User login
Long-Term Follow-Up Emphasizes HPV Vaccination Importance
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I want to briefly discuss a critically important topic that cannot be overly emphasized. It is the relevance, the importance, the benefits, and the outcome of HPV vaccination.
The paper I’m referring to was published in Pediatrics in October 2023. It’s titled, “Ten-Year Follow-up of 9-Valent Human Papillomavirus Vaccine: Immunogenicity, Effectiveness, and Safety.”
Let me emphasize that we’re talking about a 10-year follow-up. In this particular paper and analysis, 301 boys — I emphasize boys — were included and 971 girls at 40 different sites in 13 countries, who received the 9-valent vaccine, which includes HPV 16, 18, and seven other types.
Most importantly, there was not a single case. Not one. Let me repeat this: There was not a single case of high-grade intraepithelial neoplasia, or worse, or condyloma in either males or females. There was not a single case in over 1000 individuals with a follow-up of more than 10 years.
It is difficult to overstate the magnitude of the benefit associated with HPV vaccination for our children and young adults on their risk of developing highly relevant, life-changing, potentially deadly cancers.
For those of you who are interested in this topic — which should include almost all of you, if not all of you — I encourage you to read this very important follow-up paper, again, demonstrating the simple, overwhelming magnitude of the benefit of HPV vaccination. I thank you for your attention.
Dr. Markman is a professor in the department of medical oncology and therapeutics research, City of Hope, Duarte, California, and president of medicine and science, City of Hope Atlanta, Chicago, and Phoenix. He disclosed ties with GlaxoSmithKline; AstraZeneca.
A version of this article appeared on Medscape.com.
Physicians as First Responders II
I recently wrote about a fledgling program here in Maine in which some emergency room physicians were being outfitted with equipment and communications gear that would allow them to respond on the fly to emergencies in the field when they weren’t working in the hospital. I questioned the rationale of using in-house personnel, already in short supply, for the few situations in which trained EMT personnel would usually be called. At the same time, I promised to return to the broader subject of the role of physicians as first responders in a future letter. And, here it is.
Have you ever been on a plane or at a large public gathering and the public addressed system crackled, “Is there a doctor on board” or in the audience? Or have you been on the highway and come upon a fresh accident in which it appears that there may have been injuries? Or at a youth soccer game in which a player has been injured and is still on the ground?
How do you usually respond in situations like this? Do you immediately identify yourself as a physician? Or, do you routinely shy away from involvement? What thoughts run through your head?
Do you feel your training and experience with emergencies is so outdated that you doubt you could be of any assistance? Has your practice become so specialized that you aren’t comfortable with anything outside of your specialty? Maybe getting involved is likely to throw your already tight travel schedule into disarray? Or are you afraid that should something go wrong while you were helping out you could be sued?
Keeping in mind that I am a retired septuagenarian pediatrician more than a decade removed from active practice, I would describe my usual response to these situations as “attentive hovering.” I position myself to have a good view of the victim and watch to see if there are any other responders. Either because of their personality or their experience, often there is someone who steps forward to help. Trained EMTs seem to have no hesitancy going into action. If I sense things aren’t going well, or the victim is a child, I will identify myself as a retired pediatrician and offer my assistance. Even if the response given by others seems appropriate, I may still eventually identify myself, maybe to lend an air of legitimacy to the process.
What are the roots of my hesitancy? I have found that I generally have little to add when there is a trained first responder on hand. They have been-there-and-done-that far more recently than I have. They know how to stabilize potential or obvious fractures. They know how to position the victim for transport. Even when I am in an environment where my medical background is already known, I yield to the more recently experienced first responders.
I don’t particularly worry about being sued. Every state has Good Samaritan laws. Although the laws vary from state to state, here in Maine I feel comfortable with the good sense of my fellow citizens. I understand if you live or practice in a more litigious environment you may be more concerned. On an airplane there is the Aviation Medical Assistant Act, which became law in 1998, and provides us with some extra protection.
What if there is a situation in which even with my outdated skills I seem to be the only show in town? Fortunately, that situation hasn’t occurred for me in quite a few years, but the odds are that one might occur. In almost 1 out of 600 airline flights, there is an inflight emergency. I tend to hang out with other septuagenarians and octogenarians doing active things. And I frequent youth athletic events where there is unlikely to be a first responder assigned to the event.
Should I be doing more to update my skills? It’s been a while since I refreshed by CPR techniques. I can’t recall the last time I handled a defibrillator. Should I be learning more about exsanguination prevention techniques?
Every so often there are some rumblings to mandate that all physicians should be required to update these first responder skills to maintain their license or certification. That wouldn’t cover those of us who are retired or who no longer practice medicine. And, I’m not sure we need to add another layer to the system. I think there are enough of us out there who would like to add ourselves to the first responder population, maybe not as fully trained experts but as folks who would like to be more ready to help by updating old or seldom-used skills.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I recently wrote about a fledgling program here in Maine in which some emergency room physicians were being outfitted with equipment and communications gear that would allow them to respond on the fly to emergencies in the field when they weren’t working in the hospital. I questioned the rationale of using in-house personnel, already in short supply, for the few situations in which trained EMT personnel would usually be called. At the same time, I promised to return to the broader subject of the role of physicians as first responders in a future letter. And, here it is.
Have you ever been on a plane or at a large public gathering and the public addressed system crackled, “Is there a doctor on board” or in the audience? Or have you been on the highway and come upon a fresh accident in which it appears that there may have been injuries? Or at a youth soccer game in which a player has been injured and is still on the ground?
How do you usually respond in situations like this? Do you immediately identify yourself as a physician? Or, do you routinely shy away from involvement? What thoughts run through your head?
Do you feel your training and experience with emergencies is so outdated that you doubt you could be of any assistance? Has your practice become so specialized that you aren’t comfortable with anything outside of your specialty? Maybe getting involved is likely to throw your already tight travel schedule into disarray? Or are you afraid that should something go wrong while you were helping out you could be sued?
Keeping in mind that I am a retired septuagenarian pediatrician more than a decade removed from active practice, I would describe my usual response to these situations as “attentive hovering.” I position myself to have a good view of the victim and watch to see if there are any other responders. Either because of their personality or their experience, often there is someone who steps forward to help. Trained EMTs seem to have no hesitancy going into action. If I sense things aren’t going well, or the victim is a child, I will identify myself as a retired pediatrician and offer my assistance. Even if the response given by others seems appropriate, I may still eventually identify myself, maybe to lend an air of legitimacy to the process.
What are the roots of my hesitancy? I have found that I generally have little to add when there is a trained first responder on hand. They have been-there-and-done-that far more recently than I have. They know how to stabilize potential or obvious fractures. They know how to position the victim for transport. Even when I am in an environment where my medical background is already known, I yield to the more recently experienced first responders.
I don’t particularly worry about being sued. Every state has Good Samaritan laws. Although the laws vary from state to state, here in Maine I feel comfortable with the good sense of my fellow citizens. I understand if you live or practice in a more litigious environment you may be more concerned. On an airplane there is the Aviation Medical Assistant Act, which became law in 1998, and provides us with some extra protection.
What if there is a situation in which even with my outdated skills I seem to be the only show in town? Fortunately, that situation hasn’t occurred for me in quite a few years, but the odds are that one might occur. In almost 1 out of 600 airline flights, there is an inflight emergency. I tend to hang out with other septuagenarians and octogenarians doing active things. And I frequent youth athletic events where there is unlikely to be a first responder assigned to the event.
Should I be doing more to update my skills? It’s been a while since I refreshed by CPR techniques. I can’t recall the last time I handled a defibrillator. Should I be learning more about exsanguination prevention techniques?
Every so often there are some rumblings to mandate that all physicians should be required to update these first responder skills to maintain their license or certification. That wouldn’t cover those of us who are retired or who no longer practice medicine. And, I’m not sure we need to add another layer to the system. I think there are enough of us out there who would like to add ourselves to the first responder population, maybe not as fully trained experts but as folks who would like to be more ready to help by updating old or seldom-used skills.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I recently wrote about a fledgling program here in Maine in which some emergency room physicians were being outfitted with equipment and communications gear that would allow them to respond on the fly to emergencies in the field when they weren’t working in the hospital. I questioned the rationale of using in-house personnel, already in short supply, for the few situations in which trained EMT personnel would usually be called. At the same time, I promised to return to the broader subject of the role of physicians as first responders in a future letter. And, here it is.
Have you ever been on a plane or at a large public gathering and the public addressed system crackled, “Is there a doctor on board” or in the audience? Or have you been on the highway and come upon a fresh accident in which it appears that there may have been injuries? Or at a youth soccer game in which a player has been injured and is still on the ground?
How do you usually respond in situations like this? Do you immediately identify yourself as a physician? Or, do you routinely shy away from involvement? What thoughts run through your head?
Do you feel your training and experience with emergencies is so outdated that you doubt you could be of any assistance? Has your practice become so specialized that you aren’t comfortable with anything outside of your specialty? Maybe getting involved is likely to throw your already tight travel schedule into disarray? Or are you afraid that should something go wrong while you were helping out you could be sued?
Keeping in mind that I am a retired septuagenarian pediatrician more than a decade removed from active practice, I would describe my usual response to these situations as “attentive hovering.” I position myself to have a good view of the victim and watch to see if there are any other responders. Either because of their personality or their experience, often there is someone who steps forward to help. Trained EMTs seem to have no hesitancy going into action. If I sense things aren’t going well, or the victim is a child, I will identify myself as a retired pediatrician and offer my assistance. Even if the response given by others seems appropriate, I may still eventually identify myself, maybe to lend an air of legitimacy to the process.
What are the roots of my hesitancy? I have found that I generally have little to add when there is a trained first responder on hand. They have been-there-and-done-that far more recently than I have. They know how to stabilize potential or obvious fractures. They know how to position the victim for transport. Even when I am in an environment where my medical background is already known, I yield to the more recently experienced first responders.
I don’t particularly worry about being sued. Every state has Good Samaritan laws. Although the laws vary from state to state, here in Maine I feel comfortable with the good sense of my fellow citizens. I understand if you live or practice in a more litigious environment you may be more concerned. On an airplane there is the Aviation Medical Assistant Act, which became law in 1998, and provides us with some extra protection.
What if there is a situation in which even with my outdated skills I seem to be the only show in town? Fortunately, that situation hasn’t occurred for me in quite a few years, but the odds are that one might occur. In almost 1 out of 600 airline flights, there is an inflight emergency. I tend to hang out with other septuagenarians and octogenarians doing active things. And I frequent youth athletic events where there is unlikely to be a first responder assigned to the event.
Should I be doing more to update my skills? It’s been a while since I refreshed by CPR techniques. I can’t recall the last time I handled a defibrillator. Should I be learning more about exsanguination prevention techniques?
Every so often there are some rumblings to mandate that all physicians should be required to update these first responder skills to maintain their license or certification. That wouldn’t cover those of us who are retired or who no longer practice medicine. And, I’m not sure we need to add another layer to the system. I think there are enough of us out there who would like to add ourselves to the first responder population, maybe not as fully trained experts but as folks who would like to be more ready to help by updating old or seldom-used skills.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Healing From Trauma
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
“You’ll never walk alone.” — Nettie Fowler, Carousel
A few winters ago, a young man and his fiancée were driving on the 91 freeway in southern California during a torrential downpour when their Honda Civic hydroplaned, slamming into the jersey barrier. They were both unhurt. Unsure what to do next, they made the catastrophic decision to exit the vehicle. As the man walked around the back of the car he was nearly hit by a black sedan sliding out of control trying to avoid them. When he came around the car, his fiancé was nowhere to be found. She had been struck at highway speed and lay crushed under the sedan hundreds of feet away.
I know this poor man because he was referred to me. Not as a dermatologist, but as a fellow human healing from trauma. On January 1, 2019, at about 9:30 PM, while we were home together, my beloved wife of 24 years took her own life. Even 5 years on it is difficult to believe that she isn’t proofing this paragraph like she had done for every one of my Derm News columns for years. We had been together since teenagers and had lived a joy-filled life. There isn’t any medical reason to share. But that day I joined the community of those who have carried unbearable heaviness of grief and survived. Sometimes others seek me out for help.
At first, my instinct was to guide them, to give advice, to tell them what to do and where to go. But I’ve learned that people in this dark valley don’t need a guide. They need someone to accompany them. To walk with them for a few minutes on their lonely journey. I recently read David Brooks’s new book, How to Know a Person. I’ve been a fan of his since he joined the New York Times in 2003 and have read almost everything he’s written. I sometimes even imagine how he might approach a column whenever I’m stuck (thank you, David). His The Road to Character book is in my canon of literature for self-growth. This latest book is an interesting digression from that central theme. He argues that our society is in acute need of forming better connections and that an important way we can be moral is to learn, and to practice, how to know each other. He shares an emotional experience of losing a close friend to suicide and writes a poignant explanation of what it means to accompany someone in need. It particularly resonated with me. We are doctors and are wired to find the source of a problem, like quickly rotating through the 4X, 10X, 40X on a microscope. Once identified, we spend most of our time creating and explaining treatments. I see how this makes me a great dermatologist but just an average human.
Brooks tells the story of a woman with a brain tumor who often finds herself on the ground surrounded by well-meaning people trying to help. She explains later that what she really needs in those moments is just for someone to get on the ground and lie with her. To accompany her.
Having crossed the midpoint of life, I see with the benefit of perspective how suffering has afforded me wisdom: I am more sensitive and attuned to others. It also gave me credibility: I know how it feels to walk life’s loneliest journey. I’ve also learned to make myself vulnerable for someone to share their story with me. I won’t be afraid to hear the details. I won’t judge them for weeping too little or for sobbing too much. I don’t answer whys. I won’t say what they should do next. But for a few minutes I can walk beside them as a person who cares.
I do not try to remember the hours and days after Susan’s death, but one moment stands out and makes my eyes well when I think of it. That following day my dear brother flew across the country on the next flight out. I was sitting in a psychiatry waiting room when he came down the hall with his luggage in tow. He hugged me as only a brother could, then looked me in my eyes, which were bloodshot from tears just as his were, and he said, “We’re going to be OK.” And with that he walked with me into the office.
We physicians are blessed to have so many intimate human interactions. This book reminded me that sometimes my most important job is not to be the optimized doctor, but just a good human walking alongside.
I have no conflict of interest and purchased these books.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on X. Write to him at [email protected].
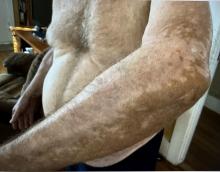
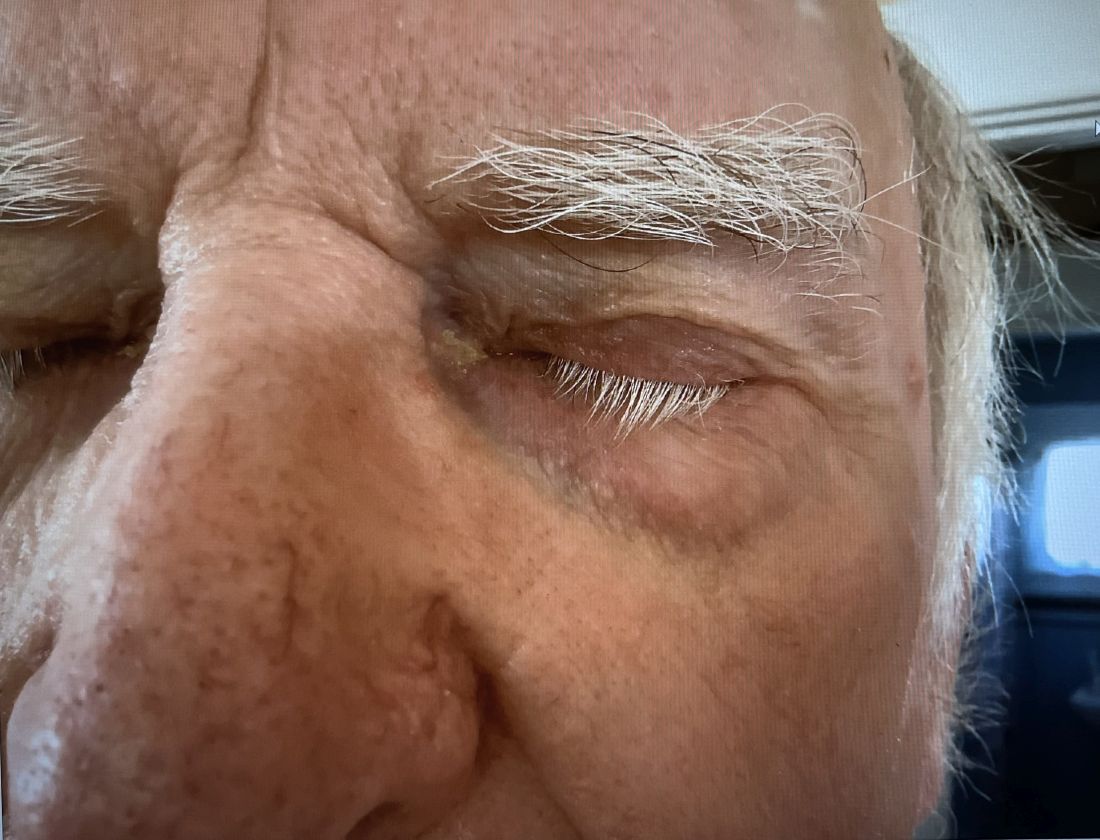
A 74-year-old White male presented with a 1-year history of depigmented patches on the hands, arms, and face, as well as white eyelashes and eyebrows
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
This patient showed no evidence of recurrence in the scar where the melanoma was excised, and had no enlarged lymph nodes on palpation. His complete blood count and liver function tests were normal. A positron emission tomography (PET) scan was ordered by Dr. Nasser that revealed hypermetabolic right paratracheal, right hilar, and subcarinal lymph nodes, highly suspicious for malignant lymph nodes. The patient was referred to oncology for metastatic melanoma treatment and has been doing well on ipilimumab and nivolumab.
Vitiligo is an autoimmune condition characterized by the progressive destruction of melanocytes resulting in hypopigmentation or depigmentation of the skin. Vitiligo has been associated with cutaneous melanoma. Melanoma-associated leukoderma occurs in a portion of patients with melanoma and is correlated with a favorable prognosis. Additionally, leukoderma has been described as a side effect of melanoma treatment itself. However, cases such as this one have also been reported of vitiligo-like depigmentation presenting prior to the diagnosis of metastatic melanoma.
Melanoma, like vitiligo, is considered highly immunogenic, and cytotoxic T lymphocytes (CTLs) can recognize antigens in melanoma. Furthermore, studies have shown a vitiligo-like halo around melanoma tumors, likely caused by T-cell recruitment, and this may lead to tumor destruction, but rarely total clearance. It seems that the CTL infiltrate in both diseases is similar, but regulatory T cells are decreased in vitiligo, whereas they are present in melanomas and may contribute to the immunosuppressive tumor microenvironment found at the margin of these lesions.
Leukoderma is also associated with melanoma immunotherapy which may be described as drug-induced leukoderma. Additionally, the frequency of recognition of melanoma cells by CTLs leading to hypopigmentation appears to be higher in those with metastatic disease. High immune infiltrate with CTLs and interferon-gamma (IFN-gamma) expression by type 1 T helper cells is associated with favorable prognosis. Immunotherapy with checkpoint inhibitors has shown promise in treatment augmentation for melanoma, but not all patients fully respond to therapy. Nonetheless, development of leukoderma with these treatments has been significantly associated with good therapeutic response. Depigmentation of hair and retinal epithelium has also been reported. However, drug-induced leukoderma and vitiligo seem to have clinical and biological differences, including family history of disease and serum chemokine levels. Vaccines are in production to aid in the treatment of melanoma, but researchers must first identify the appropriate antigen(s) to include.
Conversely, vitiligo-like depigmentation has been reported as a harbinger of metastatic melanoma. Patients with previous excision of primary melanoma have presented months or years later with depigmentation and, upon further evaluation, have been diagnosed with metastatic melanoma. The prevalence of depigmentation in melanoma patients is about 3%-6%, and is estimated to be 7-10 times more common in those with melanoma than in the general population. In most cases, hypopigmentation follows the diagnosis of melanoma, with an average of 4.8 years after the initial diagnosis and 1-2 years after lymph node or distant metastases. It is unclear whether hypopigmentation occurs before or after the growth of metastatic lesions, but this clinical finding in a patient with previous melanoma may serve as an important clue to conduct further investigation for metastasis.
This case and the photos were submitted by Lucas Shapiro, BS, of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Florida, and Natalie Y. Nasser, MD, Kaiser Permanente Riverside Medical Center; Riverside, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cerci FB et al. Cutis. 2017 Jun;99(6):E1-E2. PMID: 28686764.
Cho EA et al. Ann Dermatol. 2009 May;21(2):178-181.
Failla CM et al. Int J Mol Sci. 2019 Nov 15;20(22):5731.
When Babies ‘Stop Breathing,’ Who Needs Admission and a Workup?
Many infants have experienced an episode of apnea, defined as a pause in respiration of 20 seconds or more. Most episodes remain unexplained, and no underlying cause can be found. Historically, these were referred to as “near-miss SIDS,” episodes, but that label suggested that all of these events would have ended in death had someone not intervened. New descriptive terminology was needed.
In the mid-1980s, the term “apparent life-threatening event” (ALTE) was adopted. But that term, too, was an overstatement, because although scary for parents, these brief apnea episodes were not, in most cases, truly life-threatening.
In 2013, authors of a systematic review coined the term “brief resolved unexplained event” (BRUE). This review also addressed the history and physical exam features associated with risk for a subsequent episode. It was felt that hospitalization and testing might be warranted if certain infants could be identified as high risk for recurrence.
What Is Considered a BRUE?
In the current working definition of BRUE, the child must be < 1 year old. The episode must be a sudden, brief, and resolved, with one or more of these characteristics:
- Cyanosis or pallor (but not turning red)
- A change in breathing (absent, decreased, or irregular)
- A change in tone (hypertonia or hypotonia)
- A change in responsiveness.
Furthermore, to qualify as a BRUE, no explanation can be found for the event based on the history and physical examination but before any laboratory testing is done. The definition also excludes children with known potential explanatory diagnoses (such as gastroesophageal reflux or bronchiolitis) and those who are otherwise symptomatically ill at the time of the event.
Decision to Admit and Recurrence Risk
An apnea event in an otherwise healthy infant, regardless of what it’s called, puts providers and parents in a difficult position. Should the infant be hospitalized for further monitoring and potentially more invasive testing to determine the cause of the episode? And what are the chances that the episode will be repeated?
A clinical practice guideline (CPG) for BRUE, widely adopted in 2016, resulted in significant reductions in healthcare utilization. The CPG attempted to identify low-risk infants who could safely be discharged from the emergency department. Although the CPG improved outcomes, experts acknowledged that an underlying problem was not likely to be identified even among infants deemed high risk, and these infants would be hospitalized unnecessarily.
Available data were simply insufficient to support this decision. So, with the goal of identifying factors that could help predict recurrent BRUE risk, a 15-hospital collaborative study was undertaken, followed by the development and validation of a clinical decision rule for predicting the risk for a serious underlying diagnosis or event recurrence among infants presenting with BRUE.
Here’s what we learned from more than 3000 cases of BRUE.
First, it turns out that it’s not easy to determine whether an infant is at low or high risk for recurrence of BRUE. Initially, 91.5% of patients enrolled in the study would have been labeled high risk.
Furthermore, a BRUE recurred in 14.3% of the cohort, and 4.8% of high-risk infants were found to have a serious undiagnosed condition. Seizures, airway anomalies, and gastroesophageal reflux were the top three causes of BRUE, but the spectrum of underlying pathology was quite considerable.
The problem was that 4.6% of the entire cohort were found to have a serious underlying condition, nearly identical to the proportion of high-risk infants with these conditions. This prompted the question of whether simply labeling infants “high risk” was really appropriate any longer.
Revised BRUE Management
Although it hasn’t been possible to group infants neatly in low and high-risk categories, the data from that large cohort led to the development of the BRUE 2.0 criteria, which enabled more focused risk assessment of an infant who experienced a BRUE. With an app on MDCalc, these criteria allow providers to ascertain, and show families, a visual representation of their infant’s individualized risk for a subsequent BRUE and of having a serious underlying condition.
The cohort study also identified red flags from the history or physical exam of infants who experienced a BRUE: weight loss, failure to thrive, or a history of feeding problems. Exam findings such as a bulging fontanelle, forceful or bilious emesis, and evidence of gastrointestinal (GI) bleeding suggest a medical diagnosis rather than a BRUE. If GI-related causes are high on the differential, a feeding evaluation can be helpful. A feeding evaluation can be done in the outpatient setting and does not require hospitalization.
For suspicion of an underlying neurological condition (such as seizures), experts recommend obtaining a short EEG, which is highly sensitive for detecting infantile spasms and encephalopathy. They recommend reserving MRI for infants with abnormalities on EEG or physical exam. Metabolic or genetic testing should be done only if the infant looks ill, because most patients with genetic or inborn errors of metabolism will continue to have symptoms as they become older.
The approach to BRUE has moved into the realm of shared decision-making with families. The likelihood of identifying a serious diagnosis is low for most of these children. And unfortunately, no single test can diagnose the full spectrum of potential explanatory diagnoses. For example, data from 2023 demonstrate that only 1.1% of lab tests following a BRUE contributed to a diagnosis, and most of the time that was a positive viral test. Similarly, imaging was helpful in only 1.5% of cases. So, explaining the evidence and deciding along with parents what is reasonable to do (or not do) is the current state of affairs.
My Take
As I reflect back on two and a half decades of caring for these patients, I believe that recent data have helped us a great deal. We do less testing and admit fewer infants to the hospital than we did 20 years ago, and that’s a good thing. Nevertheless, looking for a few red flags, having a high index of suspicion when the clinical exam is abnormal, and engaging in shared decision-making with families can help make the caring for these challenging patients more bearable and lead to better outcomes for all involved.
Dr. Basco is Professor, Department of Pediatrics, Medical University of South Carolina (MUSC); Director, Division of General Pediatrics, Department of Pediatrics, MUSC Children’s Hospital, Charleston, South Carolina. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many infants have experienced an episode of apnea, defined as a pause in respiration of 20 seconds or more. Most episodes remain unexplained, and no underlying cause can be found. Historically, these were referred to as “near-miss SIDS,” episodes, but that label suggested that all of these events would have ended in death had someone not intervened. New descriptive terminology was needed.
In the mid-1980s, the term “apparent life-threatening event” (ALTE) was adopted. But that term, too, was an overstatement, because although scary for parents, these brief apnea episodes were not, in most cases, truly life-threatening.
In 2013, authors of a systematic review coined the term “brief resolved unexplained event” (BRUE). This review also addressed the history and physical exam features associated with risk for a subsequent episode. It was felt that hospitalization and testing might be warranted if certain infants could be identified as high risk for recurrence.
What Is Considered a BRUE?
In the current working definition of BRUE, the child must be < 1 year old. The episode must be a sudden, brief, and resolved, with one or more of these characteristics:
- Cyanosis or pallor (but not turning red)
- A change in breathing (absent, decreased, or irregular)
- A change in tone (hypertonia or hypotonia)
- A change in responsiveness.
Furthermore, to qualify as a BRUE, no explanation can be found for the event based on the history and physical examination but before any laboratory testing is done. The definition also excludes children with known potential explanatory diagnoses (such as gastroesophageal reflux or bronchiolitis) and those who are otherwise symptomatically ill at the time of the event.
Decision to Admit and Recurrence Risk
An apnea event in an otherwise healthy infant, regardless of what it’s called, puts providers and parents in a difficult position. Should the infant be hospitalized for further monitoring and potentially more invasive testing to determine the cause of the episode? And what are the chances that the episode will be repeated?
A clinical practice guideline (CPG) for BRUE, widely adopted in 2016, resulted in significant reductions in healthcare utilization. The CPG attempted to identify low-risk infants who could safely be discharged from the emergency department. Although the CPG improved outcomes, experts acknowledged that an underlying problem was not likely to be identified even among infants deemed high risk, and these infants would be hospitalized unnecessarily.
Available data were simply insufficient to support this decision. So, with the goal of identifying factors that could help predict recurrent BRUE risk, a 15-hospital collaborative study was undertaken, followed by the development and validation of a clinical decision rule for predicting the risk for a serious underlying diagnosis or event recurrence among infants presenting with BRUE.
Here’s what we learned from more than 3000 cases of BRUE.
First, it turns out that it’s not easy to determine whether an infant is at low or high risk for recurrence of BRUE. Initially, 91.5% of patients enrolled in the study would have been labeled high risk.
Furthermore, a BRUE recurred in 14.3% of the cohort, and 4.8% of high-risk infants were found to have a serious undiagnosed condition. Seizures, airway anomalies, and gastroesophageal reflux were the top three causes of BRUE, but the spectrum of underlying pathology was quite considerable.
The problem was that 4.6% of the entire cohort were found to have a serious underlying condition, nearly identical to the proportion of high-risk infants with these conditions. This prompted the question of whether simply labeling infants “high risk” was really appropriate any longer.
Revised BRUE Management
Although it hasn’t been possible to group infants neatly in low and high-risk categories, the data from that large cohort led to the development of the BRUE 2.0 criteria, which enabled more focused risk assessment of an infant who experienced a BRUE. With an app on MDCalc, these criteria allow providers to ascertain, and show families, a visual representation of their infant’s individualized risk for a subsequent BRUE and of having a serious underlying condition.
The cohort study also identified red flags from the history or physical exam of infants who experienced a BRUE: weight loss, failure to thrive, or a history of feeding problems. Exam findings such as a bulging fontanelle, forceful or bilious emesis, and evidence of gastrointestinal (GI) bleeding suggest a medical diagnosis rather than a BRUE. If GI-related causes are high on the differential, a feeding evaluation can be helpful. A feeding evaluation can be done in the outpatient setting and does not require hospitalization.
For suspicion of an underlying neurological condition (such as seizures), experts recommend obtaining a short EEG, which is highly sensitive for detecting infantile spasms and encephalopathy. They recommend reserving MRI for infants with abnormalities on EEG or physical exam. Metabolic or genetic testing should be done only if the infant looks ill, because most patients with genetic or inborn errors of metabolism will continue to have symptoms as they become older.
The approach to BRUE has moved into the realm of shared decision-making with families. The likelihood of identifying a serious diagnosis is low for most of these children. And unfortunately, no single test can diagnose the full spectrum of potential explanatory diagnoses. For example, data from 2023 demonstrate that only 1.1% of lab tests following a BRUE contributed to a diagnosis, and most of the time that was a positive viral test. Similarly, imaging was helpful in only 1.5% of cases. So, explaining the evidence and deciding along with parents what is reasonable to do (or not do) is the current state of affairs.
My Take
As I reflect back on two and a half decades of caring for these patients, I believe that recent data have helped us a great deal. We do less testing and admit fewer infants to the hospital than we did 20 years ago, and that’s a good thing. Nevertheless, looking for a few red flags, having a high index of suspicion when the clinical exam is abnormal, and engaging in shared decision-making with families can help make the caring for these challenging patients more bearable and lead to better outcomes for all involved.
Dr. Basco is Professor, Department of Pediatrics, Medical University of South Carolina (MUSC); Director, Division of General Pediatrics, Department of Pediatrics, MUSC Children’s Hospital, Charleston, South Carolina. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Many infants have experienced an episode of apnea, defined as a pause in respiration of 20 seconds or more. Most episodes remain unexplained, and no underlying cause can be found. Historically, these were referred to as “near-miss SIDS,” episodes, but that label suggested that all of these events would have ended in death had someone not intervened. New descriptive terminology was needed.
In the mid-1980s, the term “apparent life-threatening event” (ALTE) was adopted. But that term, too, was an overstatement, because although scary for parents, these brief apnea episodes were not, in most cases, truly life-threatening.
In 2013, authors of a systematic review coined the term “brief resolved unexplained event” (BRUE). This review also addressed the history and physical exam features associated with risk for a subsequent episode. It was felt that hospitalization and testing might be warranted if certain infants could be identified as high risk for recurrence.
What Is Considered a BRUE?
In the current working definition of BRUE, the child must be < 1 year old. The episode must be a sudden, brief, and resolved, with one or more of these characteristics:
- Cyanosis or pallor (but not turning red)
- A change in breathing (absent, decreased, or irregular)
- A change in tone (hypertonia or hypotonia)
- A change in responsiveness.
Furthermore, to qualify as a BRUE, no explanation can be found for the event based on the history and physical examination but before any laboratory testing is done. The definition also excludes children with known potential explanatory diagnoses (such as gastroesophageal reflux or bronchiolitis) and those who are otherwise symptomatically ill at the time of the event.
Decision to Admit and Recurrence Risk
An apnea event in an otherwise healthy infant, regardless of what it’s called, puts providers and parents in a difficult position. Should the infant be hospitalized for further monitoring and potentially more invasive testing to determine the cause of the episode? And what are the chances that the episode will be repeated?
A clinical practice guideline (CPG) for BRUE, widely adopted in 2016, resulted in significant reductions in healthcare utilization. The CPG attempted to identify low-risk infants who could safely be discharged from the emergency department. Although the CPG improved outcomes, experts acknowledged that an underlying problem was not likely to be identified even among infants deemed high risk, and these infants would be hospitalized unnecessarily.
Available data were simply insufficient to support this decision. So, with the goal of identifying factors that could help predict recurrent BRUE risk, a 15-hospital collaborative study was undertaken, followed by the development and validation of a clinical decision rule for predicting the risk for a serious underlying diagnosis or event recurrence among infants presenting with BRUE.
Here’s what we learned from more than 3000 cases of BRUE.
First, it turns out that it’s not easy to determine whether an infant is at low or high risk for recurrence of BRUE. Initially, 91.5% of patients enrolled in the study would have been labeled high risk.
Furthermore, a BRUE recurred in 14.3% of the cohort, and 4.8% of high-risk infants were found to have a serious undiagnosed condition. Seizures, airway anomalies, and gastroesophageal reflux were the top three causes of BRUE, but the spectrum of underlying pathology was quite considerable.
The problem was that 4.6% of the entire cohort were found to have a serious underlying condition, nearly identical to the proportion of high-risk infants with these conditions. This prompted the question of whether simply labeling infants “high risk” was really appropriate any longer.
Revised BRUE Management
Although it hasn’t been possible to group infants neatly in low and high-risk categories, the data from that large cohort led to the development of the BRUE 2.0 criteria, which enabled more focused risk assessment of an infant who experienced a BRUE. With an app on MDCalc, these criteria allow providers to ascertain, and show families, a visual representation of their infant’s individualized risk for a subsequent BRUE and of having a serious underlying condition.
The cohort study also identified red flags from the history or physical exam of infants who experienced a BRUE: weight loss, failure to thrive, or a history of feeding problems. Exam findings such as a bulging fontanelle, forceful or bilious emesis, and evidence of gastrointestinal (GI) bleeding suggest a medical diagnosis rather than a BRUE. If GI-related causes are high on the differential, a feeding evaluation can be helpful. A feeding evaluation can be done in the outpatient setting and does not require hospitalization.
For suspicion of an underlying neurological condition (such as seizures), experts recommend obtaining a short EEG, which is highly sensitive for detecting infantile spasms and encephalopathy. They recommend reserving MRI for infants with abnormalities on EEG or physical exam. Metabolic or genetic testing should be done only if the infant looks ill, because most patients with genetic or inborn errors of metabolism will continue to have symptoms as they become older.
The approach to BRUE has moved into the realm of shared decision-making with families. The likelihood of identifying a serious diagnosis is low for most of these children. And unfortunately, no single test can diagnose the full spectrum of potential explanatory diagnoses. For example, data from 2023 demonstrate that only 1.1% of lab tests following a BRUE contributed to a diagnosis, and most of the time that was a positive viral test. Similarly, imaging was helpful in only 1.5% of cases. So, explaining the evidence and deciding along with parents what is reasonable to do (or not do) is the current state of affairs.
My Take
As I reflect back on two and a half decades of caring for these patients, I believe that recent data have helped us a great deal. We do less testing and admit fewer infants to the hospital than we did 20 years ago, and that’s a good thing. Nevertheless, looking for a few red flags, having a high index of suspicion when the clinical exam is abnormal, and engaging in shared decision-making with families can help make the caring for these challenging patients more bearable and lead to better outcomes for all involved.
Dr. Basco is Professor, Department of Pediatrics, Medical University of South Carolina (MUSC); Director, Division of General Pediatrics, Department of Pediatrics, MUSC Children’s Hospital, Charleston, South Carolina. He has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Weighing the Big Decisions
In my mind’s calendar, two dates stand out. Both far enough away that I don’t have to think about them too much right now, but near enough that they can’t be forgotten about, either.
On September 30, 2028, my office lease ends, and in 2029 my neurology board certification has to be renewed. I’ll be in my early 60s then and I’ve been a practicing neurologist for 30 years.
I have no idea what I’m going to do. Of course, a lot can happen between now and then, and a lot of variables come into the calculus of when to retire.
After all these years, I still enjoy my job. It gives me the purpose that I wanted so long ago when I applied to medical school. The late William Pancoe, associate dean when I was at Creighton, always told us to remember how we felt when we got that acceptance letter — we’d need it to keep us going through medical school.
And, even now, I still remember the call from my dad that it had arrived. What a moment that was. I have no regrets. I can’t imagine doing anything else.
But in 4 years how much longer will I want to practice? Hopefully I’ll be faced with that decision. Will I want to renew the lease for 2 years? 5 years? I like my little office. It’s far from gleaming, there’s no TV or Keurig in the lobby, the carpet, paint, and furnishings are still from the early 90s when the place was built. But it’s my home away from home. I spend anywhere from 40-60 hours/week there. It’s quiet and (at least for me) cozy. Would I want to give that up and move to a smaller, shared place, for the remainder of my career? Or just close down?
Likewise, will I want to renew my board certification? Granted, that isn’t necessary to practice, but it certainly looks better to have it. To do that I’ll have to fork over a decent chunk of change to take the test, more money for a review course, and spend some time studying. Strange to think that at 63 I might be back at my desk (same desk, by the way) studying for a test like I did in college and medical school. But, if I want to keep playing doctor, that’s what I’ll have to do.
Four years to think about this. The same amount of time I spent each in high school, medical school, and residency. For that matter, the same amount of time since we all went into quarantine.
Doesn’t seem that long, does it?
I guess I’ve got some thinking to do.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In my mind’s calendar, two dates stand out. Both far enough away that I don’t have to think about them too much right now, but near enough that they can’t be forgotten about, either.
On September 30, 2028, my office lease ends, and in 2029 my neurology board certification has to be renewed. I’ll be in my early 60s then and I’ve been a practicing neurologist for 30 years.
I have no idea what I’m going to do. Of course, a lot can happen between now and then, and a lot of variables come into the calculus of when to retire.
After all these years, I still enjoy my job. It gives me the purpose that I wanted so long ago when I applied to medical school. The late William Pancoe, associate dean when I was at Creighton, always told us to remember how we felt when we got that acceptance letter — we’d need it to keep us going through medical school.
And, even now, I still remember the call from my dad that it had arrived. What a moment that was. I have no regrets. I can’t imagine doing anything else.
But in 4 years how much longer will I want to practice? Hopefully I’ll be faced with that decision. Will I want to renew the lease for 2 years? 5 years? I like my little office. It’s far from gleaming, there’s no TV or Keurig in the lobby, the carpet, paint, and furnishings are still from the early 90s when the place was built. But it’s my home away from home. I spend anywhere from 40-60 hours/week there. It’s quiet and (at least for me) cozy. Would I want to give that up and move to a smaller, shared place, for the remainder of my career? Or just close down?
Likewise, will I want to renew my board certification? Granted, that isn’t necessary to practice, but it certainly looks better to have it. To do that I’ll have to fork over a decent chunk of change to take the test, more money for a review course, and spend some time studying. Strange to think that at 63 I might be back at my desk (same desk, by the way) studying for a test like I did in college and medical school. But, if I want to keep playing doctor, that’s what I’ll have to do.
Four years to think about this. The same amount of time I spent each in high school, medical school, and residency. For that matter, the same amount of time since we all went into quarantine.
Doesn’t seem that long, does it?
I guess I’ve got some thinking to do.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In my mind’s calendar, two dates stand out. Both far enough away that I don’t have to think about them too much right now, but near enough that they can’t be forgotten about, either.
On September 30, 2028, my office lease ends, and in 2029 my neurology board certification has to be renewed. I’ll be in my early 60s then and I’ve been a practicing neurologist for 30 years.
I have no idea what I’m going to do. Of course, a lot can happen between now and then, and a lot of variables come into the calculus of when to retire.
After all these years, I still enjoy my job. It gives me the purpose that I wanted so long ago when I applied to medical school. The late William Pancoe, associate dean when I was at Creighton, always told us to remember how we felt when we got that acceptance letter — we’d need it to keep us going through medical school.
And, even now, I still remember the call from my dad that it had arrived. What a moment that was. I have no regrets. I can’t imagine doing anything else.
But in 4 years how much longer will I want to practice? Hopefully I’ll be faced with that decision. Will I want to renew the lease for 2 years? 5 years? I like my little office. It’s far from gleaming, there’s no TV or Keurig in the lobby, the carpet, paint, and furnishings are still from the early 90s when the place was built. But it’s my home away from home. I spend anywhere from 40-60 hours/week there. It’s quiet and (at least for me) cozy. Would I want to give that up and move to a smaller, shared place, for the remainder of my career? Or just close down?
Likewise, will I want to renew my board certification? Granted, that isn’t necessary to practice, but it certainly looks better to have it. To do that I’ll have to fork over a decent chunk of change to take the test, more money for a review course, and spend some time studying. Strange to think that at 63 I might be back at my desk (same desk, by the way) studying for a test like I did in college and medical school. But, if I want to keep playing doctor, that’s what I’ll have to do.
Four years to think about this. The same amount of time I spent each in high school, medical school, and residency. For that matter, the same amount of time since we all went into quarantine.
Doesn’t seem that long, does it?
I guess I’ve got some thinking to do.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Long COVID: Another Great Pretender
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
Has COVID replaced syphilis as the great imitator? At the turn of the 20th century, Sir William Osler was quoted as saying, “He who knows syphilis knows medicine.”
If you have any doubts about the “imitator” analogy, simply use any broad search engine or AI portal and enter “what is the differential diagnosis of primary syphilis, or secondary syphilis, or tertiary syphilis?” A plethora of diseases across many body systems appears.
Another Great Pretender?
Did you or do you now have long COVID? How do you know? Do you even know what long COVID is? How would you diagnosis it?
When asked about long COVID, this is what large language model source bard.google.com had to say:
Long COVID, also known as post-COVID conditions or PASC, is a complex condition that can occur in people who have had COVID-19, even if their initial illness was mild or even asymptomatic. It’s characterized by a wide range of symptoms that can last for weeks, months, or even years after the initial infection.
Symptoms of long COVID can include: fatigue, shortness of breath, brain fog (trouble thinking or concentrating), loss of smell or taste, sleep problems, muscle pain, joint pain, headache, dizziness, depression or anxiety, heart palpitations, gastrointestinal problems.
Duh!!! It reads much like a continuation of the symptoms of acute COVID. Bard continued:
The exact cause of long COVID is still unknown, but there are several theories. One possibility is that the virus itself can damage organs and tissues, leading to ongoing symptoms. Another possibility is that the immune system’s response to the virus can continue to cause inflammation even after the virus is gone.
Human intelligence source Wikipedia says this:
Long COVID or long-haul COVID is a group of health problems persisting or developing after an initial COVID-19 infection. Symptoms can last weeks, months or years and are often debilitating. The World Health Organization defines long COVID as starting three months after infection, but other definitions put the start of long COVID at four weeks.
Highly varied, including post-exertional malaise (symptoms made worse with effort), fatigue, muscle pain, shortness of breath, chest pain, and cognitive dysfunction (brain fog).
Acute COVID to Long COVID
The World Health Organization estimates that 36 million people in the European region have developed long COVID in the first 3 years of the pandemic. That›s a lot.
We all know that the common signs and symptoms of acute COVID-19 include fever or chills, a dry cough and shortness of breath, feeling very tired, muscle or body aches, headache, loss of taste or smell, sore throat, congestion, runny nose, nausea, vomiting, and diarrhea. Except for the taste and smell findings, every one of these symptoms or signs could indicate a different virus infection or even some type of allergy. My point is the nonspecificity in this list.
Uncommon signs and symptoms of acute COVID include a flat skin rash covered with small bumps, discolored swollen areas on the fingers and toes (COVID toes), and hives. The skin of hands, wrists, or ankles also can be affected. Blisters, itchiness, rough skin, or pus can be seen.
Severe confusion (delirium) might be the main or only symptom of COVID-19 in older people. This COVID-19 symptom is linked with a high risk for poor outcomes, including death. Pink eye (conjunctivitis) can be a COVID-19 symptom. Other eye problems linked to COVID-19 are light sensitivity, sore eyes, and itchy eyes. Acute myocarditis, tinnitus, vertigo, and hearing loss have been reported. And 1-4 weeks after the onset of COVID-19 infection, a patient may experience de novo reactive synovitis and arthritis of any joints.
So, take your pick. Myriad symptoms, signs, diseases, diagnoses, and organ systems — still present, recurring, just appearing, apparently de novo, or after asymptomatic infection. We have so much still to learn.
What big-time symptoms, signs, and major diseases are not on any of these lists? Obviously, cancer, atherosclerotic cardiovascular diseases, obesity, bone diseases, and competitive infections. But be patient; the lingering effects of direct tissue invasion by the virus as well as a wide range of immunologic reactions may just be getting started. Mitochondrial damage, especially in muscles, is increasingly a pathophysiologic suspect.
Human diseases can be physical or mental; and in COVID, that twain not only meet but mix and mingle freely, and may even merge into psychosoma. Don’t ever forget that. Consider “fatigue.” Who among us, COVID or NOVID, does not experience that from time to time?
Or consider brain fog as a common reported symptom of COVID. What on earth is that actually? How can a person know they have brain fog, or whether they had it and are over it?
We need one or more lab or other diagnostic tests that can objectively confirm the diagnosis of long COVID.
Useful Progress?
A recent research paper in Science reported intriguing chemical findings that seemed to point a finger at some form of complement dysregulation as a potential disease marker for long COVID. Unfortunately, some critics have pointed out that this entire study may be invalid or irrelevant because the New York cohort was recruited in 2020, before vaccines were available. The Zurich cohort was recruited up until April 2021, so some may have been vaccinated.
Then this news organization came along in early January 2024 with an article about COVID causing not only more than a million American deaths but also more than 5000 deaths from long COVID. We physicians don’t really know what long COVID even is, but we have to sign death certificates blaming thousands of deaths on it anyway? And rolling back the clock to 2020: Are patients dying from COVID or with COVID, according to death certificates?Now, armed with the knowledge that “documented serious post–COVID-19 conditions include cardiovascular, pulmonary, neurological, renal, endocrine, hematological, and gastrointestinal complications, as well as death,” CDC has published clear and fairly concise instructions on how to address post-acute COVID sequelae on death certificates.
In late January, this news organization painted a hopeful picture by naming four phenotypes of long COVID, suggesting that such divisions might further our understanding, including prognosis, and even therapy for this condition. Among the clinical phenotypes of (1) chronic fatigue–like syndrome, headache, and memory loss; (2) respiratory syndrome (which includes cough and difficulty breathing); (3) chronic pain; and (4) neurosensorial syndrome (which causes an altered sense of taste and smell), overlap is clearly possible but isn›t addressed.
I see these recent developments as needed and useful progress, but we are still left with…not much. So, when you tell me that you do or do not have long COVID, I will say to you, “How do you know?”
I also say: She/he/they who know COVID know medicine.
A version of this article first appeared on Medscape.com.
How to Prescribe Physical Activity in Patients With Obesity
Exercise should no longer be a mere “complement” or a standard recommendation within healthy lifestyle guidelines, say experts. Recent evidence confirms its physiological importance and endorses its beneficial and therapeutic effects on overall health, particularly in the case of obesity and its comorbidities. These findings emphasized the reasons to include exercise prescription in addressing this condition. This conclusion emerged from discussions among experts in Physical Activity and Sports Sciences during the XIX Congress of the Spanish Society for Obesity, where the role of physical exercise as a therapeutic strategy was analyzed from various perspectives.
Javier Butragueño, PhD, coordinator of the Exercise Working Group at the Spanish Society of Obesity, emphasized the need to “reposition” the role of exercise and the message conveyed to the population. “We must move beyond the typical recommendation to ‘just walk’ and rethink this message. When working with patients with obesity, you realize that, for example, the guideline of 10,000 steps per day makes little sense for those who weigh 140 kg, have been sedentary for a long time, and have not reached 2000 daily steps. Clinically, it becomes evident that current recommendations may not align with the needs of these patients,” he said.
Precision Focus
Dr. Butragueño highlighted the necessity of shifting the central focus from weight-related variables alone. While weight is crucial, evidence suggests that it should be evaluated along with other strategies, such as nutrition and pharmacology.
“The approach must change to view exercise as a metabolism regulator,” said Dr. Butragueño. “For specialists, this means educating the population about the need to stay active for overall health. This is a disruptive message because the prevailing idea, almost obsessive, associates exercise primarily with weight loss, a completely incorrect approach that can even be detrimental in some cases.”
Dr. Butragueño emphasized the supportive role of physical exercise in interventions for these patients. “Data show that it is both an enhancer and a co-adjuvant in strategies that also include psychology and endocrinology. It should be part of the approach to obesity but individualized and phenotyped to give physical activity the necessary dimension in each specific case.”
As an example of this adaptability in therapeutic strategy, Dr. Butragueño referred to addressing binge eating disorder. “In this case, specialists must acknowledge that sports are a third-line option, always behind the psychologist, who plays a primary role. Exercise is used to enhance the emotions triggered through its practice, considering that many of these patients maintain a very negative relationship with their bodies.”
Spanish ‘Prescription Guide’
During his presentation, Dr. Butragueño introduced the positioning document from the Exercise Group of the Spanish Society of Obesity, which is aimed at designing physical activity programs for patients with obesity. He emphasized its importance as a much-needed effort at proposing intervention strategies to guide health professionals and establish a reference framework for collaboration across different approaches to obesity.
Among the noteworthy aspects of the guidelines outlined in this document, Dr. Butragueño highlighted the assessment and classification of physical activity into four levels based on each patient’s physical condition. “This aspect should be studied by the scientific community because ‘humanizing’ exercise prescription by understanding individuals’ needs beyond their BMI is crucial.”
He also discussed the strategy outlined in the document that he said is crucial for implementing an exercise program. “Essentially, it involves two guidelines: First, engage in physical activity for at least 30-60 minutes in what we call zone 2. This includes activities like walking, cycling, or rowing, where one can speak easily with another person or sing without getting out of breath. This is a fundamental part of addressing obesity, as it improves mitochondrial biogenesis, the correct utilization of fatty acids, which is a significant concern in the pathophysiology of obesity and other diseases like cancer.”
The second strategy involves strength training alone or combined with aerobic-cardiovascular exercise. “Studies show that just 20 minutes of strength training 1 day a week for 10 consecutive weeks significantly improves strength levels in sedentary individuals.”
Dr. Butragueño emphasized that to date, there is no doubt that the most effective approach is to combine strength exercises with cardiorespiratory exercises. “This is not only to address obesity but also because, beyond weight impact, this training has proven additional benefits, such as increased oxygenation and improved cognitive capacity.”
Finally, regarding the challenges this shift in focus poses for exercise specialists, Dr. Butragueño pointed out, “Synergies in obesity treatment require sports experts to receive training in other disciplines, elevating our knowledge level and communication with the medical community to emphasize that we are indeed talking about exercise physiology applied to a condition like obesity.”
“In addition, as scientists, we must challenge ourselves to disseminate information at the societal level, surpassing the typical and outdated message of ‘eat less and move more,’ which we know is incorrect. This simplistic formula doesn’t help many patients resolve their issues like fatty liver, diabetes, and other metabolic disorders,” he concluded.
Active Breaks
Other topics debated during the congress included the importance of making exercise prescription a de facto reality in clinical practice and the challenge of achieving therapeutic compliance.
According to experts, one of the well-positioned trends in this regard is the concept of “active breaks” or “exercise snacks.” These breaks involve engaging in short-duration, moderate- to high-intensity activities throughout the day or working hours.
César Bustos, a board member of the Spanish Society of Obesity, mentioned that several studies have demonstrated that simple activities like climbing three flights of stairs or engaging in 1-minute training sessions can increase the metabolic equivalent of cardiovascular capacity and cardiorespiratory fitness. This approach could help reduce cardiovascular disease risk and all-cause mortality by 13%-15%.
“Cardiorespiratory fitness is the ability to engage in physical activity. It has been proven to be a more powerful predictor of mortality risk than traditional risk factors such as hypertension, smoking, obesity, hyperlipidemia, and type 2 diabetes,” said Mr. Bustos.
The expert added that these findings on the benefits of exercise snacks are particularly relevant in the current context, where lack of time is the primary obstacle cited by individuals with obesity for not engaging in regular physical activity. In addition, exercise prescription is considered the primary preventive measure for obesity and its associated diseases.
“Exercise is an essential complement to various treatments and strategies aimed at managing obesity and maintaining long-term weight reductions. However, patient compliance with recommended measures to stay active remains low. This deficiency can be overcome with the adoption of exercise snacks or small doses of exercise, which have become the most effective tool for achieving this goal,” he emphasized.
Also, in line with other experts, Mr. Bustos emphasized the importance of combined strength and cardiovascular training within the same session. “Undoubtedly, this is the most effective modality, as recent meta-analyses reflect. There is also a second effective modality for improving cardiometabolic parameters in patients with obesity: Hybrid training, including games, skipping ropes, and various devices.”
Exerkines and Poly Pills
Antonio García-Hermoso, PhD, a specialist in physical activity and sports at Navarrabiomed, University Hospital of Navarra in Pamplona, Spain, provided an update on the latest evidence regarding exerkines, which are molecules released during exercise. Research into these molecules attempts to analyze and understand the complex network of interactions between various exercise response systems.
Dr. García-Hermoso said that in the case of obesity and type 2 diabetes, research focuses on how exercise can affect patients’ exerkine levels and how these molecules can affect cardiometabolic control.
“The results demonstrate that these molecules are associated with multiple benefits, including improved insulin sensitivity and glucose homeostasis,” said Dr. García-Hermoso. “Concerning obesity, regular exercise has been shown to reduce interleukin-6 levels, positively affecting inflammation in these patients, also being associated with increased lipolysis and fatty acid utilization.”
Dr. García-Hermoso considered that studying exerkines supports the importance of individualized exercise prescription, like prescription of diet or medications.
He emphasized the importance of intensity, “which is even more crucial than the type of physical activity. Intense exercise activates physiological mechanisms, such as increased blood lactate levels, favoring the inhibition of ghrelin signaling associated with appetite. Therefore, higher exercise intensity leads to more lactate and greater inhibition of post-training hunger.”
“It is essential to understand that exercise is a poly pill with many advantages, and one of them is that even in small amounts, if intensity is increased, health benefits increase considerably,” Dr. García-Hermoso concluded.
Dr. Butragueño, Mr. Bustos, and Dr. García-Hermoso declared no relevant economic conflicts of interest.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
Exercise should no longer be a mere “complement” or a standard recommendation within healthy lifestyle guidelines, say experts. Recent evidence confirms its physiological importance and endorses its beneficial and therapeutic effects on overall health, particularly in the case of obesity and its comorbidities. These findings emphasized the reasons to include exercise prescription in addressing this condition. This conclusion emerged from discussions among experts in Physical Activity and Sports Sciences during the XIX Congress of the Spanish Society for Obesity, where the role of physical exercise as a therapeutic strategy was analyzed from various perspectives.
Javier Butragueño, PhD, coordinator of the Exercise Working Group at the Spanish Society of Obesity, emphasized the need to “reposition” the role of exercise and the message conveyed to the population. “We must move beyond the typical recommendation to ‘just walk’ and rethink this message. When working with patients with obesity, you realize that, for example, the guideline of 10,000 steps per day makes little sense for those who weigh 140 kg, have been sedentary for a long time, and have not reached 2000 daily steps. Clinically, it becomes evident that current recommendations may not align with the needs of these patients,” he said.
Precision Focus
Dr. Butragueño highlighted the necessity of shifting the central focus from weight-related variables alone. While weight is crucial, evidence suggests that it should be evaluated along with other strategies, such as nutrition and pharmacology.
“The approach must change to view exercise as a metabolism regulator,” said Dr. Butragueño. “For specialists, this means educating the population about the need to stay active for overall health. This is a disruptive message because the prevailing idea, almost obsessive, associates exercise primarily with weight loss, a completely incorrect approach that can even be detrimental in some cases.”
Dr. Butragueño emphasized the supportive role of physical exercise in interventions for these patients. “Data show that it is both an enhancer and a co-adjuvant in strategies that also include psychology and endocrinology. It should be part of the approach to obesity but individualized and phenotyped to give physical activity the necessary dimension in each specific case.”
As an example of this adaptability in therapeutic strategy, Dr. Butragueño referred to addressing binge eating disorder. “In this case, specialists must acknowledge that sports are a third-line option, always behind the psychologist, who plays a primary role. Exercise is used to enhance the emotions triggered through its practice, considering that many of these patients maintain a very negative relationship with their bodies.”
Spanish ‘Prescription Guide’
During his presentation, Dr. Butragueño introduced the positioning document from the Exercise Group of the Spanish Society of Obesity, which is aimed at designing physical activity programs for patients with obesity. He emphasized its importance as a much-needed effort at proposing intervention strategies to guide health professionals and establish a reference framework for collaboration across different approaches to obesity.
Among the noteworthy aspects of the guidelines outlined in this document, Dr. Butragueño highlighted the assessment and classification of physical activity into four levels based on each patient’s physical condition. “This aspect should be studied by the scientific community because ‘humanizing’ exercise prescription by understanding individuals’ needs beyond their BMI is crucial.”
He also discussed the strategy outlined in the document that he said is crucial for implementing an exercise program. “Essentially, it involves two guidelines: First, engage in physical activity for at least 30-60 minutes in what we call zone 2. This includes activities like walking, cycling, or rowing, where one can speak easily with another person or sing without getting out of breath. This is a fundamental part of addressing obesity, as it improves mitochondrial biogenesis, the correct utilization of fatty acids, which is a significant concern in the pathophysiology of obesity and other diseases like cancer.”
The second strategy involves strength training alone or combined with aerobic-cardiovascular exercise. “Studies show that just 20 minutes of strength training 1 day a week for 10 consecutive weeks significantly improves strength levels in sedentary individuals.”
Dr. Butragueño emphasized that to date, there is no doubt that the most effective approach is to combine strength exercises with cardiorespiratory exercises. “This is not only to address obesity but also because, beyond weight impact, this training has proven additional benefits, such as increased oxygenation and improved cognitive capacity.”
Finally, regarding the challenges this shift in focus poses for exercise specialists, Dr. Butragueño pointed out, “Synergies in obesity treatment require sports experts to receive training in other disciplines, elevating our knowledge level and communication with the medical community to emphasize that we are indeed talking about exercise physiology applied to a condition like obesity.”
“In addition, as scientists, we must challenge ourselves to disseminate information at the societal level, surpassing the typical and outdated message of ‘eat less and move more,’ which we know is incorrect. This simplistic formula doesn’t help many patients resolve their issues like fatty liver, diabetes, and other metabolic disorders,” he concluded.
Active Breaks
Other topics debated during the congress included the importance of making exercise prescription a de facto reality in clinical practice and the challenge of achieving therapeutic compliance.
According to experts, one of the well-positioned trends in this regard is the concept of “active breaks” or “exercise snacks.” These breaks involve engaging in short-duration, moderate- to high-intensity activities throughout the day or working hours.
César Bustos, a board member of the Spanish Society of Obesity, mentioned that several studies have demonstrated that simple activities like climbing three flights of stairs or engaging in 1-minute training sessions can increase the metabolic equivalent of cardiovascular capacity and cardiorespiratory fitness. This approach could help reduce cardiovascular disease risk and all-cause mortality by 13%-15%.
“Cardiorespiratory fitness is the ability to engage in physical activity. It has been proven to be a more powerful predictor of mortality risk than traditional risk factors such as hypertension, smoking, obesity, hyperlipidemia, and type 2 diabetes,” said Mr. Bustos.
The expert added that these findings on the benefits of exercise snacks are particularly relevant in the current context, where lack of time is the primary obstacle cited by individuals with obesity for not engaging in regular physical activity. In addition, exercise prescription is considered the primary preventive measure for obesity and its associated diseases.
“Exercise is an essential complement to various treatments and strategies aimed at managing obesity and maintaining long-term weight reductions. However, patient compliance with recommended measures to stay active remains low. This deficiency can be overcome with the adoption of exercise snacks or small doses of exercise, which have become the most effective tool for achieving this goal,” he emphasized.
Also, in line with other experts, Mr. Bustos emphasized the importance of combined strength and cardiovascular training within the same session. “Undoubtedly, this is the most effective modality, as recent meta-analyses reflect. There is also a second effective modality for improving cardiometabolic parameters in patients with obesity: Hybrid training, including games, skipping ropes, and various devices.”
Exerkines and Poly Pills
Antonio García-Hermoso, PhD, a specialist in physical activity and sports at Navarrabiomed, University Hospital of Navarra in Pamplona, Spain, provided an update on the latest evidence regarding exerkines, which are molecules released during exercise. Research into these molecules attempts to analyze and understand the complex network of interactions between various exercise response systems.
Dr. García-Hermoso said that in the case of obesity and type 2 diabetes, research focuses on how exercise can affect patients’ exerkine levels and how these molecules can affect cardiometabolic control.
“The results demonstrate that these molecules are associated with multiple benefits, including improved insulin sensitivity and glucose homeostasis,” said Dr. García-Hermoso. “Concerning obesity, regular exercise has been shown to reduce interleukin-6 levels, positively affecting inflammation in these patients, also being associated with increased lipolysis and fatty acid utilization.”
Dr. García-Hermoso considered that studying exerkines supports the importance of individualized exercise prescription, like prescription of diet or medications.
He emphasized the importance of intensity, “which is even more crucial than the type of physical activity. Intense exercise activates physiological mechanisms, such as increased blood lactate levels, favoring the inhibition of ghrelin signaling associated with appetite. Therefore, higher exercise intensity leads to more lactate and greater inhibition of post-training hunger.”
“It is essential to understand that exercise is a poly pill with many advantages, and one of them is that even in small amounts, if intensity is increased, health benefits increase considerably,” Dr. García-Hermoso concluded.
Dr. Butragueño, Mr. Bustos, and Dr. García-Hermoso declared no relevant economic conflicts of interest.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
Exercise should no longer be a mere “complement” or a standard recommendation within healthy lifestyle guidelines, say experts. Recent evidence confirms its physiological importance and endorses its beneficial and therapeutic effects on overall health, particularly in the case of obesity and its comorbidities. These findings emphasized the reasons to include exercise prescription in addressing this condition. This conclusion emerged from discussions among experts in Physical Activity and Sports Sciences during the XIX Congress of the Spanish Society for Obesity, where the role of physical exercise as a therapeutic strategy was analyzed from various perspectives.
Javier Butragueño, PhD, coordinator of the Exercise Working Group at the Spanish Society of Obesity, emphasized the need to “reposition” the role of exercise and the message conveyed to the population. “We must move beyond the typical recommendation to ‘just walk’ and rethink this message. When working with patients with obesity, you realize that, for example, the guideline of 10,000 steps per day makes little sense for those who weigh 140 kg, have been sedentary for a long time, and have not reached 2000 daily steps. Clinically, it becomes evident that current recommendations may not align with the needs of these patients,” he said.
Precision Focus
Dr. Butragueño highlighted the necessity of shifting the central focus from weight-related variables alone. While weight is crucial, evidence suggests that it should be evaluated along with other strategies, such as nutrition and pharmacology.
“The approach must change to view exercise as a metabolism regulator,” said Dr. Butragueño. “For specialists, this means educating the population about the need to stay active for overall health. This is a disruptive message because the prevailing idea, almost obsessive, associates exercise primarily with weight loss, a completely incorrect approach that can even be detrimental in some cases.”
Dr. Butragueño emphasized the supportive role of physical exercise in interventions for these patients. “Data show that it is both an enhancer and a co-adjuvant in strategies that also include psychology and endocrinology. It should be part of the approach to obesity but individualized and phenotyped to give physical activity the necessary dimension in each specific case.”
As an example of this adaptability in therapeutic strategy, Dr. Butragueño referred to addressing binge eating disorder. “In this case, specialists must acknowledge that sports are a third-line option, always behind the psychologist, who plays a primary role. Exercise is used to enhance the emotions triggered through its practice, considering that many of these patients maintain a very negative relationship with their bodies.”
Spanish ‘Prescription Guide’
During his presentation, Dr. Butragueño introduced the positioning document from the Exercise Group of the Spanish Society of Obesity, which is aimed at designing physical activity programs for patients with obesity. He emphasized its importance as a much-needed effort at proposing intervention strategies to guide health professionals and establish a reference framework for collaboration across different approaches to obesity.
Among the noteworthy aspects of the guidelines outlined in this document, Dr. Butragueño highlighted the assessment and classification of physical activity into four levels based on each patient’s physical condition. “This aspect should be studied by the scientific community because ‘humanizing’ exercise prescription by understanding individuals’ needs beyond their BMI is crucial.”
He also discussed the strategy outlined in the document that he said is crucial for implementing an exercise program. “Essentially, it involves two guidelines: First, engage in physical activity for at least 30-60 minutes in what we call zone 2. This includes activities like walking, cycling, or rowing, where one can speak easily with another person or sing without getting out of breath. This is a fundamental part of addressing obesity, as it improves mitochondrial biogenesis, the correct utilization of fatty acids, which is a significant concern in the pathophysiology of obesity and other diseases like cancer.”
The second strategy involves strength training alone or combined with aerobic-cardiovascular exercise. “Studies show that just 20 minutes of strength training 1 day a week for 10 consecutive weeks significantly improves strength levels in sedentary individuals.”
Dr. Butragueño emphasized that to date, there is no doubt that the most effective approach is to combine strength exercises with cardiorespiratory exercises. “This is not only to address obesity but also because, beyond weight impact, this training has proven additional benefits, such as increased oxygenation and improved cognitive capacity.”
Finally, regarding the challenges this shift in focus poses for exercise specialists, Dr. Butragueño pointed out, “Synergies in obesity treatment require sports experts to receive training in other disciplines, elevating our knowledge level and communication with the medical community to emphasize that we are indeed talking about exercise physiology applied to a condition like obesity.”
“In addition, as scientists, we must challenge ourselves to disseminate information at the societal level, surpassing the typical and outdated message of ‘eat less and move more,’ which we know is incorrect. This simplistic formula doesn’t help many patients resolve their issues like fatty liver, diabetes, and other metabolic disorders,” he concluded.
Active Breaks
Other topics debated during the congress included the importance of making exercise prescription a de facto reality in clinical practice and the challenge of achieving therapeutic compliance.
According to experts, one of the well-positioned trends in this regard is the concept of “active breaks” or “exercise snacks.” These breaks involve engaging in short-duration, moderate- to high-intensity activities throughout the day or working hours.
César Bustos, a board member of the Spanish Society of Obesity, mentioned that several studies have demonstrated that simple activities like climbing three flights of stairs or engaging in 1-minute training sessions can increase the metabolic equivalent of cardiovascular capacity and cardiorespiratory fitness. This approach could help reduce cardiovascular disease risk and all-cause mortality by 13%-15%.
“Cardiorespiratory fitness is the ability to engage in physical activity. It has been proven to be a more powerful predictor of mortality risk than traditional risk factors such as hypertension, smoking, obesity, hyperlipidemia, and type 2 diabetes,” said Mr. Bustos.
The expert added that these findings on the benefits of exercise snacks are particularly relevant in the current context, where lack of time is the primary obstacle cited by individuals with obesity for not engaging in regular physical activity. In addition, exercise prescription is considered the primary preventive measure for obesity and its associated diseases.
“Exercise is an essential complement to various treatments and strategies aimed at managing obesity and maintaining long-term weight reductions. However, patient compliance with recommended measures to stay active remains low. This deficiency can be overcome with the adoption of exercise snacks or small doses of exercise, which have become the most effective tool for achieving this goal,” he emphasized.
Also, in line with other experts, Mr. Bustos emphasized the importance of combined strength and cardiovascular training within the same session. “Undoubtedly, this is the most effective modality, as recent meta-analyses reflect. There is also a second effective modality for improving cardiometabolic parameters in patients with obesity: Hybrid training, including games, skipping ropes, and various devices.”
Exerkines and Poly Pills
Antonio García-Hermoso, PhD, a specialist in physical activity and sports at Navarrabiomed, University Hospital of Navarra in Pamplona, Spain, provided an update on the latest evidence regarding exerkines, which are molecules released during exercise. Research into these molecules attempts to analyze and understand the complex network of interactions between various exercise response systems.
Dr. García-Hermoso said that in the case of obesity and type 2 diabetes, research focuses on how exercise can affect patients’ exerkine levels and how these molecules can affect cardiometabolic control.
“The results demonstrate that these molecules are associated with multiple benefits, including improved insulin sensitivity and glucose homeostasis,” said Dr. García-Hermoso. “Concerning obesity, regular exercise has been shown to reduce interleukin-6 levels, positively affecting inflammation in these patients, also being associated with increased lipolysis and fatty acid utilization.”
Dr. García-Hermoso considered that studying exerkines supports the importance of individualized exercise prescription, like prescription of diet or medications.
He emphasized the importance of intensity, “which is even more crucial than the type of physical activity. Intense exercise activates physiological mechanisms, such as increased blood lactate levels, favoring the inhibition of ghrelin signaling associated with appetite. Therefore, higher exercise intensity leads to more lactate and greater inhibition of post-training hunger.”
“It is essential to understand that exercise is a poly pill with many advantages, and one of them is that even in small amounts, if intensity is increased, health benefits increase considerably,” Dr. García-Hermoso concluded.
Dr. Butragueño, Mr. Bustos, and Dr. García-Hermoso declared no relevant economic conflicts of interest.
This article was translated from the Medscape Spanish edition. A version of this article appeared on Medscape.com.
Freedom of Speech and Gender-Affirming Care
Blue Hill is a small idyllic town a little less than two and a half hours Down East the coast from where I am sitting here in Harpswell. Thanks to gentrification it tends to lean left politically, but like the rest of Maine most folks in the surrounding communities often don’t know or care much about their neighbor’s party affiliation. Its library, founded in 1796, is well funded and a source of civic pride.
One day a couple of years ago, the library director received a donated book from a patron. Although he personally didn’t agree with the book’s message, he felt it deserved a space in their collection dealing with the subject. What happened in the wake of this donation is an ugly tale. Some community members objected to the book and asked that it be removed from the shelves, or at least kept under the desk and loaned out only on request.
The objectors, many of whom knew the director, were confrontational. The collections committee unanimously supported his decision. Some committee members also received similar responses from community members. Remember, this is a small town.
A request for support sent to the American Library Association was basically ignored. Over the next 2 years things have quieted, but fractured friendships and relationships in this quiet coastal Maine town have not been repaired. However, as the librarian has observed, “intellectual freedom or the freedom of speech isn’t there just to protect the ideas that we like.”
While the title of the book may feel inflammatory to some, every publisher hopes to grab the market’s attention with a hot title. The cause of this sad situation in Blue Hill was not a white supremacist’s polemic offering specific ways to create genocide. This was a book suggesting that gender dysphoria presenting in adolescence may have multiple causes and raises concerns about the wisdom of the pace of some gender-affirming care.
Clearly the topic of gender dysphoria in adolescence has become a third rail that must be approached with caution or completely avoided. A recent opinion piece in the New York Times provides even more concerning examples of this peril. Again, the eye-catching title of the article — As Kids, They Thought They Were Trans. They No Longer Do — draws in the audience eager to read about some unfortunate individuals who have regretted their decision to transition and are now detransitioning.
If you are interested in hearing anecdotal evidence and opinions supporting the notion that there is such a thing as rapid-onset gender dysphoria, I suggest you read the entire piece. However, the article’s most troubling message for me comes when I read about the professionals who were former gender-related care providers who left the field because of “pushback, the accusations of being transphobic, from being pro-assessment and wanting a more thorough process.”
One therapist trained in gender-affirming care who began to have doubts about the model and spoke out in favor of a more measured approach was investigated by her licensing board after transgender advocates threatened to report her. Ultimately, her case was dismissed, but she continues to fear for her safety.
Gender-related healthcare is another sad example of how in this country it is the noise coming from the advocates on the extremes of the issue that is drowning out the “vast ideological middle” that is seeking civil and rational discussions.
In this situation there are those who want to make it illegal for the healthcare providers to help patients who might benefit from transitioning. On the other end of the spectrum are those advocates who are unwilling to acknowledge that there may be some adolescents with what has been called by some “rapid-onset gender dysphoria.”
The landscape on which this tragedy is being played out is changing so quickly that there will be no correct answers in the short term. There just isn’t enough data. However, there is enough anecdotal evidence from professionals who were and still are practicing gender-related care to raise a concern that something is happening in the adolescent population that suggests some individuals with gender dysphoria should be managed in a different way than the currently accepted gender-affirming model. The size of this subgroup is up for debate and we may never learn it because of reporting bias and privacy concerns.
The American Academy of Pediatrics has recently authorized a systematic review of gender-affirming care. I hope that, like the librarian in Blue Hill, it will have the courage to include all the evidence available even though, as we have seen here in Maine, some of it may spark a firestorm of vehement responses.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Blue Hill is a small idyllic town a little less than two and a half hours Down East the coast from where I am sitting here in Harpswell. Thanks to gentrification it tends to lean left politically, but like the rest of Maine most folks in the surrounding communities often don’t know or care much about their neighbor’s party affiliation. Its library, founded in 1796, is well funded and a source of civic pride.
One day a couple of years ago, the library director received a donated book from a patron. Although he personally didn’t agree with the book’s message, he felt it deserved a space in their collection dealing with the subject. What happened in the wake of this donation is an ugly tale. Some community members objected to the book and asked that it be removed from the shelves, or at least kept under the desk and loaned out only on request.
The objectors, many of whom knew the director, were confrontational. The collections committee unanimously supported his decision. Some committee members also received similar responses from community members. Remember, this is a small town.
A request for support sent to the American Library Association was basically ignored. Over the next 2 years things have quieted, but fractured friendships and relationships in this quiet coastal Maine town have not been repaired. However, as the librarian has observed, “intellectual freedom or the freedom of speech isn’t there just to protect the ideas that we like.”
While the title of the book may feel inflammatory to some, every publisher hopes to grab the market’s attention with a hot title. The cause of this sad situation in Blue Hill was not a white supremacist’s polemic offering specific ways to create genocide. This was a book suggesting that gender dysphoria presenting in adolescence may have multiple causes and raises concerns about the wisdom of the pace of some gender-affirming care.
Clearly the topic of gender dysphoria in adolescence has become a third rail that must be approached with caution or completely avoided. A recent opinion piece in the New York Times provides even more concerning examples of this peril. Again, the eye-catching title of the article — As Kids, They Thought They Were Trans. They No Longer Do — draws in the audience eager to read about some unfortunate individuals who have regretted their decision to transition and are now detransitioning.
If you are interested in hearing anecdotal evidence and opinions supporting the notion that there is such a thing as rapid-onset gender dysphoria, I suggest you read the entire piece. However, the article’s most troubling message for me comes when I read about the professionals who were former gender-related care providers who left the field because of “pushback, the accusations of being transphobic, from being pro-assessment and wanting a more thorough process.”
One therapist trained in gender-affirming care who began to have doubts about the model and spoke out in favor of a more measured approach was investigated by her licensing board after transgender advocates threatened to report her. Ultimately, her case was dismissed, but she continues to fear for her safety.
Gender-related healthcare is another sad example of how in this country it is the noise coming from the advocates on the extremes of the issue that is drowning out the “vast ideological middle” that is seeking civil and rational discussions.
In this situation there are those who want to make it illegal for the healthcare providers to help patients who might benefit from transitioning. On the other end of the spectrum are those advocates who are unwilling to acknowledge that there may be some adolescents with what has been called by some “rapid-onset gender dysphoria.”
The landscape on which this tragedy is being played out is changing so quickly that there will be no correct answers in the short term. There just isn’t enough data. However, there is enough anecdotal evidence from professionals who were and still are practicing gender-related care to raise a concern that something is happening in the adolescent population that suggests some individuals with gender dysphoria should be managed in a different way than the currently accepted gender-affirming model. The size of this subgroup is up for debate and we may never learn it because of reporting bias and privacy concerns.
The American Academy of Pediatrics has recently authorized a systematic review of gender-affirming care. I hope that, like the librarian in Blue Hill, it will have the courage to include all the evidence available even though, as we have seen here in Maine, some of it may spark a firestorm of vehement responses.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Blue Hill is a small idyllic town a little less than two and a half hours Down East the coast from where I am sitting here in Harpswell. Thanks to gentrification it tends to lean left politically, but like the rest of Maine most folks in the surrounding communities often don’t know or care much about their neighbor’s party affiliation. Its library, founded in 1796, is well funded and a source of civic pride.
One day a couple of years ago, the library director received a donated book from a patron. Although he personally didn’t agree with the book’s message, he felt it deserved a space in their collection dealing with the subject. What happened in the wake of this donation is an ugly tale. Some community members objected to the book and asked that it be removed from the shelves, or at least kept under the desk and loaned out only on request.
The objectors, many of whom knew the director, were confrontational. The collections committee unanimously supported his decision. Some committee members also received similar responses from community members. Remember, this is a small town.
A request for support sent to the American Library Association was basically ignored. Over the next 2 years things have quieted, but fractured friendships and relationships in this quiet coastal Maine town have not been repaired. However, as the librarian has observed, “intellectual freedom or the freedom of speech isn’t there just to protect the ideas that we like.”
While the title of the book may feel inflammatory to some, every publisher hopes to grab the market’s attention with a hot title. The cause of this sad situation in Blue Hill was not a white supremacist’s polemic offering specific ways to create genocide. This was a book suggesting that gender dysphoria presenting in adolescence may have multiple causes and raises concerns about the wisdom of the pace of some gender-affirming care.
Clearly the topic of gender dysphoria in adolescence has become a third rail that must be approached with caution or completely avoided. A recent opinion piece in the New York Times provides even more concerning examples of this peril. Again, the eye-catching title of the article — As Kids, They Thought They Were Trans. They No Longer Do — draws in the audience eager to read about some unfortunate individuals who have regretted their decision to transition and are now detransitioning.
If you are interested in hearing anecdotal evidence and opinions supporting the notion that there is such a thing as rapid-onset gender dysphoria, I suggest you read the entire piece. However, the article’s most troubling message for me comes when I read about the professionals who were former gender-related care providers who left the field because of “pushback, the accusations of being transphobic, from being pro-assessment and wanting a more thorough process.”
One therapist trained in gender-affirming care who began to have doubts about the model and spoke out in favor of a more measured approach was investigated by her licensing board after transgender advocates threatened to report her. Ultimately, her case was dismissed, but she continues to fear for her safety.
Gender-related healthcare is another sad example of how in this country it is the noise coming from the advocates on the extremes of the issue that is drowning out the “vast ideological middle” that is seeking civil and rational discussions.
In this situation there are those who want to make it illegal for the healthcare providers to help patients who might benefit from transitioning. On the other end of the spectrum are those advocates who are unwilling to acknowledge that there may be some adolescents with what has been called by some “rapid-onset gender dysphoria.”
The landscape on which this tragedy is being played out is changing so quickly that there will be no correct answers in the short term. There just isn’t enough data. However, there is enough anecdotal evidence from professionals who were and still are practicing gender-related care to raise a concern that something is happening in the adolescent population that suggests some individuals with gender dysphoria should be managed in a different way than the currently accepted gender-affirming model. The size of this subgroup is up for debate and we may never learn it because of reporting bias and privacy concerns.
The American Academy of Pediatrics has recently authorized a systematic review of gender-affirming care. I hope that, like the librarian in Blue Hill, it will have the courage to include all the evidence available even though, as we have seen here in Maine, some of it may spark a firestorm of vehement responses.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
‘It’s Time’ to Empower Care for Patients With Obesity
A few weeks ago, I made a patient who lost 100 pounds following a sleeve gastrectomy 9 months prior feel bad because I told her she lost too much weight. As I spoke to her, I realized that she found it hard to make life changes and that the surgery was a huge aide in changing her life and her lifestyle. I ended up apologizing for initially saying she lost too much weight.
For the first time in her life, she was successful in losing weight and keeping it off. The surgery allowed her body to defend a lower body weight by altering the secretion of gut hormones that lead to satiety in the brain. It’s not her fault that her body responded so well!
I asked her to be on my next orientation virtual meeting with prospective weight management patients to urge those with a body mass index (BMI) > 40 to consider bariatric surgery as the most effective durable and safe treatment for their degree of obesity.
Metabolic bariatric surgery, primarily sleeve gastrectomy and Roux-en-Y gastric bypass , alters the gut hormone milieu such that the body defends a lower mass of adipose tissue and a lower weight. We have learned what it takes to alter body weight defense to a healthy lower weight by studying why metabolic bariatric surgery works so well. It turns out that there are several hormones secreted by the gut that allow the brain to register fullness.
One of these gut hormones, glucagon-like peptide (GLP)-1, has been researched as an analog to help reduce body weight by 16% and has also been shown to reduce cardiovascular risk in the SELECT trial, as published in The New England Journal of Medicine (NEJM).
It’s the first weight loss medication to be shown in a cardiovascular outcomes trial to be superior to placebo in reduction of major cardiovascular events, including cardiovascular deaths, nonfatal myocardial infarction, and nonfatal stroke. The results presented at the 2023 American Heart Association meetings in Philadelphia ended in wholehearted applause by a “standing only” audience even before the presentation’s conclusion.
As we pave the way for nutrient-stimulated hormone (NuSH) therapies to be prescribed to all Americans with a BMI > 30 to improve health, we need to remember what these medications actually do. We used to think that metabolic bariatric surgery worked by restricting the stomach contents and malabsorbing nutrients. We now know that the surgeries work by altering NuSH secretion, allowing for less secretion of the hunger hormone ghrelin and more secretion of GLP-1, glucose-dependent insulinotropic polypeptide (GIP), peptide YY (PYY), cholecystokinin (CCK), oxyntomodulin (OXM), and other satiety hormones with less food ingestion.
They have pleiotropic effects on many organ systems in the body, including the brain, heart, adipose tissue, and liver. They decrease inflammation and also increase satiety and delay gastric emptying. None of these effects automatically produce weight loss, but they certainly aid in the adoption of a healthier body weight and better health. The weight loss occurs because these medications steer the body toward behavioral changes that promote weight loss.
As we delve into the SELECT trial results, a 20% reduction in major cardiovascular events was accompanied by an average weight loss of 9.6%, without a behavioral component added to either the placebo or intervention arms, as is usual in antiobesity agent trials.
Does this mean that primary care providers (PCPs) don’t have to educate patients on behavior change, diet, and exercise therapy? Well, if we consider obesity a disease as we do type 2 diabetes and dyslipidemia or hypertension, then no — PCPs don’t have to, just like they don’t in treating these other diseases.
However, we should rethink this practice. The recently published SURMOUNT-3 trial looked at another NuSH, tirzepatide, with intensive behavioral therapy; it resulted in a 26.6% weight loss, which is comparable to results with bariatric surgery. The SURMOUNT-1 trial of tirzepatide with nonintensive behavioral therapy resulted in a 20.9% weight loss, which is still substantial, but SURMOUNT-3 showed how much more is achievable with robust behavior-change therapy.
In other words, it’s time that PCPs provide education on behavior change to maximize the power of the medications prescribed in practice for the most common diseases suffered in the United States: obesity, type 2 diabetes, cardiovascular disease, and hypertension. These are all chronic, relapsing diseases. Medication alone will improve numbers (weight, blood glucose, A1c, and blood pressure), but a relapsing disease continues relentlessly as patients age to overcome the medications prescribed.
Today I made another patient feel bad because she lost over 100 pounds on semaglutide (Wegovy) 2.4 mg over 1 year, reducing her BMI from 57 to 36. She wanted to keep losing, so I recommended sleeve gastrectomy to lose more weight. I told her she could always restart the Wegovy after the procedure if needed.
We really don’t have an answer to this issue of NuSH therapy not getting to goal and bariatric surgery following medication therapy. The reality is that bariatric surgery should be considered a safe, effective treatment for extreme obesity somewhere along the trajectory of treatments starting with behavior (diet, exercise) and medications. It is still considered a last resort, and for some, just too aggressive.
We have learned much about the incretin hormones and what they can accomplish for obesity from studying bariatric — now called metabolic — surgery. Surgery should be seen as we see stent placement for angina, only more effective for longevity. The COURAGE trial, published in 2007 in NEJM, showed that when compared with medication treatment alone for angina, stent placement plus medications resulted in no difference in mortality after a 7-year follow-up period. Compare this to bariatric surgery, which in many retrospective analyses shows a 20% reduction in cardiovascular mortality after 20-year follow-up (Swedish Obesity Study). In the United States, there are 2 million stent procedures performed per year vs 250,000 bariatric surgical procedures. There are millions of Americans with a BMI > 40 and, yes, millions of Americans with angina. I think I make my point that we need to do more bariatric surgeries to effectively treat extreme obesity.
The solution to this negligent medical practice in obesity treatment is to empower PCPs to treat obesity (at least uncomplicated obesity) and refer to obesity medicine practices for complicated obesity with multiple complications, such as type 2 diabetes and cardiovascular disease, and to refer to obesity medicine practices with a surgical component for BMIs > 40 or > 35 with type 2 diabetes, sleep apnea, and/or cardiovascular disease or other serious conditions.
How do we empower PCPs? Insurance coverage of NuSH therapies due to life-saving properties — as evidenced by the SELECT trial — without prior authorizations; and education on how and why metabolic surgery works, as well as education on behavioral approaches, such as healthy diet and exercise, as a core therapy for all BMI categories.
It’s time.
Caroline Apovian, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Altimmune; Cowen and Company; Currax Pharmaceuticals; EPG Communication Holdings; Gelesis, Srl; L-Nutra; and NeuroBo Pharmaceuticals. Received research grant from: National Institutes of Health; Patient-Centered Outcomes Research Institute; and GI Dynamics. Received income in an amount equal to or greater than $250 from: Altimmune; Cowen and Company; NeuroBo Pharmaceuticals; and Novo Nordisk.
A version of this article appeared on Medscape.com.
A few weeks ago, I made a patient who lost 100 pounds following a sleeve gastrectomy 9 months prior feel bad because I told her she lost too much weight. As I spoke to her, I realized that she found it hard to make life changes and that the surgery was a huge aide in changing her life and her lifestyle. I ended up apologizing for initially saying she lost too much weight.
For the first time in her life, she was successful in losing weight and keeping it off. The surgery allowed her body to defend a lower body weight by altering the secretion of gut hormones that lead to satiety in the brain. It’s not her fault that her body responded so well!
I asked her to be on my next orientation virtual meeting with prospective weight management patients to urge those with a body mass index (BMI) > 40 to consider bariatric surgery as the most effective durable and safe treatment for their degree of obesity.
Metabolic bariatric surgery, primarily sleeve gastrectomy and Roux-en-Y gastric bypass , alters the gut hormone milieu such that the body defends a lower mass of adipose tissue and a lower weight. We have learned what it takes to alter body weight defense to a healthy lower weight by studying why metabolic bariatric surgery works so well. It turns out that there are several hormones secreted by the gut that allow the brain to register fullness.
One of these gut hormones, glucagon-like peptide (GLP)-1, has been researched as an analog to help reduce body weight by 16% and has also been shown to reduce cardiovascular risk in the SELECT trial, as published in The New England Journal of Medicine (NEJM).
It’s the first weight loss medication to be shown in a cardiovascular outcomes trial to be superior to placebo in reduction of major cardiovascular events, including cardiovascular deaths, nonfatal myocardial infarction, and nonfatal stroke. The results presented at the 2023 American Heart Association meetings in Philadelphia ended in wholehearted applause by a “standing only” audience even before the presentation’s conclusion.
As we pave the way for nutrient-stimulated hormone (NuSH) therapies to be prescribed to all Americans with a BMI > 30 to improve health, we need to remember what these medications actually do. We used to think that metabolic bariatric surgery worked by restricting the stomach contents and malabsorbing nutrients. We now know that the surgeries work by altering NuSH secretion, allowing for less secretion of the hunger hormone ghrelin and more secretion of GLP-1, glucose-dependent insulinotropic polypeptide (GIP), peptide YY (PYY), cholecystokinin (CCK), oxyntomodulin (OXM), and other satiety hormones with less food ingestion.
They have pleiotropic effects on many organ systems in the body, including the brain, heart, adipose tissue, and liver. They decrease inflammation and also increase satiety and delay gastric emptying. None of these effects automatically produce weight loss, but they certainly aid in the adoption of a healthier body weight and better health. The weight loss occurs because these medications steer the body toward behavioral changes that promote weight loss.
As we delve into the SELECT trial results, a 20% reduction in major cardiovascular events was accompanied by an average weight loss of 9.6%, without a behavioral component added to either the placebo or intervention arms, as is usual in antiobesity agent trials.
Does this mean that primary care providers (PCPs) don’t have to educate patients on behavior change, diet, and exercise therapy? Well, if we consider obesity a disease as we do type 2 diabetes and dyslipidemia or hypertension, then no — PCPs don’t have to, just like they don’t in treating these other diseases.
However, we should rethink this practice. The recently published SURMOUNT-3 trial looked at another NuSH, tirzepatide, with intensive behavioral therapy; it resulted in a 26.6% weight loss, which is comparable to results with bariatric surgery. The SURMOUNT-1 trial of tirzepatide with nonintensive behavioral therapy resulted in a 20.9% weight loss, which is still substantial, but SURMOUNT-3 showed how much more is achievable with robust behavior-change therapy.
In other words, it’s time that PCPs provide education on behavior change to maximize the power of the medications prescribed in practice for the most common diseases suffered in the United States: obesity, type 2 diabetes, cardiovascular disease, and hypertension. These are all chronic, relapsing diseases. Medication alone will improve numbers (weight, blood glucose, A1c, and blood pressure), but a relapsing disease continues relentlessly as patients age to overcome the medications prescribed.
Today I made another patient feel bad because she lost over 100 pounds on semaglutide (Wegovy) 2.4 mg over 1 year, reducing her BMI from 57 to 36. She wanted to keep losing, so I recommended sleeve gastrectomy to lose more weight. I told her she could always restart the Wegovy after the procedure if needed.
We really don’t have an answer to this issue of NuSH therapy not getting to goal and bariatric surgery following medication therapy. The reality is that bariatric surgery should be considered a safe, effective treatment for extreme obesity somewhere along the trajectory of treatments starting with behavior (diet, exercise) and medications. It is still considered a last resort, and for some, just too aggressive.
We have learned much about the incretin hormones and what they can accomplish for obesity from studying bariatric — now called metabolic — surgery. Surgery should be seen as we see stent placement for angina, only more effective for longevity. The COURAGE trial, published in 2007 in NEJM, showed that when compared with medication treatment alone for angina, stent placement plus medications resulted in no difference in mortality after a 7-year follow-up period. Compare this to bariatric surgery, which in many retrospective analyses shows a 20% reduction in cardiovascular mortality after 20-year follow-up (Swedish Obesity Study). In the United States, there are 2 million stent procedures performed per year vs 250,000 bariatric surgical procedures. There are millions of Americans with a BMI > 40 and, yes, millions of Americans with angina. I think I make my point that we need to do more bariatric surgeries to effectively treat extreme obesity.
The solution to this negligent medical practice in obesity treatment is to empower PCPs to treat obesity (at least uncomplicated obesity) and refer to obesity medicine practices for complicated obesity with multiple complications, such as type 2 diabetes and cardiovascular disease, and to refer to obesity medicine practices with a surgical component for BMIs > 40 or > 35 with type 2 diabetes, sleep apnea, and/or cardiovascular disease or other serious conditions.
How do we empower PCPs? Insurance coverage of NuSH therapies due to life-saving properties — as evidenced by the SELECT trial — without prior authorizations; and education on how and why metabolic surgery works, as well as education on behavioral approaches, such as healthy diet and exercise, as a core therapy for all BMI categories.
It’s time.
Caroline Apovian, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Altimmune; Cowen and Company; Currax Pharmaceuticals; EPG Communication Holdings; Gelesis, Srl; L-Nutra; and NeuroBo Pharmaceuticals. Received research grant from: National Institutes of Health; Patient-Centered Outcomes Research Institute; and GI Dynamics. Received income in an amount equal to or greater than $250 from: Altimmune; Cowen and Company; NeuroBo Pharmaceuticals; and Novo Nordisk.
A version of this article appeared on Medscape.com.
A few weeks ago, I made a patient who lost 100 pounds following a sleeve gastrectomy 9 months prior feel bad because I told her she lost too much weight. As I spoke to her, I realized that she found it hard to make life changes and that the surgery was a huge aide in changing her life and her lifestyle. I ended up apologizing for initially saying she lost too much weight.
For the first time in her life, she was successful in losing weight and keeping it off. The surgery allowed her body to defend a lower body weight by altering the secretion of gut hormones that lead to satiety in the brain. It’s not her fault that her body responded so well!
I asked her to be on my next orientation virtual meeting with prospective weight management patients to urge those with a body mass index (BMI) > 40 to consider bariatric surgery as the most effective durable and safe treatment for their degree of obesity.
Metabolic bariatric surgery, primarily sleeve gastrectomy and Roux-en-Y gastric bypass , alters the gut hormone milieu such that the body defends a lower mass of adipose tissue and a lower weight. We have learned what it takes to alter body weight defense to a healthy lower weight by studying why metabolic bariatric surgery works so well. It turns out that there are several hormones secreted by the gut that allow the brain to register fullness.
One of these gut hormones, glucagon-like peptide (GLP)-1, has been researched as an analog to help reduce body weight by 16% and has also been shown to reduce cardiovascular risk in the SELECT trial, as published in The New England Journal of Medicine (NEJM).
It’s the first weight loss medication to be shown in a cardiovascular outcomes trial to be superior to placebo in reduction of major cardiovascular events, including cardiovascular deaths, nonfatal myocardial infarction, and nonfatal stroke. The results presented at the 2023 American Heart Association meetings in Philadelphia ended in wholehearted applause by a “standing only” audience even before the presentation’s conclusion.
As we pave the way for nutrient-stimulated hormone (NuSH) therapies to be prescribed to all Americans with a BMI > 30 to improve health, we need to remember what these medications actually do. We used to think that metabolic bariatric surgery worked by restricting the stomach contents and malabsorbing nutrients. We now know that the surgeries work by altering NuSH secretion, allowing for less secretion of the hunger hormone ghrelin and more secretion of GLP-1, glucose-dependent insulinotropic polypeptide (GIP), peptide YY (PYY), cholecystokinin (CCK), oxyntomodulin (OXM), and other satiety hormones with less food ingestion.
They have pleiotropic effects on many organ systems in the body, including the brain, heart, adipose tissue, and liver. They decrease inflammation and also increase satiety and delay gastric emptying. None of these effects automatically produce weight loss, but they certainly aid in the adoption of a healthier body weight and better health. The weight loss occurs because these medications steer the body toward behavioral changes that promote weight loss.
As we delve into the SELECT trial results, a 20% reduction in major cardiovascular events was accompanied by an average weight loss of 9.6%, without a behavioral component added to either the placebo or intervention arms, as is usual in antiobesity agent trials.
Does this mean that primary care providers (PCPs) don’t have to educate patients on behavior change, diet, and exercise therapy? Well, if we consider obesity a disease as we do type 2 diabetes and dyslipidemia or hypertension, then no — PCPs don’t have to, just like they don’t in treating these other diseases.
However, we should rethink this practice. The recently published SURMOUNT-3 trial looked at another NuSH, tirzepatide, with intensive behavioral therapy; it resulted in a 26.6% weight loss, which is comparable to results with bariatric surgery. The SURMOUNT-1 trial of tirzepatide with nonintensive behavioral therapy resulted in a 20.9% weight loss, which is still substantial, but SURMOUNT-3 showed how much more is achievable with robust behavior-change therapy.
In other words, it’s time that PCPs provide education on behavior change to maximize the power of the medications prescribed in practice for the most common diseases suffered in the United States: obesity, type 2 diabetes, cardiovascular disease, and hypertension. These are all chronic, relapsing diseases. Medication alone will improve numbers (weight, blood glucose, A1c, and blood pressure), but a relapsing disease continues relentlessly as patients age to overcome the medications prescribed.
Today I made another patient feel bad because she lost over 100 pounds on semaglutide (Wegovy) 2.4 mg over 1 year, reducing her BMI from 57 to 36. She wanted to keep losing, so I recommended sleeve gastrectomy to lose more weight. I told her she could always restart the Wegovy after the procedure if needed.
We really don’t have an answer to this issue of NuSH therapy not getting to goal and bariatric surgery following medication therapy. The reality is that bariatric surgery should be considered a safe, effective treatment for extreme obesity somewhere along the trajectory of treatments starting with behavior (diet, exercise) and medications. It is still considered a last resort, and for some, just too aggressive.
We have learned much about the incretin hormones and what they can accomplish for obesity from studying bariatric — now called metabolic — surgery. Surgery should be seen as we see stent placement for angina, only more effective for longevity. The COURAGE trial, published in 2007 in NEJM, showed that when compared with medication treatment alone for angina, stent placement plus medications resulted in no difference in mortality after a 7-year follow-up period. Compare this to bariatric surgery, which in many retrospective analyses shows a 20% reduction in cardiovascular mortality after 20-year follow-up (Swedish Obesity Study). In the United States, there are 2 million stent procedures performed per year vs 250,000 bariatric surgical procedures. There are millions of Americans with a BMI > 40 and, yes, millions of Americans with angina. I think I make my point that we need to do more bariatric surgeries to effectively treat extreme obesity.
The solution to this negligent medical practice in obesity treatment is to empower PCPs to treat obesity (at least uncomplicated obesity) and refer to obesity medicine practices for complicated obesity with multiple complications, such as type 2 diabetes and cardiovascular disease, and to refer to obesity medicine practices with a surgical component for BMIs > 40 or > 35 with type 2 diabetes, sleep apnea, and/or cardiovascular disease or other serious conditions.
How do we empower PCPs? Insurance coverage of NuSH therapies due to life-saving properties — as evidenced by the SELECT trial — without prior authorizations; and education on how and why metabolic surgery works, as well as education on behavioral approaches, such as healthy diet and exercise, as a core therapy for all BMI categories.
It’s time.
Caroline Apovian, MD, has disclosed the following relevant financial relationships: Serve(d) as a director, officer, partner, employee, adviser, consultant, or trustee for Altimmune; Cowen and Company; Currax Pharmaceuticals; EPG Communication Holdings; Gelesis, Srl; L-Nutra; and NeuroBo Pharmaceuticals. Received research grant from: National Institutes of Health; Patient-Centered Outcomes Research Institute; and GI Dynamics. Received income in an amount equal to or greater than $250 from: Altimmune; Cowen and Company; NeuroBo Pharmaceuticals; and Novo Nordisk.
A version of this article appeared on Medscape.com.