User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Expert shares practical considerations when prescribing dupilumab
.
This scenario was illustrated in a 2020 retrospective study of 179 adults with AD who were cared for at the University of Pittsburgh Medical Center, which found that 37% did not start dupilumab, mainly due to insurance denial (19%) and high copay (11%).
“We’ve all seen this in our practice,” Amy S. Paller, MD, said during the Revolutionizing Atopic Dermatitis symposium. “We’ve also seen the denials until we get step therapy in there, so if I have a child whom I want to treat with dupilumab for safety reasons, I don’t like being told that I’m going to have to use cyclosporine or methotrexate or a medication that I think may have higher risks and certainly [would] require blood monitoring–yet that’s the state for some patients.”
Dupilumab, an interleukin-4 receptor alpha antagonist, is approved for treatment of moderate to severe AD in patients ages 6 and older.
When working to obtain insurance approval of dupilumab, Dr. Paller reminded dermatologists to document that the patient has moderate to severe AD “and document the negative effect on quality of life in order to try to help make it easier to get these medications for our patients.”
Starting patients on dupilumab
Dr. Paller, the Walter J. Hamlin Chair and Professor of Dermatology at Northwestern University, Chicago, said that if patients are on another systemic medication prior to starting dupilumab, she allows a transition period of 1-2 months. “Don’t just stop that drug because it’s ‘not working,’ ” she said. “I usually do a full dose for the first month, and a half dose for the next month before starting dupilumab. Also, don’t stop the use of topical corticosteroids. They can increase treatment response by 10%-20%, even when patients are on dupilumab.”
She recommends a 3- to 4-month trial of dupilumab while monitoring changes in disease severity, itch, and quality of life. “Usually there’s evidence of early improvement by 2 months in those who are going to do well enough to stay on the drug by about 4 months out,” she said. “In my experience, most pediatric patients do very well. In those with an inadequate response, about 50% will do better if you can increase the dose or frequency. Flares can still occur in those who do well. I usually push topicals when that happens.”
If patients respond well after starting dupilumab, Dr. Paller recommends that they continue on the drug for at least a year before considering a taper with the hope of “resetting” the immune system and having sustained improvement off drug. “Some parents and patients don’t want to stop the drug,” but for those who do, she tells them that she does not want to abruptly stop treatment, but to “space out the dosing” instead. “If someone is pretty much clear with the medication and is able to continue with topicals as you dial down, that’s great. But don’t even think about taking them off if somebody’s not clear or virtually clear, particularly if they start to flare with lower frequency.”
Data on effectiveness
Real-world data suggest that the effectiveness of dupilumab is similar to the efficacy seen in clinical trials. For example, a recently published systematic review and meta-analysis of 3,303 AD patients on dupilumab found that after 16 weeks of therapy, 60% achieved a 75% improvement in the Eczema Area and Severity (EASI75) score, and 27% achieved an EASI90. In a Dutch study of 210 adults treated with dupilumab for 52 weeks, enrolled in a Dutch registry, the mean percent reduction in EASI score was 70% at 16 weeks and 76.6% by 52 weeks.
In addition, there was at least a 4-point improvement in the Patient-Oriented Eczema Measure (POEM) score and at least a 4-point improvement in the Itch Numeric Rating Scale (NRS), said Dr. Paller, who was not involved in the study. “These patient-reported improvements were seen very early on,” she noted.
What about drug survival at 1 year? In a retrospective cohort study that drew from insurance databases, 1,963 adults given dupilumab were studied for a mean of 315 days. The rate of persistence was 92% at 6 months and 77% at 12 months. “That means that it’s still effective,” Dr. Paller said.
While that is a short period of time, she compared these results with long-term survival of nonsteroid systemic immunosuppressants such as cyclosporine, referring to a study of adults with AD treated with systemic immunosuppressants, which found “a 32% persistence rate at 12 months in drugs that require more monitoring, so more burden.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for dupilumab (Dupixent) manufacturers Regeneron and Sanofi, AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, and RAPT Therapeutics.
.
This scenario was illustrated in a 2020 retrospective study of 179 adults with AD who were cared for at the University of Pittsburgh Medical Center, which found that 37% did not start dupilumab, mainly due to insurance denial (19%) and high copay (11%).
“We’ve all seen this in our practice,” Amy S. Paller, MD, said during the Revolutionizing Atopic Dermatitis symposium. “We’ve also seen the denials until we get step therapy in there, so if I have a child whom I want to treat with dupilumab for safety reasons, I don’t like being told that I’m going to have to use cyclosporine or methotrexate or a medication that I think may have higher risks and certainly [would] require blood monitoring–yet that’s the state for some patients.”
Dupilumab, an interleukin-4 receptor alpha antagonist, is approved for treatment of moderate to severe AD in patients ages 6 and older.
When working to obtain insurance approval of dupilumab, Dr. Paller reminded dermatologists to document that the patient has moderate to severe AD “and document the negative effect on quality of life in order to try to help make it easier to get these medications for our patients.”
Starting patients on dupilumab
Dr. Paller, the Walter J. Hamlin Chair and Professor of Dermatology at Northwestern University, Chicago, said that if patients are on another systemic medication prior to starting dupilumab, she allows a transition period of 1-2 months. “Don’t just stop that drug because it’s ‘not working,’ ” she said. “I usually do a full dose for the first month, and a half dose for the next month before starting dupilumab. Also, don’t stop the use of topical corticosteroids. They can increase treatment response by 10%-20%, even when patients are on dupilumab.”
She recommends a 3- to 4-month trial of dupilumab while monitoring changes in disease severity, itch, and quality of life. “Usually there’s evidence of early improvement by 2 months in those who are going to do well enough to stay on the drug by about 4 months out,” she said. “In my experience, most pediatric patients do very well. In those with an inadequate response, about 50% will do better if you can increase the dose or frequency. Flares can still occur in those who do well. I usually push topicals when that happens.”
If patients respond well after starting dupilumab, Dr. Paller recommends that they continue on the drug for at least a year before considering a taper with the hope of “resetting” the immune system and having sustained improvement off drug. “Some parents and patients don’t want to stop the drug,” but for those who do, she tells them that she does not want to abruptly stop treatment, but to “space out the dosing” instead. “If someone is pretty much clear with the medication and is able to continue with topicals as you dial down, that’s great. But don’t even think about taking them off if somebody’s not clear or virtually clear, particularly if they start to flare with lower frequency.”
Data on effectiveness
Real-world data suggest that the effectiveness of dupilumab is similar to the efficacy seen in clinical trials. For example, a recently published systematic review and meta-analysis of 3,303 AD patients on dupilumab found that after 16 weeks of therapy, 60% achieved a 75% improvement in the Eczema Area and Severity (EASI75) score, and 27% achieved an EASI90. In a Dutch study of 210 adults treated with dupilumab for 52 weeks, enrolled in a Dutch registry, the mean percent reduction in EASI score was 70% at 16 weeks and 76.6% by 52 weeks.
In addition, there was at least a 4-point improvement in the Patient-Oriented Eczema Measure (POEM) score and at least a 4-point improvement in the Itch Numeric Rating Scale (NRS), said Dr. Paller, who was not involved in the study. “These patient-reported improvements were seen very early on,” she noted.
What about drug survival at 1 year? In a retrospective cohort study that drew from insurance databases, 1,963 adults given dupilumab were studied for a mean of 315 days. The rate of persistence was 92% at 6 months and 77% at 12 months. “That means that it’s still effective,” Dr. Paller said.
While that is a short period of time, she compared these results with long-term survival of nonsteroid systemic immunosuppressants such as cyclosporine, referring to a study of adults with AD treated with systemic immunosuppressants, which found “a 32% persistence rate at 12 months in drugs that require more monitoring, so more burden.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for dupilumab (Dupixent) manufacturers Regeneron and Sanofi, AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, and RAPT Therapeutics.
.
This scenario was illustrated in a 2020 retrospective study of 179 adults with AD who were cared for at the University of Pittsburgh Medical Center, which found that 37% did not start dupilumab, mainly due to insurance denial (19%) and high copay (11%).
“We’ve all seen this in our practice,” Amy S. Paller, MD, said during the Revolutionizing Atopic Dermatitis symposium. “We’ve also seen the denials until we get step therapy in there, so if I have a child whom I want to treat with dupilumab for safety reasons, I don’t like being told that I’m going to have to use cyclosporine or methotrexate or a medication that I think may have higher risks and certainly [would] require blood monitoring–yet that’s the state for some patients.”
Dupilumab, an interleukin-4 receptor alpha antagonist, is approved for treatment of moderate to severe AD in patients ages 6 and older.
When working to obtain insurance approval of dupilumab, Dr. Paller reminded dermatologists to document that the patient has moderate to severe AD “and document the negative effect on quality of life in order to try to help make it easier to get these medications for our patients.”
Starting patients on dupilumab
Dr. Paller, the Walter J. Hamlin Chair and Professor of Dermatology at Northwestern University, Chicago, said that if patients are on another systemic medication prior to starting dupilumab, she allows a transition period of 1-2 months. “Don’t just stop that drug because it’s ‘not working,’ ” she said. “I usually do a full dose for the first month, and a half dose for the next month before starting dupilumab. Also, don’t stop the use of topical corticosteroids. They can increase treatment response by 10%-20%, even when patients are on dupilumab.”
She recommends a 3- to 4-month trial of dupilumab while monitoring changes in disease severity, itch, and quality of life. “Usually there’s evidence of early improvement by 2 months in those who are going to do well enough to stay on the drug by about 4 months out,” she said. “In my experience, most pediatric patients do very well. In those with an inadequate response, about 50% will do better if you can increase the dose or frequency. Flares can still occur in those who do well. I usually push topicals when that happens.”
If patients respond well after starting dupilumab, Dr. Paller recommends that they continue on the drug for at least a year before considering a taper with the hope of “resetting” the immune system and having sustained improvement off drug. “Some parents and patients don’t want to stop the drug,” but for those who do, she tells them that she does not want to abruptly stop treatment, but to “space out the dosing” instead. “If someone is pretty much clear with the medication and is able to continue with topicals as you dial down, that’s great. But don’t even think about taking them off if somebody’s not clear or virtually clear, particularly if they start to flare with lower frequency.”
Data on effectiveness
Real-world data suggest that the effectiveness of dupilumab is similar to the efficacy seen in clinical trials. For example, a recently published systematic review and meta-analysis of 3,303 AD patients on dupilumab found that after 16 weeks of therapy, 60% achieved a 75% improvement in the Eczema Area and Severity (EASI75) score, and 27% achieved an EASI90. In a Dutch study of 210 adults treated with dupilumab for 52 weeks, enrolled in a Dutch registry, the mean percent reduction in EASI score was 70% at 16 weeks and 76.6% by 52 weeks.
In addition, there was at least a 4-point improvement in the Patient-Oriented Eczema Measure (POEM) score and at least a 4-point improvement in the Itch Numeric Rating Scale (NRS), said Dr. Paller, who was not involved in the study. “These patient-reported improvements were seen very early on,” she noted.
What about drug survival at 1 year? In a retrospective cohort study that drew from insurance databases, 1,963 adults given dupilumab were studied for a mean of 315 days. The rate of persistence was 92% at 6 months and 77% at 12 months. “That means that it’s still effective,” Dr. Paller said.
While that is a short period of time, she compared these results with long-term survival of nonsteroid systemic immunosuppressants such as cyclosporine, referring to a study of adults with AD treated with systemic immunosuppressants, which found “a 32% persistence rate at 12 months in drugs that require more monitoring, so more burden.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for dupilumab (Dupixent) manufacturers Regeneron and Sanofi, AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, and RAPT Therapeutics.
FROM REVOLUTIONIZING AD 2021
Malignancy risk: Secukinumab shows long-term safety for psoriasis, PsA, ankylosing spondylitis
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
that included 49 clinical trials.
Secukinumab (Cosentyx), an interleukin-17A antagonist, is approved for several conditions: moderate to severe psoriasis in children and adults, PsA, ankylosing spondylitis (AS), and nonradiographic axial spondyloarthritis.
Although secukinumab has demonstrated safety and tolerability, data on long-term malignancy rates are limited, wrote Mark Lebwohl, MD, professor of dermatology at the Icahn School of Medicine at Mount Sinai, New York, and coauthors.
In a study published in the British Journal of Dermatology, they analyzed the combined safety data from clinical trials and postmarketing surveillance. The study population included 10,685 patients with psoriasis, 2,523 patients with PsA, and 1,311 patients with ankylosing spondylitis who received at least one approved dose of secukinumab (300 mg or 150 mg). The maximum follow-up was 5 years. The exposure-adjusted incidence rate was defined as the incidence rates per 100 patient treatment-years (PTY). The cumulative exposure for patients with psoriasis, PsA, and AS was 16,482, 4,944, and 2,668 PTY, respectively, with average follow-up times of 1.54, 1.96, and 2.03 years, respectively.
The observed and the expected number of malignancies were comparable, with a standardized incidence ratio (SIR) for malignancy of 0.99 across all treatment indications, the researchers said. In further analysis of malignancy by indication, the SIR was 0.87, 1.16, and 1.61 for psoriasis, PsA, and AS, respectively.
Data from postmarketing surveillance showed similar results: The estimated crude cumulative incidence reporting rate per 100 PTY was 0.27 for malignancy across all indications. The cumulative exposure was 285,811 PTY.
The study findings were limited by several factors including the post hoc design, differences in clinical trial methodologies, and lack of controlling for confounding variables, such as smoking status and previous exposure to systemic and biologic treatments, the researchers noted. In addition, the analysis did not include postexposure follow-up data, or data on patients who discontinued clinical trials, they said.
Overall, the analysis is the largest to date and supports the low risk of malignancy in patients with psoriasis, PsA, and AS treated with secukinumab, the researchers noted.
However, “while this assessment provides a broader understanding of the safety of secukinumab and supports its long-term use in these chronic systemic inflammatory conditions, registry data are further warranted to fully understand the real-world effect of biologics on malignancy risk,” they concluded.
“Secukinumab is a relatively newer biologic, approved in 2015, and there is currently a lack of longer-term data on the incidence of malignancy in secukinumab-treated patients, so it’s important to look at the data we have so far on this topic so we can better understand the long-term risks and counsel our psoriasis and psoriatic arthritis patients,” Flavia Fedeles, MD, of the department of dermatology at Massachusetts General Hospital, Boston, said in an interview.
Dr. Fedeles, who was not involved with the study, said that she was not surprised by the study results. “Data reported in the past from phase 3 clinical trials of secukinumab compared with placebo did not show an increase in risk of malignancy, though at that time no long-term safety data or data from patients with history of malignancy was available,” she said. “This study is reassuring in that there wasn’t a signal of increased malignancy events up to 5 years of secukinumab treatment,” said Dr. Fedeles.
However, she noted that the study has a number of limitations, including the use of clinical trials data, which have stringent inclusion/exclusion criteria that can lead to selection bias, the use of postmarketing surveillance data, the post hoc nature of the analysis, and the fact that the sponsor of the trial was the manufacturer of secukinumab, which “potentially can lead to bias to this study.”
She added that “registry data are needed to fully understand the real-world long-term effect of secukinumab on malignancy risk.”
The study was funded by Novartis. Lead author Dr. Lebwohl disclosed participating in advisory boards and/or as an investigator and/or speaker and receiving grants and/or honoraria from multiple companies including Novartis. Several study coauthors are employees of Novartis.
Dr. Fedeles had no financial conflicts to disclose.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
A pacemaker that 'just disappears' and a magnetic diet device
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
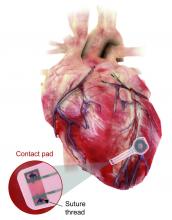
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
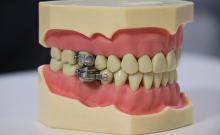
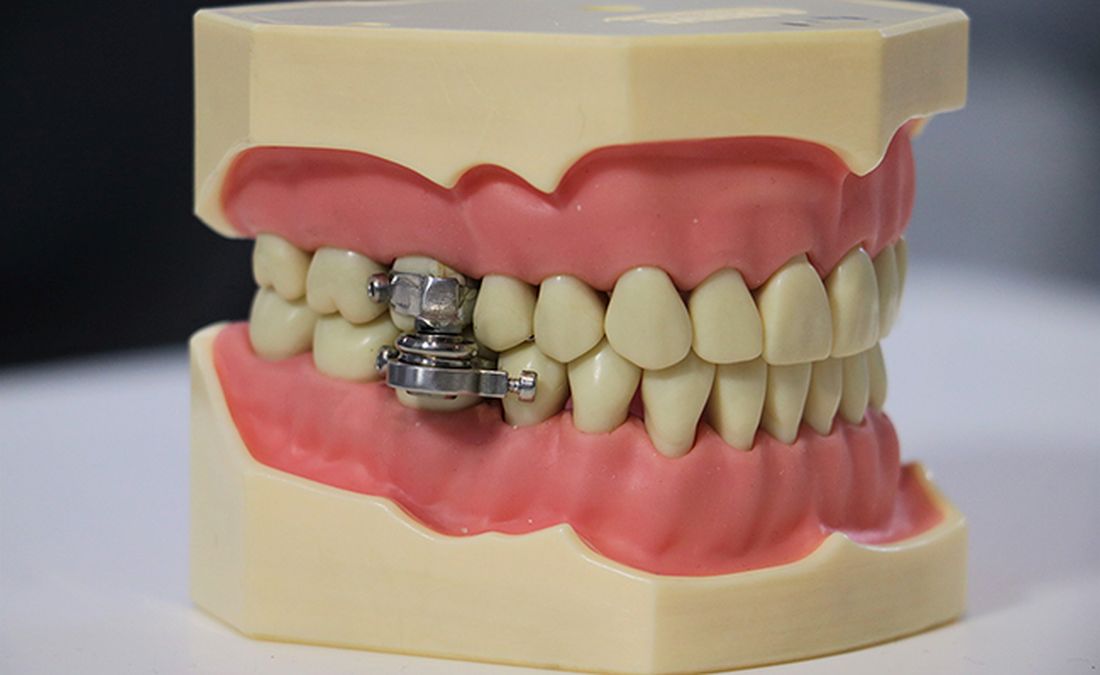
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Efficacy of Etanercept in the Treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.
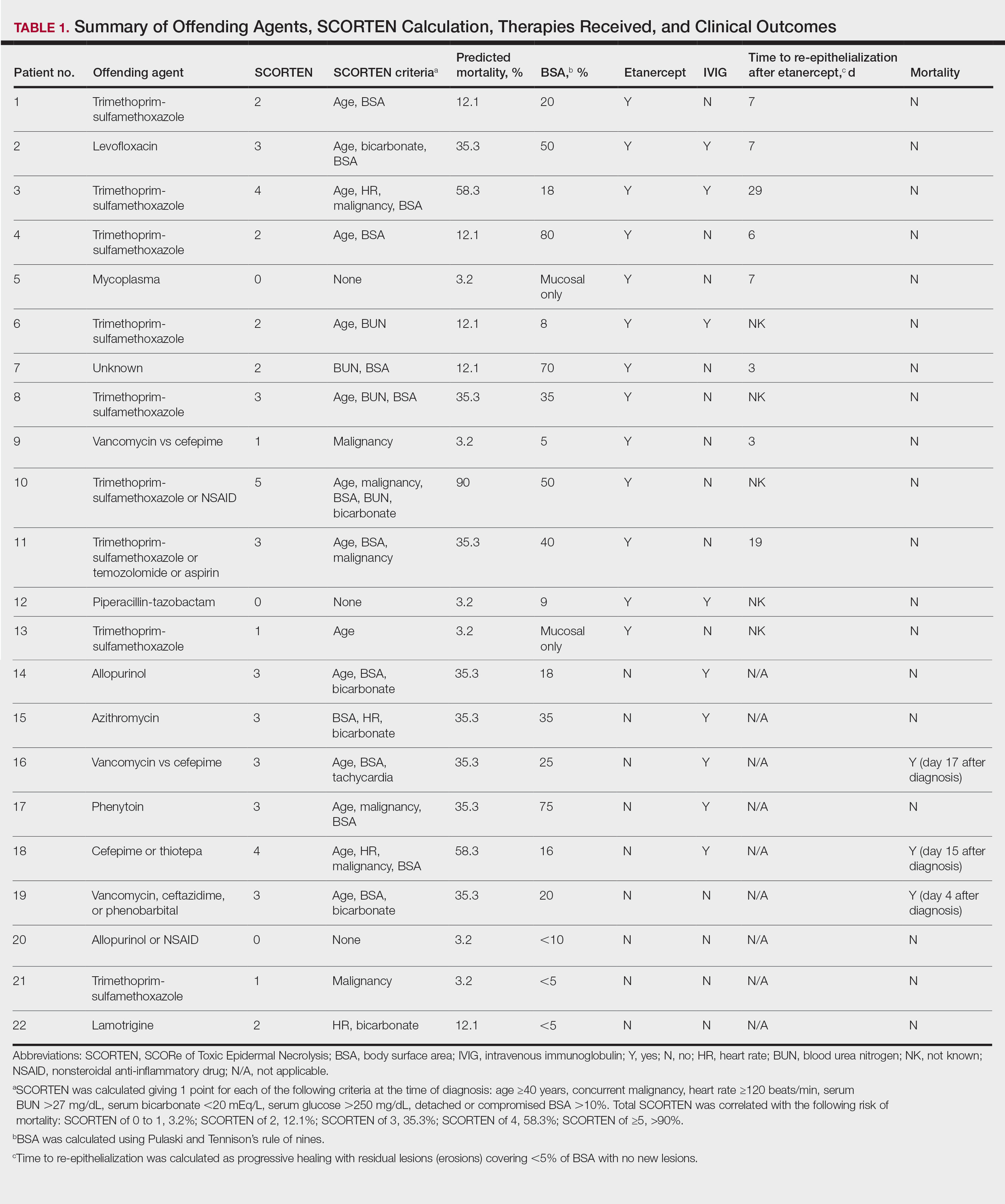
Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.
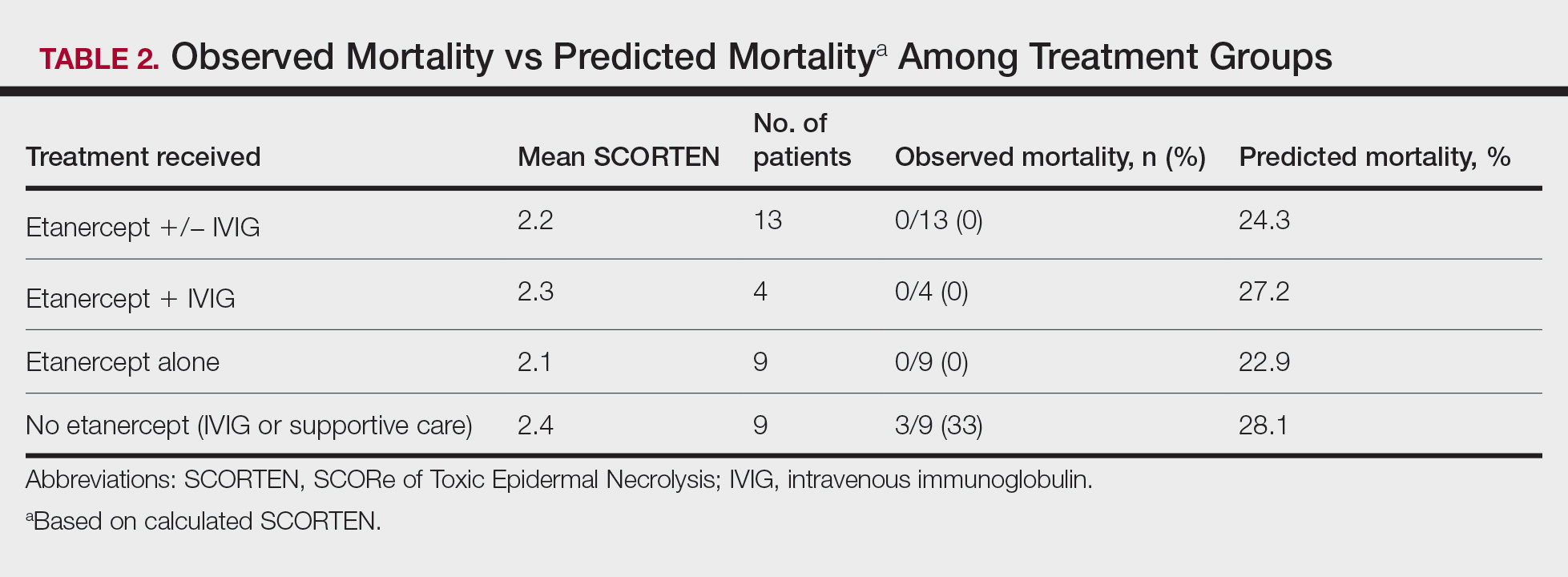
Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
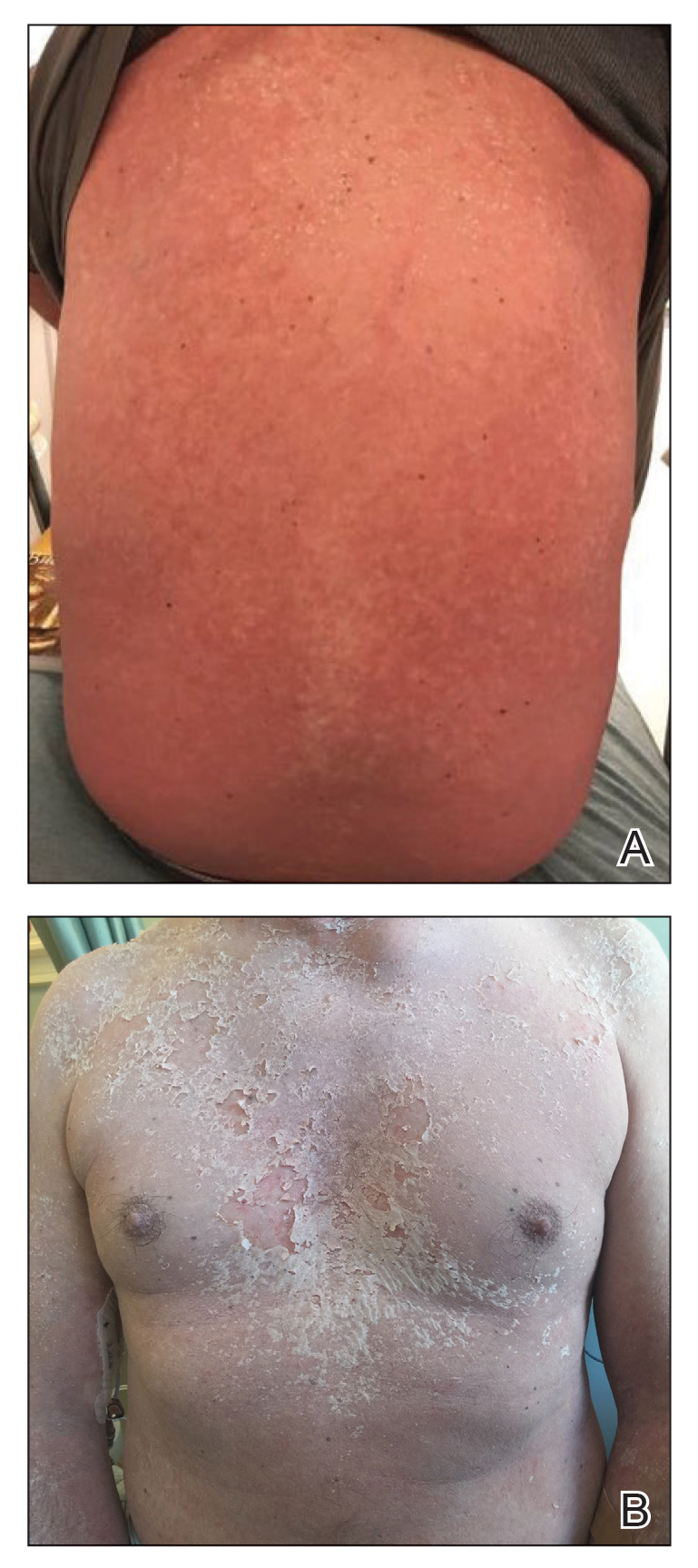
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.
Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.

Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.

Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.

Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.

Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.

Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.

Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
Practice Points
- Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening dermatologic emergencies without a universally accepted treatment.
- Results of this study support the use of single-dose subcutaneous etanercept 50 mg as a potentially lifesaving therapy for patients with SJS/TEN.
Dermatopathology Etiquette 101
The Accreditation Council for Graduate Medical Education has established core competencies to serve as a foundation for the training received in a dermatology residency program.1 Although programs are required to have the same concentrations—patient care, medical knowledge, practice-based learning and improvement, interpersonal and communication skills, professionalism, and systems-based practice—no specific guidelines are in place regarding how each of these competencies should be reached within a training period.2 Instead, it remains the responsibility of each program to formulate an individualized curriculum to facilitate proficiency in the multiple areas encompassed by a residency.
In many dermatology residency programs, dermatopathology is a substantial component of educational objectives and the curriculum.1 Residents may spend as much as 25% of their training on dermatopathology. However, there is great variability among programs in methods of teaching dermatopathology. When Hinshaw3 surveyed 52 of 109 dermatology residency programs, they identified differences in dermatopathology teaching that included, but was not limited to, utilization of problem-based learning (in 40.4% of programs), integration of journal reviews (53.8%), and computer-based learning (19.2%). In addition, differences were identified in the recommended primary textbook and the makeup of faculty who taught dermatopathology.3
Although residency programs vary in their methods of teaching this important component of dermatology, most use a multiheaded microscope in some capacity for didactics or sign-out. For most trainees, the dermatopathology laboratory is a new environment compared to the clinical space that medical students and residents become accustomed to throughout their education, thus creating a knowledge gap for trainees on proper dermatopathology etiquette and universal guidelines.
With medical students, residents, and fellows in mind, we have prepared a basic “dermatopathology etiquette” reference for trainees. Just as there are universal rules in the operating room for surgery (eg, sterile technique), we want to establish a code of conduct at the microscope. We hope that these 10 tips will, first, be useful to those who are unsure how to approach their first experience with dermatopathology and, second, serve as a guideline to aid development of appropriate communication skills and functioning within this novel setting. This list also can serve as a resource for dermatopathology attendings to provide to rotating residents and students.
1. New to pathology? It’s okay to ask. Do not hesitate to ask upper-year residents, fellows, and attendings for instructions on such matters as how to adjust your eyepiece to get the best resolution.
2. If a slide drops on the floor, do not move! Your first instinct might be to move your chair to look for the dropped slide, but you might roll over it and break it.
3. When the attending is looking through the scope, you look through the scope. Dermatopathology is a visual exercise. Getting in your “optic mileage” is best done under the guidance of an experienced dermatopathologist.
4. Rules regarding food and drink at the microscope vary by pathologist. It’s best to ask what each attending prefers. Safe advice is to avoid foods that make noise, such as chewing gum and chips, and food that has a strong odor, such as microwaved leftovers.
5. Limit use of a laptop, cell phone, and smartwatch. If you think that using any of these is necessary, it generally is best to announce that you are looking up something related to the case and then share your findings (but not the most recent post on your Facebook News Feed).
6. If you notice that something needs correcting on the report, speak up! We are all human; we all make typos. Do not hesitate to mention this as soon as possible, especially before the case is signed out. You will likely be thanked by your attending because it is harder to rectify once the report has been signed out.
7. Small talk often is welcome during large excisions. This is a great time to ask what others are doing next weekend or what happened in clinic earlier that day, or just to tell a good (clean) joke that is making the rounds. Conversely, if the case is complex, it often is best to wait until it is completed before asking questions.
8. When participating in a roundtable diagnosis, you are welcome to directly state the diagnosis for bread-and-butter cases, such as basal cell carcinomas and seborrheic keratoses. It is appropriate to be more descriptive and methodical in more complex cases. When evaluating a rash, give the general inflammatory pattern first. For example, is it spongiotic? Psoriasiform? Interface? Or a mixed pattern?
9. Extra points for identifying special sites! These include mucosal, genital, and acral sites. You might even get bonus points if you can determine something about the patient (child or adult) based on the pathologic features, such as variation in collagen patterns.
10. Whenever you are in doubt, just describe what you see. You can use the traditional top-down approach or start with stating the most evident finding, then proceed to a top-down description. If it is a neoplasm, describe the overall architecture; then, what you see at a cellular level will get you some points as well.
We acknowledge that this list of 10 tips is not comprehensive and might vary by attending and each institution’s distinctive training format. We are hopeful, however, that these 10 points of etiquette can serve as a guideline.
- Hinshaw M, Hsu P, Lee L-Y, et al. The current state of dermatopathology education: a survey of the Association of Professors of Dermatology. J Cutan Pathol. 2009;36:620-628. doi:10.1111/j.1600-0560.2008.01128.x
- Hinshaw MA, Stratman EJ. Core competencies in dermatopathology. J Cutan Pathol. 2006;33:160-165. doi:10.1111/j.0303-6987.2006.00442.x
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826. doi:10.1016/j.det.2012.06.003
The Accreditation Council for Graduate Medical Education has established core competencies to serve as a foundation for the training received in a dermatology residency program.1 Although programs are required to have the same concentrations—patient care, medical knowledge, practice-based learning and improvement, interpersonal and communication skills, professionalism, and systems-based practice—no specific guidelines are in place regarding how each of these competencies should be reached within a training period.2 Instead, it remains the responsibility of each program to formulate an individualized curriculum to facilitate proficiency in the multiple areas encompassed by a residency.
In many dermatology residency programs, dermatopathology is a substantial component of educational objectives and the curriculum.1 Residents may spend as much as 25% of their training on dermatopathology. However, there is great variability among programs in methods of teaching dermatopathology. When Hinshaw3 surveyed 52 of 109 dermatology residency programs, they identified differences in dermatopathology teaching that included, but was not limited to, utilization of problem-based learning (in 40.4% of programs), integration of journal reviews (53.8%), and computer-based learning (19.2%). In addition, differences were identified in the recommended primary textbook and the makeup of faculty who taught dermatopathology.3
Although residency programs vary in their methods of teaching this important component of dermatology, most use a multiheaded microscope in some capacity for didactics or sign-out. For most trainees, the dermatopathology laboratory is a new environment compared to the clinical space that medical students and residents become accustomed to throughout their education, thus creating a knowledge gap for trainees on proper dermatopathology etiquette and universal guidelines.
With medical students, residents, and fellows in mind, we have prepared a basic “dermatopathology etiquette” reference for trainees. Just as there are universal rules in the operating room for surgery (eg, sterile technique), we want to establish a code of conduct at the microscope. We hope that these 10 tips will, first, be useful to those who are unsure how to approach their first experience with dermatopathology and, second, serve as a guideline to aid development of appropriate communication skills and functioning within this novel setting. This list also can serve as a resource for dermatopathology attendings to provide to rotating residents and students.
1. New to pathology? It’s okay to ask. Do not hesitate to ask upper-year residents, fellows, and attendings for instructions on such matters as how to adjust your eyepiece to get the best resolution.
2. If a slide drops on the floor, do not move! Your first instinct might be to move your chair to look for the dropped slide, but you might roll over it and break it.
3. When the attending is looking through the scope, you look through the scope. Dermatopathology is a visual exercise. Getting in your “optic mileage” is best done under the guidance of an experienced dermatopathologist.
4. Rules regarding food and drink at the microscope vary by pathologist. It’s best to ask what each attending prefers. Safe advice is to avoid foods that make noise, such as chewing gum and chips, and food that has a strong odor, such as microwaved leftovers.
5. Limit use of a laptop, cell phone, and smartwatch. If you think that using any of these is necessary, it generally is best to announce that you are looking up something related to the case and then share your findings (but not the most recent post on your Facebook News Feed).
6. If you notice that something needs correcting on the report, speak up! We are all human; we all make typos. Do not hesitate to mention this as soon as possible, especially before the case is signed out. You will likely be thanked by your attending because it is harder to rectify once the report has been signed out.
7. Small talk often is welcome during large excisions. This is a great time to ask what others are doing next weekend or what happened in clinic earlier that day, or just to tell a good (clean) joke that is making the rounds. Conversely, if the case is complex, it often is best to wait until it is completed before asking questions.
8. When participating in a roundtable diagnosis, you are welcome to directly state the diagnosis for bread-and-butter cases, such as basal cell carcinomas and seborrheic keratoses. It is appropriate to be more descriptive and methodical in more complex cases. When evaluating a rash, give the general inflammatory pattern first. For example, is it spongiotic? Psoriasiform? Interface? Or a mixed pattern?
9. Extra points for identifying special sites! These include mucosal, genital, and acral sites. You might even get bonus points if you can determine something about the patient (child or adult) based on the pathologic features, such as variation in collagen patterns.
10. Whenever you are in doubt, just describe what you see. You can use the traditional top-down approach or start with stating the most evident finding, then proceed to a top-down description. If it is a neoplasm, describe the overall architecture; then, what you see at a cellular level will get you some points as well.
We acknowledge that this list of 10 tips is not comprehensive and might vary by attending and each institution’s distinctive training format. We are hopeful, however, that these 10 points of etiquette can serve as a guideline.
The Accreditation Council for Graduate Medical Education has established core competencies to serve as a foundation for the training received in a dermatology residency program.1 Although programs are required to have the same concentrations—patient care, medical knowledge, practice-based learning and improvement, interpersonal and communication skills, professionalism, and systems-based practice—no specific guidelines are in place regarding how each of these competencies should be reached within a training period.2 Instead, it remains the responsibility of each program to formulate an individualized curriculum to facilitate proficiency in the multiple areas encompassed by a residency.
In many dermatology residency programs, dermatopathology is a substantial component of educational objectives and the curriculum.1 Residents may spend as much as 25% of their training on dermatopathology. However, there is great variability among programs in methods of teaching dermatopathology. When Hinshaw3 surveyed 52 of 109 dermatology residency programs, they identified differences in dermatopathology teaching that included, but was not limited to, utilization of problem-based learning (in 40.4% of programs), integration of journal reviews (53.8%), and computer-based learning (19.2%). In addition, differences were identified in the recommended primary textbook and the makeup of faculty who taught dermatopathology.3
Although residency programs vary in their methods of teaching this important component of dermatology, most use a multiheaded microscope in some capacity for didactics or sign-out. For most trainees, the dermatopathology laboratory is a new environment compared to the clinical space that medical students and residents become accustomed to throughout their education, thus creating a knowledge gap for trainees on proper dermatopathology etiquette and universal guidelines.
With medical students, residents, and fellows in mind, we have prepared a basic “dermatopathology etiquette” reference for trainees. Just as there are universal rules in the operating room for surgery (eg, sterile technique), we want to establish a code of conduct at the microscope. We hope that these 10 tips will, first, be useful to those who are unsure how to approach their first experience with dermatopathology and, second, serve as a guideline to aid development of appropriate communication skills and functioning within this novel setting. This list also can serve as a resource for dermatopathology attendings to provide to rotating residents and students.
1. New to pathology? It’s okay to ask. Do not hesitate to ask upper-year residents, fellows, and attendings for instructions on such matters as how to adjust your eyepiece to get the best resolution.
2. If a slide drops on the floor, do not move! Your first instinct might be to move your chair to look for the dropped slide, but you might roll over it and break it.
3. When the attending is looking through the scope, you look through the scope. Dermatopathology is a visual exercise. Getting in your “optic mileage” is best done under the guidance of an experienced dermatopathologist.
4. Rules regarding food and drink at the microscope vary by pathologist. It’s best to ask what each attending prefers. Safe advice is to avoid foods that make noise, such as chewing gum and chips, and food that has a strong odor, such as microwaved leftovers.
5. Limit use of a laptop, cell phone, and smartwatch. If you think that using any of these is necessary, it generally is best to announce that you are looking up something related to the case and then share your findings (but not the most recent post on your Facebook News Feed).
6. If you notice that something needs correcting on the report, speak up! We are all human; we all make typos. Do not hesitate to mention this as soon as possible, especially before the case is signed out. You will likely be thanked by your attending because it is harder to rectify once the report has been signed out.
7. Small talk often is welcome during large excisions. This is a great time to ask what others are doing next weekend or what happened in clinic earlier that day, or just to tell a good (clean) joke that is making the rounds. Conversely, if the case is complex, it often is best to wait until it is completed before asking questions.
8. When participating in a roundtable diagnosis, you are welcome to directly state the diagnosis for bread-and-butter cases, such as basal cell carcinomas and seborrheic keratoses. It is appropriate to be more descriptive and methodical in more complex cases. When evaluating a rash, give the general inflammatory pattern first. For example, is it spongiotic? Psoriasiform? Interface? Or a mixed pattern?
9. Extra points for identifying special sites! These include mucosal, genital, and acral sites. You might even get bonus points if you can determine something about the patient (child or adult) based on the pathologic features, such as variation in collagen patterns.
10. Whenever you are in doubt, just describe what you see. You can use the traditional top-down approach or start with stating the most evident finding, then proceed to a top-down description. If it is a neoplasm, describe the overall architecture; then, what you see at a cellular level will get you some points as well.
We acknowledge that this list of 10 tips is not comprehensive and might vary by attending and each institution’s distinctive training format. We are hopeful, however, that these 10 points of etiquette can serve as a guideline.
- Hinshaw M, Hsu P, Lee L-Y, et al. The current state of dermatopathology education: a survey of the Association of Professors of Dermatology. J Cutan Pathol. 2009;36:620-628. doi:10.1111/j.1600-0560.2008.01128.x
- Hinshaw MA, Stratman EJ. Core competencies in dermatopathology. J Cutan Pathol. 2006;33:160-165. doi:10.1111/j.0303-6987.2006.00442.x
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826. doi:10.1016/j.det.2012.06.003
- Hinshaw M, Hsu P, Lee L-Y, et al. The current state of dermatopathology education: a survey of the Association of Professors of Dermatology. J Cutan Pathol. 2009;36:620-628. doi:10.1111/j.1600-0560.2008.01128.x
- Hinshaw MA, Stratman EJ. Core competencies in dermatopathology. J Cutan Pathol. 2006;33:160-165. doi:10.1111/j.0303-6987.2006.00442.x
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826. doi:10.1016/j.det.2012.06.003
Almost all U.S. COVID-19 deaths now in the unvaccinated
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.
Physician fired after slurs, including ‘cannibalism,’ against Israel
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Wiping Away Cellulitis: A Case of Factitious Disorder
To the Editor:
Patients with psychocutaneous disorders present unique challenges to physicians. We illustrate the critical role that dermoscopy may play to illuminate exogenous skin pathology.
A 50-year-old woman with a reported medical history of systemic lupus erythematosus, chronic pain, and nonhealing leg ulcers presented to the emergency department with severe pain of the left lower leg and redness that was concerning for cellulitis. She sought treatment at an outside hospital for cellulitis 2 weeks prior but left against medical advice. Symptomatic review revealed chest pain, shortness of breath, nausea, vomiting, and diarrhea. The primary team started her on intravenous clindamycin and vancomycin for the presumed infection and scheduled narcotic medications due to concerns of intractable pain in the left leg. The dermatology department was consulted after failure to improve with 1 week of systemic antibiotics.
Physical examination revealed a geometric, atrophic, purple plaque on the left anterior shin from a prior leg ulcer as well as a diffuse red-pink patch extending from the knee to the ankle. Notably, the cellulitis spared the left posterior calf resting against the sheet and had a sharp line of demarcation at the distal shin. The leg was cool to the touch while the patient was distractible. She later reported that the leg was extremely tender to palpation. Dermoscopy revealed linear red pigments within skin furrows that accentuated skin lines (Figure). These findings raised suspicions of an external manipulation. The skin was wiped with an alcohol pad that removed a shimmering pink substance consistent in appearance to a cosmetic product. The skin beneath the cellulitis appeared normal.
On further review of the patient’s medical record, it was noted that she was admitted several months ago for ulcers of the left leg. She had been to multiple hospitals and had numerous rounds of antibiotics. Biopsy of an ulcer revealed dermal fibrosis consistent with scarring. Aerobic bacteria, atypical mycobacteria, and fungal cultures were all negative. The physicians suspected a self-induced etiology consistent with dermatitis artefacta. The patient emphasized multiple psychosocial stressors as well as having frequent lupus flares despite repeated negative workup. Given the exaggerated symptoms and unnecessary hospital visits, she was given the diagnosis of factitious disorder (malingering or Munchausen syndrome). After extensive discussion, the patient was amenable to outpatient mental health counseling.
Dermoscopy is not a standard method to diagnose cellulitis of the skin; however, when patients present with an atypical response to appropriate care, the presumed diagnosis must be challenged. This patient had dramatized symptoms, false medical history, and numerous hospitalizations that were suspicious for factitious disorder.1 Furthermore, the physical examination was inconsistent with the classic course of cellulitis. In this case, dermoscopy had advantages over biopsies because it was noninvasive, gave immediate feedback, and provided a macroscopic view of the morphology. Via dermoscopy, we had an objective lens to distinguish cellulitis from cosmetic product and to obtain the correct diagnosis.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
To the Editor:
Patients with psychocutaneous disorders present unique challenges to physicians. We illustrate the critical role that dermoscopy may play to illuminate exogenous skin pathology.
A 50-year-old woman with a reported medical history of systemic lupus erythematosus, chronic pain, and nonhealing leg ulcers presented to the emergency department with severe pain of the left lower leg and redness that was concerning for cellulitis. She sought treatment at an outside hospital for cellulitis 2 weeks prior but left against medical advice. Symptomatic review revealed chest pain, shortness of breath, nausea, vomiting, and diarrhea. The primary team started her on intravenous clindamycin and vancomycin for the presumed infection and scheduled narcotic medications due to concerns of intractable pain in the left leg. The dermatology department was consulted after failure to improve with 1 week of systemic antibiotics.
Physical examination revealed a geometric, atrophic, purple plaque on the left anterior shin from a prior leg ulcer as well as a diffuse red-pink patch extending from the knee to the ankle. Notably, the cellulitis spared the left posterior calf resting against the sheet and had a sharp line of demarcation at the distal shin. The leg was cool to the touch while the patient was distractible. She later reported that the leg was extremely tender to palpation. Dermoscopy revealed linear red pigments within skin furrows that accentuated skin lines (Figure). These findings raised suspicions of an external manipulation. The skin was wiped with an alcohol pad that removed a shimmering pink substance consistent in appearance to a cosmetic product. The skin beneath the cellulitis appeared normal.

On further review of the patient’s medical record, it was noted that she was admitted several months ago for ulcers of the left leg. She had been to multiple hospitals and had numerous rounds of antibiotics. Biopsy of an ulcer revealed dermal fibrosis consistent with scarring. Aerobic bacteria, atypical mycobacteria, and fungal cultures were all negative. The physicians suspected a self-induced etiology consistent with dermatitis artefacta. The patient emphasized multiple psychosocial stressors as well as having frequent lupus flares despite repeated negative workup. Given the exaggerated symptoms and unnecessary hospital visits, she was given the diagnosis of factitious disorder (malingering or Munchausen syndrome). After extensive discussion, the patient was amenable to outpatient mental health counseling.
Dermoscopy is not a standard method to diagnose cellulitis of the skin; however, when patients present with an atypical response to appropriate care, the presumed diagnosis must be challenged. This patient had dramatized symptoms, false medical history, and numerous hospitalizations that were suspicious for factitious disorder.1 Furthermore, the physical examination was inconsistent with the classic course of cellulitis. In this case, dermoscopy had advantages over biopsies because it was noninvasive, gave immediate feedback, and provided a macroscopic view of the morphology. Via dermoscopy, we had an objective lens to distinguish cellulitis from cosmetic product and to obtain the correct diagnosis.
To the Editor:
Patients with psychocutaneous disorders present unique challenges to physicians. We illustrate the critical role that dermoscopy may play to illuminate exogenous skin pathology.
A 50-year-old woman with a reported medical history of systemic lupus erythematosus, chronic pain, and nonhealing leg ulcers presented to the emergency department with severe pain of the left lower leg and redness that was concerning for cellulitis. She sought treatment at an outside hospital for cellulitis 2 weeks prior but left against medical advice. Symptomatic review revealed chest pain, shortness of breath, nausea, vomiting, and diarrhea. The primary team started her on intravenous clindamycin and vancomycin for the presumed infection and scheduled narcotic medications due to concerns of intractable pain in the left leg. The dermatology department was consulted after failure to improve with 1 week of systemic antibiotics.
Physical examination revealed a geometric, atrophic, purple plaque on the left anterior shin from a prior leg ulcer as well as a diffuse red-pink patch extending from the knee to the ankle. Notably, the cellulitis spared the left posterior calf resting against the sheet and had a sharp line of demarcation at the distal shin. The leg was cool to the touch while the patient was distractible. She later reported that the leg was extremely tender to palpation. Dermoscopy revealed linear red pigments within skin furrows that accentuated skin lines (Figure). These findings raised suspicions of an external manipulation. The skin was wiped with an alcohol pad that removed a shimmering pink substance consistent in appearance to a cosmetic product. The skin beneath the cellulitis appeared normal.

On further review of the patient’s medical record, it was noted that she was admitted several months ago for ulcers of the left leg. She had been to multiple hospitals and had numerous rounds of antibiotics. Biopsy of an ulcer revealed dermal fibrosis consistent with scarring. Aerobic bacteria, atypical mycobacteria, and fungal cultures were all negative. The physicians suspected a self-induced etiology consistent with dermatitis artefacta. The patient emphasized multiple psychosocial stressors as well as having frequent lupus flares despite repeated negative workup. Given the exaggerated symptoms and unnecessary hospital visits, she was given the diagnosis of factitious disorder (malingering or Munchausen syndrome). After extensive discussion, the patient was amenable to outpatient mental health counseling.
Dermoscopy is not a standard method to diagnose cellulitis of the skin; however, when patients present with an atypical response to appropriate care, the presumed diagnosis must be challenged. This patient had dramatized symptoms, false medical history, and numerous hospitalizations that were suspicious for factitious disorder.1 Furthermore, the physical examination was inconsistent with the classic course of cellulitis. In this case, dermoscopy had advantages over biopsies because it was noninvasive, gave immediate feedback, and provided a macroscopic view of the morphology. Via dermoscopy, we had an objective lens to distinguish cellulitis from cosmetic product and to obtain the correct diagnosis.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
Practice Points
- Consider exogenous factors or alternative diagnoses when a patient does not respond to appropriate care.
- Although dermoscopy is not used to diagnose cellulitis, it could be helpful in distinguishing cosmetic products used in dermatitis artefacta.
Wrong-site surgery doc says he can’t be sued
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
Rate of cutaneous toxicities from ICIs may be lower than previously reported
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
FROM SID 2021