User login
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).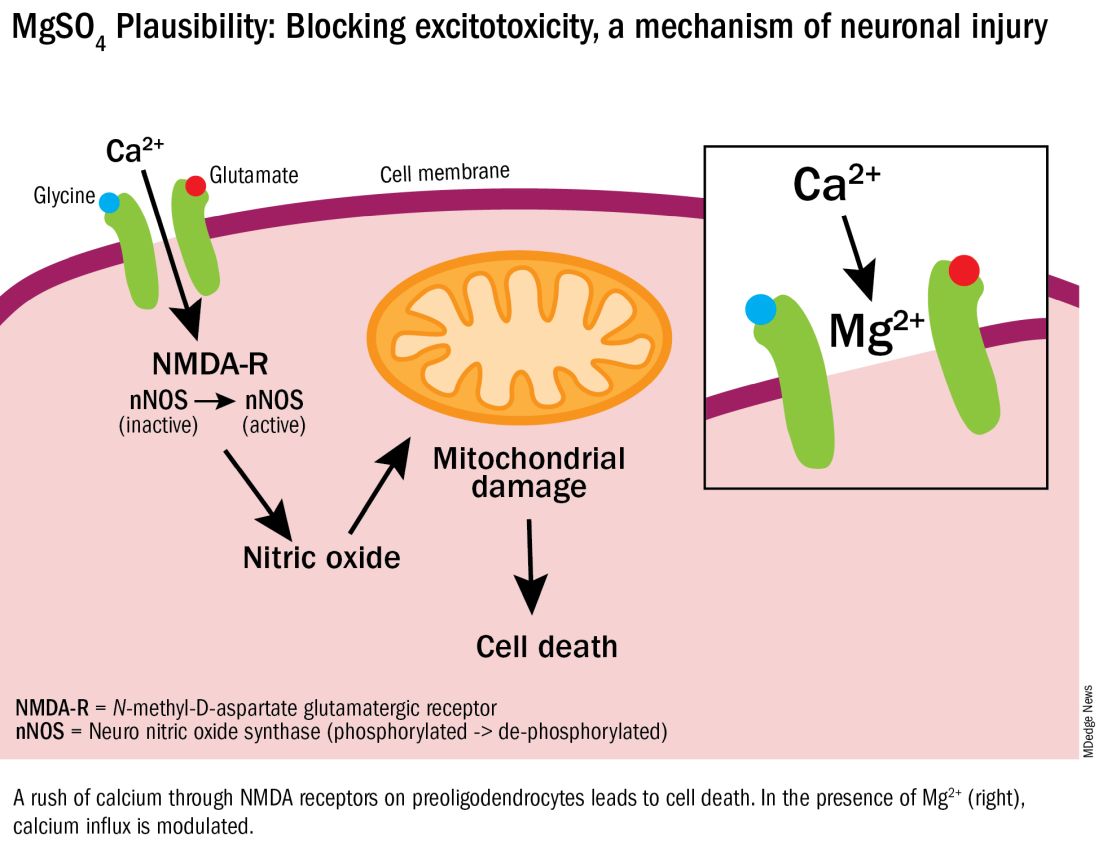
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.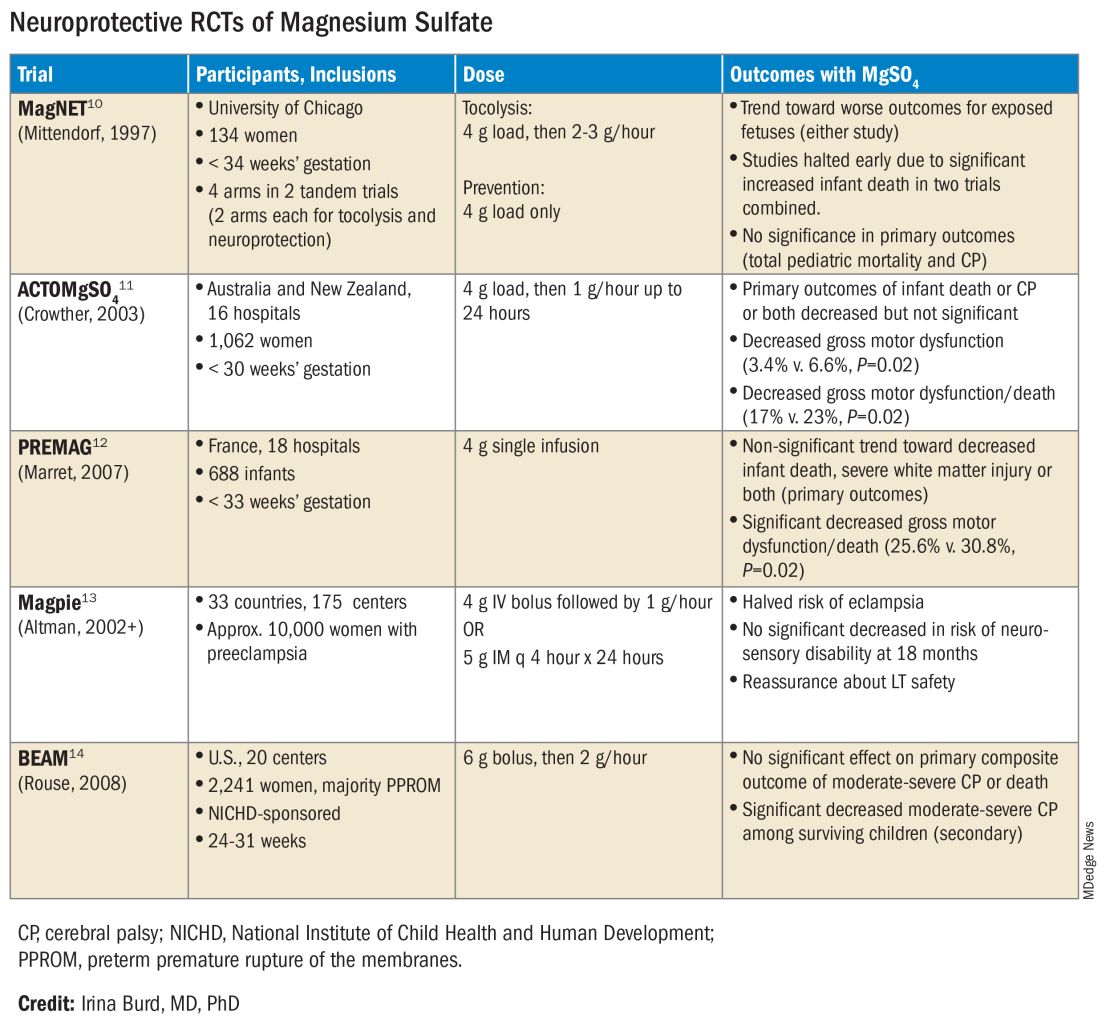
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
Introduction: The Many Lanes of Research on Magnesium Sulfate
The research that improves human health in the most expedient and most impactful ways is multitiered, with basic or fundamental research, translational research, interventional studies, and retrospective research often occurring simultaneously. There should be no “single lane” of research and one type of research does not preclude the other.
Too often, we fall short in one of these lanes. While we have achieved many moonshots in obstetrics and maternal-fetal medicine, we have tended not to place a high priority on basic research, which can provide a strong understanding of the biology of major diseases and conditions affecting women and their offspring. When conducted with proper commitment and funding, such research can lead to biologically directed therapy.
Within our specialty, research on how we can effectively prevent preterm birth, prematurity, and preeclampsia has taken a long road, with various types of therapies being tried, but none being overwhelmingly effective — with an ongoing need for more basic or fundamental research. Nevertheless, we can benefit and gain great insights from retrospective and interventional studies associated with clinical therapies used to treat premature labor and preeclampsia when these therapies have an unanticipated and important secondary benefit.
This month our Master Class is focused on the neuroprotection of prematurity. Magnesium sulfate is a valuable tool for the treatment of both premature labor and preeclampsia, and more recently, also for neuroprotection of the fetus. Interestingly, this use stemmed from researchers looking retrospectively at outcomes in women who received the compound for other reasons. It took many years for researchers to prove its neuroprotective value through interventional trials, while researchers simultaneously strove to understand on a basic biologic level how magnesium sulfate works to prevent outcomes such as cerebral palsy.
Basic research underway today continues to improve our understanding of its precise mechanisms of action. Combined with other tiers of research — including more interventional studies and more translational research — we can improve its utility for the neuroprotection of prematurity. Alternatively, ongoing research may lead to different, even more effective treatments.
Our guest author is Irina Burd, MD, PhD, Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine.* Dr. Burd is also a physician-scientist. She recounts the important story of magnesium sulfate and what is currently known about its biologic plausibility in neuroprotection — including through her own studies – as well as what may be coming in the future.
E. Albert Reece, MD, PhD, MBA, a maternal-fetal medicine specialist, is dean emeritus of the University of Maryland School of Medicine, former university executive vice president; currently the endowed professor and director of the Center for Advanced Research Training and Innovation (CARTI), and senior scientist in the Center for Birth Defects Research. Dr. Reece reported no relevant disclosures. He is the medical editor of this column. Contact him at [email protected].
Magnesium Sulfate for Fetal Neuroprotection in Preterm Birth
Without a doubt, magnesium sulfate (MgSO4) given before anticipated preterm birth reduces the risk of cerebral palsy. It is a valuable tool for fetal neuroprotection at a time when there are no proven alternatives. Yet without the persistent research that occurred over more than 20 years, it may not have won the endorsement of the American College of Obstetrics and Gynecologists in 2010 and worked its way into routine practice.
Its history is worthy of reflection. It took years of observational trials (not all of which showed neuroprotective effects), six randomized controlled trials (none of which met their primary endpoint), three meta-analyses, and a Cochrane Database Systematic Review to arrive at the conclusion that antenatal magnesium sulfate therapy given to women at risk of preterm birth has definitive neuroprotective benefit.
This history also holds lessons for our specialty given the dearth of drugs approved for use in pregnancy and the recent withdrawal from the market of Makena — one of only nine drugs to ever be approved by the Food and Drug Administration for use in pregnancy — after a second trial showed lack of benefit in preventing recurrent preterm birth. The story of MgSO4 tells us it’s acceptable to have major stumbling blocks: At one point, MgSO4 was considered to be not only not helpful, but harmful, causing neonatal death. Further research disproved this initial finding.
Moreover, the MgSO4 story is one that remains unfinished, as my laboratory and other researchers work to better understand its biologic plausibility and to discover additional neuroprotective agents for anticipated preterm birth that may further reduce the risk of cerebral palsy. This leading cause of chronic childhood disability is estimated by the United Cerebral Palsy Foundation to affect approximately 800,000 people in the United States.
Origins and Biologic Plausibility
The MgSO4 story is rooted in the late seventeenth century discovery by physician Nehemiah Grew that the compound was the key component of the then-famous medicinal spring waters in Epsom, England.1 MgSO4 was first used for eclampsia in 1906,2 and was first reported in the American literature for eclampsia in 1925.3 In 1959, its effect as a tocolytic agent was reported.4
More than 30 years later, in 1995, an observational study coauthored by Karin B. Nelson, MD, and Judith K. Grether, PhD of the National Institutes of Health, showed a reduced risk of cerebral palsy in very-low-birth-weight infants (VLBW).5 The report marked a turning point in research interest on neuroprotection for anticipated preterm birth.
The precise molecular mechanisms of action of MgSO4 for neuroprotection are still not well understood. However, research findings from the University of Maryland and other institutions have provided biologic plausibility for its use to prevent cerebral palsy. Our current thinking is that it involves the prevention of periventricular white matter injury and/or the prevention of oxidative stress and a neuronal injury mechanism called excitotoxicity.
Periventricular white matter injury involving injury to preoligodendrocytes before 32 weeks’ gestation is the most prevalent injury seen in cerebral palsy; preoligodendrocytes are precursors of myelinating oligodendrocytes, which constitute a major glial population in the white matter. Our research in a mouse model demonstrated that the intrauterine inflammation frequently associated with preterm birth can lead to neuronal injury as well as white matter damage, and that MgSO4 may ameliorate both.6,7
Excitotoxicity results from excessive stimulation of N-methyl-D-aspartate (NMDA) glutamatergic receptors on preoligodendrocytes and a rush of calcium through the voltage-gated channels. This calcium influx leads to the production of nitric oxide, oxidative stress, and subsequent mitochondrial damage and cell death. As a bivalent ion, MgSO4 sits in the voltage-gated channels of the NMDA receptors and reduces glutamatergic signaling, thus serving as a calcium antagonist and modulating calcium influx (See Figure).
In vitro research in our laboratory has also shown that MgSO4 may dampen inflammatory reactions driven by intrauterine infections, which, like preterm birth, increase the risk of cerebral palsy and adverse neurodevelopmental outcomes.8 MgSO4 appears to do so by blocking the voltage-gated P2X7 receptor in umbilical vein endothelial cells, thus blocking endothelial secretion of the proinflammatory cytokine interleukin (IL)–1beta. Much more research is needed to determine whether MgSO4 could help prevent cerebral palsy through this mechanism.
The Long Route of Research
The 1995 Nelson-Grether study compared VLBW (< 1500 g) infants who survived and developed moderate/severe cerebral palsy within 3 years to randomly selected VLBW controls with respect to whether their mothers had received MgSO4 to prevent seizures in preeclampsia or as a tocolytic agent.5 In a population of more than 155,000 children born between 1983 and 1985, in utero exposure to MgSO4 was reported in 7.1% of 42 VLBW infants with cerebral palsy and 36% of 75 VLBW controls (odds ratio [OR], 0.14; 95% CI, 0.05-0.51). In women without preeclampsia the OR increased to 0.25.
This motivating study had been preceded by several observational studies showing that infants born to women with preeclampsia who received MgSO4 had significantly lower risks of developing intraventricular hemorrhage (IVH) and germinal matrix hemorrhage (GMH). In one of these studies, published in 1992, Karl C. Kuban, MD, and coauthors reported that “maternal receipt of magnesium sulfate was associated with diminished risk of GMH-IVH even in those babies born to mothers who apparently did not have preeclampsia.”9
In the several years following the 1995 Nelson-Grether study, several other case-control/observational studies were reported, with conflicting conclusions, and investigators around the world began designing and conducting needed randomized controlled trials.
The six published randomized controlled trials looking at MgSO4 and neuroprotection varied in their inclusion and exclusion criteria, their recruitment and enrollment style, the gestational ages for MgSO4 administration, loading and maintenance doses, how cerebral palsy or neuroprotection was assessed, and other factors (See Table for RCT characteristics and main outcomes).10-14 One of the trials aimed primarily at evaluating the efficacy of MgSO4 for preventing preeclampsia.
Again, none of the randomized controlled trials demonstrated statistical significance for their primary outcomes or concluded that there was a significant neuroprotective effect for cerebral palsy. Rather, most suggested benefit through secondary analyses. Moreover, as mentioned earlier, research that proceeded after the first published randomized controlled trial — the Magnesium and Neurologic Endpoints (MAGnet) trial — was suspended early when an interim analysis showed a significantly increased risk of mortality in MgSO4-exposed fetuses. All told, it wasn’t until researchers obtained unpublished data and conducted meta-analyses and systematic reviews that a significant effect of MgSO4 on cerebral palsy could be seen.
The three systematic reviews and the Cochrane review, each of which used slightly different methodologies, were published in rapid succession in 2009. One review calculated a relative risk of cerebral palsy of 0.71 (95% CI, 0.55-0.91) — and a relative risk for the combined outcome of death and cerebral palsy at 0.85 (95% CI, 0.74-0.98) — when women at risk of preterm birth were given MgSO4.15 The number needed to treat (NNT) to prevent one case of cerebral palsy was 63, investigators determined, and the NNT to prevent one case of cerebral palsy or infant death was 44.
Another review estimated the NNT for prevention of one case of cerebral palsy at 52 when MgSO4 is given at less than 34 weeks’ gestation, and similarly concluded that MgSO4 is associated with a significantly “reduced risk of moderate/severe CP and substantial gross motor dysfunction without any statistically significant effect on the risk of total pediatric mortality.”16
A third review, from the National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU), estimated an NNT of 46 to prevent one case of cerebral palsy in infants exposed to MgSO4 before 30 weeks, and an NNT of 56 when exposure occurs before 32-34 weeks.17
The Cochrane Review, meanwhile, reported a relative reduction in the risk of cerebral palsy of 0.68 (95% CI, 0.54-0.87) when antenatal MgSO4 is given at less than 37 weeks’ gestation, as well as a significant reduction in the rate of substantial gross motor dysfunction (RR, 0.61; 95% CI, 0.44-0.85).18 The NNT to avoid one case of cerebral palsy, researchers reported, was 63.
Moving Forward
The NNTs calculated in these reviews — ranging from 44 to 63 — are convincing, and are comparable with evidence-based medicine data for prevention of other common diseases.19 For instance, the NNT for a life saved when aspirin is given immediately after a heart attack is 42. Statins given for 5 years in people with known heart disease have an NNT of 83 to save one life, an NNT of 39 to prevent one nonfatal heart attack, and an NNT of 125 to prevent one stroke. For oral anticoagulants used in nonvalvular atrial fibrillation for primary stroke prevention, the NNTs to prevent one stroke, and one death, are 22 and 42, respectively.19
In its 2010 Committee Opinion on Magnesium Sulfate Before Anticipated Preterm Birth for Neuroprotection (reaffirmed in 2020), the American College of Obstetricians and Gynecologists left it to institutions to develop their own guidelines “regarding inclusion criteria, treatment regimens, concurrent tocolysis, and monitoring in accordance with one of the larger trials.”20
Not surprisingly, most if not all hospitals have chosen a higher dose of MgSO4 administered up to 31 weeks’ gestation in keeping with the protocols employed in the NICHD-sponsored BEAM trial (See Table).
The hope moving forward is to expand treatment options for neuroprotection in cases of imminent preterm birth. Researchers have been assessing the ability of melatonin to provide neuroprotection in cases of growth restriction and neonatal asphyxia. Melatonin has anti-inflammatory and antioxidant properties and is known to mediate neuronal generation and synaptic plasticity.21
N-acetyl-L-cysteine is another potential neuroprotective agent. It acts as an antioxidant, a precursor to glutathione, and a modulator of the glutamate system and has been studied as a neuroprotective agent in cases of maternal chorioamnionitis.21 Both melatonin and N-acetyl-L-cysteine are regarded as safe in pregnancy, but much more clinical study is needed to prove their neuroprotective potential when given shortly before birth or earlier.
Dr. Burd is the Sylvan Freiman, MD Endowed Professor and Chair of the department of obstetrics, gynecology, and reproductive sciences at the University of Maryland School of Medicine, Baltimore. She has no conflicts of interest.
References
1. Clio Med. 1984;19(1-2):1-21.
2. Medicinsk Rev. (Bergen) 1906;32:264-272.
3. Am J Obstet Gynecol. 1996;174(4):1390-1391.
4. Am J Obstet Gynecol. 1959;78(1):27-32.
5. Pediatrics. 1995;95(2):263-269.
6. Am J Obstet Gynecol. 2009;201(3):279.e1-279.e8.
7. Am J Obstet Gynecol. 2010;202(3):292.e1-292.e9.
8. Pediatr Res. 2020;87(3):463-471.
9. J Child Neurol. 1992;7(1):70-76.
10. Lancet. 1997;350:1517-1518.
11. JAMA. 2003;290:2669-2676.
12. BJOG. 2007;114(3):310-318.
13. Lancet. 2002;359(9321):1877-1890.
14. N Engl J Med. 2008;359:895-905.
15. Obstet Gynecol. 2009;113(6):1327-1333.
16. Am J Obstet Gynecol. 2009;200(6):595-609.
17. Obstet Gynecol 2009;114:354-364.
18. Cochrane Database Syst Rev. 2009 Jan 21:(1):CD004661.
19. www.thennt.com.
20. Obstet Gynecol. 2010;115:669-671.
21. Front Synaptic Neurosci. 2012;13:680899.
*This story was corrected on June 10, 2024.
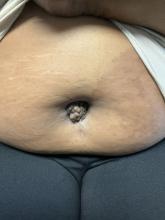
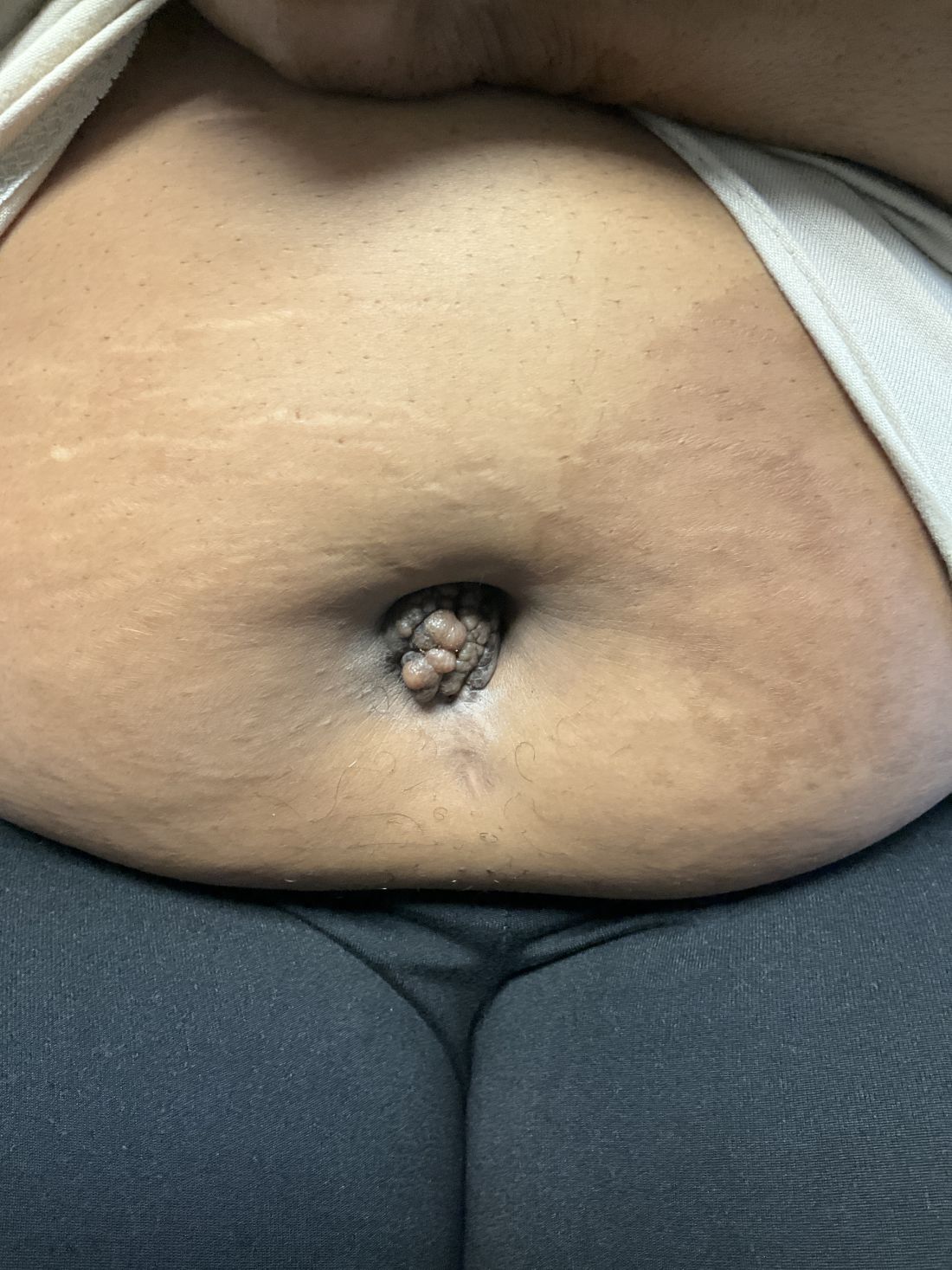
A 27-year-old Haitian woman presented with a painful umbilical mass which had been growing in size for 5 months
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Endometriosis is defined as the presence of endometrial tissue outside of the uterine cavity, commonly occurring in women of reproductive age. The condition usually affects the adnexa (ovaries, Fallopian tubes, and associated ligaments and connective tissue) but can also be seen in extrapelvic structures.
Cutaneous endometriosis is an uncommon subtype that accounts for 1% of endometriosis cases and occurs when endometrial tissue is found on the surface of the skin. It is divided into primary and secondary cutaneous endometriosis. The that may lead to seeding of endometrial tissue on the skin. In the case of our patient, it appears that her laparoscopic procedure 2 years ago was the cause of endometrial seeding in the umbilicus.
Clinically, the condition may present with a palpable mass, cyclic pain, and bloody discharge from the affected area. Due to the rarity of cutaneous endometriosis, it may be hard to distinguish from other diagnoses such as keloids, dermatofibromas, hernias, or cutaneous metastasis of cancers (Sister Mary Joseph nodules).
The definitive diagnosis can be made by biopsy and histopathological assessment showing a mixture of endometrial glands and stromal tissue. Imaging studies such as computed tomography (CT) scan and magnetic resonance imaging (MRI) are helpful in excluding more common diagnoses such as hernia or cutaneous metastasis. In this patient, the mass was surgically excised. Histopathological assessment established the diagnosis of cutaneous endometriosis.
Treatment options include surgical excision and medical therapy. Medical therapy entails the use of hormonal agents such as gonadotropin-releasing hormone agonists, danazol (a pituitary gonadotropin inhibitor), and oral contraceptives, which reduce the cyclical proliferation of endothelial tissue. These agents can be used preoperatively to reduce the size of the cutaneous mass before surgical excision, or as an alternative treatment for patients who wish to avoid surgery. The rate of recurrence is observed to be higher with medical therapy rather than surgical treatment.
The case and photo were submitted by Mina Ahmed, MBBS, Brooke Resh Sateesh MD, and Nathan Uebelhoer MD, of San Diego Family Dermatology, San Diego, California. The column was edited by Donna Bilu Martin, MD.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Gonzalez RH et al. Am J Case Rep. 2021;22:e932493-1–e932493-4.
2. Raffi L et al. Int J Womens Dermatol. 2019 Dec;5(5):384-386.
3. Sharma A, Apostol R. Cutaneous endometriosis. Treasure Island, Fla: Statpearls Publishing, 2023.
Pet Peeves About the State of Primary Care – Part 2
I have received lots of notes from readers about other pet peeves they have about practicing primary care in our current environment and wanted to share some of them. I appreciate all the emails I received on this topic.
- The rapid increase in the number of hospital administrators in the last 50 years
This has increased health system costs without providing any relief for practicing physicians, and often has led to policies that have been harmful and detrimental. This would be a great place to start cutting back to get true savings without affecting quality of care.
- Emergency physicians and specialists who refer my patient elsewhere for a service we provide in our office
It is expensive for patients to go to a specialty provider for a simple procedure that can be easily done in a primary care practice, or to be referred to see a specialist for a problem that does not need specialty care. This creates further problems accessing specialists.
- Online reviews of practices, including reviews from people who have never been patients
I am concerned about the accuracy and intent of online reviews. If a patient is upset because they did not receive an antibiotic or narcotic, they can vent their frustration in a review, when what the medical professional was actually doing was good medicine. More concerning to me is that some organizations use these reviews to determine compensation, promotion, and support. These reviews are not evidence based or accurately collected.
- Offices and organizations being dropped by insurance carriers
Insurance companies are running amok. They make their own rules, which can devastate practices and patients. They can change fees paid unilaterally, and drop practices without explanation or valid reasons. Patients suffer terribly because they now cannot see their long-time physicians or they have to pay much more to see them as they are suddenly “out of network.”
- The lack of appreciation by organizations as well as the general public of the enormous cost savings primary care professionals contribute to the healthcare system
There are many studies showing that patients who see a primary care physician save the system money and have better health outcomes. US adults who regularly see a primary care physician have 33% lower healthcare costs and 19% lower odds of dying prematurely than those who see only a specialist.1
In one study, for every $1 invested in primary care, there was $13 in savings in healthcare costs.2 I had a patient a few years ago complain about the “enormous” bill she received for a visit where I had done an annual exam, cryotherapy for three actinic keratoses, and a steroid injection for her ailing knee. The cost savings was well over $700 (the new patient cost for two specialty visits). There is no doubt that patients who have stable primary care save money themselves and for the whole medical system.
- The stress of being witness to a dysfunctional system
It is really hard to see the hurt and difficulty our patients go through on a daily basis while trying to navigate a broken system. We bear witness to them and listen to all the stories when things have gone wrong. This also takes its toll on us, as we are part of the system, and our patients’ frustrations sometimes boil over. We are also the ones who care for the whole patient, so every bad experience with a specialty clinic is shared with us.
Many thanks extended to those who wrote to share their ideas (Drs. Sylvia Androne, Bhawna Bahethi, Pierre Ghassibi, Richard Katz, Louis Kasunic, Rebecca Keenan, David Kosnosky, Gregory Miller, and James Wilkens).
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington, Seattle. Contact Dr. Paauw at [email protected].
References
1. Forbes.com. Why Primary Care Matters, and What We Can Do To Increase It. 2023 Nov 27.
2. Washingtonpost.com. A Health Care Solution We Can’t Afford to Ignore: Primary Care.
I have received lots of notes from readers about other pet peeves they have about practicing primary care in our current environment and wanted to share some of them. I appreciate all the emails I received on this topic.
- The rapid increase in the number of hospital administrators in the last 50 years
This has increased health system costs without providing any relief for practicing physicians, and often has led to policies that have been harmful and detrimental. This would be a great place to start cutting back to get true savings without affecting quality of care.
- Emergency physicians and specialists who refer my patient elsewhere for a service we provide in our office
It is expensive for patients to go to a specialty provider for a simple procedure that can be easily done in a primary care practice, or to be referred to see a specialist for a problem that does not need specialty care. This creates further problems accessing specialists.
- Online reviews of practices, including reviews from people who have never been patients
I am concerned about the accuracy and intent of online reviews. If a patient is upset because they did not receive an antibiotic or narcotic, they can vent their frustration in a review, when what the medical professional was actually doing was good medicine. More concerning to me is that some organizations use these reviews to determine compensation, promotion, and support. These reviews are not evidence based or accurately collected.
- Offices and organizations being dropped by insurance carriers
Insurance companies are running amok. They make their own rules, which can devastate practices and patients. They can change fees paid unilaterally, and drop practices without explanation or valid reasons. Patients suffer terribly because they now cannot see their long-time physicians or they have to pay much more to see them as they are suddenly “out of network.”
- The lack of appreciation by organizations as well as the general public of the enormous cost savings primary care professionals contribute to the healthcare system
There are many studies showing that patients who see a primary care physician save the system money and have better health outcomes. US adults who regularly see a primary care physician have 33% lower healthcare costs and 19% lower odds of dying prematurely than those who see only a specialist.1
In one study, for every $1 invested in primary care, there was $13 in savings in healthcare costs.2 I had a patient a few years ago complain about the “enormous” bill she received for a visit where I had done an annual exam, cryotherapy for three actinic keratoses, and a steroid injection for her ailing knee. The cost savings was well over $700 (the new patient cost for two specialty visits). There is no doubt that patients who have stable primary care save money themselves and for the whole medical system.
- The stress of being witness to a dysfunctional system
It is really hard to see the hurt and difficulty our patients go through on a daily basis while trying to navigate a broken system. We bear witness to them and listen to all the stories when things have gone wrong. This also takes its toll on us, as we are part of the system, and our patients’ frustrations sometimes boil over. We are also the ones who care for the whole patient, so every bad experience with a specialty clinic is shared with us.
Many thanks extended to those who wrote to share their ideas (Drs. Sylvia Androne, Bhawna Bahethi, Pierre Ghassibi, Richard Katz, Louis Kasunic, Rebecca Keenan, David Kosnosky, Gregory Miller, and James Wilkens).
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington, Seattle. Contact Dr. Paauw at [email protected].
References
1. Forbes.com. Why Primary Care Matters, and What We Can Do To Increase It. 2023 Nov 27.
2. Washingtonpost.com. A Health Care Solution We Can’t Afford to Ignore: Primary Care.
I have received lots of notes from readers about other pet peeves they have about practicing primary care in our current environment and wanted to share some of them. I appreciate all the emails I received on this topic.
- The rapid increase in the number of hospital administrators in the last 50 years
This has increased health system costs without providing any relief for practicing physicians, and often has led to policies that have been harmful and detrimental. This would be a great place to start cutting back to get true savings without affecting quality of care.
- Emergency physicians and specialists who refer my patient elsewhere for a service we provide in our office
It is expensive for patients to go to a specialty provider for a simple procedure that can be easily done in a primary care practice, or to be referred to see a specialist for a problem that does not need specialty care. This creates further problems accessing specialists.
- Online reviews of practices, including reviews from people who have never been patients
I am concerned about the accuracy and intent of online reviews. If a patient is upset because they did not receive an antibiotic or narcotic, they can vent their frustration in a review, when what the medical professional was actually doing was good medicine. More concerning to me is that some organizations use these reviews to determine compensation, promotion, and support. These reviews are not evidence based or accurately collected.
- Offices and organizations being dropped by insurance carriers
Insurance companies are running amok. They make their own rules, which can devastate practices and patients. They can change fees paid unilaterally, and drop practices without explanation or valid reasons. Patients suffer terribly because they now cannot see their long-time physicians or they have to pay much more to see them as they are suddenly “out of network.”
- The lack of appreciation by organizations as well as the general public of the enormous cost savings primary care professionals contribute to the healthcare system
There are many studies showing that patients who see a primary care physician save the system money and have better health outcomes. US adults who regularly see a primary care physician have 33% lower healthcare costs and 19% lower odds of dying prematurely than those who see only a specialist.1
In one study, for every $1 invested in primary care, there was $13 in savings in healthcare costs.2 I had a patient a few years ago complain about the “enormous” bill she received for a visit where I had done an annual exam, cryotherapy for three actinic keratoses, and a steroid injection for her ailing knee. The cost savings was well over $700 (the new patient cost for two specialty visits). There is no doubt that patients who have stable primary care save money themselves and for the whole medical system.
- The stress of being witness to a dysfunctional system
It is really hard to see the hurt and difficulty our patients go through on a daily basis while trying to navigate a broken system. We bear witness to them and listen to all the stories when things have gone wrong. This also takes its toll on us, as we are part of the system, and our patients’ frustrations sometimes boil over. We are also the ones who care for the whole patient, so every bad experience with a specialty clinic is shared with us.
Many thanks extended to those who wrote to share their ideas (Drs. Sylvia Androne, Bhawna Bahethi, Pierre Ghassibi, Richard Katz, Louis Kasunic, Rebecca Keenan, David Kosnosky, Gregory Miller, and James Wilkens).
Dr. Paauw is professor of medicine in the Division of General Internal Medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington, Seattle. Contact Dr. Paauw at [email protected].
References
1. Forbes.com. Why Primary Care Matters, and What We Can Do To Increase It. 2023 Nov 27.
2. Washingtonpost.com. A Health Care Solution We Can’t Afford to Ignore: Primary Care.
Zoom: Convenient and Imperfect
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Making eye contact is important in human interactions. It shows attention and comprehension. It also helps us read the nuances of another’s facial expressions when interacting.
Although the idea of video phone calls isn’t new — I remember it from my childhood in “house of the future” TV shows — it certainly didn’t take off until the advent of high-speed Internet, computers, and phones with cameras. Then Facetime, Skype, Zoom, Teams, and others.
Of course, it all still took a back seat to actually seeing people and having meetings in person. Until the pandemic made that the least attractive option. Then the adoption of such things went into hyperdrive and has stayed there ever since.
And ya know, I don’t have too many complaints. Between clinical trials and legal cases, both of which involve A LOT of meetings, it’s made my life easier. I no longer have to leave the office, allow time to drive somewhere and back, fight traffic, burn gas, and find parking. I move from a patient to the meeting and back to a patient from the cozy confines of my office, all without my tea getting cold.
But you can’t really make eye contact on Zoom. Instinctively, we generally look directly at the eyes of the person we’re speaking to, but in the virtual world we really don’t do that. On their end you’re on a screen, your gaze fixed somewhere below the level of your camera.
Try talking directly to the camera on Zoom — or any video platform. It doesn’t work. You feel like Dave addressing HAL’s red light in 2001. Inevitably your eyes are drawn back to the other person’s face, which is what you’re programmed to do. If they’re speaking you look at them, even though the sound is really coming from your speakers.
Interestingly, though, it seems something is lost in there. A recent perspective noted that Zoom meetings seemed to stifle creativity and produced fewer ideas than in person.
An interesting study compared neural response signals of people seeing a presentation on Zoom versus the same talk in person. When looking at a “real” speaker, there was synchronized neural activity, a higher level of engagement, and increased activation of the dorsal-parietal cortex.
Without actual eye contact it’s harder to read subtle facial expressions. Hand gestures and other body language may be out of the camera frame, or absent altogether. The nuances of voice pitch, timbre, and tone may not be the same over the speaker.
Our brains have spent several million of years developing facial recognition and reading, knowing friend from foe, and understanding what’s meant not just in what sounds are used but how they’re conveyed.
I’m not saying we should stop using Zoom altogether — it makes meetings more convenient for most people, including myself. But we also need to keep in mind that what it doesn’t convey is as important as what it does, and that virtual is never a perfect substitute for reality.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A Counterintuitive Approach to Lowering Cholesterol in Children
With the flip of the calendar a few short weeks ago, gyms and fitness centers began ramping up their advertising campaigns in hopes of attracting the horde of resolution makers searching for a place where they can inject some exercise into their sedentary lives. A recent survey by C.S. Mott’s Children’s Hospital found that even young people are setting health-related goals with more than half of the parents of 11- to 18-year-olds reporting their children were setting personal goals for themselves. More than 40% of the young people listed more exercise as a target.
However, our personal and professional experiences have taught us that achieving goals, particularly when it comes to exercise, is far more difficult than setting the target. Finding an exercise buddy can be an important motivator on the days when just lacing up one’s sneakers is a stumbling block. Investing in a gym membership and sweating with a peer group can help. However, it is an investment that rarely pays a dividend. Exercise isn’t fun for everyone. For adults, showing up at a gym may be just one more reminder of how they have already lost their competitive edge over their leaner and fitter peers. If they aren’t lucky enough to find a sport or activity that they enjoy, the loneliness of the long-distance runner has little appeal.
A recent study on children in the United Kingdom suggests that at least when it comes to teens and young adults we as physicians may actually have been making things worse for our obese patients by urging them to accept unrealistic activity goals. While it is already known that sedentary time is responsible for 70% of the total increase in cholesterol as children advance to young adulthood an unqualified recommendation for more exercise may not be the best advice.
In an interview with the study author, Andre O. Agbaje MD, MPH, said that in his large study population “light physical activity outperforms moderate to vigorous physical activity by five to eight times in lowering lipids”. While we may be surprised by this counterintuitive finding, Dr. Agbaje points out that an increase in sedentariness from 6 to 9 hours per day translates into a loss of 3 hours of light physical activity. In other words if you’re not sedentary you must be standing at attention or engaged in some light activity.
In my experience, and I suspect yours, it is difficult to get adults to do something, particularly if that something involves exerting energy, even a small amount of energy. The general admonishment of “be more active” is often met with a blank stare and the sometimes unspoken question “Like what?”
You could fall into a bottomless trap with them by suggesting a long list of activities, many of which are probably ones you do or would enjoy but don’t happen to fit with any of their interests or capabilities. Your chances of hitting on a perfect activity that the patient will attempt, let alone adopt, is very slim. Those of you with more patience than I have may choose to persist with this strategy. You could argue that even if the patient only dabbles briefly in one of your recommended activities, this is a minor victory worth celebrating. Who knows? The brief jolt of energy they received from this activity may prompt them to seek and find something else that works.
My interpretation of Dr. Agbaje’s findings is this: If we are going to suggest more activity, aim low. Don’t even mention the heavily weighted words “sport” or “exercise,” which are likely to dredge up bad memories. For adults, “Go shopping” or “Visit a friend” may be sufficient to at least get the person off the couch and on their feet and moving, even if very briefly.
The second message from this study applies more to children and adolescents and is one of those unusual instances in which a negative intervention may be more effective than a positive approach. Acknowledging that we are likely to have difficulty finding even a light activity that the child enjoys, why not pivot to the other side of the equation? Make a list of the child’s primary sedentary “activities.” Then suggest the parents put the child on a couch potato diet by immediately cutting in half the time he or she spends being sedentary. By definition, this will automatically increase his or her light physical activity by 50%. According to Dr. Agbaje’s data, this should be more effective in lowering lipids than in the unlikely event of finding a moderate activity the child accepts.
You can argue that the child will hound his or her parents unmercifully asking to be entertained. This may be true and this persistent complaining will be more likely to come from the older the child and the longer that the child has been allowed to be sedentary. Although the child may appear to have lost the ability to self amuse, I contend this isn’t a permanent loss and, This is another example of how saying “No!” in the right circumstances is often the most effective remedy for an unhealthy situation. I would never claim saying “No” is easy and helping parents to learn how to say “No” is one of our most difficult challenges. But, nothing else seems to be working.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
With the flip of the calendar a few short weeks ago, gyms and fitness centers began ramping up their advertising campaigns in hopes of attracting the horde of resolution makers searching for a place where they can inject some exercise into their sedentary lives. A recent survey by C.S. Mott’s Children’s Hospital found that even young people are setting health-related goals with more than half of the parents of 11- to 18-year-olds reporting their children were setting personal goals for themselves. More than 40% of the young people listed more exercise as a target.
However, our personal and professional experiences have taught us that achieving goals, particularly when it comes to exercise, is far more difficult than setting the target. Finding an exercise buddy can be an important motivator on the days when just lacing up one’s sneakers is a stumbling block. Investing in a gym membership and sweating with a peer group can help. However, it is an investment that rarely pays a dividend. Exercise isn’t fun for everyone. For adults, showing up at a gym may be just one more reminder of how they have already lost their competitive edge over their leaner and fitter peers. If they aren’t lucky enough to find a sport or activity that they enjoy, the loneliness of the long-distance runner has little appeal.
A recent study on children in the United Kingdom suggests that at least when it comes to teens and young adults we as physicians may actually have been making things worse for our obese patients by urging them to accept unrealistic activity goals. While it is already known that sedentary time is responsible for 70% of the total increase in cholesterol as children advance to young adulthood an unqualified recommendation for more exercise may not be the best advice.
In an interview with the study author, Andre O. Agbaje MD, MPH, said that in his large study population “light physical activity outperforms moderate to vigorous physical activity by five to eight times in lowering lipids”. While we may be surprised by this counterintuitive finding, Dr. Agbaje points out that an increase in sedentariness from 6 to 9 hours per day translates into a loss of 3 hours of light physical activity. In other words if you’re not sedentary you must be standing at attention or engaged in some light activity.
In my experience, and I suspect yours, it is difficult to get adults to do something, particularly if that something involves exerting energy, even a small amount of energy. The general admonishment of “be more active” is often met with a blank stare and the sometimes unspoken question “Like what?”
You could fall into a bottomless trap with them by suggesting a long list of activities, many of which are probably ones you do or would enjoy but don’t happen to fit with any of their interests or capabilities. Your chances of hitting on a perfect activity that the patient will attempt, let alone adopt, is very slim. Those of you with more patience than I have may choose to persist with this strategy. You could argue that even if the patient only dabbles briefly in one of your recommended activities, this is a minor victory worth celebrating. Who knows? The brief jolt of energy they received from this activity may prompt them to seek and find something else that works.
My interpretation of Dr. Agbaje’s findings is this: If we are going to suggest more activity, aim low. Don’t even mention the heavily weighted words “sport” or “exercise,” which are likely to dredge up bad memories. For adults, “Go shopping” or “Visit a friend” may be sufficient to at least get the person off the couch and on their feet and moving, even if very briefly.
The second message from this study applies more to children and adolescents and is one of those unusual instances in which a negative intervention may be more effective than a positive approach. Acknowledging that we are likely to have difficulty finding even a light activity that the child enjoys, why not pivot to the other side of the equation? Make a list of the child’s primary sedentary “activities.” Then suggest the parents put the child on a couch potato diet by immediately cutting in half the time he or she spends being sedentary. By definition, this will automatically increase his or her light physical activity by 50%. According to Dr. Agbaje’s data, this should be more effective in lowering lipids than in the unlikely event of finding a moderate activity the child accepts.
You can argue that the child will hound his or her parents unmercifully asking to be entertained. This may be true and this persistent complaining will be more likely to come from the older the child and the longer that the child has been allowed to be sedentary. Although the child may appear to have lost the ability to self amuse, I contend this isn’t a permanent loss and, This is another example of how saying “No!” in the right circumstances is often the most effective remedy for an unhealthy situation. I would never claim saying “No” is easy and helping parents to learn how to say “No” is one of our most difficult challenges. But, nothing else seems to be working.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
With the flip of the calendar a few short weeks ago, gyms and fitness centers began ramping up their advertising campaigns in hopes of attracting the horde of resolution makers searching for a place where they can inject some exercise into their sedentary lives. A recent survey by C.S. Mott’s Children’s Hospital found that even young people are setting health-related goals with more than half of the parents of 11- to 18-year-olds reporting their children were setting personal goals for themselves. More than 40% of the young people listed more exercise as a target.
However, our personal and professional experiences have taught us that achieving goals, particularly when it comes to exercise, is far more difficult than setting the target. Finding an exercise buddy can be an important motivator on the days when just lacing up one’s sneakers is a stumbling block. Investing in a gym membership and sweating with a peer group can help. However, it is an investment that rarely pays a dividend. Exercise isn’t fun for everyone. For adults, showing up at a gym may be just one more reminder of how they have already lost their competitive edge over their leaner and fitter peers. If they aren’t lucky enough to find a sport or activity that they enjoy, the loneliness of the long-distance runner has little appeal.
A recent study on children in the United Kingdom suggests that at least when it comes to teens and young adults we as physicians may actually have been making things worse for our obese patients by urging them to accept unrealistic activity goals. While it is already known that sedentary time is responsible for 70% of the total increase in cholesterol as children advance to young adulthood an unqualified recommendation for more exercise may not be the best advice.
In an interview with the study author, Andre O. Agbaje MD, MPH, said that in his large study population “light physical activity outperforms moderate to vigorous physical activity by five to eight times in lowering lipids”. While we may be surprised by this counterintuitive finding, Dr. Agbaje points out that an increase in sedentariness from 6 to 9 hours per day translates into a loss of 3 hours of light physical activity. In other words if you’re not sedentary you must be standing at attention or engaged in some light activity.
In my experience, and I suspect yours, it is difficult to get adults to do something, particularly if that something involves exerting energy, even a small amount of energy. The general admonishment of “be more active” is often met with a blank stare and the sometimes unspoken question “Like what?”
You could fall into a bottomless trap with them by suggesting a long list of activities, many of which are probably ones you do or would enjoy but don’t happen to fit with any of their interests or capabilities. Your chances of hitting on a perfect activity that the patient will attempt, let alone adopt, is very slim. Those of you with more patience than I have may choose to persist with this strategy. You could argue that even if the patient only dabbles briefly in one of your recommended activities, this is a minor victory worth celebrating. Who knows? The brief jolt of energy they received from this activity may prompt them to seek and find something else that works.
My interpretation of Dr. Agbaje’s findings is this: If we are going to suggest more activity, aim low. Don’t even mention the heavily weighted words “sport” or “exercise,” which are likely to dredge up bad memories. For adults, “Go shopping” or “Visit a friend” may be sufficient to at least get the person off the couch and on their feet and moving, even if very briefly.
The second message from this study applies more to children and adolescents and is one of those unusual instances in which a negative intervention may be more effective than a positive approach. Acknowledging that we are likely to have difficulty finding even a light activity that the child enjoys, why not pivot to the other side of the equation? Make a list of the child’s primary sedentary “activities.” Then suggest the parents put the child on a couch potato diet by immediately cutting in half the time he or she spends being sedentary. By definition, this will automatically increase his or her light physical activity by 50%. According to Dr. Agbaje’s data, this should be more effective in lowering lipids than in the unlikely event of finding a moderate activity the child accepts.
You can argue that the child will hound his or her parents unmercifully asking to be entertained. This may be true and this persistent complaining will be more likely to come from the older the child and the longer that the child has been allowed to be sedentary. Although the child may appear to have lost the ability to self amuse, I contend this isn’t a permanent loss and, This is another example of how saying “No!” in the right circumstances is often the most effective remedy for an unhealthy situation. I would never claim saying “No” is easy and helping parents to learn how to say “No” is one of our most difficult challenges. But, nothing else seems to be working.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Perinatal Psychiatry in 2024: Helping More Patients Access Care
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
The past year has been a challenging time for many, both at the local level and globally, with divisive undercurrents across many communities. Many times, the end of the year is an opportunity for reflection. As I reflect on the state of perinatal psychiatry in the new year, I see several evolving issues that I’d like to share in this first column of 2024.
In 2023, the American College of Obstetricians and Gynecologists published new recommendations meant to enhance the well-being of pregnant and postpartum women and families. A main message from discussion papers borne out of these recommendations was that as a field, we should be doing more than identifying perinatal illness. We should be screening women at risk for postpartum psychiatric illness and see that those suffering from posttraumatic stress disorder (PTSD) have access to care and “wrap-around services” from clinicians with varying expertise.
Screening is a primary way we identify patients at risk for psychiatric illness and also those who are suffering at the time of a screen. One problem I see in the near future is our disparate collection and management of data. When we look closely across health care systems, it’s not clear how screening data are captured, let alone managed. What is being done in one hospital system may be very different from what is being done elsewhere. Some clinicians are adopting digital platforms to identify those with postpartum depression, while others are practicing as they always have, either through a paper screening process or with queries as part of a clinical encounter.
Given this amalgam of methods for collecting and storing information, there does not appear to be a systematic way clinicians and researchers are recording whether women are meeting criteria for significant depressive symptoms or frank postpartum psychiatric illness. It is clear a more cohesive method for collection and management is needed to optimize the likelihood that next steps can be taken to get patients the care they need.
However, screening is only one part of the story. Certainly, in our own center, one of our greatest interests, both clinically and on the research side, is what happens after screening. Through our center’s initiation of the Screening and Treatment Enhancement for Postpartum Depression (STEPS for PPD) project funded by the Marriott Foundation, we are evaluating the outcomes of women who are screened at 6 weeks postpartum with significant depressive symptoms, and who are then given an opportunity to engage with a perinatal social worker who can assist with direct psychotherapy, arranging for referrals, and navigating care for a new mother.
What we are learning as we enroll women through the initial stages of STEPS for PPD is that screening and identifying women who likely suffer from PPD simply is not enough. In fact, once identified with a depression screening tool, women who are suffering from postpartum depression can be very challenging to engage clinically. What I am learning decades after starting to work with perinatal patients is that even with a screening system and effective tools for treatment of PPD, optimizing engagement with these depressed women seems a critical and understudied step on the road to optimizing positive clinical outcomes.
A recent study published in the Journal of Women’s Health explored gaps in care for perinatal depression and found that patients without a history of psychiatric illness prior to pregnancy were less likely to be screened for depression and 80% less likely to receive care if they developed depression compared with women with a previous history of psychiatric illness (J Womens Health (Larchmt). 2023 Oct;32[10]:1111-9).
That history may help women navigate to care, while women for whom psychiatric illness is a new experience may be less likely to engage, be referred for care, and receive appropriate treatment. The study indicates that, as a field, we must strive to ensure universal screening for depression in perinatal populations.
While we have always been particularly interested in populations of patients at highest risk for PPD, helping women at risk for PPD in the general population without a history of psychiatric illness is a large public health issue and will be an even larger undertaking. As women’s mental health is gaining more appropriate focus, both at the local level and even in the recent White House Initiative on Women’s Health Research, the focus has been on screening and developing new treatments.
We are not lacking in pharmacologic agents nor nonpharmacologic options as treatments for women experiencing PPD. Newer alternative treatments are being explored, such as transcranial magnetic stimulation (TMS) and even psychedelics as a potential therapy for PPD. But perhaps what we’ve learned in 2023 and as we move into a new year, is that the problem of tackling PPD is not only about having the right tools, but is about helping women navigate to the care that they need.
The COVID-19 pandemic brought with it an explosion of telehealth options that have enhanced the odds women can find support during such a challenging time; as society has returned to some semblance of normal, nearly all support groups for postpartum women have remained online.
When we set up Virtual Rounds at the Center for Women’s Mental Health at the beginning of the pandemic, I was struck by the community of colleagues at various stages of their careers dedicated to mitigating the suffering associated with perinatal psychiatric illness. As I’ve often said, it takes a village to care for these patients. We need help from colleagues with varying expertise — from lactation consultants, psychiatrists, psychologists, obstetricians, nurse practitioners, support group leaders, and a host of others — who can help reach these women.
At the end of the day, helping depressed women find resources is a challenge that we have not met in this country. We should be excited that we have so many treatment options to offer patients — whether it be a new first-in-class medication, TMS, or digital apps to ensure patients are receiving effective treatment. But there should also be a focus on reaching women who still need treatment, particularly in underserved communities where resources are sparse or nonexistent. Identifying the path to reaching these women where they are and getting them well should be a top priority in 2024.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital (MGH) in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. STEPS for PPD is funded by the Marriott Foundation. Full disclosure information for Dr. Cohen is available at womensmentalhealth.org. Email Dr. Cohen at [email protected].
JAMA Internal Medicine Editor Recaps 2023’s High-Impact Research
Harvard Medical School’s Sharon K. Inouye, MD, MPH, is editor in chief of JAMA Internal Medicine and a leading voice in American gerontology. We asked her to choose five of the influential journal’s most impactful studies from 2023 and highlight important take-home messages for internists and their colleagues.
Q: One of the studies you chose suggests that the antiviral nirmatrelvir (Paxlovid) can ward off long COVID. Could you recap the findings?
A: Researchers followed a group of more than 280,000 Department of Veterans Affairs patients who were seen in 2022, had a positive COVID test, and had at least one risk factor for severe COVID. They focused on those who survived to 30 days after their COVID infection and compared those who received the drug within the first 5 days of a positive test with an equivalent control group.
They found that 13 long COVID symptoms were all significantly less common (relative risk = 0.74) in those who received nirmatrelvir. This was true no matter whether they’d ever had a COVID vaccination.
Q: How should this research affect clinical practice?
A: You can’t generalize from this to everyone because, of course, not everyone was included in this study. But it is highly suggestive that this drug is very effective for preventing long COVID.
Nirmatrelvir was touted as being able to shorten duration of illness and prevent hospitalization. But if you were low risk or you were already well into your COVID course, it wasn’t like rush, rush, rush to the doctor to get it.
This changes that equation because we know long COVID is such a huge issue. The vast majority of doctors who work with COVID patients and know this are now being more aggressive about prescribing it.
Q: What about patients whom the CDC considers to be at less risk — people with up-to-date vaccinations who are under 50 with mild-to-moderate COVID and no higher-risk medical conditions? Should they take nirmatrelvir?
A: The evidence is not 100% in yet. A study like this one needs to be repeated and include younger people without any risk factors to see if we see the same thing. So it’s a personal choice, and a personal calculus needs to be done. A lot of people are making that choice [to take the drug], and it can be a rational decision.
Q: You also chose a study that links high thyroid hormone levels to higher rates of dementia. What did it reveal?
A: This study looks at patients who had thyrotoxicosis — a thyroid level that’s too high — from hormone produced endogenously, and exogenously. Researchers tracked almost 66,000 patients aged 65 and older and found that thyrotoxicosis from all causes, whether it was endogenous or exogenous, was linked to an increased risk of dementia in a dose-response relationship (adjusted hazard ratio = 1.39).
Q: Is there a clinical take-home message here?
A: When we start patients on thyroid medication, they don’t always get reassessed on a regular basis. Given this finding, a TSH [thyroid-stimulating hormone] level is indicated during the annual wellness check that patients on Medicare can get every year.
Q: Is TSH measured as part of routine blood tests?
A: No it’s not. It has to be ordered. I think that’s why we’re seeing this problem to begin with — because it’s not something we all have awareness about. I wasn’t aware myself that mildly high levels of thyroid could increase the risk of cognitive impairment. Certainly, I’m going to be much more aware in my practice.
Q: You also picked a study about silicosis in workers who are exposed to dust when they make engineered stone countertops, also known as quartz countertops. What were the findings?
A: Silicosis is a very serious lung condition that develops from exposure to crystalline silica. Essentially, sand gets inhaled into the lungs. Workers can be exposed when they’re making engineered stone countertops, the most popular countertops now in the United States.
This study is based on statewide surveys from 2019 to 2022 that the California Department of Public Health does routinely. They gathered cases of silicosis and found 52 — all men with an average age of 45. All but one were Latino immigrants, and most either had no insurance or very poor insurance.
Q: The study found that “diagnosis was delayed in 58%, with 38% presenting with advanced disease (progressive massive fibrosis), and 19% died.” What does that tell you?
A: It’s a very serious condition. Once it gets to the advanced stage, it will just continue to progress, and the person will die. That’s why it’s so important to know that it’s absolutely preventable.
Q: Is there a message here for internists?
A: If you treat a lot of immigrants or work in an area where there are a lot of industrial workers, you’re going to want to have a very high suspicion about it. If you see an atypical pattern on the chest x-ray or via diffusion scoring, have a low threshold for getting a pulmonary function test.
Doctors need to be aware and diagnose this very quickly. When patients present, you can pull them out of that work environment or put mitigation systems into place.
Q: California regulators were expected to put emergency rules into place in late December to protect workers. Did this study play a role in focusing attention on the problem?
A: This article, along with a commentary and podcast that we put out, really helped with advocacy to improve health and safety for workers at stone-cutting and fabrication shops.
Q: You were impressed by another study about airborne dangers, this one linking air pollution to dementia. What did researchers discover?
A: [This analysis] of more than 27,000 people in the Health and Retirement Study, a respected and rich database, found that exposure to air pollution was associated with greater rates of dementia — an increase of about 8% a year. Exposure to agricultural emissions and wildfire smoke were most robustly associated with a greater risk of dementia.
Q: How are these findings important, especially in light of the unhealthy air spawned by recent wildfires in the United States and Canada?
A: Studies like this will make it even more compelling that we are better prepared for air quality issues.
I grew up in Los Angeles, where smog and pollution were very big issues. I was constantly hearing about various mitigation strategies that were going into place. But after I moved to the East Coast, I almost never heard about prevention.
Now, I’m hoping we can keep this topic in the national conversation.
Q: You also highlighted a systematic review of the use of restraints in the emergency department. Why did you choose this research?
A: At JAMA Internal Medicine, we’re really focused on ways we can address health disparities and raise awareness of potential unconscious bias.
This review looked at 10 studies that included more than 2.5 million patient encounters, including 24,000 incidents of physical restraint use. They found that the overall rate of use of restraints was low at below 1%.
But when they are used, Black patients were 1.3 times more likely to be restrained than White patients.
Q: What’s the message here?
A: This is an important start to recognizing these differences and then changing our behavior. Perhaps restraints don’t need to be used as often in light of evidence, for example, of increased rates of misdiagnosis of psychosis in the Black population.
Q: How should physicians change their approach to restraints?
A: Restraints are not to be used to control disruption — wild behavior or verbal outbursts. They’re for when someone is a danger to themselves or others.
Dr. Inouye has no conflicts of interest.
Harvard Medical School’s Sharon K. Inouye, MD, MPH, is editor in chief of JAMA Internal Medicine and a leading voice in American gerontology. We asked her to choose five of the influential journal’s most impactful studies from 2023 and highlight important take-home messages for internists and their colleagues.
Q: One of the studies you chose suggests that the antiviral nirmatrelvir (Paxlovid) can ward off long COVID. Could you recap the findings?
A: Researchers followed a group of more than 280,000 Department of Veterans Affairs patients who were seen in 2022, had a positive COVID test, and had at least one risk factor for severe COVID. They focused on those who survived to 30 days after their COVID infection and compared those who received the drug within the first 5 days of a positive test with an equivalent control group.
They found that 13 long COVID symptoms were all significantly less common (relative risk = 0.74) in those who received nirmatrelvir. This was true no matter whether they’d ever had a COVID vaccination.
Q: How should this research affect clinical practice?
A: You can’t generalize from this to everyone because, of course, not everyone was included in this study. But it is highly suggestive that this drug is very effective for preventing long COVID.
Nirmatrelvir was touted as being able to shorten duration of illness and prevent hospitalization. But if you were low risk or you were already well into your COVID course, it wasn’t like rush, rush, rush to the doctor to get it.
This changes that equation because we know long COVID is such a huge issue. The vast majority of doctors who work with COVID patients and know this are now being more aggressive about prescribing it.
Q: What about patients whom the CDC considers to be at less risk — people with up-to-date vaccinations who are under 50 with mild-to-moderate COVID and no higher-risk medical conditions? Should they take nirmatrelvir?
A: The evidence is not 100% in yet. A study like this one needs to be repeated and include younger people without any risk factors to see if we see the same thing. So it’s a personal choice, and a personal calculus needs to be done. A lot of people are making that choice [to take the drug], and it can be a rational decision.
Q: You also chose a study that links high thyroid hormone levels to higher rates of dementia. What did it reveal?
A: This study looks at patients who had thyrotoxicosis — a thyroid level that’s too high — from hormone produced endogenously, and exogenously. Researchers tracked almost 66,000 patients aged 65 and older and found that thyrotoxicosis from all causes, whether it was endogenous or exogenous, was linked to an increased risk of dementia in a dose-response relationship (adjusted hazard ratio = 1.39).
Q: Is there a clinical take-home message here?
A: When we start patients on thyroid medication, they don’t always get reassessed on a regular basis. Given this finding, a TSH [thyroid-stimulating hormone] level is indicated during the annual wellness check that patients on Medicare can get every year.
Q: Is TSH measured as part of routine blood tests?
A: No it’s not. It has to be ordered. I think that’s why we’re seeing this problem to begin with — because it’s not something we all have awareness about. I wasn’t aware myself that mildly high levels of thyroid could increase the risk of cognitive impairment. Certainly, I’m going to be much more aware in my practice.
Q: You also picked a study about silicosis in workers who are exposed to dust when they make engineered stone countertops, also known as quartz countertops. What were the findings?
A: Silicosis is a very serious lung condition that develops from exposure to crystalline silica. Essentially, sand gets inhaled into the lungs. Workers can be exposed when they’re making engineered stone countertops, the most popular countertops now in the United States.
This study is based on statewide surveys from 2019 to 2022 that the California Department of Public Health does routinely. They gathered cases of silicosis and found 52 — all men with an average age of 45. All but one were Latino immigrants, and most either had no insurance or very poor insurance.
Q: The study found that “diagnosis was delayed in 58%, with 38% presenting with advanced disease (progressive massive fibrosis), and 19% died.” What does that tell you?
A: It’s a very serious condition. Once it gets to the advanced stage, it will just continue to progress, and the person will die. That’s why it’s so important to know that it’s absolutely preventable.
Q: Is there a message here for internists?
A: If you treat a lot of immigrants or work in an area where there are a lot of industrial workers, you’re going to want to have a very high suspicion about it. If you see an atypical pattern on the chest x-ray or via diffusion scoring, have a low threshold for getting a pulmonary function test.
Doctors need to be aware and diagnose this very quickly. When patients present, you can pull them out of that work environment or put mitigation systems into place.
Q: California regulators were expected to put emergency rules into place in late December to protect workers. Did this study play a role in focusing attention on the problem?
A: This article, along with a commentary and podcast that we put out, really helped with advocacy to improve health and safety for workers at stone-cutting and fabrication shops.
Q: You were impressed by another study about airborne dangers, this one linking air pollution to dementia. What did researchers discover?
A: [This analysis] of more than 27,000 people in the Health and Retirement Study, a respected and rich database, found that exposure to air pollution was associated with greater rates of dementia — an increase of about 8% a year. Exposure to agricultural emissions and wildfire smoke were most robustly associated with a greater risk of dementia.
Q: How are these findings important, especially in light of the unhealthy air spawned by recent wildfires in the United States and Canada?
A: Studies like this will make it even more compelling that we are better prepared for air quality issues.
I grew up in Los Angeles, where smog and pollution were very big issues. I was constantly hearing about various mitigation strategies that were going into place. But after I moved to the East Coast, I almost never heard about prevention.
Now, I’m hoping we can keep this topic in the national conversation.
Q: You also highlighted a systematic review of the use of restraints in the emergency department. Why did you choose this research?
A: At JAMA Internal Medicine, we’re really focused on ways we can address health disparities and raise awareness of potential unconscious bias.
This review looked at 10 studies that included more than 2.5 million patient encounters, including 24,000 incidents of physical restraint use. They found that the overall rate of use of restraints was low at below 1%.
But when they are used, Black patients were 1.3 times more likely to be restrained than White patients.
Q: What’s the message here?
A: This is an important start to recognizing these differences and then changing our behavior. Perhaps restraints don’t need to be used as often in light of evidence, for example, of increased rates of misdiagnosis of psychosis in the Black population.
Q: How should physicians change their approach to restraints?
A: Restraints are not to be used to control disruption — wild behavior or verbal outbursts. They’re for when someone is a danger to themselves or others.
Dr. Inouye has no conflicts of interest.
Harvard Medical School’s Sharon K. Inouye, MD, MPH, is editor in chief of JAMA Internal Medicine and a leading voice in American gerontology. We asked her to choose five of the influential journal’s most impactful studies from 2023 and highlight important take-home messages for internists and their colleagues.
Q: One of the studies you chose suggests that the antiviral nirmatrelvir (Paxlovid) can ward off long COVID. Could you recap the findings?
A: Researchers followed a group of more than 280,000 Department of Veterans Affairs patients who were seen in 2022, had a positive COVID test, and had at least one risk factor for severe COVID. They focused on those who survived to 30 days after their COVID infection and compared those who received the drug within the first 5 days of a positive test with an equivalent control group.
They found that 13 long COVID symptoms were all significantly less common (relative risk = 0.74) in those who received nirmatrelvir. This was true no matter whether they’d ever had a COVID vaccination.
Q: How should this research affect clinical practice?
A: You can’t generalize from this to everyone because, of course, not everyone was included in this study. But it is highly suggestive that this drug is very effective for preventing long COVID.
Nirmatrelvir was touted as being able to shorten duration of illness and prevent hospitalization. But if you were low risk or you were already well into your COVID course, it wasn’t like rush, rush, rush to the doctor to get it.
This changes that equation because we know long COVID is such a huge issue. The vast majority of doctors who work with COVID patients and know this are now being more aggressive about prescribing it.
Q: What about patients whom the CDC considers to be at less risk — people with up-to-date vaccinations who are under 50 with mild-to-moderate COVID and no higher-risk medical conditions? Should they take nirmatrelvir?
A: The evidence is not 100% in yet. A study like this one needs to be repeated and include younger people without any risk factors to see if we see the same thing. So it’s a personal choice, and a personal calculus needs to be done. A lot of people are making that choice [to take the drug], and it can be a rational decision.
Q: You also chose a study that links high thyroid hormone levels to higher rates of dementia. What did it reveal?
A: This study looks at patients who had thyrotoxicosis — a thyroid level that’s too high — from hormone produced endogenously, and exogenously. Researchers tracked almost 66,000 patients aged 65 and older and found that thyrotoxicosis from all causes, whether it was endogenous or exogenous, was linked to an increased risk of dementia in a dose-response relationship (adjusted hazard ratio = 1.39).
Q: Is there a clinical take-home message here?
A: When we start patients on thyroid medication, they don’t always get reassessed on a regular basis. Given this finding, a TSH [thyroid-stimulating hormone] level is indicated during the annual wellness check that patients on Medicare can get every year.
Q: Is TSH measured as part of routine blood tests?
A: No it’s not. It has to be ordered. I think that’s why we’re seeing this problem to begin with — because it’s not something we all have awareness about. I wasn’t aware myself that mildly high levels of thyroid could increase the risk of cognitive impairment. Certainly, I’m going to be much more aware in my practice.
Q: You also picked a study about silicosis in workers who are exposed to dust when they make engineered stone countertops, also known as quartz countertops. What were the findings?
A: Silicosis is a very serious lung condition that develops from exposure to crystalline silica. Essentially, sand gets inhaled into the lungs. Workers can be exposed when they’re making engineered stone countertops, the most popular countertops now in the United States.
This study is based on statewide surveys from 2019 to 2022 that the California Department of Public Health does routinely. They gathered cases of silicosis and found 52 — all men with an average age of 45. All but one were Latino immigrants, and most either had no insurance or very poor insurance.
Q: The study found that “diagnosis was delayed in 58%, with 38% presenting with advanced disease (progressive massive fibrosis), and 19% died.” What does that tell you?
A: It’s a very serious condition. Once it gets to the advanced stage, it will just continue to progress, and the person will die. That’s why it’s so important to know that it’s absolutely preventable.
Q: Is there a message here for internists?
A: If you treat a lot of immigrants or work in an area where there are a lot of industrial workers, you’re going to want to have a very high suspicion about it. If you see an atypical pattern on the chest x-ray or via diffusion scoring, have a low threshold for getting a pulmonary function test.
Doctors need to be aware and diagnose this very quickly. When patients present, you can pull them out of that work environment or put mitigation systems into place.
Q: California regulators were expected to put emergency rules into place in late December to protect workers. Did this study play a role in focusing attention on the problem?
A: This article, along with a commentary and podcast that we put out, really helped with advocacy to improve health and safety for workers at stone-cutting and fabrication shops.
Q: You were impressed by another study about airborne dangers, this one linking air pollution to dementia. What did researchers discover?
A: [This analysis] of more than 27,000 people in the Health and Retirement Study, a respected and rich database, found that exposure to air pollution was associated with greater rates of dementia — an increase of about 8% a year. Exposure to agricultural emissions and wildfire smoke were most robustly associated with a greater risk of dementia.
Q: How are these findings important, especially in light of the unhealthy air spawned by recent wildfires in the United States and Canada?
A: Studies like this will make it even more compelling that we are better prepared for air quality issues.
I grew up in Los Angeles, where smog and pollution were very big issues. I was constantly hearing about various mitigation strategies that were going into place. But after I moved to the East Coast, I almost never heard about prevention.
Now, I’m hoping we can keep this topic in the national conversation.
Q: You also highlighted a systematic review of the use of restraints in the emergency department. Why did you choose this research?
A: At JAMA Internal Medicine, we’re really focused on ways we can address health disparities and raise awareness of potential unconscious bias.
This review looked at 10 studies that included more than 2.5 million patient encounters, including 24,000 incidents of physical restraint use. They found that the overall rate of use of restraints was low at below 1%.
But when they are used, Black patients were 1.3 times more likely to be restrained than White patients.
Q: What’s the message here?
A: This is an important start to recognizing these differences and then changing our behavior. Perhaps restraints don’t need to be used as often in light of evidence, for example, of increased rates of misdiagnosis of psychosis in the Black population.
Q: How should physicians change their approach to restraints?
A: Restraints are not to be used to control disruption — wild behavior or verbal outbursts. They’re for when someone is a danger to themselves or others.
Dr. Inouye has no conflicts of interest.
Taking Stock, With Gratitude
Christmas, like New Year’s Day, Thanksgiving, birthdays, and anniversaries, is one of those times that we use to mark where we were and how far we’ve come.
I’m in a mixed marriage, so we celebrate both Hanukkah and Christmas. Twenty-five years ago I was a newly minted attending neurologist, not even 6 months out of fellowship.
My wife was pregnant with our first child and had invited my Jewish family over for Christmas dinner. This was our first December in our first house and she wanted to do something special for them.
Being the low person on the totem pole, it was my first Christmas on call, covering for myself and two other neurologists.
So I was driving. A lot. My wife was on her own to get things ready, and I was hoping to be home for dinner.
It was, as always seems to be the case with holidays, quite busy. I was up long before dawn to start, driving a circular route to cover four hospitals scattered around Phoenix. At least the roads were empty.
At some point the planned pattern breaks down as new consults and urgent patient status changes happen. You try to start by going from A to B to C to D for rounds, but within a few hours I was going from A to B to C, then back to A, then D, then B, then A again, and so on. All the while I was returning patient calls. Wash, rinse, repeat.
At some point I dialed my wife to see how she was doing and she gave me a list of last-minute things she needed picked up (which included some dairy products and more Christmas lights for her tree). I found a small store that was still open. For the rest of my day on call a grocery bag full of dairy products was carried from hospital to hospital with me, being put in the doctor’s lounge refrigerator with my name on it (this is Phoenix, even in winter you can’t leave it in the car). This added another trip from C back to A when I realized I’d left the groceries there.
I got home a few minutes before my family came over, after 14-15 hours of driving between hospitals. I was putting up the new lights when they came in. Fortunately I wasn’t called back in that night, and turned things over to my call partners in the morning.
Now? Since early 2020 my hospital days are behind me. My kids have their own lives, jobs, and school, but still all came over to see us.
I didn’t have to leave the house. I spent most of the day in a robe and pajamas, working at my desk on this and that, sometimes wandering to another table to futz with my current jigsaw puzzle or chat with my kids or go soak in my hot tub.
In 1998 I weighed 50 pounds less (still working on losing it), had no kids, or dogs. Now I’m in another house, have three grown kids, and in the interim have enjoyed seven awesome dogs (currently only one). My wife still invited my family over for Christmas dinner, but now it’s my mom and uncle. My dad and aunt are gone.
The changes are mostly good, though, as with all passages of time there is sadness and loss. When all is said and done I wouldn’t have done much differently even if I could.
I’m lucky, and I know it. To quote Sheryl Crow, “It’s not having what you want, it’s wanting what you’ve got.”
Happy New Year to all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Christmas, like New Year’s Day, Thanksgiving, birthdays, and anniversaries, is one of those times that we use to mark where we were and how far we’ve come.
I’m in a mixed marriage, so we celebrate both Hanukkah and Christmas. Twenty-five years ago I was a newly minted attending neurologist, not even 6 months out of fellowship.
My wife was pregnant with our first child and had invited my Jewish family over for Christmas dinner. This was our first December in our first house and she wanted to do something special for them.
Being the low person on the totem pole, it was my first Christmas on call, covering for myself and two other neurologists.
So I was driving. A lot. My wife was on her own to get things ready, and I was hoping to be home for dinner.
It was, as always seems to be the case with holidays, quite busy. I was up long before dawn to start, driving a circular route to cover four hospitals scattered around Phoenix. At least the roads were empty.
At some point the planned pattern breaks down as new consults and urgent patient status changes happen. You try to start by going from A to B to C to D for rounds, but within a few hours I was going from A to B to C, then back to A, then D, then B, then A again, and so on. All the while I was returning patient calls. Wash, rinse, repeat.
At some point I dialed my wife to see how she was doing and she gave me a list of last-minute things she needed picked up (which included some dairy products and more Christmas lights for her tree). I found a small store that was still open. For the rest of my day on call a grocery bag full of dairy products was carried from hospital to hospital with me, being put in the doctor’s lounge refrigerator with my name on it (this is Phoenix, even in winter you can’t leave it in the car). This added another trip from C back to A when I realized I’d left the groceries there.
I got home a few minutes before my family came over, after 14-15 hours of driving between hospitals. I was putting up the new lights when they came in. Fortunately I wasn’t called back in that night, and turned things over to my call partners in the morning.
Now? Since early 2020 my hospital days are behind me. My kids have their own lives, jobs, and school, but still all came over to see us.
I didn’t have to leave the house. I spent most of the day in a robe and pajamas, working at my desk on this and that, sometimes wandering to another table to futz with my current jigsaw puzzle or chat with my kids or go soak in my hot tub.
In 1998 I weighed 50 pounds less (still working on losing it), had no kids, or dogs. Now I’m in another house, have three grown kids, and in the interim have enjoyed seven awesome dogs (currently only one). My wife still invited my family over for Christmas dinner, but now it’s my mom and uncle. My dad and aunt are gone.
The changes are mostly good, though, as with all passages of time there is sadness and loss. When all is said and done I wouldn’t have done much differently even if I could.
I’m lucky, and I know it. To quote Sheryl Crow, “It’s not having what you want, it’s wanting what you’ve got.”
Happy New Year to all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Christmas, like New Year’s Day, Thanksgiving, birthdays, and anniversaries, is one of those times that we use to mark where we were and how far we’ve come.
I’m in a mixed marriage, so we celebrate both Hanukkah and Christmas. Twenty-five years ago I was a newly minted attending neurologist, not even 6 months out of fellowship.
My wife was pregnant with our first child and had invited my Jewish family over for Christmas dinner. This was our first December in our first house and she wanted to do something special for them.
Being the low person on the totem pole, it was my first Christmas on call, covering for myself and two other neurologists.
So I was driving. A lot. My wife was on her own to get things ready, and I was hoping to be home for dinner.
It was, as always seems to be the case with holidays, quite busy. I was up long before dawn to start, driving a circular route to cover four hospitals scattered around Phoenix. At least the roads were empty.
At some point the planned pattern breaks down as new consults and urgent patient status changes happen. You try to start by going from A to B to C to D for rounds, but within a few hours I was going from A to B to C, then back to A, then D, then B, then A again, and so on. All the while I was returning patient calls. Wash, rinse, repeat.
At some point I dialed my wife to see how she was doing and she gave me a list of last-minute things she needed picked up (which included some dairy products and more Christmas lights for her tree). I found a small store that was still open. For the rest of my day on call a grocery bag full of dairy products was carried from hospital to hospital with me, being put in the doctor’s lounge refrigerator with my name on it (this is Phoenix, even in winter you can’t leave it in the car). This added another trip from C back to A when I realized I’d left the groceries there.
I got home a few minutes before my family came over, after 14-15 hours of driving between hospitals. I was putting up the new lights when they came in. Fortunately I wasn’t called back in that night, and turned things over to my call partners in the morning.
Now? Since early 2020 my hospital days are behind me. My kids have their own lives, jobs, and school, but still all came over to see us.
I didn’t have to leave the house. I spent most of the day in a robe and pajamas, working at my desk on this and that, sometimes wandering to another table to futz with my current jigsaw puzzle or chat with my kids or go soak in my hot tub.
In 1998 I weighed 50 pounds less (still working on losing it), had no kids, or dogs. Now I’m in another house, have three grown kids, and in the interim have enjoyed seven awesome dogs (currently only one). My wife still invited my family over for Christmas dinner, but now it’s my mom and uncle. My dad and aunt are gone.
The changes are mostly good, though, as with all passages of time there is sadness and loss. When all is said and done I wouldn’t have done much differently even if I could.
I’m lucky, and I know it. To quote Sheryl Crow, “It’s not having what you want, it’s wanting what you’ve got.”
Happy New Year to all.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The Art of Seeing
People are surprised when they learn I was an art history major in college. Most folks assume I had majored in biology or chemistry. Their assumption was based on strong odds. The U.S. Bureau of Labor Statistics reports that nearly half of all physicians practicing in this country were biology majors.
I headed off to college clueless about my future. I was hoping to succeed as a walk-on to the football team and beyond that I figured someone or something would guide me toward a career. Had you asked me, “physician” it would have been a definite “Never.”
I flirted with a psychology major, but after a semester I realized that the department was more interested in the behavior of rats rather than humans. I got an “easy A” in the intro to art history and that was the open door I was looking for.
By my senior year I was applying for fellowships to study in faraway places. However, the world situation in 1965 was unsettling for a young man in this country. I had had a strong high school science education and had continued to take a some science courses. Fortunately, I had banked just enough credits so that I could apply to medical school, again without really planning to become a physician.
Even during the sharpest turns in my circuitous path to becoming a small town pediatrician, including a year doing research in exercise physiology in Denmark, I never once regretted my years spent studying art history. I credit them with making me a more sensitive observer.
You can probably understand why I was intrigued by an article I recently read that described a program in which the radiology residents that the Brigham and Women’s Hospital in Boston take a year-long course in art history using the Art Museum at Harvard University as a resource. Titled “Seeing in Art and Medical Imaging,” the program is now 6 years old. Hyewon Hyun, MD, a radiologist and one of its cofounders, observes that “art is the starting point for in-depth conversations about medicine, humanity, and different ways of seeing the world.”
Radiology and dermatology are obviously the two specialties in which the physician relies most heavily on his or her powers of observation. However, every doctor can benefit from learning to really “see” what they are looking at. Looking and seeing are two very different activities. There is obviously the forest-from the-trees phenomenon. Can the physician in a hurried clinical situation muster up the discipline to shift focus back and forth from the lesion or painful body part to the entire patient and beyond? How is the parent responding to the child’s discomfort? How are they dressed? Does this wider view suggest some additional questions to ask that may help you understand how this patient or family will be able to cope with diagnosis or follow up with your treatment plan?
The art historian sees every object in its historical context. What has come before? How have the societal conditions influenced the artist choice of subject and use of materials? How has his or her emotions at the time of creation influenced his or her style? The astute physician must likewise see the patients and their complaints in the broader context of their emotional health and socioeconomic situation. This requires sensitive listening and careful observation.
One doesn’t have to major in art history or spend years roaming through the sometimes dark and dusty halls of the world’s museums to progress from being one who simply looks to a person who really sees the environment and its inhabitants. It is really a state of mind and a commitment to improvement.
As physicians, we often complain or sometimes brag about how many patients we “see” in a day. I fear that too often we mean “looked at.” How frequently did we make the effort to really see the patient?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
People are surprised when they learn I was an art history major in college. Most folks assume I had majored in biology or chemistry. Their assumption was based on strong odds. The U.S. Bureau of Labor Statistics reports that nearly half of all physicians practicing in this country were biology majors.
I headed off to college clueless about my future. I was hoping to succeed as a walk-on to the football team and beyond that I figured someone or something would guide me toward a career. Had you asked me, “physician” it would have been a definite “Never.”
I flirted with a psychology major, but after a semester I realized that the department was more interested in the behavior of rats rather than humans. I got an “easy A” in the intro to art history and that was the open door I was looking for.
By my senior year I was applying for fellowships to study in faraway places. However, the world situation in 1965 was unsettling for a young man in this country. I had had a strong high school science education and had continued to take a some science courses. Fortunately, I had banked just enough credits so that I could apply to medical school, again without really planning to become a physician.
Even during the sharpest turns in my circuitous path to becoming a small town pediatrician, including a year doing research in exercise physiology in Denmark, I never once regretted my years spent studying art history. I credit them with making me a more sensitive observer.
You can probably understand why I was intrigued by an article I recently read that described a program in which the radiology residents that the Brigham and Women’s Hospital in Boston take a year-long course in art history using the Art Museum at Harvard University as a resource. Titled “Seeing in Art and Medical Imaging,” the program is now 6 years old. Hyewon Hyun, MD, a radiologist and one of its cofounders, observes that “art is the starting point for in-depth conversations about medicine, humanity, and different ways of seeing the world.”
Radiology and dermatology are obviously the two specialties in which the physician relies most heavily on his or her powers of observation. However, every doctor can benefit from learning to really “see” what they are looking at. Looking and seeing are two very different activities. There is obviously the forest-from the-trees phenomenon. Can the physician in a hurried clinical situation muster up the discipline to shift focus back and forth from the lesion or painful body part to the entire patient and beyond? How is the parent responding to the child’s discomfort? How are they dressed? Does this wider view suggest some additional questions to ask that may help you understand how this patient or family will be able to cope with diagnosis or follow up with your treatment plan?
The art historian sees every object in its historical context. What has come before? How have the societal conditions influenced the artist choice of subject and use of materials? How has his or her emotions at the time of creation influenced his or her style? The astute physician must likewise see the patients and their complaints in the broader context of their emotional health and socioeconomic situation. This requires sensitive listening and careful observation.
One doesn’t have to major in art history or spend years roaming through the sometimes dark and dusty halls of the world’s museums to progress from being one who simply looks to a person who really sees the environment and its inhabitants. It is really a state of mind and a commitment to improvement.
As physicians, we often complain or sometimes brag about how many patients we “see” in a day. I fear that too often we mean “looked at.” How frequently did we make the effort to really see the patient?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
People are surprised when they learn I was an art history major in college. Most folks assume I had majored in biology or chemistry. Their assumption was based on strong odds. The U.S. Bureau of Labor Statistics reports that nearly half of all physicians practicing in this country were biology majors.
I headed off to college clueless about my future. I was hoping to succeed as a walk-on to the football team and beyond that I figured someone or something would guide me toward a career. Had you asked me, “physician” it would have been a definite “Never.”
I flirted with a psychology major, but after a semester I realized that the department was more interested in the behavior of rats rather than humans. I got an “easy A” in the intro to art history and that was the open door I was looking for.
By my senior year I was applying for fellowships to study in faraway places. However, the world situation in 1965 was unsettling for a young man in this country. I had had a strong high school science education and had continued to take a some science courses. Fortunately, I had banked just enough credits so that I could apply to medical school, again without really planning to become a physician.
Even during the sharpest turns in my circuitous path to becoming a small town pediatrician, including a year doing research in exercise physiology in Denmark, I never once regretted my years spent studying art history. I credit them with making me a more sensitive observer.
You can probably understand why I was intrigued by an article I recently read that described a program in which the radiology residents that the Brigham and Women’s Hospital in Boston take a year-long course in art history using the Art Museum at Harvard University as a resource. Titled “Seeing in Art and Medical Imaging,” the program is now 6 years old. Hyewon Hyun, MD, a radiologist and one of its cofounders, observes that “art is the starting point for in-depth conversations about medicine, humanity, and different ways of seeing the world.”
Radiology and dermatology are obviously the two specialties in which the physician relies most heavily on his or her powers of observation. However, every doctor can benefit from learning to really “see” what they are looking at. Looking and seeing are two very different activities. There is obviously the forest-from the-trees phenomenon. Can the physician in a hurried clinical situation muster up the discipline to shift focus back and forth from the lesion or painful body part to the entire patient and beyond? How is the parent responding to the child’s discomfort? How are they dressed? Does this wider view suggest some additional questions to ask that may help you understand how this patient or family will be able to cope with diagnosis or follow up with your treatment plan?
The art historian sees every object in its historical context. What has come before? How have the societal conditions influenced the artist choice of subject and use of materials? How has his or her emotions at the time of creation influenced his or her style? The astute physician must likewise see the patients and their complaints in the broader context of their emotional health and socioeconomic situation. This requires sensitive listening and careful observation.
One doesn’t have to major in art history or spend years roaming through the sometimes dark and dusty halls of the world’s museums to progress from being one who simply looks to a person who really sees the environment and its inhabitants. It is really a state of mind and a commitment to improvement.
As physicians, we often complain or sometimes brag about how many patients we “see” in a day. I fear that too often we mean “looked at.” How frequently did we make the effort to really see the patient?
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
New Insights, New Standards: How 2023 Changed Care for Internists
The past year brought major changes in preventive standards for anxiety, HIV, and RSV along with new guidelines for the treatment of atrial fibrillation. For insight into the effect on internal medicine, we turned to Sarah Candler, MD, MPH, a Houston internist who specializes in the care of high-risk older adults.
Q: Which new prevention guidelines had the most impact on you over the past year?
A: I’m a primary care doctor, and most of the internal medicine updates that are interesting to me focus on how we can keep people from getting sick in the first place. That’s especially important in light of the fact that we had a decrease in life expectancy of 2 years [it finally rose slightly in 2022] and widening of the gender gap in life expectancy for men and women.
I’m excited to see new recommendations from the U.S. Preventive Services Task Force, including a new one about using PREP [pre-exposure prophylaxis] to preventively treat anyone who’s at risk for getting HIV. That’s a big one because it’s one of the first times that we’ve identified at-risk groups for screening based on social risk factors, not gender, age, or genetics.
The new recommendation is PREP for anyone who’s at risk for getting HIV because they have a partner with HIV, had an sexually transmitted infection in the last 6 months, or a history of inconsistent or no condom use with partners with unknown HIV status.
PREP therapy is something that most primary care physicians can either do or learn how to do pretty easily. But the treatment does require maintenance and monitoring.
Q: How firm is this recommendation?
A: The task force gives different grades for their recommendations based on how strong the evidence is. For the guidelines about PREP, they give a grade of A. That means this is top of the class: You should definitely do this.
Q: What are the best strategies to ask patients personal questions about their sex lives in order to evaluate their risk?
A: A lot of internal medicine physicians are getting pretty good at this. We see it as part of our job just the same way as we asked things like, “How often are you walking?” and “Have you been feeling down?”
There’s no one right way to have a conversation like that. But it’s key to say, as I do to my patients, that “I’m not here to judge anything. I am truly here to gather information and make recommendations to you as a partner in your care.”
Q: What other guidelines made an impact in 2023?
A: The U.S. Preventive Services Task Force made a recommendation to screen adults aged 18-64 for anxiety, and this guidance got a B grade. [The task force said there’s not enough evidence to support routine anxiety screening in adults 65 and older.]
The new recommendations is a sign that we’re doing a better job at making treatment of those diseases more acceptable. This is also another example of the medical community recognizing that internal medicine physicians are pretty good at identifying and treating mental health.
Q: How do you figure out whether to treat depression/anxiety yourself or refer patients to specialists?
A: As a primary care physician, I feel comfortable diagnosing and managing some mental health disease in my own practice. There are FDA-approved medications for both anxiety and depression that are easily managed by a primary care physician.
And there’s something to the therapeutic relationship, to naming and identifying these conditions with your patients. Some patients feel a bit of relief just knowing that they have a diagnosis.
Q: What should internists know about the new CDC guidelines that promote discussing RSV vaccines with patients who are over 60?
A: The vaccines are recommended for folks who have underlying conditions like lung disease or heart disease. Those are the ones who end up getting really, really sick. There are two adult vaccines that are available, and there’s not a preference for one over the other.
The vaccines are both protein-based, like the old-school versions of vaccines, not the mRNA vaccines that we’ve all been hearing more about through COVID. Anybody who’s reluctant to take an mRNA vaccine can rest assured that the RSV is not protein-based. And they are single-dose vaccines, which is helpful.
Q: What else should internists know about that was new in 2023?
A: I’m super excited about how cardiologists are thinking about atrial fibrillation. In 2023, the American College of Cardiology and the American Heart Association came up with a giant overhaul of how they look at atrial fibrillation. They classify it in stages and allows us to think about stopping it before it starts.
They’re talking about something they’re calling preclinical or subclinical atrial fibrillation, which you may detect on wearables like somebody’s watch or another tool used to monitor heart rate or exercise. It might be the first harbinger that there’s something wrong with the heart rate, and they may not even have symptoms of it. [A 2023 study in The New England Journal of Medicine linked the anticoagulant apixaban, or Eliquis, to a 37% lower risk of stroke and systemic embolism rates in older patients with subclinical atrial fibrillation but an 80% higher risk of major bleeding vs. aspirin therapy.]
And they’re now recommending early rhythm control.
Q: What does early rhythm control mean for patients and physicians?
A: For the longest time, we have thought about atrial fibrillation treatment in terms of rate control and not worrying too much about the rhythm. But now we recognize that it’s actually really important that we get the rhythm under control because physical changes to the heart can lead to permanent damage.
So now they’re recommending catheter ablation as first-line therapy in some patients as a class 1 recommendation because heart function is already decreased. Improving the ability of the heart to beat with a regular rhythm can lead to improvement of function. This was unheard of even 5 years ago.
Q: Should internists be more willing to refer patients with atrial fibrillation to cardiologists?
A: Yes, I think so. One of the biggest changes for me is that I am going to refer new diagnoses of atrial fibrillation to a cardiologist. And I’m going to ask patients if they have wearable devices because sometimes those things might tell me about something like subclinical atrial fibrillation.
Q: There’s also detailed data about atrial fibrillation risk factors, which include older age, smoking, sedentary lifestyle, alcohol use, diabetes, height, obesity, diabetes, and others. Is this information useful?
A: It’s a really great tool to have in the arsenal because it helps me have shared decision-making conversations with my patients in a way that’s much more convincing. A patient might say, “Why do you care if I drink so much? My liver levels are fine.” And I can say, “It’s going to be a risk factor for having problems with your heart.”
For better or worse, people really take the heart very seriously, I am an internal medicine physician, so I love all the organs equally. But man, people get pretty scared when you tell them something can affect their heart. So when I talk to patients about their risk factors, it’s going to really be helpful that I can remind them of the impact that some of these lifestyle behaviors can have on their heart health.
Dr. Candler has no disclosures.
The past year brought major changes in preventive standards for anxiety, HIV, and RSV along with new guidelines for the treatment of atrial fibrillation. For insight into the effect on internal medicine, we turned to Sarah Candler, MD, MPH, a Houston internist who specializes in the care of high-risk older adults.
Q: Which new prevention guidelines had the most impact on you over the past year?
A: I’m a primary care doctor, and most of the internal medicine updates that are interesting to me focus on how we can keep people from getting sick in the first place. That’s especially important in light of the fact that we had a decrease in life expectancy of 2 years [it finally rose slightly in 2022] and widening of the gender gap in life expectancy for men and women.
I’m excited to see new recommendations from the U.S. Preventive Services Task Force, including a new one about using PREP [pre-exposure prophylaxis] to preventively treat anyone who’s at risk for getting HIV. That’s a big one because it’s one of the first times that we’ve identified at-risk groups for screening based on social risk factors, not gender, age, or genetics.
The new recommendation is PREP for anyone who’s at risk for getting HIV because they have a partner with HIV, had an sexually transmitted infection in the last 6 months, or a history of inconsistent or no condom use with partners with unknown HIV status.
PREP therapy is something that most primary care physicians can either do or learn how to do pretty easily. But the treatment does require maintenance and monitoring.
Q: How firm is this recommendation?
A: The task force gives different grades for their recommendations based on how strong the evidence is. For the guidelines about PREP, they give a grade of A. That means this is top of the class: You should definitely do this.
Q: What are the best strategies to ask patients personal questions about their sex lives in order to evaluate their risk?
A: A lot of internal medicine physicians are getting pretty good at this. We see it as part of our job just the same way as we asked things like, “How often are you walking?” and “Have you been feeling down?”
There’s no one right way to have a conversation like that. But it’s key to say, as I do to my patients, that “I’m not here to judge anything. I am truly here to gather information and make recommendations to you as a partner in your care.”
Q: What other guidelines made an impact in 2023?
A: The U.S. Preventive Services Task Force made a recommendation to screen adults aged 18-64 for anxiety, and this guidance got a B grade. [The task force said there’s not enough evidence to support routine anxiety screening in adults 65 and older.]
The new recommendations is a sign that we’re doing a better job at making treatment of those diseases more acceptable. This is also another example of the medical community recognizing that internal medicine physicians are pretty good at identifying and treating mental health.
Q: How do you figure out whether to treat depression/anxiety yourself or refer patients to specialists?
A: As a primary care physician, I feel comfortable diagnosing and managing some mental health disease in my own practice. There are FDA-approved medications for both anxiety and depression that are easily managed by a primary care physician.
And there’s something to the therapeutic relationship, to naming and identifying these conditions with your patients. Some patients feel a bit of relief just knowing that they have a diagnosis.
Q: What should internists know about the new CDC guidelines that promote discussing RSV vaccines with patients who are over 60?
A: The vaccines are recommended for folks who have underlying conditions like lung disease or heart disease. Those are the ones who end up getting really, really sick. There are two adult vaccines that are available, and there’s not a preference for one over the other.
The vaccines are both protein-based, like the old-school versions of vaccines, not the mRNA vaccines that we’ve all been hearing more about through COVID. Anybody who’s reluctant to take an mRNA vaccine can rest assured that the RSV is not protein-based. And they are single-dose vaccines, which is helpful.
Q: What else should internists know about that was new in 2023?
A: I’m super excited about how cardiologists are thinking about atrial fibrillation. In 2023, the American College of Cardiology and the American Heart Association came up with a giant overhaul of how they look at atrial fibrillation. They classify it in stages and allows us to think about stopping it before it starts.
They’re talking about something they’re calling preclinical or subclinical atrial fibrillation, which you may detect on wearables like somebody’s watch or another tool used to monitor heart rate or exercise. It might be the first harbinger that there’s something wrong with the heart rate, and they may not even have symptoms of it. [A 2023 study in The New England Journal of Medicine linked the anticoagulant apixaban, or Eliquis, to a 37% lower risk of stroke and systemic embolism rates in older patients with subclinical atrial fibrillation but an 80% higher risk of major bleeding vs. aspirin therapy.]
And they’re now recommending early rhythm control.
Q: What does early rhythm control mean for patients and physicians?
A: For the longest time, we have thought about atrial fibrillation treatment in terms of rate control and not worrying too much about the rhythm. But now we recognize that it’s actually really important that we get the rhythm under control because physical changes to the heart can lead to permanent damage.
So now they’re recommending catheter ablation as first-line therapy in some patients as a class 1 recommendation because heart function is already decreased. Improving the ability of the heart to beat with a regular rhythm can lead to improvement of function. This was unheard of even 5 years ago.
Q: Should internists be more willing to refer patients with atrial fibrillation to cardiologists?
A: Yes, I think so. One of the biggest changes for me is that I am going to refer new diagnoses of atrial fibrillation to a cardiologist. And I’m going to ask patients if they have wearable devices because sometimes those things might tell me about something like subclinical atrial fibrillation.
Q: There’s also detailed data about atrial fibrillation risk factors, which include older age, smoking, sedentary lifestyle, alcohol use, diabetes, height, obesity, diabetes, and others. Is this information useful?
A: It’s a really great tool to have in the arsenal because it helps me have shared decision-making conversations with my patients in a way that’s much more convincing. A patient might say, “Why do you care if I drink so much? My liver levels are fine.” And I can say, “It’s going to be a risk factor for having problems with your heart.”
For better or worse, people really take the heart very seriously, I am an internal medicine physician, so I love all the organs equally. But man, people get pretty scared when you tell them something can affect their heart. So when I talk to patients about their risk factors, it’s going to really be helpful that I can remind them of the impact that some of these lifestyle behaviors can have on their heart health.
Dr. Candler has no disclosures.
The past year brought major changes in preventive standards for anxiety, HIV, and RSV along with new guidelines for the treatment of atrial fibrillation. For insight into the effect on internal medicine, we turned to Sarah Candler, MD, MPH, a Houston internist who specializes in the care of high-risk older adults.
Q: Which new prevention guidelines had the most impact on you over the past year?
A: I’m a primary care doctor, and most of the internal medicine updates that are interesting to me focus on how we can keep people from getting sick in the first place. That’s especially important in light of the fact that we had a decrease in life expectancy of 2 years [it finally rose slightly in 2022] and widening of the gender gap in life expectancy for men and women.
I’m excited to see new recommendations from the U.S. Preventive Services Task Force, including a new one about using PREP [pre-exposure prophylaxis] to preventively treat anyone who’s at risk for getting HIV. That’s a big one because it’s one of the first times that we’ve identified at-risk groups for screening based on social risk factors, not gender, age, or genetics.
The new recommendation is PREP for anyone who’s at risk for getting HIV because they have a partner with HIV, had an sexually transmitted infection in the last 6 months, or a history of inconsistent or no condom use with partners with unknown HIV status.
PREP therapy is something that most primary care physicians can either do or learn how to do pretty easily. But the treatment does require maintenance and monitoring.
Q: How firm is this recommendation?
A: The task force gives different grades for their recommendations based on how strong the evidence is. For the guidelines about PREP, they give a grade of A. That means this is top of the class: You should definitely do this.
Q: What are the best strategies to ask patients personal questions about their sex lives in order to evaluate their risk?
A: A lot of internal medicine physicians are getting pretty good at this. We see it as part of our job just the same way as we asked things like, “How often are you walking?” and “Have you been feeling down?”
There’s no one right way to have a conversation like that. But it’s key to say, as I do to my patients, that “I’m not here to judge anything. I am truly here to gather information and make recommendations to you as a partner in your care.”
Q: What other guidelines made an impact in 2023?
A: The U.S. Preventive Services Task Force made a recommendation to screen adults aged 18-64 for anxiety, and this guidance got a B grade. [The task force said there’s not enough evidence to support routine anxiety screening in adults 65 and older.]
The new recommendations is a sign that we’re doing a better job at making treatment of those diseases more acceptable. This is also another example of the medical community recognizing that internal medicine physicians are pretty good at identifying and treating mental health.
Q: How do you figure out whether to treat depression/anxiety yourself or refer patients to specialists?
A: As a primary care physician, I feel comfortable diagnosing and managing some mental health disease in my own practice. There are FDA-approved medications for both anxiety and depression that are easily managed by a primary care physician.
And there’s something to the therapeutic relationship, to naming and identifying these conditions with your patients. Some patients feel a bit of relief just knowing that they have a diagnosis.
Q: What should internists know about the new CDC guidelines that promote discussing RSV vaccines with patients who are over 60?
A: The vaccines are recommended for folks who have underlying conditions like lung disease or heart disease. Those are the ones who end up getting really, really sick. There are two adult vaccines that are available, and there’s not a preference for one over the other.
The vaccines are both protein-based, like the old-school versions of vaccines, not the mRNA vaccines that we’ve all been hearing more about through COVID. Anybody who’s reluctant to take an mRNA vaccine can rest assured that the RSV is not protein-based. And they are single-dose vaccines, which is helpful.
Q: What else should internists know about that was new in 2023?
A: I’m super excited about how cardiologists are thinking about atrial fibrillation. In 2023, the American College of Cardiology and the American Heart Association came up with a giant overhaul of how they look at atrial fibrillation. They classify it in stages and allows us to think about stopping it before it starts.
They’re talking about something they’re calling preclinical or subclinical atrial fibrillation, which you may detect on wearables like somebody’s watch or another tool used to monitor heart rate or exercise. It might be the first harbinger that there’s something wrong with the heart rate, and they may not even have symptoms of it. [A 2023 study in The New England Journal of Medicine linked the anticoagulant apixaban, or Eliquis, to a 37% lower risk of stroke and systemic embolism rates in older patients with subclinical atrial fibrillation but an 80% higher risk of major bleeding vs. aspirin therapy.]
And they’re now recommending early rhythm control.
Q: What does early rhythm control mean for patients and physicians?
A: For the longest time, we have thought about atrial fibrillation treatment in terms of rate control and not worrying too much about the rhythm. But now we recognize that it’s actually really important that we get the rhythm under control because physical changes to the heart can lead to permanent damage.
So now they’re recommending catheter ablation as first-line therapy in some patients as a class 1 recommendation because heart function is already decreased. Improving the ability of the heart to beat with a regular rhythm can lead to improvement of function. This was unheard of even 5 years ago.
Q: Should internists be more willing to refer patients with atrial fibrillation to cardiologists?
A: Yes, I think so. One of the biggest changes for me is that I am going to refer new diagnoses of atrial fibrillation to a cardiologist. And I’m going to ask patients if they have wearable devices because sometimes those things might tell me about something like subclinical atrial fibrillation.
Q: There’s also detailed data about atrial fibrillation risk factors, which include older age, smoking, sedentary lifestyle, alcohol use, diabetes, height, obesity, diabetes, and others. Is this information useful?
A: It’s a really great tool to have in the arsenal because it helps me have shared decision-making conversations with my patients in a way that’s much more convincing. A patient might say, “Why do you care if I drink so much? My liver levels are fine.” And I can say, “It’s going to be a risk factor for having problems with your heart.”
For better or worse, people really take the heart very seriously, I am an internal medicine physician, so I love all the organs equally. But man, people get pretty scared when you tell them something can affect their heart. So when I talk to patients about their risk factors, it’s going to really be helpful that I can remind them of the impact that some of these lifestyle behaviors can have on their heart health.
Dr. Candler has no disclosures.