User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
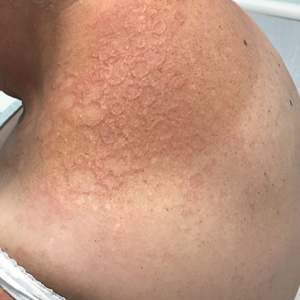
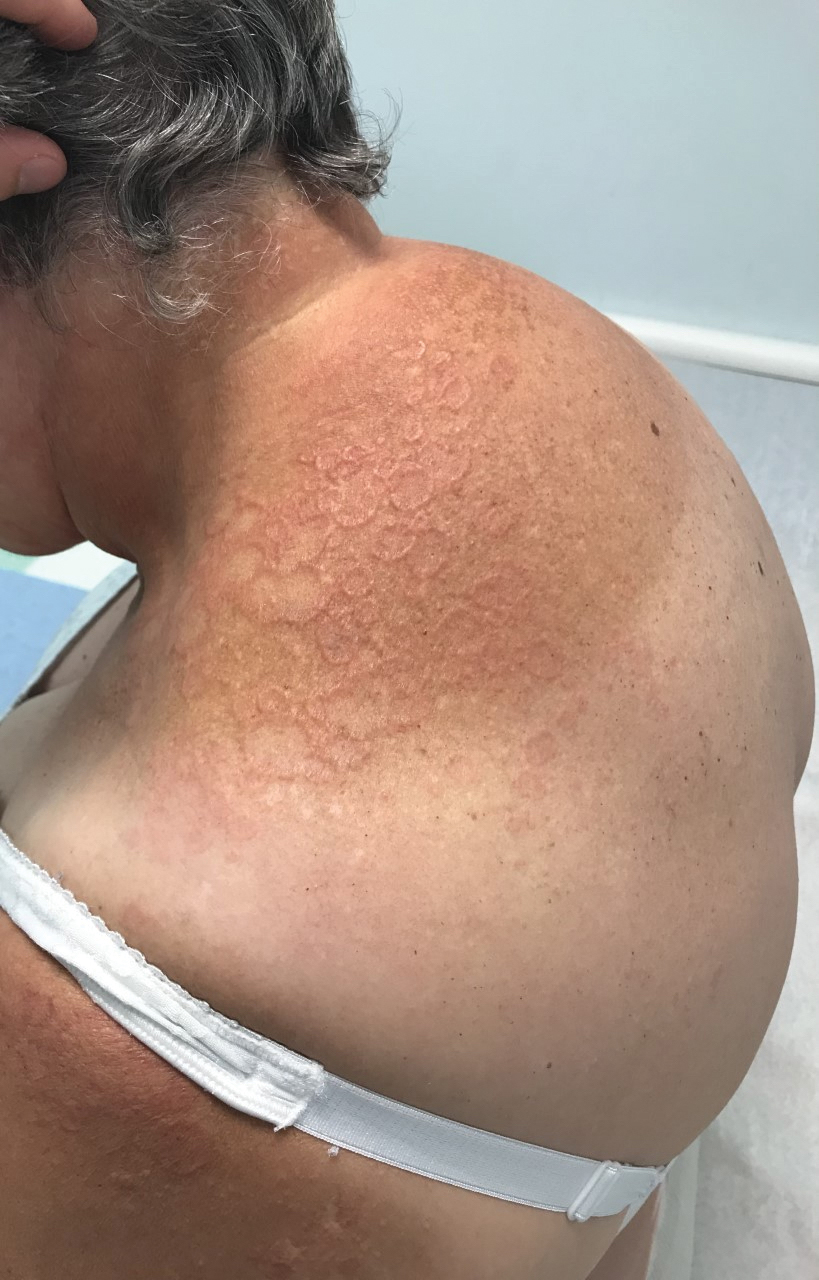
Annular Erythematous Plaques With Central Hypopigmentation on Sun-Exposed Skin
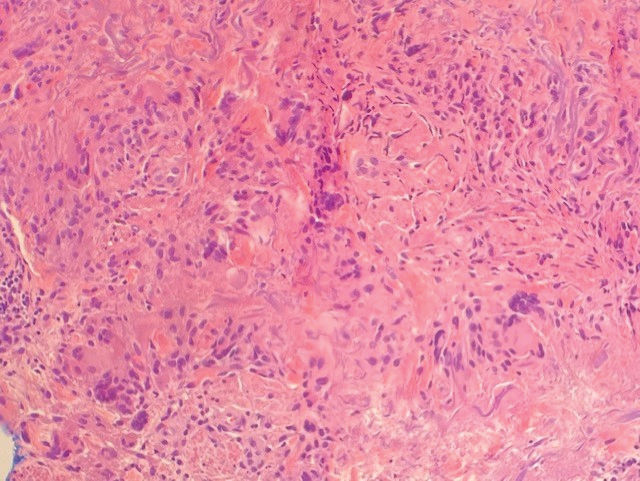
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.
Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.

Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
A biopsy showed a markedly elastotic dermis consisting of a palisading granulomatous inflammatory infiltrate and numerous multinucleated histiocytes (Figure). These histopathologic findings along with the clinical presentation confirmed a diagnosis of annular elastolytic granuloma (AEG). Treatment consisting of 3 months of oral minocycline, 2 months of oral doxycycline, and clobetasol ointment all failed. At that point, oral hydroxychloroquine was recommended. Our patient was lost to follow-up by dermatology, then subsequently was placed on hydroxychloroquine by rheumatology to treat both the osteoarthritis and AEG. A follow-up appointment with dermatology was planned for 3 months to monitor hydroxychloroquine treatment and monitor treatment progress; however, she did not follow-up or seek further treatment.

Annular elastolytic granuloma clinically is similar to granuloma annulare (GA), with both presenting as annular plaques surrounded by an elevated border.1 Although AEG clinically is distinct with hypopigmented atrophied plaque centers,2 a biopsy is required to confirm the lack of elastic tissue in zones of atrophy and the presence of multinucleated histiocytes.1,3 Lesions most commonly are seen clinically on sun-exposed areas in middle-aged White women; however, they rarely have been seen on frequently covered skin.4 Our case illustrates the striking photodistribution of AEG, especially on the posterior neck area. The clinical diagnoses of AEG, annular elastolytic giant cell granuloma, and GA in sun-exposed areas are synonymous and can be used interchangeably.5,6
Pathologies considered in the diagnosis of AEG include but are not limited to tinea corporis, annular lichen planus, erythema annulare centrifugum, and necrobiosis lipoidica. Scaling typically is absent in AEG, while tinea corporis presents with hyphae within the stratum corneum of the plaques.7 Papules along the periphery of annular lesions are more typical of annular lichen planus than AEG, and they tend to have a more purple hue.8 Erythema annulare centrifugum has annular erythematous plaques similar to those found in AEG but differs with scaling on the inner margins of these plaques. Histopathology presenting with a lymphocytic infiltrate surrounding vasculature and no indication of elastolytic degradation would further indicate a diagnosis of erythema annulare centrifugum.9 Histopathology showing necrobiosis, lipid depositions, and vascular wall thickenings is indicative of necrobiosis lipoidica.10
Similar to GA,11 the cause of AEG is idiopathic.2 Annular elastolytic granuloma and GA differ in the fact that elastin degradation is characteristic of AEG compared to collagen degradation in GA. It is suspected that elastin degradation in AEG patients is caused by an immune response triggering phagocytosis of elastin by multinucleated histiocytes.2 Actinic damage also is considered a possible cause of elastin fiber degradation in AEG.12 Granuloma annulare can be ruled out and the diagnosis of AEG confirmed with the absence of elastin fibers and mucin on pathology.13
Although there is no established first-line treatment of AEG, successful treatment has been achieved with antimalarial drugs paired with topical steroids.14 Treatment recommendations for AEG include minocycline, chloroquine, hydroxychloroquine, tranilast, and oral retinoids, as well as oral and topical steroids. In clinical cases where AEG occurs in the setting of a chronic disease such as diabetes mellitus, vascular occlusion, arthritis, or hypertension, treatment of underlying disease has been shown to resolve AEG symptoms.14
Although light therapy is not common for AEG, UV light radiation has demonstrated success in treating AEG.15,16 One study showed complete clearance of granulomatous papules after narrowband UVB treatment.15 Another study showed that 2 patients treated with psoralen plus UVA therapy reached complete clearance of AEG lasting at least 3 months after treatment.16
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
1. Lai JH, Murray SJ, Walsh NM. Evolution of granuloma annulare to mid-dermal elastolysis: report of a case and review of the literature. J Cutan Pathol. 2014;41:462-468. doi:10.1111/cup.12292 2. Klemke CD, Siebold D, Dippel E, et al. Generalised annular elastolytic giant cell granuloma. Dermatology. 2003;207:420-422. doi:10.1159/000074132 3. Limas C. The spectrum of primary cutaneous elastolytic granulomas and their distinction from granuloma annulare: a clinicopathological analysis. Histopathology. 2004;44:277-282. doi:10.1111/j.0309-0167.2004.01755.x 4. Revenga F, Rovira I, Pimentel J, et al. Annular elastolytic giant cell granuloma—actinic granuloma? Clin Exp Dermatol. 1996;21:51-53. 5. Hawryluk EB, Izikson L, English JC 3rd. Non-infectious granulomatous diseases of the skin and their associated systemic diseases: an evidence-based update to important clinical questions. Am J Clin Dermatol. 2010;11:171-181. doi:10.2165/11530080-000000000-00000 6. Berliner JG, Haemel A, LeBoit PE, et al. The sarcoidal variant of annular elastolytic granuloma. J Cutan Pathol. 2013;40:918-920. doi:10.1111/cup.12237 7. Pflederer RT, Ahmed S, Tonkovic-Capin V, et al. Annular polycyclic plaques on the chest and upper back [published online April 24, 2018]. JAAD Case Rep. 2018;4:405-407. doi:10.1016/j.jdcr.2017.07.022 8. Trayes KP, Savage K, Studdiford JS. Annular lesions: diagnosis and treatment. Am Fam Physician. 2018;98:283-291. 9. Weyers W, Diaz-Cascajo C, Weyers I. Erythema annulare centrifugum: results of a clinicopathologic study of 73 patients. Am J Dermatopathol. 2003;25:451-462. doi:10.1097/00000372-200312000-00001 10. Dowling GB, Jones EW. Atypical (annular) necrobiosis lipoidica of the face and scalp. a report of the clinical and histological features of 7 cases. Dermatologica. 1967;135:11-26. doi:10.1159/000254156 11. Piette EW, Rosenbach M. Granuloma annulare: pathogenesis, disease associations and triggers, and therapeutic options. J Am Acad Dermatol. 2016;75:467-479. doi:10.1016/j.jaad.2015 .03.055 12. O’Brien JP, Regan W. Actinically degenerate elastic tissue is the likely antigenic basis of actinic granuloma of the skin and of temporal arteritis [published correction appears in J Am Acad Dermatol. 2000; 42(1 pt 1):148]. J Am Acad Dermatol. 1999;40(2 pt 1):214-222. doi:10.1016/s0190-9622(99)70191-x 13. Rencic A, Nousari CH. Other rheumatologic diseases. In: Bolognia JL, Jorizzo JL, Rapini RP, et al, eds. Dermatology. 2nd ed. Elsevier Limited; 2008:600-601. 14. Burlando M, Herzum A, Cozzani E, et al. Can methotrexate be a successful treatment for unresponsive generalized annular elastolytic giant cell granuloma? case report and review of the literature. Dermatol Ther. 2021;34:E14705. doi:10.1111/dth.14705 15. Takata T, Ikeda M, Kodama H, et al. Regression of papular elastolytic giant cell granuloma using narrow-band UVB irradiation. Dermatology. 2006;212:77-79. doi:10.1159/000089028 16. Pérez-Pérez L, García-Gavín J, Allegue F, et al. Successful treatment of generalized elastolytic giant cell granuloma with psoralenultraviolet A. Photodermatol Photoimmunol Photomed. 2012;28:264-266. doi:10.1111/j.1600-0781.2012.00680.x
A 67-year-old White woman presented to our dermatology clinic with pruritic annular erythematous plaques with central hypopigmentation on the forearms, dorsal aspect of the hands, neck, and fingers of 3 to 4 months’ duration. The patient rated the severity of pruritus an 8 on a 10-point scale. A review of symptoms was positive for fatigue, joint pain, and headache. The patient had a history of type 2 diabetes mellitus, osteoarthritis, thyroid disease, and stage 3 renal failure. A punch biopsy from the left forearm was performed.
Limited treatment options exist for brittle nail syndrome
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
NEW ORLEANS – .
“The mainstay of treatment is irritant avoidance and moisturization,” Shari R. Lipner, MD, PhD, associate professor of clinical dermatology and director of the nail division at Weill Cornell Medicine, New York, said at the annual meeting of the American Academy of Dermatology. “This works well if patients are religious about doing it.”
Brittle nail syndrome affects about 20% of adults, she said, and is more common in females, particularly those older than age 50. Most cases are idiopathic, but some are secondary to dermatologic diseases including nail psoriasis and nail lichen planus, and systemic diseases such as hyperthyroidism and hypothyroidism. They are more common in patients in certain occupations such as carpentry. “The pathogenesis is poorly understood but is thought to be due to weakened intercellular keratinocyte bridges, decreased cholesterol sulphate in the nail plate, and reduced water content in the nail plate,” Dr. Lipner said.
Key clinical findings include onychoschizia (peeling of the nail plate), onychorrhexis (an increase in the longitudinal ridges and furrows, sometimes leading to splitting), and superficial granulation of keratin. Treatment involves general measures. “You want to treat the underlying cause and recommend that the patient avoid water and irritant exposure,” she said. Her general instructions for affected patients are to wear latex gloves for wet work and cotton gloves for dry work, avoid triclosan-based hand sanitizers, avoid nail cosmetics, minimize nail trauma, and foster moisturization.“It’s important to give these instructions verbally and in written form,” she said. “In our practice, we designed a QR code that links to our patient handout.”
According to Dr. Lipner, the promotion of vitamins and supplements such as biotin, vitamin D, amino acids, and chromium for treating brittle nail syndrome is rampant on the Internet and on social media, but no rigorously designed clinical trials have shown efficacy for any of them. “Very few people are deficient in biotin, except for those with inherited enzyme deficiencies,” and most people “can get all the biotin they need from a regular diet,” she said.
The initial rationale for using biotin for nails comes from the veterinary literature, she continued. In the 1940s, chickens with biotin deficiency developed fissures in their feet and parrot-like beaks. In the 1970s, pigs with biotin deficiency developed friable hooves, which was corrected with biotin supplementation. “By the 1980s it was standard practice to supplement the feet of pigs with biotin,” she said.
In a human trial from 1989, German researchers enrolled 71 patients with brittle nail syndrome who took oral biotin, 2.5 mg daily. Of the 45 patients evaluated, 41 (91%) showed improvement in firmness and hardness of the fingernails over the course of 5.5 months, but there was no good control group, Dr. Lipner said. In a follow-up study, the same German researchers used scanning electron microscopy to evaluate 22 patients with brittle nails who took oral biotin 2.5 mg daily and compared them with 10 patients with normal nails who did not take biotin. They found a 25% increase in nail plate thickness in the biotin group and onychoschizia resolved in 50% of patients who received biotin. “But again, there was no good control group,” Dr. Lipner said.
In a third study on the topic, researchers surveyed 46 patients who presented with onychorrhexis and/or onychoschizia on clinical exam and took 2.5 mg of biotin daily. Of the 35 survey respondents, 63% subjectively reported improvement in their nails at a mean of 2 months. “This is where we are today: There have been studies of only 80 patients that were done 25 years ago,” Dr. Lipner said. “That’s all of our evidence for biotin for the treatment of brittle nail syndrome.”
FDA warning about biotin
Additional cause for concern, she continued, is the safety communication issued by the FDA in 2017, stating that the use of biotin may interfere with certain lab tests such as thyroid tests and cardiac enzymes, in some cases leading to death. The safety communication was updated in 2019.
In 2018, Dr. Lipner and colleagues administered an anonymous survey to 447 patients at their clinic asking about their use of biotin supplements. Of the 447 patients, 34% reported current use of biotin. Among biotin users, 7% were aware of the FDA warning, 29% of respondents reported that it was recommended by either a primary care physician or a dermatologist, and 56% underwent laboratory testing while taking biotin. “It’s our duty to warn our patients about the evidence for biotin for treating brittle nails, and about this interference on laboratory tests,” Dr. Lipner said.
Other treatment options for brittle nail syndrome include two lacquers that are available by prescription. One contains hydroxypropyl chitosan, Equisetum arvense, and methylsulphonylmethane; the other contains 16% poly-ureaurethane, but has not been well studied. “These products can be very expensive if not covered by insurance,” Dr. Lipner said.
As an alternative, she recommends Nail Tek CITRA 2 Nail Strengthener, which is available for less than $10 from Walmart and other retailers.
Cyclosporine emulsion also has been studied for brittle nail syndrome, but results to date have been underwhelming. Dr. Lipner and colleagues are exploring the effect of platelet rich plasma for treating brittle nails on the premise that it will improve nail growth and promote healing, in a 16-week trial that has enrolled 10 patients and includes both a Physician Global Improvement Assessment (PGIA) and a Physician Global Assessment (PGA) score. “Our data is being analyzed by three independent nail experts, and we hope to report the findings next year,” she said.
Dr. Lipner reported having no disclosures relevant to her presentation.
AT AAD 2023
FDA Advisory panels consider easing isotretinoin requirements
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
for patients, pharmacies, and prescribers.
Isotretinoin, previously called Accutane, is marketed as Absorica, Absorica LD, Claravis, Amnesteem, Myorisan, and Zenatane.
In a joint meeting of the FDA’s Drug Safety and Risk Management Advisory Committee and Dermatologic and Ophthalmic Drugs Advisory Committee, experts addressed ways to improve the modified iPLEDGE Risk Evaluation and Mitigation Strategy (iPLEDGE REMS) for isotretinoin that caused chaos after its rollout at the end of 2021.
In January 2022, problems were multiplying with the program for clinicians, pharmacists, and patients, causing extensive delays and prescription denials. In response, the FDA said it would continue to meet with the Isotretinoin Products Manufacturers Group (IPMG) to resolve problems.
March 28 was the first day of a 2-day meeting addressing what can be done to reduce burden with the iPLEDGE REMS while maintaining safety and preventing fetal exposure to the drug.
Key areas of concern
The meeting focused on several key areas.
The 19-day lockout period
The lockout is a current restriction for patients who can become pregnant and do not pick up their first prescription of isotretinoin within the specified 7-day prescription window. Currently, those who miss the window must wait 19 days from the date of the first pregnancy test to take an additional pregnancy test to be eligible to receive the drug.
Lindsey Crist, PharmD, a risk management analyst for the FDA, who presented the FDA review committee’s analysis, acknowledged that the lockout period causes delays in treatment and adds frustration and costs.
She said it’s important to remember that the lockout applies only to the first prescription. “It’s intended as an additional layer of screening to detect pregnancy,” she said.
“At least 12 pregnancies have been identified during the 19-day lockout from March 2017–September of 2022,” she noted.
The FDA is looking to the advisory committee to provide recommendations on whether the lockout period should be changed.
Home testing
During the pandemic, iPLEDGE rules have been relaxed from having a pregnancy test done only at a Clinical Laboratory Improvement Amendments–certified laboratory and home pregnancy tests have been allowed. The question now is whether home tests should continue to be allowed.
Ms. Crist said that the FDA’s review committee recommends ending the allowance of home tests, citing insufficient data on use and the discovery of instances of falsification of pregnancy tests.
“One study at an academic medical center reviewed the medical records of 89 patients who used home pregnancy tests while taking isotretinoin during the public health emergency. It found that 15.7% submitted falsified pregnancy test results,” she said.
Ms. Crist added, however, that the review committee recommends allowing the tests to be done in a provider’s office as an alternative.
Documenting counseling patients who cannot get pregnant
Currently, this documentation must be done monthly, primarily to counsel patients against drug sharing or giving blood. Proposed changes include extending the intervals for attestation or eliminating it to reduce burden on clinicians.
IPMG representative Gregory Wedin, PharmD, pharmacovigilance and risk management director for Upsher-Smith Laboratories, said, “while we cannot support eliminating or extending the confirmation interval to a year, the [iPLEDGE] sponsors are agreeable [to] a 120-day confirmation interval.”
He said that while extending to 120 days would reduce burden on prescribers, it comes with risk in reducing oversight by a certified iPLEDGE prescriber and potentially increasing the risk for drug sharing.
“A patient may be more likely to share their drug with another person the further along with therapy they get as their condition improves,” Mr. Wedin said.
On March 29, the panel will hear more recommendations for and against modifications to iPLEDGE REMS and will vote on select modifications at the end of the meeting.
A version of this article first appeared on Medscape.com.
Commentary: IL-31 inhibitor, e-cigarettes, and upadacitinib in AD, April 2023
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
Good news! There's not a lot to say about this. Dupilumab is so easy. No blood work, no immunosuppression. Dupilumab is highly effective and very safe. It's safe enough for children as young as 6 months! It's so effective that if it is not working, I question my diagnosis (Could it be contact dermatitis or mycosis fungoides instead?) and whether the patient is taking the medication properly.
Boesjes and colleagues describe in Acta Dermato-Venereologica the Dutch experience with upadacitinib in patients who have not been successfully treated with dupilumab or baricitinib. Presumably, such patients, because treatment with dupilumab or baricitinib or both was unsuccessful, have very resistant atopic dermatitis (either due to strong genetic propensity or perhaps because they don't take their medications). Despite having such refractory disease, most patients did well on the treatment with rapid disease improvement. Upadacitinib didn't work for everyone, though. About 30% of the patients discontinued upadacitinib treatment due to ineffectiveness, adverse events, or both (8.5%, 14.9%, and 6.4%, respectively).
How much of that ineffectiveness was due to poor adherence to taking the treatment was not assessed. Upadacitinib is extraordinarily effective for atopic dermatitis. I didn't think I would ever see a drug more effective than dupilumab for atopic dermatitis, but a low dose of upadacitinib (15 mg/day) seems about twice as effective as dupilumab for complete clearing of atopic dermatitis. The higher dose of 30 mg may be 3.5 times as effective as dupiliumab at getting atopic dermatitis completely clear.1
I dislike the word significant. Significant is ambiguous. It could mean that an observed association would not be likely to occur by chance, or it could mean that an observed association is clinically meaningful. Smith and colleagues in "Association between electronic cigarette use and atopic dermatitis among United States adults" reported finding a "significant" association between e-cigarette use and atopic dermatitis. A total of 23% of 2119 e-cigarette users had atopic dermatitis vs 17.1% of 26,444 nonusers. Clearly, the observed association was statistically significant (the 6% difference was not likely to occur due to chance alone). Is the finding clinically meaningful? I don't think it would affect our practice in any way.
The authors made the point that the study doesn't tell us whether e-cigarette use causes atopic dermatitis or if atopic dermatitis causes people to smoke. I wonder if just being younger (or some other factor) might make people more likely to use e-cigarettes and more likely to have atopic dermatitis (assuming atopic dermatitis gradually subsides over time, a dogma that may not be true).
Kabashima and colleagues report on the efficacy of the interleukin (IL)–31 antagonist nemolizumab. IL-31 mediates itch and having a new drug to block IL-31 may be a great treatment for our itchy patients. In this study, patients who had greater itch reduction had greater improvement in eczema and in quality of life. I'm quite sure that reducing itch improves patients' quality of life. But when it comes to the itch and the inflammation, I'm not sure which comes first. Does controlling the itch make the inflammation better? Maybe. Does controlling inflammation make itch better? Certainly.
For atopic patients with inflammation, controlling that inflammation seems to me to be the best approach, and we don't need more new treatments to accomplish that. For those patients who have a lot of itch and little inflammation, an IL-31 antagonist may be a revolutionary addition to our treatment options.
Additional References
1. Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. doi: 10.1001/jamadermatol.2021.3023. Erratum in: JAMA Dermatol. 2022;158:219. doi: 10.1001/jamadermatol.2021.5451
JAK inhibitor ivarmacitinib shows efficacy for atopic dermatitis in a pivotal trial
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
NEW ORLEANS – The presented as a late-breaker at the annual meeting of the American Academy of Dermatology.
Two doses were studied in the placebo-controlled trial and both demonstrated “a favorable benefit-to-risk profile in patients with moderate to severe AD,” reported Yan Zhao, MD, a clinician and researcher in the department of dermatology, Peking University People’s Hospital, Beijing.
In the study, called QUARTZ3, 336 patients aged 12 and older at 51 sites in China and Canada were randomized to 4 mg once-daily ivarmacitinib, 8 mg once-daily QD ivarmacitinib, or placebo. The mean age of the population was 32 years and approximately one-third were female.
The mean duration of AD for participants was 10 years. The mean baseline Eczema Area and Severity Index (EASI) score was near 30. On the Investigator Global Assessment (IGA) tool, approximately 40% had a score of 4, which is the highest score on the scale and indicates severe disease. The remaining patients had an IGA score of 3.
The co-primary endpoints were change in IGA and EASI scores at 16 weeks, and both improved rapidly, showing statistical significance relative to placebo by 4 weeks with no plateauing effect at the end of the 16-week trial. By week 16, the proportion of patients with an EASI score of 75, signifying a 75% improvement, was 66%, 54%, and 22% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups (P < .001 versus placebo for both doses of active therapy), respectively.
The pattern of the IGA response was similar. By week 16, the proportion of patients achieving an IGA score of 0 (clear) or 1 (almost clear) was 42%, 36%, and 9% for the 8-mg dose of ivarmacitinib, 4-mg dose of ivarmacitinib, and placebo groups, respectively. The advantage of either dose over placebo was highly significant (P < .001) at 8, 12, and 16 weeks.
For the WI-NRS (Worst Itch – Numeric Rating Scale), the advantage of the 8-mg dose relative to placebo was significant (P < .001) at the 1-week evaluation. By 2 weeks, the 4-mg dose had gained the same degree of statistical significance relative to placebo. After week 4, when the maximum proportion of patients with a WI-NRS score ≤ 4 was reached (50%, 35%, and 10% in the 8-mg, 4-mg, and placebo groups), and the relative advantage of active treatment persisted until the end of the 16-week study.
Two scales were used to evaluate change in quality of life. On the DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure), improvements were again rapid and sustained. By week 4, improvement with the 8-mg dose was about fourfold greater (P < .001) than improvement with placebo for DLQI and about sixfold greater (P < .001) for POEM. For the 4-mg dose, the relative differences were approximately threefold and fourfold greater, and both were significant (P <.001).
There was no further gain in these quality-of-life scales from week 4 to week 16, but the advantages relative to placebo were generally sustained, Dr. Zhao reported.
Ivarmacitinib was safe and well-tolerated, according to Dr. Zhao. The proportion of patients with a treatment-emergent adverse event that led to drug discontinuation was numerically higher (5.4%) in the placebo group than in the 8-mg (3.6%) or 4-mg group (2.7%). Rates of infection in the three groups were similar, and there were no major adverse cardiovascular events (MACE) or thromboembolism observed in any group.
Ivarmacitinib, which has about a 10-fold greater selectivity for JAK1 than JAK2 and a more than 70-fold greater selectivity for JAK1 than JAK3, is being tested for rheumatoid arthritis, inflammatory bowel disease, and alopecia areata in addition to AD, Dr. Zhao said. She also reported that an application for new drug approval has been submitted in China. Efforts to pursue regulatory approval elsewhere are anticipated.
Currently, there are three JAK inhibitors licensed for the treatment of AD in the United States. Upadacitinib (Rinvoq) and abrocitinib (Cibinqo) are also once-daily oral JAK1-selective inhibitors. Regulatory approval for AD by the Food and Drug Administration was granted to both in early 2022 and both now have an indication for moderate to severe disease in patients ages 12 years and older.
In September 2021, the first U.S. approval of a drug in this class for AD was granted for a topical formulation of ruxolitinib (Opzelura), which has selectivity for both JAK1 and JAK2. The indication is for mild to moderate AD in patients aged 12 years and older.
In the phase 3 clinical trial that led to approval of abrocitinib for AD, the comparator groups included placebo and active treatment with 300 mg dupilumab administered subcutaneously every other week. The higher of two doses of abrocitinib (100 mg) was numerically superior to dupilumab in terms of EASI 75 response at week 12 and was statistically superior for relief of itch at week 2.
Relative to the first-generation JAK inhibitor tofacitinib (Xeljanz), both of the approved oral JAK inhibitors for AD, abrocitinib and upadacitinib, have greater JAK1-selectivity. However, selectivity for all JAK inhibitors is relative rather than absolute, according to a recent review article on oral JAK inhibitors for AD. Efficacy and safety are likely determined by relative inhibition of each of the four JAK enzymes (JAK1, JAK2, JAK3, and TYK2). Although JAK1 appears to be an important target for AD treatment, the clinical significance of the degree of selectivity among oral JAK inhibitors is not yet clear.
In an interview, the senior author of that review article, Emma Guttman-Yassky, MD, PhD, emphasized this point. She said there is no evidence and no basis on which to speculate that any one drug in this class is better than another for AD. Dr. Guttman-Yassky is a professor and system chair of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York.
“The efficacy [of ivarmacitinib] seems, in general, to be in line with other JAK inhibitors,” said Dr. Guttman-Yassky, who attended the late-breaker session during which these data were presented. Although she acknowledged that rapid control of pruritus is important clinically, she said the speed of itch relief as reported in the phase 3 ivarmacitinib trial does not distinguish it from other oral drugs in the class.
Shawn Kwatra, MD, director of the Johns Hopkins Itch Center, Johns Hopkins University, Baltimore, agreed.
“The rapid effects on itch of ivarmacitinib are consistent with those observed by the already approved JAK1-selective inhibitors abrocitinib and upadacitinib,” he said in an interview.
This suggests that head-to-head trials will be needed to draw any conclusions about the relative efficacy and safety of existing and emerging oral JAK inhibitors for AD.
Dr. Zhao has reported a financial relationship with Reistone Biopharma, which is developing ivarmacitinib and provided funding for the trial. Dr. Guttman-Yassky has reported financial relationships with more than 20 pharmaceutical companies, including companies that make JAK inhibitors. Dr. Kwatra has reported financial relationships with AbbVie, Aslan, Arcutis Biotherapeutics, Castle Biosciences, Celldex, Galderma, Genzada, Incyte, Johnson & Johnson, Leo Pharma, Novartis, Pfizer, Regeneron, and Sanofi.
A version of this article first appeared on Medscape.com.
AT AAD 2023
COVID-19 potentially induced adult-onset IgA vasculitis
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Plasma exchange successfully improved symptoms of immunoglobulin A vasculitis in an adult female patient who developed the condition after infection with COVID-19, according to a case report published in Cureus.
Immunoglobulin A (IgA) vasculitis can affect all ages, but is relatively rare in adults, and the etiology remains unclear, wrote Hassan Alwafi, MD, of Umm Al-Qura University, Makkah, Saudi Arabia, and colleagues.
COVID-19 has been associated with pulmonary and extrapulmonary complications, but , the authors wrote.
The authors described a case of a 41-year-old otherwise healthy Saudi Arabian woman who presented with an ascending rash on both lower extremities, along with arthralgia. Blood tests showed high blood urea nitrogen, creatinine, and inflammatory markers, and a negative immune panel. The patient had been infected with COVID-19 approximately 2 weeks before the onset of symptoms, but she was treated with supportive care and required no antiviral therapy of dexamethasone.
In addition, the patient’s urinalysis showed proteinuria and hematuria. After a kidney biopsy revealed additional abnormalities, the patient was started on intravenous methylprednisolone pulse therapy.
A few days after the initiation of therapy, the patient experienced nosebleeds and coughing up blood. After a chest x-ray showed bilateral pleural effusion, the patient was transferred to the ICU. The patient was started on intravenous piperacillin-tazobactam, and received two doses of intravenous immunoglobulin and plasma exchange after consultation with a nephrologist. Ultimately, the initial rash and other clinical symptoms improved, and the patient was discharged with a tapering schedule of oral prednisolone.
In this case, COVID-19 may have played a role in the development of IgA vasculitis, the authors said.
The authors also listed 21 cases of IgA vasculitis following COVID-19 infection, including 14 children and 7 adults. Of these, three cases had combined kidney and lung involvement, the two pediatric cases died from respiratory failure, while the adult case was successfully treated with steroid monotherapy.
“As COVID-19 is a novel disease and its pathogenic mechanism of causing IgA vasculitis is not well understood, every patient who is infected with or recently recovered from COVID-19 and presents with a skin rash or arthralgia should have baseline blood and urine tests done and should be treated promptly to avoid the emergence of irreversible consequences,” the authors wrote in their discussion.
Although case reports cannot prove a cause-and-effect link, the data from the cases in the current review suggest that COVID-19 infection may be an indirect trigger for IgA vasculitis, including cases associated with pulmonary renal syndrome, they said. However, more research is needed, especially on the efficacy of treatments in adults, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM CUREUS
Topical delgocitinib shows promise for chronic hand eczema, pivotal trial shows
NEW ORLEANS – , compared with those who received vehicle cream, results from a pivotal phase 3 trial showed.
“Chronic hand eczema is the most frequent chronic inflammatory disorder affecting the hands,” Robert Bissonnette, MD, a dermatologist who is founder and CEO of Innovaderm Research, said at the annual meeting of the American Academy of Dermatology, where the study was presented during a late-breaking research session. “It’s associated with pain, pruritus, and has a huge impact on quality of life,” and results with current topical treatments are often unsatisfactory, he noted.
Delgocitinib is an investigational topical pan-JAK inhibitor that inhibits activation of the JAK-STAT pathway and targets key mediators of chronic hand eczema. In a phase 2b dose-ranging trial, twice-daily treatment with delgocitinib cream demonstrated significantly greater efficacy, compared with the cream vehicle, and was well tolerated in adults with mild to severe chronic hand eczema.
For the phase 3 study, known as DELTA 1, researchers randomized 487 adults with moderate to severe chronic hand eczema to receive twice-daily applications of delgocitinib cream 20 mg/g or cream vehicle for 16 weeks. After week 16, patients had the option to enter a long-term extension trial, which is currently ongoing. DELTA 1 was limited to adults with a diagnosis of chronic hand eczema defined as hand eczema that had persisted for more than 3 months or had returned more than twice within the past 12 months; an Investigator’s Global Assessment for chronic hand eczema (IGA-CHE) score of 3 (moderate) or 4 (severe); a weekly average Hand Eczema Symptom Diary (HESD) itch score of 4 or more points, and a medical history of inadequate response to topical corticosteroids within the past 12 months or for whom treatment with topical corticosteroids was not medically advisable.
The IGA-CHE scale used in the trial was new, “where, in order to be almost clear, the only sign that could be present on the skin was barely perceptible erythema,” Dr. Bissonnette said. He noted that he has used many IGA scales over the more than 25 years he has been involved with clinical trials, and “this was the first that used a scale with a bar so high.” Key secondary endpoints include a 75% and 90% improvement in Hand Eczema Severity Index (HECSI) from baseline at week 16 and a 4-point or greater improvement in the Dermatology Life Quality Index (DLQI) from baseline at week 16.
The median age of patients was 44 years, 88% were White, 4% were Asian, 1% were Black, and the remainder were from other racial groups. One-third of patients (33%) had severe hand eczema based on their IGA-CHE score, the median HECSI was 65 (in line with severe disease), and the median DLQI was 12. As for previous chronic hand eczema treatments, 19% had undergone phototherapy, 14% had tried oral retinoids, and 12% had tried oral corticosteroids.
In the study, a greater proportion of delgocitinib-treated patients achieved the primary endpoint of IGA-CHE 0/1, compared with the cream vehicle group at week 4 (15.4% vs. 4.9%; P < .001); week 8 (22.8% vs. 10.5%; P = .001), and week 16 (19.7% vs. 9.9%; P = .006). “As early as week 2, there is a separation between cream and vehicle,” Dr. Bissonnette said. When reviewing the results and the patients in the trial, he said that, in his personal opinion, “I don’t think this is uniquely representative of the efficacy of the drug,” because of the IGA scale that was used, which set such a high bar for efficacy.
As for secondary endpoints, a greater proportion of delgocitinib-treated patients than those in the vehicle group achieved a HESCI-75 (49.2% vs. 23.5%), a HECSI-90 (29.5% vs. 12.3%), and a 4-point or greater improvement on the DLQI (74.4% vs 50%; P < .001 for all endpoints).
Delgocitinib had a similar safety profile as the vehicle over 16 weeks, with no difference between the delgocitinib and vehicle arms in the proportion of patients who had adverse events (45.2% vs. 50.6%, respectively) and serious adverse events (1.8% vs. 1.9%). The most common adverse events (defined as 5% or greater in any treatment group) during the study were COVID-19 infections and nasopharyngitis; rates were comparable in the two arms.
Raj Chovatiya, MD, PhD, a dermatologist who directs the Center for Eczema and Itch at Northwestern University, Chicago, who was asked to comment on the study, said that chronic hand eczema can be functionally limiting for many patients. “Given its focal symptoms but multifaceted immunopathogenesis, topical JAK inhibition represents a rational strategy for targeted treatment,” Dr. Chovatiya told this news organization. He was not an investigator in the trial.
“In the phase 3 DELTA 1 study, topical delgocitinib cream was superior to vehicle control with nearly one out of five patients achieving clear or almost clear skin, with no difference in total adverse events between groups. While both comparative and long-term data would be helpful to better assess how delgocitinib cream stacks up against common topical anti-inflammatories and how it may be used for a chronic condition that typically requires ongoing treatment, these findings move us closer to a potential first-in-class approved therapy for chronic hand eczema.”
Dr. Bissonnette disclosed that he served as a consultant and investigator for the developer of delgocitinib, LEO Pharma, on this study. He has also received grants and research funding from many other pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies, including LEO Pharma.
NEW ORLEANS – , compared with those who received vehicle cream, results from a pivotal phase 3 trial showed.
“Chronic hand eczema is the most frequent chronic inflammatory disorder affecting the hands,” Robert Bissonnette, MD, a dermatologist who is founder and CEO of Innovaderm Research, said at the annual meeting of the American Academy of Dermatology, where the study was presented during a late-breaking research session. “It’s associated with pain, pruritus, and has a huge impact on quality of life,” and results with current topical treatments are often unsatisfactory, he noted.
Delgocitinib is an investigational topical pan-JAK inhibitor that inhibits activation of the JAK-STAT pathway and targets key mediators of chronic hand eczema. In a phase 2b dose-ranging trial, twice-daily treatment with delgocitinib cream demonstrated significantly greater efficacy, compared with the cream vehicle, and was well tolerated in adults with mild to severe chronic hand eczema.
For the phase 3 study, known as DELTA 1, researchers randomized 487 adults with moderate to severe chronic hand eczema to receive twice-daily applications of delgocitinib cream 20 mg/g or cream vehicle for 16 weeks. After week 16, patients had the option to enter a long-term extension trial, which is currently ongoing. DELTA 1 was limited to adults with a diagnosis of chronic hand eczema defined as hand eczema that had persisted for more than 3 months or had returned more than twice within the past 12 months; an Investigator’s Global Assessment for chronic hand eczema (IGA-CHE) score of 3 (moderate) or 4 (severe); a weekly average Hand Eczema Symptom Diary (HESD) itch score of 4 or more points, and a medical history of inadequate response to topical corticosteroids within the past 12 months or for whom treatment with topical corticosteroids was not medically advisable.
The IGA-CHE scale used in the trial was new, “where, in order to be almost clear, the only sign that could be present on the skin was barely perceptible erythema,” Dr. Bissonnette said. He noted that he has used many IGA scales over the more than 25 years he has been involved with clinical trials, and “this was the first that used a scale with a bar so high.” Key secondary endpoints include a 75% and 90% improvement in Hand Eczema Severity Index (HECSI) from baseline at week 16 and a 4-point or greater improvement in the Dermatology Life Quality Index (DLQI) from baseline at week 16.
The median age of patients was 44 years, 88% were White, 4% were Asian, 1% were Black, and the remainder were from other racial groups. One-third of patients (33%) had severe hand eczema based on their IGA-CHE score, the median HECSI was 65 (in line with severe disease), and the median DLQI was 12. As for previous chronic hand eczema treatments, 19% had undergone phototherapy, 14% had tried oral retinoids, and 12% had tried oral corticosteroids.
In the study, a greater proportion of delgocitinib-treated patients achieved the primary endpoint of IGA-CHE 0/1, compared with the cream vehicle group at week 4 (15.4% vs. 4.9%; P < .001); week 8 (22.8% vs. 10.5%; P = .001), and week 16 (19.7% vs. 9.9%; P = .006). “As early as week 2, there is a separation between cream and vehicle,” Dr. Bissonnette said. When reviewing the results and the patients in the trial, he said that, in his personal opinion, “I don’t think this is uniquely representative of the efficacy of the drug,” because of the IGA scale that was used, which set such a high bar for efficacy.
As for secondary endpoints, a greater proportion of delgocitinib-treated patients than those in the vehicle group achieved a HESCI-75 (49.2% vs. 23.5%), a HECSI-90 (29.5% vs. 12.3%), and a 4-point or greater improvement on the DLQI (74.4% vs 50%; P < .001 for all endpoints).
Delgocitinib had a similar safety profile as the vehicle over 16 weeks, with no difference between the delgocitinib and vehicle arms in the proportion of patients who had adverse events (45.2% vs. 50.6%, respectively) and serious adverse events (1.8% vs. 1.9%). The most common adverse events (defined as 5% or greater in any treatment group) during the study were COVID-19 infections and nasopharyngitis; rates were comparable in the two arms.
Raj Chovatiya, MD, PhD, a dermatologist who directs the Center for Eczema and Itch at Northwestern University, Chicago, who was asked to comment on the study, said that chronic hand eczema can be functionally limiting for many patients. “Given its focal symptoms but multifaceted immunopathogenesis, topical JAK inhibition represents a rational strategy for targeted treatment,” Dr. Chovatiya told this news organization. He was not an investigator in the trial.
“In the phase 3 DELTA 1 study, topical delgocitinib cream was superior to vehicle control with nearly one out of five patients achieving clear or almost clear skin, with no difference in total adverse events between groups. While both comparative and long-term data would be helpful to better assess how delgocitinib cream stacks up against common topical anti-inflammatories and how it may be used for a chronic condition that typically requires ongoing treatment, these findings move us closer to a potential first-in-class approved therapy for chronic hand eczema.”
Dr. Bissonnette disclosed that he served as a consultant and investigator for the developer of delgocitinib, LEO Pharma, on this study. He has also received grants and research funding from many other pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies, including LEO Pharma.
NEW ORLEANS – , compared with those who received vehicle cream, results from a pivotal phase 3 trial showed.
“Chronic hand eczema is the most frequent chronic inflammatory disorder affecting the hands,” Robert Bissonnette, MD, a dermatologist who is founder and CEO of Innovaderm Research, said at the annual meeting of the American Academy of Dermatology, where the study was presented during a late-breaking research session. “It’s associated with pain, pruritus, and has a huge impact on quality of life,” and results with current topical treatments are often unsatisfactory, he noted.
Delgocitinib is an investigational topical pan-JAK inhibitor that inhibits activation of the JAK-STAT pathway and targets key mediators of chronic hand eczema. In a phase 2b dose-ranging trial, twice-daily treatment with delgocitinib cream demonstrated significantly greater efficacy, compared with the cream vehicle, and was well tolerated in adults with mild to severe chronic hand eczema.
For the phase 3 study, known as DELTA 1, researchers randomized 487 adults with moderate to severe chronic hand eczema to receive twice-daily applications of delgocitinib cream 20 mg/g or cream vehicle for 16 weeks. After week 16, patients had the option to enter a long-term extension trial, which is currently ongoing. DELTA 1 was limited to adults with a diagnosis of chronic hand eczema defined as hand eczema that had persisted for more than 3 months or had returned more than twice within the past 12 months; an Investigator’s Global Assessment for chronic hand eczema (IGA-CHE) score of 3 (moderate) or 4 (severe); a weekly average Hand Eczema Symptom Diary (HESD) itch score of 4 or more points, and a medical history of inadequate response to topical corticosteroids within the past 12 months or for whom treatment with topical corticosteroids was not medically advisable.
The IGA-CHE scale used in the trial was new, “where, in order to be almost clear, the only sign that could be present on the skin was barely perceptible erythema,” Dr. Bissonnette said. He noted that he has used many IGA scales over the more than 25 years he has been involved with clinical trials, and “this was the first that used a scale with a bar so high.” Key secondary endpoints include a 75% and 90% improvement in Hand Eczema Severity Index (HECSI) from baseline at week 16 and a 4-point or greater improvement in the Dermatology Life Quality Index (DLQI) from baseline at week 16.
The median age of patients was 44 years, 88% were White, 4% were Asian, 1% were Black, and the remainder were from other racial groups. One-third of patients (33%) had severe hand eczema based on their IGA-CHE score, the median HECSI was 65 (in line with severe disease), and the median DLQI was 12. As for previous chronic hand eczema treatments, 19% had undergone phototherapy, 14% had tried oral retinoids, and 12% had tried oral corticosteroids.
In the study, a greater proportion of delgocitinib-treated patients achieved the primary endpoint of IGA-CHE 0/1, compared with the cream vehicle group at week 4 (15.4% vs. 4.9%; P < .001); week 8 (22.8% vs. 10.5%; P = .001), and week 16 (19.7% vs. 9.9%; P = .006). “As early as week 2, there is a separation between cream and vehicle,” Dr. Bissonnette said. When reviewing the results and the patients in the trial, he said that, in his personal opinion, “I don’t think this is uniquely representative of the efficacy of the drug,” because of the IGA scale that was used, which set such a high bar for efficacy.
As for secondary endpoints, a greater proportion of delgocitinib-treated patients than those in the vehicle group achieved a HESCI-75 (49.2% vs. 23.5%), a HECSI-90 (29.5% vs. 12.3%), and a 4-point or greater improvement on the DLQI (74.4% vs 50%; P < .001 for all endpoints).
Delgocitinib had a similar safety profile as the vehicle over 16 weeks, with no difference between the delgocitinib and vehicle arms in the proportion of patients who had adverse events (45.2% vs. 50.6%, respectively) and serious adverse events (1.8% vs. 1.9%). The most common adverse events (defined as 5% or greater in any treatment group) during the study were COVID-19 infections and nasopharyngitis; rates were comparable in the two arms.
Raj Chovatiya, MD, PhD, a dermatologist who directs the Center for Eczema and Itch at Northwestern University, Chicago, who was asked to comment on the study, said that chronic hand eczema can be functionally limiting for many patients. “Given its focal symptoms but multifaceted immunopathogenesis, topical JAK inhibition represents a rational strategy for targeted treatment,” Dr. Chovatiya told this news organization. He was not an investigator in the trial.
“In the phase 3 DELTA 1 study, topical delgocitinib cream was superior to vehicle control with nearly one out of five patients achieving clear or almost clear skin, with no difference in total adverse events between groups. While both comparative and long-term data would be helpful to better assess how delgocitinib cream stacks up against common topical anti-inflammatories and how it may be used for a chronic condition that typically requires ongoing treatment, these findings move us closer to a potential first-in-class approved therapy for chronic hand eczema.”
Dr. Bissonnette disclosed that he served as a consultant and investigator for the developer of delgocitinib, LEO Pharma, on this study. He has also received grants and research funding from many other pharmaceutical companies. Dr. Chovatiya disclosed that he is a consultant to, a speaker for, and/or a member of the advisory board for several pharmaceutical companies, including LEO Pharma.
AT AAD 2023
Luxe vacations, private jets: Medical device maker, surgeon to pay $46 million penalty in kickback scheme
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
according to experts familiar with the federal Anti-Kickback Statute.
Historically, enforcement actions have primarily focused on the person or organization offering the perks – and not necessarily the physicians accepting it, Steven W. Ortquist, founder and principal of Arete Compliance Solutions, LLC, in Phoenix, told this news organization.
But that’s changing.
“In recent years, we are seeing a trend toward holding physicians and others on the receiving end of the inducement accountable as well,” said Mr. Ortquist, who is a past board member and president of the Health Care Compliance Association. He noted that authorities usually pursue the inducing company first before moving on to individual clinicians or practices.
The Department of Justice followed a similar pattern in a recently announced kickback settlement that ensnared an intraocular lens distributor, an ophthalmology equipment supplier, two CEOs, and a surgeon. Precision Lens must pay more than $43 million for offering high-end vacations and other expensive perks to surgeons who used its cataract products.
The verdict marks the end of a 6-week civil jury trial, where evidence emerged that Paul Ehlen, owner of Precision Lens and its parent company, Cameron-Ehlen Group, maintained a secret “slush fund” for paying kickbacks to ophthalmic surgeons. The inducement scheme netted the Minnesota-based company millions in sales and led to the submission of 64,575 false Medicare claims from 2006 to 2015, a violation of the Anti-Kickback Statute and the False Claims Act.
According to court documents, physicians received luxury travel and entertainment packages, including skiing, fishing, and golfing excursions at exclusive destinations, often traveling via private jet to attend Broadway musicals and major sporting events. Mr. Ehlen and company representatives also sold frequent flyer miles to physicians at a steep discount, allowing them to take personal and business trips below fair market value.
Federal authorities initially announced an investigation into the business practices of Precision Lens in 2017 after receiving a whistleblower complaint from Kipp Fesenmaier, a former executive at Sightpath Medical, an ophthalmology supplier and “corporate partner” of Precision Lens. Mr. Fesenmaier alleged that both companies were involved in an inducement scheme.
Sightpath Medical and its CEO, James Tiffany, agreed to a $12 million settlement to resolve the kickback allegations.
The Department of Justice subsequently investigated Jitendra Swarup, MD, an ophthalmologist and cataract surgeon who allegedly received “unlawful remuneration from Sightpath, Precision, and Ehlen” and filed false insurance claims. In addition to accepting expensive hunting and fishing trips from the medical device companies, Dr. Swarup was paid more than $100,000 per year for consulting services he did not fully render.
Dr. Swarup agreed to a nearly $3 million settlement and participation in a 3-year corporate integrity agreement with the Office of Inspector General. In exchange for compliance with such contracts, the OIG permits physicians to continue participating in Medicare, Medicaid, and other federal health care programs.
In a statement from attorneys, Precision Lens and Mr. Ehlen pledged to appeal the verdict and “defend ... our wholly appropriate actions” while remaining focused on their commitment to health care clinicians and manufacturers.
‘Endless’ opportunities for inducement
Unfortunately, opportunities for inducement are “endless,” experts say. Extravagant trips, dinners, and gifts can trigger a violation, but so can nearly anything of value.
Just last year, Biotronik reached a $12.95 million settlement amid allegations that company representatives wined and dined physicians to induce their use of its pacemakers and defibrillators. To date, no physicians have been charged.
But after a record-breaking number of whistleblower judgments last fiscal year totaling more than $2 billion, physicians should take note, Radha Bhatnagar, Esq, director of compliance at The CM Group, told the news organization.
“When manufacturers offer physicians kickbacks with the added element of fraudulent Medicare or Medicaid reimbursements, that is typically when manufacturers and individuals face civil and criminal liability,” said Ms. Bhatnagar, something the Department of Justice alluded to when announcing a settlement involving 15 Texas physicians last year.
In another case, Kingsley R. Chin, an orthopedic surgeon and designer of a spinal implant, was indicted in 2021 for paying millions of dollars in sham consulting fees to physicians who used his products. At least six surgeons who accepted money from Dr. Chin were later named in a civil case and ordered to pay $3.3 million in penalties.
Jason Montone, DO, an orthopedic surgeon who accepted the illicit payments, agreed to a plea deal with a reduced prison sentence, 1 year of supervised release, and a fine of $379,000.
Although Dr. Chin’s sentencing hasn’t been announced, violating kickback laws can result in a sentence of up to 10 years.
A version of this article originally appeared on Medscape.com.
Air pollution may be causing eczema
The finding points scientists toward how to better treat the skin ailment. There are now more than three times as many eczema cases as there were in the 1970s, and it now affects as many as 20% of children and 10% of adults.
“I think these authors are spot-on in recognizing that the incidence of allergic conditions is increasing concurrently with how different pollutants are increasing in our environment,” said Denver-based pediatric allergist and immunologist Jessica Hui, MD, according to NBC News. “We’re finally understanding more about why people are getting eczema.”
Some people get eczema because of genetics, but the new research built on the previous understanding of how chemicals called diisocyanates can trigger the eczema symptoms of severe itching, skin redness, and oozing or painful rashes. An experiment on mice showed that exposure to a specific part of diisocyanates, called isocyanates, disrupted oil production that the skin needs to stay healthy.
Researchers at the National Institutes of Health “found that when bacteria that live on healthy skin are exposed to isocyanate, they must adapt to survive,” the agency summarized in a news release. “When they adapt, these bacteria shift their metabolism away from making the lipids, or oils, that skin needs to stay healthy. This finding suggests that eczema may be treatable by replacing the modified skin bacteria with healthy bacteria.”
The study was published in the journal Science Advances.
The chemicals also trigger a message to the brain that causes skin inflammation and itching, lead researcher Ian Myles, MD, told NBC News. Dr. Myles is also chief of the Epithelial Research Unit in the National Institute of Allergy and Infectious Diseases Laboratory of Clinical Immunology and Microbiology.
“So much of this is out of our control. I mean, you can’t shut the highways down,” he said of the environmental sources.
Previous research that explored attempting to restore healthy skin bacteria called Roseomonas mucosa to treat eczema symptoms had mixed results. The NIH says it has made the bacteria available “for commercial, nontherapeutic development ... as a potentially beneficial probiotic.”
A version of this article first appeared on WebMD.com.
The finding points scientists toward how to better treat the skin ailment. There are now more than three times as many eczema cases as there were in the 1970s, and it now affects as many as 20% of children and 10% of adults.
“I think these authors are spot-on in recognizing that the incidence of allergic conditions is increasing concurrently with how different pollutants are increasing in our environment,” said Denver-based pediatric allergist and immunologist Jessica Hui, MD, according to NBC News. “We’re finally understanding more about why people are getting eczema.”
Some people get eczema because of genetics, but the new research built on the previous understanding of how chemicals called diisocyanates can trigger the eczema symptoms of severe itching, skin redness, and oozing or painful rashes. An experiment on mice showed that exposure to a specific part of diisocyanates, called isocyanates, disrupted oil production that the skin needs to stay healthy.
Researchers at the National Institutes of Health “found that when bacteria that live on healthy skin are exposed to isocyanate, they must adapt to survive,” the agency summarized in a news release. “When they adapt, these bacteria shift their metabolism away from making the lipids, or oils, that skin needs to stay healthy. This finding suggests that eczema may be treatable by replacing the modified skin bacteria with healthy bacteria.”
The study was published in the journal Science Advances.
The chemicals also trigger a message to the brain that causes skin inflammation and itching, lead researcher Ian Myles, MD, told NBC News. Dr. Myles is also chief of the Epithelial Research Unit in the National Institute of Allergy and Infectious Diseases Laboratory of Clinical Immunology and Microbiology.
“So much of this is out of our control. I mean, you can’t shut the highways down,” he said of the environmental sources.
Previous research that explored attempting to restore healthy skin bacteria called Roseomonas mucosa to treat eczema symptoms had mixed results. The NIH says it has made the bacteria available “for commercial, nontherapeutic development ... as a potentially beneficial probiotic.”
A version of this article first appeared on WebMD.com.
The finding points scientists toward how to better treat the skin ailment. There are now more than three times as many eczema cases as there were in the 1970s, and it now affects as many as 20% of children and 10% of adults.
“I think these authors are spot-on in recognizing that the incidence of allergic conditions is increasing concurrently with how different pollutants are increasing in our environment,” said Denver-based pediatric allergist and immunologist Jessica Hui, MD, according to NBC News. “We’re finally understanding more about why people are getting eczema.”
Some people get eczema because of genetics, but the new research built on the previous understanding of how chemicals called diisocyanates can trigger the eczema symptoms of severe itching, skin redness, and oozing or painful rashes. An experiment on mice showed that exposure to a specific part of diisocyanates, called isocyanates, disrupted oil production that the skin needs to stay healthy.
Researchers at the National Institutes of Health “found that when bacteria that live on healthy skin are exposed to isocyanate, they must adapt to survive,” the agency summarized in a news release. “When they adapt, these bacteria shift their metabolism away from making the lipids, or oils, that skin needs to stay healthy. This finding suggests that eczema may be treatable by replacing the modified skin bacteria with healthy bacteria.”
The study was published in the journal Science Advances.
The chemicals also trigger a message to the brain that causes skin inflammation and itching, lead researcher Ian Myles, MD, told NBC News. Dr. Myles is also chief of the Epithelial Research Unit in the National Institute of Allergy and Infectious Diseases Laboratory of Clinical Immunology and Microbiology.
“So much of this is out of our control. I mean, you can’t shut the highways down,” he said of the environmental sources.
Previous research that explored attempting to restore healthy skin bacteria called Roseomonas mucosa to treat eczema symptoms had mixed results. The NIH says it has made the bacteria available “for commercial, nontherapeutic development ... as a potentially beneficial probiotic.”
A version of this article first appeared on WebMD.com.
FROM SCIENCE ADVANCES
FDA approves new formulation of Hyrimoz adalimumab biosimilar
The Food and Drug Administration has approved a citrate-free, 100 mg/mL formulation of the biosimilar adalimumab-adaz (Hyrimoz), according to a statement from manufacturer Sandoz.
Hyrimoz, a tumor necrosis factor (TNF) blocker that is biosimilar to its reference product Humira, was approved by the FDA in 2018 at a concentration of 50 mg/mL for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The high-concentration formula is indicated for these same conditions.
Sandoz said that it intends to launch the citrate-free formulation in the United States on July 1. It will be one of up to nine other adalimumab biosimilars that are expected to launch in July. On January 31, Amjevita (adalimumab-atto) became the first adalimumab biosimilar to launch in the United States.
The current label for Hyrimoz contains a black box warning emphasizing certain risks, notably the increased risk for serious infections, such as tuberculosis or sepsis, and an increased risk of malignancy, particularly lymphomas.
Adverse effects associated with Hyrimoz with an incidence greater than 10% include upper respiratory infections and sinusitis, injection-site reactions, headache, and rash.
The approval for the high-concentration formulation was based on data from a phase 1 pharmacokinetics bridging study that compared Hyrimoz 50 mg/mL and citrate-free Hyrimoz 100 mg/mL.
“This study met all of the primary objectives, demonstrating comparable pharmacokinetics and showing similar safety and immunogenicity of the Hyrimoz 50 mg/mL and Hyrimoz [100 mg/mL],” according to Sandoz, a division of Novartis.
The approval for Hyrimoz 50 mg/mL in 2018 was based on preclinical and clinical research comparing Hyrimoz and Humira. In a phase 3 trial published in the British Journal of Dermatology, which included adults with clinically stable but active moderate to severe chronic plaque psoriasis, Hyrimoz and Humira showed a similar percentage of patients met the primary endpoint of a 75% reduction or more in Psoriasis Area and Severity Index (PASI 75) score at 16 weeks, compared with baseline (66.8% and 65%, respectively).
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved a citrate-free, 100 mg/mL formulation of the biosimilar adalimumab-adaz (Hyrimoz), according to a statement from manufacturer Sandoz.
Hyrimoz, a tumor necrosis factor (TNF) blocker that is biosimilar to its reference product Humira, was approved by the FDA in 2018 at a concentration of 50 mg/mL for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The high-concentration formula is indicated for these same conditions.
Sandoz said that it intends to launch the citrate-free formulation in the United States on July 1. It will be one of up to nine other adalimumab biosimilars that are expected to launch in July. On January 31, Amjevita (adalimumab-atto) became the first adalimumab biosimilar to launch in the United States.
The current label for Hyrimoz contains a black box warning emphasizing certain risks, notably the increased risk for serious infections, such as tuberculosis or sepsis, and an increased risk of malignancy, particularly lymphomas.
Adverse effects associated with Hyrimoz with an incidence greater than 10% include upper respiratory infections and sinusitis, injection-site reactions, headache, and rash.
The approval for the high-concentration formulation was based on data from a phase 1 pharmacokinetics bridging study that compared Hyrimoz 50 mg/mL and citrate-free Hyrimoz 100 mg/mL.
“This study met all of the primary objectives, demonstrating comparable pharmacokinetics and showing similar safety and immunogenicity of the Hyrimoz 50 mg/mL and Hyrimoz [100 mg/mL],” according to Sandoz, a division of Novartis.
The approval for Hyrimoz 50 mg/mL in 2018 was based on preclinical and clinical research comparing Hyrimoz and Humira. In a phase 3 trial published in the British Journal of Dermatology, which included adults with clinically stable but active moderate to severe chronic plaque psoriasis, Hyrimoz and Humira showed a similar percentage of patients met the primary endpoint of a 75% reduction or more in Psoriasis Area and Severity Index (PASI 75) score at 16 weeks, compared with baseline (66.8% and 65%, respectively).
A version of this article originally appeared on Medscape.com.
The Food and Drug Administration has approved a citrate-free, 100 mg/mL formulation of the biosimilar adalimumab-adaz (Hyrimoz), according to a statement from manufacturer Sandoz.
Hyrimoz, a tumor necrosis factor (TNF) blocker that is biosimilar to its reference product Humira, was approved by the FDA in 2018 at a concentration of 50 mg/mL for rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The high-concentration formula is indicated for these same conditions.
Sandoz said that it intends to launch the citrate-free formulation in the United States on July 1. It will be one of up to nine other adalimumab biosimilars that are expected to launch in July. On January 31, Amjevita (adalimumab-atto) became the first adalimumab biosimilar to launch in the United States.
The current label for Hyrimoz contains a black box warning emphasizing certain risks, notably the increased risk for serious infections, such as tuberculosis or sepsis, and an increased risk of malignancy, particularly lymphomas.
Adverse effects associated with Hyrimoz with an incidence greater than 10% include upper respiratory infections and sinusitis, injection-site reactions, headache, and rash.
The approval for the high-concentration formulation was based on data from a phase 1 pharmacokinetics bridging study that compared Hyrimoz 50 mg/mL and citrate-free Hyrimoz 100 mg/mL.
“This study met all of the primary objectives, demonstrating comparable pharmacokinetics and showing similar safety and immunogenicity of the Hyrimoz 50 mg/mL and Hyrimoz [100 mg/mL],” according to Sandoz, a division of Novartis.
The approval for Hyrimoz 50 mg/mL in 2018 was based on preclinical and clinical research comparing Hyrimoz and Humira. In a phase 3 trial published in the British Journal of Dermatology, which included adults with clinically stable but active moderate to severe chronic plaque psoriasis, Hyrimoz and Humira showed a similar percentage of patients met the primary endpoint of a 75% reduction or more in Psoriasis Area and Severity Index (PASI 75) score at 16 weeks, compared with baseline (66.8% and 65%, respectively).
A version of this article originally appeared on Medscape.com.