User login
Patients with migraine face an elevated risk for Parkinson's disease
Key clinical point: Patients with migraine have increased risk for incident Parkinson's disease (PD), with younger age and underlying dyslipidemia aggravating the risk for PD among women and men with migraine, respectively.
Major finding: The risk of incident PD was 1.35-fold (adjusted hazard ratio 1.35; 95% CI 1.29-1.41) higher in patients with vs without migraine, with the risk of PD being significantly higher among younger vs older women (age < 65 years vs ≥ 65 years; P = .038) and men with vs without dyslipidemia (P = .012).
Study details: This retrospective, nationwide, population-based cohort study included 214,193 individuals with migraine and 5,879,711 individuals without migraine, of whom 1973 (0.92%) and 30,664 (0.52%) individuals with and without migraine, respectively, were diagnosed with PD.
Disclosures: This research was supported by a grant from the National Research Foundation, Technology Development Program, and Technology Innovation Program. Korea. The authors declared no conflicts of interest.
Source: Ha WS et al. The association between migraine and Parkinson's disease: A nationwide cohort study. Epidemiol Health. 2023 (Dec 18). doi: 10.4178/epih.e2024010
Key clinical point: Patients with migraine have increased risk for incident Parkinson's disease (PD), with younger age and underlying dyslipidemia aggravating the risk for PD among women and men with migraine, respectively.
Major finding: The risk of incident PD was 1.35-fold (adjusted hazard ratio 1.35; 95% CI 1.29-1.41) higher in patients with vs without migraine, with the risk of PD being significantly higher among younger vs older women (age < 65 years vs ≥ 65 years; P = .038) and men with vs without dyslipidemia (P = .012).
Study details: This retrospective, nationwide, population-based cohort study included 214,193 individuals with migraine and 5,879,711 individuals without migraine, of whom 1973 (0.92%) and 30,664 (0.52%) individuals with and without migraine, respectively, were diagnosed with PD.
Disclosures: This research was supported by a grant from the National Research Foundation, Technology Development Program, and Technology Innovation Program. Korea. The authors declared no conflicts of interest.
Source: Ha WS et al. The association between migraine and Parkinson's disease: A nationwide cohort study. Epidemiol Health. 2023 (Dec 18). doi: 10.4178/epih.e2024010
Key clinical point: Patients with migraine have increased risk for incident Parkinson's disease (PD), with younger age and underlying dyslipidemia aggravating the risk for PD among women and men with migraine, respectively.
Major finding: The risk of incident PD was 1.35-fold (adjusted hazard ratio 1.35; 95% CI 1.29-1.41) higher in patients with vs without migraine, with the risk of PD being significantly higher among younger vs older women (age < 65 years vs ≥ 65 years; P = .038) and men with vs without dyslipidemia (P = .012).
Study details: This retrospective, nationwide, population-based cohort study included 214,193 individuals with migraine and 5,879,711 individuals without migraine, of whom 1973 (0.92%) and 30,664 (0.52%) individuals with and without migraine, respectively, were diagnosed with PD.
Disclosures: This research was supported by a grant from the National Research Foundation, Technology Development Program, and Technology Innovation Program. Korea. The authors declared no conflicts of interest.
Source: Ha WS et al. The association between migraine and Parkinson's disease: A nationwide cohort study. Epidemiol Health. 2023 (Dec 18). doi: 10.4178/epih.e2024010
Meta-analysis shows comorbid association between migraine and epilepsy
Key clinical point: In this meta-analysis, migraine was more frequent among patients with vs without epilepsy and epilepsy was more frequent among patients with vs without migraine, highlighting a co-morbid association between migraine and epilepsy.
Major finding: The lifetime prevalence of migraine was 80% higher in patients with epilepsy compared with those without epilepsy (odds ratio [OR]/relative risk [RR] 1.80; P < .001). Similarly, the lifetime prevalence of epilepsy was 80% higher in patients with migraine compared with those without migraine (OR/RR 1.80; P < .001).
Study details: The data come from a meta-analysis of 13 studies that evaluated the association between migraine and epilepsy.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Wu X and Zhuang J. Association between migraine and epilepsy: A meta-analysis. Front Neurol. 2024;14:1276663 (Jan 5). doi: 10.3389/fneur.2023.1276663
Key clinical point: In this meta-analysis, migraine was more frequent among patients with vs without epilepsy and epilepsy was more frequent among patients with vs without migraine, highlighting a co-morbid association between migraine and epilepsy.
Major finding: The lifetime prevalence of migraine was 80% higher in patients with epilepsy compared with those without epilepsy (odds ratio [OR]/relative risk [RR] 1.80; P < .001). Similarly, the lifetime prevalence of epilepsy was 80% higher in patients with migraine compared with those without migraine (OR/RR 1.80; P < .001).
Study details: The data come from a meta-analysis of 13 studies that evaluated the association between migraine and epilepsy.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Wu X and Zhuang J. Association between migraine and epilepsy: A meta-analysis. Front Neurol. 2024;14:1276663 (Jan 5). doi: 10.3389/fneur.2023.1276663
Key clinical point: In this meta-analysis, migraine was more frequent among patients with vs without epilepsy and epilepsy was more frequent among patients with vs without migraine, highlighting a co-morbid association between migraine and epilepsy.
Major finding: The lifetime prevalence of migraine was 80% higher in patients with epilepsy compared with those without epilepsy (odds ratio [OR]/relative risk [RR] 1.80; P < .001). Similarly, the lifetime prevalence of epilepsy was 80% higher in patients with migraine compared with those without migraine (OR/RR 1.80; P < .001).
Study details: The data come from a meta-analysis of 13 studies that evaluated the association between migraine and epilepsy.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Wu X and Zhuang J. Association between migraine and epilepsy: A meta-analysis. Front Neurol. 2024;14:1276663 (Jan 5). doi: 10.3389/fneur.2023.1276663
Erenumab demonstrates more favorable efficacy than rimegepant for migraine prevention
Key clinical point: Erenumab demonstrated a more favorable efficacy profile than rimegepant for the prevention of episodic and chronic migraine.
Major finding: Compared with 75 mg rimegepant, 70 mg erenumab significantly reduced monthly migraine days (MMD) by 0.90 days at 3 months (P = .042) and 140 mg erenumab significantly reduced MMD by 0.94 (P = .014) and 1.28 (P = .005) days at 1 month and 3 months, respectively. Erenumab showed advantages over rimegepant in improving Migraine-Specific Quality-of-life role function-restrictive domain and Migraine Disability Assessment scores (MIDAS) at 3 months.
Study details: This study performed anchored matching-adjusted indirect comparison of the relative efficacy of two erenumab regimens (70 mg and 140 mg) with rimegepant (75 mg) for migraine prevention using data from two phase 2/3 trials for erenumab (295 and STRIVE) and a phase 2/3 trial for rimegepant.
Disclosures: This study was funded by Novartis Healthcare Pvt. Ltd. Several authors declared being employees of and holding stocks or stock options in Novartis.
Source: Mahon R et al. Comparative effectiveness of erenumab versus rimegepant for migraine prevention using matching-adjusted indirect comparison. J Comp Eff Res. 2024 (Jan 4). doi: 10.57264/cer-2023-0122
Key clinical point: Erenumab demonstrated a more favorable efficacy profile than rimegepant for the prevention of episodic and chronic migraine.
Major finding: Compared with 75 mg rimegepant, 70 mg erenumab significantly reduced monthly migraine days (MMD) by 0.90 days at 3 months (P = .042) and 140 mg erenumab significantly reduced MMD by 0.94 (P = .014) and 1.28 (P = .005) days at 1 month and 3 months, respectively. Erenumab showed advantages over rimegepant in improving Migraine-Specific Quality-of-life role function-restrictive domain and Migraine Disability Assessment scores (MIDAS) at 3 months.
Study details: This study performed anchored matching-adjusted indirect comparison of the relative efficacy of two erenumab regimens (70 mg and 140 mg) with rimegepant (75 mg) for migraine prevention using data from two phase 2/3 trials for erenumab (295 and STRIVE) and a phase 2/3 trial for rimegepant.
Disclosures: This study was funded by Novartis Healthcare Pvt. Ltd. Several authors declared being employees of and holding stocks or stock options in Novartis.
Source: Mahon R et al. Comparative effectiveness of erenumab versus rimegepant for migraine prevention using matching-adjusted indirect comparison. J Comp Eff Res. 2024 (Jan 4). doi: 10.57264/cer-2023-0122
Key clinical point: Erenumab demonstrated a more favorable efficacy profile than rimegepant for the prevention of episodic and chronic migraine.
Major finding: Compared with 75 mg rimegepant, 70 mg erenumab significantly reduced monthly migraine days (MMD) by 0.90 days at 3 months (P = .042) and 140 mg erenumab significantly reduced MMD by 0.94 (P = .014) and 1.28 (P = .005) days at 1 month and 3 months, respectively. Erenumab showed advantages over rimegepant in improving Migraine-Specific Quality-of-life role function-restrictive domain and Migraine Disability Assessment scores (MIDAS) at 3 months.
Study details: This study performed anchored matching-adjusted indirect comparison of the relative efficacy of two erenumab regimens (70 mg and 140 mg) with rimegepant (75 mg) for migraine prevention using data from two phase 2/3 trials for erenumab (295 and STRIVE) and a phase 2/3 trial for rimegepant.
Disclosures: This study was funded by Novartis Healthcare Pvt. Ltd. Several authors declared being employees of and holding stocks or stock options in Novartis.
Source: Mahon R et al. Comparative effectiveness of erenumab versus rimegepant for migraine prevention using matching-adjusted indirect comparison. J Comp Eff Res. 2024 (Jan 4). doi: 10.57264/cer-2023-0122
Genetic factors influence response to anti-CGRP antibodies in migraine
Key clinical point: Monoclonal antibodies targeting the anti-calcitonin gene-related peptide (CGRP) showed persistent and comparable outcomes within a real-world cohort of patients with migraine leading to a reduction in migraine days per month (MDM) among responders with large effect sizes; however, the response was influenced by genetic factors.
Major finding: Patients responding to anti-CGRP monoclonal antibodies demonstrated persistent reduction in MDM (usually ≥50% reduction from baseline) at first (η2 = 0.26) and second (η2 = 0.22) follow-up, with all treatments showing similar effects and large effect sizes. Non-responders vs responders had a lower mean genetic risk score (P = .041) without any difference in polygenic risk score.
Study details: This retrospective clinical and genetic study included 481 patients with migraine who were prescribed preventive erenumab (n = 166), galcanezumab (n = 164), or fremanezumab (n = 151).
Disclosures: This study was funded by the US Agency for Healthcare Research and Quality. S Meyers declared serving on the speakers’ bureau for Biohaven Pharmaceuticals and Allergan.
Source: Chase BA et al. Characteristics associated with response to subcutaneously administered anti-CGRP monoclonal antibody medications in a real-world community cohort of persons living with migraine: A retrospective clinical and genetic study. Headache. 2023 (Dec 10). doi:
Key clinical point: Monoclonal antibodies targeting the anti-calcitonin gene-related peptide (CGRP) showed persistent and comparable outcomes within a real-world cohort of patients with migraine leading to a reduction in migraine days per month (MDM) among responders with large effect sizes; however, the response was influenced by genetic factors.
Major finding: Patients responding to anti-CGRP monoclonal antibodies demonstrated persistent reduction in MDM (usually ≥50% reduction from baseline) at first (η2 = 0.26) and second (η2 = 0.22) follow-up, with all treatments showing similar effects and large effect sizes. Non-responders vs responders had a lower mean genetic risk score (P = .041) without any difference in polygenic risk score.
Study details: This retrospective clinical and genetic study included 481 patients with migraine who were prescribed preventive erenumab (n = 166), galcanezumab (n = 164), or fremanezumab (n = 151).
Disclosures: This study was funded by the US Agency for Healthcare Research and Quality. S Meyers declared serving on the speakers’ bureau for Biohaven Pharmaceuticals and Allergan.
Source: Chase BA et al. Characteristics associated with response to subcutaneously administered anti-CGRP monoclonal antibody medications in a real-world community cohort of persons living with migraine: A retrospective clinical and genetic study. Headache. 2023 (Dec 10). doi:
Key clinical point: Monoclonal antibodies targeting the anti-calcitonin gene-related peptide (CGRP) showed persistent and comparable outcomes within a real-world cohort of patients with migraine leading to a reduction in migraine days per month (MDM) among responders with large effect sizes; however, the response was influenced by genetic factors.
Major finding: Patients responding to anti-CGRP monoclonal antibodies demonstrated persistent reduction in MDM (usually ≥50% reduction from baseline) at first (η2 = 0.26) and second (η2 = 0.22) follow-up, with all treatments showing similar effects and large effect sizes. Non-responders vs responders had a lower mean genetic risk score (P = .041) without any difference in polygenic risk score.
Study details: This retrospective clinical and genetic study included 481 patients with migraine who were prescribed preventive erenumab (n = 166), galcanezumab (n = 164), or fremanezumab (n = 151).
Disclosures: This study was funded by the US Agency for Healthcare Research and Quality. S Meyers declared serving on the speakers’ bureau for Biohaven Pharmaceuticals and Allergan.
Source: Chase BA et al. Characteristics associated with response to subcutaneously administered anti-CGRP monoclonal antibody medications in a real-world community cohort of persons living with migraine: A retrospective clinical and genetic study. Headache. 2023 (Dec 10). doi:
Agomelatine offers an effective preventive treatment for episodic migraine without aura
Key clinical point: Agomelatine appeared to be an effective preventive treatment for episodic migraine without aura.
Major finding: After 3 months of treatment, patients receiving agomelatine vs placebo had significant reduction in headache frequency (4.8 vs 5.82; P = .009) and severity (─4.1 vs ─0.71; P < .001), mean monthly migraine days (8.86 vs 10.63; P = .025), and Migraine Disability Assessment Score (MIDAS; ─1.06 vs ─0.36; P < .001).
Study details: Findings are from a parallel randomized controlled trial that included 99 patients with episodic migraine without aura who were randomly assigned to receive either agomelatine (n = 49) or placebo (vitamin B1 tablets; n = 50).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Farzin K et al. The effectiveness of agomelatine on headache severity and frequency in episodic migraine without aura; a parallel randomized controlled trial study. BMC Neurol. 2024;24:2 (Jan 2). doi: 10.1186/s12883-023-03516-9
Key clinical point: Agomelatine appeared to be an effective preventive treatment for episodic migraine without aura.
Major finding: After 3 months of treatment, patients receiving agomelatine vs placebo had significant reduction in headache frequency (4.8 vs 5.82; P = .009) and severity (─4.1 vs ─0.71; P < .001), mean monthly migraine days (8.86 vs 10.63; P = .025), and Migraine Disability Assessment Score (MIDAS; ─1.06 vs ─0.36; P < .001).
Study details: Findings are from a parallel randomized controlled trial that included 99 patients with episodic migraine without aura who were randomly assigned to receive either agomelatine (n = 49) or placebo (vitamin B1 tablets; n = 50).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Farzin K et al. The effectiveness of agomelatine on headache severity and frequency in episodic migraine without aura; a parallel randomized controlled trial study. BMC Neurol. 2024;24:2 (Jan 2). doi: 10.1186/s12883-023-03516-9
Key clinical point: Agomelatine appeared to be an effective preventive treatment for episodic migraine without aura.
Major finding: After 3 months of treatment, patients receiving agomelatine vs placebo had significant reduction in headache frequency (4.8 vs 5.82; P = .009) and severity (─4.1 vs ─0.71; P < .001), mean monthly migraine days (8.86 vs 10.63; P = .025), and Migraine Disability Assessment Score (MIDAS; ─1.06 vs ─0.36; P < .001).
Study details: Findings are from a parallel randomized controlled trial that included 99 patients with episodic migraine without aura who were randomly assigned to receive either agomelatine (n = 49) or placebo (vitamin B1 tablets; n = 50).
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Farzin K et al. The effectiveness of agomelatine on headache severity and frequency in episodic migraine without aura; a parallel randomized controlled trial study. BMC Neurol. 2024;24:2 (Jan 2). doi: 10.1186/s12883-023-03516-9
Gut microbiota and migraine: Is there a link?
Key clinical point: Gut microbiota may have a potential casual association with migraine symptoms, with evidence indicating a connection between gut microbiota and increased or decreased risk for migraine.
Major finding: A higher abundance of genus Lactobacillus (inverse variance weighted [IVW] odds ratio [OR] 1.10; P = .004) and family Prevotellaceae (IVW OR 0.89; P = .02) were causally associated with a higher and lower risk for migraine, respectively.
Study details: This study used a two-sample Mendelian randomization framework to assess the causal effects of gut microbiota on migraine risk using 2651 single nucleotide polymorphisms as instrumental variables. Large-scale genome-wide association studies consisting of 18,340 participants from 24 cohorts provided the summary-level statistics for gut microbiota.
Disclosures: This study was funded by the National Key Research and Development Program. The authors declared no conflicts of interest.
Source: Meng X et al. Exploring the role of gut microbiota in migraine risk: A two-sample Mendelian randomization study. Scand J Gastroenterol. 2023 (Dec 27). doi: 10.1080/00365521.2023.2298370
Key clinical point: Gut microbiota may have a potential casual association with migraine symptoms, with evidence indicating a connection between gut microbiota and increased or decreased risk for migraine.
Major finding: A higher abundance of genus Lactobacillus (inverse variance weighted [IVW] odds ratio [OR] 1.10; P = .004) and family Prevotellaceae (IVW OR 0.89; P = .02) were causally associated with a higher and lower risk for migraine, respectively.
Study details: This study used a two-sample Mendelian randomization framework to assess the causal effects of gut microbiota on migraine risk using 2651 single nucleotide polymorphisms as instrumental variables. Large-scale genome-wide association studies consisting of 18,340 participants from 24 cohorts provided the summary-level statistics for gut microbiota.
Disclosures: This study was funded by the National Key Research and Development Program. The authors declared no conflicts of interest.
Source: Meng X et al. Exploring the role of gut microbiota in migraine risk: A two-sample Mendelian randomization study. Scand J Gastroenterol. 2023 (Dec 27). doi: 10.1080/00365521.2023.2298370
Key clinical point: Gut microbiota may have a potential casual association with migraine symptoms, with evidence indicating a connection between gut microbiota and increased or decreased risk for migraine.
Major finding: A higher abundance of genus Lactobacillus (inverse variance weighted [IVW] odds ratio [OR] 1.10; P = .004) and family Prevotellaceae (IVW OR 0.89; P = .02) were causally associated with a higher and lower risk for migraine, respectively.
Study details: This study used a two-sample Mendelian randomization framework to assess the causal effects of gut microbiota on migraine risk using 2651 single nucleotide polymorphisms as instrumental variables. Large-scale genome-wide association studies consisting of 18,340 participants from 24 cohorts provided the summary-level statistics for gut microbiota.
Disclosures: This study was funded by the National Key Research and Development Program. The authors declared no conflicts of interest.
Source: Meng X et al. Exploring the role of gut microbiota in migraine risk: A two-sample Mendelian randomization study. Scand J Gastroenterol. 2023 (Dec 27). doi: 10.1080/00365521.2023.2298370
Considering high-dose EPA/DHA as a primary option for migraine prevention
Key clinical point: High-dose eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA) supplementation demonstrated highest efficacy and acceptability among all studied treatments, emphasizing its potential as a first-choice treatment for migraine prophylaxis.
Major finding: Among all prophylactic treatments, high-dose EPA/DHA supplementation showed the highest decrease in migraine frequency (standardized mean difference [SMD] ─1.36; 95% CI ─2.32 to ─0.39) and severity (SMD ─2.23; 95% CI ─3.17 to ─1.30) and the most favorable acceptability rate (odds ratio 1.00; 95% CI 0.06 to 17.41) compared with placebo.
Study details: The data come from a network meta-analysis of 40 randomized controlled trials that included 6616 patients with either episodic or chronic migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Tseng PT et al. Efficacy of high dosage anti-inflammatory eicosapentaenoic acid/docosahexaenoic acid for migraine prophylaxis: A network meta-analysis. Adv Nutr. 2023;15(2):100163 (Dec 16). doi: 10.1016/j.advnut.2023.100163
Key clinical point: High-dose eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA) supplementation demonstrated highest efficacy and acceptability among all studied treatments, emphasizing its potential as a first-choice treatment for migraine prophylaxis.
Major finding: Among all prophylactic treatments, high-dose EPA/DHA supplementation showed the highest decrease in migraine frequency (standardized mean difference [SMD] ─1.36; 95% CI ─2.32 to ─0.39) and severity (SMD ─2.23; 95% CI ─3.17 to ─1.30) and the most favorable acceptability rate (odds ratio 1.00; 95% CI 0.06 to 17.41) compared with placebo.
Study details: The data come from a network meta-analysis of 40 randomized controlled trials that included 6616 patients with either episodic or chronic migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Tseng PT et al. Efficacy of high dosage anti-inflammatory eicosapentaenoic acid/docosahexaenoic acid for migraine prophylaxis: A network meta-analysis. Adv Nutr. 2023;15(2):100163 (Dec 16). doi: 10.1016/j.advnut.2023.100163
Key clinical point: High-dose eicosapentaenoic acid (EPA)/docosahexaenoic acid (DHA) supplementation demonstrated highest efficacy and acceptability among all studied treatments, emphasizing its potential as a first-choice treatment for migraine prophylaxis.
Major finding: Among all prophylactic treatments, high-dose EPA/DHA supplementation showed the highest decrease in migraine frequency (standardized mean difference [SMD] ─1.36; 95% CI ─2.32 to ─0.39) and severity (SMD ─2.23; 95% CI ─3.17 to ─1.30) and the most favorable acceptability rate (odds ratio 1.00; 95% CI 0.06 to 17.41) compared with placebo.
Study details: The data come from a network meta-analysis of 40 randomized controlled trials that included 6616 patients with either episodic or chronic migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Tseng PT et al. Efficacy of high dosage anti-inflammatory eicosapentaenoic acid/docosahexaenoic acid for migraine prophylaxis: A network meta-analysis. Adv Nutr. 2023;15(2):100163 (Dec 16). doi: 10.1016/j.advnut.2023.100163
Elevated odds of motor vehicle crashes in older adults after newly diagnosed migraine
Key clinical point: The likelihood of motor vehicle crashes (MVC) in the year following diagnosis is significantly higher among older drivers who have recently been diagnosed with migraine, emphasizing the necessity for driving safety interventions for these individuals.
Major finding: The risk for an MVC in the year after migraine onset was > 3 times higher among older adult drivers with new-onset migraine compared with those who never had a migraine (adjusted odds ratio [aOR] 3.27; P = .019). Prevalent migraine was not associated with MVC in the subsequent 2 years (aOR 0.97; P = .88).
Study details: Findings are from the AAA Longitudinal Research on Aging Drivers study, which included 2589 older drivers (age 65-79 years) with a valid driver’s license, of whom 12.5% and 1.3% reported prevalent and incident migraines, respectively.
Disclosures: This study was funded by the AAA Foundation for Traffic Safety and National Center for Advancing Translational Sciences. The authors declared no conflicts of interest.
Source: DiGuiseppi CG et al. Migraine headaches are associated with motor vehicle crashes and driving habits among older drivers: Prospective cohort study. J Am Geriatr Soc. 2023 (Dec 22). doi: 10.1111/jgs.18719
Key clinical point: The likelihood of motor vehicle crashes (MVC) in the year following diagnosis is significantly higher among older drivers who have recently been diagnosed with migraine, emphasizing the necessity for driving safety interventions for these individuals.
Major finding: The risk for an MVC in the year after migraine onset was > 3 times higher among older adult drivers with new-onset migraine compared with those who never had a migraine (adjusted odds ratio [aOR] 3.27; P = .019). Prevalent migraine was not associated with MVC in the subsequent 2 years (aOR 0.97; P = .88).
Study details: Findings are from the AAA Longitudinal Research on Aging Drivers study, which included 2589 older drivers (age 65-79 years) with a valid driver’s license, of whom 12.5% and 1.3% reported prevalent and incident migraines, respectively.
Disclosures: This study was funded by the AAA Foundation for Traffic Safety and National Center for Advancing Translational Sciences. The authors declared no conflicts of interest.
Source: DiGuiseppi CG et al. Migraine headaches are associated with motor vehicle crashes and driving habits among older drivers: Prospective cohort study. J Am Geriatr Soc. 2023 (Dec 22). doi: 10.1111/jgs.18719
Key clinical point: The likelihood of motor vehicle crashes (MVC) in the year following diagnosis is significantly higher among older drivers who have recently been diagnosed with migraine, emphasizing the necessity for driving safety interventions for these individuals.
Major finding: The risk for an MVC in the year after migraine onset was > 3 times higher among older adult drivers with new-onset migraine compared with those who never had a migraine (adjusted odds ratio [aOR] 3.27; P = .019). Prevalent migraine was not associated with MVC in the subsequent 2 years (aOR 0.97; P = .88).
Study details: Findings are from the AAA Longitudinal Research on Aging Drivers study, which included 2589 older drivers (age 65-79 years) with a valid driver’s license, of whom 12.5% and 1.3% reported prevalent and incident migraines, respectively.
Disclosures: This study was funded by the AAA Foundation for Traffic Safety and National Center for Advancing Translational Sciences. The authors declared no conflicts of interest.
Source: DiGuiseppi CG et al. Migraine headaches are associated with motor vehicle crashes and driving habits among older drivers: Prospective cohort study. J Am Geriatr Soc. 2023 (Dec 22). doi: 10.1111/jgs.18719
Superficial Vascular Anomaly of the Glabella Mimicking a Cutaneous Cyst
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
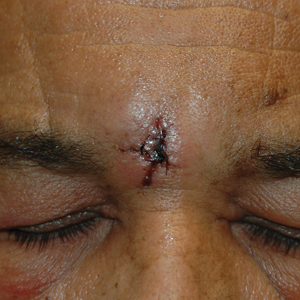
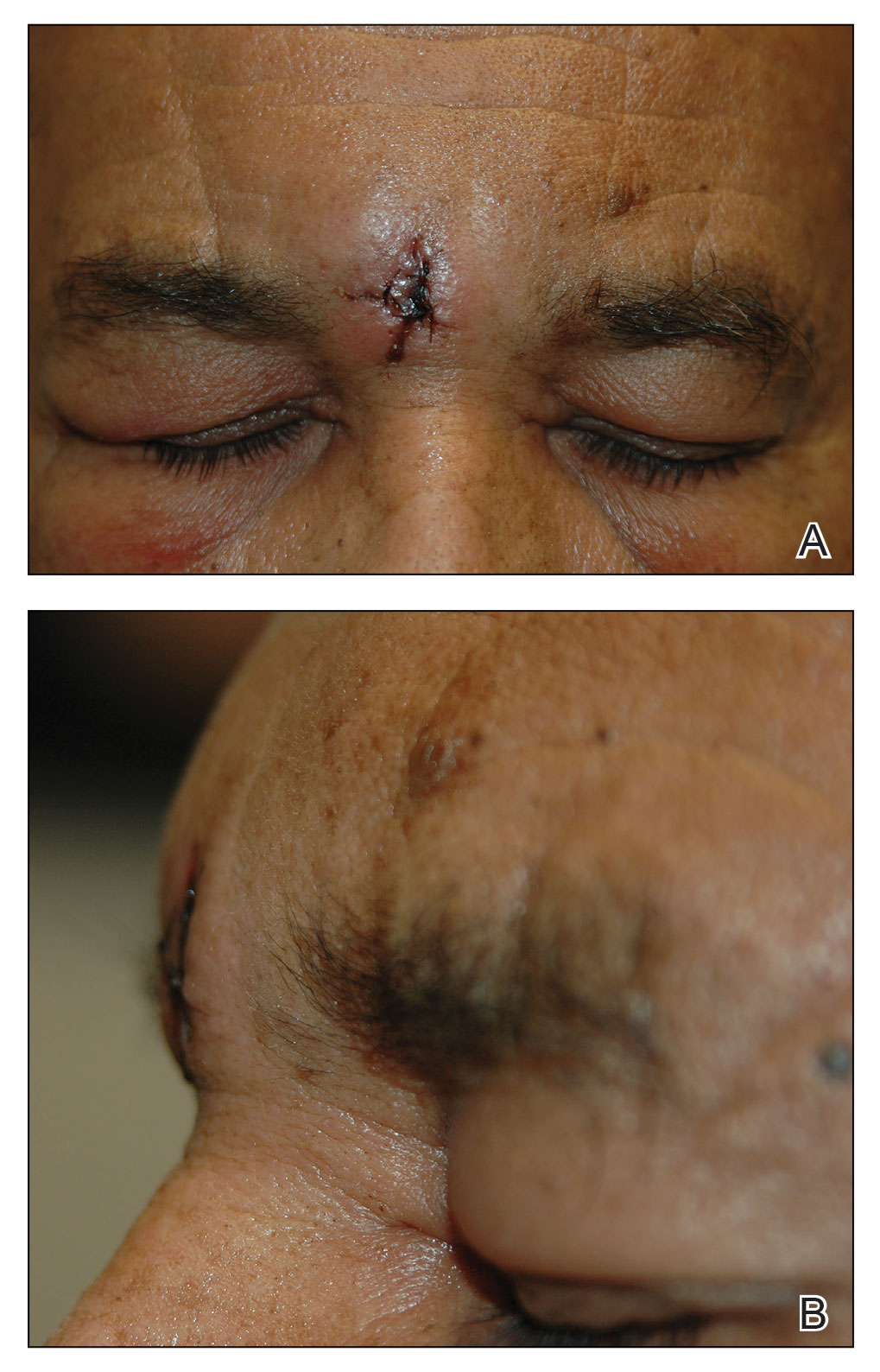
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.
A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
To the Editor:
Cutaneous cysts commonly are treated by dermatologists and typically are diagnosed clinically, followed by intraoperative or histologic confirmation; however, cyst mimickers can be misdiagnosed due to similar appearance and limited diagnostic guidelines.1 Vascular anomalies (VAs) of the face such as a facial aneurysm are rare.2 Preoperative assessment of findings suggestive of vascular etiology vs other common cutaneous tumors such as an epidermal inclusion cyst (EIC) and lipoma can help guide dermatologic management. We present a case of a VA of the glabella manifesting as a flesh-colored nodule that clinically mimicked a cyst and discuss the subsequent surgical management.
A 61-year-old man with a history of benign prostatic hyperplasia was evaluated at our dermatology clinic for an enlarging forehead mass of 1 year’s duration. Physical examination yielded a soft, flesh-colored, 2.5-cm nodule located superficially in the midline glabellar region without pulsation or palpable thrill. The differential diagnosis at the time included lipoma or EIC.
Excision of the lesion was performed utilizing superficial incisions with a descending depth of 1-mm increments to safely reach the target, identify the type of tumor, and prevent rupture of the suspected EIC. After the third incision to the level of the dermis, nonpulsatile bleeding was more than expected for a cyst. Digital pressure was applied, and the area was explored with blunt dissection to identify the source of bleeding. A fusiform, thin-walled aneurysm was identified in the dermal plane with additional tributaries coursing deep into the subcutaneous plane. The visualized tributaries were ligated with 3-0 polyglactin, figure-of-eight sutures resulting in hemostasis. The wound was closed with 5-0 nylon simple interrupted sutures. The patient was closely followed postoperatively for 1 week (Figure) and was referred for head imaging to evaluate for a possible associated intracranial aneurysm. Based on the thin vessel wall and continuous nonpulsatile hemorrhage, this VA was most consistent with venous aneurysm.

A VA can be encountered unexpectedly during dermatologic surgery. An aneurysm is a type of VA and is defined as an abnormal dilatation of a blood vessel that can be arterial, venous, or an arteriovenous malformation. Most reported aneurysms of the head and neck are cirsoid aneurysms or involve the superficial temporal artery.2,3 Reports of superficial venous aneurysms are rare.4 Preoperatively, cutaneous nodules can be evaluated for findings suggestive of a VA in the dermatologist’s office through physical examination. Arterial aneurysms may reveal palpable pulsation and audible bruit, while a venous aneurysm may exhibit a blue color, a size reduction with compression, and variable size with Valsalva maneuver.
The gold standard diagnostic tool for most dermatologic conditions is histopathology; however, dermatologic ultrasonography can provide noninvasive, real-time, important diagnostic characteristics of cutaneous pathologies as well as VA.5-7 Crisan et al6 outlined specific sonographic findings of lipomas, EICs, trichilemmal cysts, and other dermatologic conditions as well as the associated surgical pertinence. Ultrasonography of a venous aneurysm may show a heterogeneous, contiguous, echoic lesion with an adjacent superficial vein, which may be easily compressed by the probe.8 Advanced imaging such as computed tomography with contrast or magnetic resonance imaging may be performed, but these are more costly than ultrasonography. Additionally, point-of-care ultrasonography is becoming more popular and accessible for physicians to carry at bedside with portable tablet options available. Dermatologists may want to consider incorporating it into the outpatient setting to improve procedural planning.9
In conclusion, VAs should be included in the differential diagnosis of soft cutaneous nodules, as management differs from a cyst or lipoma. Dermatologists should use their clinical judgment preoperatively—including a comprehensive history, physical examination, and consideration of color Doppler ultrasonography to assess for findings of VA. We do not recommend intentional surgical exploration of cutaneous aneurysms in the ambulatory setting due to risk for hemorrhage. Furthermore, when clinical suspicion of EIC or lipoma is high, it still is preferable to descend the incision slowly at 1 to 2 mm per cut until the tumor is visualized.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
- Ring CM, Kornreich DA, Lee JB. Clinical simulators of cysts. J Am Acad Dermatol. 2016;75:1255-1257.
- Evans CC, Larson MJ, Eichhorn PJ, et al. Traumatic pseudoaneurysm of the superficial temporal artery: two cases and review of the literature. J Am Acad Dermatol. 2003;49(5 suppl):S286-S288.
- Sofela A, Osunronbi T, Hettige S. Scalp cirsoid aneurysms: case illustration and systematic review of literature. Neurosurgery. 2020;86:E98-E107.
- McKesey J, Cohen PR. Spontaneous venous aneurysm: report of a non-traumatic superficial venous aneurysm on the distal arm. Cureus. 2018;10:E2641.
- Wortsman X, Alfageme F, Roustan G, et al. Guidelines for performing dermatologic ultrasound examinations by the DERMUS Group. J Ultrasound Med. 2016;35:577-580.
- Crisan D, Wortsman X, Alfageme F, et al. Ultrasonography in dermatologic surgery: revealing the unseen for improved surgical planning [published online May 26, 2022]. J Dtsch Dermatol Ges. doi:10.1111/ddg.14781
- Corvino A, Catalano O, Corvino F, et al. Superficial temporal artery pseudoaneurysm: what is the role of ultrasound. J Ultrasound. 2016;19:197-201.
- Lee HY, Lee W, Cho YK, et al. Superficial venous aneurysm: reports of 3 cases and literature review. J Ultrasound Med. 2006;25:771-776.
- Hadian Y, Link D, Dahle SE, et al. Ultrasound as a diagnostic and interventional aid at point-of-care in dermatology clinic: a case report. J Dermatolog Treat. 2020;31:74-76.
Practice Points
- Vascular anomalies should be included in the differential diagnosis of soft cutaneous nodules, as management differs from cysts or lipomas.
- Preoperative evaluation for a cutaneous cyst excision on the head and neck should include ruling out findings of a vascular lesion through history, physical examination, and consideration of color Doppler ultrasonography in unclear cases.
- Surgical technique should involve sequential superficial incisions, descending at 1 to 2 mm per cut, until the suspected capsule is identified to minimize the risk for inadvertent injury to a cyst mimicker such as a vascular anomaly.
Acidogenic diet may be negative in patients with PsA
Key clinical point: In patients with psoriatic arthritis (PsA), a high dietary acid load (DAL), evaluated through potential renal acid load (PRAL) and net endogenous acid production (NEAP), was associated with increased disease activity and inflammation.
Major finding: The mean Disease Activity Index for PsA scores were higher in patients with PsA who had high vs low PRAL (19.8 vs 14.0; P = .006) and high vs low NEAP (20.3 vs 13.5; P = .001). In addition, patients in the high vs low PRAL and NEAP groups had significantly higher C-reactive protein levels (P = .024 and P = .020, respectively), indicating increased inflammation.
Study details: Findings are from a cross-sectional study that included 58 patients with overweight or obesity and a diagnosis of PsA.
Disclosures: This study did not disclose any funding. The authors declared no conflicts of interest.
Source: Öteleş S et al. The dietary acid load is associated with disease severity in psoriatic arthritis. Mod Rheumatol. 2023 (Nov 10). doi: 10.1093/mr/road107
Key clinical point: In patients with psoriatic arthritis (PsA), a high dietary acid load (DAL), evaluated through potential renal acid load (PRAL) and net endogenous acid production (NEAP), was associated with increased disease activity and inflammation.
Major finding: The mean Disease Activity Index for PsA scores were higher in patients with PsA who had high vs low PRAL (19.8 vs 14.0; P = .006) and high vs low NEAP (20.3 vs 13.5; P = .001). In addition, patients in the high vs low PRAL and NEAP groups had significantly higher C-reactive protein levels (P = .024 and P = .020, respectively), indicating increased inflammation.
Study details: Findings are from a cross-sectional study that included 58 patients with overweight or obesity and a diagnosis of PsA.
Disclosures: This study did not disclose any funding. The authors declared no conflicts of interest.
Source: Öteleş S et al. The dietary acid load is associated with disease severity in psoriatic arthritis. Mod Rheumatol. 2023 (Nov 10). doi: 10.1093/mr/road107
Key clinical point: In patients with psoriatic arthritis (PsA), a high dietary acid load (DAL), evaluated through potential renal acid load (PRAL) and net endogenous acid production (NEAP), was associated with increased disease activity and inflammation.
Major finding: The mean Disease Activity Index for PsA scores were higher in patients with PsA who had high vs low PRAL (19.8 vs 14.0; P = .006) and high vs low NEAP (20.3 vs 13.5; P = .001). In addition, patients in the high vs low PRAL and NEAP groups had significantly higher C-reactive protein levels (P = .024 and P = .020, respectively), indicating increased inflammation.
Study details: Findings are from a cross-sectional study that included 58 patients with overweight or obesity and a diagnosis of PsA.
Disclosures: This study did not disclose any funding. The authors declared no conflicts of interest.
Source: Öteleş S et al. The dietary acid load is associated with disease severity in psoriatic arthritis. Mod Rheumatol. 2023 (Nov 10). doi: 10.1093/mr/road107