User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
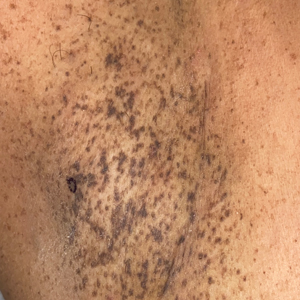
Progressive Axillary Hyperpigmentation
The Diagnosis: Dowling-Degos Disease
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.

Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.
The Diagnosis: Dowling-Degos Disease
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.

Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
The Diagnosis: Dowling-Degos Disease
Histopathology demonstrated elongation of the epidermal rete ridges with increased basal pigmentation, suprapapillary epithelial thinning, dermal melanophages, and a mild lymphocytic infiltrate (Figure). Given the clinical and histologic findings, a diagnosis of Dowling-Degos disease (DDD) was made. The patient was counseled on the increased risk for her children developing DDD. Treatment with the erbium:YAG (Er:YAG) laser subsequently was initiated.

Dowling-Degos disease (also known as reticulate pigmented anomaly of the flexures) is an uncommon autosomal-dominant condition characterized by reticular hyperpigmentation involving the flexural and intertriginous sites. Classic DDD commonly is caused by lossof-function mutations in the keratin 5 gene, KRT51; however, DDD also may result from loss-of-function mutations in the protein O-fucosyltransferase 1, POFUT1, and protein O-glucosyltransferase 1, POGLUT1, genes.2
Rare cases of DDD associated with hidradenitis suppurativa are caused by mutations in the presenilin enhancer protein 2 gene, PSENEN.3
Of note, a missense mutation in KRT5 is implicated in epidermolysis bullosa simplex with mottled pigmentation. Onset of DDD typically occurs during the third to fourth decades of life. Reticulated hyperpigmented macules initially occur in the axillae and groin and progressively increase over time to involve the neck, inframammary folds, trunk, and flexural surfaces of the arms and thighs. Patients additionally may present with pitted perioral scars, comedolike lesions on the back and neck, epidermoid cysts, and hidradenitis suppurativa. Keratoacanthoma and squamous cell carcinoma rarely have been reported in association with classic DDD.4,5
Dowling-Degos disease usually is asymptomatic, though pruritus seldom may occur in the affected flexural areas. Histologically, the epidermal rete ridges are elongated in a filiform or antlerlike pattern with increased pigmentation of the basal layer and thinning of the suprapapillary epithelium. Dermal melanosis and a mild perivascular lymphohistiocytic infiltrate also are present with no increase in the number of melanocytes.6,7 Galli-Galli disease is a variant of DDD that shares similar clinical and histologic features of DDD but is distinguished from DDD by suprabasilar nondyskeratotic acantholysis on histology.8
Regarding other differential diagnoses for our patient, acanthosis nigricans may be distinguished clinically by the presence of velvety and/or verrucous plaques, commonly in the neck folds and axillae. Histologically, acanthosis nigricans is distinct from DDD and involves hyperkeratosis, acanthosis, and epidermal papillomatosis. Our patient had no history of diabetes mellitus or insulin resistance. Granular parakeratosis presents with hyperpigmented hyperkeratotic papules and plaques classically confined to the axillary region; however, the involvement of other intertriginous areas may occur. Histologically, granular parakeratosis demonstrates compact parakeratosis with small bluish keratohyalin granules within the stratum corneum. Confluent and reticulated papillomatosis presents with red-brown keratotic papules that initially appear in the intermammary region and spread laterally forming a reticulated pattern. Histology is similar to acanthosis nigricans and demonstrates hyperkeratosis, acanthosis, and papillomatosis. Inverse psoriasis presents with symmetric and sharply demarcated, erythematous, nonscaly plaques in the intertriginous areas. The plaques of inverse psoriasis may be pruritic and/or sore and occasionally may become macerated. Inverse psoriasis shares similar histologic findings compared to classic plaque psoriasis but may have less confluent parakeratosis.
Treatment of DDD essentially is reserved for cosmetic reasons. Topical hydroquinone, tretinoin, and corticosteroids have been used with limited to no success.5,9 Beneficial results after treatment with the Er:YAG laser have been reported.10
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.
- Betz RC, Planko L, Eigelshoven S, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006;78:510-519.
- Basmanav FB, Oprisoreanu AM, Pasternack SM, et al. Mutations in POGLUT1, encoding protein O-glucosyltransferase 1, cause autosomaldominant Dowling-Degos disease. Am J Hum Genet. 2014;94:135-143.
- Pavlovsky M, Sarig O, Eskin-Schwartz M, et al. A phenotype combining hidradenitis suppurativa with Dowling-Degos disease caused by a founder mutation in PSENEN. Br J Dermatol. 2018;178:502-508.
- Ujihara M, Kamakura T, Ikeda M, et al. Dowling-Degos disease associated with squamous cell carcinomas on the dappled pigmentation. Br J Dermatol. 2002;147:568-571.
- Weber LA, Kantor GR, Bergfeld WF. Reticulate pigmented anomaly of the flexures (Dowling-Degos disease): a case report associated with hidradenitis suppurativa and squamous cell carcinoma. Cutis. 1990;45:446-450.
- Jones EW, Grice K. Reticulate pigmented anomaly of the flexures. Dowing Degos disease, a new genodermatosis. Arch Dermatol. 1978;114:1150-1157.
- Kim YC, Davis MD, Schanbacher CF, et al. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462-467.
- Reisenauer AK, Wordingham SV, York J, et al. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br J Dermatol. 2014;170:1362-1365.
- Oppolzer G, Schwarz T, Duschet P, et al. Dowling-Degos disease: unsuccessful therapeutic trial with retinoids [in German]. Hautarzt. 1987;38:615-618.
- Wenzel G, Petrow W, Tappe K, et al. Treatment of Dowling-Degos disease with Er:YAG-laser: results after 2.5 years. Dermatol Surg. 2003;29:1161-1162.

A 50-year-old Hispanic woman presented with asymptomatic, progressive, brown hyperpigmentation involving the axillae, neck, upper back, and inframammary areas of 5 years’ duration. She had no other notable medical history; family history was unremarkable. She had been treated with topical hydroquinone and tretinoin by an outside physician without improvement. Physical examination revealed reticulated hyperpigmented macules and patches involving the inverse regions of the neck, axillae, and inframammary regions. Additionally, acneform pitted scars involving the perioral region were seen. A 4.0-mm punch biopsy of the right axilla was performed.
Musical instruments can throw skin out of tune
Violin and viola players can pay a price for the music they create: Many suffer from skin irritation and inflammation where the instruments touch their necks and upper bodies.
“These skin conditions are disfiguring, and they also carry so much psychological burden. Not only are these patients under constant pressure to perform at their maximum at all times, it really is troublesome when there is a barrier between you and performing art that you absolutely love,” lead author Henry Lim, an osteopathic medical student at the University of North Texas Health Science Center at Fort Worth, said in an interview.
The results of the literature review were presented in a poster at the Inaugural Symposium for Inflammatory Skin Disease.
Mr. Lim, who has a special interest in skin, said his own musical experience inspired the research. “Throughout my experience as a violinist, I faced many dermatologic issues because of my violin, and it affected my performance,” he said. “As time went on, I recognized that many other stringed instrumentalists were dealing with similar issues but chose to live with it because it came with the territory.”
One physician told Mr. Lim that he needed to quit in order to permanently treat his skin problems. He didn’t accept this answer and instead launched the literature review with colleagues Marshall Hall, MPH, also an osteopathic medical student with an interest in dermatology, and Sajid Surve, DO, codirector of the UNT Texas Center for Performing Arts Health.
Mr. Lim and colleagues evaluated 23 articles, which included case studies and literature reviews, about dermatitis in violinists, violists, cellists, bassists, guitarists and harpists. “Stringed instrumentalists are the highest at-risk population compared to performers who play other types of instruments,” Mr. Lim said.
The poster he presented at the meeting largely focuses on fiddler’s neck, which he defined as “simply dermatitis related to friction and allergic irritation from playing violin or viola.” Many people, he noted, are allergic to nickel, and the bracket that secures the violin’s chin rest “most often contains nickel. Even a very small concentration of nickel can cause massive reactions, and we found that the C string of a viola – the thickest, lowest-sounding string – contains a nickel concentration of up to 37%.”
Gold-coated strings are an alternative option, he said, but they’re more expensive.
Stringed instrumentalists may also be allergic to rosin applied to “bow hairs,” which is the hair – typically from horses – that is used to string bows, also described in the poster. “We found that there is an overall common allergy to the main ingredient called colophony,” Mr. Lim said. The legendary violin maker Antonio Stradivari “was rumored to have used colophony and another irritating ingredient called propolis in the wood varnish of his instruments. Because he was such a great influence on the art of violin crafting, his technique is still used in the modern era, which may be another contributing factor to the allergic reactions seen in stringed instrumentalists.”
(In the poster, the authors refer to one of the articles in the review, which described a violin maker allergic to colophony and propolis, who was treated with cetirizine, mild corticosteroids, and avoidance.)
What should dermatologists know about skin conditions in these musicians? Mr. Hall, one of the coauthors of the report, suggested they invite the patients to play their instruments during a visit. “The musicians may not understand that they are doing certain things with their movements, but looking from a clinical lens, we are able to see how their biomechanics and posture [are] contributing to their dermatitis,” he said.
Dr. Surve, the other coauthor, also suggested speaking to the patient’s teacher, coach, or mentor. “Keeping that person in the loop regarding what you are seeing and recommending will go a long way towards helping your patient,” he said. “If the teacher doesn’t understand or agree with what you’re trying to accomplish, they may try to undermine your plan of care. But if they are on board, they become a valuable tool for facilitating and reinforcing it.”
As for treatments, avoidance of the instruments is the most effective, but is simply not feasible for many musicians. “Certain interventions like creating a barrier between the musician and the instrument can reduce the risk of contact dermatitis without compromising the quality [of playing] as much,” Mr. Hall said. The poster reported that a handkerchief was used for this purpose in one case attributed to nickel sulfate in a 16-year-old .
Purchasing more expensive instrument materials to prevent reactions is another option, he said, and players can also purchase stands. But musicians may be resistant to any treatment that changes how the instruments sound or forces them to adjust the way they do things, he cautioned.
No funding for the study or author disclosures were reported.
Violin and viola players can pay a price for the music they create: Many suffer from skin irritation and inflammation where the instruments touch their necks and upper bodies.
“These skin conditions are disfiguring, and they also carry so much psychological burden. Not only are these patients under constant pressure to perform at their maximum at all times, it really is troublesome when there is a barrier between you and performing art that you absolutely love,” lead author Henry Lim, an osteopathic medical student at the University of North Texas Health Science Center at Fort Worth, said in an interview.
The results of the literature review were presented in a poster at the Inaugural Symposium for Inflammatory Skin Disease.
Mr. Lim, who has a special interest in skin, said his own musical experience inspired the research. “Throughout my experience as a violinist, I faced many dermatologic issues because of my violin, and it affected my performance,” he said. “As time went on, I recognized that many other stringed instrumentalists were dealing with similar issues but chose to live with it because it came with the territory.”
One physician told Mr. Lim that he needed to quit in order to permanently treat his skin problems. He didn’t accept this answer and instead launched the literature review with colleagues Marshall Hall, MPH, also an osteopathic medical student with an interest in dermatology, and Sajid Surve, DO, codirector of the UNT Texas Center for Performing Arts Health.
Mr. Lim and colleagues evaluated 23 articles, which included case studies and literature reviews, about dermatitis in violinists, violists, cellists, bassists, guitarists and harpists. “Stringed instrumentalists are the highest at-risk population compared to performers who play other types of instruments,” Mr. Lim said.
The poster he presented at the meeting largely focuses on fiddler’s neck, which he defined as “simply dermatitis related to friction and allergic irritation from playing violin or viola.” Many people, he noted, are allergic to nickel, and the bracket that secures the violin’s chin rest “most often contains nickel. Even a very small concentration of nickel can cause massive reactions, and we found that the C string of a viola – the thickest, lowest-sounding string – contains a nickel concentration of up to 37%.”
Gold-coated strings are an alternative option, he said, but they’re more expensive.
Stringed instrumentalists may also be allergic to rosin applied to “bow hairs,” which is the hair – typically from horses – that is used to string bows, also described in the poster. “We found that there is an overall common allergy to the main ingredient called colophony,” Mr. Lim said. The legendary violin maker Antonio Stradivari “was rumored to have used colophony and another irritating ingredient called propolis in the wood varnish of his instruments. Because he was such a great influence on the art of violin crafting, his technique is still used in the modern era, which may be another contributing factor to the allergic reactions seen in stringed instrumentalists.”
(In the poster, the authors refer to one of the articles in the review, which described a violin maker allergic to colophony and propolis, who was treated with cetirizine, mild corticosteroids, and avoidance.)
What should dermatologists know about skin conditions in these musicians? Mr. Hall, one of the coauthors of the report, suggested they invite the patients to play their instruments during a visit. “The musicians may not understand that they are doing certain things with their movements, but looking from a clinical lens, we are able to see how their biomechanics and posture [are] contributing to their dermatitis,” he said.
Dr. Surve, the other coauthor, also suggested speaking to the patient’s teacher, coach, or mentor. “Keeping that person in the loop regarding what you are seeing and recommending will go a long way towards helping your patient,” he said. “If the teacher doesn’t understand or agree with what you’re trying to accomplish, they may try to undermine your plan of care. But if they are on board, they become a valuable tool for facilitating and reinforcing it.”
As for treatments, avoidance of the instruments is the most effective, but is simply not feasible for many musicians. “Certain interventions like creating a barrier between the musician and the instrument can reduce the risk of contact dermatitis without compromising the quality [of playing] as much,” Mr. Hall said. The poster reported that a handkerchief was used for this purpose in one case attributed to nickel sulfate in a 16-year-old .
Purchasing more expensive instrument materials to prevent reactions is another option, he said, and players can also purchase stands. But musicians may be resistant to any treatment that changes how the instruments sound or forces them to adjust the way they do things, he cautioned.
No funding for the study or author disclosures were reported.
Violin and viola players can pay a price for the music they create: Many suffer from skin irritation and inflammation where the instruments touch their necks and upper bodies.
“These skin conditions are disfiguring, and they also carry so much psychological burden. Not only are these patients under constant pressure to perform at their maximum at all times, it really is troublesome when there is a barrier between you and performing art that you absolutely love,” lead author Henry Lim, an osteopathic medical student at the University of North Texas Health Science Center at Fort Worth, said in an interview.
The results of the literature review were presented in a poster at the Inaugural Symposium for Inflammatory Skin Disease.
Mr. Lim, who has a special interest in skin, said his own musical experience inspired the research. “Throughout my experience as a violinist, I faced many dermatologic issues because of my violin, and it affected my performance,” he said. “As time went on, I recognized that many other stringed instrumentalists were dealing with similar issues but chose to live with it because it came with the territory.”
One physician told Mr. Lim that he needed to quit in order to permanently treat his skin problems. He didn’t accept this answer and instead launched the literature review with colleagues Marshall Hall, MPH, also an osteopathic medical student with an interest in dermatology, and Sajid Surve, DO, codirector of the UNT Texas Center for Performing Arts Health.
Mr. Lim and colleagues evaluated 23 articles, which included case studies and literature reviews, about dermatitis in violinists, violists, cellists, bassists, guitarists and harpists. “Stringed instrumentalists are the highest at-risk population compared to performers who play other types of instruments,” Mr. Lim said.
The poster he presented at the meeting largely focuses on fiddler’s neck, which he defined as “simply dermatitis related to friction and allergic irritation from playing violin or viola.” Many people, he noted, are allergic to nickel, and the bracket that secures the violin’s chin rest “most often contains nickel. Even a very small concentration of nickel can cause massive reactions, and we found that the C string of a viola – the thickest, lowest-sounding string – contains a nickel concentration of up to 37%.”
Gold-coated strings are an alternative option, he said, but they’re more expensive.
Stringed instrumentalists may also be allergic to rosin applied to “bow hairs,” which is the hair – typically from horses – that is used to string bows, also described in the poster. “We found that there is an overall common allergy to the main ingredient called colophony,” Mr. Lim said. The legendary violin maker Antonio Stradivari “was rumored to have used colophony and another irritating ingredient called propolis in the wood varnish of his instruments. Because he was such a great influence on the art of violin crafting, his technique is still used in the modern era, which may be another contributing factor to the allergic reactions seen in stringed instrumentalists.”
(In the poster, the authors refer to one of the articles in the review, which described a violin maker allergic to colophony and propolis, who was treated with cetirizine, mild corticosteroids, and avoidance.)
What should dermatologists know about skin conditions in these musicians? Mr. Hall, one of the coauthors of the report, suggested they invite the patients to play their instruments during a visit. “The musicians may not understand that they are doing certain things with their movements, but looking from a clinical lens, we are able to see how their biomechanics and posture [are] contributing to their dermatitis,” he said.
Dr. Surve, the other coauthor, also suggested speaking to the patient’s teacher, coach, or mentor. “Keeping that person in the loop regarding what you are seeing and recommending will go a long way towards helping your patient,” he said. “If the teacher doesn’t understand or agree with what you’re trying to accomplish, they may try to undermine your plan of care. But if they are on board, they become a valuable tool for facilitating and reinforcing it.”
As for treatments, avoidance of the instruments is the most effective, but is simply not feasible for many musicians. “Certain interventions like creating a barrier between the musician and the instrument can reduce the risk of contact dermatitis without compromising the quality [of playing] as much,” Mr. Hall said. The poster reported that a handkerchief was used for this purpose in one case attributed to nickel sulfate in a 16-year-old .
Purchasing more expensive instrument materials to prevent reactions is another option, he said, and players can also purchase stands. But musicians may be resistant to any treatment that changes how the instruments sound or forces them to adjust the way they do things, he cautioned.
No funding for the study or author disclosures were reported.
FROM SISD 2021
When can your hypochondria help patients?
Hypochondria has been useful to my patients. I mean my own hypochondria. It may not take one to know one, but we hypochondriacs understand each other.
Hypochondriacs worry that we are sick, worry that our fears are foolish and that we will be mocked for worrying about nothing, and worry even more that this time, we finally worried about something after all. Reassurance leaves us sheepish, then elated. Elation soon fades, and a new worry appears. Worry, rinse, repeat. . Some folks are just needy. Soothing the needs of the needy can feel like trying to drain the seven seas with a teaspoon. Those who work with people must either find ways to cope with the spectrum of neediness, or find another kind of work to do.
Some patient needs call for diagnosis and treatment. Other needs go beyond the strictly medical. Beyond knowing whether they are ill, patients have questions like, “Will this get worse?” “Will I be ashamed to go out in public?” “Can I visit my grandchildren, or will my daughter-in-law throw me out as contagious?” “Is this the beginning of the end?” or, worst of all, “Am I losing my hair?”
The list of possible patient needs is long, though not endless. Lining them up one after the other can make them sound melodramatic, even silly. (Other people’s worries often sound silly; your own never do.) Can a small growth or slight itch really cause existential agitation? Anyone who deals with complaints like these knows that the answer is yes.
Hypochondriacs with medical degrees cannot reassure themselves, but we can bring useful experience to help other members of the worry club. Doing so means paying attention not just to what doctors worry about but what patients do.
Sometimes a patient is terrified, the doctor not at all. Gentle sympathy may be enough. But the reverse can also be true: The doctor is concerned, but the patient thinks there is no problem. Sometimes I am worried enough to ask a patient to call or email an update. Patients who have already stopped worrying may not bother to answer the phone or shoot back an email. Failure to respond may mean they are fine, or in intensive care. Silence is hard to interpret.
Skin doctors have one advantageous disadvantage: Few tests help us beyond a skin scraping, the odd blood test, or a biopsy. Otherwise, most of the time all we can do is look, and perhaps apply “tincture of time,” watching the clinical course. We cannot send patients for the complex and expensive tests our colleagues use “just to be sure,” because we have no such tests to send them for.
Practice and experience help us recognize needs and worries that patients might not express. For instance, a man may show up with pimples on his back. His concerns seem intense. “What worries you?” we ask. The patient whispers, “It couldn’t be ... shingles, could it?” No, it couldn’t be shingles, because it is bilateral and for many other reasons.
The question is not whether he has shingles but why he thinks he does. Maybe his aunt suggested it. Or an article told him to watch out for it. Or his pharmacy is promoting zoster vaccination by showing huge, full-color photos of shingles cases worthy of horror movies. (Shingles the 13th!) Because he wants to visit his grandkids and his daughter is in her fourth month of pregnancy. In other words, along with the fear of cancer, fear of shingles is just out there. There are other such public concerns. Over time, we come to recognize them.
Anyone can worry, but anxiety paralyzes some to such an extent that referral to a mental health professional seems reasonable. The problem with advising it is that patients who somaticize may take exception to suggestions, however delicately put, that make us sound dismissive, locating their concern “all in the head.” Over the years, my attempts to make such referrals have met with limited success.
Dealing with needs – and neediness – can take up more of a doctor’s day than making specific diagnoses and prescribing helpful treatments. Besides, addressing needs and neediness demands skills not always stressed at school.
Practice at noting neediness makes you better at it, but no doctor nails the true wellsprings of worry all the time. We hypochondriacs can be devilishly inventive.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His latest book, “Doctoring from the Outside In,” was recently published. Write to him at [email protected].
Hypochondria has been useful to my patients. I mean my own hypochondria. It may not take one to know one, but we hypochondriacs understand each other.
Hypochondriacs worry that we are sick, worry that our fears are foolish and that we will be mocked for worrying about nothing, and worry even more that this time, we finally worried about something after all. Reassurance leaves us sheepish, then elated. Elation soon fades, and a new worry appears. Worry, rinse, repeat. . Some folks are just needy. Soothing the needs of the needy can feel like trying to drain the seven seas with a teaspoon. Those who work with people must either find ways to cope with the spectrum of neediness, or find another kind of work to do.
Some patient needs call for diagnosis and treatment. Other needs go beyond the strictly medical. Beyond knowing whether they are ill, patients have questions like, “Will this get worse?” “Will I be ashamed to go out in public?” “Can I visit my grandchildren, or will my daughter-in-law throw me out as contagious?” “Is this the beginning of the end?” or, worst of all, “Am I losing my hair?”
The list of possible patient needs is long, though not endless. Lining them up one after the other can make them sound melodramatic, even silly. (Other people’s worries often sound silly; your own never do.) Can a small growth or slight itch really cause existential agitation? Anyone who deals with complaints like these knows that the answer is yes.
Hypochondriacs with medical degrees cannot reassure themselves, but we can bring useful experience to help other members of the worry club. Doing so means paying attention not just to what doctors worry about but what patients do.
Sometimes a patient is terrified, the doctor not at all. Gentle sympathy may be enough. But the reverse can also be true: The doctor is concerned, but the patient thinks there is no problem. Sometimes I am worried enough to ask a patient to call or email an update. Patients who have already stopped worrying may not bother to answer the phone or shoot back an email. Failure to respond may mean they are fine, or in intensive care. Silence is hard to interpret.
Skin doctors have one advantageous disadvantage: Few tests help us beyond a skin scraping, the odd blood test, or a biopsy. Otherwise, most of the time all we can do is look, and perhaps apply “tincture of time,” watching the clinical course. We cannot send patients for the complex and expensive tests our colleagues use “just to be sure,” because we have no such tests to send them for.
Practice and experience help us recognize needs and worries that patients might not express. For instance, a man may show up with pimples on his back. His concerns seem intense. “What worries you?” we ask. The patient whispers, “It couldn’t be ... shingles, could it?” No, it couldn’t be shingles, because it is bilateral and for many other reasons.
The question is not whether he has shingles but why he thinks he does. Maybe his aunt suggested it. Or an article told him to watch out for it. Or his pharmacy is promoting zoster vaccination by showing huge, full-color photos of shingles cases worthy of horror movies. (Shingles the 13th!) Because he wants to visit his grandkids and his daughter is in her fourth month of pregnancy. In other words, along with the fear of cancer, fear of shingles is just out there. There are other such public concerns. Over time, we come to recognize them.
Anyone can worry, but anxiety paralyzes some to such an extent that referral to a mental health professional seems reasonable. The problem with advising it is that patients who somaticize may take exception to suggestions, however delicately put, that make us sound dismissive, locating their concern “all in the head.” Over the years, my attempts to make such referrals have met with limited success.
Dealing with needs – and neediness – can take up more of a doctor’s day than making specific diagnoses and prescribing helpful treatments. Besides, addressing needs and neediness demands skills not always stressed at school.
Practice at noting neediness makes you better at it, but no doctor nails the true wellsprings of worry all the time. We hypochondriacs can be devilishly inventive.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His latest book, “Doctoring from the Outside In,” was recently published. Write to him at [email protected].
Hypochondria has been useful to my patients. I mean my own hypochondria. It may not take one to know one, but we hypochondriacs understand each other.
Hypochondriacs worry that we are sick, worry that our fears are foolish and that we will be mocked for worrying about nothing, and worry even more that this time, we finally worried about something after all. Reassurance leaves us sheepish, then elated. Elation soon fades, and a new worry appears. Worry, rinse, repeat. . Some folks are just needy. Soothing the needs of the needy can feel like trying to drain the seven seas with a teaspoon. Those who work with people must either find ways to cope with the spectrum of neediness, or find another kind of work to do.
Some patient needs call for diagnosis and treatment. Other needs go beyond the strictly medical. Beyond knowing whether they are ill, patients have questions like, “Will this get worse?” “Will I be ashamed to go out in public?” “Can I visit my grandchildren, or will my daughter-in-law throw me out as contagious?” “Is this the beginning of the end?” or, worst of all, “Am I losing my hair?”
The list of possible patient needs is long, though not endless. Lining them up one after the other can make them sound melodramatic, even silly. (Other people’s worries often sound silly; your own never do.) Can a small growth or slight itch really cause existential agitation? Anyone who deals with complaints like these knows that the answer is yes.
Hypochondriacs with medical degrees cannot reassure themselves, but we can bring useful experience to help other members of the worry club. Doing so means paying attention not just to what doctors worry about but what patients do.
Sometimes a patient is terrified, the doctor not at all. Gentle sympathy may be enough. But the reverse can also be true: The doctor is concerned, but the patient thinks there is no problem. Sometimes I am worried enough to ask a patient to call or email an update. Patients who have already stopped worrying may not bother to answer the phone or shoot back an email. Failure to respond may mean they are fine, or in intensive care. Silence is hard to interpret.
Skin doctors have one advantageous disadvantage: Few tests help us beyond a skin scraping, the odd blood test, or a biopsy. Otherwise, most of the time all we can do is look, and perhaps apply “tincture of time,” watching the clinical course. We cannot send patients for the complex and expensive tests our colleagues use “just to be sure,” because we have no such tests to send them for.
Practice and experience help us recognize needs and worries that patients might not express. For instance, a man may show up with pimples on his back. His concerns seem intense. “What worries you?” we ask. The patient whispers, “It couldn’t be ... shingles, could it?” No, it couldn’t be shingles, because it is bilateral and for many other reasons.
The question is not whether he has shingles but why he thinks he does. Maybe his aunt suggested it. Or an article told him to watch out for it. Or his pharmacy is promoting zoster vaccination by showing huge, full-color photos of shingles cases worthy of horror movies. (Shingles the 13th!) Because he wants to visit his grandkids and his daughter is in her fourth month of pregnancy. In other words, along with the fear of cancer, fear of shingles is just out there. There are other such public concerns. Over time, we come to recognize them.
Anyone can worry, but anxiety paralyzes some to such an extent that referral to a mental health professional seems reasonable. The problem with advising it is that patients who somaticize may take exception to suggestions, however delicately put, that make us sound dismissive, locating their concern “all in the head.” Over the years, my attempts to make such referrals have met with limited success.
Dealing with needs – and neediness – can take up more of a doctor’s day than making specific diagnoses and prescribing helpful treatments. Besides, addressing needs and neediness demands skills not always stressed at school.
Practice at noting neediness makes you better at it, but no doctor nails the true wellsprings of worry all the time. We hypochondriacs can be devilishly inventive.
Dr. Rockoff, who wrote the Dermatology News column “Under My Skin,” is now semiretired, after 40 years of practice in Brookline, Mass. He served on the clinical faculty at Tufts University, Boston, and taught senior medical students and other trainees for 30 years. His latest book, “Doctoring from the Outside In,” was recently published. Write to him at [email protected].
What’s my number? Do I really need $10 million to retire from my medical practice?
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
“What’s my number?” When I hear this from my financial planning clients, I know they mean: In my 20-year career, this “magic number” is by far the most common thing physicians want to know.
If you look online, articles may recommend having a portfolio valued at $2 million, $5 million, and not uncommonly $10 million or more to retire. Really? $10 million? You might be thinking that surely not everyone needs that amount. Luckily, that’s true.
There’s no magic number your portfolio should be – just your number.
It’s human nature to want a simple, clear target to shoot for. But unfortunately, there’s no generic answer when it comes to saving for retirement. Even after a comprehensive hour-long review of a client’s financial plan – including insurance, investments, estate planning, and other items – the most honest answer I can give is: “It depends.” Not satisfying, I know. But there are still too many holes to fill.
By far the most important factor in getting beyond “it depends” is having an accurate estimate of annual retirement expenses. I have clients who live comfortably on $50,000 a year in retirement and others who need $250,000 or more. Knowing how much you need – your personal number – depends on the individual’s unique dream for retirement and calculating what that dream will cost.
Form a guesstimate based on savings and anticipated expenses
The total portfolio value needed to sustain an annual expense of $50,000 a year in retirement spending versus the portfolio size needed for $250,000 or more, blows apart the fiction of a universal “magic number.” It’s just not that simple. While it’s hard to gauge exactly what you will need, the right information can lead to a logical guesstimate about what size portfolio will provide you with financial independence.
In the end, it’s up to you to determine your desired retirement lifestyle. Then, the only way to get there is to calculate how much it will cost and save up for it by following a well-informed financial plan. This plan will be based on strategy that shifts from the middle to the later stages of your medical career and into retirement.
Let’s see how it works.
Early to mid-career: Focus on building up retirement savings
We ultimately want to save enough to meet our retirement expenses. But figuring out how much to save when you’re in your 40s and 50s is difficult. A mid-career physician likely has significant family- and child-related expenses. When we become empty-nesters, those expenses will decline. In retirement they may disappear entirely, but new expenses may arise.
With large variations in expenses at different life stages, it’s hard to calculate exactly how much you will need to save. Early on, the most sensible thing is putting aside a “reasonable” percentage of gross income for retirement savings.
What is a ‘reasonable’ savings goal for retirement?
As is often the case with high-income earners, many of our clients don’t have a budget or a clear picture of their current expenses and spending habits. That’s alright as long as they are building up a reasonable nest egg for the future – which begs the question of what is reasonable.
For mid-career docs, a reasonable goal to aim for is putting aside 20% of gross income for retirement. What you spend the rest of your money on is less important than how much you’re saving.
This is quite different from how you’ll handle expenses during retirement, when you no longer have a steady stream of income; rather, you have a pot of money that needs to last you another 20, 30, or even 40 years. At that point, thinking about specific expenses becomes more important (more on this topic later). That said, if you’re a mid-career doctor who is not meeting this 20% savings goal, it’s time to make a plan that will free up cash for retirement savings and investments.
Later-career docs: Calculate your spending level in retirement
Financial success means having a portfolio that can support your retirement dreams – with the confidence that your money will last and you won’t need to watch every dollar you spend. As you near retirement, your focus will shift away from accumulating savings to calculating the annual expenses you will have to meet in retirement.
A good place to start is figuring out which expenses will be necessary and which will be more flexible. To do this, separate your anticipated spending into these two categories:
- Fixed expenses: You can confidently forecast your “must-have” fixed expenses – such as property taxes, property/casualty insurance, health care costs, utilities, and groceries – because they remain steady from month to month.
- Discretionary expenses: These “like-to-have” expenses vary from month to month. This makes them harder to predict but easier to control. They might include dining out, travel, and charitable contributions.
As a retiree, understanding your fixed and discretionary expenses can help you prepare for a bear market, when the stock market can decline by 20% or more. Your portfolio won’t consist entirely of stocks, so it shouldn’t drop to that degree. Still, it will decline significantly. You may need to cut back on spending for a year or 2 to allow your portfolio to recover, particularly if the portfolio declines early in retirement.
Are you ready for retirement?
During the long bull market preceding the great recession of 2007 and 2009, many physicians retired –only to return to their practices when their portfolio values plummeted. In the exuberance of the moment, many failed to heed the warnings of many economists and got caught flat-footed.
Right now it’s a bull market, but we’re seeing concerning signs, such as an out-of-control housing market and rumblings about inflation and rising consumer costs. Sound familiar? If you hope to retire soon, take the time to objectively look around the corner so you can plan appropriately – whether your goal is to retire completely, stay in practice part-time, or even take on a new opportunity.
In an “it-depends” world, don’t be lured by a fictitious magic number, no matter what comes up when you Google: “When can I retire?” Instead, save early, imagine your dream retirement, and calculate expenses later to see what’s possible.
Dr. Greenwald is a graduate of the Albert Einstein College of Medicine, New York. Dr. Greenwald completed his internal medicine residency at the University of Minnesota, Minneapolis. He practiced internal medicine in the Twin Cities for 11 years before making the transition to financial planning for physicians, beginning in 1998.
A version of this article first appeared on Medscape.com.
Antimicrobial resistance threat continues during COVID-19
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.
The stark realities of antimicrobial resistance – including rising rates of difficult-to-treat infections, lack of a robust pipeline of future antimicrobials, and COVID-19 treatments that leave people more vulnerable to infections – remain urgent priorities, experts say.
For some patients, the pandemic and antimicrobial resistance (AMR) are intertwined.
“One patient I’m seeing now in service really underscores how the two interact,” Vance Fowler, MD, said during a June 30 media briefing sponsored by the Infectious Diseases Society of America (IDSA). A man in his mid-40s, married with a small child, developed COVID-19 in early January 2021. He was intubated, spent about 1 month in the ICU, and managed to survive.
“But since then he has been struck with a series of progressively more drug resistant bacteria,” said Dr. Fowler, professor of medicine at Duke University, Durham, N.C., and chair of the IDSA Antimicrobial Resistance Committee.
The patient acquired Pseudomonas ventilator-associated pneumonia. Although the infection initially responded to standard antibiotics, he has experienced relapses over the past few months. Through these multiple infections the Pseudomonas grew increasingly pan-resistant to treatment.
The only remaining antimicrobial agent for this patient, Dr. Fowler said, is “a case study in what we are describing ... a drug that is used relatively infrequently, that is fairly expensive, but for that particular patient is absolutely vital.”
A ‘terrifying’ personal experience
Tori Kinamon, a Duke University medical student and Food and Drug Administration antibacterial drug resistance fellow, joined Dr. Fowler at the IDSA briefing. She shared her personal journey of surviving a methicillin-resistant Staphylococcus aureus (MRSA) infection, one that sparked her interest in becoming a physician.
“I had a very frightening and unexpected confrontation with antimicrobial resistance when I was a freshman in college,” Ms. Kinamon said.
A few days after competing in a Division One gymnastics championship, she felt a gradual onset of pain in her left hamstring. The pain grew acutely worse and, within days, her leg become red, swollen, and painful to the touch.
Ms. Kinamon was admitted to the hospital for suspected cellulitis and put on intravenous antibiotics.
“However, my clinical condition continued to decline,” she recalled. “Imaging studies revealed a 15-cm abscess deep in my hamstring.”
The limb- and life-threatening infection left her wondering if she would come out of surgery with both legs.
“Ultimately, I had eight surgeries in 2 weeks,” she said.
“As a 19-year-old collegiate athlete, that’s terrifying. And I never imagined that something like that would happen to me – until it did,” said Ms. Kinamon, who is an NCAA infection prevention advocate.
When Ms. Kinamon’s kidneys could no longer tolerate vancomycin, she was switched to daptomycin.
“I reflect quite frequently on how having that one extra drug in the stockpile had a significant impact on my outcome,” she said.
Incentivizing new antimicrobial agents
A lack of new antimicrobials in development is not a new story.
“There’s been a chill that’s been sustained on the antibiotic development field. Most large pharmaceutical companies have left the area of anti-infectants and the bulk of research and development is now in small pharmaceutical companies,” Dr. Fowler said. “And they’re struggling.”
One potential solution is the Pasteur Act, a bipartisan bill reintroduced in Congress and supported by IDSA. The bill encourages pharmaceutical companies to develop new antimicrobial agents with funding not linked to sales or use of the drugs.
Furthermore, the bill emphasizes appropriate use of these agents through effective stewardship programs.
Although some institutions shifted resources away from AMR out of necessity when COVID-19 struck, “I can say certainly from our experience at Duke that at least stewardship was alive and well. It was not relegated to the side,” Dr. Fowler said.
“In fact,” he added, “if anything, COVID really emphasized the importance of stewardship” by helping clinicians with guidance on the use of remdesivir and other antivirals during the pandemic.
Also, in some instances, treatments used to keep people with COVID-19 alive can paradoxically place them at higher risk for other infections, Dr. Fowler said, citing corticosteroids as an example.
Everyone’s concern
AMR isn’t just an issue in hospital settings, either. Ms. Kinamon reiterated that she picked up the infection in an athletic environment.
“Antimicrobial resistance is not just a problem for ICU patients in the hospital. I was the healthiest I had ever been and just very nearly escaped death due to one of these infections,” she said. ”As rates of resistance rise as these pathogens become more virulent, AMR is becoming more and more of a community threat,” she added.
Furthermore, consumers are partially to blame as well, Dr. Fowler noted.
“It’s interesting when you look at the surveys of the numbers of patients that have used someone else’s antibiotics” or leftover antimicrobial agents from a prior infection.
“It’s really startling ... that’s the sort of antibiotic overuse that directly contributes to antibacterial resistance,” he said.
Reasons for optimism
Promising advances in diagnostics, treatment, and prevention of AMRs are underway, Dr. Fowler said.
“It always gets me really excited to talk about it. It’s amazing what technology and scientific discovery can bring to this discussion and to this threat,” he said.
For example, there is a “silent revolution” in diagnostics with the aim to rapidly provide life-saving actionable data on a real patient in nearly real time.
Traditionally, “you start off by treating what should be there” while awaiting results of tests to narrow down therapy, Dr. Fowler said. However, a whole host of new platforms are in development to reduce the time to susceptibility results. This kind of technology has “the potential to transform our ability to take care of patients, giving them the right drug at the right time and no more,” he said.
Another promising avenue of research involves bacteriophages. Dr. Fowler is principal investigator on a clinical trial underway to evaluate bacteriophages as adjunct therapy for MRSA bacteremia.
When it comes to prevention on AMR infections in the future, “I continue to be optimistic about the possibility of vaccines to prevent many of these infections,” Dr. Fowler said, adding that companies are working on vaccines against these kinds of infections caused by MRSA or Escherichia coli, for example.
Patient outcomes
The man in his 40s with the multidrug resistant Pseudomonas infections “is now to the point where he’s walking in the halls and I think he’ll get out of the hospital eventually,” Dr. Fowler said.
“But his life is forever changed,” he added.
Ms. Kinamon’s recovery from MRSA included time in the ICU, 1 month in a regular hospital setting, and 5 months at home.
“It sparked my interest in antibiotic research and development because I see myself as a direct beneficiary of the stockpile of antibiotics that were available to treat my infection,” Ms. Kinamon said. “Now as a medical student working with patients who have similar infections, I feel a deep empathy and connectedness to them because they ask the same questions that I did.”
A version of this article first appeared on WebMD.com.
How well do JAK inhibitors work for atopic dermatitis?
largely because of the heterogeneous nature of the disease.
“Atopic dermatitis patients have different complaints,” Jacob P. Thyssen, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Some of them have repeated infections. Some have psychiatric symptoms. Others have widespread eczema. When you talk about how well they work, it really depends on what aspects of AD, what subgroups of AD, and how well they work with comorbidities of AD.”
Baricitinib, a JAK1/JAK2 inhibitor in 2-mg and 4-mg tablets, is available in the European Union, and is under Food and Drug Administration review for AD in the United States. Two JAK1 inhibitors continue to be evaluated in AD clinical trials and are also under FDA review for AD: abrocitinib (100 mg and 200 mg) and upadacitinib (15 mg and 30 mg). None of these agents have been tested in head-to-head trials and only one (abrocitinib) has been compared with the interleukin-4 receptor–alpha antagonist dupilumab, which makes meaningful direct comparisons impossible. (Baricitinib and upadacitinib are approved for treating RA in the United States.)
In his informal assessment from clinical trial data of how these three JAK inhibitors compare with the biologic agents dupilumab and tralokinumab, with potency as an indication, Dr. Thyssen, professor of dermatology at the University of Copenhagen, observed that abrocitinib and dupilumab “are somewhere in the middle,” tralokinumab and baricitinib are “slightly weaker,” while upadacitinib is “very potent.” (Dupilumab is approved by the FDA for treating AD ages 6 and older, and tralokinumab, a fully human monoclonal antibody that binds to IL-13, is under FDA review for AD.)
However, he cautioned that making direct comparisons of these drugs is limited by differences in clinical trial designs, trial length, severity of disease at baseline, and demographics. “Placebo effects also differ between trials, and the speed of onset is different between JAK inhibitors and biologic agents. Because of this, efficacy can be difficult to assess over 12-16 weeks. That’s why long-term studies are necessary.”
It’s also tricky to compare safety signals with baricitinib, abrocitinib, and upadacitinib, “because some of them are JAK1 inhibitors; others are JAK1/JAK2 inhibitors,” he continued. “Even the molecules that inhibit JAK1 are different, so making a comparison between abrocitinib and upadacitinib requires studies that do this is in the best way and over a long period of time.”
Safety signals
Common safety signals in this drug class include nasopharyngitis, nausea, and headache. “Many of these are short lasting, meaning that patients will perhaps have a headache for a day or two and then it will be over,” said Dr. Thyssen, who is also a consultant dermatologist at Bispebjerg Hospital in Copenhagen. “This means that even though we see high proportions of safety signals, this is probably not going to limit the use of JAK inhibitors in most of our patients. Then we have an acne signal in higher proportions for abrocitinib and upadacitinib than for baricitinib, so perhaps this is related to the potency.”
There is also an increased risk for infections, including herpes zoster. “Is this a class effect?” he asked. “We see quite a bit for baricitinib, particularly when it’s used for rheumatoid arthritis. We also see it in AD patients, but we don’t know to what degree yet. We need the real-world evidence before we can make any conclusions.” Routine blood monitoring tests are also required in patients taking JAK inhibitors, because of the risk for leukopenia and effects on liver enzymes.
Then there’s the risk of deep vein thrombosis/pulmonary embolism. “This is mostly linked to baricitinib use, but is this a class effect or is it specific to baricitinib?” he asked. “We’ll have to wait and see, but I think overall, this is not something I have great fear of because we see that AD patients are young, usually with a normal [body mass index], at least in Europe. But we have to study this closely.”
From a clinical standpoint, JAK1/2 inhibitors work well on every measurable aspect of AD, he said, including eczema severity, itch, skin pain, sleep, and quality of life. “Based on conference abstracts and publications, they seem to work equally well independent of race, BMI, atopy status, age, and whether their AD is extrinsic or intrinsic,” Dr. Thyssen added. “One thing we haven’t learned from the companies is, what patients have the highest likelihood of getting a good treatment response? We don’t have good biomarkers yet, but anything the companies can do to help us identify the patients with the greatest chance of success would be so welcome.”
The best available data suggest that JAK inhibitors benefit AD patients with certain comorbidities, including inflammatory bowel disease (with upadacitinib), RA (with both baricitinib and upadacitinib), and alopecia areata (with baricitinib). “These drugs also have been shown to work well for the psychiatric symptoms of disease,” he said.
“As for patients with type 2 inflammation in the airways such as asthma and rhinitis, dupilumab works, but do the JAK inhibitors work? It’s possible from a mode of action standpoint, but we don’t know.” It also remains unclear how JAK inhibitors will fare in the treatment of chronic hand eczema and ocular surface disease, like allergic conjunctivitis, he said.
Despite the unknowns, Dr. Thyssen emphasized the promise that JAK inhibitors hold for AD patients. “We know they provide good AD control,” he said. “For some, like baricitinib, you may need to instruct the patient to use topical corticosteroids as well, but this does not seem to be necessary for upadacitinib and abrocitinib. You really have a single bullet here that will take away most of the problems for many patients, with very fast onset of action, which is important for our patients.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
largely because of the heterogeneous nature of the disease.
“Atopic dermatitis patients have different complaints,” Jacob P. Thyssen, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Some of them have repeated infections. Some have psychiatric symptoms. Others have widespread eczema. When you talk about how well they work, it really depends on what aspects of AD, what subgroups of AD, and how well they work with comorbidities of AD.”
Baricitinib, a JAK1/JAK2 inhibitor in 2-mg and 4-mg tablets, is available in the European Union, and is under Food and Drug Administration review for AD in the United States. Two JAK1 inhibitors continue to be evaluated in AD clinical trials and are also under FDA review for AD: abrocitinib (100 mg and 200 mg) and upadacitinib (15 mg and 30 mg). None of these agents have been tested in head-to-head trials and only one (abrocitinib) has been compared with the interleukin-4 receptor–alpha antagonist dupilumab, which makes meaningful direct comparisons impossible. (Baricitinib and upadacitinib are approved for treating RA in the United States.)
In his informal assessment from clinical trial data of how these three JAK inhibitors compare with the biologic agents dupilumab and tralokinumab, with potency as an indication, Dr. Thyssen, professor of dermatology at the University of Copenhagen, observed that abrocitinib and dupilumab “are somewhere in the middle,” tralokinumab and baricitinib are “slightly weaker,” while upadacitinib is “very potent.” (Dupilumab is approved by the FDA for treating AD ages 6 and older, and tralokinumab, a fully human monoclonal antibody that binds to IL-13, is under FDA review for AD.)
However, he cautioned that making direct comparisons of these drugs is limited by differences in clinical trial designs, trial length, severity of disease at baseline, and demographics. “Placebo effects also differ between trials, and the speed of onset is different between JAK inhibitors and biologic agents. Because of this, efficacy can be difficult to assess over 12-16 weeks. That’s why long-term studies are necessary.”
It’s also tricky to compare safety signals with baricitinib, abrocitinib, and upadacitinib, “because some of them are JAK1 inhibitors; others are JAK1/JAK2 inhibitors,” he continued. “Even the molecules that inhibit JAK1 are different, so making a comparison between abrocitinib and upadacitinib requires studies that do this is in the best way and over a long period of time.”
Safety signals
Common safety signals in this drug class include nasopharyngitis, nausea, and headache. “Many of these are short lasting, meaning that patients will perhaps have a headache for a day or two and then it will be over,” said Dr. Thyssen, who is also a consultant dermatologist at Bispebjerg Hospital in Copenhagen. “This means that even though we see high proportions of safety signals, this is probably not going to limit the use of JAK inhibitors in most of our patients. Then we have an acne signal in higher proportions for abrocitinib and upadacitinib than for baricitinib, so perhaps this is related to the potency.”
There is also an increased risk for infections, including herpes zoster. “Is this a class effect?” he asked. “We see quite a bit for baricitinib, particularly when it’s used for rheumatoid arthritis. We also see it in AD patients, but we don’t know to what degree yet. We need the real-world evidence before we can make any conclusions.” Routine blood monitoring tests are also required in patients taking JAK inhibitors, because of the risk for leukopenia and effects on liver enzymes.
Then there’s the risk of deep vein thrombosis/pulmonary embolism. “This is mostly linked to baricitinib use, but is this a class effect or is it specific to baricitinib?” he asked. “We’ll have to wait and see, but I think overall, this is not something I have great fear of because we see that AD patients are young, usually with a normal [body mass index], at least in Europe. But we have to study this closely.”
From a clinical standpoint, JAK1/2 inhibitors work well on every measurable aspect of AD, he said, including eczema severity, itch, skin pain, sleep, and quality of life. “Based on conference abstracts and publications, they seem to work equally well independent of race, BMI, atopy status, age, and whether their AD is extrinsic or intrinsic,” Dr. Thyssen added. “One thing we haven’t learned from the companies is, what patients have the highest likelihood of getting a good treatment response? We don’t have good biomarkers yet, but anything the companies can do to help us identify the patients with the greatest chance of success would be so welcome.”
The best available data suggest that JAK inhibitors benefit AD patients with certain comorbidities, including inflammatory bowel disease (with upadacitinib), RA (with both baricitinib and upadacitinib), and alopecia areata (with baricitinib). “These drugs also have been shown to work well for the psychiatric symptoms of disease,” he said.
“As for patients with type 2 inflammation in the airways such as asthma and rhinitis, dupilumab works, but do the JAK inhibitors work? It’s possible from a mode of action standpoint, but we don’t know.” It also remains unclear how JAK inhibitors will fare in the treatment of chronic hand eczema and ocular surface disease, like allergic conjunctivitis, he said.
Despite the unknowns, Dr. Thyssen emphasized the promise that JAK inhibitors hold for AD patients. “We know they provide good AD control,” he said. “For some, like baricitinib, you may need to instruct the patient to use topical corticosteroids as well, but this does not seem to be necessary for upadacitinib and abrocitinib. You really have a single bullet here that will take away most of the problems for many patients, with very fast onset of action, which is important for our patients.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
largely because of the heterogeneous nature of the disease.
“Atopic dermatitis patients have different complaints,” Jacob P. Thyssen, MD, PhD, said during the Revolutionizing Atopic Dermatitis symposium. “Some of them have repeated infections. Some have psychiatric symptoms. Others have widespread eczema. When you talk about how well they work, it really depends on what aspects of AD, what subgroups of AD, and how well they work with comorbidities of AD.”
Baricitinib, a JAK1/JAK2 inhibitor in 2-mg and 4-mg tablets, is available in the European Union, and is under Food and Drug Administration review for AD in the United States. Two JAK1 inhibitors continue to be evaluated in AD clinical trials and are also under FDA review for AD: abrocitinib (100 mg and 200 mg) and upadacitinib (15 mg and 30 mg). None of these agents have been tested in head-to-head trials and only one (abrocitinib) has been compared with the interleukin-4 receptor–alpha antagonist dupilumab, which makes meaningful direct comparisons impossible. (Baricitinib and upadacitinib are approved for treating RA in the United States.)
In his informal assessment from clinical trial data of how these three JAK inhibitors compare with the biologic agents dupilumab and tralokinumab, with potency as an indication, Dr. Thyssen, professor of dermatology at the University of Copenhagen, observed that abrocitinib and dupilumab “are somewhere in the middle,” tralokinumab and baricitinib are “slightly weaker,” while upadacitinib is “very potent.” (Dupilumab is approved by the FDA for treating AD ages 6 and older, and tralokinumab, a fully human monoclonal antibody that binds to IL-13, is under FDA review for AD.)
However, he cautioned that making direct comparisons of these drugs is limited by differences in clinical trial designs, trial length, severity of disease at baseline, and demographics. “Placebo effects also differ between trials, and the speed of onset is different between JAK inhibitors and biologic agents. Because of this, efficacy can be difficult to assess over 12-16 weeks. That’s why long-term studies are necessary.”
It’s also tricky to compare safety signals with baricitinib, abrocitinib, and upadacitinib, “because some of them are JAK1 inhibitors; others are JAK1/JAK2 inhibitors,” he continued. “Even the molecules that inhibit JAK1 are different, so making a comparison between abrocitinib and upadacitinib requires studies that do this is in the best way and over a long period of time.”
Safety signals
Common safety signals in this drug class include nasopharyngitis, nausea, and headache. “Many of these are short lasting, meaning that patients will perhaps have a headache for a day or two and then it will be over,” said Dr. Thyssen, who is also a consultant dermatologist at Bispebjerg Hospital in Copenhagen. “This means that even though we see high proportions of safety signals, this is probably not going to limit the use of JAK inhibitors in most of our patients. Then we have an acne signal in higher proportions for abrocitinib and upadacitinib than for baricitinib, so perhaps this is related to the potency.”
There is also an increased risk for infections, including herpes zoster. “Is this a class effect?” he asked. “We see quite a bit for baricitinib, particularly when it’s used for rheumatoid arthritis. We also see it in AD patients, but we don’t know to what degree yet. We need the real-world evidence before we can make any conclusions.” Routine blood monitoring tests are also required in patients taking JAK inhibitors, because of the risk for leukopenia and effects on liver enzymes.
Then there’s the risk of deep vein thrombosis/pulmonary embolism. “This is mostly linked to baricitinib use, but is this a class effect or is it specific to baricitinib?” he asked. “We’ll have to wait and see, but I think overall, this is not something I have great fear of because we see that AD patients are young, usually with a normal [body mass index], at least in Europe. But we have to study this closely.”
From a clinical standpoint, JAK1/2 inhibitors work well on every measurable aspect of AD, he said, including eczema severity, itch, skin pain, sleep, and quality of life. “Based on conference abstracts and publications, they seem to work equally well independent of race, BMI, atopy status, age, and whether their AD is extrinsic or intrinsic,” Dr. Thyssen added. “One thing we haven’t learned from the companies is, what patients have the highest likelihood of getting a good treatment response? We don’t have good biomarkers yet, but anything the companies can do to help us identify the patients with the greatest chance of success would be so welcome.”
The best available data suggest that JAK inhibitors benefit AD patients with certain comorbidities, including inflammatory bowel disease (with upadacitinib), RA (with both baricitinib and upadacitinib), and alopecia areata (with baricitinib). “These drugs also have been shown to work well for the psychiatric symptoms of disease,” he said.
“As for patients with type 2 inflammation in the airways such as asthma and rhinitis, dupilumab works, but do the JAK inhibitors work? It’s possible from a mode of action standpoint, but we don’t know.” It also remains unclear how JAK inhibitors will fare in the treatment of chronic hand eczema and ocular surface disease, like allergic conjunctivitis, he said.
Despite the unknowns, Dr. Thyssen emphasized the promise that JAK inhibitors hold for AD patients. “We know they provide good AD control,” he said. “For some, like baricitinib, you may need to instruct the patient to use topical corticosteroids as well, but this does not seem to be necessary for upadacitinib and abrocitinib. You really have a single bullet here that will take away most of the problems for many patients, with very fast onset of action, which is important for our patients.”
Dr. Thyssen disclosed that he is a speaker, advisory board member, and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
FROM REVOLUTIONIZING AD 2021
Indoor tanning ICD-10 codes may be underused, study finds
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
FROM SID 2021
Female doctors of color say they feel pressure to change their look
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
It started when a Latina doctor tweeted that she lost points on a practical exam in medical school because of her hoop earrings, with the evaluator writing “earrings, unprofessional.”
That led other female doctors to cite their own experiences, reported The Lily, a Washington Post publication aimed at millennial women. Many women posted photos of themselves wearing hoops, which have long been associated with Latina and African American women, the outlet said.
“There’s a big movement to police women of color and how they present themselves in medical spaces,” said Briana Christophers, an MD-PhD student at the Tri-Institutional MD-PhD Program in New York. “I think in part it’s a way of trying to make people who don’t usually fit the mold, fit the mold.”
Ms. Christophers, who identifies as Latina, said she was urged to wear a black or navy suit when interviewing for doctorate programs. She wore a black suit with a lavender blouse and received comments about that – some positive, some not, she said.
“Sometimes you don’t know how to interpret those sorts of comments,” Ms. Christophers said. “Do you remember because you like the shirt, or because you don’t think I should have done that?”
Doctors of color still stand out in American medicine. The Lily cited the Association of American Medical Colleges as saying that in 2018, Hispanics made up 5.8% of active American doctors and African Americans made up 5%.
Studies show that medical professionals of color often don’t receive the same respect as their White counterparts, with some people questioning whether they’re actually doctors.
“At work, wearing my white coat that has my name pretty big on it with a badge that says doctor on it, I still get asked if I’m the environmental services staff,” Alexandra Sims, MD, a pediatrician in Cincinnati, told The Lily. “I think it just demonstrates how deeply ingrained bias, racism, and sexism are in society and that we have a lot of work to do to disrupt that.”
Dr. Sims said the tweet about hoop earrings led her to wonder about daily decisions she makes about dress.
“Am I too much? Is this too much? Is this earring too big? Is this nail polish color too loud? And how will that be received at work?” she said, noting that she may opt not to wear hoops in certain situations, such as when she’s dealing with a grabby baby.
Monica Verduzco-Gutierrez, MD, professor and chair of the department of rehabilitation medicine at University of Texas Health, San Antonio, said doctors should be judged on the care they provide, not their appearance.
“Judging someone based on their earrings or their jumpsuit or whatever else that they’re noticing about the student is not an appropriate way to judge the student’s ability to take care of a patient,” Dr. Verduzco-Gutierrez said, noting that she was not speaking on behalf of the school.
A version of this article was first published on WebMD.com .
New analysis puts U.S. psoriasis prevalence at 3%
, according to an analysis of national survey data from 2011 to 2014.
“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
, according to an analysis of national survey data from 2011 to 2014.

“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
, according to an analysis of national survey data from 2011 to 2014.

“The adult prevalence rate of 3.0% continues to place psoriasis as one of the most common immune-mediated diseases affecting adults” in the United States, April W. Armstrong, MD, MPH, and associates said in a report published in JAMA Dermatology. At that rate, approximately 7,560,000 Americans aged 20 years or older have psoriasis.
That overall rate among adults aged 20 years and older, based on data from the 2011-2012 and 2013-2014 cycles of the National Health and Nutrition Examination Survey (NHANES), did not change significantly when compared with the 2003-2004 NHANES, when it was 3.15% among those aged 20-59, said Dr. Armstrong, professor of dermatology, University of Southern California, Los Angeles, and associates.
For the 2011-2014 period, psoriasis prevalence was similar between women (3.2%) and men (2.8%) but was significantly associated with older age and White/non-White status. Those aged 50-59 years had the highest prevalence of any age group at 4.3% and those aged 70 and older had a rate of 3.9%, while those aged 20-29 were the lowest at 1.6%, the investigators reported.
The prevalence in non-Hispanic Whites in the United States was 3.6% over the study period, and their odds ratio for having psoriasis was 1.92, compared with non-White individuals. Asian respondents had a prevalence of 2.5%, with the Hispanic population at 1.9%, non-Hispanic Black respondents at 1.5%, and those identifying as other (including multiracial persons) at 3.1%, they said.
The NHANES sample consisted of 12,638 people who had participated in the question that asked if they had ever been diagnosed with psoriasis by a physician or other health care professional, of whom 12,625 gave a definitive yes or no answer, the investigators noted.
A much smaller number, 329, also answered a question about the severity of their disease: Fifty-six percent had little or no psoriasis, almost 22% reported 1-2 palms of involvement, 16% had 3-10 palms of involvement, and 5.5% said the coverage was more than 10 palms. Since the survey did not distinguish between treated and untreated patients, however, some “of those reporting low body surface area involvement may be receiving treatments that are controlling their otherwise more extensive disease,” they wrote.
Dr. Armstrong and another investigator said that they have received grants, personal fees, and honoraria from a number of pharmaceutical companies; two other investigators are employees of the National Psoriasis Foundation.
FROM JAMA DERMATOLOGY
New details of myocarditis linked to COVID vaccines
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.
Further details from multiple cases of myocarditis linked to the Pfizer and Moderna mRNA COVID vaccines have been described in recent papers in the medical literature.
The cases appear to occur almost exclusively in males and most often in younger age groups. While symptoms and signs of myocarditis mostly resolved with a few days of supportive care, long-term effects are unknown at present.
The authors of all the reports and of two accompanying editorials in JAMA Cardiology are unanimous in their opinion that the benefits of vaccination still outweigh the risks.
The Centers for Disease Control and Prevention’s but committee members delivered a strong endorsement for continuing to vaccinate young people with the mRNA vaccines.
The current case reports are published in two papers in JAMA Cardiology and in three in Circulation.
U.S. military reports 23 cases
In one report in JAMA Cardiology, authors led by Jay Montgomery, MD, from Walter Reed National Military Medical Center in Bethesda, Md., described 23 cases from the U.S. Military Health System of individuals with acute myocarditis who presented within 4 days after mRNA-based COVID-19 vaccination (7 Pfizer and 16 Moderna).
All patients were male, 22 of 23 were on active duty, and the median age was 25 years (range, 20-51); 20 of the 23 cases occurred after receipt of a second dose of an mRNA COVID-19 vaccine.
The patients all presented with acute onset of marked chest pain. All patients had significantly elevated cardiac troponin levels. Among eight patients who underwent cardiac MRI (cMRI), all had findings consistent with the clinical diagnosis of myocarditis.
Additional testing did not identify other possible causes of myocarditis. All patients received brief supportive care and were recovered or recovering.
The authors reported that the military administered more than 2.8 million doses of mRNA COVID-19 vaccine in this period, and while the observed number of myocarditis cases was small, the number was “substantially higher” than expected among male military members after a second vaccine dose.
They noted that, based on historical data, among the 544,000 second doses to military members there may have been 0-10 expected myocarditis cases, but they observed 19 cases.
“All patients in this series reflect substantial similarities in demographic characteristics, proximate vaccine dose, onset interval, and character of vaccine-associated myocarditis. The consistent pattern of clinical presentation, rapid recovery, and absence of evidence of other causes support the diagnosis of hypersensitivity myocarditis,” they stated.
They added that presentation after a second vaccine dose or, in three patients, when vaccination followed SARS-CoV-2 infection, suggests that prior exposure was relevant in the hypersensitivity response.
“The spectrum of clinical presentation and reliance on patients seeking health care and on health care professionals recognizing a rare vaccine-associated adverse event limits determination of the true incidence of this condition,” the authors wrote.
They stressed that recognition of vaccine-associated myocarditis is clinically important because diagnosis impacts management, recommendations for exercise, and monitoring for cardiomyopathy.
But the authors also acknowledged that it is important to frame concerns about potential vaccine-associated myocarditis within the context of the current pandemic.
“Infection with SARS-CoV-2 is a clear cause of serious cardiac injury in many patients. ... Prevalence of cardiac injury may be as high as 60% in seriously ill patients. Notably, nearly 1% of highly fit athletes with mild COVID-19 infection have evidence of myocarditis on cMRI,” they wrote.
“Given that COVID-19 vaccines are remarkably effective at preventing infection, any risk of rare adverse events following immunization must be carefully weighed against the very substantial benefit of vaccination,” they concluded.
Four cases at Duke
In the second paper in JAMA Cardiology, a group led by Han W. Kim, MD, reported four patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination (two Pfizer and two Moderna) in patients treated at Duke University Medical Center, Durham, N.C. The hospital courses of the four patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2-4 days.
The authors said that, although a causal relationship cannot be established, none of the patients had a viral prodrome or had coincident testing that revealed an alternative explanation.
They stated that these four patients represent the majority of patients with acute myocarditis identified in the past 3 months at their institution, and this led to the highest total number of patients with acute myocarditis, compared with the same 3-month period for the past 5 years.
“Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination,” they wrote.
Further case reports
In one of the papers in Circulation, a group led by Kathryn F. Larson, MD, from the Mayo Clinic in Rochester, Minn., described eight patients hospitalized with chest pain who were diagnosed with myocarditis within 2-4 days of receiving either the Pfizer or Moderna vaccine.
Two of the patients had previously been infected by SARS-CoV-2 without need for hospitalization. All individuals were otherwise healthy males between the ages of 21 and 56 years. All but one patient developed symptoms after their second dose, and the one patient who developed myocarditis after the first vaccine dose had previously been infected with SARS-CoV-2.
Systemic symptoms began within 24 hours after vaccine administration in five of eight patients, with chest pain presenting between 48 and 96 hours later. Troponin values were elevated in all individuals and appeared to peak the day after admission, whereas none had eosinophilia.
Cardiac MRI revealed findings consistent with myocarditis in all patients. All patients had resolution of their chest pain and were discharged from the hospital in stable condition.
“The patients presented here demonstrated typical signs, symptoms, and diagnostic features of acute myocarditis. The temporal association between receiving an mRNA-based COVID-19 vaccine and the development of myocarditis is notable,” the authors said.
They added that they would consider the use of corticosteroids in these patients but cautioned that this could reduce the specific immune response against SARS-COV-2 triggered by the vaccine. “Thus, the duration of corticosteroid administration should be limited to the resolution of the symptoms or ventricular arrhythmias or the recovery of the left ventricular ejection fraction.”
Pending publication of long-term outcome data after SARS-CoV-2 vaccine–related myocarditis, they suggest adherence to the current consensus recommendation to abstain from competitive sports for a period of 3-6 months with reevaluation prior to sports participation.
In another of the Circulation papers, a group led by Carolyn M. Rosner, MSN, presented a case series of seven patients hospitalized for acute myocarditis-like illness following COVID-19 vaccination, from two U.S. medical centers, in Falls Church, Va., and Dallas. All patients were males below the age of 40 years and of White or Hispanic race/ethnicity. Only one patient reported prior history of COVID-19 infection. Six patients received mRNA (Moderna or Pfizer) and one received the adenovirus (Johnson & Johnson) vaccine. All patients presented 3-7 days post vaccination with acute onset chest pain and biochemical evidence of myocardial injury.
Hospital length of stay was 3 days, and all patients’ symptoms resolved by hospital discharge.
And finally, the third paper in Circulation reported a detailed description of one patient – a 52-year-old, previously healthy male who presented with acute myocarditis 3 days after the administration of the second dose of Moderna’s COVID-19 vaccine. The symptoms resolved, and there was a gradual improvement in cMRI findings. Ischemic injury and other potential causes of acute myocardial injury were excluded, as were other potential infectious causes of myocarditis, and there was no evidence of systemic autoimmune disease.
“Clinicians should be aware that myocarditis may be present in patients exhibiting cardiac signs and symptoms 2-4 days after COVID-19 vaccination,” the authors said.
They added that additional surveillance of such adverse events post–COVID-19 vaccination will help identify subgroups at higher risk for this vaccine-related effect, and whether additional precautions are necessary.
‘Benefits outweigh risk’
In an accompanying editorial in JAMA Cardiology, three doctors from the CDC cite several other reports of myocarditis after mRNA COVID vaccination. These include a case report published in Pediatrics of seven male adolescents aged 14-19 years who presented with myocarditis or myopericarditis within 4 days after receipt of a second dose of the Pfizer vaccine.
But the editorialists noted that the most comprehensive data about the risk for myocarditis following immunization with mRNA vaccines comes from Israel.
The Israeli Ministry of Health recently posted data describing 121 myocarditis cases occurring within 30 days of a second dose of mRNA vaccine among 5,049,424 persons, suggesting a crude incidence rate of approximately 24 cases per million.
On the current case reports, the CDC doctors wrote: “The striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization.”
They said that acute onset of chest pain 3-5 days after vaccine administration, usually after a second dose, is a typical feature of reported cases and suggests an immune-mediated mechanism.
But SARS-CoV-2 infection also causes cardiac injury which may result in severe outcomes, and based on currently available data, myocarditis following immunization with current mRNA-based vaccines is rare.
“At present, the benefits of immunization in preventing severe morbidity favors continued COVID-19 vaccination, particularly considering the increasing COVID-19 hospitalization rates among adolescents reported during spring 2021,” the editorialists stated.
But they added that many questions remain. These include whether modifications are needed to the vaccine schedule among persons with a history of possible or confirmed myocarditis after COVID vaccine, how should postvaccine myocarditis be managed, how often should follow-up assessments be performed, how might follow-up assessments affect recommendations to avoid vigorous physical activity following the diagnosis of myocarditis, and do all likely cases of acute myocarditis that appear to be uncomplicated require cardiac MRI for more definitive diagnosis?
“While the data needed to answer such questions are being collected, there is an opportunity for researchers with expertise in myocarditis to develop a comprehensive, national assessment of the natural history, pathogenesis, and treatment of acute myocarditis associated with receipt of mRNA-based COVID-19 vaccines,” they concluded.
In a second editorial in JAMA Cardiology, a group of editors from the journal acknowledged that publication of the current case reports may contribute to additional public concern regarding immunization. But they added that clinicians discussing immunization with patients should recognize that these case series suggest that the symptomatic events consistent with myocarditis are still very rare and appear to be self-limiting.
“Given the risks of COVID-19, including the risk of myocarditis from COVID-19 infection, the editors do not believe these case reports are sufficient to interrupt the march toward maximal vaccination against SARS-CoV-2 as expeditiously as possible,” they said.
A version of this article first appeared on Medscape.com.