User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
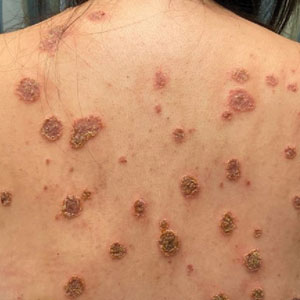
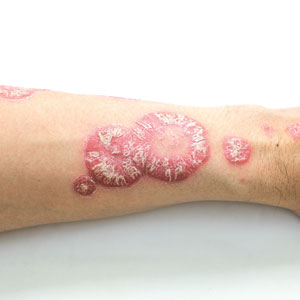
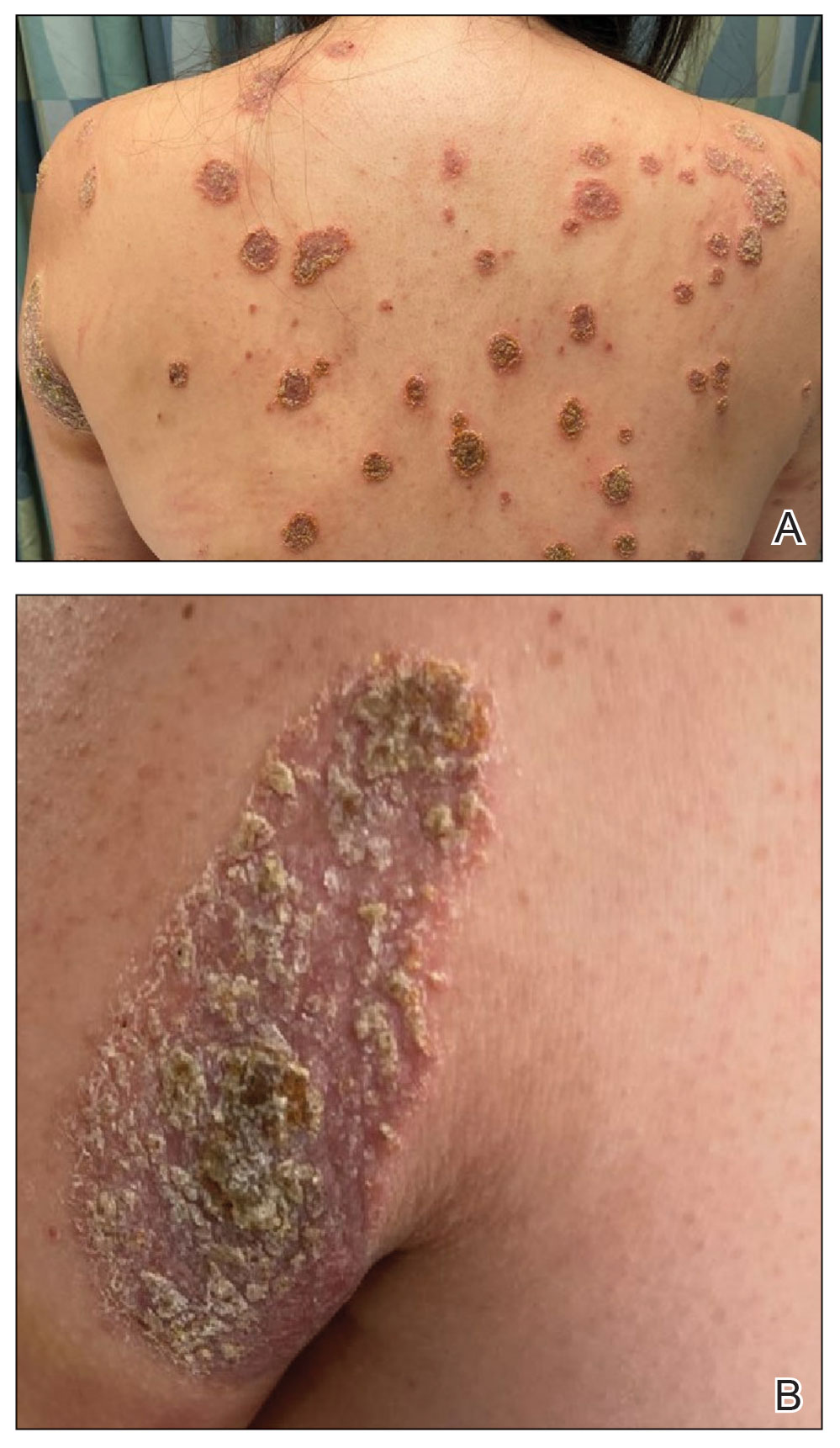
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.
Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
To the Editor:
In dermatology, rupioid describes dirty-appearing scale. The term is derived from the Greek word rhupos, which translates to “dirty” or “filthy.” This type of scale also is called ostraceous, owing to its resemblance to an oyster shell. Histopathologically, rupioid or ostraceous scale corresponds to epidermal hyperplasia and hyperkeratosis. Therefore, the presence of rupioid scale is believed to reflect an exuberant inflammatory response. Several dermatologic conditions have been associated with rupioid scale, including psoriasis, secondary syphilis, reactive arthritis, histoplasmosis, and Norwegian scabies.1-4 Peripheral eosinophilia has been reported in eczematous dermatoses such as atopic dermatitis and contact dermatitis,5,6 but our review of the literature did not find it described in the context of id reactions. We report the case of a patient who developed a rupioid id reaction with peripheral eosinophilia.
An otherwise healthy 40-year-old woman presented with a generalized pruritic eruption of 1 month’s duration. Prior to onset, she was bitten by a bug on the left arm and covered the site with a bandage. She subsequently noticed an erythematous papulopustular rash corresponding to the shape of the bandage adhesive. Shortly thereafter, a generalized eruption developed, prompting the patient to present for evaluation 1 month later. A review of systems was negative for fevers, chills, headaches, vision changes, and joint symptoms. She denied having a history of atopy.
Physical examination revealed numerous pink papules and plaques with rupioid scale scattered over the trunk and extremities (Figure). The palms, soles, and mucous membranes were spared. Laboratory studies revealed peripheral eosinophilia (9% eosinophils [reference range, 1%-6%] and an absolute eosinophil count of 600/µL [reference range, 0-400/µL]). A 3-mm punch biopsy of a representative lesion revealed a superficial perivascular infiltrate of lymphocytes, histiocytes, and eosinophils along with epidermal hyperplasia, spongiosis, and mounds of parakeratosis. Clinicopathologic correlation led to the diagnosis of a rupioid id reaction secondary to an arthropod assault and/or a reaction to the bandage adhesive.

Treatment with topical corticosteroids was avoided at the patient’s request. Instead, a ceramide-based emollient and oral antihistamines (fexofenadine 180 mg in the morning and cetirizine 10 mg in the evening) were recommended and resulted in resolution of the eruption with postinflammatory hyperpigmentation at 2-week follow-up. The patient was advised to avoid further exposure to bandage adhesives.
An id reaction, or autoeczematization, is a cutaneous immunologic response to antigen(s) released from an initial, often distant site of inflammation.7,8 Clinically, it typically manifests as a pruritic, symmetrically distributed papulovesicular eruption. Although the pathogenesis of id reactions is uncertain, overactivation of T lymphocytes responding to the initial inflammatory insult has been implicated.7 A variety of noninfectious (eg, stasis dermatitis, contact dermatitis) and infectious dermatoses (eg, fungal, bacterial, viral, parasitic) may trigger id reactions.7,9-13 In this case, we believe an arthropod assault and/or reaction to the bandage adhesive was the primary insult, and the id reaction that ensued was so exuberant that it resulted not only in rupioid scale but also in peripheral eosinophilia—similar to how more severe forms of atopic dermatitis have been associated with peripheral eosinophilia.5 As such presentations of id reactions not have been widely described in the literature, this report expands our understanding of this condition to include rupioid scale and peripheral eosinophilia.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Chung HJ, Marley-Kemp D, Keller M. Rupioid psoriasis and other skin diseases with rupioid manifestations. Cutis. 2014;94:119-121.
- Costa JB, de Sousa VLLR, da Trindade Neto PB, et al. Norwegian scabies mimicking rupioid psoriasis. An Bras Dermatol. 2012;87:910-913. doi:10.1590/S0365-05962012000600016
- Ip KH-K, Cheng HS, Oliver FG. Rupioid psoriasis. JAMA Dermatol. 2021;157:859. doi:10.1001/jamadermatol.2021.0451
- Wang Y, Wen Y. An AIDS patient with recurrent multiple skin crusted ulcerations. AIDS Res Hum Retroviruses. 2021;37:1-3. doi:10.1089/aid.2020.0212
- Staumont-Sallé D, Barbarot S, Bouaziz JD, et al. Effect of abrocitinib and dupilumab on eosinophil levels in patients with moderate-to-severe atopic dermatitis. JEADV Clin Pract. 2023;2:518-530. doi:10.1002/jvc2.192
- Savjani P. An unusual cause of eosinophilia—hypereosinophilia due to contact dermatitis. J Allergy Clin Immunol. 2016;137:AB168. doi:10.1016/j.jaci.2015.12.685
- Bertoli M, Schwartz RA, Janniger CK. Autoeczematization: a strange id reaction of the skin. Cutis. 2021;108:163-166. doi:10.12788/cutis.0342
- Ilkit M, Durdu M, Karakas¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202. doi:10.3109/1040841X.2011.645520
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129. doi:10.1111/j.1365-4362.1993.tb01454.x
- Jordan L, Jackson NAM, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;10:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6. doi:10.1067/mjd.2003.110
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:e1072-e1075. doi:10.1542/peds.2011-1054
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
Rupioid Id Reaction With Peripheral Eosinophilia
Rupioid Id Reaction With Peripheral Eosinophilia
Practice Points
- Consider a rupioid id reaction when a patient presents with lesions featuring scale that is dirty appearing and resembles an oyster shell.
- Recognize that exuberant id reactions can manifest with peripheral eosinophilia; its presence should not lead you to automatically rule out an id reaction in favor of other eosinophilic eruptions.
- Focus on uncovering the source of an id reaction (eg, contactants, infections, bites); resolving the primary insult is essential for rapid clearance of even dramatic rupioid eruptions.
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

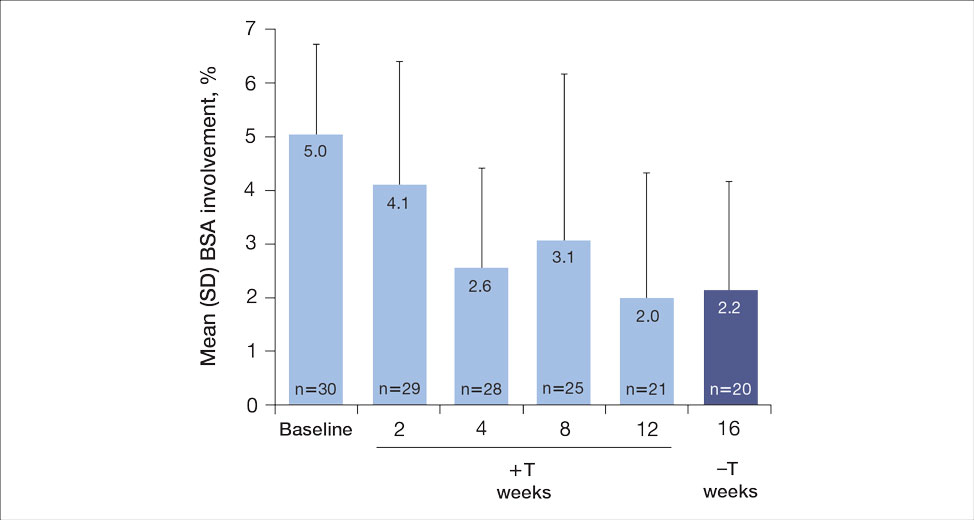
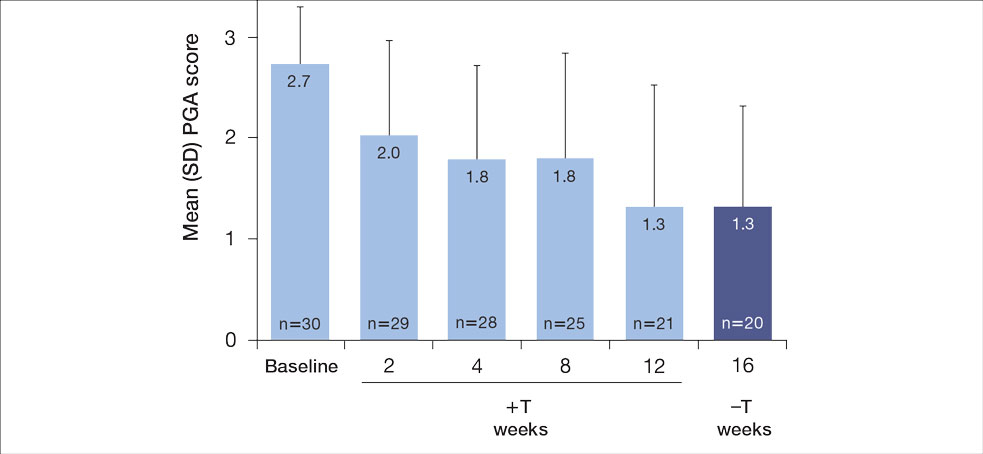
For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.
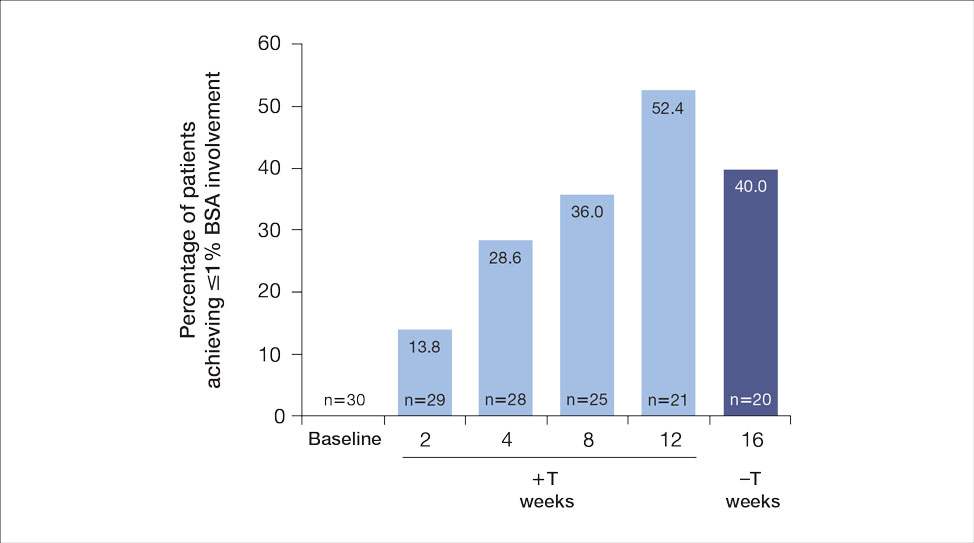
After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
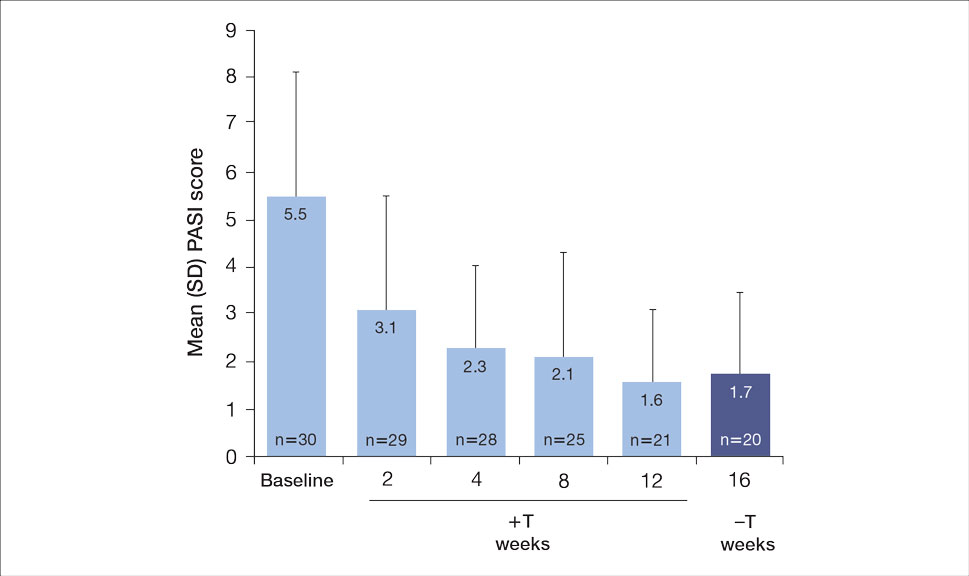
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.
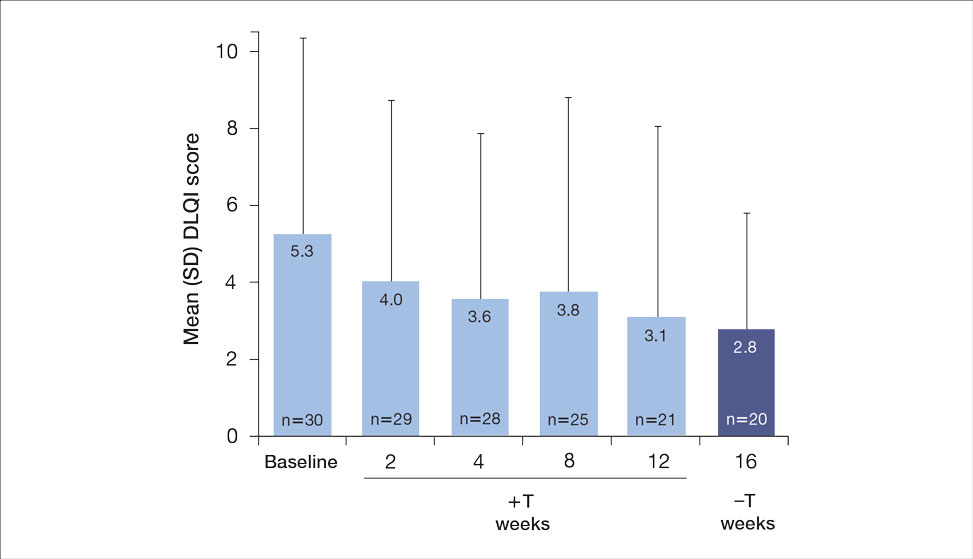
Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.

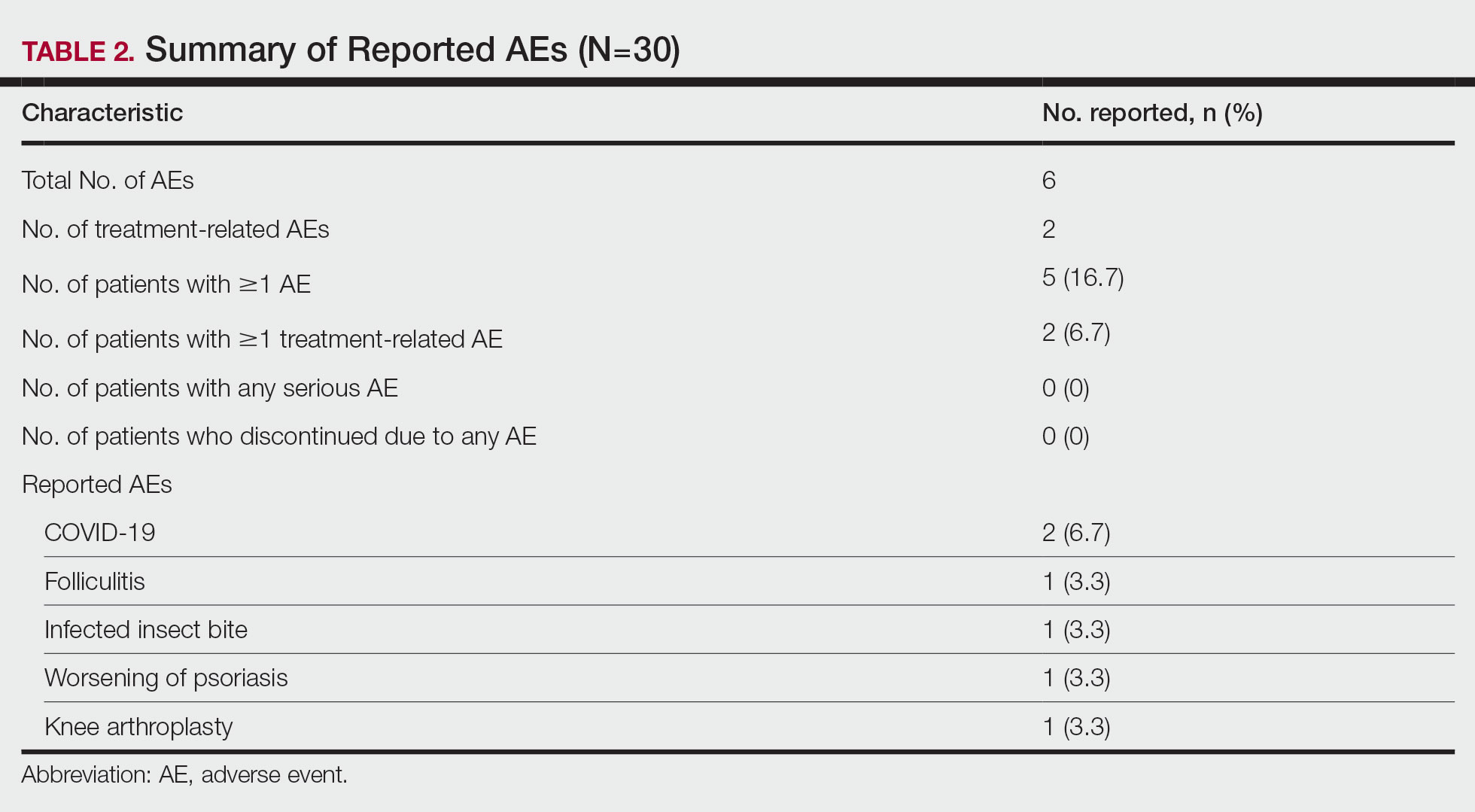
A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.
Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
The estimated prevalence of psoriasis in individuals older than 20 years in the United States has been reported at approximately 3%, or more than 7.5 million people.1 There currently is no cure for psoriasis, and available therapeutics, including phototherapy,2 topical therapies,3 systemic medications,4 and biologic agents,5 are focused only on controlling symptoms. The National Psoriasis Foundation defines an acceptable treatment response for plaque psoriasis as 3% or lower body surface area (BSA) involvement after 3 months of therapy, with a treat-to-target (TTT) goal of 1% or less BSA involvement.6
Cytokines are known to mediate psoriasis pathology, and biologic therapies target the signaling cascade of various cytokines. Biologics approved to treat moderate to severe plaque psoriasis include IgG monoclonal antibodies binding and inhibiting the activity of interleukin (IL)-17 (ixekizumab,7 secukinumab8), IL-23 (guselkumab,9 risankizumab,10 tildrakizumab11), and IL-12/23 (ustekinumab12). Despite targeting these cytokines, biologics may not sufficiently suppress the symptoms of psoriatic disease and their severity in all patients. Adding a topical treatment to biologic therapy can augment clinical response without increasing the incidence of adverse effects13-15 and may reduce the need to switch biologics due to ineffectiveness. Switching biologics likely would increase cost burden to the health care system and/or patient depending on their insurance plan and possibly introduce new safety and/or tolerability issues.16,17
In patients who do not adequately respond to biologics, better responses were reported when topical medications including halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17,18 were administered. In randomized or open-label, real-world studies, patients with psoriasis responded well when topical medications were added to a biologic, such as tildrakizumab combined with halcinonide ointment 0.1%,19 etanercept combined with topical clobetasol propionate foam,20 or adalimumab combined with calcipotriene/betamethasone dipropionate foam.21 No additional safety concerns were observed with the topical add-ons in any of these studies.
Tapinarof is an aryl hydrocarbon receptor agonist approved by the US Food and Drug Administration for topical treatment of plaque psoriasis in adults.22 It is a first-in-class small molecule with a novel mechanism of action that downregulates IL-17A and IL-17F and normalizes the skin barrier through expression of filaggrin, loricrin, and involucrin; it also has antioxidant activity.23 In the phase 3 PSOARING 1 and 2 trials, daily application of tapinarof cream was safe and efficacious in patients with plaque psoriasis,24,25 with a remittive (maintenance) effect of a median of approximately 4 months after discontinuation.25 In these 2 phase 3 studies, tapinarof significantly (P<0.01 at week 12) relieved itch, which was seen rapidly (P<0.05 at week 2),26 improved quality of life,27 and led to high patient satisfaction.27 When tapinarof cream was combined with deucravacitinib in a patient with severe plaque psoriasis, symptoms rapidly cleared, with a 75% decrease in disease severity after 4 weeks.28
The objective of this prospective, open-label, real-world, single-center study was to assess the effectiveness, safety, and remittive (or maintenance) effect of nonsteroidal tapinarof cream 1% added to ongoing biologic therapy in patients with plaque psoriasis who were not adequately responding to a biologic alone.
Methods
Study Design and Participants—This prospective, open-label, real-world, single-center study assessed the safety and effectiveness of
Eligible participants were otherwise healthy males and females aged 18 years and older with moderate to severe plaque psoriasis (BSA involvement ≥3%) who had been treated with a biologic for 24 weeks or more. Patients were recruited from the Psoriasis Treatment Center of New Jersey (East Windsor, New Jersey). Exclusion criteria were recent use of oral systemic therapies (within 4 weeks of baseline) or topical therapies (within 2 weeks) to treat psoriasis, recent use of UVB (within 2 weeks) or psoralen plus UVA (within 4 weeks) phototherapy, or use of any investigational drug within 4 weeks of baseline (or within 5 pharmacokinetic/pharmacodynamic half-lives, whichever was longer). Patients who were pregnant or breastfeeding or who had any known hypersensitivity to the excipients of tapinarof cream also were excluded from the study.
Eligible participants received tapinarof cream 1% once daily plus their ongoing biologic for 12 weeks, after which tapinarof was discontinued and the biologic was continued for an additional 4 weeks. A remittive (maintenance) effect was assessed at week 16.
Study Outcomes—Safety and efficacy were evaluated at baseline and weeks 2, 4, 8, 12, and 16. The primary end point was the proportion of patients who reached the TTT goal of 1% or less BSA involvement at week 12. Secondary end points included the proportion of patients with 1% or less BSA involvement at weeks 2, 4, 8, and 16; and PGA scores, composite PGA multiplied by mean percentage of BSA involvement (PGA×BSA), and PASI scores at baseline and weeks 2, 4, 8, 12, and 16. The patient-reported outcomes of Dermatology Life Quality Index (DLQI) and Worst Itch Numeric Rating Scale (WI-NRS) scores also were evaluated at baseline and weeks 2, 4, 8, 12, and 16. In patients who had disease involvement on the scalp or genital region at baseline, Psoriasis Scalp Severity Index (PSSI) and Static Physician’s Global Assessment of Genitalia scores, respectively, were assessed at baseline and weeks 2, 4, 8, 12, and 16. Safety was determined by the incidence, severity, and relatedness of adverse events (AEs) and serious AEs.
Statistical Analysis—Approximately 30 participants were planned for enrollment and recruited consecutively as they were identified during screening against inclusion and exclusion criteria. Changes from baseline in all outcomes were summarized descriptively. Missing data were not imputed. Given the sample size, no formal statistical analyses were conducted. Safety was summarized by descriptively collating AEs and serious AEs, including their frequency, severity, and treatment relatedness.
Results
Thirty participants were enrolled in the study, and 20 fully completed the study. Nine discontinued treatment before week 12 (6 were lost to follow-up, 2 were terminated early by the investigators, and 1 voluntarily withdrew); 1 additional participant was lost to follow-up after week 12. Patients were predominantly male (20/30 [66.7%]) and White (21/30 [70.0%]); the mean age of all participants was 55.4 years, and the mean (SD) duration of psoriasis was 21.4 (15.0) years (Table 1). The mean baseline percentage of BSA involvement and mean baseline PGA, PASI, and DLQI scores are shown in Table 1. Most (19/30 [63.3%]) patients received biologics that inhibited IL-23 activity (guselkumab, risankizumab, tildrakizumab), approximately one-third (9/30 [30.0%]) received biologics that inhibited IL-17 activity (ixekizumab, secukinumab), and 2 (6.7%) received biologics that inhibited IL-12/IL-23 activity (ustekinumab)(Table 1).

For the primary end point, 52.4% (11/21) of patients reached the TTT goal (BSA involvement ≤1% after 12 weeks of treatment with tapinarof cream added to a prescribed biologic). The proportion of patients reaching the TTT goal increased over time with the combined treatment (eFigure 1). Additionally, the mean percentage of BSA involvement (eFigure 2) as well as the mean values for PGA (eFigure 3) and PGA×BSA decreased over time. The mean percentage of BSA involvement was 5.0% at baseline and dropped to 2.0% by week 12. Similar reductions were observed for PGA and PGA×BSA scores at week 12.



After discontinuing tapinarof cream at week 12 and receiving only the biologic for 4 weeks, the proportion of patients maintaining 1% or less BSA involvement fell to 40.0% (8/20) at week 16, which was closer to that observed at week 8 (36% [9/25]) than at week 12 (52.4% [11/21])(eFigure 1).
The mean PASI score was 5.5 at baseline, then decreased over time when tapinarof cream was combined with a biologic (eFigure 4), falling to 3.1 by week 2 and 1.6 by week 12; it was maintained at 1.7 at week 16. Nine (30.0%) participants had psoriasis on the scalp at baseline with a mean PSSI score of 2.6, which decreased to 0.83 by week 2. By week 12, the mean PSSI score remained stable at 0.95 in the 2 (9.5%) participants who still had scalp involvement. The mean PSSI score increased slightly to 1.45 after patients received only the biologic for 4 weeks. At baseline, 3 (10.0%) patients had genital involvement (mean Static Physician’s Global Assessment of Genitalia score, 0.27). Symptoms resolved in 2 (66.7%) of these patients at week 2 and stayed consistent until week 16; the third patient withdrew at week 2.

Both DLQI and WI-NRS scores decreased with use of tapinarof cream added to a biologic up to week 12 (eFigures 5 and 6). Mean DLQI scores were 5.3 at baseline and 3.1 at week 12. At week 16, the mean DLQI score remained stable at 2.8. Mean WI-NRS scores decreased from 4.0 at baseline to 2.7 at week 12 with the therapy combination; at week 16, the mean WI-NRS score fell further to 1.8.


A total of 6 AEs were reported in 5 (16.7%) patients (Table 2). The majority (4/6 [67.0%]) of AEs were considered mild. Two reported cases of COVID-19 were both considered mild and unrelated. Mild folliculitis and moderate worsening of psoriasis in 2 (6.7%) different patients were the only AEs considered related to treatment. No serious AEs were reported, and no patient withdrew from the study due to an AE.

Comment
Disease activity improvements we observed with the nonsteroidal tapinarof cream were consistent with those reported when topical steroidal therapies were given to patients responding poorly to their current biologic. Our primary end point (proportion of patients with BSA involvement ≤1% after 12 weeks) showed that half (52% [11/21]) of patients whose BSA involvement was 3% or greater with a biologic for 24 weeks or more reached the TTT goal after 12 weeks of tapinarof-biologic treatment. Other studies of halobetasol propionate–tazarotene lotion16 and calcipotriene/betamethasone dipropionate foam17,18 added to the current biologic of poor responders found 60% to 68% of patients had reductions in their percentage BSA to 1% or lower at 12 to 16 weeks of treatment. Randomized studies showed etanercept plus topical clobetasol propionate foam20 or adalimumab plus calcipotriene/betamethasone dipropionate foam21 similarly enhanced treatment effects vs biologic alone.
A phase 3 PSOARING trial demonstrated benefit from treatment with tapinarof alone, with a remittive effect of approximately 4 months after discontinuation.25 Our data are consistent with these findings, with 40% (8/20) of patients demonstrating a remittive effect 4 weeks after discontinuing tapinarof while receiving a biologic. A similar maintenance effect was reported in another study in 50% (9/18) of patients treated with a biologic plus halobetasol propionate–tazarotene lotion.16 Additionally, when halcinonide ointment was given to patients receiving tildrakizumab, mean percentage of BSA involvement, PGA scores, PGA×BSA, and DLQI scores improved and were maintained 4 weeks after halcinonide ointment was stopped.19 Thus, topical therapy can augment and extend a biologic’s effect for up to 4 weeks.
In our study, tapinarof cream added to a biologic had a good safety and tolerability profile. Few AEs were recorded, with most being mild in nature, and no serious AEs or discontinuations due to AEs were reported. Only 1 case of mild folliculitis and 1 case of moderate worsening of psoriasis were considered treatment related. Further, no unexpected or new safety signals with the tapinarof-biologic combination were observed compared with tapinarof alone.27Prior studies have found that supplementing a biologic with topical therapy can reduce the probability of patients switching to another biologic.16,19 We previously found that adding halobetasol propionate–tazarotene lotion16 or calcipotriene/betamethasone dipropionate foam17 to a biologic helped reduce the probability of switching biologics from 88% to 90% at baseline to 12% to 24% after 12 weeks of combined therapy. Such combinations also could prevent a less responsive patient from being prescribed a higher biologic dose.19 These are important research findings, as patients—even when not responding well to their current biologic—are more likely to be tolerating that biologic well, and switching to a new biologic may introduce new safety or tolerability concerns. Thus, by enhancing the effect of a biologic with a topical therapy, one can avoid increasing the dose of the current biologic or switching to a new biologic, either of which may increase safety and/or tolerability risks. Switching biologics also has increased cost implications to the health care system and/or the patient. When comparing the cost of adding halobetasol propionate–tazarotene lotion to a biologic compared with switching to another biologic, the cost was 1.2 to 2.9 times higher to switch, depending on the biologic, compared with a smaller incremental cost increase to add a topical to the current biologic.16 Similar observations were reported with calcipotriene/betamethasone dipropionate foam plus a biologic.17 Although we did not evaluate biologic switching here, we anticipate a similar clinical scenario with a tapinarof-biologic combination.
Limitations of our study included the open-label design, lack of a control arm, and the relatively small study population; however, for studies investigating the safety and effectiveness of a treatment in a real-world setting, these limitations are common and are not unexpected. Our results also are consistent with the overall improvement seen in other studies16-21 examining the effects of adding a topical to a biologic. Future research is warranted to investigate a longer remittive effect and potential health care system and patient cost savings without having to switch biologics due to lack of effectiveness.
Conclusion
This study demonstrated that adjunctive use of nonsteroidal tapinarof cream 1% may enhance a biologic treatment effect in patients with moderate to severe plaque psoriasis, providing an adequate response for many patients who were not responding well to a biologic alone. Clinical outcomes improved with the tapinarof-biologic combination, and a remittive effect was noted 4 weeks after tapinarof discontinuation without any new safety signals. Adding tapinarof cream to a biologic also may prevent the need to switch biologics when patients do not sufficiently respond, preserving the safety and cost associated with a patient’s current biologic.
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
- Armstrong AW, Mehta MD, Schupp CW, et al. Psoriasis prevalence in adults in the United States. JAMA Dermatol. 2021;157:940-946. doi:10.1001/jamadermatol.2021.2007
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804. doi:10.1016/j.jaad.2019.04.042
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470. doi:10.1016/j.jaad.2020.07.087
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiological therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072. doi:10.1016/j.jaad.2018.11.057
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis.J Am Acad Dermatol. 2017;76:290-298. doi:10.1016/j.jaad.2016.10.017
- Taltz. Prescribing information. Eli Lilly and Company; 2024.
- Cosentyx. Prescribing information. Novartis Pharmaceuticals Corporation; 2023.
- Tremfya. Prescribing information. Janssen Biotech, Inc; 2023.
- Skyrizi. Prescribing information. AbbVie Inc; 2024.
- Ilumya. Prescribing information. Sun Pharmaceutical Industries, Inc; 2020.
- Stelara. Prescribing information. Janssen Biotech, Inc; 2022.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Jensen JD, Delcambre MR, Nguyen G, et al. Biologic therapy with or without topical treatment in psoriasis: what does the current evidence say? Am J Clin Dermatol. 2014;15:379-385. doi:10.1007/s40257-014-0089-1
- Gustafson CJ, Watkins C, Hix E, et al. Combination therapy in psoriasis: an evidence-based review. Am J Clin Dermatol. 2013;14:9-25. doi:10.1007/s40257-012-0003-7
- Bagel J, Novak K, Nelson E. Adjunctive use of halobetasol propionate-tazarotene in biologic-experienced patients with psoriasis. Cutis. 2022;109:103-109. doi:10.12788/cutis.0451
- Bagel J, Nelson E, Zapata J, et al. Adjunctive use of calcipotriene/betamethasone dipropionate foam in a real-world setting curtails the cost of biologics without reducing efficacy in psoriasis. Dermatol Ther (Heidelb). 2020;10:1383-1396. doi:10.1007/s13555-020-00454-z
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:611-616.
- Bagel J, Novak K, Nelson E. Tildrakizumab in combination with topical halcinonide 0.1% ointment for treating moderate to severe plaque psoriasis. J Drugs Dermatol. 2023;22:766-772. doi:10.36849/jdd.6830
- Lebwohl MG, Kircik L, Callis Duffin K, et al. A randomized study to evaluate the efficacy and safety of adding topical therapy to etanercept in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2013;69:385-392. doi:10.1016/j.jaad.2013.03.031
- Thaci D, Ortonne JP, Chimenti S, et al. A phase IIIb, multicentre, randomized, double-blind, vehicle-controlled study of the efficacy and safety of adalimumab with and without calcipotriol/betamethasone topical treatment in patients with moderate to severe psoriasis: the BELIEVE study. Br J Dermatol. 2010;163:402-411. doi:10.1111/j.1365-2133.2010.09791.x
- Vtama. Prescribing information. Dermavant Sciences, Inc; 2022.
- Bobonich M, Gorelick J, Aldredge L, et al. Tapinarof, a novel, first-in-class, topical therapeutic aryl hydrocarbon receptor agonist for the management of psoriasis. J Drugs Dermatol. 2023;22:779-784. doi:10.36849/jdd.7317
- Lebwohl MG, Stein Gold L, Strober B, et al. Phase 3 trials of tapinarof cream for plaque psoriasis. N Engl J Med. 2021;385:2219-2229. doi:10.1056/NEJMoa2103629
- Strober B, Stein Gold L, Bissonnette R, et al. One-year safety and efficacy of tapinarof cream for the treatment of plaque psoriasis: results from the PSOARING 3 trial. J Am Acad Dermatol. 2022;87:800-806. doi:10.1016/j.jaad.2022.06.1171
- Kircik L, Zirwas M, Kwatra SG, et al. Rapid improvements in itch with tapinarof cream 1% once daily in two phase 3 trials in adults with mild to severe plaque psoriasis. Dermatol Ther (Heidelb). 2024;14:201-211. doi:10.1007/s13555-023-01068-x
- Bagel J, Gold LS, Del Rosso J, et al. Tapinarof cream 1% once daily for the treatment of plaque psoriasis: patient-reported outcomes from the PSOARING 3 trial. J Am Acad Dermatol. 2023;89:936-944. doi:10.1016/j.jaad.2023.04.061
- Abdin R, Kircik L, Issa NT. First use of combination oral deucravacitinib with tapinarof cream for treatment of severe plaque psoriasis. J Drugs Dermatol. 2024;23:192-194. doi:10.36849/jdd.8091
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Safety and Effectiveness of Nonsteroidal Tapinarof Cream 1% Added to Ongoing Biologic Therapy for Treatment of Moderate to Severe Plaque Psoriasis
Practice Points
- Patients with moderate to severe psoriasis do not always reach treatment goals with biologic therapy alone.
- Adjunctive use of nonsteroidal tapinarof cream 1% may enhance the effects of ongoing biologic therapy in patients with moderate to severe plaque psoriasis, possibly avoiding the need to switch to another biologic.
- Patients with moderate to severe plaque psoriasis who are not adequately responding to biologics may benefit from adding tapinarof cream 1% to their current regimen.
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).
The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).
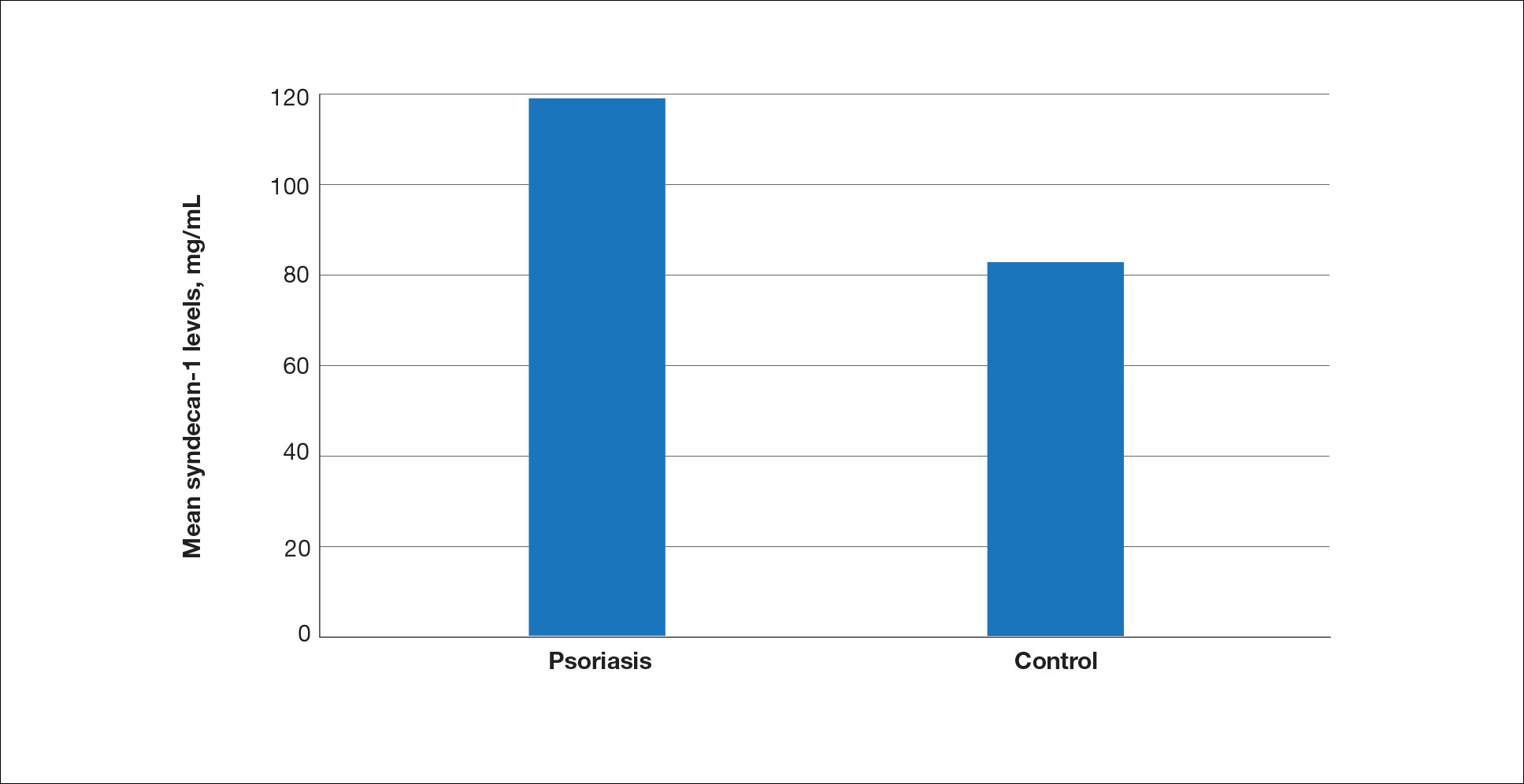
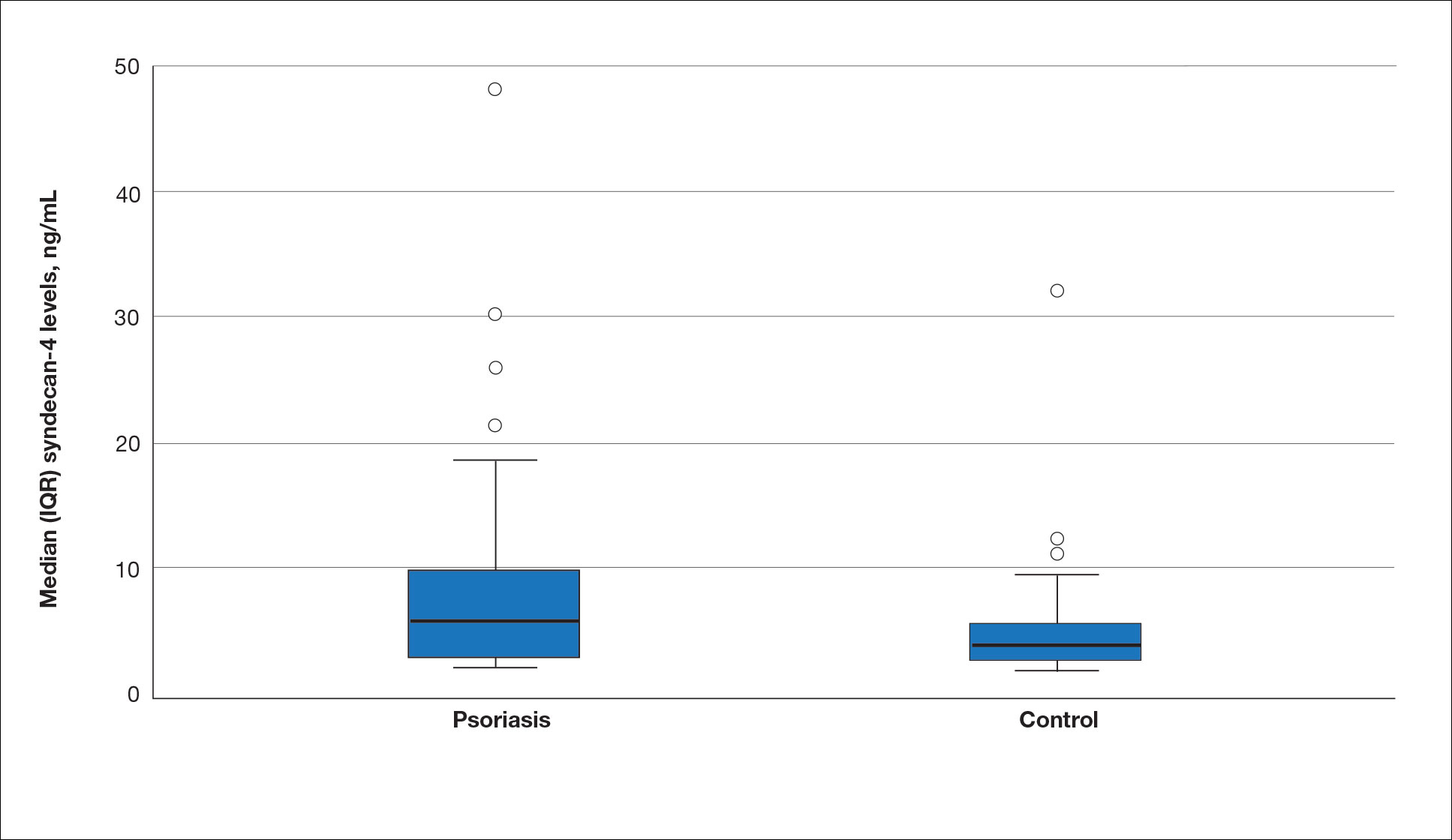
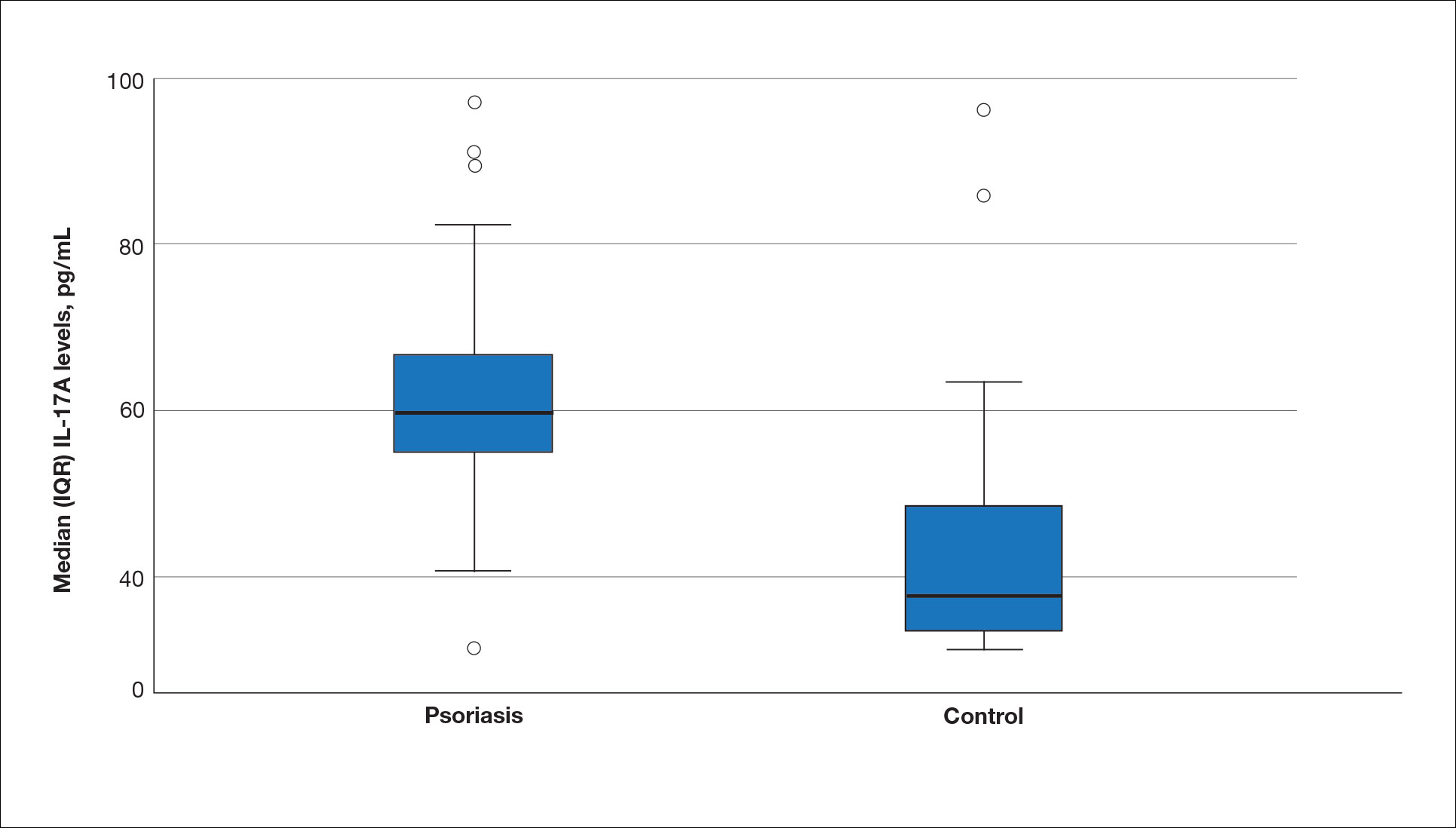
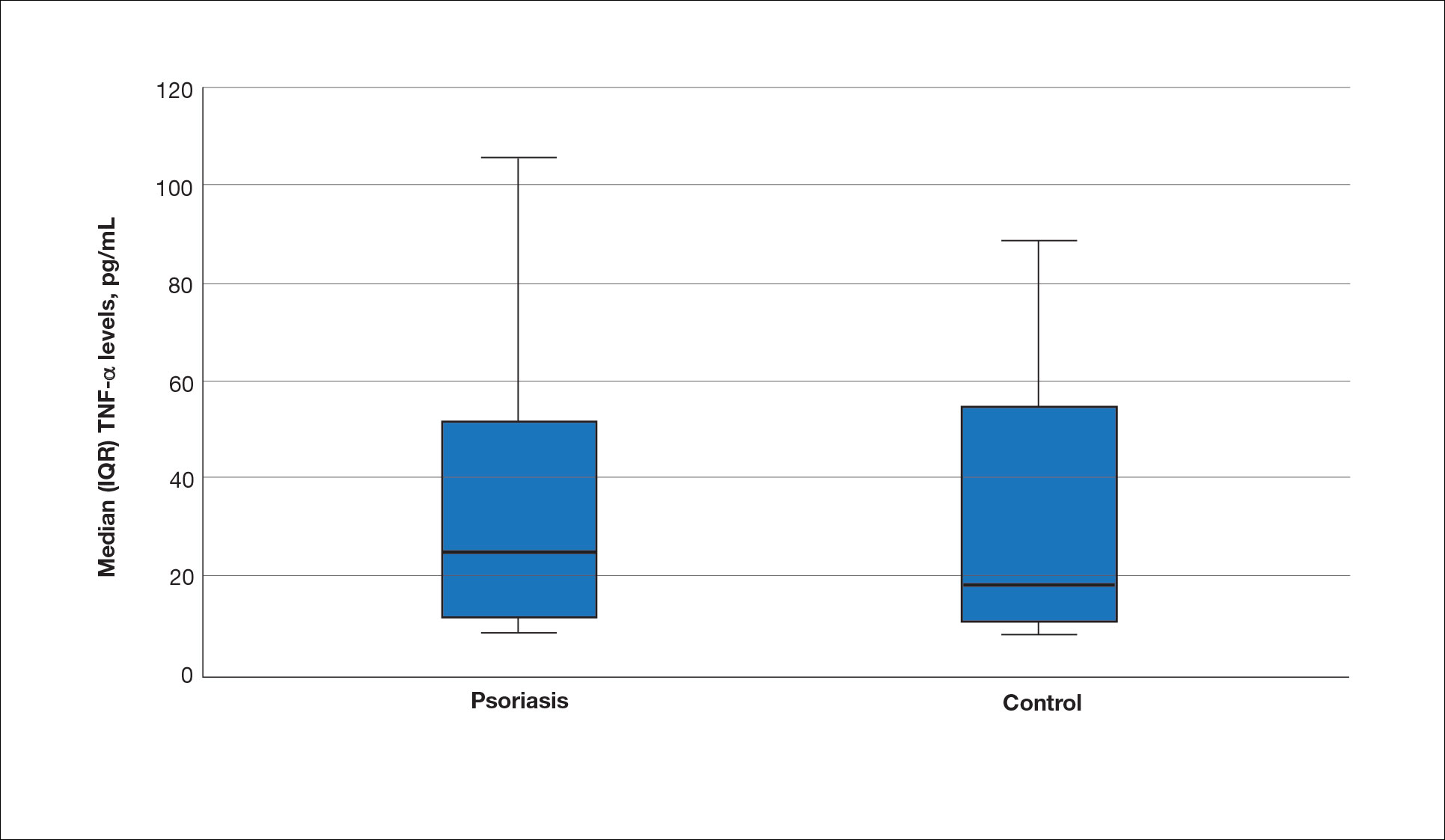
A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).
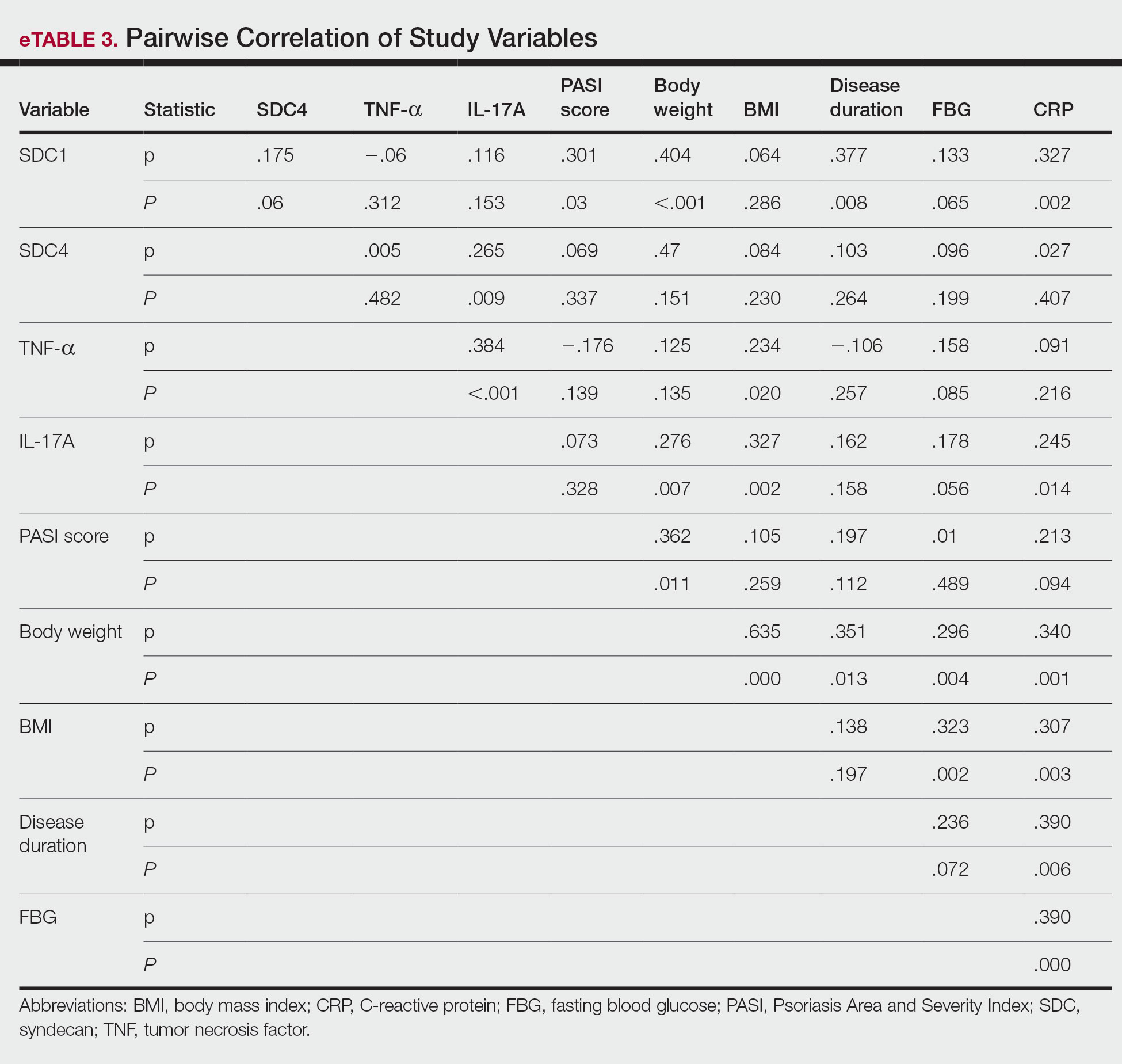
Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).
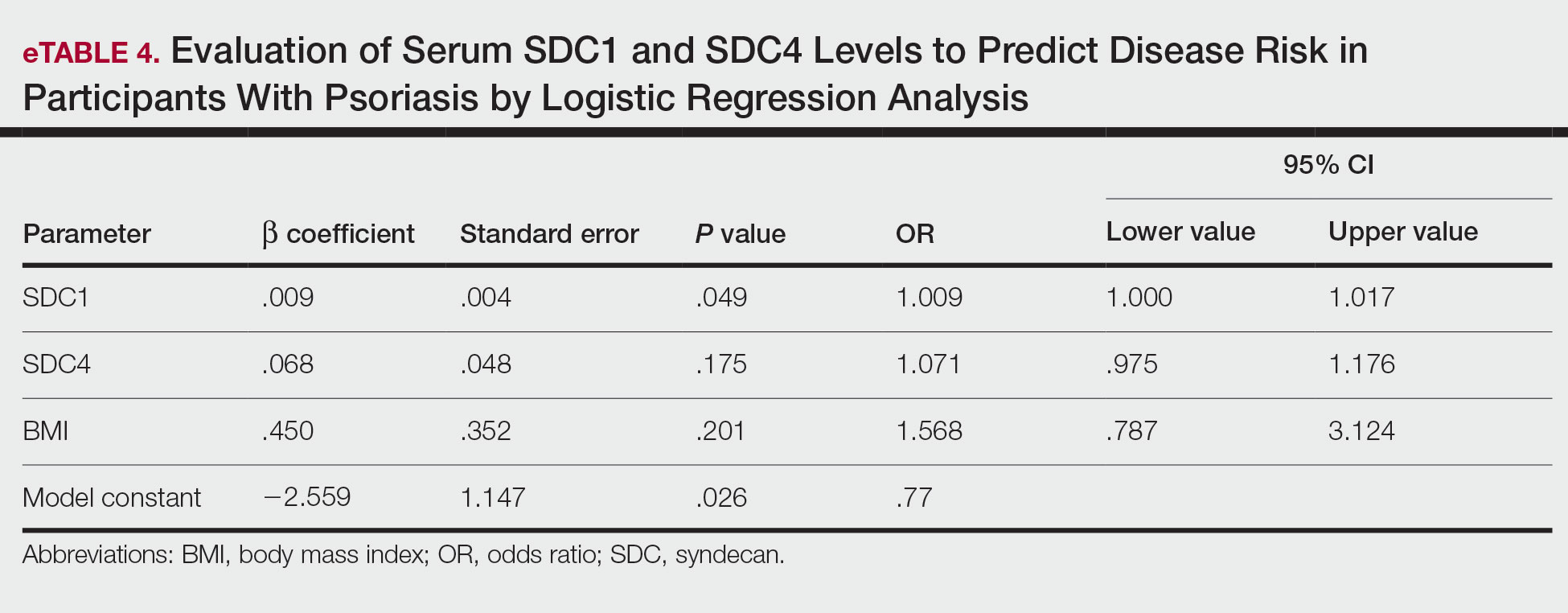
Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).

The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).





A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).

Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).

Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Psoriasis, one of the most researched diseases in dermatology, has a complex pathogenesis that is not yet fully understood. One of the most important stages of psoriasis pathogenesis is the proliferation of T helper (Th) 17 cells by IL-23 released from myeloid dendritic cells. Cytokines such as tumor necrosis factor (TNF) α released from Th1 cells and IL-17 and IL-22 released from Th17 cells are known to induce the proliferation of keratinocytes and the release of chemokines responsible for neutrophil chemotaxis.1
Although secondary messengers such as cytokines and chemokines, which provide cell interaction with the extracellular matrix (ECM), have their own specific receptors, it is known that syndecans (SDCs) play a role in ECM and cell interactions and have receptor or coreceptor functions.2 In humans, 4 types of SDCs have been identified (SDC1-SDC4), which are type I transmembrane proteoglycans found in all nucleated cells. Syndecans consist of heparan sulfate glycosaminoglycan chains that are structurally linked to a core protein sequence. The molecule has cytoplasmic, transmembrane, and extracellular domains.2,3 While SDCs often are described as coreceptors for integrins and growth factor and hormone receptors, they also are capable of acting as signaling receptors by engaging intracellular messengers, including actin-related proteins and protein kinases.4
Prior research has indicated that the release of heparanase from the lysosomes of leukocytes during infection, inflammation, and endothelial damage causes cleavage of heparan sulfate glycosaminoglycans from the extracellular domains of SDCs. The peptide chains at the SDC core then are separated by matrix metalloproteinases in a process known as shedding. The shed SDCs may have either a stimulating or a suppressive effect on their receptor activity. Several cytokines are known to cause SDC shedding.5,6 Many studies in recent years have reported that SDCs play a role in the pathogenesis of inflammatory diseases, for which serum levels of soluble SDCs can be biomarkers.7
In this study, we aimed to evaluate and compare serum SDC1, SDC4, TNF-α, and IL-17A levels in patients with psoriasis vs healthy controls. Additionally, by reviewing the literature data, we analyzed whether SDCs can be implicated in the pathogenesis of psoriasis and their potential role in this process.
Methods
The study population consisted of 40 patients with psoriasis and 40 healthy controls. Age and sex characteristics were similar between the 2 groups, but weight distribution was not. The psoriasis group included patients older than 18 years who had received a clinical and/or histologic diagnosis, had no systemic disease other than psoriasis in their medical history, and had not used any systemic treatment or phototherapy for the past 3 months. Healthy patients older than 18 years who had no medical history of inflammatory disease were included in the control group. Participants provided signed consent.
Data such as medical history, laboratory findings, and physical specifications were recorded. A Psoriasis Area and Severity Index (PASI) score of 10 or lower was considered mild disease, and a score higher than 10 was considered moderate to severe disease. An enzyme-linked immunosorbent assay was used to measure SDC1, SDC4, TNF-α, and IL-17A levels.
The data were evaluated using the IBM SPSS Statistics V22.0 statistical package program. A P value of <.05 was considered statistically significant. The conformity of the data to a normal distribution was examined using a Shapiro-Wilk test. Normally distributed variables were expressed as mean (SD) and nonnormally distributed variables were expressed as median (interquartile range [IQR]). Data were compared between the 2 study groups using either a student t test (normal distribution) or Mann-Whitney U test (nonnormal distribution). Categorical variables were expressed as numbers and percentages. Categorical data were compared using a χ2 test. Associations among SDC1, SDC4, TNF-α, IL-17A, and other variables were assessed using Spearman rank correlation. A binary logistic regression analysis was used to determine whether serum SDC1 and SDC4 levels were independent risk factors for psoriasis.
Results
The 2 study groups showed similar demographic characteristics in terms of sex (P=.67) and age (P=.22) distribution. The mean (SD) PASI score in the psoriasis group was 12.33 (7.62); the mean (SD) disease duration was 11.10 (8.00) years. Body weight and BMI were both significantly higher in the psoriasis group (P=.027 and P=.029, respectively) compared with the control group (eTable 1).

The mean (SD) serum SDC1 level was 119.52 ng/mL (69.53 ng/mL) in the psoriasis group, which was significantly higher than the control group (82.81 ng/mL [51.85 ng/mL])(P=.011)(eTable 2)(eFigure 1). The median (IQR) serum SDC4 level also was significantly higher in the psoriasis group compared with the control group (5.78 ng/mL [7.09 ng/mL] vs 3.92 ng/mL [2.88 ng/mL])(P=.030)(eTable 2)(eFigure 2). The median (IQR) IL-17A value was 59.94 pg/mL (12.97 pg/mL) in the psoriasis group, which was significantly higher than the control group (37.74 pg/mL [15.10 pg/mL])(P<.001)(eTable 2)(eFigure 3). The median (IQR) serum TNF-α level was 25.07 pg/mL (41.70 pg/mL) in the psoriasis group and 18.21 pg/mL (48.51 pg/mL) in the control group; however, the difference was not statistically significance (P=.444)(eTable 2)(eFigure 4).





A significant positive correlation was found between serum SDC1 and PASI score (p=0.064; P=.03). Furthermore, significant positive correlations were identified between serum SDC1 and body weight (p=0.404; P<.001), disease duration (p=0.377; P=.008), and C-reactive protein (p=0.327; P=.002). A significant positive correlation also was identified between SDC4 and IL-17A (p=0.265; P=.009). Serum TNF-α was positively correlated with IL-17A (p=0.384; P<.001) and BMI (p=0.234; P=.020)(eTable 3).

Logistic regression analysis showed that high SDC1 levels were independently associated with the development of psoriasis (odds ratio [OR], 1.009; 95% CI, 1.000-1.017; P=.049)(eTable 4).

Comment
Tumor necrosis factor α and IL-17A are key cytokines whose roles in the pathogenesis of psoriasis are well established. Arican et al,8 Kyriakou et al,9 and Xuan et al10 previously reported a lack of any correlation between TNF-α and IL-17A in the pathogenesis of psoriasis; however, we observed a positive correlation between TNF-α and IL-17A in our study. This finding may be due to the abundant TNF-α production by myeloid dendritic cells involved in the transformation of naive T lymphocytes into IL-17A–secreting Th17 lymphocytes, which can also secrete TNF-α.
After the molecular cloning of SDCs by Saunders et al11 in 1989, SDCs gained attention and have been the focus of many studies for their part in the pathogenesis of conditions such as inflammatory diseases, carcinogenesis, infections, sepsis, and trauma.6,12 Among the inflammatory diseases sharing similar pathogenetic features to psoriasis, serum SDC4 levels are found to be elevated in rheumatoid arthritis and are correlated with disease activity.13 Cekic et al14 reported that serum SDC1 levels were significantly higher in patients with Crohn disease than controls (P=.03). Additionally, serum SDC1 levels were higher in patients with active disease compared with those who were in remission. Correlations between SDC1 and disease severity and C-reactive protein also have been found.14 Serum SDC-1 levels found to be elevated in patients with systemic lupus erythematosus were compared to the controls and were correlated with disease activity.15 Nakao et al16 reported that the serum SDC4 levels were significantly higher in patients with atopic dermatitis compared to controls (P<.01); further, SDC4 levels were correlated with severity of the disease.
Jaiswal et al17 reported that SDC1 is abundant on the surface of IL-17A–secreting γδ T lymphocytes (Tγδ17), whose contribution to psoriasis pathogenesis is known. When subjected to treatment with imiquimod, SDC1-suppressed mice displayed increased psoriasiform dermatitis compared with wild-type counterparts. The authors stated that SDC1 may play a role in controlling homeostasis of Tγδ17
In a study examining changes in the ECM in patients with psoriasis, it was observed that the expression of
A study conducted by Koliakou et al20 showed that, in healthy skin, SDC1 was expressed in almost the full thickness of the epidermis, but lowest expression was in the basal-layer keratinocytes. In a psoriatic epidermis, unlike the normal epidermis, SDC1 was found to be more intensely expressed in the keratinocytes of the basal layer, where keratinocyte proliferation occurs. In this study, SDC4 was expressed mainly at lower levels in a healthy epidermis, especially in the spinous and the basal layers. In a psoriatic epidermis, SDC4 was absent from all the layers. In the same study, gelatin-based carriers containing anti–TNF-α and anti–IL-17A were applied to a full-thickness epidermis with psoriatic lesions, after which SDC1 expression was observed to decrease almost completely in the psoriatic epidermis; there was no change in SDC4 expression, which also was not seen in the psoriatic epidermis. The authors claimed the application of these gelatin-based carriers could be a possible treatment modality for psoriasis, and the study provides evidence for the involvement of SDC1 and/or SDC4 in the pathogenesis of psoriasis
Limitations of the current study include small sample size, lack of longitudinal data, lack of tissue testing of these molecules, and lack of external validation.
Conclusion
Overall, research has shown that SDCs play important roles in inflammatory processes, and more widespread inflammation has been associated with increased shedding of these molecules into the ECM and higher serum levels. In our study, serum SDC1, SDC4, and IL-17A levels were increased in patients with psoriasis compared to the healthy controls. A logistic regression analysis indicated that high serum SDC1 levels may be an independent risk factor for development of psoriasis. The increase in serum SDC1 and SDC4 levels and the positive correlation between SDC1 levels and disease severity observed in our study strongly implicate SDCs in the inflammatory disease psoriasis. The precise role of SDCs in the pathogenesis of psoriasis and the implications of targeting these molecules are the subject of more in-depth studies in the future.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Griffiths CEM, Armstrong AW, Gudjonsson JE, et al. Psoriasis. Lancet. 2021;397:1301-1315.
Uings IJ, Farrow SN. Cell receptors and cell signaling. Mol Pathol. 2000;53:295-299.
Kirkpatrick CA, Selleck SB. Heparan sulfate proteoglycans at a glance.J Cell Sci. 2007;120:1829-1832.
Stepp MA, Pal-Ghosh S, Tadvalkar G, et al. Syndecan-1 and its expanding list of contacts. Adv Wound Care (New Rochelle). 2015;4:235-249.
Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced shedding of syndecan-1 and its role in driving disease pathogenesis and progression. J Histochem Cytochem. 2020;68:823-840.
Gopal S, Arokiasamy S, Pataki C, et al. Syndecan receptors: pericellular regulators in development and inflammatory disease. Open Biol. 2021;11:200377.
Bertrand J, Bollmann M. Soluble syndecans: biomarkers for diseases and therapeutic options. Br J Pharmacol. 2019;176:67-81.
Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005;2005:273-279.
Kyriakou A, Patsatsi A, Vyzantiadis TA, et al. Serum levels of TNF-α, IL12/23 p40, and IL-17 in psoriatic patients with and without nail psoriasis: a cross-sectional study. ScientificWorldJournal. 2014;2014:508178.
Xuan ML, Lu CJ, Han L, et al. Circulating levels of inflammatory cytokines in patients with psoriasis vulgaris of different Chinese medicine syndromes. Chin J Integr Med. 2015;21:108-114.
Saunders S, Jalkanen M, O’Farrell S, et al. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989;108:1547-1556.
Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. FEBS J. 2010;277:3876-3889.
Zhao J, Ye X, Zhang Z. Syndecan-4 is correlated with disease activity and serological characteristic of rheumatoid arthritis. Adv Rheumatol. 2022;62:21.
Cekic C, Kırcı A, Vatansever S, et al. Serum syndecan-1 levels and its relationship to disease activity in patients with Crohn’s disease. Gastroenterol Res Pract. 2015;2015:850351.
Minowa K, Amano H, Nakano S, et al. Elevated serum level of circulating syndecan-1 (CD138) in active systemic lupus erythematosus. Autoimmunity. 2011;44:357-362.
Nakao M, Sugaya M, Takahashi N, et al. Increased syndecan-4 expression in sera and skin of patients with atopic dermatitis. Arch Dermatol Res. 2016;308:655-660.
Jaiswal AK, Sadasivam M, Archer NK, et al. Syndecan-1 regulates psoriasiform dermatitis by controlling homeostasis of IL-17-producing γδ T cells. J Immunol. 2018;201:1651-1661
Wagner MFMG, Theodoro TR, Filho CASM, et al. Extracellular matrix alterations in the skin of patients affected by psoriasis. BMC Mol Cell Biol. 2021;22:55.
Peters F, Rahn S, Mengel M, et al. Syndecan-1 shedding by meprin β impairs keratinocyte adhesion and differentiation in hyperkeratosis. Matrix Biol. 2021;102:37-69.
Koliakou E, Eleni MM, Koumentakou I, et al. Altered distribution and expression of syndecan-1 and -4 as an additional hallmark in psoriasis. Int J Mol Sci. 2022;23:6511.
Doss RW, El-Rifaie AA, Said AN, et al. Cutaneous syndecan-1 expression before and after phototherapy in psoriasis. Indian J Dermatol Venereol Leprol. 2020;86:439-440.
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
Pathogenic Significance of Serum Syndecan-1 and Syndecan-4 in Psoriasis
PRACTICE POINTS
- Improved understanding of psoriasis pathogenesis has enabled the development of targeted treatments, although the mediators driving the disease have not yet been fully identified.
- Based on the findings of this study and existing literature, we suggest that syndecan-1 and syndecan-4 may play a role in the pathogenesis of psoriasis; however, further studies are needed to elucidate their precise mechanisms of action.
Antibiotic Stewardship in Acne: Practical Tips From Dr. Lorraine L. Rosamilia
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
Dark-Brown Macule on the Periumbilical Skin
Dark-Brown Macule on the Periumbilical Skin
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.
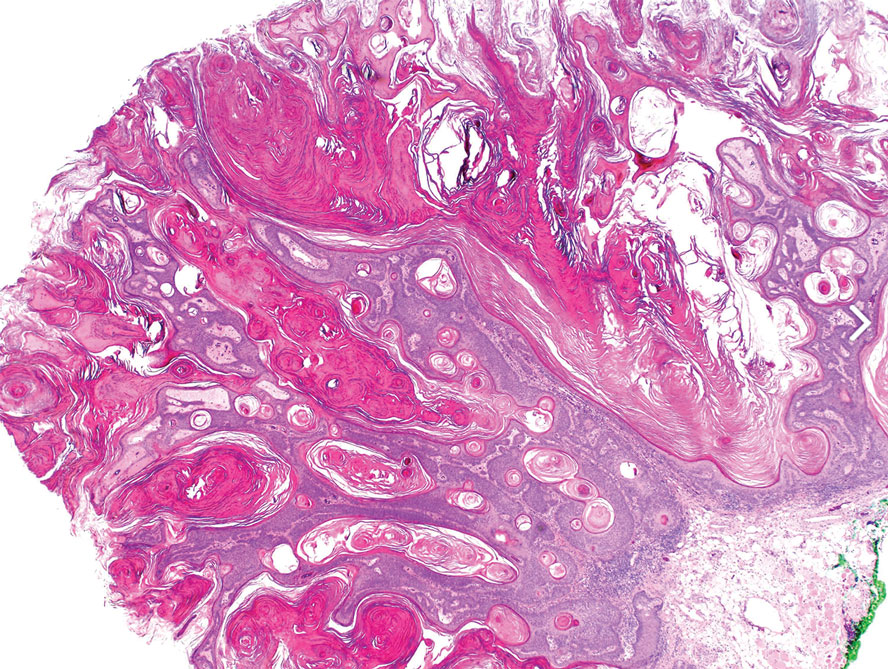
Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.

Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
THE DIAGNOSIS: Seborrheic Keratosis
Histopathology revealed epidermal hyperplasia and hyperkeratosis with no notation of atypical melanocytic activity (Figure). There were no Kamino bodies, junctional nesting, or cytologic atypia. Based on these features as well as the clinical and dermoscopic findings, a diagnosis of an inflamed seborrheic keratosis (SK) was made. No further treatment was required following the shave biopsy, and the patient was reassured regarding the benign nature of the lesion.

Seborrheic keratoses are benign epidermal growths that can manifest on any area of the skin except the palms and soles. They present clinically as tan, yellow, gray, brown, or black with a smooth, waxy, or verrucous surface. They range from 1 mm to several centimeters in diameter. Although SKs traditionally manifest more frequently in individuals with lighter skin tones, pigmented variants, such as dermatosis papulosa nigra, have been reported to occur more commonly and at younger ages in patients with skin of color.1
Dermoscopy of SK in patients with skin of color can present diagnostic challenges, as these lesions may display atypical pigmented patterns that overlap with melanocytic lesions, including Spitz nevi, particularly when starburstlike or globular structures are present.2 What sets inflamed SKs apart from other SKs is the lack of a heavily keratinized surface on both clinical and dermoscopic evaluation. Common histopathologic diagnostic criteria for Spitz nevi include Kamino bodies, uniform nuclear enlargement, and spindled or epithelioid nevus cells, which were not noted in our patient.3 Therefore, in presentations such as this, histopathology remains the gold standard for diagnosis.
The differential diagnosis in this case included benign nevus, dysplastic nevus, melanoma, and Spitz nevus. Benign nevi typically demonstrate uniform pigmentation and symmetric dermoscopic patterns. Dysplastic nevi may show architectural disorder and cytologic atypia but lack invasive features.3 Melanoma often exhibits asymmetry, atypical network patterns, and irregular pigmentation.4 Spitz nevi characteristically demonstrate large epithelioid or spindle cells with Kamino bodies on histopathology, which were absent in our patient.
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
- Greco MJ, Bhutta BS. Seborrheic keratosis. StatPearls [Internet]. StatPearls Publishing; 2025. Updated May 6, 2024. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK545285/
- Emanuel P, Cheng, H. Spitz naevus pathology. Accessed November 25, 2025. https://dermnetnz.org/topics/spitz-naevus-pathology.
- Wensley KE, Zito PM. Atypical mole. StatPearls [Internet]. StatPearls Publishing; 2025. Updated July 3, 2023. Accessed December 19, 2025. https://www.ncbi.nlm.nih.gov/books/NBK560606/
- Valenzuela FI, Hohnadel M. Dermatoscopic characteristics of melanoma versus benign lesions and nonmelanoma cancers. StatPearls [Internet]. StatPearls Publishing; 2025. Updated August 10, 2024. Accessed December 19, 2025. https://www.ncbi.nlm .nih.gov/books/NBK606113/
Dark-Brown Macule on the Periumbilical Skin
Dark-Brown Macule on the Periumbilical Skin
A 33-year-old man with moderately to deeply pigmented skin presented to the dermatology department with a dark-brown macule in the periumbilical area of more than 1 year’s duration. The patient was otherwise healthy and reported no personal or family history of atypical nevi, nonmelanoma skin cancer, or melanoma. Dermoscopy of the lesion showed a dark brown macule less than 2 mm in diameter with a starburst like pattern and a blue-hued border. A shave biopsy of the lesion was performed.
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24
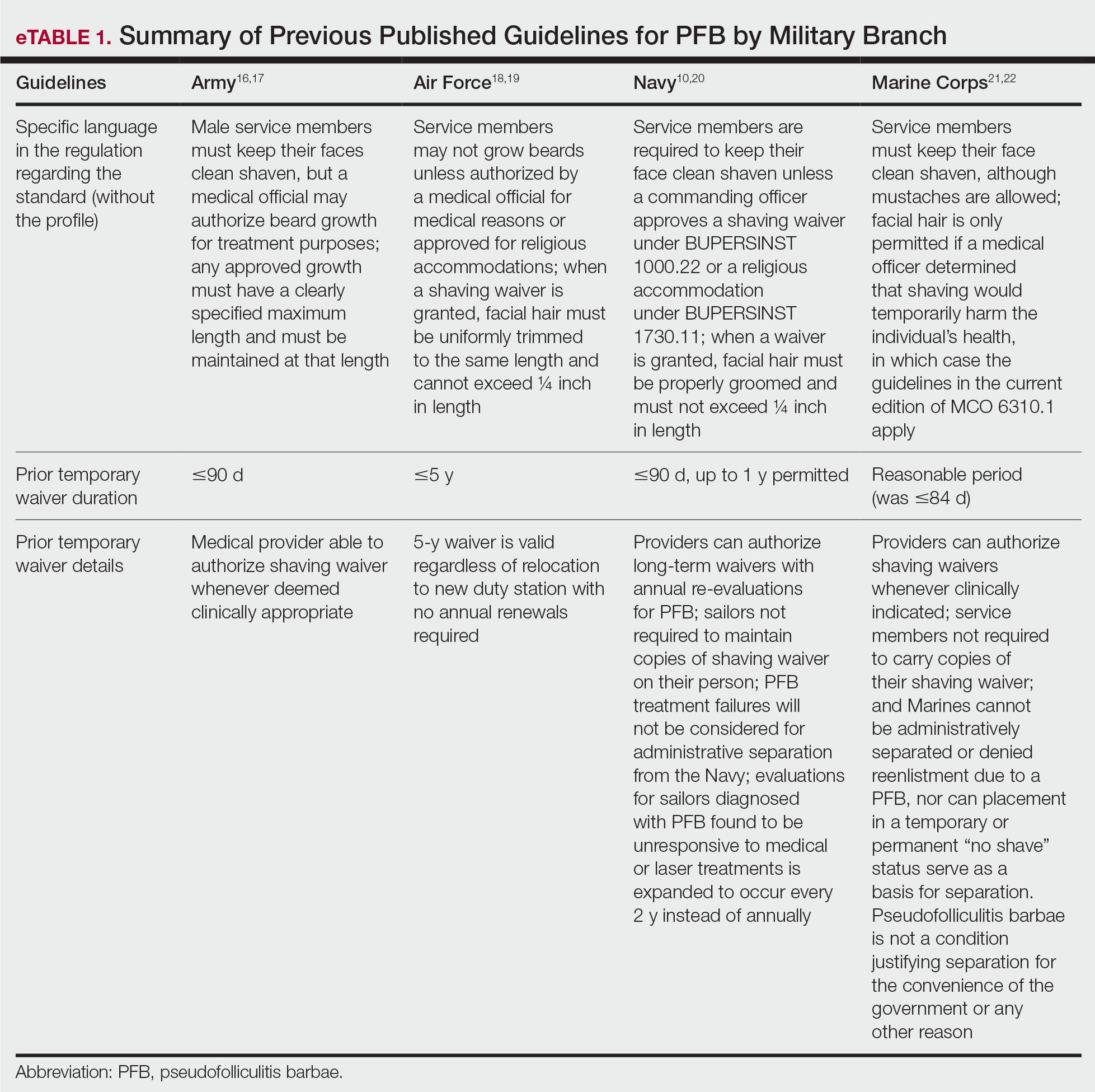
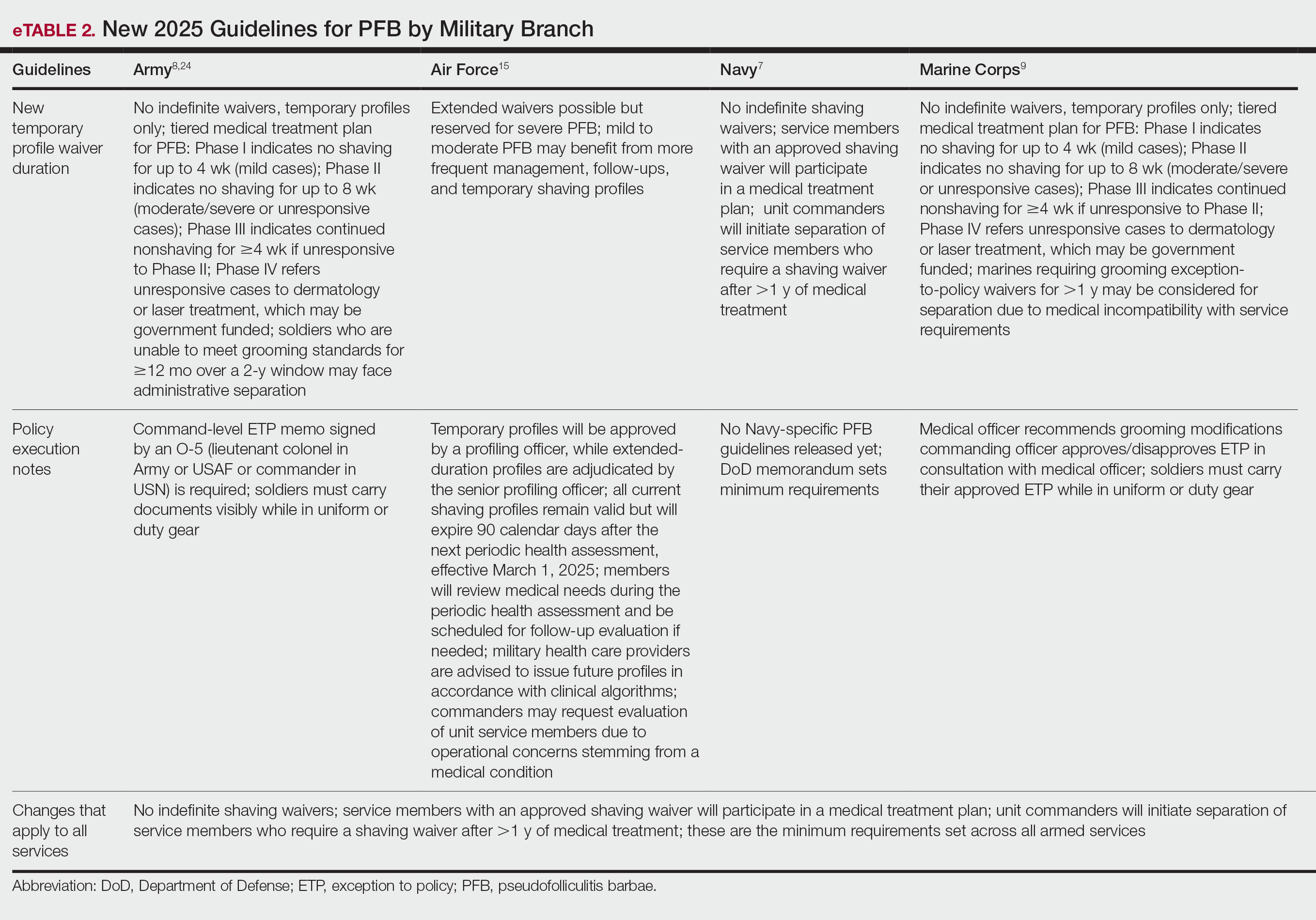
The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24


The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
According to the US Department of Defense (DoD), proper wear of the military uniform and adherence to grooming standards are essential components of military discipline and unit cohesion.1,2 The DoD posits that personal appearance reflects the professionalism, integrity, and accountability expected of all service members. These standards promote a shared identity and reinforce the discipline required for military organizations to operate as cohesive, unified, mission-oriented teams. Personal appearance embodies integrity, commitment to duty, and respect for institutional norms.1,2 In some situations, grooming standards also carry critical operational relevance; for example, the DoD states that a clean-shaven face is necessary to ensure a proper seal for gas masks and other personal protective equipment used in combat environments, especially when chemical or biological weapons are used.3 The Uniform Code of Military Justice states that service members who fail to comply with grooming standards, unless exempted, are subject to disciplinary action.4
In early March 2025, new directives from the DoD prompted a comprehensive review of personal grooming standards and wear of military uniforms across the uniformed services. The stated goal of these revisions was to enhance discipline, professionalism, and military readiness.5,6 These policy updates reversed several grooming accommodations introduced in prior administrations that allowed greater flexibility in personal appearance and hair-grooming practices for service members. The 2025 revised standards entail re-examination and rewriting regulations that govern grooming standards.
The new grooming regulations are likely to have major effects on service members with pseudofolliculitis barbae (PFB), a chronic inflammatory condition of the facial skin that often occurs due to and is aggravated by repeated close shaving. Through most of their histories, each US military branch has required a clean, smooth-shaven facial appearance that entailed regular (usually daily) shaving of facial hair; however, service-specific grooming instructions and medical guidelines have permitted commanders to authorize temporary or permanent exemptions or waivers for service members with PFB. To obtain a shaving waiver, individuals with PFB work closely with a military medical officer to design a shaving strategy that will not exacerbate PFB. If medical management was unsuccessful, the medical officer usually prepared a recommendation for a shaving waiver that also required approval from the service member’s commanding officer. Waivers were handled on a case-by-case basis and could be temporary (eg, for 3 months), recurring/renewable, or permanent.
The recent policy shifts make it difficult for service members to obtain renewable and permanent shaving waivers, raising concerns about medical outcomes and readiness implications. In this article, we examine the updated facial hair grooming standards across the uniformed services with a focus on the medical, regulatory, and administrative management of PFB.
Background and Policy Shifts
In March 2025, the Secretary of Defense ordered a widespread review of grooming standards in the armed forces.6 In accordance with this directive, the Army, Navy, Air Force, and Marine Corps made revisions to their uniform and grooming regulations. In August 2025, the Secretary of Defense issued a memorandum that reinforced the expectation that service members remain clean shaven and introduced additional limits on medical waivers.7 Under this policy, medical officers must provide written recommendations, while commanders remain the final approval authority. Service members with approved shaving waivers for PFB also must participate in a medical treatment plan for the condition. Importantly, the memorandum directed unit commanders to initiate separation for service members in any branch who continue to require a shaving waiver after more than 1 year of medical management. This directive underscores the DoD’s emphasis on uniformity and cohesion as visible markers of professionalism and the “warrior ethos.”7
Regulatory Framework and Enforcement
Beginning in March 2025, centrally mandated revisions to existing directives introduced more restrictive grooming and appearance standards across all military services. A key area of enforcement involves strict management of medical shaving waivers, particularly those related to PFB, which indicates a reversal of previous accommodations. Because of the lack of effective treatment for intractable PFB, the DoD previously has permitted service members to obtain permanent shaving waivers. The use of long-term waivers reduced administrative burden by removing the need for repeated evaluations and routine renewal paperwork, thereby decreasing the workload for service members, medical officers, and commanders. In the Army and Marine Corps, new grooming standards8,9 eliminate permanent waivers and prohibit pro forma renewals or extensions of existing waivers. Service members with PFB must seek a medical provider who will conduct a new full clinical evaluation, prepare new documentation requesting another temporary shaving waiver, and submit the application for the commander’s review and approval.
The Air Force also has adopted a stricter stance on shaving waivers. Under previous guidelines, service members diagnosed with PFB were eligible for a 5-year waiver that did not require annual renewal.10 However, the new 2025 guidelines eliminated this option. Now, waivers are subject to increased scrutiny and may be extended only for service members with severe, well-documented cases of PFB. In addition, the waiver must be approved by the commanding officer.11 The updated policy does not specify whether an existing waiver can be continued (ie, rolled over) or if a complete de novo waiver is required.
The new policies that eliminate long-term waivers introduce logistical and administrative requirements that are likely to be time consuming, at multiple levels of the military. In the Army and Marine Corps, it is immaterial whether the request comes from a new recruit or from a seasoned service member who has had a shaving waiver for their entire career. Under the new policy, every waiver requires a formal medical appointment with a licensed health care provider, documentation and case review, completion of a standardized waiver form with the provider’s signature, and signed approval by the commanding officer.8
Across military services, available data indicate a substantial rise in shaving waivers over the past decade. Between 2021 and 2023, the number of active-duty Air Force personnel with PFB-related shaving waivers increased from 10,965 to 18,991.12 Meanwhile, the Army has reported that more than 40,000 new shaving waivers were issued in 2024.13 While Black service members comprise roughly 15% of the active-duty force, they account for 66% of shaving waiver holders.14
Implications and Perspectives
Shaving waivers had provided a medically and administratively supported avenue for managing PFB within the relevant service requirements; however, the new policies have mandated a shift toward more regulated timelines for waiver evaluation and renewal, prohibition of permanent shaving waivers, and shortened durations of temporary shaving waivers.15 These changes impose higher time demands and administrative responsibilities on affected service members, on the chain of command, and on the US Army Medical Department.
The new guidelines reintroduced a command-level policy for PFB that differs from the clinically focused recommendations outlined in the Army’s official medical guidance on PFB.8,15 The new directives also explicitly tie an individual’s potential eligibility to remain in the Army—across active, reserve, and National Guard components—to their ability to meet the new facial-hair grooming standards.8 The policy sets a clear benchmark for retention: failing to meet grooming standards for 12 or more months within a 24-month period automatically launches a process that leads to administrative separation. Similarly, a new Marine Corps directive authorizes administrative separation for Marines who require a medical grooming waiver for more than 1 year.11 These branch-specific changes appear to implement a broader DoD policy outlined in the August 2025 memorandum, which represents a tightening of medical shaving waivers across all branches by limiting them to no more than 1 year in duration before triggering a review for administrative separation.7 Additional implications also may include increased utilization of laser hair removal (LHR) for service members for whom conservative management has failed and who wish to pursue more definitive options. Given the potential career implications of PFB, LHR may become a more frequently considered intervention among military and civilian dermatologists. In the civilian sector, TRICARE covers LHR for active-duty service members when deemed medically necessary and unavailable at their military treatment facility.14 Consequently, civilian dermatologists may see an increase in referrals from military personnel seeking LHR to maintain compliance with grooming standards under the new policy framework.
Final Thoughts
Military personnel, their chain of command, and the military medical system are keenly aware of the DoD’s newly mandated policy changes regarding grooming standards. There are many circumstances in which military personnel (eg, active-duty service members, reservists, National Guard members) receive medical care from civilian providers, who may not be up to date on changes in the military’s approach toward grooming. Civilian dermatologists may be the first to diagnose or treat PFB in prospective recruits and should be aware that under current DoD policy, failure to meet grooming standards can lead to premature separation from military service. Civilian providers who are aware that the DoD’s policies on shaving and waivers have changed dramatically can discuss these implications when evaluating or counseling patients with a history of or risk for PFB. Previously published guidelines for service members seeking a shaving waiver for PFB are listed in eTable 1.10,16-23 The current changes, which remove various accommodations that previously had been introduced, are detailed in eTable 2.7-9,15,24


The grooming policy changes, particularly in the Army and Marines, require de novo waivers, which are likely to increase health care costs as measured in time and dollars. Each waiver cycle involves medical evaluation, documentation, and chain-of-command review. The cumulative work of these recurring requirements becomes considerable when scaled across the force.
As the military’s grooming policies evolve, ongoing evaluation of their effects on service members and unit readiness remains important. Continued data collection, transparent communication, and collaboration among military institutions and health care providers may help ensure that future policy updates maintain operational standards while also supporting the health and well-being of the force.
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
- Department of the Air Force. Air Force Instruction 1-1: Air Forcestandards. August 18, 2023. Accessed November 14, 2025. https://static.e-publishing.af.mil/production/1/af_cc/publication/afi1-1/afi1-1.pdf
- Department of the Air Force. Air Force Instruction 1-2: Commander’s responsibilities. May 8, 2014. Accessed November 14, 2025. https://www.af.mil/Portals/1/documents/csaf/afi1_2.pdf
- Tshudy MT, Cho S. Pseudofolliculitis barbae in the US military, a review. Mil Med. 2021;186:e52-e57. doi:10.1093/milmed/usaa243
- Uniform Code of Military Justice. 892. Article 92. Failure to obey order or regulation. Accessed November 14, 2025. https://ucmj.us/892-article-92-failure-to-obey-order-or-regulation/
- The White House. Restoring America’s fighting force. The White House Newsletter. January 27, 2025. Accessed November 14, 2025. https://www.whitehouse.gov/presidential-actions/2025/01/restoring-americas-fighting-force/
- Nava V. Hegseth orders review of US military standards, including grooming, after they were loosened under Biden. New York Post. March 12, 2025. Accessed November 14, 2025. https://nypost.com/2025/03/12/us-news/hegseth-orders-review-of-us-military-standards-including-grooming/
- Secretary of Defense. Grooming standards for facial hair. Memorandum for senior Pentagon leadership, commanders of the combatant commands, defense agency and DoD field activity directors. August 20, 2025. Accessed November 14, 2025. https://media.defense.gov/2025/Sep/15/2003799859/-1/-1/1/GROOMING-STANDARDS-FOR-FACIAL-HAIR.PDF
- Driscoll D. Army Directive 2025-13 (Facial Hair Grooming Standards). Secretary of the Army. July 7, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44307-ARMY_DIR_2025-13-000-WEB-1.pdf
- US Marine Corps. MARADMIN 124/25: uniform and grooming standards for medical conditions. March 13, 2025. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/4119098/uniform-and-grooming-standards-for-medical-conditions/
- United States Navy uniform regulations NAVPERS 15665J. MyNavy HR. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/References/US-Navy-Uniforms/Uniform-Regulations/
- Novelly T. Medical beard waivers nearly double in Air Force and Space Force in just 3 years. Military.com. April 8, 2024. Accessed November 17, 2025. https://www.military.com/daily-news/2024/04/08/medical-beard-waivers-nearly-double-air-force-and-space-force-just-3-years.html
- Slayton N. Medical shaving waivers could soon get you kicked out of the Army. Task & Purpose. June 28, 2025. Accessed November 17, 2025. https://taskandpurpose.com/military-life/army-medical-shaving-waivers-separation/
- Keller E. Razor bumps can now get you kicked out of the marines. Black men will likely suffer the most. The Independent. May 27, 2025. Accessed November 17, 2025. https://www.the-independent.com/news/world/americas/us-politics/marines-grooming-shaving-waiver-black-men-b2758653.html
- Defense Health Agency. 2.3.2.4.8. Laser therapy for pseudofolliculitis barbae (PFB) of the face and neck. In: TRICARE Operations Manual 6010-59.M. April 1, 2015. Revised May 15, 2024. Accessed November 17, 2025. https://manuals.health.mil/pages/DisplayManualHtmlFile/2024-06-10/AsOf/TO15/C17S3.html
- Degoes JJ. Medical guidance for shaving protocols. Department of the Air Force. Accessed November 17, 2025. https://www.af.mil/Portals/1/documents/2025SAF/Tab_4_Medical_Guidance_for_Shaving_Profiles.pdf
- Department of the Army. Army Regulation 670-1. Uniform and insignia: wear and appearance of Army uniforms and insignia.January 26, 2021. Accessed November 14, 2025. https://cdn.shopify.com/s/files/1/0468/8107/9449/files/ARN30302-AR_670-1-26-JAN-2021.pdf?v=1615263762
- Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 14, 2025. https://api.army.mil/e2/c/downloads/2025/09/29/89dfa985/tb-med-287-jul2025.pdf
- DeFilippi GR. Department of the Air Force guidance memorandum to DAFI 36-2903, dress and personal appearance of Department of the Air Force personnel. Department of the Air Force. July 11, 2025. Accessed November 17, 2025. https://static.e-publishing.af.mil/production/1/af_a1/publication/dafi36-2903/dafi36-2903.pdf
- Miller RI. Air Force guidance memorandum to AFI44-102, Medical Care Management. Office of the Surgeon General. September 5, 2023. Accessed November 17, 2025. https://milreg.com/File.aspx?id=3068
- Department of the Navy. BUPERS Instruction 1000.22C: management of Navy uniformed personnel diagnosed with pseudofolliculitis barbae (PFB) update. Published March 2022. Accessed November 17, 2025. https://www.mynavyhr.navy.mil/Portals/55/Messages/NAVADMIN/NAV2022/NAV22064txt?ver=bc2HUJnvp6q1y2E5vOSp-g%3D%3D
- Headquarters, US Marine Corps. Marine Corps uniform regulations. May 1, 2018. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137
- US Marine Corps. Advance notification of change to MCO 6310.1C (pseudofolliculitis barbae), MCO 1900.16 CH2 (Marine Corps Retirement and Separation Manual), and MCO 1040.31 (Enlisted Retention and Career Development Program. January 21, 2022. Accessed November 17, 2025. https://www.marines.mil/News/Messages/Messages-Display/Article/2907104/advance-notification-of-change-to-mco-63101c-pseudofolliculitis-barbae-mco-1900/#:~:text=No%20Marine%20shall%20be%20processed,4
- Commandant of the Marine Corps. Marine Corps order 6310.1C. Pseudofolliculitis barbae. Department of the Navy. October 9, 2012. Accessed November 17, 2025. https://www.marines.mil/portals/1/Publications/MCO%206310.1C.pdf
- Headquarters, Department of the Army. TB MED 287. Pseudofolliculitis of the beard and acne keloidalis nuchae. July 16, 2025. Accessed November 17, 2025. https://armypubs.army.mil/epubs/DR_pubs/DR_a/ARN44381-TB_MED_287-000-WEB-1.pdf
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Military Grooming Policy Changes Affecting Service Members With Pseudofolliculitis Barbae
Practice Points
- Revised US Department of Defense grooming policies eliminate permanent shaving waivers and limit medical waivers for pseudofolliculitis barbae (PFB) to no more than 1 year, after which administrative separation may be initiated if grooming standards cannot be met.
- These changes impose increased administrative and clinical demands on service members, military medical personnel, and commanders, requiring recurrent evaluation, documentation, and approvals for temporary shaving waivers.
- Civilian dermatologists should be aware of these policy changes and their potential career implications to appropriately counsel active-duty personnel and prospective military recruits.
- Laser hair removal may see increased utilization as a treatment option for service members for whom conservative management fails.
Noncompete Agreements and Their Impact on the Medical Landscape
In April 2024, the Federal Trade Commission (FTC) issued a nationwide rule to ban most employee noncompete agreements, including many used in health care1; however, that rule never took effect. In August 2024, a federal district court ruled that the FTC had exceeded its statutory authority and blocked the ban,2 and subsequent litigation and agency actions followed. On September 5, 2025, the FTC formally moved to accede to vacatur—in other words, it will not enforce the rule and backed away from defending it on appeal.3 As of December 2025, there is no active federal ban on physician noncompetes. The obligations of the physician employee are dictated by state law and the precise language of the contract that is signed.
In this article, we discuss the historical origins of noncompetes, employer and physician perspectives, and the downstream consequences for patient continuity, access, and health care costs.
Background
The concept of noncompete agreements is not new—this legal principle dates back several centuries, but it was not until several hundred years later, between the 1950s and 1980s, that noncompete agreements became routine in physician contracts. This trend emerged, at least in part, from the growing commoditization of medicine, the expansion of hospital infrastructure, and the rise of physicians employed by entities rather than owning a private practice. Medical practices, hospitals, and increasingly large private groups began using noncompete agreements to prevent physicians from leaving and establishing competing practices nearby. Since then, noncompetes have remained a contentious issue within both the legal system and the broader physician-employer relationship.
Employer vs Employee Perspective
From the employer’s perspective, health care systems and medical groups argue that noncompete agreements are necessary to protect legitimate business interests, citing physician training, established patient relationships, and proprietary information gained from employment with that entity as supporting reasons. Additionally, employers maintain that recouping the cost of recruitment and onboarding investments as well as sustaining continuity of care within the organization should take precedence. On occasion, health care systems will invest time and financial resources in recruiting physicians, provide administrative and clinical support, and integrate new employees into established referral pathways and patient populations. In this view, noncompetes serve as a tool to ensure stability within the health care system, discouraging abrupt departures that could fracture patient care or lead to unfair competition using institutional resources. While these arguments hold merit in certain cases, many physicians do not receive employer-funded education or training beyond what is required in residency and fellowship. As a result, the financial justifications for noncompetes often are overstated; on the contrary, the cost of a “buy-out” or the financial barrier imposed by a noncompete clause can amount to a considerable portion of a physician’s annual salary—sometimes multiple times that amount—creating an imbalance that favors the employer and limits professional mobility.
When a physician is prohibited from practicing in a specific area after leaving an employer, a complex web of adverse consequences can arise, impacting both the physician and the patients they serve. Physician mobility and career choice become restricted, effectively constraining the physicians’ livelihood and ability to provide for themselves and their dependents; in single-earner physician families, this can have devastating financial consequences. These limitations contribute to growing burnout and dissatisfaction within the medical profession, which already is facing unprecedented levels of stress and physician workforce shortages.4
Effect on Patients
When a physician is forced to relocate to a new geographic region because of a noncompete clause, their patients can experience substantial disruptions in care. Access to medical services may be affected, leading to longer wait-times and fewer available appointments, especially in areas that already have a shortage of providers. Patients may lose longstanding relationships with doctors who know their medical histories, which can interrupt treatment plans and increase the risk of complications. Those with chronic illnesses, complex conditions, or time-sensitive treatments are particularly vulnerable to adverse outcomes. Many patients must travel farther—sometimes out of their insurance network—to find replacement care, increasing both financial and logistical burdens. These abrupt transitions also can raise health care costs due to emergency department use, inefficient handoffs, and higher incidence of morbidity/mortality.5 Noncompete restrictions often prevent physicians from informing patients where they are relocating, creating confusion and fragmentation of care. As a result, trust in the health care system may decline when patients perceive that business agreements are being prioritized above their wellbeing. The impact may be even more severe in rural or underserved communities where alternative providers are scarce.
Final Thoughts
In recent years, noncompete agreements in health care have come under intensified scrutiny for their potential to stifle physician mobility, reduce competition, and inflate health care costs by limiting where and how physicians can practice. The trajectory of noncompetes in physician employment reflects broader shifts in how medicine is structured and delivered in the United States. In the latter half of the 20th century, what began as a centuries-old legal concept became a standard feature of physician employment contracts. That evolution largely was driven by the corporatization of medicine and large hospital group/private equity employment of physicians. As these agreements proliferated, public policy questions emerged: What does restricting a physician’s mobility do to patient access? To competition in provider markets? To the cost and availability of care? To the current epidemic of physician burnout?
These questions moved from the legal sidelines to center stage in the 2020s, when the FTC sought to tackle noncompetes across the entire economy—physicians included—on the theory they suppressed labor mobility, entrepreneurship, and competition. In February 2020, the American Medical Association submitted comments to the FTC on the utility of noncompete agreements in employee contracts stating that they restrict competition, can disrupt continuity of care, and may limit access to care.6 Although the FTC’s regulatory attempt in April 2024 provoked strong policy signals, it was challenged and ultimately blocked. Rather than a clear federal prohibition, the outcome is a more incremental state-based shift in rules governing physician noncompetes. For physicians today, this means more awareness and more leverage, but also more complexity. Whether a noncompete will be enforceable depends heavily on the state, the wording of the contract, the structure of the employer, and the specialty. From a negotiation standpoint, physicians need more guidance and awareness on the exact ramifications of their employee contract. For newly minted physicians, many of whom enter the workforce with considerable training debt, the priority often is securing employment to work toward financial stability, building a family, or both; however, all physicians should press for shorter durations, tighter geographic limits, narrower scopes of service, clear buy-out options, and explicit patient-continuity protections. Better yet, physicians can exercise the right of refusal to any noncompete clause at all. Becoming involved with a local medical organization or foundation can provide immense support, both in reviewing contracts as well as learning how to become advocates for physicians in this environment. As more physicians stand together to protect both practice autonomy and the right to quality care, we all become closer to rediscovering the beauty and fulfillment in the purest form of medicine.
- Federal Trade Commission. FTC announces rule banning noncompetes. April 23, 2024. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-announces-rule-banning-noncompetes
- US Chamber of Commerce. Ryan LLC v FTC. August 20, 2024. Accessed December 1, 2025. https://www.uschamber.com/cases/antitrust-and-competition-law/ryan-llc-v.-ftc
- Federal Trade Commission. Federal Trade Commission files to accede to vacatur of non-compete clause rule. September 5, 2025. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2025/09/federal-trade-commission-files-accede-vacatur-non-compete-clause-rule
- Marshall JJ, Ashwath ML, Jefferies JL, et al. Restrictive covenants and noncompete clauses for physicians. JACC Adv. 2023;2:100547.
- Sabety A. The value of relationships in healthcare. J Publich Economics. 2023;225:104927.
- American Medical Association. AMA provides comment to FTC on non-compete agreements. National Advocacy Update. February 14, 2020. Accessed November 25, 2025. https://www.ama-assn.org/health-care-advocacy/advocacy-update/feb-14-2020-national-advocacy-update
In April 2024, the Federal Trade Commission (FTC) issued a nationwide rule to ban most employee noncompete agreements, including many used in health care1; however, that rule never took effect. In August 2024, a federal district court ruled that the FTC had exceeded its statutory authority and blocked the ban,2 and subsequent litigation and agency actions followed. On September 5, 2025, the FTC formally moved to accede to vacatur—in other words, it will not enforce the rule and backed away from defending it on appeal.3 As of December 2025, there is no active federal ban on physician noncompetes. The obligations of the physician employee are dictated by state law and the precise language of the contract that is signed.
In this article, we discuss the historical origins of noncompetes, employer and physician perspectives, and the downstream consequences for patient continuity, access, and health care costs.
Background
The concept of noncompete agreements is not new—this legal principle dates back several centuries, but it was not until several hundred years later, between the 1950s and 1980s, that noncompete agreements became routine in physician contracts. This trend emerged, at least in part, from the growing commoditization of medicine, the expansion of hospital infrastructure, and the rise of physicians employed by entities rather than owning a private practice. Medical practices, hospitals, and increasingly large private groups began using noncompete agreements to prevent physicians from leaving and establishing competing practices nearby. Since then, noncompetes have remained a contentious issue within both the legal system and the broader physician-employer relationship.
Employer vs Employee Perspective
From the employer’s perspective, health care systems and medical groups argue that noncompete agreements are necessary to protect legitimate business interests, citing physician training, established patient relationships, and proprietary information gained from employment with that entity as supporting reasons. Additionally, employers maintain that recouping the cost of recruitment and onboarding investments as well as sustaining continuity of care within the organization should take precedence. On occasion, health care systems will invest time and financial resources in recruiting physicians, provide administrative and clinical support, and integrate new employees into established referral pathways and patient populations. In this view, noncompetes serve as a tool to ensure stability within the health care system, discouraging abrupt departures that could fracture patient care or lead to unfair competition using institutional resources. While these arguments hold merit in certain cases, many physicians do not receive employer-funded education or training beyond what is required in residency and fellowship. As a result, the financial justifications for noncompetes often are overstated; on the contrary, the cost of a “buy-out” or the financial barrier imposed by a noncompete clause can amount to a considerable portion of a physician’s annual salary—sometimes multiple times that amount—creating an imbalance that favors the employer and limits professional mobility.
When a physician is prohibited from practicing in a specific area after leaving an employer, a complex web of adverse consequences can arise, impacting both the physician and the patients they serve. Physician mobility and career choice become restricted, effectively constraining the physicians’ livelihood and ability to provide for themselves and their dependents; in single-earner physician families, this can have devastating financial consequences. These limitations contribute to growing burnout and dissatisfaction within the medical profession, which already is facing unprecedented levels of stress and physician workforce shortages.4
Effect on Patients
When a physician is forced to relocate to a new geographic region because of a noncompete clause, their patients can experience substantial disruptions in care. Access to medical services may be affected, leading to longer wait-times and fewer available appointments, especially in areas that already have a shortage of providers. Patients may lose longstanding relationships with doctors who know their medical histories, which can interrupt treatment plans and increase the risk of complications. Those with chronic illnesses, complex conditions, or time-sensitive treatments are particularly vulnerable to adverse outcomes. Many patients must travel farther—sometimes out of their insurance network—to find replacement care, increasing both financial and logistical burdens. These abrupt transitions also can raise health care costs due to emergency department use, inefficient handoffs, and higher incidence of morbidity/mortality.5 Noncompete restrictions often prevent physicians from informing patients where they are relocating, creating confusion and fragmentation of care. As a result, trust in the health care system may decline when patients perceive that business agreements are being prioritized above their wellbeing. The impact may be even more severe in rural or underserved communities where alternative providers are scarce.
Final Thoughts
In recent years, noncompete agreements in health care have come under intensified scrutiny for their potential to stifle physician mobility, reduce competition, and inflate health care costs by limiting where and how physicians can practice. The trajectory of noncompetes in physician employment reflects broader shifts in how medicine is structured and delivered in the United States. In the latter half of the 20th century, what began as a centuries-old legal concept became a standard feature of physician employment contracts. That evolution largely was driven by the corporatization of medicine and large hospital group/private equity employment of physicians. As these agreements proliferated, public policy questions emerged: What does restricting a physician’s mobility do to patient access? To competition in provider markets? To the cost and availability of care? To the current epidemic of physician burnout?
These questions moved from the legal sidelines to center stage in the 2020s, when the FTC sought to tackle noncompetes across the entire economy—physicians included—on the theory they suppressed labor mobility, entrepreneurship, and competition. In February 2020, the American Medical Association submitted comments to the FTC on the utility of noncompete agreements in employee contracts stating that they restrict competition, can disrupt continuity of care, and may limit access to care.6 Although the FTC’s regulatory attempt in April 2024 provoked strong policy signals, it was challenged and ultimately blocked. Rather than a clear federal prohibition, the outcome is a more incremental state-based shift in rules governing physician noncompetes. For physicians today, this means more awareness and more leverage, but also more complexity. Whether a noncompete will be enforceable depends heavily on the state, the wording of the contract, the structure of the employer, and the specialty. From a negotiation standpoint, physicians need more guidance and awareness on the exact ramifications of their employee contract. For newly minted physicians, many of whom enter the workforce with considerable training debt, the priority often is securing employment to work toward financial stability, building a family, or both; however, all physicians should press for shorter durations, tighter geographic limits, narrower scopes of service, clear buy-out options, and explicit patient-continuity protections. Better yet, physicians can exercise the right of refusal to any noncompete clause at all. Becoming involved with a local medical organization or foundation can provide immense support, both in reviewing contracts as well as learning how to become advocates for physicians in this environment. As more physicians stand together to protect both practice autonomy and the right to quality care, we all become closer to rediscovering the beauty and fulfillment in the purest form of medicine.
In April 2024, the Federal Trade Commission (FTC) issued a nationwide rule to ban most employee noncompete agreements, including many used in health care1; however, that rule never took effect. In August 2024, a federal district court ruled that the FTC had exceeded its statutory authority and blocked the ban,2 and subsequent litigation and agency actions followed. On September 5, 2025, the FTC formally moved to accede to vacatur—in other words, it will not enforce the rule and backed away from defending it on appeal.3 As of December 2025, there is no active federal ban on physician noncompetes. The obligations of the physician employee are dictated by state law and the precise language of the contract that is signed.
In this article, we discuss the historical origins of noncompetes, employer and physician perspectives, and the downstream consequences for patient continuity, access, and health care costs.
Background
The concept of noncompete agreements is not new—this legal principle dates back several centuries, but it was not until several hundred years later, between the 1950s and 1980s, that noncompete agreements became routine in physician contracts. This trend emerged, at least in part, from the growing commoditization of medicine, the expansion of hospital infrastructure, and the rise of physicians employed by entities rather than owning a private practice. Medical practices, hospitals, and increasingly large private groups began using noncompete agreements to prevent physicians from leaving and establishing competing practices nearby. Since then, noncompetes have remained a contentious issue within both the legal system and the broader physician-employer relationship.
Employer vs Employee Perspective
From the employer’s perspective, health care systems and medical groups argue that noncompete agreements are necessary to protect legitimate business interests, citing physician training, established patient relationships, and proprietary information gained from employment with that entity as supporting reasons. Additionally, employers maintain that recouping the cost of recruitment and onboarding investments as well as sustaining continuity of care within the organization should take precedence. On occasion, health care systems will invest time and financial resources in recruiting physicians, provide administrative and clinical support, and integrate new employees into established referral pathways and patient populations. In this view, noncompetes serve as a tool to ensure stability within the health care system, discouraging abrupt departures that could fracture patient care or lead to unfair competition using institutional resources. While these arguments hold merit in certain cases, many physicians do not receive employer-funded education or training beyond what is required in residency and fellowship. As a result, the financial justifications for noncompetes often are overstated; on the contrary, the cost of a “buy-out” or the financial barrier imposed by a noncompete clause can amount to a considerable portion of a physician’s annual salary—sometimes multiple times that amount—creating an imbalance that favors the employer and limits professional mobility.
When a physician is prohibited from practicing in a specific area after leaving an employer, a complex web of adverse consequences can arise, impacting both the physician and the patients they serve. Physician mobility and career choice become restricted, effectively constraining the physicians’ livelihood and ability to provide for themselves and their dependents; in single-earner physician families, this can have devastating financial consequences. These limitations contribute to growing burnout and dissatisfaction within the medical profession, which already is facing unprecedented levels of stress and physician workforce shortages.4
Effect on Patients
When a physician is forced to relocate to a new geographic region because of a noncompete clause, their patients can experience substantial disruptions in care. Access to medical services may be affected, leading to longer wait-times and fewer available appointments, especially in areas that already have a shortage of providers. Patients may lose longstanding relationships with doctors who know their medical histories, which can interrupt treatment plans and increase the risk of complications. Those with chronic illnesses, complex conditions, or time-sensitive treatments are particularly vulnerable to adverse outcomes. Many patients must travel farther—sometimes out of their insurance network—to find replacement care, increasing both financial and logistical burdens. These abrupt transitions also can raise health care costs due to emergency department use, inefficient handoffs, and higher incidence of morbidity/mortality.5 Noncompete restrictions often prevent physicians from informing patients where they are relocating, creating confusion and fragmentation of care. As a result, trust in the health care system may decline when patients perceive that business agreements are being prioritized above their wellbeing. The impact may be even more severe in rural or underserved communities where alternative providers are scarce.
Final Thoughts
In recent years, noncompete agreements in health care have come under intensified scrutiny for their potential to stifle physician mobility, reduce competition, and inflate health care costs by limiting where and how physicians can practice. The trajectory of noncompetes in physician employment reflects broader shifts in how medicine is structured and delivered in the United States. In the latter half of the 20th century, what began as a centuries-old legal concept became a standard feature of physician employment contracts. That evolution largely was driven by the corporatization of medicine and large hospital group/private equity employment of physicians. As these agreements proliferated, public policy questions emerged: What does restricting a physician’s mobility do to patient access? To competition in provider markets? To the cost and availability of care? To the current epidemic of physician burnout?
These questions moved from the legal sidelines to center stage in the 2020s, when the FTC sought to tackle noncompetes across the entire economy—physicians included—on the theory they suppressed labor mobility, entrepreneurship, and competition. In February 2020, the American Medical Association submitted comments to the FTC on the utility of noncompete agreements in employee contracts stating that they restrict competition, can disrupt continuity of care, and may limit access to care.6 Although the FTC’s regulatory attempt in April 2024 provoked strong policy signals, it was challenged and ultimately blocked. Rather than a clear federal prohibition, the outcome is a more incremental state-based shift in rules governing physician noncompetes. For physicians today, this means more awareness and more leverage, but also more complexity. Whether a noncompete will be enforceable depends heavily on the state, the wording of the contract, the structure of the employer, and the specialty. From a negotiation standpoint, physicians need more guidance and awareness on the exact ramifications of their employee contract. For newly minted physicians, many of whom enter the workforce with considerable training debt, the priority often is securing employment to work toward financial stability, building a family, or both; however, all physicians should press for shorter durations, tighter geographic limits, narrower scopes of service, clear buy-out options, and explicit patient-continuity protections. Better yet, physicians can exercise the right of refusal to any noncompete clause at all. Becoming involved with a local medical organization or foundation can provide immense support, both in reviewing contracts as well as learning how to become advocates for physicians in this environment. As more physicians stand together to protect both practice autonomy and the right to quality care, we all become closer to rediscovering the beauty and fulfillment in the purest form of medicine.
- Federal Trade Commission. FTC announces rule banning noncompetes. April 23, 2024. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-announces-rule-banning-noncompetes
- US Chamber of Commerce. Ryan LLC v FTC. August 20, 2024. Accessed December 1, 2025. https://www.uschamber.com/cases/antitrust-and-competition-law/ryan-llc-v.-ftc
- Federal Trade Commission. Federal Trade Commission files to accede to vacatur of non-compete clause rule. September 5, 2025. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2025/09/federal-trade-commission-files-accede-vacatur-non-compete-clause-rule
- Marshall JJ, Ashwath ML, Jefferies JL, et al. Restrictive covenants and noncompete clauses for physicians. JACC Adv. 2023;2:100547.
- Sabety A. The value of relationships in healthcare. J Publich Economics. 2023;225:104927.
- American Medical Association. AMA provides comment to FTC on non-compete agreements. National Advocacy Update. February 14, 2020. Accessed November 25, 2025. https://www.ama-assn.org/health-care-advocacy/advocacy-update/feb-14-2020-national-advocacy-update
- Federal Trade Commission. FTC announces rule banning noncompetes. April 23, 2024. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2024/04/ftc-announces-rule-banning-noncompetes
- US Chamber of Commerce. Ryan LLC v FTC. August 20, 2024. Accessed December 1, 2025. https://www.uschamber.com/cases/antitrust-and-competition-law/ryan-llc-v.-ftc
- Federal Trade Commission. Federal Trade Commission files to accede to vacatur of non-compete clause rule. September 5, 2025. Accessed December 1, 2025. https://www.ftc.gov/news-events/news/press-releases/2025/09/federal-trade-commission-files-accede-vacatur-non-compete-clause-rule
- Marshall JJ, Ashwath ML, Jefferies JL, et al. Restrictive covenants and noncompete clauses for physicians. JACC Adv. 2023;2:100547.
- Sabety A. The value of relationships in healthcare. J Publich Economics. 2023;225:104927.
- American Medical Association. AMA provides comment to FTC on non-compete agreements. National Advocacy Update. February 14, 2020. Accessed November 25, 2025. https://www.ama-assn.org/health-care-advocacy/advocacy-update/feb-14-2020-national-advocacy-update
PRACTICE POINTS
- There is no active federal ban on physician noncompete agreements as of late 2025.
- Physician noncompetes have expanded alongside the corporatization of medicine but raise serious concerns about physician mobility, burnout, workforce shortages, and patient access to care, particularly in underserved areas.
- Physicians should critically evaluate noncompetes prior to signing an agreement, advocating for narrower limits or refusal altogether to protect professional autonomy, continuity of care, and patient welfare.
Illuminating the Role of Visible Light in Dermatology
Illuminating the Role of Visible Light in Dermatology
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
Visible light is part of the electromagnetic spectrum and is confined to a range of 400 to 700 nm. Visible light phototherapy can be delivered across various wavelengths within this spectrum, with most research focusing on blue light (BL)(400-500 nm) and red light (RL)(600-700 nm). Blue light commonly is used to treat acne as well as actinic keratosis and other inflammatory disorders,1,2 while RL largely targets signs of skin aging and fibrosis.2,3 Because of its shorter wavelength, the clinically meaningful skin penetration of BL reaches up to1 mm and is confined to the epidermis; in contrast, RL can access the dermal adnexa due to its penetration depth of more than 2 mm.4 Therapeutically, visible light can be utilized alone (eg, photobiomodulation [PBM]) or in combination with a photosensitizing agent (eg, photodynamic therapy [PDT]).5,6
Our laboratory’s prior research has contributed to a greater understanding of the safety profile of visible light at various wavelengths.1,3 Specifically, our work has shown that BL (417 nm [range, 412-422 nm]) and RL (633 nm [range, 627-639 nm]) demonstrated no evidence of DNA damage—via no formation of cyclobutane pyrimidine dimers and/or 6-4 photoproducts, the hallmark photolesions caused by UV exposure—in human dermal fibroblasts following visible light exposure at all fluences tested.1,3 This evidence reinforces the safety of visible light at clinically relevant wavelengths, supporting its integration into dermatologic practice. In this editorial, we highlight the key clinical applications of PBM and PDT and outline safety considerations for visible light-based therapies in dermatologic practice.
Photobiomodulation
Photobiomodulation is a noninvasive treatment in which low-level lasers or light-emitting diodes deliver photons from a nonionizing light source to endogenous photoreceptors, primarily cytochrome C oxidase.7-9 On the visible light spectrum, PBM primarily encompasses RL.7-9 Photoactivation leads to production of reactive oxygen species as well as mitochondrial alterations, with resulting modulation of cellular activity.7-9 Upregulation of cellular activity generally occurs at lower fluences (ie, energy delivered per unit area) of light, whereas higher fluences cause downregulation of cellular activity.5
Recent consensus guidelines, established with expert colleagues, define additional key parameters that are crucial to optimizing PBM treatment, including distance from the light source, area of the light beam, wavelength, length of treatment time, and number of treatments.5 Understanding the effects of different parameter combinations is essential for clinicians to select the best treatment regimen for each patient. Our laboratory has conducted National Institutes of Health–funded phase 1 and phase 2 clinical trials to determine the safety and efficacy of red-light PBM.10-13 Additionally, we completed several pilot phase 2 clinical studies with commercially available light-emitting diode face masks using PBM technology, which demonstrated a favorable safety profile and high patient satisfaction across multiple self-reported measures.14,15 These findings highlight PBM as a reliable and well-tolerated therapeutic approach that can be administered in clinical settings or by patients at home.
Adverse effects of PBM therapy generally are mild and transient, most commonly manifesting as slight irritation and erythema.5 Overall, PBM is widely regarded as safe with a favorable and nontoxic profile across treatment settings. Growing evidence supports the role of PBM in managing wound healing, acne, alopecia, and skin aging, among other dermatologic concerns.8
Photodynamic Therapy
Photodynamic therapy is a noninvasive procedure during which a photosensitizer—typically 5-aminolevulinic acid (5-ALA) or a derivative, methyl aminolevulinate—reacts with a light source and oxygen, resulting in reactive oxygen species.6,16 This reaction ultimately triggers targeted cellular destruction of the intended lesional skin but with negligible effects on adjacent nonlesional tissue.6 The efficacy of PDT is determined by several parameters, including composition and concentration of the photosensitizer, photosensitizer incubation temperature, and incubation time with the photosensitizer. Methyl aminolevulinate is a lipophilic molecule and may promote greater skin penetration and cellular uptake than 5-ALA, which is a hydrophilic molecule.6
Our research further demonstrated that apoptosis increases in a dose- and temperature-dependent manner following 5-ALA exposure, both in cutaneous and mucosal squamous cell carcinoma cells and in human dermal fibroblasts.17,18 Our mechanistic insights have clinical relevance, as evidenced by an independent pilot study demonstrating that temperature-modulated PDT significantly improved actinic keratosis lesion clearance rates (P<.0001).19 Additionally, we determined that even short periods of incubation with 5-ALA (ie, 15-30 minutes) result in statistically significant increases in apoptosis (P<.05).20 Thus, these findings highlight that the choice of photosensitizing agent and the administration parameters are critical in determining PDT efficacy as well as the need to optimize clinical protocols.
Photodynamic therapy also has demonstrated general clinical and genotoxic safety, with the most common potential adverse events limited to temporary inflammation, erythema, and discomfort.21 A study in murine skin and human keratinocytes revealed that 5-ALA PDT had a photoprotective effect against previous irradiation with UVB (a known inducer of DNA damage) via removal of cyclobutane pyrimidine dimers.22 Thus, PDT has been recognized as a safe and effective therapeutic modality with broad applications in dermatology, including treatment of actinic keratosis and nonmelanoma skin cancers.16
Clinical Safety, Photoprotection, and Precautions
While visible light has shown substantial therapeutic potential in dermatology, there are several safety measures and precautions to be aware of. Visible light constitutes approximately 44% of the solar output; therefore, precautions against both UV and visible light are recommended for the general population.23 Cumulative exposure to visible light has been shown to trigger melanogenesis, resulting in persistent erythema, hyperpigmentation, and uneven skin tones across all Fitzpatrick skin types.24 Individuals with skin of color are more photosensitive to visible light due to increased baseline melanin levels.24 Similarly, patients with pigmentary conditions such as melasma and postinflammatory hyperpigmentation may experience worsening of their dermatologic symptoms due to underlying visible light photosensitivity.25
Patients undergoing PBM or PDT could benefit from visible light protection. The primary form of photoprotection against visible light is tinted sunscreen, which contains iron oxides and titanium dioxide.26 Iron (III) oxide is capable of blocking nearly all visible light damage.26 Use of physical barriers such as wavelength-specific sunglasses and wide-brimmed hats also is important for preventing photodamage from visible light.26
Final Thoughts
Visible light has a role in the treatment of a variety of skin conditions, including actinic keratosis, nonmelanoma skin cancers, acne, wound healing, skin fibrosis, and photodamage. Photobiomodulation and PDT represent 2 noninvasive phototherapeutic options that utilize visible light to enact cellular changes necessary to improve skin health. Integrating visible light phototherapy into standard clinical practice is important for enhancing patient outcomes. Clinicians should remain mindful of the rare pigmentary risks associated with visible light therapy devices. Future research should prioritize optimization of standardized protocols and expansion of clinical indications for visible light phototherapy.
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
- Kabakova M, Wang J, Stolyar J, et al. Visible blue light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2025;18:E202400510. doi:10.1002/jbio.202400510
- Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145-163. doi:10.2147/CCID.S35334
- Wang JY, Austin E, Jagdeo J. Visible red light does not induce DNA damage in human dermal fibroblasts. J Biophotonics. 2022;15:E202200023. doi:10.1002/jbio.202200023
- Opel DR, Hagstrom E, Pace AK, et al. Light-emitting diodes: a brief review and clinical experience. J Clin Aesthet Dermatol. 2015;8:36-44.
- Maghfour J, Mineroff J, Ozog DM, et al. Evidence-based consensus on the clinical application of photobiomodulation. J Am Acad Dermatol. 2025;93:429-443. doi:10.1016/j.jaad.2025.04.031
- Ozog DM, Rkein AM, Fabi SG, et al. Photodynamic therapy: a clinical consensus guide. Dermatol Surg. 2016;42:804-827. doi:10.1097/DSS.0000000000000800
- Maghfour J, Ozog DM, Mineroff J, et al. Photobiomodulation CME part I: overview and mechanism of action. J Am Acad Dermatol. 2024;91:793-802. doi:10.1016/j.jaad.2023.10.073
- Mineroff J, Maghfour J, Ozog DM, et al. Photobiomodulation CME part II: clinical applications in dermatology. J Am Acad Dermatol. 2024;91:805-815. doi:10.1016/j.jaad.2023.10.074
- Mamalis A, Siegel D, Jagdeo J. Visible red light emitting diode photobiomodulation for skin fibrosis: key molecular pathways. Curr Dermatol Rep. 2016;5:121-128. doi:10.1007/s13671-016-0141-x
- Kurtti A, Nguyen JK, Weedon J, et al. Light emitting diode-red light for reduction of post-surgical scarring: results from a dose-ranging, split-face, randomized controlled trial. J Biophotonics. 2021;14:E202100073. doi:10.1002/jbio.202100073
- Nguyen JK, Weedon J, Jakus J, et al. A dose-ranging, parallel group, split-face, single-blind phase II study of light emitting diode-red light (LED-RL) for skin scarring prevention: study protocol for a randomized controlled trial. Trials. 2019;20:432. doi:10.1186/s13063-019-3546-6
- Ho D, Kraeva E, Wun T, et al. A single-blind, dose escalation, phase I study of high-fluence light-emitting diode-red light (LED-RL) on human skin: study protocol for a randomized controlled trial. Trials. 2016;17:385. doi:10.1186/s13063-016-1518-7
- Wang EB, Kaur R, Nguyen J, et al. A single-blind, dose-escalation, phase I study of high-fluence light-emitting diode-red light on Caucasian non-Hispanic skin: study protocol for a randomized controlled trial. Trials. 2019;20:177. doi:10.1186/s13063-019-3278-7
- Wang JY, Kabakova M, Patel P, et al. Outstanding user reported satisfaction for light emitting diodes under-eye rejuvenation. Arch Dermatol Res. 2024;316:511. doi:10.1007/s00403-024-03254-z
- Mineroff J, Austin E, Feit E, et al. Male facial rejuvenation using a combination 633, 830, and 1072 nm LED face mask. Arch Dermatol Res. 2023;315:2605-2611. doi:10.1007/s00403-023-02663-w
- Wang JY, Zeitouni N, Austin E, et al. Photodynamic therapy: clinical applications in dermatology. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2024.12.050
- Austin E, Koo E, Jagdeo J. Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells. Sci Rep. 2018;8:12599. doi:10.1038/s41598-018-30908-6
- Mamalis A, Koo E, Sckisel GD, et al. Temperature-dependent impact of thermal aminolaevulinic acid photodynamic therapy on apoptosis and reactive oxygen species generation in human dermal fibroblasts. Br J Dermatol. 2016;175:512-519. doi:10.1111/bjd.14509
- Willey A, Anderson RR, Sakamoto FH. Temperature-modulated photodynamic therapy for the treatment of actinic keratosis on the extremities: a pilot study. Dermatol Surg. 2014;40:1094-1102. doi:10.1097/01.DSS.0000452662.69539.57
- Koo E, Austin E, Mamalis A, et al. Efficacy of ultra short sub-30 minute incubation of 5-aminolevulinic acid photodynamic therapy in vitro. Lasers Surg Med. 2017;49:592-598. doi:10.1002/lsm.22648
- Austin E, Wang JY, Ozog DM, et al. Photodynamic therapy: overview and mechanism of action. J Am Acad Dermatol. Published online February 20, 2025. doi:10.1016/j.jaad.2025.02.037
- Hua H, Cheng JW, Bu WB, et al. 5-aminolaevulinic acid-based photodynamic therapy inhibits ultraviolet B-induced skin photodamage. Int J Biol Sci. 2019;15:2100-2109. doi:10.7150/ijbs.31583
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132:1901-1907. doi:10.1038/jid.2011.476
- Austin E, Geisler AN, Nguyen J, et al. Visible light. part I: properties and cutaneous effects of visible light. J Am Acad Dermatol. 2021;84:1219-1231. doi:10.1016/j.jaad.2021.02.048
- Fatima S, Braunberger T, Mohammad TF, et al. The role of sunscreen in melasma and postinflammatory hyperpigmentation. Indian J Dermatol. 2020;65:5-10. doi:10.4103/ijd.IJD_295_18
- Geisler AN, Austin E, Nguyen J, et al. Visible light. part II: photoprotection against visible and ultraviolet light. J Am Acad Dermatol. 2021;84:1233-1244. doi:10.1016/j.jaad.2020.11.074
Illuminating the Role of Visible Light in Dermatology
Illuminating the Role of Visible Light in Dermatology
Dermatology on Duty: Pathways to a Career in Military Medicine
Dermatology on Duty: Pathways to a Career in Military Medicine
Serving those who serve has been one of the most meaningful parts of my career. A career in military medicine offers dermatologists not only a chance to practice within a unique and diverse patient population but also an opportunity to contribute to something larger than themselves. Whether working with active-duty service members and their families within the Military Health System (MHS) or caring for veterans through the Department of Veterans Affairs (VA), the experience can be both enriching and rewarding. This article will explore the various pathways available to dermatologists to serve military communities, whether they are at the start of their careers or are looking for a change of pace within their established practice.
Care Pathways for Military and Veterans
To care for uniformed service members, their families, and retired personnel, dermatologists typically serve within the MHS—a global, integrated network of military hospitals and clinics dedicated to delivering health care to this population.1 TRICARE is the health insurance program that covers those eligible for care within the system, including active-duty and retired service members.2 In this context, it is important to clarify what the term retired actually means, as it differs from the term veteran when it comes to accessing health care options, and these terms frequently are conflated. A retired service member is an individual who completed at least 20 years of active-duty service or who has been medically retired because of a condition or injury incurred while on active duty.3 In contrast, a veteran may not have completed 20 years of service but has separated honorably after serving at least 24 continuous months.4 Veterans typically receive care through the VA system.5
Serving on Active Duty
In general, there are 2 main pathways to serve as a dermatologist within the MHS. The first is to commission in the military and serve on active duty. Most often, this pathway begins with a premedical student applying to medical school. Those considering military service typically explore scholarship programs such as the Health Professions Scholarship Program (HPSP)(https://www.medicineandthemilitary.com/applying-and-what-to-expect/medical-school-programs/hpsp) or the Health Services Collegiate Program (HSCP), or they apply to the Uniformed Services University of the Health Sciences (USU)(https://www.usuhs.edu/about). The HPSP and HSCP programs financially support medical students training at civilian medical schools, though in different ways—the HPSP covers tuition and fees, while the HSCP provides a salary during training but does not cover tuition.6 In contrast, students of USU attend the nation’s only military medical school, serving in uniform for 4 years while earning the pay and benefits of a junior officer in their respective service branch. Any premedical student considering the HPSP, HSCP or USU routes for service must meet the commissioning standards of their chosen branch—Army, Navy, or Air Force—and enter service as an officer before beginning medical school.
While direct commission prior to medical school is the most common route to active-duty service, board-certified dermatologists also can join a military branch later through what is called Direct Accession or Direct Commission; for example, the Navy offers a Residency to Direct Accession program, which commissions residents in their final year of training to join the Navy upon graduation. In some cases, commissioning at this stage includes a bonus of up to $600,000 in exchange for a 4-year active-duty commitment.7 The Army and Air Force offer similar direct commission programs, though specific incentives vary.8 Interested residents or practitioners can contact a local recruiting office within their branch of interest to learn more. Direct accession is open at many points in a dermatologist’s career—after residency, after fellowship, or even as an established civilian practitioner—and the initial commissioning rank and bonus generally reflect one’s level of experience.
Serving as a Civilian
Outside of uniformed service, dermatologists can find opportunities to provide care for active-duty service members, veterans, and military families through employment as General Schedule (GS) employees. The GS is a role classification and pay system that covers most federal employees in professional, administrative, and technical positions (eg, physicians). The GS system classifies most of these employees based on the complexity, responsibility, and qualifications required for their role.9 Such positions often are at the highest level of the GS pay scale, reflecting the expertise and years of education required to become a dermatologist, though pay varies by location and experience. In contrast, physicians employed through the VA system are classified as Title 38 federal employees, governed by a different pay structure and regulatory framework under the US Code of Federal Regulations.10 These regulations govern the hiring, retention, and firing guidelines for VA physicians, which differ from those of GS physicians. A full explanation is outside of the scope of this article, however.
Final Thoughts
In summary, uniformed or federal service as a dermatologist offers a meaningful and impactful way to give back to those who have served our country. Opportunities exist throughout the United States for dermatologists interested in serving within the MHS or VA. The most transparent and up-to-date resource for identifying open positions in both large metropolitan areas and smaller communities is USAJOBS.gov. While financial compensation may not always match that of private practice, the intangible benefits are considerable—stable employment, comprehensive benefits, malpractice coverage, and secure retirement, among others. There is something deeply fulfilling about using one’s medical skills in service of a larger mission. The relationships built with service members, the sense of shared purpose, and the opportunity to contribute to the readiness and well-being of those who serve all make this career path profoundly rewarding. For dermatologists seeking a practice that combines professional growth with purpose and patriotism, military medicine offers a truly special calling.
- Military Health System. Elements of the military health system. Accessed October 11, 2025. https://www.health.mil/About-MHS/MHS-Elements
- TRICARE. Plans and eligibility. Accessed October 11, 2025. https://tricare.mil/Plans/Eligibility
- Military Benefit. TRICARE for retirees. Accessed October 11, 2025. https://www.militarybenefit.org/get-educated/tricareforretirees/
- US Department of Veterans Affairs. Eligibility for VA health care. Accessed October 11, 2025. https://www.va.gov/health-care/eligibility/
- US Department of Veterans Affairs. VA priority groups. Accessed October 11, 2025. https://www.va.gov/health-care/eligibility/priority-groups/
- Navy Medicine. Health Professions Scholarship Program (HPSP) and Financial Assistance Program (FAP). Accessed October 12, 2025. https://www.med.navy.mil/Accessions/Health-Professions-Scholarship-Program-HPSP-and-Financial-Assistance-Program-FAP/
- US Navy. Navy Medicine R2DA program. Accessed October 12, 2025. https://www.navy.com/navy-medicine
- US Army Medical Department. Student programs. Accessed October 12, 2025. https://goamedd.com/student-programs
- US Office of Personnel Management. General Schedule. Accessed October 12, 2025. https://www.opm.gov/policy-data-oversight/pay-leave/pay-systems/general-schedule/
- Pines Federal Employment Attorneys. Title 38 employees: medical professionals. Accessed October 12, 2025. https://www.pinesfederal.com/va-federal-employees/title-38-employees-medical-professionals/
Serving those who serve has been one of the most meaningful parts of my career. A career in military medicine offers dermatologists not only a chance to practice within a unique and diverse patient population but also an opportunity to contribute to something larger than themselves. Whether working with active-duty service members and their families within the Military Health System (MHS) or caring for veterans through the Department of Veterans Affairs (VA), the experience can be both enriching and rewarding. This article will explore the various pathways available to dermatologists to serve military communities, whether they are at the start of their careers or are looking for a change of pace within their established practice.
Care Pathways for Military and Veterans
To care for uniformed service members, their families, and retired personnel, dermatologists typically serve within the MHS—a global, integrated network of military hospitals and clinics dedicated to delivering health care to this population.1 TRICARE is the health insurance program that covers those eligible for care within the system, including active-duty and retired service members.2 In this context, it is important to clarify what the term retired actually means, as it differs from the term veteran when it comes to accessing health care options, and these terms frequently are conflated. A retired service member is an individual who completed at least 20 years of active-duty service or who has been medically retired because of a condition or injury incurred while on active duty.3 In contrast, a veteran may not have completed 20 years of service but has separated honorably after serving at least 24 continuous months.4 Veterans typically receive care through the VA system.5
Serving on Active Duty
In general, there are 2 main pathways to serve as a dermatologist within the MHS. The first is to commission in the military and serve on active duty. Most often, this pathway begins with a premedical student applying to medical school. Those considering military service typically explore scholarship programs such as the Health Professions Scholarship Program (HPSP)(https://www.medicineandthemilitary.com/applying-and-what-to-expect/medical-school-programs/hpsp) or the Health Services Collegiate Program (HSCP), or they apply to the Uniformed Services University of the Health Sciences (USU)(https://www.usuhs.edu/about). The HPSP and HSCP programs financially support medical students training at civilian medical schools, though in different ways—the HPSP covers tuition and fees, while the HSCP provides a salary during training but does not cover tuition.6 In contrast, students of USU attend the nation’s only military medical school, serving in uniform for 4 years while earning the pay and benefits of a junior officer in their respective service branch. Any premedical student considering the HPSP, HSCP or USU routes for service must meet the commissioning standards of their chosen branch—Army, Navy, or Air Force—and enter service as an officer before beginning medical school.
While direct commission prior to medical school is the most common route to active-duty service, board-certified dermatologists also can join a military branch later through what is called Direct Accession or Direct Commission; for example, the Navy offers a Residency to Direct Accession program, which commissions residents in their final year of training to join the Navy upon graduation. In some cases, commissioning at this stage includes a bonus of up to $600,000 in exchange for a 4-year active-duty commitment.7 The Army and Air Force offer similar direct commission programs, though specific incentives vary.8 Interested residents or practitioners can contact a local recruiting office within their branch of interest to learn more. Direct accession is open at many points in a dermatologist’s career—after residency, after fellowship, or even as an established civilian practitioner—and the initial commissioning rank and bonus generally reflect one’s level of experience.
Serving as a Civilian
Outside of uniformed service, dermatologists can find opportunities to provide care for active-duty service members, veterans, and military families through employment as General Schedule (GS) employees. The GS is a role classification and pay system that covers most federal employees in professional, administrative, and technical positions (eg, physicians). The GS system classifies most of these employees based on the complexity, responsibility, and qualifications required for their role.9 Such positions often are at the highest level of the GS pay scale, reflecting the expertise and years of education required to become a dermatologist, though pay varies by location and experience. In contrast, physicians employed through the VA system are classified as Title 38 federal employees, governed by a different pay structure and regulatory framework under the US Code of Federal Regulations.10 These regulations govern the hiring, retention, and firing guidelines for VA physicians, which differ from those of GS physicians. A full explanation is outside of the scope of this article, however.
Final Thoughts
In summary, uniformed or federal service as a dermatologist offers a meaningful and impactful way to give back to those who have served our country. Opportunities exist throughout the United States for dermatologists interested in serving within the MHS or VA. The most transparent and up-to-date resource for identifying open positions in both large metropolitan areas and smaller communities is USAJOBS.gov. While financial compensation may not always match that of private practice, the intangible benefits are considerable—stable employment, comprehensive benefits, malpractice coverage, and secure retirement, among others. There is something deeply fulfilling about using one’s medical skills in service of a larger mission. The relationships built with service members, the sense of shared purpose, and the opportunity to contribute to the readiness and well-being of those who serve all make this career path profoundly rewarding. For dermatologists seeking a practice that combines professional growth with purpose and patriotism, military medicine offers a truly special calling.
Serving those who serve has been one of the most meaningful parts of my career. A career in military medicine offers dermatologists not only a chance to practice within a unique and diverse patient population but also an opportunity to contribute to something larger than themselves. Whether working with active-duty service members and their families within the Military Health System (MHS) or caring for veterans through the Department of Veterans Affairs (VA), the experience can be both enriching and rewarding. This article will explore the various pathways available to dermatologists to serve military communities, whether they are at the start of their careers or are looking for a change of pace within their established practice.
Care Pathways for Military and Veterans
To care for uniformed service members, their families, and retired personnel, dermatologists typically serve within the MHS—a global, integrated network of military hospitals and clinics dedicated to delivering health care to this population.1 TRICARE is the health insurance program that covers those eligible for care within the system, including active-duty and retired service members.2 In this context, it is important to clarify what the term retired actually means, as it differs from the term veteran when it comes to accessing health care options, and these terms frequently are conflated. A retired service member is an individual who completed at least 20 years of active-duty service or who has been medically retired because of a condition or injury incurred while on active duty.3 In contrast, a veteran may not have completed 20 years of service but has separated honorably after serving at least 24 continuous months.4 Veterans typically receive care through the VA system.5
Serving on Active Duty
In general, there are 2 main pathways to serve as a dermatologist within the MHS. The first is to commission in the military and serve on active duty. Most often, this pathway begins with a premedical student applying to medical school. Those considering military service typically explore scholarship programs such as the Health Professions Scholarship Program (HPSP)(https://www.medicineandthemilitary.com/applying-and-what-to-expect/medical-school-programs/hpsp) or the Health Services Collegiate Program (HSCP), or they apply to the Uniformed Services University of the Health Sciences (USU)(https://www.usuhs.edu/about). The HPSP and HSCP programs financially support medical students training at civilian medical schools, though in different ways—the HPSP covers tuition and fees, while the HSCP provides a salary during training but does not cover tuition.6 In contrast, students of USU attend the nation’s only military medical school, serving in uniform for 4 years while earning the pay and benefits of a junior officer in their respective service branch. Any premedical student considering the HPSP, HSCP or USU routes for service must meet the commissioning standards of their chosen branch—Army, Navy, or Air Force—and enter service as an officer before beginning medical school.
While direct commission prior to medical school is the most common route to active-duty service, board-certified dermatologists also can join a military branch later through what is called Direct Accession or Direct Commission; for example, the Navy offers a Residency to Direct Accession program, which commissions residents in their final year of training to join the Navy upon graduation. In some cases, commissioning at this stage includes a bonus of up to $600,000 in exchange for a 4-year active-duty commitment.7 The Army and Air Force offer similar direct commission programs, though specific incentives vary.8 Interested residents or practitioners can contact a local recruiting office within their branch of interest to learn more. Direct accession is open at many points in a dermatologist’s career—after residency, after fellowship, or even as an established civilian practitioner—and the initial commissioning rank and bonus generally reflect one’s level of experience.
Serving as a Civilian
Outside of uniformed service, dermatologists can find opportunities to provide care for active-duty service members, veterans, and military families through employment as General Schedule (GS) employees. The GS is a role classification and pay system that covers most federal employees in professional, administrative, and technical positions (eg, physicians). The GS system classifies most of these employees based on the complexity, responsibility, and qualifications required for their role.9 Such positions often are at the highest level of the GS pay scale, reflecting the expertise and years of education required to become a dermatologist, though pay varies by location and experience. In contrast, physicians employed through the VA system are classified as Title 38 federal employees, governed by a different pay structure and regulatory framework under the US Code of Federal Regulations.10 These regulations govern the hiring, retention, and firing guidelines for VA physicians, which differ from those of GS physicians. A full explanation is outside of the scope of this article, however.
Final Thoughts
In summary, uniformed or federal service as a dermatologist offers a meaningful and impactful way to give back to those who have served our country. Opportunities exist throughout the United States for dermatologists interested in serving within the MHS or VA. The most transparent and up-to-date resource for identifying open positions in both large metropolitan areas and smaller communities is USAJOBS.gov. While financial compensation may not always match that of private practice, the intangible benefits are considerable—stable employment, comprehensive benefits, malpractice coverage, and secure retirement, among others. There is something deeply fulfilling about using one’s medical skills in service of a larger mission. The relationships built with service members, the sense of shared purpose, and the opportunity to contribute to the readiness and well-being of those who serve all make this career path profoundly rewarding. For dermatologists seeking a practice that combines professional growth with purpose and patriotism, military medicine offers a truly special calling.
- Military Health System. Elements of the military health system. Accessed October 11, 2025. https://www.health.mil/About-MHS/MHS-Elements
- TRICARE. Plans and eligibility. Accessed October 11, 2025. https://tricare.mil/Plans/Eligibility
- Military Benefit. TRICARE for retirees. Accessed October 11, 2025. https://www.militarybenefit.org/get-educated/tricareforretirees/
- US Department of Veterans Affairs. Eligibility for VA health care. Accessed October 11, 2025. https://www.va.gov/health-care/eligibility/
- US Department of Veterans Affairs. VA priority groups. Accessed October 11, 2025. https://www.va.gov/health-care/eligibility/priority-groups/
- Navy Medicine. Health Professions Scholarship Program (HPSP) and Financial Assistance Program (FAP). Accessed October 12, 2025. https://www.med.navy.mil/Accessions/Health-Professions-Scholarship-Program-HPSP-and-Financial-Assistance-Program-FAP/
- US Navy. Navy Medicine R2DA program. Accessed October 12, 2025. https://www.navy.com/navy-medicine
- US Army Medical Department. Student programs. Accessed October 12, 2025. https://goamedd.com/student-programs
- US Office of Personnel Management. General Schedule. Accessed October 12, 2025. https://www.opm.gov/policy-data-oversight/pay-leave/pay-systems/general-schedule/
- Pines Federal Employment Attorneys. Title 38 employees: medical professionals. Accessed October 12, 2025. https://www.pinesfederal.com/va-federal-employees/title-38-employees-medical-professionals/
- Military Health System. Elements of the military health system. Accessed October 11, 2025. https://www.health.mil/About-MHS/MHS-Elements
- TRICARE. Plans and eligibility. Accessed October 11, 2025. https://tricare.mil/Plans/Eligibility
- Military Benefit. TRICARE for retirees. Accessed October 11, 2025. https://www.militarybenefit.org/get-educated/tricareforretirees/
- US Department of Veterans Affairs. Eligibility for VA health care. Accessed October 11, 2025. https://www.va.gov/health-care/eligibility/
- US Department of Veterans Affairs. VA priority groups. Accessed October 11, 2025. https://www.va.gov/health-care/eligibility/priority-groups/
- Navy Medicine. Health Professions Scholarship Program (HPSP) and Financial Assistance Program (FAP). Accessed October 12, 2025. https://www.med.navy.mil/Accessions/Health-Professions-Scholarship-Program-HPSP-and-Financial-Assistance-Program-FAP/
- US Navy. Navy Medicine R2DA program. Accessed October 12, 2025. https://www.navy.com/navy-medicine
- US Army Medical Department. Student programs. Accessed October 12, 2025. https://goamedd.com/student-programs
- US Office of Personnel Management. General Schedule. Accessed October 12, 2025. https://www.opm.gov/policy-data-oversight/pay-leave/pay-systems/general-schedule/
- Pines Federal Employment Attorneys. Title 38 employees: medical professionals. Accessed October 12, 2025. https://www.pinesfederal.com/va-federal-employees/title-38-employees-medical-professionals/
Dermatology on Duty: Pathways to a Career in Military Medicine
Dermatology on Duty: Pathways to a Career in Military Medicine
PRACTICE POINTS
- Dermatologists have diverse pathways to serve the military and veteran communities, either in uniform or as civilians.
- For those considering a military career, options include medical school scholarships or direct commission after residency.
- Those who prefer to remain civilians can find employment opportunities with the Military Heath System or the Department of Veterans Affairs that provide a way to care for this population without a service commitment.
The Habit of Curiosity: How Writing Shapes Clinical Thinking in Medical Training
The Habit of Curiosity: How Writing Shapes Clinical Thinking in Medical Training
I was accepted into my fellowship almost 1 year ago: major milestones on my curriculum vitae are now met, fellowship application materials are complete, and the stress of the match is long gone. At the start of my fellowship, I had 2 priorities: (1) to learn as much as I could about dermatologic surgery and (2) to be the best dad possible to my newborn son, Jay. However, most nights I still find myself up late editing a manuscript draft or chasing down references, long after the “need” to publish has passed. Recently, my wife asked me why—what’s left to prove?
I’ll be the first to admit it: early on, publishing felt almost purely transactional. Each project was little more than a line on an application or a way to stand out or meet a new mentor. I have reflected before on how easily that mindset can slip into a kind of research arms race, in which productivity overshadows purpose.1 This time, I wanted to explore the other side of that equation: the “why” behind it all.
I have learned that writing forces me to slow down and actually think about what I am seeing every day. It turns routine work into something I must understand well enough to explain. Even a small write-up can make me notice details I would otherwise skim past in clinic or surgery. These days, most of my projects start small: a case that taught me something, an observation that made me pause and think. Those seemingly small questions are what eventually grow into bigger ones. The clinical trial I am designing now did not begin as a grand plan—it started because I could not stop thinking about how we manage pain and analgesia after Mohs surgery. That curiosity, shaped by the experience of writing those earlier “smaller” papers, evolved into a study that might actually help improve patient care one day. Still, most of what I write will not revolutionize the field. It is not cutting-edge science or paradigm-shifting data; it is mostly modest analyses with a few interesting conclusions or surgical pearls that might cut down on a patient’s procedural time or save a dermatologist somewhere a few sutures. But it still feels worth doing.
While rotating with Dr. Anna Bar at Oregon Health & Science University, Portland, I noticed a poster hanging on the wall titled, “Top 10 Reasons Why Our Faculty Are Dedicated to Academics and Teaching,” based on the wisdom of Dr. Jane M. Grant-Kels.2 My favorite line on the poster reads, “Residents make us better by asking questions.” I think this philosophy is the main reason why I still write. Even though I am not a resident anymore, I am still asking questions. But if I had to sum up my “why” into a neat list, here is what it might look like:
Because asking questions keeps your brain wired for curiosity. Even small projects train us to remain curious, and this curiosity can mean the difference between just doing your job and continuing to evolve within it. As Dr. Rodolfo Neirotti reminds us, “Questions are useful tools—they open communication, improve understanding, and drive scientific research. In medicine, doing things without knowing why is risky.”3
Because the small stuff builds the culture. Dermatology is a small world. Even short case series, pearls, or “how we do it” pieces can shape how we practice. They may not change paradigms, but they can refine them. Over time, those small practical contributions become part of the field’s collective muscle memory.
Because it preserves perspective. Residency, fellowship, and early practice can blur together. A tiny project can become a timestamp of what you were learning or caring about at that specific moment. Years later, you may remember the case through the paper.
Because the act of writing is the point. Writing forces clarity. You cannot hide behind saying, “That’s just how I do things,” when you have to explain it to others. The discipline of organizing your thoughts sharpens your clinical reasoning and keeps you honest about what you actually know.
Because sometimes it is simply about participating. Publishing, even small pieces, is a way of staying in touch with your field. It says, “I’m still here. I’m still paying attention.”
I think about how Dr. Frederic Mohs developed the technique that now bears his name while he was still a medical student.4 He could have said, “I already made it into medical school. That’s enough.” But he did not. I guess my point is not that we are all on the verge of inventing something revolutionary; it is that innovation happens only when curiosity keeps moving us forward. So no, I do not write to check boxes anymore. I write because it keeps me curious, and I have realized that curiosity is a habit I never want to outgrow.
Or maybe it’s because Jay keeps me up at night, and I have nothing better to do.
- Jeha GM. A roadmap to research opportunities for dermatology residents. Cutis. 2024;114:E53-E56.
- Grant-Kels J. The gift that keeps on giving. UConn Health Dermatology. Accessed November 24, 2025. https://health.uconn.edu/dermatology/education/
- Neirotti RA. The importance of asking questions and doing things for a reason. Braz J Cardiovasc Surg. 2021;36:I-II.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011;29:135-139, vii.
I was accepted into my fellowship almost 1 year ago: major milestones on my curriculum vitae are now met, fellowship application materials are complete, and the stress of the match is long gone. At the start of my fellowship, I had 2 priorities: (1) to learn as much as I could about dermatologic surgery and (2) to be the best dad possible to my newborn son, Jay. However, most nights I still find myself up late editing a manuscript draft or chasing down references, long after the “need” to publish has passed. Recently, my wife asked me why—what’s left to prove?
I’ll be the first to admit it: early on, publishing felt almost purely transactional. Each project was little more than a line on an application or a way to stand out or meet a new mentor. I have reflected before on how easily that mindset can slip into a kind of research arms race, in which productivity overshadows purpose.1 This time, I wanted to explore the other side of that equation: the “why” behind it all.
I have learned that writing forces me to slow down and actually think about what I am seeing every day. It turns routine work into something I must understand well enough to explain. Even a small write-up can make me notice details I would otherwise skim past in clinic or surgery. These days, most of my projects start small: a case that taught me something, an observation that made me pause and think. Those seemingly small questions are what eventually grow into bigger ones. The clinical trial I am designing now did not begin as a grand plan—it started because I could not stop thinking about how we manage pain and analgesia after Mohs surgery. That curiosity, shaped by the experience of writing those earlier “smaller” papers, evolved into a study that might actually help improve patient care one day. Still, most of what I write will not revolutionize the field. It is not cutting-edge science or paradigm-shifting data; it is mostly modest analyses with a few interesting conclusions or surgical pearls that might cut down on a patient’s procedural time or save a dermatologist somewhere a few sutures. But it still feels worth doing.
While rotating with Dr. Anna Bar at Oregon Health & Science University, Portland, I noticed a poster hanging on the wall titled, “Top 10 Reasons Why Our Faculty Are Dedicated to Academics and Teaching,” based on the wisdom of Dr. Jane M. Grant-Kels.2 My favorite line on the poster reads, “Residents make us better by asking questions.” I think this philosophy is the main reason why I still write. Even though I am not a resident anymore, I am still asking questions. But if I had to sum up my “why” into a neat list, here is what it might look like:
Because asking questions keeps your brain wired for curiosity. Even small projects train us to remain curious, and this curiosity can mean the difference between just doing your job and continuing to evolve within it. As Dr. Rodolfo Neirotti reminds us, “Questions are useful tools—they open communication, improve understanding, and drive scientific research. In medicine, doing things without knowing why is risky.”3
Because the small stuff builds the culture. Dermatology is a small world. Even short case series, pearls, or “how we do it” pieces can shape how we practice. They may not change paradigms, but they can refine them. Over time, those small practical contributions become part of the field’s collective muscle memory.
Because it preserves perspective. Residency, fellowship, and early practice can blur together. A tiny project can become a timestamp of what you were learning or caring about at that specific moment. Years later, you may remember the case through the paper.
Because the act of writing is the point. Writing forces clarity. You cannot hide behind saying, “That’s just how I do things,” when you have to explain it to others. The discipline of organizing your thoughts sharpens your clinical reasoning and keeps you honest about what you actually know.
Because sometimes it is simply about participating. Publishing, even small pieces, is a way of staying in touch with your field. It says, “I’m still here. I’m still paying attention.”
I think about how Dr. Frederic Mohs developed the technique that now bears his name while he was still a medical student.4 He could have said, “I already made it into medical school. That’s enough.” But he did not. I guess my point is not that we are all on the verge of inventing something revolutionary; it is that innovation happens only when curiosity keeps moving us forward. So no, I do not write to check boxes anymore. I write because it keeps me curious, and I have realized that curiosity is a habit I never want to outgrow.
Or maybe it’s because Jay keeps me up at night, and I have nothing better to do.
I was accepted into my fellowship almost 1 year ago: major milestones on my curriculum vitae are now met, fellowship application materials are complete, and the stress of the match is long gone. At the start of my fellowship, I had 2 priorities: (1) to learn as much as I could about dermatologic surgery and (2) to be the best dad possible to my newborn son, Jay. However, most nights I still find myself up late editing a manuscript draft or chasing down references, long after the “need” to publish has passed. Recently, my wife asked me why—what’s left to prove?
I’ll be the first to admit it: early on, publishing felt almost purely transactional. Each project was little more than a line on an application or a way to stand out or meet a new mentor. I have reflected before on how easily that mindset can slip into a kind of research arms race, in which productivity overshadows purpose.1 This time, I wanted to explore the other side of that equation: the “why” behind it all.
I have learned that writing forces me to slow down and actually think about what I am seeing every day. It turns routine work into something I must understand well enough to explain. Even a small write-up can make me notice details I would otherwise skim past in clinic or surgery. These days, most of my projects start small: a case that taught me something, an observation that made me pause and think. Those seemingly small questions are what eventually grow into bigger ones. The clinical trial I am designing now did not begin as a grand plan—it started because I could not stop thinking about how we manage pain and analgesia after Mohs surgery. That curiosity, shaped by the experience of writing those earlier “smaller” papers, evolved into a study that might actually help improve patient care one day. Still, most of what I write will not revolutionize the field. It is not cutting-edge science or paradigm-shifting data; it is mostly modest analyses with a few interesting conclusions or surgical pearls that might cut down on a patient’s procedural time or save a dermatologist somewhere a few sutures. But it still feels worth doing.
While rotating with Dr. Anna Bar at Oregon Health & Science University, Portland, I noticed a poster hanging on the wall titled, “Top 10 Reasons Why Our Faculty Are Dedicated to Academics and Teaching,” based on the wisdom of Dr. Jane M. Grant-Kels.2 My favorite line on the poster reads, “Residents make us better by asking questions.” I think this philosophy is the main reason why I still write. Even though I am not a resident anymore, I am still asking questions. But if I had to sum up my “why” into a neat list, here is what it might look like:
Because asking questions keeps your brain wired for curiosity. Even small projects train us to remain curious, and this curiosity can mean the difference between just doing your job and continuing to evolve within it. As Dr. Rodolfo Neirotti reminds us, “Questions are useful tools—they open communication, improve understanding, and drive scientific research. In medicine, doing things without knowing why is risky.”3
Because the small stuff builds the culture. Dermatology is a small world. Even short case series, pearls, or “how we do it” pieces can shape how we practice. They may not change paradigms, but they can refine them. Over time, those small practical contributions become part of the field’s collective muscle memory.
Because it preserves perspective. Residency, fellowship, and early practice can blur together. A tiny project can become a timestamp of what you were learning or caring about at that specific moment. Years later, you may remember the case through the paper.
Because the act of writing is the point. Writing forces clarity. You cannot hide behind saying, “That’s just how I do things,” when you have to explain it to others. The discipline of organizing your thoughts sharpens your clinical reasoning and keeps you honest about what you actually know.
Because sometimes it is simply about participating. Publishing, even small pieces, is a way of staying in touch with your field. It says, “I’m still here. I’m still paying attention.”
I think about how Dr. Frederic Mohs developed the technique that now bears his name while he was still a medical student.4 He could have said, “I already made it into medical school. That’s enough.” But he did not. I guess my point is not that we are all on the verge of inventing something revolutionary; it is that innovation happens only when curiosity keeps moving us forward. So no, I do not write to check boxes anymore. I write because it keeps me curious, and I have realized that curiosity is a habit I never want to outgrow.
Or maybe it’s because Jay keeps me up at night, and I have nothing better to do.
- Jeha GM. A roadmap to research opportunities for dermatology residents. Cutis. 2024;114:E53-E56.
- Grant-Kels J. The gift that keeps on giving. UConn Health Dermatology. Accessed November 24, 2025. https://health.uconn.edu/dermatology/education/
- Neirotti RA. The importance of asking questions and doing things for a reason. Braz J Cardiovasc Surg. 2021;36:I-II.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011;29:135-139, vii.
- Jeha GM. A roadmap to research opportunities for dermatology residents. Cutis. 2024;114:E53-E56.
- Grant-Kels J. The gift that keeps on giving. UConn Health Dermatology. Accessed November 24, 2025. https://health.uconn.edu/dermatology/education/
- Neirotti RA. The importance of asking questions and doing things for a reason. Braz J Cardiovasc Surg. 2021;36:I-II.
- Trost LB, Bailin PL. History of Mohs surgery. Dermatol Clin. 2011;29:135-139, vii.
The Habit of Curiosity: How Writing Shapes Clinical Thinking in Medical Training
The Habit of Curiosity: How Writing Shapes Clinical Thinking in Medical Training
Practice Points
- Writing about everyday clinical experiences forces trainees to slow down, think more carefully, and better understand why they do what they do. Being able to write clearly about a clinical scenario reflects true understanding.
- The act of writing sharpens clinical judgment by requiring clarity, honesty, and reflection rather than relying on habit or routine.
- Writing fosters habits of curiosity that support continued professional growth and ongoing engagement with one’s field beyond formal training milestones.