User login
What to tell your patients about anti-amyloids for Alzheimer’s disease
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Recorded October 13, 2023. This transcript has been edited for clarity.
Kathrin LaFaver, MD: I’ll be talking today with Dr. Meredith Wicklund, senior associate consultant and behavioral neurologist specialist at Mayo Clinic in Arizona. Welcome, Meredith.
Meredith Wicklund, MD: Thank you.
Lecanemab data
Dr. LaFaver: I’m very excited about our topic. Could you give us a brief overview of why there has been so much research interest in this topic of anti-amyloid antibodies?
Dr. Wicklund: The pathologic component of what defines something as Alzheimer’s disease is, by definition, presence of amyloid plaques and tau tangles. When it was first discovered in the 1980s that the component of the plaques was actually the amyloid protein – beta amyloid specifically – interest went right from there to developing therapies to directly target the pathology that is Alzheimer’s disease.
Dr. LaFaver: Lecanemab is the first FDA-approved disease-modifying antibody in that realm. Could you review the study data, especially as it applies to both of us in daily neurology clinic?
Dr. Wicklund: The study data from a phase 3 trial did show, for the primary outcome, that there was a 27% slowing of decline compared with individuals on placebo. It’s important to point out that this was slowing of decline. It was not stabilizing decline. It was not improving decline.
I think it’s important that we inform our patients that really, even with this therapy, there’s no prospect of stabilizing or restoring cognition or function. We do progress at a slower rate compared with individuals not on this treatment, which, given that this medication is for individuals in mild disease who have relatively preserved functional status, that can be potentially very meaningful to families.
The overall benefit was small. It essentially amounts to half a point on an 18-point scale, which is statistically significant. How much clinical meaningfulness that actually leads to is unclear. Finding clinical meaningfulness cannot be defined by a particular test. It really can only be defined on the individual level, what is meaningful to them.
Recommended tests
Dr. LaFaver: It is my understanding that, to qualify for lecanemab use, one needs to have a biomarker-supported diagnosis of Alzheimer’s disease, either via an amyloid PET scan or CSF biomarkers. What would your recommendation be for a neurologist in practice to go about these requirements?
Dr. Wicklund: Since this medication is directly targeting the amyloid pathology, and it does convey a potential risk, we want to make sure that the actual pathology is present in the individuals before we treat them and potentially expose them to risk. The best way of doing that is through either an amyloid PET scan or spinal fluid testing of beta amyloid and tau.
There are several plasma-based biomarkers in development. However, I would avoid using those currently. There are still many unknowns in terms of what exactly is the right species of tau that we should be looking at, the right mechanism of the lab test, how minority status may influence it, and how different comorbidities may influence it.
I would recommend, at this time, sticking with amyloid PET or CSF testing. Given that amyloid PET is not widely available in many community practices, generally only available at academic centers, and is quite costly, many insurances do not cover it – although Medicare has a proposal to potentially start covering it – I generally go with spinal fluid testing, which is more widely available. There are several labs across the country that can process that testing in a reliable way.
Amyloid-related imaging abnormalities
Dr. LaFaver: That’s very helpful to know. There’s been a large amount of buzz just these past couple of weeks about the blood biomarker coming up. I think, as you point out, this wasn’t the marker used in the clinical studies and there are still unknowns. Maybe it’s not quite time for clinical use, unfortunately.
We also have learned that there are significant potential risks involved. One issue that’s really been a focus is ARIA – amyloid-related imaging abnormalities. Could you speak a bit about that and requirements for monitoring?
Dr. Wicklund: ARIA essentially amounts to either vasogenic edema, microhemorrhages, or superficial siderosis that develops as a result of treatment. It relates to activation of the immune system with these passive monoclonal antibodies that’s going to occur with targeting against the plaques. In the parenchyma, it will cause edema. If you have amyloid in the walls of the blood vessels, it can cause microhemorrhages.
While the term “ARIA” implies an imaging-related abnormality, and it largely is purely an imaging finding, it’s not solely an imaging-related finding. It can cause symptoms, including very serious symptoms.
Overall, with lecanemab, the incidence of ARIA within the treatment group in the phase 3 study, combined between both ARIA-E (edema/effusion) and ARIA-H (hemorrhage), was 21.5%, with about 17% being ARIA-H and about 12.5% being ARIA-E. Of course, they can occur at the same time.
Overall, in terms of people in the clinical trials, for most it was purely an imaging-related finding. About 3% developed symptomatic ARIA. Some of those were very serious symptoms, including things like seizures and need to be hospitalized. A couple of deaths have been attributed to ARIA as well.
Patients on anticoagulation
Dr. LaFaver: Along those lines, any additional words to say for people who might be on anticoagulation or might require medications for a stroke, for example?
Dr. Wicklund: While individuals on anticoagulation were allowed in the clinical trials, the current, published appropriate-use guideline is recommending against its use, as several of the serious adverse effects, including the deaths, were for the most part attributed to anticoagulation use.
When it comes to acute stroke treatment, one must carefully consider use of tPA, as two of the three deaths were tPA associated in the clinical trials. It shouldn’t necessarily be an absolute contraindication, but it can make the clinical picture very muddy. If an individual is on lecanemab and comes to the ER with acute stroke-like symptoms, it’s more likely that they’re going to be having an ARIA side effect rather than an acute stroke.
A general recommendation would be to obtain an acute head CT with a CTA, and if there is a large vessel occlusion, proceed to thrombectomy. However, if there isn’t a large vessel occlusion, if you have the ability to get a rapid MRI with diffusion-weighted imaging to screen for acute stroke changes or tissue flair with acute edema changes suggestive of ARIA, that would be preferred before proceeding with thrombolysis. These are all relative contraindications and are going to depend on what’s available near you.
Donanemab approval pending
Dr. LaFaver: This will be an issue because the population we’re talking about is definitely at risk for stroke as well as Alzheimer’s disease. Where do you see this field going as far as amyloid antibody therapy is concerned, with another agent, donanemab, possibly getting FDA approval later this year as well?
Dr. Wicklund: We’re anticipating that donanemab will get FDA approval in the next coming months. Donanemab also targets the amyloid in the brain, although lecanemab and donanemab target different aspects of the production of the amyloid plaque. They were both shown to have roughly equal efficacy in their phase 3 clinical trials. Donanemab has the benefit of being a once-monthly infusion as opposed to twice-monthly infusions with lecanemab. It does have a slightly higher risk for ARIA compared with lecanemab.
Those are just some things to take into consideration when talking with your patients. In terms of where we’re going from here, we’re moving even earlier in terms of disease state. The lecanemab and donanemab phase 3 trials were done in individuals with mild cognitive impairment or mild dementia due to Alzheimer’s disease. They should not be used in individuals with moderate or more advanced Alzheimer’s disease.
There are ongoing, large, national, multicenter clinical trials of both lecanemab and donanemab in a preclinical state of Alzheimer’s disease. These individuals have evidence of amyloidosis, either through PET imaging or through CSF, but are clinically asymptomatic and do not yet have any signs of cognitive impairment or functional decline. We look forward to those results in the next few years. Hopefully, they’ll be able to show even greater benefit when moving into these early disease states in terms of delaying or even preventing cognitive decline.
Dr. LaFaver: That’s definitely very interesting to hear about. Where can people go for more information?
Dr. Wicklund: There’s a guideline on the use of lecanemab through the American Academy of Neurology. I encourage you to look at that. Also, look at the appropriate-use recommendations that were published this year in The Journal of Prevention of Alzheimer’s Disease.
Dr. LaFaver: Wonderful. With that being said, thank you so much for talking to me. I learned a lot. Thanks, everyone, for listening.
Dr. LaFaver is a neurologist at Saratoga Hospital Medical Group, Saratoga Springs, N.Y. She disclosed having no relevant financial relationships. Dr. Wicklund is senior associate consultant in the department of Neurology at Mayo Clinic, Phoenix, Ariz. She disclosed having no relevant financial relationships.
A version of this article appeared on Medscape.com.
Revisiting the role of hydrocortisone, fludrocortisone in septic shock
Earlier this year, I stumbled across a podcast in a content update email from the Journal of the American Medical Association. The moderator was interviewing the first author of a study comparing hydrocortisone and fludrocortisone (hydro/fludro) to hydrocortisone alone for treatment of septic shock. In the introduction,
I thought this issue had been settled with publication of the COIITSS trial in 2010. This study randomly assigned 509 patients with septic shock to hydro/fludro versus hydrocortisone alone. There was a nonsignificant reduction in mortality with hydro/fludro and everyone I knew stopped adding fludrocortisone for septic shock. It wasn’t included in guidelines (and still isn›t). I figured the only docs still using it were also prescribing ivermectin and vitamin C – another treatment touted to work in an apocryphal podcast.
It wasn’t just COIITSS that killed fludrocortisone for me. Back in 2002, I was a loyal adherent. That year, a randomized controlled trial (RCT) published by “the lord of corticosteroids for critical illness” doctor, Djillali Annane, found benefit to hydro/fludro in septic shock . Everyone in that study had a cosyntropin stim test and only certain subgroups had better outcomes. As a medical resident paying obeisance to all things evidence-based medicine, I rigidly adopted their protocol for all septic patients. I also kept their insulin between 80 and 110 mg/dL, prescribed drotrecogin alfa, and made sure they were floating in crystalloid. But those are topics for another time.
Subsequent trials and meta-analyses cast doubt on the need for the stim test, and a consensus around hydrocortisone at moderate doses for patients with septic shock emerged. Because one part of the Annane protocol was already deemed unnecessary (the cosyntropin stim test), it was easy to dismiss fludrocortisone after COIITTS was published. Yes, I read Annane’s 2018 APROCCHSS trial, and I’m aware that it found that hydro/fludro reduced 90-day mortality. Like others, I rationalized this finding by framing it as a function of baseline mortality. The two Annane RCTs that found that hydro/fludro reduced mortality in enrolled patients who were considerably more likely to die than those enrolled in RCTs of hydrocortisone alone were negative. It was the target population mortality rate and not the addition of fludrocortisone that made the difference, right?
Rethinking hydro/fludro
The author interviewed for the recent JAMA podcast forced me to rethink my blithe dismissal of fludrocortisone. He contended that the COIITTS trial was underpowered and the two Annane RCTs that used fludrocortisone supply the evidence that shows corticosteroids reduce septic shock mortality. As discussed earlier, he found clinical equipoise among his colleagues. Last, he invoked pleiotropic mineralocorticoid effects, such as activation of innate immunity and clearance of alveolar fluid, to support the need to reexamine hydro/fludro.
In his study, he used Big Data to compare hospital records from 2016 to 2020. He analyzed a total of 88,275 patients with septic shock. Most were prescribed hydrocortisone alone (85,995 [97.4%] vs. only 2.6% hydro/fludro). After a number of statistical adjustments and sensitivity analyses, the authors concluded that the addition of fludrocortisone to hydrocortisone for patients with septic shock provides a 3.7% absolute risk reduction in mortality (or discharge to hospice) when compared with hydrocortisone alone. That’s a number needed to treat of 28 to prevent one death (or discharge to hospice).
Key takeaways
The study isn’t perfect. In their methods section they use terms like “ensemble machine learner (super learner)” and “immortal time bias.” The first is a fancy way of saying they did a form of propensity scoring, which in turn is a fancy way of saying they tried to control for confounding. The second is a way to adjust for time delays between drug administration. Both are attempts to compensate for the observational design, as is their argument for biologic plausibility. Here they’re on particularly thin ice when trying to prove causal inference. Biologic plausibility is never hard to find; after all, what compound doesn’t have pleiotropic effects? Furthermore, the analysis lacks any data to support their biologic plausibility hypothesis that fludrocortisone’s effect on mortality is mediated via activation of innate immunity and/or clearance of alveolar fluid.
The editorial accompanying this Big Data study endorsed adding fludrocortisone. We have very little that reduces ICU mortality so the low number needed to treat is enticing, especially in light of the low risk from adverse events, so I’m going to start using it. Do I think I’ll save one life for every 28 patients with septic shock to whom I give hydro/fludro instead of hydrocortisone alone? I sure don’t. No way an oral mineralocorticoid at that dose has that type of impact on top of hydrocortisone alone. I still believe that the Annane studies are positive because of the mortality rate in the population enrolled and not because fludrocortisone was added. It all comes full circle, though – 20 years after I abandoned hydro/fludro, I’m going back to it.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/critical care and sleep medicine physician at MedStar Washington Hospital Center in Washington, D.C.
A version of this article first appeared on Medscape.com.
Earlier this year, I stumbled across a podcast in a content update email from the Journal of the American Medical Association. The moderator was interviewing the first author of a study comparing hydrocortisone and fludrocortisone (hydro/fludro) to hydrocortisone alone for treatment of septic shock. In the introduction,
I thought this issue had been settled with publication of the COIITSS trial in 2010. This study randomly assigned 509 patients with septic shock to hydro/fludro versus hydrocortisone alone. There was a nonsignificant reduction in mortality with hydro/fludro and everyone I knew stopped adding fludrocortisone for septic shock. It wasn’t included in guidelines (and still isn›t). I figured the only docs still using it were also prescribing ivermectin and vitamin C – another treatment touted to work in an apocryphal podcast.
It wasn’t just COIITSS that killed fludrocortisone for me. Back in 2002, I was a loyal adherent. That year, a randomized controlled trial (RCT) published by “the lord of corticosteroids for critical illness” doctor, Djillali Annane, found benefit to hydro/fludro in septic shock . Everyone in that study had a cosyntropin stim test and only certain subgroups had better outcomes. As a medical resident paying obeisance to all things evidence-based medicine, I rigidly adopted their protocol for all septic patients. I also kept their insulin between 80 and 110 mg/dL, prescribed drotrecogin alfa, and made sure they were floating in crystalloid. But those are topics for another time.
Subsequent trials and meta-analyses cast doubt on the need for the stim test, and a consensus around hydrocortisone at moderate doses for patients with septic shock emerged. Because one part of the Annane protocol was already deemed unnecessary (the cosyntropin stim test), it was easy to dismiss fludrocortisone after COIITTS was published. Yes, I read Annane’s 2018 APROCCHSS trial, and I’m aware that it found that hydro/fludro reduced 90-day mortality. Like others, I rationalized this finding by framing it as a function of baseline mortality. The two Annane RCTs that found that hydro/fludro reduced mortality in enrolled patients who were considerably more likely to die than those enrolled in RCTs of hydrocortisone alone were negative. It was the target population mortality rate and not the addition of fludrocortisone that made the difference, right?
Rethinking hydro/fludro
The author interviewed for the recent JAMA podcast forced me to rethink my blithe dismissal of fludrocortisone. He contended that the COIITTS trial was underpowered and the two Annane RCTs that used fludrocortisone supply the evidence that shows corticosteroids reduce septic shock mortality. As discussed earlier, he found clinical equipoise among his colleagues. Last, he invoked pleiotropic mineralocorticoid effects, such as activation of innate immunity and clearance of alveolar fluid, to support the need to reexamine hydro/fludro.
In his study, he used Big Data to compare hospital records from 2016 to 2020. He analyzed a total of 88,275 patients with septic shock. Most were prescribed hydrocortisone alone (85,995 [97.4%] vs. only 2.6% hydro/fludro). After a number of statistical adjustments and sensitivity analyses, the authors concluded that the addition of fludrocortisone to hydrocortisone for patients with septic shock provides a 3.7% absolute risk reduction in mortality (or discharge to hospice) when compared with hydrocortisone alone. That’s a number needed to treat of 28 to prevent one death (or discharge to hospice).
Key takeaways
The study isn’t perfect. In their methods section they use terms like “ensemble machine learner (super learner)” and “immortal time bias.” The first is a fancy way of saying they did a form of propensity scoring, which in turn is a fancy way of saying they tried to control for confounding. The second is a way to adjust for time delays between drug administration. Both are attempts to compensate for the observational design, as is their argument for biologic plausibility. Here they’re on particularly thin ice when trying to prove causal inference. Biologic plausibility is never hard to find; after all, what compound doesn’t have pleiotropic effects? Furthermore, the analysis lacks any data to support their biologic plausibility hypothesis that fludrocortisone’s effect on mortality is mediated via activation of innate immunity and/or clearance of alveolar fluid.
The editorial accompanying this Big Data study endorsed adding fludrocortisone. We have very little that reduces ICU mortality so the low number needed to treat is enticing, especially in light of the low risk from adverse events, so I’m going to start using it. Do I think I’ll save one life for every 28 patients with septic shock to whom I give hydro/fludro instead of hydrocortisone alone? I sure don’t. No way an oral mineralocorticoid at that dose has that type of impact on top of hydrocortisone alone. I still believe that the Annane studies are positive because of the mortality rate in the population enrolled and not because fludrocortisone was added. It all comes full circle, though – 20 years after I abandoned hydro/fludro, I’m going back to it.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/critical care and sleep medicine physician at MedStar Washington Hospital Center in Washington, D.C.
A version of this article first appeared on Medscape.com.
Earlier this year, I stumbled across a podcast in a content update email from the Journal of the American Medical Association. The moderator was interviewing the first author of a study comparing hydrocortisone and fludrocortisone (hydro/fludro) to hydrocortisone alone for treatment of septic shock. In the introduction,
I thought this issue had been settled with publication of the COIITSS trial in 2010. This study randomly assigned 509 patients with septic shock to hydro/fludro versus hydrocortisone alone. There was a nonsignificant reduction in mortality with hydro/fludro and everyone I knew stopped adding fludrocortisone for septic shock. It wasn’t included in guidelines (and still isn›t). I figured the only docs still using it were also prescribing ivermectin and vitamin C – another treatment touted to work in an apocryphal podcast.
It wasn’t just COIITSS that killed fludrocortisone for me. Back in 2002, I was a loyal adherent. That year, a randomized controlled trial (RCT) published by “the lord of corticosteroids for critical illness” doctor, Djillali Annane, found benefit to hydro/fludro in septic shock . Everyone in that study had a cosyntropin stim test and only certain subgroups had better outcomes. As a medical resident paying obeisance to all things evidence-based medicine, I rigidly adopted their protocol for all septic patients. I also kept their insulin between 80 and 110 mg/dL, prescribed drotrecogin alfa, and made sure they were floating in crystalloid. But those are topics for another time.
Subsequent trials and meta-analyses cast doubt on the need for the stim test, and a consensus around hydrocortisone at moderate doses for patients with septic shock emerged. Because one part of the Annane protocol was already deemed unnecessary (the cosyntropin stim test), it was easy to dismiss fludrocortisone after COIITTS was published. Yes, I read Annane’s 2018 APROCCHSS trial, and I’m aware that it found that hydro/fludro reduced 90-day mortality. Like others, I rationalized this finding by framing it as a function of baseline mortality. The two Annane RCTs that found that hydro/fludro reduced mortality in enrolled patients who were considerably more likely to die than those enrolled in RCTs of hydrocortisone alone were negative. It was the target population mortality rate and not the addition of fludrocortisone that made the difference, right?
Rethinking hydro/fludro
The author interviewed for the recent JAMA podcast forced me to rethink my blithe dismissal of fludrocortisone. He contended that the COIITTS trial was underpowered and the two Annane RCTs that used fludrocortisone supply the evidence that shows corticosteroids reduce septic shock mortality. As discussed earlier, he found clinical equipoise among his colleagues. Last, he invoked pleiotropic mineralocorticoid effects, such as activation of innate immunity and clearance of alveolar fluid, to support the need to reexamine hydro/fludro.
In his study, he used Big Data to compare hospital records from 2016 to 2020. He analyzed a total of 88,275 patients with septic shock. Most were prescribed hydrocortisone alone (85,995 [97.4%] vs. only 2.6% hydro/fludro). After a number of statistical adjustments and sensitivity analyses, the authors concluded that the addition of fludrocortisone to hydrocortisone for patients with septic shock provides a 3.7% absolute risk reduction in mortality (or discharge to hospice) when compared with hydrocortisone alone. That’s a number needed to treat of 28 to prevent one death (or discharge to hospice).
Key takeaways
The study isn’t perfect. In their methods section they use terms like “ensemble machine learner (super learner)” and “immortal time bias.” The first is a fancy way of saying they did a form of propensity scoring, which in turn is a fancy way of saying they tried to control for confounding. The second is a way to adjust for time delays between drug administration. Both are attempts to compensate for the observational design, as is their argument for biologic plausibility. Here they’re on particularly thin ice when trying to prove causal inference. Biologic plausibility is never hard to find; after all, what compound doesn’t have pleiotropic effects? Furthermore, the analysis lacks any data to support their biologic plausibility hypothesis that fludrocortisone’s effect on mortality is mediated via activation of innate immunity and/or clearance of alveolar fluid.
The editorial accompanying this Big Data study endorsed adding fludrocortisone. We have very little that reduces ICU mortality so the low number needed to treat is enticing, especially in light of the low risk from adverse events, so I’m going to start using it. Do I think I’ll save one life for every 28 patients with septic shock to whom I give hydro/fludro instead of hydrocortisone alone? I sure don’t. No way an oral mineralocorticoid at that dose has that type of impact on top of hydrocortisone alone. I still believe that the Annane studies are positive because of the mortality rate in the population enrolled and not because fludrocortisone was added. It all comes full circle, though – 20 years after I abandoned hydro/fludro, I’m going back to it.
Aaron B. Holley, MD, is a professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/critical care and sleep medicine physician at MedStar Washington Hospital Center in Washington, D.C.
A version of this article first appeared on Medscape.com.
A better way to control blood pressure
My Bing AI engine, when prompted, tells me that there are about 87 journals, 45 conferences, and 53 workshops presently dedicated exclusively to hypertension. All of that attention, and yet ...
What is going on?
The top killers of Americans remain coronary artery heart disease (26%), cancer (22%), and stroke (6%). The precursors and attributable risk factors for coronary artery heart disease include hypertension (40%), obesity (20%), diabetes (15%), and combustible tobacco use (15%). The key precursors and attributable risk factors for stroke are hypertension (53%), obesity (37%), diabetes (9%), and combustible tobacco use (11%). Obviously, these are estimates, with substantial overlap.
It’s pretty obvious that
We have addressed improving tobacco control and preventing obesity and diabetes on these pages many times, and lamented the medical, public health, and societal failings. Today we turn our attention to the control of hypertension. That is much easier and far less expensive.
All physicians and medical organizations know that hypertension is a major attributable cause of many serious, expensive, and fatal illnesses. As many as 119 million (48%) of American adults have hypertension. The American Heart Association (AHA), American Medical Association (AMA), American College of Cardiology (ACC), and hundreds of other organizations have set a new target of 130/80 (revised from 140/90) for blood pressure control and have launched a major initiative, Target: BP, to reach it.
That is just great. We all wish this massive effort to succeed where few others have. But do AHA, AMA, ACC, and others understand why most efforts to this point have failed? The blame is typically aimed at patients failing to adhere to their instructions. Maybe, but why? And how does Target: BP intend to convert chronic failure into success if it just continues to do everything they have been trying to do that doesn’t work?
At this point, the Centers for Disease Control and Prevention reports that fewer than 48% of American patients with hypertension meet even the less stringent historical 140/90 goal.
A group practice in Ohio, PriMed Physicians, has consistently exceeded 90% or even 95% blood pressure control for its patients with hypertension for more than 10 years. Exemplary. How do they do it? This video of the 13th annual Lundberg Institute lecture describes this unique and successful program.
PriMed’s clinicians use the MedsEngine AI tool from MediSync and the NICaS (noninvasive cardiac system with impedance cardiography) to determine each patient’s unique blood pressure pathophysiology. Clinicians and patients understand that the simplest explanation of this pathophysiology encompasses three factors: (1) the volume of “water” (blood) in the system; (2) the strength of the pumping (pulsatile) process; and (3) the tightness (resistance) of the tubes that carry the blood. Patients “get it” when it is explained this way, and they cooperate.
At the first patient encounter, the Food and Drug Administration–approved PhysioFlow is employed to assess those three vital hemodynamic factors. The individual patient’s data are loaded into a tightly programed EHR-based algorithm with 37 clinical factors and five classes of drugs, providing multiple ways to influence the three key pathophysiologic processes. In this way, they arrive at the precise drug(s) and dosages for that patient. During the second visit, most patients are already showing improvement. By the third visit, the blood pressures of most patients have reached target control. After that, it is maintenance and tweaking.
These factors summarize why it works:
- Senior management belief, commitment, and leadership
- Informed buy-in from clinicians and patients
- A test that determines root causes of too much fluid, too strong pump action, or too tight pipes, and their proportionality
- An AI tool that matches those three pathophysiologic factors and 35 other clinical factors with the best drug or drugs (of many, not just a few) and dosages
- Persistent clinician-patient follow-up
- Refusal to accept failure
Since this approach is so successful, why is its use not everywhere?
It is not as if nobody noticed, even if you and many organizations have not. The American Medical Group Association recognized the program’s success by giving its top award to PriMed in 2015.
Klepper and Rodis wrote about this approach for managing multiple chronic conditions in 2021. Here’s a background article and an explainer, Clinical use of impedance cardiography for hemodynamic assessment of early cardiovascular disease and management of hypertension.
I found one pragmatic controlled clinical trial of impedance cardiography with a decision-support system from Beijing that did demonstrate clinical and statistical significance.
Frankly, we do need more rigorous, unbiased, large, controlled clinical trials assessing the MedsEngine and NICaS approach to managing blood pressure to facilitate a massive switch from the old and established (but failing) approach to a starkly better way.
Almost no one ever “completes a database.” All decision makers must act based upon the best data to which they have access. Data are often incomplete. The difference between success and mediocrity is often the ability of an individual or system to decide when enough information is enough and act accordingly.
Cost-effectiveness studies in three countries (United Kingdom, United States, and China) confirm sharply lower lifelong costs when blood pressure is well controlled. Of course.
For the American medical-industrial complex, lowered costs for managing common serious diseases may be an undesired rather than a good thing. In money-driven medicine, lower costs to the payer and purchaser translate to less revenue for the providers. Imagine all of those invasive and noninvasive diagnostic and therapeutic procedures forgone by prevention of hypertension. Is it possible that such an underlying truth is the real reason why American medicine is habitually unsuccessful at controlling blood pressure?
Right now, if my blood pressure were not well controlled (it is), I would find my way to Cincinnati, to give PriMed physicians, MediSync, and MedsEngine a crack at prolonging my useful life.
Dr. Lundberg is editor in chief of Cancer Commons. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
My Bing AI engine, when prompted, tells me that there are about 87 journals, 45 conferences, and 53 workshops presently dedicated exclusively to hypertension. All of that attention, and yet ...
What is going on?
The top killers of Americans remain coronary artery heart disease (26%), cancer (22%), and stroke (6%). The precursors and attributable risk factors for coronary artery heart disease include hypertension (40%), obesity (20%), diabetes (15%), and combustible tobacco use (15%). The key precursors and attributable risk factors for stroke are hypertension (53%), obesity (37%), diabetes (9%), and combustible tobacco use (11%). Obviously, these are estimates, with substantial overlap.
It’s pretty obvious that
We have addressed improving tobacco control and preventing obesity and diabetes on these pages many times, and lamented the medical, public health, and societal failings. Today we turn our attention to the control of hypertension. That is much easier and far less expensive.
All physicians and medical organizations know that hypertension is a major attributable cause of many serious, expensive, and fatal illnesses. As many as 119 million (48%) of American adults have hypertension. The American Heart Association (AHA), American Medical Association (AMA), American College of Cardiology (ACC), and hundreds of other organizations have set a new target of 130/80 (revised from 140/90) for blood pressure control and have launched a major initiative, Target: BP, to reach it.
That is just great. We all wish this massive effort to succeed where few others have. But do AHA, AMA, ACC, and others understand why most efforts to this point have failed? The blame is typically aimed at patients failing to adhere to their instructions. Maybe, but why? And how does Target: BP intend to convert chronic failure into success if it just continues to do everything they have been trying to do that doesn’t work?
At this point, the Centers for Disease Control and Prevention reports that fewer than 48% of American patients with hypertension meet even the less stringent historical 140/90 goal.
A group practice in Ohio, PriMed Physicians, has consistently exceeded 90% or even 95% blood pressure control for its patients with hypertension for more than 10 years. Exemplary. How do they do it? This video of the 13th annual Lundberg Institute lecture describes this unique and successful program.
PriMed’s clinicians use the MedsEngine AI tool from MediSync and the NICaS (noninvasive cardiac system with impedance cardiography) to determine each patient’s unique blood pressure pathophysiology. Clinicians and patients understand that the simplest explanation of this pathophysiology encompasses three factors: (1) the volume of “water” (blood) in the system; (2) the strength of the pumping (pulsatile) process; and (3) the tightness (resistance) of the tubes that carry the blood. Patients “get it” when it is explained this way, and they cooperate.
At the first patient encounter, the Food and Drug Administration–approved PhysioFlow is employed to assess those three vital hemodynamic factors. The individual patient’s data are loaded into a tightly programed EHR-based algorithm with 37 clinical factors and five classes of drugs, providing multiple ways to influence the three key pathophysiologic processes. In this way, they arrive at the precise drug(s) and dosages for that patient. During the second visit, most patients are already showing improvement. By the third visit, the blood pressures of most patients have reached target control. After that, it is maintenance and tweaking.
These factors summarize why it works:
- Senior management belief, commitment, and leadership
- Informed buy-in from clinicians and patients
- A test that determines root causes of too much fluid, too strong pump action, or too tight pipes, and their proportionality
- An AI tool that matches those three pathophysiologic factors and 35 other clinical factors with the best drug or drugs (of many, not just a few) and dosages
- Persistent clinician-patient follow-up
- Refusal to accept failure
Since this approach is so successful, why is its use not everywhere?
It is not as if nobody noticed, even if you and many organizations have not. The American Medical Group Association recognized the program’s success by giving its top award to PriMed in 2015.
Klepper and Rodis wrote about this approach for managing multiple chronic conditions in 2021. Here’s a background article and an explainer, Clinical use of impedance cardiography for hemodynamic assessment of early cardiovascular disease and management of hypertension.
I found one pragmatic controlled clinical trial of impedance cardiography with a decision-support system from Beijing that did demonstrate clinical and statistical significance.
Frankly, we do need more rigorous, unbiased, large, controlled clinical trials assessing the MedsEngine and NICaS approach to managing blood pressure to facilitate a massive switch from the old and established (but failing) approach to a starkly better way.
Almost no one ever “completes a database.” All decision makers must act based upon the best data to which they have access. Data are often incomplete. The difference between success and mediocrity is often the ability of an individual or system to decide when enough information is enough and act accordingly.
Cost-effectiveness studies in three countries (United Kingdom, United States, and China) confirm sharply lower lifelong costs when blood pressure is well controlled. Of course.
For the American medical-industrial complex, lowered costs for managing common serious diseases may be an undesired rather than a good thing. In money-driven medicine, lower costs to the payer and purchaser translate to less revenue for the providers. Imagine all of those invasive and noninvasive diagnostic and therapeutic procedures forgone by prevention of hypertension. Is it possible that such an underlying truth is the real reason why American medicine is habitually unsuccessful at controlling blood pressure?
Right now, if my blood pressure were not well controlled (it is), I would find my way to Cincinnati, to give PriMed physicians, MediSync, and MedsEngine a crack at prolonging my useful life.
Dr. Lundberg is editor in chief of Cancer Commons. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
My Bing AI engine, when prompted, tells me that there are about 87 journals, 45 conferences, and 53 workshops presently dedicated exclusively to hypertension. All of that attention, and yet ...
What is going on?
The top killers of Americans remain coronary artery heart disease (26%), cancer (22%), and stroke (6%). The precursors and attributable risk factors for coronary artery heart disease include hypertension (40%), obesity (20%), diabetes (15%), and combustible tobacco use (15%). The key precursors and attributable risk factors for stroke are hypertension (53%), obesity (37%), diabetes (9%), and combustible tobacco use (11%). Obviously, these are estimates, with substantial overlap.
It’s pretty obvious that
We have addressed improving tobacco control and preventing obesity and diabetes on these pages many times, and lamented the medical, public health, and societal failings. Today we turn our attention to the control of hypertension. That is much easier and far less expensive.
All physicians and medical organizations know that hypertension is a major attributable cause of many serious, expensive, and fatal illnesses. As many as 119 million (48%) of American adults have hypertension. The American Heart Association (AHA), American Medical Association (AMA), American College of Cardiology (ACC), and hundreds of other organizations have set a new target of 130/80 (revised from 140/90) for blood pressure control and have launched a major initiative, Target: BP, to reach it.
That is just great. We all wish this massive effort to succeed where few others have. But do AHA, AMA, ACC, and others understand why most efforts to this point have failed? The blame is typically aimed at patients failing to adhere to their instructions. Maybe, but why? And how does Target: BP intend to convert chronic failure into success if it just continues to do everything they have been trying to do that doesn’t work?
At this point, the Centers for Disease Control and Prevention reports that fewer than 48% of American patients with hypertension meet even the less stringent historical 140/90 goal.
A group practice in Ohio, PriMed Physicians, has consistently exceeded 90% or even 95% blood pressure control for its patients with hypertension for more than 10 years. Exemplary. How do they do it? This video of the 13th annual Lundberg Institute lecture describes this unique and successful program.
PriMed’s clinicians use the MedsEngine AI tool from MediSync and the NICaS (noninvasive cardiac system with impedance cardiography) to determine each patient’s unique blood pressure pathophysiology. Clinicians and patients understand that the simplest explanation of this pathophysiology encompasses three factors: (1) the volume of “water” (blood) in the system; (2) the strength of the pumping (pulsatile) process; and (3) the tightness (resistance) of the tubes that carry the blood. Patients “get it” when it is explained this way, and they cooperate.
At the first patient encounter, the Food and Drug Administration–approved PhysioFlow is employed to assess those three vital hemodynamic factors. The individual patient’s data are loaded into a tightly programed EHR-based algorithm with 37 clinical factors and five classes of drugs, providing multiple ways to influence the three key pathophysiologic processes. In this way, they arrive at the precise drug(s) and dosages for that patient. During the second visit, most patients are already showing improvement. By the third visit, the blood pressures of most patients have reached target control. After that, it is maintenance and tweaking.
These factors summarize why it works:
- Senior management belief, commitment, and leadership
- Informed buy-in from clinicians and patients
- A test that determines root causes of too much fluid, too strong pump action, or too tight pipes, and their proportionality
- An AI tool that matches those three pathophysiologic factors and 35 other clinical factors with the best drug or drugs (of many, not just a few) and dosages
- Persistent clinician-patient follow-up
- Refusal to accept failure
Since this approach is so successful, why is its use not everywhere?
It is not as if nobody noticed, even if you and many organizations have not. The American Medical Group Association recognized the program’s success by giving its top award to PriMed in 2015.
Klepper and Rodis wrote about this approach for managing multiple chronic conditions in 2021. Here’s a background article and an explainer, Clinical use of impedance cardiography for hemodynamic assessment of early cardiovascular disease and management of hypertension.
I found one pragmatic controlled clinical trial of impedance cardiography with a decision-support system from Beijing that did demonstrate clinical and statistical significance.
Frankly, we do need more rigorous, unbiased, large, controlled clinical trials assessing the MedsEngine and NICaS approach to managing blood pressure to facilitate a massive switch from the old and established (but failing) approach to a starkly better way.
Almost no one ever “completes a database.” All decision makers must act based upon the best data to which they have access. Data are often incomplete. The difference between success and mediocrity is often the ability of an individual or system to decide when enough information is enough and act accordingly.
Cost-effectiveness studies in three countries (United Kingdom, United States, and China) confirm sharply lower lifelong costs when blood pressure is well controlled. Of course.
For the American medical-industrial complex, lowered costs for managing common serious diseases may be an undesired rather than a good thing. In money-driven medicine, lower costs to the payer and purchaser translate to less revenue for the providers. Imagine all of those invasive and noninvasive diagnostic and therapeutic procedures forgone by prevention of hypertension. Is it possible that such an underlying truth is the real reason why American medicine is habitually unsuccessful at controlling blood pressure?
Right now, if my blood pressure were not well controlled (it is), I would find my way to Cincinnati, to give PriMed physicians, MediSync, and MedsEngine a crack at prolonging my useful life.
Dr. Lundberg is editor in chief of Cancer Commons. He disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
A mid-marathon cardiac arrest, an MD’s crisis of confidence
I was running my 25th New York City Marathon. It was 2018, and I almost pulled out of running that year. I wasn’t myself, and maybe that’s an understatement.
A month earlier, I had been involved in a malpractice case. I was found liable for $10 million. My colleagues didn’t think I had done anything wrong, but the jury did. And the local newspapers made me look like a villain.
I was devastated. But my priest, my friends, and my family all told me, “You can’t quit.” So, I decided to run for them.
I started on the Verrazzano-Narrows Bridge that morning with some friends from work. I usually listen to music as I’m running, but I didn’t that year. I was just in my zone, enjoying the crowds. They’re huge. Millions of people on the streets.
I was running well. I did half the race in an hour and 57 minutes. My family always meets me at mile 17, and I was almost there. I had reached 59th Street and was about to make the turn onto First Avenue.
That’s one of the noisiest places in the marathon. There’s a kind of tunnel, and with the crowd and the throng of runners, it’s incredibly loud. But somehow, I heard somebody yell, “Help!”
Now, how I heard that, I don’t know. And if I’d been listening to music like I always do, no way I would’ve heard it. I could swear it was an angel on my shoulder that said, “Turn around, dummy. You’ve got a person that needs your help to your left.”
I turned around and about 30 feet behind me, I saw a woman waving her hands and a runner on the ground. I thought, Somebody fainted. I pushed through the crowd to get to them. The woman was crying, saying, “My friend went down to tie her shoe and she fell back. I think she’s seizing or something.”
I got down and tried to wake the other woman up. I lifted her legs up. But I quickly realized there was more to the story.
Some volunteers and police started coming toward us. The police officers looked at me like, What’s this guy doing? I explained that I was a physician, and one of them began helping me with the CPR. As we did that, someone brought a defibrillator.
Meanwhile, runners were going past, almost over us. The police officers were trying to create a barrier.
The machine gave the woman a shock, but we didn’t get a response, so we resumed CPR. At that point, my legs began to cramp so badly I couldn’t go on. So the police officer took over, and I yelled, “I need an ambu bag!” Somebody brought one, and I started giving her oxygen.
At that point, a paramedic team arrived with a bigger defibrillator. We shocked her again. And again. That time we got results, but she quickly went out again. The fourth time, we got her heart back and she started breathing on her own.
We finally got her into an ambulance. I wanted to go with them, but the woman’s friend needed to get in, so there wasn’t enough room.
And then they were gone, and I was just standing there.
A police officer put his arm around me. He said, “Doc, you’re amazing. What do you need? Where can I take you?”
I said, “Take me? My wife is waiting for me at mile 17.”
I took off and ran. When I got to my wife and kids, they were so worried. We all wear tracking devices, and they could see that I had stopped for more than 20 minutes.
I fell into my wife’s arms and told her what had happened. I was crying. “I don’t know what to do. I need to get to the hospital.”
And she said, “No, you need to go finish the race.”
So, I did. It was painful because of the cramps, but I was numb at that point. I was thinking about the woman the whole way. My time was 5 hours and 20 minutes.
As soon as I finished, I went to every police officer I could find, but nobody knew anything. Suddenly, I remembered my cousin. He had previously been the head of EMS for New York City. I called him. “Abdo, it’s Ted, you’ve got to do me a favor.”
“What?” he said. “Are you delirious from running the marathon?”
I told him what I needed. He called me back 5 minutes later and said, “Ted, what’d you do? Everybody wants to know who you are and where you are! The woman just went out again at New York Cornell. But they got her back, and they’re bringing her up to the cath lab.”
After every marathon that I run, we host a big party at our house. My family and friends and neighbors all celebrate while I’m dying on the couch. That night, my daughter told everyone the story of what happened.
But I was still not right. Still thinking about the malpractice suit.
Yes, I just did something great. But I’d recently been called the worst physician in the world. The distraction of the marathon was gone, and I was back to thinking, What am I going to do with my life? Who’s ever going to want to see me again? I’m a pariah.
Everybody said, “Ted, what happened a month ago isn’t you. What happened today was you.”
I told them to leave it alone, but my daughter and my neighbor started calling people anyway. The next day I got a call from the local newspaper. It was the same journalist who had written about me from the trial. I told him I didn’t want to talk. I was actually pretty nasty.
But my wife said, “Ted, what are you doing? That guy was trying to help you.” So, I called back and apologized.
“Dr. Strange, we knew that story wasn’t right,” he said. “We have to write this story.”
After the article came out, I started getting more calls from the media. Channel 7 News and CBS News did segments. The New York Knicks invited us to a game and presented me with a watch. It was incredible. But I was also really embarrassed by it.
People started calling me a hero. I’m not a hero. I just did what I’m supposed to do, what I’m trained to do. Shame on me if I don’t do that. Good guy and hopefully good physician, sure, but not a hero.
I also give credit to the City of New York Police Department, the FDNY, and the volunteers. Without them, I couldn’t have done what I did. It was a true team effort.
A few weeks later, the woman went home to Minnesota. She’ll never run a marathon again, but she’s still alive to this day. It turned out she had a single lesion called the “widow-maker” lesion. She was in perfect health and had just completed an ultramarathon a few months before; but she had a genetic predisposition. She still calls me every December to thank me for another Christmas.
There’s more.
One year after this whole thing, almost to the date, I got a call from my attorney. “The court just threw out the malpractice verdict,” he said. “You didn’t do anything wrong.”
I’m a man of faith. And I believe all this happened for a reason. Maybe God was sending me a message, and that’s why I heard a call for help on 59th Street in my 25th marathon among millions of people in a crowd.
I ran the marathon the next year. And when I got to that spot, I stopped and reflected. Nobody knew why I was standing there, but I knew. To this day, I could take you to that spot.
I turn 65 next July, and I plan to keep on running the race.
Dr. Strange is chair of medicine at Staten Island University Hospital, associate ambulatory physician executive of the Staten Island Region, and an internal medicine and geriatric medicine physician with Northwell Health.
A version of this article first appeared on Medscape.com.
I was running my 25th New York City Marathon. It was 2018, and I almost pulled out of running that year. I wasn’t myself, and maybe that’s an understatement.
A month earlier, I had been involved in a malpractice case. I was found liable for $10 million. My colleagues didn’t think I had done anything wrong, but the jury did. And the local newspapers made me look like a villain.
I was devastated. But my priest, my friends, and my family all told me, “You can’t quit.” So, I decided to run for them.
I started on the Verrazzano-Narrows Bridge that morning with some friends from work. I usually listen to music as I’m running, but I didn’t that year. I was just in my zone, enjoying the crowds. They’re huge. Millions of people on the streets.
I was running well. I did half the race in an hour and 57 minutes. My family always meets me at mile 17, and I was almost there. I had reached 59th Street and was about to make the turn onto First Avenue.
That’s one of the noisiest places in the marathon. There’s a kind of tunnel, and with the crowd and the throng of runners, it’s incredibly loud. But somehow, I heard somebody yell, “Help!”
Now, how I heard that, I don’t know. And if I’d been listening to music like I always do, no way I would’ve heard it. I could swear it was an angel on my shoulder that said, “Turn around, dummy. You’ve got a person that needs your help to your left.”
I turned around and about 30 feet behind me, I saw a woman waving her hands and a runner on the ground. I thought, Somebody fainted. I pushed through the crowd to get to them. The woman was crying, saying, “My friend went down to tie her shoe and she fell back. I think she’s seizing or something.”
I got down and tried to wake the other woman up. I lifted her legs up. But I quickly realized there was more to the story.
Some volunteers and police started coming toward us. The police officers looked at me like, What’s this guy doing? I explained that I was a physician, and one of them began helping me with the CPR. As we did that, someone brought a defibrillator.
Meanwhile, runners were going past, almost over us. The police officers were trying to create a barrier.
The machine gave the woman a shock, but we didn’t get a response, so we resumed CPR. At that point, my legs began to cramp so badly I couldn’t go on. So the police officer took over, and I yelled, “I need an ambu bag!” Somebody brought one, and I started giving her oxygen.
At that point, a paramedic team arrived with a bigger defibrillator. We shocked her again. And again. That time we got results, but she quickly went out again. The fourth time, we got her heart back and she started breathing on her own.
We finally got her into an ambulance. I wanted to go with them, but the woman’s friend needed to get in, so there wasn’t enough room.
And then they were gone, and I was just standing there.
A police officer put his arm around me. He said, “Doc, you’re amazing. What do you need? Where can I take you?”
I said, “Take me? My wife is waiting for me at mile 17.”
I took off and ran. When I got to my wife and kids, they were so worried. We all wear tracking devices, and they could see that I had stopped for more than 20 minutes.
I fell into my wife’s arms and told her what had happened. I was crying. “I don’t know what to do. I need to get to the hospital.”
And she said, “No, you need to go finish the race.”
So, I did. It was painful because of the cramps, but I was numb at that point. I was thinking about the woman the whole way. My time was 5 hours and 20 minutes.
As soon as I finished, I went to every police officer I could find, but nobody knew anything. Suddenly, I remembered my cousin. He had previously been the head of EMS for New York City. I called him. “Abdo, it’s Ted, you’ve got to do me a favor.”
“What?” he said. “Are you delirious from running the marathon?”
I told him what I needed. He called me back 5 minutes later and said, “Ted, what’d you do? Everybody wants to know who you are and where you are! The woman just went out again at New York Cornell. But they got her back, and they’re bringing her up to the cath lab.”
After every marathon that I run, we host a big party at our house. My family and friends and neighbors all celebrate while I’m dying on the couch. That night, my daughter told everyone the story of what happened.
But I was still not right. Still thinking about the malpractice suit.
Yes, I just did something great. But I’d recently been called the worst physician in the world. The distraction of the marathon was gone, and I was back to thinking, What am I going to do with my life? Who’s ever going to want to see me again? I’m a pariah.
Everybody said, “Ted, what happened a month ago isn’t you. What happened today was you.”
I told them to leave it alone, but my daughter and my neighbor started calling people anyway. The next day I got a call from the local newspaper. It was the same journalist who had written about me from the trial. I told him I didn’t want to talk. I was actually pretty nasty.
But my wife said, “Ted, what are you doing? That guy was trying to help you.” So, I called back and apologized.
“Dr. Strange, we knew that story wasn’t right,” he said. “We have to write this story.”
After the article came out, I started getting more calls from the media. Channel 7 News and CBS News did segments. The New York Knicks invited us to a game and presented me with a watch. It was incredible. But I was also really embarrassed by it.
People started calling me a hero. I’m not a hero. I just did what I’m supposed to do, what I’m trained to do. Shame on me if I don’t do that. Good guy and hopefully good physician, sure, but not a hero.
I also give credit to the City of New York Police Department, the FDNY, and the volunteers. Without them, I couldn’t have done what I did. It was a true team effort.
A few weeks later, the woman went home to Minnesota. She’ll never run a marathon again, but she’s still alive to this day. It turned out she had a single lesion called the “widow-maker” lesion. She was in perfect health and had just completed an ultramarathon a few months before; but she had a genetic predisposition. She still calls me every December to thank me for another Christmas.
There’s more.
One year after this whole thing, almost to the date, I got a call from my attorney. “The court just threw out the malpractice verdict,” he said. “You didn’t do anything wrong.”
I’m a man of faith. And I believe all this happened for a reason. Maybe God was sending me a message, and that’s why I heard a call for help on 59th Street in my 25th marathon among millions of people in a crowd.
I ran the marathon the next year. And when I got to that spot, I stopped and reflected. Nobody knew why I was standing there, but I knew. To this day, I could take you to that spot.
I turn 65 next July, and I plan to keep on running the race.
Dr. Strange is chair of medicine at Staten Island University Hospital, associate ambulatory physician executive of the Staten Island Region, and an internal medicine and geriatric medicine physician with Northwell Health.
A version of this article first appeared on Medscape.com.
I was running my 25th New York City Marathon. It was 2018, and I almost pulled out of running that year. I wasn’t myself, and maybe that’s an understatement.
A month earlier, I had been involved in a malpractice case. I was found liable for $10 million. My colleagues didn’t think I had done anything wrong, but the jury did. And the local newspapers made me look like a villain.
I was devastated. But my priest, my friends, and my family all told me, “You can’t quit.” So, I decided to run for them.
I started on the Verrazzano-Narrows Bridge that morning with some friends from work. I usually listen to music as I’m running, but I didn’t that year. I was just in my zone, enjoying the crowds. They’re huge. Millions of people on the streets.
I was running well. I did half the race in an hour and 57 minutes. My family always meets me at mile 17, and I was almost there. I had reached 59th Street and was about to make the turn onto First Avenue.
That’s one of the noisiest places in the marathon. There’s a kind of tunnel, and with the crowd and the throng of runners, it’s incredibly loud. But somehow, I heard somebody yell, “Help!”
Now, how I heard that, I don’t know. And if I’d been listening to music like I always do, no way I would’ve heard it. I could swear it was an angel on my shoulder that said, “Turn around, dummy. You’ve got a person that needs your help to your left.”
I turned around and about 30 feet behind me, I saw a woman waving her hands and a runner on the ground. I thought, Somebody fainted. I pushed through the crowd to get to them. The woman was crying, saying, “My friend went down to tie her shoe and she fell back. I think she’s seizing or something.”
I got down and tried to wake the other woman up. I lifted her legs up. But I quickly realized there was more to the story.
Some volunteers and police started coming toward us. The police officers looked at me like, What’s this guy doing? I explained that I was a physician, and one of them began helping me with the CPR. As we did that, someone brought a defibrillator.
Meanwhile, runners were going past, almost over us. The police officers were trying to create a barrier.
The machine gave the woman a shock, but we didn’t get a response, so we resumed CPR. At that point, my legs began to cramp so badly I couldn’t go on. So the police officer took over, and I yelled, “I need an ambu bag!” Somebody brought one, and I started giving her oxygen.
At that point, a paramedic team arrived with a bigger defibrillator. We shocked her again. And again. That time we got results, but she quickly went out again. The fourth time, we got her heart back and she started breathing on her own.
We finally got her into an ambulance. I wanted to go with them, but the woman’s friend needed to get in, so there wasn’t enough room.
And then they were gone, and I was just standing there.
A police officer put his arm around me. He said, “Doc, you’re amazing. What do you need? Where can I take you?”
I said, “Take me? My wife is waiting for me at mile 17.”
I took off and ran. When I got to my wife and kids, they were so worried. We all wear tracking devices, and they could see that I had stopped for more than 20 minutes.
I fell into my wife’s arms and told her what had happened. I was crying. “I don’t know what to do. I need to get to the hospital.”
And she said, “No, you need to go finish the race.”
So, I did. It was painful because of the cramps, but I was numb at that point. I was thinking about the woman the whole way. My time was 5 hours and 20 minutes.
As soon as I finished, I went to every police officer I could find, but nobody knew anything. Suddenly, I remembered my cousin. He had previously been the head of EMS for New York City. I called him. “Abdo, it’s Ted, you’ve got to do me a favor.”
“What?” he said. “Are you delirious from running the marathon?”
I told him what I needed. He called me back 5 minutes later and said, “Ted, what’d you do? Everybody wants to know who you are and where you are! The woman just went out again at New York Cornell. But they got her back, and they’re bringing her up to the cath lab.”
After every marathon that I run, we host a big party at our house. My family and friends and neighbors all celebrate while I’m dying on the couch. That night, my daughter told everyone the story of what happened.
But I was still not right. Still thinking about the malpractice suit.
Yes, I just did something great. But I’d recently been called the worst physician in the world. The distraction of the marathon was gone, and I was back to thinking, What am I going to do with my life? Who’s ever going to want to see me again? I’m a pariah.
Everybody said, “Ted, what happened a month ago isn’t you. What happened today was you.”
I told them to leave it alone, but my daughter and my neighbor started calling people anyway. The next day I got a call from the local newspaper. It was the same journalist who had written about me from the trial. I told him I didn’t want to talk. I was actually pretty nasty.
But my wife said, “Ted, what are you doing? That guy was trying to help you.” So, I called back and apologized.
“Dr. Strange, we knew that story wasn’t right,” he said. “We have to write this story.”
After the article came out, I started getting more calls from the media. Channel 7 News and CBS News did segments. The New York Knicks invited us to a game and presented me with a watch. It was incredible. But I was also really embarrassed by it.
People started calling me a hero. I’m not a hero. I just did what I’m supposed to do, what I’m trained to do. Shame on me if I don’t do that. Good guy and hopefully good physician, sure, but not a hero.
I also give credit to the City of New York Police Department, the FDNY, and the volunteers. Without them, I couldn’t have done what I did. It was a true team effort.
A few weeks later, the woman went home to Minnesota. She’ll never run a marathon again, but she’s still alive to this day. It turned out she had a single lesion called the “widow-maker” lesion. She was in perfect health and had just completed an ultramarathon a few months before; but she had a genetic predisposition. She still calls me every December to thank me for another Christmas.
There’s more.
One year after this whole thing, almost to the date, I got a call from my attorney. “The court just threw out the malpractice verdict,” he said. “You didn’t do anything wrong.”
I’m a man of faith. And I believe all this happened for a reason. Maybe God was sending me a message, and that’s why I heard a call for help on 59th Street in my 25th marathon among millions of people in a crowd.
I ran the marathon the next year. And when I got to that spot, I stopped and reflected. Nobody knew why I was standing there, but I knew. To this day, I could take you to that spot.
I turn 65 next July, and I plan to keep on running the race.
Dr. Strange is chair of medicine at Staten Island University Hospital, associate ambulatory physician executive of the Staten Island Region, and an internal medicine and geriatric medicine physician with Northwell Health.
A version of this article first appeared on Medscape.com.
Diagnosing patients with sarcoidosis
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.
A 40-year-old women is evaluated for liver abnormalities. She had elevated transaminases and alkaline phosphatase. A liver ultrasound showed multiple lesions. She underwent liver biopsy, which showed granulomas. What test results, if abnormal, would be most suggestive of sarcoidosis?
A. Erythrocyte sedimentation rate
B. C-reactive protein
C. Lymphocyte count
D. Antinuclear antibodies
The correct answer here is lymphocyte count. Sarcoidosis is in just about every differential diagnosis, as it can involve every organ system. I will share with you a few pearls I have learned over 30 years of taking care of patients with sarcoidosis. Lymphocyte counts drop with active sarcoidosis. Sarcoidosis should always be part of the differential when you see lymphopenia. El Jammal et al. studied 90 patients referred for possible granulomatous hepatitis.1 Seventy-three patients had a final diagnosis of granulomatous hepatitis, and 38 of those patients had sarcoidosis. Lymphopenia had a high specificity (85.7%) for the diagnosis of sarcoidosis, with a specificity of 100% in the patients under 50 years old.
Morell and colleagues looked at whether low lymphocyte counts and low lymphocyte percentage were markers of active sarcoidosis.2 Forty patients with biopsy-proven sarcoidosis were prospectively evaluated every 6 months. A low lymphocyte count and a low lymphocyte percentage (< 20%) were detected more frequently in patients with active sarcoidosis than in the patients with asymptomatic sarcoidosis (P < .02 and P < .0001).
Jones et al. looked at lymphopenia as a marker of sarcoidosis in patients presenting with uveitis.3 The study was a retrospective case-control study (112 patients with sarcoidosis-associated uveitis and 398 controls with other forms of uveitis). The mean lymphocyte count for patients with sarcoidosis was 1.43 vs. 2.04 for other causes of uveitis (P ≤ .0001).
Patients with sarcoidosis are at risk of hypercalciuria, hypercalcemia, and kidney stones. These are common in patients with sarcoidosis, with up to 50% of such patients having hypercalciuria. This is because in sarcoidosis patients 25(OH) vitamin D is converted in granulomas by activated macrophages to 1,25(OH)2 vitamin D, which is the active form of vitamin D.
Several studies have looked at the diagnostic utility of 1,25(OH)2 vitamin D levels in patients with suspected sarcoidosis. Rohmer and colleagues looked at whether 1,25(OH)2 vitamin D levels could help with the diagnosis of sarcoidosis as the cause of uveitis.4 They found that the level of 25(OH) vitamin D in sarcoidosis patients with uveitis was lower than in patients with uveitis without sarcoidosis, 34 vs. 43 nmol/mL (P < .02), whereas the 1,25(OH)2 vitamin D level was higher in patients with sarcoidosis than in those with uveitis without sarcoidosis, 132 vs. 108 pmol/L (P = .02). They looked at the 1,25(OH)2D/25(OH)D ratio; a ratio > 3.5 was strongly associated with an abnormal chest CT-scan (OR = 5.7, P = .003) and granulomas on bronchial biopsy (OR = 14.7, P = .007).
Kavathia et al. looked at whether elevated 1,25(OH)2 vitamin D levels predicted chronicity of sarcoidosis.5 A total of 59 sarcoidosis patients were recruited for the study. Higher serum 1,25(OH)2 vitamin D levels were associated with patients requiring repeated systemic immunosuppressive therapy or > 1 year of therapy. Increasing quartiles of serum 1,25(OH)2 vitamin D level were associated with increased odds of patients having chronic sarcoidosis (OR = 1.82; 95% CI, 1.11-2.99, P = .019).
Because of the higher activated vitamin D levels in sarcoidosis patients, they are at risk for problems with vitamin D supplementation. I have seen two patients develop large numbers of kidney stones after receiving high-dose vitamin D. Sodhi and Aldrich reported on a cohort of 196 sarcoidosis patients who had received vitamin D and compared them with 196 control patients with sarcoidosis who were not receiving vitamin D.6 Hypercalcemia was more frequent in the group that received vitamin D (42.3%) than in the group that did not (18.3%, P < .0001). In this study, only a minority (23%) of patients receiving vitamin D had their 1,25(OH)2 vitamin D level checked.
Pearl: Lymphocyte count and 1,25(OH)2 vitamin D levels can be helpful tests in assessing sarcoidosis activity. Patients with sarcoidosis who receive vitamin D should have their 1.25(OH)2 vitamin D levels monitored.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. El Jammal et al. Sarcoidosis Vasc Diffuse Lung Dis. 2023 Sep 13;40(3):e2023031.
2. Morell F et al. Chest. 2002 Apr;121(4):1239-44.
3. Jones NP et al. Br J Ophthalmol. 2016 Oct;100(10):1393-6.
4. Rohmer J et al. Ocul Immunol Inflamm. 2020 Apr 2;28(3):341-7.
5. Kavathia D et al. Respir Med. 2010 Apr;104(4):564–70.
6. Sodhi A and Aldrich T. Am J Med Sci. 2016 Sep;352(3):252-7.
Life in the woods
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“I went to the woods because I wished to live deliberately, to front only the essential facts of life, and see if I could not learn what it had to teach.” – Henry David Thoreau
I have many patients like Maxine. Tall, with a shock of white hair. Old, but still in charge. When you try to make eye contact, she looks right through you. First with her left eye. Then her right. Her face is inscrutable. What’s she thinking? Unlike many of my patients, however, this Maxine was a llama. Every morning my daughter and I tried to coax her into moving as we leaned on the cold steel gate that kept her in her pasture. We were visiting family in October and chose to stay on a working New England farm. The kids will love the animals, we thought, and we’ll appreciate the extra bedrooms.
Airbnb helped us find this charming fiber-farm in Rhode Island where they raise Leicester Longwool sheep, a historic breed that once roamed George Washington’s pastures, along with a few goats, ducks, chickens, and Maxine. It’s situated deep in the woods, which were yellow, orange, and red that week. As it happens, we were just a short drive due south of Walden Pond where Henry David Thoreau spent 2 years, 2 months and 2 days escaping “overcivilization” nearly 175 years ago. Hoisting our overweight bags over the uneven granite stone steps when we arrived, I realized this was going to be more like the Thoreau experiment than I intended. The farmhouse dated to the 1790s. There were wide, creaky floorboards, low ceilings, one staircase to the bedrooms (which could have aptly been called a ladder) and loads of book-laden shelves. Instructions posted in the kitchen warned that the heat is tricky to regulate – a redundant admonition as we watched our 3-year-old putting on her socks and shoes as she got into bed.
Now, if you’ve ever been on vacation with little kids, you know that it’s basically just childcare in a novel location. After barricading the staircase with luggage and unplugging lamps from their dicey outlets we set out to feed the chickens and try to pet a sheep. Walking the perimeter of the farm we saw stone walls that needed mending and stumbled across two ancient cemeteries, one had been for family, the other for slaves. I wondered how many farmers and weavers and menders had walked this trail with their kids over the generations.
The next morning, we learned that roosters do not in fact crow at dawn, they crow before dawn (which could also aptly be called nighttime). There were no commutes or late patients here. But there was work to be done. Chickens don’t care that it’s Sunday. It downpoured. Watching the sheep from the kitchen as I sipped my coffee, they didn’t seem to mind. Nor did our farmer hosts who trudged past them in tall boots, just as they had every other day of their farmer lives.
By the fifth day, we had fallen into the rhythms of the homestead. We cracked the blue, green, and brown eggs that our hosts placed outside our door in the early hours and made omelets that were as orange as the foliage. We finally learned to adjust the heat so we neither got chilblains nor had to open the windows and strip naked to cool down. The sky was a brilliant blue that last morning and Sloan ran around trying to catch leaves as they blew off the trees. She had no objective. No counting. No contest. Just chasing leaves as they fell. It was the ultimate atelic activity, done just for doing it. I joined her and found I was no better at this than a 3-year-old.
We might all benefit from a little time in the woods.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
How to develop a patient referral program
Here is how old I am: When I graduated from medical school in 1977, marketing was prohibited. It was the legal profession that challenged the ban on advertising by professionals, leading to a landmark Supreme Court decision (Bates v State Bar of Arizona, 1977), which opened the door to marketing in the legal and medical professions.
Since then, Strategies range from the basic Internet website through postings on the major social media sites, and occasionally to larger-budget campaigns involving local radio, television, or billboard advertising.
All these methods are effective, to varying degrees; but nothing provides as much benefit – relative to its comparatively low cost – as the original marketing tool, word-of-mouth patient referrals. According to one survey, a clear majority of Americans still consider word-of-mouth recommendations to be the most influential element driving purchase decisions. Of course, some of your new patients already come from such referrals; but you can get a lot more by actively encouraging your existing patients to sing your praises, rather than waiting for them to do it on their own.
Soliciting current patients for referrals does take a little planning, structure, and a basic understanding of exactly how patient referral programs work. When executed correctly, a patient referral program can add substantial growth to your practice at minimal cost.
Your first step, as with any new project, should be to identify your goals: Clearly define what kind of patients you are looking to attract. Do you want more patients for cosmetic procedures, medical treatment, skin cancer screenings, a specific diagnosis (such as psoriasis), or a general mix? Design your announcements, brochures, and other literature (more on that in a minute) with those goals in mind.
Next, identify any applicable federal or state laws that dictate what you can and cannot legally do to encourage such referrals. It might be tempting, for example, to offer discounts on future services for successful referrals; but some medical groups frown on it, some states prohibit it, and the Federal Anti-Kickback Statute makes it illegal to pay anyone to refer Medicare or Medicaid patients to you if you file a claim for your services. In my experience, most patients are happy to recommend someone whom they believe provides excellent care to a friend or relative without any sort of monetary incentive; but if you plan to offer a material reward of any kind, run it by your attorney first.
Once your legal ducks are in order, make patients aware that you are accepting new patients and would welcome referrals by posting notices to that effect around your office and on your website and social media pages. Outline exactly what sort of patients (based on your goals, above) you are looking for, how to refer someone, whom to contact, and what kind of information is needed. Make it clear why existing patients should refer someone to your practice. Remind them of your specialized training, advanced technology, and patient-focused approach to health care. Highlight the benefits of the program and encourage your patients to participate.
Before implementation, you will need to educate your employees about the referral program and its benefits. All staff members should understand the program and be able to answer basic questions about it from patients or referring professionals. Encourage staffers to actively promote the program during patient interactions.
Then, start making some decisions. How, specifically, will you be requesting referrals in the office? Many physicians are not comfortable asking patients themselves. If you are going to let your assistants or receptionists do it, you will need to write a script for them to follow. An example of a basic script might be, “If you are happy with the care you are receiving here, we would love for you to tell your friends and family about us.” Your staff can then hand out cards, brochures, or both to reinforce the message, and perhaps send a follow-up email to remind them.
A referral system isn’t worth the effort if you don’t know whether it is working. Establish a system to track and monitor referrals. This could be as simple as a spreadsheet or purchasing a more sophisticated software program. Ensure that you can accurately identify and credit the referring patients for their referrals.
Make sure to thank referring patients with a thank-you note or email. Expressing gratitude will encourage continued participation in the program.
A successful referral program does not happen overnight. It relies on providing exceptional patient care and building strong relationships with your existing patients. By implementing such a program, you can leverage the satisfaction and loyalty of your patients to attract new patients and grow your private practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Here is how old I am: When I graduated from medical school in 1977, marketing was prohibited. It was the legal profession that challenged the ban on advertising by professionals, leading to a landmark Supreme Court decision (Bates v State Bar of Arizona, 1977), which opened the door to marketing in the legal and medical professions.
Since then, Strategies range from the basic Internet website through postings on the major social media sites, and occasionally to larger-budget campaigns involving local radio, television, or billboard advertising.
All these methods are effective, to varying degrees; but nothing provides as much benefit – relative to its comparatively low cost – as the original marketing tool, word-of-mouth patient referrals. According to one survey, a clear majority of Americans still consider word-of-mouth recommendations to be the most influential element driving purchase decisions. Of course, some of your new patients already come from such referrals; but you can get a lot more by actively encouraging your existing patients to sing your praises, rather than waiting for them to do it on their own.
Soliciting current patients for referrals does take a little planning, structure, and a basic understanding of exactly how patient referral programs work. When executed correctly, a patient referral program can add substantial growth to your practice at minimal cost.
Your first step, as with any new project, should be to identify your goals: Clearly define what kind of patients you are looking to attract. Do you want more patients for cosmetic procedures, medical treatment, skin cancer screenings, a specific diagnosis (such as psoriasis), or a general mix? Design your announcements, brochures, and other literature (more on that in a minute) with those goals in mind.
Next, identify any applicable federal or state laws that dictate what you can and cannot legally do to encourage such referrals. It might be tempting, for example, to offer discounts on future services for successful referrals; but some medical groups frown on it, some states prohibit it, and the Federal Anti-Kickback Statute makes it illegal to pay anyone to refer Medicare or Medicaid patients to you if you file a claim for your services. In my experience, most patients are happy to recommend someone whom they believe provides excellent care to a friend or relative without any sort of monetary incentive; but if you plan to offer a material reward of any kind, run it by your attorney first.
Once your legal ducks are in order, make patients aware that you are accepting new patients and would welcome referrals by posting notices to that effect around your office and on your website and social media pages. Outline exactly what sort of patients (based on your goals, above) you are looking for, how to refer someone, whom to contact, and what kind of information is needed. Make it clear why existing patients should refer someone to your practice. Remind them of your specialized training, advanced technology, and patient-focused approach to health care. Highlight the benefits of the program and encourage your patients to participate.
Before implementation, you will need to educate your employees about the referral program and its benefits. All staff members should understand the program and be able to answer basic questions about it from patients or referring professionals. Encourage staffers to actively promote the program during patient interactions.
Then, start making some decisions. How, specifically, will you be requesting referrals in the office? Many physicians are not comfortable asking patients themselves. If you are going to let your assistants or receptionists do it, you will need to write a script for them to follow. An example of a basic script might be, “If you are happy with the care you are receiving here, we would love for you to tell your friends and family about us.” Your staff can then hand out cards, brochures, or both to reinforce the message, and perhaps send a follow-up email to remind them.
A referral system isn’t worth the effort if you don’t know whether it is working. Establish a system to track and monitor referrals. This could be as simple as a spreadsheet or purchasing a more sophisticated software program. Ensure that you can accurately identify and credit the referring patients for their referrals.
Make sure to thank referring patients with a thank-you note or email. Expressing gratitude will encourage continued participation in the program.
A successful referral program does not happen overnight. It relies on providing exceptional patient care and building strong relationships with your existing patients. By implementing such a program, you can leverage the satisfaction and loyalty of your patients to attract new patients and grow your private practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Here is how old I am: When I graduated from medical school in 1977, marketing was prohibited. It was the legal profession that challenged the ban on advertising by professionals, leading to a landmark Supreme Court decision (Bates v State Bar of Arizona, 1977), which opened the door to marketing in the legal and medical professions.
Since then, Strategies range from the basic Internet website through postings on the major social media sites, and occasionally to larger-budget campaigns involving local radio, television, or billboard advertising.
All these methods are effective, to varying degrees; but nothing provides as much benefit – relative to its comparatively low cost – as the original marketing tool, word-of-mouth patient referrals. According to one survey, a clear majority of Americans still consider word-of-mouth recommendations to be the most influential element driving purchase decisions. Of course, some of your new patients already come from such referrals; but you can get a lot more by actively encouraging your existing patients to sing your praises, rather than waiting for them to do it on their own.
Soliciting current patients for referrals does take a little planning, structure, and a basic understanding of exactly how patient referral programs work. When executed correctly, a patient referral program can add substantial growth to your practice at minimal cost.
Your first step, as with any new project, should be to identify your goals: Clearly define what kind of patients you are looking to attract. Do you want more patients for cosmetic procedures, medical treatment, skin cancer screenings, a specific diagnosis (such as psoriasis), or a general mix? Design your announcements, brochures, and other literature (more on that in a minute) with those goals in mind.
Next, identify any applicable federal or state laws that dictate what you can and cannot legally do to encourage such referrals. It might be tempting, for example, to offer discounts on future services for successful referrals; but some medical groups frown on it, some states prohibit it, and the Federal Anti-Kickback Statute makes it illegal to pay anyone to refer Medicare or Medicaid patients to you if you file a claim for your services. In my experience, most patients are happy to recommend someone whom they believe provides excellent care to a friend or relative without any sort of monetary incentive; but if you plan to offer a material reward of any kind, run it by your attorney first.
Once your legal ducks are in order, make patients aware that you are accepting new patients and would welcome referrals by posting notices to that effect around your office and on your website and social media pages. Outline exactly what sort of patients (based on your goals, above) you are looking for, how to refer someone, whom to contact, and what kind of information is needed. Make it clear why existing patients should refer someone to your practice. Remind them of your specialized training, advanced technology, and patient-focused approach to health care. Highlight the benefits of the program and encourage your patients to participate.
Before implementation, you will need to educate your employees about the referral program and its benefits. All staff members should understand the program and be able to answer basic questions about it from patients or referring professionals. Encourage staffers to actively promote the program during patient interactions.
Then, start making some decisions. How, specifically, will you be requesting referrals in the office? Many physicians are not comfortable asking patients themselves. If you are going to let your assistants or receptionists do it, you will need to write a script for them to follow. An example of a basic script might be, “If you are happy with the care you are receiving here, we would love for you to tell your friends and family about us.” Your staff can then hand out cards, brochures, or both to reinforce the message, and perhaps send a follow-up email to remind them.
A referral system isn’t worth the effort if you don’t know whether it is working. Establish a system to track and monitor referrals. This could be as simple as a spreadsheet or purchasing a more sophisticated software program. Ensure that you can accurately identify and credit the referring patients for their referrals.
Make sure to thank referring patients with a thank-you note or email. Expressing gratitude will encourage continued participation in the program.
A successful referral program does not happen overnight. It relies on providing exceptional patient care and building strong relationships with your existing patients. By implementing such a program, you can leverage the satisfaction and loyalty of your patients to attract new patients and grow your private practice.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The future of medicine is RNA
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.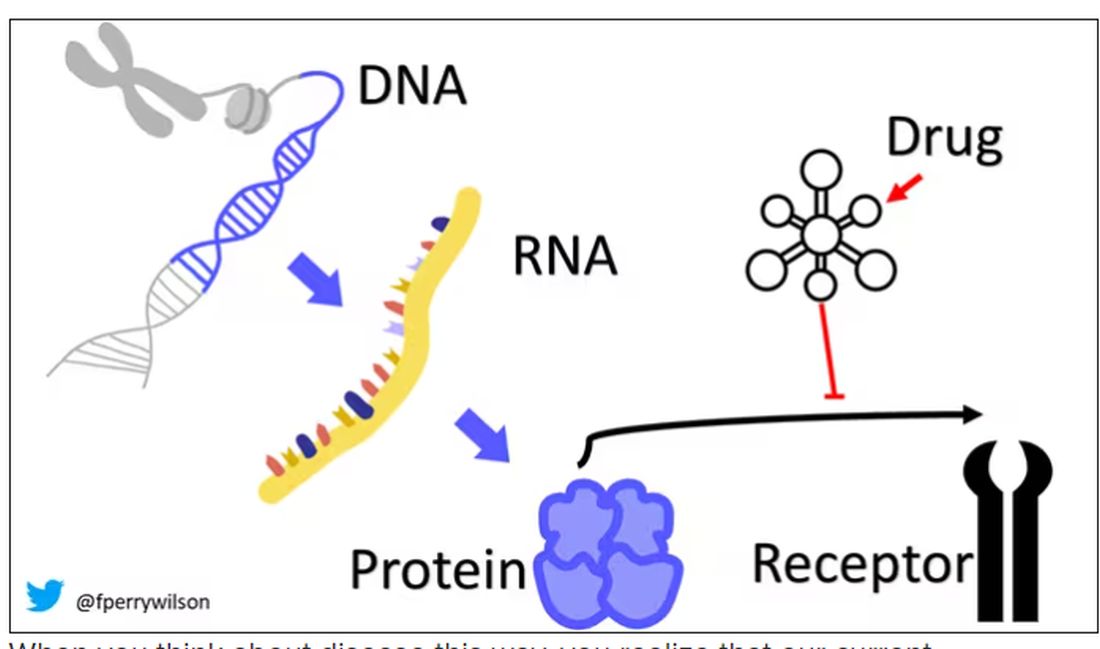
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.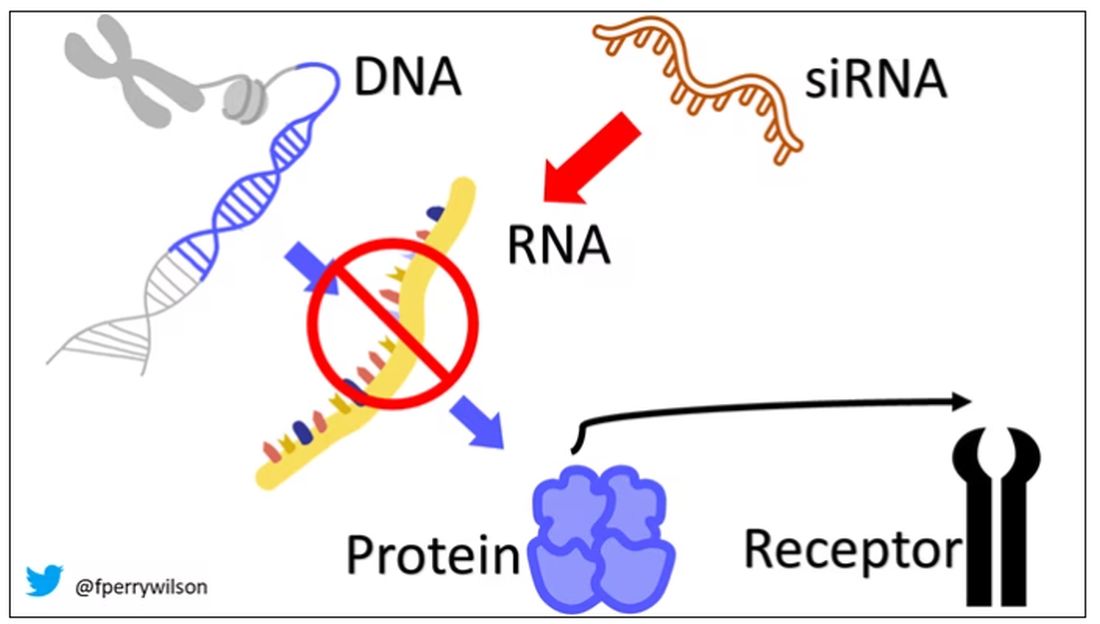
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.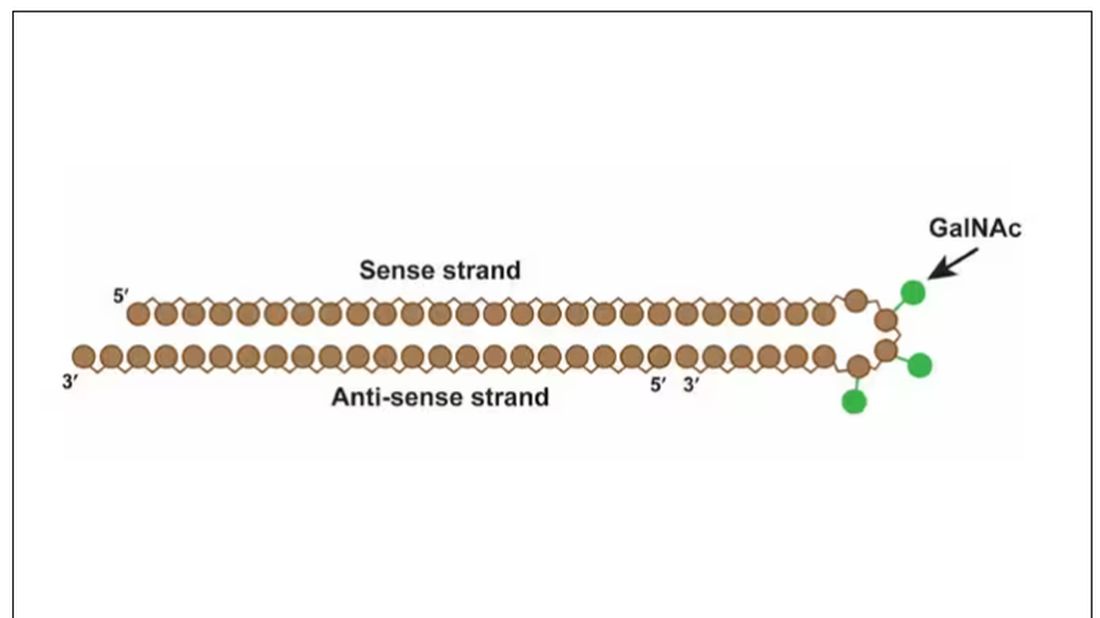
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.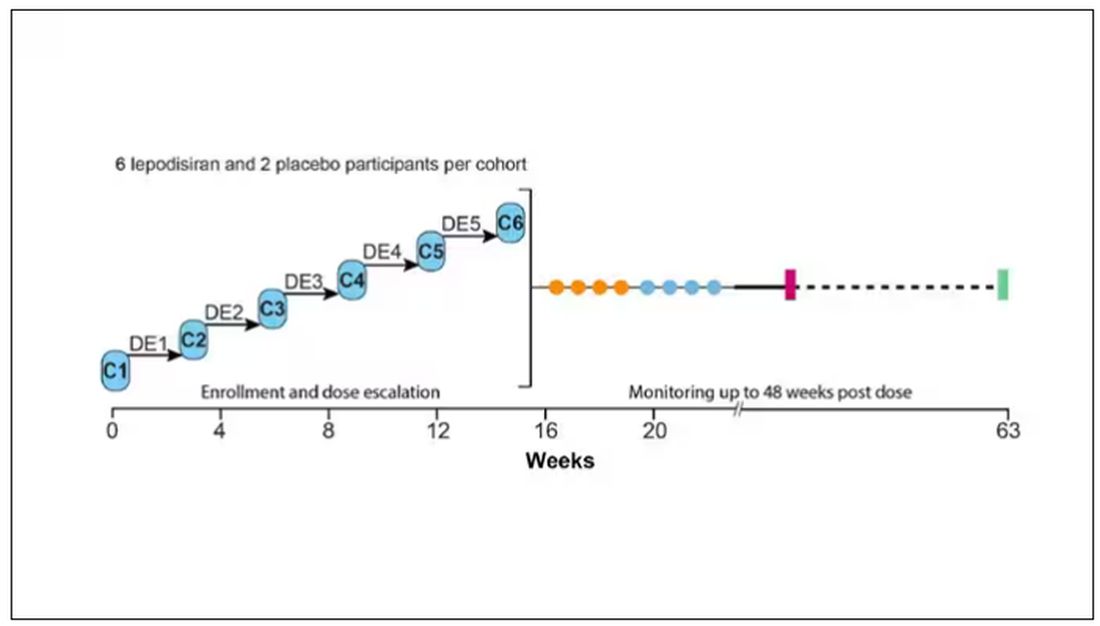
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.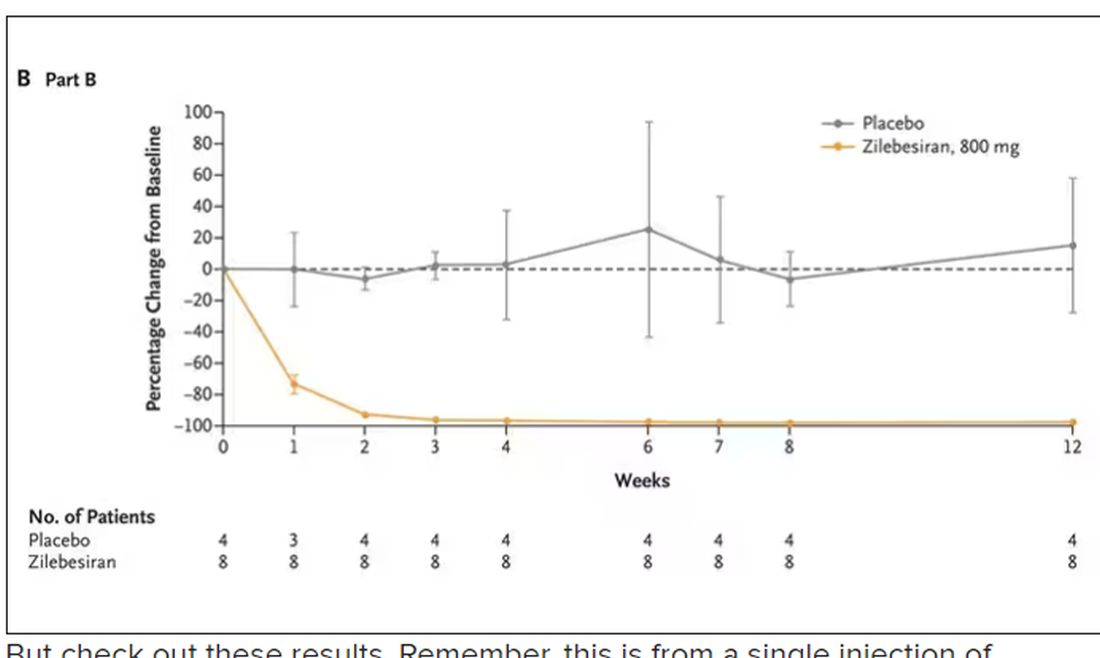
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.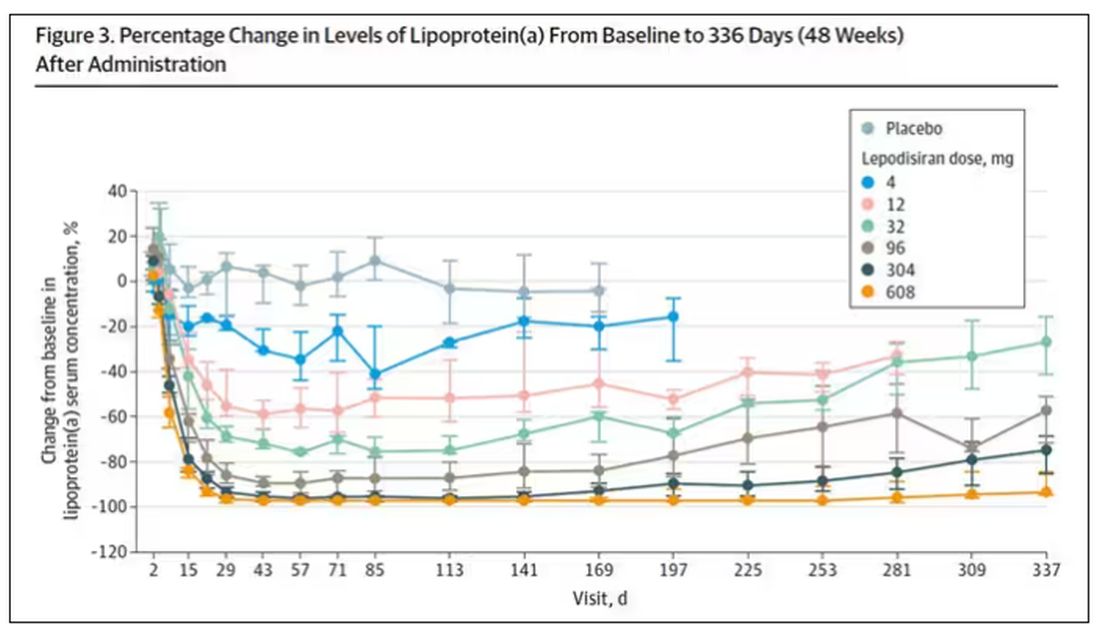
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.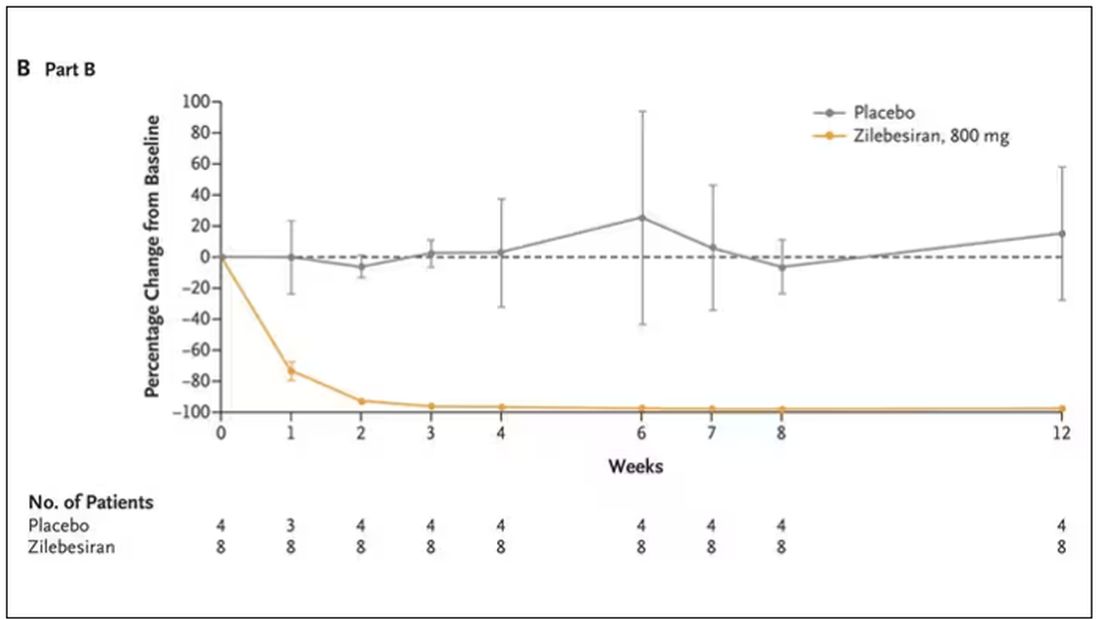
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr F. Perry Wilson of the Yale School of Medicine.
Every once in a while, medicine changes in a fundamental way, and we may not realize it while it’s happening. I wasn’t around in 1928 when Fleming discovered penicillin; or in 1953 when Watson, Crick, and Franklin characterized the double-helical structure of DNA.
But looking at medicine today, there are essentially two places where I think we will see, in retrospect, that we were at a fundamental turning point. One is artificial intelligence, which gets so much attention and hype that I will simply say yes, this will change things, stay tuned.
The other is a bit more obscure, but I suspect it may be just as impactful. That other thing is
I want to start with the idea that many diseases are, fundamentally, a problem of proteins. In some cases, like hypercholesterolemia, the body produces too much protein; in others, like hemophilia, too little.
When you think about disease this way, you realize that our current medications take effect late in the disease game. We have these molecules that try to block a protein from its receptor, prevent a protein from cleaving another protein, or increase the rate that a protein is broken down. It’s all distal to the fundamental problem: the production of the bad protein in the first place.
Enter small inhibitory RNAs, or siRNAs for short, discovered in 1998 by Andrew Fire and Craig Mello at UMass Worcester. The two won the Nobel prize in medicine just 8 years later; that’s a really short time, highlighting just how important this discovery was. In contrast, Karikó and Weissman won the Nobel for mRNA vaccines this year, after inventing them 18 years ago.
siRNAs are the body’s way of targeting proteins for destruction before they are ever created. About 20 base pairs long, siRNAs seek out a complementary target mRNA, attach to it, and call in a group of proteins to destroy it. With the target mRNA gone, no protein can be created.
You see where this is going, right? How does high cholesterol kill you? Proteins. How does Staphylococcus aureus kill you? Proteins. Even viruses can’t replicate if their RNA is prevented from being turned into proteins.
So, how do we use siRNAs? A new paper appearing in JAMA describes a fairly impressive use case.
The background here is that higher levels of lipoprotein(a), an LDL-like protein, are associated with cardiovascular disease, heart attack, and stroke. But unfortunately, statins really don’t have any effect on lipoprotein(a) levels. Neither does diet. Your lipoprotein(a) level seems to be more or less hard-coded genetically.
So, what if we stop the genetic machinery from working? Enter lepodisiran, a drug from Eli Lilly. Unlike so many other medications, which are usually found in nature, purified, and synthesized, lepodisiran was created from scratch. It’s not hard. Thanks to the Human Genome Project, we know the genetic code for lipoprotein(a), so inventing an siRNA to target it specifically is trivial. That’s one of the key features of siRNA – you don’t have to find a chemical that binds strongly to some protein receptor, and worry about the off-target effects and all that nonsense. You just pick a protein you want to suppress and you suppress it.
Okay, it’s not that simple. siRNA is broken down very quickly by the body, so it needs to be targeted to the organ of interest – in this case, the liver, since that is where lipoprotein(a) is synthesized. Lepodisiran is targeted to the liver by this special targeting label here.
The report is a standard dose-escalation trial. Six patients, all with elevated lipoprotein(a) levels, were started with a 4-mg dose (two additional individuals got placebo). They were intensely monitored, spending 3 days in a research unit for multiple blood draws followed by weekly, and then biweekly outpatient visits. Once they had done well, the next group of six people received a higher dose (two more got placebo), and the process was repeated – six times total – until the highest dose, 608 mg, was reached.
This is an injection, of course; siRNA wouldn’t withstand the harshness of the digestive system. And it’s only one injection. You can see from the blood concentration curves that within about 48 hours, circulating lepodisiran was not detectable.
But check out these results. Remember, this is from a single injection of lepodisiran.
Lipoprotein(a) levels start to drop within a week of administration, and they stay down. In the higher-dose groups, levels are nearly undetectable a year after that injection.
It was this graph that made me sit back and think that there might be something new under the sun. A single injection that can suppress protein synthesis for an entire year? If it really works, it changes the game.
Of course, this study wasn’t powered to look at important outcomes like heart attacks and strokes. It was primarily designed to assess safety, and the drug was pretty well tolerated, with similar rates of adverse events in the drug and placebo groups.
As crazy as it sounds, the real concern here might be that this drug is too good; is it safe to drop your lipoprotein(a) levels to zero for a year? I don’t know. But lower doses don’t have quite as strong an effect.
Trust me, these drugs are going to change things. They already are. In July, The New England Journal of Medicine published a study of zilebesiran, an siRNA that inhibits the production of angiotensinogen, to control blood pressure. Similar story: One injection led to a basically complete suppression of angiotensinogen and a sustained decrease in blood pressure.
I’m not exaggerating when I say that there may come a time when you go to your doctor once a year, get your RNA shots, and don’t have to take any other medication from that point on. And that time may be, like, 5 years from now. It’s wild.
Seems to me that that rapid Nobel Prize was very well deserved.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships. This transcript has been edited for clarity.
A version of this article appeared on Medscape.com.
Can vitamin and mineral supplementation prevent cancer or cardiovascular disease?
Patients often come to us with questions about vitamin and mineral supplements. Sometimes they come to us with bags full of the things they are taking. The Internet is full of the wonders these nutritional supplements can do, from turmeric curing cancer to vitamin D curing COVID. It is hard to keep up with medicine itself without learning a whole new field of nutritional supplements.
However, for cardiovascular disease (CVD) and cancer prevention, the answer is pretty easy according to USPSTF (United States Preventative Services Task Force) guidelines. They evaluated 17,459 unique citations as well as 379 full-text articles that included randomized clinical trials and observational cohort studies. The conclusions of their research showed that there was little to no benefit in taking vitamin or mineral supplements to prevent CVD, cancer, or death. In fact, beta-carotene supplementation was associated with increased risk of lung cancer and other adverse outcomes in patients at increased risk of lung cancer.
Although they are often marketed like drugs, nutritional supplements are regulated as foods, with less stringent standards.* Our current medical culture pushes us to practice evidence-based medicine. Without evidence, we simply cannot counsel patients about supplements because there is little evidence to support their use.
Additionally, many patients assume that they are safe. While this may be true for many of them, some of them can be harmful in several ways. They can interact with medications the patient may be taking for medical conditions. Some of them have been shown to cause liver and other organ damage. When they are used to replace traditional medicine, they can also lead to harm by delaying appropriate medical care. For example, a patient who believes a supplement can treat cancer when it does nothing is delaying care that might save their life. By the time they realize it is not working, the cancer may have advanced too far to be treatable.
While there may be a few studies that do show some efficacy for vitamins and minerals in certain diseases, these guidelines are looking only at use in terms of preventing cancer and CVD. As primary care physicians, we all know the screening guidelines for cancer prevention. We are better off recommending screening mammograms and colon cancer screening tests. And we all know the risk factors for CVD and how to mitigate these risks.
What can we do when patients come to us with false claims regarding supplements?
- Hear what they are saying. They don’t know who to trust. We will never become their trusted source of medical information if we don’t listen to their concerns.
- Answer their questions, no matter how ridiculous they may seem to us. Many people who sell supplements sound convincing. That is how they sell their products. Our advice may seem just as ridiculous to them. We need to explain the facts clearly and be sure the patient understands.
- Give the patient resources. Know what websites to direct them to so that they can get accurate information.
- Know what’s out there. I was once surprised when a patient told me she was going to try turmeric as a treatment for uterine cancer. We cannot combat misinformation when we don’t know what’s being said.
- Become a voice for medical information. There is so much misinformation being spread. We need more doctors to speak up about the right medical information.
Currently, patients often look for medical information online. We do them a disservice when we brush aside their questions regarding supplements, no matter how trivial they seem. We need to take a firm stand and tell them the evidence regarding these supplements: They are neither FDA approved nor studied for safety and efficacy. Anyone can sell a supplement and make any claim regarding it that they want. It is much better to eat a healthy, balanced diet to get the vitamins and minerals that you need. Not only do we need to show them the evidence, we need to convince them that it is true.
*Correction, 12/4: An earlier version of this article misstated the regulatory requirements for nutritional supplements.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
Patients often come to us with questions about vitamin and mineral supplements. Sometimes they come to us with bags full of the things they are taking. The Internet is full of the wonders these nutritional supplements can do, from turmeric curing cancer to vitamin D curing COVID. It is hard to keep up with medicine itself without learning a whole new field of nutritional supplements.
However, for cardiovascular disease (CVD) and cancer prevention, the answer is pretty easy according to USPSTF (United States Preventative Services Task Force) guidelines. They evaluated 17,459 unique citations as well as 379 full-text articles that included randomized clinical trials and observational cohort studies. The conclusions of their research showed that there was little to no benefit in taking vitamin or mineral supplements to prevent CVD, cancer, or death. In fact, beta-carotene supplementation was associated with increased risk of lung cancer and other adverse outcomes in patients at increased risk of lung cancer.
Although they are often marketed like drugs, nutritional supplements are regulated as foods, with less stringent standards.* Our current medical culture pushes us to practice evidence-based medicine. Without evidence, we simply cannot counsel patients about supplements because there is little evidence to support their use.
Additionally, many patients assume that they are safe. While this may be true for many of them, some of them can be harmful in several ways. They can interact with medications the patient may be taking for medical conditions. Some of them have been shown to cause liver and other organ damage. When they are used to replace traditional medicine, they can also lead to harm by delaying appropriate medical care. For example, a patient who believes a supplement can treat cancer when it does nothing is delaying care that might save their life. By the time they realize it is not working, the cancer may have advanced too far to be treatable.
While there may be a few studies that do show some efficacy for vitamins and minerals in certain diseases, these guidelines are looking only at use in terms of preventing cancer and CVD. As primary care physicians, we all know the screening guidelines for cancer prevention. We are better off recommending screening mammograms and colon cancer screening tests. And we all know the risk factors for CVD and how to mitigate these risks.
What can we do when patients come to us with false claims regarding supplements?
- Hear what they are saying. They don’t know who to trust. We will never become their trusted source of medical information if we don’t listen to their concerns.
- Answer their questions, no matter how ridiculous they may seem to us. Many people who sell supplements sound convincing. That is how they sell their products. Our advice may seem just as ridiculous to them. We need to explain the facts clearly and be sure the patient understands.
- Give the patient resources. Know what websites to direct them to so that they can get accurate information.
- Know what’s out there. I was once surprised when a patient told me she was going to try turmeric as a treatment for uterine cancer. We cannot combat misinformation when we don’t know what’s being said.
- Become a voice for medical information. There is so much misinformation being spread. We need more doctors to speak up about the right medical information.
Currently, patients often look for medical information online. We do them a disservice when we brush aside their questions regarding supplements, no matter how trivial they seem. We need to take a firm stand and tell them the evidence regarding these supplements: They are neither FDA approved nor studied for safety and efficacy. Anyone can sell a supplement and make any claim regarding it that they want. It is much better to eat a healthy, balanced diet to get the vitamins and minerals that you need. Not only do we need to show them the evidence, we need to convince them that it is true.
*Correction, 12/4: An earlier version of this article misstated the regulatory requirements for nutritional supplements.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
Patients often come to us with questions about vitamin and mineral supplements. Sometimes they come to us with bags full of the things they are taking. The Internet is full of the wonders these nutritional supplements can do, from turmeric curing cancer to vitamin D curing COVID. It is hard to keep up with medicine itself without learning a whole new field of nutritional supplements.
However, for cardiovascular disease (CVD) and cancer prevention, the answer is pretty easy according to USPSTF (United States Preventative Services Task Force) guidelines. They evaluated 17,459 unique citations as well as 379 full-text articles that included randomized clinical trials and observational cohort studies. The conclusions of their research showed that there was little to no benefit in taking vitamin or mineral supplements to prevent CVD, cancer, or death. In fact, beta-carotene supplementation was associated with increased risk of lung cancer and other adverse outcomes in patients at increased risk of lung cancer.
Although they are often marketed like drugs, nutritional supplements are regulated as foods, with less stringent standards.* Our current medical culture pushes us to practice evidence-based medicine. Without evidence, we simply cannot counsel patients about supplements because there is little evidence to support their use.
Additionally, many patients assume that they are safe. While this may be true for many of them, some of them can be harmful in several ways. They can interact with medications the patient may be taking for medical conditions. Some of them have been shown to cause liver and other organ damage. When they are used to replace traditional medicine, they can also lead to harm by delaying appropriate medical care. For example, a patient who believes a supplement can treat cancer when it does nothing is delaying care that might save their life. By the time they realize it is not working, the cancer may have advanced too far to be treatable.
While there may be a few studies that do show some efficacy for vitamins and minerals in certain diseases, these guidelines are looking only at use in terms of preventing cancer and CVD. As primary care physicians, we all know the screening guidelines for cancer prevention. We are better off recommending screening mammograms and colon cancer screening tests. And we all know the risk factors for CVD and how to mitigate these risks.
What can we do when patients come to us with false claims regarding supplements?
- Hear what they are saying. They don’t know who to trust. We will never become their trusted source of medical information if we don’t listen to their concerns.
- Answer their questions, no matter how ridiculous they may seem to us. Many people who sell supplements sound convincing. That is how they sell their products. Our advice may seem just as ridiculous to them. We need to explain the facts clearly and be sure the patient understands.
- Give the patient resources. Know what websites to direct them to so that they can get accurate information.
- Know what’s out there. I was once surprised when a patient told me she was going to try turmeric as a treatment for uterine cancer. We cannot combat misinformation when we don’t know what’s being said.
- Become a voice for medical information. There is so much misinformation being spread. We need more doctors to speak up about the right medical information.
Currently, patients often look for medical information online. We do them a disservice when we brush aside their questions regarding supplements, no matter how trivial they seem. We need to take a firm stand and tell them the evidence regarding these supplements: They are neither FDA approved nor studied for safety and efficacy. Anyone can sell a supplement and make any claim regarding it that they want. It is much better to eat a healthy, balanced diet to get the vitamins and minerals that you need. Not only do we need to show them the evidence, we need to convince them that it is true.
*Correction, 12/4: An earlier version of this article misstated the regulatory requirements for nutritional supplements.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. She was paid by Pfizer as a consultant on Paxlovid and is the editor in chief of Physician’s Weekly.
A nurse’s view: Women desperately need information about pelvic floor disorders
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.
Pelvic floor disorders are embarrassing, annoying, painful, and extremely disruptive to a woman’s life, often resulting in depression, anxiety, and a poor self-image. According to a 2021 study, approximately 75% of peripartum women and 68% of postmenopausal women feel insufficiently informed about pelvic floor disorders.1
Consequently, a large majority of women are not seeking care for these disorders. This drives health care costs higher as women wait until their symptoms are unbearable until finally seeking help. Many of these women don’t know they have options.
Who is at risk?
To understand the scope of this growing problem, it is vital to see who is most at risk. Parity, age, body mass index, and race are significant factors, although any woman can have a pelvic floor disorder (PFD).
Urinary incontinence (UI), pelvic floor prolapses (POP), and fecal incontinence (FI) are three of the most common pelvic floor disorders. Pregnancy and childbirth, specifically a vaginal birth, greatly contribute to this population’s risk. In pregnancy, the increase in plasma volume and glomerular filtration rate, along with hormone changes impacting urethral pressure and the growing gravid uterus, cause urinary frequency and nocturia. This can result in urinary incontinence during and after pregnancy.
Indeed, 76% of women with urinary incontinence at 3 months postpartum report it 12 years later.1 Third- and fourth-degree lacerations during delivery are uncommon (3.3%), but can cause fecal incontinence, often requiring surgery.1 Independently, all of these symptoms have been correlated with sexual dysfunction and postpartum depression.
One-third of all women and 50% of women over the age of 55 are currently affected by a PFD. Contributing factors include hormone changes with menopause that affect the pelvic floor muscles and connective tissue, prior childbirth and pregnancy, constipation, heavy lifting, prior pelvic surgery, and obesity. These women are vulnerable to pelvic organ prolapse from the weakened pelvic floor muscles. They will often present with a vague complaint of “something is protruding out of my vagina.” These women also present with urinary incontinence or leakage, proclaiming they have to wear a diaper or a pad. Without proper knowledge, aging women think these issues are normal and nothing can be done.
The woman with a BMI above 30 may have damaged tissues supporting the uterus and bladder, weakening those organs, and causing a prolapse. Incontinence is a result of poor muscle and connective tissue of the vagina that support the urethra. Obese women can suffer from both urinary and bowel incontinence. By the year 2030, it is projected that one in two adults will be obese.2 This will greatly impact health care costs.
To date, there is little conclusive evidence on the impact of race on pelvic floor disorders. A study in Scientific Reports did find that Asian women have a significantly lower risk for any PFD.2 Some research has found that Black and Hispanic women have less risk for UI but are at higher risk for FI and other PFDs.3 Understandably, women of certain cultures and demographics may be less likely to report incontinence to their clinicians and may be less informed as well.
What can we do?
The American College of Obstetricians and Gynecologists (ACOG) has acknowledged the deficiencies and lack of standard care of pelvic health in pregnancy and postpartum.1 There are differences in definitions across clinical practice and in the medical literature. Inconsistent patient reporting of PFD symptoms occurs due to nonstandard methods (questionnaire, interview, physical exam). With the often-short time allotted for visits with health care providers, women may neglect to discuss their symptoms, especially if they have other more pressing matters to address.
At the first OB appointment, a pregnant woman should be given information on what are normal and abnormal symptoms, from the beginning through postpartum. At each visit, she should be given ample opportunity to discuss symptoms of pelvic health. Clinicians should continue assessing, questioning, and discussing treatment options as applicable. Women need to know that early recognition and treatment can have a positive affect on their pelvic health for years to come.
ACOG recommends all postpartum patients see an obstetric provider within 3 weeks of delivery.1 Most are seen at 6 weeks. Pelvic health should be discussed at this final postpartum appointment, including normal and abnormal symptoms within the next few months and beyond.
Regardless of pregnancy status, women need a safe and supportive place to describe their pelvic floor issues. There is a validated questionnaire tool available for postpartum, but one is desperately needed for all women, especially women at risk. A pelvic health assessment must be included in every annual exam.
Women need to know there are multiple treatment modalities including simple exercises, physical therapy, a variety of pessaries, medications, and surgery. Sometimes, all that is needed are a few lifestyle changes: avoiding pushing or straining while urinating or having a bowel movement, maintaining a healthy diet rich in high fiber foods, and drinking plenty of fluids.
The National Public Health Service in the United Kingdom recently announced a government-funded program for pelvic health services to begin in April 2024.4 This program will address the pelvic floor needs, assessment, education and treatment for women after childbirth.
There are multiple clinics in the United States focusing on women’s health that feature urogynecologists – specialists in pelvic floor disorders. These specialists do a thorough health and physical assessment, explain types of pelvic floor disorders, and suggest appropriate treatment options. Most importantly, urogynecologists listen and address a woman’s concerns and fears.
There is no reason for women to feel compromised at any age. We, as health care providers, just need to assess, educate, treat, and follow up.
Ms. Barnett is a registered nurse in the department of obstetrics, Mills-Peninsula Medical Center, Burlingame, Calif. She has disclosed no relevant financial relationships.
References
1. Madsen AM et al. Recognition and management of pelvic floor disorders in pregnancy and the postpartum period. Obstet Gynecol Clin North Am. 2021 Sep;48(3):571-84. doi: 10.1016/j.ogc.2021.05.009.
2. Kenne KA et al. Prevalence of pelvic floor disorders in adult women being seen in a primary care setting and associated risk factors. Sci Rep. 2022 June; (12):9878. doi: 10.1038/s41598-022-13501-w.
3. Nygaard I et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311-6. doi: 10.1001/jama.300.11.1311.
4. United Kingdom Department of Health and Social Care. “National pelvic health service to support women.” 2023 Oct 19.










