User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
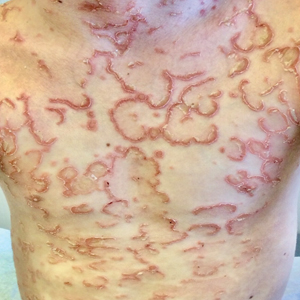
Sustained response at 2 years reported for newly approved oral psoriasis agent
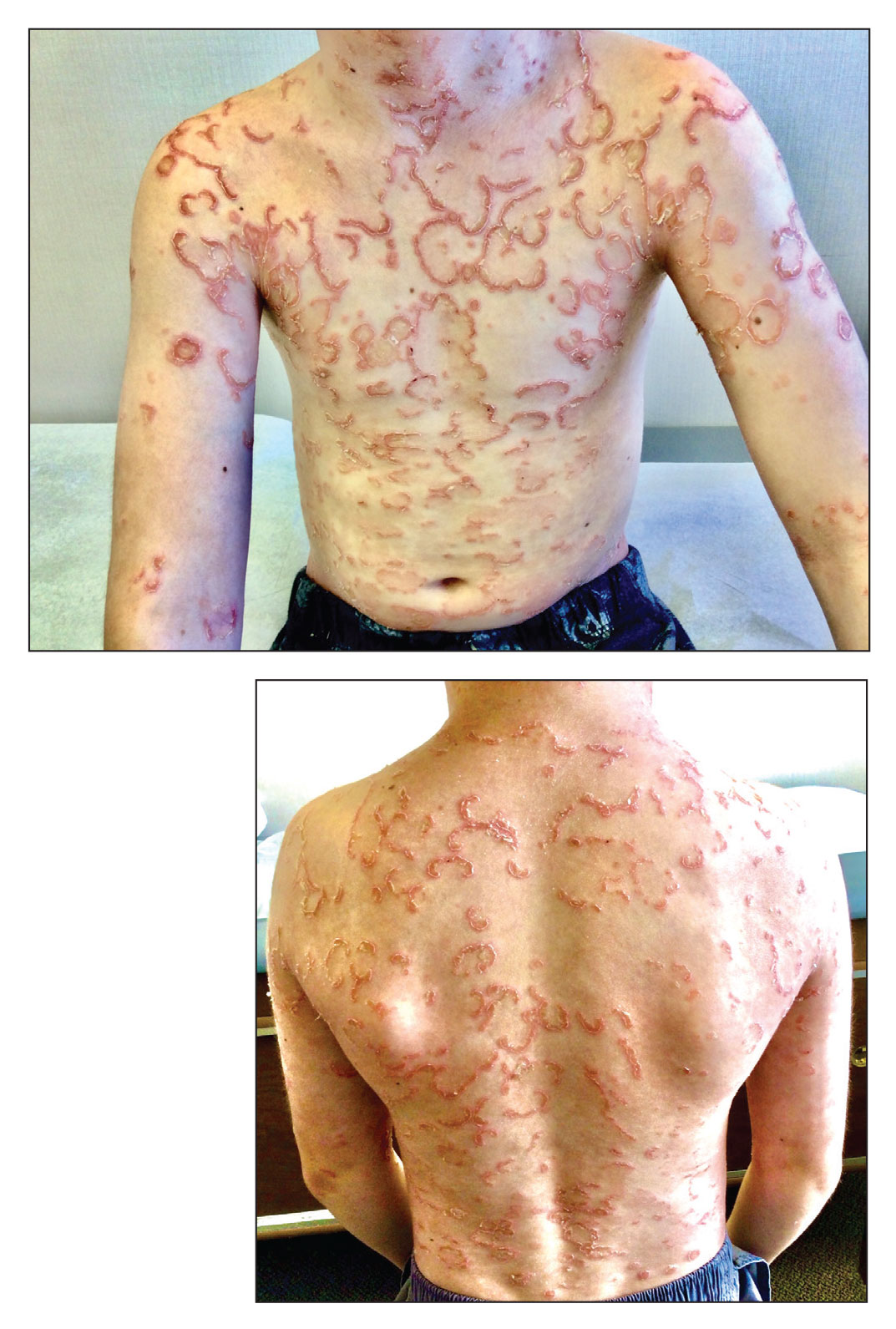
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
MILAN – The day after deucravacitinib became the first TYK2 inhibitor approved for the treatment of moderate to severe psoriasis, long-term data were presented at the annual congress of the European Academy of Dermatology and Venereology, suggesting that a high degree of benefit persists for at least 2 years, making this oral drug a potential competitor for biologics.
,” said Mark G. Lebwohl, MD, professor of dermatology and dean of clinical therapeutics, Icahn School of Medicine at Mount Sinai, New York.
Just 2 months after the 52-week data from the phase 3 POETYK PSO-1 trial were published online in the Journal of the American Academy of Dermatology, a long-term extension study found essentially no loss of benefit at 112 weeks, according to Dr. Lebwohl.
One of the two co-primary endpoints was a 75% clearance on the Psoriasis and Severity Index (PASI75) score. At 52 weeks, 80.2% of patients on deucravacitinib had met this criterion of benefit. At 112 weeks, the proportion was 84.4%.
The other primary endpoint was a static Physician’s Global Assessment (sPGA) score of clear or almost clear skin. The proportion of patients meeting this criterion at weeks 52 and 112 weeks were 65.6% and 67.6%, respectively.
When assessed by Treatment Failure Rule (TFR) or modified nonresponder imputation (mNRI), results were similar. For both, the primary endpoints at every time interval were just one or two percentage points lower but not clinically meaningfully different, according to Dr. Lebwohl.
The same type of sustained response out to 112 weeks was observed in multiple analyses. When the researchers isolated the subgroup of patients who had achieved a PASI 75 response at 16 weeks (100%), there was a modest decline in the PASI 75 rate at week 52 (90.2%) but then no additional decline at week 112 (91.3%).
There were essentially no changes in the PASI 90 rates at week 16 (63%), week 52 (65.3%), and week 112 (63.1%), Dr. Lebwohl reported. PASI 100 rates, once achieved, were sustained long term.
The target, TYK2, is one of four Janus kinase (JAK) inhibitors. Until now, almost all JAK inhibitors have had greater relative specificity for JAK 1, JAK 2, and JAK 3, but several inhibitors of TYK2 inhibitors other than deucravacitinib are in development for inflammatory diseases. Deucravacitinib (Sotyktu), approved by the Food and Drug Administration on Sept. 9, is the only TYK2 inhibitor with regulatory approval for plaque psoriasis.
In the POETYK PSO-1 trial, 666 patients were initially randomized in a 2:1:1 ratio to 6 mg deucravacitinib (now the approved dose), placebo, or the oral phosphodiesterase 4 inhibitor apremilast. At week 16, patients on placebo were switched over to deucravacitinib. At week 24, patients who did not achieve a PASI 50 on apremilast (which had been titrated to 10 mg daily to 30 mg twice a day over the first 5 days of dosing) were switched to deucravacitinib.
In the previously reported data, deucravacitinib was superior for all efficacy endpoints at week 16, including an analysis of quality of life when compared with placebo (P < .0001) or apremilast (P = .0088). At week 52, after having been switched to deucravacitinib at week 16, patients on placebo achieved comparable responses on the efficacy measures in this study, including PASI75.
Relative to JAK inhibitors commonly used in rheumatoid arthritis and other inflammatory diseases, the greater specificity of deucravacitinib for TYK2 appears to have meaningful safety advantages, according to Dr. Lebwohl. Targeted mostly on the TYK2 regulatory domain, deucravacitinib largely avoids inhibition of the JAK 1, 2, and 3 subtypes. Dr. Lebwohl said this explains why deucravacitinib labeling does not share the boxed warnings about off-target effects, such as those on the cardiovascular system, that can be found in the labeling of other JAK inhibitors.
In the published 52-week data, the discontinuation rate for adverse events was lower in the group randomized to deucravacitinib arm than in the placebo arm. In the extended follow-up, there were no new signals for adverse events, including those involving the CV system or immune function.
The key message so far from the long-term follow-up, which is ongoing, is that “continuous treatment with deucravacitinib is associated with durable efficacy,” Dr. Lebwohl said. It is this combination of sustained efficacy and safety that led Dr. Lebwohl to suggest it as a reasonable oral competitor to injectable biologics.
“Patients now have a choice,” he said.
Jashin J. Wu, MD, a board member of the National Psoriasis Foundation and an associate professor in the department of dermatology, University of Miami, has been following the development of deucravacitinib. He said that the recent FDA approval validates the clinical evidence of benefit and safety, while the long-term data presented at the EADV congress support its role in expanding treatment options.
“Deucravacitinib is a very effective oral agent for moderate to severe plaque psoriasis with strong maintenance of effect through week 112,” he said. Differentiating it from other JAK inhibitors, the FDA approval “confirms the safety of this agent as there is no boxed warning,” he added.
Dr. Lebwohl reports financial relationships with more than 30 pharmaceutical companies, including Bristol-Myers Squibb, the manufacturer of deucravacitinib. Dr. Wu has financial relationships with 14 pharmaceutical companies, including Bristol-Myers Squibb, but he was not an investigator for the phase 3 trials of deucravacitinib.
AT THE EADV CONGRESS
‘Dr. Caveman’ had a leg up on amputation
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
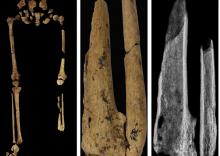
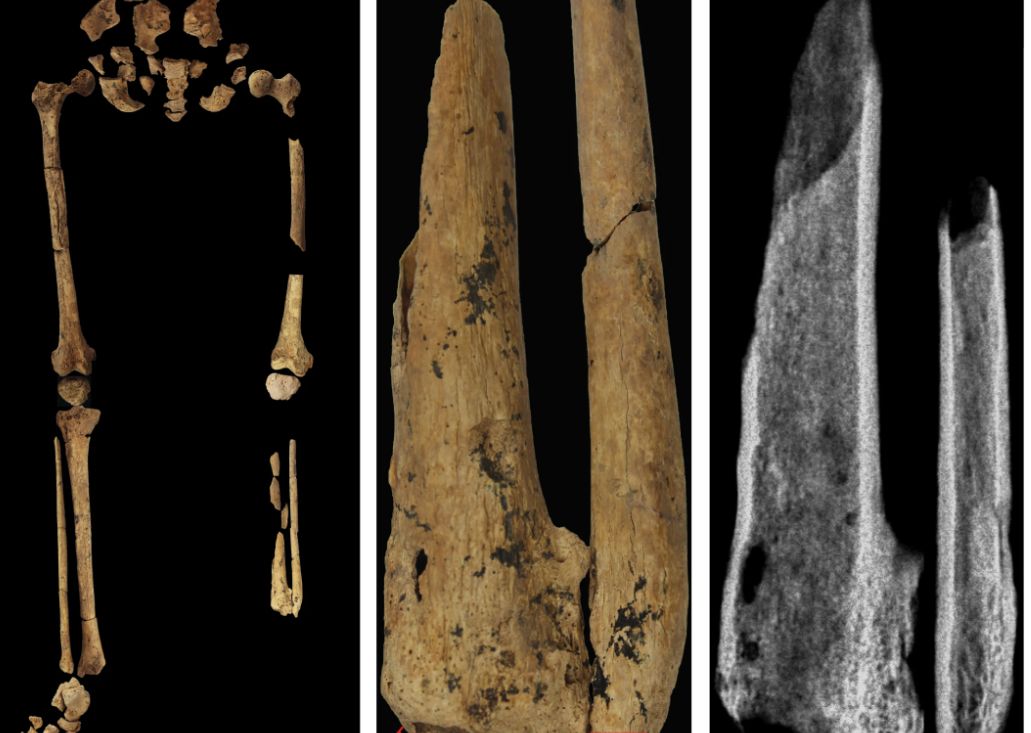
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
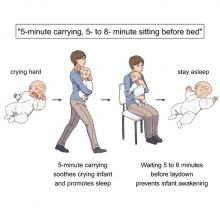
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Monkey see, monkey do (advanced medical procedures)
We don’t tend to think too kindly of our prehistoric ancestors. We throw around the word “caveman” – hardly a term of endearment – and depictions of Paleolithic humans rarely flatter their subjects. In many ways, though, our conceptions are correct. Humans of the Stone Age lived short, often brutish lives, but civilization had to start somewhere, and our prehistoric ancestors were often far more capable than we give them credit for.
Case in point is a recent discovery from an archaeological dig in Borneo: A young adult who lived 31,000 years ago was discovered with the lower third of their left leg amputated. Save the clever retort about the person’s untimely death, because this individual did not die from the surgery. The amputation occurred when the individual was a child and the subject lived for several years after the operation.
Amputation is usually unnecessary given our current level of medical technology, but it’s actually quite an advanced procedure, and this example predates the previous first case of amputation by nearly 25,000 years. Not only did the surgeon need to cut at an appropriate place, they needed to understand blood loss, the risk of infection, and the need to preserve skin in order to seal the wound back up. That’s quite a lot for our Paleolithic doctor to know, and it’s even more impressive considering the, shall we say, limited tools they would have had available to perform the operation.
Rocks. They cut off the leg with a rock. And it worked.
This discovery also gives insight into the amputee’s society. Someone knew that amputation was the right move for this person, indicating that it had been done before. In addition, the individual would not have been able to spring back into action hunting mammoths right away, they would require care for the rest of their lives. And clearly the community provided, given the individual’s continued life post operation and their burial in a place of honor.
If only the American health care system was capable of such feats of compassion, but that would require the majority of politicians to be as clever as cavemen. We’re not hopeful on those odds.
The first step is admitting you have a crying baby. The second step is … a step
Knock, knock.
Who’s there?
Crying baby.
Crying baby who?
Crying baby who … umm … doesn’t have a punchline. Let’s try this again.
A priest, a rabbi, and a crying baby walk into a bar and … nope, that’s not going to work.
Why did the crying baby cross the road? Ugh, never mind.
Clearly, crying babies are no laughing matter. What crying babies need is science. And the latest innovation – it’s fresh from a study conducted at the RIKEN Center for Brain Science in Saitama, Japan – in the science of crying babies is … walking. Researchers observed 21 unhappy infants and compared their responses to four strategies: being held by their walking mothers, held by their sitting mothers, lying in a motionless crib, or lying in a rocking cot.
The best strategy is for the mother – the experiment only involved mothers, but the results should apply to any caregiver – to pick up the crying baby, walk around for 5 minutes, sit for another 5-8 minutes, and then put the infant back to bed, the researchers said in a written statement.
The walking strategy, however, isn’t perfect. “Walking for 5 minutes promoted sleep, but only for crying infants. Surprisingly, this effect was absent when babies were already calm beforehand,” lead author Kumi O. Kuroda, MD, PhD, explained in a separate statement from the center.
It also doesn’t work on adults. We could not get a crying LOTME writer to fall asleep no matter how long his mother carried him around the office.
New way to detect Parkinson’s has already passed the sniff test
We humans aren’t generally known for our superpowers, but a woman from Scotland may just be the Smelling Superhero. Not only was she able to literally smell Parkinson’s disease (PD) on her husband 12 years before his diagnosis; she is also the reason that scientists have found a new way to test for PD.
Joy Milne, a retired nurse, told the BBC that her husband “had this musty rather unpleasant smell especially round his shoulders and the back of his neck and his skin had definitely changed.” She put two and two together after he had been diagnosed with PD and she came in contact with others with the same scent at a support group.
Researchers at the University of Manchester, working with Ms. Milne, have now created a skin test that uses mass spectroscopy to analyze a sample of the patient’s sebum in just 3 minutes and is 95% accurate. They tested 79 people with Parkinson’s and 71 without using this method and found “specific compounds unique to PD sebum samples when compared to healthy controls. Furthermore, we have identified two classes of lipids, namely, triacylglycerides and diglycerides, as components of human sebum that are significantly differentially expressed in PD,” they said in JACS Au.
This test could be available to general physicians within 2 years, which would provide new opportunities to the people who are waiting in line for neurologic consults. Ms. Milne’s husband passed away in 2015, but her courageous help and amazing nasal abilities may help millions down the line.
The power of flirting
It’s a common office stereotype: Women flirt with the boss to get ahead in the workplace, while men in power sexually harass women in subordinate positions. Nobody ever suspects the guys in the cubicles. A recent study takes a different look and paints a different picture.
The investigators conducted multiple online and lab experiments in how social sexual identity drives behavior in a workplace setting in relation to job placement. They found that it was most often men in lower-power positions who are insecure about their roles who initiate social sexual behavior, even though they know it’s offensive. Why? Power.
They randomly paired over 200 undergraduate students in a male/female fashion, placed them in subordinate and boss-like roles, and asked them to choose from a series of social sexual questions they wanted to ask their teammate. Male participants who were placed in subordinate positions to a female boss chose social sexual questions more often than did male bosses, female subordinates, and female bosses.
So what does this say about the threat of workplace harassment? The researchers found that men and women differ in their strategy for flirtation. For men, it’s a way to gain more power. But problems arise when they rationalize their behavior with a character trait like being a “big flirt.”
“When we take on that identity, it leads to certain behavioral patterns that reinforce the identity. And then, people use that identity as an excuse,” lead author Laura Kray of the University of California, Berkeley, said in a statement from the school.
The researchers make a point to note that the study isn’t about whether flirting is good or bad, nor are they suggesting that people in powerful positions don’t sexually harass underlings. It’s meant to provide insight to improve corporate sexual harassment training. A comment or conversation held in jest could potentially be a warning sign for future behavior.
Targeted anti-IgE therapy found safe and effective for chronic urticaria
MILAN – The therapeutic .
Both doses of ligelizumab evaluated met the primary endpoint of superiority to placebo for a complete response at 16 weeks of therapy, reported Marcus Maurer, MD, director of the Urticaria Center for Reference and Excellence at the Charité Hospital, Berlin.
The data from the two identically designed trials, PEARL 1 and PEARL 2, were presented at the annual congress of the European Academy of Dermatology and Venereology. The two ligelizumab experimental arms (72 mg or 120 mg administered subcutaneously every 4 weeks) and the active comparative arm of omalizumab (300 mg administered subcutaneously every 4 weeks) demonstrated similar efficacy, all three of which were highly superior to placebo.
The data show that “another anti-IgE therapy – ligelizumab – is effective in CSU,” Dr. Maurer said.
“While the benefit was not different from omalizumab, ligelizumab showed remarkable results in disease activity and by demonstrating just how many patients achieved what we want them to achieve, which is to have no more signs and symptoms,” he added.
Majority of participants with severe urticaria
All of the patients entered into the two trials had severe (about 65%) or moderate (about 35%) symptoms at baseline. The results of the two trials were almost identical. In the randomization arms, a weekly Urticaria Activity Score (UAS7) of 0, which was the primary endpoint, was achieved at week 16 by 31.0% of those receiving 72-mg ligelizumab, 38.3% of those receiving 120-mg ligelizumab, and 34.1% of those receiving omalizumab (Xolair). The placebo response was 5.7%.
The UAS7 score is drawn from two components, wheals and itch. The range is 0 (no symptoms) to 42 (most severe). At baseline, the average patients’ scores were about 30, which correlates with a substantial symptom burden, according to Dr. Maurer.
The mean reduction in the UAS7 score in PEARL 2, which differed from PEARL 1 by no more than 0.4 points for any treatment group, was 19.2 points in the 72-mg ligelizumab group, 19.3 points in the 120-mg ligelizumab group, 19.6 points in the omalizumab group, and 9.2 points in the placebo group. There were no significant differences between any active treatment arm.
Complete symptom relief, meaning a UAS7 score of 0, was selected as the primary endpoint, because Dr. Maurer said that this is the goal of treatment. Although he admitted that a UAS7 score of 0 is analogous to a PASI score in psoriasis of 100 (complete clearing), he said, “Chronic urticaria is a debilitating disease, and we want to eliminate the symptoms. Gone is gone.”
Combined, the two phase 3 trials represent “the biggest chronic urticaria program ever,” according to Dr. Maurer. The 1,034 patients enrolled in PEARL 1 and the 1,023 enrolled in PEARL 2 were randomized in a 3:3:3:1 ratio with placebo representing the smaller group.
The planned follow-up is 52 weeks, but the placebo group will be switched to 120 mg ligelizumab every 4 weeks at the end of 24 weeks. The switch is required because “you cannot maintain patients with this disease on placebo over a long period,” Dr. Maurer said.
Ligelizumab associated with low discontinuation rate
Adverse events overall and stratified by severity have been similar across treatment arms, including placebo. The possible exception was a lower rate of moderate events (16.5%) in the placebo arm relative to the 72-mg ligelizumab arm (19.8%), the 120-mg ligelizumab arm (21.6%), and the omalizumab arm (22.3%). Discontinuations because of an adverse event were under 4% in every treatment arm.
Although Dr. Maurer did not present outcomes at 52 weeks, he did note that “only 15% of those who enrolled in these trials have discontinued treatment.” He considered this remarkable in that the study was conducted in the midst of the COVID-19 pandemic, and it appears that at least some of those left the trial did so because of concern for clinic visits.
Despite the similar benefit provided by ligelizumab and omalizumab, Dr. Maurer said that subgroup analyses will be coming. The possibility that some patients benefit more from one than the another cannot yet be ruled out. There are also, as of yet, no data to determine whether at least some patients respond to one after an inadequate response to the other.
Still, given the efficacy and the safety of ligelizumab, Dr. Maurer indicated that the drug is likely to find a role in routine management of CSU if approved.
“We only have two options for chronic spontaneous urticaria. There are antihistamines, which do not usually work, and omalizumab,” he said. “It is very important we develop more treatment options.”
Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, agreed.
“More therapeutic options, especially for disease states that have a small armament – even if equivalent in efficacy to established therapies – is always a win for patients as it almost always increases access to treatment,” Dr. Friedman said in an interview.
“Furthermore, the heterogeneous nature of inflammatory skin diseases is often not captured in even phase 3 studies. Therefore, having additional options could offer relief where previous therapies have failed,” he added.
Dr. Maurer reports financial relationships with more than 10 pharmaceutical companies, including Novartis, which is developing ligelizumab. Dr. Friedman has a financial relationship with more than 20 pharmaceutical companies but has no current financial association with Novartis and was not involved in the PEARL 1 and 2 trials.
MILAN – The therapeutic .
Both doses of ligelizumab evaluated met the primary endpoint of superiority to placebo for a complete response at 16 weeks of therapy, reported Marcus Maurer, MD, director of the Urticaria Center for Reference and Excellence at the Charité Hospital, Berlin.
The data from the two identically designed trials, PEARL 1 and PEARL 2, were presented at the annual congress of the European Academy of Dermatology and Venereology. The two ligelizumab experimental arms (72 mg or 120 mg administered subcutaneously every 4 weeks) and the active comparative arm of omalizumab (300 mg administered subcutaneously every 4 weeks) demonstrated similar efficacy, all three of which were highly superior to placebo.
The data show that “another anti-IgE therapy – ligelizumab – is effective in CSU,” Dr. Maurer said.
“While the benefit was not different from omalizumab, ligelizumab showed remarkable results in disease activity and by demonstrating just how many patients achieved what we want them to achieve, which is to have no more signs and symptoms,” he added.
Majority of participants with severe urticaria
All of the patients entered into the two trials had severe (about 65%) or moderate (about 35%) symptoms at baseline. The results of the two trials were almost identical. In the randomization arms, a weekly Urticaria Activity Score (UAS7) of 0, which was the primary endpoint, was achieved at week 16 by 31.0% of those receiving 72-mg ligelizumab, 38.3% of those receiving 120-mg ligelizumab, and 34.1% of those receiving omalizumab (Xolair). The placebo response was 5.7%.
The UAS7 score is drawn from two components, wheals and itch. The range is 0 (no symptoms) to 42 (most severe). At baseline, the average patients’ scores were about 30, which correlates with a substantial symptom burden, according to Dr. Maurer.
The mean reduction in the UAS7 score in PEARL 2, which differed from PEARL 1 by no more than 0.4 points for any treatment group, was 19.2 points in the 72-mg ligelizumab group, 19.3 points in the 120-mg ligelizumab group, 19.6 points in the omalizumab group, and 9.2 points in the placebo group. There were no significant differences between any active treatment arm.
Complete symptom relief, meaning a UAS7 score of 0, was selected as the primary endpoint, because Dr. Maurer said that this is the goal of treatment. Although he admitted that a UAS7 score of 0 is analogous to a PASI score in psoriasis of 100 (complete clearing), he said, “Chronic urticaria is a debilitating disease, and we want to eliminate the symptoms. Gone is gone.”
Combined, the two phase 3 trials represent “the biggest chronic urticaria program ever,” according to Dr. Maurer. The 1,034 patients enrolled in PEARL 1 and the 1,023 enrolled in PEARL 2 were randomized in a 3:3:3:1 ratio with placebo representing the smaller group.
The planned follow-up is 52 weeks, but the placebo group will be switched to 120 mg ligelizumab every 4 weeks at the end of 24 weeks. The switch is required because “you cannot maintain patients with this disease on placebo over a long period,” Dr. Maurer said.
Ligelizumab associated with low discontinuation rate
Adverse events overall and stratified by severity have been similar across treatment arms, including placebo. The possible exception was a lower rate of moderate events (16.5%) in the placebo arm relative to the 72-mg ligelizumab arm (19.8%), the 120-mg ligelizumab arm (21.6%), and the omalizumab arm (22.3%). Discontinuations because of an adverse event were under 4% in every treatment arm.
Although Dr. Maurer did not present outcomes at 52 weeks, he did note that “only 15% of those who enrolled in these trials have discontinued treatment.” He considered this remarkable in that the study was conducted in the midst of the COVID-19 pandemic, and it appears that at least some of those left the trial did so because of concern for clinic visits.
Despite the similar benefit provided by ligelizumab and omalizumab, Dr. Maurer said that subgroup analyses will be coming. The possibility that some patients benefit more from one than the another cannot yet be ruled out. There are also, as of yet, no data to determine whether at least some patients respond to one after an inadequate response to the other.
Still, given the efficacy and the safety of ligelizumab, Dr. Maurer indicated that the drug is likely to find a role in routine management of CSU if approved.
“We only have two options for chronic spontaneous urticaria. There are antihistamines, which do not usually work, and omalizumab,” he said. “It is very important we develop more treatment options.”
Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, agreed.
“More therapeutic options, especially for disease states that have a small armament – even if equivalent in efficacy to established therapies – is always a win for patients as it almost always increases access to treatment,” Dr. Friedman said in an interview.
“Furthermore, the heterogeneous nature of inflammatory skin diseases is often not captured in even phase 3 studies. Therefore, having additional options could offer relief where previous therapies have failed,” he added.
Dr. Maurer reports financial relationships with more than 10 pharmaceutical companies, including Novartis, which is developing ligelizumab. Dr. Friedman has a financial relationship with more than 20 pharmaceutical companies but has no current financial association with Novartis and was not involved in the PEARL 1 and 2 trials.
MILAN – The therapeutic .
Both doses of ligelizumab evaluated met the primary endpoint of superiority to placebo for a complete response at 16 weeks of therapy, reported Marcus Maurer, MD, director of the Urticaria Center for Reference and Excellence at the Charité Hospital, Berlin.
The data from the two identically designed trials, PEARL 1 and PEARL 2, were presented at the annual congress of the European Academy of Dermatology and Venereology. The two ligelizumab experimental arms (72 mg or 120 mg administered subcutaneously every 4 weeks) and the active comparative arm of omalizumab (300 mg administered subcutaneously every 4 weeks) demonstrated similar efficacy, all three of which were highly superior to placebo.
The data show that “another anti-IgE therapy – ligelizumab – is effective in CSU,” Dr. Maurer said.
“While the benefit was not different from omalizumab, ligelizumab showed remarkable results in disease activity and by demonstrating just how many patients achieved what we want them to achieve, which is to have no more signs and symptoms,” he added.
Majority of participants with severe urticaria
All of the patients entered into the two trials had severe (about 65%) or moderate (about 35%) symptoms at baseline. The results of the two trials were almost identical. In the randomization arms, a weekly Urticaria Activity Score (UAS7) of 0, which was the primary endpoint, was achieved at week 16 by 31.0% of those receiving 72-mg ligelizumab, 38.3% of those receiving 120-mg ligelizumab, and 34.1% of those receiving omalizumab (Xolair). The placebo response was 5.7%.
The UAS7 score is drawn from two components, wheals and itch. The range is 0 (no symptoms) to 42 (most severe). At baseline, the average patients’ scores were about 30, which correlates with a substantial symptom burden, according to Dr. Maurer.
The mean reduction in the UAS7 score in PEARL 2, which differed from PEARL 1 by no more than 0.4 points for any treatment group, was 19.2 points in the 72-mg ligelizumab group, 19.3 points in the 120-mg ligelizumab group, 19.6 points in the omalizumab group, and 9.2 points in the placebo group. There were no significant differences between any active treatment arm.
Complete symptom relief, meaning a UAS7 score of 0, was selected as the primary endpoint, because Dr. Maurer said that this is the goal of treatment. Although he admitted that a UAS7 score of 0 is analogous to a PASI score in psoriasis of 100 (complete clearing), he said, “Chronic urticaria is a debilitating disease, and we want to eliminate the symptoms. Gone is gone.”
Combined, the two phase 3 trials represent “the biggest chronic urticaria program ever,” according to Dr. Maurer. The 1,034 patients enrolled in PEARL 1 and the 1,023 enrolled in PEARL 2 were randomized in a 3:3:3:1 ratio with placebo representing the smaller group.
The planned follow-up is 52 weeks, but the placebo group will be switched to 120 mg ligelizumab every 4 weeks at the end of 24 weeks. The switch is required because “you cannot maintain patients with this disease on placebo over a long period,” Dr. Maurer said.
Ligelizumab associated with low discontinuation rate
Adverse events overall and stratified by severity have been similar across treatment arms, including placebo. The possible exception was a lower rate of moderate events (16.5%) in the placebo arm relative to the 72-mg ligelizumab arm (19.8%), the 120-mg ligelizumab arm (21.6%), and the omalizumab arm (22.3%). Discontinuations because of an adverse event were under 4% in every treatment arm.
Although Dr. Maurer did not present outcomes at 52 weeks, he did note that “only 15% of those who enrolled in these trials have discontinued treatment.” He considered this remarkable in that the study was conducted in the midst of the COVID-19 pandemic, and it appears that at least some of those left the trial did so because of concern for clinic visits.
Despite the similar benefit provided by ligelizumab and omalizumab, Dr. Maurer said that subgroup analyses will be coming. The possibility that some patients benefit more from one than the another cannot yet be ruled out. There are also, as of yet, no data to determine whether at least some patients respond to one after an inadequate response to the other.
Still, given the efficacy and the safety of ligelizumab, Dr. Maurer indicated that the drug is likely to find a role in routine management of CSU if approved.
“We only have two options for chronic spontaneous urticaria. There are antihistamines, which do not usually work, and omalizumab,” he said. “It is very important we develop more treatment options.”
Adam Friedman, MD, professor and chair of dermatology, George Washington University, Washington, agreed.
“More therapeutic options, especially for disease states that have a small armament – even if equivalent in efficacy to established therapies – is always a win for patients as it almost always increases access to treatment,” Dr. Friedman said in an interview.
“Furthermore, the heterogeneous nature of inflammatory skin diseases is often not captured in even phase 3 studies. Therefore, having additional options could offer relief where previous therapies have failed,” he added.
Dr. Maurer reports financial relationships with more than 10 pharmaceutical companies, including Novartis, which is developing ligelizumab. Dr. Friedman has a financial relationship with more than 20 pharmaceutical companies but has no current financial association with Novartis and was not involved in the PEARL 1 and 2 trials.
AT THE EADV CONGRESS
One in three MS patients reports chronic itch
, according to investigators.
Itch is historically underrecognized as a symptom of MS, but physicians should know that it is common and may negatively impact quality of life, reported lead author Giuseppe Ingrasci, MD, a dermatology research fellow at the University of Miami, Miller School of Medicine, and colleagues.
While previous publications suggest that pruritus occurs in just 2%-6% of patients with MS, principal author Gil Yosipovitch, MD, professor, Stiefel Chair of Medical Dermatology, and director of the Miami Itch Center in the Dr. Phillip Frost department of dermatology and cutaneous surgery at the University of Miami Miller School of Medicine, encountered itch in enough patients with MS that he presented his observations to a group of neurologists.
Most of them dismissed him, he recalled in an interview: “The neurologists said, ‘Very interesting, but we don’t really see it.’ ”
One of those neurologists, however, decided to take a closer look.
Andrew Brown, MD, assistant professor of clinical neurology and chief of the general neurology division at the University of Miami, Miller School of Medicine, began asking his patients with MS if they were experiencing itch and soon found that it was “a very common problem,” according to Dr. Yosipovitch.
Dr. Yosipovitch, who was the first to report pruritus in patients with psoriasis, launched the present investigation with Dr. Brown to determine if itch is also a blind spot in the world of MS. Their results, and their uphill battle to publication, suggest that it very well could be.
After being rejected from six neurology journals, with one editor suggesting that itch is “not relevant at all to neurology,” their findings were published in the Journal of the European Academy of Dermatology & Venereology.
A common problem that may indicate more severe disease
At the Multiple Sclerosis Center of Excellence in Miami, 27 out of 79 outpatients with MS (35%) reported pruritus, with an average severity of 5.42 out of 10. Among those with itch, the extremities were affected in about half of the patients, while the face, scalp, and trunk were affected in about one-third of the patients. Many described paroxysmal itch that was aggravated by heat, and about half experienced itch on a weekly basis.
Further investigation showed that itch was associated with more severe MS. Compared with patients not experiencing itch, those with itch were significantly more likely to report fatigue (77% vs. 44%), anxiety or depression (48% vs. 16%), and cognitive impairment (62% vs. 26%).
MRI findings backed up these clinical results. Compared with patients not experiencing itch, patients with itch had significantly more T2 hyperintensities in the posterior cervical cord (74.1% vs. 46.0%) and anterior pons/ventromedial medulla (62% vs. 26%). These hyperintensities in the medulla were also associated with an 11-fold increased rate of itch on the face or scalp (odds ratio, 11.3; 95% confidence interval, 1.6-78.6, P = 0.025).
“Health care providers should be aware of episodes of localized, neuropathic itch in MS patients, as they appear to be more prevalent than previously thought and may impair these patients’ quality of life,” the investigators concluded.
Challenges with symptom characterization, management
“This is an important study for both patients and clinicians,” said Justin Abbatemarco, MD, of Cleveland Clinic’s Mellen Center for Multiple Sclerosis, in a written comment. “As the authors mention, many of our patients experience transient symptoms, including many different types of sensory disturbance (that is, pins & needles, burning, electrical shocks, and itching). These symptoms can be really distressing for patients and their caregivers.”
While Dr. Abbatemarco has encountered severe itching in “several patients” with MS, he maintained that it is “relatively uncommon” and noted that MS symptomatology is an inherently cloudy subject.
“I think it is difficult to be definite in any opinion on this topic,” Dr. Abbatemarco said. “How patients experience these symptoms is very subjective and can be difficult to describe/characterize.”
Dr. Abbatemarco emphasized that transient symptoms “do not usually represent MS relapse/flare or new inflammatory disease activity. Instead, we believe these symptoms are related to old areas of injury or demyelination.”
Symptom management can be challenging, he added. He recommended setting realistic expectations, and in the case of pruritus, asking dermatologists to rule out other causes of itch, and to offer “unique treatment approaches.”
Cool the itch?
Noting how heat appears to aggravate itch in patients with MS, Dr. Yosipovitch suggested that one of those unique – and simple – treatment approaches may be cooling itchy areas. Alternatively, clinicians may consider oral agents, like gabapentin to dampen neural transmission, or compounded formulations applied to the skin to reduce neural sensitivity, such as topical ketamine. Finally, Dr. Yosipovitch speculated that newer antibody agents for MS could potentially reduce itch.
All these treatment suggestions are purely hypothetical, he said, and require further investigation before they can be recommended with confidence.
The investigators disclosed relationships with Galderma, Pfizer, Novartis, and others. Dr. Abbatemarco disclosed no conflicts of interest.
Correction, 9/19/22: An earlier version of this article misidentified the photo of Dr. Justin Abbatemarco.
, according to investigators.
Itch is historically underrecognized as a symptom of MS, but physicians should know that it is common and may negatively impact quality of life, reported lead author Giuseppe Ingrasci, MD, a dermatology research fellow at the University of Miami, Miller School of Medicine, and colleagues.
While previous publications suggest that pruritus occurs in just 2%-6% of patients with MS, principal author Gil Yosipovitch, MD, professor, Stiefel Chair of Medical Dermatology, and director of the Miami Itch Center in the Dr. Phillip Frost department of dermatology and cutaneous surgery at the University of Miami Miller School of Medicine, encountered itch in enough patients with MS that he presented his observations to a group of neurologists.
Most of them dismissed him, he recalled in an interview: “The neurologists said, ‘Very interesting, but we don’t really see it.’ ”
One of those neurologists, however, decided to take a closer look.
Andrew Brown, MD, assistant professor of clinical neurology and chief of the general neurology division at the University of Miami, Miller School of Medicine, began asking his patients with MS if they were experiencing itch and soon found that it was “a very common problem,” according to Dr. Yosipovitch.
Dr. Yosipovitch, who was the first to report pruritus in patients with psoriasis, launched the present investigation with Dr. Brown to determine if itch is also a blind spot in the world of MS. Their results, and their uphill battle to publication, suggest that it very well could be.
After being rejected from six neurology journals, with one editor suggesting that itch is “not relevant at all to neurology,” their findings were published in the Journal of the European Academy of Dermatology & Venereology.
A common problem that may indicate more severe disease
At the Multiple Sclerosis Center of Excellence in Miami, 27 out of 79 outpatients with MS (35%) reported pruritus, with an average severity of 5.42 out of 10. Among those with itch, the extremities were affected in about half of the patients, while the face, scalp, and trunk were affected in about one-third of the patients. Many described paroxysmal itch that was aggravated by heat, and about half experienced itch on a weekly basis.
Further investigation showed that itch was associated with more severe MS. Compared with patients not experiencing itch, those with itch were significantly more likely to report fatigue (77% vs. 44%), anxiety or depression (48% vs. 16%), and cognitive impairment (62% vs. 26%).
MRI findings backed up these clinical results. Compared with patients not experiencing itch, patients with itch had significantly more T2 hyperintensities in the posterior cervical cord (74.1% vs. 46.0%) and anterior pons/ventromedial medulla (62% vs. 26%). These hyperintensities in the medulla were also associated with an 11-fold increased rate of itch on the face or scalp (odds ratio, 11.3; 95% confidence interval, 1.6-78.6, P = 0.025).
“Health care providers should be aware of episodes of localized, neuropathic itch in MS patients, as they appear to be more prevalent than previously thought and may impair these patients’ quality of life,” the investigators concluded.
Challenges with symptom characterization, management
“This is an important study for both patients and clinicians,” said Justin Abbatemarco, MD, of Cleveland Clinic’s Mellen Center for Multiple Sclerosis, in a written comment. “As the authors mention, many of our patients experience transient symptoms, including many different types of sensory disturbance (that is, pins & needles, burning, electrical shocks, and itching). These symptoms can be really distressing for patients and their caregivers.”
While Dr. Abbatemarco has encountered severe itching in “several patients” with MS, he maintained that it is “relatively uncommon” and noted that MS symptomatology is an inherently cloudy subject.
“I think it is difficult to be definite in any opinion on this topic,” Dr. Abbatemarco said. “How patients experience these symptoms is very subjective and can be difficult to describe/characterize.”
Dr. Abbatemarco emphasized that transient symptoms “do not usually represent MS relapse/flare or new inflammatory disease activity. Instead, we believe these symptoms are related to old areas of injury or demyelination.”
Symptom management can be challenging, he added. He recommended setting realistic expectations, and in the case of pruritus, asking dermatologists to rule out other causes of itch, and to offer “unique treatment approaches.”
Cool the itch?
Noting how heat appears to aggravate itch in patients with MS, Dr. Yosipovitch suggested that one of those unique – and simple – treatment approaches may be cooling itchy areas. Alternatively, clinicians may consider oral agents, like gabapentin to dampen neural transmission, or compounded formulations applied to the skin to reduce neural sensitivity, such as topical ketamine. Finally, Dr. Yosipovitch speculated that newer antibody agents for MS could potentially reduce itch.
All these treatment suggestions are purely hypothetical, he said, and require further investigation before they can be recommended with confidence.
The investigators disclosed relationships with Galderma, Pfizer, Novartis, and others. Dr. Abbatemarco disclosed no conflicts of interest.
Correction, 9/19/22: An earlier version of this article misidentified the photo of Dr. Justin Abbatemarco.
, according to investigators.
Itch is historically underrecognized as a symptom of MS, but physicians should know that it is common and may negatively impact quality of life, reported lead author Giuseppe Ingrasci, MD, a dermatology research fellow at the University of Miami, Miller School of Medicine, and colleagues.
While previous publications suggest that pruritus occurs in just 2%-6% of patients with MS, principal author Gil Yosipovitch, MD, professor, Stiefel Chair of Medical Dermatology, and director of the Miami Itch Center in the Dr. Phillip Frost department of dermatology and cutaneous surgery at the University of Miami Miller School of Medicine, encountered itch in enough patients with MS that he presented his observations to a group of neurologists.
Most of them dismissed him, he recalled in an interview: “The neurologists said, ‘Very interesting, but we don’t really see it.’ ”
One of those neurologists, however, decided to take a closer look.
Andrew Brown, MD, assistant professor of clinical neurology and chief of the general neurology division at the University of Miami, Miller School of Medicine, began asking his patients with MS if they were experiencing itch and soon found that it was “a very common problem,” according to Dr. Yosipovitch.
Dr. Yosipovitch, who was the first to report pruritus in patients with psoriasis, launched the present investigation with Dr. Brown to determine if itch is also a blind spot in the world of MS. Their results, and their uphill battle to publication, suggest that it very well could be.
After being rejected from six neurology journals, with one editor suggesting that itch is “not relevant at all to neurology,” their findings were published in the Journal of the European Academy of Dermatology & Venereology.
A common problem that may indicate more severe disease
At the Multiple Sclerosis Center of Excellence in Miami, 27 out of 79 outpatients with MS (35%) reported pruritus, with an average severity of 5.42 out of 10. Among those with itch, the extremities were affected in about half of the patients, while the face, scalp, and trunk were affected in about one-third of the patients. Many described paroxysmal itch that was aggravated by heat, and about half experienced itch on a weekly basis.
Further investigation showed that itch was associated with more severe MS. Compared with patients not experiencing itch, those with itch were significantly more likely to report fatigue (77% vs. 44%), anxiety or depression (48% vs. 16%), and cognitive impairment (62% vs. 26%).
MRI findings backed up these clinical results. Compared with patients not experiencing itch, patients with itch had significantly more T2 hyperintensities in the posterior cervical cord (74.1% vs. 46.0%) and anterior pons/ventromedial medulla (62% vs. 26%). These hyperintensities in the medulla were also associated with an 11-fold increased rate of itch on the face or scalp (odds ratio, 11.3; 95% confidence interval, 1.6-78.6, P = 0.025).
“Health care providers should be aware of episodes of localized, neuropathic itch in MS patients, as they appear to be more prevalent than previously thought and may impair these patients’ quality of life,” the investigators concluded.
Challenges with symptom characterization, management
“This is an important study for both patients and clinicians,” said Justin Abbatemarco, MD, of Cleveland Clinic’s Mellen Center for Multiple Sclerosis, in a written comment. “As the authors mention, many of our patients experience transient symptoms, including many different types of sensory disturbance (that is, pins & needles, burning, electrical shocks, and itching). These symptoms can be really distressing for patients and their caregivers.”
While Dr. Abbatemarco has encountered severe itching in “several patients” with MS, he maintained that it is “relatively uncommon” and noted that MS symptomatology is an inherently cloudy subject.
“I think it is difficult to be definite in any opinion on this topic,” Dr. Abbatemarco said. “How patients experience these symptoms is very subjective and can be difficult to describe/characterize.”
Dr. Abbatemarco emphasized that transient symptoms “do not usually represent MS relapse/flare or new inflammatory disease activity. Instead, we believe these symptoms are related to old areas of injury or demyelination.”
Symptom management can be challenging, he added. He recommended setting realistic expectations, and in the case of pruritus, asking dermatologists to rule out other causes of itch, and to offer “unique treatment approaches.”
Cool the itch?
Noting how heat appears to aggravate itch in patients with MS, Dr. Yosipovitch suggested that one of those unique – and simple – treatment approaches may be cooling itchy areas. Alternatively, clinicians may consider oral agents, like gabapentin to dampen neural transmission, or compounded formulations applied to the skin to reduce neural sensitivity, such as topical ketamine. Finally, Dr. Yosipovitch speculated that newer antibody agents for MS could potentially reduce itch.
All these treatment suggestions are purely hypothetical, he said, and require further investigation before they can be recommended with confidence.
The investigators disclosed relationships with Galderma, Pfizer, Novartis, and others. Dr. Abbatemarco disclosed no conflicts of interest.
Correction, 9/19/22: An earlier version of this article misidentified the photo of Dr. Justin Abbatemarco.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY & VENEREOLOGY
Not just what, but when: Neoadjuvant pembrolizumab in melanoma
PARIS – “It’s not just what you give, it’s when you give it,” said the investigator reporting “that the same treatment for resectable melanoma given in a different sequence can generate lower rates of melanoma recurrence.”
Sapna Patel, MD, associate professor of melanoma medical oncology at The University of Texas MD Anderson Cancer Center, Houston, reported the results from the SWOG S1801 trial, which showed that than patients who received pembrolizumab after surgery only.
At a median follow-up of almost 15 months, there was a 42% lower rate of recurrence or death.
“Compared to the same treatment given entirely in the adjuvant setting, neoadjuvant pembrolizumab followed by adjuvant pembrolizumab improves event-free survival in resectable melanoma,” Dr. Patel commented.
She suggested that the explanation for the findings was that “inhibiting the PD-1/PD-L1 immune checkpoints before surgery gives an antitumor response at local and distant sites, and this occurs before resection of the tumor bed. This approach tends to leave behind a larger number of anti-tumor T cells ... [and] these T cells can be activated and circulated systematically to recognize and attack micro-metastatic melanoma tumors.”
The findings were presented during a presidential symposium at the European Society for Medical Oncology (ESMO) Congress 2022, Paris.
“This trial provides us with more evidence of when one strategy may be preferred over the other,” commented Maya Dimitrova, MD, medical oncologist at NYU Langone Perlmutter Cancer Center. She was not involved with the trial.
“Neoadjuvant immunotherapy has elicited impressive complete pathologic responses, which thus far have proven to be associated with a durable response. Neoadjuvant therapy may help identify patients who will respond well to checkpoint inhibitors and allow for de-escalation of therapy,” she told this news organization when approached for comment.
“As with all neoadjuvant therapy, we don’t want the treatment to compromise the outcomes of surgery when the intent is curative, and we once again have evidence that this is not the case when it comes to immune therapy,” she said. However, she added that “we will need further survival data to really change the standard of practice in high-risk melanoma and demonstrate whether there is a superior sequence of therapy and surgery.”
Details of the new results
The S1801 clinical trial enrolled 345 participants with stage IIIB through stage IV melanoma considered resectable. The cohort was randomized to receive either upfront surgery followed by 18 doses of pembrolizumab 200 mg every 3 weeks for a total of 18 doses or neoadjuvant therapy with pembrolizumab 200 mg (3 doses) followed by 15 doses of adjuvant pembrolizumab.
The primary endpoint was event-free survival (EFS), defined as the time from randomization to the occurrence of one of the following: disease progression or toxicity that resulted in not receiving surgery, failure to begin adjuvant therapy within 84 days of surgery, melanoma recurrence after surgery, or death from any cause.
At a median follow-up of 14.7 months, EFS was significantly higher for patients in the neoadjuvant group, compared with those receiving adjuvant therapy only (HR, 0.58; one-sided log-rank P = .004). A total of 36 participants died in the neoadjuvant and adjuvant groups (14 and 22 patients, extrapolating to a hazard ratio of 0.63; one-sided P = .091).
“With a limited number of events, overall survival is not statistically different at this time,” Dr. Patel said. “Landmark 2-year survival was 72% in the neoadjuvant arm and 49% in the adjuvant arm.”
The authors note that the benefit of neoadjuvant therapy remained consistent across a range of factors, including patient age, sex, performance status, stage of disease, ulceration, and BRAF status. The same proportion of patients in both groups received adjuvant pembrolizumab following surgery.
Rates of adverse events were similar in both groups, and neoadjuvant pembrolizumab did not result in an increase in adverse events related to surgery. In the neoadjuvant group, 28 patients (21%) with submitted pathology reports were noted to have had a complete pathologic response (0% viable tumor) on local review.
Questions remain
Invited discussant James Larkin, PhD, FRCP, FMedSci, a clinical researcher at The Royal Marsden Hospital, London, noted that the study had “striking results” and was a landmark trial with a simple but powerful design.
However, he pointed to some questions which need to be addressed in the future. “One important question is what is the optimal duration of neoadjuvant treatment, and can we individualize it?”
Another question is just how much postoperative treatment is really needed and whether pathology help determine that. “Can surgery be safely avoided altogether?” he asked. “Another issue is the need for anti-CTL4 therapy – which patients might benefit from anti-CTL4, in addition to anti-PD-1?”
“And by extension, this paradigm provides a great platform for testing new agents, including combinations in cases where PD-1 is not sufficient to achieve a sufficient response,” said Dr. Larkin. “In the future, trials addressing these questions hand us a major opportunity to individualize and rationally de-escalate treatment.”
Also weighing in on the study, another expert pointed out that neoadjuvant therapy in this setting is already being considered as an option. “The use of immunotherapy before surgery has been reported in some trials such as the OPACIN-neo and PRADO trials,” said Anthony J. Olszanski, RPh, MD, Vice Chair of Research at the Fox Chase Cancer Center, Philadelphia. “Results have been quite exciting and have led the NCCN to list this as a potential option for some patients in the current melanoma guidelines.”
S1801 is funded by the NIH/NCI and in part by MSD through a Cooperative Research and Development Agreement with the NCI. Pembrolizumab (KEYTRUDA) is Merck’s anti-PD-1 therapy. Dr. Patel has declared multiple relationships with industry as noted in the abstract; several co-authors have also made disclosures. Dr. Olszanski has reported participating in advisory boards for BMS, Merck, and InstilBio and running trials for them.
A version of this article first appeared on Medscape.com.
PARIS – “It’s not just what you give, it’s when you give it,” said the investigator reporting “that the same treatment for resectable melanoma given in a different sequence can generate lower rates of melanoma recurrence.”
Sapna Patel, MD, associate professor of melanoma medical oncology at The University of Texas MD Anderson Cancer Center, Houston, reported the results from the SWOG S1801 trial, which showed that than patients who received pembrolizumab after surgery only.
At a median follow-up of almost 15 months, there was a 42% lower rate of recurrence or death.
“Compared to the same treatment given entirely in the adjuvant setting, neoadjuvant pembrolizumab followed by adjuvant pembrolizumab improves event-free survival in resectable melanoma,” Dr. Patel commented.
She suggested that the explanation for the findings was that “inhibiting the PD-1/PD-L1 immune checkpoints before surgery gives an antitumor response at local and distant sites, and this occurs before resection of the tumor bed. This approach tends to leave behind a larger number of anti-tumor T cells ... [and] these T cells can be activated and circulated systematically to recognize and attack micro-metastatic melanoma tumors.”
The findings were presented during a presidential symposium at the European Society for Medical Oncology (ESMO) Congress 2022, Paris.
“This trial provides us with more evidence of when one strategy may be preferred over the other,” commented Maya Dimitrova, MD, medical oncologist at NYU Langone Perlmutter Cancer Center. She was not involved with the trial.
“Neoadjuvant immunotherapy has elicited impressive complete pathologic responses, which thus far have proven to be associated with a durable response. Neoadjuvant therapy may help identify patients who will respond well to checkpoint inhibitors and allow for de-escalation of therapy,” she told this news organization when approached for comment.
“As with all neoadjuvant therapy, we don’t want the treatment to compromise the outcomes of surgery when the intent is curative, and we once again have evidence that this is not the case when it comes to immune therapy,” she said. However, she added that “we will need further survival data to really change the standard of practice in high-risk melanoma and demonstrate whether there is a superior sequence of therapy and surgery.”
Details of the new results
The S1801 clinical trial enrolled 345 participants with stage IIIB through stage IV melanoma considered resectable. The cohort was randomized to receive either upfront surgery followed by 18 doses of pembrolizumab 200 mg every 3 weeks for a total of 18 doses or neoadjuvant therapy with pembrolizumab 200 mg (3 doses) followed by 15 doses of adjuvant pembrolizumab.
The primary endpoint was event-free survival (EFS), defined as the time from randomization to the occurrence of one of the following: disease progression or toxicity that resulted in not receiving surgery, failure to begin adjuvant therapy within 84 days of surgery, melanoma recurrence after surgery, or death from any cause.
At a median follow-up of 14.7 months, EFS was significantly higher for patients in the neoadjuvant group, compared with those receiving adjuvant therapy only (HR, 0.58; one-sided log-rank P = .004). A total of 36 participants died in the neoadjuvant and adjuvant groups (14 and 22 patients, extrapolating to a hazard ratio of 0.63; one-sided P = .091).
“With a limited number of events, overall survival is not statistically different at this time,” Dr. Patel said. “Landmark 2-year survival was 72% in the neoadjuvant arm and 49% in the adjuvant arm.”
The authors note that the benefit of neoadjuvant therapy remained consistent across a range of factors, including patient age, sex, performance status, stage of disease, ulceration, and BRAF status. The same proportion of patients in both groups received adjuvant pembrolizumab following surgery.
Rates of adverse events were similar in both groups, and neoadjuvant pembrolizumab did not result in an increase in adverse events related to surgery. In the neoadjuvant group, 28 patients (21%) with submitted pathology reports were noted to have had a complete pathologic response (0% viable tumor) on local review.
Questions remain
Invited discussant James Larkin, PhD, FRCP, FMedSci, a clinical researcher at The Royal Marsden Hospital, London, noted that the study had “striking results” and was a landmark trial with a simple but powerful design.
However, he pointed to some questions which need to be addressed in the future. “One important question is what is the optimal duration of neoadjuvant treatment, and can we individualize it?”
Another question is just how much postoperative treatment is really needed and whether pathology help determine that. “Can surgery be safely avoided altogether?” he asked. “Another issue is the need for anti-CTL4 therapy – which patients might benefit from anti-CTL4, in addition to anti-PD-1?”
“And by extension, this paradigm provides a great platform for testing new agents, including combinations in cases where PD-1 is not sufficient to achieve a sufficient response,” said Dr. Larkin. “In the future, trials addressing these questions hand us a major opportunity to individualize and rationally de-escalate treatment.”
Also weighing in on the study, another expert pointed out that neoadjuvant therapy in this setting is already being considered as an option. “The use of immunotherapy before surgery has been reported in some trials such as the OPACIN-neo and PRADO trials,” said Anthony J. Olszanski, RPh, MD, Vice Chair of Research at the Fox Chase Cancer Center, Philadelphia. “Results have been quite exciting and have led the NCCN to list this as a potential option for some patients in the current melanoma guidelines.”
S1801 is funded by the NIH/NCI and in part by MSD through a Cooperative Research and Development Agreement with the NCI. Pembrolizumab (KEYTRUDA) is Merck’s anti-PD-1 therapy. Dr. Patel has declared multiple relationships with industry as noted in the abstract; several co-authors have also made disclosures. Dr. Olszanski has reported participating in advisory boards for BMS, Merck, and InstilBio and running trials for them.
A version of this article first appeared on Medscape.com.
PARIS – “It’s not just what you give, it’s when you give it,” said the investigator reporting “that the same treatment for resectable melanoma given in a different sequence can generate lower rates of melanoma recurrence.”
Sapna Patel, MD, associate professor of melanoma medical oncology at The University of Texas MD Anderson Cancer Center, Houston, reported the results from the SWOG S1801 trial, which showed that than patients who received pembrolizumab after surgery only.
At a median follow-up of almost 15 months, there was a 42% lower rate of recurrence or death.
“Compared to the same treatment given entirely in the adjuvant setting, neoadjuvant pembrolizumab followed by adjuvant pembrolizumab improves event-free survival in resectable melanoma,” Dr. Patel commented.
She suggested that the explanation for the findings was that “inhibiting the PD-1/PD-L1 immune checkpoints before surgery gives an antitumor response at local and distant sites, and this occurs before resection of the tumor bed. This approach tends to leave behind a larger number of anti-tumor T cells ... [and] these T cells can be activated and circulated systematically to recognize and attack micro-metastatic melanoma tumors.”
The findings were presented during a presidential symposium at the European Society for Medical Oncology (ESMO) Congress 2022, Paris.
“This trial provides us with more evidence of when one strategy may be preferred over the other,” commented Maya Dimitrova, MD, medical oncologist at NYU Langone Perlmutter Cancer Center. She was not involved with the trial.
“Neoadjuvant immunotherapy has elicited impressive complete pathologic responses, which thus far have proven to be associated with a durable response. Neoadjuvant therapy may help identify patients who will respond well to checkpoint inhibitors and allow for de-escalation of therapy,” she told this news organization when approached for comment.
“As with all neoadjuvant therapy, we don’t want the treatment to compromise the outcomes of surgery when the intent is curative, and we once again have evidence that this is not the case when it comes to immune therapy,” she said. However, she added that “we will need further survival data to really change the standard of practice in high-risk melanoma and demonstrate whether there is a superior sequence of therapy and surgery.”
Details of the new results
The S1801 clinical trial enrolled 345 participants with stage IIIB through stage IV melanoma considered resectable. The cohort was randomized to receive either upfront surgery followed by 18 doses of pembrolizumab 200 mg every 3 weeks for a total of 18 doses or neoadjuvant therapy with pembrolizumab 200 mg (3 doses) followed by 15 doses of adjuvant pembrolizumab.
The primary endpoint was event-free survival (EFS), defined as the time from randomization to the occurrence of one of the following: disease progression or toxicity that resulted in not receiving surgery, failure to begin adjuvant therapy within 84 days of surgery, melanoma recurrence after surgery, or death from any cause.
At a median follow-up of 14.7 months, EFS was significantly higher for patients in the neoadjuvant group, compared with those receiving adjuvant therapy only (HR, 0.58; one-sided log-rank P = .004). A total of 36 participants died in the neoadjuvant and adjuvant groups (14 and 22 patients, extrapolating to a hazard ratio of 0.63; one-sided P = .091).
“With a limited number of events, overall survival is not statistically different at this time,” Dr. Patel said. “Landmark 2-year survival was 72% in the neoadjuvant arm and 49% in the adjuvant arm.”
The authors note that the benefit of neoadjuvant therapy remained consistent across a range of factors, including patient age, sex, performance status, stage of disease, ulceration, and BRAF status. The same proportion of patients in both groups received adjuvant pembrolizumab following surgery.
Rates of adverse events were similar in both groups, and neoadjuvant pembrolizumab did not result in an increase in adverse events related to surgery. In the neoadjuvant group, 28 patients (21%) with submitted pathology reports were noted to have had a complete pathologic response (0% viable tumor) on local review.
Questions remain
Invited discussant James Larkin, PhD, FRCP, FMedSci, a clinical researcher at The Royal Marsden Hospital, London, noted that the study had “striking results” and was a landmark trial with a simple but powerful design.
However, he pointed to some questions which need to be addressed in the future. “One important question is what is the optimal duration of neoadjuvant treatment, and can we individualize it?”
Another question is just how much postoperative treatment is really needed and whether pathology help determine that. “Can surgery be safely avoided altogether?” he asked. “Another issue is the need for anti-CTL4 therapy – which patients might benefit from anti-CTL4, in addition to anti-PD-1?”
“And by extension, this paradigm provides a great platform for testing new agents, including combinations in cases where PD-1 is not sufficient to achieve a sufficient response,” said Dr. Larkin. “In the future, trials addressing these questions hand us a major opportunity to individualize and rationally de-escalate treatment.”
Also weighing in on the study, another expert pointed out that neoadjuvant therapy in this setting is already being considered as an option. “The use of immunotherapy before surgery has been reported in some trials such as the OPACIN-neo and PRADO trials,” said Anthony J. Olszanski, RPh, MD, Vice Chair of Research at the Fox Chase Cancer Center, Philadelphia. “Results have been quite exciting and have led the NCCN to list this as a potential option for some patients in the current melanoma guidelines.”
S1801 is funded by the NIH/NCI and in part by MSD through a Cooperative Research and Development Agreement with the NCI. Pembrolizumab (KEYTRUDA) is Merck’s anti-PD-1 therapy. Dr. Patel has declared multiple relationships with industry as noted in the abstract; several co-authors have also made disclosures. Dr. Olszanski has reported participating in advisory boards for BMS, Merck, and InstilBio and running trials for them.
A version of this article first appeared on Medscape.com.
Novel cell therapy beats immunotherapy in melanoma
PARIS – Cell therapies have already had a huge impact on the treatment of blood cancers, but progress in solid tumors has proved more difficult. Now, in a first multicenter randomized trial to compare the two,
The cell therapy used in this trial was composed of adoptive tumor infiltrating lymphocytes (TIL), which were made individually for each patient, just as chimeric antigen receptor T cells (CAR T cells) are for patients with blood cancers. However, the process involved is somewhat different, as TILs are made from lymphocytes that have infiltrated the patient’s tumor and are obtained by surgery in the tumor, whereas CAR T cells are made from circulating blood cells.
The phase 3 trial involved 168 patients with unresectable stage IIIC-4 melanoma and showed that patients who were treated with TILs achieved a significantly improved progression-free survival (PFS) when compared with standard immunotherapy with ipilimumab (Yervoy).
The median PFS was more than doubled to 7.2 months with TILs versus 3.1 months with ipilimumab (hazard ratio, 0.50; P < .001).
“We do think that TIL could possibly become a new treatment option for patients with advanced stage melanoma,” commented lead author John Haanen, MD, PhD, research group leader at the Netherlands Cancer Institute in Amsterdam and a professor in translational immunotherapy of cancer at Leiden (the Netherlands) University Medical Center.
He presented the findings at a presidential symposium during the European Society for Medical Oncology Annual Congress, Paris.
“The results of this trial may fuel further research of TIL in other cancer types, potentially demonstrating benefit in many other solid tumors and expanding available treatments for patients,” said Maya Dimitrova, MD, medical oncologist at NYU Langone Perlmutter Cancer Center. She was approached for comment by this news organization and was not involved in the research.
Immune checkpoint inhibitors and targeted therapies have become the standard of care for advanced melanoma and greatly improved patient outcomes, she said. But as about half of patients treated with these agents will not achieve a durable benefit, there remains a need for new treatment options.
“Although immunotherapy can yield impressive long-term responses, a substantial percentage of patients will have no response, or no durable response, to checkpoint inhibitors,” said Dr. Dimitrova. “TIL therapy has proven effectiveness in melanoma. However, no phase III trials have been done to date to compare its effectiveness to a standard of care regimen.”
She noted that these results are consistent with past reports of an approximately 50% response rate with an impressive 20% complete response rate in the TIL group. Data from a phase 2 trial reported last year, for example, showed an objective response rate of 36.4%.
“It will be important to determine the persistence of antitumor activity and whether there are biomarkers that could help with patient selection given the resource intensity of the therapy,” Dr. Dimitrova said. “TIL therapy will likely become a new standard of care in metastatic melanoma refractory to immune checkpoint inhibitors.”
Superior to immunotherapy
In the current study, Dr. Haanen and colleagues randomly assigned 168 patients to TIL or ipilimumab (3 mg/kg every 3 weeks, maximum 4 doses). Patients were stratified for BRAFV600 mutation status, treatment line and center, and the majority (86%) were refractory to anti–PD-1 treatment.
Patients in the TIL group underwent resection of a melanoma lesion (2-3 cm) for the ex vivo outgrowth and expansion of tumor-resident T cells. Before the cultured TILs were infused back into the patients from which they were made, the patient underwent nonmyeloablative, lymphodepleting chemotherapy with cyclophosphamide plus fludarabine that was followed by high-dose interleukin-2.
The study’s primary endpoint was progression-free survival, and secondary endpoints included overall and complete response rate, overall survival, and safety.
At a median follow-up of 33 months, TIL significantly improved progression-free survival, compared with ipilimumab. The overall response rate also favored TIL, compared with ipilimumab (49% vs. 21%), with 20% versus 7% complete responses, respectively.
The median overall survival was 25.8 months for TIL and 18.9 months for ipilimumab (HR, 0.83; P = 0.39).
Grade 3 or higher treatment-related adverse events occurred in all TIL and 57% of ipilimumab patients, although Dr. Haanen noted they were manageable and, in most cases, resolved by the time patients were discharged from the hospital.
“There were no new safety concerns with TIL,” said Dr. Haanen, “And these toxicities are driven by the chemotherapy and interleukin-2 that are part of the TIL regimen. There were no long-term sequelae in patients treated with TIL, and health-related quality of life was higher in patients treated with TIL.”
Ultra-personalized
Also commenting on the study, Anthony J. Olszanski, MD, RPh, associate professor and vice chair of clinical research, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, agreed that the treatment of patients with melanoma who do not respond to or progress after receiving treatment with immunotherapy is “challenging and represents an unmet need.”
“TIL therapy is, in some ways, ultra-personalized therapy, because we harvest immune cells from the patient’s tumor, expand them outside of the body, and then re-infuse them,” he said. “This trial, which randomized patients between TIL versus the CTLA-4 inhibitor, ipilimumab, has shown an impressive progression-free survival and overall response rate benefit and will help establish TIL therapy as a viable treatment strategy for some patients.”
The study was supported by the Dutch Cancer Society, the Netherlands Organization for Health Research and Development, the Dutch Ministry of Health, Stichting Avento, Copenhagen University Hospital, Herlev, the Danish Cancer Society, and Capital Region of Denmark Research Foundation.
Dr. Haanen and several of the co-authors have declared multiple relationships with industry as noted in the abstract. Dr. Olszanski reports participation in advisory boards for BMS, Merck, and Instil Bio, and he reports running trials for them.
A version of this article first appeared on Medscape.com.
PARIS – Cell therapies have already had a huge impact on the treatment of blood cancers, but progress in solid tumors has proved more difficult. Now, in a first multicenter randomized trial to compare the two,
The cell therapy used in this trial was composed of adoptive tumor infiltrating lymphocytes (TIL), which were made individually for each patient, just as chimeric antigen receptor T cells (CAR T cells) are for patients with blood cancers. However, the process involved is somewhat different, as TILs are made from lymphocytes that have infiltrated the patient’s tumor and are obtained by surgery in the tumor, whereas CAR T cells are made from circulating blood cells.
The phase 3 trial involved 168 patients with unresectable stage IIIC-4 melanoma and showed that patients who were treated with TILs achieved a significantly improved progression-free survival (PFS) when compared with standard immunotherapy with ipilimumab (Yervoy).
The median PFS was more than doubled to 7.2 months with TILs versus 3.1 months with ipilimumab (hazard ratio, 0.50; P < .001).
“We do think that TIL could possibly become a new treatment option for patients with advanced stage melanoma,” commented lead author John Haanen, MD, PhD, research group leader at the Netherlands Cancer Institute in Amsterdam and a professor in translational immunotherapy of cancer at Leiden (the Netherlands) University Medical Center.
He presented the findings at a presidential symposium during the European Society for Medical Oncology Annual Congress, Paris.
“The results of this trial may fuel further research of TIL in other cancer types, potentially demonstrating benefit in many other solid tumors and expanding available treatments for patients,” said Maya Dimitrova, MD, medical oncologist at NYU Langone Perlmutter Cancer Center. She was approached for comment by this news organization and was not involved in the research.
Immune checkpoint inhibitors and targeted therapies have become the standard of care for advanced melanoma and greatly improved patient outcomes, she said. But as about half of patients treated with these agents will not achieve a durable benefit, there remains a need for new treatment options.
“Although immunotherapy can yield impressive long-term responses, a substantial percentage of patients will have no response, or no durable response, to checkpoint inhibitors,” said Dr. Dimitrova. “TIL therapy has proven effectiveness in melanoma. However, no phase III trials have been done to date to compare its effectiveness to a standard of care regimen.”
She noted that these results are consistent with past reports of an approximately 50% response rate with an impressive 20% complete response rate in the TIL group. Data from a phase 2 trial reported last year, for example, showed an objective response rate of 36.4%.
“It will be important to determine the persistence of antitumor activity and whether there are biomarkers that could help with patient selection given the resource intensity of the therapy,” Dr. Dimitrova said. “TIL therapy will likely become a new standard of care in metastatic melanoma refractory to immune checkpoint inhibitors.”
Superior to immunotherapy
In the current study, Dr. Haanen and colleagues randomly assigned 168 patients to TIL or ipilimumab (3 mg/kg every 3 weeks, maximum 4 doses). Patients were stratified for BRAFV600 mutation status, treatment line and center, and the majority (86%) were refractory to anti–PD-1 treatment.
Patients in the TIL group underwent resection of a melanoma lesion (2-3 cm) for the ex vivo outgrowth and expansion of tumor-resident T cells. Before the cultured TILs were infused back into the patients from which they were made, the patient underwent nonmyeloablative, lymphodepleting chemotherapy with cyclophosphamide plus fludarabine that was followed by high-dose interleukin-2.
The study’s primary endpoint was progression-free survival, and secondary endpoints included overall and complete response rate, overall survival, and safety.
At a median follow-up of 33 months, TIL significantly improved progression-free survival, compared with ipilimumab. The overall response rate also favored TIL, compared with ipilimumab (49% vs. 21%), with 20% versus 7% complete responses, respectively.
The median overall survival was 25.8 months for TIL and 18.9 months for ipilimumab (HR, 0.83; P = 0.39).
Grade 3 or higher treatment-related adverse events occurred in all TIL and 57% of ipilimumab patients, although Dr. Haanen noted they were manageable and, in most cases, resolved by the time patients were discharged from the hospital.
“There were no new safety concerns with TIL,” said Dr. Haanen, “And these toxicities are driven by the chemotherapy and interleukin-2 that are part of the TIL regimen. There were no long-term sequelae in patients treated with TIL, and health-related quality of life was higher in patients treated with TIL.”
Ultra-personalized
Also commenting on the study, Anthony J. Olszanski, MD, RPh, associate professor and vice chair of clinical research, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, agreed that the treatment of patients with melanoma who do not respond to or progress after receiving treatment with immunotherapy is “challenging and represents an unmet need.”
“TIL therapy is, in some ways, ultra-personalized therapy, because we harvest immune cells from the patient’s tumor, expand them outside of the body, and then re-infuse them,” he said. “This trial, which randomized patients between TIL versus the CTLA-4 inhibitor, ipilimumab, has shown an impressive progression-free survival and overall response rate benefit and will help establish TIL therapy as a viable treatment strategy for some patients.”
The study was supported by the Dutch Cancer Society, the Netherlands Organization for Health Research and Development, the Dutch Ministry of Health, Stichting Avento, Copenhagen University Hospital, Herlev, the Danish Cancer Society, and Capital Region of Denmark Research Foundation.
Dr. Haanen and several of the co-authors have declared multiple relationships with industry as noted in the abstract. Dr. Olszanski reports participation in advisory boards for BMS, Merck, and Instil Bio, and he reports running trials for them.
A version of this article first appeared on Medscape.com.
PARIS – Cell therapies have already had a huge impact on the treatment of blood cancers, but progress in solid tumors has proved more difficult. Now, in a first multicenter randomized trial to compare the two,
The cell therapy used in this trial was composed of adoptive tumor infiltrating lymphocytes (TIL), which were made individually for each patient, just as chimeric antigen receptor T cells (CAR T cells) are for patients with blood cancers. However, the process involved is somewhat different, as TILs are made from lymphocytes that have infiltrated the patient’s tumor and are obtained by surgery in the tumor, whereas CAR T cells are made from circulating blood cells.
The phase 3 trial involved 168 patients with unresectable stage IIIC-4 melanoma and showed that patients who were treated with TILs achieved a significantly improved progression-free survival (PFS) when compared with standard immunotherapy with ipilimumab (Yervoy).
The median PFS was more than doubled to 7.2 months with TILs versus 3.1 months with ipilimumab (hazard ratio, 0.50; P < .001).
“We do think that TIL could possibly become a new treatment option for patients with advanced stage melanoma,” commented lead author John Haanen, MD, PhD, research group leader at the Netherlands Cancer Institute in Amsterdam and a professor in translational immunotherapy of cancer at Leiden (the Netherlands) University Medical Center.
He presented the findings at a presidential symposium during the European Society for Medical Oncology Annual Congress, Paris.
“The results of this trial may fuel further research of TIL in other cancer types, potentially demonstrating benefit in many other solid tumors and expanding available treatments for patients,” said Maya Dimitrova, MD, medical oncologist at NYU Langone Perlmutter Cancer Center. She was approached for comment by this news organization and was not involved in the research.
Immune checkpoint inhibitors and targeted therapies have become the standard of care for advanced melanoma and greatly improved patient outcomes, she said. But as about half of patients treated with these agents will not achieve a durable benefit, there remains a need for new treatment options.
“Although immunotherapy can yield impressive long-term responses, a substantial percentage of patients will have no response, or no durable response, to checkpoint inhibitors,” said Dr. Dimitrova. “TIL therapy has proven effectiveness in melanoma. However, no phase III trials have been done to date to compare its effectiveness to a standard of care regimen.”
She noted that these results are consistent with past reports of an approximately 50% response rate with an impressive 20% complete response rate in the TIL group. Data from a phase 2 trial reported last year, for example, showed an objective response rate of 36.4%.
“It will be important to determine the persistence of antitumor activity and whether there are biomarkers that could help with patient selection given the resource intensity of the therapy,” Dr. Dimitrova said. “TIL therapy will likely become a new standard of care in metastatic melanoma refractory to immune checkpoint inhibitors.”
Superior to immunotherapy
In the current study, Dr. Haanen and colleagues randomly assigned 168 patients to TIL or ipilimumab (3 mg/kg every 3 weeks, maximum 4 doses). Patients were stratified for BRAFV600 mutation status, treatment line and center, and the majority (86%) were refractory to anti–PD-1 treatment.
Patients in the TIL group underwent resection of a melanoma lesion (2-3 cm) for the ex vivo outgrowth and expansion of tumor-resident T cells. Before the cultured TILs were infused back into the patients from which they were made, the patient underwent nonmyeloablative, lymphodepleting chemotherapy with cyclophosphamide plus fludarabine that was followed by high-dose interleukin-2.
The study’s primary endpoint was progression-free survival, and secondary endpoints included overall and complete response rate, overall survival, and safety.
At a median follow-up of 33 months, TIL significantly improved progression-free survival, compared with ipilimumab. The overall response rate also favored TIL, compared with ipilimumab (49% vs. 21%), with 20% versus 7% complete responses, respectively.
The median overall survival was 25.8 months for TIL and 18.9 months for ipilimumab (HR, 0.83; P = 0.39).
Grade 3 or higher treatment-related adverse events occurred in all TIL and 57% of ipilimumab patients, although Dr. Haanen noted they were manageable and, in most cases, resolved by the time patients were discharged from the hospital.
“There were no new safety concerns with TIL,” said Dr. Haanen, “And these toxicities are driven by the chemotherapy and interleukin-2 that are part of the TIL regimen. There were no long-term sequelae in patients treated with TIL, and health-related quality of life was higher in patients treated with TIL.”
Ultra-personalized
Also commenting on the study, Anthony J. Olszanski, MD, RPh, associate professor and vice chair of clinical research, department of hematology/oncology at Fox Chase Cancer Center, Philadelphia, agreed that the treatment of patients with melanoma who do not respond to or progress after receiving treatment with immunotherapy is “challenging and represents an unmet need.”
“TIL therapy is, in some ways, ultra-personalized therapy, because we harvest immune cells from the patient’s tumor, expand them outside of the body, and then re-infuse them,” he said. “This trial, which randomized patients between TIL versus the CTLA-4 inhibitor, ipilimumab, has shown an impressive progression-free survival and overall response rate benefit and will help establish TIL therapy as a viable treatment strategy for some patients.”
The study was supported by the Dutch Cancer Society, the Netherlands Organization for Health Research and Development, the Dutch Ministry of Health, Stichting Avento, Copenhagen University Hospital, Herlev, the Danish Cancer Society, and Capital Region of Denmark Research Foundation.
Dr. Haanen and several of the co-authors have declared multiple relationships with industry as noted in the abstract. Dr. Olszanski reports participation in advisory boards for BMS, Merck, and Instil Bio, and he reports running trials for them.
A version of this article first appeared on Medscape.com.
‘Smoking gun–level’ evidence found linking air pollution with lung cancer
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
PARIS – Air pollution has been recognized as a risk factor for lung cancer for about 2 decades, and already present in normal lung cells to cause cancer.
Think of it as “smoking gun–level” evidence that may explain why many nonsmokers still develop non–small cell lung cancer, said Charles Swanton, PhD, from the Francis Crick Institute and Cancer Research UK Chief Clinician, London.
“What this work shows is that air pollution is directly causing lung cancer but through a slightly unexpected pathway,” he said at a briefing prior to his presentation of the data in a presidential symposium held earlier this month in Paris at the European Society for Medical Oncology Congress 2022.
Importantly, he and his team also propose a mechanism for blocking the effects of air pollution with monoclonal antibodies directed against the inflammatory cytokine interleukein-1 beta.
Carcinogenesis explored
Lung cancer in never-smokers has a low mutational burden, with about 5- to 10-fold fewer mutations in a nonsmoker, compared with an ever smoker or current smoker, Dr. Swanton noted.
“The other thing to say about never-smokers is that they don’t have a clear environmental carcinogenic signature. So how do you square the circle? You’ve got the problem that you know that air pollution is associated with lung cancer – we don’t know if it causes it – but we also see that we’ve got no DNA mutations due to an environmental carcinogen,” he said during his symposium presentation.
The traditional model proposed to explain how carcinogens cause cancer holds that exposure to a carcinogen causes DNA mutations that lead to clonal expansion and tumor growth.
“But there are some major problems with this model,” Dr. Swanton said.
For example, normal skin contains a “patchwork of mutant clones,” but skin cancer is still uncommon, he said, and in studies in mice, 17 of 20 environmental carcinogens did not induce DNA mutations. He also noted that a common melanoma driver mutation, BRAF V600E, is not induced by exposure to a ultraviolet light.
“Any explanation for never-smoking lung cancer would have to fulfill three criteria: one, you have to explain why geographic variation exists; two, you have to prove causation; and three, you have to explain how cancers can be initiated without directly causing DNA mutations,” he said.
Normal lung tissues in nonsmoking adults can harbor pre-existing mutations, with the number of mutations increasing likely as a consequence of aging. In fact, more than 50% of normal lung biopsy tissues have been shown to harbor driver KRAS and/or EGFR mutations, Dr. Swanton said.
“In our research, these mutations alone only weakly potentiated cancer in laboratory models. However, when lung cells with these mutations were exposed to air pollutants, we saw more cancers and these occurred more quickly than when lung cells with these mutations were not exposed to pollutants, suggesting that air pollution promotes the initiation of lung cancer in cells harboring driver gene mutations. The next step is to discover why some lung cells with mutations become cancerous when exposed to pollutants while others don’t,” he said.
Geographical exposures
Looking at data on 447,932 participants in the UK Biobank, the investigators found that increasing exposure to ambient air particles smaller than 2.5 mcm (PM2.5) was significantly associated with seven cancer types, including lung cancer. They also saw an association between PM2.5 exposure levels and EGFR-mutated lung cancer incidence in the United Kingdom, South Korea, and Taiwan.
And crucially, as Dr. Swanton and associates showed in mouse models, exposure of lung cells bearing somatic EGFR and KRAS mutations to PM2.5 causes recruitment of macrophages that in turn secrete IL-1B, resulting in a transdifferentiation of EGFR-mutated cells into a cancer stem cell state, and tumor formation.
Importantly, pollution-induced tumor formation can be blocked by antibodies directed against IL-1B, Dr. Swanton said.
He pointed to a 2017 study in The Lancet suggesting that anti-inflammatory therapy with the anti–IL-1 antibody canakinumab (Ilaris) could reduce incident lung cancer and lung cancer deaths.
‘Elegant first demonstration’
“This is a very meaningful demonstration, from epidemiological data to preclinical models of the role of PM2.5 air pollutants in the promotion of lung cancer, and it provides us with very important insights into the mechanism through which nonsmokers can get lung cancer,” commented Suzette Delaloge, MD, from the cancer interception program at Institut Goustave Roussy in Villejuif, France, the invited discussant.
“But beyond that, it also has a great impact on our vision of carcinogenesis, with this very elegant first demonstration of the alternative nonmutagenic, carcinogenetic promotion hypothesis for fine particulate matter,” she said.
Questions still to be answered include whether PM2.5 pollutants could also be mutagenic, is the oncogenic pathway ubiquitous in tissue, which components of PM2.5 might drive the effect, how long of an exposure is required to promote lung cancer, and why and how persons without cancer develop specific driver mutations such as EGFR, she said.
“This research is intriguing and exciting as it means that we can ask whether, in the future, it will be possible to use lung scans to look for precancerous lesions in the lungs and try to reverse them with medicines such as interleukin-1B inhibitors,” said Tony Mok, MD, a lung cancer specialist at the Chinese University of Hong Kong, who was not involved in the study.
“We don’t yet know whether it will be possible to use highly sensitive EGFR profiling on blood or other samples to find nonsmokers who are predisposed to lung cancer and may benefit from lung scanning, so discussions are still very speculative,” he said in a statement.
The study was supported by Cancer Research UK, the Lung Cancer Research Foundations, Rosetrees Trust, the Mark Foundation for Cancer Research and the Ruth Strauss Foundation. Dr. Swanton disclosed grants/research support, honoraria, and stock ownership with multiple entities. Dr. Delaloge disclosed institutional financing and research funding from multiple companies. Dr. Mok disclosed stock ownership and honoraria with multiple companies.
AT ESMO CONGRESS 2022
Optimizing Narrowband UVB Phototherapy: Is It More Challenging for Your Older Patients?
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—
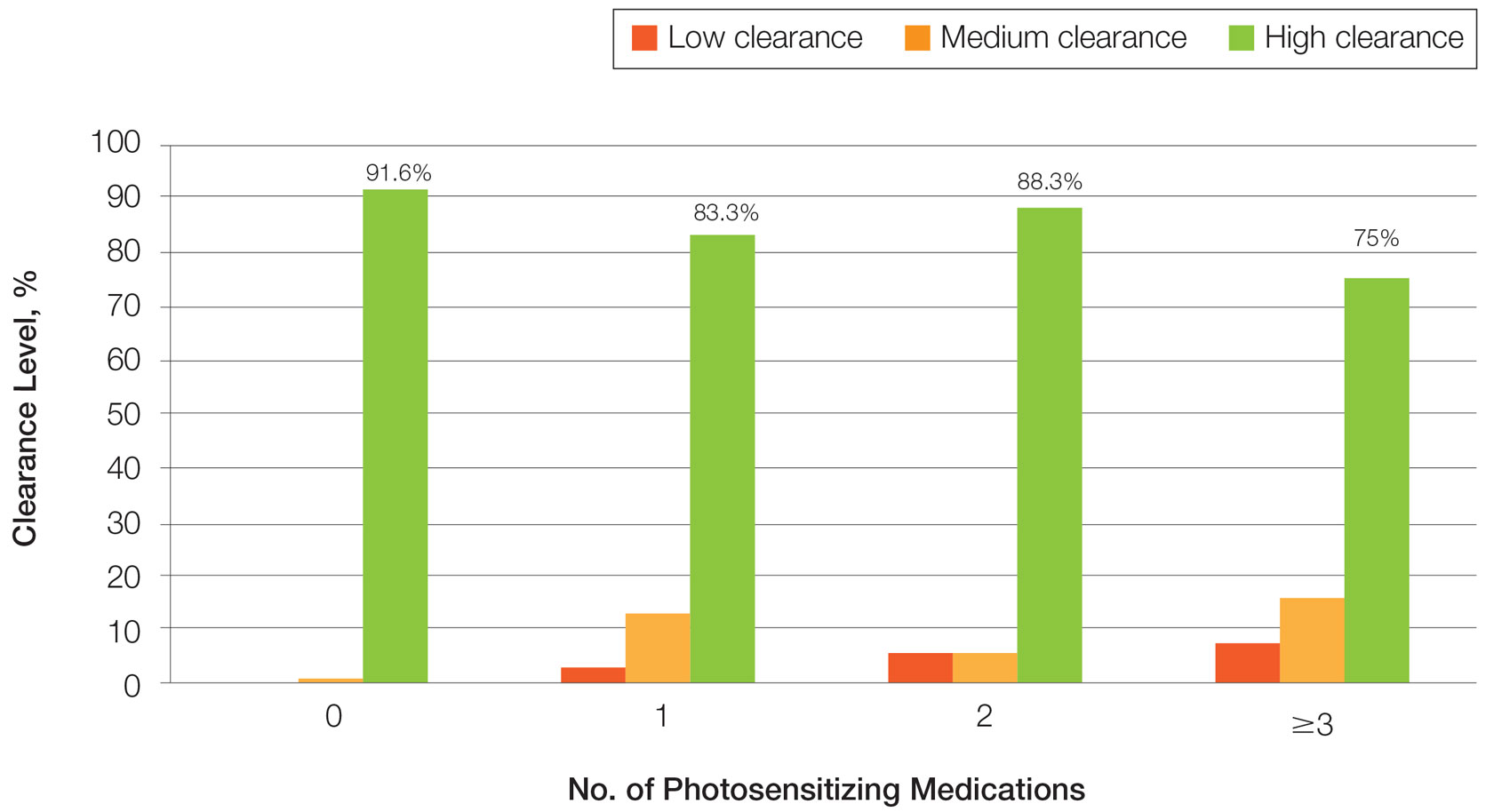
Photosensitizing Medications, Clearance Levels, and Clearance Rates—
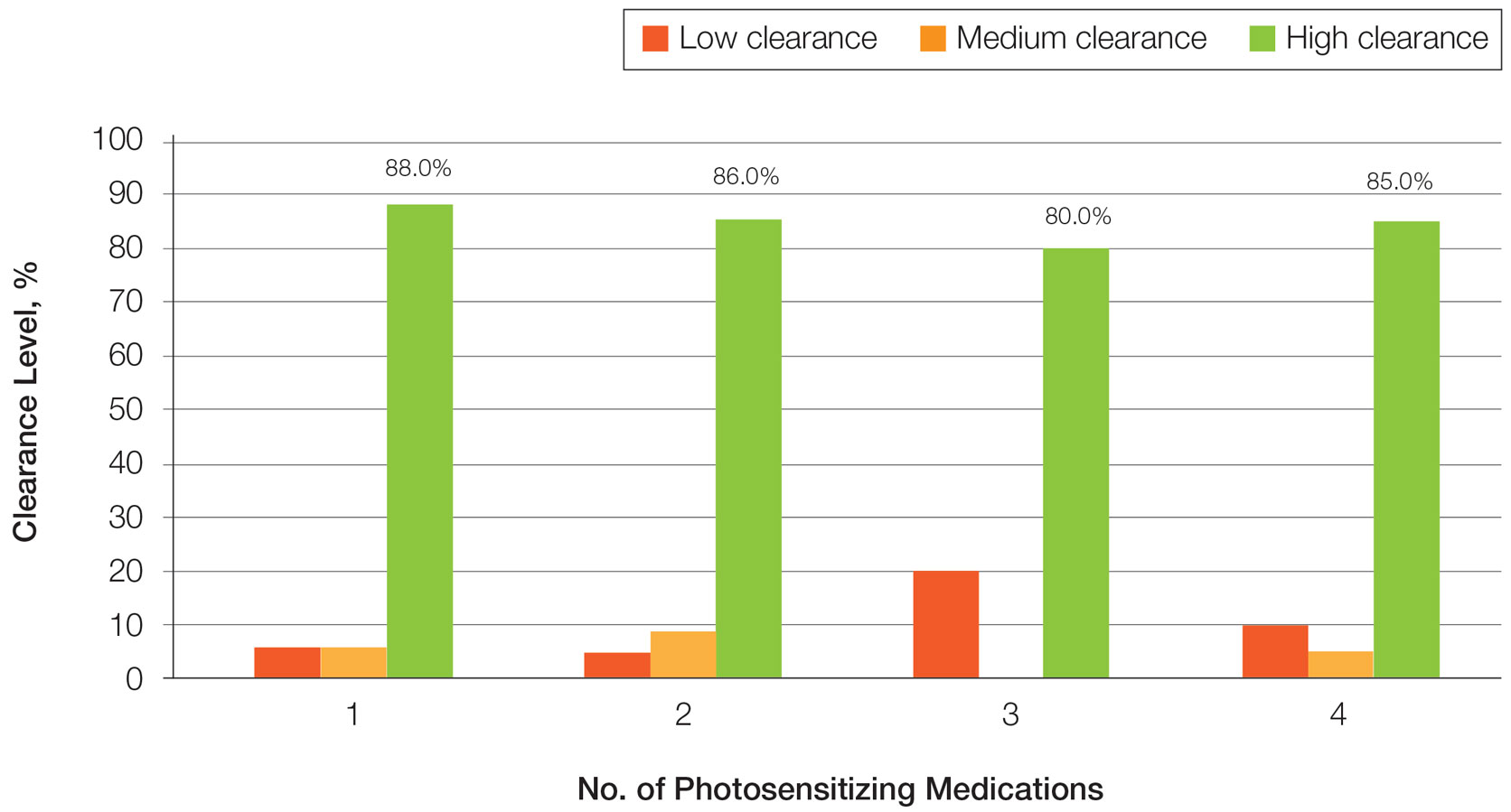
Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.
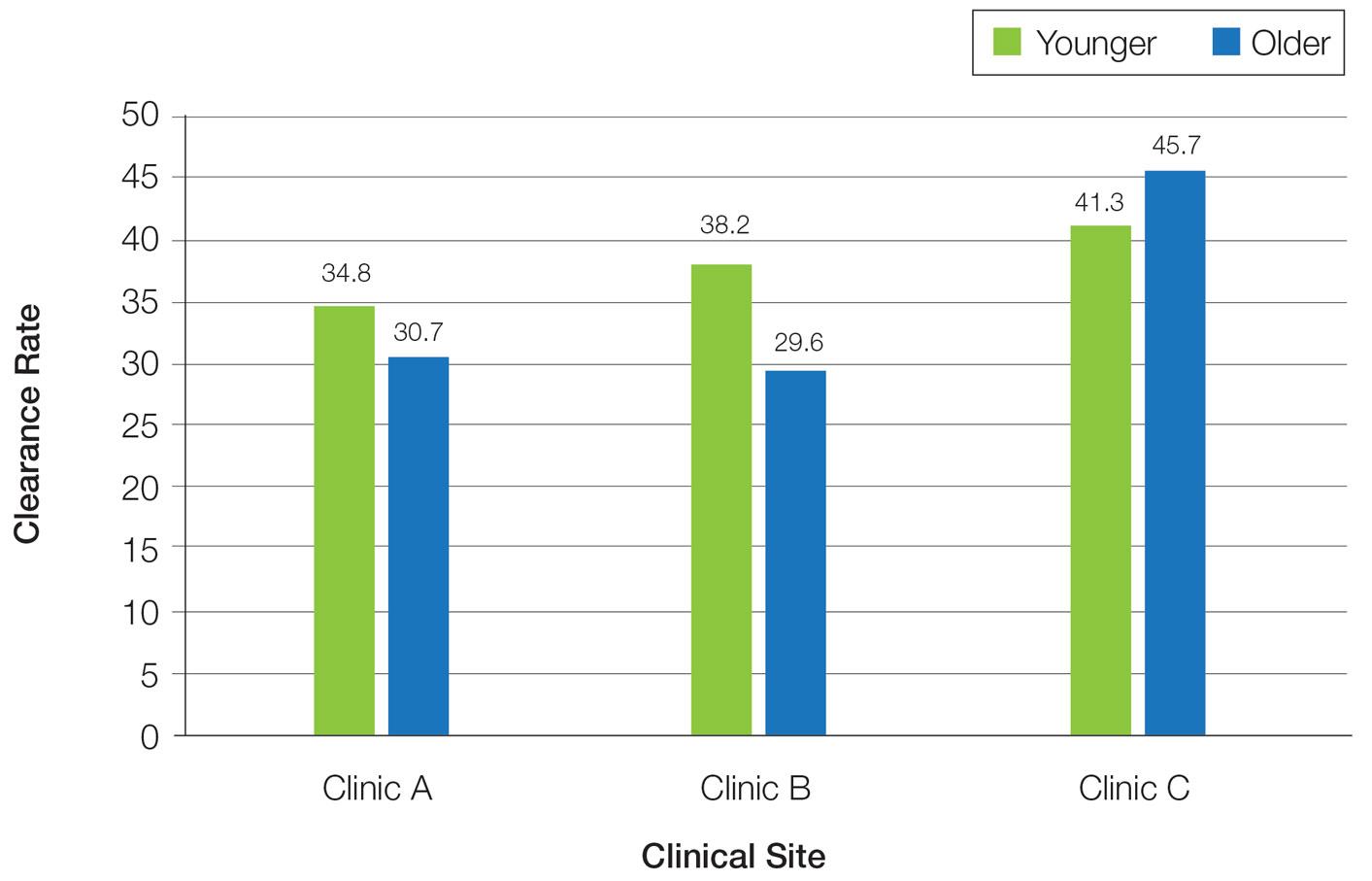
Photosensitizing Medications and Erythema Rates—
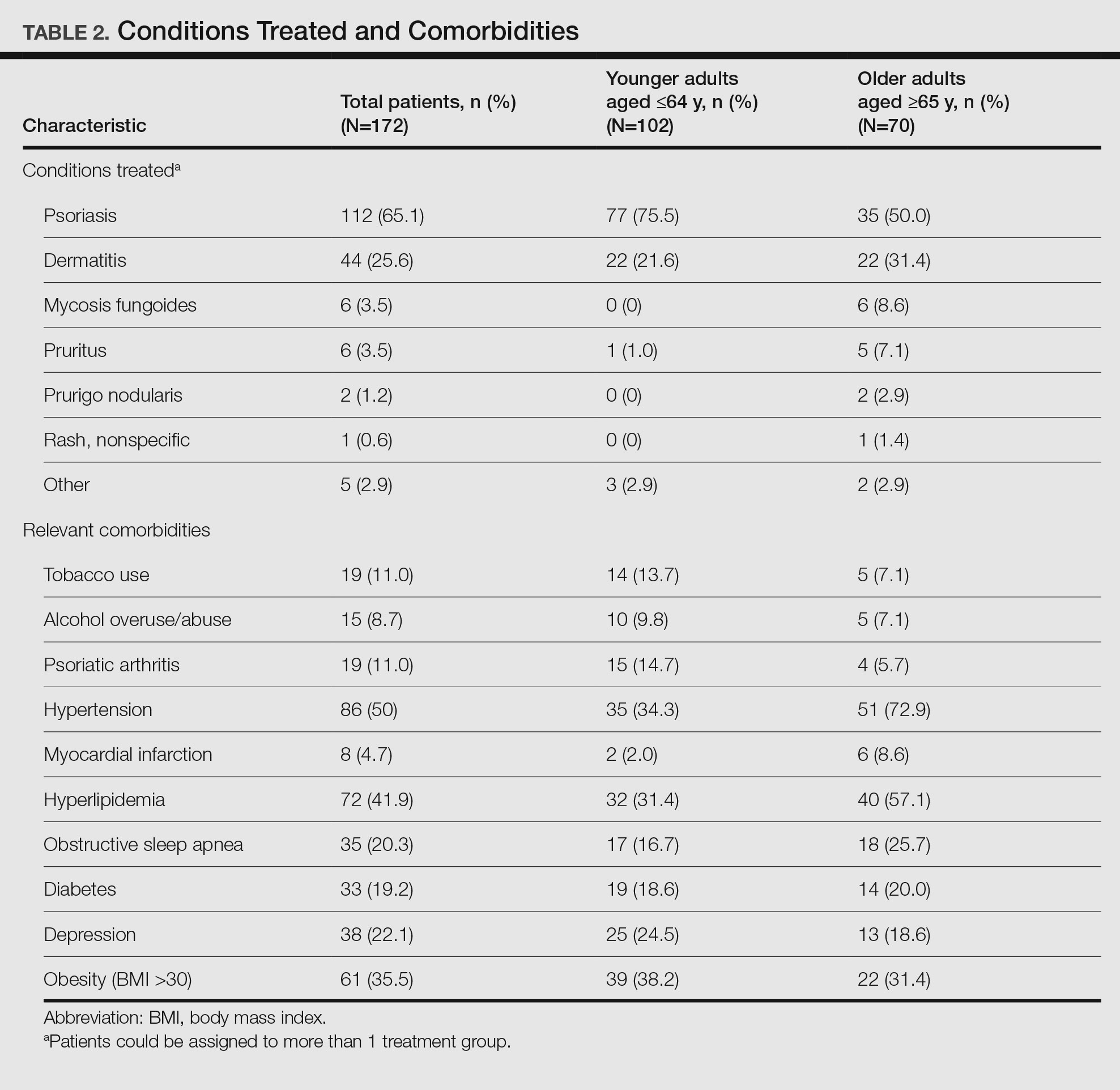
Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).
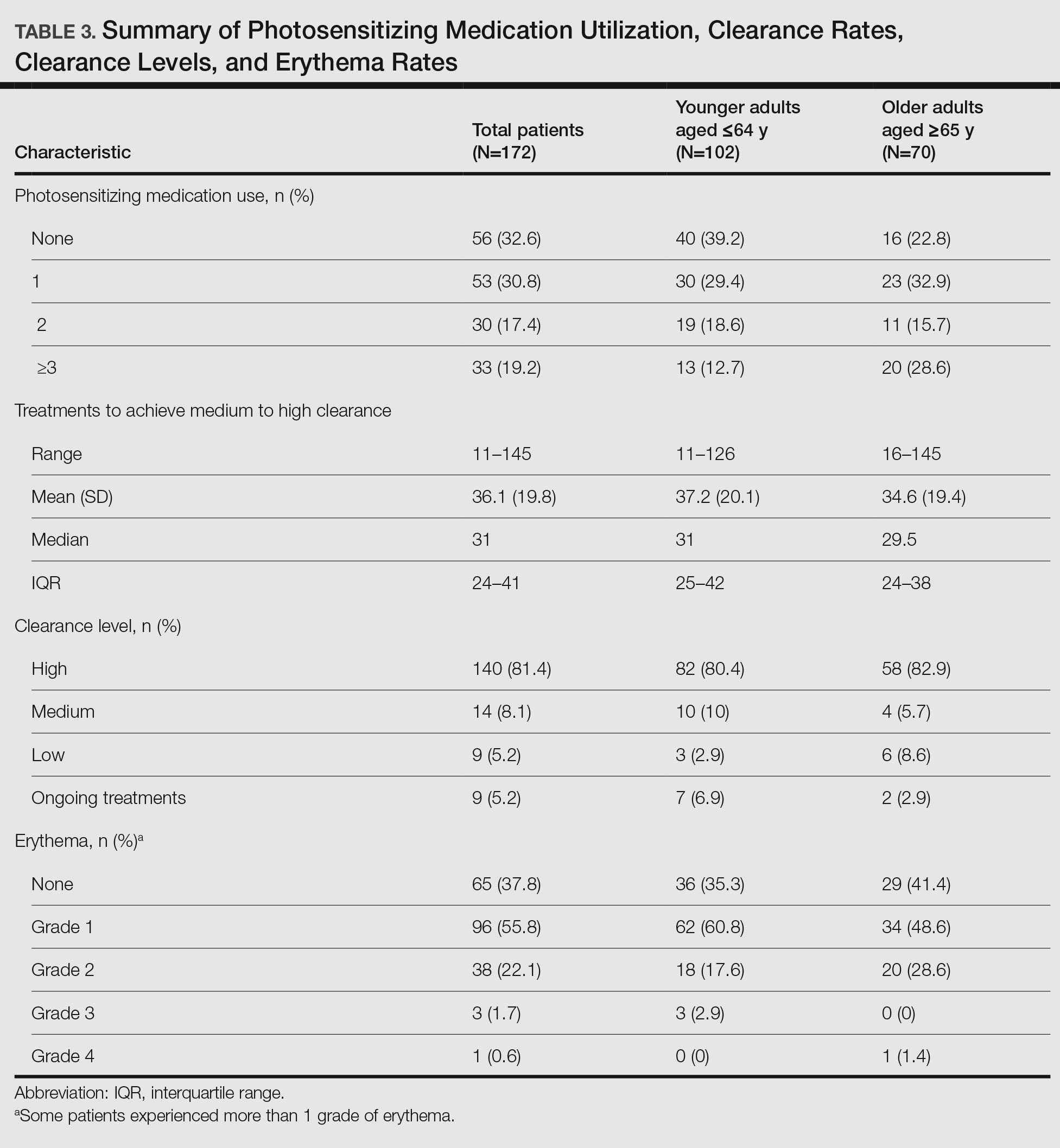
Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
Even with recent pharmacologic treatment advances, narrowband UVB (NB-UVB) phototherapy remains a versatile, safe, and efficacious adjunctive or exclusive treatment for multiple dermatologic conditions, including psoriasis and atopic dermatitis.
In a prior study, Matthews et al13 reported that 96% (50/52) of patients older than 65 years achieved medium to high levels of clearance with NB-UVB phototherapy. Nonetheless, 2 other findings in this study related to the number of treatments required to achieve clearance (ie, clearance rates) and erythema rates prompted further investigation. The first finding was higher-than-expected clearance rates. Older adults had a clearance rate with a mean of 33 treatments compared to prior studies featuring mean clearance rates of 20 to 28 treatments.7,8,14-16 This finding resembled a study in the United Kingdom17 with a median clearance rate in older adults of 30 treatments. In contrast, the median clearance rate from a study in Turkey18 was 42 treatments in older adults. We hypothesized that more photosensitizing medications used in older vs younger adults prompted more dose adjustments with NB-UVB phototherapy to avoid burning (ie, erythema) at baseline and throughout the treatment course. These dose adjustments may have increased the overall clearance rates. If true, we predicted that younger adults treated with the same protocol would have cleared more quickly, either because of age-related differences or because they likely had fewer comorbidities and therefore fewer medications.
The second finding from Matthews et al13 that warranted further investigation was a higher erythema rate compared to the older adult study from the United Kingdom.17 We hypothesized that potentially greater use of photosensitizing medications in the United States could explain the higher erythema rates. Although medication-induced photosensitivity is less likely with NB-UVB phototherapy than with UVA, certain medications can cause UVB photosensitivity, including thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.8,19,20 Therefore, photosensitizing medication use either at baseline or during a course of NB-UVB phototherapy could increase the risk for erythema. Age-related skin changes also have been considered as a
This retrospective study aimed to determine if NB-UVB phototherapy is equally effective in both older and younger adults treated with the same protocol; to examine the association between the use of photosensitizing medications and clearance rates in both older and younger adults; and to examine the association between the use of photosensitizing medications and erythema rates in older vs younger adults.
Methods
Study Design and Patients—This retrospective cohort study used billing records to identify patients who received NB-UVB phototherapy at 3 different clinical sites within a large US health care system in Washington (Group Health Cooperative, now Kaiser Permanente Washington), serving more than 600,000 patients between January 1, 2012, and December 31, 2016. The institutional review board of Kaiser Permanente Washington Health Research Institute approved this study (IRB 1498087-4). Younger adults were classified as those 64 years or younger and older adults as those 65 years and older at the start of their phototherapy regimen. A power analysis determined that the optimal sample size for this study was 250 patients.
Individuals were excluded if they had fewer than 6 phototherapy treatments; a diagnosis of vitiligo, photosensitivity dermatitis, morphea, or pityriasis rubra pilaris; and/or treatment of the hands or feet only.
Phototherapy Protocol—Using a 48-lamp NB-UVB unit, trained phototherapy nurses provided all treatments following standardized treatment protocols13 based on previously published phototherapy guidelines.24 Nurses determined each patient’s disease clearance level using a 3-point clearance scale (high, medium, low).13 Each patient’s starting dose was determined based on the estimated MED for their skin phototype.
Statistical Analysis—Data were analyzed using Stata statistical software (StataCorp LLC). Univariate analyses were used to examine the data and identify outliers, bad values, and missing data, as well as to calculate descriptive statistics. Pearson χ2 and Fisher exact statistics were used to calculate differences in categorical variables. Linear multivariate regression models and logistic multivariate models were used to examine statistical relationships between variables. Statistical significance was defined as P≤.05.
Results
Patient Characteristics—Medical records were reviewed for 172 patients who received phototherapy between 2012 and 2016. Patients ranged in age from 23 to 91 years, with 102 patients 64 years and younger and 70 patients 65 years and older. Tables 1 and 2 outline the patient characteristics and conditions treated.

Phototherapy Effectiveness—

Photosensitizing Medications, Clearance Levels, and Clearance Rates—

Frequency of Treatments and Clearance Rates—Older adults more consistently completed the recommended frequency of treatments—3 times weekly—compared to younger adults (74.3% vs 58.5%). However, all patients who completed 3 treatments per week required a similar number of treatments to clear (older adults, mean [SD]: 35.7 [21.6]; younger adults, mean [SD]: 34.7 [19.0]; P=.85). Among patients completing 2 or fewer treatments per week, older adults required a mean (SD) of only 31 (9.0) treatments to clear vs 41.5 (21.3) treatments to clear for younger adults, but the difference was not statistically significant (P=.08). However, even those with suboptimal frequency ultimately achieved similar clearance levels.


Photosensitizing Medications and Erythema Rates—

Overall, phototherapy nurses adjusted the starting dose according to the phototype-based protocol an average of 69% of the time for patients on medications with photosensitivity listed as a potential side effect. However, the frequency depended significantly on the clinic (clinic A, 24%; clinic B, 92%; clinic C, 87%)(P≤.001). Nurses across all clinics consistently decreased the treatment dose when patients reported starting new photosensitizing medications. Patients with adjusted starting doses had slightly but not significantly higher clearance rates compared to those without (mean, 37.8 vs 35.5; t(104)=0.58; P=.56).

Comment
Impact of Photosensitizing Medications on Clearance—Photosensitizing medications and treatment frequency were 2 factors that might explain the slower clearance rates in younger adults. In this study, both groups of patients used similar numbers of photosensitizing medications, but more older adults were taking 3 or more medications (Table 3). We found no statistically significant relationship between taking photosensitizing medications and either the clearance rates or the level of clearance achieved in either age group.
Impact of Treatment Frequency—Weekly treatment frequency also was examined. One prior study demonstrated that treatments 3 times weekly led to a faster clearance time and higher clearance levels compared with twice-weekly treatment.7 When patients completed treatments twice weekly, it took an average of 1.5 times more days to clear, which impacted cost and clinical resource availability. The patients ranged in age from 17 to 80 years, but outcomes in older patients were not described separately.7 Interestingly, our study seemed to find a difference between age groups when the impact of treatment frequency was examined. Older adults completed nearly 4 fewer mean treatments to clear when treating less often, with more than 80% achieving high levels of clearance, whereas the younger adults required almost 7 more treatments to clear when they came in less frequently, with approximately 80% achieving a high level of clearance. As a result, our study found that in both age groups, slowing the treatment frequency extended the treatment time to clearance—more for the younger adults than the older adults—but did not significantly change the percentage of individuals reaching full clearance in either group.
Erythema Rates—There was no association between photosensitizing medications and erythema rates except when patients were taking at least 3 medications. Most medications that listed photosensitivity as a possible side effect did not specify their relevant range of UV radiation; therefore, all such medications were examined during this analysis. Prior research has shown UVB range photosensitizing medications include thiazides, quinidine, calcium channel antagonists, phenothiazines, and nonsteroidal anti-inflammatory drugs.19 A sensitivity analysis that focused only on these medications found no association between them and any particular grade of erythema. However, patients taking 3 or more of any medications listing photosensitivity as a side effect had an increased risk for grade 2 erythema.
Erythema rates in this study were consistent with a 2013 systematic review that reported 57% of patients with asymptomatic grade 1 erythema.25 In the 2 other comparative older adult studies, erythema rates varied widely: 35% in a study from Turkey18compared to only1.89% in a study from the United Kingdom.17
The starting dose for NB-UVB may drive erythema rates. The current study’s protocols were based on an estimated MED that is subjectively determined by the dermatology provider’s assessment of the patient’s skin sensitivity via examination and questions to the patient about their response to environmental sun exposure (ie, burning and tanning)26 and is frequently used to determine the starting dose and subsequent dose escalation. Certain medications have been found to increase photosensitivity and erythema,20 which can change an individual’s MED. If photosensitizing medications are started prior to or during a course of NB-UVB without a pretreatment MED, they might increase the risk for erythema. This study did not identify specific erythema-inducing medications but did find that taking 3 or more photosensitizing medications was associated with increased episodes of grade 2 erythema. Similarly, Harrop et al8 found that patients who were taking photosensitizing medications were more likely to have grade 2 or higher erythema, despite baseline MED testing, which is an established safety mechanism to reduce the risk and severity of erythema.14,20,27 The authors of a recent study of older adults in Taiwan specifically recommended MED testing due to the unpredictable influence of polypharmacy on MED calculations in this population.28 Therefore, this study’s use of an estimated MED in older adults may have influenced the starting dose as well as the incidence and severity of erythemic events. Age-related skin changes likely are ruled out as a consideration for mild erythema by the similarity of grade 1 erythema rates in both older and younger adults. Other studies have identified differences between the age groups, where older patients experienced more intense erythema in the late phase of UVB treatments.22,23 This phenomenon could increase the risk for a grade 2 erythema, which may correspond with this study’s findings.
Other potential causes of erythema were ruled out during our study, including erythema related to missed treatments and shielding mishaps. Other factors, however, may impact the level of sensitivity each patient has to phototherapy, including genetics, epigenetics, and cumulative sun damage. With NB-UVB, near-erythemogenic doses are optimal to achieve effective treatments but require a delicate balance to achieve, which may be more problematic for older adults, especially those taking several medications.
Study Limitations—Our study design made it difficult to draw conclusions about rarer dermatologic conditions. Some patients received treatments over years that were not included in the study period. Finally, power calculations suggested that our actual sample size was too small, with approximately one-third of the required sample missing.
Practical Implications—The goals of phototherapy are to achieve a high level of disease clearance with the fewest number of treatments possible and minimal side effects.
The extra staff training and patient monitoring required for MED testing likely is to add value and preserve resources if faster clearance rates could be achieved and may warrant further investigation. Phototherapy centers require standardized treatment protocols, diligent well-trained staff, and program monitoring to ensure consistent care to all patients. This study highlighted the ongoing opportunity for health care organizations to conduct evidence-based practice inquiries to continually optimize care for their patients.
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
- Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2016;29:19-23.
- Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS One. 2017;12:e0181813.
- Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593.e581-584.
- Cameron H, Dawe RS, Yule S, et al. A randomized, observer-blinded trial of twice vs. three times weekly narrowband ultraviolet B phototherapy for chronic plaque psoriasis. Br J Dermatol. 2002;147:973-978.
- Harrop G, Dawe RS, Ibbotson S. Are photosensitizing medications associated with increased risk of important erythemal reactions during ultraviolet B phototherapy? Br J Dermatol. 2018;179:1184-1185.
- Torres AE, Lyons AB, Hamzavi IH, et al. Role of phototherapy in the era of biologics. J Am Acad Dermatol. 2021;84:479-485.
- Bukvic´ć Mokos Z, Jovic´ A, Cˇeovic´ R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Expert Opin Biol Ther. 2018;18:897-903.
- Oliveira C, Torres T. More than skin deep: the systemic nature of atopic dermatitis. Eur J Dermatol. 2019;29:250-258.
- Matthews S, Pike K, Chien A. Phototherapy: safe and effective for challenging skin conditions in older adults. Cutis. 2021;108:E15-E21.
- Rodríguez-Granados MT, Estany-Gestal A, Pousa-Martínez M, et al. Is it useful to calculate minimal erythema dose before narrowband UV-B phototherapy? Actas Dermosifiliogr. 2017;108:852-858.
- Parlak N, Kundakci N, Parlak A, et al. Narrowband ultraviolet B phototherapy starting and incremental dose in patients with psoriasis: comparison of percentage dose and fixed dose protocols. Photodermatol Photoimmunol Photomed. 2015;31:90-97.
- Kleinpenning MM, Smits T, Boezeman J, et al. Narrowband ultraviolet B therapy in psoriasis: randomized double-blind comparison of high-dose and low-dose irradiation regimens. Br J Dermatol. 2009;161:1351-1356.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Dawe RS, Ibbotson SH. Drug-induced photosensitivity. Dermatol Clin. 2014;32:363-368, ix.
- Cameron H, Dawe RS. Photosensitizing drugs may lower the narrow-band ultraviolet B (TL-01) minimal erythema dose. Br J Dermatol. 2000;142:389-390.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Gloor M, Scherotzke A. Age dependence of ultraviolet light-induced erythema following narrow-band UVB exposure. Photodermatol Photoimmunol Photomed. 2002;18:121-126.
- Cox NH, Diffey BL, Farr PM. The relationship between chronological age and the erythemal response to ultraviolet B radiation. Br J Dermatol. 1992;126:315-319.
- Morrison W. Phototherapy and Photochemotherapy for Skin Disease. 2nd ed. Informa Healthcare; 2005.
- Almutawa F, Alnomair N, Wang Y, et al. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14:87-109.
- Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27:615-619.
- Kwon IH, Kwon HH, Na SJ, et al. Could colorimetric method replace the individual minimal erythemal dose (MED) measurements in determining the initial dose of narrow-band UVB treatment for psoriasis patients with skin phototype III-V? J Eur Acad Dermatol Venereol. 2013;27:494-498.
- Chen WA, Chang CM. The minimal erythema dose of narrowband ultraviolet B in elderly Taiwanese [published online September 1, 2021]. Photodermatol Photoimmunol Photomed. doi:10.1111/phpp.12730
Practice Points
- Narrowband UVB (NB-UVB) phototherapy remains a safe and efficacious nonpharmacologic treatment for dermatologic conditions in older and younger adults.
- Compared to younger adults, older adults using the same protocols need similar or even fewer treatments to achieve high levels of clearance.
- Individuals taking 3 or more photosensitizing medications, regardless of age, may be at higher risk for substantial erythema with NB-UVB phototherapy.
- Phototherapy program monitoring is important to ensure quality care and investigate opportunities for care optimization.
FDA warns of cancer risk in scar tissue around breast implants
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
.
The FDA safety communication is based on several dozen reports of these cancers occurring in the capsule or scar tissue around breast implants. This issue differs from breast implant–associated anaplastic large-cell lymphoma (BIA-ALCL) – a known risk among implant recipients.
“After preliminary review of published literature as part of our ongoing monitoring of the safety of breast implants, the FDA is aware of less than 20 cases of SCC and less than 30 cases of various lymphomas in the capsule around the breast implant,” the agency’s alert explains.
One avenue through which the FDA has identified cases is via medical device reports. As of Sept. 1, the FDA has received 10 medical device reports about SCC related to breast implants and 12 about various lymphomas.
The incidence rate and risk factors for these events are currently unknown, but reports of SCC and various lymphomas in the capsule around the breast implants have been reported for both textured and smooth breast implants, as well as for both saline and silicone breast implants. In some cases, the cancers were diagnosed years after breast implant surgery.
Reported signs and symptoms included swelling, pain, lumps, or skin changes.
Although the risks of SCC and lymphomas in the tissue around breast implants appears rare, “when safety risks with medical devices are identified, we wanted to provide clear and understandable information to the public as quickly as possible,” Binita Ashar, MD, director of the Office of Surgical and Infection Control Devices, FDA Center for Devices and Radiological Health, explained in a press release.
Patients and providers are strongly encouraged to report breast implant–related problems and cases of SCC or lymphoma of the breast implant capsule to MedWatch, the FDA’s adverse event reporting program.
The FDA plans to complete “a thorough literature review” as well as “identify ways to collect more detailed information regarding patient cases.”
A version of this article first appeared on Medscape.com.
Polycyclic Scaly Eruption
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
The Diagnosis: Netherton Syndrome
A punch biopsy from the right lower back supported the clinical diagnosis of ichthyosis linearis circumflexa. The patient underwent genetic testing and was found to have a heterozygous mutation in the serine protease inhibitor Kazal type 5 gene, SPINK5, that was consistent with a diagnosis of Netherton syndrome.
Netherton syndrome is an autosomal-recessive genodermatosis characterized by a triad of congenital ichthyosis, hair shaft abnormalities, and atopic diatheses.1,2 It affects approximately 1 in 200,000 live births2,3; however, it is considered by many to be underdiagnosed due to the variability in the clinical appearance. Therefore, the incidence of Netherton syndrome may actually be closer 1 in 50,000 live births.1 The manifestations of the disease are caused by a germline mutation in the SPINK5 gene, which encodes the serine protease inhibitor LEKTI.1,2 Dysfunctional LEKTI results in increased proteolytic activity of the lipid-processing enzymes in the stratum corneum, resulting in a disruption in the lipid bilayer.1 Dysfunctional LEKTI also results in a loss of the antiinflammatory and antimicrobial function of the stratum corneum. Clinical features of Netherton syndrome usually present at birth or shortly thereafter.1 Congenital ichthyosiform erythroderma, or the continuous peeling of the skin, is a common presentation seen at birth and in the neonatal period.2 As the patient ages, the dermatologic manifestations evolve into serpiginous and circinate, erythematous plaques with a characteristic peripheral, double-edged scaling.1,2 This distinctive finding is termed ichthyosis linearis circumflexa and is pathognomonic for the syndrome.2 Lesions often affect the trunk and extremities and demonstrate an undulating course.1 Because eczematous and lichenified plaques in flexural areas as well as pruritus are common clinical features, this disease often is misdiagnosed as atopic dermatitis,1,3 as was the case in our patient.
Patients with Netherton syndrome can present with various hair abnormalities. Trichorrhexis invaginata, known as bamboo hair, is the intussusception of the hair shaft and is characteristic of the disease.3 It develops from a reduced number of disulfide bonds, which results in cortical softening.1 Trichorrhexis invaginata may not be present at birth and often improves with age.1,3 Other hair shaft abnormalities such as pili torti, trichorrhexis nodosa, and helical hair also may be observed in Netherton syndrome.1 Extracutaneous manifestations also are typical. There is immune dysregulation of memory B cells and natural killer cells, which manifests as frequent respiratory and skin infections as well as sepsis.1,2 Patients also may have increased levels of serum IgE and eosinophilia resulting in atopy and allergic reactions to various triggers such as foods.1 The neonatal period also may be complicated by dehydration, electrolyte imbalances, inability to regulate body temperature, and failure to thrive.1,3
When there is an extensive disruption of the skin barrier during the neonatal period, there may be severe electrolyte imbalances and thermoregulatory challenges necessitating treatment in the neonatal intensive care unit. Cutaneous disease can be treated with topical therapies with variable success.1 Topical therapies for symptom management include emollients, corticosteroids, calcineurin inhibitors, calcipotriene, and retinoids; however, utmost caution must be employed with these therapies due to the increased risk for systemic absorption resulting from the disturbance of the skin barrier. When therapy with topical tacrolimus is implemented, monitoring of serum drug levels is required.1 Pruritus may be treated symptomatically with oral antihistamines. Intravenous immunoglobulin has been shown to decrease the frequency of infections and improve skin inflammation. Systemic retinoids have unpredictable effects and result in improvement of disease in some patients but exacerbation in others. Phototherapy with narrowband UVB, psoralen plus UVA, UVA1, and balneophototherapy also are effective treatments for cutaneous disease.1 Dupilumab has been shown to decrease pruritus, improve hair abnormalities, and improve skin disease, thereby demonstrating its effectiveness in treating the atopy and ichthyosis in Netherton syndrome.4
The differential diagnosis includes other figurate erythemas including erythema marginatum and erythrokeratodermia variabilis. Erythema marginatum is a cutaneous manifestation of acute rheumatic fever and is characterized by migratory polycyclic erythematous plaques without overlying scale, usually on the trunk and proximal extremities.5 Erythrokeratodermia variabilis is caused by heterozygous mutations in gap junction protein beta 3, GJB3, and gap junction protein beta 4, GJB4, and is characterized by transient geographic and erythematous patches and stable scaly plaques; however, double-edged scaling is not a feature.1 Acrodermatitis enteropathica is an autosomal-recessive disorder caused by mutations in the zinc transporter SLC39A4. Cutaneous manifestations occur after weaning from breast milk and are characterized by erythematous plaques with erosions, vesicles, and scaling, which characteristically occur in the perioral and perianal locations.6 Neonatal lupus is a form of subacute cutaneous lupus erythematosus. Typical skin lesions are erythematous annular plaques with overlying scaling, which may be present at birth and have a predilection for the face and other sun-exposed areas. Lesions generally resolve after clearance of the pathogenic maternal antibodies.7
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
- Richard G, Ringpfeil F. Ichthyoses, erythrokeratodermas, and related disorders. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:888-923.
- Garza JI, Herz-Ruelas ME, Guerrero-González GA, et al. Netherton syndrome: a diagnostic and therapeutic challenge. J Am Acad Dermatol. 2016;74(suppl 1):AB129.
- Heymann W. Appending the appendages: new perspectives on Netherton syndrome and green nail syndrome. J Am Acad Dermatol. 2020;83:735-736.
- Murase C, Takeichi T, Taki T, et al. Successful dupilumab treatment for ichthyotic and atopic features of Netherton syndrome. J Dermatol Sci. 2021;102:126-129.
- España A. Figurate erythemas. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:320-331.
- Noguera-Morel L, McLeish Schaefer S, Hivnor C. Nutritional diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:793-809.
- Lee L, Werth V. Lupus erythematosus. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:662-680.
A 9-year-old boy presented to the dermatology clinic with a scaly eruption distributed throughout the body that had been present since birth. He had been diagnosed with atopic dermatitis by multiple dermatologists prior to the current presentation and had been treated with various topical steroids with minimal improvement. He had no family history of similar eruptions and no personal history of asthma or allergies. Physical examination revealed erythematous, serpiginous, polycyclic plaques with peripheral, double-edged scaling. Decreased hair density of the lateral eyebrows also was observed.