User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Physician couples draft wills, face tough questions amid COVID-19
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”
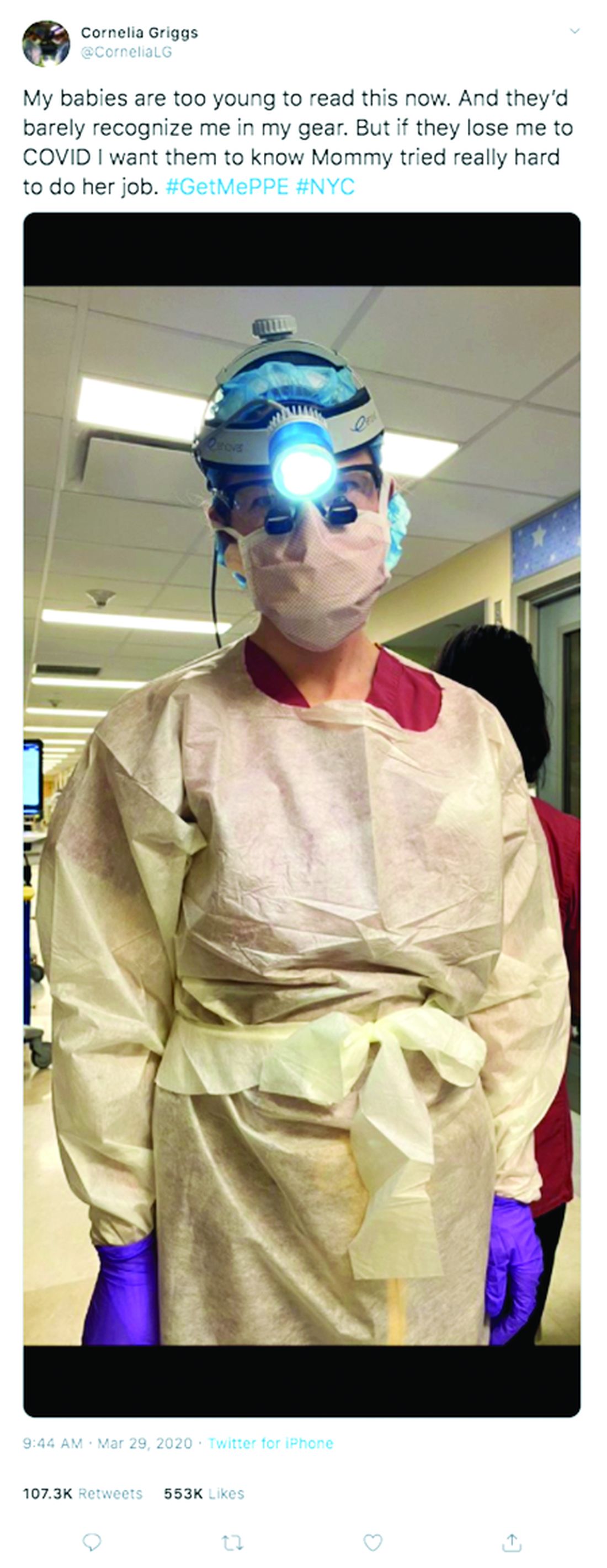
“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Not long ago, weekends for Cornelia Griggs, MD, meant making trips to the grocery store, chasing after two active toddlers, and eating brunch with her husband after a busy work week. But life has changed dramatically for the family since the spread of COVID-19. On a recent weekend, Dr. Griggs and her husband, Robert Goldstone, MD, spent their days off drafting a will.
“We’re both doctors, and we know that health care workers have an increased risk of contracting COVID,” said Dr. Griggs, a pediatric surgery fellow at Columbia University Irving Medical Center in New York. “It felt like the responsible thing to do: Have a will in place to make sure our wishes are clear about who would manage our property and assets, and who would take care of our kids – God forbid.”
Outlining their final wishes is among many difficult decisions the doctors, both 36, have been forced to make in recent weeks. Dr. Goldstone, a general surgeon at Massachusetts General Hospital in Boston, is no longer returning to New York during his time off, said Dr. Griggs, who has had known COVID-19 exposures. The couple’s children, aged 4 and almost 2, are temporarily living with their grandparents in Connecticut to decrease their exposure risk.
“I felt like it was safer for all of them to be there while I was going back and forth from the hospital,” Dr. Griggs said. “My husband is in Boston. The kids are in Connecticut and I’m in New York. That inherently is hard because our whole family is split up. I don’t know when it will be safe for me to see them again.”
Health professional couples across the country are facing similar challenges as they navigate the risk of contracting COVID-19 at work, while trying to protect their families at home. From childcare dilemmas to quarantine quandaries to end-of-life considerations, partners who work in health care are confronting tough questions as the pandemic continues.
The biggest challenge is the uncertainty, says Angela Weyand, MD, an Ann Arbor, Mich.–based pediatric hematologist/oncologist who shares two young daughters with husband Ted Claflin, MD, a physical medicine and rehabilitation physician. Dr. Weyand said she and her husband are primarily working remotely now, but she knows that one or both could be deployed to the hospital to help care for patients, if the need arises. Nearby Detroit has been labeled a coronavirus “hot spot” by the U.S. Surgeon General.
“Right now, I think our biggest fear is spreading coronavirus to those we love, especially those in higher risk groups,” she said. “At the same time, we are also concerned about our own health and our future ability to be there for our children, a fear that, thankfully, neither one of us has ever had to face before. We are trying to take things one day at a time, acknowledging all that we have to be grateful for, and also learning to accept that many things right now are outside of our control.”
Dr. Weyand, 38, and her husband, 40, finalized their wills in March.
“We have been working on them for quite some time, but before now, there has never been any urgency,” Dr. Weyand said. “Hearing about the high rate of infection in health care workers and the increasing number of deaths in young healthy people made us realize that this should be a priority.”
Dallas internist Bethany Agusala, MD, 36, and her husband, Kartik Agusala, MD, 41, a cardiologist, recently spent time engaged in the same activity. The couple, who work for the University of Texas Southwestern Medical Center, have two children, aged 2 and 4.
“The chances are hopefully small that something bad would happen to either one of us, but it just seemed like a good time to get [a will] in place,” Dr. Bethany Agusala said in an interview. “It’s never an easy thing to think about.
Pediatric surgeon Chethan Sathya, MD, 34, and his wife, 31, a physician assistant, have vastly altered their home routine to prevent the risk of exposure to their 16-month-old daughter. Dr. Sathya works for the Northwell Health System in New York, which has hundreds of hospitalized patients with COVID-19, Dr. Sathya said in an interview. He did not want to disclose his wife's name or institution, but said she works in a COVID-19 unit at a New York hospital.
When his wife returns home, she removes all of her clothes and places them in a bag, showers, and then isolates herself in the bedroom. Dr. Sathya brings his wife meals and then remains in a different room with their baby.
“It’s only been a few days,” he said. “We’re going to decide: Does she just stay in one room at all times or when she doesn’t work for a few days then after 1 day, can she come out? Should she get a hotel room elsewhere? These are the considerations.”
They employ an older nanny whom they also worry about, and with whom they try to limit contact, said Dr. Sathya, who practices at Cohen Children’s Medical Center. In a matter of weeks, Dr. Sathya anticipates he will be called upon to assist in some form with the COVID crisis.
“We haven’t figured that out. I’m not sure what we’ll do,” he said. “There is no perfect solution. You have to adapt. It’s very difficult to do so when you’re living in a condo in New York.”
For Dr. Griggs, life is much quieter at home without her husband and two “laughing, wiggly,” toddlers. Weekends are now defined by resting, video calls with her family, and exercising, when it’s safe, said Dr. Griggs, who recently penned a New York Times opinion piece about the pandemic and is also active on social media regarding personal protective equipment. She calls her husband her “rock” who never fails to put a smile on her face when they chat from across the miles. Her advice for other health care couples is to take it “one day at a time.”

“Don’t try to make plans weeks in advance or let your mind go to a dark place,” she said. “It’s so easy to feel overwhelmed. The only way to get through this is to focus on surviving each day.”
Editor's Note, 3/31/20: Due to incorrect information provided, the hospital where Dr. Sathya's wife works was misidentified. We have removed the name of that hospital. The story does not include his wife's employer, because Dr. Sathya did not have permission to disclose her workplace and she wishes to remain anonymous.
Renal denervation shown safe and effective in pivotal trial
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
Catheter-based renal denervation took a step closer to attaining legitimacy as a nonpharmacologic treatment for hypertension with presentation of the primary results of the SPYRAL HTN-OFF MED pivotal trial at the joint scientific sessions of the American College of Cardiology and the World Heart Federation. The meeting was conducted online after its cancellation because of the COVID-19 pandemic.
“We saw clinically meaningful blood pressure reductions at 3 months,” reported Michael Boehm, MD, chief of cardiology at Saarland University Hospital in Homburg, Germany.
That’s encouraging news, as renal denervation (RDN) was nearly abandoned as a potential treatment for hypertension in the wake of the unexpectedly negative results of the SYMPLICITY HTN-3 trial (N Engl J Med. 2014;370:1393-401). However, post hoc analysis of the trial revealed significant shortcomings in design and execution, and a more rigorous development program for the percutaneous device-based therapy is well underway.
The SPYRAL HTN-OFF MED pivotal trial was designed under Food and Drug Administration guidance to show whether RDN reduces blood pressure in patients with untreated hypertension. The prospective study included 331 off-medication patients in nine countries who were randomized to RDN or a sham procedure, then followed in double-blind fashion for 3 months.
The primary outcome was change in 24-hour ambulatory systolic blood pressure from baseline to 3 months. From a mean baseline 24-hour ambulatory blood pressure of 151.4/98 mm Hg, patients in the RDN group averaged a 4.7 mm Hg decrease in 24-hour SBP, which was 4 mm Hg more than in sham-treated controls. Statistically, this translated to a greater than 99.9% probability that RDN was superior to sham therapy. The RDN group also experienced a mean 3.7–mm Hg reduction in 24-hour DBP, compared with a 0.8–mm Hg decrease in controls.
Office SBP – the secondary endpoint – decreased by a mean of 9.2 mm Hg with RDN, compared with 2.5 mm Hg in controls.
These results probably understate the true antihypertensive effect of RDN for two reasons, Dr. Boehm noted. For one, previous studies have shown that the magnitude of blood pressure lowering continues to increase for up to 1-2 years following the procedure, whereas the off-medication assessment in SPYRAL HTN-OFF MED ended at 3 months for ethical and safety reasons. Also, 17% of patients in the control arm were withdrawn from the study and placed on antihypertensive medication because their office SBP reached 180 mm Hg or more, as compared to 9.6% of the RDN group.
A key finding was that RDN lowered blood pressure around the clock, including nighttime and early morning, the hours of greatest cardiovascular risk and a time when some antihypertensive medications are less effective at blood pressure control, the cardiologist observed.
The RDN safety picture was reassuring, with no strokes, myocardial infarctions, major bleeding, or acute deterioration in kidney function.
A surprising finding was that, even though participants underwent blood and urine testing for the presence of antihypertensive drugs at baseline to ensure they were off medication, and were told they would be retested at 3 months, 5%-9% nonetheless tested positive at the second test.
That elicited a comment from session chair Richard A. Chazal, MD, of Fort Myers, Fla.: “I must say, as a clinician who sometimes has trouble getting his patients to take antihypertensives, it’s fascinating that some of the people that you asked not to take the medications were taking them.”
While the primary outcome in SPYRAL HTN-OFF MED was the 3-month reduction in blood pressure while off of antihypertensive medication, the ongoing second phase of the trial may have greater clinical relevance. At 3 months, participants are being placed on antihypertensive medication and uptitrated to target, with unblinding at 6 months. The purpose is to see how many RDN recipients don’t need antihypertensive drugs, as well as whether those that do require less medication than the patients who didn’t undergo RDN.
Dr. Boehm characterized RDN as a work in progress. Two major limitations that are the focus of intense research are the lack of a predictor as to which patients are most likely to respond to what is after all an invasive procedure, and the current inability intraprocedurally to tell if sufficient RDN has been achieved.
“Frankly speaking, there is no technology during the procedure to see how efficacious the procedure was,” he explained.
Discussant Dhanunaja Lakkireddy, MD, deemed the mean 4.7–mm Hg reduction in 24-hour SBP “reasonably impressive – that’s actually a pretty good number for an antihypertensive clinical trial.” He was also favorably impressed by RDN’s safety in a 44-site study.
“The drops in blood pressure are not enough to really make a case for renal denervation to be a standalone therapy. But adding it as an adjunct to standard medications may be a very reasonable strategy to adopt. This is a fantastic signal for something that can be brought along as a long-term add-on to antihypertensive medications,” commented Dr. Lakkireddy, chair of the ACC Electrophysiology Council and medical director of the Kansas City Heart Rhythm Institute.
Simultaneous with Dr. Boehm’s presentation, the SPYRAL HTN-OFF MED Pivotal Trial details were published online (Lancet 2020 Mar 29. doi: 10.1016/S0140-6736(20)30554-7).
The study was sponsored by Medtronic. Dr. Boehm reported serving as a consultant to that company and Abbott, Amgen, Astra, Boehringer-Ingelheim, Cytokinetics, Novartis, ReCor, Servier, and Vifor.
SOURCE: Boehm M. ACC 2020, Abstract 406-15.
REPORTING FROM ACC 20
Before the COVID-19 surge hits your facility, take steps to boost capacity
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
, according to a physician leader and a health workforce expert.
Polly Pittman, PhD, is hearing a lot of concern among health care workers that it’s difficult to find definitive and accurate information about how best to protect themselves and their families, she said during a webinar by the Alliance for Health Policy titled Health System Capacity: Protecting Frontline Health Workers. “The knowledge base is evolving very quickly,” said Dr. Pittman, Fitzhugh Mullan Professor of Health Workforce Equity at the Milken Institute School of Public Health, George Washington University, Washington.
Stephen Parodi, MD, agreed that effective communication is job one in the health care workplace during the crisis. “I can’t stress enough ... that communications are paramount and you can’t overcommunicate,” said Dr. Parodi, executive vice president of external affairs, communications, and brand at the Permanente Federation and associate executive director of the Permanente Medical Group, Vallejo, Calif.
“We’re in a situation of confusion and improvisation right now,” regarding protection of health care workers, said Dr. Pittman. The potential exists for “a downward spiral where you have the lack of training, the shortages in terms of protective gear, weakening of guidelines, and confusion regarding guidelines at federal level, creating a potential cascade” that may result in “moral distress and fatigue. ... That’s not occurring now, but that’s the danger” unless the personal protective equipment (PPE) situation is adequately addressed very soon, she said.
Dr. Pittman also pointed out the concerns that many of the 18 million U.S. health care workers have for their families should they themselves fall ill or transmit coronavirus to family members. “The danger exists of a mass exodus. People don’t have to show up at work, and they won’t show up at work if they don’t feel supported and safe.”
Dr. Parodi said that the Permanente organization is on a better footing than many workplaces. “We actually had an early experience because of the work that we did to support the Diamond Princess cruise ship evacuees from Yokahama in February.” That ship was quarantined upon arrival in Yokahama on Feb. 3 because a passenger had a confirmed test for SARS-CoV-2 infection, and a quarter of the 428 Americans on board subsequently tested positive. Most of them were evacuated to California or Texas. “That actually gave us the experience for providing care within the hospital setting – and also for containment strategies,” he said.
“We quickly understood that we needed to move to a mitigation strategy,” said Dr. Parodi. Use of PPE has been “tailored for how the virus is spread.” In the absence of the risk of aerosol transmission from certain procedures, health care workers use gowns, gloves, surgical masks, and goggles.
Because of anticipated “supply chain shortfalls,” Dr. Parodi said that his organization implemented Centers for Disease Control and Prevention guidelines for reuse and extended use of N95 respirators early on. “Even if you’re not in a locale that’s been hit, you need to be on wartime footing for preserving PPE.”
Telehealth, said Dr. Parodi, has been implemented “in a huge way” throughout the Permanente system. “We have reduced primary care visits by 90% in the past week, and also subspecialty visits by 50%. … A large amount of the workforce can work from home. We turned off elective surgeries more than a week ago to reduce the number of patients who are requiring intensive care.” Making these changes means the organization is more prepared now for a surge they expect in the coming weeks.
Dr. Pittman voiced an opinion widely shared by those who are implementing large-scale telehealth efforts “We’re going to learn a lot. Many of the traditional doctor-patient visits can be done by telemedicine in the future.”
Knowledge about local trends in infection rates is key to preparedness. “We’ve ramped up testing, to understand what’s happening in the community,” said Dr. Parodi, noting that test turnaround time is currently running 8-24 hours. Tightening up this window can free up resources when an admitted patient’s test is negative.
Still, some national projections forecast a need for hospital beds at two to three times current capacity – or even more, said Dr. Parodi.
He noted that Permanente is “working hand in glove with state authorities throughout the country.” Efforts include establishing alternative sites for assessment and testing, as well as opening up closed hospitals and working with the National Guard and the Department of Defense to prepare mobile hospital units that can be deployed in areas with peak infection rates. “Having all of those options available to us is critically important,” he said.
To mitigate potential provider shortages, Dr. Pittman said, “All members of the care team could potentially do more” than their current licenses allow. Expanding the scope of practice for pharmacists, clinical laboratory staff, licensed practical nurses, and medical assistants can help with efficient care delivery.
Other measures include expedited licensing for near-graduates and nonpracticing foreign medical graduates, as well as relicensing for retired health care personnel and those who are not currently working directly with patients, she said.
Getting these things done “requires leadership on behalf of the licensing bodies,” as well as coordination with state regulatory authorities, Dr. Pittman pointed out.
Dr. Parodi called for state and federal governments to implement emergency declarations that suspend some existing health codes to achieve repurposing of staff. Getting these measures in place now will allow facilities “to be able to provide that in-time training now before the surge occurs. ... We are actively developing plans knowing that there’s going to be a need for more critical care.”
The game plan at Permanente, he said, is to repurpose critical care physicians to provide consultations to multiple hospitalists who are providing the bulk of frontline care. At the same time, they plan to repurpose other specialists to backfill the hospitalists, and to repurpose family medicine physicians to supplement staff in emergency departments and other frontline intake areas.
All the organizational measures being taken won’t be in vain if they increase preparedness for the long battle ahead, he said. “We need to double down on the work. ... We need to continue social distancing, and we’ve got to ramp up testing. Until we do that we have to hold the line on basic public health measures.”
Dr. Parodi is employed by Permanente. The panelists reported no disclosures relevant to the presentation, which was sponsored by the Alliance for Health Policy, the Commonwealth Fund, and the National Institute for Health Care Management Foundation.
REPORTING FROM AN ALLIANCE FOR HEALTH POLICY WEBINAR
FDA okays emergency use of convalescent plasma for seriously ill COVID-19 patients
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
Wilkie and the VA vs COVID-19: Who’s Winning?
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”
US Department of Veterans Affairs (VA) Secretary Robert Wilkie is finding out what it means to be on wartime footing against a virus. He is overseeing the VA’s internal response to COVID-19 while deciding how to fulfil the VA’s fourth mission: providing reinforcement for the nation’s healthcare system in a national emergency. Meanwhile, he’s facing hostilities on a third front: criticism of his efforts so far.
In late February, when lawmakers asked whether the VA needed more resources to fight COVID-19, Wilkie said no. He told NPR on March 19 that “we are poised for the onslaught.” But on March 13, 2020, the VA was being attacked for not releasing a comprehensive emergency response to the incipient pandemic. Wilkie countered, “Before there was a single confirmed case in the US,” he wrote in a recent op-ed piece for Military Times, “the VA was already conducting emergency preparedness exercises.”
In the NPR interview, Wilkie said the VA had undertaken “a very aggressive public health response at an early stage.” Now, the VA has added other measures. The VA, he said, was the first health system to stop people from entering its facilities without being questioned or tested, and the first to adopt the “hard decision” of a no-visitor rule for veterans in nursing homes. Every veteran who comes to a VA facility with flu-like symptoms is screened. Further, via tweets and blog posts, Wilkie is “inviting” retired medical personnel back to work to help deal with the pandemic.
The VA is also the “buttress force,” Wilkie says, for the Federal Emergency Management Agency and the US Department of Health and Human Services if they need medical professionals for crises. “We plan for that every day,” he says. “We are gaming out emergency preparedness scenarios and we stand ready when the President needs us to expand our mission.” But in The American Prospect, Suzanne Gordon and Jasper Craven, both fellows at the Veterans Healthcare Policy Institute, write that “one quiet action is ominous”—the VA website has deleted any mention of the department’s credo of caring for civilians in times of crisis.
According to Gordon and Craven, on Wednesday Wilkie “came out of the woodwork” to express the department’s readiness to help in the crisis. The VA has established 19 emergency operations centers across the country, Wilkie says, and has stopped elective surgeries to free up thousands of beds. He touts the agency’s flexibility, saying it’s prepared to move resources around the country as needed. “Some veterans hospitals have not been impacted [by the virus],” Wilkie said. “So, I’m not going to keep 500 respirators in the middle of a state that has one veteran with the infection, when I can use that in Seattle or New Orleans, or New York City.”
Wilkie says the VA has stockpiled equipment and its supply chain is stable. However, in the NPR interview, Mary Louise Kelly said the NPR VA correspondent had been hearing complaints about lack of gear, such as masks. When pressed on his claim that the VA had adequate protective supplies, Wilkie said those complaints “have not reached us.” In fact, he said, “I can tell you that the arrangements that we have made on both the masks side and also on the testing side—we’re in a very good place.”
Nonetheless, on March 16, the employee unions representing nearly 350,000 VA healthcare workers issued a joint statement that called on VHA management to “work with us to ensure the nation’s VA health facilities can safely handle COVID-19.” It’s time, said Everett Kelley, National President of the American Federation of Government Employees, “for the VA to invite our members to the table, instead of kicking them off the property, so we can finally work together on a solution….”
“Instead of relaxing standards and efforts,” the unions said, “like we have seen the CDC do [in allowing healthcare workers to reuse facemasks and rely on simple surgical facemasks], “we need to be stepping it up.”
It all takes money. After weeks of debate, the US Senate has just released details of the $2 trillion coronavirus aid package. The US Department of Defense (DoD) seems about to get $10.5 billion in emergency funding and the VA another $19.6 billion. The money includes funding for National Guard deployments to help state governments respond to emerging health needs, the expansion of military hospitals and mobile medical centers if needed, and help with production of medical supplies. Nearly $16 billion will be used for direct care specifically in response to veterans’ health needs, covering treatment for COVID-19 in VA hospitals, community urgent care clinics and emergency departments; overtime for clinical staff; and purchase of protective equipment, tests, and other supplies.
Despite having one of the best telehealth systems in the US, the VA has also come under fire for its telehealth preparations to meet the current pandemic-related demand. Former VA Under Secretary of Health Kenneth Kizer wrote in an op-ed for Military Times, “Regrettably, so far, there is no coordinated strategy for ramping up and optimizing the use of telehealth to combat the growing epidemic in the US.” The relief package proposes $3 billion for new telemedicine efforts, including staffing and equipping mobile treatment sites.
In mid-March, the VA had 3,000 coronavirus test kits but still had not used roughly 90%, an article in Mother Jones charged. At a White house press conference around that time, Wilkie was asked how many veterans of those who needed to be tested had been. “We believe we’ve caught most of them,” he replied.
But that was in the early days of the crisis.
With results from the 322 tests administered by Mar. 18, the VA had confirmed five positive cases, was tracking 33 presumptive cases, and acknowledged the first veteran death linked to COVID-19. As of Mar. 26, the VA had administered roughly 7,500 COVID-19 tests nationwide.
Secretary Wilkie has promised that the department’s first focus will always be caring for veterans. In an interview with Military Times, he said, “We don’t release any beds if veterans are needing them. The veterans still are primary. We are a [health] bridge for the larger community, but that’s only after veterans are taken care of.”
Reports suggest possible in utero transmission of novel coronavirus 2019
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
FROM JAMA
Doctors sound off about future of medical meetings
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
As most 2020 medical conferences have, one by one, been canceled or rescheduled as virtual meetings in the time of a pandemic, some physicians and other healthcare professionals are wondering if this is the year that will change the scene forever.
Amid the choruses of resignation (“Unfortunately, it’s the right thing to do.”) and optimism (“See you next year!”), there have been plenty of voices describing another broad sentiment – that all was not well with medical meetings even before the coronavirus.
One dominant criticism is that there are too many meetings.
Indeed, there are many, many meetings. During 2005–2015, there were 30,000-plus medical meetings in the United States, according to a report from the Healthcare Convention and Exhibitors Association.
Most of those are of little value, tweeted Dhruv Khullar, MD, an internist at Weill Cornell Medicine, New York City (@DhruvKhullar): “One possible consequence of cancelling so many meetings due to #COVID19 is that we realize we probably don’t need most of them.”
The tweet was liked 1.9K times, which is high for a medical post. Comments were mostly in agreement, with some skepticism.
Michaela West, MD, PhD, a surgeon at North Memorial Health, Minneapolis, Minnesota, responded (@MichaelaWst): “Agree. COVID-19 may forever change our perspective regarding medical professional meetings.”
Nwando Olayiwola, MD, chair of family medicine, Ohio State University, Columbus, strongly agreed (@DrNwando): “This is the tweet I wish I tweeted.”
However, Kelly Swords, MD, MPH, urologist, University of California, San Diego, in a dissenting opinion, stated the obvious (@k_dagger): “Except there is no substitute for human interaction.”
Worth the Effort?
The cancellation of medical meetings has given those who regularly attend an opportunity to reassess their value and to question the worth of the effort involved in attending in person.
David Steensma, MD, hematologist-oncologist, Harvard Medical School, Boston, (@DavidSteensma) tweeted that he would like to scale back: “The present crisis is an opportunity to reassess what is actually necessary and rebalance [in terms of meetings].”
Travel to meetings is often unpleasant, said others.
Chris Palatucci, life sciences executive recruiter, Coulter Partners, Boston, tweeted (@LifeSciRcruitr): “I will die a happy man if I never get on another plane. Glorified bus travel.” He also believes that once the coronavirus crisis is over, its “silver lining” will be the realization that “40% of all meetings are unnecessary.”
Many professionals have welcomed the announcements that major conferences have been canceled and will be conducted virtually.
The latest change is from the American Society of Clinical Oncology (ASCO), whose annual meeting was to be held in Chicago at the end of May but will now be held online.
Virtual ASCO will be more manageable – and comfy, said Fumiko Ladd Chino, MD, radiation oncologist, Memorial Sloan Kettering Cancer Center, New York City.
She (@fumikochino) explained why in a recent tweet: “1) I will be finally able to see ALL OF THE PRESENTATIONS I wanted to see instead of wandering around feeling overwhelmed. 2) I will be able to FOCUS on the presentations and not searching for a power outlet. 3) PAJAMAS.”
Virtual meetings already beat real meetings, added Adriana Scheliga, MD, hematologist-oncologist, Brazilian National Cancer Institute (@linfopedia): “I’ve been saying this for a while. For me the best ASCO Meetings, for example, are the “virtual meetings!”
However, meetings in place are also very much about professional community and mutual support, reminds Susan E. Sedory, MA, executive director, Society of Interventional Radiology, which canceled its meeting March 6 in a multifaceted process described by Medscape Medical News.
Is This the Time to Evaluate Meetings?
Coming up soon is the first major conference to go virtual after being canceled – the American College of Cardiology (ACC), which has been one of the top 20 largest meetings in the United States by attendance.
This meeting, which was to have taken place in Chicago on March 28–30, will now occur online on those days. The ACC says it will stream all “live” sessions on demand and provide access to additional videos, abstracts, and slides for at least 90 days after the meeting. And it will be free to anyone with an Internet connection.
Medical meetings in distant locales may bounce back, as they have grown into a very big business. ASCO is illustrative.
The group’s first scientific annual meeting was held in 1965 in Philadelphia, with about 70 members and invited guests in attendance. Fast forward 50-plus years to 2019: there were 42,500 attendees, a 4.4% increase from 2018. Notably, the top countries in attendance in 2019 were the United States and China.
Not everyone is happy that canceled meetings are being held online in the middle of a pandemic.
“In a COVID-19 world, the brain cannot focus on nonviral topics,” said commentator John Mandrola, MD, Baptist Health, Louisville, Kentucky, in his regular column for Medscape Cardiology/theheart.org.
The virtual ACC meeting should be canceled or delayed – to mirror what is happening in the world, he argues. “In hospitals, we have postponed the elective to make room for the coming surge. Shouldn’t ACC do the same? After the crisis passes, we can have a virtual meeting with a proper discussion of the science,” he writes.
But #MedTwitter, with its collective constructive criticism of medical meetings, is perhaps proof that the brain can function – and arrive at clarity – when under pandemic duress.
“Am I the only one experiencing a certain relief at the cancellation of multiple trips and meetings, and vowing to let this revelation affect my decision making in the future,” tweeted Steven Joffe, MD, MPH, University of Pennsylvania, Philadelphia (@Steve Joffe).
Louise Perkins King, MD, a bioethicist at Harvard Medical School, responded to Joffe. Hoping not to “belittle” the suffering from the COVID-19 pandemic, she (@louise_p_king) addressed her healthcare colleagues: “...there is potential for us all to learn what is essential travel and burden and what is not from this. I hope it leads to lasting change.”
This article first appeared on Medscape.com.
The power and promise of person-generated health data (Part II)
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1
A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
In Part I of our discussion we introduced the concept of person-generated health data (PGHD), defined as wellness and/or health-related data created, recorded, or gathered by individuals.
Such rich, longitudinal information is now being used in combination with traditional clinical information to predict, diagnose, and formulate treatment plans for diseases, as well as understand the safety and effectiveness of medical interventions.
Identifying a disease early
One novel example of digital technologies being used for early identification of disease was a promising 2019 study by Eli Lilly (in collaboration with Apple and Evidation Health) called the Lilly Exploratory Digital Assessment Study.
In this study, the feasibility of using PGHD for identifying physiological and behavioral signatures of cognitive impairment was examined for the purpose of seeking new methods to detect mild cognitive impairment (MCI) in a timely and cost-effective manner. The study enrolled 31 study participants with cognitive impairment and 82 without cognitive impairment. It used consumer-grade sensor technologies (the iPhone, Apple Watch, iPad, and Beddit sleep monitor) to continuously and unobtrusively collect data. Among the information the researchers collected were interaction with the phone keyboard, accelerometer data from the Apple Watch, volume of messages sent/received, and sleep cycles.1

A total of 16 terabytes of data were collected over the course of 12 weeks. Data were organized into a behaviorgram (See Figure 1) that gives a holistic picture of a day in a patient’s life. A machine learning model was used to distinguish between behaviorgrams of symptomatic versus healthy controls, identifying typing speed, circadian rhythm shifts, and reliance on helper apps, among other things, as differentiating cognitively impaired from healthy controls. These behaviorgrams may someday serve as “fingerprints” of different diseases, with specific diseases displaying predictable patterns. In the near future, digital measures like the ones investigated in this study are likely to be used to help clinicians predict and diagnose disease, as well as to better understand disease progression and treatment response.
Leading to better health outcomes
The potential of PGHD to detect diseases early and lead to better health outcomes is being investigated in the Heartline study, a collaboration between Johnson & Johnson and Apple, which is supported by Evidation.2
This study aims to enroll 150,000 adults age 65 years and over to analyze the impact of Apple Watch–based early detection of irregular heart rhythms consistent with atrial fibrillation (AFib). The researchers’ hypothesis is that jointly detecting atrial fibrillation early and providing cardiovascular health programs to new AFib patients, will lead to patients being treated by a medical provider for AFib that otherwise would not have been detected. This, in turn, would lead to these AFib patients decreasing their risks of stroke and other serious cardiovascular events, including death, the study authors speculated.
Presenting new challenges
While PGHD has the potential to help people, it also presents new challenges. It is highly sensitive and personal – it can be as identifying as DNA.3
The vast amount of data that PGHD can collect from interaction with consumer wearable devices poses serious privacy risks if done improperly. To address those risks, companies like Evidation have built in protections. Evidation has an app, Achievement, that has enlisted a connected population of more than 3.5 million members who earn rewards for performing health-related actions, as tracked by wearables devices and apps. Through the Achievement app (See Figure 2.), members are provided opportunities to join research studies. As part of these studies, data collected from sensors and apps is used by permission of the member so that it is clear how their data are contributing to specific research questions or use cases.
This is a collaborative model of data collection built upon trust and permission and is substantially different than the collection of data from electronic health records (EHRs) – which is typically aggregated, deidentified, and commercialized, often without the patients’ knowledge or consent. Stringent protections, explicit permission, and transparency are absolutely imperative until privacy frameworks for data outside of HIPAA regulation catches up and protects patients from discrimination and unintended uses of their data.
Large connected cohorts can help advance our understanding of public health. In one study run on Achievement during the 2017-2018 flu season, a survey was sent to the Achievement population every week asking about symptoms of influenza-like illness and requesting permission to access historical data from their wearable around the influenza-like illness event.4 With the data, it was possible to analyze patterns of activity, sleep, and resting heart rate change around flu events. Resting heart rate, in particular, is shown to increase during fever and at the population level. In fact, through the use of PGHD, it is possible to use the fraction of people with resting heart rate above their usual baseline as a proxy to quantify the number of infected people in a region.5 This resting heart rate–informed flu surveillance method, if refined to increased accuracy, can work in near real time. This means it may be able detect influenza outbreaks days earlier than current epidemiological methods.
Health data generated by connected populations are in the early stages of development. It is clear that it will yield novel insights into health and disease. Only time will tell if it will be able to help clinicians and patients better predict, diagnose, and formulate treatment plans for disease.
Neil Skolnik, M.D. is a professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, and associate director of the Family Medicine Residency Program at Abington Jefferson Health. Luca Foschini PhD, is co-founder & chief data scientist at Evidation Health. Bray Patrick-Lake, MFS, is a patient thought leader and director of strategic partnerships at Evidation Health.
References
1. Chen R et al. Developing measures of cognitive impairment in the real world from consumer-grade multimodal sensor streams. KDD ’19. August 4–8, 2019 Aug 4-8.
2. The Heartline Study. https://www.heartline.com.
3. Foschini L. Privacy of Wearable and Sensors Data (or, the Lack Thereof?). Data Driven Investor, Medium. 2019.
4. Bradshaw B et al. Influenza surveillance using wearable mobile health devices. Online J Public Health Inform. 2019;11(1):e249.
5. Radin JM et al. Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a population-based study. Lancet Digital Health. 2020. doi: 10.1016/S2589-7500(19)30222-5.
Targeting gut bacteria may improve levodopa uptake
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
Differences in metabolism of levodopa between patients with Parkinson’s disease may be caused by variations in gut bacteria, according to investigators.
Specifically, patients with a higher abundance of Enterococcus faecalis may be converting levodopa into dopamine via decarboxylation before it can cross the blood-brain barrier, reported Emily P. Balskus, PhD, of Harvard University in Cambridge, Mass.
Although existing decarboxylase inhibitors, such as carbidopa, can reduce metabolism of levodopa by host enzymes, these drugs are unable to inhibit the enzymatic activity of E. faecalis in the gut, Dr. Balskus said at the annual Gut Microbiota for Health World Summit, sponsored by the American Gastroenterological Association and the European Society for Neurogastroenterology and Motility.
“[Carbidopa] is actually completely ineffective at inhibiting decarboxylation in human fecal suspension,” Dr. Balskus said, referring to research led by PhD student Vayu Maini Rekdal. “We think that this could indicate that patients who are taking carbidopa are not inhibiting any bacterial metabolism that they may have.”
While previous research showed that E. faecalis could decarboxylate levodopa, Dr. Balskus and colleagues linked this process with the tyrosine decarboxylase gene (TyrDC), and showed that the of abundance E. faecalis and TyrDC correlate with levodopa metabolism.
Unlike the human enzyme responsible for decarboxylation of levodopa, the E. faecalis enzyme preferentially binds with L-tyrosine. This could explain why existing decarboxylase inhibitors have little impact on decarboxylation in the gut, Dr. Balskus said.
She also noted that this unique characteristic may open doors to new therapeutics. In the lab, Dr. Balskus and colleagues tested a decarboxylase inhibitor that resembled L-tyrosine, (S)-alpha-fluoromethyltyrosine (AFMT). Indeed, AFMT completely inhibited of decarboxylation of levodopa in both E. faecalis cells and complex human microbiome samples.
“We think this is pretty exciting,” Dr. Balskus said.
Early animal studies support this enthusiasm, as germ-free mice colonized with E. faecalis maintain higher serum levels of levodopa with concurrent administration of AFMT.
“We think that this could indicate that a promising way to improve levodopa therapy for Parkinson’s patients would be to develop compounds that inhibit bacterial drug metabolism activity,” Dr. Balskus said.
Concluding her presentation, Dr. Balskus emphasized the importance of a biochemical approach to microbiome research. “Studying enzymes opens up new, exciting opportunities for microbiome manipulation. We can design or develop inhibitors of enzymes, use those inhibitors as tools to study the roles of individual metabolic activities, and potentially use them as therapeutics. We are very excited about the possibility of treating or preventing human disease not just by manipulating processes in our own cells, but by targeting activities in the microbiota.”
Dr. Balskus reported funding from HHMI, the Bill and Melinda Gates Foundation, the David and Lucile Packard Foundation, and Merck.
FROM GMFH 2020
Despite strict controls, some infants born to mothers with COVID-19 appear infected
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
FROM JAMA PEDIATRICS