User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Adverse events reported in one-quarter of inpatient admissions
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Best estimates made for hydroxychloroquine retinopathy risk
A new study likely makes the best estimate yet of the degree of retinopathy risk that patients who take the antimalarial drug hydroxychloroquine (HCQ) can expect, deriving mainly from the cumulative dose taken during the first 5 years of use, according to a study published in Annals of Internal Medicine.
HCQ works to decrease activity in a patient’s immune system, which is effective in many cases of systemic lupus erythematosus, one of the most common indications for the drug. However, an adverse outcome of treatment can be HCQ retinopathy, a progressive form of vision loss in patients taking HCQ over an extended period (mostly for longer than 5 years). The disease is often asymptomatic, although some patients do present a paracentral scotoma and a decrease in color vision. Patients may also notice flashing shapes in their vision and find that they have difficulty reading. Eventually, HCQ retinopathy can lead to loss of visual acuity, loss of peripheral vision, and loss of night vision.
Researchers from Kaiser Permanente Northern California and Harvard Medical School analyzed 3,325 persons who received HCQ for 5 or more years between 2004 and 2020. Their goal was to both characterize the long-term risk for incident HCQ retinopathy and examine the degree to which average HCQ dose within the first 5 years of treatment serves as a prediction of the risk.
The researchers then estimated the risk for developing retinopathy after 15 years, according to patients’ average dosing levels during the first 5 years of therapy. Overall, 81 participants developed HCQ retinopathy with overall cumulative incidences of 2.5% after 10 years and 8.6% after 15 years; the risk was greater for those given a higher dose during the first 5 years of treatment.
The mechanism of how HCQ toxicity may occur is still not completely known. There is evidence that toxicity happens because HCQ binds to melanin in both the retinal pigment epithelium and uvea in high concentrations. HCQ can interfere with lysosomal function, leading to oxidation and accumulation of lysosomes, which can cause dysfunction of the retinal pigment epithelium.
Progressive retinopathy can continue even after the drug is stopped. “It’s thought to be a very mild but important risk,” said Nilanjana Bose, MD, MBA, a rheumatologist with Memorial Hermann Health System in Houston. “Patients taking HCQ must be screened for retinal issues, most certainly elderly patients and patients with any kind of comorbidities.”
A 2021 joint position statement from the American College of Rheumatology, American Academy of Dermatology, the Rheumatologic Dermatology Society, and the American Academy of Ophthalmology recommends a baseline eye exam within a few months after starting therapy, then additional screening at 5 years on HCQ and annually thereafter.
“Early detection of retinopathy is important in overall visual prognosis, because toxicity can continue even after discontinuation of the medication,” said Rukhsana G. Mirza, MD, professor of ophthalmology and medical education at Northwestern University in Chicago.
“Examination alone is not sufficient to evaluate early changes, and specialized testing must be done. These include color photos, visual field tests, optical coherence tomography, fundus autofluorescence and in some cases, multifocal electroretinogram. Also, the AAO [American Academy of Ophthalmology] has specific recommendations related to Asian patients as they may have a different pattern of retinopathy that must also be considered.”
More accurate risk measurements
This news organization asked study coauthor April Jorge, MD, assistant professor of medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, Boston, to discuss the study, how it correlates to past research, and what it adds that’s new and useful to rheumatologists and ophthalmologists:
Question: Your research found that a higher dose of HCQ in the first 5 years of treatment led to a greater risk of retinopathy. Is there any indication that a lower dose given more frequently, either within that 5-year period or longer, would pose a similar risk?
Answer: In our study, we assessed the HCQ dose in the first 5 years of use but followed patients who continued the medication longer than 5 years, through up to 15 years of use. Therefore, we compared the risk of HCQ retinopathy associated with different HCQ dosages but for the same duration of use. We found that for any dose of HCQ, the risk of retinopathy increases the longer the medication is used. However, patients who used a higher dose of HCQ had a higher risk of developing retinopathy over time.
Although current guidelines recommend avoiding any HCQ dose over 5 mg/kg per day to reduce the risk of retinopathy, we found a higher risk of retinopathy associated with dosing over 6 mg/kg per day than between 5 and 6 mg/kg per day and the lowest risk with dosing under 5 mg/kg per day.
Q: How does your study align with and/or expand upon previous research regarding HCQ risk?
A: An important prior study of hydroxychloroquine retinopathy was the 2014 study by Ronald B. Melles, MD, and Michael F. Marmor, MD, published in JAMA Ophthalmology. Prior to our present study, that was the largest study to use the modern screening method (optical coherence tomography) to detect HCQ retinopathy. That screening tool is more sensitive than older methods, so it can detect early/mild cases of retinopathy that are typically asymptomatic. Compared to older studies, that 2014 study found a much higher risk of HCQ retinopathy than was previously appreciated.
However, that 2014 study did have some key limitations that could affect the risk estimates, such as using prevalent cases. A key feature of our present study is that we took several important steps to generate more accurate risk estimates. This included using an incident user cohort and detecting incident retinopathy cases through serial review of optical coherence tomography (screening) studies.
To achieve a high degree of methodologic rigor in correctly identifying retinopathy outcomes, we had expert ophthalmologists perform masked adjudication of all screening studies, and we assessed the intra-rater reliability of these study interpretations. Therefore, our study adds to the literature more accurate estimates of retinopathy risk. We found a lower cumulative incidence of retinopathy than was identified in the 2014 study, but the risk is still noteworthy.
Also unique to our study, we graded the severity of HCQ retinopathy outcomes. This was important, as we found that the majority of retinopathy cases detected through routine screening are mild and presumed to be asymptomatic. This will likely be reassuring news for patients that we can screen for this adverse event to detect it early and prevent vision loss.
Another important difference was that we assessed the risk of retinopathy associated with using over 6 mg/kg per day, between 5 and 6 mg/kg per day, and less than 5 mg/kg per day, whereas the highest dosing group assessed in the 2014 study included all patients using over 5 mg/kg per day. The risk was considerably higher in the > 6 mg/kg per day group than in the 5-6 mg/kg per day group.
Q: How can rheumatologists and ophthalmologists use this new information specifically to better treat their patients?
A: Our study provides more accurate estimates of the risk of HCQ retinopathy than in prior studies. These risk estimates can be used when rheumatologists (and other clinicians who prescribe HCQ) consider the risks and benefits of this otherwise important and well-tolerated medication. The risk associated with different dose ranges could also inform dosing decisions, since dosing over 6 mg/kg per day may be more of a concern than using doses in the 5-6 mg/kg range. Ophthalmologists can also use these new risk estimates to counsel patients of the importance of HCQ retinopathy screening and can also hopefully provide some reassurance to patients that the risk of severe retinopathy is low as long as they are being monitored.
The study authors were supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rheumatology Research Foundation. The authors report no relevant financial relationships. Dr. Bose and Dr. Mirza had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A new study likely makes the best estimate yet of the degree of retinopathy risk that patients who take the antimalarial drug hydroxychloroquine (HCQ) can expect, deriving mainly from the cumulative dose taken during the first 5 years of use, according to a study published in Annals of Internal Medicine.
HCQ works to decrease activity in a patient’s immune system, which is effective in many cases of systemic lupus erythematosus, one of the most common indications for the drug. However, an adverse outcome of treatment can be HCQ retinopathy, a progressive form of vision loss in patients taking HCQ over an extended period (mostly for longer than 5 years). The disease is often asymptomatic, although some patients do present a paracentral scotoma and a decrease in color vision. Patients may also notice flashing shapes in their vision and find that they have difficulty reading. Eventually, HCQ retinopathy can lead to loss of visual acuity, loss of peripheral vision, and loss of night vision.
Researchers from Kaiser Permanente Northern California and Harvard Medical School analyzed 3,325 persons who received HCQ for 5 or more years between 2004 and 2020. Their goal was to both characterize the long-term risk for incident HCQ retinopathy and examine the degree to which average HCQ dose within the first 5 years of treatment serves as a prediction of the risk.
The researchers then estimated the risk for developing retinopathy after 15 years, according to patients’ average dosing levels during the first 5 years of therapy. Overall, 81 participants developed HCQ retinopathy with overall cumulative incidences of 2.5% after 10 years and 8.6% after 15 years; the risk was greater for those given a higher dose during the first 5 years of treatment.
The mechanism of how HCQ toxicity may occur is still not completely known. There is evidence that toxicity happens because HCQ binds to melanin in both the retinal pigment epithelium and uvea in high concentrations. HCQ can interfere with lysosomal function, leading to oxidation and accumulation of lysosomes, which can cause dysfunction of the retinal pigment epithelium.
Progressive retinopathy can continue even after the drug is stopped. “It’s thought to be a very mild but important risk,” said Nilanjana Bose, MD, MBA, a rheumatologist with Memorial Hermann Health System in Houston. “Patients taking HCQ must be screened for retinal issues, most certainly elderly patients and patients with any kind of comorbidities.”
A 2021 joint position statement from the American College of Rheumatology, American Academy of Dermatology, the Rheumatologic Dermatology Society, and the American Academy of Ophthalmology recommends a baseline eye exam within a few months after starting therapy, then additional screening at 5 years on HCQ and annually thereafter.
“Early detection of retinopathy is important in overall visual prognosis, because toxicity can continue even after discontinuation of the medication,” said Rukhsana G. Mirza, MD, professor of ophthalmology and medical education at Northwestern University in Chicago.
“Examination alone is not sufficient to evaluate early changes, and specialized testing must be done. These include color photos, visual field tests, optical coherence tomography, fundus autofluorescence and in some cases, multifocal electroretinogram. Also, the AAO [American Academy of Ophthalmology] has specific recommendations related to Asian patients as they may have a different pattern of retinopathy that must also be considered.”
More accurate risk measurements
This news organization asked study coauthor April Jorge, MD, assistant professor of medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, Boston, to discuss the study, how it correlates to past research, and what it adds that’s new and useful to rheumatologists and ophthalmologists:
Question: Your research found that a higher dose of HCQ in the first 5 years of treatment led to a greater risk of retinopathy. Is there any indication that a lower dose given more frequently, either within that 5-year period or longer, would pose a similar risk?
Answer: In our study, we assessed the HCQ dose in the first 5 years of use but followed patients who continued the medication longer than 5 years, through up to 15 years of use. Therefore, we compared the risk of HCQ retinopathy associated with different HCQ dosages but for the same duration of use. We found that for any dose of HCQ, the risk of retinopathy increases the longer the medication is used. However, patients who used a higher dose of HCQ had a higher risk of developing retinopathy over time.
Although current guidelines recommend avoiding any HCQ dose over 5 mg/kg per day to reduce the risk of retinopathy, we found a higher risk of retinopathy associated with dosing over 6 mg/kg per day than between 5 and 6 mg/kg per day and the lowest risk with dosing under 5 mg/kg per day.
Q: How does your study align with and/or expand upon previous research regarding HCQ risk?
A: An important prior study of hydroxychloroquine retinopathy was the 2014 study by Ronald B. Melles, MD, and Michael F. Marmor, MD, published in JAMA Ophthalmology. Prior to our present study, that was the largest study to use the modern screening method (optical coherence tomography) to detect HCQ retinopathy. That screening tool is more sensitive than older methods, so it can detect early/mild cases of retinopathy that are typically asymptomatic. Compared to older studies, that 2014 study found a much higher risk of HCQ retinopathy than was previously appreciated.
However, that 2014 study did have some key limitations that could affect the risk estimates, such as using prevalent cases. A key feature of our present study is that we took several important steps to generate more accurate risk estimates. This included using an incident user cohort and detecting incident retinopathy cases through serial review of optical coherence tomography (screening) studies.
To achieve a high degree of methodologic rigor in correctly identifying retinopathy outcomes, we had expert ophthalmologists perform masked adjudication of all screening studies, and we assessed the intra-rater reliability of these study interpretations. Therefore, our study adds to the literature more accurate estimates of retinopathy risk. We found a lower cumulative incidence of retinopathy than was identified in the 2014 study, but the risk is still noteworthy.
Also unique to our study, we graded the severity of HCQ retinopathy outcomes. This was important, as we found that the majority of retinopathy cases detected through routine screening are mild and presumed to be asymptomatic. This will likely be reassuring news for patients that we can screen for this adverse event to detect it early and prevent vision loss.
Another important difference was that we assessed the risk of retinopathy associated with using over 6 mg/kg per day, between 5 and 6 mg/kg per day, and less than 5 mg/kg per day, whereas the highest dosing group assessed in the 2014 study included all patients using over 5 mg/kg per day. The risk was considerably higher in the > 6 mg/kg per day group than in the 5-6 mg/kg per day group.
Q: How can rheumatologists and ophthalmologists use this new information specifically to better treat their patients?
A: Our study provides more accurate estimates of the risk of HCQ retinopathy than in prior studies. These risk estimates can be used when rheumatologists (and other clinicians who prescribe HCQ) consider the risks and benefits of this otherwise important and well-tolerated medication. The risk associated with different dose ranges could also inform dosing decisions, since dosing over 6 mg/kg per day may be more of a concern than using doses in the 5-6 mg/kg range. Ophthalmologists can also use these new risk estimates to counsel patients of the importance of HCQ retinopathy screening and can also hopefully provide some reassurance to patients that the risk of severe retinopathy is low as long as they are being monitored.
The study authors were supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rheumatology Research Foundation. The authors report no relevant financial relationships. Dr. Bose and Dr. Mirza had no relevant disclosures.
A version of this article first appeared on Medscape.com.
A new study likely makes the best estimate yet of the degree of retinopathy risk that patients who take the antimalarial drug hydroxychloroquine (HCQ) can expect, deriving mainly from the cumulative dose taken during the first 5 years of use, according to a study published in Annals of Internal Medicine.
HCQ works to decrease activity in a patient’s immune system, which is effective in many cases of systemic lupus erythematosus, one of the most common indications for the drug. However, an adverse outcome of treatment can be HCQ retinopathy, a progressive form of vision loss in patients taking HCQ over an extended period (mostly for longer than 5 years). The disease is often asymptomatic, although some patients do present a paracentral scotoma and a decrease in color vision. Patients may also notice flashing shapes in their vision and find that they have difficulty reading. Eventually, HCQ retinopathy can lead to loss of visual acuity, loss of peripheral vision, and loss of night vision.
Researchers from Kaiser Permanente Northern California and Harvard Medical School analyzed 3,325 persons who received HCQ for 5 or more years between 2004 and 2020. Their goal was to both characterize the long-term risk for incident HCQ retinopathy and examine the degree to which average HCQ dose within the first 5 years of treatment serves as a prediction of the risk.
The researchers then estimated the risk for developing retinopathy after 15 years, according to patients’ average dosing levels during the first 5 years of therapy. Overall, 81 participants developed HCQ retinopathy with overall cumulative incidences of 2.5% after 10 years and 8.6% after 15 years; the risk was greater for those given a higher dose during the first 5 years of treatment.
The mechanism of how HCQ toxicity may occur is still not completely known. There is evidence that toxicity happens because HCQ binds to melanin in both the retinal pigment epithelium and uvea in high concentrations. HCQ can interfere with lysosomal function, leading to oxidation and accumulation of lysosomes, which can cause dysfunction of the retinal pigment epithelium.
Progressive retinopathy can continue even after the drug is stopped. “It’s thought to be a very mild but important risk,” said Nilanjana Bose, MD, MBA, a rheumatologist with Memorial Hermann Health System in Houston. “Patients taking HCQ must be screened for retinal issues, most certainly elderly patients and patients with any kind of comorbidities.”
A 2021 joint position statement from the American College of Rheumatology, American Academy of Dermatology, the Rheumatologic Dermatology Society, and the American Academy of Ophthalmology recommends a baseline eye exam within a few months after starting therapy, then additional screening at 5 years on HCQ and annually thereafter.
“Early detection of retinopathy is important in overall visual prognosis, because toxicity can continue even after discontinuation of the medication,” said Rukhsana G. Mirza, MD, professor of ophthalmology and medical education at Northwestern University in Chicago.
“Examination alone is not sufficient to evaluate early changes, and specialized testing must be done. These include color photos, visual field tests, optical coherence tomography, fundus autofluorescence and in some cases, multifocal electroretinogram. Also, the AAO [American Academy of Ophthalmology] has specific recommendations related to Asian patients as they may have a different pattern of retinopathy that must also be considered.”
More accurate risk measurements
This news organization asked study coauthor April Jorge, MD, assistant professor of medicine in the division of rheumatology, allergy, and immunology at Massachusetts General Hospital and Harvard Medical School, Boston, to discuss the study, how it correlates to past research, and what it adds that’s new and useful to rheumatologists and ophthalmologists:
Question: Your research found that a higher dose of HCQ in the first 5 years of treatment led to a greater risk of retinopathy. Is there any indication that a lower dose given more frequently, either within that 5-year period or longer, would pose a similar risk?
Answer: In our study, we assessed the HCQ dose in the first 5 years of use but followed patients who continued the medication longer than 5 years, through up to 15 years of use. Therefore, we compared the risk of HCQ retinopathy associated with different HCQ dosages but for the same duration of use. We found that for any dose of HCQ, the risk of retinopathy increases the longer the medication is used. However, patients who used a higher dose of HCQ had a higher risk of developing retinopathy over time.
Although current guidelines recommend avoiding any HCQ dose over 5 mg/kg per day to reduce the risk of retinopathy, we found a higher risk of retinopathy associated with dosing over 6 mg/kg per day than between 5 and 6 mg/kg per day and the lowest risk with dosing under 5 mg/kg per day.
Q: How does your study align with and/or expand upon previous research regarding HCQ risk?
A: An important prior study of hydroxychloroquine retinopathy was the 2014 study by Ronald B. Melles, MD, and Michael F. Marmor, MD, published in JAMA Ophthalmology. Prior to our present study, that was the largest study to use the modern screening method (optical coherence tomography) to detect HCQ retinopathy. That screening tool is more sensitive than older methods, so it can detect early/mild cases of retinopathy that are typically asymptomatic. Compared to older studies, that 2014 study found a much higher risk of HCQ retinopathy than was previously appreciated.
However, that 2014 study did have some key limitations that could affect the risk estimates, such as using prevalent cases. A key feature of our present study is that we took several important steps to generate more accurate risk estimates. This included using an incident user cohort and detecting incident retinopathy cases through serial review of optical coherence tomography (screening) studies.
To achieve a high degree of methodologic rigor in correctly identifying retinopathy outcomes, we had expert ophthalmologists perform masked adjudication of all screening studies, and we assessed the intra-rater reliability of these study interpretations. Therefore, our study adds to the literature more accurate estimates of retinopathy risk. We found a lower cumulative incidence of retinopathy than was identified in the 2014 study, but the risk is still noteworthy.
Also unique to our study, we graded the severity of HCQ retinopathy outcomes. This was important, as we found that the majority of retinopathy cases detected through routine screening are mild and presumed to be asymptomatic. This will likely be reassuring news for patients that we can screen for this adverse event to detect it early and prevent vision loss.
Another important difference was that we assessed the risk of retinopathy associated with using over 6 mg/kg per day, between 5 and 6 mg/kg per day, and less than 5 mg/kg per day, whereas the highest dosing group assessed in the 2014 study included all patients using over 5 mg/kg per day. The risk was considerably higher in the > 6 mg/kg per day group than in the 5-6 mg/kg per day group.
Q: How can rheumatologists and ophthalmologists use this new information specifically to better treat their patients?
A: Our study provides more accurate estimates of the risk of HCQ retinopathy than in prior studies. These risk estimates can be used when rheumatologists (and other clinicians who prescribe HCQ) consider the risks and benefits of this otherwise important and well-tolerated medication. The risk associated with different dose ranges could also inform dosing decisions, since dosing over 6 mg/kg per day may be more of a concern than using doses in the 5-6 mg/kg range. Ophthalmologists can also use these new risk estimates to counsel patients of the importance of HCQ retinopathy screening and can also hopefully provide some reassurance to patients that the risk of severe retinopathy is low as long as they are being monitored.
The study authors were supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Rheumatology Research Foundation. The authors report no relevant financial relationships. Dr. Bose and Dr. Mirza had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ANNALS OF INTERNAL MEDICINE
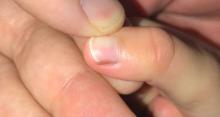
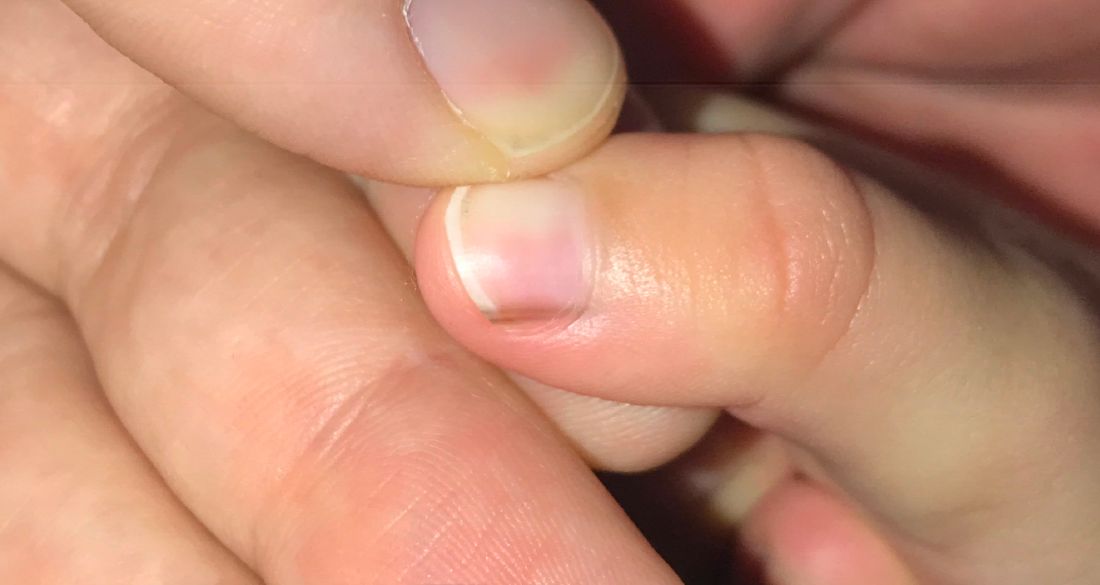
A toddler presents with a dark line on a fingernail
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Given the over 1-year history of an unchanging longitudinal band of pigment without extension to the proximal or lateral nailfolds or any other nail findings, the most likely diagnosis is benign longitudinal melanonychia.
Longitudinal melanonychia, also known as melanonychia striata, describes a brown to black streak of pigment extending from the nail matrix to the free edge of the nail.1,2
This disorder can occur secondary to a wide variety of benign and pathologic causes including lentigines, nevi, melanoma, chronic trauma, inflammatory skin diseases, systemic diseases, iatrogenic causes, and genetic syndromes.3 In melanocytic causes of longitudinal melanonychia, either melanocytic activation or hyperplasia drive pigmentary development leading to the brown to black band seen in the nail.4 Benign causes of longitudinal melanonychia include benign melanocyte activation, lentigo, and benign nevus.1
What’s the differential diagnosis?
The differential diagnosis for longitudinal melanonychia can include a wide variety of local and systemic causes. For our discussion, we will limit our differential to other locally involved disorders of the nail including subungual melanoma, subungual hematoma, onychomycosis, and glomus tumor.
Subungual melanoma is a rare subtype of acral lentiginous melanoma that most often presents as longitudinal melanonychia. Subungual melanoma is more common in those aged 50-70 years, individuals with personal or family history of melanoma or dysplastic nevus syndrome, and persons with African American, Native American, and Asian descent. Longitudinal melanonychia features that can be concerning for subungual melanoma include the presence of multiple colors, width greater than or equal to 3 mm, blurry borders, rapid increase in size, and extension to the proximal or lateral nailfolds (Hutchinson’s sign). Biopsy is required to make the diagnosis of subungual melanoma but is not necessary for melanonychia without atypical features.
Treatment of subungual melanoma depends on disease stage and can range from wide local excision of the nail apparatus to amputation of the affected digit and management with a medical oncologist. Given the absence of concerning neoplastic findings or personal or family history of melanoma, subungual melanoma is unlikely in this patient.
Subungual hematoma is an accumulation of blood underneath the nail plate that is typically the result of acute or chronic trauma to the distal phalanx. It can present as purple, red, pink, brown, or black discoloration under the nail plate and is most commonly found on the first toe. With acute trauma, pain is usually present upon initial injury. Subungual hematomas typically resolve on their own with normal nail growth. The absence of a history of trauma or pain, and the linear appearance of the lesion in our patient are inconsistent with a subungual hematoma.
Onychomycosis is a fungal infection of the nail caused by dermatophytes, nondermatophytes, or yeasts. It may present with longitudinal melanonychia; however, it more often presents with other nail abnormalities such as nail thickening, yellow discoloration, onycholysis, splitting, subungual hyperkeratosis, and nail plate destruction, which are not present in this patient. Furthermore, onychomycosis is more common in adults than children. Diagnosis is usually made with potassium hydroxide (KOH) preparations, histopathologic examination of nail clippings with a periodic acid-Schiff stain, fungal culture, or PCR.
Glomus tumor is a rare, benign neoplasm originating from cells of the glomus body. It is often found in the subungual region, in addition to other areas rich in glomus bodies such as the fingertips, palms, wrists, and forearms. Subungual glomus tumors present as a red, purple, or blueish lesions under the nail plate. Distal notching or an overlying longitudinal fissure may be present. Subungual glomus tumors are typically associated with pinpoint tenderness, paroxysmal pain, and cold sensitivity, features that are not present in our patient. The history and examination of our patient are much more consistent with benign longitudinal melanonychia.
It appears that melanoma associated with longitudinal melanonychia is very rare in children. According to one review published in 2020, only 12 cases of pediatric subungual melanoma have been reported.5 Recent series have observed longitudinal melanonychia in large sets of children, with findings that demonstrate that the vast majority of longitudinal melanonychia either stops progressing or regresses. These investigations therefore recommend serial observation of longitudinal melanonychia except in rare circumstances.6,7
Given the lack of troubling findings or concerning history, our patient was managed with observation. On follow-up 6 months later, he was found to have no change in his nail pigmentation.
Dr. Haft is an inflammatory skin disease fellow in the division of pediatric and adolescent dermatology; Ms. Sui is a research associate in the department of dermatology, division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Mannava KA et al. Hand Surg. 2013;18(1):133-9.
2. Leung AKC et al. Int J Dermatol. 2019;58(11):1239-45.
3. Andre J and Lateur N. Dermatol Clin. 2006;24(3):329-39.
4. Lee DK and Lipner SR. Ann Med. 2022;54(1):694-712.
5. Smith RJ and Rubin AI. Curr Opin Pediatr. 2020;32(4):506-15. .
6. Matsui Y et al. J Am Acad Dermatol. 2022;86(4):946-8.
7. Lee JS et al. J Am Acad Dermatol. 2022;87(2):366-72.
Examination findings reveal a 2-mm brown longitudinal band on the radial aspect of the right thumbnail that does not extend into the proximal or lateral nailfolds. The rest of the skin and nail exam is unremarkable.
AD outcomes improved with lebrikizumab and topical steroids
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
, according to results of the 16-week phase 3 ADhere trial.
“Lebrikizumab, a monoclonal antibody inhibiting interleukin-13, combined with TCS was associated with reduced overall disease severity of moderate to severe AD in adolescents and adults, and had a safety profile consistent with previous lebrikizumab AD studies,” noted lead author Eric L. Simpson, MD, professor of dermatology at Oregon Health & Science University, Portland, and coauthors in their article on the study, which was published in JAMA Dermatology.
The double-blind trial, conducted at 54 sites across Germany, Poland, Canada, and the United States, included 211 patients, mean age 37.2 years, of whom 48.8% were female and roughly 22% were adolescents. Almost 15% were Asian, and about 13% were Black.
At baseline, participants had a score of 16 or higher on the Eczema Area and Severity Index (EASI), a score of 3 or higher on the Investigator’s Global Assessment (IGA) scale, AD covering a body surface area of 10% or greater, and a history of inadequate response to treatment with topical medications.
After a minimum 1-week washout period from topical and systemic therapy, participants were randomized in a 2:1 ratio to receive lebrikizumab plus TCS (n = 145) or placebo plus TCS (n = 66) for 16 weeks.
Lebrikizumab or placebo was administered by subcutaneous injection every 2 weeks; the loading and week-2 doses of lebrikizumab were 500 mg, followed by 250 mg thereafter. All patients were instructed to use low- to mid-potency TCS at their own discretion. Study sites provided a mid-potency TCS (triamcinolone acetonide 0.1% cream) and a low-potency TCS (hydrocortisone 1% cream), with topical calcineurin inhibitors permitted for sensitive skin areas.
Primary outcomes at 16 weeks included a 2-point or more reduction in IGA score from baseline and EASI-75 response. Patients in the lebrikizumab arm had superior responses on both of these outcomes, with statistical significance achieved as early as week 8 and week 4, respectively, and maintained through week 16. Specifically, 41.2% of those treated with lebrikizumab had an IGA reduction of 2 points or more, compared with 22.1% of those receiving placebo plus TCS (P = .01), and the proportion of patients achieving EASI-75 responses was 69.5% vs. 42.2%, respectively (P < .001).
Patients treated with lebrikizumab also showed statistically significant improvements, compared with TCS alone in all key secondary endpoints, “including skin clearance, improvement in itch, itch interference on sleep, and enhanced QoL [quality of life],” noted the authors. “This study captured the clinical benefit of lebrikizumab through the combined end point of physician-assessed clinical sign of skin clearance (EASI-75) and patient-reported outcome of improvement in itch (Pruritus NRS).”
The percentage of patients who achieved the combined endpoint was more than double for the lebrikizumab plus TCS group vs. the group on TCS alone, indicating that patients treated with lebrikizumab plus TCS “were more likely to experience improvement in skin symptoms and itch,” the investigators added.
The authors noted that most treatment-emergent adverse events “were nonserious, mild, or moderate in severity, and did not lead to study discontinuation.” These included conjunctivitis (4.8%), headache (4.8%), hypertension (2.8%), injection-site reactions (2.8%), and herpes infection (3.4%) – all of which occurred in 1.5% or less of patients in the placebo group.
“The higher incidence of conjunctivitis has also been reported in other biologics inhibiting IL [interleukin]–13 and/or IL-4 signaling, as well as lebrikizumab monotherapy studies,” they noted. The 4.8% rate of conjunctivitis reported in the combination study, they added, is “compared with 7.5% frequency in 16-week data from the lebrikizumab monotherapy studies. Although the mechanism remains unclear, it has been reported that conjunctival goblet cell scarcity due to IL-13 and IL-4 inhibition, and subsequent effects on the homeostasis of the conjunctival mucosal surface, results in ocular AEs [adverse events].”
“This truly is a time of great hope and promise for our patients with AD,” commented Zelma Chiesa Fuxench, MD, who was not involved in the study. “The advent of newer, targeted therapeutic agents for AD continues to revolutionize the treatment experience for our patients, offering the possibility of greater AD disease control with a favorable risk profile and less need for blood work monitoring compared to traditional systemic agents.”
On the basis of the study results, Dr. Chiesa Fuxench, of the department of dermatology at the University of Pennsylvania, Philadelphia, said in an interview that “lebrikizumab represents an additional option in the treatment armamentarium for providers who care for patients with AD.” She added that, “while head-to-head trials comparing lebrikizumab to dupilumab, the first FDA-approved biologic for AD, would be beneficial, to the best of my knowledge this data is currently lacking. However, based on the results of this study, we would expect lebrikizumab to work at least similarly to dupilumab, based on the reported improvements in IGA and EASI score.”
Additionally, lebrikizumab showed a favorable safety profile, “with most treatment-emergent adverse effects reported as nonserious and not leading to drug discontinuation,” she said. “Of interest to clinicians may be the reported rates of conjunctivitis in this study. Rates of conjunctivitis for lebrikizumab appear to be lower than those reported in the LIBERTY AD CHRONOS study for dupilumab – a finding that merits further scrutiny in my opinion, as this one of the most frequent treatment-emergent adverse events that I encounter in my clinical practice.”
The study was funded by Dermira, a subsidiary of Eli Lilly. Dr. Simpson reported personal fees and grants from multiple sources, including Dermira and Eli Lilly, the companies developing lebrikizumab. Several authors were employees of Eli Lilly. Dr. Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
A version of this article first appeared on Medscape.com.
FROM JAMA DERMATOLOGY
Manicure gone wrong leads to cancer diagnosis
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
. Now, she and her doctor are spreading the word about her ordeal as a lesson that speed and persistence in seeking treatment are the keys that make her type of cancer – squamous cell carcinoma – completely curable.
“She cut me, and the cut wasn’t just a regular cuticle cut. She cut me deep, and that was one of the first times that happened to me,” Grace Garcia, 50, told TODAY.com, recalling the November 2021 incident.
Ms. Garcia had been getting her nails done regularly for 20 years, she said, but happened to go to a different salon than her usual spot because she couldn’t get an appointment during the busy pre-Thanksgiving season. She doesn’t recall whether the technician opened packaging that signals unused tools.
She put antibiotic ointment on the cut, but it didn’t heal after a few days. Eventually, the skin closed and a darkened bump formed. It was painful. She went to her doctor, who said it was a “callus from writing,” she told TODAY.com. But it was on her ring finger, which didn’t seem connected to writing. Her doctor said to keep an eye on it.
Five months after the cut occurred, she mentioned it during a gynecology appointment and was referred to a dermatologist, who also advised keeping an eye on it. A wart developed. She went back to her primary care physician and then to another dermatologist. The spot was biopsied.
Squamous cell carcinoma is a common type of skin cancer, according to the American Academy of Dermatology. It can have many causes, but the cause in Ms. Garcia’s case was both very common and very rare: human papillomavirus, or HPV. HPV is a virus that infects millions of people every year, but it’s not a typical cause of skin cancer.
“It’s pretty rare for several reasons. Generally speaking, the strains that cause cancer from an HPV standpoint tend to be more sexually transmitted,” dermatologist Teo Soleymani told TODAY.com. “In Grace’s case, she had an injury, which became the portal of entry. So that thick skin that we have on our hands and feet that acts as a natural barrier against infections and things like that was no longer the case, and the virus was able to infect her skin.”
Dr. Soleymani said Ms. Garcia’s persistence to get answers likely saved her from losing a finger.
“Your outcomes are entirely dictated by how early you catch them, and very often they’re completely curable,” he said. “Her persistence – not only was she able to have a great outcome, she probably saved herself from having her finger amputated.”
What the FTC’s proposed ban on noncompete agreements could mean for physicians, other clinicians
The proposed rule seeks to ban companies from enforcing noncompete clauses in employment contracts, a practice that represents an “unfair method of competition” with “exploitative and widespread” impacts, including suppression of wages, innovation, and entrepreneurial spirit, the FTC said. The public has 60 days to submit comments on the proposal before the FTC issues the final rule.
Employers often include noncompete clauses in physician contracts because they want to avoid having patients leave their health care system and follow a doctor to a competitor. A 2018 survey of primary care physicians found that about half of office-based physicians and 37% of physicians employed at hospitals or freestanding care centers were bound by restrictive covenants.
“A federal ban on noncompete agreements will ensure that physicians nationwide can finally change jobs without fear of being sued,” Erik B. Smith, MD, JD, clinical assistant professor of anesthesiology at the University of Southern California, Los Angeles, said in an interview.
Many doctors would like to see noncompete agreements vanish, but some physicians still favor them.
“As a small-practice owner, I am personally against this. The noncompete helps me take a risk and hire a physician. It typically takes 2-3 years for me to break even. I think this will further consolidate employment with large hospital systems unfortunately,” Texas cardiologist Rishin Shah, MD, recently tweeted in response to the FTC announcement.
Dr. Smith, who has advocated for noncompete reform, said about half of states currently allow the controversial clauses.
However, several states have recently passed laws restricting their use. California, North Dakota, and Oklahoma ban noncompetes, although some narrowly defined exceptions, such as the sale of a business, remain.
Other states, like Colorado, Illinois, and Oregon, broadly ban noncompete clauses, except for workers earning above a certain threshold. For example, in Colorado, noncompete agreements are permitted for highly compensated employees earning more than $101,250.
Despite additional restrictions on noncompete agreements for workers in the District of Columbia, the new legislation does not apply to physicians earning total compensation of $250,000 or more. However, their employers must define the geographic parameters of the noncompete and limit postemployment restrictions to 2 years.
Restrictive covenants are “uniquely challenging to family medicine’s emphasis on longitudinal care and the patient-physician relationship,” said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians. The limitations imposed by noncompete agreements “potentially reduce patient choice, lower the quality of care for patients, and ultimately harm the foundation of family medicine – our relationships with our patients.”
Although the proposed rule aligns with President Biden’s executive order promoting economic competition, Dr. Smith said a national ban on noncompete agreements may push the limits of FTC authority.
“This new rule will certainly result in a ‘major questions doctrine’ Supreme Court challenge,” said Dr. Smith, and possibly be struck down if the court determines an administrative overstep into areas of “vast economic or political significance.”
A controversial policy
The American Medical Association’s code of ethics discourages covenants that “unreasonably restrict” the ability of physicians to practice following contract termination. And in 2022, the AMA cited “overly broad” noncompete language as a red flag young physicians should watch out for during contract negotiations.
But in 2020, the AMA asked the FTC not to use its rulemaking authority to regulate noncompete clauses in physician employment contracts, and instead, relegate enforcement of such agreements to each state. The American Hospital Association expressed similar views.
Still, the FTC said that eliminating noncompete clauses will increase annual wages by $300 billion, allow 30 million Americans to pursue better job opportunities, and encourage hiring competition among employers. It will also save consumers up to $148 billion in health care costs annually.
“Noncompetes block workers from freely switching jobs, depriving them of higher wages and better working conditions, and depriving businesses of a talent pool that they need to build and expand,” Lina M. Khan, FTC chair, said in a press release about the proposal.
A national ban on noncompetes would keep more physicians in the industry and practicing in their communities, a win for patients and providers, said Dr. Smith. It could also compel employers to offer more competitive employment packages, including fair wages, better work conditions, and a culture of well-being and patient safety.
“Whatever the final rule is, I’m certain it will be legally challenged,” said Dr. Smith, adding that the nation’s most prominent business lobbying group, the Chamber of Commerce, has already issued a statement calling the rule “blatantly unlawful."
A version of this article first appeared on Medscape.com.
The proposed rule seeks to ban companies from enforcing noncompete clauses in employment contracts, a practice that represents an “unfair method of competition” with “exploitative and widespread” impacts, including suppression of wages, innovation, and entrepreneurial spirit, the FTC said. The public has 60 days to submit comments on the proposal before the FTC issues the final rule.
Employers often include noncompete clauses in physician contracts because they want to avoid having patients leave their health care system and follow a doctor to a competitor. A 2018 survey of primary care physicians found that about half of office-based physicians and 37% of physicians employed at hospitals or freestanding care centers were bound by restrictive covenants.
“A federal ban on noncompete agreements will ensure that physicians nationwide can finally change jobs without fear of being sued,” Erik B. Smith, MD, JD, clinical assistant professor of anesthesiology at the University of Southern California, Los Angeles, said in an interview.
Many doctors would like to see noncompete agreements vanish, but some physicians still favor them.
“As a small-practice owner, I am personally against this. The noncompete helps me take a risk and hire a physician. It typically takes 2-3 years for me to break even. I think this will further consolidate employment with large hospital systems unfortunately,” Texas cardiologist Rishin Shah, MD, recently tweeted in response to the FTC announcement.
Dr. Smith, who has advocated for noncompete reform, said about half of states currently allow the controversial clauses.
However, several states have recently passed laws restricting their use. California, North Dakota, and Oklahoma ban noncompetes, although some narrowly defined exceptions, such as the sale of a business, remain.
Other states, like Colorado, Illinois, and Oregon, broadly ban noncompete clauses, except for workers earning above a certain threshold. For example, in Colorado, noncompete agreements are permitted for highly compensated employees earning more than $101,250.
Despite additional restrictions on noncompete agreements for workers in the District of Columbia, the new legislation does not apply to physicians earning total compensation of $250,000 or more. However, their employers must define the geographic parameters of the noncompete and limit postemployment restrictions to 2 years.
Restrictive covenants are “uniquely challenging to family medicine’s emphasis on longitudinal care and the patient-physician relationship,” said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians. The limitations imposed by noncompete agreements “potentially reduce patient choice, lower the quality of care for patients, and ultimately harm the foundation of family medicine – our relationships with our patients.”
Although the proposed rule aligns with President Biden’s executive order promoting economic competition, Dr. Smith said a national ban on noncompete agreements may push the limits of FTC authority.
“This new rule will certainly result in a ‘major questions doctrine’ Supreme Court challenge,” said Dr. Smith, and possibly be struck down if the court determines an administrative overstep into areas of “vast economic or political significance.”
A controversial policy
The American Medical Association’s code of ethics discourages covenants that “unreasonably restrict” the ability of physicians to practice following contract termination. And in 2022, the AMA cited “overly broad” noncompete language as a red flag young physicians should watch out for during contract negotiations.
But in 2020, the AMA asked the FTC not to use its rulemaking authority to regulate noncompete clauses in physician employment contracts, and instead, relegate enforcement of such agreements to each state. The American Hospital Association expressed similar views.
Still, the FTC said that eliminating noncompete clauses will increase annual wages by $300 billion, allow 30 million Americans to pursue better job opportunities, and encourage hiring competition among employers. It will also save consumers up to $148 billion in health care costs annually.
“Noncompetes block workers from freely switching jobs, depriving them of higher wages and better working conditions, and depriving businesses of a talent pool that they need to build and expand,” Lina M. Khan, FTC chair, said in a press release about the proposal.
A national ban on noncompetes would keep more physicians in the industry and practicing in their communities, a win for patients and providers, said Dr. Smith. It could also compel employers to offer more competitive employment packages, including fair wages, better work conditions, and a culture of well-being and patient safety.
“Whatever the final rule is, I’m certain it will be legally challenged,” said Dr. Smith, adding that the nation’s most prominent business lobbying group, the Chamber of Commerce, has already issued a statement calling the rule “blatantly unlawful."
A version of this article first appeared on Medscape.com.
The proposed rule seeks to ban companies from enforcing noncompete clauses in employment contracts, a practice that represents an “unfair method of competition” with “exploitative and widespread” impacts, including suppression of wages, innovation, and entrepreneurial spirit, the FTC said. The public has 60 days to submit comments on the proposal before the FTC issues the final rule.
Employers often include noncompete clauses in physician contracts because they want to avoid having patients leave their health care system and follow a doctor to a competitor. A 2018 survey of primary care physicians found that about half of office-based physicians and 37% of physicians employed at hospitals or freestanding care centers were bound by restrictive covenants.
“A federal ban on noncompete agreements will ensure that physicians nationwide can finally change jobs without fear of being sued,” Erik B. Smith, MD, JD, clinical assistant professor of anesthesiology at the University of Southern California, Los Angeles, said in an interview.
Many doctors would like to see noncompete agreements vanish, but some physicians still favor them.
“As a small-practice owner, I am personally against this. The noncompete helps me take a risk and hire a physician. It typically takes 2-3 years for me to break even. I think this will further consolidate employment with large hospital systems unfortunately,” Texas cardiologist Rishin Shah, MD, recently tweeted in response to the FTC announcement.
Dr. Smith, who has advocated for noncompete reform, said about half of states currently allow the controversial clauses.
However, several states have recently passed laws restricting their use. California, North Dakota, and Oklahoma ban noncompetes, although some narrowly defined exceptions, such as the sale of a business, remain.
Other states, like Colorado, Illinois, and Oregon, broadly ban noncompete clauses, except for workers earning above a certain threshold. For example, in Colorado, noncompete agreements are permitted for highly compensated employees earning more than $101,250.
Despite additional restrictions on noncompete agreements for workers in the District of Columbia, the new legislation does not apply to physicians earning total compensation of $250,000 or more. However, their employers must define the geographic parameters of the noncompete and limit postemployment restrictions to 2 years.
Restrictive covenants are “uniquely challenging to family medicine’s emphasis on longitudinal care and the patient-physician relationship,” said Tochi Iroku-Malize, MD, MPH, president of the American Academy of Family Physicians. The limitations imposed by noncompete agreements “potentially reduce patient choice, lower the quality of care for patients, and ultimately harm the foundation of family medicine – our relationships with our patients.”
Although the proposed rule aligns with President Biden’s executive order promoting economic competition, Dr. Smith said a national ban on noncompete agreements may push the limits of FTC authority.
“This new rule will certainly result in a ‘major questions doctrine’ Supreme Court challenge,” said Dr. Smith, and possibly be struck down if the court determines an administrative overstep into areas of “vast economic or political significance.”
A controversial policy
The American Medical Association’s code of ethics discourages covenants that “unreasonably restrict” the ability of physicians to practice following contract termination. And in 2022, the AMA cited “overly broad” noncompete language as a red flag young physicians should watch out for during contract negotiations.
But in 2020, the AMA asked the FTC not to use its rulemaking authority to regulate noncompete clauses in physician employment contracts, and instead, relegate enforcement of such agreements to each state. The American Hospital Association expressed similar views.
Still, the FTC said that eliminating noncompete clauses will increase annual wages by $300 billion, allow 30 million Americans to pursue better job opportunities, and encourage hiring competition among employers. It will also save consumers up to $148 billion in health care costs annually.
“Noncompetes block workers from freely switching jobs, depriving them of higher wages and better working conditions, and depriving businesses of a talent pool that they need to build and expand,” Lina M. Khan, FTC chair, said in a press release about the proposal.
A national ban on noncompetes would keep more physicians in the industry and practicing in their communities, a win for patients and providers, said Dr. Smith. It could also compel employers to offer more competitive employment packages, including fair wages, better work conditions, and a culture of well-being and patient safety.
“Whatever the final rule is, I’m certain it will be legally challenged,” said Dr. Smith, adding that the nation’s most prominent business lobbying group, the Chamber of Commerce, has already issued a statement calling the rule “blatantly unlawful."
A version of this article first appeared on Medscape.com.
Pay an annual visit to your office
that your patients might see?
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing; laminate peels off the edges of desks and cabinets.
When did you last take a good look at your waiting room? How clean is it? Patients expect cleanliness in doctor’s offices, and they expect the reception area to be neat. How are the carpeting and upholstery holding up? Sit in your chairs; how do they feel? Patients don’t appreciate a sore back or bottom from any chairs, especially in a medical office. Consider investing in new furniture that will be attractive and comfortable for your patients.
Look at the decor itself; is it dated or just plain “old-looking?” Any interior designer will tell you they can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ‘90s, it’s probably time for a change. Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring under stationary furniture and compare them to exposed floors.
If your color scheme is hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality; paint should be high-quality “eggshell” finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) Consider updating your overhead lighting. The harsh glow of fluorescent lights amid an uninspired decor creates a sterile, uninviting atmosphere.
During renovation, get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible. This is also a good time to clear out old textbooks, journals, and files that you will never open again, in this digital age.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition; a few years ago, I redecorated my exam room walls with framed photos from my travel adventures – to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite a family member, or local artists or talented patients, to display some of their creations on your walls. If you get too many contributions, you can rotate them on a periodic basis.
Plants are great aesthetic accents, yet many offices have little or no plant life. Plants naturally aerate an office suite and help make it feel less stuffy. Also, multiple studies have found that plants promote productivity among office staff and create a sense of calm for apprehensive patients. Improvements like this can make a big difference. They show an attention to detail and a willingness to make your practice as inviting as possible for patients and employees alike.
Spruce-up time is also an excellent opportunity to inventory your medical equipment. We’ve all seen “vintage” offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice; but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part; many – particularly younger ones – assume that doctors who don’t keep up with technological innovations don’t keep up with anything else, either.
If you’re planning a vacation this year (and I hope you are), that would be the perfect time for a re-do. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
that your patients might see?
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing; laminate peels off the edges of desks and cabinets.
When did you last take a good look at your waiting room? How clean is it? Patients expect cleanliness in doctor’s offices, and they expect the reception area to be neat. How are the carpeting and upholstery holding up? Sit in your chairs; how do they feel? Patients don’t appreciate a sore back or bottom from any chairs, especially in a medical office. Consider investing in new furniture that will be attractive and comfortable for your patients.
Look at the decor itself; is it dated or just plain “old-looking?” Any interior designer will tell you they can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ‘90s, it’s probably time for a change. Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring under stationary furniture and compare them to exposed floors.
If your color scheme is hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality; paint should be high-quality “eggshell” finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) Consider updating your overhead lighting. The harsh glow of fluorescent lights amid an uninspired decor creates a sterile, uninviting atmosphere.
During renovation, get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible. This is also a good time to clear out old textbooks, journals, and files that you will never open again, in this digital age.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition; a few years ago, I redecorated my exam room walls with framed photos from my travel adventures – to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite a family member, or local artists or talented patients, to display some of their creations on your walls. If you get too many contributions, you can rotate them on a periodic basis.
Plants are great aesthetic accents, yet many offices have little or no plant life. Plants naturally aerate an office suite and help make it feel less stuffy. Also, multiple studies have found that plants promote productivity among office staff and create a sense of calm for apprehensive patients. Improvements like this can make a big difference. They show an attention to detail and a willingness to make your practice as inviting as possible for patients and employees alike.
Spruce-up time is also an excellent opportunity to inventory your medical equipment. We’ve all seen “vintage” offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice; but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part; many – particularly younger ones – assume that doctors who don’t keep up with technological innovations don’t keep up with anything else, either.
If you’re planning a vacation this year (and I hope you are), that would be the perfect time for a re-do. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
that your patients might see?
We tend not to notice gradual deterioration in the workplace we inhabit every day: Carpets fade and dull with constant traffic and cleaning; wallpaper and paint accumulate dirt, stains, and damage; furniture gets dirty and dented, fabric rips, hardware goes missing; laminate peels off the edges of desks and cabinets.
When did you last take a good look at your waiting room? How clean is it? Patients expect cleanliness in doctor’s offices, and they expect the reception area to be neat. How are the carpeting and upholstery holding up? Sit in your chairs; how do they feel? Patients don’t appreciate a sore back or bottom from any chairs, especially in a medical office. Consider investing in new furniture that will be attractive and comfortable for your patients.
Look at the decor itself; is it dated or just plain “old-looking?” Any interior designer will tell you they can determine quite accurately when a space was last decorated, simply by the color and style of the materials used. If your office is stuck in the ‘90s, it’s probably time for a change. Even if you don’t find anything obvious, it’s wise to check periodically for subtle evidence of age: Find some patches of protected carpeting and flooring under stationary furniture and compare them to exposed floors.
If your color scheme is hopelessly out of date and style, or if you are just tired of it, change it. Wallpaper and carpeting should be long-wearing industrial quality; paint should be high-quality “eggshell” finish to facilitate cleaning, and everything should be professionally applied. (This is neither the time nor place for do-it-yourself experiments.) Consider updating your overhead lighting. The harsh glow of fluorescent lights amid an uninspired decor creates a sterile, uninviting atmosphere.
During renovation, get your building’s maintenance crew to fix any nagging plumbing, electrical, or heating/air conditioning problems while pipes, ducts, and wires are more readily accessible. This is also a good time to clear out old textbooks, journals, and files that you will never open again, in this digital age.
If your wall decorations are dated and unattractive, now would be a good time to replace at least some of them. This need not be an expensive proposition; a few years ago, I redecorated my exam room walls with framed photos from my travel adventures – to very positive responses from patients and staff alike. If you’re not an artist or photographer, invite a family member, or local artists or talented patients, to display some of their creations on your walls. If you get too many contributions, you can rotate them on a periodic basis.
Plants are great aesthetic accents, yet many offices have little or no plant life. Plants naturally aerate an office suite and help make it feel less stuffy. Also, multiple studies have found that plants promote productivity among office staff and create a sense of calm for apprehensive patients. Improvements like this can make a big difference. They show an attention to detail and a willingness to make your practice as inviting as possible for patients and employees alike.
Spruce-up time is also an excellent opportunity to inventory your medical equipment. We’ve all seen “vintage” offices full of gadgets that were state-of-the-art decades ago. Nostalgia is nice; but would you want to be treated by a physician whose office could be a Smithsonian exhibit titled, “Doctor’s Office Circa 1975?” Neither would your patients, for the most part; many – particularly younger ones – assume that doctors who don’t keep up with technological innovations don’t keep up with anything else, either.
If you’re planning a vacation this year (and I hope you are), that would be the perfect time for a re-do. Your patients will be spared the dust and turmoil, tradespeople won’t have to work around your office hours, and you won’t have to cancel any hours that weren’t already canceled. Best of all, you’ll come back to a clean, fresh environment.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Exophytic Firm Papulonodule on the Labia in a Patient With Nonspecific Gastrointestinal Symptoms
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).
In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.
Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
The Diagnosis: Cutaneous Crohn Disease
Kinyoun and Grocott-Gomori methenamine-silver staining of the labial biopsy were negative for mycobacteria and fungi, respectively. A complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, celiac disease serologies, stool occult blood, and stool calprotectin laboratory test results were within reference range. Magnetic resonance imaging of the pelvis demonstrated an anal fissure extending from the anal verge at the 6 o’clock position, abnormal T2 bright signal in the skin of the buttocks and perineum extending to the labia, and mild mucosal enhancement of the rectal and anal mucosa. Esophagogastroduodenoscopy and magnetic resonance elastography were unremarkable. Colonoscopy demonstrated scattered superficial erythematous patches and erosions in the rectum. Histologically, there was mild to moderately active colitis in the rectum with no evidence of chronicity. Given our patient’s labial edema and exophytic papulonodule (Figure 1) in the setting of nonspecific gastrointestinal symptoms and granulomatous dermatitis seen on pathology (Figure 2), she was diagnosed with cutaneous Crohn disease (CD).

In our patient, labial biopsy was necessary to definitively diagnose CD. Prior to biopsy of the lesion, our patient was diagnosed with irritable bowel syndrome with constipation leading to an anal fissure and skin tag due to lack of laboratory, imaging, and colonoscopy findings commonly associated with CD. Her biopsy results and gastrointestinal symptoms made these diagnoses, as well as condyloma or a large sentinel skin tag, less likely.

Extraintestinal findings of CD, especially cutaneous manifestations, are relatively frequent and may be present in as many as 44% of patients.1,2 Cutaneous CD often is characterized based on pathogenic mechanisms as either reactive, associated, or CD specific. Reactive cutaneous manifestations include erythema nodosum, pyoderma gangrenosum, and oral aphthae. Associated cutaneous manifestations include vitiligo, palmar erythema, and palmoplantar pustulosis.2 Crohn disease–specific manifestations, including genital or extragenital metastatic CD (MCD), fistulas, and oral involvement, are granulomatous in nature, similar to intestinal CD. Genital manifestations of MCD include edema, erythema, fissures, and/or ulceration of the vulva, penis, or scrotum. Labial swelling is the most common presenting symptom of MCD in females in both pediatric and adult age groups.2 Lymphedema, skin tags, and condylomalike growths also can be seen but are relatively less common.2
Given the labial edema, exophytic papulonodule, and granulomatous dermatitis seen on histopathology, our patient likely fit into the MCD category.2 In adults, most instances of MCD arise in the setting of well-established intestinal CD disease,3 whereas in children 86% of cases occur in patients without concurrent intestinal CD.2
Given the nonspecific and variable presentation of MCD, the differential diagnosis is broad. The differential diagnosis could include infectious etiologies such as condyloma acuminatum (human papillomavirus); syphilitic chancre; or mycobacterial, bacterial, fungal, or parasitic vulvovaginitis. Sexual abuse, sarcoidosis, Behçet disease, or hidradenitis suppurativa, among other diagnoses, also should be considered. Diagnostic workup should include biopsy of the lesion with special stains, polarizing microscopy, and tissue cultures.4 A thorough evaluation for gastrointestinal CD should be completed after diagnosis.3
The clinical course of vulvar CD can be unpredictable, with some cases healing spontaneously but most persisting despite treatment and sometimes prompting surgical removal.2,4 Early recognition is crucial, as long-standing MCD lesions can be therapy resistant.5 Due to the rarity of the condition and lack of data, there is a lack of treatment consensus for MCD. In 2014, the American Academy of Dermatology published treatment guidelines recommending superpotent topical steroids or topical tacrolimus as first-line therapy. Next-line therapy includes oral metronidazole, followed by prednisolone if still symptomatic.3 Treatment-resistant disease can warrant treatment with immunomodulators or tumor necrosis factor α inhibitors. Our patient was started on adalimumab; after just 2 months of therapy, the labial swelling decreased and the exophytic nodule was less firm and smaller.
Metastatic CD is a rare manifestation of cutaneous CD and can be present in the absence of gastrointestinal disease.3 This case demonstrates the importance of recognizing the cutaneous signs of CD and the necessity of lesional biopsy for the diagnosis of MCD, as our patient presented with nonspecific gastrointestinal symptoms and a diagnostic workup, including endoscopies, that proved inconclusive for the diagnosis of CD.
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
- Antonelli E, Bassotti G, Tramontana M, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10:1-16. doi:10.3390/JCM10020364
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574. doi:10.1111/PDE.13565
- Kurtzman DJB, Jones T, Lian F, et al. Metastatic Crohn’s disease: a review and approach to therapy. J Am Acad Dermatol. 2014;71:804-813. doi:10.1016/J.JAAD.2014.04.002
- Barret M, De Parades V, Battistella M, et al. Crohn’s disease of the vulva. J Crohns Colitis. 2014;8:563-570. doi:10.1016/J.CROHNS.2013.10.009
- Aberumand B, Howard J, Howard J. Metastatic Crohn’s disease: an approach to an uncommon but important cutaneous disorder [published online January 3, 2017]. Biomed Res Int. 2017;2017:8192150. doi:10.1155/2017/8192150
An 18-year-old woman with chronic constipation presented with an enlarging, painful, and edematous “lump” in the perineum of 1 year’s duration. The lesion became firmer and more painful with bowel movements. Physical examination revealed an enlarged right labia majora, as well as a pink to flesh-colored, exophytic, firm papulonodule in the perineum posterior to the right labia. The patient concomitantly was following with gastroenterology due to abdominal pain that worsened with eating, as well as constipation, nausea, weight loss, and rectal bleeding of 5 years’ duration. The patient denied rash, joint arthralgia, or oral ulcers. A biopsy from the labial lesion was performed.
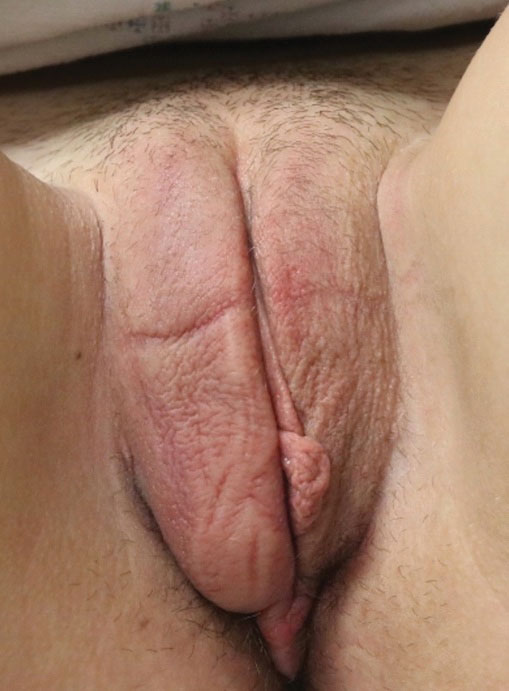
Buccal fat pad removal
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
The buccal fat pads, previously known as Bichat’s fat pads, were first described in 1802. Everyone has them and their size is predominantly related to genetics, but similar to other facial fat pockets, they can shrink or shift over time. Buccal fat pads are often resistant to weight loss and stubbornly persist because of a slower lipolytic rate than subcutaneous fat. Some patients with round facial shapes may seek removal of the midface volume to create a more angular cheek and jawline, which enhances the zygomatic prominence and mandibular angle, creating a more contoured face.
The buccal fat pad is a submuscular fat pad surrounded by a capsule that contains three lobes with four extensions. The anterior lobe rests in front of the anterior border of the masseter muscle. The intermediate lobe extends between the masseter and buccinator muscles. The posterior lobe extends between the temporal masticatory space. These pads range from 7-11 mL in volume and grow from ages 10 to 20 years, declining in size after age 20. Given their location in the central face, they contain a rich vascular supply and are surrounded by the facial nerves, salivary glands, the parotid gland, and muscles of mastication.
The aesthetic contour of the lower face is defined by the mandibular prominence, the masseter muscle, subcutaneous fat, and the buccal fat pad. Surgically, removal is a simple and safe procedure. Complications can include damage to the parotid gland, vessels, salivary duct, or facial nerve. Temporary numbness, swelling, and facial asymmetry are the most common complications.
Increasing popularity, controversy
Removal of the buccal fat pads has become popular because of celebrity media exposure, particularly among young women seeking a slim appearance to their face and jawlines. Although the procedure is relatively simple, there have been no long term studies evaluating the effects of buccal fat pad removal on facial aging.
The shrinking or shifting of fat that occurs with aging makes the removal of these fat pads in young women controversial because when removed, they cannot be effectively replaced. Shrinking of the fat pads with age, loss of midface volume, and solar elastosis can make the cheeks appear gaunt and “sucked in.”
An experienced surgeon will reduce and contour the fat pads – and will not completely remove them – to prevent a complete hollowing of the cheeks over time. Complete removal is not recommended and in men, overzealous removal in men can feminize the face.
In middle-aged men and women, the buccal fat pad can shift to the lower face and often drops below the angle of the mandible giving the appearance of jowls. Complete removal of the shifted buccal fat pad will help align the jawline; however, residual skin laxity is a complication and must be addressed to fully correct the jowls.
In my experience, the best approach to reducing buccal pads as an alternative to surgical removal is “melting” the buccal fat in a systematic, controlled manner over several sessions with either radiofrequency laser or deoxycholic acid injections. This slow, controlled method allows me to contour the cheeks appropriately in concordance with the patient’s anatomy. In younger patients or those with little skin laxity, I choose treatments with deoxycholic acid to remove the pads (which I also use to treat the jowls, as outlined in my 2020 column on treating the jowl overhang with deoxycholic acid).
In patients with more skin laxity, I perform sequential radiofrequency laser treatment over the fat pockets to simultaneously melt the fat pockets and tighten the overlying skin. Both of these methods often require three to six treatments. The controlled, cautious, treatments gradually shrink the fat pockets while preventing the overhollowing of the face.
Dr. Talakoub and Dr. Naissan O. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
References
Dubin B et al. Plast Reconstr Surg. 1989 Feb;83(2):257-64
Jackson IT. Plast Reconstr Surg. 1999 Jun;103(7):2059-60.
Matarasso A. Ann Plast Surg. 1991 May;26(5):413-8.
Belimumab for pregnant women with lupus: B-cell concerns remain
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
The largest combined analysis of birth outcome data for women with systemic lupus erythematosus (SLE) who took belimumab (Benlysta) during pregnancy appears to indicate that the biologic is “unlikely to cause very frequent birth defects,” but the full extent of possible risk remains unknown. The drug’s effect on B cells, immune function, and infections in exposed offspring were not captured in the data, but a separate case report published after the belimumab pregnancy data report indicates that the drug does cross the placenta and builds up in the blood of the newborn, reducing B cells at birth.
Children of women with SLE have increased birth defect risks, and standard SLE therapeutic agents (for example, cyclophosphamide, methotrexate, mycophenolate mofetil) have been implicated in birth defects and pregnancy loss, but birth defect data for biologic drugs such as belimumab are limited. While belimumab animal data revealed no evidence of fetal harm or pregnancy loss rates, there was evidence of immature and mature B-cell count reductions.
Belimumab is approved by the Food and Drug Administration for use in patients aged 5 years and older with active, autoantibody-positive SLE who are taking standard therapy, and also for those with lupus nephritis.
Michelle Petri, MD, of Johns Hopkins University, Baltimore, and coauthors reported in Annals of the Rheumatic Diseases on data they compiled through March 8, 2020, from belimumab clinical trials, the Belimumab Pregnancy Registry (BPR), and postmarketing/spontaneous reports that encompassed 319 pregnancies with known outcomes.
Across 18 clinical trials with 223 live births, birth defects occurred in 4 of 72 (5.6%) belimumab-exposed pregnancies and in 0 of 9 in placebo-exposed pregnancies. Pregnancy loss (excluding elective terminations) occurred in 31.8% (35 of 110) of belimumab-exposed women and 43.8% (7 of 16) of placebo-exposed women in clinical trials. In the BPR retrospective cohort, 4.2% had pregnancy loss. Postmarketing and spontaneous reports had a pregnancy loss rate of 31.4% (43 of 137). Concomitant medications, confounding factors, and/or missing data were noted in all belimumab-exposed women in clinical trials and the BPR cohort. Dr. Petri and colleagues reported no consistent pattern of birth defects across datasets but stated: “Low numbers of exposed pregnancies, presence of confounding factors/other biases, and incomplete information preclude informed recommendations regarding risk of birth defects and pregnancy loss with belimumab use.”
In an interview, coauthor Megan E. B. Clowse, MD, MPH, associate professor of medicine and director of the division of rheumatology and immunology at Duke University, Durham, N.C., said that “the Annals of the Rheumatic Diseases article provides some reassurance that belimumab is unlikely to cause very frequent birth defects. It is clearly not in the risk-range for thalidomide or mycophenolate. However, due to the complexity of collecting these data, this manuscript can’t explore the full extent of possible risks. It also did not provide information about B cells, immune function, or infection risks in exposed offspring.”
A separate case report by Helle Bitter of the department of rheumatology at Sorlandet Hospital Kristiansand (Norway) in Annals of the Rheumatic Diseases is the first to show transplacental passage of belimumab in humans. Other prior reports have shown such transplacental passage for monoclonal IgG antibodies (tumor necrosis factor inhibitors and rituximab). Even though the last infusion was given late in the second trimester, belimumab was present in cord serum at birth, suggesting much higher concentrations before treatment was stopped. While B-cell numbers were reduced at birth, they returned to normal ranges by 4 months post partum when they were undetectable. In the mother, B-cell numbers remained low throughout the study period extending to 7 months after delivery. The authors stated that the child had a normal vaccination response, and except for the reduced B-cell levels at birth, had no adverse effects of prenatal exposure to maternal medication through age 6 years.
“The belimumab transfer in the case report is the level that we would anticipate based on similar studies in infant/mother pairs on other IgG1 antibody biologics like adalimumab – about 60% higher than the maternal level at birth,” Dr. Clowse said. “That the baby has very low B cells at birth is worrisome to me, demonstrating the lasting effect of maternal belimumab on the infant’s immune system, even when the drug was stopped 14 weeks prior to delivery. While this single infant did not have problems with infections, with more widespread use it seems possible that infants would be found to have higher rates of infections after in utero belimumab exposure.”
The field of lupus research greatly needs controlled studies of newer biologics in pregnancy, Dr. Clowse said. “Women with active lupus in pregnancy – particularly with active lupus nephritis – continue to suffer tragic outcomes at an alarming rate. Newer treatments for lupus nephritis provide some hope that we might be able to control lupus nephritis in pregnancy more effectively. The available data suggests the risks of these medications are not so large as to make studies unreasonable. Our current data doesn’t allow us to sufficiently balance the potential risks and benefits in a way that provides clinically useful guidance. Trials of these medications, however, would enable us to identify improved treatment strategies that could result in healthier women, pregnancies, and babies.”
GlaxoSmithKline funded the study. Dr. Clowse reported receiving consulting fees and grants from UCB and GlaxoSmithKline that relate to pregnancy in women with lupus.
FROM ANNALS OF THE RHEUMATIC DISEASES