User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Why exercise doesn’t help people with long COVID
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
When Joel Fram woke up on the morning of March 12, 2020, he had a pretty good idea why he felt so lousy.
He lives in New York, where the first wave of the coronavirus was tearing through the city. “I instantly knew,” said the 55-year-old Broadway music director. It was COVID-19.
What started with a general sense of having been hit by a truck soon included a sore throat and such severe fatigue that he once fell asleep in the middle of sending a text to his sister. The final symptoms were chest tightness and trouble breathing.
And then he started to feel better. “By mid-April, my body was feeling essentially back to normal,” he said.
So he did what would have been smart after almost any other illness: He began working out. That didn’t last long. “It felt like someone pulled the carpet out from under me,” he remembered. “I couldn’t walk three blocks without getting breathless and fatigued.”
That was the first indication Mr. Fram had long COVID.
According to the National Center for Health Statistics, at least 7.5% of American adults – close to 20 million people – have symptoms of long COVID.
COVID-19 patients who had the most severe illness will struggle the most with exercise later, according to a review published in June from researchers at the University of California, San Francisco. But even people with mild symptoms can struggle to regain their previous levels of fitness.
“We have participants in our study who had relatively mild acute symptoms and went on to have really profound decreases in their ability to exercise,” said Matthew S. Durstenfeld, MD, a cardiologist at UCSF and principal author of the review.
Most people with long COVID will have lower-than-expected scores on tests of aerobic fitness, as shown by Yale researchers in a study published in August 2021.
“Some amount of that is due to deconditioning,” Dr. Durstenfeld said. “You’re not feeling well, so you’re not exercising to the same degree you might have been before you got infected.”
In a study published in April, people with long COVID told researchers at Britain’s University of Leeds they spent 93% less time in physical activity than they did before their infection.
But multiple studies have found deconditioning is not entirely – or even mostly – to blame.
A 2021 study found that 89% of participants with long COVID had postexertional malaise (PEM), which happens when a patient’s symptoms get worse after they do even minor physical or mental activities. According to the CDC, postexertional malaise can hit as long as 12-48 hours after the activity, and it can take people up to 2 weeks to fully recover.
Unfortunately, the advice patients get from their doctors sometimes makes the problem worse.
How long COVID defies simple solutions
Long COVID is a “dynamic disability” that requires health professionals to go off script when a patient’s symptoms don’t respond in a predictable way to treatment, said David Putrino, PhD, a neuroscientist, physical therapist, and director of rehabilitation innovation for the Mount Sinai Health System in New York.
“We’re not so good at dealing with somebody who, for all intents and purposes, can appear healthy and nondisabled on one day and be completely debilitated the next day,” he said.
Dr. Putrino said more than half of his clinic’s long-COVID patients told his team they had at least one of these persistent problems:
- Fatigue (82%).
- Brain fog (67%).
- Headache (60%).
- Sleep problems (59%).
- Dizziness (54%).
And 86% said exercise worsened their symptoms.
The symptoms are similar to what doctors see with illnesses such as lupus, Lyme disease, and chronic fatigue syndrome – something many experts compare long COVID to. Researchers and medical professionals still don’t know exactly how COVID-19 causes those symptoms. But there are some theories.
Potential causes of long-COVID symptoms
Dr. Putrino said it is possible the virus enters a patient’s cells and hijacks the mitochondria – a part of the cell that provides energy. It can linger there for weeks or months – something known as viral persistence.
“All of a sudden, the body’s getting less energy for itself, even though it’s producing the same amount, or even a little more,” he said. And there is a consequence to this extra stress on the cells. “Creating energy isn’t free. You’re producing more waste products, which puts your body in a state of oxidative stress,” Dr. Putrino said. Oxidative stress damages cells as molecules interact with oxygen in harmful ways.
“The other big mechanism is autonomic dysfunction,” Dr. Putrino said. It’s marked by breathing problems, heart palpitations, and other glitches in areas most healthy people never have to think about. About 70% of long-COVID patients at Mount Sinai’s clinic have some degree of autonomic dysfunction, he said.
For a person with autonomic dysfunction, something as basic as changing posture can trigger a storm of cytokines, a chemical messenger that tells the immune system where and how to respond to challenges like an injury or infection.
“Suddenly, you have this on-off switch,” Dr. Putrino said. “You go straight to ‘fight or flight,’ ” with a surge of adrenaline and a spiking heart rate, “then plunge back to ‘rest or digest.’ You go from fired up to so sleepy, you can’t keep your eyes open.”
A patient with viral persistence and one with autonomic dysfunction may have the same negative reaction to exercise, even though the triggers are completely different.
So how can doctors help long-COVID patients?
The first step, Dr. Putrino said, is to understand the difference between long COVID and a long recovery from COVID-19 infection.
Many of the patients in the latter group still have symptoms 4 weeks after their first infection. “At 4 weeks, yeah, they’re still feeling symptoms, but that’s not long COVID,” he said. “That’s just taking a while to get over a viral infection.”
Fitness advice is simple for those people: Take it easy at first, and gradually increase the amount and intensity of aerobic exercise and strength training.
But that advice would be disastrous for someone who meets Dr. Putrino’s stricter definition of long COVID: “Three to 4 months out from initial infection, they’re experiencing severe fatigue, exertional symptoms, cognitive symptoms, heart palpitations, shortness of breath,” he said.
“Our clinic is extraordinarily cautious with exercise” for those patients, he said.
In Dr. Putrino’s experience, about 20%-30% of patients will make significant progress after 12 weeks. “They’re feeling more or less like they felt pre-COVID,” he said.
The unluckiest 10%-20% won’t make any progress at all. Any type of therapy, even if it’s as simple as moving their legs from a flat position, worsens their symptoms.
The majority – 50%-60% – will have some improvement in their symptoms. But then progress will stop, for reasons researchers are still trying to figure out.
“My sense is that gradually increasing your exercise is still good advice for the vast majority of people,” UCSF’s Dr. Durstenfeld said.
Ideally, that exercise will be supervised by someone trained in cardiac, pulmonary, and/or autonomic rehabilitation – a specialized type of therapy aimed at resyncing the autonomic nervous system that governs breathing and other unconscious functions, he said. But those therapies are rarely covered by insurance, which means most long-COVID patients are on their own.
Dr. Durstenfeld said it’s important that patients keep trying and not give up. “With slow and steady progress, a lot of people can get profoundly better,” he said.
Mr. Fram, who’s worked with careful supervision, says he’s getting closer to something like his pre-COVID-19 life.
But he’s not there yet. Long COVID, he said, “affects my life every single day.”
A version of this article first appeared on WebMD.com.
Waking up at night could be your brain boosting your memory
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
FROM NATURE NEUROSCIENCE
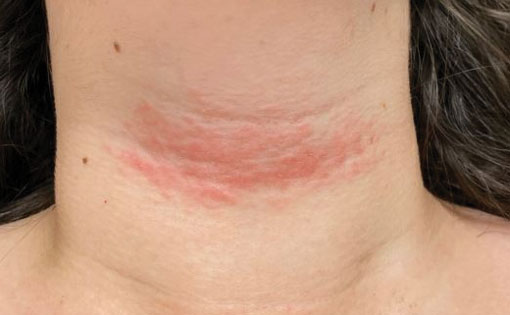
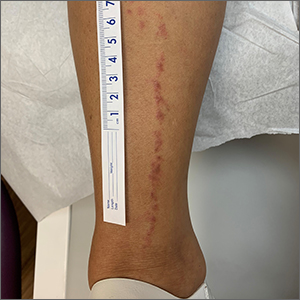
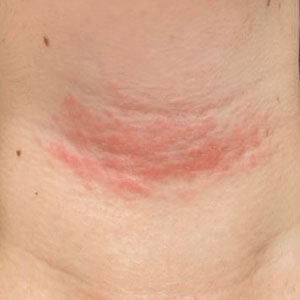
Linear leg rash

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A 4-mm punch biopsy confirmed that this was a case of blaschkitis. This uncommon condition is referred to as adult blaschkitis because it resembles lichen striatus, a linear erythematous papular eruption usually seen in children younger than 15 years of age that erupts along Blaschko lines. The biopsy in this case helped to rule out lichen planus, which can also manifest with an erythematous papular eruption along Blaschko lines.
Adult blaschkitis is thought to be a hypersensitivity reaction involving T cells. It has been linked to medication use, insect stings, trauma, and autoimmune disease.1 The characteristic linear pattern is due to the inflammatory response following the Blaschko lines of keratinocytes that migrated during the embryonic phase.1 Post-inflammatory hyperpigmentation is a frequent complication. Topical steroids often help with the itching, but do not usually make the lesions go away. There have been better results in reducing itching and lesion prominence with intralesional steroid injections, topical calcipotriol, or calcineurin inhibitors.1 The inflammation usually spontaneously resolves over 3 to 12 months.
The patient was advised that the condition is benign and would likely resolve on its own over time. She was counseled that since the clobetasol was helping with her itching, she could use it (sparingly) as needed. She was cautioned that prolonged usage could lead to skin atrophy.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846
1. Al-Balbeesi A. Adult blaschkitis with lichenoid features and blood eosinophilia. Cureus. 2021;13:e16846. doi: 10.7759/cureus.16846
COVID-19 and IPF: Fundamental similarities found
An AI-guided analysis of more than 1,000 human lung transcriptomic datasets found that COVID-19 resembles idiopathic pulmonary fibrosis (IPF) at a fundamental level, according to a study published in eBiomedicine, part of The Lancet Discovery Science.
In the aftermath of COVID-19, a significant number of patients develop a fibrotic lung disease, for which insights into pathogenesis, disease models, or treatment options are lacking, according to researchers Dr. Sinha and colleagues. This long-haul form of the disease culminates in a fibrotic type of interstitial lung disease (ILD). While the actual prevalence of post–COVID-19 ILD (PCLD) is still emerging, early analysis indicates that more than a third of COVID-19 survivors develop fibrotic abnormalities, according to the authors.
Previous research has shown that one of the important determinants for PCLD is the duration of disease. Among patients who developed fibrosis, approximately 4% of patients had a disease duration of less than 1 week; approximately 24% had a disease duration between 1 and 3 weeks; and around 61% had a disease duration longer than 3 weeks, the authors stated.
The lung transcriptomic datasets compared in their study were associated with various lung conditions. The researchers used two viral pandemic signatures (ViP and sViP) and one COVID lung-derived signature. They found that the resemblances included that COVID-19 recapitulates the gene expression patterns (ViP and IPF signatures), cytokine storm (IL15-centric), and the AT2 cytopathic changes, for example, injury, DNA damage, arrest in a transient, damage-induced progenitor state, and senescence-associated secretory phenotype (SASP).
In laboratory experiments, Dr. Sinha and colleagues were able to induce these same immunocytopathic features in preclinical COVID-19 models (human adult lung organoid and hamster) and to reverse them in the hamster model with effective anti–CoV-2 therapeutics.
PPI-network analyses pinpointed endoplasmic reticulum (ER) stress as one of the shared early triggers of both IPF and COVID-19, and immunohistochemistry studies validated the same in the lungs of deceased subjects with COVID-19 and the SARS-CoV-2–challenged hamster lungs. Additionally, lungs from transgenic mice, in which ER stress was induced specifically in the AT2 cells, faithfully recapitulated the host immune response and alveolar cytopathic changes that are induced by SARS-CoV-2.
stated corresponding author Pradipta Ghosh, MD, professor in the departments of medicine and cellular and molecular medicine, University of California, San Diego. “If proven in prospective studies, this biomarker could indicate who is at greatest risk for progressive fibrosis and may require lung transplantation,” she said in an interview.
Dr. Ghosh stated further, “When it comes to therapeutics in COVID lung or IPF, we also found that shared fundamental pathogenic mechanisms present excellent opportunities for developing therapeutics that can arrest the fibrogenic drivers in both diseases. One clue that emerged is a specific cytokine that is at the heart of the smoldering inflammation which is invariably associated with fibrosis. That is interleukin 15 [IL-15] and its receptor.” Dr. Ghosh observed that there are two Food and Drug Administration–approved drugs for IPF. “None are very effective in arresting this invariably fatal disease. Hence, finding better options to treat IPF is an urgent and an unmet need.”
Preclinical testing of hypotheses, Dr. Ghosh said, is next on the path to clinical trials. “We have the advantage of using human lung organoids (mini-lungs grown using stem cells) in a dish, adding additional cells to the system (like fibroblasts and immune cells), infecting them with the virus, or subjecting them to the IL-15 cytokine and monitoring lung fibrosis progression in a dish. Anti–IL-15 therapy can then be initiated to observe reversal of the fibrogenic cascade.” Hamsters have also been shown to provide appropriate models for mimicking lung fibrosis, Dr. Ghosh said.
“The report by Sinha and colleagues describes the fascinating similarities between drivers of post-COVID lung disease and idiopathic pulmonary fibrosis,” stated David Bowton, MD, professor emeritus, section on critical care, department of anesthesiology, Wake Forest University, Winston-Salem, N.C., in an interview. He added that, “Central to the mechanisms of induction of fibrosis in both disorders appears to be endoplasmic reticulum stress in alveolar type II cells (AT2). ER stress induces the unfolded protein response (UPR) that halts protein translation and promotes the degradation of misfolded proteins. Prolonged UPR can reprogram the cell or trigger apoptosis pathways. ER stress in the lung has been reported in a variety of cell lines including AT2 in IPF, bronchial and alveolar epithelial cells in asthma and [chronic obstructive pulmonary disease], and endothelial cells in pulmonary hypertension.”
Dr. Bowton commented further, including a caution, “Sinha and colleagues suggest that the identification of these gene signatures and mechanisms will be a fruitful avenue for developing effective therapeutics for IPF and other fibrotic lung diseases. I am hopeful that these data may offer clues that expedite this process. However, the redundancy of triggers for effector pathways in biologic systems argues that, even if successful, this will be [a] long and fraught process.”
The research study was supported by National Institutes of Health grants and funding from the Tobacco-Related Disease Research Program.
Dr. Sinha, Dr. Ghosh, and Dr. Bowton reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
An AI-guided analysis of more than 1,000 human lung transcriptomic datasets found that COVID-19 resembles idiopathic pulmonary fibrosis (IPF) at a fundamental level, according to a study published in eBiomedicine, part of The Lancet Discovery Science.
In the aftermath of COVID-19, a significant number of patients develop a fibrotic lung disease, for which insights into pathogenesis, disease models, or treatment options are lacking, according to researchers Dr. Sinha and colleagues. This long-haul form of the disease culminates in a fibrotic type of interstitial lung disease (ILD). While the actual prevalence of post–COVID-19 ILD (PCLD) is still emerging, early analysis indicates that more than a third of COVID-19 survivors develop fibrotic abnormalities, according to the authors.
Previous research has shown that one of the important determinants for PCLD is the duration of disease. Among patients who developed fibrosis, approximately 4% of patients had a disease duration of less than 1 week; approximately 24% had a disease duration between 1 and 3 weeks; and around 61% had a disease duration longer than 3 weeks, the authors stated.
The lung transcriptomic datasets compared in their study were associated with various lung conditions. The researchers used two viral pandemic signatures (ViP and sViP) and one COVID lung-derived signature. They found that the resemblances included that COVID-19 recapitulates the gene expression patterns (ViP and IPF signatures), cytokine storm (IL15-centric), and the AT2 cytopathic changes, for example, injury, DNA damage, arrest in a transient, damage-induced progenitor state, and senescence-associated secretory phenotype (SASP).
In laboratory experiments, Dr. Sinha and colleagues were able to induce these same immunocytopathic features in preclinical COVID-19 models (human adult lung organoid and hamster) and to reverse them in the hamster model with effective anti–CoV-2 therapeutics.
PPI-network analyses pinpointed endoplasmic reticulum (ER) stress as one of the shared early triggers of both IPF and COVID-19, and immunohistochemistry studies validated the same in the lungs of deceased subjects with COVID-19 and the SARS-CoV-2–challenged hamster lungs. Additionally, lungs from transgenic mice, in which ER stress was induced specifically in the AT2 cells, faithfully recapitulated the host immune response and alveolar cytopathic changes that are induced by SARS-CoV-2.
stated corresponding author Pradipta Ghosh, MD, professor in the departments of medicine and cellular and molecular medicine, University of California, San Diego. “If proven in prospective studies, this biomarker could indicate who is at greatest risk for progressive fibrosis and may require lung transplantation,” she said in an interview.
Dr. Ghosh stated further, “When it comes to therapeutics in COVID lung or IPF, we also found that shared fundamental pathogenic mechanisms present excellent opportunities for developing therapeutics that can arrest the fibrogenic drivers in both diseases. One clue that emerged is a specific cytokine that is at the heart of the smoldering inflammation which is invariably associated with fibrosis. That is interleukin 15 [IL-15] and its receptor.” Dr. Ghosh observed that there are two Food and Drug Administration–approved drugs for IPF. “None are very effective in arresting this invariably fatal disease. Hence, finding better options to treat IPF is an urgent and an unmet need.”
Preclinical testing of hypotheses, Dr. Ghosh said, is next on the path to clinical trials. “We have the advantage of using human lung organoids (mini-lungs grown using stem cells) in a dish, adding additional cells to the system (like fibroblasts and immune cells), infecting them with the virus, or subjecting them to the IL-15 cytokine and monitoring lung fibrosis progression in a dish. Anti–IL-15 therapy can then be initiated to observe reversal of the fibrogenic cascade.” Hamsters have also been shown to provide appropriate models for mimicking lung fibrosis, Dr. Ghosh said.
“The report by Sinha and colleagues describes the fascinating similarities between drivers of post-COVID lung disease and idiopathic pulmonary fibrosis,” stated David Bowton, MD, professor emeritus, section on critical care, department of anesthesiology, Wake Forest University, Winston-Salem, N.C., in an interview. He added that, “Central to the mechanisms of induction of fibrosis in both disorders appears to be endoplasmic reticulum stress in alveolar type II cells (AT2). ER stress induces the unfolded protein response (UPR) that halts protein translation and promotes the degradation of misfolded proteins. Prolonged UPR can reprogram the cell or trigger apoptosis pathways. ER stress in the lung has been reported in a variety of cell lines including AT2 in IPF, bronchial and alveolar epithelial cells in asthma and [chronic obstructive pulmonary disease], and endothelial cells in pulmonary hypertension.”
Dr. Bowton commented further, including a caution, “Sinha and colleagues suggest that the identification of these gene signatures and mechanisms will be a fruitful avenue for developing effective therapeutics for IPF and other fibrotic lung diseases. I am hopeful that these data may offer clues that expedite this process. However, the redundancy of triggers for effector pathways in biologic systems argues that, even if successful, this will be [a] long and fraught process.”
The research study was supported by National Institutes of Health grants and funding from the Tobacco-Related Disease Research Program.
Dr. Sinha, Dr. Ghosh, and Dr. Bowton reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
An AI-guided analysis of more than 1,000 human lung transcriptomic datasets found that COVID-19 resembles idiopathic pulmonary fibrosis (IPF) at a fundamental level, according to a study published in eBiomedicine, part of The Lancet Discovery Science.
In the aftermath of COVID-19, a significant number of patients develop a fibrotic lung disease, for which insights into pathogenesis, disease models, or treatment options are lacking, according to researchers Dr. Sinha and colleagues. This long-haul form of the disease culminates in a fibrotic type of interstitial lung disease (ILD). While the actual prevalence of post–COVID-19 ILD (PCLD) is still emerging, early analysis indicates that more than a third of COVID-19 survivors develop fibrotic abnormalities, according to the authors.
Previous research has shown that one of the important determinants for PCLD is the duration of disease. Among patients who developed fibrosis, approximately 4% of patients had a disease duration of less than 1 week; approximately 24% had a disease duration between 1 and 3 weeks; and around 61% had a disease duration longer than 3 weeks, the authors stated.
The lung transcriptomic datasets compared in their study were associated with various lung conditions. The researchers used two viral pandemic signatures (ViP and sViP) and one COVID lung-derived signature. They found that the resemblances included that COVID-19 recapitulates the gene expression patterns (ViP and IPF signatures), cytokine storm (IL15-centric), and the AT2 cytopathic changes, for example, injury, DNA damage, arrest in a transient, damage-induced progenitor state, and senescence-associated secretory phenotype (SASP).
In laboratory experiments, Dr. Sinha and colleagues were able to induce these same immunocytopathic features in preclinical COVID-19 models (human adult lung organoid and hamster) and to reverse them in the hamster model with effective anti–CoV-2 therapeutics.
PPI-network analyses pinpointed endoplasmic reticulum (ER) stress as one of the shared early triggers of both IPF and COVID-19, and immunohistochemistry studies validated the same in the lungs of deceased subjects with COVID-19 and the SARS-CoV-2–challenged hamster lungs. Additionally, lungs from transgenic mice, in which ER stress was induced specifically in the AT2 cells, faithfully recapitulated the host immune response and alveolar cytopathic changes that are induced by SARS-CoV-2.
stated corresponding author Pradipta Ghosh, MD, professor in the departments of medicine and cellular and molecular medicine, University of California, San Diego. “If proven in prospective studies, this biomarker could indicate who is at greatest risk for progressive fibrosis and may require lung transplantation,” she said in an interview.
Dr. Ghosh stated further, “When it comes to therapeutics in COVID lung or IPF, we also found that shared fundamental pathogenic mechanisms present excellent opportunities for developing therapeutics that can arrest the fibrogenic drivers in both diseases. One clue that emerged is a specific cytokine that is at the heart of the smoldering inflammation which is invariably associated with fibrosis. That is interleukin 15 [IL-15] and its receptor.” Dr. Ghosh observed that there are two Food and Drug Administration–approved drugs for IPF. “None are very effective in arresting this invariably fatal disease. Hence, finding better options to treat IPF is an urgent and an unmet need.”
Preclinical testing of hypotheses, Dr. Ghosh said, is next on the path to clinical trials. “We have the advantage of using human lung organoids (mini-lungs grown using stem cells) in a dish, adding additional cells to the system (like fibroblasts and immune cells), infecting them with the virus, or subjecting them to the IL-15 cytokine and monitoring lung fibrosis progression in a dish. Anti–IL-15 therapy can then be initiated to observe reversal of the fibrogenic cascade.” Hamsters have also been shown to provide appropriate models for mimicking lung fibrosis, Dr. Ghosh said.
“The report by Sinha and colleagues describes the fascinating similarities between drivers of post-COVID lung disease and idiopathic pulmonary fibrosis,” stated David Bowton, MD, professor emeritus, section on critical care, department of anesthesiology, Wake Forest University, Winston-Salem, N.C., in an interview. He added that, “Central to the mechanisms of induction of fibrosis in both disorders appears to be endoplasmic reticulum stress in alveolar type II cells (AT2). ER stress induces the unfolded protein response (UPR) that halts protein translation and promotes the degradation of misfolded proteins. Prolonged UPR can reprogram the cell or trigger apoptosis pathways. ER stress in the lung has been reported in a variety of cell lines including AT2 in IPF, bronchial and alveolar epithelial cells in asthma and [chronic obstructive pulmonary disease], and endothelial cells in pulmonary hypertension.”
Dr. Bowton commented further, including a caution, “Sinha and colleagues suggest that the identification of these gene signatures and mechanisms will be a fruitful avenue for developing effective therapeutics for IPF and other fibrotic lung diseases. I am hopeful that these data may offer clues that expedite this process. However, the redundancy of triggers for effector pathways in biologic systems argues that, even if successful, this will be [a] long and fraught process.”
The research study was supported by National Institutes of Health grants and funding from the Tobacco-Related Disease Research Program.
Dr. Sinha, Dr. Ghosh, and Dr. Bowton reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM eBIOMEDICINE
Haven’t had COVID yet? Wanna bet?
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
We all have friends or relatives who, somehow, have managed to avoid catching COVID-19, which has infected more than 91.5 million Americans. You may even be one of the lucky ones yourself.
But health experts are saying: Not so fast. because they didn’t have symptoms or had mild cases they mistook for a cold or allergies.
The upshot: These silent COVID-19 cases reflect a hidden side of the pandemic that may be helping to drive new surges and viral variants.
Still, infectious disease experts say there is little doubt that some people have indeed managed to avoid COVID-19 infection altogether, and they are trying to understand why.
Several recent studies have suggested certain genetic and immune system traits may better protect this group of people against the coronavirus, making them less likely than others to be infected or seriously sickened. Researchers around the world are now studying these seemingly super-immune people for clues to what makes them so special, with an eye toward better vaccines, treatments, and prevention strategies.
Infectious disease specialists say both types of cases – those unknowingly infected by COVID-19 and people who’ve avoided the virus altogether – matter greatly to public health, more than 2 years into the pandemic.
“It’s definitely true that some people have had COVID and don’t realize it,” says Stephen Kissler, PhD, an infectious disease researcher with the Harvard T.H. Chan School of Public Health, Boston. “It is potentially good news if there’s more immunity in the population than we realize.”
But he says that being able to identify genetic and other factors that may offer some people protection against COVID-19 is an “exciting prospect” that could help find out who’s most at risk and improve efforts to get the pandemic under control.
Some studies have found a person’s genetic profile, past exposure to other COVID-like viruses, allergies, and even drugs they take for other conditions may all provide some defense – even for people who have not been vaccinated, don’t use masks, or don’t practice social distancing.
A person’s medical history and genetics may help decide their risk from new diseases, meaning “we may be able to help identify people who are at especially high risk from infection,” Dr. Kissler says. “That knowledge could help those people better shield themselves from infection and get quicker access to treatment and vaccines, if necessary. … We don’t yet know, but studies are ongoing for these things.”
Amesh Adalja, MD, an infectious disease specialist with the Johns Hopkins Center for Health Security, Baltimore, agrees that emerging research on people who’ve avoided infection offers the chance of new public health strategies to combat COVID-19.
“I’m sure there is some subset of people who are [COVID] negative,” he says. “So what explains that phenomenon, especially if that person was out there getting significant exposures?”
Have you had COVID without knowing it?
In a media briefing late last month, White House COVID-19 Response Coordinator Ashish Jha, MD, said more than 70% of the U.S. population has had the virus, according to the latest CDC data. That’s up from 33.5% in December.
But the actual number of people in the U.S. who have been infected with SARS-CoV-2, the scientific name for the virus that causes COVID-19, is likely to be much higher due to cases without symptoms that are unreported, experts say.
Since the early days of the pandemic, researchers have tried to put a number on these hidden cases, but that figure has been evolving and a clear consensus has not emerged.
In September 2020, a study published in the Annals of Internal Medicine said “approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic.”
A follow-up analysis of 95 studies, published last December, reached similar findings, estimating that more than 40% of COVID-19 infections didn’t come with symptoms.
To get a better handle on the issue, CDC officials have been working with the American Red Cross and other blood banks to track COVID-19 antibodies – proteins your body makes after exposure to the virus to fight off an infection – in donors who said they have never had COVID-19.
While that joint effort is still ongoing, early findings say the number of donors with antibodies from COVID-19 infection increased in blood donors from 3.5% in July 2020 to at least 20.2% in May 2021. Since then, those percentages have soared, in part due to the introduction of vaccines, which also make the body produce COVID-19 antibodies.
The most current findings show that 83.3% of donors have combined COVID infection– and vaccine-induced antibodies in their blood. Those findings are based on 1.4 million blood donations.
Health experts say all of these studies are strong evidence that many COVID-19 cases continue to go undetected. In fact, the University of Washington Institute for Health Metrics and Evaluation estimates that only 7% of positive COVID-19 cases in the U.S. are being detected. That means case rates are actually 14.5 times higher than the official count of 131,000 new COVID infections each day, according to the Centers for Disease Control and Prevention, which reports the virus is still killing about 440 Americans daily.
So, why is all this important, in terms of public health?
Experts say people are more likely to be cautious if they know COVID-19 cases are high where they live, work, and play. On the other hand, if they believe case rates in their communities are lower than they actually are, they may be less likely to get vaccinated and boosted, wear masks indoors, avoid crowded indoor spaces, and take other precautions to fend off infection.
How do some avoid infection altogether?
In addition to tracking cases that go unreported and don’t have symptoms, infectious disease experts have also been trying to figure out why some people have managed to avoid getting the highly contagious virus.
Several leading lines of research have produced promising early results – suggesting that a person’s genetic makeup, past exposure to less-lethal coronaviruses, allergies, and even certain drugs they take for other conditions may all provide at least some protection against COVID.
“Our study showed that there are many human genes – hundreds of genes – that can impact SARS-CoV-2 infection,” says Neville Sanjana, PhD, a geneticist at New York University and the New York Genome Center who co-led the study. “With a better understanding of host genetic factors, we can find new kinds of therapies that target these host factors to block infection.”
In addition, he says several studies show some drugs that regulate genes, such as the breast cancer drug tamoxifen, also appear to knock down COVID-19 risk. He suggests such drugs, already approved by the Food and Drug Administration, might be “repurposed” to target the virus.
Studies in other countries show that patients taking tamoxifen before the pandemic were protected against severe COVID-19, Dr. Sanjana says. “That was a really cool thing, highlighting the power of harnessing host genetics. The virus critically depends on our genes to complete key parts of its life cycle.”
The NYU research findings echo other studies that have been published in recent months.
In July, a team of researchers led by the National Cancer Institute identified a genetic factor that appears to determine how severe an infection will be. In a study involving 3,000 people, they found that two gene changes, or mutations, that decrease the expression of a gene called OAS1 boosted the risk of hospitalization from COVID-19. OAS1 is part of the immune system’s response to viral infections.
As a result, developing a genetic therapy designed to increase the OAS1 gene’s expression might reduce the risk of severe disease.
“It’s very natural to get infected once you are exposed. There’s no magic bullet for that. But after you get infected, how you’re going to respond to this infection, that’s what is going to be affected by your genetic variants,” said Ludmila Prokunina-Olsson, PhD, the study’s lead researcher and chief of the National Cancer Institute’s Laboratory of Translational Genomics, Bethesda, Md., in an interview with NBC News.
Benjamin tenOever, PhD, a New York University virologist who co-authored the 2020 research, says the new genetic research is promising, but he believes it’s unlikely scientists will be able to identify a single gene responsible for actually preventing a COVID-19 infection.
“On the flip side, we have identified many genes that makes the disease worse,” he says.
T cells ‘remember’ past viral infections
As Dr. tenOever and Dr. Sanjana suggest, another intriguing line of research has found that prior viral infections may prime the body’s immune system to fight COVID-19.
Four other common coronaviruses – aside from SARS-CoV-2 – infect people worldwide, typically causing mild to moderate upper respiratory illnesses like the common cold, says Alessandro Sette, PhD, an infectious disease expert and vaccine researcher with the La Jolla (Calif.) Institute for Immunology.
In a recent study published in Science, he and his team found past infection with these other coronaviruses may give some protection against SARS-CoV-2.
T cells – white blood cells that act like immunological ninjas to ferret out and fight infections – appear to maintain a kind of “biological memory” of coronaviruses they have seen before and can mount an attack on similar pathogens, such SARS-CoV-2, Dr. Sette says.
The new work builds on a prior research he helped lead that found 40%-60% of people never exposed to SARS-CoV-2 had T cells that reacted to the virus – with their immune systems recognizing fragments of a virus they had never seen before.
Dr. Sette says his research shows that people whose T cells have this “preexisting memory” of past coronavirus exposures also tend to respond better to vaccination for reasons not yet well understood.
“The question is, at which point will there be enough immunity from vaccination, repeated infections from other coronaviruses, but also some of the variants of the SARS-CoV-2 … where infections become less frequent? We’re not there yet,” he says.
In addition to these exciting genetic and T-cell findings, other research has suggested low-grade inflammation from allergies – a key part of the body’s immune response to foreign substances – may also give some people an extra leg up, in terms of avoiding COVID infection.
Last May, a study of 1,400 households published in The Journal of Allergy and Clinical Immunology found that having a food allergy cut the risk of COVID-19 infection in half.
The researchers said it’s unclear why allergies may reduce the risk of infection, but they noted that people with food allergies express fewer ACE2 receptors on the surface of their airway cells, making it harder for the virus to enter cells.
The big picture: Prevention still your best bet
So, what’s the takeaway from all of this emerging research?
New York University’s Dr. tenOever says that while genes, T cells and allergies may offer some protection against COVID, tried-and-true precautions – vaccination, wearing masks, avoiding crowded indoor spaces, and social distancing – are likely to provide a greater defense.
He believes these precautions are likely why he and his family have never contracted COVID-19.
“I was tested weekly, as were my kids at school,” he says. “We definitely never got COVID, despite the fact that we live in New York City and I worked in a hospital every single day of the pandemic.”
Ziyad Al-Aly, MD, an infectious disease specialist and director of clinical epidemiology at Washington University in St. Louis, agrees that the new research on COVID-19 is intriguing but won’t likely result in practical changes in the approach to fighting the virus in the near term.
“Getting a deeper understanding of potential genetic factors or other characteristics – that could really help us understand why the virus just comes and goes without any ill effects in some people, and in other people it produces really serious disease,” he says. “That will really help us eventually to design better vaccines to prevent it or reduce severity or even [treat] people who get severe disease.”
In the meantime, Dr. Al-Aly says, “it’s still best to do everything you can to avoid infection in the first place – even if you’re vaccinated or previously infected, you should really try to avoid reinfection.”
That means sit outside if you can when visiting a restaurant. Wear a mask on a plane, even though it’s not required. And get vaccinated and boosted.
“In the future, there may be more tools to address this pandemic, but that’s really the best advice for now,” Dr. Al-Aly says.
A version of this article first appeared on WebMD.com.
Long COVID comes in three forms: Study
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
, according to a new preprint study published on MedRxiv that hasn’t yet been peer-reviewed.
Long COVID has been hard to define due to its large number of symptoms, but researchers at King’s College London have identified three distinct profiles – with long-term symptoms focused on neurological, respiratory, or physical conditions. So far, they also found patterns among people infected with the original coronavirus strain, the Alpha variant, and the Delta variant.
“These data show clearly that post-COVID syndrome is not just one condition but appears to have several subtypes,” Claire Steves, PhD, one of the study authors and a senior clinical lecturer in King’s College London’s School of Life Course & Population Sciences, said in a statement.
“Understanding the root causes of these subtypes may help in finding treatment strategies,” she said. “Moreover, these data emphasize the need for long-COVID services to incorporate a personalized approach sensitive to the issues of each individual.”
The research team analyzed ZOE COVID app data for 1,459 people who have had symptoms for more than 84 days, or 12 weeks, according to their definition of long COVID or post-COVID syndrome.
They found that the largest group had a cluster of symptoms in the nervous system, such as fatigue, brain fog, and headaches. It was the most common subtype among the Alpha variant, which was dominant in winter 2020-2021, and the Delta variant, which was dominant in 2021.
The second group had respiratory symptoms, such as chest pain and severe shortness of breath, which could suggest lung damage, the researchers wrote. It was the largest cluster for the original coronavirus strain in spring 2020, when people were unvaccinated.
The third group included people who reported a diverse range of physical symptoms, including heart palpitations, muscle aches and pain, and changes to their skin and hair. This group had some of the “most severe and debilitating multi-organ symptoms,” the researchers wrote.
The researchers found that the subtypes were similar in vaccinated and unvaccinated people based on the variants investigated so far. But the data showed that the risk of long COVID was reduced by vaccination.
In addition, although the three subtypes were present in all the variants, other symptom clusters had subtle differences among the variants, such as symptoms in the stomach and intestines. The differences could be due to other things that changed during the pandemic, such as the time of year, social behaviors, and treatments, the researchers said.
“Machine learning approaches, such as clustering analysis, have made it possible to start exploring and identifying different profiles of post-COVID syndrome,” Marc Modat, PhD, who led the analysis and is a senior lecturer at King’s College London’s School of Biomedical Engineering & Imaging Sciences, said in the statement.
“This opens new avenues of research to better understand COVID-19 and to motivate clinical research that might mitigate the long-term effects of the disease,” he said.
A version of this article first appeared on WebMD.com.
Many saw ‘meaningful’ weight loss from 12-week online program
developed by researchers at Brown University.
Primary care doctors offered the free obesity treatment program during routine care. Each week, people reported weight changes and activity and calorie consumption; attended online lessons; and received personalized feedback.
The 464 people who took part for at least 1 week lost an average of 5% of their body weight. And those who followed the plan all 12 weeks lost an average of 7%.
The researchers point out this short-term weight loss was achieved without any face-to-face counseling, which can limit weight management in busy primary care settings.
“Obesity is a highly stigmatized condition,” says lead investigator J. Graham Thomas, PhD.
People take part in the Rx Weight Loss program in the privacy of their own homes. He says this not only makes it more convenient but could be an advantage for people who feel uncomfortable managing their weight around others.
Ideally, health care providers could offer the online program as an opportunity to patients “as opposed to something punitive,” says Dr. Thomas, a researcher at the Weight Control and Diabetes Research Center at Miriam Hospital in Providence, R.I.
The study was published online in the journal Obesity.
In three previous controlled clinical trials led by the same research team, the weight-loss program was linked to average weight losses of 4.2% to 5.8%. In the current study, the researchers were not directly involved, and Dr. Thomas says he was encouraged that the doctor-led initiative led to similar results.
About 11 pounds lost
Patients were offered the program during routine care by doctors in the Rhode Island Primary Care Physicians Corporation, which includes 100 doctors at 60 sites. To be eligible, people had to be 18-75 years old, have Internet access, be fluent in English, and have a body mass index (BMI) of 25 kg/m2 or greater.
The average age of the people in the study was 53, 70% were women, and the average BMI was 36.2.
A BMI of 25 or above means you are overweight, while those with a BMI of 30 or higher are considered obese.
The average 5.1% decrease in body weight at 12 weeks translated to just more than 11 pounds of average weight loss.
‘Very encouraging’
The results of the study are “very encouraging,” says Gareth R. Dutton, PhD, who was not affiliated with the study.
Previous strategies had limits, he says.
“Fully automated interventions that have no staff contact with participants often achieve modest weight loss,” says Dr. Dutton, a professor of medicine and investigator in the Nutrition Obesity Research Center at the University of Alabama at Birmingham.
Weight-loss programs recommended by primary care doctors have often performed even worse, he says.
“Weight-loss interventions delivered through primary care are challenging because of many barriers, including limited resources and time,” says Dr. Dutton, who is also lead investigator of a study that aims to enroll 400 primary care patients to compare daily self-weighing with standard care.
Letting doctors and their staff refer patients to an evidence-based weight-loss program has great potential, he says.
Looking to improve uptake
The Rx Weight Loss program was offered to 1,721 primary care patients overall.
When asked why only 26% of people offered the program agreed to participate, Dr. Thomas replied, “No matter how good the program is, it’s just never going to be the right time for a lot of people to add this to their lives, particularly given the last couple of years where folks are experiencing a lot of challenges and a lot of stressors.”
“Even though it’s an online program, addressing obesity always involves making substantial changes to eating and activity patterns,” he said.
Future steps
The investigators plan to look into ways to get more people to take part in the program.
It is not yet available for widespread use by others, but that’s the goal. Dr. Thomas said they learned ways during the study to make the fully automated, online program easier for others to adopt.
Measuring any effect on weight loss at 1 year is the primary aim of the study. “I think we expect to find something similar to what we see in previous studies, which is that a certain amount of weight regain will be the norm” at 1 year, Dr. Thomas said.
“But a certain amount of weight loss and associated health benefits will persist, making it worthwhile even if, on average, some gradual regain occurs.”A version of this article first appeared on WebMD.com.
developed by researchers at Brown University.
Primary care doctors offered the free obesity treatment program during routine care. Each week, people reported weight changes and activity and calorie consumption; attended online lessons; and received personalized feedback.
The 464 people who took part for at least 1 week lost an average of 5% of their body weight. And those who followed the plan all 12 weeks lost an average of 7%.
The researchers point out this short-term weight loss was achieved without any face-to-face counseling, which can limit weight management in busy primary care settings.
“Obesity is a highly stigmatized condition,” says lead investigator J. Graham Thomas, PhD.
People take part in the Rx Weight Loss program in the privacy of their own homes. He says this not only makes it more convenient but could be an advantage for people who feel uncomfortable managing their weight around others.
Ideally, health care providers could offer the online program as an opportunity to patients “as opposed to something punitive,” says Dr. Thomas, a researcher at the Weight Control and Diabetes Research Center at Miriam Hospital in Providence, R.I.
The study was published online in the journal Obesity.
In three previous controlled clinical trials led by the same research team, the weight-loss program was linked to average weight losses of 4.2% to 5.8%. In the current study, the researchers were not directly involved, and Dr. Thomas says he was encouraged that the doctor-led initiative led to similar results.
About 11 pounds lost
Patients were offered the program during routine care by doctors in the Rhode Island Primary Care Physicians Corporation, which includes 100 doctors at 60 sites. To be eligible, people had to be 18-75 years old, have Internet access, be fluent in English, and have a body mass index (BMI) of 25 kg/m2 or greater.
The average age of the people in the study was 53, 70% were women, and the average BMI was 36.2.
A BMI of 25 or above means you are overweight, while those with a BMI of 30 or higher are considered obese.
The average 5.1% decrease in body weight at 12 weeks translated to just more than 11 pounds of average weight loss.
‘Very encouraging’
The results of the study are “very encouraging,” says Gareth R. Dutton, PhD, who was not affiliated with the study.
Previous strategies had limits, he says.
“Fully automated interventions that have no staff contact with participants often achieve modest weight loss,” says Dr. Dutton, a professor of medicine and investigator in the Nutrition Obesity Research Center at the University of Alabama at Birmingham.
Weight-loss programs recommended by primary care doctors have often performed even worse, he says.
“Weight-loss interventions delivered through primary care are challenging because of many barriers, including limited resources and time,” says Dr. Dutton, who is also lead investigator of a study that aims to enroll 400 primary care patients to compare daily self-weighing with standard care.
Letting doctors and their staff refer patients to an evidence-based weight-loss program has great potential, he says.
Looking to improve uptake
The Rx Weight Loss program was offered to 1,721 primary care patients overall.
When asked why only 26% of people offered the program agreed to participate, Dr. Thomas replied, “No matter how good the program is, it’s just never going to be the right time for a lot of people to add this to their lives, particularly given the last couple of years where folks are experiencing a lot of challenges and a lot of stressors.”
“Even though it’s an online program, addressing obesity always involves making substantial changes to eating and activity patterns,” he said.
Future steps
The investigators plan to look into ways to get more people to take part in the program.
It is not yet available for widespread use by others, but that’s the goal. Dr. Thomas said they learned ways during the study to make the fully automated, online program easier for others to adopt.
Measuring any effect on weight loss at 1 year is the primary aim of the study. “I think we expect to find something similar to what we see in previous studies, which is that a certain amount of weight regain will be the norm” at 1 year, Dr. Thomas said.
“But a certain amount of weight loss and associated health benefits will persist, making it worthwhile even if, on average, some gradual regain occurs.”A version of this article first appeared on WebMD.com.
developed by researchers at Brown University.
Primary care doctors offered the free obesity treatment program during routine care. Each week, people reported weight changes and activity and calorie consumption; attended online lessons; and received personalized feedback.
The 464 people who took part for at least 1 week lost an average of 5% of their body weight. And those who followed the plan all 12 weeks lost an average of 7%.
The researchers point out this short-term weight loss was achieved without any face-to-face counseling, which can limit weight management in busy primary care settings.
“Obesity is a highly stigmatized condition,” says lead investigator J. Graham Thomas, PhD.
People take part in the Rx Weight Loss program in the privacy of their own homes. He says this not only makes it more convenient but could be an advantage for people who feel uncomfortable managing their weight around others.
Ideally, health care providers could offer the online program as an opportunity to patients “as opposed to something punitive,” says Dr. Thomas, a researcher at the Weight Control and Diabetes Research Center at Miriam Hospital in Providence, R.I.
The study was published online in the journal Obesity.
In three previous controlled clinical trials led by the same research team, the weight-loss program was linked to average weight losses of 4.2% to 5.8%. In the current study, the researchers were not directly involved, and Dr. Thomas says he was encouraged that the doctor-led initiative led to similar results.
About 11 pounds lost
Patients were offered the program during routine care by doctors in the Rhode Island Primary Care Physicians Corporation, which includes 100 doctors at 60 sites. To be eligible, people had to be 18-75 years old, have Internet access, be fluent in English, and have a body mass index (BMI) of 25 kg/m2 or greater.
The average age of the people in the study was 53, 70% were women, and the average BMI was 36.2.
A BMI of 25 or above means you are overweight, while those with a BMI of 30 or higher are considered obese.
The average 5.1% decrease in body weight at 12 weeks translated to just more than 11 pounds of average weight loss.
‘Very encouraging’
The results of the study are “very encouraging,” says Gareth R. Dutton, PhD, who was not affiliated with the study.
Previous strategies had limits, he says.
“Fully automated interventions that have no staff contact with participants often achieve modest weight loss,” says Dr. Dutton, a professor of medicine and investigator in the Nutrition Obesity Research Center at the University of Alabama at Birmingham.
Weight-loss programs recommended by primary care doctors have often performed even worse, he says.
“Weight-loss interventions delivered through primary care are challenging because of many barriers, including limited resources and time,” says Dr. Dutton, who is also lead investigator of a study that aims to enroll 400 primary care patients to compare daily self-weighing with standard care.
Letting doctors and their staff refer patients to an evidence-based weight-loss program has great potential, he says.
Looking to improve uptake
The Rx Weight Loss program was offered to 1,721 primary care patients overall.
When asked why only 26% of people offered the program agreed to participate, Dr. Thomas replied, “No matter how good the program is, it’s just never going to be the right time for a lot of people to add this to their lives, particularly given the last couple of years where folks are experiencing a lot of challenges and a lot of stressors.”
“Even though it’s an online program, addressing obesity always involves making substantial changes to eating and activity patterns,” he said.
Future steps
The investigators plan to look into ways to get more people to take part in the program.
It is not yet available for widespread use by others, but that’s the goal. Dr. Thomas said they learned ways during the study to make the fully automated, online program easier for others to adopt.
Measuring any effect on weight loss at 1 year is the primary aim of the study. “I think we expect to find something similar to what we see in previous studies, which is that a certain amount of weight regain will be the norm” at 1 year, Dr. Thomas said.
“But a certain amount of weight loss and associated health benefits will persist, making it worthwhile even if, on average, some gradual regain occurs.”A version of this article first appeared on WebMD.com.
FROM OBESITY
Cultural humility required to optimize treatment of eczema patients with skin of color
INDIANAPOLIS – Treating atopic dermatitis (AD) in children and adolescents with skin of color requires an acumen that extends well beyond the skin, said Candrice R. Heath, MD, at the annual meeting of the Society for Pediatric Dermatology.
This involves the practice of cultural humility, which Dr. Heath defined as a commitment to learn about all aspects of patients to truly understand them, including their race, access to health care, and socioeconomic status.
“We can continue to prioritize learning about all different types of skin tones and hair types, but we really have to commit to advocating for what our patients deserve in every way,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said during her presentation at the meeting.
“That means advocating for kids to have access to better housing and for increasing health literacy programs in our hospitals, so that all our patients can understand what’s happening and how to navigate the health system,” she said. “It also means increasing diversity in our clinical trials by taking a few extra moments with the patient and family of color who might be eligible to participate in a clinical trial. We have work to do.”
To illustrate her points, she discussed the case of a 6-year-old Black patient, whose parents bring him into the clinic complaining about dark marks on the skin. The areas are itchy and the doctor figures, “this is a slam dunk; this is AD,” Dr. Heath said. “You talk about the diagnosis, and you give your treatment plan.
“But the issue is, in the parking lot when the patient’s family leaves, they feel like you didn’t help them at all,” she continued. “You didn’t understand what they came in for. They didn’t receive a treatment for what they came in for, because the initial complaint was dark marks on the skin, which is postinflammatory hyperpigmentation. We know that patients are distressed by this.”
As evidence, she cited a cross-sectional study that assessed the impact of hyperpigmentation and hyperchromia on quality of life in adults, published in the Journal of the American Academy of Dermatology. People who reported the highest levels of distress were women, those with postinflammatory hyperpigmentation, those with fewer formal years of education, and those who had higher out-of-pocket spending on skin-enhancing products.
“So, when you see hyperpigmentation in your AD patients of color, acknowledge it; say, ‘I see this pigmentation change,’ ” Dr. Heath advised. “Talk about how controlling the AD with a topical steroid or other treatment option can have a positive impact on that.”
However, she added that sometimes patients have steroid phobia, possibly because they believe the topical steroids are causing the pigmentation changes, “especially in cases of hypopigmentation, so I take the time to reassure patients so that they will not be fearful about using the medication.”
Parents of patients with skin of color who have AD may harbor other “invisible” concerns during office visits, she continued, including prior experiences with dermatologists that may not have been positive, difficulty accessing pediatric dermatologists, or a general mistrust of the health care system.
“All of that is going on in the room with your patients, particularly those with skin of color and those who feel marginalized,” said Dr. Heath, who is also a faculty scholar at Temple University medical school’s office of health equity, diversity and inclusion. “Of course, we can’t fix everything. But we can commit to approaching our visits with cultural humility.”
For patients with skin of color, she pointed out, other upstream effects impact AD care and outcomes, including well-documented socioeconomic factors.
“One of the equalizing factors is that we as pediatric dermatologists can think about increasing our education regarding skin of color,” Dr. Heath said.
For example, an analysis of data from the 2002 to 2012 National Inpatient Sample found that the main risk factors for inpatient hospitalization for AD were being non-White, having lowest-quartile household income, and having Medicaid or no insurance, researchers reported in 2018.
A separate multicenter study of 1,437 mother-child pairs with known AD found that non-Hispanic Black children and Hispanic children had greater odds of persistent AD than non-Hispanic White children, according to a 2019 study. Another large prospective cohort study published in 2019 found that AD prevalence and persistence is highest in U.S. urban children who are female or Black, and urban children with AD are more likely to have poor quality of life and asthma.
A few months after that study was published, researchers reported results from an analysis of data from the 2007-2008 National Survey of Children’s Health, which found that children who perceive the neighborhood they lived in as unsafe, unsupportive, or underdeveloped had a higher prevalence of AD and a higher severity of AD. The same year, a study of the social and economic risk factors for moderate to severe AD found that Black children were more likely to come from homes with a lower household income, lower parental education attainment, lack of home ownership, and live between two residences, and have exposure to smoke.
“Disease recognition is one thing, but we also want everyone to be aware of these other factors,” she said, “because some patients do need a little bit more care and help to be able to access the medications that they need and gain access to us.”
Follicular, nummular eczema
In her clinical experience, the most common clinical variants of AD in patients with skin of color is follicular eczema. “Examine the patient, apply your hand to the affected area, and you can feel the papules beneath your fingertips,” she advised.
“That’s what I teach my residents and medical students,” she said. “If you are looking for erythema to seal your diagnosis of AD, it may not happen. You may see more of a violaceous hue and sometimes you may not find it at all, depending on the patient’s skin tone. If I find an area of normal appearing skin and then look back at the area of active skin disease, I go back and forth until I’m able to train my eye to be able to see those violaceous and erythematous hues more easily.”
Nummular eczema can also be a challenge in AD patients with skin of color.
“I like to listen to buzz words,” Dr. Heath said. “If a parent says, ‘my child has been diagnosed with ringworm multiple times,’ I zoom in on that. We know that kids can get tinea corporis, but usually not multiple times. I ask about all the things that can be associated with AD, and often we do see these nummular plaques on the skin and do some education about that. I also talk to their pediatrician or send information to that person so that they can be aware that nummular eczema is a form of AD.”
She noted that AD of the scalp may be confused with tinea capitis, especially in young Black children with moderate to severe AD. In her experience, triamcinolone 0.1% ointment works well for AD of the scalp.
She concluded her presentation by noting that there is no easy solution to treating AD in young patients with skin of color. “It’s way more than just eczema. We can help people see AD in a different way. My goal is to see the value in challenging ourselves to understand the impact of what happens outside of the exam room on these patients.”
Dr. Heath disclosed that she has served as a consultant for several pharmaceutical companies, including Regeneron, Janssen, Arcutis, Johnson and Johnson, Cassiopea, and Lilly.
INDIANAPOLIS – Treating atopic dermatitis (AD) in children and adolescents with skin of color requires an acumen that extends well beyond the skin, said Candrice R. Heath, MD, at the annual meeting of the Society for Pediatric Dermatology.
This involves the practice of cultural humility, which Dr. Heath defined as a commitment to learn about all aspects of patients to truly understand them, including their race, access to health care, and socioeconomic status.
“We can continue to prioritize learning about all different types of skin tones and hair types, but we really have to commit to advocating for what our patients deserve in every way,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said during her presentation at the meeting.
“That means advocating for kids to have access to better housing and for increasing health literacy programs in our hospitals, so that all our patients can understand what’s happening and how to navigate the health system,” she said. “It also means increasing diversity in our clinical trials by taking a few extra moments with the patient and family of color who might be eligible to participate in a clinical trial. We have work to do.”
To illustrate her points, she discussed the case of a 6-year-old Black patient, whose parents bring him into the clinic complaining about dark marks on the skin. The areas are itchy and the doctor figures, “this is a slam dunk; this is AD,” Dr. Heath said. “You talk about the diagnosis, and you give your treatment plan.
“But the issue is, in the parking lot when the patient’s family leaves, they feel like you didn’t help them at all,” she continued. “You didn’t understand what they came in for. They didn’t receive a treatment for what they came in for, because the initial complaint was dark marks on the skin, which is postinflammatory hyperpigmentation. We know that patients are distressed by this.”
As evidence, she cited a cross-sectional study that assessed the impact of hyperpigmentation and hyperchromia on quality of life in adults, published in the Journal of the American Academy of Dermatology. People who reported the highest levels of distress were women, those with postinflammatory hyperpigmentation, those with fewer formal years of education, and those who had higher out-of-pocket spending on skin-enhancing products.
“So, when you see hyperpigmentation in your AD patients of color, acknowledge it; say, ‘I see this pigmentation change,’ ” Dr. Heath advised. “Talk about how controlling the AD with a topical steroid or other treatment option can have a positive impact on that.”
However, she added that sometimes patients have steroid phobia, possibly because they believe the topical steroids are causing the pigmentation changes, “especially in cases of hypopigmentation, so I take the time to reassure patients so that they will not be fearful about using the medication.”
Parents of patients with skin of color who have AD may harbor other “invisible” concerns during office visits, she continued, including prior experiences with dermatologists that may not have been positive, difficulty accessing pediatric dermatologists, or a general mistrust of the health care system.
“All of that is going on in the room with your patients, particularly those with skin of color and those who feel marginalized,” said Dr. Heath, who is also a faculty scholar at Temple University medical school’s office of health equity, diversity and inclusion. “Of course, we can’t fix everything. But we can commit to approaching our visits with cultural humility.”
For patients with skin of color, she pointed out, other upstream effects impact AD care and outcomes, including well-documented socioeconomic factors.
“One of the equalizing factors is that we as pediatric dermatologists can think about increasing our education regarding skin of color,” Dr. Heath said.
For example, an analysis of data from the 2002 to 2012 National Inpatient Sample found that the main risk factors for inpatient hospitalization for AD were being non-White, having lowest-quartile household income, and having Medicaid or no insurance, researchers reported in 2018.
A separate multicenter study of 1,437 mother-child pairs with known AD found that non-Hispanic Black children and Hispanic children had greater odds of persistent AD than non-Hispanic White children, according to a 2019 study. Another large prospective cohort study published in 2019 found that AD prevalence and persistence is highest in U.S. urban children who are female or Black, and urban children with AD are more likely to have poor quality of life and asthma.
A few months after that study was published, researchers reported results from an analysis of data from the 2007-2008 National Survey of Children’s Health, which found that children who perceive the neighborhood they lived in as unsafe, unsupportive, or underdeveloped had a higher prevalence of AD and a higher severity of AD. The same year, a study of the social and economic risk factors for moderate to severe AD found that Black children were more likely to come from homes with a lower household income, lower parental education attainment, lack of home ownership, and live between two residences, and have exposure to smoke.
“Disease recognition is one thing, but we also want everyone to be aware of these other factors,” she said, “because some patients do need a little bit more care and help to be able to access the medications that they need and gain access to us.”
Follicular, nummular eczema
In her clinical experience, the most common clinical variants of AD in patients with skin of color is follicular eczema. “Examine the patient, apply your hand to the affected area, and you can feel the papules beneath your fingertips,” she advised.
“That’s what I teach my residents and medical students,” she said. “If you are looking for erythema to seal your diagnosis of AD, it may not happen. You may see more of a violaceous hue and sometimes you may not find it at all, depending on the patient’s skin tone. If I find an area of normal appearing skin and then look back at the area of active skin disease, I go back and forth until I’m able to train my eye to be able to see those violaceous and erythematous hues more easily.”
Nummular eczema can also be a challenge in AD patients with skin of color.
“I like to listen to buzz words,” Dr. Heath said. “If a parent says, ‘my child has been diagnosed with ringworm multiple times,’ I zoom in on that. We know that kids can get tinea corporis, but usually not multiple times. I ask about all the things that can be associated with AD, and often we do see these nummular plaques on the skin and do some education about that. I also talk to their pediatrician or send information to that person so that they can be aware that nummular eczema is a form of AD.”
She noted that AD of the scalp may be confused with tinea capitis, especially in young Black children with moderate to severe AD. In her experience, triamcinolone 0.1% ointment works well for AD of the scalp.
She concluded her presentation by noting that there is no easy solution to treating AD in young patients with skin of color. “It’s way more than just eczema. We can help people see AD in a different way. My goal is to see the value in challenging ourselves to understand the impact of what happens outside of the exam room on these patients.”
Dr. Heath disclosed that she has served as a consultant for several pharmaceutical companies, including Regeneron, Janssen, Arcutis, Johnson and Johnson, Cassiopea, and Lilly.
INDIANAPOLIS – Treating atopic dermatitis (AD) in children and adolescents with skin of color requires an acumen that extends well beyond the skin, said Candrice R. Heath, MD, at the annual meeting of the Society for Pediatric Dermatology.
This involves the practice of cultural humility, which Dr. Heath defined as a commitment to learn about all aspects of patients to truly understand them, including their race, access to health care, and socioeconomic status.
“We can continue to prioritize learning about all different types of skin tones and hair types, but we really have to commit to advocating for what our patients deserve in every way,” Dr. Heath, director of pediatric dermatology at Temple University, Philadelphia, said during her presentation at the meeting.
“That means advocating for kids to have access to better housing and for increasing health literacy programs in our hospitals, so that all our patients can understand what’s happening and how to navigate the health system,” she said. “It also means increasing diversity in our clinical trials by taking a few extra moments with the patient and family of color who might be eligible to participate in a clinical trial. We have work to do.”
To illustrate her points, she discussed the case of a 6-year-old Black patient, whose parents bring him into the clinic complaining about dark marks on the skin. The areas are itchy and the doctor figures, “this is a slam dunk; this is AD,” Dr. Heath said. “You talk about the diagnosis, and you give your treatment plan.
“But the issue is, in the parking lot when the patient’s family leaves, they feel like you didn’t help them at all,” she continued. “You didn’t understand what they came in for. They didn’t receive a treatment for what they came in for, because the initial complaint was dark marks on the skin, which is postinflammatory hyperpigmentation. We know that patients are distressed by this.”
As evidence, she cited a cross-sectional study that assessed the impact of hyperpigmentation and hyperchromia on quality of life in adults, published in the Journal of the American Academy of Dermatology. People who reported the highest levels of distress were women, those with postinflammatory hyperpigmentation, those with fewer formal years of education, and those who had higher out-of-pocket spending on skin-enhancing products.
“So, when you see hyperpigmentation in your AD patients of color, acknowledge it; say, ‘I see this pigmentation change,’ ” Dr. Heath advised. “Talk about how controlling the AD with a topical steroid or other treatment option can have a positive impact on that.”
However, she added that sometimes patients have steroid phobia, possibly because they believe the topical steroids are causing the pigmentation changes, “especially in cases of hypopigmentation, so I take the time to reassure patients so that they will not be fearful about using the medication.”
Parents of patients with skin of color who have AD may harbor other “invisible” concerns during office visits, she continued, including prior experiences with dermatologists that may not have been positive, difficulty accessing pediatric dermatologists, or a general mistrust of the health care system.
“All of that is going on in the room with your patients, particularly those with skin of color and those who feel marginalized,” said Dr. Heath, who is also a faculty scholar at Temple University medical school’s office of health equity, diversity and inclusion. “Of course, we can’t fix everything. But we can commit to approaching our visits with cultural humility.”
For patients with skin of color, she pointed out, other upstream effects impact AD care and outcomes, including well-documented socioeconomic factors.
“One of the equalizing factors is that we as pediatric dermatologists can think about increasing our education regarding skin of color,” Dr. Heath said.
For example, an analysis of data from the 2002 to 2012 National Inpatient Sample found that the main risk factors for inpatient hospitalization for AD were being non-White, having lowest-quartile household income, and having Medicaid or no insurance, researchers reported in 2018.
A separate multicenter study of 1,437 mother-child pairs with known AD found that non-Hispanic Black children and Hispanic children had greater odds of persistent AD than non-Hispanic White children, according to a 2019 study. Another large prospective cohort study published in 2019 found that AD prevalence and persistence is highest in U.S. urban children who are female or Black, and urban children with AD are more likely to have poor quality of life and asthma.
A few months after that study was published, researchers reported results from an analysis of data from the 2007-2008 National Survey of Children’s Health, which found that children who perceive the neighborhood they lived in as unsafe, unsupportive, or underdeveloped had a higher prevalence of AD and a higher severity of AD. The same year, a study of the social and economic risk factors for moderate to severe AD found that Black children were more likely to come from homes with a lower household income, lower parental education attainment, lack of home ownership, and live between two residences, and have exposure to smoke.
“Disease recognition is one thing, but we also want everyone to be aware of these other factors,” she said, “because some patients do need a little bit more care and help to be able to access the medications that they need and gain access to us.”
Follicular, nummular eczema
In her clinical experience, the most common clinical variants of AD in patients with skin of color is follicular eczema. “Examine the patient, apply your hand to the affected area, and you can feel the papules beneath your fingertips,” she advised.
“That’s what I teach my residents and medical students,” she said. “If you are looking for erythema to seal your diagnosis of AD, it may not happen. You may see more of a violaceous hue and sometimes you may not find it at all, depending on the patient’s skin tone. If I find an area of normal appearing skin and then look back at the area of active skin disease, I go back and forth until I’m able to train my eye to be able to see those violaceous and erythematous hues more easily.”
Nummular eczema can also be a challenge in AD patients with skin of color.
“I like to listen to buzz words,” Dr. Heath said. “If a parent says, ‘my child has been diagnosed with ringworm multiple times,’ I zoom in on that. We know that kids can get tinea corporis, but usually not multiple times. I ask about all the things that can be associated with AD, and often we do see these nummular plaques on the skin and do some education about that. I also talk to their pediatrician or send information to that person so that they can be aware that nummular eczema is a form of AD.”
She noted that AD of the scalp may be confused with tinea capitis, especially in young Black children with moderate to severe AD. In her experience, triamcinolone 0.1% ointment works well for AD of the scalp.
She concluded her presentation by noting that there is no easy solution to treating AD in young patients with skin of color. “It’s way more than just eczema. We can help people see AD in a different way. My goal is to see the value in challenging ourselves to understand the impact of what happens outside of the exam room on these patients.”
Dr. Heath disclosed that she has served as a consultant for several pharmaceutical companies, including Regeneron, Janssen, Arcutis, Johnson and Johnson, Cassiopea, and Lilly.
AT SPD 2022
Summer flu, RSV in July, ‘super colds?’
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Richard Martinello, MD, a professor of medicine and pediatric infectious diseases at Yale University, New haven, Conn., doesn’t expect to see a child hospitalized with respiratory syncytial virus (RSV) in the middle of summer. The illness, which can strike infants and older adults especially hard, is known as a “winter virus.”
But not this year. Over the last several weeks, he says, admissions for children with RSV have increased at the Yale New Haven Children’s Hospital. While the numbers aren’t large, they are out of the ordinary, he says, “because usually, at this time of year, we see zero. For lack of a better term, it’s weird.”
Likewise, William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville, says RSV is on the rise there. Tennessee is one of 10 states taking part in a Centers for Disease Control and Prevention surveillance system that tracks influenza, RSV, and COVID-19.
He says RSV cases have risen by at least a third during the past week, including all age ranges. At this time of year, he says, “We aren’t supposed to have any RSV.”
RSV isn’t the only virus thriving out of season or otherwise acting strangely. Since the pandemic began, flu seasons have been out of whack – sometimes nearly nonexistent and other times extending well beyond “normal” seasons. Some experts say one influenza “B” strain may now be extinct, while others say it will be back.
Severe colds – what some call “super colds” – also seem to be on the rise in recent warm-weather months, although that evidence is mostly based on personal experience, not science.
Trying to explain these out-of-season variations has sparked much discussion among epidemiologists and virologists, Dr. Schaffner says, with debates ongoing about whether human behavior and habits or the seasons play a bigger role in the transmission of viral illness.
On top of that, scientists are also looking at the interactions between the SARS-CoV-2 virus that causes COVID-19 and other viruses. When people get hit with COVID-19 and other viruses at the same time, does that make COVID-19 more severe, or less?
Research is conflicting.
Summer of 2022: A repeat of 2021?
RSV. Most children contract the virus by age 2, and while it’s generally mild, about 58,000 children under age 5 years are hospitalized each year. During the pandemic, RSV cases decreased from January to April 2020, the CDC reported, and then remained at “historically low levels”: less than 1% positive RSV results a week, for the next year.
But cases began rising in April 2021.
“Last year, we did have an unusual summer,” Dr. Schaffner says. After lockdown ended, to everyone’s surprise, RSV infections rose.
That increase triggered a CDC health advisory in June 2021, telling doctors and caregivers about the increase in “interseasonal” RSV cases across parts of the Southern United States, recommending broader testing for RSV in patients who had a respiratory illness but tested negative for COVID.
Because of the reduced circulation of RSV during the winter of 2020 to 2021, the CDC warned, older infants and toddlers might have a higher risk of RSV since they weren’t exposed to typical levels of RSV for the previous 15 months.
What about 2022? “At the moment,” Dr. Schaffner says, “it looks like we are having a repeat [of 2021].”
On Twitter, other pediatricians, including those from Maine and Texas, have reported an increase in RSV cases this summer.
Influenza. From October 2020 until May 2021, flu activity was lower than during any previous flu season since at least 1997, according to the CDC.
In late 2021, researchers suggested that one line of influenza known as B/Yamagata may have become extinct.
The 2021-2022 flu season has been mild, the CDC says, but it has come in two waves, with the second wave lingering longer than previous ones. While flu activity is decreasing, last week the CDC said doctors should be alert to flu infections throughout the summer.
Colds. In reports on colds that aren’t based on science, several doctors say they are seeing more colds than usual in the summer, and they’re more severe than usual. According to the CDC, common coronaviruses and respiratory adenoviruses have been increasing since early 2021, and rhinoviruses since June 2020.
Behavior vs. seasons
In explaining the spread of viral respiratory diseases, infectious disease doctors consider two things. “One is that temperature and humidity in the winter favors longer survival of some viruses, leading to longer periods of possible transmission,” says Dean Blumberg, MD, a professor of pediatrics and chief of pediatric infectious disease at University of California Davis Health.
“The other is differences in human behavior, with people spending more time outside in the summer, which results in more distancing and [less] virus concentration due to very large air volume,” he says, and vice versa in winter.
What about the “super colds?” COVID-19 lockdowns and social distancing greatly reduced people’s exposure to common viruses like those that cause colds, says Neil A. Mabbott, PhD, a professor of immunopathology at the University of Edinburgh (Scotland).
“Immunity to these common cold viruses gained through natural infection is considered to last around 8 or 9 months or so,” he says. “Each winter, when we are exposed to the new circulating variants of these viruses, our immunity receives a natural boost.”
That explains why most people get a cold that’s relatively mild. But with all the pandemic lockdowns and the use of hand sanitizers, most people had limited exposure to other viruses, including the common cold. When people emerged from lockdown, the common cold viruses were beginning to circulate again.
“Our immune systems were less able to clear the infection than previously,” Dr. Mabbott says. “As a consequence, some may have experienced increased symptoms, giving the impression of being infected with a ‘super cold.’ ”
“The colds themselves are probably not different to those we got prepandemic,” says Ian Mackay, PhD, a virologist at the University of Queensland, Brisbane, Australia. “But there might be more of them. So I doubt they are ‘super colds’ as much as they are ‘super-perfect circumstances.’ ”
The colds themselves are probably not different to those we got prepandemic. But there might be more of them.
Those super-perfect circumstances, he says, include people gathering after lockdown; a lack of immunity in new babies; viruses that have remained, even if at low levels, but continue to mutate; and our waning immunity to the range of viruses we’d normally encounter.
While lack of exposure may partly explain why some viruses become rampant out of season, it’s likely not the only reason. For example, the reduced circulation of RSV in the population as a whole also may have reduced the transfer of immunity from mothers to infants, some researchers say, making those infants more vulnerable than usual.
Interactions of viruses
Another thing that may be driving the different behavior of viruses is that the SARS-CoV-2 virus could somehow be interacting with other respiratory viruses, Dr. Schaffner says. “And if so, what sort of interactions?”
Many researchers are looking into that, and how coinfections with other respiratory diseases, including the common cold and flu, may affect the course of COVID-19. Some studies have found that the T cells – a source of deeper, cellular immunity in people – generated after a common cold “may also provide cross-protection in some people against COVID-19.”
But another study found immunity against common cold–causing coronaviruses might make COVID-19 more severe.
When researchers in the United Kingdom studied nearly 7,000 patients infected with COVID-19, including 583 also infected with RSV, flu, or adenoviruses (causing flulike or coldlike illness), those with flu or adenovirus, compared with the others, were at higher risk of death.
To be continued …
Exactly how COVID-19 will be changing what we know of other viruses is yet to be determined, too.
Even before the pandemic, Dr. Martinello says, there were already some shifts in RSV. Florida, for instance, has an RSV season longer than the rest of the country, mimicking the pattern in the tropics.
Will the atypical patterns continue? “My guess is that this will settle out,” he says, with some sort of pattern developing. At this point, there are many unknowns. “We still can’t answer whether there will be some seasonality to COVID.”
A version of this article first appeared on WebMD.com.
Solitary Pink Plaque on the Neck
The Diagnosis: Plaque-type Syringoma
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
The Diagnosis: Plaque-type Syringoma
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
The Diagnosis: Plaque-type Syringoma
A biopsy demonstrated multiple basaloid islands of tumor cells in the reticular dermis with ductal differentiation, some with a commalike tail. The ducts were lined by 2 to 3 layers of small uniform cuboidal cells without atypia and contained inspissated secretions within the lumina of scattered ducts. There was an associated fibrotic collagenous stroma. There was no evidence of perineural invasion and no deep dermal or subcutaneous extension (Figure 1). Additional cytokeratin immunohistochemical staining highlighted the adnexal proliferation (Figure 2). A diagnosis of plaque-type syringoma (PTS) was made.

Syringomas are benign dermal sweat gland tumors that typically present as flesh-colored papules on the cheeks or periorbital area of young females. Plaque-type tumors as well as papulonodular, eruptive, disseminated, urticaria pigmentosa–like, lichen planus–like, or milialike syringomas also have been reported. Syringomas may be associated with certain medical conditions such as Down syndrome, Nicolau-Balus syndrome, and both scarring and nonscarring alopecias.1 The clear cell variant of syringoma often is associated with diabetes mellitus.2 Kikuchi et al3 first described PTS in 1979. Plaque-type syringomas rarely are reported in the literature, and sites of involvement include the head and neck region, upper lip, chest, upper extremities, vulva, penis, and scrotum.4-6

Histologically, syringomatous lesions are composed of multiple small ducts lined by 2 to 3 layers of cuboidal epithelium. The ducts may be arranged in nests or strands of basaloid cells surrounded by a dense fibrotic stroma. Occasionally, the ducts will form a comma- or teardropshaped tail; however, this also may be observed in desmoplastic trichoepithelioma (DTE).7 Perineural invasion is absent in syringomas. Syringomas exhibit a lateral growth pattern that typically is limited to the upper half of the reticular dermis and spares the underlying subcutis, muscle, and bone. The growth pattern may be discontinuous with proliferations juxtaposed by normal-appearing skin.8 Syringomas usually express progesterone receptors and are known to proliferate at puberty, suggesting that these neoplasms are under hormonal control.9 Although syringomas are benign, various treatment options that may be pursued for cosmetic purposes include radiofrequency, staged excision, laser ablation, and oral isotretinoin.8,10 If only a superficial biopsy is obtained, syringomas may display features of other adnexal neoplasms, including microcystic adnexal carcinoma (MAC), DTE, morpheaform basal cell carcinoma (BCC), and inflammatory linear verrucous epidermal nevus (ILVEN).
Microcystic adnexal carcinoma is a locally aggressive neoplasm first described by Goldstein et al11 in 1982 an indurated, ill-defined plaque or nodule on the face with a predilection for the upper and lower lip. Prior radiation therapy and immunosuppression are risk factors for the development of MAC.12 Histologically, the superficial portion displays small cornifying cysts interspersed with islands of basaloid cells and may mimic a syringoma. However, the deeper portions demonstrate ducts lined by a single layer of cells with a background of hyalinized and sclerotic stroma. The tumor cells may occupy the deep dermis and underlying subcutis, muscle, or bone and demonstrate an infiltrative growth pattern and perineural invasion. Treatment includes Mohs micrographic surgery.
Desmoplastic trichoepitheliomas most commonly present as solitary white to yellowish annular papules or plaques with a central dell located on sun-exposed areas of the face, cheeks, or chin. This benign neoplasm has a bimodal age distribution, primarily affecting females either in childhood or adulthood.13 Histologically, strands and nests of basaloid epithelial cells proliferate in a dense eosinophilic desmoplastic stroma. The basaloid islands are narrow and cordlike with growth parallel to the surface epidermis and do not dive deeply into the deep dermis or subcutis. Ductal differentiation with associated secretions typically is not seen in DTE.1 Calcifications and foreign body granulomatous infiltrates may be present. Merkel cells also are present in this tumor and may be highlighted by immunohistochemistry with cytokeratin 20.14 Rarely, desmoplastic trichoepitheliomas may transform into trichoblastic carcinomas. Treatment may consist of surgical excision or Mohs micrographic surgery.
Morpheaform BCC also is included in the clinical and histopathologic differential diagnosis of infiltrative basaloid neoplasms. It is one of the more aggressive variants of BCC. The use of immunohistochemical staining may aid in differentiating between these sclerosing adnexal neoplasms.15 For example, pleckstrin homologylike domain family A member 1 (PHLDA1) is a stem cell marker that is heavily expressed in DTE as a specific follicular bulge marker but is not present in a morpheaform BCC. This highlights the follicular nature of DTEs at the molecular level. BerEP4 is a monoclonal antibody that serves as an epithelial marker for 2 glycopolypeptides: 34 and 39 kDa. This antibody may demonstrate positivity in morpheaform BCC but does not stain cells of interest in MAC.
Inflammatory linear verrucous epidermal nevus clinically presents with erythematous and warty papules in a linear distribution following the Blaschko lines. The papules often are reported to be intensely pruritic and usually are localized to one extremity.16 Although adultonset forms of ILVEN have been described,17 it most commonly is diagnosed in young children. Histologically, ILVEN consists of psoriasiform epidermal hyperplasia with alternating areas of parakeratosis and orthokeratosis with underlying agranulosis and hypergranulosis, respectively.18 The upper dermis contains a perivascular lymphocytic infiltrate. Treatment with laser therapy and surgical excision has led to both symptomatic and clinical improvement of ILVEN.16
Plaque-type syringomas are a rare variant of syringomas that clinically may mimic other common inflammatory and neoplastic conditions. An adequate biopsy is imperative to differentiate between adnexal neoplasms, as a small superficial biopsy of a syringoma may demonstrate features observed in other malignant or locally aggressive neoplasms. In our patient, the small ducts lined by cuboidal epithelium with no cellular atypia and no deep dermal growth or perineural invasion allowed for the diagnosis of PTS. Therapeutic options were reviewed with our patient, including oral isotretinoin, laser therapy, and staged excision. Ultimately, our patient elected not to pursue treatment, and she is being monitored clinically for any changes in appearance or symptoms.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma [published online November 12, 2007]. J Cutan Pathol. 2008;35:570-574.
- Furue M, Hori Y, Nakabayashi Y. Clear-cell syringoma. association with diabetes mellitus. Am J Dermatopathol. 1984;6:131-138.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Cohen PR, Tschen JA, Rapini RP. Penile syringoma: reports and review of patients with syringoma located on the penis. J Clin Aesthet Dermatol. 2013;6:38-42.
- Okuda H, Tei N, Shimizu K, et al. Chondroid syringoma of the scrotum. Int J Urol. 2008;15:944-945.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaquetype syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Mainitz M, Schmidt JB, Gebhart W. Response of multiple syringomas to isotretinoin. Acta Derm Venereol. 1986;66:51-55.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Pujol RM, LeBoit PE, Su WP. Microcystic adnexal carcinoma with extensive sebaceous differentiation. Am J Dermatopathol. 1997;19:358-362.
- Rahman J, Tahir M, Arekemase H, et al. Desmoplastic trichoepithelioma: histopathologic and immunohistochemical criteria for differentiation of a rare benign hair follicle tumor from other cutaneous adnexal tumors. Cureus. 2020;12:E9703.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Sellheyer K, Nelson P, Kutzner H, et al. The immunohistochemical differential diagnosis of microcystic adnexal carcinoma, desmoplastic trichoepithelioma and morpheaform basal cell carcinoma using BerEP4 and stem cell markers. J Cutan Pathol. 2013;40:363-370.
- Gianfaldoni S, Tchernev G, Gianfaldoni R, et al. A case of “inflammatory linear verrucous epidermal nevus” (ILVEN) treated with CO2 laser ablation. Open Access Maced J Med Sci. 2017;5:454-457.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus [published online October 27, 1999]. J Dermatol. 1999;26:599-602.
- Patterson JW, Hosler GA, Prenshaw KL, et al. The psoriasiform reaction pattern. In: Patterson JW. Weedon’s Skin Pathology. 5th ed. Elsevier; 2021:99-120.
A 17-year-old adolescent girl presented with a solitary, 8-cm, pink plaque on the anterior aspect of the neck of 5 years’ duration. No similar skin findings were present elsewhere on the body. The rash was not painful or pruritic, and she denied prior trauma to the site. The patient previously had tried a salicylic acid bodywash as well as mupirocin cream 2% and mometasone ointment with no improvement. Her medical history was unremarkable, and she had no known allergies. There was no family history of a similar rash. Physical examination revealed no palpable subcutaneous lumps or masses and no lymphadenopathy of the head or neck. An incisional biopsy was performed.