User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
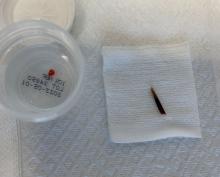
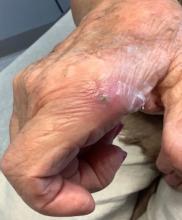
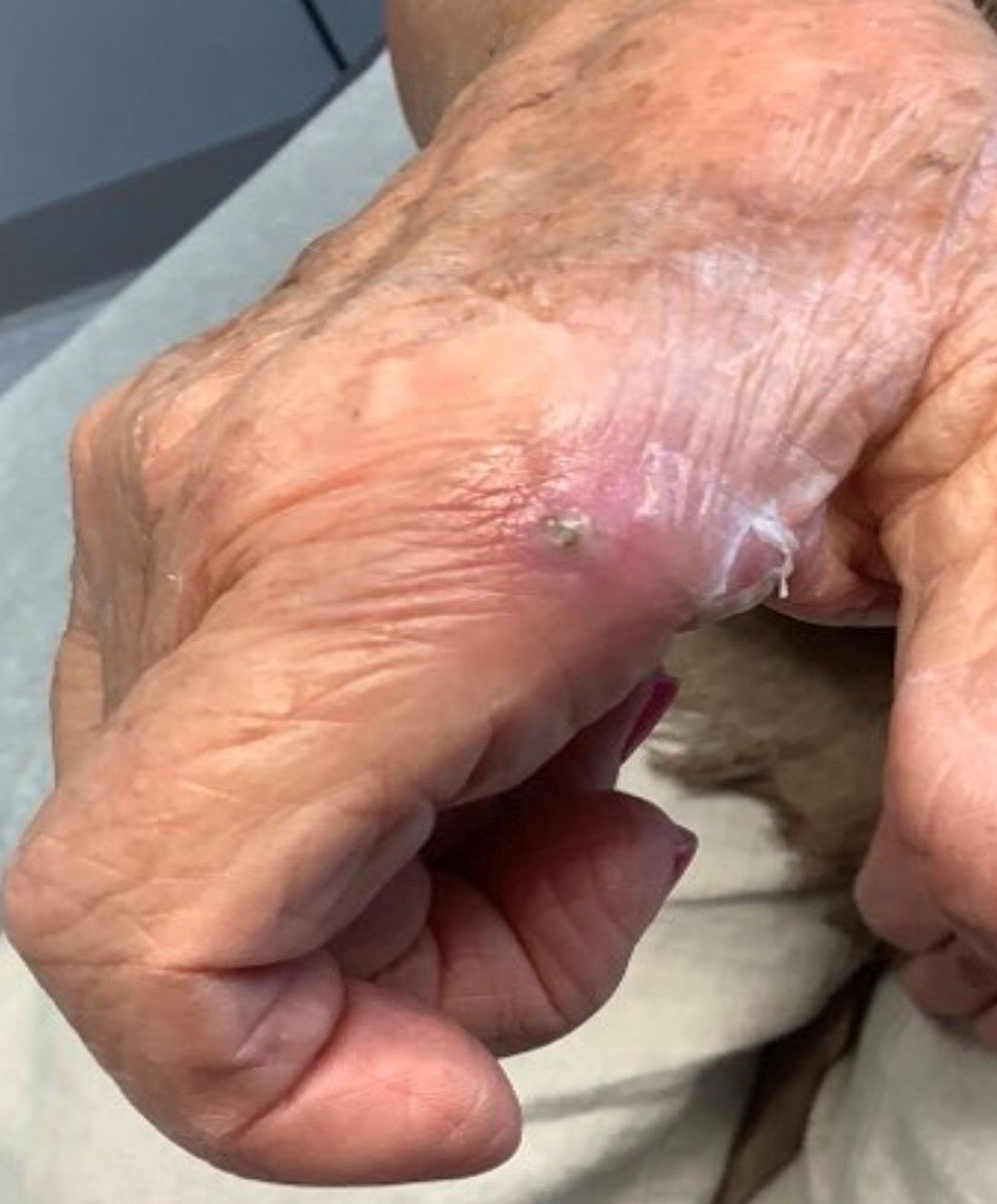
An 80-year-old female developed a painful purulent nodule a day after gardening
. There are more than 100 species of dematiaceous fungi that can cause phaeohyphomycosis, including Alternaria, Exophiala, Phialophora, Wangiella, Bipolaris, Curvularia, and Exserohilum.1,2 The causative fungi are found in plants and soil, so they are commonly seen after activities such as gardening or walking barefoot. Trauma, such as a splinter, typically incites the infection. Infections can present with superficial, cutaneous and subcutaneous involvement.
Sporotrichosis, also called Rose gardener’s disease, is a mycosis caused by Sporothrix schenckii. A typical presentation is when a gardener gets pricked by a rose thorn. Classically, a pustule will develop at the site of inoculation, with additional lesions forming along the path of lymphatic drainage (called a “sporotrichoid” pattern) weeks later. Atypical mycobacterial infections, mainly Mycobacterium marinum, may also present in this way. Histopathology and tissue cultures help to differentiate the two.
An incision and drainage with pathology was performed in the office. Upon opening the nodule, a large wood splinter was extracted. Both the foreign body and a punch biopsy of skin were sent in for examination. Pathology revealed polarizable foreign material in association with suppurative inflammation and dematiaceous fungi. PAS (Periodic-acid Schiff) and GMS (Grocott methenamine silver) stain highlighted fungal forms. Cultures were negative.
Local disease may be treated with excision alone. Oral antifungals, such as itraconazole, fluconazole, or ketoconazole may be used, although may require long treatment courses for months. Amphotericin B and flucytosine may be required in systemic cases. Almost all cases of disseminated disease occur in immunocompromised patients. Our patient’s hand resolved after removal of the causative thorn.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kradin R. Diagnostic Pathology of Infectious Disease, 1st edition (Saunders, Feb. 2, 2010).
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
. There are more than 100 species of dematiaceous fungi that can cause phaeohyphomycosis, including Alternaria, Exophiala, Phialophora, Wangiella, Bipolaris, Curvularia, and Exserohilum.1,2 The causative fungi are found in plants and soil, so they are commonly seen after activities such as gardening or walking barefoot. Trauma, such as a splinter, typically incites the infection. Infections can present with superficial, cutaneous and subcutaneous involvement.
Sporotrichosis, also called Rose gardener’s disease, is a mycosis caused by Sporothrix schenckii. A typical presentation is when a gardener gets pricked by a rose thorn. Classically, a pustule will develop at the site of inoculation, with additional lesions forming along the path of lymphatic drainage (called a “sporotrichoid” pattern) weeks later. Atypical mycobacterial infections, mainly Mycobacterium marinum, may also present in this way. Histopathology and tissue cultures help to differentiate the two.
An incision and drainage with pathology was performed in the office. Upon opening the nodule, a large wood splinter was extracted. Both the foreign body and a punch biopsy of skin were sent in for examination. Pathology revealed polarizable foreign material in association with suppurative inflammation and dematiaceous fungi. PAS (Periodic-acid Schiff) and GMS (Grocott methenamine silver) stain highlighted fungal forms. Cultures were negative.
Local disease may be treated with excision alone. Oral antifungals, such as itraconazole, fluconazole, or ketoconazole may be used, although may require long treatment courses for months. Amphotericin B and flucytosine may be required in systemic cases. Almost all cases of disseminated disease occur in immunocompromised patients. Our patient’s hand resolved after removal of the causative thorn.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kradin R. Diagnostic Pathology of Infectious Disease, 1st edition (Saunders, Feb. 2, 2010).
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
. There are more than 100 species of dematiaceous fungi that can cause phaeohyphomycosis, including Alternaria, Exophiala, Phialophora, Wangiella, Bipolaris, Curvularia, and Exserohilum.1,2 The causative fungi are found in plants and soil, so they are commonly seen after activities such as gardening or walking barefoot. Trauma, such as a splinter, typically incites the infection. Infections can present with superficial, cutaneous and subcutaneous involvement.
Sporotrichosis, also called Rose gardener’s disease, is a mycosis caused by Sporothrix schenckii. A typical presentation is when a gardener gets pricked by a rose thorn. Classically, a pustule will develop at the site of inoculation, with additional lesions forming along the path of lymphatic drainage (called a “sporotrichoid” pattern) weeks later. Atypical mycobacterial infections, mainly Mycobacterium marinum, may also present in this way. Histopathology and tissue cultures help to differentiate the two.
An incision and drainage with pathology was performed in the office. Upon opening the nodule, a large wood splinter was extracted. Both the foreign body and a punch biopsy of skin were sent in for examination. Pathology revealed polarizable foreign material in association with suppurative inflammation and dematiaceous fungi. PAS (Periodic-acid Schiff) and GMS (Grocott methenamine silver) stain highlighted fungal forms. Cultures were negative.
Local disease may be treated with excision alone. Oral antifungals, such as itraconazole, fluconazole, or ketoconazole may be used, although may require long treatment courses for months. Amphotericin B and flucytosine may be required in systemic cases. Almost all cases of disseminated disease occur in immunocompromised patients. Our patient’s hand resolved after removal of the causative thorn.
This case and these photos were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Kradin R. Diagnostic Pathology of Infectious Disease, 1st edition (Saunders, Feb. 2, 2010).
2. Bolognia J et al. Dermatology (St. Louis: Mosby/Elsevier, 2008).
Worse impact of PsA and associated comorbidities on QoL
Key clinical point: Patients with psoriatic arthritis (PsA) performed poorly in all dimensions of the European Quality of Life (EQ-5D) scoring of quality of life (QoL) as compared with the general population, thus reflecting the detrimental effect of PsA on QoL.
Major finding: Overall, 87.3% and 69.8% of patients with PsA experienced pain/discomfort and anxiety/depression, respectively. Patients with PsA presented worse results in all dimensions of the EQ-5D scale and had a lower mean QoL score compared to the general population (0.651 vs. 0.793; P < .001) with worsened QoL in patients with concomitant use of nonsteroidal anti-inflammatory drugs (P = .035) and comorbidities (P = .003).
Study details: Findings are from a cross-sectional study including 212 adult patients with PsA from a single-center pharmacy in Minas Gerais, Brazil.
Disclosures: This study was supported by Minas Gerais Research Support Foundation and National Council for Scientific and Technological Development. Several of the authors declared receiving educational scholarships, grants, or personal fees from several sources.
Source: Moraes FA et al. Value Health Reg Issues. 2021 Aug 12. doi: 10.1016/j.vhri.2021.06.003.
Key clinical point: Patients with psoriatic arthritis (PsA) performed poorly in all dimensions of the European Quality of Life (EQ-5D) scoring of quality of life (QoL) as compared with the general population, thus reflecting the detrimental effect of PsA on QoL.
Major finding: Overall, 87.3% and 69.8% of patients with PsA experienced pain/discomfort and anxiety/depression, respectively. Patients with PsA presented worse results in all dimensions of the EQ-5D scale and had a lower mean QoL score compared to the general population (0.651 vs. 0.793; P < .001) with worsened QoL in patients with concomitant use of nonsteroidal anti-inflammatory drugs (P = .035) and comorbidities (P = .003).
Study details: Findings are from a cross-sectional study including 212 adult patients with PsA from a single-center pharmacy in Minas Gerais, Brazil.
Disclosures: This study was supported by Minas Gerais Research Support Foundation and National Council for Scientific and Technological Development. Several of the authors declared receiving educational scholarships, grants, or personal fees from several sources.
Source: Moraes FA et al. Value Health Reg Issues. 2021 Aug 12. doi: 10.1016/j.vhri.2021.06.003.
Key clinical point: Patients with psoriatic arthritis (PsA) performed poorly in all dimensions of the European Quality of Life (EQ-5D) scoring of quality of life (QoL) as compared with the general population, thus reflecting the detrimental effect of PsA on QoL.
Major finding: Overall, 87.3% and 69.8% of patients with PsA experienced pain/discomfort and anxiety/depression, respectively. Patients with PsA presented worse results in all dimensions of the EQ-5D scale and had a lower mean QoL score compared to the general population (0.651 vs. 0.793; P < .001) with worsened QoL in patients with concomitant use of nonsteroidal anti-inflammatory drugs (P = .035) and comorbidities (P = .003).
Study details: Findings are from a cross-sectional study including 212 adult patients with PsA from a single-center pharmacy in Minas Gerais, Brazil.
Disclosures: This study was supported by Minas Gerais Research Support Foundation and National Council for Scientific and Technological Development. Several of the authors declared receiving educational scholarships, grants, or personal fees from several sources.
Source: Moraes FA et al. Value Health Reg Issues. 2021 Aug 12. doi: 10.1016/j.vhri.2021.06.003.
PsA patients under anti-TNF therapy show improvement in work productivity
Key clinical point: Significant improvement in work productivity was observed in a real-world cohort of patients with psoriatic arthritis (PsA) treated with a tumor necrosis factor inhibitor (anti-TNF).
Major finding: At the final follow-up visit, the overall activity impairment decreased from 55.0 ± 21.5 to 16.3 ± 18.2 (P < .001). Moreover, absenteeism, mean presenteeism, and work productivity loss showed significant improvement upon treatment at the 9-month follow-up visit (all P < .001).
Study details: Findings are from a noninterventional, prospective, and observational cohort study including 120 patients with PsA who were receiving anti-TNF treatment.
Disclosures: This study was supported by AbbVie. Some of the authors declared receiving speaker’s fees, consulting fees, or research grants from various sources including AbbVie.
Source: Karadag O et al. Clin Rheumatol. 2021 Sep 3. doi: 10.1007/s10067-021-05893-3.
Key clinical point: Significant improvement in work productivity was observed in a real-world cohort of patients with psoriatic arthritis (PsA) treated with a tumor necrosis factor inhibitor (anti-TNF).
Major finding: At the final follow-up visit, the overall activity impairment decreased from 55.0 ± 21.5 to 16.3 ± 18.2 (P < .001). Moreover, absenteeism, mean presenteeism, and work productivity loss showed significant improvement upon treatment at the 9-month follow-up visit (all P < .001).
Study details: Findings are from a noninterventional, prospective, and observational cohort study including 120 patients with PsA who were receiving anti-TNF treatment.
Disclosures: This study was supported by AbbVie. Some of the authors declared receiving speaker’s fees, consulting fees, or research grants from various sources including AbbVie.
Source: Karadag O et al. Clin Rheumatol. 2021 Sep 3. doi: 10.1007/s10067-021-05893-3.
Key clinical point: Significant improvement in work productivity was observed in a real-world cohort of patients with psoriatic arthritis (PsA) treated with a tumor necrosis factor inhibitor (anti-TNF).
Major finding: At the final follow-up visit, the overall activity impairment decreased from 55.0 ± 21.5 to 16.3 ± 18.2 (P < .001). Moreover, absenteeism, mean presenteeism, and work productivity loss showed significant improvement upon treatment at the 9-month follow-up visit (all P < .001).
Study details: Findings are from a noninterventional, prospective, and observational cohort study including 120 patients with PsA who were receiving anti-TNF treatment.
Disclosures: This study was supported by AbbVie. Some of the authors declared receiving speaker’s fees, consulting fees, or research grants from various sources including AbbVie.
Source: Karadag O et al. Clin Rheumatol. 2021 Sep 3. doi: 10.1007/s10067-021-05893-3.
Obesity, an added misery in patients with PsA
Key clinical point: Obesity was associated with a higher disease activity and poorer quality of life (QoL) in patients with psoriatic arthritis (PsA), thus emphasizing the need to consider obesity during the management of patients with PsA.
Major finding: Patients with obesity had a significantly higher PsA QoL questionnaire and psychological status measured by the Hospital Anxiety and Depression Scale compared with nonobese patients (P < .001). Even the disease activity index for PsA score was higher in patients with obesity (P < .05), whereas Psoriasis Area and Severity Index was similar between both groups (P = .154).
Study details: Findings are from a cross-sectional study including 1,033 patients with PsA, of which 62.9% of patients were nonobese and 37.1% were obese.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Gok K et al. Rheumatol Int. 2021 Aug 28. doi: 10.1007/s00296-021-04971-8.
Key clinical point: Obesity was associated with a higher disease activity and poorer quality of life (QoL) in patients with psoriatic arthritis (PsA), thus emphasizing the need to consider obesity during the management of patients with PsA.
Major finding: Patients with obesity had a significantly higher PsA QoL questionnaire and psychological status measured by the Hospital Anxiety and Depression Scale compared with nonobese patients (P < .001). Even the disease activity index for PsA score was higher in patients with obesity (P < .05), whereas Psoriasis Area and Severity Index was similar between both groups (P = .154).
Study details: Findings are from a cross-sectional study including 1,033 patients with PsA, of which 62.9% of patients were nonobese and 37.1% were obese.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Gok K et al. Rheumatol Int. 2021 Aug 28. doi: 10.1007/s00296-021-04971-8.
Key clinical point: Obesity was associated with a higher disease activity and poorer quality of life (QoL) in patients with psoriatic arthritis (PsA), thus emphasizing the need to consider obesity during the management of patients with PsA.
Major finding: Patients with obesity had a significantly higher PsA QoL questionnaire and psychological status measured by the Hospital Anxiety and Depression Scale compared with nonobese patients (P < .001). Even the disease activity index for PsA score was higher in patients with obesity (P < .05), whereas Psoriasis Area and Severity Index was similar between both groups (P = .154).
Study details: Findings are from a cross-sectional study including 1,033 patients with PsA, of which 62.9% of patients were nonobese and 37.1% were obese.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Gok K et al. Rheumatol Int. 2021 Aug 28. doi: 10.1007/s00296-021-04971-8.
Depressive and endothelial dysfunction in PsA: Is there a link?
Key clinical point: Endothelial dysfunction (ED) was inversely correlated with the severity of depressive symptoms in patients with psoriatic arthritis (PsA).
Major finding: Overall, 40% of PsA patients experienced depressive symptoms according to the Hospital Anxiety and Depression Scale (HDS). ED as measured by flow-mediated dilatation was negatively correlated with HDS score (Pearson’s coefficient [ρ] −0.339; P = .016), intensity of pain, and disease activity in PsA score (both ρ, −0.507; P = .001).
Study details: Findings are from a cross-sectional study including 50 patients with PsA between 30 and 75 years of age and without any previous history of heart disease or diabetes.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: De Lorenzis E et al. Front Med. 2021 Aug 27. doi: 10.3389/fmed.2021.669397.
Key clinical point: Endothelial dysfunction (ED) was inversely correlated with the severity of depressive symptoms in patients with psoriatic arthritis (PsA).
Major finding: Overall, 40% of PsA patients experienced depressive symptoms according to the Hospital Anxiety and Depression Scale (HDS). ED as measured by flow-mediated dilatation was negatively correlated with HDS score (Pearson’s coefficient [ρ] −0.339; P = .016), intensity of pain, and disease activity in PsA score (both ρ, −0.507; P = .001).
Study details: Findings are from a cross-sectional study including 50 patients with PsA between 30 and 75 years of age and without any previous history of heart disease or diabetes.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: De Lorenzis E et al. Front Med. 2021 Aug 27. doi: 10.3389/fmed.2021.669397.
Key clinical point: Endothelial dysfunction (ED) was inversely correlated with the severity of depressive symptoms in patients with psoriatic arthritis (PsA).
Major finding: Overall, 40% of PsA patients experienced depressive symptoms according to the Hospital Anxiety and Depression Scale (HDS). ED as measured by flow-mediated dilatation was negatively correlated with HDS score (Pearson’s coefficient [ρ] −0.339; P = .016), intensity of pain, and disease activity in PsA score (both ρ, −0.507; P = .001).
Study details: Findings are from a cross-sectional study including 50 patients with PsA between 30 and 75 years of age and without any previous history of heart disease or diabetes.
Disclosures: No information on funding was available. The authors declared no conflicts of interest.
Source: De Lorenzis E et al. Front Med. 2021 Aug 27. doi: 10.3389/fmed.2021.669397.
Frequent treatment changes in PsA patients treated with DMARDs in routine clinical care
Key clinical point: Treatment modification was frequently observed in a cohort of patients with psoriatic arthritis (PsA) receiving disease-modifying antirheumatic drugs (DMARD), highlighting the need for more effective therapies.
Major finding: Overall, 57.3% of patients were treated with biologic DMARDs either as monotherapy or in combination with conventional synthetic DMARDs (csDMARD), whereas 37.7% and 4.4% of patients were treated with csDMARDs and targeted synthetic DMARDs, respectively. Treatment modifications in the previous year were reported by 48.4% of patients, with major reasons being lack of efficacy (38%) and remission or major improvement in the disease (14%).
Study details: Findings are from a retrospective observational cross-sectional study including 316 adults with established PsA and psoriasis who received DMARD treatment for at least 183 days in the previous year.
Disclosures: This work was funded by Bristol Myers Squibb, Germany. Some of the authors declared receiving speaker’s fees and compensation for consultancy or board memberships from Bristol Myers Squibb. Dr. Daamen and Dr. Rothnie declared being current or previous employees of Bristol Myers Squibb.
Source: Behrens F et al. Mod Rheumatol. 2021 Aug 26. doi: 10.1080/14397595.2020.1816597.
Key clinical point: Treatment modification was frequently observed in a cohort of patients with psoriatic arthritis (PsA) receiving disease-modifying antirheumatic drugs (DMARD), highlighting the need for more effective therapies.
Major finding: Overall, 57.3% of patients were treated with biologic DMARDs either as monotherapy or in combination with conventional synthetic DMARDs (csDMARD), whereas 37.7% and 4.4% of patients were treated with csDMARDs and targeted synthetic DMARDs, respectively. Treatment modifications in the previous year were reported by 48.4% of patients, with major reasons being lack of efficacy (38%) and remission or major improvement in the disease (14%).
Study details: Findings are from a retrospective observational cross-sectional study including 316 adults with established PsA and psoriasis who received DMARD treatment for at least 183 days in the previous year.
Disclosures: This work was funded by Bristol Myers Squibb, Germany. Some of the authors declared receiving speaker’s fees and compensation for consultancy or board memberships from Bristol Myers Squibb. Dr. Daamen and Dr. Rothnie declared being current or previous employees of Bristol Myers Squibb.
Source: Behrens F et al. Mod Rheumatol. 2021 Aug 26. doi: 10.1080/14397595.2020.1816597.
Key clinical point: Treatment modification was frequently observed in a cohort of patients with psoriatic arthritis (PsA) receiving disease-modifying antirheumatic drugs (DMARD), highlighting the need for more effective therapies.
Major finding: Overall, 57.3% of patients were treated with biologic DMARDs either as monotherapy or in combination with conventional synthetic DMARDs (csDMARD), whereas 37.7% and 4.4% of patients were treated with csDMARDs and targeted synthetic DMARDs, respectively. Treatment modifications in the previous year were reported by 48.4% of patients, with major reasons being lack of efficacy (38%) and remission or major improvement in the disease (14%).
Study details: Findings are from a retrospective observational cross-sectional study including 316 adults with established PsA and psoriasis who received DMARD treatment for at least 183 days in the previous year.
Disclosures: This work was funded by Bristol Myers Squibb, Germany. Some of the authors declared receiving speaker’s fees and compensation for consultancy or board memberships from Bristol Myers Squibb. Dr. Daamen and Dr. Rothnie declared being current or previous employees of Bristol Myers Squibb.
Source: Behrens F et al. Mod Rheumatol. 2021 Aug 26. doi: 10.1080/14397595.2020.1816597.
Upadacitinib in a 15 mg dose could achieve robust efficacy in PsA with limited adverse events
Key clinical point: Exposure-response analysis predicted 15 mg upadacitinib daily would achieve robust efficacy in patients with psoriatic arthritis (PsA) with a limited decrease in hemoglobin or occurrence of serious infections.
Major finding: The potential benefits of increasing upadacitinib plasma exposure beyond 15 mg daily were not consistent, with 8% and 7% higher percentage of patients predicted to achieve 50% and 70% improvement in American College of Rheumatology response levels, respectively, with 30 mg upadacitinib compared to 15 mg at week 12 but not at week 24. At week 24, the percentage of patients with serious infection was 2% for both upadacitinib doses, and the percentage of patients with hemoglobin decrease >2 g/dL was 3% and 4% for 15 mg and 30 mg upadacitinib, respectively.
Study details: Findings are from an analysis of two phase 3 studies, SELECT-PsA 1 and SELECT-PsA 2, including 1,916 patients with PsA with an inadequate response to biologic or nonbiologic disease-modifying antirheumatic drugs.
Disclosures: This work was funded by AbbVie. The authors declared being current/former employees of AbbVie and may hold stocks/stock options.
Source: Muensterman E et al. Clin Transl Sci. 2021 Aug 31. doi: 10.1111/cts.13146.
Key clinical point: Exposure-response analysis predicted 15 mg upadacitinib daily would achieve robust efficacy in patients with psoriatic arthritis (PsA) with a limited decrease in hemoglobin or occurrence of serious infections.
Major finding: The potential benefits of increasing upadacitinib plasma exposure beyond 15 mg daily were not consistent, with 8% and 7% higher percentage of patients predicted to achieve 50% and 70% improvement in American College of Rheumatology response levels, respectively, with 30 mg upadacitinib compared to 15 mg at week 12 but not at week 24. At week 24, the percentage of patients with serious infection was 2% for both upadacitinib doses, and the percentage of patients with hemoglobin decrease >2 g/dL was 3% and 4% for 15 mg and 30 mg upadacitinib, respectively.
Study details: Findings are from an analysis of two phase 3 studies, SELECT-PsA 1 and SELECT-PsA 2, including 1,916 patients with PsA with an inadequate response to biologic or nonbiologic disease-modifying antirheumatic drugs.
Disclosures: This work was funded by AbbVie. The authors declared being current/former employees of AbbVie and may hold stocks/stock options.
Source: Muensterman E et al. Clin Transl Sci. 2021 Aug 31. doi: 10.1111/cts.13146.
Key clinical point: Exposure-response analysis predicted 15 mg upadacitinib daily would achieve robust efficacy in patients with psoriatic arthritis (PsA) with a limited decrease in hemoglobin or occurrence of serious infections.
Major finding: The potential benefits of increasing upadacitinib plasma exposure beyond 15 mg daily were not consistent, with 8% and 7% higher percentage of patients predicted to achieve 50% and 70% improvement in American College of Rheumatology response levels, respectively, with 30 mg upadacitinib compared to 15 mg at week 12 but not at week 24. At week 24, the percentage of patients with serious infection was 2% for both upadacitinib doses, and the percentage of patients with hemoglobin decrease >2 g/dL was 3% and 4% for 15 mg and 30 mg upadacitinib, respectively.
Study details: Findings are from an analysis of two phase 3 studies, SELECT-PsA 1 and SELECT-PsA 2, including 1,916 patients with PsA with an inadequate response to biologic or nonbiologic disease-modifying antirheumatic drugs.
Disclosures: This work was funded by AbbVie. The authors declared being current/former employees of AbbVie and may hold stocks/stock options.
Source: Muensterman E et al. Clin Transl Sci. 2021 Aug 31. doi: 10.1111/cts.13146.
PsA: Golimumab effective under long-term real-life clinical setting
Key clinical point: Golimumab was effective for both musculoskeletal and cutaneous manifestations along with good drug persistence in patients with moderate-to-severe psoriatic arthritis (PsA) and concomitant psoriasis in a long-term real-life clinical setting.
Major finding: Disease activity in PsA score (P < .0001) and psoriasis activity and severity index score (P < .01) improved significantly after 6, 12, 24, 36, and 48 months of treatment. The retention rate of golimumab was 82.8%, 73.4%, 62.0%, and 54.4% at 6, 12, 24, and 48 months, respectively. The major reasons for drug discontinuation were primary/secondary inefficacy.
Study details: Findings are from a retrospective observational study including 105 patients with moderate-to-severe PsA and concomitant psoriasis with high disease activity and elevated prevalence of comorbidities and who started treatment with golimumab.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chimenti MS et al. Clin Rheumatol. 2021 Aug 19. doi: 10.1007/s10067-021-05874-6.
Key clinical point: Golimumab was effective for both musculoskeletal and cutaneous manifestations along with good drug persistence in patients with moderate-to-severe psoriatic arthritis (PsA) and concomitant psoriasis in a long-term real-life clinical setting.
Major finding: Disease activity in PsA score (P < .0001) and psoriasis activity and severity index score (P < .01) improved significantly after 6, 12, 24, 36, and 48 months of treatment. The retention rate of golimumab was 82.8%, 73.4%, 62.0%, and 54.4% at 6, 12, 24, and 48 months, respectively. The major reasons for drug discontinuation were primary/secondary inefficacy.
Study details: Findings are from a retrospective observational study including 105 patients with moderate-to-severe PsA and concomitant psoriasis with high disease activity and elevated prevalence of comorbidities and who started treatment with golimumab.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chimenti MS et al. Clin Rheumatol. 2021 Aug 19. doi: 10.1007/s10067-021-05874-6.
Key clinical point: Golimumab was effective for both musculoskeletal and cutaneous manifestations along with good drug persistence in patients with moderate-to-severe psoriatic arthritis (PsA) and concomitant psoriasis in a long-term real-life clinical setting.
Major finding: Disease activity in PsA score (P < .0001) and psoriasis activity and severity index score (P < .01) improved significantly after 6, 12, 24, 36, and 48 months of treatment. The retention rate of golimumab was 82.8%, 73.4%, 62.0%, and 54.4% at 6, 12, 24, and 48 months, respectively. The major reasons for drug discontinuation were primary/secondary inefficacy.
Study details: Findings are from a retrospective observational study including 105 patients with moderate-to-severe PsA and concomitant psoriasis with high disease activity and elevated prevalence of comorbidities and who started treatment with golimumab.
Disclosures: This study did not report any source of funding. The authors declared no conflicts of interest.
Source: Chimenti MS et al. Clin Rheumatol. 2021 Aug 19. doi: 10.1007/s10067-021-05874-6.
Rates of relevant counseling/education lower at dermatology vs. primary care PsA outpatient visits
Key clinical point: The chances of counseling or education for modifiable lifestyle risk factors were rare during psoriatic arthritis (PsA) or psoriasis outpatient visits, with rates being even lower among dermatologists compared to nondermatologists.
Major finding: Overall, low rates of counseling were observed for any modifiable lifestyle risk factor (11.1%; 95% CI 7.9%-15.3%), tobacco (4.8%; 95% CI 2.8%-8.0%), and obesity (2.8%; 95% CI 1.7%-4.5%). Moreover, counseling rates for any modifiable risk factor were lower for dermatologists compared to nondermatologists visits (0.9% vs. 22.6%; P < .001).
Study details: This study used the National Ambulatory Medical Care Survey (2002-2016) and the National Hospital Ambulatory Medical Care Survey (2002-2011) conducted in the United States to assess the frequency of education/counseling for modifiable risk factors in an estimated 41.8 million psoriasis or PsA outpatient visits.
Disclosures: Dr. Barbieri is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and receives partial support from Pfizer. The authors declared no conflicts of interest.
Source: Taylor MT et al. J Am Acad Dermatol. 2021 Aug 24. doi: 10.1016/j.jaad.2021.08.034.
Key clinical point: The chances of counseling or education for modifiable lifestyle risk factors were rare during psoriatic arthritis (PsA) or psoriasis outpatient visits, with rates being even lower among dermatologists compared to nondermatologists.
Major finding: Overall, low rates of counseling were observed for any modifiable lifestyle risk factor (11.1%; 95% CI 7.9%-15.3%), tobacco (4.8%; 95% CI 2.8%-8.0%), and obesity (2.8%; 95% CI 1.7%-4.5%). Moreover, counseling rates for any modifiable risk factor were lower for dermatologists compared to nondermatologists visits (0.9% vs. 22.6%; P < .001).
Study details: This study used the National Ambulatory Medical Care Survey (2002-2016) and the National Hospital Ambulatory Medical Care Survey (2002-2011) conducted in the United States to assess the frequency of education/counseling for modifiable risk factors in an estimated 41.8 million psoriasis or PsA outpatient visits.
Disclosures: Dr. Barbieri is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and receives partial support from Pfizer. The authors declared no conflicts of interest.
Source: Taylor MT et al. J Am Acad Dermatol. 2021 Aug 24. doi: 10.1016/j.jaad.2021.08.034.
Key clinical point: The chances of counseling or education for modifiable lifestyle risk factors were rare during psoriatic arthritis (PsA) or psoriasis outpatient visits, with rates being even lower among dermatologists compared to nondermatologists.
Major finding: Overall, low rates of counseling were observed for any modifiable lifestyle risk factor (11.1%; 95% CI 7.9%-15.3%), tobacco (4.8%; 95% CI 2.8%-8.0%), and obesity (2.8%; 95% CI 1.7%-4.5%). Moreover, counseling rates for any modifiable risk factor were lower for dermatologists compared to nondermatologists visits (0.9% vs. 22.6%; P < .001).
Study details: This study used the National Ambulatory Medical Care Survey (2002-2016) and the National Hospital Ambulatory Medical Care Survey (2002-2011) conducted in the United States to assess the frequency of education/counseling for modifiable risk factors in an estimated 41.8 million psoriasis or PsA outpatient visits.
Disclosures: Dr. Barbieri is supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health and receives partial support from Pfizer. The authors declared no conflicts of interest.
Source: Taylor MT et al. J Am Acad Dermatol. 2021 Aug 24. doi: 10.1016/j.jaad.2021.08.034.
Affected body surface area predicts risk of PsA in patients with psoriasis
Key clinical point: Patients with psoriasis with higher vs. lower affected body surface area (BSA) were at an increased risk of developing psoriatic arthritis (PsA).
Major finding: During a mean follow-up of 4.2 years, the incidence of PsA was 5.4 cases per 1,000 person years. Compared with BSA < 3%, BSA > 10% (hazard ratio [HR] 2.01; 95% CI 1.29-3.13) and BSA = 3%-10% (HR 1.44; 95% CI 1.02-2.03) were associated with incident PsA.
Study details: Findings are from a prospective, population-based cohort study including 9,056 patients with at least 1 code for psoriasis (mild to severe) and 90,547 matched general population controls.
Disclosures: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Some authors declared serving as consultant or co-patent holder or receiving grants, honoraria, or payments for medical education from several sources.
Source: Ogdie A et al. Rheumatology. 2021 Sep 11. doi: 10.1093/rheumatology/keab622.
Key clinical point: Patients with psoriasis with higher vs. lower affected body surface area (BSA) were at an increased risk of developing psoriatic arthritis (PsA).
Major finding: During a mean follow-up of 4.2 years, the incidence of PsA was 5.4 cases per 1,000 person years. Compared with BSA < 3%, BSA > 10% (hazard ratio [HR] 2.01; 95% CI 1.29-3.13) and BSA = 3%-10% (HR 1.44; 95% CI 1.02-2.03) were associated with incident PsA.
Study details: Findings are from a prospective, population-based cohort study including 9,056 patients with at least 1 code for psoriasis (mild to severe) and 90,547 matched general population controls.
Disclosures: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Some authors declared serving as consultant or co-patent holder or receiving grants, honoraria, or payments for medical education from several sources.
Source: Ogdie A et al. Rheumatology. 2021 Sep 11. doi: 10.1093/rheumatology/keab622.
Key clinical point: Patients with psoriasis with higher vs. lower affected body surface area (BSA) were at an increased risk of developing psoriatic arthritis (PsA).
Major finding: During a mean follow-up of 4.2 years, the incidence of PsA was 5.4 cases per 1,000 person years. Compared with BSA < 3%, BSA > 10% (hazard ratio [HR] 2.01; 95% CI 1.29-3.13) and BSA = 3%-10% (HR 1.44; 95% CI 1.02-2.03) were associated with incident PsA.
Study details: Findings are from a prospective, population-based cohort study including 9,056 patients with at least 1 code for psoriasis (mild to severe) and 90,547 matched general population controls.
Disclosures: This study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Heart, Lung, and Blood Institute of the National Institutes of Health. Some authors declared serving as consultant or co-patent holder or receiving grants, honoraria, or payments for medical education from several sources.
Source: Ogdie A et al. Rheumatology. 2021 Sep 11. doi: 10.1093/rheumatology/keab622.