant women along with making weight gain a part of the discussion prior to pregnancy and at every prenatal visit.”
Dr. Badell added, “Ideally, the weight gain for an individual pregnant women would be tracked and discussed with her during each prenatal visit. If she is below or above the recommendations, the risks associated with this could be discussed along with strategies to get/stay on track. In an ideal world, women struggling with weight gain goals in pregnancy would have access to a dietitian. However, in reality, ob.gyn. offices will likely need to come up with patient education handouts or staff education.”
Another useful avenue for research would be assessing the effects of patient education, Dr. Badell said. “The next best step would be implementing a study to assess if education of women during pregnancy about their individual weight gain at each visit and discussion regarding perinatal risks affects ultimate weight gain and reduces risks. Additionally, education could begin in the preconception phase as this knowledge is likely important even prior to pregnancy. Finally, studies are needed on interventions such as working with dietitians or patient education classes once a woman has been identified as not being within weight gain goals to evaluate if these can alter weight gain and improve outcomes.”
Dr. Badell is a maternal-fetal medicine specialist in the department of gynecology and obstetrics at Emory University, Atlanta. She was asked to comment on the findings of Kominiarek MA et al. Dr. Badell had no relevant financial conflicts to disclose.
Gestational weight gain above or below the level recommended by the Institute of Medicine (IOM) guidelines resulted in significantly worse outcomes for mothers and babies, according to data from nearly 30,000 women.
Previous studies of the relationship between gestational weight gain and maternal and neonatal outcomes have been limited by “small sample sizes, single sites, restricted reporting of outcomes, and a lack of racial-ethnic diversity,” Michelle A. Kominiarek, MD, of Northwestern University in Chicago and her colleagues wrote . To determine the effects of gestational weight gain on a large and more diverse population, the researchers conducted a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network’s Assessment of Perinatal Excellence study. The findings were published in Obstetrics & Gynecology.
Gestational weight gain above the amount recommended by IOM guidelines was significantly associated with adverse outcomes in neonates, including macrosomia (adjusted odds ratio, 2.66), shoulder dystocia (aOR, 1.74), and neonatal hypoglycemia (aOR, 1.60).
In further multivariate analysis, adverse maternal outcomes associated with gestational weight gain above that recommended by the guidelines included hypertensive diseases of pregnancy for any parity (aOR, 1.84) and increased risk of cesarean delivery in nulliparous and multiparous women (aORs, 1.44 and 1.26, respectively).
Gestational weight gain below the recommended amount was associated with both spontaneous (aOR, 1.50) and indicated (aOR, 1.34) preterm birth. Weight gain above the guidelines was associated with a greater risk of indicated preterm birth only (aOR, 1.24).
The study population included 29,861 women at 25 hospitals over a 3-year period. Of these, 51% had gestational weight gains above the amount recommended by the IOM guidelines and 21% had gestational weight gains below it. The researchers calculated gestational weight gain by subtracting prepregnancy weight from delivery weight or, if prepregnancy weight was not available, by subtracting weight at the first prenatal visit at 13 weeks of gestation or earlier from delivery weight.
The study findings were limited by the use of self-reported prepregnancy weight and the possible effects of changes to the guidelines with respect to obese patients, the researchers said. However, the results support those from previous studies, and the “noted strengths include analysis of 29,861 women representative of the United States with rigorous ascertainment of outcomes and calculation of gestational weight gain to account for the wide range of gestational ages at delivery,” Dr. Kominiarek and her associates wrote.
Overall, the data support efforts to educate women on health behaviors and how gestational weight gain affects them and their infants, and additional research is needed to help women meet their goals for appropriate gestational weight, the researchers concluded.
SOURCE: Kominiarek MA et al. Obstet Gynecol. 2018 Oct;132(4):875-81.
Gestational weight gain above or below the level recommended by the Institute of Medicine (IOM) guidelines resulted in significantly worse outcomes for mothers and babies, according to data from nearly 30,000 women.
Previous studies of the relationship between gestational weight gain and maternal and neonatal outcomes have been limited by “small sample sizes, single sites, restricted reporting of outcomes, and a lack of racial-ethnic diversity,” Michelle A. Kominiarek, MD, of Northwestern University in Chicago and her colleagues wrote . To determine the effects of gestational weight gain on a large and more diverse population, the researchers conducted a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network’s Assessment of Perinatal Excellence study. The findings were published in Obstetrics & Gynecology.
Gestational weight gain above the amount recommended by IOM guidelines was significantly associated with adverse outcomes in neonates, including macrosomia (adjusted odds ratio, 2.66), shoulder dystocia (aOR, 1.74), and neonatal hypoglycemia (aOR, 1.60).
In further multivariate analysis, adverse maternal outcomes associated with gestational weight gain above that recommended by the guidelines included hypertensive diseases of pregnancy for any parity (aOR, 1.84) and increased risk of cesarean delivery in nulliparous and multiparous women (aORs, 1.44 and 1.26, respectively).
Gestational weight gain below the recommended amount was associated with both spontaneous (aOR, 1.50) and indicated (aOR, 1.34) preterm birth. Weight gain above the guidelines was associated with a greater risk of indicated preterm birth only (aOR, 1.24).
The study population included 29,861 women at 25 hospitals over a 3-year period. Of these, 51% had gestational weight gains above the amount recommended by the IOM guidelines and 21% had gestational weight gains below it. The researchers calculated gestational weight gain by subtracting prepregnancy weight from delivery weight or, if prepregnancy weight was not available, by subtracting weight at the first prenatal visit at 13 weeks of gestation or earlier from delivery weight.
The study findings were limited by the use of self-reported prepregnancy weight and the possible effects of changes to the guidelines with respect to obese patients, the researchers said. However, the results support those from previous studies, and the “noted strengths include analysis of 29,861 women representative of the United States with rigorous ascertainment of outcomes and calculation of gestational weight gain to account for the wide range of gestational ages at delivery,” Dr. Kominiarek and her associates wrote.
Overall, the data support efforts to educate women on health behaviors and how gestational weight gain affects them and their infants, and additional research is needed to help women meet their goals for appropriate gestational weight, the researchers concluded.
SOURCE: Kominiarek MA et al. Obstet Gynecol. 2018 Oct;132(4):875-81.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Gestational weight gain or loss is a significant risk factor for adverse maternal and neonatal outcomes.
Major finding: Gestational weight gain above the recommended amount was significantly associated with adverse outcomes in neonates, including macrosomia (adjusted odds ratio, 2.66), shoulder dystocia (aOR, 1.74), and neonatal hypoglycemia (aOR, 1.60).
Study details: The data came from 29,861 women who delivered at 25 hospitals across the United States on randomly selected days during a 3-year period.
Disclosures: The researchers had no financial conflicts to disclose. The study was supported in part by various grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Center for Research Resources.
Source: Kominiarek MA et al. Obstet Gynecol. 2018 Oct;132(4):875-81.
Congenital syphilis rates continue skyrocketing alongside other STDs
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Rapidly increasing cases of newborn syphilis have reached their highest prevalence in 2 decades, according to a new report by the Centers for Disease Control and Prevention on sexually transmitted disease surveillance in 2017.
Newborn syphilis incidence has more than doubled, from 362 cases in 2013 to 918 cases in 2017, resulting in 64 syphilitic stillbirths and 13 infant deaths that year, according to data published in Sexually Transmitted Disease Surveillance 2017.
At least one case was reported in 37 states last year, and the greatest burden of cases occurred in California, Arizona, Texas, Louisiana, and Florida, together accounting for 70% of all 2017 cases.
“The resurgence of syphilis, and particularly congenital syphilis, is not an arbitrary event, but rather a symptom of a deteriorating public health infrastructure and lack of access to health care,” wrote Gail Bolan, MD, director of the Division of STD Prevention at the CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD and TB Prevention. “It is exposing hidden, fragile populations in need that are not getting the health care and preventive services they deserve.”
Dr. Bolan recommends modernizing surveillance to capture more of the cases in populations without ready access to diagnosis and treatment and in those choosing not to access care.
“It is imperative that federal, state, and local programs employ strategies that maximize long-term population impact by reducing STD incidence and promoting sexual, reproductive, maternal, and infant health,” she wrote. “Further, it will be important for us to measure and monitor the adverse health consequences of STDs, such as ocular and neurosyphilis, pelvic inflammatory disease, ectopic pregnancy, infertility, HIV, congenital syphilis, and neonatal herpes.”
Multiple sources contributed data to the report: state and local STD programs’ notifiable disease reporting, private and federal national surveys, and specific projects that collect STD prevalence data, including the National Job Training Program, the STD Surveillance Network and the Gonococcal Isolate Surveillance Project.
The four nationally notifiable STDs are chlamydia, gonorrhea, syphilis, and chancroid.
The rise in newborn syphilis cases, currently at 23.3 cases per 100,000 live births, mirrors the increased U.S. prevalence of both primary and secondary syphilis in 2017, with 9.5 cases per 100,000 people. Syphilis has increased every year since 2000-2001, when prevalence was at a record low.
Chlamydia and gonorrhea rates climb too
The report also noted increases in the prevalence of other STDs. Chlamydia, the most common STD, increased 6.9% as compared to 2016, with 528.8 cases per 100,000 people. This increase occurred in all U.S. regions and independently of sex, race, or ethnicity, though rates were highest in teens and young adults. Nearly two-thirds of chlamydia cases in 2017 occurred in people ages 15-24 years old.
Reported rates were higher in women than in men, likely due to women’s increased likelihood of undergoing screening, the report suggested. Better surveillance may also partly explain the climb in men’s cases.
“Increases in rates among men may reflect an increased number of men, including gay, bisexual and other men who have sex with men (collectively referred to as MSM) being tested and diagnosed with a chlamydial infection due to increased availability of urine testing and extragenital screening,” according to the report.
The CDC received reports of more than a half million gonorrhea infections in 2017 (555,608 cases), an increase of 18.6% since the previous year, including a 19.3% increase among men and a 17.8% increase among women.
“The magnitude of the increase among men suggests either increased transmission, increased case ascertainment (e.g., through increased extra-genital screening among MSM), or both,” the authors wrote. “The concurrent increase in cases reported among women suggests parallel increases in heterosexual transmission, increased screening among women, or both.”
Overall, gonorrhea cases have skyrocketed 75.2% since their historic low in 2009, compounding the problem of antibiotic resistance that has limited CDC-recommended treatment to just ceftriaxone and azithromycin.
The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
SOURCE: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017; https://www.cdc.gov/std/stats
Key clinical point: Newborn syphilis cases have more than doubled in 5 years along with substantial increases in chlamydia, gonorrhea, and syphilis.
Major finding: 918 cases of newborn syphilis were reported in 37 states in 2017.
Study details: The findings are based on data from public health notifiable disease reports and multiple federal and private surveillance projects.
Disclosures: The report was supported by the Centers for Disease Control and Prevention. The authors did not report having any disclosures.
Source: Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2017.
Obesity plays role in sleep-disordered breathing in pregnancy
The relationship between hypertension during pregnancy and sleep-disordered breathing may be partly mediated by obesity, new research suggests.
An article published in the Journal of Sleep Research details the results of a case-control study in 80 pregnant women – 40 normotensive and 40 with either gestational hypertension or preeclampsia – who were matched on body mass index.
Nearly half of the women in the study (45%) met the criteria for sleep-disordered breathing – defined as a respiratory disturbance index of 5 or above. The incidence was higher among women with hypertension (53%) than among women in the normotensive control group (38%), but the difference was not statistically significant.
There were also no significant differences in median respiratory disturbance index or apnea-hypopnea index between the hypertension and control groups.
However, the incidence of more severe sleep-disordered breathing – a respiratory disturbance index of at least 10 – was significantly greater in the hypertensive group (35% vs. 14%; P = .04). The women with pregnancy-related hypertension also had significantly higher respiratory disturbance index during non–rapid eye movement sleep and when they were sleeping on their back.
The severity of hypertensive disease did not affect the prevalence of sleep-disordered breathing.
Danielle L. Wilson of the Institute for Breathing and Sleep at Austin Health in Melbourne and her coauthors wrote that, while previous research has pointed to a link between hypertension in pregnancy and sleep-disordered breathing, this is the first study to explore the potential confounding role of obesity.
“We found SDB [sleep-disordered breathing] to be more common in our control group than in previous studies, confirming that BMI is an important covariate that requires evaluation in future studies exploring the relationship between SDB and HDP [hypertension during pregnancy],” they reported. “SDB may be a mechanism by which obesity and adverse perinatal outcomes are linked, but given the important contribution of obesity to both SDB and HDP, failing to adjust for this covariate will overestimate the strength of association between SDB and HDP.”
However, they acknowledged there was a significant association between moderate to severe sleep-disordered breathing and hypertension in pregnancy. They suggested this might be a means to increase women’s uptake of clinical review with a sleep physician, which was very low in the study despite it being offered to all women.
“Better engagement may be more likely for women with more severe disease if stronger links with adverse pregnancy outcome are demonstrated,” they wrote.
The study was supported by the Austin Medical Research Foundation and the Medical Research Foundation for Women and Babies. One author declared a scholarship from a research funding body, and two declared unrelated research support from private industry. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Sleep Research. 2018 Oct;27(5):e12656.
The relationship between hypertension during pregnancy and sleep-disordered breathing may be partly mediated by obesity, new research suggests.
An article published in the Journal of Sleep Research details the results of a case-control study in 80 pregnant women – 40 normotensive and 40 with either gestational hypertension or preeclampsia – who were matched on body mass index.
Nearly half of the women in the study (45%) met the criteria for sleep-disordered breathing – defined as a respiratory disturbance index of 5 or above. The incidence was higher among women with hypertension (53%) than among women in the normotensive control group (38%), but the difference was not statistically significant.
There were also no significant differences in median respiratory disturbance index or apnea-hypopnea index between the hypertension and control groups.
However, the incidence of more severe sleep-disordered breathing – a respiratory disturbance index of at least 10 – was significantly greater in the hypertensive group (35% vs. 14%; P = .04). The women with pregnancy-related hypertension also had significantly higher respiratory disturbance index during non–rapid eye movement sleep and when they were sleeping on their back.
The severity of hypertensive disease did not affect the prevalence of sleep-disordered breathing.
Danielle L. Wilson of the Institute for Breathing and Sleep at Austin Health in Melbourne and her coauthors wrote that, while previous research has pointed to a link between hypertension in pregnancy and sleep-disordered breathing, this is the first study to explore the potential confounding role of obesity.
“We found SDB [sleep-disordered breathing] to be more common in our control group than in previous studies, confirming that BMI is an important covariate that requires evaluation in future studies exploring the relationship between SDB and HDP [hypertension during pregnancy],” they reported. “SDB may be a mechanism by which obesity and adverse perinatal outcomes are linked, but given the important contribution of obesity to both SDB and HDP, failing to adjust for this covariate will overestimate the strength of association between SDB and HDP.”
However, they acknowledged there was a significant association between moderate to severe sleep-disordered breathing and hypertension in pregnancy. They suggested this might be a means to increase women’s uptake of clinical review with a sleep physician, which was very low in the study despite it being offered to all women.
“Better engagement may be more likely for women with more severe disease if stronger links with adverse pregnancy outcome are demonstrated,” they wrote.
The study was supported by the Austin Medical Research Foundation and the Medical Research Foundation for Women and Babies. One author declared a scholarship from a research funding body, and two declared unrelated research support from private industry. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Sleep Research. 2018 Oct;27(5):e12656.
The relationship between hypertension during pregnancy and sleep-disordered breathing may be partly mediated by obesity, new research suggests.
An article published in the Journal of Sleep Research details the results of a case-control study in 80 pregnant women – 40 normotensive and 40 with either gestational hypertension or preeclampsia – who were matched on body mass index.
Nearly half of the women in the study (45%) met the criteria for sleep-disordered breathing – defined as a respiratory disturbance index of 5 or above. The incidence was higher among women with hypertension (53%) than among women in the normotensive control group (38%), but the difference was not statistically significant.
There were also no significant differences in median respiratory disturbance index or apnea-hypopnea index between the hypertension and control groups.
However, the incidence of more severe sleep-disordered breathing – a respiratory disturbance index of at least 10 – was significantly greater in the hypertensive group (35% vs. 14%; P = .04). The women with pregnancy-related hypertension also had significantly higher respiratory disturbance index during non–rapid eye movement sleep and when they were sleeping on their back.
The severity of hypertensive disease did not affect the prevalence of sleep-disordered breathing.
Danielle L. Wilson of the Institute for Breathing and Sleep at Austin Health in Melbourne and her coauthors wrote that, while previous research has pointed to a link between hypertension in pregnancy and sleep-disordered breathing, this is the first study to explore the potential confounding role of obesity.
“We found SDB [sleep-disordered breathing] to be more common in our control group than in previous studies, confirming that BMI is an important covariate that requires evaluation in future studies exploring the relationship between SDB and HDP [hypertension during pregnancy],” they reported. “SDB may be a mechanism by which obesity and adverse perinatal outcomes are linked, but given the important contribution of obesity to both SDB and HDP, failing to adjust for this covariate will overestimate the strength of association between SDB and HDP.”
However, they acknowledged there was a significant association between moderate to severe sleep-disordered breathing and hypertension in pregnancy. They suggested this might be a means to increase women’s uptake of clinical review with a sleep physician, which was very low in the study despite it being offered to all women.
“Better engagement may be more likely for women with more severe disease if stronger links with adverse pregnancy outcome are demonstrated,” they wrote.
The study was supported by the Austin Medical Research Foundation and the Medical Research Foundation for Women and Babies. One author declared a scholarship from a research funding body, and two declared unrelated research support from private industry. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Sleep Research. 2018 Oct;27(5):e12656.
FROM JOURNAL OF SLEEP RESEARCH
Key clinical point:
Major finding: The relationship between hypertension in pregnancy and sleep-disordered breathing is only significant for more severe sleep-disordered breathing.
Study details: Prospective case-control study in 80 pregnant women.
Disclosures: The study was supported by the Austin Medical Research Foundation and the Medical Research Foundation for Women and Babies. One author declared a scholarship from a research funding body, and two declared unrelated research support from private industry. No other conflicts of interest were declared.
Source: Wilson D et al. J Sleep Research. 2018 Oct;27(5):e12656.