User login
Adult ADHD: Tips for an accurate diagnosis
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
With the diagnosis of attention-deficit/hyperactivity disorder (ADHD) on the rise1 and a surge in prescriptions to treat the disorder leading to stimulant shortages,2 ensuring that patients are appropriately evaluated for ADHD is more critical than ever. ADHD is a clinical diagnosis that can be established by clinical interview, although the results of neuropsychological testing and collateral information from family members are helpful. Assessing adults for ADHD can be challenging when they appear to want to convince the clinician that they have the disorder. In this article, I provide tips to help you accurately diagnose ADHD in adult patients.
Use an ADHD symptom scale
An ADHD symptom checklist, such as the Adult ADHD Self-Report Scale, is an effective tool to establish the presence of ADHD symptoms. A patient can complete this self-assessment tool before their visit, and you can use the results as a springboard to ask them about ADHD symptoms. It is important to elicit specific examples of the ADHD symptoms the patient reports, and to understand how these symptoms affect their functioning and quality of life.
Review the prescription drug monitoring program
Review your state’s prescription drug monitoring program to explore the patient’s prior and current prescriptions of stimulants and other controlled substances. Discern if, when, and by whom a patient was previously treated for ADHD, and rule out the rare possibility that the patient has obtained multiple prescriptions for controlled substances from multiple clinicians, which suggests the patient may have a substance use disorder.
Begin the assessment at your initial contact with the patient
How patients present on an initial screening call or how they compose emails can reveal clues about their level of organization and overall executive functioning. The way patients complete intake forms (eg, using a concise vs a meandering writing style) as well as their punctuality when presenting to appointments can also be telling.
Conduct a mental status examination
Patients can have difficulty focusing and completing tasks for reasons other than having ADHD. A mental status examination can sometimes provide objective clues that an individual has ADHD. A digressive thought process, visible physical restlessness, and instances of a patient interrupting the evaluator are suggestive of ADHD, although all these symptoms can be present in other conditions (eg, mania). However, signs of ADHD in the mental status examination do not confirm an ADHD diagnosis, nor does their absence rule it out.
Maintain an appropriate diagnostic threshold
Per DSM-5, an ADHD diagnosis requires that the symptoms cause a significant impairment in functioning.3 It is up to the clinician to determine if this threshold is met. It is imperative to thoughtfully consider this because stimulants are first-line treatment for ADHD and are commonly misused. Psychiatrists are usually motivated to please their patients in order to maintain them as patients and develop a positive therapeutic relationship, which improves outcomes.4 However, it is important to demonstrate integrity, provide an accurate diagnosis, and not be unduly swayed by a patient’s wish to receive an ADHD diagnosis. If you sense that a prospective patient is hoping they will receive an ADHD diagnosis and be prescribed a stimulant, it may be prudent to emphasize that the patient will be assessed for multiple mental health conditions, including ADHD, and that treatment will depend on the outcome of the evaluation.
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
1. Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. doi:10.1001/jamanetworkopen.2019.14344
2. Danielson ML, Bohm MK, Newsome K, et al. Trends in stimulant prescription fills among commercially insured children and adults - United States, 2016-2021. MMWR Morb Mortal Wkly Rep. 2023;72(13):327-332. doi:10.15585/mmwr.mm7213a1
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:59-63.
4. Totura CMW, Fields SA, Karver MS. The role of the therapeutic relationship in psychopharmacological treatment outcomes: a meta-analytic review. Pyschiatr Serv. 2018;69(1):41-47. doi:10.1176/appi.ps.201700114
Childbirth-related PTSD: How it differs and who’s at risk
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
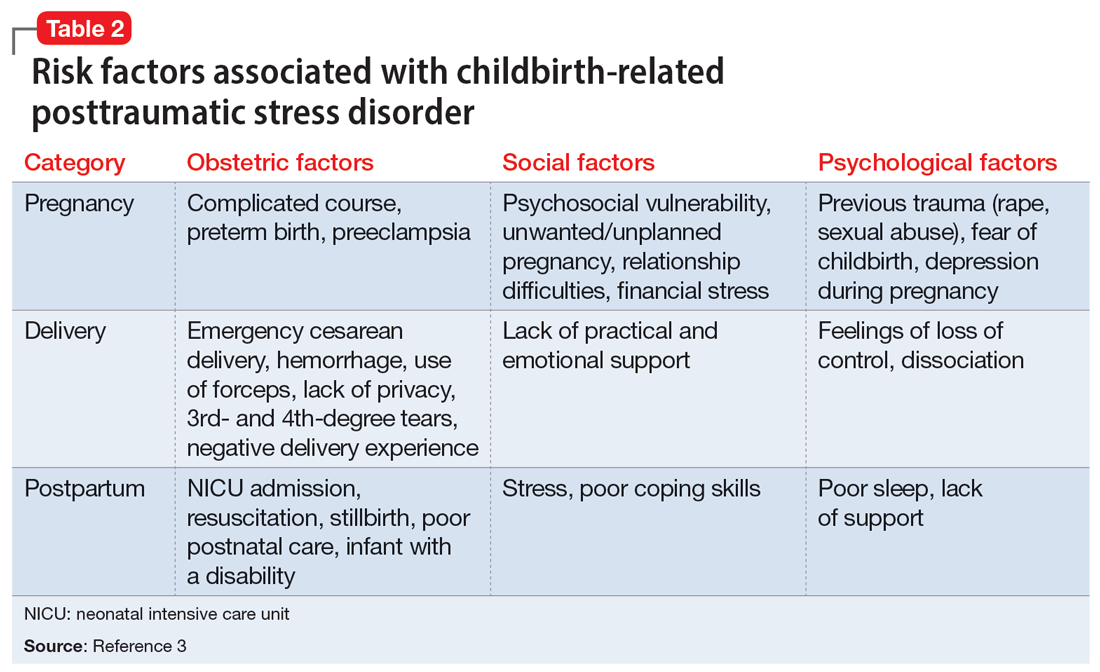
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.
Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.

Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
Childbirth-related posttraumatic stress disorder (CB-PTSD) is a form of PTSD that can develop related to trauma surrounding the events of giving birth. It affects approximately 5% of women after any birth, which is similar to the rate of PTSD after experiencing a natural disaster.1 Up to 17% of women may have posttraumatic symptoms in the postpartum period.1 Despite the high prevalence of CB-PTSD, many psychiatric clinicians have not incorporated screening for and management of CB-PTSD into their practice.
This is partly because childbirth has been conceptualized as a “stressful but positive life event.”2 Historically, childbirth was not recognized as a traumatic event; for example, in DSM-III-R, the criteria for trauma in PTSD required an event outside the range of usual human experience, and childbirth was implicitly excluded as being too common to be traumatic. In the past decade, this clinical phenomenon has been more formally recognized and studied.2

CB-PTSD presents with symptoms similar to those of other forms of PTSD, with some nuances, as outlined in Table 1.3 Avoidance can be the predominant symptom; this can affect mothers’ engagement in postnatal care and is a major risk factor for postpartum depression.3
Many risk factors in the peripartum period can impact the development of CB-PTSD (Table 23). The most significant risk factor is whether the patient views the delivery of their baby as a subjectively negative experience, regardless of the presence or lack of peripartum complications.1 However, parents of infants who require treatment in a neonatal intensive care unit and women who require emergency medical treatment following delivery are at higher risk.

Screening and treatment
Ideally, every woman should be screened for CB-PTSD by their psychiatrist or obstetrician during a postpartum visit at least 1 month after delivery. In particular, high-risk populations and women with subjectively negative birth experiences should be screened, as well as women with postpartum depression that may have been precipitated or perpetuated by a traumatic experience. The City Birth Trauma Scale is a free 31-item self-report scale that can be used for such screening. It addresses both general and birth-related symptoms and is validated in multiple languages.4
Selective serotonin reuptake inhibitors and prazosin may be helpful for symptomatic treatment of CB-PTSD. Ongoing research studying the efficacy of cognitive-behavioral therapy and eye movement desensitization and reprocessing for CB-PTSD has yielded promising results but is limited in its generalizability.
Many women who develop CB-PTSD choose to get pregnant again. Psychiatrists can apply the principles of trauma-informed care and collaborate with obstetric and pediatric physicians to reduce the risk of retraumatization. It is critical to identify at-risk women and educate and prepare them for their next delivery experience. By focusing on communication, informed consent, and emotional support, we can do our best to prevent the recurrence of CB-PTSD.
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
1. Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: a systematic review of prevalence and risk factors. Front Psych. 2017;8:560. doi:10.3389/fpsyg.2017.00560
2. Horesh D, Garthus-Niegel S, Horsch A. Childbirth-related PTSD: is it a unique post-traumatic disorder? J Reprod Infant Psych. 2021;39(3):221-224. doi:10.1080/02646838.2021.1930739
3. Kranenburg L, Lambregtse-van den Berg M, Stramrood C. Traumatic childbirth experience and childbirth-related post-traumatic stress disorder (PTSD): a contemporary overview. Int J Environ Res Public Health. 2023;20(4):2775. doi:10.3390/ijerph20042775
4. Ayers S, Wright DB, Thornton A. Development of a measure of postpartum PTSD: The City Birth Trauma Scale. Front Psychiatry. 2018;9:409. doi:10.3389/fpsyt.2018.00409
Perinatal psychiatric screening: What to ask
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
Perinatal psychiatry focuses on the evaluation, diagnosis, and treatment of mental health disorders during the preconception, pregnancy, and postpartum periods. Mood disorders, anxiety disorders, and posttraumatic stress disorder are the most common mental health conditions that arise during the perinatal period.1 Mediating factors include hormone fluctuations, sleep deprivation, trauma exposure, financial stress, having a history of psychiatric illness, and factors related to newborn care.
During the perinatal period, a comprehensive psychiatric interview is crucial. Effective screening and identification of maternal mental health conditions necessitate more than merely checking off boxes on a questionnaire. It requires compassionate, informed, and individualized conversations between physicians and their patients.
In addition to asking about pertinent positive and negative psychiatric symptoms, the following screening questions could be asked during a structured interview to identify perinatal issues during pregnancy and the postpartum period.
During pregnancy
- How do you feel about your pregnancy?
- Was this pregnancy planned or unplanned, desired or not?
- Was fertility treatment needed or used?
- Did you think about stopping the pregnancy? If so, was your decision influenced by laws that restrict abortion in your state?
- Do you feel connected to the fetus?
- Do you have a room or crib at home for the baby? A car seat? Clothing? Baby supplies?
- Are you planning on breastfeeding?
- Do you have thoughts on future desired fertility and/or contraception?
- Who is your support system at home?
- How is your relationship with the baby’s father?
- How is the baby’s father’s mental well-being?
- Have you been subject to any abuse, intimate partner violence, or neglect?
- Are your other children being taken care of properly? What is the plan for them during delivery days at the hospital?
During the postpartum period
- Was your baby born prematurely?
- Did you have a vaginal or cesarean delivery?
- Did you encounter any delivery complications?
- Did you see the baby after the delivery?
- Do you feel connected to or able to bond with the baby?
- Do you have access to maternity leave from work?
- Have you had scary or upsetting thoughts about hurting your baby?
- Do you have any concerns about your treatment plan, such as medication use?
- In case of an emergency, are you aware of perinatal psychiatry resources in your area or the national maternal mental health hotline (833-852-6262)?
The American College of Obstetricians and Gynecologists clinical practice guidelines recommend that clinicians conduct depression and anxiety screening at least once during the perinatal period by using a standardized, validated tool.2 Psychiatry residents should receive adequate guidance and education about perinatal psychiatric evaluation, risk assessment, and treatment counseling. Early detection of mental health symptoms allows for early referral, close surveillance during episodes of vulnerability, and better access to mental health care during the perinatal period.
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
1. Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19(3):313-327. doi:10.1002/wps.20769
2. American College of Obstetricians and Gynecologists. Screening and diagnosis of mental health conditions during pregnancy and postpartum. Clinical Practice Guideline Number 4. June 2023. Accessed November 3, 2023. https://www.acog.org/clinical/clinical-guidance/clinical-practice-guideline/articles/2023/06/screening-and-diagnosis-of-mental-health-conditions-during-pregnancy-and-postpartum
Brick and mortar: Changes in the therapeutic relationship in a postvirtual world
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.
My colleagues and I entered the realm of outpatient psychiatry during residency at a logistically and dynamically interesting time. At the beginning of our third year in training (July 2022), almost all of the outpatients we were treating were still being seen virtually. For much of the year, they remained that way. However, with the reinstatement of the Ryan Haight Act in May 2023, I began to meet patients in person for the first time—the same patients whom I had known only virtually for the first 10 months of our therapeutic relationship. I observed vast changes in the dynamic of the room; many of these patients opened up more in their first in-person session than they had all year over Zoom.
Once in-person sessions resumed, patients who during virtual visits had assured me for almost a year that their home situation was optimized had a plethora of new things to share about their seemingly straightforward living situations. Relationships that appeared stable had more layers to reveal once the half of the relationship I was treating was now comfortably seated within the walls of my office. Problems that had previously seemed biologically based suddenly had complex sociocultural elements that were divulged for the first time. Some patients felt freer to be unrestricted in their affect, in contrast to the logistical (and metaphorical) buttoned-up virtual environment. Emotions ranged from cathartic (“It’s so great to see you in person!”) to bemused (“You’re taller/shorter, older/younger than I thought!”). The screen was gone, and the tangibility of it all breathed a different air into the room.
Virtual vs in-person: Crabs on a beach
The virtual treatment space could be envisioned as crabs in shells scattered on a beach, in which 2 crabs situated in their own shells, not necessarily adjacent to each other, could communicate. This certainly had benefits, such as the convenience of not having to move to another shell, as well as the brief but telling opportunity to gaze into their home shell environment. However, sometimes there would be disadvantages, such as interference with the connection due to static in the sand; at other times, there was the potential for other crabs to overhear and inadvertently learn of each other’s presence, thus affecting the openness of the communication. In this analogy, perhaps the equivalent of an in-person meeting would be 1 crab meandering over and the 2 crabs cohabiting a conch for the first time—it’s spacious (enough), all-enveloping, and within the harkened privacy of a shared sacred space.
A unique training experience
My co-residents and I are uniquely positioned to observe this novel phenomenon due to the timing of having entered our outpatient psychiatry training during the COVID-19 pandemic. Previous generations of residents—as well as practicing psychiatrists who had initially met their patients in person and were forced to switch to virtual sessions during the pandemic—had certain perspectives and challenges of their own, but they had a known dynamic of in-person interactions at baseline. Accordingly, residents who practiced peak- and mid-pandemic and graduated without being required to treat patients face-to-face (the classes of 2022 and 2023) might have spent entire therapeutic relationships having never met their patients in person. My class (2024) was situated in this time- and situation-bound frame in which we started virtually, and by requirements of the law, later met our patients in person. Being not only an observer but an active participant in a treatment dyad within the context of this phenomenon taught me astutely about transference, countertransference, and the holding environment. Training in psychodynamic psychotherapy has taught me about the act of listening deeply and qualities of therapeutic communication. Having the opportunity to enact these principles in such a dichotomy of treatment settings has been invaluable in my education, in getting to know different facets of my patients, and in understanding the nuances of the human experience.
A new doctor in a COVID mask
As a 2020 graduate, my medical school experience was largely untouched by the coronavirus. However, when I transitioned to residency, the world was 4 months into the COVID-19 pandemic, and I was required to wear an N95 mask. Just as I started calling myself Dr. Petteruti, I stopped seeing my patients’ entire face, and they stopped seeing mine. In this article, I share my reflections on wearing a mask during residency.
Even after 3 years of daily practice, I have found that wearing a mask brings an acute awareness of my face. As a community physician, the spheres of personal and public life intersect as I treat patients. Learning to navigate this is an important and shared experience across many community-based residency programs. However, during the first few years of residency, I have been able to shop at a local grocery store or eat at a nearby restaurant without any concerns of being recognized by a patient. Until recently, my patients had never seen my face. That has now changed.
For a new intern, a mask can be a savior. It can hide most of what is on your face from your patient. It is remarkable how the brain fills in the gaps of the visage and, by extension, aspects of the person. Many times, I was thankful to have my morning yawn or facial expression covered during provoking conversations with patients. Furthermore, masks gave me an opportunity to examine my own reactions, emotions, affect, and countertransference of each interaction on my own time.
The mask mandate also protected some features that illustrated my youth. For the patient, a mask can add a dry, clinical distance to the physician, often emitting a professional interpretation to the encounter. For the physician, the mask serves as a concrete barrier to the otherwise effortless acts of observation. Early in my career, I had to set reminders to have patients who were taking antipsychotic medications remove their masks to assess for tardive dyskinesia. Sometimes this surprised the patient, who was hesitant to expose themselves physically and psychologically. Alternatively, mask wearing has proved to be an additional data point on some patients, such as those with disorganized behavior. If the mask is located on the patient’s head, chin, or eyes, or is otherwise inappropriately placed, this provides the clinician with supplemental information.
After spending most of my third year of residency in an outpatient office diligently learning how to build a sturdy therapeutic patient alliance, the mask mandate was lifted. Patients’ transference began to change right before my newly bared face. People often relate age to wisdom and experience, so my lack of age—and thus, possible perceived lack of knowledge—became glaringly apparent. During our initial encounters without masks, patients I had known for most of the year began discussing their symptoms and treatments with more hesitancy. My established patients suddenly had a noticeable change in the intensity of their eye contact. Some even asked if I had cut my hair or what had changed about my appearance since our previous visit. This change in affect and behavior offers a unique experience for the resident; renovating the patient-doctor relationship based on the physician’s appearance.
As psychiatrists, we would generally assume mask wearing has an undesirable effect on the therapeutic alliance and increases skewed inferences in our evaluations. This held true for my experience in residency. In psychotherapy, we work to help patients remove their own metaphorical “masks” of defense and security in self-exploration. However, as young physicians, rather than creating barriers between us and our patients, the mask mandate seemed to have created a sense of credibility in our practice and trustworthiness in our decisions.
Some questions remain. As clinicians, what are we missing when we can only see our patient’s eyes and forehead? How will the COVID-19 pandemic affect my training and career as a psychiatrist? These may remain unanswered for my generation of trainees for some time, as society will look back and contemplate this period for decades. Though we entered our career in uncertain times, with an increased risk of morbidity and death and high demand for proper personal protective equipment, we were and still are thankful for our masks and for the limited infection exposure afforded by the nature of our specialty.
As a 2020 graduate, my medical school experience was largely untouched by the coronavirus. However, when I transitioned to residency, the world was 4 months into the COVID-19 pandemic, and I was required to wear an N95 mask. Just as I started calling myself Dr. Petteruti, I stopped seeing my patients’ entire face, and they stopped seeing mine. In this article, I share my reflections on wearing a mask during residency.
Even after 3 years of daily practice, I have found that wearing a mask brings an acute awareness of my face. As a community physician, the spheres of personal and public life intersect as I treat patients. Learning to navigate this is an important and shared experience across many community-based residency programs. However, during the first few years of residency, I have been able to shop at a local grocery store or eat at a nearby restaurant without any concerns of being recognized by a patient. Until recently, my patients had never seen my face. That has now changed.
For a new intern, a mask can be a savior. It can hide most of what is on your face from your patient. It is remarkable how the brain fills in the gaps of the visage and, by extension, aspects of the person. Many times, I was thankful to have my morning yawn or facial expression covered during provoking conversations with patients. Furthermore, masks gave me an opportunity to examine my own reactions, emotions, affect, and countertransference of each interaction on my own time.
The mask mandate also protected some features that illustrated my youth. For the patient, a mask can add a dry, clinical distance to the physician, often emitting a professional interpretation to the encounter. For the physician, the mask serves as a concrete barrier to the otherwise effortless acts of observation. Early in my career, I had to set reminders to have patients who were taking antipsychotic medications remove their masks to assess for tardive dyskinesia. Sometimes this surprised the patient, who was hesitant to expose themselves physically and psychologically. Alternatively, mask wearing has proved to be an additional data point on some patients, such as those with disorganized behavior. If the mask is located on the patient’s head, chin, or eyes, or is otherwise inappropriately placed, this provides the clinician with supplemental information.
After spending most of my third year of residency in an outpatient office diligently learning how to build a sturdy therapeutic patient alliance, the mask mandate was lifted. Patients’ transference began to change right before my newly bared face. People often relate age to wisdom and experience, so my lack of age—and thus, possible perceived lack of knowledge—became glaringly apparent. During our initial encounters without masks, patients I had known for most of the year began discussing their symptoms and treatments with more hesitancy. My established patients suddenly had a noticeable change in the intensity of their eye contact. Some even asked if I had cut my hair or what had changed about my appearance since our previous visit. This change in affect and behavior offers a unique experience for the resident; renovating the patient-doctor relationship based on the physician’s appearance.
As psychiatrists, we would generally assume mask wearing has an undesirable effect on the therapeutic alliance and increases skewed inferences in our evaluations. This held true for my experience in residency. In psychotherapy, we work to help patients remove their own metaphorical “masks” of defense and security in self-exploration. However, as young physicians, rather than creating barriers between us and our patients, the mask mandate seemed to have created a sense of credibility in our practice and trustworthiness in our decisions.
Some questions remain. As clinicians, what are we missing when we can only see our patient’s eyes and forehead? How will the COVID-19 pandemic affect my training and career as a psychiatrist? These may remain unanswered for my generation of trainees for some time, as society will look back and contemplate this period for decades. Though we entered our career in uncertain times, with an increased risk of morbidity and death and high demand for proper personal protective equipment, we were and still are thankful for our masks and for the limited infection exposure afforded by the nature of our specialty.
As a 2020 graduate, my medical school experience was largely untouched by the coronavirus. However, when I transitioned to residency, the world was 4 months into the COVID-19 pandemic, and I was required to wear an N95 mask. Just as I started calling myself Dr. Petteruti, I stopped seeing my patients’ entire face, and they stopped seeing mine. In this article, I share my reflections on wearing a mask during residency.
Even after 3 years of daily practice, I have found that wearing a mask brings an acute awareness of my face. As a community physician, the spheres of personal and public life intersect as I treat patients. Learning to navigate this is an important and shared experience across many community-based residency programs. However, during the first few years of residency, I have been able to shop at a local grocery store or eat at a nearby restaurant without any concerns of being recognized by a patient. Until recently, my patients had never seen my face. That has now changed.
For a new intern, a mask can be a savior. It can hide most of what is on your face from your patient. It is remarkable how the brain fills in the gaps of the visage and, by extension, aspects of the person. Many times, I was thankful to have my morning yawn or facial expression covered during provoking conversations with patients. Furthermore, masks gave me an opportunity to examine my own reactions, emotions, affect, and countertransference of each interaction on my own time.
The mask mandate also protected some features that illustrated my youth. For the patient, a mask can add a dry, clinical distance to the physician, often emitting a professional interpretation to the encounter. For the physician, the mask serves as a concrete barrier to the otherwise effortless acts of observation. Early in my career, I had to set reminders to have patients who were taking antipsychotic medications remove their masks to assess for tardive dyskinesia. Sometimes this surprised the patient, who was hesitant to expose themselves physically and psychologically. Alternatively, mask wearing has proved to be an additional data point on some patients, such as those with disorganized behavior. If the mask is located on the patient’s head, chin, or eyes, or is otherwise inappropriately placed, this provides the clinician with supplemental information.
After spending most of my third year of residency in an outpatient office diligently learning how to build a sturdy therapeutic patient alliance, the mask mandate was lifted. Patients’ transference began to change right before my newly bared face. People often relate age to wisdom and experience, so my lack of age—and thus, possible perceived lack of knowledge—became glaringly apparent. During our initial encounters without masks, patients I had known for most of the year began discussing their symptoms and treatments with more hesitancy. My established patients suddenly had a noticeable change in the intensity of their eye contact. Some even asked if I had cut my hair or what had changed about my appearance since our previous visit. This change in affect and behavior offers a unique experience for the resident; renovating the patient-doctor relationship based on the physician’s appearance.
As psychiatrists, we would generally assume mask wearing has an undesirable effect on the therapeutic alliance and increases skewed inferences in our evaluations. This held true for my experience in residency. In psychotherapy, we work to help patients remove their own metaphorical “masks” of defense and security in self-exploration. However, as young physicians, rather than creating barriers between us and our patients, the mask mandate seemed to have created a sense of credibility in our practice and trustworthiness in our decisions.
Some questions remain. As clinicians, what are we missing when we can only see our patient’s eyes and forehead? How will the COVID-19 pandemic affect my training and career as a psychiatrist? These may remain unanswered for my generation of trainees for some time, as society will look back and contemplate this period for decades. Though we entered our career in uncertain times, with an increased risk of morbidity and death and high demand for proper personal protective equipment, we were and still are thankful for our masks and for the limited infection exposure afforded by the nature of our specialty.
Worsening mania while receiving low-dose quetiapine: A case report
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1A antagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1A antagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The second-generation antipsychotic quetiapine is commonly used to treat several psychiatric disorders, including bipolar disorder (BD) and insomnia. In this case report, we discuss a patient with a history of unipolar depression and initial signs of mania who experienced an exacerbation of manic symptoms following administration of low-dose quetiapine. This case underscores the need for careful monitoring of patients receiving quetiapine, especially at lower doses, and the potential limitations of its efficacy in controlling manic symptoms.
Depressed with racing thoughts
Mr. X, age 58, is an Army veteran who lives with his wife of 29 years and works as a contractor. He has a history of depression and a suicide attempt 10 years ago by self-inflicted gunshot wound to the head, which left him with a bullet lodged in his sinus cavity and residual dysarthria after tongue surgery. After the suicide attempt, Mr. X was medically hospitalized, but not psychiatrically hospitalized. Shortly after, he self-discontinued all psychotropic medications and follow-up.
Mr. X has no other medical history and takes no other medications or supplements. His family history includes a mother with schizoaffective disorder, 1 brother with BD, and another brother with developmental delay.
Mr. X remained euthymic until his brother died. Soon after, he began to experience low mood, heightened anxiety, racing thoughts, tearfulness, and mild insomnia. He was prescribed quetiapine 25 mg/d at bedtime and instructed to titrate up to 50 mg/d.
Ten days later, Mr. X was brought to the hospital by his wife, who reported that after starting quetiapine, her husband began to act erratically. He had disorganized and racing thoughts, loose associations, labile affect, hyperactivity/restlessness, and was not sleeping. In the morning before presenting to the hospital, Mr. X had gone to work, laid down on the floor, began mumbling to himself, and would not respond to coworkers. Upon evaluation, Mr. X was noted to have pressured speech, disorganized speech, delusions, anxiety, and hallucinations. A CT scan of his head was normal, and a complete blood count, comprehensive metabolic panel, thyroid-stimulating hormone, B12, folate, and hemoglobin A1c were within normal limits. Mr. X’s vitamin D level was low at 22 ng/mL, and a syphilis screen was negative.
Mr. X was admitted to the hospital for his safety. The treatment team discontinued quetiapine and started risperidone 3 mg twice a day for psychotic symptoms and mood stabilization. At the time of discharge 7 days later, Mr. X was no longer experiencing any hallucinations or delusions, his thought process was linear and goal-directed, his mood was stable, and his insomnia had improved. Based on the temporal relationship between the initiation of quetiapine and the onset of Mr. X’s manic symptoms, along with an absence of organic causes, the treatment team suspected Mr. X had experienced a worsening of manic symptoms induced by quetiapine. Before starting quetiapine, he had presented with an initial manic symptom of racing thoughts.
At his next outpatient appointment, Mr. X exhibited significant akathisia. The treatment team initiated propranolol 20 mg twice a day but Mr. X did not experience much improvement. Risperidone was reduced to 1 mg twice a day and Mr. X was started on clonazepam 0.5 mg twice a day. The akathisia resolved. The treatment team decided to discontinue all medications and observe Mr. X for any recurrence of symptoms. One year after his manic episode. Mr. X remained euthymic. He was able to resume full-time work and began psychotherapy to process the grief over the loss of his brother.
Quetiapine’s unique profile
This case sheds light on the potential limitations of quetiapine, especially at lower doses, for managing manic symptoms. Quetiapine exhibits antidepressant effects, even at doses as low as 50 mg/d.1 At higher doses, quetiapine acts as an antagonist at serotonin (5-HT1A and 5-HT2A), dopamine (D1 and D2), histamine H1, and adrenergic receptors.2 At doses <300 mg/d, there is an absence of dopamine receptor blockade and a higher affinity for 5-HT2A receptors, which could explain why higher doses are generally necessary for treating mania and psychotic symptoms.3-5 High 5-HT2A antagonism may disinhibit the dopaminergic system and paradoxically increase dopaminergic activity, which could be the mechanism responsible for lack of control of manic symptoms with low doses of quetiapine.2 Another possible explanation is that the metabolite of quetiapine, N-desalkylquetiapine, acts as a norepinephrine reuptake blocker and partial 5-HT1A antagonist, which acts as an antidepressant, and antidepressants are known to induce mania in vulnerable patients.4
The antimanic property of most antipsychotics (except possibly clozapine) is attributed to their D2 antagonistic potency. Because quetiapine is among the weaker D2 antagonists, its inability to prevent the progression of mania, especially at 50 mg/d, is not unexpected. Mr. X’s subsequent need for a stronger D2 antagonist—risperidone—at a significant dose further supports this observation. A common misconception is that quetiapine’s sedating effects make it effective for treating mania, but that is not the case. Clinicians should be cautious when prescribing quetiapine, especially at lower doses, to patients who exhibit signs of mania. Given the potential risk, clinicians should consider alternative treatments before resorting to low-dose quetiapine for insomnia. Regular monitoring for manic symptoms is crucial for all patients receiving quetiapine. If patients present with signs of mania or hypomania, a therapeutic dose range of 600 to 800 mg/d is recommended.6
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
- Weisler R, Joyce M, McGill L, et al. Extended release quetiapine fumarate monotherapy for major depressive disorder: results of a double-blind, randomized, placebo-controlled study. CNS Spectr. 2009;14(6):299-313. doi:10.1017/s1092852900020307
- Khalil RB, Baddoura C. Quetiapine induced hypomania: a case report and a review of the literature. Curr Drug Saf. 2012;7(3):250-253. doi:10.2174/157488612803251333
- Benyamina A, Samalin L. Atypical antipsychotic-induced mania/hypomania: a review of recent case reports and clinical studies. Int J Psychiatry Clin Pract. 2012;16(1):2-7. doi:10.3109/13651501.2011.605957
- Gnanavel S. Quetiapine-induced manic episode: a paradox for contemplation. BMJ Case Rep. 2013;2013:bcr2013201761. doi:10.1136/bcr-2013-201761
- Pacchiarotti I, Manfredi G, Kotzalidis GD, et al. Quetiapine-induced mania. Aust N Z J Psychiatry. 2003;37(5):626.
- Millard HY, Wilson BA, Noordsy DL. Low-dose quetiapine induced or worsened mania in the context of possible undertreatment. J Am Board Fam Med. 2015;28(1):154-158. doi:10.3122/jabfm.2015.01.140105
Navigating the challenges of patients with substance use disorders who leave AMA
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Working closely with individuals with substance use disorders (SUDs), we’ve observed a worrisome trend of patients leaving the hospital against medical advice (AMA). This issue is not only prevalent in psychiatric settings, but also in emergency departments, medical and surgical floors, and even intensive care units.1
Compared to individuals without such disorders, individuals with SUDs—particularly those with opioid use disorders—are up to 3 times more likely to leave the hospital AMA.1,2 Leaving AMA can lead to multiple complications, including an increased risk of readmission, suboptimal treatment outcomes, and an increased use of health care resources.1-3
It is critical to understand why patients elect to leave a hospital AMA. In a qualitative study, Simon et al1 found that individuals with SUDs often leave AMA due to uncontrolled withdrawal symptoms and pain, perceived stigma and discrimination, and dissatisfaction with care. Predictors of patients leaving the hospital AMA include the severity of their drug dependence and previous negative treatment experiences.4 A systematic review found housing instability and a lack of social support influence an individual’s decision to leave AMA.5
Recommendations for managing patients who leave AMA
Enhancing your understanding of withdrawal symptoms may allow you to offer patients more effective symptom control, possibly with methadone or buprenorphine.2 Injectable opioid agonist treatment may also help to retain a patient in care. In a case report, a 47-year-old man with a severe opioid use disorder who had left the hospital AMA due to uncontrolled opioid withdrawal was readmitted, treated with IV hydromorphone, and enrolled in ongoing community injectable opioid agonist treatment.6
Clinicians must address the stigma and discrimination patients with SUDs often face in health care institutions. Additional training for clinicians to improve their understanding of these disorders and foster a more compassionate and nonjudgmental approach to care may be beneficial.
Like most medicolegal conflicts, leaving AMA is often a clinical and interpersonal problem disguised as a legal one. When assessing these patients’ decision-making capacity, we often find they are angry and dissatisfied with the care they have (or have not) received. The most useful intervention may be to restore communication between the patient and their treatment team.
Even after a patient leaves AMA, the treatment team may experience countertransference issues, such as heightened emotional reactions or biases, that could compromise their clinical judgment. Addressing these dynamics may require team debriefings, supervision, or further training in managing transference and countertransference, particularly since patients who leave AMA may return for subsequent care.7
Integrated care models, which feature close collaboration between clinicians from different specialties, can help ensure that a patient’s diverse health needs are met and reduce the likelihood of them leaving AMA. Integrated care models may be particularly effective for patients with co-occurring conditions such as HIV and SUDs.8
Implementing these recommendations can be challenging. Barriers to addressing AMA departures span several domains, including patient-specific barriers (eg, stigma and discrimination), clinical barriers (eg, lack of resources and training for clinicians), institutional hurdles (eg, systemic inefficiencies), and broader social barriers (eg, housing instability and inadequate social support). Overcoming these barriers requires a multifaceted approach involving clinicians, policymakers, and the community that considers medical, psychological, and social factors.
1. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525.
2. Kenne DR, Boros AP, Fischbein RL. Characteristics of opiate users leaving detoxification treatment against medical advice. J Addict Dis. 2010;29(3):383-394.
3. Mahajan RK, Gautam PL, Paul G, et al. Retrospective evaluation of patients leaving against medical advice in a tertiary care teaching hospital. Indian J Crit Care Med. 2019;23(3):139-142.
4. Armenian SH, Chutuape MA, Stitzer ML. Predictors of discharges against medical advice from a short-term hospital detoxification unit. Drug Alcohol Depend. 1999;56(1):1-8.
5. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59.
6. McAdam M, Brar R, Young S. Initiation of injectable opioid agonist treatment in hospital: a case report. Drug Alcohol Rev. 2020;39(2):138-141.
7. Schouten R, Weintraub BR. Legal aspects of consultation. In: Stern TA, Freudenreich O, Smith FA, et al, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 7th ed. Elsevier; 2018:578-579.
8. Vallecillo G, Robles MJ, Fonseca F, et al. Integrated care on leaving hospital against medical advice among HIV-infected people with substance use disorders. AIDS Res Hum Retroviruses. 2018;34(12):1044-1049.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Working closely with individuals with substance use disorders (SUDs), we’ve observed a worrisome trend of patients leaving the hospital against medical advice (AMA). This issue is not only prevalent in psychiatric settings, but also in emergency departments, medical and surgical floors, and even intensive care units.1
Compared to individuals without such disorders, individuals with SUDs—particularly those with opioid use disorders—are up to 3 times more likely to leave the hospital AMA.1,2 Leaving AMA can lead to multiple complications, including an increased risk of readmission, suboptimal treatment outcomes, and an increased use of health care resources.1-3
It is critical to understand why patients elect to leave a hospital AMA. In a qualitative study, Simon et al1 found that individuals with SUDs often leave AMA due to uncontrolled withdrawal symptoms and pain, perceived stigma and discrimination, and dissatisfaction with care. Predictors of patients leaving the hospital AMA include the severity of their drug dependence and previous negative treatment experiences.4 A systematic review found housing instability and a lack of social support influence an individual’s decision to leave AMA.5
Recommendations for managing patients who leave AMA
Enhancing your understanding of withdrawal symptoms may allow you to offer patients more effective symptom control, possibly with methadone or buprenorphine.2 Injectable opioid agonist treatment may also help to retain a patient in care. In a case report, a 47-year-old man with a severe opioid use disorder who had left the hospital AMA due to uncontrolled opioid withdrawal was readmitted, treated with IV hydromorphone, and enrolled in ongoing community injectable opioid agonist treatment.6
Clinicians must address the stigma and discrimination patients with SUDs often face in health care institutions. Additional training for clinicians to improve their understanding of these disorders and foster a more compassionate and nonjudgmental approach to care may be beneficial.
Like most medicolegal conflicts, leaving AMA is often a clinical and interpersonal problem disguised as a legal one. When assessing these patients’ decision-making capacity, we often find they are angry and dissatisfied with the care they have (or have not) received. The most useful intervention may be to restore communication between the patient and their treatment team.
Even after a patient leaves AMA, the treatment team may experience countertransference issues, such as heightened emotional reactions or biases, that could compromise their clinical judgment. Addressing these dynamics may require team debriefings, supervision, or further training in managing transference and countertransference, particularly since patients who leave AMA may return for subsequent care.7
Integrated care models, which feature close collaboration between clinicians from different specialties, can help ensure that a patient’s diverse health needs are met and reduce the likelihood of them leaving AMA. Integrated care models may be particularly effective for patients with co-occurring conditions such as HIV and SUDs.8
Implementing these recommendations can be challenging. Barriers to addressing AMA departures span several domains, including patient-specific barriers (eg, stigma and discrimination), clinical barriers (eg, lack of resources and training for clinicians), institutional hurdles (eg, systemic inefficiencies), and broader social barriers (eg, housing instability and inadequate social support). Overcoming these barriers requires a multifaceted approach involving clinicians, policymakers, and the community that considers medical, psychological, and social factors.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Working closely with individuals with substance use disorders (SUDs), we’ve observed a worrisome trend of patients leaving the hospital against medical advice (AMA). This issue is not only prevalent in psychiatric settings, but also in emergency departments, medical and surgical floors, and even intensive care units.1
Compared to individuals without such disorders, individuals with SUDs—particularly those with opioid use disorders—are up to 3 times more likely to leave the hospital AMA.1,2 Leaving AMA can lead to multiple complications, including an increased risk of readmission, suboptimal treatment outcomes, and an increased use of health care resources.1-3
It is critical to understand why patients elect to leave a hospital AMA. In a qualitative study, Simon et al1 found that individuals with SUDs often leave AMA due to uncontrolled withdrawal symptoms and pain, perceived stigma and discrimination, and dissatisfaction with care. Predictors of patients leaving the hospital AMA include the severity of their drug dependence and previous negative treatment experiences.4 A systematic review found housing instability and a lack of social support influence an individual’s decision to leave AMA.5
Recommendations for managing patients who leave AMA
Enhancing your understanding of withdrawal symptoms may allow you to offer patients more effective symptom control, possibly with methadone or buprenorphine.2 Injectable opioid agonist treatment may also help to retain a patient in care. In a case report, a 47-year-old man with a severe opioid use disorder who had left the hospital AMA due to uncontrolled opioid withdrawal was readmitted, treated with IV hydromorphone, and enrolled in ongoing community injectable opioid agonist treatment.6
Clinicians must address the stigma and discrimination patients with SUDs often face in health care institutions. Additional training for clinicians to improve their understanding of these disorders and foster a more compassionate and nonjudgmental approach to care may be beneficial.
Like most medicolegal conflicts, leaving AMA is often a clinical and interpersonal problem disguised as a legal one. When assessing these patients’ decision-making capacity, we often find they are angry and dissatisfied with the care they have (or have not) received. The most useful intervention may be to restore communication between the patient and their treatment team.
Even after a patient leaves AMA, the treatment team may experience countertransference issues, such as heightened emotional reactions or biases, that could compromise their clinical judgment. Addressing these dynamics may require team debriefings, supervision, or further training in managing transference and countertransference, particularly since patients who leave AMA may return for subsequent care.7
Integrated care models, which feature close collaboration between clinicians from different specialties, can help ensure that a patient’s diverse health needs are met and reduce the likelihood of them leaving AMA. Integrated care models may be particularly effective for patients with co-occurring conditions such as HIV and SUDs.8
Implementing these recommendations can be challenging. Barriers to addressing AMA departures span several domains, including patient-specific barriers (eg, stigma and discrimination), clinical barriers (eg, lack of resources and training for clinicians), institutional hurdles (eg, systemic inefficiencies), and broader social barriers (eg, housing instability and inadequate social support). Overcoming these barriers requires a multifaceted approach involving clinicians, policymakers, and the community that considers medical, psychological, and social factors.
1. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525.
2. Kenne DR, Boros AP, Fischbein RL. Characteristics of opiate users leaving detoxification treatment against medical advice. J Addict Dis. 2010;29(3):383-394.
3. Mahajan RK, Gautam PL, Paul G, et al. Retrospective evaluation of patients leaving against medical advice in a tertiary care teaching hospital. Indian J Crit Care Med. 2019;23(3):139-142.
4. Armenian SH, Chutuape MA, Stitzer ML. Predictors of discharges against medical advice from a short-term hospital detoxification unit. Drug Alcohol Depend. 1999;56(1):1-8.
5. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59.
6. McAdam M, Brar R, Young S. Initiation of injectable opioid agonist treatment in hospital: a case report. Drug Alcohol Rev. 2020;39(2):138-141.
7. Schouten R, Weintraub BR. Legal aspects of consultation. In: Stern TA, Freudenreich O, Smith FA, et al, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 7th ed. Elsevier; 2018:578-579.
8. Vallecillo G, Robles MJ, Fonseca F, et al. Integrated care on leaving hospital against medical advice among HIV-infected people with substance use disorders. AIDS Res Hum Retroviruses. 2018;34(12):1044-1049.
1. Simon R, Snow R, Wakeman S. Understanding why patients with substance use disorders leave the hospital against medical advice: a qualitative study. Subst Abus. 2020;41(4):519-525.
2. Kenne DR, Boros AP, Fischbein RL. Characteristics of opiate users leaving detoxification treatment against medical advice. J Addict Dis. 2010;29(3):383-394.
3. Mahajan RK, Gautam PL, Paul G, et al. Retrospective evaluation of patients leaving against medical advice in a tertiary care teaching hospital. Indian J Crit Care Med. 2019;23(3):139-142.
4. Armenian SH, Chutuape MA, Stitzer ML. Predictors of discharges against medical advice from a short-term hospital detoxification unit. Drug Alcohol Depend. 1999;56(1):1-8.
5. Ti L, Ti L. Leaving the hospital against medical advice among people who use illicit drugs: a systematic review. Am J Public Health. 2015;105(12):e53-e59.
6. McAdam M, Brar R, Young S. Initiation of injectable opioid agonist treatment in hospital: a case report. Drug Alcohol Rev. 2020;39(2):138-141.
7. Schouten R, Weintraub BR. Legal aspects of consultation. In: Stern TA, Freudenreich O, Smith FA, et al, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 7th ed. Elsevier; 2018:578-579.
8. Vallecillo G, Robles MJ, Fonseca F, et al. Integrated care on leaving hospital against medical advice among HIV-infected people with substance use disorders. AIDS Res Hum Retroviruses. 2018;34(12):1044-1049.
New Therapies in Melanoma: Current Trends, Evolving Paradigms, and Future Perspectives
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2
In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.
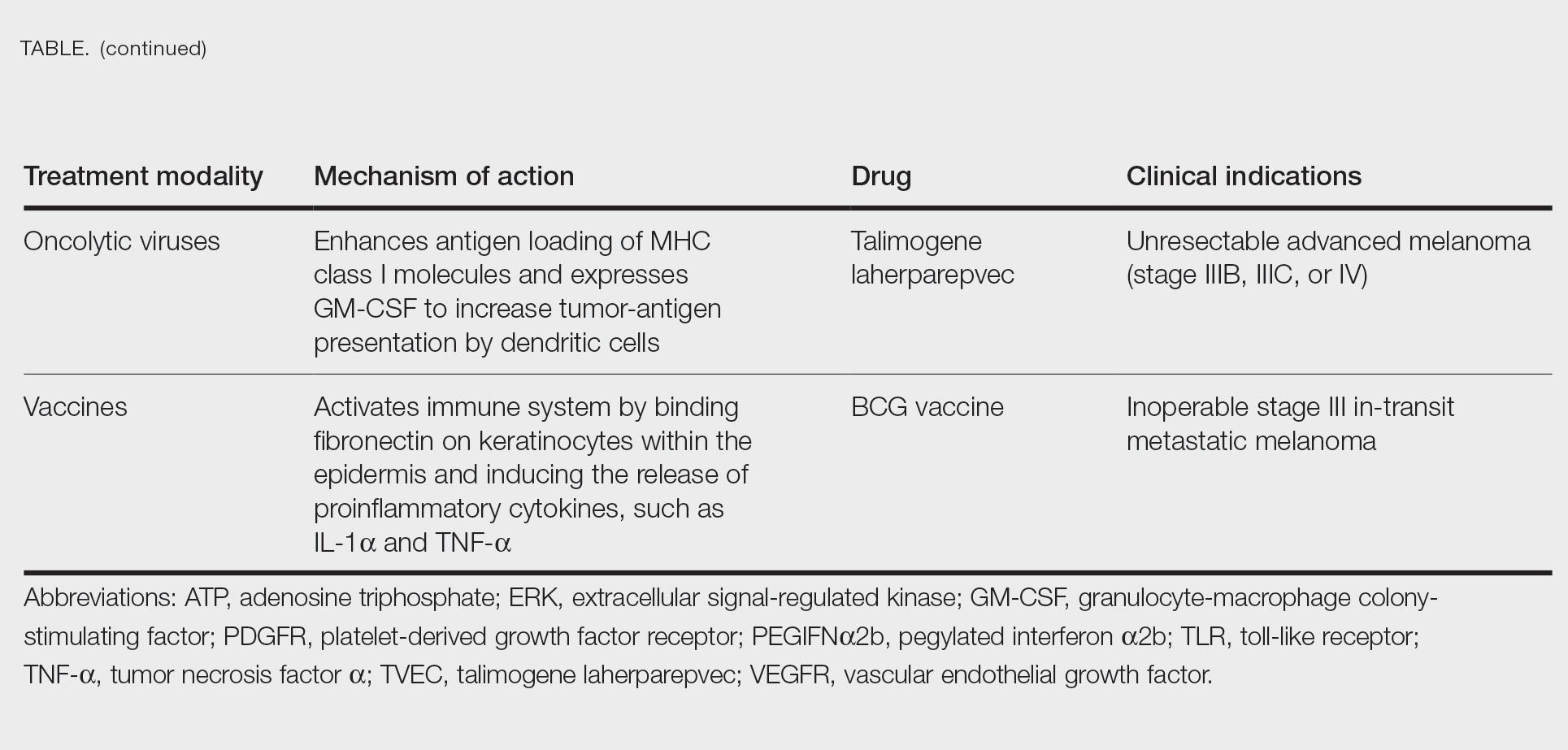
Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8
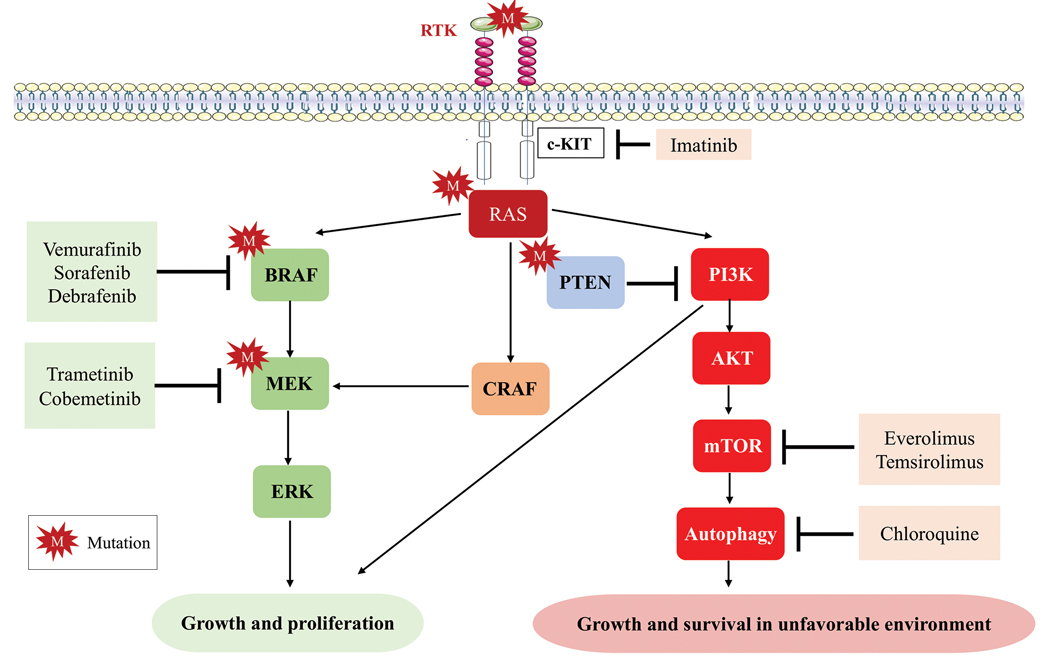
Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2

In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.


Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8

Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
Cutaneous malignant melanoma represents an aggressive form of skin cancer, with 132,000 new cases of melanoma and 50,000 melanoma-related deaths diagnosed worldwide each year.1 In recent decades, major progress has been made in the treatment of melanoma, especially metastatic and advanced-stage disease. Approval of new treatments, such as immunotherapy with anti–PD-1 (pembrolizumab and nivolumab) and anti–CTLA-4 (ipilimumab) antibodies, has revolutionized therapeutic strategies (Figure 1). Molecularly, melanoma has the highest mutational burden among solid tumors. Approximately 40% of melanomas harbor the BRAF V600 mutation, leading to constitutive activation of the mitogen-activated protein kinase (MAPK) signaling pathway.2 The other described genomic subtypes are mutated RAS (accounting for approximately 28% of cases), mutated NF1 (approximately 14% of cases), and triple wild type, though these other subtypes have not been as successfully targeted with therapy to date.3 Dual inhibition of this pathway using combination therapy with BRAF and MEK inhibitors confers high response rates and survival benefit, though efficacy in metastatic patients often is limited by development of resistance. The US Food and Drug Administration (FDA) has approved 3 combinations of targeted therapy in unresectable tumors: dabrafenib and trametinib, vemurafenib and cobimetinib, and encorafenib and binimetinib. The oncolytic herpesvirus talimogene laherparepvec also has received FDA approval for local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in patients with recurrent melanoma after initial surgery.2

In this review, we explore new therapeutic agents and novel combinations that are being tested in early-phase clinical trials (Table). We discuss newer promising tools such as nanotechnology to develop nanosystems that act as drug carriers and/or light absorbents to potentially improve therapy outcomes. Finally, we highlight challenges such as management after resistance and intervention with novel immunotherapies and the lack of predictive biomarkers to stratify patients to targeted treatments after primary treatment failure.


Targeted Therapies
Vemurafenib was approved by the FDA in 2011 and was the first BRAF-targeted therapy approved for the treatment of melanoma based on a 48% response rate and a 63% reduction in the risk for death vs dacarbazine chemotherapy.4 Despite a rapid and clinically significant initial response, progression-free survival (PFS) was only 5.3 months, which is indicative of the rapid development of resistance with monotherapy through MAPK reactivation. As a result, combined BRAF and MEK inhibition was introduced and is now the standard of care for targeted therapy in melanoma. Treatment with dabrafenib and trametinib, vemurafenib and cobimetinib, or encorafenib and binimetinib is associated with prolonged PFS and overall survival (OS) compared to BRAF inhibitor monotherapy, with response rates exceeding 60% and a complete response rate of 10% to 18%.5 Recently, combining atezolizumab with vemurafenib and cobimetinib was shown to improve PFS compared to combined targeted therapy.6 Targeted therapy usually is given as first-line treatment to symptomatic patients with a high tumor burden because the response may be more rapid than the response to immunotherapy. Ultimately, most patients with advanced BRAF-mutated melanoma receive both targeted therapy and immunotherapy.
Mutations of KIT (encoding proto-oncogene receptor tyrosine kinase) activate intracellular MAPK and phosphatidylinositol 3-kinase (PI3K) pathways (Figure 2).7 KIT mutations are found in mucosal and acral melanomas as well as chronically sun-damaged skin, with frequencies of 39%, 36%, and 28%, respectively. Imatinib was associated with a 53% response rate and PFS of 3.9 months among patients with KIT-mutated melanoma but failed to cause regression in melanomas with KIT amplification.8

Anti–CTLA-4 Immune Checkpoint Inhibition
CTLA-4 is a protein found on T cells that binds with another protein, B7, preventing T cells from killing cancer cells. Hence, blockade of CTLA-4 antibody avoids the immunosuppressive state of lymphocytes, strengthening their antitumor action.9 Ipilimumab, an anti–CTLA-4 antibody, demonstrated improvement in median OS for management of unresectable or metastatic stage IV melanoma, resulting in its FDA approval.8 A combination of ipilimumab with dacarbazine in stage IV melanoma showed notable improvement of OS.10 Similarly, tremelimumab showed evidence of tumor regression in a phase 1 trial but with more severe immune-related side effects compared with ipilimumab.11 A second study on patients with stage IV melanoma treated with tremelimumab as first-line therapy in comparison with dacarbazine demonstrated differences in OS that were not statistically significant, though there was a longer duration of an objective response in patients treated with tremelimumab (35.8 months) compared with patients responding to dacarbazine (13.7 months).12
Anti–PD-1 Immune Checkpoint Inhibition
PD-1 is a transmembrane protein with immunoreceptor tyrosine-based inhibitory signaling, identified as an apoptosis-associated molecule.13 Upon activation, it is expressed on the cell surface of CD4, CD8, B lymphocytes, natural killer cells, monocytes, and dendritic cells.14 PD-L1, the ligand of PD-1, is constitutively expressed on different hematopoietic cells, as well as on fibroblasts, endothelial cells, mesenchymal cells, neurons, and keratinocytes.15,16 Reactivation of effector T lymphocytes by PD-1:PD-L1 pathway inhibition has shown clinically significant therapeutic relevance.17 The PD-1:PD-L1 interaction is active only in the presence of T- or B-cell antigen receptor cross-link. This interaction prevents PI3K/AKT signaling and MAPK/extracellular signal-regulated kinase pathway activation with the net result of lymphocytic functional exhaustion.18,19 PD-L1 blockade is shown to have better clinical benefit and minor toxicity compared to anti–CTLA-4 therapy. Treatment with anti-PD1 nivolumab in a phase 1b clinical trial (N=107) demonstrated highly specific action, durable tumor remission, and long-term safety in 32% of patients with advanced melanoma.20 These promising results led to the FDA approval of nivolumab for the treatment of patients with advanced and unresponsive melanoma. A recent clinical trial combining ipilimumab and nivolumab resulted in an impressive increase of PFS compared with ipilimumab monotherapy (11.5 months vs 2.9 months).21 Similarly, treatment with pembrolizumab in advanced melanoma demonstrated improvement in PFS and OS compared with anti–CTLA-4 therapy,22,23 which resulted in FDA approval of pembrolizumab for the treatment of advanced melanoma in patients previously treated with ipilimumab or BRAF inhibitors in BRAF V600 mutation–positive patients.24
Lymphocyte-Activated Gene 3–Targeted Therapies
Nanotechnology in Melanoma Therapy
The use of nanotechnology represents one of the newer alternative therapies employed for treatment of melanoma and is especially gaining interest due to reduced adverse effects in comparison with other conventional treatments for melanoma. Nanotechnology-based drug delivery systems precisely target tumor cells and improve the effect of both the conventional and innovative antineoplastic treatment.27,31 Tumor vasculature differs from normal tissues by being discontinuous and having interspersed small gaps/holes that allow nanoparticles to exit the circulation and enter and accumulate in the tumor tissue, leading to enhanced and targeted release of the antineoplastic drug to tumor cells.32 This mechanism is called the enhanced permeability and retention effect.33
Another mechanism by which nanoparticles work is ligand-based targeting in which ligands such as monoclonal antibodies, peptides, and nucleic acids located on the surface of nanoparticles can bind to receptors on the plasma membrane of tumor cells and lead to targeted delivery of the drug.34 Nanomaterials used for melanoma treatment include vesicular systems such as liposomes and niosomes, polymeric nanoparticles, noble metal-based nanoparticles, carbon nanotubes, dendrimers, solid lipid nanoparticles and nanostructures, lipid carriers, and microneedles. In melanoma, nanoparticles can be used to enhance targeted delivery of drugs, including immune checkpoint inhibitors (ICIs). Cai et al35 described usage of scaffolds in delivery systems. Tumor-associated antigens, adjuvant drugs, and chemical agents that influence the tumor microenvironment can be loaded onto these scaffolding agents. In a study by Zhu et al,36 photosensitizer chlorin e6 and immunoadjuvant aluminum hydroxide were used as a novel nanosystem that effectively destroyed tumor cells and induced a strong systemic antitumor response. IL-2 is a cytokine produced by B or T lymphocytes. Its use in melanoma has been limited by a severe adverse effect profile and lack of complete response in most patients. Cytokine-containing nanogels have been found to selectively release IL-2 in response to activation of T-cell receptors, and a mouse model in melanoma showed better response compared to free IL-1 and no adverse systemic effects.37
Nanovaccines represent another interesting novel immunotherapy modality. A study by Conniot et al38 showed that nanoparticles can be used in the treatment of melanoma. Nanoparticles made of biodegradable polymer were loaded with Melan-A/MART-1 (26–35 A27L) MHC class I-restricted peptide (MHC class I antigen), and the limited peptide MHC class II Melan-A/MART-1 51–73 (MHC class II antigen) and grafted with mannose that was then combined with an anti–PD-L1 antibody and injected into mouse models. This combination resulted in T-cell infiltration at early stages and increased infiltration of myeloid-derived suppressor cells. Ibrutinib, a myeloid-derived suppressor cell inhibitor, was added and demonstrated marked tumor remission and prolonged survival.38
Overexpression of certain microRNAs (miRNAs), especially miR-204-5p and miR-199b-5p, has been shown to inhibit growth of melanoma cells in vitro, both alone and in combination with MAPK inhibitors, but these miRNAs are easily degradable in body fluids. Lipid nanoparticles can bind these miRNAs and have been shown to inhibit tumor cell proliferation and improve efficacy of BRAF and MEK inhibitors.39
Triple-Combination Therapy
Immune checkpoint inhibitors such as anti–PD-1 or anti–CTLA-4 drugs have become the standard of care in treatment of advanced melanoma. Approximately 40% to 50% of cases of melanoma harbor BRAF mutations, and patients with these mutations could benefit from BRAF and MEK inhibitors. Data from clinical trials on BRAF and MEK inhibitors even showed initial high objective response rates, but the response was short-lived, and there was frequent acquired resistance.40 With ICIs, the major limitation was primary resistance, with only 50% of patients initially responding.41 Studies on murine models demonstrated that BRAF-mutated tumors had decreased expression of IFN-γ, tumor necrosis factor α, and CD40 ligand on CD4+ tumor-infiltrating lymphocytes and increased accumulation of regulatory T cells and myeloid-derived suppressor cells, leading to a protumor microenvironment. BRAF and MEK pathway inhibition were found to improve intratumoral CD4+ T-cell activity, leading to improved antitumor T-cell responses.42 Because of this enhanced immune response by BRAF and MEK inhibitors, it was hypothesized and later supported by clinical research that a combination of these targeted treatments and ICIs can have a synergistic effect, leading to increased antitumor activity.43 A randomized phase 2 clinical trial (KEYNOTE-022) in which the treatment group was given pembrolizumab, dabrafenib, and trametinib and the control group was treated with dabrafenib and trametinib showed increased medial OS in the treatment group vs the control group (46.3 months vs 26.3 months) and more frequent complete response in the treatment group vs the control group (20% vs 15%).44 In the IMspire150 phase 3 clinical trial, patients with advanced stage IIIC to IV BRAF-mutant melanoma were treated with either a triple combination of the PDL-1 inhibitor atezolizumab, vemurafenib, and cobimetinib or vemurafenib and cobimetinib. Although the objective response rate was similar in both groups, the median duration of response was longer in the triplet group compared with the doublet group (21 months vs 12.6 months). Given these results, the FDA approved the triple-combination therapy with atezolizumab, vemurafenib, and cobimetinib. Although triple-combination therapy has shown promising results, it is expected that there will be an increase in the frequency of treatment-related adverse effects. In the phase 3 COMBi-I study, patients with advanced stage IIIC to IV BRAF V600E mutant cutaneous melanoma were treated with either a combination of spartalizumab, dabrafenib, and trametinib or just dabrafenib and trametinib. Although the objective response rates were not significantly different (69% vs 64%), there was increased frequency of treatment-related adverse effects in patients receiving triple-combination therapy.43 As more follow-up data come out of these ongoing clinical trials, benefits of triple-combination therapy and its adverse effect profile will be more definitely established.
Challenges and Future Perspectives
One of the major roadblocks in the treatment of melanoma is the failure of response to ICI with CTLA-4 and PD-1/PD-L1 blockade in a large patient population, which has resulted in the need for new biomarkers that can act as potential therapeutic targets. Further, the main underlying factor for both adjuvant and neoadjuvant approaches remains the selection of patients, optimizing therapeutic outcomes while minimizing the number of patients exposed to potentially toxic treatments without gaining clinical benefit. Clinical and pathological factors (eg, Breslow thickness, ulceration, the number of positive lymph nodes) play a role in stratifying patients as per risk of recurrence.45 Similarly, peripheral blood biomarkers have been proposed as prognostic tools for high-risk stage II and III melanoma, including markers of systemic inflammation previously explored in the metastatic setting.46 However, the use of these parameters has not been validated for clinical practice. Currently, despite promising results of BRAF and MEK inhibitors and therapeutic ICIs, as well as IL-2 or interferon alfa, treatment options in metastatic melanoma are limited because of its high heterogeneity, problematic patient stratification, and high genetic mutational rate. Recently, the role of epigenetic modifications andmiRNAs in melanoma progression and metastatic spread has been described. Silencing of CDKN2A locus and encoding for p16INK4A and p14ARF by DNA methylation are noted in 27% and 57% of metastatic melanomas, respectively, which enables melanoma cells to escape from growth arrest and apoptosis generated by Rb protein and p53 pathways.47 Demethylation of these and other tumor suppressor genes with proapoptotic function (eg, RASSF1A and tumor necrosis factor–related apoptosis-inducing ligand) can restore cell death pathways, though future clinical studies in melanoma are warranted.48
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
- Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. J Clin Oncol. 2013;31:4172-4178.
- Moreira A, Heinzerling L, Bhardwaj N, et al. Current melanoma treatments: where do we stand? Cancers (Basel). 2021;13:221.
- Watson IR, Wu C-J, Zou L, et al. Genomic classification of cutaneous melanoma. Cancer Res. 2015;75(15 Suppl):2972.
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516.
- Hamid O, Cowey CL, Offner M, et al. Efficacy, safety, and tolerability of approved combination BRAF and MEK inhibitor regimens for BRAF-mutant melanoma. Cancers (Basel). 2019;11:1642.
- Gutzmer R, Stroyakovskiy D, Gogas H, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2020;395:1835-1844.
- Reddy BY, Miller DM, Tsao H. Somatic driver mutations in melanoma. Cancer. 2017;123(suppl 11):2104-2117.
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31:3182-3190.
- Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65-97.
- Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma—a review of current and future treatment options. Acta Derm Venereol. 2015;95:516-524.
- Ribas A, Chesney JA, Gordon MS, et al. Safety profile and pharmacokinetic analyses of the anti-CTLA4 antibody tremelimumab administered as a one hour infusion. J Transl Med. 2012;10:1-6.
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
- BG Neel, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284-293.
- Ishida Y, Agata Y, Shibahara K, et al. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887-3895.
- Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538-5545.
- Keir ME, Butte MJ, Freeman GJ et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677-704.
- Blank C, Kuball J, Voelkl S, et al. Blockade of PD‐L1 (B7‐H1) augments human tumor‐specific T cell responses in vitro. Int J Cancer. 2006;119:317-327.
- Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553.
- Patsoukis N, Brown J, Petkova V, et al. Selective effects of PD-1 on Akt and Ras pathways regulate molecular components of the cell cycle and inhibit T cell proliferation. Sci Signal. 2012;5:ra46.
- Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020-1030.
- Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320-330.
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006-2017.
- Burns MC, O’Donnell A, Puzanov I. Pembrolizumab for the treatment of advanced melanoma. Exp Opin Orphan Drugs. 2016;4:867-873.
- F Triebel. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619-622.
- Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer. 2020;8:e001014.
- Shi J, Kantoff PW, Wooster R, et al. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. 2017;17:20-37.
- Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386:24-34.
- US Food and Drug Administration approves first LAG-3-blocking antibody combination, Opdualag™ (nivolumab and relatlimab-rmbw), as treatment for patients with unresectable or metastatic melanoma. Press release. Bristol Myers Squibb. March 18, 2022. Accessed November 7, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-First-LAG-3-Blocking-Antibody-Combination-Opdualag-nivolumab-and-relatlimab-rmbw-as-Treatment-for-Patients-with-Unresectable-or-Metastatic-Melanoma/default.aspx
- Zhao B-W, Zhang F-Y, Wang Y, et al. LAG3-PD1 or CTLA4-PD1 inhibition in advanced melanoma: indirect cross comparisons of the CheckMate-067 and RELATIVITY-047 trials. Cancers (Basel). 2022;14:4975.
- Jin C, Wang K, Oppong-Gyebi A, et al. Application of nanotechnology in cancer diagnosis and therapy-a mini-review. Int J Med Sci. 2020;17:2964-2973.
- Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Del Rev. 2015;91:3-6.
- Iyer AK, Khaled G, Fang J, et al. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812-818.
- Beiu C, Giurcaneanu C, Grumezescu AM, et al. Nanosystems for improved targeted therapies in melanoma. J Clin Med. 2020;9:318.
- Cai L, Xu J, Yang Z, et al. Engineered biomaterials for cancer immunotherapy. MedComm. 2020;1:35-46.
- Zhu Y, Xue J, Chen W, et al. Albumin-biomineralized nanoparticles to synergize phototherapy and immunotherapy against melanoma. J Control Release. 2020;322:300-311.
- Zhang Y, Li N, Suh H, et al. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6.
- Conniot J, Scomparin A, Peres C, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891-901.
- Fattore L, Campani V, Ruggiero CF, et al. In vitro biophysical and biological characterization of lipid nanoparticles co-encapsulating oncosuppressors miR-199b-5p and miR-204-5p as potentiators of target therapy in metastatic melanoma. Int J Mol Sci. 2020;21:1930.
- Welti M, Dimitriou F, Gutzmer R, et al. Triple combination of immune checkpoint inhibitors and BRAF/MEK inhibitors in BRAF V600 melanoma: current status and future perspectives. Cancers (Basel). 2022;14:5489.
- Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol. 2019;10:453.
- Ho P-C, Meeth KM, Tsui Y-C, et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγBRAF inhibitor-induced antitumor immunity. Cancer Res. 2014;74:3205-3217.
- Dummer R, Sandhu SK, Miller WH, et al. A phase II, multicenter study of encorafenib/binimetinib followed by a rational triple-combination after progression in patients with advanced BRAF V600-mutated melanoma (LOGIC2). J Clin Oncol. 2020;38(15 suppl):10022.
- Ferrucci PF, Di Giacomo AM, Del Vecchio M, et al. KEYNOTE-022 part 3: a randomized, double-blind, phase 2 study of pembrolizumab, dabrafenib, and trametinib in BRAF-mutant melanoma. J Immunother Cancer. 2020;8:e001806.
- Madu MF, Schopman JH, Berger DM, et al. Clinical prognostic markers in stage IIIC melanoma. J Surg Oncol. 2017;116:244-251.
- Davis JL, Langan RC, Panageas KS, et al. Elevated blood neutrophil-to-lymphocyte ratio: a readily available biomarker associated with death due to disease in high risk nonmetastatic melanoma. Ann Surg Oncol. 2017;24:1989-1996.
- Freedberg DE, Rigas SH, Russak J, et al. Frequent p16-independent inactivation of p14ARF in human melanoma. J Natl Cancer Inst. 2008;100:784-795.
- Sigalotti L, Covre A, Fratta E, et al. Epigenetics of human cutaneous melanoma: setting the stage for new therapeutic strategies. J Transl Med. 2010;8:1-22.
Practice Points
- Immune checkpoint inhibition has resulted in a paradigm shift for the treatment of metastatic melanoma.
- Alternative therapies with novel targets such as lymphocyte-activated gene 3 aim to overcome resistance to the usual immune targets such asPD-1/PD-L1 and CTLA-4.
- Newer promising tools such as nanotechnology are being added to the growing armamentarium of melanoma treatment strategies.
Neutrophilic Dermatosis of the Dorsal Hand: A Distinctive Variant of Sweet Syndrome
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.
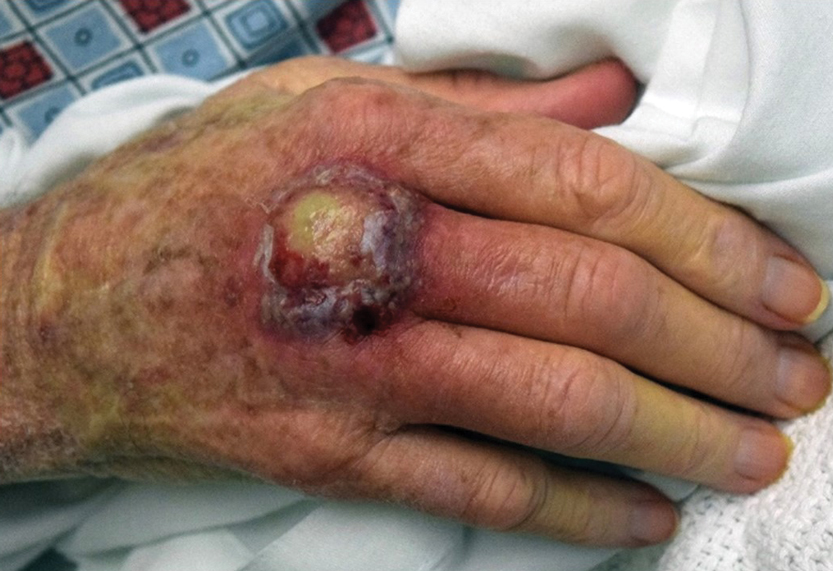
A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.
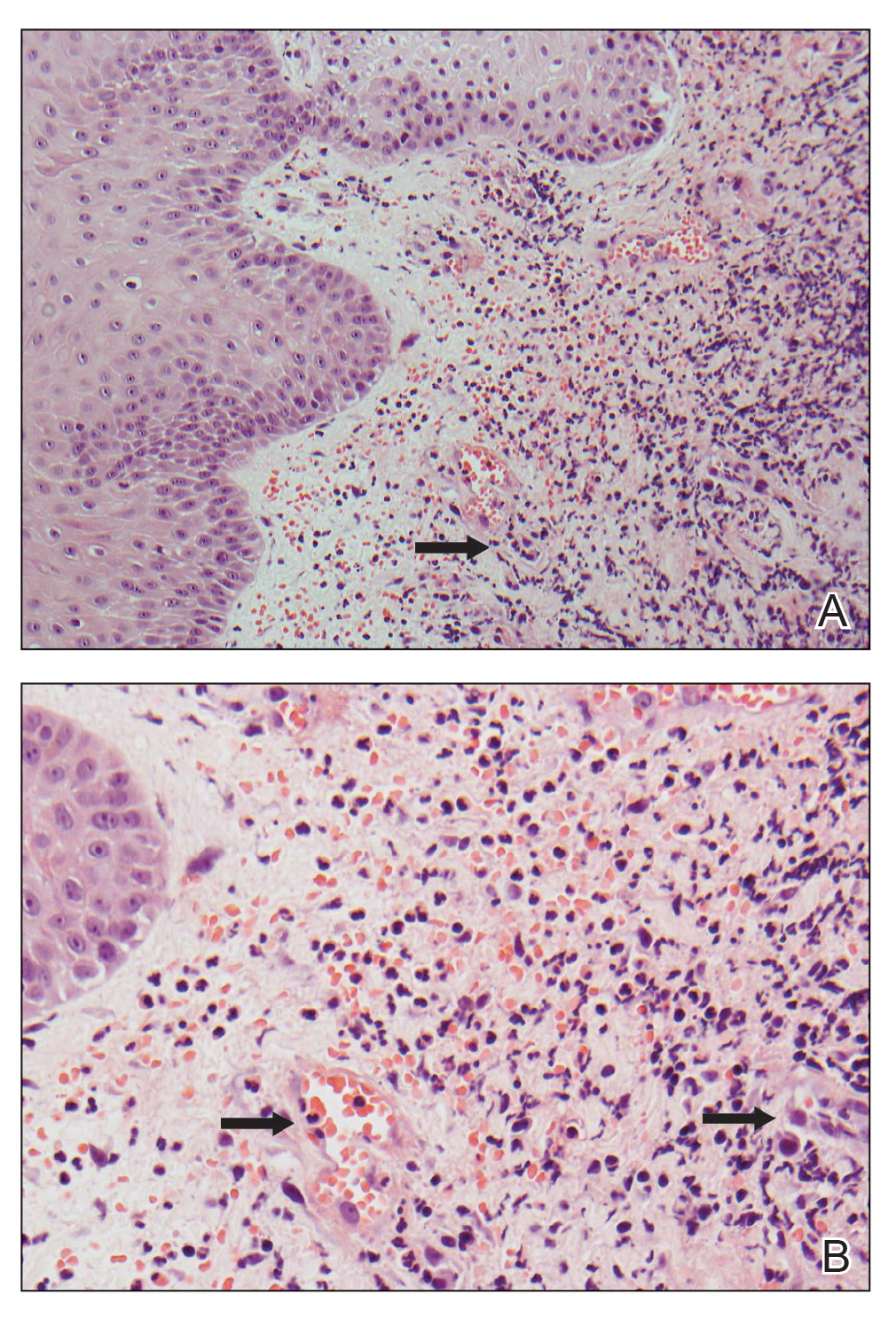
Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.

A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.

Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
To the Editor:
Neutrophilic dermatosis of the dorsal hand (NDDH) is an uncommon reactive neutrophilic dermatosis that presents as a painful, enlarging, ulcerative nodule. It often is misdiagnosed and initially treated as an infection. Similar to other neutrophilic dermatoses, it is associated with underlying infections, inflammatory conditions, and malignancies. Neutrophilic dermatosis of the dorsal hand is considered a subset of Sweet syndrome (SS); we highlight similarities and differences between NDDH and SS, reporting the case of a 66-year-old man without systemic symptoms who developed NDDH on the right hand.

A 66-year-old man presented with a progressively enlarging, painful, ulcerative, 2-cm nodule on the right hand following mechanical trauma 2 weeks prior (Figure 1). He was afebrile with no remarkable medical history. Laboratory evaluation revealed an erythrocyte sedimentation rate (ESR) of 20 mm/h (reference range, 0-10 mm/h) and C-reactive protein (CRP) level of 3.52 mg/dL (reference range, 0-0.5 mg/dL) without leukocytosis; both were not remarkably elevated when adjusted for age.1,2 The clinical differential diagnosis was broad and included pyoderma with evolving cellulitis, neutrophilic dermatosis, atypical mycobacterial infection, subcutaneous or deep fungal infection, squamous cell carcinoma, cutaneous lymphoma, and metastasis. Due to the rapid development of the lesion, initial treatment focused on a bacterial infection, but there was no improvement on antibiotics and wound cultures were negative. The ulcerative nodule was biopsied, and histopathology demonstrated abundant neutrophilic inflammation, endothelial swelling, and leukocytoclasis without microorganisms (Figure 2). Tissue cultures for bacteria, fungi, and atypical mycobacteria were negative. A diagnosis of NDDH was made based on clinical and histologic findings. The wound improved with a 3-week course of oral prednisone.

Neutrophilic dermatosis of the dorsal hand is a subset of reactive neutrophilic dermatoses, which includes SS (acute febrile neutrophilic dermatosis) and pyoderma gangrenosum. It is described as a localized variant of SS, with similar associated underlying inflammatory, neoplastic conditions and laboratory findings.3 However, NDDH has characteristic features that differ from classic SS. Neutrophilic dermatosis of the dorsal hand typically presents as painful papules, pustules, or ulcers that progress to become larger ulcers, plaques, and nodules. The clinical appearance may more closely resemble pyoderma gangrenosum or atypical SS, with ulceration frequently present. Pathergy also may be demonstrated in NDDH, similar to our patient. The average age of presentation for NDDH is 60 years, which is older than the average age for SS or pyoderma gangrenosum.3 Similar to other neutrophilic dermatoses, NDDH responds well to oral steroids or steroid-sparing immunosuppressants such as dapsone, colchicine, azathioprine, or tetracycline antibiotics.4
The criteria for SS are well established5,6 and may be used for the diagnosis of NDDH, taking into account the localization of lesions to the dorsal aspect of the hands. The diagnostic criteria for SS include fulfillment of both major and at least 2 of 4 minor criteria. The 2 major criteria include rapid presentation of skin lesions and neutrophilic dermal infiltrate on biopsy. Minor criteria are defined as the following: (1) preceding nonspecific respiratory or gastrointestinal tract infection, inflammatory conditions, underlying malignancy, or pregnancy; (2) fever; (3) excellent response to steroids; and (4) 3 of the 4 of the following laboratory abnormalities: elevated CRP, ESR, leukocytosis, or left shift in complete blood cell count. Our patient met both major criteria and only 1 minor criterion—excellent response to systemic corticosteroids. Nofal et al7 advocated for revised diagnostic criteria for SS, with one suggestion utilizing only the 2 major criteria being necessary for diagnosis. Given that serum inflammatory markers may not be as elevated in NDDH compared to SS,3,7,8 meeting the major criteria alone may be a better way to diagnose NDDH, as in our patient.
Our patient presented with an expanding ulcerating nodule on the hand that elicited a wide list of differential diagnoses to include infections and neoplasms. Rapid development, localization to the dorsal aspect of the hand, and treatment resistance to antibiotics may help the clinician consider a diagnosis of NDDH, which should be confirmed by a biopsy. Similar to other neutrophilic dermatoses, an underlying malignancy or inflammatory condition should be sought out. Neutrophilic dermatosis of the dorsal hand responds well to systemic steroids, though recurrences may occur.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med (Clinical Res Ed). 1983;286:226.
- Wyczalkowska-Tomasik A, Czarkowska-Paczek B, Zielenkiewicz M, et al. Inflammatory markers change with age, but do not fall beyond reported normal ranges. Arch Immunol Ther Exp (Warsz). 2016;64:249-254.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Gaulding J, Kohen LL. Neutrophilic dermatosis of the dorsal hands. J Am Acad Dermatol. 2017; 76(6 suppl 1):AB178.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Nofal A, Abdelmaksoud A, Amer H, et al. Sweet’s syndrome: diagnostic criteria revisited. J Dtsch Dermatol Ges. 2017;15:1081-1088.
- Wolf R, Tüzün Y. Acral manifestations of Sweet syndrome (neutrophilic dermatosis of the hands). Clin Dermatol. 2017;35:81-84.
Practice Points
- Neutrophilic dermatosis of the dorsal hand (NDDH) is a reactive neutrophilic dermatosis that includes Sweet syndrome (SS) and pyoderma gangrenosum.
- Localization to the dorsal aspect of the hand, presence of ulcerative nodules, and older age at onset are characteristic features of NDDH.
- Meeting the major criteria alone for SS may be a more sensitive way to diagnose NDDH, as serum inflammatory markers may not be remarkably elevated in this condition.