User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Vitiligo
THE COMPARISON
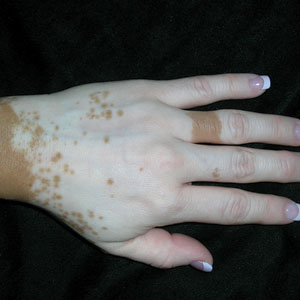
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
THE COMPARISON
A Vitiligo in a young Hispanic female, which spared the area under a ring. The patient has spotty return of pigment on the hand after narrowband UVB treatment.
B Vitiligo on the hand in a young Hispanic male.

Vitiligo is a chronic autoimmune disorder characterized by areas of depigmented white patches on the skin due to the loss of melanocytes in the epidermis. Various theories on the pathogenesis of vitiligo exist; however, autoimmune destruction of melanocytes remains the leading hypothesis, followed by intrinsic defects in melanocytes.1 Vitiligo is associated with various autoimmune diseases but is most frequently reported in conjunction with thyroid disorders.2
Epidemiology
Vitiligo affects approximately 1% of the US population and up to 8% worldwide.2 There is no difference in prevalence between races or genders. Females typically acquire the disease earlier than males. Onset may occur at any age, although about half of patients will have vitiligo by 20 years of age.1
Key clinical features in people with darker skin tones
Bright white patches are characteristic of vitiligo. The patches typically are asymptomatic and often affect the hands (Figures A and B), perioral skin, feet, and scalp, as well as areas more vulnerable to friction and trauma, such as the elbows and knees.2 Trichrome lesions—consisting of varying zones of white (depigmented), lighter brown (hypopigmented), and normal skin—are most commonly seen in individuals with darker skin. Trichrome vitiligo is considered an actively progressing variant of vitiligo.2
An important distinction when diagnosing vitiligo is evaluating for segmental vs nonsegmental vitiligo. Although nonsegmental vitiligo—the more common subtype—is characterized by symmetric distribution and a less predictable course, segmental vitiligo manifests in a localized and unilateral distribution, often avoiding extension past the midline. Segmental vitiligo typically manifests at a younger age and follows a more rapidly stabilizing course.3
Worth noting
Given that stark contrasts between pigmented and depigmented lesions are more prominent in darker skin tones, vitiligo can be more socially stigmatizing and psychologically devastating in these patients.4,5
Treatment of vitiligo includes narrowband UVB (NB-UVB) light phototherapy, excimer laser, topical corticosteroids, topical calcineurin inhibitors such as tacrolimus and pimecrolimus, and surgical melanocyte transplantation.1 In July 2022, ruxolitinib cream 1.5% was approved by the US Food and Drug Administration (FDA) for nonsegmental vitiligo in patients 12 years and older.6,7 It is the only FDA-approved therapy for vitiligo. It is thought to work by inhibiting the Janus kinase– signal transducers and activators of the transcription pathway.6 However, topical ruxolitinib is expensive, costing more than $2000 for 60 g.8
Health disparity highlight
A 2021 study reviewing the coverage policies of 15 commercial health care insurance companies, 50 BlueCross BlueShield plans, Medicaid, Medicare, and Veterans Affairs plans found inequities in the insurance coverage patterns for therapies used to treat vitiligo. There were 2 commonly cited reasons for denying coverage for therapies: vitiligo was considered cosmetic and therapies were not FDA approved.7 In comparison, NB-UVB light phototherapy for psoriasis is not considered cosmetic and has a much higher insurance coverage rate.9,10 The out-of-pocket cost for a patient to purchase their own NB-UVB light phototherapy is more than $5000.11 Not all patients of color are economically disadvantaged, but in the United States, Black and Hispanic populations experience disproportionately higher rates of poverty (19% and 17%, respectively) compared to their White counterparts (8%).12
Final thoughts
US Food and Drug Administration approval of new drugs or new treatment indications comes after years of research discovery and large-scale trials. This pursuit of new discovery, however, is uneven. Vitiligo has historically been understudied and underfunded for research; this is common among several conditions adversely affecting people of color in the United States.13
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
- Rashighi M, Harris JE. Vitiligo pathogenesis and emerging treatments. Dermatol Clin. 2017;35:257-265. doi:10.1016/j.det.2016.11.014
- Alikhan A, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016/j.jaad.2010.11.061
- van Geel N, Speeckaert R. Segmental vitiligo. Dermatol Clin. 2017; 35:145-150. doi:10.1016/j.det.2016.11.005
- Grimes PE, Miller MM. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4:32-37. doi:10.1016/j.ijwd.2017.11.005
- Ezzedine K, Eleftheriadou V, Jones H, et al. Psychosocial effects of vitiligo: a systematic literature review [published online September 23, 2021]. Am J Clin Dermatol. 2021;22:757-774. doi:10.1007/s40257 -021-00631-6
- FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. News release. US Food and Drug Administration; July 19, 2022. Accessed December 27, 2022. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients -aged-12-and-older
- Blundell A, Sachar M, Gabel CK, et al. The scope of health insurance coverage of vitiligo treatments in the United States: implications for health care outcomes and disparities in children of color [published online July 16, 2021]. Pediatr Dermatol. 2021; 38(suppl 2):79-85. doi:10.1111/pde.14714
- Opzelura prices, coupons, and patient assistance programs. Drugs.com. Accessed January 10, 2023. https://www.drugs.com /price-guide/opzelura#:~:text=Opzelura%20Prices%2C%20 Coupons%20and%20Patient,on%20the%20pharmacy%20you%20visit
- Bhutani T, Liao W. A practical approach to home UVB phototherapy for the treatment of generalized psoriasis. Pract Dermatol. 2010;7:31-35.
- Castro Porto Silva Lopes F, Ahmed A. Insurance coverage for phototherapy for vitiligo in comparison to psoriasis and atopic dermatitis. SKIN The Journal of Cutaneous Medicine. 2022;6:217-224. https://doi.org/10.25251/skin.6.3.6
- Smith MP, Ly K, Thibodeaux Q, et al. Home phototherapy for patients with vitiligo: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:451-459. doi:10.2147/CCID.S185798
- Shrider EA, Kollar M, Chen F, et al. Income and poverty in the United States: 2020. US Census Bureau. September 14, 2021. Accessed December 27, 2022. https://www.census.gov/library/publications/2021/demo/p60-273.html
- Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2010;(1):CD003263. doi:10.1002/14651858.CD003263.pub4
Janus Kinase Inhibitors: A Promising Therapeutic Option for Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29
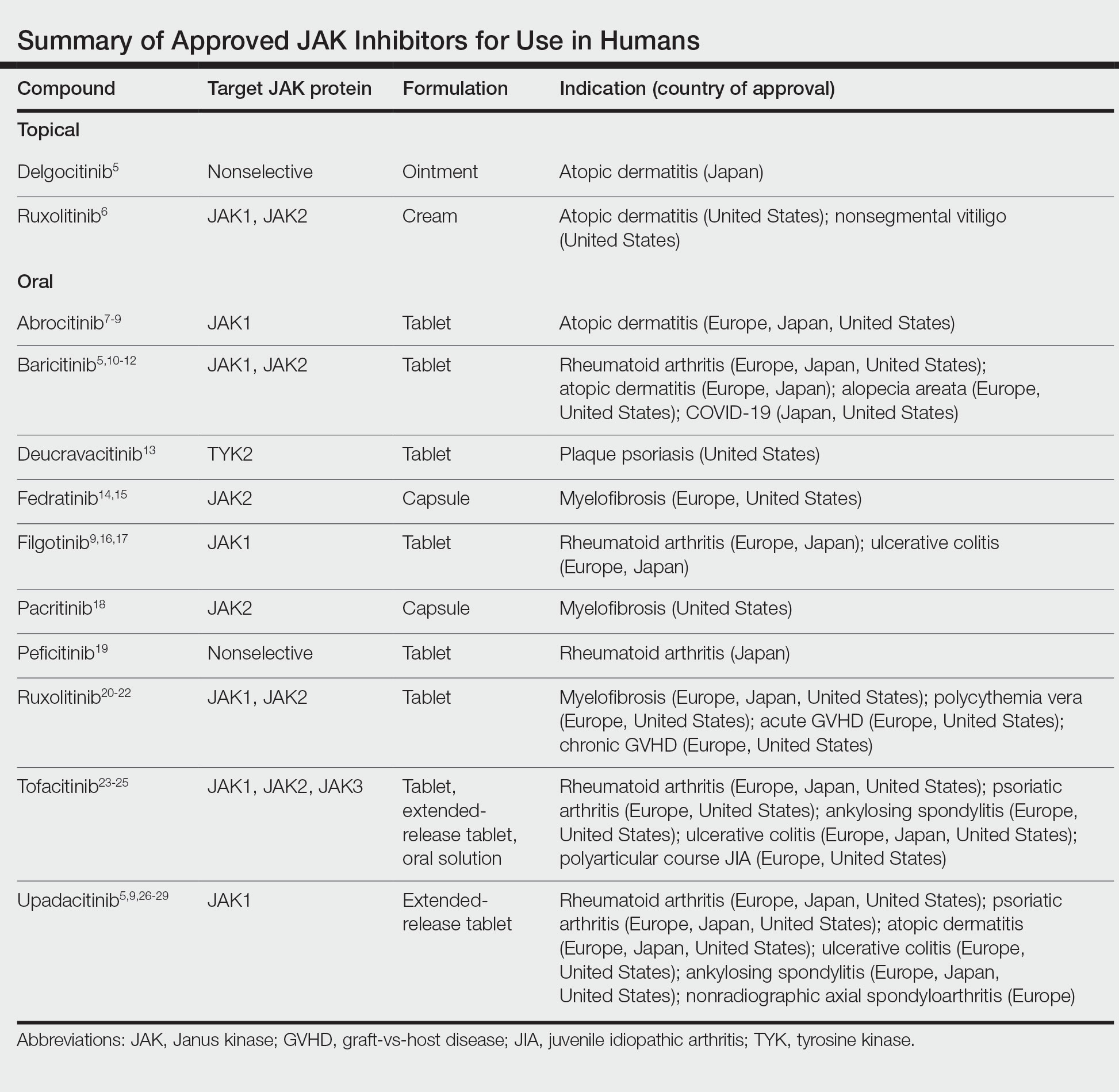
Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
Allergic contact dermatitis (ACD) is a delayed type IV hypersensitivity reaction that usually manifests with eczematous lesions within hours to days after exposure to a contact allergen. The primary treatment of ACD consists of allergen avoidance, but medications also may be necessary to manage symptoms, particularly in cases where avoidance alone does not lead to resolution of dermatitis. At present, no medical therapies are explicitly approved for use in the management of ACD. Janus kinase (JAK) inhibitors are a class of small molecule inhibitors that are used for the treatment of a range of inflammatory diseases, such as rheumatoid arthritis and psoriatic arthritis. Several oral and topical JAK inhibitors also have recently been approved by the US Food and Drug Administration (FDA) for atopic dermatitis (AD). In this article, we discuss this important class of medications and the role that they may play in the off-label management of refractory ACD.
JAK/STAT Signaling Pathway
The JAK/signal transducer and activator of transcription (STAT) pathway plays a crucial role in many biologic processes. Notably, JAK/STAT signaling is involved in the development and regulation of the immune system.1 The cascade begins when a particular transmembrane receptor binds a ligand, such as an interferon or interleukin.2 Upon ligand binding, the receptor dimerizes or oligomerizes, bringing the relevant JAK proteins into close approximation to each other.3 This allows the JAK proteins to autophosphorylate or transphosphorylate.2-4 Phosphorylation activates the JAK proteins and increases their kinase activity.3 In humans, there are 4 JAK proteins: JAK1, JAK2, JAK3, and tyrosine kinase 2.4 When activated, the JAK proteins phosphorylate specific tyrosine residues on the receptor, which creates a docking site for STAT proteins. After binding, the STAT proteins then are phosphorylated, leading to their dimerization and translocation to the nucleus.2,3 Once in the nucleus, the STAT proteins act as transcription factors for target genes.3
JAK Inhibitors
Janus kinase inhibitors are immunomodulatory medications that work through inhibition of 1 or more of the JAK proteins in the JAK/STAT pathway. Through this mechanism, JAK inhibitors can impede the activity of proinflammatory cytokines and T cells.4 A brief overview of the commercially available JAK inhibitors in Europe, Japan, and the United States is provided in the Table.5-29

Of the approved JAK inhibitors, more than 40% are indicated for AD. The first JAK inhibitor to be approved in the topical form was delgocitinib in 2020 in Japan.5 In a phase 3 trial, delgocitinib demonstrated significant reductions in modified Eczema Area and Severity Index (EASI) score (P<.001) as well as Peak Pruritus Numerical Rating Scale (P<.001) when compared with vehicle.30 Topical ruxolitinib soon followed when its approval for AD was announced by the FDA in 2021.31 Results from 2 phase 3 trials found that significantly more patients achieved investigator global assessment (IGA) treatment success (P<.0001) and a significant reduction in itch as measured by the Peak Pruritus Numerical Rating Scale (P<.001) with topical ruxolitinib vs vehicle.32
The first oral JAK inhibitor to attain approval for AD was baricitinib in Europe and Japan, but it is not currently approved for this indication in the United States by the FDA.11,12,33 Consistent findings across phase 3 trials revealed that baricitinib was more effective at achieving IGA treatment success and improved EASI scores compared with placebo.33
Upadacitinib, another oral JAK inhibitor, was subsequently approved for AD in Europe and Japan in 2021 and in the United States in early 2022.5,9,26,27 Two replicate phase 3 trials demonstrated significant improvement in EASI score, itch, and quality of life with upadacitinib compared with placebo (P<.0001).34 Abrocitinib was granted FDA approval for AD in the same time period, with phase 3 trials exhibiting greater responses in IGA and EASI scores vs placebo.35
Potential for Use in ACD
Given the successful use of JAK inhibitors in the management of AD, there is optimism that these medications also may have potential application in ACD. Recent literature suggests that the 2 conditions may be more closely related mechanistically than previously understood. As a result, AD and ACD often are managed with the same therapeutic agents.36
Although the exact etiology of ACD is still being elucidated, activation of T cells and cytokines plays an important role.37 Notably, more than 40 cytokines exert their effects through the JAK/STAT signaling pathway, including IL-2, IL-6, IL-17, IL-22, and IFN-γ.37,38 A study on nickel contact allergy revealed that JAK/STAT activation may regulate the balance between IL-12 and IL-23 and increase type 1 T-helper (TH1) polarization.39 Skin inflammation and chronic pruritus, which are major components of ACD, also are thought to be mediated in part by JAK signaling.34,40
Animal studies have suggested that JAK inhibitors may show benefit in the management of ACD. Rats with oxazolone-induced ACD were found to have less swelling and epidermal thickening in the area of induced dermatitis after treatment with oral tofacitinib, comparable to the effects of cyclosporine. Tofacitinib was presumed to exert its effects through cytokine suppression, particularly that of IFN-γ, IL-22, and tumor necrosis factor α.41 In a separate study on mice with toluene-2,4-diisocyanate–induced ACD, both tofacitinib and another JAK inhibitor, oclacitinib, demonstrated inhibition of cytokine production, migration, and maturation of bone marrow–derived dendritic cells. Both topical and oral formulations of these 2 JAK inhibitors also were found to decrease scratching behavior; only the topicals improved ear thickness (used as a marker of skin inflammation), suggesting potential benefits to local application.42 In a murine model, oral delgocitinib also attenuated contact hypersensitivity via inhibition of antigen-specific T-cell proliferation and cytokine production.37 Finally, in a randomized clinical trial conducted on dogs with allergic dermatitis (of which 10% were presumed to be from contact allergy), oral oclacitinib significantly reduced pruritus and clinical severity scores vs placebo (P<.0001).43
There also are early clinical studies and case reports highlighting the effective use of JAK inhibitors in the management of ACD in humans. A 37-year-old man with occupational airborne ACD to Compositae saw full clearance of his dermatitis with daily oral abrocitinib after topical corticosteroids and dupilumab failed.44 Another patient, a 57-year-old woman, had near-complete resolution of chronic Parthenium-induced airborne ACD after starting twice-daily oral tofacitinib. Allergen avoidance, as well as multiple medications, including topical and oral corticosteroids, topical calcineurin inhibitors, and azathioprine, previously failed in this patient.45 Finally, a phase 2 study on patients with irritant and nonirritant chronic hand eczema found that significantly more patients achieved treatment success (as measured by the physician global assessment) with topical delgocitinib vs vehicle (P=.009).46 Chronic hand eczema may be due to a variety of causes, including AD, irritant contact dermatitis, and ACD. Thus, these studies begin to highlight the potential role for JAK inhibitors in the management of refractory ACD.
Side Effects of JAK Inhibitors
The safety profile of JAK inhibitors must be taken into consideration. In general, topical JAK inhibitors are safe and well tolerated, with the majority of adverse events (AEs) seen in clinical trials considered mild or unrelated to the medication.30,32 Nasopharyngitis, local skin infection, and acne were reported; a systematic review found no increased risk of AEs with topical JAK inhibitors compared with placebo.30,32,47 Application-site reactions, a common concern among the existing topical calcineurin and phosphodiesterase 4 inhibitors, were rare (approximately 2% of patients).47 The most frequent AEs seen in clinical trials of oral JAK inhibitors included acne, nasopharyngitis/upper respiratory tract infections, nausea, and headache.33-35 Herpes simplex virus infection and worsening of AD also were seen. Although elevations in creatine phosphokinase levels were reported, patients often were asymptomatic and elevations were related to exercise or resolved without treatment interruption.33-35
As a class, JAK inhibitors carry a boxed warning for serious infections, malignancy, major adverse cardiovascular events, thrombosis, and mortality. The FDA placed this label on JAK inhibitors because of the results of a randomized controlled trial of oral tofacitinib vs tumor necrosis factor α inhibitors in RA.48,49 Notably, participants in the trial had to be 50 years or older and have at least 1 additional cardiovascular risk factor. Postmarket safety data are still being collected for patients with AD and other dermatologic conditions, but the findings of safety analyses have been reassuring to date.50,51 Regular follow-up and routine laboratory monitoring are recommended for any patient started on an oral JAK inhibitor, which often includes monitoring of the complete blood cell count, comprehensive metabolic panel, and lipids, as well as baseline screening for tuberculosis and hepatitis.52,53 For topical JAK inhibitors, no specific laboratory monitoring is recommended.
Finally, it must be considered that the challenges of off-label prescribing combined with high costs may limit access to JAK inhibitors for use in ACD.
Final Interpretation
Early investigations, including studies on animals and humans, suggest that JAK inhibitors are a promising option in the management of treatment-refractory ACD. Patients and providers should be aware of both the benefits and known side effects of JAK inhibitors prior to treatment initiation.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
- Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273-287.
- Bousoik E, Montazeri Aliabadi H. “Do we know Jack” about JAK? a closer look at JAK/STAT signaling pathway. Front Oncol. 2018;8:287.
- Jatiani SS, Baker SJ, Silverman LR, et al. Jak/STAT pathways in cytokine signaling and myeloproliferative disorders: approaches for targeted therapies. Genes Cancer. 2010;1:979-993.
- Seif F, Khoshmirsafa M, Aazami H, et al. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal. 2017;15:23.
- Traidl S, Freimooser S, Werfel T. Janus kinase inhibitors for the therapy of atopic dermatitis. Allergol Select. 2021;5:293-304.
- Opzelura (ruxolitinib) cream. Prescribing information. Incyte Corporation; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215309s001lbl.pdf
- Cibinqo (abrocitinib) tablets. Prescribing information. Pfizer Labs; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/213871s000lbl.pdf
- Cibinqo. Product information. European Medicines Agency. Published December 17, 2021. Updated November 10, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/cibinqo
- New drugs approved in FY 2021. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000246734.pdf
- Olumiant (baricitinib) tablets. Prescribing information. Eli Lilly and Company; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/207924s007lbl.pdf
- Olumiant. Product information. European Medicines Agency. Published March 16, 2017. Updated June 29, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/olumiant
- Review report: Olumiant. Pharmaceuticals and Medical Devices Agency. April 21, 2021. Accessed January 20, 2023. https://www.pmda.go.jp/files/000243207.pdf
- Sotyktu (deucravacitinib) tablets. Prescribing information. Bristol-Myers Squibb Company; 2022. Accessed January 20, 2023.https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214958s000lbl.pdf
- Inrebic (fedratinib) capsules. Prescribing information. Celgene Corporation; 2019. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212327s000lbl.pdf
- Inrebic. Product information. European Medicines Agency. Published March 3, 2021. Updated December 8, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/inrebic
- Jyseleca. Product information. European Medicines Agency. Published September 28, 2020. Updated November 9, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/jyseleca-epar-product-information_en.pdf
- Review report: Jyseleca. Pharmaceuticals and Medical Devices Agency. September 8, 2020. Accessed January 20, 2023. https://www.pmda.go.jp/files/000247830.pdf
- Vonjo (pacritinib) capsules. Prescribing information. CTI BioPharma Corp; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208712s000lbl.pdf
- Review report: Smyraf. Pharmaceuticals and Medical Devices Agency. February 28, 2019. Accessed January 20, 2023. https://www.pmda.go.jp/files/000233074.pdf
- Jakafi (ruxolitinib) tablets. Prescribing information. Incyte Corporation; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/202192s023lbl.pdf
- Jakavi. Product information. European Medicines Agency. Published October 4, 2012. Updated May 18, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/jakavi
- New drugs approved in FY 2014. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000229076.pdf
- Xeljanz (tofacitinib). Prescribing information. Pfizer Labs; 2021. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203214s028,208246s013,213082s003lbl.pdf
- Xeljanz. Product information. European Medicines Agency. Accessed January 20, 2023. https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
- Review report: Xeljanz. Pharmaceuticals and Medical Devices Agency. January 20, 2023. https://www.pmda.go.jp/files/000237584.pdf
- Rinvoq (upadacitinib) extended-release tablets. Prescribing information. AbbVie Inc; 2022. Accessed January 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/211675s003lbl.pdf
- Rinvoq. Product information. European Medicines Agency. Published December 18, 2019. Updated December 7, 2022. Accessed January 20, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/rinvoq
- New drugs approved in FY 2019. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000235289.pdfs
- New drugs approved in May 2022. Pharmaceuticals and Medical Devices Agency. Accessed January 20, 2023. https://www.pmda.go.jp/files/000248626.pdf
- Nakagawa H, Nemoto O, Igarashi A, et al. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823-831. Erratum appears in J Am Acad Dermatol. 2021;85:1069.
- Sideris N, Paschou E, Bakirtzi K, et al. New and upcoming topical treatments for atopic dermatitis: a review of the literature. J Clin Med. 2022;11:4974.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Radi G, Simonetti O, Rizzetto G, et al. Baricitinib: the first Jak inhibitor approved in Europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel). 2021;9:1575.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. Erratum appears in Lancet. 2021;397:2150.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Johnson H, Novack DE, Adler BL, et al. Can atopic dermatitis and allergic contact dermatitis coexist? Cutis. 2022;110:139-142.
- Amano W, Nakajima S, Yamamoto Y, et al. JAK inhibitor JTE-052 regulates contact hypersensitivity by downmodulating T cell activation and differentiation. J Dermatol Sci. 2016;84:258-265.
- O’Shea JJ, Schwartz DM, Villarino AV, et al. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311-328.
- Bechara R, Antonios D, Azouri H, et al. Nickel sulfate promotes IL-17A producing CD4+ T cells by an IL-23-dependent mechanism regulated by TLR4 and JAK-STAT pathways. J Invest Dermatol. 2017;137:2140-2148.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- Fujii Y, Sengoku T. Effects of the Janus kinase inhibitor CP-690550 (tofacitinib) in a rat model of oxazolone-induced chronic dermatitis. Pharmacology. 2013;91:207-213.
- Fukuyama T, Ehling S, Cook E, et al. Topically administered Janus-kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther. 2015;354:394-405.
- Cosgrove SB, Wren JA, Cleaver DM, et al. Efficacy and safety of oclacitinib for the control of pruritus and associated skin lesions in dogs with canine allergic dermatitis. Vet Dermatol. 2013;24:479, E114.
- Baltazar D, Shinamoto SR, Hamann CP, et al. Occupational airborne allergic contact dermatitis to invasive Compositae species treated with abrocitinib: a case report. Contact Dermatitis. 2022;87:542-544.
- Muddebihal A, Sardana K, Sinha S, et al. Tofacitinib in refractory Parthenium-induced airborne allergic contact dermatitis [published online October 12, 2022]. Contact Dermatitis. doi:10.1111/cod.14234
- Worm M, Bauer A, Elsner P, et al. Efficacy and safety of topical delgocitinib in patients with chronic hand eczema: data from a randomized, double-blind, vehicle-controlled phase IIa study. Br J Dermatol. 2020;182:1103-1110.
- Chen J, Cheng J, Yang H, et al. The efficacy and safety of Janus kinase inhibitors in patients with atopic dermatitis: a systematic review and meta-analysis. J Am Acad Dermatol. 2022;87:495-496.
- Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316-326.
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Updated December 7, 2021. Accessed January 20, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chen TL, Lee LL, Huang HK, et al. Association of risk of incident venous thromboembolism with atopic dermatitis and treatment with Janus kinase inhibitors: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:1254-1261.
- King B, Maari C, Lain E, et al. Extended safety analysis of baricitinib 2 mg in adult patients with atopic dermatitis: an integrated analysis from eight randomized clinical trials. Am J Clin Dermatol. 2021;22:395-405.
- Nash P, Kerschbaumer A, Dörner T, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80:71-87.
- Narla S, Silverberg JI. The suitability of treating atopic dermatitis with Janus kinase inhibitors. Exp Rev Clin Immunol. 2022;18:439-459.
PRACTICE POINTS
- Janus kinase (JAK) inhibitors are a novel class of small molecule inhibitors that modulate the JAK/signal transducer and activator of transcription signaling pathway.
- Select JAK inhibitors have been approved by the US Food and Drug Administration for the management of atopic dermatitis. Their use in allergic contact dermatitis is under active investigation.
- Regular follow-up and laboratory monitoring for patients on oral JAK inhibitors is recommended, given the potential for treatment-related adverse effects.
Dome-Shaped Periorbital Papule
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
A 76-year-old woman presented with a slowly growing, asymptomatic, 5-mm, pink-brown, dome-shaped papule adjacent to the left lateral canthus of several years’ duration. Dermoscopic examination revealed fine linear peripheral blood vessels. The lesional cells were positive with cytokeratin 7, estrogen receptor, progesterone receptor, chromogranin, synaptophysin, and neuron-specific enolase. Cytokeratin 20 and p63 were negative, and the Ki-67 proliferative index was less than 5%.
Camellia japonica
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
The various Camellia species originated in Eastern Asia and are believed to have been introduced in northwestern Spain in the 18th century. Camellia japonica, a flowering evergreen tree with various medical and cosmetic applications, is found throughout Galicia, Spain, where it is cultivated as an ornamental plant, and is native to Japan, South Korea, and China.1-4 The flowers and seeds of C. japonica have been used in traditional medicine and cosmetics in East Asia, with the oil of C. japonica used there to restore skin elasticity and to enhance skin health.4-6
While the use of C. sinensis in traditional and modern medicine is much better researched, understood, and characterized, C. japonica is now being considered for various health benefits. This column will focus on the bioactivity and scientific support for dermatologic applications of C. japonica. It is worth noting that a dry oil known as tsubaki oil, derived from C. japonica and rich in oleic acid, polyphenols, as well as vitamins A, C, D, and E, is used for skin and hair care in moisturizers produced primarily in Japan.
Antioxidant activity
In 2005, Lee and colleagues determined that C. japonica leaf and flower extracts display antioxidant, antifungal, and antibacterial activities (with the latter showing greater gram-positive than gram-negative activity).8 Investigating the antioxidant characteristics of the ethanol extract of the C. japonica flower in 2011, Piao and colleagues reported that the botanical exerted scavenging activity against reactive oxygen species in human HaCaT keratinocytes and enhanced protein expression and function of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase.9
Less than a decade later, Yoon and colleagues determined that C. japonica leaf extract contains high concentrations of vitamin E and rutin as well as other active constituents and that it exhibits antioxidant and antihyperuricemic activity in vitro and in vivo.4
Since then, Kim and colleagues have demonstrated, using cultured normal human dermal fibroblasts, that C. japonica flower extract effectively hindered urban air pollutants–induced reactive oxygen species synthesis. In ex vivo results, the investigators showed that the botanical agent suppressed matrix metalloproteinase (MMP)-1 expression, fostered collagen production, and decreased levels of pollutants-induced malondialdehyde. The authors concluded that C. japonica flower extract shows promise as a protective agent against pollutant-induced cutaneous damage.10
Anti-inflammatory and wound-healing activity
In 2012, Kim and colleagues found that C. japonica oil imparts anti-inflammatory activity via down-regulation of iNOS and COX-2 gene expression by suppressing of NF-KB and AP-1 signaling.6
Jeon and colleagues determined, in a 2018 investigation of 3,695 native plant extracts, that extracts from C. japonica fruit and stems improved induced pluripotent stem cell (iPSC) generation in mouse and human skin and enhanced wound healing in an in vivo mouse wound model. They suggested that their findings may point toward more effective approaches to developing clinical-grade iPSCs and wound-healing therapies.11
Cosmeceutical potential
Among the important bioactive ingredients present in C. japonica are phenolic compounds, terpenoids, and fatty acids, which are thought to account for the anti-inflammatory, antioxidant, antimicrobial, and anticancer activity associated with the plant.1 The high concentration of polyphenolic substances, in particular, is thought to at least partly account for the inclusion of C. japonica leaf extracts in antiaging cosmetics and cosmeceuticals.12 Specifically, some of the antioxidant substances found in C. japonica extracts include quercetin, quercetin-3-O-glucoside, quercitrin, and kaempferol.9
Wrinkle reduction and moisturization
In 2007, Jung and colleagues found that C. japonica oil activated collagen 1A2 promotion in human dermal fibroblast cells in a concentration-dependent fashion. The oil also suppressed MMP-1 functions and spurred the production of human type I procollagen. On human skin, C. japonica oil was tested on the upper back of 30 volunteers and failed to provoke any adverse reactions. The oil also diminished transepidermal water loss on the forearm. The researchers concluded that C. japonica oil merits consideration as an antiwrinkle ingredient in topical formulations.13
More recently, Choi and colleagues showed that ceramide nanoparticles developed through the use of natural oils derived from Korean traditional plants (including C. japonica, along with Panax ginseng, C. sinensis, Glycine max napjakong, and Glycine max seoritae) improve skin carrier functions and promote gene expressions needed for epidermal homeostasis. The expressions of the FLG, CASP14, and INV genes were notably enhanced by the tested formulation. The researchers observed from in vivo human studies that the application of the ceramide nanoparticles yielded more rapid recovery in impaired skin barriers than the control formulation. Amelioration of stratum corneum cohesion was also noted. The investigators concluded that this and other natural oil–derived ceramide nanoparticle formulations may represent the potential for developing better moisturizers for enhancing skin barrier function.14
Hair-growth promotion and skin-whitening activity
Early in 2021, Cho and colleagues demonstrated that C. japonica phytoplacenta extract spurred the up-regulation of the expression of hair growth–marker genes in human follicle dermal papilla cells in vitro. In clinical tests with 42 adult female volunteers, a solution with 0.5% C. japonica placenta extract raised moisture content of the scalp and reduced sebum levels, dead scalp keratin, and redness. The researchers concluded that C. japonica phytoplacenta extract displays promise as a scalp treatment and hair growth–promoting agent.2
Later that year, Ha and colleagues reported on their findings regarding the tyrosinase inhibitory activity of the essential oil of C. japonica seeds. They identified hexamethylcyclotrisiloxane (42.36%) and octamethylcyclotetrasiloxane (23.28%) as the main constituents of the oil, which demonstrated comparable inhibitory activity to arbutin (positive control) against mushroom tyrosinase. Melanogenesis was also significantly suppressed by C. japonica seed essential oil in B16F10 melanoma cells. The investigators concluded that the essential oil of C. japonica seeds exhibits robust antityrosinase activity and, therefore, warrants consideration as a skin-whitening agent.15
Conclusion
C. japonica is not as popular or well researched as another Camellia species, C. sinensis (the primary tea plant consumed globally and highly touted and appreciated for its multitude of health benefits), but it has its own history of traditional uses for medical and cosmetic purposes and is a subject of increasing research interest along with popular applications. Its antioxidant and anti-inflammatory properties are thought to be central in conferring the ability to protect the skin from aging. Its effects on the skin barrier help skin hydration. More research is necessary to elucidate the apparently widespread potential of this botanical agent that is already found in some over-the-counter products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in office and as an ecommerce solution. Write to her at [email protected].
References
1. Pereira AG et al. Food Chem X. 2022 Feb 17;13:100258.
2. Cho WK et al. FEBS Open Bio. 2021 Mar;11(3):633-51.
3. Chung MY et al. Evolution. 2003 Jan;57(1):62-73.
4. Yoon IS et al. Int J Mol Med. 2017 Jun;39(6):1613-20.
5. Lee HH et al. Evid Based Complement Alternat Med. 2016;2016:9679867.
6. Kim S et al. BMB Rep. 2012 Mar;45(3):177-82.
7. Majumder S et al. Bull Nat Res Cen. 2020 Dec;44(1):1-4.
8. Lee SY et al. Korean Journal of Medicinal Crop Science. 2005;13(3):93-100.
9. Piao MJ et al. Int J Mol Sci. 2011;12(4):2618-30.
10. Kim M et al. BMC Complement Altern Med. 2019 Jan 28;19(1):30.
11. Jeon H et al. J Clin Med. 2018 Nov 20;7(11):449.
12. Mizutani T, Masaki H. Exp Dermatol. 2014 Oct;23 Suppl 1:23-6.
13. Jung E et al. J Ethnopharmacol. 2007 May 30;112(1):127-31.
14. Choi HK et al. J Cosmet Dermatol. 2022 Oct;21(10):4931-41.
15. Ha SY et al. Evid Based Complement Alternat Med. 2021 Nov 16;2021:6328767.
Topical gene therapy heals dystrophic epidermolysis bullosa wounds
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
.
In a phase 3 study of patients with DEB, “we found that repeated topical application of B-VEC [beremagene geperpavec], an HSV-1–based gene therapy, resulted in a greater likelihood of complete wound healing than the topical application of placebo at up to 6 months,” the authors wrote. The study was published in The New England Journal of Medicine. “Longer and larger trials are warranted to determine the durability of effect and risks of this approach,” the authors noted.
“The results prove that B-VEC, the first topical in vivo gene therapy to reach late-stage development, can heal DEB,” senior author M. Peter Marinkovich, MD, associate professor of dermatology at Stanford University, Redwood City, Calif., said in an interview.
“In the past, DEB was a very specialized disease that only a handful of dermatologists would see but could not do much to treat,” he said. “With gene therapy, many more dermatologists who may not be familiar with DEB will be able to treat these patients in their offices.” It is expected that nurses will be able to administer the treatment to patients at home, he added.
Rare, life-threatening, genetic blistering disease
DEB, a rare disease that affects one to three persons per million in the United States, is caused by mutations in the COL7A1 gene that encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue.
COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile it tears with the slightest touch. This has led to patients being called “butterfly children.” Epithelial tissues blister and scar, causing esophageal and genitourinary strictures, adhesion of digits, malnutrition, anemia, infection, and bothersome itch and pain. Morbidity and mortality are high. The leading cause of death in adults is chronic wounds leading to aggressive squamous cell cancers.
The first therapy for DEB, under FDA review
B-VEC restores C7 protein by using an engineered replication-defective herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells to restore functional C7 protein fibrils that stabilize the skin structure.
On the basis of manufacturing information submitted to the FDA in December 2022, the agency extended the date for a decision on approval by 3 months, to May 19, 2023, according to a statement from Krystal Biotech, the developer of B-VEC and the sponsor of the NEJM study.
Dr. Marinkovich and his colleagues conducted the double-blind, randomized, controlled GEM-3 trial of B-VEC at three sites in the United States. The 31 study participants ranged in age from 1 to 44 years (median age, 16 years) and had genetically confirmed DEB (30 with the recessive form and 1 with the dominant form).
For each participant, a pair of wounds was chosen that were matched in size, region, and appearance. The wounds within each pair were randomly allocated to receive weekly applications of either B-VEC or placebo gel for 26 weeks.
The results of the study included the following:
- Complete healing at 6 months occurred in 67% of the wounds treated with B-VEC (including a wound in the patient with dominant DEB), vs. 22% of those who received placebo (95% confidence interval [CI], 24-68; P = .002).
- Complete healing at 3 months occurred in 71% of the wounds treated with B-VEC, vs. 20% of those who received placebo (95% CI, 29-73; P < .001).
- The mean change from baseline to week 22 in pain severity during wound-dressing changes for patients aged 6 years and older, as determined on the basis of a visual analogue scale, was –0.88 with B-VEC, vs. –0.71 with placebo (adjusted least-squares mean difference, –0.61; 95% CI, –1.10 to –0.13); similar mean changes were seen at weeks 24 and 26.
- Among all patients, 58% had at least one adverse event. Most adverse events were mild or moderate. The most common were pruritus, chills, and squamous cell carcinoma (SCC), which were reported in three patients each (SCC cases occurred at wound sites that had not been exposed to B-VEC or placebo). Serious adverse events, which were unrelated to the treatment, occurred in three patients: diarrhea, anemia, cellulitis, and a positive blood culture related to a hemodialysis catheter.
“With the ability to treat patients with topical gene therapy, dermatology is entering a new age of treatment possibilities,” Dr. Marinkovich said in the interview.
The researchers were surprised that the redosable in vivo gene therapy worked so well, he added. In vivo gene therapy has been plagued by the occurrence of immune reactions against the viral vectors used, Dr. Marinkovich explained. But because the herpes simplex virus has evolved to evade the immune system, his team could use the viral vector every week for 6 months without inflammatory reactions.
“The immune system’s inability to fight off or get rid of the herpes simplex vector makes it bad as a disease, but as a gene therapy vector, it provides a huge advantage,” he added.
Asked to comment on the results, Christen Ebens, MD, MPH, assistant professor in the department of pediatrics at the University of Minnesota, Minneapolis, whose clinical and research interests include EB, called the results exciting for patients, families, and doctors.
“Side effects were minimal, and importantly, use of the replication-incompetent HSV vector means that the payload gene does not integrate into the patient’s DNA,” Dr. Ebens, who was not involved in the study, said in an interview. “B-VEC is not a lifelong cure but potentially an effective maintenance therapy requiring repeated doses,” she added.
Although the researchers found no clinically important immune reactions to B-VEC, Dr. Ebens said she would like to see results from longer studies of the treatment. “We will want to see that patients do not produce neutralizing antibodies against B-VEC or its components, as such antibodies may yield the treatment ineffective or cause significant side effects.”
In an interview, Vanessa R. Holland, MD, associate clinical professor in the division of dermatology at UCLA Health, Burbank, Calif., who was not involved in the study, said that “topical replication-defective HSV-1 is a brilliant vector to deliver the depleted collagen.” She added that “such a vehicle may significantly alter management of these disorders and improve or extend lives by minimizing potentially fatal complications.”
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, who was not involved in the study, was surprised by the high percentage of healed wounds and wounds that remained healed over time.
In an interview, Dr. Vakharia said that he’d like to know whether patients develop antibodies against HSV and C7 with long-term treatment and whether problems will arise related to drug availability.
B-VEC for treating other conditions
Dr. Marinkovich noted that an ongoing phase 1 clinical trial, also sponsored by Krystal Biotech, is using the HSV-1 vector to deliver a different biologic (KB105) to establish dose and safety in the treatment of ichthyosis. He added that he would like to explore the use of B-VEC to treat DEB at mucosal surfaces, including inside the mouth, the eye, and the esophagus.
Authors of two editorials that accompanied the study also referred to other conditions B-VEC might treat.
This study “highlights potential future investigations,” David V. Schaffer, PhD, professor of chemical and biomolecular engineering, bioengineering, and molecular and cell biology at the University of California, Berkeley, wrotes in one of the editorials.
Important considerations he mentioned include the likelihood of the treatment becoming lifelong; the inability of HSV to penetrate intact skin, making B-VEC unsuitable for preventing the development of new wounds; and the inability of this treatment to treat EB lesions along the digestive tract. “This important trial builds on and extends gene-therapy successes to new targets and vectors, an advance for patients,” he added.
In the second editorial, Aimee S. Payne, MD, PhD, professor of dermatology at the University of Pennsylvania, Philadelphia, raised the question of whether B-VEC’s clinical success for treating DEB can translate to other genetic diseases.
“Formulations for ophthalmic, oral-gastrointestinal, or respiratory delivery would be of great value to address the extracutaneous manifestations of epidermolysis bullosa and other genetic diseases,” she wrote.
Referring to an ongoing trial of a topical gene therapy for cystic fibrosis that is delivered by a nebulizer, Dr. Payne noted, “Ultimately, the completion of clinical trials such as this one will be required to determine whether HSV-1–mediated gene delivery can go more than skin deep.”
Earlier data and more details of the study were presented in a poster at the annual meeting of the Society for Pediatric Dermatology in July 2022.
Dr. Marinkovich has disclosed no relevant financial relationships. Several coauthors are employees of or have other financial relationships with Krystal Biotech, the study’s sponsor and the developer of beremagene geperpavec. Dr. Schaffer and Dr. Payne have financial relationships with the pharmaceutical industry. Dr. Ebens, Dr. Holland, and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Three wishes: The changes health professionals want
As physicians well know, magic wands don’t exist. If they did, every patient would recover in the exam room, prior authorization wouldn’t exist, and continuing medical education credits would be printed on bearer bonds.
But Because, hey – we all need to dream.
Suzanne C. Boulter, MD, adjunct professor of pediatrics and community and family medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H.
Patients: An end to gun violence.
Practice/hospital: Adequate staffing and pediatric bed availability.
Health system: Universal access to health insurance.
Sarah G. Candler, MD, MPH, care team medical director and director of academic relations, Iora Primary Care, Northside Clinic, Houston
Patients: Systems of health that start with communities of safety, including access to affordable housing, food, transportation, and health care.
Practice/hospital: I.N.T.E.R.O.P.E.R.A.B.I.L.I.T.Y.
Health system: Clinician leadership that has the power (often aka funding) to do what’s right, not just what’s right in front of us.
Arthur L. Caplan, PhD, bioethicist, New York University Langone Health
Patients: I wish for patients in the United States greater access to affordable primary care. There are still too many people without insurance or a reasonably accessible quality provider. And I especially wish for the rapid expansion of affordable training programs to meet staffing needs, including more scholarships, 3-year programs, and more new primary care–oriented schools.
Hospital: Increased staffing, especially nursing. There are too many retirements, too much burnout, and too much privatization into boutique practices to ensure the ability to provide high-quality, safe, patient-oriented care.
Health system: I wish for health systems to seriously move into electronic medicine. While billing has become electronic, there is still much to be done to supplement diagnosis, training, and standardized data collection on key metrics. Systems are not yet behaving in a manner consistent with the hype in this regard.
Stephen Devries, MD, executive director, Gaples Institute (nonprofit) and adjunct associate professor of nutrition, Harvard School of Public Health, Boston
Patients: Patients continue to demand more from their health care professionals and insist that they are offered evidence-based counseling on nutrition and lifestyle strategies.
Practice: Quality-based reimbursement for medical services will take hold that will incentivize much-needed preventive care.
Hospital: Hospitals will more fully embrace the role of serving as true centers of health and focus as much on preventive medicine as on the more lucrative areas of high-tech treatment.
Peter D. Friedmann, MD, MPH, chief research officer, Baystate Health, Springfield, Mass.
Seconded by: Elisabeth Poorman, MD, general internist, University of Washington Clinic, Kent
Patients: Don’t forget the ongoing epidemic of substance use disorder, a major cause of premature mortality. Descheduling of cannabis and expungement of cannabis-related convictions.
Practice/hospital: Commitment of hospitals and practices to address stigma and ensure delivery of medications for opioid use disorder in primary care, the emergency department, and inpatient settings.
Health system: Reform of antiquated methadone regulations to permit office-based prescription and pharmacy dispensing to treat opioid use disorder, as is the case in most of the world.
Robert Glatter, MD, emergency physician, New York
Patients: I want all patients to understand the enormous strain the health care system has been under – not just with the pandemic, the tripledemic, and mpox [previously called monkeypox], but well before the onset of these public health crises.
Hospital: The medical profession has endured not only burnout but a growing mental health crisis, staffing shortages, a physician addiction crisis, and increased attrition in the decade leading up to the pandemic. The pandemic was like a punch in the gut, occurring at the most inopportune time one could imagine.
Health system: The intersection of health and the state of our public health deserves important mention. Unless we take action to bolster our public health infrastructure, our health care system will be in jeopardy, unable to handle the next pandemic, which could be just around the corner.
William E. Golden, MD, medical director of Arkansas Medicaid, professor of medicine and public health, University of Arkansas for Medical Sciences, Little Rock
Patients: Affordable options for diabetes and obesity management.
Health system: Greater investment by health systems and third-party payers in primary care infrastructure.
Gregory A. Hood, MD, Baptist Health, Lexington, Ky.
Patients: To embrace the gift of getting out in the world, being active, and connecting with others – having put down the screens.
Health system: To be freed from the financial gamesmanship of the insurers as they continue to serve their goals of promoting their hedge fund investing over meaningful and productive partnering with primary care physicians, and that they gain insight that they are one of the main reasons they can’t find PCPs to connect with to render care in disadvantaged environments – because they made it economically impossible to do so.
Robert H. Hopkins Jr., MD, associate professor of internal medicine and pediatrics and director of the division of general internal medicine, University of Arkansas for Medical Sciences, Little Rock
Patients/Health system: I would wish for staged implementation of universal basic health coverage for all, perhaps closest to the French or Canadian model. This would need to be coupled with expanded funding for nursing education, graduate medical education, and tracing of other health-related professionals.
Harvey Hsu, MD, Banner Health, Phoenix
Patients: More clear guidelines that are simple to understand. This can apply to colonoscopy (now age 45), immunizations, blood pressure goals. I wish medications were not as expensive so patients can take the best medicine for them and not stop taking them when they hit their donut hole in coverage.
Practice: We have been functioning on a leaner basis to cut down costs. When the pandemic hit, turnover was high and we lost PAs, nurses, front-office staff, and physicians. Having adequate staffing is probably number one on many lists. One way we dealt with lack of staffing was converting in-person visits to telehealth. Video visits are paid the same as in-person visits, but if the patient could not get their video to work, then it would be a telephone visit. Now many insurances do not even pay for telephone visits. So I would wish that we could still be reimbursed for telehealth visits.
Health system: I would wish for our health system to recognize the extra work required to take care of patients while improving quality and meeting quality measures. Allowing more time for patient visits could be one way to meet those goals or having more support staff to make sure patients get their colonoscopy/mammograms done, improve their sugars, and take their medications.
Jan L. Shifren, MD, Vincent Trustees Professor, obstetrics, gynecology, and reproductive biology, Harvard Medical School, and director of the Midlife Women’s Health Center at Massachusetts General Hospital, Boston
Patients: I wish for patients to be actively involved in all aspects of their care, well informed with shared decision-making.
Practice: I wish for the enormous time demands of electronic medical records and documentation to not distract from the pleasure of caring for patients.
Health system: Patient care remains at the center of decisions and programs.
Timothy J. Joos, MD, MPH, internal medicine/pediatrics, Seattle
Health system: I wish someone could figure out how we could be reimbursed for the quality of care we provide instead of the volume of patients we see. I wish EMRs could become less complicated and more user-friendly rather than needing advanced training to use.
Peter Kovacs, MD, medical director, Kaali Institute IVF Center, Budapest
Patients: I work as an infertility specialist, so when we talk about infectious diseases and associated risks, we talk about a minimum of two (female and male partner) and ideally three (plus the pregnancy) individuals. We have learned that SARS-CoV-2 affects reproductive health. It may compromise sperm production, could delay fertility treatment, could be associated with lower success rates; and if the treatment is successful, it may harm the pregnant woman/fetus/newborn. The best preventive measure that we can offer is vaccination. One cannot overemphasize the importance of preventive measures, paying attention to personal hygiene and social distancing. Therefore, I wish those planning to become pregnant to listen to their health care provider and accept the recommended vaccines to minimize the risk of getting infected and to minimize the risk for severe disease, especially if one undergoes successful fertility treatment and achieves a long-desired pregnancy.
Practice: During the 2022 calendar year we had many days when one or more employees were out of work on sick leave. This puts extra stress on the others to allow uncompromised work in the clinic. In addition, we all have to work in a less-comfortable environment if we consider mask use every day, all day. For health care workers, vaccination is mandated but many still are affected by milder forms of coronavirus infection and other respiratory diseases. Therefore, I wish my colleagues patience toward the preventive measures to lower the individual risk for infections. As a result, hopefully we will have a less stressful 2023.
Health system: Many resources had to be delegated to dealing with acute and chronic COVID, and this was at the expense of routine daily elective and preventive medical services. I wish the health care system to return to normal daily operations, to have the personnel and financial resources to carry on with the required preventive and elective medical services to avoid long-term consequences of not being able to provide such services. It would be sad if we had to treat otherwise preventable illnesses in the upcoming years that went undiagnosed and/or were not properly managed due to limited resources as the result of the pandemic.
Alan R. Nelson, MD, internist-endocrinologist, retired
Patients: Expansion of the FDA’s authority into over-the-counter drugs, including the veracity of their advertising claims.
Practice: Make diabetes drugs available at a reasonable cost.
Health system: With the expansion of Medicaid eligibility during COVID-19 coming to a close, federal government actions are necessary for those who once again have been dropped from coverage to have their legitimate needs met.
Kevin Powell, MD, PhD, St. Louis
Patients: To be cared for and about, and not just medically, even when illness strikes and health fails.
Hospitals: To hear the thankfulness of a grateful public for the care you provide, and to hear that above the angry noise of outraged individuals who spout vitriol and focus on how they believe others have harmed them.
Health system: A truer understanding of mercy and justice.
Margaret Thew, DNP, FNP-BC, director, department of adolescent medicine, Children’s Hospital of Wisconsin, Milwaukee
Seconded by: M. Susan Jay, MD, professor of pediatrics, chief of adolescent medicine, Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee
My wish for patients, hospital, and system: health, calm, and grace.
Mark P. Trolice, MD, director of Fertility CARE, the IVF Center, Winter Park, Fla.
Patients: To be proactive in their health care and be their own advocates. Question when unclear and only consult credible resources.
Practice/hospital: Improve support of physicians and all health care providers to allow more input in their practice operations and growth.
Health system: Reduce interference of the “business of medicine” and ensure that the patient experience is the priority.
Charles P. Vega, MD, University of California, Irvine
Three minutes on a routine basis for everyone in health care to reflect on our blessings and the honor and gravity – as well as joy – that are integral to health care. Three minutes that will also help us to recognize our challenges and put them in the proper context. I know 3 minutes is not meeting any standard for reflective practice. But it’s 3 minutes more than I have right now.
Karen Breach Washington, MD, medical director of WellCare of North Carolina/Centene, Charlotte
Seconded by: Lillian M. Beard, MD, physician director, Children’s Pediatricians and Associates, Silver Spring, Md.
Patients: Access to affordable health care.
Hospital: Resources to care for patients (sufficient number of beds and a healthy staff).
Health system: Equity for all.
Andrew Wilner, MD, host of the podcast “The Art of Medicine with Dr. Andrew Wilner,” www.andrewwilner.com
Let’s put patients first! Too many extraneous considerations other than the patient’s best interest obstruct optimal patient care.
Here are just a few examples of patients coming last instead of first.
- If a patient needs to start a new medication in hospital, we shouldn’t have to wait until the patient is an outpatient because “that’s when insurance will pay.”
- If there’s a new medication that’s better than the old medication, we shouldn’t be forced to choose the old medication and provide inferior care because “that’s when insurance will pay.”
- If patients need to stay in hospital, we shouldn’t be pressured to discharge them because the hospital has decided that decreasing “length of stay” is its highest priority.
Dr. Francis Peabody said it best in 1927: “The secret of the care of the patient is in caring for the patient.” How hard is that?
In 2023, why don’t we follow Dr. Peabody’s sage advice from nearly 100 years ago and see what happens?
James M. Wooten, PharmD, University of Missouri–Kansas City, University Health, Kansas City, Mo.
Patients: I want patients to understand and properly realize the advantage of vaccinations – not only for COVID-19 but also for influenza. There is so much misinformation that I spend a lot of time trying to convince patients to get vaccinated. Most patients don’t realize that through their lives, most of them have already been vaccinated for something just to be able to attend school. How the COVID-19 vaccine created so much stigma makes little sense to me. I also want patients to understand that COVID-19 vaccination and boosters do not always prevent infection but will many times prevent severe infection. I believe that better patient communication and education is the key and will always be the key to improving vaccination numbers. Not only communicating and educating patients on vaccination itself but also making patients realize that personal vaccination decisions may affect what happens to your neighbor. Allowing infection means that you may be more likely to infect someone else. As a society, we must take care of each other.
Health system: It will be interesting to see what happens when vaccines are no longer reimbursed by the federal government. Understanding which vaccines work best and are better tolerated will be key to choosing appropriate vaccine brands. Health care providers will need to be very selective regarding which vaccines are selected for formulary inclusion. Thorough meta-analysis studies must be done to provide more evaluable information to allow for appropriate selection. “Knowledge is power!” Appropriate knowledge will help distinguish which vaccines work best for various patient populations.
A version of this article first appeared on Medscape.com.
As physicians well know, magic wands don’t exist. If they did, every patient would recover in the exam room, prior authorization wouldn’t exist, and continuing medical education credits would be printed on bearer bonds.
But Because, hey – we all need to dream.
Suzanne C. Boulter, MD, adjunct professor of pediatrics and community and family medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H.
Patients: An end to gun violence.
Practice/hospital: Adequate staffing and pediatric bed availability.
Health system: Universal access to health insurance.
Sarah G. Candler, MD, MPH, care team medical director and director of academic relations, Iora Primary Care, Northside Clinic, Houston
Patients: Systems of health that start with communities of safety, including access to affordable housing, food, transportation, and health care.
Practice/hospital: I.N.T.E.R.O.P.E.R.A.B.I.L.I.T.Y.
Health system: Clinician leadership that has the power (often aka funding) to do what’s right, not just what’s right in front of us.
Arthur L. Caplan, PhD, bioethicist, New York University Langone Health
Patients: I wish for patients in the United States greater access to affordable primary care. There are still too many people without insurance or a reasonably accessible quality provider. And I especially wish for the rapid expansion of affordable training programs to meet staffing needs, including more scholarships, 3-year programs, and more new primary care–oriented schools.
Hospital: Increased staffing, especially nursing. There are too many retirements, too much burnout, and too much privatization into boutique practices to ensure the ability to provide high-quality, safe, patient-oriented care.
Health system: I wish for health systems to seriously move into electronic medicine. While billing has become electronic, there is still much to be done to supplement diagnosis, training, and standardized data collection on key metrics. Systems are not yet behaving in a manner consistent with the hype in this regard.
Stephen Devries, MD, executive director, Gaples Institute (nonprofit) and adjunct associate professor of nutrition, Harvard School of Public Health, Boston
Patients: Patients continue to demand more from their health care professionals and insist that they are offered evidence-based counseling on nutrition and lifestyle strategies.
Practice: Quality-based reimbursement for medical services will take hold that will incentivize much-needed preventive care.
Hospital: Hospitals will more fully embrace the role of serving as true centers of health and focus as much on preventive medicine as on the more lucrative areas of high-tech treatment.
Peter D. Friedmann, MD, MPH, chief research officer, Baystate Health, Springfield, Mass.
Seconded by: Elisabeth Poorman, MD, general internist, University of Washington Clinic, Kent
Patients: Don’t forget the ongoing epidemic of substance use disorder, a major cause of premature mortality. Descheduling of cannabis and expungement of cannabis-related convictions.
Practice/hospital: Commitment of hospitals and practices to address stigma and ensure delivery of medications for opioid use disorder in primary care, the emergency department, and inpatient settings.
Health system: Reform of antiquated methadone regulations to permit office-based prescription and pharmacy dispensing to treat opioid use disorder, as is the case in most of the world.
Robert Glatter, MD, emergency physician, New York
Patients: I want all patients to understand the enormous strain the health care system has been under – not just with the pandemic, the tripledemic, and mpox [previously called monkeypox], but well before the onset of these public health crises.
Hospital: The medical profession has endured not only burnout but a growing mental health crisis, staffing shortages, a physician addiction crisis, and increased attrition in the decade leading up to the pandemic. The pandemic was like a punch in the gut, occurring at the most inopportune time one could imagine.
Health system: The intersection of health and the state of our public health deserves important mention. Unless we take action to bolster our public health infrastructure, our health care system will be in jeopardy, unable to handle the next pandemic, which could be just around the corner.
William E. Golden, MD, medical director of Arkansas Medicaid, professor of medicine and public health, University of Arkansas for Medical Sciences, Little Rock
Patients: Affordable options for diabetes and obesity management.
Health system: Greater investment by health systems and third-party payers in primary care infrastructure.
Gregory A. Hood, MD, Baptist Health, Lexington, Ky.
Patients: To embrace the gift of getting out in the world, being active, and connecting with others – having put down the screens.
Health system: To be freed from the financial gamesmanship of the insurers as they continue to serve their goals of promoting their hedge fund investing over meaningful and productive partnering with primary care physicians, and that they gain insight that they are one of the main reasons they can’t find PCPs to connect with to render care in disadvantaged environments – because they made it economically impossible to do so.
Robert H. Hopkins Jr., MD, associate professor of internal medicine and pediatrics and director of the division of general internal medicine, University of Arkansas for Medical Sciences, Little Rock
Patients/Health system: I would wish for staged implementation of universal basic health coverage for all, perhaps closest to the French or Canadian model. This would need to be coupled with expanded funding for nursing education, graduate medical education, and tracing of other health-related professionals.
Harvey Hsu, MD, Banner Health, Phoenix
Patients: More clear guidelines that are simple to understand. This can apply to colonoscopy (now age 45), immunizations, blood pressure goals. I wish medications were not as expensive so patients can take the best medicine for them and not stop taking them when they hit their donut hole in coverage.
Practice: We have been functioning on a leaner basis to cut down costs. When the pandemic hit, turnover was high and we lost PAs, nurses, front-office staff, and physicians. Having adequate staffing is probably number one on many lists. One way we dealt with lack of staffing was converting in-person visits to telehealth. Video visits are paid the same as in-person visits, but if the patient could not get their video to work, then it would be a telephone visit. Now many insurances do not even pay for telephone visits. So I would wish that we could still be reimbursed for telehealth visits.
Health system: I would wish for our health system to recognize the extra work required to take care of patients while improving quality and meeting quality measures. Allowing more time for patient visits could be one way to meet those goals or having more support staff to make sure patients get their colonoscopy/mammograms done, improve their sugars, and take their medications.
Jan L. Shifren, MD, Vincent Trustees Professor, obstetrics, gynecology, and reproductive biology, Harvard Medical School, and director of the Midlife Women’s Health Center at Massachusetts General Hospital, Boston
Patients: I wish for patients to be actively involved in all aspects of their care, well informed with shared decision-making.
Practice: I wish for the enormous time demands of electronic medical records and documentation to not distract from the pleasure of caring for patients.
Health system: Patient care remains at the center of decisions and programs.
Timothy J. Joos, MD, MPH, internal medicine/pediatrics, Seattle
Health system: I wish someone could figure out how we could be reimbursed for the quality of care we provide instead of the volume of patients we see. I wish EMRs could become less complicated and more user-friendly rather than needing advanced training to use.
Peter Kovacs, MD, medical director, Kaali Institute IVF Center, Budapest
Patients: I work as an infertility specialist, so when we talk about infectious diseases and associated risks, we talk about a minimum of two (female and male partner) and ideally three (plus the pregnancy) individuals. We have learned that SARS-CoV-2 affects reproductive health. It may compromise sperm production, could delay fertility treatment, could be associated with lower success rates; and if the treatment is successful, it may harm the pregnant woman/fetus/newborn. The best preventive measure that we can offer is vaccination. One cannot overemphasize the importance of preventive measures, paying attention to personal hygiene and social distancing. Therefore, I wish those planning to become pregnant to listen to their health care provider and accept the recommended vaccines to minimize the risk of getting infected and to minimize the risk for severe disease, especially if one undergoes successful fertility treatment and achieves a long-desired pregnancy.
Practice: During the 2022 calendar year we had many days when one or more employees were out of work on sick leave. This puts extra stress on the others to allow uncompromised work in the clinic. In addition, we all have to work in a less-comfortable environment if we consider mask use every day, all day. For health care workers, vaccination is mandated but many still are affected by milder forms of coronavirus infection and other respiratory diseases. Therefore, I wish my colleagues patience toward the preventive measures to lower the individual risk for infections. As a result, hopefully we will have a less stressful 2023.
Health system: Many resources had to be delegated to dealing with acute and chronic COVID, and this was at the expense of routine daily elective and preventive medical services. I wish the health care system to return to normal daily operations, to have the personnel and financial resources to carry on with the required preventive and elective medical services to avoid long-term consequences of not being able to provide such services. It would be sad if we had to treat otherwise preventable illnesses in the upcoming years that went undiagnosed and/or were not properly managed due to limited resources as the result of the pandemic.
Alan R. Nelson, MD, internist-endocrinologist, retired
Patients: Expansion of the FDA’s authority into over-the-counter drugs, including the veracity of their advertising claims.
Practice: Make diabetes drugs available at a reasonable cost.
Health system: With the expansion of Medicaid eligibility during COVID-19 coming to a close, federal government actions are necessary for those who once again have been dropped from coverage to have their legitimate needs met.
Kevin Powell, MD, PhD, St. Louis
Patients: To be cared for and about, and not just medically, even when illness strikes and health fails.
Hospitals: To hear the thankfulness of a grateful public for the care you provide, and to hear that above the angry noise of outraged individuals who spout vitriol and focus on how they believe others have harmed them.
Health system: A truer understanding of mercy and justice.
Margaret Thew, DNP, FNP-BC, director, department of adolescent medicine, Children’s Hospital of Wisconsin, Milwaukee
Seconded by: M. Susan Jay, MD, professor of pediatrics, chief of adolescent medicine, Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee
My wish for patients, hospital, and system: health, calm, and grace.
Mark P. Trolice, MD, director of Fertility CARE, the IVF Center, Winter Park, Fla.
Patients: To be proactive in their health care and be their own advocates. Question when unclear and only consult credible resources.
Practice/hospital: Improve support of physicians and all health care providers to allow more input in their practice operations and growth.
Health system: Reduce interference of the “business of medicine” and ensure that the patient experience is the priority.
Charles P. Vega, MD, University of California, Irvine
Three minutes on a routine basis for everyone in health care to reflect on our blessings and the honor and gravity – as well as joy – that are integral to health care. Three minutes that will also help us to recognize our challenges and put them in the proper context. I know 3 minutes is not meeting any standard for reflective practice. But it’s 3 minutes more than I have right now.
Karen Breach Washington, MD, medical director of WellCare of North Carolina/Centene, Charlotte
Seconded by: Lillian M. Beard, MD, physician director, Children’s Pediatricians and Associates, Silver Spring, Md.
Patients: Access to affordable health care.
Hospital: Resources to care for patients (sufficient number of beds and a healthy staff).
Health system: Equity for all.
Andrew Wilner, MD, host of the podcast “The Art of Medicine with Dr. Andrew Wilner,” www.andrewwilner.com
Let’s put patients first! Too many extraneous considerations other than the patient’s best interest obstruct optimal patient care.
Here are just a few examples of patients coming last instead of first.
- If a patient needs to start a new medication in hospital, we shouldn’t have to wait until the patient is an outpatient because “that’s when insurance will pay.”
- If there’s a new medication that’s better than the old medication, we shouldn’t be forced to choose the old medication and provide inferior care because “that’s when insurance will pay.”
- If patients need to stay in hospital, we shouldn’t be pressured to discharge them because the hospital has decided that decreasing “length of stay” is its highest priority.
Dr. Francis Peabody said it best in 1927: “The secret of the care of the patient is in caring for the patient.” How hard is that?
In 2023, why don’t we follow Dr. Peabody’s sage advice from nearly 100 years ago and see what happens?
James M. Wooten, PharmD, University of Missouri–Kansas City, University Health, Kansas City, Mo.
Patients: I want patients to understand and properly realize the advantage of vaccinations – not only for COVID-19 but also for influenza. There is so much misinformation that I spend a lot of time trying to convince patients to get vaccinated. Most patients don’t realize that through their lives, most of them have already been vaccinated for something just to be able to attend school. How the COVID-19 vaccine created so much stigma makes little sense to me. I also want patients to understand that COVID-19 vaccination and boosters do not always prevent infection but will many times prevent severe infection. I believe that better patient communication and education is the key and will always be the key to improving vaccination numbers. Not only communicating and educating patients on vaccination itself but also making patients realize that personal vaccination decisions may affect what happens to your neighbor. Allowing infection means that you may be more likely to infect someone else. As a society, we must take care of each other.
Health system: It will be interesting to see what happens when vaccines are no longer reimbursed by the federal government. Understanding which vaccines work best and are better tolerated will be key to choosing appropriate vaccine brands. Health care providers will need to be very selective regarding which vaccines are selected for formulary inclusion. Thorough meta-analysis studies must be done to provide more evaluable information to allow for appropriate selection. “Knowledge is power!” Appropriate knowledge will help distinguish which vaccines work best for various patient populations.
A version of this article first appeared on Medscape.com.
As physicians well know, magic wands don’t exist. If they did, every patient would recover in the exam room, prior authorization wouldn’t exist, and continuing medical education credits would be printed on bearer bonds.
But Because, hey – we all need to dream.
Suzanne C. Boulter, MD, adjunct professor of pediatrics and community and family medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H.
Patients: An end to gun violence.
Practice/hospital: Adequate staffing and pediatric bed availability.
Health system: Universal access to health insurance.
Sarah G. Candler, MD, MPH, care team medical director and director of academic relations, Iora Primary Care, Northside Clinic, Houston
Patients: Systems of health that start with communities of safety, including access to affordable housing, food, transportation, and health care.
Practice/hospital: I.N.T.E.R.O.P.E.R.A.B.I.L.I.T.Y.
Health system: Clinician leadership that has the power (often aka funding) to do what’s right, not just what’s right in front of us.
Arthur L. Caplan, PhD, bioethicist, New York University Langone Health
Patients: I wish for patients in the United States greater access to affordable primary care. There are still too many people without insurance or a reasonably accessible quality provider. And I especially wish for the rapid expansion of affordable training programs to meet staffing needs, including more scholarships, 3-year programs, and more new primary care–oriented schools.
Hospital: Increased staffing, especially nursing. There are too many retirements, too much burnout, and too much privatization into boutique practices to ensure the ability to provide high-quality, safe, patient-oriented care.
Health system: I wish for health systems to seriously move into electronic medicine. While billing has become electronic, there is still much to be done to supplement diagnosis, training, and standardized data collection on key metrics. Systems are not yet behaving in a manner consistent with the hype in this regard.
Stephen Devries, MD, executive director, Gaples Institute (nonprofit) and adjunct associate professor of nutrition, Harvard School of Public Health, Boston
Patients: Patients continue to demand more from their health care professionals and insist that they are offered evidence-based counseling on nutrition and lifestyle strategies.
Practice: Quality-based reimbursement for medical services will take hold that will incentivize much-needed preventive care.
Hospital: Hospitals will more fully embrace the role of serving as true centers of health and focus as much on preventive medicine as on the more lucrative areas of high-tech treatment.
Peter D. Friedmann, MD, MPH, chief research officer, Baystate Health, Springfield, Mass.
Seconded by: Elisabeth Poorman, MD, general internist, University of Washington Clinic, Kent
Patients: Don’t forget the ongoing epidemic of substance use disorder, a major cause of premature mortality. Descheduling of cannabis and expungement of cannabis-related convictions.
Practice/hospital: Commitment of hospitals and practices to address stigma and ensure delivery of medications for opioid use disorder in primary care, the emergency department, and inpatient settings.
Health system: Reform of antiquated methadone regulations to permit office-based prescription and pharmacy dispensing to treat opioid use disorder, as is the case in most of the world.
Robert Glatter, MD, emergency physician, New York
Patients: I want all patients to understand the enormous strain the health care system has been under – not just with the pandemic, the tripledemic, and mpox [previously called monkeypox], but well before the onset of these public health crises.
Hospital: The medical profession has endured not only burnout but a growing mental health crisis, staffing shortages, a physician addiction crisis, and increased attrition in the decade leading up to the pandemic. The pandemic was like a punch in the gut, occurring at the most inopportune time one could imagine.
Health system: The intersection of health and the state of our public health deserves important mention. Unless we take action to bolster our public health infrastructure, our health care system will be in jeopardy, unable to handle the next pandemic, which could be just around the corner.
William E. Golden, MD, medical director of Arkansas Medicaid, professor of medicine and public health, University of Arkansas for Medical Sciences, Little Rock
Patients: Affordable options for diabetes and obesity management.
Health system: Greater investment by health systems and third-party payers in primary care infrastructure.
Gregory A. Hood, MD, Baptist Health, Lexington, Ky.
Patients: To embrace the gift of getting out in the world, being active, and connecting with others – having put down the screens.
Health system: To be freed from the financial gamesmanship of the insurers as they continue to serve their goals of promoting their hedge fund investing over meaningful and productive partnering with primary care physicians, and that they gain insight that they are one of the main reasons they can’t find PCPs to connect with to render care in disadvantaged environments – because they made it economically impossible to do so.
Robert H. Hopkins Jr., MD, associate professor of internal medicine and pediatrics and director of the division of general internal medicine, University of Arkansas for Medical Sciences, Little Rock
Patients/Health system: I would wish for staged implementation of universal basic health coverage for all, perhaps closest to the French or Canadian model. This would need to be coupled with expanded funding for nursing education, graduate medical education, and tracing of other health-related professionals.
Harvey Hsu, MD, Banner Health, Phoenix
Patients: More clear guidelines that are simple to understand. This can apply to colonoscopy (now age 45), immunizations, blood pressure goals. I wish medications were not as expensive so patients can take the best medicine for them and not stop taking them when they hit their donut hole in coverage.
Practice: We have been functioning on a leaner basis to cut down costs. When the pandemic hit, turnover was high and we lost PAs, nurses, front-office staff, and physicians. Having adequate staffing is probably number one on many lists. One way we dealt with lack of staffing was converting in-person visits to telehealth. Video visits are paid the same as in-person visits, but if the patient could not get their video to work, then it would be a telephone visit. Now many insurances do not even pay for telephone visits. So I would wish that we could still be reimbursed for telehealth visits.
Health system: I would wish for our health system to recognize the extra work required to take care of patients while improving quality and meeting quality measures. Allowing more time for patient visits could be one way to meet those goals or having more support staff to make sure patients get their colonoscopy/mammograms done, improve their sugars, and take their medications.
Jan L. Shifren, MD, Vincent Trustees Professor, obstetrics, gynecology, and reproductive biology, Harvard Medical School, and director of the Midlife Women’s Health Center at Massachusetts General Hospital, Boston
Patients: I wish for patients to be actively involved in all aspects of their care, well informed with shared decision-making.
Practice: I wish for the enormous time demands of electronic medical records and documentation to not distract from the pleasure of caring for patients.
Health system: Patient care remains at the center of decisions and programs.
Timothy J. Joos, MD, MPH, internal medicine/pediatrics, Seattle
Health system: I wish someone could figure out how we could be reimbursed for the quality of care we provide instead of the volume of patients we see. I wish EMRs could become less complicated and more user-friendly rather than needing advanced training to use.
Peter Kovacs, MD, medical director, Kaali Institute IVF Center, Budapest
Patients: I work as an infertility specialist, so when we talk about infectious diseases and associated risks, we talk about a minimum of two (female and male partner) and ideally three (plus the pregnancy) individuals. We have learned that SARS-CoV-2 affects reproductive health. It may compromise sperm production, could delay fertility treatment, could be associated with lower success rates; and if the treatment is successful, it may harm the pregnant woman/fetus/newborn. The best preventive measure that we can offer is vaccination. One cannot overemphasize the importance of preventive measures, paying attention to personal hygiene and social distancing. Therefore, I wish those planning to become pregnant to listen to their health care provider and accept the recommended vaccines to minimize the risk of getting infected and to minimize the risk for severe disease, especially if one undergoes successful fertility treatment and achieves a long-desired pregnancy.
Practice: During the 2022 calendar year we had many days when one or more employees were out of work on sick leave. This puts extra stress on the others to allow uncompromised work in the clinic. In addition, we all have to work in a less-comfortable environment if we consider mask use every day, all day. For health care workers, vaccination is mandated but many still are affected by milder forms of coronavirus infection and other respiratory diseases. Therefore, I wish my colleagues patience toward the preventive measures to lower the individual risk for infections. As a result, hopefully we will have a less stressful 2023.
Health system: Many resources had to be delegated to dealing with acute and chronic COVID, and this was at the expense of routine daily elective and preventive medical services. I wish the health care system to return to normal daily operations, to have the personnel and financial resources to carry on with the required preventive and elective medical services to avoid long-term consequences of not being able to provide such services. It would be sad if we had to treat otherwise preventable illnesses in the upcoming years that went undiagnosed and/or were not properly managed due to limited resources as the result of the pandemic.
Alan R. Nelson, MD, internist-endocrinologist, retired
Patients: Expansion of the FDA’s authority into over-the-counter drugs, including the veracity of their advertising claims.
Practice: Make diabetes drugs available at a reasonable cost.
Health system: With the expansion of Medicaid eligibility during COVID-19 coming to a close, federal government actions are necessary for those who once again have been dropped from coverage to have their legitimate needs met.
Kevin Powell, MD, PhD, St. Louis
Patients: To be cared for and about, and not just medically, even when illness strikes and health fails.
Hospitals: To hear the thankfulness of a grateful public for the care you provide, and to hear that above the angry noise of outraged individuals who spout vitriol and focus on how they believe others have harmed them.
Health system: A truer understanding of mercy and justice.
Margaret Thew, DNP, FNP-BC, director, department of adolescent medicine, Children’s Hospital of Wisconsin, Milwaukee
Seconded by: M. Susan Jay, MD, professor of pediatrics, chief of adolescent medicine, Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee
My wish for patients, hospital, and system: health, calm, and grace.
Mark P. Trolice, MD, director of Fertility CARE, the IVF Center, Winter Park, Fla.
Patients: To be proactive in their health care and be their own advocates. Question when unclear and only consult credible resources.
Practice/hospital: Improve support of physicians and all health care providers to allow more input in their practice operations and growth.
Health system: Reduce interference of the “business of medicine” and ensure that the patient experience is the priority.
Charles P. Vega, MD, University of California, Irvine
Three minutes on a routine basis for everyone in health care to reflect on our blessings and the honor and gravity – as well as joy – that are integral to health care. Three minutes that will also help us to recognize our challenges and put them in the proper context. I know 3 minutes is not meeting any standard for reflective practice. But it’s 3 minutes more than I have right now.
Karen Breach Washington, MD, medical director of WellCare of North Carolina/Centene, Charlotte
Seconded by: Lillian M. Beard, MD, physician director, Children’s Pediatricians and Associates, Silver Spring, Md.
Patients: Access to affordable health care.
Hospital: Resources to care for patients (sufficient number of beds and a healthy staff).
Health system: Equity for all.
Andrew Wilner, MD, host of the podcast “The Art of Medicine with Dr. Andrew Wilner,” www.andrewwilner.com
Let’s put patients first! Too many extraneous considerations other than the patient’s best interest obstruct optimal patient care.
Here are just a few examples of patients coming last instead of first.
- If a patient needs to start a new medication in hospital, we shouldn’t have to wait until the patient is an outpatient because “that’s when insurance will pay.”
- If there’s a new medication that’s better than the old medication, we shouldn’t be forced to choose the old medication and provide inferior care because “that’s when insurance will pay.”
- If patients need to stay in hospital, we shouldn’t be pressured to discharge them because the hospital has decided that decreasing “length of stay” is its highest priority.
Dr. Francis Peabody said it best in 1927: “The secret of the care of the patient is in caring for the patient.” How hard is that?
In 2023, why don’t we follow Dr. Peabody’s sage advice from nearly 100 years ago and see what happens?
James M. Wooten, PharmD, University of Missouri–Kansas City, University Health, Kansas City, Mo.
Patients: I want patients to understand and properly realize the advantage of vaccinations – not only for COVID-19 but also for influenza. There is so much misinformation that I spend a lot of time trying to convince patients to get vaccinated. Most patients don’t realize that through their lives, most of them have already been vaccinated for something just to be able to attend school. How the COVID-19 vaccine created so much stigma makes little sense to me. I also want patients to understand that COVID-19 vaccination and boosters do not always prevent infection but will many times prevent severe infection. I believe that better patient communication and education is the key and will always be the key to improving vaccination numbers. Not only communicating and educating patients on vaccination itself but also making patients realize that personal vaccination decisions may affect what happens to your neighbor. Allowing infection means that you may be more likely to infect someone else. As a society, we must take care of each other.
Health system: It will be interesting to see what happens when vaccines are no longer reimbursed by the federal government. Understanding which vaccines work best and are better tolerated will be key to choosing appropriate vaccine brands. Health care providers will need to be very selective regarding which vaccines are selected for formulary inclusion. Thorough meta-analysis studies must be done to provide more evaluable information to allow for appropriate selection. “Knowledge is power!” Appropriate knowledge will help distinguish which vaccines work best for various patient populations.
A version of this article first appeared on Medscape.com.
Bone Wax as a Physical Hemostatic Agent
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.
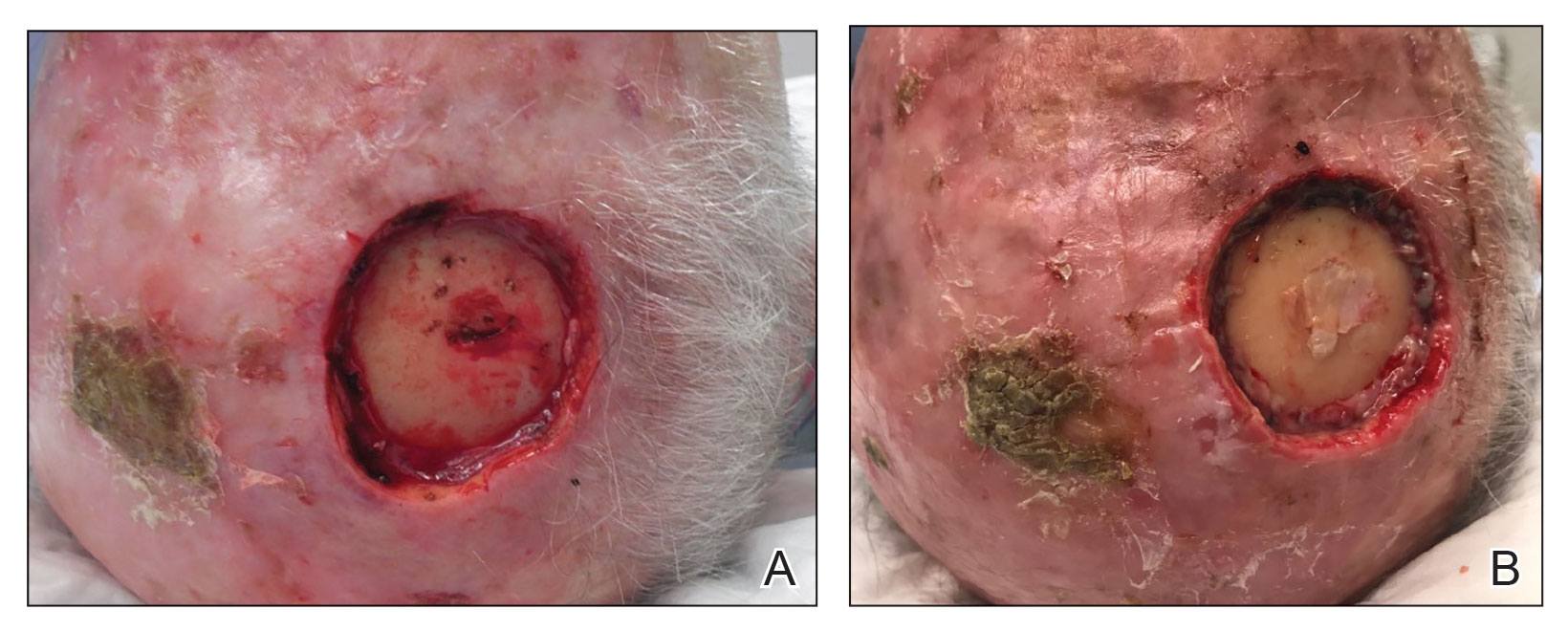
Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.

Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
Practice Gap
Hemostasis after cutaneous surgery typically can be aided by mechanical occlusion with petrolatum and gauze known as a pressure bandage. However, in certain scenarios such as bone bleeding or irregularly shaped areas (eg, conchal bowl), difficulty applying a pressure bandage necessitates alternative hemostatic measures.1 In those instances, physical hemostatic agents, such as gelatin, oxidized cellulose, microporous polysaccharide spheres, hydrophilic polymers with potassium salts, microfibrillar collagen, and chitin, also can be used.2 However, those agents are expensive and often adhere to wound edges, inducing repeat trauma with removal. To avoid such concerns, we propose the use of bone wax as an effective hemostatic technique.
The Technique
When secondary intention healing is chosen or a temporary bandage needs to be placed, we offer the use of bone wax as an alternative to help achieve hemostasis. Bone wax—a combination of beeswax, isopropyl palmitate, and a stabilizing agent such as almond oils or sterilized salicylic acid3—helps achieve hemostasis by purely mechanical means. It is malleable and can be easily adapted to the architecture of the surgical site (Figure 1). The bone wax can be applied immediately following surgery and removed during bandage change.

Practice Implications
Use of bone wax as a physical hemostatic agent provides a practical alternative to other options commonly used in dermatologic surgery for deep wounds or irregular surfaces. It offers several advantages.
Bone wax is not absorbed and does not adhere to wound surfaces, which makes removal easy and painless. Furthermore, bone wax allows for excellent growth of granulation tissue2 (Figure 2), most likely due to the healing and emollient properties of the beeswax and the moist occlusive environment created by the bone wax.

Additional advantages are its low cost, especially compared to other hemostatic agents, and long shelf-life (approximately 5 years).2 Furthermore, in scenarios when cutaneous tumors extend into the calvarium, bone wax can prevent air emboli from entering noncollapsible emissary veins.4
When bone wax is used as a temporary measure in a dermatologic setting, complications inherent to its use in bone healing (eg, granulomatous reaction, infection)—for which it is left in place indefinitely—are avoided.
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
- Perandones-González H, Fernández-Canga P, Rodríguez-Prieto MA. Bone wax as an ideal dressing for auricle concha. J Am Acad Dermatol. 2021;84:e75-e76. doi:10.1016/j.jaad.2019.08.002
- Palm MD, Altman JS. Topical hemostatic agents: a review. Dermatol Surg. 2008;34:431-445. doi:10.1111/j.1524-4725.2007.34090.x
- Alegre M, Garcés JR, Puig L. Bone wax in dermatologic surgery. Actas Dermosifiliogr. 2013;104:299-303. doi:10.1016/j.adengl.2013.03.001
- Goldman G, Altmayer S, Sambandan P, et al. Development of cerebral air emboli during Mohs micrographic surgery. Dermatol Surg. 2009;35:1414-1421. doi:10.1111/j.1524-4725.2009.01250.x
Inflammation and immunity troubles top long-COVID suspect list
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
Using live pigs in residency training sparks heated debate
Pigs have been long used in medical schools to teach surgical techniques and, more recently, in research trials and experimental xenotransplantation procedures. But
Just last month, the Physicians Committee for Responsible Medicine, a nonprofit group with a decades-long stance against the use of animals in medical education and research, placed billboards around the Portland, Ore., area demanding that Oregon Health and Science University stop using pigs to teach surgical residents.
Undergraduate medical programs no longer use live animals. But a small number of graduate medical education programs still use animals, predominantly pigs, to train physicians in subspecialties like internal medicine, emergency medicine, surgery, and anesthesiology, John Pippin, MD, FACC, director of academic affairs at PCRM, told this news organization.
Dr. Pippin says residents practice establishing emergency airways, inserting chest tubes, and accessing blood vessels on anesthetized pigs before euthanizing them.
Swine lab advocates say pigs make ideal training subjects because of their similarities to humans, including comparably sized organs like the heart, lungs, and kidneys. Pigs share about 85% of their DNA with people. Where pig skin alternatives may suffice for less invasive procedures, supporters say residents’ experiences with live tissue are irreplaceable.
In a statement, Sara Hottman, associate director of media relations at Oregon Health and Science University, told this news organization the school “only uses animal models in its surgical training program when nonanimal methods are inadequate or too dangerous for human participants.”
“We believe that the education and experience surgical trainees gain through the use of relevant animal models are essential to ensuring future surgeons have the knowledge and skills necessary to provide safe, high-quality care.”
Ms. Hottman also noted that the university continues to evaluate alternatives and looks forward to when nonanimal “surgical training methods are capable of faithfully modeling the complexity of a living system,” such as in the management of critical internal complications.
But Dr. Pippin argues that residents can gain sufficient expertise through simulators and hands-on training in the operating room, and that the differences between humans and pigs are too vast to provide meaningful clinical data or skills.
“Pigs have different genetic influences and very thick, tough skin,” he said. If you use the same pressure on a human that you learned on a pig, he added, “you’d slice right through the trachea. Whatever you think you find out in animals, you have to learn all over again with humans.”
Undergraduate medical education programs in the United States and Canada abandoned the practice of using live animals, including pigs, by 2016, with Johns Hopkins University, Baltimore, and the University of Tennessee, Chattanooga, last to announce their shift away from the controversial teaching model following campaigns by PCRM.
Today, most residency training programs have followed suit. Pippin said that pediatric residencies no longer use animals, and all trauma and anesthesiology programs have ceased such practices except two. Just 3% of emergency medicine programs continue to use animals, as do about 21% of surgical residencies, he said, based on PCRM’s latest surveys.
A public debate
Occasionally, PCRM goes public with a campaign against a residency program “if that’s the only way to win,” Dr. Pippin said.
In addition to billboards, the group has held protests, circulated petitions, and filed complaints with the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service, the entity responsible for overseeing the health and welfare of animals used in medical training and research.
In 2021, spurred by a complaint from PCRM, APHIS launched an investigation into the University of Cincinnati’s surgical residency program. At the time, a university spokesperson acknowledged the school’s limited use of pigs to train “highly-skilled, well-prepared surgeons in the most advanced, complex, real-world needs, procedures, and techniques,” adding that the training methods were endorsed by the American College of Surgeons and in compliance with federal guidelines.
Residency programs have caught the attention of state lawmakers, too. In 2020, bills introduced in both the Rhode Island House and Senate sought to ban the use of live animals in medical training when “there is an alternate teaching method that teaches the medical procedure or lesson without the use of an animal.” Violators would incur misdemeanor charges and monetary fines of up to $1,000 per animal.
The bills – backed by PCRM – targeted Brown University’s emergency medicine residency program, Providence, R.I., which sponsoring legislators said was the last program in New England still using the “outdated” and “unnecessary” method.
In testimony before lawmakers, the school said fewer than 15 pigs participate in the annual training, and faculty spoke about the benefits of the experience.
“If it was your brother or sister, or your mother or father who had to come in and get this procedure done, would you want the physician who’s doing it to be the one who does it for the very first time on a human being, on live tissue? Or do you want that provider to have only practiced on plastic and rubber?” said Nicholas Musisca, MD, an assistant program director with Brown University’s emergency medicine residency, NBC affiliate WJAR reported.
The bills have since stalled, and PCRM held a protest at Brown University in October 2022. In response, a university spokesperson told the Brown Daily Herald, “effective synthetic model alternatives simply do not exist for every complex medical procedure that an emergency physician must be prepared to perform,” including establishing an airway in adults and pediatric patients with severe facial trauma.
By the numbers
Annual reports from APHIS do not show the number of pigs dedicated solely to residency training. Instead, reporting indicates the number of animals “upon which experiments, teaching, research, surgery, or tests were conducted involving accompanying pain or distress to the animals and for which appropriate anesthetic, analgesic, or tranquilizing drugs were used.”
For fiscal year 2021 – the most recent data available – Oregon Health and Science University had 154 pigs under its control, while the University of Cincinnati and Brown University had 118 and 71 pigs, respectively, according to APHIS. Primates were more commonly used at Oregon Health and Science University and guinea pigs at the University of Cincinnati.
Similarly, the Association of American Medical Colleges supports the “use of animals to meet essential educational objectives [across] the medical education continuum. ... Further restrictions on the use of animals in biomedical and behavioral research and education threatens progress in health care and disease prevention.”
The debate will likely rage on. “The one thing we don’t do is give up,” Dr. Pippin said.
A version of this article originally appeared on Medscape.com.
Pigs have been long used in medical schools to teach surgical techniques and, more recently, in research trials and experimental xenotransplantation procedures. But
Just last month, the Physicians Committee for Responsible Medicine, a nonprofit group with a decades-long stance against the use of animals in medical education and research, placed billboards around the Portland, Ore., area demanding that Oregon Health and Science University stop using pigs to teach surgical residents.
Undergraduate medical programs no longer use live animals. But a small number of graduate medical education programs still use animals, predominantly pigs, to train physicians in subspecialties like internal medicine, emergency medicine, surgery, and anesthesiology, John Pippin, MD, FACC, director of academic affairs at PCRM, told this news organization.
Dr. Pippin says residents practice establishing emergency airways, inserting chest tubes, and accessing blood vessels on anesthetized pigs before euthanizing them.
Swine lab advocates say pigs make ideal training subjects because of their similarities to humans, including comparably sized organs like the heart, lungs, and kidneys. Pigs share about 85% of their DNA with people. Where pig skin alternatives may suffice for less invasive procedures, supporters say residents’ experiences with live tissue are irreplaceable.
In a statement, Sara Hottman, associate director of media relations at Oregon Health and Science University, told this news organization the school “only uses animal models in its surgical training program when nonanimal methods are inadequate or too dangerous for human participants.”
“We believe that the education and experience surgical trainees gain through the use of relevant animal models are essential to ensuring future surgeons have the knowledge and skills necessary to provide safe, high-quality care.”
Ms. Hottman also noted that the university continues to evaluate alternatives and looks forward to when nonanimal “surgical training methods are capable of faithfully modeling the complexity of a living system,” such as in the management of critical internal complications.
But Dr. Pippin argues that residents can gain sufficient expertise through simulators and hands-on training in the operating room, and that the differences between humans and pigs are too vast to provide meaningful clinical data or skills.
“Pigs have different genetic influences and very thick, tough skin,” he said. If you use the same pressure on a human that you learned on a pig, he added, “you’d slice right through the trachea. Whatever you think you find out in animals, you have to learn all over again with humans.”
Undergraduate medical education programs in the United States and Canada abandoned the practice of using live animals, including pigs, by 2016, with Johns Hopkins University, Baltimore, and the University of Tennessee, Chattanooga, last to announce their shift away from the controversial teaching model following campaigns by PCRM.
Today, most residency training programs have followed suit. Pippin said that pediatric residencies no longer use animals, and all trauma and anesthesiology programs have ceased such practices except two. Just 3% of emergency medicine programs continue to use animals, as do about 21% of surgical residencies, he said, based on PCRM’s latest surveys.
A public debate
Occasionally, PCRM goes public with a campaign against a residency program “if that’s the only way to win,” Dr. Pippin said.
In addition to billboards, the group has held protests, circulated petitions, and filed complaints with the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service, the entity responsible for overseeing the health and welfare of animals used in medical training and research.
In 2021, spurred by a complaint from PCRM, APHIS launched an investigation into the University of Cincinnati’s surgical residency program. At the time, a university spokesperson acknowledged the school’s limited use of pigs to train “highly-skilled, well-prepared surgeons in the most advanced, complex, real-world needs, procedures, and techniques,” adding that the training methods were endorsed by the American College of Surgeons and in compliance with federal guidelines.
Residency programs have caught the attention of state lawmakers, too. In 2020, bills introduced in both the Rhode Island House and Senate sought to ban the use of live animals in medical training when “there is an alternate teaching method that teaches the medical procedure or lesson without the use of an animal.” Violators would incur misdemeanor charges and monetary fines of up to $1,000 per animal.
The bills – backed by PCRM – targeted Brown University’s emergency medicine residency program, Providence, R.I., which sponsoring legislators said was the last program in New England still using the “outdated” and “unnecessary” method.
In testimony before lawmakers, the school said fewer than 15 pigs participate in the annual training, and faculty spoke about the benefits of the experience.
“If it was your brother or sister, or your mother or father who had to come in and get this procedure done, would you want the physician who’s doing it to be the one who does it for the very first time on a human being, on live tissue? Or do you want that provider to have only practiced on plastic and rubber?” said Nicholas Musisca, MD, an assistant program director with Brown University’s emergency medicine residency, NBC affiliate WJAR reported.
The bills have since stalled, and PCRM held a protest at Brown University in October 2022. In response, a university spokesperson told the Brown Daily Herald, “effective synthetic model alternatives simply do not exist for every complex medical procedure that an emergency physician must be prepared to perform,” including establishing an airway in adults and pediatric patients with severe facial trauma.
By the numbers
Annual reports from APHIS do not show the number of pigs dedicated solely to residency training. Instead, reporting indicates the number of animals “upon which experiments, teaching, research, surgery, or tests were conducted involving accompanying pain or distress to the animals and for which appropriate anesthetic, analgesic, or tranquilizing drugs were used.”
For fiscal year 2021 – the most recent data available – Oregon Health and Science University had 154 pigs under its control, while the University of Cincinnati and Brown University had 118 and 71 pigs, respectively, according to APHIS. Primates were more commonly used at Oregon Health and Science University and guinea pigs at the University of Cincinnati.
Similarly, the Association of American Medical Colleges supports the “use of animals to meet essential educational objectives [across] the medical education continuum. ... Further restrictions on the use of animals in biomedical and behavioral research and education threatens progress in health care and disease prevention.”
The debate will likely rage on. “The one thing we don’t do is give up,” Dr. Pippin said.
A version of this article originally appeared on Medscape.com.
Pigs have been long used in medical schools to teach surgical techniques and, more recently, in research trials and experimental xenotransplantation procedures. But
Just last month, the Physicians Committee for Responsible Medicine, a nonprofit group with a decades-long stance against the use of animals in medical education and research, placed billboards around the Portland, Ore., area demanding that Oregon Health and Science University stop using pigs to teach surgical residents.
Undergraduate medical programs no longer use live animals. But a small number of graduate medical education programs still use animals, predominantly pigs, to train physicians in subspecialties like internal medicine, emergency medicine, surgery, and anesthesiology, John Pippin, MD, FACC, director of academic affairs at PCRM, told this news organization.
Dr. Pippin says residents practice establishing emergency airways, inserting chest tubes, and accessing blood vessels on anesthetized pigs before euthanizing them.
Swine lab advocates say pigs make ideal training subjects because of their similarities to humans, including comparably sized organs like the heart, lungs, and kidneys. Pigs share about 85% of their DNA with people. Where pig skin alternatives may suffice for less invasive procedures, supporters say residents’ experiences with live tissue are irreplaceable.
In a statement, Sara Hottman, associate director of media relations at Oregon Health and Science University, told this news organization the school “only uses animal models in its surgical training program when nonanimal methods are inadequate or too dangerous for human participants.”
“We believe that the education and experience surgical trainees gain through the use of relevant animal models are essential to ensuring future surgeons have the knowledge and skills necessary to provide safe, high-quality care.”
Ms. Hottman also noted that the university continues to evaluate alternatives and looks forward to when nonanimal “surgical training methods are capable of faithfully modeling the complexity of a living system,” such as in the management of critical internal complications.
But Dr. Pippin argues that residents can gain sufficient expertise through simulators and hands-on training in the operating room, and that the differences between humans and pigs are too vast to provide meaningful clinical data or skills.
“Pigs have different genetic influences and very thick, tough skin,” he said. If you use the same pressure on a human that you learned on a pig, he added, “you’d slice right through the trachea. Whatever you think you find out in animals, you have to learn all over again with humans.”
Undergraduate medical education programs in the United States and Canada abandoned the practice of using live animals, including pigs, by 2016, with Johns Hopkins University, Baltimore, and the University of Tennessee, Chattanooga, last to announce their shift away from the controversial teaching model following campaigns by PCRM.
Today, most residency training programs have followed suit. Pippin said that pediatric residencies no longer use animals, and all trauma and anesthesiology programs have ceased such practices except two. Just 3% of emergency medicine programs continue to use animals, as do about 21% of surgical residencies, he said, based on PCRM’s latest surveys.
A public debate
Occasionally, PCRM goes public with a campaign against a residency program “if that’s the only way to win,” Dr. Pippin said.
In addition to billboards, the group has held protests, circulated petitions, and filed complaints with the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service, the entity responsible for overseeing the health and welfare of animals used in medical training and research.
In 2021, spurred by a complaint from PCRM, APHIS launched an investigation into the University of Cincinnati’s surgical residency program. At the time, a university spokesperson acknowledged the school’s limited use of pigs to train “highly-skilled, well-prepared surgeons in the most advanced, complex, real-world needs, procedures, and techniques,” adding that the training methods were endorsed by the American College of Surgeons and in compliance with federal guidelines.
Residency programs have caught the attention of state lawmakers, too. In 2020, bills introduced in both the Rhode Island House and Senate sought to ban the use of live animals in medical training when “there is an alternate teaching method that teaches the medical procedure or lesson without the use of an animal.” Violators would incur misdemeanor charges and monetary fines of up to $1,000 per animal.
The bills – backed by PCRM – targeted Brown University’s emergency medicine residency program, Providence, R.I., which sponsoring legislators said was the last program in New England still using the “outdated” and “unnecessary” method.
In testimony before lawmakers, the school said fewer than 15 pigs participate in the annual training, and faculty spoke about the benefits of the experience.
“If it was your brother or sister, or your mother or father who had to come in and get this procedure done, would you want the physician who’s doing it to be the one who does it for the very first time on a human being, on live tissue? Or do you want that provider to have only practiced on plastic and rubber?” said Nicholas Musisca, MD, an assistant program director with Brown University’s emergency medicine residency, NBC affiliate WJAR reported.
The bills have since stalled, and PCRM held a protest at Brown University in October 2022. In response, a university spokesperson told the Brown Daily Herald, “effective synthetic model alternatives simply do not exist for every complex medical procedure that an emergency physician must be prepared to perform,” including establishing an airway in adults and pediatric patients with severe facial trauma.
By the numbers
Annual reports from APHIS do not show the number of pigs dedicated solely to residency training. Instead, reporting indicates the number of animals “upon which experiments, teaching, research, surgery, or tests were conducted involving accompanying pain or distress to the animals and for which appropriate anesthetic, analgesic, or tranquilizing drugs were used.”
For fiscal year 2021 – the most recent data available – Oregon Health and Science University had 154 pigs under its control, while the University of Cincinnati and Brown University had 118 and 71 pigs, respectively, according to APHIS. Primates were more commonly used at Oregon Health and Science University and guinea pigs at the University of Cincinnati.
Similarly, the Association of American Medical Colleges supports the “use of animals to meet essential educational objectives [across] the medical education continuum. ... Further restrictions on the use of animals in biomedical and behavioral research and education threatens progress in health care and disease prevention.”
The debate will likely rage on. “The one thing we don’t do is give up,” Dr. Pippin said.
A version of this article originally appeared on Medscape.com.
Dermatology Articles in Preprint Servers: A Cross-sectional Study
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).
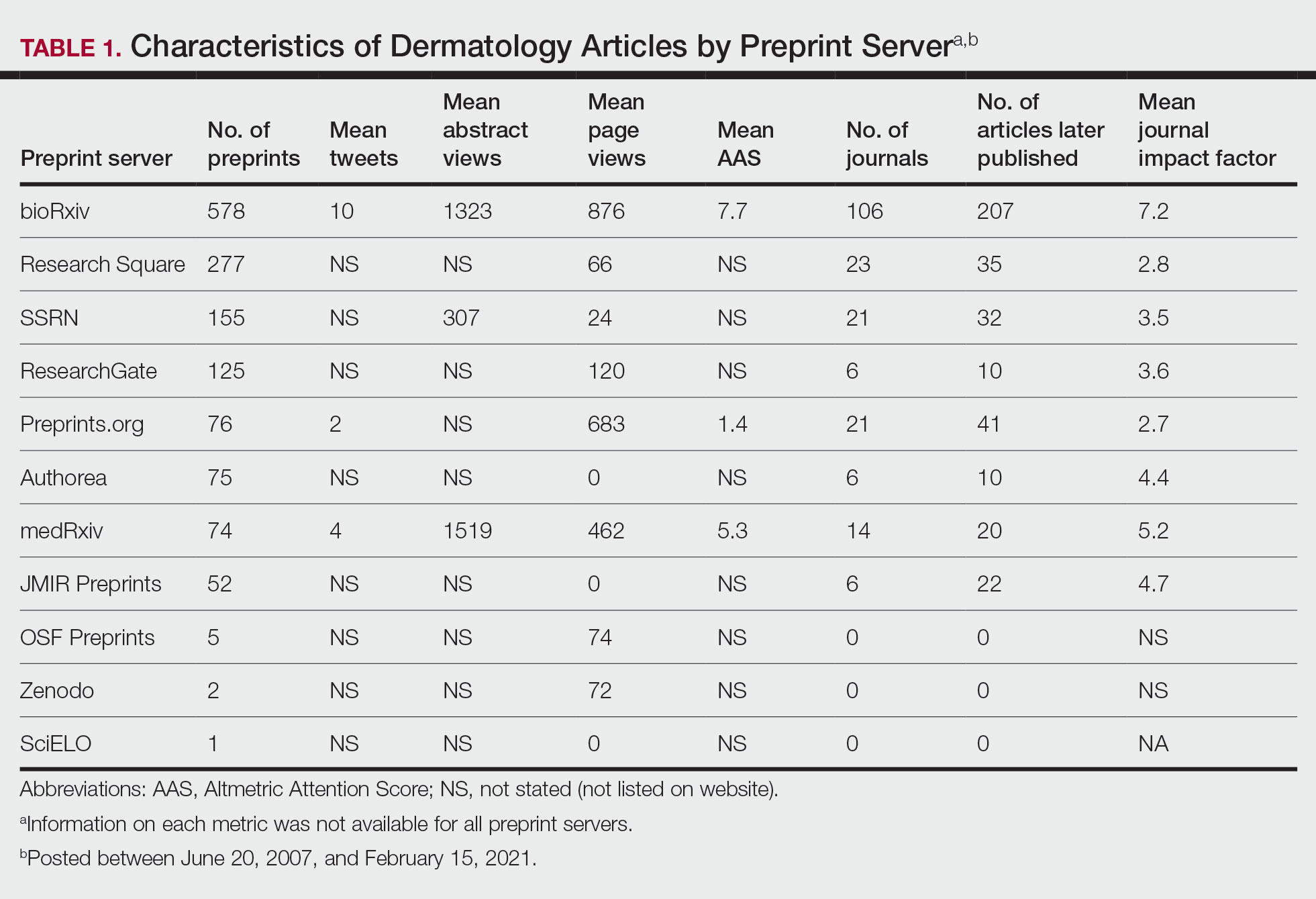
A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.
Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.
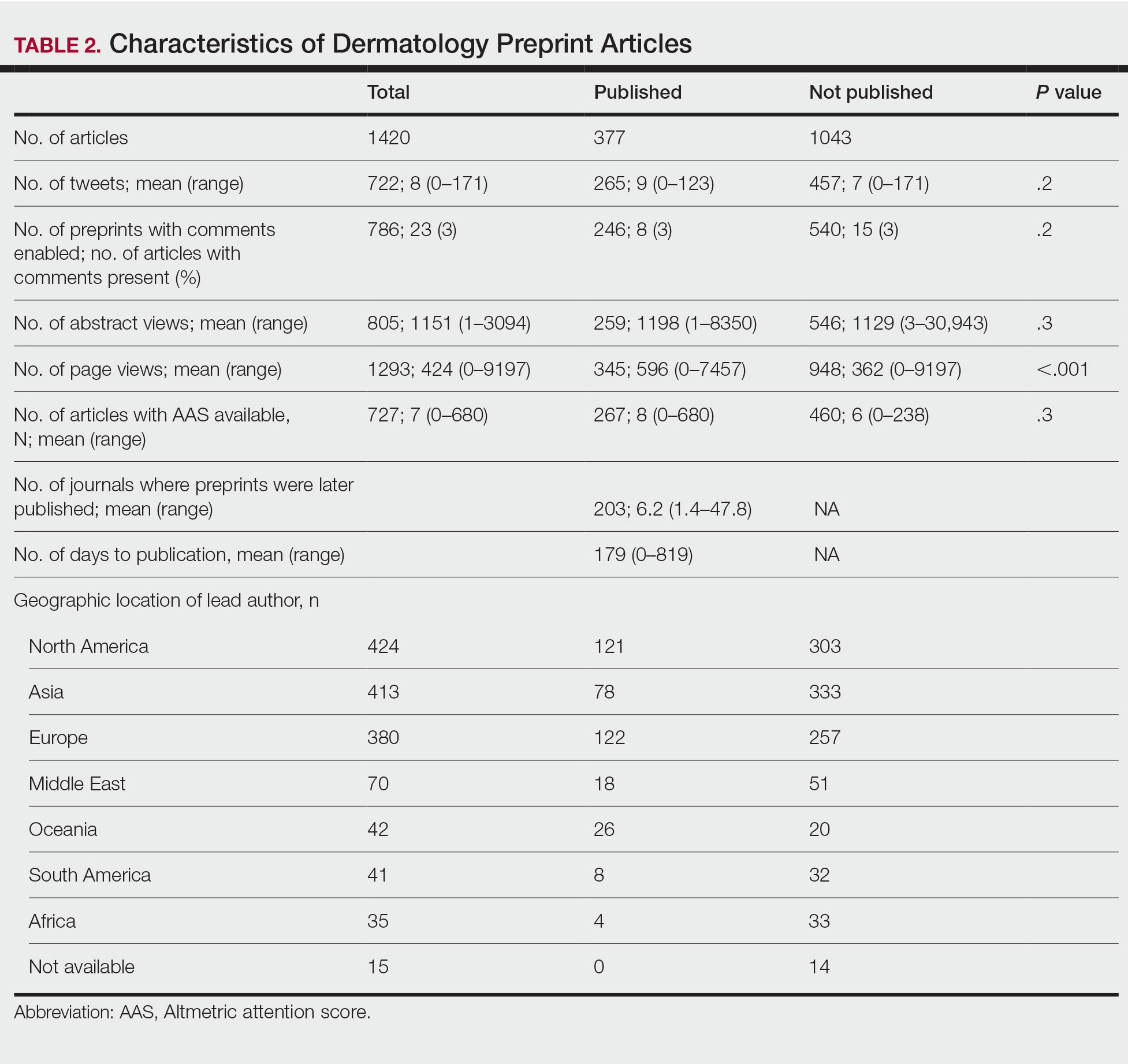
Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
To the Editor:
Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals. As of January 2022, 41 public preprint servers accepted medicine/science submissions.1 We sought to analyze characteristics of dermatology manuscripts in preprint servers and assess preprint publication policies in top dermatology journals.
Thirty-five biology/health sciences preprint servers1 were searched (March 3 to March 24, 2021) with keywords dermatology, skin, and cutaneous. Preprint server, preprint post date, location, metrics, journal, impact factor (IF), and journal publication date were recorded. Preprint policies of the top 20 dermatology journals—determined by impact factor of the journal (https://www.scimagojr.com/)—were reviewed. Two-tailed t tests and χ2 tests were performed (P<.05).

A total of 1420 articles were posted to 11 preprint servers between June 20, 2007, and February 15, 2021 (Table 1); 377 (27%) were published in peer-reviewed journals, with 350 (93%) of those published within 1 year of preprint post. Preprints were published in 203 journals with a mean IF of 6.2. Growth in preprint posts by year (2007-2020) was exponential (R2=0.78)(Figure). On average, preprints were viewed 424 times (Table 2), with published preprints viewed more often than unpublished preprints (596 vs 362 views)(P<.001). Only 23 of 786 (3%) preprints with comments enabled had feedback. Among the top 20 dermatology journals, 18 (90%) allowed preprints, 1 (5%) evaluated case by case, and 1 (5%) prohibited preprints.

Our study showed exponential growth in dermatology preprints, a low proportion published in peer-reviewed journals with high IFs, and a substantial number of page views for both published and unpublished preprints. Very few preprints had feedback. We found that most of the top 20 dermatology journals accept preprints. An analysis of 61 dermatology articles in medRxiv found only 51% (31/61) of articles were subsequently published.2 The low rate of publication may be due to the quality of preprints that do not meet criteria to be published following peer review.

Preprint servers are fairly novel, with a majority launched within the last 5 years.1 The goal of preprints is to claim conception of an idea, solicit feedback prior to submission for peer review, and expedite research distribution.3 Because preprints are uploaded without peer review, manuscripts may lack quality and accuracy. An analysis of 57 of thelargest preprint servers found that few provided guidelines on authorship, image manipulation, or reporting of study limitations; however, most preprint servers do perform some screening.4 medRxiv requires full scientific research reports and absence of obscenity, plagiarism, and patient identifiers. In its first year, medRxiv rejected 34% of 176 submissios; reasons were not disclosed.5
The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission. Almost all of the top 20 dermatologyjournals accept preprints. Therefore, dermatologists may use these preprint servers to assert project ideas and disseminate research quickly and freely but may not receive constructive criticism.
Our study is subject to several limitations. Although our search was extensive, it is possible manuscripts were missed. Article metrics also were not available on all servers, and we could not account for accepted articles that were not yet indexed.
There has been a surge in posting of dermatology preprints in recent years. Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice. Utilization of preprint servers by dermatologists is increasing, but because the impact is still unknown, further studies on accuracy and reliability of preprints are warranted.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
1. List of preprint servers: policies and practices across platforms. ASAPbio website. Accessed January 25, 2023. https://asapbio.org/preprint-servers
2. Jia JL, Hua VJ, Sarin KY. Journal attitudes and outcomes of preprints in dermatology. Br J Dermatol. 2021;185:230-232.
3. Chiarelli A, Johnson R, Richens E, et al. Accelerating scholarly communication: the transformative role of preprints. Copyright, Fair Use, Scholarly Communication, etc. 127. September 20, 2019. Accessed January 18, 2023. https://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1128&context=scholcom
4. Malicki M, Jeroncic A, Riet GT, et al. Preprint servers’ policies, submission requirements, and transparency in reporting and research integrity recommendations. JAMA. 2020;324:1901-1903.
5. Krumholz HM, Bloom T, Sever R, et al. Submissions and downloads of preprints in the first year of medRxiv. JAMA. 2020;324:1903-1905.
PRACTICE POINTS
- Preprint servers allow researchers to post manuscripts before publication in peer-reviewed journals.
- The low rate of on-site comments suggests that preprint servers may not be effective for obtaining feedback to improve dermatology manuscripts prior to journal submission; therefore, dermatologists may use these servers to disseminate research quickly and freely but may not receive constructive criticism.
- Preprints have not been peer reviewed, and data should be corroborated before incorporating new diagnostics or treatments into clinical practice.