User login
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
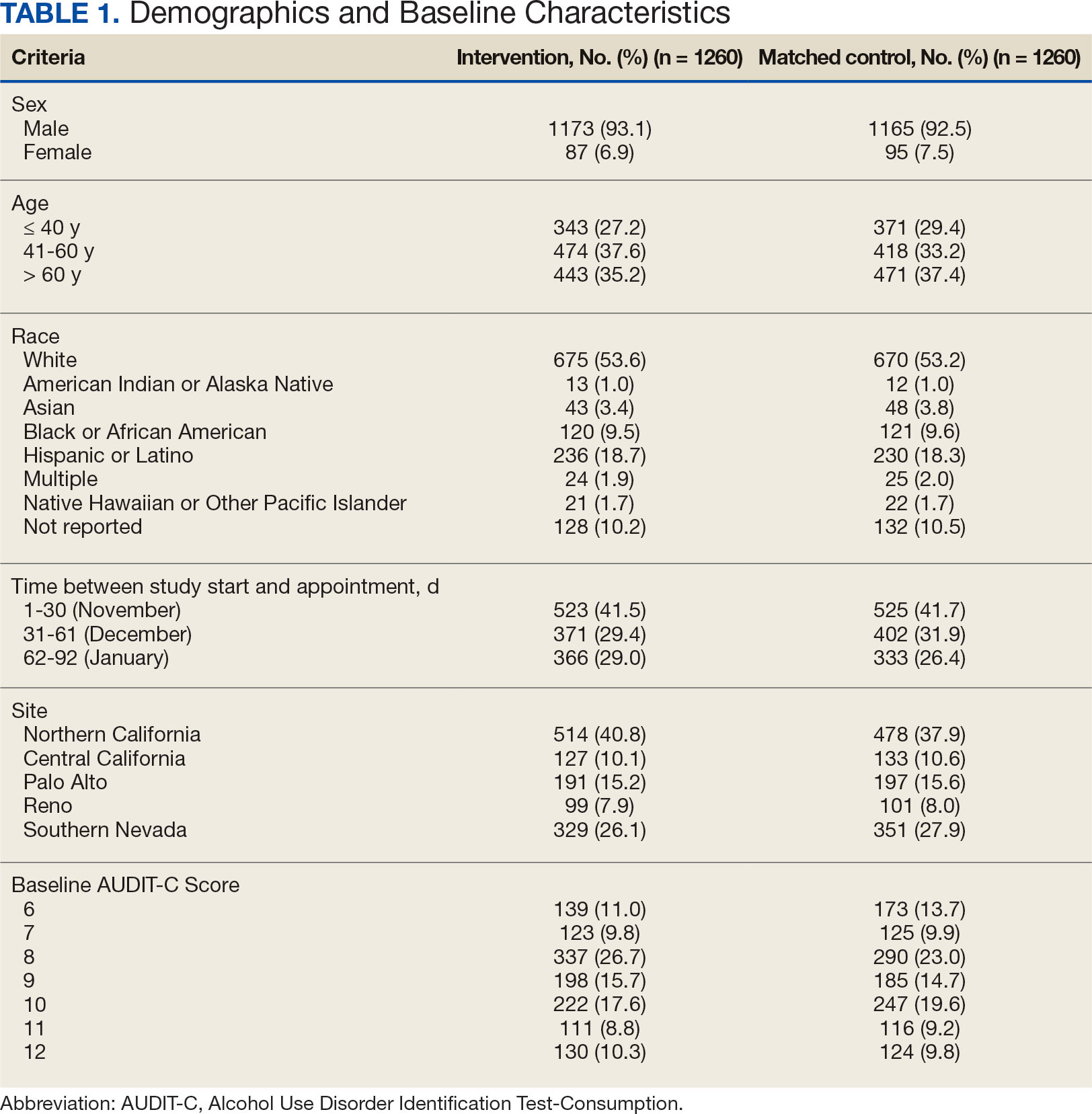
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
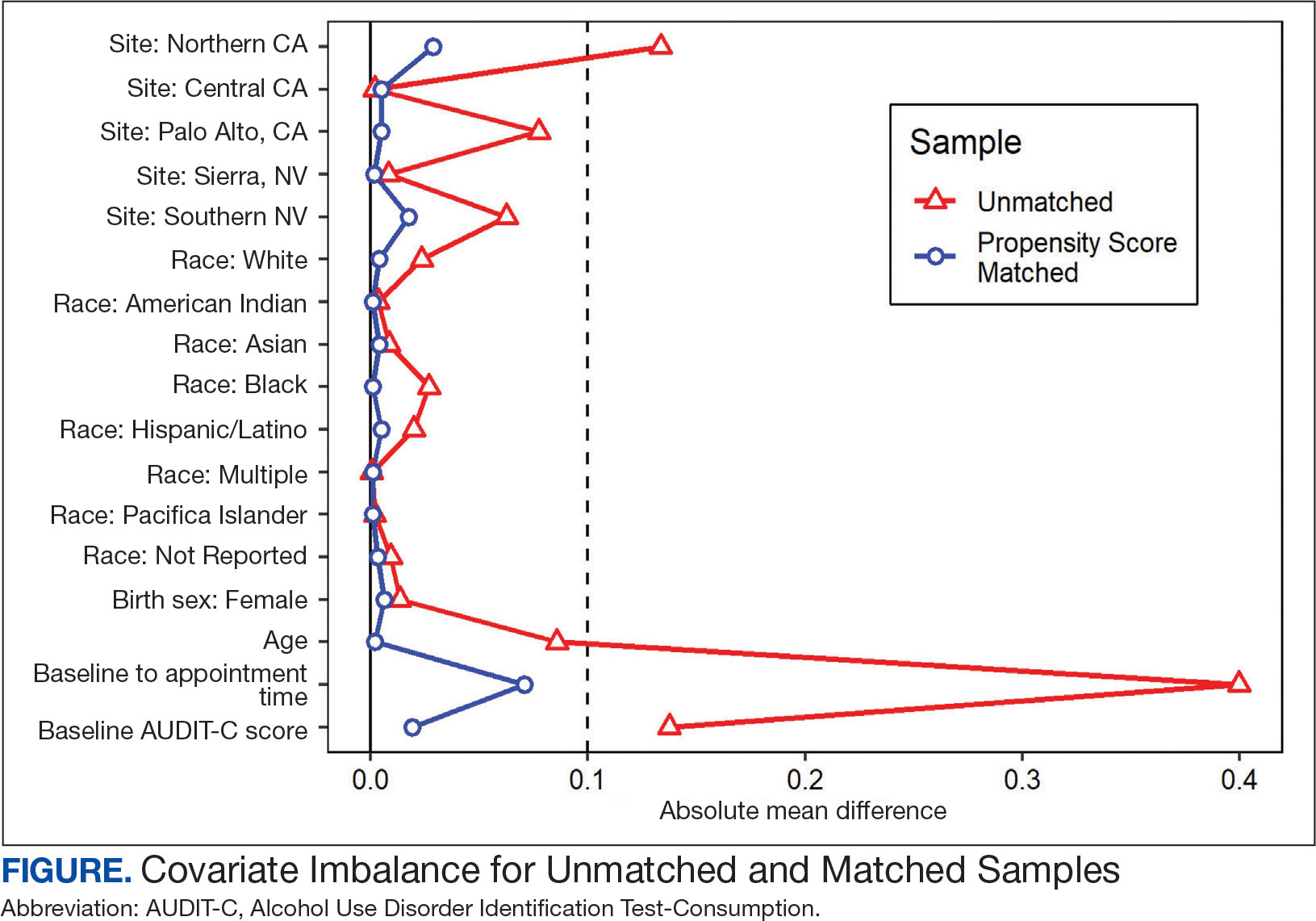
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19
A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
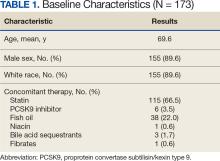
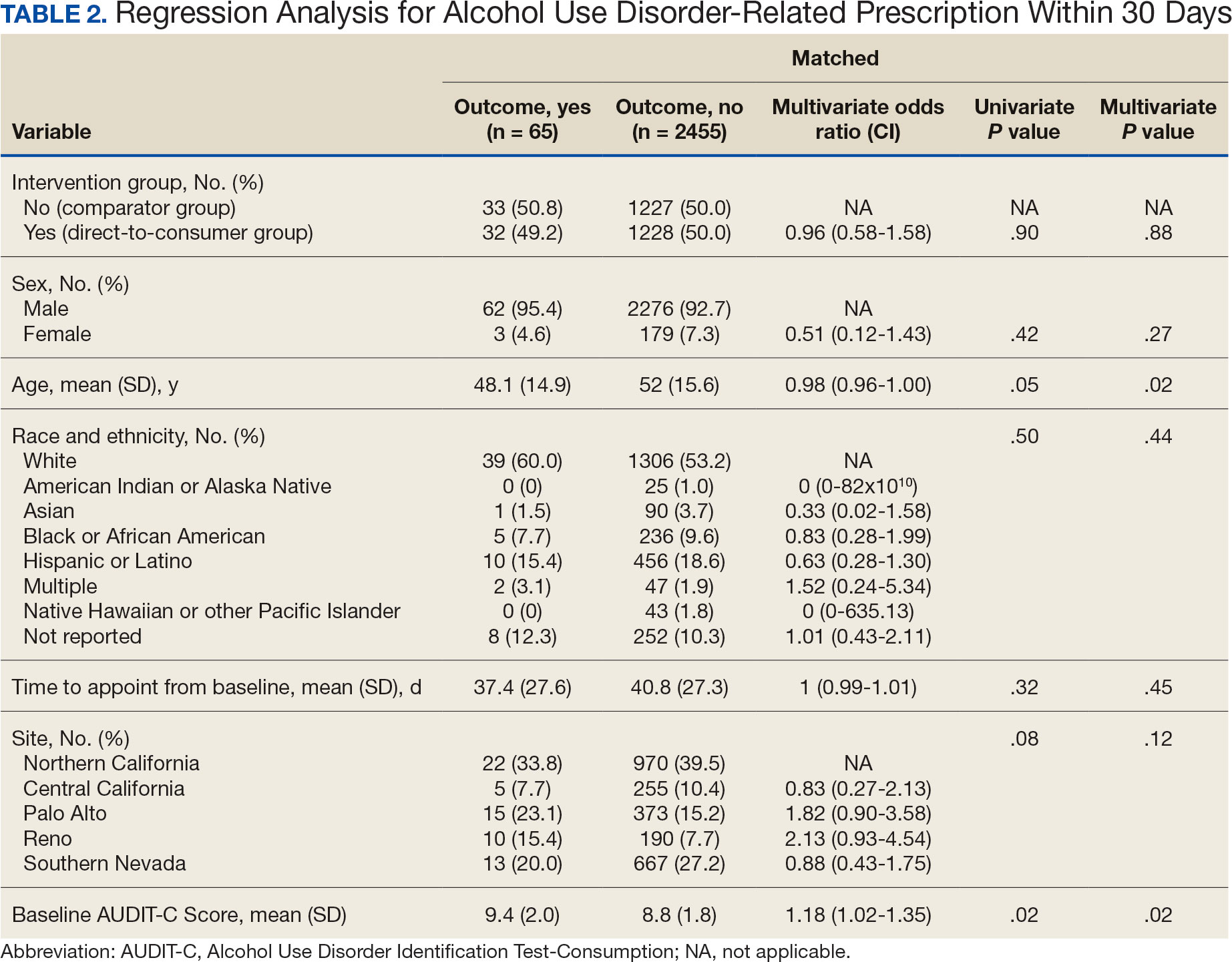
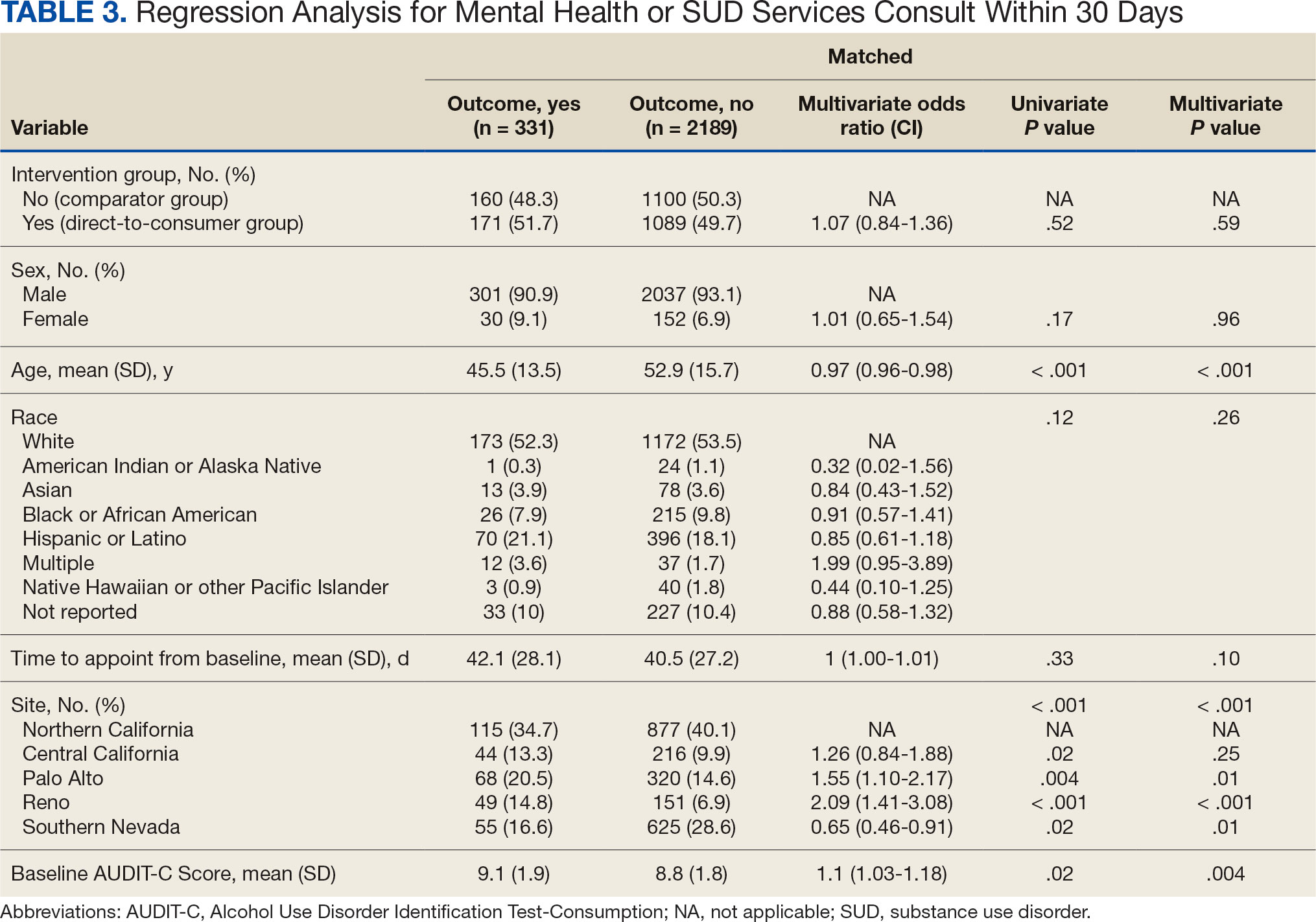
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).
DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19

A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).



DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
Excessive alcohol use is one of the leading preventable causes of death in the United States, responsible for about 178,000 deaths annually and an average of 488 daily deaths in 2020 and 2021.1Alcohol-related deaths increased by 49% between 2006 and 2019.2 This trend continued during the COVID-19 pandemic, with death certificates that listed alcohol increasing by > 25% from 2019 to 2020, and another 10% in 2021.3 This increase of alcohol-related deaths includes those as a direct result of chronic alcohol use, such as alcoholic cardiomyopathy, alcoholic hepatitis and cirrhosis, and alcohol-induced pancreatitis, as well as a result of acute use such as alcohol poisoning, suicide by exposure to alcohol, and alcohol-impaired driving fatalities.4
Excessive alcohol consumption poses other serious risks, including cases when intake is abruptly reduced without proper management. Alcohol withdrawal syndrome (AWS) can vary in severity, with potentially life-threatening complications such as hallucinations, seizures, and delirium tremens.5
These risks highlight the importance of professional intervention and support, not only to mitigate risks associated with AWS, but provide a pathway towards recovery from alcohol use disorder (AUD).
According to the 2022 National Survey on Drug Use and Health, 28.8 million US adults had AUD in the prior year, yet only 7.6% of these individuals received treatment and an even smaller group (2.2%) received medication-assisted treatment for alcohol.6,7 This is despite American Psychiatric Association guidelines for the pharmacological treatment of patients with AUD, including the use of naltrexone, acamprosate, disulfiram, topiramate, or gabapentin, depending on therapy goals, past medication trials, medication contraindications, and patient preference.8 Several of these medications are approved by the US Food and Drug Administration (FDA) for the treatment of AUD and have support for effectiveness from randomized controlled trials and meta-analyses.9-11
Clinical practice guidelines for the management of substance use disorders (SUDs) from the US Department of Veterans Affairs (VA) and US Department of Defense have strong recommendations for naltrexone and topiramate as first-line pharmacotherapies for moderate to severe AUD. Acamprosate and disulfiram are weak recommendations as alternative options. Gabapentin is a weak recommendation for cases where first-line treatments are contraindicated or ineffective. The guidelines emphasize the importance of a comprehensive approach to AUD treatment, including psychosocial interventions in addition to pharmacotherapy.12
A 2023 national survey found veterans reported higher alcohol consumption than nonveterans.13 At the end of fiscal year 2023, > 4.4 million veterans—6% of Veterans Health Administration patients—had been diagnosed with AUD.14 However, > 87% of these patients nationally, and 88% of Veterans Integrated Service Network (VISN) 21 patients, were not receiving naltrexone, acamprosate, disulfiram, or topiramate as part of their treatment. The VA Academic Detailing Service (ADS) now includes AUD pharmacotherapy as a campaign focus, highlighting its importance. The ADS is a pharmacy educational outreach program that uses unbiased clinical guidelines to promote aligning prescribing behavior with best practices. Academic detailing methods include speaking with health care practitioners (HCPs), and direct-to-consumer (DTC) patient education.
ADS campaigns include DTC educational handouts. Past ADS projects and research using DTC have demonstrated a significant improvement in outcomes and positively influencing patients’ pharmacotherapy treatment. 15,16 A VA quality improvement project found a positive correlation between the initiation of AUD pharmacotherapy and engagement with mental health care following the distribution of AUD DTC patient education. 17 This project aimed to apply the same principles of prior research to explore the use of DTC across multiple facilities within VISN 21 to increase AUD pharmacotherapy. VISN 21 includes VA facilities and clinics across the Pacific Islands, Nevada, and California and serves about 350,000 veterans.
METHODS
A prospective cohort of VISN 21 veterans with or at high risk for AUD was identified using the VA ADS AUD Dashboard. The cohort included those not on acamprosate, disulfiram, naltrexone, topiramate, or gabapentin for treatment of AUD and had an elevated Alcohol Use Disorder Identification Test-Consumption (AUDIT-C) score of ≥ 6 (high risk) with an AUD diagnosis or ≥ 8 (severe risk) without a diagnosis. The AUDIT-C scores used in the dashboard are supported by the VA AUD clinician guide as the minimum scores when AUD pharmacotherapy should be offered to patients.18 Prescriptions filled outside the VA were not included in this dashboard.
Data and patient information were collected using the VA Corporate Data Warehouse. To be eligible, veterans needed a valid mailing address within the VISN 21 region and a primary care, mental health, or SUD clinician prescriber visit scheduled between October 1, 2023, and January 31, 2024. Veterans were excluded if they were in hospice, had a 1-year mortality risk score > 50% based on their Care Assessment Need (CAN) score, or facility leadership opted out of project involvement. Patients with both severe renal and hepatic impairments were excluded because they were ineligible for AUD pharmacotherapy. However, veterans with either renal or hepatic impairment (but not both) were included, as they could be potential candidates for ≥ 1 AUD pharmacotherapy option.
Initial correspondence with facilities was initiated through local academic detailers. A local champion was identified for the 1 facility without an academic detailer. Facilities could opt in or out of the project. Approval was provided by the local pharmacy and therapeutics committee, pharmacy, primary care, or psychiatry leadership. Approval process and clinician involvement varied by site.
Education
The selected AUD patient education was designed and approved by the national VA ADS (eappendix). The DTC patient education provided general knowledge about alcohol, including what constitutes a standard amount of alcohol, what is considered heavy drinking, risks of heavy drinking, creating a plan with a clinician to reduce and manage withdrawal symptoms, and additional resources. The DTC was accompanied by a cover letter that included a local facility contact number.
A centralized mailing facility was used for all materials. VA Northern California Health Care System provided the funding to cover the cost of postage. The list of veterans to be contacted was updated on a rolling basis and DTC education was mailed 2 weeks prior to their scheduled prescriber visit.
The eligible cohort of 1260 veterans received DTC education. A comparator group of 2048 veterans that did not receive DTC education was obtained retrospectively by using the same inclusion and exclusion criteria with a scheduled primary care, mental health, or SUD HCP visit from October 1, 2022, to January 31, 2023. The outcomes assessed were within 30 days of the scheduled visit, with the primary outcome as the initiation of AUD-related pharmacotherapy and the secondary outcome as the placement of a consultation for mental health or SUD services. Any consultations sent to Behavioral Health, Addiction, Mental Health, Psychiatric, and SUD services following the HCP visit, within the specified time frame, were used for the secondary outcome.
Matching and Analysis
A 1-to-1 nearest neighbor propensity score (PS) matching without replacement was used to pair the 1260 veterans from the intervention group with similarly scored comparator group veterans for a PS-matched final dataset of 2520 veterans. The PS model was a multivariate logistic regression with the outcome being exposure and comparator group status. Baseline characteristics used in the PS model were age, birth sex, race, facility of care, baseline AUDIT-C score, and days between project start and scheduled appointment. Covariate imbalance for the PS-matched sample was assessed to ensure the standardized mean difference for all covariates fell under a 0.1 threshold (Figure).19

A frequency table was provided to compare the discrete distributions of the baseline characteristics in the intervention and comparator groups. Logistic regression analysis was performed to evaluate the association between DTC education exposure and pharmacotherapy initiation, while controlling for potential confounders. Univariate and multivariate P value results for each variable included in the model were reported along with the multivariate odds ratios (ORs) and their associated 95% CIs. Logistic regression analyses were run for both outcomes. Each model included the exposure and comparator group status as well as the baseline characteristics included in the PS model. Statistical significance was set at P < .05. All statistical analyses were performed with R version 4.2.1.
RESULTS
Two of 7 VISN 21 sites did not participate, and 3 had restrictions on participation. DTC education was mailed about 2 weeks prior to scheduled visit for 1260 veterans; 53.6% identified as White, 37.6% were aged 41 to 60 years, and 79.2% had an AUDIT-C ≥ 8 (Table 1). Of those mailed education, there were 173 no-show appointments (13.7%). Thirty-two veterans (2.5%) in the DTC group and 33 veterans (2.6%) in the comparator group received an AUD-related pharmacotherapy prescription (P = .88) (Table 2). One hundred seventy-one veterans (13.6%) in the DTC group and 160 veterans (12.7%) in the comparator group had a consult placed for mental health or SUD services within 30 days of their appointment (P = .59) (Table 3).



DISCUSSION
This project did not yield statistically significant differences in either the primary or secondary outcomes within the 30-day follow-up window and found limited impact from the DTC educational outreach to veterans. The percentage of veterans that received AUD-related pharmacotherapy or consultations for mental health or SUD services was similarly low in the DTC and comparator groups. These findings suggest that although DTC education may raise awareness, it may not be sufficient on its own to drive changes in prescribing behavior or referral patterns without system-level support.
Addiction is a complex disease faced with stigma and requiring readiness by both the HCP and patient to move forward in support and treatment. The consequences of stigma can be severe: the more stigma perceived by a person with AUD, the less likely they are to seek treatment.20 Stigma may exist even within HCPs and may lead to compromised care including shortened visits, less engagement, and less empathy.19 Cultural attitude towards alcohol use and intoxication can also be influenced through a wide range of sources including social media, movies, music, and television. Studies have shown targeted alcohol marketing may result in the development of positive beliefs about drinking and expand environments where alcohol use is socially acceptable and encouraged.21 These factors can impact drinking behavior, including the onset of drinking, binge drinking, and increased alcohol consumption.22
Three VISN 21 sites in this study had restrictions on or excluded primary care from participation. Leadership at some of these facilities were concerned that primary care teams did not have the bandwidth to take on additional items and/or there was variable primary care readiness for initiating AUD pharmacotherapy. Further attempts should be made to integrate primary care into the process of initiating AUD treatment as significant research suggests that integrated care models for AUD may be associated with improved process and outcome measures of care.23
There are several differences between this quality improvement project and prior research investigating the impact of DTC education for other conditions, such as the EMPOWER randomized controlled trial and VISN 22 project, which both demonstrated effectiveness of DTC education for reducing benzodiazepine use in geriatric veterans. 15,16 These studies focused on reducing or stopping pharmacotherapy use, whereas this project sought to promote the initiation of AUD pharmacotherapy. These studies evaluated outcomes at least 6 months postindex date, whereas this project evaluated outcomes within 30 days postappointment. Furthermore, the educational content varied significantly. Other projects provided patients with information focused on specific medications and interventions, such as benzodiazepine tapering, while this project mailed general information on heavy drinking, its risks, and strategies for cutting back, without mentioning pharmacotherapy. The DTC material used in this project was chosen because it was a preapproved national VA ADS resource, which expedited the project timeline by avoiding the need for additional approvals at each participating site. These differences may impact the observed effectiveness of DTC education in this project, especially regarding the primary outcome.
Strengths and Limitations
This quality improvement project sent a large sample of veterans DTC education in a clinical setting across multiple sites. Additionally, PS matching methods were used to balance covariates between the comparator and DTC education group, thereby simulating a randomized controlled trial and reducing selection bias. The project brought attention to the VISN 21 AUD treatment rates, stimulated conversation across sites about available treatments and resources for AUD, and sparked collaboration between academic detailing, mental health, and primary care services. The time frame for visits was selected during the winter; the National Institute on Alcohol Abuse and Alcoholism notes this is a time when people may be more likely to engage in excessive alcohol consumption than at other times of the year.24
The 30-day time frame for outcomes may have been too short to observe changes in prescribing or referral patterns. Additionally, the comparator group was comprised of veterans seen from October 1, 2022, to January 31, 2023, where seasonal timing may have influenced alcohol consumption behaviors and skewed the results. There were also no-show appointments in the DTC education group (13.7%), though it is likely some patients rescheduled and still received AUD pharmacotherapy within 30 days of the original appointment. Finally, it was not possible to confirm whether a patient opened and read the education that was mailed to them. This may be another reason to explore electronic distribution of DTC education. This all may have contributed to the lack of statistically significant differences in both the primary and secondary outcomes.
There was a high level of variability between facility participation in the project. Two of 7 sites did not participate, and 3 sites restricted primary care engagement. This represents a significant limitation, particularly for the secondary outcome of placing consultations for MH or SUD services. Facilities that only included mental health or SUD HCPs may have resulted in lower consultation rates due to their inherent specialization, reducing the likelihood of self-referrals.
The project may overestimate prescribed AUD pharmacotherapy in the primary outcome due to potential misclassification of medications. While the project adhered to the national VA ADS AUD dashboard’s definition of AUD pharmacotherapy, including acamprosate, disulfiram, naltrexone, topiramate, and gabapentin, some of these medications have multiple indications. For example, gabapentin is commonly prescribed for peripheral neuropathy, and topiramate is used to treat migraines and seizures. The multipurpose use adds uncertainty about whether they were prescribed specifically for AUD treatment, especially in cases where the HCP is responsible for treating a broad range of disease states, as in primary care.
CONCLUSIONS
Results of this quality improvement project did not show a statistically significant difference between patients sent DTC education and the comparator group for the initiation of AUD pharmacotherapy or placement of a consult to mental health or SUD services within 30 days of their scheduled visit. Future studies may seek to implement stricter criteria to confirm the intended use of topiramate and gabapentin, such as looking for keywords in the prescription instructions for use, performing chart reviews, and/or only including these medications if prescribed by a mental health or SUD HCP. Alternatively, future studies may consider limiting the analysis to only FDA-approved AUD medications: acamprosate, disulfiram, and naltrexone. It is vital to continue to enhance primary care HCP readiness to treat AUD, given the existing relationships and trust they often have with patients. Electronic methods for distributing DTC education could also be advantageous, as these methods may have the ability to track whether a message has been opened and read. Despite a lack of statistical significance, this project sparked crucial conversations and collaboration around AUD, available treatments, and addressing potential barriers to connecting patients to care within VISN 21.
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
- Centers for Disease Control and Prevention. Facts about U.S. deaths from excessive alcohol use. August 6, 2024. Accessed February 5, 2025. https://www.cdc.gov/alcohol/facts-stats/
- State Health Access Data Assistance Center. Escalating alcohol-involved death rates: trends and variation across the nation and in the states from 2006 to 2019. April 19, 2021. Accessed February 5, 2025. https://www.shadac.org/escalating-alcohol-involved-death-rates-trends-and-variation-across-nation-and-states-2006-2019
- National Institute on Alcohol Abuse and Alcoholism. Alcohol- related emergencies and deaths in the United States. Updated November 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-related-emergencies-and-deaths-united-states
- Esser MB, Sherk A, Liu Y, Naimi TS. Deaths from excessive alcohol use - United States, 2016- 2021. MMWR Morb Mortal Wkly Rep. 2024;73(8):154-161. doi:10.15585/mmwr.mm7308a1
- Canver BR, Newman RK, Gomez AE. Alcohol Withdrawal Syndrome. In: StatPearls. StatPearls Publishing; 2024.
- National Institute on Alcohol Abuse and Alcoholism. Alcohol treatment in the United States. Updated January 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-treatment-united-states
- National Institute on Alcohol Abuse and Alcoholism. Alcohol use disorder (AUD) in the United States: age groups and demographic characteristics. Updated September 2024. Accessed February 5, 2025. https://www.niaaa.nih.gov/alcohols-effects-health/alcohol-topics/alcohol-facts-and-statistics/alcohol-use-disorder-aud-united-states-age-groups-and-demographic-characteristics
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
- Blodgett JC, Del Re AC, Maisel NC, Finney JW. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293. doi:10.1111/j.1360-0443.2012.04054.x
- Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
- US Department of Veterans Affairs, Department of Defense. VA/DoD clinical practice guideline for the management of substance use disorders. August 2021. Accessed February 5, 2025. https://www.healthquality.va.gov/guidelines/MH/sud/VADODSUDCPG.pdf
- Ranney RM, Bernhard PA, Vogt D, et al. Alcohol use and treatment utilization in a national sample of veterans and nonveterans. J Subst Use Addict Treat. 2023;146:208964. doi:10.1016/j.josat.2023.208964
- US Department of Veterans Affairs, Pharmacy Benefit Management Service, Academic Detailing Service. AUD Trend Report. https://vaww.pbi.cdw.va.gov/PBIRS/Pages/ReportViewer.aspx?/GPE/PBM_AD/SSRS/AUD/AUD_TrendReport
- Mendes MA, Smith JP, Marin JK, et al. Reducing benzodiazepine prescribing in older veterans: a direct-to-consumer educational brochure. Fed Pract. 2018;35(9):36-43.
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890-898. doi:10.1001/jamainternmed.2014.949
- Maloney R, Funmilayo M. Acting on the AUDIT-C: implementation of direct-to-consumer education on unhealth alcohol use. Presented on March 31, 2023; Central Virginia Veterans Affairs Health Care System, Richmond, Virginia.
- US Department of Veterans Affairs, Pharmacy Benefit Management Service. Alcohol use disorder (AUD) – leading the charge in the treatment of AUD: a VA clinician’s guide. February 2022. Accessed February 5, 2025. https://www.pbm.va.gov/PBM/AcademicDetailingService/Documents/508/10-1530_AUD_ClinicianGuide_508Conformant.pdf
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi:10.1080/00273171.2011.568786
- National Institute on Alcohol Abuse and Alcoholism. Stigma: overcoming a pervasive barrier to optimal care. Updated January 6, 2025. Accessed February 5, 2025. https://www.niaaa.nih.gov/health-professionals-communities/core-resource-on-alcohol/stigma-overcoming-pervasive-barrier-optimal-care
- Sudhinaraset M, Wigglesworth C, Takeuchi DT. Social and cultural contexts of alcohol use: influences in a socialecological framework. Alcohol Res. 2016;38(1):35-45.
- Tanski SE, McClure AC, Li Z, et al. Cued recall of alcohol advertising on television and underage drinking behavior. JAMA Pediatr. 2015;169(3):264-271. doi:10.1001/jamapediatrics.2014.3345
- Hyland CJ, McDowell MJ, Bain PA, Huskamp HA, Busch AB. Integration of pharmacotherapy for alcohol use disorder treatment in primary care settings: a scoping review. J Subst Abuse Treat. 2023;144:108919. doi:10.1016/j.jsat.2022.108919
- National Institute on Alcohol Abuse and Alcoholism. The truth about holiday spirits. Updated November 2023. Accessed February 5, 2025. ,a href="https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits">https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/truth-about-holiday-spirits
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
Impact of Multisite Patient Education on Pharmacotherapy for Veterans With Alcohol Use Disorder
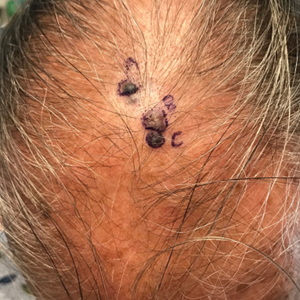
Pigmented Cystic Masses on the Scalp
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
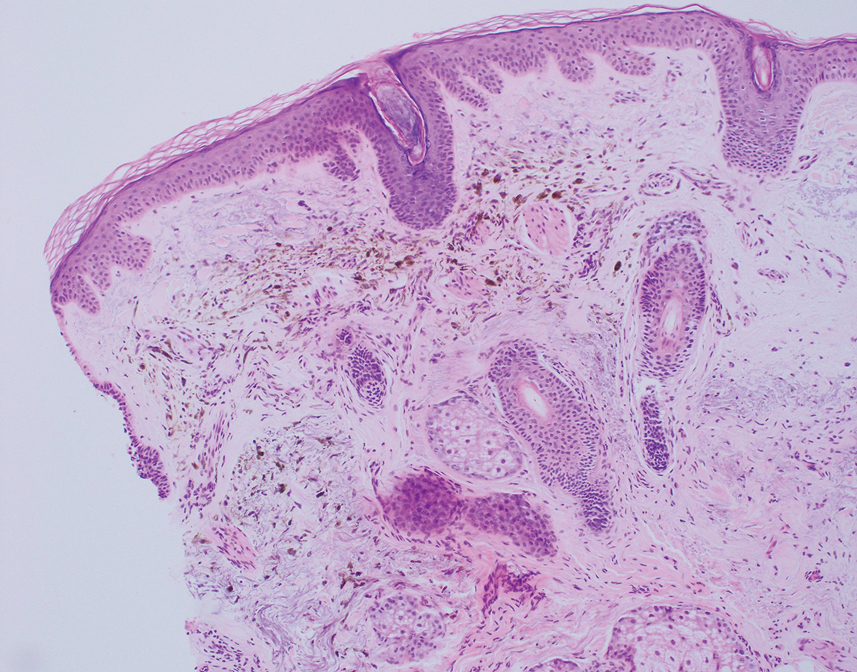
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7
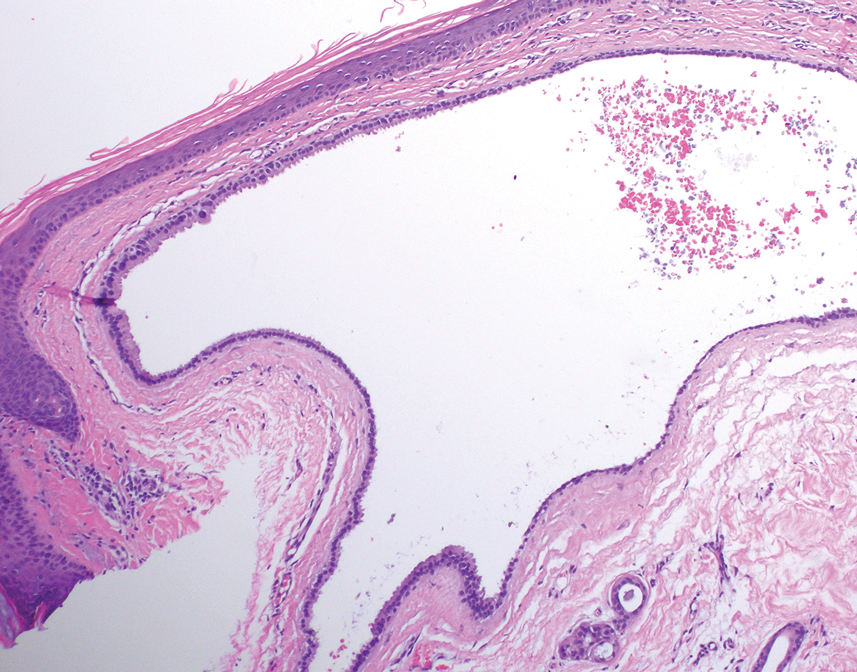
The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.
Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.
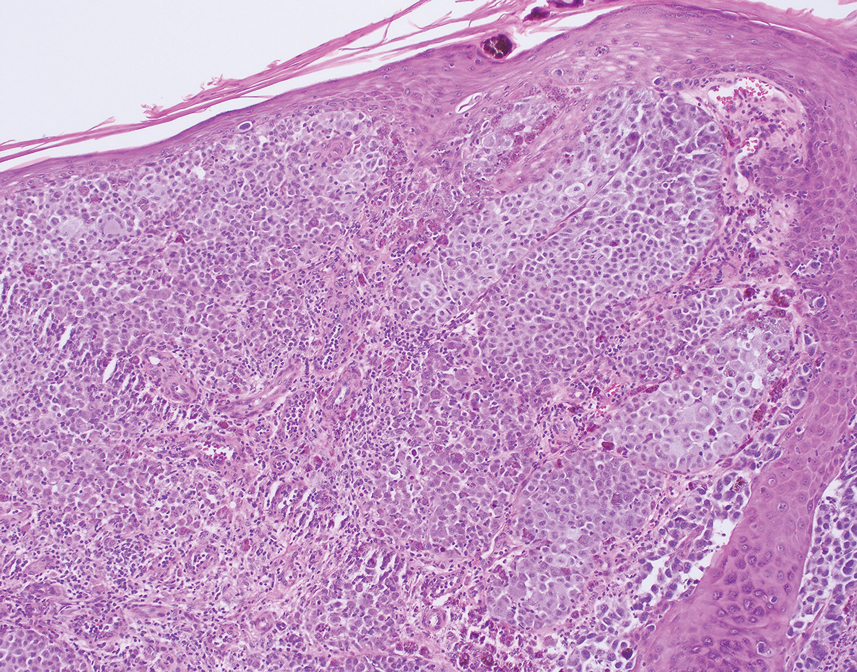
Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7

The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.

Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.

Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
THE DIAGNOSIS: Apocrine Hidrocystoma
Histology for all 3 lesions demonstrated similar cystic structures lined by a dual layer of epithelial cells, with the outermost layer composed of flattened myoepithelial cells and the inner layer composed of cells with apocrine features (Figure 1). Based on these findings, a diagnosis of apocrine hidrocystoma was made. The patient underwent successful surgical excision shortly thereafter without recurrence at follow-up 1 year later.
Apocrine hidrocystomas are rare benign cystic lesions that are considered to be adenomatous proliferations of apocrine glands. They typically manifest as solitary asymptomatic lesions measuring 3 to 15 mm.1 They tend to appear on the face, usually in the periorbital region, but also have been described on the neck, scalp, trunk, arms, and legs.2-4 Multiple apocrine hidrocystomas can be a marker of 2 rare inherited disorders: Gorlin-Goltz syndrome and Schopf-Schulz-Passarge syndrome.5 Apocrine hidrocystomas may be flesh colored or may have a blue, black, or brown appearance due to the Tyndall effect, in which light with shorter wavelengths is scattered by the contents of the lesions.2 Histologically, apocrine hidrocystomas are cysts lined by a dual layer of epithelial cells. The inner layer is composed of cells with apocrine features, and the outer layer is composed of flattened myoepithelial cells. Due to their range of colors and predilection for sun-exposed surfaces, apocrine hidrocystomas may be mistaken for various malignant neoplasms, including melanoma.6,7

The differential diagnosis for our patient included agminated blue nevi, melanoma, pigmented basal cell carcinoma (BCC), and seborrheic keratosis. A blue nevus is a dermal melanocytic lesion that manifests as a well-demarcated, blue to blue-black papule that typically appears on the face, scalp, arms, legs, lower back, and buttocks. Although there are several histologic subtypes, the common blue nevus usually manifests as a solitary lesion measuring less than 1 cm, often developing during childhood to young adulthood.8 Histologically, common blue nevi are characterized by a dermal proliferation of deeply pigmented bipolar spindled melanocytes embedded in thickened collagen bundles, often with scattered epithelioid melanophages, and no conspicuous mitotic activity (Figure 2).9 There are other types of blue nevi, including cellular blue nevi, which tend to be larger and manifest commonly on the buttocks and sacrococcygeal region in early adulthood.9 Histologically, cellular blue nevi contain oval to spindled melanocytes with scattered melanophages forming a well-demarcated nodule typically in the reticular dermis. There may be bulbous extension into the subcutaneous adipose tissue. Occasional mitoses may be seen.9,10 Melanoma can arise from common or cellular blue nevi, though it more frequently occurs with cellular blue nevi. Other subtypes of blue nevi have been described, including the sclerosing, plaque-type, combined, hypomelanotic/amelanotic, and pigmented epithelioid melanocytoma.11 However, they typically have features of the common blue nevus or cellular blue nevus, such as oval/spindle cell morphology, some degree of melanin, and biphasic architecture, but are classified according to their dominant histologic characteristics.

Given the location of our patient’s lesions on the scalp and his extensive history of sun exposure, malignancy was high in the differential. Multiple synchronous primary melanomas including nodular melanoma, blue nevus–like metastatic melanoma, and metastatic melanoma were considered. The leg and the scalp have the highest reported incidence of cutaneous metastases of melanoma, with many cases presenting as dermal or subcutaneous nodules and eruptive blue nevus–like papules, similar to our patient’s clinical presentation.12,13 Nodular melanoma (NM) is one of 4 major types of melanoma, accounting for approximately 15% to 30% of cases in the United States.14 Nodular melanoma typically manifests as a smooth, raised, symmetric, well-circumscribed lesion with variable pigmentation, from very dark to amelanotic. Histologically, NM is defined as a dermal mass, either in isolation or with an epidermal component, not to exceed 3 rete ridges beyond the dermal component.15 Tumor cells have a high cell density with pleomorphism, usually with atypical epithelioid cells with vesicular nuclei and irregular cytoplasm, and occasionally spindle cells (Figure 2).16 Mitoses and necrosis are frequent. Scalp location independently is responsible for worse survival, both overall and melanoma specific.17 Nodular melanoma tends to have greater Breslow thickness at diagnosis than other melanoma subtypes and often carries a worse prognosis.

Malignant melanomas that develop from or in conjunction with or bear histologic resemblance to blue nevi are termed blue nevus–like melanoma or blue nevus–associated melanoma. These malignancies are exceedingly rare, accounting for only 0.3% of melanomas in one Turkey-based multicenter study.18 The histologic criteria for diagnosing blue nevus–like melanoma are poorly defined, and terminology of these lesions has led to some debate in naming conventions.19 Nevertheless, unlike blue nevus, blue nevus–like melanoma demonstrates histologic features of malignancy, including pleomorphism, prominent nucleoli, mitotic activity, vascular invasion, and potential necrosis.10 The lack of an inflammatory infiltrate, surrounding fibrosis, junctional activity, and pre-existing nevus can help distinguish cutaneous melanoma metastases from primary nodular melanoma. Immunohistochemical stains such as S100, Melan-A/MART1, or SOX-10 can help confirm melanocytic lineage.12
Pigmented BCC is a clinical and histologic variant of BCC characterized by increased melanin pigmentation due to melanocytes admixed with tumor cells. Dermoscopically, the pigment can have a maple leaf–like appearance with spoke-wheel areas, in-focus dots, and concentric structures at the dermoepidermal junction, which is more characteristic of superficial and infiltrating BCC.20 In nodular BCC, the pigment occurs as blue-gray ovoid nests and globules in deeper layers of the dermis.20
Seborrheic keratoses (SKs) can vary widely in clinical appearance, with pigmentation ranging from flesh colored to yellow to brown to black. Melanoacanthomas are acanthotic SKs that are highly pigmented due to intermixed epidermal melanocytes and subepidermal melanophages.21 Dermoscopy can help distinguish cutaneous malignancies from SKs, which often demonstrate fissures and ridges, comedolike openings, and milialike cysts. Biopsy sometimes is required to assess for malignancy, as was the case in our patient. The classic histologic features of SKs include acanthosis, papillomatosis, and hyperkeratosis.22
This case highlights the need to consider apocrine hidrocystoma, along with malignancy, in the differential diagnosis of pigmented cystic masses of the face and scalp. Because apocrine hidrocystomas are benign, they do not need to be treated but often are surgically excised for cosmesis or complete histopathologic examination. Destruction via electrodessication, carbon dioxide ablation, trichloroacetic acid chemical ablation, botulinum toxin injection, and anticholinergic creams sometimes is used, especially for cosmetic treatment of multiple small lesions.5 Our patient was treated with surgical excision with no evidence of recurrence on follow-up 1 year later.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
- Ioannidis DG, Drivas EI, Papadakis CE, et al. Hidrocystoma of the external auditory canal: a case report. Cases J. 2009;2:79. doi:10.1186/1757- 1626-2-79
- Nguyen HP, Barker HS, Bloomquist L, et al. Giant pigmented apocrine hidrocystoma of the scalp. Dermatol Online J. 2020;26. doi:10.5070/D3268049895
- Mendoza-Cembranos MD, Haro R, Requena L, et al. Digital apocrine hidrocystoma: the exception confirms the rule. Am J Dermatopathol. 2019;41:79. doi:10.1097/DAD.0000000000001044
- May C, Chang O, Compton N. A giant apocrine hidrocystoma of the trunk. Dermatol Online J. 2017;23. doi:10.5070/D3239036497
- Sarabi K, Khachemoune A. Hidrocystomas—a brief review. Medscape Gen Med. 2006;8:57.
- Kruse ALD, Zwahlen R, Bredell MG, et al. Apocrine hidrocystoma of the cheek. J Craniofac Surg. 2010;21:594-596. doi:10.1097 /SCS.0b013e3181d08c77
- Zaballos P, Bañuls J, Medina C, et al. Dermoscopy of apocrine hidrocystomas: a morphological study. J Eur Acad Dermatol Venereol. 2014;28:378-381. doi:10.1111/jdv.12044
- Rodriguez HA, Ackerman LV. Cellular blue nevus. clinicopathologic study of forty-five cases. Cancer. 1968;21:393-405. doi:10.1002 /1097-0142(196803)21:3<393::aid-cncr2820210309>3.0.co;2-k
- Murali R, McCarthy SW, Scolyer RA. Blue nevi and related lesions: a review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv Anat Pathol. 2009;16:365. doi:10.1097/PAP.0b013e3181bb6b53
- Borgenvik TL, Karlsvik TM, Ray S, et al. Blue nevus-like and blue nevusassociated melanoma: a comprehensive review of the literature. ANZ J Surg. 2017;87:345-349. doi:10.1111/ans.13946
- de la Fouchardiere A. Blue naevi and the blue tumour spectrum. Pathology. 2023;55:187-195. doi:10.1016/j.pathol.2022.12.342
- Lowe L. Metastatic melanoma and rare melanoma variants: a review. Pathology (Phila). 2023;55:236-244. doi:10.1016/j.pathol.2022.11.006
- Plaza JA, Torres-Cabala C, Evans H, et al. Cutaneous metastases of malignant melanoma: a clinicopathologic study of 192 cases with emphasis on the morphologic spectrum. Am J Dermatopathol. 2010;32:129-136. doi:10.1097/DAD.0b013e3181b34a19
- Shaikh WR, Xiong M, Weinstock MA. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Archives of Dermatology. 2012;148:30-36. doi:10.1001/archdermatol.2011.264
- Clark WH, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705-727.
- Bobos M. Histopathologic classification and prognostic factors of melanoma: a 2021 update. Ital J Dermatol Venereol. 2021;156:300-321. doi:10.23736/S2784-8671.21.06958-3
- Ozao-Choy J, Nelson DW, Hiles J, et al. The prognostic importance of scalp location in primary head and neck melanoma. J Surg Oncol. 2017;116:337-343. doi:10.1002/jso.24679
- Gamsizkan M, Yilmaz I, Buyukbabani N, et al. A retrospective multicenter evaluation of cutaneous melanomas in Turkey. Asian Pac J Cancer Prev APJCP. 2014;15:10451-10456. doi:10.7314 /apjcp.2014.15.23.10451
- Mones JM, Ackerman AB. “Atypical” blue nevus, “malignant” blue nevus, and “metastasizing” blue nevus: a critique in historical perspective of three concepts flawed fatally. Am J Dermatopathol. 2004;26:407-430. doi:10.1097/00000372-200410000-00012
- Tanese K. Diagnosis and management of basal cell carcinoma Curr Treat Options Oncol. 2019;20:13. doi:10.1007/s11864 -019-0610-0
- Barthelmann S, Butsch F, Lang BM, et al. Seborrheic keratosis. JDDG J Dtsch Dermatol Ges. 2023;21:265-277. doi:10.1111/ddg.14984
- Taylor S. Advancing the understanding of seborrheic keratosis. J Drugs Dermatol. 2017;16:419-424.
A 67-year-old man presented to the dermatology clinic with 3 asymptomatic pigmented papules on the scalp. The patient reported that he was unaware of the lesions until they were pointed out weeks earlier by his primary care physician during a routine visit. He then was referred to dermatology for follow-up. Physical examination at the current presentation revealed clustered firm, smooth, well-circumscribed, pigmented papules on the scalp measuring 5 to 8 mm. The patient reported no personal or family history of skin cancer but stated that he spent a lot of time outdoors and had a history of 6 blistering sunburns in his life. A punch biopsy of each lesion was performed.
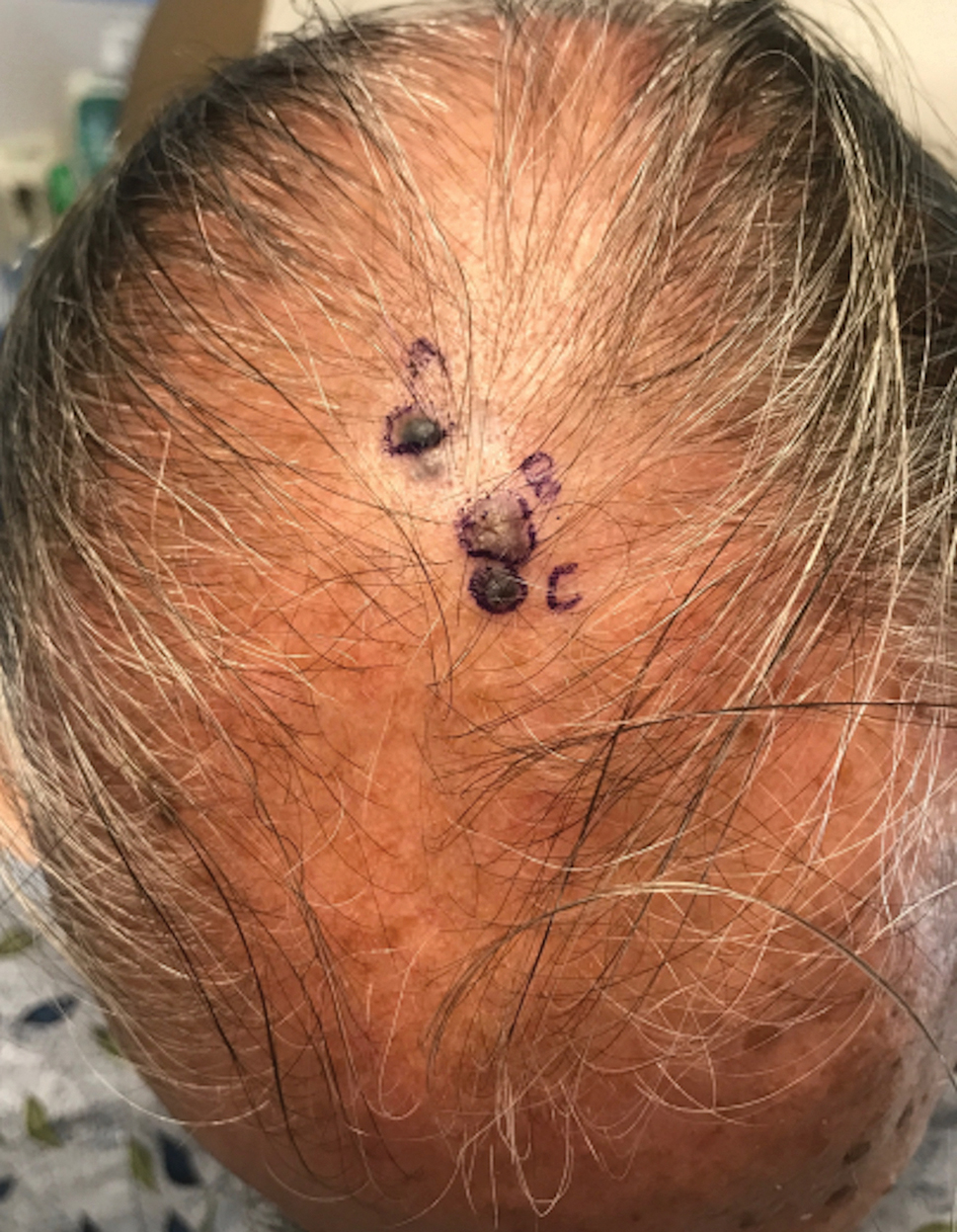
Low-Dose Oral Naltrexone for Darier Disease
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.
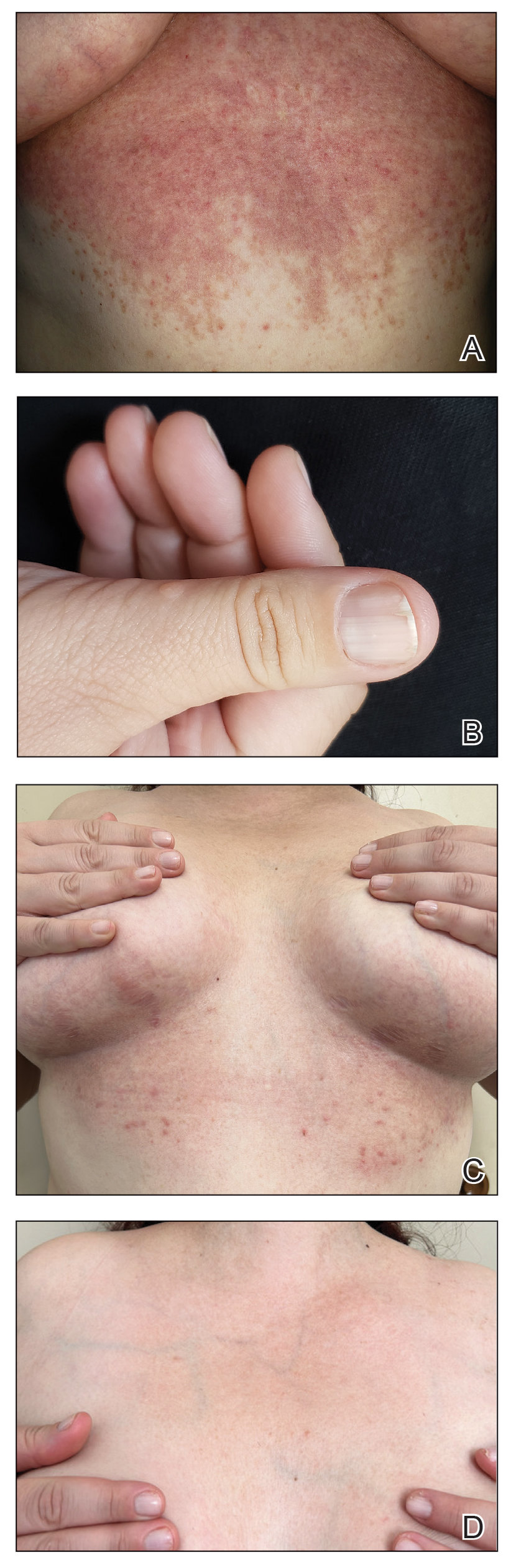
Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2
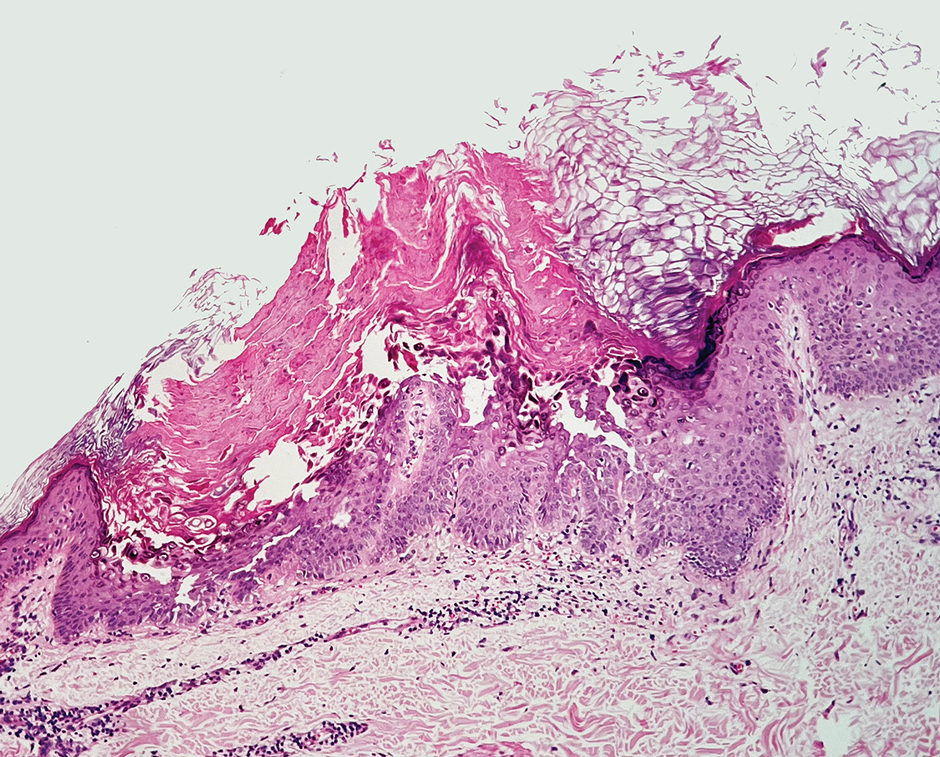
Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.

Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2

Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
To the Editor:
A 34-year-old Brazilian woman presented to the dermatology department with pruritic lesions on the neck and chest that had been present since adolescence. She reported a family history of Darier disease in her father. Physical examination revealed erythematous follicular papules on the neck, inframammary region, and abdomen (Figure 1A), as well as longitudinal bandlike leukonychia and distal nail splits on the fingernails (Figure 1B). Histopathology of a lesion on the back revealed compact hyperkeratosis and parakeratosis above an acantholytic cleft accompanied by dyskeratotic keratinocytes, including some corps ronds and grains, which supported the clinical impression of Darier disease (Figure 2). The typical clinical presentation along with the family history and histopathology confirmed the diagnosis. After therapeutic failure with topical corticosteroids and oral antibiotics for 3 months, low-dose oral naltrexone (4.5 mg/d) as monotherapy noticeably improved the lesions and pruritus within 2 months, with near-complete regression at 6 months, achieving disease stability (Figures 1C and 1D). The patient remained stable with no recurrence after 1 year of follow-up.

Darier disease is an autosomal-dominant genodermatosis caused by a mutation in the ATP2A2 gene, which encodes the sarco/endoplasmic reticulum calcium ATPase, leading to defective intracellular calcium signaling and alterations in epidermal adhesion and keratinization.1 Darier disease typically begins in adolescence and is aggravated by exposure to heat and friction. It is characterized by seborrheic distribution of painful and pruritic red-brown keratotic papules. Nail manifestations include longitudinal ridges—erythronychia and/or leukonychia—and grooves that end in a V-shaped notch. The differential diagnosis includes Hailey-Hailey disease, psoriasis, and pityriasis rubra pilaris.1,2 The diagnosis is clinical and is confirmed by histopathology, which reveals suprabasal cleavage, acantholytic dyskeratosis, corps ronds, and grains. Treatment options are limited and include corticosteroids, oral and/or topical antibiotics, and systemic retinoids.2

Oral naltrexone has been used in Darier disease based on its observed effectiveness in Hailey-Hailey disease, considering the histopathologic similarities and alterations in calcium homeostasis in both conditions. Low-dose oral naltrexone (1-5 mg/d) increases the expression of opioid receptors (δ, μ, κ), enhancing its immunomodulatory and antinociceptive effects. The δ opioid receptor regulates the expression of desmoglein, improving epidermal differentiation and wound healing.3 Activation of the δ and μ receptors increases intracellular calcium through the inositol phosphate pathway, which contributes to calcium homeostasis.4 Naltrexone blocks the nonopioid toll-like receptor 4 found in keratinocytes and macrophages, exerting an anti-inflammatory effect by reducing proinflammatory cytokines.3 Adverse events associated with low-dose naltrexone are minimal, mostly mild, and often related to sleep disorders3,5; however, patients should undergo screening for prior opioid dependence, recent opioid usage, and signs of opioid withdrawal before initiating naltrexone treatment.5
Boehmer et al6 used naltrexone (4.5 mg/d) and oral magnesium (200 mg/d) in 6 patients with inconsistent results, except for 1 case that concurrently used acitretin (25 mg/d) with satisfactory improvement. Pessoa et al7 added naltrexone (4.5 mg/d) to oral isotretinoin (0.5 mg/kg/d) in 1 patient, resulting in notable improvement of lesions within 3 months.
In our patient with Darier disease, low-dose naltrexone demonstrated a substantial response as monotherapy after 2 months of treatment and nearly complete regression of lesions within 6 months, with no reported side effects after 1 year of follow-up. The use of low-dose naltrexone could be a promising and safe treatment option as monotherapy or in combination with conventional therapy for Darier disease; however, further studies are needed.
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
Sakuntabhai A, Ruiz-Perez V, Carter S, et al. Mutations in ATP2A2, encoding a Ca2+ pump, cause Darier disease. Nat Genet. 1999;21:271-277. doi:10.1038/6784
Burge SM, Wilkinson JD. Darier-White disease: a review of the clinical features in 163 patients. J Am Acad Dermatol. 1992;27:40-50. doi:10.1016/0190-9622(92)70154-8
Lee B, Elston DM. The uses of naltrexone in dermatologic conditions. Am Acad Dermatol. 2019;80:1746-1752. doi:10.1016/j.jaad.2018.12.031
Samways DSK, Henderson G. Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance. Cell Signal. 2006;18:151-161. doi:10.1016/j.cellsig.2005.08.005
Ekelem C, Juhasz M, Khera P, et al. Utility of naltrexone treatment for chronic inflammatory dermatologic conditions: a systematic review. JAMA Dermatol. 2019;155:229-236. doi:10.1001/jamadermatol.2018.4093
Boehmer D, Eyerich K, Darsow U, et al. Variable response to low‐dose naltrexone in patients with Darier disease: a case series. J Eur Acad Dermatol Venereol. 2019;33:950-953. doi:10.1111/jdv.15457
Pessoa T, Rebelo C, Gabriela Marques Pinto, et al. Combination of naltrexone and isotretinoin for the treatment of Darier disease. Cureus. 2023;15:E33321. doi:10.7759/cureus.33321
Practice Points
- Consider low-dose naltrexone as a potential treatment option for patients with Darier disease, as it regulates opioid receptors and has shown benefits in enhancing epidermal differentiation, wound healing, and anti-inflammatory effects.
- Further research is needed to validate the efficacy and safety of low-dose naltrexone in treating Darier disease considering its observed clinical improvement in this single patient case.
Improved Pharmacogenomic Testing Process for Veterans in Outpatient Settings by Clinical Pharmacist Practitioners
Peer-review, evidence-based, detailed gene/drug clinical practice guidelines suggest that genetic variations can impact how individuals metabolize medications, which is sometimes included in medication prescribing information.1-3 Pharmacogenomic testing identifies genetic markers so medication selection and dosing can be tailored to each individual by identifying whether a specific medication is likely to be safe and effective prior to prescribing.4
Pharmacogenomics can be a valuable tool for personalizing medicine but has had suboptimal implementation since its discovery. The US Department of Veterans Affairs (VA) health care system reviewed the implementation of the Pharmacogenomic Testing for Veterans (PHASER) program. This review identified clinician barriers pre- and post-PHASER program implementation; staffing issues, competing clinical priorities, and inadequate PHASER program resources were the most frequently reported barriers to implementation of pharmacogenomic testing.5
Another evaluation of the implementation of the PHASER program that surveyed VA patients found that patients could be separated into 3 groups. Acceptors of pharmacogenomic testing emphasized potential health benefits of testing. Patients that declined testing often cited concerns for genetic information affecting insurance coverage, being misused, or being susceptible to data breach. The third group—identified as contemplators—reported the need for clinician outreach to impact their decision on whether or not to receive pharmacogenomic testing.6 These studies suggest that removing barriers by providing ample pharmacogenomics resources to clinicians, in addition to detailed training on how to offer and follow up with patients regarding pharmacogenomic testing, is crucial to successful implementation of the PHASER program.
PHASER
In 2019, the VA began working with Sanford Health to establish the PHASER program and offer pharmacogenomic testing. PHASER has since expanded to 25 VA medical centers, including the VA Central Ohio Healthcare System (VACOHCS).7,8 Pharmacogenomic testing through PHASER is conducted using a standardized laboratory panel that includes 12 different medication classes.9 The drug classes include certain anti-infective, anticoagulant, antiplatelet, cardiovascular, cholesterol, gastrointestinal, mental health, neurological, oncology, pain, transplant, and other miscellaneous medications. Medications are correlated to each class and assessed for therapeutic impacts based on gene panel results.
Clinical recommendations for medication-gene interactions can range from monitoring for increased risk of adverse effects or therapeutic failure to recommending avoiding a medication. For example, patients who test positive for the HLA-B gene have significantly increased risk of hypersensitivity to abacavir, an HIV treatment.10
Similarly, patients who cannot adequately metabolize cytochrome P450 2C19 should consider avoiding clopidogrel as they are unlikely to convert clopidogrel to its active prodrug, which reduces its effectiveness.11 Pharmacists can play a critical role educating patients about pharmacogenomic testing, especially within hematology and oncology.12 Patients can benefit from this testing even if they are not currently taking medications with known concerns as they could be prescribed in the future. The SLCO1B1 gene-drug test, for example, can identify risk for statin-associated muscle symptoms.13
Clinical pharmacist practitioners (CPPs) can increase access to genetic testing because they interact with patients in a variety of settings and can order this laboratory test.12,14 Recent research has demonstrated that most VA patients carry ≥ 1 genetic variant that may influence medication decisions and that half of veterans are prescribed a medication with known gene-drug interactions.15 CPP ordering of pharmacogenomic tests at the VACOHCS outpatient clinic was evaluated through collection of baseline data from March 8, 2023, to September 8, 2023. A goal was identified to increase orders by 50% for a patient care quality improvement initiative and use CPPs to increase access to pharmacogenomic testing. The purpose of this quality improvement initiative was to expand access to pharmacogenomic testing through process implementation and improvement within CPP-led clinic settings.
Gap Analysis
Lean Six Sigma A3 methodology was used to identify ways to increase the use of pharmacogenomic testing for veterans at VACOHCS and develop an improved process for increased ordering of pharmacogenomic testing. Lean Six Sigma A3 methodology is a stepwise approach to process improvement that helps identify gaps in efficiency, sustainable changes, and eliminate waste.16 Baseline data were collected from March 8, 2023, to September 8, 2023, to determine the frequency of CPPs ordering pharmacogenomic laboratory panels during clinic appointments. The ordering of pharmacogenomic panels was monitored by the VACOHCS PHASER coordinator.
CPPs were surveyed to identify perceived barriers to PHASER implementation. A gap analysis was conducted using Lean Six Sigma A3 methodology. Gap analyses use lean tools such as a Fishbone Diagram to illustrate and identify the gap between current state and ideal state. (Figure 1).The following barriers were identified: lack of clinician education materials, lack of a standardized patient screening process, time constraints on patient education and ordering, higher priority clinical needs, forgetting to order, lack of comfort with pharmacogenomics ordering and education, lack of support for the initiative, and increased workload and burnout. Among these perceived barriers, higher priority clinical needs, forgetting to order, and time constraints ranked highest in importance among CPPs.
In line with Lean Six Sigma A3 methodology, several tests of change were used to improve pharmacogenomic testing ordering. These changes focused on increasing patient and clinician awareness, facilitating discussion, educating clinicians, and simplifying documentation to ease time constraints. Several strategies were employed postimplementation (Figure 2). Prefilled templates simplified documentation. These templates helped identify patients without pharmacogenomic testing, provided reminders, and saved documentation time during visits. CPPs also received training and materials on PHASER ordering and documentation within encounter notes. Additionally, patient-directed advertisements were displayed in CPP examination rooms to help inspire and facilitate discussion between veterans and CPPs.
Process Improvement Data
The quality improvement project goal was to increase PHASER orders by 50% after 3 months. PHASER orders increased from 87 at baseline (March 8, 2023, to September 8, 2023) to 196 during the intervention (November 16, 2023, to February 16, 2024), a 125% increase. Changes were consistent and sustained with 65 orders the first month, 67 orders the second month, and 64 orders the third month.
Discussion
Using Lean Six Sigma A3 methodology for a quality improvement process to increase PHASER orders by CPPs revealed barriers and guided potential solutions to overcome these barriers. Interventions included additional CPP training and ordering, tools for easier identification of potential patients, documentation best practices, patient-directed advertisements to facilitate conversations. These interventions required about 8 hours for preparation, distribution, development, and interpretation of surveys, education, and documentation materials. The financial impact of these interventions was already included in allotted office materials budgeted and provided. Additional funding was not needed to provide patient-directed advertisements or education materials. The VACOHCS pharmacogenomics CPP discusses PHASER test results with patients at a separate appointment.
Future directions include educating other CPPs to assist in discussing results with veterans. Overall, the changes implemented to improve the PHASER ordering process were low effort and exemplify the ease of streamlining future initiatives, allowing for sustained optimal implementation of pharmacogenomic testing.
Conclusions
A quality improvement initiative resulted in increased PHASER orders and a clearly defined process, allowing for a continued increase and sustained support. Perceived barriers were identified, and the changes implemented were often low effort but exhibited a sustained impact. The insights gleaned from this process will shape future process development initiatives and continue to sustain pharmacogenomic testing ordering by CPPs. This process will be extended to other VACOHCS clinical departments to further support increased access to pharmacogenomic testing, reduce medication trial and error, and reduce hospitalizations from adverse effects for veterans.
Cecchin E, Stocco G. Pharmacogenomics and personalized medicine. Genes (Basel). 2020;11(6):679. doi:10.3390/genes11060679
Guidelines. CPIC. Accessed April 16, 2025. https://cpicpgx.org/guidelines/
PharmGKB. PharmGKB. 2025. Accessed April 16, 2025. https://www.pharmgkb.org
Centers for Disease Control and Prevention. Pharmacogenomics. Updated November 13, 2024. Accessed April 16, 2024. https://www.cdc.gov/genomics-and-health/pharmacogenomics/
Dong OM, Roberts MC, Wu RR, et al. Evaluation of the Veterans Affairs Pharmacogenomic Testing for Veterans (PHASER) clinical program at initial test sites. Pharmacogenomics. 2021;22(17):1121-1133. doi:10.2217/pgs-2021-0089
Melendez K, Gutierrez-Meza D, Gavin KL, et al. Patient perspectives of barriers and facilitators for the uptake of pharmacogenomic testing in Veterans Affairs’ pharmacogenomic testing for the veterans (PHASER) program. J Pers Med. 2023;13(9):1367. doi:10.3390/jpm13091367
Sanford Health Imagenetics. FREQUENTLY ASKED QUESTIONS (FAQs) about the “Pharmacogenomic Teting for Vetans” (PHASER) Program. US Department of Veterans Affairs. December 20, 2019. Accessed April 16, 2025. https://www.va.gov/opa/publications/factsheets/PHASER-FLYER-VA-Patient-FAQ.pdf
Peterson H. PHASER program testing informs how you respond to medicines. VA News. September 6, 2022. Accessed April 16, 2025. https://news.va.gov/108091/phaser-program-testing-respond-medicines/
Pharmacogenomics (PGx). Sanford Health Imagenetics. 2025. Accessed April 16, 2025. https://imagenetics.sanfordhealth.org/pharmacogenomics/
Martin MA, Hoffman JM, Freimuth RR, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing: 2014 update. Clin Pharmacol Ther. 2014;95(5):499-500. doi:10.1038/clpt.2014.38
Lee CR, Luzum JA, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112(5):959-967. doi:10.1002/cpt.2526
Dreischmeier E, Hecht H, Crocker E, et al. Integration of a clinical pharmacist practitioner-led pharmacogenomics service in a Veterans Affairs hematology/oncology clinic. Am J Health Syst Pharm. 2024;81(19):e634-e639. doi:10.1093/ajhp/zxae122
Tomcsanyi KM, Tran KA, Bates J, et al. Veterans Health Administration: implementation of pharmacogenomic clinical decision support with statin medications and the SLCO1B1 gene as an exemplar. Am J Health Syst Pharm. 2023;80(16):1082-1089. doi:10.1093/ajhp/zxad111
Gammal RS, Lee YM, Petry NJ, et al. Pharmacists leading the way to precision medicine: updates to the core pharmacist competencies in genomics. Am J Pharm Educ. 2022;86(4):8634. doi:10.5688/ajpe8634
Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345. doi:10.1001/jamanetworkopen.2019.5345
Shaffie S, Shahbazi S. The McGraw-Hill 36-Hour Course: Lean Six Sigma. McGraw-Hill; 2012.
Peer-review, evidence-based, detailed gene/drug clinical practice guidelines suggest that genetic variations can impact how individuals metabolize medications, which is sometimes included in medication prescribing information.1-3 Pharmacogenomic testing identifies genetic markers so medication selection and dosing can be tailored to each individual by identifying whether a specific medication is likely to be safe and effective prior to prescribing.4
Pharmacogenomics can be a valuable tool for personalizing medicine but has had suboptimal implementation since its discovery. The US Department of Veterans Affairs (VA) health care system reviewed the implementation of the Pharmacogenomic Testing for Veterans (PHASER) program. This review identified clinician barriers pre- and post-PHASER program implementation; staffing issues, competing clinical priorities, and inadequate PHASER program resources were the most frequently reported barriers to implementation of pharmacogenomic testing.5
Another evaluation of the implementation of the PHASER program that surveyed VA patients found that patients could be separated into 3 groups. Acceptors of pharmacogenomic testing emphasized potential health benefits of testing. Patients that declined testing often cited concerns for genetic information affecting insurance coverage, being misused, or being susceptible to data breach. The third group—identified as contemplators—reported the need for clinician outreach to impact their decision on whether or not to receive pharmacogenomic testing.6 These studies suggest that removing barriers by providing ample pharmacogenomics resources to clinicians, in addition to detailed training on how to offer and follow up with patients regarding pharmacogenomic testing, is crucial to successful implementation of the PHASER program.
PHASER
In 2019, the VA began working with Sanford Health to establish the PHASER program and offer pharmacogenomic testing. PHASER has since expanded to 25 VA medical centers, including the VA Central Ohio Healthcare System (VACOHCS).7,8 Pharmacogenomic testing through PHASER is conducted using a standardized laboratory panel that includes 12 different medication classes.9 The drug classes include certain anti-infective, anticoagulant, antiplatelet, cardiovascular, cholesterol, gastrointestinal, mental health, neurological, oncology, pain, transplant, and other miscellaneous medications. Medications are correlated to each class and assessed for therapeutic impacts based on gene panel results.
Clinical recommendations for medication-gene interactions can range from monitoring for increased risk of adverse effects or therapeutic failure to recommending avoiding a medication. For example, patients who test positive for the HLA-B gene have significantly increased risk of hypersensitivity to abacavir, an HIV treatment.10
Similarly, patients who cannot adequately metabolize cytochrome P450 2C19 should consider avoiding clopidogrel as they are unlikely to convert clopidogrel to its active prodrug, which reduces its effectiveness.11 Pharmacists can play a critical role educating patients about pharmacogenomic testing, especially within hematology and oncology.12 Patients can benefit from this testing even if they are not currently taking medications with known concerns as they could be prescribed in the future. The SLCO1B1 gene-drug test, for example, can identify risk for statin-associated muscle symptoms.13
Clinical pharmacist practitioners (CPPs) can increase access to genetic testing because they interact with patients in a variety of settings and can order this laboratory test.12,14 Recent research has demonstrated that most VA patients carry ≥ 1 genetic variant that may influence medication decisions and that half of veterans are prescribed a medication with known gene-drug interactions.15 CPP ordering of pharmacogenomic tests at the VACOHCS outpatient clinic was evaluated through collection of baseline data from March 8, 2023, to September 8, 2023. A goal was identified to increase orders by 50% for a patient care quality improvement initiative and use CPPs to increase access to pharmacogenomic testing. The purpose of this quality improvement initiative was to expand access to pharmacogenomic testing through process implementation and improvement within CPP-led clinic settings.
Gap Analysis
Lean Six Sigma A3 methodology was used to identify ways to increase the use of pharmacogenomic testing for veterans at VACOHCS and develop an improved process for increased ordering of pharmacogenomic testing. Lean Six Sigma A3 methodology is a stepwise approach to process improvement that helps identify gaps in efficiency, sustainable changes, and eliminate waste.16 Baseline data were collected from March 8, 2023, to September 8, 2023, to determine the frequency of CPPs ordering pharmacogenomic laboratory panels during clinic appointments. The ordering of pharmacogenomic panels was monitored by the VACOHCS PHASER coordinator.
CPPs were surveyed to identify perceived barriers to PHASER implementation. A gap analysis was conducted using Lean Six Sigma A3 methodology. Gap analyses use lean tools such as a Fishbone Diagram to illustrate and identify the gap between current state and ideal state. (Figure 1).The following barriers were identified: lack of clinician education materials, lack of a standardized patient screening process, time constraints on patient education and ordering, higher priority clinical needs, forgetting to order, lack of comfort with pharmacogenomics ordering and education, lack of support for the initiative, and increased workload and burnout. Among these perceived barriers, higher priority clinical needs, forgetting to order, and time constraints ranked highest in importance among CPPs.
In line with Lean Six Sigma A3 methodology, several tests of change were used to improve pharmacogenomic testing ordering. These changes focused on increasing patient and clinician awareness, facilitating discussion, educating clinicians, and simplifying documentation to ease time constraints. Several strategies were employed postimplementation (Figure 2). Prefilled templates simplified documentation. These templates helped identify patients without pharmacogenomic testing, provided reminders, and saved documentation time during visits. CPPs also received training and materials on PHASER ordering and documentation within encounter notes. Additionally, patient-directed advertisements were displayed in CPP examination rooms to help inspire and facilitate discussion between veterans and CPPs.
Process Improvement Data
The quality improvement project goal was to increase PHASER orders by 50% after 3 months. PHASER orders increased from 87 at baseline (March 8, 2023, to September 8, 2023) to 196 during the intervention (November 16, 2023, to February 16, 2024), a 125% increase. Changes were consistent and sustained with 65 orders the first month, 67 orders the second month, and 64 orders the third month.
Discussion
Using Lean Six Sigma A3 methodology for a quality improvement process to increase PHASER orders by CPPs revealed barriers and guided potential solutions to overcome these barriers. Interventions included additional CPP training and ordering, tools for easier identification of potential patients, documentation best practices, patient-directed advertisements to facilitate conversations. These interventions required about 8 hours for preparation, distribution, development, and interpretation of surveys, education, and documentation materials. The financial impact of these interventions was already included in allotted office materials budgeted and provided. Additional funding was not needed to provide patient-directed advertisements or education materials. The VACOHCS pharmacogenomics CPP discusses PHASER test results with patients at a separate appointment.
Future directions include educating other CPPs to assist in discussing results with veterans. Overall, the changes implemented to improve the PHASER ordering process were low effort and exemplify the ease of streamlining future initiatives, allowing for sustained optimal implementation of pharmacogenomic testing.
Conclusions
A quality improvement initiative resulted in increased PHASER orders and a clearly defined process, allowing for a continued increase and sustained support. Perceived barriers were identified, and the changes implemented were often low effort but exhibited a sustained impact. The insights gleaned from this process will shape future process development initiatives and continue to sustain pharmacogenomic testing ordering by CPPs. This process will be extended to other VACOHCS clinical departments to further support increased access to pharmacogenomic testing, reduce medication trial and error, and reduce hospitalizations from adverse effects for veterans.
Peer-review, evidence-based, detailed gene/drug clinical practice guidelines suggest that genetic variations can impact how individuals metabolize medications, which is sometimes included in medication prescribing information.1-3 Pharmacogenomic testing identifies genetic markers so medication selection and dosing can be tailored to each individual by identifying whether a specific medication is likely to be safe and effective prior to prescribing.4
Pharmacogenomics can be a valuable tool for personalizing medicine but has had suboptimal implementation since its discovery. The US Department of Veterans Affairs (VA) health care system reviewed the implementation of the Pharmacogenomic Testing for Veterans (PHASER) program. This review identified clinician barriers pre- and post-PHASER program implementation; staffing issues, competing clinical priorities, and inadequate PHASER program resources were the most frequently reported barriers to implementation of pharmacogenomic testing.5
Another evaluation of the implementation of the PHASER program that surveyed VA patients found that patients could be separated into 3 groups. Acceptors of pharmacogenomic testing emphasized potential health benefits of testing. Patients that declined testing often cited concerns for genetic information affecting insurance coverage, being misused, or being susceptible to data breach. The third group—identified as contemplators—reported the need for clinician outreach to impact their decision on whether or not to receive pharmacogenomic testing.6 These studies suggest that removing barriers by providing ample pharmacogenomics resources to clinicians, in addition to detailed training on how to offer and follow up with patients regarding pharmacogenomic testing, is crucial to successful implementation of the PHASER program.
PHASER
In 2019, the VA began working with Sanford Health to establish the PHASER program and offer pharmacogenomic testing. PHASER has since expanded to 25 VA medical centers, including the VA Central Ohio Healthcare System (VACOHCS).7,8 Pharmacogenomic testing through PHASER is conducted using a standardized laboratory panel that includes 12 different medication classes.9 The drug classes include certain anti-infective, anticoagulant, antiplatelet, cardiovascular, cholesterol, gastrointestinal, mental health, neurological, oncology, pain, transplant, and other miscellaneous medications. Medications are correlated to each class and assessed for therapeutic impacts based on gene panel results.
Clinical recommendations for medication-gene interactions can range from monitoring for increased risk of adverse effects or therapeutic failure to recommending avoiding a medication. For example, patients who test positive for the HLA-B gene have significantly increased risk of hypersensitivity to abacavir, an HIV treatment.10
Similarly, patients who cannot adequately metabolize cytochrome P450 2C19 should consider avoiding clopidogrel as they are unlikely to convert clopidogrel to its active prodrug, which reduces its effectiveness.11 Pharmacists can play a critical role educating patients about pharmacogenomic testing, especially within hematology and oncology.12 Patients can benefit from this testing even if they are not currently taking medications with known concerns as they could be prescribed in the future. The SLCO1B1 gene-drug test, for example, can identify risk for statin-associated muscle symptoms.13
Clinical pharmacist practitioners (CPPs) can increase access to genetic testing because they interact with patients in a variety of settings and can order this laboratory test.12,14 Recent research has demonstrated that most VA patients carry ≥ 1 genetic variant that may influence medication decisions and that half of veterans are prescribed a medication with known gene-drug interactions.15 CPP ordering of pharmacogenomic tests at the VACOHCS outpatient clinic was evaluated through collection of baseline data from March 8, 2023, to September 8, 2023. A goal was identified to increase orders by 50% for a patient care quality improvement initiative and use CPPs to increase access to pharmacogenomic testing. The purpose of this quality improvement initiative was to expand access to pharmacogenomic testing through process implementation and improvement within CPP-led clinic settings.
Gap Analysis
Lean Six Sigma A3 methodology was used to identify ways to increase the use of pharmacogenomic testing for veterans at VACOHCS and develop an improved process for increased ordering of pharmacogenomic testing. Lean Six Sigma A3 methodology is a stepwise approach to process improvement that helps identify gaps in efficiency, sustainable changes, and eliminate waste.16 Baseline data were collected from March 8, 2023, to September 8, 2023, to determine the frequency of CPPs ordering pharmacogenomic laboratory panels during clinic appointments. The ordering of pharmacogenomic panels was monitored by the VACOHCS PHASER coordinator.
CPPs were surveyed to identify perceived barriers to PHASER implementation. A gap analysis was conducted using Lean Six Sigma A3 methodology. Gap analyses use lean tools such as a Fishbone Diagram to illustrate and identify the gap between current state and ideal state. (Figure 1).The following barriers were identified: lack of clinician education materials, lack of a standardized patient screening process, time constraints on patient education and ordering, higher priority clinical needs, forgetting to order, lack of comfort with pharmacogenomics ordering and education, lack of support for the initiative, and increased workload and burnout. Among these perceived barriers, higher priority clinical needs, forgetting to order, and time constraints ranked highest in importance among CPPs.
In line with Lean Six Sigma A3 methodology, several tests of change were used to improve pharmacogenomic testing ordering. These changes focused on increasing patient and clinician awareness, facilitating discussion, educating clinicians, and simplifying documentation to ease time constraints. Several strategies were employed postimplementation (Figure 2). Prefilled templates simplified documentation. These templates helped identify patients without pharmacogenomic testing, provided reminders, and saved documentation time during visits. CPPs also received training and materials on PHASER ordering and documentation within encounter notes. Additionally, patient-directed advertisements were displayed in CPP examination rooms to help inspire and facilitate discussion between veterans and CPPs.
Process Improvement Data
The quality improvement project goal was to increase PHASER orders by 50% after 3 months. PHASER orders increased from 87 at baseline (March 8, 2023, to September 8, 2023) to 196 during the intervention (November 16, 2023, to February 16, 2024), a 125% increase. Changes were consistent and sustained with 65 orders the first month, 67 orders the second month, and 64 orders the third month.
Discussion
Using Lean Six Sigma A3 methodology for a quality improvement process to increase PHASER orders by CPPs revealed barriers and guided potential solutions to overcome these barriers. Interventions included additional CPP training and ordering, tools for easier identification of potential patients, documentation best practices, patient-directed advertisements to facilitate conversations. These interventions required about 8 hours for preparation, distribution, development, and interpretation of surveys, education, and documentation materials. The financial impact of these interventions was already included in allotted office materials budgeted and provided. Additional funding was not needed to provide patient-directed advertisements or education materials. The VACOHCS pharmacogenomics CPP discusses PHASER test results with patients at a separate appointment.
Future directions include educating other CPPs to assist in discussing results with veterans. Overall, the changes implemented to improve the PHASER ordering process were low effort and exemplify the ease of streamlining future initiatives, allowing for sustained optimal implementation of pharmacogenomic testing.
Conclusions
A quality improvement initiative resulted in increased PHASER orders and a clearly defined process, allowing for a continued increase and sustained support. Perceived barriers were identified, and the changes implemented were often low effort but exhibited a sustained impact. The insights gleaned from this process will shape future process development initiatives and continue to sustain pharmacogenomic testing ordering by CPPs. This process will be extended to other VACOHCS clinical departments to further support increased access to pharmacogenomic testing, reduce medication trial and error, and reduce hospitalizations from adverse effects for veterans.
Cecchin E, Stocco G. Pharmacogenomics and personalized medicine. Genes (Basel). 2020;11(6):679. doi:10.3390/genes11060679
Guidelines. CPIC. Accessed April 16, 2025. https://cpicpgx.org/guidelines/
PharmGKB. PharmGKB. 2025. Accessed April 16, 2025. https://www.pharmgkb.org
Centers for Disease Control and Prevention. Pharmacogenomics. Updated November 13, 2024. Accessed April 16, 2024. https://www.cdc.gov/genomics-and-health/pharmacogenomics/
Dong OM, Roberts MC, Wu RR, et al. Evaluation of the Veterans Affairs Pharmacogenomic Testing for Veterans (PHASER) clinical program at initial test sites. Pharmacogenomics. 2021;22(17):1121-1133. doi:10.2217/pgs-2021-0089
Melendez K, Gutierrez-Meza D, Gavin KL, et al. Patient perspectives of barriers and facilitators for the uptake of pharmacogenomic testing in Veterans Affairs’ pharmacogenomic testing for the veterans (PHASER) program. J Pers Med. 2023;13(9):1367. doi:10.3390/jpm13091367
Sanford Health Imagenetics. FREQUENTLY ASKED QUESTIONS (FAQs) about the “Pharmacogenomic Teting for Vetans” (PHASER) Program. US Department of Veterans Affairs. December 20, 2019. Accessed April 16, 2025. https://www.va.gov/opa/publications/factsheets/PHASER-FLYER-VA-Patient-FAQ.pdf
Peterson H. PHASER program testing informs how you respond to medicines. VA News. September 6, 2022. Accessed April 16, 2025. https://news.va.gov/108091/phaser-program-testing-respond-medicines/
Pharmacogenomics (PGx). Sanford Health Imagenetics. 2025. Accessed April 16, 2025. https://imagenetics.sanfordhealth.org/pharmacogenomics/
Martin MA, Hoffman JM, Freimuth RR, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing: 2014 update. Clin Pharmacol Ther. 2014;95(5):499-500. doi:10.1038/clpt.2014.38
Lee CR, Luzum JA, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112(5):959-967. doi:10.1002/cpt.2526
Dreischmeier E, Hecht H, Crocker E, et al. Integration of a clinical pharmacist practitioner-led pharmacogenomics service in a Veterans Affairs hematology/oncology clinic. Am J Health Syst Pharm. 2024;81(19):e634-e639. doi:10.1093/ajhp/zxae122
Tomcsanyi KM, Tran KA, Bates J, et al. Veterans Health Administration: implementation of pharmacogenomic clinical decision support with statin medications and the SLCO1B1 gene as an exemplar. Am J Health Syst Pharm. 2023;80(16):1082-1089. doi:10.1093/ajhp/zxad111
Gammal RS, Lee YM, Petry NJ, et al. Pharmacists leading the way to precision medicine: updates to the core pharmacist competencies in genomics. Am J Pharm Educ. 2022;86(4):8634. doi:10.5688/ajpe8634
Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345. doi:10.1001/jamanetworkopen.2019.5345
Shaffie S, Shahbazi S. The McGraw-Hill 36-Hour Course: Lean Six Sigma. McGraw-Hill; 2012.
Cecchin E, Stocco G. Pharmacogenomics and personalized medicine. Genes (Basel). 2020;11(6):679. doi:10.3390/genes11060679
Guidelines. CPIC. Accessed April 16, 2025. https://cpicpgx.org/guidelines/
PharmGKB. PharmGKB. 2025. Accessed April 16, 2025. https://www.pharmgkb.org
Centers for Disease Control and Prevention. Pharmacogenomics. Updated November 13, 2024. Accessed April 16, 2024. https://www.cdc.gov/genomics-and-health/pharmacogenomics/
Dong OM, Roberts MC, Wu RR, et al. Evaluation of the Veterans Affairs Pharmacogenomic Testing for Veterans (PHASER) clinical program at initial test sites. Pharmacogenomics. 2021;22(17):1121-1133. doi:10.2217/pgs-2021-0089
Melendez K, Gutierrez-Meza D, Gavin KL, et al. Patient perspectives of barriers and facilitators for the uptake of pharmacogenomic testing in Veterans Affairs’ pharmacogenomic testing for the veterans (PHASER) program. J Pers Med. 2023;13(9):1367. doi:10.3390/jpm13091367
Sanford Health Imagenetics. FREQUENTLY ASKED QUESTIONS (FAQs) about the “Pharmacogenomic Teting for Vetans” (PHASER) Program. US Department of Veterans Affairs. December 20, 2019. Accessed April 16, 2025. https://www.va.gov/opa/publications/factsheets/PHASER-FLYER-VA-Patient-FAQ.pdf
Peterson H. PHASER program testing informs how you respond to medicines. VA News. September 6, 2022. Accessed April 16, 2025. https://news.va.gov/108091/phaser-program-testing-respond-medicines/
Pharmacogenomics (PGx). Sanford Health Imagenetics. 2025. Accessed April 16, 2025. https://imagenetics.sanfordhealth.org/pharmacogenomics/
Martin MA, Hoffman JM, Freimuth RR, et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing: 2014 update. Clin Pharmacol Ther. 2014;95(5):499-500. doi:10.1038/clpt.2014.38
Lee CR, Luzum JA, Sangkuhl K, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112(5):959-967. doi:10.1002/cpt.2526
Dreischmeier E, Hecht H, Crocker E, et al. Integration of a clinical pharmacist practitioner-led pharmacogenomics service in a Veterans Affairs hematology/oncology clinic. Am J Health Syst Pharm. 2024;81(19):e634-e639. doi:10.1093/ajhp/zxae122
Tomcsanyi KM, Tran KA, Bates J, et al. Veterans Health Administration: implementation of pharmacogenomic clinical decision support with statin medications and the SLCO1B1 gene as an exemplar. Am J Health Syst Pharm. 2023;80(16):1082-1089. doi:10.1093/ajhp/zxad111
Gammal RS, Lee YM, Petry NJ, et al. Pharmacists leading the way to precision medicine: updates to the core pharmacist competencies in genomics. Am J Pharm Educ. 2022;86(4):8634. doi:10.5688/ajpe8634
Chanfreau-Coffinier C, Hull LE, Lynch JA, et al. Projected prevalence of actionable pharmacogenetic variants and level A drugs prescribed among US Veterans Health Administration pharmacy users. JAMA Netw Open. 2019;2(6):e195345. doi:10.1001/jamanetworkopen.2019.5345
Shaffie S, Shahbazi S. The McGraw-Hill 36-Hour Course: Lean Six Sigma. McGraw-Hill; 2012.
Safety and Efficacy of Ezetimibe in Patients With and Without Chronic Kidney Disease at a Pharmacist-Managed Clinic
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Statins are widely used to reduce low-density lipoprotein (LDL) and non-high-density lipoprotein (HDL) levels for the prevention of atherosclerotic cardiovascular disease (ASCVD).1 However, despite maximally tolerated statin therapy, many patients may not reach their LDL and non-HDL goals. Some patients may experience adverse events (AEs), particularly muscle-related AEs, which can limit the use of these medications.
The 2022 American College of Cardiology (ACC) expert consensus pathway recommends a goal LDL of < 55 mg/dL in very high-risk patients, defined as those with a history of multiple major ASCVD events or 1 major ASCVD event and multiple high-risk conditions.2 Major ASCVD events include acute coronary syndrome within 12 months, history of myocardial infarction (MI) or ischemic stroke, and symptomatic peripheral arterial disease (ie, claudication with ankle-brachial index < 0.85 or previous revascularization or amputation). Factors for being considered high risk include age > 65 years, heterozygous familial hypercholesterolemia, history of prior coronary artery bypass surgery or percutaneous coronary intervention outside the major ASCVD events, diabetes, hypertension, chronic kidney disease (CKD) (estimated glomerular filtration rate [eGFR] 15-59 mL/min/1.73 m2), current smoking, persistently elevated LDL cholesterol (LDL-C) levels despite maximally tolerated statin therapy and ezetimibe, and history of congestive heart failure.2 For these patients, statin therapy alone may not achieve LDL goal.
The ACC recommends ezetimibe as the initial nonstatin therapy in patients who are not at their goal LDL.2 Ezetimibe works by inhibiting Niemann-Pick C1-Like 1 protein, which causes reduced cholesterol absorption in the small intestine.2,3 Previous studies have shown the benefit of ezetimibe for LDL reduction and ASCVD prevention.4-7 The 2015 IMPROVE-IT study found the combination of simvastatin and ezetimibe resulted in a significantly lower risk of cardiovascular events than simvastatin monotherapy. IMPROVE-IT also reported a further clinical benefit when lower LDL targets (ie, < 55 mg/dL) are achieved, which aligns with the expert consensus pathway recommendations for a lower LDL goal for very high-risk patients.2,5
The RACING trial found that treatment with a moderate-intensity statin and ezetimibe was noninferior to treatment with a high-intensity statin for the primary outcome of occurrence of cardiovascular death, major cardiovascular events, or nonfatal stroke within 3 years. The combination of moderate-intensity statin and ezetimibe achieved lower LDL-C levels and lower incidence of drug intolerance compared to high intensity statin monotherapy.6 The SHARP-CKD study assessed major atherosclerotic events in patients with CKD who had no history of MI or coronary revascularization. The study found that lowering LDL-C with the combination of simvastatin plus ezetimibe safely reduces the risk of major atherosclerotic events in a wide range of patients with CKD.7
Lastly, the 2019 EWTOPIA 75 study found that ezetimibe noted a statistically significant reduction in the incidence of the composite of sudden cardiac death, MI, coronary revascularization, or stroke compared to placebo. Ezetimibe showed benefits in preventing ASCVD events independently of statin therapy.8 These clinical trials provided evidence for the efficacy of ezetimibe for secondary or primary prevention of ASCVD, patients with CKD, and patients who are not at their LDL goal despite maximally tolerated statin therapy.
Reductions in LDL levels with ezetimibe are reported to be 15% to 19% for monotherapy and 13% to 25% when used in combination with a statin.4 Given that the ACC now recommends lower LDL goals, patients may need additional lowering despite taking maximally tolerated statin therapy.2 Additionally, the package insert for ezetimibe reports increased area under the curve (AUC) values of ezetimibe and its metabolites in patients with severe renal disease. It is anticipated that ezetimibe may show an increased reduction of LDL and non-HDL, but there may also be an increased risk for muscle-related AEs.3
This quality-assurance quality improvement project investigated the use of ezetimibe in patients with CKD to determine whether there is further LDL and non-HDL reduction in this patient population. It sought to determine the LDL and non-HDL percentage reduction in patients with and without CKD at the Wilkes-Barre Veterans Affairs Medical Center (WBVAMC) and whether there is an increased risk for muscle-related AEs. Determining the percentage reduction of LDL and non-HDL within this population can help increase use of ezetimibe in patients not at their LDL or non-HDL goal or for those patients unable to tolerate statin therapy.
Methods
This single-center retrospective chart review investigated patients prescribed ezetimibe by a patient aligned care team (PACT) pharmacist at WBVAMC between September 1, 2021, and September 1, 2023. This project was determined to be nonresearch by the Veterans Integrated Service Network 4 multisite institutional review board. Patients were excluded from the review if they started taking ezetimibe outside of the prespecified time frame, if ezetimibe was initiated by a non-WBVAMC PACT pharmacist, or if there was no follow-up lipid panel obtained within 6 months of initiation of ezetimibe.
The primary outcomes were to determine the percentage mean change in LDL and non-HDL reduction and the incidence of muscle-related AEs after initiation of ezetimibe in patients without CKD. The secondary outcomes were to determine the percentage mean change in LDL and non-HDL levels and the incidence of muscle-related AEs after initiation of ezetimibe in patients with CKD. For this study, CKD was defined as a patient having an eGFR 15 to 60 ml/min/1.73 m2. Non-HDL is the combination of LDL-C and very LDL-C and represents all potentially atherogenic particles. The 2022 Expert Consensus Pathway included non-HDL goals in addition to LDL goals.2 Non-HDL cholesterol levels can be used for patients with elevated triglycerides where LDL levels may not be as accurate. To account for instances of elevated triglycerides, this study assessed changes in both LDL and non-HDL levels.
Data were collected from the US Department of Veterans Affairs (VA) Computerized Patient Record System (CPRS) and recorded in a spreadsheet. Collected data included age, sex, race, concomitant cholesterol-lowering medications (statin, proprotein convertase subtilisin/kexin type 9 [PCSK9] inhibitor, bempedoic acid, fish oil, niacin, bile acid sequestrants, and fibrates), baseline lipid panel, lipid panel within 6 months of ezetimibe initiation, and eGFR level. If the patient’s LDL or non-HDL levels worsened on the follow-up lipid panel, their baseline LDL and non-HDL levels were used to calculate the percentage reduction; thus, the percentage reduction would be 0%. This strategy was used in prior research, notably the IMPROVE-IT and SHARP-CKD trials.
Ezetimibe 5 mg once daily was used in this study based on a 2008 VA study that evaluated the use of ezetimibe 5 mg vs ezetimibe 10 mg and the percentage reduction of LDL with each dose. The study found no significant difference between the 5 mg and 10 mg dose.9 Most patients included in this study received the 5 mg dose.
Results
This retrospective chart review consisted of 173 patients, 137 (79.2%) without CKD and 36 (20.8%) with CKD at baseline. The mean age was 69.6 years, 155 (89.6%) patients were male, and 18 (10.4%) were female. There were 164 concomitant medications, including 115 patients prescribed a statin and 38 patients prescribed fish oil (Table 1).
Patients without CKD had mean reductions in LDL levels of 23.5% and non-HDL levels of 21.7% (Figure). Patients who had an increase in LDL and non-HDL levels were excluded to control for potential confounding factors such as dietary changes, discontinuation of ezetimibe therapy, nonadherence to ezetimibe, and medication changes that impacted follow-up laboratory tests such as discontinuation of a statin. Fifteen patients experienced an increase in LDL or non-HDL levels. After excluding these patients, those without CKD had a mean reduction in LDL levels of 28.0% and non-HDL levels of 25.5%. Nineteen (13.9%) patients without CKD experienced a muscle-related AE (Table 2). One patient discontinued ezetimibe and statin use following a Lyme disease diagnosis due to concerns over potential muscle-related AEs.
Patients with CKD had a mean reduction in LDL and non-HDL levels of 27.0% and 24.8%, respectively. Patients with an increase in LDL or non-HDL levels were also excluded to help control for potential confounding factors. After excluding 4 patients with increased LDL and non-HDL levels, the mean reduction in LDL and non-HDL levels was 30.5% and 27.5%, respectively. Five (13.9%) patients with CKD experienced muscle-related AEs thought to be due to ezetimibe. Other AEs (eg, urticaria, diarrhea, reflux, dizziness, headache, upset stomach) were reported that led to discontinuation of ezetimibe, but only muscle-related AEs were analyzed.
Discussion
This retrospective chart review found larger reductions in LDL and non-HDL levels for patients with CKD than reported in the literature.4 Based on the findings that indicate a greater cholesterol reduction with ezetimibe, the results suggest an underutilization of ezetimibe in clinical practice, which may be due to clinicians favoring statin therapy and overlooking ezetimibe as a viable option based on recommendation in earlier guidelines. The 2022 guidelines transitioned from a statin focus to a focus on LDL targets and goals.2
According to the ACC, there is evidence to support a direct relationship between LDL-C levels, atherosclerosis progression, and ASCVD event risk.2 Absolute LDL-C level reduction is directly associated with ASCVD risk reduction which supports the LDL hypothesis. There appears to be no specific LDL-C level below which benefit ceases.2 This suggests that lower LDL-C targets (< 55 mg/dL) should be used when clinically indicated. Many patients are either unable to reach their goal LDL levels with statin monotherapy or are unable to tolerate statin therapy at higher doses, which may require additional pharmacotherapy to reach goal LDL-C. The ACC expert consensus pathway recommends ezetimibe as the initial add-on treatment to statins.2 The RACING trial showed the benefit of adding ezetimibe to a moderate-intensity statin vs increasing to a high-intensity statin dose. This trial found patients had lower LDL levels and lower rates of intolerances, which further supports ezetimibe use.6
This quality improvement project assessed LDL and non-HDL level reduction in patients with CKD. As anticipated, there was greater reduction in LDL and non-HDL levels seen in patients with CKD. The SHARP-CKD trial also found reductions in LDL levels with ezetimibe in patients with CKD.7 Given the reduction in LDL and non-HDL levels with ezetimibe in patients with or without CKD, add-on therapy of ezetimibe should be recommended for patients who do not achieve their LDL goals with statin therapy or for patients who intolerant to statin therapy.
The ezetimibe package insert reports myalgias incidence to be < 5% in patients and research has shown up to a 20% incidence of muscle-related AEs with statin therapy.3,10 Based on the package information reporting increased AUC values of ezetimibe and its metabolites in patients with severe renal disease, it was anticipated there may be an increased risk of muscle-related AEs in patients with CKD.3 However, this study found the same incidence of muscle-related AEs in patients with and without CKD. Previous research on statin-intolerant patients found the incidence of muscle-related AEs with ezetimibe to be 23.0% and 28.8%.11,12 This increased incidence of muscle-related AEs may be the result of including patients with a history of statin intolerance. Collectively, data from clinical trials and this study indicate that patients with prior intolerances to statins appear to have a higher likelihood of developing a muscle-related AEs with ezetimibe.11,12 Clinicians and patients should be educated on the potential for these AEs and be aware that the likelihood may be greater if there is a history of statin intolerance. To our knowledge, this was the first study to evaluate muscle-related AEs with ezetimibe in patients with and without CKD.
Limitations
This retrospective chart review was performed over a prespecified period and only patients initiated on ezetimibe by a PACT pharmacist were included. This study did not assess the percentage of LDL reduction in patients on concomitant statins vs those who were not on concomitant statins. The study only included 173 patients. Additionally, the study was primarily composed of White men and may not be representative of other populations. In addition, veterans may not be representative of the general population given their high comorbidity burden and other exposures. Some reported muscle-related AEs associated with ezetimibe may be attributed to the nocebo effect.
Conclusions
The results of this retrospective chart review suggest there may be a larger mean reduction in LDL and non-HDL levels seen with ezetimibe therapy than reported within the literature. There was a larger mean reduction in LDL and non-HDL levels in patients with CKD than in patients without CKD. Additionally, there were the same rates of muscle-related AEs with ezetimibe therapy in patients with and without CKD. The rates of muscle-related AEs with ezetimibe therapy were higher than reported in the medication’s package insert, but lower than reported in literature that included statin-intolerant patients. These results indicate there may be a benefit to an increase in use of ezetimibe in clinical practice due to its increased effectiveness and safety in patients with and without CKD. Ultimately, this can help patients achieve their LDL goals as recommended by ACC clinical practice guidelines.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24) e285-e350. doi:10.1016/j.jacc.2018.11.003
Writing Committee, Lloyd-Jones DM, Morris PB, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366-1418. doi:10.1016/j.jacc.2022.07.006
US Food and Drug Administration. Ezetimibe. 2007. Accessed April 1, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021445s019lbl.pdf
Singh A, Cho LS. Nonstatin therapy to reduce low-density lipoprotein cholesterol and improve cardiovascular outcomes. Cleve Clin J Med. 2024;91(1):53-63. doi:10.3949/ccjm.91a.23058
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372(25):2387-2397. doi:10.1056/NEJMoa1410489
Kim B, Hong S, Lee Y, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400(10349):380-390. doi:10.1016/S0140-6736(22)00916-3
Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181-2192. doi:10.1016/S0140-6736(11)60739-3
Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992-1003. doi:10.1161/CIRCULATIONAHA.118.039415
Baruch L, Gupta B, Lieberman-Blum SS, Agarwal S, Eng C. Ezetimibe 5 and 10 mg for lowering LDL-C: potential billion-dollar savings with improved tolerability. Am J Manag Care. 2008;14(10):637-641. https://www.ajmc.com/view/oct08-3644p637-641
Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36(17):1012-1022. doi:10.1093/eurheartj/ehv043
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541-2548. doi:10.1016/j.jacc.2014.03.019
Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315(15):1580-1590. doi:10.1001/jama.2016.3608
Multiagent AI Systems in Health Care: Envisioning Next-Generation Intelligence
Artificial intelligence (AI) is rapidly evolving, with large language models (LLMs) marking a significant milestone in processing and generating human-like responses to natural language prompts. However, this advancement only signals the beginning of a more profound transformation in AI capabilities. The development of AI agents represents a new paradigm at the forefront of this evolution.
BACKGROUND
AI agents represent a leap forward from traditional LLM applications. While definitions may vary slightly among technology developers, the core concept remains: these agents are autonomous software entities designed to interact with their environment, make independent decisions, and execute tasks based on predefined goals.1-3 What sets AI agents apart is their combination of sophisticated components within structured architectures. At their core, AI agents incorporate an LLM for response generation, which is augmented by a suite of tools to optimize workflow and complete tasks, memory capabilities for personalized interactions, and autonomous reasoning. This combination allows AI agents to plan, create subtasks, gather information, and learn iteratively from their own experiences or other AI agents.
The true potential of this technology becomes apparent when multiple AI agents collaborate within multiagent AI systems. This concept introduces a new level of flexibility and capability in tackling complex tasks. Autogen, CrewAI, and LangChain offer various agent network configurations, including hierarchical, sequential, conditional, or even parallel task execution.4-6 This adaptability opens up a world of possibilities across various industries, but perhaps nowhere is the potential impact more exciting and profound than in health care.
AI agents in health care present an opportunity to revolutionize patient care, streamline administrative processes, and support complex clinical decision-making. This review examines 3 scenarios that illustrate the impact of AI agents in health care: a hypothetical sepsis management system, chronic disease management, and hospital patient flow optimization. This article will provide a detailed look at the technical implementation challenges, including the integration with existing health care IT systems, data privacy considerations, and the crucial role of explainable AI in maintaining trust and transparency.
It is challenging to implement AI agents in health care. Concerns include ensuring data quality and mitigating bias, seamlessly integrating these systems into existing clinical workflows, and navigating the complex ethical considerations that arise when deploying autonomous systems in health care. The integration with Internet of Things (IoT) devices for real-time patient data monitoring and the development of more sophisticated natural language interfaces to enhance future human-AI collaboration.
The adoption of AI agents in health care is only beginning, and it promises to be transformative. As AI continues to evolve, a comprehensive understanding of its applications, limitations, and ethical considerations is essential. This report provides a comprehensive overview of the current state, potential applications, and future directions of AI agents in health care, offering insights valuable to researchers, clinicians, and policymakers.
MultiAgent AI architecture
Sepsis Management
Despite advancements in broad-spectrum antibiotics, imaging, and life support systems, mortality rates associated with sepsis remain high. The complexity of optimizing care in clinical settings has hindered progress in managing sepsis. Previous attempts to develop predictive sepsis models have proven challenging.7 This report proposes a multiagent AI system designed to enhance comprehensive patient monitoring and care through coordinated AI-driven interventions.
Data Collection and Integration Agent. Powered by a controlled vocabulary to specify all data, the primary function for the data collection and integration agent is to clean, transform, and organize patient data from structured and unstructured sources. This agent prepares succinct summaries of consultant notes and formats data for human and machine consumption. All numerical data are presented graphically, including relevant historical data trends. The agent also digitally captures all orders in a structured format using a specified controlled vocabulary. This structured data feed supports the output of other agents, including documentation, treatment planning, and risk stratification, while also supplying the data structures for future training.
Diagnostic Agent. Critical illness is characterized by multiple abnormalities across a wide array of tests, ranging from plain chest X-ray, computed tomography (CT), blood cell composition, plasma chemistry, and microscopic evaluation of specimens. Additionally, life support parameters provide insights into disease severity and can inform management recommendations. These data offer a wide array of visual and numerical data to be used as input for computation, recommendation, and further training. For example, to evaluate fluid overload on chest X-rays or tissue histopathology slides, an AI agent can leverage deep learning models such as convolutional neural networks and vision transformers to analyze images like radiographs and histopathology slides.8,9 Recurrent neural networks or transformer models process sequential data like time-series vital signs. The agent also implements ensemble methods that combine multiple machine learning algorithms to enhance diagnostic accuracy.
Risk Stratification Agent. This assesses severity and predicts potential outcomes. Morbidity and mortality risks are calculated using an established scoring system and individualized based on the history of other agents’ conditional patients. These are presented graphically, with major risk factors highlighted for explainability.
Treatment Recommendation Agent. Using a reinforcement learning framework supplemented by up-to-date clinical guidelines, this system leverages historical data structured with standardized vocabulary to analyze patients with similar clinical features. Training is also conducted on the patient’s physiological data. All recommendations are presented via a dedicated user interface in a readable format, along with recommendations for editable, orderable items, references, and full-text snippets from previous research. Stop rules end computing if confidence in recommendations is too broad or no clear pathway can be computed with certainty, prompting human mitigation.
Resource Management Agent. This agent coordinates hospital resources using constraint programming techniques for optimal resource allocation, uses queueing theory models to predict and manage patient flow, and implements genetic algorithms for complex scheduling problems.10,11
Monitoring and Alert Agent. By tracking patients’ progress and alerting staff to changes, this agent uses anomaly detection algorithms to identify unusual patterns in patient data and implement time-series forecasting models, such as autoregressive integrated moving average and prophet, to predict future patient states. The agent also uses stream-processing techniques for real-time data analysis.12,13
Documentation and Reporting Agent. This agent maintains comprehensive medical records and generates reports. It employs advanced natural language processing techniques for automated report generation, uses advanced LLMs fine-tuned on medical corpora for narrative creation, and implements information-retrieval techniques to efficiently query patient records.
CLINICAL CASE STUDIES
To illustrate the functionality of a multiagent system, this report examines its application for managing sepsis. The data collection and integration agent continuously aggregates patient data from various sources, normalizing and timestamping it for consistent processing. The diagnostic agent analyzes this integrated data in real time, applying sepsis criteria and utilizing a deep learning model trained on a large sepsis dataset to detect subtle patterns.
The risk stratification agent calculates severity scores, such as the Sepsis-related Organ Failure Assessment (SOFA), quick SOFA (qSOFA), and Acute Physiology and Chronic Health Evaluation II, upon detecting a possible sepsis case.14 It predicts the likelihood of specific outcomes and estimates the potential trajectory of the patient’s condition for the next 24 to 48 hours. Based on this assessment, the treatment recommendation agent suggests an initial treatment plan, including appropriate antibiotics, fluid resuscitation protocols, and vasopressor recommendations, recommendations when indicated.
Concurrently, the resource management agent checks the availability of necessary resources and prioritizes allocation based on the severity. The monitoring agent tracks the patient’s response to interventions in real time, alerting the care team to any concerning changes or lack of expected improvement. Throughout this process, the documentation agent ensures that all actions, responses, and outcomes are meticulously recorded in a structured format and generates real-time updates for the patient’s electronic health record (EHR) and preparing summary reports for handoffs between care teams.
Administrative Workflow Support
Modern health care operations are resource-intensive, requiring coordination of advanced imaging, procedures, laboratory testing, and professional consultations.15 AI-powered health care administrative workflow systems are revolutionizing how medical facilities coordinate patient care. For patients with chronic cough, these systems seamlessly integrate scheduling, imaging, diagnostics, and follow-up care into a cohesive process that reduces administrative burden while improving patient outcomes. Through an intuitive interface and automated assistance, health care practitioners (HCPs) can track patient progress from initial consultation through diagnosis and treatment.
The process begins when an HCP enters a patient into the system, which triggers an automated CT scan scheduling system. The system considers factors like urgency, facility availability, and patient preferences to suggest optimal appointment times. Once imaging is complete, AI agents analyze the radiology reports, extract key findings, and generate structured summaries that highlight critical information such as “mild bronchial wall thickening with patchy ground-glass opacities” or “findings consistent with chronic bronchitis.”
Based on these findings, the system automatically generates evidence-based recommendations for follow-up care, such as pulmonology consultations or follow-up imaging in 3 months. These recommendations are presented to the ordering clinician, along with suggested appointment slots for specialist consultations. The system then manages the coordination of multiple appointments, ensuring each step in the patient’s care plan is properly sequenced and scheduled.
The entire process is monitored through a comprehensive dashboard that provides real-time updates on patient status, appointment schedules, and clinical recommendations. HCPs can track which patients require immediate attention, view upcoming appointments, and monitor the progress of ongoing care plans.
Multiagent AI Operation Optimization
Hospitals are complex entities that must function at different scales and respond in an agile, timely manner at all hours, deploying staff at various positions.16 A system of AI agents can receive signals from sensors monitoring foot traffic in the emergency department and trauma unit, as well as the availability of operating room staff, equipment, and intensive care unit beds. Smart sensors enable this monitoring through IoT networks. These networks benefit from advances in adaptive and consensus networking algorithms, along with recent advances in bioengineering and biocomputing.17
For example, in the case of imaging for suspected abdominal obstruction, an AI agent tasked with scheduling CTs could time the patient’s arrival based on acuity. Another AI agent could alert staff transporting the patient to the CT appointment, with the next location contingent on a clinical decision to proceed to the operating room. Yet another AI agent could summarize radiology interpretations and alert the surgery and anesthesia teams to a potential case, while others could notify operating room staff of equipment needs or reserve a bed. In this paradigm, AI agents facilitate more precise and timely communication between multiple staff members.
TECHNICAL IMPLEMENTATION
Large Language Models
Each agent uses a different LLM optimized for its specific task. For example, the diagnostic agent uses an LLM pretrained on a large corpus of biomedical literature and fine-tuned on a dataset of confirmed sepsis cases and their presentations.18 It implements few-shot learning techniques to adapt to rare or atypical presentations. The treatment recommendation agent also uses an LLM, employing a retrieval-augmented generation approach to access the latest clinical guidelines during inference. The documentation agent uses another advanced language model, fine-tuned on a large corpus of high-quality medical documentation, implementing controlled text generation techniques and utilizing a separate smaller model for real-time error checking and correction.
Interagent Quality Control
Agents learn from their own experience and the experience of other agents. They are equipped with user-defined rule-based and model-based systems for quality assurance, with clear stopping rules for human involvement and mitigation.
Sophisticated quality control measures bolster the system’s reliability, including ensemble techniques for result comparison, redundancy for critical tasks, and automatic human review for disagreements above a certain threshold. Each agent provides a calibrated confidence score with its output, used to weigh inputs in downstream tasks and trigger additional checks for low-confidence outputs.
A dedicated quality control agent monitors output from all agents, employing both supervised and unsupervised anomaly detection techniques. Feedback loops allow agents to evaluate the quality and utility of information received from other agents. The system implements a multiarmed bandit approach to dynamically adjust the influence of different agents based on their performance and periodically retrains agent models using federated learning techniques.19
Electronic Health Record Integration
Seamless EHR integration is crucial for practical implementation. The system has secure application programming interface access to various EHR platforms, implements OAuth 2.0 for authentication, and use HTTPS with perfect forward secrecy for all communications.20 It works with HL7 FHIR to ensure interoperability and uses SNOMED CT for clinical terminology to ensure semantic interoperability across different EHRs.21,22
The system implements a multilevel approval system for write-backs to EHRs, with different thresholds based on the information’s criticality. It uses digital signatures to ensure the integrity and nonrepudiation of AI-generated entries and implements blockchain technology to create an immutable and distributed ledger of all AI system actions.23
Decision Transparency
To ensure transparency in decision-making processes, the system applies techniques (eg, local interpretable model-agnostic explanations and Shapley additive explanations) to provide insights into agent decision-making processes.24-26 It provides customized visualizations for different stakeholders and allows users to explore alternative decision paths through what-if scenario modeling.27
The system provides calibrated confidence indicators for each recommendation or decision, implementing a novel confidence calibration agent that continuously monitors and adjusts confidence scores based on observed outcomes.
Continuous Learning and Adaptation
The system employs several techniques to remain current with evolving medical knowledge. Federated learning includes information from diverse datasets across multiple institutions without compromising patient privacy.28 A/B testing is used to safely deploy and compare new agent versions in controlled settings, implementing multiarmed bandit algorithms to efficiently explore new models while minimizing potential negative impacts. Human-in-the-loop learning and active learning techniques are used to incorporate feedback from HCPs and efficiently solicit expert input on the most informative data.29
CLINICAL IMPLICATIONS
The implementation of multiagent AI systems in health care has several potential benefits: enhanced diagnostic accuracy, personalized treatment, improved efficiency, continuous monitoring, and resource optimization. A recent review of AI sepsis predictive models exhibited superior results to standard clinical scoring methods like qSOFA.30 In oncology, such systems can result in more tailored treatments, enhancing outcomes.31 The implementation of an ambient dictation system can improve workflow and prevent HCP burnout.32
ETHICAL CONSIDERATIONS AND AI OVERSIGHT
Integrating AI agents into health care raises significant ethical considerations that must be carefully addressed to ensure equitable and effective care delivery. One primary concern involves cultural and linguistic competency, as AI systems may struggle with cultural nuances, idioms, and context-specific communication patterns. This becomes particularly challenging in regions with diverse ethnic populations or immigrant communities, where medical terminology may not have direct translations and cultural beliefs significantly influence health care decisions. AI systems also may inherit and amplify existing biases in health care delivery, whether through HCP bias reflected in training data, patient bias affecting acceptance of AI-assisted care, or demographic underrepresentation during system development.
AI agents present unique opportunities for improving health care access and outcomes through community engagement, though such initiatives require thoughtful implementation. Predictive analytics can identify high-risk individuals within communities who may benefit from preventive care, while analysis of social determinants of health can enable more targeted interventions. However, these capabilities must be balanced with privacy concerns and the risk of surveillance, particularly in communities that distrust health care institutions. The potential for AI to bridge health care gaps must be weighed against the need to maintain cultural sensitivity and community trust.
The governance and oversight of health care AI systems requires a multistakeholder approach with clear lines of responsibility and accountability. This includes involvement from government health care agencies, professional medical associations, ethics boards, and independent auditors, all working together to establish and enforce standards while monitoring system performance and addressing potential biases. Health care organizations must maintain transparent policies about AI use, implement regular monitoring and evaluation protocols, and establish precise mechanisms for patient feedback and grievance resolution. Ongoing assessment and adjustment of these systems, informed by community feedback and outcomes data, will be crucial for their ethical implementation, ensuring that AI agents complement, rather than replace, human judgment and cultural sensitivity.
FUTURE DIRECTIONS
Despite the potential benefits, implementing multiagent AI systems in health care faces significant challenges that require careful consideration. Beyond the fundamental issues such as data quality and bias mitigation, health care organizations struggle with fragmented systems, inconsistent data formats, and varying quality. Technical infrastructure requirements are substantial, particularly in rural or underserved areas that lack robust networks and cybersecurity. HCPs already face significant cognitive load and time pressures, making integrating AI agents into existing workflows particularly challenging. There is also the critical issue of transparency and interpretability, as health care decisions require clear reasoning and accountability that many black-box AI systems struggle to provide.
The legal landscape introduces another layer of complexity, particularly regarding liability, consent, and privacy questions. When AI agents contribute to medical decisions, establishing clear lines of responsibility becomes crucial. There are also serious concerns about algorithmic fairness and the potential for AI systems to perpetuate or amplify existing inequities. The cost of implementation remains a significant barrier, requiring substantial investment in technology, training, and ongoing maintenance while ensuring resources are not diverted from direct patient care. Moreover, HCPs may resist adoption due to concerns about job security, loss of autonomy, or skepticism about AI capabilities while paradoxically facing risks of overreliance on AI systems that could lead to the degradation of human clinical skills.
Addressing these challenges requires a multifaceted approach that combines technical solutions with organizational and policy changes. Health care organizations must implement rigorous data validation processes and interoperability standards while developing hybrid models that balance sophisticated AI capabilities with interpretable techniques. Extensive research and iterative design processes, with direct input from HCPs, are essential for successful integration. Establishing independent ethics boards to oversee system development and deployment, conducting multicenter randomized controlled trials, and creating clear regulatory frameworks will ensure safe and effective implementation. Success will ultimately depend on ongoing collaboration between technology developers, HCPs, policymakers, and patients, maintaining a steady focus on improving patient care and outcomes while carefully navigating the complex challenges of AI integration in health care.33-35
As multiagent AI systems in health care evolve, several exciting directions emerge. These include the integration of IoT and wearable devices, the development of more sophisticated natural language interfaces, and applying these systems to predictive maintenance of medical equipment.
CONCLUSIONS
The advent of multiagent AI systems in health care represents a paradigm shift in the approach to patient care, clinical decision making, and health care management. While these systems offer immense potential to transform health care delivery, their development and implementation must be guided by rigorous scientific validation, ethical considerations, and a patient-centered approach. The ultimate goal remains clear: harnessing the power of AI to improve patient outcomes, enhance the efficiency of health care delivery, and ultimately advance the health and well-being of patients.
Amazon Web Services, Inc. What are AI agents? Agents in artificial intelligence explained. Accessed April 7, 2025. https://aws.amazon.com/what-is/ai-agents/
Gutowska A. What are AI agents? IBM. Accessed April 7, 2025. https://www.ibm.com/think/topics/ai-agents
Agent AI. Microsoft Research. Accessed April 7, 2025. https://www.microsoft.com/en-us/research/project/agent-ai
Microsoft. AutoGen. Accessed April 7, 2025. https://microsoft.github.io/autogen/
Crew AI. The Leading Multi-Agent Platform. CrewAI. Accessed April 7, 2025. https://www.crewai.com/
LangChain. Accessed April 7, 2025. https://www.langchain.com/
Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070. doi:10.1001/jamainternmed.2021.2626
Willemink MJ, Roth HR, Sandfort V. Toward foundational deep learning models for medical imaging in the new era of transformer networks. Radiol Artif Intell. 2022;4(6):e210284. doi:10.1148/ryai.210284
Waqas A, Bui MM, Glassy EF, et al. Revolutionizing digital pathology with the power of generative artificial intelligence and foundation models. Lab Invest. 2023;103(11):100255. doi:10.1016/j.labinv.2023.100255
Moreno-Carrillo A, Arenas LMÁ, Fonseca JA, Caicedo CA, Tovar SV, Muñoz-Velandia OM. Application of queuing theory to optimize the triage process in a tertiary emergency care (“ER”) department. J Emerg Trauma Shock. 2019;12(4):268-273. doi:10.4103/JETS.JETS_42_19
Pongcharoen P, Hicks C, Braiden PM, Stewardson DJ. Determining optimum genetic algorithm parameters for scheduling the manufacturing and assembly of complex products. Int J Prod Econ. 2002;78(3):311-322. doi:10.1016/S0925-5273(02)00104-4
Sardar I, Akbar MA, Leiva V, Alsanad A, Mishra P. Machine learning and automatic ARIMA/Prophet models-based forecasting of COVID-19: methodology, evaluation, and case study in SAARC countries. Stoch Environ Res Risk Assess. 2023;37(1):345-359. doi:10.1007/s00477-022-02307-x
Samosir J, Indrawan-Santiago M, Haghighi PD. An evaluation of data stream processing systems for data driven applications. Procedia Comput Sci. 2016;80:439-449. doi:10.1016/j.procs.2016.05.322
Asmarawati TP, Suryantoro SD, Rosyid AN, et al. Predictive value of sequential organ failure assessment, quick sequential organ failure assessment, acute physiology and chronic health evaluation II, and new early warning signs scores estimate mortality of COVID-19 patients requiring intensive care unit. Indian J Crit Care Med. 2022;26(4):466-473. doi:10.5005/jp-journals-10071-24170
Khan S, Vandermorris A, Shepherd J, et al. Embracing uncertainty, managing complexity: applying complexity thinking principles to transformation efforts in healthcare systems. BMC Health Serv Res. 2018;18(1):192. doi:10.1186/s12913-018-2994-0
Plsek PE, Greenhalgh T. The challenge of complexity in health care. BMJ. 2001;323(7313):625-628. doi:10.1136/bmj.323.7313.625
Kouchaki S, Ding X, Sanei S. AI- and IoT-enabled solutions for healthcare. Sensors. 2024;24(8):2607. doi:10.3390/s24082607
Saab K, Tu T, Weng WH, et al. Capabilities of Gemini Models in Medicine. arXiv. doi:10.48550/arXiv.2404.18416
Villar SS, Bowden J, Wason J. Multi-armed bandit models for the optimal design of clinical trials: benefits and challenges. Stat Sci. 2015;30(2):199-215. doi:10.1214/14-STS504
Auth0. What is OAuth 2.0. Accessed April 7, 2025. https://auth0.com/intro-to-iam/what-is-oauth-2
HL7. Welcome to FHIR. Updated March 26, 2025. Accessed April 7, 2025. https://www.hl7.org/fhir/
SNOMED International. Accessed April 7, 2025. https://www.snomed.org
Hasselgren A, Kralevska K, Gligoroski D, Pedersen SA, Faxvaag A. Blockchain in healthcare and health sciences—a scoping review. Int J Med Inf. 2020;134:104040. doi:10.1016/j.ijmedinf.2019.104040
Ribeiro MT, Singh S, Guestrin C. “Why Should I Trust You?”: Explaining the predictions of any classifier. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. 2016:1135-1144. doi:10.1145/2939672.2939778
Ekanayake IU, Meddage DPP, Rathnayake U. A novel approach to explain the black-box nature of machine learning in compressive strength predictions of concrete using Shapley additive explanations (SHAP). Case Stud Constr Mater. 2022;16:e01059. doi:10.1016/j.cscm.2022.e01059
Alabi RO, Elmusrati M, Leivo I, Almangush A, Mäkitie AA. Machine learning explainability in nasopharyngeal cancer survival using LIME and SHAP. Sci Rep. 2023;13(1):8984. doi:10.1038/s41598-023-35795-0
Otto E, Culakova E, Meng S, et al. Overview of sankey flow diagrams: focusing on symptom trajectories in older adults with advanced cancer. J Geriatr Oncol. 2022;13(5):742-746. doi:10.1016/j.jgo.2021.12.017
Fereidooni H, Marchal S, Miettinen M, et al. SAFELearn: secure aggregation for private federated learning. In: 2021 IEEE security and privacy workshops (SPW). 2021:56-62. doi:10.1109/SPW53761.2021.00017
Linton DL, Pangle WM, Wyatt KH, Powell KN, Sherwood RE. Identifying key features of effective active learning: the effects of writing and peer discussion. Life Sci Educ. 2014;13(3):469-477. doi:10.1187/cbe.13-12-0242
Yang HS. Machine learning for sepsis prediction: prospects and challenges. Clin Chem. 2024;70(3):465-467. doi:10.1093/clinchem/hvae006
Liao J, Li X, Gan Y, et al. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol. 2023;12. doi:10.3389/fonc.2022.998222
Tierney AA, Gayre G, Hoberman B, et al. Ambient artificial intelligence scribes to alleviate the burden of clinical documentation. NEJM Catal. 2024;5(3):CAT.23.0404. doi:10.1056/CAT.23.0404
Borkowski AA, Jakey CE, Thomas LB, Viswanadhan N, Mastorides SM. Establishing a hospital artificial intelligence committee to improve patient care. Fed Pract. 2022;39(8):334-336. doi:10.12788/fp.0299
Isaacks DB, Borkowski AA. Implementing trustworthy AI in VA high reliability health care organizations. Fed Pract.2024;41(2):40-43. doi:10.12788/fp.0454
Han R, Acosta JN, Shakeri Z, Ioannidis JPA, Topol EJ, Rajpurkar P. Randomized controlled trials evaluating artificial intelligence in clinical practice: a scoping review. Lancet Digit Health. 2024;6(5):e367-e373. doi:10.1016/S2589-7500(24)00047-5
Artificial intelligence (AI) is rapidly evolving, with large language models (LLMs) marking a significant milestone in processing and generating human-like responses to natural language prompts. However, this advancement only signals the beginning of a more profound transformation in AI capabilities. The development of AI agents represents a new paradigm at the forefront of this evolution.
BACKGROUND
AI agents represent a leap forward from traditional LLM applications. While definitions may vary slightly among technology developers, the core concept remains: these agents are autonomous software entities designed to interact with their environment, make independent decisions, and execute tasks based on predefined goals.1-3 What sets AI agents apart is their combination of sophisticated components within structured architectures. At their core, AI agents incorporate an LLM for response generation, which is augmented by a suite of tools to optimize workflow and complete tasks, memory capabilities for personalized interactions, and autonomous reasoning. This combination allows AI agents to plan, create subtasks, gather information, and learn iteratively from their own experiences or other AI agents.
The true potential of this technology becomes apparent when multiple AI agents collaborate within multiagent AI systems. This concept introduces a new level of flexibility and capability in tackling complex tasks. Autogen, CrewAI, and LangChain offer various agent network configurations, including hierarchical, sequential, conditional, or even parallel task execution.4-6 This adaptability opens up a world of possibilities across various industries, but perhaps nowhere is the potential impact more exciting and profound than in health care.
AI agents in health care present an opportunity to revolutionize patient care, streamline administrative processes, and support complex clinical decision-making. This review examines 3 scenarios that illustrate the impact of AI agents in health care: a hypothetical sepsis management system, chronic disease management, and hospital patient flow optimization. This article will provide a detailed look at the technical implementation challenges, including the integration with existing health care IT systems, data privacy considerations, and the crucial role of explainable AI in maintaining trust and transparency.
It is challenging to implement AI agents in health care. Concerns include ensuring data quality and mitigating bias, seamlessly integrating these systems into existing clinical workflows, and navigating the complex ethical considerations that arise when deploying autonomous systems in health care. The integration with Internet of Things (IoT) devices for real-time patient data monitoring and the development of more sophisticated natural language interfaces to enhance future human-AI collaboration.
The adoption of AI agents in health care is only beginning, and it promises to be transformative. As AI continues to evolve, a comprehensive understanding of its applications, limitations, and ethical considerations is essential. This report provides a comprehensive overview of the current state, potential applications, and future directions of AI agents in health care, offering insights valuable to researchers, clinicians, and policymakers.
MultiAgent AI architecture
Sepsis Management
Despite advancements in broad-spectrum antibiotics, imaging, and life support systems, mortality rates associated with sepsis remain high. The complexity of optimizing care in clinical settings has hindered progress in managing sepsis. Previous attempts to develop predictive sepsis models have proven challenging.7 This report proposes a multiagent AI system designed to enhance comprehensive patient monitoring and care through coordinated AI-driven interventions.
Data Collection and Integration Agent. Powered by a controlled vocabulary to specify all data, the primary function for the data collection and integration agent is to clean, transform, and organize patient data from structured and unstructured sources. This agent prepares succinct summaries of consultant notes and formats data for human and machine consumption. All numerical data are presented graphically, including relevant historical data trends. The agent also digitally captures all orders in a structured format using a specified controlled vocabulary. This structured data feed supports the output of other agents, including documentation, treatment planning, and risk stratification, while also supplying the data structures for future training.
Diagnostic Agent. Critical illness is characterized by multiple abnormalities across a wide array of tests, ranging from plain chest X-ray, computed tomography (CT), blood cell composition, plasma chemistry, and microscopic evaluation of specimens. Additionally, life support parameters provide insights into disease severity and can inform management recommendations. These data offer a wide array of visual and numerical data to be used as input for computation, recommendation, and further training. For example, to evaluate fluid overload on chest X-rays or tissue histopathology slides, an AI agent can leverage deep learning models such as convolutional neural networks and vision transformers to analyze images like radiographs and histopathology slides.8,9 Recurrent neural networks or transformer models process sequential data like time-series vital signs. The agent also implements ensemble methods that combine multiple machine learning algorithms to enhance diagnostic accuracy.
Risk Stratification Agent. This assesses severity and predicts potential outcomes. Morbidity and mortality risks are calculated using an established scoring system and individualized based on the history of other agents’ conditional patients. These are presented graphically, with major risk factors highlighted for explainability.
Treatment Recommendation Agent. Using a reinforcement learning framework supplemented by up-to-date clinical guidelines, this system leverages historical data structured with standardized vocabulary to analyze patients with similar clinical features. Training is also conducted on the patient’s physiological data. All recommendations are presented via a dedicated user interface in a readable format, along with recommendations for editable, orderable items, references, and full-text snippets from previous research. Stop rules end computing if confidence in recommendations is too broad or no clear pathway can be computed with certainty, prompting human mitigation.
Resource Management Agent. This agent coordinates hospital resources using constraint programming techniques for optimal resource allocation, uses queueing theory models to predict and manage patient flow, and implements genetic algorithms for complex scheduling problems.10,11
Monitoring and Alert Agent. By tracking patients’ progress and alerting staff to changes, this agent uses anomaly detection algorithms to identify unusual patterns in patient data and implement time-series forecasting models, such as autoregressive integrated moving average and prophet, to predict future patient states. The agent also uses stream-processing techniques for real-time data analysis.12,13
Documentation and Reporting Agent. This agent maintains comprehensive medical records and generates reports. It employs advanced natural language processing techniques for automated report generation, uses advanced LLMs fine-tuned on medical corpora for narrative creation, and implements information-retrieval techniques to efficiently query patient records.
CLINICAL CASE STUDIES
To illustrate the functionality of a multiagent system, this report examines its application for managing sepsis. The data collection and integration agent continuously aggregates patient data from various sources, normalizing and timestamping it for consistent processing. The diagnostic agent analyzes this integrated data in real time, applying sepsis criteria and utilizing a deep learning model trained on a large sepsis dataset to detect subtle patterns.
The risk stratification agent calculates severity scores, such as the Sepsis-related Organ Failure Assessment (SOFA), quick SOFA (qSOFA), and Acute Physiology and Chronic Health Evaluation II, upon detecting a possible sepsis case.14 It predicts the likelihood of specific outcomes and estimates the potential trajectory of the patient’s condition for the next 24 to 48 hours. Based on this assessment, the treatment recommendation agent suggests an initial treatment plan, including appropriate antibiotics, fluid resuscitation protocols, and vasopressor recommendations, recommendations when indicated.
Concurrently, the resource management agent checks the availability of necessary resources and prioritizes allocation based on the severity. The monitoring agent tracks the patient’s response to interventions in real time, alerting the care team to any concerning changes or lack of expected improvement. Throughout this process, the documentation agent ensures that all actions, responses, and outcomes are meticulously recorded in a structured format and generates real-time updates for the patient’s electronic health record (EHR) and preparing summary reports for handoffs between care teams.
Administrative Workflow Support
Modern health care operations are resource-intensive, requiring coordination of advanced imaging, procedures, laboratory testing, and professional consultations.15 AI-powered health care administrative workflow systems are revolutionizing how medical facilities coordinate patient care. For patients with chronic cough, these systems seamlessly integrate scheduling, imaging, diagnostics, and follow-up care into a cohesive process that reduces administrative burden while improving patient outcomes. Through an intuitive interface and automated assistance, health care practitioners (HCPs) can track patient progress from initial consultation through diagnosis and treatment.
The process begins when an HCP enters a patient into the system, which triggers an automated CT scan scheduling system. The system considers factors like urgency, facility availability, and patient preferences to suggest optimal appointment times. Once imaging is complete, AI agents analyze the radiology reports, extract key findings, and generate structured summaries that highlight critical information such as “mild bronchial wall thickening with patchy ground-glass opacities” or “findings consistent with chronic bronchitis.”
Based on these findings, the system automatically generates evidence-based recommendations for follow-up care, such as pulmonology consultations or follow-up imaging in 3 months. These recommendations are presented to the ordering clinician, along with suggested appointment slots for specialist consultations. The system then manages the coordination of multiple appointments, ensuring each step in the patient’s care plan is properly sequenced and scheduled.
The entire process is monitored through a comprehensive dashboard that provides real-time updates on patient status, appointment schedules, and clinical recommendations. HCPs can track which patients require immediate attention, view upcoming appointments, and monitor the progress of ongoing care plans.
Multiagent AI Operation Optimization
Hospitals are complex entities that must function at different scales and respond in an agile, timely manner at all hours, deploying staff at various positions.16 A system of AI agents can receive signals from sensors monitoring foot traffic in the emergency department and trauma unit, as well as the availability of operating room staff, equipment, and intensive care unit beds. Smart sensors enable this monitoring through IoT networks. These networks benefit from advances in adaptive and consensus networking algorithms, along with recent advances in bioengineering and biocomputing.17
For example, in the case of imaging for suspected abdominal obstruction, an AI agent tasked with scheduling CTs could time the patient’s arrival based on acuity. Another AI agent could alert staff transporting the patient to the CT appointment, with the next location contingent on a clinical decision to proceed to the operating room. Yet another AI agent could summarize radiology interpretations and alert the surgery and anesthesia teams to a potential case, while others could notify operating room staff of equipment needs or reserve a bed. In this paradigm, AI agents facilitate more precise and timely communication between multiple staff members.
TECHNICAL IMPLEMENTATION
Large Language Models
Each agent uses a different LLM optimized for its specific task. For example, the diagnostic agent uses an LLM pretrained on a large corpus of biomedical literature and fine-tuned on a dataset of confirmed sepsis cases and their presentations.18 It implements few-shot learning techniques to adapt to rare or atypical presentations. The treatment recommendation agent also uses an LLM, employing a retrieval-augmented generation approach to access the latest clinical guidelines during inference. The documentation agent uses another advanced language model, fine-tuned on a large corpus of high-quality medical documentation, implementing controlled text generation techniques and utilizing a separate smaller model for real-time error checking and correction.
Interagent Quality Control
Agents learn from their own experience and the experience of other agents. They are equipped with user-defined rule-based and model-based systems for quality assurance, with clear stopping rules for human involvement and mitigation.
Sophisticated quality control measures bolster the system’s reliability, including ensemble techniques for result comparison, redundancy for critical tasks, and automatic human review for disagreements above a certain threshold. Each agent provides a calibrated confidence score with its output, used to weigh inputs in downstream tasks and trigger additional checks for low-confidence outputs.
A dedicated quality control agent monitors output from all agents, employing both supervised and unsupervised anomaly detection techniques. Feedback loops allow agents to evaluate the quality and utility of information received from other agents. The system implements a multiarmed bandit approach to dynamically adjust the influence of different agents based on their performance and periodically retrains agent models using federated learning techniques.19
Electronic Health Record Integration
Seamless EHR integration is crucial for practical implementation. The system has secure application programming interface access to various EHR platforms, implements OAuth 2.0 for authentication, and use HTTPS with perfect forward secrecy for all communications.20 It works with HL7 FHIR to ensure interoperability and uses SNOMED CT for clinical terminology to ensure semantic interoperability across different EHRs.21,22
The system implements a multilevel approval system for write-backs to EHRs, with different thresholds based on the information’s criticality. It uses digital signatures to ensure the integrity and nonrepudiation of AI-generated entries and implements blockchain technology to create an immutable and distributed ledger of all AI system actions.23
Decision Transparency
To ensure transparency in decision-making processes, the system applies techniques (eg, local interpretable model-agnostic explanations and Shapley additive explanations) to provide insights into agent decision-making processes.24-26 It provides customized visualizations for different stakeholders and allows users to explore alternative decision paths through what-if scenario modeling.27
The system provides calibrated confidence indicators for each recommendation or decision, implementing a novel confidence calibration agent that continuously monitors and adjusts confidence scores based on observed outcomes.
Continuous Learning and Adaptation
The system employs several techniques to remain current with evolving medical knowledge. Federated learning includes information from diverse datasets across multiple institutions without compromising patient privacy.28 A/B testing is used to safely deploy and compare new agent versions in controlled settings, implementing multiarmed bandit algorithms to efficiently explore new models while minimizing potential negative impacts. Human-in-the-loop learning and active learning techniques are used to incorporate feedback from HCPs and efficiently solicit expert input on the most informative data.29
CLINICAL IMPLICATIONS
The implementation of multiagent AI systems in health care has several potential benefits: enhanced diagnostic accuracy, personalized treatment, improved efficiency, continuous monitoring, and resource optimization. A recent review of AI sepsis predictive models exhibited superior results to standard clinical scoring methods like qSOFA.30 In oncology, such systems can result in more tailored treatments, enhancing outcomes.31 The implementation of an ambient dictation system can improve workflow and prevent HCP burnout.32
ETHICAL CONSIDERATIONS AND AI OVERSIGHT
Integrating AI agents into health care raises significant ethical considerations that must be carefully addressed to ensure equitable and effective care delivery. One primary concern involves cultural and linguistic competency, as AI systems may struggle with cultural nuances, idioms, and context-specific communication patterns. This becomes particularly challenging in regions with diverse ethnic populations or immigrant communities, where medical terminology may not have direct translations and cultural beliefs significantly influence health care decisions. AI systems also may inherit and amplify existing biases in health care delivery, whether through HCP bias reflected in training data, patient bias affecting acceptance of AI-assisted care, or demographic underrepresentation during system development.
AI agents present unique opportunities for improving health care access and outcomes through community engagement, though such initiatives require thoughtful implementation. Predictive analytics can identify high-risk individuals within communities who may benefit from preventive care, while analysis of social determinants of health can enable more targeted interventions. However, these capabilities must be balanced with privacy concerns and the risk of surveillance, particularly in communities that distrust health care institutions. The potential for AI to bridge health care gaps must be weighed against the need to maintain cultural sensitivity and community trust.
The governance and oversight of health care AI systems requires a multistakeholder approach with clear lines of responsibility and accountability. This includes involvement from government health care agencies, professional medical associations, ethics boards, and independent auditors, all working together to establish and enforce standards while monitoring system performance and addressing potential biases. Health care organizations must maintain transparent policies about AI use, implement regular monitoring and evaluation protocols, and establish precise mechanisms for patient feedback and grievance resolution. Ongoing assessment and adjustment of these systems, informed by community feedback and outcomes data, will be crucial for their ethical implementation, ensuring that AI agents complement, rather than replace, human judgment and cultural sensitivity.
FUTURE DIRECTIONS
Despite the potential benefits, implementing multiagent AI systems in health care faces significant challenges that require careful consideration. Beyond the fundamental issues such as data quality and bias mitigation, health care organizations struggle with fragmented systems, inconsistent data formats, and varying quality. Technical infrastructure requirements are substantial, particularly in rural or underserved areas that lack robust networks and cybersecurity. HCPs already face significant cognitive load and time pressures, making integrating AI agents into existing workflows particularly challenging. There is also the critical issue of transparency and interpretability, as health care decisions require clear reasoning and accountability that many black-box AI systems struggle to provide.
The legal landscape introduces another layer of complexity, particularly regarding liability, consent, and privacy questions. When AI agents contribute to medical decisions, establishing clear lines of responsibility becomes crucial. There are also serious concerns about algorithmic fairness and the potential for AI systems to perpetuate or amplify existing inequities. The cost of implementation remains a significant barrier, requiring substantial investment in technology, training, and ongoing maintenance while ensuring resources are not diverted from direct patient care. Moreover, HCPs may resist adoption due to concerns about job security, loss of autonomy, or skepticism about AI capabilities while paradoxically facing risks of overreliance on AI systems that could lead to the degradation of human clinical skills.
Addressing these challenges requires a multifaceted approach that combines technical solutions with organizational and policy changes. Health care organizations must implement rigorous data validation processes and interoperability standards while developing hybrid models that balance sophisticated AI capabilities with interpretable techniques. Extensive research and iterative design processes, with direct input from HCPs, are essential for successful integration. Establishing independent ethics boards to oversee system development and deployment, conducting multicenter randomized controlled trials, and creating clear regulatory frameworks will ensure safe and effective implementation. Success will ultimately depend on ongoing collaboration between technology developers, HCPs, policymakers, and patients, maintaining a steady focus on improving patient care and outcomes while carefully navigating the complex challenges of AI integration in health care.33-35
As multiagent AI systems in health care evolve, several exciting directions emerge. These include the integration of IoT and wearable devices, the development of more sophisticated natural language interfaces, and applying these systems to predictive maintenance of medical equipment.
CONCLUSIONS
The advent of multiagent AI systems in health care represents a paradigm shift in the approach to patient care, clinical decision making, and health care management. While these systems offer immense potential to transform health care delivery, their development and implementation must be guided by rigorous scientific validation, ethical considerations, and a patient-centered approach. The ultimate goal remains clear: harnessing the power of AI to improve patient outcomes, enhance the efficiency of health care delivery, and ultimately advance the health and well-being of patients.
Artificial intelligence (AI) is rapidly evolving, with large language models (LLMs) marking a significant milestone in processing and generating human-like responses to natural language prompts. However, this advancement only signals the beginning of a more profound transformation in AI capabilities. The development of AI agents represents a new paradigm at the forefront of this evolution.
BACKGROUND
AI agents represent a leap forward from traditional LLM applications. While definitions may vary slightly among technology developers, the core concept remains: these agents are autonomous software entities designed to interact with their environment, make independent decisions, and execute tasks based on predefined goals.1-3 What sets AI agents apart is their combination of sophisticated components within structured architectures. At their core, AI agents incorporate an LLM for response generation, which is augmented by a suite of tools to optimize workflow and complete tasks, memory capabilities for personalized interactions, and autonomous reasoning. This combination allows AI agents to plan, create subtasks, gather information, and learn iteratively from their own experiences or other AI agents.
The true potential of this technology becomes apparent when multiple AI agents collaborate within multiagent AI systems. This concept introduces a new level of flexibility and capability in tackling complex tasks. Autogen, CrewAI, and LangChain offer various agent network configurations, including hierarchical, sequential, conditional, or even parallel task execution.4-6 This adaptability opens up a world of possibilities across various industries, but perhaps nowhere is the potential impact more exciting and profound than in health care.
AI agents in health care present an opportunity to revolutionize patient care, streamline administrative processes, and support complex clinical decision-making. This review examines 3 scenarios that illustrate the impact of AI agents in health care: a hypothetical sepsis management system, chronic disease management, and hospital patient flow optimization. This article will provide a detailed look at the technical implementation challenges, including the integration with existing health care IT systems, data privacy considerations, and the crucial role of explainable AI in maintaining trust and transparency.
It is challenging to implement AI agents in health care. Concerns include ensuring data quality and mitigating bias, seamlessly integrating these systems into existing clinical workflows, and navigating the complex ethical considerations that arise when deploying autonomous systems in health care. The integration with Internet of Things (IoT) devices for real-time patient data monitoring and the development of more sophisticated natural language interfaces to enhance future human-AI collaboration.
The adoption of AI agents in health care is only beginning, and it promises to be transformative. As AI continues to evolve, a comprehensive understanding of its applications, limitations, and ethical considerations is essential. This report provides a comprehensive overview of the current state, potential applications, and future directions of AI agents in health care, offering insights valuable to researchers, clinicians, and policymakers.
MultiAgent AI architecture
Sepsis Management
Despite advancements in broad-spectrum antibiotics, imaging, and life support systems, mortality rates associated with sepsis remain high. The complexity of optimizing care in clinical settings has hindered progress in managing sepsis. Previous attempts to develop predictive sepsis models have proven challenging.7 This report proposes a multiagent AI system designed to enhance comprehensive patient monitoring and care through coordinated AI-driven interventions.
Data Collection and Integration Agent. Powered by a controlled vocabulary to specify all data, the primary function for the data collection and integration agent is to clean, transform, and organize patient data from structured and unstructured sources. This agent prepares succinct summaries of consultant notes and formats data for human and machine consumption. All numerical data are presented graphically, including relevant historical data trends. The agent also digitally captures all orders in a structured format using a specified controlled vocabulary. This structured data feed supports the output of other agents, including documentation, treatment planning, and risk stratification, while also supplying the data structures for future training.
Diagnostic Agent. Critical illness is characterized by multiple abnormalities across a wide array of tests, ranging from plain chest X-ray, computed tomography (CT), blood cell composition, plasma chemistry, and microscopic evaluation of specimens. Additionally, life support parameters provide insights into disease severity and can inform management recommendations. These data offer a wide array of visual and numerical data to be used as input for computation, recommendation, and further training. For example, to evaluate fluid overload on chest X-rays or tissue histopathology slides, an AI agent can leverage deep learning models such as convolutional neural networks and vision transformers to analyze images like radiographs and histopathology slides.8,9 Recurrent neural networks or transformer models process sequential data like time-series vital signs. The agent also implements ensemble methods that combine multiple machine learning algorithms to enhance diagnostic accuracy.
Risk Stratification Agent. This assesses severity and predicts potential outcomes. Morbidity and mortality risks are calculated using an established scoring system and individualized based on the history of other agents’ conditional patients. These are presented graphically, with major risk factors highlighted for explainability.
Treatment Recommendation Agent. Using a reinforcement learning framework supplemented by up-to-date clinical guidelines, this system leverages historical data structured with standardized vocabulary to analyze patients with similar clinical features. Training is also conducted on the patient’s physiological data. All recommendations are presented via a dedicated user interface in a readable format, along with recommendations for editable, orderable items, references, and full-text snippets from previous research. Stop rules end computing if confidence in recommendations is too broad or no clear pathway can be computed with certainty, prompting human mitigation.
Resource Management Agent. This agent coordinates hospital resources using constraint programming techniques for optimal resource allocation, uses queueing theory models to predict and manage patient flow, and implements genetic algorithms for complex scheduling problems.10,11
Monitoring and Alert Agent. By tracking patients’ progress and alerting staff to changes, this agent uses anomaly detection algorithms to identify unusual patterns in patient data and implement time-series forecasting models, such as autoregressive integrated moving average and prophet, to predict future patient states. The agent also uses stream-processing techniques for real-time data analysis.12,13
Documentation and Reporting Agent. This agent maintains comprehensive medical records and generates reports. It employs advanced natural language processing techniques for automated report generation, uses advanced LLMs fine-tuned on medical corpora for narrative creation, and implements information-retrieval techniques to efficiently query patient records.
CLINICAL CASE STUDIES
To illustrate the functionality of a multiagent system, this report examines its application for managing sepsis. The data collection and integration agent continuously aggregates patient data from various sources, normalizing and timestamping it for consistent processing. The diagnostic agent analyzes this integrated data in real time, applying sepsis criteria and utilizing a deep learning model trained on a large sepsis dataset to detect subtle patterns.
The risk stratification agent calculates severity scores, such as the Sepsis-related Organ Failure Assessment (SOFA), quick SOFA (qSOFA), and Acute Physiology and Chronic Health Evaluation II, upon detecting a possible sepsis case.14 It predicts the likelihood of specific outcomes and estimates the potential trajectory of the patient’s condition for the next 24 to 48 hours. Based on this assessment, the treatment recommendation agent suggests an initial treatment plan, including appropriate antibiotics, fluid resuscitation protocols, and vasopressor recommendations, recommendations when indicated.
Concurrently, the resource management agent checks the availability of necessary resources and prioritizes allocation based on the severity. The monitoring agent tracks the patient’s response to interventions in real time, alerting the care team to any concerning changes or lack of expected improvement. Throughout this process, the documentation agent ensures that all actions, responses, and outcomes are meticulously recorded in a structured format and generates real-time updates for the patient’s electronic health record (EHR) and preparing summary reports for handoffs between care teams.
Administrative Workflow Support
Modern health care operations are resource-intensive, requiring coordination of advanced imaging, procedures, laboratory testing, and professional consultations.15 AI-powered health care administrative workflow systems are revolutionizing how medical facilities coordinate patient care. For patients with chronic cough, these systems seamlessly integrate scheduling, imaging, diagnostics, and follow-up care into a cohesive process that reduces administrative burden while improving patient outcomes. Through an intuitive interface and automated assistance, health care practitioners (HCPs) can track patient progress from initial consultation through diagnosis and treatment.
The process begins when an HCP enters a patient into the system, which triggers an automated CT scan scheduling system. The system considers factors like urgency, facility availability, and patient preferences to suggest optimal appointment times. Once imaging is complete, AI agents analyze the radiology reports, extract key findings, and generate structured summaries that highlight critical information such as “mild bronchial wall thickening with patchy ground-glass opacities” or “findings consistent with chronic bronchitis.”
Based on these findings, the system automatically generates evidence-based recommendations for follow-up care, such as pulmonology consultations or follow-up imaging in 3 months. These recommendations are presented to the ordering clinician, along with suggested appointment slots for specialist consultations. The system then manages the coordination of multiple appointments, ensuring each step in the patient’s care plan is properly sequenced and scheduled.
The entire process is monitored through a comprehensive dashboard that provides real-time updates on patient status, appointment schedules, and clinical recommendations. HCPs can track which patients require immediate attention, view upcoming appointments, and monitor the progress of ongoing care plans.
Multiagent AI Operation Optimization
Hospitals are complex entities that must function at different scales and respond in an agile, timely manner at all hours, deploying staff at various positions.16 A system of AI agents can receive signals from sensors monitoring foot traffic in the emergency department and trauma unit, as well as the availability of operating room staff, equipment, and intensive care unit beds. Smart sensors enable this monitoring through IoT networks. These networks benefit from advances in adaptive and consensus networking algorithms, along with recent advances in bioengineering and biocomputing.17
For example, in the case of imaging for suspected abdominal obstruction, an AI agent tasked with scheduling CTs could time the patient’s arrival based on acuity. Another AI agent could alert staff transporting the patient to the CT appointment, with the next location contingent on a clinical decision to proceed to the operating room. Yet another AI agent could summarize radiology interpretations and alert the surgery and anesthesia teams to a potential case, while others could notify operating room staff of equipment needs or reserve a bed. In this paradigm, AI agents facilitate more precise and timely communication between multiple staff members.
TECHNICAL IMPLEMENTATION
Large Language Models
Each agent uses a different LLM optimized for its specific task. For example, the diagnostic agent uses an LLM pretrained on a large corpus of biomedical literature and fine-tuned on a dataset of confirmed sepsis cases and their presentations.18 It implements few-shot learning techniques to adapt to rare or atypical presentations. The treatment recommendation agent also uses an LLM, employing a retrieval-augmented generation approach to access the latest clinical guidelines during inference. The documentation agent uses another advanced language model, fine-tuned on a large corpus of high-quality medical documentation, implementing controlled text generation techniques and utilizing a separate smaller model for real-time error checking and correction.
Interagent Quality Control
Agents learn from their own experience and the experience of other agents. They are equipped with user-defined rule-based and model-based systems for quality assurance, with clear stopping rules for human involvement and mitigation.
Sophisticated quality control measures bolster the system’s reliability, including ensemble techniques for result comparison, redundancy for critical tasks, and automatic human review for disagreements above a certain threshold. Each agent provides a calibrated confidence score with its output, used to weigh inputs in downstream tasks and trigger additional checks for low-confidence outputs.
A dedicated quality control agent monitors output from all agents, employing both supervised and unsupervised anomaly detection techniques. Feedback loops allow agents to evaluate the quality and utility of information received from other agents. The system implements a multiarmed bandit approach to dynamically adjust the influence of different agents based on their performance and periodically retrains agent models using federated learning techniques.19
Electronic Health Record Integration
Seamless EHR integration is crucial for practical implementation. The system has secure application programming interface access to various EHR platforms, implements OAuth 2.0 for authentication, and use HTTPS with perfect forward secrecy for all communications.20 It works with HL7 FHIR to ensure interoperability and uses SNOMED CT for clinical terminology to ensure semantic interoperability across different EHRs.21,22
The system implements a multilevel approval system for write-backs to EHRs, with different thresholds based on the information’s criticality. It uses digital signatures to ensure the integrity and nonrepudiation of AI-generated entries and implements blockchain technology to create an immutable and distributed ledger of all AI system actions.23
Decision Transparency
To ensure transparency in decision-making processes, the system applies techniques (eg, local interpretable model-agnostic explanations and Shapley additive explanations) to provide insights into agent decision-making processes.24-26 It provides customized visualizations for different stakeholders and allows users to explore alternative decision paths through what-if scenario modeling.27
The system provides calibrated confidence indicators for each recommendation or decision, implementing a novel confidence calibration agent that continuously monitors and adjusts confidence scores based on observed outcomes.
Continuous Learning and Adaptation
The system employs several techniques to remain current with evolving medical knowledge. Federated learning includes information from diverse datasets across multiple institutions without compromising patient privacy.28 A/B testing is used to safely deploy and compare new agent versions in controlled settings, implementing multiarmed bandit algorithms to efficiently explore new models while minimizing potential negative impacts. Human-in-the-loop learning and active learning techniques are used to incorporate feedback from HCPs and efficiently solicit expert input on the most informative data.29
CLINICAL IMPLICATIONS
The implementation of multiagent AI systems in health care has several potential benefits: enhanced diagnostic accuracy, personalized treatment, improved efficiency, continuous monitoring, and resource optimization. A recent review of AI sepsis predictive models exhibited superior results to standard clinical scoring methods like qSOFA.30 In oncology, such systems can result in more tailored treatments, enhancing outcomes.31 The implementation of an ambient dictation system can improve workflow and prevent HCP burnout.32
ETHICAL CONSIDERATIONS AND AI OVERSIGHT
Integrating AI agents into health care raises significant ethical considerations that must be carefully addressed to ensure equitable and effective care delivery. One primary concern involves cultural and linguistic competency, as AI systems may struggle with cultural nuances, idioms, and context-specific communication patterns. This becomes particularly challenging in regions with diverse ethnic populations or immigrant communities, where medical terminology may not have direct translations and cultural beliefs significantly influence health care decisions. AI systems also may inherit and amplify existing biases in health care delivery, whether through HCP bias reflected in training data, patient bias affecting acceptance of AI-assisted care, or demographic underrepresentation during system development.
AI agents present unique opportunities for improving health care access and outcomes through community engagement, though such initiatives require thoughtful implementation. Predictive analytics can identify high-risk individuals within communities who may benefit from preventive care, while analysis of social determinants of health can enable more targeted interventions. However, these capabilities must be balanced with privacy concerns and the risk of surveillance, particularly in communities that distrust health care institutions. The potential for AI to bridge health care gaps must be weighed against the need to maintain cultural sensitivity and community trust.
The governance and oversight of health care AI systems requires a multistakeholder approach with clear lines of responsibility and accountability. This includes involvement from government health care agencies, professional medical associations, ethics boards, and independent auditors, all working together to establish and enforce standards while monitoring system performance and addressing potential biases. Health care organizations must maintain transparent policies about AI use, implement regular monitoring and evaluation protocols, and establish precise mechanisms for patient feedback and grievance resolution. Ongoing assessment and adjustment of these systems, informed by community feedback and outcomes data, will be crucial for their ethical implementation, ensuring that AI agents complement, rather than replace, human judgment and cultural sensitivity.
FUTURE DIRECTIONS
Despite the potential benefits, implementing multiagent AI systems in health care faces significant challenges that require careful consideration. Beyond the fundamental issues such as data quality and bias mitigation, health care organizations struggle with fragmented systems, inconsistent data formats, and varying quality. Technical infrastructure requirements are substantial, particularly in rural or underserved areas that lack robust networks and cybersecurity. HCPs already face significant cognitive load and time pressures, making integrating AI agents into existing workflows particularly challenging. There is also the critical issue of transparency and interpretability, as health care decisions require clear reasoning and accountability that many black-box AI systems struggle to provide.
The legal landscape introduces another layer of complexity, particularly regarding liability, consent, and privacy questions. When AI agents contribute to medical decisions, establishing clear lines of responsibility becomes crucial. There are also serious concerns about algorithmic fairness and the potential for AI systems to perpetuate or amplify existing inequities. The cost of implementation remains a significant barrier, requiring substantial investment in technology, training, and ongoing maintenance while ensuring resources are not diverted from direct patient care. Moreover, HCPs may resist adoption due to concerns about job security, loss of autonomy, or skepticism about AI capabilities while paradoxically facing risks of overreliance on AI systems that could lead to the degradation of human clinical skills.
Addressing these challenges requires a multifaceted approach that combines technical solutions with organizational and policy changes. Health care organizations must implement rigorous data validation processes and interoperability standards while developing hybrid models that balance sophisticated AI capabilities with interpretable techniques. Extensive research and iterative design processes, with direct input from HCPs, are essential for successful integration. Establishing independent ethics boards to oversee system development and deployment, conducting multicenter randomized controlled trials, and creating clear regulatory frameworks will ensure safe and effective implementation. Success will ultimately depend on ongoing collaboration between technology developers, HCPs, policymakers, and patients, maintaining a steady focus on improving patient care and outcomes while carefully navigating the complex challenges of AI integration in health care.33-35
As multiagent AI systems in health care evolve, several exciting directions emerge. These include the integration of IoT and wearable devices, the development of more sophisticated natural language interfaces, and applying these systems to predictive maintenance of medical equipment.
CONCLUSIONS
The advent of multiagent AI systems in health care represents a paradigm shift in the approach to patient care, clinical decision making, and health care management. While these systems offer immense potential to transform health care delivery, their development and implementation must be guided by rigorous scientific validation, ethical considerations, and a patient-centered approach. The ultimate goal remains clear: harnessing the power of AI to improve patient outcomes, enhance the efficiency of health care delivery, and ultimately advance the health and well-being of patients.
Amazon Web Services, Inc. What are AI agents? Agents in artificial intelligence explained. Accessed April 7, 2025. https://aws.amazon.com/what-is/ai-agents/
Gutowska A. What are AI agents? IBM. Accessed April 7, 2025. https://www.ibm.com/think/topics/ai-agents
Agent AI. Microsoft Research. Accessed April 7, 2025. https://www.microsoft.com/en-us/research/project/agent-ai
Microsoft. AutoGen. Accessed April 7, 2025. https://microsoft.github.io/autogen/
Crew AI. The Leading Multi-Agent Platform. CrewAI. Accessed April 7, 2025. https://www.crewai.com/
LangChain. Accessed April 7, 2025. https://www.langchain.com/
Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070. doi:10.1001/jamainternmed.2021.2626
Willemink MJ, Roth HR, Sandfort V. Toward foundational deep learning models for medical imaging in the new era of transformer networks. Radiol Artif Intell. 2022;4(6):e210284. doi:10.1148/ryai.210284
Waqas A, Bui MM, Glassy EF, et al. Revolutionizing digital pathology with the power of generative artificial intelligence and foundation models. Lab Invest. 2023;103(11):100255. doi:10.1016/j.labinv.2023.100255
Moreno-Carrillo A, Arenas LMÁ, Fonseca JA, Caicedo CA, Tovar SV, Muñoz-Velandia OM. Application of queuing theory to optimize the triage process in a tertiary emergency care (“ER”) department. J Emerg Trauma Shock. 2019;12(4):268-273. doi:10.4103/JETS.JETS_42_19
Pongcharoen P, Hicks C, Braiden PM, Stewardson DJ. Determining optimum genetic algorithm parameters for scheduling the manufacturing and assembly of complex products. Int J Prod Econ. 2002;78(3):311-322. doi:10.1016/S0925-5273(02)00104-4
Sardar I, Akbar MA, Leiva V, Alsanad A, Mishra P. Machine learning and automatic ARIMA/Prophet models-based forecasting of COVID-19: methodology, evaluation, and case study in SAARC countries. Stoch Environ Res Risk Assess. 2023;37(1):345-359. doi:10.1007/s00477-022-02307-x
Samosir J, Indrawan-Santiago M, Haghighi PD. An evaluation of data stream processing systems for data driven applications. Procedia Comput Sci. 2016;80:439-449. doi:10.1016/j.procs.2016.05.322
Asmarawati TP, Suryantoro SD, Rosyid AN, et al. Predictive value of sequential organ failure assessment, quick sequential organ failure assessment, acute physiology and chronic health evaluation II, and new early warning signs scores estimate mortality of COVID-19 patients requiring intensive care unit. Indian J Crit Care Med. 2022;26(4):466-473. doi:10.5005/jp-journals-10071-24170
Khan S, Vandermorris A, Shepherd J, et al. Embracing uncertainty, managing complexity: applying complexity thinking principles to transformation efforts in healthcare systems. BMC Health Serv Res. 2018;18(1):192. doi:10.1186/s12913-018-2994-0
Plsek PE, Greenhalgh T. The challenge of complexity in health care. BMJ. 2001;323(7313):625-628. doi:10.1136/bmj.323.7313.625
Kouchaki S, Ding X, Sanei S. AI- and IoT-enabled solutions for healthcare. Sensors. 2024;24(8):2607. doi:10.3390/s24082607
Saab K, Tu T, Weng WH, et al. Capabilities of Gemini Models in Medicine. arXiv. doi:10.48550/arXiv.2404.18416
Villar SS, Bowden J, Wason J. Multi-armed bandit models for the optimal design of clinical trials: benefits and challenges. Stat Sci. 2015;30(2):199-215. doi:10.1214/14-STS504
Auth0. What is OAuth 2.0. Accessed April 7, 2025. https://auth0.com/intro-to-iam/what-is-oauth-2
HL7. Welcome to FHIR. Updated March 26, 2025. Accessed April 7, 2025. https://www.hl7.org/fhir/
SNOMED International. Accessed April 7, 2025. https://www.snomed.org
Hasselgren A, Kralevska K, Gligoroski D, Pedersen SA, Faxvaag A. Blockchain in healthcare and health sciences—a scoping review. Int J Med Inf. 2020;134:104040. doi:10.1016/j.ijmedinf.2019.104040
Ribeiro MT, Singh S, Guestrin C. “Why Should I Trust You?”: Explaining the predictions of any classifier. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. 2016:1135-1144. doi:10.1145/2939672.2939778
Ekanayake IU, Meddage DPP, Rathnayake U. A novel approach to explain the black-box nature of machine learning in compressive strength predictions of concrete using Shapley additive explanations (SHAP). Case Stud Constr Mater. 2022;16:e01059. doi:10.1016/j.cscm.2022.e01059
Alabi RO, Elmusrati M, Leivo I, Almangush A, Mäkitie AA. Machine learning explainability in nasopharyngeal cancer survival using LIME and SHAP. Sci Rep. 2023;13(1):8984. doi:10.1038/s41598-023-35795-0
Otto E, Culakova E, Meng S, et al. Overview of sankey flow diagrams: focusing on symptom trajectories in older adults with advanced cancer. J Geriatr Oncol. 2022;13(5):742-746. doi:10.1016/j.jgo.2021.12.017
Fereidooni H, Marchal S, Miettinen M, et al. SAFELearn: secure aggregation for private federated learning. In: 2021 IEEE security and privacy workshops (SPW). 2021:56-62. doi:10.1109/SPW53761.2021.00017
Linton DL, Pangle WM, Wyatt KH, Powell KN, Sherwood RE. Identifying key features of effective active learning: the effects of writing and peer discussion. Life Sci Educ. 2014;13(3):469-477. doi:10.1187/cbe.13-12-0242
Yang HS. Machine learning for sepsis prediction: prospects and challenges. Clin Chem. 2024;70(3):465-467. doi:10.1093/clinchem/hvae006
Liao J, Li X, Gan Y, et al. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol. 2023;12. doi:10.3389/fonc.2022.998222
Tierney AA, Gayre G, Hoberman B, et al. Ambient artificial intelligence scribes to alleviate the burden of clinical documentation. NEJM Catal. 2024;5(3):CAT.23.0404. doi:10.1056/CAT.23.0404
Borkowski AA, Jakey CE, Thomas LB, Viswanadhan N, Mastorides SM. Establishing a hospital artificial intelligence committee to improve patient care. Fed Pract. 2022;39(8):334-336. doi:10.12788/fp.0299
Isaacks DB, Borkowski AA. Implementing trustworthy AI in VA high reliability health care organizations. Fed Pract.2024;41(2):40-43. doi:10.12788/fp.0454
Han R, Acosta JN, Shakeri Z, Ioannidis JPA, Topol EJ, Rajpurkar P. Randomized controlled trials evaluating artificial intelligence in clinical practice: a scoping review. Lancet Digit Health. 2024;6(5):e367-e373. doi:10.1016/S2589-7500(24)00047-5
Amazon Web Services, Inc. What are AI agents? Agents in artificial intelligence explained. Accessed April 7, 2025. https://aws.amazon.com/what-is/ai-agents/
Gutowska A. What are AI agents? IBM. Accessed April 7, 2025. https://www.ibm.com/think/topics/ai-agents
Agent AI. Microsoft Research. Accessed April 7, 2025. https://www.microsoft.com/en-us/research/project/agent-ai
Microsoft. AutoGen. Accessed April 7, 2025. https://microsoft.github.io/autogen/
Crew AI. The Leading Multi-Agent Platform. CrewAI. Accessed April 7, 2025. https://www.crewai.com/
LangChain. Accessed April 7, 2025. https://www.langchain.com/
Wong A, Otles E, Donnelly JP, et al. External validation of a widely implemented proprietary sepsis prediction model in hospitalized patients. JAMA Intern Med. 2021;181(8):1065-1070. doi:10.1001/jamainternmed.2021.2626
Willemink MJ, Roth HR, Sandfort V. Toward foundational deep learning models for medical imaging in the new era of transformer networks. Radiol Artif Intell. 2022;4(6):e210284. doi:10.1148/ryai.210284
Waqas A, Bui MM, Glassy EF, et al. Revolutionizing digital pathology with the power of generative artificial intelligence and foundation models. Lab Invest. 2023;103(11):100255. doi:10.1016/j.labinv.2023.100255
Moreno-Carrillo A, Arenas LMÁ, Fonseca JA, Caicedo CA, Tovar SV, Muñoz-Velandia OM. Application of queuing theory to optimize the triage process in a tertiary emergency care (“ER”) department. J Emerg Trauma Shock. 2019;12(4):268-273. doi:10.4103/JETS.JETS_42_19
Pongcharoen P, Hicks C, Braiden PM, Stewardson DJ. Determining optimum genetic algorithm parameters for scheduling the manufacturing and assembly of complex products. Int J Prod Econ. 2002;78(3):311-322. doi:10.1016/S0925-5273(02)00104-4
Sardar I, Akbar MA, Leiva V, Alsanad A, Mishra P. Machine learning and automatic ARIMA/Prophet models-based forecasting of COVID-19: methodology, evaluation, and case study in SAARC countries. Stoch Environ Res Risk Assess. 2023;37(1):345-359. doi:10.1007/s00477-022-02307-x
Samosir J, Indrawan-Santiago M, Haghighi PD. An evaluation of data stream processing systems for data driven applications. Procedia Comput Sci. 2016;80:439-449. doi:10.1016/j.procs.2016.05.322
Asmarawati TP, Suryantoro SD, Rosyid AN, et al. Predictive value of sequential organ failure assessment, quick sequential organ failure assessment, acute physiology and chronic health evaluation II, and new early warning signs scores estimate mortality of COVID-19 patients requiring intensive care unit. Indian J Crit Care Med. 2022;26(4):466-473. doi:10.5005/jp-journals-10071-24170
Khan S, Vandermorris A, Shepherd J, et al. Embracing uncertainty, managing complexity: applying complexity thinking principles to transformation efforts in healthcare systems. BMC Health Serv Res. 2018;18(1):192. doi:10.1186/s12913-018-2994-0
Plsek PE, Greenhalgh T. The challenge of complexity in health care. BMJ. 2001;323(7313):625-628. doi:10.1136/bmj.323.7313.625
Kouchaki S, Ding X, Sanei S. AI- and IoT-enabled solutions for healthcare. Sensors. 2024;24(8):2607. doi:10.3390/s24082607
Saab K, Tu T, Weng WH, et al. Capabilities of Gemini Models in Medicine. arXiv. doi:10.48550/arXiv.2404.18416
Villar SS, Bowden J, Wason J. Multi-armed bandit models for the optimal design of clinical trials: benefits and challenges. Stat Sci. 2015;30(2):199-215. doi:10.1214/14-STS504
Auth0. What is OAuth 2.0. Accessed April 7, 2025. https://auth0.com/intro-to-iam/what-is-oauth-2
HL7. Welcome to FHIR. Updated March 26, 2025. Accessed April 7, 2025. https://www.hl7.org/fhir/
SNOMED International. Accessed April 7, 2025. https://www.snomed.org
Hasselgren A, Kralevska K, Gligoroski D, Pedersen SA, Faxvaag A. Blockchain in healthcare and health sciences—a scoping review. Int J Med Inf. 2020;134:104040. doi:10.1016/j.ijmedinf.2019.104040
Ribeiro MT, Singh S, Guestrin C. “Why Should I Trust You?”: Explaining the predictions of any classifier. In: Proceedings of the 22nd ACM SIGKDD international conference on knowledge discovery and data mining. 2016:1135-1144. doi:10.1145/2939672.2939778
Ekanayake IU, Meddage DPP, Rathnayake U. A novel approach to explain the black-box nature of machine learning in compressive strength predictions of concrete using Shapley additive explanations (SHAP). Case Stud Constr Mater. 2022;16:e01059. doi:10.1016/j.cscm.2022.e01059
Alabi RO, Elmusrati M, Leivo I, Almangush A, Mäkitie AA. Machine learning explainability in nasopharyngeal cancer survival using LIME and SHAP. Sci Rep. 2023;13(1):8984. doi:10.1038/s41598-023-35795-0
Otto E, Culakova E, Meng S, et al. Overview of sankey flow diagrams: focusing on symptom trajectories in older adults with advanced cancer. J Geriatr Oncol. 2022;13(5):742-746. doi:10.1016/j.jgo.2021.12.017
Fereidooni H, Marchal S, Miettinen M, et al. SAFELearn: secure aggregation for private federated learning. In: 2021 IEEE security and privacy workshops (SPW). 2021:56-62. doi:10.1109/SPW53761.2021.00017
Linton DL, Pangle WM, Wyatt KH, Powell KN, Sherwood RE. Identifying key features of effective active learning: the effects of writing and peer discussion. Life Sci Educ. 2014;13(3):469-477. doi:10.1187/cbe.13-12-0242
Yang HS. Machine learning for sepsis prediction: prospects and challenges. Clin Chem. 2024;70(3):465-467. doi:10.1093/clinchem/hvae006
Liao J, Li X, Gan Y, et al. Artificial intelligence assists precision medicine in cancer treatment. Front Oncol. 2023;12. doi:10.3389/fonc.2022.998222
Tierney AA, Gayre G, Hoberman B, et al. Ambient artificial intelligence scribes to alleviate the burden of clinical documentation. NEJM Catal. 2024;5(3):CAT.23.0404. doi:10.1056/CAT.23.0404
Borkowski AA, Jakey CE, Thomas LB, Viswanadhan N, Mastorides SM. Establishing a hospital artificial intelligence committee to improve patient care. Fed Pract. 2022;39(8):334-336. doi:10.12788/fp.0299
Isaacks DB, Borkowski AA. Implementing trustworthy AI in VA high reliability health care organizations. Fed Pract.2024;41(2):40-43. doi:10.12788/fp.0454
Han R, Acosta JN, Shakeri Z, Ioannidis JPA, Topol EJ, Rajpurkar P. Randomized controlled trials evaluating artificial intelligence in clinical practice: a scoping review. Lancet Digit Health. 2024;6(5):e367-e373. doi:10.1016/S2589-7500(24)00047-5
Blue Subcutaneous Nodules in a Young Service Member
Blue Subcutaneous Nodules in a Young Service Member
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
DISCUSSION
A diagnosis of familial glomangiomatosis was made based on the clinical history and histopathologic findings from the punch biopsy. Glomus tumors are comprised of glomus cells, or undifferentiated smooth muscle cells responsible for thermoregulation.1 Glomus tumors are classified into 3 categories: solid (predominantly glomus cells), glomangiomas (predominantly blood vessels), and glomangiomyomas (predominantly smooth muscle cells).2 Glomangiomas, which comprise up to 20% of glomus tumors, typically present as bluish-purple, papular or nodular, hyperkeratotic lesions that are 2 to 10 mm in diameter.1 These lesions are tender to palpation and pain may worsen with exposure to cold. Glomangiomas are associated with a classic triad of symptoms that include hypersensitivity, intermittent pain, and pinpoint pain, but patients rarely present with all 3.3
Glomangiomas tend to occur in areas rich with glomus bodies—the distal extremities—specifically the palms, wrists, forearms, feet, and subungual regions; visceral organ involvement including the GI tract is very rare.1,4,5 About 38% to 68% of these lesions are hereditary or can be sporadic. If these lesions are hereditary, a patient is said to have familial glomangiomatosis. In familial glomangiomatosis, the glomulin gene is mutated in an autosomal dominant inheritance pattern with incomplete penetrance and variable expressivity. Inherited glomangiomas may present at birth or puberty similar to other vascular anomalies.4
Histopathology of glomangiomas shows rows of glomus cells (modified smooth muscle cells) surrounding distorted venous channels.6,7 These lesions stain positive for CD34, vimentin, calponin, and α-smooth muscle actin, but are negative for desmin, S-100, and von Willebrand factor.1,8 Although the patient’s medical history and physical examination are important in establishing the diagnosis, histopathology is confirmatory.
While the punch biopsy results were pending, a complete blood count (CBC) and fecal occult blood test (FOBT) were ordered due to concerns for blue rubber bleb nevus syndrome (BRBNS), a rare disorder with about 200 reported cases. Patients present with multiple blue to violaceous compressible nodules that feel rubbery in consistency and may be painful with compression. Lesions may be up to 5 cm in diameter and with time, the GI tract may also become involved.9 In the GI tract, the small bowel is the most common site of involvement and patients may present with severe iron deficiency anemia due to hemorrhage.10 Histopathologic features are nonspecific and have features of venous malformations but may include large, tortuous, dilated vessels with a single endothelial lining with possible smooth muscle in vessel walls or calcifications.11 Due to concerns of BRBNS, laboratory studies (CBC and FOBT) were obtained but did not indicate the patient was experiencing a GI hemorrhage.
The differential diagnosis included Maffucci syndrome, also known as dyschondrodysplasia with hemangiomas, enchondromatosis with cavernous hemangiomas, or hemangiomatosis chondrodystrophic. Patients with Maffucci syndrome present with multiple enchondromas, soft tissue hemangiomas or lymphangiomas, and gliomas. These lesions tend to undergo malignant transformation from enchondromas to chondrosarcomas and hemangiomas to vascular sarcomas.12 This diagnosis was less likely in the patient in this case as there were no concerns of skeletal involvement upon history and physical examination.
Lastly, Klippel-Trénaunay syndrome can be associated with similar cutaneous vascular manifestations.13,14 This syndrome occurs due to somatic mutations altering angiogenesis during embryological development. This results in varicosities of superficial and deep venous systems, persistent embryonic veins, and valvular incompetence. However, these patients typically have capillary manifestations such as a flat, red, or purple port-wine stain present at birth and associated limb hypertrophy predominantly affecting a single lower limb.15,16 The patient reported not having the lesions present at birth and because bilateral upper/lower extremity and trunk involvement is rare in this syndrome, a Klippel-Trénaunay syndrome diagnosis was unlikely even in the absence of biopsy results.
Treatment
Based on pathology results, the patient was diagnosed with familial glomangiomatosis and a discussion of treatment options ensued. Asymptomatic lesions can be periodically managed. In addition, there are several treatments for symptomatic lesions. Symptomatic lesions may be tender to palpation and or hypersensitive to temperature change (cold). Though they exhibit slow growth, they can invade surrounding tissues including nerve sheaths which can worsen pain.
Surgical resection, sclerotherapy, laser therapy, and electron beam radiation have been used on patients with symptomatic lesions.8,17 Sclerotherapy involves introducing sterile solutions into a blood vessel’s lumen or into the vascular lesion itself to induce permanent endofibrosis and ablation.17 Hypertonic saline, sodium tetradecyl sulfate (STS), and absolute alcohol have been used to treat vascular anomalies as well as glomangiomas.17 Though case reports have noticed significant improvement in symptomatic lesions, sclerotherapy has been shown to be more effective in treating venous malformations than glomangiomas.18,19
A long-pulsed 1064-nm neodymium-doped yttrium aluminum garnet (Nd:YAG) laser has also been effective in treating larger glomangiomas that would otherwise be difficult to excise.20 The Nd:YAG laser has successfully treated lesions in patients with familial glomangiomatosis.21,22
Our patient opted for sclerotherapy with STS on symptomatic lesions of the bilateral upper extremities and trunk. The patient reported moderate improvement of some lesions at a 4-week follow-up appointment and sclerotherapy with STS was repeated.
It is important to note that if a glomangioma is fully excised, the prognosis is favorable; however, recurrence after surgical excision is seen in 10% to 33% of cases.23,24 Our patient had symptomatic lesions excised on the face, but they recurred. Glomangiomas confer a low risk of malignancy but some risk factors include lesions > 2 cm in size, deep lesions, muscle and/or bone invasion, and high mitotic activity.17,25 If left untreated, high-risk glomangiomas can potentially be life-threatening due to growth, bleeding, or vital organ obstruction.26
Primary Care Role
This patient was referred by his PCP assuming that these were symptomatic vascular lesions or telangiectasias (spider veins). Glomus cell tumors are classified as neurovascular neoplasms which may appear similar to vascular malformations or hemangiomas. 27 PCPs serve an important role in performing cutaneous biopsies to increase patient access to dermatologic care, increase patient awareness of skin conditions including skin cancer, and to potentially diagnose a malignant lesion.28 However, the PCP ultimately referred the patient to dermatology due to the number of growing, painful lesions. If the patient had a single lesion, it may have been appropriate to biopsy for diagnostic clarity.
A retrospective review found that the clinical diagnosis of glomus tumor showed concordance with histopathological diagnosis in 45.4% of cases. The most common alternate histopathological diagnoses were vascular tumors (25.9%) followed by other skin or soft tissue tumors like neuromas, leiomyomas, lipomas, or nevi.29 Even if the PCP performed an initial biopsy with high clinical suspicion of a vascular malformation, some glomus cell tumors may be vascular tumors and vice versa.
Though the patient’s history was consistent with the classic triad of glomangiomas including hypersensitivity, intermittent pain, and pinpoint pain, histopathology was necessary to confirm the diagnosis. Given that these appeared to be similar to telangiectasias to the PCP, a rare condition like BRBNS was likely not considered upon initial presentation. Furthermore, the patient had a negative review of systems to include GI symptoms like melena or hematochezia. The PCP had no concern of GI hemorrhage as these lesions can involve the GI tract. If the patient were to endorse additional symptoms, a CBC to evaluate for anemia as well as a GI referral would be warranted.
CONCLUSIONS
This case exhibits the importance of differentiating glomus cell tumors from other more common vascular anomalies via a patient’s history and histopathological findings. Diagnosis and treatment may be difficult depending on the extent of lesions.
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
- Brouillard P, Boon LM, Mulliken JB, et al. Mutations in a novel factor, glomulin, are responsible for glomuvenous malformations (“glomangiomas”). Am J Hum Genet. 2002;70(4):866- 874. doi:10.1086/339492
- Chatterjee JS, Youssef AH, Brown RM, Nishikawa H. Congenital nodular multiple glomangioma: a case report. J Clin Pathol. 2005;58(1):102-103. doi:10.1136/jcp.2003.014324
- Larsen DK, Madsen PV. Ugeskr Laeger. 2018;180(30):V10170807.
- Boon LM, Brouillard P, Irrthum A, et al. A gene for inherited cutaneous venous anomalies (“glomangiomas”) localizes to chromosome 1p21-22. Am J Hum Genet. 1999;65(1):125-133. doi:10.1086/302450
- Tewattanarat N, Srinakarin J, Wongwiwatchai J, et al. Imaging of a glomus tumor of the liver in a child. Radiol Case Rep. 2020;15(4):311-315. doi:10.1016/j.radcr.2019.12.014
- Bolognia J, Schaffer JV, Cerroni L. Dermatology. 5th ed. Elsevier; 2024.
- Elston D, Ferringer T, Ko CJ, Peckham S, High WA, DiCaudo DJ. Dermatopathology. 3rd ed. Elsevier; 2018.
- Leger M, Patel U, Mandal R, et al. Glomangioma. Dermatol Online J. 2010;16(11):11.
- Jin XL, Wang ZH, Xiao XB, Huang LS, Zhao XY. Blue rub ber bleb nevus syndrome: a case report and literature review. World J Gastroenterol. 2014;20(45):17254-17259. doi:10.3748/wjg.v20.i45.17254
- Aravindan U, Ganesan R, Thamarai Kannan M. Surgery for blue rubber bleb nevus syndrome-a case report. Indian J Surg. 2018;80(3):272-274. doi:10.1007/s12262-017-1715-y
- Dobru D, Seuchea N, Dorin M, Careianu V. Blue rubber bleb nevus syndrome: case report and literature review. Rom J Gastroenterol. 2004;13(3):237-240.
- Prokopchuk O, Andres S, Becker K, Holzapfel K, Hartmann D, Friess H. Maffucci syndrome and neoplasms: a case report and review of the literature. BMC Res Notes. 2016;9:126. doi:10.1186/s13104-016-1913-x
- Wang SK, Drucker NA, Gupta AK, Marshalleck FE, Dalsing MC. Diagnosis and management of the venous malformations of Klippel-Trénaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2017;5(4):587-595. doi:10.1016/j.jvsv.2016.10.084
- Yamaki T, Konoeda H, Fujisawa D, et al. Prevalence of various congenital vascular malformations in patients with Klippel- Trenaunay syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(2):187-193. doi:10.1016/j.jvsv.2012.07.010
- Alwalid O, Makamure J, Cheng QG, et al. Radiological aspect of Klippel-Trénaunay Syndrome: a case series with review of literature. Curr Med Sci. 2018;38(5):925-931. doi:10.1007/s11596-018-1964-4
- Sung HM, Chung HY, Lee SJ, et al. Clinical experience of the Klippel-Trenaunay Syndrome. Arch Plast Surg. Sep 2015;42(5):552-558. doi:10.5999/aps.2015.42.5.552
- Jha A, Khunger N, Malarvizhi K, Ramesh V, Singh A. Familial disseminated cutaneous glomuvenous malformation: treatment with polidocanol sclerotherapy. J Cutan Aesthet Surg. 2016;9(4):266-269. doi:10.4103/0974-2077.197083
- Enjolras O, Ciabrini D, Mazoyer E, Laurian C, Herbreteau D. Extensive pure venous malformations in the upper or lower limb: a review of 27 cases. J Am Acad Dermatol. 1997;36(2 Pt 1):219-225. doi:10.1016/s0190-9622(97)70284-6
- Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: complications and results. Plast Reconstr Surg. 1999;104(1):1-15.
- Rivers JK, Rivers CA, Li MK, Martinka M. Laser therapy for an acquired glomuvenous malformation (glomus tumour): a nonsurgical approach. J Cutan Med Surg. 2016;20(1):80-183. doi:10.1177/1203475415596121
- Phillips CB, Guerrero C, Theos A. Nd:YAG laser offers promising treatment option for familial glomuvenous malformation. Dermatol Online J. 2015;21(4).
- Jha A, Ramesh V, Singh A. Disseminated cutaneous glomuvenous malformation. Indian J Dermatol Venereol Leprol. 2014;80(6):556-558. doi:10.4103/0378-6323.144200
- Gonçalves R, Lopes A, Júlio C, Durão C, de Mello RA. Knee glomangioma: a rare location for a glomus tumor. Rare Tumors. 2014;6(4):5588. doi:10.4081/rt.2014.5588
- Cabral CR, Oliveira Filho J, Matsumoto JL, Cignachi S, Tebet AC, Nasser KaR. Type 2 segmental glomangioma- -Case report. An Bras Dermatol. 2015;90(3 Suppl 1):97-100. doi:10.1590/abd1806-4841.20152483
- Tony G, Hauxwell S, Nair N, Harrison DA, Richards PJ. Large plaque-like glomangioma in a patient with multiple glomus tumours: review of imaging and histology. Clin Exp Dermatol. 2013;38(7):693-700. doi:10.1111/ced.12122
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971-976. doi:10.1001/archderm.140.8.971
- Honsawek S, Kitidumrongsook P, Luangjarmekorn P, Pataradool K, Thanakit V, Patradul A. Glomus tumors of the fingers: Expression of vascular endothelial growth factor. World J Orthop. 2016;7(12):843-846. doi:10.5312/wjo.v7.i12.843
- Jones TP, Boiko PE, Piepkorn MW. Skin biopsy indications in primary care practice: a population-based study. J Am Board Fam Pract. 1996;9(6):397-404.
- Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23(3):181-188. doi:10.1177/1066896914567330
Blue Subcutaneous Nodules in a Young Service Member
Blue Subcutaneous Nodules in a Young Service Member
A 26-year-old male with Fitzpatrick skin type II presented for evaluation in the dermatology clinic after being referred by his primary care practitioner (PCP) with a complaint of spider veins. The patient reported a lifelong history of blue subcutaneous nodules that initially appeared on his face during childhood but have since involved his trunk and upper and lower extremities. The patient reported that some of the nodules were painful and increased in size with exercise. His medical history was unremarkable with no other chronic conditions or daily medication use. The patient reported no gastrointestinal (GI) symptoms, melena, or hematochezia. The patient’s mother had similar nodules but his 7 siblings did not.
Upon physical examination, numerous blue subcutaneous nodules, 2 to 8 mm in size, were scattered across his trunk, and proximal and distal extremities were present (Figure 1). The physical examination was otherwise unremarkable. Upon discussing differential diagnosis of these lesions with the patient, he was amenable to a punch biopsy for further diagnostic clarity (Figure 2).
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).
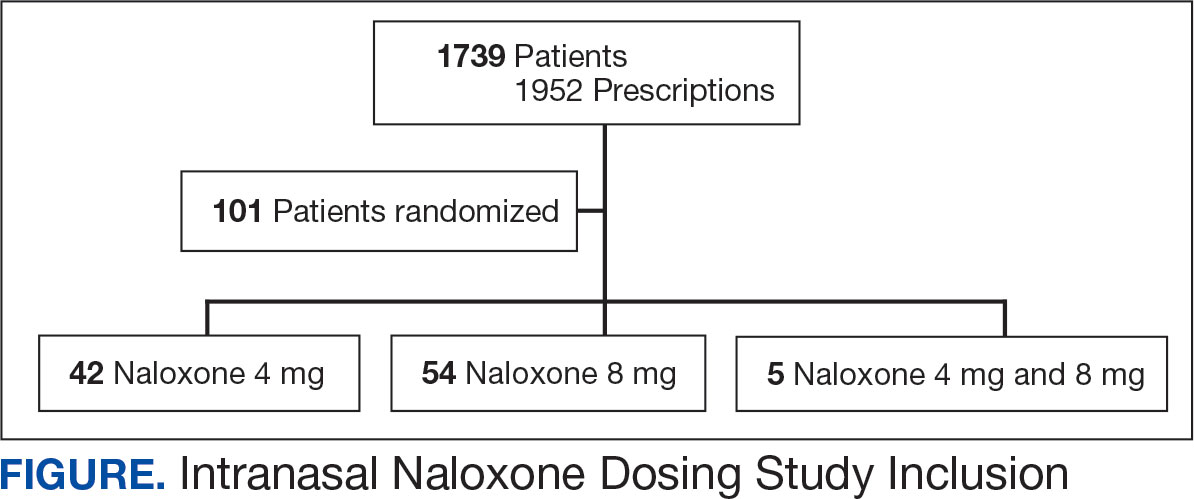
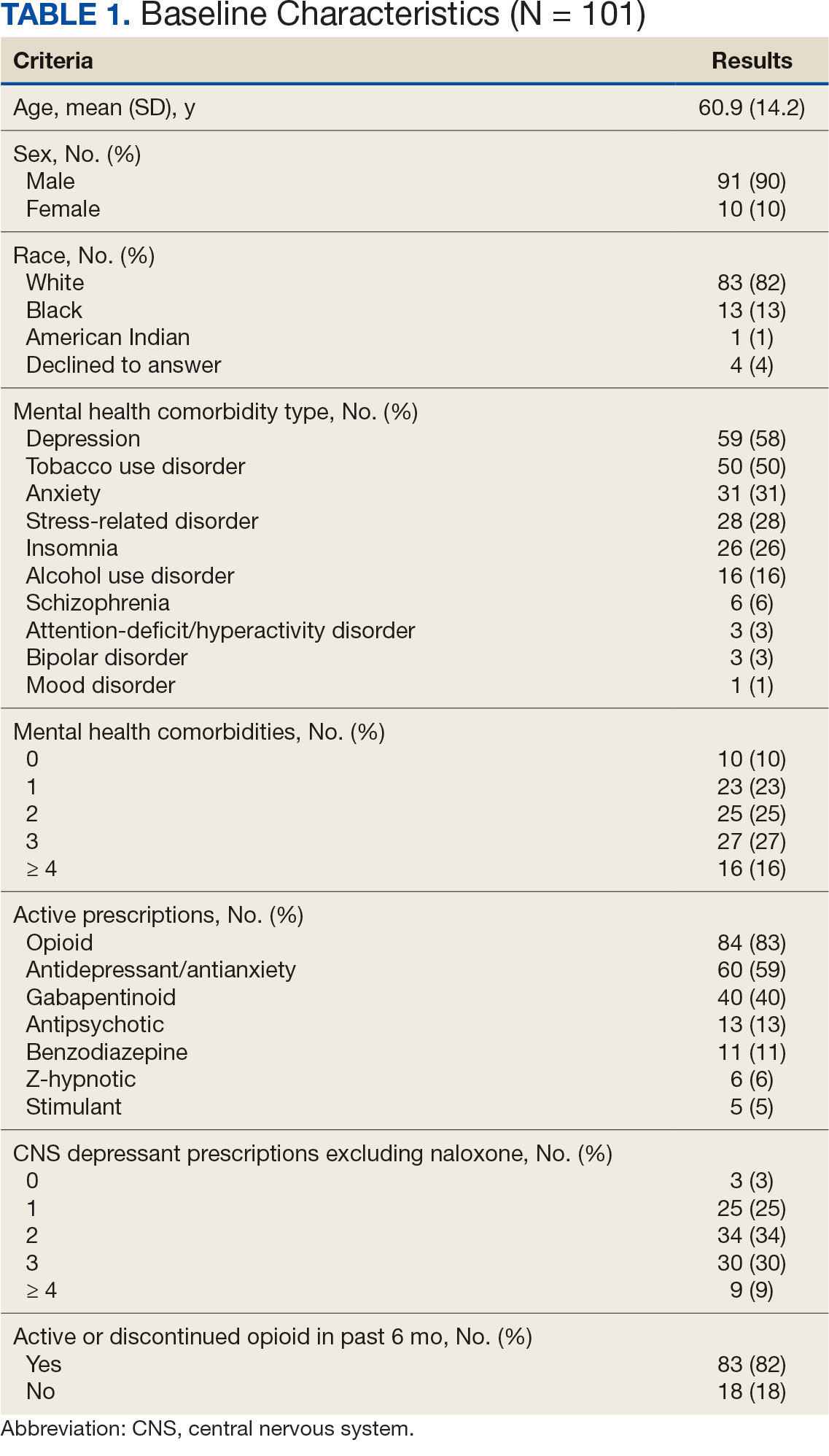
The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).
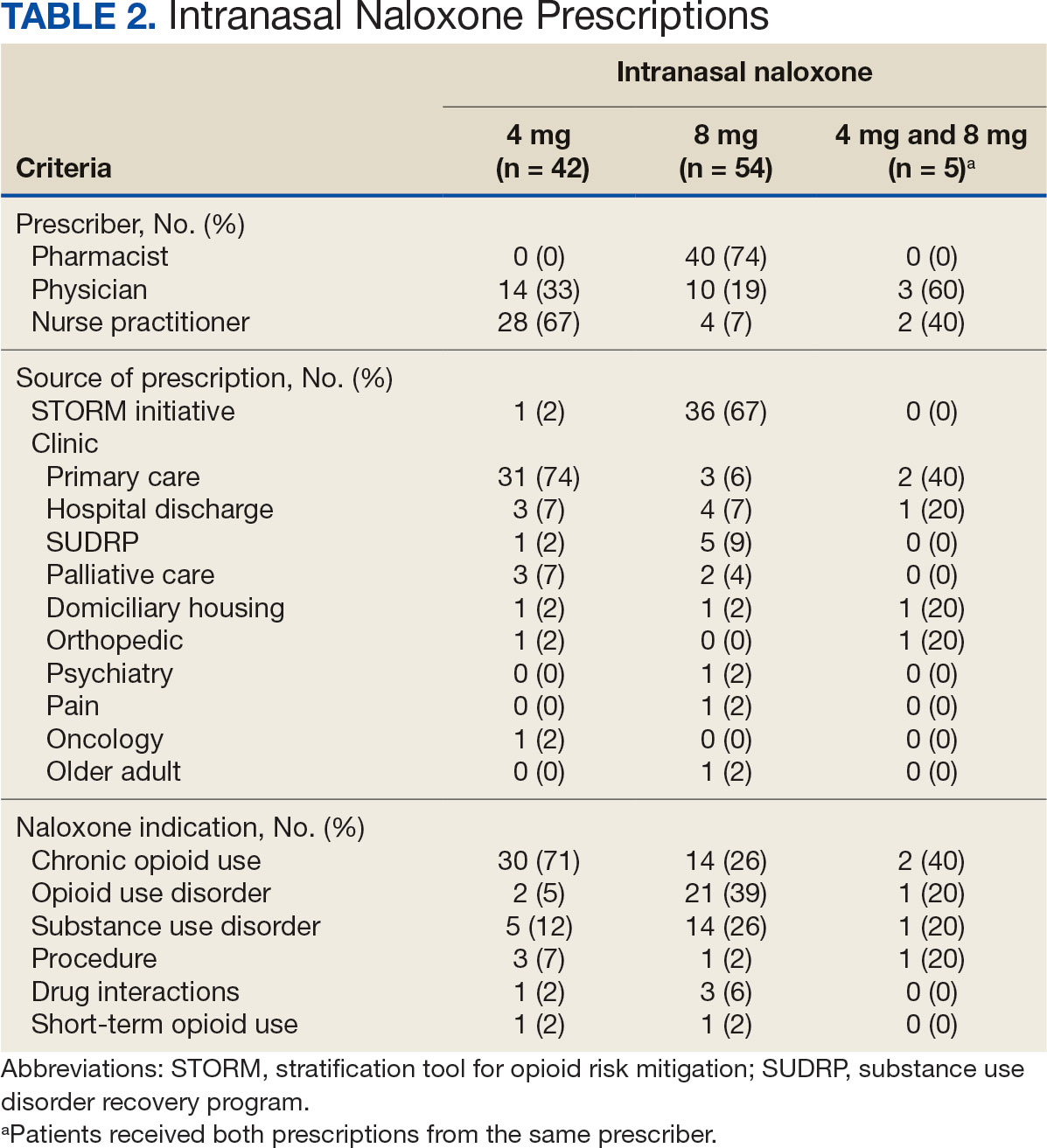
Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).


The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).

Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
Since 1999, annual deaths attributed to opioid overdose in the United States have increased from about 10,000 to about 50,000 in 2019.1 During the COVID-19 pandemic > 74,000 opioid overdose deaths occurred in the US from April 2020 to April 2021.2,3 Opioid-related overdoses now account for about 75% of all drug-related overdose deaths.1 In 2017, the cost of opioid overdose deaths and opioid use disorder (OUD) reached $1.02 trillion in the United States and $26 million in Indiana.4 The total deaths and costs would likely be higher if it were not for naloxone.
Naloxone hydrochloride was first patented in the 1960s and approved by the US Food and Drug Administration (FDA) in 1971 to treat opioid-related toxicity.1 It is the most frequently prescribed antidote for opioid toxicity due to its activity as a pure υ-opioid receptor competitive antagonist. Naloxone formulations include intramuscular, intravenous, subcutaneous, and intranasal delivery methods.5 According to the Centers for Disease Control and Prevention, clinicians should offer naloxone to patients at high risk for opioid-related adverse events. Risk factors include a history of overdose, opioid dosages of ≥ 50 morphine mg equivalents/day, and concurrent use of opioids with benzodiazepines.6
Intranasal naloxone 4 mg has become more accessible following the classification of opioid use as a public health emergency in 2017 and its over-the-counter availability since 2023. Intranasal naloxone 4 mg was approved by the FDA in 2015 for the prevention of opioid overdoses (accidental or intentional), which can be caused by heroin, fentanyl, carfentanil, hydrocodone, oxycodone, methadone, and other substances. 7 Fentanyl has most recently been associated with xylazine, a nonopioid tranquilizer linked to increased opioid overdose deaths.8 Recent data suggest that 34% of opioid overdose reversals involved ≥ 2 doses of intranasal naloxone 4 mg, which led to FDA approval of an intranasal naloxone 8 mg spray in April 2021.9-11
Veteran Health Indiana (VHI) has implemented several initiatives to promote naloxone prescribing. Established in 2020, the Opioid Overdose Education and Naloxone Distribution (OEND) program sought to prevent opioid-related deaths through education and product distribution. These criteria included an opioid prescription for ≥ 30 days. In 2021, the Stratification Tool for Opioid Risk Mitigation (STORM) was created to identify patients at high risk of opioid overdose and allowing pharmacists to prescribe naloxone for at-risk patients without restrictions, increasing accessibility.12
Recent cases of fentanyl-related overdoses involving stronger fentanyl analogues highlight the need for higher naloxone dosing to prevent overdose. A pharmacokinetic comparison of intranasal naloxone 8 mg vs 4 mg demonstrated maximum plasma concentrations of 10.3 ng/mL and 5.3 ng/mL, respectively. 13 Patients may be at an increased risk of precipitated opioid withdrawal when using intranasal naloxone 8 mg over 4 mg; however, some patients may benefit from achieving higher serum concentrations and therefore require larger doses of naloxone.
No clinical trials have demonstrated a difference in reversal rates between naloxone doses. No clinical practice guidelines support a specific naloxone formulation, and limited US Department of Veterans Affairs (VA)-specific guidance exists. VA Naloxone Rescue: Recommendations for Use states that selection of naloxone 8 mg should be based on shared decision-making between the patient and clinician and based on individual risk factors.12 The purpose of this study is to analyze data to determine if there is a difference in prescribing patterns of intranasal naloxone 4 mg and intranasal naloxone 8 mg.
METHODS
A retrospective chart reviews using the VA Computerized Patient Record System (CPRS) analyzed patients prescribed intranasal naloxone 4 mg or intranasal naloxone 8 mg at VHI. A patient list was generated based on active naloxone prescriptions between April 1, 2022, and April 1, 2023. Data were obtained exclusively through CPRS and patients were not contacted. This study was reviewed and deemed exempt by the Indiana University Health Institutional Review Board and the VHI Research and Development Committee.
Patients were included if they were aged ≥ 18 years and had an active prescription for intranasal naloxone 4 mg or intranasal naloxone 8 mg during the trial period. Patients were excluded if their naloxone prescription was written by a non-VHI clinician, if the dose was not 4 mg or 8 mg, or if the dosage form was other than intranasal spray.
The primary endpoint was the comparison for prescribing patterns for intranasal naloxone 4 mg and intranasal naloxone 8 mg during the study period. Secondary endpoints included total naloxone prescriptions; monthly prescriptions; number of patients with repeated naloxone prescriptions; prescriber type by naloxone dose; clinic type by naloxone dose; and documented indication for naloxone use by dose.
Demographic data collected included baseline age, sex, race, comorbid mental health conditions, and active central nervous system depressant medications on patient profile (ie, opioids, gabapentinoids, benzodiazepines, antidepressants, antipsychotics). Opioid prescriptions that were active or discontinued within the last 3 months were also recorded. Comorbid mental health conditions were collected based on the most recent clinical note before initiating medication.
Prescription-related data included strength of medication prescribed (4 mg, 8 mg, or both), documented use of medication, prescriber name, prescriber discipline, prescription entered by, number of times naloxone was filled or refilled during the study period, indication, clinic location, and clinic name. If > 1 prescription was active during the study period, the number of refills, prescriber name and clinic location of the first prescription in the study period was recorded. Additionally, the indication of OUD was differentiated from substance use disorder (SUD) if the patient was only dependent on opioids, excluding tobacco or alcohol. Patients with SUDs may include opioid dependence in addition to other substance dependence (eg, cannabis, stimulants, gabapentinoids, or benzodiazepines).
Basic descriptive statistics, including mean, ranges, and percentages were used to characterize the study subjects. For nominal data, X2 tests were used. A 2-sided 5% significance level was used for all statistical tests.
RESULTS
A total of 1952 active naloxone prescriptions from 1739 patients met the inclusion criteria; none were eliminated based on the exclusion criteria and some were included multiple times because data were collected for each active prescription during the study period. One hundred one patients were randomized and included in the final analysis (Figure). Most patients identified as White (81%), male (90%), and had a mean (SD) age of 60.9 (14.2) years. Common mental health comorbidities included 59 patients with depression, 50 with tobacco use disorder, and 31 with anxiety. Eighty-four patients had opioid and 60 had antidepressants/antianxiety, and 40 had gabapentinoids prescriptions. Forty-three patients had ≥ 3 mental health comorbidities. Thirty-four patients had 2 active central nervous system depressant prescriptions, 30 had 3 active prescriptions, and 9 had ≥ 4 active prescriptions. Most patients (n = 83) had an active or recently discontinued opioid prescription (Table 1).


The 101 patients received 54 prescriptions for naloxone 8 mg and 47 for 4 mg (Table 2). Five patients received prescriptions for both the 4 mg and 8 mg intranasal naloxone formulations. Sixty-six patients had naloxone filled once (66%) during the study period. Intranasal naloxone 4 mg was prescribed to 30 patients by nurse practitioners, 17 patients by physicians, and not prescribed by pharmacists. Intranasal naloxone 8 mg was prescribed to 40 patients by pharmacists, 13 patients by physicians, and 6 patients by nurses. Patients who received prescriptions for both intranasal naloxone 4 mg and 8 mg were most routinely ordered by physicians (n = 3; 60%) in primary care (n = 2; 40%) for chronic opioid use (n = 2; 40%).

Patients access naloxone from many different VHI clinics. Primary care clinics prescribed the 4 mg formulation to 31 patients, 8 mg to 3 patients, and both to 2 patients. The STORM initiative was used for 37 of 106 prescriptions (35%): 4 mg intranasal naloxone was prescribed to 1 patient, 8 mg to 36 patients, and no patients received both formulations. Chronic opioid use was the most common indication (46%) with 30 patients prescribed intranasal naloxone 4 mg, 14 patients prescribed 8 mg, and 2 patients prescribed both. OUD was the indication for 24% of patients: 2 patients prescribed intranasal naloxone 4 mg, 21 patients prescribed 8 mg, and 1 patient prescribed both.
The 106 intranasal naloxone prescriptions were equally distributed across each month from April 1, 2022, to April 1, 2023. Of the 101 patients, 34 had multiple naloxone prescriptions filled during the study period. Pharmacists wrote 40 of 106 naloxone prescriptions (38%), all for the 8 mg formulation. Nurse practitioners prescribed naloxone 4 mg 30 times and 8 mg 6 times for 36 of 106 prescriptions (34%). Physicians prescribed 30 of 106 prescriptions (28%), including intranasal naloxone 4 mg 17 times and 8 mg 13 times.
Statistics were analyzed using a X2 test; however, it was determined that the expected frequencies made the tests inappropriate. Differences in prescribing patterns between naloxone doses, prescriber disciplines, source of the prescription, or indications were not statistically significant.
DISCUSSION
Many pharmacists possess a scope of practice under state law and/or institution policy to prescribe naloxone. In this study, pharmacists prescribed the most naloxone prescriptions compared to physicians and nurse practitioners. Initiatives such as OEND and STORM have given pharmacists at VHI an avenue to combat the growing opioid epidemic while expanding their scope of practice. A systematic review of 67 studies found that pharmacist-led OEND programs showed a statistically significant increase in naloxone orders. A statistical significance was likely met given the large sample sizes ranging from 10 to 217,000 individuals, whereas this study only assessed a small portion of patients.14 This study contributes to the overwhelming amount of data that highlights pharmacists’ impact on overall naloxone distribution.
The STORM initiative and primary care clinics were responsible for large portions of naloxone prescriptions in this study. STORM was used by pharmacists and contributed to more than half of the higher dose naloxone prescriptions. Following a discussion with members of the pain management team, pharmacists involved in STORM prescribing were revealed to exclusively prescribe intranasal naloxone 8 mg as opposed to 4 mg. At the risk of precipitating withdrawal from higher doses of naloxone, it was agreed that this risk was heavily outweighed by the benefit of successful opioid reversal. In this context, it is expected for this avenue of prescribing to influence naloxone prescribing patterns at VHI.
Prescribing in primary care clinics was shown to be equally as substantial. Primary care-based multidisciplinary transition clinics have been reported to be associated with increased access to OUD treatment.15 Primary care clinics at VHI, or patient aligned care teams (PACT), largely consist of multidisciplinary health care teams. PACT clinicians are heavily involved in transitions of care because one system provides patients with comprehensive acute and chronic care. Continuing to encourage naloxone distribution through primary care and using STORM affords various patient populations access to high-level care.
Notable differences were observed between indications for naloxone use and the corresponding dose. Patients with OUD or SUD were more likely to receive intranasal naloxone 8 mg as opposed to patients receiving intranasal naloxone for chronic opioid use, who were more likely to receive the 4 mg dose. This may be due to a rationale to provide a higher dose of naloxone to combat overdoses in the case of ingesting substances mixed with fentanyl or xylazine.12,13 Without standard of care guidelines, concerns remain for varying outcomes in opioid overdose prevention within vulnerable populations.
Limitations
Chart data were dependent on documentation, which may have omitted pertinent baseline characteristics and risk factors. Additional data collection could have further assessed a patient’s specific risk factors (eg, opioid dose in morphine equivalents) to draw conclusions to the dose of naloxone prescribed. The sample size was small, and the patient population was largely White and male, which minimized the generalizability of the results.
CONCLUSIONS
This study evaluated the differences in intranasal naloxone prescribing patterns within a veteran population at VHI over 12 months. Findings revealed that most prescriptions were written for intranasal naloxone 8 mg, by a pharmacist, in a primary care setting, and for chronic opioid use. The results revealed evidence of differing naloxone prescribing practices, which emphasize the need for clinical guidelines and better defined recommendations in relation to naloxone dosing.
The most evident gap in patient care could be addressed by urging the VA Pharmacy Benefits Management group to update naloxone recommendations for use to include more concrete dosing recommendations. Furthermore, it would be beneficial to re-educate clinicians on naloxone prescribing to increase awareness of different doses and the importance of equipping patients with the correct amount of naloxone in an emergency. Additional research assessing change in prescribing patterns is warranted as the use of higher dose naloxone becomes more routine.
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
- Britch SC, Walsh SL. Treatment of opioid overdose: current approaches and recent advances. Psychopharmacology (Berl). 2022;239(7):2063-2081. doi:10.1007/s00213-022-06125-5
- Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional Drug Overdose Death Counts. National Center for Health Statistics, Centers for Disease Control and Prevention; 2023. Accessed April 10, 2025. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls — United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740-1746. doi:10.15585/mmwr.mm7050e3
- Luo F, Li M, Florence C. State-level economic costs of opioid use disorder and fatal opioid overdose — United States, 2017. MMWR Morb Mortal Wkly Rep. 2021;70:541-546. doi:10.15585/mmwr.mm7015a1
- Lexicomp. Lexicomp Online. Accessed April 10, 2025. http://online.lexi.com
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC Clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi:10.15585/mmwr.rr7103a1
- Narcan (naloxone) FDA approval history. Drugs.com. Accessed April 10, 2025. https://www.drugs.com/history/narcan.html
- Centers for Disease Control and Prevention. What you should know about xylazine. May 16, 2024. Accessed April 10, 2025. https://www.cdc.gov/overdose-prevention/about/what-you-should-know-about-xylazine.html
- Avetian GK, Fiuty P, Mazzella S, Koppa D, Heye V, Hebbar P. Use of naloxone nasal spray 4 mg in the community setting: a survey of use by community organizations. Curr Med Res Opin. 2018;34(4):573-576. doi:10.1080/03007995.2017.1334637
- Kloxxado [package insert]. Hikma Pharmaceuticals USA Inc; 2021.
- FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. News release. FDA. April 30, 2021. Accessed April 10, 2025. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose
- US Department of Veterans Affairs, Pharmacy Benefits Management Services and National Formulary Committee in Collaboration with the VA National Harm Reduction Support & Development Workgroup. Naloxone Rescue: Recommendations for Use. June 2014. Updated March 2024. Accessed April 10, 2025. https://www.va.gov/formularyadvisor/DOC_PDF/CRE_Naloxone_Rescue_Guidance_March_2024.pdf
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243-1253. doi:10.1002/jcph.759
- Rawal S, Osae SP, Cobran EK, Albert A, Young HN. Pharmacists’ naloxone services beyond community pharmacy settings: a systematic review. Res Social Adm Pharm. 2023;19(2):243-265. doi:10.1016/j.sapharm.2022.09.002
- Incze MA, Sehgal SL, Hansen A, Garcia L, Stolebarger L. Evaluation of a primary care-based multidisciplinary transition clinic for patients newly initiated on buprenorphine in the emergency department. Subst Abus. 2023;44(3):220-225. doi:10.1177/08897077231188592
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Comparison of Prescribing Patterns of Intranasal Naloxone in a Veteran Population
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
The uselessness of men above sixty years of age and the incalculable benefit it would be in commercial, in political, and in professional life, if as a matter of course, men stopped working at this age.
Sir William Osler1
The first time I remember hearing the word retirement was when I was 5 or 6 years old. My mother told me that my father had been given new orders: either be promoted to general and move to oversee a hospital somewhere far away, or retire from the Army. He was a scholar, teacher, and physician with no interest or aptitude for military politics and health care administration. Reluctantly, he resigned himself to retirement before he had planned. I recall being angry with him, because in my solipsistic child mind he was depriving me of the opportunity to live in a big house across from the parade field, where the generals lived or having a reserved parking spot in front of the post exchange. As a psychiatrist, I suspect that the anger was a primitive defense against the fear of leaving the only home I had ever known on an Army base.
I recently finished reading Michael Bliss’s seminal biography of Sir William Osler (1848-1919), the great Anglo-American physician and medical educator.2 Bliss found few blemishes on Osler’s character or missteps in his stellar career, but one of the few may be his views on retirement. The epigraph is from an address Osler gave before leaving Johns Hopkins for semiretirement in Oxford, England. The farewell speech caused a media controversy with his comments reflecting attitudes that seem ageist today, when many people are active, productive, and happy long past the age of 60 years.3 I do not endorse Osler’s philosophy of aging, nor his exclusion of women (if I did, I would not be around to write this editorial). Not even Osler himself followed his advice: he was active in medicine almost until his death at 70 years old.2
Yet like many of my fellow federal health care practitioners (HCPs), I have been thinking about and planning for retirement earlier than expected, given the memos and directives about voluntary early retirement, deferred resignation, and reductions in force.4,5 The COVID-19 pandemic sadly compelled many burned-out and traumatized HCPs to cross the retirement Rubicon far sooner than they imagined.6
A Google search for information about HCP retirement, particularly among physicians, produces a cascade of advisory articles. They primarily focus on finances, with many pushing their own commercial agenda for retirement planning.7 Although money is a necessary piece of the retirement puzzle, for HCPs it may not be sufficient to ensure a healthy and satisfying retirement. Two other considerations may be even more important to weigh in making the retirement decision, namely timing and meaning.8
For earlier generations of HCPs, work was almost their sole identity. Although younger practitioners are more likely to embrace a better work-life balance, it is still a driving factor for many in the decision to retire.9 It is not just about the cliché of being a workaholic, rather many clinicians continue to enjoy lifelong learning, the rewards of helping people in need, and professional satisfaction. HCPs also spend a longer time training than many other professions; perhaps since we waited so long to practice, we want to stay a little longer.10 For those whose motivation for federal practice was a commitment to service, these may be even more powerful incentives to continue working.
When a nurse, physician, pharmacist, or social worker no longer finds the same gratification and stimulation in their work, whether due to unwelcome changes in the clinical setting or the profession at large, declining health or emotional exhaustion, or the very human need to move onto another phase of life (what Osler likely really meant), then that may be a signal to think hard about retiring. Of course, there have always been—and will continue to be—professionals of all stripes who, even in the most agreeable situation, just cannot wait to retire. Simply because there are so many other ways they want to spend their remaining energy and time: travel, grandchildren, hobbies, even a second career. Because none of us knows how far out our life extends, it is prudent to periodically ask what is the optimal path that combines both purpose and well-being.
All of us as HCPs, and even more as human beings with desires and duties far beyond our respective professions, face a dilemma: a choice between 2 goods that cannot both be fulfilled simultaneously. This is likely why HCPs frequently do what is technically called a phased retirement, a fancy name for working part-time, or retiring from 1 position and taking up another. This temporizes the decision and tempers the bittersweet emotional experience of leaving the profession in one way, and in another, it delays the inevitable.
Over the last few years, I have learned 2 important lessons while watching many of my closest friends retire. First, for those who are still working and those who are retired may seem to inhabit a separate country; hence, special efforts must be made to both appreciate them while they are in our immediate circle of concern and to make efforts to stay in contact once they are emeriti. It is almost as if after being a daily integral aspect of the workplace they have passed into a different dimension of existence. In terms of priorities and mindsets, many of them have. Second, what makes retirement a reality with peace and growth rather than regret and stagnation is owning the decision to retire. There are always constraints: financial, medical, and familial. However, those who retire on their own terms and not primarily in response to fear or uncertainty appear to fare better than those feeling the same pressures who give away their power.11 Having read about retirement in the last months, the best advice I have seen is from Harry Emerson Fosdick, a Protestant minister in the early 20th century: “Don’t simply retire from something; have something to retire to.”12
I have not yet decided about my retirement. Whatever decision you make, remember it is solely yours. After a lifetime of caring for others, retirement is all about caring for yourself.
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
- Osler W. The Fixed Period. In: Osler W, ed. Aequanimitas With Other Addresses to Medical Students, Nurses and Practitioners of Medicine. 3rd ed. The Blakiston Company; 1932:373-393.
- Bliss M. William Osler: A Life in Medicine. Oxford University Press; 1999.
- Anderson M, Scofield RH. The “Fixed period,” the wildfire news, and an unpublished manuscript: Osler’s farewell speech revisited in geographical breadth and emotional depth. Am J Med Sci. Published online February 11, 2025. doi:10.1016/j.amjms.2025.02.005
- Obis A. What federal workers should consider before accepting deferred resignation. Federal News Network. April 8, 2025. Accessed April 25, 2025. https://federalnewsnetwork.com/workforce/2025/04/what-federal-workers-should-consider-before-accepting-deferred-resignation/
- Dyer J. VA exempts clinical staff from OPM deferred resignation program. Federal Practitioner. February 11, 2025. Accessed April 28, 2025. https://www.mdedge.com/content/va-exempts-clinical-staff-opm-deferred-resignation-program
- Shyrock T. Retirement planning secrets for physicians. Medical Economics. 2024;101(8). Accessed April 28, 2025. https:// www.medicaleconomics.com/view/retirement-planningsecrets-for-physicians
- Sinsky CA, Brown RL, Stillman MJ, Linzer M. COVID-related stress and work intentions in a sample of US health care workers. Mayo Clin Proc Innov Qual Outcomes. 2021;5(6):1165-1173. doi:10.1016/j.mayocpiqo.2021.08.007
- Tabloski PA. Life after retirement. American Nurse. March 3, 2022. Accessed April 25, 2025. https://www.myamericannurse.com/life-after-retirement/
- Chen T-P. Young doctors want work-life balance. Older doctors say that’s not the job. The Wall Street Journal. November 3, 2024. Accessed April 25, 2025. https://www.wsj.com/lifestyle/careers/young-doctors-want-work-life-balance-older-doctors-say-thats-not-the-job-6cb37d48
- Sweeny JF. Physician retirement: Why it’s hard for doctors to retire. Medical Economics. 2019;96(4). Accessed April 25, 2025. https://www.medicaleconomics.com/view/physician-retirement-why-its-hard-doctors-retire
- Nelson J. Wisdom for Our Time. W.W. Norton; 1961.
- Silver MP, Hamilton AD, Biswas A, Williams SA. Life after medicine: a systematic review of studies physician’s adjustment to retirement. Arch Community Med Public Health. 2016;2(1):001-007. doi:10.17352/2455-5479.000006
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Should I Stay or Should I Go? Federal Health Care Professional Retirement Dilemmas
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).
VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6
Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).
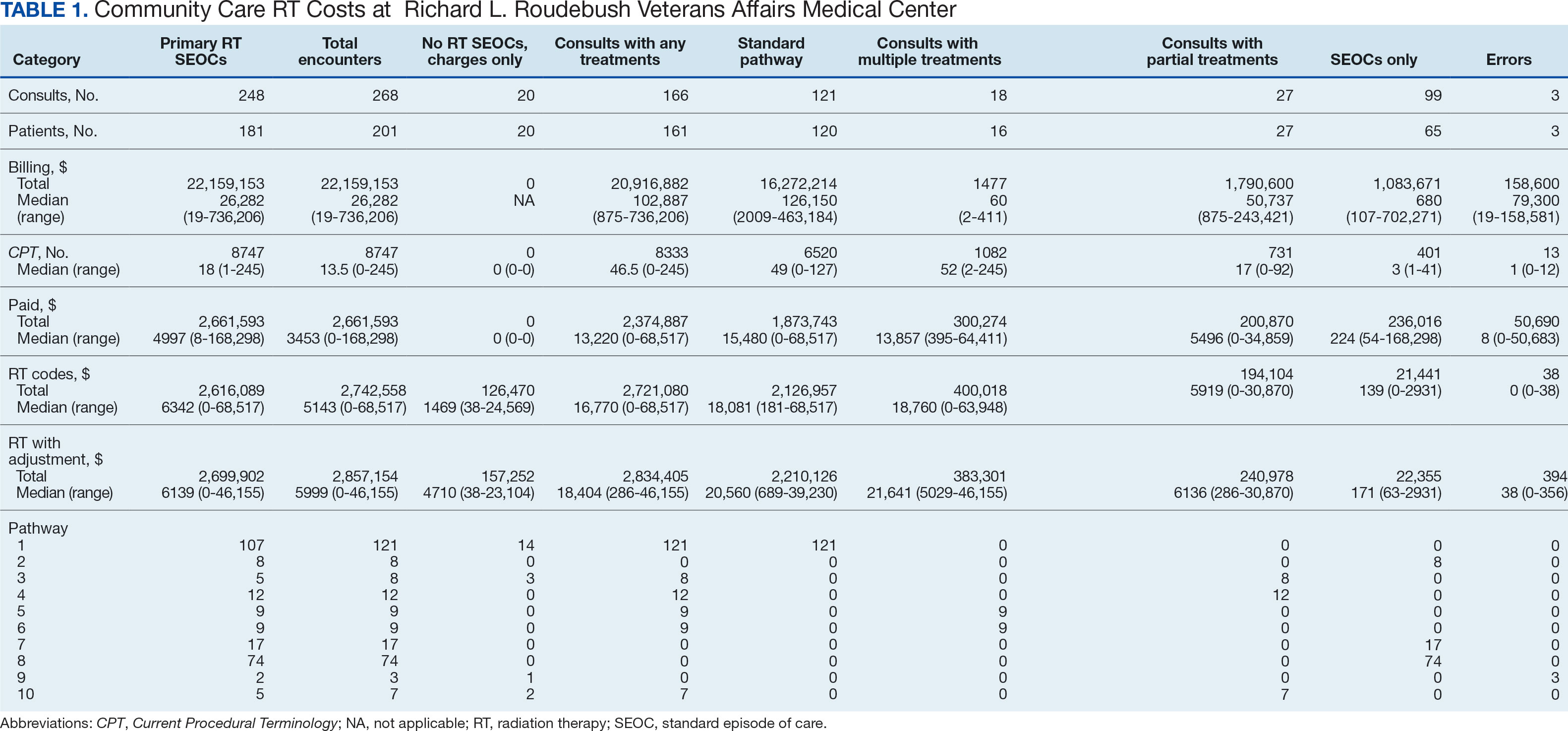
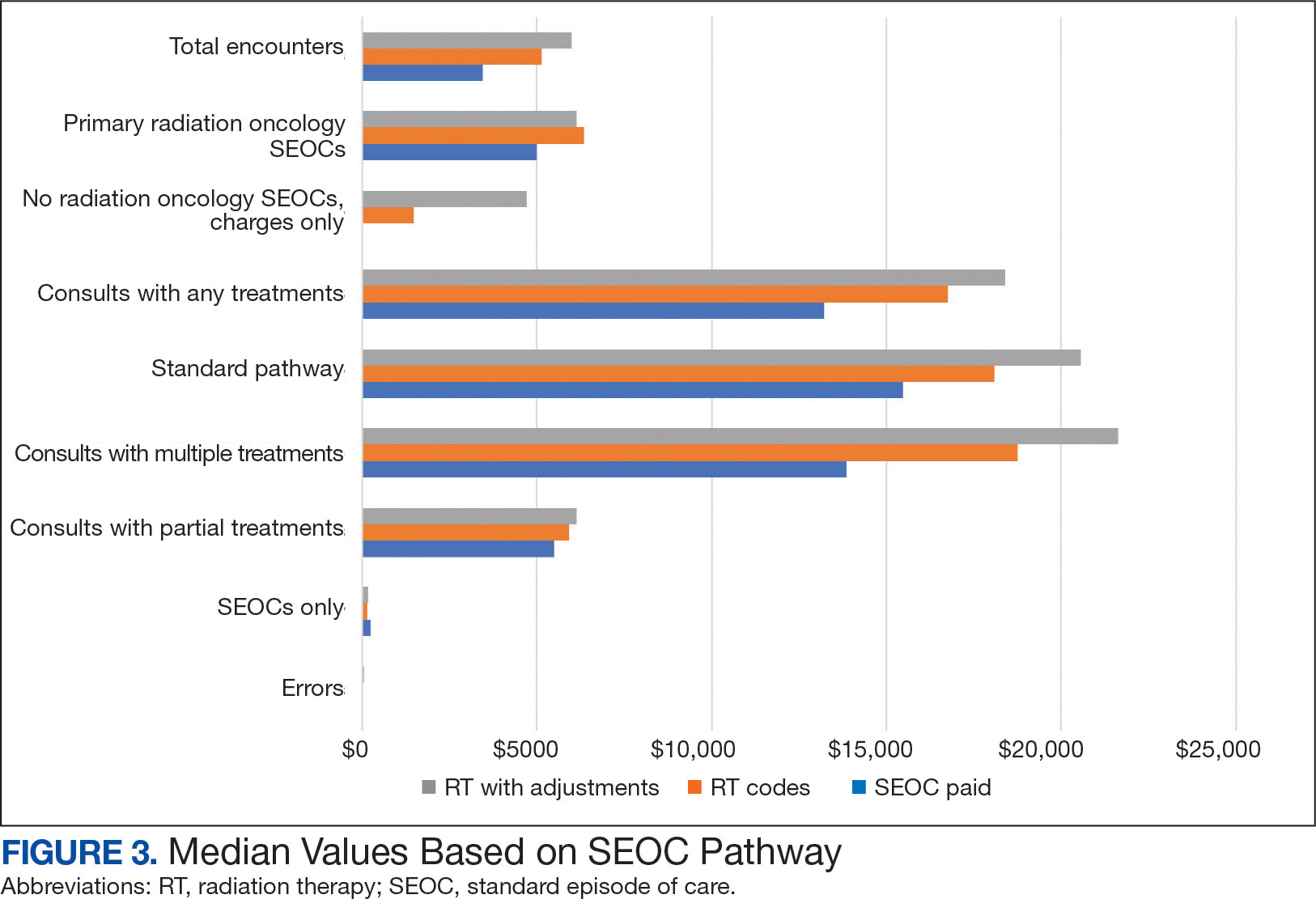
After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).
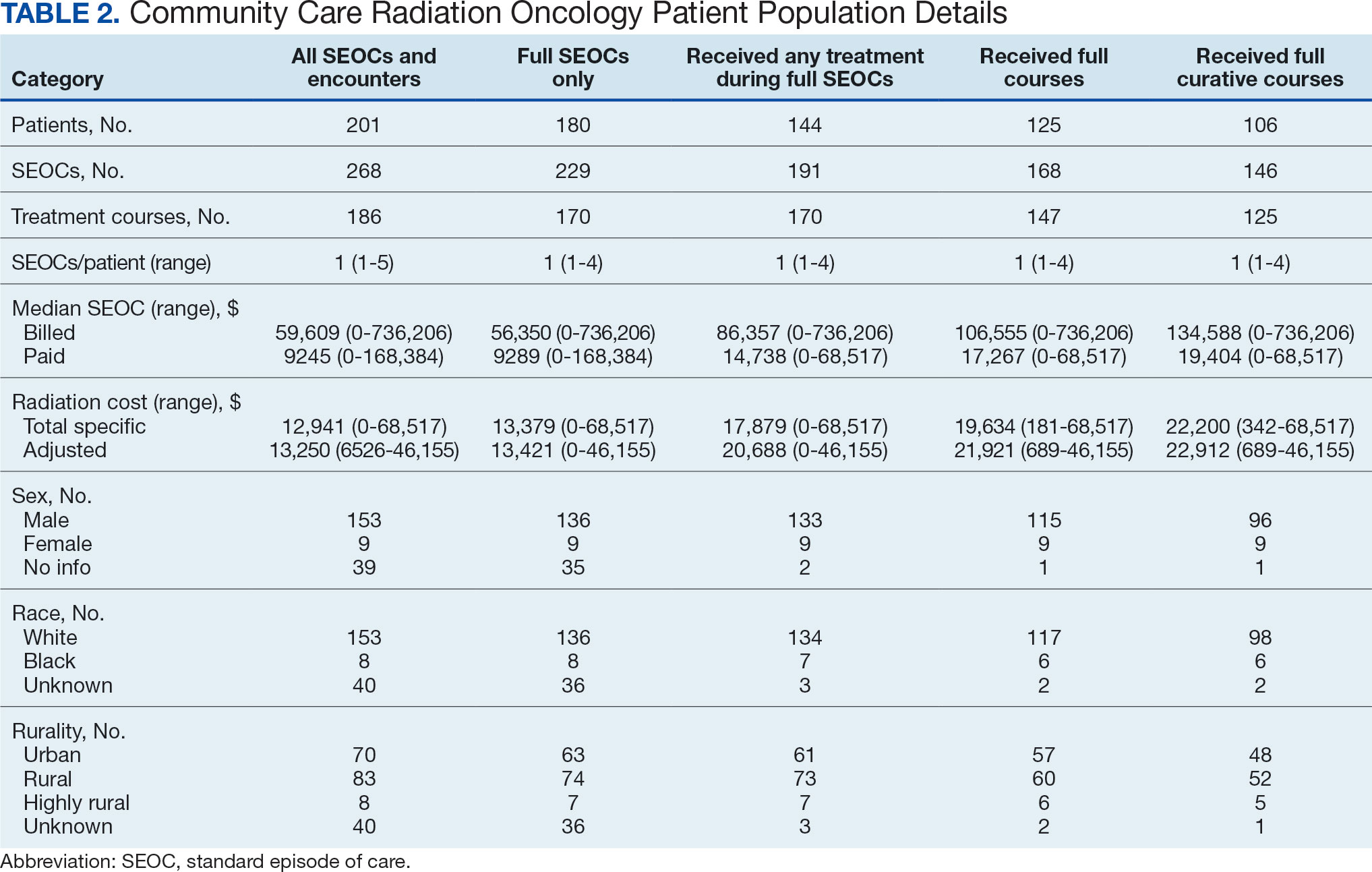
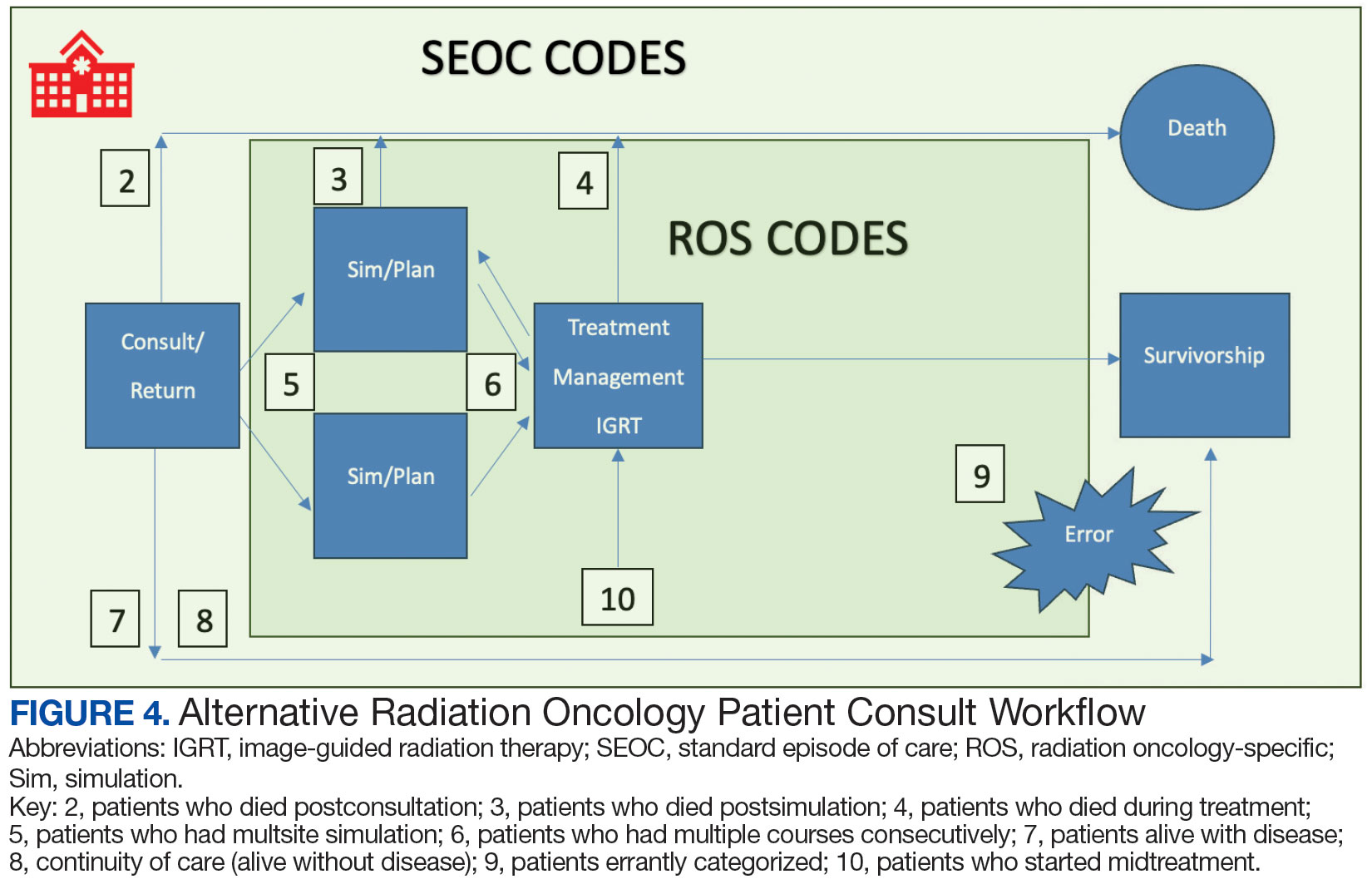
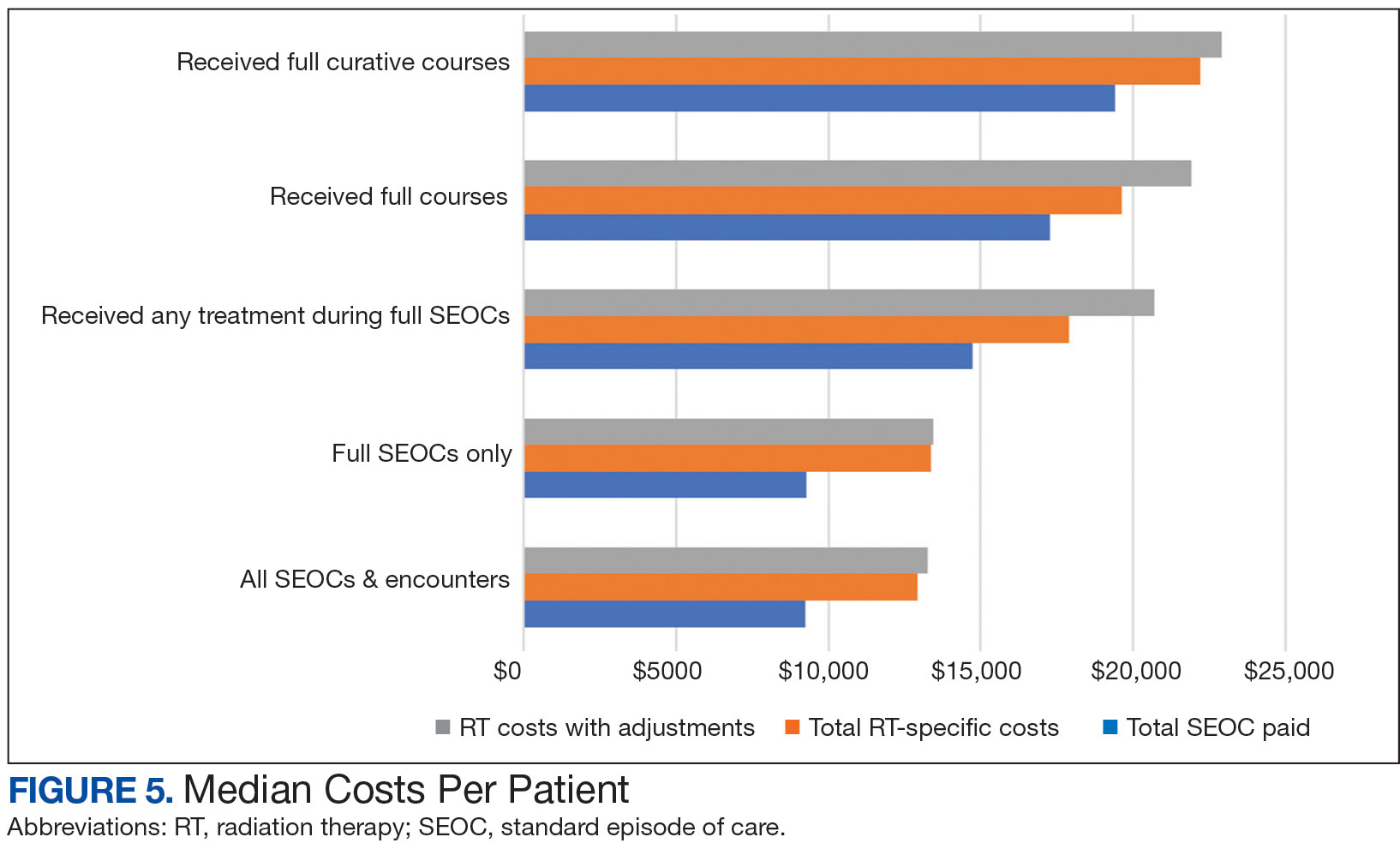
Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.
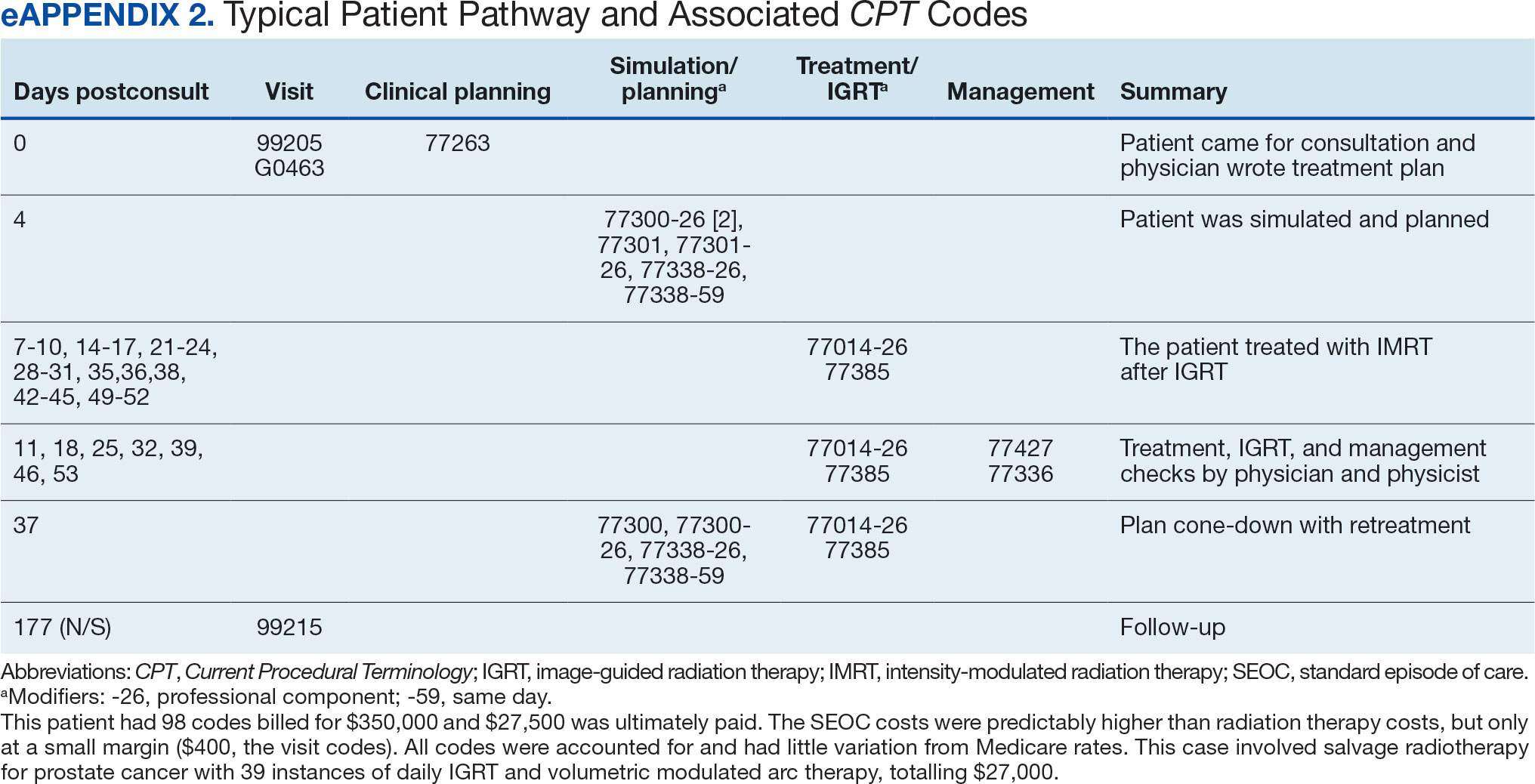

Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).
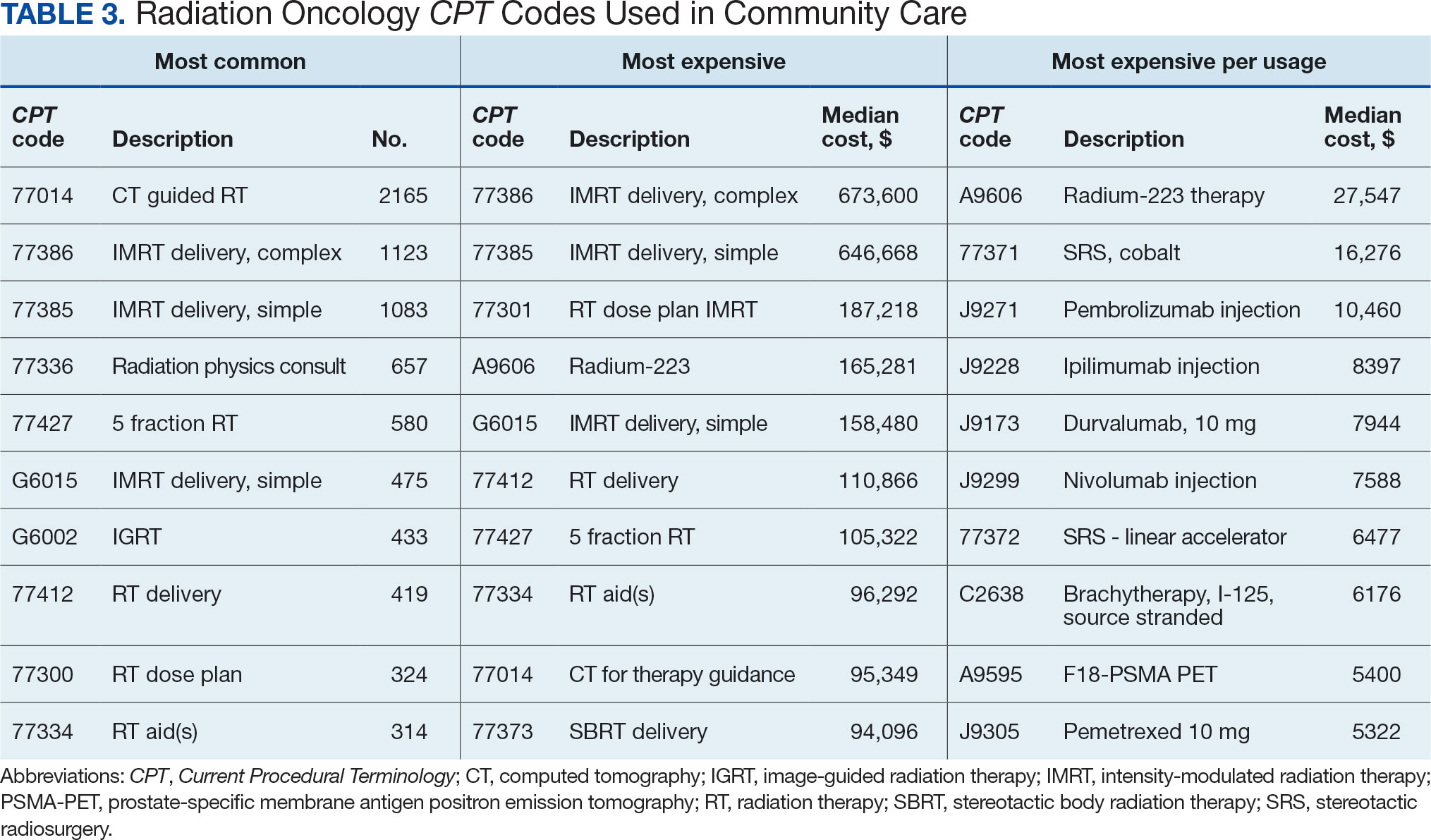

DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).

VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6

Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).



After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).



Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.


Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).



DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
William Kissick’s description of health care’s iron triangle in 1994 still resonates. Access, quality, and cost will always come at the expense of the others.1 In 2018, Congress passed the VA MISSION Act, allowing patients to pursue community care options for extended waits (> 28 days) or longer distance drive times of > 60 minutes for specialty care services, such as radiation oncology. According to Albanese et al, the VA MISSION Act sought to address gaps in care for veterans living in rural and underserved areas.2 The Veterans Health Administration (VHA) continues to increase community care spending, with a 13.8% increase in fiscal year 2024 and an expected cost of > $40 billion for 2025.3 One could argue this pays for access for remote patients and quality when services are unavailable, making it a direct application of the iron triangle.
The VA MISSION Act also bolstered the expansion of existing community care department staff to expediently facilitate and coordinate care and payments.2 Cost management and monitoring have become critical in predicting future staff requirements, maintaining functionality, and ensuring patients receive optimal care. The VHA purchases care through partner networks and defines these bundled health care services as standard episodes of care (SEOCs), which are “clinically related health care services for a specific unique illness or medical condition… over a defined period of time.”4 Medicare publishes its rates quarterly, and outpatient procedure pricing is readily available online.5 Along these same lines, the US Department of Veterans Affairs (VA) publishes a current list of available procedures and associated Current Procedure Technology (CPT) codes that are covered under its VA fee schedule for community care.
Unique challenges persist when using this system to accurately account for radiation oncology expenditures. This study was based on the current practices at the Richard L. Roudebush VA Medical Center (RLRVAMC), a large 1a hospital. A detailed analysis reveals the contemporaneous cost of radiation oncology cancer care from October 1, 2021, through February 1, 2024, highlights the challenges in SEOC definition and duration, communication issues between RLRVAMC and purchase partners, inconsistencies in billing, erroneous payments, and difficulty of cost categorization.
METHODS
Community care radiation oncology-related costs were examined from October 1, 2021, to February 1, 2024 for RLRVAMC, 6 months prior to billing data extraction. Figure 1 shows a simple radiation oncology patient pathway with consultation or visit, simulation and planning, and treatment, with codes used to check billing. It illustrates the expected relationships between the VHA (radiation oncology, primary, and specialty care) and community care (clinicians and radiation oncology treatment sites).

VHA standard operating procedures for a patient requesting community-based radiation oncology care require a board-certified radiation oncologist at RLRVAMC to review and approve the outside care request. Community care radiation oncology consultation data were accessed from the VA Corporate Data Warehouse (CDW) using Pyramid Analytics (V25.2). Nurses, physicians, and community care staff can add comments, forward consultations to other services, and mark them as complete or discontinued, when appropriate. Consultations not completed within 91 days are automatically discontinued. All community care requests from 2018 through 2024 were extracted; analysis began April 1, 2021, 6 months prior to the cost evaluation date of October 1, 2021.
An approved consultation is reviewed for eligibility by a nurse in the community care department and assigned an authorization number (a VA prefix followed by 12 digits). Billing codes are approved and organized by the community care networks, and all procedure codes should be captured and labeled under this number. The VAMC Community Care department obtains initial correspondence from the treating clinicians. Subsequent records from the treating radiation oncologist are expected to be scanned into the electronic health record and made accessible via the VA Joint Legacy Viewer (JLV) and Computerized Patient Record System (CPRS).
Radiation Oncology SEOC
The start date of the radiation oncology SEOC is determined by the community care nurse based on guidance established by the VA. It can be manually backdated or delayed, but current practice is to start at first visit or procedure code entry after approval from the VAMC Radiation Oncology department. Approved CPT codes from SEOC versions between October 1, 2021, and February 1, 2024, are in eAppendix 1 (available at doi:10.12788/fp.0585). These generally include 10 types of encounters, about 115 different laboratory tests, 115 imaging studies, 25 simulation and planning procedures, and 115 radiation treatment codes. The radiation oncology SEOCs during the study period had an approval duration of 180 days. Advanced Medical Cost Management Solutions software (AMCMS) is the VHA data analytics platform for community care medical service costs. AMCMS includes all individual CPT codes billed by specific radiation oncology SEOC versions. Data are refreshed monthly, and all charges were extracted on September 12, 2024, > 6 months after the final evaluated service date to allow for complete billing returns.6

Radiation Oncology-Specific Costs
The VA Close to Me (CTM) program was used to find 84 specific radiation oncology CPT codes, nearly all within the 77.XXX or G6.XXX series, which included all radiation oncology-specific (ROS) codes (except visits accrued during consultation and return appointments). ROS costs are those that could not be performed by any other service and include procedures related to radiation oncology simulation, treatment planning, treatment delivery (with or without image guidance), and physician or physicist management. All ROS costs should be included in a patient’s radiation oncology SEOC. Other costs that may accompany operating room or brachytherapy administration did not follow a 77.XXX or G6.XXX pattern but were included in total radiation therapy operating costs.
Data obtained from AMCMS and CTM included patient name and identifier; CPT billed amount; CPT paid amount; dates of service; number of claims; International Classification of Diseases, Tenth Revision (ICD) diagnosis; and VA authorization numbers. Only CTM listed code modifiers. Only items categorized as paid were included in the analysis. Charges associated with discontinued consultations that had accrued costs also were included. Codes that were not directly related to ROS were separately characterized as other and further subcategorized.
Deep Dive Categorization
All scanned documents tagged to the community consultation were accessed and evaluated for completeness by a radiation oncologist (RS). The presence or absence of consultation notes and treatment summaries was evaluated based on necessity (ie, not needed for continuation of care or treatment was not given). In the absence of a specific completion summary or follow-up note detailing the treatment modality, number of fractions, and treatment sites, available documentation, including clinical notes and billing information, was used. Radical or curative therapies were identified as courses expected to eradicate disease, including stereotactic ablative radiotherapy to the brain, lung, liver, and other organs. Palliative therapies included whole-brain radiotherapy or other low-dose treatments. If the patient received the intended course, this was categorized as full. If incomplete, it was considered partial.
Billing Deviations
The complete document review allowed for close evaluation of paid therapy and identification of gaps in billing (eg, charges not found in extracted data that should have occurred) for external beam radiotherapy patients. Conversely, extra charges, such as an additional weekly treatment management charge (CPT code 77427), would be noted. Patients were expected to have the number of treatments specified in the summary, a clinical treatment planning code, and weekly treatment management notes from physicians and physicists every 5 fractions. Consultations and follow-up visits were expected to have 1 visit code; CPT codes 99205 and 99215, respectively, were used to estimate costs in their absence.
Costs were based on Medicare rates as of January 1 of the year in which they were accrued. 7-10 Duplicates were charges with the same code, date, billed quantity, and paid amounts for a given patient. These would always be considered erroneous. Medicare treatment costs for procedures such as intensity modulated radiotherapy (CPT code 77385 or 77386) are available on the Medicare website. When reviewing locality deviations for 77427, there was a maximum of 33% increase in Medicare rates. Therefore, for treatment codes, one would expect the range to be at least the Medicare rate and maximally 33% higher. These rates are negotiated with insurance companies, but this range was used for the purpose of reviewing and adjusting large data sets.
RESULTS
Since 2018, > 500 community care consults have been placed by radiation oncology for treatment in the community, with more following implementation of the VA MISSION Act. Use of radiation oncology community care services annually increased during the study period for this facility (Table 1, Figure 2). Of the 325 community care consults placed from October 1, 2021, to February 1, 2024, 248 radiation oncology SEOCs were recorded with charges for 181 patients (range, 1-5 SEOCs). Long drive time was the rationale for > 97% of patients directed to community care (Supplemental materials, available at doi:10.12788/fp.0585). Based on AMCMS data, $22.2 million was billed and $2.7 million was paid (20%) for 8747 CPT codes. Each community care interval cost the VA a median (range) of $5000 ($8-$168,000 (Figure 3).



After reviewing ROS charges extracted from CTM, 20 additional patients had radiation oncology charges but did not have a radiation oncology SEOC for 268 episodes of care for 201 unique patients. In addition to the 20 patients who did not have a SEOC, 42 nonradiation oncology SEOCs contained 1148 radiation oncology codes, corresponding to almost $500,000 paid. Additional charges of about $416,000, which included biologic agents (eg, durvalumab, nivolumab), procedures (eg, mastectomies), and ambulance rides were inappropriately added to radiation oncology SEOCs.
While 77% of consultations were scanned into CPRS and JLV, only 54% of completion summaries were available with an estimated $115,000 in additional costs. The total adjusted costs was about $2.9 million. Almost 37% of SEOCs were for visits only. For the 166 SEOCs where patients received any radiation treatment or planning, the median cost was $18,000. Differences in SEOC pathways are shown in Figure 4. One hundred twenty-one SEOCs (45%) followed the standard pathway, with median SEOC costs of $15,500; when corrected for radiation-specific costs, the median cost increased to $18,000. When adjusted for billing irregularities, the median cost was $20,600. Ninety-nine SEOCs (37%) were for consultation/ follow-up visits only, with a median cost of $220. When omitting shared scans and nonradiation therapy costs and correcting for billing gaps, the median cost decreased to $170. A median of $9200 was paid per patient, with $12,900 for radiation therapy-specific costs and $13,300 adjusted for billing deviations. Narrowing to the 106 patients who received full, radical courses, the median SEOC, ROS, and adjusted radiation therapy costs increased to $19,400, $22,200, and $22,900, respectively (Table 2, Figure 5). Seventy-one SEOCs (26%) had already seen a radiation oncologist before the VA radiation oncology department was aware, and 49 SEOCs (18%) had retroactive approvals (Supplemental materials available at doi:10.12788/fp.0585).



Every consultation charge was reviewed. A typical patient following the standard pathway (eAppendix 2, available at doi:10.12788/ fp.0585) exhibited a predictable pattern of consultation payment, simulation and planning, multiple radiation treatments interspersed with treatment management visits and a cone-down phase, and finishing with a follow-up visit. A less predictable case with excess CPT codes, gaps in charges, and an additional unexpected palliative course is shown in eAppendix 3 (available at doi:10.12788/fp.0585). Gaps occurred in 42% of SEOCs with missed bills costing as much as $12,000. For example, a patient with lung cancer had a treatment summary note for lung cancer after completion that showed the patient received 30 fractions of 2 Gy, a typical course. Only 10 treatment codes and 3 of 6 weekly treatment management codes were available. There was a gap of 20 volumetric modulated arc therapy treatments, 3 physics weekly status checks, 3 physician managements notes, and a computed tomography simulation charge.


Between AMCMS and CTM, 10,005 CPT codes were evaluated; 1255 (12.5%) were unique to AMCMS (either related to the radiation oncology course, such as Evaluation and Management CPT codes or “other” unrelated codes) while 1158 (11.6%) were unique to CTM. Of the 7592 CPT codes shared between AMCMS and CTM, there was a discrepancy in 135 (1.8%); all were duplicates (CTM showed double payment while AMCMS showed $0 paid). The total CPT code costs came to $3.2 million with $560,000 unique to SEOCs and $500,000 unique to CTM. Treatment codes were the most common (33%) as shown in Table 3 and accounted for 55% of the cost ($1.8 million). About 700 CPT codes were considered “other,” typically for biologic therapeutic agents (Table 4 and eAppendix 4, available at doi:10.12788/fp.0585).



DISCUSSION
The current method of reporting radiation oncology costs used by VA is insufficient and misleading. Better data are needed to summarize purchased care costs to guide decisions about community care at the VA. Investigations into whether the extra costs for quality care (ie, expensive capital equipment, specialized staff, mandatory accreditations) are worthwhile if omitted at other facilities patients choose for their health care needs. No study has defined specialty care-specific costs by evaluating billing receipts from the CDW to answer the question. Kenamond et al highlight the need for radiation oncology for rural patients.11 Drive time was cited as the reason for community care referral for 97% of veterans, many of whom lived in rural locations. Of patients with rurality information who enrolled in community care, 57% came from rural or highly rural counties, and this ratio held for those who received full curative therapies. An executive administrator relying on AMCMS reports would see a median SEOC cost of $5000, but without ROS knowledge in coding, the administrator would miss many additional costs. For example, 2 patients who each had 5 SEOCs during the evaluated period, incurred a total cost of only $1800.
Additionally, an administrator could include miscategorized costs with significant ramifications. The 2 most expensive SEOCs were not typical radiation oncology treatments. A patient undergoing radium-223 dichloride therapy incurred charges exceeding $165,000, contributing disproportionately to the overall median cost analysis; this would normally be administered by the nuclear medicine department. Immunotherapy and chemotherapy are uniformly overseen by medical oncology services, but drug administration codes were still found in radiation oncology SEOCs. A patient (whose SEOC was discontinued but accrued charges) had an electrocardiogram interpretation for $8 as the SEOC cost; 3 other SEOCs continued to incur costs after being discontinued. There were 24 empty SEOCs for patients that had consults to the community, and 2 had notes stating treatment had been delivered yet there was no ROS costs or SEOC costs. Of the 268 encounters, 43% had some sort of billing irregularities (ie, missing treatment costs) that would be unlikely for a private practice to omit; it would be much more likely that the CDW miscategorized the payment despite confirmation of the 2 retrieval systems.
It would be inadvisable to make staffing decisions or forecast costs based on current SEOC reports without specialized curation. A simple yet effective improvement to the cost attribution process would be to restrict the analysis to encounters containing primary radiation treatment codes. This targeted approach allows more accurate identification of patients actively receiving radiation oncology treatment, while excluding those seen solely for consultations or follow-up visits. Implementing this refinement leads to a substantial increase in the median payment—from $5000 to $13,000—without requiring additional coding or data processing, thereby enhancing the accuracy of cost estimates with minimal effort.
Clarifying radiation oncology service costs requires addressing the time frame and services included, given laxity and interpretation of the SEOCs. VA community care departments have streamlined the reimbursement process at the expense of medical cost organization and accuracy; 86% of VA practitioners reported that ≥ 1 potential community health care partners had refused to work with the VA because of payment delays.12 Payments are contingent on correspondence from outside practices for community work. For radiation oncology, this includes the consultation but also critical radiation-related details of treatment, which were omitted nearly half the time. SEOC approval forms have many costly laboratory tests, imaging, and procedures that have little to do with radiation oncology cancer treatments but may be used in the workup and staging process; this creates noise when calculating radiation oncology fiscal cost.
The presumption that an episode of care equates to a completed radiation therapy course is incorrect; this occurs less than half of the time. An episode often refers to a return visit, or conversely, multiple treatment courses. As the patients’ medical homes are their VHA primary care practitioners, it would be particularly challenging to care for the patients without full treatment information, especially if adverse effects from therapy were to arise. As a tertiary specialty, radiation oncology does not seek out patients and are sent consultations from medical oncology, surgical, and medical oncologic specialties. Timesensitive processes such as workup, staging, and diagnosis often occur in parallel. This analysis revealed that patients see outside radiation oncologists prior to the VA. There are ≥ 100 patients who had radiation oncology codes without a radiation oncology SEOC or community care consultation, and in many cases, the consultation was placed after the patient was seen.
Given the lack of uniformity and standardization of patient traffic, the typical and expected pathways were insufficient to find the costs. Too many opportunities for errors and incorrect categorization of costs meant a different method would be necessary. Starting at the inception of the community care consult, only 1 diagnosis code can be entered. For patients with multiple diagnoses, one would not be able to tell what was treated without chart access. Radiation oncology consults come from primary and specialty care practitioners and nurses throughout the VA. Oftentimes, the referral would be solicited by the community radiation oncology clinic, diagnosing community specialty (ie, urology for a patient with prostate cancer), or indirectly from the patient through primary care. Many cases were retroactively approved as the veteran had already been consulted by the community care radiation oncologist. If the patient is drive-time eligible, it would be unlikely that they would leave and choose to return to the VA. There is no way for a facility VA service chief or administrator to mitigate VA community costs of care, especially as shown by the miscategorization of several codes. Database challenges exacerbate the issue: 1 patient changed her first and last name during this time frame, and 2 patients had the same name but different social security numbers. In order to strictly find costs between 2 discrete timepoints, 39 (15%) SEOCs were split and incomplete, and 6 SEOCs contained charges for 2 different patients. This was corrected, and all inadvertent charges were cancelled. Only 1 ICD code is allowed per community care consultation, so an investigation is required to find costs for patients with multiple sites of disease. Additionally, 5 of the patients marked for drive time were actually patients who received Gamma Knife and brachytherapy, services not available at the VA.
Hanks et al first attempted to calculate cost of radiation oncology services. External beam prostate cancer radiotherapy at 3 suburban California centers cost $6750 ($20,503 inflation adjusted) per patient before October 1984 and $5600 ($17,010 inflation adjusted) afterwards.13 According to the American Society for Radiation Oncology, Advocacy Radiation Oncology Case Rate Program Curative radiation courses should cost $20,000 to $30,000 and palliative courses should cost $10,000 to $15,000. These costs are consistent with totals demonstrated in this analysis and similar to the inflation-adjusted Hanks et al figures. Preliminary findings suggest that radiation treatment constituted more than half of the total expenditures, with a notable $4 million increase in adjusted cost compared to the Medicare rates, indicating significant variation. Direct comparisons with Medicaid or commercial payer rates remain unexplored.
Future Directions
During the study period, 201 patients received 186 courses of radiation therapy in the community, while 1014 patients were treated in-house for a total of 833 courses. A forthcoming analysis will directly compare the cost of in-house care with that of communitybased treatment, specifically breaking down expenditure differences by diagnosis. Future research should investigate strategies to align reimbursement with quality metrics, including the potential role of tertiary accreditation in incentivizing high-value care. Additional work is also warranted to assess patient out-ofpocket expenses across care settings and to benchmark VA reimbursement against Medicare, Medicaid, and private insurance rates. In any case, with the increasing possibility of fewer fractions for treatments such as stereotactic radiotherapy or palliative care therapy, there is a clear financial incentive to treat as frequently as allowed despite equal clinical outcomes.
CONCLUSIONS
Veterans increasingly choose to receive care closer to home if the option is available. In the VA iron triangle, cost comes at the expense of access but quantifying this has proved elusive in the cost accounting model currently used at the VA.1 The inclusion of all charges loosely associated with SEOCs significantly impairs the ability to conduct meaningful cost analyses. The current VA methodology not only introduces substantial noise into the data but also leads to a marked underestimation of the true cost of care delivered in community settings. Such misrepresentation risks driving policy decisions that could inappropriately reduce or eliminate in-house radiation oncology services. Categorizing costs effectively in the VA could assist in making managerial and administrative decisions and would prevent damaging service lines based on misleading or incorrect data. A system which differentiates between patients who have received any treatment codes vs those who have not would increase accuracy.
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
- Kissick W. Medicine’s Dilemmas: Infinite Needs Versus Finite Resources. 1st ed. Yale University Press; 1994.
- Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
- Office of Management and Budget (US). Budget of the United States Government, Fiscal Year 2025. Washington, DC: US Government Publishing Office; 2024. Available from: US Department of Veterans Affairs FY 2025 Budget Submission: Budget in Brief.
- US Department of Veterans Affairs. Veteran care claims. Accessed April 3, 2025. https://www.va.gov/COMMUNITYCARE/revenue-ops/Veteran-Care-Claims.asp
- US Centers for Medicare and Medicaid Services. Accessed April 3, 2025. Procedure price lookup https://www.medicare.gov/procedure-price-lookup
- US Department of Veterans Affairs. WellHive -Enterprise. Accessed April 3, 2025. https://department.va.gov/privacy/wp-content/uploads/sites/5/2023/05/FY23WellHiveEnterprisePIA.pdf
- US Centers for Medicare and Medicaid Services. RVU21a physician fee schedule, January 2021 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu21a
- US Centers for Medicare and Medicaid Services. RVU22a physician fee schedule, January 2022 release. Accessed April 3, 2025. https://www.cms.gov/medicaremedicare-fee-service-paymentphysicianfeeschedpfs-relative-value-files/rvu22a
- US Centers for Medicare and Medicaid Services. RVU23a physician fee schedule, January 2023 release. Accessed April 3, 2025. https://www.cms.gov/medicare/medicare-fee-service-payment/physicianfeesched/pfs-relative-value-files/rvu23a
- US Centers for Medicare and Medicaid Services. RVU23a Medicare Physician Fee Schedule rates effective January 1, 2024, through March 8, 2024. Accessed on April 3, 2025. https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files/rvu24a
- Kenamond MC, Mourad WF, Randall ME, Kaushal A. No oncology patient left behind: challenges and solutions in rural radiation oncology. Lancet Reg Health Am. 2022;13:100289. doi:10.1016/j.lana.2022.100289
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Hanks GE, Dunlap K. A comparison of the cost of various treatment methods for early cancer of the prostate. Int J Radiat Oncol Biol Phys. 1986;12(10):1879-1881. doi:10.1016/0360-3016(86)90334-2
- American Society of Radiation Oncology. Radiation oncology case rate program (ROCR). Accessed April 3, 2025. https://www.astro.org/advocacy/key-issues-8f3e5a3b76643265ee93287d79c4fc40/rocr
Community Care Radiation Oncology Cost Calculations for a VA Medical Center
Community Care Radiation Oncology Cost Calculations for a VA Medical Center