User login
Newer brand-name drugs fuel spending on antiseizure medications
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
NASHVILLE, TENN. – , pointing to a major shift to newer, costlier, brand-name drugs – a trend in spending that may not be sustainable, the lead author of a study of drug costs said.
The study, presented at the 2022 annual meeting of the American Epilepsy Society, evaluated claims data for prescriptions for common antiseizure medications in the Medicare Part D and Medicaid databases from 2012 to 2020. The study excluded gabapentin and pregabalin because they’re frequently prescribed for other indications in addition to epileptic seizures.
“We found that third-generation medications, even though they accounted for the smallest percentage of claims in 2020, took up the most astronomical portion of the money that was spent,” lead author Deepti Zutshi, MD, an associate professor of neurology at Wayne State University in Detroit, said in an interview.
The study found that Medicare Part D spending on antiseizure medications increased from $1.16 billion in 2012 to $2.68 billion in 2020. In Medicaid, spending followed a similar trend, increasing from $973 million in 2012 to $1.05 billion in 2020.
Analyzing Medicare/Medicaid claims data
The study categorized drugs two ways: by brand or generic; and by first, second, or third generation, Dr. Zutshi said. First-generation drugs include medications such as phenobarbital, phenytoin, valproate, and carbamazepine. Second-generation medications were released in the early 2000s and include medications such as lamotrigine and levetiracetam. Examples of third-generation drugs include lacosamide, vigabatrin, clobazam, and perampanel.
Prescribers shifted significantly to third-generation treatments, Dr. Zutshi said. In Medicare Part D, the total spent on third-generation antiseizure medications went from $124 million in 2012 to $1.08 billion in 2020, representing a quadrupling in percentage of costs, from 10.7% to 40.4%. The total number of claims for third-generation antiseizure medications was 240,000 in 2012 (1.3%) and 1.1 million in 2020 (4.4%).
When looking at brand versus generic, the total spent on brand-name antiseizure medications increased nearly threefold from $546 million in 2012 to $1.62 million in 2020, with the share of all funding spent on brand-name antiseizure medications jumping from 46.8% to 60.2%. However, the proportion of total claims for branded antiseizure medications actually dropped, from 9.24% in 2012 to 6.62% in 2020.
Medicaid trends followed a similar pattern. Third-generation antiseizure medications accounted for 1.7% of total claims in 2012 and 6% in 2020. Spending on third-generation antiseizure medications grew nearly eight times: from $147 million, or 15.1% of funding spent on antiseizure medications, in 2012 to $1.15 billion in 2020, a 56.1% share of costs. The total spend of branded antiseizure medications in Medicaid was $605 million in 2012 and $1.46 billion in 2020 – a jump in the share of total spending from 62.2% to 71.3%. As in Medicare Part D, the percentage of total claims for branded antiseizure medications in Medicaid also dropped from 2012 to 2020, from 12.1% to 6.8%.
Why the substantial increase in spending?
“The reason we are prescribing these more expensive medications may be that the third-generation medications have better side-effect profiles, improved safety and outcomes in pregnancy, or that they have less drug interactions with other medications,” Dr. Zutshi said.
That’s desirable for older patients on Medicare who are more likely to have comorbidities and be on other medications, or women of child-bearing age on Medicaid, Dr. Zutshi said. “But I don’t think people realize what the cost is to Medicare and Medicaid,” she said, “so this was a bit of a shocking finding in our paper when we looked at this. I wasn’t expecting to see the substantial increase of spending focusing on just a few medications.”
Neurologists and other providers have to be more aware of individual patients’ needs as well as cost when prescribing branded or third-generation antiseizure medications, Dr. Zutshi said. “We have to do what’s best for all of our patients, but it has to be sustainable. If not, we could start losing the ability to prescribe these medications in these vulnerable population groups, so we have to use them judiciously,” Dr. Zutshi said.
Controlling costs versus managing seizures
Timothy E. Welty, PharmD, a professor of pharmacy at Drake University in Des Moines, Iowa, noted some potential issues with the study’s methodology, namely that, while it excluded gabapentin and pregabalin, it did include other antiseizure medications that are used for other indications without accounting for them. Additionally, the pharmacy claims data the study used didn’t cross match with any diagnostic data.
Controlling drug costs is noteworthy, he said, but managing seizures is equally important. “You have to think not only in terms of preventing seizures and what impact that has on health care costs specifically, but what impact that has on overall costs to society,” Dr. Welty said. “Doing the best we can to get their seizures under control as quickly as possible has great benefits for the patient outside of health care costs.”
He added, “We just really need to educate pharmacists and decision makers within third-party payers, be it Medicare, Medicaid, private insurance, whatever, on the advances that are being made in the use of seizure medications to treat epilepsy and stop seizures, but it’s a far broader issue than just how many dollars are we spending on seizure medication.”
Dr. Zutshi and Dr. Welty have no relevant disclosures to report.
AT AES 2022
Know the right resuscitation for right-sided heart failure
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Amado Alejandro Baez, MD, said in a presentation at the 2022 scientific assembly of the American College of Emergency Physicians.
The patient arrived on day 20 after a radical cystoprostatectomy. He had driven 4 hours from another city for a urology follow-up visit. On arrival, he developed respiratory distress symptoms and presented to the emergency department, said Dr. Baez, professor of emergency medicine and epidemiology at the Medical College of Georgia/Augusta University and triple-board certified in EMS, emergency medicine, and critical care.
The patient developed a massive pulmonary embolism with acute cor pulmonale (right-sided heart failure). An electrocardiogram showed an S1Q3T3, demonstrating the distinctive nature of right ventricular failure, said Dr. Baez.
Research has demonstrated the differences in physiology between the right and left ventricles, he said.
Dr. Baez highlighted some of the features of right ventricle (RV) failure and how to manage it. Notably, the RV is thinner and less resilient. “RV failure patients may fall off the Starling curve,” in contrast to patients with isolated left ventricle (LV) failure.
RV pressure overload is associated with a range of conditions, such as pericardial disease, pulmonary embolism, acute respiratory distress syndrome, and pulmonary arterial hypertension. When combined with RV overload, patients may develop intracardiac shunting or coronary heart disease, Dr. Baez said. Decreased contractility associated with RV failure can result from sepsis, right ventricular myocardial infarction, myocarditis, and arrhythmia.
Dr. Baez cited the 2018 scientific statement from the American Heart Association on the evaluation and management of right-sided heart failure. The authors of the statement noted that the complicated geometry of the right heart makes functional assessment a challenge. They wrote that various hemodynamic and biochemical markers can help guide clinical assessment and therapeutic decision-making.
Increased RV afterload drives multiple factors that can ultimately lead to cardiogenic shock and death, said Dr. Baez. These factors include decreased RV oxygen delivery, decreased RV coronary perfusion, decreased systemic blood pressure, and low carbon monoxide levels. RV afterload also leads to decreased RV contractility, an increase in RV oxygen demand, and tension in the RV wall, and it may contribute to tricuspid valve insufficiency, neurohormonal activation, and RV ischemia.
Treatment strategies involve improving symptoms and stopping disease progression, said Baez. In its scientific statement, the AHA recommends steps for assessing RV and LV function so as to identify RV failure as soon as possible, he said. After excluding pericardial disease, the AHA advises diagnosis and treatment of etiology-specific causes, such as right ventricular MI, pulmonary embolism, and sepsis. For arrhythmias, it recommends maintaining sinus rhythm when possible and considering a pacemaker to maintain atrioventricular synchrony and to avoid excessive bradycardia.
In its statement, the AHA also recommends optimizing preload with right arterial pressure/central venous pressure of 8-12 mm Hg, said Dr. Baez. Preload optimization combined with afterload reduction and improved contractility are hallmarks of care for patients with RV failure.
Avoiding systemic hypotension can prevent sequelae, such as myocardial ischemia and further hypotension, he said.
Optimization of fluid status is another key to managing RV failure, said Dr. Baez. Right heart coronary perfusion pressure can be protected by maintaining mean arterial pressure, and consideration should be given to reducing the RV afterload. Other strategies include inotropic medications and rhythm stabilization.
In general, for RV failure patients, “correct hypoxia, hypercarbia, and acidosis and avoid intubation when possible,” he said. Extracorporeal membrane oxygenation (ECMO) may be an option, depending on how many mechanical ventilator settings need to be adjusted.
In a study by Dr. Baez and colleagues published in Critical Care Medicine, the authors presented a Bayesian probability model for plasma lactate and severity of illness in cases of acute pulmonary embolism. “This Bayesian model demonstrated that the combination of shock index and lactate yield superior diagnostic gains than those compare to the sPESI and lactate,” Dr. Baez said.
The care model needs to be specific to the etiology, he added. Volume management in congested pulmonary hypertension involves a “squeeze and diurese” strategy.
According to the Internet Book of Critical Care, for patients with mean arterial pressure (MAP) of 60 mm Hg, central venous pressure (CVP) of 25 mm Hg, renal perfusion pressure of 25 mm Hg, and no urine output, a vasopressor should be added to treatment, Dr. Baez said. In cases in which the MAP 75 mm Hg, the CVP is 25 mm Hg, the renal perfusion pressure is 50 mm Hg, and the patient has good urine output, vasopressors should be continued and fluid should be removed through use of a diuretic. For patients with a MAP of 75 mm Hg, a CVP of 12 mm Hg, and renal perfusion pressure of 63 mm Hg who have good urine output, the diuretic and the vasopressor should be discontinued.
Dr. Baez also reviewed several clinical studies of the utility of acute mechanical circulatory support systems for RV failure.
In two small studies involving a heart pump and a right ventricular assistive device, the 30-day survival rate was approximately 72%-73%. A study of 179 patients involving ECMO showed an in-hospital mortality rate of 38.6%, he said.
Overall, “prompt diagnosis, hemodynamic support, and initiation of specific treatment” are the foundations of managing RV failure, he concluded.
Dr. Baez disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACEP 2022
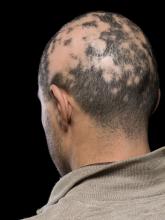
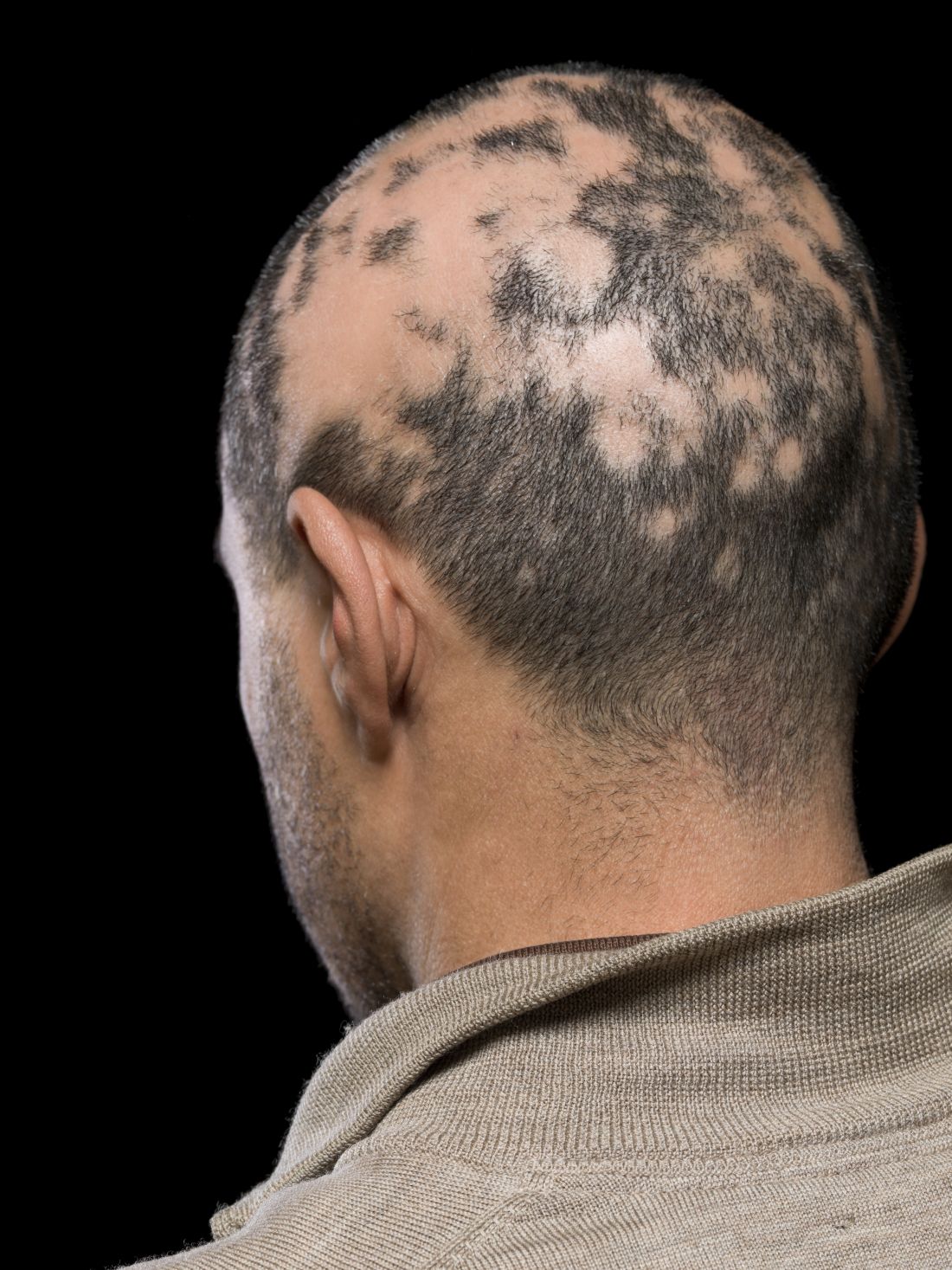
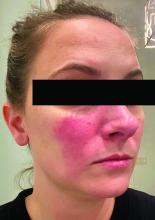
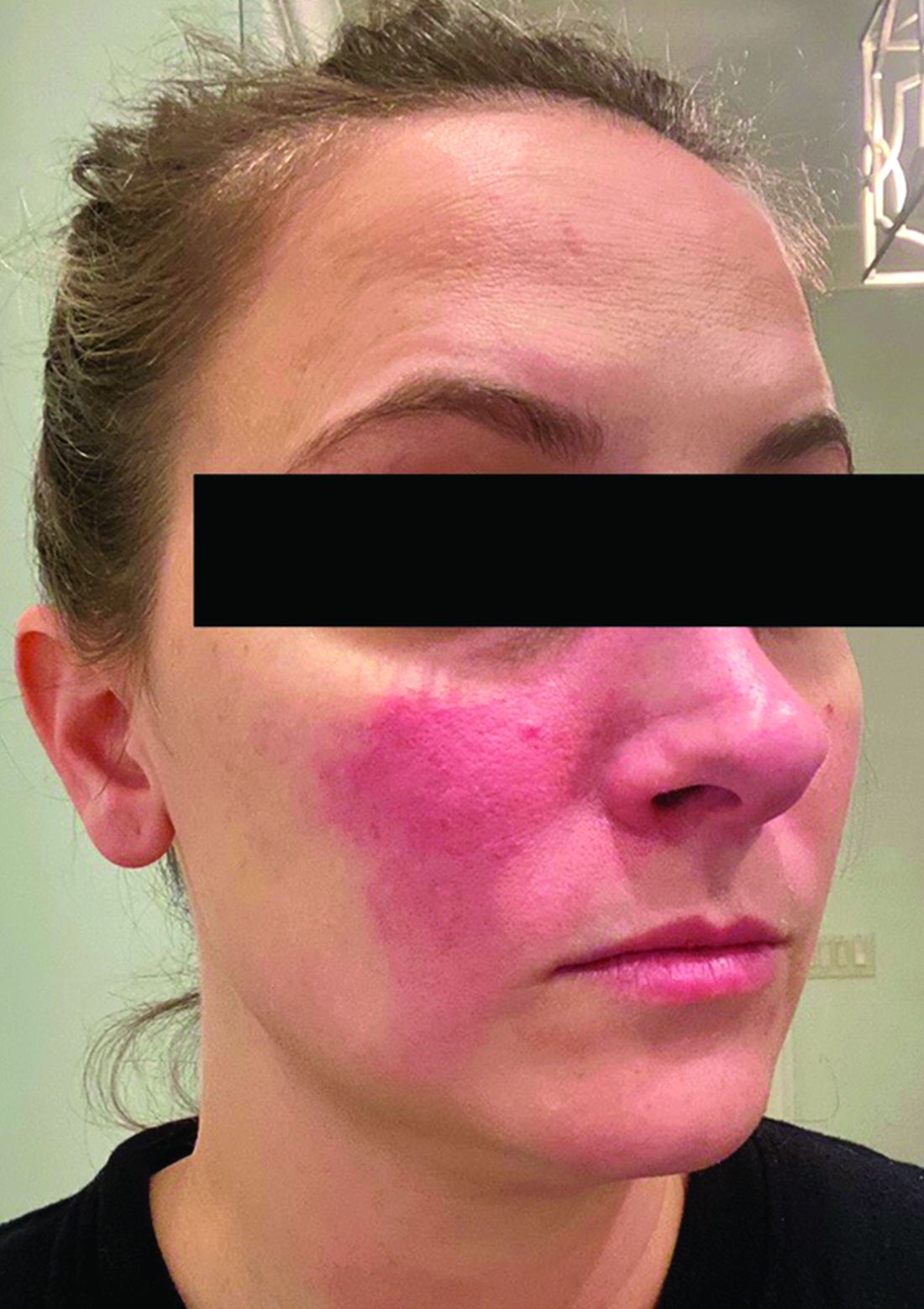
Current alopecia areata options include old and new therapies
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
“Some patients don’t have alopecia, but they have been managed for it,” he said. “Whenever there is an ounce of doubt, take a biopsy,” he advised.
Assessing disease severity in patients with alopecia areata (AA) is especially important as new therapies become available, said Dr. King, associate professor of dermatology at Yale University, New Haven, Conn. The Severity of Alopecia Tool (SALT) Score has been available since 2004, and remains a useful tool to estimate percent hair loss. The SALT Score divides the scalp into four sections: 18% each for the right and left sides, 40% for the top of the head, and 24% for the back of the head, said Dr. King. However, the SALT Score can be enhanced or modified based on a holistic approach to disease severity that categorizes alopecia as mild (scalp hair loss of 20% or less), moderate (scalp hair loss of 21 to 49%), or severe (scalp hair loss of 50% or more).
For example, if a patient’s hair loss based on SALT Score is mild or moderate, increase the severity by 1 level (from mild to moderate, or moderate to severe) if any of the following conditions apply: Noticeable eyebrow or eyelash involvement, inadequate treatment response after 6 months, diffuse positive hair pull test consistent with rapid progression of AA, or a negative impact on psychosocial functioning because of AA, he said.
Treatment advances
Understanding of the pathogenesis of AA has been slow to evolve, Dr. King noted. “We haven’t been able to shake this concept that people are causing the disease by being depressed,” as noted in the literature from the 1950s.
In 2014, breakthrough research changed the game by identifying the roles of interferon gamma and interleukin 15, Dr. King said. Since then, more research has been conducted on Janus kinase (JAK) inhibitors for AA. Dr. King was a coinvestigator on a 2014 case report in which a patient with psoriasis and alopecia universalis experienced regrowth of most of his body hair after 8 months of daily oral tofacitinib, a JAK inhibitor.
However, despite the dramatic results in some patients, “tofacitinib doesn’t always work,” said Dr. King. In his experience, patients for whom tofacitinib didn’t work were those with complete or nearly complete scalp hair loss for more than 10 years.
Approval of baricitinib
Dr. King’s recent work supported the approval in June 2022 of oral baricitinib, a JAK inhibitor, for AA. He reviewed data from his late-breaker abstract presented at the annual meeting of the American Academy of Dermatology in March 2022, where he reported that almost 40% of adults with AA treated with 4 mg of baricitinib daily had significant hair regrowth over 52 weeks.
Two other oral JAK inhibitors in the pipeline for AA are deuruxolitinib and ritlecitinib, which significantly increased the proportion of patients achieving SALT scores of 20 or less, compared with patients on placebo in early clinical trials. Data on both were presented at the annual meeting of the European Academy of Dermatology and Venereology.
So far, topical JAK inhibitors have not shown success in hair regrowth for AA patients, said Dr. King. Phase 2 studies of both ruxolitinib 1.5% cream and delgocitinib ointment were ineffective for AA.
Emerging role for oral minoxidil
Oral minoxidil has had a recent resurgence as an adjunct therapy to the new JAK inhibitors. A study published in 1987 found that, with oral minoxidil monotherapy, a cosmetic response was seen in 18% of patients with AA, Dr. King said.
In a study published in the Journal of the American Academy of Dermatology, Dr. King and colleagues noted that dose escalation is sometimes needed for effective treatment of AA with tofacitinib. They examined the effect of adding oral minoxidil to tofacitinib in patients with severe AA as a way to increase efficacy without increasing tofacitinib dosage. They reviewed data from 12 patients ages 18-51 years who were prescribed 5 mg of tofacitinib twice daily, plus 2.5 mg oral minoxidil daily for women and 2.5 mg of minoxidil twice daily for men; women received a lower dose to minimize the side effect of hypertrichosis.
After 6 months, 67% (eight patients) achieved at least 75% hair regrowth; of those eight patients, seven (58% of the total) had hair regrowth on a twice-daily dose of 5 mg tofacitinib with no need for dose escalation, Dr. King said.
More research is needed, but oral minoxidil may be a useful adjunct treatment for some patients with AA, he added.
During a question and answer session, Dr. King was asked to elaborate on the mechanism of minoxidil in combination with JAK inhibitors. “The truth is that I just don’t know” why the combination works for some patients. However, the majority of patients who succeed with this combination regrow hair by 4 months. “There is something special about that combination.”
Dr. King disclosed serving as a consultant or adviser for AbbVie, AltruBio, Almirall, AnaptysBio, Arena Pharmaceuticals, Bioniz, Bristol Myers Squibb, Concert Pharmaceuticals, Horizon, Incyte, Leo Pharma, Eli Lilly, Otsuka, Pfizer, Regeneron, Sanofi Genzyme, Twi Biotechnology, Viela Bio, and Visterra; serving as a speaker or as a member of the speakers bureau for Incyte, Pfizer, Regeneron, Sanofi Genzyme; and receiving research funding from Concert Pharmaceuticals, Eli Lilly, and Pfizer.
MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Paxlovid has been free so far. Next year, sticker shock awaits
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Nearly 6 million Americans have taken Paxlovid for free, courtesy of the federal government. The Pfizer pill has helped prevent many people infected with COVID-19 from being hospitalized or dying, and it may even reduce the risk of developing long COVID.
And that means fewer people will get the potentially lifesaving treatments, experts said.
“I think the numbers will go way down,” said Jill Rosenthal, director of public health policy at the Center for American Progress, a left-leaning think tank. A bill for several hundred dollars or more would lead many people to decide the medication isn’t worth the price, she said.
In response to the unprecedented public health crisis caused by COVID, the federal government spent billions of dollars on developing new vaccines and treatments, to swift success: Less than a year after the pandemic was declared, medical workers got their first vaccines. But as many people have refused the shots and stopped wearing masks, the virus still rages and mutates. In 2022 alone, 250,000 Americans have died from COVID, more than from strokes or diabetes.
But soon the Department of Health & Human Services will stop supplying COVID treatments, and pharmacies will purchase and bill for them the same way they do for antibiotic pills or asthma inhalers. Paxlovid is expected to hit the private market in mid-2023, according to HHS plans shared in an October meeting with state health officials and clinicians. Merck’s Lagevrio, a less-effective COVID treatment pill, and AstraZeneca’s Evusheld, a preventive therapy for the immunocompromised, are on track to be commercialized sooner, sometime in the winter.
The U.S. government has so far purchased 20 million courses of Paxlovid, priced at about $530 each, a discount for buying in bulk that Pfizer CEO Albert Bourla called “really very attractive” to the federal government in a July earnings call. The drug will cost far more on the private market, although in a statement to Kaiser Health News, Pfizer declined to share the planned price. The government will also stop paying for the company’s COVID vaccine next year – those shots will quadruple in price, from the discount rate the government pays of $30 to about $120.
Mr. Bourla told investors in November that he expects the move will make Paxlovid and its COVID vaccine “a multibillion-dollars franchise.”
Nearly 9 in 10 people dying from the virus now are 65 or older. Yet federal law restricts Medicare Part D – the prescription drug program that covers nearly 50 million seniors – from covering the COVID treatment pills. The medications are meant for those most at risk of serious illness, including seniors.
Paxlovid and the other treatments are currently available under an emergency use authorization from the FDA, a fast-track review used in extraordinary situations. Although Pfizer applied for full approval in June, the process can take anywhere from several months to years. And Medicare Part D can’t cover any medications without that full stamp of approval.
Paying out-of-pocket would be “a substantial barrier” for seniors on Medicare – the very people who would benefit most from the drug, wrote federal health experts.
“From a public health perspective, and even from a health care capacity and cost perspective, it would just defy reason to not continue to make these drugs readily available,” said Dr. Larry Madoff, medical director of Massachusetts’s Bureau of Infectious Disease and Laboratory Sciences. He’s hopeful that the federal health agency will find a way to set aside unused doses for seniors and people without insurance.
In mid-November, the White House requested that Congress approve an additional $2.5 billion for COVID therapeutics and vaccines to make sure people can afford the medications when they’re no longer free. But there’s little hope it will be approved – the Senate voted that same day to end the public health emergency and denied similar requests in recent months.
Many Americans have already faced hurdles just getting a prescription for COVID treatment. Although the federal government doesn’t track who’s gotten the drug, a Centers for Disease Control and Prevention study using data from 30 medical centers found that Black and Hispanic patients with COVID were much less likely to receive Paxlovid than White patients. (Hispanic people can be of any race or combination of races.) And when the government is no longer picking up the tab, experts predict that these gaps by race, income, and geography will widen.
People in Northeastern states used the drug far more often than those in the rest of the country, according to a KHN analysis of Paxlovid use in September and October. But it wasn’t because people in the region were getting sick from COVID at much higher rates – instead, many of those states offered better access to health care to begin with and created special programs to get Paxlovid to their residents.
About 10 mostly Democratic states and several large counties in the Northeast and elsewhere created free “test-to-treat” programs that allow their residents to get an immediate doctor visit and prescription for treatment after testing positive for COVID. In Massachusetts, more than 20,000 residents have used the state’s video and phone hotline, which is available 7 days a week in 13 languages. Massachusetts, which has the highest insurance rate in the country and relatively low travel times to pharmacies, had the second-highest Paxlovid usage rate among states this fall.
States with higher COVID death rates, like Florida and Kentucky, where residents must travel farther for health care and are more likely to be uninsured, used the drug less often. Without no-cost test-to-treat options, residents have struggled to get prescriptions even though the drug itself is still free.
“If you look at access to medications for people who are uninsured, I think that there’s no question that will widen those disparities,” Ms. Rosenthal said.
People who get insurance through their jobs could face high copays at the register, too, just as they do for insulin and other expensive or brand-name drugs.
Most private insurance companies will end up covering COVID therapeutics to some extent, said Sabrina Corlette, a research professor at Georgetown University’s Center on Health Insurance Reforms. After all, the pills are cheaper than a hospital stay. But for most people who get insurance through their jobs, there are “really no rules at all,” she said. Some insurers could take months to add the drugs to their plans or decide not to pay for them.
And the additional cost means many people will go without the medication. “We know from lots of research that when people face cost sharing for these drugs that they need to take, they will often forgo or cut back,” Ms. Corlette said.
One group doesn’t need to worry about sticker shock. Medicaid, the public insurance program for low-income adults and children, will cover the treatments in full until at least early 2024.
HHS officials could set aside any leftover taxpayer-funded medication for people who can’t afford to pay the full cost, but they haven’t shared any concrete plans to do so. The government purchased 20 million courses of Paxlovid and 3 million of Lagevrio. Fewer than a third have been used, and usage has fallen in recent months, according to KHN’s analysis of the data from HHS.
Sixty percent of the government’s supply of Evusheld is also still available, although the COVID prevention therapy is less effective against new strains of the virus. The health department in one state, New Mexico, has recommended against using it.
HHS did not make officials available for an interview or answer written questions about the commercialization plans.
The government created a potential workaround when they moved bebtelovimab, another COVID treatment, to the private market this summer. It now retails for $2,100 per patient. The agency set aside the remaining 60,000 government-purchased doses that hospitals could use to treat uninsured patients in a convoluted dose-replacement process. But it’s hard to tell how well that setup would work for Paxlovid: Bebtelovimab was already much less popular, and the FDA halted its use on Nov. 30 because it’s less effective against current strains of the virus.
Federal officials and insurance companies would have good reason to make sure patients can continue to afford COVID drugs: They’re far cheaper than if patients land in the emergency room.
“The medications are so worthwhile,” said Dr. Madoff, the Massachusetts health official. “They’re not expensive in the grand scheme of health care costs.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Novel platform harnesses 3D laser technology for skin treatments
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
in all skin types, according to speakers at a virtual course on laser and aesthetic skin therapy.
The products feature “focal point technology,” which pairs 3D laser targeting with an integrated high-resolution imaging system (IntelliView), to help the user guide treatments at selectable depths. They have been cleared by the Food and Drug Administration for use in skin resurfacing procedures, and to treat benign pigmented lesions of the skin, including hyperpigmentation, and were created by Dieter Manstein, MD, PhD, Rox Anderson, MD, and Henry Chan, MD, of the Wellman Center for Photomedicine at Massachusetts General Hospital, and Irina Erenburg, PhD, CEO of AVAVA, the company that markets the products.
dermally focused treatment with Focal Point Technology. The coagulation zone, in dark purple, shows a deep conical lesion that extends 1.3 mm deep with significant epidermal sparing.
At the meeting, Mathew M. Avram, MD, JD, director of the Massachusetts General Hospital Dermatology Laser & Cosmetic Center, described focal point technology as an adjustable intradermally focused laser platform guided by real-time visual mapping to ensure the precise dose and depth of energy as the user performs treatments. “This is the key for rejuvenation,” he said. “You can go to different depths of the skin. You can be superficial for dyschromia and maybe a little bit different for wrinkles. If you want to treat scars, you go a little bit deeper. Coagulation occurs at these different depths.”
The collimated beam from conventional lasers affects all tissue in its path. The laser beam from the AVAVA product, however, creates a cone-shaped profile of injury in the dermis that minimizes the area of epidermal damage, making it safe in skin of color, according to Dr. Avram. “The beam comes to a focal point in the dermis at the depth that you want it to,” he explained during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “That’s where the energy is going to focus and it bypasses the dermal/epidermal junction, which traditional fractional lasers cannot. What’s interesting about this platform is that you have a wavelength for skin rejuvenation, then you have wavelengths for pigment, which allows you to treat conditions like melasma at different depths.”
The AVAVA high-speed IntelliView imaging system features 10-micron resolution, “so you get exquisite imaging that can help guide your treatments,” he said. It also features image acquisition and storage with artificial intelligence algorithm interrogation and the ability to personalize treatments to the patient’s specific skin type. Commercial availability is expected in the first half of 2023, Dr. Avram said.
In a separate presentation, New York-based cosmetic dermatologist Roy G. Geronemus, MD, who has been involved in clinical trials of AVAVA’s focal point technology, said that patients “feel less pain and have less down time than we saw previously with other nonablative, fractional technologies.”
Downtime involves “just some mild redness,” he said, adding that he is encouraged by early results seen to date, and that “there appears to be some unique capabilities that will be borne out as the clinical studies progress.”
Dr. Avram disclosed that he has received consulting fees from Allergan, Galderma, and Revelle. He is an investigator for Endo and holds ownership and/or shareholder interest in Cytrellis and La Jolla NanoMedical. Dr. Geronemus disclosed having financial relationships with numerous device and pharmaceutical companies.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Rosacea and the gut: Looking into SIBO
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
, according to speakers at the annual Integrative Dermatology Symposium.
“SIBO is definitely something we test for and treat,” Raja Sivamani, MD, said in an interview after the meeting. Dr. Sivamani practices as an integrative dermatologist at the Pacific Skin Institute in Sacramento and is the director of clinical research at the institute’s research unit, Integrative Skin Science and Research. He led a panel discussion on rosacea and acne at the meeting.
Associations between SIBO and several dermatologic conditions, including systemic sclerosis, have been reported, but the strongest evidence to date involves rosacea. “There’s associative epidemiological evidence showing higher rates of SIBO among those with rosacea, and there are prospective studies” showing clearance of rosacea in patients treated for SIBO, said Dr. Sivamani, also adjunct associate professor of clinical dermatology at the University of California, Davis.
Studies are small, but are “well done and well-designed,” he said in the interview. “Do we need more studies? Absolutely. But what we have now is compelling [enough] for us to take a look at it.”
Findings of rosacea clearance
SIBO’s believed contribution to the pathophysiology of rosacea is part of the increasingly described gut microbiome-skin axis. SIBO has been recognized as a medical phenomenon for many decades and has been defined as an excessive bacterial load in the small bowel that causes gastrointestinal symptoms, according to the 2020 American College of Gastroenterology clinical guideline on SIBO.
Symptoms commonly associated with SIBO overlap with the cardinal symptoms of irritable bowel syndrome (IBS): abdominal pain; diarrhea, constipation, or both; bloating; and flatulence. SIBO can be diagnosed with several validated carbohydrate substrate (glucose or lactulose)–based breath tests that measure hydrogen and/or methane.
Hydrogen-positive breath tests suggest bacterial overgrowth, and methane-positive breath tests suggest small intestinal methanogen overgrowth. Methane is increasingly important and recognized, the AGA guideline says, though it creates a “nomenclature problem in the SIBO framework” because methanogens are not bacteria, the authors note.
In conventional practice, SIBO is typically treated with antibiotics such as rifaximin, and often with short-term dietary modification as well. Integrative medicine typically considers the use of supplements and botanicals in addition to or instead of antibiotics, as well as dietary change and increasingly, a close look at SIBO risk factors to prevent recurrence, Dr. Sivamani said. (His research unit is currently studying the use of herbal protocols as an alternative to antibiotics in patients with SIBO and dermatologic conditions.)
During a presentation on rosacea at the meeting, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research, a dermatology treatment and research center in San Diego, said that currently available breath tests for SIBO “are very interesting tools for understanding what may be happening in the gut” and that the “rifaximin data are good.”
He referred to a study reported in the Journal of the American Academy of Dermatology showing that patients with rosacea were significantly more likely to have SIBO (41.7% of 48 patients vs. 5.0% of 40 controls; P < .001), and that 64.5% of rosacea patients who completed treatment with rifaximin had remission of rosacea at a 3-year follow-up.
An earlier crossover study is also notable, he said. This study enrolled 113 consecutive patients with rosacea and 60 age- and sex-matched controls, and randomized those with SIBO (52 of the 113 with rosacea vs. 3 of the 60 controls) to rifaximin or placebo. Rosacea cleared in 20 of the 28 patients in the rifaximin group and greatly improved in 6 of the 28. Of 20 patients in the placebo group, rosacea remained unchanged in 18 and worsened in 2. When patients in the placebo group were switched to rifaximin, SIBO was eradicated in 17 of the 20, and rosacea completely resolved in 15 of those patients, Dr. Bhatia said.
In his view, it will take more time, greater awareness of the rosacea-SIBO link, and a willingness “to take chances” for more dermatologists to consider SIBO during rosacea care. “Breath tests are not something used in the [typical dermatology] clinic right now, but they may make their way in,” he said at the meeting.
In a follow-up interview, Dr. Bhatia emphasized that “it’s really a question of uptake, which always takes a while” and of willingness to “think through the disease from another angle ... especially in patients who are recalcitrant.”
Treatment
Dr. Sivamani said in the interview that a third type of SIBO – hydrogen sulfide–dominant SIBO – is now documented and worth considering when glucose and lactulose breath tests are negative in patients with rosacea who have gastrointestinal symptoms.
The use of breath tests to objectively diagnose SIBO is always best, Dr. Sivamani said, but he will consider empiric therapy in some patients. “I always tell patients [about] the benefits of testing, but if they can’t get the test covered or are unable to pay for the test, and they have symptoms consistent with SIBO, I’m okay doing a trial with therapy,” he said.
Rifaximin, one of the suggested antibiotics listed in the AGA guideline, is a nonabsorbable antibiotic that is FDA-approved for IBS with diarrhea (IBS-D); it has been shown to not negatively affect the growth of beneficial bacteria in the colon.
However, herbals are also an attractive option – alone or in combination with rifaximin or other antibiotics – speakers at the meeting said. In a multicenter retrospective chart review led by investigators at the Johns Hopkins Hospital, herbal therapies were at least as effective as rifaximin for treating SIBO, with similar safety profiles. The response rate for normalizing breath hydrogen testing in patients with SIBO was 46% for herbal therapies and 34% for rifaximin.
Dietary change is also part of treatment, with the reduction of fermentable carbohydrates – often through the Low FODMAP Diet and Specific Carbohydrate Diet – being the dominant theme in dietary intervention for SIBO, according to the AGA guideline.
“There are definitely some food choices you can shift,” said Dr. Sivamani. “I’ll work with patients on FODMAP, though it’s hard to sustain over the long-term and can induce psychological issues. You have to provide other options.”
Dr. Sivamani works with patients on using “a restrictive diet for a short amount of time, with the gradual reintroduction of foods to see [what] foods are and aren’t [causing] flares.” He also works to identify and eliminate risk factors and predisposing factors for SIBO so that recurrence will be less likely.
“SIBO is definitely an entity that is not on the fringes anymore ... it adds to inflammation in the body ... and if you have an inflamed gut, there’s a domino effect that will lead to inflammation elsewhere,” Dr. Sivamani said.
“You want to know, do your patients have SIBO? What subset do they have? Do they have risk factors you can eliminate?” he said. “And then what therapies will you use – pharmaceuticals, supplements and botanicals, or a combination? And finally, what will you do with diet?”
Dr. Bhatia disclosed he has affiliations with Abbvie, Almirall, Arcutis, Arena, Biofrontera, BMS, BI, Brickell, Dermavant, EPI Health, Ferndale, Galderma, Genentech, InCyte, ISDIN, Johnson & Johnson, LaRoche-Posay, Leo, Lilly, Novartis, Ortho, Pfizer, Proctor & Gamble, Regeneron, Sanofi, Stemline, SunPharma, and Verrica. Dr. Sivamani did not provide a disclosure statement.
REPORTING FROM IDS 2022
Applications for laser-assisted drug delivery on the horizon, expert says
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
For those who view fractional ablative laser–assisted drug delivery as a pie-in-the-sky procedure that will take years to work its way into routine clinical practice, think again.
According to Merete Haedersdal, MD, PhD, DMSc, .
“The groundwork has been established over a decade with more than 100 publications available on PubMed,” Dr. Haedersdal, professor of dermatology at the University of Copenhagen, said during a virtual course on laser and aesthetic skin therapy. “There is no doubt that by drilling tiny little holes or channels with ablative fractional lasers, we enhance drug delivery to the skin, and we also empower different topical treatment regimens. Also, laser-assisted drug delivery holds the potential to bring new innovations into established medicine.”
Many studies have demonstrated that clinicians can enhance drug uptake into the skin with the fractional 10,600 nm CO2 laser, the fractional 2,940 nm erbium:YAG laser, and the 1,927 nm thulium laser, but proper tuning of the devices is key. The lower the density, the better, Dr. Haedersdal said.
“Typically, we use 5% density or 5% coverage, sometimes 10%-15%, but don’t go higher in order to avoid the risk of having a systemic uptake,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “Also, the pulse energy for channel depth needs to be tailored to the specific dermatologic disease being treated,” she said, noting that for melasma, for example, “very low pulse energies” would be used, but they would be higher for treating thicker lesions, such as a hypertrophic scar.
Treatment with ablative fractional lasers enhances drug accumulation in the skin of any drug or substance applied to the skin, and clinical indications are expanding rapidly. Established indications include combining ablative fractional lasers and photodynamic therapy (PDT) for AKs and combining ablative fractional lasers and triamcinolone or 5-FU for scars. “Although we have a good body of evidence, particularly for AKs, it’s still an off-label use,” she emphasized.
Evolving indications include concomitant use of ablative fractional laser and vitamins and cosmeceuticals for rejuvenation; lidocaine for local anesthetics; tranexamic acid and hydroquinone for melasma; antifungals for onychomycosis; Botox for hyperhidrosis; minoxidil for alopecia; and betamethasone for vitiligo. A promising treatment for skin cancer “on the horizon,” she said, is the “combination of ablative fractional laser with PD1 inhibitors and chemotherapy.”
Data on AKs
Evidence supporting laser-assisted drug delivery for AKs comes from more than 10 randomized, controlled trials in the dermatology literature involving 400-plus immunocompetent and immunosuppressed patients. These trials have found ablative fractional laser–assisted PDT to be significantly more efficacious than PDT alone up to 12 months postoperatively and to foster lower rates of AK recurrence.
In a meta-analysis and systematic review, German researchers concluded that PDT combined with ablative laser treatment for AKs is more efficient but not more painful than either therapy alone. They recommended the combined regimen for patients with severe photodamage, field cancerization, and multiple AKs.
In 2020, an international consensus panel of experts, including Dr. Haedersdal, published recommendations regarding laser treatment of traumatic scars and contractures. The panel members determined that laser-assisted delivery of corticosteroids and antimetabolites was recommended for hypertrophic scars and cited triamcinolone acetonide suspension (TAC) as the most common corticosteroid used in combination with ablative fractional lasers. “It can be applied in concentrations of 40 mg/mL or less depending on the degree of hypertrophy,” they wrote.
In addition, they stated that 5-FU solution is “most commonly applied in a concentration of 50 mg/mL alone, or mixed with TAC in ratios of 9:1 or 3:1.”
According to the best available evidence, the clinical approach for hypertrophic scars supports combination treatment with ablative fractional laser and triamcinolone acetonide either alone or in combination with 5-FU. For atrophic scars, laser-assisted delivery of poly-L-lactic acid has been shown to be efficient. “Both of these treatments improve texture and thickness but also dyschromia and scar functionality,” said Dr. Haedersdal, who is also a visiting scientist at the Wellman Center for Photomedicine, Boston.
Commenting on patient safety with laser-assisted drug delivery, “the combination of lasers and topicals can be a powerful cocktail,” she said. “You can expect intensified local skin reactions. When treating larger areas, consider the risk of systemic absorption and the risk of potential toxicity. There is also the potential for infection with pathogens such as Staphylococcus aureus. The take-home message here is that you should only use the type and amount of drug no higher than administered during intradermal injection.”
Dr. Haedersdal disclosed that she has received equipment from Cherry Imaging, Cynosure-Hologic, MiraDry, and PerfAction Technologies. She has also received research grants from Leo Pharma, Lutronic, Mirai Medical, Novoxel, and Venus Concept.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
CRT boosts heart failure survival in extended follow-up
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
CHICAGO – Extended follow-up of patients with heart failure enrolled in the RAFT trial strengthens the case for starting treatment early with a cardiac resynchronization therapy plus defibrillation (CRT-D) device in appropriate patients.
RAFT, which compared CRT-D with treatment with an implantable cardioverter defibrillator (ICD) alone, showed that the early survival benefit produced by CRT-D during an average 40-month follow-up in the original trial persisted during an additional mean follow-up of about 5 years. This result strengthens the case for starting treatment early with a CRT-D device in appropriate patients with heart failure.
During extended follow-up of more than half of the enrolled patients, out to an average of 7.6 years overall and to an average of 12.9 years among survivors, patients who received a CRT-D device had a significant 21% relative reduction in their rate of all-cause mortality compared with randomized patients who received an ICD and no cardiac resynchronization, John L. Sapp, MD, reported at the American Heart Association scientific sessions.
The primary results of RAFT were first reported in 2010.
This magnitude of a survival benefit among the patients originally randomized to CRT is “dramatic,” given that many of the comparator patients who initially received no CRT likely crossed over to receive a CRT-D device once the initial, randomized 4 years of the study finished, commented Lynne W. Stevenson, MD, director of cardiomyopathy and the Lisa M. Jacobson Professor of Cardiology at Vanderbilt University Medical Center in Nashville, Tenn., who was not involved with the study.
‘CRT can remap heart failure trajectory’
The new findings “strengthen our conviction that CRT can remap the trajectory” of selected patients with heart failure, and that “candidates for CRT should be vigorously identified,” Dr. Stevenson said in an interview.
She also noted that the benefit with extended follow-up was “strikingly parallel” to that seen at 12 years after the addition of an ACE inhibitor for mild heart failure during the 4 years of the landmark SOLVD trial. The new RAFT extended follow-up, as well as the 12-year follow-up of the SOLVD trial, “support the concept that longer follow-up reveals vital information not provided by the relatively short randomized trial period,” she said.
“The new data say ‘don’t delay starting CRT in appropriate patients with heart failure,’ and ‘don’t think of CRT as just a treatment that makes patients feel better.’
“The totality of these data shows that CRT also treats the underlying heart muscle weakness, which helps patients live longer. Previous data showed that patients with left bundle branch block eligible for CRT are unlikely to respond well to the usual, recommended heart medications so it is important to start treatment with CRT-D early,” declared Dr. Stevenson, who cochaired the session where Dr. Sapp gave his report.
RAFT randomized 1,798 patients with New York Heart Association (NYHA) class II or III heart failure, a left ventricular ejection fraction of 30% or less, and an intrinsic QRS duration of at least 120 msec to receive either a CRT-D or ICD device. The study’s primary endpoint was death from any cause or hospitalization for heart failure. After an average 40 months of randomized follow-up, the primary endpoint occurred in 40% of the patients with an ICD and in 33% of those with a CRT-D device, a significant 25% relative reduction linked with CRT-D use. Both endpoint components contributed to the combined result significantly and to about the same extent, and the incremental benefit from CRT-D was significant for patients with NYHA class II heart failure as well as for those with class III.
However, prespecified subgroup analyses showed that the incremental benefit from CRT-D was significantly limited to patients with an intrinsic QRS duration of at least 150 msec, while in those with a duration of 120-149 msec CRT-D had a neutral effect compared with ICD. The same pattern also appeared when the analysis split patients into those with a left bundle branch block, who significantly benefited from CRT-D, but the initial benefit was not apparent in patients with right bundle branch block.
A study subgroup with extended follow-up
The new, extended follow-up analysis presented by Dr. Sapp included 1,050 of the original 1,798 patients (58%) enrolled at any of eight participating Canadian centers that each enrolled at least 100 patients and followed them through the end of 2021 (the full study cohort came from 34 centers, including 10 centers outside Canada). This subgroup included 520 patients randomized to receive CRT-D and 530 who received an ICD. Although this was a post hoc subgroup analysis, the CRT-D and ICD arms matched closely in all measured baseline characteristics.
The prespecified primary outcome of this follow-up analysis was the rate of all-cause mortality. Because of their longer disease trajectory, this pared-down study cohort included many more patients with NYHA class II function, 803, and in this subgroup CRT-D exerted a significant 23% incremental reduction in mortality compared with ICD treatment. CRT-D also produced a 17% relative reduction in long-term mortality among patients with NYHA class III function at baseline, but this point estimate of relative benefit was not significant in this subgroup of just 247 patients, said Dr. Sapp, a cardiologist and professor at Dalhousie University & Nova Scotia Health in Halifax.
Based on the original RAFT results from 2010, as well as on evidence from several other trials, the current heart failure management guideline from the AHA, the American College of Cardiology, and the Heart Failure Society of America give the highest level of recommendation, level 1, for CRT in patients with a left ventricular ejection fraction of 35% or less, sinus rhythm with left bundle branch block, a QRS duration of at least 150 msec, and NYHA class II, III, or ambulatory IV symptoms while on guideline-directed medical therapy.
The guideline also gives class 2a (“can be useful”) or 2b (“may be considered”) recommendation for certain other heart failure patients, including those with a QRS duration of 120-149 msec, a left ventricular ejection fraction as high as 50%, no left bundle branch block, or NYHA class I symptoms.
Don’t wait to start CRT
Although this 2022 guideline, as well as earlier versions that had roughly similar recommendations for CRT for about a decade, have led to “common” use of CRT in appropriate patients in U.S. practice, “it has not been used as much as it should be, in part because there’s been a feeling that CRT mostly treats symptoms and so perhaps you can wait” to start it, said Dr. Stevenson.
The findings from the new, extended follow-up RAFT analysis give increased urgency to starting CRT “as soon as possible” in appropriate patients with heart failure, even before they stabilize on guideline-directed medical therapy, said Dr. Stevenson. She also downplayed any ambiguity in the RAFT findings about optimal medical therapy, which during the RAFT study included traditional triple therapy at a time before treatment with sacubitril/valsartan (Entresto) and sodium-glucose cotransporter 2 (SGLT2) inhibitors became recommended.
“There is no reason to think that these treatments will negate the benefit of CRT for patients with heart failure with reduced ejection fraction and a wide left bundle branch block,” Dr. Stevenson said.
She also believes that the extended follow-up results, which showed clear efficacy for CRT-D in patients with NYHA class II function, support the case for upgrading the current 2b recommendation for using CRT treatment in patients with NYHA class I function and ischemic heart failure to a 2a recommendation regardless of whether or not patients have coronary artery disease. “The difference between class I and class II depends more on a patient’s lifestyle rather than on the severity of their heart failure,” Dr. Stevenson noted. “The RAFT study results encourage us to reexamine the clinical class and timing for CRT” in the current heart failure guideline.
RAFT received partial sponsorship from Medtronic. Dr. Sapp has been a consultant to Abbott, Biosense Webster, Medtronic, and Varian and has received research funding from Abbott and Biosense Webster. Dr. Stevenson had no disclosures.
AT AHA 2022
ASH 2022: New clinical data challenge long-held assumptions
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
The conference starts in New Orleans on Saturday, Dec. 10, , but a sample of what is to come was given last week in a preview media briefing, moderated by Mikkael A. Sekeres, MD, from the University of Miami. Dr. Sekeres, who recently authored a book on the FDA and how it regulates drug approvals, also serves as chair of the ASH Committee on Communications.
“Feeding Our Patients Gruel”
Dr. Sekeres expressed particular excitement about a multicenter randomized trial done in Italy. It showed that patients who have neutropenia after a stem cell transplant need not be required to eat a bland diet (Abstract 169).
“We for years have been essentially feeding our patients gruel in the hospital, and these are folks who have to be hospitalized for a stem cell transplant or in my case – I’m a leukemia specialist – for acute leukemia, for 4-6 weeks. The neutropenic diet consists of the blandest food you can imagine, with nothing to really spice it up.”
He noted that a neutropenic diet is so unpalatable that family members often sneak food into patient rooms, and “for years we’ve never seen adverse outcomes in any of those folks who instead of having mashed potatoes and oatmeal ate a corned beef sandwich for dinner.”
Now, the results from this trial “actually give us license to finally allow patients to eat whatever they want,” Dr. Sekeres said.
Practice-changing data
ASH experts pointed to two more presentations that are expected to change clinical practice. These include the finding that high-dose methotrexate does not reduce the risk for central nervous system relapse in children with acute lymphoblastic leukemia and lymphoblastic lymphoma (Abstract 214).
Another new study that seems to defy conventional wisdom showed that in adults with relapsed or refractory acute myeloid leukemia, intensive chemotherapy in an effort to achieve remission before a stem cell transplant did not result in better outcomes, compared with sequential conditioning and immediate transplant (Abstract 4).
Premature aging in HL survivors
ASH President Jane N. Winter, MD, from Northwestern University, Chicago, who also spoke at the briefing, highlighted a study that followed adult survivors of pediatric Hodgkin lymphoma. This study, from St. Jude Children’s Research Hospital in Memphis and the Wilmot Cancer Institute at the University of Rochester (N.Y), found that these adult survivors are at significantly elevated risk for epigenetic age acceleration accompanied by neurocognitive deficits when compared with controls (Abstract 902).
“This is an area that is very near and dear to my heart,” she said. “Much of my career has focused on reducing the therapy to reduce the long-term consequences of treatments. Pediatricians have been very much wedded to very intensive therapies and tend to incorporate radiation more commonly in their treatment strategies for children than we do in adults.”
Dr. Winter noted that, although clinicians focus primarily on the link between mediastinal radiation and long-term adverse events such as breast cancer, “now we’re shedding a light on the neurocognitive deficits, which I think are underappreciated. Being able to screen for this impact of our treatment, and perhaps then develop strategies to deal with it or prevent it, will have very wide-ranging impact.”
Inherited thrombophilia and miscarriage
Cynthia E. Dunbar, MD, chief of the translational stem cell biology branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., who also spoke at the briefing, said that one of the abstracts most important to her practice is a study concerning pregnancy. It showed that low-molecular-weight heparin did not prevent miscarriage in pregnant women with confirmed inherited thrombophilia who had two or more prior pregnancy losses, compared with standard surveillance (Abstract LBA-5).
“This is not my field at all; on the other hand, as a hematologist and a woman, that’s what my emails in the middle of the night and my panicked phone calls are often about. Once somebody has one miscarriage, especially if they feel like they’re already over 30 and the clock is ticking, there’s a huge emphasis and a huge amount of pressure on obstetricians to basically work up for everything, kind of a shotgun [approach],” she said.
Those workups may reveal genetic mutations that are associated with mild elevations in risk for clotting. As a result, some pregnant women are put on anticoagulation therapy, which can cause complications for both pregnancy and delivery. These study findings don’t solve the problem of spontaneous pregnancy loss, but they at least rule out inherited thrombophilia as a preventable cause of miscarriages, Dr. Dunbar said.
Another potentially practice-changing abstract is a study showing that, in younger adults with mantle cell lymphoma, the addition of the Bruton’s tyrosine kinase inhibitor ibrutinib (Imbruvica) to induction therapy and as maintenance with or without autologous stem cell transplant had strong efficacy and acceptable toxicity (Abstract 1).
“The results show that the ibrutinib-containing regimen without transplant is at least as good as the current standard of care with transplant.” Dr. Winter said. “Additional follow-up will be required to show definitively that an autotransplant is unnecessary if ibrutinib is included in this treatment regimen.”
A version of this article first appeared on Medscape.com.
FROM ASH 2022
Ask knee OA patients about stair climbing difficulty
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
Asking knee osteoarthritis patients a simple question – do you have difficulty climbing stairs? – may predict the risk of future functional limitation, according to research presented at the annual meeting of the American College of Rheumatology. Finding out that the patient has difficulty also opens avenues for further evaluation and intervention, said Jason Jakiela, a PhD candidate at the University of Delaware, Newark, who led the study. “We like to view it as a kind of yellow flag,” Mr. Jakiela said in an interview.
Another expert agreed. “I think this is useful for clinical rheumatologists,” said C. Kent Kwoh, MD, professor of medicine and medical imaging at the University of Arizona, Tucson, and director of the University of Arizona Arthritis Center. He commented on the study findings but was not involved in the study. Another common question asked of OA patients, about pain, may not be as useful as asking about difficulty climbing stairs, he said. “Their pain level can go up and down and can be quite varied.”
Osteoarthritis affects more than 32.5 million adults, according to the CDC, and the knee is a common site.
Study details, results
Mr. Jakiela and his team, including Daniel White, PT, ScD, MSC, associate professor of physical therapy at the University of Delaware, Newark, used data from the Osteoarthritis Initiative (OAI). They assessed stair climbing difficulty at baseline with the question: Does your health now limit you in climbing several flights of stairs? Respondents could answer that they were limited a lot, a little, or not at all.
The researchers evaluated functional limitation using two measures: Walking speed and Western Ontario and McMaster Universities Osteoarthritis Index physical function (WOMAC-PF) scores. A walking speed of < 1.22 m/s over 20 meters, the speed needed to safely cross a timed intersection, represented poor function. A WOMAC-PF score of 28/68 or more was also used to define low functioning.
The analyses included only people free of functional limitations at baseline. Each measure was conducted at the start and then at 12, 24, 36, 48, 72, and 96 months’ follow-up visits.
While 2,952 participants (mean age 60.1, 54% female, mean body mass index 27.9) were in the walking speed sample, 3,983 participants (mean age 61.2, 57% female, mean BMI 28.2) were in the WOMAC-PF sample.
When compared with people who had no limitations, those limited a little had a 47% greater risk of gait speed functional limitation and those limited a lot had a 61% greater risk at follow-up. There was a 70% greater risk for functional limitation defined by WOMAC-PF score at follow-up among people who were limited a little in stair climbing when compared with those not limited at all, and people with a lot of limitations had 161% greater risk. Slow gait speed has been linked with mortality.
Over the 8-year follow-up, 973 in the walking speed sample and 578 in the WOMAC-PF sample developed functional limitation.
Starting the conversation
The question about stair climbing difficulty is a good “jumping-off point,” Mr. Jakiela said. “It opens up a line of questioning.” With knee OA, stair climbing difficulty is often the first reported limitation. That difficulty could capture a variety of issues, he said. Patients could be struggling with strength issues, cardiovascular problems, or balance deficits, for instance.
It signals there may be a trajectory of slow decline coming in this patient, Mr. Jakiela said.
“It’s a signal that something is not right,” Dr. White said in an interview. “We don’t know what is wrong.” While questions about stairs have routinely been asked of OA patients, the study findings suggest the answer to the question about having difficulty could help predict a patient’s future course, he said.
After patients reported a little or a lot of difficulty with stair climbing, the average time to reach functional limitation status was about 3 years, Mr. Jakiela said. That gives health care providers time to ask more questions about the patient’s condition and potentially intervene, depending on the details of the difficulty. If it’s a balance issue, physical therapy might help, for example.
While gait speed is a tried-and-true indication, collecting answers about stair climbing difficulty is easier and quicker for clinicians than assessing gait speed, which requires more time as well as office space, Mr. Jakiela said. It’s also intuitive for the patients to recall, the researchers said.
More practical takeaways
Finding out whether functional limitation is likely, based on the stair question, can help health care providers consider nonpharmacologic interventions, Dr. Kwoh agreed, such as physical therapy or braces. “It doesn’t have to be drugs. We have limited drugs for OA at the moment. We don’t have a so-called DMARD drug [for OA].”
NSAIDs have side effects, and people are very familiar with the issues of opioids, he said. It’s important, he added, for the health care provider, if referring to a physical therapist, to find the right one. To help those dealing with knee OA, a PT in sports medicine might be a good choice, he said.
Mr. Jakiela has no disclosures. Dr. Kwoh and Dr. White have no relevant disclosures.
FROM ACR 2022