User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Does using A1c to diagnose diabetes miss some patients?
The introduction of hemoglobin A1c as an option for diagnosing type 2 diabetes over a decade ago may have resulted in underdiagnosis, new research indicates.
In 2011, the World Health Organization advised that A1c measurement, with a cutoff value of 6.5%, could be used to diagnose diabetes. The American Diabetes Association had issued similar guidance in 2010.
Prior to that time, the less-convenient 2-hour oral glucose tolerance test (OGTT) and fasting blood glucose (FBG) were the only recommended tests. While WHO made no recommendations for interpreting values below 6.5%, the ADA designated 5.7%-6.4% as prediabetes.
The new study, published online in The Lancet Regional Health–Europe, showed that the incidence of type 2 diabetes in Denmark had been increasing prior to the 2012 adoption of A1c as a diagnostic option but declined thereafter. And all-cause mortality among people with type 2 diabetes, which had been dropping, began to increase after that time.
“Our findings suggest that fewer patients have been diagnosed with [type 2 diabetes] since A1c testing was introduced as a convenient diagnostic option. We may thus be missing a group with borderline increased A1c values that is still at high metabolic and cardiovascular risk,” Jakob S. Knudsen, MD, of the department of clinical epidemiology, Aarhus (Denmark) University Hospital, and colleagues wrote.
Therefore, Dr. Knudsen said in an interview, clinicians should “consider testing with FBG or OGTT when presented with borderline A1c values.”
The reason for the increase in mortality after incident type 2 diabetes diagnosis, he said, “is that the patients who would have reduced the average mortality are no longer diagnosed...This does not reflect that we are treating already diagnosed patients any worse, rather some patients are not diagnosed.”
But M. Sue Kirkman, MD, emeritus professor of medicine at the University of North Carolina at Chapel Hill, who was part of the writing group for the 2010 ADA guidelines, isn’t convinced.
“This is an interesting paper, but it is a bit hard to believe that a change in WHO recommendations would have such a large and almost immediate impact on incidence and mortality. It seems likely that ... factors [other] than just the changes in recommendations for the diagnostic test account for these findings,” she said.
Dr. Kirkman pointed to new data just out from the Centers for Disease Control and Prevention on Jan. 26 that don›t show evidence of a higher proportion of people in the United States who have undiagnosed diabetes, “which would be expected if more cases were being ‘missed’ by A1c.”
She added that the CDC incidence data “show a continuing steady rate of decline in incidence that began in 2008, before any organizations recommended using A1c to screen for or diagnose diabetes.” Moreover, “there is evidence that type 2 diabetes incidence has fallen or plateaued in many countries since 2006, well before the WHO recommendation, with most of the studies from developed countries.”
But Dr. Knudsen also cited other data, including a study that showed a drop or stabilization in diagnosed diabetes incidence in high-income countries since 2010.
“That study concluded that the reasons for the declines in the incidence of diagnosed diabetes warrant further investigation with appropriate data sources, which was a main objective of our study,” wrote Dr. Knudsen and coauthors.
Dr. Knudsen said in an interview: “We are not the first to make the point that this sudden change is related to A1c introduction...but we are the first to have the data to clearly show that is the case.”
Diabetes incidence dropped but mortality rose after 2010
The population-based longitudinal study used four Danish medical databases and included 415,553 patients treated for type 2 diabetes for the first time from 1995-2018 and 2,060,279 matched comparators not treated for diabetes.
From 1995 until the 2012 introduction of A1c as a diagnostic option, the annual standardized incidence rates of type 2 diabetes more than doubled, from 193 per 100,000 population to 396 per 100,000 population, at a rate of 4.1% per year.
But from 2011 to 2018, the annual standardized incidence rate declined by 36%, to 253 per 100,000 population, a 5.7% annualized decrease.
The increase prior to 2011 occurred in both men and women and in all age groups, while the subsequent decline was seen primarily in the older age groups. The all-cause mortality risk within the first year after diabetes diagnosis was higher than subsequent 1-year mortality risks and not different between men and women.
From the periods 1995-1997 to 2010-2012, the adjusted mortality rate among those with type 2 diabetes decreased by 44%, from 72 deaths per 1000 person-years to 40 deaths per 1000 person-years (adjusted mortality rate ratio, 0.55). After that low level in 2010-2012, mortality increased by 27% to 48 per 1000 person-years (adjusted mortality rate ratio 0.69, compared with 1995-1997).
The reversed mortality trend after 2010-2012 was caused almost entirely by the increase in the first year after diabetes diagnosis, Dr. Knudsen and colleagues noted.
According to Dr. Kirkman, “A1c is strongly predictive of complications and mortality. That plus its ease of use and the fact that more people may be screened mean it’s still a good option. But for any of these tests, people who are slightly below the cut-point should not be considered normal or low risk.”
Indeed, Dr. Knudsen and colleagues said, “these findings may have implications for clinical practice and suggest that a more multifactorial view of metabolic risk is needed.”
Dr. Knudsen and Dr. Kirkman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The introduction of hemoglobin A1c as an option for diagnosing type 2 diabetes over a decade ago may have resulted in underdiagnosis, new research indicates.
In 2011, the World Health Organization advised that A1c measurement, with a cutoff value of 6.5%, could be used to diagnose diabetes. The American Diabetes Association had issued similar guidance in 2010.
Prior to that time, the less-convenient 2-hour oral glucose tolerance test (OGTT) and fasting blood glucose (FBG) were the only recommended tests. While WHO made no recommendations for interpreting values below 6.5%, the ADA designated 5.7%-6.4% as prediabetes.
The new study, published online in The Lancet Regional Health–Europe, showed that the incidence of type 2 diabetes in Denmark had been increasing prior to the 2012 adoption of A1c as a diagnostic option but declined thereafter. And all-cause mortality among people with type 2 diabetes, which had been dropping, began to increase after that time.
“Our findings suggest that fewer patients have been diagnosed with [type 2 diabetes] since A1c testing was introduced as a convenient diagnostic option. We may thus be missing a group with borderline increased A1c values that is still at high metabolic and cardiovascular risk,” Jakob S. Knudsen, MD, of the department of clinical epidemiology, Aarhus (Denmark) University Hospital, and colleagues wrote.
Therefore, Dr. Knudsen said in an interview, clinicians should “consider testing with FBG or OGTT when presented with borderline A1c values.”
The reason for the increase in mortality after incident type 2 diabetes diagnosis, he said, “is that the patients who would have reduced the average mortality are no longer diagnosed...This does not reflect that we are treating already diagnosed patients any worse, rather some patients are not diagnosed.”
But M. Sue Kirkman, MD, emeritus professor of medicine at the University of North Carolina at Chapel Hill, who was part of the writing group for the 2010 ADA guidelines, isn’t convinced.
“This is an interesting paper, but it is a bit hard to believe that a change in WHO recommendations would have such a large and almost immediate impact on incidence and mortality. It seems likely that ... factors [other] than just the changes in recommendations for the diagnostic test account for these findings,” she said.
Dr. Kirkman pointed to new data just out from the Centers for Disease Control and Prevention on Jan. 26 that don›t show evidence of a higher proportion of people in the United States who have undiagnosed diabetes, “which would be expected if more cases were being ‘missed’ by A1c.”
She added that the CDC incidence data “show a continuing steady rate of decline in incidence that began in 2008, before any organizations recommended using A1c to screen for or diagnose diabetes.” Moreover, “there is evidence that type 2 diabetes incidence has fallen or plateaued in many countries since 2006, well before the WHO recommendation, with most of the studies from developed countries.”
But Dr. Knudsen also cited other data, including a study that showed a drop or stabilization in diagnosed diabetes incidence in high-income countries since 2010.
“That study concluded that the reasons for the declines in the incidence of diagnosed diabetes warrant further investigation with appropriate data sources, which was a main objective of our study,” wrote Dr. Knudsen and coauthors.
Dr. Knudsen said in an interview: “We are not the first to make the point that this sudden change is related to A1c introduction...but we are the first to have the data to clearly show that is the case.”
Diabetes incidence dropped but mortality rose after 2010
The population-based longitudinal study used four Danish medical databases and included 415,553 patients treated for type 2 diabetes for the first time from 1995-2018 and 2,060,279 matched comparators not treated for diabetes.
From 1995 until the 2012 introduction of A1c as a diagnostic option, the annual standardized incidence rates of type 2 diabetes more than doubled, from 193 per 100,000 population to 396 per 100,000 population, at a rate of 4.1% per year.
But from 2011 to 2018, the annual standardized incidence rate declined by 36%, to 253 per 100,000 population, a 5.7% annualized decrease.
The increase prior to 2011 occurred in both men and women and in all age groups, while the subsequent decline was seen primarily in the older age groups. The all-cause mortality risk within the first year after diabetes diagnosis was higher than subsequent 1-year mortality risks and not different between men and women.
From the periods 1995-1997 to 2010-2012, the adjusted mortality rate among those with type 2 diabetes decreased by 44%, from 72 deaths per 1000 person-years to 40 deaths per 1000 person-years (adjusted mortality rate ratio, 0.55). After that low level in 2010-2012, mortality increased by 27% to 48 per 1000 person-years (adjusted mortality rate ratio 0.69, compared with 1995-1997).
The reversed mortality trend after 2010-2012 was caused almost entirely by the increase in the first year after diabetes diagnosis, Dr. Knudsen and colleagues noted.
According to Dr. Kirkman, “A1c is strongly predictive of complications and mortality. That plus its ease of use and the fact that more people may be screened mean it’s still a good option. But for any of these tests, people who are slightly below the cut-point should not be considered normal or low risk.”
Indeed, Dr. Knudsen and colleagues said, “these findings may have implications for clinical practice and suggest that a more multifactorial view of metabolic risk is needed.”
Dr. Knudsen and Dr. Kirkman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The introduction of hemoglobin A1c as an option for diagnosing type 2 diabetes over a decade ago may have resulted in underdiagnosis, new research indicates.
In 2011, the World Health Organization advised that A1c measurement, with a cutoff value of 6.5%, could be used to diagnose diabetes. The American Diabetes Association had issued similar guidance in 2010.
Prior to that time, the less-convenient 2-hour oral glucose tolerance test (OGTT) and fasting blood glucose (FBG) were the only recommended tests. While WHO made no recommendations for interpreting values below 6.5%, the ADA designated 5.7%-6.4% as prediabetes.
The new study, published online in The Lancet Regional Health–Europe, showed that the incidence of type 2 diabetes in Denmark had been increasing prior to the 2012 adoption of A1c as a diagnostic option but declined thereafter. And all-cause mortality among people with type 2 diabetes, which had been dropping, began to increase after that time.
“Our findings suggest that fewer patients have been diagnosed with [type 2 diabetes] since A1c testing was introduced as a convenient diagnostic option. We may thus be missing a group with borderline increased A1c values that is still at high metabolic and cardiovascular risk,” Jakob S. Knudsen, MD, of the department of clinical epidemiology, Aarhus (Denmark) University Hospital, and colleagues wrote.
Therefore, Dr. Knudsen said in an interview, clinicians should “consider testing with FBG or OGTT when presented with borderline A1c values.”
The reason for the increase in mortality after incident type 2 diabetes diagnosis, he said, “is that the patients who would have reduced the average mortality are no longer diagnosed...This does not reflect that we are treating already diagnosed patients any worse, rather some patients are not diagnosed.”
But M. Sue Kirkman, MD, emeritus professor of medicine at the University of North Carolina at Chapel Hill, who was part of the writing group for the 2010 ADA guidelines, isn’t convinced.
“This is an interesting paper, but it is a bit hard to believe that a change in WHO recommendations would have such a large and almost immediate impact on incidence and mortality. It seems likely that ... factors [other] than just the changes in recommendations for the diagnostic test account for these findings,” she said.
Dr. Kirkman pointed to new data just out from the Centers for Disease Control and Prevention on Jan. 26 that don›t show evidence of a higher proportion of people in the United States who have undiagnosed diabetes, “which would be expected if more cases were being ‘missed’ by A1c.”
She added that the CDC incidence data “show a continuing steady rate of decline in incidence that began in 2008, before any organizations recommended using A1c to screen for or diagnose diabetes.” Moreover, “there is evidence that type 2 diabetes incidence has fallen or plateaued in many countries since 2006, well before the WHO recommendation, with most of the studies from developed countries.”
But Dr. Knudsen also cited other data, including a study that showed a drop or stabilization in diagnosed diabetes incidence in high-income countries since 2010.
“That study concluded that the reasons for the declines in the incidence of diagnosed diabetes warrant further investigation with appropriate data sources, which was a main objective of our study,” wrote Dr. Knudsen and coauthors.
Dr. Knudsen said in an interview: “We are not the first to make the point that this sudden change is related to A1c introduction...but we are the first to have the data to clearly show that is the case.”
Diabetes incidence dropped but mortality rose after 2010
The population-based longitudinal study used four Danish medical databases and included 415,553 patients treated for type 2 diabetes for the first time from 1995-2018 and 2,060,279 matched comparators not treated for diabetes.
From 1995 until the 2012 introduction of A1c as a diagnostic option, the annual standardized incidence rates of type 2 diabetes more than doubled, from 193 per 100,000 population to 396 per 100,000 population, at a rate of 4.1% per year.
But from 2011 to 2018, the annual standardized incidence rate declined by 36%, to 253 per 100,000 population, a 5.7% annualized decrease.
The increase prior to 2011 occurred in both men and women and in all age groups, while the subsequent decline was seen primarily in the older age groups. The all-cause mortality risk within the first year after diabetes diagnosis was higher than subsequent 1-year mortality risks and not different between men and women.
From the periods 1995-1997 to 2010-2012, the adjusted mortality rate among those with type 2 diabetes decreased by 44%, from 72 deaths per 1000 person-years to 40 deaths per 1000 person-years (adjusted mortality rate ratio, 0.55). After that low level in 2010-2012, mortality increased by 27% to 48 per 1000 person-years (adjusted mortality rate ratio 0.69, compared with 1995-1997).
The reversed mortality trend after 2010-2012 was caused almost entirely by the increase in the first year after diabetes diagnosis, Dr. Knudsen and colleagues noted.
According to Dr. Kirkman, “A1c is strongly predictive of complications and mortality. That plus its ease of use and the fact that more people may be screened mean it’s still a good option. But for any of these tests, people who are slightly below the cut-point should not be considered normal or low risk.”
Indeed, Dr. Knudsen and colleagues said, “these findings may have implications for clinical practice and suggest that a more multifactorial view of metabolic risk is needed.”
Dr. Knudsen and Dr. Kirkman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET REGIONAL HEALTH–EUROPE
No COVID vax, no transplant: Unfair or good medicine?
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Right now, more than 106,600 people in the United States are on the national transplant waiting list, each hoping to hear soon that a lung, kidney, heart, or other vital organ has been found for them. It’s the promise not just of a new organ, but a new life.
Well before they are placed on that list, transplant candidates, as they’re known, are evaluated with a battery of tests and exams to be sure they are infection free, their other organs are healthy, and that all their vaccinations are up to date.
In January, a 31-year-old Boston father of two declined to get the COVID-19 vaccine, and Brigham and Women’s Hospital officials removed him from the heart transplant waiting list. And in North Carolina, a 38-year-old man in need of a kidney transplant said he, too, was denied the organ when he declined to get the vaccination.
Those are just two of the most recent cases. The decisions by the transplant centers to remove the candidates from the waiting list have set off a national debate among ethicists, family members, doctors, patients, and others.
On social media and in conversation, the question persists: Is removing them from the list unfair and cruel, or simply business as usual to keep the patient as healthy as possible and the transplant as successful as possible?
Two recent tweets sum up the debate.
“The people responsible for this should be charged with attempted homicide,” one Twitter user said, while another suggested that the more accurate way to headline the news about a transplant candidate refusing the COVID-19 vaccine would be: “Patient voluntarily forfeits donor organ.”
Doctors and ethics experts, as well as other patients on the waiting list, say it’s simply good medicine to require the COVID vaccine, along with a host of other pretransplant requirements.
Transplant protocols
“Transplant medicine has always been a strong promoter of vaccination,” said Silas Prescod Norman, MD, a clinical associate professor of nephrology and internal medicine at the University of Michigan, Ann Arbor. He is a kidney specialist who works in the university’s transplant clinic.
Requiring the COVID vaccine is in line with requirements to get numerous other vaccines, he said.“Promoting the COVID vaccine among our transplant candidates and recipients is just an extension of our usual practice.
“In transplantation, first and foremost is patient safety,” Dr. Norman said. “And we know that solid organ transplant patients are at substantially higher risk of contracting COVID than nontransplant patients.”
After the transplant, they are placed on immunosuppressant drugs, that weaken the immune system while also decreasing the body’s ability to reject the new organ.
“We know now, because there is good data about the vaccine to show that people who are on transplant medications are less likely to make detectable antibodies after vaccination,” said Dr. Norman, who’s also a medical adviser for the American Kidney Fund, a nonprofit that provides kidney health information and financial assistance for dialysis.
And this is not a surprise because of the immunosuppressive effects, he said. “So it only makes sense to get people vaccinated before transplantation.”
Researchers compared the cases of more than 17,000 people who had received organ transplants and were hospitalized from April to November 2020, either for COVID (1,682 of them) or other health issues. Those who had COVID were more likely to have complications and to die in the hospital than those who did not have it.
Vaccination guidelines, policies
Federal COVID-19 treatment guidelines from the National Institutes of Health state that transplant patients on immunosuppressant drugs used after the procedure should be considered at a higher risk of getting severe COVID if infected.
In a joint statement from the American Society of Transplant Surgeons, the American Society of Transplantation, and the International Society for Heart and Lung Transplantation, the organizations say they “strongly recommend that all eligible children and adult transplant candidates and recipients be vaccinated with a COVID-19 vaccine [and booster] that is approved or authorized in their jurisdiction. Whenever possible, vaccination should occur prior to transplantation.” Ideally, it should be completed at least 2 weeks before the transplant.
The organizations also “support the development of institutional policies regarding pretransplant vaccination. We believe that this is in the best interest of the transplant candidate, optimizing their chances of getting through the perioperative and posttransplant periods without severe COVID-19 disease, especially at times of greater infection prevalence.”
Officials at Brigham and Women’s Hospital, where the 31-year-old father was removed from the list, issued a statement that reads, in part: “Our Mass General Brigham health care system requires several [Centers for Disease Control and Prevention]-recommended vaccines, including the COVID-19 vaccine, and lifestyle behaviors for transplant candidates to create both the best chance for a successful operation and to optimize the patient’s survival after transplantation, given that their immune system is drastically suppressed. Patients are not active on the wait list without this.”
Ethics amid organ shortage
“Organs are scarce,” said Arthur L. Caplan, PhD, director of the division of medical ethics at New York University Langone Medical Center. That makes the goal of choosing the very best candidates for success even more crucial.
“You try to maximize the chance the organ will work,” he said. Pretransplant vaccination is one way.
The shortage is most severe for kidney transplants. In 2020, according to federal statistics, more than 91,000 kidney transplants were needed, but fewer than 23,000 were received. During 2021, 41,354 transplants were done, an increase of nearly 6% over the previous year. The total includes kidneys, hearts, lungs, and other organs, with kidneys accounting for more than 24,000 of the total.
Even with the rise in transplant numbers, supply does not meet demand. According to federal statistics, 17 people in the United States die each day waiting for an organ transplant. Every 9 minutes, someone is added to the waiting list.
“This isn’t and it shouldn’t be a fight about the COVID vaccine,” Dr. Caplan said. “This isn’t an issue about punishing non-COVID vaccinators. It’s deciding who is going to get a scarce organ.”
“A lot of people [opposed to removing the nonvaccinated from the list] think: ‘Oh, they are just killing those people who won’t take a COVID vaccine.’ That’s not what is going on.”
The transplant candidate must be in the best possible shape overall, Dr. Caplan and doctors agreed. Someone who is smoking, drinking heavily, or abusing drugs isn’t going to the top of the list either. And for other procedures, such as bariatric surgery or knee surgery, some patients are told first to lose weight before a surgeon will operate.
The worry about side effects from the vaccine, which some patients have cited as a concern, is misplaced, Dr. Caplan said. What transplant candidates who refuse the COVID vaccine may not be thinking about is that they are facing a serious operation and will be on numerous anti-rejection drugs, with side effects, after the surgery.
“So to be worried about the side effects of a COVID vaccine is irrational,” he said.
Transplants: The process
The patients who were recently removed from the transplant list could seek care and a transplant at an alternate center, said Anne Paschke, a spokesperson for the United Network for Organ Sharing, a nonprofit group that is under contract with the federal government and operates the national Organ Procurement and Transplantation Network (OPTN).
“Transplant hospitals decide which patients to add to the wait list based on their own criteria and medical judgment to create the best chance for a positive transplant outcome,” she said. That’s done with the understanding that patients will help with their medical care.
So, if one program won’t accept a patient, another may. But, if a patient turned down at one center due to refusing to get the COVID vaccine tries another center, the requirements at that hospital may be the same, she said.
OPTN maintains a list of transplant centers. As of Jan. 28, there were 251 transplant centers, according to UNOS, which manages the waiting list, matches donors and recipients, and strives for equity, among other duties.
Pretransplant refusers not typical
“The cases we are seeing are outliers,” Dr. Caplan said of the handful of known candidates who have refused the vaccine. Most ask their doctor exactly what they need to do to live and follow those instructions.
Dr. Norman agreed. Most of the kidney patients he cares for who are hoping for a transplant have been on dialysis, “which they do not like. They are doing whatever they can to make sure they don’t go back on dialysis. As a group, they tend to be very adherent, very safety conscious because they understand their risk and they understand the gift they have received [or will receive] through transplantation. They want to do everything they can to respect and protect that gift.”
Not surprisingly, some on the transplant list who are vaccinated have strong opinions about those who refuse to get the vaccine. Dana J. Ufkes, 61, a Seattle realtor, has been on the kidney transplant list – this time – since 2003, hoping for her third transplant. When asked if potential recipients should be removed from the list if they refuse the COVID vaccine, her answer was immediate: “Absolutely.”
At age 17, Ms. Ufkes got a serious kidney infection that went undiagnosed and untreated. Her kidney health worsened, and she needed a transplant. She got her first one in 1986, then again in 1992.
“They last longer than they used to,” she said. But not forever. (According to the American Kidney Fund, transplants from a living kidney donor last about 15-20 years; from a deceased donor, 10-15.)
The decision to decline the vaccine is, of course, each person’s choice, Ms. Ufkes said. But “if they don’t want to be vaccinated [and still want to be on the list], I think that’s BS.”
Citing the lack of organs, “it’s not like they are handing these out like jellybeans.”
A version of this article first appeared on WebMD.com.
Disseminated Erythematous-Violet Edematous Plaques and Necrotic Nodules
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

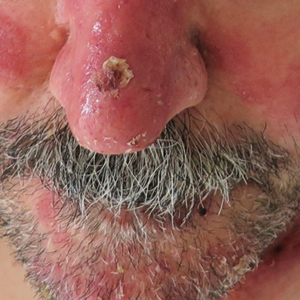
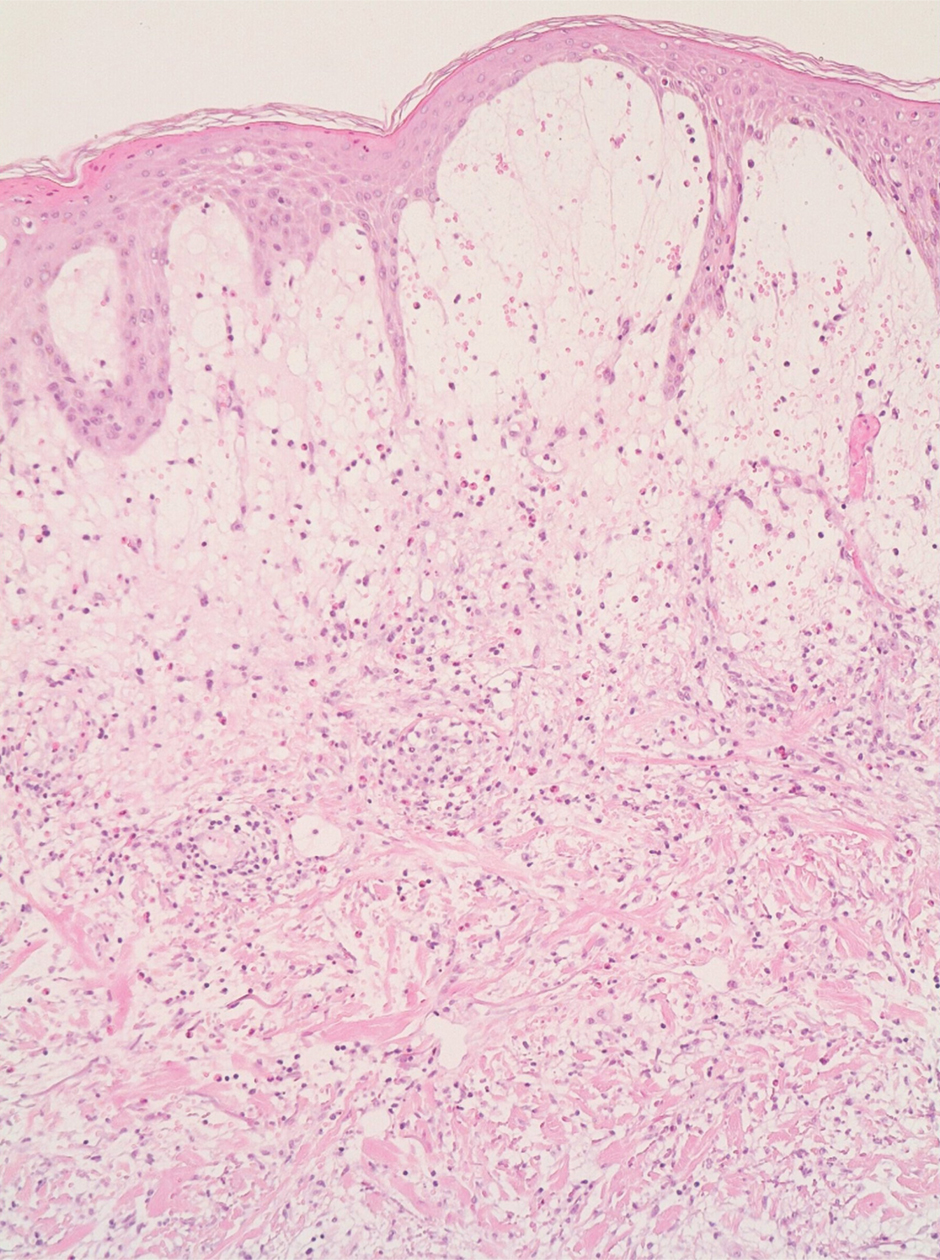
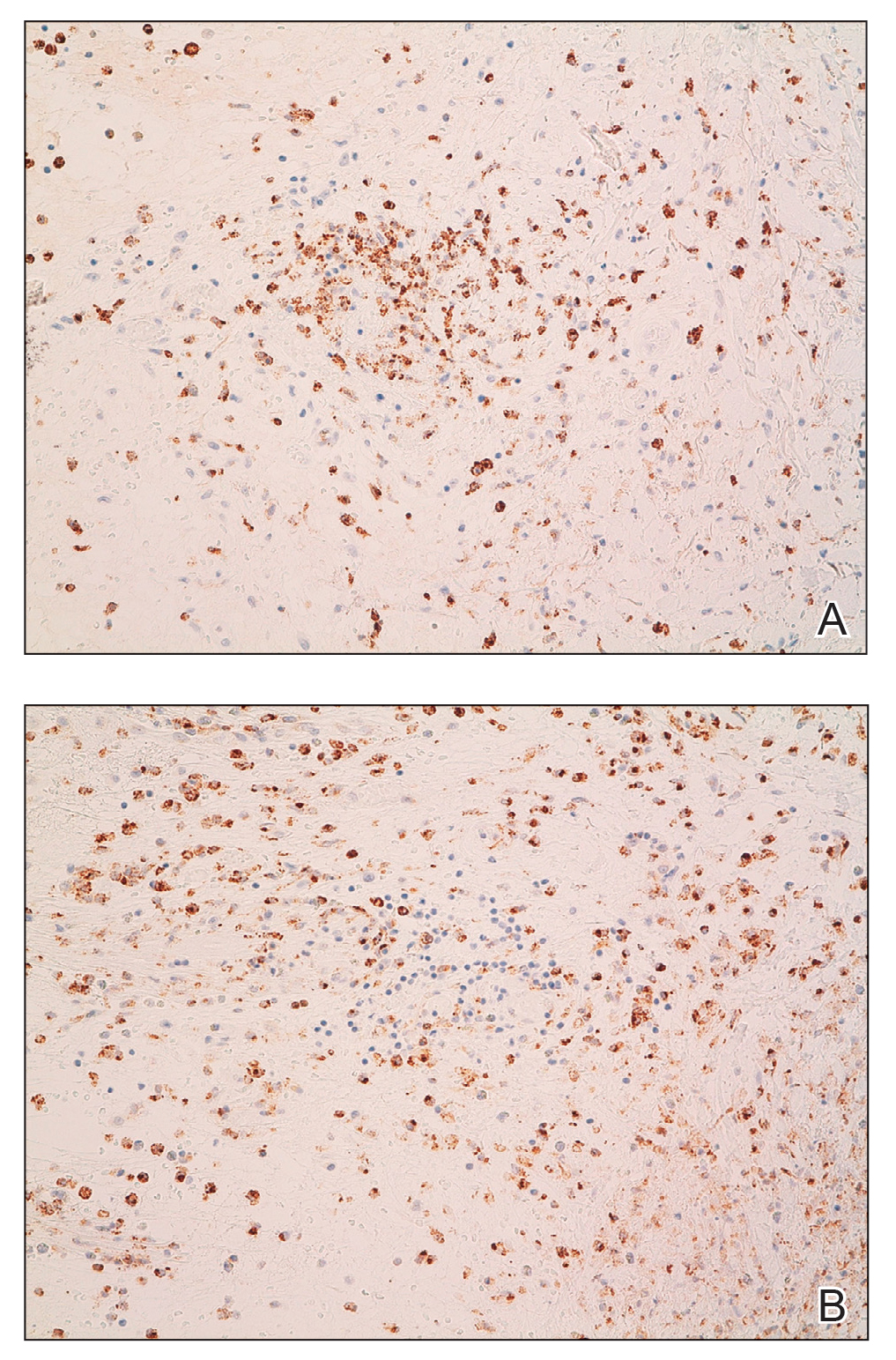
A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.
Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).
The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.

Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).

The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
The Diagnosis: Histiocytoid Sweet Syndrome
The patient was admitted for clinical study and treatment monitoring. During the first 72 hours of admittance, the lesions and general malaise further developed along with C-reactive protein elevation (126 mg/L). Administration of intravenous prednisone at a dosage of 1 mg/kg daily was accompanied by substantial improvement after 1 week of treatment, with subsequent follow-up and outpatient monitoring. An underlying neoplasia was ruled out after review of medical history, physical examination, complete blood cell count, chest radiography, abdominal ultrasonography, colonoscopy, and bone marrow aspiration.

A 4-mm skin biopsy was performed from a lesion on the neck (Figure 1). Histology revealed a dermis with prominent edema alongside superficial, deep, and periadnexal perivascular inflammatory infiltrates, as well as predominant lymphocytes and cells with a histiocytoid profile (Figure 2). These findings were accompanied by isolated neutrophil foci. The absence of leukocytoclastic vasculitis was noted. Immunohistochemistry demonstrated that the histiocyte population was positive for myeloperoxidase and CD68, which categorized them as immature cells of myeloid origin (Figure 3). Clinical and histopathologic findings led to a definitive diagnosis of histiocytoid Sweet syndrome (SS). Sweet syndrome consists of a neutrophilic dermatosis profile. Clinically, it manifests as a sudden onset of painful nodules and plaques accompanied by fever, malaise, and leukocytosis.

Histiocytoid SS is a rare histologic variant of SS initially described by Requena et al1 in 2005. In histiocytoid SS, the main inflammatory infiltrates are promyelocytes and myelocytes.2 Immunohistochemistry shows positivity for myeloperoxidase, CD15, CD43, CD45, CD68, MAC-386, and HAM56.1 The diagnosis is determined by exclusion after adequate clinical and histopathologic correlation, which also should exclude other diagnoses such as leukemia cutis and interstitial granulomatous dermatitis.3 Histiocytoid SS may be related to an increased risk for underlying malignancy. Haber et al4 performed a systematic review in which they concluded that approximately 40% of patients newly diagnosed with histiocytoid SS subsequently were diagnosed or already were diagnosed with a hematologic or solid cancer vs 21% in the classical neutrophilic infiltrate of SS (NSS). Histiocytoid SS more commonly was associated with myelodysplastic syndrome (46% vs 2.5% in NSS) and hematologic malignancies (42.5% vs 25% in SS).

The initial differential diagnoses include inflammatory dermatoses, infections, neoplasms, and systemic diseases. In exudative erythema multiforme, early lesions are composed of typical target lesions with mucosal involvement in 25% to 60% of patients.5 Erythema elevatum diutinum is a chronic dermatosis characterized by asymptomatic papules and red-violet nodules. The most characteristic histologic finding is leukocytoclastic vasculitis.6 The absence of vasculitis is part of the major diagnostic criteria for SS.7 Wells syndrome is associated with general malaise, and edematous and erythematous-violet plaques or nodules appear on the limbs; however, it frequently is associated with eosinophilia in peripheral blood, and histology shows that the main cell population of the inflammatory infiltrate also is eosinophilic.8 Painful, superficial, and erosive blisters appear preferentially on the face and backs of the arms in bullous pyoderma gangrenosum. It usually is not associated with the typical systemic manifestations of SS (ie, fever, arthralgia, damage to target organs). On histopathology, the neutrophilic infiltrate is accompanied by subepidermal vesicles.9
Histiocytoid SS responds dramatically to corticosteroids. Other first-line treatments that avoid use of corticosteroids are colchicine, dapsone, and potassium iodide. Multiple treatments were attempted in our patient, including corticosteroids, methotrexate, dapsone, colchicine, and anakinra. Despite patients responding well to treatment, a possible underlying neoplasm, most frequently of hematologic origin, must be excluded.10
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842. doi:10.1001/archderm.141.7.834
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659. doi:10.1001/jamadermatol.2016.6092
- Llamas-Velasco M, Concha-Garzón MJ, Fraga J, et al. Histiocytoid Sweet syndrome related to bortezomib: a mimicker of cutaneous infiltration by myeloma. Indian J Dermatol Venereol Leprol. 2015; 81:305-306. doi:10.4103/0378-6323.152743
- Haber R, Feghali J, El Gemayel M. Risk of malignancy in histiocytoid Sweet syndrome: a systematic review and reappraisal [published online February 21, 2020]. J Am Acad Dermatol. 2020;83:661-663. doi:10.1016/j.jaad.2020.02.048
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902. doi:10.1111/j.1365-4632.2011.05348.x
- Newburger J, Schmieder GJ. Erythema elevatum diutinum. StatPearls. StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov /books/NBK448069/
- Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
- Weins AB, Biedermann T, Weiss T, et al. Wells syndrome. J Dtsch Dermatol Ges. 2016;14:989-993. doi:10.1111/ddg.13132
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409; quiz 410-412. doi:10.1016/s0190-9622(96)90428-4
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378. doi:10.1016/j.ad.2015.12.001
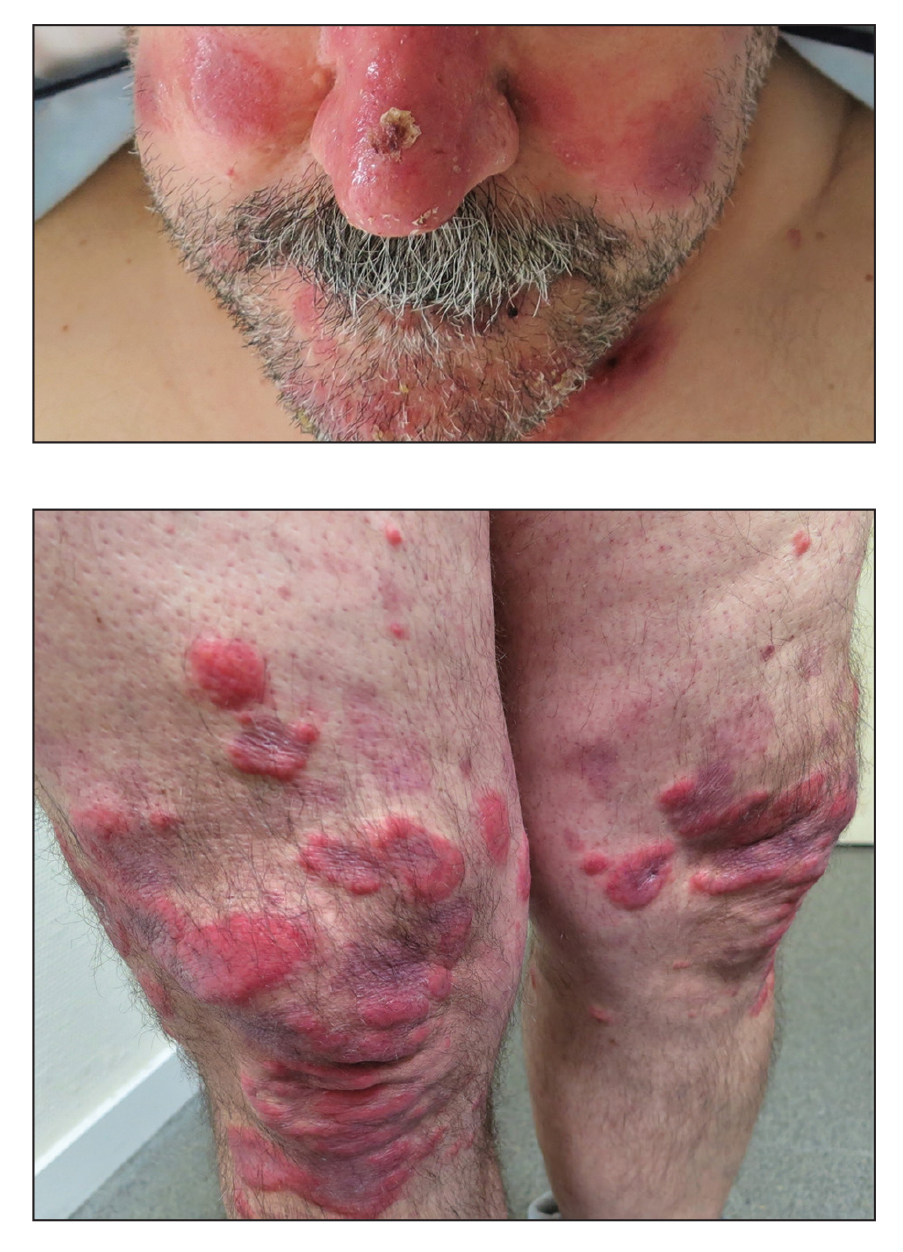
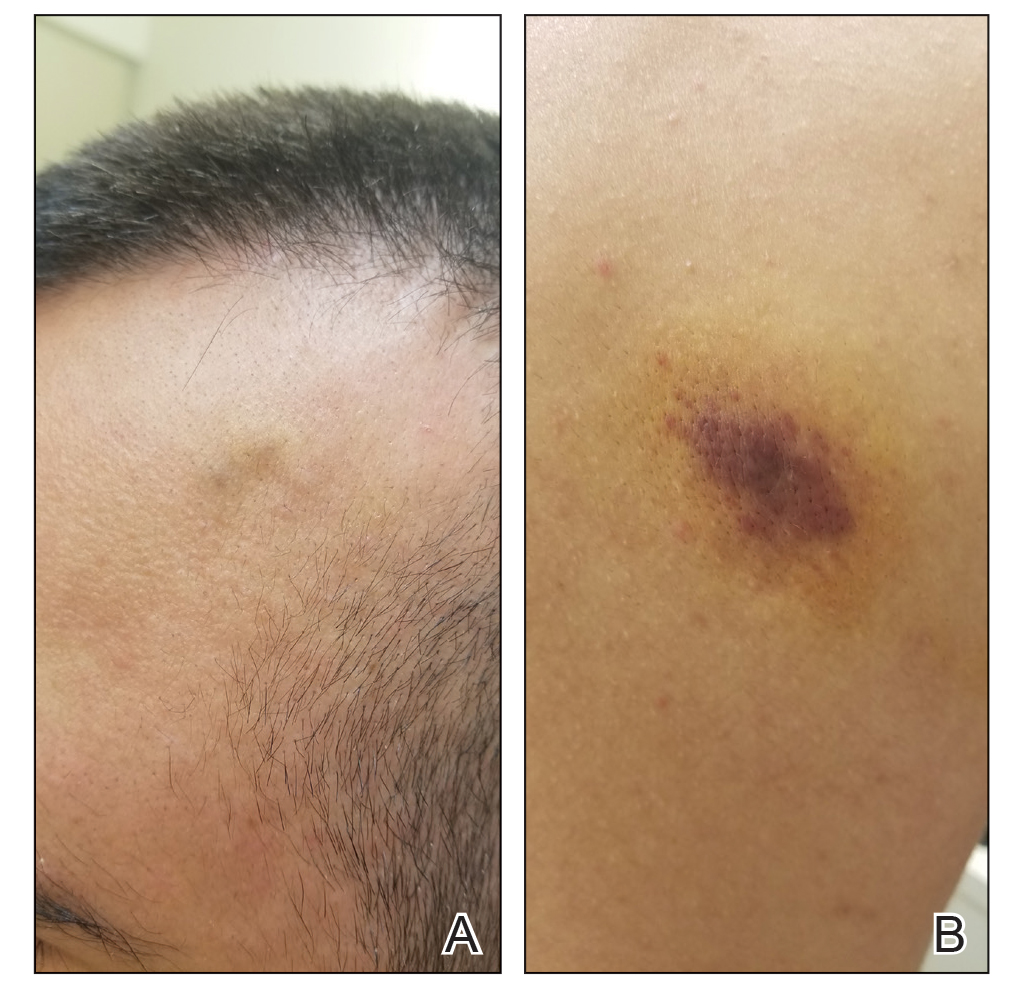
A 53-year-old man presented to the emergency department with a fever and painful skin lesions of 2 days’ duration. He reported a medical history of an upper respiratory infection 4 weeks prior. Physical examination was notable for erythematous-violet edematous papules, necrotic lesions, and pseudovesicles located on the face (top), head, neck, arms, and legs (bottom). Hemorrhagic splinters were evidenced in multiple nail sections. Urgent blood work revealed microcytic anemia (hemoglobin, 12.6 g/dL [reference range, 14.0–17.5 g/dL]) and elevated C-reactive protein (58 mg/L [reference range, 0.0–5.0 mg/L]).
Indurated Violaceous Lesions on the Face, Trunk, and Legs
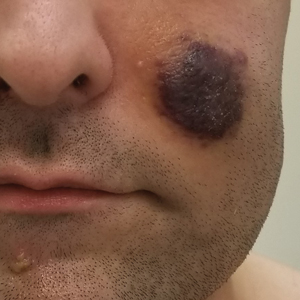
The Diagnosis: Kaposi Sarcoma
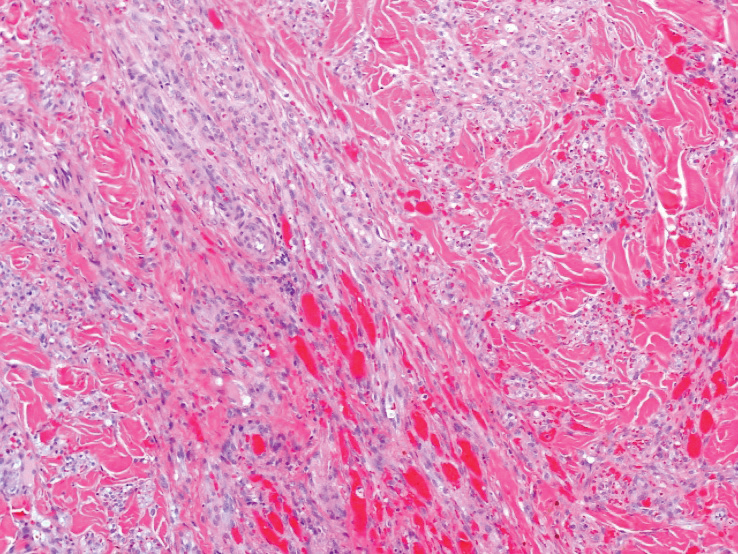
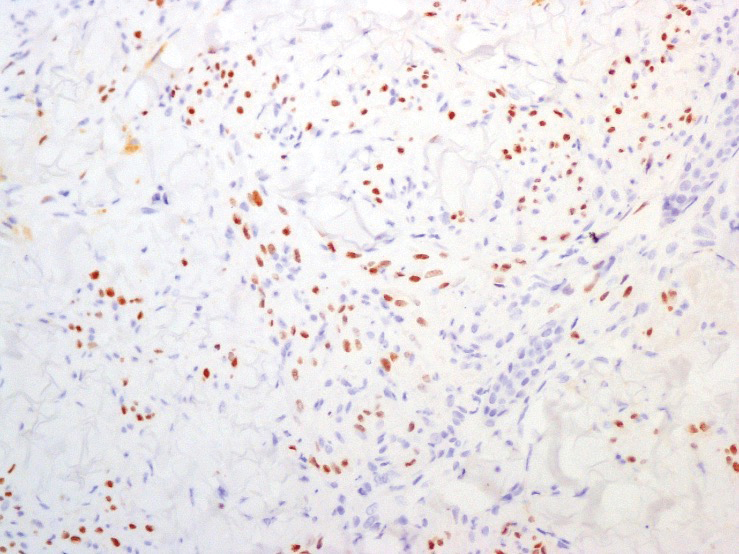
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.
Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4
Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.
The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.

Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4

Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.

The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
The Diagnosis: Kaposi Sarcoma
A punch biopsy of a lesion on the right side of the back revealed a diffuse, poorly circumscribed, spindle cell neoplasm of the papillary and reticular dermis with associated vascular and pseudovascular spaces distended by erythrocytes (Figure 1). Immunostaining was positive for human herpesvirus 8 (HHV-8)(Figure 2), ETS-related gene, CD31, and CD34 and negative for pan cytokeratin, confirming the diagnosis of Kaposi sarcoma (KS). Bacterial, fungal, and mycobacterial tissue cultures were negative. The patient was tested for HIV and referred to infectious disease and oncology. He subsequently was found to have HIV with a viral load greater than 1 million copies. He was started on antiretroviral therapy and Pneumocystis jirovecii pneumonia prophylaxis. Computed tomography of the chest, abdomen, and pelvis showed bilateral, multifocal, perihilar, flame-shaped consolidations suggestive of KS. The patient later disclosed having an intermittent dry cough of more than a year’s duration with occasional bright red blood per rectum after bowel movements. After workup, the patient was found to have cytomegalovirus esophagitis/gastritis and candidal esophagitis that were treated with valganciclovir and fluconazole, respectively.

Kaposi sarcoma is an angioproliferative, AIDSdefining disease associated with HHV-8. There are 4 types of KS as defined by the populations they affect. AIDS-associated KS occurs in individuals with HIV, as seen in our patient. It often is accompanied by extensive mucocutaneous and visceral lesions, as well as systemic symptoms such as fever, weight loss, and diarrhea.1 Classic KS is a variant that presents in older men of Mediterranean, Eastern European, and South American descent. Cutaneous lesions typically are distributed on the lower extremities.2,3 Endemic (African) KS is seen in HIV-negative children and young adults in equatorial Africa. It most commonly affects the lower extremities or lymph nodes and usually follows a more aggressive course.2 Lastly, iatrogenic KS is associated with immunosuppressive medications or conditions, such as organ transplantation, chemotherapy, and rheumatologic disorders.3,4

Kaposi sarcoma commonly presents as violaceous or dark red macules, patches, papules, plaques, and nodules on various parts of the body (Figure 3). Lesions typically begin as macules and progress into plaques or nodules. Our patient presented as a deceptively healthy young man with lesions at various stages of development. In addition to the skin and oral mucosa, the lungs, lymph nodes, and gastrointestinal tract commonly are involved in AIDS-associated KS.5 Patients may experience symptoms of internal involvement, including bleeding, hematochezia, odynophagia, or dyspnea.

The differential diagnosis includes conditions that can mimic KS, including bacillary angiomatosis, angioinvasive fungal disease, sarcoid, and other malignancies. A skin biopsy is the gold standard for definitive diagnosis of KS. Histopathology shows a vascular proliferation in the dermis and spindle cell proliferation.6 Kaposi sarcoma stains positively for factor VIII–related antigen, CD31, and CD34.2 Additionally, staining for HHV-8 gene products, such as latency-associated nuclear antigen 1, is helpful in differentiating KS from other conditions.7
In HIV-associated KS, the mainstay of treatment is initiation of highly active antiretroviral therapy. Typically, as the CD4 count rises with treatment, the tumor burden classic KS, effective treatment options include recurrent cryotherapy or intralesional chemotherapeutics, such as vincristine, for localized lesions; for widespread disease, pegylated liposomal doxorubicin or radiation have been found to be effective options. Lastly, for patients with iatrogenic KS, reducing immunosuppressive medications is a reasonable first step in management. If this does not yield adequate improvement, transitioning from calcineurin inhibitors (eg, cyclosporine) to proliferation signal inhibitors (eg, sirolimus) may lead to resolution.7
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
- Friedman-Kien AE, Saltzman BR. Clinical manifestations of classical, endemic African, and epidemic AIDS-associated Kaposi’s sarcoma. J Am Acad Dermatol. 1990;22:1237-1250.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Vangipuram R, Tyring SK. Epidemiology of Kaposi sarcoma: review and description of the nonepidemic variant. Int J Dermatol. 2019;58:538-542.
- Klepp O, Dahl O, Stenwig JT. Association of Kaposi’s sarcoma and prior immunosuppressive therapy. a 5‐year material of Kaposi’s sarcoma in Norway. Cancer. 1978;42:2626-2630.
- Lemlich G, Schwam L, Lebwohl M. Kaposi’s sarcoma and acquired immunodeficiency syndrome: postmortem findings in twenty-four cases. J Am Acad Dermatol. 1987;16:319-325.
- Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:10.
- Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi’s sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. 2016;6:465-470.
A 25-year-old man with no notable medical history presented to the dermatology clinic with growing selfdescribed cysts on the face, trunk, and legs of 6 months’ duration. The lesions started as bruiselike discolorations and progressed to become firm nodules and inflamed masses. Some were minimally itchy and sensitive to touch, but there was no history of bleeding or drainage. The patient denied any new or recent environmental or animal exposures, use of illicit drugs, or travel correlating with the rash onset. He denied any prior treatments. He reported being in his normal state of health and was not taking any medications. Physical examination revealed indurated, violaceous, purpuric subcutaneous nodules, plaques, and masses on the forehead, cheek (top), jaw, flank, axillae (bottom), and back.
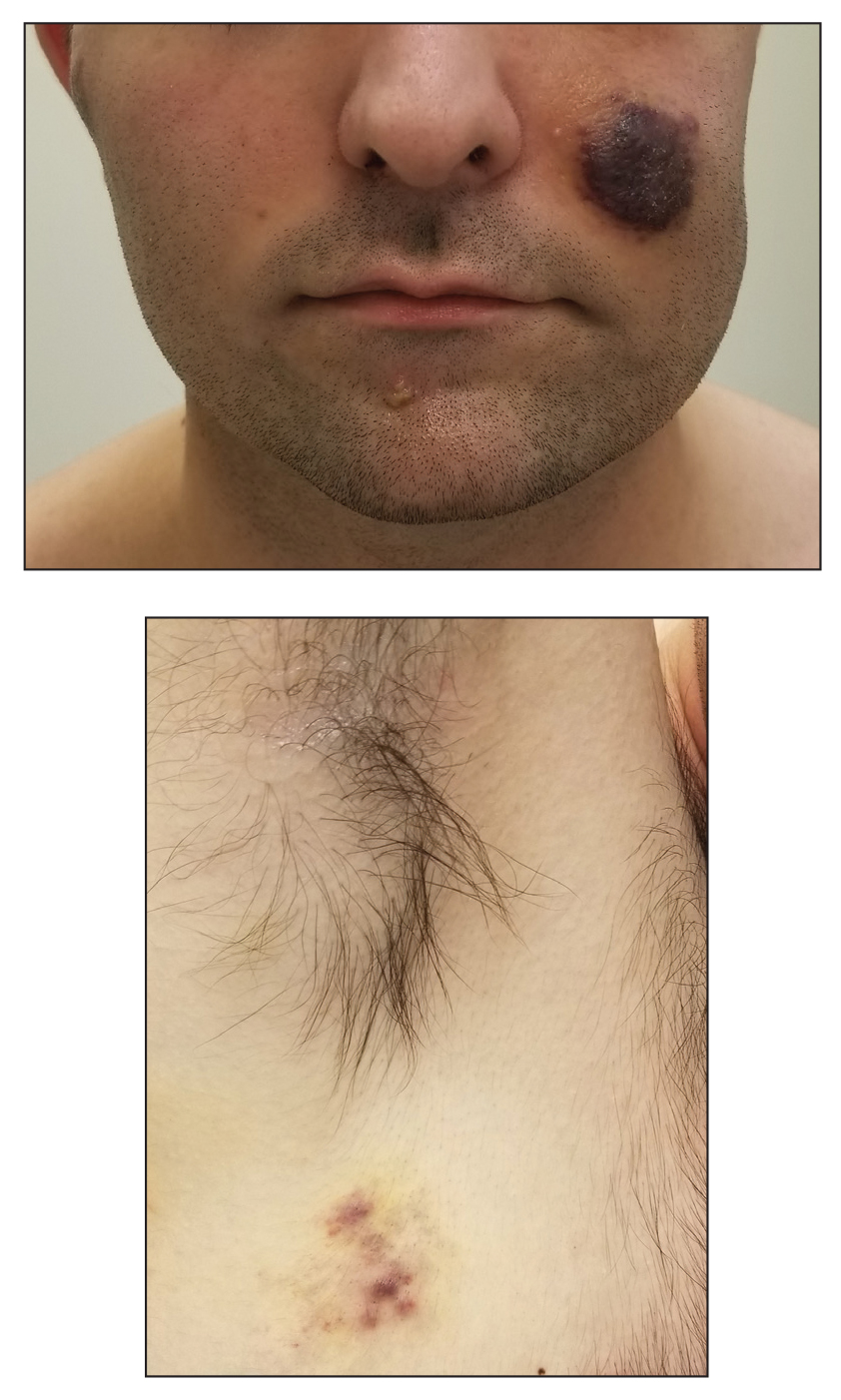
FDA approves 2-month dosing of injectable HIV drug Cabenuva
Cabenuva was first approved by the FDA in January 2021 to be administered once monthly to treat HIV-1 infection in virologically suppressed adults. The medication was the first injectable complete antiretroviral regimen approved by the FDA.
Cabenuva can replace a current treatment in virologically suppressed adults on a stable antiretroviral regimen with no history of treatment failure and no known or suspected resistance to rilpivirine and cabotegravir, the Janssen Pharmaceutical Companies of Johnson & Johnson said in a press release. Janssen and ViiV Healthcare codeveloped the injectable antiretroviral medication Cabenuva.
The expanded label approval “marks an important step forward in advancing the treatment landscape for people living with HIV,” said Candice Long, the president of infectious diseases and vaccines at Janssen Therapeutics, in a Feb. 1 press release. “With this milestone, adults living with HIV have a treatment option that further reduces the frequency of medication.”
This expanded approval was based on global clinical trial of 1,045 adults with HIV-1, which found Cabenuva administered every 8 weeks (3 mL dose of both cabotegravir and rilpivirine) to be noninferior to the 4-week regimen (2 mL dose of both medicines). At week 48 of the trial, the proportion of participants with viral loads above 50 copies per milliliter was 1.7% in the 2-month arm and 1.0% in the 1-month arm. The study found that rates of virological suppression were similar for both the 1-month and 2-month regimens (93.5% and 94.3%, respectively).
The most common side effects were injection site reactions, pyrexia, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. Adverse reactions reported in individuals receiving the regimen every 2 months or once monthly were similar. Cabenuva is contraindicated for patients with a hypersensitivity reaction to cabotegravir or rilpivirine or for those receiving carbamazepine, oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, St. John’s wort, and more than one dose of systemic dexamethasone.
A version of this article first appeared on Medscape.com.
Cabenuva was first approved by the FDA in January 2021 to be administered once monthly to treat HIV-1 infection in virologically suppressed adults. The medication was the first injectable complete antiretroviral regimen approved by the FDA.
Cabenuva can replace a current treatment in virologically suppressed adults on a stable antiretroviral regimen with no history of treatment failure and no known or suspected resistance to rilpivirine and cabotegravir, the Janssen Pharmaceutical Companies of Johnson & Johnson said in a press release. Janssen and ViiV Healthcare codeveloped the injectable antiretroviral medication Cabenuva.
The expanded label approval “marks an important step forward in advancing the treatment landscape for people living with HIV,” said Candice Long, the president of infectious diseases and vaccines at Janssen Therapeutics, in a Feb. 1 press release. “With this milestone, adults living with HIV have a treatment option that further reduces the frequency of medication.”
This expanded approval was based on global clinical trial of 1,045 adults with HIV-1, which found Cabenuva administered every 8 weeks (3 mL dose of both cabotegravir and rilpivirine) to be noninferior to the 4-week regimen (2 mL dose of both medicines). At week 48 of the trial, the proportion of participants with viral loads above 50 copies per milliliter was 1.7% in the 2-month arm and 1.0% in the 1-month arm. The study found that rates of virological suppression were similar for both the 1-month and 2-month regimens (93.5% and 94.3%, respectively).
The most common side effects were injection site reactions, pyrexia, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. Adverse reactions reported in individuals receiving the regimen every 2 months or once monthly were similar. Cabenuva is contraindicated for patients with a hypersensitivity reaction to cabotegravir or rilpivirine or for those receiving carbamazepine, oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, St. John’s wort, and more than one dose of systemic dexamethasone.
A version of this article first appeared on Medscape.com.
Cabenuva was first approved by the FDA in January 2021 to be administered once monthly to treat HIV-1 infection in virologically suppressed adults. The medication was the first injectable complete antiretroviral regimen approved by the FDA.
Cabenuva can replace a current treatment in virologically suppressed adults on a stable antiretroviral regimen with no history of treatment failure and no known or suspected resistance to rilpivirine and cabotegravir, the Janssen Pharmaceutical Companies of Johnson & Johnson said in a press release. Janssen and ViiV Healthcare codeveloped the injectable antiretroviral medication Cabenuva.
The expanded label approval “marks an important step forward in advancing the treatment landscape for people living with HIV,” said Candice Long, the president of infectious diseases and vaccines at Janssen Therapeutics, in a Feb. 1 press release. “With this milestone, adults living with HIV have a treatment option that further reduces the frequency of medication.”
This expanded approval was based on global clinical trial of 1,045 adults with HIV-1, which found Cabenuva administered every 8 weeks (3 mL dose of both cabotegravir and rilpivirine) to be noninferior to the 4-week regimen (2 mL dose of both medicines). At week 48 of the trial, the proportion of participants with viral loads above 50 copies per milliliter was 1.7% in the 2-month arm and 1.0% in the 1-month arm. The study found that rates of virological suppression were similar for both the 1-month and 2-month regimens (93.5% and 94.3%, respectively).
The most common side effects were injection site reactions, pyrexia, fatigue, headache, musculoskeletal pain, nausea, sleep disorders, dizziness, and rash. Adverse reactions reported in individuals receiving the regimen every 2 months or once monthly were similar. Cabenuva is contraindicated for patients with a hypersensitivity reaction to cabotegravir or rilpivirine or for those receiving carbamazepine, oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, St. John’s wort, and more than one dose of systemic dexamethasone.
A version of this article first appeared on Medscape.com.
Is there a cure for aging?
Heart disease. Cancer. Diabetes. Dementia.
Researchers spend billions of dollars every year trying to eradicate these medical scourges.
Yet even if we discover cures to these and all other chronic conditions, it won’t change our ultimate prognosis: death.
“That’s because you haven’t stopped aging,” says Jay Olshansky, PhD, a professor of epidemiology and biostatistics at the University of Illinois at Chicago School of Public Health.
But what if we could?
Some scientists think so. Fueled in part by a billion dollars of investor money, they are attempting to reverse-engineer your molecular biological clock. Their goal? To eliminate not merely diseases that kill people, but to prevent death itself.
Hacking the code for immortality
Aubrey de Grey, PhD, a biomedical gerontologist, has drawn wide attention for his belief that the first person who will live to be 1,000 years old is already among us.
He believes there’s no cap on how long we can live, depending on what medicines we develop in the future.
“The whole idea is that there would not be a limit on how long we can keep people healthy,” Dr. de Grey says. He’s the chief science officer and co-founder of the SENS Research Foundation, which funds research on how to put the brakes on aging.
Dr. De Grey’s view, in theory, isn’t so far-fetched.
Scientists have studied the immortal jellyfish, Turritopsis dohrnii. It’s the only animal that can cheat death by reverting from adulthood back to its polyp stage when threatened with danger or starvation.
Other clues to possible eternal life also may exist underwater. Certain marine clams can live more than 500 years. And lobsters stock a seemingly limitless supply of a youthful enzyme that has some scientists wondering if the crustacean, under the best conditions, just might live forever.
Among humans, researchers have been studying “super-agers” – people who not only live exceptionally long, but also do so without many of the chronic diseases that plague their peers. That’s even though they share some of the same bad habits as everyone else.
“They are making it past the age of 80 with their minds completely intact. That’s what’s so unusual,” Dr. Olshansky says. The rest of their bodies are doing better than those of average 80-year-olds, too.
People who reached ages 95 to 112 got cancer, heart disease, diabetes, osteoporosis, and stroke up to 24 years later than those with average lifespans, data show. Figuring out why might pave the way for targeted gene therapy to mimic the DNA of these nonagenarians and centenarians.
“There’s likely to be secrets contained within their genome that are eventually discovered that will help us develop therapeutic interventions to mimic the effects of decelerated aging,” Dr. Olshansky says.
Treating aging this way may offer a bigger payoff than targeting individual diseases. That’s because even if you manage to dodge any illnesses, there’s ultimately no escaping old age.
“Longevity is a side effect of health,” Dr. de Grey says. “If we can keep people healthy, then their likelihood of dying is reduced.”
Aging as a preventable condition
In 2015, Michael Cantor was prescribed metformin for prediabetes. Once that was under control, his doctor said Mr. Cantor could quit the drug. But Mr. Cantor had heard about studies testing it as an anti-aging drug. The 62-year-old Connecticut-based attorney asked if he could stay on it. A year ago Cantor’s wife, Shari, who is mayor of West Hartford, Conn., started to take metformin, too.
“I read the articles, they made a lot of sense to me, and with the number of people that have been taking this drug worldwide for decades, I felt like there was nothing to lose,” he says.
The couple can’t say if their daily doses have led to any changes in how they look or feel. After all, they’re taking the pills not to treat current ailments but to prevent ones in the future.
They may have answers soon. Nir Barzilai, MD, director of the National Institutes of Health’s Nathan Shock Centers of Excellence in the Basic Biology of Aging, is leading a study that hopes to prove aging is a preventable health condition. The TAME (Targeting Aging with Metformin) study is designed to do this by demonstrating that metformin, a cheap and widely prescribed pill for diabetes, may also be an anti-aging elixir.
The TAME trial is currently in phase III – typically the final step of research into any treatment before drugmakers can apply for FDA approval.
Earlier studies found that people with type 2 diabetes who take metformin have lower death rates from any cause, compared to peers who don’t take the drug. Metformin also seems to help curb the incidence of age-related diseases, including heart disease, dementia, and Alzheimer›s. It also may lower the risk of many types of cancer as well as raise the chances of survival. Observations made since the beginning of the COVID-19 pandemic suggest that people who get the virus while taking metformin are less likely to land in the hospital or die from it.
It’s not clear exactly how metformin works to do all that. The compound was originally derived from Galega officinalis, also known as goat’s rue, a perennial plant used as medicine since medieval times.
Dr. Barzilai says he hopes to prove that aging is a preventable condition.
“If the results are what they think they will be, the whole world could go on metformin and extend life for everybody – extend your good quality of life,” Dr. Barzilai says. “That’s what we all want. Every extra year that we could get where we’re still vigorous and vital would be amazing.”
Long life versus healthy life
Some researchers argue that only the “healthspan” – the period of life free of illness – is worth extending. Of course, a healthy lifestyle can add years to most people’s lives and actually improve cellular aging. Some of the biggest payoffs come from quitting or never smoking, logging more than 5½ hours of physical activity per week, and keeping a normal weight.
Drugs may be able to do that as well by interrupting common markers of aging, including telomere length, inflammation, oxidative stress, and slower cell metabolism.
“You don’t have to target all of these hallmarks to get improvement” in healthspans, says Dr. Barzilai, who also is director of the Institute for Aging Research at the Albert Einstein College of Medicine in the Bronx and scientific director of the American Federation for Aging Research.
“If you target one, you show benefit in the others.”
The medical term for growing old is senescence. Buffeted by DNA damage and stresses, your cells deteriorate and eventually stop multiplying, but don’t die.
That slowdown may have big consequences for your health. Your genes become more likely to get mutations, which can pave the way for cancer. Mitochondria, which produce energy in the cell, struggle to fuel your body. That can damage cells and cause chronic inflammation, which plays a part in diabetes, arthritis, ulcerative colitis, and many other diseases.
One major hallmark of aging is the growing stockpile of these senescent cells. Damaged cells become deactivated as a way to protect your body from harmful or uncontrolled cell division. But like the rotten apple that spoils the whole bunch, senescent cells encourage their neighbors to turn dysfunctional, too. They also emit proteins that trigger inflammation. Your body naturally removes these dormant cells. But older immune systems have a harder time cleaning up, so the senescent cells are more likely to hang around.
Flushing out this accumulated debris may be one way to avert aging, some experts say.
Dr. De Grey also believes that could be done with drugs.
“These therapies would actually repair [cellular] damage,” he says. “They’ll eliminate damage from the body by resetting or turning back the clock.”
James Kirkland, MD, PhD, of the Mayo Clinic, is one researcher exploring this theory. He gave a mixture of the cancer drug dasatinib and a plant pigment called quercetin to people with diabetic kidney disease. Quercetin is an antioxidant that gives grapes, tomatoes, and other fruits and vegetables their flavor.
A small phase I clinical trial showed that the dasatinib-quercetin combination got rid of senescent cells in the tissues of people with the disease.
The researchers don’t know yet if the results will translate into prolonged youth. They also don’t know how high a dosage is needed and what long-term problems the treatment might cause. People with chronic leukemia take dasatinib for years with few serious ill effects.
In another recent study, scientists used oxygen therapy to tackle senescent cells. Thirty-five adults ages 64 and older received oxygen therapy in a pressurized chamber. After 60 daily sessions, they showed a decrease in senescent cells and improvement in the length of DNA segments called telomeres. Shortened segments of telomeres are thought to be another marker of aging.
Researchers are also looking to the gene-editing technology CRISPR for anti-aging treatments, but the testing is only in mice so far.
Dr. Barzilai hopes that if the metformin trial succeeds, it will open the floodgates to a wave of new drugs that can stop or reverse human aging. Some of the major players in this field include Juvenescence, AgeX Therapeutics, LyGenesis, and Life Biosciences, which Dr. Barzilai founded.
“Until aging is seen as preventable, health plans won’t have to pay for this type of treatment,” he says. And if health plans won’t cover aging, pharmaceutical companies have little incentive to invest in drug development.
That may be the only thing standing between humans and unprecedented lifespans. The Census Bureau projects that Americans born in 2060 should live an average of 85.6 years, up from 78.7 years in 2018. Dr. De Grey’s prediction tops that mark by a factor of about 50. He believes that the life expectancy for someone born in 2100 may well be 5,000 years.
Dr. Barzilai, for his part, has a prediction that’s seemingly more modest.
“We die at 80. Getting an additional 35 years is relatively low-hanging fruit,” he says. “But I don’t believe that is a fixed limit.”
A version of this article first appeared on WebMD.com.
Heart disease. Cancer. Diabetes. Dementia.
Researchers spend billions of dollars every year trying to eradicate these medical scourges.
Yet even if we discover cures to these and all other chronic conditions, it won’t change our ultimate prognosis: death.
“That’s because you haven’t stopped aging,” says Jay Olshansky, PhD, a professor of epidemiology and biostatistics at the University of Illinois at Chicago School of Public Health.
But what if we could?
Some scientists think so. Fueled in part by a billion dollars of investor money, they are attempting to reverse-engineer your molecular biological clock. Their goal? To eliminate not merely diseases that kill people, but to prevent death itself.
Hacking the code for immortality
Aubrey de Grey, PhD, a biomedical gerontologist, has drawn wide attention for his belief that the first person who will live to be 1,000 years old is already among us.
He believes there’s no cap on how long we can live, depending on what medicines we develop in the future.
“The whole idea is that there would not be a limit on how long we can keep people healthy,” Dr. de Grey says. He’s the chief science officer and co-founder of the SENS Research Foundation, which funds research on how to put the brakes on aging.
Dr. De Grey’s view, in theory, isn’t so far-fetched.
Scientists have studied the immortal jellyfish, Turritopsis dohrnii. It’s the only animal that can cheat death by reverting from adulthood back to its polyp stage when threatened with danger or starvation.
Other clues to possible eternal life also may exist underwater. Certain marine clams can live more than 500 years. And lobsters stock a seemingly limitless supply of a youthful enzyme that has some scientists wondering if the crustacean, under the best conditions, just might live forever.
Among humans, researchers have been studying “super-agers” – people who not only live exceptionally long, but also do so without many of the chronic diseases that plague their peers. That’s even though they share some of the same bad habits as everyone else.
“They are making it past the age of 80 with their minds completely intact. That’s what’s so unusual,” Dr. Olshansky says. The rest of their bodies are doing better than those of average 80-year-olds, too.
People who reached ages 95 to 112 got cancer, heart disease, diabetes, osteoporosis, and stroke up to 24 years later than those with average lifespans, data show. Figuring out why might pave the way for targeted gene therapy to mimic the DNA of these nonagenarians and centenarians.
“There’s likely to be secrets contained within their genome that are eventually discovered that will help us develop therapeutic interventions to mimic the effects of decelerated aging,” Dr. Olshansky says.
Treating aging this way may offer a bigger payoff than targeting individual diseases. That’s because even if you manage to dodge any illnesses, there’s ultimately no escaping old age.
“Longevity is a side effect of health,” Dr. de Grey says. “If we can keep people healthy, then their likelihood of dying is reduced.”
Aging as a preventable condition
In 2015, Michael Cantor was prescribed metformin for prediabetes. Once that was under control, his doctor said Mr. Cantor could quit the drug. But Mr. Cantor had heard about studies testing it as an anti-aging drug. The 62-year-old Connecticut-based attorney asked if he could stay on it. A year ago Cantor’s wife, Shari, who is mayor of West Hartford, Conn., started to take metformin, too.
“I read the articles, they made a lot of sense to me, and with the number of people that have been taking this drug worldwide for decades, I felt like there was nothing to lose,” he says.
The couple can’t say if their daily doses have led to any changes in how they look or feel. After all, they’re taking the pills not to treat current ailments but to prevent ones in the future.
They may have answers soon. Nir Barzilai, MD, director of the National Institutes of Health’s Nathan Shock Centers of Excellence in the Basic Biology of Aging, is leading a study that hopes to prove aging is a preventable health condition. The TAME (Targeting Aging with Metformin) study is designed to do this by demonstrating that metformin, a cheap and widely prescribed pill for diabetes, may also be an anti-aging elixir.
The TAME trial is currently in phase III – typically the final step of research into any treatment before drugmakers can apply for FDA approval.
Earlier studies found that people with type 2 diabetes who take metformin have lower death rates from any cause, compared to peers who don’t take the drug. Metformin also seems to help curb the incidence of age-related diseases, including heart disease, dementia, and Alzheimer›s. It also may lower the risk of many types of cancer as well as raise the chances of survival. Observations made since the beginning of the COVID-19 pandemic suggest that people who get the virus while taking metformin are less likely to land in the hospital or die from it.
It’s not clear exactly how metformin works to do all that. The compound was originally derived from Galega officinalis, also known as goat’s rue, a perennial plant used as medicine since medieval times.
Dr. Barzilai says he hopes to prove that aging is a preventable condition.
“If the results are what they think they will be, the whole world could go on metformin and extend life for everybody – extend your good quality of life,” Dr. Barzilai says. “That’s what we all want. Every extra year that we could get where we’re still vigorous and vital would be amazing.”
Long life versus healthy life
Some researchers argue that only the “healthspan” – the period of life free of illness – is worth extending. Of course, a healthy lifestyle can add years to most people’s lives and actually improve cellular aging. Some of the biggest payoffs come from quitting or never smoking, logging more than 5½ hours of physical activity per week, and keeping a normal weight.
Drugs may be able to do that as well by interrupting common markers of aging, including telomere length, inflammation, oxidative stress, and slower cell metabolism.
“You don’t have to target all of these hallmarks to get improvement” in healthspans, says Dr. Barzilai, who also is director of the Institute for Aging Research at the Albert Einstein College of Medicine in the Bronx and scientific director of the American Federation for Aging Research.
“If you target one, you show benefit in the others.”
The medical term for growing old is senescence. Buffeted by DNA damage and stresses, your cells deteriorate and eventually stop multiplying, but don’t die.
That slowdown may have big consequences for your health. Your genes become more likely to get mutations, which can pave the way for cancer. Mitochondria, which produce energy in the cell, struggle to fuel your body. That can damage cells and cause chronic inflammation, which plays a part in diabetes, arthritis, ulcerative colitis, and many other diseases.
One major hallmark of aging is the growing stockpile of these senescent cells. Damaged cells become deactivated as a way to protect your body from harmful or uncontrolled cell division. But like the rotten apple that spoils the whole bunch, senescent cells encourage their neighbors to turn dysfunctional, too. They also emit proteins that trigger inflammation. Your body naturally removes these dormant cells. But older immune systems have a harder time cleaning up, so the senescent cells are more likely to hang around.
Flushing out this accumulated debris may be one way to avert aging, some experts say.
Dr. De Grey also believes that could be done with drugs.
“These therapies would actually repair [cellular] damage,” he says. “They’ll eliminate damage from the body by resetting or turning back the clock.”
James Kirkland, MD, PhD, of the Mayo Clinic, is one researcher exploring this theory. He gave a mixture of the cancer drug dasatinib and a plant pigment called quercetin to people with diabetic kidney disease. Quercetin is an antioxidant that gives grapes, tomatoes, and other fruits and vegetables their flavor.
A small phase I clinical trial showed that the dasatinib-quercetin combination got rid of senescent cells in the tissues of people with the disease.
The researchers don’t know yet if the results will translate into prolonged youth. They also don’t know how high a dosage is needed and what long-term problems the treatment might cause. People with chronic leukemia take dasatinib for years with few serious ill effects.
In another recent study, scientists used oxygen therapy to tackle senescent cells. Thirty-five adults ages 64 and older received oxygen therapy in a pressurized chamber. After 60 daily sessions, they showed a decrease in senescent cells and improvement in the length of DNA segments called telomeres. Shortened segments of telomeres are thought to be another marker of aging.
Researchers are also looking to the gene-editing technology CRISPR for anti-aging treatments, but the testing is only in mice so far.
Dr. Barzilai hopes that if the metformin trial succeeds, it will open the floodgates to a wave of new drugs that can stop or reverse human aging. Some of the major players in this field include Juvenescence, AgeX Therapeutics, LyGenesis, and Life Biosciences, which Dr. Barzilai founded.
“Until aging is seen as preventable, health plans won’t have to pay for this type of treatment,” he says. And if health plans won’t cover aging, pharmaceutical companies have little incentive to invest in drug development.
That may be the only thing standing between humans and unprecedented lifespans. The Census Bureau projects that Americans born in 2060 should live an average of 85.6 years, up from 78.7 years in 2018. Dr. De Grey’s prediction tops that mark by a factor of about 50. He believes that the life expectancy for someone born in 2100 may well be 5,000 years.
Dr. Barzilai, for his part, has a prediction that’s seemingly more modest.
“We die at 80. Getting an additional 35 years is relatively low-hanging fruit,” he says. “But I don’t believe that is a fixed limit.”
A version of this article first appeared on WebMD.com.
Heart disease. Cancer. Diabetes. Dementia.
Researchers spend billions of dollars every year trying to eradicate these medical scourges.
Yet even if we discover cures to these and all other chronic conditions, it won’t change our ultimate prognosis: death.
“That’s because you haven’t stopped aging,” says Jay Olshansky, PhD, a professor of epidemiology and biostatistics at the University of Illinois at Chicago School of Public Health.
But what if we could?
Some scientists think so. Fueled in part by a billion dollars of investor money, they are attempting to reverse-engineer your molecular biological clock. Their goal? To eliminate not merely diseases that kill people, but to prevent death itself.
Hacking the code for immortality
Aubrey de Grey, PhD, a biomedical gerontologist, has drawn wide attention for his belief that the first person who will live to be 1,000 years old is already among us.
He believes there’s no cap on how long we can live, depending on what medicines we develop in the future.
“The whole idea is that there would not be a limit on how long we can keep people healthy,” Dr. de Grey says. He’s the chief science officer and co-founder of the SENS Research Foundation, which funds research on how to put the brakes on aging.
Dr. De Grey’s view, in theory, isn’t so far-fetched.
Scientists have studied the immortal jellyfish, Turritopsis dohrnii. It’s the only animal that can cheat death by reverting from adulthood back to its polyp stage when threatened with danger or starvation.
Other clues to possible eternal life also may exist underwater. Certain marine clams can live more than 500 years. And lobsters stock a seemingly limitless supply of a youthful enzyme that has some scientists wondering if the crustacean, under the best conditions, just might live forever.
Among humans, researchers have been studying “super-agers” – people who not only live exceptionally long, but also do so without many of the chronic diseases that plague their peers. That’s even though they share some of the same bad habits as everyone else.
“They are making it past the age of 80 with their minds completely intact. That’s what’s so unusual,” Dr. Olshansky says. The rest of their bodies are doing better than those of average 80-year-olds, too.
People who reached ages 95 to 112 got cancer, heart disease, diabetes, osteoporosis, and stroke up to 24 years later than those with average lifespans, data show. Figuring out why might pave the way for targeted gene therapy to mimic the DNA of these nonagenarians and centenarians.
“There’s likely to be secrets contained within their genome that are eventually discovered that will help us develop therapeutic interventions to mimic the effects of decelerated aging,” Dr. Olshansky says.
Treating aging this way may offer a bigger payoff than targeting individual diseases. That’s because even if you manage to dodge any illnesses, there’s ultimately no escaping old age.
“Longevity is a side effect of health,” Dr. de Grey says. “If we can keep people healthy, then their likelihood of dying is reduced.”
Aging as a preventable condition
In 2015, Michael Cantor was prescribed metformin for prediabetes. Once that was under control, his doctor said Mr. Cantor could quit the drug. But Mr. Cantor had heard about studies testing it as an anti-aging drug. The 62-year-old Connecticut-based attorney asked if he could stay on it. A year ago Cantor’s wife, Shari, who is mayor of West Hartford, Conn., started to take metformin, too.
“I read the articles, they made a lot of sense to me, and with the number of people that have been taking this drug worldwide for decades, I felt like there was nothing to lose,” he says.
The couple can’t say if their daily doses have led to any changes in how they look or feel. After all, they’re taking the pills not to treat current ailments but to prevent ones in the future.
They may have answers soon. Nir Barzilai, MD, director of the National Institutes of Health’s Nathan Shock Centers of Excellence in the Basic Biology of Aging, is leading a study that hopes to prove aging is a preventable health condition. The TAME (Targeting Aging with Metformin) study is designed to do this by demonstrating that metformin, a cheap and widely prescribed pill for diabetes, may also be an anti-aging elixir.
The TAME trial is currently in phase III – typically the final step of research into any treatment before drugmakers can apply for FDA approval.
Earlier studies found that people with type 2 diabetes who take metformin have lower death rates from any cause, compared to peers who don’t take the drug. Metformin also seems to help curb the incidence of age-related diseases, including heart disease, dementia, and Alzheimer›s. It also may lower the risk of many types of cancer as well as raise the chances of survival. Observations made since the beginning of the COVID-19 pandemic suggest that people who get the virus while taking metformin are less likely to land in the hospital or die from it.
It’s not clear exactly how metformin works to do all that. The compound was originally derived from Galega officinalis, also known as goat’s rue, a perennial plant used as medicine since medieval times.
Dr. Barzilai says he hopes to prove that aging is a preventable condition.
“If the results are what they think they will be, the whole world could go on metformin and extend life for everybody – extend your good quality of life,” Dr. Barzilai says. “That’s what we all want. Every extra year that we could get where we’re still vigorous and vital would be amazing.”
Long life versus healthy life
Some researchers argue that only the “healthspan” – the period of life free of illness – is worth extending. Of course, a healthy lifestyle can add years to most people’s lives and actually improve cellular aging. Some of the biggest payoffs come from quitting or never smoking, logging more than 5½ hours of physical activity per week, and keeping a normal weight.
Drugs may be able to do that as well by interrupting common markers of aging, including telomere length, inflammation, oxidative stress, and slower cell metabolism.
“You don’t have to target all of these hallmarks to get improvement” in healthspans, says Dr. Barzilai, who also is director of the Institute for Aging Research at the Albert Einstein College of Medicine in the Bronx and scientific director of the American Federation for Aging Research.
“If you target one, you show benefit in the others.”
The medical term for growing old is senescence. Buffeted by DNA damage and stresses, your cells deteriorate and eventually stop multiplying, but don’t die.
That slowdown may have big consequences for your health. Your genes become more likely to get mutations, which can pave the way for cancer. Mitochondria, which produce energy in the cell, struggle to fuel your body. That can damage cells and cause chronic inflammation, which plays a part in diabetes, arthritis, ulcerative colitis, and many other diseases.
One major hallmark of aging is the growing stockpile of these senescent cells. Damaged cells become deactivated as a way to protect your body from harmful or uncontrolled cell division. But like the rotten apple that spoils the whole bunch, senescent cells encourage their neighbors to turn dysfunctional, too. They also emit proteins that trigger inflammation. Your body naturally removes these dormant cells. But older immune systems have a harder time cleaning up, so the senescent cells are more likely to hang around.
Flushing out this accumulated debris may be one way to avert aging, some experts say.
Dr. De Grey also believes that could be done with drugs.
“These therapies would actually repair [cellular] damage,” he says. “They’ll eliminate damage from the body by resetting or turning back the clock.”
James Kirkland, MD, PhD, of the Mayo Clinic, is one researcher exploring this theory. He gave a mixture of the cancer drug dasatinib and a plant pigment called quercetin to people with diabetic kidney disease. Quercetin is an antioxidant that gives grapes, tomatoes, and other fruits and vegetables their flavor.
A small phase I clinical trial showed that the dasatinib-quercetin combination got rid of senescent cells in the tissues of people with the disease.
The researchers don’t know yet if the results will translate into prolonged youth. They also don’t know how high a dosage is needed and what long-term problems the treatment might cause. People with chronic leukemia take dasatinib for years with few serious ill effects.
In another recent study, scientists used oxygen therapy to tackle senescent cells. Thirty-five adults ages 64 and older received oxygen therapy in a pressurized chamber. After 60 daily sessions, they showed a decrease in senescent cells and improvement in the length of DNA segments called telomeres. Shortened segments of telomeres are thought to be another marker of aging.
Researchers are also looking to the gene-editing technology CRISPR for anti-aging treatments, but the testing is only in mice so far.
Dr. Barzilai hopes that if the metformin trial succeeds, it will open the floodgates to a wave of new drugs that can stop or reverse human aging. Some of the major players in this field include Juvenescence, AgeX Therapeutics, LyGenesis, and Life Biosciences, which Dr. Barzilai founded.
“Until aging is seen as preventable, health plans won’t have to pay for this type of treatment,” he says. And if health plans won’t cover aging, pharmaceutical companies have little incentive to invest in drug development.
That may be the only thing standing between humans and unprecedented lifespans. The Census Bureau projects that Americans born in 2060 should live an average of 85.6 years, up from 78.7 years in 2018. Dr. De Grey’s prediction tops that mark by a factor of about 50. He believes that the life expectancy for someone born in 2100 may well be 5,000 years.
Dr. Barzilai, for his part, has a prediction that’s seemingly more modest.
“We die at 80. Getting an additional 35 years is relatively low-hanging fruit,” he says. “But I don’t believe that is a fixed limit.”
A version of this article first appeared on WebMD.com.
Updated guidance for COVID vaccination in rheumatology patients arrives amid continued hesitancy
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
As rheumatologists contend with vaccine hesitancy among certain subsets of patients, the American College of Rheumatology has released updated clinical guidelines on COVID-19 vaccination for patients with rheumatic and musculoskeletal diseases (RMDs), including new recommendations on supplemental and booster doses.
The revised guidance from this fifth version of the ACR guidelines includes strongly recommending that all RMD patients receive a booster after their primary vaccine series, regardless of whether they have been naturally infected with COVID-19. In addition, they strongly recommend third supplemental doses for patients with autoimmune inflammatory rheumatic diseases (AIIRDs) who likely mounted an inadequate vaccine response, which would then be followed by a fourth booster dose as advised by the Centers for Disease Control and Prevention for immunocompromised individuals.
Other recommendations include pre-exposure prophylaxis monoclonal antibody treatment for high-risk AIIRD patients, defined as those with moderate to severely compromised immune systems who may not mount an adequate immune response to COVID-19 vaccination, when it is available and authorized for emergency use by the Food and Drug Administration, as well as monoclonal antibody therapy for postexposure prophylaxis of asymptomatic, recently exposed high-risk AIIRD patients or as treatment for newly symptomatic, high-risk AIIRD patients. The ACR guidance notes that, currently, neither the monoclonal antibodies bamlanivimab and etesevimab (administered together) nor casirivimab and imdevimab (REGEN-COV), are licensed or available under an emergency use authorization given their lack of activity against the Omicron variant, the dominant strain of SARS-CoV-2 circulating in the United States.
Finally, the guidance clarified that the timing of intravenous immunoglobulin doses does not need to be modified around the administration of COVID vaccine doses, based on moderate consensus among task force members.
Vaccine hesitancy in community rheumatology practices
The revised guidelines were released just as Arthritis & Rheumatology published a new study that assessed vaccine hesitancy among rheumatology patients on immunomodulatory therapies. A three-item electronic survey was conducted at 101 offices within a community practice–based rheumatology research network and ultimately collected responses from 58,529 patients, 20,987 of whom had an AIIRD and were receiving targeted therapies like biologics or Janus kinase inhibitors.
Of the total respondents, 77% (n = 43,675) had been vaccinated, 16.9% were not vaccinated and did not plan to be, and 6.1% were not vaccinated but planned to be. However, AIIRD patients were 16% less likely to be vaccinated, compared with the other patients, such as those with osteoarthritis or osteoporosis who were not receiving disease-modifying antirheumatic drugs (76.9% vs. 87%; odds ratio, 0.84; 95% confidence interval, 0.77-0.92; P < .001). Multivariable analysis also found that older patients (OR, 1.49 per 10 years) and Asians (OR, 2.42; 95% CI, 1.77-3.33) were more likely to be vaccinated.
“Rheumatologists need to be asking their patients more than just: ‘Are you vaccinated?’ ” Jeffrey Curtis, MD, MPH, head of the ACR COVID-19 vaccine task force and a coauthor of the vaccine hesitancy study, said in an interview. “A year ago, that was a fine approach, but now they need to be asking whether you’ve been vaccinated, and with what, and how many times, and how recently. There are a whole lot of subtleties there; ‘vaccinated: yes or no’ is just the tip of the iceberg.”
His research into the vaccine hesitant includes recent anecdotal data from thousands of patients treated in local rheumatology community practices, many of whom cited long-term safety data and potential side effects as reasons why they were unwilling to get vaccinated. But despite their on-paper responses, he cautioned rheumatologists to think critically when determining which patients may truly be open to vaccination.
“If you’re designing strategies to affect vaccine hesitancy, you may be wasting your time with some people,” said Dr. Curtis, professor of medicine at the University of Alabama at Birmingham. “A critical need is to figure out who are the patients who may be amendable to more information or an intervention or a little bit more time and care, and who are the people where you know, this is a lost cause: You don’t get a flu shot, you haven’t been vaccinated for shingles, [and] you’re not going to get this one either.
“In terms of a research agenda, how do we develop efficient, simple, short screening tools?” he added. “Something with a few helpful questions, on a patient portal or an iPad, that will do a good job identifying your patients at risk who haven’t had vaccination but that you might be able to spend time with, intervene, and actually change their mind. If you spend gobs of time with everyone, you’ll help some people, but clinicians don’t have an infinite amount of time.”
One of the authors of the vaccine hesitancy study acknowledged being employed by the rheumatology research network that hosted the survey. Several others, including Dr. Curtis, reported receiving grants and consulting fees from various pharmaceutical companies.
FROM ARTHRITIS & RHEUMATOLOGY
Boosted Americans 97 times less likely to die of COVID-19 than unvaccinated
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.
according to a new update from the CDC.
In addition, fully vaccinated Americans — meaning those with up to two doses, but no booster — are 14 times less likely to die from COVID-19 than unvaccinated people.
“These data confirm that vaccination and boosting continues to protect against severe illness and hospitalization, even during the Omicron surge,” Rochelle Walensky, MD, director of the CDC, said during a briefing by the White House COVID-19 Response Team.
“If you are not up to date on your COVID-19 vaccinations, you have not optimized your protection against severe disease and death, and you should get vaccinated and boosted if you are eligible,” she said.
Dr. Walensky presented the latest numbers on Feb. 2 based on reports from 25 jurisdictions in early December. The number of average weekly deaths for those who were unvaccinated was 9.7 per 100,000 people, as compared with 0.7 of those who were vaccinated and 0.1 of those who had received a booster.
“The data are really stunningly obvious why a booster is really very important,” Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said during the briefing.
Dr. Fauci also encouraged vaccination for those who are pregnant and couples who may want to conceive in the near feature. He highlighted two recent studies that found vaccination in either partner didn’t affect fertility, including in vitro fertilization.
Meanwhile, fertility fell temporarily among men who were infected with the coronavirus. Couples were 18% less likely to conceive if the male partner had contracted the coronavirus within 60 days before a menstrual cycle.
“New data adds to previous studies that indicate that COVID-19 vaccination does not negatively impact fertility,” Dr. Fauci said. “Vaccination is recommended for people who are trying to get pregnant now or might become pregnant in the future, as well as their partners.”
About 80% of eligible Americans have received at least one vaccine dose, and 68% are fully vaccinated, according to the latest CDC data. About 51% of those who are eligible for a booster dose have received one.
The FDA could authorize the Pfizer vaccine for children under age 5 later this month. When that happens, about 18 million children will qualify for a shot, Jeff Zients, coordinator of the White House COVID-19 Response Team, said during the briefing. The Biden administration is already working on distribution plans for the shot for young kids, he added.
“We’ll be ready to start getting shots in arms soon after FDA and CDC make their decisions,” he said.
A version of this article first appeared on WebMD.com.
Global pediatric oncology workforce hit hard, but resilient amid pandemic
according to a study that surveyed workers from more than 200 institutions in 79 countries.
A snapshot of the extensive findings reveals that half of participating institutions experienced staffing shortages that had a “major impact” on pediatric cancer care. On the financial front, many respondents pointed to instances of unpaid leave and diminished salary, and others highlighted the psychological toll of providing care, including high rates of burnout and stress. The challenges were evident across high- and low-income countries.
Despite these barriers, pediatric oncology clinicians demonstrated incredible perseverance.
Health care professionals “caring for children with cancer across the world were shown to be incredibly resilient, coming together to continue to provide care even in the direst circumstances,” Elizabeth R. Sniderman, MSN, APRN, of St. Jude Children’s Research Hospital, Memphis, and colleagues concluded.
The findings, published online Jan. 24, 2022, in Cancer, highlight the global impact of COVID-19 on pediatric oncology clinicians early in the pandemic.
The survey, conducted in summer 2020, included responses from 311 pediatric oncology clinicians who completed a 60-item questionnaire about their experiences of clinical care, resources, and support. The investigators also convened 19 multidisciplinary focus groups who answered questions related to teamwork, communication, and changes to care. Respondents practiced in low- to high-income countries, and included pediatric hematologists and oncologists, nurses, and infectious disease physicians.
Overall, the investigators found that just over half of institutions experienced “major” shortages of clinical staff (108 of 213), and two-thirds experienced reductions in staffing availability (141 of 213). Notably, national income was not associated with this reduction; rather, staffing shortages were more likely to occur in countries with greater COVID-19 incidence and mortality rates.
Respondents reported experiencing threats to their physical health, with half pointing to a lack of necessary personal protective equipment. The financial and psychological toll of the pandemic represented another major stressor, with the effects described across all income levels.
One respondent from Belarus commented on financial concerns, noting that “people don’t really want to admit that they don’t feel well ... they know, that if infected, unpaid self-isolation is waiting for them. Either you don’t go to work for 2 weeks, unpaid, or you go to work for 2 weeks, paid, and endanger all of your colleagues with your infection.”
A respondent from Mexico described the psychological stress: “Honestly, I think that sometimes we put aside the mental health of all of us involved, myself included. I think we were all on the verge of collapse ... practically all the residents who were rotating here told us that they had anxiety attacks, panic attacks, they could not sleep, [and] many of them needed psychiatric medicine.”
Others highlighted feelings of guilt about their ability to provide the highest level of care. An oncologist in the United States noted: “This was a major stress for many providers because [we are] feeling unable to provide the same level of care which we used to provide. And this is what eventually takes a toll.”
And despite these pandemic-related challenges, the study authors found that only 46% of institutions (99 of 213) made psychological support available to staff.
Rays of hope
But it was not all bad news.
Participants also described a greater sense of teamwork, communication, and collegiality throughout the pandemic – “stabilizing elements,” which helped mitigate the many physical, psychological, and financial stressors.
An infection-control physician in Belarus highlighted the importance of receiving “support and encouragement” from colleagues: “When a person gets tired and they have no more enthusiasm, it’s easy to give up and say: ‘I can’t do this anymore.’ But when you see a colleague who tries ... to share the work, and help each other, then you get extra strength.”
An oncologist in South Africa agreed, noting that “everyone has got their sleeves rolled up and are doing the work ... and that’s a testament to everyone that we work with. There was no one that shied away from work or used this as an excuse to do less work.”
An oncologist in Spain described practicing during the pandemic being “one of the best experiences I have had,” explaining that “I have been working in this hospital for ... 25 years, [and] I have never had the feeling of being so informed at all levels.”
Overall, the findings paint a picture of a resilient workforce, and offer lessons about preparedness for future crises, the investigators concluded.
“To protect pediatric oncology providers and their patients, organizations must pay attention to interventions that increase physical, psychological, and financial safety,” the authors stressed. For instance, providing adequate personal protective equipment and vaccines, allowing for time off and rest, and setting up professional psychology services as well as access to peer-support programs can help protect staff.
Although this survey took place relatively early in the pandemic, organizations should take heed of the findings, Lorena V. Baroni, MD, of Hospital J P Garrahan, Buenos Aires, and Eric Bouffet, MD, of The Hospital for Sick Children, Toronto, wrote in an accompanying editorial.
“The results presented in this study should not be taken lightly,” Dr. Baroni and Dr. Bouffet wrote. “The most concerning findings are the physical and psychological impact experienced by pediatric oncology providers.” And perhaps most surprisingly, “the survey did not identify any difference based on country income groups. Participants in both low- and high-income countries described similar oncologic care limitations.”
Overall, these findings “reflect a serious risk that can ultimately affect the care of children and compromise the success of their treatment,” Dr. Baroni and Dr. Bouffet wrote.
This study was supported by the American Lebanese Syrian Associated Charities. The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study that surveyed workers from more than 200 institutions in 79 countries.
A snapshot of the extensive findings reveals that half of participating institutions experienced staffing shortages that had a “major impact” on pediatric cancer care. On the financial front, many respondents pointed to instances of unpaid leave and diminished salary, and others highlighted the psychological toll of providing care, including high rates of burnout and stress. The challenges were evident across high- and low-income countries.
Despite these barriers, pediatric oncology clinicians demonstrated incredible perseverance.
Health care professionals “caring for children with cancer across the world were shown to be incredibly resilient, coming together to continue to provide care even in the direst circumstances,” Elizabeth R. Sniderman, MSN, APRN, of St. Jude Children’s Research Hospital, Memphis, and colleagues concluded.
The findings, published online Jan. 24, 2022, in Cancer, highlight the global impact of COVID-19 on pediatric oncology clinicians early in the pandemic.
The survey, conducted in summer 2020, included responses from 311 pediatric oncology clinicians who completed a 60-item questionnaire about their experiences of clinical care, resources, and support. The investigators also convened 19 multidisciplinary focus groups who answered questions related to teamwork, communication, and changes to care. Respondents practiced in low- to high-income countries, and included pediatric hematologists and oncologists, nurses, and infectious disease physicians.
Overall, the investigators found that just over half of institutions experienced “major” shortages of clinical staff (108 of 213), and two-thirds experienced reductions in staffing availability (141 of 213). Notably, national income was not associated with this reduction; rather, staffing shortages were more likely to occur in countries with greater COVID-19 incidence and mortality rates.
Respondents reported experiencing threats to their physical health, with half pointing to a lack of necessary personal protective equipment. The financial and psychological toll of the pandemic represented another major stressor, with the effects described across all income levels.
One respondent from Belarus commented on financial concerns, noting that “people don’t really want to admit that they don’t feel well ... they know, that if infected, unpaid self-isolation is waiting for them. Either you don’t go to work for 2 weeks, unpaid, or you go to work for 2 weeks, paid, and endanger all of your colleagues with your infection.”
A respondent from Mexico described the psychological stress: “Honestly, I think that sometimes we put aside the mental health of all of us involved, myself included. I think we were all on the verge of collapse ... practically all the residents who were rotating here told us that they had anxiety attacks, panic attacks, they could not sleep, [and] many of them needed psychiatric medicine.”
Others highlighted feelings of guilt about their ability to provide the highest level of care. An oncologist in the United States noted: “This was a major stress for many providers because [we are] feeling unable to provide the same level of care which we used to provide. And this is what eventually takes a toll.”
And despite these pandemic-related challenges, the study authors found that only 46% of institutions (99 of 213) made psychological support available to staff.
Rays of hope
But it was not all bad news.
Participants also described a greater sense of teamwork, communication, and collegiality throughout the pandemic – “stabilizing elements,” which helped mitigate the many physical, psychological, and financial stressors.
An infection-control physician in Belarus highlighted the importance of receiving “support and encouragement” from colleagues: “When a person gets tired and they have no more enthusiasm, it’s easy to give up and say: ‘I can’t do this anymore.’ But when you see a colleague who tries ... to share the work, and help each other, then you get extra strength.”
An oncologist in South Africa agreed, noting that “everyone has got their sleeves rolled up and are doing the work ... and that’s a testament to everyone that we work with. There was no one that shied away from work or used this as an excuse to do less work.”
An oncologist in Spain described practicing during the pandemic being “one of the best experiences I have had,” explaining that “I have been working in this hospital for ... 25 years, [and] I have never had the feeling of being so informed at all levels.”
Overall, the findings paint a picture of a resilient workforce, and offer lessons about preparedness for future crises, the investigators concluded.
“To protect pediatric oncology providers and their patients, organizations must pay attention to interventions that increase physical, psychological, and financial safety,” the authors stressed. For instance, providing adequate personal protective equipment and vaccines, allowing for time off and rest, and setting up professional psychology services as well as access to peer-support programs can help protect staff.
Although this survey took place relatively early in the pandemic, organizations should take heed of the findings, Lorena V. Baroni, MD, of Hospital J P Garrahan, Buenos Aires, and Eric Bouffet, MD, of The Hospital for Sick Children, Toronto, wrote in an accompanying editorial.
“The results presented in this study should not be taken lightly,” Dr. Baroni and Dr. Bouffet wrote. “The most concerning findings are the physical and psychological impact experienced by pediatric oncology providers.” And perhaps most surprisingly, “the survey did not identify any difference based on country income groups. Participants in both low- and high-income countries described similar oncologic care limitations.”
Overall, these findings “reflect a serious risk that can ultimately affect the care of children and compromise the success of their treatment,” Dr. Baroni and Dr. Bouffet wrote.
This study was supported by the American Lebanese Syrian Associated Charities. The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a study that surveyed workers from more than 200 institutions in 79 countries.
A snapshot of the extensive findings reveals that half of participating institutions experienced staffing shortages that had a “major impact” on pediatric cancer care. On the financial front, many respondents pointed to instances of unpaid leave and diminished salary, and others highlighted the psychological toll of providing care, including high rates of burnout and stress. The challenges were evident across high- and low-income countries.
Despite these barriers, pediatric oncology clinicians demonstrated incredible perseverance.
Health care professionals “caring for children with cancer across the world were shown to be incredibly resilient, coming together to continue to provide care even in the direst circumstances,” Elizabeth R. Sniderman, MSN, APRN, of St. Jude Children’s Research Hospital, Memphis, and colleagues concluded.
The findings, published online Jan. 24, 2022, in Cancer, highlight the global impact of COVID-19 on pediatric oncology clinicians early in the pandemic.
The survey, conducted in summer 2020, included responses from 311 pediatric oncology clinicians who completed a 60-item questionnaire about their experiences of clinical care, resources, and support. The investigators also convened 19 multidisciplinary focus groups who answered questions related to teamwork, communication, and changes to care. Respondents practiced in low- to high-income countries, and included pediatric hematologists and oncologists, nurses, and infectious disease physicians.
Overall, the investigators found that just over half of institutions experienced “major” shortages of clinical staff (108 of 213), and two-thirds experienced reductions in staffing availability (141 of 213). Notably, national income was not associated with this reduction; rather, staffing shortages were more likely to occur in countries with greater COVID-19 incidence and mortality rates.
Respondents reported experiencing threats to their physical health, with half pointing to a lack of necessary personal protective equipment. The financial and psychological toll of the pandemic represented another major stressor, with the effects described across all income levels.
One respondent from Belarus commented on financial concerns, noting that “people don’t really want to admit that they don’t feel well ... they know, that if infected, unpaid self-isolation is waiting for them. Either you don’t go to work for 2 weeks, unpaid, or you go to work for 2 weeks, paid, and endanger all of your colleagues with your infection.”
A respondent from Mexico described the psychological stress: “Honestly, I think that sometimes we put aside the mental health of all of us involved, myself included. I think we were all on the verge of collapse ... practically all the residents who were rotating here told us that they had anxiety attacks, panic attacks, they could not sleep, [and] many of them needed psychiatric medicine.”
Others highlighted feelings of guilt about their ability to provide the highest level of care. An oncologist in the United States noted: “This was a major stress for many providers because [we are] feeling unable to provide the same level of care which we used to provide. And this is what eventually takes a toll.”
And despite these pandemic-related challenges, the study authors found that only 46% of institutions (99 of 213) made psychological support available to staff.
Rays of hope
But it was not all bad news.
Participants also described a greater sense of teamwork, communication, and collegiality throughout the pandemic – “stabilizing elements,” which helped mitigate the many physical, psychological, and financial stressors.
An infection-control physician in Belarus highlighted the importance of receiving “support and encouragement” from colleagues: “When a person gets tired and they have no more enthusiasm, it’s easy to give up and say: ‘I can’t do this anymore.’ But when you see a colleague who tries ... to share the work, and help each other, then you get extra strength.”
An oncologist in South Africa agreed, noting that “everyone has got their sleeves rolled up and are doing the work ... and that’s a testament to everyone that we work with. There was no one that shied away from work or used this as an excuse to do less work.”
An oncologist in Spain described practicing during the pandemic being “one of the best experiences I have had,” explaining that “I have been working in this hospital for ... 25 years, [and] I have never had the feeling of being so informed at all levels.”
Overall, the findings paint a picture of a resilient workforce, and offer lessons about preparedness for future crises, the investigators concluded.
“To protect pediatric oncology providers and their patients, organizations must pay attention to interventions that increase physical, psychological, and financial safety,” the authors stressed. For instance, providing adequate personal protective equipment and vaccines, allowing for time off and rest, and setting up professional psychology services as well as access to peer-support programs can help protect staff.
Although this survey took place relatively early in the pandemic, organizations should take heed of the findings, Lorena V. Baroni, MD, of Hospital J P Garrahan, Buenos Aires, and Eric Bouffet, MD, of The Hospital for Sick Children, Toronto, wrote in an accompanying editorial.
“The results presented in this study should not be taken lightly,” Dr. Baroni and Dr. Bouffet wrote. “The most concerning findings are the physical and psychological impact experienced by pediatric oncology providers.” And perhaps most surprisingly, “the survey did not identify any difference based on country income groups. Participants in both low- and high-income countries described similar oncologic care limitations.”
Overall, these findings “reflect a serious risk that can ultimately affect the care of children and compromise the success of their treatment,” Dr. Baroni and Dr. Bouffet wrote.
This study was supported by the American Lebanese Syrian Associated Charities. The study authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CANCER
Hyperpigmented plaque on back
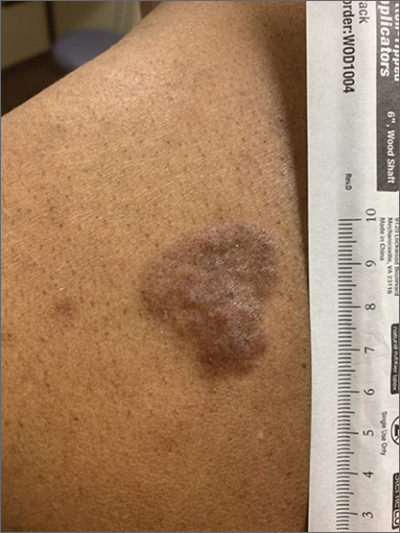
Based on the thickness and size of this irregular lesion, a punch biopsy was performed and confirmed the diagnosis of dermatofibrosarcoma protuberans (DFSP).
DFSPs are usually found on the trunk and proximal extremities; they are most often located on the chest and shoulders. The lesion usually manifests as an asymptomatic, firm, and sometimes nodular plaque that may go undiagnosed for years. DFSP is an uncommon mesenchymal tumor with uncertain etiology. It is thought that prior injury to the affected skin may result in a translocation of chromosomes 17 and 22 in skin cells, as this molecular change characterizes the vast majority of DFSPs.
Black patients are more likely than other ethnic populations to develop DFSP and its variants; there is also a slight female predominance.1 Known variants of DFSP include violaceous plaques with telangiectatic atrophic skin, and plaques with dark brown pigmentation called Bednar tumors.1,2
DFSPs are rarely metastatic, but can be locally invasive, so primary treatment consists of wide local excision or Mohs micrographic surgery (MMS). There is a higher probability for cure when MMS is utilized to treat DFSPs with the added benefit of minimizing surgical margins and preserving healthy surrounding skin. In the rarer cases of advanced local, unresectable, or metastatic disease, inhibitor therapy with imatinib or radiation therapy may be considered.2 Due to the risk of local recurrence, patients with DFSP should have regular clinical follow-up every 6 months for 5 years, followed by annual lifelong surveillance.1
This patient was referred for MMS and has not yet returned for follow-up evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Morgan Haynes, BS, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Mendenhall WM, Scarborough MT, Flowers FP. Dermatofibrosarcoma protuberans: epidemiology, pathogenesis, clinical presentation, diagnosis, and staging. UpToDate. Updated March 31, 2021. Accessed February 2, 2022. www.uptodate.com/contents/dermatofibrosarcoma-protuberans-epidemiology-pathogenesis-clinical-presentation-diagnosis-and-staging
2. Brooks J, Ramsey ML. Dermatofibrosarcoma Protuberans. StatPearls. Updated November 14, 2021. Accessed January 27, 2022. www.ncbi.nlm.nih.gov/books/NBK513305

Based on the thickness and size of this irregular lesion, a punch biopsy was performed and confirmed the diagnosis of dermatofibrosarcoma protuberans (DFSP).
DFSPs are usually found on the trunk and proximal extremities; they are most often located on the chest and shoulders. The lesion usually manifests as an asymptomatic, firm, and sometimes nodular plaque that may go undiagnosed for years. DFSP is an uncommon mesenchymal tumor with uncertain etiology. It is thought that prior injury to the affected skin may result in a translocation of chromosomes 17 and 22 in skin cells, as this molecular change characterizes the vast majority of DFSPs.
Black patients are more likely than other ethnic populations to develop DFSP and its variants; there is also a slight female predominance.1 Known variants of DFSP include violaceous plaques with telangiectatic atrophic skin, and plaques with dark brown pigmentation called Bednar tumors.1,2
DFSPs are rarely metastatic, but can be locally invasive, so primary treatment consists of wide local excision or Mohs micrographic surgery (MMS). There is a higher probability for cure when MMS is utilized to treat DFSPs with the added benefit of minimizing surgical margins and preserving healthy surrounding skin. In the rarer cases of advanced local, unresectable, or metastatic disease, inhibitor therapy with imatinib or radiation therapy may be considered.2 Due to the risk of local recurrence, patients with DFSP should have regular clinical follow-up every 6 months for 5 years, followed by annual lifelong surveillance.1
This patient was referred for MMS and has not yet returned for follow-up evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Morgan Haynes, BS, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Based on the thickness and size of this irregular lesion, a punch biopsy was performed and confirmed the diagnosis of dermatofibrosarcoma protuberans (DFSP).
DFSPs are usually found on the trunk and proximal extremities; they are most often located on the chest and shoulders. The lesion usually manifests as an asymptomatic, firm, and sometimes nodular plaque that may go undiagnosed for years. DFSP is an uncommon mesenchymal tumor with uncertain etiology. It is thought that prior injury to the affected skin may result in a translocation of chromosomes 17 and 22 in skin cells, as this molecular change characterizes the vast majority of DFSPs.
Black patients are more likely than other ethnic populations to develop DFSP and its variants; there is also a slight female predominance.1 Known variants of DFSP include violaceous plaques with telangiectatic atrophic skin, and plaques with dark brown pigmentation called Bednar tumors.1,2
DFSPs are rarely metastatic, but can be locally invasive, so primary treatment consists of wide local excision or Mohs micrographic surgery (MMS). There is a higher probability for cure when MMS is utilized to treat DFSPs with the added benefit of minimizing surgical margins and preserving healthy surrounding skin. In the rarer cases of advanced local, unresectable, or metastatic disease, inhibitor therapy with imatinib or radiation therapy may be considered.2 Due to the risk of local recurrence, patients with DFSP should have regular clinical follow-up every 6 months for 5 years, followed by annual lifelong surveillance.1
This patient was referred for MMS and has not yet returned for follow-up evaluation.
Photo courtesy of Daniel Stulberg, MD. Text courtesy of Morgan Haynes, BS, University of New Mexico School of Medicine and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Mendenhall WM, Scarborough MT, Flowers FP. Dermatofibrosarcoma protuberans: epidemiology, pathogenesis, clinical presentation, diagnosis, and staging. UpToDate. Updated March 31, 2021. Accessed February 2, 2022. www.uptodate.com/contents/dermatofibrosarcoma-protuberans-epidemiology-pathogenesis-clinical-presentation-diagnosis-and-staging
2. Brooks J, Ramsey ML. Dermatofibrosarcoma Protuberans. StatPearls. Updated November 14, 2021. Accessed January 27, 2022. www.ncbi.nlm.nih.gov/books/NBK513305
1. Mendenhall WM, Scarborough MT, Flowers FP. Dermatofibrosarcoma protuberans: epidemiology, pathogenesis, clinical presentation, diagnosis, and staging. UpToDate. Updated March 31, 2021. Accessed February 2, 2022. www.uptodate.com/contents/dermatofibrosarcoma-protuberans-epidemiology-pathogenesis-clinical-presentation-diagnosis-and-staging
2. Brooks J, Ramsey ML. Dermatofibrosarcoma Protuberans. StatPearls. Updated November 14, 2021. Accessed January 27, 2022. www.ncbi.nlm.nih.gov/books/NBK513305