User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Wuhan data link COVID-19 with myocardial damage
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
The first data on myocardial injury linked with COVID-19 disease during the start of the pandemic in Wuhan, China serves as a “wake up call” for clinicians and the general public on what the United States and other Western countries can expect as the SARS-CoV-2 virus spreads and case numbers mount: a potentially “daunting” toll of deaths as an infection with a tendency to be most severe in patients with underlying cardiovascular disease hits populations that include large numbers of such patients.
“A consistent picture emerges” from two reports on a total of 603 COVID-19 patients treated at two academic hospitals in Wuhan, which described “remarkably similar characteristics of patients who develop myocardial injury” associated with their infection. “Patients who develop myocardial injury with COVID-19 have clinical evidence of higher acuity, with a higher incidence of acute respiratory distress syndrome and more frequent need for assisted ventilation than those without myocardial injury, and the patients who are more prone to have myocardial injury are “older patients with preexisting cardiovascular complications and diabetes,” Robert O. Bonow, MD, and coauthors wrote in an editorial published online (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1105).
These new findings have special relevance to the United States and other Western countries because of their substantial numbers of elderly patients with cardiovascular diseases, said Dr. Bonow, professor of medicine at Northwestern University, Chicago, and coauthors.
One of the two reports cited in the editorial reviewed 416 patients hospitalized at Renmin Hospital in Wuhan during the period of Jan. 20 to Feb. 10, 2020, with confirmed COVID-19 disease, and found that 20% of the cohort had evidence of cardiac injury, defined as blood levels of the high-sensitivity troponin I cardiac biomarker above the 99th-percentile upper reference limit, regardless of new abnormalities in electrocardiography and echocardiography.
The analysis also showed that patients with myocardial injury had a significantly higher in-hospital mortality rate, 51%, compared with a 5% mortality rate among patients without myocardial injury, and among patients with myocardial injury those with elevated high-sensitivity troponin I had an even higher mortality rate (JAMA Cardiol. 2020 Mar 25. doi: 10.1001/jamacardio.2020.0950).
A second review of 187 confirmed COVID-19 cases at Seventh Hospital in Wuhan during the period of Jan. 23 to Feb. 23, 2020, showed similar findings, with a 28% prevalence of myocardial injury at admission based on an elevated level of plasma troponin T (TnT), and 35% had cardiovascular disease (CVD) including hypertension, coronary heart disease, and cardiomyopathy. Elevated TnT levels and CVD at entry each linked with substantially increased mortality. The incidence of death among patients with elevated TnT and no underlying CVD was 38% compared with 8% among patients without elevated TnT or underlying CVD. Among patients admitted with underlying CVD those who also had an elevated TnT had a 69% death rate during hospitalization compared with a 13% rate in those without TnT elevation (JAMA Cardiol. 2020 Mar 27. doi: 10.1001/jamacardio.2020.1017).
Dr. Bonow and coauthors noted that patients with chronic coronary artery disease have a heightened risk for developing acute coronary syndrome during acute infection, potentially resulting from a severe increase in myocardial demand during infection, or severe systemic inflammatory stress that could result in atherosclerotic plaque instability and rupture as well as vascular and myocardial inflammation.
In addition, patients with heart failure are prone to hemodynamic instability during severe infection. “Thus it is anticipated that patients with underlying cardiovascular diseases, which are more prevalent in older adults, would be susceptible to higher risks of adverse outcomes and death during the severe and aggressive inflammatory responses to COVID-19 than individuals who are younger and healthier,” they wrote.
They also cited the potential for acute or fulminant myocarditis as well as new-onset heart failure caused by the SARS-CoV-2 virus that causes COVID-19 disease based on experience with the related Middle East respiratory syndrome coronavirus. Another concerning observation is that the SARS-CoV-2 virus binds to the angiotensin-converting enzyme 2 protein on cell surfaces as its main entry receptor, “raising the possibility of direct viral infection of vascular endothelium and myocardium,” a process that itself could produce myocardial injury and myocarditis.
These new findings from COVID-19 patients in Wuhan represent early data from what has become a global pandemic, and raise questions about generalizability, but for the time being a key message from these early cases is that prevention of SARS-CoV-2 infection is paramount. “Until we know more, the populations described in these primary data reports should be most observant of strict hand hygiene, social distancing, and, where available, COVID-19 testing,” the authors said.
Dr. Bonow and coauthors had no disclosures.
FROM JAMA CARDIOLOGY
Rheumatologists seek to reassure amid hydroxychloroquine shortage
Physicians and pharmacists are reporting shortages of hydroxychloroquine and chloroquine following President Trump’s promotion of the medications as potential COVID-19 treatments, leaving patients with rheumatic diseases wondering how it will impact their access.
The American Medical Association, the American Pharmacists Association, and the American Society of Health-System Pharmacists, issued a joint statement that strongly opposed prophylactic prescribing of these medications for COVID-19 or stockpiling them in anticipation of use for COVID-19. The concerns over shortages have also prompted the American College of Rheumatology, American Academy of Dermatology, Arthritis Foundation, and Lupus Foundation of America to send a joint statement to the Trump administration and the nation’s governors highlighting critical hydroxychloroquine access issues and asking policymakers to work together with health care providers and patient communities to ensure continued availability of these drugs.
Now
In a Q and A interview, NYU Langone Health rheumatology division director and Lupus Center director Jill P. Buyon, MD, and associate professor of rheumatology, Peter M. Izmirly, MD, noted that, while shortages have been reported across the United States because of large increases in off-label prescribing, many of the drugs’ manufacturers have committed to donating millions of doses and/or stepping up production to meet demand.
Later in this article, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Langone Health, New York, answered questions about a new multicenter study called COLCORONA getting underway to test the anti-inflammatory drug colchicine. The answers in this Q&A have been edited for length and clarity.
Questions about hydroxychloroquine shortage
Q: What is the current situation with hydroxychloroquine in your practice?
A: We have been getting calls from our patients asking about getting refills for hydroxychloroquine. Our group has been calling local pharmacies asking about the availability of hydroxychloroquine, and we are compiling a list of pharmacies in New York with current availabilities to share with patients. We are somewhat limited by our electronic health record system, Epic, which can only send a prescription to one pharmacy, so that has placed some limitations on knowing where it is available. Some pharmacies have not had hydroxychloroquine available, while others have. We have also been encouraging patients to check online and look for mail-order possibilities for 90-day supplies.
Nearly all prescriptions are for generic hydroxychloroquine. Branded hydroxychloroquine (Plaquenil) is much more expensive, and we can run into obstacles with getting it approved by insurers, too.
Q: What are you telling patients who seek to refill their prescription or call with concerns? Is it feasible for patients to stop hydroxychloroquine or cut their dosage if necessary?
A: If someone’s been on hydroxychloroquine and has benefited from its use there’s no reason to come off it at this time, and given the possibility that it may have an effect on COVID-19, that is all the better. But we want to reassure patients that they can get the drug and that it is not difficult to manufacture.
Given the significantly higher risk of disease flare that was first described in lupus patients who discontinued hydroxychloroquine in the Canadian Hydroxychloroquine Study Group’s 1991 randomized, controlled trial, it is not advisable for patients to stop the drug.
Some patients do split their dosage day-to-day if they are taking less than 400 mg daily, such that someone taking 300 mg daily may take two 200-mg tablets one day and just one 200-mg tablet the next day, and so on. To avoid eye toxicity that can occur after years of taking the drug, hydroxychloroquine is generally prescribed based on weight at 5 mg/kg.
The drug also stays in the body for quite a while [often up to 3 months and even longer], so that is helpful for patients to know.
Given the current situation and the possibility of its effectiveness against COVID-19, it is ironic that we are actually trying to recruit older lupus patients who have had long-term stable disease while on hydroxychloroquine to a trial of stopping the drug to reduce the risk of developing the side effect of retinopathy. We want to see if patients can safely withdraw hydroxychloroquine without flaring, so we hope to not run into enrollment difficulties based on the current situation with COVID-19.
Q: How do you view the balance between having enough hydroxychloroquine for patients with lupus or other rheumatic diseases and its use in COVID-19 patients?
A: We want to reassure patients that hydroxychloroquine will be available, and there is no reason to hoard the drug or to worry excessively about being unable to obtain it. Efforts to increase production by Mylan, Teva, Sanofi, Novartis, and other manufacturers of hydroxychloroquine should really help out.
Q: Are there pharmacy restrictions on prescription amounts?
A: This is not universal at this time, but some institutions are cutting back and offering only 1-month supplies.
Colchicine COVID-19 trial underway
Dr. Pillinger, of NYU Langone Health, explored the COLCORONA study of colchicine as a treatment for people infected with COVID-19 and the worry that shortage concerns may arise for it, too.
Q: What is the general availability of colchicine and its susceptibility to shortage?
A: There are two major manufacturers of colchicine in the United States, Takeda and Hikma, who together manufacture the majority of the drug.
The greatest use of colchicine in the United States is for gout, which affects approximately 4 million Americans, but the drug is not used chronically, so a much smaller number of patients are using colchicine at any one time. Colchicine is also used for other inflammatory conditions, primarily calcium pyrophosphate crystal disease and familial Mediterranean fever (FMF is rare in the United States). Cardiologists also regularly prescribe colchicine in pericarditis for short-term use. Physicians may use it off label for other purposes, too.
Overall, the number of patients using colchicine is much larger than that for the use of hydroxychloroquine, for example, suggesting that the immediate risk of shortage could be lower. However, if individuals started using it off label, or prescribing inappropriately for the COVID-19 indication, the supply would rapidly run short.
Q: What other points are there to consider regarding the use of colchicine to treat COVID-19?
A: There is no evidence – zero – that colchicine has any benefit for COVID-19, not even case reports. There is some rationale that it might be beneficial, but that is exactly why the COLCORONA trial would be logical to try.
The COLCORONA trial is exactly the kind of trial that would be needed for assessing colchicine, and it is big enough and happening quickly enough to get an answer. But if people start to use colchicine off label, we may never know the truth.
While colchicine can be used safely in most people, it can be very problematic and requires an experienced doctor’s supervision. Overdoses can be fatal, and colchicine interacts with many drugs, all of which require dose adjustment and some of which must be stopped in order to use colchicine – it isn’t candy. Some of the other drugs being looked at for COVID-19 in fact may interact with colchicine.
Colchicine must also be dose adjusted for kidney disease, and, in some of the COVID-19 patients, kidney function changes rapidly. So again, its use would require expert supervision even if there were evidence for its utility.
The side effects of colchicine, if mis-dosed, can be very unpleasant, including nausea, vomiting, and diarrhea. Even at the apparent right dose, some people will get these side effects, so colchicine has to be something that works to make the risk/benefit ratio worth it.
Some preparations of colchicine are made combined with probenecid, a gout drug. This is even more problematic because probenecid can raise the level of drugs excreted by the kidney and could affect other treatments.
So in sum, what may be a good idea in theory can turn out to be a disastrous idea in practice, and here we have nothing but theory. This is not an agent to use randomly; the studies will be rushed out quickly and hopefully will give us the knowledge to know what to do.
Dr. Izmirly and Dr. Buyon said they have research grants with the National Institutes of Health to study hydroxychloroquine in patients with lupus and in anti–SSA/Ro-positive pregnant women with a previous child with congenital heart block. Dr. Pillinger reports that he has an investigator-initiated grant from Hikma to study colchicine in osteoarthritis.
This article was reformatted on 3/30/2020 for clarity.
Physicians and pharmacists are reporting shortages of hydroxychloroquine and chloroquine following President Trump’s promotion of the medications as potential COVID-19 treatments, leaving patients with rheumatic diseases wondering how it will impact their access.
The American Medical Association, the American Pharmacists Association, and the American Society of Health-System Pharmacists, issued a joint statement that strongly opposed prophylactic prescribing of these medications for COVID-19 or stockpiling them in anticipation of use for COVID-19. The concerns over shortages have also prompted the American College of Rheumatology, American Academy of Dermatology, Arthritis Foundation, and Lupus Foundation of America to send a joint statement to the Trump administration and the nation’s governors highlighting critical hydroxychloroquine access issues and asking policymakers to work together with health care providers and patient communities to ensure continued availability of these drugs.
Now
In a Q and A interview, NYU Langone Health rheumatology division director and Lupus Center director Jill P. Buyon, MD, and associate professor of rheumatology, Peter M. Izmirly, MD, noted that, while shortages have been reported across the United States because of large increases in off-label prescribing, many of the drugs’ manufacturers have committed to donating millions of doses and/or stepping up production to meet demand.
Later in this article, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Langone Health, New York, answered questions about a new multicenter study called COLCORONA getting underway to test the anti-inflammatory drug colchicine. The answers in this Q&A have been edited for length and clarity.
Questions about hydroxychloroquine shortage
Q: What is the current situation with hydroxychloroquine in your practice?
A: We have been getting calls from our patients asking about getting refills for hydroxychloroquine. Our group has been calling local pharmacies asking about the availability of hydroxychloroquine, and we are compiling a list of pharmacies in New York with current availabilities to share with patients. We are somewhat limited by our electronic health record system, Epic, which can only send a prescription to one pharmacy, so that has placed some limitations on knowing where it is available. Some pharmacies have not had hydroxychloroquine available, while others have. We have also been encouraging patients to check online and look for mail-order possibilities for 90-day supplies.
Nearly all prescriptions are for generic hydroxychloroquine. Branded hydroxychloroquine (Plaquenil) is much more expensive, and we can run into obstacles with getting it approved by insurers, too.
Q: What are you telling patients who seek to refill their prescription or call with concerns? Is it feasible for patients to stop hydroxychloroquine or cut their dosage if necessary?
A: If someone’s been on hydroxychloroquine and has benefited from its use there’s no reason to come off it at this time, and given the possibility that it may have an effect on COVID-19, that is all the better. But we want to reassure patients that they can get the drug and that it is not difficult to manufacture.
Given the significantly higher risk of disease flare that was first described in lupus patients who discontinued hydroxychloroquine in the Canadian Hydroxychloroquine Study Group’s 1991 randomized, controlled trial, it is not advisable for patients to stop the drug.
Some patients do split their dosage day-to-day if they are taking less than 400 mg daily, such that someone taking 300 mg daily may take two 200-mg tablets one day and just one 200-mg tablet the next day, and so on. To avoid eye toxicity that can occur after years of taking the drug, hydroxychloroquine is generally prescribed based on weight at 5 mg/kg.
The drug also stays in the body for quite a while [often up to 3 months and even longer], so that is helpful for patients to know.
Given the current situation and the possibility of its effectiveness against COVID-19, it is ironic that we are actually trying to recruit older lupus patients who have had long-term stable disease while on hydroxychloroquine to a trial of stopping the drug to reduce the risk of developing the side effect of retinopathy. We want to see if patients can safely withdraw hydroxychloroquine without flaring, so we hope to not run into enrollment difficulties based on the current situation with COVID-19.
Q: How do you view the balance between having enough hydroxychloroquine for patients with lupus or other rheumatic diseases and its use in COVID-19 patients?
A: We want to reassure patients that hydroxychloroquine will be available, and there is no reason to hoard the drug or to worry excessively about being unable to obtain it. Efforts to increase production by Mylan, Teva, Sanofi, Novartis, and other manufacturers of hydroxychloroquine should really help out.
Q: Are there pharmacy restrictions on prescription amounts?
A: This is not universal at this time, but some institutions are cutting back and offering only 1-month supplies.
Colchicine COVID-19 trial underway
Dr. Pillinger, of NYU Langone Health, explored the COLCORONA study of colchicine as a treatment for people infected with COVID-19 and the worry that shortage concerns may arise for it, too.
Q: What is the general availability of colchicine and its susceptibility to shortage?
A: There are two major manufacturers of colchicine in the United States, Takeda and Hikma, who together manufacture the majority of the drug.
The greatest use of colchicine in the United States is for gout, which affects approximately 4 million Americans, but the drug is not used chronically, so a much smaller number of patients are using colchicine at any one time. Colchicine is also used for other inflammatory conditions, primarily calcium pyrophosphate crystal disease and familial Mediterranean fever (FMF is rare in the United States). Cardiologists also regularly prescribe colchicine in pericarditis for short-term use. Physicians may use it off label for other purposes, too.
Overall, the number of patients using colchicine is much larger than that for the use of hydroxychloroquine, for example, suggesting that the immediate risk of shortage could be lower. However, if individuals started using it off label, or prescribing inappropriately for the COVID-19 indication, the supply would rapidly run short.
Q: What other points are there to consider regarding the use of colchicine to treat COVID-19?
A: There is no evidence – zero – that colchicine has any benefit for COVID-19, not even case reports. There is some rationale that it might be beneficial, but that is exactly why the COLCORONA trial would be logical to try.
The COLCORONA trial is exactly the kind of trial that would be needed for assessing colchicine, and it is big enough and happening quickly enough to get an answer. But if people start to use colchicine off label, we may never know the truth.
While colchicine can be used safely in most people, it can be very problematic and requires an experienced doctor’s supervision. Overdoses can be fatal, and colchicine interacts with many drugs, all of which require dose adjustment and some of which must be stopped in order to use colchicine – it isn’t candy. Some of the other drugs being looked at for COVID-19 in fact may interact with colchicine.
Colchicine must also be dose adjusted for kidney disease, and, in some of the COVID-19 patients, kidney function changes rapidly. So again, its use would require expert supervision even if there were evidence for its utility.
The side effects of colchicine, if mis-dosed, can be very unpleasant, including nausea, vomiting, and diarrhea. Even at the apparent right dose, some people will get these side effects, so colchicine has to be something that works to make the risk/benefit ratio worth it.
Some preparations of colchicine are made combined with probenecid, a gout drug. This is even more problematic because probenecid can raise the level of drugs excreted by the kidney and could affect other treatments.
So in sum, what may be a good idea in theory can turn out to be a disastrous idea in practice, and here we have nothing but theory. This is not an agent to use randomly; the studies will be rushed out quickly and hopefully will give us the knowledge to know what to do.
Dr. Izmirly and Dr. Buyon said they have research grants with the National Institutes of Health to study hydroxychloroquine in patients with lupus and in anti–SSA/Ro-positive pregnant women with a previous child with congenital heart block. Dr. Pillinger reports that he has an investigator-initiated grant from Hikma to study colchicine in osteoarthritis.
This article was reformatted on 3/30/2020 for clarity.
Physicians and pharmacists are reporting shortages of hydroxychloroquine and chloroquine following President Trump’s promotion of the medications as potential COVID-19 treatments, leaving patients with rheumatic diseases wondering how it will impact their access.
The American Medical Association, the American Pharmacists Association, and the American Society of Health-System Pharmacists, issued a joint statement that strongly opposed prophylactic prescribing of these medications for COVID-19 or stockpiling them in anticipation of use for COVID-19. The concerns over shortages have also prompted the American College of Rheumatology, American Academy of Dermatology, Arthritis Foundation, and Lupus Foundation of America to send a joint statement to the Trump administration and the nation’s governors highlighting critical hydroxychloroquine access issues and asking policymakers to work together with health care providers and patient communities to ensure continued availability of these drugs.
Now
In a Q and A interview, NYU Langone Health rheumatology division director and Lupus Center director Jill P. Buyon, MD, and associate professor of rheumatology, Peter M. Izmirly, MD, noted that, while shortages have been reported across the United States because of large increases in off-label prescribing, many of the drugs’ manufacturers have committed to donating millions of doses and/or stepping up production to meet demand.
Later in this article, Michael H. Pillinger, MD, a rheumatologist and professor of medicine, biochemistry, and molecular pharmacology at NYU Langone Health, New York, answered questions about a new multicenter study called COLCORONA getting underway to test the anti-inflammatory drug colchicine. The answers in this Q&A have been edited for length and clarity.
Questions about hydroxychloroquine shortage
Q: What is the current situation with hydroxychloroquine in your practice?
A: We have been getting calls from our patients asking about getting refills for hydroxychloroquine. Our group has been calling local pharmacies asking about the availability of hydroxychloroquine, and we are compiling a list of pharmacies in New York with current availabilities to share with patients. We are somewhat limited by our electronic health record system, Epic, which can only send a prescription to one pharmacy, so that has placed some limitations on knowing where it is available. Some pharmacies have not had hydroxychloroquine available, while others have. We have also been encouraging patients to check online and look for mail-order possibilities for 90-day supplies.
Nearly all prescriptions are for generic hydroxychloroquine. Branded hydroxychloroquine (Plaquenil) is much more expensive, and we can run into obstacles with getting it approved by insurers, too.
Q: What are you telling patients who seek to refill their prescription or call with concerns? Is it feasible for patients to stop hydroxychloroquine or cut their dosage if necessary?
A: If someone’s been on hydroxychloroquine and has benefited from its use there’s no reason to come off it at this time, and given the possibility that it may have an effect on COVID-19, that is all the better. But we want to reassure patients that they can get the drug and that it is not difficult to manufacture.
Given the significantly higher risk of disease flare that was first described in lupus patients who discontinued hydroxychloroquine in the Canadian Hydroxychloroquine Study Group’s 1991 randomized, controlled trial, it is not advisable for patients to stop the drug.
Some patients do split their dosage day-to-day if they are taking less than 400 mg daily, such that someone taking 300 mg daily may take two 200-mg tablets one day and just one 200-mg tablet the next day, and so on. To avoid eye toxicity that can occur after years of taking the drug, hydroxychloroquine is generally prescribed based on weight at 5 mg/kg.
The drug also stays in the body for quite a while [often up to 3 months and even longer], so that is helpful for patients to know.
Given the current situation and the possibility of its effectiveness against COVID-19, it is ironic that we are actually trying to recruit older lupus patients who have had long-term stable disease while on hydroxychloroquine to a trial of stopping the drug to reduce the risk of developing the side effect of retinopathy. We want to see if patients can safely withdraw hydroxychloroquine without flaring, so we hope to not run into enrollment difficulties based on the current situation with COVID-19.
Q: How do you view the balance between having enough hydroxychloroquine for patients with lupus or other rheumatic diseases and its use in COVID-19 patients?
A: We want to reassure patients that hydroxychloroquine will be available, and there is no reason to hoard the drug or to worry excessively about being unable to obtain it. Efforts to increase production by Mylan, Teva, Sanofi, Novartis, and other manufacturers of hydroxychloroquine should really help out.
Q: Are there pharmacy restrictions on prescription amounts?
A: This is not universal at this time, but some institutions are cutting back and offering only 1-month supplies.
Colchicine COVID-19 trial underway
Dr. Pillinger, of NYU Langone Health, explored the COLCORONA study of colchicine as a treatment for people infected with COVID-19 and the worry that shortage concerns may arise for it, too.
Q: What is the general availability of colchicine and its susceptibility to shortage?
A: There are two major manufacturers of colchicine in the United States, Takeda and Hikma, who together manufacture the majority of the drug.
The greatest use of colchicine in the United States is for gout, which affects approximately 4 million Americans, but the drug is not used chronically, so a much smaller number of patients are using colchicine at any one time. Colchicine is also used for other inflammatory conditions, primarily calcium pyrophosphate crystal disease and familial Mediterranean fever (FMF is rare in the United States). Cardiologists also regularly prescribe colchicine in pericarditis for short-term use. Physicians may use it off label for other purposes, too.
Overall, the number of patients using colchicine is much larger than that for the use of hydroxychloroquine, for example, suggesting that the immediate risk of shortage could be lower. However, if individuals started using it off label, or prescribing inappropriately for the COVID-19 indication, the supply would rapidly run short.
Q: What other points are there to consider regarding the use of colchicine to treat COVID-19?
A: There is no evidence – zero – that colchicine has any benefit for COVID-19, not even case reports. There is some rationale that it might be beneficial, but that is exactly why the COLCORONA trial would be logical to try.
The COLCORONA trial is exactly the kind of trial that would be needed for assessing colchicine, and it is big enough and happening quickly enough to get an answer. But if people start to use colchicine off label, we may never know the truth.
While colchicine can be used safely in most people, it can be very problematic and requires an experienced doctor’s supervision. Overdoses can be fatal, and colchicine interacts with many drugs, all of which require dose adjustment and some of which must be stopped in order to use colchicine – it isn’t candy. Some of the other drugs being looked at for COVID-19 in fact may interact with colchicine.
Colchicine must also be dose adjusted for kidney disease, and, in some of the COVID-19 patients, kidney function changes rapidly. So again, its use would require expert supervision even if there were evidence for its utility.
The side effects of colchicine, if mis-dosed, can be very unpleasant, including nausea, vomiting, and diarrhea. Even at the apparent right dose, some people will get these side effects, so colchicine has to be something that works to make the risk/benefit ratio worth it.
Some preparations of colchicine are made combined with probenecid, a gout drug. This is even more problematic because probenecid can raise the level of drugs excreted by the kidney and could affect other treatments.
So in sum, what may be a good idea in theory can turn out to be a disastrous idea in practice, and here we have nothing but theory. This is not an agent to use randomly; the studies will be rushed out quickly and hopefully will give us the knowledge to know what to do.
Dr. Izmirly and Dr. Buyon said they have research grants with the National Institutes of Health to study hydroxychloroquine in patients with lupus and in anti–SSA/Ro-positive pregnant women with a previous child with congenital heart block. Dr. Pillinger reports that he has an investigator-initiated grant from Hikma to study colchicine in osteoarthritis.
This article was reformatted on 3/30/2020 for clarity.
Physicians pessimistic despite increased COVID-19 test kits
according to a survey.
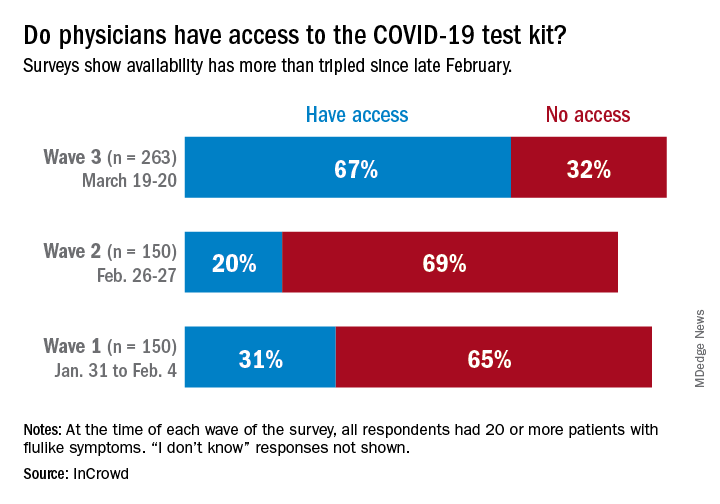
One positive finding from the physicians who participated in this survey March 19-20 was that the availability of COVID-19 test kits has more than doubled since late February.
Reported access to test kits went from 31% in the first wave of a series of surveys (Jan. 31–Feb. 4), down to 20% in the second (Feb. 26-27), and then jumped to 67% by the third wave (March 19-20), InCrowd reported March 26.
Views on several other COVID-related topics were negative among the majority of responding physicians – all of whom had or were currently treating 20 or more patients with flu-like symptoms at the time of the survey.
“Their frustrations and concerns about their ability to protect themselves while meeting upcoming patient care levels has increased significantly in the last 3 months,” Daniel S. Fitzgerald, CEO and president of InCrowd, said in a written statement.
In the third wave, 78% of respondents were “concerned for the safety of loved ones due to my exposure as a physician to COVID-19” and only 16% believed that their facility was “staffed adequately to treat the influx of patients anticipated in the next 30 days,” InCrowd said.
One primary care physician from California elaborated on the issue of safety equipment: “First, [the CDC] said we need N95 masks and other masks would not protect us. As those are running out then they said just use regular surgical masks. Now they are saying use bandannas and scarves! It’s like they don’t care about the safety of the people who will be treating the ill! We don’t want to bring it home to our families!”
“Overall, morale appears low, with few optimistic about the efficacy of public-private collaboration (21%), their own safety given current PPE [personal protective equipment] supply (13%), and the U.S.’s ability to ‘flatten the curve’ (12%),” InCrowd noted in the report.
The first two waves each had 150 respondents, but the number increased to 263 for wave 3, with similar proportions – about 50% emergency medicine or critical care specialists, 25% pediatricians, and 25% primary care physicians – in all three.
according to a survey.

One positive finding from the physicians who participated in this survey March 19-20 was that the availability of COVID-19 test kits has more than doubled since late February.
Reported access to test kits went from 31% in the first wave of a series of surveys (Jan. 31–Feb. 4), down to 20% in the second (Feb. 26-27), and then jumped to 67% by the third wave (March 19-20), InCrowd reported March 26.
Views on several other COVID-related topics were negative among the majority of responding physicians – all of whom had or were currently treating 20 or more patients with flu-like symptoms at the time of the survey.
“Their frustrations and concerns about their ability to protect themselves while meeting upcoming patient care levels has increased significantly in the last 3 months,” Daniel S. Fitzgerald, CEO and president of InCrowd, said in a written statement.
In the third wave, 78% of respondents were “concerned for the safety of loved ones due to my exposure as a physician to COVID-19” and only 16% believed that their facility was “staffed adequately to treat the influx of patients anticipated in the next 30 days,” InCrowd said.
One primary care physician from California elaborated on the issue of safety equipment: “First, [the CDC] said we need N95 masks and other masks would not protect us. As those are running out then they said just use regular surgical masks. Now they are saying use bandannas and scarves! It’s like they don’t care about the safety of the people who will be treating the ill! We don’t want to bring it home to our families!”
“Overall, morale appears low, with few optimistic about the efficacy of public-private collaboration (21%), their own safety given current PPE [personal protective equipment] supply (13%), and the U.S.’s ability to ‘flatten the curve’ (12%),” InCrowd noted in the report.
The first two waves each had 150 respondents, but the number increased to 263 for wave 3, with similar proportions – about 50% emergency medicine or critical care specialists, 25% pediatricians, and 25% primary care physicians – in all three.
according to a survey.

One positive finding from the physicians who participated in this survey March 19-20 was that the availability of COVID-19 test kits has more than doubled since late February.
Reported access to test kits went from 31% in the first wave of a series of surveys (Jan. 31–Feb. 4), down to 20% in the second (Feb. 26-27), and then jumped to 67% by the third wave (March 19-20), InCrowd reported March 26.
Views on several other COVID-related topics were negative among the majority of responding physicians – all of whom had or were currently treating 20 or more patients with flu-like symptoms at the time of the survey.
“Their frustrations and concerns about their ability to protect themselves while meeting upcoming patient care levels has increased significantly in the last 3 months,” Daniel S. Fitzgerald, CEO and president of InCrowd, said in a written statement.
In the third wave, 78% of respondents were “concerned for the safety of loved ones due to my exposure as a physician to COVID-19” and only 16% believed that their facility was “staffed adequately to treat the influx of patients anticipated in the next 30 days,” InCrowd said.
One primary care physician from California elaborated on the issue of safety equipment: “First, [the CDC] said we need N95 masks and other masks would not protect us. As those are running out then they said just use regular surgical masks. Now they are saying use bandannas and scarves! It’s like they don’t care about the safety of the people who will be treating the ill! We don’t want to bring it home to our families!”
“Overall, morale appears low, with few optimistic about the efficacy of public-private collaboration (21%), their own safety given current PPE [personal protective equipment] supply (13%), and the U.S.’s ability to ‘flatten the curve’ (12%),” InCrowd noted in the report.
The first two waves each had 150 respondents, but the number increased to 263 for wave 3, with similar proportions – about 50% emergency medicine or critical care specialists, 25% pediatricians, and 25% primary care physicians – in all three.
Keep calm: Under 25s with diabetes aren't being hospitalized for COVID-19
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.
Reports from pediatric endocrinologists in COVID-19 hot spots globally indicate that children, adolescents, and young adults with diabetes have so far not shown a different disease pattern with the virus compared to children and younger people who do not have diabetes.
Indeed, to date [as of March 24]” according to a new statement from the International Society for Pediatric and Adolescent Diabetes (ISPAD), which currently has about 1,300 members around the globe and has instituted a discussion forum about the topic of treating children with both diabetes and COVID-19.
“We find these reports [from colleagues around the world], though anecdotal, to be reassuring,” it notes. However, there are real worries regarding other potentially dangerous effects. ISPAD has expressed concern, for example, that the COVID-19 pandemic will prevent youngsters with existing diabetes who are having diabetic emergencies from seeking hospital care.
Chinese physicians have reported to ISPAD a number of cases of delayed hospital admissions for diabetic ketoacidosis (DKA) in children with known type 1 diabetes because hospital services were closed for non–COVID-19 care.
Andrea Scaramuzza, MD, a pediatric endocrinologist at Ospedale Maggiore di Cremona, Italy, has similarly reported multiple cases of patients presenting to emergency services there with severe DKA.
“These experiences reinforce the importance of continued attentiveness to standard diabetes care to avoid the need for hospitalization and emergency or urgent care visits,” says ISPAD, under the strapline: “Keep calm and mind your diabetes care.”
But it nevertheless stresses that these resources should be used “if needed.”
Worries that new-onset diabetes will be missed during COVID-19
Dr. Scaramuzza said in an interview that there also are concerns regarding delays in diagnoses of new cases of type 1 diabetes “due to the fear families have to go to the emergency department because of COVID-19.”
Indeed, in Italy, a few patients have arrived with very serious DKA, he said. Dr. Scaramuzza noted a colleague from Naples, Dario Iafusco, MD, and colleagues have made a video to keep awareness high regarding new-onset diabetes.
“This coronavirus pandemic can be defeated if you stay at home, but if you know of a child who has excessive thirst, frequent urination, or who starts vomiting,” seek health care advice immediately. “This child could have [type 1] diabetes. Prevent severe DKA, or worse, death,” Dr. Iafusco of the Regional Centre of Paediatric Diabetology G.Stoppoloni Via S. Andrea delle Dame, Naples, said in the video.
Physicians from China have similar observations, reporting to ISPAD several cases of delayed admissions of newly diagnosed type 1 diabetes because hospital services were closed for non–COVID-19 care.
Keep calm and mind your diabetes care; physicians use telemedicine
Meanwhile, last week ISPAD issued guidance for young people with diabetes and their carers about what to do if COVID-19 infection is suspected.
Most advice is the same as for the general public because reports of COVID-19 infection suggest it is much less severe in children and adolescents, and the summary currently serves “as reassurance that youth with diabetes are not more affected by COVID-19 than peers,” it adds.
“Our approach to treating a child with diabetes would be to follow the ISPAD sick-day guidelines, which provide generalized diabetes management in any flu-like illness. We wouldn’t do anything very different right now,” one of the authors, Jamie Wood, MD, associate professor of clinical pediatrics at Case Western Reserve University, Cleveland, said in an interview.
“Any illness makes diabetes more difficult to manage and can increase the risk of DKA,” she emphasized.
“We would reinforce frequent monitoring of blood glucose and ketone levels, to never stop insulin – in fact, when most people are ill, the body is stressed and requires more insulin – and to stay hydrated and treat the underlying symptoms.”
And make sure to “treat the fever,” she stressed. “When patients with type 1 diabetes get fever, they have a tendency to make more ketones, so we recommend aggressive control of fever.”
ISPAD recommends young people aim to keep blood glucose levels between 4 and 10 mmol/L (72-180 mg/dL) and blood ketones below 0.6 mmol/L (10.8 mg/dL) during illness and to never stop insulin.
Guidance is provided on when to seek urgent specialist advice with possible referral to emergency care, for example, in cases in which the patient has DKA symptoms, such as persistent and/or worsened fruity breath odor or vomiting.
Dr. Scaramuzza said in an interview that, in Italy, he and his colleagues have increased their use of telemedicine to keep monitoring their patients with diabetes even from a distance and that it was working very well.
“Technology – such as downloading [records from] insulin pumps, continuous glucose monitoring systems, and the possibility to use Skype or other platforms – really helps,” he noted.
“There has been a rapid increase in telehealth as a way to continue to care for youth with diabetes and decrease risk for infection,” said ISPAD.
“Communication between patients, families, and health care teams is vitally important. Methods to do so that avoid visits to clinics or hospitals can provide needed diabetes advice and reduce risk for COVID-19 transmission.”
A version of this article originally appeared on Medscape.com.
Elderly Americans carry heavier opioid burden
according to the Agency for Healthcare Quality and Research.
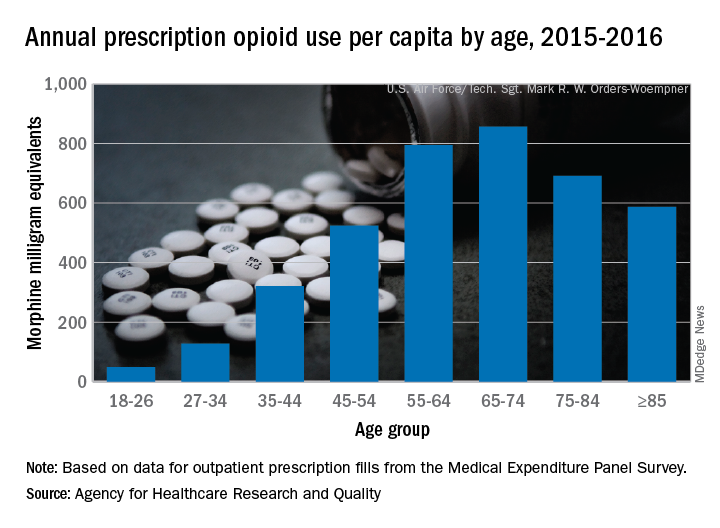
Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
according to the Agency for Healthcare Quality and Research.

Elderly adults with chronic and acute pain obtained an average of 774 morphine milligram equivalents (MMEs) of prescription opioids annually during 2015-2016 from outpatient clinicians, compared with 376 MMEs a year for nonelderly adults, said Asako S. Moriya, PhD, and G. Edward Miller, PhD, of the AHRQ.
Narrowing the age groups shows that opioid MMEs increased with age, starting at 49 MMEs for 18- to 26-year-olds and rising to a high of 856 MMEs in the 65- to 74-year-old group, before dropping off in the oldest adults, the investigators said in a Medical Expenditure Panel Survey (MEPS) research findings report.
The analysis included “all opioid medications that are commonly used to treat pain” and excluded respiratory agents, antitussives, and drugs used for medication-assisted treatment, they noted. The MEPS data cover prescriptions purchased or obtained in outpatient settings but not those administered in inpatient settings or in clinics or physician offices.
Guidelines on delaying cancer surgery during COVID-19
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cancer surgeries may need to be delayed as hospitals are forced to allocate resources to a surge of COVID-19 patients, says the American College of Surgeons, as it issues a new set of recommendations in reaction to the crisis.
Most surgeons have already curtailed or have ceased to perform elective operations, the ACS notes, and recommends that surgeons continue to do so in order to preserve the necessary resources for care of critically ill patients during the COVID-19 pandemic. The new clinical guidance for elective surgical case triage during the pandemic includes recommendations for cancer surgery as well as for procedures that are specific to certain cancer types.
“These triage guidelines and joint recommendations are being issued as we appear to be entering a new phase of the COVID-19 pandemic with more hospitals facing a potential push beyond their resources to care for critically ill patients,” commented ACS Executive Director David B. Hoyt, MD, in a statement.
“ACS will continue to monitor the landscape for surgical care but we feel this guidance document provides a good foundation for surgeons to begin enacting these triage recommendations today to help them make the best decisions possible for their patients during COVID-19,” he said.
For cancer surgery, which is often not elective but essential to treatment, ACS has issued general guidance for triaging patients, taking into account the acuity of the local COVID-19 situation.
First, decisions about whether to proceed with elective surgeries must consider the available resources of local facilities. The parties responsible for preparing the facility to manage coronavirus patients should be sharing information at regular intervals about constraints on local resources, especially personal protective equipment (PPE), which is running low in many jurisdictions. For example, if an elective case has a high likelihood of needing postoperative ICU care, it is imperative to balance the risk of delay against the need of availability for patients with COVID-19.
Second, cancer care coordination should use virtual technologies as much as possible, and facilities with tumor boards may find it helpful to locate multidisciplinary experts by virtual means, to assist with decision making and establishing triage criteria.
Three Phases of Pandemic
The ACS has also organized decision making into three phases that reflect the acuity of the local COVID-19 situation:
- Phase I. Semi-Urgent Setting (Preparation Phase) – few COVID-19 patients, hospital resources not exhausted, institution still has ICU ventilator capacity and COVID-19 trajectory not in rapid escalation phase
- Phase II. Urgent Setting – many COVID-19 patients, ICU and ventilator capacity limited, operating room supplies limited
- Phase III. Hospital resources are all routed to COVID-19 patients, no ventilator or ICU capacity, operating room supplies exhausted; patients in whom death is likely within hours if surgery is deferred
Breast Cancer Surgery
The ACS also issued specific guidance for several tumor types, including guidance for breast cancer surgery.
For phase I, surgery should be restricted to patients who are likely to experience compromised survival if it is not performed within next 3 months. This includes patients completing neoadjuvant treatment, those with clinical stage T2 or N1 ERpos/PRpos/HER2-negative tumors, patients with triple negative or HER2-positive tumors, discordant biopsies that are likely to be malignant, and removal of a recurrent lesion.
Phase II would be restricted to patients whose survival is threatened if surgery is not performed within the next few days. These would include incision and drainage of breast abscess, evacuating a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
In Phase III, surgical procedures would be restricted to patients who may not survive if surgery is not performed within a few hours. This includes incision and drainage of breast abscess, evacuation of a hematoma, revision of an ischemic mastectomy flap, and revascularization/revision of an autologous tissue flap (autologous reconstruction should be deferred).
Colorectal Cancer Surgery
Guidance for colorectal cancer surgery is also split into the three phases of the pandemic.
Phase I would include cases needing surgical intervention as soon as feasible, while recognizing that the status of each hospital is likely to evolve over the next week or two. These patients would include those with nearly obstructing colon cancer or rectal cancer; cancers that require frequent transfusions; asymptomatic colon cancers; rectal cancers that do not respond to neoadjuvant chemoradiation; malignancies with a risk of local perforation and sepsis; and those with early stage rectal cancers that are not candidates for adjuvant therapy.
Phase II comprises patients needing surgery as soon as feasible, but recognizing that hospital status is likely to progress over the next few days. These cases include patients with a nearly obstructing colon cancer where stenting is not an option; those with nearly obstructing rectal cancer (should be diverted); cancers with high (inpatient) transfusion requirements; and cancers with pending evidence of local perforation and sepsis.
All colorectal procedures typically scheduled as routine should be delayed.
In Phase III, if the status of the facility is likely to progress within hours, the only surgery that should be performed would be for perforated, obstructed, or actively bleeding (inpatient transfusion dependent) cancers or those with sepsis. All other surgeries should be deferred.
Thoracic Cancer Surgery
Thoracic cancer surgery guidelines follow those for breast cancer. Phase I should be restricted to patients whose survival may be impacted if surgery is not performed within next 3 months. These include:
- Cases with solid or predominantly solid (>50%) lung cancer or presumed lung cancer (>2 cm), clinical node negative
- Node positive lung cancer
- Post-induction therapy cancer
- Esophageal cancer T1b or greater
- Chest wall tumors that are potentially aggressive and not manageable by alternative means
- Stenting for obstructing esophageal tumor
- Staging to start treatment (mediastinoscopy, diagnostic VATS for pleural dissemination)
- Symptomatic mediastinal tumors
- Patients who are enrolled in therapeutic clinical trials.
Phase II would permit surgery if survival will be impacted by a delay of a few days. These cases would include nonseptic perforated cancer of esophagus, a tumor-associated infection, and management of surgical complications in a hemodynamically stable patient.
All thoracic procedures considered to be routine/elective would be deferred.
Phase III restricts surgery to patients whose survival will be compromised if they do not undergo surgery within the next few hours. This group would include perforated cancer of esophagus in a septic patient, a patient with a threatened airway, sepsis associated with the cancer, and management of surgical complications in an unstable patient (active bleeding that requires surgery, dehiscence of airway, anastomotic leak with sepsis).
All other cases would be deferred.
Other Cancer Types
Although the ACS doesn’t have specific guidelines for all cancer types, a few are included in their general recommendations for the specialty.
For gynecologic surgeries, ACS lists cancer or suspected cancer as indications where significantly delayed surgery could cause “significant harm.”
Delays, in general, are not recommended for neurosurgery, which would include brain cancers. In pediatrics, most cancer surgery is considered “urgent,” where a delay of days to weeks could prove detrimental to the patient. This would comprise all solid tumors, including the initial biopsy and resection following neoadjuvant therapy.
This article first appeared on Medscape.com.
Cardiac symptoms can be first sign of COVID-19
In about 7% of people with confirmed novel coronavirus disease 2019 (COVID-19), and 22% of the critically ill, the virus injures the heart, probably by either attacking it directly or causing a cytokine storm that leads to myocyte apoptosis, according to a report from the Columbia University Division of Cardiology in New York.
Reports from China document patients presenting with palpitations and chest pain without the typical fever and cough.
The exact mechanism of injury is uncertain, but for now, “it appears that the incidence of fulminant myocarditis and profound cardiogenic shock is low; however, the rate of recovery and mode of treatment are yet to be determined,” wrote authors led by Kevin Clerkin, MD, a cardiologist and assistant professor of medicine at Columbia.
High-sensitivity cardiac troponin I (hs-cTnI) might be prognostic. In one Chinese study of hospitalized patients, median hs-cTnI levels were 2.5 pg/mL in survivors on day 4 of symptoms and did not change significantly during follow-up. Among people who died, day 4 hs-cTnI was 8.8 pg/mL and climbed to 290.6 pg/mL by day 22.
“The rise in hs-cTnI tracks with other inflammatory biomarkers ... raising the possibility that this reflects cytokine storm or secondary hemophagocytic lymphohistiocytosis more than isolated myocardial injury,” Dr. Clerkin and colleagues wrote.
But there are also acute heart injury reports out of China, including one man who presented with chest pain and ST-segment elevation, but no coronary obstruction, and another who presented with fulminant myocarditis in addition to severe respiratory manifestations, but with no cardiac history.
Both had depressed left ventricular ejection fractions, enlarged left ventricles, and elevated cardiac biomarkers, and both responded to intravenous immunoglobulin and steroids, among other treatments.
Amid a surge of COVID-19 cases at Columbia, “we have seen both forms of cardiac presentations: those presenting with cardiac predominant symptoms (none have had true [ST-segment elevation myocardial infarctions] yet, but most fall in the myopericarditis group), some of which have required mechanical circulatory support, and those who seem to have secondary myocardial injury with globally elevated inflammatory biomarkers (e.g., ferritin, interleukin-6, lactate dehydrogenase, hs-cTnI, and D-dimer),” Dr. Clerkin said in an interview.
“We are discussing each of these cases in a multidisciplinary fashion with our infectious disease, pulmonary, interventional cardiology, and cardiac surgery colleagues to try to make the best decision based on what we know and as our knowledge evolves,” he said.
The exact cardiac effect of COVID-19 is unknown for now, but it is known already that it rides along with cardiovascular issues. There’s a high prevalence of hypertension, diabetes, and diagnosed cardiovascular disease among patients, but it’s unclear at this point if it’s because the virus favors older people who happen to be more likely to have those problems or if it attacks people with those conditions preferentially.
It might be the latter. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), invades cells through angiotensin converting enzyme (ACE) 2 receptors, which are highly expressed in the heart.
That raises the question of whether ACE inhibitors or angiotensin receptor blockers might help. However, “at this time, nearly all major societies have recommended against adding or stopping ... antagonists in this setting, unless done on clinical grounds independently of COVID-19, given the lack of evidence,” Dr. Clerkin and his colleagues wrote.
As for heart transplants, the current thinking is to continue them without changes in immunosuppression so long as recipients test negative and haven’t been around anyone who has tested positive for a month. If a donor had COVID-19, they should have been free of the virus by polymerase chain reaction for at least 14 days. The concern is that it might be in the donor heart.
If transplant patients come down with COVID-19, the “data to date [indicate that management] is supportive care and continuation of immunosuppression for mild COVID-19 with reduction of the antimetabolite (mycophenolate or azathioprine), and further treatment based on disease severity and drug availability. Notably, one potential treatment option for COVID-19 is protease inhibitors,” the authors said, but it’s important to remember that they will increase the levels of cyclosporine, tacrolimus, and other calcineurin inhibitor transplant drugs.
At Columbia, “our processes have been adjusted” for heart transplants. “For instance, non-urgent testing (pre- and post-transplant) has been tabled, we have predominantly shifted to noninvasive screening for rejection, and each potential transplant requires more scrutiny for urgency, donor screening/risk for COVID-19, and perioperative management,” Dr. Clerkin said in the interview.
A study out of Wuhan, China, the outbreak epicenter, was reassuring. It found that routine prevention efforts were enough to protect heart transplant patients.
There was no funding, and the authors had no disclosures.
SOURCE: Clerkin KJ et al. Circulation. 2020 Mar 21. doi: 10.1161/CIRCULATIONAHA.120.046941
In about 7% of people with confirmed novel coronavirus disease 2019 (COVID-19), and 22% of the critically ill, the virus injures the heart, probably by either attacking it directly or causing a cytokine storm that leads to myocyte apoptosis, according to a report from the Columbia University Division of Cardiology in New York.
Reports from China document patients presenting with palpitations and chest pain without the typical fever and cough.
The exact mechanism of injury is uncertain, but for now, “it appears that the incidence of fulminant myocarditis and profound cardiogenic shock is low; however, the rate of recovery and mode of treatment are yet to be determined,” wrote authors led by Kevin Clerkin, MD, a cardiologist and assistant professor of medicine at Columbia.
High-sensitivity cardiac troponin I (hs-cTnI) might be prognostic. In one Chinese study of hospitalized patients, median hs-cTnI levels were 2.5 pg/mL in survivors on day 4 of symptoms and did not change significantly during follow-up. Among people who died, day 4 hs-cTnI was 8.8 pg/mL and climbed to 290.6 pg/mL by day 22.
“The rise in hs-cTnI tracks with other inflammatory biomarkers ... raising the possibility that this reflects cytokine storm or secondary hemophagocytic lymphohistiocytosis more than isolated myocardial injury,” Dr. Clerkin and colleagues wrote.
But there are also acute heart injury reports out of China, including one man who presented with chest pain and ST-segment elevation, but no coronary obstruction, and another who presented with fulminant myocarditis in addition to severe respiratory manifestations, but with no cardiac history.
Both had depressed left ventricular ejection fractions, enlarged left ventricles, and elevated cardiac biomarkers, and both responded to intravenous immunoglobulin and steroids, among other treatments.
Amid a surge of COVID-19 cases at Columbia, “we have seen both forms of cardiac presentations: those presenting with cardiac predominant symptoms (none have had true [ST-segment elevation myocardial infarctions] yet, but most fall in the myopericarditis group), some of which have required mechanical circulatory support, and those who seem to have secondary myocardial injury with globally elevated inflammatory biomarkers (e.g., ferritin, interleukin-6, lactate dehydrogenase, hs-cTnI, and D-dimer),” Dr. Clerkin said in an interview.
“We are discussing each of these cases in a multidisciplinary fashion with our infectious disease, pulmonary, interventional cardiology, and cardiac surgery colleagues to try to make the best decision based on what we know and as our knowledge evolves,” he said.
The exact cardiac effect of COVID-19 is unknown for now, but it is known already that it rides along with cardiovascular issues. There’s a high prevalence of hypertension, diabetes, and diagnosed cardiovascular disease among patients, but it’s unclear at this point if it’s because the virus favors older people who happen to be more likely to have those problems or if it attacks people with those conditions preferentially.
It might be the latter. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), invades cells through angiotensin converting enzyme (ACE) 2 receptors, which are highly expressed in the heart.
That raises the question of whether ACE inhibitors or angiotensin receptor blockers might help. However, “at this time, nearly all major societies have recommended against adding or stopping ... antagonists in this setting, unless done on clinical grounds independently of COVID-19, given the lack of evidence,” Dr. Clerkin and his colleagues wrote.
As for heart transplants, the current thinking is to continue them without changes in immunosuppression so long as recipients test negative and haven’t been around anyone who has tested positive for a month. If a donor had COVID-19, they should have been free of the virus by polymerase chain reaction for at least 14 days. The concern is that it might be in the donor heart.
If transplant patients come down with COVID-19, the “data to date [indicate that management] is supportive care and continuation of immunosuppression for mild COVID-19 with reduction of the antimetabolite (mycophenolate or azathioprine), and further treatment based on disease severity and drug availability. Notably, one potential treatment option for COVID-19 is protease inhibitors,” the authors said, but it’s important to remember that they will increase the levels of cyclosporine, tacrolimus, and other calcineurin inhibitor transplant drugs.
At Columbia, “our processes have been adjusted” for heart transplants. “For instance, non-urgent testing (pre- and post-transplant) has been tabled, we have predominantly shifted to noninvasive screening for rejection, and each potential transplant requires more scrutiny for urgency, donor screening/risk for COVID-19, and perioperative management,” Dr. Clerkin said in the interview.
A study out of Wuhan, China, the outbreak epicenter, was reassuring. It found that routine prevention efforts were enough to protect heart transplant patients.
There was no funding, and the authors had no disclosures.
SOURCE: Clerkin KJ et al. Circulation. 2020 Mar 21. doi: 10.1161/CIRCULATIONAHA.120.046941
In about 7% of people with confirmed novel coronavirus disease 2019 (COVID-19), and 22% of the critically ill, the virus injures the heart, probably by either attacking it directly or causing a cytokine storm that leads to myocyte apoptosis, according to a report from the Columbia University Division of Cardiology in New York.
Reports from China document patients presenting with palpitations and chest pain without the typical fever and cough.
The exact mechanism of injury is uncertain, but for now, “it appears that the incidence of fulminant myocarditis and profound cardiogenic shock is low; however, the rate of recovery and mode of treatment are yet to be determined,” wrote authors led by Kevin Clerkin, MD, a cardiologist and assistant professor of medicine at Columbia.
High-sensitivity cardiac troponin I (hs-cTnI) might be prognostic. In one Chinese study of hospitalized patients, median hs-cTnI levels were 2.5 pg/mL in survivors on day 4 of symptoms and did not change significantly during follow-up. Among people who died, day 4 hs-cTnI was 8.8 pg/mL and climbed to 290.6 pg/mL by day 22.
“The rise in hs-cTnI tracks with other inflammatory biomarkers ... raising the possibility that this reflects cytokine storm or secondary hemophagocytic lymphohistiocytosis more than isolated myocardial injury,” Dr. Clerkin and colleagues wrote.
But there are also acute heart injury reports out of China, including one man who presented with chest pain and ST-segment elevation, but no coronary obstruction, and another who presented with fulminant myocarditis in addition to severe respiratory manifestations, but with no cardiac history.
Both had depressed left ventricular ejection fractions, enlarged left ventricles, and elevated cardiac biomarkers, and both responded to intravenous immunoglobulin and steroids, among other treatments.
Amid a surge of COVID-19 cases at Columbia, “we have seen both forms of cardiac presentations: those presenting with cardiac predominant symptoms (none have had true [ST-segment elevation myocardial infarctions] yet, but most fall in the myopericarditis group), some of which have required mechanical circulatory support, and those who seem to have secondary myocardial injury with globally elevated inflammatory biomarkers (e.g., ferritin, interleukin-6, lactate dehydrogenase, hs-cTnI, and D-dimer),” Dr. Clerkin said in an interview.
“We are discussing each of these cases in a multidisciplinary fashion with our infectious disease, pulmonary, interventional cardiology, and cardiac surgery colleagues to try to make the best decision based on what we know and as our knowledge evolves,” he said.
The exact cardiac effect of COVID-19 is unknown for now, but it is known already that it rides along with cardiovascular issues. There’s a high prevalence of hypertension, diabetes, and diagnosed cardiovascular disease among patients, but it’s unclear at this point if it’s because the virus favors older people who happen to be more likely to have those problems or if it attacks people with those conditions preferentially.
It might be the latter. The virus that causes COVID-19, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), invades cells through angiotensin converting enzyme (ACE) 2 receptors, which are highly expressed in the heart.
That raises the question of whether ACE inhibitors or angiotensin receptor blockers might help. However, “at this time, nearly all major societies have recommended against adding or stopping ... antagonists in this setting, unless done on clinical grounds independently of COVID-19, given the lack of evidence,” Dr. Clerkin and his colleagues wrote.
As for heart transplants, the current thinking is to continue them without changes in immunosuppression so long as recipients test negative and haven’t been around anyone who has tested positive for a month. If a donor had COVID-19, they should have been free of the virus by polymerase chain reaction for at least 14 days. The concern is that it might be in the donor heart.
If transplant patients come down with COVID-19, the “data to date [indicate that management] is supportive care and continuation of immunosuppression for mild COVID-19 with reduction of the antimetabolite (mycophenolate or azathioprine), and further treatment based on disease severity and drug availability. Notably, one potential treatment option for COVID-19 is protease inhibitors,” the authors said, but it’s important to remember that they will increase the levels of cyclosporine, tacrolimus, and other calcineurin inhibitor transplant drugs.
At Columbia, “our processes have been adjusted” for heart transplants. “For instance, non-urgent testing (pre- and post-transplant) has been tabled, we have predominantly shifted to noninvasive screening for rejection, and each potential transplant requires more scrutiny for urgency, donor screening/risk for COVID-19, and perioperative management,” Dr. Clerkin said in the interview.
A study out of Wuhan, China, the outbreak epicenter, was reassuring. It found that routine prevention efforts were enough to protect heart transplant patients.
There was no funding, and the authors had no disclosures.
SOURCE: Clerkin KJ et al. Circulation. 2020 Mar 21. doi: 10.1161/CIRCULATIONAHA.120.046941
FROM CIRCULATION
FDA approves ozanimod for relapsing and secondary progressive forms of MS
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
The Food and Drug Administration has approved the oral medication ozanimod (Zeposia) for relapsing forms of multiple sclerosis (MS), including clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, according to a release from Bristol-Myers Squibb.
Ozanimod is a sphingosine 1-phosphate (S1P) receptor modulator that binds with high affinity to S1P receptors 1 and 5. It blocks the capacity of lymphocytes to egress from lymph nodes, reducing the number of lymphocytes in peripheral blood. Although its therapeutic mechanism of action in MS is unknown, it may involve the reduction of lymphocyte migration into the central nervous system. A genetic test is not required to start the drug, and no patient observation is required for the first dose, although up-titration of initial doses are required to reach the maintenance dose because a transient decrease in heart rate and atrioventricular conduction delays may occur, according to the company.
The approval is based on a pair of head-to-head studies that compared it with interferon beta-1a (Avonex) and together included more than 2,600 patients. It delivered better efficacy in terms of relative reduction in annualized relapse rate (48% at 1 year and 38% at 2 years). It also demonstrated better relative reduction of the number of T1-weighted gadolinium-enhanced brain lesions (63% fewer at 1 year and 53% fewer at 2 years) and number of new or enlarging T2 lesions (48% fewer at 1 year and 42% at 2 years).
Ozanimod is contraindicated in patients who, in the past 6 months, experienced a myocardial infarction, unstable angina, stroke, or other conditions. It is associated with other health risks, including infections, liver injury, additive immunosuppressive effects from prior immune-modulating therapies, and increased blood pressure. Certain assessments, such as recent complete blood count, ECG, liver function test, and current and prior medications and vaccinations, are required before initiation of treatment.
In its announcement, Bristol-Myers Squibb said that it has decided to delay the commercial launch of ozanimod during the COVID-19 pandemic until a later date.
The drug is also in development for additional immune-inflammatory indications, including ulcerative colitis and Crohn’s disease.
The full prescribing information can be found on the company’s website.
At U.S. Ground Zero for coronavirus, a hospital is transformed
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.
The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.

The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
David Baker, MD, a hospitalist at EvergreenHealth in Kirkland, Wash., had just come off a 7-day stretch of work and was early into his usual 7 days off. He’d helped care for some patients from a nearby assisted living facility who had been admitted with puzzlingly severe viral pneumonia that wasn’t influenza.
Though COVID-19, the novel coronavirus that was sickening tens of thousands in the Chinese province of Hubei, was in the back of everyone’s mind in late February, he said he wasn’t really expecting the call notifying him that two of the patients with pneumonia had tested positive for COVID-19.
Michael Chu, MD, was coming onto EvergreenHealth’s hospitalist service at about the time Dr. Baker was rotating off. He recalled learning of the first two positive COVID-19 tests on the evening of Feb. 28 – a Friday. He and his colleagues took in this information, coming to the realization that they were seeing other patients from the same facility who had viral pneumonia and negative influenza tests. “The first cohort of coronavirus patients all came from Life Care,” the Kirkland assisted living facility that was the epicenter of the first identified U.S. outbreak of community-transmitted coronavirus, said Dr. Chu. “They all fit a clinical syndrome” and many of them were critically ill or failing fast, since they were aged and with multiple risk factors, he said during the interviews he and his colleagues participated in.
As he processed the news of the positive tests and his inadvertent exposure to COVID-19, Dr. Baker realized that his duty schedule worked in his favor, since he wasn’t expected back for several more days. When he did come back to work after remaining asymptomatic, he found a much-changed environment as the coronavirus cases poured in and continual adaptations were made to accommodate these patients – and to keep staff and other patients safe.

The hospital adapts to a new normal
The usual protocol in EvergreenHealth’s ICU is for the nocturnist hospitalists, such as Dr. Baker, to staff that unit, with intensivists readily available for phone consultation. However, as the numbers of critically ill, ventilated COVID-19 patients climbed, the facility switched to 24/7 staffing with intensivists to augment the hospitalist team, said Nancy Marshall, MD, the director of EvergreenHealth’s hospitalist service.
Dr. Marshall related how the entire hospital rallied to create appropriate – but flexible – staffing and environmental adaptations to the influx of coronavirus patients. “Early on, we established a separate portion of the emergency department to evaluate and test persons under investigation,” for COVID-19, she said. When they realized that they were seeing the nation’s first cluster of community coronavirus transmission, they used “appropriate isolation precautions” when indicated. Triggers for clinical suspicion included not just fever or cough, but also a new requirement for supplemental oxygen and new abnormal findings on chest radiographs.
Patients with confirmed or suspected coronavirus, once admitted, were placed in negative-pressure rooms, and droplet precautions were used with these patients. In the absence of aerosol-generating procedures, those caring for these patients used a standard surgical mask, goggles or face shield, an isolation gown, and gloves. For intubations, bronchoscopies, and other aerosol-generating procedures, N95 masks were used; the facility also has some powered and controlled air-purifying respirators.
In short order, once the size of the outbreak was appreciated, said Dr. Marshall, the entire ICU and half of another general medical floor in the hospital were converted to negative-pressure rooms.
Dr. Marshall said that having daily team debriefings has been essential. The hospitalist team room has a big whiteboard where essential information can be put up and shared. Frequent video conferencing has allowed physicians and advanced practice clinicians on the hospitalist team to ask questions, share concerns, and develop a shared knowledge base and vocabulary as they confronted this novel illness.
The rapid adaptations that EvergreenHealth successfully made depended on a responsive administration, good communication among physician services and with nursing staff, and the active participation of engineering and environmental services teams in adjusting to shifting patient needs, said Dr. Marshall.
“Preparedness is key,” Dr. Chu noted. “Managing this has required a unified effort” that addresses everything from the supply chain for personal protective equipment, to cleaning procedures, to engineering fixes that quickly added negative-pressure rooms.
“I can’t emphasize enough that this is a team sport,” said Dr. Marshall.
The unpredictable clinical course of COVID-19
The chimeric clinical course of COVID-19 means clinicians need to keep an open mind and be ready to act nimbly, said the EvergreenHealth hospitalists. Pattern recognition is a key to competent clinical management of hospitalized patients, but the course of coronavirus thus far defies any convenient application of heuristics.
Those first two patients had some characteristics in common, aside from their arrival from the same long-term care facility They each had unexplained acute respiratory distress syndrome and ground-glass opacities seen on chest CT, said Dr. Marshall. But all agreed it is still not clear who will fare well, and who will do poorly once they are admitted with coronavirus.
“We have noticed that these patients tend to have a rough course,” said Dr. Marshall. The “brisk inflammatory response” seen in some patients manifests in persistent fevers, big C-reactive protein (CRP) elevations, and likely is part of the picture of yet-unknown host factors that contribute to a worse disease course for some, she said. “These patients look toxic for a long time.”
Dr. Chu said that he’s seen even younger, healthier-looking patients admitted from the emergency department who are already quite dyspneic and may be headed for ventilation. These patients may have a low procalcitonin, and will often turn out to have an “impressive-looking” chest x-ray or CT that will show prominent bilateral infiltrates.
On the other hand, said Dr. Marshall, she and her colleagues have admitted frail-appearing nonagenarians who “just kind of sleep it off,” with little more than a cough and intermittent fevers.
Dr. Chu concurred: “So many of these patients had risk factors for severe disease and only had mild illness. Many were really quite stable.”
In terms of managing respiratory status, Dr. Baker said that the time to start planning for intubation is when the supplemental oxygen demands of COVID-19 patients start to go up. Unlike with patients who may be in some respiratory distress from other causes, once these patients have increased Fi02 needs, bridging “doesn’t work. ... They need to be intubated. Early intubation is important.” Clinicians’ level of concern should spike when they see increased work of breathing in a coronavirus patient, regardless of what the numbers are saying, he added.
For coronavirus patients with acute respiratory distress syndrome (ARDS), early proning also seems to provide some benefit, he said. At EvergreenHealth, standard ARDS ventilation protocols are being followed, including low tidal volume ventilation and positive end-expiratory pressure (PEEP) ladders. Coronavirus ventilation management has thus far been “pretty similar to standard practice with ARDS patients,” he said.
The hospitalist team was able to tap into the building knowledge base in China: Two of the EvergreenHealth hospitalists spoke fluent Mandarin, and one had contacts in China that allowed her to connect with Chinese physicians who had been treating COVID-19 patients since that outbreak had started. They established regular communication on WeChat, checking in frequently for updates on therapies and diagnostics being used in China as well.
One benefit of being in communication with colleagues in China, said Dr. Baker, was that they were able to get anecdotal evidence that elevated D-dimer levels and highly elevated CRP levels can portend a worse illness course. These findings seem to have held generally true for EvergreenHealth patients, he said. Dr. Marshall also spoke to the value of early communication with Chinese teams, who confirmed that the picture of a febrile illness with elevated CRP and leukopenia should raise the index of suspicion for coronavirus.
“Patients might improve over a few days, and then in the final 24 hours of their lives, we see changes in hemodynamics,” including reduced ejection fraction consistent with cardiogenic shock, as well as arrhythmias, said Dr. Baker. Some of the early patient deaths at EvergreenHealth followed this pattern, he said, noting that others have called for investigation into whether viral myocarditis is at play in some coronavirus deaths.
Moderately and severely ill coronavirus patients at EvergreenHealth currently receive a course of hydroxychloroquine of approximately 4-5 days’ duration. The hospital obtained remdesivir from Gilead through its compassionate-use program early on, and now is participating in a clinical trial for COVID-19 patients in the ICU.
By March 23, the facility had seen 162 confirmed COVID-19 cases, and 30 patients had died. Twenty-two inpatients had been discharged, and an additional 58 who were seen in the emergency department had been discharged home without admission.
Be suspicious – and prepared
When asked what he’d like his colleagues around the country to know as they diagnose and admit their first patients who are ill with coronavirus, Dr. Baker advised maintaining a high index of suspicion and a low threshold for testing. “I’ve given some thought to this,” he said. “From our reading and what information is out there, we are geared to pick up on the classic symptoms of coronavirus – cough, fever, some gastrointestinal symptoms.” However, many elderly patients “are not good historians. Some may have advanced dementia. ... When patients arrive with no history, we do our best to gather information,” but sometimes a case can still take clinicians by surprise, he said.
Dr. Baker told a cautionary tale of one of his patients, a woman who was admitted for a hip fracture after a fall at an assisted living facility. The patient was mildly hypoxic, but had an unremarkable physical exam, no fever, and a clear chest x-ray. She went to surgery and then to a postoperative floor with no isolation measures. When her respiratory status unexpectedly deteriorated, she was tested for COVID-19 – and was positive.
“When in doubt, isolate,” said Dr. Baker.
Dr. Chu concurred: “As soon as you suspect, move them, rather than testing first.”
Dr. Baker acknowledged, though, that when testing criteria and availability of personal protective equipment and test materials may vary by region, “it’s a challenge, especially with limited resources.”
Dr. Chu said that stringent isolation, though necessary, creates great hardship for patients and families. “It’s really important for us to check in with family members,” he said; patients are alone and afraid, and family members feel cut off – and also afraid on behalf of their ill loved ones. Workflow planning should acknowledge this and allocate extra time for patient connection and a little more time on the phone with families.
Dr. Chu offered a sobering final word. Make sure family members know their ill loved one’s wishes for care, he said: “There’s never been a better time to clarify code status on admission.”
Physicians at EvergreenHealth have created a document that contains consolidated information on what to anticipate and how to prepare for the arrival of COVID-19+ patients, recommendations on maximizing safety in the hospital environment, and key clinical management considerations. The document will be updated as new information arises.
Correction, 3/27/20: An earlier version of this article referenced white blood counts, presence of lymphopenia, and elevated hepatic enzymes for patients at EvergreenHealth when in fact that information pertained to patients in China. That paragraph has been deleted.
Perspective from the heartland: Cancer care and research during a public health crisis
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
I have no knowledge of, or experience with, managing a cancer patient during a pandemic. However, from the published and otherwise shared experience of others, we should not allow ourselves to underestimate the voracity of the coronavirus pandemic on our patients, communities, and health care systems.
Data from China suggest cancer patients infected with SARS-CoV-2 face a 3.5 times higher risk of mechanical ventilation, intensive care unit admission, or death, compared with infected patients without cancer (Lancet Oncol 2020;21:335-7).
Health care workers in Seattle have also shared their experiences battling coronavirus infections in cancer patients (J Natl Compr Canc Netw. 2020 Mar 20. doi: 10.6004/jnccn.2020.7560). Masumi Ueda, MD, of Seattle Cancer Care Alliance, and colleagues reviewed their decisions in multiple domains over a 7-week period, during which the state of Washington went from a single case of SARS-CoV-2 infection to nearly 650 cases and 40 deaths.
Making tough treatment decisions
Dr. Ueda and colleagues contrasted their customary resource-rich, innovation-oriented, cancer-combatting environment with their current circumstance, in which they must prioritize treatment for patients for whom the risk-reward balance has tilted substantially toward “risk.”
The authors noted that their most difficult decisions were those regarding delay of cancer treatment. They suggested that plans for potentially curative adjuvant therapy should likely proceed, but, for patients with metastatic disease, the equation is more nuanced.
In some cases, treatment should be delayed or interrupted with recognition of how that could result in worsening performance status and admission for symptom palliation, further stressing inpatient resources.
The authors suggested scenarios for prioritizing cancer surgery. For example, several months of systemic therapy (ideally, low-risk systemic therapy such as hormone therapy for breast or prostate cancer) and surgical delay may be worthwhile, without compromising patient care.
Patients with aggressive hematologic malignancy requiring urgent systemic treatment (potentially stem cell transplantation and cellular immunotherapies) should be treated promptly. However, even in those cases, opportunities should be sought to lessen immunosuppression and transition care as quickly as possible to the outpatient clinic, according to guidelines from the American Society of Transplantation and Cellular Therapy.
See one, do one, teach one
Rendering patient care during a pandemic would be unique for me. However, I, like all physicians, am familiar with feelings of inadequacy at times of professional challenge. On countless occasions, I have started my day or walked into a patient’s room wondering whether I will have the fortitude, knowledge, creativity, or help I need to get through that day or make that patient “better” by any definition of that word.
We all know the formula: “Work hard. Make evidence-based, personalized decisions for those who have entrusted their care to us. Learn from those encounters. Teach from our knowledge and experience – that is, ‘See one, do one, teach one.’ ”
The Seattle oncologists are living the lives of first responders and deserve our admiration for putting pen to paper so we can learn from their considerable, relevant experience.
Similar admiration is due to Giuseppe Curigliano, MD, of the European Institute of Oncology in Milan. In the ASCO Daily News, Dr. Curigliano described an epidemic that, within 3 weeks, overloaded the health care system across northern Italy.
Hospitalization was needed for over 60% of infected patients, and nearly 15% of those patients needed intensive care unit services for respiratory distress. The Italians centralized oncology care in specialized hubs, with spokes of institutions working in parallel to provide cancer-specific care in a COVID-free environment.
To build upon cancer-specific information from Italy and other areas hard-hit by COVID-19, more than 30 cancer centers have joined together to form the COVID-19 and Cancer Consortium. The consortium’s website hosts a survey designed to “capture details related to cancer patients presumed to have COVID-19.”
Calculating deaths and long-term consequences for cancer care delivery
It is proper that the authors from China, Italy, and Seattle did not focus attention on the case fatality rate from the COVID-19 pandemic among cancer patients. To say the least, it would be complicated to tally the direct mortality – either overall or in clinically important subsets of patients, including country-specific cohorts.
What we know from published reports is that, in Italy, cancer patients account for about 20% of deaths from coronavirus. In China, the case-fatality rate for patients with cancer was 5.6% (JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648).
However, we know nothing about the indirect death toll from malignancy (without coronavirus infection) that was untreated or managed less than optimally because of personnel and physical resources that were diverted to COVID-19–associated cases.
Similarly, we cannot begin to estimate indirect consequences of the pandemic to oncology practices, such as accelerated burnout and posttraumatic stress disorder, as well as the long-range effects of economic turmoil on patients, health care workers, and provider organizations.
What happens to cancer trials?
From China, Italy, and Seattle, thus far, there is little information about how the pandemic will affect the vital clinical research endeavor. The Seattle physicians did say they plan to enroll patients on clinical trials only when the trial offers a high chance of benefiting the patient over standard therapy alone.
Fortunately, the National Institutes of Health and Food and Drug Administration have released guidance documents related to clinical trials.
The National Cancer Institute (NCI) has also released guidance documents (March 13 guidance; March 23 guidance) for patients on clinical trials supported by the NCI Cancer Therapy Evaluation Program (CTEP) and the NCI Community Oncology Research Program (NCORP).
CTEP and NCORP are making reasonable accommodations to suspend monitoring visits and audits, allow tele–follow-up visits for patients, and permit local physicians to provide care for patients on study. In addition, with appropriate procedural adherence and documentation, CTEP and NCORP will allow oral investigational medicines to be mailed directly to patients’ homes.
Planned NCI National Clinical Trials Network meetings will be conducted via remote access webinars, conference calls, and similar technology. These adjustments – and probably many more to come – are geared toward facilitating ongoing care to proceed safely and with minimal risk for patients currently receiving investigational therapies and for the sites and investigators engaged in those studies.
Each of us has probably faced a personal “defining professional moment,” when we had to utilize every skill in our arsenal and examine the motivations that led us to a career in oncology. However, it is clear from the forgoing clinical and research processes and guidelines that the COVID-19 pandemic is such a defining professional moment for each of us, in every community we serve.
Critical junctures like this cause more rapid behavior change and innovation than the slow-moving pace that characterizes our idealized preferences. As oncologists who embrace new data and behavioral change, we stand to learn processes that will facilitate more perfected systems of care than the one that preceded this unprecedented crisis, promote more efficient sharing of high-quality information, and improve the outcome for our future patients.
Dr. Lyss was an oncologist and researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers, as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.











