User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
COVID emergency orders ending: What’s next?
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
Developments in wound healing include different treatment options
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
ORLANDO – , Hadar Lev-Tov, MD, said at the ODAC Dermatology, Aesthetic & Surgery Conference.
At the meeting, Dr. Lev-Tov, associate professor of dermatology at the University of Miami, reviewed some of the latest developments in several conditions involving wound care.
Pyoderma gangrenosum (PG): In this condition, pustules or nodules become large ulcerations, and one-third of patients with PG have pathergy, exaggerated skin injury after a mild trauma such as a bump or a bruise.
“You want to look at the clues in the history because 20% of these patients had histories of PG elsewhere,” Dr. Lev-Tov said. “Ask them about other ulcers, maybe they had some wound dehiscence history.”
Criteria have been developed to help with the diagnosis of ulcerative PG, which includes one major criterion, a biopsy of the ulcer edge showing neutrophilic infiltrate, along with minor criteria, including exclusion of an infection, pathergy, and a history of inflammatory bowel disease or inflammatory arthritis.
“This is no longer a diagnosis of exclusion,” Dr. Lev-Tov said.
Cyclosporine and oral steroids have been found to work well, but it typically takes many months before healing occurs. Tacrolimus or topical steroids can work as well, but healing also takes a fairly long time with those medications, Dr. Lev-Tov said.
The tumor necrosis factor (TNF) blocker infliximab is another option. He had a patient who was referred to him who had been treated with cyclosporine for 3 years for PG on his feet, even though it had not been effective. Dr. Lev-Tov tried infliximab, and the wounds finally cleared, he said.
Apremilast, a phosphodiesterase 4 (PDE4)-inhibitor, is another option for treating PG, he said. “Anecdotally, I used apremilast on three patients with recurrent PG for long-term suppression, with success,” he noted.
Epidermal grafting using suction and heat is an approach that might deserve further exploration for PG, Dr. Lev-Tov suggested. With this procedure, described in an article in 2014, heat and suction are used to induce blistering to separate and remove the epidermis from the dermis at the dermal-epidermal junction, creating an epidermal graft is placed over the wound to promote healing. Patients with PG who are immunosuppressed but demonstrate pathergy do not tend to experience pathergy when epidermal skin grafting is performed, he said.
The heat-suction procedure is simple, painless, and scarless, but better controlled data on this approach are needed, he said.
Corona phlebectatica: This disease involving abnormally dilated veins near the ankle has received formal recognition as a sign of venous insufficiency, in a 2020 update of a classification system for describing patients with chronic venous disorders, Dr. Lev-Tov said.
“We knew about it for years, but now there’s some data that can actually predict the severity of disease,” and, he said, it is now a part of the diagnostic criteria for venous insufficiency .
Venous leg ulcers: These often painful sores on the inside of the leg typically take more than a month to heal. A systematic review of placebo-controlled studies of pentoxifylline as a treatment for venous leg ulcers, published in 2021, supports its use for healing venous leg ulcers, Dr. Lev-Tov said. “It improved the healing rate and increased what [the researchers] called ‘significant improvement,’ ” a category they created to account for the varying methods across the studies, he said.
Topical beta-blockers can improve epithelialization and fibroblast migration in wound healing, he said. A study on topical timolol for various wounds found that a 0.5% formulation of topical timolol, with one drop applied per square centimeter as frequently as possible, was effective in healing. But the healing process was prolonged – a median of 90 days, said Dr. Lev-Tov, one of the study authors.
“When you start this, I don’t want you to expect the wound to heal tomorrow,” he said. “You’ve got to educate your patient.”
Dr. Lev-Tov reports relevant financial relationships with Abbvie, Novartis, Pfizer and other companies.
AT ODAC 2023
The long-range thrombolysis forecast calls for tiny ultrasonic tornadoes
Sticks and stones may break my bones, but clots will never hurt me
You’ve probably seen “Ghostbusters” or at least heard the theme song. Maybe you even know about the Discovery Channel’s “Mythbusters.” But now there’s a new buster in town, and it eats platitudes for breakfast: Meet Cliche-busters, LOTME’s new recurring feature.
This week, Cliche-busters takes on “Two wrongs don’t make a right.” Yum.
We start with blood clots, which are bad. Doctors go to a lot of trouble to get rid of the things because they are dangerous. A blood clot, then, is a bodily function gone wrong.
Tornadoes are also bad. Out there in the world, these violently rotating columns of air can destroy buildings, toss large objects long distances, and inspire mediocre action movies. They are examples of nature gone wrong.
Seemingly, these two wrongs – blood clots and tornadoes – are not about to make a right. Has Cliche-busters bitten off more than it can chew?
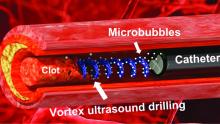
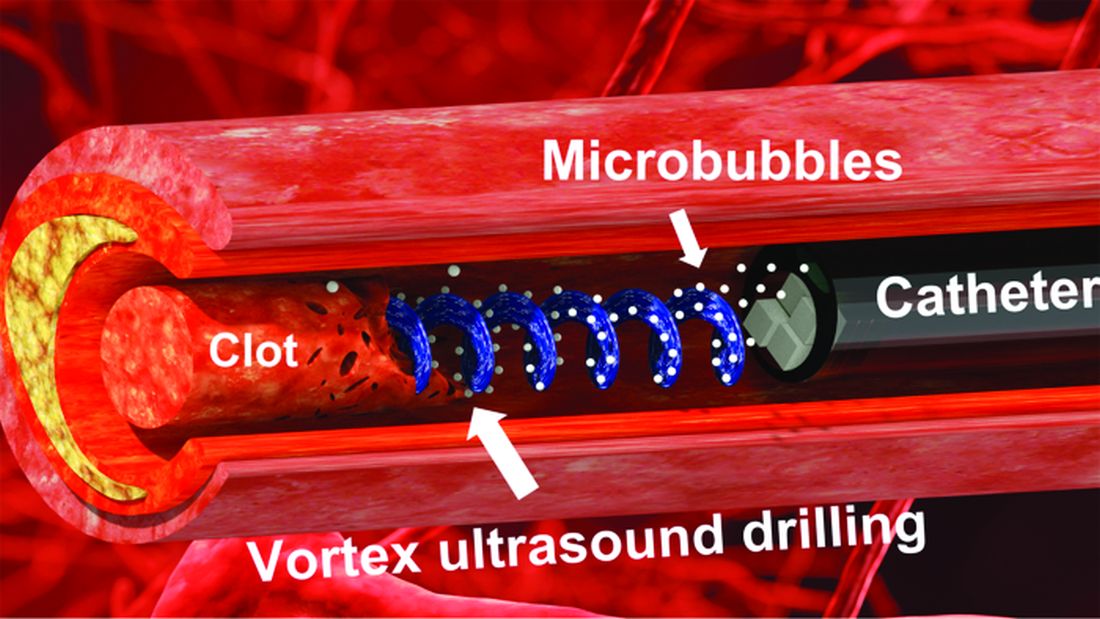
Not according to Xiaoning Jiang of North Carolina State University, Raleigh, and his team of researchers. They’ve figured out a way to use a tiny ultrasonic tornado to break down clots in the brain. “Our new work uses vortex ultrasound, where the ultrasound waves have a helical wavefront. In other words, the ultrasound is swirling as it moves forward,” he said in a statement from the university.
Their new tool’s single transducer is small enough to fit in a catheter, and its “vortex ultrasound-induced shear force has the potential to break down clots safely and improve the efficacy of thrombolysis,” they explained in the open-access journal Research.
The investigators used cow blood in a 3D-printed model of the cerebral venous sinus for the proof-of-concept study and were able to dissolve an acute blood clot in less than 30 minutes, compared with the 15-30 hours needed with a pharmaceutical intervention, according to the written statement.
Can you hear the sound of two wrongs making a right? We can, and that closes the curtain on this cliche.
With age does not come wisdom
We’ve all met this person before. The sort of person who takes a 10-minute IQ test on a shifty-looking website and then proceeds to brag about a 180 IQ until the heat death of the universe. The one who worships at the altar of Mensa. Yeah, that guy. They’re never as smart as they think they are, but they’ll never, ever admit it.
It’s not exactly a secret that IQ as a measurement of intelligence is highly overrated. A lot of scientists doubt we should bother measuring it at all. That said, a higher IQ is associated with greater success in academic and financial endeavors, so it’s not absolutely worthless. And if we’re stuck with it, we may as well study it.
That brings us neatly to new research published in Brain and Behavior. Most studies into IQ and self-estimated intelligence have focused on younger adults, and the author of this study was curious if the stereotype of young men inflating their IQ, a stereotype backed up by research, persisted into older adulthood. So she conducted a survey of 159 younger adults and 152 older adults to find out.
The results in younger adults were not surprising: Younger men overestimated their actual IQ by 5-15 points, which tracks with previous research. We’re in for a bit of a surprise with the older adults, though, because the older men were more humble about their intelligence, with their estimation falling in line with their actual IQ. Older women, however, not so much. In fact, they overestimated their intelligence just as much as the younger men.
In addition, older women who perceived themselves as more attractive reported the highest self-estimated intelligence of all. That isn’t how intelligence works, but honestly, if Grandma’s out and about thinking she looks good and has the brains to go and win “Jeopardy!” do you really have the heart to tell her otherwise?
Fight temptation with empathy … and shoes
Relationships are tough. They all go through their respective ups and downs, but what happens when one person is feeling so down in the partnership that cheating comes to mind? Is there any way to stop it from happening?
Well, a recent study suggests that there is, and it’s as simple as putting yourself in the other person’s shoes. By observing 408 heterosexual, monogamous participants in a series of experiments, psychologists in Israel and New York found that practicing empathy and “perspective taking” doesn’t necessarily stop people from cheating but it does reduces the desire.
People cheat on their significant others for many different reasons – men for a lack of sexual needs being met and women for shortfalls regarding emotional needs – but prioritizing the other person’s perspective gives the idea of being unfaithful a different view and could make one act differently, the investigators said.
Perspective taking also promotes other positive attributes to the relationship, such as the promotion of compassion and the feeling of being understood, lead author Gurit Birnbaum of Reichman University in Herzliya, Israel, said in a written statement. These things ultimately help couples navigate the rough patches and strengthen bonds, making them even less likely to cheat.
The researchers noted that even people in satisfying relationships do cheat, but this approach does encourage people to stop and think before they act. It could ultimately prevent what might be a huge mistake.
Think before they act. Hmm, that’s kind of like look before they leap, right? Sounds like a job for the Cliche-busters.
Sticks and stones may break my bones, but clots will never hurt me
You’ve probably seen “Ghostbusters” or at least heard the theme song. Maybe you even know about the Discovery Channel’s “Mythbusters.” But now there’s a new buster in town, and it eats platitudes for breakfast: Meet Cliche-busters, LOTME’s new recurring feature.
This week, Cliche-busters takes on “Two wrongs don’t make a right.” Yum.
We start with blood clots, which are bad. Doctors go to a lot of trouble to get rid of the things because they are dangerous. A blood clot, then, is a bodily function gone wrong.
Tornadoes are also bad. Out there in the world, these violently rotating columns of air can destroy buildings, toss large objects long distances, and inspire mediocre action movies. They are examples of nature gone wrong.
Seemingly, these two wrongs – blood clots and tornadoes – are not about to make a right. Has Cliche-busters bitten off more than it can chew?
Not according to Xiaoning Jiang of North Carolina State University, Raleigh, and his team of researchers. They’ve figured out a way to use a tiny ultrasonic tornado to break down clots in the brain. “Our new work uses vortex ultrasound, where the ultrasound waves have a helical wavefront. In other words, the ultrasound is swirling as it moves forward,” he said in a statement from the university.
Their new tool’s single transducer is small enough to fit in a catheter, and its “vortex ultrasound-induced shear force has the potential to break down clots safely and improve the efficacy of thrombolysis,” they explained in the open-access journal Research.
The investigators used cow blood in a 3D-printed model of the cerebral venous sinus for the proof-of-concept study and were able to dissolve an acute blood clot in less than 30 minutes, compared with the 15-30 hours needed with a pharmaceutical intervention, according to the written statement.
Can you hear the sound of two wrongs making a right? We can, and that closes the curtain on this cliche.
With age does not come wisdom
We’ve all met this person before. The sort of person who takes a 10-minute IQ test on a shifty-looking website and then proceeds to brag about a 180 IQ until the heat death of the universe. The one who worships at the altar of Mensa. Yeah, that guy. They’re never as smart as they think they are, but they’ll never, ever admit it.
It’s not exactly a secret that IQ as a measurement of intelligence is highly overrated. A lot of scientists doubt we should bother measuring it at all. That said, a higher IQ is associated with greater success in academic and financial endeavors, so it’s not absolutely worthless. And if we’re stuck with it, we may as well study it.
That brings us neatly to new research published in Brain and Behavior. Most studies into IQ and self-estimated intelligence have focused on younger adults, and the author of this study was curious if the stereotype of young men inflating their IQ, a stereotype backed up by research, persisted into older adulthood. So she conducted a survey of 159 younger adults and 152 older adults to find out.
The results in younger adults were not surprising: Younger men overestimated their actual IQ by 5-15 points, which tracks with previous research. We’re in for a bit of a surprise with the older adults, though, because the older men were more humble about their intelligence, with their estimation falling in line with their actual IQ. Older women, however, not so much. In fact, they overestimated their intelligence just as much as the younger men.
In addition, older women who perceived themselves as more attractive reported the highest self-estimated intelligence of all. That isn’t how intelligence works, but honestly, if Grandma’s out and about thinking she looks good and has the brains to go and win “Jeopardy!” do you really have the heart to tell her otherwise?
Fight temptation with empathy … and shoes
Relationships are tough. They all go through their respective ups and downs, but what happens when one person is feeling so down in the partnership that cheating comes to mind? Is there any way to stop it from happening?
Well, a recent study suggests that there is, and it’s as simple as putting yourself in the other person’s shoes. By observing 408 heterosexual, monogamous participants in a series of experiments, psychologists in Israel and New York found that practicing empathy and “perspective taking” doesn’t necessarily stop people from cheating but it does reduces the desire.
People cheat on their significant others for many different reasons – men for a lack of sexual needs being met and women for shortfalls regarding emotional needs – but prioritizing the other person’s perspective gives the idea of being unfaithful a different view and could make one act differently, the investigators said.
Perspective taking also promotes other positive attributes to the relationship, such as the promotion of compassion and the feeling of being understood, lead author Gurit Birnbaum of Reichman University in Herzliya, Israel, said in a written statement. These things ultimately help couples navigate the rough patches and strengthen bonds, making them even less likely to cheat.
The researchers noted that even people in satisfying relationships do cheat, but this approach does encourage people to stop and think before they act. It could ultimately prevent what might be a huge mistake.
Think before they act. Hmm, that’s kind of like look before they leap, right? Sounds like a job for the Cliche-busters.
Sticks and stones may break my bones, but clots will never hurt me
You’ve probably seen “Ghostbusters” or at least heard the theme song. Maybe you even know about the Discovery Channel’s “Mythbusters.” But now there’s a new buster in town, and it eats platitudes for breakfast: Meet Cliche-busters, LOTME’s new recurring feature.
This week, Cliche-busters takes on “Two wrongs don’t make a right.” Yum.
We start with blood clots, which are bad. Doctors go to a lot of trouble to get rid of the things because they are dangerous. A blood clot, then, is a bodily function gone wrong.
Tornadoes are also bad. Out there in the world, these violently rotating columns of air can destroy buildings, toss large objects long distances, and inspire mediocre action movies. They are examples of nature gone wrong.
Seemingly, these two wrongs – blood clots and tornadoes – are not about to make a right. Has Cliche-busters bitten off more than it can chew?
Not according to Xiaoning Jiang of North Carolina State University, Raleigh, and his team of researchers. They’ve figured out a way to use a tiny ultrasonic tornado to break down clots in the brain. “Our new work uses vortex ultrasound, where the ultrasound waves have a helical wavefront. In other words, the ultrasound is swirling as it moves forward,” he said in a statement from the university.
Their new tool’s single transducer is small enough to fit in a catheter, and its “vortex ultrasound-induced shear force has the potential to break down clots safely and improve the efficacy of thrombolysis,” they explained in the open-access journal Research.
The investigators used cow blood in a 3D-printed model of the cerebral venous sinus for the proof-of-concept study and were able to dissolve an acute blood clot in less than 30 minutes, compared with the 15-30 hours needed with a pharmaceutical intervention, according to the written statement.
Can you hear the sound of two wrongs making a right? We can, and that closes the curtain on this cliche.
With age does not come wisdom
We’ve all met this person before. The sort of person who takes a 10-minute IQ test on a shifty-looking website and then proceeds to brag about a 180 IQ until the heat death of the universe. The one who worships at the altar of Mensa. Yeah, that guy. They’re never as smart as they think they are, but they’ll never, ever admit it.
It’s not exactly a secret that IQ as a measurement of intelligence is highly overrated. A lot of scientists doubt we should bother measuring it at all. That said, a higher IQ is associated with greater success in academic and financial endeavors, so it’s not absolutely worthless. And if we’re stuck with it, we may as well study it.
That brings us neatly to new research published in Brain and Behavior. Most studies into IQ and self-estimated intelligence have focused on younger adults, and the author of this study was curious if the stereotype of young men inflating their IQ, a stereotype backed up by research, persisted into older adulthood. So she conducted a survey of 159 younger adults and 152 older adults to find out.
The results in younger adults were not surprising: Younger men overestimated their actual IQ by 5-15 points, which tracks with previous research. We’re in for a bit of a surprise with the older adults, though, because the older men were more humble about their intelligence, with their estimation falling in line with their actual IQ. Older women, however, not so much. In fact, they overestimated their intelligence just as much as the younger men.
In addition, older women who perceived themselves as more attractive reported the highest self-estimated intelligence of all. That isn’t how intelligence works, but honestly, if Grandma’s out and about thinking she looks good and has the brains to go and win “Jeopardy!” do you really have the heart to tell her otherwise?
Fight temptation with empathy … and shoes
Relationships are tough. They all go through their respective ups and downs, but what happens when one person is feeling so down in the partnership that cheating comes to mind? Is there any way to stop it from happening?
Well, a recent study suggests that there is, and it’s as simple as putting yourself in the other person’s shoes. By observing 408 heterosexual, monogamous participants in a series of experiments, psychologists in Israel and New York found that practicing empathy and “perspective taking” doesn’t necessarily stop people from cheating but it does reduces the desire.
People cheat on their significant others for many different reasons – men for a lack of sexual needs being met and women for shortfalls regarding emotional needs – but prioritizing the other person’s perspective gives the idea of being unfaithful a different view and could make one act differently, the investigators said.
Perspective taking also promotes other positive attributes to the relationship, such as the promotion of compassion and the feeling of being understood, lead author Gurit Birnbaum of Reichman University in Herzliya, Israel, said in a written statement. These things ultimately help couples navigate the rough patches and strengthen bonds, making them even less likely to cheat.
The researchers noted that even people in satisfying relationships do cheat, but this approach does encourage people to stop and think before they act. It could ultimately prevent what might be a huge mistake.
Think before they act. Hmm, that’s kind of like look before they leap, right? Sounds like a job for the Cliche-busters.
Washington medical board charges doctor with spreading COVID misinformation
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Expert gives tips on less-discussed dermatologic diseases
ORLANDO – , according to Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
These semi-forsaken diseases are important not to miss and can “also be quite challenging when we think about their management,” he said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Friedman, also director of the GW dermatology residency program, reviewed several of these diseases – along with tips for management – during a session at the meeting.
. It does not always have the classic ring pattern for which it is best known, he said. And in patients with darker skin tones, it is characterized by more of a brown or black color, rather than the pink-red color.
Dr. Friedman said that despite a kind of “Pavlovian response” linking GA with diabetes, this link might not be as strong as the field has come to believe, since the studies on which this belief was based included a patient population with narrow demographics. “Maybe GA and type 1 diabetes aren’t necessarily connected,” he said.
Dyslipidemia, on the other hand, has a strong connection with GA, he said. The disease is also linked to thyroid disease and is linked with malignancy, especially in older patients with generalized or atypical presentations of GA, he said.
Spontaneous resolution of the disease is seen within 2 years for 50% to 75% of patients, so “no treatment may be the best treatment,” but antimalarials can be effective, Dr. Friedman said. “I use antimalarials frequently in my practice,” he said. “The key is, they take time to work (4-5 months),” which should be explained to patients.
Antibiotics, he said, can be “somewhat effective,” but in the case of doxycycline at least, the disease can resolve within weeks but then may return when treatment is stopped.
There is some evidence to support using biologics and more recently, Janus kinase (JAK) inhibitors, off-label, to treat GA. Efficacy has been seen with the tumor necrosis factor (TNF) blocker infliximab and with the JAK inhibitor tofacitinib, he said.
Lichen planus (LP). This is another common disease that can go off-script with its presentation. The disease is often described with the “six P’s” indicating the following characteristics: pruritic, polygonal, planar or flat-topped, purple papules, and plaques. But LP “didn’t read the textbook,” Dr. Friedman said.
“The clinical presentation of lichen planus can be quite broad,” he said. “The P’s aren’t always followed as there are a variety of colors and configurations which can be witnessed.”
With LP, there is a clear association with dyslipidemia and diabetes, so “asking the right questions is going to be important” when talking to the patient. There is also a higher risk of autoimmune diseases, especially of the thyroid type, associated with LP, he said.
No treatment has been Food and Drug Administration approved for LP, but some are expected in the future, he said.
For now, he emphasized creativity in the management of patients with LP. “I love oral retinoids for this,” he said. Antimalarials and methotrexate are also options.
In one case Dr. Friedman saw, nothing seemed to work: light therapy for a year; metronidazole; isotretinoin; halobetasol/tazarotene lotion; and the TNF-blocker adalimumab either weren’t effective or resulted in complications in the patient.
Knowing the recent implication of the interleukin (IL)-17 pathway in the pathophysiology of LP, he then tried the anti-IL17 antibody secukinumab. “This patient had a pretty robust response to treatment,” Dr. Friedman said. “He was very excited. The problem, as always, is access, especially for off-label therapies.”
Tumid lupus erythematosus. This disease is characterized by erythematous, edematous, nonscarring plaques on sun-exposed sites. For treatment, Dr. Friedman said antimalarials can be up to 90% effective, sometimes with rapid resolution of the lesions.
“You want to dose below that 5 mg per kg of true body weight to limit the small potential for ocular toxicity over time,” he said. And, he emphasized, “always combine treatment with good sun-protective measures.”
Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
ORLANDO – , according to Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
These semi-forsaken diseases are important not to miss and can “also be quite challenging when we think about their management,” he said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Friedman, also director of the GW dermatology residency program, reviewed several of these diseases – along with tips for management – during a session at the meeting.
. It does not always have the classic ring pattern for which it is best known, he said. And in patients with darker skin tones, it is characterized by more of a brown or black color, rather than the pink-red color.
Dr. Friedman said that despite a kind of “Pavlovian response” linking GA with diabetes, this link might not be as strong as the field has come to believe, since the studies on which this belief was based included a patient population with narrow demographics. “Maybe GA and type 1 diabetes aren’t necessarily connected,” he said.
Dyslipidemia, on the other hand, has a strong connection with GA, he said. The disease is also linked to thyroid disease and is linked with malignancy, especially in older patients with generalized or atypical presentations of GA, he said.
Spontaneous resolution of the disease is seen within 2 years for 50% to 75% of patients, so “no treatment may be the best treatment,” but antimalarials can be effective, Dr. Friedman said. “I use antimalarials frequently in my practice,” he said. “The key is, they take time to work (4-5 months),” which should be explained to patients.
Antibiotics, he said, can be “somewhat effective,” but in the case of doxycycline at least, the disease can resolve within weeks but then may return when treatment is stopped.
There is some evidence to support using biologics and more recently, Janus kinase (JAK) inhibitors, off-label, to treat GA. Efficacy has been seen with the tumor necrosis factor (TNF) blocker infliximab and with the JAK inhibitor tofacitinib, he said.
Lichen planus (LP). This is another common disease that can go off-script with its presentation. The disease is often described with the “six P’s” indicating the following characteristics: pruritic, polygonal, planar or flat-topped, purple papules, and plaques. But LP “didn’t read the textbook,” Dr. Friedman said.
“The clinical presentation of lichen planus can be quite broad,” he said. “The P’s aren’t always followed as there are a variety of colors and configurations which can be witnessed.”
With LP, there is a clear association with dyslipidemia and diabetes, so “asking the right questions is going to be important” when talking to the patient. There is also a higher risk of autoimmune diseases, especially of the thyroid type, associated with LP, he said.
No treatment has been Food and Drug Administration approved for LP, but some are expected in the future, he said.
For now, he emphasized creativity in the management of patients with LP. “I love oral retinoids for this,” he said. Antimalarials and methotrexate are also options.
In one case Dr. Friedman saw, nothing seemed to work: light therapy for a year; metronidazole; isotretinoin; halobetasol/tazarotene lotion; and the TNF-blocker adalimumab either weren’t effective or resulted in complications in the patient.
Knowing the recent implication of the interleukin (IL)-17 pathway in the pathophysiology of LP, he then tried the anti-IL17 antibody secukinumab. “This patient had a pretty robust response to treatment,” Dr. Friedman said. “He was very excited. The problem, as always, is access, especially for off-label therapies.”
Tumid lupus erythematosus. This disease is characterized by erythematous, edematous, nonscarring plaques on sun-exposed sites. For treatment, Dr. Friedman said antimalarials can be up to 90% effective, sometimes with rapid resolution of the lesions.
“You want to dose below that 5 mg per kg of true body weight to limit the small potential for ocular toxicity over time,” he said. And, he emphasized, “always combine treatment with good sun-protective measures.”
Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
ORLANDO – , according to Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington.
These semi-forsaken diseases are important not to miss and can “also be quite challenging when we think about their management,” he said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dr. Friedman, also director of the GW dermatology residency program, reviewed several of these diseases – along with tips for management – during a session at the meeting.
. It does not always have the classic ring pattern for which it is best known, he said. And in patients with darker skin tones, it is characterized by more of a brown or black color, rather than the pink-red color.
Dr. Friedman said that despite a kind of “Pavlovian response” linking GA with diabetes, this link might not be as strong as the field has come to believe, since the studies on which this belief was based included a patient population with narrow demographics. “Maybe GA and type 1 diabetes aren’t necessarily connected,” he said.
Dyslipidemia, on the other hand, has a strong connection with GA, he said. The disease is also linked to thyroid disease and is linked with malignancy, especially in older patients with generalized or atypical presentations of GA, he said.
Spontaneous resolution of the disease is seen within 2 years for 50% to 75% of patients, so “no treatment may be the best treatment,” but antimalarials can be effective, Dr. Friedman said. “I use antimalarials frequently in my practice,” he said. “The key is, they take time to work (4-5 months),” which should be explained to patients.
Antibiotics, he said, can be “somewhat effective,” but in the case of doxycycline at least, the disease can resolve within weeks but then may return when treatment is stopped.
There is some evidence to support using biologics and more recently, Janus kinase (JAK) inhibitors, off-label, to treat GA. Efficacy has been seen with the tumor necrosis factor (TNF) blocker infliximab and with the JAK inhibitor tofacitinib, he said.
Lichen planus (LP). This is another common disease that can go off-script with its presentation. The disease is often described with the “six P’s” indicating the following characteristics: pruritic, polygonal, planar or flat-topped, purple papules, and plaques. But LP “didn’t read the textbook,” Dr. Friedman said.
“The clinical presentation of lichen planus can be quite broad,” he said. “The P’s aren’t always followed as there are a variety of colors and configurations which can be witnessed.”
With LP, there is a clear association with dyslipidemia and diabetes, so “asking the right questions is going to be important” when talking to the patient. There is also a higher risk of autoimmune diseases, especially of the thyroid type, associated with LP, he said.
No treatment has been Food and Drug Administration approved for LP, but some are expected in the future, he said.
For now, he emphasized creativity in the management of patients with LP. “I love oral retinoids for this,” he said. Antimalarials and methotrexate are also options.
In one case Dr. Friedman saw, nothing seemed to work: light therapy for a year; metronidazole; isotretinoin; halobetasol/tazarotene lotion; and the TNF-blocker adalimumab either weren’t effective or resulted in complications in the patient.
Knowing the recent implication of the interleukin (IL)-17 pathway in the pathophysiology of LP, he then tried the anti-IL17 antibody secukinumab. “This patient had a pretty robust response to treatment,” Dr. Friedman said. “He was very excited. The problem, as always, is access, especially for off-label therapies.”
Tumid lupus erythematosus. This disease is characterized by erythematous, edematous, nonscarring plaques on sun-exposed sites. For treatment, Dr. Friedman said antimalarials can be up to 90% effective, sometimes with rapid resolution of the lesions.
“You want to dose below that 5 mg per kg of true body weight to limit the small potential for ocular toxicity over time,” he said. And, he emphasized, “always combine treatment with good sun-protective measures.”
Dr. Friedman reported financial relationships with Sanova, Pfizer, Novartis, and other companies.
AT ODAC 2023
Expert offers insights on pediatric dermatology emergencies
ORLANDO – The eruption spread away from the head and her transaminase levels were “dramatic,” in the 700s, said Kalyani S. Marathe, MD, MPH, associate professor of dermatology and pediatrics at the University of Cincinnati.
Dr. Marathe, director of the division of dermatology at Cincinnati Children’s Hospital, reviewed this case in a presentation on pediatric dermatologic emergencies at the ODAC Dermatology, Aesthetic & Surgery Conference, pointing out potential pitfalls and important aspects that might require swift action.
The patient was diagnosed with drug reaction with eosinophilia and systemic symptoms (DRESS).
Facial involvement is common in pediatric cases of DRESS, but edema of the face is less common in children than adults, Dr. Marathe said.
Antiepileptic medications are the most common cause of DRESS, followed by antibiotics – most often, vancomycin and trimethoprim/sulfamethoxazole, she said. But sometimes the trigger is not clear, she noted, recalling a vexing case she once saw in which IV contrast was eventually identified as the cause.
When DRESS is suspected, she said, lab work should be done during the acute eruption and after resolution. This should include CBC, liver function tests, creatinine, and urinalysis, and human herpesvirus 6 (HHV-6) and thyroid testing.
Treatment typically includes supportive care, unless symptoms are systemic, or if there is impending liver failure, when steroids, cyclosporine, or IVIG can be used.
Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN): Mortality rates when these diseases overlap is 4%, Dr. Marathe said. Clues to diagnosing this other medication-induced condition include involvement of the palms and the soles of the feet; presence of the Nikolsky sign in which the top layers of the skin slip away from the lower layers when rubbed; mucosal involvement, which often precedes cutaneous involvement; and these symptoms occurring within the first 8 weeks of taking a medication, which are most commonly antibiotics and anti-epileptics.
Dr. Marathe underscored how important it is to get ophthalmology involved right away, because of the risk of vision loss. Amniotic membrane transfer to the eye at the time of diagnosis has been found to produce dramatically better outcomes, she said. The membrane has anti-inflammatory and antiscarring properties and can promote wound healing on the surface of the eye.
“I would recommend getting your ophthalmology team on board early because they have to advocate for these patients,” she said.
Corticosteroids and IVIG can improve ocular outcomes, but cyclosporine is associated with better mortality outcomes, she said. Emerging data on etanercept has also led to more use of that drug, she said.
Erythema multiforme (EM): unlike urticaria, multiforme EM can have mucosal involvement, Dr. Marathe said. Clinicians should look for three zones of color: A central duskiness, a rim of pallor, and a ring of erythema.
EM is triggered by a virus, which is usually herpes simplex virus (HSV). But she added that HSV is not always found. “So, there are certainly other triggers out there that we just haven’t identified,” she said.
If HSV is suspected, oral acyclovir is effective, she noted.
Other cases might not be as straightforward. Dr. Marathe said that during her fellowship, she saw a patient with EM that was controlled only by IVIG, so it was administered every 3 months. In that case, the trigger was never found.
Multisystem inflammatory syndrome in children (MIS-C): This syndrome can follow COVID-19 infection, and usually presents with 3-5 days of fever after COVID has resolved. It can include gastrointestinal, cardiorespiratory, and neurocognitive symptoms.
The skin presentation is mainly a morbilliform pattern, but clinicians might also see conjunctival involvement, mucosal involvement, and “COVID toes,” painful red or purple lesions on the toes.
Treatment is usually IVIG and systemic corticosteroids, with the treatment course depending on the severity.
MIS-C was initially thought to be Kawasaki’s disease, another autoinflammatory disorder, which is related but distinct, Dr. Marathe said.
Patients with MIS-C “are usually going to have COVID-positive antibodies,” she said. But since almost everybody may have COVID antibodies, “it’s not usually a helpful test for you now. But early on, that’s what we used as helpful indicator.”
Dr. Marathe reported no relevant financial relationships.
ORLANDO – The eruption spread away from the head and her transaminase levels were “dramatic,” in the 700s, said Kalyani S. Marathe, MD, MPH, associate professor of dermatology and pediatrics at the University of Cincinnati.
Dr. Marathe, director of the division of dermatology at Cincinnati Children’s Hospital, reviewed this case in a presentation on pediatric dermatologic emergencies at the ODAC Dermatology, Aesthetic & Surgery Conference, pointing out potential pitfalls and important aspects that might require swift action.
The patient was diagnosed with drug reaction with eosinophilia and systemic symptoms (DRESS).
Facial involvement is common in pediatric cases of DRESS, but edema of the face is less common in children than adults, Dr. Marathe said.
Antiepileptic medications are the most common cause of DRESS, followed by antibiotics – most often, vancomycin and trimethoprim/sulfamethoxazole, she said. But sometimes the trigger is not clear, she noted, recalling a vexing case she once saw in which IV contrast was eventually identified as the cause.
When DRESS is suspected, she said, lab work should be done during the acute eruption and after resolution. This should include CBC, liver function tests, creatinine, and urinalysis, and human herpesvirus 6 (HHV-6) and thyroid testing.
Treatment typically includes supportive care, unless symptoms are systemic, or if there is impending liver failure, when steroids, cyclosporine, or IVIG can be used.
Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN): Mortality rates when these diseases overlap is 4%, Dr. Marathe said. Clues to diagnosing this other medication-induced condition include involvement of the palms and the soles of the feet; presence of the Nikolsky sign in which the top layers of the skin slip away from the lower layers when rubbed; mucosal involvement, which often precedes cutaneous involvement; and these symptoms occurring within the first 8 weeks of taking a medication, which are most commonly antibiotics and anti-epileptics.
Dr. Marathe underscored how important it is to get ophthalmology involved right away, because of the risk of vision loss. Amniotic membrane transfer to the eye at the time of diagnosis has been found to produce dramatically better outcomes, she said. The membrane has anti-inflammatory and antiscarring properties and can promote wound healing on the surface of the eye.
“I would recommend getting your ophthalmology team on board early because they have to advocate for these patients,” she said.
Corticosteroids and IVIG can improve ocular outcomes, but cyclosporine is associated with better mortality outcomes, she said. Emerging data on etanercept has also led to more use of that drug, she said.
Erythema multiforme (EM): unlike urticaria, multiforme EM can have mucosal involvement, Dr. Marathe said. Clinicians should look for three zones of color: A central duskiness, a rim of pallor, and a ring of erythema.
EM is triggered by a virus, which is usually herpes simplex virus (HSV). But she added that HSV is not always found. “So, there are certainly other triggers out there that we just haven’t identified,” she said.
If HSV is suspected, oral acyclovir is effective, she noted.
Other cases might not be as straightforward. Dr. Marathe said that during her fellowship, she saw a patient with EM that was controlled only by IVIG, so it was administered every 3 months. In that case, the trigger was never found.
Multisystem inflammatory syndrome in children (MIS-C): This syndrome can follow COVID-19 infection, and usually presents with 3-5 days of fever after COVID has resolved. It can include gastrointestinal, cardiorespiratory, and neurocognitive symptoms.
The skin presentation is mainly a morbilliform pattern, but clinicians might also see conjunctival involvement, mucosal involvement, and “COVID toes,” painful red or purple lesions on the toes.
Treatment is usually IVIG and systemic corticosteroids, with the treatment course depending on the severity.
MIS-C was initially thought to be Kawasaki’s disease, another autoinflammatory disorder, which is related but distinct, Dr. Marathe said.
Patients with MIS-C “are usually going to have COVID-positive antibodies,” she said. But since almost everybody may have COVID antibodies, “it’s not usually a helpful test for you now. But early on, that’s what we used as helpful indicator.”
Dr. Marathe reported no relevant financial relationships.
ORLANDO – The eruption spread away from the head and her transaminase levels were “dramatic,” in the 700s, said Kalyani S. Marathe, MD, MPH, associate professor of dermatology and pediatrics at the University of Cincinnati.
Dr. Marathe, director of the division of dermatology at Cincinnati Children’s Hospital, reviewed this case in a presentation on pediatric dermatologic emergencies at the ODAC Dermatology, Aesthetic & Surgery Conference, pointing out potential pitfalls and important aspects that might require swift action.
The patient was diagnosed with drug reaction with eosinophilia and systemic symptoms (DRESS).
Facial involvement is common in pediatric cases of DRESS, but edema of the face is less common in children than adults, Dr. Marathe said.
Antiepileptic medications are the most common cause of DRESS, followed by antibiotics – most often, vancomycin and trimethoprim/sulfamethoxazole, she said. But sometimes the trigger is not clear, she noted, recalling a vexing case she once saw in which IV contrast was eventually identified as the cause.
When DRESS is suspected, she said, lab work should be done during the acute eruption and after resolution. This should include CBC, liver function tests, creatinine, and urinalysis, and human herpesvirus 6 (HHV-6) and thyroid testing.
Treatment typically includes supportive care, unless symptoms are systemic, or if there is impending liver failure, when steroids, cyclosporine, or IVIG can be used.
Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN): Mortality rates when these diseases overlap is 4%, Dr. Marathe said. Clues to diagnosing this other medication-induced condition include involvement of the palms and the soles of the feet; presence of the Nikolsky sign in which the top layers of the skin slip away from the lower layers when rubbed; mucosal involvement, which often precedes cutaneous involvement; and these symptoms occurring within the first 8 weeks of taking a medication, which are most commonly antibiotics and anti-epileptics.
Dr. Marathe underscored how important it is to get ophthalmology involved right away, because of the risk of vision loss. Amniotic membrane transfer to the eye at the time of diagnosis has been found to produce dramatically better outcomes, she said. The membrane has anti-inflammatory and antiscarring properties and can promote wound healing on the surface of the eye.
“I would recommend getting your ophthalmology team on board early because they have to advocate for these patients,” she said.
Corticosteroids and IVIG can improve ocular outcomes, but cyclosporine is associated with better mortality outcomes, she said. Emerging data on etanercept has also led to more use of that drug, she said.
Erythema multiforme (EM): unlike urticaria, multiforme EM can have mucosal involvement, Dr. Marathe said. Clinicians should look for three zones of color: A central duskiness, a rim of pallor, and a ring of erythema.
EM is triggered by a virus, which is usually herpes simplex virus (HSV). But she added that HSV is not always found. “So, there are certainly other triggers out there that we just haven’t identified,” she said.
If HSV is suspected, oral acyclovir is effective, she noted.
Other cases might not be as straightforward. Dr. Marathe said that during her fellowship, she saw a patient with EM that was controlled only by IVIG, so it was administered every 3 months. In that case, the trigger was never found.
Multisystem inflammatory syndrome in children (MIS-C): This syndrome can follow COVID-19 infection, and usually presents with 3-5 days of fever after COVID has resolved. It can include gastrointestinal, cardiorespiratory, and neurocognitive symptoms.
The skin presentation is mainly a morbilliform pattern, but clinicians might also see conjunctival involvement, mucosal involvement, and “COVID toes,” painful red or purple lesions on the toes.
Treatment is usually IVIG and systemic corticosteroids, with the treatment course depending on the severity.
MIS-C was initially thought to be Kawasaki’s disease, another autoinflammatory disorder, which is related but distinct, Dr. Marathe said.
Patients with MIS-C “are usually going to have COVID-positive antibodies,” she said. But since almost everybody may have COVID antibodies, “it’s not usually a helpful test for you now. But early on, that’s what we used as helpful indicator.”
Dr. Marathe reported no relevant financial relationships.
AT ODAC 2023
First Humira biosimilar launches in U.S.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
The Ins and Outs of Transferring Residency Programs
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
Transferring from one residency program to another is rare but not unheard of. According to the most recent Accreditation Council for Graduate Medical Education Data Resource Book, there were 1020 residents who transferred residency programs in the 2020-2021 academic year.1 With a total of 126,759 active residents in specialty programs, the percentage of transferring residents was less than 1%. The specialties with the highest number of transferring residents included psychiatry, general surgery, internal medicine, and family medicine. In dermatology programs, there were only 2 resident transfers during the 2019-2020 academic year and 6 transfers in the 2020-2021 academic year.1,2 A resident contemplating transferring training programs must carefully consider the advantages and disadvantages before undertaking the uncertain transfer process, but transferring residency programs can be achieved successfully with planning and luck.
Deciding to Transfer
The decision to transfer residency programs may be a difficult one that is wrought with anxiety. There are many reasons why a trainee may wish to pursue transferring training programs. A transfer to another geographic area may be necessary for personal or family reasons, such as to reunite with a spouse and children or to care for a sick family member. A resident may find their program to be a poor fit and may wish to train in a different educational environment. Occasionally, a program can lose its accreditation, and its residents will be tasked with finding a new position elsewhere. A trainee also may realize that the specialty they matched into initially does not align with their true passions. It is important for the potential transfer applicant to be levelheaded about their decision. Residency is a demanding period for every trainee; switching programs may not be the best solution for every problem and should only be considered if essential.
Transfer Timing
A trainee may have thoughts of leaving a program soon after starting residency or perhaps even before starting if their National Resident Matching Program (NRMP) Match result was a disappointment; however, there are certain rules related to transfer timing. The NRMP Match represents a binding commitment for both the applicant and program. If for any reason an applicant will not honor the binding commitment, the NRMP requires the applicant to initiate a waiver review, which can be requested for unanticipated serious and extreme hardship, change of specialty, or ineligibility. According to the NRMP rules and regulations, applicants cannot apply for, discuss, interview for, or accept a position in another program until a waiver has been granted.3 Waivers based on change of specialty must be requested by mid-January prior to the start of training, which means most applicants who match to positions that begin in the same year of the Match do not qualify for change of specialty waivers. However, those who matched to an advanced position and are doing a preliminary year position may consider this option if they have a change of heart during their internship. The NRMP may consider a 1-year deferral to delay training if mutually agreed upon by both the matched applicant and the program.3 The binding commitment is in place for the first 45 days of training, and applicants who resign within 45 days or a program that tries to solicit the transfer of a resident prior to that date could be in violation of the Match and can face consequences such as being barred from entering the matching process in future cycles. Of the 1020 transfers that occurred among residents in specialty programs during the 2020-2021 academic year, 354 (34.7%) occurred during the first year of the training program; 228 (22.4%) occurred during the second year; 389 (38.1%) occurred during the third year; and 49 (4.8%) occurred in the fourth, fifth, or sixth year of the program.1 Unlike other jobs/occupations in which one can simply give notice, in medical training even if a transfer position is accepted, the transition date between programs must be mutually agreed upon. Often, this may coincide with the start of the new academic year.
The Transfer Process
Transferring residency programs is a substantial undertaking. Unlike the Match, a trainee seeking to transfer programs does so without a standardized application system or structured support through the process; the transfer applicant must be prepared to navigate the transfer process on their own. The first step after making the decision to transfer is for the resident to meet with the program leadership (ie, program director[s], coordinator, designated official) at their home program to discuss the decision—a nerve-wracking but imperative first step. A receiving program may not favor an applicant secretly applying to a new program without the knowledge of their home program and often will require the home program’s blessing to proceed. The receiving program also would want to ensure the applicant is in good standing and not leaving due to misconduct. Once given the go-ahead, the process is largely in the hands of the applicant. The transfer applicant should identify locations or programs of interest and then take initiative to reach out to potential programs. FREIDA (Fellowship and Residency Electronic Interactive Database Access) is the American Medical Association’s residency and fellowship database that allows vacant position listings to be posted online.4 Additionally, the Association of American Medical Colleges’ FindAResident website is a year-round search tool designed to help find open residency and fellowship positions.5 Various specialties also may have program director listserves that communicate vacant positions. On occasion, there are spots in the main NRMP Match that are reserved positions (“R”). These are postgraduate year 2 positions in specialty programs that begin in the year of the Match and are reserved for physicians with prior graduate medical education; these also are known as “Physician Positions.”6 Ultimately, advertisements for vacancies may be few and far between, requiring the resident to send unsolicited emails with curriculum vitae attached to the program directors at programs of interest to inquire about any vacancies and hope for a favorable response. Even if the transfer applicant is qualified, luck that the right spot will be available at the right time may be the deciding factor in transferring programs.
The next step is interviewing for the position. There likely will be fewer candidates interviewing for an open spot but that does not make the process less competitive. The candidate should highlight their strengths and achievements and discuss why the new program would be a great fit both personally and professionally. Even if an applicant is seeking a transfer due to discontent with a prior program, it is best to act graciously and not speak poorly about another training program.
Prior to selection, the candidate may be asked to provide information such as diplomas, US Medical Licensing Examination Step and residency in-service training examination scores, and academic reviews from their current residency program. The interview process may take several weeks as the graduate medical education office often will need to officially approve of an applicant before a formal offer to transfer is extended.
Finally, once an offer is made and accepted, there still is a great amount of paperwork to complete before the transition. The applicant should stay on track with all off-boarding and on-boarding requirements, such as signing a contract, obtaining background checks, and applying for a new license to ensure the switch is not delayed.
Disadvantages of Transferring Programs
The transfer process is not easy to navigate and can be a source of stress for the applicant. It is natural to fear resentment from colleagues and co-residents. Although transferring programs might be in the best interest of the trainee, it may leave a large gap in the program that they are leaving, which can place a burden on the remaining residents.
There are many adjustments to be made after transferring programs. The transferring resident will again start from scratch, needing to learn the ropes and adapt to the growing pains of being at a new institution. This may require learning a completely new electronic medical record, adapting to a new culture, and in many cases stepping in as a senior resident without fully knowing the ins and outs of the program.
Advantages of Transferring Programs
Successfully transferring programs is something to celebrate. There may be great benefits to transferring to a program that is better suited to the trainee—either personally or professionally. Ameliorating the adversity that led to the decision to transfer such as reuniting a long-distance family or realizing one’s true passion can allow the resident to thrive as a trainee and maximize their potential. Transferring programs can give a resident a more well-rounded training experience, as different programs may have different strengths, patient populations, and practice settings. Working with different faculty members with varied niches and practice styles can create a more comprehensive residency experience.
Final Thoughts
Ultimately, transferring residency programs is not easy but also is not impossible. Successfully switching residency programs can be a rewarding experience providing greater well-being and fulfillment.
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2021-2022. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2021-2022_acgme__databook_document.pdf
- Accreditation Council for Graduate Medical Education. Data Resource Book, Academic Year 2020-2021. Accreditation Council for Graduate Medical Education. Accessed January 20, 2023. https://www.acgme.org/globalassets/pfassets/publicationsbooks/2020-2021_acgme_databook_document.pdf
- After the Match. National Resident Matching Program website. Accessed January 23, 2023. https://www.nrmp.org/fellowship-applicants/after-the-match/
- FREIDA vacant position listings. American Medical Association website. Accessed January 23, 2023. https://freida.ama-assn.org/vacant-position
- FindAResident. Association of American Medical Colleges website. Accessed January 23, 2023. https://students-residents.aamc.org/findaresident/findaresident
- What are the types of program positions in the main residency match? National Resident Matching Program website. Published August 5, 2021. Accessed January 23, 2023. https://www.nrmp.org/help/item/what-types-of-programs-participate-in-the-main-residency-match/
RESIDENT PEARL
- Transferring residency programs is difficult but possible. The decision to transfer residencies may be anxiety producing, but with substantial motives, the rewards of transferring can be worthwhile.
Hemorrhagic Lacrimation and Epistaxis: Rare Findings in Acute Hemorrhagic Edema of Infancy
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.
A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.
Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.

A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.

Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
To the Editor:
Hemorrhagic lacrimation and epistaxis are dramatic presentations with a narrow differential diagnosis. It rarely has been reported to present alongside the more typical features of acute hemorrhagic edema of infancy (AHEI), which is a benign self-limited leukocytoclastic vasculitis most often seen in children aged 4 months to 2 years. Extracutaneous involvement rarely is seen in AHEI, though joint, gastrointestinal tract, and renal involvement have been reported.1 Most patients present with edematous, annular, or cockade purpuric vasculitic lesions classically involving the face and distal extremities with relative sparing of the trunk. We present a case of a well-appearing, 10-month-old infant boy with hemorrhagic vasculitic lesions, acral edema, and an associated episode of hemorrhagic lacrimation and epistaxis.

A 10-month-old infant boy who was otherwise healthy presented to the emergency department (ED) with an acute-onset, progressively worsening cutaneous eruption of 2 days’ duration. A thorough history revealed that the eruption initially had presented as several small, bright-red papules on the thighs. The eruption subsequently spread to involve the buttocks, legs, and arms (Figures 1 and 2). The parents also noted that the patient had experienced an episode of bloody tears and epistaxis that lasted a few minutes at the pediatrician’s office earlier that morning, a finding that prompted the urgent referral to the ED.

Dermatology was then consulted. A review of systems was notable for rhinorrhea and diarrhea during the week leading to the eruption. The patient’s parents denied fevers, decreased oral intake, or a recent course of antibiotics. The patient’s medical history was notable only for atopic dermatitis treated with emollients and occasional topical steroids. The parents denied recent travel or vaccinations. Physical examination showed an afebrile, well-appearing infant with multiple nontender, slightly edematous, circular, purpuric papules and plaques scattered on the buttocks and extremities with edema on the dorsal feet. The remainder of the patient’s workup in the ED was notable for mild elevations in C-reactive protein levels (1.4 mg/dL [reference range, 0–1.2 mg/dL]) and an elevated erythrocyte sedimentation rate (22 mm/h [reference range, 2–12 mm/h]). A complete blood cell count; liver function tests; urinalysis; and coagulation studies, including prothrombin, partial thromboplastin time, and international normalized ratio, were unremarkable. Acute hemorrhagic edema of infancy was diagnosed based on the clinical manifestations.
Acute hemorrhagic edema of infancy (also known as Finkelstein disease, medallionlike purpura, Seidemayer syndrome, infantile postinfectious irislike purpura and edema, and purpura en cocarde avec oedeme) is believed to result from an immune complex–related reaction, often in the setting of an upper respiratory tract infection; medications, especially antibiotics; or vaccinations. The condition previously was considered a benign form of Henoch-Schönlein purpura; however, it is now recognized as its own clinical entity. Acute hemorrhagic edema of infancy commonly affects children between the ages of 4 months and 2 years. The incidence peaks in the winter months, and males tend to be more affected than females.1
Acute hemorrhagic edema of infancy is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Edema can develop on the hands, feet, and genitalia. Importantly, facial edema has been noted to precede skin lesions.2 Coin-shaped or targetoid hemorrhagic and purpuric lesions in a cockade or rosette pattern with scalloped margins typically begin on the distal extremities and tend to spread proximally. The lesions are variable in size but have been reported to be as large as 5 cm in diameter. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.3 Hemorrhagic lacrimation and epistaxis—both present in our patient—are rare findings with AHEI. It is likely that most providers, including dermatologists, may be unfamiliar with these striking clinical findings. Although the pathophysiology of hemorrhagic lacrimation and epistaxis has not been formally investigated, we postulate that it likely is related to the formation of immune complexes that lead to small vessel vasculitis, underpinning the characteristic findings in AHEI.4,5 This reasoning is supported by the complete resolution of symptoms corresponding with clinical clearance of the cutaneous vasculitis in 2 prior cases4,5 as well as in our patient who did not have a relapse of symptoms following cessation of the cutaneous eruption at a pediatric follow-up appointment 2 weeks later.
Acute hemorrhagic edema of infancy is a clinical diagnosis; however, a skin biopsy can be performed to confirm the clinical suspicion and rule out more serious conditions. Histopathologic examination reveals a leukocytoclastic vasculitis involving the capillaries and postcapillary venules of the upper and mid dermis. Laboratory test results usually are nonspecific but can help distinguish AHEI from more serious diseases. The erythrocyte sedimentation rate and C-reactive protein level may be slightly elevated in infants with AHEI. Urinalysis and stool guaiac tests also can be performed to evaluate for any renal or gastrointestinal involvement.6
The differential diagnosis includes IgA vasculitis, erythema multiforme, acute meningococcemia, urticarial vasculitis, Kawasaki disease, and child abuse. IgA vasculitis often presents with more systemic involvement, with abdominal pain, vomiting, hematemesis, diarrhea, and hematochezia occurring in up to 50% of patients. The cutaneous findings of erythema multiforme classically are confined to the limbs and face, and edema of the extremities typically is not seen. Patients with acute meningococcemia appear toxic with high fevers, malaise, and possible septic shock.5
Acute hemorrhagic edema of infancy is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.7 Antibiotics should be given to treat concurrent bacterial infections, and antihistamines and steroids may be useful for symptomatic relief. Importantly, however, systemic corticosteroids do not appear to conclusively alter the disease course.8
Acute hemorrhagic edema of infancy is a rare benign leukocytoclastic vasculitis with a striking presentation often seen following an upper respiratory tract infection or course of antibiotics. Our case demonstrates that on rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis—findings that can be quite alarming to both parents and medical providers. Nonetheless, patients and their caretakers should be assured that the condition is self-limited and resolves without permanent sequalae.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
- Emerich PS, Prebianchi PA, Motta LL, et al. Acute hemorrhagic edema of infancy: report of three cases. An Bras Dermatol. 2011;86:1181-1184.
- Avhad G, Ghuge P, Jerajani H. Acute hemorrhagic edema of infancy. Indian Dermatol Online J. 2014;5:356-357.
- Krause I, Lazarov A, Rachmel A, et al. Acute haemorrhagic oedema of infancy, a benign variant of leucocytoclastic vasculitis. Acta Paediatr. 1996;85:114-117.
- Sneller H, Vega C, Zemel L, et al. Acute hemorrhagic edema of infancy with associated hemorrhagic lacrimation. Pediatr Emerg Care. 2021;37:E70-E72. doi:10.1097/PEC.0000000000001542
- Mreish S, Al-Tatari H. Hemorrhagic lacrimation and epistaxis in acute hemorrhagic edema of infancy. Case Rep Pediatr. 2016;2016:9762185. doi:10.1155/2016/9762185
- Savino F, Lupica MM, Tarasco V, et al. Acute hemorrhagic edema of infancy: a troubling cutaneous presentation with a self-limiting course. Pediatr Dermatol. 2013;30:E149-E152.
- Fiore E, Rizzi M, Ragazzi M, et al. Acute hemorrhagic edema of young children (cockade purpura and edema): a case series and systematic review. J Am Acad Dermatol. 2008;59:684-695.
- Acute hemorrhagic edema of young children: a concise narrative review. Eur J Pediatr. 2011;170:1507-1511.
PRACTICE POINTS
- Acute hemorrhagic edema of infancy (AHEI) is clinically characterized by a triad of large purpuric lesions, low-grade fever, and peripheral acral edema. Although joint pain, bloody diarrhea, hematuria, and proteinuria can accompany AHEI, most cases are devoid of systemic symptoms.
- It is a self-limited condition typically lasting 1 to 3 weeks and requires only supportive care.
- On rare occasions, AHEI may be accompanied by hemorrhagic lacrimation and epistaxis. Patients should be assured that the condition is self-limited and resolves without permanent sequalae.
Skin of Color Society Scientific Symposium Winners: 2022
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.
The 18th Annual Skin of Color Society Scientific Symposium was held in March 2022 in Boston, Massachusetts. With a theme of Diversity in Action: Science, Healthcare & Society, researchers gathered to present new findings, share key insights, and discuss the continuing evolution of the field. Three awards were presented from the scientific posters at the symposium.
The Best Poster Presentation Award was presented to Brandyn M. White, BS, for “A Preliminary Analysis of the DDB1 Gene: Genome-Wide Association Studies in African and Admixed African American Populations—Is Our Skin Different?” authored by Brandyn M. White, BS; Chidubem A.V. Okeke, BS; Raveena Khanna, MD; Ginette A. Okoye, MD; Michael C. Campbell, PhD; and Angel S. Byrd, MD, PhD. Their research evaluated the association of variant DNA damage binding protein 1, DDB1, with African populations and highlighted the possible phenotypic variations between African and admixed African American populations. Further, it discussed the advantages of conducting future genome-wide association studies in the Washington metropolitan area to better understand dermatological diseases that disproportionately affect skin of color patients.
The Best Oral Presentation Award was presented to Erica Ogwumike, BA, for “Matching into Dermatology Residency: The Impact of Research Fellowships” authored by Erica Ogwumike, BA; Chine Chime, MS, MPH; and Rebecca Vasquez, MD. The aim of this study was to explore what variables were important for 2 events: taking a research fellowship and matching into dermatology. The authors analyzed Electronic Residency Application Service (ERAS) applications for all medical students applying to the UT Southwestern Dermatology Residency Program in the 2014-2015 cycle. They found that 1 of 5 students participated in a research fellowship prior to applying to dermatology residency, and it was not associated with increased odds of matching. They also discovered that students more likely to take a research fellowship were Latinx, attended a medical school ranked in the Top 25, and were not Alpha Omega Alpha members. Nevertheless, total publications did increase the odds of matching; therefore, the authors concluded that when looking for a research fellowship, applicants should look for one that allows productivity so that this measure can be achieved. Further investigation is needed to substantiate these results, but this study was a starting point to examine the characteristics involved in taking a research fellowship in dermatology.
Finally, the Crowd Favorite Award was presented to Jennifer Cucalon, BS, for “Non-invasive, In-Vivo RCM Monitoring of Lentigines Treated With Cryotherapy to Establish Minimum Freeze Time in Seconds (Dose) in Skin of Color” authored by Jennifer Cucalon, BS, and Babar K. Rao, MD. This pilot study showed a minimum freezing time of 3 seconds to be effective in removing lentigines in darker skin; increasing the dose to 6 and 9 seconds had no added benefit. The authors also demonstrated reflectance confocal microscopy to be an appropriate, noninvasive, in vivo tool to visualize pigmentary changes and monitor the effectiveness of treatments for various skin conditions.
The 19th Annual Scientific Symposium will take place on March 16, 2023, in New Orleans, Louisiana. The theme will be Where Science, Innovation & Inclusion Meet. For more information, visit https://skinofcolorsociety.org/19th-annual-skin-of-color-society-scientific-symposium/.