User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Inhaled, systemic steroids linked to changes in brain structure
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
New research links the use of glucocorticoids with changes in white matter microstructure – which may explain the development of anxiety, depression, and other neuropsychiatric side effects related to these drugs, investigators say.
Results from a cross-sectional study showed that use of both systemic and inhaled glucocorticoids was associated with widespread reductions in fractional anisotropy (FA) and increases in mean diffusivity.
Glucocorticoids have “a whole catalogue” of adverse events, and effects on brain structure “adds to the list,” co-investigator Onno C. Meijer, PhD, professor of molecular neuroendocrinology of corticosteroids, department of medicine, Leiden University Medical Center, the Netherlands, told this news organization.
The findings should encourage clinicians to consider whether doses they are prescribing are too high, said Dr. Meijer. He added that the negative effect of glucocorticoids on the brain was also found in those using inhalers, such as patients with asthma.
The findings were published online in the BMJ Open.
Serious side effects
Glucocorticoids, a class of synthetic steroids with immunosuppressive properties, are prescribed for a wide range of conditions, including rheumatoid arthritis and asthma.
However, they are also associated with potentially serious metabolic, cardiovascular, and musculoskeletal side effects as well as neuropsychiatric side effects such as depression, mania, and cognitive impairment.
About 1 in 3 patients exposed to “quite a lot of these drugs” will experience neuropsychiatric symptoms, Dr. Meijer said.
Most previous studies that investigated effects from high levels of glucocorticoids on brain structure have been small and involved selected populations, such as those with Cushing disease.
The new study included participants from the UK Biobank, a large population-based cohort. Participants had undergone imaging and did not have a history of psychiatric disease – although they could have conditions associated with glucocorticoid use, including anxiety, depression, mania, or delirium.
The analysis included 222 patients using oral or parenteral glucocorticoids at the time of imaging (systemic group), 557 using inhaled glucocorticoids, and 24,106 not using glucocorticoids (the control group).
Inhaled steroids target the lungs, whereas a steroid in pill form “travels in the blood and reaches each and every organ and cell in the body and typically requires higher doses,” Dr. Meijer noted.
The groups were similar with respect to sex, education, and smoking status. However, the systemic glucocorticoid group was slightly older (mean age, 66.1 years vs. 63.3 years for inhaled glucocorticoid users and 63.5 years for the control group).
In addition to age, researchers adjusted for sex, education level, head position in the scanner, head size, assessment center, and year of imaging.
Imaging analyses
Imaging analyses showed systemic glucocorticoid use was associated with reduced global FA (adjusted mean difference, -3.7e-3; 95% confidence interval, -6.4e-3 to 1.0e-3), and reductions in regional FA in the body and genu of the corpus callosum versus the control group.
Inhaled glucocorticoid use was associated with reduced global FA (AMD, -2.3e-3; 95% CI, -4.0e-3 to -5.7e-4), and lower FA in the splenium of the corpus callosum and the cingulum of the hippocampus.
Global mean diffusivity was higher in systemic glucocorticoid users (AMD, 7.2e-6; 95% CI, 3.2e-6 to 1.1e-5) and inhaled glucocorticoid users (AMD, 2.7e-6; 95% CI, 1.7e-7 to 5.2e-6), compared with the control group.
The effects of glucocorticoids on white matter were “pervasive,” and the “most important finding” of the study, Dr. Meijer said. “We were impressed by the fact white matter is so sensitive to these drugs.”
He noted that it is likely that functional connectivity between brain regions is affected by use of glucocorticoids. “You could say communication between brain regions is probably somewhat impaired or challenged,” he said.
Subgroup analyses among participants using glucocorticoids chronically, defined as reported at two consecutive visits, suggested a potential dose-dependent or duration-dependent effect of glucocorticoids on white matter microstructure.
Systemic glucocorticoid use was also associated with an increase in total and grey matter volume of the caudate nucleus.
In addition, there was a significant association between inhaled glucocorticoid use and decreased grey matter volume of the amygdala, which Dr. Meijer said was surprising because studies have shown that glucocorticoids “can drive amygdala big time.”
Move away from ‘one dose for all’?
Another surprise was that the results showed no hippocampal volume differences with steroid use, Dr. Meijer noted.
The modest association between glucocorticoid use and brain volumes could indicate that white matter integrity is more sensitive to glucocorticoids than is grey matter volume, “at least at the structural level,” he said.
He added that longer use or higher doses may be necessary to also induce volumetric changes.
Participants also completed a questionnaire to assess mood over the previous 2 weeks. Systemic glucocorticoid users had more depressive symptoms, disinterest, tenseness/restlessness, and tiredness/lethargy, compared with the control group. Inhaled glucocorticoid users only reported more tiredness/lethargy.
The investigators note that mood-related effects could be linked to the condition for which glucocorticoids were prescribed: for example, rheumatoid arthritis or chronic obstructive pulmonary disease.
In terms of cognition, systemic glucocorticoid users performed significantly worse on the symbol digit substitution task, compared with participants in the control group.
In light of these findings, pharmaceutical companies that make inhaled corticosteroids “should perhaps find out if glucocorticoids can be dosed by kilogram body weight rather than simply one dose fits all,” which is currently the case, Dr. Meijer said.
Impressive, but several limitations
Commenting on the findings, E. Sherwood Brown, MD, PhD, Distinguished Chair in Psychiatric Research and professor and vice chair for clinical research, department of psychiatry, The University of Texas Southwestern Medical Center, Dallas, called the study sample size “impressive.”
In addition, the study is the first to look at systemic as well as inhaled corticosteroids, said Dr. Brown, who was not involved with the research. He noted that previously, there had been only case reports of psychiatric symptoms with inhaled corticosteroids.
That results are in the same direction but greater with systemic, compared with inhaled corticosteroids, is “particularly interesting” because this might suggest dose-dependent effects, Dr. Brown said.
He noted that cognitive differences were also only observed with systemic corticosteroids.
Some study observations, such as smaller amygdala volume with inhaled but not systemic corticosteroids, “are harder to understand,” said Dr. Brown.
However, he pointed out some study limitations. For example, data were apparently unavailable for verbal and declarative memory test data, despite corticosteroids probably affecting the hippocampus and causing memory changes.
Other drawbacks were that the dose and duration of corticosteroid use, as well as the medical histories of study participants, were not available, Dr. Brown said.
No study funding was reported. Dr. Meijer has received research grants and honorariums from Corcept Therapeutics and a speakers’ fee from Ipsen. Dr. Brown is on an advisory board for Sage Pharmaceuticals, which is developing neurosteroids (not corticosteroids) for mood disorders. He is also on a Medscape advisory board related to bipolar disorder.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN
Omega-3 fatty acids and depression: Are they protective?
New research is suggesting that there are “meaningful” associations between higher dietary intake of omega-3 fatty acids and lower risk for depressive episodes.
In addition, consumption of total fatty acids and alpha-linolenic acid was associated with a reduced risk for incident depressive episodes (9% and 29%, respectively).
“Our results showed an important protective effect from the consumption of omega-3,” Maria de Jesus Mendes da Fonseca, University of the State of Rio de Janeiro, and colleagues write.
The findings were published online in Nutrients.
Mixed bag of studies
Epidemiologic evidence suggests that deficient dietary omega-3 intake is a modifiable risk factor for depression and that individuals with low consumption of omega-3 food sources have more depressive symptoms.
However, the results are inconsistent, and few longitudinal studies have addressed this association, the investigators note.
The new analysis included 13,879 adults (aged 39-65 years or older) participating in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) from 2008 to 2014.
Data on depressive episodes were obtained with the Clinical Interview Schedule Revised (CIS-R), and food consumption was measured with the Food Frequency Questionnaire (FFQ).
The target dietary components were total polyunsaturated fatty acids (PUFA) and the omega-3 fatty acids: alpha-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA).
The majority of participants had adequate dietary intake of omega-3 fatty acids, and none was taking omega-3 supplements.
In the fully adjusted model, consumption of fatty acids from the omega-3 family had a protective effect against maintenance of depressive episodes, showing “important associations, although the significance levels are borderline, possibly due to the sample size,” the researchers report.
In regard to onset of depressive episodes, estimates from the fully adjusted model suggest that a higher consumption of omega-3 acids (total and subtypes) is associated with lower risk for depressive episodes – with significant associations for omega-3 and alpha-linolenic acid.
The investigators note that strengths of the study include “its originality, as it is the first to assess associations between maintenance and incidence of depressive episodes and consumption of omega-3, besides the use of data from the ELSA-Brasil Study, with rigorous data collection protocols and reliable and validated instruments, thus guaranteeing the quality of the sample and the data.”
A study limitation, however, was that the ELSA-Brasil sample consists only of public employees, with the potential for a selection bias such as healthy worker phenomenon, the researchers note. Another was the use of the FFQ, which may underestimate daily intake of foods and depends on individual participant recall – all of which could possibly lead to a differential classification bias.
Interpret cautiously
Commenting on the study, David Mischoulon, MD, PhD, professor of psychiatry, Harvard Medical School, and director of the depression clinical and research program at Massachusetts General Hospital, both in Boston, said that data on omega-3s in depression are “very mixed.”
“A lot of the studies don’t necessarily agree with each other. Certainly, in studies that try to seek an association between omega-3 use and depression, it’s always complicated because it can be difficult to control for all variables that could be contributing to the result that you get,” said Dr. Mischoulon, who is also a member of the Anxiety and Depression Association of America and was not involved in the research.
A caveat to the current study was that diet was assessed only at baseline, “so we don’t really know whether there were any substantial dietary changes over time, he noted.
He also cautioned that it is hard to draw any firm conclusions from this type of study.
“In general, in studies with a large sample, which this study has, it’s easier to find statistically significant differences. But you need to ask yourself: Does it really matter? Is it enough to have a clinical impact and make a difference?” Dr. Mischoulon said.
The ELSA-Brasil study was funded by the Brazilian Ministry of Science, Technology, and Innovation and by the Ministry of Health. The investigators have reported no relevant financial relationships. Dr. Mischoulon has received research support from Nordic Naturals and heckel medizintechnik GmbH and honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He also works with the MGH Clinical Trials Network and Institute, which has received research funding from multiple pharmaceutical companies and the National Institute of Mental Health.
A version of this article first appeared on Medscape.com.
New research is suggesting that there are “meaningful” associations between higher dietary intake of omega-3 fatty acids and lower risk for depressive episodes.
In addition, consumption of total fatty acids and alpha-linolenic acid was associated with a reduced risk for incident depressive episodes (9% and 29%, respectively).
“Our results showed an important protective effect from the consumption of omega-3,” Maria de Jesus Mendes da Fonseca, University of the State of Rio de Janeiro, and colleagues write.
The findings were published online in Nutrients.
Mixed bag of studies
Epidemiologic evidence suggests that deficient dietary omega-3 intake is a modifiable risk factor for depression and that individuals with low consumption of omega-3 food sources have more depressive symptoms.
However, the results are inconsistent, and few longitudinal studies have addressed this association, the investigators note.
The new analysis included 13,879 adults (aged 39-65 years or older) participating in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) from 2008 to 2014.
Data on depressive episodes were obtained with the Clinical Interview Schedule Revised (CIS-R), and food consumption was measured with the Food Frequency Questionnaire (FFQ).
The target dietary components were total polyunsaturated fatty acids (PUFA) and the omega-3 fatty acids: alpha-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA).
The majority of participants had adequate dietary intake of omega-3 fatty acids, and none was taking omega-3 supplements.
In the fully adjusted model, consumption of fatty acids from the omega-3 family had a protective effect against maintenance of depressive episodes, showing “important associations, although the significance levels are borderline, possibly due to the sample size,” the researchers report.
In regard to onset of depressive episodes, estimates from the fully adjusted model suggest that a higher consumption of omega-3 acids (total and subtypes) is associated with lower risk for depressive episodes – with significant associations for omega-3 and alpha-linolenic acid.
The investigators note that strengths of the study include “its originality, as it is the first to assess associations between maintenance and incidence of depressive episodes and consumption of omega-3, besides the use of data from the ELSA-Brasil Study, with rigorous data collection protocols and reliable and validated instruments, thus guaranteeing the quality of the sample and the data.”
A study limitation, however, was that the ELSA-Brasil sample consists only of public employees, with the potential for a selection bias such as healthy worker phenomenon, the researchers note. Another was the use of the FFQ, which may underestimate daily intake of foods and depends on individual participant recall – all of which could possibly lead to a differential classification bias.
Interpret cautiously
Commenting on the study, David Mischoulon, MD, PhD, professor of psychiatry, Harvard Medical School, and director of the depression clinical and research program at Massachusetts General Hospital, both in Boston, said that data on omega-3s in depression are “very mixed.”
“A lot of the studies don’t necessarily agree with each other. Certainly, in studies that try to seek an association between omega-3 use and depression, it’s always complicated because it can be difficult to control for all variables that could be contributing to the result that you get,” said Dr. Mischoulon, who is also a member of the Anxiety and Depression Association of America and was not involved in the research.
A caveat to the current study was that diet was assessed only at baseline, “so we don’t really know whether there were any substantial dietary changes over time, he noted.
He also cautioned that it is hard to draw any firm conclusions from this type of study.
“In general, in studies with a large sample, which this study has, it’s easier to find statistically significant differences. But you need to ask yourself: Does it really matter? Is it enough to have a clinical impact and make a difference?” Dr. Mischoulon said.
The ELSA-Brasil study was funded by the Brazilian Ministry of Science, Technology, and Innovation and by the Ministry of Health. The investigators have reported no relevant financial relationships. Dr. Mischoulon has received research support from Nordic Naturals and heckel medizintechnik GmbH and honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He also works with the MGH Clinical Trials Network and Institute, which has received research funding from multiple pharmaceutical companies and the National Institute of Mental Health.
A version of this article first appeared on Medscape.com.
New research is suggesting that there are “meaningful” associations between higher dietary intake of omega-3 fatty acids and lower risk for depressive episodes.
In addition, consumption of total fatty acids and alpha-linolenic acid was associated with a reduced risk for incident depressive episodes (9% and 29%, respectively).
“Our results showed an important protective effect from the consumption of omega-3,” Maria de Jesus Mendes da Fonseca, University of the State of Rio de Janeiro, and colleagues write.
The findings were published online in Nutrients.
Mixed bag of studies
Epidemiologic evidence suggests that deficient dietary omega-3 intake is a modifiable risk factor for depression and that individuals with low consumption of omega-3 food sources have more depressive symptoms.
However, the results are inconsistent, and few longitudinal studies have addressed this association, the investigators note.
The new analysis included 13,879 adults (aged 39-65 years or older) participating in the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) from 2008 to 2014.
Data on depressive episodes were obtained with the Clinical Interview Schedule Revised (CIS-R), and food consumption was measured with the Food Frequency Questionnaire (FFQ).
The target dietary components were total polyunsaturated fatty acids (PUFA) and the omega-3 fatty acids: alpha-linolenic acid, eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and docosapentaenoic acid (DPA).
The majority of participants had adequate dietary intake of omega-3 fatty acids, and none was taking omega-3 supplements.
In the fully adjusted model, consumption of fatty acids from the omega-3 family had a protective effect against maintenance of depressive episodes, showing “important associations, although the significance levels are borderline, possibly due to the sample size,” the researchers report.
In regard to onset of depressive episodes, estimates from the fully adjusted model suggest that a higher consumption of omega-3 acids (total and subtypes) is associated with lower risk for depressive episodes – with significant associations for omega-3 and alpha-linolenic acid.
The investigators note that strengths of the study include “its originality, as it is the first to assess associations between maintenance and incidence of depressive episodes and consumption of omega-3, besides the use of data from the ELSA-Brasil Study, with rigorous data collection protocols and reliable and validated instruments, thus guaranteeing the quality of the sample and the data.”
A study limitation, however, was that the ELSA-Brasil sample consists only of public employees, with the potential for a selection bias such as healthy worker phenomenon, the researchers note. Another was the use of the FFQ, which may underestimate daily intake of foods and depends on individual participant recall – all of which could possibly lead to a differential classification bias.
Interpret cautiously
Commenting on the study, David Mischoulon, MD, PhD, professor of psychiatry, Harvard Medical School, and director of the depression clinical and research program at Massachusetts General Hospital, both in Boston, said that data on omega-3s in depression are “very mixed.”
“A lot of the studies don’t necessarily agree with each other. Certainly, in studies that try to seek an association between omega-3 use and depression, it’s always complicated because it can be difficult to control for all variables that could be contributing to the result that you get,” said Dr. Mischoulon, who is also a member of the Anxiety and Depression Association of America and was not involved in the research.
A caveat to the current study was that diet was assessed only at baseline, “so we don’t really know whether there were any substantial dietary changes over time, he noted.
He also cautioned that it is hard to draw any firm conclusions from this type of study.
“In general, in studies with a large sample, which this study has, it’s easier to find statistically significant differences. But you need to ask yourself: Does it really matter? Is it enough to have a clinical impact and make a difference?” Dr. Mischoulon said.
The ELSA-Brasil study was funded by the Brazilian Ministry of Science, Technology, and Innovation and by the Ministry of Health. The investigators have reported no relevant financial relationships. Dr. Mischoulon has received research support from Nordic Naturals and heckel medizintechnik GmbH and honoraria for speaking from the Massachusetts General Hospital Psychiatry Academy. He also works with the MGH Clinical Trials Network and Institute, which has received research funding from multiple pharmaceutical companies and the National Institute of Mental Health.
A version of this article first appeared on Medscape.com.
FROM NUTRIENTS
‘Doomscrolling’ may be a significant driver of poor mental health
The past 2 years have been filled with worrisome global events, from the pandemic to the war in Ukraine, large-scale protests, mass shootings, and devastating wildfires. The 24-hour media coverage of these events can take a toll on “news addicts” who have an excessive urge to constantly check the news, researchers note.
Results from an online survey of more than 1,000 adults showed that nearly 17% showed signs of “severely problematic” news consumption.
These “doomscrollers” or “doomsurfers” scored high on all five problematic news consumption dimensions: being absorbed in news content, being consumed by thoughts about the news, attempting to alleviate feelings of threat by consuming more news, losing control over news consumption, and having news consumption interfere in daily life.
“We anticipated that a sizable portion of our sample would show signs of problematic news consumption. However, we were surprised to find that 17% of study participants suffer from the most severe level of problematic news consumption,” lead author Bryan McLaughlin, PhD, Texas Tech University, Lubbock, told this news organization. “This is certainly concerning and suggests the problem may be more widespread than we expected,” he said.
In addition, 74% of those with severe levels of problematic news consumption reported experiencing mental problems, and 61% reported physical problems.
“It’s important for health care providers to be aware that problematic news consumption may be a significant driver of mental and physical ill-being, especially because a lot of people might be unaware of the negative impact the news is having on their health,” Dr. McLaughlin said.
The findings were published online in Health Communication.
Emotionally invested
The researchers assessed data from an online survey of 1,100 adults (mean age, 40.5 years; 51% women) in the United States who were recruited in August 2021.
Among those surveyed, 27.3% reported “moderately problematic” news consumption, 27.5% reported minimally problematic news consumption, and 28.7% reported no problematic news consumption.
Perhaps not surprisingly, respondents with higher levels of problematic news consumption were significantly more likely to experience mental and physical ill-being than those with lower levels, even after accounting for demographics, personality traits, and overall news use, the researchers note.
Nearly three-quarters (74%) of those with severe levels of problematic news consumption reported experiencing mental ill-being “quite a bit” or “very much” – whereas frequent symptoms were only reported by 8% of all other study participants.
In addition, 61% of adults with severe problematic news consumption reported experiencing physical ill-being “quite a bit” or “very much,” compared with only 6.1% for all other study participants.
Dr. McLaughlin noted that one way to combat this problem is to help individuals develop a healthier relationship with the news – and mindfulness training may be one way to accomplish that.
“We have some preliminary evidence that individuals with high levels of mindfulness are much less susceptible to developing higher levels of problematic news consumption,” he said.
“Given this, mindfulness-based training could potentially help problematic news consumers follow the news without becoming so emotionally invested in it. We hope to examine the effectiveness of a mindfulness intervention in our future research,” he added.
Increased distress
Commenting on the study, Steven R. Thorp, PhD, ABPP, a professor at California School of Professional Psychology, Alliant International University, San Diego, said that he and his colleagues have noticed an increase in clients reporting distress about news consumption.
The survey by Dr. McLaughlin and colleagues “appears to be representative and has sufficient statistical power to address the issues,” said Dr. Thorp, who was not involved with the research.
“However, as the researchers note, it is a cross-sectional and correlational survey. So it’s possible that, as implied, people who ‘doomscroll’ are more likely to have physical and mental health problems that interfere with their functioning,” he added.
It is also possible that individuals with physical and mental health problems are more likely to be isolated and have restricted activities, thus leading to greater news consumption, Dr. Thorp noted. Alternatively, there could be an independent link between health and news consumption.
Most news is “sensational and not representative,” Dr. Thorp pointed out.
For example, “we are far more likely to hear about deaths from terrorist attacks or plane crashes than from heart attacks, though deaths from heart attacks are far more common,” he said.
“News also tends to be negative, rather than uplifting, and most news is not directly relevant to a person’s day-to-day functioning. Thus, for most people, the consumption of news may have more downsides than upsides,” Dr. Thorp added.
Still, many people want to stay informed about national and international events. So rather than following a “cold turkey” or abstinence model of stopping all news consumption, individuals could consider a “harm reduction” model of reducing time spent consuming news, Dr. Thorp noted.
Another thing to consider is the news source. “Some outlets and social media sites are designed to instill outrage, fear, or anger and to increase polarization, while others have been shown to provide balanced and less sensational coverage,” Dr. Thorp said.
“I also think it’s a good idea for providers to regularly ask about news consumption, along with learning about other daily activities that may enhance or diminish mental and physical health,” he added.
The research had no specific funding. Dr. McLaughlin and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The past 2 years have been filled with worrisome global events, from the pandemic to the war in Ukraine, large-scale protests, mass shootings, and devastating wildfires. The 24-hour media coverage of these events can take a toll on “news addicts” who have an excessive urge to constantly check the news, researchers note.
Results from an online survey of more than 1,000 adults showed that nearly 17% showed signs of “severely problematic” news consumption.
These “doomscrollers” or “doomsurfers” scored high on all five problematic news consumption dimensions: being absorbed in news content, being consumed by thoughts about the news, attempting to alleviate feelings of threat by consuming more news, losing control over news consumption, and having news consumption interfere in daily life.
“We anticipated that a sizable portion of our sample would show signs of problematic news consumption. However, we were surprised to find that 17% of study participants suffer from the most severe level of problematic news consumption,” lead author Bryan McLaughlin, PhD, Texas Tech University, Lubbock, told this news organization. “This is certainly concerning and suggests the problem may be more widespread than we expected,” he said.
In addition, 74% of those with severe levels of problematic news consumption reported experiencing mental problems, and 61% reported physical problems.
“It’s important for health care providers to be aware that problematic news consumption may be a significant driver of mental and physical ill-being, especially because a lot of people might be unaware of the negative impact the news is having on their health,” Dr. McLaughlin said.
The findings were published online in Health Communication.
Emotionally invested
The researchers assessed data from an online survey of 1,100 adults (mean age, 40.5 years; 51% women) in the United States who were recruited in August 2021.
Among those surveyed, 27.3% reported “moderately problematic” news consumption, 27.5% reported minimally problematic news consumption, and 28.7% reported no problematic news consumption.
Perhaps not surprisingly, respondents with higher levels of problematic news consumption were significantly more likely to experience mental and physical ill-being than those with lower levels, even after accounting for demographics, personality traits, and overall news use, the researchers note.
Nearly three-quarters (74%) of those with severe levels of problematic news consumption reported experiencing mental ill-being “quite a bit” or “very much” – whereas frequent symptoms were only reported by 8% of all other study participants.
In addition, 61% of adults with severe problematic news consumption reported experiencing physical ill-being “quite a bit” or “very much,” compared with only 6.1% for all other study participants.
Dr. McLaughlin noted that one way to combat this problem is to help individuals develop a healthier relationship with the news – and mindfulness training may be one way to accomplish that.
“We have some preliminary evidence that individuals with high levels of mindfulness are much less susceptible to developing higher levels of problematic news consumption,” he said.
“Given this, mindfulness-based training could potentially help problematic news consumers follow the news without becoming so emotionally invested in it. We hope to examine the effectiveness of a mindfulness intervention in our future research,” he added.
Increased distress
Commenting on the study, Steven R. Thorp, PhD, ABPP, a professor at California School of Professional Psychology, Alliant International University, San Diego, said that he and his colleagues have noticed an increase in clients reporting distress about news consumption.
The survey by Dr. McLaughlin and colleagues “appears to be representative and has sufficient statistical power to address the issues,” said Dr. Thorp, who was not involved with the research.
“However, as the researchers note, it is a cross-sectional and correlational survey. So it’s possible that, as implied, people who ‘doomscroll’ are more likely to have physical and mental health problems that interfere with their functioning,” he added.
It is also possible that individuals with physical and mental health problems are more likely to be isolated and have restricted activities, thus leading to greater news consumption, Dr. Thorp noted. Alternatively, there could be an independent link between health and news consumption.
Most news is “sensational and not representative,” Dr. Thorp pointed out.
For example, “we are far more likely to hear about deaths from terrorist attacks or plane crashes than from heart attacks, though deaths from heart attacks are far more common,” he said.
“News also tends to be negative, rather than uplifting, and most news is not directly relevant to a person’s day-to-day functioning. Thus, for most people, the consumption of news may have more downsides than upsides,” Dr. Thorp added.
Still, many people want to stay informed about national and international events. So rather than following a “cold turkey” or abstinence model of stopping all news consumption, individuals could consider a “harm reduction” model of reducing time spent consuming news, Dr. Thorp noted.
Another thing to consider is the news source. “Some outlets and social media sites are designed to instill outrage, fear, or anger and to increase polarization, while others have been shown to provide balanced and less sensational coverage,” Dr. Thorp said.
“I also think it’s a good idea for providers to regularly ask about news consumption, along with learning about other daily activities that may enhance or diminish mental and physical health,” he added.
The research had no specific funding. Dr. McLaughlin and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The past 2 years have been filled with worrisome global events, from the pandemic to the war in Ukraine, large-scale protests, mass shootings, and devastating wildfires. The 24-hour media coverage of these events can take a toll on “news addicts” who have an excessive urge to constantly check the news, researchers note.
Results from an online survey of more than 1,000 adults showed that nearly 17% showed signs of “severely problematic” news consumption.
These “doomscrollers” or “doomsurfers” scored high on all five problematic news consumption dimensions: being absorbed in news content, being consumed by thoughts about the news, attempting to alleviate feelings of threat by consuming more news, losing control over news consumption, and having news consumption interfere in daily life.
“We anticipated that a sizable portion of our sample would show signs of problematic news consumption. However, we were surprised to find that 17% of study participants suffer from the most severe level of problematic news consumption,” lead author Bryan McLaughlin, PhD, Texas Tech University, Lubbock, told this news organization. “This is certainly concerning and suggests the problem may be more widespread than we expected,” he said.
In addition, 74% of those with severe levels of problematic news consumption reported experiencing mental problems, and 61% reported physical problems.
“It’s important for health care providers to be aware that problematic news consumption may be a significant driver of mental and physical ill-being, especially because a lot of people might be unaware of the negative impact the news is having on their health,” Dr. McLaughlin said.
The findings were published online in Health Communication.
Emotionally invested
The researchers assessed data from an online survey of 1,100 adults (mean age, 40.5 years; 51% women) in the United States who were recruited in August 2021.
Among those surveyed, 27.3% reported “moderately problematic” news consumption, 27.5% reported minimally problematic news consumption, and 28.7% reported no problematic news consumption.
Perhaps not surprisingly, respondents with higher levels of problematic news consumption were significantly more likely to experience mental and physical ill-being than those with lower levels, even after accounting for demographics, personality traits, and overall news use, the researchers note.
Nearly three-quarters (74%) of those with severe levels of problematic news consumption reported experiencing mental ill-being “quite a bit” or “very much” – whereas frequent symptoms were only reported by 8% of all other study participants.
In addition, 61% of adults with severe problematic news consumption reported experiencing physical ill-being “quite a bit” or “very much,” compared with only 6.1% for all other study participants.
Dr. McLaughlin noted that one way to combat this problem is to help individuals develop a healthier relationship with the news – and mindfulness training may be one way to accomplish that.
“We have some preliminary evidence that individuals with high levels of mindfulness are much less susceptible to developing higher levels of problematic news consumption,” he said.
“Given this, mindfulness-based training could potentially help problematic news consumers follow the news without becoming so emotionally invested in it. We hope to examine the effectiveness of a mindfulness intervention in our future research,” he added.
Increased distress
Commenting on the study, Steven R. Thorp, PhD, ABPP, a professor at California School of Professional Psychology, Alliant International University, San Diego, said that he and his colleagues have noticed an increase in clients reporting distress about news consumption.
The survey by Dr. McLaughlin and colleagues “appears to be representative and has sufficient statistical power to address the issues,” said Dr. Thorp, who was not involved with the research.
“However, as the researchers note, it is a cross-sectional and correlational survey. So it’s possible that, as implied, people who ‘doomscroll’ are more likely to have physical and mental health problems that interfere with their functioning,” he added.
It is also possible that individuals with physical and mental health problems are more likely to be isolated and have restricted activities, thus leading to greater news consumption, Dr. Thorp noted. Alternatively, there could be an independent link between health and news consumption.
Most news is “sensational and not representative,” Dr. Thorp pointed out.
For example, “we are far more likely to hear about deaths from terrorist attacks or plane crashes than from heart attacks, though deaths from heart attacks are far more common,” he said.
“News also tends to be negative, rather than uplifting, and most news is not directly relevant to a person’s day-to-day functioning. Thus, for most people, the consumption of news may have more downsides than upsides,” Dr. Thorp added.
Still, many people want to stay informed about national and international events. So rather than following a “cold turkey” or abstinence model of stopping all news consumption, individuals could consider a “harm reduction” model of reducing time spent consuming news, Dr. Thorp noted.
Another thing to consider is the news source. “Some outlets and social media sites are designed to instill outrage, fear, or anger and to increase polarization, while others have been shown to provide balanced and less sensational coverage,” Dr. Thorp said.
“I also think it’s a good idea for providers to regularly ask about news consumption, along with learning about other daily activities that may enhance or diminish mental and physical health,” he added.
The research had no specific funding. Dr. McLaughlin and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HEALTH COMMUNICATION
Distorted time perception during the pandemic tied to stress, poor mental health
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL TRAUMA: THEORY, RESEARCH, PRACTICE, AND POLICY
How do you live with COVID? One doctor’s personal experience
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Stable, long-term opioid therapy safer than tapering?
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.

The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.

The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed data for almost 200,000 patients who did not have signs of opioid use disorder (OUD) and were receiving opioid treatment. The investigators compared three dosing strategies: abrupt withdrawal, gradual tapering, and continuation of the current stable dosage.
Results showed a higher adjusted cumulative incidence of opioid overdose or suicide events 11 months after baseline among participants for whom a tapered dosing strategy was utilized, compared with those who continued taking a stable dosage. The risk difference was 0.15% between taper and stable dosage and 0.33% between abrupt discontinuation and stable dosage.
“This study identified a small absolute increase in risk of harms associated with opioid tapering compared with a stable opioid dosage,” Marc LaRochelle, MD, MPH, assistant professor, Boston University, and colleagues write.
“These results do not suggest that policies of mandatory dosage tapering for individuals receiving a stable long-term opioid dosage without evidence of opioid misuse will reduce short-term harm via suicide and overdose,” they add.
The findings were published online in JAMA Network Open.
Benefits vs. harms
The investigators note that the Centers for Disease Control and Prevention, in its 2016 Guideline for Prescribing Opioids for Chronic Pain, “recommended tapering opioid dosages if benefits no longer outweigh harms.”
In response, “some health systems and U.S. states enacted stringent dose limits that were applied with few exceptions, regardless of individual patients’ risk of harms,” they write. By contrast, there have been “increasing reports of patients experiencing adverse effects from forced opioid tapers.”
Previous studies that identified harms associated with opioid tapering and discontinuation had several limitations, including a focus on discontinuation, which is “likely more destabilizing than gradual tapering,” the researchers write. There is also “a high potential for confounding” in these studies, they add.
The investigators sought to fill the research gap by drawing on 8-year data (Jan. 1, 2010, to Dec. 31, 2018) from a large database that includes adjudicated pharmacy, outpatient, and inpatient medical claims for individuals with commercial or Medicare Advantage insurance encompassing all 50 states, the District of Columbia, and Puerto Rico.
Notably, individuals who had received a diagnosis of substance use, abuse, or dependence or for whom there were indicators consistent with OUD were excluded.
The researchers compared the three treatment strategies during a 4-month treatment strategy assignment period (“grace period”) after baseline. Tapering was defined as “2 consecutive months with a mean MME [morphine milligram equivalent] reduction of 15% or more compared with the baseline month.”
All estimates were adjusted for potential confounders, including demographic and treatment characteristics, baseline year, region, insurance plan type, comorbid psychiatric and medical conditions, and the prescribing of other psychiatric medications, such as benzodiazepines, gabapentin, or pregabalin.
Patient-centered approaches
The final cohort that met inclusion criteria consisted of 199,836 individuals (45.1% men; mean age, 56.9 years). Of the total group, 57.6% were aged 45-64 years. There were 415,123 qualifying long-term opioid therapy episodes.
The largest percentage of the cohort (41.2%) were receiving a baseline mean MME of 50-89 mg/day, while 34% were receiving 90-199 mg/day and 23.5% were receiving at least 200 mg/day.
During the 6-month eligibility assessment period, 34.8% of the cohort were receiving benzodiazepine prescriptions, 18% had been diagnosed with comorbid anxiety, and 19.7% had been diagnosed with comorbid depression.
After the treatment assignment period, most treatment episodes (87.1%) were considered stable, 11.1% were considered a taper, and 1.8% were considered abrupt discontinuation.
Eleven months after baseline, the adjusted cumulative incidence of opioid overdose or suicide events was lowest for those who continued to receive a stable dose.

The risk differences between taper vs. stable dosage were 0.15% (95% confidence interval, 0.03%-0.26%), and the risk differences between abrupt discontinuation and stable dose were 0.33% (95% CI, −0.03%-0.74%). The risk ratios associated with taper vs. stable dosage and abrupt discontinuation vs. stable dosage were 1.15 (95% CI, 1.04-1.27) and 1.34 (95% CI, 0.97-1.79), respectively.
The adjusted cumulative incidence curves for overdose or suicide diverged at month 4 when comparing stable dosage and taper, with a higher incidence associated with the taper vs. stable dosage treatment strategies thereafter. However, when the researchers compared stable dosage with abrupt discontinuation, the event rates were similar.
A per protocol analysis, in which the researchers censored episodes involving lack of adherence to assigned treatment, yielded results similar to those of the main analysis.
“Policies establishing dosage thresholds or mandating tapers for all patients receiving long-term opioid therapy are not supported by existing data in terms of anticipated benefits even if, as we found, the rate of adverse outcomes is small,” the investigators write.
Instead, they encourage health care systems and clinicians to “continue to develop and implement patient-centered approaches to pain management for patients with established long-term opioid therapy.”
Protracted withdrawal?
Commenting on the study, A. Benjamin Srivastava, MD, assistant professor of clinical psychiatry, division on substance use disorders, Columbia University Medical Center, New York State Psychiatric Institute, New York, called the study “an important contribution to the literature” that “sheds further light on the risks associated with tapering.”
Dr. Srivastava, who was not involved with the research, noted that previous studies showing an increased prevalence of adverse events with tapering included participants with OUD or signs of opioid misuse, “potentially confounding findings.”
By contrast, the current study investigators specifically excluded patients with OUD/opioid misuse but still found a “slight increase in risk for opioid overdose and suicide, even when excluding for potential confounders,” he said.
Although causal implications require further investigation, “a source of these adverse outcomes may be unmanaged withdrawal that may be protracted,” Dr. Srivastava noted.
While abrupt discontinuation “may result in significant acute withdrawal symptoms, these should subside by 1-2 weeks at most,” he said.
Lowering the dose without discontinuation may lead to patients’ entering into “a dyshomeostatic state characterized by anxiety and dysphoria ... that may not be recognized by the prescribing clinician,” he added.
The brain “is still being primed by opioids [and] ‘wanting’ a higher dose. Thus, particular attention to withdrawal symptoms, both physical and psychiatric, is prudent when choosing to taper opioids vs. maintaining or discontinuing,” Dr. Srivastava said.
The study was funded by a grant from the CDC and a grant from the National Institute on Drug Abuse to one of the investigators. Dr. LaRochelle received grants from the CDC and NIDA during the conduct of the study and has received consulting fees for research paid to his institution from OptumLabs outside the submitted work. The other investigators’ disclosures are listed in the original article. Dr. Srivastava reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Infographic: Is physician behavior on social media really so bad?
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.
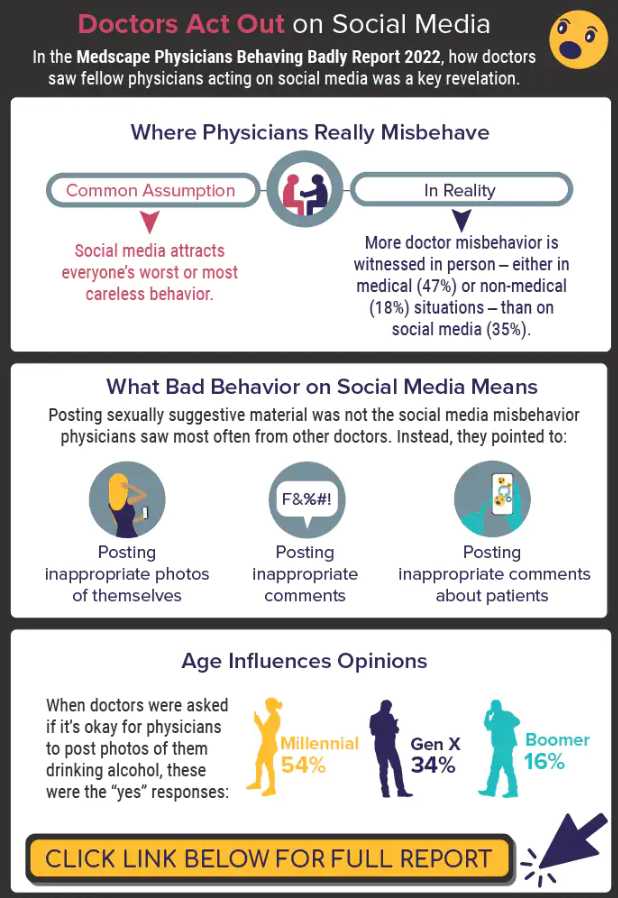
A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.

A version of this article first appeared on Medscape.com.
The medical profession is held to a high standard of personal conduct, so physicians keep a sharp eye out for how fellow doctors behave. That goes for social media as well as in-person conduct.
(and it’s not as egregious as you might think). If you’re interested in delving deeper into the data, check out the Medscape Physicians Behaving Badly Report 2022.

A version of this article first appeared on Medscape.com.
TikTok’s impact on adolescent mental health
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
For younger generations, TikTok is a go-to site for those who like short and catchy video clips. As a social media platform that allows concise video sharing, TikTok has over 1 billion monthly global users. Because of its platform size, a plethora of resources, and influence on media discourse, TikTok is the place for content creators to share visual media. Its cursory, condensed content delivery with videos capped at 1-minute focuses on high-yield information and rapid identification of fundamental points that are both engaging and entertaining.
Currently, on TikTok, 40 billion views are associated with the hashtag #mentalhealth. Content creators and regular users are employing this platform to share their own experiences, opinions, and strategies to overcome their struggles. While it is understandable for creators to share their personal stories that may be abusive, traumatic, or violent, they may not be prepared for their video to “go viral.”
Like any other social media platform, hateful speech such as racism, sexism, or xenophobia can accumulate on TikTok, which may cause more self-harm than self-help. Oversharing about personal strategies may lead to misconceived advice for TikTok viewers, while watching these TikTok videos can have negative mental health effects, even though there are no malicious intentions behind the creators who post these videos.
Hence, public health should pay more attention to the potential health-related implications this platform can create, as the quality of the information and the qualifications of the creators are mostly unrevealed. The concerns include undisclosed conflicts of interest, unchecked spread of misinformation, difficulty identifying source credibility, and excessive false information that viewers must filter through.1,2
Individual TikTok users may follow accounts and interpret these content creators as therapists and the content they see as therapy. They may also believe that a close relationship with the content creator exists when it does not. Specifically, these relationships may be defined as parasocial relationships, which are one-sided relationships where one person (the TikTok viewer) extends emotional energy, interest, and time, and the other party (the content creator) is completely unaware of the other’s existence.3 Additionally, Americans who are uninsured/underinsured may turn to this diluted version of therapy to compensate for the one-on-one or group therapy they need.
While TikTok may seem like a dangerous platform to browse through or post on, its growing influence cannot be underestimated. With 41% of TikTok users between the ages of 16 and 24, this is an ideal platform to disseminate public health information pertaining to this age group (for example, safe sex practices, substance abuse, and mental health issues).4 Because younger generations have incorporated social media into their daily lives, the medical community can harness TikTok’s potential to disseminate accurate information to potential patients for targeted medical education.
For example, Jake Goodman, MD, MBA, and Melissa Shepard, MD, each have more than a million TikTok followers and are notable psychiatrists who post a variety of content ranging from recognizing signs of depression to reducing stigma around mental health. Similarly, Justin Puder, PhD, is a licensed psychologist who advocates for ways to overcome mental health issues. By creating diverse content with appealing strategies, spreading accurate medical knowledge, and answering common medical questions for the public, these ‘mental health influencers’ educate potential patients to create patient-centered interactions.
While there are many pros and cons to social media platforms, it is undeniable that these platforms – such as TikTok – are here to stay. It is crucial for members of the medical community to recognize the outlets that younger generations use to express themselves and to exploit these media channels therapeutically.
Ms. Wong is a fourth-year medical student at the New York Institute of Technology College of Osteopathic Medicine in Old Westbury, N.Y. Dr. Chua is a psychiatrist with the department of child and adolescent psychiatry and behavioral sciences at Children’s Hospital of Philadelphia, and assistant professor of clinical psychiatry at the University of Pennsylvania, also in Philadelphia.
References
1. Gottlieb M and Dyer S. Information and Disinformation: Social Media in the COVID-19 Crisis. Acad Emerg Med. 2020 Jul;27(7):640-1. doi: 10.1111/acem.14036.
2. De Veirman M et al. Front Psychol. 2019;10:2685. doi: 10.3389/fpsyg.2019.02685.
3. Bennett N-K et al. “Parasocial Relationships: The Nature of Celebrity Fascinations.” National Register of Health Service Psychologists. https://www.findapsychologist.org/parasocial-relationships-the-nature-of-celebrity-fascinations/.
4. Eghtesadi M and Florea A. Can J Public Health. 2020 Jun;111(3):389-91. doi: 10.17269/s41997-020-00343-0.
Sacubitril/valsartan shows cognitive safety in heart failure: PERSPECTIVE
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
BARCELONA – Treatment of patients with chronic heart failure with sacubitril/valsartan (Entresto), a mainstay agent for people with this disorder, produced no hint of incremental adverse cognitive effects during 3 years of treatment in a prospective, controlled, multicenter study with nearly 600 patients, although some experts note that possible adverse cognitive effects of sacubitril were not an issue for many heart failure clinicians, even before the study ran.
The potential for an adverse effect of sacubitril on cognition had arisen as a hypothetical concern because sacubitril inhibits the human enzyme neprilysin. This activity results in beneficial effects for patients with heart failure by increasing levels of several endogenous vasoactive peptides. But neprilysin also degrades amyloid beta peptides and so inhibition of this enzyme could possibly result in accumulation of amyloid peptides in the brain with potential neurotoxic effects, which raised concern among some cardiologists and patients that sacubitril/valsartan could hasten cognitive decline.
Results from the new study, PERSPECTIVE, showed “no evidence that neprilysin inhibition increased the risk of cognitive impairment due to the accumulation of beta amyloid” in patients with heart failure with either mid-range or preserved ejection fraction,” John McMurray, MD, said at the annual congress of the European Society of Cardiology.
Dr. McMurray, professor of medical cardiology at the University of Glasgow, highlighted that the study enrolled only patients with heart failure with a left ventricular ejection fraction of greater than 40% because the study designers considered it “unethical” to withhold treatment with sacubitril/valsartan from patients with an ejection fraction of 40% or less (heart failure with reduced ejection fraction, HFrEF), whereas “no mandate” exists in current treatment guidelines for using sacubitril/valsartan in patients with heart failure and higher ejection fractions. He added that he could see no reason why the results seen in patients with higher ejection fractions would not also apply to those with HFrEF.
Reassuring results, but cost still a drag on uptake
“This was a well-designed trial” with results that are “very reassuring” for a lack of harm from sacubitril/valsartan, commented Biykem Bozkurt, MD, PhD, the study’s designated discussant and professor of medicine at Baylor College of Medicine, Houston. The findings “solidify the lack of risk and are very exciting for the heart failure community because the question has bothered a large number of people, especially older patients” with heart failure.
Following these results, “hopefully more patients with heart failure will receive” sacubitril/valsartan, agreed Dr. McMurray, but he added the caveat that the relatively high cost of the agent (which has a U.S. list price of roughly $6,000/year) has been the primary barrier to wider uptake of the drug for patients with heart failure. Treatment with sacubitril/valsartan is recommended in several society guidelines as a core intervention for patients with HFrEF and as a treatment option for patients with heart failure and higher ejection fractions.
“Cost remains the single biggest deterrent for use” of sacubitril/valsartan, agreed Dipti N. Itchhaporia, MD, director of disease management at the Hoag Heart and Vascular Institute in Newport Beach, Calif. “Concerns about cognitive impairment has not been why people have not been using sacubitril/valsartan,” Dr. Itchhaporia commented in an interview.
PERSPECTIVE enrolled patients with heart failure with an ejection fraction greater than 40% and at least 60 years old at any of 137 sites in 20 countries, with about a third of enrolled patients coming from U.S. centers. The study, which ran enrollment during January 2017–May 2019, excluded people with clinically discernible cognitive impairment at the time of entry.
Researchers randomized patients to either a standard regimen of sacubitril/valsartan (295) or valsartan (297) on top of their background treatment, with most patients also receiving a beta-blocker, a diuretic, and a statin. The enrolled patients averaged about 72 years of age, and more than one-third were at least 75 years old.
The study’s primary endpoint was the performance of these patients in seven different tests of cognitive function using a proprietary metric, the CogState Global Cognitive Composite Score, measured at baseline and then every 6 months during follow-up designed to run for 3 years on treatment (the researchers collected data for at least 30 months of follow-up from 71%-73% of enrolled patients). Average changes in these scores over time tracked nearly the same in both treatment arms and met the study’s prespecified criteria for noninferiority of the sacubitril valsartan treatment, Dr. McMurray reported. The results also showed that roughly 60% of patients in both arms had “some degree of cognitive impairment” during follow-up.
A secondary outcome measure used PET imaging to quantify cerebral accumulation of beta amyloid, and again the results met the study’s prespecified threshold for noninferiority for the patients treated with sacubitril/valsartan, said Dr. McMurray.
Another concern raised by some experts was the relatively brief follow-up of 3 years, and the complexity of heart failure patients who could face several other causes of cognitive decline. The findings “help reassure, but 3 years is not long enough, and I’m not sure the study eliminated all the other possible variables,” commented Dr. Itchhaporia.
But Dr. McMurray contended that 3 years represents robust follow-up in patients with heart failure who notoriously have limited life expectancy following their diagnosis. “Three years is a long time for patients with heart failure.”
The findings also raise the prospect of developing sacubitril/valsartan as an antihypertensive treatment, an indication that has been avoided until now because of the uncertain cognitive effects of the agent and the need for prolonged use when the treated disorder is hypertension instead of heart failure.
PERSPECTIVE was funded by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. McMurray has received consulting and lecture fees from Novartis and he and his institution have received research funding from Novartis. Dr. Bozkurt has been a consultant to numerous companies but has no relationship with Novartis. Dr. Itchhaporia had no disclosures.
AT ESC CONGRESS 2022
Psychedelic drug therapy a potential ‘breakthrough’ for alcohol dependence
Results from the first randomized, placebo-controlled trial of psilocybin for alcohol dependence showed that during the 8 months after first treatment dose, participants who received psilocybin had less than half as many heavy drinking days as their counterparts who received placebo.
In addition, 7 months after the last dose of medication, twice as many psilocybin-treated patients as placebo-treated patients were abstinent.
The effects observed with psilocybin were “considerably larger” than those of currently approved treatments for AUD, senior investigator Michael Bogenschutz, MD, psychiatrist and director of the NYU Langone Center for Psychedelic Medicine, New York, said during an Aug. 24 press briefing.
If the findings hold up in future trials, psilocybin will be a “real breakthrough” in the treatment of the condition, Dr. Bogenschutz said.
The findings were published online in JAMA Psychiatry.
83% reduction in drinking days
The study included 93 adults (mean age, 46 years) with alcohol dependence who consumed an average of seven drinks on the days they drank and had had at least four heavy drinking days during the month prior to treatment.
Of the participants, 48 were randomly assigned to receive two doses of psilocybin, and 45 were assigned to receive an antihistamine (diphenhydramine) placebo. Study medication was administered during 2 day-long sessions at week 4 and week 8.
The participants also received 12 psychotherapy sessions over a 12-week period. All were assessed at intervals from the beginning of the study until 32 weeks after the first medication session.
The primary outcome was percentage of days in which the patient drank heavily during the 32-week period following first medication dose. Heavy drinking was defined as having five or more drinks in a day for a man and four or more drinks in a day for a woman.
The percentage of heavy drinking days during the 32-week period was 9.7% for the psilocybin group and 23.6% for the placebo group, for a mean difference of 13.9% (P = .01).
“Compared to their baseline before the study, after receiving medication, the psilocybin group decreased their heavy drinking days by 83%, while the placebo group reduced their heavy drinking by 51%,” Dr. Bogenschutz reported.
During the last month of follow-up, which was 7 months after the final dose of study medication, 48% of the psilocybin group were entirely abstinent vs. 24% of the placebo group.
“It is remarkable that the effects of psilocybin treatment persisted for 7 months after people received the last dose of medication. This suggests that psilocybin is treating the underlying disorder of alcohol addiction rather than merely treating symptoms,” Dr. Bogenschutz noted.
Total alcohol consumption and problems related to alcohol use were also significantly less in the psilocybin group.
‘Encouraged and hopeful’
Adverse events related to psilocybin were mostly mild, self-limiting, and consistent with other recent trials that evaluated the drug’s effects in various conditions.
However, the current investigators note that they implemented measures to ensure safety, including careful medical and psychiatric screening, therapy, and monitoring that was provided by well-trained therapists, including a licensed psychiatrist. In addition, medications were available to treat acute psychiatric reactions.
A cited limitation of the study was that blinding was not maintained because the average intensity of experience with psilocybin was high, whereas it was low with diphenhydramine.
This difference undermined the masking of treatment such that more than 90% of participants and therapists correctly guessed the treatment assignment.
Another limitation was that objective measures to validate self-reported drinking outcomes were available for only 54% of study participants.
Despite these limitations, the study builds on earlier work by the NYU team that showed that two doses of psilocybin taken over a period of 8 weeks significantly reduced alcohol use and cravings in patients with AUD.
“We’re very encouraged by these findings and hopeful about where they could lead. Personally, it’s been very meaningful and rewarding for me to do this work and inspiring to witness the remarkable recoveries that some of our participants have experienced,” Dr. Bogenschutz told briefing attendees.
Urgent need
The authors of an accompanying editorial note that novel medications for alcohol dependence are “sorely needed. Recent renewed interest in the potential of hallucinogens for treating psychiatric disorders, including AUD, represents a potential move in that direction.”
Henry Kranzler, MD, and Emily Hartwell, PhD, both with the Center for Studies of Addiction, University of Pennsylvania, Philadelphia, write that the new findings “underscore the potential of developing psilocybin as an addition to the alcohol treatment pharmacopeia.”
They question, however, the feasibility of using hallucinogens in routine clinical practice because intensive psychotherapy, such as that provided in this study, requires a significant investment of time and labor.
“Such concomitant therapy, if necessary to realize the therapeutic benefits of psilocybin for treating AUD, could limit its uptake by clinicians,” Dr. Kranzler and Dr. Hartwell write.
The study was funded by the Heffter Research Institute and by individual donations from Carey and Claudia Turnbull, Dr. Efrem Nulman, Rodrigo Niño, and Cody Swift. Dr. Bogenschutz reports having received research funds from and serving as a consultant to Mind Medicine, the Multidisciplinary Association for Psychedelic Studies, B. More, AJNA Labs, Beckley Psytech, Journey Colab, and Bright Minds Biosciences. Dr. Kranzler and Dr. Hartwell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the first randomized, placebo-controlled trial of psilocybin for alcohol dependence showed that during the 8 months after first treatment dose, participants who received psilocybin had less than half as many heavy drinking days as their counterparts who received placebo.
In addition, 7 months after the last dose of medication, twice as many psilocybin-treated patients as placebo-treated patients were abstinent.
The effects observed with psilocybin were “considerably larger” than those of currently approved treatments for AUD, senior investigator Michael Bogenschutz, MD, psychiatrist and director of the NYU Langone Center for Psychedelic Medicine, New York, said during an Aug. 24 press briefing.
If the findings hold up in future trials, psilocybin will be a “real breakthrough” in the treatment of the condition, Dr. Bogenschutz said.
The findings were published online in JAMA Psychiatry.
83% reduction in drinking days
The study included 93 adults (mean age, 46 years) with alcohol dependence who consumed an average of seven drinks on the days they drank and had had at least four heavy drinking days during the month prior to treatment.
Of the participants, 48 were randomly assigned to receive two doses of psilocybin, and 45 were assigned to receive an antihistamine (diphenhydramine) placebo. Study medication was administered during 2 day-long sessions at week 4 and week 8.
The participants also received 12 psychotherapy sessions over a 12-week period. All were assessed at intervals from the beginning of the study until 32 weeks after the first medication session.
The primary outcome was percentage of days in which the patient drank heavily during the 32-week period following first medication dose. Heavy drinking was defined as having five or more drinks in a day for a man and four or more drinks in a day for a woman.
The percentage of heavy drinking days during the 32-week period was 9.7% for the psilocybin group and 23.6% for the placebo group, for a mean difference of 13.9% (P = .01).
“Compared to their baseline before the study, after receiving medication, the psilocybin group decreased their heavy drinking days by 83%, while the placebo group reduced their heavy drinking by 51%,” Dr. Bogenschutz reported.
During the last month of follow-up, which was 7 months after the final dose of study medication, 48% of the psilocybin group were entirely abstinent vs. 24% of the placebo group.
“It is remarkable that the effects of psilocybin treatment persisted for 7 months after people received the last dose of medication. This suggests that psilocybin is treating the underlying disorder of alcohol addiction rather than merely treating symptoms,” Dr. Bogenschutz noted.
Total alcohol consumption and problems related to alcohol use were also significantly less in the psilocybin group.
‘Encouraged and hopeful’
Adverse events related to psilocybin were mostly mild, self-limiting, and consistent with other recent trials that evaluated the drug’s effects in various conditions.
However, the current investigators note that they implemented measures to ensure safety, including careful medical and psychiatric screening, therapy, and monitoring that was provided by well-trained therapists, including a licensed psychiatrist. In addition, medications were available to treat acute psychiatric reactions.
A cited limitation of the study was that blinding was not maintained because the average intensity of experience with psilocybin was high, whereas it was low with diphenhydramine.
This difference undermined the masking of treatment such that more than 90% of participants and therapists correctly guessed the treatment assignment.
Another limitation was that objective measures to validate self-reported drinking outcomes were available for only 54% of study participants.
Despite these limitations, the study builds on earlier work by the NYU team that showed that two doses of psilocybin taken over a period of 8 weeks significantly reduced alcohol use and cravings in patients with AUD.
“We’re very encouraged by these findings and hopeful about where they could lead. Personally, it’s been very meaningful and rewarding for me to do this work and inspiring to witness the remarkable recoveries that some of our participants have experienced,” Dr. Bogenschutz told briefing attendees.
Urgent need
The authors of an accompanying editorial note that novel medications for alcohol dependence are “sorely needed. Recent renewed interest in the potential of hallucinogens for treating psychiatric disorders, including AUD, represents a potential move in that direction.”
Henry Kranzler, MD, and Emily Hartwell, PhD, both with the Center for Studies of Addiction, University of Pennsylvania, Philadelphia, write that the new findings “underscore the potential of developing psilocybin as an addition to the alcohol treatment pharmacopeia.”
They question, however, the feasibility of using hallucinogens in routine clinical practice because intensive psychotherapy, such as that provided in this study, requires a significant investment of time and labor.
“Such concomitant therapy, if necessary to realize the therapeutic benefits of psilocybin for treating AUD, could limit its uptake by clinicians,” Dr. Kranzler and Dr. Hartwell write.
The study was funded by the Heffter Research Institute and by individual donations from Carey and Claudia Turnbull, Dr. Efrem Nulman, Rodrigo Niño, and Cody Swift. Dr. Bogenschutz reports having received research funds from and serving as a consultant to Mind Medicine, the Multidisciplinary Association for Psychedelic Studies, B. More, AJNA Labs, Beckley Psytech, Journey Colab, and Bright Minds Biosciences. Dr. Kranzler and Dr. Hartwell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results from the first randomized, placebo-controlled trial of psilocybin for alcohol dependence showed that during the 8 months after first treatment dose, participants who received psilocybin had less than half as many heavy drinking days as their counterparts who received placebo.
In addition, 7 months after the last dose of medication, twice as many psilocybin-treated patients as placebo-treated patients were abstinent.
The effects observed with psilocybin were “considerably larger” than those of currently approved treatments for AUD, senior investigator Michael Bogenschutz, MD, psychiatrist and director of the NYU Langone Center for Psychedelic Medicine, New York, said during an Aug. 24 press briefing.
If the findings hold up in future trials, psilocybin will be a “real breakthrough” in the treatment of the condition, Dr. Bogenschutz said.
The findings were published online in JAMA Psychiatry.
83% reduction in drinking days
The study included 93 adults (mean age, 46 years) with alcohol dependence who consumed an average of seven drinks on the days they drank and had had at least four heavy drinking days during the month prior to treatment.
Of the participants, 48 were randomly assigned to receive two doses of psilocybin, and 45 were assigned to receive an antihistamine (diphenhydramine) placebo. Study medication was administered during 2 day-long sessions at week 4 and week 8.
The participants also received 12 psychotherapy sessions over a 12-week period. All were assessed at intervals from the beginning of the study until 32 weeks after the first medication session.
The primary outcome was percentage of days in which the patient drank heavily during the 32-week period following first medication dose. Heavy drinking was defined as having five or more drinks in a day for a man and four or more drinks in a day for a woman.
The percentage of heavy drinking days during the 32-week period was 9.7% for the psilocybin group and 23.6% for the placebo group, for a mean difference of 13.9% (P = .01).
“Compared to their baseline before the study, after receiving medication, the psilocybin group decreased their heavy drinking days by 83%, while the placebo group reduced their heavy drinking by 51%,” Dr. Bogenschutz reported.
During the last month of follow-up, which was 7 months after the final dose of study medication, 48% of the psilocybin group were entirely abstinent vs. 24% of the placebo group.
“It is remarkable that the effects of psilocybin treatment persisted for 7 months after people received the last dose of medication. This suggests that psilocybin is treating the underlying disorder of alcohol addiction rather than merely treating symptoms,” Dr. Bogenschutz noted.
Total alcohol consumption and problems related to alcohol use were also significantly less in the psilocybin group.
‘Encouraged and hopeful’
Adverse events related to psilocybin were mostly mild, self-limiting, and consistent with other recent trials that evaluated the drug’s effects in various conditions.
However, the current investigators note that they implemented measures to ensure safety, including careful medical and psychiatric screening, therapy, and monitoring that was provided by well-trained therapists, including a licensed psychiatrist. In addition, medications were available to treat acute psychiatric reactions.
A cited limitation of the study was that blinding was not maintained because the average intensity of experience with psilocybin was high, whereas it was low with diphenhydramine.
This difference undermined the masking of treatment such that more than 90% of participants and therapists correctly guessed the treatment assignment.
Another limitation was that objective measures to validate self-reported drinking outcomes were available for only 54% of study participants.
Despite these limitations, the study builds on earlier work by the NYU team that showed that two doses of psilocybin taken over a period of 8 weeks significantly reduced alcohol use and cravings in patients with AUD.
“We’re very encouraged by these findings and hopeful about where they could lead. Personally, it’s been very meaningful and rewarding for me to do this work and inspiring to witness the remarkable recoveries that some of our participants have experienced,” Dr. Bogenschutz told briefing attendees.
Urgent need
The authors of an accompanying editorial note that novel medications for alcohol dependence are “sorely needed. Recent renewed interest in the potential of hallucinogens for treating psychiatric disorders, including AUD, represents a potential move in that direction.”
Henry Kranzler, MD, and Emily Hartwell, PhD, both with the Center for Studies of Addiction, University of Pennsylvania, Philadelphia, write that the new findings “underscore the potential of developing psilocybin as an addition to the alcohol treatment pharmacopeia.”
They question, however, the feasibility of using hallucinogens in routine clinical practice because intensive psychotherapy, such as that provided in this study, requires a significant investment of time and labor.
“Such concomitant therapy, if necessary to realize the therapeutic benefits of psilocybin for treating AUD, could limit its uptake by clinicians,” Dr. Kranzler and Dr. Hartwell write.
The study was funded by the Heffter Research Institute and by individual donations from Carey and Claudia Turnbull, Dr. Efrem Nulman, Rodrigo Niño, and Cody Swift. Dr. Bogenschutz reports having received research funds from and serving as a consultant to Mind Medicine, the Multidisciplinary Association for Psychedelic Studies, B. More, AJNA Labs, Beckley Psytech, Journey Colab, and Bright Minds Biosciences. Dr. Kranzler and Dr. Hartwell have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY

















