User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Improving health care with simulation
QI is for clinicians too
Simulation is commonly used in the education and training of health care professionals, but more recently it’s entering the quality improvement world.
“Instead of just thinking about training individuals and teams, people are starting to use simulation to look at the physical layout of resuscitation bays, to map work flows of a patient journey through hospitals, to identify latent safety threats,” said Victoria Brazil, MD, MBA, lead author of a study on the subject in BMJ Quality & Safety. “These are great things to do, but many of the people doing it didn’t have quality improvement skills or knowledge – that’s why we wrote this article.”
Dr. Brazil, a specialist in health care simulation at Gold Coast (Australia) Hospital and Health Service, explained that, “in terms of the top takeaways, for quality improvement teams – and I’m including everyday clinicians in this: Think about simulation as one of the tools that can be utilized when looking at the questions of how we make our performance better, whether that’s a team performance, environmental, investigational impacts, or one of my key interests, whether that’s about exploring and shaping culture in hospitals, which we’ve done a lot of work on using simulation.”
Quality improvement has become a very specialized field, she added, so hospitalists may think it’s outside their purview. “As clinicians, we don’t think about ourselves as being engaged in quality improvement. I think that’s a shame, because many of the things that we can do bit by bit to make our patient outcomes better, we need to be thinking about finding better ways to do those things. I suggest simulation is one way, and that doesn’t need to be a massive simulation center. It can be simulating the kind of things that are important to you, your teams, and your patients and using those to both explore improved performance.”
Dr. Brazil said that Gold Coast Hospital has used simulation as a way of getting people from different departments and different professions together to shape culture through understanding shared knowledge and goals around patient journeys.
“That’s been pretty successful for us, and I think it’s really important that quality improvement has that understanding of context and culture as well as the idea of having specific interventions – maybe like a simulation – to try and improve an outcome,” she said.
Reference
1. Brazil V et al.. Connecting simulation and quality improvement: How can healthcare simulation really improve patient care? BMJ Qual Saf. 2019 Jul 18. doi: 10.1136/bmjqs-2019-009767.
QI is for clinicians too
QI is for clinicians too
Simulation is commonly used in the education and training of health care professionals, but more recently it’s entering the quality improvement world.
“Instead of just thinking about training individuals and teams, people are starting to use simulation to look at the physical layout of resuscitation bays, to map work flows of a patient journey through hospitals, to identify latent safety threats,” said Victoria Brazil, MD, MBA, lead author of a study on the subject in BMJ Quality & Safety. “These are great things to do, but many of the people doing it didn’t have quality improvement skills or knowledge – that’s why we wrote this article.”
Dr. Brazil, a specialist in health care simulation at Gold Coast (Australia) Hospital and Health Service, explained that, “in terms of the top takeaways, for quality improvement teams – and I’m including everyday clinicians in this: Think about simulation as one of the tools that can be utilized when looking at the questions of how we make our performance better, whether that’s a team performance, environmental, investigational impacts, or one of my key interests, whether that’s about exploring and shaping culture in hospitals, which we’ve done a lot of work on using simulation.”
Quality improvement has become a very specialized field, she added, so hospitalists may think it’s outside their purview. “As clinicians, we don’t think about ourselves as being engaged in quality improvement. I think that’s a shame, because many of the things that we can do bit by bit to make our patient outcomes better, we need to be thinking about finding better ways to do those things. I suggest simulation is one way, and that doesn’t need to be a massive simulation center. It can be simulating the kind of things that are important to you, your teams, and your patients and using those to both explore improved performance.”
Dr. Brazil said that Gold Coast Hospital has used simulation as a way of getting people from different departments and different professions together to shape culture through understanding shared knowledge and goals around patient journeys.
“That’s been pretty successful for us, and I think it’s really important that quality improvement has that understanding of context and culture as well as the idea of having specific interventions – maybe like a simulation – to try and improve an outcome,” she said.
Reference
1. Brazil V et al.. Connecting simulation and quality improvement: How can healthcare simulation really improve patient care? BMJ Qual Saf. 2019 Jul 18. doi: 10.1136/bmjqs-2019-009767.
Simulation is commonly used in the education and training of health care professionals, but more recently it’s entering the quality improvement world.
“Instead of just thinking about training individuals and teams, people are starting to use simulation to look at the physical layout of resuscitation bays, to map work flows of a patient journey through hospitals, to identify latent safety threats,” said Victoria Brazil, MD, MBA, lead author of a study on the subject in BMJ Quality & Safety. “These are great things to do, but many of the people doing it didn’t have quality improvement skills or knowledge – that’s why we wrote this article.”
Dr. Brazil, a specialist in health care simulation at Gold Coast (Australia) Hospital and Health Service, explained that, “in terms of the top takeaways, for quality improvement teams – and I’m including everyday clinicians in this: Think about simulation as one of the tools that can be utilized when looking at the questions of how we make our performance better, whether that’s a team performance, environmental, investigational impacts, or one of my key interests, whether that’s about exploring and shaping culture in hospitals, which we’ve done a lot of work on using simulation.”
Quality improvement has become a very specialized field, she added, so hospitalists may think it’s outside their purview. “As clinicians, we don’t think about ourselves as being engaged in quality improvement. I think that’s a shame, because many of the things that we can do bit by bit to make our patient outcomes better, we need to be thinking about finding better ways to do those things. I suggest simulation is one way, and that doesn’t need to be a massive simulation center. It can be simulating the kind of things that are important to you, your teams, and your patients and using those to both explore improved performance.”
Dr. Brazil said that Gold Coast Hospital has used simulation as a way of getting people from different departments and different professions together to shape culture through understanding shared knowledge and goals around patient journeys.
“That’s been pretty successful for us, and I think it’s really important that quality improvement has that understanding of context and culture as well as the idea of having specific interventions – maybe like a simulation – to try and improve an outcome,” she said.
Reference
1. Brazil V et al.. Connecting simulation and quality improvement: How can healthcare simulation really improve patient care? BMJ Qual Saf. 2019 Jul 18. doi: 10.1136/bmjqs-2019-009767.
Echoes of SARS mark 2019 novel coronavirus outbreak
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
The current outbreak of severe respiratory infections caused by the 2019 novel coronarvirus (2019-nCoV) has a clinical presentation resembling the Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) outbreak that began in 2002, Chinese investigators caution.

By Jan. 2, 2020, 41 patients with confirmed 2019-nCoV had been admitted to a designated hospital in the city of Wuhan, Hubei Province, in central China. Thirteen required ICU admission and six died, reported Chaolin Huang, MD, from Jin Yin-tan Hospital in Wuhan, and colleagues.
“2019-nCoV still needs to be studied deeply in case it becomes a global health threat. Reliable quick pathogen tests and feasible differential diagnosis based on clinical description are crucial for clinicians in their first contact with suspected patients. Because of the pandemic potential of 2019-nCoV, careful surveillance is essential to monitor its future host adaption, viral evolution, infectivity, transmissibility, and pathogenicity,” they wrote in a review published online by The Lancet.
According to the U.S. Centers for Disease Control and Prevention, as of Jan. 28, 2020, the total number of 2019-nCoV cases reported in the United States stood at five, but further cases of the infection – which Chinese health officials have confirmed can be transmitted person-to-person – are expected.
Dr. Huang and colleagues note that although most human coronavirus infections are mild, SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) were responsible for more than 10,000 infections, with mortality rates ranging from 10% with SARS to 37% with MERS. To date, 2019-nCoV has “caused clusters of fatal pneumonia greatly resembling SARS-CoV,” they write.
The authors studied the epidemiological, clinical, laboratory, and radiological characteristics as well as treatments and clinical outcomes of 41 patients admitted or transferred to the Jin Yin-tan Hospital with laboratory-confirmed 2019-nCoV infections.
The median patient age was 49 years. Thirty of the 41 patients (73%) were male. Comorbid conditions included diabetes in 13 of the 41 patients (32%), hypertension in 6 (15%), and cardiovascular disease in 6.
In all 27 of the 41 patients had been exposed to the Huanan seafood market in Wuhan, the suspected epicenter of the outbreak that was shut down by health authorities on Jan. 1 of this year.
The most common symptoms at the onset of the illness were fever in all but one of the 41 patients, cough in 31, and myalgia or fatigue in 18. Other, less frequent symptoms included sputum production in 11, headache in three, hemoptysis in two, and diarrhea in one.
“In this cohort, most patients presented with fever, dry cough, dyspnoea, and bilateral ground-glass opacities on chest CT scans. These features of 2019-nCoV infection bear some resemblance to SARS-CoV and MERS-CoV infections. However, few patients with 2019-nCoV infection had prominent upper respiratory tract signs and symptoms (e.g., rhinorrhoea, sneezing, or sore throat), indicating that the target cells might be located in the lower airway. Furthermore, 2019-nCoV patients rarely developed intestinal signs and symptoms (e.g., diarrhoea), whereas about 20%-25% of patients with MERS-CoV or SARS-CoV infection had diarrhoea.”
In all, 22 patients developed dyspnea, with a median time from illness onset to dyspnea of 8 days. The median time from illness onset to admission was 7 days, median time to shortness of breath was 8 days, median time to acute respiratory distress syndrome (ARDS) was 9 days, and median time to both mechanical ventilation and ICU admission was 10.5 days.
All of the patients developed pneumonia with abnormal findings on chest CT scan. In addition, 12 patients developed ARDS, six had RNAaemia, five developed acute cardiac injury, and four developed a secondary infection. As noted before, 13 of the 14 patients were admitted to an ICU, and six died. RNAaemia is a positive result for real-time polymerase chain reaction in plasma samples. Patients admitted to the ICU had higher initial concentrations of multiple inflammatory cytokines than patients who did not need ICU care, “suggesting that the cytokine storm was associated with disease severity.”
All of the patients received empirical antibiotics, 38 were treated with oseltamivir (Tamiflu), and 9 received systemic corticosteroids.
The investigators have initiated a randomized controlled trial of the antiviral agents lopinavir and ritonavir for patients hospitalized with 2019-nCoV infection.
The study was funded by the Chinese Ministry of Science and Technology, Chinese Academy of Medical Sciences, National Natural Science Foundation of China, and Beijing Municipal Science and Technology Commission. All authors declared having no competing interests.
SOURCE: Huang C et al. Lancet. 2020 Jan 24. doi: 10.1016/S0140-6736(20)30183-5.
FROM THE LANCET
CDC: Five confirmed 2019-nCoV cases in the U.S.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Five cases of the new infectious coronavirus, 2019-nCoV, have been confirmed in the United States, Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, said during a Jan. 27 press briefing.
A total of 110 individuals are under investigation in 26 states, she said. While five cases have been confirmed positive for the virus, 32 cases were confirmed negative. There have been no new cases overnight.
Last week, CDC scientists developed a real-time polymerase chain reaction (PCR) test that can diagnose the virus in respiratory and serum samples from clinical specimens. On Jan. 24, the protocol for this test was publicly posted. “This is essentially a blueprint to make the test,” Dr. Messonnier explained. “Currently, we are refining the use of the test so that it can provide optimal guidance to states and labs on how to use it. We are working on a plan so that priority states get these test kits as soon as possible. In the coming weeks, we will share these tests with domestic and international partners so they can test for this virus themselves.”
The CDC uploaded the entire genome of the virus from the first two cases in the United States to GenBank. It was similar to the one that China had previously posted. “Right now, based on CDC’s analysis of the available data, it doesn’t look like the virus has mutated,” she said. “And we are growing the virus in cell culture, which is necessary for further studies, including the additional genetic characterization.”
As of today, 16 international locations, including the United States, have identified cases of the virus. CDC officials are continuing to screen passengers from Wuhan, China, at five designated airports. “This serves two purposes: first to detect the illness and rapidly respond to [affected] people entering the country,” Dr. Messonnier said. “The second purpose is to educate travelers about the symptoms of this new virus, and what to do if they develop symptoms. I expect that in the coming days, our travel recommendations will change. Risk depends on exposure. Right now, we have an handful of new patients with this new virus here in the U.S. However, at this time in the U.S., this virus is not spreading in the community. For that reason, we believe that the immediate health risk of the new virus to the general American public is low.”
The CDC is asking its clinical lab partners to send virus samples to the CDC to ensure that results are analyzed as accurately as possible.
Zika virus: Birth defects rose fourfold in U.S. hardest-hit areas
according to the Centers for Disease Control and Prevention.
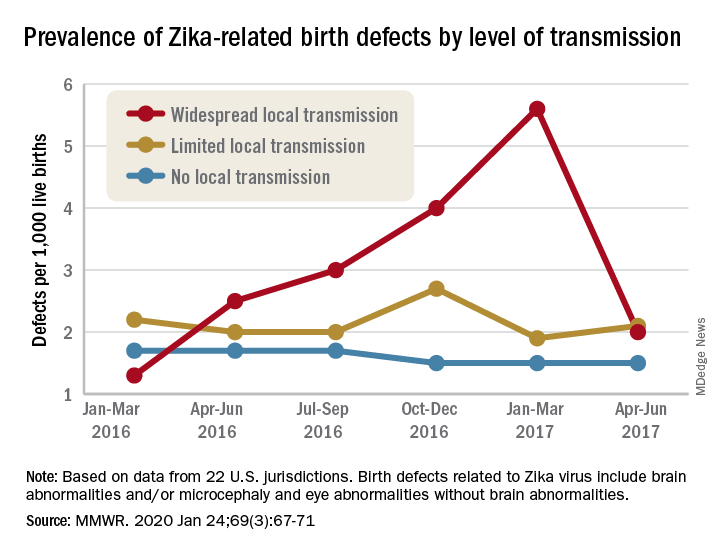
That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
FROM MMWR
IBD: Inpatient opioids linked with outpatient use
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
, based on a retrospective analysis of more than 800 patients.
Awareness of this dose-dependent relationship and IBD-related risks of opioid use should encourage physicians to consider alternative analgesics, according to lead author Rahul S. Dalal, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
“Recent evidence has demonstrated that opioid use is associated with severe infections and increased mortality among IBD patients,” the investigators wrote in Clinical Gastroenterology and Hepatology. “Despite these concerns, opioids are commonly prescribed to IBD patients in the outpatient setting and to as many as 70% of IBD patients who are hospitalized.”
To look for a possible relationship between inpatient and outpatient opioid use, the investigators reviewed electronic medical records of 862 IBD patients who were treated at three urban hospitals in the University of Pennsylvania Health System. The primary outcome was opioid prescription within 12 months of discharge, including prescriptions at time of hospital dismissal.
During hospitalization, about two-thirds (67.6%) of patients received intravenous opioids. Of the total population, slightly more than half (54.6%) received intravenous hydromorphone and about one-quarter (25.9%) received intravenous morphine. Following discharge, almost half of the population (44.7%) was prescribed opioids, and about 3 out of 4 patients (77.9%) received an additional opioid prescription within the same year.
After accounting for confounders such as IBD severity, preadmission opioid use, pain scores, and psychiatric conditions, data analysis showed that inpatients who received intravenous opioids had a threefold (odds ratio [OR], 3.3) increased likelihood of receiving postdischarge opioid prescription, compared with patients who received no opioids while hospitalized. This association was stronger among those who had IBD flares (OR, 5.4). Furthermore, intravenous dose was positively correlated with postdischarge opioid prescription.
Avoiding intravenous opioids had no impact on the relationship between inpatient and outpatient opioid use. Among inpatients who received only oral or transdermal opioids, a similarly increased likelihood of postdischarge opioid prescription was observed (OR, 4.2), although this was a small cohort (n = 67).
Compared with other physicians, gastroenterologists were the least likely to prescribe opioids. Considering that gastroenterologists were also most likely aware of IBD-related risks of opioid use, the investigators concluded that more interdisciplinary communication and education are needed.
“Alternative analgesics such as acetaminophen, dicyclomine, hyoscyamine, and celecoxib could be advised, as many of these therapies have been deemed relatively safe and effective in this population,” they wrote.The investigators disclosed relationships with Abbott, Gilead, Romark, and others.
SOURCE: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Patients with inflammatory bowel disease (IBD) who receive opioids while hospitalized are three times as likely to be prescribed opioids after discharge.
Major finding: Patients who were given intravenous opioids while hospitalized were three times as likely to receive a postdischarge opioid prescription, compared with patients who did not receive inpatient intravenous opioids (odds ratio, 3.3).
Study details: A retrospective cohort study involving 862 patients with inflammatory bowel disease.
Disclosures: The investigators disclosed relationships Abbott, Gilead, Romark, and others.
Source: Dalal RS et al. Clin Gastro Hepatol. 2019 Dec 27. doi: 10.1016/j.cgh.2019.12.024.
Wuhan virus: What clinicians need to know
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
EVALI update warns of chemicals in vaping products
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
A report issued by the Centers for Disease Control and Prevention confirms that 82% of patients presenting with e-cigarette– or vaping product use–associated lung injury (EVALI) used products containing tetrahydrocannabinol (THC).
Another report published in the CDC’s Morbidity and Mortality Weekly Report assessed cases in which the patients reported using only nicotine-containing vaping products.
“As of Jan. 14, 2020, a total of 2,668 hospitalized EVALI cases had been reported to CDC,” based on data from the National Syndromic Surveillance Program (NSSP), wrote Vikram P. Krishnasamy, MD, of the National Center for Injury Prevention and Control at the CDC, Atlanta, and colleagues. Cases have occurred in all 50 states, the District of Columbia, the U.S. Virgin Islands, and Puerto Rico. The age of the patients ranged from 13 to 85 years, with an average age of 24 years; 66% were male, and 73% were non-Hispanic white.
In addition, 57% of the patients reported using any nicotine-containing product, and 14% of these reported use of nicotine products exclusively.
Previous studies have shown that vitamin E acetate is associated with the EVALI outbreak, which peaked during the week of Sept. 15, 2019, with 215 reported hospital admissions, Dr. Krishnasamy and associates noted. “However, evidence is not sufficient to rule out the contribution of other chemicals of concern, including chemicals in either THC- or non-THC–containing products, in some reported EVALI cases,” they said.
The study findings were limited by several factors, including incomplete data on product use, increased reporting of vaping product use at emergency department visits after increased public awareness of risk, and inconsistency in the health care facilities contributing data via the NSSP, the researchers wrote.
The decline in EVALI cases since September 2019 may be related to factors including the rapid public health response to increase awareness of the risks of vaping, and the possible removal of vitamin E acetate as a diluent in THC-containing products, but clinicians and public health professionals should remain on alert for new EVALI cases and continue to discourage the use of THC-containing e-cigarette or vaping products, Dr. Krishnasamy and associates concluded.
Nicotine-only vaping products
In a second report published in MMWR, Isaac Ghinai, MBBS, of the Illinois Department of Public Health and CDC researchers examined characteristics of EVALI patients in Illinois who reported using only nicotine-containing vaping products.
A total of 9 of 121 (7%) EVALI patients surveyed in Illinois reported no indication of THC use. These patients were more likely than those who reported any use of THC-containing products to be female (78% vs. 25%) and aged 45 years and older (33% vs. 2%); P less than .01 in both cases.
In addition, EVALI patients with no indication of THC-containing product use were less likely than THC product users to present with constitutional symptoms (56% vs. 96%) or initial leukocytosis (38% vs. 91%), or to have previously visited an outpatient provider or ED before being hospitalized (25% vs. 80%).
Other presenting characteristics including initial vital signs and lab results, as well as the frequency of severe outcomes such as death or respiratory failure, were not significantly different between users and nonusers of THC-containing vaping products.
The study findings were limited by factors including the use of self-reports, the small sample size, and lack of initial and follow-up interviews for all EVALI patients, the researchers noted. However, the results support the CDC’s recommendation that “persons should not use THC-containing e-cigarette, or vaping, products, particularly those obtained from informal sources such as friends, family members, or from in-person or online dealers,” and should not add vitamin E acetate or other substances to these products, they said.
In addition, users of nicotine-containing e-cigarette or vaping products as an alternative to cigarettes should not return to cigarettes, but should explore other options to help them quit, Dr. Ghinai, and associates said.
The studies were supported by the CDC. The researchers in both studies had no financial conflicts to disclose.
SOURCES: Krishnasamy VP et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e2; Ghinai I et al. MMWR Morb Mortal Wkly Rep. 17 Jan 2020. doi: 10.15585/mmwr.mm6903e1.
FROM MMWR
Hospitalist movers and shakers – January 2020
Hyung (Harry) Cho, MD, SFHM, and Christopher Moriates, MD, SFHM, have been honored by Modern Healthcare as two of 25 emerging young executives in health care management.
Dr. Cho is chief value officer for NYC Health and Hospitals, where his focus is on eliminating unnecessary testing and treatments within the New York City public health system, which includes 11 hospitals and five post-acute care facilities. Before landing with NYC Health and Hospitals, Dr. Cho was director of quality, safety and value at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Moriates is assistant dean for health care and value at the University of Texas at Austin’s Dell Medical School, where he has created the Discovering Value-Based Health Care online learning platform. In addition, Dr. Moriates has helped design a care model to enhance the treatment of patients who suffer from opioid use disorder. Prior to arriving at Dell, he helped create curriculum to educate students about costs and value at the University of California, San Francisco.
Trina Abla, DO, was appointed chief medical officer at Mercy Catholic Medical Center in Darby, Pa. A practicing hospitalist, Dr. Abla will be in charge of the hospital budget, the recruiting and training of physicians, and maintaining safety standards and quality care at the facility.
Prior to taking the position at Mercy Catholic, Dr. Abla was chief quality officer and associate CMO at Penn State Health St. Joseph in Reading, Pa.
Ghania El Akiki, MD, has been named to the board of advisors at Beth Israel Deaconess Hospital in Needham, Mass. Dr. Akiki is chief of hospitalist services at Beth Israel Deaconess, landing there after a fellowship in geriatrics at Beth Israel Deaconess Medical Center.
Dr. Akiki completed a physician leadership program at BID Medical Center in 2018, and serves as instructor of medicine at Harvard Medical School, Boston.
Michael Schandorf-Lartey, MD, has been named the chief medical officer at Doctors Hospital in Sarasota, Fla. Dr. Schandorf-Lartey has been a hospitalist at Doctors Hospital for the past 12 years.
In his time at Doctors, Dr. Schandorf-Lartey also has been chief of medicine, president-elect, and president of the medical staff. A native of Ghana, he has had experience working in rural and urban hospitals in Africa before coming to the United States.
Michael Roberts, MD, was named chief of staff at East Alabama Medical Center in Opeleika, Ala. He has been part of EAMC since 2008, when he became a hospitalist there through Internal Medicine Associates.
As chief of staff, Dr. Roberts will work with different components of the medical staff and serve as a liaison between the hospital board and its staff; assist in developing policies alongside the chief medical officer; and serve on many of the medical staff’s committees.
Brian Dawson, MD, has been named chief medical officer for Ballad Health, Southwest Region, based in Johnson City, Tenn. Dr. Dawson will lead Ballad Health locations in Washington County, which include Franklin Woods Community Hospital, Johnson City Medical Center, Niswonger Children Hospital, and Woodridge Hospital.
Dr. Dawson comes to Ballad Health after serving as vice president at VEP Healthcare, where he focused on contract management for the emergency medicine and hospitalist firm. Previously, he was chief of staff and Northeast regional director for emergency medicine at Johnston Memorial Hospital, Abington, Va.
Eagle Telemedicine (Atlanta, Ga.) recently agreed to begin a telehospitalist program at Jersey Community Hospital in Jerseyville, Ill. Eagle Telemedicine offers telehospitalist services to more than 150 hospitals nationwide.
A rural facility with fewer than 50 beds, JCH will use Eagle to make up for the lack of a full-time, onsite hospitalist program, taking strain off of physicians handling emergency calls. At JCH, telehospitalists work closely with onsite nurse practitioners to guide patients through their hospital stay.
Hyung (Harry) Cho, MD, SFHM, and Christopher Moriates, MD, SFHM, have been honored by Modern Healthcare as two of 25 emerging young executives in health care management.
Dr. Cho is chief value officer for NYC Health and Hospitals, where his focus is on eliminating unnecessary testing and treatments within the New York City public health system, which includes 11 hospitals and five post-acute care facilities. Before landing with NYC Health and Hospitals, Dr. Cho was director of quality, safety and value at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Moriates is assistant dean for health care and value at the University of Texas at Austin’s Dell Medical School, where he has created the Discovering Value-Based Health Care online learning platform. In addition, Dr. Moriates has helped design a care model to enhance the treatment of patients who suffer from opioid use disorder. Prior to arriving at Dell, he helped create curriculum to educate students about costs and value at the University of California, San Francisco.
Trina Abla, DO, was appointed chief medical officer at Mercy Catholic Medical Center in Darby, Pa. A practicing hospitalist, Dr. Abla will be in charge of the hospital budget, the recruiting and training of physicians, and maintaining safety standards and quality care at the facility.
Prior to taking the position at Mercy Catholic, Dr. Abla was chief quality officer and associate CMO at Penn State Health St. Joseph in Reading, Pa.
Ghania El Akiki, MD, has been named to the board of advisors at Beth Israel Deaconess Hospital in Needham, Mass. Dr. Akiki is chief of hospitalist services at Beth Israel Deaconess, landing there after a fellowship in geriatrics at Beth Israel Deaconess Medical Center.
Dr. Akiki completed a physician leadership program at BID Medical Center in 2018, and serves as instructor of medicine at Harvard Medical School, Boston.
Michael Schandorf-Lartey, MD, has been named the chief medical officer at Doctors Hospital in Sarasota, Fla. Dr. Schandorf-Lartey has been a hospitalist at Doctors Hospital for the past 12 years.
In his time at Doctors, Dr. Schandorf-Lartey also has been chief of medicine, president-elect, and president of the medical staff. A native of Ghana, he has had experience working in rural and urban hospitals in Africa before coming to the United States.
Michael Roberts, MD, was named chief of staff at East Alabama Medical Center in Opeleika, Ala. He has been part of EAMC since 2008, when he became a hospitalist there through Internal Medicine Associates.
As chief of staff, Dr. Roberts will work with different components of the medical staff and serve as a liaison between the hospital board and its staff; assist in developing policies alongside the chief medical officer; and serve on many of the medical staff’s committees.
Brian Dawson, MD, has been named chief medical officer for Ballad Health, Southwest Region, based in Johnson City, Tenn. Dr. Dawson will lead Ballad Health locations in Washington County, which include Franklin Woods Community Hospital, Johnson City Medical Center, Niswonger Children Hospital, and Woodridge Hospital.
Dr. Dawson comes to Ballad Health after serving as vice president at VEP Healthcare, where he focused on contract management for the emergency medicine and hospitalist firm. Previously, he was chief of staff and Northeast regional director for emergency medicine at Johnston Memorial Hospital, Abington, Va.
Eagle Telemedicine (Atlanta, Ga.) recently agreed to begin a telehospitalist program at Jersey Community Hospital in Jerseyville, Ill. Eagle Telemedicine offers telehospitalist services to more than 150 hospitals nationwide.
A rural facility with fewer than 50 beds, JCH will use Eagle to make up for the lack of a full-time, onsite hospitalist program, taking strain off of physicians handling emergency calls. At JCH, telehospitalists work closely with onsite nurse practitioners to guide patients through their hospital stay.
Hyung (Harry) Cho, MD, SFHM, and Christopher Moriates, MD, SFHM, have been honored by Modern Healthcare as two of 25 emerging young executives in health care management.
Dr. Cho is chief value officer for NYC Health and Hospitals, where his focus is on eliminating unnecessary testing and treatments within the New York City public health system, which includes 11 hospitals and five post-acute care facilities. Before landing with NYC Health and Hospitals, Dr. Cho was director of quality, safety and value at the Icahn School of Medicine at Mount Sinai, New York.
Dr. Moriates is assistant dean for health care and value at the University of Texas at Austin’s Dell Medical School, where he has created the Discovering Value-Based Health Care online learning platform. In addition, Dr. Moriates has helped design a care model to enhance the treatment of patients who suffer from opioid use disorder. Prior to arriving at Dell, he helped create curriculum to educate students about costs and value at the University of California, San Francisco.
Trina Abla, DO, was appointed chief medical officer at Mercy Catholic Medical Center in Darby, Pa. A practicing hospitalist, Dr. Abla will be in charge of the hospital budget, the recruiting and training of physicians, and maintaining safety standards and quality care at the facility.
Prior to taking the position at Mercy Catholic, Dr. Abla was chief quality officer and associate CMO at Penn State Health St. Joseph in Reading, Pa.
Ghania El Akiki, MD, has been named to the board of advisors at Beth Israel Deaconess Hospital in Needham, Mass. Dr. Akiki is chief of hospitalist services at Beth Israel Deaconess, landing there after a fellowship in geriatrics at Beth Israel Deaconess Medical Center.
Dr. Akiki completed a physician leadership program at BID Medical Center in 2018, and serves as instructor of medicine at Harvard Medical School, Boston.
Michael Schandorf-Lartey, MD, has been named the chief medical officer at Doctors Hospital in Sarasota, Fla. Dr. Schandorf-Lartey has been a hospitalist at Doctors Hospital for the past 12 years.
In his time at Doctors, Dr. Schandorf-Lartey also has been chief of medicine, president-elect, and president of the medical staff. A native of Ghana, he has had experience working in rural and urban hospitals in Africa before coming to the United States.
Michael Roberts, MD, was named chief of staff at East Alabama Medical Center in Opeleika, Ala. He has been part of EAMC since 2008, when he became a hospitalist there through Internal Medicine Associates.
As chief of staff, Dr. Roberts will work with different components of the medical staff and serve as a liaison between the hospital board and its staff; assist in developing policies alongside the chief medical officer; and serve on many of the medical staff’s committees.
Brian Dawson, MD, has been named chief medical officer for Ballad Health, Southwest Region, based in Johnson City, Tenn. Dr. Dawson will lead Ballad Health locations in Washington County, which include Franklin Woods Community Hospital, Johnson City Medical Center, Niswonger Children Hospital, and Woodridge Hospital.
Dr. Dawson comes to Ballad Health after serving as vice president at VEP Healthcare, where he focused on contract management for the emergency medicine and hospitalist firm. Previously, he was chief of staff and Northeast regional director for emergency medicine at Johnston Memorial Hospital, Abington, Va.
Eagle Telemedicine (Atlanta, Ga.) recently agreed to begin a telehospitalist program at Jersey Community Hospital in Jerseyville, Ill. Eagle Telemedicine offers telehospitalist services to more than 150 hospitals nationwide.
A rural facility with fewer than 50 beds, JCH will use Eagle to make up for the lack of a full-time, onsite hospitalist program, taking strain off of physicians handling emergency calls. At JCH, telehospitalists work closely with onsite nurse practitioners to guide patients through their hospital stay.
Second U.S. coronavirus patient confirmed
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
at a Jan. 24, 2020, press briefing.
The first U.S. case, a traveler who entered the United States at Seattle-Tacoma International Airport, was confirmed on Jan. 20.
A Chicago resident returning from Wuhan, China, on Jan. 13, 2020, developed symptoms of the disease and contacted her health care clinician and is currently being treated in isolation at an unnamed hospital, according to Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases at the CDC. The patient, a woman in her 60s, is in stable condition and remains hospitalized. She was not symptomatic on her flight to Chicago but developed symptoms in the following days after her return from Wuhan. She had limited contacts after her return, and all potential contacts are being tracked.
Dr. Messonnier said the CDC expects more cases in the United States but stressed that, although this is a serious public health threat, the risk to the American public is low. She noted that the situation is evolving rapidly and that the CDC is following the developments hour by hour.
Jennifer Layden, MD, PhD, chief medical officer and state epidemiologist with the Illinois Department of Public Health, said public health preparations made it possible to quickly identify and arrange appropriate hospitalization for this patient. Allison Arwady, MD, Chicago Department of Health commissioner, said the Illinois Department of Health partnered with the CDC to test specimens quickly, which led to the diagnosis in this patient.
So far, 63 U.S. patients have been investigated for possible infection with the 2019-nCoV; 11 so far have tested negative and 2 have tested positive. Testing of the remaining potential cases and others is ongoing.
Currently, samples from patients with suspected 2010-nCoV infections are being sent to the CDC for testing, Dr. Messonnier said. The turnaround for testing is currently 4-6 hours. Respiratory samples and some blood samples are being tested by the CDC labs.
The CDC is developing diagnostic kits for public health authorities in the United States for local testing and will work with the World Health Organization to make these kits available to the international community when possible.
Dr. Messonnier said that, at present, the incubation period for this disease appears to be about 14 days, but she suggested that further study will be required to identify the range of time for contagion. She also said it is premature to compare the 2019-nCoV with previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) or Middle East respiratory syndrome (MERS), in terms of contagion or fatality rates.
Meanwhile, Andrew D. Mesecar, PhD, the Walther Professor in Cancer Structural Biology and head of the department of biochemistry at Purdue University, West Lafayette, Ind., said on Jan. 24 in a news release that 2019-nCoV is genetically similar to the SARS variant. “MERS virus and the SARS virus are more different genetically,” noted Dr. Mesecar, whose team received the genome of 2019-nCoV on Jan. 17 and analyzed it the next day. “But the Wuhan virus is genetically almost identical to the SARS virus and, therefore, it is expected to look and act nearly the same. In another week or two, we’ll be able to begin to see if the virus is mutating.”
Dr. Messonnier said that nonessential travel to Wuhan is not recommended. In addition, she said, and all other visitors to China need to take appropriate precautions, such as handwashing and avoiding other individuals with respiratory illness.
Screenings at five U.S. airports will continue. So far, approximately 200 flights and 2,000 travelers have been screened as of Jan. 23. No cases were reported, but one traveler has been identified for further for evaluation. Possible contacts with those suspected of infection have been identified and alerted in 22 states.
The CDC will continue to update the public and will post information on the CDC newsroom website.
Suicide rate higher than average for female clinicians
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.
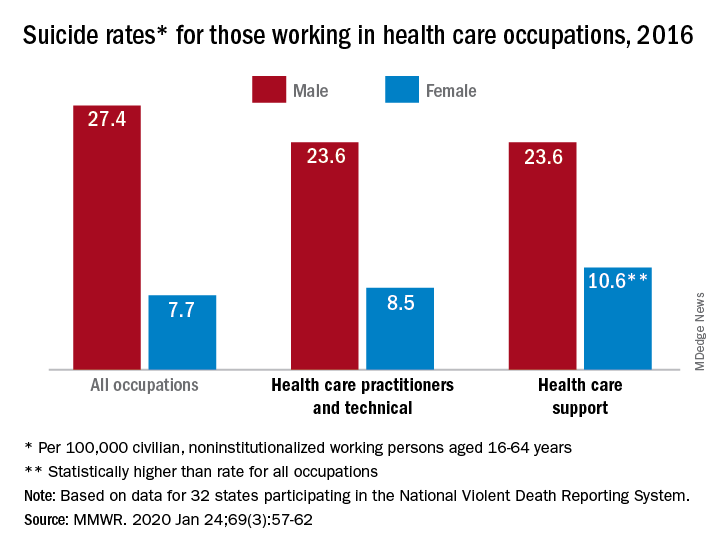
In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.

In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
The suicide rate for women who provide health care is higher than that of all women of working age, while male health care practitioners are less likely to end their lives than working-age men as a whole, according to the Centers for Disease Control and Prevention.

In 2016, the suicide rate for women classified as “healthcare practitioners and technical” – a category that includes physicians and surgeons, as well as chiropractors, physician assistants, and nurse practitioners – was 8.5 per 100,000 population, compared with 7.7 per 100,000 for all working women aged 16-64 years. That difference, however, was not statistically significant, Cora Peterson, PhD, and associates at the CDC said in the Morbidity and Mortality Weekly Report.
For females classified as “healthcare support” – medical assistants and transcriptionists, phlebotomists, and pharmacy aides – the suicide rate of 10.6 per 100,000 was significantly higher than that of all working women, the investigators noted.
The suicide rate for males in each of the two occupation categories was 23.6 per 100,000 population in 2016, lower than the rate of 27.4 per 100,000 for males of all occupations, they said, based on data from 32 states that participated in the 2016 National Violent Death Reporting System.
For males, the highest suicide rates in occupations meeting criteria for sample size were “construction and extraction” (49.4 per 100,000); “installation, maintenance, and repair” (36.9); and “arts, design, entertainment, sports, and media” (32.0). Among females, the highest rates were seen in “construction and extraction” (25.5 per 100,000), “protective service” (14.0), and “transportation and material moving” (12.5), with healthcare support next, Dr. Peterson and associates reported.
“Although relative comparisons of suicide rates in this manner are useful for prevention purposes, Therefore, all industry sectors and occupational groups can contribute to reducing suicide incidence,” they wrote.
SOURCE: Peterson C et al. MMWR. 2020 Jan 24;69(3):57-62.
FROM MMWR