User login
Mapping Pathology Work Associated With Precision Oncology Testing
Mapping Pathology Work Associated With Precision Oncology Testing
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
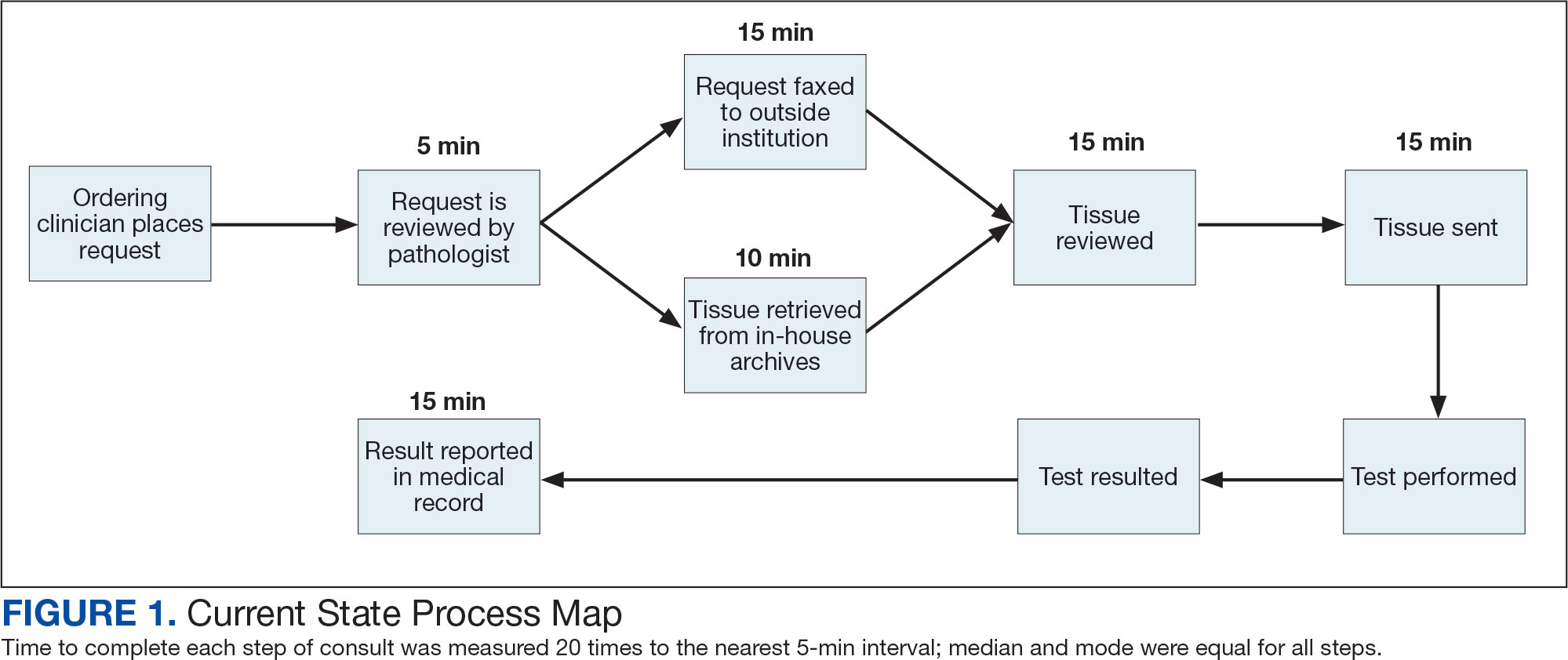
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.
The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.
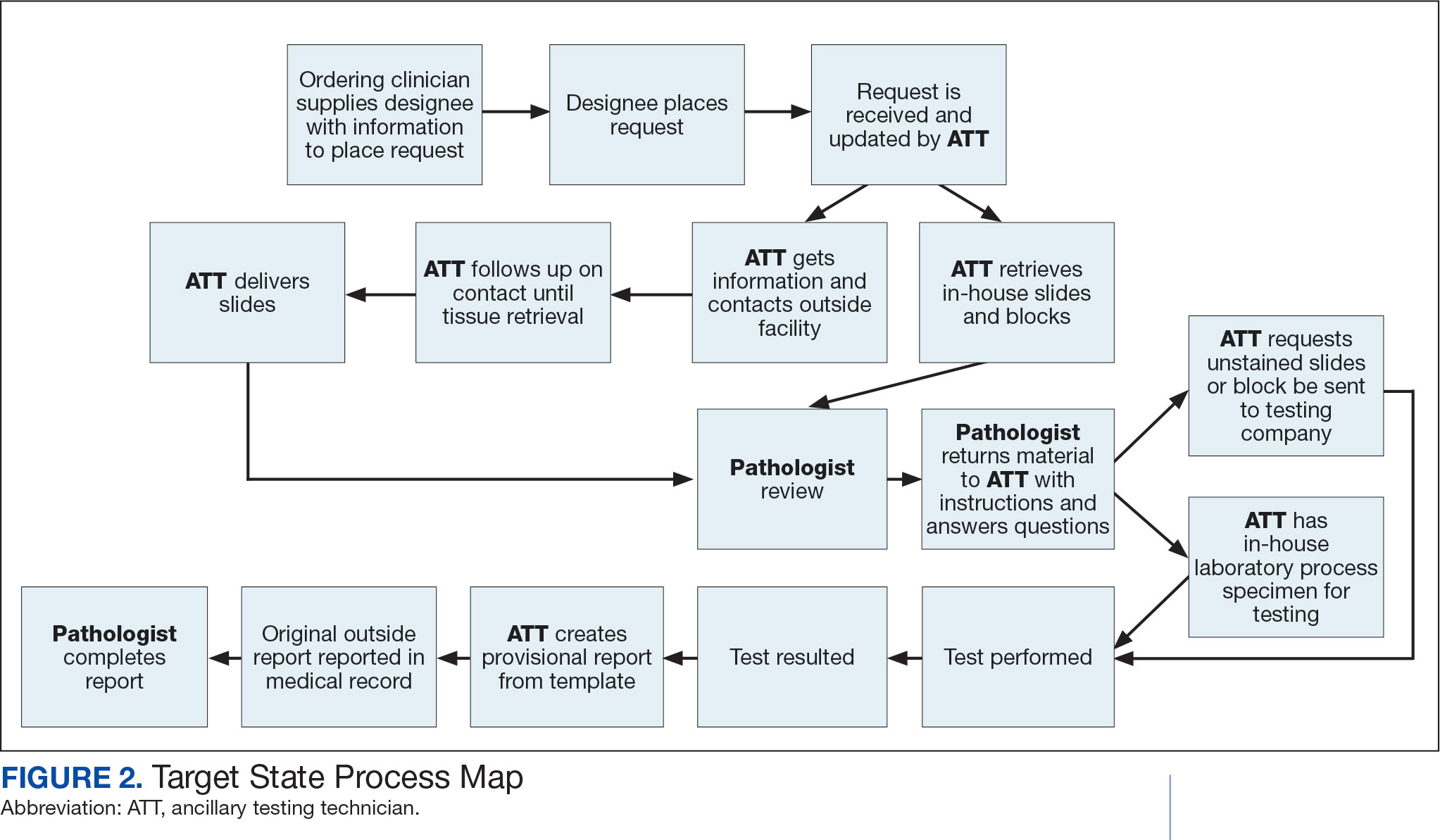
The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.

The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.

The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
Comprehensive genomic profiling (CGP) is becoming progressively common and appropriate as the array of molecular targets expands. However, most hospital laboratories in the United States do not perform CGP assays in-house; instead, these tests are sent to reference laboratories. As evidenced by Inal et al, only a minority of guideline-indicated molecular testing is performed.1
The workload associated with referral testing is a barrier to increased use of such tests; streamlined processes in pathology might increase molecular test use. At 6 high-complexity US Department of Veterans Affairs (VA) medical centers (VAMCs) (Manhattan, Los Angeles, San Diego, Denver, Kansas City, and Salisbury, Maryland) ranging from 150 to 750 beds, a consult process for anatomic pathology molecular testing has increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care. This report comprehensively describes and maps the anatomic pathology molecular testing consult process at a VAMC. We present areas of inefficiency and a target state process map that incorporates best practices.
MOLECULAR TESTING CONSULT PROCESS
At the Kansas City VAMC (KCVAMC), a consult process for anatomic pathology molecular testing was introduced in 2021. Prior to this, requesting anatomic pathology molecular testing was not standardized. A variety of opportunities and methods were used for requests (eg, phone, page, Teams message, email, Computerized Patient Record System alert; or in-person during tumor board, an office meeting, or in passing). Requests were not documented in a standardized way, resulting in duplicate requests. Testing status and updates were documented outside the medical record, so requests for status updates (via various opportunities and methods) were common and redundant. Data from the year preceding consult implementation and the year following consult implementation have demonstrated increased test utilization, appropriateness of orders, standardization of reporting, and efficiency of care.
Consult Request
The precision oncology testing process starts with a health care practitioner (HCP) request on behalf of any physician or advanced practice registered nurse. It can be placed by any health care employee and directed to a designated employee in the pathology department. The request is ultimately reviewed by a pathologist (Figure 1). At KCVAMC, this request comes in the form of a consult in the electronic health record (EHR) from the ordering HCP to a pathologist. The KCVAMC pathology consult form was previously published with a discussion of the rationale for this process as opposed to a laboratory order process.2 This consult form ensures ordering HCPs supply all necessary information for the pathologist to approve the request and order the test without needing to, in most cases, contact the ordering HCP for clarification or additional information. The form asks the ordering HCP to specify which test is being requested and why. Within the Veterans Health Administration (VHA) there are local and national contracts with many laboratories with hundreds of precision oncology tests to choose from. Consulting with a pathologist is necessary to determine which test is most appropriate.

The precision oncology consult form cannot be submitted without completing all required fields. It also contains indications for the test the ordering HCP selects to minimize unintentionally inappropriate orders. The form asks which tissue the requestor expects the test to be performed on. The requestor must provide contact information for the originating institution when the tissue was collected outside the VHA. The consult form also asks whether another anatomic site is accessible and could be biopsied without unacceptable risk or impracticality, should all previously collected tissue be insufficient. For CGP requests, this allows the pathologist to determine the appropriateness of liquid biopsy without having to reach out to the ordering HCP or wait for the question to be addressed at a tumor board. When a companion diagnostic is available for a test, the ordering HCP is asked which drug will be used so that the most appropriate assay is chosen.
Consult Review
Pathology service involvement begins with pathologist review of the consult form to ensure that the correct test is indicated. Depending on the resources and preferences at a site, consults can be directed to and reviewed by the pathologist associated with the corresponding pathology specimen or to a single pathologist or group of pathologists charged with attending to consults.
The patient’s EHR is reviewed to verify that the test has not already been performed and to determine which tissue to review. Previous surgical pathology reports are examined to assess whether sufficient tissue is available for testing, which may be determined without the need for direct slide examination. Pathologists often use wording such as “rare cells” or in some cases specify that there are not enough lesional cells for ancillary testing. In biopsy reports, the percentage of tissue occupied by lesional cells or the greatest linear length of tumor cells is often documented. As for quality, pathologists may note that a specimen is largely necrotic, and gross descriptions will indicate if a specimen was compromised for molecular analysis by exposure to fixatives such as Bouin’s solution, B-5, or decalcifying agents that contain strong acids.
Tissue Retrieval
If, after such evaluation, the test is indicated and there is tissue that could be sufficient for testing, retrieval of the tissue is pursued. For in-house cases, the pathologist reviews the corresponding surgical pathology report to determine which blocks and slides to pull from the archives. In the cancer checklist, some pathologists specify the best block for subsequent ancillary studies. From the final diagnosis and gross description, the pathologist can determine which blocks are most likely to contain lesional tissue. These slides are retrieved from the archives.
For cases collected at an outside institution (other VHA facility or non-VHA facility/institution), the outside institution must be contacted to retrieve the needed slides and blocks. The phone numbers, fax numbers, email addresses, and mailing addresses for outside institutions are housed in an electronic file and are specific to the point of contact for such requests. Maintaining a record of contacts increases efficiency of the overall process; gathering contact information and successfully requesting tissue often involves multiple automated answering systems, misdirected calls, and failed attempts.
Tissue Review
After retrieving in-house tissue, the pathologist can proceed directly to slide review. For outside cases, the case must first be accessioned so that after review of the slides the pathologist can issue a report to confirm the outside diagnosis. In reviewing the slides, the pathologist looks to see that the diagnosis is correct, that there is a sufficient number of lesional cells in a section, that the lesional cells are of a sufficient concentration in a section, or subsection of the section that could be dissected, and that the cells are viable. Depending on the requested assay and the familiarity of the pathologist with that assay, the pathologist may need to look up the technical requirements of the assay and capabilities of the testing company. Assays vary in sensitivity and require differing amounts and concentrations of tumor. Some companies will dissect tissue, others will not.
If there is sufficient tissue in the material reviewed, the corresponding blocks are retrieved from in-house archives or requests are placed for outside blocks or unstained slides. If there was not enough tissue for testing, the same process is repeated to retrieve and evaluate any other specimens the patient may have. If there are no other specimens to review, this is simply communicated to the ordering HCP via the consult. If the patient is a candidate for liquid biopsy—ie, current specimens are of insufficient quality and/or quantity and a new tissue sample cannot be obtained due to unacceptable risk or impracticality—the order is placed at this time.
Tissue Transport and Testing
Unstained slides need to be cut unless blocks are sent. Slides, blocks, reports, and requisition forms are packaged for transport. An accession number is created for the precision oncology molecular laboratory test in the clinical laboratory section of the EHR system. The clinical laboratory accession number provides a way of tracking sendout testing status. The case is accessioned just prior to placement in the mail so that when an accession number appears in the EHR, the ordering HCP knows the case has been sent out. When results are received, the clinical laboratory accession is completed and a comment is added to indicate where in the EHR to find the report or, when applicable, notes that testing failed.
RESULT REPORTING
When a result becomes available, the report file is downloaded from the vendor portal. This full report is securely transmitted to the ordering HCP. The file is then scanned into the EHR. Additionally, salient findings from the report are abstracted by the pathologist for inclusion as a supplement to the anatomic pathology case. This step ensures that this information travels with the anatomic pathology report if the patient’s care is transferred elsewhere. Templates are used to ensure essential data is captured based on the type of test. The template reminds the pathologist to comment on things such as variants that may represent clonal hematopoiesis, variants that may be germline, and variants that qualify a patient for germline testing. Even with the template, the pathologist must spend significant time reviewing the chart for things such as personal cancer history, other medical history, other masses on imaging, family history, previous surgical pathology reports, and previous molecular testing.
If results are suboptimal, recommendations for repeat testing are made based on the consult response to the question of repeat biopsy feasibility and review of previous pathology reports. The final consult report is added as a consult note, the consult is completed, and the original vendor report file is associated with the consult note in the EHR.
Ancillary Testing Technician
Due to chronic KCVAMC understaffing in the clerical office, gross room, and histology, most of the consult tasks are performed by a pathologist. In an ideal scenario, the pathology staff would divide its time between a pathologist and another dedicated laboratory position, such as an ancillary testing technician (ATT). The ATT can assume responsibilities that do not require the expertise of a pathologist (Figure 2). In such a process, the only steps that would require a pathologist would be review of requests and slides and completion of the interpretive report. All other steps could be accomplished by someone who lacks certifications, laboratory experience, or postsecondary education.

The ATT can receive the requests and retrieve slides and blocks. After slides have been reviewed by a pathologist, the pathologist can inform the ATT which slides or blocks testing will be performed on, provide any additional necessary information for completing the order, and answer any questions. For send-out tests, this allows the ATT to independently complete online portal forms and all other physical requirements prior to delivery of the slides and blocks to specimen processors in the laboratory.
ATTs can keep the ordering HCPs informed of status and be identified as the point of contact for all status inquiries. ATTs can receive results and get outside reports scanned into the EHR. Finally, ATTs can use pathologistdesigned templates to transpose information from outside reports such that a provisional report is prepared and a pathologist does not spend time duplicating information from the outside report. The pathologist can then complete the report with information requiring medical judgment that enhances care.
Optimal Pathologist Involvement
Only 3 steps in the process (request review, tissue review, and completion of an interpretive report) require a pathologist, which are necessary for optimal care and to address barriers to precision oncology.3 While the laboratory may consume only 5% of a health system budget, optimal laboratory use could prevent as much as 30% of avoidable costs.4 These estimates are widely recognized and addressed by campaigns such as Choosing Wisely, as well as programming of alerts and hard stops in EHR systems to reduce duplicate or otherwise inappropriate orders. The tests associated with precision oncology, such as CGP assays, require more nuanced consideration that is best achieved through pathology consultation. In vetting requests for such tests, the pathologist needs information that ordering HCPs do not routinely provide when ordering other tests. A consult asking for such information allows an ordering HCP to efficiently convey this information without having to call the laboratory to circumvent a hard stop.
Regardless of whether a formal electronic consult is used, pathologists must be involved in the review of requests. Creation of an original in-house report also provides an opportunity for pathologists to offer their expertise and maximize the contribution of pathology to patient care. If outside (other VHA facility or non-VHA facility/institution) reports are simply scanned into the EHR without review and issuance of an interpretive report by an in-house pathologist, then an interpretation by a pathologist with access to the patient’s complete chart is never provided. Testing companies are not provided with every patient diagnosis, so in patients with multiple neoplastic conditions, a report may seem to indicate that a detected mutation is from 1 tumor when it is actually from another. Even when all known diagnoses are considered, a variant may be detected that the medical record could reveal to indicate a new diagnosis.
Variation in reporting between companies necessitates pathologist review to standardize care. Some companies indicate which variants may represent clonal hematopoiesis, while others will simply list the pathogenic variants. An oncologist who sees a high volume of hematolymphoid neoplasia may recognize which variants may represent clonal hematopoiesis, but others may not. Reports from the same company may vary, and their interpretation often requires a pathologist's expertise. For example, even if a sample meets the technical requirements for analysis, the report may indicate that the quality or quantity of DNA has reduced the sensitivity for genomic alteration detection. A pathologist would know how to use this information in deciding how to proceed. In a situation where quantity was the issue, the pathologist may know there is additional tissue that could be sent for testing. If quality is the issue, the pathologist may know that additional blocks from the same case likely have the same quality of DNA and would also be unsuitable for testing.
Pathologist input is necessary for precision oncology testing. Some tasks that would ideally be completed by a molecular pathologist (eg, creation of reports to indicate which variants may represent clonal hematopoiesis of indeterminate potential) may be sufficiently completed by a pathologist without fellowship training in molecular pathology.
There are about 15,000 full-time pathologists in the US.4 In the 20 years since molecular genetic pathology was formally recognized as a specialty, there have been < 500 pathologists who have pursued fellowship training in this specialty.5 With the inundation of molecular variants uncovered by routine next-generation sequencing (NGS), there are too few fellowship-trained molecular pathologists to provide all such aforementioned input; it is incumbent on surgical pathologists in general to take on such responsibilities.
Consult Implementation Data
These results support the feasibility and effectiveness of the consult process. Prior to consult implementation, many requests were not compliant with VHA National Precision Oncology Program (NPOP) testing guidelines. Since enactment of the consult, > 90% of requests have been in compliance. In the year preceding the consult (January 2020 to December 2021), 55 of 211 (26.1%) metastatic lung and prostate cancers samples eligible for NGS were tested and 126 (59.7%) NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 151 days. In the year following enactment of the consult (January 2021 to December 2022), 168 of 224 (75.0%) of metastatic lung and prostate cancers eligible for NGS were tested and all 224 NGS vendor reports were scanned into the EHR. The mean time from metastasis to NGS result was 83 days. These data indicate that the practices recommended increase test use, appropriateness of orders, standardization of reporting, and efficiency of care.
CONCLUSIONS
Processing precision oncology testing requires substantial work for pathology departments. Laboratory workforce shortages and ever-expanding indications necessitate additional study of pathology processes to manage increasing workload and maintain the highest quality of cancer care through maximal efficiency and the development of appropriate staffing models. The use of a consult for anatomic pathology molecular testing is one process that can increase test use, appropriateness of orders, standardization of reporting, and efficiency of care. This report provides a comprehensive description and mapping of the process, highlights best practices, identifies inefficiencies, and provides a description and mapping of a target state.
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
- Inal C, Yilmaz E, Cheng H, et al. Effect of reflex testing by pathologists on molecular testing rates in lung cancer patients: experience from a community-based academic center. J Clin Oncol. 2014;32(15 suppl):8098. doi:10.1200/jco.2014.32.15_suppl.8098
- Mettman D, Goodman M, Modzelewski J, et al. Streamlining institutional pathway processes: the development and implementation of a pathology molecular consult to facilitate convenient and efficient ordering, fulfillment, and reporting for tissue molecular tests. J Clin Pathw.Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633 2022;8(1):28-33.
- Ersek JL, Black LJ, Thompson MA, Kim ES. Implementing precision medicine programs and clinical trials in the community-based oncology practice: barriers and best practices. Am Soc Clin Oncol Educ Book. 2018;38:188- 196. doi:10.1200/EDBK_200633
- Robboy SJ, Gupta S, Crawford JM, et al. The pathologist workforce in the United States: II. An interactive modeling tool for analyzing future qualitative and quantitative staffing demands for services. Arch Pathol Lab Med. 2015;139(11):1413-1430. doi:10.5858/arpa.2014-0559-OA doi:10.25270/jcp.2022.02.1
- Robboy SJ, Gross D, Park JY, et al. Reevaluation of the US pathologist workforce size. JAMA Netw Open. 2020;3(7): e2010648. doi:10.1001/jamanetworkopen.2020.10648
Mapping Pathology Work Associated With Precision Oncology Testing
Mapping Pathology Work Associated With Precision Oncology Testing
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).
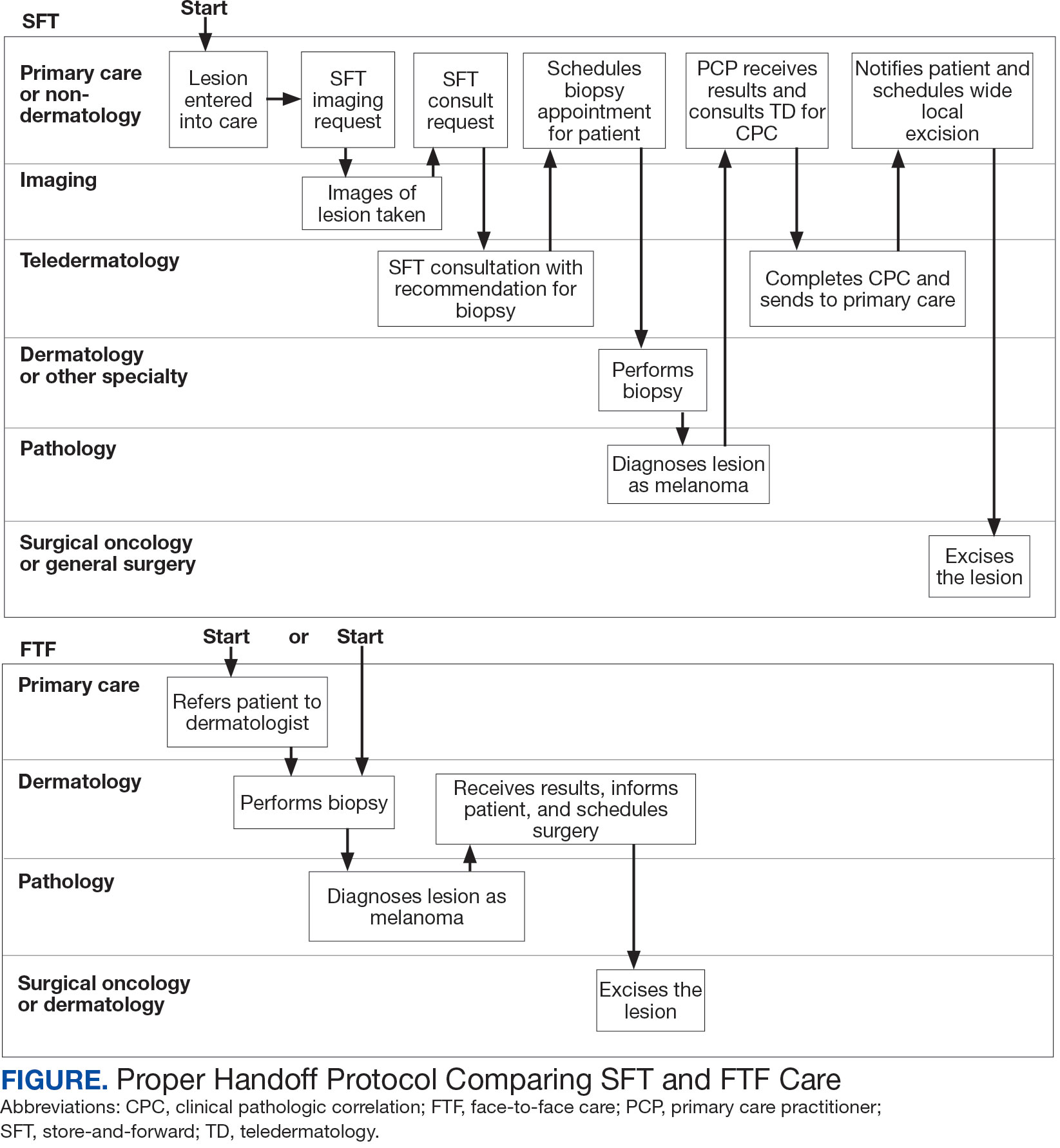
This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.
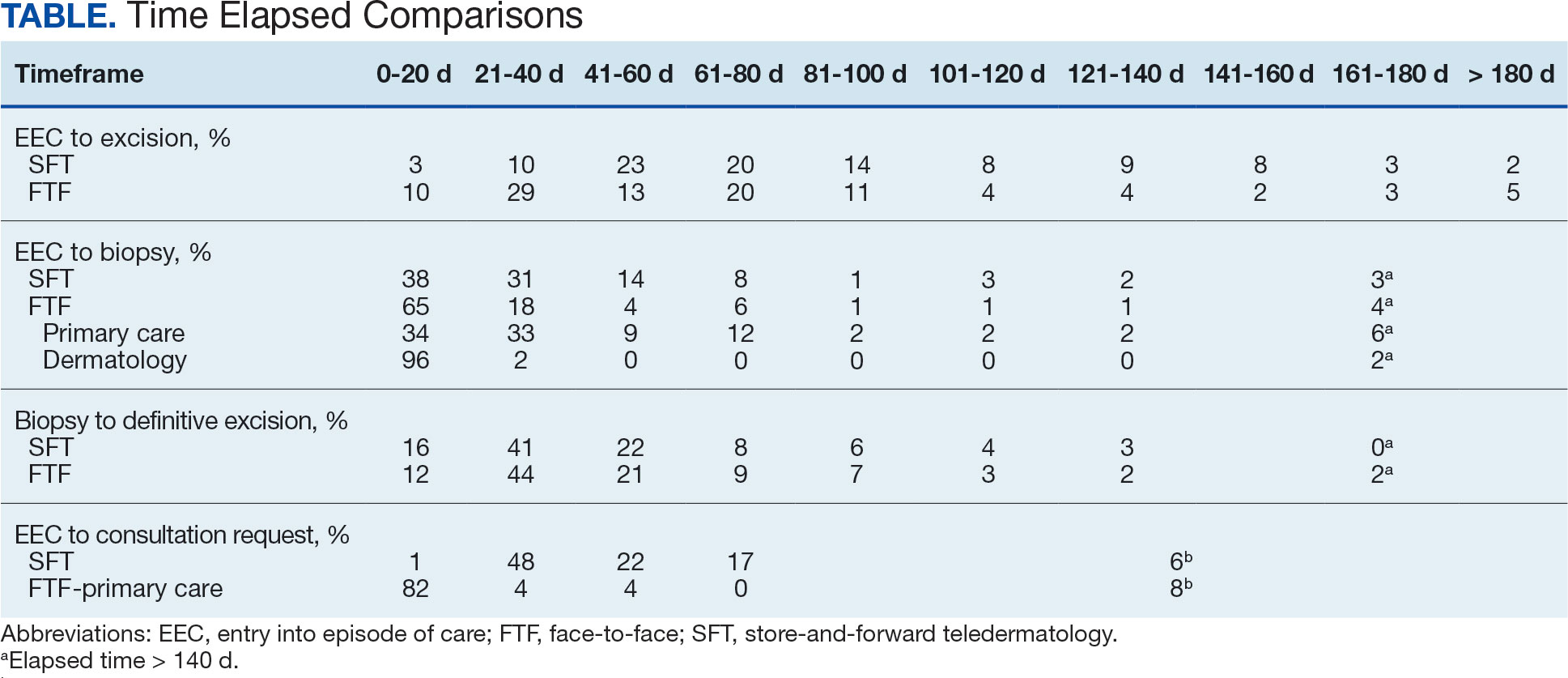
EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).

This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.

EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
Store-and-forward teledermatology (SFT) allows clinical images and information to be sent to a dermatologist for evaluation. In fiscal year (FY) 2018, 117,780 SFT consultations were completed in the Veterans Health Administration. Continued growth is expected since SFT has proven to be an effective method for improving access to face-to-face (FTF) dermatology care.1 In the same period, the US Department of Veterans Affairs (VA) Puget Sound Health Care System (VAPSHCS) completed 12,563 consultations in a mean 1.1 days from entry into episode of care (EEC), according to data reported by VA Teledermatology Program Administrator Chris Foster.
Obtaining a prompt consultation is reported to be an overwhelming advantage of using SFT.2-5 Rapid turnaround may appear to make SFT specialist care more accessible to veterans, yet this is an oversimplification. The process of delivering care (rather than consultation) through SFT is more complex than reading the images and reporting the findings. When a skin condition is identified by a primary care clinician and that person decides to request an SFT consultation, a complex set of tasks and handoffs is set into motion. A swim-lane diagram illustrates the numerous steps and handoffs that go into delivering care to a patient with a malignant melanoma on the SFT platform compared to FTF care, which requires fewer handoffs (Figure).

This process improvement project examined whether handoffs necessitated by SFT care lengthened the timeline of care for biopsy-proven primary cutaneous malignant melanoma. The stakes of delay in care are high. A 2018 study using the National Cancer Database found that a delay of > 30 days from biopsy to definitive excision (the date definitive surgical procedure for the condition is performed) resulted in a measurable increase in melanoma-related mortality. 6 This study sought to identify areas where the SFT timeline of care could be shortened.
Methods
This retrospective cohort study was approved by the VAPSHCS Institutional Review Board. The study drew from secondary data obtained from VistA, the VA Corporate Data Warehouse, the Veterans Integrated Service Network (VISN) 20 database, the American Academy of Dermatology Teledermatology Program database, and the VA Computerized Patient Record System.
Patients registered for ≥ 1 year at VAPSHCS with a diagnosis of primary cutaneous malignant melanoma by the Pathology service between January 1, 2006, and December 31, 2013, were included. Patients with metastatic or recurrent melanoma were excluded.
Cases were randomly selected from a melanoma database previously validated and used for another quality improvement project.7 There were initially 115 patient cases extracted from this database for both the FTF and SFT groups. Eighty-seven SFT and 107 FTF cases met inclusion criteria. To further analyze these groups, we split the FTF group into 2 subgroups: FTF dermatology (patients whose melanomas were entered into care in a dermatology clinic) and FTF primary care (patients whose melanomas were entered into care in primary care or a nondermatology setting).
The timeline of care was divided into 2 major time intervals: (1) entry into episode of care (EEC; the date a lesion was first documented in the electronic health record) to biopsy; and (2) biopsy to definitive excision. The SFT process was divided into the following intervals: EEC to imaging request (the date a clinician requested imaging); imaging request to imaging completion (the date an imager photographed a patient’s lesion); imaging completion to SFT consultation request (the date the SFT consultation was requested); SFT consultation request to consultation completion (the date an SFT reader completed the consultation request for a patient); and SFT consultation completion to biopsy. Mean and median interval lengths were compared between groups and additional analyses identified steps that may have contributed to delays in care.
To address potential bias based on access to care for rural veterans, SFT and FTF primary care cases were categorized into groups based on their location: (1) EEC and biopsy conducted at the same facility; (2) EEC and biopsy conducted at different facilities within the same health care system (main health care facility and its community-based outpatient clinics); and (3) EEC and biopsy conducted at different health care systems.
Statistics
Means, medians, and SDs were calculated in Excel. The Mann-Whitney U test was used to compare SFT medians to the FTF data and X2 test was used to compare proportions for secondary analyses.
Results
The median (mean) interval from EEC to definitive excision was 73 days (85) for SFT and 58 days (73) for FTF (P = .004) (Table). To understand this difference, the distribution of intervals from EEC to biopsy and biopsy to definitive excision were calculated. Only 38% of SFT cases were biopsied within 20 days compared to 65% of FTF cases (P < .001). The difference in time from biopsy to definitive excision distributions were not statistically significant, suggesting that the difference is actually a reflection of the differences seen in the period between EEC and biopsy.

EEC and biopsy occurred at the same facility in 85% and 82% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different facilities within the same health care system in 15% and 16% of FTF primary care and SFT cases, respectively. EEC and biopsy occurred at different health care systems in 0% and 2% of FTF primary care and SFT cases, respectively. Geographic bias did not impact results for either group of veterans.
The interval between EEC and biopsy was shorter for FTF dermatology cases than for FTF primary care cases. For FTF dermatology cases, 96% were biopsied within 20 days compared with 34% of FTF primary care cases (P < .001).
To further analyze the difference in the EEC to biopsy interval duration between SFT and FTF primary care the timeline was divided into smaller steps: EEC to imaging completion, imaging completion to SFT consult completion, and SFT consult completion to biopsy. From EEC to SFT consult completion, SFT cases took a median of 6.0 days and a mean of 12.3 days, reflecting the administrative handoffs that must occur in SFT. A total of 82% of FTF primary care cases were entered into care and consultation was requested on the same day, while this was true for only 1% of SFT cases.
Since mortality data were not collected, the frequency of in situ melanomas and invasive melanomas (pathologic stage pT1a or greater) was used as a proxy for comparing outcomes. No significant difference was found in the frequency of in situ vs invasive melanomas in the SFT and FTF dermatology groups; however, there was a much higher frequency of invasive melanomas in the FTF primary care group (P = .007).
Discussion
This study compared the time to treatment for SFT vs FTF and identified important differences. The episode of care for melanomas diagnosed by SFT was statistically significantly longer (15 days) than those diagnosed by FTF. The interval between biopsy and definitive excision was a median of 34 and 38 days, and a mean of 48 and 44 days for SFT and FTF, respectively, which were not statistically significant. The difference in the total duration of the interval between EEC and definitive excision was accounted for by the duration of the interval from EEC to biopsy. When excluding dermatology clinic cases from the FTF group, there was no difference in the interval between EEC and biopsy for SFT and FTF primary care. The handoffs in SFT accounted for a median of 6 days and mean of 12 days, a significant portion of the timeline, and is a target for process improvement. The delay necessitated by handoffs did not significantly affect the distribution of in situ and invasive melanomas in the SFT and FTF dermatology groups. This suggests that SFT may have better outcomes than FTF primary care.
There has been extensive research on the timeline from the patient initially noticing a lesion to the EEC.8-11 There is also a body of research on the timeline from biopsy to definitive excision. 6,12-16 However, there has been little research on the timeline between EEC and biopsy, which comprises a large portion of the overall timeline of both SFT care and FTF care. This study analyzed the delays that can occur in this interval. When patients first enter FTF dermatology care, this timeline is quite short because lesions are often biopsied on the same day. When patients enter into care with their primary or nondermatology clinician, there can be significant delays.
Since the stakes are high when it comes to treating melanoma, it is important to minimize the overall timeline. A 6-day median and 12-day mean were established as targets for teledermatology handoffs. Ideally, a lesion should be entered into an episode of care, imaged, and sent for consultation on the same day. To help further understand delays in administrative handoffs, we stratified the SFT cases by VISN 20 sites and spoke with an administrator at a top performing site. Between 2006 and 2013, this site had a dedicated full-time imager as well as a backup imager that ensured images were taken quickly, usually on the same day the lesion was entered into care. Unfortunately, this is not the standard at all VISN 20 sites and certainly contributes to the overall delay in care in SFT
Minimizing the timeline of care is possible, as shown by the Danish health system, which developed a fast-track referral system after recognizing the need to minimize delays between the presentation, diagnosis, and treatment of cutaneous melanomas. In Denmark, a patient who presents to a general practitioner with a suspicious lesion is referred to secondary care for excision biopsy within 6 days. Diagnosis is made within 2 weeks, and, if necessary, definitive excision is offered within 9 days of the diagnosis. This translates into a maximum 20-day EEC to biopsy timeline and maximum 29-day EEC to definitive excision timeline. Although an intervention such as this may be difficult to implement in the United States due to its size and decentralized health care system, it would, however, be more realistic within the VA due to its centralized structure. The Danish system shows that with appropriate resource allocation and strict timeframes for treatment referrals, the timeline can be minimized.17
Despite the delay in the SFT timeline, this study found no significant difference between the distribution of in situ vs invasive melanomas in FTF dermatology and SFT groups. One possible explanation for this is that SFT increases access to dermatologist care, meaning clinicians may be more willing to consult SFT for less advanced– appearing lesions.
The finding that SFT diagnosed a larger proportion of in situ melanomas than FTF primary care is consistent with the findings of Ferrándiz et al, who reported that the mean Breslow thickness was significantly lower among patients in an SFT group compared to patients in an FTF group consisting of general practitioners. 18 However, the study population was not randomized and the results may have been impacted by ascertainment bias. Ferrándiz et al hypothesized that clinicians may have a lower threshold for consulting teledermatology, resulting in lower mean Breslow thicknesses.18 Karavan et al found the opposite results, with a higher mean Breslow thickness in SFT compared to a primary care FTF group.19 The data presented here suggest that SFT has room for process improvement yet is essentially equivalent to FTF dermatology in terms of outcomes.
Limitations
The majority of patients in this study were aged > 50 years, White, and male. The results may not be representative for other populations. The study was relatively small compared to studies that looked at other aspects of the melanoma care timeline. The study was not powered to ascertain mortality, the most important metric for melanoma.
Conclusions
The episode of care was significantly longer for melanomas diagnosed by SFT than those diagnosed by FTF; however, timelines were not statistically different when FTF lesions entered into care in dermatology were excluded. A median 6-day and mean 12.3-day delay in administrative handoffs occurred at the beginning of the SFT process and is a target for process improvement. Considering the high stakes of melanoma, the SFT timeline could be reduced if EEC, imaging, and SFT consultation all happened in the same day.
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
- Raugi GJ, Nelson W, Miethke M, et al. Teledermatology implementation in a VHA secondary treatment facility improves access to face-to-face care. Telemed J E Health. 2016;22(1):12-17. doi:10.1089/tmj.2015.0036
- Moreno-Ramirez D, Ferrandiz L, Nieto-Garcia A, et al. Store-and-forward teledermatology in skin cancer triage: experience and evaluation of 2009 teleconsultations. Arch Dermatol. 2007;143(4):479-484. doi:10.1001/archderm.143.4.479
- Landow SM, Oh DH, Weinstock MA. Teledermatology within the Veterans Health Administration, 2002–2014. Telemed J E Health. 2015;21(10):769-773. doi:10.1089/tmj.2014.0225
- Whited JD, Hall RP, Foy ME, et al. Teledermatology’s impact on time to intervention among referrals to a dermatology consult service. Telemed J E Health. 2002;8(3):313-321. doi:10.1089/15305620260353207
- Hsiao JL, Oh DH. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol. 2008;59(2):260-267. doi:10.1016/j.jaad.2008.04.011
- Conic RZ, Cabrera CI, Khorana AA, Gastman BR. Determination of the impact of melanoma surgical timing on survival using the National Cancer Database. J Am Acad Dermatol. 2018;78(1):40-46.e7. doi:10.1016/j.jaad.2017.08.039
- Dougall B, Gendreau J, Das S, et al. Melanoma registry underreporting in the Veterans Health Administration. Fed Pract. 2016;33(suppl 5):55S-59S
- Xavier MHSB, Drummond-Lage AP, Baeta C, Rocha L, Almeida AM, Wainstein AJA. Delay in cutaneous melanoma diagnosis: sequence analyses from suspicion to diagnosis in 211 patients. Medicine (Baltimore). 2016;95(31):e4396. doi:10.1097/md.0000000000004396
- Schmid-Wendtner MH, Baumert J, Stange J, Volkenandt M. Delay in the diagnosis of cutaneous melanoma: an analysis of 233 patients. Melanoma Res. 2002;12(4):389-394. doi:10.1097/00008390-200208000-00012
- Betti, R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003;13(2):183-188.
- Blum A, Brand CU, Ellwanger U, et al. Awareness and early detection of cutaneous melanoma: An analysis of factors related to delay in treatment. Br J Dermatol. 1999;141(5):783-787. doi:10.1046/j.1365-2133.1999.03196.x
- Brian T, Adams B, Jameson M. Cutaneous melanoma: an audit of management timeliness against New Zealand guidelines. N Z Med J. 2017;130(1462):54-61. https://pubmed.ncbi.nlm.nih.gov/28934768
- Adamson AS, Zhou L, Baggett CD, Thomas NE, Meyer AM. Association of delays in surgery for melanoma with Insurance type. JAMA Dermatol. 2017;153(11):1106-1113. doi:https://doi.org/10.1001/jamadermatol.2017.3338
- Niehues NB, Evanson B, Smith WA, Fiore CT, Parekh P. Melanoma patient notification and treatment timelines. Dermatol Online J. 2019;25(4)13. doi:10.5070/d3254043588
- Lott JP, Narayan D, Soulos PR, Aminawung J, Gross CP. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151(7):731-741. doi:10.1001/jamadermatol.2015.119
- Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81(4):908-916. doi:10.1016/j.jaad.2019.05.079
- Jarjis RD, Hansen LB, Matzen SH. A fast-track referral system for skin lesions suspicious of melanoma: population-based cross-sectional study from a plastic surgery center. Plast Surg Int. 2016;2016:2908917. doi:10.1155/2016/2908917
- Ferrándiz L, Ruiz-de-Casas A, Martin-Gutierrez FJ, et al. Effect of teledermatology on the prognosis of patients with cutaneous melanoma. Arch Dermatol. 2012;148(9):1025-1028. doi:10.1001/archdermatol.2012.778
- Karavan M, Compton N, Knezevich S, et al. Teledermatology in the diagnosis of melanoma. J Telemed Telecare. 2014;20(1):18-23. doi:10.1177/1357633x13517354
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Handoff Delays in Teledermatology Lengthen Timeline of Care for Veterans With Melanoma
Fatigue and brain fog
Early-onset AD (EOAD) is the most likely diagnosis for this patient. Her symptoms — cognitive decline, executive function deficits, and visuospatial dysfunction — and brain imaging results are consistent with EOAD. But importantly, her father’s diagnosis of EOAD at age 58 suggests a hereditary component, which greatly increases the genetic risk for this patient. Moreover, neuroimaging shows cortical atrophy in the temporal lobes. A key radiologic feature of AD in this patient is the large increase in the subarachnoid spaces affecting the parietal region.
Between one third to just over one half of patients with EOAD have at least one first-degree relative with the disease. Given the patient’s neuroimaging results and family history of EOAD, she was sent for genetic testing, which revealed mutations in the PSEN1 gene; one the most common genetic causes of EOAD (along with mutations in the APP gene). For those who do have an autosomal dominant familial form of EOAD, clinical presentation is often atypical and includes headaches, myoclonus, seizures, hyperreflexia, and gait abnormalities. This patient did experience headaches but not the other symptoms.
Traumatic brain injury (TBI) is an insult to the brain from an outside mechanical force. It is both non-congenital and non-degenerative but can lead to permanent physical, cognitive, and/or psychosocial functioning. Patients often experience an altered or diminished state of consciousness in the aftermath of such an event. TBI is a diagnostic consideration for this patient, given that she was in a car accident and has experienced unusual cognitive and behavioral symptoms — eg, memory loss and visuospatial dysfunction. However, imaging does not reveal evidence of TBI, with no concussion or cerebral hemorrhage. Thus, TBI is not an accurate diagnosis for this patient.
Parkinson’s disease (PD) is one of the most common neurologic disorders that is marked by three hallmark features: resting tremor, rigidity, and bradykinesia that generally affects people over the age of 60. Even though many patients with PD exhibit some measure of executive function impairment early in the course of the disease, substantial impairment and dementia usually manifest about 8 years after the onset of motor symptoms. Dementia occurs in approximately 20%-40% of patients with PD. Although patients with PD demonstrate executive function deficits, memory loss, and visuospatial dysfunction, they do not experience aphasia. This patient does not have any of the cardinal features of PD, which rules it out as a diagnosis.
Frontotemporal dementia is a progressive dysfunction of the frontal lobes of the brain, primarily manifesting as language abnormalities, including reduced speech, perseveration, mutism, and echolalia, also known as primary progressive aphasia (PPA). Over time, patients develop other psychiatric symptoms: disinhibition, impulsivity, loss of social awareness, neglect of personal hygiene, mental rigidity, and utilization behavior. Given the patient’s language difficulties, frontotemporal dementia may be an initial diagnostic consideration. However, per the neurologic imaging results, brain abnormalities are located in the temporal regions. Additionally, the patient does not exhibit any of the psychiatric symptoms associated with frontotemporal dementia but does experience short-term memory loss and visuospatial dysfunction, which are not typical of this diagnosis. This patient does not have frontotemporal dementia.
EOAD is characterized by deficits in language, visuospatial skills and executive function. Often, these patients do not exhibit amnestic disorder early in the disease. While their memory recognition and semantic memory is higher than for patients who present with late-onset (normal course) AD, their attention scores are typically lower. As a result of this atypical presentation, patients with EOAD tend to have a longer duration of disease before diagnosis (~1.6 years). They are also likely to have a history of TBI, which is a risk factor for dementia.
In comparison to late-onset AD (LOAD), EOAD has a larger genetic predisposition (92%-100% vs 70%-80%), a more aggressive course, a more frequent delay in initial diagnosis, higher prevalence of TBI, and less memory impairment. However, EOAD has greater decline in other cognitive domains, and because of the young age of onset, greater psychosocial impairment. Overall disease progression in these patients is much faster compared with patients who have LOAD. Neuroimaging in these patients generally features greater hippocampal sparing and posterior neocortical atrophy, with brain changes that affect the frontoparietal networks rather than the classic presentation found in LOAD.
An important aspect of the workup of patients for whom EOAD is a diagnostic consideration is to thoroughly determine their family history and to perform genetic testing along with counseling.
The pharmacological treatment of patients with early-onset AD is identical to patients who have normal course or late-onset AD. Cholinesterase inhibitors (ChEIs) — eg, donepezil, galantamine, and rivastigmine, with the usual titration schedules — are indicated in these patients. Although these medications target memory, they also provide support to patients with other variants of EOAD — eg, logopenic variant PPA. However, it is imperative that providers monitor these patients carefully, as ChEIs may exacerbate some behaviors.
Management of patients with EOAD varies based on the patient's specific variant. It is vital for clinicians to coordinate patient-centered care individually. For example, patients with logopenic variant PPA should be referred for speech therapy assessment and treatment should focus on improving communication, while patients with posterior cortical atrophy benefit most from interventions for those who experience vision impairments. As this patient’s symptoms revolve primarily around cognition and visuospatial dysfunction, her treatment will focus on interventions to improve coordination, balance, and cognitive function.
Perhaps the most important part of managing a patient with EOAD is providing adequate and appropriate psychosocial support. These patients are most often in their most productive time of life, balancing careers and families. EOAD can bring about feelings of loss of independence, anticipatory grief, and anxiety about the future and the increased difficulty in managing the tasks of daily life. It is vital that these patients — and their families — receive adequate education and psychiatric support via therapists, support groups, and community resources that are age appropriate.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Early-onset AD (EOAD) is the most likely diagnosis for this patient. Her symptoms — cognitive decline, executive function deficits, and visuospatial dysfunction — and brain imaging results are consistent with EOAD. But importantly, her father’s diagnosis of EOAD at age 58 suggests a hereditary component, which greatly increases the genetic risk for this patient. Moreover, neuroimaging shows cortical atrophy in the temporal lobes. A key radiologic feature of AD in this patient is the large increase in the subarachnoid spaces affecting the parietal region.
Between one third to just over one half of patients with EOAD have at least one first-degree relative with the disease. Given the patient’s neuroimaging results and family history of EOAD, she was sent for genetic testing, which revealed mutations in the PSEN1 gene; one the most common genetic causes of EOAD (along with mutations in the APP gene). For those who do have an autosomal dominant familial form of EOAD, clinical presentation is often atypical and includes headaches, myoclonus, seizures, hyperreflexia, and gait abnormalities. This patient did experience headaches but not the other symptoms.
Traumatic brain injury (TBI) is an insult to the brain from an outside mechanical force. It is both non-congenital and non-degenerative but can lead to permanent physical, cognitive, and/or psychosocial functioning. Patients often experience an altered or diminished state of consciousness in the aftermath of such an event. TBI is a diagnostic consideration for this patient, given that she was in a car accident and has experienced unusual cognitive and behavioral symptoms — eg, memory loss and visuospatial dysfunction. However, imaging does not reveal evidence of TBI, with no concussion or cerebral hemorrhage. Thus, TBI is not an accurate diagnosis for this patient.
Parkinson’s disease (PD) is one of the most common neurologic disorders that is marked by three hallmark features: resting tremor, rigidity, and bradykinesia that generally affects people over the age of 60. Even though many patients with PD exhibit some measure of executive function impairment early in the course of the disease, substantial impairment and dementia usually manifest about 8 years after the onset of motor symptoms. Dementia occurs in approximately 20%-40% of patients with PD. Although patients with PD demonstrate executive function deficits, memory loss, and visuospatial dysfunction, they do not experience aphasia. This patient does not have any of the cardinal features of PD, which rules it out as a diagnosis.
Frontotemporal dementia is a progressive dysfunction of the frontal lobes of the brain, primarily manifesting as language abnormalities, including reduced speech, perseveration, mutism, and echolalia, also known as primary progressive aphasia (PPA). Over time, patients develop other psychiatric symptoms: disinhibition, impulsivity, loss of social awareness, neglect of personal hygiene, mental rigidity, and utilization behavior. Given the patient’s language difficulties, frontotemporal dementia may be an initial diagnostic consideration. However, per the neurologic imaging results, brain abnormalities are located in the temporal regions. Additionally, the patient does not exhibit any of the psychiatric symptoms associated with frontotemporal dementia but does experience short-term memory loss and visuospatial dysfunction, which are not typical of this diagnosis. This patient does not have frontotemporal dementia.
EOAD is characterized by deficits in language, visuospatial skills and executive function. Often, these patients do not exhibit amnestic disorder early in the disease. While their memory recognition and semantic memory is higher than for patients who present with late-onset (normal course) AD, their attention scores are typically lower. As a result of this atypical presentation, patients with EOAD tend to have a longer duration of disease before diagnosis (~1.6 years). They are also likely to have a history of TBI, which is a risk factor for dementia.
In comparison to late-onset AD (LOAD), EOAD has a larger genetic predisposition (92%-100% vs 70%-80%), a more aggressive course, a more frequent delay in initial diagnosis, higher prevalence of TBI, and less memory impairment. However, EOAD has greater decline in other cognitive domains, and because of the young age of onset, greater psychosocial impairment. Overall disease progression in these patients is much faster compared with patients who have LOAD. Neuroimaging in these patients generally features greater hippocampal sparing and posterior neocortical atrophy, with brain changes that affect the frontoparietal networks rather than the classic presentation found in LOAD.
An important aspect of the workup of patients for whom EOAD is a diagnostic consideration is to thoroughly determine their family history and to perform genetic testing along with counseling.
The pharmacological treatment of patients with early-onset AD is identical to patients who have normal course or late-onset AD. Cholinesterase inhibitors (ChEIs) — eg, donepezil, galantamine, and rivastigmine, with the usual titration schedules — are indicated in these patients. Although these medications target memory, they also provide support to patients with other variants of EOAD — eg, logopenic variant PPA. However, it is imperative that providers monitor these patients carefully, as ChEIs may exacerbate some behaviors.
Management of patients with EOAD varies based on the patient's specific variant. It is vital for clinicians to coordinate patient-centered care individually. For example, patients with logopenic variant PPA should be referred for speech therapy assessment and treatment should focus on improving communication, while patients with posterior cortical atrophy benefit most from interventions for those who experience vision impairments. As this patient’s symptoms revolve primarily around cognition and visuospatial dysfunction, her treatment will focus on interventions to improve coordination, balance, and cognitive function.
Perhaps the most important part of managing a patient with EOAD is providing adequate and appropriate psychosocial support. These patients are most often in their most productive time of life, balancing careers and families. EOAD can bring about feelings of loss of independence, anticipatory grief, and anxiety about the future and the increased difficulty in managing the tasks of daily life. It is vital that these patients — and their families — receive adequate education and psychiatric support via therapists, support groups, and community resources that are age appropriate.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Early-onset AD (EOAD) is the most likely diagnosis for this patient. Her symptoms — cognitive decline, executive function deficits, and visuospatial dysfunction — and brain imaging results are consistent with EOAD. But importantly, her father’s diagnosis of EOAD at age 58 suggests a hereditary component, which greatly increases the genetic risk for this patient. Moreover, neuroimaging shows cortical atrophy in the temporal lobes. A key radiologic feature of AD in this patient is the large increase in the subarachnoid spaces affecting the parietal region.
Between one third to just over one half of patients with EOAD have at least one first-degree relative with the disease. Given the patient’s neuroimaging results and family history of EOAD, she was sent for genetic testing, which revealed mutations in the PSEN1 gene; one the most common genetic causes of EOAD (along with mutations in the APP gene). For those who do have an autosomal dominant familial form of EOAD, clinical presentation is often atypical and includes headaches, myoclonus, seizures, hyperreflexia, and gait abnormalities. This patient did experience headaches but not the other symptoms.
Traumatic brain injury (TBI) is an insult to the brain from an outside mechanical force. It is both non-congenital and non-degenerative but can lead to permanent physical, cognitive, and/or psychosocial functioning. Patients often experience an altered or diminished state of consciousness in the aftermath of such an event. TBI is a diagnostic consideration for this patient, given that she was in a car accident and has experienced unusual cognitive and behavioral symptoms — eg, memory loss and visuospatial dysfunction. However, imaging does not reveal evidence of TBI, with no concussion or cerebral hemorrhage. Thus, TBI is not an accurate diagnosis for this patient.
Parkinson’s disease (PD) is one of the most common neurologic disorders that is marked by three hallmark features: resting tremor, rigidity, and bradykinesia that generally affects people over the age of 60. Even though many patients with PD exhibit some measure of executive function impairment early in the course of the disease, substantial impairment and dementia usually manifest about 8 years after the onset of motor symptoms. Dementia occurs in approximately 20%-40% of patients with PD. Although patients with PD demonstrate executive function deficits, memory loss, and visuospatial dysfunction, they do not experience aphasia. This patient does not have any of the cardinal features of PD, which rules it out as a diagnosis.
Frontotemporal dementia is a progressive dysfunction of the frontal lobes of the brain, primarily manifesting as language abnormalities, including reduced speech, perseveration, mutism, and echolalia, also known as primary progressive aphasia (PPA). Over time, patients develop other psychiatric symptoms: disinhibition, impulsivity, loss of social awareness, neglect of personal hygiene, mental rigidity, and utilization behavior. Given the patient’s language difficulties, frontotemporal dementia may be an initial diagnostic consideration. However, per the neurologic imaging results, brain abnormalities are located in the temporal regions. Additionally, the patient does not exhibit any of the psychiatric symptoms associated with frontotemporal dementia but does experience short-term memory loss and visuospatial dysfunction, which are not typical of this diagnosis. This patient does not have frontotemporal dementia.
EOAD is characterized by deficits in language, visuospatial skills and executive function. Often, these patients do not exhibit amnestic disorder early in the disease. While their memory recognition and semantic memory is higher than for patients who present with late-onset (normal course) AD, their attention scores are typically lower. As a result of this atypical presentation, patients with EOAD tend to have a longer duration of disease before diagnosis (~1.6 years). They are also likely to have a history of TBI, which is a risk factor for dementia.
In comparison to late-onset AD (LOAD), EOAD has a larger genetic predisposition (92%-100% vs 70%-80%), a more aggressive course, a more frequent delay in initial diagnosis, higher prevalence of TBI, and less memory impairment. However, EOAD has greater decline in other cognitive domains, and because of the young age of onset, greater psychosocial impairment. Overall disease progression in these patients is much faster compared with patients who have LOAD. Neuroimaging in these patients generally features greater hippocampal sparing and posterior neocortical atrophy, with brain changes that affect the frontoparietal networks rather than the classic presentation found in LOAD.
An important aspect of the workup of patients for whom EOAD is a diagnostic consideration is to thoroughly determine their family history and to perform genetic testing along with counseling.
The pharmacological treatment of patients with early-onset AD is identical to patients who have normal course or late-onset AD. Cholinesterase inhibitors (ChEIs) — eg, donepezil, galantamine, and rivastigmine, with the usual titration schedules — are indicated in these patients. Although these medications target memory, they also provide support to patients with other variants of EOAD — eg, logopenic variant PPA. However, it is imperative that providers monitor these patients carefully, as ChEIs may exacerbate some behaviors.
Management of patients with EOAD varies based on the patient's specific variant. It is vital for clinicians to coordinate patient-centered care individually. For example, patients with logopenic variant PPA should be referred for speech therapy assessment and treatment should focus on improving communication, while patients with posterior cortical atrophy benefit most from interventions for those who experience vision impairments. As this patient’s symptoms revolve primarily around cognition and visuospatial dysfunction, her treatment will focus on interventions to improve coordination, balance, and cognitive function.
Perhaps the most important part of managing a patient with EOAD is providing adequate and appropriate psychosocial support. These patients are most often in their most productive time of life, balancing careers and families. EOAD can bring about feelings of loss of independence, anticipatory grief, and anxiety about the future and the increased difficulty in managing the tasks of daily life. It is vital that these patients — and their families — receive adequate education and psychiatric support via therapists, support groups, and community resources that are age appropriate.
Shaheen E. Lakhan, MD, PhD, MS, MEd, Chief of Pain Management, Carilion Clinic and Virginia Tech Carilion School of Medicine, Roanoke, Virginia.
Disclosure: Shaheen E. Lakhan, MD, PhD, MS, MEd, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 52-year-old female presents complaining of fatigue, brain fog, and headaches. She reports that, over the last year, she feels increasingly more irritable, anxious, overwhelmed, and sometimes disoriented. When prompted, she admits to having trouble keeping track of things — dates, important deadlines, objects like her keys, and things she uses each day. She needs multiple reminder events or tasks and must write everything down. She also reports that she often loses her train of thought and sometimes struggles to find words. She works in a medical office, is married, and has two children in college. She helps with caring for her in-laws on the weekend by doing their bills and grocery shopping. She admits to being moody and does not want to socialize, which she attributes to fatigue and having a busy life. She also complains of muscle stiffness and general clumsiness. Eight months ago, the patient was in an automobile accident in which her car was totaled, but she did not sustain serious injury and opted not to seek medical evaluation.
Physical exam reveals slight visuospatial dysfunction, with issues with both coordination and balance. The patient appears fatigued and has some trouble maintaining her attention during the conversation. Further questioning reveals mild anomic aphasia and some lapses in recent memory. Her reflexes are otherwise normal, as are heart, lung, and breath sounds. All systemic lymph nodes are normal as are liver and spleen on palpation. The patient recently discovered that her estranged father had been diagnosed with Alzheimer’s disease (AD) at 58 years of age and passed away 5 years later.
Laboratory testing is performed and reveals nothing remarkable. Considering the car accident and new cognitive/behavioral changes experienced by the patient, a CT of the brain is ordered. Results are negative for concussion and cerebral hemorrhage but show cortical atrophy of the temporal territories and a large increase in the size of the subarachnoid spaces predominantly affecting the parietal region.
Alzheimer's Disease and Athletes
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).
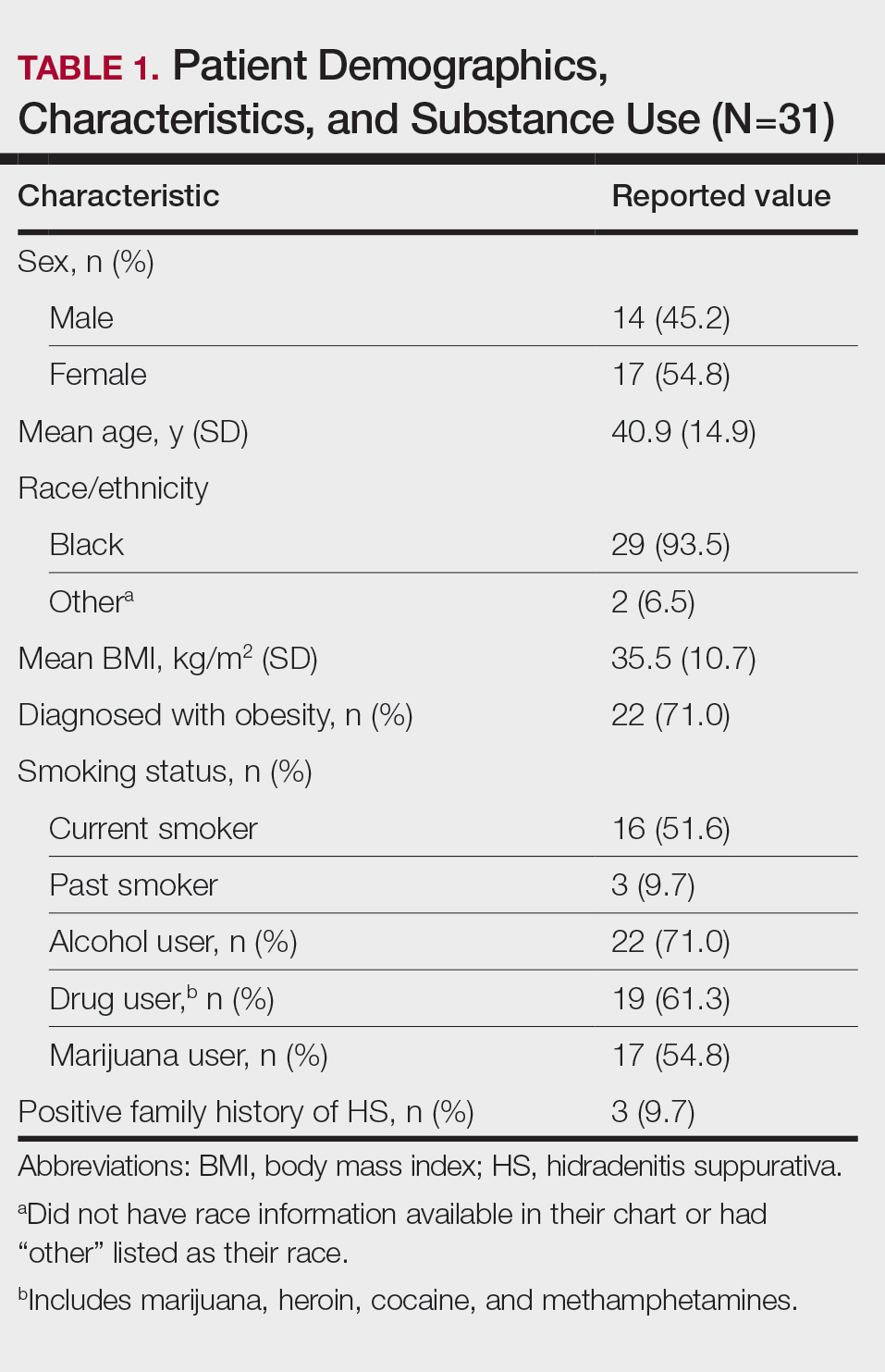
Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.
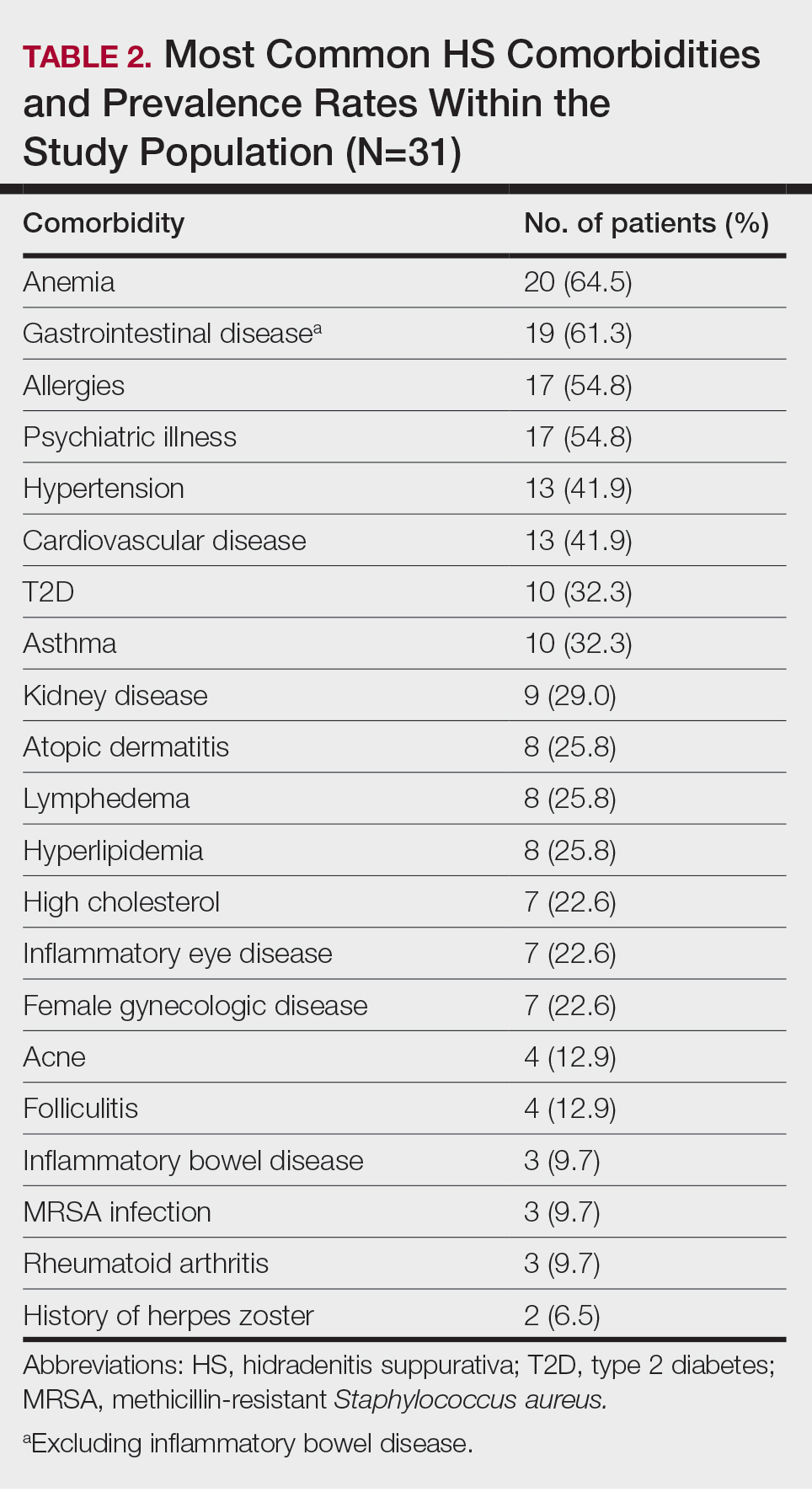
The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.
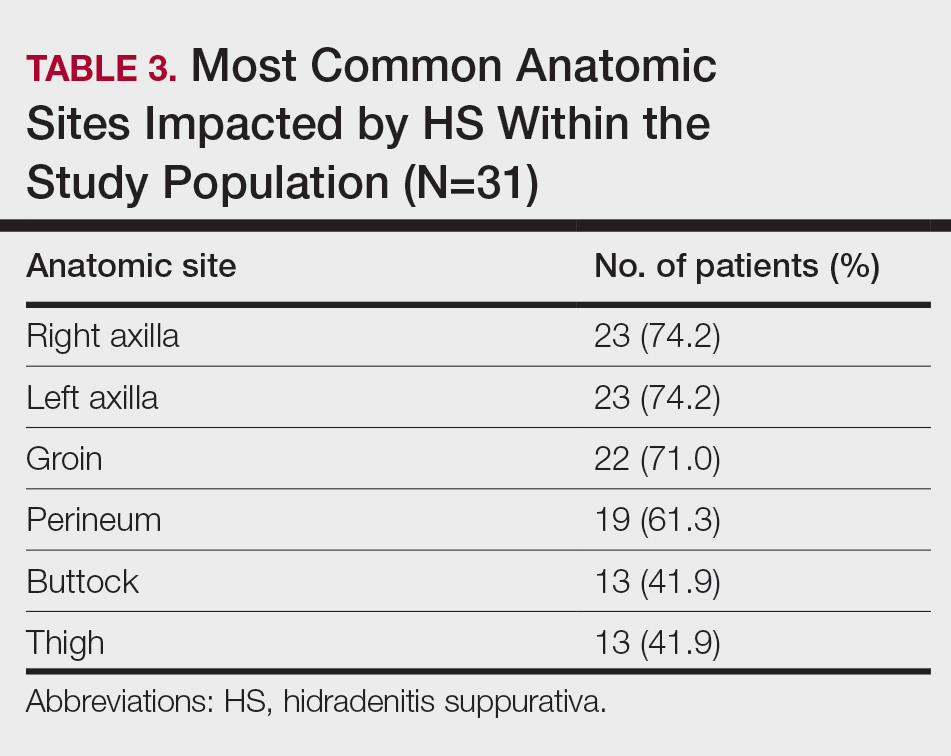
Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).
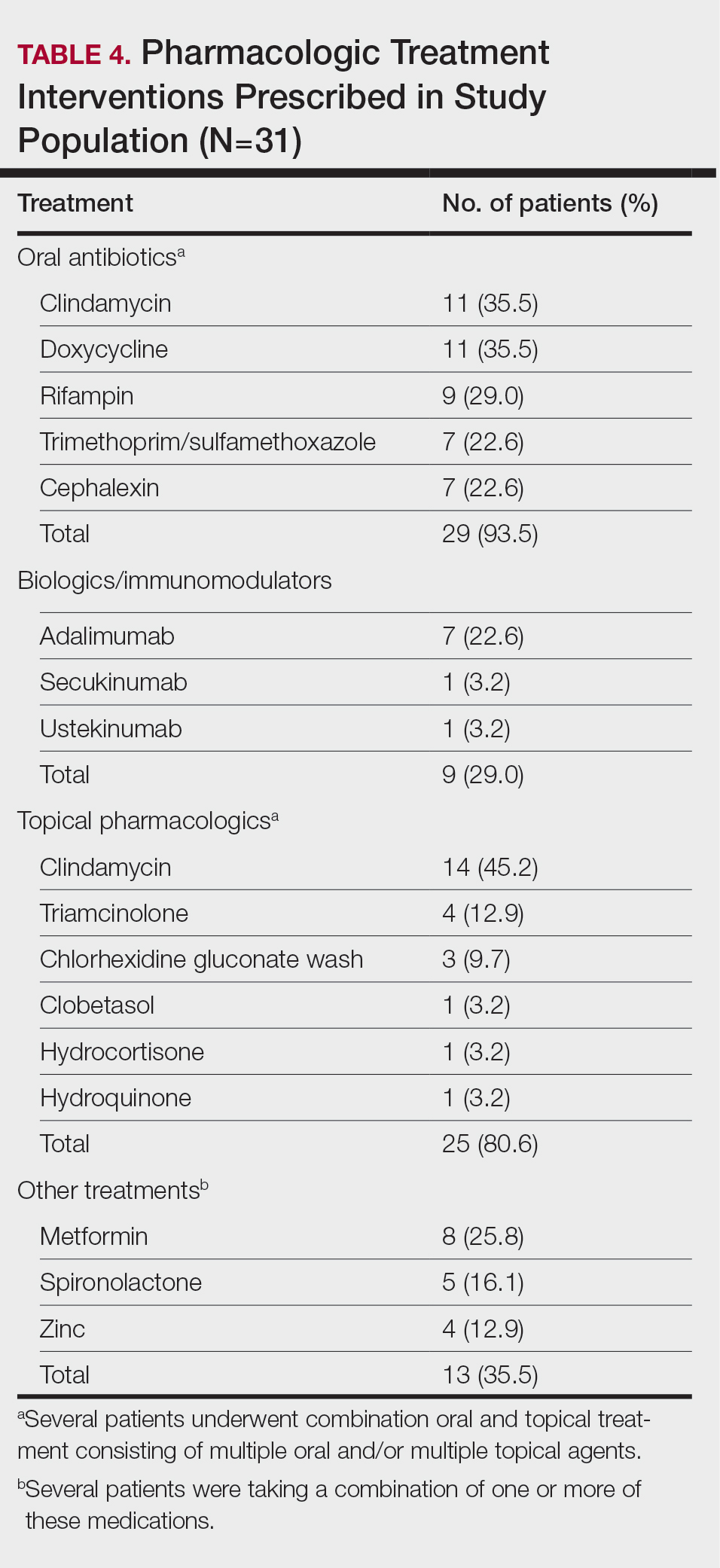
Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).

Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.

The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.

Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).

Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
Hidradenitis suppurativa (HS) is a debilitating chronic skin disease that often affects apocrinebearing regions of the skin such as the axillae, perineum, and groin.1 Although current research on the etiology and pathogenesis of HS is limited, the disease is known to have a considerable psychosocial impact on patient quality of life.
Clinically, HS lesions manifest as tender subcutaneous nodules that rupture to form painful and deep dermal abscesses.2 These lesions typically develop due to hair follicle occlusion, followed by a cyclic process of inflammation, healing, re-inflammation, and scarring. Often, they are mistaken for cysts or a simple abscess in the early stages of the disease, leading to a delay in diagnosis.1 Disease severity is categorized based on Hurley staging: stage 1 involves abscess formation without scarring; stage 2 involves limited sinus tracts and recurrent abscesses with scarring and/or multiple separated lesions; and stage 3 is the most advanced stage, with diffuse involvement or multiple interconnected sinus tracts across an area with scarring. The condition primarily is medically managed with antibiotics and immunomodulators, but patients who have refractory disease can benefit from surgical excision.1,2
The prevalence of HS in the United States ranges from 0.77% to 1.19%, and individuals who self-identify as Black have 3-fold higher odds of having this condition compared with all other racial groups.3-5 Black patients also are thought to have a greater number and size of apocrine glands compared with patients who self-identify as White, suggesting an anatomic predisposition to developing HS and greater disease severity.6 However, despite HS disproportionately impacting individuals with skin of color (SOC), the majority of published HS research includes predominantly White patient cohorts.5 There is insufficient research assessing HS epidemiology, comorbidities, and treatment responses in patients with SOC.
A 2020 review reported the notable lack of clinical trials that sufficiently examine systemic medication treatment response in HS patients with SOC.7 Of the 15 HS treatment trials published from 2000 to 2019, only 16.4% (138/840) of the patient population were of African descent.7 Clinical trials investigating the efficacy of adalimumab in reducing HS burden also did not adequately evaluate clinical response in patients with SOC. One clinical trial did not include any Black patients as part of the cohort,8 and in 3 other studies, 80% to 85% of the study participants self-identified as White.9 The current literature does not reflect the patient populations most affected by HS, as several studies have reported that 65% of patients diagnosed with HS in the United States annually are Black.5,7 These results emphasize the underrepresentation of SOC populations in the current HS literature and the need for more research that investigates the disease processes, comorbidities, and treatment outcomes of the diverse patient population impacted by HS.
Methods
Study Population and Data Extraction—Following a protocol reviewed and approved by the MedStar Health/Georgetown University institutional review board (IRB #00006783), a retrospective chart review of 31 adult patients with HS who underwent surgery at a regional verified burn center from April 2014 to April 2023 was conducted. The following variables were collected from the electronic medical record (EMR): baseline demographics including age, sex, body mass index (BMI), obesity status, race, ethnicity, Fitzpatrick skin type, smoking status, substance use, employment status, and family history of HS; HS-specific details including Hurley staging, affected areas, and age at initial diagnosis; comorbidities such as dermatologic conditions, autoimmune disorders, infectious diseases, cardiovascular and associated diseases, ovarian disorders, gastrointestinal diseases, and othother common chronic comorbidities (psychiatric illness, kidney disease, type 2 diabetes [T2D], asthma, allergies, lymphedema, and inflammatory eye disease); and use of pharmacologics such as topical medications, oral antibiotics, immunomodulators, and steroids.
Study Definitions—Obesity was defined as both a continuous and categorical variable. Each patient’s BMI at the surgery date was recorded from the EMR as a continuous variable. Patients with obesity also had this condition listed under their complaints and problem list in the EMR, which was recorded as a categorical variable. Race and ethnicity were self-reported by patients. Comorbidity data, including T2D and hyperlipidemia, were defined by previously diagnosed diseases listed in the EMR. Pharmacologic medication data were included in the study if a patient was recommended/prescribed a medication and they had confirmed use of the medication in a subsequent office visit.
Statistical Analysis—Descriptive statistics were calculated for demographics, HS characteristics (eg, location, Hurley stage), and comorbidities. Continuous variables were presented as mean and standard deviation or median and interquartile range and were evaluated using a t test or Mann-Whitney U test when appropriate. Categorical variables were presented as frequencies and percentages and tested for associations using the X2 or Fisher exact test. Data analyses were performed using SAS software version 9.4 (SAS Institute Inc.).
Results
Thirty-one patients (17 females, 14 males; mean age, 40.9 years) were included in the study. Twenty-nine (93.5%) patients identified as Black. All study patients had at least 1 comorbidity. Obesity was diagnosed in 22 (71.0%) patients (mean BMI, 35.5 kg/m2). A total of 16 (51.6%) patients were current smokers, 3 (9.7%) were past smokers, 22 (71%) reported alcohol use, and 17 (54.8%) were active marijuana users. Only 3 (9.7%) patients had a family history of HS (Table 1).

Other common comorbidities associated with HS were anemia (64.5% [20/31]), a non–inflammatory bowel disease gastrointestinal disease (61.3% [19/31]), allergies (54.8% [17/31]), hypertension (41.9% [13/31]), cardiovascular disease (41.9% [13/31]), T2D (32.3% [10/31]), asthma (32.3% [10/31]), kidney disease (29.0% [9/31]), and atopic dermatitis (25.8% [8/31]). More than half (54.8% [17/31]) of patients were diagnosed with psychiatric illnesses, including depression, anxiety, bipolar depression, psychosis, anorexia, impulsive anger, hallucinations, delusion, attention deficit-hyperactivity disorder, and panic disorder (Table 2). Depression was diagnosed in 38.7% (12/31) of patients, and 22.6% (7/31) were diagnosed with anxiety.

The most common anatomic locations for HS were the right axilla (74.2% [23/31]), left axilla (74.2% [23/31]), groin (71% [22/31]), perineum (61.3% [19/31]), buttocks (41.9% [13/31]), and thigh (41.9% [13/31]). Other locations included the breast, lower back, posterior neck, dorsal foot, and scalp (all 3.2% [1/31])(Table 3). Twenty (64.5%) patients had Hurley staging recorded in the EMR. Seventeen (54.8%) were categorized as Hurley stage 3, and 3 (9.7%) were categorized as Hurley stage 2.

Twenty-nine (93.5%) patients were prescribed an oral antibiotic regimen. The most common oral antibiotics were clindamycin (35.5% [11/31]), doxycycline (35.5% [11/31]), rifampin (29% [9/31]), trimethoprim/sulfamethoxazole (22.6% [7/31]), and cephalexin (22.6% [7/31]). Of the patients who were prescribed rifampin, 87.5% (8/9) also were prescribed an adjunct oral clindamycin regimen. Twenty-nine percent (9/31) of patients were prescribed a biologic regimen; 22.6% (7/31) were prescribed adalimumab, 3.2% (1/31) were prescribed secukinumab, and 3.2% (1/31) were prescribed ustekinumab (Table 4).

Twenty-five (80.6%) patients were prescribed a topical treatment regimen, the most common being topical clindamycin (45.2% [14/31]). Other topical medications included triamcinolone (12.9% [4/31]), chlorhexidine gluconate wash (9.7% [3/31]), clobetasol (3.2% [1/31]), hydrocortisone (3.2% [1/31]), and hydroquinone (3.2% [1/31])(Table 4).
Other medical treatments for HS included metformin (25.8% [8/31]), spironolactone (16.1% [5/31]), and zinc supplements (12.9% [4/31]). Four patients (12.9%) were prescribed clindamycin plus rifampin as well as a combination of metformin, spironolactone, and/or zinc (Table 4).
Twenty-two (71.0%) patients had a history of receiving incision and drainage procedures as treatment for HS. All 31 patients underwent excisional surgery followed by appropriate reconstruction. The total number of excisional surgeries a single patient underwent for HS treatment ranged from 1 to 9, with a mean of 2 excisional surgeries per patient.
Comment
Our regional verified burn center in Washington, DC, serves a large population of patients with SOC, making it a unique and important sample to study for HS. Our results suggest that Black patients with HS may be at a higher risk for depression and anxiety. Twelve (38.7%) of our patients were diagnosed with depression, which is substantially higher than the 17% to 21% depression prevalence rate among all HS patients reported in meta-analyses.10,11 Additionally, 22.6% (7/31) of our patients were diagnosed with anxiety, which is higher than the 5% to 12% prevalence rate of anxiety among HS patients reported in meta-analyses.10,11 The stress of chronic disease management, psychosocial impact of living with HS, social stigma, sexual dysfunction, pain, and financial concerns make mental illness a debilitating yet common comorbidity for patients with HS. The results of our study suggest that anxiety and depression are highly prevalent among Black patients with HS. It is important to identify if this finding is due to the interplay of health care disparities and social determinants of health; the cause likely is multifactorial, as race and ethnicity may be potential predictors for increased disease severity. Hidradenitis suppurativa is known to be a major economic burden on patients, and race-dependent structural and societal inequalities may be influencing the increased prevalence of anxiety and depression among Black patients with HS.12 Therefore, clinicians must be vigilant for the signs and symptoms of mental illnesses to refer patients for psychiatric treatment when appropriate. Implementing self-report Patient Health Questionnaire-9, General Anxiety Disorder-7 depression and anxiety screening tools, and Dermatology Life Quality Index questionnaires at primary care and dermatology office visits may be a beneficial step toward identifying patients who could benefit from additional mental health resources.13
The patients included in our study predominantly self-identified as Black, and the current smoker prevalence rate was 51.6% (16/31). This percentage is lower than the smoking rates of other published HS studies conducted in predominantly White patient populations, which report up to a 76.5% smoking prevalence rate.14-16 One review article published in 2022 reported that approximately 90% of HS patients are current or former smokers.17 Additionally, a retrospective cohort analysis identifying HS cases among 3,924,310 tobacco smokers in the United States reported that tobacco smokers diagnosed with HS most commonly racially self-identified as White (66.2%).18 Tobacco chemicals and smoke can increase inflammatory cytokine levels, and the activation of nicotinic acetylcholine receptors surrounding pilosebaceous-apocrine units can increase follicular occlusion.14 While several studies1-3,14,19,20 support the strong correlation between tobacco smoking and HS, there are very few that specifically investigate the association between smoking and HS disease in SOC populations. It is possible that smoking rates may be lower in Black patients with HS compared with White patients with HS, which would suggest a multifactorial nature of HS disease pathophysiology. Future large, multicenter studies are needed that investigate smoking rates and HS disease severity in patients across various racial groups.
Prior research has shown a strong correlation between cigarette smoking and HS, but there is minimal data on the role of use of marijuana and other illicit drugs in HS disease pathophysiology.21 A total of 54.8% of our patients were active marijuana users with daily or weekly usage. Further research is needed to investigate whether marijuana use is linked with HS disease pathophysiology and severity or if patients with HS may be using marijuana to relieve pain, anxiety, and depression. Additional studies that survey the method of marijuana use (eg, joint, vape devices, or edibles) would clarify the relationship between not only HS and marijuana but also a potential link between disease severity and the process of inhaling large amounts of smoke vs a link with the active ingredients in the marijuana plant itself.
Approximately 61% (19/31) of our patients were diagnosed with a gastrointestinal disease in addition to HS. Current research reports the link between HS and inflammatory bowel disease, but few studies have investigated if a relationship exists between the gut microbiome and HS, as well as the incidence of general gastrointestinal disease among Black patients with HS.14,22 Our patients were diagnosed with gastrointestinal conditions such as colonic polyps, gastroesophageal reflux disease, benign neoplasms of the cecum and sigmoid colons, small bowel obstruction and perforation, biliary tract diseases, ileus, abdominal hernia, peritonitis, and diverticulosis. Further research is warranted to identify if there is a true relationship between gastrointestinal disease, the gut microbiome, and skin conditions such as HS.22 Biochemical research on the common genetic and inflammatory cytokine pathways involved in HS and gastrointestinal manifestations could help predict disease severity and management in HS patients with SOC.
Several research studies have reported the association between obesity and HS, likely due to adipose cells producing increased estrogen and leading to an estrogen-dominant hormone profile and increased local androgen production in adipose tissue.14,23,24 Antiandrogenic drugs such as finasteride and spironolactone lead to positive results in HS treatment compared to oral antibiotics alone.24 While 71.9% (22/31) of our patients were diagnosed with obesity, only 16.1% (5/31) were prescribed antiandrogen therapy such as spironolactone. It is unclear if this result reflects a health disparity due to insufficient insurance coverage and low prescribing rates or if there is patient hesitancy to taking antiandrogen medications. Additional clinical trials are needed to investigate the efficacy of antiandrogen therapies for HS. If proven to be efficacious, providers should consider adding these medications to the pharmacologic regimen of HS patients with SOC prior to recommending wide-excision surgeries. Furthermore, in addition to antiandrogen medication, weight-management interventions may be helpful in reducing HS disease. The results of a survey conducted in 35 HS patients who underwent bariatric surgery reported 48.6% (17/35) experienced complete disease remission after more than a 15% weight reduction.25,26 Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
Limitations
Our study was limited by the constraints of a retrospective chart review and small sample size. Retrospective chart reviews are susceptible to recall bias, variability in providers’ charting practices, and human error from data collectors. We acknowledge that a control group of non-HS patients should be the next step in furthering our research on HS disease comorbidities. Also, since 35.5% (11/31) of our patients did not have Hurley staging recorded in the EMR, it would be beneficial to conduct a future study comprehensive of all 3 Hurley stages. Since 93.5% (29/31) of the patients in our study racially identified as Black, having a control group of racially diverse HS patients would help further our understanding of HS pathophysiology. Lastly, since the inclusion criteria required patients to have undergone excisional surgery for HS, future studies that consider comorbidities among both surgical and nonsurgical patients with HS will aid in our understanding of HS patients with SOC.
Conclusion
The results of our study demonstrate a descriptive analysis of the demographics, most common comorbidities, lesion sites, pharmacologic treatments, and surgical profiles in patients with SOC who underwent surgical treatment for HS. Our data show that HS patients with SOC may be more likely to experience anxiety, depression, and gastrointestinal disease than other HS patients. Additionally, our patients had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology. Furthermore, despite published research on the efficacy of immunomodulator therapy for HS, most of our HS patients with SOC underwent surgical intervention without first attempting biologic treatment regimens, indicating possible gaps in health care access for minority patients that may be impacting disease severity and outcomes. Studies such as this one that investigate disease pathophysiology and risk factors in SOC patient populations with HS are imperative in minimizing the health care disparity gap, improving disease outcomes, and providing more equitable health care for all patients.
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
- Wieczorek M, Walecka I. Hidradenitis suppurativa—known and unknown disease. Reumatologia. 2018;56:337-339. doi:10.5114/reum.2018.80709
- Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60:539-563. doi:10.1016/j. jaad.2008.11.911
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Ingram JR, Jenkins-Jones S, Knipe DW, et al. Population-based Clinical Practice Research Datalink study using algorithm modelling to identify the true burden of hidradenitis suppurativa. Br J Dermatol. 2018;178:917-924. doi:10.1111/bjd.16101
- Lee DE, Clark AK, Shi VY. Hidradenitis suppurativa: disease burden and etiology in skin of color. Dermatology. 2017;233:456-461. doi:10.1159/000486741
- Brown-Korsah JB, McKenzie S, Omar D, et al. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color—part I: genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 2022;87:1239-1258. doi:10.1016/j.jaad.2022.06.1193
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Arenbergerova M, Gkalpakiotis S, Arenberger P. Effective long-term control of refractory hidradenitis suppurativa with adalimumab after failure of conventional therapy. Int J Dermatol. 2010;49:1445-1449. doi:10.1111/j.1365-4632.2010.04638.x
- Kimball AB, Okun MM, Williams DA, et al. Two phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med. 2016;375:422-434. doi:10.1056/NEJMoa1504370
- Jalenques I, Ciortianu L, Pereira B, et al. The prevalence and odds of anxiety and depression in children and adults with hidradenitis suppurativa: systematic review and meta-analysis. J Am Acad Dermatol. 2020;83:542-553. doi:10.1016/j.jaad.2020.03.041
- Machado MO, Stergiopoulos V, Maes M, et al. Depression and anxiety in adults with hidradenitis suppurativa: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:939-945. doi:10.1001 /jamadermatol.2019.0759
- Kilgour JM, Li S, Sarin KY. Hidradenitis suppurativa in patients of color is associated with increased disease severity and healthcare utilization: a retrospective analysis of 2 U.S. cohorts. JAAD Int. 2021;3:42-52. doi:10.1016/j.jdin.2021.01.007
- Rymaszewska JE, Krajewski PK, Szcze² ch J, et al. Depression and anxiety in hidradenitis suppurativa patients: a cross-sectional study among Polish patients. Postep Dermatol Alergol. 2023;40:35-39. doi:10.5114ada.2022.119080
- Johnston LA, Alhusayen R, Bourcier M, et al. Practical guidelines for managing patients with hidradenitis suppurativa: an update. J Cutan Med Surg. 2022;26(2 suppl):2S-24S. doi:10.1177/12034754221116115
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population-based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Seyed Jafari SM, Knüsel E, Cazzaniga S, et al. A retrospective cohort study on patients with hidradenitis suppurativa. Dermatology. 2018;234:71-78. doi:10.1159/000488344
- Lewandowski M, S´ wierczewska Z, Baran´ ska-Rybak W. Hidradenitis suppurativa: a review of current treatment options. Int J Dermatol. 2022;61:1152-1164. doi:10.1111/ijd.16115
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population-based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Tzellos T, Zouboulis CC. Which hidradenitis suppurativa comorbidities should I take into account? Exp Dermatol. 2022;31(suppl 1):29-32. doi:10.1111/exd.14633
- Metko D, Mehta S, Piguet V. Cannabis usage among patients with hidradenitis suppurativa: a scoping review. J Cutan Med Surg. 2024;28:307-308. doi:10.1177/12034754241238719
- Mahmud MR, Akter S, Tamanna SK, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. 2022;14:2096995. doi:10.1080/194 90976.2022.2096995
- Mair KM, Gaw R, MacLean MR. Obesity, estrogens and adipose tissue dysfunction—implications for pulmonary arterial hypertension. Pulm Circ. 2020;10:2045894020952019. doi:10.1177/2045894020952023
- Abu Rached N, Gambichler T, Dietrich JW, et al. The role of hormones in hidradenitis suppurativa: a systematic review. Int J Mol Sci. 2022;23:15250. doi:10.3390/ijms232315250
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016 /j.jaad.2019.02.067
- Choi ECE, Phan PHC, Oon HH. Hidradenitis suppurativa: racial and socioeconomic considerations in management. Int J Dermatol. 2022;61:1452-1457. doi:10.1111/ijd.16163
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
Evaluating Factors Impacting Hidradenitis Suppurativa Disease Severity in Patients With Darker Skin Types
PRACTICE POINTS
- Anxiety and depression are highly prevalent among Black patients with hidradenitis suppurativa (HS). Implementing self-report questionnaires at medical office visits are crucial to identifying patients who could benefit from additional psychiatric resources.
- Hidradenitis suppurativa patients with skin of color may have a higher incidence of comorbid gastrointestinal disease than other HS patients.
- Investigating the impact of weight-management practices on disease severity would be helpful in outlining nonpharmacologic treatments for patients with HS.
- The patient cohort described here had a high prevalence of marijuana use but lower prevalence of current cigarette use compared to studies conducted in predominantly White HS patient populations, emphasizing the multifactorial nature of HS pathophysiology.
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4
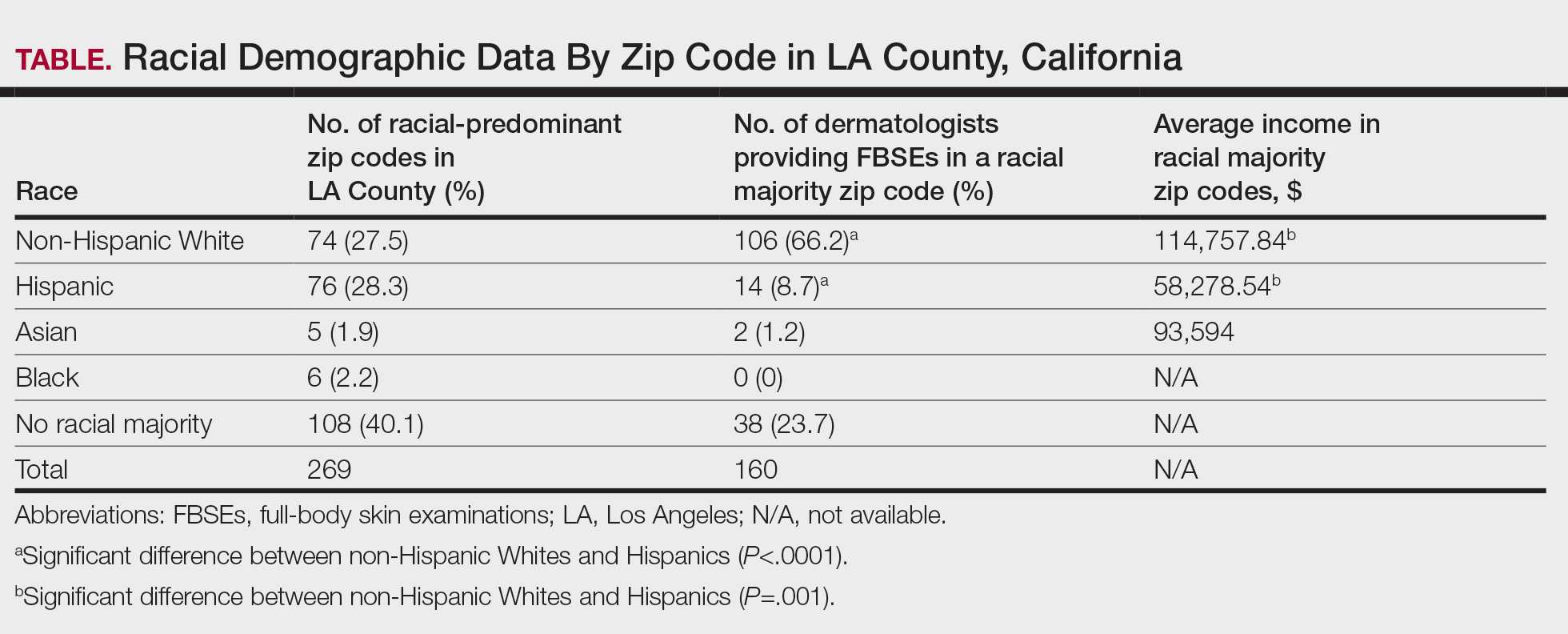
In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4

In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
To the Editor:
Early skin cancer detection improves patient outcomes1; however, socioeconomic and racial disparities may impact access to dermatologic care.2 Although non-Hispanic White individuals have a high incidence of skin cancer, they experience higher melanoma-specific survival rates than non-White patients, who often receive later-stage diagnoses and experience higher mortality.2 Furthermore, racial/ ethnic minorities often face longer surgery wait times after diagnosis and have lower socioeconomic status (SES) and less favorable health insurance coverage, contributing to poorer outcomes.2,3
To examine access to full-body skin examinations (FBSEs) by board-certified dermatologists in Los Angeles (LA) County, California, we analyzed the availability of FBSEs based on racial demographics, income, and insurance type (Medicaid [Medi-Cal] vs private [Blue Cross Blue Shield (BCBS)]). Demographic data by zip code were obtained from the US Census Bureau.4 This validated metric highlights socioeconomic disparities and minimizes data gaps5,6 and was used to assess health care access among different population subgroups. Dermatologists’ contact information was obtained from the Find a Dermatologist page on the American Academy of Dermatology website and the listed phone numbers of their practice were used to contact them. Practices with board-certified dermatologists accepting new patients were included in the study; practices were not included if they had exclusive insurance plans; were pediatric, cosmetic, or research only; or were nonresponsive to calls. From August 2022 to September 2022, each practice was called twice within a 36-hour period—once by a simulated patient with Medi-Cal and once by a simulated patient with BCBS—and were asked about availability for new patient FBSE appointments and accepted insurance types. Data were analyzed using SAS software (SAS Institute Inc.).
Los Angeles County comprises 269 zip codes, of which 82 (30.5%) have dermatology practices. Of 213 total dermatologists in LA County listed on the American Academy of Dermatology website, 193 (90.6%) met preliminary criteria, and 169 (79.3%) were successfully contacted. Almost all (94.6% [160/169]) accepted new patients for FBSEs; of those, 63.1% (101/160) accepted only private insurance, 16.9% (27/160) accepted both private insurance and Medi-Cal, and 16.2% (26/160) did not accept any insurance. Racial predominance for each dermatology practice was analyzed by zip code (Table). Dermatologists included in our study were significantly more concentrated in predominantly non- Hispanic White areas of LA County vs predominantly Hispanic areas (P<.0001). Notably, the average income in predominantly non-Hispanic White zip codes ($114,757.74) was significantly higher than in predominantly Hispanic areas ($58,278.54)(P=.001)(Table).4

In LA County, 40.1% (108/269) of zip codes have no racial majority, 28.2% (76/269) are predominantly Hispanic, 27.5% (74/269) are predominantly non-Hispanic White, 2.2% (6/269) are predominantly Black, and 1.9% (5/269) are predominantly Asian.4 There are no dermatologists in predominantly Black zip codes, 2 in predominantly Asian zip codes, 14 in predominantly Hispanic zip codes, 38 in zip codes with no racial majority, and 106 in predominantly non-Hispanic White zip codes. There are significantly more dermatologists in predominantly non-Hispanic White zip codes compared to predominantly Hispanic zip codes (P<.0001). In LA County, the average income in predominantly Asian, non-Hispanic White, and Hispanic zip codes was $93,594, $114,757.84, and $58,278.54, respectively, in 2021.4 The average income in predominantly non-Hispanic White zip codes was significantly higher than in predominantly Hispanic zip codes (P=.001). There were no income data available for predominantly Black zip codes or zip codes with no racial majority.
The results from our study revealed potential barriers to FBSEs for racial and ethnic minorities in LA County, which supports previous research on the impact of SES, race, and insurance on access to dermatologic care.2,3 Predominantly Hispanic zip codes have significantly lower income (P<.0001) and fewer dermatologists (P=.001) compared to zip codes that are predominantly non-Hispanic White, reflecting how lower SES correlates with worse health outcomes and higher melanoma mortality. Conversely, predominantly non-Hispanic White areas with higher income have better access to dermatologists, which may contribute to the improved melanoma survival rates among White patients. Additionally, most dermatologists accept only private insurance, further highlighting the disparity in FBSE access for non-White patients across LA County. While our study focused on FBSE access, our findings may point to a wider barrier to dermatologic care, especially in zip codes with fewer dermatologists. Further studies are needed to determine whether these areas also face barriers to accessing primary care.
Our study was limited by the exclusion of nonphysician providers (eg, nurse practitioners, physician assistants), a small sample size, and lack of available economic data for predominantly Black zip codes.4 Additionally, the exclusion of practices with exclusive insurance plans (eg, Kaiser Permanente) limited the generalizability of our findings, as our results did not account for the populations served by these practices. Furthermore, our analysis did not account for variations in practice size or the proportion of care provided to patients with different insurance types, which could impact overall accessibility. Additional studies are needed to explore the impact of these factors on access to general dermatologic care and not just FBSEs.
Racial/ethnic minorities and lower SES populations face major barriers to FBSE access in LA County, such as difficulty finding a dermatologist in their area or one who accepts Medi-Cal. Addressing these disparities is crucial for improving skin cancer outcomes. Further research is needed to develop strategies to eliminate these barriers to dermatologic care, such as increasing access to teledermatology, offering mobile dermatology clinics, and improving insurance coverage.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
- Chiaravalloti AJ, Laduca JR. Melanoma screening by means of complete skin exams for all patients in a dermatology practice reduces the thickness of primary melanomas at diagnosis. J Clin Aesthet Dermatol. 2014;7:18-22.
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016). J Am Acad Dermatol. 2021;84:1585-1593.
- Baranowski MLH, Yeung H, Chen SC, et al. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. J Am Acad Dermatol. 2019;81:908-916.
- United States Census Bureau. Explore census data. Accessed March 17, 2025. https://data.census.gov/all?q=los+angeles+county
- Berkowitz SA, Traore CY, Singer DE, et al. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50:398-417.
- Jacobs B, Ir P, Bigdeli M, et al. Addressing access barriers to health services: an analytical framework for selecting appropriate interventions in lowincome Asian countries. Health Policy Plan. 2012;27:288-300.
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
Evaluating Access to Full-Body Skin Examinations in Los Angeles County, California
PRACTICE POINTS
- Socioeconomic and racial disparities impact access to full-body skin examinations (FBSEs) in Los Angeles County.
- Most dermatologists included in this study were accepting new patients for a FBSE.
- There are significantly more dermatologists in predominantly non-Hispanic White zip codes than in predominantly Hispanic zip codes in Los Angeles County.
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.
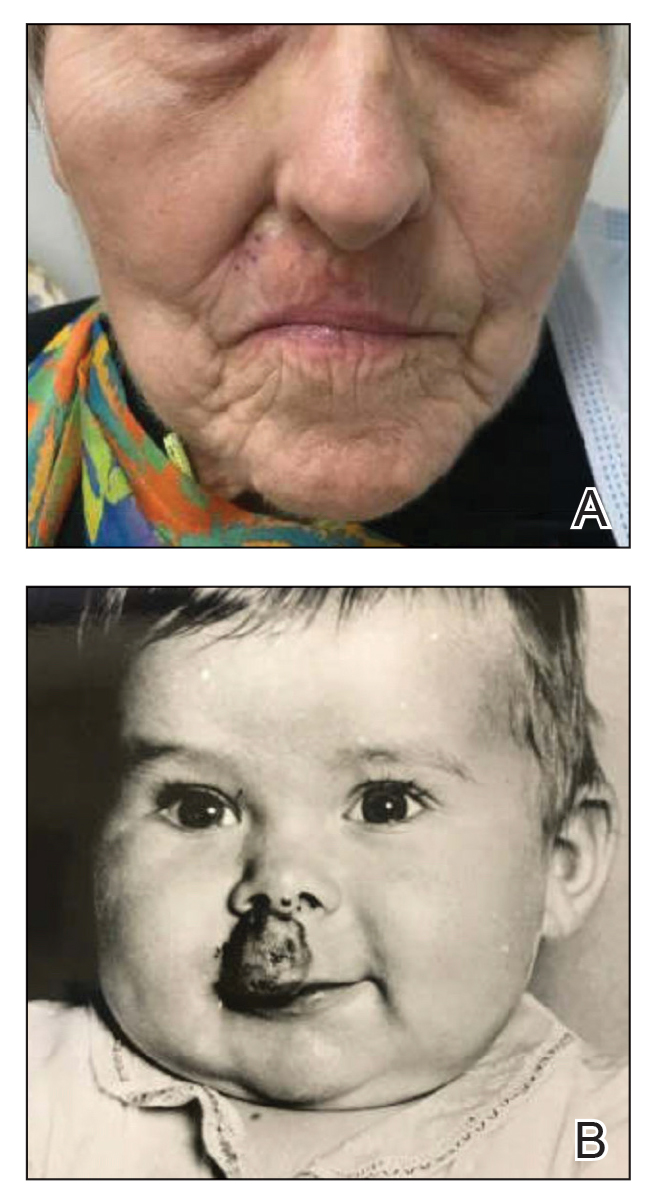
Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.
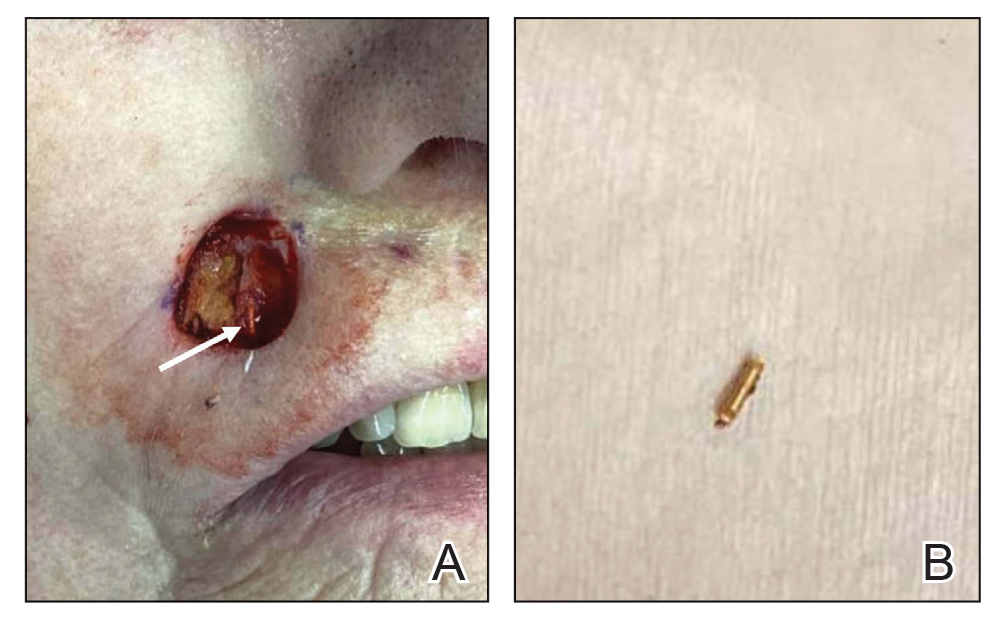
Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.

Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.

Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
To the Editor:
Basal cell carcinoma (BCC), which is the most common type of skin cancer, typically arises on sun-damaged skin as a result of long-term exposure to UV radiation. Another known risk factor for BCC is exposure to ionizing radiation, though this is less commonly encountered.1 We present a unique case of a BCC arising at the site of an involuted infantile hemangioma that had been treated with implanted and retained gold radon seeds more than 7 decades prior. This case highlights the importance of obtaining a detailed history of radiation exposures to better counsel patients about skin cancer risk and manage disease in complex skin locations.
A 75-year-old woman presented to an outside dermatologist for evaluation of a pink papule on the right upper cutaneous lip that had enlarged over several months (Figure 1). The patient’s medical history was remarkable for an infantile hemangioma present since shortly after birth in the same location that had been treated with 10 implanted gold radon seeds when she was 6 years old. Over her lifetime, several seeds had self-extruded from the area, but some remained within the subcutaneous tissue as confirmed by dental radiographs. A shave biopsy of the papule demonstrated a superficial BCC, and the patient was referred to our institution for Mohs micrographic surgery.

Intraoperative frozen sections revealed both superficial and nodular BCC, and the tumor was cleared in 3 stages. During surgery, a gold radon seed was visualized at the base of the excised BCC and was removed from the subcutaneous tissue (Figure 2). The primary defect on the upper lip was closed with a rotation flap. The patient returned for follow-up 2 months later and showed good healing and cosmetic outcome.

Although not commonly encountered, ionizing radiation is a known risk factor for BCC.1 Basal cell carcinoma arising from implanted gold radon seeds represents a minority of reported cases.2,3 Radium was first used to treat skin disease in the early 1900s.1 The radioactive decay of radium produced tissue destruction via alpha, beta, and gamma particles, which slowly released over weeks when radium was packaged into a capsule.4 Following implantation of the capsule, DNA damage occurred due to double-stranded breaks, chromosomal aberrations, and generation of reactive oxygen species. The downstream effect of these cellular insults resulted in cell-cycle shortening, apoptosis, and carcinogenesis.5
Gold radon seeds were used to treat infantile hemangiomas in the United States and Europe from the early 1940s to the 1960s; their use declined dramatically in the 1950s due to adverse effects and discovery of the potential for future malignancies as well as the development of safer and more effective treatments.1,3 Our patient received a substantial dose of ionizing radiation from the implantation of gold radon seeds at the site of the infantile hemangioma, which dramatically increased her risk for BCC in this location.
Infantile hemangiomas are the most common vascular tumors in children. Most infantile hemangiomas regress spontaneously and are stably involuted by about 5 or 6 years of age.6 Treatment is indicated for rapidly growing hemangiomas that are at risk for ulceration or are located by critical structures (eg, the eyes or airway). Hemangiomas located on or near the lips should be treated to avoid disfigurement and loss of function as a consequence of rapid growth and involution.7 The treatment of choice for large or high-risk infantile hemangiomas over the past 10 to 15 years has been beta blockers.6-8 Propranolol hydrochloride, a systemic beta blocker, was approved by the US Food and Drug Administration in 2014 for the treatment of infantile hemangiomas and has demonstrated safety and effectiveness in promoting involution in these lesions.8 Unlike radiation therapy from implanted gold radon seeds, propranolol does not increase the risk for BCC. Although other risk factors such as skin type and cumulative UV exposure contribute to the development of BCC, the exact location of the BCC overlying the residual gold radon seeds was highly suggestive of ionizing radiation playing a major role in the carcinogenesis of the tumor in our patient.
Our case highlights the importance of screening elderly patients for exposures that may increase the risk for skin carcinogenesis. Dermatologists are accustomed to asking about history of UV exposure, sunburns, and use of sun-protective measures; however, direct questioning about less common sources of radiation exposure also may help stratify a patient’s risk for developing BCC. Although the US Preventive Services Task Force 2023 guidelines determined there is insufficient evidence to recommend visual skin cancer screening examinations in asymptomatic adults,9 we advocate for verbal screening of radiation exposure in both primary care and dermatology office settings. At a time when access to care, particularly dermatology services, is challenging, determining the appropriate interval for follow-up based on the patient’s skin cancer risk is imperative.
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Fürst CJ, Lundell M, Holm LE. Radiation therapy of hemangiomas, 1909- 1959. a cohort based on 50 years of clinical practice at Radiumhemmet, Stockholm. Acta Oncol. 1987;26:33-36. doi:10.3109/02841868709092974
- Bräuner EV, Loft S, Sørensen M, et al. Residential radon exposure and skin cancer incidence in a prospective Danish cohort. PLoS ONE. 2015;10:E0135642. doi:10.1371/journal.pone.0135642
- Weiss E, Sukal SA, Zimbler MS, et al. Basal cell carcinoma arising 57 years after interstitial radiotherapy of a nasal hemangioma. Dermatol Surg. 2008;34:1137-1140. doi:10.1111/j.1524-4725.2008.34229.x
- Lavery MJ, Lorenzelli D, Crema J. A radon seed identified during skin surgery: an unusual finding. Clin Exp Dermatol. 2021;46:604-606. doi:10.1111/ced.14454
- Robertson A, Allen J, Laney R, et al. The cellular and molecular carcinogenic effects of radon exposure: a review. Int J Mol Sci. 2013;14:14024-14063. doi:10.3390/ijms140714024
- Rodríguez Bandera AI, Sebaratnam DF, et al. Infantile hemangioma. part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379-1392. doi:10.1016 /j.jaad.2021.08.019
- Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:E20183475. doi:10.1542/peds.2018-3475
- Sebaratnam DF, Rodríguez Bandera AL, Wong LF, et al. Infantile hemangioma. part 2: management. J Am Acad Dermatol. 2021;85: 1395-1404. doi:10.1016/j.jaad.2021.08.020
- US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
Basal Cell Carcinoma Arising From an Infantile Hemangioma Treated With Gold Radon Seeds
PRACTICE POINTS
- Historical use of ionizing radiation to treat skin disease is a risk factor for basal cell carcinoma (BCC).
- Mohs micrographic surgery is the treatment of choice for BCC in high-risk areas such as the nose, eyelids, and lips, where tissue conservation and complete margin control are essential.
- Elderly patients should be screened for less common sources of radiation exposure for better risk stratification and to determine appropriate intervals for follow-up with a dermatologist.
Multiple Firm Papules on the Wrists and Forearms
Multiple Firm Papules on the Wrists and Forearms
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).
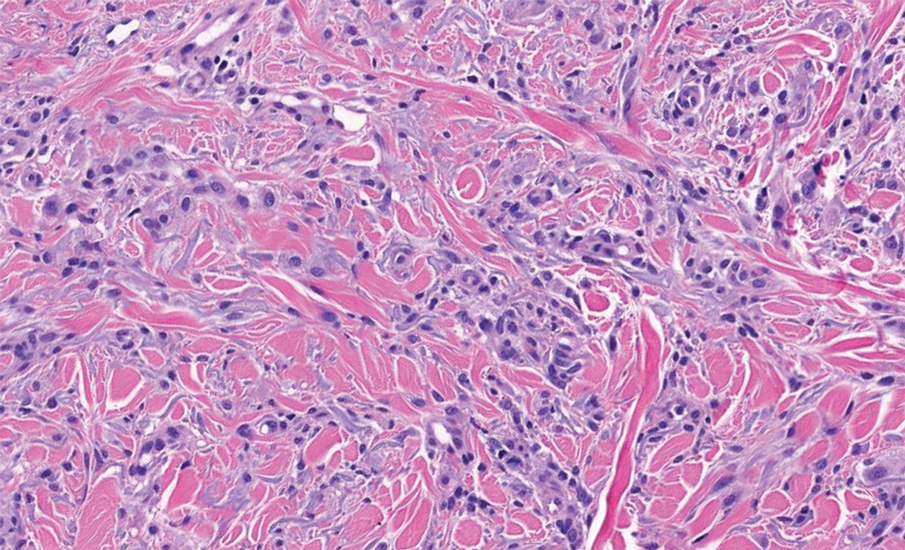
The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.
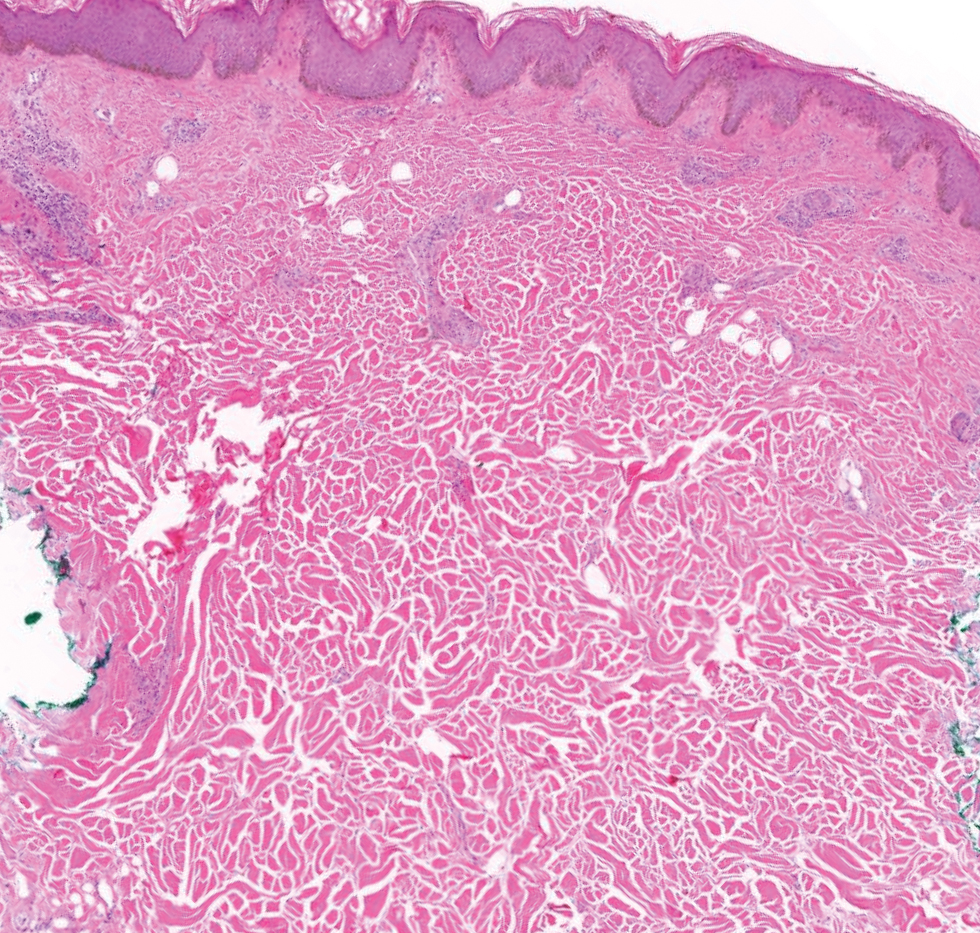
Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.
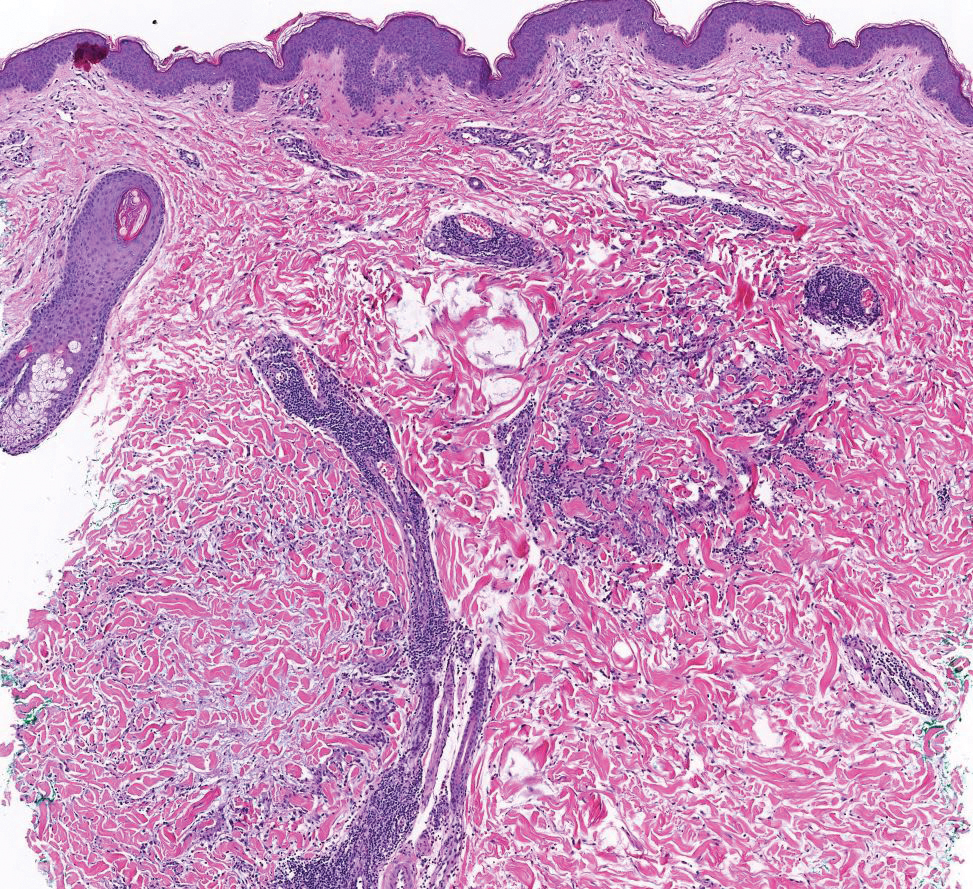
Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11
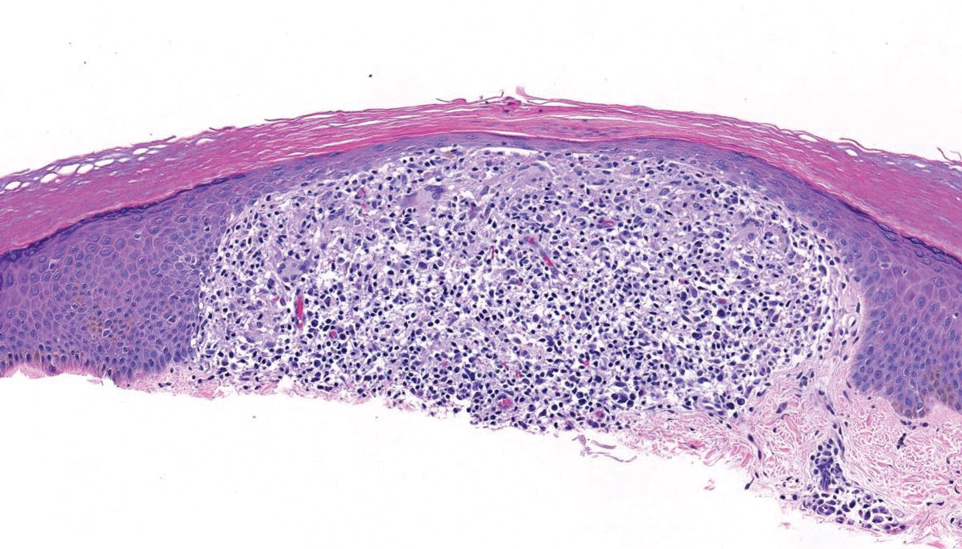
Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).

The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.

Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.

Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11

Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
THE DIAGNOSIS: Acral Persistent Papular Mucinosis
Histopathologic analysis revealed conspicuous interstitial mucin deposition throughout the upper to mid reticular dermis in the absence of a cellular infiltrate or fibroplasia. Colloidal iron staining confirmed the presence of mucin. In correlation with the clinical presentation, a diagnosis of acral persistent papular mucinosis (APPM) was made. The patient was counseled on the benign disease course and lack of associated comorbidities, and additional treatment was not pursued.
Acral persistent papular mucinosis is a rare distinct subtype of cutaneous mucinosis that initially was described by Rongioletti et al1 in 1986. As a localized form of lichen myxedematosus, APPM is characterized by mucin deposition in the dermis with no systemic involvement. The precise pathogenesis remains unclear, although some investigators have suggested that cytokine-mediated stimulation of glycosaminoglycan production may contribute to increased mucin accumulation in the dermis.2 Acral persistent papular mucinosis predominantly affects middle-aged women with a 5:1 female-to-male predominance.3 Clinically, patients present with discrete, nonfollicular, waxy papules that typically measure 2 to 5 mm and are distributed symmetrically on the extensor surfaces of the wrists and forearms. While the lesions generally are asymptomatic, some patients may report mild pruritus. The condition is chronic, with lesions seldom resolving and often increasing in number over time.3
Histologically, APPM is characterized by focal deposits of mucin in the upper reticular dermis with no evidence of increased fibroblast proliferation or fibrosis.4 This feature is pivotal in differentiating APPM from other subtypes of localized lichen myxedematosus and similar dermatoses. Diagnosis of APPM requires exclusion of systemic involvement, including thyroid abnormalities and monoclonal gammopathy, aligning with its classification as a purely cutaneous condition.5 Management of APPM is unclear due to its rarity. Reassurance for patients of its benign nature as well as clinical observation are recommended, though some reports cite benefits of treatment with topical corticosteroids or calcineurin inhibitors.6,7 The long-term prognosis for patients with APPM is favorable, although the persistence of and potential increase in lesions over time can be a cosmetic concern.
The differential diagnoses for APPM include scleromyxedema, scleredema, and other cutaneous eruptions that manifest as smooth flesh-colored papules, such as granuloma annulare and lichen nitidus.3 Scleromyxedema is a systemic cutaneous mucinosis that is part of the same disease spectrum as lichen myxedematosus. The papular eruption of scleromyxedema is much more widespread, and coalescing of the lesions may lead to characteristic skin thickening, creating leonine facies and deep furrowing over the trunk.8 Extracutaneous manifestations are frequent in scleromyxedema, and up to 90% of patients exhibit evidence of an underlying plasma cell dyscrasia.2 Histopathologically, scleromyxedema shows extensive fibroblast proliferation and fibrosis, in contrast to the findings of APPM (Figure 1).

The histopathology of APPM is most similar to scleredema, a rare fibromucinous disorder of the skin associated with diabetes, infection (especially poststreptococcal), or monoclonal gammopathy.9 Biopsy evaluation of scleredema reveals a normal epidermis with mucin deposition between collagen bundles predominantly in the deep reticular dermis as well as absent fibroblast proliferation (Figure 2). Unlike APPM, scleredema manifests with diffuse woody induration with erythema and hyperpigmentation on the posterior neck and upper back.9 On physical examination, the distinct clinical features of scleredema distinguish this condition from APPM and scleromyxedema.

Papular granuloma annulare also was considered in our patient due to the presence of small flesh-colored papules. Histologically, granuloma annulare is characterized by palisading granulomas and mucin deposition in the dermis.10 However, the pattern of mucin deposition differs from that seen in APPM. In granuloma annulare, mucin is observed around foci of degenerated collagen (Figure 3), which was not observed in our patient.10 Additionally, the absence of an inflammatory infiltrate in our patient further ruled out this diagnosis.

Lichen nitidus also could be considered in the differential diagnosis for ACCM. It typically manifests with minute, clustered, monomorphous papules with a predilection for the chest, abdomen, flexural forearms, and genitalia. The histology of lichen nitidus is distinct, showing a well-circumscribed lymphohistiocytic infiltrate in the papillary dermis bordered by epidermal ridges, resembling a ball and clutch appearance (Figure 4).11

Although the clinical differential diagnosis in our patient was broad, histopathologic evaluation played a crucial role in confirming the diagnosis of APPM. This benign condition could be overlooked by patients and physicians; thorough clinical evaluation is necessary to rule out systemic mucinoses, which are associated with higher risks of morbidity and mortality.
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
- Rongioletti F, Rebora A. Acral persistent papular mucinosis: a new entity. Arch Dermatol. 1986;122:1237-1239. doi:10.1001 /archderm.1986.01660230027002
- Christman MP, Sukhdeo K, Kim RH, et al. Papular mucinosis, or localized lichen myxedematosus (LM)(discrete papular type). Dermatol Online J. 2017;23:13030/qt3xp109qd.
- Rongioletti F, Ferreli C, Atzori L. Acral persistent papular mucinosis. Clin Dermatol. 2021;39:211-214. doi:10.1016/j.clindermatol.2020.10.001
- Rongioletti F, Rebora A. Cutaneous mucinoses: microscopic criteria for diagnosis. Am J Dermatopathol. 2001;23:257-267. doi:10.1097/00000372- 200106000-00022
- Rongioletti F. Lichen myxedematosus (papular mucinosis): new concepts and perspectives for an old disease. Semin Cutan Med Surg. 2006;25:100-104. doi:10.1016/j.sder.2006.04.001
- Jun JY, Oh SH, Shim JH, et al. Acral persistent papular mucinosis with partial response to tacrolimus ointment. Ann Dermatol. 2016;28:517-519. doi:10.5021/ad.2016.28.4.517
- Rongioletti F, Zaccaria E, Cozzani E, et al. Treatment of localized lichen myxedematosus of discrete type with tacrolimus ointment. J Am Acad Dermatol. 2008;58:530-532. doi:10.1016/j.jaad.2006.10.021
- Rongioletti F, Merlo G, Cinotti E, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol. 2013;69:66-72. doi:10.1016 /j.jaad.2013.01.007
- Rongioletti F, Kaiser F, Cinotti E, et al. Scleredema. a multicentre study of characteristics, comorbidities, course and therapy in 44 patients. J Eur Acad Dermatol Venereol. 2015;29:2399-2404. doi:10.1111/jdv.13272
- Piette EW, Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75:457-465. doi:10.1016/j.jaad.2015.03.054
- Al-Mutairi N, Hassanein A, Nour-Eldin O, et al. Generalized lichen nitidus. Pediatr Dermatol. 2005;22:158-160. doi:10.1111 /j.1525-1470.2005.22215.x
Multiple Firm Papules on the Wrists and Forearms
Multiple Firm Papules on the Wrists and Forearms
A 69-year-old woman presented to the dermatology department with persistent asymptomatic skin lesions on the wrists and forearms of several months’ duration. The lesions had slowly grown in number over the past few months with no identifiable triggers. The patient reported no known history of injury or trauma to the affected sites and was not taking any prescription medications other than daily vitamins. She denied any family history of similar lesions and was otherwise healthy. Physical examination revealed multiple waxy, firm, hypopigmented, 3- to 5-mm papules located exclusively on the dorsal wrists and forearms. No extracutaneous involvement was observed. A 4-mm punch biopsy from the forearm was obtained.
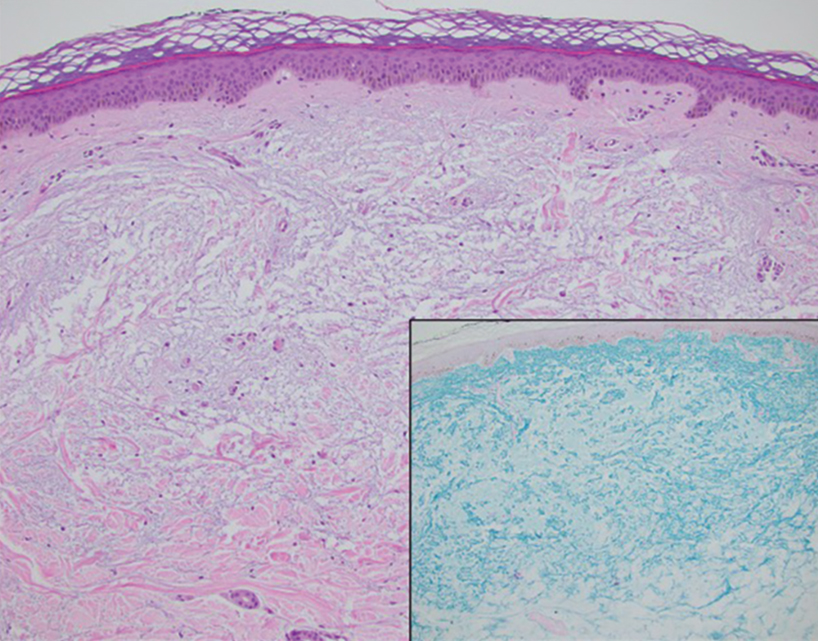
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Click to view more from Gastroenterology Data Trends 2025.
- Vantanasiri K, Kamboj AK, Kisiel JB, Iyer PG. Advances in Screening for Barrett Esophagus and Esophageal Adenocarcinoma. Mayo Clin Proc. 2024;99(3):459-473. doi:10.1016/j.mayocp.2023.07.014
- Cancer Stat Facts: Esophageal Cancer. NIH National Cancer Institute: Survival, Epidemiology, and End Results Program web site. Accessed March 12, 2025. https://seer.cancer.gov/statfacts/html/esoph.html
- Seer*Explorer: Esophagus. NIH National Cancer Institute: Survival, Epidemiology, and End Results Program web site. Accessed March 4, 2025. https://seer.cancer.gov/statistics-network/explorer/application.html
- Kolb JM, Chen M, Tavakkoli A, et al. Understanding Compliance, Practice Patterns, and Barriers Among Gastroenterologists and Primary Care Providers Is Crucial for Developing Strategies to Improve Screening for Barrett’s Esophagus. Gastroenterology. 2022;162(6):1568-1573.e4. doi:10.1053/j.gastro.2022.02.003
- Kunzmann AT, Thrift AP, Cardwell CR, et al. Model for Identifying Individuals at Risk for Esophageal Adenocarcinoma. Clin Gastroenterol Hepatol. 2018;16(8):1229-1236.e4. doi:10.1016/j.cgh.2018.03.014
- Rubenstein JH, Evans RR, Burns JA, et al. Patients With Adenocarcinoma of the Esophagus or Esophagogastric Junction Frequently Have Potential Screening Opportunities. Gastroenterology. 2022;162(4):1349-1351.e5. doi:10.1053/j.gastro.2021.12.255
- Xie S-H, Ness-Jensen E, Medefelt N, Lagergren J. Assessing the feasibility of targeted screening for esophageal adenocarcinoma based on individual risk assessment in a population-based cohort study in Norway (The HUNT Study). Am J Gastroenterol. 2018;113(6):829-835. doi:10.1038/s41395-018-0069-9
- Rubenstein JH, Fontaine S, MacDonald PW, et al. Predicting Incident Adenocarcinoma of the Esophagus or Gastric Cardia Using Machine Learning of Electronic Health Records. Gastroenterology. 2023;165(6):1420-1429.e10. doi:10.1053/j.gastro.2023.08.011
- Fitzgerald RC, di Pietro M, O’Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396(10247):333-344. doi:10.1016/S0140-6736(20)31099-0
- Moinova HR, Verma S, Dumot J, et al. Multicenter, Prospective Trial of Nonendoscopic
Biomarker-Driven Detection of Barrett’s Esophagus and Esophageal Adenocarcinoma. Am J Gastroenterol. 2024;119(11):2206-2214. doi:10.14309/ajg.0000000000002850 - Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am J Gastroenterol. 2022;117(4):559-587. doi:10.14309/ajg.0000000000001680
- ASGE STANDARDS OF PRACTICE COMMITTEE; Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc. 2019;90(3):335-359.e2. doi:10.1016/j.gie.2019.05.012
- Muthusamy VR, Wani S, Gyawali CP, Komanduri S. CGIT Barrett’s Esophagus Consensus Conference Participants. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett’s Esophagus: Expert review. Clin Gastroenterol Hepatol. 2022;20(12):2696-2706. doi:10.1016/j.cgh.2022.06.003
- Xie SH, Lagergren J. A model for predicting individuals’ absolute risk of esophageal adenocarcinoma: Moving toward tailored screening and prevention. Int J Cancer. 2016;138(12):2813-2819. doi:10.1002/ijc.29988
- Rubenstein JH, McConnell D, Waljee AK, et al. Validation and Comparison of Tools for Selecting Individuals to Screen for Barrett’s Esophagus and Early Neoplasia. Gastroenterology. 2020;158(8):2082-2092. doi:10.1053/j.gastro.2020.02.037
- Iyer PG, Sachdeva K, Leggett CL, et al. Development of Electronic Health Record–Based Machine Learning Models to Predict Barrett’s Esophagus and Esophageal Adenocarcinoma Risk. Clin Transl Gastroenterol. 2023;14(10):e00637. doi:10.14309/ctg.0000000000000637
- Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al; BEST2 Study Group. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multicenter case-control study. PLoS Med. 2015;12(1):e1001780. doi:10.1371/journal.pmed.1001780
Click to view more from Gastroenterology Data Trends 2025.
Click to view more from Gastroenterology Data Trends 2025.
- Vantanasiri K, Kamboj AK, Kisiel JB, Iyer PG. Advances in Screening for Barrett Esophagus and Esophageal Adenocarcinoma. Mayo Clin Proc. 2024;99(3):459-473. doi:10.1016/j.mayocp.2023.07.014
- Cancer Stat Facts: Esophageal Cancer. NIH National Cancer Institute: Survival, Epidemiology, and End Results Program web site. Accessed March 12, 2025. https://seer.cancer.gov/statfacts/html/esoph.html
- Seer*Explorer: Esophagus. NIH National Cancer Institute: Survival, Epidemiology, and End Results Program web site. Accessed March 4, 2025. https://seer.cancer.gov/statistics-network/explorer/application.html
- Kolb JM, Chen M, Tavakkoli A, et al. Understanding Compliance, Practice Patterns, and Barriers Among Gastroenterologists and Primary Care Providers Is Crucial for Developing Strategies to Improve Screening for Barrett’s Esophagus. Gastroenterology. 2022;162(6):1568-1573.e4. doi:10.1053/j.gastro.2022.02.003
- Kunzmann AT, Thrift AP, Cardwell CR, et al. Model for Identifying Individuals at Risk for Esophageal Adenocarcinoma. Clin Gastroenterol Hepatol. 2018;16(8):1229-1236.e4. doi:10.1016/j.cgh.2018.03.014
- Rubenstein JH, Evans RR, Burns JA, et al. Patients With Adenocarcinoma of the Esophagus or Esophagogastric Junction Frequently Have Potential Screening Opportunities. Gastroenterology. 2022;162(4):1349-1351.e5. doi:10.1053/j.gastro.2021.12.255
- Xie S-H, Ness-Jensen E, Medefelt N, Lagergren J. Assessing the feasibility of targeted screening for esophageal adenocarcinoma based on individual risk assessment in a population-based cohort study in Norway (The HUNT Study). Am J Gastroenterol. 2018;113(6):829-835. doi:10.1038/s41395-018-0069-9
- Rubenstein JH, Fontaine S, MacDonald PW, et al. Predicting Incident Adenocarcinoma of the Esophagus or Gastric Cardia Using Machine Learning of Electronic Health Records. Gastroenterology. 2023;165(6):1420-1429.e10. doi:10.1053/j.gastro.2023.08.011
- Fitzgerald RC, di Pietro M, O’Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396(10247):333-344. doi:10.1016/S0140-6736(20)31099-0
- Moinova HR, Verma S, Dumot J, et al. Multicenter, Prospective Trial of Nonendoscopic
Biomarker-Driven Detection of Barrett’s Esophagus and Esophageal Adenocarcinoma. Am J Gastroenterol. 2024;119(11):2206-2214. doi:10.14309/ajg.0000000000002850 - Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am J Gastroenterol. 2022;117(4):559-587. doi:10.14309/ajg.0000000000001680
- ASGE STANDARDS OF PRACTICE COMMITTEE; Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc. 2019;90(3):335-359.e2. doi:10.1016/j.gie.2019.05.012
- Muthusamy VR, Wani S, Gyawali CP, Komanduri S. CGIT Barrett’s Esophagus Consensus Conference Participants. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett’s Esophagus: Expert review. Clin Gastroenterol Hepatol. 2022;20(12):2696-2706. doi:10.1016/j.cgh.2022.06.003
- Xie SH, Lagergren J. A model for predicting individuals’ absolute risk of esophageal adenocarcinoma: Moving toward tailored screening and prevention. Int J Cancer. 2016;138(12):2813-2819. doi:10.1002/ijc.29988
- Rubenstein JH, McConnell D, Waljee AK, et al. Validation and Comparison of Tools for Selecting Individuals to Screen for Barrett’s Esophagus and Early Neoplasia. Gastroenterology. 2020;158(8):2082-2092. doi:10.1053/j.gastro.2020.02.037
- Iyer PG, Sachdeva K, Leggett CL, et al. Development of Electronic Health Record–Based Machine Learning Models to Predict Barrett’s Esophagus and Esophageal Adenocarcinoma Risk. Clin Transl Gastroenterol. 2023;14(10):e00637. doi:10.14309/ctg.0000000000000637
- Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al; BEST2 Study Group. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multicenter case-control study. PLoS Med. 2015;12(1):e1001780. doi:10.1371/journal.pmed.1001780
- Vantanasiri K, Kamboj AK, Kisiel JB, Iyer PG. Advances in Screening for Barrett Esophagus and Esophageal Adenocarcinoma. Mayo Clin Proc. 2024;99(3):459-473. doi:10.1016/j.mayocp.2023.07.014
- Cancer Stat Facts: Esophageal Cancer. NIH National Cancer Institute: Survival, Epidemiology, and End Results Program web site. Accessed March 12, 2025. https://seer.cancer.gov/statfacts/html/esoph.html
- Seer*Explorer: Esophagus. NIH National Cancer Institute: Survival, Epidemiology, and End Results Program web site. Accessed March 4, 2025. https://seer.cancer.gov/statistics-network/explorer/application.html
- Kolb JM, Chen M, Tavakkoli A, et al. Understanding Compliance, Practice Patterns, and Barriers Among Gastroenterologists and Primary Care Providers Is Crucial for Developing Strategies to Improve Screening for Barrett’s Esophagus. Gastroenterology. 2022;162(6):1568-1573.e4. doi:10.1053/j.gastro.2022.02.003
- Kunzmann AT, Thrift AP, Cardwell CR, et al. Model for Identifying Individuals at Risk for Esophageal Adenocarcinoma. Clin Gastroenterol Hepatol. 2018;16(8):1229-1236.e4. doi:10.1016/j.cgh.2018.03.014
- Rubenstein JH, Evans RR, Burns JA, et al. Patients With Adenocarcinoma of the Esophagus or Esophagogastric Junction Frequently Have Potential Screening Opportunities. Gastroenterology. 2022;162(4):1349-1351.e5. doi:10.1053/j.gastro.2021.12.255
- Xie S-H, Ness-Jensen E, Medefelt N, Lagergren J. Assessing the feasibility of targeted screening for esophageal adenocarcinoma based on individual risk assessment in a population-based cohort study in Norway (The HUNT Study). Am J Gastroenterol. 2018;113(6):829-835. doi:10.1038/s41395-018-0069-9
- Rubenstein JH, Fontaine S, MacDonald PW, et al. Predicting Incident Adenocarcinoma of the Esophagus or Gastric Cardia Using Machine Learning of Electronic Health Records. Gastroenterology. 2023;165(6):1420-1429.e10. doi:10.1053/j.gastro.2023.08.011
- Fitzgerald RC, di Pietro M, O’Donovan M, et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: a multicentre, pragmatic, randomised controlled trial. Lancet. 2020;396(10247):333-344. doi:10.1016/S0140-6736(20)31099-0
- Moinova HR, Verma S, Dumot J, et al. Multicenter, Prospective Trial of Nonendoscopic
Biomarker-Driven Detection of Barrett’s Esophagus and Esophageal Adenocarcinoma. Am J Gastroenterol. 2024;119(11):2206-2214. doi:10.14309/ajg.0000000000002850 - Shaheen NJ, Falk GW, Iyer PG, et al. Diagnosis and Management of Barrett’s Esophagus: An Updated ACG Guideline. Am J Gastroenterol. 2022;117(4):559-587. doi:10.14309/ajg.0000000000001680
- ASGE STANDARDS OF PRACTICE COMMITTEE; Qumseya B, Sultan S, Bain P, et al. ASGE guideline on screening and surveillance of Barrett’s esophagus. Gastrointest Endosc. 2019;90(3):335-359.e2. doi:10.1016/j.gie.2019.05.012
- Muthusamy VR, Wani S, Gyawali CP, Komanduri S. CGIT Barrett’s Esophagus Consensus Conference Participants. AGA Clinical Practice Update on New Technology and Innovation for Surveillance and Screening in Barrett’s Esophagus: Expert review. Clin Gastroenterol Hepatol. 2022;20(12):2696-2706. doi:10.1016/j.cgh.2022.06.003
- Xie SH, Lagergren J. A model for predicting individuals’ absolute risk of esophageal adenocarcinoma: Moving toward tailored screening and prevention. Int J Cancer. 2016;138(12):2813-2819. doi:10.1002/ijc.29988
- Rubenstein JH, McConnell D, Waljee AK, et al. Validation and Comparison of Tools for Selecting Individuals to Screen for Barrett’s Esophagus and Early Neoplasia. Gastroenterology. 2020;158(8):2082-2092. doi:10.1053/j.gastro.2020.02.037
- Iyer PG, Sachdeva K, Leggett CL, et al. Development of Electronic Health Record–Based Machine Learning Models to Predict Barrett’s Esophagus and Esophageal Adenocarcinoma Risk. Clin Transl Gastroenterol. 2023;14(10):e00637. doi:10.14309/ctg.0000000000000637
- Ross-Innes CS, Debiram-Beecham I, O’Donovan M, et al; BEST2 Study Group. Evaluation of a minimally invasive cell sampling device coupled with assessment of trefoil factor 3 expression for diagnosing Barrett’s esophagus: a multicenter case-control study. PLoS Med. 2015;12(1):e1001780. doi:10.1371/journal.pmed.1001780
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Advances in Screening for Barrett’s Esophagus and Esophageal Adenocarcinoma
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Click to view more from Gastroenterology Data Trends.
- Jones JM. LGBTQ+ identification in U.S. rises to 9.3%. Gallup.com website. February 20, 2025. Accessed March 4, 2025. https://news.gallup.com/poll/656708/lgbtq-identification-rises.aspx.
- Newman KL, Vélez C, Paul S, Radix AE, Streed CG Jr, Targownik LE. Research Considerations in Digestive and Liver Disease in Transgender and Gender-Diverse Populations. Gastroenterology. 2023;165(3):523-528.e1. doi:10.1053/j.gastro.2023.07.011
- Vélez C, Newman KL, Paul S, Berli JU, Tangpricha V, Targownik LE. Approaching Digestive Health Care in Transgender and Gender-Diverse Communities. Clin Gastroenterol Hepatol. 2024;22(3):441-447.e2. doi:10.1016/j.cgh.2023.12.001
- Condray CD, Newman KL, Chedid VG. Consequences of bathroom restriction on transgender individuals with gastrointestinal conditions in the United States. Nat Rev Gastroenterol Hepatol. 2024;21(10):662-663. doi:10.1038/s41575-024-00975-4
- Tsai C, Abdelhalim S, Wong S-Y, Xie X, Agrawal M, Keefer LA. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct 24:S1542-3565(24)00953-4. doi:10.1016/j.cgh.2024.09.015
- Newman KL, Chedid VG, Boden EK. A Systematic Review of Inflammatory Bowel Disease Epidemiology and Health Outcomes in Sexual and Gender Minority Individuals. Gastroenterology. 2023;164(6):866-871. doi:10.1053/j.gastro.2022.11.048
- Hassan B, Suchan A, Brown M, Kishan A, Liang F, Truta B. The Impact of Hormone Therapy on Inflammatory Bowel Disease in Transgender and Nonbinary Individuals. Inflamm Bowel Dis. 2024 Oct 16:izae236. doi:10.1093/ibd/izae236
- Elhence H, Dodge JL, Kahn JA, Lee BP. Characteristics and Outcomes Among US Commercially Insured Transgender Adults With Cirrhosis: A National Cohort Study. Am J Gastroenterol. 2024;119(12):2455-2461. doi:10.14309/ajg.0000000000002907
- Stier EA, Clarke MA, Deshmukh AA, et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int J Cancer. 2024;154(10):1694-1702. doi:10.1002/ijc.34850
- Nash R, Ward KC, Jemal A, Sandberg DE, Tangpricha V, Goodman M. Frequency and distribution of primary site among gender minority cancer patients: An analysis of U.S. national surveillance data. Cancer Epidemiol. 2018;54:1-6. doi:10.1016/j.canep.2018.02.008
Click to view more from Gastroenterology Data Trends.
Click to view more from Gastroenterology Data Trends.
- Jones JM. LGBTQ+ identification in U.S. rises to 9.3%. Gallup.com website. February 20, 2025. Accessed March 4, 2025. https://news.gallup.com/poll/656708/lgbtq-identification-rises.aspx.
- Newman KL, Vélez C, Paul S, Radix AE, Streed CG Jr, Targownik LE. Research Considerations in Digestive and Liver Disease in Transgender and Gender-Diverse Populations. Gastroenterology. 2023;165(3):523-528.e1. doi:10.1053/j.gastro.2023.07.011
- Vélez C, Newman KL, Paul S, Berli JU, Tangpricha V, Targownik LE. Approaching Digestive Health Care in Transgender and Gender-Diverse Communities. Clin Gastroenterol Hepatol. 2024;22(3):441-447.e2. doi:10.1016/j.cgh.2023.12.001
- Condray CD, Newman KL, Chedid VG. Consequences of bathroom restriction on transgender individuals with gastrointestinal conditions in the United States. Nat Rev Gastroenterol Hepatol. 2024;21(10):662-663. doi:10.1038/s41575-024-00975-4
- Tsai C, Abdelhalim S, Wong S-Y, Xie X, Agrawal M, Keefer LA. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct 24:S1542-3565(24)00953-4. doi:10.1016/j.cgh.2024.09.015
- Newman KL, Chedid VG, Boden EK. A Systematic Review of Inflammatory Bowel Disease Epidemiology and Health Outcomes in Sexual and Gender Minority Individuals. Gastroenterology. 2023;164(6):866-871. doi:10.1053/j.gastro.2022.11.048
- Hassan B, Suchan A, Brown M, Kishan A, Liang F, Truta B. The Impact of Hormone Therapy on Inflammatory Bowel Disease in Transgender and Nonbinary Individuals. Inflamm Bowel Dis. 2024 Oct 16:izae236. doi:10.1093/ibd/izae236
- Elhence H, Dodge JL, Kahn JA, Lee BP. Characteristics and Outcomes Among US Commercially Insured Transgender Adults With Cirrhosis: A National Cohort Study. Am J Gastroenterol. 2024;119(12):2455-2461. doi:10.14309/ajg.0000000000002907
- Stier EA, Clarke MA, Deshmukh AA, et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int J Cancer. 2024;154(10):1694-1702. doi:10.1002/ijc.34850
- Nash R, Ward KC, Jemal A, Sandberg DE, Tangpricha V, Goodman M. Frequency and distribution of primary site among gender minority cancer patients: An analysis of U.S. national surveillance data. Cancer Epidemiol. 2018;54:1-6. doi:10.1016/j.canep.2018.02.008
- Jones JM. LGBTQ+ identification in U.S. rises to 9.3%. Gallup.com website. February 20, 2025. Accessed March 4, 2025. https://news.gallup.com/poll/656708/lgbtq-identification-rises.aspx.
- Newman KL, Vélez C, Paul S, Radix AE, Streed CG Jr, Targownik LE. Research Considerations in Digestive and Liver Disease in Transgender and Gender-Diverse Populations. Gastroenterology. 2023;165(3):523-528.e1. doi:10.1053/j.gastro.2023.07.011
- Vélez C, Newman KL, Paul S, Berli JU, Tangpricha V, Targownik LE. Approaching Digestive Health Care in Transgender and Gender-Diverse Communities. Clin Gastroenterol Hepatol. 2024;22(3):441-447.e2. doi:10.1016/j.cgh.2023.12.001
- Condray CD, Newman KL, Chedid VG. Consequences of bathroom restriction on transgender individuals with gastrointestinal conditions in the United States. Nat Rev Gastroenterol Hepatol. 2024;21(10):662-663. doi:10.1038/s41575-024-00975-4
- Tsai C, Abdelhalim S, Wong S-Y, Xie X, Agrawal M, Keefer LA. Trauma-Informed Care in Gastroenterology: A Survey of Provider Attitudes, Knowledge, and Skills. Clin Gastroenterol Hepatol. 2024 Oct 24:S1542-3565(24)00953-4. doi:10.1016/j.cgh.2024.09.015
- Newman KL, Chedid VG, Boden EK. A Systematic Review of Inflammatory Bowel Disease Epidemiology and Health Outcomes in Sexual and Gender Minority Individuals. Gastroenterology. 2023;164(6):866-871. doi:10.1053/j.gastro.2022.11.048
- Hassan B, Suchan A, Brown M, Kishan A, Liang F, Truta B. The Impact of Hormone Therapy on Inflammatory Bowel Disease in Transgender and Nonbinary Individuals. Inflamm Bowel Dis. 2024 Oct 16:izae236. doi:10.1093/ibd/izae236
- Elhence H, Dodge JL, Kahn JA, Lee BP. Characteristics and Outcomes Among US Commercially Insured Transgender Adults With Cirrhosis: A National Cohort Study. Am J Gastroenterol. 2024;119(12):2455-2461. doi:10.14309/ajg.0000000000002907
- Stier EA, Clarke MA, Deshmukh AA, et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int J Cancer. 2024;154(10):1694-1702. doi:10.1002/ijc.34850
- Nash R, Ward KC, Jemal A, Sandberg DE, Tangpricha V, Goodman M. Frequency and distribution of primary site among gender minority cancer patients: An analysis of U.S. national surveillance data. Cancer Epidemiol. 2018;54:1-6. doi:10.1016/j.canep.2018.02.008
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities
Optimizing the Delivery of GI Care in Transgender and Gender-Diverse Communities