User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Chronic inflammatory diseases vary widely in CHD risk
Not all chronic systemic inflammatory diseases are equal enhancers of atherosclerotic cardiovascular disease risk, according to a large case-control study.
Current AHA/American College of Cardiology guidelines cite three chronic inflammatory diseases as atherosclerotic cardiovascular disease risk enhancers: rheumatoid arthritis, psoriasis, and HIV infection. But this study of those three diseases, along with three others marked by elevated high sensitivity C-reactive protein (systemic sclerosis, inflammatory bowel disease, and systemic lupus erythematosus [SLE]), showed that chronic inflammatory diseases are not monolithic in terms of their associated risk of incident coronary heart disease (CHD).
Indeed, two of the six inflammatory diseases – psoriasis and inflammatory bowel disease – turned out to be not at all associated with increased cardiovascular risk in the 37,117-patient study. The highest-risk disease was SLE, not specifically mentioned in the guidelines, Arjun Sinha, MD, a cardiology fellow at Northwestern University, Chicago, noted in his presentation at the virtual American Heart Association scientific sessions.
The study included 18,129 patients with one of the six chronic inflammatory diseases and 18,988 matched controls, none with CHD at baseline. All regularly received outpatient care at Northwestern during 2000-2019. There were 1,011 incident CHD events during a median of 3.5 years of follow-up.
In a Cox proportional hazards analysis adjusted for demographics, insurance status, hypertension, diabetes, current smoking, total cholesterol, and estimated glomerular filtration rate, here’s how the chronic inflammatory diseases stacked up in terms of incident CHD and MI risks:
- SLE: hazard ratio for CHD, 2.85; for MI, 4.76.
- Systemic sclerosis: HR for CHD, 2.14; for MI, 3.19.
- HIV: HR for CHD, 1.38; for MI, 1.69.
- Rheumatoid arthritis: HR for CHD, 1.22; for MI, 1.45.
- Psoriasis: no significant increase.
- Inflammatory bowel disease: no significant increase.
In an exploratory analysis, Dr. Sinha and coinvestigators evaluated the risk of incident CHD stratified by disease severity. For lack of standardized disease severity scales, the investigators relied upon tertiles of CD4 T cell count in the HIV group and CRP in the others. The HR for new-onset CHD in the more than 5,000 patients with psoriasis didn’t vary by CRP tertile. However, there was a nonsignificant trend for greater disease severity, as reflected by CRP tertile, to be associated with increased incident CHD risk in the HIV and inflammatory bowel disease groups.
In contrast, patients with rheumatoid arthritis or systemic sclerosis who were in the top CRP tertile had a significantly greater risk of developing CHD than that of controls, with HRs of 2.11 in the rheumatoid arthritis group and 4.59 with systemic sclerosis, although patients in the other two tertiles weren’t at significantly increased risk. But all three tertiles of CRP in patients with SLE were associated with significantly increased CHD risk: 3.17-fold in the lowest tertile of lupus severity, 5.38-fold in the middle tertile, and 4.04-fold in the top tertile for inflammation.
These findings could be used in clinical practice to fine-tune atherosclerotic cardiovascular disease risk assessment based upon chronic inflammatory disease type and severity. That’s information which in turn can help guide the timing and intensity of preventive therapy for patients with each disease type.
But studying the association between chronic systemic inflammatory diseases and CHD risk can be useful in additional ways, according to Dr. Sinha. These inflammatory diseases can serve as models of atherosclerosis that shed light on the non–lipid-related mechanisms involved in cardiovascular disease.
“The gradient in risk may be hypothesis-generating with respect to which specific inflammatory pathways may contribute to CHD,” he explained.
Each of these six chronic inflammatory diseases is characterized by a different form of major immune dysfunction, Dr. Sinha continued. A case in point is SLE, the inflammatory disease associated with the highest risk of CHD and MI. Lupus is characterized by a form of neutrophil dysfunction marked by increased formation and reduced degradation of neutrophil extracellular traps, or NETs, as well as by an increase in autoreactive B cells and dysfunctional CD4+ T helper cells. The increase in NETs of of particular interest because NETs have also been shown to contribute to the development of atherosclerosis, endothelial dysfunction, plaque erosion, and thrombosis.
In another exploratory analysis, Dr. Sinha and coworkers found that SLE patients with a neutrophil count above the median level were twice as likely to develop CHD than were those with a neutrophil count below the median.
A better understanding of the upstream pathways linking NET formation in SLE and atherosclerosis could lead to development of new or repurposed medications that target immune dysfunction in order to curb atherosclerosis, said Dr. Sinha, whose study won the AHA’s Samuel A. Levine Early Career Clinical Investigator Award.
He reported having no financial conflicts regarding his study.
Not all chronic systemic inflammatory diseases are equal enhancers of atherosclerotic cardiovascular disease risk, according to a large case-control study.
Current AHA/American College of Cardiology guidelines cite three chronic inflammatory diseases as atherosclerotic cardiovascular disease risk enhancers: rheumatoid arthritis, psoriasis, and HIV infection. But this study of those three diseases, along with three others marked by elevated high sensitivity C-reactive protein (systemic sclerosis, inflammatory bowel disease, and systemic lupus erythematosus [SLE]), showed that chronic inflammatory diseases are not monolithic in terms of their associated risk of incident coronary heart disease (CHD).
Indeed, two of the six inflammatory diseases – psoriasis and inflammatory bowel disease – turned out to be not at all associated with increased cardiovascular risk in the 37,117-patient study. The highest-risk disease was SLE, not specifically mentioned in the guidelines, Arjun Sinha, MD, a cardiology fellow at Northwestern University, Chicago, noted in his presentation at the virtual American Heart Association scientific sessions.
The study included 18,129 patients with one of the six chronic inflammatory diseases and 18,988 matched controls, none with CHD at baseline. All regularly received outpatient care at Northwestern during 2000-2019. There were 1,011 incident CHD events during a median of 3.5 years of follow-up.
In a Cox proportional hazards analysis adjusted for demographics, insurance status, hypertension, diabetes, current smoking, total cholesterol, and estimated glomerular filtration rate, here’s how the chronic inflammatory diseases stacked up in terms of incident CHD and MI risks:
- SLE: hazard ratio for CHD, 2.85; for MI, 4.76.
- Systemic sclerosis: HR for CHD, 2.14; for MI, 3.19.
- HIV: HR for CHD, 1.38; for MI, 1.69.
- Rheumatoid arthritis: HR for CHD, 1.22; for MI, 1.45.
- Psoriasis: no significant increase.
- Inflammatory bowel disease: no significant increase.
In an exploratory analysis, Dr. Sinha and coinvestigators evaluated the risk of incident CHD stratified by disease severity. For lack of standardized disease severity scales, the investigators relied upon tertiles of CD4 T cell count in the HIV group and CRP in the others. The HR for new-onset CHD in the more than 5,000 patients with psoriasis didn’t vary by CRP tertile. However, there was a nonsignificant trend for greater disease severity, as reflected by CRP tertile, to be associated with increased incident CHD risk in the HIV and inflammatory bowel disease groups.
In contrast, patients with rheumatoid arthritis or systemic sclerosis who were in the top CRP tertile had a significantly greater risk of developing CHD than that of controls, with HRs of 2.11 in the rheumatoid arthritis group and 4.59 with systemic sclerosis, although patients in the other two tertiles weren’t at significantly increased risk. But all three tertiles of CRP in patients with SLE were associated with significantly increased CHD risk: 3.17-fold in the lowest tertile of lupus severity, 5.38-fold in the middle tertile, and 4.04-fold in the top tertile for inflammation.
These findings could be used in clinical practice to fine-tune atherosclerotic cardiovascular disease risk assessment based upon chronic inflammatory disease type and severity. That’s information which in turn can help guide the timing and intensity of preventive therapy for patients with each disease type.
But studying the association between chronic systemic inflammatory diseases and CHD risk can be useful in additional ways, according to Dr. Sinha. These inflammatory diseases can serve as models of atherosclerosis that shed light on the non–lipid-related mechanisms involved in cardiovascular disease.
“The gradient in risk may be hypothesis-generating with respect to which specific inflammatory pathways may contribute to CHD,” he explained.
Each of these six chronic inflammatory diseases is characterized by a different form of major immune dysfunction, Dr. Sinha continued. A case in point is SLE, the inflammatory disease associated with the highest risk of CHD and MI. Lupus is characterized by a form of neutrophil dysfunction marked by increased formation and reduced degradation of neutrophil extracellular traps, or NETs, as well as by an increase in autoreactive B cells and dysfunctional CD4+ T helper cells. The increase in NETs of of particular interest because NETs have also been shown to contribute to the development of atherosclerosis, endothelial dysfunction, plaque erosion, and thrombosis.
In another exploratory analysis, Dr. Sinha and coworkers found that SLE patients with a neutrophil count above the median level were twice as likely to develop CHD than were those with a neutrophil count below the median.
A better understanding of the upstream pathways linking NET formation in SLE and atherosclerosis could lead to development of new or repurposed medications that target immune dysfunction in order to curb atherosclerosis, said Dr. Sinha, whose study won the AHA’s Samuel A. Levine Early Career Clinical Investigator Award.
He reported having no financial conflicts regarding his study.
Not all chronic systemic inflammatory diseases are equal enhancers of atherosclerotic cardiovascular disease risk, according to a large case-control study.
Current AHA/American College of Cardiology guidelines cite three chronic inflammatory diseases as atherosclerotic cardiovascular disease risk enhancers: rheumatoid arthritis, psoriasis, and HIV infection. But this study of those three diseases, along with three others marked by elevated high sensitivity C-reactive protein (systemic sclerosis, inflammatory bowel disease, and systemic lupus erythematosus [SLE]), showed that chronic inflammatory diseases are not monolithic in terms of their associated risk of incident coronary heart disease (CHD).
Indeed, two of the six inflammatory diseases – psoriasis and inflammatory bowel disease – turned out to be not at all associated with increased cardiovascular risk in the 37,117-patient study. The highest-risk disease was SLE, not specifically mentioned in the guidelines, Arjun Sinha, MD, a cardiology fellow at Northwestern University, Chicago, noted in his presentation at the virtual American Heart Association scientific sessions.
The study included 18,129 patients with one of the six chronic inflammatory diseases and 18,988 matched controls, none with CHD at baseline. All regularly received outpatient care at Northwestern during 2000-2019. There were 1,011 incident CHD events during a median of 3.5 years of follow-up.
In a Cox proportional hazards analysis adjusted for demographics, insurance status, hypertension, diabetes, current smoking, total cholesterol, and estimated glomerular filtration rate, here’s how the chronic inflammatory diseases stacked up in terms of incident CHD and MI risks:
- SLE: hazard ratio for CHD, 2.85; for MI, 4.76.
- Systemic sclerosis: HR for CHD, 2.14; for MI, 3.19.
- HIV: HR for CHD, 1.38; for MI, 1.69.
- Rheumatoid arthritis: HR for CHD, 1.22; for MI, 1.45.
- Psoriasis: no significant increase.
- Inflammatory bowel disease: no significant increase.
In an exploratory analysis, Dr. Sinha and coinvestigators evaluated the risk of incident CHD stratified by disease severity. For lack of standardized disease severity scales, the investigators relied upon tertiles of CD4 T cell count in the HIV group and CRP in the others. The HR for new-onset CHD in the more than 5,000 patients with psoriasis didn’t vary by CRP tertile. However, there was a nonsignificant trend for greater disease severity, as reflected by CRP tertile, to be associated with increased incident CHD risk in the HIV and inflammatory bowel disease groups.
In contrast, patients with rheumatoid arthritis or systemic sclerosis who were in the top CRP tertile had a significantly greater risk of developing CHD than that of controls, with HRs of 2.11 in the rheumatoid arthritis group and 4.59 with systemic sclerosis, although patients in the other two tertiles weren’t at significantly increased risk. But all three tertiles of CRP in patients with SLE were associated with significantly increased CHD risk: 3.17-fold in the lowest tertile of lupus severity, 5.38-fold in the middle tertile, and 4.04-fold in the top tertile for inflammation.
These findings could be used in clinical practice to fine-tune atherosclerotic cardiovascular disease risk assessment based upon chronic inflammatory disease type and severity. That’s information which in turn can help guide the timing and intensity of preventive therapy for patients with each disease type.
But studying the association between chronic systemic inflammatory diseases and CHD risk can be useful in additional ways, according to Dr. Sinha. These inflammatory diseases can serve as models of atherosclerosis that shed light on the non–lipid-related mechanisms involved in cardiovascular disease.
“The gradient in risk may be hypothesis-generating with respect to which specific inflammatory pathways may contribute to CHD,” he explained.
Each of these six chronic inflammatory diseases is characterized by a different form of major immune dysfunction, Dr. Sinha continued. A case in point is SLE, the inflammatory disease associated with the highest risk of CHD and MI. Lupus is characterized by a form of neutrophil dysfunction marked by increased formation and reduced degradation of neutrophil extracellular traps, or NETs, as well as by an increase in autoreactive B cells and dysfunctional CD4+ T helper cells. The increase in NETs of of particular interest because NETs have also been shown to contribute to the development of atherosclerosis, endothelial dysfunction, plaque erosion, and thrombosis.
In another exploratory analysis, Dr. Sinha and coworkers found that SLE patients with a neutrophil count above the median level were twice as likely to develop CHD than were those with a neutrophil count below the median.
A better understanding of the upstream pathways linking NET formation in SLE and atherosclerosis could lead to development of new or repurposed medications that target immune dysfunction in order to curb atherosclerosis, said Dr. Sinha, whose study won the AHA’s Samuel A. Levine Early Career Clinical Investigator Award.
He reported having no financial conflicts regarding his study.
FROM AHA 2020
A novel method for assessing attractiveness and beauty
While Phi (or the Golden Ratio) and Leonardo da Vinci’s neoclassical canons have been used as traditional mathematical approaches to assess and calculate beauty, there may be more than meets the eye.
This model was created to denote “natural beauty,” both at baseline and after cosmetic procedures, which is what many physicians and patients ideally want to achieve after any aesthetic procedure.
In this model, when all three variables are at a maximum, a desirable attractive appearance is achieved that can be interpreted as “natural.” In his paper introducing this novel model, Dr. Dayan wrote that similar to the time-space dilemma, attractiveness “is relative, dynamic, and highly dependent on the position of the projector and the interpreter.” The 3-D cube of attractiveness “is therefore contained within a fourth dimension that takes into account the perspective of the judger.”
Similarly, in a pilot study,2 Dr. Dayan and colleagues also demonstrated that visually blind individuals can detect beauty. “This study further isolates the nature of beauty as a primal form of messaging that is subconsciously appreciated via embodied senses other than vision,” he and his coauthors wrote.
This observational study consisted of 8 blind and 10 nonblind test subjects and 6 models who were categorized into predetermined beauty categories. Test subjects were blindfolded and unblindfolded during their assessments. All groups rated those models, who were preselected as more beautiful, higher, except for the blindfolded, nonblind group – demonstrating a primal or neural pathway ability to perceive attractiveness in blind individuals. The study, “revealed that beauty is not only detected by visual sense but also through embodied senses other than sight,” the authors commented.
It should be noted that sometimes ethnic features and features that are unique outside of the neoclassical canons or golden ratio can also uniquely make people look more attractive. Ethnic variations in beauty standards exist and need to be further studied and celebrated. There is certainly high expertise and an art required to perceiving aesthetics and performing aesthetic procedures, further exemplified by the complex nature of the different models and mathematical approaches of assessing it. These newer models account for attractiveness that may also start on the inside or beyond purely visual perception.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References:
1. Dayan S, Romero DH. J Cosmet Dermatol. 2018 Oct;17(5):925-30.
2. Dayan SH et al. Dermatol Surg. 2020 Oct;46(10):1317-22.
While Phi (or the Golden Ratio) and Leonardo da Vinci’s neoclassical canons have been used as traditional mathematical approaches to assess and calculate beauty, there may be more than meets the eye.
This model was created to denote “natural beauty,” both at baseline and after cosmetic procedures, which is what many physicians and patients ideally want to achieve after any aesthetic procedure.
In this model, when all three variables are at a maximum, a desirable attractive appearance is achieved that can be interpreted as “natural.” In his paper introducing this novel model, Dr. Dayan wrote that similar to the time-space dilemma, attractiveness “is relative, dynamic, and highly dependent on the position of the projector and the interpreter.” The 3-D cube of attractiveness “is therefore contained within a fourth dimension that takes into account the perspective of the judger.”
Similarly, in a pilot study,2 Dr. Dayan and colleagues also demonstrated that visually blind individuals can detect beauty. “This study further isolates the nature of beauty as a primal form of messaging that is subconsciously appreciated via embodied senses other than vision,” he and his coauthors wrote.
This observational study consisted of 8 blind and 10 nonblind test subjects and 6 models who were categorized into predetermined beauty categories. Test subjects were blindfolded and unblindfolded during their assessments. All groups rated those models, who were preselected as more beautiful, higher, except for the blindfolded, nonblind group – demonstrating a primal or neural pathway ability to perceive attractiveness in blind individuals. The study, “revealed that beauty is not only detected by visual sense but also through embodied senses other than sight,” the authors commented.
It should be noted that sometimes ethnic features and features that are unique outside of the neoclassical canons or golden ratio can also uniquely make people look more attractive. Ethnic variations in beauty standards exist and need to be further studied and celebrated. There is certainly high expertise and an art required to perceiving aesthetics and performing aesthetic procedures, further exemplified by the complex nature of the different models and mathematical approaches of assessing it. These newer models account for attractiveness that may also start on the inside or beyond purely visual perception.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References:
1. Dayan S, Romero DH. J Cosmet Dermatol. 2018 Oct;17(5):925-30.
2. Dayan SH et al. Dermatol Surg. 2020 Oct;46(10):1317-22.
While Phi (or the Golden Ratio) and Leonardo da Vinci’s neoclassical canons have been used as traditional mathematical approaches to assess and calculate beauty, there may be more than meets the eye.
This model was created to denote “natural beauty,” both at baseline and after cosmetic procedures, which is what many physicians and patients ideally want to achieve after any aesthetic procedure.
In this model, when all three variables are at a maximum, a desirable attractive appearance is achieved that can be interpreted as “natural.” In his paper introducing this novel model, Dr. Dayan wrote that similar to the time-space dilemma, attractiveness “is relative, dynamic, and highly dependent on the position of the projector and the interpreter.” The 3-D cube of attractiveness “is therefore contained within a fourth dimension that takes into account the perspective of the judger.”
Similarly, in a pilot study,2 Dr. Dayan and colleagues also demonstrated that visually blind individuals can detect beauty. “This study further isolates the nature of beauty as a primal form of messaging that is subconsciously appreciated via embodied senses other than vision,” he and his coauthors wrote.
This observational study consisted of 8 blind and 10 nonblind test subjects and 6 models who were categorized into predetermined beauty categories. Test subjects were blindfolded and unblindfolded during their assessments. All groups rated those models, who were preselected as more beautiful, higher, except for the blindfolded, nonblind group – demonstrating a primal or neural pathway ability to perceive attractiveness in blind individuals. The study, “revealed that beauty is not only detected by visual sense but also through embodied senses other than sight,” the authors commented.
It should be noted that sometimes ethnic features and features that are unique outside of the neoclassical canons or golden ratio can also uniquely make people look more attractive. Ethnic variations in beauty standards exist and need to be further studied and celebrated. There is certainly high expertise and an art required to perceiving aesthetics and performing aesthetic procedures, further exemplified by the complex nature of the different models and mathematical approaches of assessing it. These newer models account for attractiveness that may also start on the inside or beyond purely visual perception.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
References:
1. Dayan S, Romero DH. J Cosmet Dermatol. 2018 Oct;17(5):925-30.
2. Dayan SH et al. Dermatol Surg. 2020 Oct;46(10):1317-22.
Escalate HIV adherence strategies amid COVID-19
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
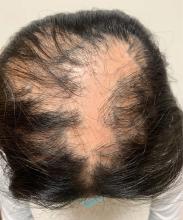
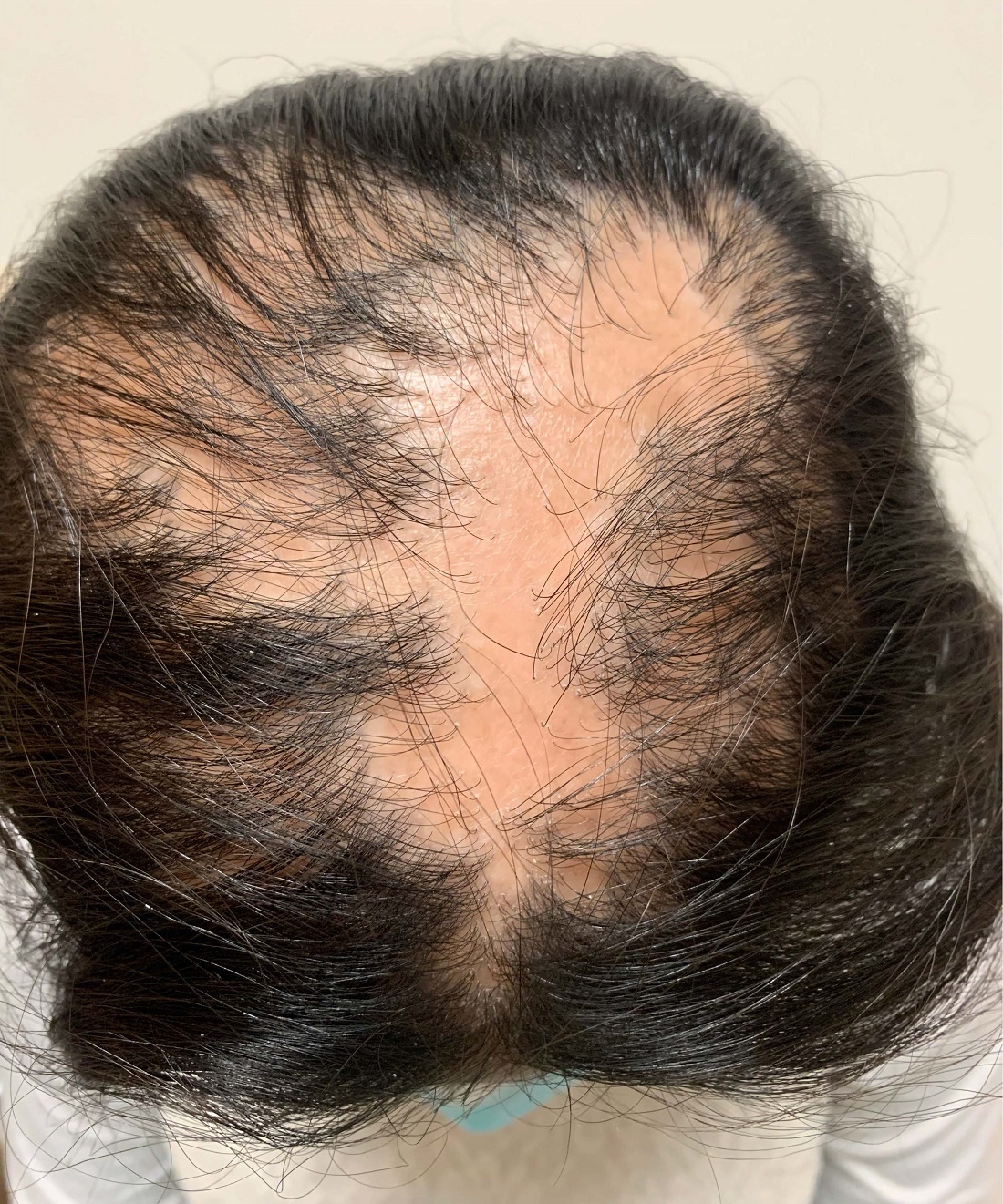
An 11-year-old female with a 3-year history of alopecia
Given the longstanding scarring alopecia, with negative fungal cultures and with perifollicular erythema and scaling, this diagnosis is most consistent with lichen planopilaris.
Lichen planopilaris (LPP) is considered one of the primary scarring alopecias, a group of diseases characterized by inflammation and subsequent irreversible hair loss.1 LPP specifically is believed to be caused by dysfunction of cell-mediated immunity, resulting in T lymphocytes attacking follicular hair stem cells.2 It typically presents with hair loss, pruritus, scaling, burning pain, and tenderness of the scalp when active,1,3 with exam showing perifollicular scale and erythema on the borders of the patches of alopecia.4,5 Over time, scarring of the scalp develops with loss of follicular ostia.1 Definitive diagnosis typically requires punch biopsy of the affected scalp, as such can determine the presence or absence of inflammation in affected areas of the scalp.1
What’s the treatment plan?
Given that LPP is an autoimmune inflammatory disease process, the goal of treatment is to calm down the inflammation of the scalp to prevent further progression of a patient’s hair loss. This is typically achieved with superpotent topical corticosteroids, such as clobetasol applied directly to the scalp, and/or intralesional corticosteroids, such as triamcinolone acetonide suspension injected directly to the affected scalp.3,6,7 Other treatment options include systemic agents, such as hydroxychloroquine, methotrexate, mycophenolate mofetil, pioglitazone, and doxycycline.3,6 Hair loss is not reversible as loss of follicular ostia and hair stem cells results in permanent scarring.1 Management often requires a referral to dermatology for aggressive treatment to prevent further hair loss.
What’s the differential diagnosis?
The differential diagnosis of lichen planopilaris includes other scarring alopecias, including central centrifugal cicatricial alopecia, discoid lupus erythematosus, folliculitis decalvans. While nonscarring, alopecia areata, trichotillomania, and telogen effluvium are discussed below as well.
Central centrifugal cicatricial alopecia is very rare in pediatrics, and is a type of asymptomatic scarring alopecia that begins at the vertex of the scalp, spreading centrifugally and resulting in shiny plaque development. Treatment involves reduction of hair grooming as well as topical and intralesional steroids.
Discoid lupus erythematosus presents as scaling erythematous plaques on the face and scalp that result in skin pigment changes and atrophy over time. Scalp involvement results in scarring alopecia. Treatment includes the use of high-potency topical corticosteroids, topical calcineurin inhibitors, and hydroxychloroquine.
Folliculitis decalvans is another form of scarring alopecia believed to be caused by an inflammatory response to Staphylococcus aureus in the scalp, resulting in the formation of scarring of the scalp and perifollicular pustules. Treatment is topical antibiotics and intralesional steroids.
Alopecia areata is a form of nonscarring alopecia resulting in small round patches of partially reversible hair loss characterized by the pathognomonic finding of so-called exclamation point hairs that are broader distally and taper toward the scalp on physical exam. Considered an autoimmune disorder, it varies greatly in extent and course. While focal hair loss is the hallmark of this disease, usually hair follicles are present.
Trichotillosis, also known as trichotillomania (hair pulling), results in alopecia with irregular borders and broken hairs of different lengths secondary to the urge to remove or pull one’s own hair, resulting in nonscarring alopecia. It may be associated with stress or anxiety, obsessive-compulsive disorders, or other repetitive body-altering behaviors. Treatments include reassurance and education as it can be self-limited in some, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs.
Our patient underwent scalp punch biopsy to confirm the diagnosis and was started on potent topical corticosteroids with good disease control.
Dr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university, and he is chief of pediatric and adolescent dermatology at the hospital. Neither of the doctors had any relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2005 Jul. doi: 10.1016/j.jaad.2004.06.015.
2. J Pathol. 2013 Oct. doi: 10.1002/path.4233.
3. Pediatr Dermatol. 2015 Sep-Oct. doi: 10.1111/pde.12624.
4. J Am Acad Dermatol. 2004 Jan. doi: 10.1016/j.jaad.2003.04.001.
5. J Am Acad Dermatol. 1992 Dec. doi: 10.1016/0190-9622(92)70290-v.
6. Clin Cosmet Investig Dermatol. 2018 Feb 27. doi: 10.2147/CCID.S137870.
7. Semin Cutan Med Surg. 2009 Mar. doi: 10.1016/j.sder.2008.12.006.
Given the longstanding scarring alopecia, with negative fungal cultures and with perifollicular erythema and scaling, this diagnosis is most consistent with lichen planopilaris.
Lichen planopilaris (LPP) is considered one of the primary scarring alopecias, a group of diseases characterized by inflammation and subsequent irreversible hair loss.1 LPP specifically is believed to be caused by dysfunction of cell-mediated immunity, resulting in T lymphocytes attacking follicular hair stem cells.2 It typically presents with hair loss, pruritus, scaling, burning pain, and tenderness of the scalp when active,1,3 with exam showing perifollicular scale and erythema on the borders of the patches of alopecia.4,5 Over time, scarring of the scalp develops with loss of follicular ostia.1 Definitive diagnosis typically requires punch biopsy of the affected scalp, as such can determine the presence or absence of inflammation in affected areas of the scalp.1
What’s the treatment plan?
Given that LPP is an autoimmune inflammatory disease process, the goal of treatment is to calm down the inflammation of the scalp to prevent further progression of a patient’s hair loss. This is typically achieved with superpotent topical corticosteroids, such as clobetasol applied directly to the scalp, and/or intralesional corticosteroids, such as triamcinolone acetonide suspension injected directly to the affected scalp.3,6,7 Other treatment options include systemic agents, such as hydroxychloroquine, methotrexate, mycophenolate mofetil, pioglitazone, and doxycycline.3,6 Hair loss is not reversible as loss of follicular ostia and hair stem cells results in permanent scarring.1 Management often requires a referral to dermatology for aggressive treatment to prevent further hair loss.
What’s the differential diagnosis?
The differential diagnosis of lichen planopilaris includes other scarring alopecias, including central centrifugal cicatricial alopecia, discoid lupus erythematosus, folliculitis decalvans. While nonscarring, alopecia areata, trichotillomania, and telogen effluvium are discussed below as well.
Central centrifugal cicatricial alopecia is very rare in pediatrics, and is a type of asymptomatic scarring alopecia that begins at the vertex of the scalp, spreading centrifugally and resulting in shiny plaque development. Treatment involves reduction of hair grooming as well as topical and intralesional steroids.
Discoid lupus erythematosus presents as scaling erythematous plaques on the face and scalp that result in skin pigment changes and atrophy over time. Scalp involvement results in scarring alopecia. Treatment includes the use of high-potency topical corticosteroids, topical calcineurin inhibitors, and hydroxychloroquine.
Folliculitis decalvans is another form of scarring alopecia believed to be caused by an inflammatory response to Staphylococcus aureus in the scalp, resulting in the formation of scarring of the scalp and perifollicular pustules. Treatment is topical antibiotics and intralesional steroids.
Alopecia areata is a form of nonscarring alopecia resulting in small round patches of partially reversible hair loss characterized by the pathognomonic finding of so-called exclamation point hairs that are broader distally and taper toward the scalp on physical exam. Considered an autoimmune disorder, it varies greatly in extent and course. While focal hair loss is the hallmark of this disease, usually hair follicles are present.
Trichotillosis, also known as trichotillomania (hair pulling), results in alopecia with irregular borders and broken hairs of different lengths secondary to the urge to remove or pull one’s own hair, resulting in nonscarring alopecia. It may be associated with stress or anxiety, obsessive-compulsive disorders, or other repetitive body-altering behaviors. Treatments include reassurance and education as it can be self-limited in some, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs.
Our patient underwent scalp punch biopsy to confirm the diagnosis and was started on potent topical corticosteroids with good disease control.
Dr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university, and he is chief of pediatric and adolescent dermatology at the hospital. Neither of the doctors had any relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2005 Jul. doi: 10.1016/j.jaad.2004.06.015.
2. J Pathol. 2013 Oct. doi: 10.1002/path.4233.
3. Pediatr Dermatol. 2015 Sep-Oct. doi: 10.1111/pde.12624.
4. J Am Acad Dermatol. 2004 Jan. doi: 10.1016/j.jaad.2003.04.001.
5. J Am Acad Dermatol. 1992 Dec. doi: 10.1016/0190-9622(92)70290-v.
6. Clin Cosmet Investig Dermatol. 2018 Feb 27. doi: 10.2147/CCID.S137870.
7. Semin Cutan Med Surg. 2009 Mar. doi: 10.1016/j.sder.2008.12.006.
Given the longstanding scarring alopecia, with negative fungal cultures and with perifollicular erythema and scaling, this diagnosis is most consistent with lichen planopilaris.
Lichen planopilaris (LPP) is considered one of the primary scarring alopecias, a group of diseases characterized by inflammation and subsequent irreversible hair loss.1 LPP specifically is believed to be caused by dysfunction of cell-mediated immunity, resulting in T lymphocytes attacking follicular hair stem cells.2 It typically presents with hair loss, pruritus, scaling, burning pain, and tenderness of the scalp when active,1,3 with exam showing perifollicular scale and erythema on the borders of the patches of alopecia.4,5 Over time, scarring of the scalp develops with loss of follicular ostia.1 Definitive diagnosis typically requires punch biopsy of the affected scalp, as such can determine the presence or absence of inflammation in affected areas of the scalp.1
What’s the treatment plan?
Given that LPP is an autoimmune inflammatory disease process, the goal of treatment is to calm down the inflammation of the scalp to prevent further progression of a patient’s hair loss. This is typically achieved with superpotent topical corticosteroids, such as clobetasol applied directly to the scalp, and/or intralesional corticosteroids, such as triamcinolone acetonide suspension injected directly to the affected scalp.3,6,7 Other treatment options include systemic agents, such as hydroxychloroquine, methotrexate, mycophenolate mofetil, pioglitazone, and doxycycline.3,6 Hair loss is not reversible as loss of follicular ostia and hair stem cells results in permanent scarring.1 Management often requires a referral to dermatology for aggressive treatment to prevent further hair loss.
What’s the differential diagnosis?
The differential diagnosis of lichen planopilaris includes other scarring alopecias, including central centrifugal cicatricial alopecia, discoid lupus erythematosus, folliculitis decalvans. While nonscarring, alopecia areata, trichotillomania, and telogen effluvium are discussed below as well.
Central centrifugal cicatricial alopecia is very rare in pediatrics, and is a type of asymptomatic scarring alopecia that begins at the vertex of the scalp, spreading centrifugally and resulting in shiny plaque development. Treatment involves reduction of hair grooming as well as topical and intralesional steroids.
Discoid lupus erythematosus presents as scaling erythematous plaques on the face and scalp that result in skin pigment changes and atrophy over time. Scalp involvement results in scarring alopecia. Treatment includes the use of high-potency topical corticosteroids, topical calcineurin inhibitors, and hydroxychloroquine.
Folliculitis decalvans is another form of scarring alopecia believed to be caused by an inflammatory response to Staphylococcus aureus in the scalp, resulting in the formation of scarring of the scalp and perifollicular pustules. Treatment is topical antibiotics and intralesional steroids.
Alopecia areata is a form of nonscarring alopecia resulting in small round patches of partially reversible hair loss characterized by the pathognomonic finding of so-called exclamation point hairs that are broader distally and taper toward the scalp on physical exam. Considered an autoimmune disorder, it varies greatly in extent and course. While focal hair loss is the hallmark of this disease, usually hair follicles are present.
Trichotillosis, also known as trichotillomania (hair pulling), results in alopecia with irregular borders and broken hairs of different lengths secondary to the urge to remove or pull one’s own hair, resulting in nonscarring alopecia. It may be associated with stress or anxiety, obsessive-compulsive disorders, or other repetitive body-altering behaviors. Treatments include reassurance and education as it can be self-limited in some, behavior modification, or systemic therapy including tricyclic antidepressants or SSRIs.
Our patient underwent scalp punch biopsy to confirm the diagnosis and was started on potent topical corticosteroids with good disease control.
Dr. Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and a professor of dermatology and pediatrics at the university, and he is chief of pediatric and adolescent dermatology at the hospital. Neither of the doctors had any relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2005 Jul. doi: 10.1016/j.jaad.2004.06.015.
2. J Pathol. 2013 Oct. doi: 10.1002/path.4233.
3. Pediatr Dermatol. 2015 Sep-Oct. doi: 10.1111/pde.12624.
4. J Am Acad Dermatol. 2004 Jan. doi: 10.1016/j.jaad.2003.04.001.
5. J Am Acad Dermatol. 1992 Dec. doi: 10.1016/0190-9622(92)70290-v.
6. Clin Cosmet Investig Dermatol. 2018 Feb 27. doi: 10.2147/CCID.S137870.
7. Semin Cutan Med Surg. 2009 Mar. doi: 10.1016/j.sder.2008.12.006.
An 11-year-old female is seen in clinic with a 3-year history of alopecia. The patient recently immigrated to the United States from Afghanistan. Prior to immigrating, she was evaluated for "scarring alopecia" and had been treated with oral and topical steroids as well as oral and topical antifungals. When active, she had itching and tenderness. She is not actively losing any hair at this time, but she has not regrown any of her hair. The patient has no family members with alopecia. She reports some burning pain and itching of her scalp, and denies any muscle pain or weakness or sun sensitivity.
On physical exam, you see 50% loss of hair on the superior scalp with preservation of the anterior hair line. Patches of hair can be seen throughout, with segments of smooth-skinned alopecia, without pustules. There is a loss of the follicle pattern in scarred areas, and magnification or "dermoscopy" shows perifollicular erythema and scaling at the border of the affected scalp. Labs are all within normal limits. Bacterial and fungal cultures of the scalp do not grow organisms.
Situation ‘dire’ as COVID spike in West, Midwest worsens, experts say
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.
Nearly one in five develop mental illness following COVID-19
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
New reports guide return to play in athletes with COVID-19
Increasingly, clinicians are being called upon to advise athletes who have recovered from COVID-19 on when it is safe for them to return to play.
Now, they have two reports that offer more insights into the cardiotoxic effects of COVID-19 on the athletic heart.
In the first report, researchers report a high prevalence of pericardial involvement in college-student athletes who have recovered from COVID-19 and give their practical advice on how to let these athletes return to play safely.
In the second report, an expert panel of sports cardiologists provides a comprehensive guide to the appropriate imaging of athletes who may have cardiovascular complications from COVID-19.
Both are published in JACC: Cardiovascular Imaging.
“We were asked by the editors of JACC to submit this paper, and the impetus for it was the fact that there are so many athletes returning after being infected with COVID-19, we need to try and give guidance to cardiologists as to how best to evaluate these athletes,” Dermot Phelan, MD, PhD, Sanger Heart and Vascular Institute, Atrium Health, Charlotte, N.C., and lead author of the consensus statement, said in an interview.
The consensus statement acknowledges that information about the cardiovascular complications of COVID-19 continues to evolve. Meanwhile, pathologies such as myocarditis, pericarditis, and right ventricular dysfunction, in the absence of significant clinical symptoms, in athletes who have been affected by COVID-19 remain of considerable concern.
It also emphasizes the unique challenges the average cardiologist faces in distinguishing between what is normal for an athlete’s heart and what is true pathology after COVID-19 infection; details how different imaging modalities can help in screening, evaluating, and monitoring athletes with suspected cardiovascular complications of COVID-19 infection; and discusses the strengths and limitations of these modalities.
Finally, the consensus statement provides some well-needed guidance on return-to-play decision-making, for both the athlete and the clinician.
Athletic remodeling or covid-19 damage?
Athletes can develop certain cardiovascular characteristics because of their athletic activity, and sometimes, this can cloud the diagnostic picture.
“Is this change due to the effects of COVID-19, or is it just because this is an athlete’s heart? This was an international expert consensus, made up of sports cardiologists from all over the world who have a lot of experience in dealing with athletes,” Dr. Phelan said. “We were trying to relay the important information to the cardiologist who is not used to dealing with athletes on a day-to-day basis, as to what they might expect to find in that athlete, and what is not an expected finding and should be tested further.”
Phelan, a sports cardiologist, is familiar with what is normal for an athlete’s heart and what is pathology.
“We know that athletes, particularly long-term endurance athletes, develop changes in the heart that can affect not only the electrics but the structure of the heart, and sometimes, that overlaps with abnormalities with pathology. This can be a challenge for the nonsports cardiologist to differentiate,” he said.
Phelan and his group have written two other consensus documents on the management of cardiovascular problems that develop in some athletes who have been infected with COVID-19.
The first was published in May in JAMA Cardiology, and the second, which revised some of the original recommendations made in the first document, was published online Oct. 26 in JAMA Cardiology.
The first set of recommendations called for imaging studies to be done in all athletes, but the second set states that athletes who recover and are asymptomatic do not need extensive (and expensive) imaging tests.
“These two papers work hand in hand,” Dr. Phelan said. “In May, we had very little experience with COVID, and there was a lot of concern about hospitalized patients having a very high incidence of heart disease. We published those recommendations, but we recognized at the time that we had very little data and that we would reconsider once we had more experience with data.
“This current set of recommendations that we have put forth here are for those athletes who do need to get further testing, so it’s a step beyond,” Dr. Phelan added. “So the second iteration states that young athletes who had mild or no symptoms didn’t need to go through all of that cardiac testing, but others do need it.”
To do widespread cardiovascular imaging for many individuals would be very costly. Realistically, there are not that many centers in the United States that have all the sophisticated equipment required to do such testing, Dr. Phelan noted.
“One of our major points is difficulty obtaining the test, but also the cost; these are very expensive tests. There are limitations. They are useful when used in the correct context,” he said.
To play or not to play, that is the question
Partho P. Sengupta, MD, DM, had to answer that question for more than 50 young athletes who were returning to college at West Virginia University, anxious to be back with their teams and on the playing field. They had been infected with COVID-19 and needed to know when they could return to play.
Dr. Sengupta, who is also an author for the Phelan et al consensus statement on imaging, said there was a lot of pressure – from all the various stakeholders, and from anxious parents, worried college athletes, their teammates, and the university – to determine if the youngsters could return to play.
The fear was that COVID-19 infection left the young athlete’s heart vulnerable to myocarditis and, thus, sudden death on the playing field after strenuous activity.
“At the time we were doing this imaging, there was a lot of concern in the media, and papers were coming out reporting a lot of cardiac involvement or myocarditis associated with COVID-19. Nobody really knew what to do,” he explained.
“There were all kinds of questions, concerns. The parents were putting pressure on us, the athletes wanted to know, the teams, the university. So we put together a team and completed all of the examinations, including testing of blood markers, within a 2-week period. These young athletes, they’re scared, they’re worried and anxious, they don’t know what’s going to happen with their scholarship, so there was some urgency to this work,” Dr. Sengupta said.
“We had to screen all comers within a very short period. We had 54 consecutive patients, gave them full screening, full battery of tests, blood tests, all in a 2-week period,” he said.
Speed was of the essence, and Dr. Sengupta and his team rolled up their sleeves and got to work “We had to know who was safe to clear to return to play and who might need extra follow-up.”
Screening echocardiograms
They performed screening echocardiograms on 54 consecutive college athletes who had tested positive for COVID-19 on reverse transcription polymerase chain reaction nasal swab testing or who showed that they had IgG antibodies against COVID-19. The screening echocardiograms were done after the athletes had quarantined for at least 14 days and were no longer infectious.
Most (85%) were male, and the mean age was 19 years. A total of 16 (30%) athletes were asymptomatic, 36 (66%) reported mild COVID-19 related symptoms, and two (4%) reported moderate symptoms.
Of the 54 athletes who were initially screened with echocardiography, 48 (11 asymptomatic, 37 symptomatic), went on to have cardiac magnetic resonance imaging.
Results showed that more than half the athletes (27; 56.3%), showed some cardiac abnormality. The most common was pericardial late enhancement with associated pericardial effusion, affecting 19 (39.5%) athletes.
Of these, six (12.5%) had reduced global longitudinal strain (GLS) or an increased native T1.
One patient showed myocardial enhancement.
Additionally, seven athletes (14.6%) had reduced left ventricular ejection fraction or reduced GLS with or without increased native T1. Native T2 levels were normal in all subjects and no specific imaging features of myocardial inflammation were identified.
Participants were brought back to receive the results of their tests and to get an individualized plan about their safe return to play 3 to 5 weeks after they had ceased to be infectious with COVID-19.
“We saw pericardial inflammation that was resolving. We did not see any blood biomarkers to suggest that there was active inflammation going on,” he said. “We also did not see any muscle inflammation, but we did see pockets of fluid in over a third of our athletes.”
Fortunately, most were deemed able to get back to playing safely, despite having evidence of pericardial inflammation.
This was on strict condition that they be monitored very closely for any adverse events that might occur as they began to exercise again.
“Once they go back to the field to start exercising and practicing, it is under great supervision. We instructed all of our sports physicians and other team managers that these people need to be observed very carefully. So as long as they were asymptomatic, even though the signs of pericardial inflammation were there, if there were no signs of inflammation in the blood, we let them go back to play, closely monitored,” Dr. Sengupta said.
A small number remained very symptomatic at the end of the 5 weeks and were referred to cardiac rehabilitation, Dr. Sengupta said. “They were tired, fatigued, short of breath, even 5 weeks after they got over COVID, so we sent them for cardiac rehab to help them get conditioned again.”
The researchers plan to reevaluate and reimage all of the athletes in another 3 months to monitor their cardiac health.
Dr. Sengupta acknowledged the limitations of this single-center, nonrandomized, controlled report, but insists reports such as this add a bit more to what we are learning about COVID-19 every day.
“These kids were coming to us and asking questions. You have to use the best science you have available to you at that point in time. Some people ask why we did not have a control group, but how do you design a control population in the midst of a pandemic? The science may or may not be perfect, I agree, but the information we obtained is important,” he said.
“Right now, I don’t think we have enough science, and we are still learning. It is very difficult to predict who will develop the heart muscle disease or the pericardial disease,” Dr. Sengupta said. “We had to do our work quickly to give answers to the young athletes, their parents, their teammates, their university, as soon as possible, and we were doing this under pandemic conditions.”
The work was supported by the National Science Foundation National Institute of General Medical Sciences of the National Institutes of Health. Dr. Phelan reported no relevant financial relationships. Dr. Sengupta reported that he is a consultant for HeartSciences, Kencor Health, and Ultromics.
This article first appeared on Medscape.com.
Increasingly, clinicians are being called upon to advise athletes who have recovered from COVID-19 on when it is safe for them to return to play.
Now, they have two reports that offer more insights into the cardiotoxic effects of COVID-19 on the athletic heart.
In the first report, researchers report a high prevalence of pericardial involvement in college-student athletes who have recovered from COVID-19 and give their practical advice on how to let these athletes return to play safely.
In the second report, an expert panel of sports cardiologists provides a comprehensive guide to the appropriate imaging of athletes who may have cardiovascular complications from COVID-19.
Both are published in JACC: Cardiovascular Imaging.
“We were asked by the editors of JACC to submit this paper, and the impetus for it was the fact that there are so many athletes returning after being infected with COVID-19, we need to try and give guidance to cardiologists as to how best to evaluate these athletes,” Dermot Phelan, MD, PhD, Sanger Heart and Vascular Institute, Atrium Health, Charlotte, N.C., and lead author of the consensus statement, said in an interview.
The consensus statement acknowledges that information about the cardiovascular complications of COVID-19 continues to evolve. Meanwhile, pathologies such as myocarditis, pericarditis, and right ventricular dysfunction, in the absence of significant clinical symptoms, in athletes who have been affected by COVID-19 remain of considerable concern.
It also emphasizes the unique challenges the average cardiologist faces in distinguishing between what is normal for an athlete’s heart and what is true pathology after COVID-19 infection; details how different imaging modalities can help in screening, evaluating, and monitoring athletes with suspected cardiovascular complications of COVID-19 infection; and discusses the strengths and limitations of these modalities.
Finally, the consensus statement provides some well-needed guidance on return-to-play decision-making, for both the athlete and the clinician.
Athletic remodeling or covid-19 damage?
Athletes can develop certain cardiovascular characteristics because of their athletic activity, and sometimes, this can cloud the diagnostic picture.
“Is this change due to the effects of COVID-19, or is it just because this is an athlete’s heart? This was an international expert consensus, made up of sports cardiologists from all over the world who have a lot of experience in dealing with athletes,” Dr. Phelan said. “We were trying to relay the important information to the cardiologist who is not used to dealing with athletes on a day-to-day basis, as to what they might expect to find in that athlete, and what is not an expected finding and should be tested further.”
Phelan, a sports cardiologist, is familiar with what is normal for an athlete’s heart and what is pathology.
“We know that athletes, particularly long-term endurance athletes, develop changes in the heart that can affect not only the electrics but the structure of the heart, and sometimes, that overlaps with abnormalities with pathology. This can be a challenge for the nonsports cardiologist to differentiate,” he said.
Phelan and his group have written two other consensus documents on the management of cardiovascular problems that develop in some athletes who have been infected with COVID-19.
The first was published in May in JAMA Cardiology, and the second, which revised some of the original recommendations made in the first document, was published online Oct. 26 in JAMA Cardiology.
The first set of recommendations called for imaging studies to be done in all athletes, but the second set states that athletes who recover and are asymptomatic do not need extensive (and expensive) imaging tests.
“These two papers work hand in hand,” Dr. Phelan said. “In May, we had very little experience with COVID, and there was a lot of concern about hospitalized patients having a very high incidence of heart disease. We published those recommendations, but we recognized at the time that we had very little data and that we would reconsider once we had more experience with data.
“This current set of recommendations that we have put forth here are for those athletes who do need to get further testing, so it’s a step beyond,” Dr. Phelan added. “So the second iteration states that young athletes who had mild or no symptoms didn’t need to go through all of that cardiac testing, but others do need it.”
To do widespread cardiovascular imaging for many individuals would be very costly. Realistically, there are not that many centers in the United States that have all the sophisticated equipment required to do such testing, Dr. Phelan noted.
“One of our major points is difficulty obtaining the test, but also the cost; these are very expensive tests. There are limitations. They are useful when used in the correct context,” he said.
To play or not to play, that is the question
Partho P. Sengupta, MD, DM, had to answer that question for more than 50 young athletes who were returning to college at West Virginia University, anxious to be back with their teams and on the playing field. They had been infected with COVID-19 and needed to know when they could return to play.
Dr. Sengupta, who is also an author for the Phelan et al consensus statement on imaging, said there was a lot of pressure – from all the various stakeholders, and from anxious parents, worried college athletes, their teammates, and the university – to determine if the youngsters could return to play.
The fear was that COVID-19 infection left the young athlete’s heart vulnerable to myocarditis and, thus, sudden death on the playing field after strenuous activity.
“At the time we were doing this imaging, there was a lot of concern in the media, and papers were coming out reporting a lot of cardiac involvement or myocarditis associated with COVID-19. Nobody really knew what to do,” he explained.
“There were all kinds of questions, concerns. The parents were putting pressure on us, the athletes wanted to know, the teams, the university. So we put together a team and completed all of the examinations, including testing of blood markers, within a 2-week period. These young athletes, they’re scared, they’re worried and anxious, they don’t know what’s going to happen with their scholarship, so there was some urgency to this work,” Dr. Sengupta said.
“We had to screen all comers within a very short period. We had 54 consecutive patients, gave them full screening, full battery of tests, blood tests, all in a 2-week period,” he said.
Speed was of the essence, and Dr. Sengupta and his team rolled up their sleeves and got to work “We had to know who was safe to clear to return to play and who might need extra follow-up.”
Screening echocardiograms
They performed screening echocardiograms on 54 consecutive college athletes who had tested positive for COVID-19 on reverse transcription polymerase chain reaction nasal swab testing or who showed that they had IgG antibodies against COVID-19. The screening echocardiograms were done after the athletes had quarantined for at least 14 days and were no longer infectious.
Most (85%) were male, and the mean age was 19 years. A total of 16 (30%) athletes were asymptomatic, 36 (66%) reported mild COVID-19 related symptoms, and two (4%) reported moderate symptoms.
Of the 54 athletes who were initially screened with echocardiography, 48 (11 asymptomatic, 37 symptomatic), went on to have cardiac magnetic resonance imaging.
Results showed that more than half the athletes (27; 56.3%), showed some cardiac abnormality. The most common was pericardial late enhancement with associated pericardial effusion, affecting 19 (39.5%) athletes.
Of these, six (12.5%) had reduced global longitudinal strain (GLS) or an increased native T1.
One patient showed myocardial enhancement.
Additionally, seven athletes (14.6%) had reduced left ventricular ejection fraction or reduced GLS with or without increased native T1. Native T2 levels were normal in all subjects and no specific imaging features of myocardial inflammation were identified.
Participants were brought back to receive the results of their tests and to get an individualized plan about their safe return to play 3 to 5 weeks after they had ceased to be infectious with COVID-19.
“We saw pericardial inflammation that was resolving. We did not see any blood biomarkers to suggest that there was active inflammation going on,” he said. “We also did not see any muscle inflammation, but we did see pockets of fluid in over a third of our athletes.”
Fortunately, most were deemed able to get back to playing safely, despite having evidence of pericardial inflammation.
This was on strict condition that they be monitored very closely for any adverse events that might occur as they began to exercise again.
“Once they go back to the field to start exercising and practicing, it is under great supervision. We instructed all of our sports physicians and other team managers that these people need to be observed very carefully. So as long as they were asymptomatic, even though the signs of pericardial inflammation were there, if there were no signs of inflammation in the blood, we let them go back to play, closely monitored,” Dr. Sengupta said.
A small number remained very symptomatic at the end of the 5 weeks and were referred to cardiac rehabilitation, Dr. Sengupta said. “They were tired, fatigued, short of breath, even 5 weeks after they got over COVID, so we sent them for cardiac rehab to help them get conditioned again.”
The researchers plan to reevaluate and reimage all of the athletes in another 3 months to monitor their cardiac health.
Dr. Sengupta acknowledged the limitations of this single-center, nonrandomized, controlled report, but insists reports such as this add a bit more to what we are learning about COVID-19 every day.
“These kids were coming to us and asking questions. You have to use the best science you have available to you at that point in time. Some people ask why we did not have a control group, but how do you design a control population in the midst of a pandemic? The science may or may not be perfect, I agree, but the information we obtained is important,” he said.
“Right now, I don’t think we have enough science, and we are still learning. It is very difficult to predict who will develop the heart muscle disease or the pericardial disease,” Dr. Sengupta said. “We had to do our work quickly to give answers to the young athletes, their parents, their teammates, their university, as soon as possible, and we were doing this under pandemic conditions.”
The work was supported by the National Science Foundation National Institute of General Medical Sciences of the National Institutes of Health. Dr. Phelan reported no relevant financial relationships. Dr. Sengupta reported that he is a consultant for HeartSciences, Kencor Health, and Ultromics.
This article first appeared on Medscape.com.
Increasingly, clinicians are being called upon to advise athletes who have recovered from COVID-19 on when it is safe for them to return to play.
Now, they have two reports that offer more insights into the cardiotoxic effects of COVID-19 on the athletic heart.
In the first report, researchers report a high prevalence of pericardial involvement in college-student athletes who have recovered from COVID-19 and give their practical advice on how to let these athletes return to play safely.
In the second report, an expert panel of sports cardiologists provides a comprehensive guide to the appropriate imaging of athletes who may have cardiovascular complications from COVID-19.
Both are published in JACC: Cardiovascular Imaging.
“We were asked by the editors of JACC to submit this paper, and the impetus for it was the fact that there are so many athletes returning after being infected with COVID-19, we need to try and give guidance to cardiologists as to how best to evaluate these athletes,” Dermot Phelan, MD, PhD, Sanger Heart and Vascular Institute, Atrium Health, Charlotte, N.C., and lead author of the consensus statement, said in an interview.
The consensus statement acknowledges that information about the cardiovascular complications of COVID-19 continues to evolve. Meanwhile, pathologies such as myocarditis, pericarditis, and right ventricular dysfunction, in the absence of significant clinical symptoms, in athletes who have been affected by COVID-19 remain of considerable concern.
It also emphasizes the unique challenges the average cardiologist faces in distinguishing between what is normal for an athlete’s heart and what is true pathology after COVID-19 infection; details how different imaging modalities can help in screening, evaluating, and monitoring athletes with suspected cardiovascular complications of COVID-19 infection; and discusses the strengths and limitations of these modalities.
Finally, the consensus statement provides some well-needed guidance on return-to-play decision-making, for both the athlete and the clinician.
Athletic remodeling or covid-19 damage?
Athletes can develop certain cardiovascular characteristics because of their athletic activity, and sometimes, this can cloud the diagnostic picture.
“Is this change due to the effects of COVID-19, or is it just because this is an athlete’s heart? This was an international expert consensus, made up of sports cardiologists from all over the world who have a lot of experience in dealing with athletes,” Dr. Phelan said. “We were trying to relay the important information to the cardiologist who is not used to dealing with athletes on a day-to-day basis, as to what they might expect to find in that athlete, and what is not an expected finding and should be tested further.”
Phelan, a sports cardiologist, is familiar with what is normal for an athlete’s heart and what is pathology.
“We know that athletes, particularly long-term endurance athletes, develop changes in the heart that can affect not only the electrics but the structure of the heart, and sometimes, that overlaps with abnormalities with pathology. This can be a challenge for the nonsports cardiologist to differentiate,” he said.
Phelan and his group have written two other consensus documents on the management of cardiovascular problems that develop in some athletes who have been infected with COVID-19.
The first was published in May in JAMA Cardiology, and the second, which revised some of the original recommendations made in the first document, was published online Oct. 26 in JAMA Cardiology.
The first set of recommendations called for imaging studies to be done in all athletes, but the second set states that athletes who recover and are asymptomatic do not need extensive (and expensive) imaging tests.
“These two papers work hand in hand,” Dr. Phelan said. “In May, we had very little experience with COVID, and there was a lot of concern about hospitalized patients having a very high incidence of heart disease. We published those recommendations, but we recognized at the time that we had very little data and that we would reconsider once we had more experience with data.
“This current set of recommendations that we have put forth here are for those athletes who do need to get further testing, so it’s a step beyond,” Dr. Phelan added. “So the second iteration states that young athletes who had mild or no symptoms didn’t need to go through all of that cardiac testing, but others do need it.”
To do widespread cardiovascular imaging for many individuals would be very costly. Realistically, there are not that many centers in the United States that have all the sophisticated equipment required to do such testing, Dr. Phelan noted.
“One of our major points is difficulty obtaining the test, but also the cost; these are very expensive tests. There are limitations. They are useful when used in the correct context,” he said.
To play or not to play, that is the question
Partho P. Sengupta, MD, DM, had to answer that question for more than 50 young athletes who were returning to college at West Virginia University, anxious to be back with their teams and on the playing field. They had been infected with COVID-19 and needed to know when they could return to play.
Dr. Sengupta, who is also an author for the Phelan et al consensus statement on imaging, said there was a lot of pressure – from all the various stakeholders, and from anxious parents, worried college athletes, their teammates, and the university – to determine if the youngsters could return to play.
The fear was that COVID-19 infection left the young athlete’s heart vulnerable to myocarditis and, thus, sudden death on the playing field after strenuous activity.
“At the time we were doing this imaging, there was a lot of concern in the media, and papers were coming out reporting a lot of cardiac involvement or myocarditis associated with COVID-19. Nobody really knew what to do,” he explained.
“There were all kinds of questions, concerns. The parents were putting pressure on us, the athletes wanted to know, the teams, the university. So we put together a team and completed all of the examinations, including testing of blood markers, within a 2-week period. These young athletes, they’re scared, they’re worried and anxious, they don’t know what’s going to happen with their scholarship, so there was some urgency to this work,” Dr. Sengupta said.
“We had to screen all comers within a very short period. We had 54 consecutive patients, gave them full screening, full battery of tests, blood tests, all in a 2-week period,” he said.
Speed was of the essence, and Dr. Sengupta and his team rolled up their sleeves and got to work “We had to know who was safe to clear to return to play and who might need extra follow-up.”
Screening echocardiograms
They performed screening echocardiograms on 54 consecutive college athletes who had tested positive for COVID-19 on reverse transcription polymerase chain reaction nasal swab testing or who showed that they had IgG antibodies against COVID-19. The screening echocardiograms were done after the athletes had quarantined for at least 14 days and were no longer infectious.
Most (85%) were male, and the mean age was 19 years. A total of 16 (30%) athletes were asymptomatic, 36 (66%) reported mild COVID-19 related symptoms, and two (4%) reported moderate symptoms.
Of the 54 athletes who were initially screened with echocardiography, 48 (11 asymptomatic, 37 symptomatic), went on to have cardiac magnetic resonance imaging.
Results showed that more than half the athletes (27; 56.3%), showed some cardiac abnormality. The most common was pericardial late enhancement with associated pericardial effusion, affecting 19 (39.5%) athletes.
Of these, six (12.5%) had reduced global longitudinal strain (GLS) or an increased native T1.
One patient showed myocardial enhancement.
Additionally, seven athletes (14.6%) had reduced left ventricular ejection fraction or reduced GLS with or without increased native T1. Native T2 levels were normal in all subjects and no specific imaging features of myocardial inflammation were identified.
Participants were brought back to receive the results of their tests and to get an individualized plan about their safe return to play 3 to 5 weeks after they had ceased to be infectious with COVID-19.
“We saw pericardial inflammation that was resolving. We did not see any blood biomarkers to suggest that there was active inflammation going on,” he said. “We also did not see any muscle inflammation, but we did see pockets of fluid in over a third of our athletes.”
Fortunately, most were deemed able to get back to playing safely, despite having evidence of pericardial inflammation.
This was on strict condition that they be monitored very closely for any adverse events that might occur as they began to exercise again.
“Once they go back to the field to start exercising and practicing, it is under great supervision. We instructed all of our sports physicians and other team managers that these people need to be observed very carefully. So as long as they were asymptomatic, even though the signs of pericardial inflammation were there, if there were no signs of inflammation in the blood, we let them go back to play, closely monitored,” Dr. Sengupta said.
A small number remained very symptomatic at the end of the 5 weeks and were referred to cardiac rehabilitation, Dr. Sengupta said. “They were tired, fatigued, short of breath, even 5 weeks after they got over COVID, so we sent them for cardiac rehab to help them get conditioned again.”
The researchers plan to reevaluate and reimage all of the athletes in another 3 months to monitor their cardiac health.
Dr. Sengupta acknowledged the limitations of this single-center, nonrandomized, controlled report, but insists reports such as this add a bit more to what we are learning about COVID-19 every day.
“These kids were coming to us and asking questions. You have to use the best science you have available to you at that point in time. Some people ask why we did not have a control group, but how do you design a control population in the midst of a pandemic? The science may or may not be perfect, I agree, but the information we obtained is important,” he said.
“Right now, I don’t think we have enough science, and we are still learning. It is very difficult to predict who will develop the heart muscle disease or the pericardial disease,” Dr. Sengupta said. “We had to do our work quickly to give answers to the young athletes, their parents, their teammates, their university, as soon as possible, and we were doing this under pandemic conditions.”
The work was supported by the National Science Foundation National Institute of General Medical Sciences of the National Institutes of Health. Dr. Phelan reported no relevant financial relationships. Dr. Sengupta reported that he is a consultant for HeartSciences, Kencor Health, and Ultromics.
This article first appeared on Medscape.com.
AMA creates COVID-19 CPT codes for Pfizer, Moderna vaccines
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
Nearly 10% of hospitalized patients with COVID-19 later readmitted
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
About 1 in 11 patients discharged after COVID-19 treatment is readmitted to the same hospital, according to researchers from the Centers for Disease Control and Prevention (CDC).
Older age and chronic diseases are associated with increased risk, said senior author Adi V. Gundlapalli, MD, PhD, chief public health informatics officer of the CDC’s Center for Surveillance, Epidemiology, and Laboratory Services.
Gundlapalli and colleagues published the finding November 9 in Morbidity and Mortality Weekly Report.
To get a picture of readmission after COVID-19 hospitalization, the researchers analyzed records of 126,137 patients hospitalized with COVID-19 between March and July and included in the Premier Healthcare Database, which covers discharge records from 865 nongovernmental, community, and teaching hospitals.
Overall, 15% of the patients died during hospitalization. Of those who survived to discharge, 9% were readmitted to the same hospital within 2 months of discharge; 1.6% of patients were readmitted more than once. The median interval from discharge to first readmission was 8 days (interquartile range, 3-20 days). This short interval suggests that patients are probably not suffering a relapse, Gundlapalli said in an interview. More likely they experienced some adverse event, such as difficulty breathing, that led their caretakers to send them back to the hospital.
Forty-five percent of the primary discharge diagnoses after readmission were infectious and parasitic diseases, primarily COVID-19. The next most common were circulatory system symptoms (11%) and digestive symptoms (7%).
After controlling for covariates, the researchers found that patients were more likely to be readmitted if they had chronic obstructive pulmonary disease (odds ratio [OR], 1.4), heart failure (OR, 1.6), diabetes (OR, 1.2), or chronic kidney disease (OR, 1.6).
They also found increased odds among patients discharged from the index hospitalization to a skilled nursing facility (OR, 1.4) or with home health organization support (OR, 1.3), compared with being discharged to home or self-care. Looked at another way, the rate of readmission was 15% among those discharged to a skilled nursing facility, 12% among those needing home health care and 7% of those discharged to home or self-care.
The researchers also found that people who had been hospitalized within 3 months prior to the index hospitalization were 2.6 times more likely to be readmitted than were those without prior inpatient care.
Further, the odds of readmission increased significantly among people over 65 years of age, compared with people aged 18 to 39 years.
“The results are not surprising,” Gundlapalli said. “We have known from before that elderly patients, especially with chronic conditions, certain clinical conditions, and those who have been hospitalized before, are at risk for readmission.”
But admitting COVID-19 patients requires special planning because they must be isolated and because more personal protective equipment (PPE) is required, he pointed out.
One unexpected finding from the report is that non-Hispanic White people were more likely to be readmitted than were people of other racial or ethnic groups. This contrasts with other research showing Hispanic and Black individuals are more severely affected by COVID-19 than White people. More research is needed to explain this result, Gundlapalli said.
The authors have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Should our patients really go home for the holidays?
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].
As an East Coast transplant residing in Texas, I look forward to the annual sojourn home to celebrate the holidays with family and friends – as do many of our patients and their families. But this is 2020. SARS-CoV-2, the causative agent of COVID-19, is still circulating. To make matters worse, cases are rising in 45 states and internationally. The day of this writing 102,831 new cases were reported in the United States. 
Social distancing, wearing masks, and hand washing have been strategies recommended to help mitigate the spread of the virus. We know adherence is not always 100%. The reality is that several families will consider traveling and gathering with others over the holidays. Their actions may lead to increased infections, hospitalizations, and even deaths. It behooves us to at least remind them of the potential consequences of the activity, and if travel and/or holiday gatherings are inevitable, to provide some guidance to help them look at both the risks and benefits and offer strategies to minimize infection and spread.
What should be considered prior to travel?
Here is a list of points to ponder:
- Is your patient is in a high-risk group for developing severe disease or visiting someone who is in a high-risk group?
- What is their mode of transportation?
- What is their destination?
- How prevalent is the disease at their destination, compared with their community?
- What will be their accommodations?
- How will attendees prepare for the gathering, if at all?
- Will multiple families congregate after quarantining for 2 weeks or simply arrive?
- At the destination, will people wear masks and socially distance?
- Is an outdoor venue an option?
All of these questions should be considered by patients.
Review high-risk groups
In terms of high-risk groups, we usually focus on underlying medical conditions or extremes of age, but Black and LatinX children and their families have been diagnosed with COVID-19 and hospitalized more frequently than other racial/ ethnic groups in the United States. Of 277,285 school-aged children infected between March 1 and Sept. 19, 2020, 42% were LatinX, 32% White, and 17% Black, yet they comprise 18%, 60%, and 11% of the U.S. population, respectively. Of those hospitalized, 45% were LatinX, 22% White, and 24% Black. LatinX and Black children also have disproportionately higher mortality rates.
Think about transmission and how to mitigate it
Many patients erroneously think combining multiple households for small group gatherings is inconsequential. These types of gatherings serve as a continued source of SARS-CoV-2 spread. For example, a person in Illinois with mild upper respiratory infection symptoms attended a funeral; he reported embracing the family members after the funeral. He dined with two people the evening prior to the funeral, sharing the meal using common serving dishes. Four days later, he attended a birthday party with nine family members. Some of the family members with symptoms subsequently attended church, infecting another church attendee. A cluster of 16 cases of COVID-19 was subsequently identified, including three deaths likely resulting from this one introduction of COVID-19 at these two family gatherings.
In Tennessee and Wisconsin, household transmission of SARS-CoV-2 was studied prospectively. A total of 101 index cases and 191 asymptomatic household contacts were enrolled between April and Sept. 2020; 102 of 191 (53%) had SARS-CoV-2 detected during the 14-day follow-up. Most infections (75%) were identified within 5 days and occurred whether the index case was an adult or child.
Lastly, one adolescent was identified as the source for an outbreak at a family gathering where 15 persons from five households and four states shared a house between 8 and 25 days in July 2020. Six additional members visited the house. The index case had an exposure to COVID-19 and had a negative antigen test 4 days after exposure. She was asymptomatic when tested. She developed nasal congestion 2 days later, the same day she and her family departed for the gathering. A total of 11 household contacts developed confirmed, suspected, or probable COVID-19, and the teen developed symptoms. This report illustrates how easily SARS-CoV-2 is transmitted, and how when implemented, mitigation strategies work because none of the six who only visited the house was infected. It also serves as a reminder that antigen testing is indicated only for use within the first 5-12 days of onset of symptoms. In this case, the adolescent was asymptomatic when tested and had a false-negative test result.
Ponder modes of transportation
How will your patient arrive to their holiday destination? Nonstop travel by car with household members is probably the safest way. However, for many families, buses and trains are the only options, and social distancing may be challenging. Air travel is a must for others. Acquisition of COVID-19 during air travel appears to be low, but not absent based on how air enters and leaves the cabin. The challenge is socially distancing throughout the check in and boarding processes, as well as minimizing contact with common surfaces. There also is loss of social distancing once on board. Ideally, masks should be worn during the flight. Additionally, for those with international destinations, most countries now require a negative polymerase chain reaction COVID-19 test within a specified time frame for entry.
Essentially the safest place for your patients during the holidays is celebrating at home with their household contacts. The risk for disease acquisition increases with travel. You will not have the opportunity to discuss holiday plans with most parents. However, you can encourage them to consider the pros and cons of travel with reminders via telephone, e-mail, and /or social messaging directly from your practices similar to those sent for other medically necessary interventions. As for me, I will be celebrating virtually this year. There is a first time for everything.
For additional information that also is patient friendly, the Centers for Disease Control and Prevention offers information about travel within the United States and international travel.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures. Email her at [email protected].