User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
For SCC, legs are a high-risk anatomic site in women
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
When Maryam M. Asgari, MD, reviewed results from a large population-based study published in 2017, which found that a large proportion of cutaneous squamous cell carcinomas were being detected on the lower extremities of women, it caused her to reflect on her own clinical practice as a Mohs surgeon.
“I was struck by the number of times I was seeing women present with lower extremity SCCs,” Dr. Asgari, professor of dermatology, Harvard Medical School, Boston, said during a virtual forum on cutaneous malignancies jointly presented by Postgraduate Institute for Medicine and Global Academy for Medical Education. “When female patients push you for a waist-up skin exam, try to convince them that the legs are an important area to look at as well.”
In an effort to ascertain if there are sex differences in the anatomic distribution of cutaneous SCC, she and her postdoctoral fellow, Yuhree Kim, MD, MPH, used an institutional registry to identify 618 non-Hispanic White patients diagnosed with 2,111 SCCs between 2000 and 2016. They found that men were more likely to have SCCs arise on the head and neck (52% vs. 21% among women, respectively), while women were more likely to have SCCs develop on the lower extremity (41% vs. 10% in men).
“When we looked at whether these tumors were in situ or invasive, in women, the majority of these weren’t just your run-of-the-mill in situ SCCs; 44% were actually invasive SCCs,” Dr. Asgari said. “What this is getting at is to make sure that you’re examining the lower extremities when you’re doing these skin exams. Many times, especially in colder weather, your patients will come in and request a waist-up exam. For women, you absolutely have to examine their lower extremities. That’s their high-risk area for SCCs.”
, she continued. According to 2020 data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results SEER program, the incidence of KC in the United States is estimated to be 3.5 million cases per year, while all other cancers account for approximately 1.8 million cases per year.
To make matters worse, while the incidence of many other cancers have plateaued or even declined over time in the United States, data from a population-based cohort at Kaiser Permanente Northern California show that the incidence of BCCs rose between 1998 and 2012, estimated to occur in about 2 million Americans each year.
Dr. Asgari noted that the incidence of KCs can be difficult to quantify and study. “Part of the reason is that they’re not reported to traditional cancer registries like the SEER program,” she said. “You can imagine why. The sheer volume of KC dwarfs all other cancers, and oftentimes KCs are biopsied in dermatology offices. Sometimes, dermatologists even read their own biopsy specimens, so they don’t go to a central pathology repository like other cancers do.”
The best available research suggests that patients at the highest risk of KC include men and women between the ages of 60 and 89. Dr. Asgari said that she informs her patients that people in their 80s have about a 20-fold risk of BCC or SCC compared with people in their 30s. “I raise this because a lot of time the people who come in for skin cancer screenings are the ‘worried well,’ ” she said. “They can be at risk, but they’re not our highest risk subgroup. They come in proactively wanting to have those full skin screens done, but where we really need to be focusing is in people in their 60s to 80s.”
Risk factors can be shared or unique to each tumor type. Extrinsic factors include chronic UV exposure, ionizing radiation, and tanning bed use. “Acute UV exposures that give you a blistering sunburn puts you at risk for BCC, whereas chronic sun exposures puts you at risk for SCC,” she said. “Tanning bed use can increase the risk for both types, as can ionizing radiation, although it ups the risk for BCCs much more than it does for SCCs.” Intrinsic risk factors for both tumor types include fair skin, blue/green eyes, blond/red hair, male gender, having pigment gene variants, and being immunosuppressed.
By race/ethnicity, the highest risk for KC in the United States falls to non-Hispanic Whites (a rate of 150-360 per 100,000 individuals), while the rate among blacks is 3 per 100,000 individuals. “In darker skin phenotypes, sun exposure tends to be less of a risk factor,” Dr. Asgari said. “They can rise on sun-protected areas and are frequently associated with chronic inflammation, chronic wounds, or scarring.”
In a soon-to-be published study, Dr. Asgari and colleagues sought to examine the association between genetic ancestry and SCC risk. The found that people with northwestern European ancestry faced the highest risk of SCC, especially those with Irish/Scottish ancestry. Among people of Hispanic/Latino descent, the highest risk of SCC came in those who had the most European ancestry.
Global Academy for Medical Education and this news organization are owned by the same parent company.
Dr. Asgari disclosed that she receives royalties from UpToDate.
FROM THE CUTANEOUS MALIGNANCIES FORUM
Pfizer’s COVID-19 vaccine 95% effective in final phase 3 results
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
After initial promising interim results on Nov. 9, Pfizer and BioNTech today announced that their mRNA vaccine, in development to prevent COVID-19, is 95% effective.
Final analysis of the randomized, phase 3 study of more than 43,000 people yielded 170 confirmed cases of COVID-19 – with 162 positive cases in the placebo group versus 8 in the BNT162b2 vaccine group.
Researchers reported 10 severe cases of COVID-19 in the trial, 9 of which occurred in the placebo group.
The study was ethnically diverse, and results were consistent across gender and age groups, with a 94% efficacy reported among participants aged older than 65 years.
Pfizer plans to file for an emergency-use authorization with the Food and Drug Administration “within days,” having now met all the FDA data endpoints, according to a news release from the two companies.
The vaccine was well tolerated with no serious safety concerns, the company stated. Two grade 3 adverse events were reported – fatigue in 3.8% of participants and headache in 2%.
The 95% efficacy places the Pfizer vaccine in the same neighborhood as the interim results of the Moderna vaccine, reported at 94.5%. Both products are two-dose mRNA vaccines.
As of Nov. 13, of 43,661 total participants in the Pfizer vaccine phase 3 trial, 41,135 received a second dose. The final results are based on two outcomes measured 7 days after the second dose: vaccine efficacy in people without prior SARS-CoV-2 infection as well as a secondary outcome in people both with and without prior SARS-CoV-2 infection.
The 95% vaccine efficacy was statistically significant, compared with placebo (P < .0001).
‘Historic 8-month journey’
The BNT162b2 vaccine candidate is a joint effort between Pfizer and BioNTech. “The study results mark an important step in this historic 8-month journey to bring forward a vaccine capable of helping to end this devastating pandemic,” Albert Bourla, DVM, PhD, Pfizer chairman and CEO, said in a statement. “With hundreds of thousands of people around the globe infected every day, we urgently need to get a safe and effective vaccine to the world.”
Ugur Sahin, MD, PhD, cofounder and CEO of BioNTech, added, “we are grateful that the first global trial to reach the final efficacy analysis mark indicates that a high rate of protection against COVID-19 can be achieved very fast after the first 30-mcg dose, underscoring the power of BNT162 in providing early protection.”
The two companies expect to produce up to 50 million vaccine doses in 2020 for global distribution. Projections for 2021 include up to 1.3 billion doses.
The companies also designed temperature-controlled thermal shipping containers with dry ice to maintain the required, approximate –70° C (–94° F) conditions. Clinicians can use the containers as temporary storage units for up to 15 days by replacing the dry ice.
This article first appeared on Medscape.com.
GLIMMER of hope for itch in primary biliary cholangitis
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Patients with primary biliary cholangitis experienced rapid improvements in itch and quality of life after treatment with linerixibat in a randomized, placebo-controlled trial of the safety, efficacy, and tolerability of the small-molecule drug.
Moderate to severe pruritus “affects patients’ quality of life and is a huge burden for them,” said investigator Cynthia Levy, MD, from the University of Miami Health System.
“Finally having a medication that controls those symptoms is really important,” she said in an interview.
With a twice-daily mid-range dose of the drug for 12 weeks, patients with moderate to severe itch reported significantly less itch and better social and emotional quality of life, Dr. Levy reported at the Liver Meeting, where she presented findings from the phase 2 GLIMMER trial.
After a single-blind 4-week placebo run-in period for patients with itch scores of at least 4 on a 10-point rating scale, those with itch scores of at least 3 were then randomly assigned to one of five treatment regimens – once-daily linerixibat at doses of 20 mg, 90 mg, or 180 mg, or twice-daily doses of 40 mg or 90 mg – or to placebo.
After 12 weeks of treatment, all 147 participants once again received placebo for 4 weeks.
During the trial, participants recorded itch levels twice daily. The worst of these daily scores was averaged every 7 days to determine the mean worst daily itch.
The primary study endpoint was the change in worst daily itch from baseline after 12 weeks of treatment. Participants whose self-rated itch improved by 2 points on the 10-point scale were considered to have had a response to the drug.
Participants also completed the PBC-40, an instrument to measure quality of life in patients with primary biliary cholangitis, answering questions about itch and social and emotional status.
Reductions in worst daily itch from baseline to 12 weeks were steepest in the 40-mg twice-daily group, at 2.86 points, and in the 90-mg twice-daily group, at 2.25 points. In the placebo group, the mean decrease was 1.73 points.
During the subsequent 4 weeks of placebo, after treatment ended, the itch relief faded in all groups.
Scores on the PBC-40 itch domain improved significantly in every group, including placebo. However, only those in the twice-daily 40-mg group saw significant improvements on the social (P = .0016) and emotional (P = .0025) domains.
‘Between incremental and revolutionary’
The results are on a “kind of continuum between incremental and revolutionary,” said Jonathan A. Dranoff, MD, from the University of Arkansas for Medical Sciences, Little Rock, who was not involved in the study. “It doesn’t hit either extreme, but it’s the first new drug for this purpose in forever, which by itself is a good thing.”
The placebo effect suggests that “maybe the actual contribution of the noncognitive brain to pruritus is bigger than we thought, and that’s worth noting,” he added. Nevertheless, “the drug still appears to have effects that are statistically different from placebo.”
The placebo effect in itching studies is always high but tends to wane over time, said Dr. Levy. This trial had a 4-week placebo run-in period to allow that effect to fade somewhat, she explained.
About 10% of the study cohort experienced drug-related diarrhea, which was expected, and about 10% dropped out of the trial because of drug-related adverse events.
Linerixibat is an ileal sodium-dependent bile acid transporter inhibitor, so the gut has to deal with the excess bile acid fallout, but the diarrhea is likely manageable with antidiarrheals, said Dr. Levy.
It is unlikely that diarrhea will deter patients with severe itch from using an effective drug when other drugs have failed them. “These patients are consumed by itch most of the time,” said Dr. Dranoff. “I think for people who don’t regularly treat patients with primary biliary cholangitis, it’s one of the underappreciated aspects of the disease.”
The improvements in social and emotional quality of life seen with linerixibat are not only statistically significant, they are also clinically significant, said Dr. Levy. “We are really expecting this to impact the lives of our patients and are looking forward to phase 3.”
Dr. Levy disclosed support from GlaxoSmithKline. Dr. Dranoff disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Dermatologists and the history of skin care and beauty devices: Part 4
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
In this series on the role dermatologists have played in the history of skin care, I have covered dermatologists who developed cosmeceutical ingredients, dermatologists who consulted for the skin care industry, and those who developed a novel and successful skin care line. In this column, part 4 of the series, I will continue to discuss .
Dermatologists and Stiefel Laboratories
The Stiefel Medicinal Soap Company, founded in 1847, later became Stiefel Laboratories and was sold to GlaxoSmithKline in 2009. Stiefel Laboratories made many contributions over the years to the field of dermatology as chronicled in the excellent book, “Skin Saga” written by Charles Stiefel and published in 2018. The company was first known for soaps and groundbreaking products, such as “Freckle Soap” that sped epidermal turnover, resulting in a more even toned complexion.
Many dermatologists were involved in developing products and providing advice to the company. Herman Sharlit, MD, in New York, had the idea for a moisturizing soap (Oilatum), a detergent soap (Acne Aid detergent soap), and a coal tar soap (Polytar). Eugene Farber, MD, who was professor and chairman of the department of dermatology at Stanford (Calif.) University, consulted for Stiefel Laboratories and helped them identify and develop many products over the years.1 Stiefel Labs came out with the first facial scrub called Brasivol, an abrasive cream with aluminum oxide particles – the predecessor to modern day microdermabrasion. This facial scrub was conceived by dermatologist Rose Saperstein, MD, Los Angeles, who published a report2 on this in 1960 and also received a patent for it in 1963.3 Brasivol became the company’s first million dollar product.1
Stiefel Laboratories worked with many dermatologists to help them develop their ideas. They included Cleveland White, MD, who patented a highly absorbent foot and body powder known as Zeasorb powder. William Pace, MD, was a Canadian dermatologist who patented an acne treatment containing benzoyl peroxide and sulfur that Stiefel Labs marketed as Sulfoxyl Lotion. Dr. Pace is lovingly referred to as “the father of benzoyl peroxide” because his idea led Stiefel Labs to develop more benzoyl peroxide products. Benzoyl peroxide remains the most popular OTC ingredient to treat acne.
Comedone extractors
Many dermatologists have developed ways to extract comedones. There are publications on using paper clips,4,5safety pins,6 and medicine droppers,7 but some dermatologists have developed special comedone extractors, which include the following: Jay Schamberg, MD, developed a comedone extractor with a loop at each end. He disapproved of cutting a comedone, so did not include a needle or scalpel in his extractor.8
- Leonard Savitt, MD,9 attached a scalpel to one end of the Schamberg extractor.
- Alan Shalita, MD, developed a comedone extractor with a large, keyhole-shaped extracting orifice that made the tool easier to clean.10
The Saalfield comedone extractor combines a fixed pointed blade at one end and a small spoon-shaped expressor foot at the other end. (However, I have not been able to determine if Saalfield was a dermatologist.)
Dermatologist who developed methods for lesion excisions
Robert Segal, MD, a dermatologist at the University of Arizona, Tucson, invented the Dermablade. Although this is technically not a beauty device, I am including it because it has made the removal of unsightly moles and lesions much easier. He holds six patents on this device.
Dermatologists developed dermabrasion and microneedling
Ernst Kromayer, MD,11 a dermatologist in Germany, first described microneedling in 1905 when he mounted dental burrs on motor-driven flexible cord equipment to treat scars. Abner Kurtin, MD, a New York dermatologist, learned about Dr. Kromayer’s technique and modified it using stainless wireless brushes. Dr. Kurtin is known as the “father of dermabrasion.” His work was noted by Nobel Laureate Alexis Carrel, MD, who moved to New York City and began using the technique. Dr. Carrel’s protege, New York dermatologist, Norman Orentreich, MD, began using hypodermic needles instead of wire brushes. Microneedling has gained much popularity over the last decade and has been combined with platelet rich plasma injections.
Dermatologist-developed injection to shrink fat
Adam Rotunda, MD, was a dermatology resident at the University of California, Los Angeles, when he and his professor Michael Kolodney, MD, PhD, had the idea to develop deoxycholate as an injectable to reduce fat deposits. They filed a patent in 2004, conducted clinical trials, and it worked! In 2009, the patent for deoxycholic acid (ATX-10), marketed as Kybella, was granted. The rights to the drug were purchased by Aestherx, which later became Kythera Biopharmaceuticals. Kybella received Food and Drug Administration approval in 2015, and 6 months later, Kythera was acquired by Allergan.
Development of FDA-approved drugs to improve skin appearance
In 2004, dermatologists Stuart Shanler, MD, and Andrew Ondo, MD, filed a patent for the use of topical oxymetazoline for the treatment of the erythema of rosacea. They published their observations in 2007, noting that oxymetazoline improved facial flushing and erythema.11 Dr. Shanler then teamed up with dermatologist Neal Walker, MD, to form a start-up pharmaceutical company, Vicept Therapeutics, and took this compound through phase 2 clinical trials, while Dr. Shanler filed additional patents on oxymetazoline compositions and their uses. Once they successfully demonstrated the efficacy of topical oxymetazoline for rosacea, Allergan acquired the rights of the drug, successfully completed the phase 3 clinical trials, and Rhofade was approved by the FDA in 2017. It is the only topical drug invented and developed by a dermatologist to receive FDA approval since tretinoin (Renova) was developed by Albert Kligman, MD, and approved by the FDA for the improvement in appearance of fine wrinkling, mottled hyperpigmentation and roughness associated with photodamage in 1992.
The development of lasers
The last dermatologist I will discuss in this history series is R. Rox Anderson, MD, professor of dermatology at Harvard University, and director of the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. It is impossible to list all his contributions in such a limited space. It would take a book. Building on efforts pioneered by Leon Goldman, MD, Dr. Anderson and his associates pioneered the use of lasers in dermatology and invented the idea of photothermolysis when they filed a patent on using light to remove hair in 1995.Dieter Manstein, MD, PhD,Dr. Anderson and others filed many patents that led to devices such as hair removal lasers, resurfacing lasers, and Fraxel lasers. They also made discoveries related to using cold to shrink fat. One of their inventions is known as CoolSculpting. They were so influential in the development of cosmetic dermatology that it is hard to imagine the field without their contributions.
This concludes my four-part series on the history of dermatologists’ role in the development of the skin care industry. I hope I have not forgotten anyone; if I did, I apologize. I have asked for ideas on Dermchat, Facebook and LinkedIn. Feel free to reach out if I missed one of your contributions. I will be giving lectures on this topic in the future and would be happy to include anyone I missed.
As the year 2020 ends, I want to say, Happy 50th Anniversary Dermatology News! I hope you enjoyed this historical series in honor of this anniversary.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Stiefel, CW. (n.d.). Skin Saga: How a Tiny Family Soap Business Evolved Over Six Generations Into the #1 Dermatology Company in the World. United States: Smart Business Network.
2. Saperstein, RB. Arch Dermatol. 1960 Apr;81:601.
3. Saperstein, RB, and Stiefel, WK (1963). U.S. Patent No. 3,092,111. Washington, DC: U.S. Patent and Trademark Office.
4. George DE et al. J Am Acad Dermatol. 2006 Feb;54(2):326.
5. Cvancara JL, Meffert JJ. J Am Acad Dermatol. 1999 Mar;40(3):477-8.
6. Mukhtar M., Sharma R. Int J Dermatol. 2004 Dec;43(12):967-8.
7. Shellow, H. JAMA. 1951;147(18):1777.
8. Wright CS. Arch Dermatol. 1961;84(3):515.
9. Savitt LE. Arch Dermatol. 1961 Apr;83:660-1.
10. Shalita AR, Harris H. Arch Dermatol. 1972 May;105(5):759-60.
11. Shanler SD, Ondo AL. Arch Dermatol. 2007 Nov;143(11):1369-71.
‘Hospital at home’ increases COVID capacity in large study
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
A “hospital at home” (HaH) program at Atrium Health, a large integrated delivery system in the Southeast, expanded its hospital capacity during the early phase of the COVID-19 pandemic by providing hospital-level acute care to COVID-19 patients at home, according to a new study in Annals of Internal Medicine.
“Virtual hospital programs have the potential to provide health systems with additional inpatient capacity during the COVID-19 pandemic and beyond,” wrote Kranthi Sitammagari, MD, from the Atrium Health Hospitalist Group, Monroe, N.C., and colleagues.
Whereas most previous HaH programs have relied on visiting nurses and physicians, the new study uses telemedicine to connect with patients. Advocate Health Care researchers published the only other study using the telemedicine-powered model in 2015.
The new Atrium Health study evaluated 1,477 patients who received care in the HaH program between March 23 and May 7 of this year after having been diagnosed with COVID-19. The program provided home monitoring and hospital-level care in a home-based virtual observation unit (VOU) and a virtual acute care unit (VACU).
Patients were tested for the virus in Atrium emergency departments, primary care clinics, urgent care centers, and external testing sites. Those who tested positive were invited to be cared for either in the VOU, if they had mild to moderate symptoms, or in the VACU, if they were sick enough to be admitted to the hospital.
Patients hop onboard
Nearly all COVID-positive patients tested in these sites agreed to be admitted to the hospital at home, coauthor Stephanie Murphy, DO, medical director of the Atrium Health HaH program, said in an interview.
Patients with moderate symptoms were glad to be monitored at home, she said. When they got to the point where the nurse supervising their care felt they needed escalation to acute care, they were asked whether they wanted to continue to be cared for at home. Most opted to stay home rather than be admitted to the hospital, where their loved ones couldn’t visit them.
Low-acuity patients in the VOU received daily telemonitoring by a nurse to identify disease progression and escalate care as needed. For those who required more care and were admitted to the VACU, a team of paramedics and registered nurses (RNs; mobile clinicians) visited the patient’s home within 24 hours, setting up a hospital bed, other necessary medical equipment, videoconferencing gear, and a remote-monitoring kit that included a blood pressure cuff, a pulse oximeter, and a thermometer.
Dedicated hospitalists and nurses managed patients with 24/7 coverage and monitoring, bringing in other specialties as needed for virtual consults. Mobile clinician and virtual provider visits continued daily until a patient’s condition improved to the point where they could be deescalated back to the VOU. After that, patients received mobile app-driven symptom monitoring and telephone follow-up with a nurse until they got better.
Few patients go to hospital
Overall, patients had a median length of stay of 11 days in the VOU or the VACU or both. The vast majority, 1,293 patients (88%), received care in the VOU only. In that cohort, just 40 patients (3%) required hospitalization in an Atrium facility. Sixteen of those patients spent time in an ICU, seven required ventilator support, and two died in the hospital.
A total of 184 patients (12%) were admitted to the VACU. Twenty-one (11%) required intravenous fluids, 16 (9%) received antibiotics, 40 (22%) required inhaler or nebulizer treatments, 41 (22%) used supplemental oxygen, and 24 (13%) were admitted to a conventional hospital. Of the latter patients, 10 were admitted to an ICU, one required a ventilator, and none died in the hospital.
Dr. Sitammagari, a hospitalist and comedical director for quality at Atrium Health, told this news organization that, overall, the outcomes for patients in the system’s HaH were comparable to those seen in the literature among other COVID-19 cohorts.
Augmenting hospital capacity
The authors note that treating the 160 VACU patients within the HaH saved hospital beds for other patients. The HaH maintained a consistent census of between 20 and 30 patients for the first 6 weeks as COVID-19 cases spread.
Since last spring, Dr. Murphy said, the Atrium HaH’s daily census has grown to between 30 and 45 patients. “We could absorb 50 patients if our hospitals required it.”
How much capacity does that add to Atrium Health? While there are 50 hospitals in the health system, the HaH was set up mainly to care for COVID-19 patients who would otherwise have been admitted to the 10 acute-care hospitals in the Charlotte, N.C., area. In the 4 weeks ending Nov. 16, these facilities carried an average daily census of around 160 COVID-19 patients, Dr. Murphy noted. “During that time, the Atrium Health HaH has carried, on average, about 20%-25% of that census.”
If the pandemic were to overwhelm area hospitals, she added, “the structure would support flexing up our staffing and supplies to expand to crisis capacity,” which could be up to 200 patients a day.
For the nurses who make most of the phone calls to patients, patients average about 12 to 15 per RN, Dr. Murphy said, and there’s one mobile clinician for every six to nine patients. That’s pretty consistent with the staffing on med-surg floors in hospitals, she said.
The physicians in the program include hospitalists dedicated to telemedicine and some doctors who can’t work in the regular hospital because they’re immunocompromised. The physicians round virtually, covering 12-17 HaH patients per day, according to Dr. Murphy.
Prior planning paid off
Unlike some other health care systems that have launched HaH programs with the aid of outside vendors, Atrium Health developed its own HaH and brought it online just 2 weeks after deciding to launch the program. Atrium was able to do this, Dr. Sitammagari explained, because before the pandemic its hospitalist program was already developing an HaH model to improve the care of high-risk patients after hospital discharge to prevent readmission.
While Atrium’s electronic health record system wasn’t designed for hospital at home, its health information technology department and clinicians collaborated in rewriting some of the workflows and order sets in the EHR. For example, they set up a nursing questionnaire to administer after VACU admission, and they created another form for automatic admission to the HaH after a patient tested positive for COVID-19. Atrium staff also modified a patient-doctor communications app to help clinicians monitor HaH patients, Dr. Murphy noted.
Other hospital systems have gotten up to speed on HaH pretty quickly by using platforms supplied by outside vendors. Adventist Health in Los Angeles, for example, started admitting patients to its hospital at home just a month after approaching a vendor called Medically Home.
COVID vs. non-COVID patients
Atrium’s decision to focus its HaH effort on COVID-19 patients is unusual among the small but growing number of health systems that have adopted the HaH model to increase their capacity. (Atrium is now transferring some hospitalized patients with other conditions to its HaH, but is still focusing mainly on COVID-19 in its HaH program.)
Bruce Leff, MD, a professor of health policy and management at Johns Hopkins Bloomberg School of Public Health, Baltimore, a leading expert on the HaH model, agrees that it can increase hospital capacity significantly.
Dr. Leff praised the Atrium Health study. “It proves that within an integrated delivery system you can quickly deploy and implement a virtual hospital in the specific-use case of COVID, and help patients and help the system at scale,” he said. “They took a bunch of people into the virtual observation unit and thereby kept people from overwhelming their [emergency department] and treated those people safely at home.”
Dr. Leff had no problem with Atrium’s focus on patients with COVID-19 rather than other conditions. “My guess is that they have the ability to take what they developed and apply it to other conditions. Once you have the ability to do acute care at home, you can do a lot at home.”
The biggest barrier to the spread of hospital at home remains the lack of insurer coverage. Dr. Murphy said that health plans are covering virtual physician consultations with patients in the HaH, as well as some other bits and pieces, but not the entire episode of acute care.
Dr. Leff believes that this will start changing soon. COVID-19 has altered the attitudes of physicians and hospitals toward telehealth, he noted, “and it has moved policy makers and payers to start thinking about the new models – home-based care in general and hospital at home in particular. For the first time in 25 years, payers are starting to get interested.”
Most of the authors are employees of Atrium Health. In addition, one coauthor reports being the cofounder of a digital health company, iEnroll, and receiving grants from The Heineman Foundation. Dr. Leff is an advisor to Medically Home, which provides support to hospital at home programs.
A version of this article originally appeared on Medscape.com.
Painful ethical choices in 2020 vs. 2010: How has thinking changed?
Much has changed in the 10 years since Medscape’s first survey on what physicians would do when faced with painful choices in patient care.
A new report, Ethics 2020: Life, Death, and Painful Dilemmas, shows that physicians’ value judgments have shifted in many respects, sometimes as a result of increased regulations and fears of litigation.
End-of-life decisions
Several of the questions in the survey revolved around end-of-life decisions, and in some cases, the differences seen in just a decade were striking. One example concerned life support decisions in the context of a family’s choices.
Age also seemed to play a role in the 2020 answers to that question: Physicians younger than 45 were more likely (28%) to answer “yes” (that they would withdraw life support in that instance) than were those 45 and older (16%).
A critical care physician said, “If the family appears to have an underlying motivation that may not be in the patient’s best interest, I might be inclined to pursue a legal decision prior to withdrawing support.”
A cardiologist had a more pointed response to the question: “To me, that would be murder.”
Another example of how perspectives have changed over the past 10 years concerns whether physician-aided dying should be legal for terminally ill patients. The practice is now mandated by law in eight states and the District of Columbia, and it is mandated by court ruling in two additional states.
In 2010, 41% said “no.” That number dropped to 28% in 2020.
On legalization, a psychiatrist said, “Yes, when there is truly no hope and the quality of remaining life is too poor. We show more compassion to our sick animals than we do to our human population.”
Conversely, a neurologist answered, “No, I see younger physicians already becoming comfortable with the idea of deciding ASAP whether there is a reasonable chance of survival and then pressing for the right code status. This change would make things worse.”
Assisted death and incurable suffering
Far fewer physicians supported physician-assisted death for those who had years to live but faced incurable suffering: Thirty-seven percent said “yes,” 34% said “no,” and 29% said “it depends.”
However, support was significantly higher than it was just 2 years ago, in 2018, when only 27% supported the concept, the report authors noted.
“The shift reflects movements by many states to legalize assisted dying for the terminally ill,” Arthur Caplan, PhD, director of the division of medical ethics, New York University, said in the report. “Legalization has not been abused, so some doctors are more willing to press further beyond terminal illness as a trigger to suffering.”
Conversely, many more physicians (44% vs. 24% a decade ago) said they would provide life-sustaining therapy if the family requested it, even if the physician thought it was futile.
“Concerns over a malpractice lawsuit and potential negative patient/family online reviews are factors that play into this change,” the survey authors wrote.
Shared decision making also increased in the past decade.
Would you undertreat pain?
Primary care physicians fear the consequences of what they consider adequate pain management more than specialists do (24% vs. 17%), the survey authors noted.
Ten years ago, Medscape asked physicians whether they would undertreat a patient’s pain because of fear of repercussions or the patient’s becoming addicted: Eighty-four percent said “no,” and 6% said “yes.” The rest said “it depends.”
In 2020, the question was asked slightly differently: “Would you undertreat a patient’s pain for fear of addiction or Drug Enforcement Administration or medical board scrutiny?” This year, three times as many said “yes” (18%); 63% said “no.”
“Respondents this year talked about investigations and reprimands by medical boards, and how much they wanted to avoid that,” the survey authors wrote.
Should physicians be required to treat COVID-19 patients?
Some questions were new this year, including one on whether physicians should be required to treat COVID-19 patients. Fewer than half (47%) answered “yes,” 24% said “no,” and 29% answered “it depends.”
Doctors’ answers to this question differed slightly by gender: Fifty percent of men and 43% of women said “yes.” In their responses, many physicians said consideration should be given to risk factors, such as age, underlying conditions, risk of family members, and availability of personal protective equipment (PPE).
Another pandemic-related question asked whether physicians felt they should correct physicians who post misinformation about the pandemic on social media. Half (50%) said “yes,” 19% said “no,” and 31% said “it depends.”
Speaking out against the workplace
This year, many physicians have felt betrayed when they didn’t have adequate PPE during the pandemic.
Asked, “Is it right to speak out against your hospital or workplace when they don’t give you what you need?” 53% of physicians said “yes,” 8% said “no,” and 40% said “it depends.”
A cardiologist made the value judgment this way: “Speaking out just because you had an argument with your boss is inappropriate. Bringing to the public repeated failures to correct situations that have been brought through the proper channels is necessary to incite change.”
Random drug testing for physicians?
Another question in the survey asked whether physicians should be subjected to random drug testing for alcohol and drug abuse. About one-third (34%) said yes, 43% said no, and 23% said “it depends.” A study found that between 10% and 15% of physicians have abused a substance at some point in their careers.
The subject continues to hit a nerve in medicine.
A family physician wrote, “This should not be done unless a particular physician had a problem with drug or alcohol abuse and shows signs of impairment.”
An internist took a different view, saying, “Military service men and women, police, firefighters, airline pilots, and other professions that have responsibilities affecting people’s lives are subject to testing; why not physicians?”
A version of this article originally appeared on Medscape.com.
Much has changed in the 10 years since Medscape’s first survey on what physicians would do when faced with painful choices in patient care.
A new report, Ethics 2020: Life, Death, and Painful Dilemmas, shows that physicians’ value judgments have shifted in many respects, sometimes as a result of increased regulations and fears of litigation.
End-of-life decisions
Several of the questions in the survey revolved around end-of-life decisions, and in some cases, the differences seen in just a decade were striking. One example concerned life support decisions in the context of a family’s choices.
Age also seemed to play a role in the 2020 answers to that question: Physicians younger than 45 were more likely (28%) to answer “yes” (that they would withdraw life support in that instance) than were those 45 and older (16%).
A critical care physician said, “If the family appears to have an underlying motivation that may not be in the patient’s best interest, I might be inclined to pursue a legal decision prior to withdrawing support.”
A cardiologist had a more pointed response to the question: “To me, that would be murder.”
Another example of how perspectives have changed over the past 10 years concerns whether physician-aided dying should be legal for terminally ill patients. The practice is now mandated by law in eight states and the District of Columbia, and it is mandated by court ruling in two additional states.
In 2010, 41% said “no.” That number dropped to 28% in 2020.
On legalization, a psychiatrist said, “Yes, when there is truly no hope and the quality of remaining life is too poor. We show more compassion to our sick animals than we do to our human population.”
Conversely, a neurologist answered, “No, I see younger physicians already becoming comfortable with the idea of deciding ASAP whether there is a reasonable chance of survival and then pressing for the right code status. This change would make things worse.”
Assisted death and incurable suffering
Far fewer physicians supported physician-assisted death for those who had years to live but faced incurable suffering: Thirty-seven percent said “yes,” 34% said “no,” and 29% said “it depends.”
However, support was significantly higher than it was just 2 years ago, in 2018, when only 27% supported the concept, the report authors noted.
“The shift reflects movements by many states to legalize assisted dying for the terminally ill,” Arthur Caplan, PhD, director of the division of medical ethics, New York University, said in the report. “Legalization has not been abused, so some doctors are more willing to press further beyond terminal illness as a trigger to suffering.”
Conversely, many more physicians (44% vs. 24% a decade ago) said they would provide life-sustaining therapy if the family requested it, even if the physician thought it was futile.
“Concerns over a malpractice lawsuit and potential negative patient/family online reviews are factors that play into this change,” the survey authors wrote.
Shared decision making also increased in the past decade.
Would you undertreat pain?
Primary care physicians fear the consequences of what they consider adequate pain management more than specialists do (24% vs. 17%), the survey authors noted.
Ten years ago, Medscape asked physicians whether they would undertreat a patient’s pain because of fear of repercussions or the patient’s becoming addicted: Eighty-four percent said “no,” and 6% said “yes.” The rest said “it depends.”
In 2020, the question was asked slightly differently: “Would you undertreat a patient’s pain for fear of addiction or Drug Enforcement Administration or medical board scrutiny?” This year, three times as many said “yes” (18%); 63% said “no.”
“Respondents this year talked about investigations and reprimands by medical boards, and how much they wanted to avoid that,” the survey authors wrote.
Should physicians be required to treat COVID-19 patients?
Some questions were new this year, including one on whether physicians should be required to treat COVID-19 patients. Fewer than half (47%) answered “yes,” 24% said “no,” and 29% answered “it depends.”
Doctors’ answers to this question differed slightly by gender: Fifty percent of men and 43% of women said “yes.” In their responses, many physicians said consideration should be given to risk factors, such as age, underlying conditions, risk of family members, and availability of personal protective equipment (PPE).
Another pandemic-related question asked whether physicians felt they should correct physicians who post misinformation about the pandemic on social media. Half (50%) said “yes,” 19% said “no,” and 31% said “it depends.”
Speaking out against the workplace
This year, many physicians have felt betrayed when they didn’t have adequate PPE during the pandemic.
Asked, “Is it right to speak out against your hospital or workplace when they don’t give you what you need?” 53% of physicians said “yes,” 8% said “no,” and 40% said “it depends.”
A cardiologist made the value judgment this way: “Speaking out just because you had an argument with your boss is inappropriate. Bringing to the public repeated failures to correct situations that have been brought through the proper channels is necessary to incite change.”
Random drug testing for physicians?
Another question in the survey asked whether physicians should be subjected to random drug testing for alcohol and drug abuse. About one-third (34%) said yes, 43% said no, and 23% said “it depends.” A study found that between 10% and 15% of physicians have abused a substance at some point in their careers.
The subject continues to hit a nerve in medicine.
A family physician wrote, “This should not be done unless a particular physician had a problem with drug or alcohol abuse and shows signs of impairment.”
An internist took a different view, saying, “Military service men and women, police, firefighters, airline pilots, and other professions that have responsibilities affecting people’s lives are subject to testing; why not physicians?”
A version of this article originally appeared on Medscape.com.
Much has changed in the 10 years since Medscape’s first survey on what physicians would do when faced with painful choices in patient care.
A new report, Ethics 2020: Life, Death, and Painful Dilemmas, shows that physicians’ value judgments have shifted in many respects, sometimes as a result of increased regulations and fears of litigation.
End-of-life decisions
Several of the questions in the survey revolved around end-of-life decisions, and in some cases, the differences seen in just a decade were striking. One example concerned life support decisions in the context of a family’s choices.
Age also seemed to play a role in the 2020 answers to that question: Physicians younger than 45 were more likely (28%) to answer “yes” (that they would withdraw life support in that instance) than were those 45 and older (16%).
A critical care physician said, “If the family appears to have an underlying motivation that may not be in the patient’s best interest, I might be inclined to pursue a legal decision prior to withdrawing support.”
A cardiologist had a more pointed response to the question: “To me, that would be murder.”
Another example of how perspectives have changed over the past 10 years concerns whether physician-aided dying should be legal for terminally ill patients. The practice is now mandated by law in eight states and the District of Columbia, and it is mandated by court ruling in two additional states.
In 2010, 41% said “no.” That number dropped to 28% in 2020.
On legalization, a psychiatrist said, “Yes, when there is truly no hope and the quality of remaining life is too poor. We show more compassion to our sick animals than we do to our human population.”
Conversely, a neurologist answered, “No, I see younger physicians already becoming comfortable with the idea of deciding ASAP whether there is a reasonable chance of survival and then pressing for the right code status. This change would make things worse.”
Assisted death and incurable suffering
Far fewer physicians supported physician-assisted death for those who had years to live but faced incurable suffering: Thirty-seven percent said “yes,” 34% said “no,” and 29% said “it depends.”
However, support was significantly higher than it was just 2 years ago, in 2018, when only 27% supported the concept, the report authors noted.
“The shift reflects movements by many states to legalize assisted dying for the terminally ill,” Arthur Caplan, PhD, director of the division of medical ethics, New York University, said in the report. “Legalization has not been abused, so some doctors are more willing to press further beyond terminal illness as a trigger to suffering.”
Conversely, many more physicians (44% vs. 24% a decade ago) said they would provide life-sustaining therapy if the family requested it, even if the physician thought it was futile.
“Concerns over a malpractice lawsuit and potential negative patient/family online reviews are factors that play into this change,” the survey authors wrote.
Shared decision making also increased in the past decade.
Would you undertreat pain?
Primary care physicians fear the consequences of what they consider adequate pain management more than specialists do (24% vs. 17%), the survey authors noted.
Ten years ago, Medscape asked physicians whether they would undertreat a patient’s pain because of fear of repercussions or the patient’s becoming addicted: Eighty-four percent said “no,” and 6% said “yes.” The rest said “it depends.”
In 2020, the question was asked slightly differently: “Would you undertreat a patient’s pain for fear of addiction or Drug Enforcement Administration or medical board scrutiny?” This year, three times as many said “yes” (18%); 63% said “no.”
“Respondents this year talked about investigations and reprimands by medical boards, and how much they wanted to avoid that,” the survey authors wrote.
Should physicians be required to treat COVID-19 patients?
Some questions were new this year, including one on whether physicians should be required to treat COVID-19 patients. Fewer than half (47%) answered “yes,” 24% said “no,” and 29% answered “it depends.”
Doctors’ answers to this question differed slightly by gender: Fifty percent of men and 43% of women said “yes.” In their responses, many physicians said consideration should be given to risk factors, such as age, underlying conditions, risk of family members, and availability of personal protective equipment (PPE).
Another pandemic-related question asked whether physicians felt they should correct physicians who post misinformation about the pandemic on social media. Half (50%) said “yes,” 19% said “no,” and 31% said “it depends.”
Speaking out against the workplace
This year, many physicians have felt betrayed when they didn’t have adequate PPE during the pandemic.
Asked, “Is it right to speak out against your hospital or workplace when they don’t give you what you need?” 53% of physicians said “yes,” 8% said “no,” and 40% said “it depends.”
A cardiologist made the value judgment this way: “Speaking out just because you had an argument with your boss is inappropriate. Bringing to the public repeated failures to correct situations that have been brought through the proper channels is necessary to incite change.”
Random drug testing for physicians?
Another question in the survey asked whether physicians should be subjected to random drug testing for alcohol and drug abuse. About one-third (34%) said yes, 43% said no, and 23% said “it depends.” A study found that between 10% and 15% of physicians have abused a substance at some point in their careers.
The subject continues to hit a nerve in medicine.
A family physician wrote, “This should not be done unless a particular physician had a problem with drug or alcohol abuse and shows signs of impairment.”
An internist took a different view, saying, “Military service men and women, police, firefighters, airline pilots, and other professions that have responsibilities affecting people’s lives are subject to testing; why not physicians?”
A version of this article originally appeared on Medscape.com.
One-third of critical illness survivors emerge from ICU with functional deterioration
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
More patients are surviving critical illnesses requiring ICU care but many emerge with physical debility that may or may not eventually resolve.
Over the past decade, functional status deterioration after critical illness has become more common and of greater magnitude, despite concurrent efforts to reduce post–intensive care syndrome, based on a retrospective analysis of more than 100,000 patients.
Almost one-third of patients who survived nonsurgical ICU admission had evidence of functional status decline, reported lead author Nicholas E. Ingraham, MD, of the University of Minnesota, Minneapolis, and colleagues.
“Increasing capacity and decreasing mortality have created an evolving and diverse population of ICU survivors,” the investigators wrote in Critical Care Medicine. “Today’s survivors of critical illness are increasingly burdened by extensive physical and psychological comorbidities, often resulting in reduced quality of life.”
To determine trends in post–intensive care syndrome from 2008 to 2016, Dr. Ingraham and colleagues analyzed data from the Cerner Acute Physiology and Chronic Health Evaluation outcomes database, a national prospective cohort. Out of 202,786 adult patients admitted to the ICU, 129,917 were eligible for the study. Patients were excluded because of surgical admission, death, lack of functional status documentation, or inadequate hospital size or duration of participation. The final dataset had a median age of 63 years, with a slight predominance of male patients (54.0%). Most patients (80.9%) were White.
The primary outcome was defined as presence or absence of functional status deterioration, based on functional status at admission versus time of discharge. The secondary outcome was magnitude of deterioration over time.
The analysis, which controlled for age and severity of illness, revealed concerning trends for both outcomes.
Across the entire cohort 38,116 patients (29.3%) had functional status deterioration, with a 15% increase in prevalence over the course of the decade that spanned all disease categories (prevalence rate ratio, 1.15; 95% confidence interval, 1.13-1.17; P < .001). The magnitude of functional status decline also increased by 4% (odds ratio, 1.04; P < .001), with all but nonsurgical trauma patients showing greater deterioration over time.
“However, despite the decreasing magnitude of functional status deterioration in nonsurgical trauma, many admission diagnoses in this category remain in the top quartile of higher risk for functional status deterioration,” the investigators noted.
Functional status decline was most common among patients with head and polytrauma (OR, 3.39), followed closely by chest and spine trauma (OR, 3.38), and spine trauma (OR, 3.19). The top quartile of categories for prevalence of deterioration included nonsurgical trauma, neurologic, pulmonary, and gastrointestinal diseases.
Functional status decline was least common among patients diagnosed with diabetic ketoacidosis (OR, 0.27) or asthma (OR, 0.35).
“We believe our study provides important information that can be used in beginning to identify patients at high risk of functional status decline,” the investigators concluded. “Improving the identification of these patients and targeting appropriate interventions to mitigate this decline will be important directions for future studies in this area.”
According to David L. Bowton, MD, FCCP, professor emeritus, section on critical care, Wake Forest Baptist Health, Winston-Salem, N.C., the findings show just how common functional decline is after critical illness, and may actually underestimate prevalence.
“Because the authors employed a course evaluation tool employing only three categories of ability/disability and abstracted the level of disability from the medical record, they likely underestimated the frequency of clinically important, though not detected, disability at the time of hospital discharge,” Dr. Bowton said. “The study did not address cognitive impairment which can be detected in half of patients at 3 months following critical illness, and which significantly affects patients’ quality of life (Am J Respir Crit Care Med. 2020;202[2]:193-201).”
Dr. Bowton suggested that evidence-based methods of preventing post–intensive care syndrome are limited.
“Current efforts to improve post-ICU functional and cognitive outcomes suffer from the lack of proven effective interventions (Crit Care Med. 2019;47[11]:1607-18),” he said. “Observational data indicates that compliance with the ABCDEF bundle decreases the duration and incidence of delirium, ICU length of stay, duration of mechanical ventilation, and mortality (Crit Care Med. 2019;47[1]:3-14). However, the implications of these improvements on postdischarge functional outcomes are unknown as area the relative importance of individual elements of the bundle. Early mobility and patient and family diaries appear to improve functional status at discharge and postdischarge anxiety and depression, though the evidence supporting this is thin.”
Appropriate intervention may be especially challenging during the COVID-19 pandemic, he added.
“The impact of COVID on ICU staffing adequacy and stress is significant and the impact on quality bundle compliance and the availability of support services is currently not clear, but likely to be detrimental, especially to support services such as physical therapy that are already commonly understaffed,” Dr. Bowton said.
The study was supported by grants from the University of Minnesota’s Critical Care Research and Programmatic Development Program; the National Heart, Lung, and Blood Institute; and the University of Minnesota Clinical and Translational Science via the National Center for Advancing Translational Sciences. The investigators reported financial relationships with no other relevant organizations. Dr. Bowton reported no conflicts of interest.
SOURCE: Ingraham NE et al. Crit Care Med. 2020 Nov. doi: 10.1097/CCM.0000000000004524.
FROM CRITICAL CARE MEDICINE
COVID-19 burdens follow patients after discharge
COVID-19 patients who survive their hospitalization don’t leave the disease behind upon discharge, as a significant percentage died within 60 days of discharge, with an ICU admission heightening the risk, according to an observational study of 38 Michigan hospitals. What’s more, many of them were burdened with health and emotional challenges ranging from hospital readmission to job loss and financial problems.
“These data confirm that the toll of COVID-19 extends well beyond hospitalization, a finding consistent with long-term sequelae from sepsis and other severe respiratory viral illnesses,” wrote lead author Vineet Chopra, MBBS, of the University of Michigan, Ann Arbor, and colleagues (Ann Intern Med. 2020 Nov 11: doi: 10.7326/M20-5661)
The researchers found that 29.2% of all patients hospitalized for COVID-19 from March 16 to July 1 died. The observational cohort study included 1,648 COVID-19 patients hospitalized at 38 Michigan hospitals participating in a statewide collaborative.
The bulk of those deaths occurred during hospitalization: 24.2% of patients (n = 398). Of the 1,250 patients discharged, 78% (n = 975) went home and 12.6% (n = 158) went to a skilled nursing facility, with the remainder unaccounted for. Within 60 days of discharge, 6.7% (n = 84) of hospitalized survivors had died and 15.2% (n = 189) were readmitted. The researchers gathered 60-day postdischarge data via a telephone survey, contacting 41.8% (n = 488) of discharged patients.
Outcomes were even worse for discharged patients who spent time in the ICU. The death rate among this group was 10.4% (17 of 165) after discharge. That resulted in an overall study death rate of 63.5% (n = 257) for the 405 patients who were in the ICU.
While the study data were in the first wave of the novel coronavirus, the findings have relevance today, said Mary Jo Farmer, MD, PhD, FCCP, directory of pulmonary hypertension services at Baystate Health in Springfield, Mass.
“This is the best information we have to date,” she said. “We have to continue to have an open mind and expect that this information may change as the virus possibly mutates as it spreads, and we should continue doing these types of outcomes studies at 90 days, 120 days, etc.”
The median age of study patients was 62, with a range of 50-72. The three leading comorbidities among discharged patients were hypertension (n = 800, 64%), diabetes (34.9%, n = 436), and cardiovascular disease (24.1%, n = 301).
Poor postdischarge outcomes weren’t limited to mortality and readmission. Almost 19% (n = 92) reported new or worsening cardiopulmonary symptoms such as cough and dyspnea, 13.3% had a persistent loss of taste or smell, and 12% (n = 58) reported more difficulty with daily living tasks.
The after-effects were not only physical. Nearly half of discharged patients (48.7%, n = 238) reported emotional effects and almost 6% (n = 28) sought mental health care. Among the 40% (n = 195) employed before they were hospitalized, 36% (n = 78) couldn’t return to work because of health issues or layoffs. Sixty percent (n = 117) of the pre-employed discharged patients did return to work, but 25% (n = 30) did so with reduced hours or modified job duties because of health problems.
Financial problems were also a burden. More than a third, 36.7% (n = 179), reported some financial impact from their hospitalization. About 10% (n = 47) said they used most or all of their savings, and 7% (n = 35) said they resorted to rationing necessities such as food or medications.
The researchers noted that one in five patients had no primary care follow-up at 2 months post discharge. “Collectively, these findings suggest that better models to support COVID-19 survivors are necessary,” said Dr. Chopra and colleagues.
The postdischarge course for patients involves two humps, said Sachin Gupta, MD, FCCP a pulmonary and critical care specialist at Alameda Health System in Oakland, Calif.: Getting over the hospitalization itself and the recovery phase. “As you look at the median age of the survivors, elderly patients who survive a hospital stay are still going to have a period of recovery, and like any viral illness that leads to someone being hospitalized, when you have an elderly patient with comorbidities, not all of them can make it over that final hump.”
He echoed the study authors’ call for better postdischarge support for COVID-19 patients. “There’s typically, although not at every hospital, a one-size-fits-all discharge planning process,” Dr. Gupta said. “For older patients, particularly with comorbid conditions, close follow-up after discharge is important.”
Dr. Farmer noted that one challenge in discharge support may be a matter of personnel. “The providers of this care might be fearful of patients who have had COVID-19 – Do the patients remain contagious? What if symptoms of COVID-19 return such as dry cough, fever? – and of contracting the disease themselves,” she said.
The findings regarding the emotional status of discharged patients should factor into discharge planning, she added. “Providers of posthospital care need to be educated in the emotional impact of this disease (e.g., the patients may feel ostracized or that no one wants to be around them) to assist in their recovery.”
Dr. Chopra and Dr. Farmer have no financial relationships to disclose. Dr. Gupta is an employee and shareholder of Genentech.
SOURCE: Chopra V et al. Ann Intern Med. 2020 Nov 11. doi: 10.7326/M20-5661.
COVID-19 patients who survive their hospitalization don’t leave the disease behind upon discharge, as a significant percentage died within 60 days of discharge, with an ICU admission heightening the risk, according to an observational study of 38 Michigan hospitals. What’s more, many of them were burdened with health and emotional challenges ranging from hospital readmission to job loss and financial problems.
“These data confirm that the toll of COVID-19 extends well beyond hospitalization, a finding consistent with long-term sequelae from sepsis and other severe respiratory viral illnesses,” wrote lead author Vineet Chopra, MBBS, of the University of Michigan, Ann Arbor, and colleagues (Ann Intern Med. 2020 Nov 11: doi: 10.7326/M20-5661)
The researchers found that 29.2% of all patients hospitalized for COVID-19 from March 16 to July 1 died. The observational cohort study included 1,648 COVID-19 patients hospitalized at 38 Michigan hospitals participating in a statewide collaborative.
The bulk of those deaths occurred during hospitalization: 24.2% of patients (n = 398). Of the 1,250 patients discharged, 78% (n = 975) went home and 12.6% (n = 158) went to a skilled nursing facility, with the remainder unaccounted for. Within 60 days of discharge, 6.7% (n = 84) of hospitalized survivors had died and 15.2% (n = 189) were readmitted. The researchers gathered 60-day postdischarge data via a telephone survey, contacting 41.8% (n = 488) of discharged patients.
Outcomes were even worse for discharged patients who spent time in the ICU. The death rate among this group was 10.4% (17 of 165) after discharge. That resulted in an overall study death rate of 63.5% (n = 257) for the 405 patients who were in the ICU.
While the study data were in the first wave of the novel coronavirus, the findings have relevance today, said Mary Jo Farmer, MD, PhD, FCCP, directory of pulmonary hypertension services at Baystate Health in Springfield, Mass.
“This is the best information we have to date,” she said. “We have to continue to have an open mind and expect that this information may change as the virus possibly mutates as it spreads, and we should continue doing these types of outcomes studies at 90 days, 120 days, etc.”
The median age of study patients was 62, with a range of 50-72. The three leading comorbidities among discharged patients were hypertension (n = 800, 64%), diabetes (34.9%, n = 436), and cardiovascular disease (24.1%, n = 301).
Poor postdischarge outcomes weren’t limited to mortality and readmission. Almost 19% (n = 92) reported new or worsening cardiopulmonary symptoms such as cough and dyspnea, 13.3% had a persistent loss of taste or smell, and 12% (n = 58) reported more difficulty with daily living tasks.
The after-effects were not only physical. Nearly half of discharged patients (48.7%, n = 238) reported emotional effects and almost 6% (n = 28) sought mental health care. Among the 40% (n = 195) employed before they were hospitalized, 36% (n = 78) couldn’t return to work because of health issues or layoffs. Sixty percent (n = 117) of the pre-employed discharged patients did return to work, but 25% (n = 30) did so with reduced hours or modified job duties because of health problems.
Financial problems were also a burden. More than a third, 36.7% (n = 179), reported some financial impact from their hospitalization. About 10% (n = 47) said they used most or all of their savings, and 7% (n = 35) said they resorted to rationing necessities such as food or medications.
The researchers noted that one in five patients had no primary care follow-up at 2 months post discharge. “Collectively, these findings suggest that better models to support COVID-19 survivors are necessary,” said Dr. Chopra and colleagues.
The postdischarge course for patients involves two humps, said Sachin Gupta, MD, FCCP a pulmonary and critical care specialist at Alameda Health System in Oakland, Calif.: Getting over the hospitalization itself and the recovery phase. “As you look at the median age of the survivors, elderly patients who survive a hospital stay are still going to have a period of recovery, and like any viral illness that leads to someone being hospitalized, when you have an elderly patient with comorbidities, not all of them can make it over that final hump.”
He echoed the study authors’ call for better postdischarge support for COVID-19 patients. “There’s typically, although not at every hospital, a one-size-fits-all discharge planning process,” Dr. Gupta said. “For older patients, particularly with comorbid conditions, close follow-up after discharge is important.”
Dr. Farmer noted that one challenge in discharge support may be a matter of personnel. “The providers of this care might be fearful of patients who have had COVID-19 – Do the patients remain contagious? What if symptoms of COVID-19 return such as dry cough, fever? – and of contracting the disease themselves,” she said.
The findings regarding the emotional status of discharged patients should factor into discharge planning, she added. “Providers of posthospital care need to be educated in the emotional impact of this disease (e.g., the patients may feel ostracized or that no one wants to be around them) to assist in their recovery.”
Dr. Chopra and Dr. Farmer have no financial relationships to disclose. Dr. Gupta is an employee and shareholder of Genentech.
SOURCE: Chopra V et al. Ann Intern Med. 2020 Nov 11. doi: 10.7326/M20-5661.
COVID-19 patients who survive their hospitalization don’t leave the disease behind upon discharge, as a significant percentage died within 60 days of discharge, with an ICU admission heightening the risk, according to an observational study of 38 Michigan hospitals. What’s more, many of them were burdened with health and emotional challenges ranging from hospital readmission to job loss and financial problems.
“These data confirm that the toll of COVID-19 extends well beyond hospitalization, a finding consistent with long-term sequelae from sepsis and other severe respiratory viral illnesses,” wrote lead author Vineet Chopra, MBBS, of the University of Michigan, Ann Arbor, and colleagues (Ann Intern Med. 2020 Nov 11: doi: 10.7326/M20-5661)
The researchers found that 29.2% of all patients hospitalized for COVID-19 from March 16 to July 1 died. The observational cohort study included 1,648 COVID-19 patients hospitalized at 38 Michigan hospitals participating in a statewide collaborative.
The bulk of those deaths occurred during hospitalization: 24.2% of patients (n = 398). Of the 1,250 patients discharged, 78% (n = 975) went home and 12.6% (n = 158) went to a skilled nursing facility, with the remainder unaccounted for. Within 60 days of discharge, 6.7% (n = 84) of hospitalized survivors had died and 15.2% (n = 189) were readmitted. The researchers gathered 60-day postdischarge data via a telephone survey, contacting 41.8% (n = 488) of discharged patients.
Outcomes were even worse for discharged patients who spent time in the ICU. The death rate among this group was 10.4% (17 of 165) after discharge. That resulted in an overall study death rate of 63.5% (n = 257) for the 405 patients who were in the ICU.
While the study data were in the first wave of the novel coronavirus, the findings have relevance today, said Mary Jo Farmer, MD, PhD, FCCP, directory of pulmonary hypertension services at Baystate Health in Springfield, Mass.
“This is the best information we have to date,” she said. “We have to continue to have an open mind and expect that this information may change as the virus possibly mutates as it spreads, and we should continue doing these types of outcomes studies at 90 days, 120 days, etc.”
The median age of study patients was 62, with a range of 50-72. The three leading comorbidities among discharged patients were hypertension (n = 800, 64%), diabetes (34.9%, n = 436), and cardiovascular disease (24.1%, n = 301).
Poor postdischarge outcomes weren’t limited to mortality and readmission. Almost 19% (n = 92) reported new or worsening cardiopulmonary symptoms such as cough and dyspnea, 13.3% had a persistent loss of taste or smell, and 12% (n = 58) reported more difficulty with daily living tasks.
The after-effects were not only physical. Nearly half of discharged patients (48.7%, n = 238) reported emotional effects and almost 6% (n = 28) sought mental health care. Among the 40% (n = 195) employed before they were hospitalized, 36% (n = 78) couldn’t return to work because of health issues or layoffs. Sixty percent (n = 117) of the pre-employed discharged patients did return to work, but 25% (n = 30) did so with reduced hours or modified job duties because of health problems.
Financial problems were also a burden. More than a third, 36.7% (n = 179), reported some financial impact from their hospitalization. About 10% (n = 47) said they used most or all of their savings, and 7% (n = 35) said they resorted to rationing necessities such as food or medications.
The researchers noted that one in five patients had no primary care follow-up at 2 months post discharge. “Collectively, these findings suggest that better models to support COVID-19 survivors are necessary,” said Dr. Chopra and colleagues.
The postdischarge course for patients involves two humps, said Sachin Gupta, MD, FCCP a pulmonary and critical care specialist at Alameda Health System in Oakland, Calif.: Getting over the hospitalization itself and the recovery phase. “As you look at the median age of the survivors, elderly patients who survive a hospital stay are still going to have a period of recovery, and like any viral illness that leads to someone being hospitalized, when you have an elderly patient with comorbidities, not all of them can make it over that final hump.”
He echoed the study authors’ call for better postdischarge support for COVID-19 patients. “There’s typically, although not at every hospital, a one-size-fits-all discharge planning process,” Dr. Gupta said. “For older patients, particularly with comorbid conditions, close follow-up after discharge is important.”
Dr. Farmer noted that one challenge in discharge support may be a matter of personnel. “The providers of this care might be fearful of patients who have had COVID-19 – Do the patients remain contagious? What if symptoms of COVID-19 return such as dry cough, fever? – and of contracting the disease themselves,” she said.
The findings regarding the emotional status of discharged patients should factor into discharge planning, she added. “Providers of posthospital care need to be educated in the emotional impact of this disease (e.g., the patients may feel ostracized or that no one wants to be around them) to assist in their recovery.”
Dr. Chopra and Dr. Farmer have no financial relationships to disclose. Dr. Gupta is an employee and shareholder of Genentech.
SOURCE: Chopra V et al. Ann Intern Med. 2020 Nov 11. doi: 10.7326/M20-5661.
FROM ANNALS OF INTERNAL MEDICINE
Pediatric dermatology: Reflecting on 50 years
As part of the 50th anniversary of Dermatology News, it is intriguing to think about where a time machine journey 5 decades back would find the field of pediatric dermatology, and to assess the changes in the specialty during the time that Dermatology News (operating then as “Skin & Allergy News”) has been reporting on innovations and changes in the practice of dermatology.
So, starting . It was not until 3 years later, in October 1973 in Mexico City, that the first international symposium on Pediatric Dermatology was held, and the International Society for Pediatric Dermatology was founded. I reached out to Andrew Margileth, MD, 100 years old this past July, and still active voluntary faculty in pediatric dermatology at the University of Miami, to help me “reach back” to those days. Dr. Margileth commented on how the first symposium was “brilliantly orchestrated by Ramon Ruiz-Maldonado,” from the National Institute of Paediatrics in Mexico, and that it was his “Aha moment for future practice!” That meeting spurred discussions on the development of the Society for Pediatric Dermatology the next year, with Alvin Jacobs, MD; Samuel Weinberg, MD; Nancy Esterly, MD; Sidney Hurwitz, MD; William Weston, MD; and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers,” and the society was officially established in 1975.
The field of pediatric dermatology was fairly “infantile” 50 years ago, with few practitioners. But the early leaders in the field recognized that up to 30% of pediatric primary care visits included skin problems, and that there was limited training for dermatologists, as well as pediatricians, about skin diseases in children. There were clearly clinical and educational needs to establish a subspecialty of pediatric dermatology, and over the next 1-2 decades, the field expanded. The journal Pediatric Dermatology was established (in 1982), the Section on Dermatology was established by the American Academy of Pediatrics (in 1986), and fellowship programs were launched at select academic centers. And it was 30 years into our timeline before the formal subspecialty of pediatric dermatology was established through the American Board of Dermatology (2000).
The field of pediatric dermatology has evolved and matured rapidly. Standard reference textbooks have been developed in the United States and around the world (and of course, online). Pediatric dermatology is an essential part of the core curriculum for dermatologist trainees. Organizations promoting pediatric research have developed to influence basic, translational, and clinical research in conditions in neonates through adolescents, such as the Pediatric Dermatology Research Alliance (PeDRA). And meetings throughout the world now feature pediatric dermatology sessions and help to spread the advances in the diagnosis and management of pediatric skin disorders.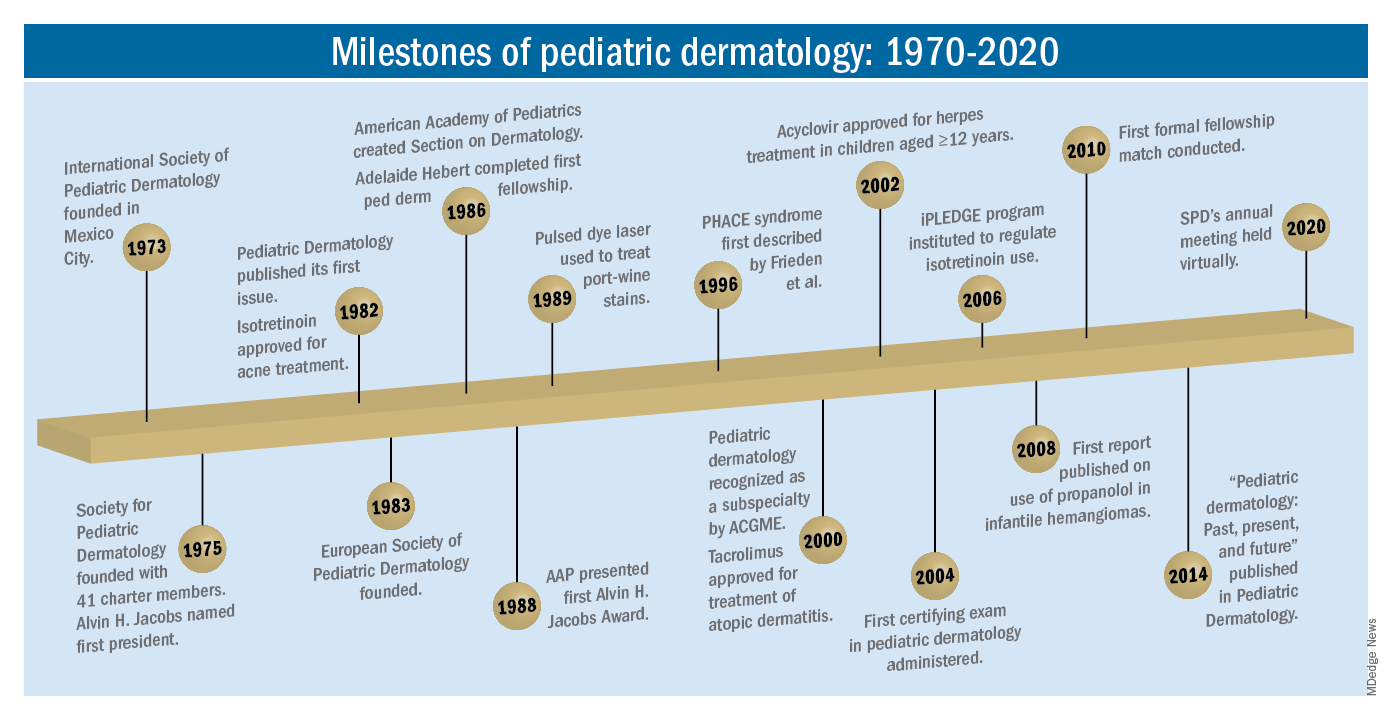
The practice of pediatric dermatology: How has it changed?
It is beyond the scope of this article to try to comprehensively review all of the changes in pediatric dermatology practice. But review of the evolution of a few disease states (choices influenced by my discussions with my 100-year old history guide, Dr. Margileth) displays examples of where we have been, and where we are going in our next 5, 10, or 50 years.
Hemangiomas and vascular malformations
Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that standard cutaneous hemangiomas had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of interest, the hallmark article’s first author was Dr. Margileth, published in 1965 in JAMA!.This was still at a time when the identification of hemangiomas of infancy (or “HOI” as we say in the trade) was confused with vascular malformations, and no one had recognized the distinct variant tumors such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, or hemangioendotheliomas. PHACE syndrome was not yet described (that was done in 1996 by Ilona Frieden, MD, and colleagues). And for a time, hemangiomas were treated with x-rays, before the negative impact of such radiation was acknowledged. It seems that, as a consequence of the use of x-ray therapy and as a backlash from the radiation therapy side effects and potential toxicities, even deforming and functionally significant lesions were “followed clinically” for natural involution, with a sensibility that doing nothing might be better than doing the wrong thing.
Over the next 15 years, the recognition of functionally significant hemangiomas, deformation associated with their proliferation, and the recognition of PHACE syndrome made hemangiomas of infancy an area of concern, with systemic steroids and occasionally chemotherapeutic agents (such as vincristine) being used for problematic lesions.
It has now been 12 years since the work of Christine Léauté-Labrèze, MD, et al., from the University of Bordeaux (France), led to the breakthrough of propranolol for hemangioma treatment, profoundly changing hemangioma management to an incredibly effective medical therapy extensively studied, tested in formal clinical trials, and approved by regulatory authorities. And how intriguing that this was pursued after the chance (but skilled) observation that a child who developed hypertension as a side effect of systemic steroids for nasal hemangioma treatment was prescribed propranolol for the hypertension and had his nasal hemangioma rapidly shrink, with a response superior and much quicker than that to corticosteroids.
The evolution of management of hemangiomas has another story within it, that of collaborative research. The Hemangioma Investigator Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development, and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas. Our knowledge of these lesions is now evidence based and broad, and the impact on care tremendous! The HIG has also influenced the practice of pediatricians and other specialists, including otorhinolaryngologists, hematologist/oncologists, and surgeons, is partnering with advocacy groups to support patients and families, and is helping guide patients and families to contribute to ongoing research.
Vascular malformations (VM) reflect an incredible change in our understanding of the developmental pathways and pathophysiology of blood vessel tumors, and, in fact, birthmarks other than vascular lesions! First, important work separated out hemangiomas of infancy and hemangiomalike tumors from vascular malformations, with the thought being that hemangiomas had a rapid growth phase, often arising from lesions that were minimally evident or not evident at birth, unlike malformations, which were “programing errors,” all present at birth and expected to be fairly static with proportionate growth over a lifetime. Approaches to vascular malformations were limited to sclerotherapy, laser, and/or surgery. While this general schema of classification is still useful, our sense of the “why and how” of vascular malformations is remarkably different. Vascular malformations – still usefully subdivided into capillary, lymphatic, venous arteriovenous, or mixed malformations – are mostly associated with inherited or somatic mutations. Mutations are most commonly found in two signal pathways: RAS/MAPK/ERK and PI3K/AKT/mTOR pathways, with specific sets of mutations seen in both localized and multifocal lesions, with or without overgrowth or other systemic anomalies. The discovery of specific mutations has led to the possibility of small-molecule inhibitors, many already existing as anticancer drugs, being utilized as targeted therapies for VM.
And similar advances in understanding of other birthmarks, with or without syndromic features, are being made steadily. The mutations in congenital melanocytic nevi, epidermal nevi, acquired tumors (pilomatricomas), and other lesions, along with steady epidemiologic, translational, and clinical work, evolves our knowledge and potential therapies.
Inflammatory skin disorders: Acne, psoriasis, and atopic dermatitis
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris is now recognized as much more common under age 12 than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later, with expert recommendations formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013. Over the past few years, there has been a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance. There are unanswered questions as we evolve our care: How will the new topical antiandrogens be used? Will spironolactone become part of hormonal therapy under age 18? Will the insights on certain strains of Cutibacterium acnes being associated with worse acne translate to microbiome or vaccine-based strategies?
Pediatric psoriasis has suffered, being “behind in the revolution” of biologic agents because of delayed approval of any biologic agent for treatment of pediatric psoriasis in the United States until just a few years ago, and lags behind Europe and elsewhere in the world by almost a decade. Only this year have we expanded beyond one biologic agent approved for under age 12 and two for ages 12 and older, with other approvals expected including interleukin (IL)-17 and IL-23 agents. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, with new recommendations on how to screen children with psoriasis, supplied first by PeDRA and then in the new American Academy of Dermatology-National Psoriasis Foundation pediatric psoriasis guidelines .
Pediatric atopic dermatitis (AD) is in its early years of revolution. In the 50-year period of our thought experiment, AD has increased in prevalence from 5% or less of the pediatric population to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, and management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their use of these useful agents. But newer studies are markedly reassuring about their safe use in children.
Steroid phobia, as well as concerns about potential side effects of the TCIs, has resulted in undertreatment of childhood AD. It is quite common to see multiple children during pediatric dermatology office hours with poorly controlled eczema, high body-surface areas of eczema, compromised sleep, secondary infections, and anxiety and depression, especially in our moderate to severe adolescents. The field is “hot” with new topical and systemic agents, including our few years’ experience with topical crisaborole, a phosphodiesterase (PDE)-4 inhibitor; and dupilumab, an IL-4-alpha blocker – the first biologic agent approved for AD and the first systemic agent (other than oral corticosteroids), just extended from 12 years to 6 years of age! As dupilumab gets studied for younger children, other biologics (including IL-13 and IL-31 blockers) are undertaking pediatric and/or adolescent trials, oral and topical JAK inhibitors are including adolescents in core clinical trials, and other novel topical agents are under study, including an aryl-hydrocarbon receptor–modulating agent and other PDE-4 inhibitors.
Procedural pediatric dermatology: From liquid nitrogen to laser, surgery, and multimodal skin care
The first generation of pediatric dermatologists were considered medical dermatologist specialists. The care of the conditions discussed above, as well as genodermatoses, diagnostic dilemmas, and management of dermatologic manifestations of systemic disease and other conditions, was the “bread and butter” of pediatric dermatology care. When I was in training, my mentor Paul Honig, MD, at the Children’s Hospital of Philadelphia had a procedure half-day each week, where he would care for a few patients who needed liquid nitrogen therapy for warts, or who needed biopsies. It was uncommon to have a large procedural/surgical part of pediatric dermatology practice. But this is now a routine part of many specialists in the field. How did this change occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser for treatment of port-wine birthmarks in children with minimal scarring, and a seminal article published in the New England Journal of Medicine in 1989. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists developed the exposure and skill to do this work. An added advantage was having the knowledge of how to handle children and adolescents in an age-appropriate manner, with consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota, for example), hair laser, and combinations of lasers, including fractionated CO2 technology, to treat hypertrophic, constrictive and/or deforming scars.
The future
What will pediatric dermatology be like in 10, 20, or 50 years?
I have not yet discussed some of the most challenging diseases in our field, including epidermolysis bullosa, ichthyosis, and neurocutaneous disorders and other genetic skin disorders that have an incredible impact on the lives of affected children and their families, with incredible morbidity and with many conditions that shorten lifespans. But these are the conditions where “the future is happening now,” and we are looking forward to our new gene therapy techniques helping to transform our care.
And other aspects of practice? Will we be doing a large percentage of practice over the phone (or whatever devices we have then – remember, the first iPhone was only released 13 years ago)?
Will our patients be using their own imaging systems to evaluate their nevi and skin growths, and perhaps to diagnose and manage their rashes?
Will we have prevented our inflammatory skin disorders, or “turned them off” in early life with aggressive therapy in infantile life?
I project only that all of us in dermatology will still be a resource to our pediatric patients, from neonate through young adult, through our work of preventing, caring, healing and minimizing disease impact, and hopefully enjoying the pleasures of seeing our patients healthfully develop and evolve! As will our field.
Dr. Eichenfield is professor of dermatology and pediatrics and vice-chair of the department of dermatology at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. Dr. Eichenfield reports financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including products for the diseases discussed here.
As part of the 50th anniversary of Dermatology News, it is intriguing to think about where a time machine journey 5 decades back would find the field of pediatric dermatology, and to assess the changes in the specialty during the time that Dermatology News (operating then as “Skin & Allergy News”) has been reporting on innovations and changes in the practice of dermatology.
So, starting . It was not until 3 years later, in October 1973 in Mexico City, that the first international symposium on Pediatric Dermatology was held, and the International Society for Pediatric Dermatology was founded. I reached out to Andrew Margileth, MD, 100 years old this past July, and still active voluntary faculty in pediatric dermatology at the University of Miami, to help me “reach back” to those days. Dr. Margileth commented on how the first symposium was “brilliantly orchestrated by Ramon Ruiz-Maldonado,” from the National Institute of Paediatrics in Mexico, and that it was his “Aha moment for future practice!” That meeting spurred discussions on the development of the Society for Pediatric Dermatology the next year, with Alvin Jacobs, MD; Samuel Weinberg, MD; Nancy Esterly, MD; Sidney Hurwitz, MD; William Weston, MD; and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers,” and the society was officially established in 1975.
The field of pediatric dermatology was fairly “infantile” 50 years ago, with few practitioners. But the early leaders in the field recognized that up to 30% of pediatric primary care visits included skin problems, and that there was limited training for dermatologists, as well as pediatricians, about skin diseases in children. There were clearly clinical and educational needs to establish a subspecialty of pediatric dermatology, and over the next 1-2 decades, the field expanded. The journal Pediatric Dermatology was established (in 1982), the Section on Dermatology was established by the American Academy of Pediatrics (in 1986), and fellowship programs were launched at select academic centers. And it was 30 years into our timeline before the formal subspecialty of pediatric dermatology was established through the American Board of Dermatology (2000).
The field of pediatric dermatology has evolved and matured rapidly. Standard reference textbooks have been developed in the United States and around the world (and of course, online). Pediatric dermatology is an essential part of the core curriculum for dermatologist trainees. Organizations promoting pediatric research have developed to influence basic, translational, and clinical research in conditions in neonates through adolescents, such as the Pediatric Dermatology Research Alliance (PeDRA). And meetings throughout the world now feature pediatric dermatology sessions and help to spread the advances in the diagnosis and management of pediatric skin disorders.
The practice of pediatric dermatology: How has it changed?
It is beyond the scope of this article to try to comprehensively review all of the changes in pediatric dermatology practice. But review of the evolution of a few disease states (choices influenced by my discussions with my 100-year old history guide, Dr. Margileth) displays examples of where we have been, and where we are going in our next 5, 10, or 50 years.
Hemangiomas and vascular malformations
Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that standard cutaneous hemangiomas had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of interest, the hallmark article’s first author was Dr. Margileth, published in 1965 in JAMA!.This was still at a time when the identification of hemangiomas of infancy (or “HOI” as we say in the trade) was confused with vascular malformations, and no one had recognized the distinct variant tumors such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, or hemangioendotheliomas. PHACE syndrome was not yet described (that was done in 1996 by Ilona Frieden, MD, and colleagues). And for a time, hemangiomas were treated with x-rays, before the negative impact of such radiation was acknowledged. It seems that, as a consequence of the use of x-ray therapy and as a backlash from the radiation therapy side effects and potential toxicities, even deforming and functionally significant lesions were “followed clinically” for natural involution, with a sensibility that doing nothing might be better than doing the wrong thing.
Over the next 15 years, the recognition of functionally significant hemangiomas, deformation associated with their proliferation, and the recognition of PHACE syndrome made hemangiomas of infancy an area of concern, with systemic steroids and occasionally chemotherapeutic agents (such as vincristine) being used for problematic lesions.
It has now been 12 years since the work of Christine Léauté-Labrèze, MD, et al., from the University of Bordeaux (France), led to the breakthrough of propranolol for hemangioma treatment, profoundly changing hemangioma management to an incredibly effective medical therapy extensively studied, tested in formal clinical trials, and approved by regulatory authorities. And how intriguing that this was pursued after the chance (but skilled) observation that a child who developed hypertension as a side effect of systemic steroids for nasal hemangioma treatment was prescribed propranolol for the hypertension and had his nasal hemangioma rapidly shrink, with a response superior and much quicker than that to corticosteroids.
The evolution of management of hemangiomas has another story within it, that of collaborative research. The Hemangioma Investigator Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development, and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas. Our knowledge of these lesions is now evidence based and broad, and the impact on care tremendous! The HIG has also influenced the practice of pediatricians and other specialists, including otorhinolaryngologists, hematologist/oncologists, and surgeons, is partnering with advocacy groups to support patients and families, and is helping guide patients and families to contribute to ongoing research.
Vascular malformations (VM) reflect an incredible change in our understanding of the developmental pathways and pathophysiology of blood vessel tumors, and, in fact, birthmarks other than vascular lesions! First, important work separated out hemangiomas of infancy and hemangiomalike tumors from vascular malformations, with the thought being that hemangiomas had a rapid growth phase, often arising from lesions that were minimally evident or not evident at birth, unlike malformations, which were “programing errors,” all present at birth and expected to be fairly static with proportionate growth over a lifetime. Approaches to vascular malformations were limited to sclerotherapy, laser, and/or surgery. While this general schema of classification is still useful, our sense of the “why and how” of vascular malformations is remarkably different. Vascular malformations – still usefully subdivided into capillary, lymphatic, venous arteriovenous, or mixed malformations – are mostly associated with inherited or somatic mutations. Mutations are most commonly found in two signal pathways: RAS/MAPK/ERK and PI3K/AKT/mTOR pathways, with specific sets of mutations seen in both localized and multifocal lesions, with or without overgrowth or other systemic anomalies. The discovery of specific mutations has led to the possibility of small-molecule inhibitors, many already existing as anticancer drugs, being utilized as targeted therapies for VM.
And similar advances in understanding of other birthmarks, with or without syndromic features, are being made steadily. The mutations in congenital melanocytic nevi, epidermal nevi, acquired tumors (pilomatricomas), and other lesions, along with steady epidemiologic, translational, and clinical work, evolves our knowledge and potential therapies.
Inflammatory skin disorders: Acne, psoriasis, and atopic dermatitis
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris is now recognized as much more common under age 12 than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later, with expert recommendations formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013. Over the past few years, there has been a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance. There are unanswered questions as we evolve our care: How will the new topical antiandrogens be used? Will spironolactone become part of hormonal therapy under age 18? Will the insights on certain strains of Cutibacterium acnes being associated with worse acne translate to microbiome or vaccine-based strategies?
Pediatric psoriasis has suffered, being “behind in the revolution” of biologic agents because of delayed approval of any biologic agent for treatment of pediatric psoriasis in the United States until just a few years ago, and lags behind Europe and elsewhere in the world by almost a decade. Only this year have we expanded beyond one biologic agent approved for under age 12 and two for ages 12 and older, with other approvals expected including interleukin (IL)-17 and IL-23 agents. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, with new recommendations on how to screen children with psoriasis, supplied first by PeDRA and then in the new American Academy of Dermatology-National Psoriasis Foundation pediatric psoriasis guidelines .
Pediatric atopic dermatitis (AD) is in its early years of revolution. In the 50-year period of our thought experiment, AD has increased in prevalence from 5% or less of the pediatric population to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, and management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their use of these useful agents. But newer studies are markedly reassuring about their safe use in children.
Steroid phobia, as well as concerns about potential side effects of the TCIs, has resulted in undertreatment of childhood AD. It is quite common to see multiple children during pediatric dermatology office hours with poorly controlled eczema, high body-surface areas of eczema, compromised sleep, secondary infections, and anxiety and depression, especially in our moderate to severe adolescents. The field is “hot” with new topical and systemic agents, including our few years’ experience with topical crisaborole, a phosphodiesterase (PDE)-4 inhibitor; and dupilumab, an IL-4-alpha blocker – the first biologic agent approved for AD and the first systemic agent (other than oral corticosteroids), just extended from 12 years to 6 years of age! As dupilumab gets studied for younger children, other biologics (including IL-13 and IL-31 blockers) are undertaking pediatric and/or adolescent trials, oral and topical JAK inhibitors are including adolescents in core clinical trials, and other novel topical agents are under study, including an aryl-hydrocarbon receptor–modulating agent and other PDE-4 inhibitors.
Procedural pediatric dermatology: From liquid nitrogen to laser, surgery, and multimodal skin care
The first generation of pediatric dermatologists were considered medical dermatologist specialists. The care of the conditions discussed above, as well as genodermatoses, diagnostic dilemmas, and management of dermatologic manifestations of systemic disease and other conditions, was the “bread and butter” of pediatric dermatology care. When I was in training, my mentor Paul Honig, MD, at the Children’s Hospital of Philadelphia had a procedure half-day each week, where he would care for a few patients who needed liquid nitrogen therapy for warts, or who needed biopsies. It was uncommon to have a large procedural/surgical part of pediatric dermatology practice. But this is now a routine part of many specialists in the field. How did this change occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser for treatment of port-wine birthmarks in children with minimal scarring, and a seminal article published in the New England Journal of Medicine in 1989. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists developed the exposure and skill to do this work. An added advantage was having the knowledge of how to handle children and adolescents in an age-appropriate manner, with consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota, for example), hair laser, and combinations of lasers, including fractionated CO2 technology, to treat hypertrophic, constrictive and/or deforming scars.
The future
What will pediatric dermatology be like in 10, 20, or 50 years?
I have not yet discussed some of the most challenging diseases in our field, including epidermolysis bullosa, ichthyosis, and neurocutaneous disorders and other genetic skin disorders that have an incredible impact on the lives of affected children and their families, with incredible morbidity and with many conditions that shorten lifespans. But these are the conditions where “the future is happening now,” and we are looking forward to our new gene therapy techniques helping to transform our care.
And other aspects of practice? Will we be doing a large percentage of practice over the phone (or whatever devices we have then – remember, the first iPhone was only released 13 years ago)?
Will our patients be using their own imaging systems to evaluate their nevi and skin growths, and perhaps to diagnose and manage their rashes?
Will we have prevented our inflammatory skin disorders, or “turned them off” in early life with aggressive therapy in infantile life?
I project only that all of us in dermatology will still be a resource to our pediatric patients, from neonate through young adult, through our work of preventing, caring, healing and minimizing disease impact, and hopefully enjoying the pleasures of seeing our patients healthfully develop and evolve! As will our field.
Dr. Eichenfield is professor of dermatology and pediatrics and vice-chair of the department of dermatology at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. Dr. Eichenfield reports financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including products for the diseases discussed here.
As part of the 50th anniversary of Dermatology News, it is intriguing to think about where a time machine journey 5 decades back would find the field of pediatric dermatology, and to assess the changes in the specialty during the time that Dermatology News (operating then as “Skin & Allergy News”) has been reporting on innovations and changes in the practice of dermatology.
So, starting . It was not until 3 years later, in October 1973 in Mexico City, that the first international symposium on Pediatric Dermatology was held, and the International Society for Pediatric Dermatology was founded. I reached out to Andrew Margileth, MD, 100 years old this past July, and still active voluntary faculty in pediatric dermatology at the University of Miami, to help me “reach back” to those days. Dr. Margileth commented on how the first symposium was “brilliantly orchestrated by Ramon Ruiz-Maldonado,” from the National Institute of Paediatrics in Mexico, and that it was his “Aha moment for future practice!” That meeting spurred discussions on the development of the Society for Pediatric Dermatology the next year, with Alvin Jacobs, MD; Samuel Weinberg, MD; Nancy Esterly, MD; Sidney Hurwitz, MD; William Weston, MD; and Coleman Jacobson, MD, as some of the initial “founding mothers and fathers,” and the society was officially established in 1975.
The field of pediatric dermatology was fairly “infantile” 50 years ago, with few practitioners. But the early leaders in the field recognized that up to 30% of pediatric primary care visits included skin problems, and that there was limited training for dermatologists, as well as pediatricians, about skin diseases in children. There were clearly clinical and educational needs to establish a subspecialty of pediatric dermatology, and over the next 1-2 decades, the field expanded. The journal Pediatric Dermatology was established (in 1982), the Section on Dermatology was established by the American Academy of Pediatrics (in 1986), and fellowship programs were launched at select academic centers. And it was 30 years into our timeline before the formal subspecialty of pediatric dermatology was established through the American Board of Dermatology (2000).
The field of pediatric dermatology has evolved and matured rapidly. Standard reference textbooks have been developed in the United States and around the world (and of course, online). Pediatric dermatology is an essential part of the core curriculum for dermatologist trainees. Organizations promoting pediatric research have developed to influence basic, translational, and clinical research in conditions in neonates through adolescents, such as the Pediatric Dermatology Research Alliance (PeDRA). And meetings throughout the world now feature pediatric dermatology sessions and help to spread the advances in the diagnosis and management of pediatric skin disorders.
The practice of pediatric dermatology: How has it changed?
It is beyond the scope of this article to try to comprehensively review all of the changes in pediatric dermatology practice. But review of the evolution of a few disease states (choices influenced by my discussions with my 100-year old history guide, Dr. Margileth) displays examples of where we have been, and where we are going in our next 5, 10, or 50 years.
Hemangiomas and vascular malformations
Some of the first natural history studies on hemangiomas were done in the early 1960s, establishing that standard cutaneous hemangiomas had a typical clinical course of fairly rapid growth, plateau, and involution over time. Of interest, the hallmark article’s first author was Dr. Margileth, published in 1965 in JAMA!.This was still at a time when the identification of hemangiomas of infancy (or “HOI” as we say in the trade) was confused with vascular malformations, and no one had recognized the distinct variant tumors such as rapidly involuting and noninvoluting congenital hemangiomas (RICHs or NICHs), tufted angiomas, or hemangioendotheliomas. PHACE syndrome was not yet described (that was done in 1996 by Ilona Frieden, MD, and colleagues). And for a time, hemangiomas were treated with x-rays, before the negative impact of such radiation was acknowledged. It seems that, as a consequence of the use of x-ray therapy and as a backlash from the radiation therapy side effects and potential toxicities, even deforming and functionally significant lesions were “followed clinically” for natural involution, with a sensibility that doing nothing might be better than doing the wrong thing.
Over the next 15 years, the recognition of functionally significant hemangiomas, deformation associated with their proliferation, and the recognition of PHACE syndrome made hemangiomas of infancy an area of concern, with systemic steroids and occasionally chemotherapeutic agents (such as vincristine) being used for problematic lesions.
It has now been 12 years since the work of Christine Léauté-Labrèze, MD, et al., from the University of Bordeaux (France), led to the breakthrough of propranolol for hemangioma treatment, profoundly changing hemangioma management to an incredibly effective medical therapy extensively studied, tested in formal clinical trials, and approved by regulatory authorities. And how intriguing that this was pursued after the chance (but skilled) observation that a child who developed hypertension as a side effect of systemic steroids for nasal hemangioma treatment was prescribed propranolol for the hypertension and had his nasal hemangioma rapidly shrink, with a response superior and much quicker than that to corticosteroids.
The evolution of management of hemangiomas has another story within it, that of collaborative research. The Hemangioma Investigator Group was formed to take a collaborative approach to characterize and study hemangiomas and related tumors. Beginning with energetic, insightful pediatric dermatologists and little funding, they changed our knowledge base of how hemangiomas present, the risk factors for their development, and the characteristics and multiple organ findings associated with PHACE and other syndromic hemangiomas. Our knowledge of these lesions is now evidence based and broad, and the impact on care tremendous! The HIG has also influenced the practice of pediatricians and other specialists, including otorhinolaryngologists, hematologist/oncologists, and surgeons, is partnering with advocacy groups to support patients and families, and is helping guide patients and families to contribute to ongoing research.
Vascular malformations (VM) reflect an incredible change in our understanding of the developmental pathways and pathophysiology of blood vessel tumors, and, in fact, birthmarks other than vascular lesions! First, important work separated out hemangiomas of infancy and hemangiomalike tumors from vascular malformations, with the thought being that hemangiomas had a rapid growth phase, often arising from lesions that were minimally evident or not evident at birth, unlike malformations, which were “programing errors,” all present at birth and expected to be fairly static with proportionate growth over a lifetime. Approaches to vascular malformations were limited to sclerotherapy, laser, and/or surgery. While this general schema of classification is still useful, our sense of the “why and how” of vascular malformations is remarkably different. Vascular malformations – still usefully subdivided into capillary, lymphatic, venous arteriovenous, or mixed malformations – are mostly associated with inherited or somatic mutations. Mutations are most commonly found in two signal pathways: RAS/MAPK/ERK and PI3K/AKT/mTOR pathways, with specific sets of mutations seen in both localized and multifocal lesions, with or without overgrowth or other systemic anomalies. The discovery of specific mutations has led to the possibility of small-molecule inhibitors, many already existing as anticancer drugs, being utilized as targeted therapies for VM.
And similar advances in understanding of other birthmarks, with or without syndromic features, are being made steadily. The mutations in congenital melanocytic nevi, epidermal nevi, acquired tumors (pilomatricomas), and other lesions, along with steady epidemiologic, translational, and clinical work, evolves our knowledge and potential therapies.
Inflammatory skin disorders: Acne, psoriasis, and atopic dermatitis
The care of pediatric inflammatory skin disorders has evolved, but more slowly for some diseases than others. Acne vulgaris is now recognized as much more common under age 12 than previously, presumably reflecting earlier pubertal changes in our preteens. Over the past 30 years, therapy has evolved with the use of topical retinoids (still underused by pediatricians, considered a “practice gap”), hormonal therapy with combined oral contraceptives, and oral isotretinoin, a powerful but highly effective systemic agent for severe and refractory acne. Specific pediatric guidelines came much later, with expert recommendations formulated by the American Acne and Rosacea Society and endorsed by the American Academy of Pediatrics in 2013. Over the past few years, there has been a push by experts for more judicious use of antibiotics for acne (oral and topical) to minimize the emergence of bacterial resistance. There are unanswered questions as we evolve our care: How will the new topical antiandrogens be used? Will spironolactone become part of hormonal therapy under age 18? Will the insights on certain strains of Cutibacterium acnes being associated with worse acne translate to microbiome or vaccine-based strategies?
Pediatric psoriasis has suffered, being “behind in the revolution” of biologic agents because of delayed approval of any biologic agent for treatment of pediatric psoriasis in the United States until just a few years ago, and lags behind Europe and elsewhere in the world by almost a decade. Only this year have we expanded beyond one biologic agent approved for under age 12 and two for ages 12 and older, with other approvals expected including interleukin (IL)-17 and IL-23 agents. Adult psoriasis has been recognized to be associated with a broad set of comorbidities, including obesity and early heart disease, and there is now research on how children are at risk as well, with new recommendations on how to screen children with psoriasis, supplied first by PeDRA and then in the new American Academy of Dermatology-National Psoriasis Foundation pediatric psoriasis guidelines .
Pediatric atopic dermatitis (AD) is in its early years of revolution. In the 50-year period of our thought experiment, AD has increased in prevalence from 5% or less of the pediatric population to 10%-15%. Treatment of most individuals has remained the same over the decades: Good skin care, frequent moisturizers, topical corticosteroids for flares, and management of infection if noted. The topical calcineurin inhibitors (TCIs) broadened the therapeutic approach when introduced in 2000 and 2001, but the boxed warning resulted in some practitioners minimizing their use of these useful agents. But newer studies are markedly reassuring about their safe use in children.
Steroid phobia, as well as concerns about potential side effects of the TCIs, has resulted in undertreatment of childhood AD. It is quite common to see multiple children during pediatric dermatology office hours with poorly controlled eczema, high body-surface areas of eczema, compromised sleep, secondary infections, and anxiety and depression, especially in our moderate to severe adolescents. The field is “hot” with new topical and systemic agents, including our few years’ experience with topical crisaborole, a phosphodiesterase (PDE)-4 inhibitor; and dupilumab, an IL-4-alpha blocker – the first biologic agent approved for AD and the first systemic agent (other than oral corticosteroids), just extended from 12 years to 6 years of age! As dupilumab gets studied for younger children, other biologics (including IL-13 and IL-31 blockers) are undertaking pediatric and/or adolescent trials, oral and topical JAK inhibitors are including adolescents in core clinical trials, and other novel topical agents are under study, including an aryl-hydrocarbon receptor–modulating agent and other PDE-4 inhibitors.
Procedural pediatric dermatology: From liquid nitrogen to laser, surgery, and multimodal skin care
The first generation of pediatric dermatologists were considered medical dermatologist specialists. The care of the conditions discussed above, as well as genodermatoses, diagnostic dilemmas, and management of dermatologic manifestations of systemic disease and other conditions, was the “bread and butter” of pediatric dermatology care. When I was in training, my mentor Paul Honig, MD, at the Children’s Hospital of Philadelphia had a procedure half-day each week, where he would care for a few patients who needed liquid nitrogen therapy for warts, or who needed biopsies. It was uncommon to have a large procedural/surgical part of pediatric dermatology practice. But this is now a routine part of many specialists in the field. How did this change occur?
The fundamental shift began to occur with the introduction of the pulsed dye laser for treatment of port-wine birthmarks in children with minimal scarring, and a seminal article published in the New England Journal of Medicine in 1989. Vascular lesions including port-wine stains were common, and pediatric dermatologists managed these patients for both diagnosis and medical management. Also, dermatology residencies at this time offered training in cutaneous surgery, excisions (including Mohs surgery) and repairs, and trainees in pediatric dermatology were “trained up” to high levels of expertise. As lasers were incorporated into dermatology residency work and practices, pediatric dermatologists developed the exposure and skill to do this work. An added advantage was having the knowledge of how to handle children and adolescents in an age-appropriate manner, with consideration of methods to minimize the pain and anxiety of procedures. Within a few years, pediatric dermatologists were at the forefront of the use of topical anesthetics (EMLA and liposomal lidocaine) and had general anesthesia privileges for laser and excisional surgery.
So while pediatric dermatologists still do “small procedures” every hour in most practices (cryotherapy for warts, cantharidin for molluscum, shave and punch biopsies), a subset now have extensive procedural practices, which in recent years has extended to pigment lesion lasers (to treat nevus of Ota, for example), hair laser, and combinations of lasers, including fractionated CO2 technology, to treat hypertrophic, constrictive and/or deforming scars.
The future
What will pediatric dermatology be like in 10, 20, or 50 years?
I have not yet discussed some of the most challenging diseases in our field, including epidermolysis bullosa, ichthyosis, and neurocutaneous disorders and other genetic skin disorders that have an incredible impact on the lives of affected children and their families, with incredible morbidity and with many conditions that shorten lifespans. But these are the conditions where “the future is happening now,” and we are looking forward to our new gene therapy techniques helping to transform our care.
And other aspects of practice? Will we be doing a large percentage of practice over the phone (or whatever devices we have then – remember, the first iPhone was only released 13 years ago)?
Will our patients be using their own imaging systems to evaluate their nevi and skin growths, and perhaps to diagnose and manage their rashes?
Will we have prevented our inflammatory skin disorders, or “turned them off” in early life with aggressive therapy in infantile life?
I project only that all of us in dermatology will still be a resource to our pediatric patients, from neonate through young adult, through our work of preventing, caring, healing and minimizing disease impact, and hopefully enjoying the pleasures of seeing our patients healthfully develop and evolve! As will our field.
Dr. Eichenfield is professor of dermatology and pediatrics and vice-chair of the department of dermatology at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. Dr. Eichenfield reports financial relationships with 20 pharmaceutical companies that manufacture dermatologic products, including products for the diseases discussed here.
Escaping the daily grind
Few films have universal appeal these days, but one that comes close is the 1993 classic Groundhog Day, in which the protagonist is trapped in a time loop, doomed to living the same day over and over for many years.
One reason that this story resonates with so many, I think, is that we are all living a similar life. Not as a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. . And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of one a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lessons; or a long weekend away with my wife.
And until COVID-19 put a temporary stop to them earlier this year, we have embarked on at least one longer adventure each year, some of which have been shared in these pages. Our 2019 expedition to Easter Island remains among the most memorable, and fulfilled a dream I’ve had since I read Thor Heyerdahl’s Aku Aku in grade school. As we explored the giant stone moai – which are found nowhere else in the world – I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out. When our trip was over, I returned ready to take on the world, and my practice, anew.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money; this is breaking the routine, clearing the cobwebs, living your life. Trust me, your practice will still be there when you return. And while COVID-19 will not last forever, there are plenty of other “sharpeners” while we wait.
More than once I’ve recounted the story of Alex Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few films have universal appeal these days, but one that comes close is the 1993 classic Groundhog Day, in which the protagonist is trapped in a time loop, doomed to living the same day over and over for many years.
One reason that this story resonates with so many, I think, is that we are all living a similar life. Not as a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. . And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of one a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lessons; or a long weekend away with my wife.
And until COVID-19 put a temporary stop to them earlier this year, we have embarked on at least one longer adventure each year, some of which have been shared in these pages. Our 2019 expedition to Easter Island remains among the most memorable, and fulfilled a dream I’ve had since I read Thor Heyerdahl’s Aku Aku in grade school. As we explored the giant stone moai – which are found nowhere else in the world – I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out. When our trip was over, I returned ready to take on the world, and my practice, anew.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money; this is breaking the routine, clearing the cobwebs, living your life. Trust me, your practice will still be there when you return. And while COVID-19 will not last forever, there are plenty of other “sharpeners” while we wait.
More than once I’ve recounted the story of Alex Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Few films have universal appeal these days, but one that comes close is the 1993 classic Groundhog Day, in which the protagonist is trapped in a time loop, doomed to living the same day over and over for many years.
One reason that this story resonates with so many, I think, is that we are all living a similar life. Not as a same-day loop, of course; but each week seems eerily similar to the last, as does each month, each year – on and on, ad infinitum. That’s why it is so important, every so often, to step out of the “loop” and reassess the bigger picture.
I write this reminder every couple of years because it’s so easy to lose sight of the overall landscape among the pressures of our daily routines. . And we are too busy to sit down and think about what we might do to break that vicious cycle. This is detrimental to our own well being, as well as that of our patients.
There are many ways to maintain your intellectual and emotional health, but here’s how I do it: I take individual days off (average of one a month) to catch up on journals or taking a CME course; or to try something new – something I’ve been thinking about doing “someday, when there is time” – such as a guitar, bass, or sailing lessons; or a long weekend away with my wife.
And until COVID-19 put a temporary stop to them earlier this year, we have embarked on at least one longer adventure each year, some of which have been shared in these pages. Our 2019 expedition to Easter Island remains among the most memorable, and fulfilled a dream I’ve had since I read Thor Heyerdahl’s Aku Aku in grade school. As we explored the giant stone moai – which are found nowhere else in the world – I didn’t have the time – or the slightest inclination – to worry about the office. But I did accumulate some great ideas – practical, medical, and literary. Original thoughts are hard to chase down during the daily grind; but in a refreshing environment, they will seek you out. When our trip was over, I returned ready to take on the world, and my practice, anew.
I know how some of you feel about “wasting” a day – or, God forbid, a week. Patients might go elsewhere while you’re gone, and every day the office is idle you “lose money.” That whole paradigm is wrong. You bring in a given amount of revenue per year – more on some days, less on other days, none on weekends and vacations; it all averages out in the end.
Besides, this is much more important than money; this is breaking the routine, clearing the cobwebs, living your life. Trust me, your practice will still be there when you return. And while COVID-19 will not last forever, there are plenty of other “sharpeners” while we wait.
More than once I’ve recounted the story of Alex Müller and J. Georg Bednorz, the Swiss Nobel Laureates whose superconductivity research ground to a halt in 1986. The harder they pressed, the more elusive progress became. So Müller decided to take a break to read a new book on ceramics – a subject that had always interested him.
Nothing could have been less relevant to his work, of course; ceramics are among the poorest conductors known. But in that lower-pressure environment, Müller realized that a unique property of ceramics might apply to their project.
Back in the lab, the team created a ceramic compound that became the first successful “high-temperature” superconductor, which in turn triggered an explosion of research leading to breakthroughs in computing, electricity transmission, magnetically-elevated trains, and many applications yet to be realized.
Sharpening your saw may not change the world, but it will change you; any nudge out of your comfort zone will give you fresh ideas and help you look at seemingly insoluble problems in completely new ways.
And to those who still can’t bear the thought of taking time off, remember the dying words that no one has spoken, ever: “I wish I had spent more time in my office!”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].